CGMP training english
Stability(cGMP培训系列7)

• Microbial微生物
– Understand impurities/degradation products
5
Stability Testing适用范围
• Stability Testing
– Drugs and Cosmetics – Combination Drug/Devices where the drug component is the regulating factor
– Package Integrity – Color, odor, taste, etc
• Chemical化学性能
– Active ingredient – pH, specific gravity, viscosity, etc – Adhesive, polymer residuals, etc
15
QP 101 – Cosmetics
• Cosmetic products requiring stability studies will be allowed to proceed to market after a minimum of 3 months testing has been successfully achieved at accelerated conditions (as per Section 5.1). The stability study protocol will continue to its planned conclusion. Freeze/thaw studies may be a part of the stability protocol.
8
ICH Guidelines ICH纲要
• Three production lots or scalable pilot lots that simulate the conditions of production.三批次 • ICH Guidelines温度和湿度 – International Committee on Harmonization • Accelerated - 40º C/75% RH – 3 months = 1 year – 6 months = 2 years • Real Time - 25º C/60% RH • Intermediate - 30º C/65% RH
GMP专业英语

COS(Certificate of Suitability)指的是欧洲药典适用性认证,目的是考察欧洲药典是否能够有效地控制进口药品的质量,这是中国的原料药合法地被欧盟的最终用户使用的另一种注册方式。
这种注册途径的优点是不依赖于最终用户,可以由原料药生产厂商独立地提出申请。
中国的原料药生产厂商可以向欧盟药品质量指导委员会(EDQM)提交产品的COS认证文件(COS Dossier),申请COS证书,同时生产厂商必须要承诺产品生产的质量管理严格遵循GMP标准,在文件审查和可能的现场考察通过之后,EDQM会向原料药品的生产厂商颁发COS证书。
如果作为最终用户的欧盟成员国制剂生产企业准备采用中国生产的原料时,只要在注册文件或变更文件中附上该产品的COS证书复印件即可非常容易地获得批准。
随着美国、欧盟和日本三方在药品注册程序和法规上的相互协调,欧盟在进口的原料药注册中逐步接近美国FDA的偏重现场GMP检查的办法,今后有可能对每一家提出COS认证的生产厂家进行现场的GMP检查。
自1999年开始,原料药生产企业在申请COS认证的技术文件后面必须要附加两封承诺信,一封信承诺说产品是按照GMP规范进行生产的,另一封信要承诺同意欧盟的相关审查机构进行现场检查。
如果欧盟EDQM的GMP审查越来越频繁,甚至最终变成为一种必要的审查手段,生产厂家就应当对此做出充分的准备,以使自身的GMP管理状况能够适应欧盟的检查。
欧盟的GMP检查与国内的GMP认证有以下差别:首先,欧盟的GMP检查依据的ICH Q7A的指导纲要,厂家要参照此指导进行自身检查;其次,所有的质量管理文件、操作规范(SOP)和各种生产管理表格、标牌、标签和生产记录都应当具备中英文对照,能够让国外的审查官员看懂;其三,要对员工进行GMP的全员培训,了解并适应国外检查的特点。
COS认证过程对企业是有积极意义的,会使企业的GMP管理达到国际水平,而且随着美、欧、日三方协调的进一步发展,通过欧盟的GMP检查和COS认证最终有可能直接进入美国和日本市场,至少会使美国FDA的注册变得更为容易。
2024年英语培训指南

In response to the current problems in English education, developing English training guidelines can help promote English education reform and improve the level of English teaching.
Training instructors
This guide is applicable to teachers, foreign teachers, volunteers, etc. engaged in English teaching.
Training target
This guide is suitable for English learners of different age groups, English proficiency levels, and learning needs.
teacher team
目录
• English training and teaching management
• Marketing Strategies for English Training
• Prediction of Development Trends in the English Training Industry
03
English training course des and content
• Listening skills: By using diverse listening materials such as news, movies, lectures, etc., students can improve their listening comprehension abilities.
膳食补充剂CGMP知识培训1

