VRT752271_DataSheet_MedChemExpress
Genotoxicity A Standard Battery for Genotoxicity Testing

The European Agency for the Evaluation of Medicinal ProductsHuman Medicines Evaluation UnitICH - Technical Coordination - R. Bass 7 Westferry Circus, Canary Wharf, London E14 4HB, UK ICH Topic S 2 BGenotoxicity: A Standard Battery forGenotoxicity Testing of PharmaceuticalsStep 4, Consensus guideline, 16 July 1997NOTE FOR GUIDANCE ON GENOTOXICITY:A STANDARD BATTERY FOR GENOTOXICITY TESTINGOF PHARMACEUTICALS(CPMP/ICH/174/95)TRANSMISSION TO CPMPOctober 1996TRANSMISSION TO INTERESTED PARTIESOctober 1996COMMENTS REQUESTED BEFOREApril 1997FINAL APPROVAL BY CPMPSeptember 1997DATE FOR COMING INTO OPERATION March 1998GENOTOXICITY: A STANDARD BATTERY FORGENOTOXICITY TESTING OF PHARMACEUTICALS[ICH Harmonised Tripartite Guideline]TABLE OF CONTENTS1.INTRODUCTION (2)2.GENERAL PURPOSE OF GENOTOXICITY TESTING (2)3.THE STANDARD TEST BATTERY FOR GENOTOXICITY (2)4.MODIFICATIONS OF THE 3-TEST BATTERY (4)4.1Limitations to the use of bacterial test organisms (4)4.2Compounds bearing structural alerts for genotoxic activity (4)4.3Limitations to the use of standard in vivo tests (4)4.4Additional genotoxicity testing in relation to the carcinogenicity bioassay (5)4.4.1 Evidence for tumour response (5)4.4.2 Structurally unique chemical classes (5)5.STANDARD PROCEDURES FOR IN VITRO TESTS (5)6.NOTES (6)7.GLOSSARY (8)1.INTRODUCTIONTwo fundamental areas in which harmonisation of genotoxicity testing for pharmaceuticals is considered necessary are the scope of this guideline: i) Identification of a standard set of tests to be conducted for registration. ii) The extent of confirmatory experimentation in in vitro genotoxicity tests in the standard battery. Further issues that were considered necessary for harmonisation can be found in the ICH guideline "Specific Aspects of Regulatory Genotoxicity Tests for Pharmaceuticals" (ICH topic S2A). The two ICH guidelines on genotoxicity complement each other and therefore should be used together as ICH guidance principles for testing of a pharmaceutical for potential genotoxicity.2.GENERAL PURPOSE OF GENOTOXICITY TESTINGGenotoxicity tests can be defined as in vitro and in vivo tests designed to detect compounds which induce genetic damage directly or indirectly by various mechanisms. These tests should enable a hazard identification with respect to damage to DNA and its fixation. Fixation of damage to DNA in the form of gene mutations, larger scale chromosomal damage, recombination and numerical chromosome changes is generally considered to be essential for heritable effects and in the multi-step process of malignancy, a complex process in which genetic changes may play only a part. Compounds which are positive in tests that detect such kinds of damage have the potential to be human carcinogens and/or mutagens, i.e. may induce cancer and/or heritable defects. Because the relationship between exposure to particular chemicals and carcinogenesis is established for man, whilst a similar relationship has been difficult to prove for heritable diseases, genotoxicity tests have been used mainly for the prediction of carcinogenicity. Nevertheless, because germ line mutations are clearly associated with human disease, the suspicion that a compound may induce heritable effects is considered to be just as serious as the suspicion that a compound may induce cancer. In addition, the outcome of such tests may be valuable for the interpretation of carcinogenicity studies.3.THE STANDARD TEST BATTERY FOR GENOTOXICITYRegistration of pharmaceuticals requires a comprehensive assessment of their genotoxic potential. It is clear that no single test is capable of detecting all relevant genotoxic agents. Therefore, the usual approach should be to carry out a battery of in vitro and in vivo tests for genotoxicity. Such tests are complementary rather than representing different levels of hierarchy.The general features of a standard test battery can be outlined as follows:i)It is appropriate to assess genotoxicity in a bacterial reverse mutation test. This test hasbeen shown to detect relevant genetic changes and the majority of genotoxic rodent carcinogens.ii)DNA damage considered to be relevant for mammalian cells and not adequately measured in bacteria should be evaluated in mammalian cells. Several mammalian cell systems are in use: systems that detect gross chromosomal damage (in vitro tests forstructural and numerical chromosomal aberrations), systems that detect primarily gene mutations (see Note 1), and a system that detects gene mutations and clastogenic effects (mouse lymphoma tk assay) (see Note 2). The information given in Notes 3 and4 demonstrate that with appropriate test protocols (see Section 5) the various in vitrotests for chromosomal damage and the mouse lymphoma tk assay yield results with a high level of congruence for compounds that are regarded as genotoxic but yield negative results in the bacterial reverse mutation assay. Therefore, these systems are currently considered interchangeable when used together with other genotoxicity tests in a standard battery for genotoxicity testing of pharmaceuticals, if these test protocols are used.iii)An in vivo test for genetic damage should usually be a part of the test battery to provide a test model in which additional relevant factors (absorption, distribution metabolism, excretion) that may influence the genotoxic activity of a compound are included. As a result, in vivo tests permit the detection of some additional genotoxic agents (see Note 5). An in vivo test for chromosomal damage in rodent hematopoietic cells fulfills this need. This in vivo test for chromosomal damage in rodents could be either an analysis of chromosomal aberrations in bone marrow cells or an analysis of micronuclei in bone marrow or peripheral blood erythrocytes.The following standard test battery is recommended based upon the considerations mentioned above:i) A test for gene mutation in bacteria.ii)An in vitro test with cytogenetic evaluation of chromosomal damage with mammalian cells or an in vitro mouse lymphoma tk assay.iii)An in vivo test for chromosomal damage using rodent hematopoietic cells.For compounds giving negative results, the completion of this 3-test battery, performed and evaluated in accordance with current recommendations, will usually provide a sufficient level of safety to demonstrate the absence of genotoxic activity (see Note 6). Compounds giving positive results in the standard test battery may, depending on their therapeutic use, need to be tested more extensively (see ICH Guideline “Specific Aspects of Regulatory Genotoxicity Tests for Pharmaceuticals”).The suggested standard set of tests does not imply that other genotoxicity tests are generally considered as inadequate or inappropriate (e.g. tests for measurement of DNA adducts, DNA strand breaks, DNA repair or recombination). Such tests serve as options in addition to the standard battery for further investigation of genotoxicity test results obtained in the standard battery. Furthermore, molecular techniques to study mechanisms of genotoxicity in the standard battery systems may be useful for risk assessment. Only under extreme conditions in which one or more tests comprising the standard battery cannot be employed for technical reasons, alternative validated tests can serve as substitutes. For this to occur, sufficient scientific justification should be provided to support the argument that a given standard battery test is not appropriate.The standard battery does not include an independent test designed specifically to test for aneuploidy. However, information on this type of damage may be derived from the tests for chromosomal damage in vitro and in vivo. Elements of the standard protocols that provide such information are elevations in the mitotic index, polyploidy induction and micronucleus evaluation. There is also limited experimental evidence that aneuploidy inducers can be detected in the mouse lymphoma tk assay (see Note 4). In such cases, further testing may be needed.4.MODIFICATIONS OF THE 3-TEST BATTERYThe following sections give situations where the standard 3-test battery may need modification.4.1Limitations to the use of bacterial test organismsThere are circumstances where the performance of the bacterial reverse mutation test does not provide appropriate or sufficient information for the assessment of genotoxicity. This may be the case for compounds that are excessively toxic to bacteria (e.g. some antibiotics) and compounds thought or known to interfere with the mammalian cell replication system (e.g. topoisomerase inhibitors, nucleoside analogues, or inhibitors of DNA metabolism). For these cases, usually two in vitro mammalian cell tests should be performed using two different cell types and of two different endpoints [gene mutation (see Note 1) and chromosomal damage]. Nevertheless, it is still important to perform the bacterial reverse mutation test (see Note 7); either a full test or a limited (range-finding) test (see Section 5) may be appropriate.4.2Compounds bearing structural alerts for genotoxic activityStructurally alerting compounds (see Note 8) are usually detectable in the standard 3-test battery. However, compounds bearing structural alerts that have given negative results in the standard 3-test battery may necessitate limited additional testing. The choice of additional test(s) or protocol modification(s) depend on the chemical nature, the known reactivity and metabolism data on the structurally alerting compound under question (see Note 9 and ICH “Guidance on Specific Aspects of Regulatory Genotoxicity Tests for Pharmaceuticals”).4.3Limitations to the use of standard in vivo testsThere are compounds for which standard in vivo tests do not provide additional useful information. This includes compounds for which data from