Synthesis and Reactions of Thioether-Thiolate Compounds of 1,3-Dithiole-2-thione-4,5-dithiolate
核苷合成

手性季鏻盐相转移催化剂在不对称反应中的应用_喻理德

3
氮磷型手性季鏻盐相转移催化剂
2007 年, Ooi 小 组 首 次 以 L -缬 氨 酸 衍 生 的 手 性
2
早期工作
Shioiri 等率先将手性季鏻盐相转移催化剂 应 用
[15]
二胺和五氯化磷为原料合成了具有螺手性的高效螺 环季鏻盐催化剂 4 , 开 拓 了 氮 -磷 型 手 性 季 鏻 盐 催 化 剂的新篇章 。 随后, 对催化剂 4 进行了简单的修饰, 6 。 然 后, 合成了手性 季 鏻 盐 催 化 剂 5 、 又将手性二 胺骨架扩展到二苯基乙二胺 、 联萘衍生的二胺等, 合 高活性手 性 季 鏻 盐 催 化 剂 8 、 成了多种类型的高效 、 9、 10 。 此 外, 该课题组还设计合成了二氨基二羟基 12 ( 图式 3 ) 。 这 些 催 化 剂 在 多 种 季鏻盐催化剂 11 、 不对称转化中均表 现 出 高 效 的 催 化 活 性 和 选 择 性, Mannich 反 应 、 如不 对 称 Henry 反 应 、 烷 基 化 反 应、 氢磷酰化反应和 Michael 加成反应等 。 3. 1 不对称 Henry 反应 不对 称 Henry 反 应 是 有 效 构 建 手 性 β -硝 基 醇 Ooi 小组首 类化合物的直接 、 快捷的方法 。2007 年, 次将螺手性季鏻盐 催 化 剂 4a 应 用 于 醛 与 硝 基 烷 烃 的不对称 Henry 反应
[16]
, 并应用于环戊 酮 -β -羰 基 酯 的 不 对 称 烷 基 化 反
仅得到中等的对映选择性( 图式 2 ) 。 研究发现, 应, 产率随反应时间的延长明显增加, 在 20 ℃ 反应时 间 由 24 h 增 加 为 168 h , 产 率 由 43% 增 加 到 80% , 产 物的 ee 值 基 本 不 变 。 此 外, 产 物 的 ee 值 受 温 度 的
The Chemistry of Green Synthesis and Catalysis

The Chemistry of Green Synthesis andCatalysisIntroductionThe modern world is facing many environmental problems caused by human activities. Environmental pollution, climate change, and depletion of natural resources are the major concerns. To address these issues, scientists are exploring ways to develop sustainable technologies and practices. The field of green chemistry aims to reduce the environmental impact of chemical processes by minimizing waste, using renewable resources, and reducing toxic substances. Green synthesis and catalysis are two important subfields of green chemistry, which have great potential for sustainable development.What is Green Synthesis?Green synthesis refers to the development of chemical reactions that are environmentally benign and sustainable. It involves using renewable resources, reducing the use of hazardous chemicals, and minimizing waste. Green synthesis is important because traditional chemical processes are often resource-intensive, produce large amounts of waste, and use toxic solvents, which can have adverse effects on human health and the environment.Green synthesis can be achieved by several methods, including using bio-based feedstocks, replacing hazardous solvents with benign ones, using microwave or ultrasound-assisted reactions, and using catalysts. Catalysts are an important tool in green synthesis, as they often allow the reaction to proceed more efficiently and with fewer environmental impacts.What is Catalysis?Catalysis is the process of accelerating a chemical reaction by adding a substance known as a catalyst. A catalyst works by lowering the activation energy required for the reaction to occur. It does not affect the thermodynamics of the reaction and is notconsumed in the reaction. Therefore, catalysts can be used repeatedly to speed up reactions without being depleted.Catalysis plays a crucial role in many industrial processes, including the production of fuels, polymers, and pharmaceuticals. However, traditional catalytic processes often use high temperatures and pressures, require toxic solvents, and produce hazardous waste. This is where green catalysis comes in.What is Green Catalysis?Green catalysis is a branch of green chemistry that focuses on developing sustainable catalysts and catalytic processes. Its goal is to reduce the environmental impact of catalytic reactions by using renewable resources, minimizing waste and toxicity, and improving efficiency.Green catalysis has many benefits over traditional catalysis. For example, it can reduce energy consumption and therefore lower greenhouse gas emissions. It can also use non-toxic and renewable resources, which can reduce the environmental impact of the reaction. In addition, green catalysis often uses less hazardous solvents, which improves the safety of the reaction for workers and reduces the risk of contaminated waste.Green Synthesis and Catalysis in ActionThere are many examples of green synthesis and catalysis being used in industrial processes. For example, the production of biodiesel is a green synthesis process that uses vegetable oil or animal fat as a renewable feedstock. The reaction is catalyzed by sodium hydroxide or potassium hydroxide, which are both inexpensive and non-toxic catalysts.Another example is the production of pharmaceuticals using enzyme catalysis. Enzymes are biocatalysts that work under mild conditions of temperature and pressure and produce little or no waste. Their use in the production of pharmaceuticals can reduce the environmental impact of the process and improve the safety for workers.ConclusionGreen synthesis and catalysis are two important subfields of green chemistry that have great potential for sustainable development. By using renewable resources, minimizing waste and toxicity, and improving efficiency, these processes can reduce the environmental impact of chemical reactions. Green synthesis and catalysis are already being used in many industrial processes, and their use is likely to increase in the future as the demand for sustainable technologies and practices grows.。
相转移催化在精细有机合成中的进展

相转移催化在精细有机合成中的进展摘要:相转移催化技术是一种重要的非均相反应方法,本文综述了相转移催化反应的概念,原理,杂多酸有机盐催化剂的作用。
文中着重介绍了近年来该技术的新发展,同时讨论了其在精细有机合成领域的应用和存在的不足。
关键词:相转移催化技术;发展;有机合成相转移催化(Phase transfer),简称PT,是20 世纪70 年代以来在有机合成中应用日趋广泛的一种新的合成技术。
在有机合成中常遇到非均相有机反应,这类反应的通常速度很慢,收率低。
20 世纪70 年代初,相转移催化技术发展起来。
泛应用于医药、农药、香料、造纸、制革等行业,带来了令人瞩目的经济效益和社会效益。
一.相转移催化的定义。
相转移催化作用是指:一种催化剂能加速或者能使分别处于互不相溶的两种溶剂(液-液两相体系或固-液两相体系)中的物质发生反应。
反应时,催化剂把一种实际参加反应的实体(如负离子)从一相转移到另一相中,以便使它与底物相遇而发生反应。
相转移催化作用能使离子化合物与不溶于水的有机物质在低极性溶剂中进行反应,或加速这些反应。
相转移催化剂把一种实际参加反应的化合物,从一相转移到另一相中,以便使它与底物相遇而发生反应。
目前相转移催化剂已广泛应用于有机反应的绝大多数领域,如卡宾反应、取代反应、氧化反应、还原反应、重氮化反应、置换反应、烷基化反应、酰基化反应、聚合反应,甚至高聚物修饰等,同时相转移催化反应在工业上也广泛应用于医药、农药、香料、造纸、制革等行业,带来了令人瞩目的经济效益和社会效益。
二.相转移催化的原理。
是指在反应中使用一种能将反应实体从一相转移到另一相的相转移催化剂,使实体与底物相遇而发生反应的一种方法。
以卤代烷与氰化钠的反应为例,相转移催化反应的过程大致如下:(1)水相反应NaCN+Q+X-→NaX+QCN(Q+X-为相转移催化剂);(2)QCN进入有机相;(3)有机相反应RX+QCN→RCN+Q+X-;(4)Q+X-返回水相。
芳香硫醚的制备

科研开发2018·01161Chenmical Intermediate当代化工研究芳香硫醚的制备*路丹丹(西北民族大学 甘肃 730100)摘要:二甲亚砜是一个极佳的溶剂,可以溶解许多其他溶剂不能溶解的物质。
二甲亚砜作为极性非质子溶剂,对于有机化学反应特别是双分子亲和反应有促进作用。
它本身也可以参加许多化学反应,是化学合成中的重要试剂。
在有机合成领域,芳香硫醚是一类重要的中间体和反应试剂。
本试验以羟甲基亚磺酸钠和苯乙酮为原料,二甲亚枫作为硫化剂,经过多种反应制备芳香硫醚。
选择催化剂I2、反应温度和反应时间为优化因素,进行实验,通过实验确定了较好的芳香硫醚反应条件:I2(equiv)1.5,反应温度100℃,反应时间8h。
关键词:芳香硫醚;催化剂;优化中图分类号:O 文献标识码:APreparation of Aromatic Sulfur EtherLu Dandan(Northwest Minzu University, Gansu, 730100)Abstract:Dimethyl sulfoxide is an excellent solvent that can dissolve many other solvents that cannot be dissolved. Dimethyl sulfoxide as a polar aprotic solvent, for organic chemical reactions, especially bimolecular affinity reaction has a catalytic role. It itself can participate in many chemical reactions, it is an important reagent in chemical synthesis.In the field of organic synthesis, aromatic thioethers are an important class of intermediates and reagents. In this test, sodium hydroxymethanesulfinate and acetophenone were used as raw materials, and dimethylsulfoxidewas used as a vulcanizing agent to prepare aromatic sulfides through various reactions. In this experiment, catalyst I2, reaction temperature, andreaction time were selected as optimization factors to conduct experiments. Through experiment, the best reaction condition of aromatic thioether wasdetermined: I2 (equiv) 1.5, reaction temperature 100℃, reaction time 8h.Key words:aromatic thioether;catalyst;optimization引言二甲亚砜是用二甲硫醚合成的,在医药学方面,人们发现了它的许多作用,如抗炎症、利尿、止痛、血管舒畅等。
合成溴苄

