LARG-20010118-Natasha
glenair-band-master-ats-banding-tools-601-100-and-

Band-Master™ ATSBanding Tools 601-100 and 601-101 Operating InstructionsShield Termination Preparation ProcessNOTES:1. Use only genuine Band-Master™ ATS bands. Other manufacturer’s bands may damage tool.2. Use only .240" wide bands with 601-100 tool and .120" wide bands with 601-101 tool. 3 Callibrate the standard band tool to 150 ±5 lbs. and the micro band tool to 80 ±5 lbs.Band-Master ™ ATS Standard, and Micro Overview1 Calibration Access Plug (See Note 3)2 Tensioning Lever:Squeeze with short gentle strokes to tighten band to the proper tension. Lever will lock to 3 Handle with final full stroke.4 Cut-Off Lever:Squeeze to lock band buckle and trim excess band material.5 Calibration Counter6 Band Insertion and Release Lever:Depress lever to insert or release band from tool.7 Serial Number8 Tension Release Lever72Double-coil the band prior to use:IMPORTANT: Due to connector/adapter circumference, it may be necessary to double-coil the band in place around the cable or retention area.A. Insert leading edge of band through the buckle slot twice. (Bands must be double-coiled.)B. Tighten the coil until the indicator mark ( ) is approximately .250 inch(6.4mm) shy of the buckle slot (see illustration below). This will ensure sufficient band tail length for insertion into tool..250 in.(6.4 mm)Tail Length Indicator Mark ()Depress the band insertion and release lever (6), and insert the band into the front end opening of the tool, with the loop positioned outward as shown.The band termination area on all backshells is wider than the band. Position the band near the rear lip of the banding platform, allowing room for buckle. For eliptical cable entries position the buckle off center of the peak of the circle. NOTE: Overly rapid tightening of the band may result in uneven compression. If alignment of the band and shield is unsatisfactory, tension can be relaxed by pulling up tension lever (2) and pushing the tension release lever (8) forward on top of the tool. Make adjustments as necessary and finish tightening with tensioning lever (2) as described above. Instructional videos are available on the Glenair website:Complete the clamping process by depressing the cut-off lever (4), allowingTool CalibrationBand-Master™ ATS tools are factory-calibrated and are supplied with a calibration certificate. Glenair recommends that tool calibration be checked after 500 terminations. Actual calibration interval can be determined by tool users. Glenair also provides calibration services. A portable kit is available for on-site calibration. Factory calibration values are 150± 5 lbs for standard band tool 601-100, and80 ± 5 lbs for the micro band tool 601-101.601-200 Calibration Kit For Banding ToolsThe 601-200 Calibration Kit provides fast, easy, accurate calibration of all Banding Tools.Kit includes the (1) 601-200-3 calibration device, (2) fifty 601-203 micro test bands, (3) fifty 601-202 standard test bands, (4) fifty 601-217 nano test bands (5) 601-205 calibration key, (6) 601-218 tool adapter for 601-100 and 600-058 hand tools (installed), (7) 601-219 tool adapter for 600-067 and 601-104. (8) 601-220 tool adapter for 600-061, 600-068, 601-101, 601-105, 601-108. Range of the calibration device is 45 to 170 lbs, and accuracy is calibrated to ±1 lb. At factory. Dimensions: 6.65 In. X 2.47 In. X 2.83 In. (168.9 X 62.7 X 71.9Mm). Weight: 2.10 Lbs. (1.0Kg).Tool Repair and RefurbishmentGlenair provides repair and refurbishment services for Band-Master ™ ATS tools. Typical services include calibration along with replacements of cutter knife and cut-off blade. Simply send the tool to Glenair:ATTN: Customer ServiceGlenair, Inc.1211 Air Way Glendale CA 91201Pneumatic Tools for High Volume ProductionPneumatic Band-Master ™ ATS tools speed up band installation and reduce operator fatigue.Band-Master ™ ATSPneumatic Banding ToolsThe Band-Master ™ ATSPneumatic Banding Tools provide fast, easy band installation.Weight of the tool is 2.52 lbs (1.14 Kg); the control box weighs 2.74 lbs (1.24 Kg).601-104 for use with standard bands. 601-105 for use with micro bands.601-400 Foot Pedal Control for Pneumatic Banding Toolsfrees both hands to help assure more accurate, reliable and faster shield terminations.Visit the Glenair website for additional information on backshell assembly tools,banding tools and accessories:Training videos on Band-Master™ ATS termination procedures are available onthe Glenair website:/banding/Consult factory for additional recommendations for technical information on overall shields with distributed individual shields on common terminations.。
B5A-(基本)

⚩⢗㕷澔〩⍰Ṧἀ䒩⬊⋔㇌㚻㆗iPhone ⍃斆孵㖏ᷧɝ /pro/mobile/global/ ⍰徛彈⬊⋔㇌㚻㆗iPhone ᶋ䘅㳐奉◩㝦䚌䥼∩䒩ㆸ㊈⋘ɝ
⚩⺁⥌ἀ䒩᷌⇎澔學斆孼Ṧᶌ℆⬺澢 ゠宣〩岮ᷱ学KWDḨ⑂ɝ ⚩㑎ờ㚭㚻⇎澔學ṕ乇斆孼孵㖏Ṧ䟯㚁Ỵ門偾ɝ ἀ䒩Ḩ⑂⇎學ṕ乇斆孼㚭ἀ䒩孵㖏ᷧ澔ⷷ學⤦┅䬢ɝ
1
连接外部设备
连接外部显示器 ...................................... 20 将剪辑加载到个人电脑中 ........................ 21
把手装置 K
1
镜头罩
1
* 目镜和镜头保护盖已安装至摄像机。
网络功能 K 网络连接功能 K .................................. 22
其他
菜单屏幕层次结构图 ............................... 23 菜单屏幕中的基本操作 ........................... 24 显示屏幕 ................................................. 25 状态屏幕 ................................................. 32 故障排除 ................................................. 33 妥善使用本机的注意事项 ........................ 35 规格 ........................................................ 39 软件使用许可合同 ................................... 41 关于软件的重要通知 ............................... 42
惠威HR70遥控器使用说明书

HR070 Series Remote Control Programming Guide
6. Repeat steps 1 to 5 for the other components you want to control. For future reference, write down each working component code below:
HR Series Remote Control Programming
Assigned Push-button Component Program Code
TV
CBL
SAT
AUX
DVR
Push-buttons available for Programming
Flashes during Programming
4
Used to activate Programming Mode
with the most popular code first. If the component
responds, go to step 7.
