Triamterene_396-01-0_DataSheet_MedChemExpress
艾森生物 实验室耗材和试剂 目录t
21127-030
P0018 31458
20W 500ml 500ml 100ml PL009 PL017 353002 3516
11644807001
2227S/100ul 3175S/100ul 7002s/100ul 7004s/100ul 9803s/15ml G7126-1KG
胎牛血清FBS MEM
FX 384 30ul 枪头
Horse serum马血清
DMSO
PMSF
25ml移液管
DMEM 细胞筛网 70um
细胞培养皿 65×15mm
384孔板
真空抽气泵
BSA牛血清蛋白
EGF Receptor (D38B1) XP® Rabbit mAb
western 一抗二抗去除液(弱碱性)
96孔细胞培养板 细胞网筛
25ml移液管 96孔板
无酚红1640 0.1-20ul排枪枪头 10-250ul排枪枪头 1ml盒装蓝色枪头 Trypsin EDTA,0.05%
DMSO 医用酒精棉球
医用酒精棉 麻面无粉手套 1ml灭菌盒装蓝枪头
DMEM L-15 384 HT assay plates
P3563-10PAK
PL017
SM0671/2x250ul
PL009 4685s/100ul 9271s/100ul
9101s/200ul
9102s/200ul 7002s/100ul 7004s/100ul 9803s/15ml
3777s/100ul
21127-022
11644807001 11966-025 6570 SD6031 华东 CU50
Gibco 华东 华东 Roche Gibco CORNING 上海生工 M号 中新 迪申 sigma AXYGEN Gibco
碧云天生物技术产品说明书.pdf_1694034956.947723
碧云天生物技术/Beyotime Biotechnology订货热线:400-168-3301或800-8283301订货e-mail:******************技术咨询:*****************网址:碧云天网站微信公众号Cyclodextrin Glucanotransferase (Crude Enzyme)产品编号产品名称包装P3618-100ml Cyclodextrin Glucanotransferase (Crude Enzyme) 100mlP3618-1L Cyclodextrin Glucanotransferase (Crude Enzyme) 1L产品简介:Cyclodextrin Glucanotransferase,中文名为环糊精糖基转移酶,催化淀粉转移葡萄糖基生成环糊精。
本产品为大肠杆菌表达的环糊精糖基转移酶,并经简单纯化获得的可以确保达到一定酶活力的粗酶液。
由于本产品需新鲜制备,确认订购后约6个工作日才可以发货。
个别情况首次制备出现问题,发货时间会顺延。
本产品可以提供十公斤、百公斤乃至吨级的粗酶液,如有需求请联系碧云天。
In enzymology, a cyclomaltodextrin glucanotransferase is an enzyme that catalyzes the chemical reaction of cyclizing part of a 1,4-alpha-D-glucan molecule through the formation of a 1,4-alpha-D-glucosidic bond.This enzyme belongs to the family of glycosyltransferases, specifically the hexosyltransferases.This product with the indicated enzyme activity was briefly purified from engineered E.coli.产品详细信息见下表:About this productName Cyclodextrin Glucanotransferase (Crude Enzyme);环糊精糖基转移酶(粗酶液)Application 催化淀粉转移葡萄糖基生成环糊精Appearance Clear to translucent yellow solutionEnzyme activity UndeterminedAbout this enzymeName Cyclodextrin Glucanotransferase;环糊精糖基转移酶CAS Number 9030-09-5EC Number 2.4.1.19Enzymatic Reaction Cyclizes part of a (1→4)-α-D-glucan chain by formation of a (1→4)-α-D-glucosidic bondSynonyms Bacillus macerans amylase; cyclodextrin glucanotransferase; α-cyclodextrin glucanotransferase; α-cyclodextrin glycosyltransferase; β-cyclodextrin glucanotransferase; β-cyclodextrin glycosyltransferase; γ-cyclodextrin glycosyltransferase; cyclodextrin glycosyltransferase; cyclomaltodextrin glucotransferase; cyclomaltodextrin glycosyltransferase; konchizaimu; α-1,4-glucan 4-glycosyltransferase, cyclizing; BMA; CGTase; neutral-cyclodextrin glycosyltransferase; 1,4-α-D-glucan 4-α-D-(1,4-α-D-glucano)-transferase (cyclizing)Systematic name (1→4)-α-D-glucan:(1→4)-α-D-glucan 4-α-D-[(1→4)-α-D-glucano]-transferase (cyclizing)Cofactor(s) -Application industry; synthesis; nutrition; pharmacology包装清单:产品编号产品名称包装P3618-100ml Cyclodextrin Glucanotransferase (Crude Enzyme) 100mlP3618-1L Cyclodextrin Glucanotransferase (Crude Enzyme) 1L-说明书1份保存条件:-20ºC或更低温度保存,至少1个月有效。
美蓓亚三美株式会社

美蓓亚三美集团绿色采购管理要领EM10507 附属文件环境化学物质清单Ver.2.00Ⅰ 禁用物质Ⅱ 预计禁用物质Ⅲ 顾客要求禁用物质Ⅳ 管理物质发行:2018年02月01日实行:2018年04月02日美蓓亚三美株式会社Ⅰ禁用物质国内外法律规定、国际条约等禁止或限制使用的化学物质中,本公司产品中可能含有的化学物质。
* 如未指定限制值单位,则单位为ppm。
所有限制要求均为低于限制数值。
No. 化学物质(群)名 限制对象(分类)美蓓亚三美集团限制值(单位:ppm)备注主要参照的法律规定1 镉及其化合物・树脂、树脂产品、树脂材料(包括橡胶、胶膜等)・涂料、油墨、颜料、染料、润滑油脂、油脂、胶合剂(无挥发成分状态)5 RoHS指令(2011/65/EU)EU REACH规则AnnexⅩⅦ<适用豁免>RoHS适用豁免品 ・无铅焊料(焊锡棒、焊线、松香芯焊锡、焊锡膏、焊锡球)・印刷线路板焊接部・部件的焊锡电镀部位(如引线端子等)・部件的镀锡部位(热浸镀除外)20・黄铜、锌及锌合金・铝及铝合金・部件的镀锡以外的金属电镀部位・无电解镀镍部位・厚膜浆料材料、电阻75其他 752 铅及其化合物・树脂、树脂产品、树脂材料(包括橡胶、胶膜等)・涂料、油墨、颜料、染料、润滑油脂、油脂、胶合剂(无挥发成分状态)100 RoHS指令(2011/65/EU)<适用豁免>RoHS适用豁免品 ・无铅焊料(焊锡棒、焊线、松香芯焊锡、焊锡膏、焊锡球)500・印刷线路板焊接部・部件的焊接电镀部分(包括引线端等熔焊电镀在内)・部件的镀锡部分・部件镀锡之外的金属电镀部分・化学镀镍部分1,000其他 1,0003 六价铬化合物所有用途 1,000 RoHS指令(2011/65/EU)<适用豁免>RoHS适用豁免品4 汞极其化合物所有用途 禁止有意添加且限制值为1,000RoHS指令(2011/65/EU)<适用豁免>RoHS适用豁免品5 PBB;聚溴联苯 所有用途 1,000 RoHS指令(2011/65/EU) 6 PBDE;聚溴联苯醚 所有用途 1,000 RoHS指令(2011/65/EU)7 PCB;多氯化联苯 所有用途 禁止有意添加且限制值为50 化审法EU POPs规则 AnnexⅠ8 PCN;聚氯化萘(氯数 1以上) 所有用途 禁止有意添加且限制值为50化审法EU POPs规则AnnexⅠ9 PCT;聚氯三联苯 所有用途 禁止有意添加且限制值为50 化审法EU REACH规则 AnnexⅩⅦ10 石棉类 所有用途 禁止有意添加 安卫法德国化学品禁止规则EU REACH规则AnnexⅩⅦ11 短链氯化石蜡(碳数10-13)(CAS No.85535-84-8) 所有用途 禁止有意添加且限制值为1,000化审法EU POPs规则AnnexⅠ12 臭氧层消耗物质*蒙特利尔协议附属文件A(类别Ⅰ、Ⅱ)附属文件B(类别Ⅰ、Ⅱ、Ⅲ)附属文件C(类别Ⅰ、Ⅱ、Ⅲ)附属文件E(类别Ⅰ) 所有用途 禁止有意添加 臭氧层保护法EU规则((EC) No1005/2009)13 氢氟烃(HFC)、全氟碳(PFC)、六氟化硫(SF6) 所有用途 禁止有意添加 EU规则((EU) No517/2014)14 双(三丁基锡)=氧化物;TBTO(CAS No. 56-35-9) 所有用途 禁止有意添加且锡元素1,000化审法EU REACH规则AnnexⅩⅦ15 三取代有机锡化合物(三丁基锡(TBT)化合物、三苯基锡(TPT)化合物等) 所有用途 禁止有意添加且锡元素1,000化审法EU REACH规则AnnexⅩⅦ16 二丁基锡(DBT)化合物 所有用途 锡元素1,000 EU REACH规则AnnexⅩⅦ17 二辛基锡(DOT)化合物 仅适用于以下物品・接触皮肤的纤维产品・玩具、儿童用品、育儿产品・双组分室温固化(RTV-2)成型试剂盒 锡元素1,000 EU REACH规则AnnexⅩⅦ18 特定胺化合物及生成特定胺类的部分偶氮染料及颜料(着色剂)(*1) 所有用途 30EU REACH规则AnnexⅩⅦ19 甲醛;福尔马林(CAS No. 50-00-0) 使用纤维板、刨花板以及复合板的木工产品(扬声器、架子等)0.1(测量值密闭小室法)德国化学品禁止规则20 镍及其化合物 长期接触皮肤的用途(耳机、耳麦等) 0.5μg/cm2/week试验标准EN1811:2011+A1:2015 EU指令(94/27/EC) EU REACH规则 AnnexⅩⅦ21 砷及其化合物(包括三氧化二砷、五氧化二砷) 仅适用于木材防腐剤、玻璃消泡剂、澄清剂等用途1,000 EU REACH规则AnnexⅩⅦPRTR法22 放射性物质所有用途 禁止有意添加 放射线障害防止法23 全氟辛烷磺酸(PFOS)及其盐 所有用途 禁止有意添加且限制值为1000 化审法EU POPs规则 AnnexⅠ24 特定苯并三唑2-(2H-1,2,3-苯并三唑-2-基)-4,6-二叔丁基苯酚(UV-320)(CAS No. 3846-71-7) 所有用途 禁止有意添加化审法25 氯化钴(CAS No. 7646-79-9) 所有用途 禁止有意添加且限制值为1,000化审法EU规则((EC) No1272/2008)26 氧化铍(CAS No. 1304-56-9) 所有用途 禁止有意添加且限制值为1,000劳动安全卫生法EU规则((EC) No1272/2008)27 富马酸二甲酯(DMF)(CAS No. 624-49-7) 所有用途 0.1 欧州委员会决议(2009/251/EC)EU REACH规则AnnexⅩⅦ28 磷酸三(2-氯乙基)酯(TCEP)(CAS No. 115-96-8) 所有用途 1,000 EU REACH规则AnnexⅩⅣ佛蒙特州 法律规定29 磷酸三(1-氯-2-丙基)酯(TCPP)(CAS No. 13674-84-5)用于树脂、纤维的阻燃剂用途 1,000 佛蒙特州 法律规定30 磷酸三(1,3-二氯-2-丙基)酯(TDCPP)(CAS No. 13674-87-8)用于树脂、纤维的阻燃剂用途 1,000 佛蒙特州 法律规定31 六溴环十二烷(HBCDD)以及所有主要非对映体 所有用途 禁止有意添加且限制值为100化审法EU REACH规则AnnexⅩⅣEU POPs规则Annex I32 二苯胺与苯乙烯和 2,4,4-三甲基戊烯的反应产物(BNST)(CAS No.68921-45-9) 除橡胶材料外的所有用途(但,轮胎橡胶材料属于限制对象)禁止有意添加 加拿大环境保护法33 全氟辛酸(PFOA)及其盐和酯(*2) 所有用途 1,000 关于使用挪威特定有害化学物质等的规则34 PAHs (下述8种物质) 长期或反复直接接触皮肤或口腔的橡胶或塑料部件玩具 各成分0.5 EU REACH规则AnnexⅩⅦ苯并[a]芘(CAS No. 50-32-8)苯并[e]芘(CAS No. 192-97-2)苯并[a]蒽(CAS No. 56-55-3)屈(CAS No. 218-01-9)苯并[b]荧蒽(CAS No. 205-99-2)玩具以外的成形品各成分1苯并[j]荧蒽(CAS No. 205-82-3)苯并[k]荧蒽(CAS No. 207-08-9)二苯并[a,h]蒽(CAS No. 53-70-3)35 卤代二苯甲烷(*3)所有用途 禁止有意添加 EU REACH规则AnnexⅩⅦ36 邻苯二甲酸二(2-乙基己基)酯(DEHP)(CAS No. 117-81-7) 玩具、儿童产品禁止有意添加且6种物质总量1,000台湾CNS4797(玩具安全标准)美国消费者产品安全法新法(CPSIA)日本玩具安全标准(ST标准)EU REACH规则AnnexⅩⅦ邻苯二甲酸二丁酯(DBP) (CAS No. 84-74-2)邻苯二甲酸丁苄酯(BBP) (CAS No. 85-68-7)邻苯二甲酸二异壬酯(DINP) (CAS No. 28553-12-0) (CAS No.68515-48-0)邻苯二甲酸二异癸酯(DIDP) (CAS No. 26761-40-0)邻苯二甲酸二正辛酯(DNOP)(CAS No. 117-84-0)37 苯(CAS No. 71-43-2) 玩具、儿童产品 5 EU REACH规则AnnexⅩⅦ物质或混合物 1,00038 三(1-氮丙啶基)氧化膦(TAPO)(CAS No. 545-55-1) 直接接触皮肤的纤维产品 禁止有意添加 EU REACH规则AnnexⅩⅦ39 三(2,3-二溴丙基)磷酸酯(TBPP)(CAS No. 126-72-7) 直接接触皮肤的纤维产品 禁止有意添加 EU REACH规则AnnexⅩⅦ40 高氯酸盐 所有用途产品的 0.006 加利福尼亚州高氯酸盐法规41 2,4,6-三-叔-丁基苯酚(CAS No. 732-26-3)所有用途 禁止有意添加 化审法42 汞、镉、六价铬、铅 捆包材料 总量:100 EU指令(94/62/EC) (*1) 特定胺化合物一览No. 化学物质名 CAS No.1 4-氨基偶氮苯 60-09-32 邻氨基苯甲醚 90-04-03 2-萘胺 91-59-84 3,3'-二氯联苯胺 91-94-15 4-氨基二苯基 92-67-16 联苯胺 92-87-57 邻甲苯胺 95-53-48 4-氯 - 邻 - 甲苯胺 95-69-29 2,4-甲苯二胺 95-80-710 邻氨基偶氮甲苯 97-56-311 5-硝基邻甲苯胺 99-55-812 4,4'-亚甲基双(2-氯苯胺) 101-14-413 4,4'-二氨基二苯基甲烷 101-77-914 4,4'-氧基二苯胺及其盐 101-80-415 P-氯苯胺 106-47-816 3,3'-二甲氧基联苯胺 119-90-417 3,3'-二甲基联苯胺 119-93-718 邻氨基对甲苯甲醚 120-71-819 2,4,5-三甲基苯胺 137-17-720 4,4'-硫代二苯胺 139-65-121 2,4-二氨基茴香醚 615-05-422 3,3'-二甲基-4,4'-二氨基二苯基甲烷 838-88-0(*2)全氟辛酸(PFOA)及其盐和酯No. 化学物质名 CAS No.1 全氟辛酸(PFOA) 335-67-12 全氟辛酸铵(APFO) 3825-26-13 全氟辛酸钠盐 335-95-54 全氟辛酸钾盐 2395-00-85 全氟辛酸银盐 335-93-36 全氟辛酸氟化物 335-66-07 全氟辛酸甲酯 376-27-28 全氟辛酸乙酯 3108-24-5(*3) 卤代二苯甲烷No. 化学物质(群)名 CAS No.1 四氯二苯甲烷单甲基酯 76253-60-62 单二氯二苯基甲烷 81161-70-83 单甲基二溴二苯基甲烷 99688-47-8II 预计禁用物质相关法律法规等规定了使用期限的物质。
自噬研究方法

MDC:取12 mg粉末溶于720 nl DMSO使其浓度为50 mmol/L,分装后-20冰箱保存。
临用前用MEM稀释到终浓度50 umol/L;Rapamycin:用MEM培养基配成终浓度为1 umol/L,现用现配;400ng/ml喹乙醇:称取4 mg喹乙醇,DMSO预溶(体积<0.1%)后加入10 ml MEM培养液至完全溶解,现用现配,避光保存;3-MA:首先用PBS溶解粉末,临用前加热至完全溶解后再加入MEM培养基至终浓度10mmol/L; PI3K抑制剂(3-MA,Wortmannin)可干扰或阻断自噬体的形成用RAPAMYCIN诱导自噬我也查过一部分文献,有用无血清的,也有用,一般培养基的,浓度从25nM到100nM都有,用的是50nM的雷帕霉素,加入一般的培养基中,目的是排除无血清所诱导出来的自噬。
