1-s2.0-S0927775701010718-main
ASCII码对照表
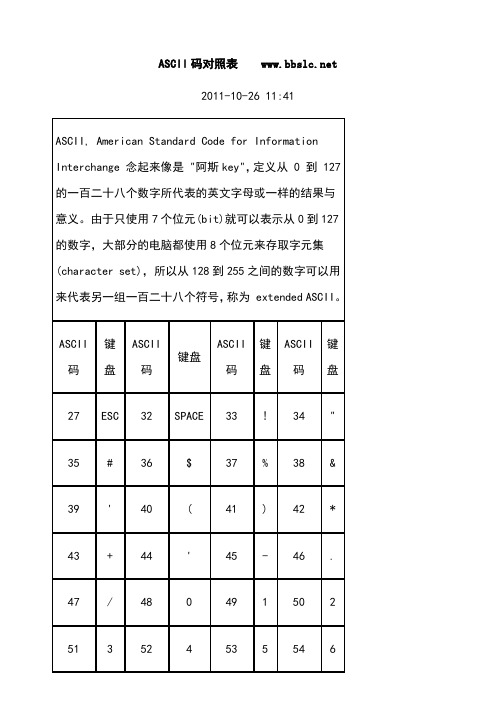
ASCII码对照表 2011-10-26 11:41目前计算机中用得最广泛的字符集及其编码,是由美国国家标准局(ANSI)制定的ASCII码(American Standard Code for Information Interchange,美国标准信息交换码),它已被国际标准化组织(ISO)定为国际标准,称为ISO 646标准。
适用于所有拉丁文字字母,ASCII码有7位码和8位码两种形式。
因为1位二进制数可以表示(21=)2种状态:0、1;而2位二进制数可以表示(22)=4种状态:00、01、10、11;依次类推,7位二进制数可以表示(27=)128种状态,每种状态都唯一地编为一个7位的二进制码,对应一个字符(或控制码),这些码可以排列成一个十进制序号0~127。
所以,7位ASCII码是用七位二进制数进行编码的,可以表示128个字符。
第0~32号及第127号(共34个)是控制字符或通讯专用字符,如控制符:LF(换行)、CR(回车)、FF(换页)、DEL(删除)、BEL(振铃)等;通讯专用字符:SOH(文头)、EOT(文尾)、ACK(确认)等;第33~126号(共94个)是字符,其中第48~57号为0~9十个阿拉伯数字;65~90号为26个大写英文字母,97~122号为26个小写英文字母,其余为一些标点符号、运算符号等。
注意:在计算机的存储单元中,一个ASCII码值占一个字节(8个二进制位),其最高位(b7)用作奇偶校验位。
所谓奇偶校验,是指在代码传送过程中用来检验是否出现错误的一种方法,一般分奇校验和偶校验两种。
奇校验规定:正确的代码一个字节中1的个数必须是奇数,若非奇数,则在最高位b7添1;偶校验规定:正确的代码一个字节中1的个数必须是偶数,若非偶数,则在最高位b7添1。
附:ASCII表键盘常用ASCII码ESC键 VK_ESCAPE (27)回车键: VK_RETURN (13)Caps Lock键: VK_CAPITAL (20) Shift键: VK_SHIFT ($10)Ctrl键: VK_CONTROL (17)Alt键: VK_MENU (18)空格键: VK_SPACE ($20/32)退格键: VK_BACK (8)左徽标键: VK_LWIN (91)右徽标键: VK_LWIN (92)鼠标右键快捷键:VK_APPS (93) Insert键: VK_INSERT (45) Home键: VK_HOME (36)Page Up: VK_PRIOR (33) PageDown: VK_NEXT (34)End键: VK_END (35)Delete键: VK_DELETE (46)方向键(←): VK_LEFT (37)方向键(↑): VK_UP (38)方向键(→): VK_RIGHT (39)方向键(↓): VK_DOWN (40)F1键: VK_F1 (112)F2键: VK_F2 (113)F4键: VK_F4 (115)F5键: VK_F5 (116)F6键: VK_F6 (117)F7键: VK_F7 (118)F8键: VK_F8 (119)F9键: VK_F9 (120)F10键: VK_F10 (121)F11键: VK_F11 (122)F12键: VK_F12 (123)Num Lock键: VK_NUMLOCK (144) 小键盘0: VK_NUMPAD0 (96)小键盘1: VK_NUMPAD0 (97)小键盘2: VK_NUMPAD0 (98)小键盘3: VK_NUMPAD0 (99)小键盘4: VK_NUMPAD0 (100)小键盘5: VK_NUMPAD0 (101)小键盘6: VK_NUMPAD0 (102)小键盘7: VK_NUMPAD0 (103)小键盘8: VK_NUMPAD0 (104)小键盘9: VK_NUMPAD0 (105)小键盘.: VK_DECIMAL (110)。
ASCII码表二进制十进制十六进制

ASCII码表(二进制十进制十六进制)控制字符二进制十进制十六进制缩写解释0000 0000 0 0 NUL 空字符(Null)0000 0001 1 1 SOH 标题开始0000 0010 2 2 STX 正文开始0000 0011 3 3 ETX 正文结束0000 0100 4 4 EOT 传输结束0000 0101 5 5 ENQ 请求0000 0110 6 6 ACK 收到通知0000 0111 7 7 BEL 响铃0000 1000 8 8 BS 退格0000 1001 9 9 HT 水平制表符0000 1010 10 0A LF 换行键0000 1011 11 0B VT 垂直制表符0000 1100 12 0C FF 换页键0000 1101 13 0D CR 回车键0000 1110 14 0E SO 不用切换0000 1111 15 0F SI 启用切换0001 0000 16 10 DLE 数据链路转义0001 0001 17 11 DC1 设备控制10001 0010 18 12 DC2 设备控制20001 0011 19 13 DC3 设备控制30001 0100 20 14 DC4 设备控制40001 0101 21 15 NAK 拒绝接收0001 0110 22 16 SYN 同步空闲0001 0111 23 17 ETB 传输块结束0001 1000 24 18 CAN 取消0001 1001 25 19 EM 介质中断0001 1010 26 1A SUB 替补0001 1011 27 1B ESC 溢出0001 1100 28 1C FS 文件分割符0001 1101 29 1D GS 分组符0001 1110 30 1E RS 记录分离符0001 1111 31 1F US 单元分隔符0111 1111 127 7F DEL 删除可显示字符二进制十进制十六进制字符0010 0000 32 20 空格0010 0001 33 21 !0010 0010 34 22 "0010 0011 35 23 #0010 0100 36 24 $0010 0101 37 25 %0010 0110 38 26 &0010 0111 39 27 '0010 1000 40 28 (0010 1001 41 29 )0010 1010 42 2A *0010 1011 43 2B +0010 1100 44 2C ,0010 1101 45 2D -0010 1110 46 2E .0010 1111 47 2F /0011 0000 48 30 00011 0001 49 31 10011 0010 50 32 20011 0011 51 33 30011 0100 52 34 40011 0101 53 35 50011 0110 54 36 60011 0111 55 37 70011 1000 56 38 80011 1001 57 39 90011 1010 58 3A :0011 1011 59 3B ;0011 1100 60 3C <0011 1101 61 3D =0011 1110 62 3E >0011 1111 63 3F ?可显示字符二进制十进制十六进制字符0100 0001 65 41 A0100 0010 66 42 B0100 0011 67 43 C0100 0100 68 44 D0100 0101 69 45 E0100 0110 70 46 F0100 0111 71 47 G0100 1000 72 48 H0100 1001 73 49 I0100 1010 74 4A J0100 1011 75 4B K0100 1100 76 4C L0100 1101 77 4D M0100 1110 78 4E N0100 1111 79 4F O0101 0000 80 50 P0101 0001 81 51 Q0101 0010 82 52 R0101 0011 83 53 S0101 0100 84 54 T0101 0101 85 55 U0101 0110 86 56 V0101 0111 87 57 W0101 1000 88 58 X0101 1001 89 59 Y0101 1010 90 5A Z0101 1011 91 5B [0101 1100 92 5C \0101 1101 93 5D ]0101 1110 94 5E ^0101 1111 95 5F _0110 0000 96 60 `可显示字符二进制十进制十六进制字符0110 0001 97 61 a0110 0011 99 63 c 0110 0100 100 64 d 0110 0101 101 65 e 0110 0110 102 66 f 0110 0111 103 67 g 0110 1000 104 68 h 0110 1001 105 69 i 0110 1010 106 6A j 0110 1011 107 6B k 0110 1100 108 6C l 0110 1101 109 6D m 0110 1110 110 6E n 0110 1111 111 6F o 0111 0000 112 70 p 0111 0001 113 71 q 0111 0010 114 72 r 0111 0011 115 73 s 0111 0100 116 74 t 0111 0101 117 75 u 0111 0110 118 76 v 0111 0111 119 77 w 0111 1000 120 78 x 0111 1001 121 79 y 0111 1010 122 7A z 0111 1011 123 7B { 0111 1100 124 7C | 0111 1101 125 7D } 0111 1110 126 7E ~。
ascii码全集

029 035 01D 00011101 GS (Group Separator)
030 036 01E 00011110 RS(RequesttoSendRecordSeparator)
031 037 01F 00011111 US (Unit Separator)
024 030 018 00011000 CAN (Cancel)
025 031 019 00011001 EM (End of Medium)
026 032 01A 00011010 SUB (Substitute)
027 033 01B 00011011 ESC (Escape)
016 020 010 00010000 DLE (Data Link Escape)
017 021 011 00010001 DC1 (XON) (Device Control 1)
018 022 012 00010010 DC2 (Device Control 2)
019 023 013 00010011 DC3 (XOFF)(Device Control 3)
070 106 046 01000110 F
071 107 047 01000111 G
072 110 048 01001000 H
073 111 049 01001001 I
074 112 04A 01001010 J
075 113 04B 01001011 K
076 114 04C 01001100 L
101 145 065 01100101 e
102 146 066 01100110 f
ASCII编码对照表

0010 1110 56 46 2E . 句号 00101111 57 47 2F / 斜杠 00110000 60 48 30 0 数字0 00110001 61 49 31 1 数字1 00110010 62 50 32 2 数字2 00110011 63 51 33 3 数字3 00110100 64 52 34 4 数字4 00110101 65 53 35 5 数字5 00110110 66 54 36 6 数字6 00110111 67 55 37 7 数字7 00111000 70 56 38 8 数字8 00111001 71 57 39 9 数字9 00111010 72 58 3A : 冒号 00111011 73 59 3B ; 分号 00111100 74 60 3C < 小于 0011175 61 3D = 等号
01101100 154 108 6C l 小写字母l 01101101 155 109 6D m 小写字母m 01101110 156 110 6E n 小写字母n 01101111 157 111 6F o 小写字母o 01110000 160 112 70 p 小写字母p 01110001 161 113 71 q 小写字母q 01110010 162 114 72 r 小写字母r 01110011 163 115 73 s 小写字母s 01110100 164 116 74 t 小写字母t 01110101 165 117 75 u 小写字母u 01110110 166 118 76 v 小写字母v 01110111 167 119 77 w 小写字母w 01111000 170 120 78 x 小写字母x 01111001 171 121 79 y 小写字母y 01111010 172 122 7A z 小写字母z 01111173 123 7B { 开花括号
