Anisomycin_DataSheet_MedChemExpress
Ionomycin-SDS-MedChemExpress

Inhibitors, Agonists, Screening LibrariesSafety Data Sheet Revision Date:Sep.-19-2018Print Date:Sep.-19-20181. PRODUCT AND COMPANY IDENTIFICATION1.1 Product identifierProduct name :IonomycinCatalog No. :HY-13434CAS No. :56092-81-01.2 Relevant identified uses of the substance or mixture and uses advised againstIdentified uses :Laboratory chemicals, manufacture of substances.1.3 Details of the supplier of the safety data sheetCompany:MedChemExpress USATel:609-228-6898Fax:609-228-5909E-mail:sales@1.4 Emergency telephone numberEmergency Phone #:609-228-68982. HAZARDS IDENTIFICATION2.1 Classification of the substance or mixtureGHS Classification in accordance with 29 CFR 1910 (OSHA HCS)Acute toxicity, oral (Category 4),H3022.2 GHS Label elements, including precautionary statementsPictogramSignal word WarningHazard statement(s)H302 Harmful if swallowedPrecautionary statement(s)P264 Wash skin thoroughly after handlingP270 Do not eat, drink, or smoke when using this product.P301+P312 IF SWALLOWED: Call a POISON CENTER or doctor ⁄physician if you feel unwell.P330 Rinse mouth.P501 Dispose of contents ⁄container to an approved waste disposal plant.2.3 Other hazardsNone.3. COMPOSITION/INFORMATION ON INGREDIENTS3.1 SubstancesSynonyms:NoneFormula:C41H72O9Molecular Weight:709.01CAS No. :56092-81-04. FIRST AID MEASURES4.1 Description of first aid measuresEye contactRemove any contact lenses, locate eye-wash station, and flush eyes immediately with large amounts of water. Separate eyelids with fingers to ensure adequate flushing. Promptly call a physician.Skin contactRinse skin thoroughly with large amounts of water. Remove contaminated clothing and shoes and call a physician.InhalationImmediately relocate self or casualty to fresh air. If breathing is difficult, give cardiopulmonary resuscitation (CPR). Avoid mouth-to-mouth resuscitation.IngestionWash out mouth with water; Do NOT induce vomiting; call a physician.4.2 Most important symptoms and effects, both acute and delayedThe most important known symptoms and effects are described in the labelling (see section 2.2).4.3 Indication of any immediate medical attention and special treatment neededTreat symptomatically.5. FIRE FIGHTING MEASURES5.1 Extinguishing mediaSuitable extinguishing mediaUse water spray, dry chemical, foam, and carbon dioxide fire extinguisher.5.2 Special hazards arising from the substance or mixtureDuring combustion, may emit irritant fumes.5.3 Advice for firefightersWear self-contained breathing apparatus and protective clothing.6. ACCIDENTAL RELEASE MEASURES6.1 Personal precautions, protective equipment and emergency proceduresUse full personal protective equipment. Avoid breathing vapors, mist, dust or gas. Ensure adequate ventilation. Evacuate personnel to safe areas.Refer to protective measures listed in sections 8.6.2 Environmental precautionsTry to prevent further leakage or spillage. Keep the product away from drains or water courses.6.3 Methods and materials for containment and cleaning upAbsorb solutions with finely-powdered liquid-binding material (diatomite, universal binders); Decontaminate surfaces and equipment by scrubbing with alcohol; Dispose of contaminated material according to Section 13.7. HANDLING AND STORAGE7.1 Precautions for safe handlingAvoid inhalation, contact with eyes and skin. Avoid dust and aerosol formation. Use only in areas with appropriate exhaust ventilation.7.2 Conditions for safe storage, including any incompatibilitiesKeep container tightly sealed in cool, well-ventilated area. Keep away from direct sunlight and sources of ignition.Recommended storage temperature:Powder-20°C 3 yearsIn solvent-80°C 6 months-20°C 1 monthShipping at room temperature if less than 2 weeks.7.3 Specific end use(s)No data available.8. EXPOSURE CONTROLS/PERSONAL PROTECTION8.1 Control parametersComponents with workplace control parametersThis product contains no substances with occupational exposure limit values.8.2 Exposure controlsEngineering controlsEnsure adequate ventilation. Provide accessible safety shower and eye wash station.Personal protective equipmentEye protection Safety goggles with side-shields.Hand protection Protective gloves.Skin and body protection Impervious clothing.Respiratory protection Suitable respirator.Environmental exposure controls Keep the product away from drains, water courses or the soil. Cleanspillages in a safe way as soon as possible.9. PHYSICAL AND CHEMICAL PROPERTIES9.1 Information on basic physical and chemical propertiesAppearance Colorless to light yellow (Ethanol Solution)Odor No data availableOdor threshold No data availablepH No data availableMelting/freezing point No data availableBoiling