化学工程与工艺专业英语课后习题答案重点
济南大学成人教育《化学工程与工艺专业英语(224201)》期末考试复习题及参考答案

9.钠:( )
A、sodium,Na B、iron,Fe C、gold,Au D、iodine,I E、nitrogen,N F、tin,Sn
答案: A
10.氧:( )
A、calcium,Ca B、carbon,C C、oxygen,O D、silver,Ag E、hydrogen,H F、chlorine,Cl
氧 答案: 二 化硫
13. aluminum oxide:( )
氧 铝 答案: 化
四、 句式改写
请 两 简单 为 导 1. 将 个 句合并 which或that引 的从句
The peak of graphene oxide was shifted to 22.5°. This is due to partial reduction of graphene oxide to graphene caused by coprecipitation reaction of iron ions.
苯 答案: 三甲基
3. calcium hypochlorite:( )
氯 钙 答案: 次 酸
4. sodium perchlorate:( )
氯 钠 答案: 高 酸
5. copper sulphate:( )
铜 答案: 硫酸
6. 2-hexene:( )
烯 答案: 2-己
7. dichloromethane:( )
单词 两 答案: therefore、hence、consequently、thus,在表示“因此”的 任意 个
4. replace:( )、( )
单词 两 答案: displace、substitute,在表示“替代”的 任意 个
5. in addition to:( )、( )
化学工程与工艺专业英语课后习题参考答案
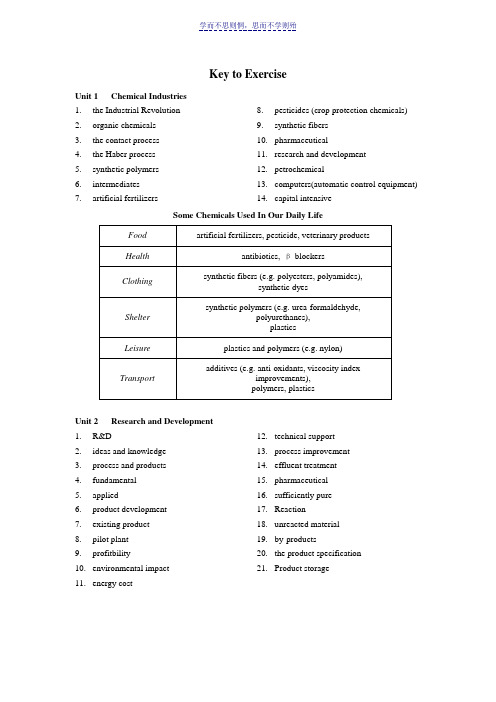
学而不思则惘,思而不学则殆Key to Exercise Unit 1 Chemical Industries1.the Industrial Revolutionanic chemicals3.the contact process4.the Haber process5.synthetic polymers6.intermediates7.artificial fertilizers 8.pesticides (crop protection chemicals)9.synthetic fibers10.pharmaceutical11.research and development12.petrochemicalputers(automatic control equipment)14.capital intensiveSome Chemicals Used In Our Daily LifeUnit 2 Research and Development1.R&D2.ideas and knowledge3.process and products4.fundamental5.applied6.product development7.existing product8.pilot plant9.profitbility10.environmental impact11.energy cost 12.technical support13.process improvement14.effluent treatment15.pharmaceutical16.sufficiently pure17.Reaction18.unreacted material19.by-products20.the product specification21.Product storageUnit 3 Typical Activities of Chemical Engineers1.Mechanical2.electrical3.civil4.scale-upmercial-size6.reactors7.distillation columns8.pumps9.control and instrumentation10.mathematics11.industry12.academia13.steam 14.cooling water15.an economical16.to improve17.P&I Drawings18.Equipment Specification Sheets19.Construction20.capacity and performance21.bottlenecks22.Technical Sales23.new or improved24.engineering methods25.configurationsUnit 4 Sources of Chemicals1.inorganic chemicals2.derive from (originate from)3.petrochemical processes4.Metallic ores5.extraction process6.non-renewable resource7.renewable sources8.energy source9.fermentation process10.selective 11.raw material12.separation and purification13.food industry14.to be wetted15.Key to success16.Crushing and grinding17.Sieving18.Stirring and bubbling19.Surface active agents20.OverflowingUnit 5 Basic Chemicals 1. Ethylene 2. acetic acid 3.4. Polyvinyl acetate5. Emulsion paintUnit 6 Chlor-Alkali and Related Processes 1. Ammonia 2. ammonia absorber 3. NaCl & NH 4OH 4.5. NH 4Cl6. Rotary drier7. Light Na 2CO 3Unit 7 Ammonia, Nitric Acid and Urea 1. kinetically inert 2. some iron compounds 3. exothermic 4. conversion 5. a reasonable speed 6. lower pressures 7. higher temperatures 8.9. energy 10. steam reforming 11. carbon monoxide 12. secondary reformer 13. the shift reaction 14. methane 15. 3:1Unit 8 Petroleum Processing 1. organic chemicals 2. H:C ratios3. high temperature carbonization4. crude tar5. pyrolysis6. poor selectivity7. consumption of hydrogen8. the pilot stage9. surface and underground 10.fluidized bed 11. Biotechnology 12. sulfur speciesUnit 9 PolymersUnit 10 What Is Chemical EngineeringMicroscale (≤10-3m)●Atomic and molecular studies of catalysts●Chemical processing in the manufacture of integrated circuits●Studies of the dynamics of suspensions and microstructured fluidsMesoscale (10-3-102m)●Improving the rate and capacity of separations equipment●Design of injection molding equipment to produce car bumpers madefrom polymers●Designing feedback control systems for bioreactorsMacroscale (>10m)●Operability analysis and control system synthesis for an entire chemicalplant●Mathematical modeling of transport and chemical reactions ofcombustion-generated air pollutants●Manipulating a petroleum reservoir during enhanced oil recoverythrough remote sensing of process data, development and use of dynamicmodels of underground interactions, and selective injection of chemicalsto improve efficiency of recoveryUnit 12 What Do We Mean by Transport Phenomena?1.density2.viscosity3.tube diameter4.Reynolds5.eddiesminar flow7.turbulent flow 8.velocity fluctuations9.solid surface10.ideal fluids11.viscosity12.Prandtl13.fluid dynamicsUnit 13 Unit Operations in Chemical Engineering 1. physical 2. unit operations 3. identical 4. A. D. Little 5. fluid flow6. membrane separation7. crystallization8. filtration9. material balance 10. equilibrium stage model 11. Hydrocyclones 12. Filtration 13. Gravity 14. VaccumUnit 14 Distillation Operations 1. relative volatilities 2. contacting trays 3. reboiler4. an overhead condenser5. reflux6. plates7. packing8.9. rectifying section 10. energy-input requirement 11. overall thermodynamic efficiency 12. tray efficiencies 13. Batch operation 14. composition 15. a rectifying batch 1 < 2 < 3Unit 15 Solvent Extraction, Leaching and Adsorption 1. a liquid solvent 2. solubilities 3. leaching 4. distillation 5. extract 6. raffinate 7. countercurrent 8. a fluid 9. adsorbed phase 10. 400,000 11. original condition 12. total pressure 13. equivalent numbers 14. H + or OH –15. regenerant 16. process flow rates17. deterioration of performance 18. closely similar 19. stationary phase 20. mobile phase21. distribution coefficients 22. selective membranes 23. synthetic24. ambient temperature 25. ultrafiltration26. reverse osmosis (RO).Unit 16 Evaporation, Crystallization and Drying 1. concentrate solutions 2. solids 3. circulation 4. viscosity 5. heat sensitivity 6. heat transfer surfaces 7. the long tube8. multiple-effect evaporators 9.10. condensers 11. supersaturation 12. circulation pump 13. heat exchanger 14. swirl breaker 15. circulating pipe 16. Product17. non-condensable gasUnit 17 Chemical Reaction Engineering1.design2.optimization3.control4.unit operations (UO)5.many disciplines6.kinetics7.thermodynamics,8.fluid mechanics9.microscopic10.chemical reactions 11.more valuable products12.harmless products13.serves the needs14.the chemical reactors15.flowchart16.necessarily17.tail18.each reaction19.temperature and concentrations20.linearUnit 18 Chemical Engineering Modeling1.optimization2.mathematical equations3.time4.experiments5.greater understanding6.empirical approach7.experimental design8.differing process condition9.control systems 10.feeding strategies11.training and education12.definition of problem13.mathematical model14.numerical methods15.tabulated or graphical16.experimental datarmation1.the preliminary economics2.technological changes3.pilot-plant data4.process alternatives5.trade-offs6.Off-design7.Feedstocks 8.optimize9.plant operations10.energy11.bottlenecking12.yield and throughput13.Revamping14.new catalystUnit 19 Introduction to Process Design1. a flowsheet2.control scheme3.process manuals4.profit5.sustainable industrial activities6.waste7.health8.safety9. a reactor10.tradeoffs11.optimizations12.hierarchyUnit 20 Materials Science and Chemical Engineering1.the producing species2.nutrient medium3.fermentation step4.biomass5.biomass separation6.drying agent7.product8.water9.biological purificationUnit 21 Chemical Industry and Environment1.Atmospheric chemistry2.stratospheric ozone depletion3.acid rain4.environmentally friendly products5.biodegradable6.harmful by-product7.efficiently8.power plant emissions 9.different plastics10.recycled or disposed11.acidic waste solutionsanic components13.membrane technology14.biotechnology15.microorganisms。
化学工程与工艺专业英语(1)

