BMS-303141_DataSheet_MedChemExpress
利用甲型流感病毒阳性临床标本制备质控品

利用甲型流感病毒阳性临床标本制备质控品李瑜霞;石翰铂;余斐;郑书发;崔大伟;曹红翠【摘要】目的制备一种能适用于甲型流感病毒核酸提取和PCR检测全过程的质控品.方法采用TRIzol试剂灭活高、中、低3种浓度的甲型流感病毒阳性的痰液标本,分析TRIzol试剂对检测结果的影响;放置于35℃以及反复冻融,评估灭活后标本的稳定性;并在不同实验室间采用不同试剂进行适用性评价.结果 TRIzol试剂对核酸检测无影响,处理后的高、中、低浓度标本反复冻融40次病毒浓度无明显降低,变异系数分别为1.26%、1.54%、1.54%,均低于试剂盒批内精密度.TRIzol试剂处理的标本在35℃环境保存40 d后高、中、低浓度变异系数分别为3.13%、2.77%、2.20%,变异系数均低于试剂盒批间精密度.在不同单位采用不同试剂均能检测出高、中、低不同浓度质控品.结论采用TRIzol试剂灭活甲型流感病毒阳性标本制备的质控品在瓶间差、热稳定和长期保存时效等方面性能较优,可成为日后临床实验室开展甲型流感病毒核酸检测的理想质控品.【期刊名称】《临床检验杂志》【年(卷),期】2018(036)008【总页数】3页(P634-636)【关键词】甲型流感病毒;TRIzol;病毒灭活;质控品【作者】李瑜霞;石翰铂;余斐;郑书发;崔大伟;曹红翠【作者单位】浙江大学医学院附属第一医院检验科,杭州310003;浙江大学医学院附属第一医院浙江省肝胆胰肿瘤精准诊治研究重点实验室,杭州310003;浙江大学医学院附属第一医院检验科,杭州310003;浙江大学医学院附属第一医院检验科,杭州310003;浙江大学医学院附属第一医院输血科,杭州310003;浙江大学医学院附属第一医院传染病诊治协同创新中心,杭州310003【正文语种】中文【中图分类】R446核酸检测技术是目前临床实验室检测流感病毒较常使用的方法,具有灵敏度高、特异性好等优点[1],但检测样本中的抑制物、核酸提取过程中的残留试剂等可能会影响PCR扩增的效率,造成结果的偏差,因此需要对检测全过程进行质量控制[2]。
奥美沙坦酯原料药的稳定性试验研究

奥美沙坦酯原料药的稳定性试验研究
沈静远;梁毅
【期刊名称】《机电信息》
【年(卷),期】2017(0)32
【摘要】针对奥美沙坦酯原料药注册申报的要求,对申报注册生产的连续三个批次的奥美沙坦酯进行稳定性试验,包括影响因素试验、加速稳定性试验、长期稳定性试验.根据影响因素试验结果可知,本品在高温、高湿和强光照射条件下的稳定性较好;根据6个月的加速稳定性试验和长期稳定性试验数据汇总结果,建议奥美沙坦酯原料药的有效期暂定为2年;根据分析方法验证中的强降解试验结果,建议采用乙腈作为溶剂进行样品检测时,样品溶液需避光,可使用棕色瓶配制溶液.
【总页数】4页(P46-49)
【作者】沈静远;梁毅
【作者单位】中国药科大学,江苏南京211198;中国药科大学,江苏南京211198【正文语种】中文
【相关文献】
1.海洋星虫多糖原料药稳定性试验研究 [J], 刘玉明;钱甜甜;蒋定文;何颖;沈先荣;江叔奇
2.盐酸林可霉素原料药稳定性研究 [J], 刘学威;王灵灵;薛娟;李贵文;王留国;秦宝福
3.索非布韦原料药的质量控制及稳定性研究 [J], 严宾; 冯成亮
4.索非布韦原料药的质量控制及稳定性研究 [J], 严宾; 冯成亮
5.奥美沙坦酯原料药的稳定性试验研究 [J], 沈静远;梁毅
因版权原因,仅展示原文概要,查看原文内容请购买。
阿芬太尼调节SphK1

阿芬太尼调节SphK 1/S 1P 信号通路对急性心肌梗死大鼠心肌纤维化的影响Δ肖锦亮*,邹雪,但家朋 #[荆州市中心医院(长江大学附属荆州医院)麻醉科,湖北 荆州 434000]中图分类号 R 965;R 542.2+2 文献标志码 A 文章编号 1001-0408(2024)08-0955-06DOI 10.6039/j.issn.1001-0408.2024.08.10摘要 目的 探究阿芬太尼(ALF )调节鞘氨醇激酶1(SphK 1)/1-磷酸鞘氨醇(S 1P )信号通路对急性心肌梗死(AMI )大鼠心肌纤维化的影响。
方法 取雄性SD 大鼠,采用左冠状动脉前降支结扎法构建AMI 模型,并将造模成功的大鼠随机分为AMI 模型组(Model 组)和低剂量ALF 组(ALF-L 组,予0.25 mg/kg ALF )、高剂量ALF 组(ALF-H 组,予0.5 mg/kg ALF )、高剂量ALF+SphK 1激活剂组(ALF-H+K 6PC-5组,予0.5 mg/kg ALF+1 μg/g K 6PC-5),同时设置仅进行开/关胸操作而不作左冠状动脉前降支结扎的假手术组(Sham 组),每组15只。
各药物组大鼠腹腔注射相应药液,每天1次,连续4周。
末次给药12 h 后,检测各组大鼠的心功能指标[左室收缩压(LVSP )、左室射血分数(LVEF )、左室收缩期内径(LVSD )、左室短轴缩短率(LVFS )],观察其心肌梗死情况、心肌组织病理改变和纤维化程度,检测其血清脑钠肽(BNP )、心肌肌钙蛋白Ⅰ(cTn Ⅰ)水平以及心肌组织中Ⅰ型胶原蛋白(collagen Ⅰ)、collagen Ⅲ、基质金属蛋白酶2(MMP-2)、SphK 1、S 1P 蛋白的表达水平。
结果 与Sham 组比较,Model 组大鼠心肌组织细胞排列紊乱,可见大量炎症细胞浸润;LVSP 、LVFS 、LVEF 均显著降低(P <0.05);LVSD ,心肌梗死面积、心肌组织胶原容积分数,血清BNP 、cTn Ⅰ水平,心肌组织中collagen Ⅰ、collagen Ⅲ、MMP-2、SphK 1、S 1P 蛋白的表达水平均显著升高或增大(P <0.05)。
Gelucire-14-44-SDS-MedChemExpress