新提案要求食品工厂人员对卫生问题调查负责,并对食品污染有相关的教育经历。 食品处理者和监管人必须要经过适当的食品处理技术培训,在操作前需要告知其不卫生 操作有可能带来的危险。
这项新提案要求工厂的员工最好有能力胜任这份工作并且经过良好的培训。
cGMP:英文Current Good Manufacturing Practice 的缩写,是“良好操作规范”。 cGMP是指加工生产膳食补充剂、食品、医药材料、药品、生物制品以及兽医产品要 求的方法、系统、设备、设施及控制手段的规定和法规。 cGMP是食品生产企业实现生产工艺合理化、科学化、现代化的首要条件。 2007 年FDA 发布了21CFR Part 111是膳食补充剂的cGMP最终规范要求。
第一部法案:1906年美国颁布的《纯净食品和化妆品法》,此法律主要是禁止冒用商 标和洲际间及国外贸易运输掺假食品。
第一次世界大战期间,美国新闻界披露美国食品工业的不良状况和药品生产的欺骗行 径之后,美国1938年《食品、药品和化妆品法》取代了《纯净食品和化妆品法》,开 始以法律形式来保证食品、药品的质量,建立了世界上第一个国家级的食品药品管理 机构——美国食品药品管理局(FDA)。
2011年1月4日由奥巴马总统签署《食品安全现代法》后, 《食品安全现代化 法》赋予FDA前所未有的授权。 按照《规定》要求,美国在法规公布后3年内,对所有生产和销售维生素/矿 物质制剂、植物类制剂和各种膳食补充剂的美国本土公司强制实施cGMP改造。 最迟在5年里,要全部完成对cGMP改造工作。
凡是出口到美国的食品原料,都必须要有当地权威机构的检测报告,而这一规定是过 去所没有的。
制作人:蒋江红
二战间数次较大药物灾难→成品抽样分析有缺陷,不能保证药品安全。
欧盟化妆品良好操作规范(EU-CGMP)》中文版

Council of Europe欧盟理事会Guidelines forGood Manufacturing Practice of Cosmetic Products化妆品的良好生产规范应用指南(GMPC)1995内容序言 (5)I. 术语 (6)II. 质量体系 (10)II.1 简介 (10)II.2. 员工 (10)II.3. 厂房 (10)II.4. 设备 (11)II.5. 程序和过程 (11)II.5.1. 程序和指导书 (12)II.5.2. 过程 (12)III. 采购 (13)III.1. 简介 (13)III.2. 合同要求................................................. . (13)III.3. 采购文件 (13)IV. 制造 (14)IV.1. 简介 (14)IV.2. 来料接收 (14)IV.2.1. 原料,包装材料,和散装产品 (14)IV.2.2. 水 (14)IV.2.3. 仓库和储存 (15)IV.3. 制造过程 (15)IV.3.1. 准备 (15)IV.3.2. 实际制造过程 (16)IV.3.3. 散装产品的储存 (16)IV.4. 包装 (16)IV.5. 成品的储存 (16)V.制造分包 (17)VI.质量管理 (18)VI.1. 简介 (18)VI.2. 质量控制 (18)VI.2.1. 简介 (18)VI.2.2. 仪器和试剂 (18)VI.2.3. 控制活动 (19)VI.2.4. 控制记录 (20)VI.2.5. 采样和样品室 (20)VI.3. 数据监控和使用 (21)VI.4. 文件控制 (21)VI.4.1. 跟踪文件 (21)VI.4.2. 文件管理 (21)VI.5. 不合格产品的管理 (22)VI.6. 卫生 (22)VI.6.1. 工厂卫生 (22)VI.6.2. 个人卫生 (22)VI.7. 审核 (23)参考文献 (24)前言1949年5月, 由10个国家参与成立了欧洲国家政治联盟: 欧盟理事会。
CGMP--Current Good Manufacturing Practice(中英文对照版)