Synthesis of benzyl bromides with hexabromoacetone:an alternative path to drug intermediatesKara M.Joseph,Isabel Larraza-Sanchez ⇑Department of Chemistry and Physics,Saint Mary’s College,Notre Dame,IN 46556,USAa r t i c l e i n f o Article history:Received 4September 2010Revised 22October 2010Accepted 26October 2010Available online 31October 2010Keywords:Hexabromoacetone Benzyl bromides Benzyl alcohols Drug intermediatesa b s t r a c tA series of benzyl bromides were efficiently prepared from the corresponding alcohols with Br 3CCOCBr 3/PPh 3at low temperatures and under neutral conditions.The present protocol was applied to the hetero-cyclic analogues and to the successful synthesis of the precursor of the antiulcer drug omeprazole,thus furnishing an alternate,mild method for the preparation of these drug intermediates.A significant steric factor was observed throughout both series supporting a S N 2mechanism.Ó2010Elsevier Ltd.All rights reserved.1.IntroductionBenzyl halides can be considered as versatile intermediates or final products in organic synthesis.1Not only are they extensively used as reactive electrophiles in nucleophilic substitution reac-tions,but they have also been utilized as starting materials in the preparation of aldehydes and ketones,2acids,3amides,4labelled aminoacids,5oxazoles and triazoles,6amongst others;in metal-catalysed couplings to form more complex molecules;7or as build-ing blocks of biologically active compounds such as antivirals,8antifungal agents 9anticonvulsants,10etc.Alkyl halides that have a halogen atom at the ‘benzylic’position of diverse heteroaromatic rings have also received wide attention as precursors in the synthesis of active pharmaceuticals.11Of par-ticular importance to our study are those derivatives that possess a pyridine ring and which are commonly involved in the preparation of proton pump inhibitors (PPI),such as omeprazole 1(Prilosec Ò),pantoprazole 2(Protonix Ò),etc.These represent some of the most potent antiulcer drugs available today;omeprazole,in particular,is a widely prescribed drug internationally.To date many of the syntheses for these compounds are based on the coupling of the sodium salt of a 2-mercaptobenzimidazole with a substituted 2-chloromethylpyridine,followed by oxidation of the resulting thi-oether to the sulfoxide (Scheme 1).The 2-chloromethylpyridines have traditionally been prepared by the reaction of the correspond-ing alcohol with harsh,corrosive chlorinating agents like SOCl 2,in dichloromethane at reflux temperature,with the evolution of toxic gasses.Since benzyl bromides are more reactive than chlorides,12we considered that the design of an efficient,milder protocol for the preparation of such bromides would provide a valuable alter-native to the chemical and pharmaceutical industries.132.Results and DiscussionEven though Gilbert described an accessible preparation of hex-abromoacetone (HBA)in high yields as early as 1969,14a its appli-cation as an efficient reagent for the bromination of alcohols and0040-4039/$-see front matter Ó2010Elsevier Ltd.All rights reserved.doi:10.1016/j.tetlet.2010.10.133Corresponding author.Tel.:+15742844660;fax:+15742844988.E-mail address:isanchez@ (I.Larraza-Sanchez).acids has been recognized only recently.14b,c In 2008,Chavasiri and co-workers published a new method for the synthesis of alkyl bro-mides from alcohols which uses HBA (now commercially available)and triphenylphosphine (PPh 3).14b,d This reaction occurs under neutral conditions,at 30°C,and the yields are usually very high even when only 0.3equiv of HBA are pared to other methods that include highly toxic reagents such as Br 2and HBr gas or coupling reagents like CBr 4/PPh 3,Br 2/PPh 3,Br 2PPh 3that require high temperatures and generate the strong acid HBr as a byproduct,15Chavasiri’s conditions not only comply with achemistry that is less energy and materials intensive,but the chemical compounds involved are also less hazardous to humans and the environment.Chavasiri experimented with various primary,secondary and tertiary alcohols.Although benzyl alcohol was one of his sub-strates,he did not include alcohols with substituent groups on the aromatic ring.The objective of the present research was to examine the bromination of a wider range of benzyl alcohols that contained electron-withdrawing and electron-releasing groups,and to develop a reproducible protocol that could be extrapolatedTable 1Bromination of benzyl alcoholsEntry AlcoholTemp (°C)Reaction time Conversion (%)1OHrt 200–1h 812rt Overnight 1003401h 904OHMert1h1005OHMert 1h 7564070min 1007OHMeOrt2h1008OH OMert Overnight 609402h 45min 10010OHOMeMeOrt Overnight 8011402h 15min 10012OHCl401h10013OHBr402h 10014OHO 2N401h 7515OHNO 2401h 10016OH 401h 10017OH402h 10018N OH4015min10019NOHMe MeOMe4050min 9520O OH 4050min 9021SOH4070min100aDetermined by 1H NMR.14K.M.Joseph,rraza-Sanchez /Tetrahedron Letters 52(2011)13–16to pyridilcarbinols and other heterocyclic analogues.Our results are summarized in Table1.It is worth mentioning that the conditions shown in Table1dif-fer from Chavasiri’s.After an optimization study we found that 0.3equiv of HBA furnished good results,but the method provided better yields with0.5equiv.Likewise,we achieved a higher degree of reproducibility when we used the greener solvent acetonitrile in our studies,rather than dichloromethane.16From Table1it is evident that there is a highly influential steric factor:at room temperature para-derivatives afforded higher con-version rates and/or lower reaction times than the corresponding ortho compounds.For example,para-methylbenzyl alcohol con-verted to the bromide in quantitative yield in1h,while the ortho-derivative rendered only75%conversion during the same period of time(entries4and5).This pattern was consistently ob-served with all other substituents(entries7–8,12–13).Fortu-nately,the steric impediment can be overcome by raising the temperature to40°C(entries6,9and11)and,in general,shorter reaction times and good yields were obtained at this temperature. The present results also indicate that secondary benzylic alcohols are converted successfully to their bromides(entry16).It is interesting to note that both,activating and deactivating groups furnished surprisingly good conversions.These results suggest there is no significant charge developed on the central ben-zylic carbon in the transition state and thus no dependence upon the ring substituents’electronic nature,17therefore,supporting a second order mechanism similar to the Appel reaction between alcohols and PPh3/CCl418(Scheme2).The initial step is the reaction between PPh3and HBA to form intermediate3,which subse-quently reacts with the alcohol yielding an alkoxyphosphonium salt4;the latter then transforms to the corresponding bromide by S N2displacement.An opposite steric-related reactivity was observed for the nitro compounds(entries14and15)where the2-nitrobenzyl alcohol converted quantitatively into the bromide in one hour and the3-nitro derivative only in75%under the same conditions.We attrib-uted this difference in reactivity to an anchimeric assistance of the nitro group in the ortho position on the oxy-phosphonium moiety of intermediate5(Scheme3).In much the same vein,the heterocyclic rings we wanted to analyse can be classified as electron-rich or electron-poor systems, so it was foreseen that they would react in a comparable fashion to the substituted benzenes.Indeed,this method furnished excellent outcomes in yield and reaction time for the heterocyclic series(en-tries18–21).As with the aromatic substrates,we observed the same steric trend(entries18and19).We were able to test the viability of our procedure and apply it successfully to the synthesis of omeprazole’s precursor6in high yield(Scheme4).19Incidentally,the furan(entry20)and thiophene (entry21)derivatives offer a good alternative for the synthesis of another type of antiulcer agents,specifically histamine H2blockers like ranitidine(ZantacÒ)and famotidine(PepcidÒ).Considering that furan and thiophene act themselves as acti-vated rings,we were prompted to study the chemoselectivity of this reaction by isolating20derivative7in almost quantitative yield and then comparing its spectroscopic features with a sample pre-pared independently by a different route.We observed no compet-ing substitution on the ring(Scheme5).We extended our study to other solvents in order to assess their effect on reaction time and/or need for heating conditions(Table2). We carried out comparable experiments using benzyl alcohol,in which we measured the reaction progress every20min by1H NMR until no change was observed.From Table2it can be seen that,like acetonitrile,tetrahydrofuran and especially toluene(entries1and 3)are effective,green alternatives to the chlorinated solvents (entries2and7).16We also observed that the positive effect of higher temperature relies on heating the reaction mixture from the beginning(entry6).Entries4,5and7,8displayed slight or noTable2Solvent studyEntry Solvent Temp(°C)Reaction time Conversion(%)1THF rt40min9221,2-Dichloroethane rt1h893Toluene rt20min1004Acetonitrile b rt200–1h815Acetonitrile c rt then4080min846Acetonitrile d401h907Dichloromethane rt1h818Dichloromethane rt then402h80a Reaction progress was measured every20min by1H NMR until no change wasobserved.b Conversion was the same at20,40or60min.c Reaction was run at rt for1h,then200at40°.d Reaction was run at40°from the start.K.M.Joseph,rraza-Sanchez/Tetrahedron Letters52(2011)13–1615improvement when the reaction was started at room temperature and then heated at40°C.Similarly,in an effort to evaluate the ef-fect of the solvent and the temperature on HBA’s stability,we pre-pared several solutions in acetonitrile with the same concentration used in our protocol and monitored them over a period of3h at different temperatures(room temperature to52°C).As expected, we did not observe any decomposition of HBA by13C NMR or IR spectroscopy and recovered quantitative amounts of HBA once the solvent was removed.In conclusion,we have developed a highly reproducible proto-col for the preparation of benzyl bromides from alcohols.The pres-ent process can be applied to a wide range of starting materials, aromatic and heteroaromatic,with high conversion rates and short reaction times.Likewise,it can be considered as a mild alternative to the synthesis of drug intermediates that have a benzyl unit in their structure.3.General procedure for the synthesis of benzyl bromidesA mixture of alcohol(1.2mmol)and triphenylphosphine(1.8mmol)was stirred in dry acetonitrile(6mL)for15min.Hex-abromoacetone(0.6mmol)was added and the stirring continued at40°under a nitrogen atmosphere.Conversion of the alcohol into the bromide was followed by1H NMR analysis on a sample previ-ously quenched with cold water.4.Synthesis of omeprazole’s precursor6A solution of4-methoxy-3,5-dimethyl-2-pyridinemethyl bro-mide(1.2mmol)in CH3CN(6mL)was prepared according the above procedure and added to a suspension of2-mercapto-5-meth-oxybenzimidazole(1.2mmol)in CH3CN(2mL).The mixture was stirred for30min at room temperature,under a N2atmosphere,fol-lowed by addition of30%NaOH(0.32mL,2.4mmol)and the stirring was continued for an additional30min.The reaction was quenched with water,the CH3CN removed under reduced pressure and the residue was extracted with1M HCl(3Â30mL).The latter was then treated with1M NaOH(pH$11)and extracted with CH2Cl2.The organic phase was washed with brine and dried over anhydrous Na2SO4.After evaporation the residue was purified with a plug of neutral alumina using hexane/EtOAc1:4to give omeprazole’s precursor6(85%):IR(film)m max3375,3057,2951,1628,1591, 1568,1475,1452,1435,1271,1155,1078cmÀ1.1H NMR(CDCl3) d(ppm)2.26(s,3H),2.30(s,3H),3.76(s,3H),3.82(s,3H),4.38 (s,2H),6.72(d,J=2.8Hz,1H),6.95(dd,J=2.5Hz,J=7.3Hz,1H), 7.39(d,J=7.5Hz,1H),8.25(s,1H),10.25(br,1H). AcknowledgementsThis project was funded by the Maryjeanne R.Burke and Daughters SISTAR Grant from Saint Mary’s College and by the Van Smith family.We are also grateful for the support received from the Center for Academic Innovation and the Department of Chemistry and Physics at Saint Mary’s College,Notre Dame,IN. References and notes1.(a)Laven,G.;Kalek,M.;Jezowska,M.New J.Chem.2010,34,967–975;(b)Sharghi,H.;Khalifeh,R.;Doroodmand,M.M.Adv.Synth.Catal.2009,351,207–218;(c)Kamal,A.;Chouhan,G.Tetrahedron Lett.2005,46,1489–1491.2.(a)Kshirsagar,S.W.;Patil,N.R.;Samant,S.D.Res.J.Pharm.Biol.Chem.Sci.2010,1,48–51;(b)Heidarizadeh,F.;Asareh,n J.Chem.2009,21,949–952;(c) Liu,Q.;Lu,M.;Sun,F.;Li,J.;Zhao,mun.2008,38,4188–4197. 3.Scialdone,O.;Galia,A.;Silvestri,G.;Amatore,C.;Thouin,L.;Verpeaux,J.N.Chem.Eur.J.2006,12,7433–7447.4.(a)Troisi,L.;Granito,C.;Rosato,F.;Videtta,V.Tetrahedron Lett.2010,51,371–373;(b)Troisi,L.;Pindinelli,E.;Strusi,V.;Trinchera,P.Tetrahedron:Asymmetry 2009,20,368–374.5.Barnett,D.W.;Panigot,M.J.;Curley,R.W.Tetrahedron:Asymmetry2002,13,1893–1900.6.(a)El Kaim,L.;Grimaud,L.;Schiltz,A.Tetrahedron Lett.2009,50,5235–5237;(b)Shargi,H.;Khalifeh,R.;Doroodmand,M.M.Adv.Synth.Catal.2009,351,207–218.7.For examples of cross-coupling with alkynes see:(a)Xiao,Q.;Ma,J.;Yang,Y.;Zhang,Y.;Wang,.Lett.2009,11,4732–4735;(b)Davies,K.A.;Abel,R.C.;Wulff,.Chem.2009,74,3997–4000;For examples of Suzuki–Miyaura couplings see:(c)Fairlamb,I.J.S.;Sehnal,P.;Taylor,R.J.K.Synthesis2009,508–510;(d)Ines,B.;Moreno,I.;SanMartin,R.;Dominguez,.Chem.2008,73,8448–8451;For an example,of Negishi coupling see:(e)Bedford,R.B.;Huwe,M.;Wilkinson,M. mun.2009,600–602;For palladium-catalyzed intramolecular cyclizations see:(f)Liu,Z.;Shi,C.;Chen,Y.Synlett2008,1734–1736.8.Yan,R.Z.;Liu,X.Y.;Xu,W.F.;Pannecouque,C.;Witvrouw,M.;De Clercq,E.Arch.Pharmacol.Res.2006,29,957–962.9.(a)Godefroi,E.F.;Van Cutsem,J.;Van der Eycken,C.A.M.;Janssen,P.A.J.J.Med.Chem.1969,12,784;(b)Walker,K.A.M.;Marx,M.U.S.Patent4038,409, 1976.10.Zarghi,A.;Faizi,M.;Shafaghi,B.;Ahadian,A.;Khojastehpoor,H.R.;Zanganeh,V.;Tabatabai,S.A.;Shafiee,A.Bioorg.Med.Chem.Lett.2005,15,3126–3129.11.For an excellent,comprehensive source of information about drug synthesis,see:Lednicer,D.Strategies for Organic Drug Synthesis and Design,2nd ed.;John Wiley&Sons:New Jersey,2009.12.Munbunjong,W.;Lee,E.H.;Chavasiri,W.;Jang,D.O.Tetrahedron Lett.2005,46,8769–8771.13.For the preparation of4-benzylpyridines using manganese reagents and mildconditions see:Suh,Y.S.;Lee,J.;Kim,S.H.;Rieke,anomet.Chem.2003,684,20–36.14.(a)Gilbert,E.E.Tetrahedron1969,1801–1806;(b)Tongkate,P.;Pluempanupat,W.;Chavasiri,W.Tetrahedron Lett.2008,49,1146–1148;For the synthesis of amides using acyl bromides prepared with HBA as intermediates see:(c) Menezes,F.G.;Kolling,R.;Bortoluzzi,A.J.;Gallardo,H.;Zucco,C.Tetrahedron Lett.2009,50,2559–2561;(d)Sigma–Aldrich,catalog number70,2404.15.(a)Meyers,C.Y.;Hou,Y.;Lutfi,H.G.;Saft,.Chem.1999,64,9444–9449;(b)Deno,N.C.;Potter,N.H.J.Am.Chem.Soc.1967,89,3555–3556;(c) Wagner,A.;Heitz,M.P.;Mioskowski,C.Tetrahedron Lett.1990,31,3141–3144;(d)Schaefer,J.P.;Higgins,.Chem.1967,32,1607–1608.16.Alfonsi,K.;Colberg,J.;Dunn,P.J.;Fevig,T.;Jennings,S.;Johnson,T.A.;Kleine,H.P.;Knight,C.;Nagy,M.A.;Perry,D.A.;Stefaniak,M.Green Chem.2008,10,31–36.17.A recent study found that liquid ammonia behaved like a dipolar aproticsolvent in nucleophilic substitutions with a bimolecular S N2mechanism.Their Hammet r-values show a similar trend:Ji,P.-J.;Atherton,J.H.;Page,M.I.Faraday Discuss.2010,145,15–25.18.(a)Appel,R.;Halstenberg,M.In Organophosphorus Reagents in OrganicSynthesis;Cadogan,J.I.G.,Ed.;Academic Press:London,1979;(b)Slagle,J.D.;Huang,T.T.S.;Franzus,.Chem.1981,46,2526–2530.19.For a related synthesis of omeprazole analogues see:Vidaillac,C.;Guillon,J.;Arpin,I.;Forfar-Bares,I.;Ba,B.B.;Grellet,J.;Moreau,S.;Caignard,D.H.;Jarry,C.;Quentin,C.Antimicrob.Agents Chemother.2007,831–838.20.Mehner,A.;Montero,A.L.;Martinez,R.;Spange,S.Molecules2007,12,634–640.16K.M.Joseph,rraza-Sanchez/Tetrahedron Letters52(2011)13–16。
包合物