studies on toxicokinetics or pharmacokinetics indicate that they are not systemically absorbed and therefore are not available for the target tissues in standard in vivo genotoxicity tests. Examples of such compounds are some radioimaging agents, aluminum based antacids, and some dermally applied pharmaceuticals. In cases where a modification of the route of administration does not provide sufficient target tissue exposure, it may be appropriate to base the evaluation only on in vitro testing.4.4Additional genotoxicity testing in relation to the carcinogenicity bioassay4.4.1Evidence for tumour responseAdditional genotoxicity testing in appropriate models may be conducted for compounds that were negative in the standard 3-test battery but which have shown effects in carcinogenicity bioassay(s) with no clear evidence for a non-genotoxic mechanism. To help understand the mechanism of action, additional testing can include modified conditions for metabolic activation in in vitro tests or can include in vivo tests measuring genetic damage in target organs of tumour induction (e.g. liver UDS test, 32P-postlabelling, mutation induction in transgenes, molecular characterisation of genetic changes in tumour-related genes).4.4.2Structurally unique chemical classesOn rare occasions, a completely novel compound in a unique structural chemical class will be introduced as a pharmaceutical. When such a compound will not be tested in chronic rodent carcinogenicity bioassays, further genotoxicity evaluation may be invoked.5.STANDARD PROCEDURES FOR IN VITRO TESTSReproducibility of experimental results is an essential component of research involving novel methods or unexpected findings; however, the routine testing of chemicals with standard, widely used genotoxicity tests need not always be completely replicated. These tests are sufficiently well characterised and have sufficient internal controls that repetition can usually be avoided if protocols with built-in confirmatory elements, such as those outlined below, are used.For both bacterial and mammalian cell gene mutation tests, the results of a range-finding test can be used to guide the selection of concentrations to be used in the definitive mutagenicity test. By these means, a range-finding test may supply sufficient data to provide reassurance that the reported result is the correct one. In bacterial mutagenicity tests, preliminary range-finding tests performed on all bacterial strains, with and without metabolic activation, with appropriate positive and negative controls, and with quantification of mutants, may be considered a sufficient replication of a subsequent complete test. Similarly, a range-finding test may also be a satisfactory substitute for a complete repeat of a test in gene mutation tests with mammalian cells other than the mouse lymphoma tk assay (see below) if the range-finding test is performed with and without metabolic activation, with appropriate positive and negative controls, and with quantification of mutants (see Note 10).For the cytogenetic evaluation of chromosomal damage in vitro, the test protocol includes the conduct of tests with and without metabolic activation, with appropriate positive and negative controls, where the exposure to the test articles is 3 to 6 hours and a sampling time of approximately 1.5 normal cell cycles from the beginning of the treatment. A continuous treatment without metabolic activation up to the sampling time of approximately 1.5 normal cell cycles is needed in case of a negative result for the short treatment period without metabolic activation. Certain chemicals may be more readily detected by longer treatment or delayed sampling times, e.g. some nucleoside analogues or some nitrosamines. Negative results in the presence of a metabolic activation system may need confirmation on a case by case basis (see Note 11). In any case information on the ploidy status should be obtained byrecording the incidence of polyploid cells as a percentage of the number of metaphase cells. An elevated mitotic index or an increased incidence of polyploid cells may give an indication of the potential of a compound to induce aneuploidy. In such cases, further testing may be needed.For the mouse lymphoma tk assay, the test protocol includes the conduct of tests with and without metabolic activation, with appropriate positive and negative controls, where the exposure to the test articles is 3 to 4 hours. A continuous treatment without metabolic activation for approximately 24 hours is needed in case of a negative result for the short treatment without metabolic activation (see Note 4). Negative results in the presence of a metabolic activation system may need confirmation on a case by case basis (see Note 11). In any case, an acceptable mouse lymphoma tk assay includes (i) the incorporation of positive controls which induces mainly small colonies, (ii) colony sizing for positive controls, solvent controls and at least one positive test compound dose (should any exist), including the culture that gave the greatest mutant frequency.Following such testing, further confirmatory testing in the case of clearly negative or positive test results is not usually needed.Ideally it should be possible to declare test results as clearly negative or clearly positive. However, test results sometimes do not fit the predetermined criteria for a positive or negative call and therefore are declared “equivocal”. The application of statistical methods aids in data interpretation, however, adequate biological interpretation is of critical importance. Nonetheless, further testing is usually indicated for equivocal results.6.NOTES1)Test approaches currently accepted for the assessment of mammalian cell genemutation involve the tk locus using mouse lymphoma L5178Y cells or human lymphoblastoid TK6 cells, the hprt locus using CHO cells, V79 cells, or L5178Y cells, or the gpt locus using AS52 cells.2)The molecular dissection of mutants induced at the tk locus shows a broad range ofgenetic events including point mutations, deletions, translocations, recombinations etc.Small colony mutants have been shown to predominantly lack the tk b allele as a consequence of structural or numerical alterations or recombinational events. There is some evidence that other loci, such as hprt or gpt are also sensitive to large deletion events. However, due to the X-chromosomal origin of the hprt gene which is probably flanked by essential genes, large scale deletionevents or numerical alterations often do not give rise to mutant colonies, thus limiting the sensitivity of this genetic locus relative to the tk locus for the detection of a wide range of genetic changes.3)With respect to the cytogenetic evaluation of chromosomal damage, it is notuncommon for the systems currently in use, i.e. several systems with permanent mammalian cells in culture and human lymphocytes either isolated or in whole blood, to give different results for the same test compound. However, there is evidence that some of the differences observed have been due to protocol differences. This may be minimised by using the procedures described in Section 5.For the great majority of presumptive genotoxic compounds that were negative in a bacterial reverse mutation assay, the data on chromosomal damage in vitro and mouse lymphoma tk results are in agreement. Several reliable studies indicate that the mouse lymphoma tk assay is able to detect compounds that induce structural and numerical chromosomal damage. For safety testing of pharmaceuticals, the mouse lymphoma tk assay is considered an acceptable alternative to the direct analysis of chromosomal damage in vitro. Although colony sizing is an essential element of the mouse lymphoma tk assay test protocol, it gives only limited information on the type of damage induced in mutant colonies. Further mechanistic investigations may be used to assess the nature of cytogenetic changes induced by clastogens and aneuploidy inducers in the mouse lymphoma tk assay. Such information could be provided by studies to demonstrate the loss of the tk gene or the loss of the chromosome carrying the tk gene.4)The detection of a number of different nucleoside analogues and base analogues isenhanced for the mouse lymphoma tk assay when the treatment protocol for both agar and microtitre methods include a 24 hour treatment regimen in the absence of an exogenous metabolic activation system. Similarly, the detection of aneuploidy inducers is enhanced if a 24 hour treatment regimen is used with the microtitre method. Currently, there is no evidence to support this conclusion for the soft agar method. The specificity of the test protocol, i.e. to obtain correct test results for presumptive non-genotoxic compounds, does not change significantly using a 24 hour treatment in the microtitre method. For the soft agar method there appears to be a reduction in specificity under the same treatment regimen. Based on this information, the microtitre method is recommended for use in the standard battery.5)There are a small but significant number of genotoxic carcinogens that are reliablydetected by the bone marrow tests for chromosomal damage that have yielded negative/weak/conflicting results in the pairs of in vitro tests outlined in the standard battery options e.g. bacterial reverse mutation plus one of a selection of possible tests with cytogenetic evaluation of chromosomal damage or bacterial mutation plus the mouse lymphoma tk assay. Carcinogens such as procarbazine, hydroquinone, urethane and benzene fall into this category.6)The continuing evolution of short-term tests and test methodologies will afford new,more sensitive, more practical, more expeditious, and more economical techniques for detection of genotoxic compounds. Some of these may ultimately replace the genotoxicity tests used for regulatory purposes. Among the more promising