AM630_164178-33-0_DataSheet_MedChemExpress
磁力架说明书_Magnetic Stand Manual_MCE
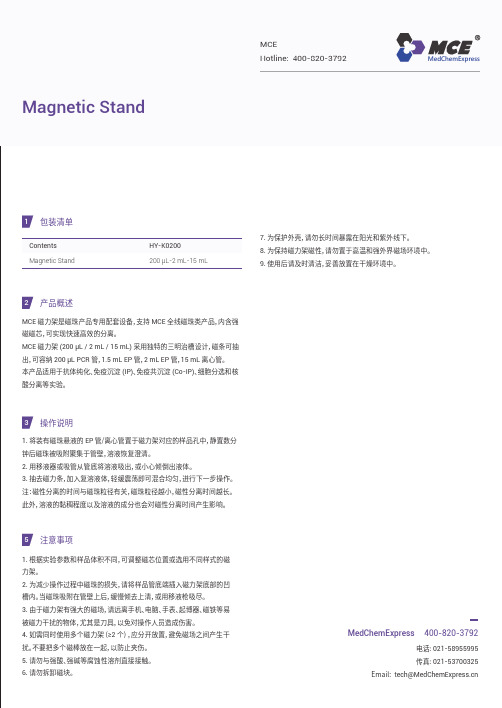
1包装清单产品概述MCE 磁力架是磁珠产品专用配套设备,支持 MCE 全线磁珠类产品,内含强磁磁芯,可实现快速高效的分离。
MCE 磁力架 (200 μL / 2 mL / 15 mL ) 采用独特的三明治槽设计,磁条可抽出,可容纳 200 μL PCR 管,1.5 mL EP 管,2 mL EP 管,15 mL 离心管。
本产品适用于抗体纯化、免疫沉淀 (IP )、免疫共沉淀 (Co-IP )、细胞分选和核酸分离等实验。
2操作说明31. 将装有磁珠悬液的 EP 管/离心管置于磁力架对应的样品孔中,静置数分钟后磁珠被吸附聚集于管壁,溶液恢复澄清。
2. 用移液器或吸管从管底将溶液吸出,或小心倾倒出液体。
3. 抽去磁力条,加入复溶液体,轻缓震荡即可混合均匀,进行下一步操作。
注:磁性分离的时间与磁珠粒径有关,磁珠粒径越小,磁性分离时间越长。
此外,溶液的黏稠程度以及溶液的成分也会对磁性分离时间产生影响。
5注意事项1. 根据实验参数和样品体积不同,可调整磁芯位置或选用不同样式的磁力架。
2. 为减少操作过程中磁珠的损失,请将样品管底端插入磁力架底部的凹槽内。
当磁珠吸附在管壁上后,缓慢倾去上清,或用移液枪吸尽。
3. 由于磁力架有强大的磁场,请远离手机、电脑、手表、起博器、磁铁等易被磁力干扰的物体,尤其是刀具,以免对操作人员造成伤害。
4. 如需同时使用多个磁力架 (≥2 个) ,应分开放置,避免磁场之间产生干扰。
不要把多个磁棒放在一起,以防止夹伤。
5. 请勿与强酸、强碱等腐蚀性溶剂直接接触。
6. 请勿拆卸磁块。
7. 为保护外壳,请勿长时间暴露在阳光和紫外线下。
8. 为保持磁力架磁性,请勿置于高温和强外界磁场环境中。
9. 使用后请及时清洁,妥善放置在干燥环境中。
Magnetic StandMedChemExpress MedChemExpress 400-820-3792 电话: ************ 传真: ************Email: t *********************MCE Hotline: 400-820-3792Contents HY-K0200Magnetic Stand 200 μL-2 mL-15 mL。
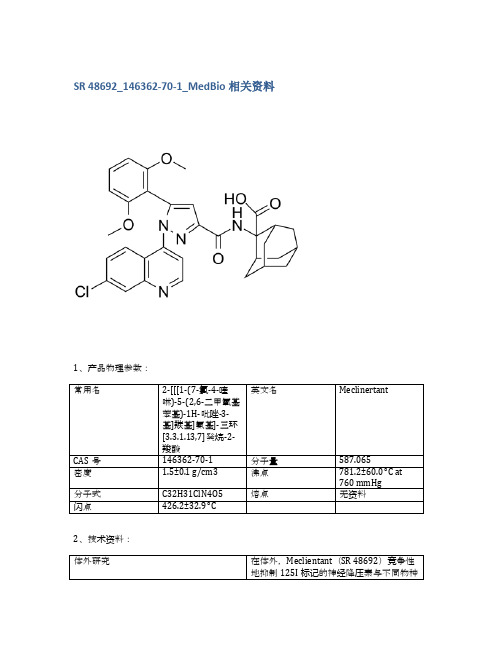
SR 48692_146362-70-1_MedBio相关资料
C32H31ClN4O5
熔点
无资料
闪点
426.2±32.9 °C
2、技术资料:
体外研究
在体外,Meclientant(SR 48692)竞争性地抑制125I标记的神经降压素与不同物种脑组织中的高亲和力结合位点的结合,IC50值分别为0.99 nM(豚鼠)、4.0 nM(大鼠中脑细胞)、7.6 nM(转染克隆的高亲和力大鼠脑受体的COS-7细胞),13.7nm(新生小鼠脑)、17.8nm(新生人脑)、8.7nm(成年人脑)和30.3nm(HT-29细胞)。Meclientant也能从低亲和力的左旋卡托斯汀敏感结合位点置换125I标记的神经降压素,但浓度较高(成年小鼠脑34.8nm,成年大鼠脑82.0nm)[1]。在豚鼠纹状体切片中,Meclientant阻断了神经降压素刺激的[3H]多巴胺的K+诱发释放,其强度(IC50=0.46 nM)与其结合亲和力相关[1]。
477313-09-0
10mg
≥98%
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
MED13083
CGS 15943
CGS 15943
104615-18-1
10mg
≥98%
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
MED13199
O-1602
O-1602
317321-41-8
25mg
≥98%
≥98%
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
MED13022
A 61603 hydrobromide
630103-23-0_MRS 2500 tetraammonium salt基本简述MedBio

145108-58-3
50mg
≥98%
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
MED13232
Rec 15/2615 dihydrochloride
Rec 15/2615 dihydrochloride
173059-17-1
50mg
≥98%
品牌
货号
中文名称
≥98%
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
MED13000
BRL 37344, sodium salt
BRL 37344, sodium salt
127299-93-8
10mg
≥98%
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
MED12866
SB 328437
SB 328437
英文名称
CAS
包装
纯度
MedBio
MED13439
N-Acetylserotonin
N-Acetylserotonin
1210-83-9
100mg
≥98%
cas
1、产品物理参数:
常用名
MRS2500 tetraammonium
英文名
MRS2500 tetraammonium
CAS号
630103-23-0
分子量
629.285
密度
无资料
沸点
无资料
分子式
C13H30IN9O8P2
朗道多项生化定值质控

0843PAGE 1 OF 24LIQUID ASSAYED CHEMISTRY CONTROL PREMIUM PLUS - LEVEL 1 (LIQ CHEM ASY PREMIUM PLUS 1)Cat. No . LAL 4213 Lot No . 153UL Size : 12 x 5 ml Expiry : 2015-02INTENDED USEThis product is intended for in vitro diagnostic use, in the quality control of diagnostic assays. The Liquid Assayed Chemistry Control Premium Plus is for the control of accuracy.DEVICE DESCRIPTIONThe Liquid Assayed Chemistry Control Premium Plus is supplied at 3 levels, level 1, 2 and 3. Target values and ranges are supplied for the analytes listed in the values section at all three levels.SAFETY PRECAUTIONS AND WARNINGSFor in vitro diagnostic use only. Do not pipette by mouth. Exercise the normal precautions required for handling laboratory reagents.Human source material from which this product has been derived has been tested at donor level for the Human Immunodeficiency Virus (HIV 1, HIV 2) antibody, Hepatitis B Surface Antigen (HbsAg), and Hepatitis C Virus (HCV) antibody and found to be NON-REACTIVE. FDA approved methods have been used to conduct these tests.However, since no method can offer complete assurance as to the absence of infectious agents, this material and all patient samples should be handled as though capable of transmitting infectious diseases and disposed of accordingly.Health and Safety Data Sheets are available on request.STORAGE AND STABILITY OPENED: Store refrigerated (+2ºC to + 8ºC). Thawed serum is stable for 7 days at +2ºC to +8ºC, with the followingexceptions: Troponin T is stable for 3 days at +2ºC to +8ºC. Only the required amount of product should be removed. After use, any residual product should NOT BE RETURNED to the original vial.UNOPENED: Store frozen at -20ºC to -70ºC. Stable to expiration date printed on individual vials (see Limitations).LIMITATIONSFor Total Acid Phosphatase, the material should be stabilised by adding 1 drop (25 µl – 30 µl) of 0.7M Acetic acid solution to 1ml of the serum after thawing. After stabilisation, Total Acid Phosphatase is stable for 7 days at +2ºC to +8ºC. Bilirubin in the serum is light sensitive and it is recommended that the serum is stored in the dark.ALT, Total Acid Phosphatase, Alkaline Phosphatase, Total and Direct Bilirubin values may gradually decrease during the products shelf life.Bacterial contamination of the thawed serum will cause reductions in the stability of many components. The control should not be used as a calibration material.PREPARATION1. Allow the frozen control to thaw at room temperature (+15ºC to +25ºC) until completely thawed. Swirl the contents to ensurehomogeneity.2. Refer to the Control section of the individual analyser application.3. Refrigerate any unused material. Prior to reuse, mix contents thoroughly.MATERIALS PROVIDEDLiquid Assayed Chemistry Control Premium Plus - Level 1 12 x 5 mlMATERIALS REQUIRED BUT NOT PROVIDED NoneASSIGNED VALUESEach lot of serum is submitted to a number of external laboratories. Values are assigned from a consensus of results obtained by these laboratories and internal testing conducted at Randox Laboratories Ltd. With each batch, a control range is provided for individual parameters and each parameter method.If an instrument specific value is not available, refer to the Mean of all Instruments section. If necessary, contact Randox Laboratories – Customer Technical Services, Northern Ireland, Tel: +44 (0) 28 9445 1070 or email Technical.Services@29 Nov 13 rwPage 2 of 2429/11/2013___________________________________________________________________________________________________RANDOX Laboratories Ltd., 55 Diamond Road, Crumlin, Co. Antrim, United Kingdom, BT29 4QYTel: +44 (0) 28 9442 2413 Fax: +44 (0) 28 9445 2912Email: applications@ Website: Page 3 of 24 29/11/2013___________________________________________________________________________________________________RANDOX Laboratories Ltd., 55 Diamond Road, Crumlin, Co. Antrim, United Kingdom, BT29 4QYTel: +44 (0) 28 9442 2413 Fax: +44 (0) 28 9445 2912Email: applications@ Website: Page 4 of 24 29/11/2013___________________________________________________________________________________________________RANDOX Laboratories Ltd., 55 Diamond Road, Crumlin, Co. Antrim, United Kingdom, BT29 4QYTel: +44 (0) 28 9442 2413 Fax: +44 (0) 28 9445 2912Email: applications@ Website: Page 5 of 2429/11/2013___________________________________________________________________________________________________RANDOX Laboratories Ltd., 55 Diamond Road, Crumlin, Co. Antrim, United Kingdom, BT29 4QYTel: +44 (0) 28 9442 2413 Fax: +44 (0) 28 9445 2912Email: applications@ Website: Page 6 of 2429/11/2013___________________________________________________________________________________________________RANDOX Laboratories Ltd., 55 Diamond Road, Crumlin, Co. Antrim, United Kingdom, BT29 4QYTel: +44 (0) 28 9442 2413 Fax: +44 (0) 28 9445 2912Email: applications@ Website: Page 7 of 2429/11/2013___________________________________________________________________________________________________RANDOX Laboratories Ltd., 55 Diamond Road, Crumlin, Co. Antrim, United Kingdom, BT29 4QYTel: +44 (0) 28 9442 2413 Fax: +44 (0) 28 9445 2912Email: applications@ Website: Page 8 of 2429/11/2013___________________________________________________________________________________________________RANDOX Laboratories Ltd., 55 Diamond Road, Crumlin, Co. Antrim, United Kingdom, BT29 4QYTel: +44 (0) 28 9442 2413 Fax: +44 (0) 28 9445 2912Email: applications@ Website: Page 9 of 2429/11/2013___________________________________________________________________________________________________RANDOX Laboratories Ltd., 55 Diamond Road, Crumlin, Co. Antrim, United Kingdom, BT29 4QYTel: +44 (0) 28 9442 2413 Fax: +44 (0) 28 9445 2912Email: applications@ Website: Page 10 of 2429/11/2013___________________________________________________________________________________________________RANDOX Laboratories Ltd., 55 Diamond Road, Crumlin, Co. Antrim, United Kingdom, BT29 4QYTel: +44 (0) 28 9442 2413 Fax: +44 (0) 28 9445 2912Email: applications@ Website: Page 11 of 2429/11/2013___________________________________________________________________________________________________Page 12 of 2429/11/2013___________________________________________________________________________________________________Page 13 of 2429/11/2013___________________________________________________________________________________________________Page 14 of 2429/11/2013___________________________________________________________________________________________________Page 15 of 2429/11/2013___________________________________________________________________________________________________Page 16 of 2429/11/2013___________________________________________________________________________________________________Page 17 of 2429/11/2013___________________________________________________________________________________________________Page 18 of 2429/11/2013___________________________________________________________________________________________________Page 19 of 2429/11/2013___________________________________________________________________________________________________Page 20 of 2429/11/2013___________________________________________________________________________________________________Page 21 of 2429/11/2013___________________________________________________________________________________________________Page 22 of 2429/11/2013___________________________________________________________________________________________________Page 23 of 2429/11/2013___________________________________________________________________________________________________Page 24 of 2429/11/2013___________________________________________________________________________________________________。
线粒体呼吸链复合体Ⅳ 细胞色素 C 氧化酶活性检测试剂盒说明书

线粒体呼吸链复合体Ⅳ/细胞色素C 氧化酶活性检测试剂盒说明书微量法注意:本产品试剂有所变动,请注意并严格按照该说明书操作。
货号:BC0945规格:100T/96S产品组成:使用前请认真核对试剂体积与瓶内体积是否一致,有疑问请及时联系索莱宝工作人员。
试剂名称规格 保存条件 提取液液体75 mL×2瓶 2-8℃保存 试剂一液体33mL×1瓶 2-8℃保存 试剂二粉剂×2瓶 -20℃保存 试剂三粉剂×2支 2-8℃保存溶液的配制:1、 试剂二:试剂放于试剂瓶内玻璃瓶中。
临用前取1支加入13.5mL 试剂一溶解,用不完的试剂-20℃分装保存2周,避免反复冻融;2、 试剂三:试剂置于试剂瓶内EP 管中;临用前取1支加入2mL 试剂一溶解,用不完的试剂-20℃保存2周,避免反复冻融;3、 工作液的配制:临用前取0.5mL 试剂三加入到溶解好的4.5mL 试剂二中混合备用(约25T ),或者按比例现用现配。
产品说明:线粒体复合体Ⅳ又称细胞色素C 氧化酶,也是线粒体呼吸电子传递链主路和支路的共有成分,负责催化还原型细胞色素C 的氧化,并最终把电子传递给氧生成水。
还原型细胞色素C 在550nm 有特征光吸收,线粒体复合体Ⅳ催化还原型细胞色素C 生成氧化型细胞色素C ,因此550nm 光吸收下降速率能够反映线粒体复合体Ⅳ酶活性。
Reduced Cytochrome C (550nm ) Oxidized Cytochrome C注意:实验之前建议选择2-3个预期差异大的样本做预实验。
如果样本吸光值不在测量范围内建议稀释或者增加样本量进行检测。
需自备的仪器和用品:可见分光光度计/酶标仪、台式离心机、水浴锅/恒温培养箱、可调式移液器、微量玻璃比色皿/96孔板、研钵/匀浆器/细胞超声破碎仪、冰和蒸馏水。
操作步骤:一、样本处理(可适当调整待测样本量,具体比例可以参考文献)1. 称取约0.1g 组织或收集500万细胞,加入1.0 mL 提取液,用冰浴匀浆器或研钵匀浆。
TMT 蛋白标记试剂盒使用说明90064

