SB-674042_483313-22-0_DataSheet_MedChemExpress
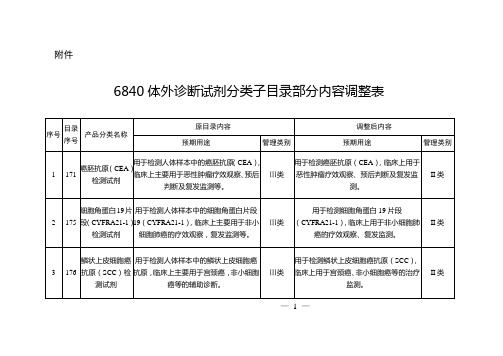
6840体外诊断试剂分类子目录部分内容调整表
用于检测泌乳素,临床上用于泌乳素瘤的治疗监测。
II类
27
206
降钙素检测试剂
用于检测人体样本中的降钙素(CT),临床上主要用于甲状腺髓样癌、小细胞肺癌的辅助诊断。
Ⅲ类
用于检测降钙素,临床上用于甲状腺髓样癌、小细胞肺癌的治疗监测。
II类
28
207
芳香基硫酸酯酶检测试剂
用于检测人体样本中的芳香基硫酸酯酶,临床上主要用于乳腺癌、结直肠癌的辅助诊断。
附件
6840体外诊断试剂分类子目录部分内容调整表
序号
目录序号
产品分类名称
原目录内容
调整后内容
预期用途
管理类别
预期用途
管理类别
1
171
癌胚抗原(CEA)检测试剂
用于检测人体样本中的癌胚抗原(CEA),临床上主要用于恶性肿瘤疗效观察、预后判断及复发监测等。
Ⅲ类
用于检测癌胚抗原(CEA),临床上用于恶性肿瘤疗效观察、预后判断及复发监测。
Ⅲ类
用于检测癌抗原15-3(CA15-3),临床上用于乳腺癌治疗疗效及预后观察。
II类
8
181
糖类抗原19-9(CA19-9)检测试剂
用于检测人体样本中的糖类抗原19-9(CA19-9),临床上主要用于胰腺等消化道恶性肿瘤的辅助诊断,疗效监测等。
Ⅲ类
用于检测糖类抗原19-9(CA19-9),临床上用于胰腺等消化道恶性肿瘤的疗效监测。
II类
21
196
胃蛋白酶原(PG)Ⅱ检测试剂
用于检测人体样本中的胃蛋白酶原(PG)Ⅱ。PGⅡ的浓度水平及PGI/Ⅱ的比值可用于胃癌的辅助诊断。
Ⅲ类
用于检测胃蛋白酶原(PG)Ⅱ,临床上用于监测PGII的浓度水平及PGI/Ⅱ的比值可用于胃癌的治疗监测。
碧云天细胞自噬染色检测试剂盒(MDC法)说明书

细胞自噬染色检测试剂盒(MDC 法)产品简介:碧云天生产的细胞自噬染色检测试剂盒(MDC 法),即Autophagy Staining Assay Kit with MDC ,是一种使用丹酰尸胺,也称单丹磺酰尸胺、丹酰尸胺或丹酰戊二胺(monodansylcadaverine, MDC)作为荧光探针快速便捷地检测细胞自噬的试剂盒。
自噬(autophagy)是一种在进化上高度保守的通过溶酶体吞噬并降解部分自身组分的细胞内分解代谢途径。
自噬与多种生理功能有关,在饥饿等环境条件下,细胞通过自噬降解多余或异常的细胞内组分,为细胞的生存提供能量及原材料,促进生物体的生长发育、细胞分化及对环境变化产生应答。
自噬异常与多种病理过程如肿瘤、神经退行性疾病、代谢疾病、病原体感染等都有密切关系。
由于细胞自噬在生理和病理过程中都有重要作用,自噬已经成为细胞生物学领域的一个研究热点。
MDC 是细胞自噬检测最常用的荧光探针之一。
MDC 可以通过离子捕获(ion trapping)和与膜脂的特异性结合,从而特异性标记自噬体(autophagosome),也称autophagic vacuole ,因而常用于细胞自噬的检测。
MDC 是一种嗜酸性荧光探针,很多酸性膜性结构也会被MDC 染色,因此MDC 染色时正常的细胞也会有一定的染色背景。
本产品的染色原理决定了本产品只能用于培养的细胞或者组织的细胞自噬荧光染色检测,不能用于冻存的或固定的细胞、组织或者组织切片的染色检测。
使用本产品染色后可以通过荧光显微镜拍照观察,也可以通过荧光酶标仪或流式细胞仪进行荧光检测。
荧光显微镜观察时可以使用紫外区激发光激发,发出绿色荧光。
荧光酶标仪或流式细胞仪推荐的激发波长为335nm (330-360nm 均可),发射波长为512nm (510-540nm 均可)。
本产品用于细胞自噬染色的效果参考图1。
图1. 细胞自噬染色检测试剂盒(MDC 法)的染色效果图。
碧云天生物技术 Beyotime Biotechnology 生物素标记EMSA探针说明书

碧云天生物技术/Beyotime Biotechnology订货热线:400-168-3301或800-8283301订货e-mail:******************技术咨询:*****************网址:碧云天网站微信公众号生物素标记EMSA探针-β-Catenin/TCF (0.2μM)产品编号产品名称包装GS018B 生物素标记EMSA探针-β-Catenin/TCF (0.2µM) 200µl产品简介:生物素标记EMSA探针-β-Catenin/TCF是用于EMSA(也称gel shift)研究的生物素(Biotin)标记的β-Catenin/TCF consensus oligonucleotide。
这个生物素标记的双链寡核苷酸含有公认的β-Catenin/TCF结合位点,可以用作EMSA研究时的探针。
β-Catenin/TCF consensus oligo的序列如下:5'-CCC TTT GAT CTT ACC-3'3'-GGG AAA CTA GAA TGG-5'本生物素标记EMSA探针已经过纯化,可以直接用于EMSA结合反应。
本生物素标记EMSA探针可以和碧云天的化学发光法EMSA试剂盒(GS009)配套使用。
一个包装的生物素标记探针可以进行约200-400个样品的EMSA检测。
包装清单:产品编号产品名称包装GS018B 生物素标记EMSA探针-β-Catenin/TCF (0.2µM) 200µl—说明书1份保存条件:-20ºC保存,一年有效。
注意事项:避免加热到40ºC以上,温度过高会导致双链DNA探针解聚成单链。
而单链无法用于EMSA研究。
对于基于生物素标记的EMSA检测的详细操作可以参考碧云天的化学发光法EMSA试剂盒(GS009)的使用说明。
本产品仅限于专业人员的科学研究用,不得用于临床诊断或治疗,不得用于食品或药品,不得存放于普通住宅内。
Trigonox B(滴苷但羊水)产品数据表单说明书

Product Data SheetTrigonox BDi-tert-butyl peroxideTrigonox® B is a pure peroxide in liquid form.CAS number110-05-4EINECS/ELINCS No. 203-733-6TSCA statuslisted on inventory Molecular weight 146.2Active oxygen contentperoxide10.94%SpecificationsAppearance Clear liquidAssay≥ 99.0 %ApplicationsTrigonox® B (Di-tert-butyl peroxide) can be used for the market segments: polymer production, polymer crosslinking and acrylics production with their different applications/functions. For more information please check our website and/or contact us.Half-life dataThe reactivity of an organic peroxide is usually given by its half-life (t½) at various temperatures. For Trigonox® B in chlorobenzene half-life at other temperatures can be calculated by using the equations and constants mentioned below:0.1 hr at 164°C (327°F)1 hr at 141°C (286°F)10 hr at 121°C (250°F)Formula 1kd = A·e-Ea/RTFormula 2t½ = (ln2)/kdEa153.46 kJ/moleA 4.20E+15 s-1R8.3142 J/mole·KT(273.15+°C) KThermal stabilityOrganic peroxides are thermally unstable substances which may undergo self-accelerating decomposition. The lowest temperature at which self-accelerating decomposition may occur with a substance in the packaging as used for transport is the Self-Accelerating Decomposition Temperature (SADT). The SADT is determined on the basis of the Heat Accumulation Storage Test.SADT80°C (176°F)Method The Heat Accumulation Storage Test is a recognized test method for thedetermination of the SADT of organic peroxides (see Recommendations on theTransport of Dangerous Goods, Manual of Tests and Criteria - United Nations, NewYork and Geneva).StorageDue to the relatively unstable nature of organic peroxides, a loss of quality will occur over a period of time. To minimize the loss of quality, Nouryon recommends a maximum storage temperature (Ts max. ) for each organic peroxide product.Ts Max.40°C (104°F) andTs Min.-30°C (-22°F) to prevent crystallizationNote When stored according to these recommended storage conditions, Trigonox® Bwill remain within the Nouryon specifications for a period of at least 6 months afterdelivery.Packaging and transportIn North America Trigonox® B is packed in non-returnable, five gallon polyethylene containers of 30 lb net weight and steel drums of 100 or 340 lb net weight. In other regions the standard packaging is a 30-liter HDPE can (Nourytainer®) for 20 kg peroxide. Delivery in a 200 l steel drum for 150 kg peroxide is also possible in a number of countries. Both packaging and transport meet the international regulations. For the availability of other packed quantities consult your Nouryon representative. Trigonox® B is classified as Organic peroxide type E; liquid, Division 5. 