tests, the in vitro micronucleus test appears to offer potential for screening purposes.7)Some antibacterial agents, albeit highly toxic to the tester strains, are detected asgenotoxic at very low, sub-lethal concentrations in the bacterial reverse mutation test(e.g. nitrofuran antibiotics).8)Certain structurally alerting molecular entities are recognised as being causally relatedto the carcinogenic and/or mutagenic potential of chemicals. Examples of structural alerts include alkylating electrophilic centers, unstable epoxides, aromatic amines, azo-structures, N-nitroso-groups, aromatic nitro-groups.9)For some classes of compounds with specific structural alerts, it is established thatspecific protocol modifications/additional tests are necessary for optimum detection of genotoxicity (e.g. molecules containing an azo-group, glycosides, compounds such as nitroimidazoles requiring nitroreduction for activation, compounds such as phenacetin requiring another rodent S9 for metabolic activation). The additional testing needed when the chosen 3-test battery yields negative results for a structurally alerting test compound could consist of such modifications.10)The dose range-finding study should (i) give information on the shape of the toxicitydose-response curve if the test compound exhibits toxicity, (ii) include highly toxic concentrations, (iii) include quantification of mutants in the cytotoxic range. If a compound were not toxic, then mutants should nevertheless be quantified.11) A repetition of a test using the identical source and concentration of the metabolicactivation system is usually not necessary. A modification of the metabolic activation system may be indicated for certain chemical classes where knowledge is available on specific requirements of metabolism. This would usually invoke the use of an external metabolising system which is known to be competent for the metabolism/activation of the class of compound under test.7.GLOSSARYCytogenetic evaluation: chromosome structure analysis in mitosis or meiosis by light microscopyDNA adduct: (covalent) binding of chemicals to DNADNA repair: reconstitution of damaged DNA sequenceDNA strand breaks: single or double strand scissions in the DNANumerical chromosome changes: chromosome numbers different from the original haploid or diploid set of chromosomes; for cell lines, chromosome numbers different from the modal chromosome setRecombination: breakage and balanced or unbalanced rejoining of DNATransgene: an exogenous or foreign gene inserted into the host genome, either into somatic cells or germ lline cells。
HT-2157_DataSheet_MedChemExpress

Inhibitors, Agonists, Screening Libraries Data SheetBIOLOGICAL ACTIVITY:HT–2157 (SNAP 37889) is a selective, high–affinity, competitive antagonists of galanin–3 receptor (Gal 3).IC50 & Target: Galanin–3 receptor [1]In Vitro: HT–2157 (SNAP 37889) binds with high affinity to membranes from transiently transfected LMTK – cells expressing the human Gal 3 receptor (K i =17.44±0.01 nM; n>100) and is highly selective for Gal 3 over the Gal 1 and Gal 2 subtypes (K i >10,000 nM for each subtype; n=46 of each subtype). When tested for the antagonism of galanin–evoked inhibition of adenylyl cyclase, HT–2157 (0.1–10μM) produces concentration–dependent rightward shifts of the concentration–effect curve to galanin [1].In Vivo: The galanin–3 receptor antagonist, HT–2157 (SNAP 37889), reduces operant responding for ethanol in alcohol–preferring rats. The novel selective GALR3 antagonist, HT–2157, to reduce anxiety–like behaviour and voluntary ethanol consumption in the iP (alcohol–preferring) rat. Male iP rats treated with HT–2157 at a dose of 30 mg/kg (i.p.) do not show altered locomotor activity or changes in anxiety–like behaviour in the elevated plus maze or light–dark paradigms. Treatment with HT–2157 (30 mg/kg, i.p.)reduces operant responding for solutions containing ethanol, sucrose and saccharin. Collectively, results from the current study shows that HT–2157 (30 mg/kg, i.p.) is effective in reducing operant responding for ethanol, independent of a sedative effect [2].PROTOCOL (Extracted from published papers and Only for reference)Kinase Assay:[1]Binding affinities for HT–2157 (SNAP 37889) and SNAP 398299 at the human Gal 1, Gal 2, and Gal 3 receptors are determined by using the 125I–galanin displacement assay. Additionally, is tested for binding in a broad cross–reactivity panel that included G–protein–coupled receptors, ion channels, enzymes, and transporters. The ability of HT–2157 to antagonize functional responses to galanin is examined in modified HEK–293 cells (PEAK rapid cells) transiently cotransfected with the Gal 3 receptor and Gαz by measuring the inhibition of adenylyl cyclase activity [1].Animal Administration: HT–2157 (SNAP 37889) is prepared in 5% DMSO and 1% HMC in saline [2].[2]Rat [2]The effect of HT–2157 on locomotor activity is examined using adult male iP rats (n=12; 418–467 g). Locomotor activity is tested to ensure that any potential change in anxiety or ethanol consumption in subsequent tests is not due to any sedative property of the drug. Rats are habituated to the locomotor cells (26×26×40 cm) for 60 minute sessions which are conducted daily for threeconsecutive days. To minimise the effect of habituation during the treatment phase, rats are divided into two even groups which received a single injection of either HT–2157 (30 mg/kg, i.p.) or vehicle (1 ml/kg i.p.) 30 min prior to being placed in the locomotor cells for a 60 minute session. The following day the treatments are reversed so that all rats received treatment with both the vehicle and SNAP 37889 compound. Movements in terms of number of moves, move time (s) and total distance travelled (cm), are recorded automatically using TruScan 2.0 software.References:Product Name:HT–2157Cat. No.:HY-100717CAS No.:303149-14-6Molecular Formula:C 21H 13F 3N 2O Molecular Weight:366.34Target:Neuropeptide Y Receptor Pathway:GPCR/G Protein Solubility:DMSO: ≥ 29 mg/mL[1]. Swanson CJ, et al. Anxiolytic– and antidepressant–like profiles of the galanin–3 receptor (Gal3) antagonists SNAP 37889 and SNAP 398299. Proc Natl Acad Sci U S A. 2005 Nov 29;102(48):17489–94.[2]. Ash BL, et al. The galanin–3 receptor antagonist, SNAP 37889, reduces operant responding for ethanol in alcohol–preferring rats. Regul Pept. 2011 Jan 17;166(1–3):59–67.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
Amtech Tacky 助焊膏系列安全数据表说明书

Inventec Performance Chemicals USA, LLCSAFETY DATA SHEET (SDS)SECTION 1: PRODUCT AND COMPANY IDENTIFICATIONPRODUCT NAME: Amtech Tacky Paste Flux Series: 200, 400, 500, 600, 4000, SynTECH, WSFC-305L and #61 SYNONYMS:Tacky FluxMANUFACTURER: Inventec Performance Chemicals USA, LLCADDRESS:PO Box 989 Deep River, CT 06417 USAPHONE:860-526-8300FAX:860-526-8243EMERGENCY:Infotrac-(800)535-5035REVISION DATE:December 19, 2014REVISION DATE: 3DOCUMENT NAME:SDS-Tacky Flux-008PRODUCT USE:Bonding solder joints in production and repair of circuit boardsSECTION 2: HAZARDS IDENTIFICATIONCHEMICAL NAME:N/ACHEMICAL FAMILY:MixtureCHEMICAL FORMULA:N/AROUTES OF ENTRY: Inhalation, Ingestion, Skin/Eye ContactGHS:Signal Word: WarningHazard statement(s)H302 Harmful if swallowedH317 May cause an allergic skin reactionH320 Causes eye irritationH335 May cause respiratory irritationPrecautionary statement(s)P102 Keep out of reach of childrenP233 Keep container tightly closedP264 Wash hands thoroughly after handlingP270 Do not eat, drink or smoke when using this productP280 Wear protective gloves/protective clothing/eye protection/face protectionP302+P352 IF ON SKIN: Wash with plenty of soap and waterP305+P351 IF IN EYES: Rinse continuously with water for several minutesP404 Store in a closed containerP501 Dispose of contents/containers in accordance with Federal, State/Provincial, and/or local regulations POTENTIAL HEALTH EFFECTS:EYE CONTACT: May cause moderate irritation. Do not allow material to come in contact with eyes.SKIN CONTACT: May cause moderate skin irritation.INHALATION: May cause irritation to the respiratory tract.INGESTION: Harmful if swallowed. May cause irritation to the mouth, throat, and stomach. May cause abdominal discomfort, nausea, vomiting, and/or diarrhea.CHRONIC: Not established.SECTION 2 NOTES:Inventec Performance Chemicals USA, LLC does not recommend, manufacture, market, or endorse any of its products for human consumption.SECTION 3: COMPOSITION/INFORMATION ON INGREDIENTSIngredient CAS Number Exposure LimitsModified Rosins N/A N/APine Oil Derivatives 8000-41-7 N/AProprietary Ingredients N/A N/AMixed Carboxylic Acids N/A N/ASECTION 3 NOTES:Percentages of individual components are not listed as this information is considered a trade secret.SECTION 4: FIRST AID MEASURESEYES: Flush with plenty of water, contact a physician. If contact lenses can be removed easily, flush eyes without contact lenses. SKIN: Wash affected area with plenty of warm, soapy water. If irritation persists, seek medical attention.INGESTION: Call a physician or Poison Control Center immediately. Do not induce vomiting.INHALATION: Remove to fresh air. If not breathing, seek immediate medical attention.SECTION 5: FIRE-FIGHTING MEASURESEXTINGUISHING MEDIA: Dry chemical, foamSPECIAL FIRE FIGHTING PROCEDURES: Do not use water. Use NIOSH-approved self-contained Breathing Apparatusand full protective clothing if involved in a fire.UNUSUAL FIRE AND EXPLOSION HAZARDS:This product does not present any unusual fire and explosion hazards. SECTION 6: ACCIDENTAL RELEASE MEASURESACCIDENTAL RELEASE MEASURES: If material spills or leaks, collect and place into a properly labeled waste container. Remove traces of tacky flux using cloth rags or paper towels moistened with Isopropyl Alcohol. Follow on-site personal protective equipment recommendations.SECTION 6 NOTES:See Sections 2, 4, and 7 for additional information.SECTION 7: HANDLING AND