Inhibitors, Agonists, Screening LibrariesSafety Data Sheet Revision Date:Nov.-23-2018Print Date:Nov.-23-20181. PRODUCT AND COMPANY IDENTIFICATION1.1 Product identifierProduct name :Gelucire 14/44Catalog No. :HY-Y1892CAS No. :121548-04-71.2 Relevant identified uses of the substance or mixture and uses advised againstIdentified uses :Laboratory chemicals, manufacture of substances.1.3 Details of the supplier of the safety data sheetCompany:MedChemExpress USATel:609-228-6898Fax:609-228-5909E-mail:sales@1.4 Emergency telephone numberEmergency Phone #:609-228-68982. HAZARDS IDENTIFICATION2.1 Classification of the substance or mixtureNot a hazardous substance or mixture.2.2 GHS Label elements, including precautionary statementsNot a hazardous substance or mixture.2.3 Other hazardsNone.3. COMPOSITION/INFORMATION ON INGREDIENTS3.1 SubstancesSynonyms:NoneFormula:N/AMolecular Weight:N/ACAS No. :121548-04-74. FIRST AID MEASURES4.1 Description of first aid measuresEye contactRemove any contact lenses, locate eye-wash station, and flush eyes immediately with large amounts of water. Separate eyelids with fingers to ensure adequate flushing. Promptly call a physician.Skin contactRinse skin thoroughly with large amounts of water. Remove contaminated clothing and shoes and call a physician.InhalationImmediately relocate self or casualty to fresh air. If breathing is difficult, give cardiopulmonary resuscitation (CPR). Avoid mouth-to-mouth resuscitation.IngestionWash out mouth with water; Do NOT induce vomiting; call a physician.4.2 Most important symptoms and effects, both acute and delayedThe most important known symptoms and effects are described in the labelling (see section 2.2).4.3 Indication of any immediate medical attention and special treatment neededTreat symptomatically.5. FIRE FIGHTING MEASURES5.1 Extinguishing mediaSuitable extinguishing mediaUse water spray, dry chemical, foam, and carbon dioxide fire extinguisher.5.2 Special hazards arising from the substance or mixtureDuring combustion, may emit irritant fumes.5.3 Advice for firefightersWear self-contained breathing apparatus and protective clothing.6. ACCIDENTAL RELEASE MEASURES6.1 Personal precautions, protective equipment and emergency proceduresUse full personal protective equipment. Avoid breathing vapors, mist, dust or gas. Ensure adequate ventilation. Evacuate personnel to safe areas.Refer to protective measures listed in sections 8.6.2 Environmental precautionsTry to prevent further leakage or spillage. Keep the product away from drains or water courses.6.3 Methods and materials for containment and cleaning upAbsorb solutions with finely-powdered liquid-binding material (diatomite, universal binders); Decontaminate surfaces and equipment by scrubbing with alcohol; Dispose of contaminated material according to Section 13.7. HANDLING AND STORAGE7.1 Precautions for safe handlingAvoid inhalation, contact with eyes and skin. Avoid dust and aerosol formation. Use only in areas with appropriate exhaust ventilation.7.2 Conditions for safe storage, including any incompatibilitiesKeep container tightly sealed in cool, well-ventilated area. Keep away from direct sunlight and sources of ignition.Recommended storage temperature:Pure form-20°C 3 years4°C 2 yearsIn solvent-80°C 6 months-20°C 1 monthShipping at room temperature if less than 2 weeks.7.3 Specific end use(s)No data available.8. EXPOSURE CONTROLS/PERSONAL PROTECTION8.1 Control parametersComponents with workplace control parametersThis product contains no substances with occupational exposure limit values.8.2 Exposure controlsEngineering controlsEnsure adequate ventilation. Provide accessible safety shower and eye wash station.Personal protective equipmentEye protection Safety goggles with side-shields.Hand protection Protective gloves.Skin and body protection Impervious clothing.Respiratory protection Suitable respirator.Environmental exposure controls Keep the product away from drains, water courses or the soil. Cleanspillages in a safe way as soon as possible.9. PHYSICAL AND CHEMICAL PROPERTIES9.1 Information on basic physical and chemical propertiesAppearance White to off-white (Oil)Odor No data availableOdor threshold No data availablepH No data availableMelting/freezing point No data availableBoiling point/range No data availableFlash point No data availableEvaporation rate No data availableFlammability (solid, gas)No data availableUpper/lower flammability or explosive limits No data availableVapor pressure No data availableVapor density No data availableRelative density No data availableWater Solubility No data availablePartition coefficient No data availableAuto-ignition temperature No data availableDecomposition temperature No data availableViscosity No data availableExplosive properties No data availableOxidizing properties No data available9.2 Other safety informationNo data available.10. STABILITY AND REACTIVITY10.1 ReactivityNo data available.10.2 Chemical stabilityStable under recommended storage conditions.10.3 Possibility of hazardous reactionsNo data available.10.4 Conditions to avoidNo data available.10.5 Incompatible materialsStrong acids/alkalis, strong oxidising/reducing agents.10.6 Hazardous decomposition productsUnder fire conditions, may decompose and emit toxic fumes.Other decomposition products - no data available.11.TOXICOLOGICAL INFORMATION11.1 Information on toxicological effectsAcute toxicityClassified based on available data. For more details, see section 2Skin corrosion/irritationClassified based on available data. For more details, see section 2Serious eye damage/irritationClassified based on available data. For more details, see section 2Respiratory or skin sensitizationClassified based on available data. For more details, see section 2Germ cell mutagenicityClassified based on available data. For more details, see section 2CarcinogenicityIARC: No component of this product present at a level equal to or greater than 0.1% is identified as probable, possible or confirmed human carcinogen by IARC.ACGIH: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by ACGIH.NTP: No component of this product present at a level equal to or greater than 0.1% is identified as a anticipated or confirmed carcinogen by NTP.OSHA: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by OSHA.Reproductive toxicityClassified based on available data. For more details, see section 2Specific target organ toxicity - single exposureClassified based on available data. For more details, see section 2Specific target organ toxicity - repeated exposureClassified based on available data. For more details, see section 2Aspiration hazardClassified based on available data. For more details, see section 212. ECOLOGICAL INFORMATION12.1 ToxicityNo data available.12.2 Persistence and degradabilityNo data available.12.3 Bioaccumlative potentialNo data available.12.4 Mobility in soilNo data available.12.5 Results of PBT and vPvB assessmentPBT/vPvB assessment unavailable as chemical safety assessment not required or not conducted.12.6 Other adverse effectsNo data available.13. DISPOSAL CONSIDERATIONS13.1 Waste treatment methodsProductDispose substance in accordance with prevailing country, federal, state and local regulations.Contaminated packagingConduct recycling or disposal in accordance with prevailing country, federal, state and local regulations.14. TRANSPORT INFORMATIONDOT (US)This substance is considered to be non-hazardous for transport.IMDGThis substance is considered to be non-hazardous for transport.IATAThis substance is considered to be non-hazardous for transport.15. REGULATORY INFORMATIONSARA 302 Components:No chemicals in this material are subject to the reporting requirements of SARA Title III, Section 302.SARA 313 Components:This material does not contain any chemical components with known CAS numbers that exceed the threshold (De Minimis) reporting levels established by SARA Title III, Section 313.SARA 311/312 Hazards:No SARA Hazards.Massachusetts Right To Know Components:No components are subject to the Massachusetts Right to Know Act.Pennsylvania Right To Know Components:No components are subject to the Pennsylvania Right to Know Act.New Jersey Right To Know Components:No components are subject to the New Jersey Right to Know Act.California Prop. 65 Components:This product does not contain any chemicals known to State of California to cause cancer, birth defects, or anyother reproductive harm.16. OTHER INFORMATIONCopyright 2018 MedChemExpress. The above information is correct to the best of our present knowledge but does not purport to be all inclusive and should be used only as a guide. The product is for research use only and for experienced personnel. It must only be handled by suitably qualified experienced scientists in appropriately equipped and authorized facilities. The burden of safe use of this material rests entirely with the user. MedChemExpress disclaims all liability for any damage resulting from handling or from contact with this product.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
液相色谱-质谱联用法检出降糖胶囊中的格列本脲和苯乙双胍