文献说饥饿初期激活的是大分子自噬,在4-6小时活力达到最大,24h后以CMA途径为主Earle's balanced salts solution (EBSS) for 48 hsigma的EBSS,货号E2888,有碳酸氢钠,有酚红的,酚红到不是很必须,只是一个PH指示作用,好看些无血清诱导自噬:EBSS 诱导6个小时就可以了。
EBSS一定可以诱导出来,只是需要说明的是时间点的设置,因为从饥饿诱导开始半个小时就可能开始自噬了,一直到24小时都持续,所以应该设置不同的时间点观察这个作用。
另外一个很大的问题是,饥饿诱导的一个很大的弊端是细胞死亡,这也是我面临的问题,就是在细胞收养的时候蛋白浓度太小了。
24小时就很少了,更不要说48小时和72小时了Hank's诱导,也就是通常所说的饥饿诱导,细胞培养到对数生长期后以Hank's替代常规完全培养基,3h后就可诱导出自噬。
我用Hank's诱导了3h后电镜观察有30%细胞都有自噬这种现象,但不如国外报道的高。
核苷类药物知识

核苷类药物知识核苷类药物的综述,免费下载的,大家给好评吧!O(∩_∩)O~1. 前言核苷和脱氧核苷是由核苷碱基分别和核糖或脱氧核糖以苷键形式而构成的,它们是组成核糖核酸(RNA)和脱氧核糖核酸(DNA)的基本元件,是遗传基因的基础。
核苷和脱氧核苷系列衍生物具有多种生物活性物质,可以直接或间接地作为药物使用,在治疗多种重大的疾病方面起到极其重要的作用,国外已经研究开发出系列化药物并商品化,国内研究与开发较晚,发展前景非常广阔.1。
1 核苷类药物的合成与生产从20世纪40年代末期,国外就开始核苷及其系列药物的合成与开发。
目前世界排名前25位制药大公司都有自己的核苷衍生物生产或加工厂,并且均有持有专利的核苷类药物上市,并且从20世纪90年代起投入巨资用于基因药物的研究。
据国外有关资料预计,2003年基因药物的市场价值将超过30亿美元.在亚洲,日本是最早开发核苷类药物和基因药物的国家,如武田、住友、味之素等公司均有相关的中间体开发机构和生产基地。
另外韩国、印度在20世纪90年代初开始投入这类产品的开发与生产.中国在核苷及其衍生物方面的开发研究与生产始于20世纪90年代末期,但是核苷及其中间体品种少,部分原料依赖进口,与目前快速发展的生命科学及相关药物研究不相适应。
1。
2 核苷类药物的应用核苷与脱氧核苷系列化合物主要用于医药领域,用途广泛,而且新产品层出不穷,应用范围不断扩大.(一)抗病毒药物。
核苷类抗病毒药物品种繁多,结构多样,主要以破坏病毒转录,干扰或终止病毒核酸的合成为目的,用于抗疱疹病毒、HIV、HBV、以及流感和呼吸系统病毒等DNA和RNA病毒。
目前在这方面应用最多,而且新出现的药物主要集中于治疗上述疾病。
(二)抗肿瘤药物。
目前用于临床和正在研究的核苷类抗肿瘤药物有数十种,它们的主要作用是干扰肿瘤的DNA合成,或者影响核酸的转录过程,抑制蛋白质的合成,从而达到治疗肿瘤的效果。
(三)抗真菌类药物.具有这方面作用的核苷类化合物已经有多种用于临床应用,其中有部分产品对多种真菌具有抑制作用,而且对哺乳动物几乎无毒性。
M5 鲑鱼精 DNA 10mg ml 说明书

北京聚合美生物科技有限公司 Mei5 Biotechnology, Co., Ltd
北京市昌平区回龙观龙域北街10号院1号楼四层422-1室(创集合大楼) 热线电话:(86)************
M5 鲑鱼精DNA 10mg/ml 使用说明书
产品名称 单位 货号 M5 鲑鱼精DNA 10mg/ml 1 ml MF475-01 M5 鲑鱼精DNA 10mg/ml 5x1 ml MF475-05
【储存条件】
-20℃保存,有效期2年。
【产品简介】
鲑鱼精DNA 溶液(10mg/ml )是经过酚氯仿抽提,超声和热变性处理的短片段的单链DNA 溶液,可直接用于Southern 、Northern 等核酸杂交中。
【操作步骤】
本产品鲑鱼精DNA 的浓度为10mg/ml ,使用时按实验具体要求操作,稀释至所需工作浓度即可。
【注意事项】
1. 如果每次的使用量很小,可以适当分装后再使用,避免反复冻融。
2. 为了您的安全和健康,请穿实验服并戴手套操作。
【备注】
本产品仅供科研使用。
在确认产品质量出现问题时,本公司承诺为客户免费更换等量的质量合格产品。
MALDI-TOF 常用基质基本信息
盐酸利多卡因注射剂遗传毒性杂质研究_NormalPdf

Journal of China Pharmaceutical University2020,51(4):466-471学报盐酸利多卡因注射剂遗传毒性杂质研究冼芷然1,孙春萌2,骆雪芳1*,钟文英1**(1中国药科大学理学院药物质量研究中心,南京211198;2中国药科大学药学院,南京211198)摘要确定2,6-二甲基苯胺为盐酸利多卡因注射液中遗传毒性杂质,N-氯乙酰-2,6-二甲基苯胺为潜在遗传毒性杂质,建立LC-MS/MS方法,用色谱柱Agilent ZORBAX Eclipse Plus C18(4.6mm×250mm,5μm)对原料、自制制剂及原研制剂进行遗传毒性杂质研究。
研究结果表明自制制剂中杂质2,6-二甲基苯胺与N-氯乙酰-2,6-二甲基苯胺除由原料引入外,可能分别由氧化条件或碱性条件下降解引入,为盐酸利多卡因注射液的遗传毒性风险评估和工艺优化提供参考与指导。
关键词盐酸利多卡因注射液;遗传毒性杂质;LC-MS/MS中图分类号R917文献标志码A文章编号1000-5048(2020)04-0466-06doi:10.11665/j.issn.1000-5048.20200412引用本文冼芷然,孙春萌,骆雪芳,等.盐酸利多卡因注射剂遗传毒性杂质研究[J].中国药科大学学报,2020,51(4):466–471.Cite this article as:XIAN Zhiran,SUN Chunmeng,LUO Xuefang,et al.Profiling of genotoxic impurities in a lidocaine hydrochloride injec‐tion[J].J China Pharm Univ,2020,51(4):466–471.Profiling of genotoxic impurities in a lidocaine hydrochloride injection XIAN Zhiran1,SUN Chunmeng2,LUO Xuefang1*,ZHONG Wenying1**1Drug Quality Research Center,College of Science,China Pharmaceutical University;2School of Pharmacy,China Pharmaceutical University,ChinaAbstract2,6-dimethylbenzenamine was determined as a genotoxic impurity in lidocaine hydrochloride injec‐tion,and2-chloro-N-(2,6-dimethylphenyl)acetamide was determined as potential genotoxic impurity.An LC-MS/ MS method was established to research the profiling of genotoxic impurities in active pharmaceutical ingredients (API),homemade preparation and reference preparation on column Agilent ZORBAX Eclipse Plus C18(4.6mm×250mm,5μm).The results show that in the homemade preparation the2,6-dimethylbenzenamine and the 2-chloro-N-(2,6-dimethylphenyl)acetamide may be degraded under oxidation condition and alkaline condition in addition to the introduction from API preparation process.This study provides guidance for genotoxic risk assess‐ment and prescription process optimization of lidocaine hydrochloride.Key words lidocaine hydrochloride injection;genotoxic impurities;LC-MS/MS盐酸利多卡因(lidocaine hydrochloride)为临床上常制成盐酸利多卡因注射剂应用于局部麻醉药[1]和抗心律失常药物等[2-3]。
安捷伦产品目录

15
Real-Time PCR
16
Mx3000P QPCR System
17
Brilliant III Ultra-Fast SYBR Green QPCR and QRT-PCR Reagents
18
Brilliant III Ultra-Fast QPCR and QRT-PCR Reagents
Agilent / STRATAGENE
Agilent website: /genomics
Welgene | Agilent Stratagene
威健股份有限公司 | Stratagene 總代理
Table of Content
Table of Contents
/ XL1-Red Competent Cells SoloPack Gold Supercompetent Cells
/ TK Competent Cells Specialty Cells
/ Classic Cells / Fine Chemicals For Competent Cells
適用於 UNG 去汙染或 bisulphite
sequencing
適用於 TA Cloning
最高敏感性
取代傳統 Taq 的好選擇
-
2
威健股份有限公司 | Stratagene 總代理
PCR Enzyme & Instrument
Agilent SureCycler 8800
市場上領先的 cycling 速度和 sample 體積 10 ~ 100 μL 簡易快速可以選擇 96 well 和 384 well 操作盤 優秀的溫控設備讓各個 well 都能保持溫度的穩定 七吋的高解析度觸控螢幕讓操作上更為簡便 可以透過網路遠端操控儀器及監控儀器 Agilent 專業的技術支援可以幫助您應對各種 PCR 的問題
3M Harvest RC 二次生物疗法制品说明书

3M™ Harvest RCSingle-stage chromatographic purification for recombinant protein therapeutic manufacturing2 | 3M ™Harvest RCThe first step in the recombinant biotherapeutic process is harvesting cell culture fluid containing the product. Conventional approaches for performing this unit operation utilize a combination of depth filtration, centrifugation, and membrane filtration. These technologies utilize differences in density and in size as the principles of separation. As cell culture processes are intensified to yield higher cell densities and product titers, the ability to effectively harvest the cell culture fluid with the consistency and scalability required becomes challenging.3M ™ Harvest RC is a harvest solution that utilizes fibrous anion exchange (AEX) chromatography to efficiently separate the cells, cell debris, and DNA from the harvest fluid containing the target product. Precisionquaternary ammonium (Q) functionalized polypropylene fiber, combined with a 0.2 µm PES membrane, provides scalable and predictable clarification from discovery to commercial manufacturing scale.Cells being captured by AEX fiberchromatographySimplify three stages into a single stageCell CultureClarification by Size3M ™ Harvest RCSterilizing MembraneSterilizing Membrane3M ™ Harvest RC | 3� Capsule format enables typical product recoveries of 95+%� R eplaces primary, secondary, and guard membrane clarification stages � P redictably scales from discovery to manufacturing in terms of clarification consistency and cell loading capacity � C apsules fit into laboratory to manufacturing scale workflows.� L ower total cost of manufacturing compared tocentrifugation and depth filtration � N o post-use cleaning required which means that there is no use of caustic or potentially toxic clean-in-place (CIP) agents � L ower consumption of buffer and water compared to depth filtrationIntroducing 3M ™ Harvest RC3M ™ Harvest RC is a new single-stage, single-use chromatographic clarification solution. It is the next generation in harvest and clarification technology and is designed as an efficient option to increase monoclonal antibody (mAb) yields while streamlining the upstream process by replacing the centrifuge and/or depth filtration process steps.ManufacturingDevelopmentScale-upDiscovery 3M ™ Harvest RC product family: laboratory, pilot, andproduction capsules.BC340CT15BC25BC4WP6BC16000BC1020BC23004 | 3M ™ Harvest RC3M ™ Harvest RC encapsulates innovative synthetic fibrous anion exchange (AEX)chromatography media and a 0.2 µm polyether sulfone (PES) membrane. This enables a single-stage clarification process of low to high-density cell culture (>40 million cells per mL) with high recovery, and high fidelity of soluble and insoluble contaminant separation. Cells are bound inside the media by electrostatic charge interaction with the AEXchromatographic fibers. This results in the efficient retention of large and small particulates without developing a surface cake layer. The media can also remove soluble impurities which results in cleaner effluent than centrifugation or depth filtration.