化学工程与工艺专业英语1. Introduction化学工程与工艺是一门涉及化学反应、化学工艺以及工程原理的学科,它在许多工业领域中起着重要的作用。
作为一个化学工程与工艺专业的学生,具备良好的英语沟通能力对于学习和就业都具有重要意义。
本文档将介绍一些化学工程与工艺专业中常用的英语术语和短语,以帮助读者更好地理解和运用这些知识。
2. Basic Terms and Definitions在开始学习化学工程与工艺专业的英语词汇之前,我们需要了解一些基本的术语和定义。
•Chemical Engineering: 化学工程•Process: 过程•Reactor: 反应器•Mass Transfer: 质量传递•Heat Transfer: 热传递•Distillation: 蒸馏•Extraction: 提取•Polymerization: 聚合•Catalysis: 催化•Reaction Kinetics: 反应动力学•Thermodynamics: 热力学•Unit Operation: 单元操作•Unit Process: 单元工艺3. Chemical Engineering Processes化学工程与工艺专业涉及许多不同的化学过程和工艺。
下面是一些常见的过程名称和定义。
3.1 Distillation蒸馏是一种通过利用不同组分的沸点差异进行分离的过程。
在蒸馏过程中,液体混合物被加热,使其沸腾,然后通过冷凝,得到不同组分的纯液体。
蒸馏在石油化工、酒精生产和石油提炼等领域中广泛应用。
3.2 Extraction提取是一种将溶质从溶剂中分离出来的过程。
提取可以通过溶剂选择性地与溶质相互作用,使得溶质从溶剂中转移到新的相中。
提取常用于药物生产、化妆品制造等领域。
3.3 Polymerization聚合是一种将单体分子结合成长链聚合物的过程。
聚合通常需要催化剂和适当的反应条件。
聚合在塑料制造、纤维生产和涂料工业等领域中被广泛应用。
化学工程与工艺专业英语Unit 12