Subpart A-General Provisions§211.1 Scopea)The regulations in this part contain theminimum current good manufacturing practice for preparation of drug products for administration to humans or animals.b)The current good manufacturing practiceregulations in this chapter, as they pertain to drug products, and in parts 600 through 680 of this chapter, as they pertain to biological products for human use, shall be considered to supplement, not supersede, the regulations in this part unless the regulations explicitly provide otherwise. In the event it is impossible to comply with applicable regulations both in this part and in other parts of this chapter or in parts 600 through 680 of this chapter, the regulation specifically applicable to the drug product in question shall supersede the regulation in this part.c)Pending consideration of a proposedexemption, published in the Federal Register of September 29, 1978, the requirements in this part shall not be enforced for OTC drug products if the products and all their ingredients are ordinarily marketed and consumed as human foods, and which products may also fall within the legal definition of drugs by virtue of their intended use. Therefore, until further notice, regulations under part 110 of this chapter, and where applicable, parts 113 to 129 of this chapter, shall be applied in determining whether these OTC drug products that are also foods are manufactured, processed, packed, or held under current good manufacturing practice.§211.3 Definitions.The definitions set forth in §210.3 of this chapter apply in this part.A.总则211.1 范围(a)本部分的条例包含人用或兽用药品制备的现行最低限度的药品生产管理规范(GMP)。
GMPWaterSystem(cGMP培训系列1)

12
Contaminants of water (3)
Problem minerals • 水的污染物 3 • 矿物质
1. Calcium and magnesium 2. Iron and manganese 3. Silicates 4. Carbon dioxide 5. Hydrogen sulfide 6. Phosphates
目标 水处理系统 存储要求 取样,测试 水的种类 微生物,消毒
23
Water system design
1. Pipes sloped so water does not pool and can drain easily 2. Sanitary fittings & connections 3. Constructed of suitable materials such as stainless steel 4. Circulate the water 5. Incorporate non-return valves (NRV)
Stagnant water inside valve
27
Water system design (3)
1. Sanitary pumps 2. Clamps and O rings
versus threaded fittings 3. Heat exchangers 4. Side arm level measuring devices are
声明 根据世界卫生组织的有关培训材料改写而成
3
Part I Water Pre-treatment
水的预处理
4
Water Introduction and treatment
国际商务英语口语实训Module 7 Exhibition Service

Module 7 Exhibition Service
Background Information
In addition, costs are incurred at the show for service such as electrical equipment, booth cleaning, internet services, and drayage (also known as material handling). Consequently, cities often promote trade shows as a means of economic development. Exhibitors attending the event are required to use an exhibitor manual or online exhibitor manual to order their required services and complete any necessary paperwork such as health and safety declarations.
Module 7.1 Pre-Exhibition
Speaking out
Task 1 Read the model dialogue. Pay attention to the highlighted sentences and then role-play it with your partner.
Module 7.1 Pre-Exhibition
Speaking out
Task 1 Read the model dialogue. Pay attention to the highlighted sentences and then role-play it with your partner. S: Well let’s do it then. It doesn’t cost much and it’ll probably strengthen our brand. Z: Absolutely it’ll be good for our business. Now we are expecting to extend our business. That’s exactly why they put on these fairs. S: I’ll give the exhibition office a call and ask for more information. (Later, Sam Brown calls the organizing office of the fair. Fang, who is a youth volunteer of the exhibition, answers the phone.)
美国 CGMP--中英文对照.12

Subpart A-General Provisions§211.1 Scopea)The regulations in this part contain theminimum current good manufacturing practice for preparation of drug products for administration to humans or animals.b)The current good manufacturing practiceregulations in this chapter, as they pertain to drug products, and in parts 600 through 680 of this chapter, as they pertain to biological products for human use, shall be considered to supplement, not supersede, the regulations in this part unless the regulations explicitly provide otherwise. In the event it is impossible to comply with applicable regulations both in this part and in other parts of this chapter or in parts 600 through 680 of this chapter, the regulation specifically applicable to the drug product in question shall supersede the regulation in this part.c)Pending consideration of a proposedexemption, published in the Federal Register of September 29, 1978, the requirements in this part shall not be enforced for OTC drug products if the products and all their ingredients are ordinarily marketed and consumed as human foods, and which products may also fall within the legal definition of drugs by virtue of their intended use. Therefore, until further notice, regulations under part 110 of this chapter, and where applicable, parts 113 to 129 of this chapter, shall be applied in determining whether these OTC drug products that are also foods are manufactured, processed, packed, or held under current good manufacturing practice.§211.3 Definitions.The definitions set forth in §210.3 of this chapter apply in this part.A.总则211.1 范围(a)本部分的条例包含人用或兽用药品制备的现行最低限度的药品生产管理规范(GMP)。
职业技能培训计划英文缩写

