50_324Phosphate Capacity of CaO–SiO2–MnO–FeO Slag Saturated with MgO
中英文对照版二氧化硅质量标准

稀氨水(R2):取14g浓氨水用水稀释到100ml。氨的含量为3.3%-3.5%,约2M。
稀盐酸:取20g浓盐酸用水稀释到100ml,含量为7.3%w/v
标准铅溶液(1ppm Pb):取10ppmPb标准铅溶液用水稀释10倍,临用前配制。
标准铅溶液(10ppm Pb):取100ppmPb标准铅溶液用水稀释10倍,临用前配制。
3.1.2 Method
值
仪器
方法
Chlorides (2.4.4)
3.2.1 Reagent
3.2.2 Method
3.2氯化物
试剂
方法
3.3Heavy metals (2.4.8)
正文
pH meter
Shake 1.0 g with 30 ml ofcarbon dioxide-free water R. The pH of the suspension is 3.5 to 5.5.
pH计
参照B.P.附录()。取本品,加入30ml不含二氧化碳的水,振摇后检查混悬液的pH值为。
Nitric Acid, Dilute:Dilute 20 g ofnitric acidto 100 ml withwater. Contains about 12.5% w/v of HNO3(about 2M).
Ammonia, Concentrated:M).
Ammonia R2, Dilute:Dilute 14 g ofconcentrated ammoniato 100 ml withwater. The solution contains not less than 3.3% and not more than 3.5% of NH3(about 2M).
三氧化二铁作为铁源制备碳包覆磷酸铁锂

三氧化二铁作为铁源制备碳包覆磷酸铁锂朱令之;韩恩山;曹吉林【摘要】Based on the optimization condition[ n(Li): n(Fe) = 1.05:1.00, adding 1.50 g glucose into 100 g precursor, sintering at 650℃ for 15 h] , by using ferric oxide(Fe2O3) as Fe source, glucose as deoxidizer and carbon source, carbon coated lithium iron phosphate (LiFePO4/C) was prepared. The product was olivine structure without obvious impurity phase; the tap density was 1.18 mg/cm3. When cycled in 4.2 ~2.5 V,its initial specific discharge capacity at 0.1 C,0.5 C and 2.0 C was 139.4 mAh/g, 120.4 mAh/g and 102.0 mAh/g,respectively,the specific discharge capacity was 138.0 mAh/g, 121.9 mAh/g and 92.4 mAh/g in the 30th cycle,respectively,there was no structure change occurred in the material.%以三氧化二铁(Fe2O3)为铁源,葡萄糖为还原剂和碳源,在优化条件[n(Li)∶ n(Fe)=1.05∶1.00,100 g前驱体加入1.50 g葡萄糖,在650 ℃下焙烧15 h]下制备碳包覆磷酸铁锂(LiFePO4/C).产物为橄榄石型晶相,无明显的杂质相,振实密度为1.18 g/cm3.在4.2~2.5V循环,0.1C、0.5C、2.0C下的首次放电比容量分别为139.4 mAh/g、120.4 mAh/g 和102.0 mAh/g,第30次循环的放电比容量分别为138.0 mAh/g、121.9mAh/g和92.4 mAh/g,材料的结构没有变化.【期刊名称】《电池》【年(卷),期】2012(042)006【总页数】3页(P318-320)【关键词】磷酸铁锂(LiFePO4);三氧化二铁(Fe2O3);振实密度;电化学性能【作者】朱令之;韩恩山;曹吉林【作者单位】河北工业大学化工学院,天津300130;河北工业大学化工学院,天津300130;河北工业大学化工学院,天津300130【正文语种】中文【中图分类】TM912.9磷酸铁锂(LiFePO4)用作锂离子正极材料,具有高温稳定性和循环性能好、价格低廉及对环境友好等优点。
浓硫酸添加点对磷矿石反浮选作业的影响

浓硫酸添加点对磷矿石反浮选作业的影响杨稳权;罗廉明;彭杰【摘要】对海口中低品位磷矿石反浮选作业在不同的加药点添加浓硫酸和捕收剂YP-3,试验表明:在同一加药点同时添加浓硫酸和捕收剂YP-3,浮选指标较差,精矿产率低,且MgO含量高.对此类矿石的反浮选作业,需要在不同的加药点分别添加浓硫酸和捕收剂YP-3,以提高浮选过程的选择性,获得更理想的选矿指标.%It was studied that sulfaric acid and collector YP-3 was added in different dosing point with Haikon low grade phosphate rock reverse flotation operation.The results of the test indicated that: in the same dosing point and adding sulfuric acid and collector YP-3, flotation index is poor, pure mineral rate is low, and content of MgO is high.Such ore reverse flotation homework needs different dosing points respectively adding sulfuric acid and collector YP-3, in order to improve the flotation process selectivity and obtain more ideal dressing indexes.【期刊名称】《武汉工程大学学报》【年(卷),期】2011(033)003【总页数】3页(P79-80,86)【关键词】中低品位磷矿;浓硫酸;反浮选;云南省海口磷矿【作者】杨稳权;罗廉明;彭杰【作者单位】云南磷化集团研发中心,云南,昆明,650113;云南磷化集团研发中心,云南,昆明,650113;云南磷化集团研发中心,云南,昆明,650113【正文语种】中文【中图分类】TD970 引言云南海口磷矿浮选厂原设计时采用正反浮选工艺流程,且正—反浮选工艺流程采用同一种捕收剂,即在碱性条件下正浮选捕收磷矿物和在酸性条件下捕收碳酸盐矿物使用同一种捕收剂YP2(脂肪酸阴离子捕收剂).故在正浮选磷精矿进行反浮选前只设计了一台矿浆搅拌槽,用于添加浓硫酸(98%)作为磷矿物的抑制剂.由于浮选药剂的研发,提高了浮选药剂性能,用YP2-1(脂肪酸阴离子捕收剂)作为碱性条件下正浮选捕收磷矿物的捕收剂,用YP2-3(脂肪酸阴离子捕收剂)作为酸性条件下捕收碳酸盐矿物的捕收剂.这就造成了在同一台矿浆搅拌槽内同时需要添加反浮选抑制剂和捕收剂.本文就在反浮选工艺流程中同时添加硫酸和捕收剂进行试验研究,考察硫酸添加点对浮选过程的影响,及改进措施和方法.1 原矿性质试验矿样为海口矿区中低品位硅钙质磷矿岩,主要有用矿物为胶磷矿,主要脉石矿物为白云石、石英和玉髓.矿样多元素分析见表1所示.表1 生产矿样多元素分析结果Table 1 Production samples of ore more element analysis results %组分P2O5MgOSiO2CaOFe2O3Al2O3Na2OK2O 质量分数22.564.0819.2937.380.991.820.410.28从表1可以看出:生产矿样为硅钙质磷矿岩,其中m(CaO)/m(P2O5)=1.66,m(SiO2)/m(CaO)=0.52.对这类矿石在选矿过程中必须同时排除大部分碳酸盐和硅酸盐杂质后富集磷矿物,才能满足后续加工的要求.对此类矿石目前以正反浮选工艺流程生产较为成熟.即在碱性介质中,采用捕收剂富集磷矿物,硅酸盐矿物留在槽内产品作为尾矿被排除,泡沫产品为得到的正浮选磷精矿.在正浮选磷精矿中添加无机酸(通常用硫酸和磷酸)作为矿浆pH值调整剂和抑制剂,在弱酸性介质中用脂肪酸捕收剂浮出白云石,槽内产品为富集的磷矿物.2 试验内容2.1 相同加药搅拌时间试验试验采用对比法:一是采用反浮抑制剂(主要是硫酸)和捕收剂分开在不同的时间添加,二是将反浮抑制剂和捕收剂同时添加.不同药剂试验工艺流程及条件如图1所示,结果如表2所示.图1 浮选药剂制度不同试验工艺及条件流程图Fig.1 Flotation reagents system of different test process flow chart and conditions从表2可知:对反浮选作业,分别在不同时间添加浮选药剂比在同一个矿浆搅拌槽中同时添加抑制剂硫酸、磷酸和捕收剂YP2-3的浮选效果(精矿选别指标、选矿效率等)要好得多.表2 不同药剂制度试验结果Table 2 Different reagent regime test results加药方式产品名称产率γ/%品位β/%P2O5MgO回收率ε/%选矿效率E(ε-γ)/%β-γ排镁率/%不同时加药反浮精矿75.8931.650.7093.8617.976.0688.79反浮尾矿24.116.5017.466.14正浮精矿100.0025.594.74100.00同时加药反浮精矿68.5930.671.4582.4313.845.1578.61反浮尾矿31.4114.2811.6417.57原矿100.0025.524.65100.002.2 不同加药搅拌时间试验试验工艺流程如图2所示,结果如表3所示.从表3可知:随着浮选药剂搅拌时间的增加,精矿产率、回收率降低,精矿中P2O5品位、选矿效率升高,MgO品位降低,排MgO效率增加.图2 不同加药搅拌时间浮选试验工艺及条件流程图Fig.2 Different dosingwhisking time flotation test technology and conditions flow chart表3 不同加药搅拌时间试验结果Table 3 Different dosing whisking time test results搅拌时间/min产品名称产率γ/%品位β/%P2O5MgO回收率ε/%选矿效率E(ε-γ)/%β-γ排镁率/%0.5反浮精矿70.1830.761.4485.2815.105.4577.10反浮尾矿29.8212.5011.4114.72正浮精矿100.0025.314.41100.001.0反浮精矿68.6630.881.3383.6414.985.5379.57反浮尾矿31.3413.2311.3516.36 正浮精矿100.0025.354.47100.00 2.0反浮精矿68.2030.761.3082.3014.105.2779.51反浮尾矿31.8013.8011.4417.70 正浮精矿100.0025.374.52100.00 3.0反浮精矿65.7430.921.2880.3914.655.6782.10反浮尾矿34.2614.5710.5619.61 正浮精矿100.0025.324.46100.003 结果讨论浮选药剂的添加和调节是浮选过程中重要的工艺因素,对提高药效、改善浮选指标有重大影响[1].从表2和表3中可以看出,硫酸和YP-3的不同添加方式浮选指标相差较大,且增加或减少搅拌时间,也不能很好的改善浮指标.另外,浮选药剂添加地点的选择与该药剂的用途及溶解度有关[1].通常在反浮选脱除碳酸盐矿物的作业中,加入的硫酸因与碳酸盐矿物反应快,故反浮选酸的搅拌时间短,搅拌时间长短对浮选指标影响小.而对于捕收剂YP2-3则需要较长的搅拌时间,以利于充分和碳酸盐矿物接触,矿化充分而利于提高浮选过程的选择性.在同一矿浆搅拌桶中同时添加浓硫酸、磷酸和捕收剂YP-3,导致浓硫酸放热时将一部分捕收剂碳化,使得碳化了的那部分捕收剂失去了捕收能力;另外,硫酸、磷酸与一部分捕收剂(脂肪酸皂)发生了反应,生成脂肪酸,也使得这部分捕收剂选择性变差.硫酸与捕收剂YP-3(脂肪酸皂)生成脂肪酸的反应式为[2]:对海口中低品位磷矿石的反浮选作业,需分别在不同地点添加浓硫酸和脂肪酸捕收剂,以利更有效地提高浮选效率.现海口浮选厂已将反浮选浓硫酸、磷酸和捕收剂YP-3的加药点分开,即浓硫酸、磷酸加入到矿浆搅拌桶中,而捕收剂YP-3加入到浮选机给矿间箱中,浮选效果很好.参考文献:[1]胡为柏.浮选[M].北京:冶金工业出版社,1988:246.[2]朱玉霜.浮选药剂的化学原理[M].长沙:中南工业大学出版社,1996:56. Abstract: It was studied that sulfaric acid and collector YP-3 was added in different dosing point with Haikon low grade phosphate rock reverse flotation operation. The results of the test indicated that: in the same dosing point and adding sulfuric acid and collector YP-3, flotation index is poor, pure mineral rate is low, and content of MgO is high. Such ore reverse flotation homework needs different dosing points respectively adding sulfuric acid and collector YP-3, in order to improve the flotation process selectivity and obtain more ideal dressing indexes.Key words: low-grade phosphorus ore; sulfuric acid; reverseflotation;dressing indexes; Yunnan Haikou grade phosphorus。
五种改性纳米纤维素吸附剂的制备及除磷性能比较

2017年第36卷第11期 CHEMICAL INDUSTRY AND ENGINEERING PROGRESS·4279·化 工 进展五种改性纳米纤维素吸附剂的制备及除磷性能比较王婷庭,刘敏,崔桂榕,陈滢(四川大学建筑与环境学院,四川 成都 610065)摘要:含磷废水的排放是造成水体富营养化的重要原因,吸附法可以有效去除废水中的磷。
开发环境友好的高效吸附剂是该法进一步推广的关键因素之一。
采用TEMPO 氧化+机械剪切结合的方法制备纳米纤维素(CNFs ),分别用Fe(OH)3、Al(OH)3、Mg(OH)2、La 2O 3和MnO 2对CNFs 进行改性。
将改性前后的CNFs 用于吸附去除废水中磷,并比较了不同pH 条件下的除磷效果。
结果表明,Fe(OH)3、Al(OH)3、Mg(OH)2、La 2O 3和MnO 2均能成功负载于CNFs 上。
经改性后的CNFs 对磷的吸附去除效果有明显提高,pH 越低吸附容量越高。
同一pH 条件下,吸附容量依次为Fe(OH)3@CNFs >Al(OH)3@CNFs >Mg(OH)2@CNFs >La 2O 3@CNFs >MnO 2@CNFs 。
Fe(OH)3@CNFs 对磷的吸附效果最好,且受pH 变化的影响不大。
在磷初始浓度为10mg/L 、pH 为4时,Fe(OH)3@CNFs 对磷的吸附容量为7.58mg/g ,为未负载CNFs 的94.75倍;当pH 升高至7时,其吸附容量仍可达到7.09mg/g 。
将其用于实际废水除磷时无需调节pH ,可节约药剂,降低处理成本。
关键词:纳米纤维素;TEMPO 氧化;改性;吸附;除磷中图分类号:X703.1 文献标志码:A 文章编号:1000–6613(2017)11–4279–07 DOI :10.16085/j.issn.1000-6613.2017-0376Preparation of several modified cellulose nanofiber hybrid adsorbents andperformance comparison of phosphate removalsWANG Tingting ,LIU Min ,CUI Guirong ,CHEN Ying(College of Architecture & Environment ,Sichuan University ,Chengdu 610065,Sichuan ,China )Abstract: The discharge of phosphorus wastewater is considered as a dominant factor for water eutrophication. Adsorption is an effective method for phosphorus removal in the wastewater treatment. The development of environment friendly adsorbents with good adsorption capacity is one of the key factors for the further a adsorption application. The TEMPO oxidation combined physical treatmentswas used to prepare the cellulose nanofiber hybrid (CNFs ). Then, Fe(OH)3, Al(OH)3,Mg(OH)2,La 2O 3 and MnO 2 were used to modify CNFs. CNFs and modified CNFs were applied in the phosphate removal from wastewater. The performance of phosphate removal at different pH was investigated. Fe(OH)3,Al(OH)3,Mg(OH)2,La 2O 3 and MnO 2 could be successfully loaded onto CNFs. While modified CNFs showed higher adsorption capacity than CNFs. The phosphate adsorption capacities of modified CNFs were as follows: Fe(OH)3@CNFs >Al(OH)3@CNFs >Mg(OH)2@CNFs >La 2O 3@CNFs >MnO 2@CNFs ,and they all had better phosphate adsorption performance in lower pH. It could be concluded that Fe(OH)3@CNFs had a superior adsorption performance. When the initial concentration of phosphate is 10mg/L ,the adsorption capacity of Fe(OH)3@CNFs was 7.58mg/g at pH 4,which is 95.75 times that of CNFs ,7.09mg/g at pH 7 for practical applications ,Fe(OH)3@ CNFs is*************。
A highly ordered nanostructured carbon–sulphur cathode for lithium–sulphur batteries