实际上,在有一些情况下,设计工程师可能直接使用传递现 象的方法和等式来进行设备设计。
An example would be a tubular reactor, (which might be illustrated as a pipe, e.g., the heat exchanger described earlier,) with a homogeneous chemical reaction occurring in the fluid within.
(1) The first requires one to recognize that heat, mass, and momentum transport occur in many kinds of engineering , (e.g., heat exchangers ,compressors ,nuclear and chemical reactors, humidifiers, air coolers ,driers , fractionaters, and absorbers.) It is important that engineers have an understanding of the physical laws (governing these transport processes) if they are to understand (what is taking place in engineering equipment) and to make wise decisions (with regard to its economical operation). 第一种要求大家认识到热量, 质量和动量传递发生在许多工程设备中, 如热交换器,压缩机,核反应堆,化学反应器,增湿器,空气冷凝器, Line 7. humidifier 增湿器 干燥器,分馏器以及吸收器。 fractionater 分馏器 如果工程师要理解工程设备中正在发生什么以及关于其经济性操作作出 英明的决策,那么理解支配这些传递过程的物理定律有是t be emphasized , (however, that while
化学工程与工艺专业英语Unit_11

Unit 10 What Is Chemical Engineering?什么是化学工程学In a wider sense, engineering may be defined as a scientific presentation of the techniques and facilities used in a particular industry. For example, mechanical engineering refers to the techniques and facilities employed to make machines. It is predominantly based on mechanical forces which are used to change the appearance and/or physical properties of the materials being worked, while their chemical properties are left unchanged. Chemical engineering encompasses the chemical processing of raw materials, based on chemical and physico-chemical phenomena of high complexity.广义来讲,工程学可以定义为对某种工业所用技术和设备的科学表达。
例如,机械工程学涉及的是制造机器的工业所用技术和设备。
它优先讨论的是机械力,这种作用力可以改变所加工对象的外表或物理性质而不改变其化学性质。
化学工程学包括原材料的化学过程,以更为复杂的化学和物理化学现象为基础。
Thus, chemical engineering is that branch of engineering which is concerned with the study of the design, manufacture, and operation of plant and machinery in industrial chemical processes.因此,化学工程学是工程学的一个分支,它涉及工业化化学过程中工厂和机器的设计、制造、和操作的研究。
化学工程与工艺专业英语课后习题参考答案
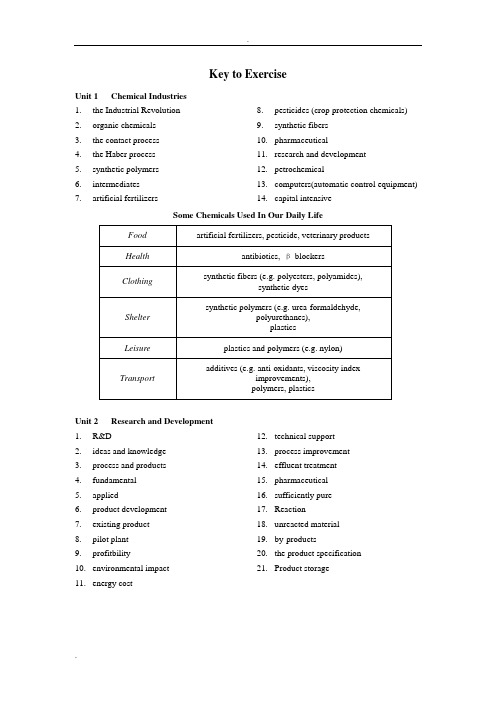
Key to Exercise Unit 1 Chemical Industries1.the Industrial Revolutionanic chemicals3.the contact process4.the Haber process5.synthetic polymers6.intermediates7.artificial fertilizers 8.pesticides (crop protection chemicals)9.synthetic fibers10.pharmaceutical11.research and development12.petrochemicalputers(automatic control equipment)14.capital intensiveSome Chemicals Used In Our Daily LifeUnit 2 Research and Development1.R&D2.ideas and knowledge3.process and products4.fundamental5.applied6.product development7.existing product8.pilot plant9.profitbility10.environmental impact11.energy cost 12.technical support13.process improvement14.effluent treatment15.pharmaceutical16.sufficiently pure17.Reaction18.unreacted material19.by-products20.the product specification21.Product storageUnit 3 Typical Activities of Chemical Engineers1.Mechanical2.electrical3.civil4.scale-upmercial-size6.reactors7.distillation columns8.pumps9.control and instrumentation10.mathematics11.industry12.academia13.steam 14.cooling water15.an economical16.to improve17.P&I Drawings18.Equipment Specification Sheets19.Construction20.capacity and performance21.bottlenecks22.Technical Sales23.new or improved24.engineering methods25.configurationsUnit 4 Sources of Chemicals1.inorganic chemicals2.derive from (originate from)3.petrochemical processes4.Metallic ores5.extraction process6.non-renewable resource7.renewable sources8.energy source9.fermentation process10.selective 11.raw material12.separation and purification13.food industry14.to be wetted15.Key to success16.Crushing and grinding17.Sieving18.Stirring and bubbling19.Surface active agents20.OverflowingUnit 5 Basic Chemicals 1. Ethylene 2. acetic acid 3.4. Polyvinyl acetate5. Emulsion paintUnit 6 Chlor-Alkali and Related Processes 1. Ammonia 2. ammonia absorber 3. NaCl & NH 4OH 4.5. NH 4Cl6. Rotary drier7. Light Na 2CO 3Unit 7 Ammonia, Nitric Acid and Urea 1. kinetically inert 2. some iron compounds 3. exothermic 4. conversion 5. a reasonable speed 6. lower pressures 7. higher temperatures 8.9. energy 10. steam reforming 11. carbon monoxide 12. secondary reformer 13. the shift reaction 14. methane 15. 3:1Unit 8 Petroleum Processing 1. organic chemicals 2. H:C ratios3. high temperature carbonization4. crude tar5. pyrolysis6. poor selectivity7. consumption of hydrogen8. the pilot stage9. surface and underground 10.fluidized bed 11. Biotechnology 12. sulfur speciesUnit 9 PolymersUnit 10 What Is Chemical EngineeringMicroscale (≤10-3m)●Atomic and molecular studies of catalysts●Chemical processing in the manufacture of integrated circuits●Studies of the dynamics of suspensions and microstructured fluidsMesoscale (10-3-102m)●Improving the rate and capacity of separations equipment●Design of injection molding equipment to produce car bumpers madefrom polymers●Designing feedback control systems for bioreactorsMacroscale (>10m)●Operability analysis and control system synthesis for an entire chemicalplant●Mathematical modeling of transport and chemical reactions ofcombustion-generated air pollutants●Manipulating a petroleum reservoir during enhanced oil recoverythrough remote sensing of process data, development and use of dynamicmodels of underground interactions, and selective injection of chemicalsto improve efficiency of recoveryUnit 12 What Do We Mean by Transport Phenomena?1.density2.viscosity3.tube diameter4.Reynolds5.eddiesminar flow7.turbulent flow 8.velocity fluctuations9.solid surface10.ideal fluids11.viscosity12.Prandtl13.fluid dynamicsUnit 13 Unit Operations in Chemical Engineering 1. physical 2. unit operations 3. identical 4. A. D. Little 5. fluid flow6. membrane separation7. crystallization8. filtration9. material balance 10. equilibrium stage model 11. Hydrocyclones 12. Filtration 13. Gravity 14. VaccumUnit 14 Distillation Operations 1. relative volatilities 2. contacting trays 3. reboiler4. an overhead condenser5. reflux6. plates7. packing8.9. rectifying section 10. energy-input requirement 11. overall thermodynamic efficiency 12. tray efficiencies 13. Batch operation 14. composition 15. a rectifying batch 1 < 2 < 3Unit 15 Solvent Extraction, Leaching and Adsorption 1. a liquid solvent 2. solubilities 3. leaching 4. distillation 5. extract 6. raffinate 7. countercurrent 8. a fluid 9. adsorbed phase 10. 400,000 11. original condition 12. total pressure 13. equivalent numbers 14. H + or OH –15. regenerant 16. process flow rates17. deterioration of performance 18. closely similar 19. stationary phase 20. mobile phase21. distribution coefficients 22. selective membranes 23. synthetic24. ambient temperature 25. ultrafiltration26. reverse osmosis (RO).Unit 16 Evaporation, Crystallization and Drying 1. concentrate solutions 2. solids 3. circulation 4. viscosity 5. heat sensitivity 6. heat transfer surfaces 7. the long tube8. multiple-effect evaporators 9.10. condensers 11. supersaturation 12. circulation pump 13. heat exchanger 14. swirl breaker 15. circulating pipe 16. Product17. non-condensable gasUnit 17 Chemical Reaction Engineering1.design2.optimization3.control4.unit operations (UO)5.many disciplines6.kinetics7.thermodynamics,8.fluid mechanics9.microscopic10.chemical reactions 11.more valuable products12.harmless products13.serves the needs14.the chemical reactors15.flowchart16.necessarily17.tail18.each reaction19.temperature and concentrations20.linearUnit 18 Chemical Engineering Modeling1.optimization2.mathematical equations3.time4.experiments5.greater understanding6.empirical approach7.experimental design8.differing process condition9.control systems 10.feeding strategies11.training and education12.definition of problem13.mathematical model14.numerical methods15.tabulated or graphical16.experimental datarmation1.the preliminary economics2.technological changes3.pilot-plant data4.process alternatives5.trade-offs6.Off-design7.Feedstocks 8.optimize9.plant operations10.energy11.bottlenecking12.yield and throughput13.Revamping14.new catalystUnit 19 Introduction to Process Design1. a flowsheet2.control scheme3.process manuals4.profit5.sustainable industrial activities6.waste7.health8.safety9. a reactor10.tradeoffs11.optimizations12.hierarchyUnit 20 Materials Science and Chemical Engineering1.the producing species2.nutrient medium3.fermentation step4.biomass5.biomass separation6.drying agent7.product8.water9.biological purificationUnit 21 Chemical Industry and Environment1.Atmospheric chemistry2.stratospheric ozone depletion3.acid rain4.environmentally friendly products5.biodegradable6.harmful by-product7.efficiently8.power plant emissions 9.different plastics10.recycled or disposed11.acidic waste solutionsanic components13.membrane technology14.biotechnology15.microorganisms。
化学工程与工艺专业英语1、2、3、4、5、6、7、10、11、12、13、20、21