液相色谱-质谱联用法检出降糖胶囊中的格列本脲和苯乙双胍郑成;向智敏
【期刊名称】《药学实践杂志》
【年(卷),期】2007(25)6
【摘要】目的:建立液相色谱-质谱联用法测定中药中非法掺入的化学降糖药格列本脲和苯乙双胍.方法:以Altima C18色谱柱为固定相,以乙腈-0.1%甲酸溶液(65∶35)为流动相,用液相色谱-质谱-质谱法进行定性分析鉴定.结果:在一批降糖胶囊中检测到非法掺入的化学药格列本脲和苯乙双胍.结论:该方法专属性强,灵敏度高,是分析检测中药制剂中添加化学药物的有效方法.
【总页数】3页(P388-390)
【作者】郑成;向智敏
【作者单位】浙江省药品检验所,浙江,杭州,310004;浙江省药品检验所,浙江,杭州,310004
【正文语种】中文
【中图分类】R917
【相关文献】
1.降糖保健食品中非法添加盐酸苯乙双胍和格列本脲的检测 [J], 张妤琳;曹玲
2.高效液相色谱-质谱联用法测定二甲双胍格列本脲胶囊(Ⅰ)中格列本脲含量 [J], 刘信奎;王萌萌;张连成;王超众
3.高效液相色谱–质谱联用法测定二甲双胍格列本脲片(Ⅰ)中N-亚硝基二甲胺 [J], 徐艳梅;程新杰;卞广利;闫凯;高燕霞
4.中药降糖制剂中西药成分盐酸二甲双胍、盐酸苯乙双胍、格列齐特和格列本脲的鉴别 [J], 李冰;王本杰;黄萍;魏春敏;郭瑞臣
5.液相色谱-质谱联用法检测中药降糖制剂中非法掺入的苯乙双胍和格列本脲 [J], 董宇;孔璋;钟大放
因版权原因,仅展示原文概要,查看原文内容请购买。
《中国药典》(2020年版)复方磺胺恶唑片中的甲氧苄啶含量测