A highly ordered nanostructured carbon–sulphur cathode for lithium–sulphur batteriesXiulei Ji,Kyu Tae Lee and Linda F.Nazar *The Li–S battery has been under intense scrutiny for over two decades,as it offers the possibility of high gravimetric capacities and theoretical energy densities ranging up to a factor of five beyond conventional Li-ion systems.Herein,we report the feasibility to approach such capacities by creating highly ordered interwoven composites.The conductive mesoporous carbon framework precisely constrains sulphur nanofiller growth within its channels and generates essential electrical contact to the insulating sulphur.The structure provides access to Li +ingress/egress for reactivity with the sulphur,and we speculate that the kinetic inhibition to diffusion within the framework and the sorption properties of the carbon aid in trapping the polysulphides formed during redox.Polymer modification of the carbon surface further provides a chemical gradient that retards diffusion of these large anions out of the electrode,thus facilitating more complete reaction.Reversible capacities up to 1,320mA h g −1are attained.The assembly process is simple and broadly applicable,conceptually providing new opportunities for materials scientists for tailored design that can be extended to many different electrode materials.Safe,low-cost,high-energy-density and long-lasting recharge-able batteries are in high demand to address pressing environmental needs for energy storage systems that can be coupled to renewable sources 1,2.These include wind,wave and solar energy,as well as regenerative braking from vehicular transport.With production of oil predicted to decline,and the number of vehicles and their pollution impact increasing globally,a transformation in transportation economy is inevitable given that we live in a carbon-constrained world.One of the most promising candidates for storage devices is the lithium–sulphur cell.Under intense scrutiny for well over two decades,the cell in its simplest configuration consists of sulphur as the positive electrode and lithium as the negative electrode 3,4.It differs from conventional lithium-ion cells,which operate on the basis of topotactic inter-calation reactions:reversible uptake of Li ions and electrons in a solid with minimal change to the structure.They typically use a lithium transition-metal oxide or phosphate as a positive electrode (cathode)that de/re-intercalates Li +at a high potential with respect to the carbon negative electrode (anode).As the reaction is topotac-tic at both electrodes,the charge storage capability is inherently limited to about 300mA h g −1for any prospective system,and maximum capacities observed so far are 180mA h g −1with high power characteristics having been reported 5.The lithium–sulphur cell operates quite differently.The redox couple,described by the reaction S 8+16Li ↔8Li 2S lies near 2.2V with respect to Li +/Li o ,a potential about 2/3of that exhibited by conventional positive electrodes 6.However,this is offset by the very high theoretical capacity afforded by the non-topotactic ‘assimilation’process,of 1,675mA h g −1.Thus,compared with intercalation batteries,Li–S cells have the opportunity to provide a significantly higher energy density (a product of capacity and voltage).Values can approach 2,500W h kg −1or 2,800W h l −1on a weight or volume basis respec-tively,assuming complete reaction to Li 2S (refs 7,8).Despite its considerable advantages,the Li–S cell is plagued with problems that have hindered its widespread practical realization.These arise from the fact that all components of the cell must be addressed as a whole,including the interfaces betweenUniversity of Waterloo,Department of Chemistry,Waterloo,Ontario N2L 3G1,Canada.*e-mail:lfnazar@uwaterloo.ca.them.Sulphur or sulphur-containing organic compounds are highly electrically and ionically insulating 9.To enable a reversible electrochemical reaction at high current rates,the sulphur must maintain intimate contact with an electrically conductive additive.Various carbon–sulphur composites have been used for this purpose,but they have limitations owing to the scale of the contact area.Typical reported capacities are between 300and 550mA h g −1at moderate rates 10.To make a sulphur-containing cathode ionically conductive,liquid electrolytes are used that act not only as a charge transport medium but also as ionic conductors within the sulphur-containing cathode 11.This presents difficulties of electrolyte access.Another major hurdle is capacity degradation on repeated discharge–charge of the cell.This is mainly due to the high solubility of the polysulphide anions formed as reaction intermediates in both discharge and charge processes in the polar organic solvents used in electrolytes 12.During cycling,the polysulphide anions can migrate through the separator to the Li negative electrode whereupon they are reduced to solid precipitates (Li 2S 2and/or Li 2S),causing active mass loss.In addition,the solid product that extensively precipitates on the surface of the positive electrode during discharge becomes electrochemically irreversible,which also contributes to active mass loss 13.In response to these considerable challenges,novel advances in materials design such as new electrolytes 14–17and protective films for the lithium anode have been developed 18–binations of electrolyte modification,additives and anode protection have resulted in some promising results,although rates are not given 21.Much of the difficulty still remains at the cathode,where the lack of breakthroughs has led to some cell configurations in which all of the sulphides are solubilized (so-called ‘catholyte’cells)22.In the opposite approach,that is,to contain the sulphides,some interesting cathode developments have been reported recently 23–26.However,they still fall short of the mark for practical electrochemical performance.They include,for example,the fabrication of disordered mesoporous carbon/sulphur 50:50composites in conjunction with ionic liquid electrolytes;systems that achieve high initial capacity,but suffer extensive capacity0.20.40.60.81.01.21.4x in Li x S CMK¬3+S mixtureCMK¬3/S 155 °CV o l t a g e (V ) v e r s u s L i /L i +V o l t a g e (V ) v e r s u s L i /L i +Specific capacity (mA h g ¬1)2004006008001,0001,200Specific capacity (mA h g ¬1)02004006008001,0001,2001.52.02.53.01.52.02.53.0Sab cd2 µm2 µmFigure 1|SEM images of CMK-3/sulphur,and its electrochemical characterization.a ,Mixture of CMK-3and elemental sulphur before heating.b ,CMK-3/S heated at 155◦C,showing the disappearance of the sulphur mass indicated by the red rectangle in a .c ,d ,Comparison of the galvanostatic discharge–charge profiles of the first cycles of the carbon–sulphur composites shown in a ,b ,at a current rate of 168mAg −1.The marked increase in capacity in d is due to the encapsulation effect.fading posites with sulphur embedded in conducting polymers have shown some promising results 27.However,a large polarization was observed,resulting in a very low operating voltage that reduces the energy density of cells.The loading of active mass in the S-polymer composite is also limited (less than 55wt%)owing to the low surface area of the conducting polymer.Here,we demonstrate that cathodes based on nanostructured sulphur/mesoporous carbon materials can overcome these challenges to a large degree,and exhibit stable,high,reversible capacities (up to 1,320mA h g −1)with good rate properties and cycling efficiency.Our proof-of-concept studies are based on CMK-3,the most well-known member of the mesoporous carbon family,although they are not limited to this material.Highly ordered mesoporous carbons exhibit a uniform pore diameter,very high pore volume,interconnected porous structure and can exhibit high conductivity 28,29.They,and their oxide analogues 30,31,have attracted much attention recently as nanoscale electrode materials in Li batteries 32,33,as supercapacitors and as supports for proton-exchange-membrane fuel-cell catalysts 34.CMK-3was synthesized by a nanocasting method that uses silaceous SBA-15as a hard template.The resulting replica comprises an assembly of hollow 6.5-nm-thick carbon rods separated by empty 3–4-nm-wide channel voids 35.The channel space is spanned by carbon microfibres that prevent the collapse of the nano-architecture of the two-dimensional hexagonally ordered carbon rods.We tuned the synthesis of the CMK-3to produce a short rod-like morphology,to optimize access to the mesoporous channels 36.The CMK-3/sulphur composite was prepared following a simple melt-diffusion strategy.A 3:7weight ratio mixture of CMK-3and sulphur was heated just above the melting point of sulphur,where the viscosity is lowest 37.The melt is imbibed into the channels by capillary forces,whereupon it solidifies and shrinks to form sulphur nanofibres that are in intimate contact with the conductive carbon walls.The scanning electron microscopy (SEM)images in Fig.1reveal the changes in the mixture of CMK-3and sulphur before and after heating.The bulk sulphur evident in the SEM image of the composite on initial mixing (Fig.1a)largely disappears at 145◦C (see Supplementary Fig.S1),and completely disappears after heat treatment at 155◦C (Fig.1b).Full incorporation of sulphur into the channels of CMK-3occurs at this latter temperature.CMK-3and sulphur are both hydrophobic materials,which accounts for the ready absorption of sulphur into the channel structure.The filling of the carbon channels with sulphur is corroborated by the transmission electron microscopy (TEM)image shown in Fig.2a,along with the magnified image shown in Fig.2b.The fibres have a similar diameter to that of the channels of the mesoporous carbon (3.3nm),and a comparable diameter to the carbon nanorods that enclose them (6–7nm).The filling of the pores with sulphur,of similar density to carbon,is also evident from the decrease in contrast in relation to CMK-3itself (shown in the inset in Fig.2b).The sulphur and carbon elemental maps (Fig.2c,d)clearly demonstrate that sulphur is homogeneously distributed in the framework of the mesoporous carbon,with no significant fraction on the external surface.The marked diminution3 nm6.5 nmC Ka1_2S Ka1S meltS xtalx abc def30 nmFigure 2|TEM image and elemental maps of a CMK-3/S-155composite particle and schematic diagrams of the structure and redox processes.a ,CMK-3/S-155composite particle.b ,Image expansion corresponding to the area outlined by the red square in a ,where the inset shows the TEM image for pristine CMK-3at the same magnification.c ,d ,Corresponding carbon and sulphur elemental maps showing the homogeneous distribution of sulphur.e ,A schematic diagram of the sulphur (yellow)confined in the interconnected pore structure of mesoporous carbon,CMK-3,formed from carbon tubes that are propped apart by carbon nanofibres.f ,Schematic diagram of composite synthesis by impregnation of molten sulphur,followed by its densification on crystallization.The lower diagram represents subsequent discharging–charging with Li,illustrating the strategy of pore-filling to tune for volume expansion/contraction.of the X-ray diffraction (XRD)peak (low-angle diffraction pattern,Fig.3a)due to long-range order in CMK-3is further proof of pore-filling,which is the result of the decrease in the scattering contrast (Fig.3a)paring the wide-angle XRD patterns in Fig.3b,the well-resolved peaks corresponding to bulk crystalline sulphur completely disappear after sulphur impregnation,and thermogravimetric analysis (TGA;Supplementary Fig.S2)shows the composites range up to 70wt%sulphur.A schematic diagram illustrating the impregnation of the CMK-3with sulphur is shown in Fig.2e,showing the alignment of the channels in comparison with the inset of Fig.2b.Note that most of the sulphur is contained within the interior of the pore structure,as the particles span hundreds of carbon channels in width.The average CMK-3particle size is of the order of 1µm (Fig.1b).Table 1summarizes the physical characteristics of the CMK-3and the CMK-3/S composite derived from Brunauer–Emmett–Teller (BET)and conductivity measurements.After imbibition of the sulphur in the channels,the pore size of the CMK-3/S composite decreases markedly,indicating that the channels of CMK-3are partially filled.Along with the presence of residual micropores in the carbon wall structure 39,this allows ingress of electrolyte within the structure.Empty volume within the pores is also necessary to accommodate the uptake of Liions,I n t e n s i t y2 (°)I n t e n s i t yi abθ2 (°)θFigure 3|XRD patterns of CMK-3/S before and after heating.a ,Low-angle XRD patterns of a mixture of CMK-3and sulphur before heating (i)and after heating at 155◦C (ii).The disappearance of the first peak is due to the loss of contrast on sulphur imbibition.b ,Wide-angle XRD patterns of a mixture of CMK-3and sulphur before heating (i)and after heating at 155◦C (ii),showing the complete incorporation of crystalline sulphur within the framework.given by the reaction S +2Li →Li 2S,because of the lower density of Li 2S (1.66g cm −3)compared with sulphur.Note that the 70wt%sulphur/composite ratio is less than the theoretical limit of 79wt%sulphur/composite based on the pore volume of CMK-3(2.1cm 3g −1)and the density of liquidized sulphur (1.82g cm −3),and is precisely tuned for the volume expansion (see the Methods section).Using even lower S/carbon ratios provides less ‘stuffed’structures and extra porosity,but at the expense of reduced active mass.Most importantly,the electrical conductivity of the composites (∼0.2S cm −1for 70wt%sulphur/composite)is the same as its mesoporous carbon counterpart.The insulating sulphur merely occupies the empty channels in the mesoporous carbon and does not block the electrical current transporting paths.Three-dimensional,multiple electronic contacts are provided by the numerous carbon interconnects that span the channels,as illustrated schematically in Fig.2e,f (ref.35).Coin cells using a metallic Li anode were assembled to evaluate the materials.All of the capacity values in this article are calculated on the basis of sulphur mass.The first discharge–charge curve for a typical nanostructured CMK-3/S cathode is shown in Fig.1d alongside its SEM image,and is compared with a simple physical (unheated)mixture of 7:3weight ratio of sulphur and CMK-3in Fig.1c.The nanostructured composite exhibits an impressive capacity of 1,005mA h g −1.In contrast,the ‘macro-mixture’exhibited a reversible capacity of 390mA h g −1(on average between 300and 420mA h g −1),similar to that reported in the literature for C–S composites 10.The capacity of CMK-3/S was3.02.52.01.5V o l t a g e (V ) v e r s u s L i +/L i3.02.52.01.5V o l t a g e (V ) v e r s u s L i +/L i3006009001,2001,500Specific capacity (mA h g ¬1)S p e c i f i c c a p a c i t y (m A h g ¬1)CMK¬3/S at 55 °C with C/10 + C/10000.20.40.60.81.01.21.41.6x in Li x S Cycle numberCycle number200406080100S u l p h u r i n e l e c t r o l y t e /t o t a l s u l p h u r (%)1,0001,400abcFigure 4|Electrochemical characterization of PEG-coated CMK-3/S and comparison to reference materials.a ,Lower panel:galvanostaticdischarge–charge profile of PEG-modified CMK-3/S-155recorded at room temperature at 168mA g −1.The reversible capacity of 1,320mA h g −1at room temperature is very close to that obtained for unmodified CMK-3/S obtained at elevated temperature under ‘quasi-equilibrium’conditions shown in the upper panel (CMK-3/S-155recorded at 55◦C at 168mA g −1on discharge to 1.0V followed by quasi-equilibrium discharge at 16.8mA g −1).The slight overcharge in the latter case is due to dissolution of some polysulphide,which is minor even at these conditions.This also indicates that storage of the cell at partial or full discharge does not lead to significant capacity loss.b ,Cycling stability comparison of CMK-3/S-PEG (upper points,in black)versus CMK-3/S (lower points,in red)at 168mA g −1at room temperature.c ,Percentage of sulphur dissolution into the electrolyte from:the CMK-3/S-PEGcomposite cathode (black curve);from the CMK-3/S composite cathode (blue curve);a cathode made of a mixture of acetylene black carbon and sulphur with the exact same C /S ratio (red curve).highly reproducible over many cells.The coulombic efficiency for CMK-3/S in the first discharge–charge cycle is 99.94%without any overcharge,with virtually no irreversibility.This indicates that a very low fraction of polysulphide anions diffuse into the electrolyte.The polarization was decreased by more than a factor of three,owing to the greatly enhanced electrical contact achieved in the nanostructure.Further unequivocal proof of the effectiveness of the contact arises from experiments in which the degree of S incorporation was varied.Nanostructured composites (CMK-3/S-145)with the same S/C ratio,but heated at 145◦C instead of 155◦C result in less complete diffusion of sulphur into the channels because of the higher viscosity at the lower temperature.These composites showed less utilization of sulphur (capacity of 780mA h g −1)in the first discharge sweep (see Supplementary Fig.S3),and an irreversible capacity of 50mA h g −1on plete imbibition prevents sulphur agglomerates on the externalsurface of the mesoporous framework that would have poorer electrical wiring of the conductive carbon phase.These results are superior to those reported for sulphur in contact with multi-walled carbon nanotubes.Such composites exhibit lower capacities and a large electrochemical hysteresis 23.Although the sulphur is apparently confined in the carbon,the contact is limited owing to the relatively large diameter (∼50nm)of the multi-walled carbon nanotubes,and hence of the sulphur fibres within them.Thus,the efficiency of electron transfer to the sulphur mass and accessibility to the Li +electrolyte has a vitally important role in determining the electrochemical behaviour.As seen in Fig.1d,there are two plateaux in the discharge process.The first,which contributes a minor part to the overall capacity from 2.4to 2.0V,corresponds to the conversion from elemental sulphur (S 8)to Li polysulphide anions (Li 2S x ;where x is typically 4–5).The kinetics of this reaction is fast 40.The second plateau atHeat flow (W g ¬1)W e i g h t (%)Temperature (°C)Figure 5|TGA of PEG-modified CMK-3.TGA and differential scanning calorimetry curves recorded in air with a heating rate of 20◦C min −1,for PEG-CMK-3(solid lines),compared with PEG itself (dashed lines),showing the shift to higher temperature of the PEG release on bonding to the CMK-3framework.around 2.0V is due to the conversion of polysulphides to Li 2S 2and then to Li 2S,which occurs at a much slower rate.As we achieve a nominal reversible capacity of Li 1.2S in the nanostructured composite,we wanted to explore the limitations to full conversion.To gain a measure of the reversible capacity under conditions where the kinetics should be a minimal concern,we carried out discharge of the CMK-3/S cathode at 55◦C at 168mA g −1to a cutoff of 1.0V,and allowed the voltage to relax to equilibrium.We then switched the discharge current to a rate of 16.8mA g −1to the end of discharge,and completed charge at 168mA g −1.The electrochemical profile is presented in Fig.4a (upper panel).Under these close-to-equilibrium conditions of full discharge,we achieve a reversible capacity of 1,400mA h g −1—84%of the theoretical capacity (1,675mA h g −1)—indicating that indeed,the kinetics of the last reaction step has a role in capacity limitation.The other factor could be a transport problem.There is progressively more limited accessibility of Li +ions and electrolyte to the sulphur mass towards the end of discharge because the pores become filled with insoluble Li x S (x =1–2)—even though at 70wt%sulphur loading,there is sufficient space for the volume expansion based on the conversion of S to Li 2S.However,we observed that in doubling the rate from 168to 336mA g −1(equivalent to C/5rate),the capacity is reduced by only a small amount to 930mA h g −1(see Supplementary Fig.S4).The mesoporous carbon clearly performs very well as a sulphur container.This is apparent from the small degree of overcharge even under rigorous (55◦C;C/100discharge)conditions as shown in Fig.4a.The complete lack of a sharp minimum in the discharge curve between the two plateaux,as observed by others and ascribed to supersaturation of the electrolyte with S 2−(refs 21,41),is also indicative of the strong extent of sulphide containment in our case.Experiments were carried out to evaluate the degree of self-discharge,by taking the cell to a voltage of 2.1V,holding it at the open-circuit voltage for 24h and then completing discharge.The discharge capacity after relaxation was 5%less than the cell taken to full discharge without the open-circuit voltage step.However,this suggests that the framework still allows for some egress of dissolved sulphur species.We propose that the complex inner pathway and porous,absorptive carbon greatly retard the diffusion of the bulky polysulphide anions out from the channels into the electrolyte,butcannot entirelyprevent it.This is evident by the very slow capacity fading shown in Fig.4b(upperred points).To further trapthe highly polar polysulphide species,we adjusted the hydrophilicity of the carbon external surface afterabcd300 nmFigure 6|Changes in surface morphology of CMK-3/S-155versusPEG-modified CMK-3/S-155on cycling.a ,b ,SEM images of CMK-3/S-155before (a )and after (b )the 15th charge.c ,d ,SEM images of PEG-modified CMK-3/S before (c )and after (d )the 15th charge.Images show the effects of ‘polymer protection’in inhibiting surface deposition.sulphur imbibition by functionalizing the surface with polyethylene glycol (PEG)chains of varying molecular weight.The attachment of the PEG to CMK-3is evident by TGA (Fig.5).The release of the PEG tethered to the CMK-3occurs at 50◦C higher than in PEG itself owing to the ester bonds.The discharge–charge profile of CMK-3/S-PEG is shown in Fig.4a (lower panel).Not only is the initial discharge capacity increased to 1,320mA h g −1(approaching the ‘equilibrium’limit for CMK-3/S of 1,400mA h g −1),and the polarization decreased to low values,but no fading is observed in the second 10cycles and the capacity is stabilized at 1,100mA h g −1on cycling (Fig.4b,upper black points).The entrapment of sulphur active mass on cycling in the polymer-modified CMK-3/S composite is demonstrated in Fig.4c.To measure the degree of sulphur retention in the cathode,a 1.0M LiPF 6solution in a sulphur-free solvent,tetra(ethylene glycol)dimethyl ether (TEGDME),was used as the electrolyte.Glyme solvents are known for their excellent ability to dissolve polysulphides,and hence represent an ‘aggressive’pared with the cathode made of a mixture of sulphur and acetylene black that loses 96%of the total active mass into the electrolyte after 30cycles,the polymer-modified composite shows significant retention of sulphur.Only 25%of the total active mass is solubilized in the electrolyte after 30cycles.The polysulphide retention is also improved in relation to CMK-3/S.We believe that the effect of the PEG-functionalized surface is twofold.First,it serves to trap the polysulphide species by providing a highly hydrophilic surface chemical gradient that preferentially solubilizes them in relation to the electrolyte.Second,by limiting the concentration of the polysulphide anions in the electrolyte,the redox shuttle mechanism is curtailed to a large degree.Deposition of insoluble sulphur species on the surface of the Li electrode and formation of irreversible Li 2S on the cathode surface are strongly inhibited.The last point is clearly demonstrated in SEM images of the PEG-functionalized CMK-3/S cathode before and after cycling,which exhibit very little change in surface morphology (Fig.6),compared with CMK-3/S,which clearly shows precipitation of insoluble products on the surface of the mesoporous carbon particles.In summary,we demonstrate that the strategy illustrated here provides a versatile route to nanostructured polymer-modified mesoporous carbon–sulphur composites that display all of the benefits of confinement effects at a small length scale.Intimate contact of the insulating sulphur and discharge-product sulphides with the retaining conductive carbon framework at nanoscaledimensions affords excellent accessibility of the active material. The carbon framework not only acts as an electronic conduit to the active mass encapsulated within,but also serves as a mini-electrochemical reaction chamber.The entrapment ensures that a more complete redox process takes place,and results in enhanced utilization of the active sulphur material.This is vital to the success of all conversion reactions to ensure full reversibility of the back-reaction.The polymer coating on the external surface of the composite further helps retard diffusion of polysulphide out of the cathode structure,minimize the loss of the active mass in the cathode and improve the cycling stability.The composite materials reported here can supply up to nearly80%of the theoretical capacity of sulphur(1,320mA h g−1),representing more than three times the energy density of lithium transition-metal oxide cathodes,at reasonable rates with good cycling stability.In our laboratory,mesoporous carbon frameworks with various wall thicknesses,conductivities and connectivities have recently been prepared to take advantage of structural and electronic variation of the constraining support.The three-dimensional variants such as CMK-1and CMK-8are particularly promising in this respect42. We will report those results in a forthcoming paper.Owing to the flexibility of the method,the high capacity of the carbon for active material incorporation and facile functionalization of the surface,we believe that a wide variety of nanostructured‘imbibed’composites could find broad application in many areas of materials science,not only as advanced electrode materials that rely on assimilation and conversion reactions.MethodsSynthesis.For the synthesis of SBA-15with controlled morphology43,2g of Pluronic P123(EO20PPO70EO20)was dissolved in60ml of2M HCl at38◦C. Tetraethylorthosilicate(4.2g)was added to the above solution with vigorous stirring.The mixture was stirred for only6min and remained quiescent for24h at38◦C.The mixture was subsequently heated at100◦C for another24h in an autoclave.The as-synthesized SBA-15with short-rod morphology was collected by filtration,dried and calcined at550◦C in air.A nanocasting method was used to fabricate CMK-3from SBA-15as a hard template44.Sucrose(1.25g)was dissolved in5.0ml of water containing0.14g H2SO4.Surfactant-free SBA-15(1.0g)was then dispersed in the above solution and the mixture was sonicated for1h;heated at100◦C for12h and at160◦C for another12h.The impregnation process was repeated once with another5.0ml aqueous solution containing0.8g sucrose and 0.09g H2SO4.The composite was completely carbonized at900◦C for5h in an argon atmosphere.To remove the SBA-15silica template,the composite was stirred in a5%HF solution at room temperature for4h,although NaOH can also be used to dissolve the silica.The CMK-3/S nanocomposite was prepared following a melt-diffusion strategy.CMK-3(1.0g)and sulphur(2.33g)were ground together,and heatedto155◦C.The weight ratio of sulphur/carbon was adjusted to be equal to or less than7:3,to allow for expansion of the pore content on full lithiation to Li2S.For example,1.0g of CMK-3can accommodate3.486g of Li2S(1.66g cm−3(density of Li2S)×2.1cm3g−1,the pore volume of the CMK-3),which corresponds to a maximum of2.425g of sulphur.To prepare the CMK-3/S-PEG composite,CMK-3was first functionalized with carboxylic groups by oxidization treatment in concentrated HNO3solution for half an hour at80◦C,before incorporation of the sulphur.To tether the PEG chains to the surface of the CMK-3/S composite,the composite was dispersedin a PEG aqueous solution and the solution was heated at58◦C and stirred continuously overnight to ensure complete reaction of the carboxylic groups on the carbon particles with the hydroxyl groups on the PEG.The mixture was sonicated for20min to completely remove physically absorbed PEG on the composite,and the CMK-3/S-PEG composite was collected by filtration and dried. Characterization.X-ray diffraction patterns at low-angle(0.75◦to4◦2θ)and wide-angle(from10◦to80◦2θ)were collected on a D8-ADVANCE powderX-ray diffractometer operating at40kV and30mA and using Cu-Kαradiation (λ=0.15406nm).Nitrogen adsorption and desorption isotherms were obtained using a Micromeritics Gemini2735system at−196◦C.Before measurement of CMK-3,the sample was degassed at150◦C on a vacuum line following a standard protocol.It was not possible to carry this out for CMK-3/S owing to the volatility of the sulphur,and so no pretreatment was used.The BET method was usedto calculate the surface area45.The total pore volumes were calculated fromthe amount adsorbed at a relative pressure of0.99.The pore size distributions were calculated by means of the Barrett–Joyner–Halenda method applied to the desorption branch46.As the mesopores of CMK-3/S are decreased to micropores on(partial)filling with sulphur,the possibility of water entrapment,and/or pore blockage means that the values represent lower estimates.The morphology of the sulphur/CMK-3composites were examined by SEM using a LEO1530field-emission SEM instrument or a Hitachi S-5200 instrument.TEM was carried out on a Hitachi HD-2000STEM.Conductivity measurements were carried out at room temperature using the four-point method.Sample bars for the measurement were cut from the pellets and then cold pressed using a force of45kN.Elemental analyses were carried out at M-H-W Laboratories,Phoenix,USA.Electrochemistry.Positive electrodes were comprised84wt%CMK-3/S composite,8wt%Super-S carbon and8wt%poly(vinylidene fluoride)binder. The cathode materials were slurry-cast from cyclopentanone onto a carbon-coated aluminium current collector(Intelicoat).The electrolyte is composed of a1.2M LiPF6solution in ethyl methyl sulphone47.Lithium metal foil was used as the counter electrode.The equivalent current density for the168mA g−1rate is0.19 and0.37mA cm−2for the336mA g−1rate.To measure the degree of sulphur retention in the cathode,a1.0M LiPF6solution in TEGDME was used as the electrolyte.Cathodes comprising CMK-3/S-PEG were compared with simple mixtures of sulphur and acetylene black at the exact same S/C ratio.We used large Swagelok-type cells that accommodate a sufficient excess of the electrolyte to dissolve sulphur species.Swagelok cells were disassembled and immersed into TEGDME to completely extract sulphur species from the electrolyte.Sulphur analysis was carried out by Galbraith Laboratories(Tennessee,USA).Received10September2008;accepted17April2009; published online17May2009References1.Winter,M.&Brodd,R.Batteries,fuel cells and supercapacitors.Chem.Rev.104,4245–4269(2004).2.Bruce,P.G.Energy storage beyond the horizon:Rechargeable lithium batteries.Solid State Ion.179,752–760(2008).3.Rauh,R.D.,Abraham,K.M.,Pearson,G.F.,Surprenant,J.K.&Brummer,S.B.A lithium/dissolved sulfur battery with an organic electrolyte.J.Electrochem.Soc.126,523–527(1979).4.Shim,J.,Striebel,K.A.&Cairns,E.J.The lithium/sulfur rechargeable cell.J.Electrochem.Soc.149,A1321–A1325(2002).5.Kang,K.,Meng,Y.S.,Bréger,J.,Grey,C.P.&Ceder,G.Electrodes withhigh power and high capacity for rechargeable lithium batteries.Science311, 977–980(2006).6.Peled,E.&Yamin,H.Lithium/sulfur organic battery.Prog.Batteries Sol.Cells5,56–58(1984).7.Chu,M.-Y.Rechargeable positive Patent US5686201(1997).8.Peramunage,D.&Licht,S.A solid sulfur cathode for aqueous batteries.Science261,1029–1032(1993).9.Dean,J.A.(ed.)Lange’s Handbook of Chemistry3rd edn,3–5(McGraw-Hill,1985).10.Cunningham,P.T.,Johnson,S.A.&Cairns,E.J.Phase equilibria inlithium–chalcogen systems:Lithium–sulfur.J.Electrochem.Soc.119,1448–1450(1972).11.Choi,J.-W.et al.Rechargeable lithium/sulfur battery with suitable mixedliquid electrolytes.Electrochim.Acta52,2075–2082(2007).12.Rauh,R.D.,Shuker,F.S.,Marston,J.M.&Brummer,S.B.Formationof lithium polysulfides in aprotic media.J.Inorg.Nucl.Chem.39,1761–1766(1977).13.Cheon,S.-E.et al.Rechargeable lithium sulfur battery II.Rate capability andcycle characteristics.J.Electrochem.Soc.150,A800–A805(2003).14.Shin,J.H.&Cairns,E.J.Characterization of N-methyl-N-butylpyrrolidiniumbis(trifluoromethanesulfonyl)imide-LiTFSI-tetra(ethylene glycol)dimethyl ether mixtures as a Li metal cell electrolyte.J.Electrochem.Soc.155,A368–A373(2008).15.Yuan,L.X.et al.Improved dischargeability and reversibility of sulfur cathodein a novel ionic liquid mun.8,610–614(2006).16.Ryu,H.-S.et al.Discharge behavior of lithium/sulfur cell with TEGDME basedelectrolyte at low temperature.J.Power Sources163,201–206(2006).17.Wang,J.et al.Sulfur-mesoporous carbon composites in conjunction with anovel ionic liquid electrolyte for lithium rechargeable batteries.Carbon46, 229–235(2008).18.Chung,K.-I.,Kim,W.-S.&Choi,Y.-K.Lithium phosphorous oxynitride as apassive layer for anodes in lithium secondary batteries.J.Electroanal.Chem.566,263–267(2004).19.Visco,S.J.,Nimon,Y.S.&Katz,B.D.Ionically conductive composites forprotection of active metal Patent7,282,296,October16(2007). 20.Skotheim,T.A.,Sheehan,C.J.,Mikhaylik,Y.V.&Affinito,J.Lithium anodesfor electrochemical patent7247,408,July24(2007).21.Akridge,J.R.,Mikhaylik,Y.V.&White,N.Li/S fundamental chemistry andapplication to high-performance rechargeable batteries.Solid State Ion.175, 243–245(2004).。
磷石膏的综合利用及其在建筑材料领域的应用研究进展