ASCII码表

q
18
DC2
50
2
82
R
114
r
19
DC3
51
3
83
XHale Waihona Puke 115s20
DC4
52
4
84
T
116
t
21
NAK
53
5
85
U
117
u
22
SYN
54
6
86
V
118
v
23
TB
55
7
87
W
119
w
24
CAN
56
8
88
X
120
x
25
EM
57
9
89
Y
121
y
26
SUB
58
:
90
Z
122
z
27
ESC
59
;
91
[
123
‹
171
«
203
Ë
234
ê
140
Œ
172
¬
204
Ì
235
ë
141
173
205
Í
236
ì
142
Ž
174
®
206
Î
237
í
143
175
¯
207
Ï
238
î
144
176
°
208
Ð
239
ï
145
‘
177
±
209
Ñ
240
ASCALL码表
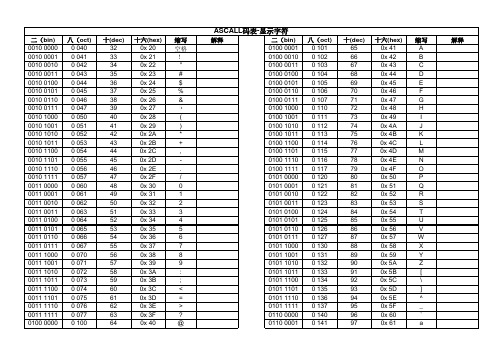
十(dec) 65 66 67 68 69 70 71 72 73 74 75 76 77 78 79 80 81 82 83 84 85 86 87 88 89 90 91 92 93 94 95 96 97
十六(hex) 0x 41 0x 42 0x 43 0x 44 0x 45 0x 46 0x 47 0x 48 0x 49 0x 4A 0x 4B 0x 4C 0x 4D 0x 4E 0x 4F 0x 50 0x 51 0x 52 0x 53 0x 54 0x 55 0x 56 0x 57 0x 58 0x 59 0x 5A 0x 5B 0x 5C 0x 5D 0x 5E 0x 5F 0x 60 0x 61
0100 1001
0100 1010
0100 1011
0100 1100
0100 1101
0100 1110
0100 1111
0101 0000
0101 0001
0101 0010
0101 0011
0101 0100
0101 0101
0101 0110
0101 0111
0101 1000
0101 1001
0 167
119
0x 77
0 170
120
0x 78
0 171
121
0x 79
0 172
122
0x 7A
0 173
123
0x 7B
0 174
124
0x 7C
0 175
125
0x 7D
0 176
126
0x 7E
缩写 b c d e f g h i j k l m n o p q r s t u v w x y z { ; # $ % &
ASCII码表和键盘码详解

ASCII码表和键盘码星期四,2015年8月13日下午 03:43ASCII非打印控制字符表ASCII 表上的数字 0–31 分配给了控制字符,用于控制像打印机等一些外围设备。
例如,数字12 代表换页/新页功能。
此命令指示打印机跳到下一页的开头。
缩写/字符解释(二进)Bin(十进)Dec(十六进)Hex0000 0000 0 0 NUL (null) 空字符0000 0001 1 1 SOH (start of handing) 标题开始0000 0010 2 2 STX (start of text) 正文开始0000 0011 3 3 ETX (end of text) 正文结束0000 0100 4 4 EOT (end of transmission) 传输结束0000 0101 5 5 ENQ (enquiry) 请求0000 0110 6 6 ACK (acknowledge) 收到通知0000 0111 7 7 BEL (bell) 响铃0000 1000 8 8 BS (backspace) 退格0000 1001 9 9 HT (horizontal tab) 水平制表符0000 1010 10 0A LF (NL line feed, new line) 换行键0000 1011 11 0B VT (vertical tab) 垂直制表符换页键0000 1100 12 0C FF (NP form feed, newpage)0000 1101 13 0D CR (carriage return) 回车键0000 1110 14 0E SO (shift out) 不用切换0000 1111 15 0F SI (shift in) 启用切换0001 0000 16 10 DLE (data link escape) 数据链路转义0001 0001 17 11 DC1 (device control 1) 设备控制1 0001 0010 18 12 DC2 (device control 2) 设备控制2 0001 0011 19 13 DC3 (device control 3) 设备控制3 0001 0100 20 14 DC4 (device control 4) 设备控制4拒绝接收0001 0101 21 15 NAK (negativeacknowledge)0001 0110 22 16 SYN (synchronous idle) 同步空闲0001 0111 23 17 ETB (end of trans. block) 传输块结束0001 1000 24 18 CAN (cancel) 取消0001 1001 25 19 EM (end of medium) 介质中断0001 1010 26 1A SUB (substitute) 替补0001 1011 27 1B ESC (escape) 溢出0001 1100 28 1C FS (file separator) 文件分割符0001 1101 29 1D GS (group separator) 分组符0001 1110 30 1E RS (record separator) 记录分离符0001 1111 31 1F US (unit separator) 单元分隔符ASCII打印字符数字 32–126 分配给了能在键盘上找到的字符,当您查看或打印文档时就会出现。
ASCII码表完整版

就是英文字符的编码,那些字符实际上的数据就是ASCII码,在计算机内部就是Ascii对应的二进制010101010这样的ASCII码表完整版Text only语言: Ascii码表(全)ASCII Table (7-bit)(ASCII = American Standard Code for Information Interchange)Decimal Octal Hex Binary Value------- ----- --- ------ -----000 000 000 00000000 NUL (Null char.)001 001 001 00000001 SOH (Start of Header)002 002 002 00000010 STX (Start of Text)003 003 003 00000011 ETX (End of Text)004 004 004 00000100 EOT (End of Transmission)005 005 005 00000101 ENQ (Enquiry)006 006 006 00000110 ACK (Acknowledgment)007 007 007 00000111 BEL (Bell)008 010 008 00001000 BS (Backspace)009 011 009 00001001 HT (Horizontal Tab)010 012 00A 00001010 LF (Line Feed)011 013 00B 00001011 VT (Vertical Tab)012 014 00C 00001100 FF (Form Feed)013 015 00D 00001101 CR (Carriage Return)014 016 00E 00001110 SO (Shift Out)015 017 00F 00001111 SI (Shift In)016 020 010 ******** DLE (Data Link Escape)017 021 011 00010001 DC1 (XON) (Device Control 1)018 022 012 00010010 DC2 (Device Control 2)019 023 013 00010011 DC3 (XOFF)(Device Control 3)020 024 014 00010100 DC4 (Device Control 4)021 025 015 00010101 NAK (Negative Acknowledgement)022 026 016 00010110 SYN (Synchronous Idle)023 027 017 00010111 ETB (End of Trans. Block)024 030 018 00011000 CAN (Cancel)025 031 019 00011001 EM (End of Medium)026 032 01A 00011010 SUB (Substitute)027 033 01B 00011011 ESC (Escape)028 034 01C 00011100 FS (File Separator)029 035 01D 00011101 GS (Group Separator)030 036 01E 00011110 RS (Request to Send)(Record Separator) 031 037 01F 00011111 US (Unit Separator)032 040 020 ******** SP (Space)033 041 021 ******** ! (exclamation mark)034 042 022 ******** " (double quote)035 043 023 ******** # (number sign)036 044 024 ******** $ (dollar sign)037 045 025 ******** % (percent)038 046 026 00100110 & (ampersand)039 047 027 ******** ' (single quote)040 050 028 ******** ( (left/opening parenthesis)041 051 029 ******** ) (right/closing parenthesis)042 052 02A 00101010 * (asterisk)043 053 02B 00101011 + (plus)044 054 02C 00101100 , (comma)045 055 02D 00101101 - (minus or dash)046 056 02E 00101110 . (dot)047 057 02F 00101111 / (forward slash)048 060 030 00110000 0049 061 031 00110001 1050 062 032 00110010 2051 063 033 00110011 3052 064 034 00110100 4053 065 035 00110101 5054 066 036 00110110 6055 067 037 00110111 7056 070 038 00111000 8057 071 039 00111001 9058 072 03A 00111010 : (colon)059 073 03B 00111011 ; (semi-colon)060 074 03C 00111100 < (less than)061 075 03D 00111101 = (equal sign)062 076 03E 00111110 > (greater than)063 077 03F 00111111 ? (question mark)064 100 040 01000000 @ (AT symbol)065 101 041 01000001 A066 102 042 01000010 B067 103 043 01000011 C068 104 044 01000100 D069 105 045 01000101 E070 106 046 01000110 F071 107 047 01000111 G072 110 048 01001000 H073 111 049 01001001 I074 112 04A 01001010 J075 113 04B 01001011 K076 114 04C 01001100 L077 115 04D 01001101 M078 116 04E 01001110 N079 117 04F 01001111 O080 120 050 01010000 P081 121 051 01010001 Q082 122 052 01010010 R083 123 053 01010011 S084 124 