INSTRUCTIONSTMT Mass Tagging Kits and90060 TMTduplex Isotopic Label Reagent Set, sufficient reagents for 5 duplex isotopic experiments Contents:TMT0 Label Reagent, 5 × 0.8mgTMT6-127 Label Reagent, 5 × 0.8mg90061 TMTsixplex Isobaric Label Reagent Set, sufficient reagents for 1 sixplex isobaric experiment Contents:TMT6-126 Label Reagent, 1 × 0.8mgTMT6-127 Label Reagent, 1 × 0.8mgTMT6-128 Label Reagent, 1 × 0.8mgTMT6-129 Label Reagent, 1 × 0.8mgTMT6-130 Label Reagent, 1 × 0.8mgTMT6-131 Label Reagent, 1 × 0.8mg90062 TMTsixplex Isobaric Label Reagent Set, sufficient reagents for 2 sixplex isobaric experiments Contents:TMT6-126 Label Reagent, 2 × 0.8mgTMT6-127 Label Reagent, 2 × 0.8mgTMT6-128 Label Reagent, 2 × 0.8mgTMT6-129 Label Reagent, 2 × 0.8mgTMT6-130 Label Reagent, 2 × 0.8mgTMT6-131 Label Reagent, 2 × 0.8mg90063TMTduplex Isobaric Mass Tagging Kit, sufficient reagents for 5 duplex isobaric experiments Contents:TMT0 Label Reagent, 5 × 0.8mgTMT2-126 Label Reagent, 5 × 0.8mgTMT2-127 Label Reagent, 5 × 0.8mgDissolution Buffer (1 M triethyl ammonium bicarbonate), 5mLDenaturing Reagent (10% SDS), 1mLReducing Reagent (0.5M TCEP), 1mLIodoacetamide, 12 × 9mgQuenching Reagent (50% hydroxylamine), 1mLPierce™Trypsin Protease, MS Grade, 2 × 20µgTrypsin Storage Solution, 250µLAlbumin, Bovine, 2.5mg90064TMTsixplex Isobaric Mass Tagging Kit, sufficient reagents for 5 sixplex isobaric experiments Contents:TMT0 Label Reagent, 5 × 0.8mgTMT6-126 Label Reagent, 5 × 0.8mgTMT6-127 Label Reagent, 5 × 0.8mgTMT6-128 Label Reagent, 5 × 0.8mgTMT6-129 Label Reagent, 5 × 0.8mgTMT6-130 Label Reagent, 5 × 0.8mgTMT6-131 Label Reagent, 5 × 0.8mgDissolution Buffer (1M triethyl ammonium bicarbonate), 5mLDenaturing Reagent (10% SDS), 1mLReducing Reagent (0.5 M TCEP), 1mLIodoacetamide, 12 × 9mgQuenching Reagent (50% hydroxylamine), 1mLPierce Trypsin Protease, MS Grade, 5 × 20µgTrypsin Storage Solution, 250µLAlbumin, Bovine, 2.5mg90065TMTduplex Isobaric Label Reagent Set, sufficient reagents for 5 duplex isobaric experiments Contents:TMT2-126 Label Reagent, 5 × 0.8mgTMT2-127 Label Reagent, 5 × 0.8mg90066TMTsixplex Label Reagent Set, sufficient reagents for 5 sixplex isobaric experimentsContents:TMT6-126 Label Reagent, 5 × 0.8mgTMT6-127 Label Reagent, 5 × 0.8mgTMT6-128 Label Reagent, 5 × 0.8mgTMT6-129 Label Reagent, 5 × 0.8mgTMT6-130 Label Reagent, 5 × 0.8mgTMT6-131 Label Reagent, 5 × 0.8mg90067TMTzero Label Reagent, 5 × 0.8mg, sufficient reagents for 5 samples90068TMTsixplex Label Reagent Set, sufficient reagents for 12 sixplex isobaric experimentsContents:TMT6-126 Label Reagent, 2 × 5mgTMT6-127 Label Reagent, 2 × 5mgTMT6-128 Label Reagent, 2 × 5mgTMT6-129 Label Reagent, 2 × 5mgTMT6-130 Label Reagent, 2 × 5mgTMT6-131 Label Reagent, 2 × 5mgStorage: Upon receipt store at -20°C. Reagents are shipped with dry ice.Note: These products are for research use only − do not use for diagnostic procedures.ContentsIntroduction (3)Procedure Summary (4)Important Product Information (4)Additional Materials Required (4)Material Preparation (5)Preparing and Labeling Peptides with the TMT Isobaric Mass Tags (5)Troubleshooting (6)Additional Information (6)A.Data Acquisition Methods (6)B.Data Analysis and Quantitation (7)rmation Available from our Website (8)Related Thermo Scientific Products (8)General References (8)IntroductionThe Thermo Scientific™ TMT™ Isobaric Mass Tagging Kits and Reagents enable multiplex relative quantitation by mass spectrometry (MS). Each mass-tagging reagent within a set has the same nominal mass (i.e., isobaric) and chemical structure composed of an amine-reactive NHS-ester group, a spacer arm and an MS/MS reporter (Figure 1). The reagent sets can be used to label two or six peptide samples prepared from cells or tissues. For each sample, a unique reporter in the low mass region of the MS/MS spectrum (i.e., 126-127Da for TMT2 and 126-131Da for TMT6 Isobaric Label Reagents) is used to measure relative protein expression levels during peptide fragmentation.The TMTduplex™ Isotopic Label Reagent Set contains TMTzero™ and one of the TMTsixplex™ Reagents (TMT6-127) to be used as “light” and “heavy” tags for MS-level peptide quantitation similar to duplex isotopic metabolic labeling (e.g., SILAC) or isotopic dimethylation labeling. These isotopic pairs can also be used in targeted quantitation strategies, including selective reaction monitoring (SRM, see the Additional Information Section). Advantages of the TMTduplex and TMTsixplex Isobaric Label Reagents include increased sample multiplexing for relative quantitation, increased sample throughput and fewer missing quantitative channels among samples.Figure 1.Chemical structure of the TMTLabel Reagents. A. Functional regions of thereagent structure, including MS/MSfragmentation sites by higher energy collisiondissociation (HCD) and electron transferdissociation (ETD). B. TMTduplex Reagentstructures and isotope positions (*); only HCDdifferentiates between these two reporters.C. TMTsixplex Reagent structures and isotopepositions (*).Procedure SummaryProtein extracts isolated from cells or tissues are reduced, alkylated and digested overnight. Samples are labeled with the TMT Reagents and then mixed before sample fractionation and clean-up. Labeled samples are analyzed by high resolutionOrbitrap LC-MS/MS before data analysis to identify peptides and quantify reporter ion relative abundance (Figure 2).Figure 2. Schematic for using the Thermo Scientific TMTsixplex Isobaric Mass Tagging Reagents.Important Product Information• The TMT Reagents are moisture-sensitive. To avoid moisture condensation onto the product, vial must be equilibrated to room temperature before opening.•Anhydrous acetonitrile is the recommended solvent to dissolve reagents. Stock solutions are stable for one week when stored at -20°C. For long term storage of unused reagent, remove all solvent by drying and store with desiccant at -20°C. Anhydrous ethanol can be used as an alternative solvent to dissolve reagents but is not recommended for stock solution storage.• The TMT Reagents are amine-reactive and modify lysine residues and the peptide N-termini. All amine-containing buffers and additives must be removed before digestion and labeling.• All samples must be digested, labeled and then mixed equally before desalting, fractionation and LC-MS/MS. For optimal results, use 25-100µg of peptide for each labeling reaction.• To avoid contamination of MS samples, always wear gloves when handling samples and gels. Use ultrapure MS-grade reagents. Perform sample preparation in a cleaned work area.• The TMTzero Label Reagent can be used to optimize methods before multiplexed analysis of samples with the TMTduplex or TMTsixplex Reagent Set.Additional Materials Required• Microcentrifuge tubes• Anhydrous acetonitrile (Thermo Scientific™ Acetonitrile HPLC grade, Product No. 51101) • Water, LC-MS Grade (Product No. 51140) • Chilled (-20°C) acetone• Protein assay (e.g., Thermo Scientific™ BCA Protein Assay Kit, Product No. 22235) • 75-300µm capillary C 18 reversed-phase column• High-resolution Orbitrap Mass Spectrometer, ion trap or time-of-flight (TOF) mass spectrometer with online or offline liquid chromatography (LC) system• Data analysis software such as Thermo Scientific™ Proteome Discoverer™ or Mascot™ Software (Matrix Science, Ltd.)• Optional: C18 spin tips or columns (e.g., Thermo Scientific™ Pierce™ C18 Spin Columns, Product No. 89870 or Pierce™ C18 Tips, Product No. 87784)Material PreparationNote: The 50% hydroxylamine and 10% SDS stock solutions provided with the kit may precipitate during storage. Warm both solutions to room temperature and vortex before use. The amounts listed below are sufficient for preparing and labeling 6 samples.Add 500µL of the Dissolution Buffer (1M TEAB) to 4.5mL of ultrapure water.100mM TEAB (triethylammonium bicarbonate)Lysis Buffer Add 200µL of the Denaturing Reagent (10% SDS) to 1.8mL of 100mM TEAB.200mM TCEP Add 70µL of the Reducing Reagent (0.5M TCEP) to 70µL of ultrapure water. Then add 35µL of the Dissolution Buffer (1M TEAB).5% Hydroxylamine Add 50µL of the Quenching Reagent (50% hydroxylamine) to 450µL of 100mM TEAB. Preparing and Labeling Peptides with the TMT Isobaric Mass TagsNote: BSA can be used as a control sample for method optimization. Dissolve BSA to 1mg/mL using 100mM TEAB. Use 25-100µg of protein per labeling reaction. The Thermo Scientific™ Pierce™ Mass Spec Sample Prep Kit for Cultured Cells can also be used to prepare peptide digests for TMT reagent labeling.A.Preparing Whole Cell Protein Extracts1.Culture cells to harvest at least 100µg of protein per condition. For best results, culture a minimum of 2 × 106 cells.Note: Rinse cells 2-3 times with 1X PBS to remove cell culture media. Pellet cells using low-speed centrifugation(i.e., < 1000 × g) to prevent premature cell lysis.2.Lyse the cells by adding five cell-pellet volumes of Lysis Buffer (i.e., 100μL of Lysis Buffer for a 20μL cell pellet).Note: Lysis buffers such as 8M urea (Product No. 29700) in 50mM TEAB or HEPES buffer, pH 8 may be used as alternative denaturing cell lysis buffers. For urea-based lysis buffer, protein samples must be diluted to < 1M urea before digestion, and the final C18 desalting step (C.6) is not optional. Addition of protease and/or phosphatase inhibitors during lysis is optional and may interfere with MS analysis.Note: Depending on the Lysis Buffer used it may be necessary to reduce sample viscosity by shearing DNA using a microtip sonicator or addition of a nuclease (e.g., Thermo Scientific™ Pierce™ Universal Nuclease for Cell Lysis, Product No. 88700)3.Centrifuge lysate at 16,000 × g for 10 minutes at 4°C.4.Carefully separate the supernatant and transfer into a new tube.5.Determine the protein concentration of the supernatant using established methods such as the BCA Protein Assay Kit(Product No. 23227).Note: Use samples at ≥ 2mg/mL. Less concentrated samples may be used; however, it might be necessary to use larger volumes of reducing/alkylating reagents.6.Transfer 100µg per condition (two for the TMTduplex or six for the TMTsixplex Label Reagents) into a newmicrocentrifuge tube and adjust to a final volume of 100µL with 100mM TEAB.7.Add 5µL of the 200mM TCEP and incubate sample at 55°C for 1 hour.8.Immediately before use, dissolve one tube of iodoacetamide (9mg) with 132µL of 100mM TEAB to make375mM iodoacetamide. Protect solution from light.9.Add 5µL of the 375mM iodoacetamide to the sample and incubate for 30 minutes protected from light at roomtemperature.10.Add six volumes (~600µL) of pre-chilled (-20°C) acetone and freeze at -20°C. Allow the precipitation to proceed for atleast 4 hours up to overnight.Note: Methanol/chloroform is the recommended solvent for precipitation of proteins derived from tissue extracts.11.Centrifuge the samples at 8000 ×g for 10 minutes at 4°C. Carefully invert the tubes to decant the acetone withoutdisturbing the white pellet. Allow the pellet to dry for 2-3 minutes.B.Protein Digestion1.Resuspend 100µg of acetone-precipitated (or lyophilized) protein pellets with 100µL of 50mM TEAB.Note: An acetone-precipitated pellet might not completely dissolve; however, after proteolysis at 37°C, all the protein (peptides) will be solubilized.2.Immediately before use, add 20µL of the Trypsin Storage Solution to the bottom of the trypsin glass vial and incubate for5 minutes. Store any remaining reagent in single-use volumes at -80°C (e.g., 2.5µg of trypsin per 100µg of protein).3.Add 2.5µL of trypsin (i.e., 2.5µg) per 100µg of protein. Digest the sample overnight at 37°C.C. Peptide Labeling1.Immediately before use, equilibrate the TMT Label Reagents to room temperature. For the 0.8mg vials, add 41µL ofanhydrous acetonitrile to each tube. For the 5mg vials, add 256µL of solvent to each tube. Allow the reagent to dissolve for 5 minutes with occasional vortexing. Briefly centrifuge the tube to gather the solution.Note: Reagents dissolved in anhydrous acetonitrile are stable for one week when stored at -20°C. Anhydrous ethanol can be used as an alternative solvent to dissolve reagents but is not recommended for stock solution storage.2.Optional: Measure protein digest concentration using Thermo Scientific™ Pierce™ Quantitative Fluorescent PeptideAssay (Product No. 23290) or Thermo Scientific™ Pierce™ Quantitative Colorimetric Peptide Assay (Product No.23275).3.Carefully add 41µL of the TMT Label Reagent to each 100µL sample (25-100µg protein digest). Alternatively, transferthe reduced and alkylated protein digest to the TMT Reagent vial.4.Note: Labeling more than 100µg of protein digest per reaction requires additional TMT Label Reagent.5.Incubate the reaction for 1 hour at room temperature.6.Add 8µL of 5% hydroxylamine to the sample and incubate for 15 minutes to quench the reaction.bine samples at equal amounts in new microcentrifuge tube and store at -80°C.Note: TMT-labeled peptide concentration can be measured using Thermo Scientific™ Pierce™ QuantitativeColorimetric Peptide Assay. The Thermo Scientific™ Pierce™ Quantitative Fluorescent Peptide Assay cannot be used to measure TMT-labeled peptide concentrations.8.Optional: Clean-up samples with C18 spin tips (Product No. 87784) or columns (Product No. 89870)before LC-MSanalysis. Peptide clean up is recommended before LC-MS analysis but is not required. Fractionation of labeled peptides using Thermo Scientific™ Pierce™ High pH Reversed-Phase Peptide Fractionation Kit (Product No. 84868) isrecommended before LC-MS analysis to increase the number of peptide identifications.TroubleshootingProblem Possible Cause SolutionPoor labeling An amine-based buffer was used Use a non-amine-based bufferIncorrect buffer pH Make sure the buffer pH is ~8.0Too much sample was used Label 25-100µg per sampleProtein precipitation Lack of detergent present Add detergent, such as 0.05% SDS to the preparationpH decreased Make sure the pH is > 7.5Additional InformationA.Data Acquisition MethodsQuantitation of peptides labeled with Thermo Scientific™ Tandem Mass Tag™ Reagents requires a mass spectrometer capable of MS/MS fragmentation, such as an ion trap, quadrupole time of flight, time of flight-time of flight (TOF-TOF) or triple quadrupole instrument. Higher energy collision dissociation (HCD) is recommended for TMT reporter ion fragmentation. Optimal HCD fragmentation energy is instrument-dependent and can be optimized using TMTzero Reagents.Electron transfer dissociation (ETD) may be used as an alternative fragmentation method for peptide identification and quantitation. The choice of MS/MS fragmentation method(s) depends on the instrument capabilities such as collisionally induced dissociation (CID), pulsed-Q dissociation (PQD), higher energy collisional dissociation (HCD), or electron transfer dissociation (ETD). TMT Reagent reporter ions are not visible in ion traps following traditional CID fragmentation.Table 1. Instruments and MS/MS fragmentation options for peptide identification and quantitation withThermo Scientific TMT Reagents.Instrument Fragmentation Method Reference(s)Thermo Scientific Orbitrap™ Fusion™ Tribrid™ Mass Spectrometer HCD/SPS-MS3 McAllister, G.C., et al. (2014), Viner,et al. (2013)Thermo Scientific Orbitrap Elite™ Mass Spectrometer HCD/MS3 McAllister, G.C., et al. (2012), Viner,et al. (2012)Thermo Scientific Q Exactive™ MassSpectrometerHCD/MS2 Wühr, et al. (2012)Thermo Scientific Orbitrap Velos Pro™, LTQ-Orbitrap™ XL, or MALDI-Orbitrap™ XL Mass Spectrometer HCD/MS2 Ting, et al. (2011), Wenger, et al(2011), Schirle, et al. (2012), Lee, etal (2011), Xiong, et al. (2011),Strupat, et al. (2008)Thermo Scientific™ Velos Pro™ ion trap Trap HCD/MS2 Biringer, et al. (2011)Thermo Scientific Orbitrap Elite ETD, Velos Pro ETD, LTQ-OrbitrapXL ETD HCD/MS2 orETD/MS2Viner, et al. (2009)Q-TOF CID Van Ulsen, et al. (2009)TOF-TOF CID Dayon, et al. (2008)Triple Quadrupole CID/SRM Stella, et al (2011), Byers, et al.(2009)B.Data Analysis and QuantitationThe masses for peptide modification by the TMT zero, duplex, and sixplex reagents are present in the UNIMOD database () and are listed below. Several software packages directly support the modifications by TMT Reagents and the relative quantitation of reporter ions released from labeled peptides, including Thermo Scientific™ Proteome Discoverer™ 1.1 and above, Matrix Science Mascot™ 2.1 and above, and Proteome Software Scaffold™ Q+. For data acquired using a combination of fragmentation methods (i.e., HCD/MS3 or HCD/ETD), Proteome Discoverer may be necessary to merge spectra for identification and quantitation.Table 2. Modification masses of the Thermo Scientific TMT Label Reagents.Label ReagentReagentReporter IonModificationMass(monoisotopic)ModificationMass(average)HCDMonoisotopicReporter Mass*ETDMonoisotopicReporter Mass**TMT0-126 126 224.152478 224.2994 126.127726 114.127725TMT2-126 126 225.155833 225.2921 126.127726 114.127725TMT2-127 127C 225.155833 225.2921 127.131081 114.127725TMT6-126 126 229.162932 229.2634 126.127726 114.127725TMT6-127 127N 229.162932 229.2634 127.124761 115.124760TMT6-128 128C 229.162932 229.2634 128.134436 116.134433TMT6-129 129N 229.162932 229.2634 129.131471 117.131468TMT6-130 130C 229.162932 229.2634 130.141145 118.141141TMT6-131 131 229.162932 229.2634 131.138180 119.138176 * HCD is a collisional fragmentation method that generates six unique reporter ions from 126 to 131Da.**ETD is a non-ergodic fragmentation method that generates six unique reporter ions from 114 to 119Da.rmation Available from our Website•Tech Tip Protocol #49: Acetone precipitation of proteins•Tech Tip Protocol #19: Remove detergent from protein samplesRelated Thermo Scientific Products90110 TMT10plex™ Isobaric Label Reagent Set, 10 × 0.8mg90113 TMT10plex Isobaric Mass Tag Labeling Kit90406 TMT10plex Isobaric Label Reagent Set, 10 × 5mg90114 1M Triethylammonium bicarbonate (TEAB), 50mL90115 50% Hydroxylamine, 5mL90100 iodoTMTzero™ Label Reagent, 5 × 0.2mg90101 iodoTMTsixplex™ Label Reagent Set, 1 × 0.2mg90103 iodoTMTsixplex Isobaric Mass Tag Labeling Kit90076 Immobilized Anti-TMT Antibody Resin90075 Anti-TMT Antibody, 0.1mL90104 TMT Elution Buffer, 20mL84840 Pierce™ Mass Spec Sample Prep Kit for Cultured Cells23227 BCA Protein Assay Kit23275 Pierce Quantitative Colorimetric Peptide Assay23290 Pierce Quantitative Fluorescent Peptide Assay90057 Pierce Trypsin Protease, MS Grade90051 Lys-C Protease, MS Grade88300 Fe-NTA Phosphopeptide Enrichment Kit88301 Pierce TiO2 Phosphopeptide Enrichment and Clean-up Kit84868 Pierce High pH Reversed-Phase Peptide Fractionation Kit88321 Pierce Peptide Retention Time Calibration Mixture, 200µL87784 Pierce C18 Tips, 100µL bed, 96 tips89870 Pierce C18 Spin Columns, 25 columns28904 Trifluoroacetic Acid, Sequanal GradeGeneral ReferencesAltelaar A.F., et al. (2012). Benchmarking stable isotope labeling based quantitative proteomics. J Proteomics Oct 22. pii: S1874-3919(12)00704-X.doi: 10.1016/j.jprot.2012.10.009.Bantscheff, M., et al. (2008). Robust and sensitive iTRAQ quantification on an LTQ Orbitrap Mass Spectrometer. Mol Cell Proteomics7:1702-13.Biringer, R.G., et al. (2011). Quantitation of TMT-Labeled Peptides Using Higher-Energy Collisional Dissociation on the Velos Pro Ion Trap Mass Spectrometer. Application note # 520. Byers, H.L. (2009). Candidate verification of iron-regulated Neisseria meningitidis proteins using isotopic versions of tandem mass tags (TMT) and single reaction monitoring, J Prot73(2):231-9.Dayon, L., et al. (2008). Relative quantification of proteins in human cerebrospinal fluids by MS/MS using 6-plex isobaric tags. Anal Chem80(8):2921-31. Dillon, R, et al. (2011). Discovery of a Novel B-Raf Fusion Protein Related to c-Met Drug Resistance. J Proteome Res10(11):5084-94.Erikson, B.K., et al. (2015). Evaluating multiplexed quantitative phosphopeptide analysis on a hybrid quadrupole mass filter/linear ion trap/orbitrap mass spectrometer. Anal Chem87(2):1241-9.Keshishian, H., et al. (2015). Multiplexed, quantitative workflow for sensitive biomarker discovery in plasma yields novel candidates for early myocardial injury. Mol Cell Proteomics. 2015 Feb 27. pii: mcp.M114.046813Lee, M.V., et al. (2011). A dynamic model of proteome changes reveals new roles for transcript alteration in yeast. Mol Syst Biol 7:514.McAllister, G.C., et al. (2014). MultiNotch MS3 enables accurate, sensitive, and multiplexed detection of differential expression across cancer cell line proteomes. Anal Chem86(14):7150-8.McAllister, G.C., et al. (2012). Increasing the multiplexing capacity of TMTs using reporter ion isotopologues with isobaric masses. Anal Chem 84(17):7469-78.Murphy, J.P., et al. (2014). Combining amine metabolomics and quantitative proteomics of cancer cells using derivatization with isobaric tags. Proteomics 86(7):3585-93.Paulo, J.A., et al. (2014). A comprehensive proteomic and phosphoproteomic analysis of yeast deletion mutants of 14-3-3 orthologs and associated effects of rapamycin. Nature(2-3):474-86.Ross, P.L., et al. (2004). Multiplexed protein quantitation in Saccharomyces cerevisiae using amine-reactive isobaric tagging reagents. Mol Cell Proteomics 3(12):1154-69.Savitski, M.M., et al. (2014). Tracking cancer drugs in living cells by thermal profiling of the proteome. Science346(6205):1255784Schirle, M., et al. (2012). Kinase inhibitor profiling using chemoproteomics. Methods Mol Biol795:161-77.Schwartz, J. et al. (2008). Relative quantitation of protein digests using tandem mass tags and pulsed-Q dissociation (PQD). Application note # 452.Stella, R., et al. (2011). Relative Quantification of Membrane Proteins in Wild-type and PrP-knockout Cerebellar Granule Neurons. J Proteome Res doi: 10.1021/pr200759m. Strupat K., et al. (2008). Accurate MS and MSn Analysis with the Thermo Scientific MALDI LTQ Orbitrap. Application note # 30150.Ting, L., et al. (2011). MS3 eliminates ratio distortion in isobaric multiplexed quantitative proteomics. Nature Methods8: 937–940.Van Ulsen, P., et al. (2009). Identification of proteins of Neisseria meningitidis induced under iron-limiting conditions using the isobaric tandem mass tag (TMT) labeling approach. Proteomics9(7):1771-81.Viner, R.I., et al. (2013). Increasing the multiplexing of protein quantitation from 6- to 10-Plex with reporter ion isotopologues.PN_ASMS_W617_RViner_R1.Viner, R.I., et al. (2012). Relative quantitation of TMT-labeled proteomes – Focus on sensitivity and precision. Application note #566.Viner, R.I., et al. (2009). Quantification of post-translationally modified peptides of bovine α-crystallin using tandem mass tags and electron transfer dissociation. J Proteomics72(5):874-85.Wenger, C.D., et al. (2011). Gas-phase purification enables accurate, multiplexed proteome quantification with isobaric tagging. Nat Methods 8(11):933-5. Xiong, L., et al. (2011). Mass spectrometric studies on epigenetic interaction networks in cell differentiation. J Biol Chem 286(15):13657-68.Zhang, T., et al. (2010). Improving quantitation of TMT-labeled peptides using stepped higher-energy collisional dissociation. Application note # 483 Products are warranted to operate or perform substantially in conformance with published Product specifications in effect at the time of sale, as set forth in the Product documentation, specifications and/or accompanying package inserts (“Documentation”). No claim of suitability for use in applications regulated by FDA is made. The warranty provided herein is valid only when used by properly trained individuals. Unless otherwise stated in the Documentation, this warranty is limited to one year from date of shipment when the Product is subjected to normal, proper and intended usage. This warranty does not extend to anyone other than Buyer. Any model or sample furnished to Buyer is merely illustrative of the general type and quality of goods and does not represent that any Product will conform to such model or sample.NO OTHER WARRANTIES, EXPRESS OR IMPLIED, ARE GRANTED, INCLUDING WITHOUT LIMITATION, IMPLIED WARRANTIES OF MERCHANTABILITY, FITNESS FOR ANY PARTICULAR PURPOSE, OR NON INFRINGEMENT. BUYER’S EXCLUSIVE REMEDY FOR NON-CONFORMING PRODUCTS DURING THE WARRANTY PERIOD IS LIMITED TO REPAIR, REPLACEMENT OF OR REFUND FOR THE NON-CONFORMING PRODUCT(S) AT SELLER’S SOLE OPTION. THERE IS NO OBLIGATION TO REPAIR, REPLACE OR REFUND FOR PRODUCTS AS THE RESULT OF (I) ACCIDENT, DISASTER OR EVENT OF FORCE MAJEURE, (II) MISUSE, FAULT OR NEGLIGENCE OF OR BY BUYER, (III) USE OF THE PRODUCTS IN A MANNER FOR WHICH THEY WERE NOT DESIGNED, OR (IV) IMPROPER STORAGE AND HANDLING OF THE PRODUCTS.Unless otherwise expressly stated on the Product or in the documentation accompanying the Product, the Product is intended for research only and is not to be used for any other purpose, including without limitation, unauthorized commercial uses, in vitro diagnostic uses, ex vivo or in vivo therapeutic uses, or any type of consumption by or application to humans or animals.Current product instructions are available at . For a faxed copy, call 800-874-3723 or contact your local distributor.© 2016 Thermo Fisher Scientific Inc. All rights reserved. Tandem Mass Tag and TMT are trademarks of Proteome Sciences plc. iTRAQ is a trademark of AB Sciex Pte. Ltd. Mascot is a trademark of Matrix Science. Scaffold is a trademark of Proteome Software. Unless otherwise indicated, all other trademarks are the property of Thermo Fisher Scientific Inc. and its subsidiaries. Printed in the USA.。
稳定性英文版

HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationFLUOXETINE HClC17H18F3NO•HClM.W. = 345.79CAS — 59333-67-4STABILITY INDICATINGA S S A Y V A L I D A T I O NMethod is suitable for:ýIn-process controlþProduct ReleaseþStability indicating analysis (Suitability - US/EU Product) CAUTIONFLUOXETINE HYDROCHLORIDE IS A HAZARDOUS CHEMICAL AND SHOULD BE HANDLED ONLY UNDER CONDITIONS SUITABLE FOR HAZARDOUS WORK.IT IS HIGHLY PRESSURE SENSITIVE AND ADEQUATE PRECAUTIONS SHOULD BE TAKEN TO AVOID ANY MECHANICAL FORCE (SUCH AS GRINDING, CRUSHING, ETC.) ON THE POWDER.ED. N0: 04Effective Date:APPROVED::HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationTABLE OF CONTENTS INTRODUCTION........................................................................................................................ PRECISION............................................................................................................................... System Repeatability ................................................................................................................ Method Repeatability................................................................................................................. Intermediate Precision .............................................................................................................. LINEARITY................................................................................................................................ RANGE...................................................................................................................................... ACCURACY............................................................................................................................... Accuracy of Standard Injections................................................................................................ Accuracy of the Drug Product.................................................................................................... VALIDATION OF FLUOXETINE HCl AT LOW CONCENTRATION........................................... Linearity at Low Concentrations................................................................................................. Accuracy of Fluoxetine HCl at Low Concentration..................................................................... System Repeatability................................................................................................................. Quantitation Limit....................................................................................................................... Detection Limit........................................................................................................................... VALIDATION FOR META-FLUOXETINE HCl (POSSIBLE IMPURITIES).................................. Meta-Fluoxetine HCl linearity at 0.05% - 1.0%........................................................................... Detection Limit for Fluoxetine HCl.............................................................................................. Quantitation Limit for Meta Fluoxetine HCl................................................................................ Accuracy for Meta-Fluoxetine HCl ............................................................................................ Method Repeatability for Meta-Fluoxetine HCl........................................................................... Intermediate Precision for Meta-Fluoxetine HCl......................................................................... SPECIFICITY - STABILITY INDICATING EVALUATION OF THE METHOD............................. FORCED DEGRADATION OF FINISHED PRODUCT AND STANDARD..................................1. Unstressed analysis...............................................................................................................2. Acid Hydrolysis stressed analysis..........................................................................................3. Base hydrolysis stressed analysis.........................................................................................4. Oxidation stressed analysis...................................................................................................5. Sunlight stressed analysis.....................................................................................................6. Heat of solution stressed analysis.........................................................................................7. Heat of powder stressed analysis.......................................................................................... System Suitability stressed analysis.......................................................................................... Placebo...................................................................................................................................... STABILITY OF STANDARD AND SAMPLE SOLUTIONS......................................................... Standard Solution...................................................................................................................... Sample Solutions....................................................................................................................... ROBUSTNESS.......................................................................................................................... Extraction................................................................................................................................... Factorial Design......................................................................................................................... CONCLUSION...........................................................................................................................ED. N0: 04Effective Date:APPROVED::HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationBACKGROUNDTherapeutically, Fluoxetine hydrochloride is a classified as a selective serotonin-reuptake inhibitor. Effectively used for the treatment of various depressions. Fluoxetine hydrochloride has been shown to have comparable efficacy to tricyclic antidepressants but with fewer anticholinergic side effects. The patent expiry becomes effective in 2001 (US). INTRODUCTIONFluoxetine capsules were prepared in two dosage strengths: 10mg and 20mg dosage strengths with the same capsule weight. The formulas are essentially similar and geometrically equivalent with the same ingredients and proportions. Minor changes in non-active proportions account for the change in active ingredient amounts from the 10 and 20 mg strength.The following validation, for the method SI-IAG-206-02 , includes assay and determination of Meta-Fluoxetine by HPLC, is based on the analytical method validation SI-IAG-209-06. Currently the method is the in-house method performed for Stability Studies. The Validation was performed on the 20mg dosage samples, IAG-21-001 and IAG-21-002.In the forced degradation studies, the two placebo samples were also used. PRECISIONSYSTEM REPEATABILITYFive replicate injections of the standard solution at the concentration of 0.4242mg/mL as described in method SI-IAG-206-02 were made and the relative standard deviation (RSD) of the peak areas was calculated.SAMPLE PEAK AREA#15390#25406#35405#45405#55406Average5402.7SD 6.1% RSD0.1ED. N0: 04Effective Date:APPROVED::HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationED. N0: 04Effective Date:APPROVED::PRECISION - Method RepeatabilityThe full HPLC method as described in SI-IAG-206-02 was carried-out on the finished product IAG-21-001 for the 20mg dosage form. The method repeated six times and the relative standard deviation (RSD) was calculated.SAMPLENumber%ASSAYof labeled amountI 96.9II 97.8III 98.2IV 97.4V 97.7VI 98.5(%) Average97.7SD 0.6(%) RSD0.6PRECISION - Intermediate PrecisionThe full method as described in SI-IAG-206-02 was carried-out on the finished product IAG-21-001 for the 20mg dosage form. The method was repeated six times by a second analyst on a different day using a different HPLC instrument. The average assay and the relative standard deviation (RSD) were calculated.SAMPLENumber% ASSAYof labeled amountI 98.3II 96.3III 94.6IV 96.3V 97.8VI 93.3Average (%)96.1SD 2.0RSD (%)2.1The difference between the average results of method repeatability and the intermediate precision is 1.7%.HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationLINEARITYStandard solutions were prepared at 50% to 200% of the nominal concentration required by the assay procedure. Linear regression analysis demonstrated acceptability of the method for quantitative analysis over the concentration range required. Y-Intercept was found to be insignificant.RANGEDifferent concentrations of the sample (IAG-21-001) for the 20mg dosage form were prepared, covering between 50% - 200% of the nominal weight of the sample.Conc. (%)Conc. (mg/mL)Peak Area% Assayof labeled amount500.20116235096.7700.27935334099.21000.39734463296.61500.64480757797.52000.79448939497.9(%) Average97.6SD 1.0(%) RSD 1.0ED. N0: 04Effective Date:APPROVED::HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationED. N0: 04Effective Date:APPROVED::RANGE (cont.)The results demonstrate linearity as well over the specified range.Correlation coefficient (RSQ)0.99981 Slope11808.3Y -Interceptresponse at 100%* 100 (%) 0.3%ACCURACYACCURACY OF STANDARD INJECTIONSFive (5) replicate injections of the working standard solution at concentration of 0.4242mg/mL, as described in method SI-IAG-206-02 were made.INJECTIONNO.PEAK AREA%ACCURACYI 539299.7II 540599.9III 540499.9IV 5406100.0V 5407100.0Average 5402.899.9%SD 6.10.1RSD, (%)0.10.1The percent deviation from the true value wasdetermined from the linear regression lineHPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationED. N0: 04Effective Date:APPROVED::ACCURACY OF THE DRUG PRODUCTAdmixtures of non-actives (placebo, batch IAG-21-001 ) with Fluoxetine HCl were prepared at the same proportion as in a capsule (70%-180% of the nominal concentration).Three preparations were made for each concentration and the recovery was calculated.Conc.(%)Placebo Wt.(mg)Fluoxetine HCl Wt.(mg)Peak Area%Accuracy Average (%)70%7079.477.843465102.27079.687.873427100.77079.618.013465100.0101.0100%10079.6211.25476397.910080.8011.42491799.610079.6011.42485498.398.6130%13079.7214.90640599.413080.3114.75632899.213081.3314.766402100.399.618079.9920.10863699.318079.3820.45879499.418080.0820.32874899.599.4Placebo, Batch Lot IAG-21-001HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationED. N0: 04Effective Date:APPROVED::VALIDATION OF FLUOXETINE HClAT LOW CONCENTRATIONLINEARITY AT LOW CONCENTRATIONSStandard solution of Fluoxetine were prepared at approximately 0.02%-1.0% of the working concentration required by the method SI-IAG-206-02. Linear regression analysis demonstrated acceptability of the method for quantitative analysis over this range.ACCURACY OF FLUOXETINE HCl AT LOW CONCENTRATIONThe peak areas of the standard solution at the working concentration were measured and the percent deviation from the true value, as determined from the linear regression was calculated.SAMPLECONC.µg/100mLAREA FOUND%ACCURACYI 470.56258499.7II 470.56359098.1III 470.561585101.3IV 470.561940100.7V 470.56252599.8VI 470.56271599.5(%) AverageSlope = 132.7395299.9SD Y-Intercept = -65.872371.1(%) RSD1.1HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationSystem RepeatabilitySix replicate injections of standard solution at 0.02% and 0.05% of working concentration as described in method SI-IAG-206-02 were made and the relative standard deviation was calculated.SAMPLE FLUOXETINE HCl AREA0.02%0.05%I10173623II11503731III10103475IV10623390V10393315VI10953235Average10623462RSD, (%) 5.0 5.4Quantitation Limit - QLThe quantitation limit ( QL) was established by determining the minimum level at which the analyte was quantified. The quantitation limit for Fluoxetine HCl is 0.02% of the working standard concentration with resulting RSD (for six injections) of 5.0%. Detection Limit - DLThe detection limit (DL) was established by determining the minimum level at which the analyte was reliably detected. The detection limit of Fluoxetine HCl is about 0.01% of the working standard concentration.ED. N0: 04Effective Date:APPROVED::HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationED. N0: 04Effective Date:APPROVED::VALIDATION FOR META-FLUOXETINE HCl(EVALUATING POSSIBLE IMPURITIES)Meta-Fluoxetine HCl linearity at 0.05% - 1.0%Relative Response Factor (F)Relative response factor for Meta-Fluoxetine HCl was determined as slope of Fluoxetine HCl divided by the slope of Meta-Fluoxetine HCl from the linearity graphs (analysed at the same time).F =132.7395274.859534= 1.8Detection Limit (DL) for Fluoxetine HClThe detection limit (DL) was established by determining the minimum level at which the analyte was reliably detected.Detection limit for Meta Fluoxetine HCl is about 0.02%.Quantitation Limit (QL) for Meta-Fluoxetine HClThe QL is determined by the analysis of samples with known concentration of Meta-Fluoxetine HCl and by establishing the minimum level at which the Meta-Fluoxetine HCl can be quantified with acceptable accuracy and precision.Six individual preparations of standard and placebo spiked with Meta-Fluoxetine HCl solution to give solution with 0.05% of Meta Fluoxetine HCl, were injected into the HPLC and the recovery was calculated.HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationED. N0: 04Effective Date:APPROVED::META-FLUOXETINE HCl[RECOVERY IN SPIKED SAMPLES].Approx.Conc.(%)Known Conc.(µg/100ml)Area in SpikedSampleFound Conc.(µg/100mL)Recovery (%)0.0521.783326125.735118.10.0521.783326825.821118.50.0521.783292021.55799.00.0521.783324125.490117.00.0521.783287220.96996.30.0521.783328526.030119.5(%) AVERAGE111.4SD The recovery result of 6 samples is between 80%-120%.10.7(%) RSDQL for Meta Fluoxetine HCl is 0.05%.9.6Accuracy for Meta Fluoxetine HClDetermination of Accuracy for Meta-Fluoxetine HCl impurity was assessed using triplicate samples (of the drug product) spiked with known quantities of Meta Fluoxetine HCl impurity at three concentrations levels (namely 80%, 100% and 120% of the specified limit - 0.05%).The results are within specifications:For 0.4% and 0.5% recovery of 85% -115%For 0.6% recovery of 90%-110%HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationED. N0: 04Effective Date:APPROVED::META-FLUOXETINE HCl[RECOVERY IN SPIKED SAMPLES]Approx.Conc.(%)Known Conc.(µg/100mL)Area in spikedSample Found Conc.(µg/100mL)Recovery (%)[0.4%]0.4174.2614283182.66104.820.4174.2614606187.11107.370.4174.2614351183.59105.36[0.5%]0.5217.8317344224.85103.220.5217.8316713216.1599.230.5217.8317341224.81103.20[0.6%]0.6261.3918367238.9591.420.6261.3920606269.81103.220.6261.3920237264.73101.28RECOVERY DATA DETERMINED IN SPIKED SAMPLESHPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationED. N0: 04Effective Date:APPROVED::REPEATABILITYMethod Repeatability - Meta Fluoxetine HClThe full method (as described in SI-IAG-206-02) was carried out on the finished drug product representing lot number IAG-21-001-(1). The HPLC method repeated serially, six times and the relative standard deviation (RSD) was calculated.IAG-21-001 20mg CAPSULES - FLUOXETINESample% Meta Fluoxetine % Meta-Fluoxetine 1 in Spiked Solution10.0260.09520.0270.08630.0320.07740.0300.07450.0240.09060.0280.063AVERAGE (%)0.0280.081SD 0.0030.012RSD, (%)10.314.51NOTE :All results are less than QL (0.05%) therefore spiked samples with 0.05% Meta Fluoxetine HCl were injected.HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationED. N0: 04Effective Date:APPROVED::Intermediate Precision - Meta-Fluoxetine HClThe full method as described in SI-IAG-206-02 was applied on the finished product IAG-21-001-(1) .It was repeated six times, with a different analyst on a different day using a different HPLC instrument.The difference between the average results obtained by the method repeatability and the intermediate precision was less than 30.0%, (11.4% for Meta-Fluoxetine HCl as is and 28.5% for spiked solution).IAG-21-001 20mg - CAPSULES FLUOXETINESample N o:Percentage Meta-fluoxetine% Meta-fluoxetine 1 in spiked solution10.0260.06920.0270.05730.0120.06140.0210.05850.0360.05560.0270.079(%) AVERAGE0.0250.063SD 0.0080.009(%) RSD31.514.51NOTE:All results obtained were well below the QL (0.05%) thus spiked samples slightly greater than 0.05% Meta-Fluoxetine HCl were injected. The RSD at the QL of the spiked solution was 14.5%HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationSPECIFICITY - STABILITY INDICATING EVALUATIONDemonstration of the Stability Indicating parameters of the HPLC assay method [SI-IAG-206-02] for Fluoxetine 10 & 20mg capsules, a suitable photo-diode array detector was incorporated utilizing a commercial chromatography software managing system2, and applied to analyze a range of stressed samples of the finished drug product.GLOSSARY of PEAK PURITY RESULT NOTATION (as reported2):Purity Angle-is a measure of spectral non-homogeneity across a peak, i.e. the weighed average of all spectral contrast angles calculated by comparing all spectra in the integrated peak against the peak apex spectrum.Purity Threshold-is the sum of noise angle3 and solvent angle4. It is the limit of detection of shape differences between two spectra.Match Angle-is a comparison of the spectrum at the peak apex against a library spectrum.Match Threshold-is the sum of the match noise angle3 and match solvent angle4.3Noise Angle-is a measure of spectral non-homogeneity caused by system noise.4Solvent Angle-is a measure of spectral non-homogeneity caused by solvent composition.OVERVIEWT he assay of the main peak in each stressed solution is calculated according to the assay method SI-IAG-206-02, against the Standard Solution, injected on the same day.I f the Purity Angle is smaller than the Purity Threshold and the Match Angle is smaller than the Match Threshold, no significant differences between spectra can be detected. As a result no spectroscopic evidence for co-elution is evident and the peak is considered to be pure.T he stressed condition study indicated that the Fluoxetine peak is free from any appreciable degradation interference under the stressed conditions tested. Observed degradation products peaks were well separated from the main peak.1® PDA-996 Waters™ ; 2[Millennium 2010]ED. N0: 04Effective Date:APPROVED::HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationFORCED DEGRADATION OF FINISHED PRODUCT & STANDARD 1.UNSTRESSED SAMPLE1.1.Sample IAG-21-001 (2) (20mg/capsule) was prepared as stated in SI-IAG-206-02 and injected into the HPLC system. The calculated assay is 98.5%.SAMPLE - UNSTRESSEDFluoxetine:Purity Angle:0.075Match Angle:0.407Purity Threshold:0.142Match Threshold:0.4251.2.Standard solution was prepared as stated in method SI-IAG-206-02 and injected into the HPLC system. The calculated assay is 100.0%.Fluoxetine:Purity Angle:0.078Match Angle:0.379Purity Threshold:0.146Match Threshold:0.4272.ACID HYDROLYSIS2.1.Sample solution of IAG-21-001 (2) (20mg/capsule) was prepared as in method SI-IAG-206-02 : An amount equivalent to 20mg Fluoxetine was weighed into a 50mL volumetric flask. 20mL Diluent was added and the solution sonicated for 10 minutes. 1mL of conc. HCl was added to this solution The solution was allowed to stand for 18 hours, then adjusted to about pH = 5.5 with NaOH 10N, made up to volume with Diluent and injected into the HPLC system after filtration.Fluoxetine peak intensity did NOT decrease. Assay result obtained - 98.8%.SAMPLE- ACID HYDROLYSISFluoxetine peak:Purity Angle:0.055Match Angle:0.143Purity Threshold:0.096Match Threshold:0.3712.2.Standard solution was prepared as in method SI-IAG-206-02 : about 22mg Fluoxetine HCl were weighed into a 50mL volumetric flask. 20mL Diluent were added. 2mL of conc. HCl were added to this solution. The solution was allowed to stand for 18 hours, then adjusted to about pH = 5.5 with NaOH 10N, made up to volume with Diluent and injected into the HPLC system.Fluoxetine peak intensity did NOT decrease. Assay result obtained - 97.2%.ED. N0: 04Effective Date:APPROVED::HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationSTANDARD - ACID HYDROLYSISFluoxetine peak:Purity Angle:0.060Match Angle:0.060Purity Threshold:0.099Match Threshold:0.3713.BASE HYDROLYSIS3.1.Sample solution of IAG-21-001 (2) (20mg/capsule) was prepared as per method SI-IAG-206-02 : An amount equivalent to 20mg Fluoxetine was weight into a 50mL volumetric flask. 20mL Diluent was added and the solution sonicated for 10 minutes. 1mL of 5N NaOH was added to this solution. The solution was allowed to stand for 18 hours, then adjusted to about pH = 5.5 with 5N HCl, made up to volume with Diluent and injected into the HPLC system.Fluoxetine peak intensity did NOT decrease. Assay result obtained - 99.3%.SAMPLE - BASE HYDROLYSISFluoxetine peak:Purity Angle:0.063Match Angle:0.065Purity Threshold:0.099Match Threshold:0.3623.2.Standard stock solution was prepared as per method SI-IAG-206-02 : About 22mg Fluoxetine HCl was weighed into a 50mL volumetric flask. 20mL Diluent was added. 2mL of 5N NaOH was added to this solution. The solution was allowed to stand for 18 hours, then adjusted to about pH=5.5 with 5N HCl, made up to volume with Diluent and injected into the HPLC system.Fluoxetine peak intensity did NOT decrease - 99.5%.STANDARD - BASE HYDROLYSISFluoxetine peak:Purity Angle:0.081Match Angle:0.096Purity Threshold:0.103Match Threshold:0.3634.OXIDATION4.1.Sample solution of IAG-21-001 (2) (20mg/capsule) was prepared as per method SI-IAG-206-02. An equivalent to 20mg Fluoxetine was weighed into a 50mL volumetric flask. 20mL Diluent added and the solution sonicated for 10 minutes.1.0mL of 30% H2O2 was added to the solution and allowed to stand for 5 hours, then made up to volume with Diluent, filtered and injected into HPLC system.Fluoxetine peak intensity decreased to 95.2%.ED. N0: 04Effective Date:APPROVED::HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationSAMPLE - OXIDATIONFluoxetine peak:Purity Angle:0.090Match Angle:0.400Purity Threshold:0.154Match Threshold:0.4294.2.Standard solution was prepared as in method SI-IAG-206-02 : about 22mg Fluoxetine HCl were weighed into a 50mL volumetric flask and 25mL Diluent were added. 2mL of 30% H2O2 were added to this solution which was standing for 5 hours, made up to volume with Diluent and injected into the HPLC system.Fluoxetine peak intensity decreased to 95.8%.STANDARD - OXIDATIONFluoxetine peak:Purity Angle:0.083Match Angle:0.416Purity Threshold:0.153Match Threshold:0.4295.SUNLIGHT5.1.Sample solution of IAG-21-001 (2) (20mg/capsule) was prepared as in method SI-IAG-206-02 . The solution was exposed to 500w/hr. cell sunlight for 1hour. The BST was set to 35°C and the ACT was 45°C. The vials were placed in a horizontal position (4mm vials, National + Septum were used). A Dark control solution was tested. A 2%w/v quinine solution was used as the reference absorbance solution.Fluoxetine peak decreased to 91.2% and the dark control solution showed assay of 97.0%. The difference in the absorbance in the quinine solution is 0.4227AU.Additional peak was observed at RRT of 1.5 (2.7%).The total percent of Fluoxetine peak with the degradation peak is about 93.9%.SAMPLE - SUNLIGHTFluoxetine peak:Purity Angle:0.093Match Angle:0.583Purity Threshold:0.148Match Threshold:0.825 ED. N0: 04Effective Date:APPROVED::HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationSUNLIGHT (Cont.)5.2.Working standard solution was prepared as in method SI-IAG-206-02 . The solution was exposed to 500w/hr. cell sunlight for 1.5 hour. The BST was set to 35°C and the ACT was 42°C. The vials were placed in a horizontal position (4mm vials, National + Septum were used). A Dark control solution was tested. A 2%w/v quinine solution was used as the reference absorbance solution.Fluoxetine peak was decreased to 95.2% and the dark control solution showed assay of 99.5%.The difference in the absorbance in the quinine solution is 0.4227AU.Additional peak were observed at RRT of 1.5 (2.3).The total percent of Fluoxetine peak with the degradation peak is about 97.5%. STANDARD - SUNLIGHTFluoxetine peak:Purity Angle:0.067Match Angle:0.389Purity Threshold:0.134Match Threshold:0.8196.HEAT OF SOLUTION6.1.Sample solution of IAG-21-001-(2) (20 mg/capsule) was prepared as in method SI-IAG-206-02 . Equivalent to 20mg Fluoxetine was weighed into a 50mL volumetric flask. 20mL Diluent was added and the solution was sonicated for 10 minutes and made up to volume with Diluent. 4mL solution was transferred into a suitable crucible, heated at 105°C in an oven for 2 hours. The sample was cooled to ambient temperature, filtered and injected into the HPLC system.Fluoxetine peak was decreased to 93.3%.SAMPLE - HEAT OF SOLUTION [105o C]Fluoxetine peak:Purity Angle:0.062Match Angle:0.460Purity Threshold:0.131Match Threshold:0.8186.2.Standard Working Solution (WS) was prepared under method SI-IAG-206-02 . 4mL of the working solution was transferred into a suitable crucible, placed in an oven at 105°C for 2 hours, cooled to ambient temperature and injected into the HPLC system.Fluoxetine peak intensity did not decrease - 100.5%.ED. N0: 04Effective Date:APPROVED::。
HC-030031_DataSheet_MedChemExpress
Inhibitors, Agonists, Screening Libraries Data SheetBIOLOGICAL ACTIVITY:HC–030031 is a potent and selective of TRPA1 inhibitor, which antagonizes AITC– and formalin–evoked calcium influx with IC 50s of6.2±0.2 and 5.3±0.2 μM, respectively.IC50 & Target: TRPA1[1]In Vitro: HC–030031 reversibly blocks TRPA1 currents with a similar potency, regardless of the agonist used; this includes blockade of currents elicited by reversible agonists, such as AITC, or irreversible agonists, such as N–methyl maleimide. HC–030031 blocks activation of TRPA1 by N–methyl maleimide, which opens the channel irreversibly through cysteine modification. HC–030031 does not block currents mediated by TRPV1, TRPV3, TRPV4, hERG, or NaV1.2 channels [1]. The potencies of HC–030031 versuscinnamaldehyde or allyl isothiocyanate (AITC or Mustard oil)–induced TRPA1 activation are 4.9±0.1 and 7.5±0.2 μM respectively (IC 50). These findings are similar to the previously reported IC 50 of 6.2 μM against AITC activation of TRPA1. The ability of HC–030031 to block TRPA1 activation is tested in a FLIPR calcium–influx assay using HEK–293 cells stably expressing human TRPA1. Concentrations of HC–030031 from 0.3 to 60 μM are incubated with cells for 10 minutes prior to addition of an EC 60 concentration of either cinnamaldehyde or AITC. HC–030031 dose–dependently blocks cinnamaldehyde– and AITC–induced calcium influx with IC 50 values of 4.9 and 7.5 μM, respectively [2].In Vivo: After injection of AITC (50 μL of 10%) into the rat hind paw, HC–030031 (300 mg/kg) significantly reduces flinching during the first 5 min. Over the remainder of the hour, HC–030031 decreases flinch frequency, a result that mirrors the effects observed on formalin–induced flinching [1]. In the rat, oral administration of HC–030031 reduces AITC–induced nocifensive behaviors at a dose of100 mg/kg. Moreover, oral HC–030031 (100 mg/kg) significantly reverses mechanical hypersensitivity in the more chronic models of Complete Freunds Adjuvant (CFA)–induced inflammatory pain and the spinal nerve ligation model of neuropathic pain. One hour post–oral administration, HC–030031 significantly reduces the lifting duration following 1% AITC injection (p<0.001)[2]. HC–030031completely reverses the enhanced mechanical firing in inflamed mice (p<0.001)[3].PROTOCOL (Extracted from published papers and Only for reference)Cell Assay: HC–030031 is prepared in DMSO and stored, and then diluted with appropriate medium (DMSO 0.4%) beforeuse [2].[2]HEK–293 cells stably expressing human TRPA1 are plated into 384–well plates at a density of 20,000 cells/well 24 hours prior to assaying. On the day of assay, cells are loaded with 4 μM Fluo–4 dye and 0.08% pluronic acid for 1 hour at room temperature in assay buffer consisting of Hank's balanced salt solution supplemented with 20 mM HEPES, 2.5 mM probenecid, and 4% TR–40.Calcium influx assays are performed using the Fluorometric Imaging Plate Reader (FLIPR) TETRA. Concentration–response curves are generated for the TRPA1 agonists cinnamaldehyde and AITC prior to antagonist testing so EC 60 concentrations could be determined.Titrations of HC–030031 are made from a DMSO stock solution and DMSO is kept to a constant of 0.4% in the assay. The antagonist is incubated with the cells for 10 minutes before the addition of an EC 60 concentration of either cinnamaldehyde (18 μM) or AITC (6μM) and calcium influx is monitored for an additional 10 minutes [2].Product Name:HC–030031Cat. No.:HY-15064CAS No.:349085-38-7Molecular Formula:C 18H 21N 5O 3Molecular Weight:355.39Target:TRP Channel Pathway:Membrane Transporter/Ion Channel Solubility:10 mM in DMSOAnimal Administration: HC–030031 is suspended in 0.5% methylcellulose (Rat)[2].HC–030031 is prepared in 0.5% DMSO and 0.25% Tween–80 in PBS (Mice)[3].[2][3]Rat[2]Male Sprague–Dawley rats (200–500 g) are used in all experiments. HC–030031 (100, 300 mg/kg) is used. For all experiments,HC–030031 is suspended in 0.5% Methylcellulose and the drug is dosed p.o. at a volume of 10 mL/kg. Naproxen (20 mg/kg) is dissolved in sterile water and dosed p.o. to serve as a positive comparator for the CFA experiment. Pregabalin (20 mg/kg) is dissolved in sterile water and dosed p.o. to serve as a positive comparator for the neuropathic pain experiment.Mice[3]Adult male C57BL/6 mice (8–12 weeks old) are used. Mice are injected with a 30 μL emulsion of undiluted CFA into the medial left plantar hind paw. The vehicle control group is injected with 30 μL of sterile 0.9% saline solution. Two days after injection, at the peak of hypersensitivity, the magnitude of inflammation is measured at the midpoint of the hind paw using digital calipers (VWR). For one experiment, the membrane–impermeable sodium channel inhibitor lidocaine N–ethyl–bromide, also known as QX–314, (0.2% in saline;30 μL) is injected with or without the TRPA1 agonist cinnamaldehyde (30 μM) into the left plantar hind paw 2 days post CFA injection. For another experiment, the TRPA1 antagonist HC–030031 (100 μg in 30 μL of 0.5% DMSO and 0.25% Tween–80 in PBS) is injected into the left plantar hind paw 2 days post CFA injection. Vehicle controls are injected with 30 μL 0.5% DMSO and 0.25% Tween–80 in PBS. All behavioral assays are completed between 1 and 4 hours following the QX–314, HC–030031 or vehicle injections. References:[1]. McNamara CR, et al. TRPA1 mediates formalin–induced pain. Proc Natl Acad Sci U S A. 2007 Aug 14;104(33):13525–30.[2]. Eid SR, et al. HC–030031, a TRPA1 selective antagonist, attenuates inflammatory– and neuropathy–induced mechanical hypersensitivity. Mol Pain. 2008 Oct 27;4:48.[3]. Lennertz RC, et al. TRPA1 mediates mechanical sensitization in nociceptors during inflammation. PLoS One. 2012;7(8):e43597.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
AM966_DataSheet_MedChemExpress