2; UN 3107.Safety and handlingKeep containers tightly closed. Store and handle Trigonox® B in a dry well-ventilated place away from sources of heat or ignition and direct sunlight. Never weigh out in the storage room. Avoid contact with reducing agents (e. g. amines), acids, alkalis and heavy metal compounds (e. g. accelerators, driers and metal soaps). Please refer to the Safety Data Sheet (SDS) for detailed information on the safe storage, use and handling of Trigonox® B. This information should be thoroughly reviewed prior to acceptance of this product. The SDS is available at /sds-search.Major decomposition productsAcetone, Methane, tert-ButanolAll information concerning this product and/or suggestions for handling and use contained herein are offered in good faith and are believed to be reliable.Nouryon, however, makes no warranty as to accuracy and/or sufficiency of such information and/or suggestions, as to the product's merchantability or fitness for any particular purpose, or that any suggested use will not infringe any patent. Nouryon does not accept any liability whatsoever arising out of the use of or reliance on this information, or out of the use or the performance of the product. Nothing contained herein shall be construed as granting or extending any license under any patent. Customer must determine for himself, by preliminary tests or otherwise, the suitability of this product for his purposes.The information contained herein supersedes all previously issued information on the subject matter covered. The customer may forward, distribute, and/or photocopy this document only if unaltered and complete, including all of its headers and footers, and should refrain from any unauthorized use. Don’t copythis document to a website.Trigonox® and Nourytainer are registered trademarks of Nouryon Functional Chemicals B.V. or affiliates in one or more territories.Contact UsPolymer Specialties Americas************************Polymer Specialties Europe, Middle East, India and Africa*************************Polymer Specialties Asia Pacific************************2022-6-30© 2022Polymer crosslinking Trigonox B。
XMD8-87_DataSheet_MedChemExpress

Inhibitors, Agonists, Screening Libraries Data SheetBIOLOGICAL ACTIVITY:XMD8–87 is a potent TNK2 inhibitor with IC 50 values of 38 and 113 nM for the D163E and R806Q mutations, respectively.IC50 & Target: IC50: 38 nM (TNK2, D163E mutation), 113 nM (TNK2, R806Q mutation)[1]In Vitro: XMD8–87 potently inhibits the growth of the TNK2 mutant expressing cell lines while having little or no effect on the control cells out to the highest tested concentrations (1,000 nM). XMD8–87 has IC 50s of 38 nM and 113 nM for the D163E and R806Q mutations. The effects of XMD8–87 on TNK2 cell lines are largely due to on–target effects on TNK2. Auto–phosphorylation of overexpressed TNK2 mutants could be blocked with TNK2 inhibitor XMD8–87[1].PROTOCOL (Extracted from published papers and Only for reference)Kinase Assay:[1]Kinase targets are tested with biochemical enzymatic kinase assays using the SelectScreen Kinase Profiling Service to determine IC 50 values. The compounds (XMD8–87) are assayed at 10 concentrations (3–fold serial dilutions starting from 1 μM) at an ATP concentration equal to the ATP Km [1].Cell Assay:[1]Cells are treated with the following inhibitors for 72 hours: dasatinib, AIM–100, XMD8–87 and XMD16–5. Cell viability is measured using a methanethiosulfonate (MTS)–based assay and absorbance (490 nm) is read at 1 and 3 hours after adding reagent [1].References:[1]. Maxson JE, et al. Identification and Characterization of Tyrosine Kinase Nonreceptor 2 Mutations in Leukemia through Integration of Kinase Inhibitor Screening and Genomic Analysis.Product Name:XMD8–87Cat. No.:HY-15811CAS No.:1234480-46-6Molecular Formula:C 24H 27N 7O 2Molecular Weight:445.52Target:Tyrosinase Pathway:Metabolic Enzyme/Protease Solubility:DMSO: ≥ 26 mg/mLCaution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@ Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
_15国超说明书用药政策的循证评价

Evidence-Based Evaluation on Off-Label Drug Use Policies in 15 Countries
ZHANG Ling-li1,2, LI You-ping1*, ZENG Li-nan2, LIANG Yi2,3, HU Die2,3, LIU Yi2,3, LV Juan2,3
CJEBM • 426 •
© 2012 Editorial Board of Chin J Evid-based Med
中国循证医学杂志 2012, 12(4): 426~435
论 著 • 二次研究
tries. The right to prescribe off-label drug was defined in Britain and Ireland; b) Medical staff had to take the responsibility of off-label drug use in the country where the duty regulations were formulated; and c) Ten countries published guidelines or statements related to off label drug use by their official departments and academic organizations. And the regulation included the following procedures: firstly, to obtain the relative information and evidence; secondly, to get the informed consent; thirdly, to be approved by the ethics committee and/or pharmacy administration committee; fourthly, to record the reasons and effectiveness of off-label use; fifthly, to monitor the adverse reactions of off-label drug use. Besides monitoring the medical institutes, the pharmaceutical companies had also be monitored which included the following 3 aspects: a) to require companies to train specialized staffs to answer the questions related to off-label drug use; b) to open the contact information of medical departments of companies; and c) to prohibit preaching and advertising the off-label drug use. Conclusion Off-label drug use has its rationality and necessity. To protect the safety of patients, avoid the risk for hospitals and medical staffs, it requires formulating relative regulations soon in order to manage the off-label drug use in China. As a developing country, China is different from the developed countries in health care system. Therefore, when formulating the regulations, it is necessary to perform evidence-based evaluation on each country’s laws, regulations and guidelines about off-label drug use, with Chinese national conditions and experts’ opinions in combination. After a regulation is preliminarily drawn up, it needs to be put into pilot practice, and then revised and spread to the whole country.
