AT-101_acetic_acid_DataSheet_MedChemExpress
Acetaminophen_Paracetamol_Tylenol_COX_CAS号103-90-2说明书_AbMole中国

分子量151.16
溶解性(25°C)
DMSO 30 mg/mL
分子式C H NO Water 13 mg/mL
CAS号103-90-2Ethanol 30 mg/mL
储存条件3年 -20°C 粉末状
生物活性
Acetaminophen是COX-2选择性抑制剂。
Acetaminophen作用于黑色素瘤细胞,如SK-MEL-28, MeWo, SK-MEL-5, B16-F0和 B16-F10具有选择毒性, IC50为100μM,作用于BJ, Saos-2, SW-620, 和PC-3非黑色素瘤细胞没有明显的毒性。
Acetaminophen通过耗尽细胞内GSH和形成ROS,诱导SK-MEL-28 细胞凋亡,且诱导线粒体毒性,双香豆素和1-溴代庚烷可增强毒性,抗环血酸, GSH, 三氟拉嗪和环孢菌素A可降低毒性。
不同实验动物依据体表面积的等效剂量转换表(数据来源于FDA指南)
小鼠大鼠兔豚鼠仓鼠狗
重量 (kg)0.020.15 1.80.40.0810
体表面积 (m)0.0070.0250.150.050.020.5
K系数36128520
动物 A (mg/kg) = 动物 B (mg/kg) ×
动物 B的K系数
动物 A的K系数
例如,依据体表面积折算法,将白藜芦醇用于小鼠的剂量22.4 mg/kg 换算成大鼠的剂量,需要将22.4 mg/kg 乘以小鼠的K系数(3),再除以大鼠的K系数(6),得到白藜芦醇用于大鼠的等效剂量为11.2 mg/kg。
Acetaminophen 目录号M3301
化学数据
892
2
m
m
m
m m。
kga101 n凯基annexin vegfp细胞凋亡检测试剂盒说明书

凯基Annexin V-EGFP细胞凋亡检测试剂盒(Annexin V-EGFP Apoptosis Detection Kit)Cat number:KGA For Research Use OnlyStore at4℃ for one yearExpire date:一、 试剂盒说明在正常细胞中,磷脂酰丝氨酸(PS)只分布在细胞膜脂质双层的内侧,而在细胞凋亡早期,细胞膜中的磷脂酰丝氨酸(PS)由脂膜内侧翻向外侧。
Annexin V是一种分子量为35~36kD的Ca2+依赖性磷脂结合蛋白,与磷脂酰丝氨酸有高度亲和力,故可通过细胞外侧暴露的磷脂酰丝氨酸与凋亡早期细胞的胞膜结合。
因此Annexin V被作为检测细胞早期凋亡的灵敏指标之一。
将Annexin V进行荧光素(EGFP、FITC)标记,以标记了的Annexin V作为荧光探针,利用荧光显微镜或流式细胞仪可检测细胞凋亡的发生。
与FITC的绿色荧光信号相比,EGFP的绿色荧光信号具有信号强,不易淬灭,稳定性高等优点,故本试剂盒采用EGFP作为荧光标记探针。
碘化丙啶(Propidium Iodide, PI)是一种核酸染料,它不能透过完整的细胞膜,但对凋亡中晚期的细胞和死细胞,PI能够透过细胞膜而使细胞核染红。
因此将Annexin V与PI匹配使用,就可以将处于不同凋亡时期的细胞区分开来。
本试剂盒可应用于培养细胞凋亡检测(不推荐用于检测组织样本)。
二、 试剂盒组份组份Cat: KGA10110 assays Cat: KGA10220 assaysCat: KGA10350 assaysCat: KGA104100 assays储存条件AnnexinV-EGFP 50μL 100μL 250μL 500μL 4℃避光Propidium Iodide 50μL 100μL 250μL 500μL 4℃避光Binding Buffer 5 mL 10.0 mL 25 mL 50 mL 4℃三、 试剂盒以外自备仪器和试剂流式细胞仪或荧光显微镜、低速离心机、微量移液器1.5m L Microtube、载玻片、盖玻片(荧光显微镜观察需用)、PBS、不含EDTA的胰酶消化液四、 使用注意事项1.微量试剂取用前请离心集液。
Trigonox 101-50D-PD 产品数据表说明书

Product Data SheetTrigonox 101-50D-PD 2,5-Dimethyl-2,5-di(tert-butylperoxy) hexaneTrigonox® 101-50D-PD is a 50% formulation on an inert carrier sytem in powder form.CAS number78-63-7EINECS/ELINCS No.201-128-1TSCA statuslisted on inventoryMolecular weight290.4Active oxygen contentperoxide11.02%Concentration5.40-5.62%SpecificationsAppearance White powderAssay49.0-51.0 %ApplicationsTrigonox® 101-50D-PD is a bifunctional peroxide which is used for the crosslinking of natural rubber and synthetic rubbers, as well as polyolefins. Rubber compounds containing Trigonox® 101-50D-PD have excellent scorch safety, and under certain conditions one step mixing is possible. Safe processing temperature: 135°C (rheometer ts2 > 20 min.). Typical crosslinking temperature: 175°C (rheometer t90 about 12 min.).Thermal stabilityOrganic peroxides are thermally unstable substances, which may undergo self-accelerating decomposition. The lowest temperature at which self-accelerating decomposition of a substance in the original packaging may occur is the Self-Accelerating Decomposition Temperature (SADT). The SADT is determined on the basis of the Heat Accumulation Storage Test.SADT80°CMethod The Heat Accumulation Storage Test is a recognized test method for thedetermination of the SADT of organic peroxides (see Recommendations on theTransport of Dangerous Goods, Manual of Tests and Criteria - United Nations, NewYork and Geneva).StorageDue to the relatively unstable nature of organic peroxides a loss of quality can be detected over a period of time. To minimize the loss of quality, Nouryon recommends a maximum storage temperature (Ts max. ) for each organic peroxide product.Ts Max.30°CTs Min.0°CNote When stored under these recommended storage conditions, Trigonox® 101-50D-PD will remain within the Nouryon specifications for a period of at least 6 monthsafter delivery.Packaging and transportThe standard packaging is a cardboard box for 20 kg peroxide formulationBoth packaging and transport meet the international regulations. For the availability of other packed quantities contact your Nouryon representative. Trigonox®101-50D-PD is classified as Organic peroxide type E; solid, Division 5. 