《中国药典》(2020年版)复方磺胺噁唑片中的甲氧苄啶含量测甲氧苄啶(Trimethoprim, TMP),又称为甲氧苄氨嘧啶、甲氧苄嘧啶,是一种抗菌增效药,与磺胺类药物联合使用时,能使磺胺类药物抗菌谱扩大、抗菌活性大大增强。
由于其独特的作用,甲氧苄啶在养殖业病害防治中被广泛应用。
迪信泰检测平台采用高效液相色谱(HPLC)和液相色谱-三重四极杆质谱(LC-MS/MS)法,可高效、精准的检测甲氧苄啶的含量变化。
此外,我们还提供其他抗生素检测服务,以满足您的不同需求。
HPLC和LC-MS测定甲氧苄啶样本要求:1. 请确保样本量大于0.2g或者0.2mL。
周期:2~3周项目结束后迪信泰检测平台将会提供详细中英文双语技术报告,报告包括:1. 实验步骤(中英文)2. 相关质谱参数(中英文)3. 质谱图片4. 原始数据5. 甲氧苄啶含量信息应用范围:本方法采用高效液相色谱法测定复方磺胺甲恶唑片中磺胺甲恶唑和甲氧苄啶的含量。
本方法适用于复方磺胺甲恶唑片。
方法原理:供试品加甲醇稀释,最终用流动相定量稀释后,进入高效液相色谱仪进行色谱分离,用紫外吸收检测器,于波长240nm处检测磺胺甲恶唑和甲氧苄啶的吸收值,计算出其含量。
试剂: 1. 0.1mol/L盐酸2. 乙腈3. 三乙胺4. 氢氧化钠试液5. 冰醋酸仪器设备: 1. 仪器1.1 高效液相色谱仪1.2 色谱柱十八烷基硅烷键合硅胶为填充剂,理论塔板数按磺胺甲恶唑峰计算不低于4000。
磺胺甲恶唑和甲氧苄啶的分离度应符合要求。
1.3 紫外吸收检测器2. 色谱条件2.1 流动相:水乙腈三乙胺=799 200 1(用氢氧化钠试液或冰醋酸调节pH 值至5.9。
)2.2 检测波长:240nm2.3 柱温:室温试样制备:1. 称取供试品取本品10片,精密称定,研细,精密称取适量(约相当于磺胺甲恶唑44mg)置100mL量瓶中。
2. 对照品溶液的制备精密称取磺胺甲恶唑对照品和甲氧苄啶对照品适量,用0.1mol/L盐酸溶液溶解并定量稀释制成每1mL中约含有磺胺甲恶唑0.44mg的和甲氧苄啶89µg的溶液。
HA14-1_DataSheet_MedChemExpress
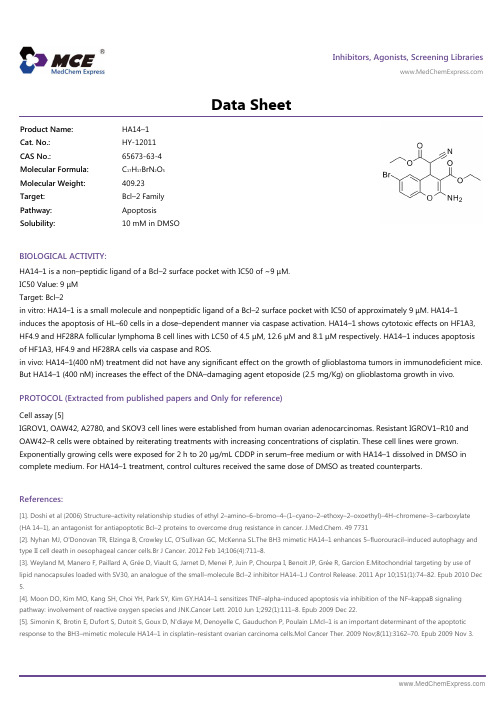
Inhibitors, Agonists, Screening Libraries Data SheetBIOLOGICAL ACTIVITY:HA14–1 is a non–peptidic ligand of a Bcl–2 surface pocket with IC50 of ~9 μM.IC50 Value: 9 μMTarget: Bcl–2in vitro: HA14–1 is a small molecule and nonpeptidic ligand of a Bcl–2 surface pocket with IC50 of approximately 9 μM. HA14–1induces the apoptosis of HL–60 cells in a dose–dependent manner via caspase activation. HA14–1 shows cytotoxic effects on HF1A3,HF4.9 and HF28RA follicular lymphoma B cell lines with LC50 of 4.5 μM, 12.6 μM and 8.1 μM respectively. HA14–1 induces apoptosis of HF1A3, HF4.9 and HF28RA cells via caspase and ROS.in vivo: HA14–1(400 nM) treatment did not have any significant effect on the growth of glioblastoma tumors in immunodeficient mice.But HA14–1 (400 nM) increases the effect of the DNA–damaging agent etoposide (2.5 mg/Kg) on glioblastoma growth in vivo.PROTOCOL (Extracted from published papers and Only for reference)Cell assay [5]IGROV1, OAW42, A2780, and SKOV3 cell lines were established from human ovarian adenocarcinomas. Resistant IGROV1–R10 and OAW42–R cells were obtained by reiterating treatments with increasing concentrations of cisplatin. These cell lines were grown.Exponentially growing cells were exposed for 2 h to 20 μg/mL CDDP in serum–free medium or with HA14–1 dissolved in DMSO in complete medium. For HA14–1 treatment, control cultures received the same dose of DMSO as treated counterparts.References:[1]. Doshi et al (2006) Structure–activity relationship studies of ethyl 2–amino–6–bromo–4–(1–cyano–2–ethoxy–2–oxoethyl)–4H–chromene–3–carboxylate (HA 14–1), an antagonist for antiapoptotic Bcl–2 proteins to overcome drug resistance in cancer. J.Med.Chem. 49 7731[2]. Nyhan MJ, O'Donovan TR, Elzinga B, Crowley LC, O'Sullivan GC, McKenna SL.The BH3 mimetic HA14–1 enhances 5–fluorouracil–induced autophagy and type II cell death in oesophageal cancer cells.Br J Cancer. 2012 Feb 14;106(4):711–8.[3]. Weyland M, Manero F, Paillard A, Grée D, Viault G, Jarnet D, Menei P, Juin P, Chourpa I, Benoit JP, Grée R, Garcion E.Mitochondrial targeting by use of lipid nanocapsules loaded with SV30, an analogue of the small–molecule Bcl–2 inhibitor HA14–1.J Control Release. 2011 Apr 10;151(1):74–82. Epub 2010 Dec 5.[4]. Moon DO, Kim MO, Kang SH, Choi YH, Park SY, Kim GY.HA14–1 sensitizes TNF–alpha–induced apoptosis via inhibition of the NF–kappaB signaling pathway: involvement of reactive oxygen species and JNK.Cancer Lett. 2010 Jun 1;292(1):111–8. Epub 2009 Dec 22.[5]. Simonin K, Brotin E, Dufort S, Dutoit S, Goux D, N'diaye M, Denoyelle C, Gauduchon P, Poulain L.Mcl–1 is an important determinant of the apoptotic response to the BH3–mimetic molecule HA14–1 in cisplatin–resistant ovarian carcinoma cells.Mol Cancer Ther. 2009 Nov;8(11):3162–70. Epub 2009 Nov 3.Product Name:HA14–1Cat. No.:HY-12011CAS No.:65673-63-4Molecular Formula:C 17H 17BrN 2O 5Molecular Weight:409.23Target:Bcl–2 Family Pathway:Apoptosis Solubility:10 mM in DMSOCaution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
HC-030031_DataSheet_MedChemExpress
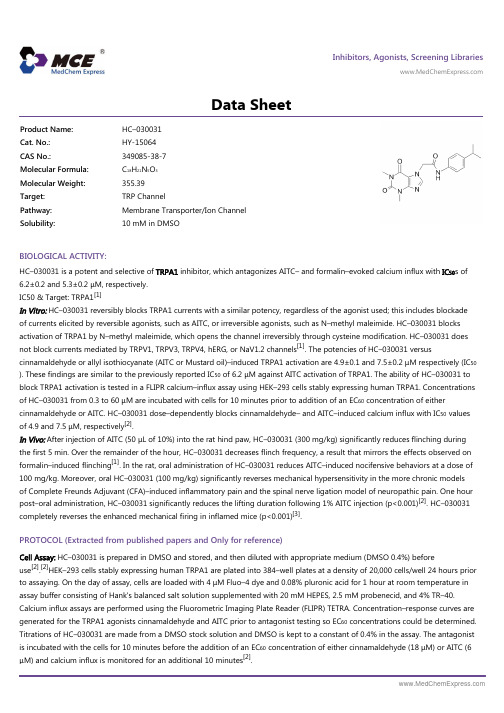
Inhibitors, Agonists, Screening Libraries Data SheetBIOLOGICAL ACTIVITY:HC–030031 is a potent and selective of TRPA1 inhibitor, which antagonizes AITC– and formalin–evoked calcium influx with IC 50s of6.2±0.2 and 5.3±0.2 μM, respectively.IC50 & Target: TRPA1[1]In Vitro: HC–030031 reversibly blocks TRPA1 currents with a similar potency, regardless of the agonist used; this includes blockade of currents elicited by reversible agonists, such as AITC, or irreversible agonists, such as N–methyl maleimide. HC–030031 blocks activation of TRPA1 by N–methyl maleimide, which opens the channel irreversibly through cysteine modification. HC–030031 does not block currents mediated by TRPV1, TRPV3, TRPV4, hERG, or NaV1.2 channels [1]. The potencies of HC–030031 versuscinnamaldehyde or allyl isothiocyanate (AITC or Mustard oil)–induced TRPA1 activation are 4.9±0.1 and 7.5±0.2 μM respectively (IC 50). These findings are similar to the previously reported IC 50 of 6.2 μM against AITC activation of TRPA1. The ability of HC–030031 to block TRPA1 activation is tested in a FLIPR calcium–influx assay using HEK–293 cells stably expressing human TRPA1. Concentrations of HC–030031 from 0.3 to 60 μM are incubated with cells for 10 minutes prior to addition of an EC 60 concentration of either cinnamaldehyde or AITC. HC–030031 dose–dependently blocks cinnamaldehyde– and AITC–induced calcium influx with IC 50 values of 4.9 and 7.5 μM, respectively [2].In Vivo: After injection of AITC (50 μL of 10%) into the rat hind paw, HC–030031 (300 mg/kg) significantly reduces flinching during the first 5 min. Over the remainder of the hour, HC–030031 decreases flinch frequency, a result that mirrors the effects observed on formalin–induced flinching [1]. In the rat, oral administration of HC–030031 reduces AITC–induced nocifensive behaviors at a dose of100 mg/kg. Moreover, oral HC–030031 (100 mg/kg) significantly reverses mechanical hypersensitivity in the more chronic models of Complete Freunds Adjuvant (CFA)–induced inflammatory pain and the spinal nerve ligation model of neuropathic pain. One hour post–oral administration, HC–030031 significantly reduces the lifting duration following 1% AITC injection (p<0.001)[2]. HC–030031completely reverses the enhanced mechanical firing in inflamed mice (p<0.001)[3].PROTOCOL (Extracted from published papers and Only for reference)Cell Assay: HC–030031 is prepared in DMSO and stored, and then diluted with appropriate medium (DMSO 0.4%) beforeuse [2].[2]HEK–293 cells stably expressing human TRPA1 are plated into 384–well plates at a density of 20,000 cells/well 24 hours prior to assaying. On the day of assay, cells are loaded with 4 μM Fluo–4 dye and 0.08% pluronic acid for 1 hour at room temperature in assay buffer consisting of Hank's balanced salt solution supplemented with 20 mM HEPES, 2.5 mM probenecid, and 4% TR–40.Calcium influx assays are performed using the Fluorometric Imaging Plate Reader (FLIPR) TETRA. Concentration–response curves are generated for the TRPA1 agonists cinnamaldehyde and AITC prior to antagonist testing so EC 60 concentrations could be determined.Titrations of HC–030031 are made from a DMSO stock solution and DMSO is kept to a constant of 0.4% in the assay. The antagonist is incubated with the cells for 10 minutes before the addition of an EC 60 concentration of either cinnamaldehyde (18 μM) or AITC (6μM) and calcium influx is monitored for an additional 10 minutes [2].Product Name:HC–030031Cat. No.:HY-15064CAS No.:349085-38-7Molecular Formula:C 18H 21N 5O 3Molecular Weight:355.39Target:TRP Channel Pathway:Membrane Transporter/Ion Channel Solubility:10 mM in DMSOAnimal Administration: HC–030031 is suspended in 0.5% methylcellulose (Rat)[2].HC–030031 is prepared in 0.5% DMSO and 0.25% Tween–80 in PBS (Mice)[3].[2][3]Rat[2]Male Sprague–Dawley rats (200–500 g) are used in all experiments. HC–030031 (100, 300 mg/kg) is used. For all experiments,HC–030031 is suspended in 0.5% Methylcellulose and the drug is dosed p.o. at a volume of 10 mL/kg. Naproxen (20 mg/kg) is dissolved in sterile water and dosed p.o. to serve as a positive comparator for the CFA experiment. Pregabalin (20 mg/kg) is dissolved in sterile water and dosed p.o. to serve as a positive comparator for the neuropathic pain experiment.Mice[3]Adult male C57BL/6 mice (8–12 weeks old) are used. Mice are injected with a 30 μL emulsion of undiluted CFA into the medial left plantar hind paw. The vehicle control group is injected with 30 μL of sterile 0.9% saline solution. Two days after injection, at the peak of hypersensitivity, the magnitude of inflammation is measured at the midpoint of the hind paw using digital calipers (VWR). For one experiment, the membrane–impermeable sodium channel inhibitor lidocaine N–ethyl–bromide, also known as QX–314, (0.2% in saline;30 μL) is injected with or without the TRPA1 agonist cinnamaldehyde (30 μM) into the left plantar hind paw 2 days post CFA injection. For another experiment, the TRPA1 antagonist HC–030031 (100 μg in 30 μL of 0.5% DMSO and 0.25% Tween–80 in PBS) is injected into the left plantar hind paw 2 days post CFA injection. Vehicle controls are injected with 30 μL 0.5% DMSO and 0.25% Tween–80 in PBS. All behavioral assays are completed between 1 and 4 hours following the QX–314, HC–030031 or vehicle injections. References:[1]. McNamara CR, et al. TRPA1 mediates formalin–induced pain. Proc Natl Acad Sci U S A. 2007 Aug 14;104(33):13525–30.[2]. Eid SR, et al. HC–030031, a TRPA1 selective antagonist, attenuates inflammatory– and neuropathy–induced mechanical hypersensitivity. Mol Pain. 2008 Oct 27;4:48.[3]. Lennertz RC, et al. TRPA1 mediates mechanical sensitization in nociceptors during inflammation. PLoS One. 2012;7(8):e43597.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
美国Medchemexpress化合物库(小分子库)_Medchemexpress_(MCE中国)

