Monensin-sodium-salt-DataSheet-MedChemExpress
新版MSDS——水杨酸钠

中国MAC(mg/m3): 前苏联MAC(mg/m3): TLVTN: TLVWN: 监测方法: 工程控制: 呼吸系统防护: 眼睛防护: 身体防护: 手防护: 其他防护:
密闭操作,局部排风。 空气中粉尘浓度超标时,必须佩戴自吸过滤式防尘口罩。紧急事态抢救或撤 离时,应该佩戴空气呼吸器。 戴化学安全防护眼镜。 穿防毒物渗透工作服。 戴橡胶手套。 工作场所禁止吸烟、进食和饮水,饭前要洗手。工作完毕,淋浴更衣。保持 良好的卫生习惯。 第九部分:理化特性 白色鳞片或粉末,无气味,久露光线中变粉红色。 无资料 无资料 160.11 无资料 无资料 无资料 无资料
外观与性状: pH: 熔点(℃): 200 相对密度(水=1): 沸点(℃): 相对蒸气密度(空气=1): 无资料 分子式: C7H5NaO3 分子量: 主要成分: 饱和蒸气压(kPa): 燃烧热(kJ/mol): 无资料 临界温度(℃): 临界压力(MPa): 无资料 辛醇/水分配系数的对数值: 无资料 闪点(℃): 爆炸上限%(V/V): 无意义 引燃温度(℃): 爆炸下限%(V/V): 无资料 溶解性: 溶于水、甘油,不溶于醚、氯仿、苯。 主要用途: 作解热、镇痛药,分析试剂,防腐剂。 其它理化性质: 第十部分:稳定性和反应活性 稳定性: 稳定 禁配物: 强氧化剂、强碱。 避免接触的条件: 光照。 聚合危害: 不聚合 分解产物: 一氧化碳、二氧化碳、氧化钠。 第十一部分:毒理学信息 LD50:1200 mg/kg(大鼠经口) 急性毒性: LC50:无资料
无资料。 起运时包装要完整,装载应稳妥。运输过程中要确保容器不泄漏、不倒塌、 不坠落、不损坏。严禁与氧化剂、碱类、食用化学品等混装混运。运输途中 应防曝晒、雨淋,防高温。运输时运输车辆应配备相应品种和数量的消防器 材及泄漏应急处理设备。装运本品的车辆排气管须有阻火装置。中途停留时 应远离火种、热源。车辆运输完毕应进行彻底清扫。公路运输时要按规定路 线行驶。 第十五部分:法规信息 化学危险物品安全管理条例 (1987年2月17日国务院发布),化学危险物品安 全管理条例实施细则 (化劳发[1992] 677号),工作场所安全使用化学品规 定 ([1996]劳部发423号)等法规,针对化学危险品的安全使用、生产、储存 、运输、装卸等方面均作了相应规定。 第十六部分:其他信息 无
化学药品中英文对照

1 阿拉伯胶(Acacia)2 乙酰舒泛钾(Acesulfame Potassium)3 冰醋酸(Acetic Acid,Glacial)4 乙酰枸橼酸三丁酯(Acetyltributyl Citrate)5 乙酰枸橼酸三乙酯(Acetyltriethyl Citrate)6 人血白蛋白(A lbumin)7 乙醇(A lcohol)8 海藻酸(Alginic Acid)9 脂肪族聚酯(A liphatic Polyesters)10 阿力糖(A litame)11 杏仁油(A lmond Oil)12 维生素E(Alpha Tocopherol)13 氨溶液(A mmonia Solution)14 维生素C(Ascorbic Acid)15 棕榈酸维生素C酯(Ascorbyl Palmitate)16 阿司帕坦(Aspartame)17 绿坡缕石(Attapulgite)18 皂土(Bentonite)19 苯扎氯铵(Benzalkonium Chloride)20 苄索氯铵(Benzethonium Chloride)21 苯甲酸(Benzoic Acid)22 苯甲醇(Benzyl Alcohol)23 苯甲酸苄酯(Benzyl Benzoate)24 溴硝丙二醇(Bronopol)25 丁羟茴醚(Butylated Hydroxyanisole)26 丁羟甲苯(Butylated Hydroxytoluene)27 羟苯丁酯(Butylparaben)28 碳酸钙(Calcium Carbonate)29 无水磷酸氢钙(Calcium Phosphate, Dibasic Anhydrous)30 磷酸氢钙二水合物(Calcium Phosphate, Dibasic Dihydrate)31 磷酸钙(Calcium Phosphate, Tribasic)32 硬脂酸钙(Calcium Stearate)33 硫酸钙(Calcium Sulfate)34 低芥酸菜籽油(Canola Oil)35 卡波姆(Carbomer)36 二氧化碳(Carbon Dioxide)37 羧甲纤维素钙(Carboxymethylcellulose Calcium)38 羧甲纤维素钠(Carboxymethylcellulose Sodium)39 角叉菜胶(Carrageenan)40 蓖麻油(Castor Oil)41 氢化蓖麻油(Castor Oil,Hydro- genated)42 微晶纤维素(Cellulose,Microcr ystalline)43 粉状纤维素(Cellulose, Powdered)44 微粉硅胶微晶纤维素(Cellulose, Silicified Microcrystalline)45 醋酸纤维素(Cellulose Acetate)46 纤维醋法酯(Cellulose Acetate Phthalate)47 角豆胶(Ceratonia)48 十八十六醇(Cetostearyl A lcohol)49 西曲溴铵(Cetrimide)50 十六醇(Cetyl Alcohol)51 壳聚糖(Chitosan)15652 氯己定(Chlorhexidine)53 三氯叔丁醇(Chlorobutanol)54 氯甲酚(Chlorocresol)55 一氯二氟乙烷(Chlorodifluoroe- thane)56 氟里昂(Chlorofluorocabons)57 对氯间二甲酚(Chloroxylenol)58 胆固醇(Cholesterol)18159 枸橼酸(Citric Acid Monohydrate)60 胶态二氧化硅(微粉硅胶)(Colloidal Silicon Dioxide)61 着色剂(Coloring Agents)62 玉米油(Corn Oil)63 棉籽油(Cottonseed Oil)64 甲酚(Cresol)20865 交联羧甲纤维素钠(Croscarmellose Sodium)66 交联聚维酮(Crospovidone)67 环糊精(Cyclodextrins)68 环甲基硅酮(Cyclomethicone)69 苯甲地那铵(Denatonium Benzoate)70 葡萄糖结合剂(Dextrates)22771 糊精(Dextrin)23072 葡萄糖(Dextrose)23373 邻苯二甲酸二丁酯(Dibutyl Phthalate)74 癸二酸二丁酯(Dibutyl Sebacate)23975 二乙醇胺(Diethanolamine)24176 邻苯二甲酸二乙酯(Diethyl Phthalate)77 二氟乙烷(Difluoroethane)78 二甲硅油(Dimethicone)79 二甲醚(Dimethyl Ether)80 邻苯二甲酸二甲酯(Dimethyl Phthalate)81 二甲亚砜(Dimethyl Sulfoxide)82 多库酯钠(Docusate Sodium)25883 依地酸(乙二胺四乙酸)(Edetic Acid)84 乙酸乙酯(Ethyl Acetate)85 