职业技能培训计划英文缩写Introduction:The Professional Skills Training Program is designed to provide participants with the essential skills and knowledge required to excel in their chosen career. The program will cover a wide range of topics including communication, leadership, time management, and problem-solving. The aim is to equip participants with the necessary skills to thrive in the current competitive job market and to progress in their career.Program Structure:The program will consist of a series of workshops, seminars, and practical exercises. Each session will focus on a specific skill set and will be delivered by experienced professionals in the field. The program will be highly interactive, encouraging participants to engage in group discussions, role-playing exercises, and case studies.Key Areas of Training:1. Communication:- Effective communication skills- Business writing and presenting- Active listening and questioning techniques2. Leadership:- Team building and motivation- Decision-making and problem-solving- Conflict management and resolution3. Time Management:- Setting priorities and managing deadlines- Planning and organizing tasks- Avoiding procrastination and time-wasting habits4. Problem-solving:- Critical thinking and analytical skills- Creative problem-solving techniques- Decision-making under pressure5. Emotional Intelligence:- Self-awareness and self-regulation- Empathy and understanding others- Managing emotions in professional settings6. Professional Development:- Networking and building professional relationships- Career advancement and goal setting- Personal branding and professional imageDelivery Format:The program will be delivered through a combination of in-person workshops and virtual webinars. Participants will have the flexibility to choose the delivery format that best suits their schedule and preferences. All sessions will be recorded for those who are unable to attend live, ensuring that everyone can access the training content.Duration:The program will run for a total of 12 weeks, with each week focusing on a different skill set. Participants will be expected to attend a minimum of 80% of the sessions in order to qualify for a certificate of completion.Assessment and Certification:Participants will be assessed through a combination of individual assignments and group projects. The assessment will focus on applying the skills learned in real-life scenarios. Those who successfully complete the program will receive a certificate of completion, recognizing their dedication to professional development.Benefits:Upon completion of the program, participants can expect to gain the following benefits:- Enhanced communication and leadership skills- Improved time management and problem-solving abilities- Increased emotional intelligence and self-awareness- A stronger professional network and personal brand- Greater confidence and readiness for career advancement opportunitiesConclusion:The Professional Skills Training Program aims to empower individuals with the essential skills and knowledge required to succeed in their professional endeavors. By focusing on key areas such as communication, leadership, time management, and problem-solving, the program will prepare participants to excel in their current roles and to progress in their career. With a flexible delivery format and a comprehensive curriculum, the program is designed to meet the diverse needs of professionals across different industries. Participants can expect to gain valuable insights and practical skills that will set them apart in today's competitive job market.。
欧盟化妆品良好操作规范(EU-CGMP)》中文版