美国贝克曼库尔特流式细胞分析仪

美国贝克曼库尔特流式细胞分析仪(Beckman coulter cell)产品型号:Cell Lab Quanta SC当前价格:0.00元产品数量:0新旧程度:全新有效期至:0000-00-00所在地:产品简介:仪器简介:T细胞亚群检测的CD45/CD4/CD8/CD3、CD45/CD56/CD19/CD3;阵发性血红蛋白尿(PNH)检测的CD55、CD59;血小板无力症(GT)检测的CD41、CD61等等详细信息仪器简介:T细胞亚群检测的CD45/CD4/CD8/CD3、CD45/CD56/CD19/CD3;阵发性血红蛋白尿(PNH)检测的CD55、CD59;血小板无力症(GT)检测的CD41、CD61等等。
但对于白血病/淋巴瘤免疫分型,国际上迄今为止也没有统一的抗体组合。
在2000年国际细胞分析学会(ISAC)大会上,临床血细胞计数协会组织了一次国际专家会议,以期对检测血液淋巴系统肿瘤所需最少、最有效的单抗数达成共识。
75%与会者一致认为,对于慢性淋巴系统增殖性疾病(CLD)有9种单抗:CD5,CD19,κ,λ,CD3,CD20,CD23,CD10,CD45对初诊来说是最基本的。
淋巴瘤和CLD相似,需要至少12-16种单抗。
对于急性白血病(AL),75%的与会者认为大约13-15种单抗是最基本的:CD10,CD19,CD79a,CD13,CD33,CD34,CD45,CD2,MPO,CD7,CD14,CD3,HLA-DR等,对初步鉴别白血病系列是必需的。
其他一些(CD16,CD56,CDw65,TdT,cyCD3)可能对某些病例有用。
几乎所有的投票者都认为,要对急性白血病完善分类所需单抗的恰当数量平均为20-24种。
但这些抗体之间组合也是一大难题,目前也无统一规定(如表二)。
大会多数发言者(11/13)指出,对已确诊病人的监护和分期来说,仅需较少单抗。
抗体的质量控制是实验的关键环节。
抗体的质量包括其特异性、灵敏度、精密度。
3-TYP_DataSheet_MedChemExpress

Inhibitors, Agonists, Screening Libraries Data SheetBIOLOGICAL ACTIVITY:3–TYP is a selective SIRT3 inhibitor, with an IC 50 of 16 nM, more potent over SIRT1 (IC 50=88 nM), SIRT2 (IC 50=92 nM).IC50 & Target: IC50: 16 nM (SIRT3), 88 nM (SIRT1), 92 nM (SIRT2)[3]In Vitro: 3–TYP inhibits melatonin–enhanced SIRT3 activity but does not affect SIRT3 protein expression. 3–TYP pretreatment reverses the protective effects of melatonin on cadmium (Cd)–induced mitochondrial–derived O2•- production and autophagic cell death.3–TYP significantly attenuates melatonin–induced increases in deacetylated–SOD2 expression and SOD2 activity in HepG2 cells exposed to Cd [1].In Vivo: 3–TYP (50 mg/kg, i.p.) does not significantly influence the LVEF, LVFS, infarct size, serum LDH levels, apoptosis, and oxidative stress compared with those of the Sham group. Moreover, 3–TYP has little effect on gp91phox, Nrf2, NQO 1, Bax, Bcl–2, Caspase–3,and cleaved Caspase–3 expression levels, compared with the Sham group. 3–TYP significantly decreases SIRT3 activity and increases the acetylation of SOD2 compared with that in the control group, without influencing SIRT3 expression. 3–TYP attenuates thecardioprotective effects of melatonin by decreasing the LVEF and LVFS after 24 hour of reperfusion. 3–TYP also increases the infarct size, serum LDH levels, and apoptotic ratio compared with those in the IR+Mel group [2].PROTOCOL (Extracted from published papers and Only for reference)Cell Assay:[1]Cell viability is analyzed using Cell Counting Kit–8. Briefly, 1×104 cells are inoculated into 96–well plates. After being treated, 90 μL of medium and 10 μL of CCK–8 solution are added to each well. The cells are then incubated at 37°C for 2 h. After incubation, the absorption at 450 nm is measured using an Infinite? M200 Microplate Reader. The results are expressed as apercentage of the control. The cell death is also evaluated using the trypan blue assay. HepG2 cells are plated in the 6–well plates (5×105 cells per well) and incubated for 24 h. After being treated with Cd or melatonin, the cells are detached with 300 μLtrypsin–EDTA solution. The mixture of detached cells is centrifugated at 300 g for 5 min. Then, the residue is combined with 800 μL trypan blue solution and dispersed. After 3 min staining, cells are counted using an automated cell counter. The dead cells are stained with the blue color. Cell mortality (%) is expressed as percentage of the dead cell number/the total cell number.Animal Administration: 3–TYP is formulated in 1% ethanol.[2]In brief, male C57BL/6 mice are anesthetized with 2% isoflurane, andmyocardial ischemia is produced by temporarily exteriorizing the heart via a left thoracic incision and placing a 6–0 silk suture slipknot around the left anterior descending coronary artery. After 30 minutes of myocardial ischemia, the slipknot is released, and the myocardium is reperfused for 3 hour (for western blot analysis and oxidative stress measurement) or 24 hour (for cardiac function,apoptotic index and infarct size determination). Sham–operated mice undergo the same surgical procedures except the suture placed under the left coronary artery is not tied. Ten minutes before reperfusion, mice are randomized to receive either vehicle (1% ethanol)or melatonin (20 mg/kg) by intraperitoneal injection. C57BL/6 mice are randomly divided into the following groups: (i) Sham group:mice underwent the sham operation and are treated with vehicle (1% ethanol); (ii) Mel group: mice are treated with melatonin (20Product Name:3–TYP Cat. No.:HY-108331CAS No.:120241-79-4Molecular Formula:C7H6N4Molecular Weight:146.15Target:Sirtuin Pathway:Cell Cycle/DNA Damage; Epigenetics Solubility:DMSO: ≥ 150 mg/mLmg/kg via intraperitoneal injection); (iii) IR+V group: mice underwent the MI/R operation and are treated with vehicle (1% ethanol); (iv) IR+Mel group: mice underwent the MI/R operation and are treated with melatonin (20 mg/kg via intraperitoneal injection 10 minutes before reperfusion); (v) IR+Mel+3–TYP group: mice are pretreated with 3–TYP (3–TYP is intraperitoneally injected at a dose of 50mg/kg every 2 days for a total of three doses prior to the MI/R surgery), subjected to the MI/R operation, and treated with melatonin (20 mg/kg via intraperitoneal injection 10 minutes before reperfusion); and (vi) IR+3–TYP group: mice are pretreated with 3–TYP and then subjected to the MI/R operation.References:[1]. Pi H, et al. SIRT3–SOD2–mROS–dependent autophagy in cadmium–induced hepatotoxicity and salvage by melatonin. Autophagy. 2015;11(7):1037–51.[2]. Zhai M, et al. Melatonin ameliorates myocardial ischemia reperfusion injury through SIRT3–dependent regulation of oxidative stress and apoptosis. J Pineal Res. 2017 Sep;63(2).[3]. Galli U, et al. Identification of a sirtuin 3 inhibitor that displays selectivity over sirtuin 1 and 2. Eur J Med Chem. 2012 Sep;55:58–66.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
SB-674042_SDS_MedChemExpress
Inhibitors, Agonists, Screening LibrariesSafety Data Sheet Revision Date:May-24-2017Print Date:May-24-20171. PRODUCT AND COMPANY IDENTIFICATION1.1 Product identifierProduct name :SB-674042Catalog No. :HY-10898CAS No. :483313-22-01.2 Relevant identified uses of the substance or mixture and uses advised againstIdentified uses :Laboratory chemicals, manufacture of substances.1.3 Details of the supplier of the safety data sheetCompany:MedChemExpress USATel:609-228-6898Fax:609-228-5909E-mail:sales@1.4 Emergency telephone numberEmergency Phone #:609-228-68982. HAZARDS IDENTIFICATION2.1 Classification of the substance or mixtureNot a hazardous substance or mixture.2.2 GHS Label elements, including precautionary statementsNot a hazardous substance or mixture.2.3 Other hazardsNone.3. COMPOSITION/INFORMATION ON INGREDIENTS3.1 SubstancesSynonyms:SB 674042; SB674042Formula:C24H21FN4O2SMolecular Weight:448.51CAS No. :483313-22-04. FIRST AID MEASURES4.1 Description of first aid measuresEye contactRemove any contact lenses, locate eye-wash station, and flush eyes immediately with large amounts of water. Separate eyelids with fingers to ensure adequate flushing. Promptly call a physician.Skin contactRinse skin thoroughly with large amounts of water. Remove contaminated clothing and shoes and call a physician.InhalationImmediately relocate self or casualty to fresh air. If breathing is difficult, give cardiopulmonary resuscitation (CPR). Avoid mouth-to-mouth resuscitation.IngestionWash out mouth with water; Do NOT induce vomiting; call a physician.4.2 Most important symptoms and