乙基麦芽酚(Ethyl Maltol)86 油酸乙酯(Ethyl Oleate)87 乙基香草醛(Ethyl Vanillin)88 乙基纤维素(Ethylcellulose)89 硬脂酸棕榈酸乙二醇酯(Ethylene Glycol Palmitostearate)90 羟苯乙酯(Ethylparaben)91 果糖(Fructose)92 富马酸(Fumaric Acid)93 明胶(Gelatin)94 液体葡萄糖(Glucose,Liquid)95 甘油(Glycerin)96 山萮酸甘油酯(Glyceryl Behenate)97 单油酸甘油酯(Glyceryl Monooleate)98 单硬脂酸甘油酯(Glyceryl Monostearate)99 硬脂酸棕榈酸甘油酯(Glyceryl Palmitostearate)311100 四氢呋喃聚乙二醇醚(Glycofurol)101 瓜耳胶(Guar Gum)102 七氟丙烷(HFC)(Heptafluoro- propane)103 海克西定(Hexetidine)104 烷烃类(HC) (Hydrocarbons)105 盐酸(Hydrochloric Acid)106 羟乙纤维素(Hydroxyethyl Cellulose)107 羟乙甲纤维素(Hydroxyethylmethyl Cellulose)108 羟丙纤维素(Hydroxypropyl Cellulose)109 低取代羟丙纤维素(Hydroxypropyl Cellulose,Low-substituted) 110 羟丙甲纤维素(Hypromellose)111 羟丙甲纤维素酞酸酯(Hypromellose Phthalate)112 咪唑烷脲(Imidurea)113 异丙醇(Isopropyl Alcohol)114 肉豆蔻酸异丙酯(Isopropyl Myristate)115 棕榈酸异丙酯(Isopropyl Palmitate)116 白陶土(Kaolin)117 乳酸(Lactic Acid)118 拉克替醇(Lactitol)119 乳糖(Lactose)120 羊毛脂(Lanolin)121 含水羊毛脂(Lanolin,Hydrous)122 羊毛醇(Lanolin Alcohols)123 卵磷脂(Lecithin)124 硅酸镁铝(Magnesium Aluminum Silicate)125 碳酸镁(Magnesium Carbonate)126 氧化镁(Magnesium Oxide)127 硅酸镁(Magnesium Silicate)128 硬脂酸镁(Magnesium Stearate)129 三硅酸镁(Magnesium Trisilicate)130 苹果酸(Malic Acid)131 麦芽糖醇(Maltitol)132 麦芽糖醇溶液(Maltitol Solution)133 麦芽糖糊精(Maltodextrin)134 麦芽酚(Maltol)135 麦芽糖(Maltose)136 甘露醇(Mannitol)137 中链脂肪酸甘油三酯(Medium-chain Triglycerides)138 葡甲胺(Meglumine)139 薄荷脑(Menthol)140 甲基纤维素(Methylcellulose)141 羟苯甲酯(Methylparaben)142 液体石蜡(Mineral Oil)143 轻质液体石蜡(Mineral Oil, Light)144 液体石蜡羊毛醇(Mineral Oil and Lanolin Alcohols)145 单乙醇胺(Monoethanolamine)146 谷氨酸一钠(Monosodium Glutamate)147 硫代甘油(Monothioglycerol)148 氮(Nitrogen)149 一氧化二氮(Nitrous Oxide)150 油酸(Oleic Acid)151 橄榄油(Olive Oil)152 石蜡(Paraffin)153 花生油(Peanut Oil)154 凡士林(Petrolatum)155 凡士林羊毛醇(Petrolatum and Lanolin A lcohols)156 苯酚(Phenol)157 苯氧乙醇(Phenoxyethanol)158 苯乙醇(Phenylethyl Alcohol)159 醋酸苯汞(Phenylmercuric Acetate)160 硼酸苯汞(Phenylmercuric Borate)161 硝酸苯汞(Phenylmercuric Nitrate)162 磷酸(Phosphoric Acid)163 波拉克林钾(Polacrilin Potassium)164 泊洛沙姆(Poloxamer)165 葡聚糖(Polydextrose)166 聚乙二醇(Polyethylene Glycol)167 聚氧乙烯(Polyethylene Oxide)168 聚(甲基)丙烯酸树脂(Polymethacr- ylates)169 聚氧乙烯烷基醚(Polyoxyethylene A lkyl Ethers)170 聚氧乙烯蓖麻油衍生物(Polyoxyeth- ylene Castor Oil Derivatives) 171 聚山梨酯(Polyoxyethylene Sorbitan Fatty Acid Esters)172 硬脂酸聚氧乙烯酯(Polyoxyethylene Stearates)173 聚醋酸乙烯酞酸酯(Polyvinyl Acetate Phthalate)174 聚乙烯醇(Polyvinyl Alcohol)175 苯甲酸钾(Potassium Benzoate)176 碳酸氢钾(Potassium Bicarbonate)177 氯化钾(Potassium Chloride)178 枸橼酸钾(Potassium Citrate)179 氢氧化钾(Potassium Hydroxide)180 焦亚硫酸钾(Potassium Metabisulfite)181 山梨酸钾(Potassium Sorbate)182 聚维酮(Povidone)183 丙酸(Propionic Acid)184 没食子酸丙酯(Propyl Gallate)185 碳酸丙烯酯(Propylene Carbonate)186 丙二醇(Propylene Glycol)187 海藻酸丙二醇酯(Propylene Glycol A lginate)188 羟苯丙酯(Propylparaben)189 糖精(Saccharin)607190 糖精钠(Saccharin Sodium)191 芝麻油(Sesame Oil)613192 虫胶(Shellac)193 二氧化硅二甲硅油(Simethi cone)194 海藻酸钠(Sodium A lginate)195 抗坏血酸钠(Sodium Ascorbate)196 苯甲酸钠(Sodium Benzoate)197 碳酸氢钠(Sodium Bicarbonate)198 氯化钠(Sodium Chloride)199 枸橼酸钠二水合物(Sodium Citrate Dihydrate)200 环拉酸钠(Sodium Cyclamate)201 氢氧化钠(Sodium Hydroxide)202 月桂硫酸钠(十二烷基硫酸钠) (Sodium Lauryl Sulfate) 203 焦亚硫酸钠(偏亚硫酸钠)(Sodium Metabisulfite)204 磷酸氢二钠(Sodium Phosphate, Dibasic)205 磷酸二氢钠(Sodium