054 01010100 T085 125 055 01010101 U086 126 056 01010110 V087 127 057 01010111 W088 130 058 01011000 X089 131 059 01011001 Y090 132 05A 01011010 Z091 133 05B 01011011 [ (left/opening bracket) 092 134 05C 01011100 \ (back slash)093 135 05D 01011101 ] (right/closing bracket) 094 136 05E 01011110 ^ (caret/circumflex) 095 137 05F 01011111 _ (underscore)096 140 060 01100000 `097 141 061 01100001 a098 142 062 01100010 b099 143 063 01100011 c100 144 064 01100100 d101 145 065 01100101 e102 146 066 01100110 f103 147 067 01100111 g104 150 068 01101000 h105 151 069 01101001 i106 152 06A 01101010 j107 153 06B 01101011 k108 154 06C 01101100 l109 155 06D 01101101 m110 156 06E 01101110 n111 157 06F 01101111 o112 160 070 01110000 p113 161 071 01110001 q114 162 072 01110010 r115 163 073 01110011 s116 164 074 01110100 t117 165 075 01110101 u118 166 076 01110110 v119 167 077 01110111 w120 170 078 01111000 x121 171 079 01111001 y122 172 07A 01111010 z123 173 07B 01111011 { (left/opening brace)124 174 07C 01111100 | (vertical bar)125 175 07D 01111101 } (right/closing brace)126 176 07E 01111110 ~ (tilde)127 177 07F 01111111 DEL (delete)------------------------------------------------------------------0 1 2 3 4 5 6 7 8 9 A B C D E F0 NUL SOH STX ETX EOT ENQ ACK BEL BS HT LF VT FF CR SO SI1 DLE DC1 DC2 DC3 DC4 NAK SYN ETB CAN EM SUB ESC FS GS RS US2 SP ! " # $ % & ' ( ) * + , - . /3 0 1 2 3456789:;<=> ?4 @ A B C D E F G H I J K L M N O5 P Q R S T U V W X Y Z [ \ ] ^ _6 ` a b c d e f g h i j k l m n o7 p q r s t u v w x y z { | } ~ DEL------------------------------------------------------------------。
ASCII码表

vk_Home Home vk_Left vk_Up Left Arrow Up Arrow
vk_Subtract --键 vk_Decimal . 键 vk_Divide /键 vk_F1 vk_F2 vk_F3 vk_F4 vk_F5 vk_F6 vk_F7 vk_F8 vk_F9 vk_F10 vk_F11 vk_F12 vk_F13 vk_F14 vk_F15 vk_F16 vk_F17 vk_F18 vk_F19 vk_F20 vk_F21 F1 键 F2 键 F3 键 F4 键 F5 键 F6 键 F7 键 F8 键 F9 键 F10 键 F11 键 F12 键 F13 键 F14 键 F15 键 F16 键 F17 键 F18 键 F19 键 F20 键 F21 键
fs( 文 件 分 01011100 割符) gs(分组符) 01011101 rs( 记 录 分 01011110 离符) us( 单 元 分 01011111 隔符) sp(空格) ! " # $ % & ` ( ) * + , . / 0 1 2 3 01100000 01100001 01100010 01100011 01100100 01100101 01100110 01100111 01101000 01101001 01101010 01101011 01101100 01101101 01101110 01101111 01110000 01110001 01110010 01110011
键盘 ASCII 码表
F1~F12 对应为 $70(112)~$7B(123) A~Z 对应为 $41(65)~$5A(90) 0~9 对应为 $30(48)~$39(57) 十六进位 十进位 说明 二进制 十六进制 十进制 Hex Decimal Binary $01 $02 $03 $04 1 2 3 4 键值 Value 解释 二进制 十六进制 十进制 Hex Decimal Binary $4F $50 $51 $52 $53 $54 $55 $56 Clear $57 $58 $59 Shift $5A 79 80 81 82 83 84 85 86 87 88 89 90 键值 Value vk_O vk_P vk_Q vk_R vk_S vk_T vk_U vk_V vk_W vk_X vk_Y vk_Z 解释 O键 P键 Q键 R键 S键 T键 U键 V键 W键 X键 Y键 Z键
ASCALL码表

0101 1010
0101 1011
0101 1100
0101 1101
0101 1110
0101 1111
0110 0000
0110 0001
八(oct) 0 101 0 102 0 103 0 104 0 105 0 106 0 107 0 110 0 111 0 112 0 113 0 114 0 115 0 116 0 117 0 120 0 121 0 122 0 123 0 124 0 125 0 126 0 127 0 130 0 131 0 132 0 133 0 134 0 135 0 136 0 137 0 140 0 141
缩写 A B C D E F G H I J K L M N O P Q R S T U V W X Y Z [ \ ] ^ _ ` a
解释
二(bin) 0110 0010 0110 0011 0110 0100 0110 0101 0110 0110 0110 0111 0110 1000 0110 1001 0110 1010 0110 1011 0110 1100 0110 1101 0110 1110 0110 1111 0111 0000 0111 0001 0111 0010 0111 0011 0111 0100 0111 0101 0111 0110 0111 0111 0111 1000 0111 1001 0111 1010 0111 1011 0111 1100 0111 1101 0111 1110
0 167
119
0x 77
0 170
120
0x 78
0 171
121
0x 79
0 172
122
ASCII码表完整版

ASCII码表完整版控制字符二进制十进制十六进制缩写解释0000 0000 0 00 NUL 空字符(Null) 0000 0001 1 01 SOH 标题开始0000 0010 2 02 STX 正文开始0000 0011 3 03 ETX 正文结束0000 0100 4 04 EOT 传输结束0000 0101 5 05 ENQ 请求0000 0110 6 06 ACK 收到通知0000 0111 7 07 BEL 响铃0000 1000 8 08 BS 退格0000 1001 9 09 HT 水平制表符0000 1010 10 0A LF 换行键0000 1011 11 0B VT 垂直制表符0000 1100 12 0C FF 换页键0000 1101 13 0D CR 回车键0000 1110 14 0E SO 不用切换0000 1111 15 0F SI 启用切换0001 0000 16 10 DLE 数据链路转义0001 0001 17 11 DC1 设备控制1 0001 0010 18 12 DC2 设备控制2 0001 0011 19 13 DC3 设备控制3 0001 0100 20 14 DC4 设备控制4 0001 0101 21 15 NAK 拒绝接收0001 0110 22 16 SYN 同步空闲0001 0111 23 17 ETB 传输块结束0001 1000 24 18 CAN 取消0001 1001 25 19 EM 介质中断0001 1010 26 1A SUB 替补0001 1011 27 1B ESC 溢出0001 1100 28 1C FS 文件分割符0001 1101 29 1D GS 分组符0001 1110 30 1E RS 记录分离符0001 1111 31 1F US 单元分隔符0111 1111 127 7F DEL 删除可显示字符二进制十进制十六进制字符0010 0000 32 20 空格0010 0001 33 21 !0010 0010 34 22 "0010 0011 35 23 #0010 0100 36 24 $0010 0101 37 25 %0010 0110 38 26 &0010 0111 39 27 '0010 1000 40 28 (0010 1001 41 29 )0010 1010 42 2A *0010 1011 43 2B +0010 1100 44 2C ,0010 1101 45 2D -0010 1110 46 2E .0011 0000 48 30 0 0011 0001 49 31 1 0011 0010 50 32 2 0011 0011 51 33 3 0011 0100 52 34 4 0011 0101 53 35 5 0011 0110 54 36 6 0011 0111 55 37 7 0011 1000 56 38 8 0011 1001 57 39 9 0011 1010 58 3A : 0011 1011 59 3B ; 0011 1100 60 3C < 0011 1101 61 3D = 0011 1110 62 3E > 0011 1111 63 3F ? 0100 0000 64 40 @可显示字符(字母)二进制十进制十六进制字符0100 0001 65 41 A 0100 0010 66 42 B 0100 0011 67 43 C 0100 0100 68 44 D 0100 0101 69 45 E 0100 0110 70 46 F0100 1000 72 48 H 0100 1001 73 49 I 0100 1010 74 4A J 0100 1011 75 4B K 0100 1100 76 4C L 0100 1101 77 4D M 0100 1110 78 4E N 0100 1111 79 4F O 0101 0000 80 50 P 0101 0001 81 51 Q 0101 0010 82 52 R 0101 0011 83 53 S 0101 0100 84 54 T 0101 0101 85 55 U 0101 0110 86 56 V 0101 0111 87 57 W 0101 1000 88 58 X 0101 1001 89 59 Y 0101 1010 90 5A Z 0101 1011 91 5B [ 0101 1100 92 5C \ 0101 1101 93 5D ] 0101 1110 94 5E ^ 0101 1111 95 5F _ 0110 0000 96 60 ` 0110 0001 97 61 a0110 0011 99 63 c 0110 0100 100 64 d 0110 0101 101 65 e 0110 0110 102 66 f 0110 0111 103 67 g 0110 1000 104 68 h 0110 1001 105 69 i 0110 1010 106 6A j 0110 1011 107 6B k 0110 1100 108 6C l 0110 1101 109 6D m 0110 1110 110 6E n 0110 1111 111 6F o 0111 0000 112 70 p 0111 0001 113 71 q 0111 0010 114 72 r 0111 0011 115 73 s 0111 0100 116 74 t 0111 0101 117 75 u 0111 0110 118 76 v 0111 0111 119 77 w 0111 1000 120 78 x 0111 1001 121 79 y 0111 1010 122 7A z 0111 1011 123 7B { 0111 1100 124 7C |0111 1110 126 7E ~ASCII码对照表下表列出了字符集中的 0 - 127。