point/range No data availableFlash point No data availableEvaporation rate No data availableFlammability (solid, gas)No data availableUpper/lower flammability or explosive limits No data availableVapor pressure No data availableVapor density No data availableRelative density No data availableWater Solubility No data availablePartition coefficient No data availableAuto-ignition temperature No data availableDecomposition temperature No data availableViscosity No data availableExplosive properties No data availableOxidizing properties No data available9.2 Other safety informationNo data available.10. STABILITY AND REACTIVITY10.1 ReactivityNo data available.10.2 Chemical stabilityStable under recommended storage conditions.10.3 Possibility of hazardous reactionsNo data available.10.4 Conditions to avoidNo data available.10.5 Incompatible materialsStrong acids/alkalis, strong oxidising/reducing agents.10.6 Hazardous decomposition productsUnder fire conditions, may decompose and emit toxic fumes.Other decomposition products - no data available.11.TOXICOLOGICAL INFORMATION11.1 Information on toxicological effectsAcute toxicityClassified based on available data. For more details, see section 2Skin corrosion/irritationClassified based on available data. For more details, see section 2Serious eye damage/irritationClassified based on available data. For more details, see section 2Respiratory or skin sensitizationClassified based on available data. For more details, see section 2Germ cell mutagenicityClassified based on available data. For more details, see section 2CarcinogenicityIARC: No component of this product present at a level equal to or greater than 0.1% is identified as probable, possible or confirmed human carcinogen by IARC.ACGIH: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by ACGIH.NTP: No component of this product present at a level equal to or greater than 0.1% is identified as a anticipated or confirmed carcinogen by NTP.OSHA: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by OSHA.Reproductive toxicityClassified based on available data. For more details, see section 2Specific target organ toxicity - single exposureClassified based on available data. For more details, see section 2Specific target organ toxicity - repeated exposureClassified based on available data. For more details, see section 2Aspiration hazardClassified based on available data. For more details, see section 2Additional informationRTECS No.: NO600000This information is based on our current knowledge. However the chemical, physical, and toxicological properties have not been completely investigated.12. ECOLOGICAL INFORMATION12.1 ToxicityNo data available.12.2 Persistence and degradabilityNo data available.12.3 Bioaccumlative potentialNo data available.12.4 Mobility in soilNo data available.12.5 Results of PBT and vPvB assessmentPBT/vPvB assessment unavailable as chemical safety assessment not required or not conducted.12.6 Other adverse effectsNo data available.13. DISPOSAL CONSIDERATIONS13.1 Waste treatment methodsProductDispose substance in accordance with prevailing country, federal, state and local regulations.Contaminated packagingConduct recycling or disposal in accordance with prevailing country, federal, state and local regulations.14. TRANSPORT INFORMATIONDOT (US)This substance is considered to be non-hazardous for transport.IMDGThis substance is considered to be non-hazardous for transport.IATAThis substance is considered to be non-hazardous for transport.15. REGULATORY INFORMATIONSARA 302 Components:SARA 302: No chemical in this material are subject to the reporting requirements of SARA Title III, Section 302.SARA 313 Components:SARA 313: This material does not contain any chemical components with known CAS numbers that exceed the threshold (De Minimis) reporting levels established by SARA Title II, Section 313.SARA 311/312 Hazards:Ethanol: Fire Hazard, Acute Health Hazard, Chronic Health Hazard16. OTHER INFORMATIONCopyright 2018 MedChemExpress. The above information is correct to the best of our present knowledge but does not purport to be all inclusive and should be used only as a guide. The product is for research use only and for experienced personnel. It mustonly be handled by suitably qualified experienced scientists in appropriately equipped and authorized facilities. The burden of safe use of this material rests entirely with the user. MedChemExpress disclaims all liability for any damage resulting from handling or from contact with this product.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
Tasquinimod_DataSheet_MedChemExpress

