Real time thermal propagtors for massive gauge bosons
法国SETARAM公司一直是全球顶级热分析及量热仪的制造

法国SETARAM公司一直是全球顶级热分析及量热仪的制造商,隶属于空中客车尖端配套商之一的法国KEP集团。
并于2006年设立法国塞塔拉姆仪器公司上海代表处。
长期以来都是空客集团SNECMA及法国原子能机构CEA的指定热分析技术及量热技术合作伙伴。
公司位于热分析和量热仪技术的发源地-法国,拥有世界上最著名的高温热分析及量热专家,是世界上第一个采用钨合金炉体制造1600°C综合热分析厂商(1965), 凭借50多年的技术传承和世界顶级用户的互动,塞塔拉姆公司在高温和超高温热分析领域以其独特的Eyraud光电天平技术、卡尔维三维量热技术及模块化设计一直处于行业领先地位。
塞塔拉姆产品在制药、生物、食品、石油和天然气、核能、过程安全和先进材料等领域正起着日益重要的作用。
可用于测试腐蚀、氧化、降解及混合反应和研究纳米材料、金属、陶瓷和合金的老化特性。
在生物制药、过程安全和能源开发研究领域,我们的系统广泛应用于多晶态、晶状体球蛋白、溶度测定、预测逃生时间、动力学研究、燃气水合物和钻井泥浆等研究过程。
塞塔拉姆通过非破坏性分析还开发出了表征核能废料的独特解决方案。
而2008年收购美国HY-Energy技术公司,也预示着储氢材料研究领域的全面涉足。
塞塔拉姆仪器公司在全球的主要市场(美国、法国、德国、意大利、瑞士和英国)都有直接销售渠道。
为满足用户的需要,2006年底在中国上海成立代表处,2008底建立技术中心及应用实验室,对销售人员的培训,技术支持和现场服务也一应俱全,同时拟通过该平台更好地向国内用户提供产品、培训应用方面的信息及支持。
热重分析仪(TGA) 测量物质的重量变化(在受控气氛内温度变化条件下)。
所有塞塔拉姆天平都满足最高的精确度和稳定性标准。
由热重分析仪(TGA) 所测的性质包括腐蚀,高温分解,吸附/解吸附,溶剂的损耗,氧化/还原反应,水合/脱水,分解,炭黑等高性能模块TGA热分析仪(室温/ 2400°C)SETSYS Evolution TGA具有单独加热炉和最宽广的温度范围(室温/ 2400°C)模块性: DTA, DSC, TGA, TMA 不同组合能在同一基本结构上互换。
梅特勒-托利多新型动态热机械分析仪DMA1及新一代热重分析仪TGA1隆重上市

梅特勒-托利多新型动态热机械分析仪DMA1及新一代热重
分析仪TGA1隆重上市
梅特勒
【期刊名称】《上海计量测试》
【年(卷),期】2012(000)006
【总页数】1页(P58-58)
【作者】梅特勒
【作者单位】托利多公司
【正文语种】中文
【相关文献】
1.梅特勒-托利多超越系列熔点仪全新上市——熔点仪新概念:自动测理与视频记录同步 [J],
2.梅特勒-托利多超越系列熔点仪全新上市——熔点仪新概念:自动测量与视频记录同步 [J],
3.2012梅特勒-托利多新型动态热机械分析仪DMA1及新一代热重分析仪TGA1隆重上市! [J],
4.梅特勒-托利多全新一代FiveEasy Plus^(TM)系列台式仪表于2012年3月隆重上市! [J],
5.梅特勒-托利多全新一代FiveEasy Plus^(TM)系列台式仪表于2012年3月隆重上市! [J],
因版权原因,仅展示原文概要,查看原文内容请购买。
聚苯乙烯ps质量法熔融指仪

聚苯乙烯ps质量法熔融指仪
聚苯乙烯PS质量法熔融指仪
聚苯乙烯PS质量法熔融指仪是一种采用聚苯乙烯PS的质量法来测定熔融指仪的仪器。
它由一个可控制聚苯乙烯PS温度的采样腔、一个用于安装聚苯乙烯PS的熔融测试仪和一个用于熔点测试的测温探头组成。
它的工作原理是:当聚苯乙烯PS温度上升时,它会在一定温度下熔化,即该聚苯乙烯PS的熔点。
在改变聚苯乙烯PS温度的同时,可以测量出该聚苯乙烯PS的熔点,从而确定该聚苯乙烯PS的质量。
聚苯乙烯PS质量法熔融指仪在实验室使用时常常与其他仪器结合起来,用于测量熔点、收缩率、晶向、光谱和拉伸强度等性能参数。
它可以用来评估聚苯乙烯PS的品质,并且可以帮助检测制造商是否达到质量标准,以及在产品开发和生产过程中起到质量控制的作用。
聚苯乙烯PS质量法熔融指仪的优点在于它可以快速准确的测量出聚苯乙烯PS的熔点,这样就可以准确判断其质量,而它的精度更高,更能满足客户的要求。
同时,由于它可以高温操作,因此它对于处理聚苯乙烯PS的耐候性、热稳定性和可持续性的测定也有极大的帮助。
- 1 -。
alsic电子封装材料热导率以及散热特性

AlSiC介绍 ALSIC微电子封装材料是西安明科微电子材料有限公司与西北工业大学合作开发的新一代电子产品。
明科公司(Xi'an Miqam Microelectronics Materials Co., Ltd)是目前国内唯一一家可以生产这种材料的企业。
铝碳化硅(AlSiC)金属基热管理复合材料,是电子元器件专用电子封装材料,主要是指将铝与高体积分数的碳化硅复合成为低密度、高导热率和低膨胀系数的电子封装材料,以解决电子电路的热失效问题。
AlSiC的性能特点■ AlSiC具有高导热率(170~200W/mK)和可调的热膨胀系数(6.5~9.5×10-6/K),因此一方面AlSiC的热膨胀系数与半导体芯片和陶瓷基片实现良好的匹配,能够防止疲劳失效的产生,甚至可以将功率芯片直接安装到AlSiC基板上;另一方面AlSiC的热导率是可伐合金的十倍,芯片产生的热量可以及时散发。
这样,整个元器件的可靠性和稳定性大大提高。
■ AlSiC是复合材料,其热膨胀系数等性能可通过改变其组成而加以调整,因此电子产品可按用户的具体要求而灵活地设计,能够真正地做到量体裁衣,这是传统的金属材料或陶瓷材料无法作到的。
■ AlSiC的密度与铝相当,比铜和Kovar轻得多,还不到Cu/W 的五分之一,特别适合于便携式器件、航空航天和其他对重量敏感领域的应用。
■ AlSiC的比刚度(刚度除以密度)是所有电子材料中最高的:是铝的3倍,是W-Cu和Kovar的5倍,是铜的25倍,另外AlSiC的抗震性比陶瓷好,因此是恶劣环境(震动较大,如航天、汽车等领域)下的首选材料。
■ AlSiC可以大批量加工,但加工的工艺取决于碳化硅的含量,可以用电火花、金刚石、激光等加工。
■ AlSiC 可以镀镍、金、锡等,表面也可以进行阳极氧化处理。
■ 金属化的陶瓷基片可以钎焊到镀好的AlSiC基板上,用粘结剂、树脂可以将印制电路板芯与AlSiC粘合。
TA Instruments产品介绍说明书