ASCII对照表

ASCII对照表2007/08/03 15:08 ASCII字符对照表
ASCII码(American Standard Code for Information Interchange,美国标准信息交换码),它已被国际标准化组织(ISO)定为国际标准,称为ISO 646标准。
适用于所有拉丁文字字母,ASCII码有7位码和8位码两种形式。
因为1位二进制数可以表示(21=)2种状态:0、1;而2位二进制数可以表示(22)=4种状态:00、01、10、11;依次类推,7位二进制数可以表示(27=)128种状态,每种状态都唯一地编为一个7位的二进制码,对应一个字符(或控制码),这些码可以排列成一个十进制序号0~127。
所以,7位ASCII码是用七位二进制数进行编码的,可以表示128个字符。
第0~32号及第127号(共34个)是控制字符或通讯专用字符,如控制符:LF (换行)、CR(回车)、FF(换页)、DEL(删除)、BEL(振铃)等;通讯专用字符:SOH(文头)、EOT(文尾)、ACK(确认)等;
第33~126号(共94个)是字符,其中第48~57号为0~9十个阿拉伯数字;65~90号为26个大写英文字母,97~122号为26个小写英文字母,其余为一些标
点符号、运算符号等。
注意:在计算机的存储单元中,一个ASCII码值占一个字节(8个二进制位),其最高位(b7)用作奇偶校验位。
所谓奇偶校验,是指在代码传送过程中用来检验是否出现错误的一种方法,一般分奇校验和偶校验两种。
奇校验规定:正确的代码一个字节中1的个数必须是奇数,若非奇数,则在最高位b7添1;偶校验规定:正确的代码一个字节中1的个数必须是偶数,若非偶数,则在最高位b7添1。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Colloids and SurfacesA:Physicochemical and Engineering Aspects202(2002)269–279Metal–metal and metal–semiconductor compositenanoclustersPrashant V.Kamat*,Mark Flumiani1,Amy DawsonNotre Dame Radiation Laboratory,Uni6ersity of Notre Dame,Notre Dame,IN46556-0579,USAReceived5December2000;accepted24August2001AbstractColloidal metal–metal and metal–semiconductor nanocomposites have been synthesized by the chemical reduction of[AuCl4]−on the surface of preformed Silver and TiO2colloids and Ag+on the surface of SnO2and SiO2colloids. Photoinduced bleaching of surface plasmon band has been monitored using a picosecond laserflash photolysis apparatus.In the case of Ag/Au nanoparticles,the magnitude of the surface plasmon bleach of the silver core can be modulated by controlling the capping layer of gold without altering the long term bleaching recovery kinetics.In the case of TiO2/Au nanoclusters,the ratio of[TiO2]/[Au]directly influences the particle size and the stability of these core-shell composite nanoparticles.Aggregation effects were observed for colloids with a TiO2:Au ratio of less than 1:ser pulse excitation(532nm)of these aggregated colloids resulted in the fusion and formation of large nanocomposite clusters.The gold capped TiO2nanoparticles were found to improve the efficiency of interfacial charge transfer process.©2002Elsevier Science B.V.All rights reserved.Keywords:Metals;Semiconductors;Transient bleaching;Composite nanoclusters;Photocatalysis/locate/colsurfa1.IntroductionThe photophysical and photochemical study of single and multicomponent metals and semicon-ductor nanoclusters plays an important role in the development of efficient light energy conversion systems,optical devices and microelectronics(see for example,[1–3]).For example,photoinduced deposition of noble metals such as Pt or Au on semiconductor nanoclusters has often been em-ployed to enhance their photocatalytic activity [4–7].A variety of nanocomposites have been synthesized in recent years.Such composite nanoparticles provides a convenient way to probe the photodynamics of metal–metal and metal–semiconductor interfaces using transient absorp-tion spectroscopy.Examples of core/shell type nanocomposites include,semiconductor/semicon-ductor[2,8–12],semiconductor/metal[4],metal/ semiconductor,[13–15]metal/metal[16–18],and metal/metal oxide[19–21].The surface plasmon absorption band of a metal cluster is dependent both on the cluster*Corresponding author.Fax:+1-219-239-8068;http:/// pkamat.E-mail address:pkamat@(P.V.Kamat).1Mark Flumiani and Amy Dawson are co-op students fromDepartment of Chemistry,University of Waterloo,Canada.0927-7757/02/$-see front matter©2002Elsevier Science B.V.All rights reserved. PII:S0927-7757(01)01071-8P.V.Kamat et al./Colloids and Surfaces A:Physicochem.Eng.Aspects202(2002)269–279 270size/shape and the surrounding media[22–24].Of particular interest are the optical properties of gold and silver nanoclusters that are dependent on the laser pulse excitation.For example,the sur-face plasmon absorption band of gold colloid is bleached in the subpicosecond timescale when excited with a UV or visible laser pulse.The evolution of the optical spectrum of passivated gold clusters in the1.4–3.2nm range( 70–900 atoms)has been demonstrated to be systematic function of size[25].Laser induced fragmentation and fusion of silver and gold clusters has been the topic of interest in many recent studies[26–36]. Depending upon the laser energy,time of irradia-tion and the nature of dispersion controlled the melting of these nanoparticles.Formation of lin-early arrayed gold nanoparticles on gold single-crystal surfaces has been achieved using infrared laser excitation[37].Particle size dependence on the optical limitation properties of gold nanoclus-ters has been investigated by Belloni and cowork-ers[38].The electron dynamics of Au and Ag nanoclusters has also been investigated by tran-sient grating spectroscopy[39]and transient ab-sorption spectroscopy[27,40–42].Recently,in a Au/CdS composite system we were able to induce surface plasmon bleach in the gold core by exciting the external CdS shell[13]. Efforts have been made earlier to synthesize gold capped silver[3]and silver capped gold colloids [17].The absorption spectrum of such composites exhibits spectral features that are significantly dif-ferent than the additive features of the two col-loids.Surface enhanced Raman scattering studies indicated that even submonolayer of silver can modulate specific chemical interactions between the Au and trans-1,2-bis(4-pyridyl)ethylene in Ag capped Au colloids[17].In our continued efforts to