� H igh mAb product recovery (Capsules: >95%; Conical Tube and Well Plate >90%)� C onsistent cell loading capacity � T urbidity reduction (<15 NTU)� D NA reduction (<500 ppb)� M inimal cell shear� 0.1 µm sterile filter protectionWell plateConical tube Laboratory scaleBefore and after using 3M ™ Harvest RC:turbidity reduction in a single stageAvailable formats:3M ™ Harvest RC | 5Performance datamAb product recovery3M ™ Harvest RC is a single stage chromatography solution that effectively clarifies Chinese Hamster Ovary (CHO) harvest cell culture fluid (HCCF) across a wide range of cell densities, packed cell volumes (PCV), and turbidities.3M Harvest RC chromatographic clarification capsulesconsistently provide >95% mAb product recovery for high cell density cultures from the laboratory to the manufacturing scale.Turbidity reduction3M Harvest RC provides consistent separation of cells, cell debris, and DNA from the target protein. Clarified cell culture fluid (CCCF) has low turbidity, typically <15 NTU. Additionally, consistently low acidified turbidity of CCCF indicates significant reduction of DNA in the clarified material. Low acidifiedCCCF turbidity is a measure of the amount of DNA present in the cell culture fluid. (Koehler et al. Biotechnology Progress. 2019;35:e2882)Scalability3M Harvest RC capsules scale linearly across laboratory, pilot, and manufacturing scales.Fibrous chromatographic clarification assures scalable performance from discovery to manufacturing scales. Performance isconsistent from laboratory capsules (BC4 and BC25), scale-up capsules (BC340 and BC1020), to production capsules (BC2300 and BC16000) within ±20% of BC25 throughput.Throughputs of 3M Harvest RC capsules are scaled by area based on packed cell volume.Figure 1A: mAb product recovery in clarification process at different packed cell volumes (N = 1 – 4)m A b P r o d u c t R e c o v e r y (%)Packed Cell Volume (%)105%4%5%6%7%8%9%10%100%95%90%85%80%75%Figure 1B: mAb product recovery in clarification process at different media surface areas (N = 1 – 3)m A b P r o d u c t R e c o v e r y (%)Media Surface Area (cm 2)105%10100100010000100000100%95%90%85%80%75%Figure 2: Turbidity Reduction by 3M ™ Harvest RC capsules (N = 3 – 6). A – E are different CHO cell cultures at 5 – 8 % PCV.T u r b i d i t y (N T U )Cell Cultures100001000100101AB CDEHCCFCCCFAc-CCCFFigure 3: Scalability from laboratory to scale-up and production capsules (N = 1 – 5, 6 cell cultures)N o r m a l i z e d T h r o u g h p u tMedia Surface Area (cm 2)120%1100101000100001000000100000100%80%60%40%20%0%Cell loading capacity3M™ Harvest RC solution utilizes advanced Q functionalized fibrous chromatography media to achieve single-stage clarification, enabling predictable and consistent cell loading capacity for CHO cell culture fluid for a wide range of packed cell volumes.Cell shearThe low-pressure chromatographic clarification relies on charge rather than size or density. This results in minimal cell shear compared to conventional depth filtration processes evenat medium and high cell densities. Cell shear was evaluatedby lactate dehydrogenase (LDH) assay (Sigma-Aldrich 11644793001).Robust sterile filter protectionDue to the highly effective chromatographic reduction of soluble and insoluble contaminants, 3M Harvest RC enables efficient clarification, and is capable of effective protection of final sterilizing grade membrane filter down to 0.1 µm pore size. Figure 4: Cell loading capacity of 3M™ Harvest RC capsules for CHO harvested cell culture fluid at different packed cell volumes (N = 2 - 3) CellLoadingCapacity(L-cell/m2)Packed Cell Volume (%)7354679108654321Figure 5: Minimal cell shear of 3M™ Harvest RC during clarification of 8% PCV CHO cell culture at 100 LMH.CellShear(%)Normalized Throughput (-)20%18%16%14%12%10%8%6%4%2%0%Harvest RC10SP02A60SPO2A05SP01AFigure 6: 0.1 µm sterile filter pressure increase at 500 L/m2. A – E are clarified fluids of CHO harvested cell culture fluids at 8%PCV by 3M™ Harvest RC capsules..1μmFilterPressureIncrease(psid)Clarified Cell Culture Fluid by 3M Harvest RC 654321A B C D E6 | 3M™ Harvest RCBiopharmaceutical purification process improvementsThis process train illustrates the potential of combining 3M products that work together to create an intensifiedmanufacturing process, eliminating several process steps.ntended for flow-through polishing3M™ Harvest RC | 7Intended Use: 3M ™ Harvest RC products are intended for use in biopharmaceutical processing applications of aqueous based pharmaceuticals (drugs) and vaccines inaccordance with the product instructions and specifications, and cGMP requirements (for BC340, BC1020, BC2300 and BC16000) or GLP requirements (for CT15, WP6, BC4 and BC25), where applicable.Since there are many factors that can affect a product’s use, the customer and user remain responsible for determining whether the 3M product is suitable and appropriate for the user’s specific application, including user conducting an appropriate risk assessment and evaluating the 3M product in user’s application.Product Selection and Use: Many factors beyond 3M’s control and uniquely within user’s knowledge and control can affect the use and performance of a 3M product in a particular application. As a result, customer is solely responsible for evaluating the product and determining whether it is appropriate and suitable for customer’s application, including completing a risk assessment that considers the product leachable characteristics and its impact on drug safety, conducting a workplace hazard assessment andreviewing all applicable regulations and standards (e.g., OSHA, ANSI, etc.). Failure to properly evaluate, select, and use a 3M product and appropriate safety products, or to meet all applicable safety regulations, may result in injury, sickness, death, and/or harm to property.Restrictions on Use: For CT15, WP6, BC4 and BC25: For laboratory use only. Not intended for use with materials that will be used on humans or animals. For all sizes: 3M advises against the use of these 3M products in any application other than the stated intended use(s), since other applications have not been evaluated by 3M and may result in an unsafe or unintended condition. Do not use in any manner whereby the 3M product, or any leachable from the 3M product, may become part of or remains in a medical device that is regulated by any agency, and/or globally exemplary agencies, including but not limited to: a) FDA, b) European Medical Device Directive (MDD), c) Japan Pharmaceuticals and Medical Devices Agency (PMDA) or in applications involving permanent implantation into the body; Life-sustaining medical applications; Applications requiring food contact compliance.Warranty, Limited Remedy, and Disclaimer: Unless a different warranty is specifically stated on the applicable 3M product packaging or product literature (in which case such warranty governs), 3M warrants that each 3M product meets the applicable 3M product specification at the time 3M ships the product.3M MAKES NO OTHER WARRANTIES OR CONDITIONS, EXPRESS OR IMPLIED, INCLUDING, BUT NOT LIMITED TO, ANY IMPLIED WARRANTY OR CONDITION OF MERCHANTABILITY, FITNESS FOR A PARTICULAR PURPOSE, OR ARISING OUT OF A COURSE OF DEALING, CUSTOM, OR USAGE OF TRADE. If a 3M product does not conform to this warranty, then the sole and exclusive remedy is, at 3M’s option, replacement of the 3M product or refund of the purchase price.Limitation of Liability: Except for the limited remedy stated above, and except to the extent prohibited by law, 3M will not be liable for any loss or damage arising from or related to the 3M product, whether direct, indirect, special, incidental, or consequential (including, but not limited to, lost profits or business opportunity), regardless of the legal or equitable theory asserted, including, but not limited to, warranty, contract, negligence, or strict liability.3M Purification Inc.3M Separation and Purification Sciences Division 400 Research Parkway Meriden, CT 06450 USA Phone: 1-800-243-6894 1-203-237-5541 Web: /bioprocessing3M and LifeASSURE are trademarks of 3M Company. Allother trademarks are property of their respective owners. © 2021 3M Company. All rights reserved. Please recycle. Printed in U.S.A.Ordering GuideFor more information about the 3M ™ Harvest RC, contact your local sales representative by calling 1-800-243-6894, option 4, or visiting us at/bioprocessing1. Cell Culture Volume Range is the estimation for CHO cell culture fluid at 5 - 8% packed cell volume.2. Fill Volume is defined as the volume of liquid that is required to fill the capsule.3. Post Blow-Down Hold-Up Volume is defined as the volume of the residual liquid after air/gas blow down.。
