CK-636_442632-72-6_DataSheet_MedChemExpress
细胞凋亡、坏死、细胞活性检查常见方式及试剂

线粒体膜电位指示染料线粒体膜电位的下降是细胞凋亡初期的一个标志性事件. 它在凋亡进程中与caspase 活化同时发生并先于磷脂酰丝氨酸(PS)的外翻。
基于以上研究,Biotium 研发了各类的新型的荧光探针用于测量线粒体膜电位。
MitoView ™ 633MitoView ™ 633 是一种新型的用于测量线粒体膜电位的深红染料(激发光/发射光622/648 nm)。
利用NucView ™ 488 和MitoView ™ 633 凋亡检测试剂盒能够在荧光显微镜(图.1)或流式细胞仪(图.2、3)下同时进行线粒体膜电位和caspase-3活性的检测。
图 1. 利用MitoView ™ 633进行活细胞染色:Hela 细胞图 2. 流式细胞仪分析:Jurkat 细胞一组用CCCP 使线粒体去极化,另一组利用staurosporine 作为凋亡诱导剂。
利用MitoView ™633 染色图3. 流式细胞仪分析:对照(A)与经staurosporine 处置(B)的Jurkat 细胞(JC-1 染色). FL1 (x- 轴) 为绿色荧光; FL2 (y-轴) 为红色荧光。
(A 图) 较高的红绿荧光比例说明线粒体膜电位未下降. (B 图)较低的红绿荧光比例说明:由于staurosporine诱导了凋亡的发生,细胞的线粒体膜电位大幅下降。
JC-1 线粒体膜电位检测试剂盒JC-1通常被用于检测细胞中线粒体膜电位的转变。
在健康细胞中,JC-1以聚合体(J-aggregates),的形式存在在线粒体基质中,能够产生红色的荧光(激发光/发射光585/590nm)。
相反,在正在凋亡或坏死的细胞中,JC-1不能聚集在基质中,以单体的形式存在,从而发出绿色的荧光( 激发光/ 发射光510/527nm),如此能够利用流式细胞仪和荧光显微镜、荧光计数仪通过测量荧光颜色的转变来检测线粒体膜电位的转变。
经常使用红绿荧光的相对照例来衡量线粒体去极化的比例。
肌酸激酶(CK)测定试剂盒(磷酸肌酸底物法)产品技术要求sainuopu
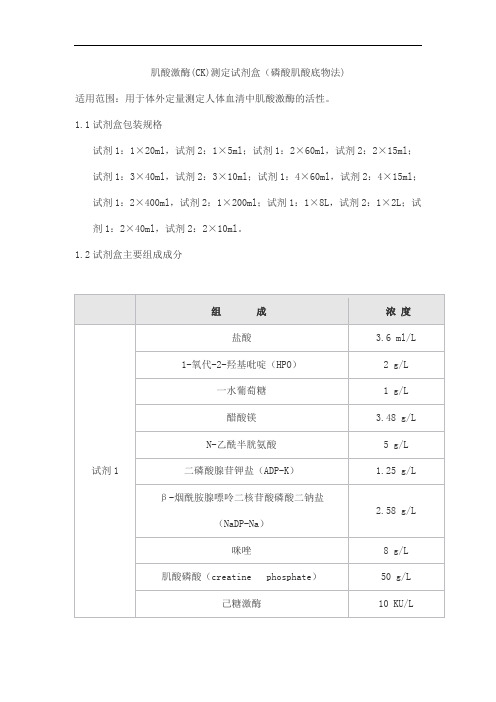
肌酸激酶(CK)测定试剂盒(磷酸肌酸底物法)适用范围:用于体外定量测定人体血清中肌酸激酶的活性。
1.1试剂盒包装规格试剂1:1×20ml,试剂2:1×5ml;试剂1:2×60ml,试剂2:2×15ml;试剂1:3×40ml,试剂2:3×10ml;试剂1:4×60ml,试剂2:4×15ml;试剂1:2×400ml,试剂2:1×200ml;试剂1:1×8L,试剂2:1×2L;试剂1:2×40ml,试剂2:2×10ml。
1.2试剂盒主要组成成分2.1 外观液体双试剂:试剂1无色至浅黄色澄清液体;试剂2无色至浅黄色澄清液体。
2.2 净含量液体试剂的净含量不得低于标示体积。
2.3 试剂空白2.3.1试剂空白吸光度:在37℃、340nm波长、1cm光径条件下,试剂空白吸光度应不大于0.5。
2.3.2试剂空白吸光度变化率:在37℃、340nm波长、1cm光径条件下,试剂空白吸光度变化率(ΔA/min)应不大于0.002。
2.4 分析灵敏度测定活性为100U/L的样本时,吸光度变化值(ΔA/min)应不小于0.014。
2.5 线性范围在(25,1000) U/L范围内:线性相关系数r不小于0.990。
在(100,1000) U/L 范围内,线性相对偏差应不大于±10%;(25,100] U/L范围内,线性绝对偏差应不大于±10U/L。
2.6 重复性重复测试两份高低浓度的样本,所得结果的变异系数(CV%)应不大于5%。
2.7 批间差不同批号试剂测试同一份样本,测定结果的批间相对极差应不大于10%。
2.8 准确度相对偏差:相对偏差应不超过±10%。
2.9 稳定性效期稳定性:试剂盒在2℃~8℃下有效期为15个月。
取失效期的试剂盒进行检测,试验结果满足2.3、2.4、2.5、2.6、2.8的要求。
多种维生素片中维生素K2含量检测研究

多种维生素片中维生素K2含量检测研究杨菊辉(哈药集团制药六厂,黑龙江哈尔滨 150056)摘 要:目的:建立快速测定多种维生素片中维生素K2含量的分析方法。
方法:样品使用美国Waters公司高效液相色谱仪检测,C18色谱柱(250 mm×4.6 mm,5 μm)分离,流动相为甲醇,流速为1.0 mL·min-1。
结果:维生素K2保留时间约为54 min,在1.25~10.00 μg·mL-1线性关系良好,相关系数为0.998 2。
重复性实验相对标准偏差为1.69%,50%加标回收率为92.21%~100.22%,检出限为0.06 μg·g-1,定量限为0.20 μg·g-1。
结论:该方法准确度和精密度良好,灵敏度高,分离度好,可为多种维生素片中维生素K2的检测研究提供参考。
关键词:高效液相色谱检测法;维生素K2;保健食品Study on Vitamin K2 Content Detection in MultivitaminTabletsYANG Juhui(Harbin Pharmaceutical Group No.6 Pharmaceutical Factory, Harbin 150056, China) Abstract: Objective: To establish an analytical method for the rapid determination of vitamin K2 content in multivitamin tablets. Method: Samples were detected using a US Waters HPLC chromatograph, separated with a C18 column (250 mm×4.6 mm, 5 μm), mobile phase in methanol at a flow rate of 1.0 mL·min-1. Result: Vitamin K2 retention time was about 54 min, with a good linear relationship in the concentration 1.25~10.00 μg·mL-1 range, the correlation coefficient was 0.998 2. The relative standard deviation of the repeatability experiments was 1.69%, and the 50% spiking recovery was 92.21%~100.22%. The limit of detection was 0.06 μg·g-1 and the limit of quantification was 0.20 μg·g-1. Conclusion: This method has good accuracy and precision, high sensitivity and good separation degree, which can provide a reference for the detection of vitamin K2 in various vitamin tablets.Keywords: HPLC detection method; vitamin K2; health food维生素K是一类具有叶绿醌生物活性的萘醌基团的衍生物。
伯乐生化质控靶值表310

CK-MB试剂盒说明书(罗氏)

11821598 322 100测试主要用途用免疫学方法定量测定人血清或血浆中肌酸激酶同工酶MB含量。
电化学发光免疫测定试剂,适用于罗氏Elecsys和cobas e免疫测定分析仪。
临床应用1、2、3肌酸激酶(CK)是一种二聚体酶,它有四种不同的形式:线粒体同工酶和胞浆同工酶CK-MM (肌型)、CK-BB(脑型)和CK-MB。
血清中CK-MB的测定值是诊断心肌缺血(例如急性心肌梗塞、心肌炎等)的重要指标。
CK-MB 在心脏症状出现3-8小时后即可检出并且可持续较长时间,这取决于病情经过。
CK-MB还可出现在其它临床条件下,例如横纹肌溶解症和中风。
实验室诊断方面,测定总体CK、肌钙蛋白T和/或肌红蛋百有助于鉴别这些临床疾病。
CK-MB测定的灵敏度与取样时间有关。
因此跟踪测定非常有意义。
Elecsys Ck-MB测定法采用了两种不同的直接对抗人CK-MB的单克隆抗体。
检测原理双抗体夹心法,总检测时间:18分钟●第一次孵育:15μl标本、生物素化的抗CK-MB单克隆抗体和钌(Ru)a标记的CK-MB特异性单克隆抗体一起反应生成抗原抗体夹心复合物。
●第二次孵育:添加包被链霉亲和素的磁珠微粒后,该复合物通过生物素和链霉素之间的反应结合到微粒上。
●将反应液吸入测量池中,通过电磁作用将磁珠吸附在电极表面。
未与磁珠结合的物质通过ProCell被去除。
给电极加以一定的电压,使复合体化学发光,并通过光电倍增器测量发光强度。
●仪器自动通过2点校正的定标曲线计算得到检测结果。
a)Tris(2,2’-bipyridyl) ruthenium(II)-complex (Ru(bpy){23}三联吡啶钌试剂-工作溶液M 包被链霉亲和素的磁珠微粒(透明瓶盖),1瓶,6.5ml;包被链霉亲和素的磁珠微粒,0.72mg/ml;防腐剂。
R1 生物素化的抗CK-MB抗体(灰盖),1瓶,10mL:生物素化抗CK-MB单克隆抗体(小鼠)1.2mg/L;磷酸盐缓冲液100mmol/L,pH值7.0;防腐剂。
糖尿病患者诊断应用血清C肽及糖化血红蛋白联合检测的价值分析

DOI:10.16658/ki.1672-4062.2023.14.085糖尿病患者诊断应用血清C肽及糖化血红蛋白联合检测的价值分析倪胜南,陈少,陈一鸣泗阳康达医院检验科,江苏宿迁223700[摘要]目的探讨糖尿病患者诊断应用血清C肽联合糖化血红蛋白检测的价值。
方法将2022年1月—2023年1月泗阳康达医院收治的74例疑似糖尿病患者作为研究对象,检测入组患者糖化血红蛋白(glycosylated hemoglobin, HbA1c)以及血清C肽水平,以口服葡萄糖耐量试验(glucose tolerance test check, OGTT)为金标准,统计血清C肽联合糖化血红蛋白检测与单一项目检测的敏感性、特异度和诊断符合率。
结果74例疑似糖尿病患者根据葡萄糖耐量试验结果,确诊患者67例,确诊率为90.54%(67/74);与血清C肽、HbA1c单一检测相比,血清C肽+HbA1c联合检测敏感度更高,差异有统计学意义(P<0.05);血清C肽+HbA1c联合检测的特异度略高于血清C肽、HbA1c单一检测,但差异无统计学意义(P>0.05);联合检测诊断符合率明显高于血清C 肽、HbA1c单项检测,差异有统计学意义(P<0.05)。
结论血清C肽与糖化血红蛋白是临床诊断糖尿病的重要参考指标,二者表达水平的变化有助于检测患者胰岛素分泌功能,评估疾病严重程度,两者联合检验灵敏性与特异度良好,有助于早期明确诊断,临床参考价值较高。
[关键词] 糖尿病;血清C肽;糖化血红蛋白;诊断价值[中图分类号] R446.1 [文献标识码] A [文章编号] 1672-4062(2023)07(b)-0085-04Analysis of the Value of the Diagnostic Application of Combined Serum C-peptide and Glycosylated Hemoglobin Testing in Patients with Diabetes MellitusNI Shengnan, CHEN Shao, CHEN YimingDepartment of Laboratory Medicine, Siyang Kangda Hospital, Suqian, Jiangsu Province, 223700 China[Abstract] Objective To explore the value of applying serum C-peptide combined with glycated hemoglobin test for the diagnosis of diabetic patients. Methods A total of 74 patients with suspected diabetes admitted to Siyang Kangda Hospital from January 2022 to January 2023 were selected as the research objects. The levels of glycosylated hemoglo‐bin (HbA1c) and serum C-peptide were detected. Oral glucose tolerance test (OGTT) was used as the gold standard. The sensitivity, specificity and diagnostic coincidence rate of serum C-peptide combined with glycosylated hemoglo‐bin detection and single item detection were statistically analyzed. Results According to the results of