Phosphate , Monobasic)206 丙酸钠(Sodium Propionate)207 羧甲淀粉钠(Sodium Starch Glycolate)208 硬脂富马酸钠(Sodium Stearyl Fumarate)209 山梨酸(Sorbic Acid)210 山梨坦酯Sorbitan Esters(Sorbitan Fatty Acid Esters) 211 山梨醇(Sorbitol)212 大豆油(Soybean Oil)213 淀粉(Starch)214 预胶化淀粉(Starch, Pregelatinized)215 灭菌玉米淀粉(Starch,Sterilizable Maize)216 硬脂酸(Stearic Acid)217 硬脂醇(Stearyl A lcohol)218 羟糖氯(Sucralose)219 蔗糖(Sucrose)220 可压性蔗糖(Sugar, Compressible)221 蔗糖粉(Sugar,Confectioner’s)222 蔗糖球形颗粒(Sugar Spheres)223 硫酸(Sulfuric Acid)224 葵花籽油(Sunflower Oil)225 氢化植物油(硬脂)栓剂基质(Sup- pository Bases,Hard Fat) 226 滑石粉(Talc)227 酒石酸(Tartaric Acid)228 四氟乙烷(HFC)(Tetrafluoroe- thane)229 硫柳汞(Thimerosal)230 二氧化钛(Titanium Dioxide)231 西黄蓍胶(Tragacanth)232 海藻糖(Trehalose)233 三醋汀(Triacetin)234 枸橼酸三丁酯(Tributyl Citrate)235 三乙醇胺(Triethanolamine)236 枸橼酸三乙酯(Triethyl Citrate)237 香草醛(Vanillin)238 氢化植物油(Vegetable Oil, Hydrogenated)239 水(Water)240 阴离子乳化蜡(Wax,Anionic Emulsifying)241 巴西棕榈蜡(Wax,Carnauba)242 十六醇酯蜡(Wax,Cetyl Esters)243 微晶蜡(Wax,Microcrystalline)244 非离子乳化蜡(聚西托醇乳化蜡)(Wax, Nonionic Emulsifying) 245 白蜡(Wax,White)246 黄蜡(Wax,Yellow)247 黄原酸胶(Xanthan Gum)248 木糖醇(Xylitol)249 玉米朊(玉米蛋白)(Zein)250 硬脂酸锌(Zinc Stearate)。
脱氧胆酸钠

脱氧胆酸钠
中文名称: 脱氧胆酸钠
分子式: C24H40O4·Na
分子量: 414.56
英文名称:3-alpha,12-alpha-dihydroxy-5-beta-cholan-24-oic aci sodium salt
性状:白色结晶性粉末。
类似胆汁气味。
有强烈苦味。
易吸湿。
易溶于水,微溶于无水醇,不溶于醚。
比旋光度约+42.5º(c=2,水中)。
低毒,半数致死量(大鼠,经口)1370mg/kg。
有刺激性。
脱氧胆酸钠
质量标准:
项目生物试剂级
砷Arsenic(%) ≤0.001
重金属Heavy Metals(%) ≤0.005
干燥失重Loss on Drying(%) ≤5.0
纯度Purity (Dry Basis)(%) >99.0
胆酸钠Sodium Cholate(%) ≤2.0
可溶性试验Solubility (10%, Water)(P/F) 合格
贮存室温保存
用途:配制细菌培养基,代替脑磷脂作胆固醇絮状试验,蛋白质分析。
裂解液中脱氧胆酸钠和原矾酸钠的作用
前者是离子型去垢剂,后者是抑制酪氨酸磷酸酶活性的。
离子型去垢剂主要作用有:1、裂解细胞;2、溶解蛋白,尤其是可以溶解一些难溶于水的蛋白,如膜蛋白等;3、很适合做WB,但在Co-IP中使用,需要谨慎。
(完整word版)药学英语 终极版 最全版

试剂、化学结构苯环取代meta—, m- 间苯环取代ortho—, 0—邻苯环取代para-, p—对苯环取代symetrical, sym—均苯环取代unsymmetrical, unsym—偏苯环取代vicinal,v—连成分cyclovirobuxine D (CVB-D) 环维黄杨星D基团tertiary butyl 叔丁基链异构iso- 异链异构neo- 新链异构normal, n—正链异构primary 伯链异构quaternary 季链异构secondary, sec—仲,另链异构tertiary, ter—, tert—叔,特手性异构dextro, d—右手性异构laevo, l—左手性异构meso- 内消旋手性异构Rectus, R—右,顺时针手性异构Siniter,S—左,逆时针顺反异构cis- 顺顺反异构cis—isomer 反式体顺反异构Entgagen, E—相反顺反异构trans- 反顺反异构trans-isomer 顺式体顺反异构zusammen, Z- 共同2—Butanone 2—丁酮2-methylprapanoate, isobutyrate 2-甲基丙酸酯4-benzoylphenol 4—羟基二苯酮;4—羟基苯基苯甲酮;(4-羟基苯基)苯基甲酮;4-羟基二苯甲酮absolute ethanol 无水乙醇(含水量1%以下)acetal 乙缩醛,乙缩醛二乙醇,俗称塑钢(港台地区有称:塑胶钢)acetaldehyde 乙醛acetate buffer 醋酸盐缓冲液acetic anhydride 醋酸酐,又称醋酐、乙酐、乙酸酐acetone 丙酮acetonitrile 乙腈,别名甲基氰acetophenone 苯乙酮acetyloxy 乙酰氧基acidified water 酸化水activated carbon, activated charcoal 活性炭Adrenaline 肾上腺素Adsorption indicator (Adsorb)吸附指示剂albendazole 阿苯达唑aldehyde free alcohol 无醛乙醇aliphatic 脂肪族的alkali hydroxide 氢氧化碱alkaline solution 碱性溶液alkyl 烷基,烃基alkyl aryl ether 烷基芳基醚alkylene 烯烃基alkyne 炔Aminoalkyl Methacrylate Copolymer E (Eudragit E100)甲基丙烯酸氨烷基酯共聚物E型aminobenzoic acid 氨基苯甲酸aminoethoxymethyl 氨乙氧甲基aminoheptane 氨基庚烷aminophenol 氨基苯酚aminophenyl 氨(基)苯(基)aminosalicyclic 氨基水杨酸aminosalicylate 氨基水杨酸盐Aminothiophenol 氨基硫酚Ammonio Methacrylate Copolymer Type A (Eudragit RL 100) 季胺基甲基丙烯酸酯共聚物A型Ammonio Methacrylate Copolymer Type B (Eudragit RS 100)季胺基甲基丙烯酸酯共聚物B型ammonium chloride 氯化铵ammonium hydroxide 氢氧化铵,即氨水。
法莫替丁