近年来用先进的分析技术(如X射线、红外光谱等)研 究碘跟淀粉生成的蓝色物,证明碘和淀粉的显色除吸附 原因外,主要是由于生成包合物的缘故。 直链淀粉是由α-葡萄糖分子缩合而成螺旋状的长长的 螺旋体,每个葡萄糖单元都仍有羟基暴露在螺旋外。碘 分子跟这些羟基作用,使碘分子嵌入淀粉螺旋体的轴心 部位。碘跟淀粉的这种作用叫做包合作用,生成物叫做 包合物。
J. Am. Chem. Soc., ASAP Article 10.1021/ja025813x S0002-7863(02)05813-4 Web Release Date: October 4, 2002
Connection of 2-H3+ and 3 is controlled by reduction/oxidation (chemical or electrochemical) of the bipyridinium unit of component 2-H3+. The luminescence of crown ether 3 is quenched by the presence of the CT excited state.
Synthesis and Characterization of a Disulfide-Linked C5-Symmetric [5]Carceplex Christoph Naumann, Samuel Place, and John C. Sherman* J. Am. Chem. Soc., 124 (1), 16 -17, 2002.
Photoinduced Electron Transfer in a Triad That Can Be Assembled/Disassembled by Two Different External Inputs. Toward Molecular-Level Electrical Extension Cables
天然气制乙烯技术进展及经济性分析

2016年第35卷第6期CHEMICAL INDUSTRY AND ENGINEERING PROGRESS ·1733·化工进展天然气制乙烯技术进展及经济性分析胡徐腾(中国化工集团公司,北京 100080)摘要:介绍了当前几种主要的天然气制乙烯技术新进展,包括天然气经甲醇制乙烯、费-托合成制乙烯、甲烷氧化偶联制乙烯技术进展及应用情况,并对这几种工艺进行了技术经济评价,结论认为:天然气制乙烯技术的大规模应用,主要取决于天然气原料供应的有效保障及其价格是否合理,在天然气供应充足、价格合理的条件下,天然气经甲醇制乙烯工艺将会得到较快发展,而费-托合成制乙烯、甲烷氧化偶联制乙烯技术目前尚未达到成熟应用阶段,需要持续加大研发力度,争取早日实现工业化应用。
关键词:天然气;乙烯;甲醇;费-托合成;甲烷氧化偶联;技术进展;经济评价中图分类号:TQ 221.21 文献标志码:A 文章编号:1000–6613(2016)06–1733–06DOI:10.16085/j.issn.1000-6613.2016.06.013Technology progress and economy analysis on natural gas to ethyleneHU Xuteng(China National Chemical Corporation,Beijng 100080,China)Abstract:The current main technology progress of natural gas to ethylene were introduced,including natural gas to ethylene through methanol,Fischer-Tropsch synthesis route,and oxidative coupling of methane. Technical and economic evaluation of these processes were performed,and the conclusions are:the large-scale application of natural gas to ethylene technology mainly depends on the natural gas supply and it’s price. Under the conditions of sufficient gas supply and reasonable price,the technology of natural gas to ethylene through methanol will get a rapid development,while the applications of Fischer-Tropsch synthesis and oxidative coupling of methane technology at present has not yet been mature and it is necessary to continue to intensify their research and development for the realization of their industrial application.Key words: natural gas;ethylene;methanol;Fischer-Tropsch synthesis; oxidative coupling of methane;technical progress;economic evaluation近年来,全球乙烯市场需求强劲,2014年全球乙烯产能达到1.53亿吨/年,需求量1.43亿吨,产量1.3亿吨,由于供应增速低于需求增速,市场供应相对偏紧[1]。
1有机合成化学复习思考题及答案