Inhibitors, Agonists, Screening Libraries Data SheetBIOLOGICAL ACTIVITY:Tasquinimod is an allosteric modulator of HDAC4 with IC 50 of 0.5 μM in 3D–HUVEC sprouting assay.IC50 & Target: HDAC4[1][2]In Vitro: Tasquinimod suppresses hypoxia induced decrease in histone acetylation without lowering HDAC expression or directly inhibiting HDAC activity. Tasquinimod binds allosterically within the regulatory Zinc domain of HDAC4[1]. Tasquinimod, an orallyactive quinoline–3–carboxamide, binds with high affinity to HDAC4 and S100A9 in cancer and infiltrating host cells within compromised tumor microenvironment inhibiting adaptive survival pathways needed for an angiogenic response [2]. At 10 μM Tasquinimod, the TSP1 mRNA expression is elevated at 6 h and peaked after 72 h. Moreover, after 72 h exposure the TSP1 mRNA levels is already elevated at a dose of 1 μM Tasquinimod, indicating that Tasquinimod–induced changes in TSP1 mRNA expression occurrs in a dose range. At higher dose levels (i.e. 50–100 μM) the mRNA levels decline at 72 h, indicating additional drug effects at these concentrations. The up–regulation of TSP1 mRNA in LNCaP cells by Tasquinimod is manifested by an increased expression of TSP1 protein, as shown by western blot analysis of cell lysates prepared from cells cultured for 24 h and 72 h. Accompanied by increased intracellular TSP1 protein levels are also a statistically significant (p<0.05) accumulation of extracellular TSP1 in the cell culture medium detected. The extracellular secretion of TSP1 is time dependent and could clearly be detected after 24 h exposure to Tasquinimod at 10 μM. Also, TSP1 mRNA levels are induced by Tasquinimod at 10 μM in the hormone insensitive cell line LNCaP19 but not in DU145 cells [3].In Vivo: The bioavailability and oral absorption of Tasquinimod is excellent when adult male mice (i.e., C57Bl/6J, or athymic nude mice) are given 0.1–30 mg/kg (i.e., 0.2–74 μmoles/kg) via gavage or the drinking water. The potency of Tasquinimod expressed as the daily oral dose of Tasquinimod which inhibits cancer growth by 50% ranges from 0.1–1.0 mg/kg/d (i.e., 0.24–2.40μmoles/kg/day) against a series (n>5) of human prostate cancer xenografts in immune–deficient mice. Tasquinimod at a chronic dose of 5 mg/kg/day via the drinking water produces > 80% inhibition (p<0.05) of TRAMP–C2 mouse prostate cancer growth in immune–competent syngeneic mice [2]. Nude mice carrying subcutaneous LNCaP tumors are treated with Tasquinimod for 3 weeks.Exposure to Tasquinimod at 1 mg/kg/day and 10 mg/kg/day started on day 7 after inoculation. There is a statistically significant dose dependent reduction in tumor weight both at 1 mg/kg/day and 10 mg/kg/day compare to the untreated control group 28days after inoculation (p<0.001), illustrating the anti–tumor effect of Tasquinimod [3].PROTOCOL (Extracted from published papers and Only for reference)Cell Assay: Tasquinimod is dissolved in DMSO and stored, and then diluted with appropriate media before use [3].[3]Two human prostate cancer cell lines, CWR–22RH and LNCaP (ATCC) are both androgen independent, but remain sensitive to androgen stimulation of growth, express PSA and a mutated androgen receptor. The hormone independent cell lines LNCaP19 and DU145are also tested for TSP1 induction after in vitro exposure to Tasquinimod (0.1–100 μM). CWR–22RH, LNCaP and DU145 are grown in RPMI Medium 1640 containing 10% FCS and L–Glutamine mixture, while LNCAP19 is cultured in RPMI–medium with 10% hormoneProduct Name:Tasquinimod Cat. No.:HY-10528CAS No.:254964-60-8Molecular Formula:C 20H 17F 3N 2O 4Molecular Weight:406.36Target:HDAC; HDAC Pathway:Epigenetics; Cell Cycle/DNA Damage Solubility:DMSO: ≥ 42 mg/mLfree (RDCC) FCS[3].Animal Administration: Tasquinimod is prepared in drinking water (Mice)[3].[3]Mice[3]Nude BALB/c mice are used for subcutaneous implantation of human prostate tumor cells LNCaP and CWR–22RH. Tumor growth is measured with a microcaliper twice a week throughout the experiment, and the final tumor burden is measured by weight on the day of termination of the experiment. Distribution of Tasquinimod at 1 mg/kg/day and 10 mg/kg/day (administered orally via the drinking water) started on day 7 after inoculation.References:[1]. Isaacs JT, et al. Tasquinimod Is an Allosteric Modulator of HDAC4 survival signaling within the compromised cancer microenvironment. Cancer Res. 2013 Feb 15;73(4):1386–99.[2]. Isaacs JT, et al. Anti–cancer potency of tasquinimod is enhanced via albumin–binding facilitating increased uptake in the tumor microenvironment. Oncotarget. 2014 Sep 30;5(18):8093–106.[3]. Olsson A, et al. Tasquinimod (ABR–215050), a quinoline–3–carboxamide anti–angiogenic agent, modulates the expression of thrombospondin–1 in human prostate tumors. Mol Cancer. 2010 May 17;9:107.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
Silibinin_SDS_MedChemExpress