explore the optical properties of nanocom-posites we have now investigated the photody-namics of Ag/Au and TiO2/Au nanoclusters (Scheme1).These two examples present two dif-ferent scenarios.In thefirst case we have investi-gated the influence of gold shell on the properties of silver core and in the second case we have probed the influence of a semiconductor(TiO2) core on the properties of a metal(gold)deposit. Synergetics of the metal and semiconductor com-ponents is important for improving the perfor-mance of nanocomposites in photocatalytic applications.2.Experimental section2.1.Preparation of Au and Ag colloidsAu colloids were prepared by the chemical re-duction method of reducing HAuCl4(0.12mM) in water with sodium citrate at near boiling tem-perature[43].Similarly,Ag colloids were prepared using AgNO3as a precursor[27].The concentra-tion of sodium citrate employed for the reduction was kept low(0.42mM)to avoid presence of excess citric acid in the colloidal suspension.2.2.Preparation of Au/Ag colloidsThe composite clusters of Ag/Au were prepared by the sequential reduction of the two metals. Known amount of HAuCl4was added to the preformed silver colloidal suspension and boiled on a hot plate.Additional(equimolar)citric acid was then added and boiled further for about5–10 min.The reduction of gold occurred on the sur-face of silver colloids thus yielding composite metal nanoclusters of core-shell geometry.2.3.Preparation of TiO2colloidal suspensionTiO2colloidal suspension(5mM)was prepared by dropwise addition of2.97ml of10%titanium isopropoxide(Aldrich,99.999%)in1-propanol to Scheme1.Photoexcitation of metal–metal and metal–semi-conductor composite nanoparticles.P.V.Kamat et al./Colloids and Surfaces A:Physicochem.Eng.Aspects202(2002)269–279271 200ml of deionized water with vigorous stirring.Prior to addition of titanium isopropoxide the pHof the water was adjusted to 1.5,with HClO4.The solution was kept stirring in a stoppered glassflask.Freshly prepared colloidal suspension wasused in all the experiments.2.4.Preparation of TiO2/Au colloidsTiO2/Au colloids were prepared by adding thedesired amount of5mM stock HAuCl4(Aldrich)solution to the colloidal TiO2suspension in waterwhile stirring vigorously.The solution was stirredfor an additional5min to allow complete adsorp-tion of[AuCl4]−ions onto the TiO2surface.Reduction of the[AuCl4]−was achieved by thedropwise addition of sodium borohydride(8–10mM)until a color change was observed.Fourdifferent suspensions of TiO2/Au nanocomposite colloids were prepared,by keeping the gold con-centration constant at0.2mM while varying the TiO2concentration from4to0.05mM.These four TiO2/Au suspensions contained[TiO2]:[Au] ratios of20:1,10:1,1:1and0.25:1. Transmission Electron Microscopy(TEM)was carried out by applying a drop of the colloid sample to the carbon-coated copper grid.Particle sizes were determined from the photographs taken at a magnification of150000×using a Hitachi H600transmission electron microscope.Absorp-tion spectra were recorded with a Shimadzu UV-3101PC spectrophotometer.Picosecond laserflash photolysis experiments were performed with355and532nm laser pulses from a mode-locked,Q-switched Continuum YG-501DP Nd:YAG laser system(output1–5mJ per pulse,pulse width 18ps).The white continuum picosecond probe pulse was generated by passing the fundamental output through a D2O/H2O solu-tion.The details of the experimental setup and its operation are described elsewhere[44,45].3.Results and discussion3.1.Ag/Au colloidsSilver colloids prepared by the citrate reduction Fig.1.(A)Ag/Au colloids.Absorption spectra of6×10−4M colloidal Ag in water containing different capping concentra-tions of gold(pathlength2mm).The molar ratio of Ag:Au was maintained at(a)1:0;(b)1:0.0175;(c)1:0.035;(d)1:0.05;(e)1:0.1;and(f)1:0.175.(B)Mixing Ag colloids with Au colloids.Absorption spectra of spectra of2.8×10−4M Ag colloids in water(2mm cell)recorded after the addition of preformed gold particles:(a)0;(b)5×10−6m M;(c)1×10−5 m M;(d)1.5×10−5m M(e)3×10−5m M;and(f)5×10−5m M Au.method produces relatively larger size particles with diameter ranging from40to60nm.The absorption spectrum of silver colloids shows ab-sorption maximum around420nm corresponding to the surface plasmon resonance(Fig.1(A)).This absorption band is rather broad and red-shifted compared with the plasmon absorption band of silver colloids prepared by radiolysis and other methods[46–50].The position and shape of the plasmon absorption of silver nanoclusters are strongly dependent on the particle size,dielectric medium and surface adsorbed species.Efforts have been made earlier to synthesize gold capped silver[3],silver capped gold colloids [17]and bimetallic alloys[51].The absorption spectrum of such composites exhibits spectral fea-tures that are significantly different from the addi-P.V.Kamat et al./Colloids and Surfaces A:Physicochem.Eng.Aspects202(2002)269–279 272tive features of the two colloids.Fig.1(A)shows the absorption spectra of Ag colloids at different Au capping concentrations.As explained in the experimental section,the gold layer was directly deposited on the preformed silver colloids by boil-ing the silver colloid suspension with known amount of HAuCl4and adding equimolar citric acid.We also probed the possibility of interaction between individually stabilized gold and silver colloids by monitoring the absorption properties of mixed systems.The gold and silver colloids were prepared separately using the citrate reduc-tion method.A known amount of gold colloidal suspension was mixed with the Ag colloidal sus-pension to achieve comparable concentrations as in Fig.1(A).The