Unit 1 Chemical Industry化学工业1.化学工业的起源尽管化学品的使用可以追溯到古代文明时代,我们所谓的现代化学工业的发展却是非常近代(才开始的)。
可以认为它起源于工业革命其间,大约在1800年,并发展成为为其它工业部门提供化学原料的产业。
比如制肥皂所用的碱,棉布生产所用的漂白粉,玻璃制造业所用的硅及Na2CO3. 我们会注意到所有这些都是无机物。
有机化学工业的开始是在十九世纪六十年代以William Henry Perkin 发现第一种合成染料—苯胺紫并加以开发利用为标志的。
20世纪初,德国花费大量资金用于实用化学方面的重点研究,到1914年,德国的化学工业在世界化学产品市场上占有75%的份额。
这要归因于新染料的发现以及硫酸的接触法生产和氨的哈伯生产工艺的发展。
而后者需要较大的技术突破使得化学反应第一次可以在非常高的压力条件下进行。
这方面所取得的成绩对德国很有帮助。
特别是由于1914年第一次世界大仗的爆发,对以氮为基础的化合物的需求飞速增长。
这种深刻的改变一直持续到战后(1918-1939)。
1940年以来,化学工业一直以引人注目的速度飞速发展。
尽管这种发展的速度近年来已大大减慢。
化学工业的发展由于1950年以来石油化学领域的研究和开发大部分在有机化学方面取得。
石油化工在60年代和70年代的迅猛发展主要是由于人们对于合成高聚物如聚乙烯、聚丙烯、尼龙、聚脂和环氧树脂的需求巨大增加。
今天的化学工业已经是制造业中有着许多分支的部门,并且在制造业中起着核心的作用。
它生产了数千种不同的化学产品,而人们通常只接触到终端产品或消费品。
这些产品被购买是因为他们具有某些性质适合(人们)的一些特别的用途,例如,用于盆的不粘涂层或一种杀虫剂。
这些化学产品归根到底是由于它们能产生的作用而被购买的。
2.化学工业的定义在本世纪初,要定义什么是化学工业是不太困难的,因为那时所生产的化学品是很有限的,而且是非常清楚的化学品,例如,烧碱,硫酸。
《化学工程与工艺专业英语》课文翻译完