Meldrum’s Acids as Acylating Agents in the CatalyticIntramolecular Friedel -Crafts ReactionEric Fillion,*Dan Fishlock,Ashraf Wilsily,and Julie M.GollDepartment of Chemistry,University of Waterloo,Waterloo,Ontario N2L 3G1,Canadaefillion@uwaterloo.caReceived September 14,2004The intramolecular Friedel -Crafts acylation of aromatics with Meldrum’s acid derivatives catalyzed by metal trifluoromethanesulfonates is reported.Meldrum’s acids are easily prepared,functionalized,handled,and purified.The synthesis of polysubstituted 1-indanones from benzyl Meldrum’s acids was investigated thoroughly,and it was shown that a variety of catalysts were effective,while accommodating a diversity of functional groups under mild conditions.The scope,limitations,and functional group tolerance (terminal alkene and alkyne,ketal,dialkyl ether,dialkyl thioether,aryl methyl ether,aryl TIPS and TBDPS ethers,nitrile-and nitro-substituted aryls,alkyl and aryl halides)for a variety of 5-benzyl (enolizable Meldrum’s acids)and 5-benzyl-5-substituted Meldrum’s acids (quaternized Meldrum’s acids),forming 1-indanones and 2-substituted-1-indanones,respec-tively,are delineated.This method was further applied to the synthesis of 1-tetralones,1-benzo-suberones,and the potent acetylcholinesterase inhibitor donepezil.Rate of cyclization as a function of ring size was established for various benzocyclic ketones via competition experiments:1-tetralones form faster than both 1-indanones and 1-benzosuberones,and 1-benzosuberones cyclize faster than 1-indanones.IntroductionThe intramolecular Friedel -Crafts acylation is the most powerful carbon -carbon forming reaction in syn-thetic organic chemistry for the synthesis of benzocyclic ketones,which comprise 1-indanones,1-tetralones,1-ben-zosuberones,and related compounds.1These structural motifs have proven synthetic utility in numerous biologi-cally active natural products 2and play a major role in medicinal chemistry and the development of pharma-ceuticals.3As illustrated in Figure 1,the antihyperten-sive drug (+)-indacrinone,4the norditerpene taiwan-iaquinol B,5and the acetylcholinesterase inhibitor(1)For monographs on the Friedel -Crafts acylation reaction,see:(a)Heaney,H.In Comprehensive Organic Synthesis ;Trost,B.M.,Fleming,I.,Eds.;Pergamon Press:Oxford,UK,1991;Vol.2,pp 733-752.(b)Olah,G.A.Friedel -Crafts Chemistry ;John Wiley and Sons:New York,1973.(c)For reviews on the intramolecular Friedel -Crafts acylation reaction,see:(d)Heaney,H.In Comprehensive Organic Synthesis ;Trost,B.M.,Fleming,I.,Eds.;Pergamon Press:Oxford,UK,1991;Vol.2,pp 753-768.(e)Sethna,S.In Friedel -Crafts and Related Reactions ;Olah,G.A.,Ed.;Interscience:New York,1964;Vol.3,pp 911-1002.(f)Gore,P.H.Chem.Rev.1955,55,229-281.(g)Johnson,.React.1944,2,114-177.(2)(a)Wipf,P.;Jung,.Chem.2000,65,6319-6337.(b)Ollero,L.;Castedo,L.;Dominguez,D.Tetrahedron Lett.1998,39,1413-1416.(c)Danheiser,R.L.;Helgason,A.L.J.Am.Chem.Soc.1994,116,9471-9479.(d)Tori,M.;Sono,M.;Nishigaki,Y.;Na-(3)(a)Catoen-Chackal,S.;Facompre ´,M.;Houssin,R.;Pommery,N.;Goossens,J.-F.;Colson,P.;Bailly,C.;He ´nichart,J.-P.J.Med.Chem.2004,47,3665-3673.(b)Musso,D.L.;Cochran,F.R.;Kelley,J.L.;McLean,E.W.;Selph,J.L.;Rigdon,G.C.;Orr,G.F.;Davis,R.G.;Cooper,B.R.;Styles,V.L.;Thompson,J.B.;Hall,W.R.J.Med.Chem.2003,46,399-408.(c)Musso,D.L.;Orr,G.F.;Cochran,F.R.;Kelley,J.L.;Selph,J.L.;Rigdon,G.C.;Cooper,B.R.;Jones,M.L.J.Med.Chem.2003,46,409-416.(d)Bauta,W. E.;Lovett, D.P.;Cantrell,W.R.,Jr.;Burke,.Chem.2003,68,5967-5973.(e)Caro,Y.;Masaguer,C.F.;Ravin ˜a,E.Tetrahedron :Asymmetry 2003,14,381-387.(f)Ghatak,A.;Dorsey,J.M.;Garner,C.M.;Pinney,K.G.Tetrahedron Lett.2003,44,4145-4148.(g)Adams,D.R.;Duncton,mun.2001,31,2029-2036.(h)Shiraishi,M.;Aramaki,Y.;Seto,M.;Imoto,H.;Nishikawa,Y.;Kanzaki,N.;Okamoto,M.;Sawada,H.;Nishimura,O.;Baba,M.;Fujino,M.J.Med.Chem.2000,43,2049-2063.(i)Bo ¨s,M.;Jenck,F.;Martin,J.R.;Moreau,J.-L.;Sleight,A.J.;Wichmann,J.;Widmer,U.J.Med.Chem.1997,40,2762-2769.(4)(a)Dolling,U.-H.;Davis,P.;Grabowski,E.J.J.J.Am.Chem.Soc.1984,106,446-447.(b)deSolms,S.J.;Woltersdorf,O.W.,Jr.;Cragoe,E.J.,Jr.J.Med.Chem.1978,21,437-443.(5)For studies on the synthesis of members of this family of natural products,see:(a)Lomberget,T.;Bentz,E.;Bouyssi,D.;Balme,.Lett.2003,5,2055-2057.(b)Banerjee,M.;Makhopadhyay,R.;Achari,B.;Banerjee,.Lett.2003,5,3931-3933.Isolation,see:(c)donepezil hydrochloride (Aricept),6used for the treatment of Alzheimer’s disease,all contain a 1-indanone core.7Conditions for the mild and catalytic acylation of aromatic compounds with broad functional group toler-ance have been elusive.Existing procedures work well with simple substrates but are rarely applicable to functionalized precursors.The classical intramolecular Friedel -Crafts acylation involves the reaction of an acyl halide or carboxylic acid with a tethered arene promoted by either Lewis or Brønsted acids.8Reacting an aromatic with an acyl chloride in combination with a strong Lewis acid such as AlCl 3,TiCl 4,or SnCl 4is one of the most common acylation procedures.However,due to catalyst inhibition by the product,via formation of a stable Lewis acid -aromatic ketone complex,stoichiometric or excess amounts of the oxophilic promoter are necessary.Fur-thermore,decomposition of this complex by aqueous workup renders product isolation tedious.Additional drawbacks of this protocol include the moisture sensitiv-ity of acyl chlorides and the generation of hydrogen chloride.Alternatively,the reaction of acyl chlorides with stoichiometric quantities of trifluromethanesulfonic acid provides good yields of benzocyclic ketones via highly reactive sulfocarboxylic acid anhydride intermediates.9Lewis acid-catalyzed intramolecular Friedel -Crafts acylation procedures with acyl halides have not been reported.10Complementary intramolecular acylation methods that directly use carboxylic acids as the electrophile suffer from the poor leaving group ability of the -OH moiety and thus require forcing conditions.Friedel -Crafts de-hydrative acylation with carboxylic acids have been promoted by polyphosphoric acid,11methanesulfonic acid,HF,or dehydrating agents such as P 2O 5,trifluoroacetic anhydride,and trifluoromethanesulfonic anhydride.8Nafion-H,an immobilized perfluorinated sulfonic acid,does not form stable complexes with aryl ketones in the acylation with acyl chlorides or carboxylic acids.12Al-though Nafion-H has been reported to effectively promote intramolecular dehydrative Friedel -Crafts acylations to yield tetralones at moderate temperature,it was inef-fective for preparing the synthetically more challenging indanones.Generally,1-tetralones are the easiest ben-zocyclic ketones to form by intramolecular Friedel -Crafts acylation.Difficulties are associated with 1-indanone synthesis and rigorous conditions are typically required for their preparation,including high temperatures and long reaction times.13The synthetic importance of the Friedel -Crafts acylation has generated interest in the development of a catalytic version under mild reaction conditions.Progress has been made toward intermolecular Lewis acid-catalyzed protocols with use of rare-earth metal triflates but cyclization precursors are still essentially limited to acid halides and anhydrides.14The intermolecular acylation of aromatics with carboxylic acids at moderate temperature by the combined use of perfluoroalkanoic acid anhydride and Bi(OTf)3or Sc(OTf)3,via the in situ generation of an anhydride intermediate,was de-scribed.15,16Dehydrative cyclization protocols catalyzed by Bi(NTf 2)3and Tb(OTf)3were reported,but elevated temperatures were required,between 180and 200°C for the synthesis of 1-tetralones 17and 250°C for the prepa-ration of 1-indanones.18,19Rather than examining reaction conditions,little at-tention has been paid to the elaboration of novel acylating agents.Operationally simple intramolecular Friedel -(6)Sugimoto,H.;Iimura,Y.;Yamanishi,Y.;Yamatsu,K.J.Med.Chem.1995,38,4821-4829.(7)For other natural products containing a 1-indanone core,see;(a)Ito,T.;Tanaka,T.;Iinuma,M.;Nakaya,K.;Takahashi,Y.;Sawa,R.;Murata,J.;Darnaedi,D.J.Nat.Prod.2004,67,932-937.(b)Nagle,D.G.;Zhou,Y.-D.;Park,P.U.;Paul,V.J.;Rajbhandari,I.;Duncan,C.J.G.;Pasco,D.S.J.Nat.Prod.2000,63,1431-1433.(8)Larock,prehensive Organic Transformations ,2nd ed.;Wiley-VCH:New York,1999;pp 1422-1433.(9)(a)Hulin,B.;Koreeda,.Chem.1984,49,207-209.Trifluoromethanesulfonic acid has been reported to catalyze intermo-lecular Friedel -Crafts acylation of aromatics with acyl chlorides,see:(b)Effenberger,F.;Epple,G.Angew.Chem.,Int.Ed.Engl.1972,11,299-300.(c)Effenberger,F.;Epple,G.Angew.Chem.,Int.Ed.Engl.1972,11,300-301.(10)Intermolecular catalytic Friedel -Crafts acylations with acyl chlorides have been reported,see:(a)ZnO-Catalyzed:Sarvari,M.H.;Sharghi,.Chem.2004,69,6953-6956.(b)Metal bis-{(trifluoromethyl)sulfonyl }amide complexes catalyzed:Earle,M.J.;Hakala,U.;McAuley,B.J.;Nieuwenhuyzen,M.;Ramani,A.;Seddon,mun.2004,1368-1369.(c)SbCl 5-benzyltriethylam-monium chloride complex:Huang,A.;Liu,X.;Li,L.;Wu,X.;Liu,W.;Liang,Y.Adv.Synth.Catal.2004,346,599-602and references therein.(d)Ga(ONf)3catalyzed:Matsuo,J.;Odashima,K.;Kobayashi,S.Synlett 2000,403-405.(e)Bi(OTf)3catalyzed:Re´pichet,S.;Le Roux,(11)Popp,F.D.;McEwen,W.E.Chem.Rev.1958,58,321-401.(12)(a)Olah,G.A.;Mathew,T.;Farnia,M.;Prakash,G.K.S.Synlett 1999,1067-1068.(b)Yamato,T.;Hideshima,C.;Prakash,G.K.S.;Olah,.Chem.1991,56,3955-3957.(13)For other approaches to 1-indanone,see:(a)Rendy,R.;Zhang,Y.;McElrea,A.;Gomez,A.;Klumpp,.Chem.2004,69,2340-2347.(b)Gagnier,S.V.;Larock,R.C .J.Am.Chem.Soc.2003,125,4804-4807.(c)Prakash,G.K.S.;Yan,P.;To ¨ro ¨k,B.;Olah,G.A.Catal.Lett.2003,87,109-112.(d)Pletnev,A.A.;Larock,.Chem.2002,67,9428-9438.(14)For a review on Sc(OTf)3in synthesis,see:(a)Kobayashi,S.;Sugiura,M.;Kitagawa,H.;Lam,W.W.-L.Chem.Rev.2002,102,2227-2302.(b)Kawada,A.;Mitamura,S.;Matsuo,J.;Tsuchiya,T.;Kobayashi,S.Bull.Chem.Soc.Jpn.2000,73,2325-2333.(c)Yon-ezawa,N.;Hino,T.;Ikeda,T.Recent .Chem.1998,1,213-223.(15)Matsushita,Y.;Sugamoto,K.;Matsui,T.Tetrahedron Lett.2004,45,4723-4727and references therein.(16)Intermolecular catalytic Friedel -Crafts acylations with car-boxylic acids have been reported,see:(a)Eu(NTf 2)3catalyzed:Kawa-mura,M.;Cui,D.-M.;Hayashi,T.;Shimada,S.Tetrahedron Lett.2003,44,7715-7717and references therein.(b)Sc(OTf)3catalyzed:Koba-yashi,S.;Moriwaki,M.;Hachiya,I.Tetrahedron Lett.1996,37,4183-4186.(17)Cui,D.-M.;Kawamura,M.;Shimada,S.;Hayashi,T.;Tanaka,M.Tetrahedron Lett.2003,44,4007-4010.(18)Cui,D.-M.;Zhang,C.;Kawamura,M.;Shimada,S.Tetrahedron Lett.2004,45,1741-1745.F IGURE 1.Bioactive 1-indanonesMeldrum’s Acids as Acylating AgentsCrafts reactions would be facilitated by the availability of a moisture-stable,highly electrophilic precursor20that is easily prepared,functionalized,and purified,preferably by recrystallization.Such a precursor should ideally provide aromatic ketones catalytically at moderate tem-peratures while generating only volatile and inert side products.This acylating agent should be sufficiently flexible for the facile and expedient modification and assembly of diverse polycyclic ring systems. Ketenes,21isocyanates,22isothiocyanates,23 -lactams,24 cyclic anhydrides,25azalactones,26carbamates,27and nitriles28have been exploited as electrophiles in intramo-lecular Friedel-Crafts acylations but with limited suc-cess and/or lack of generality.Esters and lactones have attracted little attention as acylating agents due to the predominant Friedel-Crafts alkylation pathway,29the carboxylate being an excellent leaving group when acti-vated by a Lewis acid.30A survey of the literature on intramolecular Lewis acid-promoted Friedel-Crafts acyl-ation with esters provided two examples.31Pinnick and co-workers reported a tandem Friedel-Crafts alkylation/ acylation of benzene with ethyl cyclopropanecarboxylate promoted by excess AlCl3at80°C to yield2-methyl-1-indanone in93%yield.32Gewald’s group described the formation of4-oxo-3-(1,4-dihydro-3-cinnoline)carbonitrile in64%yield from ethyl2-cyano-2-(2-phenylhydrazono)-acetate and excess AlCl3at reflux in chlorobenzene.33 In our hands,the application of Pinnick’s and Gewald’s work to a catalytic Friedel-Crafts acylation protocol with esters for the preparation of1-indanones was unfruitful. The methyl ester1,34bearing an electron-richπ-nucleo-phile,35was treated with a catalytic amount of BF3‚OEt2. The formation of indanone2with only10%conversion directly reflected the quantity of Lewis acid used and the stoichiometric nature of the process(Scheme1).Since the primary objective was to devise a catalytic acylation reaction,metal trifluoromethanesulfonate catalysts were examined.Ester1was treated with Mg(OTf)2but the starting material was quantitatively recovered after24 h at reflux in CH3NO2.Mono-and dialkylated malonates 336and4were inert in the presence of Sc(OTf)3,and it was therefore concluded that methyl esters held little promise in metal-catalyzed intramolecular Friedel-Crafts acylation reactions.Efforts were then focused on the development of a potent electrophile for these reaction conditions.Crow and McNab reported that Meldrum’s acid(2,2-dimethyl-1,3-dioxane-4,6-dione)could act as an electro-phile in Friedel-Crafts acylation;flash vacuum pyrolysis(20)For a review on superelectrophiles,see:Olah,G.A.Angew. Chem.,Int.Ed.Engl.1993,32,767-788.(21)(a)Intramolecular arylation of ketenium ions,see:Zhang,L.; Kozmin,S. A.J.Am.Chem.Soc.2004,126,10204-10205.(b) Intramolecular Friedel-Crafts acylation with chromium-carbene complex derived ketenes catalyzed by ZnCl2,see:Bueno,A.B.;Moser, W.H.;Hegedus,.Chem.1998,63,1462-1466.(22)Intramolecular acylation with isocyanates,see:(a)Bala´zs,L.; Nyerges,M.;Ka´das,I.;To¨ke,L.Synthesis1995,1373-1375.(b) Umezawa,B.;Hoshino,O.;Sawaki,S.;Mori,K.Chem.Pharm.Bull. 1980,28,1003-1005.(c)Tanaka,H.;Nagai,Y.;Irie,H.;Uyeo,S.; Kuno,A.J.Chem.Soc.,Perkin Trans.11979,874-878.(d)Umezawa, B.;Hoshino,O.;Sawaki,S.;Sashida,H.;Mori,K.Heterocycles1979, 12,1475-1478.(e)Davies,R.V.;Iddon,B.;Suschitzky,H.;Gittos,M. W.J.Chem.Soc.,Perkin Trans.11978,180-184.(f)Ohta,S.;Kimoto, S.Chem.Pharm.Bull.1976,24,2969-2976.(g)Tsuda,Y.;Isobe,K.; Toda,J.;Taga,J.Heterocycles1976,5,157-162.(h)Ohta,S.;Kimoto, S.Tetrahedron Lett.1975,16,2279-2282.(i)Hendrickson,J.B.; Bogard,T.L.;Fisch,M.E.;Grossert,S.;Yoshimura,N.J.Am.Chem. Soc.1974,96,7781-7789.(23)Intramolecular acylation with isothiocyanates,see:(a)Smith,P.A.S.;Kan,.Chem.1964,29,2261-2265.(b)Smith,P.A.S.;Kan,.Synth.1964,44,91-94.(c)Smith,P.A.S.;Kan, R.O.J.Am.Chem.Soc.1960,82,4753-4754.(24)Intramolecular acylation with -lactams,see:Anderson,K.W.; Tepe,.Lett.2002,4,459-461.(25)Intramolecular Friedel-Crafts acylation with cyclic anhydrides, see:(a)Fischer,W.;Kvita,V.Helv.Chim.Acta1985,68,854-859.(b)Cannon,J.G.;Brubaker,A.N.;Long,J.P.;Flynn,J.R.;Verimer, T.;Harnirattisai,P.;Costall,B.;Naylor,R.J.;Nohria,V.J.Med.Chem. 1981,24,149-153.(c)Horton,W.J.;Johnson,H.W.;Zollinger,J.L. J.Am.Chem.Soc.1954,76,4587-4589.(d)Campbell,A.D.J.Chem. Soc.1954,3659-3669.(e)Lloyd,H.A.;Horning,E.C.J.Am.Chem. Soc.1954,76,3651-3653.(f)Urban,R.S.;Beavers,E.M.J.Am. Chem.Soc.1954,76,3042-3043.(g)Gensler,W.J.;Samour,C.M.; Wang,S.Y.J.Am.Chem.Soc.1954,76,315-316.(h)Campbell,K. N.;Cella,J.A.;Campbell,B.K.J.Am.Chem.Soc.1953,75,4681-4684.(i)Haworth,R.D.;Sheldrick,G.;Mavin,C.R.J.Chem.Soc. 1935,636-644.(26)Intramolecular Friedel-Crafts acylation with azalactones, see:(a)Filler,R.;Rao,.Chem.1962,27,2403-2406.(b) Awad,W.I.;Hafez,.Chem.1961,26,2055-2057.(27)Intramolecular acylation with carbamates(Bischler-Napier-alsky reaction),see:(a)Wang,Y.-C.;Georghiou,P.E.Synthesis2002, 2187-2190.(b)Wang,X.;Tan,J.;Grozinger,K.Tetrahedron Lett. 1998,39,6609-6612.(c)Angle,S.R.;Boyce,J.P.Tetrahedron Lett. 1995,36,6185-6188.(d)Banwell,M.G.;Bissett,B.D.;Busato,S.; Cowden,C.J.;Hockless,D.C.R.;Holman,J.W.;Read,R.W.;Wu,A. W.J.Chem.Soc.,mun.1995,2551-2553.(e)Bandwell, M.G.;Cowden,C.J.;Gable,R.W.J.Chem.Soc.,Perkin Trans.11994, 3515-3518.(f)Bandwell,M.G.;Cowden,C.J.;Mackay,M.F.J.Chem. Soc.,mun.1994,61-62.(g)Banwell,M.G.;Wu,A.W.J. Chem.Soc.,Perkin Trans.11994,2671-2672.(h)Martin,S.F.;Tu,.Chem.1981,46,3763-3764.(28)For intramolecular versions of the Houben-Hoesch reaction (acylation with nitriles),see:(a)Sato,Y.;Yato,M.;Ohwada,T.;Saito, S.;Shudo,K.J.Am.Chem.Soc.1995,117,3037-3043.(b)Rama Rao, A.V.;Gaitonde,A.S.;Prakash,K.R.C.;Rao,S.P.Tetrahedron Lett. 1994,35,6347-6350.(c)Cameron,D.W.;Deutscher,K.R.;Feutrill,(29)For an example of intramolecular Friedel-Crafts alkylation of π-nucleophiles withγ-lactones,see:Fillion,E.;Beingessner,R.L.J. Org.Chem.2003,68,9485-9488.(30)For reviews on the Friedel-Crafts alkylation reaction,see:(a) Olah,G.A.;Krishnamurti,R.;Prakash,G.K.S.In Comprehensive Organic Synthesis;Trost,B.M.,Fleming,I.,Eds.;Pergamon Press: Oxford,UK,1991;Vol.3,pp293-339.(b)Roberts,R.M.;Khalaf,A.A.Friedel-Crafts Alkylation Chemistry:A Century of Discovery;Marcel Dekker:New York,1984.Intermolecular Friedel-Crafts acylation with esters,see:(c)Karade,N.N.;Shirodkar,S.G.;Potrekar,R.A. J.Chem.Res.(S)2003,652-654.(d)Olah,G.A.;Nishimura,J.J. Am.Chem.Soc.1974,96,2214-2220and references therein.(e) Pepper,J.M.;Robinson,B.P.Can.J.Chem.1966,44,1809-1816.(f) Pepper,J.M.;Robinson,B.P.Can.J.Chem.1963,41,2103-2106.(g)Man,E.H.;Hauser,.Chem.1952,17,397-403.(h) Takegami,Y.;Shingu,H.Bull.Inst.Chem.Res.,Kyoto Univ.1951, 24,84.(i)Illari,G.Gazz.Chim.Ital.1947,77,352-358.(j)Kursanov, D.N.;Zel’vin,pt.Rend.Acad.Sci.URSS1942,36,17-21. (k)Norris,J.F.;Arthur,P.,Jr.J.Am.Chem.Soc.1940,62,874-877. (l)Simons,J.H.;Archer,S.;Randall,D.I.J.Am.Chem.Soc.1939, 61,1821-1822.(m)Norris,J.F.;Sturgis,B.M.J.Am.Chem.Soc. 1939,61,1413-1417.(n)Kursanov,D.N.;Zel’vin,R.R.Zh.Obshch. Khim.1939,9,2173-2178.(o)Berman,N.;Lowy,A.J.Am.Chem. Soc.1938,60,2596-2597.(p)Bowden,E.J.Am.Chem.Soc.1938, 60,645-647.(q)McKenna,J.F.;Sowa,F.J.J.Am.Chem.Soc.1937, 59,1204-1205.(r)Kashtanov,L.I.Zh.Obshch.Khim.1932,2,515-523.(s)Cox,E.H.J.Am.Chem.Soc.1930,52,352-358.(t)Cryer,J. Trans.R.Soc.Can.,Sect.III1925,19,29.(31)Intramolecular Friedel-Crafts acylation with ethyl ester pro-moted by PPA,see:Poondra,R.R.;Fischer,P.M.;Turner,N.J.J. Org.Chem.2004,69,6920-6922.(32)Pinnick,H.W.;Brown,S.P.;McLean,E.A.;Zoller,L.W.,III .Chem.1981,46,3758-3760.(33)Gewald,K.;Calderon,O.;Scha¨fer,H.;Hain,U.Liebigs Ann. Chem.1984,1390-1394.(34)TenBrink,R.E.;McCall,J.M.J.Heterocycl.Chem.1981,18, 821-824.(35)Mayr,H.;Kempf,B.;Ofial,A.R.Acc.Chem.Res.2003,36,66-Fillion et al.(FVP)of 2,2-dimethyl-5-phenoxy-1,3-dioxan-4,6-dione (5)at 450°C yielded benzofuran-2(3H )-one (6)in an unde-termined (N/A)yield (Scheme 2).37Starting from the analogous toluyl derivative 7,a 6%yield of the Friedel -Crafts acylation product 8was obtained and the authors proposed that the acylation proceeded via the interme-diacy of a phenoxyketene.Other than McNab’s work,the addition of carbon-based nucleophiles to Meldrum’s acid derivatives has not been exploited.38The high acidity of Meldrum’s acid and its propensity to enolize in the presence of weak Brønsted or Lewis bases complicate nucleophilic addition to its highly electrophilic carbonyl groups.It was considered that neutral nonbasic π-nucleophiles would add to Mel-drum’s acid derivatives in the presence of a Lewis acid to further activate the carbonyl groups.39Recent workfrom our laboratories has demonstrated that Meldrum’s acid derivatives are indeed effective acylating agents in intramolecular Friedel -Crafts reactions catalyzed by Sc(OTf)3under mild reaction conditions.40Meldrum’s acid is a versatile reagent,which offers several advantages over the conventional electrophiles:the precursors are readily prepared by mono-and difunctionalization at the 5-position,41easily purified,and frequently crystalline.Meldrum’s acids are highly stable with a long shelf life at room temperature.In addition,volatile byproducts,namely carbon dioxide and acetone,are generated in the acylation process.We report herein the full account of our findings on the intramolecular Friedel -Crafts acylation of aromatics with Meldrum’s acid derivatives catalyzed by metal trifluoromethanesulfonates under mild reaction condi-tions (eq 1).The preparation of polysubstituted 1-in-danones from benzyl Meldrum’s acids was investigated thoroughly,and it was shown that a diversity of catalysts can promote the reaction and many functional groups are tolerated by these relatively mild conditions in compari-son to conventional methods.The scope,limitations,and functional group tolerance for a variety of 5-benzyl (enolizable Meldrum’s acids)and 5-benzyl-5-substituted Meldrum’s acids (quaternized Meldrum’s acids),forming 1-indanones and 2-substituted-1-indanones,respectively,in good to excellent yields,are delineated.This method was further applied to the synthesis of 1-tetralones,1-benzosuberones,and the acetylcholinesterase inhibitor donepezil.Results and DiscussionSubstrate Preparation.To examine the proposed methodology of catalytic Friedel -Crafts acylation with Meldrum’s acid derivatives,a ready supply of substrates with appropriately tethered aromatics was required.Meldrum’s acid is a poor nucleophile,yet has a high propensity to overalkylate at the 5-position.Several approaches were therefore used to access substrates with variable tether length and substituents within both the aromatic and aliphatic portions of the molecule (Schemes 3and 4).5-Benzyl Meldrum’s acids unsubstituted at the benzylic position were procured on large scale by reduc-tive alkylation of Meldrum’s acid with benzaldehydes.Reductive alkylation methods,which proceed via a tandem Knoevenagel condensation/alkylidene reduction,were previously reported in the literature with sodium hydrogen telluride,42borane ‚dimethylamine complex,43and triethylammonium formate 44as the reducing agents.45(37)Crow,W.D.;McNab,H.Aust.J.Chem.1979,32,111-121.(38)For a review on the FVP of Meldrum’s acid derivatives,see:(a)Gaber,A.E.-A.O.;McNab,H.Synthesis 2001,2059-2074.(b)Mahidol,C.;Pinyopronpanit,Y.;Radviroongit,S.;Thebtaranonth,C.;Thebtaranonth,Y.J.Chem.Soc.,mun.1998,1382-1383.(39)Few examples of Meldrum’s acid derivatives activation by Lewis (40)For a communication of our initial work,see:Fillion, E.;Fishlock,.Lett.2003,5,4653-4656.(41)(a)Chen,B.-C.Heterocycles 1991,32,529-597.(b)McNab,H.Chem.Soc.Rev.1978,7,345-358.(42)Huang,X.;Xie,mun.1986,16,1701-1707.(43)Hrubowchak,D.M.;Smith,F.X.Tetrahedron Lett.1983,24,4951-4954.S CHEME 1.Intramolecular Friedel -Crafts Acylation with Esters and MalonatesS CHEME 2.FVP of Meldrum’s Acid DerivativesMeldrum’s Acids as Acylating Agents5-Alkyl Meldrum’s acids have been prepared by reducing isopropylidene acylmalonates,via the intermediacy of an alkylidene,with either NaBH 3CN or NaBH 4in AcOH.46,47It was believed that these reported protocols could be combined such that the condensation/reduction sequence could be conveniently executed in one pot by using NaBH 3CN in a buffered medium.48Indeed,benzyl Mel-drum’s acids were successfully prepared from substituted benzaldehydes and Meldrum’s acid with NaBH 3CN at room temperature in the presence of a catalytic amount of piperidinium acetate in EtOH.In most cases,the highly crystalline products were purified,for convenience and practicality,by recrystallization from MeOH or EtOH.5-Benzyl Meldrum’s acid derivatives mono-and disub-stituted at the benzylic position were accessed via 1,4-conjugate addition of aryl Grignard’s to Meldrum’s alkylidenes following literature procedures.49,50Disub-stituted Meldrum’s alkylidenes were prepared by Knoevenagel condensation of Meldrum’s acid with ke-tones (cyclohexanone,tetrahydro-4H -pyran-4-one,tet-rahydrothiopyran-4-one,acetone)in pyridine in the presence of a catalytic amount of piperidine 51or molec-ular sieves,49a or via dehydrative condensation with TiCl 4in CH 2Cl 2.52The alkylidenes were unstable on silica gel and purified by recrystallization from MeOH or EtOH.These cyclization precursors could also be obtained by conjugate addition of alkyl and aryl Grignard’s or dialkyl-aluminum reagents 53to Meldrum’s acid arylidenes.The arylidenes were obtained by the condensation of Mel-drum’s acid with benzaldehydes in water,54or by the addition of aryl Grignards to methoxymethylene Mel-drum’s acid.Substrates with longer tether length (5-ethylbenzyl and 5-propylbenzyl)were synthesized by using Tsuka-moto’s methodology.Carboxylic acids were coupled to Meldrum’s acid with use of DCC to form the isopropy-lidene acylmalonates that were subsequently reduced with NaBH 4in AcOH to the corresponding 5-alkyl Meldrum’s acids.47Symmetrical 5,5-dibenzyl substrates were prepared in one step by reacting Medrum’s acid with 2equivalents of the appropriate benzyl bromide,using K 2CO 3in DMF (Scheme 4).55Unsymmetrical 5-benzyl-5-substituted Mel-drum’s acids were produced from monosubstituted sub-strates in an analogous manner,by alkylation with iodomethane,allyl bromide,propargyl bromide,and various benzyl bromides.56Quaternized Meldrum’s acids were easily isolated in an analytically pure form by extraction and further purified by recrystallization from MeOH.Friedel -Crafts Acylation with Enolizable Mel-drum’s Acids.To study the viability of the proposed intramolecular Friedel -Crafts strategy,substrate 9bear-ing an electron-rich π-nucleophile was selected as the initial and optimal cyclization precursor.Various reaction conditions,Lewis acids (Sc(OTf)3,Dy(OTf)3,Yb(OTf)3,and(46)(a)Rosowsky,A.;Forsch,R.;Uren,J.;Wick,M.;Kumar,A.A.;Freisheim,J.H.J.Med.Chem.1983,26,1719-1724.(b)Nutaitis,C.F.;Schultz,R.A.;Obaza,J.;Smith,.Chem.1980,45,4606-4608.(47)(a)Hin,B.;Majer,P.;Tsukamoto,.Chem.2002,67,7365-7368.(b)Smrcina,M.;Majer,P.;Majerova,E.;Guerassina,T.A.;Eissenstat,M.A.Tetrahedron 1997,53,12867-12874.(48)Following our initial publication,40a one-pot reductive alkyla-tion of Meldrum’s acid with benzaldehydes using NaBH 4was reported,see:Desai,U.V.;Pore,D.M.;Mane,R.B.;Solabannavar,S.B.;Wadgaonkar,mun.2004,34,25-32.(49)(a)Vogt,P.F.;Molino,B.F.;Robichaud,mun.2001,31,679-684.(b)Davies,A.P.;Egan,T.J.;Orchard,M.G.;Cunningham,D.;McArdle,P.Tetrahedron 1992,48,8725-8738.(c)Larcheve ˆque,M.;Tamagnan,G.;Petit,Y.J.Chem.Soc.,mun.1989,31-33.(d)Huang,X.;Chan,C.-C.;Wu,Q.-L.Synth.React.Inorg.Met.-Org.Chem.1982,12,549-556.(e)Huang,X.;Chan,C.-C.;Wu,Q.-L.Tetrahedron Lett.1982,23,75-76.(f)Haslego,M.L.;Smith,mun.1980,10,421-427.(g)For the (51)(a)Baty,J.D.;Jones,G.;Moore,.Chem.1969,34,3295-3302.(b)For the synthesis of methyl alkylidene Meldrum’s acid,see:Ziegler,F.E.;Guenther,T.;Nelson,mun.1980,10,661-665.(52)Brown,R.F.C.;Coulston,K.J.;Eastwood,F.W.;Gatehouse,B.M.;Guddatt,L.W.;Luke,W.;Pfenninger,M.;Rainbow,I.Aust.J.Chem.1984,37,2509-2524.(53)Maas,S.;Stamm,A.;Kunz,H.Synthesis 1999,1792-1798.(54)Bigi,F.;Carloni,S.;Ferrari,L.;Maggi,R.;Mazzacani,A.;Sartori,G.Tetrahedron Lett.2001,42,5203-5205.(55)(a)Desai,D.G.;Mane,R.B.Chem.Ind.(London )1982,809.For alternative methods,see:(b)Dhuru,S.P.;Mohe,N.U.;Salunkhe,mun.2001,31,3653-3657.(c)Shing,T.K.M.;Li,L.-H.;Narkunan,.Chem.1997,62,1617-1622.(d)Chen,S CHEME 3.5-Monosubstituted Meldrum’s AcidSynthesisS CHEME 4.5,5-Disubstituted Meldrum’s AcidSynthesisFillion et al.TMSOTf),Brønsted acids (TfOH and TFA),and solvents ation was catalyzed by Sc(OTf)(Table 1,entry 1).T ABLE 1.Intramolecular Friedel -Crafts Acylation with Enolizable Benzyl Meldrum’s AcidsaA 68%yield was obtained in CH 3CN (2h,8mol %catalyst),and a 72%yield in 1,2-dichloroethane (4.5h,12mol %catalyst).b 90%conversion.c The reaction was run in CH 3CN.d Powdered 5ÅMS (100wt %)were added to the reaction mixture.e The substrate was added by syringe pump,over approximately 8h,to a refluxing solution of Sc(OTf)3,followed by an additional ∼1at reflux.The one-pot procedure yielded the indanone in 36%yield.f The slow addition protocol furnished the indanone in 73%.g The one-pot procedure failed to produce 1-indanone.h A yield of 57%was obtained when the slow addition procedure was used.Meldrum’s Acids as Acylating Agents。
费托合成反应机理研究进展