Inhibitors, Agonists, Screening LibrariesSafety Data Sheet Revision Date:Jul.-07-2017Print Date:Jul.-07-20171. PRODUCT AND COMPANY IDENTIFICATION1.1 Product identifierProduct name :SilibininCatalog No. :HY-13748CAS No. :22888-70-61.2 Relevant identified uses of the substance or mixture and uses advised againstIdentified uses :Laboratory chemicals, manufacture of substances.1.3 Details of the supplier of the safety data sheetCompany:MedChemExpress USATel:609-228-6898Fax:609-228-5909E-mail:sales@1.4 Emergency telephone numberEmergency Phone #:609-228-68982. HAZARDS IDENTIFICATION2.1 Classification of the substance or mixtureGHS Classification in accordance with 29 CFR 1910 (OSHA HCS)Acute toxicity, Oral (Category 4),H302Acute aquatic toxicity (Category 1),H400Chronic aquatic toxicity (Category 1),H4102.2 GHS Label elements, including precautionary statementsPictogramSignal word WarningHazard statement(s)H302 Harmful if swallowed.H410 Very toxic to aquatic life with long lasting effects.Precautionary statement(s)P264 Wash skin thoroughly after handling.P270 Do not eat, drink or smoke when using this product.P273 Avoid release to the environment.P301 + P312 IF SWALLOWED: Call a POISON CENTER or doctor/ physician if you feel unwell.P330 Rinse mouth.P391 Collect spillage.P501 Dispose of contents/ container to an approved waste disposal plant.2.3 Other hazardsNone.3. COMPOSITION/INFORMATION ON INGREDIENTS3.1 SubstancesSynonyms:Silybin; Silibinin A; Silymarin IFormula:C25H22O10Molecular Weight:482.44CAS No. :22888-70-64. FIRST AID MEASURES4.1 Description of first aid measuresEye contactRemove any contact lenses, locate eye-wash station, and flush eyes immediately with large amounts of water. Separate eyelids with fingers to ensure adequate flushing. Promptly call a physician.Skin contactRinse skin thoroughly with large amounts of water. Remove contaminated clothing and shoes and call a physician.InhalationImmediately relocate self or casualty to fresh air. If breathing is difficult, give cardiopulmonary resuscitation (CPR). Avoid mouth-to-mouth resuscitation.IngestionWash out mouth with water; Do NOT induce vomiting; call a physician.4.2 Most important symptoms and effects, both acute and delayedThe most important known symptoms and effects are described in the labelling (see section 2.2).4.3 Indication of any immediate medical attention and special treatment neededTreat symptomatically.5. FIRE FIGHTING MEASURES5.1 Extinguishing mediaSuitable extinguishing mediaUse water spray, dry chemical, foam, and carbon dioxide fire extinguisher.5.2 Special hazards arising from the substance or mixtureDuring combustion, may emit irritant fumes.5.3 Advice for firefightersWear self-contained breathing apparatus and protective clothing.6. ACCIDENTAL RELEASE MEASURES6.1 Personal precautions, protective equipment and emergency proceduresUse full personal protective equipment. Avoid breathing vapors, mist, dust or gas. Ensure adequate ventilation. Evacuate personnel to safe areas.Refer to protective measures listed in sections 8.6.2 Environmental precautionsTry to prevent further leakage or spillage. Keep the product away from drains or water courses.6.3 Methods and materials for containment and cleaning upAbsorb solutions with finely-powdered liquid-binding material (diatomite, universal binders); Decontaminate surfaces and equipment by scrubbing with alcohol; Dispose of contaminated material according to Section 13.7. HANDLING AND STORAGE7.1 Precautions for safe handlingAvoid inhalation, contact with eyes and skin. Avoid dust and aerosol formation. Use only in areas with appropriate exhaust ventilation.7.2 Conditions for safe storage, including any incompatibilitiesKeep container tightly sealed in cool, well-ventilated area. Keep away from direct sunlight and sources of ignition.Recommended storage temperature:Powder-20°C 3 years4°C 2 yearsIn solvent-80°C 6 months-20°C 1 monthShipping at room temperature if less than 2 weeks.7.3 Specific end use(s)No data available.8. EXPOSURE CONTROLS/PERSONAL PROTECTION8.1 Control parametersComponents with workplace control parametersThis product contains no substances with occupational exposure limit values.8.2 Exposure controlsEngineering controlsEnsure adequate ventilation. Provide accessible safety shower and eye wash station.Personal protective equipmentEye protection Safety goggles with side-shields.Hand protection Protective gloves.Skin and body protection Impervious clothing.Respiratory protection Suitable respirator.Environmental exposure controls Keep the product away from drains, water courses or the soil. Cleanspillages in a safe way as soon as possible.9. PHYSICAL AND CHEMICAL PROPERTIES9.1 Information on basic physical and chemical propertiesAppearance