effects, both acute and delayedThe most important known symptoms and effects are described in the labelling (see section 2.2).4.3 Indication of any immediate medical attention and special treatment neededTreat symptomatically.5. FIRE FIGHTING MEASURES5.1 Extinguishing mediaSuitable extinguishing mediaUse water spray, dry chemical, foam, and carbon dioxide fire extinguisher.5.2 Special hazards arising from the substance or mixtureDuring combustion, may emit irritant fumes.5.3 Advice for firefightersWear self-contained breathing apparatus and protective clothing.6. ACCIDENTAL RELEASE MEASURES6.1 Personal precautions, protective equipment and emergency proceduresUse full personal protective equipment. Avoid breathing vapors, mist, dust or gas. Ensure adequate ventilation. Evacuate personnel to safe areas.Refer to protective measures listed in sections 8.6.2 Environmental precautionsTry to prevent further leakage or spillage. Keep the product away from drains or water courses.6.3 Methods and materials for containment and cleaning upAbsorb solutions with finely-powdered liquid-binding material (diatomite, universal binders); Decontaminate surfaces and equipment by scrubbing with alcohol; Dispose of contaminated material according to Section 13.7. HANDLING AND STORAGE7.1 Precautions for safe handlingAvoid inhalation, contact with eyes and skin. Avoid dust and aerosol formation. Use only in areas with appropriate exhaust ventilation.7.2 Conditions for safe storage, including any incompatibilitiesKeep container tightly sealed in cool, well-ventilated area. Keep away from direct sunlight and sources of ignition.Recommended storage temperature:Powder-20°C 3 years4°C 2 yearsIn solvent-80°C 6 months-20°C 1 monthShipping at room temperature if less than 2 weeks.7.3 Specific end use(s)No data available.8. EXPOSURE CONTROLS/PERSONAL PROTECTION8.1 Control parametersComponents with workplace control parametersThis product contains no substances with occupational exposure limit values.8.2 Exposure controlsEngineering controlsEnsure adequate ventilation. Provide accessible safety shower and eye wash station.Personal protective equipmentEye protection Safety goggles with side-shields.Hand protection Protective gloves.Skin and body protection Impervious clothing.Respiratory protection Suitable respirator.Environmental exposure controls Keep the product away from drains, water courses or the soil. Cleanspillages in a safe way as soon as possible.9. PHYSICAL AND CHEMICAL PROPERTIES9.1 Information on basic physical and chemical propertiesAppearance White to off-white (Solid)Odor No data availableOdor threshold No data availablepH No data availableMelting/freezing point No data availableBoiling point/range No data availableFlash point No data availableEvaporation rate No data availableFlammability (solid, gas)No data availableUpper/lower flammability or explosive limits No data availableVapor pressure No data availableVapor density No data availableRelative density No data availableWater Solubility No data availablePartition coefficient No data availableAuto-ignition temperature No data availableDecomposition temperature No data availableViscosity No data availableExplosive properties No data availableOxidizing properties No data available9.2 Other safety informationNo data available.10. STABILITY AND REACTIVITY10.1 ReactivityNo data available.10.2 Chemical stabilityStable under recommended storage conditions.10.3 Possibility of hazardous reactionsNo data available.10.4 Conditions to avoidNo data available.10.5 Incompatible materialsStrong acids/alkalis, strong oxidising/reducing agents.10.6 Hazardous decomposition productsUnder fire conditions, may decompose and emit toxic fumes.Other decomposition products - no data available.11.TOXICOLOGICAL INFORMATION11.1 Information on toxicological effectsAcute toxicityClassified based on available data. For more details, see section 2Skin corrosion/irritationClassified based on available data. For more details, see section 2Serious eye damage/irritationClassified based on available data. For more details, see section 2Respiratory or skin sensitizationClassified based on available data. For more details, see section 2Germ cell mutagenicityClassified based on available data. For more details, see section 2CarcinogenicityIARC: No component of this product present at a level equal to or greater than 0.1% is identified as probable, possible or confirmed human carcinogen by IARC.ACGIH: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by ACGIH.NTP: No component of this product present at a level equal to or greater than 0.1% is identified as a anticipated or confirmed carcinogen by NTP.OSHA: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by OSHA.Reproductive toxicityClassified based on available data. For more details, see section 2Specific target organ toxicity - single exposureClassified based on available data. For more details, see section 2Specific target organ toxicity - repeated exposureClassified based on available data. For more details, see section 2Aspiration hazardClassified based on available data. For more details, see section 212. ECOLOGICAL INFORMATION12.1 ToxicityNo data available.12.2 Persistence and degradabilityNo data available.12.3 Bioaccumlative potentialNo data available.12.4 Mobility in soilNo data available.12.5 Results of PBT and vPvB assessmentPBT/vPvB assessment unavailable as chemical safety assessment not required or not conducted.12.6 Other adverse effectsNo data available.13. DISPOSAL CONSIDERATIONS13.1 Waste treatment methodsProductDispose substance in accordance with prevailing country, federal, state and local regulations.Contaminated packagingConduct recycling or disposal in accordance with prevailing country, federal, state and local regulations.14. TRANSPORT INFORMATIONDOT (US)This substance is considered to be non-hazardous for transport.IMDGThis substance is considered to be non-hazardous for transport.IATAThis substance is considered to be non-hazardous for transport.15. REGULATORY INFORMATIONSARA 302 Components:No chemicals in this material are subject to the reporting requirements of SARA Title III, Section 302.SARA 313 Components:This material does not contain any chemical components with known CAS numbers that exceed the threshold (De Minimis) reporting levels established by SARA Title III, Section 313.SARA 311/312 Hazards:No SARA Hazards.Massachusetts Right To Know Components:No components are subject to the Massachusetts Right to Know Act.Pennsylvania Right To Know Components:No components are subject to the Pennsylvania Right to Know Act.New Jersey Right To Know Components:No components are subject to the New Jersey Right to Know Act.California Prop. 65 Components:This product does not contain any chemicals known to State of California to cause cancer, birth defects, or anyother reproductive harm.16. OTHER INFORMATIONCopyright 2017 MedChemExpress. The above information is correct to the best of our present knowledge but does not purport to be all inclusive and should be used only as a guide. The product is for research use only and for experienced personnel. It must only be handled by suitably qualified experienced scientists in appropriately equipped and authorized facilities. The burden of safe use of this material rests entirely with the user. MedChemExpress disclaims all liability for any damage resulting from handling or from contact with this product.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