Unit 1 Chemical Industry化学工业1.化学工业的起源尽管化学品的使用可以追溯到古代文明时代,我们所谓的现代化学工业的发展却是非常近代(才开始的)。
可以认为它起源于工业革命其间,大约在1800年,并发展成为为其它工业部门提供化学原料的产业。
比如制肥皂所用的碱,棉布生产所用的漂白粉,玻璃制造业所用的硅及Na2CO3. 我们会注意到所有这些都是无机物。
有机化学工业的开始是在十九世纪六十年代以William Henry Perkin 发现第一种合成染料—苯胺紫并加以开发利用为标志的。
20世纪初,德国花费大量资金用于实用化学方面的重点研究,到1914年,德国的化学工业在世界化学产品市场上占有75%的份额。
这要归因于新染料的发现以及硫酸的接触法生产和氨的哈伯生产工艺的发展。
而后者需要较大的技术突破使得化学反应第一次可以在非常高的压力条件下进行。
这方面所取得的成绩对德国很有帮助。
特别是由于1914年第一次世界大仗的爆发,对以氮为基础的化合物的需求飞速增长。
这种深刻的改变一直持续到战后(1918-1939)。
date bake to/from: 回溯到dated: 过时的,陈旧的stand sb. in good stead: 对。
很有帮助1940年以来,化学工业一直以引人注目的速度飞速发展。
尽管这种发展的速度近年来已大大减慢。
化学工业的发展由于1950年以来石油化学领域的研究和开发大部分在有机化学方面取得。
石油化工在60年代和70年代的迅猛发展主要是由于人们对于合成高聚物如聚乙烯、聚丙烯、尼龙、聚脂和环氧树脂的需求巨大增加。
今天的化学工业已经是制造业中有着许多分支的部门,并且在制造业中起着核心的作用。
它生产了数千种不同的化学产品,而人们通常只接触到终端产品或消费品。
这些产品被购买是因为他们具有某些性质适合(人们)的一些特别的用途,例如,用于盆的不粘涂层或一种杀虫剂。
这些化学产品归根到底是由于它们能产生的作用而被购买的。
化学工程与工艺专业英语

化学工程与工艺专业英语1. Although the use of chemicals dates back to the ancient civilizations, the evolution of what we know as the modern chemical industry started much more recently. It may be considered to have begun during the Industrial Revolution, about 1800, and developed to provide chemicals roe use by other industries.尽管化学品的使用可以追溯到古代文明时代,我们所谓的现代化学工业的发展却是非常近代(才开始的)。
可以认为它起源于工业革命其间,大约在1800年,并发展成为为其它工业部门提供化学原料的产业.2.At the start of the twentieth century the emphasis on research on the applied aspects of chemistry in Germany had paid off handsomely, and by 1914 had resulted in the German chemical industry having 75% of the world market in chemicals. This was based on the discovery of new dyestuffs plus the development of both the contact process for sulphuric acid and the Haber process for ammonia. The later required a major technological breakthrough that of being able to carry out chemical reactions under conditions of very high pressure for the first time.20世纪初,德国花费大量资金用于实用化学方面的重点研究,到1914年,德国的化学工业在世界化学产品市场上占有75%的份额。
化学工程与工艺专业英语unit1

It will be noted that these are all inorganic chemicals. The organic chemicals industry started in the 1860s with the exploitation of William Henry Perkin’s discovery of the first synthetic dyestuff—mauve. At the start of the twentieth century the emphasis on research on the applied of chemistry in Germany had paid off handsomely, and by 1914 had resulted in the German chemical industry having75% of the world market in chemicals. This was based on the discovery of new dyestuffs plus the development of both the contact process for sulphuric acid and the Haber process for ammonia.
Since 1940 the chemical industry has grown at a remarkable rate, although this has slowed significantly in recent years. The lion’s share of this growth has been in the organic chemicalssector due to the development and growth of the petrochemicals area since 1950.The explosivegrowth in petrochemicals in the 1960s and 1970s was largely due to the enormous increase in demand for synthetic polymers such as polyethylene, polypropylene, nylon, polyesters and epoxy re Chemical Industry
化学工程与工艺专业英语考试重点

(1) Clothing.The improvement in properties of modern synthetic fibers over the traditional clothing materials (e.g. cotton and wool) has been quite remarkable. Thus shirts, dresses and suits made from polyesters like Terylene and polyamides like Nylon are crease-resistant, machine-washable, and drip-dry or non-iron. They are also cheaper than natural materials.衣物。
在传统的衣服面料上,现代合成纤维性质的改善也是非常显著的。
用聚脂如涤纶或聚酰胺如尼龙所制作的T恤、上衣、衬衫抗皱、可机洗,晒干自挺或免烫,也比天然面料便宜。
Parallel developments in the discovery of modern synthetic dyes and the technology to “bond” them to the fiber has resulted in a tremendous increase in the variety of colors available to the fashion designer. Indeed they now span almost every color and hue of the visible spectrum. Indeed if a suitable shade is not available, structural modification of an existing dye to achieve this can readily be carried out, provided there is a satisfactory market for the product.与此同时,现代合成染料开发和染色技术的改善使得时装设计师们有大量的色彩可以利用。
化学工程与工艺专业英语unit1

