Rutin_LCMS_20442_MedChemExpress
超高效液相色谱-串联质谱法测定人血浆中精氨酸及衍生物含量

超高效液相色谱-串联质谱法测定人血浆中精氨酸及衍生物含量田晔;江骥;胡蓓;薛金萍;王洪允【摘要】建立了超高效液相色谱-串联质谱(UPLC-MS/MS)法同时测定使用艾普拉唑后人血浆中二甲基精氨酸(ADMA)、对称二甲基精氨酸(SDMA)、单甲基精氨酸(NMMA)、瓜氨酸(Cit)和L-精氨酸(L-Arg)的浓度.采用HILIC亲水相互作用色谱和非衍生化的蛋白沉淀法进行分离分析,色谱柱选取Waters Atlantic HILIC柱(2.1 mm×50 mm×3μm),流动相由乙腈(含0.5%乙酸和0.025%三氟乙酸)-水(含0.5%乙酸和0.025%三氟乙酸)(85:15,v/V)组成,流速0.25 mL/min.采用多反应离子监测(MRM)模式,以电喷雾离子源(ESI)正离子方式检测.结果显示,ADMA、SDMA、NMMA、L-Arg和Cit的线性关系良好,相关系数r均大于0.994 0;ADMA、SDMA和NMMA的线性范围为0.1~5 mmol/L,L-Arg和Cit的线性范围为10~250 mmol/L;5种氨基酸的日内、日间精密度均小于15%,准确度在85%~115%之间.该方法快速、简便、灵敏,可为相关疾病的临床诊断提供一种高效的检测手段.【期刊名称】《质谱学报》【年(卷),期】2016(037)005【总页数】7页(P446-452)【关键词】超高效液相色谱-串联质谱(UPLC-MS/MS);艾普拉唑;蛋白沉淀法;亲水性色谱【作者】田晔;江骥;胡蓓;薛金萍;王洪允【作者单位】福州大学化学学院,福建省功能材料工程研究中心,福建省光动力治疗药物与诊疗工程技术研究中心,福建福州350108;中国医学科学院北京协和医院临床药理中心,北京100730;中国医学科学院北京协和医院临床药理中心,北京100730;中国医学科学院北京协和医院临床药理中心,北京100730;福州大学化学学院,福建省功能材料工程研究中心,福建省光动力治疗药物与诊疗工程技术研究中心,福建福州350108;中国医学科学院北京协和医院临床药理中心,北京100730【正文语种】中文【中图分类】O657.63一氧化氮是人体重要的信使分子,L-精氨酸(L-Arg)在一氧化氮全酶(NOS)的催化下,产生一氧化氮(NO)和瓜氨酸(Cit)[1-2]。
Gelucire-14-44-SDS-MedChemExpress

Inhibitors, Agonists, Screening LibrariesSafety Data Sheet Revision Date:Nov.-23-2018Print Date:Nov.-23-20181. PRODUCT AND COMPANY IDENTIFICATION1.1 Product identifierProduct name :Gelucire 14/44Catalog No. :HY-Y1892CAS No. :121548-04-71.2 Relevant identified uses of the substance or mixture and uses advised againstIdentified uses :Laboratory chemicals, manufacture of substances.1.3 Details of the supplier of the safety data sheetCompany:MedChemExpress USATel:609-228-6898Fax:609-228-5909E-mail:sales@1.4 Emergency telephone numberEmergency Phone #:609-228-68982. HAZARDS IDENTIFICATION2.1 Classification of the substance or mixtureNot a hazardous substance or mixture.2.2 GHS Label elements, including precautionary statementsNot a hazardous substance or mixture.2.3 Other hazardsNone.3. COMPOSITION/INFORMATION ON INGREDIENTS3.1 SubstancesSynonyms:NoneFormula:N/AMolecular Weight:N/ACAS No. :121548-04-74. FIRST AID MEASURES4.1 Description of first aid measuresEye contactRemove any contact lenses, locate eye-wash station, and flush eyes immediately with large amounts of water. Separate eyelids with fingers to ensure adequate flushing. Promptly call a physician.Skin contactRinse skin thoroughly with large amounts of water. Remove contaminated clothing and shoes and call a physician.InhalationImmediately relocate self or casualty to fresh air. If breathing is difficult, give cardiopulmonary resuscitation (CPR). Avoid mouth-to-mouth resuscitation.IngestionWash out mouth with water; Do NOT induce vomiting; call a physician.4.2 Most important symptoms and effects, both acute and delayedThe most important known symptoms and effects are described in the labelling (see section 2.2).4.3 Indication of any immediate medical attention and special treatment neededTreat symptomatically.5. FIRE FIGHTING MEASURES5.1 Extinguishing mediaSuitable extinguishing mediaUse water spray, dry chemical, foam, and carbon dioxide fire extinguisher.5.2 Special hazards arising from the substance or mixtureDuring combustion, may emit irritant fumes.5.3 Advice for firefightersWear self-contained breathing apparatus and protective clothing.6. ACCIDENTAL RELEASE MEASURES6.1 Personal precautions, protective equipment and emergency proceduresUse full personal protective equipment. Avoid breathing vapors, mist, dust or gas. Ensure adequate ventilation. Evacuate personnel to safe areas.Refer to protective measures listed in sections 8.6.2 Environmental precautionsTry to prevent further leakage or spillage. Keep