DOI: 10.19906/ki.JFCT.2023034费托合成反应机理研究进展苏俊超1,刘 勒1,郝庆兰1,刘星辰2,滕波涛1,*(1. 天津科技大学 化工与材料学院, 天津 300457;2. 中国科学院山西煤炭化学研究所 煤转化国家重点实验室, 山西 太原 030001)摘 要:合成气(CO + H 2)经费托合成(Fischer-Tropsch Synthesis, FTS)转化为清洁燃料与化学品是煤炭清洁利用与保障中国能源战略安全的重要途径。
从分子水平深入研究费托合成反应机理,揭示合成气在催化剂表面活化,链增长为C n H x *与C n H x O y *中间体,链终止为烷烃、烯烃、醇、酸产物的基元反应过程是实现费托合成目的产物调节、高性能催化剂理性设计与开发的重要基础,也是催化科学研究的热点与难点。
为深入研究费托合成反应机理,科学家采用反应中间体检测、模型化合物添加、稳态机理动力学、稳态同位素瞬变动力学、第一性原理计算、反应网络等方法从不同的角度、不同层次揭示合成气转化机理。
本综述总结了近百年来费托合成反应机理研究结果,提出了合理的反应机理路线图,并对反应机理研究进行了展望。
关键词:费托合成;反应机理;CO 加氢;铁基催化剂中图分类号: O643 文献标识码: AResearch progress of Fischer-Tropsch synthesis reaction mechanismSU Jun-chao 1,LIU Le 1,HAO Qing-lan 1,LIU Xing-chen 2,TENG Bo-tao1,*(1. College of Chemical Engineering and Materials , Tianjin University of Science and Technology , Tianjin 300457, China ;2. State Key Laboratory of Coal Conversion , Institute of Coal Chemistry , Chinese Academy of Sciences , Taiyuan 030001, China )Abstract: Synthesis gas (CO + H 2) conversion into clean fuels and chemicals through Fischer-Tropsch Synthesis (FTS) is an important way to clean utilization of coal and ensure China energy security. Investigation of FTS reaction mechanism at the molecular level, including of activation of synthesis gas on catalyst surface, the chain growth via C n H x * and C n H x O y *, as well as the chain termination into alkanes, olefins, alcohols, and acids, is the key to the regulation of FTS products, the rational design and development of high-performance catalysts. It is also a hot and difficult point in catalysis science. To study FTS reaction mechanism, intermediate detection, modeling compound addition, steady-state kinetics based on reaction mechanism, steady-state isotope transient kinetic analysis (SSITKA), first-principles calculations, and reaction networks, etc. have been applied to reveal the mechanism of synthesis gas conversion. This paper systematically summarizes the research results of reaction mechanism over the past century, proposes a reasonable route map for FTS reaction, and gives a prospection of the research on FTS mechanism.Key words: Fischer-Tropsch Synthesis (FTS);reaction mechanism ;CO hydrogenation ;iron-based catalyst费托合成(Fischer-Tropsch Synthesis)是指合成气(CO + H 2)在催化剂表面转化为C 1–C n 的烯烃及烷烃的反应,同时产生醇、醛、酸、酯和酮等含氧有机化合物。
碘催化的有机化学反应