6. If the component does not respond, press LEVEL+
Push-button and the Remote Control will test
The HR70 Series Remote Controls have stored in permanent memory the necessary information to send the correct commands to the component to be controlled. By entering a five digit numeric code the commands for controlling the component is activated.
Helena 0-0-21 植物滋养剂说明书

0-0-21CONCENTRATED POTASH SOLUTION AND OXIDIZED SULFURGUARANTEED ANALYSIS :Soluble Potash (K 2O) . . . . . . . . . . . . . . . . . . . . . . ……………………………….21.00% Sulfur (S)………………………………………………………………………………13.00% 13.00% Combined Sulfur (S)Derived from potassium thiosulfate. KEEP OUT OF REACH OF CHILDREN WARNINGMay be harmful if swallowedMay be harmful in contact with skin Causes serious eye irritation Causes skin irritation May be harmful if inhaledWEIGHT PER GALLON: 11.47 lbs. (5.20 kg) @ 68°F SN 041415/0815G FREEZING TEMPERATURE:.30°F NET CONTENTS: □ 5 gal (18.93 L) □ 30 gal (113.56 L)□ 250 gal (946.25 L) □ 275 gal (1040.99 L) □ Bulk ____________Information about the components of this lot of fertilizer may be obtained by writing to Helena Chemical Company, 225 Schilling Boulevard, Suite 300, Collierville, TN 38017 and giving the lot number which is found on the container.Information regarding the contents and levels of metals in this product is available on the Internet at /metals.htm F224MANUFACTURED FORHELENA CHEMICAL COMPANY225 SCHILLING BOULEVARD, SUITE 300 COLLIERVILLE, TN 38017 901-761-0050PRECAUTIONARY STATEMENTSHAZARDS TO HUMANS AND DOMESTIC ANIMALSWARNINGBEFORE USING THIS PRODUCT, READ ALL PRECAUTIONS, DIRECTIONS FOR USE, CONDITIONS OF SALE–LIMITED WARRANTY AND LIMITATIONS OF LIABILITY AND REMEDIES.May be harmful if swallowed. May be harmful in contact with skin. Causes serious eye irritation. Causes skin irritation. May be harmful if inhaled. Keep product locked up and out of the reach of children. Wash thoroughly with soap and water after handling and before eating, drinking, chewing gum or smoking tobacco. Remove and wash contaminated clothing before reuse. Do not take internally. Avoid contact with or inhalation of spray application mist if present. Do not apply this product in such a manner as to directly expose workers or other persons. If product is being mixed with pesticides and/or spray adjuvants, follow all precautionary statements on the accompanying product(s) labeling. Not for human or animal consumption.Personal Protective Equipment (PPE): Wear protective eyewear (goggles or face shield), chemical-resistant gloves, long-sleeved shirt and long pants, and shoes plus socks when using this product. Take off any contaminated clothing and wash before reuse. FIRST AID IF IN EYES: ∙ Rinse cautiously with water for several minutes. Removecontact lenses, if present and easy to do. Continue rinsing. ∙ Call a poison control center or doctor for treatmentadvice.IF SWALLOWED: ∙ Call a POISON CENTER or doctor immediately.∙ Rinse mouth. Do NOT induce vomiting. ∙ Do not give anything by mouth to an unconscious person.∙ Immediately call a POISON CENTER or doctor.IF INHALED:∙ Move person to fresh air and keep at rest in a position comfortable for breathing if they feel unwell.∙ If not breathing, call 911 or an ambulance, then give artificial respiration, preferably mouth-to-mouth if possible.∙ Call a POISON CENTER or doctor for treatment advice. IF ON SKIN ORHAIR:∙ Take off immediately all contaminated clothing. Rinse skin with water or shower.∙ Get medical attention if irritation develops or persists. ∙Wash contaminated clothing before reuse.HOT LINE NUMBERHave the product container or label with you when calling a poison control center or doctor, or going for treatment. You may also contact 1-800-424-9300 for emergency medical treatment information.STORAGE AND DISPOSALKeep container tightly closed and do not allow water to be introduced into it. Store in a dry place. Temperatures below 25°F may result in product crystallization. The product will readily reconstitute, however, with warmer temperatures and gentle agitation of the container.Do not contaminate water sources by cleaning of equipment or disposal of spray waste.Dispose of empty containers by triple rinsing with detergent solution or puncture and discard empty containers in a landfill in accordance with current local, state, and federal