the product away from drains or water courses.6.3 Methods and materials for containment and cleaning upAbsorb solutions with finely-powdered liquid-binding material (diatomite, universal binders); Decontaminate surfaces and equipment by scrubbing with alcohol; Dispose of contaminated material according to Section 13.7. HANDLING AND STORAGE7.1 Precautions for safe handlingAvoid inhalation, contact with eyes and skin. Avoid dust and aerosol formation. Use only in areas with appropriate exhaust ventilation.7.2 Conditions for safe storage, including any incompatibilitiesKeep container tightly sealed in cool, well-ventilated area. Keep away from direct sunlight and sources of ignition.Recommended storage temperature:Pure form-20°C 3 years4°C 2 yearsIn solvent-80°C 6 months-20°C 1 monthShipping at room temperature if less than 2 weeks.7.3 Specific end use(s)No data available.8. EXPOSURE CONTROLS/PERSONAL PROTECTION8.1 Control parametersComponents with workplace control parametersThis product contains no substances with occupational exposure limit values.8.2 Exposure controlsEngineering controlsEnsure adequate ventilation. Provide accessible safety shower and eye wash station.Personal protective equipmentEye protection Safety goggles with side-shields.Hand protection Protective gloves.Skin and body protection Impervious clothing.Respiratory protection Suitable respirator.Environmental exposure controls Keep the product away from drains, water courses or the soil. Cleanspillages in a safe way as soon as possible.9. PHYSICAL AND CHEMICAL PROPERTIES9.1 Information on basic physical and chemical propertiesAppearance White to off-white (Oil)Odor No data availableOdor threshold No data availablepH No data availableMelting/freezing point No data availableBoiling point/range No data availableFlash point No data availableEvaporation rate No data availableFlammability (solid, gas)No data availableUpper/lower flammability or explosive limits No data availableVapor pressure No data availableVapor density No data availableRelative density No data availableWater Solubility No data availablePartition coefficient No data availableAuto-ignition temperature No data availableDecomposition temperature No data availableViscosity No data availableExplosive properties No data availableOxidizing properties No data available9.2 Other safety informationNo data available.10. STABILITY AND REACTIVITY10.1 ReactivityNo data available.10.2 Chemical stabilityStable under recommended storage conditions.10.3 Possibility of hazardous reactionsNo data available.10.4 Conditions to avoidNo data available.10.5 Incompatible materialsStrong acids/alkalis, strong oxidising/reducing agents.10.6 Hazardous decomposition productsUnder fire conditions, may decompose and emit toxic fumes.Other decomposition products - no data available.11.TOXICOLOGICAL INFORMATION11.1 Information on toxicological effectsAcute toxicityClassified based on available data. For more details, see section 2Skin corrosion/irritationClassified based on available data. For more details, see section 2Serious eye damage/irritationClassified based on available data. For more details, see section 2Respiratory or skin sensitizationClassified based on available data. For more details, see section 2Germ cell mutagenicityClassified based on available data. For more details, see section 2CarcinogenicityIARC: No component of this product present at a level equal to or greater than 0.1% is identified as probable, possible or confirmed human carcinogen by IARC.ACGIH: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by