GLPbio产品说明书-SB 431542 (GC11545)

Product Data SheetProduct Name:SB 431542Cat. No.:GC11545Chemical PropertiesCas No.301836-41-9化学名4-[4-(1,3-benzodioxol-5-yl)-5-pyridin-2-yl-1H-imidazol-2-yl]benzamideCanonicalSMILESC1OC2=C(O1)C=C(C=C2)C3=C(NC(=N3)C4=CC=C(C=C4)C(=O)N)C5=CC=CC=N5分子式C22H16N4O3分子量384.39溶解度≥ 19.2mg/mL in DMSO, ≥ 10.06mg/mL in EtOH with ultrasonic储存条件Store at -20°CGeneral tips For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months.Shipping Condition Evaluation sample solution : ship with blue ice All other available size: ship with RT , or blue ice upon request.StructureProtocolKinase experiment [1]:Preparation Method The kinase domain without the GS region was cloned and expressed as a GST fusion protein. Expressing the protein without the GS domain, which has been shown to regulate the kinase activity, creates a constitutively active kinase that is able to phosphorylate GST-Smad3. Test the effects of SB-431542 on ALK5 and ALK4 kinase activity with GST-Smad3 as substrate.Reaction Conditions The Kinase assays were performed with 65 nM GSTALK5 and 184 nM GST-Smad3 in 50 mM HEPES, 5 mM MgCl2, 1 mM CaCl2, 1 mM dithiothreitol, and 3 μM ATP. Reactions were incubated with 0.5 μCi of [33P]γATP for 3 h at 30°C.Applications SB-431542 is a selective ALK5 inhibitor with little activity against p38 MAPK. SB-431542 also inhibits ALK4 activity. Which is consistent with the degree of homology between these kinases, such that ALK4 is the closest related kinase to ALK5. This data clearly demonstrated that SB-431542 is a potent and selective inhibitor of ALK5 and ALK4, with slightly higher selectivity for ALK5.Cell experiment [1]:Cell lines Renal proximal tubule epithelial cells (RPTEC)Product Data SheetPreparation Method Cells were grown in Earle’s minimum essential medium supplemented with 10% fetal calfserum, penicillin (5 units/ml), and streptomycin (5 ng/ml). Cells were serum-starved for 24 hbefore treatment.Reaction Conditions Cells were treated with TGF-β1 (5 ng/ml) plus increasing concentrations of SB-431542 (50,250, 500, and 700 nM).Applications SB-431542 could be used to evaluate whether ALK5 activity is required for TGF-β1-inducedtranslocation of Smad3. SB-431542 at a concentration of 1 μM significantly reduced the TGF-β1-induced nuclear accumulation of Smad proteins. Thus, SB-431542 selectively inhibitsTGF-β1–induced Smad translocation without affecting BMP-induced Smads.Animal experiment [2]:Animal models Male Sprague-Dawley rats aged 5 weeks, weighing 200-220 gPreparation Method Rats lived in air served as control groups, and rats lived in an air condition incubatorcontaining 10% O2 to simulate chronic hypoxia animal model, and served as model groups.Model groups were treated with daily intraperitoneal injections of the SB-431542 for 28days.Dosage form 10 mg/kg; 20 mg/kgApplications SB-431542 inhibited the proliferative activity as a function of exposure time andconcentration. Treated rats with SB-431542 caused more pathological changes in vascularadventitia, and the severity of the changes varied from slight to moderate depending on concentrations. In addition, the pulmonary arteries in the hypoxia-induced model groups hadgreater amounts of collagen fibers than that of the control groups. In comparison, collagenfibers were significantly reduced after treatment with SB-431542 (P < 0.01).References:[1]. Laping NJ, et al. Inhibition of transforming growth factor (TGF)-beta1-induced extracellular matrix with a novel inhibitor of the TGF-beta type I receptor kinase activity: SB-431542. Mol Pharmacol. 2002 Jul;62(1):58-64.[2]. Yuan W, et al. SB-431542, a specific inhibitor of the TGF-β type I receptor inhibits hypoxia-induced proliferation of pulmonary artery adventitial fibroblasts. Pharmazie. 2016 Feb;71(2):94-100.BackgroundSB-431542, a small molecule inhibitor of the type I TGF-β receptor, blocks intracellular mediators of TGF-1 signaling,which leads to decreased TGF-β1–mediated proliferation, cytokines and collagen expression. In clinical settings,SB-431542 is widely used to treat respiratory asthma, and inhibits proliferation and synthesis of adventitial fibro in the process of pulmonary vascular remodeling.[1]In vitro study indicated that SB-431542 is able to inhibit ALK5 with an IC50 of 94 nM and other type I receptors, such as ALK4. Although SB-431542 inhibited ALK4 with an IC50 of 140 nM. Moreover, SB-431542 inhibited TGF-β1–induced collagen Iα1 and PAI-1 mRNA with IC50 values of 60 and 50 nM, respectively. In addition, SB-431542 inhibited TGF-β1–induced fibronectin mRNA and protein with IC50 values of 62 and 22 nM, respectively. These data demonstrate for the first time that ALK5 activity is required for TGF-β1 regulation of extracellular matrix markers FN, collagen Iα1,and PAI-1 mRNA.[1]In vivo study demonstrated that SB-431542 has the capacity to inhibit TGF-β1-induced gene expression. SB-431542is recognized as a important inhibitor of the TGF-β1 receptors in blocking TGF-β1/Smads signal pathways in vascular remodeling. Moreover, hypoxia-induced vascular remodeling can significantly increase the amount of cytokines and collagen in vascular adventitia. However, after the treatment of SB-431542, attenuation of the fibrosis promoting effects of TGF-β1, including TGF-β1-induced cell proliferation, cell motility, cell migration and cell synthesis were observed. Therefore, it is significant to the identify the potential of SB-431542 for the treatment of hypoxia-induced pulmonary hypertension.[2]References:[1]. Laping NJ, et al. Inhibition of transforming growth factor (TGF)-beta1-induced extracellular matrix with a novelProduct Data Sheetinhibitor of the TGF-beta type I receptor kinase activity: SB-431542. Mol Pharmacol. 2002 Jul;62(1):58-64.[2]. Yuan W, et al. SB-431542, a specific inhibitor of the TGF-β type I receptor inhibits hypoxia-induced proliferation of pulmonary artery adventitial fibroblasts. Pharmazie. 2016 Feb;71(2):94-100.SB-431542 是 I 型 TGF-β 受体的小分子抑制剂,可阻断 TGF-1 信号转导的细胞内介质,从而导致 TGF-β1 介导的增殖、细胞因子和胶原蛋白表达减少。
IFCC Aspartate Aminotransferase 检测手册说明书

ASTAspartate Aminotransferase IFCCMANUAL RX MONZAINTENDED USEFor the quantitative in vitro determination of AspartateAminotransferase (AST) in serum and plasma. This product is suitable for manual use and on the Rx Monza analyser.Cat. No. AS 1202 R1a. Buffer/Substrate 1 x 70 ml 20 x 2 ml R1b. Enzyme/Coenzyme/ 20 x 2 ml α-oxoglutarate GTIN: 05055273200416AS 1204 R1a. Buffer/Substrate 1 x 105 ml 10 x 10 ml R1b. Enzyme/Coenzyme/ 10 x 10 ml α-oxoglutarate GTIN: 05055273200423AS 1267 R1a. Buffer/Substrate 1 x 105 ml 5 x 20 ml R1b. Enzyme/Coenzyme/ 5 x 20 ml α-oxoglutarate GTIN: 05055273200430AS 2359 R1a. Buffer/Substrate 5 x 100 ml 5 x 100 ml R1b. Enzyme/Coenzyme/ 5 x 100 ml α-oxoglutarate GTIN: 05055273200454UV METHODThis is an optimised standard method according to the concentrations recommended by the IFCC.CLINICAL SIGNIFICANCE (1,2,3,4)The aminotransferases are a group of enzymes that catalyse the inter conversions of amino acids and α-oxoacids by transfer of amino groups. AST (aspartate aminotransferase or glutamate oxaloacetatetransaminase) has been found in the cytoplasm and the mitochondria of cells that have been studied. In cases of mild tissue damage, e.g. liver, the predominant form of serum AST is that from the cytoplasm, with a smaller amount coming from the mitochondria. Severe tissue damage will result in more mitochondrial enzyme being released. Elevated levels of AST can signal myocardial infarction, hepatic disease, muscular dystrophy and organ damage.Although heart muscle is found to have the most activity of the enzyme, significant activity has also been seen in the brain, liver, gastric mucosa, adipose tissue and kidneys of humans.The IFCC has now recommended (1980) standardised procedures for AST determinations including:-1. optimization of substrate concentrations.2. Employment of Tris buffers (instead of phosphate, which has beenshown to inhibit recombination of the apoenzyme with pyridoxal phosphate).3. Pre-incubation of combined buffer and serum to allow sidereactions with NADH to occur. 