White to off-white (Solid)Odor No data availableOdor threshold No data availablepH No data availableMelting/freezing point No data availableBoiling point/range No data availableFlash point No data availableEvaporation rate No data availableFlammability (solid, gas)No data availableUpper/lower flammability or explosive limits No data availableVapor pressure No data availableVapor density No data availableRelative density No data availableWater Solubility No data availablePartition coefficient No data availableAuto-ignition temperature No data availableDecomposition temperature No data availableViscosity No data availableExplosive properties No data availableOxidizing properties No data available9.2 Other safety informationNo data available.10. STABILITY AND REACTIVITY10.1 ReactivityNo data available.10.2 Chemical stabilityStable under recommended storage conditions.10.3 Possibility of hazardous reactionsNo data available.10.4 Conditions to avoidNo data available.10.5 Incompatible materialsStrong acids/alkalis, strong oxidising/reducing agents.10.6 Hazardous decomposition productsUnder fire conditions, may decompose and emit toxic fumes.Other decomposition products - no data available.11.TOXICOLOGICAL INFORMATION11.1 Information on toxicological effectsAcute toxicityClassified based on available data. For more details, see section 2Skin corrosion/irritationClassified based on available data. For more details, see section 2Serious eye damage/irritationClassified based on available data. For more details, see section 2Respiratory or skin sensitizationClassified based on available data. For more details, see section 2Germ cell mutagenicityClassified based on available data. For more details, see section 2CarcinogenicityIARC: No component of this product present at a level equal to or greater than 0.1% is identified as probable, possible or confirmed human carcinogen by IARC.ACGIH: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by ACGIH.NTP: No component of this product present at a level equal to or greater than 0.1% is identified as a anticipated or confirmed carcinogen by NTP.OSHA: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by OSHA.Reproductive toxicityClassified based on available data. For more details, see section 2Specific target organ toxicity - single exposureClassified based on available data. For more details, see section 2Specific target organ toxicity - repeated exposureClassified based on available data. For more details, see section 2Aspiration hazardClassified based on available data. For more details, see section 212. ECOLOGICAL INFORMATION12.1 ToxicityNo data available.12.2 Persistence and degradabilityNo data available.12.3 Bioaccumlative potentialNo data available.12.4 Mobility in soilNo data available.12.5 Results of PBT and vPvB assessmentPBT/vPvB assessment unavailable as chemical safety assessment not required or not conducted.12.6 Other adverse effectsNo data available.13. DISPOSAL CONSIDERATIONS13.1 Waste treatment methodsProductDispose substance in accordance with prevailing country, federal, state and local regulations.Contaminated packagingConduct recycling or disposal in accordance with prevailing country, federal, state and local regulations.14. TRANSPORT INFORMATIONDOT (US)This substance is considered to be non-hazardous for transportIMDGUN number: 3077Class: 9Packing group: IIIEMS-No: F-A, S-FProper shipping name: ENVIRONMENTALLY HAZARDOUS SUBSTANCE, SOLID, N.O.S.Marine pollutant: Marine pollutantIATAUN number: 3077Class: 9Packing group: IIIProper shipping name: Environmentally hazardous substance, solid, n.o.s.15. REGULATORY INFORMATIONSARA 302 Components:No chemicals in this material are subject to the reporting requirements of SARA Title III, Section 302.SARA 313 Components:This material does not contain any chemical components with known CAS numbers that exceed the threshold (De Minimis) reporting levels established by SARA Title III, Section 313.SARA 311/312 Hazards:No SARA Hazards.Massachusetts Right To Know Components:No components are subject to the Massachusetts Right to Know Act.Pennsylvania Right To Know Components:No components are subject to the Pennsylvania Right to Know Act.New Jersey Right To Know Components:No components are subject to the New Jersey Right to Know Act.California Prop. 65 Components:This product does not contain any chemicals known to State of California to cause cancer, birth defects, or anyother reproductive harm.16. OTHER INFORMATIONCopyright 2017 MedChemExpress. The above information is correct to the best of our present knowledge but does not purport to be all inclusive and should be used only as a guide. The product is for research use only and for experienced personnel. It must only be handled by suitably qualified experienced scientists in appropriately equipped and authorized facilities. The burden of safe use of this material rests entirely with the user. MedChemExpress disclaims all liability for any damage resulting from handling or from contact with this product.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