regulations. GENERAL INFORMATIONNUCLEUS® 0-0-21 is a highly concentrated water-based solution of potash and oxidized sulfur useful in the correction of nutritional deficiencies in plants. The unique formulation of NUCLEUS® 0-0-21 provides a non-corrosive liquid that is stable at cold temperatures. Applications of NUCLEUS® 0-0-21 through mixing, application, and irrigation equipment may reduce the rate and degree of corrosion that occurs when this equipment is exposed to fertilizer solutions. When used as directed this product does not supply all nutrients required by plants and is to supplement a soil fertility program based on soil tests.APPLICATION AND MIXING GUIDEApplication rates for general use are 2-6 gallons per acre.TURF: Apply 2 to 10 fl. ounces per 1,000 sq. ft. using 2 to 5 gallons of water per 1,000 sq. ft. Use lower rate during summer applications. Apply as needed.DO NOT APPLY NEAR WATER, STORM DRAINS, OR DRAINAGE DITCHES. DO NOT APPLY IF HEAVY RAIN IS EXPECTED. APPLY THIS PRODUCT ONLY TO YOUR LAWN/GARDEN. MIXING:NUCLEUS® 0-0-21 provides a neutral or slightly acidic pH value when diluted with water. This characteristic makes it compatible with most pesticides. In any mixing operation, NUCLEUS® 0-0-21 should be introduced in the following sequence: 1. Water2. NUCLEUS® 0-0-213. Other Fertilizer Products4. Pesticides5. Spray AdjuvantsCompatibility tests are always recommended prior to preparing a spray mixture of NUCLEUS® 0-0-21 with other products.CONDITIONS OF SALE–LIMITED WARRANTY AND LIMITATIONS OF LIABILITY AND REMEDIES Read the Conditions of Sale–Warranty and Limitations of Liability and Remedies before using this product. If the terms are not acceptable, return the product, unopened, and the full purchase price will be refunded.This label is believed to be reliable and must be followed carefully. Injury to the crop to which the product is applied may result from the occurrence of extraordinary or unusual weather conditions or the failure to follow the label directions or goodapplication practices, all of which are beyond the control of Helena Chemical Company (the "Company") or seller. In addition, failure to follow label directions may cause injury to crops, animals, man or the environment. The Company warrants that this product conforms to the chemical description on the label and is reasonably fit for the purpose referred to subject to the factors noted above which are beyond the control of the Company. The Company makes no other warranties or representations of any kind, express or implied, concerning the product, including no implied warranty of merchantability or fitness for any particular purpose, and no such warranty shall be implied by law.The exclusive remedy against the Company for any cause of action relating to the handling or use ofthis product shall be limited to, at Helena Chemical Company’s election, one of the following:1.Refund of the purchase price paid by buyer or user for product bought, or2.Replacement of the product usedTo the extent allowed by law, the Company shall not be liable and any and all claims against the Company are waived for special, indirect, incidental, or consequential damages or expense of any nature, including, but not limited to, loss of profits or income. The Company and the seller offer this product and the buyer and user accept it, subject to the foregoing conditions of sale and limitation of warranty, liability and remedies.© Copyright Helena Holding Company, 2015NUCLEUS® is a registered trademark of Helena Holding Company.。
In-Fusion

Please read the In-Fusion Snap Assembly User Manual before using this Protocol-At-A-Glance. This abbreviated protocol is provided for your convenience but is not intended for first-time users.Cloning more than two fragments at once (e.g., multiple inserts simultaneously into one linearized vector) requires adherence to specific considerations in experimental design and overall cloning protocol. This Protocol-At-A-Glance details these considerations and recommended modifications to ensure cloning success.Please note the following materials are required but not supplied:•Ampicillin (100 mg/ml stock) or other antibiotic required for plating the In-Fusion reaction•LB (Luria-Bertani) medium (pH 7.0)•LB/antibiotic plates•SOC mediumThe table below is a general outline of the protocol