ACGIH.NTP: No component of this product present at a level equal to or greater than 0.1% is identified as a anticipated or confirmed carcinogen by NTP.OSHA: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by OSHA.Reproductive toxicityClassified based on available data. For more details, see section 2Specific target organ toxicity - single exposureClassified based on available data. For more details, see section 2Specific target organ toxicity - repeated exposureClassified based on available data. For more details, see section 2Aspiration hazardClassified based on available data. For more details, see section 212. ECOLOGICAL INFORMATION12.1 ToxicityNo data available.12.2 Persistence and degradabilityNo data available.12.3 Bioaccumlative potentialNo data available.12.4 Mobility in soilNo data available.12.5 Results of PBT and vPvB assessmentPBT/vPvB assessment unavailable as chemical safety assessment not required or not conducted.12.6 Other adverse effectsNo data available.13. DISPOSAL CONSIDERATIONS13.1 Waste treatment methodsProductDispose substance in accordance with prevailing country, federal, state and local regulations.Contaminated packagingConduct recycling or disposal in accordance with prevailing country, federal, state and local regulations.14. TRANSPORT INFORMATIONDOT (US)This substance is considered to be non-hazardous for transport.IMDGThis substance is considered to be non-hazardous for transport.IATAThis substance is considered to be non-hazardous for transport.15. REGULATORY INFORMATIONSARA 302 Components:No chemicals in this material are subject to the reporting requirements of SARA Title III, Section 302.SARA 313 Components:This material does not contain any chemical components with known CAS numbers that exceed the threshold (De Minimis) reporting levels established by SARA Title III, Section 313.SARA 311/312 Hazards:No SARA Hazards.Massachusetts Right To Know Components:No components are subject to the Massachusetts Right to Know Act.Pennsylvania Right To Know Components:No components are subject to the Pennsylvania Right to Know Act.New Jersey Right To Know Components:No components are subject to the New Jersey Right to Know Act.California Prop. 65 Components:This product does not contain any chemicals known to State of California to cause cancer, birth defects, or anyother reproductive harm.16. OTHER INFORMATIONCopyright 2018 MedChemExpress. The above information is correct to the best of our present knowledge but does not purport to be all inclusive and should be used only as a guide. The product is for research use only and for experienced personnel. It must only be handled by suitably qualified experienced scientists in appropriately equipped and authorized facilities. The burden of safe use of this material rests entirely with the user. MedChemExpress disclaims all liability for any damage resulting from handling or from contact with this product.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
26291346_基于UPLC-MS

Abstract: A UPLC-MS/MS method was established to quantitatively determine the content of alliin in animal plasma to study whether alliin and alliin in garlic enteric preparations can react to produce the active ingredient allicin in the in vivo environment. Methods Reversed-phase C18 column (Waters ICQUITY UPLC BEH, 100 × 2.1 mm, 1.7μm), column temperature: 40 ℃, flow rate: 0.15 mL/min, injection volume: 2μl, Mobile phase: 0.1% formic acid (A)-acetonitrile (B), gradient elution; mass spectrometry ionization: ESI+, determination of allicin in rat plasma . Results The results of two parallel experiments of garlic enteric preparation and enzymatic garlic powder showed that in the garlic enteric preparation with allinase, the plasma concentration of alliin in the blood of rats was significantly lower. Conclusion A UPLC-MS/MS method for the quantitative determination of alliin in animal plasma has been established. Alliin and alliin in garlic enteric-coated preparations can react in vivo.Key words: Garlic enteric preparation; garbonine; UPLC-MS-MS基于UPLC-MS/MS大蒜肠溶制剂中蒜氨酸、蒜酶体内反应情况研究杨亮1,胡小霞4 ,宋百灵4,关明3,李新霞2*(1.新疆警察学院 新疆 乌鲁木齐 8300112.新疆医科大学药学院 新疆 乌鲁木齐 8300113.新疆师范大学化学化工学院 新疆 乌鲁木齐 8300544.新疆医科大学中心实验室 新疆 乌鲁木齐 830011)Study on the Reaction of Garlic and Uterine in the UPLC-MS / MS of Garlic SausolYANG Liang 1,HU Xiaoxia 4 ,SONG Bailing 4,GUAN Ming 3,LI Xinxia 2*(1. Xinjiang Police College, Urumqi 830054, Xinjiang China2.Chemistry and Chemical Engineering of Xinjiang Normal University College, Urumqi 830054, Xinjiang China3.School of Pharmacy, Xinjiang Medical University, Urumqi 830011, Xinjiang China4.Central Laboratory of Xinjiang Medical University, Urumqi 830011, Xinjiang China )摘要:目的 建立定量测定动物血浆中蒜氨酸含量的UPLC-MS/MS 方法,研究大蒜肠溶制剂中蒜氨酸、蒜酶能否在体内环境下反应生成活性成分大蒜辣素。
抗结核药品导致严重骨髓抑制一例并文献复习

中国防痨杂志 2021年 4 月第 43卷第 4 期C h i n J A m i t u b e r c,April 2021, Vol. 43,N〇. 4•413••短篇论著•抗结核药品导致严重骨髓抑制一例并文献复习刘鑫郭乐仵倩红【摘要】抗结核药品是引起骨髓抑制的危险因素,严重者可导致患者死亡。
本文作者报道了1例与使用抗结 核药品相关的骨髓抑制并导致死亡的患者。
患者为女性,24岁,以“发现双侧颈部包块1个月余,经外院穿刺病理 诊断为颈部淋巴结结核”住院治疗.入院后颈部包块脓液经结核分枝杆菌药物敏感性试验(M T frD S T)证实为耐多 药结核病,入院常规行血液检查未见异常后给予初治抗结核治疗(3H-R-Z-E/12H-R),全血细胞从最初的仅粒系细 胞轻度降低,逐步发展至重度降低,最终红系、粒系、巨核系细胞均严重降低,从而继发感染性休克;患者被积极抢 救后失败.最终死亡。
作者通过复习相关文献.讨论了抗结核药品引起骨髓抑制的发病机制、临床表现、诊断及治 疗方法,提醒临床医生了解抗结核药品引起的血液系统不良反应及其严重性,尽早明确原因并采取必要的干预措施。
【关键词】抗结核药;结核,抗多种药物性;骨髓疾病;死亡;综述文献(主题)A case of severe myelosuppression caused by anti-tuberculosis drugs and literature review on this issue LIU X i n,GUO L e*WJJ Q ian-hong. Shaanxi Provincial Tuberculosis Prevention and Control Institute* X i1 an 710100 <,ChinaCorresponding author:W U Qian-hong, Email :15902969^31 @126. com【Abstract】A nti-tuberculosis drug is a risk factor for causing bone m arrow suppression,which can lead to death in vSevere cases. T his article reported a patient who got bone m arrow suppression caused by the use of antituberculosis drugs and died. T he authors also did a literature review regarding to this issue. T he patient was female, 24 years old. She was found to have bilateral neck masses for m ore than 1m onth and diagnosed as cervical lymph node tuberculosis by puncture biopsy in another hospital before she came to our hospital. A fter adm ission,she was confirmed as m ultidrug-resistant tuberculosis (M DR-TB) by Mycobacterium tuberculosis drug susceptible test (M T&-DST) using the pus obtained from her neck mass. T he routine blood examinations were performed for her and the results w ere norm al, and then the initial treatm ent regimen of anti-tuberculosis (3H-R-Z-E/12H-R) was given to this patient. A fter she received the treatm ent, the granulocyte cells were starting to drop slightly and gradually progressed to severe reduction,and then further progressed to severe reductions of the erythroid,granulocyte and m egakaryocyte cells. T he patient eventually died of infectious shock and failure to resuscitate. By reviewing the relevant publications, the authors did discussions on pathogenesis, clinical m anifestations, diagnosis and treatm ent of bone m arrow suppression caused by anti-tuberculosis drugs in this article, aimed to remind clinicians to understand the adverse reactions in blood system caused by anti-tuberculosis drugs and their severity,and to emphasize that the causes of the abnormal blood test results should be identified as soon as possible and the effective intervention m easures should be taken soon.【Key words】A ntitubercular agents; Tuberculosis,m uhidrug-resistant; Bone m arrow diseases;D eath;Review literature as topic骨髓抑制是抗结核药品治疗中经常发生的且严重的药 物不良反应(adverse drug reaction,A D R),相关文献报道发 生率在3. 3%〜10%[11。