Inhibitors, Agonists, Screening Libraries Data SheetBIOLOGICAL ACTIVITY:AM966 is a high affinity, selective, oral LPA 1–antagonist, inhibits LPA–stimulated intracellular calcium release (IC 50=17 nM).IC50 & Target: LPA 1[1]In Vitro: AM966 is a potent, selective, orally bioavailable LPA 1 receptor antagonist. AM966 inhibits LPA 1–mediatedchemotaxis of human A2058 melanoma cells (IC 50=138±43 nM), IMR–90 human lung fibroblasts (IC 50=182±86 nM) and CHO mLPA 1cells (IC 50=469±54 nM)[1]. LPA–induced ERK1/2 activation is completely blocked by AM966 (100 nM), which selectively antagonizes LPA 1 over LPA 2–5, with an IC 50 value of 3.8±0.4 nM. Pre–treatment with AM966 (100 nM) completely blocks ERK1/2 phosphorylation induced by either amitriptyline or mianserin [2].In Vivo: AM966 (30 mg/kg, BID) reduces vascular leakage, inflammation and lung injury and inflammation in a 3 day bleomycin model. AM966 inhibits lung fibrosis, maintains mouse body weight and decreases lung inflammation 14 days after bleomycin lung injury. AM966 reduces vascular leakage, tissue injury and pro–fibrotic cytokine production in the 14 day bleomycin study. AM966demonstrates greater efficacy compared to pirfenidone in the 14 day bleomycin model. AM966 decreases mortality and fibrosis at late time points after bleomycin injury [1].PROTOCOL (Extracted from published papers and Only for reference)Cell Assay: AM966 (Chem Scene, Monmouth Junction, NJ, USA) is dissolved in DMSO and stored, and then diluted withappropriate media (DMSO 0.5%) before use [2].[2]CHO–K1 cells are grown to 80% confluency in 12–well plates,serum–starved for 24 h and incubated in serum–free medium with AM966. After 21 h, [3H]thymidine (0.5 μCi/well) isadded and the incubation is continued for 3 h. The medium is then removed, and the cells are placed on ice and washed twice with 1 mL of ice–cold PBS containing 5% trichloroacetic acid. Cells are solubilized and [3H]thymidine incorporation isdetermined by liquid scintillation counting. Assays are performed in triplicate [2].Animal Administration: AM966 is prepared in water (Mice)[1].[1]Mice [1]The oral exposure of AM966 is determined in fasted mice. Animals received AM966 (10 mg/kg) in vehicle (water) by oral gavage and are then killed by CO 2 inhalation at 1, 2, 4, 8 and 24 h post dose (n=2 animals per time point for each test compound).Blood (approximately 300 μL) is collected via cardiac puncture into EDTA–containing tubes and centrifuged at 1450×g for 10 min. The plasma is removed and analysed for AM966 content by liquid chromatography–mass spectrometry (LCMS).Briefly, known amounts of AM966 are added to thawed mouse plasma to yield a concentration range from 0.8 to 4000ng/mL. Mouse plasma samples are precipitated using acetonitrile (1:4, v:v) containing the internal standard buspirone. A 10μL aliquot of the analyte mixture is injected using a Leap PAL autosampler. Analyses are performed using an AgilentZorbax SB–C8 column (2.1×50 mm; 5 μm) linked to a Shimadzu LC–10AD VP with SCL–10A VP system controller. Tandem mass spectrometric detection is carried out on a PE Sciex API3200 in the positive ion mode (ESI) by multiple reactionmonitoring. The calibration curves are constructed by plotting the peak–area ratio of analysed peaks against knownProduct Name:AM966Cat. No.:HY-15277CAS No.:1228690-19-4Molecular Formula:C 27H 23ClN 2O 5Molecular Weight:490.93Target:LPL Receptor Pathway:GPCR/G Protein Solubility:DMSO: ≥ 105 mg/mLconcentrations. The lower limit of quantitation is 0.8 ng/mL. The data are subjected to linear regression analysis with 1/x2weighting.References:[1]. Swaney, JS, et al. A novel, orally active LPA1 receptor antagonist inhibits lung fibrosis in the mouse bleomycin model. Br J Pharmacol. 2010 Aug; 160(7):1699–713.[2]. Olianas MC, et al. Antidepressants activate the lysophosphatidic acid receptor LPA(1) to induce insulin–like growth factor–I receptor transactivation, stimulation of ERK1/2 signaling and cell proliferation in CHO–K1 fibroblasts. Biochem Pharmacol. 2015 JuCaution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
Pimecrolimus_SDS_MedChemExpress