第43卷第2期2024年2月硅㊀酸㊀盐㊀通㊀报BULLETIN OF THE CHINESE CERAMIC SOCIETYVol.43㊀No.2February,2024磷石膏的综合利用及其在建筑材料领域的应用研究进展周㊀武1,2,李㊀杨1,2,冯伟光3,苏㊀轶1,2,揭伟哲1,2,张㊀华1,2,倪红卫1,2 (1.武汉科技大学钢铁冶金与资源利用省部共建教育部重点实验室,武汉㊀430081;2.武汉科技大学钢铁冶金新工艺湖北省重点实验室,武汉㊀430081;3.青岛睿海兴业管理咨询服务有限公司,青岛㊀266041)摘要:磷石膏是湿法制备磷肥工艺过程中的副产物,主要物相是CaSO4㊃2H2O㊂我国磷石膏产量居世界第一,综合利用率却不到50%,堆存量已达8亿吨,对生态环境造成了严重破坏,因此,探索磷石膏的有效利用途径已迫在眉睫㊂本文对磷石膏综合利用主要领域的研究现状进行了分析,其中磷石膏在化工领域的利用率仅有5%,在农业领域的利用率也只有2%,而建筑材料领域是目前磷石膏的主要应用领域㊂目前将磷石膏应用于水泥缓凝剂㊁石膏砌块和水泥砂浆已经实现了工业化;将磷石膏应用于胶凝材料和路基材料,由于浸出毒性的问题,并未大规模应用;将磷石膏用作填充剂则因为材料强度较低,仅仅停留于实验室探索阶段㊂最后展望了未来无害化利用磷石膏技术研究的发展趋势,以期为解决磷石膏的堆存问题提供参考㊂关键词:磷石膏;综合利用;应用领域;建筑材料;研究现状;发展趋势中图分类号:X781㊀㊀文献标志码:A㊀㊀文章编号:1001-1625(2024)02-0534-09 Research Progress on Comprehensive Utilization of Phosphogypsum and Its Application in the Field of Building MaterialsZHOU Wu1,2,LI Yang1,2,FENG Weiguang3,SU Yi1,2,JIE Weizhe1,2,ZHANG Hua1,2,NI Hongwei1,2 (1.Key Laboratory for Ferrous Metallurgy and Resources Utilization of Ministry of Education,Wuhan University of Science and Technology,Wuhan430081,China;2.Hubei Provincial Key Laboratory for New Processes of Ironmaking and Steelmaking,Wuhan University of Science and Technology,Wuhan430081,China;3.Ruihai Xingye Management Consulting Service Co.,Ltd.,Qingdao266041,China)Abstract:Phosphogypsum is a byproduct in the wet process of phosphate fertilizer production,with its main phase being CaSO4㊃2H2O.Despite China being the world s largest producer of phosphogypsum,its comprehensive utilization rate is below50%.The accumulated stock has reached800million tons,causing severe ecological damage.Therefore,exploring effective utilization methods for phosphogypsum is urgently needed.This article analyzes the current research status of the comprehensive utilization of phosphogypsum in various fields.Currently,the utilization rate of phosphogypsum in the chemical industry is only5%,and in agriculture is a mere2%,while the building materials sector remains its primary application field.Industrial applications such as using phosphogypsum as a cement retarder,gypsum block,and cement mortar have been industrialized.However,its application in cementitious materials and subgrade materials has not been widely adopted due to concerns about leaching toxicity.The use of phosphogypsum as a filler is still in the experimental exploration phase due to the relatively low strength of material.The paper concludes by envisioning the future trends in the research on environmental friendly utilization of phosphogypsum,aiming to provide references for resolving the stockpile issue of phosphogypsum.Key words:phosphogypsum;comprehensive utilization;application field;building material;research status;development trend收稿日期:2023-09-11;修订日期:2023-11-10基金项目:国家自然科学基金面上项目(52374344)作者简介:周㊀武(1998 ),男,硕士研究生㊂主要从事固废回收利用的研究㊂E-mail:2528548316@通信作者:李㊀杨,博士,副教授㊂E-mail:liyang2468@第2期周㊀武等:磷石膏的综合利用及其在建筑材料领域的应用研究进展535㊀0㊀引㊀言中国作为农业大国,大多数耕地缺少作物所必需的磷元素[1],对磷肥的需求量约为850万吨/年㊂一般来说,湿法是生产磷肥最主要的工艺[2]㊂该工艺以硫酸和磷矿石为原料,将磷矿石置于硫酸中分解,经萃取㊁分离等工艺后制得磷酸;再向磷酸中加入不同种类的原料(如磷矿石㊁氨气㊁石灰等)制得磷肥,湿法制磷肥工艺流程如图1所示,反应如式(1)所示㊂Ca 3(PO 4)2+2H 2SO 4ң3CaSO 4+2H 3PO 4(1)生产经验表明,每生产1t 的磷肥就会伴生近5t 的磷石膏[3]㊂我国磷石膏产地较为集中,基本上分布在云贵地区㊁长江中下游地区以及山东地区[4](见图2)㊂据中国磷复肥工业协会统计,2021年中国磷石膏产量突破8000万吨,2022年总堆存量已达8亿吨[5],而2021年巴西的产量只有1237万吨,塞尔维亚的产量仅有81万吨[6]㊂中国磷石膏产量居世界第一,综合利用率却不到50%,相比日本等国家近100%的利用率,中国的磷石膏综合利用能力亟须增强[7](见表1)㊂图1㊀湿法制磷肥工艺流程图[2]Fig.1㊀Flowchart of wet process for phosphate fertilizer production[2]图2㊀中国磷石膏产地分布[4]Fig.2㊀Distribution of phosphogypsum producing areas in China [4]表1㊀部分国家磷石膏综合利用现状[7]Table 1㊀Comprehensive utilization status of phosphogypsum in some countries [7]地区主要处置措施比利时约90%用于石膏建筑材料,8%左右用于农业,少量临时堆存巴西主要用于农业,使用量已超过产生量加拿大磷酸生产已停止,堆场表层 人造土 后复垦种植人造林芬兰堆存为主,少量用于农业㊁道路等试点哈萨克斯坦主要用于农业土壤改良波兰少量用于农业印度约45%用于制造水泥,10%用于农业摩洛哥排入海洋菲律宾部分用于水泥㊁农业,少量进行稀土元素回收试点俄罗斯农业㊁道路应用已实现商业化美国堆存为主,少量用于农业日本100%用于石膏建筑材料及水泥缓凝剂1㊀磷石膏的性质及危害1.1㊀磷石膏的性质磷石膏一般呈灰色粉末状,受杂质的影响也可能呈黄白色㊁浅灰白色或黑灰色(见图3(a)),其粒径一般为5~150μm,晶体形貌有针状㊁单分散板状㊁多晶核和密实四种形态,但以板状晶体为主[8](见图3(b))㊂磷石膏的成分十分复杂,其主要化学组成是CaO 和SO 3,还有少量的Al 2O 3㊁Fe 2O 3㊁SiO 2等(见表2)㊂此外,磷石膏中还存在一些有机物㊁氟化物㊁重金属离子以及微量的放射性元素[9]㊂磷石膏的XRD 谱如图4所536㊀资源综合利用硅酸盐通报㊀㊀㊀㊀㊀㊀第43卷示,其主要物相结构是CaSO 4㊃2H 2O,还有少量的共晶磷(CaHPO 4㊃2H 2O)和SiO 2[10]㊂另外,部分磷石膏中还含有难溶磷(Ca 3(PO 4)2)以及难溶氟(CaSiF 6㊁CaF 2)等杂质[11]㊂图3㊀磷石膏的实物图与SEM 照片[8]Fig.3㊀Physical and SEM images of phosphogypsum [8]表2㊀磷石膏的化学成分[9]Table 2㊀Chemical composition of phosphogypsum [9]Composition CaO SiO 2Al 2O 3K 2O SO 3Fe 2O 3P 2O 5TiO 2MgO F LOIMass fraction /%27.0508.336 1.2730.81237.9490.5780.8020.1320.113 1.30321.652图4㊀磷石膏的XRD 谱[10]Fig.4㊀XRD pattern of phosphogypsum [10]1.2㊀磷石膏的危害未经处理的磷石膏含有P㊁F㊁重金属以及放射性元素,长时间的堆存对环境和人类都有较大的危害㊂研究[12]表明,磷石膏中的有毒元素会在雨水的冲刷下进入水循环,破坏生态平衡㊂此外,磷石膏中放射性元素虽然含量极低,但长期处于堆存区域仍会引起身体不适甚至癌变㊂本着废弃物资源化利用的原则,人们采用各种手段对磷石膏加以利用,均取得了不错的效果㊂例如利用磷石膏生产化工原料㊁改性土壤㊁生产建筑材料等㊂但相较于国内日渐增长的堆存量,这些利用手段对磷石膏的利用量显得杯水车薪,整个行业亟须一种更高效的方法来应对这种情况㊂2㊀磷石膏的回收利用目前磷石膏的综合利用主要在化工㊁农业以及建筑材料三个领域[13]㊂化工领域消耗量占比5%左右,农业领域消耗只占2%左右,除22%左右用于外供联营,其余磷石膏基本投入建筑材料领域㊂该领域消纳磷石膏的手段种类较多,可用于生产各种水泥㊁砌砖㊁凝胶材料㊁路基㊁填充剂等[14],虽然生产的建筑材料存在强度较低㊁浸出毒性大的问题,但该领域前景广阔,是目前解决磷石膏堆存问题的最有效手段㊂2.1㊀化工领域磷石膏在化工领域的利用一般是利用其中的Ca㊁S 等元素生产硫酸钙㊁硫酸氢钙等,但是过多的杂质使得生产成本较高,只能局限于实验室或者小规模试验生产,难以处理堆存量巨大的磷石膏㊂Xu 等[15]以磷石膏为原料,在100ħ条件下利用硫酸溶液制备出短柱状无水微米CaSO 4,该材料经NaOH-硬脂酸改性后,用作聚氯乙烯(PVC)的填充剂可显著提高其综合力学性能㊂Zdah 等[16]以磷石膏和LiOH㊃H 2O 为原料,在常温常压的水溶液中反应制得Ca(OH)2和Li 2SO 4㊃H 2O,发现反应时间为3h㊁磷石膏和LiOH㊃H 2O 浓度分别为2.0和4.1mol /L 时磷石膏的转化率最高㊂李冬丽等[17]以磷酸与磷石膏制备㊀第2期周㊀武等:磷石膏的综合利用及其在建筑材料领域的应用研究进展537的硫氢化钙为原料,合成出饲料级硫酸氢钙,为综合利用磷石膏提供了宝贵的指导意见㊂目前制约这一领域发展的最主要原因就是杂质去除问题,如何低成本㊁高效率净化磷石膏是打破瓶颈的关键因素,也是未来科学工作者们需要突破的方向㊂2.2㊀农业领域农业领域主要利用磷石膏中的酸性成分改良盐碱地,或利用其中所含的P㊁Ca等元素来增强土壤肥力㊂也有部分学者逆向思维,通过改性工艺使磷石膏能够改良酸性土壤㊂Sagna等[18]在探究磷石膏对盐碱土壤的调理效果时发现有机改良剂配合磷石膏能够显著降低盐碱土壤的碱化度㊂Panda等[19]另辟蹊径,在700ħ的条件下通过共热解香蕉花梗和磷石膏制备出一种生物炭磷石膏复合材料,该材料应用于酸性红壤可提高土壤中硫酸盐的含量,增强土壤肥力㊂此外,由于磷石膏在未经处理之前难以用于酸性土壤改良,严建立等[20]通过向磷石膏中配加石灰制得酸性土壤改良剂,发现在磷石膏掺量较少的情况下,该酸性土壤改良剂能够有效降低土壤中Cd㊁Cr㊁Pb的含量㊂综合上述分析可知,未经除杂的磷石膏不但没有调节土壤环境的能力,其中所含有的有毒元素反而会污染土壤和地下水,危害生态环境和人类健康㊂因而利用磷石膏对土壤进行改性的适用范围较窄,发展潜力不足㊂2.3㊀建筑材料领域建筑材料领域是目前回收利用磷石膏的主要途径,也可能是未来解决磷石膏堆存问题的发展方向㊂该领域主要利用磷石膏中的Ca㊁Si等有效元素,通过添加水泥或耦合其他固废,在碱性环境下发生火山灰反应制备得到各类建筑材料[21],反应原理如下:CaO+H2OңCa(OH)2ңCa2++OH-(2)Ca2++2OH-+SiO2ңC-S-H(3)Ca2++2OH-+Al2O3ңC-A-H(4)SiO2+OH-+H2Oң[H3SiO4]-(5)AlO2-+OH-+H2Oң[H3AlO4]2-(6)[H3SiO4]-+[H3AlO4]2-+Ca2+ңC-A-S-H(7)反应生成的C-S-H㊁C-A-H以及C-A-S-H具有很强的胶结作用,在脱水固化后能够大幅度提高材料的强度[22]㊂目前,磷石膏在建筑材料领域的利用有以下几种手段:制成胶凝材料以替代部分水泥的使用;制成填充剂以降低建筑材料孔隙率;直接制造水泥,降低生产成本;制造石膏砌块减少烧结砖的生产;制造路基材料㊁水泥缓凝剂等㊂2.3.1㊀胶凝材料胶凝材料能在水化反应后形成坚固的石状体,并胶结其他物料,在建筑材料领域具有广泛的应用㊂利用磷石膏制备胶凝材料不但可以减少水泥的使用,还能有效缓解磷石膏的堆存压力㊂魏兴[23]以磷石膏㊁无水石膏㊁钢渣和硅酸盐水泥(P㊃Ⅱ52.5)为原料,添加少量增效剂制备出一种复合胶凝材料,该胶凝材料28d抗压强度可达51.5MPa,一定程度上可减少P㊃C32.5水泥的使用㊂而胡修权等[24]向磷石膏基胶凝材料中掺入聚丙烯酸系高分子进行改性,发现磷石膏质量分数为40%时,样品28d抗压强度达到47.5MPa,相比未改性样品提高了25%以上㊂Gong等[25]以水泥和磷石膏为原料,探究了磷石膏制备复合胶凝材料的潜力,发现磷石膏质量分数为10%时,复合胶凝材料28d抗压强度可达49.8MPa㊂刘冬梅等[26]以质量比为20ʒ72ʒ8的磷石膏㊁磷渣㊁水泥熟料为原料,掺入质量分数为1.5%的水玻璃,制备出的复合胶凝材料28d抗压强度可达43MPa㊂虽然磷石膏制备的胶凝材料强度达标,但其对有毒元素的固化效果却不尽如人意,并不能够完全替代水泥在生产中的地位㊂2.3.2㊀建筑材料填充剂建筑材料填充剂通常不与物料组分发生反应,但又可以有效改善物料性能㊂近年来发现利用磷石膏制备建筑材料填充剂可以有效固化其中的有害元素,其固化原理如图5所示㊂黄琬等[27]以磷石膏为原料压制团粒,并对团粒的填充效应进行多角度验证,结果表明团粒填充试样较538㊀资源综合利用硅酸盐通报㊀㊀㊀㊀㊀㊀第43卷粉末在有害元素的固定效果上更为理想,90d 试样浸出液中F -浓度为0.035mg /L,P 5+浓度也仅有0.35mg /L,满足一级排放标准㊂陈秋松等[28]为比较不同温度下磷石膏充填体的浸出毒性,采用磷石膏㊁硅酸盐水泥等原料制备样品并进行浸出毒性测试,结果表明30ħ条件下P 元素浸出浓度仅有0.05mg /L,符合国家标准㊂但可惜的是该工艺生产的材料强度并不理想,且磷石膏的利用量也比较少,难以消纳大量的磷石膏㊂图5㊀磷石膏充填体对毒害离子固化示意图[28]Fig.5㊀Schematic diagram of immobilization of toxic ions by phosphogypsum filling body [28]2.3.3㊀水泥砂浆水泥砂浆在建筑工程中主要用作粘合剂以及室内外抹灰㊂研究表明,磷石膏的主要成分CaSO 4能够用于生产水泥,有望通过这一手段来减少磷石膏的堆存量㊂但不容乐观的是,磷石膏的大量掺入会导致水泥凝结时间延长,强度急剧下降㊂图6㊀不同磷石膏掺量水泥砂浆的抗压强度[30]Fig.6㊀Compressive strength of cement mortar with different phosphogypsum content [30]Gong 等[29]为考察磷石膏掺量对水泥强度的影响,采用800ħ煅烧的磷石膏替代部分水泥制备新型水泥,结果显示,煅烧磷石膏对水泥强度的影响随掺量的增加呈先增强后减弱的趋势,在磷石膏质量分数为30%时,新型水泥的28d 抗压强度达到最大值(41.0MPa)㊂张敏等[30]同样采用高温煅烧的磷石膏,探究了其掺入量对水泥强度的影响,结果如图6所示,在磷石膏质量分数为10%时,水泥强度最大,随着掺量的增加,水泥强度降幅十分明显,这显然并不符合大量利用磷石膏的预期目标㊂2.3.4㊀石膏砌块石膏砌块作为建筑石膏制品(见图7),在墙体材料应用上具有较优异的性能㊂近年来,使用磷石膏代替建筑石膏制备砌块也成为综合利用该大宗固废的热门研究方向㊂图7㊀轻质石膏砌块与普通石膏砌块Fig.7㊀Lightweight gypsum block and ordinary gypsum block㊀第2期周㊀武等:磷石膏的综合利用及其在建筑材料领域的应用研究进展539 Wu等[31]以磷石膏为原料,采用常规机械压制法制备磷石膏砌块,发现当压制压力为300MPa㊁砌块中水的质量分数为5%㊁添加单质铁或单质铝的质量分数为1%时,磷石膏砌块的3d抗折强度可达8.0MPa㊂此外,为耦合其他固废,Oubaha等[32]采用质量比为52ʒ40ʒ8的磷矿废渣㊁磷石膏与水泥进行磷石膏砌块的制备,结果表明,磷石膏砌块28d抗压强度可达8.1MPa㊂不同于以上磷石膏砌块的传统制备工艺,骆真等[33]采用 先成型 再蒸压 后湿养 的新工艺,以改性磷石膏和α-半水石膏为原料,添加发泡剂制备出一种轻质石膏砌块,结果表明,当α-半水石膏与改性磷石膏质量比为1ʒ4㊁蒸压温度为140ħ时,新型砌块7d 抗折强度可达7.0MPa㊂遗憾的是,磷石膏砌块在强度等方面虽然达到了要求,但是其浸出毒性及长期稳定性仍然存在缺陷,这对于建筑材料来说这是一个不容忽视的问题㊂2.3.5㊀路基材料路基作为路面带状构造物,是铁路和公路的基础㊂由于磷石膏主要成分与路基原料成分相近,人们开始研究利用磷石膏耦合其他冶金固废来制作路基材料,并在这方面取得了较大成果㊂Dutta等[34]以质量比为90ʒ8ʒ2的粉煤灰㊁石灰和磷石膏为原料,制备出一种复合路基材料,结果表明该材料28d抗压强度为2.2MPa,满足美国国家公路与运输官员协会采用的碎石和沙土材料的强度标准㊂Zmemla等[35]以质量比为46.5ʒ46.5ʒ7的混合沙子㊁磷石膏与水泥为原料,制备出一种新型路基材料,测试结果表明该材料的28d抗压强度达到2.2MPa,满足路基材料的要求㊂同样地,吕伟等[36]以质量比为88ʒ6ʒ6的改性磷石膏㊁水泥和矿渣粉为原料,使用团粒工艺制成的轻骨料60d筒压强度达到5.8MPa,并在实际应用中取得了一定的效果(见图8),其中B-3为普通碎石道路稳定层,B-1和B-2为轻骨料部分替代碎石制备的磷石膏基道路稳定层㊂图8㊀不同配比的磷石膏基道路稳定层试样和工程应用[36]Fig.8㊀Samples and engineering applications of phosphogypsum-based road stabilizing layer with different ratios[36] 2.3.6㊀水泥缓凝剂水泥缓凝剂能延长水泥的凝结时间,且不对其后期各项性能造成不良影响㊂使用磷石膏替代天然石膏生产水泥缓凝剂,既可为企业开源节流,又可循环利用固废㊂刘骥等[37]使用磷石膏作为水泥缓凝剂,探究了磷石膏掺量对水泥凝结时间以及强度的影响,发现在26ħ环境下,水泥初凝时间从128min延长至276min,但磷石膏中可溶磷会降低水泥早期强度㊂此外,研究发现使用不同改性工艺制备的磷石膏缓凝剂性能各有侧重,王银等[38]采用蒸压法改性磷石膏与天然石膏制备缓凝剂,测试表明该缓凝剂侧重水泥强度,掺入缓凝剂后水泥28d抗压强度可达50.3MPa,各项指标均满足P㊃O42.5水泥质量标准㊂而He等[39]使用 两步晶化法 改性磷石膏制备的缓凝剂则更侧重有害元素的去除,当一次晶化硫酸质量分数为30%㊁二次晶化固液比为7ʒ1时,改性磷石膏中P2O5的质量分数仅有540㊀资源综合利用硅酸盐通报㊀㊀㊀㊀㊀㊀第43卷0.02%,并且部分重金属元素含量为0%㊂2.3.7㊀磷石膏在建筑材料领域不同利用方法的对比磷石膏在建筑材料领域的利用方法按实际应用进度可分为工业化㊁暂未工业化和实验室探索阶段三种,其中制备水泥缓凝剂㊁石膏砌块以及水泥砂浆已实现了工业化利用,对磷石膏的利用量占比达到33%;生产路基及胶凝材料由于其浸出毒性较高,暂未大规模应用;制备建筑材料填充剂由于材料强度较低,仍在实验室探索中㊂这些利用方法的优缺点对比如表3所示㊂表3㊀磷石膏在建筑材料领域不同利用方法的对比Table3㊀Comparison of different utilization methods of phosphogypsum in the field of building materials 应用情况利用方法优点缺点水泥缓凝剂替代部分天然石膏使用,降低成本需要除杂处理,掺量过多会降低水泥性能工业化石膏砌块强度高,磷石膏用量大浸出毒性大,长期稳定性差水泥砂浆替代部分水泥的生产,减少碳排放磷石膏掺量少暂未工业化路基材料水稳性好,符合强度要求浸出毒性及长期稳定性不明胶凝材料强度高,可协同其他固废进行利用浸出毒性易超标实验室探索阶段建筑材料填充剂有毒元素固化效果好材料强度低,磷石膏用量少3㊀结语与展望磷石膏的综合利用主要在化工㊁农业以及建筑材料三个领域,其中利用磷石膏制备建筑材料是目前消纳磷石膏的最有效手段,按应用进度可分为工业化㊁暂未工业化㊁实验室探索阶段三种㊂制备水泥缓凝剂㊁石膏砌块以及水泥砂浆已经实现工业化应用,但仍存在磷石膏掺入量少㊁强度低㊁浸出毒性大的问题;生产路基及胶凝材料也同样存在浸出毒性大的问题,因而未大规模工业化应用;而制备填充剂由于材料强度低,磷石膏用量少,仅停留在实验室探索阶段㊂针对以上磷石膏回收利用的难题,为解决磷石膏堆存问题,未来可以从以下几个方向进行探索:1)寻找高效㊁低成本的磷石膏除杂工艺,尽量降低杂质元素对建筑材料强度以及浸出毒性的影响;2)综合利用其他固废,通过协同作用提升建筑材料强度以及对有害元素的固化效果㊂参考文献[1]㊀赵亚丽,杨春收,王㊀群,等.磷肥施用深度对夏玉米产量和养分吸收的影响[J].中国农业科学,2010,43(23):4805-4813.ZHAO Y L,YANG C S,WANG Q,et al.Effects of phosphorus placement depth on yield and nutrient uptake of summer maize[J].Scientia Agricultura Sinica,2010,43(23):4805-4813(in Chinese).[2]㊀何宾宾,魏立军,谢德龙,等.中国湿法磷加工产业现状与可持续发展[J].无机盐工业,2020,52(1):1-4+16.HE B B,WEI L J,XIE D L,et al.Current situation and sustainable development of wet process phosphorus processing industry in China[J].Inorganic Chemicals Industry,2020,52(1):1-4+16(in Chinese).[3]㊀HAK S A,SRAIDI A,KHALESS K,et al.Two steps leaching process for recovery of rare earths from Moroccan phosphogypsum[J].Journal ofCleaner Production,2023,427:138976.[4]㊀王㊀童.利用磷石膏制备硫铝酸盐水泥的研究[D].大连:大连理工大学,2022.WANG T.Synthesis of sulfoaluminate cement with phosphogypsum[D].Dalian:Dalian University of Technology,2022(in Chinese). [5]㊀崔荣政.2021年我国磷石膏综合利用现状及建议[J].磷肥与复肥,2022,37(11):1-3.CUI R Z.Present situation and suggestions on comprehensive utilization of phosphogypsum in China in2021[J].Phosphate&Compound Fertilizer,2022,37(11):1-3(in Chinese).[6]㊀QI J,ZHU H,ZHOU P,et al.Application of phosphogypsum in soilization:a review[J].International Journal of Environmental Science andTechnology,2023,20(9):10449-10464.[7]㊀欧志兵,杨文娟,何宾宾.国内外磷石膏综合利用现状[J].云南化工,2021,48(11):6-9.OU Z B,YANG W J,HE B B.The general introduction of phosphogypsum comprehensive utilization technology in China[J].Yunnan Chemical Technology,2021,48(11):6-9(in Chinese).[8]㊀王锦涛.磷石膏制备Ⅱ型无水石膏及应用研究[D].绵阳:西南科技大学,2023.WANG J T.Study on preparation and application of anhydriteⅡfrom phosphogypsum[D].Mianyang:Southwest University of Science and㊀第2期周㊀武等:磷石膏的综合利用及其在建筑材料领域的应用研究进展541 Technology,2023(in Chinese).[9]㊀SUN T,LI W M,XU F,et al.A new eco-friendly concrete made of high content phosphogypsum based aggregates and binder:mechanicalproperties and environmental benefits[J].Journal of Cleaner Production,2023,400:136555.[10]㊀郭㊀爽,邢冬娴,郭㊀校,等.磷石膏基建筑石膏的制备及改性研究[J/OL].无机盐工业:1-15[2023-09-24].https:///10.19964/j.issn.1006-4990.2023-0138.GUO S,XING D X,GUO X,et al.Preparation and modification of phosphogypsum-based architectural gypsum[J/OL].Inorganic Chemicals Industry:1-15[2023-09-24].https:///10.19964/j.issn.1006-4990.2023-0138(in Chinese).[11]㊀XIANG J C,QIU J P,SONG Y Y,et al.Synergistic removal of phosphorus and fluorine impurities in phosphogypsum by enzyme-inducedmodified microbially induced carbonate precipitation method[J].Journal of Environmental Management,2022,324:116300. [12]㊀EL KATEB A,STALDER C,RÜGGEBERG A,et al.Impact of industrial phosphate waste discharge on the marine environment in the Gulf ofGabes(Tunisia)[J].PLoS One,2018,13(5):e0197731.[13]㊀WEI Z Q,DENG Z B.Research hotspots and trends of comprehensive utilization of phosphogypsum:bibliometric analysis[J].Journal ofEnvironmental Radioactivity,2022,242:106778.[14]㊀张㊀峻,解维闵,董雄波,等.磷石膏材料化综合利用研究进展[J].材料导报,2023,37(16):167-178.ZHANG J,XIE W M,DONG X B,et al.Research progress on comprehensive utilization of phosphogypsum for materials:a review[J].Materials Reports,2023,37(16):167-178(in Chinese).[15]㊀XU L,FANG K N,BI Y X,et al.Preparation of anhydrous micron CaSO4with different morphologies from phosphogypsum and its reinforcing inpolyvinyl chloride[J].Construction and Building Materials,2023,365:130126.[16]㊀ZDAH I,AZIFA A,ENNACIRI Y,et al.Green method of phosphogypsum waste conversion to lithium sulfate monohydrate and calciumhydroxide[J].Sustainable Chemistry and Pharmacy,2022,30:100850.[17]㊀李冬丽,谭艳霞,姚㊀波.磷石膏制备磷酸氢钙的实验研究[J].昆明冶金高等专科学校学报,2021,37(3):97-100.LI D L,TAN Y X,YAO B.Experimental study on preparation of calcium hydrogen phosphate from phosphogypsum[J].Journal of Kunming Metallurgy College,2021,37(3):97-100(in Chinese).[18]㊀SAGNA Y P,DIEDHIOU S,GOUDIABY A O K,et al.Do phosphogypsum combined with organic amendments improve rice growth in a salineenvironment?[J].Current Journal of Applied Science and Technology,2023,42(35):52-61.[19]㊀PANDA L,KUMAR M,PRADHAN A.Leaching of sulphate from biochar and phosphogypsum-biochar for the treatment of acidic red soil[J].Asian Journal of Water,Environment and Pollution,2022,19(3):23-29.[20]㊀严建立,章明奎,王道泽.磷石膏与石灰石粉配施对新垦红壤耕地的改良效果[J].农学学报,2022,12(7):33-37.YAN J L,ZHANG M K,WANG D Z.Improvement effect of phosphogypsum combined with limestone powder on newly cultivated red soil farmland[J].Journal of Agriculture,2022,12(7):33-37(in Chinese).[21]㊀WANG C Q,CHEN S,HUANG D M,et al.Safe environmentally friendly reuse of red mud modified phosphogypsum composite cementitiousmaterial[J].Construction and Building Materials,2023,368:130348.[22]㊀MESKINI S,SAMDI A,EJJAOUANI H,et al.Valorization of phosphogypsum as a road material:stabilizing effect of fly ash and lime additiveson strength and durability[J].Journal of Cleaner Production,2021,323:129161.[23]㊀魏㊀兴.磷石膏复合胶凝材料的制备及产品研发[D].昆明:昆明理工大学,2021.WEI X.Preparation and product development of phosphogypsum composite cementitious material[D].Kunming:Kunming University of Science and Technology,2021(in Chinese).[24]㊀胡修权,张㊀立,张㊀晋,等.非煅烧磷石膏基胶凝材料的改性实验[J].无机盐工业,2022,54(4):29-33.HU X Q,ZHANG L,ZHANG J,et al.Modification experiment of non-calcined phosphogypsum based cementitious materials[J].Inorganic Chemicals Industry,2022,54(4):29-33(in Chinese).[25]㊀GONG Y Y,DONG S H,LIU L Y,et al.A sustainable composite cementitious material manufactured by phosphogypsum waste[J].AppliedSciences,2022,12(24):12718.[26]㊀刘冬梅,王玮琦,彭艳周,等.磷石膏-磷渣基复合胶凝材料强度和水化特性研究[J].金属矿山,2022(9):230-237.LIU D M,WANG W Q,PENG Y Z,et al.Study on strength and hydration characteristics of phosphogypsum-phosphoslag based composite cementitious materials[J].Metal Mine,2022(9):230-237(in Chinese).[27]㊀黄㊀琬,谈云志,陈君廉,等.磷石膏团粒的制备与填充效应验证[J/OL].土木与环境工程学报(中英文):1-10[2023-11-24]./kcms/detail/50.1218.tu.20221205.0845.001.html.HUANG W,TAN Y Z,CHEN J L,et al.Preparation and filling effect verification of phosphogypsum particles[J/OL].Journal of Civil and Environmental Engineering:1-10[2023-11-24]./kcms/detail/50.1218.tu.20221205.0845.001.html(in Chinese).[28]㊀陈秋松,张㊀琦,齐冲冲,等.磷石膏充填体强度和浸出毒性的温变规律[J].中国有色金属学报,2021,31(4):1084-1095.CHEN Q S,ZHANG Q,QI C C,et al.Temperature-depending characteristics of strength and leaching toxicity of phosphogympsum-based cemented paste backfill[J].The Chinese Journal of Nonferrous Metals,2021,31(4):1084-1095(in Chinese).542㊀资源综合利用硅酸盐通报㊀㊀㊀㊀㊀㊀第43卷[29]㊀GONG X Q,LIU J S,SUN Z G,et al.Effects of phosphogypsum and calcined phosphogypsum content on the basic physical and mechanicalproperties of Portland cement mortar[J].Journal of Testing and Evaluation,2020,48(5):20180380.[30]㊀张㊀敏,王㊀红,陈马聪,等.磷石膏煅烧温度及掺量对水泥砂浆力学性能的影响[J].四川建材,2022,48(11):1-2.ZHANG M,WANG H,CHEN M C,et al.Effect of calcination temperature and dosage of phosphogypsum on mechanical properties of cement mortar[J].Sichuan Building Materials,2022,48(11):1-2(in Chinese).[31]㊀WU F H,JIN C Y,QU G F,et al.Enhancement of phosphogypsum mechanical block with the addition of iron and aluminum salts[J].Journalof Building Engineering,2022,52:104397.[32]㊀OUBAHA S,HAKKOU R,TAHA Y,et al.Elaboration of compressed earth blocks based on phosphogypsum and phosphate mining by-products[J].Journal of Building Engineering,2022,62:105423.[33]㊀骆㊀真,马玉莹,郭元杨,等.磷石膏制备轻质石膏砌块新工艺[J].应用科技,2020,47(5):94-99.LUO Z,MA Y Y,GUO Y Y,et al.New technology for preparing lightweight gypsum block from phosphogypsum[J].Applied Science and Technology,2020,47(5):94-99(in Chinese).[34]㊀DUTTA R K,KUMAR V.Suitability of flyash-lime-phosphogypsum composite in road pavements[J].Periodica Polytechnica Civil Engineering,2016:455-469.[35]㊀ZMEMLA R,BENJDIDIA M,NAIFAR I,et al.A phosphogypsum-based road material with enhanced mechanical properties for sustainableenvironmental remediation[J].Environmental Progress&Sustainable Energy,2022,41(1):13732.[36]㊀吕㊀伟,吴赤球,龚文辉,等.改性磷石膏轻骨料在路基材料中的应用研究[J].混凝土与水泥制品,2022(6):82-86.LYU W,WU C Q,GONG W H,et al.Research on the application of modified phosphogypsum lightweight aggregate in subgrade materials[J].China Concrete and Cement Products,2022(6):82-86(in Chinese).[37]㊀刘㊀骥,唐小春,韦显文,等.浅谈磷石膏对水泥性能的影响[J].企业科技与发展,2020(9):82-83.LIU J,TANG X C,WEI X W,et al.Discussion on the influence of phosphogypsum on cement properties[J].Sci-Tech&Development of Enterprise,2020(9):82-83(in Chinese).[38]㊀王㊀银,向丛阳,段亚军,等.改性磷石膏作水泥缓凝剂的应用研究[J].水泥工程,2022(3):11-13.WANG Y,XIANG C Y,DUAN Y J,et al.Application of modified phosphogypsum as cement retarder[J].Cement Engineering,2022(3):11-13(in Chinese).[39]㊀HE S K,YANG L,HU G T,et al.Deep removal of phosphate impurities in phosphogypsum by two-step crystal transformation for use as Portlandcement retarder[J].Journal of Building Engineering,2023,79:107831.。
关于磷酸铁锂的英文文献(含中文翻译)