STORAGEHANDLING/STORAGE: Keep containers tightly closed when not in use. Use care to avoid spills. Avoid inhalation of fumes or dust. Avoid contact with eyes, skin, and clothing.OTHER PRECAUTIONS: Empty containers may retain product residues in vapor, liquid, and/or solid form. All labeled hazard precautions should be observed.WORK HYGIENIC PRACTICES: Cosmetics/Food/Drink/Tobacco should not be consumed or used in work areas. Always wash hands after handling material and before applying or using cosmetics/food/drink/tobacco.SECTION 7 NOTES:For industrial use only.SECTION 8: EXPOSURE CONTROLS/PERSONAL PROTECTIONVENTILATION: Provide sufficient mechanical (general and/or local exhaust) ventilation to maintain exposure below TLVs. RESPIRATORY PROTECTION: Use with adequate ventilation.EYE PROTECTION: Use with appropriate safety glasses.SKIN PROTECTION: Protective gloves and clothing should be worn when handling material. Wash hands thoroughly with soap and water upon leaving the work area.SECTION 9: PHYSICAL AND CHEMICAL PROPERTIESAPPEARANCE: Clear, White, or Yellow to Dark Amber gelODOR: Mild odorODOR THRESHOLD: Not establishedpH as SUPPLIED: N/ASECTION 9: PHYSICAL AND CHEMICAL PROPERTIES (continued)MELTING POINT: Not establishedFREEZING POINT: Not establishedINITIAL BOILING POINT: Not establishedBOILING RANGE: Not establishedFLASH POINT: Not establishedEVAPORATION RATE: Not establishedFLAMMABILITY (solid): Not establishedUPPER/LOWER FLAMMABILITY: Not establishedUPPER/LOWER EXPLOSIVE LIMITS:Not establishedVAPOR PRESSURE (mmHg): N/A (°F/°C)VAPOR DENSITY (AIR = 1): N/A (°F/°C)RELATIVE DENSITY: Not establishedSOLUBILITY IN WATER: PartiallyPARTITION COEFFICIENT (n-octanol/water): Not establishedAUTOIGNITION TEMPERATURE: Not establishedDECOMPOSITION TEMPERATURE: Not establishedVISCOSITY: N/A (°F/°C)SECTION 10: STABILITY AND REACTIVITYSTABILITY: StableCONDITIONS TO AVOID (STABILITY): Freezing temperatures. High temperatures. INCOMPATIBILITY (MATERIAL TO AVOID): Strong oxidizing materialsHAZARDOUS DECOMPOSITION/BY-PRODUCTS: Harmful organic fumes and toxic oxide fumes may form at elevatedtemperatures.POSSIBILITY OF HAZARDOUS REACTIONS: Will not occurSECTION 11: TOXICOLOGICAL INFORMATIONACUTE TOXICITY: Not availableSKIN CORRISION/IRRITATION: Not establishedSERIOUS EYE DAMAGE/IRRITATION: Not availableRESPIRATORY OR SKIN SENSITIZATION: Not establishedGERM CELL MUTAGENICITY: Not availableCARCINOGENICITY: Not availableREPRODUCTIVE TOXICITY: Not availableSTOT-SINGLE EXPOSURE: Not availableSTOT-REPEATED EXPOSURE: Not availableASPIRATION HAZARD: Not availableSECTION 12: ECOLOGICAL INFORMATIONTOXICITY: Product not testedPERSISTENCE AND DEGRADIBILITY: Product not testedBIOACCUMULATIVE POTENTIAL: Product not testedMOBILITY IN SOIL: Product not testedOTHER ADVERSE EFFECTS: Product not testedSECTION 13: DISPOSAL CONSIDERATIONSWASTE DISPOSAL METHOD: Scrap and waste solder should be stored in a dry, sealed container for later disposal. Disposal must be in accordance with Federal, State/Provincial, and Local Regulations.SECTION 14: TRANSPORT INFORMATIONTransport in accordance with applicable regulations and requirements.UN Number: Not availableUN Proper Shipping Name: Not availablePackaging Group:Not applicableEnvironmental Hazards:NoneTRANSPORT HAZARD CLASSES:US DOT Hazardous Material Classification: Tacky Flux is not listed as a DOT hazardous materialWater Transportation: Tacky Flux is not listed as a hazardous materialIATA Hazardous Material Classification: Tacky Flux is not listed as IATA hazardous materialSECTION 15: REGULATORY INFORMATIONAll ingredients used to manufacture this product are listed on the EPA TSCA Inventory.U.S. FEDERAL REGULATIONS: Not regulatedSTATE REGULATIONS: Not regulatedINTERNATIONAL REGULATIONS: Not regulatedSECTION 16: OTHER INFORMATIONHMIS Rating: Health=1 Flammability=1 Physical Hazard=0 Personal Protection=X KEY:N/A: Not applicableGHS: Global Harmonized SystemOSHA: Occupational Safety and Health AdministrationACGIH: American Conference of Governmental Industrial HygienistsNTP: National Toxicology ProgramIARC: International Agency for Research on CancerCAS: Chemical Abstract ServiceNIOSH: National Institute for Occupational Safety & HealthSTOT: Specific target organ toxicityTLV: Threshold limit valueUS DOT: United States Department of TransportationDOT: Department of TransportationIATA: International Air Transport AssociationEPA:Environmental Protection AgencyTSCA:Toxic Substance Control ActHMIS:Hazardous Material Identification SystemPREPARATION INFORMATION:This update supersedes all previously released documents.PREPARED BY: Wendy W. GesickAPPROVED BY: Leigh W. GesickDISCLAIMER:The information contained herein is based on data considered to be accurate but does not purport to be all-inclusive and shall be used only as a guide. No warranty is expressed or implied regarding the accuracy of this data and Inventec Performance Chemicals USA, LLC shall not be held liable for any damage resulting from any handling or contact with the above product. Liability is expressly disclaimed for loss or injury arising out of use of this information or the use of any materials designated. This material is not for resale, unauthorized distribution, or personal use.。
IRLAB产品使用指南:IRL752和IRL790临床二期管道更新说明书

IRLAB update on clinical Phase II pipeline - IRL752 and IRL790 IRLAB’s two lead development programs, IRL752 and IRL790, both addressing complications in Parkinson’s disease, are progressing according to communicated plans and are both evaluated in Phase II following successfully concluded Phase I-studies. The promising results give a solid foundation for continued development of the both candidate drugs. In parallel, the development of large scale synthesis of API and quality assurance has been optimized for both IRL752 and IRL790.ISP – a unique discovery engineIRLABs compounds in development are discovered by means of the proprietary platform ISP (Integrative Screening Platform).The ISP is a systems biology based discovery engine designed to effectively discover new candidate drugs with novel mechanisms and improved efficacy. The discovery engine is based on IRLAB’s systematically constructed in-vivo database built on data generated from numerous CNS active compounds. Based on chemometric methods and machine-learning algorithms, the combined chemical and biological data are converted to information that enable IRLAB to design new drugable chemical entities, which are developed to novel pharmacological treatment strategies in CNS disorders.Preclinical resultsIRL752IRL752 is developed for the treatment of Parkinson Disease Dementia (PD-D) ultimately affecting up to 80 % of all PD patients. Effective treatments are lacking and thus, the medical need is huge. IRL752 has the ability to increase synaptic availability of the neurotransmitters norepinephrine and dopamine in the frontal cortex and also activates expression of genes modulating synaptic activity and plasticity.IRL752 primary targets are brain 5HT7 and cortical Alpha receptors as an antagonist leading to improvement of disturbed cognitive function, antidepressant and antipsychotic effects. These are all desired properties for treatment of PD-D.Clinical research has shown that both norepinephrine and dopamine neurotransmitters are reduced in frontal cortical brain areas in PD-D. By counteracting this reduction, treatment with IRL752 may improve cognitive and psychiatric symptoms in these patients.IRL790IRL790 is developed for treatment of dyskinesia in PD (PD-LIDs), i.e. involuntary movements that often follow after a number of years of treatment with L-dopa, and psychosis in Parkinson’s disease (PD-P). In pre-clinical studies, IRL790 reduced involuntary movements occuring after a period of treatment with L-dopa. Additionally, in pre-clinical studies, IRL790 has shown antipsychotic properties. Thus, IRL790 has the potential for clinical treatment of both dyskinesia and psychosis in Parkinson’s disease.IRL790 acts as an antagonist at the dopamine D3 receptor. The D3 receptor was recently genetically linked to increased risk for dyskinesia in PD. In Parkinson’s disease patients’ basal ganglia, dopamine D3 receptor densities are elevated and in Parkinson’s disease patients with LIDs, dopamine D3 receptor density is further elevated. Thus, by inhibiting brain dopamine D3 receptors, IRL790 may reduce symptoms of dyskinesia in PD.Clinical Phase I healthy volunteer studiesRandomized, double-blind, placebo-controlled, Phase I, single and multiple dose studies were completed in 2016 and 2017 with both IRL752 and IRL790. The studies included 40 healthy volunteers each.IRL752IRL752 demonstrated good tolerability and very good safety profile over a wide dose range, up to 350 mg as single dose and up to 750mg/24h as repeat dosing over 10 days which well covers the clinically anticipated patient doses. No serious adverse events were reported in the study. There were no effects on vital signs parameters nor on safety laboratory parameters. IRL752 demonstrated dose linear pharmacokinetics and concomitant food intake did not affect pharmacokinetic parameters.The results are encouraging and indicate IRL752 to be well suited for continued clinical Phase II development.IRL790Up to 120 mg as single dose and up to 80 mg/24h repeated dosing for 10 days were administered. IRL790 was well tolerated and had a very good safety profile. Doses administered exceeded the planned dose levels in patients. No serious adverse events were reported in the study. There were no effects on vital signs nor on laboratory safety parameters. Also, IRL790 showed dose linear pharmacokinetics and uptake was not affected by food intake.The encouraging results fully support continued clinical Phase II development.Clinical studies in Parkinson disease patients - Phase Ib and Phase IIIRL752During the fall of 2017 a randomized, double-blind, placebo-controlled and four