90-95%
Bioorganic & Medicinal Chemistry Letters 17 (2007) 621–623
Iodine catalyzed preparation of amidoalkyl naphthols in solution and under solvent-free conditions
Tetrahedron Letters 47 (2006) 4065–4068
1,1-二乙酸酯的生成反应
J. Org. Chem. 2006, 71, 8283-8286
Addition
I2 as an efficient catalyst in ionic Diels–Alder reactions of unsaturated acetals
O.L.2006.Vol. 8, No. 12。2491-2494
Light-Induced, Iodine-Catalyzed Aerobic Oxidation of Unsaturated Tertiary Amines
J. Org. Chem. 1990,55, 3679-3682
Aerobic photocatalytic oxidation of activated benzylic and allylic alcohols to carbonyl compounds catalyzed by molecular iodine
Journal of Molecular Catalysis A: Chemical 278 (2007) 38–41
双键重排,构型翻转
J. Org. Chem. 2001, 66, 8135-8138
杂环的合成
手性季鏻盐相转移催化剂在不对称反应中的应用_喻理德

, 并应用于环戊 酮 -β -羰 基 酯 的 不 对 称 烷 基 化 反
仅得到中等的对映选择性( 图式 2 ) 。 研究发现, 应, 产率随反应时间的延长明显增加, 在 20 ℃ 反应时 间 由 24 h 增 加 为 168 h , 产 率 由 43% 增 加 到 80% , 产 物的 ee 值 基 本 不 变 。 此 外, 产 物 的 ee 值 受 温 度 的
Yu Lide 1 Cui Hanfeng 1 * * Fan Hao 1 Ren Shuhui 2 Lin Yan 1
( 1. College of Pharmacy ,Jiangxi University of Traditional Chinese Medicine ,Nanchang 330004 ,China ; 2. College of Clinical Medicine ,Jiangxi University of Traditional Chinese Medicine ,Nanchang 330004 ,China ) Abstract Chiral phase-tranfer catalysis plays an important role in modern asymmetric synthesis and organic
[8] aldol 反 应[9] 、 Darzens 展到 不 对 称 Michael 加 成 、
手性季鏻盐作为一类重要的手性相转移催化
[13 , 14]
图式 2 and 3
季鏻盐 2 和 3 催化的不对称烷基化反应 Asymmetric alkylation reaction catalyzed by 2
收稿: 2012 年 10 月,收修改稿: 2012 年 12 月 * 江西省青年科学基金项目( No. 20122BAB213006 ) 、 江西省 教 育 厅 青 年 基 金 项 目 ( No. GJJ12538 ) 和 江 西 中 医 学 院 博 士 启 动基金项目( No. Y049 ) 资助 ** Corresponding author e-mail : cuihanfeng@ 126. com
三氟乙基硫醚(亚砜)类化合物的设计、合成及其杀螨活性研究