4. Substrate start (α-oxoglutarate)5. Optional pyridoxal phosphate activation.This is an optimised standard method according to the recommendations of the IFCC.PRINCIPLEα-oxoglutarate reacts with L-aspartate in the presence of AST to form L-glutamate plus oxaloacetate. The indicator reaction utilises the oxaloacetate for a kinetic determination of NADH consumption. AST -oxoglutarate + L-aspartate L-glutamate + oxaloacetate MDH oxaloacetate + NADH + H + L-malate + NAD +SPECIMEN COLLECTION AND PREPARATION (5) Serum:- Use serum free from haemolysis.Plasma:- EDTA or heparin can be used as the anticoagulant.Plasma should be separated from cells within one hour after collection.Specimens should be refrigerated if not used immediately:-Specimens stored longer than 3 days should be frozen at -20︒C.REAGENT COMPOSITIONContents Concentrations in the TestR1a. Buffer/Substrate Tris buffer 80 mmol/l, pH 7.5 L-aspartate 240 mmol/l R1b. Enzyme/Coenzyme/α-oxoglutarate α-oxoglutarate 12 mmol/l MDH ≥420 U/l LD ≥600 U/l NADH 0.18 mmol/lSAFETY PRECAUTIONS AND WARNINGS For in vitro diagnostic use only. Do not pipette by mouth.Exercise the normal precautions required for handling laboratory reagents.Solution R1a contains Sodium Azide. Avoid ingestion or contact with skin or mucous membranes. In case of skin contact, flush affected area with copious amounts of water. In case of contact with eyes or if ingested, seek immediate medical attention.Sodium Azide reacts with lead and copper plumbing, to form potentially explosive azides. When disposing of such reagents flush with large volumes of water to prevent azide build up. Exposed metal surfaces should be cleaned with 10% sodium hydroxide.Health and Safety data sheets available on request.The reagents must be used only for the purpose intended by suitably qualified laboratory personnel, under appropriate laboratory conditions.STABILITY AND PREPARATION OF REAGENTS R1a. Buffer/SubstrateContents ready for use. Stable up to the expiry date when stored at +2 to +8︒C.R1b. Enzyme/Coenzyme/α-oxoglutarate Reconstitute one vial of Enzyme/Coenzyme/α-oxoglutarate R1b with the appropriate volume of Buffer/Substrate R1a: 2 ml for the 20 x 2 ml kit (AS 1202) 10 ml for the 10 x 10 ml kit (AS 1204) 20 ml for the 5 x 20 ml kit (AS 1267) Stable for 14 days at +2 to +8︒C or 24 hours at +15 to +25︒C. Cat. AS 2359 5 x 100 mlReconstitute one vial of Enzyme/Coenzyme/α-oxoglutarate R1b with a portion of Buffer/Substrate R1a and then transfer the entire contents to bottle R1a rinsing bottle R1b several times. Stable for 14 days at +2 to +8︒C or 24 hours at +15 to +25︒C.MATERIALS PROVIDED Buffer/SubstrateEnzyme/Coenzyme/ -oxoglutarateMATERIALS REQUIRED BUT NOT PROVIDEDRandox Assayed Multisera Level 2 (Cat. No. HN 1530) and Level 3 (Cat. No. HE 1532)Randox Calibration Serum Level 3 (Cat. No. CAL 2351) RX series Saline (Cat. No. SA 3854)PROCEDUREAspirate fresh ddH 2O and perform a new Gain Calibration in flow cell mode. Select AST in the Run Test screen and carry out a water blank as instructed.Pipette into a test tube:Sample 0.05 ml Reagent 0.5 mlMix and aspirate into the Rx Monza.CALIBRATION FOR RX MONZAThe use of Saline and Randox Calibration Serum Level 3 isrecommended for calibration. Calibration is recommended with change of reagent lot or as indicated by quality control procedures.FOR MANUAL USEWavelength: 340 nm (Hg 334 nm or Hg 365 nm) Cuvette: 1 cm light path Temperature: 25/30/37︒C Measurement: against airPipette into cuvette: Macro MicroSample 0.2 ml 0.1 ml Enzyme/Coenzyme/ α-oxoglutarate R1 2.0 ml 1.0 mlMix, read initial absorbance after 1 minute. Read again after 1, 2 and 3 minutes. Note: If the absorbance change per minute is between 0.11 and 0.16 at 340/Hg 334 nm 0.06 and 0.08 at Hg 365 nmuse only the values for the first 2 minutes for the calculation.MANUAL CALCULATIONTo calculate the AST activity, use the following formulae:U/l = 1746 x A 340 nm/min U/l = 1780 x A Hg 334 nm/min U/l = 3235 x A Hg 365 nm/minSTANDARDISATIONRandox Calibration Serum Level 3 is traceable to AST reference material JSCC TS01.QUALITY CONTROLRandox Assayed Multisera, Level 2 and Level 3 are recommended for daily quality control. Two levels of controls should be assayed at least once a day. Values obtained should fall within a specified range. If these values fall outside the range and repetition excludes error the following steps should be taken:1. Check instrument settings and light source.2. Check cleanliness of all equipment in use.3. Check water. Contaminants, i.e. bacterial growth, maycontribute to inaccurate results. 4. Check reaction temperature.5. Check expiry date of kit and contents.6. Contact Randox Laboratories Customer Technical Services, Northern Ireland +44 (0) 28 9445 1070.SPECIFICITY/INTERFERENCE (6,7)Gross haemolysis will produce falsely elevated test results. The effects of various drugs on AST activity should be taken intoconsideration in the case of patients receiving large doses of drugs.The analytes below were tested up to the following levels and were found not to interfere: Haemoglobin 250 mg/dl Free Bilirubin 25 mg/dl Conjugate Bilirubin 25 mg/dl Triglycerides 1000 mg/dlIntralipid ® 200 mg/dlA list of substances and conditions known to effect AST activity in vivo is given by both Young et al and Friedman et al. Norepresentation is made by Randox Laboratories Ltd regarding the completeness of these lists and the accuracy of the information contained therein.NORMAL VALUES IN SERUM (8,9) +25︒C +30︒C +37︒C Men up to 18 U/l up to 25 U/l up to 37 U/l Women up to 15 U/l up to 21 U/l up to 31 U/lIt is recommended that each laboratory establish its own reference range to reflect the age, sex, diet and geographical location of the population.SPECIFIC PERFORMANCE CHARACTERISTICS The following performance data were obtained using an Rx Monza analyser running at +37o C.LINEARITYThis method is linear up to 562 U/l. If the sample concentration exceeds this value, dilute the sample 1+9 with 0.9% NaCl solution and re-assay. Multiply the result by 10.SENSITIVITYThe minimum detectable concentration of AST with an acceptable level of precision was determined as 9.3 U/l.PRECISIONIntra AssayLevel 2 Level 3Mean (U/l) 35.6 153SD 1.66 1.47CV(%) 4.65 0.96n 20 20Inter AssayLevel 2 Level 3Mean (U/l) 35.6 153SD 1.77 7.10CV(%) 4.96 4.63n 20 20CORRELATIONThis method (Y) was compared with another commerciallyavailable method (X) and the following linear regression equationobtained:Y = 1.07X + 4.9and a correlation coefficient of r = 0.997543 patient samples were analysed spanning the range 28 to 559U/l.REFERENCES1. Wroblewski F, La Due J.S: Ann Intern Med. 1956; 45: 801.2. Wroblewski F, La Due J.S: Proc Soc Exp Biol Med 1956;91: 569.3. Bergmeyer HU, Bowers GN Jr, et al: Clin Chem 1977; 23:887.4. Bergmeyer HU, Bowers GN Jr, et al: J.Clin Chem ClinBiochem 1980; 18: 521-534.5. Tietz N W: Fundamentals of Clinical Chemistry ed 3.Philadelphia, WB Saunders Co. 1987, pg 372.6. Young D S, et al: Clin Chem 1975, 21; No5.7. Friedman RB, et al: Clin Chem 1980, 26; No4.8. Wallnofer H, Schmidt.E, Schmidt FW, eds: Synopsis derLeberkrankheiten Stuttgart, Georg Thieme Verlag, 1974.9. Thefeld W, et al: Dtsch Med Wschr 1974; 99: 343.Revised 26 Apr 16 biRev. 003THIS PAGE IS INTENTIONALLY BLANK。