week treatment Phase II study was initiated in 40 patients with PD-D. The study is executed in Sweden and Finland (EU Clinical Trials Register identifier: 2017-001673-17).The primary objective is to study safety and tolerability of IRL752 and secondarily to study effects of IRL752 on cognitive and motor symptoms. The study will also collect data from caregivers to provide information on how the treatment affects daily living.Top line data from this safety and tolerability study are expected in the second quarter of 2018.IRL790Phase IbTo optimize the design of a Phase II study on IRL790, a randomized placebo-controlled Phase Ib study in 15 patients with PD-LIDs was performed. The primary objective was to evaluate safety and tolerability but effects on symptoms were also measured and subjected to descriptive statistics for evaluation.PD-LID dyskinesia patients were randomized to placebo or IRL790 treatment (1:3 ratio) for 4 weeks. Average patient age was 70 years. Study drug was given as addition to the regular antiparkinsonian medication. Dosing was individually titrated during 2 weeks followed by stable dosing for another two weeks. The average dose after four weeks of IRL790 treatment was 18mg/day and for placebo 42 mg/day.IRL790 displayed good safety. Reported side effects were mild and transient. The pharmacokinetics of IRL790 was similar to those of the healthy volunteer Phase I study.Effects were evaluated at baseline and after 4 weeks of treatment using established and accepted rating scales; The Unified Dyskinesia Rating Scale (UDysRS) the scale accepted by regulatory bodies, Unified Parkinson’s Disease Rating Scale (UPDRS) and wrist worn electronic movement devices, PKG, was used.On the UDysRS scale a median reduction of 11,5 points vs placebo and a mean reduction of 8,2 points vs placebo was observed for theIRL790 treated group (ITT) during the 4 week study. The UPDRS scale and the PKG indicated that IRL790 did not worsen the beneficial treatment effects of patients’ standard anti parkinsonian treatment.The Phase I and Phase Ib studies with IRL790 have defined a safe and tolerable dose range (10-20 mg/day) which can reduce dyskinesia without negatively affecting the parallel standard antiparkinsonian treatment.Phase IIDuring the fall of 2017 IRLAB received approval from the Medicines & Healthcare products Regulatory Agency (MHRA) in the UK to conduct a Phase II study with the drug candidate IRL790 for treatment of L-dopa induced dyskinesias (LIDs) in about 74 patients Identifier: NCT03368170).The Phase II study aims to confirm, the Phase Ib study results and is a randomized double-blind multicentre Phase II clinical trial in Parkinson patients with LIDs. The primary objective is to investigate dyskinesia effects from day 1 to day 28 using UDysRS (the Unified Dyskinesia Rating Scale) in the dose range 10-20 mg/day. Secondary objectives are effects on the UPDRS scale plus safety and tolerability. Top line data from this study are expected in the third quarter of 2018.Chemistry, Manufacturing and Controls (CMC)In parallel with the clinical progress, development of manufacturing methods and quality assurance for API have been optimized for bothIRL790 and IRL752. For IRL752, upscaling of production is also competed. This work fulfills authority guidelines in preparation for coming Phase IIb studies as well as other subsequent studies.For further informationNicholas Waters, CEOPhone: +46 730 75 77 01E-mail: nicholas.waters@irlab.seJoakim Tedroff, CMOPhone: +46 70 760 16 91E-mail: joakim.tedroff@irlab.seAbout IRLABIRLAB is a research and development company, listed on Nasdaq First North Premier, focused on development of novel therapies for the treatment of neurodegenerative diseases, in particular Parkinson’s disease and dementia.IRLAB has two clinical candidate drugs, IRL752 and IRL790, focused on medical needs in Parkinson’s disease. IRLAB also has additional programs in pre-clinical stages.IRLAB’s research is aimed at discovery and development of new candidate drugs addressing unmet medical need in diseases of the central nervous system, using the unique and proprietary integrative screening process, ISP.IRLAB is based in Gothenburg, Sweden. The operations are mainly carried out through the subsidiary Integrative Research Laboratories Sweden AB.For more information, please visit www.irlab.se.。
TMA-DPH-SDS-MedChemExpress
Inhibitors, Agonists, Screening LibrariesSafety Data Sheet Revision Date:Mar.-25-2019Print Date:Mar.-25-20191. PRODUCT AND COMPANY IDENTIFICATION1.1 Product identifierProduct name :TMA-DPHCatalog No. :HY-D0986CAS No. :115534-33-31.2 Relevant identified uses of the substance or mixture and uses advised againstIdentified uses :Laboratory chemicals, manufacture of substances.1.3 Details of the supplier of the safety data sheetCompany:MedChemExpress USATel:609-228-6898Fax:609-228-5909E-mail:sales@1.4 Emergency telephone numberEmergency Phone #:609-228-68982. HAZARDS IDENTIFICATION2.1 Classification of the substance or mixtureNot a hazardous substance or mixture.2.2 GHS Label elements, including precautionary statementsNot a hazardous substance or mixture.2.3 Other hazardsNone.3. COMPOSITION/INFORMATION ON INGREDIENTS3.1 SubstancesSynonyms:NoneFormula:C28H31NO3SMolecular Weight:461.62CAS No. :115534-33-34. FIRST AID MEASURES4.1 Description of first aid measuresEye contactRemove any contact lenses, locate eye-wash station, and flush eyes immediately with large amounts of water. Separate eyelids with fingers to ensure adequate flushing. Promptly call a physician.Skin contactRinse skin thoroughly with large amounts of water. Remove contaminated clothing and shoes and call a physician.InhalationImmediately relocate self or casualty to fresh air. If breathing is difficult, give cardiopulmonary resuscitation (CPR). Avoid mouth-to-mouth resuscitation.IngestionWash out mouth with water; Do NOT induce vomiting; call a physician.4.2 Most important symptoms and effects, both acute and delayedThe most important known symptoms and effects are described in the labelling (see section 2.2).4.3 Indication of any immediate medical attention and special treatment neededTreat symptomatically.5. FIRE FIGHTING MEASURES5.1 Extinguishing mediaSuitable extinguishing mediaUse water spray, dry chemical, foam, and carbon dioxide fire extinguisher.5.2 Special hazards arising from the substance or mixtureDuring combustion, may emit irritant fumes.5.3 Advice for firefightersWear self-contained breathing apparatus and protective clothing.6. ACCIDENTAL RELEASE MEASURES6.1 Personal precautions, protective equipment and emergency proceduresUse full personal protective equipment. Avoid breathing vapors, mist, dust or gas. Ensure adequate ventilation. Evacuate personnel to safe areas.Refer to protective measures listed in sections 8.6.2 Environmental precautionsTry to prevent further leakage or spillage. Keep the product away from drains or water courses.6.3 Methods and materials for containment and cleaning upAbsorb solutions with finely-powdered liquid-binding material (diatomite, universal binders); Decontaminate surfaces and equipment by scrubbing with alcohol; Dispose of contaminated material according to Section 13.7. HANDLING AND STORAGE7.1 Precautions for safe handlingAvoid inhalation, contact with eyes and skin. Avoid dust and aerosol formation. Use only in areas with appropriate exhaust ventilation.7.2 Conditions for safe storage, including any incompatibilitiesKeep container tightly sealed in cool, well-ventilated area. Keep away from direct sunlight and sources of ignition.Recommended storage temperature: -20°C, protect from lightShipping at room temperature if less than 2 weeks.7.3 Specific end use(s)No data available.8. EXPOSURE CONTROLS/PERSONAL PROTECTION8.1 Control parametersComponents with workplace control parametersThis product contains no substances with occupational exposure limit values.8.2 Exposure controlsEngineering controlsEnsure adequate ventilation. Provide accessible safety shower and eye wash station.Personal protective equipmentEye protection Safety goggles with side-shields.Hand protection Protective gloves.Skin and body protection Impervious clothing.Respiratory protection Suitable respirator.Environmental exposure controls Keep the product away from drains, water courses or the soil. Cleanspillages in a safe way as soon as possible.9. PHYSICAL AND CHEMICAL PROPERTIES9.1 Information on basic physical and chemical propertiesAppearance Light yellow to yellow (Solid)Odor No data availableOdor threshold No data availablepH No data availableMelting/freezing point No data availableBoiling point/range No data availableFlash point No data availableEvaporation rate No data availableFlammability (solid, gas)No data availableUpper/lower flammability or explosive limits No data availableVapor pressure No data availableVapor density No data availableRelative density No data availableWater Solubility No data availablePartition coefficient No data availableAuto-ignition temperature No data availableDecomposition temperature No data availableViscosity No data availableExplosive properties No data availableOxidizing properties No data available9.2 Other safety informationNo data available.10. STABILITY AND REACTIVITY10.1 ReactivityNo data available.10.2 Chemical stabilityStable under recommended storage conditions.10.3 Possibility of hazardous reactionsNo data available.10.4 Conditions to avoidNo data available.10.5 Incompatible materialsStrong acids/alkalis, strong oxidising/reducing agents.10.6 Hazardous decomposition productsUnder fire conditions, may decompose and emit toxic fumes.Other decomposition products - no data available.11.TOXICOLOGICAL INFORMATION11.1 Information on toxicological effectsAcute toxicityClassified based on available data. For more details, see section 2Skin corrosion/irritationClassified based on available data. For more