Inhibitors, Agonists, Screening LibrariesSafety Data Sheet Revision Date:May-24-2017Print Date:May-24-20171. PRODUCT AND COMPANY IDENTIFICATION1.1 Product identifierProduct name :PimecrolimusCatalog No. :HY-13723CAS No. :137071-32-01.2 Relevant identified uses of the substance or mixture and uses advised againstIdentified uses :Laboratory chemicals, manufacture of substances.1.3 Details of the supplier of the safety data sheetCompany:MedChemExpress USATel:609-228-6898Fax:609-228-5909E-mail:sales@1.4 Emergency telephone numberEmergency Phone #:609-228-68982. HAZARDS IDENTIFICATION2.1 Classification of the substance or mixtureGHS Classification in accordance with 29 CFR 1910 (OSHA HCS)Acute toxicity, Oral (Category 4),H302Acute aquatic toxicity (Category 1),H400Chronic aquatic toxicity (Category 1),H4102.2 GHS Label elements, including precautionary statementsPictogramSignal word WarningHazard statement(s)H302 Harmful if swallowed.H410 Very toxic to aquatic life with long lasting effects.Precautionary statement(s)P264 Wash skin thoroughly after handling.P270 Do not eat, drink or smoke when using this product.P273 Avoid release to the environment.P301 + P312 IF SWALLOWED: Call a POISON CENTER or doctor/ physician if you feel unwell.P330 Rinse mouth.P391 Collect spillage.P501 Dispose of contents/ container to an approved waste disposal plant.2.3 Other hazardsNone.3. COMPOSITION/INFORMATION ON INGREDIENTS3.1 SubstancesSynonyms:SDZ⁻ASM 981Formula:C43H68ClNO11Molecular Weight:810.45CAS No. :137071-32-04. FIRST AID MEASURES4.1 Description of first aid measuresEye contactRemove any contact lenses, locate eye-wash station, and flush eyes immediately with large amounts of water. Separate eyelids with fingers to ensure adequate flushing. Promptly call a physician.Skin contactRinse skin thoroughly with large amounts of water. Remove contaminated clothing and shoes and call a physician.InhalationImmediately relocate self or casualty to fresh air. If breathing is difficult, give cardiopulmonary resuscitation (CPR). Avoid mouth-to-mouth resuscitation.IngestionWash out mouth with water; Do NOT induce vomiting; call a physician.4.2 Most important symptoms and effects, both acute and delayedThe most important known symptoms and effects are described in the labelling (see section 2.2).4.3 Indication of any immediate medical attention and special treatment neededTreat symptomatically.5. FIRE FIGHTING MEASURES5.1 Extinguishing mediaSuitable extinguishing mediaUse water spray, dry chemical, foam, and carbon dioxide fire extinguisher.5.2 Special hazards arising from the substance or mixtureDuring combustion, may emit irritant fumes.5.3 Advice for firefightersWear self-contained breathing apparatus and protective clothing.6. ACCIDENTAL RELEASE MEASURES6.1 Personal precautions, protective equipment and emergency proceduresUse full personal protective equipment. Avoid breathing vapors, mist, dust or gas. Ensure adequate ventilation. Evacuate personnel to safe areas.Refer to protective measures listed in sections 8.6.2 Environmental precautionsTry to prevent further leakage or spillage. Keep the product away from drains or water courses.6.3 Methods and materials for containment and cleaning upAbsorb solutions with finely-powdered liquid-binding material (diatomite, universal binders); Decontaminate surfaces and equipment by scrubbing with alcohol; Dispose of contaminated material according to Section 13.7. HANDLING AND STORAGE7.1 Precautions for safe handlingAvoid inhalation, contact with eyes and skin. Avoid dust and aerosol formation. Use only in areas with appropriate exhaust ventilation.7.2 Conditions for safe storage, including any incompatibilitiesKeep container tightly sealed in cool, well-ventilated area. Keep away from direct sunlight and sources of ignition.Recommended storage temperature:Powder-20°C 3 years4°C 2 yearsIn solvent-80°C 6 months-20°C 1 monthShipping at room temperature if less than 2 weeks.7.3 Specific end use(s)No data available.8. EXPOSURE CONTROLS/PERSONAL PROTECTION8.1 Control parametersComponents with workplace control parametersThis product contains no substances with occupational exposure limit values.8.2 Exposure controlsEngineering controlsEnsure adequate ventilation. Provide accessible safety shower and eye wash station.Personal protective equipmentEye protection Safety goggles with side-shields.Hand protection Protective gloves.Skin and body protection Impervious clothing.Respiratory protection Suitable respirator.Environmental exposure controls Keep the product away from drains, water courses or the soil. Cleanspillages in a safe way as soon as possible.9. PHYSICAL AND CHEMICAL PROPERTIES9.1 Information on basic physical and chemical propertiesAppearance White to off-white (Solid)Odor No data availableOdor threshold No data availablepH No data availableMelting/freezing point No data availableBoiling point/range No data availableFlash point No data availableEvaporation rate No data availableFlammability (solid, gas)No data availableUpper/lower flammability or explosive limits No data availableVapor pressure No data availableVapor density No data availableRelative density No data availableWater Solubility No data availablePartition coefficient No data availableAuto-ignition temperature No data availableDecomposition temperature No data availableViscosity No data availableExplosive properties No data availableOxidizing properties No data available9.2 Other safety informationNo data available.10. STABILITY AND REACTIVITY10.1 ReactivityNo data available.10.2 Chemical stabilityStable under recommended storage conditions.10.3 Possibility of hazardous reactionsNo data available.10.4 Conditions to avoidNo data available.10.5 Incompatible materialsStrong acids/alkalis, strong oxidising/reducing agents.10.6 Hazardous decomposition productsUnder fire conditions, may decompose and emit toxic fumes.Other decomposition products - no data available.11.TOXICOLOGICAL INFORMATION11.1 Information on toxicological effectsAcute toxicityClassified based on available data. For more details, see section 2Skin corrosion/irritationClassified based on available data. For more details, see section 2Serious eye damage/irritationClassified based on available data. For more details, see section 2Respiratory or skin sensitizationClassified based on available data. For more details, see section 2Germ cell mutagenicityClassified based on available data. For more details, see section 2CarcinogenicityIARC: No component of this product present at a level equal to or greater than 0.1% is identified as probable, possible or confirmed human carcinogen by IARC.ACGIH: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by ACGIH.NTP: No component of this product present at a level equal to or greater than 0.1% is identified as a anticipated or confirmed carcinogen by NTP.OSHA: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by OSHA.Reproductive toxicityClassified based on available data. For more details, see section 2Specific target organ toxicity - single exposureClassified based on available data. For more details, see section 2Specific target organ toxicity - repeated exposureClassified based on available data. For more details, see section 2Aspiration hazardClassified based on available data. For more details, see section 212. ECOLOGICAL INFORMATION12.1 ToxicityNo data available.12.2 Persistence and degradabilityNo data available.12.3 Bioaccumlative potentialNo data available.12.4 Mobility in soilNo data available.12.5 Results of PBT and vPvB assessmentPBT/vPvB assessment unavailable as chemical safety assessment not required or not conducted.12.6 Other adverse effectsNo data available.13. DISPOSAL CONSIDERATIONS13.1 Waste treatment methodsProductDispose substance in accordance with prevailing country, federal, state and local regulations.Contaminated packagingConduct recycling or disposal in accordance with prevailing country, federal, state and local regulations.14. TRANSPORT INFORMATIONDOT (US)This substance is considered to be non-hazardous for transport.IMDGUN number: 3077Class: 9Packing group: IIIEMS-No: F-A, S-FProper shipping name: ENVIRONMENTALLY HAZARDOUS SUBSTANCE, SOLID, N.O.S.Marine pollutant: Marine pollutant.IATAUN number: 3077Class: 9Packing group: IIIProper shipping name: Environmentally hazardous substance, solid, n.o.s.15. REGULATORY INFORMATIONSARA 302 Components:No chemicals in this material are subject to the reporting requirements of SARA Title III, Section 302.SARA 313 Components:This material does not contain any chemical components with known CAS numbers that exceed the threshold (De Minimis) reporting levels established by SARA Title III, Section 313.SARA 311/312 Hazards:No SARA Hazards.Massachusetts Right To Know Components:No components are subject to the Massachusetts Right to Know Act.Pennsylvania Right To Know Components:No components are subject to the Pennsylvania Right to Know Act.New Jersey Right To Know Components:No components are subject to the New Jersey Right to Know Act.California Prop. 65 Components:This product does not contain any chemicals known to State of California to cause cancer, birth defects, or anyother reproductive harm.16. OTHER INFORMATIONCopyright 2017 MedChemExpress. The above information is correct to the best of our present knowledge but does not purport to be all inclusive and should be used only as a guide. The product is for research use only and for experienced personnel. It must only be handled by suitably qualified experienced scientists in appropriately equipped and authorized facilities. The burden of safe use of this material rests entirely with the user. MedChemExpress disclaims all liability for any damage resulting from handling or from contact with this product.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
Febuxostat_DataSheet_MedChemExpress