Since 1940 the chemical industry has grown at a remarkable rate, although this has slowed significantly in recent years. The lion’s share of this growth has been in the organic chemicalssector due to the development and growth of the petrochemicals area since 1950.The explosivegrowth in petrochemicals in the 1960s and 1970s was largely due to the enormous increase in demand for synthetic polymers such as polyethylene, polypropylene, nylon, polyesters and epoxy resins.
The latter required a major technological breakthrough that of being able to carry out chemical reactions under conditions of very high pressure for the first time. The experience gained with this was to stand Germany in good stead, particularly with the rapidly increased demand for nitrogen-based compounds (ammonium salts for fertilizers and nitric acid for explosives manufacture ) with the outbreak of World War I in 1914. This initiated profound changes which continued during the inter-war years(1918-1939).
化工专业英语练习题 参考答案

练习一参考答案1将下列句子或段落翻译成英语1)A process is any operation or series of operations that causes a physical or chemical change in asubstance or a mixture of substances .The material that enters a process is referred to as input or feed the process,and that which leaves is called output or product.2)As a chemical engineer,you might be called on to design individual process units (such as reactors,distillation columns,heat exchangers),supervise the operation of a process,or modify a process design to accommodate a change in the feed or in the desired product characteristics.As a rule,to any of these things you must know the amounts,compositions,and conditions of the materials that enter and leave each process unit,and if you are working with an existing units,you must be able to measure enough of these quantities to verify that the process is doing what it was designed to do.3)Founded in 1839from a small production firm for pharmaceutical products,B.Braun has grown steadilyinto a multinational company dealing with medical products,medical technology,pharmaceutical and biotechnology.2将下列句子或段落翻译成汉语1)包括的一系列操作,如混合、蒸发、过滤,无论产物是什么,这些操作都基本同,从而导致了单元操作的概念。
化学工程与工艺专业英语 Unit 10-17 参考英文

首先,化学工程是以化学为基础的,例如,物理化学、化工热力学以及化工动力学。然而, 尽管如此,它不只是简单地复制这些学科的研究结果,而是将这些研究结果应用于大众化学品 的处理。化学的任务的目标是,去寻找操作最经济的用途,去设计最合适该操作的商业设备和 辅助设备。因此,化学工程,如果没有与经济学、物理学、数学、控制论、应用机械学和其他 技术科学的紧密联系,就难以想象。
在单元操作(intensive 密集)快速发展的这一个时期中,化学工程分析的其他传统工具开 始被采用或得到了广泛的开发,这些工具包括过程的物料和能量平衡的研究以及多组分体糸基
2
础热力学的研究。
化学工程与工艺专业英语 化工系 陈中胜
化学工程师在帮助美国及其盟军赢得二战发挥了关键作用。他们开发了天然橡胶资源的人 造橡胶的合成路线,天然橡胶资源在战争早期已经落于日本的手中。他们提供了建立原子弹所 必要的“U-235”,通过一步法将 u-235 生产过程从实验室生产放大到曾经所建立的最大的工业 化工厂。他们对优化青霉素的生产方面的帮助很大,青霉素生产挽救了成千上万的受伤士兵的 生命。
1.热力学的应用 热力学有两个主要的应用,两者对化学工程师都很重要。 (1)与过程相联系的热效应和功效应的计算,以及从过程得到的最大功或驱动过程所需 的最小功的计算。 (2)描述处于平衡的系统的各变量之间的关系的确定。 第一种应用由热力学这个名词可联想到,热力学表示运动中的热。直接利用第一和第二定 律可完成许多(热效应和功效应的)计算。例如:计算压缩气体的功,对一个完整过程或某一 过程单元的进行能量衡算,确定分离乙醇和水混合物所需的最小功,或者(evaluate)评估一 个氨合成工厂的效率。
化学工程与工艺·专业英语unite17

Unit 17 Chemical Reaction Engineering每一种工业化的化工过程的目的都是通过一系列的处理步骤从各种原料经济性地生成所需的产品。
图3-5表示一种典型的过程。
为了使原料处于能发生化学反应的形式,原料要经过(undergo)许多物理处理步骤,然后,通过反应器。
为了得到最终的所需的产品,反应的产物必须经过进一步的物理处理,如分离、纯化等。
用于物理处理步骤的设备的设计在单元操作中研究,这里我们关心的是过程的化学处理步骤。
经济上,化学处理步骤是不重要的装置,如一简单的混合槽。
然而,化学处理步骤通常是整个过程的核心,在经济方面可使过程发生或停止的因素。
反应器的设计不是例行公事(routine),对于某一过程可以提出许多其它的设计。
为了寻求最佳的设计,必须减少的费用不仅仅是反应器费用。
一种设计可以是反应器费用低,但离开该装置的物料可以是该情况:物料的处理费用比其它设计费用高得多。
所以全过程的经济性必须要考虑。
反应器的设计要运用各种领域(热力学、化学动力学、流体力学、传质、传热以及经济学)的信息、知识和经验。
化学反应工程是这些所有的因素的综合(synthesis),其目的是精确地设计化学反应器。
化学反应器的设计可能是化学工程师的独特(unique)的一种活动,这可能较其它方面更能证明化学工程作为工程学科的独特的分支的存在。
在化学反应器设计时,必须要回答两个问题:(1)我们期望发生什么变化?(2)变化发生有多快?。
第一个问题关于热力学,而第二个问题是关于各种速率过程——化学动力学、传热,等等。
把这些过程综合起来以及要确定这些过程是如何关联的,是相当难的问题(事情)。
因此我们必须从简单的情况开始,利用考虑其它的因素来增长(帮助)我们的分析,直到我们能处理更困难的问题。
1. Thermodynamics热力学给出了设计所需的两条重要的信息:反应释放(或吸收)的热量以及反应的最大的可能程度。
化学工程与工艺专业英语最全版