美国Medchemexpress化合物库(小分子库)-原装进口,现货供
应,提供组合定制服务
品牌:Medchemexpress (MCE)
保存条件:-20℃
供应商:MCE中国
数量:大量
保质期:2年
Size:
Pre-dissolved DMSO/Solid(Or dry solid)
100 uL/well (10 mM solution)
200 uL/well (10 mM solution)
MedChemExpress (MCE)专注于各种抑制剂、调节剂、API、天然产物及化合物库,总部位于美国新泽西。
MCE经过十年努力已成为全球生物活性小分子领域的一流供应商。
MedChemExpress(MCE)产品涵盖近20个热门研究领域,1000多个细分靶点,超过3000个现货抑制剂、拮抗剂和激动剂。
相关的应用成果已发表于Nature、Cell等国际知名杂志,在全球20余个国家地区设有代理机构。
上海皓元生物医药科技有限公司(MCE 中国) 是MedChemExpress (MCE) 亚洲总代理。
MCE化合物库涵盖20余种不同的类型,超过2500个化合物,进口原装,
现货供应,提供详实的生物活性信息、化学结构信息、质控图谱(NMR和HPLC 等)。
还可根据您的实际研究需要,为您度身定制任意组合、规格、布板的特殊化合物库。
/screening-libraries.html
现有特色化合物库有:。
TOK-001_DataSheet_MedChemExpress

Inhibitors, Agonists, Screening Libraries Data SheetBIOLOGICAL ACTIVITY:TOK–001 is a multifunctional antiandrogen and CYP17 inhibitor (IC 50=47 nM) in castration resistant prostate cancer (CRPC).IC50 & Target: IC50: 47 nM (CYP17)[1]In Vitro: TOK–001 affords strong CYP17 lyase inhibition, with IC 50 of 47 nM [1]. TOK–001 is both a CYP17A1 inhibitor and androgen receptor antagonist and the similarity of these binding modes is likely the reason for this dual mechanism of action.This CYP17A1binds abiraterone and TOK–001 with absorbance decreases at 402 nm and increases at 424 nm, consistent with nitrogen binding to the heme iron (type II interaction) with K d of <100 nM [2]. When LNCaP cells are cultured in medium supplemented withcharcoal–stripped serum (CSS, T<1 nM) followed by treatment with increasing concentrations of TOK–001, the steady–state levels of AR protein are markedly decreased (up to 84%, 15 μM TOK–001). In LAPC–4 cells, abiraterone alcohol reduced AR expression to a greater extent than TOK–001 at concentrations greater than or equal to 1 μM. When LNCaP cells are treated with 20 μM TOK–001for 24 h, AR mRNA levels are reduced by 38%[3].In Vivo: Mice inoculated with LAPC–4 tumors are treated subcutaneously with 0.15 mmol/kg of TOK–001 twice daily. Mice treated with TOK–001 have smaller average tumor volume on day 31 when compared to control (p= 0.0001). TOK–001 treatment alsosignificantly reduces the growth rate of tumor growth compared to control (p<0.0001). Upon excision, final tumor weights are also significantly reduced in animals treated with TOK–001 compared to animals treated with control, and castration (p<0.05)[1].PROTOCOL (Extracted from published papers and Only for reference)Kinase Assay:[2]Progesterone 17α–hydroxylation is evaluated using a modified HPLC method with UV–detection. CYP17A1 (50 pmol)and rat NADPH–cytochrome P450 reductase 1:4 are mixed, incubated on ice (20 minutes), and added to buffer (50 mM Tris, pH 7.4and 5 mM MgCl 2) containing progesterone (0–50 μM) to a total volume of 500 μL. Phosphatidylcholine (25 μg) is included for side–by–side kinetic comparisons with the full–length enzyme. For IC 50 determinations, inhibitors concentrations are 0–1500 nM for abiraterone and 0–3000 nM for TOK–001 (Shanghai Haoyuan Chemexpress Co., Shanghai, China). After warming (37°C, 3 minutes),reactions are initiated by NADPH addition (20 μL 25 mM), incubated for 10 minutes (37°C), and quenched with 20% trichloroacetic acid (300 μL) and placed on ice. The 17α–hydroxyprogesterone metabolite is identified by UV detection at 248 nm following HPLC separation and coeluted with authentic standards. The HPLC mobile phase is 40% acetonitrile, 60% water with 1% acetic acid and run at 1 mL/min (Phenomenex, Luna 5 μ, C18, 50×4.6 mm)[2].Cell Assay: TOK–001 is dissolved with DMSO and diluted with appropriate media [3].[3]LNCaP cells are seeded in 24–well plates at 70% confluence a day before transfections. Cells then are transfected with plasmid DNA (0.5 μg/well) carrying pIR–AR 5′UTR–Luc (5′UTR; test) or pIRES–Luc (IRES, control) for 6 h in serum–free and antibiotic–free conditions. Transcription of both luciferasereporter genes is under the control of the CMV promoter. Lipofectamine 2000 reagent is used in all transfections according to the manufacturer's instructions. Cells are then treated with doses of TOK–001 (0, 10, and 20 μM) in 5% FBS/T–Medium. Luciferase reporter gene activities are measured using Luciferase Assay System from Promega at 36 h post treatment using a BMG LabtechProduct Name:TOK–001Cat. No.:HY-70006CAS No.:851983-85-2Molecular Formula:C 26H 32N 2O Molecular Weight:388.55Target:Cytochrome P450Pathway:Metabolic Enzyme/Protease Solubility:DMSO: 18 mg/mLmicroplate reader. Relative luciferase units are normalized to total protein and then normalized to vector control (pIR–AR5′UTR–Luc) and the result is presented as luciferase activity. For cell proliferation studies, LNCaP cells in 96–well plate are seeded 24 h prior to drug treatment and then treated with control (mock), TOK–001 (10 μM), or abiraterone alcohol (10 μM) in 5%FBS/T–medium for 72 h. Cell proliferation is determined using MTS[3].Animal Administration: TOK–001 is dissolved in DMSO and then diluted with saline or PBS[1]. [1]Mice[1]Mice inoculated with LAPC–4 tumors are treated subcutaneously with 0.15 mmol/kg of TOK–001 twice daily. Mice treated with TOK–001 have smaller average tumor volume on day 31 when compared to control (p=0.0001). TOK–001 treatment also significantly reduced the growth rate of tumor growth compared to control (p<0.0001). Upon excision, final tumor weights are also significantly reduced in animals treated with TOK–001 compared to animals treated with control, and castration (p<0.05).References:[1]. Bruno RD, et al. Synthesis and biological evaluations of putative metabolically stable analogs of VN/124–1 (TOK–001): head to head anti–tumor efficacy evaluation of VN/124–1 (TOK–001) and abiraterone in LAPC–4 human prostate cancer xenograft model.Steroid[2]. DeVore NM, et al. Structures of cytochrome P450 17A1 with prostate cancer drugs abiraterone and TOK–001.Nature. 2012 Jan 22;482(7383):116–9.[3]. Soifer HS, et al. Direct regulation of androgen receptor activity by potent CYP17 inhibitors in prostate cancer cells.J Biol Chem. 2012 Feb 3;287(6):3777–87. Epub 2011 Dec 15.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
BGB-3111_SDS_MedChemExpress