glucose toler‐ance test, 67 patients were diagnosed in 74 patients with suspected diabetes, and the diagnosis rate was 90.54% (67/ 74). Compared with the single detection of serum C-peptide and HbA1c, the sensitivity of combined detection of se‐rum C peptide and HbA1c was higher, and the difference was statistically significant (P<0.05). The specificity of com‐bined detection of serum C-peptide and HbA1c was slightly higher than that of single detection of serum C-peptide and HbA1c, but the difference was no statistically significant (P>0.05). The diagnostic coincidence rate of combined detection was significantly higher than that of single detection of serum C-peptide and HbA1c, and the difference was statistically significant (P<0.05). Conclusion Serum C-peptide and glycosylated hemoglobin are important reference indexes for clinical diagnosis of diabetes mellitus, and changes in the expression levels of the two can help to detect the insulin secretion function of patients and assess the severity of the disease. The sensitivity and specificity of the [作者简介]倪胜南(1991-),女,本科,主管检验师,研究方向为免疫学、分子生物学检验。
纤维素酶检测试剂盒(DNS 比色法)说明书
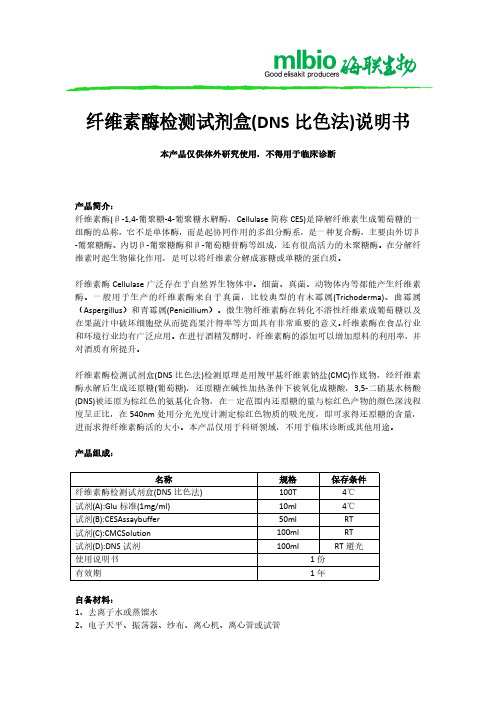
2、准备样品: 称取样品 10g(或 10ml)加入装有玻璃珠的三角瓶中,再加人一定体积的蒸馏水稀释,静置 20min,200r/min 振荡 30min,然后四层纱布过滤,滤液 3000r/min 离心 10min,上清液加 入 50ml 容量瓶中,补水定容,即为纤维素酶提取液,用于样品 CES 活力的检测。如样品酶 活力较高,应稀释至合适浓度再次检测。
产品组成:
名称 纤维素酶检测试剂盒(DNS 比色法) 试剂(A):Glu 标准(1mg/ml) 试剂(B):CESAssaybuffer 试剂(C):CMCSolution 试剂(D):DNS 试剂 使用说明书 有效期
规格 100T 10ml 50ml 100ml 100ml
保存条件 4℃ 4℃ RT RT
纤维素酶检测试剂盒(DNS 比色法)说明书
本产品仅供体外研究使用,不得用于临床诊断
产品简介: 纤维素酶(β-1,4-葡聚糖-4-葡聚糖水解酶,Cellulase 简称 CES)是降解纤维素生成葡萄糖的一 组酶的总称,它不是单体酶,而是起协同作用的多组分酶系,是一种复合酶,主要由外切β -葡聚糖酶、内切β-葡聚糖酶和β-葡萄糖苷酶等组成,还有很高活力的木聚糖酶。在分解纤 维素时起生物催化作用,是可以将纤维素分解成寡糖或单糖的蛋白质。
纤维素酶检测试剂盒(DNS 比色法)检测原理是用羧甲基纤维素钠盐(CMC)作底物,经纤维素 酶水解后生成还原糖(葡萄糖),还原糖在碱性加热条件下被氧化成糖酸,3,5-二硝基水杨酸 (DNS)被还原为棕红色的氨基化合物,在一定范围内还原糖的量与棕红色产物的颜色深浅程 度呈正比,在 540nm 处用分光光度计测定棕红色物质的吸光度,即可求得还原糖的含量, 进而求得纤维素酶活的大小。本产品仅用于科研领域,不用于临床诊断或其他用途。
伯乐1708895系列说明书

Reaction ProtocolIncubate complete reaction mix in a real-time thermal detection system as follows:cDNA synthesis:10 min at 50°CiScript reverse transcriptase inactivation: 5 min at 95°CPCR cycling and detection (30 to 45 cycles):10 to 15 sec at 95°C30 sec at 55°C to 60°C (data collection step) Recommendations for optimal results using the iScript One-Step RT-PCR Kit for ProbesProbe and primers should be designed according to standard qPCR guidelines.Suggested input quantities of template are: 1 pg to 1 µg total RNA; 10 fg to 100 ng polyA(+) RNA.First strand synthesis can be performed between 40°C and 52°C. Optimal results are generally obtained with a 10-minute incubation at 50°C. Incubation at temperatures higher than 50°C can delay or eliminate the detection of some non-specific amplification artifacts. However, this may also delay the C t for detection of specific targets. We also recommend a 5-minute incubation at 95°C to fully inactive the reverse transcriptase prior to PCR cycling.Thaw all components, except the iScript reverse transcriptase, at room temperature. Mix gently, but thoroughly, and then centrifuge at 4°C to collect contents to the bottom of the tube. Chill on ice before using. Centrifuge again briefly at 4°C if needed. Preparation of a reaction cocktail is crucial in quantitative PCR applications to reduce pipetting errors and maximize assay precision and accuracy. Assemble the reaction cocktail with all required components except sample template (total RNA) and dispense equal aliquots into each reaction tube. Add target sample to each reaction as the final step. Addition of sample as 5–10 µl volumes will improve assay precision. Replicate samples should be assembled as a master mix with a single addition of sample template.Reagents and Materials Not SuppliedGene-specific primers and probePipet tips, aerosol barrier tips, such as:Xcluda®Style B, 211-2006Nuclease-free tubes or plates, such as:0.2 ml thin-wall tubes, 223-9473 or plates, 223-9441RNA purification kit, such as:Aurum™total RNA mini kit, 732-6820, orAurum total RNA kit, 2 x 96 well, 732-6800To learn more about Bio-Rad's complete solution for Amplification, visit our website:/genomicsNOTICE TO PURCHASER: LIMITED LICENSEPractice of the patented polymerase chain reaction (PCR) process requires a license. The Bio-Rad real-time detection systems include a licensed thermal cycler and may be used with PCR licenses available from Applied Biosystems. Its use with authorized reagents also provides a limited PCR license in accordance with the label rights accompanying such reagents. Some applications may require licenses from other parties. Aurum, iScript, iTaq, iQ, iCycler,Xcluda, and MyiQ are trademarks of Bio-Rad Laboratories. SYBR Green is a registered trademark of Molecular Probes, Inc. Bio-Rad Laboratories, Inc. is licensed by Molecular Probes, Inc. to sell reagents containing SYBR Green I for use in real-time PCR, for research purposes only.A license to perform the patented 5’ Nuclease Process for research is obtained by the purchase of (i) both Authorized 5’ Nuclease Core Kit and Licensed Probe, (ii) a Licensed5’ Nuclease Kit, or (iii) license rights from Applied Biosystems.This product is an Authorized 5’ Nuclease Core Kit. Use of this product is covered by one or more of the following US patents and corresponding patent claims outside the US: 5,079,352, 5,789,224, 5,618,711, 6,127,155, 5,677,152, (Claims 1-23 only), 5,773,258, (claims 1 and 6 only), 5,407,800, 5,322,770, 5,310,652, 5,210,015, 5,487,972, and claims outside the US corresponding to US Patent No. 4,889,818. The purchase of this product includes a limited, non-transferable immunity from suit under the foregoing patent claims for using only this amount of product for the purchaser’s own internal research. Separate purchase of a Licensed Probe would convey rights under the applicable claims on US Patents Nos. 5,538,848, 5,723,591, 5,876,930, 6,030,787, 6,258,569, 5,804,375 (claims 1-12 only), and 6,214,979, and corresponding claims outside the United States. No right under any other patent claim and no right to perform commercial services of any kind, including without limitation reporting the results of purchaser’s activities for a fee or other commercial consideration, is conveyed expressly, by implication, or by estoppel. This product is for research use only. Diagnostic uses under Roche patents require a separate license from Roche. Further information on purchasing licenses may be obtained from the Director of Licensing, Applied Biosystems, 850 Lincoln Centre Drive, Foster City, California 94404, USA.Bio-Rad Laboratories2000 Alfred Nobel Drive, Hercules, CA 94547510-741-10004106270 Rev C。