超分子溶剂萃取

第42 卷第 5 期2023 年5 月Vol.42 No.5559~567分析测试学报FENXI CESHI XUEBAO(Journal of Instrumental Analysis)超分子溶剂萃取/超高效液相色谱-串联质谱法测定血浆中他克莫司含量谢以清1,2,吕悦广2,孟宪双2,雷海民1*,马强2*(1.北京中医药大学中药学院,北京102488;2.中国检验检疫科学研究院,北京100176)摘要:该文建立了血浆中免疫抑制剂他克莫司(TAC)的超分子溶剂(SUPRAS)萃取/超高效液相色谱-串联质谱分析方法。
通过单因素实验结合响应面设计对超分子溶剂组成、用量及涡旋萃取时间等关键因素进行优化后,血浆样本以正戊醇、四氢呋喃和水形成的超分子溶剂进行高效萃取。
萃取液经Waters ACQUITY UPLC BEH C18(50 mm × 2.1 mm,1.7 μm)色谱柱分离后,在电喷雾质谱正离子模式下,以多反应监测(MRM)模式对他克莫司进行测定,内标法定量。
结果表明,他克莫司在0.5 ~ 30 ng/mL质量浓度范围内的线性关系良好,相关系数(r)为0.998 6;方法检出限和定量下限分别为0.1、0.5 ng/mL;在低、中、高3个加标水平下,平均回收率(n = 3)为91.9% ~ 99.9%,相对标准偏差(RSD)为1.7% ~ 5.7%。
所建立的方法快速、灵敏、稳定,适用于血浆中他克莫司的准确测定。
关键词:他克莫司;免疫抑制剂;超分子溶剂;血浆;超高效液相色谱-串联质谱中图分类号:O657.7;R917文献标识码:A 文章编号:1004-4957(2023)05-0559-09Determination of Tacrolimus in Plasma by Supramolecular Solvent Extraction/Ultra-high Performance Liquid Chromatography-Tandem Mass SpectrometryXIE Yi-qing1,2,LÜ Yue-guang2,MENG Xian-shuang2,LEI Hai-min1*,MA Qiang2*(1.School of Chinese Materia Medica,Beijing University of Chinese Medicine,Beijing 102488,China;2.Chinese Academy of Inspection and Quarantine,Beijing 100176,China)Abstract:An analytical method for the determination of tacrolimus(TAC) in blood plasma was estab⁃lished by supramolecular solvent(SUPRAS)extraction combined with ultra-high performance liquid chromatography-tandem mass spectrometry.After optimizing the key factors such as the composition and amount of SUPRAS,and vortex extraction time through single factor experiment and response sur⁃face design,blood plasma samples were extracted efficiently with SUPRAS formed by pentanol,tetra⁃hydrofuran and water.The extract was separated on a Waters ACQUITY UPLC BEH C18column (50 mm × 2.1 mm,1.7 μm),analyzed by electrospray ionization mass spectrometry in positive ion mode under multiple reaction monitoring(MRM) mode,and quantified by internal standard method.Experimental results demonstrated that there was a good linear relationship for TAC in the concentration range of 0.5-30 ng/mL,with a correlation coefficient(r) of 0.998 6.The limit of detection(LOD)and quantitation(LOQ) were 0.1 ng/mL and 0.5 ng/mL,respectively.The average recoveries(n = 3)at low,medium and high spiked concentration levels ranged from 91.9% to 99.9%,with relative stan⁃dard deviations(RSDs) of 1.7%-5.7%.The proposed method is rapid,sensitive and stable,and it was suitable for the accurate determination of TAC in blood plasma.Key words:tacrolimus;immunosuppresive agent;supramolecular solvent;plasma;ultra-high performance liquid chromatography-tandem mass spectrometry免疫抑制剂是用于抑制机体免疫力的药物,多用于抑制肝肾移植术后的免疫反应,以及治疗变态反应性和自身免疫性疾病,如类风湿关节炎、红斑狼疮等[1-3]。
止咳水中31种常见毒品成分的快速测定

中国口岸科学技术止咳水中31种常见毒品成分的快速测定黄昌雄*1吴章杰1姚宇星1徐婷婷1罗耀1华红慧1摘要止咳水试样用50%甲醇水溶液超声提取,经有机滤膜过滤后,利用液相色谱-串联质谱(LC-MS/MS )进行检测,建立了止咳水中31种毒品成分的快速测定方法分析物采用电喷雾离子源(E S I),正离子多反应监测(MRM)模式检测,外标法定量。
可待因等28种组分在2 ~ 5(V g/L浓度范围内线性关系良好,检出限在0.2 ~ 2jig/L范围内,在10〜50jjig/ L添加浓度范围内回收率为80.1% ~109%;大麻酚、四氢大麻酚、大麻二酚物质在10 ~ 2(X V g/L浓度范围内线性关系良好,检出限均为4(jLg/L,在50〜250|x g/L添加浓度范围内回收率为81.2% ~ 109%;化合物的相对标准偏差(R S D)为 1.7% ~14.4%。
所建立的方法灵敏度高,简便快捷,稳定性好,可用于止咳水中毒品类物质的定性鉴定及定量分析,为该类物质的快速检测提供技术支撑。