Council of Europe欧盟理事会Guidelines forGood Manufacturing Practice of Cosmetic Products化妆品的良好生产规范应用指南(GMPC)1995内容序言 (5)I. 术语 (6)II. 质量体系 (10)II.1 简介 (10)II.2. 员工 (10)II.3. 厂房 (10)II.4. 设备 (11)II.5. 程序和过程 (11)II.5.1. 程序和指导书 (12)II.5.2. 过程 (12)III. 采购 (13)III.1. 简介 (13)III.2. 合同要求................................................. . (13)III.3. 采购文件 (13)IV. 制造 (14)IV.1. 简介 (14)IV.2. 来料接收 (14)IV.2.1. 原料,包装材料,和散装产品 (14)IV.2.2. 水 (14)IV.2.3. 仓库和储存 (15)IV.3. 制造过程 (15)IV.3.1. 准备 (15)IV.3.2. 实际制造过程 (16)IV.3.3. 散装产品的储存 (16)IV.4. 包装 (16)IV.5. 成品的储存 (16)V.制造分包 (17)VI.质量管理 (18)VI.1. 简介 (18)VI.2. 质量控制 (18)VI.2.1. 简介 (18)VI.2.2. 仪器和试剂 (18)VI.2.3. 控制活动 (19)VI.2.4. 控制记录 (20)VI.2.5. 采样和样品室 (20)VI.3. 数据监控和使用 (21)VI.4. 文件控制 (21)VI.4.1. 跟踪文件 (21)VI.4.2. 文件管理 (21)VI.5. 不合格产品的管理 (22)VI.6. 卫生 (22)VI.6.1. 工厂卫生 (22)VI.6.2. 个人卫生 (22)VI.7. 审核 (23)参考文献 (24)前言1949年5月, 由10个国家参与成立了欧洲国家政治联盟: 欧盟理事会。
美国cGMP中英文对照
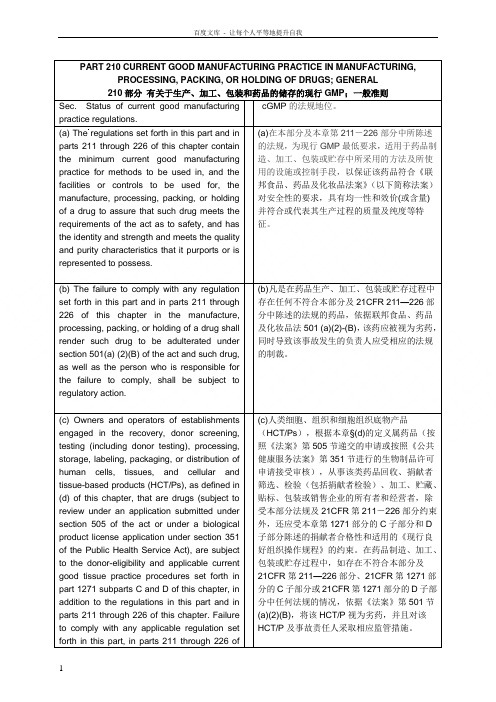
PART 210 CURRENT GOOD MANUFACTURING PRACTICE IN MANUFACTURING, PROCESSING, PACKING, OR HOLDING OF DRUGS; GENERAL 210部分有关于生产、加工、包装和药品的储存的现行GMP;一般准则Sec. Status of current good manufacturingpractice regulations.cGMP的法规地位。
(a) The regulations set forth in this part and in parts 211 through 226 of this chapter contain the minimum current good manufacturing practice for methods to be used in, and the facilities or controls to be used for, the manufacture, processing, packing, or holding of a drug to assure that such drug meets the requirements of the act as to safety, and has the identity and strength and meets the quality and purity characteristics that it purports or is represented to possess. (a)在本部分及本章第211-226部分中所陈述的法规,为现行GMP最低要求,适用于药品制造、加工、包装或贮存中所采用的方法及所使用的设施或控制手段,以保证该药品符合《联邦食品、药品及化妆品法案》(以下简称法案)对安全性的要求,具有均一性和效价(或含量)并符合或代表其生产过程的质量及纯度等特征。
密理博 除菌过滤TRAINING

MILLIPORE
•创建于1954年 •2004年销售8亿美元 •雇员5000人 •总部位于美国麻省波士顿 •全球性的销售和服务支持 •全球有7个符合ISO9000标准的制造厂
MILLIPORE
•三个部门
•市场份额在制药业市场超过50%
•2004/2003年30%增长率
MILLIPORE生物制药部服务对象
通接判性 过触定 测角湿 量来润
i. . e L n n Ho触 o tt g yh w a=oi cc l o a ed b 角 r p 接小 疏 c 较 水 H tt g yh i cc l=o g a e dl h a Hi o n n r i p c
Ho yh db r i oc p 疏 水 < 9 0 ? 9 0 ? > 9 0 ? Hi yh dl r i o p c 亲 水 N cl o tg c a o n n e t a
深层过滤器的特性
相对较厚的过滤介质 相对无序的结构 较大的颗粒承载量 相对较低的过滤效率 可能会有颗粒从下游脱落/释放出来 常作预过滤使用 内容物较多 材料:聚丙烯或者玻璃纤维
深层过滤器的制造工艺
交织---纤维被交织成密集网状结构 熔喷---纤维熔化后被挤喷到一个移动的金 属网上 缠绕---纤维被缠绕在一个芯子上 湿成型---溶解在液体中的纤维真空沉积到 网上形成密集滤膜
下 游 排 放 口
D r a i n
疏水性与亲水性
亲水性
与水有一种亲和性能,水能在上面吸附和湿润 过滤水性溶液不需要预湿润
疏水性
表现出憎水/抵抗水结合特性 当过滤水性溶液或高表面张力的液体时,需要 预湿润
疏水性与亲水性
CA t m f et o n haoti n g es W tt li a e e c s u t l r e a b i y
美国cGMP