盐酸利多卡因注射剂遗传毒性杂质研究_NormalPdf

Journal of China Pharmaceutical University2020,51(4):466-471学报盐酸利多卡因注射剂遗传毒性杂质研究冼芷然1,孙春萌2,骆雪芳1*,钟文英1**(1中国药科大学理学院药物质量研究中心,南京211198;2中国药科大学药学院,南京211198)摘要确定2,6-二甲基苯胺为盐酸利多卡因注射液中遗传毒性杂质,N-氯乙酰-2,6-二甲基苯胺为潜在遗传毒性杂质,建立LC-MS/MS方法,用色谱柱Agilent ZORBAX Eclipse Plus C18(4.6mm×250mm,5μm)对原料、自制制剂及原研制剂进行遗传毒性杂质研究。
研究结果表明自制制剂中杂质2,6-二甲基苯胺与N-氯乙酰-2,6-二甲基苯胺除由原料引入外,可能分别由氧化条件或碱性条件下降解引入,为盐酸利多卡因注射液的遗传毒性风险评估和工艺优化提供参考与指导。
关键词盐酸利多卡因注射液;遗传毒性杂质;LC-MS/MS中图分类号R917文献标志码A文章编号1000-5048(2020)04-0466-06doi:10.11665/j.issn.1000-5048.20200412引用本文冼芷然,孙春萌,骆雪芳,等.盐酸利多卡因注射剂遗传毒性杂质研究[J].中国药科大学学报,2020,51(4):466–471.Cite this article as:XIAN Zhiran,SUN Chunmeng,LUO Xuefang,et al.Profiling of genotoxic impurities in a lidocaine hydrochloride injec‐tion[J].J China Pharm Univ,2020,51(4):466–471.Profiling of genotoxic impurities in a lidocaine hydrochloride injection XIAN Zhiran1,SUN Chunmeng2,LUO Xuefang1*,ZHONG Wenying1**1Drug Quality Research Center,College of Science,China Pharmaceutical University;2School of Pharmacy,China Pharmaceutical University,ChinaAbstract2,6-dimethylbenzenamine was determined as a genotoxic impurity in lidocaine hydrochloride injec‐tion,and2-chloro-N-(2,6-dimethylphenyl)acetamide was determined as potential genotoxic impurity.An LC-MS/ MS method was established to research the profiling of genotoxic impurities in active pharmaceutical ingredients (API),homemade preparation and reference preparation on column Agilent ZORBAX Eclipse Plus C18(4.6mm×250mm,5μm).The results show that in the homemade preparation the2,6-dimethylbenzenamine and the 2-chloro-N-(2,6-dimethylphenyl)acetamide may be degraded under oxidation condition and alkaline condition in addition to the introduction from API preparation process.This study provides guidance for genotoxic risk assess‐ment and prescription process optimization of lidocaine hydrochloride.Key words lidocaine hydrochloride injection;genotoxic impurities;LC-MS/MS盐酸利多卡因(lidocaine hydrochloride)为临床上常制成盐酸利多卡因注射剂应用于局部麻醉药[1]和抗心律失常药物等[2-3]。
肌酸激酶同工酶MB测定试剂盒说明书
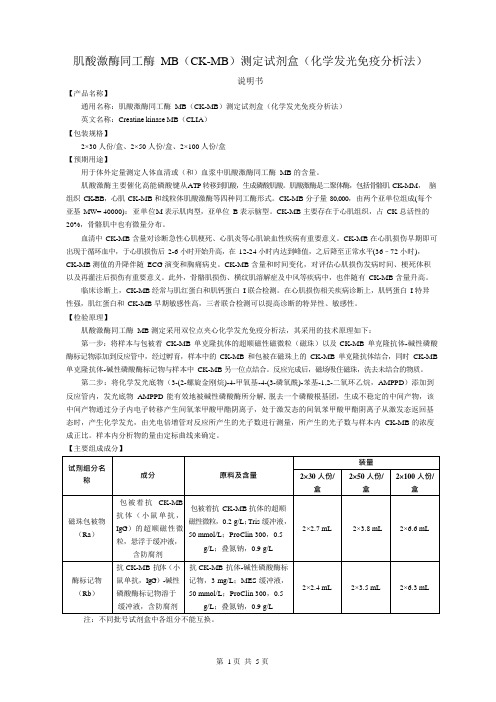
肌酸激酶同工酶MB(CK-MB)测定试剂盒(化学发光免疫分析法)说明书【产品名称】通用名称:肌酸激酶同工酶MB(CK-MB)测定试剂盒(化学发光免疫分析法)英文名称:Creatine kinase MB(CLIA)【包装规格】2×30 人份/盒、2×50 人份/盒、2×100 人份/盒【预期用途】用于体外定量测定人体血清或(和)血浆中肌酸激酶同工酶MB 的含量。
肌酸激酶主要催化高能磷酸键从ATP 转移到肌酸,生成磷酸肌酸。
肌酸激酶是二聚体酶,包括骨骼肌CK-MM,脑组织CK-BB,心肌CK-MB 和线粒体肌酸激酶等四种同工酶形式。
CK-MB 分子量80,000,由两个亚单位组成(每个亚基MW= 40000):亚单位M 表示肌肉型,亚单位B 表示脑型。
CK-MB 主要存在于心肌组织,占CK 总活性的20%,骨骼肌中也有微量分布。
血清中CK-MB 含量对诊断急性心肌梗死、心肌炎等心肌缺血性疾病有重要意义。
CK-MB 在心肌损伤早期即可出现于循环血中,于心肌损伤后2-6 小时开始升高,在12-24 小时内达到峰值,之后降至正常水平(36–72 小时),CK-MB 测值的升降伴随ECG 演变和胸痛病史。
CK-MB 含量和时间变化,对评估心肌损伤发病时间、梗死体积以及再灌注后损伤有重要意义。
此外,骨骼肌损伤、横纹肌溶解症及中风等疾病中,也伴随有CK-MB 含量升高。
临床诊断上,CK-MB 经常与肌红蛋白和肌钙蛋白I 联合检测。
在心肌损伤相关疾病诊断上,肌钙蛋白I 特异性强,肌红蛋白和CK-MB 早期敏感性高,三者联合检测可以提高诊断的特异性、敏感性。
【检验原理】肌酸激酶同工酶MB 测定采用双位点夹心化学发光免疫分析法,其采用的技术原理如下:第一步:将样本与包被着CK-MB 单克隆抗体的超顺磁性磁微粒(磁珠)以及CK-MB 单克隆抗体-碱性磷酸酶标记物添加到反应管中,经过孵育,样本中的CK-MB 和包被在磁珠上的CK-MB 单克隆抗体结合,同时CK-MB 单克隆抗体-碱性磷酸酶标记物与样本中CK-MB 另一位点结合。
安捷伦产品目录

15
Real-Time PCR
16
Mx3000P QPCR System
17
Brilliant III Ultra-Fast SYBR Green QPCR and QRT-PCR Reagents
18
Brilliant III Ultra-Fast QPCR and QRT-PCR Reagents
Agilent / STRATAGENE
Agilent website: /genomics
Welgene | Agilent Stratagene
威健股份有限公司 | Stratagene 總代理
Table of Content
Table of Contents
/ XL1-Red Competent Cells SoloPack Gold Supercompetent Cells
/ TK Competent Cells Specialty Cells
/ Classic Cells / Fine Chemicals For Competent Cells
適用於 UNG 去汙染或 bisulphite
sequencing
適用於 TA Cloning
最高敏感性
取代傳統 Taq 的好選擇
-
2
威健股份有限公司 | Stratagene 總代理
PCR Enzyme & Instrument
Agilent SureCycler 8800
市場上領先的 cycling 速度和 sample 體積 10 ~ 100 μL 簡易快速可以選擇 96 well 和 384 well 操作盤 優秀的溫控設備讓各個 well 都能保持溫度的穩定 七吋的高解析度觸控螢幕讓操作上更為簡便 可以透過網路遠端操控儀器及監控儀器 Agilent 專業的技術支援可以幫助您應對各種 PCR 的問題
高效液相色谱-串联质谱法检测泮托拉唑钠原料药中的水合肼

·药物研发·高效液相色谱-串联质谱法检测泮托拉唑钠原料药中的水合肼赵会明 张振洋 樊华军[英格尔检测技术服务(上海)有限公司 上海 201100]摘要建立了泮托拉唑钠原料药中的基因毒性杂质水合肼的高效液相色谱-串联质谱(LC-MSMS)检测方法。
采用反相色谱,以水-乙腈(含0.1%甲酸)为流动相,梯度洗脱,流速0.5 mL/min,以ESI正离子多反应监测(MRM)模式进行质谱检测。
结果显示,水合肼的检测限和定量限可达到0.23、0.47 ng/mL,其在0.47~9.37 ng/mL浓度范围内线性关系良好(r=0.999 9),准确度试验中低、中、高浓度回收率均在81.6%~90.9%之间。
在3批次泮托拉唑钠原料药中均未检出水合肼。
关键词高效液相色谱-串联质谱法基因毒性杂质泮托拉唑钠水合肼痕量检测中图分类号:R917; O657 文献标志码:A 文章编号:1006-1533(2022)11-0072-04引用本文 赵会明, 张振洋, 樊华军. 高效液相色谱-串联质谱法检测泮托拉唑钠原料药中的水合肼[J]. 上海医药, 2022, 43(11): 72-75.Determination of hydrazine hydrate in pantoprazole sodium by high performance liquid chromatography-tandem mass spectrometryZHAO Huiming, ZHANG Zhenyang, FAN Huajun[ICAS Testing Technology Service (Shanghai) CO., LTD., Shanghai 201100, China]ABSTRACT To establish a high-performance liquid chromatography-tandem mass spectrometry (LC-MSMS) method for the determination of hydrazine hydrate in active pharmaceutical ingredient (API) pantoprazole sodium. HPLC was carried out by reverse chromatography using water-acetonitrile containing 0.1% formic acid as flow phase and gradient elution at a flow rate of 0.5 mL/min. Mass spectrometry was performed with multi-reaction monitoring (MRM) in positive ESI mode. The detection and quantitative limits of hydrazine hydrate reached 0.23, 0.47 ng/mL and hydrazine hydrate showed good linear relationship in the range of 0.47-9.37 ng/mL (r=0.999 9). The recoveries of samples at low, medium and high-level concentrations reached81.6% to 90.9% in the accuracy experiment. No hydrazine hydrate was detected in 3 batches of pantoprazole sodium.KEY WORDS HPLC-tandem mass spectrometry; genotoxic impurities; pantoprazole sodium; hydrazine hydrate; trace determination上消化道出血是近年的临床疾病中常见且多发的一种疾病,其临床表现为呕血、黑便等,如得不到及时有效治疗,可能引发失血性休克。