details, see section 2Serious eye damage/irritationClassified based on available data. For more details, see section 2Respiratory or skin sensitizationClassified based on available data. For more details, see section 2Germ cell mutagenicityClassified based on available data. For more details, see section 2CarcinogenicityIARC: No component of this product present at a level equal to or greater than 0.1% is identified as probable, possible or confirmed human carcinogen by IARC.ACGIH: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by ACGIH.NTP: No component of this product present at a level equal to or greater than 0.1% is identified as a anticipated or confirmed carcinogen by NTP.OSHA: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by OSHA.Reproductive toxicityClassified based on available data. For more details, see section 2Specific target organ toxicity - single exposureClassified based on available data. For more details, see section 2Specific target organ toxicity - repeated exposureClassified based on available data. For more details, see section 2Aspiration hazardClassified based on available data. For more details, see section 212. ECOLOGICAL INFORMATION12.1 ToxicityNo data available.12.2 Persistence and degradabilityNo data available.12.3 Bioaccumlative potentialNo data available.12.4 Mobility in soilNo data available.12.5 Results of PBT and vPvB assessmentPBT/vPvB assessment unavailable as chemical safety assessment not required or not conducted.12.6 Other adverse effectsNo data available.13. DISPOSAL CONSIDERATIONS13.1 Waste treatment methodsProductDispose substance in accordance with prevailing country, federal, state and local regulations.Contaminated packagingConduct recycling or disposal in accordance with prevailing country, federal, state and local regulations.14. TRANSPORT INFORMATIONDOT (US)This substance is considered to be non-hazardous for transport.IMDGThis substance is considered to be non-hazardous for transport.IATAThis substance is considered to be non-hazardous for transport.15. REGULATORY INFORMATIONSARA 302 Components:No chemicals in this material are subject to the reporting requirements of SARA Title III, Section 302.SARA 313 Components:This material does not contain any chemical components with known CAS numbers that exceed the threshold (De Minimis) reporting levels established by SARA Title III, Section 313.SARA 311/312 Hazards:No SARA Hazards.Massachusetts Right To Know Components:No components are subject to the Massachusetts Right to Know Act.Pennsylvania Right To Know Components:No components are subject to the Pennsylvania Right to Know Act.New Jersey Right To Know Components:No components are subject to the New Jersey Right to Know Act.California Prop. 65 Components:This product does not contain any chemicals known to State of California to cause cancer, birth defects, or anyother reproductive harm.16. OTHER INFORMATIONCopyright 2019 MedChemExpress. The above information is correct to the best of our present knowledge but does not purport to be all inclusive and should be used only as a guide. The product is for research use only and for experienced personnel. It must only be handled by suitably qualified experienced scientists in appropriately equipped and authorized facilities. The burden of safe use of this material rests entirely with the user. MedChemExpress disclaims all liability for any damage resulting from handling or from contact with this product.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
CHIR-99021_DataSheet_MedChemExpress

Inhibitors, Agonists, Screening Libraries Data SheetBIOLOGICAL ACTIVITY:CHIR–99021 is a GSK–3α/β inhibitor with IC 50 of 10 nM/6.7 nM; > 500–fold selectivity for GSK–3 versus its closest homologs CDC2 and ERK2, as well as other protein kinases.IC50 & Target: IC50: 10 nM/6.7 nM (GSK–3α/β)[1]In Vitro: CHIR 99021inhibits human GSK–3β with K i values of 9.8 nM [1]. CHIR 99021 is a small organic molecule that inhibits GSK3α and GSK3β by competing for their ATP–binding sites.In vitro kinase assays reveal that CHIR 99021 specifically inhibits GSK3β (IC 50=~5 nM) and GSK3α (IC 50=~10 nM), with little effect on other kinases [2]. In the presence of CHIR–99021 the viability of the ES–D3 cells is reduced by 24.7% at 2.5 μM, 56.3% at 5 μM, 61.9% at 7.5 μM and 69.2% at 10 μM CHIR–99021 with an IC 50 of 4.9μM [3].In Vivo: In ZDF rats, a single oral dose of CHIR 99021 (16 mg/kg or 48 mg/kg) rapidly lowers plasma glucose, with a maximal reduction of nearly 150 mg/dl 3–4 h after administration [1]. CHIR99021 (2 mg/kg) given once, 4 h before irradiation, significantly improves survival after 14.5 Gy abdominal irradiation (ABI). CHIR99021 treatment significantly blocks crypt apoptosis andaccumulation of p–H2AX + cells, and improves crypt regeneration and villus height. CHIR99021 treatment increases Lgr5+ cellsurvival by blocking apoptosis, and effectively prevents the reduction of Olfm4, Lgr5 and CD44 as early as 4 h [4].PROTOCOL (Extracted from published papers and Only for reference)Kinase Assay:[2]Kinases are purified from SF9 cells through use of their His or Glu tag. Glu–tagged proteins are purified, and His–tagged proteins are purified. Kinase assays are performed in 96–well plates with appropriate peptide substrates in a 300–μL reaction buffer (variations on 50 mM Tris–HCl, pH 7.5, 10 mM MgCl 2, 1 mM EGTA, 1 mMdithiothreitol, 25 mMβ–glycerophosphate, 1mM NaF, and 0.01% bovine serum albumin). Peptides has K m values from 1 to 100 μM. CHIR 99021 or CHIR GSKIA is added in 3.5μL of Me 2SO, followed by ATP to a final concentration of 1 μM. After incubation, triplicate 100–μL aliquots are transferred to Combiplate 8 plates containing 100 μL/well of 50 μM ATP and 20 mM EDTA. After 1 hour, the wells are rinsed five times with phosphate–buffered saline, filled with 200 μL of scintillation fluid, sealed, and counted in a scintillation counter 30 min later. All of the steps are at room temperature. The percentage of inhibition is calculated as 100×(inhibitor–no enzyme control)/(Me 2SO control–no enzyme control)[2].Cell Assay: CHIR 99021 is dissolved in DMSO and stored, and then diluted with appropriate media before use [3].[3]The viability of the mouse ES cells is determined after exposure to different concentrations of GSK3 inhibitors for three days using the MTT assay.The decrease of MTT activity is a reliable metabolism–based test for quantifying cell viability; this decrease correlates with the loss of cell viability. 2,000 cells are seeded overnight on gelatine–coated 96–well plates in LIF–containing ES cell medium. On the next day the medium is changed to medium devoid of LIF and with reduced serum and supplemented with 0.1–1 μM BIO, or 1–10 μM SB–216763, CHIR–99021 or CHIR–98014. Basal medium without GSK3 inhibitors or DMSO is used as control. All tested conditions are analyzed in triplicates [3].Product Name:CHIR–99021Cat. No.:HY-10182CAS No.:252917-06-9Molecular Formula:C 22H 18Cl 2N 8Molecular Weight:465.34Target:GSK–3; GSK–3; Autophagy Pathway:Stem Cell/Wnt; PI3K/Akt/mTOR; Autophagy Solubility:DMSO: ≥ 5.1 mg/mLAnimal Administration: CHIR 99021 is formulated as solutions in 20 mM citrate–buffered 15% Captisol or as fine suspensions in0.5% carboxymethylcellulose (Rat)[1].CHIR 99021 is prepared in DMSO and diluted (Mice)[4].[1][4]Rat[1]Primary hepatocytes from male Sprague Dawley rats that weighed <140 g are prepared and used 1–3 h after isolation. Aliquotsof 1×106cells in 1 mL of DMEM/F12 medium plus 0.2% BSA and CHIR 99021(orally at 16 or 48 mg/kg) or controls are incubated in 12–well plates on a low–speed shaker for 30 min at 37°C in a CO2–enriched atmosphere, collected by centrifugation and lysed by freeze/thaw in buffer A plus 0.01% NP40; the GS assay is again performed.Mice[4]Mice 6–10 weeks old are used. The PUMA+/+ and PUMA–/– littermates on C57BL/6 background (F10) and Lgr5–EGFP(Lgr5–EGFP–IRES–creERT2) mice are subjected to whole body irradiation (TBI), or abdominal irradiation (ABI). Mice are injected intraperitoneally (i.p.) with 2 mg/kg of CHIR99021 4 h before radiation or 1 mg/kg of SB415286 28 h and 4 h before radiation. Mice are sacrificed to collect small intestines for histology analysis and western blotting. All mice are injected i.p. with 100 mg/kg of BrdU before sacrifice.References:[1]. Ring DB, et al. Selective glycogen synthase kinase 3 inhibitors potentiate insulin activation of glucose transport and utilization in vitro and in vivo. Diabetes. 2003 Mar;52(3):588–95.[2]. Bennett CN, et al. Regulation of Wnt signaling during adipogenesis. J Biol Chem. 2002 Aug 23;277(34):30998–1004.[3]. Naujok O, et al. Cytotoxicity and activation of the Wnt/beta–catenin pathway in mouse embryonic stem cells treated with four GSK3 inhibitors.BMC Res Notes. 2014 Apr 29;7:273.[4]. Wang X, et al. Pharmacologically blocking p53–dependent apoptosis protects intestinal stem cells and mice from radiation. Sci Rep. 2015 Apr 10;5:8566.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
危险化学品目录表二

有机过氧化物,C型
单过氧马来酸叔丁酯[含量≤52%,糊状物]
tert-butyl monoperoxymaleate (not more than 52% as a paste)
107-08-4
易燃液体,类别3
185
2-碘丙烷
异丙基碘;碘代异丙烷
2-iodopropane;isopropyl iodide;iodo-iso-propane
75-30-9
易燃液体,类别3
186
1-碘丁烷
正丁基碘;碘代正丁烷
1-iodobutane;n-butyl iodide;iodo-n-butane
严重眼损伤/眼刺激,类别1
特异性靶器官毒性-一次接触,类别3(呼吸道刺激)
190
碘化亚汞
一碘化汞
mercurous iodide;mercurous monoiodide
15385-57-6
急性毒性-经口,类别2*
急性毒性-经皮,类别1
急性毒性-吸入,类别2*
特异性靶器官毒性-反复接触,类别2*
危害水生环境-急性危害,类别1
遇水放出易燃气体的物质和混合物,类别3
严重眼损伤/眼刺激,类别2
皮肤致敏物,类别1
生殖毒性,类别2
危害水生环境-急性危害,类别1
危害水生环境-长期危害,类别1
171
单过氧马来酸叔丁酯[含量>52%]
tert-butyl monoperoxymaleate (more than 52%)
3112A型KX热敏电极、1双20AWG(固体)双铬+阿尔茨尔热敏电极,PVC膜薄荷色,红色标识,整

Environmental Suitability:
Flammability / Reaction to Fire:
NEC / UL Compliance:
AWM Compliance:
CEC / C(UL) Compliance:
European Directive Compliance:
APAC Compliance:
All sales of Belden products are subject to Belden's standard terms and conditions of sale.