1.The explosives growth in petrochemicals in the 1960s and 1970s was largely due to the enormous increase in demand for synthet ic polymers such as polyethylene, polypropylene, nylon, polyesters and epoxy resins.石油化工在60年代和70年代的迅猛发展主要是由于人们对于合成高聚物如聚乙烯、聚丙烯、尼龙、聚脂和环氧树脂的需求巨大增加。
2.The difficulty cones in deciding at which point in this sequence the particular operation ceases to be part of the chemical industry’s sphere of activities. 困难在于如何决定在一些特殊的生产过程中哪一个环节不再属于化学工业的活动范畴。
举一个特殊的例子来描述一下这种困境。
3.The chemical engineer must also work closely with mechanical, electrical, civil, and metallurgical engineers in order to design and operate the physical equipment in a plant--the reactors, tanks, distillation columns, heat exchangers, pumps, compressors, Control and instrumentation devices, and so on. 化学工程师还必须与机械、电子、土木建筑和冶金工程师密切协作以设计和操作工厂的机械设备—反应器、槽、蒸馏塔、热交换器、泵、压缩机、控制器和仪器设备等等。
化学工程与工艺专业英语Unit 11_2003版

Thermodynamics might be broadly defined as a means of extending our experimentally gained knowledge of a system or as a framework for viewing and correlating the behavior of the system.
Para. 2
Para. 3
Para. 3
Line. 6
Additionally, the network of relationships (among the variables of a system) allows the calculation of values of variables 【which are either unknown or difficult to determine experimentally from variables (which are either available or easier to measure).】
热力学也许可更广地定义为扩充我们在实验中所获知识体系 的一种方法或定义为考察和关联此系统行为的基本框架。
Para. 2
Line 1. macroscopic 宏观的,肉眼可见的 Line 2. matter in bulk 大批物质 macroscopic 微观的,微小的,显微镜 as opposed to 与…截然相反,对照 be assigned to 被分配给…,归属于 Line 4. recourse to 求助于,依赖 Line 6. authenticity 可靠性,真实性 validity 有效,合法性,正确,确实 Line 7. be independent of 不依赖…,独立于…之外
化学工程与工艺专业英语eighth

Eb1 : 辐射力
I
:定向辐射强度
b1
X dA1 ,dA2
dA2
cos1 r 2
cos 2
(1)
图8-2 两微元面间的辐射
同理:
X dA2 ,dA1
dA1
cos1 r 2
cos2
(2)
整理(1)、(2)式得:
X dA1 ,dA2dA1 X dA2 ,dA1dA2
(3)
两微元表面角系数的相对性表达式: dA1 X dA1 ,dA2 dA2 X dA2 ,dA1
守衡原理,从任何一个表面发射出的辐射能必 全部落到封闭系统的个表面上。因此,任何一 个表面对封闭腔各表面的角系数之间存在下列 关系:
X1,1 X1,2 X1,3 X1,n 1
n
X1,i 1
(5)
i 1
图8-3 角系数的完整性
上式称为角系数的完整性。
注:若表面1为非凹表面时,X1,1 = 0;若
A1 X 1,2 A2 X 2,1 A1 X 1,3 A3 X 3,1 A2 X 2,3 A3 X 3,2
上述方程解得:
X 1,2
A1
A2 2 A1
A3
X 1,3
A1
A3 2A3
A2
X 2,3
A2
A3 2A2
A1
由于垂直纸面方向的长度相同,则有:
X 1,2
l1
l2 l3 2l1
根据角系数的完整性:
X ab,cd 1 X ab,ac X ab,bd
X ab,ac
ab ac bc 2ab
ab bd ad
X ab,bd
2ab
(bc ad) (ac bd)
X ab,cd
化学专业英语课后练习题含答案

化学专业英语课后练习题含答案题目:1.What is the molecular formula for acetic acid?2.What is the common name for sodium chloride?3.What is HNO3? What does it do?4.What is the chemical formula for ammonium nitrate?5.What is the difference between an organic and inorganiccompound?6.What is the difference between an acid and a base?答案:1.The molecular formula for acetic acid is C2H4O2.2.The common name for sodium chloride is table salt.3.HNO3 is nitric acid. It is a strong acid and is used in theproduction of fertilizers, explosives, and dyes.4.The chemical formula for ammonium nitrate is NH4NO3.5.An organic compound contns carbon atoms while inorganiccompounds do not.6.Acids release hydrogen ions (H+) in aqueous solutions whilebases release hydroxide ions (OH-) in aqueous solutions. Acids have a pH less than 7 while bases have a pH greater than 7.解析:1.Acetic acid is a weak acid with a sweet smell and taste. Itsmolecular formula is C2H4O2 and it is also known as ethanoic acid.It is used in the manufacture of various chemicals, solvents,coatings, and plastics.2.Sodium chloride is an inorganic compound with the chemicalformula NaCl. It is commonly used as a seasoning and preservative for food. It is also used in the chemical industry for various purposes, such as the production of chlorine, sodium hydroxide, and soda ash.3.Nitric acid is a strong acid with the chemical formula HNO3.It is a highly corrosive and toxic liquid that is used in theproduction of fertilizers, explosives, and dyes. It also has other industrial and laboratory applications, such as the etching of metals and the synthesis of organic compounds.4.Ammonium nitrate is an inorganic compound with the chemicalformula NH4NO3. It is a common fertilizer that is used to supply nitrogen to plants. It is also used as an explosive in mining, quarrying, and construction.anic compounds are based on carbon atoms, while inorganiccompounds do not contn carbon atoms. Organic compounds can befound in living organisms and are usually covalently bonded.Inorganic compounds are often ionic or covalent and are found in non-living things, such as rocks and minerals.6.Acids are compounds that release hydrogen ions (H+) in aqueous solutions, while bases release hydroxide ions (OH-) in aqueous solutions. Acids are characterized by a sour taste, and have a pH less than7. Bases are characterized by a bitter taste and slippery feel. They have a pH greater than 7. The pH scale is used to measure the acidity or basicity of a substance, with 7 being neutral.。
化学工程与工艺专业英语课后习题答案