Inhibitors, Agonists, Screening LibrariesSafety Data Sheet Revision Date:Jun.-20-2017Print Date:Jun.-20-20171. PRODUCT AND COMPANY IDENTIFICATION1.1 Product identifierProduct name :BGB-3111Catalog No. :HY-101474CAS No. :1633350-06-71.2 Relevant identified uses of the substance or mixture and uses advised againstIdentified uses :Laboratory chemicals, manufacture of substances.1.3 Details of the supplier of the safety data sheetCompany:MedChemExpress USATel:609-228-6898Fax:609-228-5909E-mail:sales@1.4 Emergency telephone numberEmergency Phone #:609-228-68982. HAZARDS IDENTIFICATION2.1 Classification of the substance or mixtureNot a hazardous substance or mixture.2.2 GHS Label elements, including precautionary statementsNot a hazardous substance or mixture.2.3 Other hazardsNone.3. COMPOSITION/INFORMATION ON INGREDIENTS3.1 SubstancesSynonyms:BGB3111; BGB 3111Formula:C27H29N5O3Molecular Weight:471.55CAS No. :1633350-06-74. FIRST AID MEASURES4.1 Description of first aid measuresEye contactRemove any contact lenses, locate eye-wash station, and flush eyes immediately with large amounts of water. Separate eyelids with fingers to ensure adequate flushing. Promptly call a physician.Skin contactRinse skin thoroughly with large amounts of water. Remove contaminated clothing and shoes and call a physician.InhalationImmediately relocate self or casualty to fresh air. If breathing is difficult, give cardiopulmonary resuscitation (CPR). Avoid mouth-to-mouth resuscitation.IngestionWash out mouth with water; Do NOT induce vomiting; call a physician.4.2 Most important symptoms and effects, both acute and delayedThe most important known symptoms and effects are described in the labelling (see section 2.2).4.3 Indication of any immediate medical attention and special treatment neededTreat symptomatically.5. FIRE FIGHTING MEASURES5.1 Extinguishing mediaSuitable extinguishing mediaUse water spray, dry chemical, foam, and carbon dioxide fire extinguisher.5.2 Special hazards arising from the substance or mixtureDuring combustion, may emit irritant fumes.5.3 Advice for firefightersWear self-contained breathing apparatus and protective clothing.6. ACCIDENTAL RELEASE MEASURES6.1 Personal precautions, protective equipment and emergency proceduresUse full personal protective equipment. Avoid breathing vapors, mist, dust or gas. Ensure adequate ventilation. Evacuate personnel to safe areas.Refer to protective measures listed in sections 8.6.2 Environmental precautionsTry to prevent further leakage or spillage. Keep the product away from drains or water courses.6.3 Methods and materials for containment and cleaning upAbsorb solutions with finely-powdered liquid-binding material (diatomite, universal binders); Decontaminate surfaces and equipment by scrubbing with alcohol; Dispose of contaminated material according to Section 13.7. HANDLING AND STORAGE7.1 Precautions for safe handlingAvoid inhalation, contact with eyes and skin. Avoid dust and aerosol formation. Use only in areas with appropriate exhaust ventilation.7.2 Conditions for safe storage, including any incompatibilitiesKeep container tightly sealed in cool, well-ventilated area. Keep away from direct sunlight and sources of ignition.Recommended storage temperature:Powder-20°C 3 years4°C 2 yearsIn solvent-80°C 6 months-20°C 1 monthShipping at room temperature if less than 2 weeks.7.3 Specific end use(s)No data available.8. EXPOSURE CONTROLS/PERSONAL PROTECTION8.1 Control parametersComponents with workplace control parametersThis product contains no substances with occupational exposure limit values.8.2 Exposure controlsEngineering controlsEnsure adequate ventilation. Provide accessible safety shower and eye wash station.Personal protective equipmentEye protection Safety goggles with side-shields.Hand protection Protective gloves.Skin and body protection Impervious clothing.Respiratory protection Suitable respirator.Environmental exposure controls Keep the product away from drains, water courses or the soil. Cleanspillages in a safe way as soon as possible.9. PHYSICAL AND CHEMICAL PROPERTIES9.1 Information on basic physical and chemical propertiesAppearance White to off-white (Solid)Odor No data availableOdor threshold No data availablepH No data availableMelting/freezing point No data availableBoiling point/range No data availableFlash point No data availableEvaporation rate No data availableFlammability (solid, gas)No data availableUpper/lower flammability or explosive limits No data availableVapor pressure No data availableVapor density No data availableRelative density No data availableWater Solubility No data availablePartition coefficient No data availableAuto-ignition temperature No data availableDecomposition temperature No data availableViscosity No data availableExplosive properties No data availableOxidizing properties No data available9.2 Other safety informationNo data available.10. STABILITY AND REACTIVITY10.1 ReactivityNo data available.10.2 Chemical stabilityStable under recommended storage conditions.10.3 Possibility of hazardous reactionsNo data available.10.4 Conditions to avoidNo data available.10.5 Incompatible materialsStrong acids/alkalis, strong oxidising/reducing agents.10.6 Hazardous decomposition productsUnder fire conditions, may decompose and emit toxic fumes.Other decomposition products - no data available.11.TOXICOLOGICAL INFORMATION11.1 Information on toxicological effectsAcute toxicityClassified based on available data. For more details, see section 2Skin corrosion/irritationClassified based on available data. For more details, see section 2Serious eye damage/irritationClassified based on available data. For more details, see section 2Respiratory or skin sensitizationClassified based on available data. For more details, see section 2Germ cell mutagenicityClassified based on available data. For more details, see section 2CarcinogenicityIARC: No component of this product present at a level equal to or greater than 0.1% is identified as probable, possible or confirmed human carcinogen by IARC.ACGIH: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by ACGIH.NTP: No component of this product present at a level equal to or greater than 0.1% is identified as a anticipated or confirmed carcinogen by NTP.OSHA: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by OSHA.Reproductive toxicityClassified based on available data. For more details, see section 2Specific target organ toxicity - single exposureClassified based on available data. For more details, see section 2Specific target organ toxicity - repeated exposureClassified based on available data. For more details, see section 2Aspiration hazardClassified based on available data. For more details, see section 212. ECOLOGICAL INFORMATION12.1 ToxicityNo data available.12.2 Persistence and degradabilityNo data available.12.3 Bioaccumlative potentialNo data available.12.4 Mobility in soilNo data available.12.5 Results of PBT and vPvB assessmentPBT/vPvB assessment unavailable as chemical safety assessment not required or not conducted.12.6 Other adverse effectsNo data available.13. DISPOSAL CONSIDERATIONS13.1 Waste treatment methodsProductDispose substance in accordance with prevailing country, federal, state and local regulations.Contaminated packagingConduct recycling or disposal in accordance with prevailing country, federal, state and local regulations.14. TRANSPORT INFORMATIONDOT (US)This substance is considered to be non-hazardous for transport.IMDGThis substance is considered to be non-hazardous for transport.IATAThis substance is considered to be non-hazardous for transport.15. REGULATORY INFORMATIONSARA 302 Components:No chemicals in this material are subject to the reporting requirements of SARA Title III, Section 302.SARA 313 Components:This material does not contain any chemical components with known CAS numbers that exceed the threshold (De Minimis) reporting levels established by SARA Title III, Section 313.SARA 311/312 Hazards:No SARA Hazards.Massachusetts Right To Know Components:No components are subject to the Massachusetts Right to Know Act.Pennsylvania Right To Know Components:No components are subject to the Pennsylvania Right to Know Act.New Jersey Right To Know Components:No components are subject to the New Jersey Right to Know Act.California Prop. 65 Components:This product does not contain any chemicals known to State of California to cause cancer, birth defects, or anyother reproductive harm.16. OTHER INFORMATIONCopyright 2017 MedChemExpress. The above information is correct to the best of our present knowledge but does not purport to be all inclusive and should be used only as a guide. The product is for research use only and for experienced personnel. It must only be handled by suitably qualified experienced scientists in appropriately equipped and authorized facilities. The burden of safe use of this material rests entirely with the user. MedChemExpress disclaims all liability for any damage resulting from handling or from contact with this product.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
安捷伦产品目录