THERMAL ANALYSISDifferential Scanning Calorimetry (DSC) Q2000 4Q20 6DSC Technology 8Accessories 10Temperature Control Options 14Tzero ® & MDSC ®Technology 18Thermomechanical Analysis (TMA)Q400EM/Q400 98 Q400 Technology 100Modes of Deformation 102TMA Theory/Modes of Operation 104Applications 108Dynamic Mechanical Analysis (DMA) Deformation Modes & Sample Size 80Subambient Operation 81Q800 Technology 82Modes of Deformation 84Accessories 86DMA Theory 90Modes of Operation 91Vapor Sorption AnalysisVTI-SA + 58VTI-SA + Technology 60Q5000 SA 64Q5000 SA Technology 66Applications 72Simultaneous DSC/TGAQ600 50SDT Technology 52Applications 54Thermogravimetric Analysis (TGA)Q500 32Q50 34Q500/Q50 Technology 36TGA Accessories & Options 38Applications 44AnAlysis5958Vti-Sa + sPECiFiCATiOns Maximum Sample Weight 750 mg/5 gDynamic Range 100 mg/500 mg Weighing Accuracy +/- 0.1% Weighing Precision +/- 0.01% Sensitivity 0.1 µg/0.5 µg Signal Resolution 0.01 µg/0.05 µg Temperature Control Peltier Elements, Resistance Heaters Experimental Temperature Range 5 to 150°C Isothermal Stability +/- 0.1°C Relative Humidity Control Range See Figure Below Accuracy +/- 1% RH Humidity Control Closed Loop, Dew Point Analyzer Organic Solvent Capability Optional Camera/2.5x Microscope Accessory Optional Raman Probe Accessory Optional The VTI-SA + Vapor Sorption Analyzer is a continuous vapor flow sorption instrument for obtaining precision water and organic vapor isotherms at temperatures ranging from 5°C to 150°C at ambient pressure. The VTI-SA + combines the features of VTI’s original SGA design with almost two decades of field-proven performance: the isothermal aluminum block construction, the three isolated thermal zones and chilled-mirror dew point analyzer for primary humidity measurements with the field-proven TA Instruments thermobalance technology… all to provide precise and accurate gravimetric measurements with excellent temperature and RH stability.Temperature (˚C)R e l a t i v e H u m i d i t y (%R H )*Performance may vary slightly, depending on laboratory conditions6160Symmetrical Microbalance DesignResolution and Stability of the MicrobalancePrecision Humidity MeasurementsAs part of our standard design, the VTI-SA + employs a chilled mirror dew point analyzer (a NIST-traceable standard for humidity) to determine the absolute relative humidity at the sample. In applications where RH control is critical (as in most pharmaceutical studies), chilled-mirror dew point analyzers are the preferred method, because of the absence of drift and long term stability.Sorption Testing Using an Organic VaporThe VTI-SA + can also be configured for organic vapor sorption. In the VTI-SA +, the concentration of the organic vapor in the gas stream reaching the sample is determined by the fraction of gas going through the organic solvent evaporator and the fraction of dry gas.In competitive systems, assumptions are made that the evaporator is 100% efficient and that the temperature of the evaporator is constant from low to high concentrations. The VTI-SA + system measures the temperature of the organic solvent in the evaporator and uses this information together with the Wagner equation to control the organic vapor concentration in the gas phase. This method solves the issue of adiabatic cooling of the solvent, a major source of error in competitive systems.The solvent containers/evaporators are easily removed and exchanged so there is no need for decontamination or cleaning of the system when changing organic solvents or reverting to water sorption experiments. For safety, the evaporator compartment is purged with dry nitrogen and fitted with a combustible gas sensor with an audible alarm that, when triggered, shuts down the power to the analyzer.Simultaneous Microscope Camera or Raman Measurement Sample Chamber Design62Temperature Controlled Thermobalance Included Dynamic Range 100 mg Weighing Accuracy +/- 0.1% Weighing Precision +/- 0.01% Sensitivity < 0.1 µg Baseline Drift* < 5 µg Signal Resolution 0.01 µg Temperature Control Peltier Elements Temperature Range 5 to 85°C Isothermal Stability +/- 0.1°C Relative Humidity Control Range 0 to 98% RH Accuracy +/- 1% RH Autosampler – 10 samples** Included Platinum™ Software Included Sample PansQuartz or Metal-Coated Quartz 180 µLPlatinum 50, 100 µL Aluminum Sealed Pan 20 µL The patented Q5000 SA delivers the performanceand reliability required in a leading sorption analyzerin a compact, user-friendly design. The Q5000SA is designed for manual or automated sorptionanalysis of materials under controlled conditions oftemperature and relative humidity (RH ). Its designintegrates our latest high-sensitivity, temperature-controlled thermobalance with an innovative humiditygeneration system, multi-position autosampler,and powerful Advantage™ software with technique-specific programs and Platinum™ features. Q5000 SAsPECiFiCATiOns 65* Over 24 hours at 25˚C and 20 % RH with empty metal coated quartz pans ** Optional tray accommodates 25 samples for use with platinum and sealed aluminum pansHumidity Control Chamber67MFC N 2Thermobalance Autosampler Sample Crucibles6871Vapor Sorption analysis is an established technique for determining the effect on materials of exposure to controlled conditions of temperature and humidity. Isotherm and Isohume™ experiments are the most commonly performed analyses.All TA Instruments sorption analyzers perform a range of essential sorption experiments such as time-courses, isotherms (constant temperature, variable RH), and isohumidity (Isohume™) experiments (constant RH, variable temperature). Complex protocols with step changes in temperature and RH can be defined and saved for later use. Also, multiple experiments can be run sequentially without further operator assistance.In isothermal experiments, a weighed sample is “dried” externally, or preferably in the instrument, and exposed to a series of humidity step changes at constant temperature. The sample is staged at each humidity level until no further weight change is detected or a set time has elapsed. A data point is recorded, the humidity is changed in 5 or 10% controlled RH steps, and the process repeated in an increasing or decreasing procedure. Isohume experiments involve a series of temperature step changes at constant humidity and result in similar plots. They are used to determine how sample exposure to a given humidity results in a physiochemical change, such as a change in the sample’s hydration state. The curve shape provides useful information to this end.TA Instruments analysis software offers Sorption Analysis, BET Analysis, and GAB programs. In addition, the full power and flexibility of our renowned Universal Analysis software provides for easy data manipulation, advanced reporting, plotting, and file exporting capabilities. In addition, advanced data reduction of VTI-SA+ data can be performed using custom-designed data analysis packages. Analysis options include:• Kinetic analysis for the determination of rate constant of adsorption • Isosteric heat of adsorption using the Clausius-Clapeyron equation• Surface area calculation using the BET equation for either water or organic vaporsGraVimetric Vapor Sorption analySiS General practice73Hydrate FormationThe figure to the right contains the experimental results demonstrating the formation of a hydrate. The hydrate formation is characterized by a plateau in the desorption branch of the isotherm. In this example the hydrate is formed at around 45% RH. The sample adsorbs about 4.5% by weight water and does not lose the water of hydration until the RH is lowered below 25%. This hydrate would be considered as a labile or unstable hydrate.Characterization of Morphological StabilityExposure to elevated humidity can initiate morphological changes in some pharmaceutical materials, particularly in amorphous sugars. As the humidity is increased, the adsorbed water plasticizes the material and lowers the glass transition. When the glass transition temperature decreases to the experimental temperature, crystallization will typically occur. The data in the figure below show the behavior of amorphous lactose at 25°C under a constant increase in humidity. Note how the character in the measured weight signal is indicative of a variety of morphological changes including the glass transition and subsequent crystallization of the amorphous phase.72Evaluation of Amorphous StructurePharmaceutical scientists are often interested in determining the amount of amorphous material in a drug formulation. As the amorphous and crystalline forms are chemically identical, classical analysis techniques are often insensitive to amorphous content. The figure below shows the moisture sorption analysis of a generic drug in its amorphous and crystalline forms. As the amorphous form absorbs significantly more water, the Q5000 SA can be used to quantify relative amorphous content in drug mixtures.Analyzing Small Amounts of PharmaceuticalsWhen evaluating pharmaceuticals it is common for only small amounts of material to be available for conducting multiple analytical tests. Hence, the ability to work with small samples is critical. The low baseline drift of the Q5000 SA means that good results can be obtained on even 10-20 milligrams of a crystalline drug, such as prednisone, which adsorbs <0.1% moisture over a broad humidity range. The sorption results shown below represent about 15 micrograms of weight change full-scale. The reversibility (lack of hysteresis) in the sorption/desorption profile for prednisone (as well as the low level of moisture adsorbed) indicates that the moisture picked up by the material is adsorbed on the surface of the materialrather than being absorbed into its structure.5.00.01.02.03.04.0-1.0W e i g h t (% c h a n g e )Relative Humidity (%)W e i g h t (%)Relative Humidity (%)W e i g h t C h a n g e(%)0.000.080.060.040.02Relative Humidity (%)W e i g h t C h a n g e (%)75Packaging Film AnalysisIn addition to evaluation of the actual pharmaceutical formulations, sorption analysis can also be valuable in comparing the polymeric films which are being considered for packaging the drugs and other materials. The figure to the right shows comparative profiles for two different packaging materials undergoing temperature and relative humidity cycling. Film A adsorbs and desorbs moisture at a more rapid rate than the other film evaluated which suggests it may not be suitable for packaging moisture sensitive compounds.Rate of DiffusionThe VTI-SA + can be equipped with a diffusion cell which allows for the direct measurement of the permeability of a film or membrane for a particular solvent vapor. The cell consists of a cavity that is filled either with a desiccant or absorber, a gasketed lid for attaching the film to be tested, and a wire stirrup to hang the assembled cell on the hang-down wire of the balance. Any vapor permeating through the film gets absorbed immediately and the weight of the cell will increase until steady-state conditions are reached. The normalized rate of permeation is obtained from the slope of this line (weight per unit time) and the diameter of the permeating film.74Organic Vapor Sorption (VTI-SA +)With the organic vapor sorption capability, the VTI-SA +can obtain not only water sorption isotherms, but can also be used to measure organic vapor isotherms. The use of organic vapor increases the sensitivity of the sorption measurement for many pharmaceutical and polymer materials, and provides information on the specificity of solvent adsorption for many materials. In the first figure, the time course data for the adsorption of ethanol on activated carbon is shown. The sample is initially dried at 0% RH , then the relative pressure of the ethanol is stepped in 0.10 increments.This second figure shows the sorption isotherm plot for the carbon/ethanol experiment, excluding the initial drying step. The sample exhibits a significant adsorption at low solvent concentrations. This is typical of the particle and internal pore-size distribution of activated carbon which is designed to allow for rapid gas-phase adsorption with low pressure drop.7LPH PLQ:H L J K W-2108801006040200642TimeW e i g h t C h a n g e (%)R e l a t i v eH u m i d i t y (%)Rel Pressure (req)W e i g h t C h a n g e (%)35-5-515250600400200800Temperature (˚C)W e i g h t C h a n g e (%)R e l P r e s s (r e q )0.001.00.200.400.600.80001。
质谱 加热块英文

质谱加热块英文Mass Spectrometry and Heating Block: Revolutionizing Analytical TechniquesMass spectrometry is a powerful analytical technique that has revolutionized the field of scientific research. This technology, combined with the use of a heating block, has become an indispensable tool in various industries, from chemistry and biology to environmental science and forensics. In this comprehensive essay, we will explore the principles, applications, and advancements of mass spectrometry and its integration with heating block technology.At the heart of mass spectrometry lies the ability to separate and identify the components of a given sample based on their mass-to-charge ratio. This process involves the ionization of the sample, the separation of the ions based on their mass-to-charge ratio, and the detection of these ions by a specialized detector. The resulting mass spectrum provides a wealth of information about the chemical composition and structure of the analyte, allowing researchers to gain valuable insights into a wide range of scientific phenomena.One of the key components in mass spectrometry is the heatingblock, which plays a crucial role in the sample preparation and ionization processes. The heating block is a device that heats the sample to a specific temperature, facilitating the vaporization and ionization of the analyte. This step is particularly important for the analysis of thermally labile or high-molecular-weight compounds, as the heating block ensures the efficient conversion of the sample into a gas-phase form, which is essential for the subsequent mass spectrometric analysis.The integration of mass spectrometry and heating block technology has led to significant advancements in various fields of study. In the pharmaceutical industry, for example, mass spectrometry coupled with heating block analysis has become an indispensable tool for the characterization and quality control of drug compounds. By precisely controlling the temperature of the sample, researchers can accurately identify and quantify the active ingredients, impurities, and degradation products in drug formulations, ensuring the safety and efficacy of the final product.Similarly, in the field of environmental analysis, mass spectrometry and heating block technology have played a crucial role in the detection and quantification of pollutants, such as pesticides, heavy metals, and organic compounds, in air, water, and soil samples. The ability to vaporize and ionize these compounds using the heating block allows for their efficient separation and identification by themass spectrometer, enabling researchers to monitor environmental contamination and develop effective remediation strategies.In the realm of forensic science, mass spectrometry coupled with heating block analysis has become an invaluable tool for the identification and characterization of a wide range of evidence, including illicit drugs, explosives, and trace evidence. The heating block's ability to vaporize and ionize these complex samples, combined with the high sensitivity and selectivity of mass spectrometry, has revolutionized the way forensic investigators approach the analysis of evidence, leading to more accurate and reliable results.Beyond these traditional applications, the integration of mass spectrometry and heating block technology has also found its way into the field of biological research. Researchers have utilized this powerful combination to study the structure and function of proteins, lipids, and other biomolecules, providing insights into the underlying mechanisms of biological processes and the development of new therapeutic strategies.As technology continues to evolve, the capabilities of mass spectrometry and heating block instrumentation have also experienced significant advancements. The development of more sensitive and selective mass analyzers, coupled with the introductionof advanced ionization techniques and improved sample handling methods, has led to enhanced analytical performance and the ability to tackle increasingly complex analytical challenges.Moreover, the integration of mass spectrometry and heating block technology with other analytical techniques, such as chromatography and spectroscopy, has further expanded the scope of applications and the depth of information that can be obtained from a single analysis. This synergistic approach has enabled researchers to gain a more comprehensive understanding of the samples under investigation, leading to more accurate and reliable results.In conclusion, the integration of mass spectrometry and heating block technology has revolutionized the field of analytical science, providing researchers with a powerful tool for the identification, characterization, and quantification of a wide range of analytes. From the pharmaceutical industry to environmental monitoring and forensic investigations, this powerful combination has become an indispensable part of the scientific landscape, driving advancements and enabling groundbreaking discoveries across various disciplines. As technology continues to evolve, the future of mass spectrometry and heating block analysis promises even more exciting developments and applications that will shape the course of scientific research and discovery.。
顶尖科技 TT-N-851 恒温器使用说明书

Thermostat Quick Reference Thermostat Operation Thermostat OptionsContact Us and Warranty Registration2345Congratulations on purchasing a new thermostat. This thermostat was designed to the highest reliability and ease of use standards. Thank you for choosing TopTech.® U.S. Registered Trademark. Patents pending.Copyright © 2010 Pro1 IAQ, Inc. All rights reserved.Rev. 1026Table of ContentsPage1Need Help?For assistance with this productplease visit or call TopTech Customer Care toll-free at 888-776-1427 during normal business hours (Mon-Fri 9 AM - 6 PM Eastern).Una versión española de este manual puede ser descargadaen Battery information2On the back of the thermostat insert 2 AA Alkaline batteries (included).Pull the thermostat directly away from the wall to access the batteries. A firm tug will be required to remove the thermostat from the subbase mounted on the wall.Getting to know your thermostat2Fan Button 3System Button 4Button Access Door5Temperature Setpoint Buttons6Light Button (Glow in the dark)612345LCD1+1 will appear in the display when second stage of heat or cool is on. +2 will appear for third stage of heat.System operationindicators: The COOL, HEAT or FAN icon will display when the COOL, HEAT or FAN is on.NOTE: The compressor delay feature is active if these icons are flashing. The compressor will not turn on until the 5 minute delay has elapsed.Replace batteries when this indicator is shown.Indicates the current Displays the user selectable setpoint Use the “+” or “-” keys to select your desired room temperature. A copy of the OperatingManual can be downloaded at FanSystem3Easy to use controlsLCD Display:See page 2 for details about this display read out and icons.12345Glow in the Dark Light Button:The glow in the dark light button will self illuminate for several hours after exposure to ambient light. This button turns on the display light when pressed.Temperature Setpoint Buttons:Press the + or - buttons to select the desired room temperature.Fan Key:Select ON or AUTO . The ON key will run the fan continuously. The AUTO key will cycle the fan on only when the heating or cooling system is on.System Key:Selects the operation mode of your HVAC system. Selecting HEAT turns on the heat mode. Selecting COOL turns on the air conditioning mode. Selecting OFF turns both heating and cooling off. Selecting AUTO will turn the HEAT or COOL on as needed. (EM Heat will appear as an option if operating a heat pump. EM Heat setting will turn on Emergency Heat)12345A Note About Auto Changeover: Auto changeover will switch between heating and cooling as needed. It is very important to make sure the coolingsetpoint temperature is at least 3º above the heating setpoint temperature and that the heating setpoint temperature is at least 3º below the cooling setpoint temperature.Use the “+” or “-” keys to select your desired room temperature. A copy of the OperatingManual can be downloaded at FanSystemFilter Change ReminderIf your installing contractor has configured the thermostat to remind you when the air filter needs changed, you will see FILT in the display when your air filter needs changed. FILT will be shown in the display after your system has run long enough to require an air filter change.Resetting the filter change reminder:When FILT reminder is displayed, you should change your air filter and reset the reminder by holding down the FAN button for 3 seconds.4to reset filter reminder.5Name: ____________________________Address: ________________________________________________________City: ____________________________State: ____________________________Zip:_____________________Thermostat Model: _____________________Date Installed:_____________________Contact Us InformationTopTech Warranty Registration:TopTech by Pro11111 S. Glenstone Suite 2-100Spring eld, MO 65804Toll-free: 1-888-776-1427Toll Number (Outside the USA): 330-821-3600Web: Hours of Operation: Monday - Friday 9 AM - 6 PM EasternComplete form and mail to:TopTech by Pro11111 S. Glenstone Suite 2-100Spring eld, MO 65804。
激光导热分析仪LFA427