Preparation and characterization of carbon-coated LiFePO 4cathode materials for lithium-ion batteries with resorcinol –formaldehyde polymer as carbon precursorYachao Lan,Xiaodong Wang ⁎,Jingwei Zhang,Jiwei Zhang,Zhishen Wu,Zhijun Zhang ⁎Key Laboratory for Special Functional Materials,Henan University,Kaifeng 475004,Chinaa b s t r a c ta r t i c l e i n f o Article history:Received 8February 2011Received in revised form 26May 2011Accepted 3June 2011Available online 12June 2011Keywords:Lithium iron phosphateResorcinol –formaldehyde polymer Lithium-ion batteryLiFePO 4/C composites were synthesized by two methods using home-made amorphous nano-FePO 4as the iron precursor and soluble starch,sucrose,citric acid,and resorcinol –formaldehyde (RF)polymer as four carbon precursors,respectively.The crystalline structures,morphologies,compositions,electrochemical performances of the prepared powders were investigated with XRD,TEM,Raman,and cyclic voltammogram method.The results showed that employing soluble starch and sucrose as the carbon precursors resulted in a de ficient carbon coating on the surface of LiFePO 4particle,but employing citric acid and RF polymer as the carbon precursors realized a uniform carbon coating on the surface of LiFePO 4particle,and the corresponding thicknesses of the uniform carbon films are 2.5nm and 4.5nm,respectively.When RF polymer was used as the carbon precursor,the material showed the highest initial discharge capacity (138.4mAh g −1at 0.2C at room temperature)and the best rate performance among the four materials.©2011Elsevier B.V.All rights reserved.1.IntroductionLiFePO 4is an attractive cathode material for lithium-ion batteries because of its high theoretical capacity of 170mAh g −1,environ-mental benign,and high thermal stability.However,its poor electric conductivity of less than 10−13S cm −1limits its battery performance [1],such as the dramatic decrease in power at a high current density,which is the main drawback to commercial use.To overcome the low electric conductivity of LiFePO 4,many effective approaches have been introduced,including metal substitution [2–5],metal powder com-pounding [6],and conductive carbon coating [7–15].Among them,the preparation of LiFePO 4/carbon composite (LiFePO 4/C)is one of the attractive ways to improve the electric conductivity of the final product by forming a good conduction path.Furthermore,carbon can be also used as a reductant,which can reduce Fe 3+ions to Fe 2+ions.It should be noted that many studies involving the synthesis of nano-sized LiFePO 4employ Fe 2+salts as precursors [3,16–20],such as FeC 2O 4·2H 2O and (CH 3COO)2Fe,which are expensive.Therefore,it is necessary to use cheap materials and a convenient method.Here,we report the synthesis,characterization and electrochemical test of LiFePO 4/C composites prepared by two methods using home-made amorphous nano-FePO 4as the iron precursor and various organics as carbon precursors.The two methods using FePO 4as starting material are cheap and environmentally benign for the production of LiFePO 4material.Particularly,we present a novel method to synthesize a uniformcarbon film coated LiFePO 4cathode materials.This method involved an in situ reaction of resorcinol and formaldehyde on the surface of amorphous FePO 4.At room temperature,electrochemical tests showed that this material exhibited an initial discharge capacity of 138.4mAh g −1at 0.2C and a good cycling property at 0.5and 1.0C rate,respectively.2.Experimental2.1.Preparation of amorphous nano-FePO 4Amorphous nano-FePO 4was prepared by spontaneous precipita-tion from aqueous solutions.An equimolar solution of H 3PO 4was added to a solution of Fe(NO 3)3·9H 2O at 60°C under stirring and given amounts of PEG-400as surfactant.Then ammonia water (NH 3·H 2O)was slowly added to the mixed solution under vigorous stirring and a milk-white precipitate formed immediately.The pH of the solution was kept at 2.0.The precipitate was filtered and washed several times with distilled water.After drying in vacuum oven at 120°C for 12h,yellowish-white amorphous FePO 4was obtained.2.2.Preparation of LiFePO 4/CTwo methods were used to prepare the LiFePO 4/C composites in this study.2.2.1.Method oneA rheological phase method [21]was employed to synthesize LiFePO 4/C composite.Stoichiometric amount of amorphous FePO 4,LiOH·H 2O were used as the starting materials.The carbon precursorsPowder Technology 212(2011)327–331⁎Corresponding authors.Tel./fax:+863783881358.E-mail address:donguser@ (X.Wang).0032-5910/$–see front matter ©2011Elsevier B.V.All rights reserved.doi:10.1016/j.powtec.2011.06.005Contents lists available at ScienceDirectPowder Technologyj o u r n a l h o me p a g e :w w w.e l sev i e r.c o m /l oc a t e /pow t e care soluble starch(50.0g/1mol LiOH·H2O),sucrose(35.0g/1mol LiOH·H2O),citric acid monohydrate(21.0g/1mol LiOH·H2O),respec-tively.These carbon precursors were respectively solved in an appropri-ate amount of distilled water under stirring and heating.Then the amorphous FePO4and LiOH·H2O were added under vigorous stirring. Subsequently,the mixtures were respectively dried in an oven at120°C for6h,heated at350°C for1h in argonflow,treated at750°C for12h in argonflow,and ground.Finally,the LiFePO4/C composites were obtained and were denoted as sample A,sample B and sample C,respectively. 2.2.2.Method twoIn a typical synthesis,0.10g of CTAB was solved in30ml of distilled water solution under continuous stirring.Subsequently,1.52g FePO4·3H2O,0.055g resorcinol(R)and0.10ml formaldehyde(F)were successively added.When the temperature of water bath was up to85°C,LiOH·H2O was added.The mixture was kept stirred up in the dark for2h,dried in an oven at120°C for6h,heated at 350°C for1h in argonflow,treated at750°C for12h in argonflow, andfinally ground to obtain the LiFePO4/C composites(denoted as sample D).These four samples and their corresponding parameters are listed in Table1.The carbon contents of the samples were calculated by the loss on ignition of the four LiFePO4/C composites in air.2.3.CharacterizationThermogravimetric(TG)and differential thermal analysis(DTA) analyses were conducted with an EXSTAR6000thermal analysis system at a heating rate of10°C min−1.The powder X-ray diffraction (XRD,X'Pert Pro MPD,Philips)using Cu Ka radiation was employed to identify the crystalline phase of the prepared materials.Raman spectrum was recorded on a Renishaw RM-1000Microscopic Raman spectrometer with457.5nm excitation requiring a10mW power at room temperature.Low-magnification and high-magnification TEM images were taken on a JEM-2010transmission electron microscope (using an accelerating voltage of200kV).Electrodes were fabricated from a mixture of prepared carbon-coated LiFePO4powders(80wt.%),carbon black(12wt.%),and polyvinylidenefluoride in N-methylpyrrolidinon(8wt.%).The slurry was spread onto Al foil and dried in vacuum at120°C for12h.The carbon-coated LiFePO4loading was2mg cm−2in the experimental cells.The cells were assembled in an argon-atmosphere-filled glove box.The electrolyte was1M LiPF6in a mixture of ethylene carbonate (EC)and dimethyl carbonate(DMC)(1:1volume).The cells were galvanostatically charged and discharged at a voltage range of2.5–4.2V with LAND battery testing system at room temperature.Cyclic voltammograms were run on an IM6impedance and electrochemicalmeasurement system(Zahner,Germany)at a scan rate of0.1mV s−1 between2.5and4.0V.3.Results and discussionThe TEM images of the amorphous nano-FePO4were shown in Fig.1.The morphology of the as-prepared FePO4is an irregular particle with an average diameter of30nm.Most of the particles connected to each other because of their high surface energy which results from their small sizes.Fig.2a shows the TG/DTA curves of the FePO4·3H2O powder with a heating rate of10°C/min from room temperature to850°C in air.On the DTA curve near150°C,there is a very strong endothermic peak, associating with the sharp weight loss on the TG curve,which is related to the quick dehydration of FePO4·3H2O.During150–550°C, 26.3%weight loss on the TG curve indicates the slow elimination of residual H2O in FePO4·3H2O,exactly corresponding to the loss of crystalline water of FePO4·3H2O.And one exothermic peak is displayed at a higher temperature of590°C,which is not accompa-nied by appreciable weight loss in the TG curve,indicating the transformation of the amorphous FePO4to hexagonal FePO4crystal. The XRD patterns of FePO4·3H2O before and after calcination have been investigated in Fig.2b.As illustrated in pattern A,it can be seen that there is no evidence of diffraction peaks before calcination, indicating the synthesized FePO4·3H2O is just amorphous.While for the calcinated FePO4·3H2O at600°C for6h in air,it exhibits strong and narrow peaks revealing a well-crystallized material in pattern B. All of the diffraction peaks of the prepared FePO4are indexed to a single-phase hexagonal structure with a P3121space group and without any impurities,which is in good agreement with the standard card(JCPDS card no:72–2124).Table1Carbon precursors and residual carbon content of samples A,B,C and D.Samples A B C DCarbon precursor Starch Sucrose Citric acid RF polymer Final carbon content(wt.%) 5.48.5 4.35.1Fig.1.TEM images of the prepared amorphous nano-FePO4.n et al./Powder Technology212(2011)327–331The XRD diffraction patterns of LiFePO 4/C powders prepared with different carbon precursors were shown in Fig.3.All peaks can be indexed as a single phase with an ordered olivine structure indexed to the orthorhombic space group,Pnmb (JCPDS card no.83–2092).The obtained lattice parameters are sample A:a=10.2956Å,b=6.0367Å,and c =4.7001Å,sample B:a =10.1992Å,b =6.0483Å,andc=4.6971Å,sample C:a=10.2472Å,b=6.0208Å,and c=4.6882Åand sample D:a=10.3372Å,b=5.9993Å,and c=4.6932Å,respec-tively.There is no evidence of diffraction peaks for carbon,though some amorphous masses and films attached to the LiFePO 4particles were observed from TEM images (see Fig.4).This indicates the carbon contents are very low.Morphologies of these LiFePO 4/C composites were shown in Fig.4.It is obvious that the samples show different carbon distribution on LiFePO 4particle surface.From Fig.4a,c,e and g,we observed that the samples were composed of agglomerated particles whose sizes range from 50to 300nm.From Fig.4b and d,there is not enough carbon coating to spread throughout the substrate particles.In contrast to sample A and sample B,there are uniform carbon thin films on the grain surfaces of sample C and sample D,and the thickness of the carbon films are about 2.5nm (Fig.4f)and 4.5nm (Fig.4h),respectively.The reason may lie in that different carbon precursors have different adsorbabilities on the surface of FePO 4·3H 2O particles,resulting in different carbon distribution on the surface of LiFePO 4particle after the post treatment.Soluble starch and sucrose possess plentiful hydroxyl groups,by which soluble starch and sucrose molecules could probably weakly adsorb on the surface of FePO 4·3-H 2O particles in the hydrogen bonding.In the post treatment process,part of soluble starch and sucrose molecules desorbed from the surface of FePO 4·3H 2O particles,resulting in the de ficient carbon coating.But citric acid possesses carboxyl groups,which may be partially esteri fied by hydroxyl groups on the FePO 4·3H 2O particles,forming a tight connection.This results in more complete carbon coating after the post treatment.For sample D,we suppose that,in the present synthetic system,the surfactant CTAB may con fine the resorcinol –formaldehyde (RF)polymer molecules and FePO 4·3H 2O particles in plenty of tiny spaces,so the RF polymer molecules were tightly attached to FePO 4·3H 2O particles.After the post treatment,the RF polymer was transformed into the carbon film which tightly stuck on the surface of LiFePO 4particle.In addition,from the HRTEM image of sample D (shown in Fig.4h),the d-spacing of 0.294nm corresponds to the (211)plane of LiFePO 4.As an important aid investigating the structure of the carbon,the Raman measurement was adopted,and the results were shown in Fig.5.Every Raman spectrum consists of a small band at 940cm −1,which corresponds to the symmetric PO 4stretching vibration in LiFePO 4.The intense broad bands at 1350and 1590cm −1can be attributed to the characteristic Raman spectra of carbon.The bands at 1590cm −1mainly correspond to graphitized structured carbon of G band,while that at 1350cm −1corresponds to disordered structured carbon of D band [22,23].The graphitized carbon contains sp 2hybrid bonding,which is positively correlated with the electronic conduc-tivity of carbon,and the disordered carbon mainly corresponds to sp 3hybrid bonding.As shown in Fig.5,the integrated intensity ratios of sp 2/sp 3of the LiFePO 4/C composites synthesized with different carbon precursors are 0.865(curve A),0.857(curve B),0.856(curve C)and 0.860(curve D),respectively.So the similar sp 2/sp 3ratios of the four samples give us few clues to explain the difference in their electrochemical performances.Fig.6shows the cycling performance curves of all the samples at different rates.As shown in Fig.6,the initial discharge capacities of sample A,sample B,sample C and sample D at room temperature at 0.2C rate are 110.4,118.8,137.7and 138.4mAh g −1,respectively.The capacity of sample D gradually increases in the initial cycles.This may be due to the incomplete dispersion of the electrolyte into the electrode material at the beginning.Moreover,the capacity of sample D is highest among the four samples at 0.5C and 1.0C,indicating that method two is better than method one.The lower capacities of sample A and sample B must be due to the incomplete carbon coating on the LiFePO 4particles.The higher capacity of sample D than that of sample C may be attributed to the thicker carbon film of sample D keeping the crystal structure of LiFePO 4morestable.Fig.2.(a)TG/DTA curves of the FePO 4·3H 2O.(b)XRD patterns of the FePO 4samples before (A)and after (B)calcination inair.Fig. 3.XRD patterns of LiFePO 4/C composites synthesized with different carbon precursors.329n et al./Powder Technology 212(2011)327–331In order to further understand the electrochemical properties of the four samples,the cyclic voltammogram (CV)curves were performed at a scan rate of 0.1mV s −1at room temperature (as shown in Fig.7).Each of the CV curves consists of an oxidation peak and a reduction peak,corresponding to the charge reaction and discharge reaction of the Fe 2+/Fe 3+redox couple.In the CV pro files of the LiFePO 4cathode material,the smaller voltage difference between the charge and discharge plateaus and the higher peak current means better electrode reaction kinetics,and consequently better rate performance.Sample A and sample B electrodes have broad peaks in CV curves.In contrast,sample C and sample D electrodes demonstrate sharp redox peaks,which indicate an improvement in the kinetics of the lithium intercalation/de-intercalation at the electrode/electrolyte interface.The voltage difference of sample D is smaller than that of sample C,so sample D demonstrates a better rate performance.4.ConclusionsLiFePO 4/C composites were synthesized by two methods using home-made amorphous nano-FePO 4as the iron precursor and various organics as carbon precursors.It was found that employing soluble starch and sucrose as the carbon precursors resulted in a de ficient carbon coating on the surface of LiFePO 4particle,but employing citric acid and RF polymer as the carbon precursors realized a uniform carbon coating on the surface of LiFePO 4particle.Particularly,when RF polymer was used as the carbon precursor,the carbon film is thicker,and the material showed the highest initial discharge capacity (138.4mAh g −1at 0.2C at room temperature)and the best rate performance among the four materials.The intensities of redox peak and the voltage differences in the CV curves of the four samples are consistent with their rateperformance.Fig.4.TEM images of synthesized LiFePO 4/C composite synthesized with different carbon precursors.(a)and (b)sample A,(c)and (d)sample B,(e)and (f)sample C,(g)and (h)sampleD.Fig. 5.Raman shift of LiFePO 4/C composites synthesized with different carbonprecursors.Fig.6.The cycling performance curves of the samples with different carbon precursors at various discharge rates.n et al./Powder Technology 212(2011)327–331References[1] A.K.Padhi,K.S.Nanjundaswamy,J.B.Goodenough,Phospho-olivines as positive-electrode materials for rechargeable lithium batteries,J.Electrochem.Soc.144(1997)1188–1194.[2]T.Nakamura,Y.Miwa,M.Tabuchi,Y.Yamada,Structural and surfacemodi fications of LiFePO 4olivine particles and their electrochemical properties,J.Electrochem.Soc.153(2006)A1108–A1114.[3]S.-Y.Chung,J.T.Bloking,Y.-M.Chiang,Electronically conductive phospho-olivinesas lithium storage electrodes,Nat.Mater.1(2002)123–128.[4]P.S.Herle,B.Ellis,N.Coombs,L.F.Nazar,Nano-network electronic conduction iniron and nickel olivine phosphates,Nat.Mater.3(2004)147–152.[5]G.X.Wang,S.Bewlay,S.A.Needham,H.K.Liu,R.S.Liu,V.A.Drozd,J.-F.Lee,J.M.Chend,Synthesis and characterization of LiFePO 4and LiTi 0.01Fe 0.99PO 4cathode materials,J.Electrochem.Soc.153(2006)A25–A31.[6] F.Croce,A.D'Epifanio,J.Hasson,A.Duptula,T.Olczac,B.Scrosati,A novel conceptfor the synthesis of an improved LiFePO 4lithium battery cathode,Electrochem.Solid-State Lett.5(2002)A47–A50.[7]H.Huang,S.C.Yin,L.F.Nazar,Approaching theoretical capacity of LiFePO 4at roomtemperature at high rates,Electrochem.Solid-State Lett.4(2001)A170–A172.[8]M.Herstedt,M.Stjerndahl,A.Nyten,T.Gustafsson,H.Rensmo,H.Siegbahn,N.Ravert,M.Armand,J.O.Thomas,K.Edstroem,Surface chemistry of carbon-treated LiFePO 4particles for Li-ion battery cathodes studied by PES,Electrochem.Solid-State Lett.6(2003)A202–A206.[9]M.M.Doeff,Y.Hu,F.McLarnon,R.Kostecki,Effect of surface carbon structure onthe electrochemical performance of LiFePO 4,Electrochem.Solid-State Lett.6(2003)A207–A209.[10]Y.Hu,M.M.Doeff,R.Kostecki,R.Finones,Electrochemical performance of sol –gelsynthesized LiFePO 4in lithium batteries,J.Electrochem.Soc.151(2004)A1279–A1285.[11]X.Z.Liao,Z.Ma,L.Wang,X.Zhang,Y.Jiang,Y.He,A novel synthesis route forLiFePO 4/C cathode materials for lithium-ion batteries,Electrochem.Solid-State Lett.7(2004)A522–A525.[12] C.H.Mi,X.B.Zhao,G.S.Cao,J.P.Tu,In situ synthesis and properties of carbon-coated LiFePO 4as Li-ion battery cathodes,J.Electrochem.Soc.152(2005)A483–A487.[13]R.Dominko,M.Bele,M.Gaberscek,M.Remskar,D.Hanzel,S.Pejovnik,J.Jamnik,Impact of the carbon coating thickness on the electrochemical performance of LiFePO 4/C composites,J.Electrochem.Soc.152(2005)A607-A607.[14]K.Zaghib,J.Shim,A.Guer fi,P.Charest,K.A.Striebel,Effect of carbon source asadditives in LiFePO 4as positive electrode for lithium-ion batteries,Electrochem.Solid-State Lett.8(2005)A207–A210.[15]K.S.Park,J.T.Son,H.T.Chung,S.J.Kim,C.H.Lee,K.T.Kang,H.G.Kim,Surfacemodi fication by silver coating for improving electrochemical properties of LiFePO 4,Solid State Commun.129(2004)311–314.[16] A.Yamada,S.C.Chung,K.Hinokuma,Optimized LiFePO 4for lithium batterycathodes,J.Electrochem.Soc.148(2001)A224–A229.[17]N.Meethong,H.-Y.Shadow Huang,W.C.Carter,Y.-M.Chiang,Size-dependentlithium miscibility gap in nanoscale Li 1−x FePO 4,Electrochem.Solid-State Lett.10(2007)A134–A138.[18] C.Delacourt,P.Poizot,S.Levasseur,C.Masquelier,Size effects on carbon-freeLiFePO 4powders,Electrochem.Solid-State Lett.9(2006)A352–A355.[19] D.Choi,P.N.Kumta,Surfactant based sol –gel approach to nanostructured LiFePO 4for high rate Li-ion batteries,J.Power Sources 163(2007)1064–1069.[20]K.Zaghib,A.Mauger,F.Gendron,C.M.Julien,Surface effects on the physical andelectrochemical properties of thin LiFePO 4particles,Chem.Mater.20(2008)462–469.[21]Y.H.Huang,H.B.Ren,S.Y.Yin,Y.H.Wang,Z.H.Peng,Y.H.Zhou,Synthesis ofLiFePO 4/C composite with high-rate performance by starch sol assisted rheolog-ical phase method,J.Power Sources 195(2010)610–613.[22]M.M.Doeff,J.D.Wilcox,R.Kostecki,u,Optimization of carbon coatings onLiFePO 4,J.Power Sources 163(2006)180–184.[23]G.L.Yang,A.F.Jalbout,Y.Xu,H.Y.Yu,X.G.He,H.M.Xie,R.S.Wang,Effect ofpolyacenic semiconductors on the performance of olivine LiFePO 4,Electrochem.Solid-State Lett.11(2008)A125–A128.Fig.7.The CV pro files of the different samples at the scan rate of 0.1mV s −1.331n et al./Powder Technology 212(2011)327–331。
含稀土磷精矿湿法制磷酸过程稀土的浸出规律