used for the In-Fusion Snap Assembly cloning kits. Please refer to the specified User Manual pages for further details on performing each step.Table I. In-Fusion Snap Assembly protocol outlineStep Action User Manual pages1 Select a base vector and identify the insertion site. Linearize the5vector at the insertion site by restriction enzyme digestion orinverse PCR. Isolate and purify the linearized vector.2 Design PCR primers for your sequence(s) of interest with 20-bpextensions (5’) that are complementary to the ends of adjacent5sequences (the linearized vector or another insert).3 Amplify your sequence(s) of interest with PrimeSTAR® Max DNAPolymerase. Verify on an agarose gel that your targets have been6amplified and confirm the integrity of the PCR products.4 Spin-column purify your PCR products OR treat with Cloning7Enhancer.5Set up your In-Fusion Cloning reaction 7–86 Incubate the reaction for 15 min at 50°C, then place on ice. 87 Transform competent cells with 2.5 μl of the reaction9mixture from Step 6.I. PCR and Experimental Preparation (Section IV of the User Manual)A. Preparation of a Linearized Vector by Restriction DigestionFor vector linearization via PCR, please see primer design recommendations in the User Manual,Section IV.Complete, efficient digestion will reduce the amount of cloning background. Generally speaking, twodifferent cut sites are better than one for cloning. Efficiency of digestion will always be better if therestriction sites do not overlap and have at least 5 bases between them. (This varies with each enzyme, butthe majority digest at >90% efficiency in these conditions.)1.Incubate your restriction digest as directed by the restriction enzyme supplier. Longer reactiontimes can increase linearization and reduce background.2.After digestion, purify the linearized vector using a PCR purification kit. We recommend gelpurification using the NucleoSpin Gel and PCR Clean-Up, sold as part of the In-Fusion SnapAssembly Starter Bundle (Cat. No. 638945) and Value Bundle (Cat. No. 638946) and alsoavailable separately (Cat. No. 740609.50).3.[Control] Check the background of your vector by transforming competent cells with 5–10 ng ofthe linearized and purified vector, in the absence of In-Fusion cloning master mix. If backgroundis high, add more restriction enzyme(s) and continue digesting the vector (2 hr to overnight). Gelpurify the remainder of the vector and transform again.B. PCR Primer DesignWe recommend using our online Primer Design tool to easily design In-Fusion-compatible primers:https:///in-fusion-toolsFor more information, see Appendix A.C. PCR Amplification of Target Fragment(s)The In-Fusion method is not affected by the presence or absence of A-overhangs, so you can use anythermostable DNA polymerase for amplification, including proofreading enzymes. We recommend using our PrimeSTAR Max DNA Polymerase (included in every In-Fusion Snap Assembly Starter and Value Bundle and sold separately as Cat. No. R045A). If you are using a different polymerase, please refer to the manufacturer’s instructions. If using PrimeSTAR Max DNA Polymerase, please read the User Manual and follow the guidelines below:Table II. Recommendations for PCR with PrimeSTAR Max DNA PolymeraseTemplate type Template amount Product size Extension timeHuman genomic DNA 5–100 ng up to 6 kb 5 sec/kbE. coli genomic DNA 100 pg–100 ng up to 10 kb 5 sec/kbλ DNA10 pg–100 ng up to 15 kb 5 sec/kbPlasmid DNA 10 pg–1 ng up to 15 kb 5 sec/kbcDNA ≤ the equivalent of25–125 ng total RNA up to 6 kb 5–10 sec/kb When PCR cycling is complete, confirm your product(s) on an agarose gel.II. In-Fusion Cloning Procedure (Section V of the User Manual)1.Isolate each target fragment (insert or linearized vector) by gel extraction followed by spin-columnpurification using a silica-based purification system, such as the NucleoSpin Gel and PCR Clean-Up.2.Plan the In-Fusion cloning reaction. Good cloning efficiency is achieved when using 200 ng combinedamount of vector and inserts in a 10 μl reaction. More is not better. Use the table below for reactionrecommendations.Table III. Recommended In-Fusion reactions for purified fragmentsReaction component Cloningreaction Negative controlreactionPositive controlreactionPurified PCR fragment 10–200 ng – 2 μl of 2 kbcontrol insertLinearized vector 50–200 ng 1 