2; UN 3108.Safety and handlingKeep containers tightly closed. Store and handle Trigonox® 101-50D-PD in a dry well-ventilated place away from sources of heat or ignition and direct sunlight. Never weigh out in the storage room. Avoid contact with reducing agents (e. g. amines), acids, alkalis and heavy metal compounds (e. g. accelerators, driers and metal soaps). Please refer to the Safety Data Sheet (SDS) for further information on the safe storage, use and handling of Trigonox® 101-50D-PD. This information should be thoroughly reviewed prior to acceptance of this product. The SDS is available at /sds-search.Major decomposition productsMethane, Ethane, Acetone, tert-Butanol, tert-AmylalcoholAll information concerning this product and/or suggestions for handling and use contained herein are offered in good faith and are believed to be reliable.Nouryon, however, makes no warranty as to accuracy and/or sufficiency of such information and/or suggestions, as to the product's merchantability or fitness for any particular purpose, or that any suggested use will not infringe any patent. Nouryon does not accept any liability whatsoever arising out of the use of or reliance on this information, or out of the use or the performance of the product. Nothing contained herein shall be construed as granting or extending any license under any patent. Customer must determine for himself, by preliminary tests or otherwise, the suitability of this product for his purposes.The information contained herein supersedes all previously issued information on the subject matter covered. The customer may forward, distribute, and/or photocopy this document only if unaltered and complete, including all of its headers and footers, and should refrain from any unauthorized use. Don’t copythis document to a website.Trigonox® is a registered trademark of Nouryon Functional Chemicals B.V. or affiliates in one or more territories.Contact UsPolymer Specialties Americas************************Polymer Specialties Europe, Middle East, India and Africa*************************Polymer Specialties Asia Pacific************************2022-6-30© 2022Polymer crosslinking Trigonox 101-50D-PD。
美国药典 阿司匹林 中英文对照

About 143 °C (instantaneous method).
IDENTIFICATION
First identification A, B.
Second identification B, C, D.
A.Infrared absorption spectrophotometry {2.2.24).
Reference solution (a) Dissolve 50.0 mg of salicylic acid R in the mobile phase and dilute to 50.0 ml with the mobile phase.Dilute 1.0 ml of this solution to 100.0 ml with the mobile phase.
Limits:
--any impurity: for each impurity, not more than the area of the principal peak in the chromatogramobtained with reference solution (a) (0.1 per cent);
ASSAY
In a flask with a ground-glass stopper, dissolve 1.000 g in 10 ml of ethanol (96 per cent) R. Add 50.0 ml of 0.5 M sodium hydroxide. Close the flask and allow to stand for 1 h.
Comparison acetylsalicyiic acid CRS.
B.To 0.2 g add 4 ml of dilute sodium hydroxide solution R and boil for 3 min. Cool and add 5 ml of dilute sulphuric add R
AT-101_acetic_acid_NP-HPLC_15080_MedChemExpress
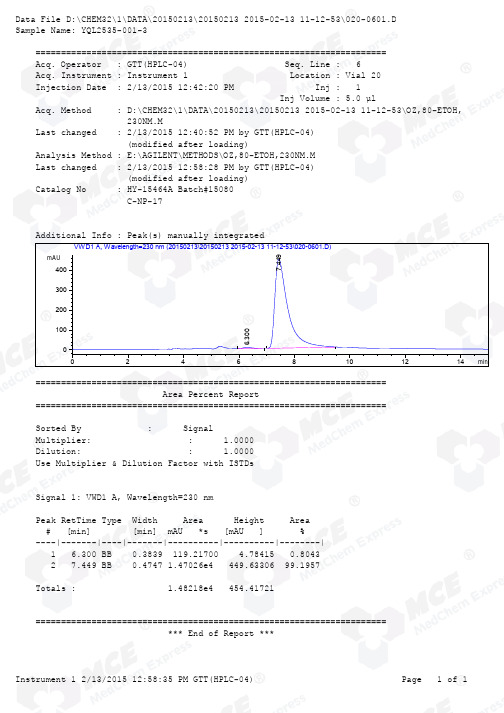
Injection Date : 2/13/2015 12:42:20 PM Inj : 1 Inj Volume : 5.0 µl Acq. Method : D:\CHEM32\1\DATA\20150213\20150213 2015-02-13 11-12-53\OZ,80-ETOH, 230NM.M Last changed : 2/13/2015 12:40:52 PM by GTT(HPLC-04) (modified after loading)Analysis Method : E:\AGILENT\METHODS\OZ,80-ETOH,230NM.M Last changed : 2/13/2015 12:58:28 PM by GTT(HPLC-04) (modified after loading)Catalog No : HY-15464A Batch#15080 C-NP-17 Additional Info : Peak(s) manually integrated min 024********mAU 0100200300400 VWD1 A, Wavelength=230 nm (20150213\20150213 2015-02-13 11-12-53\020-0601.D)6.3007.449===================================================================== Area Percent Report ===================================================================== Sorted By : Signal Multiplier: : 1.0000Dilution: : 1.0000Use Multiplier & Dilution Factor with ISTDs Signal 1: VWD1 A, Wavelength=230 nm Peak RetTime Type Width Area Height Area # [min] [min] mAU *s [mAU ] %----|-------|----|-------|----------|----------|--------| 1 6.300 BB 0.3839 119.21700 4.78415 0.8043 2 7.449 BB 0.4747 1.47026e4 449.63306 99.1957 Totals : 1.48218e4 454.41721 ===================================================================== *** End of Report ***Injection Date : 2/13/2015 12:07:11 PM Inj : 1 Inj Volume : 5.0 µl Acq. Method : D:\CHEM32\1\DATA\20150213\20150213 2015-02-13 