Belden believes this product to be in compliance with all applicable environmental programs as listed in the data sheet. The information provided is correct to the best of Belden's knowledge, information and belief at the date of its publication. This information is designed only as a general guide for the safe handling, storage, and any other operation of the product itself or the one that it becomes a part of. The Product Disclosure is not to be considered a warranty or quality specification. Regulatory information is for guidance purposes only. Product users are responsible for determining the applicability of legislation and regulations based on their individual usage of the product.
签名2750-7 pH电子技术手册说明书

Signet 3-2724-00 Flat pH Electrode
The bottom yellow nut is not used when using the 2750-7 pH electronics with the Signet 463X flow cell.
Do Not Use Lubricant or Sealing Tape on Threads. Do Not Overtighten.
China RoHS (visit for details)
23 mm (0.91 in.)
61.0 mm (2.40 in.)
Georg Fischer Signet LLC, 3401 Aero Jet Avenue, El Monte, CA 91731-2882 U.S.A. • Tel. (626) 571-2770 • Fax (626) 573-2057 For Worldwide Sales and Service, visit our website: • Or call (in the U.S.): (800) 854-4090 For the most up-to-date information, please refer to our website at
• For calibration and configuration please refer to ansmitter manual.
4. Specifications
General Compatible Electrode: Signet 2724-00 Flat pH electrode Compatible Instrument: Signet 3-8630-3P Chlorine Transmitter Mounting: DryLoc connection Materials: Valox®* (PBT)* Cable: 4.6 m (15 ft) 3 conductor shielded, 22 AWG Shipping Weight: 0.64 kg (1.41 lb)
Y-33075-dihydrochloride-DataSheet-MedChemExpress
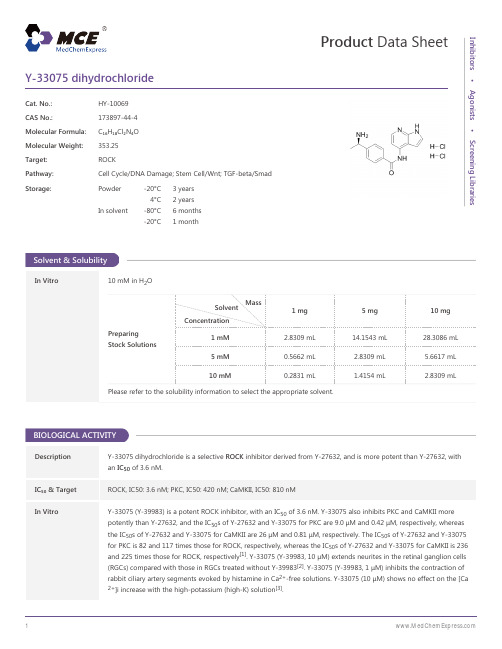
Y-33075 dihydrochloridedye embedding, rats is euthanized and the eyes is enucleated for preparation of retinal flat-mounts. The posterioreyecup is then separated from the vitreous body and postfixed with 4% paraformaldehyde solution in phosphatebuffer for around 1 hour at room temperature. Fluorescence micrographs of the labeled cells is imported using afluorescence microscope connected to a computer. Labeled cells is counted using image analysis software. As anormal group, the subsequent procedure for retrograde labeling with 4-Di-10ASP is performed without graftingsciatic nerve and administering the test drug. Statistical analysis is performed using logarithmically transformedvalues due to differences in variance among the groups. The statistical significance of differences between the normaland saline groups and the saline and Y-33075 groups is examined by t-test (onesided) and William’s test (one-sided).Findings of p < 0.05 is considered significant[2].MCE has not independently confirmed the accuracy of these methods. They are for reference only.CUSTOMER VALIDATION• Science. 2017 Dec 1;358(6367). pii: eaan4368.• Cell. 2018 Jul 26;174(3):636-648.e18.• Neurotox Res. 2013 Apr;23(3):238-48.• Patent. US20170349879A1.See more customer validations on REFERENCES[1]. Hideki Tokushige, et al. Effects of Topical Administration of Y-39983, a Selective Rho-Associated Protein Kinase Inhibitor, on Ocular Tissues in Rabbits and Monkeys Invest. Ophthalmol. Vis. Sci. July 2007 vol. 48no. 7 3216-3222[2]. Tokushige H, et al. Effects of Y-39983, a selective Rho-associated protein kinase inhibitor, on blood flow in optic nerve head in rabbits and axonal regeneration of retinal ganglion cells in rats. Curr Eye Res. 2011 Oct;36(10):964-70.[3]. Watabe H, et al. Effects of Rho-associated protein kinase inhibitors Y-27632 and Y-39983 on isolated rabbit ciliary arteries.Jpn J Ophthalmol. 2011 Jul;55(4):411-7. Epub 2011 Jun 11.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
苯磺酸氨氯地平片申报资料全

苯磺酸氨氯地平片申报资料(药学部分)目录3.2.P.1 剂型及产品组成 (2)3.2.P.2 产品开发 (3)3.2.P.2.1 处方组成 (3)3.2.P.2.1.1 原料药 (3)3.2.P.2.1.2 辅料 (4)3.2.P.2.2 制剂研究 (4)3.2.P.2.2.1 处方开发过程 (4)3.2.P.2.3 生产工艺的开发 (19)3.2.P.2.4 包装材料/容器 (22)2.3.P.2.5 相容性 (22)3.2.P.3 生产 (22)3.2.P.3.1生产商 (22)3.2.P.3.2批处方 (22)3.2.P.3.3 生产工艺和工艺控制 (23)3.2.P.3.4 关键步骤和中间体的控制 (24)3.2.P.3.5 工艺验证和评价 (25)3.2.P.4 原辅料的控制 (30)3.2.P.5 制剂的质量控制 (30)3.2.P.5.1质量标准 (30)3.2.P.5.2 分析方法 (30)3.2.P.5.3 分析方法的验证 (33)3.2.P.5.4 批检验报告 (92)3.2.P.5.5 杂质分析 (96)3.2.P.6 对照品 (100)3.2.P.7 稳定性 (100)3.2.P.7.1稳定性总结 (100)3.2.P.7.2上市后的稳定性承诺和稳定性方案 (101)3.2.P.7.3 稳定性数据 (101)申报资料正文3.2.P.1 剂型及产品组成苯磺酸氨氯地平片是一种独特的具有高度血管选择性的长效二氢吡啶类钙离子拮抗剂,是心血管治疗药物中比较理想的长效降压药,也是近几年来世界处方量最大的高血压和心绞痛治疗药物。
######最早由美国##公司研制开发成功,1990年在英国和爱尔兰首先投放市场。
1992年7月获得美国FDA批准在美国上市,后在许多国家上市,目前已在全世界几十个国家和地区上市销售。
该药于1993年投放中国市场,于1993年12月1日在中国获得药品行政保护,已于2001年6月1日期限届满。
AVE752与AVE764C联合用于尿常规检验的效果观察

.110中国医疗器械信息 | China Medical Device Information临床应用研究Clinical Application Research尿常规检验是目前医学界常规检验项目之一,不仅可以为全身性病变及脏器病变的诊断提供科学依据,还能反映出某些疾病治疗效果及预后,临床应用价值非常显著。
随着我国医疗技术的飞速发展,尿常规检验方法层出不穷,向着科学化、标准化、准确化方向发展,但不同检验方法其检验准确率存在一定的差异。
干化学检验和尿有形成分检验是尿常规检验常见方法,两种方法工作原理不同,但互有优势[1]。
因此将A VE752与A VE764C 联合使用观察其临床检验效果。
1.资料与方法1.1临床资料随机抽取2016年6月~2016年8月本院接收的尿常规样本,运用随机分组原则,将270例样本分为甲、乙、丙三组,每组各90例,其中甲组男性47例,女性43例,年龄18~75岁,平均年龄(35.87±8.22)岁;乙组男性46例,女性44例,年龄20~74岁,平均年龄(35.64±8.25)岁;丙组男性48例,女性42例,年龄19~75岁,平均年龄(35.77±8.20)岁。
三组患者基线资料,如年龄、性别等比较,无统计学意义(P >0.05),具有较高的均衡性。
1.2方法和仪器甲组采用AVE752(爱威科技股份有限公司)进行干化学法检验,乙组采用AVE764C (爱威科技股份有限公司)进行尿液有形成分检验,丙组采用AVE752与AVE764C 联合检验。
配套试剂、干化学试纸条及相应质控品均由爱威科技股份有限公司提供。
样本的采集和处理按照《全国临床检验操作规程》第4版[2]操作。
检验前使用相应质控品对仪器进行室内质控,通过后方能进行验本检验。
1.3评价指标详细收集三组尿常规检验结果,对尿常规检验白细胞、红细胞数值进行分析,评估标准[3]如下,A VE764C :(1)阴性:白细胞检出值为0.0~10.0/μL ,红细胞检出值为0.0~7.0/μL ;(2)阳性:白细胞检出值>10.0/μL ;红细胞检出值>7.0/μL 。
pBluescript II KS(-) Phagemid 212208 化学品安全技术说明书

pBluescript II KS(-) Phagemid, Part Number 212208*************(24小时)化学品安全技术说明书GHS product identifier 应急咨询电话(带值班时间)::供应商/ 制造商:安捷伦科技贸易(上海)有限公司中国(上海)外高桥自由贸易试验区英伦路412号(邮编:200131)电话号码: 800-820-3278传真号码: 0086 (21) 5048 2818pBluescript II KS(-) Phagemid, Part Number 212208化学品的推荐用途和限制用途pBluescript II KS (-) Phagemid 212208-51XL1-Blue MRF’ E.coli Strain 200301-81部件号:物质用途:分析试剂。