MED4830B

MATERIAL SAFETY DATA SHEETMED-4830 PART BNuSil Technology LLC urges each customer or recipient of this MSDS to study it carefully to become aware of and understand the hazards associated with the product. The reader should consider consulting reference works or individuals who are experts in ventilation, toxicology, and fire prevention, as necessary or appropriate to the use and understanding of the data contained in this MSDS.To promote safe handling, each customer or recipient should: (1) notify its employees, agents, contractors, and others whom it knows or believes will use this material of the information regarding hazards or safety; (2) furnish this same information to each of its customers for the product; and (3) request its customers to notify their employees, customers and other users of the product of this information.1. CHEMICAL PRODUCT AND COMPANY IDENTIFICATIONNuSil Technology LLC1050 Cindy Lane Carpinteria, California 93013 USA(805) 684-8780 EMERGENCY TELEPHONE NUMBERS: (800) 424-9300 CHEMTREC (805) 684-8780OUTSIDE OF THE USA (703) 527-3887 CHEMTRECPRODUCT NAME: MED-4830 PART BCHEMICAL NAME: N/ACHEMICAL FAMILY: SiliconeFORMULA: ProprietaryMOLECULAR WEIGHT: N/ASYNONYMS: N/ACAS # : Mixture2. HAZARDOUS INGREDIENTS% MATERIAL CAS # EXPOSURE VALUE CLASSIFICATION30 Silica, amorphous 07631-86-9 See Section 8 See Section 7<3 Dimethyl, Methylhydrogen Siloxane Copolymer68037-59-2 None Established See Section 73. HAZARDS IDENTIFICATIONEFFECTS OF SINGLE OVEREXPOSURE:SWALLOWING:Small amounts transferred to the mouth by fingers during use, etc., should not injure. Swallowing large amounts may cause digestive discomfort.SKINABSORPTION:No evidence of adverse effects from available information.INHALATION:No evidence of adverse effects from available information.SKINCONTACT:No evidence of adverse effects from available information.EYECONTACT:Direct contact may cause temporary discomfort with mild redness, dryness, and irritation.EFFECTS OF REPEATED OVEREXPOSURE:No injury from silica or dust should occur during reasonable use. If use creates respirable particles, some respiratory system injury may occur. However, since the silica in this product is compounded into the polymer matrix, it is not expected to present the same hazards as neat silica.MEDICAL CONDITIONS AGGRAVATED BY OVEREXPOSURE:A knowledge of the available toxicology information and of the physical and chemical properties of the material suggests that overexposure is unlikely to aggravate existing medical conditions.SIGNIFICANT LABORATORY DATA WITH POSSIBLE RELEVANCE TO HUMAN HEALTH HAZARD EVALUATION:None currently known.OTHER EFFECTS OF OVEREXPOSURE:None currently known.4. FIRST AID MEASURESEMERGENCY AND FIRST AID MEASURES:SWALLOWING:No emergency care anticipatedSKIN:Wash with soap and water.INHALATION:Short-term harmful health effects are not expected from vapor generated at ambient temperature.EYES:Immediately flush eyes with water for at least 15 minutes. Obtain medical attention if discomfort persists.NOTES TO PHYSICIAN:There is no specific antidote. Treatment of overexposure should be directed at the control of symptoms and the clinical condition of the patient. NOTE: Do not induce vomiting. Emesis of this material may prove difficult due to its high viscosity. Aspiration may cause lung damage.5. FIRE FIGHTING MEASURESFLASH POINT (test method(s)): >300°F (Tag Closed Cup)FLAMMABLE LIMITS IN AIR (by volume):LOWER: N/A UPPER: N/AEXTINGUISHING MEDIA:Apply alcohol-type or universal-type foams by manufacturers' recommended techniques for large fires. Use carbon dioxide or dry chemical media for small fires.SPECIAL FIRE FIGHTING PROCEDURES:Do not aim extinguisher stream directly into a pool of hot, burning liquid as this may cause frothing, and may intensify the fire. Use self-contained breathing apparatus when fighting fire in an enclosed area.UNUSUAL FIRE AND EXPLOSION HAZARDS:This product contains polydimethylsiloxane which can generate formaldehyde as a byproduct of oxidative thermal decomposition at temperatures greater than 150°C (300°F). See Section 10 for further information.6. ACCIDENTAL RELEASE MEASURESSTEPS TO BE TAKEN IF MATERIAL IS RELEASED OR SPILLED:Spills should be contained with mechanical barriers. Transfer spilled material to a suitable container for disposal. WASTE DISPOSAL METHOD:Dispose of in accordance with all Federal, State and local regulations.7. HANDLING AND STORAGEPRECAUTIONS TO BE TAKEN IN HANDLING AND STORAGE:Normal precautions common to safe manufacturing practice should be followed in handling and storage.Keep container closed, in a cool dry place. S3/S7/S8Avoid contact with skin and eyes S24/S25In case of fire, do not breathe fumes S41Any proposed use of this product in elevated-temperature processes should be thoroughly evaluated to assure that safe operating conditions are established and maintained.8. EXPOSURE CONTROLS / PERSONAL PROTECTIONOCCUPATIONAL EXPOSURE VALUES AND SOURCE:Silica, amorphous : 10 mg/m3 - 8 hours TWA (ACGIH,)6 mg/m3 - 8 hours TWA (OSHA, NIOSH)RESPIRATORY PROTECTION:Use NIOSH approved respirator or self-contained breathing apparatus as needed to maintain personnel exposure below established Occupational Exposure Values.VENTILATION:General (mechanical) room ventilation with local ventilation as needed to maintain exposure below established Occupational Exposure Value.PROTECTIVE GLOVES: PVC-coated.EYE PROTECTION: Safety glasses.OTHER PROTECTIVE EQUIPMENT: Eye bath and safety shower.9. PHYSICAL AND CHEMICAL PROPERTIES (based on typical material) BOILING POINT: N/ASPECIFIC GRAVITY (H2O=1): 1.15FREEZING POINT: N/AVAPOR PRESSURE : < 5 mm HgVAPOR DENSITY (air=1): N/AEVAPORATION RATE (Butyl Acetate=1): N/ASOLUBILITY IN WATER (By wt): < 0.1 %APPEARANCE: TranslucentODOR: Slight OdorPHYSICAL STATE : Viscous liquidPERCENT VOLATILES (by wt): See Section 15Note: The above information is not intended for use in preparing product specifications.10. STABILITY AND REACTIVITY DATASTABILITY: Stable.CONDITIONS TO AVOID: None.INCOMPATIBILITY: Oxidizing materials can cause a reaction.HAZARDOUS COMBUSTION OR DECOMPOSITION PRODUCTS:Burning can produce carbon monoxide, carbon dioxide, oxides of silicon, and hydrocarbons. Carbon monoxide is highly toxic if inhaled; carbon dioxide in sufficient concentrations can act as an asphyxiant. Acute overexposure to the products of combustion may result in irritation of the respiratory tract.Traces of formaldehyde may be generated due to oxidative thermal decomposition at temperatures greater than 150°C (300°F). Exposure to formaldehyde can cause adverse effects such as skin and respiratory sensitization and eye and throat irritation. Formaldehyde is a potential carcinogen. Evaluate and control exposure to formaldehyde when warranted by conditions of use.HAZARDOUS POLYMERIZATION: Will not occur.11. TOXICOLOGICAL INFORMATIONCOMPONENT:MED-4830 PART