11-12-53\OZ,80-ETOH, 230NM.M Last changed : 2/13/2015 12:05:25 PM by GTT(HPLC-04) (modified after loading)Analysis Method : E:\AGILENT\METHODS\OZ,80-ETOH,230NM.M Last changed : 2/13/2015 1:00:24 PM by GTT(HPLC-04) (modified after loading)Catalog No : HY-15464A Batch#15080 C-NP-17 min 024********mAU 0100200300400VWD1 A, Wavelength=230 nm (20150213\20150213 2015-02-13 11-12-53\018-0401.D)5.957 7.455 ===================================================================== Area Percent Report ===================================================================== Sorted By : Signal Multiplier: : 1.0000Dilution: : 1.0000Use Multiplier & Dilution Factor with ISTDs Signal 1: VWD1 A, Wavelength=230 nm Peak RetTime Type Width Area Height Area # [min] [min] mAU *s [mAU ] %----|-------|----|-------|----------|----------|--------| 1 5.957 BB 0.3212 9913.30078 444.72357 48.3676 2 7.455 BB 0.4301 1.05825e4 369.60165 51.6324 Totals : 2.04958e4 814.32523 ===================================================================== *** End of Report ***Injection Date : 2/13/2015 12:23:54 PM Inj : 1 Inj Volume : 5.0 µl Acq. Method : D:\CHEM32\1\DATA\20150213\20150213 2015-02-13 11-12-53\OZ,80-ETOH, 230NM.M Last changed : 2/13/2015 12:22:34 PM by GTT(HPLC-04) (modified after loading)Analysis Method : E:\AGILENT\METHODS\OZ,80-ETOH,230NM.M Last changed : 2/13/2015 12:59:31 PM by GTT(HPLC-04) (modified after loading)Catalog No : HY-15464A Batch#15080 C-NP-17 Additional Info : Peak(s) manually integrated min 024********mAU100200300400500600700VWD1 A, Wavelength=230 nm (20150213\20150213 2015-02-13 11-12-53\019-0501.D)===================================================================== Area Percent Report ===================================================================== Sorted By : Signal Multiplier: : 1.0000Dilution: : 1.0000Use Multiplier & Dilution Factor with ISTDs No peaks found ===================================================================== *** End of Report ***。
α-Estradio_57-91-0_MedBio_参考使用

ICI 204,448 hydrochloride
ICI 204,448 hydrochloride
121264-04-8
50mg
≥98%
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
MED12175
Finasteride acetate
Finasteride acetate
222989-99-3
3、α-Estradiol同类产品列表:
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
MED12185
Clomiphene citrate
Clomiphene citrate
50-41-9
1g
≥98%
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
MED12197
Erteberel (LY500307)
200mg
≥98%
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
MED12170
ODM-201
ODM-201
1297538-32-9
50mg
≥98%
体内研究
α-雌二醇(17-α-雌二醇,0.01,0.1,1μg)显着降低小鼠幼崽中央无血管/总视网膜面积的百分比。在暴露于高氧的幼鼠的视网膜中,α-雌二醇(1μg)显着降低出生后第9天,第13天和第17天的丙二醛(MDA)水平。 α-雌二醇(1μg)还减少NADPH-氧化酶阳性细胞的数量,NADPH氧化酶浓度和幼仔视网膜中的活性。在1.0-μgα-雌二醇处理的幼崽中,VEGF视网膜浓度在PND 9上较高,但在PND 14和17上较低.1.1-μgα-雌二醇处理的幼崽的视网膜中的最佳效果在PND上被ICI182780部分逆转。 14和17 [3]。
CAL-101_DataSheet_MedChemExpress

Inhibitors, Agonists, Screening Libraries Data SheetBIOLOGICAL ACTIVITY:CAL–101 is a highly selective and potent p110δ inhibitor with IC 50 of 2.5 nM, is 40– to 300–fold more selective for p110δrelative to other PI3K class I enzymes (p110α, p110β, and p110γ; IC 50 are 820, 565, and 89nM, respectively).IC50 & Target: IC50: 2.5 nM (p110δ), 89 nM (p110γ), 565 nM (p110β), 820 nM (p110α)[1]In Vitro: CAL–101 is a highly selective and potent p110δ inhibitor (EC 50=8 nM). Greater selectivity (400– to 4000–fold) is seen against related kinases C2β, hVPS34, DNA–PK, and mTOR, whereas no activity is observed against a panel of 402 diverse kinases at 10 μM.CAL–101 reduces PDGF–induced pAkt by only 25% at 10 μM. CAL–101 inhibits LPA–induced pAkt with an EC 50 of 1.9 μM. CAL–101blocks Fc?RI p110δ–mediated CD63 expression with an EC 50 of 8 nM, whereas formyl–methionyl–leucyl–phenylalanine activation of p110γ is inhibited with an EC 50 of 3 μM. Thus, in cell–based assays, CAL–101 has 240– to 2500–fold selectivity for p110δ over the other class I PI3K isoforms [1]. CAL–101–induced apoptosis of chronic lymphocytic leukemia (CLL) cells is significant compare withvehicle treatment alone (P<0.001). CAL–101 induces selective cytotoxicity in CLL cells independent of IgVH mutational status or interphase cytogenetics [2].In Vivo: A significant reduction is observed in the CD11b +Ly6G + neutrophils from brain homogenates of bothp110δD910A/D910A mice and CAL–101 (40 mg/kg, i.v.) post–treated mice [3].PROTOCOL (Extracted from published papers and Only for reference)Cell Assay: CAL–101 is dissolved in DMSO and stored, and then diluted with appropriate media before use [2].