(c)本部门有批准或驳回影响药品的均一性、效价或含量、质量及纯度的所有程序或规格标准的职责。
(d)适用于本部门的职责与程序,应成文字材料,并应遵循。
211.25人员资格
(a)每位从事药品生产、加工,包装或仓贮工作的人员,应接受培训、教育及有实践经验,
(b) Adequate laboratory facilities for the testing and approval (or rejection) of components, drug product containers, closures, packaging materials, in-process materials, and drug products shall be available to the quality control unit.
351 of the Public Health Service Act); supplement and do not supersede the regulations in this part unless the regulations
[联邦法律]
[第4卷,第21条]
[2005年4月1日修订]
(d) The responsibilities and procedures applicable to the quality control unit shall be in writing; such written procedures shall be followed.
Sec. 211.25 Personnel qualifications.
CGMP-中英文对照

C G M P中英文对照Table of Contents 目录• SUBPART B 111.10 – 111。
14: Personnel 人员• SUBPART C 111。
15 – 111.23: Physical Plant and Grounds 工厂与场所• SUBPART D 111。
25 – 111。
35: Equipment and Utensils 设备与器具• SUBPART E 111.55—111.95: Production and Process Control System 生产与过程控制系统• SUBPART F 111。
103-111。
140: Production and Process Control System:• Requirements for Quality Control 生产与过程控制系统对质量控制的要求• SUBPART G 111。
153 – 111.180: Production and Process Control System:• Requirements for components, packaging, and labels 生产与过程控制系统对成分,包装与标签的要求• SUBPART H 111.205—111。
210: Production and Process Control System:• Requirements for the Master Manufacturing Record. 生产与过程控制系统对主要制造记录的要求• SUBPART I 111。
255 – 111.260: Production and Process Control System:• Require ments for the Batch Production Record。
生产与过程控制系统对批生产记录的要求• SUBPART J 111.303 – 111.325: Production and Process Control System:• Requirements for Laboratory Operations 生产与过程控制系统对实验室操作的要求• SUBPART K 111。
进行技能培训的英文