pBluescript II KS (-) Phagemid 0.02 ml(毫升) (20 µg 1 µg/µl)XL1-Blue MRF’ E.coli Strain 0.5 ml(毫升)部件号(化学品试剂盒):212208安全技术说明书根据 GB/ T 16483-2008 和 GB/ T 17519-2013GHS化学品标识:pBluescript II KS(-) 噬菌粒,部件号 212208危险性类别信号词:pBluescript II KS (-)Phagemid无信号词。
XL1-Blue MRF’ E.coli Strain 警告危险性说明:没有明显的已知作用或严重危险。
XL1-Blue MRF’ E.coli StrainH316 - 造成轻微皮肤刺激。
H319 - 造成严重眼刺激。
:XL1-Blue MRF’ E.coli Strain防范说明GHS标签要素象形图物质或混合物的分类根据 GB13690-2009 和 GB30000-2013皮肤腐蚀/刺激 - 类别 3H319严重眼损伤/眼刺激 - 类别 2A紧急情况概述pBluescript II KS (-) Phagemid 液体。
薄层色谱扫描仪

KH-2100A法定型双波长薄层色谱扫描仪文章类别:法定型薄层色谱扫描仪发表日期:2007-5-16 21:16:44KH-2100A法定型双波长薄层色谱扫描仪----中药GMP控制专用机型上海科哲生化科技有限公司是国内最大最专业的薄层色谱仪器公司,受到上海科委的重点扶持,是国内唯一的法定型薄层色谱扫描仪生产商。
针对基层用户方法相对固定,分析任务较为繁重,对易用性、稳定性要求较高,我公司将原来的KH-2000升级为KH-2100,性能更为优越,将2000版、2005、2010版药典所有薄层定量品种、方法预置在机器内,形成药厂专用薄层色谱扫描仪,使用者只需动手,不需动脑即可完成操作;产品与日本岛津CS-9301PC薄层色谱扫描仪处于同一档次,是中药企业质控的最优选择。
友情提示:所谓法定薄层色谱扫描仪是指符合2005版药典一部薄层色谱扫描法、《中国药品检验标准操作规范》中的《薄层色谱扫描法》、《药品检验仪器检定规程》中的《薄层色谱扫描仪检定规程》的详细规定,由氘灯-钨灯组合光源、单色仪、X-Y移动平台组成,波长准确度:≤±5nm;背景漂移:≤±0.5Abs;基线噪声:≤±0.02Abs;重复性误差:RSD≤2.0%;扫描方向为沿展开方向自下而上扫描的非成像型薄层色谱扫描仪。
主要优点:1、拥有国家计量认证,完全符合有关要求,使用无风险;2、符合国家《薄层色谱扫描仪检定规程》所有要求,方法合法;3、拥有ISO9001:2000质量保证体系,性能稳定,故障少;4、药典所有薄层定量品种、方法预置在机器内,随时调用,非常方便;5、定量准确,价格合理,性价比高;6、自动化程度高,操作简单,适合成批分析;仪器组成:1、主机(含光源、光栅单色仪、移动平台);2、TStar-2000A专业薄层色谱工作站仪器附件1、SP-II电动薄层点样仪;2、TS-II超细电动喷雾器;3、TD-II自动薄层铺板机;仪器指标:1、光谱范围:190-700nm(自动设置);2、波长准确度:≤±1nm;3、重复性误差:RSD≤2.0%;4、单色器:全息光栅;5、光源:氘灯-钨灯组合光源;6、测量方法:反射法、荧光法、吸收法;7、软件环境:WIN2000/WINXP;8、定量方法:外标法、内标法、归一化法;9、记录内容:薄层色谱扫描图、峰面积、工作曲线、回归方程、相关系数、测定结果;可预置的薄层分析方法:1、2000版中国药典所有薄层扫描定量方法;2、2005版中国药典所有薄层扫描定量方法;3、2010版中国药典所有薄层扫描定量方法;更高的分析要求,请选用本公司的KH-3000型全波长波长薄层色谱扫描仪;GoodLook-1000型全自动薄层色谱成像系统文章类别:薄层色谱成像系统发表日期:2007-5-16 21:23:20GoodLook-1000型全自动薄层色谱成像系统上海科委“十五科技攻关项目”,专门为中药指纹图谱分析而打造---最直观,最快速的中药质控技术上海科哲生化科技有限公司是国内最大最专业的薄层色谱仪器生产商,为了适合2005版药典薄层色谱检视与中药薄层指纹图谱分析的要求,利用薄层色谱图像特有的直观、丰富多彩的特点,推出了GoodLook-1000型全自动薄层色谱成像系统。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Inhibitors, Agonists, Screening Libraries
Data Sheet
BIOLOGICAL ACTIVITY:
VRT752271 is a reversible, ATP–competitive inhibitor of ERK1 and ERK2 kinases, with IC 50 of <0.3 nM against ERK2.
IC50 & Target: IC50: <0.3 nM (ERK2)[2]
In Vivo: In the pharmacokinetic study, the sensitivity and specificity of the assay are found to be sufficient for accurately
characterizing the plasma pharmacokinetics of VRT752271 in Balb/C mice [1].
PROTOCOL (Extracted from published papers and Only for reference)
Kinase Assay:[2]The MEK autophosphorylation assay is performed using the ADP Glo Kinase Assay kit. Activated MEK protein is expressed and purified in–house. Enzyme and substrate solutions are prepared in assay buffer consisting of 50 mM Tris (pH 7.5), 10mM MgCl 2, 0.1 mM EGTA, 10 mM DTT and 0.01 % Tween 20. 6 nM MEK protein is prepared in assay buffer and 2 μL is dispensed into each well of a 384–well white small volume medium bind plate containing test and reference control compounds. Compound plates are dosed with a 12 point dose response curve from 10 μM down to 0.0625 nM in order to calculate compound IC 50s, with a total DMSO concentration in the assay of 1%. Following a 15 minute pre–incubation of enzyme and compound at room temperature,2 μL of a 20 μM Ultra Pure ATP solution in assay buffer is added to the wells, and the reaction is allowed to progress for 90 minutes at room temperature before the addition of 4 μL ADP Glo reagent R1 to quench the reaction. The plate is incubated at room
temperature for 45 minutes before the addition of 8 μL Kinase Detection Reagent, and then the luminescence signal is allowed to equilibrate for 60 minutes before the plates are read on a Pherastar plate reader.
Cell Assay:[2]A375 cells are cultured in cell media composed of DMEM, 10% (v/v) Foetal Calf Serum and 1% (v/v) L–Glutamine. After harvesting, cells are dispensed into black, 384–well Costar plates to give 200 cells per well in a total volume of 40 μL cell media, and are incubated overnight at 37°C, 90% relative humidity and 5% CO 2 in a rotating incubator. Test compounds and reference controls are dosed directly into the cell plates, into the inner 308 wells, sing a Labcyte Echo 555 acoustic dispenser. The cells are dosed over a 12 point range from 30 μM down to 0.03 nM in order to calculate compound IC 50s, with a total DMSO concentration in the assay of 0.3%. The cell plates are then incubated for 72 hours at 37°C. Cells are fixed and stained by the addition of 20 μL 12% formaldehyde in PBS/A (4% final concentration) and 1:2000 dilution of Hoechst 33342, with a 30 minute room temperature incubation, and then
washed with PBS/A. A cell count is performed on the stained cell plates using a Cellomics ArrayScanTM VTI imaging platform. A Day 0cell plate is also fixed, stained and read to generate a cell count baseline for determining compound cytotoxic effects as well as anti–proliferative effects.
References:
[1]. Kumar R, et al. Determination of ulixertinib in mice plasma by LC–MS/MS and its application to a pharmacokinetic study in mice. J Pharm Biomed Anal.
Product Name:
VRT752271Cat. No.:
HY-15816CAS No.:
869886-67-9Molecular Formula:
C 21H 22Cl 2N 4O 2Molecular Weight:
433.33Target:
ERK; ERK Pathway:
Stem Cell/Wnt; MAPK/ERK Pathway Solubility:
DMSO: ≥ 45 mg/mL
2016 Jun 5;125:140–4.
[2]. Ward RA, et al. Structure–Guided Design of Highly Selective and Potent Covalent Inhibitors of ERK1/2. J Med Chem. 2015 Jun 11;58(11):4790–801.
Caution: Product has not been fully validated for medical applications. For research use only.
Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@
Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