μl 1 μl of pUC19 controlvector5X In-Fusion SnapAssembly Master Mix 2 μl 2 μl 2 μlDeionized Water to 10 μl to 10 μl to 10 μlMolar Ratio RecommendationsGenerally, the molar ratio of each of the multiple inserts should be 2:1 with regard to the linearized vector,i.e., two moles of each insert for each mole of linearized vector. The molar ratio of two inserts with onevector should be 2:2:1.NOTE: A molar ratio calculator is included in our online cloning tools. The tool currently supports cloningreactions with up to five inserts: https:///molar-ratio3.Set up the In-Fusion cloning reaction:2 μl5X In-Fusion Snap Assembly Master Mixx μl*Linearized vectorx μl*Purified PCR insertx μl*Purified PCR insertx μl dH2O (as needed)10 μl Total volume*For reactions with larger combined volumes of vector and PCR insert (>7 μl of vector + insert), double theamount of enzyme premix, and add dH20 for a total volume of 20 μl.4.Adjust the total reaction volume to 10 µl using deionized H2O, and mix.5.Incubate the reaction for 15 min at 50°C, then place on ice.6.Continue to the Transformation Procedure (Section III). You can store the cloning reactions at –20°C untilyou are ready.III. Transformation Procedure Using Stellar™ Competent Cells (Section VI of the User Manual)This transformation protocol has been optimized for transformation using Stellar Competent Cells, sold inIn-Fusion Snap Assembly Starter Bundles and Value Bundles and separately in several formats. If you are not using Stellar Competent Cells, follow the protocol provided by the manufacturer. We strongly recommend the use of competent cells with a transformation efficiency ≥1 x 108 cfu/ug.For complete information on the handling of Stellar Competent Cells, please see the full protocol.1.Thaw Stellar Competent Cells on ice just before use. After thawing, mix gently to ensure even distribution,and then move 50 µl of competent cells to a 14-ml round-bottom tube (Falcon tube). Do not vortex.2.Add 2.5 µl of the In-Fusion cloning reaction to the competent cells.3.Place the tubes on ice for 30 min.4.Heat shock the cells for exactly 45 sec at 42°C.5.Place the tubes on ice for 1–2 min.6.Add SOC medium to bring the final volume to 500 µl. SOC medium should be warmed to 37°C before using.7.Incubate with shaking (160–225 rpm) for 1 hr at 37°C.8.Plate 1/5–1/3 of each transformation reaction into separate tubes and bring the volume to 100 µl with SOCmedium. Spread each diluted transformation reaction on a separate LB plate containing an antibioticappropriate for the cloning vector (e.g., the control vector included with the kit requires 100 µg/ml ofampicillin.)9.Centrifuge the remainder of each transformation reaction at 6,000 rpm x 5 min. Discard the supernatant andresuspend each pellet in 100 µl fresh SOC medium. Spread each sample on a separate antibiotic LB plate.Incubate all plates overnight at 37°C.10.The next day, pick individual isolated colonies from each experimental plate. Isolate plasmid DNA using astandard method of your choice (e.g., miniprep). To determine the presence of inserts, analyze the DNA byrestriction digest or PCR screening.IV. Expected Results (Section VII of the User Manual)The positive control plates typically develop several hundred colonies when using cells with a minimumtransformation efficiency of 1 x 108cfu/μg. The negative control plates should have few colonies.The number of colonies on your experimental plates will depend on the amount and purity of the PCR products and linearized vector used for the In-Fusion cloning reaction.•The presence of a low number of colonies on both the experimental plate and positive control plate (typically,a few dozen colonies) is indicative of either low transformation efficiency or low-quality DNA fragments.