激光导热分析仪LFA 427简介:对于材料或组分的热传导性能描述,导热系数与热扩散系数是最为重要的热物性参数。
激光闪射法是导热测试领域最为广泛使用的一种方法,用于精确测量材料的热扩散系数并计算导热系数。
而耐驰公司推出的激光导热仪 LFA 427 则代表了世界范围内同类产品的最高水平。
LFA 427 具有高精度、高重复性、测量快速、样品支架种类丰富、测试气氛可自由设定等突出优点,其总的测量温度范围为 -120℃-2800℃。
LFA 427 最新推出带高温计的特别配置版,可在室温至 2800℃的宽广温度范围内进行测量。
LFA 427 的样品适应面极广,包括陶瓷、玻璃、金属、熔融物、液体、粉末、纤维与多层材料等各种材料,从低导热材料直至最高导热系数的金刚石,都可在相同的速度与精度下进行测量。
仪器直接测试的是随温度而变的热扩散系数,若结合比热值(通常使用DSC 404 F1 Pegasus®进行测试,也可在 LFA 427 上使用比较法测得)与密度(密度随温度的变化使用热膨胀仪 DIL 402 C 测量计算),则可进一步计算导热系数。
测量所使用的激光能量、脉冲宽度、气氛与真空均可自由选择,可以针对不同的样品性质设定最佳的测量条件。
本仪器拥有完全密封的系统,设计上注重节省空间,其安全等级达到了最高级(1级),操作时不需要任何特殊的安全措施。
软件功能先进,允许仪器工作于手动或全自动模式。
并提供特殊支架,用于测试粉末,液体,矿渣,纤维和夹层样品。
LFA 427 是最强大与灵活的 LFA 系统,适用于包括汽车制造、航空航天与能源技术在内的各种领域的常规材料与新型高性能材料的表征。
LFA 427 - 技术参数•温度范围:-120—400℃, RT ... 1300℃, RT ... 1500℃, RT ... 2000℃/2800℃(四种可选的炉体类型)•升降温速率:0.01-50 K/min(取决于相应炉体)•激光能量:20 J/pulse(功率与脉冲宽度可调)•使用红外检测器,进行非接触式的样品表面温升信号测试•热扩散系数范围:0.01-1000 mm2/s•导热系数范围:0.1—2000 W/m*K•样品直径:6—12.7 mm(另可选 20 mm 特殊规格)•样品厚度:0.1—6 mm•样品支架:氧化铝,石墨•熔融金属容器:蓝宝石•液体样品容器:铂金•气氛:惰性,氧化,还原,静态,动态•高真空密闭系统,真空度 10-5mbar用于片状固体样品测试的标准样品支架LFA 427 - 软件功能LFA 427 的测量与分析软件是基于MicroSoft Windows® 系统的Proteus® 软件包,它包含了所有必要的测量功能和数据分析功能。
IRISYS IRI 1010 Thermal Imaging Radiometer

Mar 2007IPU 40125IRI 1010Thermal Imaging RadiometeraThe IRI 1010Thermal Imaging RadiometerInnovative ThermalImaging The IRI1010 is a groundbreaking thermalimager product, which brings the benefits of thisversatile technology to the professional, thetradesperson and the non-specialist alike.With the aid of the real-time thermal imagedisplay, the user can find, identify and measurethe temperature of problem areas quickly andwith confidence.The flexibility, ease of use and above all the lowcost of this product extend the normalapplication areas for thermal imaging fromprofessional use to wider use in industrial,commercial and domestic applications.Typical applications for the IRI 1010 include:• Predictive and Preventative Maintenance• Plumbing and Electrical• Research and Development• HVAC + refrigeration troubleshooting• Vehicle Maintenance• General Industrial/DomesticProduct DescriptionThe ergonomically designed imager houses the complete uncooled camera core together with a long life Li-ion battery pack. For ease of use the image is displayed on a large 31/2”colour LCD display with backlight. The thermal imaging radiometer is ideal for the engineer who is experienced in the use of conventional spot temperature measuring radiometers, but now wants to move to the next level.OperationDesigned for self-contained use, the camera is the ideal tool for all users wanting temperature measurement and display. The high capacity, rechargeable Li-ion battery allows continuous operation for up to 6 hours. The IRI 1010 is radiometric and hot spots can be identified by use of a trigger-activated laser pointer.© 2007 InfraRed Integrated Systems Limited (IRISYS). No part of this publication may be reproduced without prior permission in writing from InfraRed Integrated Systems Limited. This document gives only a general description of the product and except where expressly provided otherwise shall form no part of any contract. IRISYS have a policy of continuous product improvement and reserve the right to change the specification of the products and descriptions in this data sheet. Prior to ordering products please check with IRISYS for current specification details.SPECIFICATIONPERFORMANCETemperature range: -10o C to +300oC Field of view (FOV): 20o x 20 o Spectral Response: 8 to 14 µmSensitivity:~0.3o C @ 30o C Displayed Image: 192 x 192 pixels Detector: 16x16 pixel array Frame rate:8HzFocal Range:0.7m to infinityLASER POINTERA built in Class 2 laser is supplied to highlight the reference pixel.IMAGER POWER SUPPLYBattery: Lithium-ion field rechargeable.Operation time: Up to 6 hours continuous operationAC operation: AC adaptor suppliedMECHANICALHousing: Impact Resistant Plastic Dimensions: 230x120x90mm Weight: 0.70kg Mounting: Handheld & Tripod mounting ¼” BSWSETTINGS AND CONTROLS• On/Off soft power control • User selectable span control • User selectable level control • Auto adjust span and level• Display palettes: red-blue, green-blue and greyscale• Laser trigger switch • Readout in °C, °F or K• User selectable image integration • User selectable emissivity values • User selectable reflected temperatureFEATURES• Real-time image and temperature measurement display• Large bright 3 ½ inch display • Simple operation• Battery Charge indicator • Lightweight • Laser PointerIRI 1010 INCLUDESIR Camera, Battery, AC Adaptor, User manual, Carrying Case.Whilst IRISYS endeavor to ensure that all descriptions, weights, temperatures, dimensions and other statistics contained in this product information are correct, they are intended to give a general idea of the product only and IRISYS do not warrant their accuracy or accept liability for any reliance on them. IRISYS have a policy of continuous product improvement and reserve the right to change the specification of the products and descriptions in this data sheet. Prior to ordering products please check with IRISYS for current specification details. This product is protected by patents EP 0 853 237 B1 and US 6,239,433 B1. Other patents pending. All brands and product names are acknowledged and may be trademarks or registered trademarks of their respective holders. aInfraRed Integrated Systems Ltd,Park Circle, Tithe Barn WaySwan Valley, Northampton, NN4 9BG, UK Telephone: +44 (0) 1604 594200 Fax: +44 (0) 1604 594210 e-mail: ***************.uk web site: Innovative ThermalImagingIRI 1010。
Series 925 MicroPirani

Series 925MicroPirani™ TransducerThe Series 925 MicroPirani™ transducer is a thermal conductivity gauge based on a unique, MEMS-based (Micro-Electro-Mechanical Systems) sensor. The 925 is used for vacuum pressure measurement and offers analog voltage output, digital interface and set point relays for process controlling.The 925 Transducer offers a wide measurement range from 1x10measurement of thermal conductivity. The MicroPirani sensor consists of a silicon chip with a heated resistive element forming one surface of a cavity. A cover on top of the chip forms the other surface of the cavity. Dueto the geometry of the sensor, convection cannot take place within the cavity and consequently, the sensor is insensitive to the mounting position. Gas molecules are passed by diffusion only to the heated element wherethe heat loss of the gas is measured.ApplicationsThe 925 can be used in many differentvacuum applications within the semiconductor,analytical, and coating industries:General vacuum pressure measurementForeline and roughing pressure measurementGas backfilling measurement and controlMass spectrometer controlActivation of UHV gaugesSystem process controlControl system pressureLike all thermal conductivity sensors, the 925 is sensitive to Array gas type. To compensate for gas dependency, the MicroPiranihas a number of common gas calibrations that can beselected via the digital interface. This makes it a simplesolution for locating medium to fine leaks in vacuum systems.The 925 has RS232, RS485, and EtherCAT digitalcommunication interface for setup of transducer parametersand to provide real time pressure measurement.The 925 also has a analog pressure output of 1 VDC/decadethat can be interfaced to external analog equipment forpressure readout or controlling. Other analog outputs andcurves can be selected via the digital user interface.The 925 has up to three mechanical relays which can beused for process control, examples are interlocking valvesor pumps. The 925 compact design significantly reducesthe amount of space occupied by a vacuum gauge. This isparticularly appealling to system designers and allows for amore compact vacuum system.Dra winga lDimensionNote: Unless otherwise specified, dimensions are nominal values in inches(mm referenced).PinOutsThree (3) set point relays and dual Aout, 15 pin D Subminiature and RJ45 EtherCAT IN/OUT ConnectorsSpecificationsSensor Type MicroPirani (MEMS Thermal Conductivity) Measuring Range 1.0 x 10-5 Torr to AtmosphereSet Point Range 5.0 x 10-4 Torr to 500 TorrCalibration Gas Air, Argon, Helium, Nitrogen, Hydrogen, H2O vapor, CO2, Xenon, NeonOperating Temperature Range 0° to 40°C (32° to 104°F)Maximum Bakeout Temperature 80°C (176°F), non-operatingCommunication RS485 / RS232 (4800 to 230400 Baud)Controls Zero adjust, atmosphere adjust, pressure units, baud rate, address, factory default, gas type;set point functions: value, hysteresis, direction, enable analog output transducer status, switch,LEDtestStatus Pressure reading and units, set point, operating time, transducer temperature, user tag, model,device type, serial number, firmware and hardware versions part number, manufacturer Analog Output 1 to 9 VDC, 100W maximum output impedance, 1 volt/decadeAnalog Output Resolution 16 bitRelays (Optional) 925 - 3 relays SPDTRelay Contact Rating 1 A @ 30VAC/DC, resistiveRelay Response<100 msec maximumPower Requirements 9 to 30 VDC, < 1.5 watts maxAccuracy (Typical)1 5 x 10-4 to 10-3 Torr ±10% of Reading10-3 to 100 Torr ±5% of Reading100 Torr to atm ±25% of ReadingRepeatability (Typical)110-3 to 100 Torr ±2% of ReadingOverpressure Limit 3000 Torr absoluteInstallation Orientation AnyInternal Volume (KF16) 2.80 cm3Materials Exposed to Vacuum 304 stainless steel, Silicon, SiO2, Si3N4, Gold, Viton®,Low out gassing epoxy resinElectronic Casing and Flange 304 stainless steelWeight (KF 16) 170 gCompliance CE, ETG.5003.2080 Vacuum Pressure Gauge 1 Accuracy and repeatability are typical values measured with Nitrogen gas at ambient temperature after zero adjustment.Ordering InformationOrdering Code Example: 925-11030Code Configuration925 with Displ a yThe optional integrated touch-screen display is user configurable; the user can change pressure units, orientation and has access to set point parameters as well as gas type. The display also indicates the status of the available set point relays. Displayed reading can be seen from >5 meters away on the high contrast display.PDR900 Power Supply and DisplayThe PDR900 power supply and readout unit is a stand alone, single channel controller for use with the Series 900 digital vacuum transducers. It can be used as a stand-alone power supply readout unit or as a tool for configuration, calibration and diagnostics of system integrated transducers in OEM applications.+1-978-645-5500 I +1-800-227-8766MKS products provided subject to the US Export Regulations. Export, re-export, diversion or transfer contrary to US law (and local country law) is prohibited.mksinst ™ and MicroPirani ™ are trademarks of MKS Instruments, Inc., VCR ® is a registered trademark of Swagelok Co. Viton ® is a registered trademark of E.I Dupont Co., Inc. EtherCAT ® is a registered trademark and patented technology, licensed by Beckhoff Automation GmbH, Germany. U.S. Patent No. 6,672,171. Other patents pending.925_01/20©2020 MKS Instruments, Inc.Specifications are subject to change without notice.。
浇注料
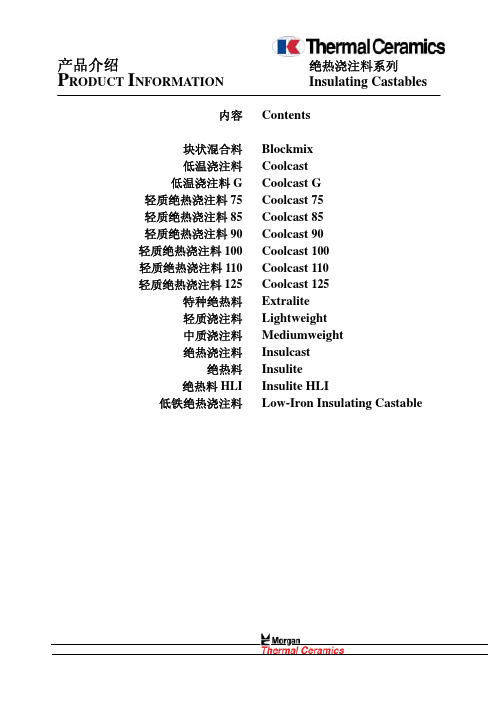
技术数据 Technical Data
结合形式 最高使用温度 耐火度(塞格锥) 110℃干燥 体积密度(kg/m3) 施工用量
Kind of Bond Maximum Service Temperature ℃ Refractoriness℃ (Seger Cone) Bulk Density (kg/m3) Dried to 110℃ Material Requirement (kg/m3)
水化 Hydraulic 1100 1250(8)
750
718
Test Temperature(℃) 检测温度(摄氏度)
110℃ 1000℃ 1100℃
Cold Crushing Strength (MPa) 冷态耐压强度 (MPa)
1.9 0.7 0.7
Linear Shrinkage (%) 线性收缩(%) NIL(微量)
水化 Hydraulic 1200 750 715
Test Temperature(℃) 检测温度(摄氏度)
110℃ 1000℃ 1200℃
Cold Crushing Strength (MPa) 冷态耐压强度 (MPa) 1.1 0.8 1.4
Linear Shrinkage (%) 线性收缩(%) NIL(微量) 0.6
Water Addition (%) 加水量 (%)
Samples Prepared By 制样方法
60~80
Casting 浇注
注:以上数据为“平均值”。 Notes:The above data are average values.
修订号:1 Revision :1
发布日期:2001 年 1 月 1 日 Date of issue:1 Jan 2001
差示扫描量热法测定钛合金的相变温度