Quant-iT
Quant-iT ™ 1X dsDNA HS Assay KitCatalog No. Q33232Product informationThe Quant-iT ™ 1X dsDNA HS (High Sensitivity) Assay Kit makes DNA quantitation easy and accurate. The kit includes a ready-to-use assay buffer and DNA standards. To perform the assay, dilute your sample (any volume from 1–20 μL is acceptable) into the 1X working solution provided, then read the concentration using a fluorescence plate reader. The assay is highly selective for double-stranded DNA (dsDNA) over RNA (Figure 4, page 6) and is accurate for initial sampleconcentrations from 10 pg/μL to 100 ng/μL, providing a core detection range of 0.2 ng to 100 ng of DNA in the assay tube. The assay is performed at room temperature, and the signal is stable for 3 hours when the samples are protected from light. Common contaminants such as salts, free nucleotides, solvents, detergents, or protein are well tolerated in the assay (Table 2, page 7).In addition to the Quant-iT ™ 1X dsDNA HS Assay Kit described here, we also offer the Quant-iT ™ 1X dsDNA BR (Broad Range) Assay Kit (Cat. No. Q33267). The Quant-iT ™ 1X dsDNA BR Assay Kit is designed for assaying samples containing 4–2000 ng of DNA. The Qubit ™ dsDNA HS Assay – Lambda standard (Cat. No. Q33233) can be used to create the standard dilution series for the Quant-iT ™ dsDNA HS assay.If you would like to use this kit with the Qubit ™ Fluorometer, we have included instructions under "Perform the Quant-iT ™ 1X dsDNA HS Assay on a Qubit ™ Fluorometer" (page 4).Table 1.Contents and storagePub. No. MAN0017526Rev. C.0Critical assay parametersAssay temperatureThe Quant-iT ™ 1X dsDNA HS Assay delivers optimal performance when all solutions are at room temperature (18–28˚C). Temperature fluctuations can influence the accuracy of the assay (Figure 5, page 6).To minimize temperature fluctuations, insert all assay tubes into the fluorescence microplate reader only for as much time as it takes for the instrument to measure the fluorescence. Do not hold the assay tubes in your hand before reading because this warms the solution and results in a different reading.Incubation timeTo allow the Quant-iT ™ 1X dsDNA HS Assay to reach optimal fluorescence, incubate the tubes for 2 minutes after mixing the sample or the standard with the working solution. After this incubation period, the fluorescence signal is stable for 3 hours at room temperature when samples are protected from light.Photostability of Quant-iT ™reagentsThe Quant-iT ™ reagents exhibit high photostability, showing <0.3% drop in fluorescence after 9 readings and <2.5% drop in fluorescence after 40 readings.Handling and disposalNo data are currently available that address the mutagenicity or toxicity of theQuant-iT ™ 1X dsDNA HS Reagent (the dye in Component A). This reagent is known to bind nucleic acids. Treat the Quant-iT ™ 1X dsDNA HS working solution with the same safety precautions as all other potential mutagens and dispose of the dye in accordance with local regulations.Figure 1. Excitation and emission maxima for the Quant-iT ™1X dsDNA HS reagent when bound to dsDNA.Perform the Quant-iT™ dsDNA HS Assay on a fluorescence microplate readerThis protocol describes the use of the Quant-iT™ 1X dsDNA HS Assay Kit with afluorescence microplate reader that is equipped with either a monochrometer orexcitation and emission filters appropriate for fluorescein or Alexa Fluor™ 488 dye(Figure 1, page 2). Some contaminating substances may interfere with the assay; formore information, see "Contaminants tolerated by the Quant-iT™ 1X dsDNA HS Assay"(page 7). For an overview of this procedure, see Figure 2.Figure 2. The Quant-iT™ dsDNA High-Sensitivity assay.Assay procedure IMPORTANT! For best results, ensure that all materials and reagents are at roomtemperature.1.1 Add 10 μL of each Quant-iT™ 1X dsDNA HS Standard to separate wells. Duplicates ortriplicates of the standards are recommended.1.2 Add 1–20 µL of each unknown DNA sample to separate wells. Duplicates or triplicatesof the unknown samples are recommended.1.3 Load 200 μL of the Quant-iT™ 1X dsDNA working solution into each microplate well.This can be done readily using a multichannel pipettor.If possible, mix your 96-well plate using a plate mixer or using the plate reader for1.4about 3–10 seconds. Following mixing, allow the plate to incubate at room temperaturefor 2 minutes..Measure the fluorescence using a microplate reader (excitation/emission maxima are1.5502/523 nm; see Figure 1, page 2). Standard fluorescein wavelengths (excitation/emission at ~480/530 nm) are appropriate for this dye. The fluorescence signal is stablefor 3 hours at room temperature when protected from light.Use a standard curve to determine the DNA amounts. For the dsDNA standards, plot1.6amount vs. fluorescence, and fit a straight line to the data points.Note: Many curve fitting programs will calculate the y-intercept. However, for bestresults, manually set the y-intercept as the RFU value obtained from the 0 ng/μLdsDNA standard.Data analysis considerations –standard curves and extendedranges The fluorescence of the Quant-iT™ 1X dsDNA HS reagent bound to dsDNA is extremelylinear from 0–100 ng. For best results at the low end of the standard curve, the lineshould be forced through the background point (or through zero, if backgroundhas been