•The presence of many (hundreds) of colonies on the negative control is indicative of incomplete vector linearization.If you do not obtain the expected results, use the guide in Section VIII of the User Manual to troubleshoot your experiment. To confirm that your kit is working properly, perform the control reactions detailed in Section IV.D of the User Manual.NOTE: Many troubleshooting topics are covered in our online In-Fusion Cloning tips and FAQs:https:///learning-centers/cloning/in-fusion-cloning-faqsAppendix A. PCR Primer DesignWhen designing In-Fusion PCR primers, consider the following:1.Every PCR primer for multi-insert cloning must be designed in such a way that it generates productscontaining 5’ ends with 20 bp of homology to the ends of the neighboring cloning fragments (either thelinearized vector or other inserts).2.The 3’ portion of each primer should:•be specific to your template•be between 18–25 bases in length, with GC-content between 40–60%•have a T m between 58–65°C; with the difference between the forward and reverse primers ≤4°C. T m should be calculated based upon the 3’ (gene-specific) end of the primer, NOT the entire primer.•not contain identical runs of nucleotides; the last five nucleotides at the 3’ end of each primer should not have more than two guanines (G) or cytosines (C)3.Avoid complementarity within each primer and between primer pairs4.Online tools are available to help with primer design:•BLAST searches can determine specificity and uniqueness of the 3’ end (athttps:///Blast.cgi)•Our online primer design tool simplifies PCR primer design for In-Fusion reactions (at/in-fusion-tools)5.Desalted oligonucleotide primers are generally recommended for PCR reactions. However, PAGEpurification may be needed for primers of poor quality or longer than 45 nucleotides.Contact UsCustomer Service/Ordering Technical Supporttel: 800.662.2566 (toll-free) tel: 800.662.2566 (toll-free)fax: 800.424.1350 (toll-free) fax: 800.424.1350 (toll-free)web: /service web: /supporte-mail: **********************e-mail: *******************************Notice to PurchaserOur products are to be used for Research Use Only. They may not be used for any other purpose, including, but not limited to, use in humans, therapeutic or diagnostic use, or commercial use of any kind. Our products may not be transferred to third parties, resold, modified for resale, or used to manufacture commercial products or to provide a service to third parties without our prior written approval.Your use of this product is also subject to compliance with any applicable licensing requirements described on the product’s web page at . It is your responsibility to review, understand and adhere to any restrictions imposed by such statements© 2020 Takara Bio Inc. All Rights Reserved.All trademarks are the property of Takara Bio Inc. or its affiliate(s) in the U.S. and/or other countries or their respective owners. Certain trademarks may not be registered in all jurisdictions. Additional product, intellectual property, and restricted use information is available at .This document has been reviewed and approved by the Quality Department.。
色卡-RAL-德国色表标准

© 2000 by Günther Productions · Zöllnerstraße 38 · 51491 Overath
RAL 2llorange RAL 2004 Reinorange
RAL 2005 Leuchtorange RAL 2007 Leuchthellorange
CMYK 100 50 0 80 CMYK 100 100 70 40 CMYK 100 40 0 40 CMYK 80 20 0 40 CMYK 60 0 0 90
RAL Farbtabelle Die Farbwerte sind Angaben der Firma
Classic 840-HR/841-GL
RAL 6000 Patinagrün
RAL 6001 Smaragdgrün RAL 6002 Laubgrün
RAL 6003 Olivgrün
RAL 6004 Blaugrün
CMYK 80 20 60 20 CMYK 90 30 90 10 CMYK 90 40 90 10 CMYK 80 50 80 20 CMYK 100 50 60 40
RAL 1018 Zinkgelb
RAL 1019 Graubeige
RAL 1020 Olivgelb
RAL 1021 Rapsgelb
RAL 1023 Verkehrsgelb
CMYK 0 0 80 0 CMYK 5 20 40 40 CMYK 1 5 30 40 CMYK 0 10 100 0 CMYK 0 10 90 0
195 Farben
Zöllnerstraße 38 · D-51491 Overath Tel.: 0 22 04/97 00 73 Fax.: 0 22 04/97 00 74 e-Mail: Guenther_Productions@t-online.de Internet: www.Guenther-Productions.de
智利抗震规范

NCh433
Index
5.8 Seismic actions on the structure 5.9 Seismic deformations 5.10 Separations between buildings or building parts 5.11 Drawings and calculation report
38
A.4 General provisions for repair methods
38Βιβλιοθήκη A.5 Requirements that must be met by the construction process of the
structural rehabilitation
39
A.6 Necessity of rehabilitation for buildings without damages
the seismic movement
6
4.3 Classification of buildings and structures according to their importance,
occupancy and failure risk
6
4.4 Seismic instruments
7
5
37
A.1 General
37
A.2 Evaluation of the seismic damage and structural rehabilitation decisions
37
A.3 Requirements to be met by the structural rehabilitation project
6
Automatic fiber processing system including method
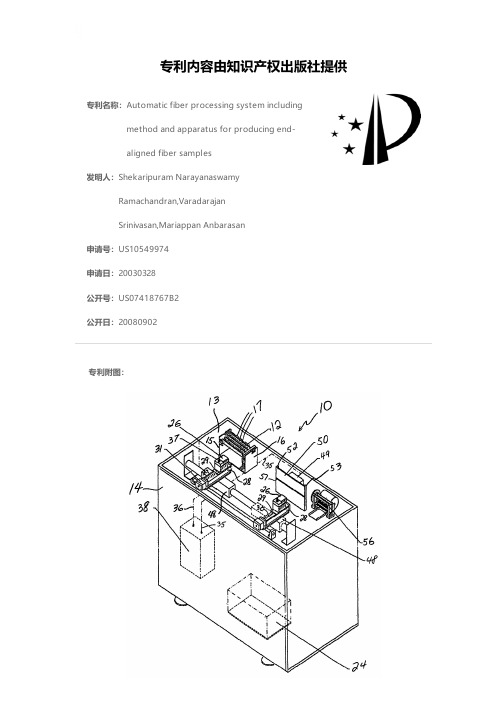