关键词止咳水;毒品成分;液相色谱-串联质谱(LC-MS/MS )Fast Determination of 31 Common Drug Ingredients inCough SyrupHUANG Chang-Xiong1WU Zhang-Jie1YAO Yu-Xing1XU Ting-Ting1LUO Yao1HUA Hong-Hui1Abstract The cough syrup sample was extracted by 50%methanol aqueous solution using sonication.After being filtered through an organic filter membrane,the sample was detected with Liquid chromatography-tandem mass(LC-MS/MS)spectrometry,and a rapid determination method for 31 drug components in the cough syrup was established.Analytes were detected by electrospray ion(E S I) source and positive ion multiple reaction monitoring(MRM)mode,and quantified by external standard method.The linear relationships of 28 components including codeine were good in the range of 2〜50 (xg/L ,and the detection limits were within 0.2〜2 |x g/L.The recoveries were between 80.1%and 109%in the added range of 10〜50 |x g/L;For Cannabind,Tetrahydrocannabinol and Cannabidiol,the linear relationships were also good within 10〜200(xg/L and the of detection 4|x g/L.The recoveries within 50〜250jxg/L were 81.2%〜109%and the relative standard deviations(RSDs)of the compounds were 1.7%〜14.4%. The sensitivity of the established method was high,simple and quick with good stability.It can be used for qualitative identification and quantitative analysis of drug substances in the cough syrup,and can provide technical support for the rapid monitoring of such substances.Keywords cough syrup;drug ingredient;liquid chromatography-tandem mass spectrometry(LC-MS/MS)基金项目:国家重点研发计划项目(2017YFC1601603 )第一作者:黄昌雄( 1994一),男,深圳,大学专科,助理工程师,主要从事食品非法添加物和毒品(物)的检测工作,E-mail: 370701783@1.深圳海关食品检验检疫技术中心深圳5180451.Food Inspection and Quarantine Technology Center of Shenzhen Customs,Shenzhen 5180459CHINA PORT SCIENCE AND TECHNOLOGY作为一种处方药,止咳水具有镇咳和镇痛等功能,其主要成分为磷酸可待因和盐酸麻黄碱等,化学结构 式如图1所示。
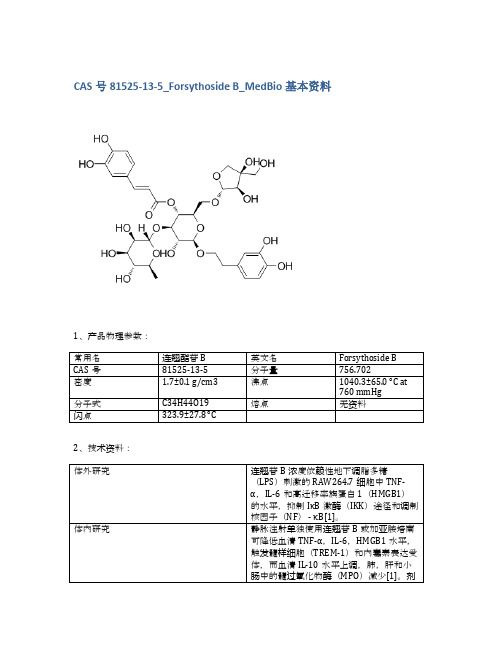
CAS号81525-13-5_Forsythoside B_MedBio基本资料
≥98%
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
MED16437
Carnosic acid
Carnosic acid
3650-09-7
20mg
≥98%
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
MED15815
Hydroxysafflor yellow A
3、同类产品列表:
品牌
货号
中文名称
英文名称CAS包装 Nhomakorabea纯度
MedBio
MED15880
Sennoside B
Sennoside B
128-57-4
20mg
≥98%
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
MED15956
Shikimic acid
Shikimic acid
138-59-0
20mg
≥98%
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
MED16069
Rhodojaponin V
Rhodojaponin V
37720-86-8
5mg
≥98%
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
MED15874
Madecassoside
Madecassoside
34540-22-2
Ginsenoside Rc
激酶抑制剂类药物

Sutent药物基本信息〖NDA申请人〗CPPY CV〖NDA原始批准日期〗2006年07月26日〖剂型/规格〗胶囊剂/12.5mg;胶囊剂/25mg;胶囊剂/50mg;胶囊剂/37.5mg〖适应证〗50mg QD,用于治疗:Ⅰ、病情恶化后或对马来酸伊马替尼不耐受的胃肠间质瘤;Ⅱ、晚期肾细胞瘤活性成分信息〖USAN名称〗Sunitinib Malate,苹果酸舒尼替尼〖CAS号〗341031-54-7(苹果酸盐);557795-19-4(游离碱)〖曾用代号〗SU-11248(苹果酸盐)〖作用类别〗激酶抑制剂类抗肿瘤药〖化学名〗(Z)-N-(2-(二乙基氨基)乙基)-5-((5-氟-2-氧代吲哚-3-亚基)甲基)-2,4-二甲基-1H-吡咯-3-羧酰胺苹果酸盐〖化学结构式〗专利信息年度销售情况(亿美元,信息来源:辉瑞公司年度财务报告及SEC报表)Tykerb药物基本信息〖NDA申请人〗Smithkline Beecham〖NDA原始批准日期〗2007年03月13日〖剂型/规格〗片剂/250mg;〖适应证〗1250mg QD+卡培他滨治疗肿瘤过度表达HER2且使用过包括蒽环类抗生素、紫杉烷类抗生素曲妥珠单抗在内的抗肿瘤药物治疗的晚期或转移性乳腺癌;1500 QD+来曲唑治疗HER2过度表达且需要进行激素治疗的绝经后妇女的激素受体阳性的转移性乳腺癌活性成分信息〖USAN名称〗Lapatinib ditosylate (monohydrate),拉帕替尼二(对甲基苯磺酸)盐(单水合物)〖CAS号〗388082-78-8〖曾用代号〗〖作用类别〗激酶抑制剂类抗肿瘤药;〖化学名〗N-[3-氯-4-[(3-氟苯基)甲氧基]苯基]-6-[5[[[2-(甲磺酰基)乙基]氨基]甲基]-2-呋喃基]-4-喹啉胺二(对甲基苯磺酸)盐单水合物〖理化性质〗黄色固体,25℃下于水中的溶解度为0.007mg/mL,于0.1N HCl中的溶解度为0.001mg/mL〖化学结构式〗专利信息年度销售情况(亿英磅)Tasigna药物基本信息〖NDA申请人〗诺华制药〖NDA原始批准日期〗2007.10.29〖剂型/规格〗片剂/200mg(按游离碱计)〖适应证〗300mg BID用于于慢性期治疗新近确认成年患者的费城染色体阳性慢性髓样白血病;400mg BID用于于慢性期或急性期治疗成年患者对包括伊马替尼在内的先前治疗方法耐药或不耐受的费城染色体阳性慢性髓样白血病。
液相色谱-串联质谱法检测血液中的美西律