subtracted). When 10 μL volumes of the standards are used, the lowestDNA-containing standard represents 5 ng of DNA; nevertheless, highly accuratedeterminations of DNA down to 0.2 ng are attained using the standard curve asdescribed above.To assess the reliability of the assay in the low range, use smaller volumes of thestandards; for example, 2 μL volumes for a standard curve ranging from 0–20 ng.Alternatively, dilute the standards in buffer for an even tighter range. Duringdevelopment of the Quant-iT™ 1X dsDNA HS assay, we were able to detect 0.05 ng ofλ DNA under ideal experimental circumstances (using calibrated pipettors, octuplicatedeterminations, the best microplate readers, and Z-factor1 analysis). Your results mayvary.If desired, the utility of the Quant-iT™ 1X dsDNA HS assay can be extended beyond100 ng, up to 200 ng. For standards in this range, use 20 μL volumes of the providedstandards. Note that the standard curve may not be linear in the range 160–200 ng, andhigh levels of RNA may now interfere slightly with the results.Perform the Quant-iT™ dsDNA HS Assay on a Qubit™ FluorometerThe Quant-iT™ 1X dsDNA Assay Kit can be adapted for use with the Qubit™Fluorometer. The protocol below is abbreviated from the Qubit™ Fluorometer userguide, which is available at /qubit. Although a step-by-step protocoland critical assay parameters are given here, more detail is available in the Qubit™Fluorometer user guide and you are encouraged to familiarize yourself with thismanual before you begin your assay. See Figure 3 for an overview of the procedure.Figure 3. Overview for using the Quant-iT™ 1X dsDNA HS assay in the Qubit™ fluorometer.Assay procedure IMPORTANT! For best results, ensure that all materials and reagents are at roomtemperature.2.1 Set up the required number of 0.5-mL tubes for standards and samples. The Quant-iT™1X dsDNA HS Assay requires 2 standards.Note: Use only thin-wall, clear, 0.5-mL PCR tubes. Acceptable tubes include Qubit™assay tubes (Cat. No. Q32856).2.2 Label the tube lids.Note: Do not label the side of the tube as this could interfere with the sample read. Labelthe lid of each standard tube correctly. Calibration of the Qubit™ Fluorometer requiresthe standards to be inserted into the instrument in the right order.2.3 Add 10 µL of the 0 ng/μL and the 10 ng/μL Quant-iT™ 1X dsDNA HS Standard to theappropriate tube2.4 Add 1–20 µL of each user sample to the appropriate tube.2.5 Add the Quant-iT™ 1X dsDNA HS Working Solution to each tube such that the finalvolume is 200 µL.Note: The final volume in each tube must be 200 µL. Each standard tube requires 190 µLof Quant-iT™ working solution, and each sample tube requires anywhere from180–199 µL.2.6 Mix each sample vigorously by vortexing for 3–5 seconds.2.7 Allow all tubes to incubate at room temperature for 2 minutes, then proceed to read thestandards and samples. Follow the procedure appropriate for your instrument:• Qubit™ Flex Fluorometer• Qubit™ 4 Fluorometer• Qubit™ 3 FluorometerNote: If you are using the Qubit™ 3 Fluorometer, download the 1X dsDNA algorithmand assay button from /qubit, then install it onto your Qubit™Fluorometer.AppendixSelectivity of the Quant-iT™ 1XdsDNA HS AssayFigure 4. DNA selectivity and sensitivity of the Quant-iT™ 1X dsDNA HS Assay (Cat. No. Q33232). Triplicate10-μL samples of λ DNA, E. coli rRNA, or a 1:1 mixture of DNA and RNA were assayed with the Quant-iT™1X dsDNA HS Assay. Fluorescence was measured at 502/532 nm and plotted versus the concentration ofthe RNA or DNA sample alone, or versus the mass of the DNA component in the 1:1 mixture. The variation(CV) of replicate DNA determinations was ≤2%. The inset is an expanded view of the low range of the assayshowing the extreme sensitivity of the assay for DNA. Background fluorescence has not been subtracted.Effect of temperature on theQuant-iT™ 1X dsDNA HSAssayFigure 5. Plot of fluorescence vs. temperature for the Quant-iT™ 1X dsDNA HS Assay. The Quant-iT™assays are designed to be performed at room temperature, as temperature fluctuations can influence theaccuracy of the assay.Contaminants tolerated by the Quant-iT ™ 1X dsDNA HSAssayNote: While the contaminant tolerances of the Quant-iT ™ 1X dsDNA HS assay and theQuant-iT ™ dsDNA HS assay are largely similar, they are not identical.Reference1. J Biomol Screen 4, 67–73 (1999).Table 2. Effect of contaminants in the Quant-iT ™1X dsDNA HS Assay*/support | /askaquestion Limited Product WarrantyLife Technologies Corporation and/or its affiliate(s) warrant their products as set forth in the Life Technologies’ General Terms and Conditions of Sale found on Life Technologies’ website at /us/en/home/global/terms-and-conditions.html . If you have any questions, please contact Life Technologies at /support .Life Technologies Corporation | 29851 Willow Creek Road | Eugene, OR 97402 USAFor descriptions of symbols on product labels or prodoct documents, go to /symbols-definition .The information in this guide is subject to change without notice.DISCLAIMER: TO THE EXTENT ALLOWED BY LAW, LIFE TECHNOLOGIES AND/OR ITS AFFILIATE(S) WILL NOT BE LIABLE FOR SPECIAL, INCIDENTAL,INDIRECT, PUNITIVE, MULTIPLE OR CONSEQUENTIAL DAMAGES IN CONNECTION WITH OR ARISING FROM THIS DOCUMENT, INCLUDING YOUR USE OF IT.Important Licensing Information: These products may be covered by one or more Limited Use Label Licenses. By use of these products, you accept the terms and conditions of all applicable Limited Use Label Licenses.Revision history:Pub. No. MAN0017526©2021 Thermo Fisher Scientific Inc. All rights reserved. All trademarks are the property of Thermo Fisher Scientific and its subsidiaries unless otherwise specified .Ordering informationCat. No. Product name Unit size Q33232Quant-iT™ 1X dsDNA HS Assay Kit.................................................................... 1 kitRelated products Q33267 Quant-iT™ 1X dsDNA BR Assay Kit.................................................................... 1 kit Q33120 Quant-iT™ dsDNA Assay Kit, High Sensitivity............................................................ 