含稀土磷精矿湿法制磷酸过程稀土的浸出规律梅吟;张泽强;张文胜;吴启海;吴健;池汝安【摘要】为查明二水法制磷酸过程稀土的走向规律,以贵州织金含稀土磷精矿为研究对象,在实验室模拟二水物湿法磷酸生产过程,研究了不同工艺条件下稀土的浸出规律.研究结果表明,在温度75 ℃,酸过量系数1.25,液固比41,反应时间4 h的条件下,稀土的浸出率最高为53.45%.当浸出率综合考虑磷和稀土的浸出率时,最佳的工艺条件为:温度75 ℃,酸过量系数1.25,液固比31,反应时间4 h的条件下,含稀土磷精矿中P2O5的浸出率为96.85%,稀土的浸出率为52.26%.%The production of (NH4)2SO4 from phosphogypsum and (NH4)2CO3 has been investigated.The factors affecting the conversion of phosphogypsum to (NH4)2SO4, such as (NH4)2CO3 to phosphogypsum ratio, reaction temperature, reaction time, stripping speed and liquid to solid ratio were studied. The optimum reaction conditions obtained at (NH4)2CO3 to phosphogypsum ratio 1.15,50℃, reaction time 120 min, stripping speed 150 r/min and liquid to solid ration 5: 1. The maximum conversion of phosphogypsum to (NH4)2SO4 obtained under these conditions was 98. 68%. A new crystallization method of (NH4)2SO4 was proposed, and the main factors affecting crystallization rate such as crystallization temperature and concentration of sulfate ion have been investigated. Results indicated that temperature and concentration of sulfate ion have obvious effects on crystallization rate of (NH4)2SO4. Suitable temperature was 25 ℃, and the higher the concentration of sulfate ion, the higher the crystallization rate of (NH4)2 SO4.【期刊名称】《武汉工程大学学报》【年(卷),期】2011(033)003【总页数】4页(P9-11,15)【关键词】含稀土磷精矿;稀土的浸出规律;浸出率【作者】梅吟;张泽强;张文胜;吴启海;吴健;池汝安【作者单位】武汉工程大学环境与城市建设学院,湖北,武汉,430074;武汉工程大学环境与城市建设学院,湖北,武汉,430074;贵州锦麟化工有限责任公司,贵州,贵阳,550005;贵州锦麟化工有限责任公司,贵州,贵阳,550005;贵州锦麟化工有限责任公司,贵州,贵阳,550005;武汉工程大学环境与城市建设学院,湖北,武汉,430074【正文语种】中文【中图分类】TD983;TD9540 引言自然界中部分磷矿床,尤其是氟磷灰石矿床伴生大量稀土[1-2].由于稀土离子与钙离子性质很相近,稀土主要以类质同象方式赋存于磷酸盐矿物中,因此分选富集磷矿时,稀土也富集到磷精矿中,具有很大的回收价值[3-4].磷矿主要用于制磷酸,从中提取伴生稀土,也在制酸过程中进行.其工艺方法可分为热法和湿法两类.因为热法存在成本高和能耗大等问题,有实用意义的还是在湿法磷酸生产过程中提取稀土[5].其中用硫酸分解磷矿的二水法工艺由于技术成熟、工艺简单、操作稳定,在湿法磷酸工艺中居主导地位,因此研究二水法制磷酸过程稀土的走向规律,对从中有效回收稀土具有一定的指导意义.1 试验磷精矿物质组成本实验研究对象为贵州织金含稀土磷精矿,表1为该磷精矿多元素化学分析结果,其中稀土钇、铈、镧和镨在稀土总量中的含量较高,分别占稀土总量的29.25%、23.77%、16.17%、12.19%;其次是钕、钆、钐和镝,含量分别占稀土总量的8.58%、2.36%、2.11%和1.32%;其它稀土元素的含量相对较低,分配率在1%以下.2 实验部分2.1 试验方法取磷精矿试验样品30 g,在不同条件下用硫酸分解磷精矿浸出磷和稀土,然后过滤固液分离,分析得到的浸出液及浸出渣中稀土及磷的走向,考查不同分解条件对磷精矿中磷和稀土浸出率的影响.表1 磷精矿多元素化学分析结果Table 1 Multielement chemical analysis of phosphate concentrates成分P2O5REOCaOSiO2MgOw/%33.560.1651.164.700.82成分Al2O3Fe2O3F酸不溶物烧失w/%0.881.144.065.754.822.2 分析方法P2O5的分析方法:磷钼酸铵容量法.方法原理:在硝酸溶液中,磷酸根与钼酸铵和柠檬酸反应生成磷钼酸铵黄色沉淀,过滤后,将沉淀溶解于碱标准溶液中,然后用盐酸标准溶液返滴过量的碱,即可求出P2O5含量.操作方法:称取P2O5 0.1 g,加80 mL沉淀剂加热搅拌至沸腾,然后冷却,将沉淀剂转移到漏斗中,用硝酸钾和蒸馏水轮流洗剂数次使滤液呈中性为止;将沉淀和脱脂棉移入原烧杯中,从滴定管中加入NaOH的浓度为0.25 mol/dm3的标准溶液,边加边搅拌,使沉淀完全溶解变成原来白色为止,然后加入酚酞呈紫色,再用浓度为0.1mol/dm3盐酸标准溶液滴至沉淀从紫色变为白色为终点.稀土的分析方法:稀土矿石化学分析方法.操作方法:称0.20 g左右样品于刚玉坩埚中,加入一定量的Na2O2,搅匀,再覆盖一层,置于已升温的高温炉中,熔融片刻,取下稍冷,置于加有100 mL水的烧杯中,在电炉上加热至微沸取下,用稀盐酸洗出坩埚,加水稀至200 mL,搅匀,澄清后过滤,用1% NaOH洗烧杯和沉淀7~8次,水洗1~2次,滤液弃去,用盐酸分次溶解沉淀于容量瓶中,定容到刻度.上机测量.测试仪器:全镨直读等离子体发射光谱仪,型号:Optima 4300DV;美国PerKinElmer 公司;主要技术指标:波长范围:165~782 nm;分辨率:<0.006 nm;重复性:优于1%;工作气体:冷却气(15 L/min)、雾化气(0.8 L/min).美国PerkinElmer公司的Optima 4300DV全谱直读型ICP-AES(双向观察).3 实验结果与分析3.1 硫酸用量对磷和稀土浸出率的影响硫酸分解磷精矿实际上是沉淀磷精矿中所有钙的反应过程.硫酸的理论用量可以根据磷精矿中的CaO的含量计算.计算出每1 kg磷精矿所需硫酸的理论用量QS为:以此理论硫酸用量做为参考,进行不同用量硫酸分解磷精矿的试验,得到浸出磷及浸出稀土总量的试验结果列于图1.试验过程固定条件为:液固比3∶1,浸出温度75 ℃,浸出时间4 h,洗涤水用量180 mL.图1 硫酸用量对稀土及磷浸出率影响试验结果Fig.1 The test results of dosage of sulfuric acid impact on sulphuric acid leaching rates of Re2O3 and P2O5 从图1可以看出,硫酸用量在1.25 kg/kg矿时,稀土的浸出率是最好的,浸出率为52.26%,而同时硫酸用量在1.46 kg/kg矿时,磷的浸出率最好为97.53%.由于硫酸用量在1.25 kg/kg矿时,磷的浸出率仍有96.85%.硫酸用量过小,磷和稀土的浸出率都较低;硫酸用量过大,矿浆粘度增大,对磷和稀土的浸出也不利,而且会造成硫酸的浪费.因此,浸出率综合考虑磷和稀土的浸出率,每1 kg磷精矿所需硫酸用量以1.25 kg为佳.3.2 液固比对磷和稀土浸出率的影响在确定硫酸用量的基础上,考查了液固比对磷和稀土浸出率的影响,得到浸出磷及稀土的试验结果列于图2.试验过程固定条件为:硫酸用量1.25 kg,浸出温度75 ℃,浸出时间4 h,洗涤水用量180 mL.图2 液固比对稀土及磷浸出率影响试验结果Fig.2 The test results of liquid-to-solid ratio impact on sulphuric acid leaching rates of Re2O3 and P2O5从图2可以看出,当液固比为4∶1时,稀土的浸出率最好,可达到53.45%,而磷的浸出率只有94.79%.而当液固比为3∶1时,稀土的浸出率为52.26%,相比53.45%下降不明显,而磷的浸出率可达到96.85%.当液固比过大或过小时,磷的浸出率都比3∶1效果要差,而稀土的浸出率相当,因此该试验应选用液固比3∶1的反应条件.3.3 浸出温度对磷和稀土浸出率的影响在确定硫酸用量的基础上,考查了浸出温度对磷和稀土浸出率的影响,所得试验结果见图3,试验过程固定条件为:硫酸用量1.25 kg,液固比3∶1,浸出时间4 h,洗涤水用量180 mL.图3 浸出温度对稀土及磷浸出率影响试验结果Fig.3 The test results of leaching temperature impact on sulphuric acid leaching rates of Re2O3 and P2O5 从图3可以看出,在反应温度为75 ℃时,稀土的浸出率最好,可达52.26%,磷的浸出率也可达96.85%,而在反应温度为90 ℃时磷的浸出率为97.58%,稍稍高于96.85%,但相差不明显,而稀土的浸出率仅45.51%.因此,在反应温度为75 ℃时,磷和稀土的浸出率相对来说是最高的,固反应温度应选择在75 ℃.3.4 浸出时间对磷和稀土浸出率的影响控制硫酸用量1.25 kg,液固比3∶1,浸出温度75 ℃,洗涤水用量180 mL,考查了浸出时间对磷和稀土浸出率的影响,试验结果见图4.图4 浸出时间对稀土及磷浸出率影响试验结果Fig.4 The test results of leaching time impact on sulphuric acid leaching rates of Re2O3 and P2O5由图4可以看出,当浸出时间为4 h时,磷和稀土的浸出率相对来说是最高的,分别为96.85%和52.26%,因此选择浸出反应时间应为4 h.综上所述,在二水物法萃取磷酸的最优浸出工艺条件即温度75 ℃,酸过量系数1.25,液固比4∶1,反应时间4 h的条件下,稀土的浸出率最好,但最优浸出率仅为53.45%,这说明大部分稀土元素损失在石膏中,对于该难题还需进一步研究.4 结语a. 采用贵州织金含稀土的磷精矿,在实验室研究了硫酸用量、液固比和浸出温度等不同工艺条件下稀土的浸出规律,由不同工艺条件下稀土浸出进入磷酸溶液和留磷石膏的规律可知:在温度75 ℃,酸过量系数1.25,液固比4∶1,反应时间4 h的条件下,稀土的浸出率最好,可达到53.45%.b. 当浸出率综合考虑磷和稀土的浸出率时,最佳的工艺条件为:温度75 ℃,酸过量系数1.25,液固比3∶1,反应时间4 h的条件下,含稀土磷精矿中P2O5的浸出率为96.85%,稀土的浸出率为52.26%.即可看出稀土的浸出规律与磷的浸出规律基本一致.参考文献:[1]Jorjani E, Bagherieh A H, Mesroghli Sh, et al. Prediction ofyttrium,lanthanum,cerium,and neodymium leaching recovery from apatite concentrate using artificial neural networks[J].Journal of University of Science and Technology Beijing,2008,15(4):367-374.[2]Preston J S, Cole P M, Craig W M, et al. The recovery of rare earth oxidesfrom a phosphoric acid by-product. Part 1 Leaching of rare earth values and recovery of a mixed rare earth oxide by solventextraction[J].Hydrometallurgy,1996,41:1-19.[3]Awadallah R M,Soltan M E,El Taher,et al.Concentration of lanthanide and actinides present in Sibaiya phosphate ores[J].Modelling, Measurement & Control,C: Energetics,Chemistry & ChemicalEngineering,Earth,Resources,Environment,BiomedicalProblems,2002,63(1):1-20.[4]施春华,胡瑞忠,王国芝.贵州织金磷矿岩稀土元素地球化学特征研究[J].矿物岩石,2004,24(4):71-75.[5]龙志奇,王良士,黄小卫,等.磷矿中微量稀土提取技术研究进展[J].稀有金属,2009,33(3):434-441.Abstract: In order to ascertain the distribution of rare earths in production of phosphate by dihydrate process, the main participants of this study is Zhijin phosphate concentrates bearing rare earths in Guizhou Province. The production process of dihydrate wet-process phosphoric acid in the laboratory is stimulated and the leaching rules of rare earth are investigated under different conditions. The test results show that when the leachin g temperature is 75 ℃, the excess coefficient of sulfuric acidis 1.25, liquid to solid ratio 3∶1, time consumption is 4 hours, the highest leaching rates of Re2O3 is 53.45%. Under the optimum conditions the leaching temperature is 75 ℃, the excess coeffici ent of sulfuric acid 1.25, liquid-to-solid ratio 3∶1 and time consumption 4 hours. The leaching rates of P2O5 and Re2O3 are 96.85% and 52.26%, respectively.Key words: phosphate concentrates bearing rare earths; leaching rule of rare earth; leaching rates。
电感耦合等离子体发射光谱法测定磷矿石中常量元素硅磷硫钙镁铝铁钛锰