差示扫描量热法测定钛合金的相变温度差示扫描量热法是一种准确测量物质相变温度的方法,其基本原理是通过测量样品的热容或热量变化来确定物质的相变温度。
本文将介绍使用差示扫描量热法测定钛合金的相变温度。
1. 实验原理首先,在样品温度恒定时,记录样品的热容变化曲线。
然后,通过对样品进行升温,记录样品的热量变化曲线。
最后,通过将升温时的热容变化曲线与热量变化曲线分别与已知的相变温度进行比较,确定钛合金的相变温度。
2. 实验步骤差示扫描量热仪量热杯样品盖2.2.1 样品制备将钛合金样品制成薄片,保证样品在加热和降温的过程中获得均匀的温度和热量变化。
(1)将热容量杯放置在量热仪中,并将待测样品置于量热杯中。
(2)将温度控制器设置在室温,并开启热容量杯的循环水;(3)根据实验要求设置升温和降温速率,并启动差示扫描量热仪;(4)记录样品在升温和降温过程中的热容量变化曲线和热量变化曲线。
2.2.3 数据处理(1)将热容量变化曲线和热量变化曲线与已知的相变温度进行比较,确定钛合金的相变温度;(2)计算样品相变时的热焓变化;(3)即可得到钛合金的相变热和相变热功率。
3. 实验结果与分析通过差示扫描量热法测定钛合金的相变温度,得到相变温度为921.6°C。
根据实验数据计算得到相变热为127.5J/g,相变热功率为2.5W。
这些数据可以帮助我们更好地理解钛合金的性质和应用。
4. 小结本实验介绍了差示扫描量热法测定钛合金的相变温度的方法和步骤。
实验结果表明,差示扫描量热法是一种准确测量物质相变温度的方法,并可以用于研究钛合金的性质和应用。
通过本实验,可以让学生更好地理解差示扫描量热法的基本原理和实验操作,同时也能够提高学生对钛合金材料性质和应用方面的认识。
Thermo-calc软件-TCCP用户指南 (有目录索引)