重组贻贝粘蛋白的表征及功效评价

生物技术进展 2023 年 第 13 卷 第 4 期 596 ~ 603Current Biotechnology ISSN 2095‑2341研究论文Articles重组贻贝粘蛋白的表征及功效评价李敏 , 魏文培 , 乔莎 , 郝东 , 周浩 , 赵硕文 , 张立峰 , 侯增淼 *西安德诺海思医疗科技有限公司,西安 710000摘要:为了推进重组贻贝粘蛋白在医疗、化妆品领域的应用,对大肠杆菌规模化发酵及纯化生产获得的重组贻贝粘蛋白进行了表征及功效评价。
经Edman 降解法、基质辅助激光解吸电离飞行时间质谱、PITC 法、非还原型SDS -聚丙烯酰胺凝胶电泳法、凝胶法、改良的Arnow 法对重组贻贝粘蛋白进行氨基酸N 端测序、相对分子量分析、氨基酸组成分析、蛋白纯度分析、内毒素含量测定、多巴含量测定;通过细胞迁移、斑马鱼尾鳍修复效果对重组贻贝粘蛋白进行功效评价。
结果显示,获得的重组贻贝粘蛋白与理论的一级结构一致,蛋白纯度达95%以上,内毒素<10 EU ·mg -1,多巴含量大于5%;重组贻贝粘蛋白浓度为60 μg ·mL -1时能够显著促进细胞增殖的活性(P <0.01);斑马鱼尾鳍面积样品组与模型对照组相比极显著增加(P <0.001)。
研究结果表明,重组贻贝粘蛋白具有显著的促细胞迁移和修复愈合的功效,具备作为生物医学材料的潜质。
关键词:贻贝粘蛋白;基因重组;生物材料;表征;功效评价DOI :10.19586/j.20952341.2023.0021 中图分类号:S985.3+1 文献标志码:ACharacterization and Efficacy Evaluation of Recombinant Mussel Adhesive ProteinLI Min , WEI Wenpei , QIAO Sha , HAO Dong , ZHOU Hao , ZHAO Shuowen , ZHANG Lifeng ,HOU Zengmiao *Xi'an DeNovo Hith Medical Technology Co., Ltd , Xi'an 710000, ChinaAbstract :In order to promote the application of recombinant mussel adhesive protein in the medical and cosmetics field , the recombi⁃nant mussel adhesive protein obtained from scale fermentation and purification of Escherichia coli was characterized and its efficacy was evaluated. Amino acid N -terminal sequencing , relative molecular weight analysis , amino acid composition analysis , protein purityanalysis , endotoxin content , dihydroxyphenylalanine (DOPA ) content of recombinant mussel adhesive protein were determined by the following methods : Edman degradation , matrix -assisted laser desorption ionization time -of -flight mass spectrometry (MALDI -TOF -MS ), phenyl -isothiocyanate (PITC ), nonreductive SDS -polyacrylamide gel electrophoresis (SDS -PAGE ), gel method , modified Ar⁃now. The efficacy of recombinant mussel adhesive protein was evaluated by cell migration and repairing effect of zebrafish tail fin. Re⁃sults showed that the obtained recombinant mussel adhesive protein was confirmed to be consistent with the theoretical primary structure , protein purity of more than 95%, endotoxin <10 EU ·mg -1, DOPA content above 5%. When the recombinant mussel adhesive protein concentration was 60 μg ·mL -1, the effect of promoting cell proliferation was the most obvious , and it had very significant activity (P <0.01). The caudal fin area of zebrafish in sample group was significantly increased compared with model control group (P <0.001). The results indicated that recombinant mussel adhesive protein can promote cell migration and repair healing and has the potential to be used as biomedical materials.Key words :mussel adhesive protein ; gene recombination ; biological materials ; representation ; efficacy evaluation贻贝粘蛋白(mussel adhesive protein , MAP )也称作贻贝足丝蛋白(mussel foot protein ,Mfps ),收稿日期:2023⁃02⁃24; 接受日期:2023⁃03⁃31联系方式:李敏 E -mail:*******************;*通信作者 侯增淼 E -mail:***********************.cn李敏,等:重组贻贝粘蛋白的表征及功效评价是海洋贝类——紫贻贝(Mytilus galloprovincalis)、厚壳贻贝(Mytilus coruscus)、翡翠贻贝(Perna viri⁃dis)等分泌的一种特殊的蛋白质,贻贝中含有多种贻贝粘蛋白,包括贻贝粘蛋白(Mfp 1~6)、前胶原蛋白(precollagens)和基质蛋白(matrix proteins)等[1]。
WHO International Standard 1st WHO International Standard for Human Papillomavirus (HPV) Type 16 DNA

WHO International Standard1st WHO International Standard for Human Papillomavirus (HPV)Type 16 DNA NIBSC code: 06/202 Instructions for use(Version 2.0, Dated 10/11/2010)1. INTENDED USEThe 1st International Standard for HPV Type 16 (HPV-16) DNA Nucleic Acid Amplification Techniques consists of a freeze-dried preparation of recombinant plasmid containing full-length HPV-16 DNA cloned via its unique BamH1 site (Quint et al., 2006). The standard has been formulated in a background of purified human genomic DNA, lyophilized in 0.5 ml aliquots and stored at -20 °C. The material was calibrated in an international collaborative study involving 19 laboratories (Wilkinson et al., 2008). The International Standard contains material that is proprietory to third parties and should be used for the sole purpose of calibrating in-house or working standards for the amplification and detection of HPV-16 DNA. The International Standard should not be used for any other purpose and should be discarded after use. 2. CAUTIONThis preparation is not for administration to humans .This material contains DNA derived from C33A cells. As with all materials of biological origin, this preparation should be regarded as potentially hazardous to health. It should be used and discarded according to your own laboratory's safety procedures. Such safety procedures should include the wearing of protective gloves and avoiding the generation of aerosols. Care should be exercised in opening ampoules or vials, to avoid cuts.3. UNITAGEThe 1st International Standard for HPV-16 DNA Nucleic Acid Amplification Techniques has been assigned a unitage of 5 x 106 International Units (IU) per ampoule.Traceability statement:It was proposed at a WHO meeting in January 2008 (WHO Meeting Report, 2008) that the instructions for use of the International Standard for HPV-16 DNA include the calculations and assumptions used in determining the theoretical HPV-16 qenome equivalents (GEq) of the bulk material used in formulating the International Standard, thus demonstrating that 1 IU is equivalent to 1 GEq for HPV-16 DNA . The definitive unitage of the 1st WHO International Standard for HPV-16 DNA therefore remains as IU while the traceability statement would allow users to equate IU with GEq.Assays for DNA concentration of the recombinant HPV-16 plasmid stock preparation were performed in Dr Cosette Wheeler‟s laboratory, University of New Mexico (UNM). DNA concentrations were determined by absorbance at 260 nm as well as spectrofluorometrically using the Picogreen assay (Invitrogen Corporation, USA). A correlation coefficient of 0.95 or higher was obtained between the two DNA measurements. 10 ng HPV-16 plasmid DNA/μl was supplied to NIBSC for formulating the bulk material for subsequent freeze-drying. The UNM laboratory also provided NIBSC with a statement indicating that 1.0 x 1011 GEq/ml for HPV-16 is equal to 1.17 ng/μl. 10 ng HPV-16 plasmid DNA/μl plasmid stock preparation is therefore equivalent to 8.547 x 1011 HPV-16 GEq/ml. NIBSC used this data in formulating the 1st International Standard for HPV Type 16 DNA.Formulation of bulk material for the 1st International Standard for HPV Type 16 DNA (NIBSC code 06/202):At NIBSC, the bulk HPV-16 plasmid DNA material was prepared according to the formula:HPV GEq/ml of bulk material = (HPV GEq/ml of plasmid stock x volume plasmid stock) / volume bulk material.Therefore,HPV-16 GEq/ml of bulk material = (8.547 x 1011 HPV-16 GEq/ml plasmid stock) x (0.02223 ml HPV-16 plasmid stock) / 1900 ml HPV-16 bulk material = 1.0 x 107 HPV-16 GEq/ml bulk materialThe HPV-16 DNA bulk material was subsequently freeze-dried in 0.5 ml aliquots.Certain assumptions are required for equating IU to GEq for the 1st International Standard for HPV-16 DNA: 1) 1.0 x 1011 GEq/ml for HPV-16 is equal to 1.17 ng/μl. 2) There is no loss in activity of the HPV-16 DNA upon lyophilization. 