2012年6月June2012岩 矿 测 试ROCKANDMINERALANALYSISVol.31,No.3446~449收稿日期:2011-12-29;接受日期:2012-03-01作者简介:郭振华,高级工程师,主要从事等离子体发射光谱和等离子体质谱分析测试工作。
E mail:gzh_415@yahoo.com.cn。
文章编号:02545357(2012)03044604电感耦合等离子体发射光谱法测定磷矿石中常量元素硅磷硫钙镁铝铁钛锰郭振华(中化地质矿山总局地质研究院分析测试中心,河北涿州 072750)摘要:以粒状氢氧化钠作熔剂,样品在银坩锅中700℃熔融,热水浸取后用盐酸酸化,溶液冷却定容后直接用电感耦合等离子体发射法测定磷矿石中硅、磷、硫、钙、镁、铝、铁、钛、锰9种常量元素,采用基体匹配和离峰扣背景等方式有效消除了测定的干扰。
对样品分解条件、试剂用量、元素分析谱线的选择、干扰的影响及消除等进行了优化处理和讨论。
方法精密度(RSD)为0.28%~1.85%(n=12),回收率为97.92%~107.1%,具有快捷、成本低等特点。
经国家一级标准物质分析验证,结果满意,适用于磷矿石中常量元素的快速分析。
关键词:磷矿石;常量元素;氢氧化钠熔融;电感耦合等离子体发射光谱法中图分类号:P578.92;O657.31文献标识码:BDeterminationofMajorComponentsinPhosphateOresbyInductivelyCoupledPlasma AtomicEmissionSpectrometryGUOZhen hua(GeologicalInstituteofBureauofGeologyandMiningofChinaChemicalIndustry,Zhuozhou 072750,China)Abstract:ThephosphateoresampleswerefusedbygrainyNaOHinasilvercrucibleat700℃,andthendissolvedwithHClacidsolution.AmethodforthedeterminationofSi,P,S,Ca,Mg,Al,Fe,TiandMninphosphateoresbyInductivelyCoupledPlasma AtomicEmissionSpectrometry(ICP AES)wasthenestablished.Theinterferenceswereeliminatedbyusingmatchedmatrixandoffpeakbackgroundcorrection.Thesampledigestionconditionsandreagentusagewereoptimized.Theselectionofanalysisspectrallines,theeffectsandeliminationofinterferencesandinstrumentparametersarepresentedinthispaper.Thismethodprovidestheadvantagesofhighrecoveriesof97.92%-107.1%withrelativestandarddeviations(RSD,n=12)of0.28%-1.85%andisrapidandinexpensive.Thepresentedmethodhasbeenappliedtotheanalysisofmajorelementsinnationalstandardreferenceofphosphateoresandtheresultsareinagreementwithcertifiedvalues.Keywords:phosphateores;majorelements;sodiunhydroxidefused;InductivelyCoupledPlasma AtomicEmissionSpectrometry磷矿石是重要的化工矿产之一,由磷矿石制取的磷肥是农业生产所必需的营养物质,随着近年来磷矿资源的大量探查与开发利用[1-5],给分析工作者提出了更高的要求,即如何提供准确、快捷、污染少的分析方法。
X射线荧光光谱法测定透辉石中氧化钙、氧化镁和二氧化硅