• 杂质 (impurity)
反应器 (reactor)
• (使)优化 (to optimize) • 纯度 (purity)
Supplementary Exercise 2: Nomenclature of Chemical Elements in the Fourth Period • 1. 钾元素的英文名称是什么?有什么含义?说出 钾原子的元素符号来源。 + ash(灰)再加上表示活泼金属后缀-ium的合 成词,表示“锅灰”;钾原子的元素符号来源于 它的拉丁文名称kalium的第一个字母。
• (7) artificial fertilizers
• (8) crop protection chemicals
• (9) technology
• (10) pharmaceutical
• (11) research and development
• (12) petrochemical
• (13) automatic controll equipment
• 答案:氦原子的英文名称helium,首次被从太阳 光谱分析中确认,使用希腊神话中太阳神 “Helios”的名字命名。
• 3. 钠原子的英文名称是什么?钠的元素符号为什 么不是英文名称的第一个字母? • 答案:钠原子的英文名称sodium来源于阿拉伯 语suda,而钠的元素符号来源于它的拉丁文名称 natron的第一个字母,所以有不同的表示。
• (5) extraction process
• (6) non-renewable resources
• (7) renewable resources
• (8) energy sources
• (9) fermentation
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
• fermenter(发酵器)
• CFC = chloroflurocarbon(氯氟化碳 或 氯氟烃)
• refrigerant(制冷剂)
• 4. Put the following into English: • 试剂 (reagent) 单体 (monomer) • 丙酮 (acetone or propanone or dimethyl ketone)
Supplementary Exercise 2: Nomenclature of Chemical Elements in the Fourth Period
• 1. 钾元素的英文名称是什么?有什么含义?说出 钾原子的元素符号来源。
• 答案:钾元素的英文名称potassium是由pot(锅) + ash(灰)再加上表示活泼金属后缀-ium的合 成词,表示“锅灰”;钾原子的元素符号来源于 它的拉丁文名称kalium的第一个字母。
• 聚合物 (polymer)
聚乙烯 (polyethylene)
• 氯化物 (chloride)
粘度 (viscosity)
• 烃 (hydrocarbon)
催化剂 (catalyst)
• 炼油厂 (refinery)
添加剂 (additives)
Supplementary Exercise 1: Nomenclature of
• silica( 硅石)
ammonium(铵离子)
• polyester(聚酯)
• the lion's share(大份额)
• 4. Put the following into English
• 钠 Na (sodium)
钾 K (potassium)
• 磷 P (phosphur)
氨 (ammonia)
• (7) artificial fertilizers • (8) crop protection chemicals • (9) technology • (10) pharmaceutical • (11) research and development • (12) petrochemical • (13) automatic controll equipment • (14) capital rather-thanollowing into Chinese:
• quantum(量子) strain(菌株)
• mould(霉菌)
phenol(苯酚)
• sulphate(硫酸盐) carbide(碳化物)
• foul(发臭的)
scrub(洗涤)
• semi-technical(半技术的)
• (7) an existing product • (8) pilot-plant • (9) profitability • (10) environmental impact • (11) pollution • (12) technical support • (13) process improvement • (14) effluent treatment • (15) pharmaceuticals
General Review
Exercise for Unit 1: Chemical Inductry
• 1. complete the summary of the text • (1) the Industrial Revolution • (2) organic chemical • (3) contact process • (4) Haber process • (5) synthetic polymers • (6) intermediates
• 3. Put the following into Chinese
• carbonate(碳酸盐) polypropylene(聚丙烯)
• epoxy(环氧基)
vinyl(乙烯基)
• acetate(醋酸盐)
• pharmaceutical(药物的)
• spectrum(光谱) formaldehyde(甲醛)
光谱分析中确认,使用希腊神话中太阳神 “Helios”的名字命名。
• 3. 钠原子的英文名称是什么?钠的元素符号为什 么不是英文名称的第一个字母?
• 答案:钠原子的英文名称sodium来源于阿拉伯 语suda,而钠的元素符号来源于它的拉丁文名称 natron的第一个字母,所以有不同的表示。
• 4. 磷的英文名称是什么?有什么含义?
chemical elements in the first three short periods
• 1. 氢原子的英文名称是什么含义? • 答案:氢原子的英文名称hydrogen是由hydro
(水)+ gen(产生)合成的单词,表示“水之 来源”。
• 2. 氦的英文名称是什么?有什么含义? • 答案:氦原子的英文名称helium,首次被从太阳
• 脉动 (fluctuation or pulsation) • 乙炔 (acetylene or ethyne) • 硫 (sulphur or sulfur) • 盐酸 (hydrochloric acid) • 停车时间 (down time or shut down time)
• 杂质 (impurity) 反应器 (reactor) • (使)优化 (to optimize) • 纯度 (purity)
• 答案:磷原子的英文名称phosphorus是由phos (光)+ phor(fer带来)的合成词,表示“带 来光”之意。反映出有些磷化合物在黑暗中发光 的事实。
Exercises for Unit 2: Research and Development
• 1. Complete the summary of the text • (1) R & D • (2) ideas and knowledge • (3) processes and products • (4) fundamental • (5) applied • (6) product development