spectra of Ag colloids mixed with different concentration of Au colloids are shown in Fig.1(B).Upon increasing the concen-tration of gold colloids,a small increase in the absorption(500–600nm region)is seen.These spectral differences are merely additive effects and are not the same as those observed with Ag/Au colloids in Fig.1(A).These observations further confirm that the absorption changes observed in Fig.1(A)result from capping of Au shell on Ag colloids.In the case of Ag/Au colloids we also observe a dampening of the surface plasmon absorption band at420nm as the interaction with the gold shell influences the optical properties of the Ag core.Since the Fermi energy of the two metals is similar( 0.5V vs.NHE),we expect a quick equilibration of electrons between the two metals. The surface plasmon absorption band of metal nanoclusters is very sensitive to the surface ad-sorbed species and dielectric of the medium [52,53].Kreibig and coworkers,[22,23,54]who studied the effect of surrounding environment on the plasmon absorption of metal nanoclusters concluded that deviations of the optical properties of metal clusters arise from the predictions of Mie theory.It yields additional information about electronic size effects,about cluster/medium inter-face and about chemical reactions at the interface. For example,one can observe dampening of the surface plasmon band by depositing electrons from radiolytically produced radicals[55]or by exciting the semiconductor deposit in a metal/ semiconductor composite[13].It is interesting to note however,that a thin layer of another noble metal can significantly influence the electronic properties of the bulk metal core.In the present study,we would expect all the gold atoms in thefirst monolayer capping to interact with adjacent silver atoms and form an alloy.Therefore,it is not surprising that we do not observe any surface plasmon absorption cor-responding to gold at this low shell coverage.On the other hand,the small alteration of the surface (alloying with gold)is sufficient enough to dampen the plasmon resonance of silver core. Alloying of Ag and Au systems have also been observed under photoirradiation[56]and room temperature melting conditions.[57]Other inter-esting properties of bimetallic composite particles have also been investigated.For example,a low frequency acoustic breathing mode has been ob-served in the case of Au/Pb colloids[58].Rapid alloy formation has also been noted in the elec-trodeposition of gold onto silver electrodes[59].3.2.TiO2/Au and SnO2/Ag colloids Electrostatic adsorption of metal ions on oppo-sitely charged metal oxide nanoparticles was fol-lowed by the reduction of the metal ions to obtain metal capped semiconductor nanoparticle suspen-sions.The TiO2colloids prepared in acidic medium were positively charged while SnO2col-loids in alkaline medium carried a net negative surface charge.Thus,AuCl4−and Ag+ions bind strongly to the TiO2and SnO2surfaces,respec-tively.Upon reduction with NaBH4we obtain stable TiO2/Au and SnO2/Ag nanocomposite par-ticles in water.The presence of metal oxide col-loid is important for achieving the stability of the suspension.Gold(or silver)reduction carried out in the absence of TiO2(or SnO2)core using same experimental procedure did not produce stable colloids.Fig.2(A)shows the ground state ab-sorbance spectra of TiO2/Au and SnO2/Ag col-loidal solutions at two different ratios of[oxide core]/[metal].We kept the metal ion concentration same and varied the concentration of metal oxide core.P .V .Kamat et al ./Colloids and Surfaces A :Physicochem .Eng .Aspects 202(2002)269–279274Fig.3.Transmission electron micrographs (TEM)of (A)silver (B)gold nanoclusters as prepared by citrate reduction method.(C)Gold capped silver nanoclusters (Ag:Au ratio of 5.7:1).sity in particle size and shape.Most of these silvercolloids exhibit facets,which indicate that thecitric acid reduction method yields fully-grownpared with uncapped silvernanocrystallites,the electron micrograph of Ag /Au sample shows no noticeable increase in size orshape of these crystallites.This in turn indicatesthat the gold layer deposited on the silvernanocrystallites is relatively thin.Even at thehighest gold concentration employed in thepresent study we expect thickness of the gold layerto be of the order of only 5nm.Citric acidreduction of HAuCl 4usually yields 20nm di-ameter spherical particles (Fig.3(B)).Absence ofsuch small spherical particles in the electron mi-crograph of Fig.3(C)rule out formation of sepa-rate gold nanoparticles.As shown in theradiolysis method,the coating of Ag by Au isfacilitated by the fact that their lattice constantsare similar [3].As a result of this lattice matchingthese researchers were not able to distinguish theelectron diffraction pattern of the two metals inAg /Au nanocrystallites.Fig.4shows the electron micrographs of TiO 2/Au and SnO 2/Ag nanoparticles.These compositenanoparticles are spherical in shape.In both thesecases metal oxide core determines the size anddistribution of the nanocomposite.The particlediameter of TiO 2/Au was in the range of 10–15nm while SnO 2/Ag was in the range of 5–10nm.The deposition of the metal layer was relativelysmall since we do not see signi ficant growth in thesize of the composite nanoparticles.3.4.Transient photobleaching of gold capped sil 6er nanoclusters The surface plasmon band of metal particles as explained on the basis of Mie theory involves dipolar oscillations of the free electrons in the conduction band that occupy energy states imme-diately above the Fermi level [22,65].Upon laser pulse excitation,these electrons do not oscillate at the same