液相色谱-串联质谱法检测血液中的美西律严慧;向平;卜俊;沈敏【摘要】目的建立测定血液中美西律(mexiletine)的液相色谱-串联质谱联用法(LC-MS/MS).方法采用简便的乙腈蛋白沉淀法对血液进行预处理,应用Allure PAP Propel液相柱分离,用电喷雾正离子模式离子化,多反应监测模式对美西律进行分析.结果美西律与内标纳洛酮分离良好,在0.02~10.00μg/mL内线性关系良好,相关系数为0.9999,回归方程为y=0.028 3x-0.015 1,日内与日间精密度的RSD均小于15%,最低检测限为0.01μg/ml.结论建立的L.C.-MS/MS方法简单、灵敏、可靠,可同时适用于美西律临床药物监测和法医毒物分析的需要.【期刊名称】《法医学杂志》【年(卷),期】2007(023)006【总页数】3页(P441-443)【关键词】LC-MS/MS;抗心律失常药;美西律;血液【作者】严慧;向平;卜俊;沈敏【作者单位】司法部司法鉴定科学技术研究所,上海,200063;复旦大学上海医学院法医学系,上海,200032;司法部司法鉴定科学技术研究所,上海,200063;司法部司法鉴定科学技术研究所,上海,200063;司法部司法鉴定科学技术研究所,上海,200063【正文语种】中文【中图分类】DF795.4美西律(Mexiletine)是常用的钠通道阻滞类抗心律失常药,能阻滞心肌细胞膜、快钠通道,对室性心律失常的疗效较好[1]。
美西律的有效血药质量浓度范围为0.7~2μg/mL[2],中毒血药质量浓度与有效血药质量浓度相近,为2μg/mL以上,因此监测其血药质量浓度极为必要。
美西律服用剂量过大时可出现心律减慢、血压下降、房室传导阻滞和视力模糊等症状,严重者可致呼吸抑制死亡。
有关美西律中毒的案例时有发生[3-5]。
目前国内外对美西律的测定方法主要有气相色谱-质谱联用法(GC-MS)[2-3]、高效液相色谱法(HPLC)[6]、毛细管电泳法(CE)[7-8]、液相色谱-串联质谱联用法(LCMS/MS)[9]等。
Chemerin对MCD饮食诱导的非酒精性脂肪肝的干预及机制研究

材料和方法一、材料1 所需主要试剂GW4064购自invitrogen公司,二甲基亚砜(DMSO)购自美国sigma公司。
11995 DMEM培养基\胎牛血清购自美国Gibco BRL公司。
进口分装牛血清白蛋白(BSA)购自上海年丰生物技术有限公司。
硝酸纤维素膜、Whatman 3MMOL/L滤纸、聚丙烯酰胶浓缩液-29:1、过硫酸铵(APS)、甘氨酸均购于华舜生物工程有限公司。
细胞裂解液RIPA及PMSF购自上海申能博彩公司。
丽春红染色液购自碧云天生物技术公司。
Trizol RNA抽提试剂,RNA逆转录酶及Olig dT购自invitrogen 公司。
SYBR® Premix Ex Taq TM荧光实时定量PCR试剂盒购自TaKaRa公司。
KOD-Plus-购自日本TOYOBO公司,限制性内切酶及PCR试剂盒、质粒提取试剂盒、质粒纯化试剂盒(MiniBEST Plasmid Purification Kit Ver.2.0 )均购自TaKaRa公司;pGEM-T Easy Vector SystemI购自Promega公司;质粒测序由上海博尚生物技术有限公司进行;脂质体2000购于Invitrogen公司羊抗人chemerin抗体购自美国R&D公司;荧光素酶报告基因测定试剂盒购自Promega公司。
鼠抗人GAPDH抗体购自KangCheng生物科技有限公司。
HRP标记抗鼠二抗购自上海普飞生物科技有限公司。
生物素偶联蛋白Ladder购自cell signal 公司。
HRP标记抗羊二抗购自Santa Cruz 公司。
HepG2细胞株购自ATCC(American Type Culture Collection)。
BCA 蛋白测定试剂盒和West Pico chemiluminescent发光底物来自美国PIERCE公司。
2 GW4064及实验所需液体的配制GW4064的配制GW4064按一定比例溶解于DMSO配成终浓度分别:0.5mM、1mM、2mM、5mM、的储存液放于-20℃避光保存。
顺铂通过增加HeLa人宫颈癌细胞中Nrf2入核促进细胞自噬和凋亡

[收稿日期]2020-09-08 [修回日期]2020-12-01[作者单位]江苏省无锡市妇幼保健院宫颈科,214000[作者简介]季 静(1985-),女,硕士,主治医师.[通信作者]詹惠英,主任医师.E⁃mail:wxzhy2013@[文章编号]1000⁃2200(2023)03⁃0301⁃06㊃基础医学㊃顺铂通过增加HeLa 人宫颈癌细胞中Nrf2入核促进细胞自噬和凋亡季 静,张 晔,陆晓红,徐 锋,詹惠英[摘要]目的:探讨顺铂对HeLa 人宫颈癌细胞中Nrf2蛋白表达及核转位的影响,并观察细胞自噬和凋亡的变化㊂方法:使用不同浓度的顺铂(0㊁2.5㊁5㊁10μmol /L)干预HeLa 人宫颈癌细胞,MTT 法检测细胞活性,Transwell 实验观察细胞迁移能力,mCherry⁃GFP⁃LC3B 融合蛋白腺病毒转染HeLa 细胞观察自噬水平的变化,流式细胞术检测细胞凋亡水平,免疫蛋白印迹检测Nrf2在细胞质和细胞核内的表达情况,免疫荧光法观察Nrf2的核转位情况㊂结果:与对照组相比,2.5㊁5㊁10μmol /L 的顺铂均可抑制HeLa 细胞的活性及细胞迁移能力(P <0.05),且随着药物浓度增高抑制作用增强(P <0.05)㊂与对照组相比,2.5㊁5㊁10μmol /L 的顺铂均可促进HeLa 细胞中LC3B 的表达㊁细胞凋亡及Nrf2的核转位(P <0.05),降低细胞质中Nrf2的蛋白表达水平(P <0.05),并升高细胞核中Nrf2的蛋白表达水平(P <0.05)㊂结论:顺铂通过增加HeLa 人宫颈癌细胞中Nrf2入核促进细胞自噬和细胞凋亡,并抑制细胞的活性和迁移能力㊂[关键词]HeLa 人宫颈癌细胞;顺铂;Nrf2;自噬;细胞凋亡[中图法分类号]R 737.33 [文献标志码]A DOI :10.13898/ki.issn.1000⁃2200.2023.03.005Cisplatin increases nuclear translocation of Nrf2and promotes autophagy and apoptosis in HeLa human cervical cancer cellsJI Jing,ZHANG Ye,LU Xiao⁃hong,XU Feng,ZHAN Hui⁃ying(Department of Cervical ,Wuxi Maternal and Child Health Care Hospital ,Jiangsu Wuxi 214000,China )[Abstract ]Objective :To investigate the effect of cisplatin on the protein expression and nuclear translocation of Nrf2,and on the changes of autophagy and apoptosis in HeLa human cervical cancer cells.Methods :After the HeLa human cervical cancer cells were treated with different concentrations of cisplatin(0,2.5,5and 10μmol /L),MTT assay was used to detect the cell activity,Transwell assay was used to observe the cell migration ability,mCherry⁃GFP⁃LC3B fusion protein adenovirus transfected HeLa cells were applied to observe the autophagy changes,flow cytometry was employed to detect the apoptosis,Western blotting was used to detect the expression of Nrf2in the cytoplasm and nucleus,and immunofluorescence staining