取本品约0.12g,精密称定,加冰醋酸20mL与醋酐5mL溶解后,加结晶紫指示液1滴,用高氯酸滴定液 (0.1mol/L)滴定至溶液显绿色,并将滴定的结果用空白试验校正。每1mL高氯酸滴定液(0.1mol/L)相当于 16.87mg的C8H15N7O2S3。
H2受体阻滞药。
遮光,密封保存。
1、法莫替丁片。 2、法莫替丁注射液。 3、法莫替丁胶囊。 4、法莫替丁颗粒。
安全信息
安全术语
风险术语
S22:Do not breathe dust. 不要吸入粉尘。 S24/25:Avoid contact with skin and eyes. 避免皮肤和眼睛接触。
R20/21/22:Harmful by inhalation, in contact with skin and if swallowed. 吸入、与皮肤接触和吞食是有害的。
摩尔折射率:79.06 摩尔体积(cm3/mol):183.5 等张比容(90.2K):576.5 表面张力(dyne/cm):97.3 极化率(10-24cm3):31.34
疏水参数计算参考值(XlogP):-0.6 氢键供体数量:4 氢键受体数量:9 可旋转化学键数量:7 拓扑分子极性表面积(TPSA):176 重原子数量:20 表面电荷:0 复杂度:469 同位素原子数量:0 确定原子立构中心数量:0 不确定原子立构中心数量:0
3
分子结构数据
4
计算化学数据
5
用途
化学式:C8H15N7O2S3 分子量:337.445 CAS号:-35-6
密度:1.83 g/cm3 熔点:163-164°C 沸点:662.4℃ 闪 点 : 3 5 4 . 4 ºC 折射率:1.808 外观:白色结晶性粉末 溶解性:在甲醇中微溶,在丙酮中极微溶解,在水或氯仿中几乎不溶,在冰醋酸中易溶
cas69-57-8_Penicillin G Sodium基本简述MedBio
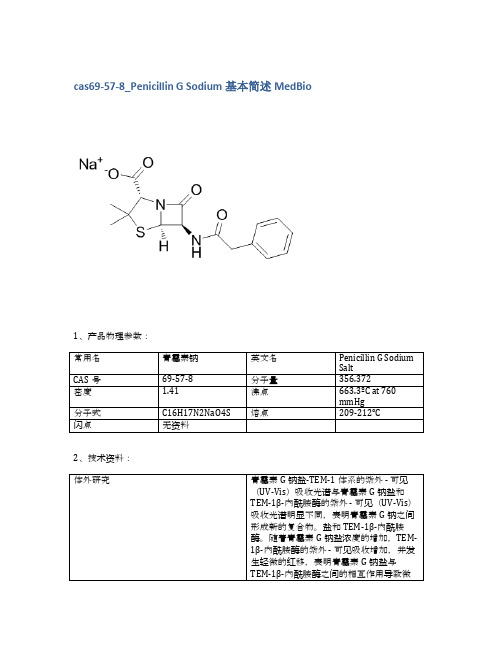
英文名称
CAS
包装
纯度
MedBio
MED13777
Capreomycin Sulfate
Capreomycin Sulfate
1405-37-4
5g
≥98%
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
MED13835
Cefprozil
Cefprozil
92665-29-7
10mg
≥98%
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
MED13836
P005672 hydrochloride
P005672 hydrochloride
1035979-44-2
5mg
≥98%
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
MED13807
Cloxacillin Sodium
Cloxacillin Sodium
1405-37-4
10mM (in 1mL H2O)
≥98%
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
MED13829
Sitafloxacin Hydrate
Sitafloxacin Hydrate
163253-35-8
50mg
≥98%
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
MED13807
体内研究
镀金药水配方
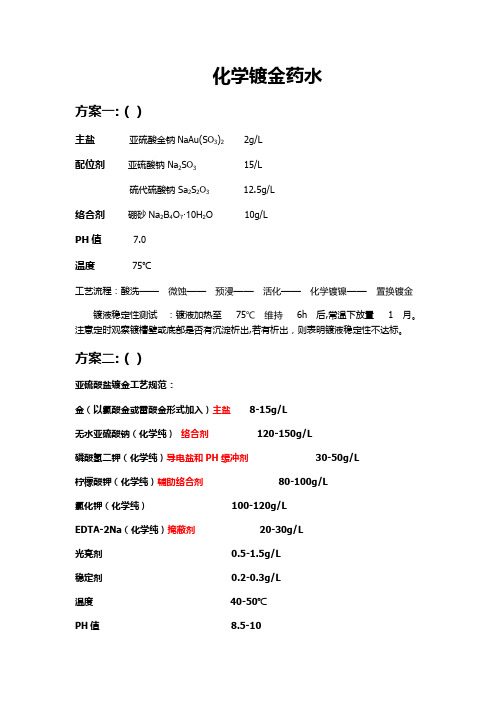
化学镀金药水方案一:()主盐亚硫酸金钠NaAu(SO3)2 2g/L配位剂亚硫酸钠 Na2SO3 15/L硫代硫酸钠 Sa2S2O3 12.5g/L络合剂硼砂Na2B4O7.10H2O 10g/LPH值 7.0温度 75℃工艺流程:酸洗——————————镀液稳定性测试:镀液加热至756h后,常温下放置1月。
注意定时观察镀槽壁或底部是否有沉淀析出,若有析出,则表明镀液稳定性不达标。
方案二:()亚硫酸盐镀金工艺规范:金(以氯酸金或雷酸金形式加入)主盐 8-15g/L无水亚硫酸钠(化学纯)络合剂 120-150g/L磷酸氢二钾(化学纯)导电盐和PH缓冲剂 30-50g/L柠檬酸钾(化学纯)辅助络合剂 80-100g/L氯化钾(化学纯) 100-120g/LEDTA-2Na(化学纯)掩蔽剂 20-30g/L光亮剂 0.5-1.5g/L稳定剂 0.2-0.3g/L温度 40-50℃PH值 8.5-101.1金盐金是镀液的主盐,在溶解纯金后以氯酸金或雷酸金形式加入镀液。
在镀液中以亚硫酸金络离子[A(SO3)3-]和柠檬酸金络离子[A(C6H5O7)]3-存在。
金含量高,允许阴极电流密度较高,沉速快;金含量低,允许阴极电流密度低,沉速慢。
正常情况下的沉积速度为0.1-0.3um/min。
1.2 亚硫酸钠亚硫酸钠是金的主要络合剂。
1mol金需要2mol以上的亚硫酸钠才能完全络合。
其作用是改善镀液的分散能力,提高镀液的导电性。
稳定PH在8.5以上,可保证亚硫酸金络离子不发生解离而缩短溶液的寿命。
1.3 柠檬酸钾柠檬酸钾是金的辅助络合剂,在镀液中生成柠檬酸金络离子有助于溶液的稳定。
1.4 氯化钾氯化钾的作用是提高镀液的导电性能和阴极电流密度,从而提高金的沉积速度。
氯化钾含量低于工艺范围则使用的电流密度范围变小。
1.5 磷酸氢二钾磷酸氢二钾是导电盐和PH缓冲剂。
当镀液的PH降低至酸性时,亚硫酸钠发生分解:SO32-+2H+→SO2↑+H2O。
WS-10001-(HD-0759)-2002
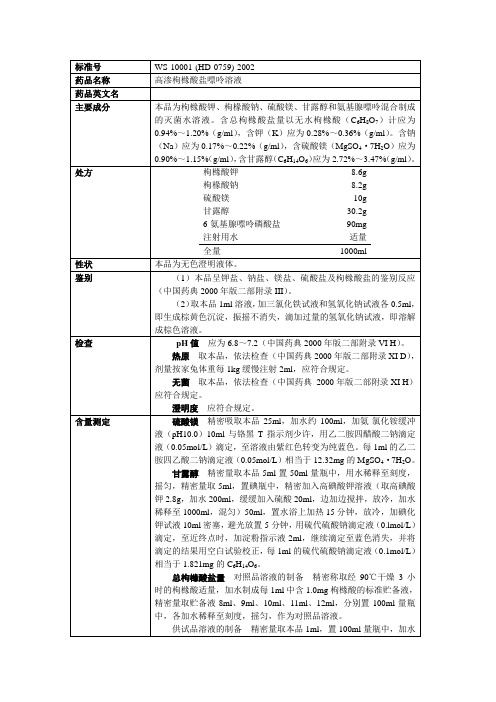
供试品溶液的制备精密量取本品1ml,置100ml量瓶中,加水稀释至刻度,摇匀。
以上操作均应采用无菌技术,所用器材均应无菌无热原。
注意
1.取供肾时,应避免对肾脏的牵拉、损伤,以防止供肾血管痉挛。
2.尽可能减少供肾的热缺血时间,以减少热缺血对肾细胞的损伤。
3.灌注器材应保持管道的畅通。
4.保存肾使用前必须用生理盐水通过肾动脉洗去本液,防止本液的高浓度钾离子进入受者的血循环,造成不良后果。
标准曲线的绘制:精密量取标准钾溶液0.5ml、1.0ml、1.5ml、2.0ml、2.5ml,分别置50ml量瓶中,用去离子水稀释至刻度,钾含量分别为0.5mmol/L、1.0mmol/L、1.5mmol/L、2.0mmol/L、2.5mmol/L。
供试品溶液配制及测定:精密量取本品1.0ml,置100ml量瓶中,用去离子水稀释至刻度,用火焰光度计在769nm的波长处(或红色滤色片)测定其吸收值,自标准曲线上查出供试品中钾含量Bmmol/L。
规格
贮藏
在凉暗处保存。
有效期
暂定1年
曾用名
离体肾保存液
起草单位
复核单位
出处
化学药品地方标准上升国家标准(第八册)
标准号
WS-10001-(HD-0759)-2002
药品名称
高渗枸橼酸盐嘌呤溶液
药品英文名
主要成分
本品为枸橼酸钾、枸椽酸钠、硫酸镁、甘露醇和氨基腺嘌呤混合制成的灭菌水溶液。含总枸橼酸盐量以无水枸橼酸(C6H8O7)计应为0.94%~1.20%(g/ml),含钾(K)应为0.28%~0.36%(g/ml)。含钠(Na)应为0.17%~0.22%(g/ml),含硫酸镁(MgSO4·7H2O)应为0.90%~1.15%(g/ml),含甘露醇(C6H14O6)应为2.72%~3.47%(g/ml)。
等渗对比剂(碘克沙醇) 精品

IOCM,碘克沙醇
*升高 SCr >25%或>0.5mg/dl 1. Jo S-H et al. J Am Coll Cardiol 2006; 48: 924-30.