frequency and thus,cause depletion in the plasmon absorption band.Both Ag and Ag /Au nanoclusters exhibit transient bleaching corre-sponding to the dampening of the plasmon absorption band when subjected to 355or 532nm laser pulse excitation (see for example,spectrum a in Fig.5(A)).This transient bleaching recovers within few nanoseconds.The transient absorption spectra that were recorded 250ps following 355nm laser pulse (pulse width 18ps)excitation are compared in Fig.5(A).The difference absorption spectra of silver and gold capped silver colloids show an intense bleaching of the surface plasmon band at 420nm as they are subjected to laser pulse excita-tion (Fig.5(A),spectrum a).With increasing con-centration of gold capping,the magnitude of transient bleaching increases with a small shift in the bleaching maximum.The maximum bleaching at 440nm is plotted versus the mole fraction of Au in Ag /Au in Fig.5(B).The magnitude of bleaching of the plasmon absorption band in-creases with increasing gold concentration upto the Ag:Au ratio of 1:0.05.Under these conditions,P.V.Kamat et al./Colloids and Surfaces A:Physicochem.Eng.Aspects202(2002)269–279275the bleaching at440nm is nearly twice as that of uncapped silver colloids.At higher gold concen-trations(Ag:Au\1:0.05),the bleaching in the420 nm region decreases and a new bleaching maxi-mum appears around520nm.This new bleaching maximum corresponds to the bleaching of the Au plasmon band.As discussed in the characteriza-tion of ground state spectra of composite clusters, at thicker gold layers the two metal layers attain their individual identity and thus contribute inde-pendently to the plasmon bleach.The results de-scribed here highlight a new approach of modulating transient plasmon bleach of silver col-loids by capping them with another metal shell. It may be noted that the gold colloids at the concentration levels employed here have relatively low absorption compared with Ag colloids at the excitation wavelength.Moreover,the transient spectra recorded following the355nm laser pulse excitation of separately mixed colloidal suspen-sions of Ag and Au colloids did not show any changes in the plasmon bleach.Since these two (Ag and Au)colloids were stabilized separately before mixing,we expect the direct interactions between the two metals to be minimal.The bleaching recovery usually consists of a fast(1–3ps)and a slow( 1ns)components. While the fast recovery corresponds to thermal-ization of hot electrons,the slow recovery has been attributed to electron–phonon and phonon–phonon relaxations.Several studies have dis-cussed in detail the dynamics of laser induced transient bleaching of the plasmon band of Au and Ag nanoclusters[39,41].In the present exper-iments,we have monitored the long term recovery of the transient bleaching at440nm for Ag/Au and510nm for TiO2/Au nanoparticles(Fig.6(A and B)).All the traces in Fig.6(A)had a very similar recovery rate with a time constant in the range of 1.25–1.40ns.Thus,the capping of the gold layer did little or no effect on the long-term bleaching recovery of the surface plasmon bleach in Ag/Au nanoparticles.Following the transient recovery of the surface plasmon band of silver,we observe a growth in the red-IR region.In our previous study[27],we have identified this transient as an aggregate of fragmented silver clusters.We expect such a fragmentation process to dominate under laser excitation conditions.3.5.Transient absorption and photofusion ofTiO2/Au colloidsThe absorption-time profiles recorded at510 nm(Fig.6(B))show the recovery kinetics of theFig.4.Transmission electron micrographs(TEM)of(A)gold capped TiO2(TiO2:Au ratio of10:1)and(B)silver capped SnO2 colloids(SnO2:Ag ratio of50:1).P.V.Kamat et al./Colloids and Surfaces A:Physicochem.Eng.Aspects202(2002)269–279 278spectral feature of this transient match the charac-teristics of the thiocyanate radicals((SCN)2− ) with an absorption maximum at480nm[71,72]. Photocatalytically produced thiocyanate radicals were found to complex with the gold surface(abs. max.390nm),the details of which can be found elsewhere[73].Since the formation of(SCN)2− radicals imme-diately after the laser pulse excitation represents the quantitative estimate of the whole oxidation process,we monitored maximum absorbance at 480nm at different TiO2:Au ratios(the TiO2:Au ratio was varied by varying the gold concentra-tion during sample preparation).The inset shows the dependence of(SCN)2− yield on the gold shell concentration.In the absence of gold capping, TiO2colloids generate(SCN2)− radicals with a quantum yield of0.09.At low concentrations of gold we see an increase in the efficiency of oxida-tion process.For a[Au]:[TiO2]ratio of0.17we see more than40%enhancement in the oxidation efficiency(b(SCN)2− =0.13).As we further in-crease the Au concentration the efficiency of thio-cyanate oxidation at gold capped TiO2 nanoparticles decreases.Inability of the photogen-erated holes to reach the electrolyte interface as well as increased absorption by the gold are the likely reasons for observing lower(SCN)2− yield at higher capping concentrations of gold.On the other hand,when low concentrations of metal is used to cap the semiconductor core we can expect the outer layer to be discontinuous.Such a configuration of core shell particles(i.e.small metal islands deposited on the TiO2core)provide a favorable geometry for facilitating the interfa-cial charge transfer under UV irradiation.The results discussed in this paper highlight some of the interesting properties of the metal–metal and semiconductor–metal nanocomposites. The examples of Ag/Au and Au/TiO2nanoparti-cles show how the composition of the two compo-nents directly influences the photophysical and photocatalytic properties of the metal and semi-conductor nanoclusters.Further studies are un-derway to assess the morphology and surface catalytic properties of nanocomposite materials.AcknowledgementsThe work described herein was supported by the Office of the Basic Energy Sciences of the US Department of Energy.This is contribution No. 4275from the Notre Dame Radiation Laboratory.References[1]A.Henglein,J.Phys.Chem.97(1993)5457.