专利名称:Automatic fiber processing system including method and apparatus for producing end-aligned fiber samples发明人:Shekaripuram NarayanaswamyRamachandran,VaradarajanSrinivasan,Mariappan Anbarasan申请号:US10549974申请日:20030328公开号:US07418767B2公开日:20080902专利内容由知识产权出版社提供专利附图:摘要:The apparatus for the preparation of an end-aligned fiber sample comprises an array of fiber combs, a first drive unit, a fiber collection device connected to the first drive unit, a suction device, a detection device and a control unit to control each of the foregoing components. The method for preparing an end-aligned fiber sample uses the control unit to operate each of the foregoing components to prepare an end-aligned fiber sample. The apparatus can be combined with testing units for testing the end-aligned fiber samples. One of the testing units can test for short fiber content. The testing for short fiber content also can employ the detection device of the apparatus.申请人:Shekaripuram Narayanaswamy Ramachandran,VaradarajanSrinivasan,Mariappan Anbarasan地址:Coimbatore IN,Coimbatore IN,Coimbatore IN国籍:IN,IN,IN代理机构:Dority & Manning, P.A.更多信息请下载全文后查看。
CHARACTER COLOR EXTRACTING DEVICE AND METHOD, RECO
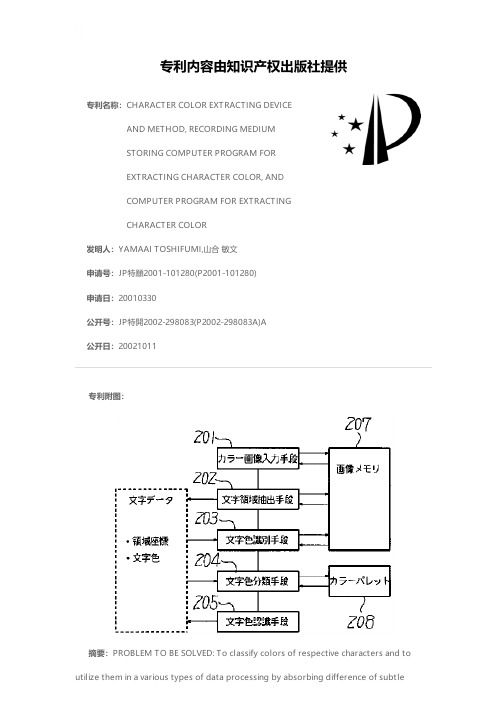
专利名称:CHARACTER COLOR EXTRACTING DEVICEAND METHOD, RECORDING MEDIUMSTORING COMPUTER PROGRAM FOREXTRACTING CHARACTER COLOR, ANDCOMPUTER PROGRAM FOR EXTRACTINGCHARACTER COLOR发明人:YAMAAI TOSHIFUMI,山合 敏文申请号:JP特願2001-101280(P2001-101280)申请日:20010330公开号:JP特開2002-298083(P2002-298083A)A公开日:20021011专利内容由知识产权出版社提供专利附图:摘要:PROBLEM TO BE SOLVED: To classify colors of respective characters and to utilize them in a various types of data processing by absorbing difference of subtlecharacter colors among characters included in colored image data. SOLUTION: A character region is extracted from the colored image data having the character region (202) and the character color of the character image data included in the character region is recognized as RGB(red, green and blue) data (203). The RGB data of the recognized character color are determined to fall under either color data out of, at least, two or more color data stored in a color pallet 208 as the data specifying the color (204), This method can recognize the color of the character image data not as live RGB data but as the color data for specifying the color and, in this case, avoid such an inconvenience as determined as a different color by the subtle reading error where it is actually same color or should be regarded as the same one color but handled as the live RGB data.申请人:RICOH CO LTD,株式会社リコー地址:東京都大田区中馬込1丁目3番6号国籍:JP代理人:柏木 慎史 (外2名)更多信息请下载全文后查看。
Production manner of the nuclear acid numerator an
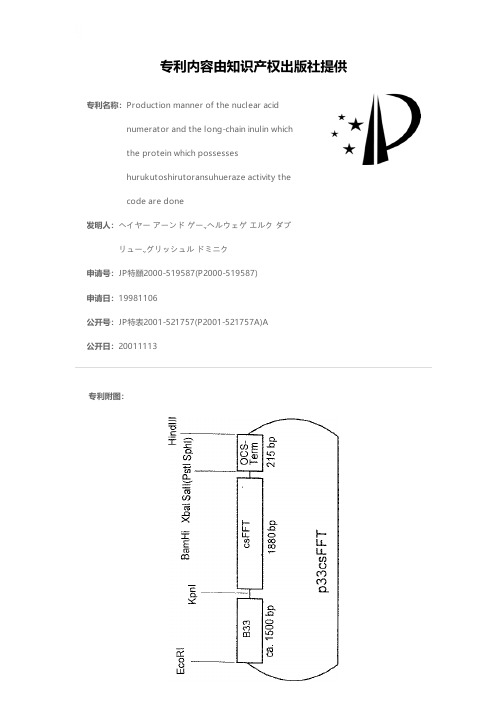
专利名称:Production manner of the nuclear acidnumerator and the long-chain inulin whichthe protein which possesseshurukutoshirutoransuhueraze activity thecode are done发明人:ヘイヤー アーンド ゲー.,ヘルウェゲ エルク ダブリュー.,グリッシュル ドミニク申请号:JP特願2000-519587(P2000-519587)申请日:19981106公开号:JP特表2001-521757(P2001-521757A)A公开日:20011113专利内容由知识产权出版社提供专利附图:摘要: (57)< Abstract > It states concerning the nuclear acid numerator which the protein which possesses the enzyme activity of hurukutoshirutoransuhueraze the code is done. These enzymes are hurukutoshirutoransuhueraze (FFT). Furthermore, the vector and the host cell which include the nuclear acid numerator of this invention, especially character the plant cell which is converted, plant system and, being reproducible from those, it states concerning the plant which reveals aforementioned FFT. Furthermore, manner in order depends on using FFT which is produced the aforementioned protein and the host, with the especially plant cell and/or those to produce the long-chain inulin is stated.申请人:マックス-プランク-ゲゼルシャフト ツール フォルデルング デル ヴィッセンシャフテン エー.ファウ.地址:ドイツ国 ベルリン (番地なし)国籍:DE代理人:清水 初志 (外1名)更多信息请下载全文后查看。
福建高分子材料重点室测试收费标准-福建师范大学
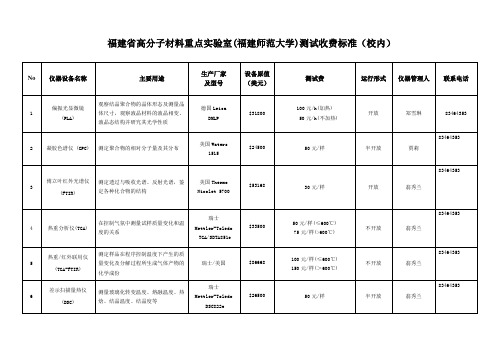
Trace GC Ultra/Trace DSQ
$84,000
50元/样
不开放
贾莉
83464353
14
热裂解仪
材料的高温裂解
美国CDS
5150
$63,000
100元/温度级
不开放
贾莉
83464353
15
浓缩仪
浓缩液、气及空气样品中的挥发性有机物及裂解产物,能集吹扫、动态顶空及热裂解析功能于一体,并能与热裂解联机或单独使用
光度计(UV/Vis)
测量物质的吸收光谱和漫反射光谱(液体透射,固体透射,固体镜面反射,固体粉末漫反射)
美国PE
Lampda 850
$53,000
100元/h
开放
郑雪琳
83464353
13
气/质谱联用仪(GC/MASS)
用于挥发性和半挥发性有机化合物的定性定量及化学结构分析,特别是复杂基体及同分异构体化合物的分析
美国CDS
8000
$63,000
100元/样
不开放
贾莉
83464353
16
比表面积及
孔隙分析仪
采用气体吸附法测定比表面积、孔体积、孔径和微孔及介孔分布
荷兰安米德
Belsorp-max
$48,000
BET: 50元/样
Full-sorption: 150元/样
不开放
赖寿莲
翁秀兰
83464353
17
场发射扫描电子显微镜(SEM)
美国Thermo