3) The recombinant HPV-16 plasmid DNA accurately mimics the activity of HPV-16 viral DNA in biological samples.Independent calculation of GEq/ml for recombinant HPV-16 plasmid DNA.NIBSC also independently calculated the genome equivalence of the HPV-16 plasmid stock preparation and bulk preparation in which the molecular weights of the full-length HPV-16 genome and pBR322 DNA were based on sequence content using BioEdit Sequence Alignment Editor v7.0.5.3 (Tom Hall, Isis Pharmaceuticals Inc., USA). The sequences used for determining the molecular weights are GenBank Accession number J01749.1 for pBR322 and the reference sequence for HPV16 (Accession K02718).BioEdit dataDNA molecule: HPV16 Accession K02718 Length = 7904 base pairsMW= 4786756.00 Daltons, double strandedDNA molecule: cloning vector pBR322 Length = 4361 base pairsMW= 2653867.00 Daltons, double strandedFormulaeGEq/ml of the HPV plasmid stock was calculated according to the formula: GEq/ml of the HPV plasmid stock = (DNA concentration of HPV plasmid stock) x (MW of HPV DNA + MW of pBR322)-1 x (Avogadro‟s Number) where Avogadro‟s Number = 6.022x1023 molecules/molGEq/ml of the bulk HPV DNA materials was calculated according to the formula:HPV GEq/ml of bulk material = (HPV GEq/ml of plasmid stock x volume plasmid stock) / volume bulk material.CalculationThe recombinant HPV-16 plasmid stock preparation was supplied to NIBSC at a concentration of 10 ng/μl. Using the MW determinations shown above, the GEq/ml of the HPV-16 plasmid stock is:= (10 x 10-9 g/μl) x (mol/(7440623 g) x (6.022x1023 molecules/mol) = 8.093 x 108 molecules/μl = 8.093 x 1011molecules/ml = 8.093 x 1011 HPV-16 GEq/ml22.23μl of the recombinant HPV-16 plasmid stock was diluted to a final volume of 1900ml, therefore,HPV-16 GEq/ml of bulk material = (8.093 x 1011 HPV-16 GEq/ml plasmid stock) x (0.02223 ml HPV-16 plasmid stock) / 1900 ml HPV-16 bulk material = 0.947 x 107 HPV-16 GEq/ml bulk material4. CONTENTSCountry of origin of biological material: United Kingdom.Each ampoule contains the lyophilized equivalent of 0.5 ml HPV-16 plasmid DNA in 10mM Tris buffer pH7.4 containing 1mM EDTA, 5 mg/ml trehalose and ~1 x 106 human GEq/ml derived from C33a cells.5. STORAGEThe ampoule should be stored at -20 °C or below on receipt.Please note: because of the inherent stability of lyophilized material, NIBSC may ship these materials at ambient temperature.6. DIRECTIONS FOR OPENINGDIN ampoules have an …easy -open‟ coloured stress point, where the narrow ampoule stem joins the wider ampoule body.Tap the ampoule gently to collect the material at the bottom (labeled) end. Ensure that the disposable ampoule safety breaker provided is pushed down on the stem of the ampoule and against the shoulder of the ampoule body. Hold the body of the ampoule in one hand and the disposable ampoule breaker covering the ampoule stem between the thumb and first finger of the other hand. Apply a bending force to open the ampoule at the coloured stress point, primarily using the hand holding the plastic collar.Care should be taken to avoid cuts and projectile glass fragments that might enter the eyes, for example, by the use of suitable gloves and an eye shield. Take care that no material is lost from the ampoule and no glass falls into the ampoule. Within the ampoule is dry nitrogen gas at slightly less than atmospheric pressure. A new disposable ampoule breaker is provided with each DIN ampoule.7. USE OF MATERIALNo attempt should be made to weigh out any portion of the freeze-dried material prior to reconstitution.The 1st International Standard for HPV-16 DNA contains high copy number template. There is a high risk of HPV-16 plasmid DNA contamination via aerosolization upon opening of the glass ampoule. The material must be opened and handled in a separate laboratory environment, away from other pre-amplification components such as reagents, labware and samples.The material is supplied lyophilized and, before use, should be reconstituted in 0.5 ml sterile nuclease-free water. Ensure that the inside surface of the ampoule is wetted with the added water so that any particles of freeze-dried material adhering to the glass are reconstituted. The reconstituted material has a final concentration of 1 X 107 IU/ml. The reconstituted material is suitable for calibration of in-house or working standards for the amplification and detection of HPV-16 DNA.. The material is not suitable for calibrating or assessing extraction, precipitation or centrifugation procedures. The material has NOT been calibrated for human DNA nucleic acid amplification techniques.8. STABILITYReference materials are held at NIBSC within assured, temperature-controlled storage facilities. The 1st International Standard for HPV-16 DNA should be stored at -20 °C or below on receipt.Studies on the stability of reconstituted standard are underway. Users should determine the stability of the reconstituted material according to their own method of preparation, storage and use.NIBSC follows the policy of WHO with respect to its reference materials.9. REFERENCESQuint, W. G. V., Pagliusi, S. R., Lelie, N., de Villiers, E. M., Wheeler, C. M. and the World Health Organization Human Papillomavirus DNA International Collaborative Study Group. (2006). Results of the First WorldHealth Organization International Collaborative Study of Detection of Human Papillomavirus DNA. J. Clin. Microbiol. 44: 571-579.Wilkinson, D.E., Baylis, S.A., Padley, D., Heath, A.B., Ferguson, M., Pagliusi, S.R., et al. Establishment of the 1st World Health Organization international standards for human papillomavirus type 16 DNA and type 18 DNA. Int J Cancer 2010 Jun 15;126(12):2969-83.WHO meeting report, on “Standardization of HPV assays and the role of HPV LabNet in supporting vaccine introduction” Geneva, Switzerland, 23-25 January 2008, in preparation.10. ACKNOWLEDGEMENTS11. FURTHER INFORMATIONFurther information can be obtained as follows; This material: enquiries@ WHO Biological Standards:http://www.who.int/biologicals/en/JCTLM Higher order reference materials: /en/committees/jc/jctlm/ Derivation of International Units:/products/biological_reference_materials/frequently _asked_questions/how_are_international_units.aspx Ordering standards from NIBSC:/products/ordering_information/frequently_asked_q uestions.aspxNIBSC Terms & Conditions:/terms_and_conditions.aspx12. CUSTOMER FEEDBACKCustomers are encouraged to provide feedback on the suitability or use of the material provided or other aspects of our service. Please send any comments to enquiries@13. CITATIONIn all publications, including data sheets, in which this material is referenced, it is important that the preparation's title, its status, the NIBSC code number, and the name and address of NIBSC are cited and cited correctly.15. LIABILITY AND LOSSInformation provided by the Institute is given after the exercise of all reasonable care and skill in its compilation, preparation and issue, but it is provided without liability to the Recipient in its application and use. It is the responsibility of the Recipient to determine the appropriateness of the standards or reference materials supplied by the Institute to the Recip ient (“the Goods”) for the proposed application and ensure that it has the necessary technical skills to determine that they are appropriate. Results obtained from the Goods are likely to be dependant on conditions of use by the Recipient and the variability of materials beyond the control of the Institute.All warranties are excluded to the fullest extent permitted by law, including without limitation that the Goods are free from infectious agents or that the supply of Goods will not infringe any rights of any third party.The Institute shall not be liable to the Recipient for any economic loss whether direct or indirect, which arise in connection with this agreement.The total liability of the Institute in connection with this agreement, whether for negligence or breach of contract or otherwise, shall in no event exceed 120% of any price paid or payable by the Recipient for the supply of the Goods.If any of the Goods supplied by the Institute should prove not to meet their specification when stored and used correctly (and provided that the Recipient has returned the Goods to the Institute together with written notification of such alleged defect within seven days of the time when the Recipient discovers or ought to have discovered the defect), the Institute shall either replace the Goods or, at its sole option, refund the handling charge provided that performance of either one of the above options shall constitute an entire discharge of the Institute‟s liability under this Condition.。