[2]MTT assays are performed to determine cytotoxicity. Briefly, 1×105 cells (CLL B cells or healthy volunteer T cells or NK cells) are incubated for 48hours with different concentrations of CAL–101 (0.1 μM, 1 μM, 5 μM, 10 μM), 25 μM LY294002, or vehicle control. MTT reagent is then added, and plates are incubated for an additional 20 hours before washing with protamine sulfate in phosphate–buffered saline. DMSO is added, and absorbance is measured by spectrophotometry at 540 nm in a Labsystems plate reader. Cell viability is also measured at various time points with the use of annexin/PI flow cytometry. Data are analyzed with Expo–ADC32 software package. At least 10,000 cells are counted for each sample. Results are expressed as the percentage of total positive cells over untreated control. Experiments examining caspase–dependent apoptosis included the addition of 100 μM Z–VAD. Experiments examining survival signals include the addition of 1 μg/mL CD40L, 800 U/mL IL–4, 50 ng/mL BAFF, 20 ng/mL TNF–α, or coculturing on fibronectin or stromal (HS–5 cell line) coated plates. Stromal coculture is done by plating a 75–cm2 flask (80%–100% confluent)per 6–well plate 24 hours before the addition of CLL cells [2].Animal Administration: CAL–101 is prepared in DMSO and then diluted [3].[3]Mice [3]For CAL–101 treatment, wild–type C57BL/6 mice are administered either 40 mg/kg CAL–101 or vehicle DMSO, by 25 μL infusion into the femoral vein, 15 min before I/R (pre–treatment), or 3 and 6 h after initiation of reperfusion (post–treatment). Controls and animals treated with CAL–101 underwent cerebral blood flow (CBF) measurements using a laser Doppler perfusion monitor. The CBFProduct Name:CAL–101Cat. No.:HY-13026CAS No.:870281-82-6Molecular Formula:C 22H 18FN 7O Molecular Weight:415.42Target:PI3K; Autophagy Pathway:PI3K/Akt/mTOR; Autophagy Solubility:DMSO: ≥ 59.7 mg/mLmeasurements obtained immediately before and after MCAO and again at 3 h after reperfusion showed an ~90–95% reduction in the blood flow to the MCAO infarct region, which does not differ between groups.References:[1]. Lannutti BJ, et al. CAL–101, a p110delta selective phosphatidylinositol–3–kinase inhibitor for the treatment of B–cell malignancies, inhibits PI3K signaling and cellular viability. Blood, 2011, 117(2), 591–594.[2]. Herman SE, et al. Phosphatidylinositol 3–kinase–δ inhibitor CAL–101 shows promising preclinical activity in chronic lymphocytic leukemia by antagonizing intrinsic and extrinsic cellular survival signals. Blood, 2010, 116(12), 2078–2088.[3]. Low PC, et al. PI3Kδ inhibition reduces TNF secretion and neuroinflammation in a mouse cerebral stroke model. Nat Commun. 2014 Mar 14;5:3450.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
MedBio_106566-58-9_AS 101技术参考

≥98%
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
MED13561
BAY-X 1005
BAY-X 1005
128253-31-6
1mg
≥98%
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
MED13608
Protease Inhibitor Cocktail (EDTA-Free, 100X in DMSO)
59937-28-9
10mM (in 1mL DMSO)
≥98%
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
MED13583
PS 1145 dihydrochloride
PS 1145 dihydrochloride
1049743-58-9
25mg
≥98%
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
MED13521
Malotilate
Malotilate
体内研究
骨化(AS101;0.5 mg/kg/天;IP;25天)通过抑制IL-10使GBM肿瘤对紫杉醇敏感,从而提高生存率[2]。动物模型:具有GBM细胞的SCID小鼠[2]剂量:0.5mg/kg给药:IP;每日;25天结果:GBM荷瘤小鼠生存率显著提高。
3、Ossirene同类产品列表:
品牌
货号
中文名称
Protease Inhibitor Cocktail (EDTA-Free, 100X in DMSO)
Alda-1_DataSheet_MedChemExpress