德国Haake PheoDrive 4
$248000
80元/h
开放
陈钦慧
83464353
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
– Logogens for each word – Accumulate evidence passively until threshold – Perceptual & contextual evidence raise activation – Lower thresholds for more frequent words
• Dual-route model (revised)
– Non-lexical route (G-P) – Non-semantic route (O-P) – Lexical-semantic route (G-L-S-P)
• Connectionist model
– Similar to IA model of word recognition – Learns by associating phonology and orthography – Patterns of activation
Second language acquisition • Factors that affect our chances of learning L2:
– – – – Individual differences Age of acquisition effects Environment of learning Style of instruction--conejo is ―rabbit‖ or
Language Representation
• What is a concept?
• Is there any such thing?
Things I Haven’t Covered
• • • • • • • Language and thought Structure of the language system Speech perception Language disorders Discourse processing Reading Orthographic (e.g., neighborhood) effects
Language is acquired
How are Language Acquisition, Representation, & Processing Related?
Language is acquired
Acquisition leads to a set of representations
What is Language Acquisition?
• The process of attaining a specific variant of human language. • The process of learning a native or a second language.
Bilingualism Defined
• Bilingualism is the ability to master the use of two languages, and multilingualism is the ability to master the use of more than two languages. Although bilingualism is relatively rare among native speakers of English, in many parts of the world it is the standard rather than the exception. For example, more than half the population of Papua New Guinea is functionally competent in both an indigenous language and Tok Pisin. People in many parts of the country have mastered two or more indigenous languages. Bilingualism and multilingualism often involve different degrees of competence in the languages involved. A person may control one language better than another, or a person might have mastered the different languages better for different purposes, using one language for speaking, for example, and another for writing.
Spoken word recognition: An example
• /d/
dog, dirt, dry, dries, drive, drip, dumb, desk
• /dr/
dry, dries, drive, drip
• /dry/
dry, dries, drive, driving, driver
Individual Differences
• Do individual differences determine the activation of multiple meanings of ambiguous words (especially the irrelevant ones)? • What is the influence of verbal ability and working memory on syntactic processing?
• Language processing
– What factors influence the processing of language?
How are Language Acquisition, Representation, & Processing Related?
How are Language Acquisition, Representation, & Processing Related?
– ―fly‖
• Imageability and other word-specific factors
– ―table‖ versus ―freedom‖
• Context effects--autonomous or interactive?
Spoken Word Recognition Models
Visual Word Recognition Models
• Interactive-Activation Model
– Accounts for the ―word superiority effect‖ – Visual features, letters, words – Facilitatory and inhibitory connections
What is Language?
• a system of symbols and rules that enable us to communicate
• a symbolic code used in communication
• the systematic, meaningful arrangement of symbols
Language Acquisition, Representation, & Processing
• Language acquisition
– How is language acquired or learned?
• Language representation
– How are the symbols of language represented in memory?
Visual Word Recognition Methods/Findings
• Word frequency effects
– “year” versus “permutation”
– “rain” versus “puddle” related effects: familiarity
Visual Word Recognition Models
• Initial contact, selection of a lexical entry, word recognition/lexical access, integration • Cohort model (Marslen-Wilson, 1973, 1975) Access stage--> cohort of items Selection stage--> one item chosen Integration stage--> syntax, semantics
– Lemma selection – Word form (lexeme) retrieval
• Phonological specification
• Speech errors
– Syntactic planning--how far ahead?
• Aphasias
Models of Language Production
Language Acquisition
Major Issues
First language acquisition • How does our general intelligence interact with our biological predispositions? • How do we learn our native language? What are the stages this process follows? • How do failures in this process occur?