IFCC Aspartate Aminotransferase 检测手册说明书

ASTAspartate Aminotransferase IFCCMANUAL RX MONZAINTENDED USEFor the quantitative in vitro determination of AspartateAminotransferase (AST) in serum and plasma. This product is suitable for manual use and on the Rx Monza analyser.Cat. No. AS 1202 R1a. Buffer/Substrate 1 x 70 ml 20 x 2 ml R1b. Enzyme/Coenzyme/ 20 x 2 ml α-oxoglutarate GTIN: 05055273200416AS 1204 R1a. Buffer/Substrate 1 x 105 ml 10 x 10 ml R1b. Enzyme/Coenzyme/ 10 x 10 ml α-oxoglutarate GTIN: 05055273200423AS 1267 R1a. Buffer/Substrate 1 x 105 ml 5 x 20 ml R1b. Enzyme/Coenzyme/ 5 x 20 ml α-oxoglutarate GTIN: 05055273200430AS 2359 R1a. Buffer/Substrate 5 x 100 ml 5 x 100 ml R1b. Enzyme/Coenzyme/ 5 x 100 ml α-oxoglutarate GTIN: 05055273200454UV METHODThis is an optimised standard method according to the concentrations recommended by the IFCC.CLINICAL SIGNIFICANCE (1,2,3,4)The aminotransferases are a group of enzymes that catalyse the inter conversions of amino acids and α-oxoacids by transfer of amino groups. AST (aspartate aminotransferase or glutamate oxaloacetatetransaminase) has been found in the cytoplasm and the mitochondria of cells that have been studied. In cases of mild tissue damage, e.g. liver, the predominant form of serum AST is that from the cytoplasm, with a smaller amount coming from the mitochondria. Severe tissue damage will result in more mitochondrial enzyme being released. Elevated levels of AST can signal myocardial infarction, hepatic disease, muscular dystrophy and organ damage.Although heart muscle is found to have the most activity of the enzyme, significant activity has also been seen in the brain, liver, gastric mucosa, adipose tissue and kidneys of humans.The IFCC has now recommended (1980) standardised procedures for AST determinations including:-1. optimization of substrate concentrations.2. Employment of Tris buffers (instead of phosphate, which has beenshown to inhibit recombination of the apoenzyme with pyridoxal phosphate).3. Pre-incubation of combined buffer and serum to allow sidereactions with NADH to occur. 4. Substrate start (α-oxoglutarate)5. Optional pyridoxal phosphate activation.This is an optimised standard method according to the recommendations of the IFCC.PRINCIPLEα-oxoglutarate reacts with L-aspartate in the presence of AST to form L-glutamate plus oxaloacetate. The indicator reaction utilises the oxaloacetate for a kinetic determination of NADH consumption. AST -oxoglutarate + L-aspartate L-glutamate + oxaloacetate MDH oxaloacetate + NADH + H + L-malate + NAD +SPECIMEN COLLECTION AND PREPARATION (5) Serum:- Use serum free from haemolysis.Plasma:- EDTA or heparin can be used as the anticoagulant.Plasma should be separated from cells within one hour after collection.Specimens should be refrigerated if not used immediately:-Specimens stored longer than 3 days should be frozen at -20︒C.REAGENT COMPOSITIONContents Concentrations in the TestR1a. Buffer/Substrate Tris buffer 80 mmol/l, pH 7.5 L-aspartate 240 mmol/l R1b. Enzyme/Coenzyme/α-oxoglutarate α-oxoglutarate 12 mmol/l MDH ≥420 U/l LD ≥600 U/l NADH 0.18 mmol/lSAFETY PRECAUTIONS AND WARNINGS For in vitro diagnostic use only. Do not pipette by mouth.Exercise the normal precautions required for handling laboratory reagents.Solution R1a contains Sodium Azide. Avoid ingestion or contact with skin or mucous membranes. In case of skin contact, flush affected area with copious amounts of water. In case of contact with eyes or if ingested, seek immediate medical attention.Sodium Azide reacts with lead and copper plumbing, to form potentially explosive azides. When disposing of such reagents flush with large volumes of water to prevent azide build up. Exposed metal surfaces should be cleaned with 10% sodium hydroxide.Health and Safety data sheets available on request.The reagents must be used only for the purpose intended by suitably qualified laboratory personnel, under appropriate laboratory conditions.STABILITY AND PREPARATION OF REAGENTS R1a. Buffer/SubstrateContents ready for use. Stable up to the expiry date when stored at +2 to +8︒C.R1b. Enzyme/Coenzyme/α-oxoglutarate Reconstitute one vial of Enzyme/Coenzyme/α-oxoglutarate R1b with the appropriate volume of Buffer/Substrate R1a: 2 ml for the 20 x 2 ml kit (AS 1202) 10 ml for the 10 x 10 ml kit (AS 1204) 20 ml for the 5 x 20 ml kit (AS 1267) Stable for 14 days at +2 to +8︒C or 24 hours at +15 to +25︒C. Cat. AS 2359 5 x 100 mlReconstitute one vial of Enzyme/Coenzyme/α-oxoglutarate R1b with a portion of Buffer/Substrate R1a and then transfer the entire contents to bottle R1a rinsing bottle R1b several times. Stable for 14 days at +2 to +8︒C or 24 hours at +15 to +25︒C.MATERIALS PROVIDED Buffer/SubstrateEnzyme/Coenzyme/ -oxoglutarateMATERIALS REQUIRED BUT NOT PROVIDEDRandox Assayed Multisera Level 2 (Cat. No. HN 1530) and Level 3 (Cat. No. HE 1532)Randox Calibration Serum Level 3 (Cat. No. CAL 2351) RX series Saline (Cat. No. SA 3854)PROCEDUREAspirate fresh ddH 2O and perform a new Gain Calibration in flow cell mode. Select AST in the Run Test screen and carry out a water blank as instructed.Pipette into a test tube:Sample 0.05 ml Reagent 0.5 mlMix and aspirate into the Rx Monza.CALIBRATION FOR RX MONZAThe use of Saline and Randox Calibration Serum Level 3 isrecommended for calibration. Calibration is recommended with change of reagent lot or as indicated by quality control procedures.FOR MANUAL USEWavelength: 340 nm (Hg 334 nm or Hg 365 nm) Cuvette: 1 cm light path Temperature: 25/30/37︒C Measurement: against airPipette into cuvette: Macro MicroSample 0.2 ml 0.1 ml Enzyme/Coenzyme/ α-oxoglutarate R1 2.0 ml 1.0 mlMix, read initial absorbance after 1 minute. Read again after 1, 2 and 3 minutes. Note: If the absorbance change per minute is between 0.11 and 0.16 at 340/Hg 334 nm 0.06 and 0.08 at Hg 365 nmuse only the values for the first 2 minutes for the calculation.MANUAL CALCULATIONTo calculate the AST activity, use the following formulae:U/l = 1746 x A 340 nm/min U/l = 1780 x A Hg 334 nm/min U/l = 3235 x A Hg 365 nm/minSTANDARDISATIONRandox Calibration Serum Level 3 is traceable to AST reference material JSCC TS01.QUALITY CONTROLRandox Assayed Multisera, Level 2 and Level 3 are recommended for daily quality control. Two levels of controls should be assayed at least once a day. Values obtained should fall within a specified range. If these values fall outside the range and repetition excludes error the following steps should be taken:1. Check instrument settings and light source.2. Check cleanliness of all equipment in use.3. Check water. Contaminants, i.e. bacterial growth, maycontribute to inaccurate results. 