1 kit Q33130 Quant-iT™ dsDNA Assay Kit, Broad Range.............................................................. 1 kit Q10213 Quant-iT™ RNA Assay Kit, Broad Range................................................................ 1 kit Q33140 Quant-iT™ RNA Assay Kit, 1000 assays ................................................................ 1 kit Q32882 Quant-iT™ microRNA Assay Kit, 1000 assays............................................................ 1 kit Q33210 Quant-iT™ Protein Assay Kit, 1000 assays .............................................................. 1 kit O11492 Quant-iT™ OliGreen™ ssDNA Assay Kit ................................................................ 1 kit Q33233 Qubit™ 1X dsDNA Assay- Lambda Standard ............................................................ 1 kit Q33238 Qubit™ 4 Fluorometer with WiFi....................................................................... 1 each Q33327 Qubit™ Flex Fluorometer ............................................................................ 1 each Q33252 Qubit™ Flex Assay Tube Strips .................................................................. 125 tube strips M33089 Microplates for fluorescence-based assays, 96-well (black-walled, clear bottom) ................................ 10 plates。
QIAstat-Dx Analyzer 1.0 Printer Setup Guide

Technical Information September 2021 QIAstat-Dx® Analyzer 1.0 Printer Setup Guide The following information provides additional guidance on how to set up printers with the QIAstat-Dx Analyzer 1.0. This document covers the following information:• R ecommended printers• G eneral recommendations for printer use• S tep-by-step instructions for setting up USB printers• S tep-by-step instructions for setting up network printers• S tep-by-step instructions for connecting directly to a Windows 7 PC• S tep-by-step instructions for connecting directly to a Windows 10 PC• F requently asked questionsFurther informationQIAstat-Dx Analyzer 1.0 User Manual: /HB-2636Technical assistance: Recommended printersNote that we have tested only the printer models listed below. If you are using a different printer model, QIAGEN cannot guarantee compatibility. Tested printer models include the following:• H P® OfficeJet® Pro 6230• H P Color LaserJet® Pro M254dw• H P Color LaserJet MFP M227dw• H P Laserjet Pro M404n• H P OfficeJet Pro 8610• B rother® MFC-9330CDW• B rother HL-L2370DNGeneral recommendations for printer useWe recommend the following:• U se only printers with displays and indicator lights that provide unambiguous information on print job status and printer failure modes.• U se generic printer drivers, such as those delivered with the system, rather than specific printer drivers.• R estart the QIAstat-Dx Analyzer by powering it OFF and then ON after Common UNIX Printing System (CUPS) configuration changes, such as adding new printers. Use the power switch on the back. The use of the standby button on the front of the instrument is insufficient.Step-by-step instructions for setting up USB printersNote that USB-connected printers may not require installation after plugging them into any USB port of the QIAstat-Dx Analyzer (refer to “CUPS printer driver installation” in the QIAstat-Dx Analyzer 1.0 User Manual). This option works only if the generic printer is enabled under the Printer settings by selecting the available option PRINTER (Options ✂ System Config ✂ Printer).Step-by-step instructions for setting up network printersFor the installation of network printers, see “List of tested printers” of the QIAstat-Dx Analyzer 1.0 User Manual and the printers recommended above. Ensure that the network printer, QIAstat-Dx Analyzer and the PC connecting to the QIAstat-Dx Analyzer are part of the same local network (see “Network settings” in the QIAstat-Dx Analyzer 1.0 User Manual). Ideally, use a local private network with static IP addresses to ensure that CUPS can be accessed as described in the “CUPS printer driver installation” section of the QIAstat Dx Analyzer 1.0 User Manual. Ask your local IT expert how to configure a local private network with static IP addresses, or follow the step-by-step guide below describing how to directly connect the QIAstat Dx Analyzer to a PC.Step-by-step instructions for connecting directly to a Windows 7 PCYou can directly connect your PC to the QIAstat-Dx Analyzer via an ethernet cable connected to the ethernet socket located at the back of the device. Once physically connected, follow the steps below to configure network connectivity.Configure a static IP address on the QIAstat-Dx Operational Module1. Click Options ✂ System Config ✂ Network.2. Enable IPv4.3. Click Save.4. Click IPv4 Setting.5. Enter the following IP Configuration:5a. Disable Obtain IPv4 address autom.5b. IPv4 Address: 192.168.1.1005c. Subnet mask: 255.255.255.05d. Default gateway: 192.168.1.15e. The remaining fields do not need to be altered.6. Click Save (Figure 1).Figure 1. Configuring a static IP Address on the QIAstat-Dx Operational Module.Figure 2. Selecting the ethernet device .Figure 3. Configuring the Local Area Connection Properties.Note : If the QIAstat-Dx Operational Module and the PC are connected via an ethernet cable and the settings are completed as described in this section, you should be able to execute the steps described above with the IP address 192.168.1.100.1. In Windows, click Start ✂ Control Panel ✂ Network and Sharing Center ✂ Change adapter settings2. Select the ethernet device (Local Area Connection ), right click, and select Properties (Figure 2).3. Select Internet Protocol Version 4 (TCP/IPv4), and click Properties (Figure 3).4. Insert the following information, and click OK (Figure 4).4a. Select Use the following IP address . 