Odanacatib_603139-19-1_DataSheet_MedChemExpress

Product Name:Odanacatib CAS No.:603139-19-1Cat. No.:HY-10042Product Data SheetMWt:525.56Formula:C25H27F4N3O3S Purity :>98%Solubility:DMSO ≥102mg/mL Watery Mechanisms:Biological Activity:Odanacatib (MK 0822)is a potent selective and neutral inhibitor of cathepsin K (human/rabbit)withPathways:Metabolism/Protease; Target:Cathepsin g <1.2mg/mL Ethanol ≥2.9mg/mLOdanacatib (MK 0822) is a potent, selective, and neutral inhibitor of cathepsin K (human/rabbit) with IC50 of 0.2 nM/1 nM, and demonstrated high selectivity versus off-target cathepsin B, L, S.IC50 value: 0.2 nM/1 nM(human/rabbi cathepsin K)in vitro: In vitro, Odanacatib shows the high inhibitory activity and selectivity on cathepsin K with IC50 values of 0.2 nM and 1 nM for human cathepsin K and rabbit cathepsin K, respectively.Furthermore, Odanacatib also shows similar potencies in whole human cell enzyme occupancy assays with corrected IC50 of 5 nM. A recent study shows that Odanacatib results in reduction ofOsteoclast (OC) resorption activity by interrupting intracellular vesicular trafficking. in vivo: n preclinical rats, Odanacatib (10 mg/kg) exhibits excellent pharmacokinetics with clearance References:[1]. Jacques Yves Gauthier, Nathalie ChauretThe discovery of odanacatib (MK-0822), a selective inhibitor of cathepsin K Bioorganic & Medicinal Chemistry Letters Volume 18, Issue 3, 1 February2008, Pages 923-928p (g g)p(Cl: 2 mL kg-1 min-1), low volume of distribution (Vdss: 1.1 L kg...g [2]. Ng KW.Potential role of odanacatib in the treatment of osteoporosis.Clin Interv Aging.2012;7:235-47. Epub 2012 Jul 12.[3]. Langdahl B, Binkley N, Bone H, Gilchrist N, Resch H, Portales JR, Denker A, Lombardi A, De Tilleghem CL, Dasilva C, Rosenberg E, Leung A.Odanacatib in the treatment of postmenopausal women with low bone mineral density: 5 years of continued therapy in a phase 2 study.J Bone MinerRes. 2012 Jul 6.[4]. Khosla S.Odanacatib: location and timing are everything.J Bone Miner Res. 2012Mar;27(3):506-8.[5]S L F i S S h t MS B idi S W lf EJ Q tit ti d t i ti f d tib i Caution: Not fully tested. For research purposes onlyMedchemexpress LLC[5]. Sun L, Forni S, Schwartz MS, Breidinger S, Woolf EJ.Quantitative determination of odanacatib in human plasma using liqui...18W i l k i n s o n W a y , P r i n c e t o n , N J 08540,U S AE m a i l : i n f o @m e d c h e m e x p r e s s .c o m W e b : w w w .m e d c h e m e x p r e s s .c o m。
VX-745_DataSheet_MedChemExpress

Inhibitors, Agonists, Screening Libraries Data SheetBIOLOGICAL ACTIVITY:VX–745 is a potent and selective inhibitor of p38α, and possesses anti–inflammatory activity.In Vitro: VX–745 exhibits PBMC IL–1β and TNFα IC 50 values of 45 and 51 nM, respectively. VX–745 is also effective in whole blood,blocking IL–1β and TNFα release with IC 50 values of 150 and 180 nM, respectively. VX–745 shows a promising selectivity profile,with 20–fold selectivity for p38α over p38β (K i =220 nM)[1]. VX–745 solutions in DMSO/DMEM inhibits the IL–6 production with IC 50of 15±9 nM [2]. VX–745 (5.0 nM) displays potent activity and 1000–fold selectivity over closely related kinases, including ERK1,JNK1–3 and MK2. VX–745 (10 nM–50 μM) increasingly inhibits the anisomycin–induced activity of p38α[3]. VX–745 (0.06 μM–20 μM)inhibits IL–6 and VEGF secretion in BMSCs. VX–745 can inhibit cytokine (TNF–α, IL–6, VEGF)–induced paracrine MM cell growth,survival, and drug resistance in the BM microenvironment. VX–745 induces modest growth inhibition of MM.1S, RPMI8226, and U266 cell lines in a dose–dependent fashion, with inhibitory concentration of 50% (IC 50) of 10 μM [4].In Vivo: VX–745 (2.5, 5, and 10 mg/kg) improves the inflammatory scores in mice by 27%, 31%, and 44%, respectively [1]. VX–745(1.06 mg/kg) significantly decreases the inflammation score from 2.07±0.29 for the control group to 1.42±0.06[2].PROTOCOL (Extracted from published papers and Only for reference)Kinase Assay:[1]VX–745 inhibits p38α and p38β. The IC 50 for the inhibition of these two p38 homologs are obtained by aspectrophotometric coupled–enzyme assay. A fixed concentration of enzyme (15 nM of p38α or p38β) is incubated with various concentrations of VX–745 in DMSO for 10 min. at 30°C in 0.1 M HEPES buffer, pH 7.5, containing 10% glycerol, 10 mM MgCl 2, 2.5mM phosphoenolpyruvate, 200 μM NADH, 150 μg/mL pyruvate kinase, 50 μg/mL lactate dehydrogenase, and 200 μM EGF receptor peptide. The reaction is initiated with 100 μM and 70 μM ATP for p38α and p38β assays, respectively. The decrease of absorbance at 340 nm is monitored to follow the rate of the reaction. IC 50 is evaluated from the rate data as a function of the inhibitor concentration.Cell Assay: VX–745 is dissolved in DMSO.