Inhibitors, Agonists, Screening Libraries Data SheetBIOLOGICAL ACTIVITY:Febuxostat(TEI 6720;TMX 67 ) is selective xanthine oxidase inhibitor with Ki of 0.6 nM.IC50 value: 0.6 nM (Ki) [1]Target: xanthine oxidasein vitro: Febuxostat displays potent mixed–type inhibition of the activity of purified bovine milk xanthine oxidase, with Ki and Ki' values of 0.6 nM and 3.1 nM respectively, indicating inhibition of both the oxidized and reduced forms of xanthine oxidase [1].in vivo: Febuxostat (5–6 mg/kg/day) combined with fructose significantly lowers blood pressure, UA, triglycerides, and insulin in rats compared with fructose alone. Febuxostat (5–6 mg/kg/day) combined with fructose also reduces glomerular pressure, renal vasoconstriction, and afferent arteriolar area in rats compared with fructose alone [2]. Febuxostat prevents hyperuricemia in 5/6nephrectomy (5/6 Nx)+oxonic acid (OA)+Febuxostat(Fx) rats and ameliorates proteinuria, preserves renal function and prevents glomerular hypertension in both 5/6 nephrectomy (5/6 Nx)+vehicle (V)+Febuxostat(Fx) and 5/6 nephrectomy (5/6 Nx)+oxonic acid (OA)+Febuxostat(Fx) groups [3]. Febuxostat (5 mg/kg/d by gavage for 8 days) treatment after transverse aortic constriction (TAC)attenuates the TAC–induced left ventricular (LV) hypertrophy and dysfunction. Febuxostat blunts the TAC–induced increases innitrotyrosine (indicating reduced myocardial oxidative stress), p–Erk(Thr202/Tyr204), and p–mTOR(Ser2488), with no effect on total Erk or total mTOR [4].References:[1]. Takano Y, et al. Selectivity of febuxostat, a novel non–purine inhibitor of xanthine oxidase/xanthine dehydrogenase. Life Sci, 2005, 76(16), 1835–1847.[2]. Sánchez–Lozada LG, et al. Effects of febuxostat on metabolic and renal alterations in rats with fructose–induced metabolic syndrome. Am J Physiol Renal Physiol, 2008, 294(4), F710–F718.[3]. Sánchez–Lozada LG, et al. Effect of febuxostat on the progression of renal disease in 5/6 nephrectomy rats with and without hyperuricemia. Nephron Physiol, 2008, 108(4), p69–p78.[4]. Xu X, et al. Xanthine oxidase inhibition with febuxostat attenuates systolic overload–induced left ventricular hypertrophy and dysfunction in mice. Card Fail, 2008, 14(9), 746–753.Product Name:Febuxostat Cat. No.:HY-14268CAS No.:144060-53-7Molecular Formula:C 16H 16N 2O 3S Molecular Weight:316.37Target:Xanthine Oxidase Pathway:Metabolic Enzyme/Protease Solubility:10 mM in DMSOCaution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@ Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
MEDICA EasyRA

REF 10213-4 4 x 24mL总蛋白 (TP)楔形瓶,每瓶含试剂可用量 24mL。
预期用途EasyRA® TP 试剂目的是用于通过 MEDICA EasyRA 临床化学分析仪进行人血清或血浆(采用肝素锂作为抗凝血剂)中总蛋白的定量测定。
仅用于体外诊断用途。
仅供专业人员使用。
摘要和说明1, 2, 3在人血清中,血清蛋白主要包括白蛋白(占总蛋白的50-60%),其余部分包括α1-、α2-、β- 和γ-球蛋白。
总蛋白的浓度对维持血液和组织之间水的正常平衡与交换是很重要的。
血清蛋白浓度较低,可能是由于吸收不良、合成受损引起的,或是由于出血或过度分解代谢导致蛋白损失而引起的,如肾病和肝病中观察到的情况,这种情况可能导致低蛋白血症。
在高免疫球蛋白血症(如多发性骨髓瘤和感染)或脱水情况下,可能发生高蛋白血症。
方法的原理本分析法采用双缩脲反应测定血清蛋白,其中碱性溶液中的二价铜离子与化合物(含 2 个或多个、与碳原子结合的酰胺基或肽基)反应,形成一种有色络合物4。
碱性 pH血清蛋白质类 + Cu++———— 紫色络合物紫色络合物以分光光度法在 550nm 处测定,以 700nm 作为空白波长。
在较宽的线性范围内,该络合物的密度与蛋白质浓度成正比5。
试剂五水合硫酸铜0.3g/dL氢氧化钠0.8g/dL碘化钾、酒石酸钾钠和叠氮化钠作为防腐剂。
注意事项1.当处理任何实验室试剂时,应遵守良好实验室安全规范(CLSI, GP17-A2)。
2.氢氧化钠具有腐蚀性,能引起烧伤。
不要用嘴吸取试剂。
如果吞服,应立即就医处理。
避免眼部和皮肤接触。
如发生眼部接触,立即用大量水清洗眼睛,并就医处理。
如果发生皮肤接触,用水清洗皮肤至少 15 分钟。
3.本试剂含叠氮化钠 <0.1%;叠氮化钠可与铅和铜水管反应,形成高爆炸性金属叠氮化物。
请参考化学品安全说明书中的危险、危害和安全信息。
4.就任何诊断试验方法而言,其结果应根据所有其它试验结果和患者的临床状况加以解释。
Vazyme-分子生物学与细胞生物学全套解决方案

ClonExpressTM 克隆流程
ClonExpressTM 应用实例
ClonExpressTM 应用实例
Positive rate >90%
ClonExpressTM 应用范围
一步法DNA片段克隆
ClonExpressTM 应用范围
一步法多DNA片段克隆
两片段拼合克隆
五片段拼合克隆
• • • • • RNA 提取 RT-PCR PCR扩增 克隆构建 分子检测
分子生物学系列产品
• Hi-Script Reverse Transcriptase
分子生物学系列产品
• Ace Taq DNA Polymircular ssDNA
Lane 1. cdsDNA control Lane 2. 0.1 U Taq Lane 3. 0.05 U Taq Lane 4. 0.025 U Taq Lane 5. 0.0125 U Taq Lane 6. 0.0625 U Taq Lane 7. 10 U modified Taq Lane 8. cssDNA control
PhantaTM 广泛的模板适应性
47 对针对人基因组设计的引物(GC含量30% ~ 75% ) 55 对针对人cDNA设计的引物(GC含量30% ~ 75% ) Polymerase PhantaTM Phusion PrimerSTAR KOD Pfu Successful amplifications from Human Genomic DNA 43/47 40/47 36/47 21/47 16/47
0.5 kb-5 kb 0.5 kb-2 kb Total 95% 80% 75% 39% 25%
Successful amplifications from Human cDNA 54/55 42/55 40/55 19/55 10/55
IFCC Aspartate Aminotransferase 检测手册说明书

ASTAspartate Aminotransferase IFCCMANUAL RX MONZAINTENDED USEFor the quantitative in vitro determination of AspartateAminotransferase (AST) in serum and plasma. This product is suitable for manual use and on the Rx Monza analyser.Cat. No. AS 1202 R1a. Buffer/Substrate 1 x 70 ml 20 x 2 ml R1b. Enzyme/Coenzyme/ 20 x 2 ml α-oxoglutarate GTIN: 05055273200416AS 1204 R1a. Buffer/Substrate 1 x 105 ml 10 x 10 ml R1b. Enzyme/Coenzyme/ 10 x 10 ml α-oxoglutarate GTIN: 05055273200423AS 1267 R1a. Buffer/Substrate 1 x 105 ml 5 x 20 ml R1b. Enzyme/Coenzyme/ 5 x 20 ml α-oxoglutarate GTIN: 05055273200430AS 2359 R1a. Buffer/Substrate 5 x 100 ml 5 x 100 ml R1b. Enzyme/Coenzyme/ 5 x 100 ml α-oxoglutarate GTIN: 05055273200454UV METHODThis is an optimised standard method according to the concentrations recommended by the IFCC.CLINICAL SIGNIFICANCE (1,2,3,4)The aminotransferases are a group of enzymes that catalyse the inter conversions of amino acids and α-oxoacids by transfer of amino groups. AST (aspartate aminotransferase or glutamate oxaloacetatetransaminase) has been found in the cytoplasm and the mitochondria of cells that have been studied. In cases of mild tissue damage, e.g. liver, the predominant form of serum AST is that from the cytoplasm, with a smaller amount coming from the mitochondria. Severe tissue damage will result in more mitochondrial enzyme being released. Elevated levels of AST can signal myocardial infarction, hepatic disease, muscular dystrophy and organ damage.Although heart muscle is found to have the most activity of the enzyme, significant activity has also been seen in the brain, liver, gastric mucosa, adipose tissue and kidneys of humans.The IFCC has now recommended (1980) standardised procedures for AST determinations including:-1. optimization of substrate concentrations.2. Employment of Tris buffers (instead of phosphate, which has beenshown to inhibit recombination of the apoenzyme with pyridoxal phosphate).3. Pre-incubation of combined buffer and serum to allow sidereactions with NADH to occur. 4. Substrate start (α-oxoglutarate)5. Optional pyridoxal phosphate activation.This is an optimised standard method according to the recommendations of the IFCC.PRINCIPLEα-oxoglutarate reacts with L-aspartate in the presence of AST to form L-glutamate plus oxaloacetate. The indicator reaction utilises the oxaloacetate for a kinetic determination of NADH consumption. AST -oxoglutarate + L-aspartate L-glutamate + oxaloacetate MDH oxaloacetate + NADH + H + L-malate + NAD +SPECIMEN COLLECTION AND PREPARATION (5) Serum:- Use serum free from haemolysis.Plasma:- EDTA or heparin can be used as the anticoagulant.Plasma should be separated from cells within one hour after collection.Specimens should be refrigerated if not used immediately:-Specimens stored longer than 3 days should be frozen at -20︒C.REAGENT COMPOSITIONContents Concentrations in the TestR1a. Buffer/Substrate Tris buffer 80 mmol/l, pH 7.5 L-aspartate 240 mmol/l R1b. Enzyme/Coenzyme/α-oxoglutarate α-oxoglutarate 12 mmol/l MDH ≥420 U/l LD ≥600 U/l NADH 0.18 mmol/lSAFETY PRECAUTIONS AND WARNINGS For in vitro diagnostic use only. Do not pipette by mouth.Exercise the normal precautions required for handling laboratory reagents.Solution R1a contains Sodium Azide. Avoid ingestion or contact with skin or mucous membranes. In case of skin contact, flush affected area with copious amounts of water. In case of contact with eyes or if ingested, seek immediate medical attention.Sodium Azide reacts with lead and copper plumbing, to form potentially explosive azides. When disposing of such reagents flush with large volumes of water to prevent azide build up. Exposed metal surfaces should be cleaned with 10% sodium hydroxide.Health and Safety data sheets available on request.The reagents must be used only for the purpose intended by suitably qualified laboratory personnel, under appropriate laboratory conditions.STABILITY AND PREPARATION OF REAGENTS R1a. Buffer/SubstrateContents ready for use. Stable up to the expiry date when stored at +2 to +8︒C.R1b. Enzyme/Coenzyme/α-oxoglutarate Reconstitute one vial of Enzyme/Coenzyme/α-oxoglutarate R1b with the appropriate volume of Buffer/Substrate R1a: 2 ml for the 20 x 2 ml kit (AS 1202) 10 ml for the 10 x 10 ml kit (AS 1204) 20 ml for the 5 x 20 ml kit (AS 1267) Stable for 14 days at +2 to +8︒C or 24 hours at +15 to +25︒C. Cat. AS 2359 5 x 100 mlReconstitute one vial of Enzyme/Coenzyme/α-oxoglutarate R1b with a portion of Buffer/Substrate R1a and then transfer the entire contents to bottle R1a rinsing bottle R1b several times. Stable for 14 days at +2 to +8︒C or 24 hours at +15 to +25︒C.MATERIALS PROVIDED Buffer/SubstrateEnzyme/Coenzyme/ -oxoglutarateMATERIALS REQUIRED BUT NOT PROVIDEDRandox Assayed Multisera Level 2 (Cat. No. HN 1530) and Level 3 (Cat. No. HE 1532)Randox Calibration Serum Level 3 (Cat. No. CAL 2351) RX series Saline (Cat. No. SA 3854)PROCEDUREAspirate fresh ddH 2O and perform a new Gain Calibration in flow cell mode. Select AST in the Run Test screen and carry out a water blank as instructed.Pipette into a test tube:Sample 0.05 ml Reagent 0.5 mlMix and aspirate into the Rx Monza.CALIBRATION FOR RX MONZAThe use of Saline and Randox Calibration Serum Level 3 isrecommended for calibration. Calibration is recommended with change of reagent lot or as indicated by quality control procedures.FOR MANUAL USEWavelength: 340 nm (Hg 334 nm or Hg 365 nm) Cuvette: 1 cm light path Temperature: 25/30/37︒C Measurement: against airPipette into cuvette: Macro MicroSample 0.2 ml 0.1 ml Enzyme/Coenzyme/ α-oxoglutarate R1 2.0 ml 1.0 mlMix, read initial absorbance after 1 minute. Read again after 1, 2 and 3 minutes. Note: If the absorbance change per minute is between 0.11 and 0.16 at 340/Hg 334 nm 0.06 and 0.08 at Hg 365 nmuse only the values for the first 2 minutes for the calculation.MANUAL CALCULATIONTo calculate the AST activity, use the following formulae:U/l = 1746 x A 340 nm/min U/l = 1780 x A Hg 334 nm/min U/l = 3235 x A Hg 365 nm/minSTANDARDISATIONRandox Calibration Serum Level 3 is traceable to AST reference material JSCC TS01.QUALITY CONTROLRandox Assayed Multisera, Level 2 and Level 3 are recommended for daily quality control. Two levels of controls should be assayed at least once a day. Values obtained should fall within a specified range. If these values fall outside the range and repetition excludes error the following steps should be taken:1. Check instrument settings and light source.2. Check cleanliness of all equipment in use.3. Check water. Contaminants, i.e. bacterial growth, maycontribute to inaccurate results. 4. Check reaction temperature.5. Check expiry date of kit and contents.6. Contact Randox Laboratories Customer Technical Services, Northern Ireland +44 (0) 28 9445 1070.SPECIFICITY/INTERFERENCE (6,7)Gross haemolysis will produce falsely elevated test results. The effects of various drugs on AST activity should be taken intoconsideration in the case of patients receiving large doses of drugs.The analytes below were tested up to the following levels and were found not to interfere: Haemoglobin 250 mg/dl Free Bilirubin 25 mg/dl Conjugate Bilirubin 25 mg/dl Triglycerides 1000 mg/dlIntralipid ® 200 mg/dlA list of substances and conditions known to effect AST activity in vivo is given by both Young et al and Friedman et al. Norepresentation is made by Randox Laboratories Ltd regarding the completeness of these lists and the accuracy of the information contained therein.NORMAL VALUES IN SERUM (8,9) +25︒C +30︒C +37︒C Men up to 18 U/l up to 25 U/l up to 37 U/l Women up to 15 U/l up to 21 U/l up to 31 U/lIt is recommended that each laboratory establish its own reference range to reflect the age, sex, diet and geographical location of the population.SPECIFIC PERFORMANCE CHARACTERISTICS The following performance data were obtained using an Rx Monza analyser running at +37o C.LINEARITYThis method is linear up to 562 U/l. If the sample concentration exceeds this value, dilute the sample 1+9 with 0.9% NaCl solution and re-assay. Multiply the result by 10.SENSITIVITYThe minimum detectable concentration of AST with an acceptable level of precision was determined as 9.3 U/l.PRECISIONIntra AssayLevel 2 Level 3Mean (U/l) 35.6 153SD 1.66 1.47CV(%) 4.65 0.96n 20 20Inter AssayLevel 2 Level 3Mean (U/l) 35.6 153SD 1.77 7.10CV(%) 4.96 4.63n 20 20CORRELATIONThis method (Y) was compared with another commerciallyavailable method (X) and the following linear regression equationobtained:Y = 1.07X + 4.9and a correlation coefficient of r = 0.997543 patient samples were analysed spanning the range 28 to 559U/l.REFERENCES1. Wroblewski F, La Due J.S: Ann Intern Med. 1956; 45: 801.2. Wroblewski F, La Due J.S: Proc Soc Exp Biol Med 1956;91: 569.3. Bergmeyer HU, Bowers GN Jr, et al: Clin Chem 1977; 23:887.4. Bergmeyer HU, Bowers GN Jr, et al: J.Clin Chem ClinBiochem 1980; 18: 521-534.5. Tietz N W: Fundamentals of Clinical Chemistry ed 3.Philadelphia, WB Saunders Co. 1987, pg 372.6. Young D S, et al: Clin Chem 1975, 21; No5.7. Friedman RB, et al: Clin Chem 1980, 26; No4.8. Wallnofer H, Schmidt.E, Schmidt FW, eds: Synopsis derLeberkrankheiten Stuttgart, Georg Thieme Verlag, 1974.9. Thefeld W, et al: Dtsch Med Wschr 1974; 99: 343.Revised 26 Apr 16 biRev. 003THIS PAGE IS INTENTIONALLY BLANK。
高效液相色谱-串联质谱法检测泮托拉唑钠原料药中的水合肼