4b. IPv4 Address : 192.168.1.101 4c. Subnet mask : 255.255.255.0 4d. Default gateway : 192.168.1.1Configure a static IP address on a Windows 7 PCStep-by-step instructions for connecting directly to a Windows 10 PCYou can directly connect your PC to the QIAstat-Dx Analyzer via an ethernet cable connected to the ethernet socket at the back of the device. Once physi-cally connected, follow the steps below to configure network connectivity.Configure a static IP address on the QIAstat-Dx Operational ModuleConfigure a static IP address on the QIAstat-Dx Operational Module following the instruction provided in the “Configure a static IP address on the QIAstat-Dx Operational Module” section above. In this example, it is assumed that the IP address of the QIAstat-DxOperational Module is statically set as 192.168.1.100. Configuring a static IP address on Windows 10 PC 1. Click the Start menu button and search for theControl Panel (Figure 5).Figure 4. Configuring Internet Protocol Version 4 (TCP/IPv4) Properties. Figure 5. Searching for the Control Panel in the Start menu.2. Click View network status and tasks under theNetwork and Internet section (Figure 6).3. In the dialog that opens, click Change adaptersettings. A new window opens, listing all availablenetwork adapters. Select the adapter related to thephysical ethernet plug on your PC, usually referredto as “ethernet” (Figure 7).4. Once the ethernet adapter is selected, clickChange Settings of this connection (Figure 8).Figure 6. Opening the View network status and tasks menu.Figure 7. Configuring ethernet settings in Change adapter settings.Figure 8. Changing the settings of the ethernet connection.5. The Ethernet Properties window appears. SelectInternet Protocol version 4 (TCP/IPv4) and tickthe corresponding check box. Click Properties(Figure 9).6. Select Use the following IP address and enter thefollowing information (Figure 10):6a. IPv4 Address: 192.168.1.1016b. Subnet mask: 255.255.255.06c. Default gateway: 192.168.1.17. Click OK.Figure 9. Ticking the Internet Protocol Version 4 (TCP IPv4) checkbox.Figure 10. Configuring the IP address settings.Frequently asked questionsThese frequently asked questions address printer setup troubleshooting and provide guidance to avoid common printer issuesI am trying to access the CUPS page from my PC, but it does not accept the password. What can I do?The CUPS password is case sensitive. If a particular CUPS password does not work despite being entered correctly, disable CUPS, save the option, re-enable CUPS and save the option to get a new password. Where can I find the IP address of the QIAstat-Dx Analyzer?You can find the IP address of your QIAstat-Dx Analyzer under Settings ✂ Network ✂ IPv4 Settings ✂ IPv4 Address.The QIAstat-Dx Analyzer is not connected to a local network. How can I connect to the CUPS admin interface from my Windows PC?• F ollow the above step-by-step guide (according to the operating system of your PC) for how to connect your PC to the QIAstat-Dx Operational Module.• E nable CUPS web interface from Network ✂ Enable Cups (enabled).• F ollow the step-by-step guide about how to configure a printer from the CUPS interface.My USB printer does not print using the generic printer driver. How can I install a custom printer driver?Try to install a more specific driver for your printer via CUPS. For this purpose, follow the instructions for set-ting up network printers (see above). Ensure the QIAstat-Dx Analyzer and connecting PC are part of the same local network(see “Network settings” of the QIAstat-Dx Analyzer 1.0 User Manual).I configured my printer via CUPS, but it does not show up in the printer settings of the QIAstat-Dx application software. What can I do?• E nsure that the printer is powered on and that the USB or network connection is established successfully.• Y our printer can be accessed via a generic printer driver that is pre-installed on the QIAstat-Dx Analyzer. Try to print via this driver (see “Printer settings” in the QIAstat-Dx Analyzer 1.0 User Manual).• I f a custom printer driver was already previously configured, power OFF the QIAstat-Dx Operational Module and then power ON again to make the driver available. To power OFF or ON, use the switch at the back of the instrument. The QIAstat-Dx application software detects the available printer drivers upon start up. When adding a new printer, the QIAstat-Dx Operational Module must be restarted before the new driver will be available.The printer settings in Options System Config of the application software do not show any printers. Also, the generic printer is missing. What can I do?Re-install the generic printer driver via the CUPS page. Contact QIAGEN Technical Service to request the generic printer driver if it is unavailable.I configured my printer via CUPS. When I click Print, no report is printed. What can I do?• C onsider that it may take a few minutes to store a PDF report, which must be completed before the printer receives the print job. Avoid pressing the print button several times in a row. This may delay the print process even further.• N ote that, after clicking the print button, the print job may be spooled. To check if a print job is still queued, connect CUPS. On the CUPS page, click Jobs to review the printer queue on the subsequent page.• E nsure that the printer being used is not reporting any error. For example, failed previous print jobs, a paper jam or an empty paper tray can cause an error. Be sure to solve these types of errors before printing.• I n CUPS, ensure that the appropriate media size and paper format as they are displayed or selected on your printer: some printers will not print if the paper format is wrong.