Thermo-Calc®User’s GuideVersion PThermo-Calc Software ABStockholm Technology ParkBjörnnäsvägen 21SE-113 47 Stockholm, SwedenCopyright © 1995-2003 Foundation of Computational ThermodynamicsStockholm, Sweden目录第1部分一般介绍 (12)1.1 计算热力学 (12)1.2 Thermo-Calc软件/数据库/界面包 (12)1.3 致谢 (13)1.4 版本历史 (13)1.5 Thermo-Calc软件包的通用结构 (13)1.6 各类硬件上Thermo-Calc软件包的有效性 (14)1.7 使用Thermo-Calc软件包的好处 (14)第2部分如何成为Thermo-Calc专家 (14)2.1 如何容易地使用本用户指南 (14)2.2 如何安装和维护Thermo-Calc软件包 (16)2.2.1 许可要求 (16)2.2.2 安装程序 (16)2.2.3 维护当前和以前版本 (16)2.2.4 使TCC执行更方便 (16)2.3 如何成为Thermo-Calc专家 (16)2.3.1 从TCSAB与其世界各地的代理获得迅速技术支持 (17)2.3.2 日常使用各种Thermo-Calc功能 (17)2.3.3 以专业的和高质量的标准提交结果 (17)2.3.4 通过各种渠道相互交换经验 (17)第3部分Thermo-Calc软件系统 (17)3.1 Thermo-Calc软件系统的目标 (17)3.2 一些热力学术语的介绍 (18)3.2.1 热力学 (18)3.2.2 体系、组元、相、组成、物种(System, component, phases, constituents and species) (18)3.2.3 结构、亚点阵和位置 (19)3.2.4 成分、构成、位置分数、摩尔分数和浓度(composition, constitution, site fractions, molefractions and concentration) (19)3.2.5 平衡态和状态变量 (19)3.2.6 导出变量 (22)3.2.7 Gibbs相规则 (25)3.2.8 状态的热力学函数 (25)3.2.9 具有多相的体系 (25)3.2.10 不可逆热力学 (26)3.2.11 热力学模型 (26)3.2.12 与各种状态变量有关的Gibbs能 (27)3.2.13 参考态与标准态 (27)3.2.14 溶解度范围 (28)3.2.15 驱动力 (28)3.2.16 化学反应 (28)3.2.17 与平衡常数方法相对的Gibbs能最小化技术 (28)3.2.18 平衡计算 (29)3.3 热力学数据 (30)3.3.1 数据结构 (30)3.3.3 数据估价 (32)3.3.6 数据加密 (33)3.4 用户界面 (34)3.4.1 普通结构 (34)3.4.2 缩写 (34)3.4.3 过程机制(history mechanism) (35)3.4.4 工作目录和目标目录(Working directory and target directory) (35)3.4.5 参数转换为命令 (36)3.4.6 缺省值 (36)3.4.7 不理解的问题 (36)3.4.8 帮助与信息 (36)3.4.9 出错消息 (36)3.4.10 控制符 (36)3.4.11 私人文件 (36)3.4.12 宏工具 (37)3.4.13 模块性 (37)3.5 Thermo-Calc中的模块 (37)3.5.1 基本模块 (37)3.7 Thermo-Calc编程界面 (39)3.7.1 Thermo-Calc作为引肇 (39)3.7.2 Thermo-Calc应用编程界面:TQ和TCAPI (40)3.7.3 在其它软件包中开发Thermo-Calc工具箱 (43)3.7.4 材料性质计算核材料工艺模拟的应用 (43)3.8 Thermo-Calc的功能 (44)3.9 Thermo-Calc应用 (44)第4部分Thermo-Calc数据库描述 (45)4.1 引言 (45)4.2 Thermo-Calc数据库描述形式 (45)第5部分数据库模块(TDB)——用户指南 (55)5.1 引言 (55)5.2 TDB模块中用户界面 (56)5.3 开始 (56)5.3.1 SWITCH-DATABASE (56)5.3.2 LIST-DATABASE ELEMENT (56)5.3.3 DEFINE_ELEMENTS (56)5.3.4 LIST_SYSTEM CONSTITUENT (56)5.3.5 REJECT PHASE (56)5.3.6 RESTORE PHASE (56)5.3.7 GET_DATA (56)5.4 所有TDB监视命令的描述 (56)5.4.1 AMEND_SELACTION (56)5.4.6 DEFINE_SPECIES (58)5.4.7 DEFINE_SYSTEM (58)5.4.8 EXCLUDE_UNUSED_SPECIES (58)5.4.9 EXIT (58)5.4.10 GET_DATA (58)5.4.11 GOTO_MODULE (59)5.4.12 HELP (59)5.4.13 INFORMA TION (59)5.4.14 LIST_DATABASE (60)5.4.15 LIST_SYSTEM (60)5.4.16 MERGE_WITH_DA TABASES (61)5.4.17 NEW_DIRECTORY_FILE (61)5.4.18 REJECT (61)5.4.19 RESTORE (62)5.4.20 SET_AUTO_APPEND_DA TABASE (62)5.4.21 SWITCH_DA TABASE (63)5.5 扩展命令 (64)第6部分数据库模块(TDB)——管理指南 (64)6.1 引言 (64)6.2 TDB模块的初始化 (65)6.3 数据库定义文件语法 (66)6.3.1 ELEMENT (67)6.3.2 SPECIES (67)6.3.3 PHASE (67)6.3.4 CONSTITUENT (67)6.3.5 ADD_CONSTITUENT (68)6.3.6 COMPOUND_PHASE (68)6.3.7 ALLOTROPIC_PHASE (68)6.3.8 TEMPERA TURE_LIMITS (68)6.3.9 DEFINE_SYSTEM_DEFAULT (69)6.3.10 DEFAULT_COMMAND (69)6.3.11 DATABASE_INFORMATION (69)6.3.12 TYPE_DEFINITION (69)6.3.13 FTP_FILE (70)6.3.14 FUNCTION (70)6.3.15 PARAMETER (72)6.3.16 OPTIONS (73)6.3.17 TABLE (73)6.3.18 ASSESSED_SYSTEMS (73)6.3.19 REFERENCE_FILE (74)6.3.20 LIST_OF_REFERENCE (75)6.3.21 CASE与ENDCASE (76)6.3.22 VERSION_DA TA (76)6.5 数据库定义文件实例 (77)6.5.1 例1:一个小的钢数据库 (77)6.5.2 例2:Sb-Sn系个人数据库 (78)第7部分制表模块(TAB) (81)7.1 引言 (81)7.2 一般命令 (81)7.2.1 HELP (81)7.2.2 GOTO_MODULE (81)7.2.3 BACK (82)7.2.4 EXIT (82)7.2.5 PATCH (82)7.3 重要命令 (82)7.3.1 TABULATE_SUBSTANCE (82)7.3.2 TABULATE_REACTION (85)7.3.3 ENTER_REACTION (86)7.3.4 SWITCH_DA TABASE (87)7.3.5 ENTER_FUNCTION (88)7.3.6 TABULATE_DERIV A TIVES (89)7.3.7 LIST_SUBSTANCE (91)7.4 其它命令 (92)7.4.1 SET_ENERGY_UNIT (92)7.4.2 SET_PLOT_FORMAT (92)7.4.3 MACRO_FILE_OPEN (92)7.4.4 SET_INTERACTIVE (93)7.5 绘制表 (93)第8部分平衡计算模块(POL Y) (94)8.1 引言 (94)8.2 开始 (95)8.3 基本热力学 (95)8.3.1 体系与相 (95)8.3.2 组元(Species) (95)8.3.3 状态变量 (96)8.3.4 组分 (97)8.3.5 条件 (98)8.4 不同类型的计算 (98)8.4.1 计算单一平衡 (98)8.4.2 性质图的Steping计算 (99)8.4.3 凝固路径模拟 (99)8.4.4 仲平衡与T0温度模拟 (99)8.4.5 相图的Mapping计算 (101)8.4.6 势图计算 (101)8.4.7 Pourbaix图计算 (101)8.4.8 绘制图 (101)8.5.4 更高阶相图 (104)8.5.5 性质图 (104)8.6 普通命令 (104)8.6.1 HELP (104)8.6.2 INFORMA TION (104)8.6.3 GOTO_MODULE (105)8.6.4 BACK (105)8.6.5 SET_INTERACTIVE (105)8.6.6 EXIT (106)8.7 基本命令 (106)8.7.1 SET_CONDITION (106)8.7.2 RESET_CONDITION (107)8.7.3 LIST_CONDITIONS (107)8.7.4 COMPUTE_EQUILIBRIUM (107)8.7.6 DEFINE_MATERIAL (108)8.7.6 DEFINE_DIAGRAM (111)8.8 保存和读取POL Y数据结构的命令 (112)8.8.1 SA VE_WORKSPACES (112)8.8.2 READ_WORKSPACES (113)8.9 计算与绘图命令 (114)8.9.1 SET_AXIS_V ARIABLE (114)8.9.2 LIST_AXIS_V ARIABLE (114)8.9.3 MAP (114)8.9.4 STEP_WITH_OPTIONS (115)8.9.5 ADD_INITIAL_EQUILIBRIUM (117)8.9.6 POST (118)8.10 其它有帮助的命令 (118)8.10.1 CHANGE_STA TUS (118)8.10.2 LIST_STA TUS (119)8.10.3 COMPUTE_TRANSITION (120)8.10.4 SET_ALL_START_V ALUES (121)8.10.5 SHOW_V ALUE (122)8.10.6 SET_INPUT_AMOUNTS (122)8.10.7 SET_REFERENCE_STA TE (122)8.10.8 ENTER_SYMBOL (123)8.10.9 LIST_SYMBOLS (124)8.10.10 EV ALUATE_FUNCTIONS (124)8.10.11 TABULATE (124)8.11 高级命令 (125)8.11.1 AMEND_STORED_EQUILIBRIA (125)8.11.3 DELETE_INITIAL_EQUILIBRIUM (126)8.11.4 LIST_INITIAL_EQUILIBRIA (126)8.11.5 LOAD_INITIAL_EQUILIBRIUM (126)8.11.10 SELECT_EQUILIBRIUM (128)8.11.11 SET_NUMERICAL_LIMITS (128)8.11.12 SET_START_CONSTITUTION (129)8.11.13 SET_START_V ALUE (129)8.11.14 PATCH (129)8.11.15 RECOVER_START_V ALUE (129)8.11.16 SPECIAL_OPTIONS (129)8.12 水溶液 (132)8.13 排除故障 (133)8.13.1 第一步 (133)8.13.2 第二步 (133)8.13.3 第三步 (133)8.14 频繁提问的问题 (134)8.14.1 程序中为什么只得到半行? (134)8.14.2 在已经保存之后为什么不能绘图? (134)8.14.3 为什么G.T不总是与-S相同? (134)8.14.4 如何获得组元偏焓 (135)8.14.5 为什么H(LIQUID) 是零而HM(LIQUID)不是零 (135)8.14.6 即使石墨是稳定的为什么碳活度小于1? (135)8.14.7 如何获得过剩Gibbs能? (135)8.14.8 当得到交叉结线而不是混溶裂隙时什么是错的? (135)8.14.9 怎么能直接计算最大混溶裂隙? (136)第9部分后处理模块(POST) (136)9.1 引言 (136)9.2 一般命令 (137)9.2.1 HELP (137)9.2.2 BACK (137)9.2.3 EXIT (137)9.3 重要命令 (137)9.3.1 SET_DIAGRAM_AXIS (137)9.3.2 SET_DIAGRAM_TYPE (138)9.3.3 SET_LABEL_CORVE_OPTION (139)9.3.5 MODIFY_LABEL_TEXT (139)9.3.6 SET_PLOT_FORMAT (140)9.3.7 PLOT_DIAGRAM (141)9.3.8 PRINT_DIAGRAM (142)9.3.9 DUMP_DIAGRAM (143)9.3.10 SET_SCALING_STA TUS (144)9.3.11 SET_TITLE (144)9.3.12 LIST_PLOT_SETTINGS (144)9.4 实验数据文件绘图命令 (144)9.4.1 APPEND_EXPERIMENTAL_DA TA (144)9.4.2 MAKE_EXPERIMENTAL_DA TAFILE (145)9.5.3 SET_AXIS_LENGTH (147)9.5.4 SET_AXIS_TEXT_STATUS (147)9.5.5 SET_AXIS_TYPE (147)9.5.6 SET_COLOR (147)9.5.7 SET_CORNER_TEXT (148)9.5.8 SET_FONT (148)9.5.9 SET_INTERACTIVE_MODE (149)9.5.10 SET_PLOT_OPTION (149)9.5.11 SET_PREFIX_SCALING (149)9.5.12 SET_REFERENCE_STA TE (149)9.5.13 SET_TIELINE_STA TE (150)9.5.14 SET_TRUE_MANUAL_SCALING (150)9.5.15 TABULATE (150)9.6 奇特的命令 (150)9.6.1 PATCH_WORKSPACE (150)9.6.2 RESTORE_PHASE_IN_PLOT (150)9.6.3 REINIATE_PLOT_SETTINGS (151)9.6.4 SET_AXIS_PLOT_STATUS (151)9.6.5 SET_PLOT_SIZE (151)9.6.6 SET_RASTER_STATUS (151)9.6.8 SUSPEND_PHASE_IN_PLOT (151)9.7 3D图标是:命令与演示 (151)9.7.1 CREATE_3D_PLOTFILE (153)9.7.2 在Cortona VRML Client阅读器中查看3D图 (154)第10部分一些特殊模块 (155)10.1 引言 (155)10.2 特殊模块生成或使用的文件 (156)10.2.1 POL Y3文件 (156)10.2.2 RCT文件 (156)10.2.3 GES5文件 (156)10.2.4 宏文件 (157)10.3 与特殊模块的交互 (157)10.4 BIN模块 (157)10.4.1 BIN模块的描述 (157)10.4.2 特定BIN模块数据库的结构 (161)10.4.3特定BIN计算的演示实例 (162)10.5 TERN 模块 (162)10.5.1 TERN 模块的描述 (162)10.5.2 特殊TERN模块数据库的结构 (166)10.5.3 TERN模块计算的演示实例 (167)10.6 POT模块 (167)10.7 POURBAIX 模块 (167)10.8 SCHAIL 模块 (167)11.2 热化学 (168)11.2.1 一些术语的定义 (168)11.2.2 元素与物种(Elements and species) (168)11.2.3 大小写模式 (169)11.2.4 相 (169)11.2.5 温度与压力的函数 (169)11.2.6 符号 (170)11.2.7 混溶裂隙 (170)11.3 热力学模型 (170)11.3.1 标准Gibbs能 (171)11.3.2 理想置换模型 (171)11.3.3 规则溶体模型 (171)11.3.4 使用组元而不是元素 (172)11.3.5 亚点阵模型—化合物能量公式 (172)11.3.6 离子液体模型,对具有有序化趋势的液体 (172)11.3.7 缔合模型 (173)11.3.8 准化学模型 (173)11.3.9 对Gibbs能的非化学贡献(如铁磁) (173)11.3.10 既有有序-无序转变的相 (173)11.3.11 CVM方法:关于有序/无序现象 (173)11.3.12 Birch-Murnaghan模型:关于高压贡献 (173)11.3.13 理想气体模型相对非理想气体/气体混合物模型 (173)11.3.14 DHLL和SIT模型:关于稀水溶液 (173)11.3.15 HKF和PITZ模型:对浓水溶液 (173)11.3.16 Flory-Huggins模型:对聚合物 (173)11.4 热力学参数 (173)11.5 数据结构 (175)11.5.1 构造 (175)11.5.2 Gibbs能参考表面 (175)11.5.3 过剩Gibbs能 (175)11.5.4 存储私有文件 (175)11.5.5 加密与不加密数据库 (176)11.6 GES系统的应用程序 (176)11.7 用户界面 (176)11.7.1 模块性和交互性 (177)11.7.2 控制符的使用 (177)11.8 帮助与信息的命令 (177)11.8.1 HELP (177)11.8.2 INFORMATION (177)11.9 改变模块与终止程序命令 (178)11.9.1 GOTO_MODULE (178)11.9.2 BACK (178)11.9.3 EXIT (178)11.10 输入数据命令 (178)11.10.4 ENTER_SYMBOL (180)11.10.5 ENTER_PARAMETER (181)11.11 列出数据的命令 (183)11.11.1 LIST_DATA (183)11.11.2 LIST_PHASE_DA TA (183)11.11.3 LIST_PARAMETER (184)11.11.4 LIST_SYMBOL (185)11.11.5 LIST_CONSTITUENT (185)11.11.6 LIST_STATUS (185)11.12 修改数据命令 (185)11.12.1 AMEND_ELEMENT_DA TA (185)11.12.2 AMEND_PHASE_DESCRIPTION (186)11.12.3 AMEND_SYMBOL (188)11.12.4 AMEND_PARAMETER (189)11.12.5 CHANGE_STATUS (191)11.12.6 PATCH_WORKSPACES (191)11.12.7 SET_R_AND_P_NORM (191)11.13 删除数据的命令 (192)11.13.1 REINITIATE (192)11.13.2 DELETE (192)11.14 存储或读取数据的命令 (192)11.14.1 SA VE_GES_WORKSPACE (192)11.14.2 READ_GES_WORKSPACE (193)11.15 其它命令 (193)11.15.1 SET_INTERACTIVE (193)第12部分优化模块(PARROT) (193)12.1 引言 (193)12.1.1 热力学数据库 (194)12.1.2 优化方法 (194)1 2.1.4 其它优化软件 (195)12.2 开始 (195)12.2.1 试验数据文件:POP文件 (195)12.2.2 图形试验文件:EXP文件 (197)12.2.3 系统定义文件:SETUP文件 (197)12.2.4 工作文件或存储文件:PAR文件 (198)12.2.5 各种文件名与其关系 (198)12.2.6 交互运行PARROT模块 (199)12.2.6.3 绘制中间结果 (199)12.2.6.4 实验数据的选择 (199)12.2.6.6 优化与连续优化 (200)12.2.7 参数修整 (200)12.2.8 交互完成的变化要求编译 (201)12.3 交替模式 (201)12.4 诀窍与处理 (201)12.4.4 参数量 (201)12.5 命令结构 (201)12.5.1 一些项的定义 (201)12.5.2 与其它模块连接的命令 (201)12.5.3 用户界面 (201)12.6 一般命令 (201)12.7 最频繁使用的命令 (202)12.8 其它命令 (203)第13部分编辑-实验模块(ED-EXP) (203)第14部分系统实用模块(SYS) (203)14.1 引言 (203)14.2 一般命令 (203)14.2.1 HELP (203)14.2.2 INFORMA TION (204)14.2.4 BACK (205)14.2.5 EXIT (205)14.2.6 SET_LOG_FILE (205)14.2.7 MACRO+FILE_OPEN (205)14.2.8 SET_PLOT_ENVIRONMENT (206)14.3 Odd命令 (207)14.3.1 SET_INTERACTIVE_MODE (207)14.3.2 SET_COMMAND_UNITS (207)14.3.4 LIST_FREE_WORKSPACE (207)14.3.5 PATCH (207)14.3.6 TRACE (207)14.3.7 STOP_ON_ERROR (208)14.3.8 OPEN_FILE (208)14.3.9 CLOSE_FILE (208)14.3.10 SET_TERMINAL (208)14.3.11 NEWS (208)14.3.12 HP_CALCULATOR (208)14.4 一般信息的显示 (209)第15部分数据绘图语言(DATAPLOT) (215)第1部分一般介绍1.1 计算热力学在近十年内与材料科学与工程相联系的计算机计算与模拟的研究与发展已经为定量设计各种材料产生了革命性的方法,热力学与动力学模型的广泛结合使预测材料成分、各种加工后的结构和性能。
BM43THA-T2-2.0

Pin Description
2 Pin Descriptions
Table1 Pin Descriptions Pin 1 2 3 4 Function GND Thermistor ThermopileThermistor Thermopile+ Output DC voltage- pin. Description
BM43THA-T2
Ambient temperature compensation resistance- pin and GND. Ambient temperature compensation resistance+ pin. Output DC voltage+ pin.
Specification
Description
1. Description
1.1 General Description
BM43THA-T2
The BM43THA-T2 is a thermopile temperature sensor based on MEMS (micro-electromechanical systems) technology. This thermopile detector consists of thermopile MEMS chip, 5-14um infrared band pass filter, a NTC thermistor for temperature compensation and a small size TO Package. 1.2 Features Non-contact surface temperature measuring TO housing with an 5-14um infrared filter Using NTC thermistor for ambient temperature compensation High sensitivity Fast response time Wide working temperature: -40℃+120℃(Temperature range can be extended to +1000℃by using specific infrared lens) 1.3 Applications Non-contact human body temperature infrared thermometer Microwave oven Automatic induction equipment Heating, Ventilation and Air Conditioning(HVAC) Appliance 1.4 Package
Emulsions乳剂