ZHAO Wei,XIA ChuanGbo,JIANG Yun,etal.Determinationofcalciumoxide,magnesiumoxideandsilicondioxidein diopsidebyXGrayfluorescencespectrometry.MetallurgicalAnalysis,2018,38(3):29G34
冶 金 分 析 ,2018,38(3):29G34 MetallurgicalAnalysis,2018,38(3):29G34
DOI:10.13228/j.boyuani.ssn1000G7571.010221
X 射线荧光光谱法测定透辉石中氧化钙、 氧化镁和二氧化硅
赵 伟1,2,夏传波 ∗1,2,姜 云1,2,王 卿1,2,张会堂1,2
收 稿 日 期 :2017G07G13 基 金 项 目 :国 土 资 源 公 益 性 行 业 科 研 专 项 (201311084) 作 者 简 介 :赵 伟 (1982— ),女 ,高 级 工 程 师 ,主 要 从 事 岩 矿 分 析 及 标 准 物 质 研 制 等 工 作 ;EGmail:workzhaowei@163.com ∗ 通讯联系人:夏传波(1981—),男,工程师,主要从事岩矿分析工作;EGmail:chuanbo007@126.com
文 献 标 志 码 :A 文 章 编 号 :1000G7571(2018)03G0029G06
透辉石是一种含钙镁的链状结构硅酸盐矿物, 化学式 为 CaMgSi2O6. 透 辉 石 能 实 现 陶 瓷 的 低 温 快 烧 ,降 低 能 耗 ,提 高 陶 瓷 的 性 能 ,广 泛 应 用 于 电 瓷 、 建筑陶瓷和日用陶 瓷 工 业,是 一 种 优 良 的 新 型 节 能 添加剂和陶瓷原料 . [1G3] 我国透辉石资源储 量 大、分 布广,对其进行 开 发 和 利 用 具 有 重 要 意 义. 天 然 透 辉石矿中常存在 石 英、方 解 石、硅 灰 石、磷 灰 石 等 共 生 矿 物 ,这 对 其 独 特 的 熔 剂 性 能 、热 膨 胀 特 性 和 工 艺 性 能 都 有 重 要 的 影 响 ,因 此 ,快 速 准 确 地 测 定 透 辉 石 中的 CaO、MgO 和 SiO2 等 主 量 成 分,可 为 透 辉 石 的 地 质 勘 查 、质 量 评 价 和 开 发 利 用 提 供 科 学 依 据 . 目 前,透 辉 石 中 CaO、MgO、SiO2 等 主 量 成 分 的测定主要参照一般硅酸盐岩石,采用 EDTA 滴定 法 、动 物 胶 凝 聚 重 量 法 等 化 学 分 析 法 ,一 般 每 项 成 分 需要单独测定,手续繁琐、分析周期长.X 射线荧光 光谱法(XRF)具 有 多 元 素 同 时 测 定、制 样 简 单、绿
《沈阳化工大学学报》2020年总目次

沈阳化工大学学报JOURNAL OF SHENYANG UNIVERSITY OF CHEMICAL TECHNOLOGY第34卷第4期2020.12Vol. 34 No. 4Dec. 20202020年总目次-化学与化学工程-CUO-WO 3纳米立方块的合成及气体传感特性研究司建朋,王明月,孟高耐碱表面活性剂的开发及在工业清洗中的应用张冬喜,李新钰,石磊,王Co/g - C 3N 4- CHIT/GCE 修饰电极的制备及其对H 2PO 4-的测定陈异构十三醇聚氧乙烯醚磷酸酯的合成及性能研究十六烷值改进剂的制备与性能研究离子液体分离乙酸甲酯-甲醇共沸物系的模拟研究离子液体-环己烷(乙醇)二元体系气液相平衡研究萃取精馏分离苯-甲醇共沸体系的模拟碳纳米管对 C u O - ZnO - Ga 2 O 3/HZSM - 5催化剂性能的影响低品位菱镁矿浮选剂实验研究均三乙苯的合成研究甲基丙烯酸混合醇酯-苯乙烯-醋酸乙烯酯三元聚合物的合成与降凝性能研究车用水蜡的研究新型银制品洗涤剂的研制间氨基乙酰苯胺的合成及分离研究岩,思,李文秀,王英文,丹,刘冬雨,赵 嘉,李玉娇,江寒峰1 (1)张志刚,郭禹含,李晓茜,许光文2 (97)刘坤,于丹舟,杨旺,姚慧2 (107)-魏田,张芮,王瑞灵,陈永杰 2 (115)宋明龙,龙小柱 2 (120)李继鹏,张羽,张志刚,张弢3 (193)-李宏辉,李文秀,张志刚,张弢3(198)-尹海鹰,李文秀,张志刚,张弢3 (205)王 开,于欣瑞,刘 楠,张雅静3 (210)康坤红,龙小柱3 (216)-马婉莹,张风雨,丁茯,王东平 4 (289)-徐妍,龙小柱,靳璐璐,于海洋4 (295)-高鹏飞,龙小柱,靳璐璐,高碌4 (301)-卢羲亚,于媛,韩英男,龙小柱 4 (306)-王瑞灵,陈永杰,曹爽,张芮4 (310)高效液相色谱法同时测定邻位香兰素、香兰素、甲基香兰素和乙基香兰素贾璇,王国胜4 (314)Pd/N 3 - SiO 2催化剂制备及其催化乙烘气相加氢性能研究王梦娇,王康军,李东楠4(319)2沈阳化工大学学报2020年-生物与环境工程-积雪草酸A环衍生物的合成及其抗肿瘤活性研究.........................李孝孝,佟贺,熊果酸衍生物的合成及体外抗肿瘤活性研究.......................................徐川东,N-金刚烷基-N,-芳杂基二酰肼类化合物的合成..............刘丹,关月月,张淑曼,齐墩果酸A环衍生物的合成与体外抗肿瘤活性研究...............................王强,模板剂对MnO”催化剂微观形貌的调控及其催化氧化甲苯性能.......................................项文杰,刘威,赵恒,齐墩果酸衍生物的合成及其与MEK靶点分子对接研究.............................张蓬勃,齐墩果酸硫脲类衍生物的合成及以VEGFR-2为靶点的分子对接研究........................................................李杰,2-(漠甲基)-3-取代丙烯酸酯的合成及生物活性研究.............................廖桥,WBS-RBS和AHP的方法在化工园区安全容量评价的应用.........................孟宇强,-材料科学与工程-以三(二乙胺基)环硼氮烷为前驱体制备六方氮化硼李宗鹏,王长松,石墨烯/二氧化锰复合材料的制备及其电化学性能的研究李静梅,不同分散剂对天然橡胶性能的影响孟唯,刘浩,武文斌,张舒雅,肉豆蔻酸/棕榈醇共晶物作为相变材料的热性能研究李蛟龙,任子真,Ni2P/Cu3P复合纳米材料的制备、表征及电催化性能研究鲍彤,祁佳音,赵国庆,g-C3N4/CeVO4/Ag纳米复合材料的制备及光催化性能的研究钱坤,邱永堃,高雨,丁茯,孙亚光,两相闭式热虹吸管的强化传热新能源集成厨用加热系统结构形式对挡板岀口截面流体力学性能的影响多孔板旋流静态混合器强化传热性能分析基于声发射技术的减速顶故障诊断三聚磷酸钠对镁合金阳极氧化膜性能的影响•机械工程•蔡长庸,'战洪仁,史胜,张倩倩,惠尧,惠尧,陈彤,翟雪发,战洪仁,张海春,周圆圆,龚斌,吴剑华,龚斌,刘海良,王巍,周圆圆,金志浩,迟展,孟艳秋1(9)孟艳秋1(18)王然1(22)孟艳秋2(125)张学军3(222)宋艳玲3(230)宋艳玲4(324)杨桂秋4(330)宫博4(334)梁兵1(25)张辉1(31)王重2(130)李贵强3(236)郭卓4(338)徐振和4(345)王立鹏1(41)曾祥福1(47)张静2(135)张静2(142)于宝刚2(147)付广艳,姜天琪,钱神华2(153)第4期《沈阳化工大学学报》2020年总目次3稳流器结构对消防直流水枪水力学性能的影响风载荷作用下倾斜塔板压降的数值模拟...... Mg-xZn合金的制备及腐蚀性能研究..........带有内螺纹的重力热管仿真模拟研究........带有开槽中性捏合块和反向螺纹双螺杆挤岀机的三维流场分析.........................张静,陈生国,张平,张丽,张平,王豪,付广艳,钱神华,许文兰,战洪仁,张倩倩,史胜,王立鹏,郭树国,于淼,王丽艳,汤霖森,陈科昊,网格类型对管内旋流特性数值计算的影响•信息与计算机工程-BP神经网络算法在“摇头”避障小车中的应用.....................................任帅男,基于GPRS DTU远程通讯技术在油气集输管线上的应用..................赵思渊,何戡,基于通信节点的WSN自主聚类非均匀分簇路由协议......................刘一珏,王军,基于冗余节点间歇性的WSN路由协议的设计..................马德朋,王军,田鹍,基于Python爬虫的电影数据可视化分析.................................高巍,孙盼盼,基于STM32的CAN总线数据采集卡设计..........................................李蛟龙,基于物联网的雾化降尘效果优化研究...................................安然然,路晨贺,基于SPA-SVDD方法对间歇过程的故障检测...........................谢彦红,薛志强,基于Labview的三容水箱液位控制系统设计.............................李凌,曹纪中,基于数据分片的WSN安全数据融合方案优化..................王军,陈羽,田鹍,基于加权优化树的WSN分簇路由算法............................................刘一珏,筛分车间矿料仓除尘优化策略.................................安然然,路晨贺,高文文,多路光功率监测系统的设计......................................................高淑芝,餐饮业液化气罐物联网智能管理系统...................................汪滢,于洋,布袋除尘器耗损件生命周期监控策略...........................路晨贺,安然然,孙晓鑫,仿海底洋流实验中水流动状况智能监控系统..........王金亮,安然然,路晨贺,孙晓鑫,基于潜隐变量自相关性子空间划分的故障检测策略......................张成,郭青秀,无混载校车路线分析模型优化实现方法.................................高巍,陈泽颖,-数理科学•非定常对流占优扩散方程的龙格库塔伽辽金有限元方法.............................冯立伟,龚斌3(239)秦然3(245)姜天琪3(250)惠尧4(352)韩彦林4(358)王宗勇4(363)王庆辉1(51)宗学军1(56)田鹍1(60)徐万一1(67)李大舟1(73)任子真1(79)张蔓蔓1(85)李元2(158)王璐2(165)赵子君2(171)王军2(178)张蔓蔓2(187)徐林涛3(255)张延华3(261)张语仙3(268)张语仙3(275)李元4(369)李大舟4(377)席伟1(91)外磁场下的双层类石墨烯系统的元激发能谱赵宇星,成泰民3(282)4沈阳化工大学学报2020年Comprehensive Table of Contents2020・Chemistry and Chemical Engineering・Synthesis and Gas Sensing Properties of CuO-WO3Nanocubes SI Jian-peng,et al1(i) Development of High Alkali-Resistant Surfactant and ItsApplication in Industrial Cleaning ZHANG Dong-xi,et al2(97) Preparation of Co/g-C;N4-CHIT/GCE Modified Electrode andDetermination of Dihydrogen Phosphate CHEN Si,et al2(i07) Study on the Synthesis and Properties of the Phosphate Ester ofIso-Tridecanol Polyoxyethylene WEI Tian,et al2(115) Preparation and Properties of Cetane Number Improver SONG Ming-long,et al2(120) Simulation Study on Separation of Methyl Acetate-MethanolAzeotrope System by Ionic Liquid LI Wen-xiu,et al3(193) Vapor-Liquid Equilibrium of Ionic Liquids with Cyclohexane orEthanol Binary System LI Hong-hui,et al3(198) Simulation of Azeotrope Separation of Benzene-Methanol byExtractive Distillation YIN Hai-ying,et al3(205) Effect of Carbon Nanotubes on the Performance ofCuO-ZnO-Ga2O3/HZSM-5Catalysts WANG Ying-wen,et al3(210) Experimental Study on Flotation Agentfor the Low Grade Magnesite KANG Kun-hong,et al3(216) The Synthesis of1,3,5-Triethylbenzene MA Wan-ying,et al4(289) Study on Synthesis and Pour Point Depressing Performance of Methyl AcrylicAcid Mixed Alcohol Ester-Styrene-Vinyl Acetate Terpolymer XU Yan,et al4(295) Study on Vehicle Water Wax GAO Peng-fei,et al4(301) Development of New Detergent for Silver Products LU Xi-ya,et al4(306) Synthesis and Separation of m-Acetamidoaniline WANG Rui-ling,et al4(310) Simultaneous Determination of o-Vanillin,Methyl Vanillin,Ethyl Vanillin andVanillin by High Performance Liquid Chromatography JIA Xuan,et al4(314) Synthesis of Pd/N s-SiO?Catalyst and its Catalytic Performance forAcetylene Hydrogenation to Ethylene WANG Meng-jiao,et al4(319)・Biological and Environmental Engineering・Synthesis and Antitumor Activity of A-Ring Derivatives of Asiatic Acid LI Xiao-xiao,et al1(9) Synthesis and Antitumor Activity in Vitro of Ursolic Acid Derivatives XU Chuan-dong,et al1(18) Synthesis of N-adamantyl-N'-arylheterodihydrazides LIU Dan,et al1(22) Synthesis and Anti-Tumor Activity of Oleanolic AcidA Ring Derivatives in Vitro WANG Qiang,et al2(125) Tunable Synthesis of Morphologies of MnO^Catalyst by Template andIts Catalytic Oxidation Performance for Toluene XIANG Wen-jie,et al3(222)第4期《沈阳化工大学学报》2020年总目次5Synthesis of Oleanolic Acid Derivatives and MolecularDocking Studies with MEK.............................................................................................ZHANG Peng-bo,et al3(230) Synthesis of Oleanolic Acid Thiourea Derivatives and MolecularDocking Study with VEGFR-2Kinase.............................................................................................LI Jie,et al4(324) Synthesis and Biological Activities of2-(bromomethyl)-3-substituted Acrylate.......................................................................................................................LIAO Qiao,et al4(330) Application of WBS-RBS and AHP in Safety Capacity Analysis ofChemical Industrial Park.................................................................................................MENG Y u-qiang,et al4(334)・Material Science and Engineering・Synthesis of the Hexagonal Boron Nitride Using Tris(diethylamino)borazine as Precursor...................................................................................................................LI Zong-peng,et al1(25) Preparation and Electrochemical Properties of Graphene/ManganeseDioxide Composites.......................................................................................................................LI Jing-mei,et al1(31) Effect of Different Dispersants on the Properties of Natural Rubber..............................................MENG Wei,et al2(130) Thermal Properties of Myristic Acid/1-hexadecanol EutecticMixture as Phase Change Material.........................................................................................LI Jiao-long,et al3(236) Hydrothermal Synthesis,Characterization and Electrocatalytic HydrogenEvolution of Nif/Cuf Nanomaterials.........................................................................................BAO Tong,et al4(338) Preparation of Photocatalytic Properties g-C3N4/CeVO q/Ag Nanocomposites........................QIAN Kun,et al4(345)・Mechanical Engineering・The Enhancement of Heat Transfer in Two-Phase Closed Thermosyphon....................ZHAN Hong-ren,et al1(41) New Energy Integrated Kitchen Heating System...................................................................CAI Chang-yong,et al1(47) Effect of the Baffle Structure on Hydrodynamic Performanceat the Outlet Section ZHANG Hai-chun,et al2(135) Analysis on Enhanced Heat Transfer Performance of Cyclone StaticMixer with the Porous PlateFault Diagnosis of Retarder in Railway Stations Based on Acoustic Emission TechnologyInfluence of Sodium Tripolyphosphate on the Properties of Anodizing Films of Magnesium AlloyEffect of the Stabilizer Structure on the Hydraulic Characteristics in the Fire Water GunGONG Bin,et al2(142) JIN Zhi-hao,et al2(147) FU Guang-yan,et al2(153) ZHANG Jing,et al3(239)Numerical Simulation of Pressure Drop of ObliqueTray under Wind Load ZHANG Ping,et al3(245)Preparation and Corrosion Properties of Mg-xZn Alloys.......... Numerical Simulation of Gravity Heat Pipe with Internal Threads Three Dimensional Flow Field Analysis of Twin Screw Extruder with Slotted Neutral Kneading Block and Reverse Thread.................■-FU Guang-yan,et al3(250) ZHAN Hong-ren,et al4(352)GUO Shu-guo,et al4(358)6沈阳化工大学学报2020年Influence of Grid Type on Numerical Calculation of SwirlCharacteristics in Tubes......................................................................................................CHEN Ke-hao,et al4(363)・I information and Computer Engineering・Application of BP Neural Network Algorithm in“Shaking Head”Vehicle forObstacle Avoidance..................................................................................................................REN Shuai-nan,et al1(51) The Application of GPRS DTU Remote Communication Technology inOil and Gas Gathering Pipeline.............................................................................................ZHAO Si-yuan,et al1(56) WSN Autonomous Cluster Heterogeneous Clustering Routing ProtocolBased on Communication Nodes.................................................................................................LIU Yi-jue,et al1(60) Design of WSN Routing Protocol Based on Redundancy Node Intermittent.............................MA De-peng,et al1(67) Visual Analysis of Film Data Based on Python Crawler...................................................................GAO Wei,et al1(73) Design of CAN Bus Data Acquisition Card Based on STM32..................................................LI Jiao-long,et al1(79) Study on Optimization of Atomization and Dust Reduction EffectBased on Internet of Things..........................................................................................................AN Ran-ran,et al1(85) Fault Detection Based on SPA-SVDD in Batch Process......................................................XIE Yan-hong,et al2(158) Design of Three Tank Level Control System Based on Labview...........................................................LI Ling,et al2(165) Optimization of WSN Secure Data Aggregation SchemeBased on Data Slice...................................................................................................................WANG Jun,et al2(171) A WSN Cluster Routing Algorithm Based on theOptimized-Weighting Tree......................................................................................................LIU Yi-jue,et al2(178) Optimization Strategy for Dust Removal of Mine MaterialWarehouse in Sieve Workshop.................................................................................................AN Ran-ran,et al2(187) Design of Multi-Channel Optical Power Monitoring System..................................................GAO Shu-zhi,et al3(255) The Internet of Things Intelligent Management System ofCatering Industry Liquefied Gas Tank.....................................................................................WANG Ying,et al3(261) Life Cycle Monitoring Strategy for Bag Filter Wearer...............................................................LU Chen-he,et al3(268) Intelligent Monitoring Scheme for Water Flow inImitation Ocean Current Experiment................................................................................WANG Jin-liang,et al3(275) Fault Detection Strategy Based on Dividing Autocorrelation ofLatent Variables.......................................................................................................................ZHANG Cheng,et al4(369) Optimization Implementation Method of No-Mixed SchoolBus Route Analysis Model..............................................................................................................GAO Wei,et al4(377)・Science of Mathematics and Physics・Rung-Kutta Galerkin FEM Method for Unsteady ConvectionDominated Diffusion Equation.................................................................................................FENG Li-wei,et al1(91) Elementary Excitation Energy Spectra of Double-Layer Graphene-LikeSystem Under External Magnetic Field ZHAO Yu-xing,et al3(282)。
Activities of SiO2 in Some CaO–Al2O3–SiO2(–10%MgO) Melts with Low SiO2 Contents at 1873 K