4. Check reaction temperature.5. Check expiry date of kit and contents.6. Contact Randox Laboratories Customer Technical Services, Northern Ireland +44 (0) 28 9445 1070.SPECIFICITY/INTERFERENCE (6,7)Gross haemolysis will produce falsely elevated test results. The effects of various drugs on AST activity should be taken intoconsideration in the case of patients receiving large doses of drugs.The analytes below were tested up to the following levels and were found not to interfere: Haemoglobin 250 mg/dl Free Bilirubin 25 mg/dl Conjugate Bilirubin 25 mg/dl Triglycerides 1000 mg/dlIntralipid ® 200 mg/dlA list of substances and conditions known to effect AST activity in vivo is given by both Young et al and Friedman et al. Norepresentation is made by Randox Laboratories Ltd regarding the completeness of these lists and the accuracy of the information contained therein.NORMAL VALUES IN SERUM (8,9) +25︒C +30︒C +37︒C Men up to 18 U/l up to 25 U/l up to 37 U/l Women up to 15 U/l up to 21 U/l up to 31 U/lIt is recommended that each laboratory establish its own reference range to reflect the age, sex, diet and geographical location of the population.SPECIFIC PERFORMANCE CHARACTERISTICS The following performance data were obtained using an Rx Monza analyser running at +37o C.LINEARITYThis method is linear up to 562 U/l. If the sample concentration exceeds this value, dilute the sample 1+9 with 0.9% NaCl solution and re-assay. Multiply the result by 10.SENSITIVITYThe minimum detectable concentration of AST with an acceptable level of precision was determined as 9.3 U/l.PRECISIONIntra AssayLevel 2 Level 3Mean (U/l) 35.6 153SD 1.66 1.47CV(%) 4.65 0.96n 20 20Inter AssayLevel 2 Level 3Mean (U/l) 35.6 153SD 1.77 7.10CV(%) 4.96 4.63n 20 20CORRELATIONThis method (Y) was compared with another commerciallyavailable method (X) and the following linear regression equationobtained:Y = 1.07X + 4.9and a correlation coefficient of r = 0.997543 patient samples were analysed spanning the range 28 to 559U/l.REFERENCES1. Wroblewski F, La Due J.S: Ann Intern Med. 1956; 45: 801.2. Wroblewski F, La Due J.S: Proc Soc Exp Biol Med 1956;91: 569.3. Bergmeyer HU, Bowers GN Jr, et al: Clin Chem 1977; 23:887.4. Bergmeyer HU, Bowers GN Jr, et al: J.Clin Chem ClinBiochem 1980; 18: 521-534.5. Tietz N W: Fundamentals of Clinical Chemistry ed 3.Philadelphia, WB Saunders Co. 1987, pg 372.6. Young D S, et al: Clin Chem 1975, 21; No5.7. Friedman RB, et al: Clin Chem 1980, 26; No4.8. Wallnofer H, Schmidt.E, Schmidt FW, eds: Synopsis derLeberkrankheiten Stuttgart, Georg Thieme Verlag, 1974.9. Thefeld W, et al: Dtsch Med Wschr 1974; 99: 343.Revised 26 Apr 16 biRev. 003THIS PAGE IS INTENTIONALLY BLANK。
药用辅料中英文对照

药用辅料中英文对照药用辅料中英文对照1 阿拉伯胶(Acacia)2 乙酰舒泛钾(Acesulfame Potassium)3 冰醋酸(Acetic Acid,Glacial)4 乙酰枸橼酸三丁酯(AcetyltributylCitrate)5 乙酰枸橼酸三乙酯(AcetyltriethylCitrate)6 人血白蛋白(Albumin)7 乙醇(Alcohol)8 海藻酸(Alginic Acid)9 脂肪族聚酯(Aliphatic Polyesters)10 阿力糖(Alitame)11 杏仁油(Almond Oil)12 维生素E(Alpha Tocopherol)13 氨溶液(Ammonia Solution)14 维生素C(Ascorbic Acid)15 棕榈酸维生素C酯(Ascorbyl Palmitate)16 阿司帕坦(Aspartame)17 绿坡缕石(Attapulgite)18 皂土(Bentonite)19 苯扎氯铵(Benzalkonium Chloride)20 苄索氯铵(Benzethonium Chloride)21 苯甲酸(Benzoic Acid)22 苯甲醇(Benzyl Alcohol)23 苯甲酸苄酯(Benzyl Benzoate)24 溴硝丙二醇(Bronopol)25 丁羟茴醚(Butylated Hydroxyanisole)26 丁羟甲苯(Butylated Hydroxytoluene)27 羟苯丁酯(Butylparaben)28 碳酸钙(Calcium Carbonate)29 无水磷酸氢钙(Calcium Phosphate,Dibasic Anhydrous)30 磷酸氢钙二水合物(Calcium Phosphate,Dibasic Dihydrate)31 磷酸钙(Calcium Phosphate,Tribasic)32 硬脂酸钙(Calcium Stearate)33 硫酸钙(Calcium Sulfate)34 低芥酸菜籽油(Canola Oil)35 卡波姆(Carbomer)36 二氧化碳(Carbon Dioxide)37 羧甲纤维素钙(Carboxymethylcellulose Calcium)38 羧甲纤维素钠(Carboxymethylcellulose Sodium)39 角叉菜胶(Carrageenan)40 蓖麻油(Castor Oil)41 氢化蓖麻油(Castor Oil,Hydro-genated)42 微晶纤维素(Cellulose,Microcr ystalline)43 粉状纤维素(Cellulose,Powdered)44 微粉硅胶微晶纤维素(Cellulose, Silicified Microcrystalline)45 醋酸纤维素(Cellulose Acetate)46 纤维醋法酯(Cellulose Acetate Phthalate)47 角豆胶(Ceratonia)48 十八十六醇(Cetostearyl Alcohol)49 西曲溴铵(Cetrimide)50 十六醇(Cetyl Alcohol)51 壳聚糖(Chitosan)52 氯己定(Chlorhexidine)53 三氯叔丁醇(Chlorobutanol)54 氯甲酚(Chlorocresol)55 一氯二氟乙烷(Chlorodifluoroe-thane)56 氟里昂(Chlorofluorocabons)57 对氯间二甲酚(Chloroxylenol)58 胆固醇(Cholesterol)59 枸橼酸(Citric Acid Monohydrate)60 胶态二氧化硅(微粉硅胶)(Colloidal Silicon Dioxide)61 着色剂(Coloring Agents)62 玉米油(Corn Oil)63 棉籽油(Cottonseed Oil)64 甲酚(Cresol)65 交联羧甲纤维素钠(Croscarmellose Sodium)66 交联聚维酮(Crospovidone)67 环糊精(Cyclodextrins)68 环甲基硅酮(Cyclomethicone)69 苯甲地那铵(Denatonium Benzoate)70 葡萄糖结合剂(Dextrates)71 糊精(Dextrin)72 葡萄糖(Dextrose)73 邻苯二甲酸二丁酯(Dibutyl Phthalate)74 癸二酸二丁酯(Dibutyl Sebacate)75 二乙醇胺(Diethanolamine)76 邻苯二甲酸二乙酯(Diethyl Phthalate)77 二氟乙烷(Difluoroethane)78 二甲硅油(Dimethicone)79 二甲醚(Dimethyl Ether)80 邻苯二甲酸二甲酯(Dimethyl Phthalate)81 二甲亚砜(Dimethyl Sulfoxide)82 多库酯钠(Docusate Sodium)83 依地酸(乙二胺四乙酸)(Edetic Acid)84 乙酸乙酯(Ethyl Acetate)85 乙基麦芽酚(Ethyl Maltol)86 油酸乙酯(Ethyl Oleate)87 乙基香草醛(Ethyl Vanillin)88 乙基纤维素(Ethylcellulose)89 硬脂酸棕榈酸乙二醇酯(Ethylene Glycol Palmitostearate)90 羟苯乙酯(Ethylparaben)91 果糖(Fructose)92 富马酸(Fumaric Acid)93 明胶(Gelatin)94 液体葡萄糖(Glucose,Liquid)95 甘油(Glycerin)96 山萮酸甘油酯(Glyceryl Behenate)97 单油酸甘油酯(Glyceryl Monooleate)98 单硬脂酸甘油酯(Glyceryl Monostearate)99 硬脂酸棕榈酸甘油酯(Glyceryl Palmitostearate)100 四氢呋喃聚乙二醇醚(Glycofurol)101 瓜耳胶(Guar Gum)102 七氟丙烷(HFC)(Heptafluoro-propane)103 海克西定(Hexetidine)104 烷烃类(HC) (Hydrocarbons)105 盐酸(Hydrochloric Acid)106 羟乙纤维素(Hydroxyethyl Cellulose)107 羟乙甲纤维素(Hydroxyethylmethyl Cellulose)108 羟丙纤维素(Hydroxypropyl Cellulose)109 低取代羟丙纤维素(Hydroxypropyl Cellulose,Low-substituted) 110 羟丙甲纤维素(Hypromellose)111 羟丙甲纤维素酞酸酯(Hypromellose Phthalate)112 咪唑烷脲(Imidurea)113 异丙醇(Isopropyl Alcohol)114 肉豆蔻酸异丙酯(Isopropyl Myristate)115 棕榈酸异丙酯(Isopropyl Palmitate)116 白陶土(Kaolin)117 乳酸(Lactic Acid)118 拉克替醇(Lactitol)119 乳糖(Lactose)120 羊毛脂(Lanolin)121 含水羊毛脂(Lanolin,Hydrous)122 羊毛醇(Lanolin Alcohols)123 卵磷脂(Lecithin)124 硅酸镁铝(Magnesium Aluminum Silicate)125 碳酸镁(Magnesium Carbonate)126 氧化镁(Magnesium Oxide)127 硅酸镁(Magnesium Silicate)128 硬脂酸镁(Magnesium Stearate)129 三硅酸镁(Magnesium Trisilicate)130 苹果酸(Malic Acid)131 麦芽糖醇(Maltitol)132 麦芽糖醇溶液(Maltitol Solution)133 麦芽糖糊精(Maltodextrin)134 麦芽酚(Maltol)135 麦芽糖(Maltose)136 甘露醇(Mannitol)137 中链脂肪酸甘油三酯(Medium-chain Triglycerides) 138 葡甲胺(Meglumine)139 薄荷脑(Menthol)140 甲基纤维素(Methylcellulose)141 羟苯甲酯(Methylparaben)142 液体石蜡(Mineral Oil)143 轻质液体石蜡(Mineral Oil,Light)144 液体石蜡羊毛醇(Mineral Oil and Lanolin Alcohols) 145 单乙醇胺(Monoethanolamine)146 谷氨酸一钠(Monosodium Glutamate)147 硫代甘油(Monothioglycerol)148 氮(Nitrogen)149 一氧化二氮(Nitrous Oxide)150 油酸(Oleic Acid)151 橄榄油(Olive Oil)152 石蜡(Paraffin)153 花生油(Peanut Oil)154 凡士林(Petrolatum)155 凡士林羊毛醇(Petrolatum and Lanolin Alcohols)156 苯酚(Phenol)157 苯氧乙醇(Phenoxyethanol)158 苯乙醇(Phenylethyl Alcohol)159 醋酸苯汞(Phenylmercuric Acetate)160 硼酸苯汞(Phenylmercuric Borate)161 硝酸苯汞(Phenylmercuric Nitrate)162 磷酸(Phosphoric Acid)163 波拉克林钾(Polacrilin Potassium)164 泊洛沙姆(Poloxamer)165 葡聚糖(Polydextrose)166 聚乙二醇(Polyethylene Glycol)167 聚氧乙烯(Polyethylene Oxide)168 聚(甲基)丙烯酸树脂(Polymethacr-ylates)169 聚氧乙烯烷基醚(Polyoxyethylene Alkyl Ethers)170 聚氧乙烯蓖麻油衍生物(Polyoxyeth-ylene Castor Oil Derivatives) 171 聚山梨酯(Polyoxyethylene Sorbitan Fatty Acid Esters)172 硬脂酸聚氧乙烯酯(Polyoxyethylene Stearates)173 聚醋酸乙烯酞酸酯(Polyvinyl Acetate Phthalate)174 聚乙烯醇(Polyvinyl Alcohol)175 苯甲酸钾(Potassium Benzoate)176 碳酸氢钾(Potassium Bicarbonate)177 氯化钾(Potassium Chloride)178 枸橼酸钾(Potassium Citrate)179 氢氧化钾(Potassium Hydroxide)180 焦亚硫酸钾(Potassium Metabisulfite)181 山梨酸钾(Potassium Sorbate)182 聚维酮(Povidone)183 丙酸(Propionic Acid)184 没食子酸丙酯(Propyl Gallate)185 碳酸丙烯酯(Propylene Carbonate)186 丙二醇(Propylene Glycol)187 海藻酸丙二醇酯(Propylene Glycol Alginate)188 羟苯丙酯(Propylparaben)189 糖精(Saccharin)190 糖精钠(Saccharin Sodium)191 芝麻油(Sesame Oil)192 虫胶(Shellac)193 二氧化硅二甲硅油(Simethicone)194 海藻酸钠(Sodium Alginate)195 抗坏血酸钠(Sodium Ascorbate)196 苯甲酸钠(Sodium Benzoate)197 碳酸氢钠(Sodium Bicarbonate)198 氯化钠(Sodium Chloride)199 枸橼酸钠二水合物(Sodium Citrate Dihydrate)200 环拉酸钠(Sodium Cyclamate)201 氢氧化钠(Sodium Hydroxide)202 月桂硫酸钠(十二烷基硫酸钠)(Sodium Lauryl Sulfate) 203 焦亚硫酸钠(偏亚硫酸钠)(Sodium Metabisulfite) 204 磷酸氢二钠(Sodium Phosphate,Dibasic)205 磷酸二氢钠(Sodium Phosphate ,Monobasic)206 丙酸钠(Sodium Propionate)207 羧甲淀粉钠(Sodium Starch Glycolate)208 硬脂富马酸钠(Sodium Stearyl Fumarate)209 山梨酸(Sorbic Acid)210 山梨坦酯Sorbitan Esters(Sorbitan Fatty Acid Esters)211 山梨醇(Sorbitol)212 大豆油(Soybean Oil)213 淀粉(Starch)214 预胶化淀粉(Starch,Pregelatinized)215 灭菌玉米淀粉(Starch,Sterilizable Maize)216 硬脂酸(Stearic Acid)217 硬脂醇(Stearyl Alcohol)218 羟糖氯(Sucralose)219 蔗糖(Sucrose)220 可压性蔗糖(Sugar,Compressible)221 蔗糖粉(Sugar,Confectioner’s)222 蔗糖球形颗粒(Sugar Spheres)223 硫酸(Sulfuric Acid)224 葵花籽油(Sunflower Oil)225 氢化植物油(硬脂)栓剂基质(Sup-pository Bases,Hard Fat) 226 滑石粉(Talc)227 酒石酸(Tartaric Acid)228 四氟乙烷(HFC)(Tetrafluoroe-thane)229 硫柳汞(Thimerosal)230 二氧化钛(Titanium Dioxide)231 西黄蓍胶(Tragacanth)232 海藻糖(Trehalose)233 三醋汀(Triacetin)234 枸橼酸三丁酯(Tributyl Citrate)235 三乙醇胺(Triethanolamine)236 枸橼酸三乙酯(Triethyl Citrate)237 香草醛(Vanillin)238 氢化植物油(Vegetable Oil,Hydrogenated)239 水(Water)240 阴离子乳化蜡(Wax,Anionic Emulsifying)241 巴西棕榈蜡(Wax,Carnauba)242 十六醇酯蜡(Wax,Cetyl Esters)243 微晶蜡(Wax,Microcrystalline)244 非离子乳化蜡(聚西托醇乳化蜡)(Wax,Nonionic Emulsifying) 245 白蜡(Wax,White)246 黄蜡(Wax,Yellow)247 黄原酸胶(Xanthan Gum)248 木糖醇(Xylitol)796249 玉米朊(玉米蛋白)(Zein)250 硬脂酸锌(Zinc Stearate)。