Conducting Skills Training: The Importance and BenefitsSkills training plays a crucial role in personal and professional development. It equips individuals with the knowledge and expertise required to excel in their chosen fields. In this document, we will explore the significance and advantages of conducting skills training in English.Introduction to Skills TrainingSkills training refers to the process of acquiring and refining specific abilities, knowledge, and competencies that are essential for carrying out tasks effectively. It encompasses a wide range of disciplines, including communication, leadership, problem-solving, and technical skills. Investing time and resources in skills training can have a significant impact on an individual’s career growth and overall success.The Importance of Skills TrainingEnhancing Professional CompetenceIn today’s competitive job market, possessing a diverse skill set is an invaluable asset. Skills training enables individuals to enhance their professional competence by gaining proficiency in various areas. Whether it is acquiring technical skills relevant to a specific job or developing soft skills like teamwork and communication, continuous training allows individuals to stay up-to-date and adapt to evolving industry standards.Fostering Personal GrowthSkills training not only empowers individuals in their professional lives but also contributes to personal growth. Learning new skills boosts self-confidence and fosters a sense of accomplishment. It encourages individuals to challenge themselves, step out of their comfort zones, and embrace continuous learning. Moreover, skills training can also help individuals discover hidden talents and passions, leading to personal fulfillment and satisfaction.Increasing EmployabilityEmployers value candidates who are willing to invest in their personal and professional development. By participating in skills training programs, individuals can demonstrate their commitment to continuous improvement, making them more desirable to potential employers. A diverse skill set enhances an ind ividual’s marketability and increases their chances of securing better job opportunities and career advancement.Improving Efficiency and ProductivitySkills training directly impacts an individual’s efficiency and productivity within their role. Acquiring new skills or refining existing ones enables individuals to perform tasks more effectively, reducing wastage of time and resources. For instance, a training program in time management can help individuals prioritize tasks, meet deadlines, and accomplish goals efficiently. By improving efficiency, skills training contributes to overall organizational success as well.Encouraging Innovation and AdaptabilityThe world is constantly evolving, and skills training equips individuals with the ability to adapt to changes in their respective fields. By staying updated with the latest industry trends and technological advancements, individuals can identify opportunities for innovation and improvement. Skills training encourages a growth mindset, enabling individuals to embrace change, think creatively, and develop innovative solutions to challenges.The Benefits of Conducting Skills Training in EnglishGlobal CommunicationEnglish is one of the most widely spoken languages globally and serves as a lingua franca in many industries. Conducting skills training in English ensures that individuals can effectively communicate and collaborate with professionals from different countries and cultures. This is particularly important in multinational organizations or industries where English is the primary language for business communication.Access to Resources and KnowledgeEnglish is the language of extensive educational resources and knowledge repositories. By conducting skills training in English, individuals gain access to a vast range of learning materials, including books, articles, and online courses. This exposure to diverse resources enhances the depth and quality of the training, enabling individuals to acquire a more comprehensive understanding of the subject matter.Enhanced Career OpportunitiesProficiency in English opens up a plethora of career opportunities on a global scale. Many multinational companies require employees who can interact and work in English-speaking environments. By conducting skills training in English, individuals develop a competitive edge, broadening their career prospects beyond their local regions.Confidence in English CommunicationEnglish skills training not only focuses on specific job-related skills but also aids in improving English language proficiency. Clear and effective communication is vital in every professional setting, and being confident in English communication can greatly boost an individual’s professional image. Skills training in English provides individuals with ample practice and guidance to develop fluency, vocabulary, and grammatical accuracy.ConclusionSkills training is an essential aspect of personal and professional growth. It enhances competence, increases employability, fosters personal development, and improves overall efficiency and productivity. Conducting skills training in English offers additional advantages, such as global communication opportunities, access to extensive resources, enhanced career prospects, and improved English language proficiency. Investing in skills training, particularly in English, is a worthwhile endeavor that paves the way for success in today’s interconnected and competitive world.。
职高英语基础模块培训计划