[2]For these experiments, cells are plated at a density of 60,000 cells/well in 96–well plates.Each condition is tested in triplicate or more. The following day, all particle suspensions, active substances or control solutions are freshly prepared, distributed and incubated for 24 h at 37°C. Then, supernatants are carefully discarded. To perform the MTT test, 50μL of 0.1% MTT solution is added to each well for 3 h. Each well is then incubated for 1 h with 200 μL of dimethyl sulfoxide.Absorbance is measured at 595 nm. Reported results are expressed as the means±SD.Animal Administration: VX–745 is prepared in 100% propylene glycol.[1]Type II collagen–induced arthritis is established in male DBA/1mice with a minor modification. 10–Week old male DBA/1 mice are immunized by two intradermal injections within a 3 week interval using 100 μL of an emulsion consisting of a 1:1 (v/v) mixture of chick type II collagen (200 μg in 10 mM acetic acid) and complete Freund's adjuvant. Following the booster immunization, the mice are left untreated for 2–3 weeks, and are randomized into five treatment groups after they exhibit focal carpal (wrist) swelling (level 2 arthritic severity score) in both front paws. The five treatment groups are: 1: water control, 10 mL/kg, p.o., bid, (n=14); 2: 100% propylene glycol (PG) vehicle control, 10 mL/kg, p.o., bid, (n=8); 3:VX–745 in PG, at 10 mg/kg, p.o., bid, (n=7); 4: VX–745 in PG, at 5 mg/kg, p.o., bid, (n=10); and 5: VX–745 in PG, at 2.5 mg/kg, p.o., bid,Product Name:VX–745Cat. No.:HY-10328CAS No.:209410-46-8Molecular Formula:C 19H 9Cl 2F 2N 3OS Molecular Weight:436.26Target:p38 MAPK Pathway:MAPK/ERK Pathway Solubility:DMSO: 13.08 mg/mL (Need ultrasonic)(n=11). Arthritic symptoms are scored every other day using a level 1 to level 5 scoring system. Paw inflammation begins with erythema at the wrist (level 1), progressing to focal swelling of the wrist (level 2), to complete swelling of the wrist (level 3), to complete swelling of wrist and palm (level 4), and finally to complete swelling of wrist, palm and fingers (level 5). The sums of the scores from both front paw scores are used for plotting disease progression curves. Mice are sacrificed on day 20 and paws are removed, sectioned sagitally, stained with hemotoxylin & eosin, and scored for inflammation. Histologically, wrist joint inflammation begins with an infiltration of the synovium into the joint space (level 1), progressing to joint cartilage erosion (level 2), to joint cartilage and bone erosion (level 3), and finally to erosion of cartilage and bone accompanied by pannus formation (level 4). References:[1]. Duffy JP, et al. The Discovery of VX–745: A Novel and Selective p38α Kinase Inhibitor. ACS Med Chem Lett. 2011 Jul 28;2(10):758–63.[2]. Pradal J, et al. Intra–articular bioactivity of a p38 MAPK inhibitor and development of an extended–release system. Eur J Pharm Biopharm. 2015 Jun; 93:110–7.[3]. Bagley MC, et al. Rapid synthesis of VX–745: p38 MAP kinase inhibition in Werner syndrome cells. Bioorg Med Chem Lett. 2007 Sep 15;17(18):5107–10. Epub 2007 Jul 13.[4]. Hideshima T, et al. Targeting p38 MAPK inhibits multiple myeloma cell growth in the bone marrow milieu. Blood, 2003, 101(2), 703–705.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
Geldanamycin_30562-34-6_DataSheet_MedChemExpress
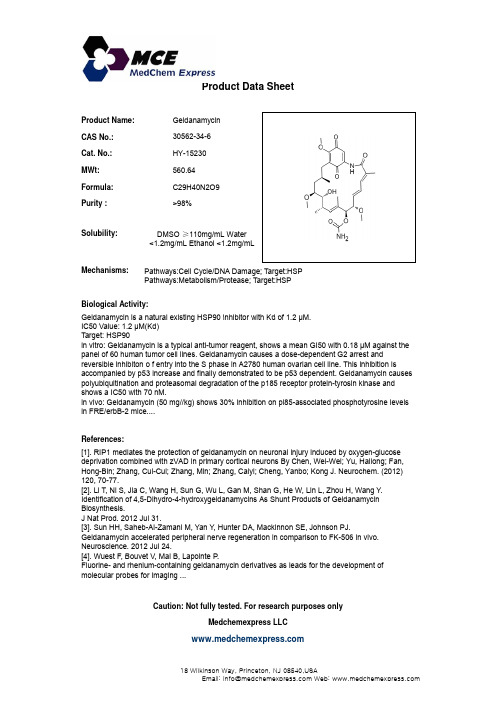
Product Name:Geldanamycin CAS No.:30562-34-6Cat. No.:HY-15230Product Data SheetMWt:560.64Formula:C29H40N2O9Purity :>98%Solubility:DMSO ≥110mg/mL Watery Mechanisms:Biological Activity:Geldanamycin is a natural existing HSP90inhibitor with Kd of 12MPathways:Cell Cycle/DNA Damage; Target:HSPPathways:Metabolism/Protease; Target:HSP g <1.2mg/mL Ethanol <1.2mg/mLGeldanamycin is a natural existing HSP90 inhibitor with Kd of 1.2 μM.IC50 Value: 1.2 μM(Kd)Target: HSP90in vitro: Geldanamycin is a typical anti-tumor reagent, shows a mean GI50 with 0.18 μM against the panel of 60 human tumor cell lines. Geldanamycin causes a dose-dependent G2 arrest and reversible inhibiton o f entry into the S phase in A2780 human ovarian cell line. This inhibition isaccompanied by p53 increase and finally demonstrated to be p53 dependent. Geldanamycin causes polyubiquitination and proteasomal degradation of the p185 receptor protein-tyrosin kinase and shows a IC50 with 70 nM. References:[1]. RIP1 mediates the protection of geldanamycin on neuronal injury induced by oxygen-glucose deprivation combined with zVAD in primary cortical neurons By Chen, Wei-Wei; Yu, Hailong; Fan,Hong-Bin; Zhang, Cui-Cui; Zhang, Min; Zhang, Caiyi; Cheng, Yanbo; Kong J. Neurochem. (2012)in vivo: Geldanamycin (50 mg//kg) shows 30% inhibition on pl85-associated phosphotyrosine levels in FRE/erbB-2 mice....120, 70-77. [2]. Li T, Ni S, Jia C, Wang H, Sun G, Wu L, Gan M, Shan G, He W, Lin L, Zhou H, Wang Y .Identification of 4,5-Dihydro-4-hydroxygeldanamycins As Shunt Products of GeldanamycinBiosynthesis.J Nat Prod. 2012 Jul 31.