• I n CUPS, check the status of your print jobs. This can be performed by navigating to Jobs or clicking the Manage Jobs button. If necessary, cancel existing and unfinished jobs, as they may block the QIAstat-Dx Analyzer from printing.• I n CUPS, try to print a test page. This can be performed by navigating to Manage printers and selecting your printer. From the maintenance drop-down, select print test page.• F or network printers, it is preferred to use direct printing (Port 9100). The connections to this port should be checked to ensure they are not blocked by connecting via the socket:9100 protocol (Direct Printing) and setting the filter to Generic PS or Generic PCL in CUPS when adding a new printer.I tried to configure a specific driver for my printer, but the list of drivers does not include my model. What can I do?Use the most generic driver listed for your printer brand. In the case that none of the listed drivers works, download the CUPS printer driver as a *PPD file from the manufacturer’s website and select PPD File before clicking Add printer.I tried to configure a driver for my printer, but the CUPS page is not accessible. What can I do?• E nsure that the printer is connected via ethernet cable. Also, check switches or other hardware devices in your network infrastructure to ensure they are working properly.• E nsure that the QIAstat-Dx Analyzer and connecting PC are part of the same network (e.g., same gateway, subnet mask).• E nsure that your network infrastructure allows communication on port 631. Also, confirm that communication via the QIAstat-Dx Analyzer, connecting PC and network printer are allowed.• E nsure that the QIAstat-Dx Analyzer and connecting PC are in the same local network. For security reasons, the accessibility of the CUPS page is limited to local networks. If possible, connect via a direct ethernet connection and assign static IPs from a private IP address range to the QIAstat-Dx Analyzer and PC.• E nsure that CUPS is enabled on the QIAstat-Dx Analyzer The current password is used as the password and expires after 24 hours.• C lear the browser cache or try a different browser to avoid login credentials from previous login being used by the PC accessing the CUPS page.CUPS shows that print jobs were canceled, but I did not cancel the print job. What does this mean?If a print job is listed as cancelled and you did not cancel it, an incompatible printer driver might have been used. Try printing via the generic printer driver instead.QIAstat-Dx Analyzer 1.0 Printer Setup Guide 09/2021 11For up-to-date licensing information and product-specific disclaimers, see the respective QIAGEN kit instructions for use or user operator manual. QIAGEN instructions for use and user manuals are available at or can be requested from QIAGEN Technical Services (or your local distributor).Trademarks: QIAGEN®, Sample to Insight®, QIAstat-Dx® (QIAGEN Group), Brother® (Brother Industries, Ltd.); HP®, OfficeJet®, LaserJet® (Hewlett Packard Company); Windows (Microsoft Corporation). Registered names, trademarks, etc. used in this document, even when not specifically marked as such, may still be protected by law.09/2021 1125913 PROM-18835-001 © 2021 QIAGEN, all rights reserved.Ordering /shop Technical Support Website 1125913 09/2021。
肌酸激酶测定试剂

(一)试剂(盒)命名的原则
试剂(盒)名称由三部分组成:第一部分被测物名称:肌酸激酶;第二部分用途;测定试剂(盒);第三部 分方法或原理:磷酸肌酸底物法。
产品名称:肌酸激酶测定试剂(盒)(磷酸肌酸底物法)
(二)试剂(盒)的结构组成
试剂(盒)的组成形式:双试剂; 试剂盒的性状:干粉或液体。
(三)反应原理
(五)产品的预期用途
本试剂(盒)用于体外定量测定人血清或血浆中的肌酸激酶的活性。 注:预期用途中的测定样本类型需经临床验证。
(六)产品的主要技术指标
目测检查,符合生产企业规定的正常外观要求。 (一般要求试剂无杂质、无絮状物,外包装完整无破损)。 用通用量具测量,液体试剂的净含量应不少于标示值。 3.1试剂空白吸光度 用指定空白样品测试试剂(盒),在测试主波长下,37℃、340nm波长、1cm光径条件下,记录测试启动时 的吸光度(A1)和约5分钟(T)后的吸光度(A2),A2测试结果即为试剂空白吸光度测定值,应不大于0.50。 3.2试剂空白吸光度变化率 记录测试启动时的吸光度(A1)和约5分钟(T)后的吸光度(A2),计算出吸光度变化值(|A2-A1|/T), 即为试剂空白吸光度变化率(ΔA/min),应不大于0.002。 用已知浓度或活性的样品进行测试,记录在试剂(盒)规定参数下产生的吸光度改变。换算为n单位吸光度 差值(ΔA)或吸光度变化(ΔA/min)。应符合生产企业给定范围。 用超出线性范围上限活性的样品和超出或等于线性范围下限活性的样品,混合成至少5个稀释浓度(xi)。
二、技术审查要点
01
(一)试剂 (盒)命名 的原则
02
(二)试剂 (盒)的结 构组成
03
(三)反应 原理
04
(四)产品 适用的相关 标准
组织半胱氨酸(cysteine)含量比色法定量检测试剂盒产品说

组织半胱氨酸(cysteine)含量比色法定量检测试剂盒产品说明书(中文版)主要用途组织半胱氨酸(cysteine)含量比色法定量检测试剂是一种旨在通过先酸性预处理,有机溶剂萃取后,在碱性条件下,与萘醌磺酸钠进行反应后,由次亚硫酸钠作用产生的红色显色产物,在分光光度仪下,即采用比色法来测定样品中半胱氨酸含量的权威而经典的技术方法。
该技术经过精心改良沙利文反应(Sullivan Reaction)、成功实验证明的。
其适用于各种组织(动物、人体等)裂解萃取液样品中半胱氨酸水平检测。
产品严格无菌,即到即用,操作简捷,性能稳定。
技术背景L-半胱氨酸(L-cysteine;Cys;C)学名为2-氨基-3-巯基丙酸,是一种疏水性非必需α-氨基酸,分子式是HO2CCH(NH2)CH2SH或C3H7NO2S,分子量121.16,编码为UGU和UGC。
其侧链是巯基(thiol),为非极性,作为亲核剂(nucleophile),参与酶类反应。
氧化后,产生二硫化物和衍生物(disulfide derivative)胱氨酸(cystine),二硫化物成为蛋白质的结构形式。
动物通过胱硫醚β合酶(cystathionine beta synthase)和胱硫醚γ裂合酶(cystathione gamma lyase)由丝氨酸(serine)和蛋氨酸(methionine)转化成同源半胱氨酸(homocysteine)、S-腺苷蛋氨酸(S-adenosylmethionine)、胱硫醚(cystathionine),最后产生半胱氨酸和α-酮基丁酸(α-ketobutyrate)。
而植物和细菌通过丝氨酸乙酰基转移酶(transacetylase)和O-乙酰丝氨酸巯基裂合酶(O-acetylserine(thio)lyase),由丝氨酸产生半胱氨酸和乙酸。
半胱氨酸,是谷胱苷肽的前体,具有抗氧化作用、抗蛋白水解、使蛋白分子之间交联、参与硫化物代谢、与各种金属原子,例如铁、锌、铜等结合、参与蛋白质的转录后修饰、中和酒精毒性等功能。
肌酸激酶(CK)活性检测试剂盒说明书__紫外分光光度法UPLC-MS-4368

肌酸激酶(CK)活性检测试剂盒说明书注意:本产品试剂有所变动,请注意并严格按照该说明书操作。
紫外分光光度法货号:UPLC-MS-4368规格:50T/48S产品组成:使用前请认真核对试剂体积与瓶内体积是否一致,有疑问请及时联系工作人员。
试剂名称规格保存条件提取液液体60mL×1瓶4℃保存试剂一粉剂×1瓶-20℃保存试剂二粉剂×1支-20℃保存试剂三粉剂×2支-20℃保存试剂四粉剂×2支-20℃保存试剂五液体15mL×1瓶4℃保存溶液的配制:1、试剂一:临用前加10mL蒸馏水溶解;用不完的分装后-20℃保存,禁止反复冻融;2、试剂二:临用前加入0.5mL蒸馏水溶解;用不完的分装后-20℃保存,禁止反复冻融;3、试剂三:临用前取1支加入0.5mL蒸馏水溶解;用不完的分装后-20℃保存,禁止反复冻融;4、试剂四:临用前加入0.65mL蒸馏水溶解;用不完的分装后-20℃保存,禁止反复冻融;5、工作液:临用前根据用量将试剂一、试剂二、试剂三、试剂四、试剂五以70:4:7:10:90的比例混合(体积比)。
现用现配。
使用前室温孵育20min(该步骤不可省略)。
产品说明:肌酸激酶(Creatine Kinase,CK)(EC2.7.3.2)也成为肌酸磷酸激酶,主要存在于心脏、肌肉以及脑等组织中,能可逆地催化肌酸与ATP之间的转磷酰基反应,是一个与细胞能量运转、肌肉收缩、ATP再生有直接关系的重要激酶。
CK催化磷酸肌酸和ADP生成肌酸和ATP,己糖激酶催化ATP与葡萄糖形成6-磷酸葡萄糖,6-磷酸葡萄糖脱氢酶催化6-磷酸葡萄糖与NADP+生成NADPH,导致340nm光吸收值增加,以此来表示CK酶活。
注意:实验之前建议选择2-3个预期差异大的样本做预实验。
如果样本吸光值不在测量范围内建议稀释或者增加样本量进行检测。
需自备的仪器和用品:天平、低温离心机、恒温水浴锅、紫外分光光度计、1mL石英比色皿、恒温水浴锅、研钵/匀浆器、蒸馏水。