职高英语基础模块培训计划1. IntroductionVocational high schools are integral to the education system as they provide specialized learning opportunities for students who want to pursue a career in a particular field. The English language is an essential tool for communication and professional success in today's globalized world. Therefore, it is crucial for vocational high school students to have a strong grasp of the English language. This training plan aims to provide a comprehensive approach to learning English at a basic level for vocational high school students.2. ObjectivesThe objectives of this training plan are as follows:- To develop basic communication skills in English, including speaking, listening, reading, and writing.- To build vocabulary and grammar knowledge to effectively communicate in English.- To provide opportunities for students to apply their English language skills in practical, real-world scenarios related to their vocational field.- To prepare students for further English language learning and professional development.3. Duration and ScheduleThe basic module training plan is designed to span over one academic year, divided into two semesters. Each semester will consist of 18 weeks of instruction, with a total of 36 weeks for the entire training plan.4. Course ContentThe course content for the basic module English training plan will cover the following areas: - Basic grammar and sentence structure- Vocabulary building and word usage- Reading and comprehension- Speaking and listening practice- Writing skills development- Practical application of English in vocational contexts5. Teaching MethodsThe training plan will employ a variety of teaching methods to cater to different learning styles and maximize student engagement. These methods may include:- Lectures and demonstrations- Group discussions and activities- Role-plays and simulations- Audio-visual aids- Individual and group projects- Real-life vocational scenarios and case studies- Guest speakers from relevant professional fields6. Assessment and EvaluationA variety of assessment methods will be used to evaluate the progress of students throughout the training plan. These may include:- Written tests and quizzes- Oral presentations and speaking assessments- Reading and listening comprehension exercises- Writing assignments and projects- Participation and engagement in class activities7. ResourcesTo support the training plan, various resources will be utilized, including:- Textbooks and workbooks specifically designed for vocational high school English learning - Audio recordings and listening materials- Online resources and interactive learning tools- Reference materials related to the students' vocational field8. Practical ApplicationTo ensure that the students can apply their English language skills in a practical context, the training plan will incorporate hands-on activities related to their vocational field. These activities may include:- Role-plays of workplace scenarios- Written and oral communication exercises specific to their vocational area- Visits to relevant workplaces or industries- Projects and presentations related to their vocational field in English9. Professional DevelopmentIn addition to the basic English language skills, the training plan will also seek to develop students' professional skills. This may include:- Networking and communication skills- Workplace etiquette and professionalism- Resume writing and interview preparation- Time management and goal setting10. ConclusionThe Vocational High School English Basic Module Training Plan aims to provide a comprehensive and practical approach to learning English for students in vocational high schools. By developing strong English language skills and practical application in their vocational field, students will be better prepared for further education or entry into the workforce. This training plan seeks to equip students with the language and professional skills necessary for success in their chosen career paths.。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
The FDA website can be accessed anywhere in the world anytime by anyone
(central site)
/cdrh/index.html
TITLE 21--FOOD AND DRUGS CHAPTER I--FOOD AND DRUG ADMINISTRATION
(1) The requirements in this part govern the methods used in, and the facilities and controls used for, the design, manufacture, packaging, labeling, storage, installation, and servicing of all finished devices intended for human use. (2) The provisions of this part shall be applicable to any finished device as defined in this part, intended for human use, that is manufactured, imported, or offered for import in any State or Territory of the United States, the District of Columbia, or the Commonwealth of Puerto Rico.
The Rules are titled “21 CFR Part 820. and apply to all medical products intended for human use.
21 CFR (Code of Federal Regulations) part 820
“The quality system regulation includes requirements related to the methods used in and the facilities and controls used for: Designing, purchasing, manufacturing, packaging, labelling, storing, installing, and servicing of medical devices intended for human use.”
§820.3 – Definitions (exactly) §820.5 - Quality system.
Each manufacturer shall establish and maintain a quality system that is appropriate for the specific medical device(s) designed or manufactured, and that meets the requirements of this part.
FDA is organized into Centers which are responsible for each type of product.
Center for Food Safety and Applied Nutrition Center for Drug Evaluation and Research Center for Devices and Radiological Health Center for Biologics Evaluation and Research Center for Veterinary Medicine Office of Regulatory Affairs National Center for Toxicological Research
This is a basic overview, it is up to the individual to read and understand the complete rule!
Who are these guys?!
US FDA CDRH
The U.S. Food and Drug Administration (FDA) is the U.S. Government agency that oversees most foods and medical products. Its job is to make sure that:
Why do these rules apply to S&N here in Suzhou?
S&N Suzhou will export direct to the US market. All products entering the US market that are regulated by the US FDA must comply regardless of the country of origin. If we don’t meet, we don’t compete.
processes.
What is the outcome of following CGMP?
The assured ability to consistently produce the same product to meet the same specifications time after time! Increase production, lower cost, reduce price to customer, faster delivery times, etc. . . Are NOT part of GMP!
DEPARTMENT OF HEALTH AND HUMAN SERVICES
SUBCHAPTER H--MEDICAL DEVICES PART 820 QUALITY SYSTEM REGULATION
Rules Subpart A--General Provisions
§820.1 - Scope.
Subpart B--Quality System Requirements
When do the rules apply?
Research – do not, not in compliance
Development – some do, compliance is considered
Manufacturing – compliance is required
Now for the fun part..............
On the next series of slides we will cover who is the FDA and what is the intent of each sub-part of the rules we must comply with at all times
food is safe, healthy, and clean medicines and medical devices are reasonably safe and effective cosmetic products are safe animal foods and drugs are safe food and medical products have proper labels
Assure that correct and APPROVED procedures are always followed. Provide controlled documentation, traceability, and accurate history records.
Overall Intent: To assure Quality is “built in” to the product and
CGMP and CGLP
CGMP: (Current Good Manufacturing Practice)
Protect the integrity and quality of manufactured product intended for human use.
CGLP: (Current Good Laboratory Practice)
Protect the integrity and quality of laboratory data used to support a products release to the customer.
Current Good Manufacturing Practices (CGMP)
US FDA CGMP INDUCTION TRAINING
GMP stands for “Current Good Manufacturing Practices” and is the bases for the US FDA Rules of Conduct for ALL medical device manufacturers that import or sell products to the US market.
Manufacturing
Must be even more Controlled . . .
CGMP takes priority
Quality and Control are critical