[3]. Sun HH, Saheb-Al-Zamani M, Yan Y , Hunter DA, Mackinnon SE, Johnson PJ. Geldanamycin accelerated peripheral nerve regeneration in comparison to FK-506 in vivo.Neuroscience. 2012 Jul 24.[4]W t F B t V M i B L i t P Caution: Not fully tested. For research purposes onlyMedchemexpress LLC[4]. Wuest F, Bouvet V, Mai B, Lapointe P.Fluorine- and rhenium-containing geldanamycin derivatives as leads for the development of molecular probes for imaging ...18W i l k i n s o n W a y , P r i n c e t o n , N J 08540,U S AE m a i l : i n f o @m e d c h e m e x p r e s s .c o m W e b : w w w .m e d c h e m e x p r e s s .c o m。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Inhibitors, Agonists, Screening Libraries
Data Sheet
BIOLOGICAL ACTIVITY:
Anisomycin is a pyrrolidine antibiotic, acts as an anti–fungal antibiotic which inhibits Protein Synthesis, also is a potent activator of SAPKs/JNKs.
Target: antibiotic
in vitro: Anisomycin inhibits EAC cell proliferation in concentration–dependent manner. [2] Anisomycin (3 μM) decreases protein synthesis in MDA16 and MDA–MB–468 cells, and reduces colony formation by MDA–MB–468 cells. Anisomycin causes an increase in the number of apoptotic cells in MDA–MB–468 cultures, but not in MDA16 cultures. Anisomycin actives JNK phosphorylation in MDA–MB–468 cells.[3]
in vivo: Peritumoral administration of anisomycin (5 mg/kg) significantly suppresses Ehrlich ascites carcinoma (EAC) growth resulting in the survival of approximately 60% of the mice 90 days after EAC inoculation. [2]
PROTOCOL (Extracted from published papers and Only for reference)
Cell assay [2]
For the assay, EAC cells are plated in 96–well plates at a density of 10,000 cells/well/200 μL of medium. The cells are treated with the different concentrations of Anisomycin for 48 h. Adriamycin (500 ng/mL) is used as a positive control. 0.5 mg/mL of MTT is added to each well. 4 h later, the formazan product of MTT reduction is dissolved in DMSO, and absorbance is measured at 570 nm using a Model 680 microplate reader.
Animal administrattion [1]
The 47 young, adult male Sprague Dawley rats weighing between 200 and 350 g. All rats were housed individually in 47 × 25 × 20.5cm polycarbonate cages for the duration of the experiment with a 12 h light/dark cycle. Anisomycin (100 μg/μl dissolved in 10 N HCl and brought to volume with PBS) was used for dorsal hippocampus infusions. On day 3 of training, animals were randomly assigned to one of the three conditions: Anisomycin (100 μg/μl dissolved in 10 N HCl and brought to volume with PBS), or a vehicle control group (PBS). Solutions were infused into both dorsal hippocampal hemispheres through 26 gauge stainless steel internal cannulae attached to a 10 μl Hamilton syringe with polyethylene tubing at a rate of 0.5 μl/min for 2 min (total infusion volume of 1 μl per hemisphere) using a double infusion pump. Internal infusion cannulae were left in the guide cannulae for one additional minute to allow for diffusion of the drug out of the cannulae. Infusions were performed on awake animals that were lightly restrained by an experimenter. Behavioral testing occurred 30 min following the start of the infusion. This corresponds to the time period of maximal neural suppression for Anisomycin.
References:
Product Name:
Anisomycin Cat. No.:
HY-18982CAS No.:
22862-76-6Molecular Formula:
C 14H 19NO 4Molecular Weight:
265.30Target:
Bacterial; JNK Pathway:
Anti–infection; MAPK/ERK Pathway Solubility:
10 mM in DMSO
[1]. Dubue JD, et al. Intrahippocampal Anisomycin Impairs Spatial Performance on the Morris Water Maze. J Neurosci. 2015 Aug 5;35(31):11118–11124.
[2]. You P, et al. In vitro and in vivo evaluation of anisomycin against Ehrlich ascites carcinoma. Oncol Rep. 2013, 29(6), 2227–2236.
[3]. Monaghan D, et al. Inhibition of protein synthesis and JNK activation are not required for cell death induced by anisomycin andanisomycin analogues. Biochem Biophys Res Commun. 2014 Jan 10;443(2):761–767.
Caution: Product has not been fully validated for medical applications. For research use only.
Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@
Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