15
Real-Time PCR
16
Mx3000P QPCR System
17
Brilliant III Ultra-Fast SYBR Green QPCR and QRT-PCR Reagents
18
Brilliant III Ultra-Fast QPCR and QRT-PCR Reagents
Agilent / STRATAGENE
Agilent website: /genomics
Welgene | Agilent Stratagene
威健股份有限公司 | Stratagene 總代理
Table of Content
Table of Contents
/ XL1-Red Competent Cells SoloPack Gold Supercompetent Cells
/ TK Competent Cells Specialty Cells
/ Classic Cells / Fine Chemicals For Competent Cells
適用於 UNG 去汙染或 bisulphite
sequencing
適用於 TA Cloning
最高敏感性
取代傳統 Taq 的好選擇
-
2
威健股份有限公司 | Stratagene 總代理
PCR Enzyme & Instrument
Agilent SureCycler 8800
市場上領先的 cycling 速度和 sample 體積 10 ~ 100 μL 簡易快速可以選擇 96 well 和 384 well 操作盤 優秀的溫控設備讓各個 well 都能保持溫度的穩定 七吋的高解析度觸控螢幕讓操作上更為簡便 可以透過網路遠端操控儀器及監控儀器 Agilent 專業的技術支援可以幫助您應對各種 PCR 的問題
高效液相色谱法测定1-氯甲酰基-2-咪唑烷酮的量

高效液相色谱法测定1-氯甲酰基-2-咪唑烷酮的量
苏为科;李景华;杨江丰;陈理
【期刊名称】《浙江工业大学学报》
【年(卷),期】2000(000)0S1
【摘要】报道了用HPLC法对 1 氯甲酰基 2 咪唑烷酮进行定量测定 ,讨论了影响分析结果的主要因素 ,如流动相。
发现样品量在 0 5~ 2 5mg范围内线性关系良好 ,精密度高。
这为该产品的工业化生产提供了一个较好的分析方法
【总页数】2页(P)
【作者】苏为科;李景华;杨江丰;陈理
【作者单位】浙江工业大学化学工程学院;杭州市药品检验所
【正文语种】中文
【中图分类】O657.72
【相关文献】
1.维格列汀中间体(S)-1-(2-氯乙酰基)吡咯烷-2-甲腈的合成 [J], 陶铸;邓瑜;彭俊;陈英杰;王安民;胡湘南
2.1-氯甲酰基-3-乙酰基咪唑烷酮合成新方法研究 [J], 孙艳;李坚军
3.1-氯甲酰基-3-甲磺酰基-2-咪唑烷酮的工艺改进 [J], 傅志慧;姜小林;吕拥军;郑育飞;应志洪
4.1-氯甲酰基-2-咪唑烷酮的合成工艺改进 [J], 金庆平;赖月琴;陈建
5.双(1-甲基咪唑-2-基)甲酮、双(1-甲基咪唑-2-基)甲烷及双(1-甲基咪唑-2-基)乙烯与第六族羰基金属化合物的反应研究 [J], 张晓燕;丁可;宋海斌;唐良富
因版权原因,仅展示原文概要,查看原文内容请购买。
BMS-303141_943962-47-8_DataSheet_MedChemExpress

Product Name:BMS-303141CAS No.:943962-47-8Cat No :HY 16107Product Data SheetCat. No.:HY-16107MWt:424.30Formula:C19H15Cl2NO4S Purity :>98%S l bilit Soluble to 10mM in DMSO andtoSolubility:Mechanisms:Biological Activity:Pathways:Others; Target:ATP citrate lyase Soluble to 10 mM in DMSO and to 50 mM in ethanolBMS-303141 is a potent ATP-citrate lyase (ACL) inhibitor with IC50 value of 0.13 uM (humanrecombinant ACL).IC50 value: 0.13 uM [1]Target: ATP citrate lyase in vitro: In HepG2 cells, BMS-303141 showed inhibition of total lipid syntheses with an IC50 of 8 μM.A cell based Alamar Blue cytotoxicity assay was used in parallel to differentiate the effect on the inhibition of lipid synthesis versus potential cytotoxicity. Under identical incubation conditions, BMS-303141 showed no cytotoxicity up to 50 μM, indicating the observed inhibition of lipid synthesis was t lt f d i d d t t i it [1]References:[1]. Li JJ, et al. 2-hydroxy-N-arylbenzenesulfonamides as ATP-citrate lyase inhibitors. Bioorg Med Ch L tt 2007J 117(11)320811 not a result of compound-induced cytotoxicity [1].in vivo: In mice, BMS-303141 showed an oral bioavailability of 55% but a relatively short half-life of2.1 h.20 We therefore decided to dose BMS-303141 admixed in the food to assure greater duration of exposure in subsequent chronic efficacy studies.There were a...Chem Lett. 2007 Jun 1;17(11):3208-11.Caution: Not fully tested. For research purposes onlyMedchemexpress LLC18 W i l k i n s o n W a y , P r i n c e t o n , N J 08540,U S AE m a i l : i n f o @m e d c h e m e x p r e s s .c o m W e b : w w w .m e d c h e m e x p r e s s .c o m。
BMS-303141_有效的ATP-柠檬酸裂合酶抑制剂_943962-47-8_Apexbio

制总脂肪合成,IC50 值为 8 μM。此外,BMS-303141 在高达 50 μM 时仍无细胞毒性[1]。 在小鼠中,BMS-303141 的口服生物利用度为 55%。在高脂肪饲喂的小鼠中,BMS-303141 降 低血浆甘油三酯和胆固醇水平 20-30%,降低葡萄糖水平 30-50%,并可抑制增重[1]。 BMS-303141 可以浓度依赖的方式抑制 ACL,对人类 ACL 的 IC50 值为 0.94 μM[2]。
BMS-303141 是一种有效的 ATP-柠檬酸裂合酶抑制剂,IC50 值为 0.13 μM[1]。 ATP-柠檬酸裂合酶(ACL)是一种胞质酶,负责生成胞质乙酰 CoA,一种生物从头合成胆固 醇和脂肪酸必需的前体。抑制 ACL 可用于治疗血脂异常和肥胖[1]。 BMS-303141 是一种有效的 ATP-柠檬酸裂合酶抑制剂。在 HepG2 细胞中,BMS-303141 可抑
参考文献: [1]. Li JJ, Wang H, Tino JA, et al. 2-hydroxy-N-arylbenzenesulfonamides as ATP-citrate lyase inhibitors. Bioorg Med Chem Lett, 2007, 17(11): 3208-3211. [2]. Ma Z, Chu CH, Cheng D. A novel direct homogeneous assay for ATP citrate lyase. J Lipid Res, 2009, 50(10): 2131-2135.
产品名: BMS-303141 修订日期: 6/30/2016
化学名:
SMILES: 溶解性: 储存条件: 一般建议:
运输条件:
3,5-dichloro-2-hydroxy-N-(4-methoxy-[1,1'-biphenyl]-3-yl)benzenes ulfonamide
Alda-1_DataSheet_MedChemExpress