1,276例接受PCI的病人使用碘克沙醇或碘帕醇的比较研究
住院期间和
Байду номын сангаас
30天的主要心脏不良事件(MACE)的比较
VICC研究
Ref. Harrison JK et al. Circulation 2003; 108 (Suppl IV): ABSTRACT 1660
典淳宁TM的等渗特性
2500
2130+
•低渗对比剂(LOCM)渗透压仍然高达人体 血液的两倍,其化学成分仍会对组织产生
2000
毒性作用(又称为:次高渗对比剂)。
1870
•等渗对比剂(IOCM)的渗透压与血液相同 ,心肾耐受性和安全性高
mOsm/kg H2O
1500 1000
500 0
915
521 290 290
HOCM
LOCM
IOCM
Blood
HOCM: 高渗对比剂; LOCM:低渗对比剂(次高渗对比剂); IOCM:等渗对比剂
血细胞和内皮细胞在不 同渗透压对比剂中的表现
红细胞
非离子型 低渗对比剂 (844mOsm/kg H2O) 离子型 高渗对比剂 (2000mOsm/kg H2O)
生理盐水 (与血浆等渗)
肝 脏 增 强 造 影
心脏介入
肿瘤介入
神经介入
产品结构及理化特性 临床的应用领域及产品优势
安全性评估及临床文献支持
ADR的表现及评价
心脏安全性
对比剂与心脏安全性
英文缩写

PNA凝集素DBA双花扁豆凝集素UEA荆豆凝集素TBS Tris-HCl缓冲盐溶液PBS NaCl KCl Na2HPO4 KH2PO4 缓冲液BSA牛血清白蛋白(bovine serum albuminICC免疫细胞化学(immunocytochemistryELISA酶联免疫吸附试验HRP辣根过氧化物酶(horseradish peroxidaseDAB二氨基联苯胺AP碱性磷酸酶TNF肿瘤坏死因子CXCL12 趋化因子CXCL12SDF-1基质细胞衍生因子-1LPS脂多糖TGF-β转化生长因子βGDF生长分化因子Growth differentiation factor 5TLRs Tolls样受体HIF-1α缺氧诱导因子1αHIF-2α缺氧诱导因子1αAS动脉粥样硬化atherosclerosisGM-CSF粒细胞集落刺激生物因子TCR T细胞抗原受体TGF-β转化生长因子-βDCs树突状细胞Dendritic cellsMIP一3p巨噬细胞炎性蛋白Macrophage inflammatory protein一3 PBS磷酸盐缓冲溶液Phosphate Buffered SolutionFCM流式细胞仪Flow CytometryHLA白细胞抗原PD1COX-2环氧化酶-2IκB NF—κB的抑制性蛋白NF—κB核因子κBHRP是应用最广的一种酶,来源于植物辣根,由无色的酶蛋白和深棕色的铁叶琳结合而成,分子量约40kDDAB本身无色,反应后呈棕色,不溶于水,不易褪色,电子密度高。
趋化因子CXCL12 又称基质细胞衍生因子-1(SDF-1)趋化因子CXCL12 又称基质细胞衍生因子-1(SDF-1)①脂质和多糖的复合物;②为革兰氏阴性细菌细胞壁的主要成分,脂多糖是内毒素和重要群特异性抗原(O抗原)。
脂多糖由三部分组成。
脂质A(Lipid A)为构成内毒素活性的糖脂,以共价键联结到杂多糖链,有两部分:一是核心多糖,在有关的株内是恒定的;另一O特异性链(O-specific chain)是高度可变的。
水杨酸钠的化学需氧量

水杨酸钠的化学需氧量水杨酸钠是一种常用的化学试剂,广泛应用于医药、化妆品和农药等领域。
它的化学需氧量(Chemical Oxygen Demand,COD)是指单位体积水中水杨酸钠被氧化消耗的氧气量。
下面将从水杨酸钠的性质、COD的测定方法和应用等方面进行探讨。
水杨酸钠(Sodium Salicylate)是一种白色结晶粉末,无臭,微苦味,易溶于水。
它的化学式为C7H5NaO3,分子量为160.1。
水杨酸钠是水杨酸(Salicylic Acid)与氢氧化钠(Sodium Hydroxide)反应生成的钠盐。
水杨酸钠具有抗菌、消炎、镇痛等药理作用,因此被广泛应用于医药领域,用于治疗炎症、疼痛等症状。
化学需氧量(COD)是衡量水体中有机物污染程度的重要指标之一。
COD的测定方法有多种,常用的方法包括高温消解-电导法、高温消解-紫外分光光度法和高温消解-滴定法等。
其中,高温消解-滴定法是一种常用的测定COD的方法。
该方法的原理是利用高温消解将水样中的有机物氧化为二氧化碳和水,在酸性条件下,将产生的二氧化碳滴定至酸碱指示剂的终点,通过滴定液的消耗量计算出COD的含量。
水杨酸钠的COD测定方法与一般有机物的COD测定方法相似。
首先,将水样与硫酸混合,使有机物在酸性条件下被氧化。
然后,通过高温消解将水样中的有机物完全氧化为二氧化碳和水。
最后,用硫酸钾溶液滴定消耗的硫酸,通过滴定液的消耗量计算出水样中水杨酸钠的COD含量。
水杨酸钠的COD测定具有一定的应用价值。
首先,COD测定可以用于评估水体中有机物的污染程度,为环境监测和水质评价提供依据。
其次,COD测定也可以用于药品和化妆品等产品的质量控制,确保其符合相关标准和要求。
此外,COD测定还可以用于研究水杨酸钠在环境中的降解和迁移特性,为环境保护和生态安全提供科学依据。
水杨酸钠的化学需氧量是指单位体积水中水杨酸钠被氧化消耗的氧气量。
水杨酸钠具有广泛的应用领域,其COD的测定方法常用高温消解-滴定法。
日本药典-孟鲁司特钠-英文

001-1409-2(仮訳).pdfMontelukast SodiumモンテルカストナトリウムC35H35ClNNaO3S: 608.17Sodium (1-{[((1R)-1-{3-[(1E)-2-(7-chloroquinolin-2-yl)ethenyl] phenyl}-3-[2-(1-hydroxy-1-methylethyl)phenyl]propyl)sulfanyl] methyl}cyclopropyl)acetate[151767-02-1]Montelukast Sodium contains not less than 98.0% and not more than 102.0% of C35H35ClNNaO3S, calcu-lated on the anhydrous basis and corrected on the amount of the residual solvent.Description Montelukast Sodium occurs as a white to pale yellowish white powder.It is very soluble in methanol and in ethanol (99.5), and freely soluble in water.It is hygroscopic.It turns yellow on exposure to light.Identification(1)Place 0.1 g of Montelukast Sodium in a crucible, and heat until a white residue is formed. To the residue add 2 mL of water, and then filter. To the filtrate add 2 mL of potassium carbonate solution (3 in 20), and heat to boiling: no precipitate is observed. To this solution add 4 mL of potassium hexahydroxoantimonate (V) TS, heat to boiling, and cool immediately in ice water: a white precipitate is formed. Rub the inside wall of the test tube with a glass rod, if necessary.(2)Determine the absorption spectrum of a solution of Montelukast Sodium in a mixture of methanol and water (3:1) (1 in 100,000) as directed under Ultraviolet-visible Spectrophotometry <2.24>, and compare the spectrum with the Reference Spectrum or the spectrum of a solution of Montelukast Sodium RS prepared in the same manner as the sample solution: both spectra exhibit similar intensities of absorption at the same wavelengths.(3)Determine the infrared absorption spectrum of Mon-telukast Sodium as directed in the paste method under Infra-red Spectrophotometry <2.25>, and compare the spectrum with the Reference Spectrum or the spectrum of Montelukast Sodium RS: both spectra exhibit similar intensities of ab-sorption at the same wave numbers. Or, perform the test by the potassium bromide disk method or ATR method, and compare the spectrum with the spectrum of Montelukast So-dium RS: both spectra exhibit similar intensities of absorp-tion at the same wave numbers.Purity (1) Heavy metals-Dissolve 0.5 g of Montelukast Sodium in 20 mL of a mixture of acetone and water (4:1), and use this solution as the sample solution. Separately, take 0.5 mL of Standard Lead Solution, add 20 mL of the mixture of acetone and water (4:1), and use this solution as the stand-ard solution. To the sample solution and the standard solution add 2 mL of acetate buffer solution, pH 3.5, and shake. To these solutions add 1.2 mL of thioacetamide-alkaline glycerin TS, shake immediately, then allow to stand for 2 minutes, and filter through a membrane filter with pore size 0.45 µm (about 13 mm in diameter). Compare the color on the mem-brane filters through which each solution is filtered: the color obtained from the sample solution is not darker than that obtained from the standard solution (not more than 10 ppm).