B:Acute Oral LD50 (mg/kg): 500-5000 (Rat) Inferred from ingredient hazard(s)Acute Dermal LD50 (mg/kg): 1000-2000 (Rbt.) Inferred from ingredient hazard(s)Acute Inhalation LC50 (mg/l): 2-20 (Rat) Inferred from ingredient hazard(s)Other:N/A.AmesTest: N/A.Refer to Section 3 for further discussion of the health hazards associated with this preparation.12. ECOLOGICAL INFORMATIONECOTOXICOLOGICAL INFORMATION: Complete information not yet available.CHEMICAL FATE INFORMATION: Complete information not yet available.13. DISPOSAL CONSIDERATIONSDispose of in accordance with all Federal, State, and local regulations.14. TRANSPORT INFORMATIONDOT HAZARD CLASSIFICATION: NoneI.A.T.A. HAZARD CLASSIFICATION: Not Regulated15. REGULATORY INFORMATIONSTATUS ON SUBSTANCE LISTS:The concentrations shown are maximum or ceiling levels (weight %) to be used for calculations for regulations. Trade Secrets are indicated by "TS".REGULATIONS C.H.I.P.Chemicals (Hazards Information and Packaging) Regulations 2008 requires physico-chemical and health hazard determination of all substances and preparations manufactured, transported, stored, modified, or consumed within the U.K. Components present in this product at a level which could require reporting under the statute are:**** NONE ****____________________________________________________________________________________________EPA FEDERALComprehensive Environmental Response Compensation and Liability Act of 1980 (CERCLA) requires notification of the National Response Center of release of quantities of Hazardous Substances equal to or greater than the reportable quantities (RQ's) in 40 CFR 302.4. Components present in this product at a level which could require reporting under the statute are: **** NONE ****____________________________________________________________________________________________ Superfund Amendments and Reauthorization Act of 1986 (SARA) Title III requires emergency planning based on Threshold Planning Quantities (TPQ's) and release reporting based on Reportable Quantities (RQ's) in 40 CFR 355 (used for SARA 302, 304, 311, and 312). Components present in this product at a level which could require reporting under the statute are: **** NONE ****____________________________________________________________________________________________Superfund Amendments and Reauthorization Act of 1986 (SARA) Title III requires submission of annual reports of release of toxic chemicals that appear in 40 CFR 372 (for SARA 313). This information must be included in all MSDS's that are copied and distributed for this material. Components present in this product at a level which could require reporting under this statute are: **** NONE ****____________________________________________________________________________________________INVENTORY STATUSThe ingredients of this product are listed on, or are exempt from listing on, the TSCA inventory.____________________________________________________________________________________________STATE-RIGHT-TO-KNOWCALIFORNIA Proposition 65This product contains no levels of listed substances, which the State of California has found to cause cancer, birth defects or other reproductive harm, which would require a warning under the statute.____________________________________________________________________________________________MASSACHUSETTS 105 CMR 670.000 Right-To-Know, Substance List (MSL)Hazardous Substances and Extraordinarily Hazardous Substances on the MSL must be identified when present in products. Components present in this product at a level which could require reporting under the statute are:UPPER BOUND MATERIALCAS NUMBER CONCENTRATION Silica, amorphous 07631-86-9 30 % ____________________________________________________________________________________________PENNSYLVANIA Right-To-Know, Hazardous Substance ListHazardous Substances and Special Hazardous Substances on the List must be identified when present in products. Components present in this product at a level which could require reporting under the statute are:UPPER BOUND MATERIALCAS NUMBER CONCENTRATION Silica, amorphous 07631-86-9 30 % ____________________________________________________________________________________________CALIFORNIA SCAQMD RULE 443.1 VOC'S:Volatile Organic Components (VOC's) = Substances with vapor pressure of ≥ 0.5 mm Hg at 104°C (219.2°F). This product contains < 1 % by weight VOC's.____________________________________________________________________________________________OTHER REGULATORY INFORMATION:EPA Hazard Categories: NoneC.H.I.P. Regulations:Designation: MED-4830 PART BSymbol: N/AIndication of Danger: N/ASafety Phrases: S3/S7/S8/S24/S25/S41(Ref. Sect. 7)16. OTHER INFORMATIONHMIS FORMAT:Health: 1 Flammability: 1 Reactivity: 0We believe that the information contained herein is current as of the date of this Material Safety Data Sheet, and is offered in good faith. Since the use of this information and of these opinions and the conditions of the use of the product are not within the control of NuSil Technology, it is the user's obligation to determine the conditions of safe use of the product.-NuSil Technology LLC Regulatory Compliance DepartmentEffective Date: January 1, 2009。
利用荧光偏振装置对细胞膜流动性进行检测

态。膜脂类分子在相变温度以上条件下主要有侧向扩散、旋转、左右摇摆、伸缩振荡、翻转 及异化运动等方式。膜蛋白的运动方式大体分为侧向及旋转运动,主要受脂质双分子层的影 响。脂肪酸不饱和键含量和链的长度明显影响着膜脂流动性,不饱和键的存在会降低膜脂分 子间排列的有序性,从而增加膜的流动性,短链能减低脂肪酸链尾部相互作用,在相变温度 下,不易于凝集,此外,脂肪酸烃链围绕 C- C 键由全反式构型到歪扭(CAUCHE)的旋转异 构运动,也可使流动性加大。
按下列公式计算荧光偏振度 P: P=(IVV-GIVHIHV)/(IVV+GIVH) 其中校正因子 G=IHV/IHH
-3-
式中 IVV:起偏和检偏器光轴同为垂直方向时测得的荧光强度; IVH:起偏和检偏器光轴分别为垂直和水平方向时测得的荧光强度。 IHV:起偏和检偏器光轴分别为水平和垂直方向时测得的荧光强度; IHH:起偏和检偏器光轴同为水平方向时测得的荧光强度。 P 值越大 ,流动性越小;P 值越小,流动性越大。
体洒落。 ● 染色液为 DMSO 溶液,冬季气温较低时在室温时为凝固状态,极易粘附在管壁、吸头壁。注意需要
加热溶解,吸头也需要放在培养箱预热,否者容易再次凝固在吸头内壁产生损耗。 ● 细胞处理需要小心操作,尽量避免人为的损伤细胞。 ● 染色后立即进行分析。 ● 标记的条件因细胞种类而异,根据不同样本的实际染色结果做相应调整,在每次实验前,请先确
定最佳条件。 ● 以下步骤仅供参考。请根据实际实验方案设计或者参考文献实验。
1、 染色工作液的配制: 用稀释液稀释 TMA-DPH 探针 1000 倍-10000 倍,配制成 TMA-DPH 染色工作液。
细胞粘附的检测方法
包被: 1、 96 孔板每孔加入 100ul 包被液。 2、 将培养板置 2-8°C 过夜。 3、 移除包被液,用洗涤液洗涤 2-3 次。
细胞接种: 1、 待测定细胞处理好后,用胰酶消化,PBS 洗涤,然后用相应培养基重悬,制成细胞
悬液。 2、 按 5×104 细胞/孔接种 96 孔板,建议设 3-5 个复孔。同时设立对照组,即孵育后不
体损失导致试剂量不够用。 ● 细胞处理需要小心操作,尽量避免人为的损伤细胞。离心力在不损失细胞的前提下尽可能小,重悬
细胞是动作要轻柔,避免多次反复的激烈吹打。不用涡旋振荡器。 ● 染色培养时间根据细胞种类的不同和每孔内细胞数量的多少而异。一般情况下,白细胞较难染色,
因此需要较长的培养时间。培养时间一般为 1- 4 小时,但在培养 30 分钟左右即可取出肉眼观察 染色程度 (根据细胞种类而定,需要摸索一下条件)。当使用标准 96 孔板时,贴壁细胞的最小接 种量至少为 1,000 个/孔 (100 μl 培养基)。检测白细胞时的灵敏度相对较低,因此推荐接种量不 低于 2,500 个/孔 (100 μl 培养基)。如果要使用 24 孔板或 6 孔板实验,请先计算每孔相应的接 种量,并按照每孔培养基总体积的 10%加入染色液 B。 ● 有条件的情况下建议采用多通道移液器,可以减少平行孔间的差异。加染色液 B 试剂时,建议斜
产品说明书
细胞粘附检测试剂盒
货号:BB-48120
V2.16
试剂盒储存条件: 2-8℃保存。
开盖后组份按要求条件保存。 Nhomakorabea试剂盒组成:
产品组成
BB-48120-1
BB-48120-2
规格
100 T
250 T
试剂 A:包被液 A
欧洲药典7.5版

INDEX
To aid users the index includes a reference to the supplement in which the latest version of a text can be found. For example : Amikacin sulfate...............................................7.5-4579 means the monograph Amikacin sulfate can be found on page 4579 of Supplement 7.5. Note that where no reference to a supplement is made, the text can be found in the principal volume.
English index ........................................................................ 4707
Latin index ................................................................................. 4739
EUROPEAN PHARMACOPபைடு நூலகம்EIA 7.5
Index
Numerics 1. General notices ................................................................... 7.5-4453 2.1.1. Droppers...................