Inhibitors, Agonists, Screening Libraries Data SheetBIOLOGICAL ACTIVITY:Alda–1 is a potent ALDH2 agonist, which significantly improves ALDH2 activity.In Vivo: Alda–1 treatment results in a significant decrease of 4–HNE–protein content in the plasma of apoE -/- mice. Alda–1administration leads to a slight increase in gene expression related to neurogenesis (Nog ), mitochondrial biogenesis (CYTB , ND1),and apoptosis (Bax , Gsk3b ) in the Hp of apoE -/- mice. Alda–1 administration leads to 2 and 10 differentially expressed proteins in theFCx and Hp of apoE -/- mice, respectively [1]. Alda–1 (1.5 mg/kg, b.w., i.p.) administration significantly increases the climbing time,tends to reduce the immobility time and increases the swimming time of the prenatally stressed rats in the forced swim test.Moreover, treatment of prenatally stressed rats with Alda–1 significantly increases number of entries into the open arms of the maze and the time spent therein, as assessed by elevated plus–maze test [2]. Alda–1 (8.5 mg/kg, i.p.) with glucose significantly lowers 4–HNE and FJB–positive cells in the cerebral cortex of Alda–1–treated rats than in DMSO–treated rats 24 h after glucose administration [3]. Alda–1 (10 mg/kg per day) treatment prevents aldehydic overload, mitochondrial dysfunction and improves ventricular function in post–MI cardiomyopathy rats [4].PROTOCOL (Extracted from published papers and Only for reference)Cell Assay:[2]Spleen cells (4×106 cells/mL) are stimulated by optimal concentrations of concanavalin A (Con A; 2.5μg/mL and 0.6 μg/mL) and lipopolysaccharide (LPS, 5 μg/mL) and are incubated in 96–well plates at final volume of 0.2mL for 72 h. Cell proliferation is determined by adding 0.5 μCi of [3H]–thymidine per well at 16 h before the end of the incubation.The cultures are harvested with an automatic cell harvester, and [3H] thymidine incorporation is assessed using a liquid scintillationcounter.Animal Administration: Alda–1 is dissolved in 1 mL/kg b.w. DMSO/water 50/50.[2]After behavioral verification at three months of age,the animals are divided into the following four groups: control, control + Alda–1, prenatally stressed and prenatally stressed + Alda–1(6 animals per group). Alda–1 injections are given intraperitoneally (i.p.) once daily at a dose of 1.5 mg/kg b.w. (dissolved in 1 mL/kg b.w. DMSO/water 50/50) for 14 days. At the same time, the control and prenatally stressed rats receive 1 mL/kg b.w. DMSO/water 50/50. The injections of Alda–1 and vehicle are given between 10 a.m and 11 a.m. In the last five days of Alda–1 treatment the behavioral parameters in the elevated plus maze test and then in the forced swim test are measured.References:[1]. Stachowicz A, et al. Proteomic Analysis of Mitochondria–Enriched Fraction Isolated from the Frontal Cortex and Hippocampus of Apolipoprotein E Knockout Mice Treated with Alda–1, an Activator of Mitochondrial Aldehyde Dehydrogenase (ALDH2). Int J Mol Sci.[2]. Stachowicz A, et al. The impact of mitochondrial aldehyde dehydrogenase (ALDH2) activation by Alda–1 on the behavioral and biochemical disturbances in animal model of depression. Brain Behav Immun. 2016 Jan;51:144–53.Product Name:Alda–1Cat. No.:HY-18936CAS No.:349438-38-6Molecular Formula:C 15H 11Cl 2NO 3Molecular Weight:324.16Target:Aldehyde Dehydrogenase (ALDH)Pathway:Metabolic Enzyme/Protease Solubility:DMSO: ≥ 51 mg/mL[3]. Ikeda T, et al. Effects of Alda–1, an Aldehyde Dehydrogenase–2 Agonist, on Hypoglycemic Neuronal Death. PLoS One. 2015 Jun 17;10(6):e0128844.[4]. Gomes KM, et al. Aldehydic load and aldehyde dehydrogenase 2 profile during the progression of post–myocardial infarction cardiomyopathy: benefits of Alda–1. Int J Cardiol. 2015 Jan 20;179:129–138.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
AT-406_DataSheet_MedChemExpress

Inhibitors, Agonists, Screening Libraries Data SheetBIOLOGICAL ACTIVITY:AT–406 is a potent and orally bioavailable Smac mimetic and an antagonist of IAPs , and it binds to XIAP, cIAP1, and cIAP2 proteins with K i of 66.4, 1.9, and 5.1 nM, respectively.IC50 & Target: Ki: 66.4 nM (XIAP), 1.9 nM (cIAP1), 5.1 nM (cIAP2)In Vitro: AT–406 mimic closely the AVPI peptide in both hydrogen bonding and hydrophobic interactions with XIAP, with additional hydrophobic contacts with W323 of XIAP. AT–406 is more sensitive to these IAPs than Smac AVPI peptide with 50–100 fold binding affinities. AT–406 (1 μM) completely restores the activity of caspase–9, which is suppressed by 500 nM XIAP BIR3 in a cell–freesystem. In MDA–MB–231 cell, AT–406 induces rapid cellular cIAP1 degradation and also pulls down the cellular XIAP protein. AT–406effectively inhibits lots of human cancer cell