(2)Related substances-Conduct this procedure using light-resistant vessels. Dissolve 50 mg of Montelukast So-dium in 50 mL of a mixture of methanol and water (9:1), and use this solution as the sample solution. Perform the test with 10 µL of the sample solution as directed under Liquid Chromatography <2.01>according to the following condi-tions. Determine each peak area by the automatic integration method, and calculate the amount of them by the area per-centage method: the amount of the peak having the relative retention time of about 1.9 to montelukast (related substance F) is not more than 0.3%, the amount of the peak having the relative retention time of about 0.4 (related substance A) is not more than 0.2%, the amounts of the peaks having the relative retention times of about 0.8 (related substance B) and about 1.2 (related substance E) are not more than 0.15%, respectively, the total amount of the two peaks having the relative retention time about 0.9 (related substances C and D) is not more than 0.15%, and the amounts of the peaks other than montelukast and the peaks mentioned above are not more than 0.10%, respectively. The total amount of the peaks other than montelukast is not more than 0.6%.Operating conditions-Detector, column, column temperature, mobile phase, and flow rate: Proceed as directed in the operating conditions in the Assay.Time span of measurement: For 16 minutes after injection, beginning after the solvent peak.System suitability-System performance: Proceed as directed in the system suitability in the Assay.Test for required detectability: Pipet 1 mL of the sample solution, add the mixture of methanol and water (9:1) to make exactly 100 mL. Pipet 1 mL of this solution, add the mixture of methanol and water (9:1) to make exactly 20 mL, and use this solution as the solution for system suitability test.001-1409-2(仮訳).pdf When the procedure is run with 10 µL of the solution forsystem suitability test under the above operating conditions,the SN ratio of the peak of montelukast is not less than 10.For the calculations mentioned above, the peak areassmaller than that of montelukast, founded in the chromato-gram obtained with 10 µL of the solution for system suitabil-ity test, are excluded.(3)Optical isomer-Conduct this procedure usinglight-resistant vessels. Dissolve 50 mg of Montelukast So-dium in 50 mL of a mixture of water and acetonitrile (1:1),and use this solution as the sample solution. Perform the testwith 10 µL of the sample solution as directed under LiquidChromatography <2.01>according to the following condi-tions. Determine each peak area by the automatic integra-tion method, and calculate the amounts of them by the areapercentage method: the amount of the peak having the rela-tive retention time of about 0.7 to montelukast is not morethan 0.2%.Operating conditions-Detector: An ultraviolet absorption photometer (wave-length: 280 nm).Column: A stainless steel column 4.0 mm in inside diame-ter and 15 cm in length, packed with α1-acid glycoproteinbinding silica gel for liquid chromatography (5 µm in particlediameter).Column temperature: A constant temperature of about30℃.Mobile phase A: Dissolve 2.3 g of ammonium acetate in1000 mL of water, and adjust to pH 5.7 with acetic acid(100).Mobile phase B: A mixture of methanol and acetonitrile(3:2).Flowing of mobile phase: Control the gradient by mixingthe mobile phases A and B as directed in the following table.Time after injection of sample (min) Mobile phase A(vol%)Mobile phase B(vol%)0 -30 70 →60 30 →4030 -35 60 40Flow rate: 0.9 mL per minute (the retention time of mon-telukast is about 25 minutes).System suitability-Test for required detectability: Pipet 1 mL of the sample solution, add the mixture of water and acetonitrile (1:1) to make exactly 100 mL. Pipet 1 mL of this solution, add the mixture of water and acetonitrile (1:1) to make exactly 10 mL. When the procedure is run with 10 µL of this solution under the above operating conditions, the SN ratio of the peak of montelukast is not less than 10.System performance: Dissolve about 5 mg of Montelukast Racemate RS in the mixture of water and acetonitrile (1:1) to make 50 mL. When the procedure is run with 10 µL of this solution under the above operating conditions, the resolution between the peak of montelukast and the peak having the relative retention time of about 0.7 to montelukast is not less than 2.9.(4)Residual solvent-Being specified separately when the drug is granted approval based on the Pharmaceutical Affairs Law.Water <2.48>Not more than 4.0% (0.3 g, volumetric titra-tion, direct titration).Assay Conduct this procedure using light-resistant vessels. Weigh accurately about 50 mg of Montelukast Sodium, and dissolve in a mixture of methanol and water (9:1) to make exactly 50 mL. Pipet 10 mL of this solution, add the mixture of methanol and water (9:1) to make exactly 100 mL, and use this solution as the sample solution. Separately, weigh accurately about 26 mg of Montelukast Dicyclohexylamine RS, dissolve in the mixture of methanol and water (9:1) to make exactly 50 mL. Pipet 5 mL of this solution, add the mixture of methanol and water (9:1) to make exactly 20 mL, and use this solution as the standard solution. Perform the test with exactly 10 µL each of the sample solution