第1$卷第1期 2019年2月现代农药Modern AgrochemicalsVol.18 No.1Feb.2019♦创制与主测!三氟乙基硫醚(亚砜2类化合物的设计、合成及其杀蜗活性研究张坡,张熹晗,张石鑫,郎钰莹,张静F,张立新G(沈阳化工大学功能分子研宄所,辽宁省绿色功能分子设计与开发重点实验室,沈阳110142)摘要:为了寻找高效、低毒、低残留的环境友好型杀螨剂,应用“骨架跃迁”策略,设计、合成了一系列结构新颖的三氟乙基硫醚(亚砚)类化合物。
其结构经1H NMR、LC-MS等确证。
初步生物活性数据表明,部分化合物对朱砂叶螨具有较好的生物活性,其中化合物FA-6及FA-7在质量浓度6.25 mg/L时防效可达70%,可作为先导化合物进行深入研究。
关键词:三氟乙基硫醚(亚砚)类;骨架跃迁;合成;杀螨活性中图分类号:TQ 450.1+1 文献标志码:A doi:10.3969/j.issn.1671-5284.2019.01.003Design and Synthesis of Trifluoro Ethyl Thioether (Sulfoxide)Derivativesand Their Acaricidal ActivityZhang Po, Zhang Xi-han, Zhang Shi-xin, Lang Yu-ying, Zhang Jing*, Zhang Li-xin* (Institute of Functional Molecules, Shenyang University of Chemical Technology, Liaoning Province Key Laboratory of Green Functional Molecular Design and Development, Shenyang 110142, China)Abstract:In order to discover new acaricides with high activity, low toxicity and low residue, a series of novel trifluoro ethyl thioether (sulfoxide) derivatives were designed and synthesized utilizing the "scaffold hopping". The targeted compounds were characterized by 1H NMR, LC-MS. Preliminary bioactivity data indicated that several compounds had good acaricidal activity against Tetranychus cinnabini.The mortality rates of compound FA-6 and FA-7 were more than 70% at a dose of6.25 mg/L, and the two compounds could be used as lead compounds for further research.Key words:trifluoro ethyl thioether (sulfoxide) derivative; scaffold hopping; synthesis; acaricidal activity农业害螨属节肢动物,大多 害螨物 害,果树、棉花、蔬菜等大量产,重_。
甲氧基乙酸甲酯的合成及应用进展

第49卷第10期2021年5月广㊀州㊀化㊀工Guangzhou Chemical Industry Vol.49No.10 May.2021甲氧基乙酸甲酯的合成及应用进展陈春玉,王少楠,胡㊀迎(西南化工研究设计院有限公司,四川㊀成都㊀610225)摘㊀要:甲氧基乙酸甲酯不仅是一种合成维生素B6㊁周效磺胺等药物的重要原材料,而且还是更经济合理合成乙二醇的重要原材料㊂当前制备工艺主要包括甲醛和甲酸甲酯偶联法㊁氯乙酸类和甲醇钠取代法㊁乙二醇单甲醚氧化法和甲缩醛羰基法㊂分析了制备甲氧基乙酸甲酯的方法的优缺点,综述了甲氧基乙酸甲酯在应用领域的研究进展,并对其发展趋势和应用前景作了展望㊂关键词:甲氧基乙酸甲酯;合成;应用㊀中图分类号:O622.5㊀文献标志码:A文章编号:1001-9677(2021)010-0014-02 Synthesis and Application of PolymethylmethacrylateCHEN Chun-yu,WANG Shao-nan,HU Ying(Southwest Research&Design Institute of the Chemical Industry Co.,Ltd.,Sichuan Chengdu610225,China)Abstract:Methyl methoxyacetateis not only an important raw material for vitamin B6,sulfanilamide and other drugs, but also an important even more economical and reasonable raw material for ethylene glycol.Current productions include mainly formaldehyde and methyl formate coupling method,chloroacetic acid and sodium methoxide substitution method, ethylene glycol monomethyl ether oxidation method and methylal carbonyl method.The advantages and disadvantages of eachmethods,the research progress on application of methyl methoxyacetate and the developing trend,as well as prospects for future application of methyl methoxyacetate,were presented.Key words:methyl methoxyacetate;synthetic;application甲氧基乙酸甲酯(下简称MMAc)是一种非常重要的精细化学品,具有酯的性质,常用于水解反应或加成反应,应用面广,比如:是手性胺类化合物的拆分剂,也是多种化工产品的中间体,同时在医药方面也具有很大用途,例如合成维生素B6㊁周效磺胺等药物;此外它也是高效合成下游产品乙二醇重要的前驱体原料㊂1㊀MMAc的合成方法MMAc的合成方法比较多,按照原料划分,有甲醛和甲酸甲酯偶联法㊁氯乙酸类和甲醇钠取代法㊁乙二醇单甲醚氧化法和甲缩醛羰基法等㊂(1)甲醛和甲酸甲酯偶联法甲醛和甲酸甲酯在酸催化剂条件下反应生成MMAc,此法分三步进行,第一步是甲酸甲酯在酸催化剂条件下分解成甲醇和一氧化碳;第二步是甲醇在酸催化剂条件下,醇羟基与氢离子结合生成佯盐的过渡态,甲醛在酸催化剂条件下,醛基与氢离子结合形成质子化的过渡态;第三步是两种过渡态分别与CO结合,发生羰基化反应,最终生成目标产物㊂基于此合成机理,2006年,王克冰等[1]报道了以三聚甲醛和甲酸甲酯为原料,在CF3SO3H酸催化条件下,110ħ反应2h,MMAc的收率为42.72%,由于此反应副产物多,所以收率很低㊂(2)氯乙酸类和甲醇钠取代法氯乙酸类化合物和甲醇钠反应生成MMAc,是卤代烃与醇钠反应制备混合醚的威廉姆逊合成法,反应机理为醇羟基在碱性条件下形成醇负离子,进攻卤代烃的碳正中心,卤代烃脱去卤素形成醚键㊂1989年,中国专利[2]报道了一种制备甲氧基乙酸的方法,采用氯乙酸和甲醇钠为原料,在40ħ条件下反应,得到甲氧基乙酸收率为91%;然后甲氧基乙酸与甲醇酯化后生成MMAc㊂CH3ONa+ClCH2COOHңCH3OCH2COOH+NaCl CH3OOH+CH3OCH2COOHңCH3OCH2COOCH3+H2O 2002年,徐志珍等[3]报道以氯乙酸甲酯和甲醇钠为原料,在80ħ条件下反应4h,合成的MMAc收率为96.2%㊂CH3ONa+ClCH2COOCH3ңCH3OCH2COOCH3+NaCl此法中使用的甲醇钠,价格比较昂贵,而且容易与空气中的水蒸气反应,不易保存,故不是一条经济合理的工业化合成路线㊂(3)乙二醇单甲醚氧化法以乙二醇单甲醚为原料合成MMAc,分两步完成,第一步是乙二醇单甲醚氧化生成甲氧基乙酸,第二步是甲氧基乙酸与甲醇发生酯化反应,生成目标产物㊂第49卷第10期陈春玉,等:甲氧基乙酸甲酯的合成及应用进展15㊀2015年,中国专利[4]报道以Pt/C为催化剂,O2为氧化剂,水为溶剂,在70ħ下反应7h,则乙二醇单甲醚氧化制得甲氧基乙酸,收率为91%;然后甲氧基乙酸再与甲醇酯化,生成MMAc㊂3CH3CO(CH2)2OH+3O2ң4CH3OCH2COOHCH3OCH2COOH+CH2OHңCH3OCH2COOCH3+H2O此氧化法中虽然反应收率较高,但是反应中使用了贵金属,成本高,同时反应时间较长,不是一条合适的工业化路线㊂(4)甲缩醛羰基法甲缩醛羰基法是迄今为止研究的最多的制备MMAc的方法㊂以甲缩醛为原料合成MMAc,是一种Koch型机理,即CO 与酸中氢正离子结合后,进攻甲缩醛中的仲碳,使仲碳失去氢正离子后,完成在仲碳上的插入CO的羰基化反应㊂3CH3OCH2OCH3+COңCH3OCH2COOCH3+2CH3OCH3+HCOOCH3 2015年,中国专利[5]报道在一价铜改性的磺酸型聚苯乙烯交联树脂催化剂条件下,甲缩醛与CO在120ħ下发生羰基化反应,生成最终产物MMAc,反应收率为87%左右㊂2016年,中国专利[6]报道在固体酸催化剂和多聚甲醛的条件下,110ħ反应6h,含水甲缩醛(含水量2%)与CO生成主产物MMAc,反应收率为72%左右㊂2020年,张晓艳[7]报道以ZSM-5分子筛为催化剂,110ħ下反应7h,甲缩醛和CO生成的MMAc收率为69%左右㊂甲缩醛简单易得且价格便宜,是合成MMAc的最佳原料,但是由于甲缩醛易发生歧化反应,副产物较多,只有通过研究不同催化剂来提高MMAc的选择性,才能走出一条清洁生产㊁经济合理的工业化路线㊂综上所述,尽管合成MMAc的路线很多,但由于反应条件苛刻,反应过程复杂,副产物比较多,收率比较低,所用催化剂难回收,分离成本高,易腐蚀设备,耗能高,污染环境,不利于工业化高质量生产㊂故迫切需要找出一种低能耗㊁高效率㊁低污染的生产MMAc的方法㊂2㊀MMAc的应用研究MMAc是一种重要的医药中间体和精细化工产品中间体,能在一定条件下转化为其衍生物维生素B6㊁周效磺胺以及乙二醇等药物或化工产品,具有广泛的用途㊂(1)医药领域MMAc经取代㊁环化等过程可以合成维生素B6,该方法是1939年Harris S A等[8]开发的,简称 吡啶酮法 ㊂维生素B6是人体必需的维生素之一,是人体内约140种酶的辅酶,参与催化80多种生化反应,是人体内许多代谢反应不可或缺的指挥者,还可以预防妇产科疾病以及在保健方面也有一定的作用,所以MMAc在制备维生素B6过程中有着悠久的历史㊂另外,由MMAc经克氏反应㊁酰胺化环合反应㊁氯化反应㊁缩合反应㊁甲氧基化反应合成周效磺胺㊂周效磺胺治疗各种细菌感染,特别适用于皮肤感染㊁肺及上呼吸道感染㊁细菌性痢疾,还治疗疟疾㊁麻疯病,与异烟肼合用治疗肺结核[9]㊂由此可见,MMAc在制备周效磺胺过程中发挥着重要作用,相信在不久的将来,越来越多的以MMAc为原料的药物将会被合成㊂(2)化工领域MMAc除了可以合成维生素B6㊁合成周效磺胺外,更多的使用价值是作为乙二醇的前体,即MMAc通过加氢㊁水解两步高效制成乙二醇㊂乙二醇是国家重要的化工原料和战略物资,可用作溶剂㊁防冻剂以及合成涤纶的原料㊂在溶剂方面,乙二醇常可代替甘油使用,在制革和制药工业中分别用作水合剂和溶剂,也可用于玻璃纸㊁纤维㊁皮革㊁粘合剂的湿润剂㊂在防冻剂方面,乙二醇60%的水溶液凝固点为-40ħ,可用作冬季汽车散热器的防冻剂和飞机发动机的致冷剂㊂乙二醇也是合成聚酯涤纶㊁纤维和化妆品的原料㊂乙二醇的高聚物聚乙二醇(PEG)是一种相转移催化剂,用于细胞融合;乙二醇的硝酸酯是一种炸药,因此,MMAc作为合成下游产品乙二醇的应用前景十分广阔㊂3㊀结㊀语通过对MMAc的合成方向及应用方面的介绍,可以看出,虽然MMAc在国内外研究较多,但是至今在工业化生产道路上还是存在,如何提高其反应收率,降低生产成本,减少环境污染等问题㊂尤其是在廉价的甲缩醛法越来越显现出其特有的优越性的条件下,但是甲缩醛法的研究工作仍然进展缓慢,且不是很理想㊂一方面,甲缩醛容易发生歧化反应,使得反应副产物多,后处理困难,不利于环保要求,如何尽可能多的得到目标产物MMAc,以此提高反应收率也是迫切需要解决的问题;另一方面,甲缩醛的羰基化反应受酸强度的影响非常大,较强的酸具有较强的催化活性,但是强酸对设备腐蚀严重,所以就需要通过寻找合适的催化剂或助催化剂来解决,还需要通过探索最佳化学计量比㊁改变反应时间或温度来提高反应的收率与纯度,以此取得较好的效果㊂因此,发展高效㊁温和的催化体系,实现生产成本低,环境污染小,适合于MMAc的工业化生产路线无论从经济利益还是环境影响两个方面,都具有重要意义㊂总之,随着科技的进步,MMAc的应用领域会越来越广,因此对其合成方向及应用领域的深入开发和研究还是十分有价值的㊂参考文献[1]㊀王克冰,姚洁,王越,等.酸催化剂在甲醛与甲酸甲酯偶联反应中的作用研究[J].天然气化工2006,31(6):19-21.[2]㊀奥戈奇㊃巴尔,瑞奇㊃劳尤什,佩伊瓦㊃耶诺,等.甲氧基乙酸的制备方法[P].中国:1039798A.1989-07-14.[3]㊀徐志珍,潘鹤林.甲氧基乙酸甲酯合成工艺研究[J].上海化工,2002,27(7):14-15.[4]㊀聂俊琦,李雄,王亦鸣,等.一种甲氧基乙酸的制备方法[P].中国:104892390A.2015-04-17.[5]㊀李晓明,吕建刚,刘波,等.甲氧基乙酸甲酯催化剂[P].中国:106582833A.2015-10-14.[6]㊀石磊,龚页境,王玉鑫.利用工业含水原料甲缩醛制备甲氧基乙酸甲酯的方法[P].中国:106518676A.2016-09-05.[7]㊀张晓艳.ZSM-5分子筛催化甲缩醛气相羰基化制备甲氧基乙酸甲酯研究[D].太原:山西大学化学化工学院,2020.[8]㊀Harris S A,Folkers K.Synthesis of vitamin B6[J].J.Am.Chem.Soc.,1939,61:1245-1247.[9]㊀上海化学工业设计院.周效磺胺设计简介[J].医药农药工业设计,1972(4):1-7.。
[1,2]-Wittig重排反应的研究及应用
![[1,2]-Wittig重排反应的研究及应用](https://img.taocdn.com/s3/m/74499e32eefdc8d376ee3271.png)
ratllerlow yields aIld t11e restricted砌ge of蚰bs仃ates,t11erefore it is lligllly desi胁le
noVel舭gy t0 deVelop
t0 improve it.
111 “s pap%、№reviewed 陀cent deVelopments of tlle 【l,2】一Winig
Vl
杭州师范大学硕士学位论文
l文献综述
l 文献综述
1.1引言
【1,2】-Wittig重排反应最早是由是Winig(1979年诺贝尔化学奖得主)和
L611n1猢在1942年发表的文章中提出来的。【l,2】一wittig重排反应是合成多取代
醇的一个较好的方法,该反应是在强碱的作用下(如丁基锂)在低温下反应再水 解之后得到高级醇。该反应在化学键形成或者断裂过程中具有独特的高立体选择 性和高效性,因此广泛应用于有机合成和药物中间体的合成,这就引起了广大化 学家的浓厚兴趣,特别是20世纪50年代至70年代许多化学家对该反应做了很 多相关的研究工作,尤其是针对该反应的机理做了大量的工作,目前被公认的 【1,2】一wittig重排反应的机理就是在那时候提出来的,可惜他们在研究过程中发 现该反应对底物的要求是相当苛刻的,并不是所有的醚类化合物都适合 【1捌-Wittig重排反应,一般来说含烯丙基或者苯甲基(苄基)的醚类化合物相 对的容易反应一些,但其产物的收率也是相当的低,所以尽管该反应在有机合成 具有很好的应该价值但其存在的缺陷,使化学家对该反应渐渐的失去了兴趣,目 前只有少数的化学家还在继续研究该反应。
inVeStigate tlle Wittig l沁觚锄gement of acet),l compounds derived丘℃m t11e
以钛酸异丙酯为原料合成Ti(Ⅳ)取代的Keggin型多金属氧酸盐