Inhibitors, Agonists, Screening Libraries Data SheetBIOLOGICAL ACTIVITY:Alda–1 is a potent ALDH2 agonist, which significantly improves ALDH2 activity.In Vivo: Alda–1 treatment results in a significant decrease of 4–HNE–protein content in the plasma of apoE -/- mice. Alda–1administration leads to a slight increase in gene expression related to neurogenesis (Nog ), mitochondrial biogenesis (CYTB , ND1),and apoptosis (Bax , Gsk3b ) in the Hp of apoE -/- mice. Alda–1 administration leads to 2 and 10 differentially expressed proteins in theFCx and Hp of apoE -/- mice, respectively [1]. Alda–1 (1.5 mg/kg, b.w., i.p.) administration significantly increases the climbing time,tends to reduce the immobility time and increases the swimming time of the prenatally stressed rats in the forced swim test.Moreover, treatment of prenatally stressed rats with Alda–1 significantly increases number of entries into the open arms of the maze and the time spent therein, as assessed by elevated plus–maze test [2]. Alda–1 (8.5 mg/kg, i.p.) with glucose significantly lowers 4–HNE and FJB–positive cells in the cerebral cortex of Alda–1–treated rats than in DMSO–treated rats 24 h after glucose administration [3]. Alda–1 (10 mg/kg per day) treatment prevents aldehydic overload, mitochondrial dysfunction and improves ventricular function in post–MI cardiomyopathy rats [4].PROTOCOL (Extracted from published papers and Only for reference)Cell Assay:[2]Spleen cells (4×106 cells/mL) are stimulated by optimal concentrations of concanavalin A (Con A; 2.5μg/mL and 0.6 μg/mL) and lipopolysaccharide (LPS, 5 μg/mL) and are incubated in 96–well plates at final volume of 0.2mL for 72 h. Cell proliferation is determined by adding 0.5 μCi of [3H]–thymidine per well at 16 h before the end of the incubation.The cultures are harvested with an automatic cell harvester, and [3H] thymidine incorporation is assessed using a liquid scintillationcounter.Animal Administration: Alda–1 is dissolved in 1 mL/kg b.w. DMSO/water 50/50.[2]After behavioral verification at three months of age,the animals are divided into the following four groups: control, control + Alda–1, prenatally stressed and prenatally stressed + Alda–1(6 animals per group). Alda–1 injections are given intraperitoneally (i.p.) once daily at a dose of 1.5 mg/kg b.w. (dissolved in 1 mL/kg b.w. DMSO/water 50/50) for 14 days. At the same time, the control and prenatally stressed rats receive 1 mL/kg b.w. DMSO/water 50/50. The injections of Alda–1 and vehicle are given between 10 a.m and 11 a.m. In the last five days of Alda–1 treatment the behavioral parameters in the elevated plus maze test and then in the forced swim test are measured.References:[1]. Stachowicz A, et al. Proteomic Analysis of Mitochondria–Enriched Fraction Isolated from the Frontal Cortex and Hippocampus of Apolipoprotein E Knockout Mice Treated with Alda–1, an Activator of Mitochondrial Aldehyde Dehydrogenase (ALDH2). Int J Mol Sci.[2]. Stachowicz A, et al. The impact of mitochondrial aldehyde dehydrogenase (ALDH2) activation by Alda–1 on the behavioral and biochemical disturbances in animal model of depression. Brain Behav Immun. 2016 Jan;51:144–53.Product Name:Alda–1Cat. No.:HY-18936CAS No.:349438-38-6Molecular Formula:C 15H 11Cl 2NO 3Molecular Weight:324.16Target:Aldehyde Dehydrogenase (ALDH)Pathway:Metabolic Enzyme/Protease Solubility:DMSO: ≥ 51 mg/mL[3]. Ikeda T, et al. Effects of Alda–1, an Aldehyde Dehydrogenase–2 Agonist, on Hypoglycemic Neuronal Death. PLoS One. 2015 Jun 17;10(6):e0128844.[4]. Gomes KM, et al. Aldehydic load and aldehyde dehydrogenase 2 profile during the progression of post–myocardial infarction cardiomyopathy: benefits of Alda–1. Int J Cardiol. 2015 Jan 20;179:129–138.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
欧洲药典7.5版

INDEX
To aid users the index includes a reference to the supplement in which the latest version of a text can be found. For example : Amikacin sulfate...............................................7.5-4579 means the monograph Amikacin sulfate can be found on page 4579 of Supplement 7.5. Note that where no reference to a supplement is made, the text can be found in the principal volume.
English index ........................................................................ 4707
Latin index ................................................................................. 4739
EUROPEAN PHARMACOPபைடு நூலகம்EIA 7.5
Index
Numerics 1. General notices ................................................................... 7.5-4453 2.1.1. Droppers...................
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Inhibitors, Agonists, Screening Libraries
Data Sheet
BIOLOGICAL ACTIVITY:
BMS–303141 is a potent, cell–permeable ATP–citrate lyase (ACL ) inhibitor with an IC 50 value of 0.13 μM.
IC50 & Target: IC50: 0.13 uM (ACL)[1]
In Vitro: In HepG2 cells, BMS–303141 shows inhibition of total lipid syntheses with an IC 50 of 8 μM. BMS–303141 shows no
cytotoxicity up to 50 lM under a cell based Alamar Blue cytotoxicity assay, indicating the observed inhibition of lipid synthesis is not a result of compound–induced cytotoxicity [1].
In Vivo: Chronic oral dosing of BMS–303141 in high–fat fed mice lowers approximate 20–30% plasma cholesterol and triglycerides,as well as 30–50% fasting plasma glucose. Chronic treatment with BMS–303141 shows a gradual inhibition of weight gain along with a reduction in adiposity without apparent changes in food intake. BMS–303141 shows an oral bioavailability of 55% but a relatively short half–life of 2.1 h [1].
PROTOCOL (Extracted from published papers and Only for reference)
Animal Administration:[1]Mouse: Effect of BMS–303141 in high–fat fed mice is studied. There are a total of four groups in the study; mice on normal diet and high–fat diet controls, and two treated groups that are supplemented with BMS–303141 in their high–fat diet to an equivalent daily dose of 10 or 100 mg/kg. The study is continued for a total of 34 days. Food consumption and body weight gain are tracked along with weekly assessment of lipid and glucose plasma chemistries [1].
References:
[1]. Li JJ, et al. 2–hydroxy–N–arylbenzenesulfonamides as ATP–citrate lyase inhibitors. Bioorg Med Chem Lett. 2007 Jun 1;17(11):3208–11.
Product Name:
BMS–303141Cat. No.:
HY-16107CAS No.:
943962-47-8Molecular Formula:
C 19H 15Cl 2NO 4S Molecular Weight:
424.30Target:
ATP Citrate Lyase Pathway:
Metabolic Enzyme/Protease Solubility:
DMSO: ≥ 47 mg/mL
Caution: Product has not been fully validated for medical applications. For research use only.
Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@ Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