and standard solution as directed under Liquid Chromatography <2.01> according to the following conditions. Determine the peak areas, A T and A S, of montelukast in each solution.Amount (mg) of C35H35ClNNaO3S=M S×A T/A S×5/2 ×0.792M S: Amount (mg) of Montelukast Dicyclohexylamine RSOperating conditions-Detector: An ultraviolet absorption photometer (wave-length: 238 nm).Column: A stainless steel column 4.6 mm in inside diame-ter and 5 cm in length, packed with phenylsilanized silica gel for liquid chromatography (1.8 µm in particle diameter). Column temperature: A constant temperature of about 30℃.Mobile phase A: A mixture of water and trifluoroacetic acid (2000:3).Mobile phase B: A mixture of acetonitrile and trifluoroa-cetic acid (2000:3).Flowing of mobile phase: Control the gradient by mixing the mobile phases A and B as directed in the following table.Time after injectionof sample (min)Mobile phase A(vol%)Mobile phase B(vol%)0 - 3 60 403 -16 60 →49 40 →51 Flow rate: 1.2 mL per minute (the retention time of mon-telukast is about 7 minutes).001-1409-2(仮訳).pdfSystem suitability-System performance: Dissolve 10 mg of Montelukast for Peak Identification RS in the mixture of methanol and water (9:1) to make 10 mL, and use this solution as the solution A for peak identification. Perform the test with 10 µL of the solution A for peak identification under the above operating conditions, and identify the peaks having the relative reten-tion times to montelukast of about 0.4 (related substance A), about 0.9 (related substances C and D), about 1.2 (related substance E), and about 1.9 (related substance F). Place 1 mL of the solution A for peak identification in a clear glass con-tainer, allow to stand for about 20 minutes, and use this solu-tion as the solution B for peak identification. When the pro-cedure is run with 10 µL of the solution B for peak identifi-cation under the above operating conditions, and identify the peak having the relative retention time of about 0.8 to mon-telukast (related substance B), the resolution between the peaks of related substance B and montelukast is not less than 2.5, and between the peaks of montelukast and related sub-stance E is not less than 1.5.System repeatability: When the test is repeated 5 times with 10 µL of the standard solution under the above operat-ing conditions, the relative standard deviation of the peak area of montelukast is not more than 0.73%.Containers and storage Containers-Tight containers Storage-Light-resistant.Related substancesRelated substance A:(1-{[(1-{3-[(1E)-2-(7-Chloroquinolin-2-yl)ethenyl]phenyl} -3-[2-(1-hydroxy-1-methylethyl)phenyl]propyl)sulfinyl] methyl}cyclopropyl)acetic acid Related substance B:(1-{[((1R)-1-{3-[(1Z)-2-(7-Chloroquinolin-2-yl)ethenyl] phenyl}-3-[2-(1-hydroxy-1-methylethyl)phenyl]propyl)sulfanyl]methyl}cyclopropyl)acetic acidRelated substance C:(1-{[((1R)-1-{3-[(1R)-1-({[1-(Carboxymethyl)cyclopropyl] methyl}sulfanyl)-2-(7-chloroquinolin-2-yl)ethyl]phenyl}-3- [2-(1-hydroxy-1-methylethyl)phenyl]propyl)sulfanyl]methyl}cyclopropyl)acetic acidRelated substance D:(1-{[((1R)-1-{3-[(1S)-1-({[1-(Carboxymethyl)cyclopropyl] methyl}sulfanyl)-2-(7-chloroquinolin-2-yl)ethyl]phenyl}-3- [2-(1-hydroxy-1-methylethyl)phenyl]propyl)sulfanyl]methyl}cyclopropyl)acetic acidRelated substance E:(1-{[((1R)-3-(2-Acetylphenyl)-1-{3-[(1E)-2-(7-chloroquinolin -2-yl)ethenyl]phenyl}propyl)sulfanyl]methyl}cyclopropyl)acetic acid001-1409-2(仮訳).pdfRelated substance F:(1-{[((1R)-1-{3-[(1E)-2-(7-Chloroquinolin-2-yl)ethenyl]phenyl}-3-[2-(1-methylethenyl)phenyl]propyl)sulfanyl]methyl}cyclopropyl)acetic acidAdd the following to 9.01 Reference Standards(1):Montelukast Sodium RSMontelukast Dicyclohexylamine RSMontelukast Racemate RSMontelukast for Peak Identification RS。
Sodium molybdate_CAS号7631-95-0说明书_AbMole中国
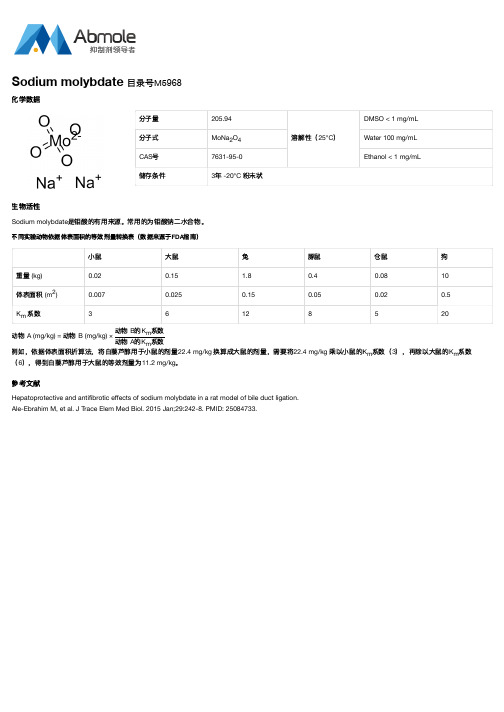
mg/kg
换算成大鼠的剂量,需要将22.4
mg/kg
乘以小鼠的Km系数(3),再除以大鼠的Km系数
参考文献
Hepatoprotective and antifibrotic effects of sodium molybdate in a rat model of bile duct ligation. Ale-Ebrahim M, et al. J Trace Elem Med Biol. 2015 Jan;29:242-8. PMID: 25084733.
Sodium molybdate 目录号M5968
化学数据
分子量 分子式 号 CAS 储存条件
205.94 MoNa2O4 7631-95-0
3年 -20°C 粉末状
溶解性(25°C)
DMSO < 1 mg/mL Water 100 mg/mL Ethanol < 1 mg/mL
生物活性
Sodium molybdate是钼酸的有用来源。常用的为钼酸钠二水合物。
不同实验动物依据体表面积的等效剂量转换表(数据来源于FDA指南)
小鼠
大鼠
兔
豚鼠
仓鼠
狗
重量 (kg)
0.02
0.15
1.8
0.4
0.08
10
体表面积 (m2)Βιβλιοθήκη 0.0070.025
0.15
0.05
0.02
0.5
Km 系数
3
6
12
8
5
20
动例物如,A依(m据g/体kg表) =面动积物折算B (法m,g/k将g白) ×藜动动芦物物醇BA用的的于KK小mm系系鼠数数的剂量22.4 (6),得到白藜芦醇用于大鼠的等效剂量为11.2 。 mg/kg
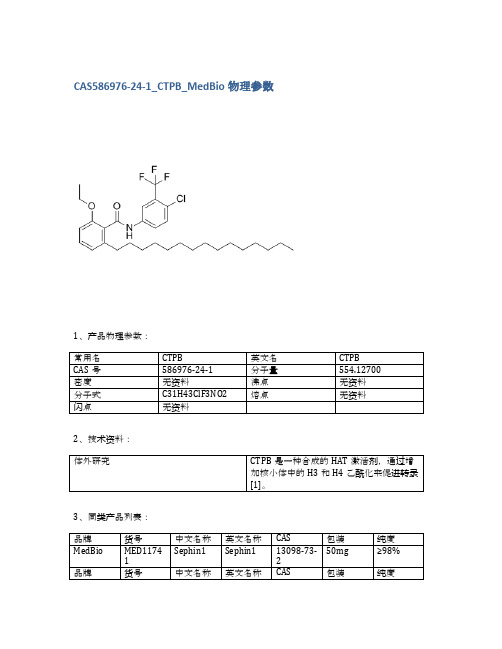
CAS586976-24-1_CTPB_MedBio物理参数
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
MED11741
Sephin1
Sephin1
13098-73-2
50mg
≥98%
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
MED11859
HAT Inhibitor II
HAT Inhibitor II
932749-62-7
10mg
≥98%
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
MED11825
HLCL-61
HLCL-61
1158279-20-9
10mM (in 1mL DMSO)
≥98%
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
MED11755
EPZ004777 HCl
EPZ004777 HCl
1380316-03-9
50mg
≥98%
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
MED11803
EPZ020411
EPZ020411
1700663-41-7
10mg
≥98%
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
MED11751
AZ505
AZ505
1035227-43品牌
货号
中文名称
英文名称