ISIJ International, Vol. 47 (2007), No. 6, pp. 805–810805©2007ISIJFig.1.Experimental apparatus.Table2.Analyzed compositions of the melts and SiO ©2007ISIJ806experiments. However, SiO 2contents slightly increased in the low SiO 2containing samples. Table 2 also presents the measured activities of SiO 2in the CaO–Al 2O 3–SiO 2Based on the obtained values, iso-SiO 2activity curves were constructed in the iso-thermal section of the 2O 3–SiO 2system at 1873K. The contour lines are presented in Fig. 2. The iso-activity curves estimated by Rein and Chipman 5)are also reproduced in the figure for comparison. The present activity values of SiO 2are higher than the suggestion by Rein and Chipman,5)in spite of the similarity of the shape. M oreover, the SiO 2activity ob-tained in the present study decreases more drastically with the increase of CaO content in comparison with the results by Rein and Chipman. Note also that significant decrease in 2activity can be seen, as the composition ap-proaches CaO-saturated line.It should be mentioned that Rein and Chipman was not successful to determine the activity of SiO 2at low SiO gion experimentally. Hence, a hypothetical point, at which CaO and 2CaO ·SiO 2are doubly saturated at 1(marked as a solid circle in Fig. 2), was considered by these 5)Using the Gibbs free energy change for the de-composition of the dicalcium silicate into CaO and SiO the activity of SiO 2at the coexisting point of CaO and SiO 2was estimated as 7.7ϫ10Ϫ4at 1873K. By ex-trapolating from the measurement result at high SiO tent region through the hypothetical point, the authors de-rived the activity of SiO 2in the rest of the low SiO 2region. However, the estimated value does not agree well807©2007ISIJFig.2.Iso-a lines in the CaO–Al O –SiO system at 1873K, Solid lines: Present work, dotted lines: Rein and Chipman.5)Fig.3.Dependence of a SiO 2on mass%CaO/mass%SiOprofound. To examine the effect of MgO on the activity of SiO 2, the activity coefficients of SiO 2is plotted against the ratio of mass%CaO/mass%Al 2O 3at constant SiO 2content for both of the ternary and quaternary systems in Fig. monly in the 2 systems, the activity coefficient of SiO 2decreases with the increase of the CaO content. It is also apparent that the activity coefficient of SiO 2in the quater-nary system with 10mass% MgO is much lower than that of the ternary system. Only 10mass% M gO would lower the activity coefficient of SiO 2significantly by modifying SiO 2network structure.Iso-activity contours of SiO 2in the CaO–Al 2O 3–SiO 2–10mass%M gO system at 1873K were drawn in the iso-thermal section in Fig. 5. The figure shows again that the activity of SiO 2decreases as CaO content increases and Al 2O 3content decrease. 3.3.Liquid Oxide Inclusions at the End of Ladle TreatmentIn previous studies,1,2,12)it was found that a large number of liquid inclusions of calcium aluminate coexist with spinel inclusions before vacuum degassing. After vacuum degassing, most of the inclusions were calcium aluminate liquid inclusions.1,2,12)An example of these inclusions is presented in Fig. 6. When dissolved Al level is low, 2 types of liquid calcium aluminate inclusions with considerably different SiO 2contents were found to coexist even after vacuum degassing.2)Since stirring is carried out during vacuum degassing for about half an hour, the inclusions have a good chance to reach or at least get close to the equi-librium with liquid steel. To understand the coexistence of the two types of liquid inclusions with different SiO 2con-808©2007ISIJFig.5.Iso-a SiO 2lines in the CaO–Al 2O 3–SiO 2–10%MgO system at 1873K.Table 3.Analyzed compositions of the melts and SiO 2activities in the CaO–Al 2O 3–SiO 2–MgO system.Fig.4.Dependence of gSiO 2of the ternary and quaternary meltson mass%CaO/mass%Al 2O 3.Fig.6.Microphotograph of a typical inclusion after vacuum de-gassing.2)tents at the end of the ladle treatment a thermodynamic consideration using the present activity data would be help-ful. To assist the discussion, the composition range of the liquid inclusions observed after vacuum degassing is pre-sented in Table 4.2)The typical steel composition after vac-uum degassing is presented in Table 5.2)The activity of SiO 2in equilibrium with the liquid steel can be calculated using the following reaction.SiO 2(s)ϭSi ϩ2O (8) (9) (10)are the activities of silicon and oxygen in liquid steel relative to a dilute solution; a SiO 2is the activity with pure solid as the standard state; [%i ] and f i are the concentration of element i and its activity coefficient on a weight percent basis with dilute solution as its reference is the first order interaction parameter of j on i .The interaction parameters employed in this study are The decrease in the dissolved oxygen level during de-gassing period may have some influence on the equilibrium between the inclusions and liquid metal. On the basis of the steel composition in Table 5 and the interaction parameter in Table 6, the activities of the dissolved Si and O after vac-for the coexistence of the two types of inclusions could be the difference in their CaO contents. The inclusions having higher SiO 2content have considerably higher CaO content.The difference between the measured activities in the last two rows in Table 3 and the value calculated from a Si and a O in the steel could be due to either or both of the follow-ing two factors. (i) The EDX analyses to determine the compositions of the inclusions 2)were possibly associated with uncertainties. As shown in the last two rows in Table 3, slight change in the composition would results consider-able difference in the a SiO 2. (ii) A final equilibrium between the inclusion with steel has not been reached. Nevertheless,the present results would suggest that the SiO the 2 types of inclusions are rather close to each other. Both types of inclusions are very close to the equilibrium with the steel after vacuum treatment.4.SummaryIn order to consider the equilibrium between non-metal-lic inclusions and liquid steel, the activities of SiO CaO–Al 2O 3–SiO 2(–MgO) liquid solutions having low SiO contents were measured. The solution was equilibrated with Cu at known oxygen potential at 1activities were evaluated using Si content in Cu and oxygen partial pressure. The present measured values were higher than the previously reported data for the CaO–Al system. Addition of 10mass% M the SiO 2activity substantially. On the basis of the experi-mental data, iso-activity contours of SiO a f i f e j i i i i j jϭϭ[%]log [%]∑log log.K a a a T8304141159ϭϭϪϩSi O2SiO 2⋅809©2007ISIJTable 6.Interaction parameters at 1873K used in this study.ASM, Metal Park, (1973), 793.9) E. T. Turkdogan: Physical Chemistry of High Temperature Technol-ogy, Academic Press, New Y ork, (1980), 7.10)O. Kubaschewski and C. B. Alcock: Metallurgical Thermochemistry,5th ed., Pergamon Press, Oxford, (1979), 382.11)Slag Atlas, 2nd ed., Verlag Stahleisen, Düsseldorf ,Germany, (1995),105.12)K. Beskow, Du Sichen and N. Sano: Iron Steel Technol., 3(2006),103.13)G. K. Sigworth and J. F. EIliott: Met. Sci., 8(1974), 298.14)The 19th Committee on Steelmaking: Steelmaking Data Source-book, the Japan Society for the Promotion of Science, Gordon andBreach Science Publishers, New Y ork, (1988), 280/282.810©2007ISIJ。
生物活性_硅酸二钙的喷雾干燥_微波快速烧结制备与性能表征_英文_

JIANG Zhen1,LI Weifeng1,QIAN Duanfen1,WAN Long1,LIU Xuanyong1,2 (1. College of Materials Science and Engineering, Hunan University, Changsha 410082; 2. Shang7 卷第 9 期
蒋震 等:生物活性 β-硅酸二钙的喷雾干燥–微波快速烧结制备与性能表征
· 1537 ·
adhesion and spreading. Dicalcium silicate is one of the most important com-
ponents in portland cement clinker. It has five normal polymorphs which are α, α′H, α′L, β, and γ. Compared with the other four normal polymorphs, β-Ca2SiO4 is more likely an excellent candidate for tissue engineering material because it is a hydraulic cement that will set and develop strength in water environments. However, β-Ca2· SiO4 is a metastable phase at ambient temperature, and easily transforms into stable γ- Ca2SiO4.[15–19] In conventional methods, it is necessary to prepare β-Ca2SiO4 ceramics at high sintering temperature (1 300–1 400 ℃) and for a long time (2–3 h).
CaO-SiO2二元系晶体和玻璃的拉曼光谱研究
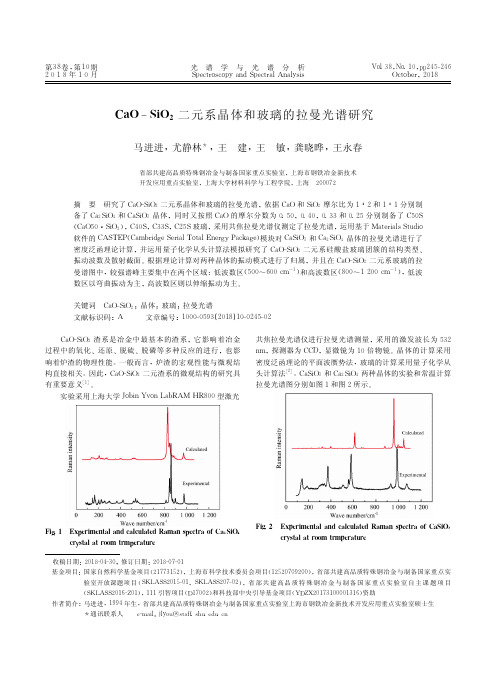
第38卷,第10期 光谱学与光谱分析Vol.38,No.10,pp245-2462 0 1 8年1 0月 Spectroscopy and Spectral Analysis October,2018 CaO-SiO2二元系晶体和玻璃的拉曼光谱研究马进进,尤静林*,王 建,王 敏,龚晓晔,王永春省部共建高品质特殊钢冶金与制备国家重点实验室,上海市钢铁冶金新技术开发应用重点实验室,上海大学材料科学与工程学院,上海 200072摘 要 研究了CaO-SiO2二元系晶体和玻璃的拉曼光谱,依据CaO和SiO2摩尔比为1∶2和1∶1分别制备了Ca2SiO4和CaSiO3晶体,同时又按照CaO的摩尔分数为0.50,0.40,0.33和0.25分别制备了C50S(CaO50·SiO2),C40S,C33S,C25S玻璃,采用共焦拉曼光谱仪测定了拉曼光谱,运用基于Materials Studio软件的CASTEP(Cambridge Serial Total Energy Package)模块对CaSiO3和Ca2SiO4晶体的拉曼光谱进行了密度泛函理论计算,并运用量子化学从头计算法模拟研究了CaO-SiO2二元系硅酸盐玻璃团簇的结构类型、振动波数及散射截面。
根据理论计算对两种晶体的振动模式进行了归属,并且在CaO-SiO2二元系玻璃的拉曼谱图中,较强谱峰主要集中在两个区域:低波数区(500~600cm-1)和高波数区(800~1 200cm-1),低波数区以弯曲振动为主,高波数区则以伸缩振动为主。
关键词 CaO-SiO2;晶体;玻璃;拉曼光谱文献标识码:A 文章编号:1000-0593(2018)10-0245-02 收稿日期:2018-04-30,修订日期:2018-07-01 基金项目:国家自然科学基金项目(21773152),上海市科学技术委员会项目(12520709200),省部共建高品质特殊钢冶金与制备国家重点实验室开放课题项目(SKLASS2015-01,SKLASS207-02),省部共建高品质特殊钢冶金与制备国家重点实验室自主课题项目(SKLASS2016-Z01),111引智项目(D17002)和科技部中央引导基金项目(YDZX20173100001316)资助 作者简介:马进进,1994年生,省部共建高品质特殊钢冶金与制备国家重点实验室上海市钢铁冶金新技术开发应用重点实验室硕士生*通讯联系人 e-mail:jlyou@staff.shu.edu.cn CaO-SiO2渣系是冶金中最基本的渣系,它影响着冶金过程中的氧化、还原、脱硫、脱磷等多种反应的进行,也影响着炉渣的物理性能。
美国A123 nanophosphate 纳米磷酸铁锂电池_Space_Appl_DCarmen

power. safety. life.™
LiFePO4 Thermal Stability
• The TGA test of LiFePO4 in contrast with NCA, or NMC, indicates the LFP less likely to release oxygen
NASA Battery Workshop 2007 Copyright © 2007 A123 Systems, Inc. All rights reserved
power. safety. life.™
Safety
Conventional Lithium Ion
• Energetic thermal runaway above 150 °C • Significant oxygen evolution • Excess lithium can plate during overcharge • Failure mode on overcharge: self-accelerating heat generation, potential explosion
A123’s doped Nanophosphate™ chemistry is more abuse tolerant.
NASA Battery Workshop 2007 Copyright © 2007 A123 Systems, Inc. All rights reserved
power. safety. life.™
88
0
异丙醇铝加压水解合成亚微米级球形氧化铝粉_赵晓媛

研究与开发异丙醇铝加压水解合成亚微米级球形氧化铝粉*赵晓媛,潘 文,宁桂玲,甘志宏,刘 杰,林 源(大连理工大学精细化工重点实验室,辽宁大连116012)摘 要:以异丙醇铝、异丙醇为原料,在反应釜中加热到250 ,压力为5M Pa ,然后迅速泄压,水解,得到粒度分布窄的亚微米级球形氧化铝颗粒。
对粉体进行了X 射线衍射(XRD )、扫描电镜(SE M )、透射电镜(TE M )和热分析(TG -DTG )等表征。
结果表明,加压水解法得到了白色蓬松状前驱物粉末,其颗粒呈球形,比表面积大,粒度分布窄,经过5002h ,800 2h ,1050 2h 阶梯式加热焙烧后,得到产物 -A l 2O 3球形度不变、粒径0.4~1.2 m 。
此法具有设备简单、无毒无害、无杂质引入的优点。
关键词:异丙醇铝;加压水解;球形氧化铝中图分类号:TQ133.1 文献标识码:A 文章编号:1006-4990(2007)11-0012-03Preparation of sub m icron spherical alu m ina pow der by high -pressurehydrolysis of alu m i n u m isopropoxideZhao X i a oyuan,Pan W en ,N i n g Gu ili n g ,Gan Zh i h ong ,L i u Ji e ,L in Yuan(State K ey Labora t ory of F i ne Che m icals ,D alian University of T echno l ogy,D alian,116012,Ch i na)Abstrac t :A lu m i nu m i sopropox i de and i sopropano l w ere hea ted to 250i n the reacto r under 5M P a .Then re l easepressure s w iftl y and t he na rrow -d i str i buting sub m icron spherical alu m i na pa rtic l es w ere prepared by hydrolysis .T he co l lected pow ders w ere cha racte rized by XRD,SE M,TE M and TG -DTG respectively .T he resu lts sho w ed t hat loo se w hite pre cursor pow ders w ere prepared by pressure hydro lysis ,w hich were spher i ca l pa rti c l es w it h l arge spec ific area and na rrow pa r ti c le size distr i buti on ;after 3-step ca lcina ti ons (500 2h ,800 2h and 1050 2h), -A l 2O 3was obta i ned w hose deg ree of sphe ricity w as no t changed and i ts particle size rang i ng from 0.4 m t o 1.2 m.The m ethod has t he advantage o f si m p l e equ i p m ents ,i nnocu ity ,and no i m purities i m ported .K ey word s :a l u m i n i u m isopropox ide ;h i gh-pressure hydrolysis ;spher i ca l alu m ina随着高精尖技术的发展,氧化铝以其高硬度、稳定性好等优点而广泛应用于精密加工制造等工业,尤其是在化学机械抛光(C M P)方面倍受青睐[1-2]。
云南安宁白泥山磷矿成因类型与成矿模式

云南安宁白泥山磷矿成因类型与成矿模式陈启良;何敏芳;郭健【摘要】白泥山磷矿属于独立风化矿床.磷矿体呈似层状面形赋存于第四系残坡积层的混杂层中,与原生下寒武统海相沉积型磷块岩矿床有着密切关系,但又相对独立,属于具有残坡积和淋滤次生双重特点的风化淋滤残积型矿床.在阐述地质背景和矿床地质特征的基础上,分析了矿床的成因类型、形成条件、成矿过程、成矿作用及机理,初步建立了该类型矿床的成矿模式.%Bainishan phosphate deposit is a independent weathered deposit.The phosphate ore bodies are bed-ded in shape and occur in the mixed layer of the Quaternary residual slope layer,It is closely related to the primary lower Cambrian marine sedimentary type phosphate rock deposit,but relatively independent,be-longs to weathering leaching residual deposit with the dual characteristics of residual slope type and seconda-ry leaching type.Based on the description of the geological background and geological characteristics of de-posit,the genetic types,formation conditions,mineralization process,mineralization and mechanism of the deposit are analyzed,the metallogenic model of this type deposit has been preliminarily established.【期刊名称】《矿产与地质》【年(卷),期】2017(031)006【总页数】8页(P1113-1120)【关键词】白泥山磷矿;风化淋滤残积型矿床;成因类型;成矿模式;云南【作者】陈启良;何敏芳;郭健【作者单位】云南省有色地质局地质地球物理化学勘查院,云南昆明 650216;云南省有色地质局地质地球物理化学勘查院,云南昆明 650216;安宁泰昇经贸有限责任公司,云南安宁 650304【正文语种】中文【中图分类】P611.2+1;P619.21+30 引言白泥山磷矿位于安宁市240°方向平距约17km处。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
ISIJ International, Vol. 50 (2010), No. 2, pp. 324–326
Fig.1.Phosphate capacity of various slags as a function of ba-
©2010ISIJ
324
Fig.2.Relationship between excess free energy of P
2O
5
and ba-
Fig.3.Schematic expression of the relationship between excess
325©2010ISIJ
by 31P MAS NMR spectra analysis. They showed that the values of the chemical shifts of the pyrophosphate com-plexes are lowered and their relative amount decrease as the ratio of MgO/SiO 2change from 0.14 to 0.54. Therefore, the excess free energy of P 2O 5can be represented with basicity as Fig. 3.
In addition, the effect of MnO on the efficiency of phos-phorus removal by various slags is shown in Fig. 4by plot-ting the phosphate capacity against the MnO to SiO 2ratio.8,19–21)It is interesting that the phosphate capacity is not affected by MnO to SiO 2ratio in the slags investigated.It increases only as the temperature decreases, viz.the phos-phate capacity decreases in order of BaO–BaF 2–MnO–SiO 2system at 1573K 19)ϾCaO–SiO 2–MnO(–MgO) at 1623K 20)ϾCaO–SiO 2–MnO–16(Ϯ1)mass%FeO–MgO satd.at 1723K (present study)ХMnO–SiO 2–9(Ϯ1)mass%FeO at 1723K 21)ϾMgO–SiO 2–MnO–16(Ϯ1)mass%FeO at 1823K .21)The phosphate capacity of the Na 2O–MgO–SiO 2–FeO–5(Ϯ1)mass%MnO system at 1873K is relatively close to those measured at about 1723 to 1823K, which could be due to highly basic characteristics of Na 2O-containing
slags.8)Because ferro-manganese smelting slags based on MnO have relatively low basicity in comparison with steel-making slags as CaO-based slags,22)the tendency of phos-phate capacity is similar to that of low basic region in Fig.1. Consequently, the ratio of basicity to phosphate stability is not significantly affected when the MnO/SiO 2ratio changes in the MnO-containing slags based on Eq. (2).
In summary, the stable phosphate ions in lime-based slags can be changed from monomeric to polymeric (e.g.dimeric) species as the basicity decreases even though the quantitative structural investigation for the silicate system containing small amounts of phosphorus is remained for fu-ture work. Furthermore, the MnO/SiO 2ratio does not affect the basicity to phosphate stability, which is important to ferromanganese smelting process.
REFERENCES
1) C. Wagner: Metall. Trans. B , 6B (1975), 405.
2)F . D. Richardson: Physical Chemistry of Melts in Metallurgy, V ol. 1,
Academic Press, London, (1974), 87.
3)R. Selin: Proc. 3rd Int. Conf. Molten Slags and Fluxes, Glasgow,
IoM, London, (1989), 317.
4) A. Tagaya, H. Chiba, F . Tsukihashi and N. Sano: Metall. Trans. B ,
22B (1991), 499.
5)H. Suito, R. Inoue and M. Takada: Trans. Iron Steel Inst. Jpn., 21
(1981), 250.
6)H. Suito and R. Inoue: Trans. Iron Steel Inst. Jpn., 22(1982), 869.7)K. Sekino and N. Sano: Tetsu-to-Hagané, 73(1987), 988.
8)K. Kunisada and H. Iwai: Trans. Iron Steel Inst. Jpn., 27(1987), 332.9)H. Ishii and R. J. Fruehan: ISS Trans., Feb. (1997), 47.
10) C. M. Lee and R. J. Fruehan: Ironmaking Steelmaking , 32(2005),
503.
11)T. Hamano and F . Tsukihashi: ISIJ Int., 45(2005), 159.
12)G. Li, T. Hamano and F . Tsukihashi: ISIJ Int., 45(2005), 12.13)M. Iwase, E. Ichise and N. Y amada: Steel Res., 56(1985), 319.
14) E. T. Turkdogan and J. P . Pearson: J. Iron Steel Inst., 175(1953), 393.15)F . D. Richardson and J. H. E. Jeffes: J. Iron Steel Inst., 160 (1948),
261.
16) C. H. P . Lupis: Chemical Thermodynamics of Materials, Prentice
Hall, New Y ork, (1993), 155.
17) D. C. Douglass, T. M. Duncan, K. L. Walker and R. Csencsits: J.
Appl. Phys., 58(1985), 197.
18)I. Wacl ⁄
awska and M. Szumera: J. Therm. Anal. Cal., 84(2006), 18519)X. Liu, O. Wijk, R. Selin and J. O. Edstorm: ISIJ Int., 38(1998), 36.20) A. Sobandi, H. G. Katayama and T. Momono: ISIJ Int., 38(1998),
781.
21)Y . Kobayashi, N. Y oshida and K. Nagai: ISIJ Int., 44(2004), 21.
22)N. Sano and W . K. Lu: Advanced Physical Chemistry for Process
Metallurgy, Academic Press, New Y ork, (1997), 48.
326
©2010
ISIJ Fig.4.Relationship between the phosphate capacity of MnO-containing slags and the ratio (mass% MnO)/(mass%SiO 2).。