Section 10EmulsionsBy Drs. Pardeep K. Gupta, Clyde M. Ofner and Roger L. SchnaareTable of Contents Emulsions (1)Table of Contents (1)Introduction and Background (3)Definitions (3)Types of Emulsions (3)Formation of an Emulsion (4)Determination of Emulsion Type (4)Miscibility or Dilution Test (4)Staining or Dye Test (4)Electrical Conductivity Test (4)Physical State of Emulsions (5)Pharmaceutical Application of Emulsions (5)Formulations (6)Typical Ingredients (6)Drug (6)Oil Phase (6)Aqueous Phase (6)Thickening Agents (6)Sweeteners (6)Preservative (6)Buffer (7)Flavor (7)Color (7)Sequestering Agents (7)Humectants (7)Antioxidants (7)Emulsifiers (7)Guidelines (7)Type of Emulsion Desired (7)Toxicity (8)Method of Preparation (8)Typical Formulas (8)Cod Liver Oil Emulsion (polysaccharide emulsifier) (8)Protective Lotion (divalent soap emulsifier) (8)Benzoyl Benzoate Emulsion (emulsifying wax emulsifier) (8)Barrier Cream (soap emulsifier) (9)Cold Cream (soap emulsifier) (9)All Purpose Cream (synthetic surfactant emulsifier) (9)Emulsifiers (10)Natural Products (10)Polysaccharides (10)Sterols (10)Phospholipids (10)Surfactants (10)Anionic Surfactants (11)Soaps (11)Detergents (11)Cationic Surfactants (11)Nonionic Surfactants (11)Finely Divided Solids (12)Methods to Prepare Emulsions (13)Classical Gum Methods (13)Dry Gum Method (13)Wet Gum Method (13)“In Situ” Soap Method (13)Lime Water/Vegetable Oil Emulsions (13)Other Soaps (13)With Synthetic Surfactants (13)Required HLB of the Oil Phase (14)HLB of Surfactant Mixtures (14)Emulsion Stability (15)Sedimentation or Creaming (15)Factors - Stoke’s Law (15)Droplet Size (15)Density Difference (15)The Gravitational Constant, g (15)Viscosity (15)Breaking or Cracking (16)Thermodynamics of Emulsions (17)Microemulsions (18)References (19)Selected Readings (19)Introduction and BackgroundDefinitionsEmulsions are pharmaceutical preparations consisting of at least two immiscible liquids.Due to the lack of mutual solubility, one liquid is dispersed as tiny droplets in the other liquid to form an emulsion. Therefore,emulsions belong to the group of prepara-tions known as disperse systems.The USP also defines several dosage forms that are essentially emulsions but historically are referred to by other names. For example;Lotions are fluid emulsions orsuspensions intended for external application.Creams are viscous liquid or semi-solid emulsions of either an oil-in-water (O/W) or the water-in-oil (W/O) type. They are ordinarily used topically. The term cream is applied most frequently to soft, cosmetically acceptable types of preparations.Microemulsions are emulsions withextremely small droplet sizes and usually require a high concentration of surfactant for stability. They can also be regarded as isotropic, swollen micellar systems.Multiple emulsions are emulsions that have been emulsified a second time,consequently containing three phases. They may be water-in-oil-in-water (W/O/W) or oil-in-water-in-oil (O/W/O).Fluid emulsions are generally composed of discrete, observable liquid droplets in a fluid media, while semi-solid emulsions generally have a complex, more disorganized structure.The liquid which is dispersed as droplets iscalled as the dispersed , discontinuous or internal phase, and the liquid in which thedispersion is suspended is the dispersion medium or the continuous or external phase.For example, if olive oil is shaken with water,it breaks up into small globules andbecomes dispersed in water. In this case the oil is the internal phase, and water is the external phase.The dispersed particles or globules can range in size from less than 1 µm up to 100 µm. An emulsion is rarely a monodis-perse system, e.g., all the particles are rarely of the same size. A typical emulsion contains a distribution of many sizes, making it a polydisperse system.Types of EmulsionsBased on the nature of the internal (or exter-nal) phase, emulsions are of two types; oil-in-water (O/W) and water-in-oil (W/O). In an O/W type the oil phase is dispersed in the aqueous phase, while the opposite is true in W/O emulsions. Figure 1 depicts these two types of emulsions.Figure 1: Representation of Two Types of EmulsionsO/W Emulsion W/O Emulsion (water black)(oil white)When two immiscible phases are shaken together, either type of emulsion can result.However, this result is not random, but is dependent primarily on two factors; most importantly the type of emulsifier used and secondly the relative ratio of the aqueous and oil phases (phase volume ratio). The emulsifiers and their role in the type of emulsion are discussed in detail later in this chapter.In terms of the phase volume ratio, the percent of the internal phase is generally less than 50%, although emulsions can have internal phase volume percent as high as 75%. Uniform spheres, when packed in a rhombohedral geometry occupy approxi-mately 75% of the total volume. Phase volumes higher than 75% require that the droplets of dispersed phase be distorted into geometric shapes other than perfect spheres. Although it is rare to find emulsions with higher than 75% internal volume, phase volumes of over 90% have been reportedin literature.Formation of an EmulsionWhen two immiscible liquids are placedin contact with each other, they form two separate layers. The liquid with higher density forms the lower layer and the one with lower density forms the upper layer. When this two-layer system is shaken vigorously, one of the layers disperses in the other liquid forming an unstable emul-sion. If left unstirred, the dispersed phase comes together and coalesces into larger drops until the layers become separate again. If no other ingredient is added, this process of separation is usually completein a matter of a few minutes to a few hours. Therefore, a liquid dispersion is inherently an unstable system.However, when an emulsifier is present in the system, it reduces the interfacial tension between the two liquids and forms a physical barrier between droplets, hence lowers the total energy of the system(see discussion on Thermodynamics of Emulsions), thereby reducing the tendency of the droplets to come together and coalesce. Consequently, the globules ofthe internal phase may remain intact for long periods of time, forming a “stable”emulsion. It should be noted, however,that even with an emulsifier, an emulsionis a thermodynamically unstable system and will eventually revert to bulk phases. The time required for this process is determined by kinetics.Determination of Emulsion TypeSeveral tests can be used to determine whether a given emulsion is an O/W or W/O type. These are as follows:Miscibility or Dilution TestThis method is based on the fact that an emulsion can be diluted freely with a liquid of the same kind as its external phase. Typically, a small amount of the emulsion is added to a relatively large volume of water and the mixture is stirred. If the emulsion disperses in water, it is considered to bean O/W type emulsion. If, however, the emulsion remains undispersed, it is a W/O type emulsion.Staining or Dye TestThis test is based on the fact that if a dye is added to an emulsion and the dye is soluble only in the internal phase, the emulsion contains colored droplets dispersed inthe colorless external phase. This can be confirmed by observing a drop of emulsion under a low power microscope. An example of such a dye is scarlet red, which is an oil soluble dye. When added to an O/W type emulsion, followed by observation under the microscope, bright red colored oil drops in an aqueous phase can be seen clearly. Electrical Conductivity TestThis test is based on the fact that onlythe aqueous phase can conduct electrical current. Thus, when a voltage is applied across a liquid, a significant amount of electrical current will flow only when the path of the current is through a continuous aqueous phase. Since oil is a non-conductor of electricity, when tested for conductivity, a W/O type emulsion will show insignificant current flow.Often times a single test may not be conclu-sive. In such circumstances, more than one test may need to be carried out to confirm the emulsion type.Physical State of EmulsionsMost emulsions are either liquid or semi-solid at room temperature. In general, due to their high viscosity, the semi-solid emulsions are relatively more physically stable. Liquid emulsions are more commonly compounded for internal use, while semisolids are usually for external use or for use in body cavities (rectal or vaginal).Other terms commonly used to describe emulsions are lotion and cream . The term lotion refers to a disperse system that flows freely under the force of gravity. A cream is a product that does not flow freely under the force of gravity. It should be noted, however,that these terms are meaningful only when the product is at room temperature. A cream product may behave like a lotion with a temperature increase of a few degrees. The physical state of the final product is also influenced by its intended use. For example suntan lotions are dispensed as lotions instead of creams because they need to be applied on large body surface. Lotion form makes it easy to pour and spread the product. For application over a small portion of skin, a cream is the preferred form of an emulsion.Pharmaceutical Applications of Emulsions There are several reasons for formulation of a product as an emulsion. These include the following:•To disguise the taste or smell of oils or oil soluble drugs. These emulsions are normally O/W types with the aqueous phase containing sweeteners and flavoring agents to mask the poor taste of oils. An O/W type of emulsionalso makes it easy to rinse off the residual dose from the mouth and does not leave an oily taste. Mineral oil and cod liver oil are emulsified for this reason.•To improve the absorption of poorly soluble drugs. Oil soluble drugs may not be soluble enough to be absorbed efficiently. An example of such a drug is cyclosporin, which is dispensed as a microemulsion. •To deliver nutrients and vitamins by intravenous injection. Intralipid is an emulsion product for administering an oil by the IV route.•To serve as a vehicle for the topical administration of a variety of drugs.Kb is the binding constant of the preservative with the surfactantSweeteners are added to emulsions to produce a more palatable preparation, toand sorbitol.AntioxidantsAntioxidants are often added to prevent oxidation of vegetable oils and/or the active drug.Table 1. Typical AntioxidantsEmulsifiersEmulsifiers are substances that have the ability to concentrate at the surface of a liquid or interface of two liquids, many of them reducing the surface or interfacial tension. Those emulsifiers that reduce surface tension are also called surfactants .Emulsifiers in general are discussed inmore detail in a later section of this chapter.GuidelinesBefore selecting a formula for an emulsion,one needs to consider several factors.These are listed below.Type of Emulsion DesiredSince O/W emulsions are more pleasant to touch and swallow, they are generally preferred. Preparations for internal use are almost always O/W type products.Externally used emulsions may be of either type. Creams and lotions that are used primarily to provide oil to the skin need to be W/O due to high concentration of oils in these preparations.The equation shows that the effective concentration in the aqueous phase will always be a fraction of the total concentration.Solvents such as alcohol, glycerin and propylene glycol are often used as apreservative at concentrations approaching 10%. See Table 5, Typical Preservatives in Section 9 of this manual.BufferMany chemical buffer systems have been used in emulsions to control the pH. The optimal pH is chosen to ensure activity of the emulsifier, control stability of the drug and to ensure compatibility and stability of other ingredients.FlavorFlavoring agents enhance patient accept-ance of the product, which is particularly important for pediatric patients.ColorColorants are intended to provide a more aesthetic appearance to the final product.Emulsions are generally not colored with the exception of some topical products. Sequestering AgentsSequestering agents may be necessary to bind metal ions in order to control oxidative degradation of either the drug or other ingredients. HumectantsHumectants are water soluble polyols that prevent or hinder the loss of water from semi-solid emulsions, i.e., topical creams.They also contribute to the solvent proper-ties of the aqueous phase and contribute to the sweetness of oral preparations. The most common are glycerin, propylene glycolToxicityMost emulsifiers are not suitable for internal use. For orally given emulsions, acacia is commonly used as an emulsifying agent.Taste is another factor in selection ofingredients. In this regard, most polysaccha-rides are tasteless and, hence, suitable from a taste standpoint.Method of PreparationSoaps and acacia are excellent forextemporaneous preparations. While soaps allow the preparation to be made by simply mixing the ingredients and shaking, acacia can be used in a pestle and mortar to prepare emulsions.Typical FormulasCod Liver Oil Emulsion (polysaccharide emulsifier)Preparationing a ratio of 4:2:1 for oil, water and gums(both combined), prepare a primary emulsion by dry gum method. (See Methods to Prepare Emulsions on page 13.)2.Dilute with water to a flowable consistency andpour in a measuring device.3.Add alcohol diluted with equal volume of water,followed by the benzaldehyde and saccharin sodium.4.Dilute to volume (200 mL) with waterPreparation1.Add benzyl benzoate to the wax in a beakerand heat in a water bath until the wax melts and the temperature reaches 60°C.2.In a separate beaker, add an appropriate volumeof water and heat to