·药物研发·高效液相色谱-串联质谱法检测泮托拉唑钠原料药中的水合肼赵会明 张振洋 樊华军[英格尔检测技术服务(上海)有限公司 上海 201100]摘要建立了泮托拉唑钠原料药中的基因毒性杂质水合肼的高效液相色谱-串联质谱(LC-MSMS)检测方法。
采用反相色谱,以水-乙腈(含0.1%甲酸)为流动相,梯度洗脱,流速0.5 mL/min,以ESI正离子多反应监测(MRM)模式进行质谱检测。
结果显示,水合肼的检测限和定量限可达到0.23、0.47 ng/mL,其在0.47~9.37 ng/mL浓度范围内线性关系良好(r=0.999 9),准确度试验中低、中、高浓度回收率均在81.6%~90.9%之间。
在3批次泮托拉唑钠原料药中均未检出水合肼。
关键词高效液相色谱-串联质谱法基因毒性杂质泮托拉唑钠水合肼痕量检测中图分类号:R917; O657 文献标志码:A 文章编号:1006-1533(2022)11-0072-04引用本文 赵会明, 张振洋, 樊华军. 高效液相色谱-串联质谱法检测泮托拉唑钠原料药中的水合肼[J]. 上海医药, 2022, 43(11): 72-75.Determination of hydrazine hydrate in pantoprazole sodium by high performance liquid chromatography-tandem mass spectrometryZHAO Huiming, ZHANG Zhenyang, FAN Huajun[ICAS Testing Technology Service (Shanghai) CO., LTD., Shanghai 201100, China]ABSTRACT To establish a high-performance liquid chromatography-tandem mass spectrometry (LC-MSMS) method for the determination of hydrazine hydrate in active pharmaceutical ingredient (API) pantoprazole sodium. HPLC was carried out by reverse chromatography using water-acetonitrile containing 0.1% formic acid as flow phase and gradient elution at a flow rate of 0.5 mL/min. Mass spectrometry was performed with multi-reaction monitoring (MRM) in positive ESI mode. The detection and quantitative limits of hydrazine hydrate reached 0.23, 0.47 ng/mL and hydrazine hydrate showed good linear relationship in the range of 0.47-9.37 ng/mL (r=0.999 9). The recoveries of samples at low, medium and high-level concentrations reached81.6% to 90.9% in the accuracy experiment. No hydrazine hydrate was detected in 3 batches of pantoprazole sodium.KEY WORDS HPLC-tandem mass spectrometry; genotoxic impurities; pantoprazole sodium; hydrazine hydrate; trace determination上消化道出血是近年的临床疾病中常见且多发的一种疾病,其临床表现为呕血、黑便等,如得不到及时有效治疗,可能引发失血性休克。
Emamectin_Benzoate_DataSheet_MedChemExpress

Inhibitors, Agonists, Screening Libraries Data SheetBIOLOGICAL ACTIVITY:Emamectin Benzoate works as a chloride channel activator by binding gamma aminobutyric acid (GABA) receptor andglutamate–gated chloride channels disrupting nerve signals within arthropods.Target: GABA ReceptorEmamectin Benzoate stimulates the release of GABA from the synapses between nerve cells and while additionally increasing GABA's affinity for its receptor on the post–junction membrane of muscle cells in insects and arthropods.PROTOCOL (Extracted from published papers and Only for reference)Animal administration [1]Emamectin benzoate was dissolved in acetone to various concentrations (200, 100, 50, 25, 10, 5.0 and 1.0 mg a.i./L). The nematicidal efficacy of Emamectin benzoate against J2 of M. incognita was determined in aqueous tests. Emamectin benzoate treatments (200,100, 50, 25, 10, 5.0 and 1.0 mg a.i./L) were prepared in acetone + distilled water (10: 90% by volume), and distilled water, as well as a mixture of water with acetone at concentrations equivalent to those in the treatment wells, were used as controls. Then 1 mL ofsolution and 1 mL of root–knot nematodes J2 (containing average 150 J2) was added to each well of a 24–well plate. Well plates were wrapped with parafilm, placed in plastic zip–lock bags and stored in aluminum foil pans covered with another pan to keep them dark.Units were kept at 25°C. After 48 h, the relative percentages of the motile and immotile J2 were evaluated using an invertedmicroscope at 40×magnification. Furthermore, nematodes were moved to distilled water after washing in tap water through a 20 μm pore screen to remove excess chemicals. To confirm the nematicidal activity of emamectin benzoate, immobile 30 J2 from each treatment were collected from the above experiments, transferred to tissue culture plates filled with water, and monitored for 12 h.The experiments had five replications and were repeated three times.References:[1]. Cheng X, et al. Effect of Emamectin Benzoate on Root–Knot Nematodes and Tomato Yield. PLoS One. 2015 Oct 28;10(10):e0141235.Product Name:Emamectin (Benzoate)Cat. No.:HY-B0837CAS No.:155569-91-8Molecular Formula:C 104H 154N 2O 28Molecular Weight:1880.33Target:GABA Receptor; GABA Receptor Pathway:Neuronal Signaling; Membrane Transporter/Ion Channel Solubility:DMSO: ≥ 31 mg/mLCaution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@ Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
凯美达157FSH产品安全数据表说明书

Version 5.0 Revision Date: 05/29/2018SDS Number: 1761653-00005Date of last issue: 02/05/2018 Date of first issue: 06/19/2017SECTION 1. IDENTIFICATIONProduct name: Krytox ™ 157FSHProduct code: D1*******SDS-Identcode: 130000031452Manufacturer or supplier's details Company name of supplier: The Chemours Company FC, LLCAddress : 1007 Market Street Wilmington, DE 19899 United States of America (USA)Telephone : 1-844-773-CHEM (outside the U.S. 1-302-773-1000) Emergency telephone : Medical emergency: 1-866-595-1473 (outside the U.S. 1-302-773-2000) ; Transport emergency: +1-800-424-9300 (outsidethe U.S. +1-703-527-3887) Recommended use of the chemical and restrictions on use Recommended use: LubricantRestrictions on use : For industrial use only. Do not use or resell Chemours™ materials in medical applica-tions involving implantation in the human body or contact withinternal body fluids or tissues unless agreed to by Seller in a written agreement covering such use. For further information, please contact your Chemours representative. SECTION 2. HAZARDS IDENTIFICATIONGHS classification in accordance with 29 CFR 1910.1200 Skin sensitization: Category 1 GHS label elements Hazard pictograms:Signal Word: WarningHazard Statements: H317 May cause an allergic skin reaction. Precautionary Statements:Prevention:P261 Avoid breathing mist or vapors.P272 Contaminated work clothing should not be allowed out ofVersion 5.0 Revision Date: 05/29/2018SDS Number: 1761653-00005Date of last issue: 02/05/2018 Date of first issue: 06/19/2017the workplace.P280 Wear protective gloves.Response:P302 + P352 IF ON SKIN: Wash with plenty of soap and water. P333 + P313 If skin irritation or rash occurs: Get medical advice/ attention.P363 Wash contaminated clothing before reuse.Disposal:P501 Dispose of contents/ container to an approved waste dis-posal plant.Other hazardsThe thermal decomposition vapors of fluorinated plastics may cause polymer fume fever with flu-like symptoms in humans, especially when smoking contaminated tobacco. SECTION 3. COMPOSITION/INFORMATION ON INGREDIENTSSubstance / Mixture: SubstanceSubstance name: Perfluoropolyether carboxylic acidCAS-No.: 51798-33-5Hazardous ingredientsSECTION 4. FIRST AID MEASURESGeneral advice: In the case of accident or if you feel unwell, seek medicaladvice immediately., When symptoms persist or in all cases of doubt seek medical advice.If inhaled: If inhaled, remove to fresh air.Get medical attention if symptoms occur.In case of skin contact: In case of contact, immediately flush skin with soap and plenty of water.Remove contaminated clothing and shoes. Get medical attention.Wash clothing before reuse.Thoroughly clean shoes before reuse.In case of eye contact: Flush eyes with water as a precaution.Get medical attention if irritation develops and persists.If swallowed: If swallowed, DO NOT induce vomiting. Get medical attention if symptoms occur. Rinse mouth thoroughly with water.Most important symptoms: Blurred visionVersion 5.0 Revision Date: 05/29/2018SDS Number: 1761653-00005Date of last issue: 02/05/2018 Date of first issue: 06/19/2017and effects, both acute and delayedRashDiscomfort IrritationSensitization Redness DermatitisMay cause an allergic skin reaction.Protection of first-aiders: First Aid responders should pay attention to self-protection, and use the recommended personal protective equipment when the potential for exposure exists.Notes to physician: Treat symptomatically and supportively.SECTION 5. FIRE-FIGHTING MEASURESSuitable extinguishing media : Not applicable Will not burnUnsuitable extinguishing media : Not applicable Will not burn Specific hazards during fire fighting: Exposure to combustion products may be a hazard to health. Hazardous combustion prod-ucts : Hydrogen fluoride carbonyl fluoride potentially toxic fluorinated compoundsaerosolized particulates Carbon oxidesSpecific extinguishing meth-ods : Use extinguishing measures that are appropriate to local cir-cumstances and the surrounding environment. Use water spray to cool unopened containers.Remove undamaged containers from fire area if it is safe to do so.Evacuate area.Special protective equipment for fire-fighters : In the event of fire, wear self-contained breathing apparatus. Use personal protective equipment. SECTION 6. ACCIDENTAL RELEASE MEASURESPersonal precautions, protec-tive equipment and emer-gency procedures: Use personal protective equipment. Follow safe handling advice and personal protectiveequipment recommendations.Environmental precautions : Discharge into the environment must be avoided. Prevent further leakage or spillage if safe to do so.Prevent spreading over a wide area (e.g., by containment or oil barriers).Retain and dispose of contaminated wash water.Local authorities should be advised if significant spillages cannot be contained.Version 5.0 Revision Date: 05/29/2018SDS Number: 1761653-00005Date of last issue: 02/05/2018 Date of first issue: 06/19/2017Methods and materials for containment and cleaning up : Soak up with inert absorbent material. For large spills, provide diking or other appropriate containment to keep material from spreading. If diked materialcan be pumped, store recovered material in appropriate container.Clean up remaining materials from spill with suitable absorbent.Local or national regulations may apply to releases anddisposal of this material, as well as those materials and items employed in the cleanup of releases. You will need to determine which regulations are applicable.Sections 13 and 15 of this SDS provide information regarding certain local or national requirements. SECTION 7. HANDLING AND STORAGETechnical measures: See Engineering measures under EXPOSURE CONTROLS/PERSONAL PROTECTION section.Local/Total ventilation: Use only with adequate ventilation.Advice on safe handling: Do not get on skin or clothing. Avoid inhalation of vapor or mist. Do not swallow.Avoid contact with eyes.Handle in accordance with good industrial hygiene and safety practice, based on the results of the workplace exposure assessmentTake care to prevent spills, waste and minimize release to the environment.Conditions for safe storage: Keep in properly labeled containers.Store in accordance with the particular national regulations.Materials to avoid: No special restrictions on storage with other products.Further information on stor-age stability: No decomposition if stored and applied as directed.SECTION 8. EXPOSURE CONTROLS/PERSONAL PROTECTIONIngredients with workplace control parametersContains no substances with occupational exposure limit values.Occupational exposure limits of decomposition productsVersion 5.0 Revision Date: 05/29/2018SDS Number: 1761653-00005Date of last issue: 02/05/2018 Date of first issue: 06/19/2017Engineering measures: Processing may form hazardous compounds (see section 10).Ensure adequate ventilation, especially in confined areas. Minimize workplace exposure concentrations.Personal protective equipment Respiratory protection : General and local exhaust ventilation is recommended to maintain vapor exposures below recommended limits. Whereconcentrations are above recommended limits or areunknown, appropriate respiratory protection should be worn. Follow OSHA respirator regulations (29 CFR 1910.134) and use NIOSH/MSHA approved respirators. Protection provided by air purifying respirators against exposure to anyhazardous chemical is limited. Use a positive pressure air supplied respirator if there is any potential for uncontrolled release, exposure levels are unknown, or any othercircumstance where air purifying respirators may not provide adequate protection.Hand protectionMaterial: Chemical-resistant glovesVersion 5.0 Revision Date: 05/29/2018SDS Number: 1761653-00005Date of last issue: 02/05/2018 Date of first issue: 06/19/2017Remarks: Choose gloves to protect hands against chemicals depending on the concentration specific to place of work. Breakthrough time is not determined for the product. Change gloves often! For special applications, we recommend clarifying the resistance to chemicals of the aforementioned protective gloves with the glove manufacturer. Wash hands before breaks and at the end of workday.Eye protection: Wear the following personal protective equipment: Safety glassesSkin and body protection: Select appropriate protective clothing based on chemical resistance data and an assessment of the local exposure potential.Skin contact must be avoided by using impervious protective clothing (gloves, aprons, boots, etc).Hygiene measures: Ensure that eye flushing systems and safety showers are located close to the working place.When using do not eat, drink or smoke. Wash contaminated clothing before re-use.SECTION 9. PHYSICAL AND CHEMICAL PROPERTIESAppearance: viscous liquidColor: clear, amber, dark grayOdor: odorlessOdor Threshold : No data availablepH: No data availableMelting point/freezing point: No data availableInitial boiling point and boiling range: No data available Flash point: does not flashEvaporation rate: No data availableFlammability (solid, gas): Not applicableFlammability (liquids): Will not burnUpper explosion limit / Upper flammability limit: No data available Lower explosion limit / Lower flammability limit: No data availableVersion 5.0 Revision Date: 05/29/2018SDS Number: 1761653-00005Date of last issue: 02/05/2018 Date of first issue: 06/19/2017Vapor pressure: No data availableRelative vapor density: No data available Relative density: 1.9 Solubility(ies)Water solubility: insolublePartition coefficient: n-octanol/water: No data available Autoignition temperature: No data availableDecomposition temperature: 338 - 392 °F / 170 - 200 °CViscosityViscosity, kinematic: No data availableExplosive properties: Not explosiveOxidizing properties: The substance or mixture is not classified as oxidizing.Particle size: Not applicableSECTION 10. STABILITY AND REACTIVITYReactivity: Not classified as a reactivity hazard.Chemical stability: Stable under normal conditions.Possibility of hazardous reac-tions: Hazardous decomposition products will be formed at elevated temperatures.Conditions to avoid: None known.Incompatible materials: None.Hazardous decomposition products Thermal decomposition : Hydrofluoric acid Carbonyl difluorideCarbon dioxide Carbon monoxide SECTION 11. TOXICOLOGICAL INFORMATIONInformation on likely routes of exposure Inhalation Skin contact Ingestion Eye contactVersion 5.0 Revision Date:05/29/2018SDS Number: 1761653-00005Date of last issue: 02/05/2018 Date of first issue: 06/19/2017Acute toxicityNot classified based on available information. Components:Perfluoropolyether carboxylic acid: Acute oral toxicity : LD50 (Rat): > 5,000 mg/kg Acute dermal toxicity : LD50 (Rat): > 5,000 mg/kg Skin corrosion/irritationNot classified based on available information. Components:Perfluoropolyether carboxylic acid: Species : Rabbit Result : No skin irritationSerious eye damage/eye irritationNot classified based on available information. Components:Perfluoropolyether carboxylic acid: Species : Rabbit Result : No eye irritationRespiratory or skin sensitization Skin sensitizationMay cause an allergic skin reaction.Respiratory sensitizationNot classified based on available information. Components:Perfluoropolyether carboxylic acid: Test Type : Local lymph node assay (LLNA) Routes of exposure : Skin contact Species : Mouse Assessment : Probability or evidence of low to moderate skin sensitizationrate in humansResult : positiveGerm cell mutagenicityNot classified based on available information. Components:Perfluoropolyether carboxylic acid: Germ cell mutagenicity - Assessment : Weight of evidence does not support classification as a germ cell mutagen.Version 5.0 Revision Date: 05/29/2018SDS Number: 1761653-00005Date of last issue: 02/05/2018 Date of first issue: 06/19/2017CarcinogenicityNot classified based on available information. IARC No ingredient of this product present at levels greater than or equal to 0.1% isidentified as probable, possible or confirmed human carcinogen by IARC.OSHA No component of this product present at levels greater than or equal to 0.1% ison OSHA’s list of regulated carcinogens.NTP No ingredient of this product present at levels greater than or equal to 0.1% isidentified as a known or anticipated carcinogen by NTP. Reproductive toxicityNot classified based on available information. STOT-single exposureNot classified based on available information. STOT-repeated exposureNot classified based on available information. Components:Perfluoropolyether carboxylic acid: Assessment : No significant health effects observed in animals at concentra-tions of 100 mg/kg bw or less.Repeated dose toxicity Components:Perfluoropolyether carboxylic acid: Species : Rat NOAEL : 1,000 mg/kg LOAEL : > 1,000 mg/kg Application Route : Ingestion Exposure time : 28 d Remarks : No significant adverse effects were reportedAspiration toxicityNot classified based on available information. SECTION 12. ECOLOGICAL INFORMATIONEcotoxicityNo data availablePersistence and degradability No data availableBioaccumulative potential No data availableVersion 5.0 Revision Date: 05/29/2018SDS Number: 1761653-00005Date of last issue: 02/05/2018 Date of first issue: 06/19/2017Mobility in soil No data available Other adverse effects No data availableSECTION 13. DISPOSAL CONSIDERATIONSDisposal methods Waste from residues : Dispose of in accordance with local regulations.Contaminated packaging: Empty containers should be taken to an approved waste handling site for recycling or disposal.If not otherwise specified: Dispose of as unused product.SECTION 14. TRANSPORT INFORMATIONInternational Regulations UNRTDG Not regulated as a dangerous good IATA-DGR Not regulated as a dangerous good IMDG-Code Not regulated as a dangerous goodTransport in bulk according to Annex II of MARPOL 73/78 and the IBC Code Not applicable for product as supplied. Domestic regulation49 CFR Not regulated as a dangerous good SECTION 15. REGULATORY INFORMATIONEPCRA - Emergency Planning and Community Right-to-Know CERCLA Reportable QuantityThis material does not contain any components with a CERCLA RQ. SARA 304 Extremely Hazardous Substances Reportable QuantityThis material does not contain any components with a section 304 EHS RQ. SARA 302 Extremely Hazardous Substances Threshold Planning Quantity This material does not contain any components with a section 302 EHS TPQ. SARA 311/312 Hazards: Respiratory or skin sensitizationSARA 313: This material does not contain any chemical components with known CAS numbers that exceed the threshold (De Minimis) reporting levels established by SARA Title III, Section 313.Version 5.0 Revision Date:05/29/2018SDS Number: 1761653-00005Date of last issue: 02/05/2018 Date of first issue: 06/19/2017US State RegulationsPennsylvania Right To Know Perfluoropolyether carboxylic acid51798-33-5California Prop. 65This product does not contain any chemicals known to the State of California to cause cancer, birth, or any other reproductive defects.SECTION 16. OTHER INFORMATIONFurther information NFPA 704:HMIS® IV:HMIS® ratings are based on a 0-4 rating scale, with 0 representing minimal haz-ards or risks, and 4 representing signifi-cant hazards or risks. The "*" represents a chronic hazard, while the "/" represents the absence of a chronic hazard.Krytox™ and any associated logos are trademarks or copyrights of The Chemours Company FC, LLC.Chemours™ and the Chemours Logo are trademarks of The Chemours Company. Before use read Chemours safety information.For further information contact the local Chemours office or nominated distributors.All chemical substances in this material are included on or exempted from listing on the TSCA Inventory of Chemical Substances.Full text of other abbreviations ACGIH : USA. ACGIH Threshold Limit Values (TLV) NIOSH REL : USA. NIOSH Recommended Exposure Limits OSHA Z-1 : USA. Occupational Exposure Limits (OSHA) - Table Z-1 Lim-its for Air Contaminants OSHA Z-2 : USA. Occupational Exposure Limits (OSHA) - Table Z-2 ACGIH / TWA : 8-hour, time-weighted average ACGIH / STEL : Short-term exposure limit ACGIH / C : Ceiling limit NIOSH REL / TWA : Time-weighted average concentration for up to a 10-hourworkday during a 40-hour workweekNIOSH REL / ST : STEL - 15-minute TWA exposure that should not be exceededat any time during a workdayNIOSH REL / C : Ceiling value not be exceeded at any time. OSHA Z-1 / TWA : 8-hour time weighted averageFlammability H e a l t hInstabilitySpecial hazard.Version 5.0 Revision Date: 05/29/2018SDS Number: 1761653-00005Date of last issue: 02/05/2018 Date of first issue: 06/19/2017OSHA Z-2 / TWA : 8-hour time weighted averageAICS - Australian Inventory of Chemical Substances; ASTM - American Society for the Testing of Materials; bw - Body weight; CERCLA - Comprehensive Environmental Response, Compensation, and Liability Act; CMR - Carcinogen, Mutagen or Reproductive Toxicant; DIN - Standard of the German Institute for Standardisation; DOT - Department of Transportation; DSL - Domestic Sub-stances List (Canada); ECx - Concentration associated with x% response; EHS - Extremely Haz-ardous Substance; ELx - Loading rate associated with x% response; EmS - Emergency Schedule; ENCS - Existing and New Chemical Substances (Japan); ErCx - Concentration associated with x% growth rate response; ERG - Emergency Response Guide; GHS - Globally Harmonized Sys-tem; GLP - Good Laboratory Practice; HMIS - Hazardous Materials Identification System; IARC - International Agency for Research on Cancer; IATA - International Air Transport Association; IBC - International Code for the Construction and Equipment of Ships carrying Dangerous Chemicals in Bulk; IC50 - Half maximal inhibitory concentration; ICAO - International Civil Aviation Organiza-tion; IECSC - Inventory of Existing Chemical Substances in China; IMDG - International Maritime Dangerous Goods; IMO - International Maritime Organization; ISHL - Industrial Safety and Health Law (Japan); ISO - International Organisation for Standardization; KECI - Korea Existing Chemi-cals Inventory; LC50 - Lethal Concentration to 50 % of a test population; LD50 - Lethal Dose to 50% of a test population (Median Lethal Dose); MARPOL - International Convention for the Pre-vention of Pollution from Ships; MSHA - Mine Safety and Health Administration; n.o.s. - Not Oth-erwise Specified; NFPA - National Fire Protection Association; NO(A)EC - No Observed (Adverse) Effect Concentration; NO(A)EL - No Observed (Adverse) Effect Level; NOELR - No Observable Effect Loading Rate; NTP - National Toxicology Program; NZIoC - New Zealand Inventory of Chemicals; OECD - Organization for Economic Co-operation and Development; OPPTS - Office of Chemical Safety and Pollution Prevention; PBT - Persistent, Bioaccumulative and Toxic sub-stance; PICCS - Philippines Inventory of Chemicals and Chemical Substances; (Q)SAR - (Quanti-tative) Structure Activity Relationship; RCRA - Resource Conservation and Recovery Act; REACH - Regulation (EC) No 1907/2006 of the European Parliament and of the Council concern-ing the Registration, Evaluation, Authorisation and Restriction of Chemicals; RQ - Reportable Quantity; SADT - Self-Accelerating Decomposition Temperature; SARA - Superfund Amend-ments and Reauthorization Act; SDS - Safety Data Sheet; TCSI - Taiwan Chemical Substance Inventory; TSCA - Toxic Substances Control Act (United States); UN - United Nations; UNRTDG - United Nations Recommendations on the Transport of Dangerous Goods; vPvB - Very Persistent and Very BioaccumulativeSources of key data used to compile the Material Safety Data Sheet : Internal technical data, data from raw material SDSs, OECD eChem Portal search results and European Chemicals Agen-cy, http://echa.europa.eu/ Revision Date : 05/29/2018Items where changes have been made to the previous version are highlighted in the body of this document by two vertical lines.The information provided in this Safety Data Sheet is correct to the best of our knowledge, information and belief at the date of its publication. The information is designed only as a guidance for safe handling, use, processing, storage, transportation, disposal and release and shall not be considered a warranty or quality specification of any type. The information provided relates only to the specific material identified at the top of this SDS and may not be valid when the SDS material is used in combination with any other materials or in any process, unless specified in the text. Material users should review the information and recommendations in the specific context of their intended manner of handling, use, processing and storage, including an assessment of the appropriateness of the SDS material in the user’s end product, if applicabl e.US / Z8。
BAOSR6x86.03 OSR General Chemistry 产品说明书