喹啉合成
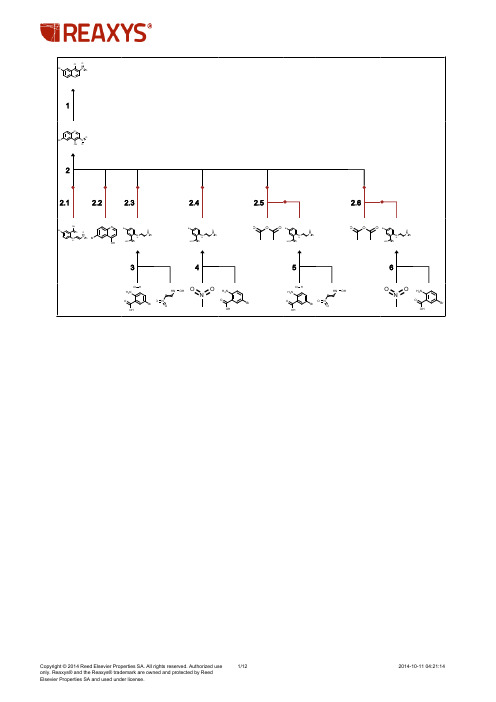
1/12
2014-10-11 04:21:14
Cl N Br O Br OH N N O
O N O
1
Rx-ID: 23335695 View in Reaxys Yield Conditions & References
93 %
Example Name 1.II.1 To a solution of 7 (15 g, 0.056 mol) in acetonitrile (80 mL) and DIPEA (15.9 g, 0.123 mol), was added POCI3 (17.1 g, 0.112 mol) dropwisely at O0C. The reaction temperature was slowly raised to 1000C for 2 hours. The mixture was cooled and poured onto ice-water. After Neutralized with aq NaHCC>3, extracted with ethyl acetate, and dried over Na2SO4, the crude product was obtained by evaporating of solution to dryness (15 g, 93percent) as a brown solid. MS (m/z) (M++H): 287, 289. With N-ethyl-N,N-diisopropylamine, trichlorophosphate in acetonitrile, Time= 2h, T= 0 - 100 °C Patent; PROGENICS PHARMACEUTICALS, INC.; WO2009/155527; (2009); (A2) English; WO 2009/155527 A2 View in Reaxys
Novex Tris-Glycine凝胶电泳说明书

Instructions are provided below for electrophoresis of Novex ® Tris-Glycine Gels using the XCell SureLock ® Mini-Cell. For details, refer to the Novex ® Technical Guide available at /manuals or contact Technical Support.Denaturing ElectrophoresisReagent Reduced SampleNon-reduced Sample Sample x μLx μL Tris-Glycine SDS Sample Buffer (2X) 5 μL5 μL NuPAGE ® Reducing Agent (10X) 1 μL--Deionized Water to 4 μLto 5 μL Total Volume 10 μL10 μLHeat samples at 85˚C for 2 minutes.Add 100 mL 10X Novex ® Tris-Glycine SDS Running Buffer to 900 mL de-ionized water to prepare 1X Tris-Glycine SDS Running Buffer.Load the appropriate concentration of your protein sample on the gel .Fill the Upper Buffer Chamber with 200 mL and the Lower Buffer Chamber with 600 mL of 1X Tris-Glycine SDS Running Buffer.Voltage: 125 V constant Run Time:90 minutes (dependent on gel percentage)Expected Current: 30–40 mA/gel (start); 8–12 mA/gel (end)PrepareSamples Prepare 1XBuffer Load Sample Load BufferRunConditions QUICK REFERENCEIntended Use: For research use only. Not for human or animal therapeuticor diagnostic use.Novex ® Tris-Glycine GelsPub. Part No. IM-6000 MAN0003681 Rev. Date 27 June 2011Non-Denaturing (Native) ElectrophoresisRe gent S a mple Sample x μLTris-Glycine Native Sample Buffer (2X) 5 μLDeionized Water to 5 μLTotal Volume 10 μLDo not heat samples for native electrophoresis.Add 100 mL 10X Tris-Glycine Native Running Buffer to 900 mL deionized water to prepare 1X Tris-Glycine Native Running Buffer. Load the appropriate concentration of your protein sample on the gel.Fill the Upper Buffer Chamber with 200 mL and the Lower Buffer Chamber with 600 mL of 1X Tris-Glycine Native Running Buffer.Voltage: 125 V constant Run Time: 1–12 hours Expected Current: 6–12 mA/gel (start); 3–6 mA/gel (end)For blotting denaturing and native gels, use 1X Tris-Glycine Transfer Buffer with 20% methanol. Perform blotting at 25 V constant for 1–2 hours using the XCell II ™ Blot Module. The expected start current is 100 mA.©2011 Life Technologies Corporation. All rights reserved. The trademarks mentioned herein are the property of Life Technologies Corporation or their respective owners.PrepareSamples Prepare 1XBuffer Load Sample Load BufferRunConditionsBlot Gel Limited Use Label License: Research Use Only: The purchase of this product conveys to the purchaser the limited, non-transferable right to use the purchased amount of the product only to perform internal research for the sole benefi t of the purchaser. No right to resell this product or any of its components is conveyed expressly, by implication, or by estoppel. This product is for internal research purposes only and is not for use in com-mercial applications of any kind, including, without limitation, quality control and commercial services such as reporting the results of purchaser’s activitiesforafeeorotherformofconsideration.Forinformationonobtainingadditionalrights,*******************************************Licensing, Life Technologies, 5791 Van Allen Way, Carlsbad, California 92008.Novex ® Tris-Glycine GelsFor support visit /support 。
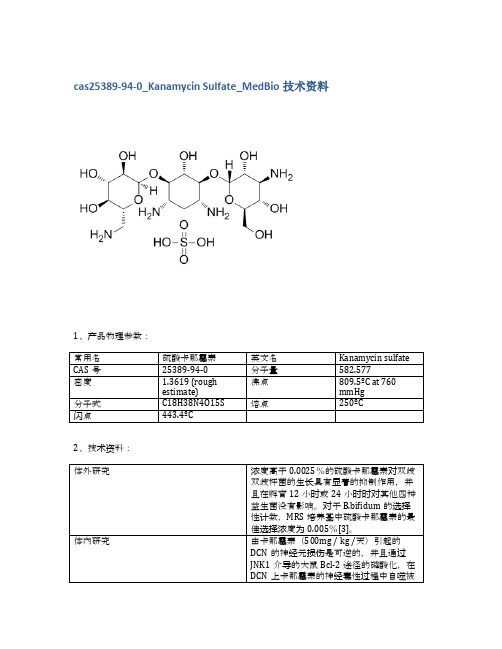
cas25389-94-0_Kanamycin Sulfate_MedBio技术资料
Fialuridine
69123-98-4
10mg
≥98%
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
MED15240
Cephalexin
Cephalexin
15686-71-2
10mM (in 1mL H2O)
≥98%
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
MED15367
155347-36-7
10mM (in 1mL DMSO)
≥98%
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
MED15228
Meropenem trihydrate
Meropenem trihydrate
119478-56-7
10mM (in 1mL H2O)
≥98%
品牌
货号
中文名称
英文名称
5mg
≥98%
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
MED15280
Pefloxacin Mesylate Dihydrate
Pefloxacin Mesylate Dihydrate
149676-40-4
100mg
≥98%
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
MED15350
CAS
包装
纯度
MedBio
MED15291
冰冻切片神经组织金属锌改良蒂姆(Neo-TIMM)染色试剂盒

冰冻切片神经组织金属锌改良蒂姆(Neo-TIMM)染色试剂盒产品说明书(中文版)主要用途冰冻切片神经组织金属锌改良蒂姆(Neo-TIMM)染色试剂是一种旨在使用新型蒂姆硫化法银染技术,分析存档中的冰冻组织切片里神经系统中含锌神经细胞结构分布的权威而经典的技术方法。
该技术经过精心改良蒂姆(Timm)方法、成功实验证明的。
主要适用于冰冻脑组织(海马齿状回门区hilus of dentate gyrus、安蒙氏角cornu ammonis、大脑皮层、丘脑、杏仁核amygdala nuclei或外周神经组织切片,例如海马(hippocampus)中苔藓纤维区(mossy fiber region)和齿状分子层(dentate molecular layer)等含锌神经细胞体,例如锥体细胞(pyramidal cells)、齿状颗粒细胞(dentate granule cells)等检测。
广泛用于脑神经病理生理,尤其是退行性神经病变包括癫痫、自闭症、精神分裂症、脑损伤、脑缺血等疾病的研究。
产品严格无菌,即到即用,操作简捷,性能稳定,显色清晰。
技术背景蒂姆硫化法银染(Timm’s sulfide silver staining)是由蒂姆建立的用于观察脑组织和其它组织中(例如胰腺、小肠、睾丸、肾脏等)微量金属元素锌,以及其它重金属元素,例如铜、镍、钴和铁等的染色技术。
主要用于观察中枢神经系统中含锌神经元,以及新轴突分枝(sprouted axon)和轴突终端(axon terminal)等结构,分析大脑灰质内部的海马中轴突终端(突触泡囊synaptic vesicle)、苔藓终端(齿状颗粒细胞dentate granule cells)中的反应性锌的分布,与突触活性和膜去极化的关系,研究神经退行性和癫痫病理性变化机制。
其原理在于使用硫化物,与组织中的游离金属元素反应成为不溶性复合物沉积,进而在还原剂的作用下,金属硫化物催化还原离子银为可见金属银沉积,呈现黑色,此为金属自显影术(autometallography,AMG)。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Caution: Not fully tested. For research purposes only Medchemexpress LLC
Байду номын сангаас
m o c . s s e r p x e m e h c d e m . w w w : b e AW Sm Uo ,c 0 4. 5s 8s 0e r p Jx Ne ,m n e oh t c e cd ne i rm P @ ,o y f an Wi : l ni oa sm n iE k l i W 8 1
Product Data Sheet
Product Name: CAS No.: Cat. No.: MWt: Formula: Purity :
Triamterene 396-01-0 HY-B0575 253.26 C12H11N7 >98%
Solubility:
DMSO 20 mg/mL; Water <1 mg/mL
Mechanisms: Pathways:Membrane Tranporter/Ion Channel; Target:Sodium Channel g y Biological Activity: Triamterene blocks epithelial Na+ channel (ENaC) in a voltage-dependent manner, which used as a mild diuretic. Target: Sodium Channel Triamterene blocked rENaC in a voltage-dependent manner, and was 100-fold less potent than amiloride at pH 7.5. At -90 mV and -40 mV, the IC50 values were 5 microM and 10 microM, respectively. The blockage by triamterene, which is a weak base with a pKa of 6.2, was dependent on the extracellular pH. The IC50 was 1 microM at pH 6.5 and only 17 microM at pH 8.5 [1]. Triamterene ( (TA) ) is partly p y eliminated by y a first-pass-effect. p The main metabolite of TA is OH-TAester, which is pharmacologically active [2]. ... References: [1]. Busch, A.E., et al., Blockade of epithelial Na+ channels by triamterenes - underlying mechanisms and molecular basis. Pflugers Arch, 1996. 432(5): p. 760-6. [2]. Gilfrich, H.J., et al., Pharmacokinetics of triamterene after i.v. administration to man: determination of bioavailability. y Eur J Clin Pharmacol, 1983. 25(2): ( ) p p. 237-41.