the same temperature.3.Add the water to the oil phase with continuousstirring.4.Continue to stir until the mixture begins tothicken and cools to room temperature.Preparation1.Mix the two powders in a mortar and trituratewell, taking care that all the lumps and large particles have been reduced.2.Then add oil slowly with constant trituration untilall the oil has been added. Triturate to form a smooth paste.3.Then add the limewater and triturate briskly toform the emulsion.Note: The emulsifier, calcium oleate (from limewater and olive oil), preferentially forms O/W emulsions.Protective Lotion (divalent soap emulsifier)Benzyl Benzoate Emulsion (emulsifying wax emulsifier)Preparation1.Mix the paraffins, cetostearyl alcohol andstearic acid in a beaker and heat in a water bath to about 60°C.2.Heat water and chlorocresol together to thesame temperature.3.Add the aqueous phase to the oil phase andstir until congealed and cooled to room temperature.Note:The emulsifier is triethanolamine stearate formed in situ.Preparation1.Melt the sorbitan monostearate and stearicacid in the liquid paraffin and cool to 60°C. 2.Mix the sorbitol solution, preservatives,polysorbate 60 and water and heat to the temperature of the oil mixture.3.Add the aqueous solution to the oil phase andstir until it has congealed and cooled to room temperature.Note:Propylene glycol serves as a solvent for the preservatives.Preparation1.Mix and melt the wax and paraffin together.2.Dissolve borax in water and heat both containerson a water bath to 70°C.3.Add the aqueous phase to the oil phase andstir until it has congealed and cooled to room temperature.Note:The fatty acid in white beeswax reacts with borax (sodium borate) to make a sodium soap which acts as an W/O type emulsifier.Barrier Cream (soap emulsifier)All Purpose Cream (synthetic surfactant emulsifier)Cold Cream (soap emulsifier)Surfactants or surface active agents are molecules that consist of two distinct parts,a hydrophobic tail and a hydrophilic head group. They are generally classified based on the hydrophilic properties of the head group (ionic charge, polarity, etc.). Since the hydrophobic chains do not vary much in their properties, the nature of surfactants is dependent mainly on the head group structure.A common problem with sterol-containing emulsifiers is that being complex mixtures of natural substances, they are prone to variability in their quality and, hence, performance. Also, these agents usually contain some degree of an odor, which varies with the purity and source. Some semi-synthetic substitutes are available that seek to overcome some of the problems associated with these agents.There are of basically three types of emulsifiers: natural products, surface active agents (surfactants), and finely divided solids. Based on whether a stable emulsion can be produced, emulsifiers are also classified either as primary emulsifying agents which produce stable emulsions by themselves, or secondary emulsifying agents (stabilizers) which help primary emulsifiers to form a more stable emulsion.of cholesterol. Cholesterol itself is a very efficient emulsifier and produces W/O type emulsions. Consequently, its use is limited to topical preparations such as Hydrophilic Petrolatum USP which readily absorbs water forming a W/O cream. Woolfat or lanolin contains a considerable amount of choles-terol esters and can absorb up to 50% of its own weight of water.This group of emulsifiers, which numbers in the hundreds, contain a polyoxyethylene chain as the polar head group. They arenonionic and, thus, are compatible with ionic compounds and are less susceptible to pH changes. There are several such surfactants official in the USP/NF , typified by sorbitan monooleate (a partial ester of lauric acid with sorbitol), polysorbate 80(polyoxyethyl-ene 20 sorbitan monooleate) which contains 20 oxyethylene units copolymerized sorbitanAmine soaps consist of an amine, such as triethanolamine, in the presence of a fatty acid. These surfactants are viscous solutions and produce O/W type emulsions. They offer the advantage that the final pH of the preparations is generally close to neutral,and, therefore, allows their use on skin for extended periods of time.monooleate) and polyoxyl 40 stearate(a mixture of stearic acid esters with mixed poloxyethylene diols equivalent to about40 oxyethylene units).The large number of nonionic emulsifiers results from the large number of possible combinations of various alkyl groups with polyoxyethylene chains of varying lengths. Compounds with saturated and/or large alkyl groups, such as stearyl, tend to be solids or semisolids while oleyl (also large, but unsaturated) compounds tend to be liquids. Also, the longer the polyoxyethylene chain, the higher the melting point.To characterize such a large number of compounds, they are each assigned an HLB number. The HLB number or hydrophile-lipophile balance, is a measure of the relative hydrophilic vs lipophilic character of the molecule as determined by the relative size of the polyoxyethylene chain vs the alkyl group. HLB numbers range from 0 for a pure hydrocarbon to 20 for a pure poly-oxyethylene chain. Some typical valuesare listed in Table 3.Ionic surfactants, such as sodium lauryl sulfate, were not included in the original definition of the HLB system but have been included as the HLB system was developed. The HLB number of 40 for sodium lauryl sulfate is outside of the range of 0 to 20 and simply means that sodium lauryl sulfate is much more soluble or hydrophilic thana pure polyoxyethylene chain.Table 3. Typical HLB Numbersof EmulsifiersFinely Divided SolidsFinely divided solids function as emulsifiers because of their small particle size. Fine particles tend to concentrate at a liquid-liquid interface, depending on their wetability, and form a particulate film around the dispersed droplets. They are seldom used as the primary emulsifier.phase. The emulsion type will depend on the type of soap formed.Basically the formula is divided into anoil phase and an aqueous phase with the ingredients dissolved in their proper phases (oil or water). The surfactant(s) is added to the phase in which it is most soluble. The oil phase is then added to the aqueous phase with mixing, and the coarse mixture passed through an homogenizer.When waxes or waxy solids are included in the formulation, the use of heat is necessary,as described above.Required HLB of the Oil Phase.It has been found that various oils and lipid materials form stable emulsions withsurfactants that have a certain HLB value.This HLB value is called the required HLB of the oil or lipid. Theoretically, any surfac-tant with the required HLB would produce a stable emulsion with the indicated oil or lipid. Some examples are given in Table 4.Table 4. Required HLB Values for Typical Oils and LipidsHLB of Surfactant MixturesIt may be difficult to find a surfactant with the exact HLB number required for a given oil phase in an emulsion. Fortunately, the HLB numbers have been shown to be additive for a mixture of surfactants. Thus, if one required a surfactant with a HLB of 10, one could use a mixture of sorbitan monooleate (HLB = 4.7) and polysorbate 80 (HLB = 15.6). Such a mixture can be calculated on the basis of a simple weighted average as follows.Suppose 5 g of surfactant mixture is required. Let = the g of sorbitanmonooleate, then 5 = the g of polysorbate 80 required.(4.7)+(5- )(15.6) = 10(5)4.7 + 78.0- 15.6= 10(5)10.9= 28= 2.57 and 5- = 2.43Thus a mixture of 2.57 g of sorbitanmonooleate and 2.43 g of polysorbate 80would have a HLB of 10.Griffin 2described an experimental approach for the formulation of emulsions using synthetic emulsifiers.1.Group the ingredients on the basis of theirsolubilities in the aqueous and oil phases.2.Determine the type of emulsion required andcalculate an approximate required HLB value.3.Blend a low HLB emulsifier and a high HLBemulsifier to the required HLB.4.Dissolve the oil soluble ingredients and the lowHLB emulsifier in the oil phase. Heat, if necessary,to approximately 5 to 10°over the melting point of the highest melting ingredient or to a maximum temperature of 70 to 80°C.5.Dissolve the water soluble ingredients (exceptacids and salts) in a sufficient quantity of water.6.Heat the aqueous phase to a temperature whichis 3 to 5°higher than that of the oil phase.7.Add the aqueous phase to the oil phase withsuitable agitation.8.If acids or salts are employed, dissolve them inwater and add the solution to the cold emulsion.9.Examine the emulsion and make adjustments inthe formulation if the product is unstable. It may be necessary to add more emulsifier, change to an emulsifier with a slightly higher or lower HLB value or to use an emulsifier with different chemical characteristics.In addition to chemical degradation of various components of an emulsion, which can happen in any liquid preparation, emulsions are subject to a variety of physical instabilities. Sedimentation or Creaming Factors - Stoke’s LawCreaming usually occurs in a liquid emulsion since the particle size is generally greater than that of a colloidal dispersion. The rate is described by Stoke’s Law for a single particle settling in an infinite container under the force of gravity as follows:d =d 2(2- 1)gdt 18where:d /d t= the sedimentation rate in distance/time d = droplet diameter 2= droplet density1= emulsion medium density g = acceleration due to gravity = viscosity of the emulsion mediumSince for most oil phases, 2< 1, then sedimentation will be negative, i.e., the oil droplets will rise forming a creamy whitelayer. While Stoke’s Law does not describe creaming quantitatively in an emulsion, it does provide a clear collection of factors and their qualitative influence on creaming.Droplet SizeReducing droplet size can have a significant effect on creaming rate. Since the diameter is squared in Stoke’s Law, a reduction in size by ¹⁄₂will reduce the creaming rate by (¹⁄₂)2or a factor of 4.Emulsion StabilityDensity DifferenceIf the difference in density between the emulsion droplet and the external phase can be matched, the creaming rate could be reduced to zero. This is almost impossi-ble with most oils and waxy solids used in emulsions.The Gravitational Constant, gThis parameter is not of much interest since it can not be controlled or changed unless in space flight.ViscosityViscosity turns out to be the most readily controllable parameter in affecting the creaming rate. While the viscosity in Stoke’s Law refers to the viscosity of the fluid through which a droplet rises, in reality the viscosity that controls creaming is the viscosity of the entire emulsion. Thus, doubling the viscosity of an emulsion will decrease the creaming rate by a factor of 2.There are three major ways to increase the viscosity of an emulsion:•Increase the concentration of the internal phase•Increase the viscosity of the internal phase by adding waxes and waxy solids to the oil phase.•Increase the viscosity of the external phase by adding a viscosity building agent. Most of the suspending agents described in the Suspensions Section in this manual have been used for this purpose.Creaming does not usually occur in a semi-solid emulsion.Breaking or CrackingThis problem arises when the dispersed globules come together and coalesce to form larger globules. As this process continues, the size of the globules increases, making it easier for them to coalesce. This eventually leads to separation of the oil and water phases. For cracking to occur, the barrier that normally holds globules apart has to break down. Some of the factorsthat contribute to cracking are as follows:•Insufficient or wrong kind of emulsifier in the system.•Addition of ingredients that inactivate the emulsifier. Incompatible ingredients may show their effect over a period of time.An example of such an incompatibilitywill be to use large anions in thepresence of cationic emulsifier.•Presence of hardness in water. The calcium and magnesium present in hard water can replace a part of the alkalisoap with divalent soap. Since thesesoaps form different kinds of emulsions, phase inversion usually takes place.•Low viscosity of the emulsion •Exposure to high temperatures can also accelerate the process of coalescence.This is due to the fact that at an elevated temperature, the collisions between theglobules can overcome the barrier tocoalescence, thereby increasing thechance that a contact between twoparticles will lead to their fusion.Temperature may have an adverse effect on the activity of emulsifiers, particularly if these are proteinaceous in nature.However, this usually happens at temper-atures higher than 50°C. Conversely, areduction in temperature to the point that the aqueous phase freezes also will break the emulsion.•An excessive amount of the internal phase makes an emulsion inherently less stable because there is a greater chance of globules coming together.Cracking is the most serious kind of physical instability of an emulsion. Cracking of an emulsion usually renders it useless. In creams, the problem of cracking may show up as tearing. This is a process where one phase separates and appears like drops on top of the cream.The basic difference between creamingand cracking is that the globules in creaming do not coalesce to form larger particles. Therefore, creaming is a less serious problem and most preparations that show creaming can be shaken to redisperse the internal phase to its original state. A com-mon example of creaming is the formation of cream on top of whole milk due to collection of emulsified fat of the milk. This problem is solved by homogenizing the milk to reduce the particle size of dispersed fat, thereby reducing the rate at which they travel tothe surface.。
碳点的功能化、自放热合成及其在生化药物分析中的应用研究