以钛 酸 异 丙 酯 为 原 料 合 成 T ( ) 代 的 iⅣ 取
Ke gn型 多 金 属 氧 酸 盐 gi
刘 红 霞。 杨 春
( 南 京 师 范 大 学 化 学 与 环 境科 学 学 院 “
周 家 宏
南京 )
南 京 20 9 ; 京 师 范 大学 分 析测 试 中 心 10 7 南
20 —00 06 1-6收稿 .0 70 -8修 回 20 -10 国家 自然 科 学 基 金 资助 项 目 (0 70 7 , 苏 省 教 育 厅 自然 科学 基 金资 助 项 目 (4 J 106 ), 家人 事 部 留学 人 员 科 技 活 动 项 目 2 4 33 ) 江 0 K B 50 9 国
摘
要
以钛酸异丙酯为钛源 , 在酸性条件 ( H=2 下合成 了一钛取代的钨磷 、 p ) 钨硅 多金属 氧酸盐 ( uN B 或
MeN 盐 )用 I PN 、H N , R、 MR MR和 T —T G D A测试技术对样 品的结构 、 阳离子 含量等进行 了表 征 , 并对 样 品存在的形态( 二聚体 、 单体和质子化单体 ) 以及形态 的转化进行 了研究 。 R光谱 显示 ,i 代的钨磷 酸盐和 I T取 钨硅酸盐均具有 T— —i 构 , 明它们是 T 取代多金属氧酸盐的二聚体 , i T结 O 表 i 或含有 T 取 代多金属氧酸盐 的二 i 聚体 , 采用 T — T G D A测得的 阳离 子( eN 含量也证实了这一点。 PN M ) MR和 MR进一步揭示 ,i HN T 取代的 钨磷酸盐是一质子化单体 和二聚体 的混合物 。实验结 果还表明 , 酸性介质是生成质子化单体和二 聚体 的必要
维普资讯
第2 4卷 第 9期
合成双硝基二苯硫醚的方便方法

合成双硝基二苯硫醚的方便方法合成双硝基二苯硫醚的方便方法1. 引言合成双硝基二苯硫醚是有机化学中一项重要的研究课题。
它具有广泛的应用领域,包括医药、材料科学和农药等。
然而,以往的研究方法存在着一些限制,如繁琐的操作步骤、低产率和高代价等问题。
本文旨在介绍一种方便且高效的方法,可用于合成双硝基二苯硫醚。
2. 方法本方法使用两步反应来合成双硝基二苯硫醚:首先是芳香硝化反应,然后是硫醚化反应。
2.1 芳香硝化反应将苯胺溶于硫酸中,并用浓硝酸缓慢滴加至反应体系中。
反应进行时,需保持温度在低于10°C的条件下,并搅拌反应混合物以提高反应的均匀性。
在芳香硝化反应中,硝化剂的选择对反应结果至关重要。
通常使用浓硝酸和浓硫酸的混合物作为硝化剂,这可以确保反应的高选择性和收率。
2.2 硫醚化反应经过芳香硝化反应后,得到的芳香硝基化合物需要进行硫醚化反应。
为此,将硝基化合物与硫化钠或硫醇反应,待反应结束后,通过过滤、结晶等方法得到目标产物。
硫醚化反应在较温和的条件下进行,反应过程中需控制温度和反应时间,以避免产物的降解或副反应的发生。
3. 结果与讨论利用本方法,合成了一系列双硝基二苯硫醚化合物,并对其进行了表征与分析。
对产物进行核磁共振谱(NMR)和质谱(MS)等分析手段的综合利用,确认了所合成的化合物的结构。
通过优化反应条件,我们还探索了反应的最佳化条件,以提高产率和减少副产物的形成。
4. 总结与展望本文介绍了一种合成双硝基二苯硫醚的方便方法。
该方法使用两步反应,包括芳香硝化反应和硫醚化反应,并在反应条件和反应物选择上进行了优化。
通过对产物的分析和表征,我们验证了本方法的有效性和可行性。
探索了反应条件的优化,将进一步提高产率和降低成本。
该方法也为研究双硝基二苯硫醚的应用提供了新的合成途径。
对于我个人来说,双硝基二苯硫醚的合成方法的研究具有重要的理论意义和应用前景。
作为有机化学领域的研究人员,我认为合成方法的优化和改进是实现高效合成和应用探索的关键。
基于Staudinger反应的荧光探针用于检测三苯基膦_赵洲
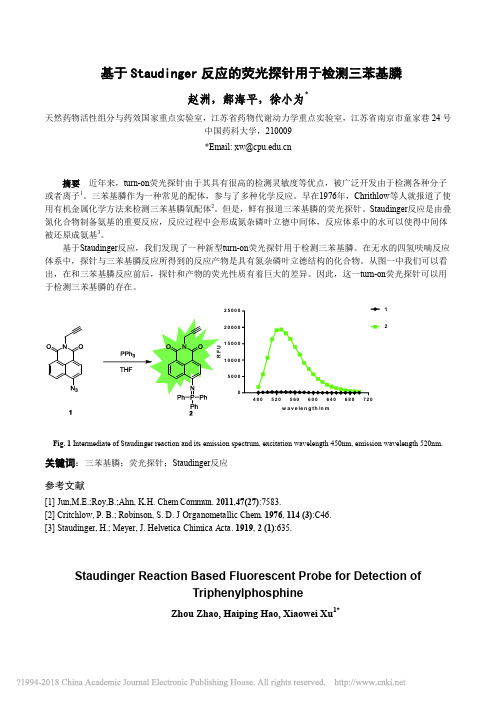
基于Staudinger 反应的荧光探针用于检测三苯基膦赵洲,郝海平,徐小为*天然药物活性组分与药效国家重点实验室,江苏省药物代谢动力学重点实验室,江苏省南京市童家巷24号中国药科大学,210009*Email: xw@摘要 近年来,turn-on 荧光探针由于其具有很高的检测灵敏度等优点,被广泛开发由于检测各种分子或者离子1。
三苯基膦作为一种常见的配体,参与了多种化学反应。
早在1976年,Chrithlow 等人就报道了使用有机金属化学方法来检测三苯基膦氧配体2。
但是,鲜有报道三苯基膦的荧光探针。
Staudinger 反应是由叠氮化合物制备氨基的重要反应,反应过程中会形成氮杂磷叶立德中间体,反应体系中的水可以使得中间体被还原成氨基3。
基于Staudinger 反应,我们发现了一种新型turn-on 荧光探针用于检测三苯基膦。
在无水的四氢呋喃反应体系中,探针与三苯基膦反应所得到的反应产物是具有氮杂磷叶立德结构的化合物。
从图一中我们可以看出,在和三苯基膦反应前后,探针和产物的荧光性质有着巨大的差异。
因此,这一turn-on 荧光探针可以用于检测三苯基膦的存在。
4805205606006406807205001000150020002500w a v e le n g th /n m 12Fig. 1 Intermediate of Staudinger reaction and its emission spectrum, excitation wavelength 450nm, emission wavelength 520nm. 关键词:三苯基膦;荧光探针;Staudinger 反应参考文献[1] Jun,M.E.;Roy,B.;Ahn, K.H. Chem Commun . 2011,47(27):7583.[2] Critchlow, P. B.; Robinson, S. D. J Organometallic Chem . 1976, 114 (3):C46.[3] Staudinger, H.; Meyer, J. Helvetica Chimica Acta . 1919, 2 (1):635.Staudinger Reaction Based Fluorescent Probe for Detection ofTriphenylphosphineZhou Zhao, Haiping Hao, Xiaowei Xu 1*State Key Laboratory of Natural Medicines, Key Lab of Drug Metabolism and Pharmacokinetics, China Pharmaceutical University, Nanjing,Tongjiaxiang 24#, 210009We have successfully developed a novel turn-on probe to detect the absence of triphenylphosphine based on Staudinger reaction. The probe has almost no fluorescence emission under the excitation of 450nm wave. After reaction with triphenylphosphine in the environment of anhydrous tetrahydrofuran, the product showed good fluorescent properties. Therefore, the probe can be used to detect triphenylphosphine.。