BAOSR6x86.03 OSR General Chemistry 2012-01 IRONOSR6186 4 x 15 mL R14 x 15 mL R2OSR6286 4 x 30 mL R1 4 x 30 mL R2Intended UseSystem reagent for the quantitative determination of Iron in human serum on Beckman Coulter AU analyzers.Summary Iron (non-heme) measurements are used in the diagnosis and treatment of diseases such as iron deficiency anemia, hemochromatosis (a disease associated with widespread deposit in the tissues of two iron-containing pigments, hemosiderin and hemofuscin, and characterized by pigmentation of the skin), and chronic renal disease. Transferrin is the major iron carrying protein in the serum.MethodologyIn 1954, Schade et al.1introduced a method for the direct determination of serum iron. The iron level was determined by incubating the serum in a phosphate buffer with ascorbic acid and terpyridine. Goodwin,2 in 1966, proposed a direct method for serum iron using an acetate buffer andbathophenanthroline. These modifications eliminated random iron contamination from phosphate buffers and enhanced color development by using a more sensitive iron chromogen.This Beckman Coulter method utilizes a variation of these methods using TPTZ [2,4,6-Tri-(2-pyridyl)-5-triazine] as the chromogen.3In an acidicmedium, transferrin-bound iron dissociates into free ferric ions and apo-transferrin. Hydrochloric acid and sodium ascorbate reduce the ferric ions to the ferrous state. The ferrous ions then react with TPTZ to form a blue colored complex which can be measured bichromatically at 600/800 nm. The increase in absorbance is directly proportional to the amount of transferrin bound iron present.Buffer Transferrin 2 (Fe 3+) 2 (Fe 3+) + Apo-transferrin2 Fe 3+ + Ascorbic Acid + 2 H 2O 2 Fe 2+ + Dehydroascorbic Acid + 2 H 30+Fe 2+ + TPTZ Iron-complex (blue colored complex)System Information For AU400/400e /480, AU600/640/640e/680 and AU2700/5400/AU5800 Beckman Coulter Analy z ers .ReagentsFinal concentration of reactive ingredients:Glycine buffer (pH 1.7) 215mmol/L L-Ascorbic Acid 4.7mmol/L 2,4,6-Tri-(2-pyridyl)-5-triazine 0.5mmol/L Also contains preservativesPrecautions1. For in vitro diagnostic use.2. WARNING! CORROSIVE! Do not pipet by mouth. Avoid contact with eyes, skin or clothing. In case of contact, immediately flush affected areawith plenty of water for 15 minutes. Obtain medical attention immediately for eye contact or ingestion.Preparation of ReagentsThe Iron Reagents are ready for use. No preparation is required.Storage and StabilityThe reagents are stable, if unopened, up to the stated expiration date when stored at 2 - 8°C. Opened reagents are stable for 60 days when stored in the refrigerated compartment of the analyzer.The color of R1 turns to brown during the course of the shelf life. This does not restrict any function of this reagent as long as reagent OD results on the analyzer are within specified limits.Indications of DeteriorationVisible signs of microbial growth, turbidity or precipitation or any change in the color of the reagent may indicate degradation and warrantdiscontinuance of use.Specimen Collection and PreparationSerum or heparinized plasma samples, free from hemolysis, are the recommended specimens. Remove serum from the red cells to minimize hemolysis as hemolyzed samples may produce erroneous results. Plasma specimens collected with EDTA, oxalate, or citrate are unsatisfactory, since they bind iron, preventing its reaction with the chromogen. Samples should be taken in the morning from patients in a fasting state, since iron values decrease by 30% during the course of the day 4 and there can be significant interference from lipemia.Sample Storage and Stability Serum iron is stable for 7 days when stored at 2 - 8°C or 4 days at room temperature (15 - 25°C) after the serum is separated from red cells.5IronInterfering SubstancesResults of studies6 show that the following substances interfere with this iron procedure when tested at 150 µg/dL Iron.The criteria for no significant interference is recovery within 10% of the initial value.Bilirubin: No significant interference up to 40 mg/dL BilirubinCopper: No significant interference up to 1 mg/dL CopperGlobulin: No significant interference up to 5 g/dL Human Gamma GlobulinLipemia: No significant interference up to 400 mg/dL Intralipid*Hemolysis*** Intralipid, manufactured by KabiVitrium Inc., is a 20% IV fat emulsion used to emulate extremely turbid samples.** Hemolyzed samples should not be tested. Hemolyzed samples may react with the reagent producing results with a negative bias.In very rare cases gammopathy, especially monoclonal IgM (Waldenström’s macroglobulinemia), may cause unreliable results10.The information presented is based on results from Beckman Coulter studies and is current at the date of publication. Beckman Coulter Inc., makes no representation about the completeness or accuracy of results generated by future studies. For further information on interfering substances, refer to Young for a compilation of reported interferences with this test.7ProcedureA complete list of test parameters and operational procedure can be found in the User’s Guide appropriate to the analyzer.Materials ProvidedIron ReagentMaterials Required But Not ProvidedChemistry Calibrator (Cat # DR0070)Stability of Final Reaction MixtureThe Beckman Coulter AU analyzer automatically computes every determination at the same time interval.CalibrationThe frequency of calibration is 30 days. Calibration of this iron procedure is accomplished by use of the Chemistry Calibrator (Cat # DR0070), which is traceable to the National Institutes of Standards and Technology (NIST) Standard Reference Material (SRM) 1598 and 937.Recalibration of this test is required when any of these conditions exist:1. A reagent lot number has changed or there is an observed shift in control values.2. A fresh bottle of reagent is used for testing.3. Major preventative maintenance was performed on the analyzer or a critical part was replaced.Quality ControlDuring operation of the Beckman Coulter AU analyzer at least two levels of an appropriate quality control material should be tested a minimum of once a day. In addition, controls should be performed after calibration, with each new lot of reagent, and after specific maintenance or troubleshooting steps described in the appropriate User’s Guide. Quality control testing should be performed in accordance with regulatory requirements and each laboratory’s standard procedure.ResultsAutomatically printed out for each sample in µg/dL at 37°C. For SI units (µmol/L) multiply result by 0.179.Dynamic RangeThe Iron procedure is linear from 10 to 1000 µg/dL. Samples exceeding the upper limit of linearity should be diluted and repeated. The sample may be diluted, repeated and multiplied by the dilution factor automatically by utilizing the AUTO REPEAT RUN.Expected ValuesAdult:850 - 212 µg/dLExpected values may vary with age, sex, diet and geographical location. Each laboratory should determine its own expected values as dictated by good laboratory practice.Specific Performance CharacteristicsThe following data was obtained using the Iron Reagent on Beckman Coulter AU analyzers according to established procedures. Results obtained in individual laboratories may differ.Precision11Estimates of precision, based on CLSI recommendations9, are consistent with typical performance. The within run precision is less than 3% CV and total precision is less than 5% CV.Assays of control sera were performed and the data reduced following CLSI guidelines above:N = 60 Within run TotalMean, µg/dL SD CV% SD CV%53.6 0.54 1.02 1.12 2.09158 1.05 0.66 2.8 1.77589 3.81 0.65 7.23 1.23OSR General Chemistry BAOSR6x86.032012-01IronBAOSR6x86.03 OSR General Chemistry2012-01 Method Comparison 11 Patient samples were used to compare this Iron Reagent. The table below demonstrates representative performance on AU analyzers. Y Method AU640X Method OSR6123/6223Slope 1.040Intercept 2.23Correlation Coeff. (r) 0.995No. of Samples (n) 96Range (µg/dL) 11.60-253SensitivityTypical change in absorbance for 1 µg/dL of Iron is 0.2 mAbsorbance.References1. Schade, A., Ogama, J., Reinhart, R., and Miller, J., Proc. Soc. Exp. Biol. Med., 87: 442, 1954.2. Goodwin, J., Murphy, B., and Guillemette, M., Clin. Chem., 12: 47, 1966.3. Diehl, H., and Smith, G.F., “The Iron Reagents, Bathophenanthroline, 2,4,6-Tripyridyl-s-triazine, Phenyl-2-pyridylocine”, GF Smith Chem Co, 1960.4. Tietz, N.W. Textbook of Clinical Chemistry, WB. Saunders, 1580, 1986.5. Henry, R.J. et al. Clinical Chemistry: Principles and Technics, Harper and Row, 1974.6. CLSI/NCCLS, Interference Testing in Clinical Chemistry EP7-P, 1986.7. Young, D.S., Effects of Drugs on Clinical Laboratory Tests, 5th Edition, AACC Press, 2000.8. Beckman Coulter Inc. data on samples collected from 200 blood donors in North Texas.9. CLSI/NCCLS Evaluation Protocol, EP5-T2, 1992.10. Bakker, A.J., Clin. Chem. 37:690,1991.11. Data is on file for specific AU analyzers.Manufactured by: Beckman Coulter, Inc., 250 S. Kraemer Blvd. Brea, CA 92821, USA。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Product Name:
AM630CAS No.:
164178-33-0
Product Data Sheet
Cat. No.:
HY-15421MWt:
504.36Formula:
C23H25IN2O3Purity :
>98%
Solubility:Mechanisms:
Biological Activity:
Pathways:GPCR/G protein; Target:Cannabinoid Receptor DMSO
g y AM630 is a selectivity CB2 antagonist with Ki of 31.2 nM; > 150 fold selectivity over CB1 receptor.
IC50 Value: 31.2 nM
Target: CB2 receptor AM630 is a selective CB2 receptor antagonist that binds to CB1 and CB2 receptors with Ki values of
5.2 uM and 31.2 nM, respectively. AM630 has been shown to display 165-fold selectivity over CB1receptors and behave as a weak partial/inverse agonist at CB1 receptors. AM630 acts as a
cannabinoid receptor antagonist in mouse brain, vas deferens and guinea pig brain, but acts as an agonist in guinea pig leum. AM630 acts as an inverse agonist on cloned human CB1 receptors....References:
[1]. Garcia-Gutierrez, Maria S.; Garcia-Bueno, Borja; Zoppi, Silvia et al. Chronic blockade of cannabinoid CB2 receptors induces anxiolytic-like actions associated with alterations in GABAA
receptors. British Journal of Pharmacology (2012), 165(4), 951-964.[2]. Patil, Mayur; Patwardhan, Amol; Salas, Margaux M. et al. Cannabinoid receptor antagonists g g p g g p []y g p g
AM251 and AM630 activate TRPA1 in sensory neurons. Neuropharmacology (2011), 61(4), 778-788.[3]. Geng, D. C.; Xu, Y. Z.; Yang, H. L. et al. Inhibition of titanium particle-induced inflammatory
osteolysis through inactivation of cannabinoid receptor 2 by AM630. Journal of Biomedical Materials
Research, Part A (2010), 95A(1), 321-326.[4]. Mukherjee, Sutapa; Adams, Monique; Whiteaker, Kristi et al. Species comparison and
pharmacological characterization of rat and human CB2 cannabinoid receptors. European Jour...
Caution: Not fully tested. For research purposes only
Medchemexpress LLC
18 W i l k i n s o n W a y , P r i n c e t o n , N J 08540,U S A
E m a i l : i n f o @m e d c h e m e x p r e s s .c o m W e b : w w w .m e d c h e m e x p r e s s .c o m。