n-羟基丁二酰亚胺硫酸钠的相对摩尔质量

n-羟基丁二酰亚胺硫酸钠的相对摩尔质量n-羟基丁二酰亚胺硫酸钠(N-Hydroxysuccinimide sodium sulfate)并不是一个常见的化学名称或标准命名。
可能您是指的某种与N-羟基丁二酰亚胺(N-Hydroxysuccinimide, NHS)和硫酸钠(Sodium sulfate)有关的化合物或混合物。
N-羟基丁二酰亚胺本身是一种常用于生化反应的交联剂,而硫酸钠则是一种常见的无机盐。
若要计算该化合物的相对摩尔质量,我们首先需要知道其确切的化学式。
然而,由于“n-羟基丁二酰亚胺硫酸钠”不是一个标准的化学名称,我们无法直接给出一个确切的相对摩尔质量。
不过,我们可以分别计算N-羟基丁二酰亚胺和硫酸钠的相对摩尔质量,以便给您一个大致的概念:N-羟基丁二酰亚胺(NHS): C4H5NO3相对摩尔质量= 12.011 (C) × 4 + 1.008 (H) × 5 + 14.007 (N) ×1 + 15.999 (O) ×3 = 115.09 g/mol硫酸钠(Na2SO4): Na2SO4相对摩尔质量= 22.990 (Na) × 2 + 32.065 (S) × 1 + 15.999 (O) ×4 = 142.04 g/mol如果您所指的“n-羟基丁二酰亚胺硫酸钠”是某种形式的结合物或复合物,其相对摩尔质量将取决于这两种化合物在其中的比例和结合方式。
这通常需要更详细的化学式或结构信息才能准确计算。
请注意,以上计算是基于各元素的原子量近似值,实际值可能会有轻微差异。
同时,由于“n-羟基丁二酰亚胺硫酸钠”并非一个标准的化学名称,这里给出的信息仅供参考。
如需更准确的信息,请提供该化合物的确切化学式或更多背景信息。
脱氧胆酸钠

脱氧胆酸钠
中文名称: 脱氧胆酸钠
分子式: C24H40O4·Na
分子量: 414.56
英文名称:3-alpha,12-alpha-dihydroxy-5-beta-cholan-24-oic aci sodium salt
性状:白色结晶性粉末。
类似胆汁气味。
有强烈苦味。
易吸湿。
易溶于水,微溶于无水醇,不溶于醚。
比旋光度约+42.5º(c=2,水中)。
低毒,半数致死量(大鼠,经口)1370mg/kg。
有刺激性。
脱氧胆酸钠
质量标准:
项目生物试剂级
砷Arsenic(%) ≤0.001
重金属Heavy Metals(%) ≤0.005
干燥失重Loss on Drying(%) ≤5.0
纯度Purity (Dry Basis)(%) >99.0
胆酸钠Sodium Cholate(%) ≤2.0
可溶性试验Solubility (10%, Water)(P/F) 合格
贮存室温保存
用途:配制细菌培养基,代替脑磷脂作胆固醇絮状试验,蛋白质分析。
裂解液中脱氧胆酸钠和原矾酸钠的作用
前者是离子型去垢剂,后者是抑制酪氨酸磷酸酶活性的。
离子型去垢剂主要作用有:1、裂解细胞;2、溶解蛋白,尤其是可以溶解一些难溶于水的蛋白,如膜蛋白等;3、很适合做WB,但在Co-IP中使用,需要谨慎。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Inhibitors, Agonists, Screening Libraries
Data Sheet
BIOLOGICAL ACTIVITY:
Monensin sodium salt is an antibiotic secreted by the bacteria Streptomyces cinnamonensis .
IC50 & Target: bacterial [1]
In Vitro: Monensin sodium salt is an antibiotic secreted by the bacteria Streptomyces cinnamonensis . Untreated cells display 2.5%apoptosis; 48 hours treatment with 1 μM Monensin sodium salt shows 4.5% apoptosis whereas 5 μM Monensin sodium salt for 48hours induces a greater apoptotic response (16.4%). Pretreatment with either 1 or 5 μM Monensin sodium salt for 24 hours followed by 10 μM erlotinib treatment for another 24 hours results in a marked increases in apoptotic events (14.6% and 38.7%, respectively)when compare with either Monensin sodium salt or erlotinib treatments alone. Combination of 5 μM Monensin sodium salt with 10μM erlotinib shows the highest percentage of apoptosis (38.7%)[1].
In Vivo: Although the numbers of tumors do not change substantially, a significant (P=0.0144) reduction in the average size of lesions is observed in Monensin sodium salt-treated Apc +/Min mice when compare with control animals (mean 0.199 mm 2 vs. 0.299 mm 2).The total tumor area estimated in one animal is decreased in individuals receiving Monensin sodium salt (mean 10.16 mm 2 vs. 16.46mm 2; P=0.0125). Monensin sodium salt treatment increases the numbers of apoptotic cells and cells expressing the p21 cell-cycle inhibitor at the surface area of the neoplastic outgrowths. No changes in the cell proliferation, differentiation, and tissue architecture
in the healthy parts of mucosa are noted after exposure to Monensin sodium salt [2].PROTOCOL (Extracted from published papers and Only for reference)
Cell Assay:[1]One million SCC25 cells are seeded in 10-cm plates and incubated overnight to allow for attachment and recovery. The following day, cells are pretreated with 0, 1, or 5 μM Monensin sodium salt for 24 hours then treated with 10 μM erlotinib alone or in combination with Monensin sodium salt for a further 24 hours. Adherent and cells in suspension are collected by centrifugation and fixed in 3 mL of cold 80% ethanol overnight at -20°C. Before analysis, cell pellets are washed with PBS resuspended in staining buffer containing 25 μg/mL propidium iodide and 40 μg/mL RNase A and incubated for a minimum of 1 hour in the dark at room
temperature [1]. Animal Administration:[2]Multiple intestinal neoplasia (Min) mice are used in this study. Four-week-old pups are
weaned, genotyped, and randomized. The animals are divided into two groups and treated with Monensin sodium salt (10 mg/kg ) or vehicle (DMSO). Daily oral applications continue for 6 weeks. In addition, six pairs of Apc +/Min mice age 7, 10, 13, 16, 19, and 22 weeks
are treated with Monensin sodium salt or vehicle for 5 weeks. The mice are sacrificed and the intestines are dissected, washed in PBS,and fixed in 4% formaldehyde (v/v) in PBS for 3 days. Fixed intestines are embedded in paraffin, sectioned and stained. The number and size of the neoplastic lesions are quantified using Ellipse software [2].References:
[1]. Dayekh K, et al. Monensin inhibits epidermal growth factor receptor trafficking and activation: synergistic cytotoxicity in combination with EGFR
Product Name:
Monensin sodium salt Cat. No.:
HY-N0150CAS No.:
22373-78-0Molecular Formula:
C 36H 61NaO 11Molecular Weight:
692.85Target:
Bacterial Pathway:
Anti-infection Solubility:
10 mM in Ethanol
inhibitors. Mol Cancer Ther. 2014 Nov;13(11):2559-71.
[2]. Tumova L, et al. Monensin inhibits canonical Wnt signaling in human colorectal cancer cells and suppresses tumor growth in multiple intestinal neoplasia mice. Mol Cancer Ther. 2014 Apr;13(4):812-22.
Caution: Product has not been fully validated for medical applications. For research use only.
Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@
Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