基金资助本论文得到国家自然科学基金:基于长程共振能量转移的生物医学成像分析基础研究(编号:21535006)的资助,在此表示衷心的感谢。
目录摘要 (I)Abstract (III)第1章论文选题依据及研究内容 (1)1.1论文选题依据 (1)1.1.1 研究意义 (1)1.1.2 研究现状 (1)1.1.2.1 碳点的分类、光致发光的起源、性质和挑战 (1)1.1.2.2 碳点的合成途径 (5)1.1.2.3 碳点在荧光分析检测领域的应用 (10)1.2研究目标、研究内容、以及拟解决的关键科学问题 (13)1.2.1 研究目标 (13)1.2.2 研究内容 (13)1.2.3 拟解决的关键科学问题 (14)1.3技术路线 (14)第2章铽掺杂碳点的一步碳化合成及其选择性的检测2,4,6-三硝基苯酚 (15)2.1前言 (15)2.2实验部分 (16)2.2.1 材料及试剂 (16)2.2.2 实验仪器 (16)2.2.3 铽掺杂碳点的合成与纯化 (16)2.2.4 TNP的荧光分析检测 (16)2.3结果与讨论 (17)2.3.1 铽掺杂碳点的合成与表征 (17)2.3.2 铽掺杂碳点的稳定性 (19)2.3.3 TNP的荧光分析检测 (20)2.3.4 水样中TNP含量的测定 (25)2.4小结 (25)第3章铽修饰碳量子点的常规水热合成及其高选择性的检测ppGpp (27)3.1前言 (27)3.2实验部分 (28)3.2.1 材料及试剂 (28)3.2.2 实验仪器 (28)3.2.3 碳量子点和铽修饰碳量子点的合成与纯化 (28)3.2.4 ppGpp的比率荧光检测 (29)3.3结果与讨论 (29)3.3.1 碳量子点的合成与表征 (29)3.3.2 铽修饰碳量子点的合成与表征 (32)3.3.3 比率荧光法检测ppGpp (32)3.4小结 (34)第4章单层石墨烯量子点的自放热合成及其高灵敏和高特异性的检测铝离子 (35)4.1前言 (35)4.2实验部分 (36)4.2.1 材料及试剂 (36)4.2.2 实验仪器 (36)4.2.3 单层石墨烯量子点的合成与纯化 (36)4.2.4 Al3+的荧光分析检测 (37)4.3结果与讨论 (37)4.3.1 单层石墨烯量子点的自催化放热合成 (37)4.3.2 单层石墨烯量子点的表征 (40)4.3.3 Al3+诱导的单层石墨烯量子点光致发光增强 (42)4.4小结 (46)第5章高光致发光碳量子点的自放热合成及其选择性的检测维生素B12 (47)5.1前言 (47)5.2实验部分 (48)5.2.1 材料及试剂 (48)5.2.2 实验仪器 (48)5.2.3 碳量子点的合成与纯化 (48)5.2.4 维生素B12的荧光分析检测 (49)5.2.5 维生素B12的固相传感 (49)5.3结果与讨论 (49)5.3.1 碳量子点的自催化放热合成 (49)5.3.2 碳量子点的表征 (53)5.3.3 碳量子点的稳定性 (54)5.3.4 维生素B12的荧光检测 (55)5.3.5 注射液中维生素B12的含量测定 (57)5.3.6 维生素B12的固相传感 (57)5.4小结 (58)第6章全文总结与展望 (59)6.1全文总结 (59)6.1.1 主要结论 (59)6.1.2 主要创新点 (60)6.2前景展望 (60)6.2.1 碳点功能化手段的开发及应用 (60)6.2.2 自放热合成在其他纳米材料领域的开发 (60)参考文献 (63)科研成果 (71)致谢 (73)碳点的功能化、自放热合成及其在生化药物分析中的应用研究专业: 药物分析学硕士研究生: 陈斌斌指导教师: 黄承志教授摘要碳点作为新类型的光致发光纳米材料,由于其优越的光稳定性、可调的光致发光、良好的生物相容性以及易于制备的特性,而被广泛应用于细胞成像、生物与化学传感、光电器件等领域。
SP136420-33Q Thermo加热板和搅拌器和加热板和搅拌器价格
SP136420-33Q Thermo 加热板和搅拌器和加热板和搅拌器价格SP136420-33QThermo 加热板和搅拌器 标题:SP136420-33Q Thermo 加热板和搅拌器Thermo Scientific RT-Elite 系列加热板和搅拌器RT-Elite 系列数字型带搅拌功能的加热板提供极为良好的温度均一性和可靠的安全性能。
nStirTrac 功能提供温和、稳定的搅拌速度和强劲的磁力耦合作用 n 样品拿走前可以通过即刻停止搅拌子转动 nHOT TOP报警系统在温度超过50℃时启用,防止过温 n 精确的温度和搅拌速度控制功能,温度设定精度为1℃,温度显示精度为0.1℃,设定增量为1℃ n 使用单旋钮可以分别控制温度和搅拌速度,锁定功能防止误操作 n 用户设定存储键可以存储3个常用的温度和搅拌速度,方便快速设定 n 用户友好型界面,3个独立的LCD显示屏可以分别显示温度、搅拌速度和时间 n 稳固的基座可以保证运行稳定 nRS232接口与PC 连接可以将运行时间、温度等信息进行存档 n 用户可以预先设定停...厂家:上海政泓 市场价格: 优惠价格:百度搜索联系 Thermo Scientific PLR386 +4℃实验室冰箱 标题:Thermo Scientific PLR386 +4℃实验室冰箱 应用领域: 常规/通用科研需求 样品、实验材料、培养基及储备溶液的储存 (生物制药/生物技术/研究所)及性能多样性(样品/实验原料/培养基及储存溶液) 产品特点: 卓越的气流稳定性: +/- 3℃ 直观操作界面的数字温度控制系统 温度过高/过低的声光报警系统 高密度,无氟绝热层 重型结构 标准轮脚 标准可锁定外门 ...厂家:上海政泓 市场价格: 优惠价格:百度搜索联系 Thermo ScientificPLR221 +4℃实验室冰箱 标题:Thermo Scientific PLR221 +4℃实验室冰箱 应用领域: 常规/通用科研需求 样品、实验材料、培养基及储备溶液的储存 (生物制药/生物技术/研究所)及性能多样性(样品/实验原料/培养基及储存溶液) 产品特点: 卓越的气流稳定性: +/- 3℃ 直观操作界面的数字温度控制系统 温度过高/过低的声光报警系统 高密度,无氟绝热层 重型结构 标准厂家:上海政泓市场价格:优惠价格:百度搜索联系轮脚标准可锁定外门 ...Revco超低温冰箱UXF系列标题:Revco超低温冰箱UXF系列UxF系列主要特点: 新型Embraco封闭式压缩机2台1HP功率压缩机。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
a r X i v :h e p -p h /9307229v 3 31 J a n 1994Imperial/TP/92-93/4312th January,1994hep-ph/9307229Real Time Thermal Propagators for Massive Gauge BosonsT.S.Evans ∗&A.C.Pearson †Blackett Laboratory,Imperial College,Prince Consort Road,London SW72BZ U.K.AbstractWe derive Feynman rules for gauge theories exhibiting spontaneous symmetry break-ing using the real-time formalism of finite temperature field theory.We also derive the thermal propagators where only the physical degrees of freedom are given thermal bound-ary conditions.We analyse the abelian Higgs model and find that these new propagators simplify the calculation of the thermal contribution to the self energy.1IntroductionSince the observation by Kirzhnits and Linde [1]that spontaneously broken symmetries may be restored at high temperatures,the subject has attracted wide attention.However,very little study has been made of theories containing spontaneous symmetry breaking at non-zero temperature using the so-called real time formalisms.This seems odd since in this formalism,dynamical quantities are more readily calculated as the thermal Green functions are given directly in terms of real times .We are only aware of one paper,by Ueda[2],that uses the real time formalism to study the abelian Higgs model at non-zero temperature.However,this work was done prior to the further development of real time methods by Niemi &Semenoff[3]and others.We therefore feel that a study of spontaneously broken gauge symmetries using the real time formalism is necessary.In this paper we begin by deriving the real time thermal propagator for a massive gauge boson in the covariant gauges.We find that there are two thermal contributions to the propagator.The first part is consistent with the interpretation of a field in a heat bath of real particles.However the second term acts as a counterterm to the finite temperature contributions from the unphysical goldstone modes.We then apply the the ideas of Landshoff&Rebhan[4]and only apply the thermal boundary conditions to the physical degrees of freedom.Previously,this method has only been applied to gauge theories where the full symmetry is maintained.In the final section ,we use the abelian Higgs model as a simple example.We analyse thecalculation ofthe one-loop corrections to the mass of the Higgs boson performed by Ueda[2].While we do not disagree with the the results therein,there were a number of mistakes in the form of the propagators used.We evaluate the propagators correctly.We also show that if we use propagators for which only the physical modes are thermalised,the calculation is greatly simplified.2Derivation of RTF PropagatorTo begin with let us consider a set of gauge fields A µa invariant under some gauge group.After symmetry breaking ,the free field lagrangian for these fields is of the form;L 0=−12M 2ab A µa A µb +1ζ−1)∂µ∂ν∆νρ(t −t ′)=g µρδC (t −t ′)(2)subject to the KMS condition[6].The subscript C denotes that we are considering the solutionto this equation along the contour in the complex time plane associated with the real time formalism[3,7,8,9](see fig.1).6ℑm (t )−ı1M 2A ( x ,t ;M 2)−A ( x ,t ;ζM 2)(3)where A ( x ,t ;M 2)=d 3¯keı k. xıexp(βω)−1(e −ıωt +e −βωe ıωt )Θ(t )+(e ıωt +e −βωe −ıωt )Θ(−t )is the solution of (2+M 2)A ( x ,t −t ′;M 2)=δC (t −t ′),ω2= k 2+M 2and∂Θ(t −t ′)M 2B∆(k )00−∆∗(k )B−k µk νk 2−M 2+ıε,Γ(k )=1e β|k 0|−1Consider the 1-1component of this propagator which is interpreted as the propagator betweenthe physical fields.Explicitly the 1-1component isı∆µν11(k )=−ık 2−ζM 2+ıε−g µν−k µk νe β|k 0|−1−k µk νe β|k 0|−1(6)It can be seen that the propgator splits into the T =0and T >0contributions.in the limitas T →0(β→∞)the T >0terms disappear and ∆µν11reduces to the zero temperature propagator.We note that there are two finite temperature contributions to the form of this propagator.The first term containing ‘δ(k 2−M 2)’may be interpreted as a contribution due to a heat bath of real particles of mass M .However,the second term contains the unphysical gauge parameter ζand its interpretation is not so straightforward.The second term acts as a counterterm to the unphysical contributions from the goldstone boson.This can be shown in two ways.Firstly,let us consider this model in the unitary gauge.In this gauge,the goldstone boson is gauged away and so we would expect there to be no counterterms in the photon propagator.The unitary gauge propagator satisfies the following equation.(2+M 2)gµν−∂µ∂ν∆νρ(t −t ′)=g µρδC (t −t ′)(7)Evaluating this equation we find∆µν(k )=−gµν−k µk νM 2B∆(k )00−∆∗(k )B−k µk νk 2−M 2+ıεgµν−(1−ζ)k µk νM 22πδ(k 2−M 2)∂M 2M 2=0(10)In momentum space this becomes,∆µν(k )=−g µν−k µk ν∂k 2+ıε.This is exactly the propagator for a photon first derived by Kobes,Semenoff,and Weiss in [5].3The abelian Higgs modelThe propagator we have derived for the massive gauge boson differs from the result used by Ueda (Eqn.3.8,[2]).However this work was done prior to the formulation of real time thermal field theory[7]using path integral methods devloped by Niemi &Semenoff[3].We would therefore like to reconsider the abelian Higgs model.The abelian Higgs model consists of a U (1)gauge field coupled to a complex scalar field described by the Lagrangian densityL =−16(Φ∗Φ)2where F µν=∂µA ν∂νA µand D µ=∂µ−ıeA µ.We choose ρ2<0so that spontaneous symmetry breaking occurs.Expanding Φabout its expectation value as Φ(x )=1√2,we may rewrite the lagrangian as L =L 0−V (A µ,φ,χ)where L 0=−12M 2A µA µ−12∂µφ∂µφ−12∂µχ∂µχ−M∂µχA µ(12)and V (A µ,φ,χ)is a potential term containing only cubic and quartic terms.M =ev and m 2=ρ2+1k 2−m 2+ıε+2πδ(k 2−m 2)k 2+ıε+2πδ(k 2)k 2−M 2+ıεgµν−(1−ζ)k µk νM 22πδ(k 2−M 2)M 22πδ(k 2−ζM 2)∂k 2ıe β|k 0|−1ı∆µνmod (k )=−ıe β|k 0|−1ζk µk νk 2−k µk νe β|k 0|−1+ıζk µk ν∂k 2+ıε+2πδ(k 2)k 2.As it was pointed out by Kobes,Semenoff&Weiss[5],these terms should instead be considered as derivatives of the delta function.As we find that the modified propagator used by Ueda is essentially correct,it is no surprisethat we agree with the result of the one-loop self energy corrections calculated in[2]and we shall not repeat the calculation here.However,we would like to consider this calculation using propagators for which only the physical degrees of freedom are given thermal boundary conditions[4].Using these propagators the modified propagators due to photon-goldstone mixing areı∆µν(k)=− ıeβ|k0|−1 gµν−kµkνk2+ıεζkµkνmodk2 ıReferences[1]D.A.Kirzhnits and A.D.Linde,Phys.Lett.42B(1972)472[2]Y.Ueda,Phys.Rev.D23(1981)1383[3]A.J.Niemi and G.W.Semenoff,Ann.Phys.(NY)152(1984)305;Nucl.Phys.B230(1984)181[4]ndshoff,A.Rebhan,Nucl.Phys.B383(1992)607.[5]R.L.Kobes,G.W.Semenoff,and N.Weiss,Z.Phys.C29(1985)371[6]R.Kubo,J.Phys.Soc.Japan12,(1957)570;P.C.Martin and J.Schwinger,Phys.Rev.115,(1959)1342[7]ndsman and Ch.G.van Weert,Phys.Rep.145(1987)141.;R.J.Rivers,Path In-tegral Methods in Quantum Field.Theory(Cambridge University Press,Cambridge, 1987);[8]A.C.Pearson,Why the real time formalism does not factorise,talk given at the3rdWorkshop on Thermal Field Theories and their applications in Physics,August1993, Banff,Canada;[9]T.S.Evans,A new time contour for thermalfield theories,Why the real time formalismdoes not factorise,talk given at the3rd Workshop on Thermal Field Theories and their applications in Physics,August1993,Banff,Canada.[10]P.Arnold,E.Braaten and S.Vokos,Phys.Rev.D46(1992)3576;P.F.Kelly,talk givenat the3rd Workshop on thermalfield theories and their applications in physics,August 1993,Banff,Canada[11]K.Takahashi,Z.Phys.C-Particles and Fields26601(1985)。