lines and shows IC 50 of 144 and 142 nM in MDA–MB–231 cell and SK–OV–3 ovarian cell, with low toxicity against normal–like human breast epithelial MCF–12F cells and primary human normal prostate epithelial cells. AT–406 induces apoptosis in MDA–MB–231 cell by inducing activation of caspase–3 and cleavage of PARP [1]. AT–406 displays single agent activity in ovarian cancer cell lines. The IC 50 values of AT–406 in these ovarian cancer cells range from 0.05–0.5 μg/mL.AT–406 exhibits anti–ovarian cancer efficacy both as a single agent and in combination with carboplatin. AT–406 (30 μg/mL)induced degradation of XIAP in the drug sensitive ovarian cancer cell lines [2].In Vivo: AT–406 has good pharmacokinetic properties and oral bioavailability in mice, rats, non–human primates, and dogs. In the MDA–MB–231 xenograft, AT–406 effectively induces cIAP1 degradation and processing of procaspase–8, cleavage of PARP in tumor tissues at 100 mg/kg with well toleration even at 200 mg/kg. AT–406 induces significant tumor growth inhibition with p of 0.0012 at 100 mg/kg [2]. SM–406 (30, 100 mg/kg, p.o.) decreases the plasma and tumor in tumor–bearing mice [3].PROTOCOL (Extracted from published papers and Only for reference)Kinase Assay:[1]MDA–MB–231 cell lysates are prepared by solubilizing cells in ice cold buffer containing KCl (50 mM), EGTA (5 mM),MgCl 2 (2 mM) DTT (1 mM), 0.2% CHAPS and HEPES, (50 mM, pH 7.5), containing cocktail protease inhibitors, incubating on ice for 10minutes, then freezing in liquid nitrogen. Cytochrome c and dATP are added to the cell lysates, which are then incubated at 30°C in a water bath for 60 minutes to activate caspase–9. Addition of recombinant XIAP BIR3 protein dose–dependently suppresses the activity of caspase–9. Different concentrations of a tested Smac mimetic (1 nM–100 μM) are added to determine the restoration of the activity of these caspases.Cell Assay:[1]Cells are seeded in 96–well flat bottom cell culture plates at a density of 3–4 ×103 cells/well with AT–406 andincubated for 4 days. The rate of cell growth inhibition after treatment with different concentrations of AT–406 is determined by assaying with (2–(2–methoxy–4–nitrophenyl)–3–(4–nitrophenyl)–5–(2,4–disulfophenyl)–2H–tetrazolium monosodium salt (WST–8).WST–8 is added to each well to a final concentration of 10%, and then the plates are incubated at 37°C for 2-3 hours. Theabsorbance of the samples is measured at 450 nm using a TECAN ULTRA reader. Concentration of AT–406 that inhibits cell growth by 50% (IC 50) is calculated by comparing absorbance in the untreated cells and the cells treated with AT–406.Product Name:AT–406Cat. No.:HY-15454CAS No.:1071992-99-8Molecular Formula:C 32H 43N 5O 4Molecular Weight:561.71Target:IAP Pathway:Apoptosis Solubility:10 mM in DMSOAnimal Administration:[1]SCID mice (8–10 per group) bearing MDA–MB–231 xenograft tumors are treated with different doses of compound 2, or 7.5 mg/kg of Taxotere or vehicle control daily, 5 days a week for 2 weeks. Tumor sizes and animal weights are measured 3 times a week during the treatment and twice a week after the treatment. Data are presented as mean tumor volumes±SEM. Statistical analyses are performed by two–way ANOVA and unpaired two–tailed t test, using Prism. P < 0.05 is considered statistically significant.References:[1]. Cai Q, et al. A potent and orally active antagonist (SM–406/AT–406) of multiple inhibitor of apoptosis proteins (IAPs) in clinical development for cancer treatment. J Med Chem. 2011 Apr 28;54(8):2714–26.[2]. Brunckhorst MK, et al. AT–406, an orally active antagonist of multiple inhibitor of apoptosis proteins, inhibits progression of human ovarian cancer. Cancer Biol Ther. 2012 Jul;13(9):804–11.[3]. Zhang T, et al. Physiologically based pharmacokinetic and pharmacodynamic modeling of an antagonist (SM–406/AT–406) of multiple inhibitor of apoptosis proteins (IAPs) in a mouse xenograft model of human breast cancer. Biopharm Drug Dispos. 2013 Sep;34(6):Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
AT-101 90141-22-3 GlpBio
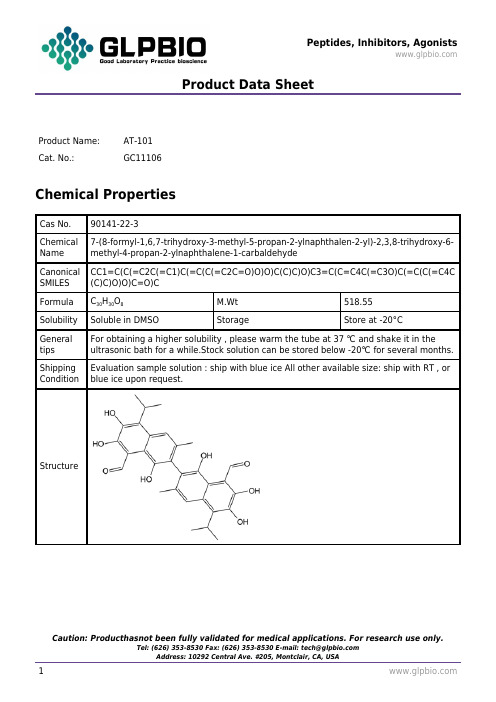