[2]P.V.Kamat,in:P.V.Kamat,D.Meisel(Eds.),Semicon-ductor Nanoclusters—Physical,Chemical and Catalytic Aspects,Elsevier Science,Amsterdam,1997,p.237. [3]P.Mulvaney,M.Giersig,A.Henglein,J.Phys.Chem.97(1993)7061.[4]B.Kraeutler,A.J.Bard,J.Am.Chem.Soc.100(1978)4317.[5]E.Borgarello,R.Harris,N.Serpone,Nouv.J.Chim.9(1985)743.[6]ls,G.Williams,J.Chem.Soc.,Faraday Trans.185(1989)503.[7]E.Amouyal,P.Koffi,J.Photochem.29(1985)227.[8]M.Ocana,W.P.Hsu,E.Matijevic,Langmuir7(1991)2911.[9]A.Haesselbarth,A.Eychmueller,R.Eichberger,M.Gier-sig,A.Mews,H.Weller,J.Phys.Chem.97(1993)5333.[10]I.Bedja,P.V.Kamat,J.Phys.Chem.99(1995)9182.[11]V.F.Kamalov,R.Little,S.L.Logunov,M.A.El-Sayed,J.Phys.Chem.100(1996)6381.[12]A.Mews,A.V.Kadavanich,U.Banin,A.P.Alivisatos,Phys.Rev.B53(1996)R13242.[13]B.Shanghavi,P.V.Kamat,J.Phys.Chem.B101(1997)7675.[14]I.Pastoriza-Santos,D.S.Koktysh,A.A.Mamedov,M.Giersig,N.A.Kotov,L.M.Liz-Marza´n,Langmuir16 (2000)2731.[15]B.Schreder,T.Schmidt,V.Ptatschek,U.Winkler,A.Materny,E.Umbach,M.Lerch,G.Mu¨ller,W.Kiefer,L.Spanhel,J.Phys.Chem.B104(2000)1677.[16]A.Henglein,J.Phys.Chem.B104(2000)6683.[17]R.G.Freeman,M.B.Hommer,K.C.Grabar,M.A.Jack-son,M.J.Natan,J.Phys.Chem.100(1996)718. [18]J.L.Marignier,J.Belloni,M.O.Delcourt,J.P.Chevalier,Nature317(1985)344.[19]wless,S.Kapoor,P.Kennepohl, D.Meisel,N.Serpone,J.Phys.Chem.98(1994)9619.[20]L.M.Liz-Marza´n,M.Giersig,P.Mulvaney,Langmuir12(1996)4329.[21]V.Hardikar,E.Matijevic,J.Coll.Interf.Sci.221(2000)133.[22]U.Kreibig,M.Vollmer,Optical Properties of MetalClusters,Springer,Berlin,1995.P.V.Kamat et al./Colloids and Surfaces A:Physicochem.Eng.Aspects202(2002)269–279279[23]U.Kreibig,M.Gartz,A.Hilger,H.Hovel,in:E.Peliz-zatti(Ed.),Fine Particles Science and Technology, Kluwer Academic Publishers,Boston,1996,p.499. [24]P.Mulvaney,in:P.V.Kamat,D.Meisel(Eds.),Semicon-ductor Nanoclusters—Physical,Chemical and Catalytic Aspects,Elsevier Science,Amsterdam,1997,p.99. [25]M.M.Alvarez,J.T.Khoury,T.G.Schaaff,M.N.Shafig-ullin,I.Vezmar,R.L.Whetten,J.Phys.Chem.B101 (1997)3706.[26]A.Takami,H.Yamada,K.Nakano,S.Koda,Jpn.J.Appl.Phys.35(1996)L781.[27]P.V.Kamat,M.Flumiani,G.Hartland,J.Phys.Chem.B102(1998)3123.[28]H.Kurita,A.Takami,S.Koda,Appl.Phys.Lett.72(1998)789.[29]A.Takami,H.Kurita,S.Koda,J.Phys.Chem.B103(1999)1226.[30]H.Fujiwara,S.Yanagida,P.V.Kamat,J.Phys.Chem.B103(1999)2589.[31]S.Link,C.Burda,M.B.Mohamed,B.Nikoobakht,M.A.El-Sayed,J.Phys.Chem.A103(1999)1165.[32]S.Link,C.Burda,B.Nikoobakht,M.A.El-Sayed,Chem.Phys.Lett.315(1999)12.[33]S.Link,C.Burda,B.Nikoobakht,M.A.El-Sayed,J.Phys.Chem.B104(2000)6152.[34]S.Link,Z.L.Wang,M.A.El-Sayed,J.Phys.Chem.B104(2000)6767.[35]Y.Niidome,A.Hori,T.Sato,S.Yamada,Chem.Lett.,310(2000).[36]C.S.Ah,H.S.Soo Han,K.Kim, D.J.Jang,J.Phys.Chem.B104(2000)8153.[37]K.Murakoshi,Y.Nakato,Adv.Mater.12(2000)791.[38]L.Franc¸ois,M.Mostafavi,J.Belloni,J.-F.Delouis,J.Delaire,P.Feneyrou,J.Phys.Chem.B104,6133(2000).[39]E.J.Heilweil,R.M.Hochstrasser,J.Chem.Phys.82(1985)179.[40]T.W.Roberti,B.A.Smith,J.Z.Zhang,J.Chem.Phys.102(1995)3860.[41]T.S.Ahmadi,S.L.Logunov,M.A.El-Sayed,J.Phys.Chem.100(1996)8053.[42]S.L.Logunov,T.S.Ahmadi,M.A.El-Sayed,J.T.Khoury,R.L.Whetten,J.Phys.Chem.B101(1997) 3713.[43]J.Turkevich,P.L.Stevenson,J.Hillier,Discuss.FaradaySoc.11(1951)55.[44]T.W.Ebbesen,Rev.Sci.Instrum.59(1988)1307.[45]P.V.Kamat,T.W.Ebbesen,N.M.Dimitrijevic, A.J.Nozik,Chem.Phys.Lett.157(1989)384.[46]M.Mostafavi,N.Keghouche,M.-O.Delcourt,J.Belloni,Chem.Phys.Lett.167(1990)193.[47]O.Platzer,J.Amblard,J.L.Marignier,J.Belloni,J.Phys.Chem.96(1992)2334.[48]A.Henglein,Chem.Phys.Lett.154(1989)473.[49]A.Henglein,T.Linnert,P.Mulvaney,Ber.Bunsenges.Phys.Chem.94(1990)1449.[50]T.Linnert,P.Mulvaney,A.Henglein,H.Weller,J.Am.Chem.Soc.112(1990)4657.[51]M.Treguer,C.de Cointet,H.Remita,J.Khatouri,M.Mostafavi,J.Amblard,J.Belloni,R.de Keyzer,J.Bel-loni,J.Phys.Chem.B102(1998)4310.[52]T.Linnert,P.Mulvaney,A.Henglein,J.Phys.Chem.97(1993)679.[53]M.Gutierrez, A.Henglein,J.Phys.Chem.97(1993)11368.[54]B.N.J.Persson,Phys.Rev.B39(1989)8220.[55]A.Henglein,P.Mulvaney,T.Linnert,Faraday Discuss.92(1991)31.[56]J.Hodak,A.Henglein,M.Giersig,G.V.Hartland,J.Phys.Chem.B104(2000)11708.[57]T.Shibata, B.Bunker,Z.Zhang, D.Meisel,J.Phys.Chem.B105,submitted(2001).[58]J.Hodak,A.Henglein,G.V.Hartland,J.Phys.Chem.104(2000)5053.[59]E.Schmidt,S.Stucki,J.Electroanal.Chem.39(1972)63.[60]R.D.Averitt,S.L.Westcott,S.J.Oldenburg,T.R.Lee,N.J.Halas,Langmuir14(1998)5396.[61]H.Giesche,E.Matijevic,J.Mater.Res.9(1994)436.[62]R.D.Averitt,S.L.Westcott,N.J.Halas,J.Opt.Soc.Am.B Opt.Phys.16(1824)1999.[63]S.J.Oldenburg,J.B.Jackson,S.L.Westcott,N.J.Halas,Appl.Phys.Lett.75(1999)2897.[64]O.V.Makarova,A.E.Ostafin,H.Miyoshi,J.R.Norris,D.Meisel,J.Phys.Chem.B103(1999)9080.[65]G.Mie,Ann.Physik B25(1908)377.[66]A.Heller,Pure Appl.Chem.58(1986)1189.[67]Y.Nosaka,Y.Ishizuka,H.Miyama,Ber.Bunsenges.Phys.Chem.90(1986)1199.[68]Y.Chen,Z.Wei,Y.Chen,H.Lin,Z.Hong,H.Liu,Y.Dong,C.Yu,W.Li,J.Mol.Catal.21(1983)275.[69]Y.Nakato,H.Tsubomura,Isr.J.Chem.22(1982)180.[70]N.R.de Tacconi,J.Carmona,K.Rajeshwar,J.Phys.Chem.B101(1997)10151.[71]P.V.Kamat,Langmuir1(1985)608.[72]D.Behar,P.L.T.Bevan,G.Scholes,J.Phys.Chem.76(1972)1537.[73]A.Dawson,P.V.Kamat,J.Phys.Chem.B104(11842)2000.。