Product Data SheetProduct Name:AT-101Cat. No.:GC11106Chemical PropertiesCas No.90141-22-3Chemical Name 7-(8-formyl-1,6,7-trihydroxy-3-methyl-5-propan-2-ylnaphthalen-2-yl)-2,3,8-trihydroxy-6-methyl-4-propan-2-ylnaphthalene-1-carbaldehydeCanonical SMILES CC1=C(C(=C2C(=C1)C(=C(C(=C2C=O)O)O)C(C)C)O)C3=C(C=C4C(=C3O)C(=C(C(=C4C (C)C)O)O)C=O)CFormula C30H30O8M.Wt518.55 Solubility Soluble in DMSO Storage Store at -20°CGeneral tips For obtaining a higher solubility , please warm the tube at 37 ℃ and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20℃ for several months.Shipping Condition Evaluation sample solution : ship with blue ice All other available size: ship with RT , or blue ice upon request.StructureProduct Data Sheet实验参考方法Cell experiment [1]:Cell lines CLL(Chronic lymphocytic leukemia) B cellPreparation method Soluble in DMSO > 10 mM. General tips for obtaining a higher concentration: Please warm the tube at 37℃ for 10 minutes and/or shake it in the ultrasonic bath for a while. Stock solution can be stored below -20℃ for several months.Reacting condition1, 3, 10, 15, 20, 30μm for 24h; 20μm for 4, 8, 24 hoursApplications AT-101 induced apoptosis in CLL B cells and overcomes microenvironment-mediated resistance while sparing normal stromal cells. AT-101 treatment resulted in cleavage of Mcl-1 (Myeloid cell leukemia-1) in a time- and dose-dependent fashion. The decrease in full-length Mcl-1 correlated well with annexin positivity and PARP(poly ADP-ribose polymerase) cleavage.Animal experiment [2]:Animal models athymic nude mice with allografted intracranial medulloblastomas from Ptch+/-; p53-/- mouseDosage form20 or 40 mg/kg, daily administered, oral gavageApplication Treatment with AT-101 obviously inhibited the growth of allografted medulloblastoma in mice. AT-101 might inhibit the growth of Hh(hedgehog)-driven medulloblastoma in vivo by suppressing the Hh pathwayOther notes Please test the solubility of all compounds indoor, and the actual solubility may slightly differ with the theoretical value. This is caused by an experimental system error and it is normal.References:[1]. Balakrishnan K,Burger J, et al, AT-101 induces apoptosis in CLL B cells and overcomes stromal cell–mediated Mcl-1 induction and drug resistance.Blood,2009,113(1): 149–153.[2]. Wang J1, Peng Y, et al, AT-101 inhibits hedgehog pathway activity and cancer growth. Cancer Chemother Pharmacol. 2015 Sep;76(3):461-9. doi: 10.1007/s00280-015-2812-x. Epub 2015 Jun 26. BackgroundAT101, a natural product from cottonseed with a BH3-mimetic structure, was identified as a small molecule inhibitor of Bcl-2/Bcl-xL/Mcl-1 that potently induces apoptosis in various cancer cell lines [1].It is one of the world's first small molecule Bcl-2 inhibitors that has entered into clinical trials and is now in phase II clinical trials for hormone-refractory prostate cancer and other types of cancers [2, 3]. Few side effects of gossypol have been reported, with the major side effects of gossypol being nausea and vomiting in the third month of treatment or rashes earlier in the course of treatment [4]. Thus, AT101 is clinically safe [2, 3] and could be used as a potential inducer of apoptosis in cancer treatment.Product Data SheetAs a BH3 mimetic, AT-101 binds to the hydrophobic surface binding groove BH3 of the anti-apoptotic proteins Bcl-2 and Bcl-xL, blocking their heterodimerization with pro-apoptotic members of the Bcl-2 family of proteins such as Bad, Bid, and Bim; this may result in the inhibition of tumor cell proliferation and the induction of tumor cell apoptosis. Preclinical studies revealed that gossypol not only interrupts the interaction between anti- and proapoptotic Bcl-2 family proteins but also induces BH3 protein (such as Puma and Noxa) up-regulation or down-regulates XIAP expression [5]. Thus, gossypol can induce apoptosis by activating apoptogenic factors other than the Bcl-2 family. AT-101 induced apoptosis in vitro through activation of caspase-9.AT101 delayed onset of androgen-independent growth of VCaP prostate cancer xenografts in vivo. Gossypol can neutralize antiapoptotic Bcl-2 proteins and induced Bax activation [1]. However, the function of gossypol was not limited to effects on the interaction between anti- and proapoptotic Bcl-2 proteins. Some studies have demonstrated that gossypol could down-regulate Bcl-2, Bcl-xL, and XIAP expression [6] or induce Puma and Noxa expression. Therefore, the apoptotic effect of gossypol has been demonstrated to be attenuated by the presence of androgen in a prostate cancer xenograft mouse model.References:[1]. Meng Y, Tang W,Dai Y, et al. Natural BH3-mimetic (-)-gossypol chemosensitizes human prostate cancer via Bcl-xL inhibition accompanied by increase of Puma and Noxa.Molecular cancer therapeutics,2008,7(7): 2192–2202.[2]. Liu G,Kelly W. K,Wilding G,et al. An Open-Label, Multicenter, Phase I/II Study of Single-AgentAT-101 in Men with Castrate-Resistant Prostate Cancer (CRPC).Clinical Cancer Research,2009,15(9): 3172–3176.[3]. Van Poznak C, Seidman A. D,Reidenberg M,et al. Oral gossypol in the treatment of patients with refractory metastatic breast cancer: a phase I/II clinical trial,Breast Cancer ResTreat,2001,66(3):239-48.[4]. Qiu J,Levin L. R,Buck J,et al. Different pathways of cell killing by gossypol enantiomers.Exp Biol Med (Maywood) ,2002,227(6):398-401.[5]. Balakrishnan K,Burger J. A,Wierda W. G,et al. AT-101 induces apoptosis in CLL B cells and overcomes stromal cell–mediated Mcl-1 induction and drug resistance.Blood,2009,113(1): 149–153. [6].Sung B,Ravindran J,Prasad S,et al.Gossypol Induces Death Receptor-5 through Activation of the ROS-ERK-CHOP Pathway and Sensitizes Colon Cancer Cells to TRAIL.J Biol Chem,2010,285(46): 35418–35427.。
Aimmune公司用于治疗花生过敏新药AR101在临床研究中表现良好

Aimmune公司用于治疗花生过敏新药AR101在临床研究中
表现良好
佚名
【期刊名称】《临床合理用药杂志》
【年(卷),期】2016(9)8
【摘要】最近,来自美国加州的生物医药公司Aimmune宣布公司开发的用于治疗花生过敏新药AR101在最新临床研究中表现良好,已扫清了该药物进入临床三期研究的障碍。
【总页数】1页(P70-70)
【正文语种】中文
【中图分类】R593.102
【相关文献】
1.新药临床开发、上市和应用-心脑血管系统药物:Astellas公司在美国申请RSD1235用于心房颤动治疗
2.抗癌新药Carfilzomib临床表现良好
3.美国从花生壳中提取新药用于临床
4.糖尿病新药利抗鲁肽全球临床试验表现良好
5.AR101治疗花生过敏Ⅲ期临床试验达到主终点
因版权原因,仅展示原文概要,查看原文内容请购买。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Inhibitors, Agonists, Screening Libraries
Data Sheet
BIOLOGICAL ACTIVITY:
AT101 acetic acid, the R–(–) enantiomer of Gossypol acetic acid, binds with Bcl–2, Bcl–xL and Mcl–1 with Ki of 0.32 μM, 0.48 μM and 0.18 μM.
IC50 Value: 0.32/0.48/0.18 uM for Bcl–2/Bcl–xL/Mcl–1
Target: Bcl–2; Bcl–xl; Mcl–1
AT–101 is orally bioavailable solvate of R–(–)–enantiomer of gossypol with potential antineoplastic activity. As a BH3 mimetic, AT–101binds to the hydrophobic surface binding groove BH3 of the anti–apoptotic proteins Bcl–2 and Bcl–xL, blocking their
heterodimerization with pro–apoptotic members of the Bcl–2 family of proteins such as Bad, Bid, and Bim; this may result in the
inhibition of tumor cell proliferation and the induction of tumor cell apoptosis. AT–101 induces apoptosis in vitro through activation of caspase–9; cytotoxic to multiple myeloma and drug–resistant cell lines. AT–101 delays onset of androgen–independent growth of VCaP prostate cancer xenografts in vivo.
References:
[1]. Loberg RD, McGregor N, Ying C et al. In vivo evaluation of AT–101 (R–(–)–gossypol acetic acid) in androgen–independent growth of VCaP prostate cancer cells in combination with surgical castration. Neoplasia. 2007 Dec;9(12):1030–7.
[2]. Kline MP, Rajkumar SV, Timm MM et al. R–(–)–gossypol (AT–101) activates programmed cell death in multiple myeloma cells. Exp Hematol. 2008 May;36(5):568–76.
[3]. Balakrishnan K, Burger JA, Wierda WG, Gandhi V. AT–101 induces apoptosis in CLL B cells and overcomes stromal cell–mediated Mcl–1 induction and drug resistance. Blood. 2009 Jan 1;113(1):149–53.
[4]. Moretti L, Li B, Kim KW et al. AT–101, a pan–Bcl–2 inhibitor, leads to radiosensitization of non–small cell lung cancer. J Thorac Oncol. 2010 May;5(5):680–7.
[5]. McGregor N, Patel L, Craig M et al. AT–101 (R–(–)–gossypol acetic acid) enhances the effectiveness of androgen deprivation therapy in the VCaP prostate cancer model. J Cell Biochem. 2010 Aug 1;110(5):1187–94.
Product Name:
AT–101 (acetic acid)Cat. No.:
HY-15464A CAS No.:
866541-93-7Molecular Formula:
C 32H 34O 10Molecular Weight:
578.61Target:
Bcl–2 Family; Autophagy Pathway:
Apoptosis; Autophagy Solubility:
DMSO; Methanol: 7.75 mg/mL
Caution: Product has not been fully validated for medical applications. For research use only.
Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@ Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