was used to observe the nuclear translocation of Nrf2.Results :Compared with the control group,2.5,5and 10μmol /L cisplatin could inhibit the activity and migration ability of HeLa cells (P <0.05),and the inhibitory effect increased with the increase of drug concentration(P <0.05).Compared with the control group,2.5,5and 10μmol /L cisplatin could promote the expression of LC3B,apoptosis and nuclear translocation of Nrf2in HeLa cells(P <0.05),reduce the protein expression level of Nrf2in the cytoplasm(P <0.05),and increase the protein expression level of Nrf2in thenucleus(P <0.05).Conclusions :Cisplatin promotes autophagy and apoptosis by increasing nuclear translocation of Nrf2in HeLa human cervical cancer cells,and inhibits cell viability and migration ability.[Key words ]HeLa human cervical cancer cells;cisplatin;Nrf2;autophagy;apoptosis 虽然近年来我国宫颈癌的死亡率大幅下降,但作为女性常见的恶性肿瘤,我国每年宫颈癌新发病例仍高达13.15万,每年约5.3万女性死于宫颈癌[1]㊂顺铂作为宫颈癌经典的化疗药物,在单药化疗㊁联合其他药物化疗或同步放化疗的应用中都显示出对宫颈癌病人的有益作用[2]㊂自噬广泛参与宫颈癌的发生㊁发展㊁药物耐受等[3],有学者[4]报道顺铂对HeLa 人宫颈癌细胞自噬具有诱导作用,但顺铂对宫颈癌自噬的调节及其可能机制仍值得深入探索㊂本研究从顺铂对HeLa 人宫颈癌细胞中Nrf2入核影响的角度,探讨顺铂通过增加Nrf2的核转位促进细胞自噬相关的细胞凋亡抑制HeLa 细胞生长和迁移的可能机制㊂1 材料与方法1.1 药物与试剂 顺铂(Sigma⁃Aldrich 公司,货号:P4394);MEM 培养基(美国Thermo Fisher Scientific 公司,货号:11095072);胎牛血清(美国ThermoFisher Scientific公司,货号:10099);细胞消化液(美国Thermo Fisher Scientific公司,货号:12605010);青霉素-链霉素(美国Thermo Fisher Scientific公司,货号:15070063);噻唑蓝(MTT)(上海碧云天生物技术有限公司,货号:ST316);结晶紫染色液(上海碧云天生物技术有限公司,货号:C0121); mCherry⁃GFP⁃LC3B融合蛋白腺病毒(上海碧云天生物技术有限公司,货号:C3011);Annexin V⁃FITC细胞凋亡检测试剂盒(上海碧云天生物技术有限公司,货号:C1062M);DAPI染色液(上海碧云天生物技术有限公司,货号:C1006);细胞核蛋白与细胞质蛋白抽提试剂盒(上海碧云天生物技术有限公司,货号:P0027);GAPDH兔多克隆抗体(武汉三鹰生物技术有限公司,货号:10494⁃1⁃AP);Nrf2兔多克隆抗体(武汉三鹰生物技术有限公司,货号:16396⁃1⁃AP);LaminB1兔多克隆抗体(武汉三鹰生物技术有限公司,货号:12987⁃1⁃AP);HRP⁃标记的山羊抗兔IgG(武汉三鹰生物技术有限公司,货号:SA00001⁃2);Fluorescein(FITC)⁃标记的山羊抗兔IgG(武汉三鹰生物技术有限公司,货号:SA00003⁃2);其他试剂为分析纯,购自国药集团化学试剂有限公司㊂1.2 仪器 高速低温离心机(德国Sartorius公司,型号:Centrisart®D⁃16C);光学显微镜(日本Olympus公司,型号:IX83);荧光显微镜(德国Leica 公司,型号:AF6000);酶标仪(美国Thermo Fisher Scientific公司,型号:Multiskan FC);流式细胞仪(美国BD公司,型号:Accuri®C6Plus);电泳电源(美国Bio⁃Rad公司,型号:PowerPac Basic);垂直电泳/转膜槽(美国Bio⁃Rad公司,型号:Mini⁃PROTEAN Tetra);凝胶成像系统(美国Bio⁃Rad公司,型号: BIO⁃RAD Gel Doc XR+)㊂1.3 HeLa人宫颈癌细胞的培养及分组 HeLa人宫颈癌细胞株(产品目录号:TCHu187)购买于中国科学院典型培养物保藏委员会细胞库㊂使用MEM 培养基(90%)和胎牛血清(10%)配制完全培养基,在95%空气和5%CO2的37℃培养箱中培养HeLa人宫颈癌细胞㊂细胞生长至80%亚融合状态时,胰酶消化,分瓶继续培养㊂所有实验使用细胞复苏后4~6代的HeLa人宫颈癌细胞㊂实验分组:对照组㊁顺铂2.5μmol/L组㊁顺铂5μmol/L组及顺铂10μmol/L组㊂1.4 MTT法检测细胞的活性 取HeLa细胞,计数后用MEM完全培养基稀释,使细胞密度为1×105个/毫升,将细胞接种于96孔板,每孔5000个细胞,每组10个复孔,共4组,12h细胞贴壁后弃去原培养液,各组加入含有对应浓度顺铂的完全培养基200μL,对照组加入MEM完全培养基200μL,培养24h后,每孔加入20μL MTT(5mg/mL)试剂,培养4h后,吸弃培养液,加入150μL二甲基亚砜,摇床震荡10min后,使用酶标仪在490nm波长下测定吸光值(OD)㊂将对照组的细胞存活率定义为100%,细胞活性=给药组OD值/对照组OD值×l00%㊂1.5 Transwell实验检测细胞迁移 用含有不同浓度顺铂(0㊁2.5㊁5㊁10μmol/L)的单培养基将HeLa 细胞孵育24h后,收集各组细胞并计数,混匀于MEM单培养基中㊂Transwell小室上室中加入各组细胞5000个,每组6个重复,Transwell小室下室中加入含10%胎牛血清的MEM完全培养基,上室底部浸入下室完全培养基㊂培养24h后,4%多聚甲醛浸泡Transwell上室固定细胞30min,PBS缓冲液清洗2次,使用结晶紫染色液染色5min,再次清洗2次,使用棉签擦去Transwell上室内侧细胞,将小室置于载玻片上,使用倒置光学显微镜,对Transwell 上室外(下)侧的细胞进行拍照㊂1.6 mCherry⁃GFP⁃LC3B融合蛋白腺病毒转染细胞检测自噬 参考mCherry⁃GFP⁃LC3B融合蛋白腺病毒使用说明书,对HeLa细胞进行转染,6孔板中放入细胞爬片,并接种HeLa细胞,每组1块6孔板㊂细胞生长至50%融合时加入1.2mL单培养液,并加入腺病毒,培养24h㊂之后弃去所有培养液并更换为完全培养液后,继续培养24h㊂各组加入含有对应浓度顺铂的完全培养基2mL,对照组加入MEM完全培养基2mL,培养24h㊂4%多聚甲醛固定细胞30min,PBS缓冲液清洗2次后,取出细胞爬片,覆盖在载玻片上后,使用荧光显微镜观察mCherry⁃GFP⁃LC3B的荧光强度并拍照㊂1.7 流式细胞术检测细胞凋亡 用含有不同浓度顺铂(0㊁2.5㊁5㊁10μmol/L)的单培养基将HeLa细胞孵育24h后,收集各组细胞㊂参考Annexin V⁃FITC细胞凋亡检测试剂盒说明书检测HeLa细胞的凋亡率㊂将5~10万重悬细胞离心弃上清后,加入195μL Annexin V⁃FITC结合液,再加入5μL Annexin V⁃FITC,混匀后加入10μL碘化丙啶(PI)染色液,孵育20min,放入流式细胞仪样本架,设置仪器通道后检测并导出结果㊂1.8 免疫蛋白印迹法检测细胞Nrf2的蛋白表达 用含有不同浓度顺铂(0㊁2.5㊁5㊁10μmol/L)的完全培养基将HeLa细胞孵育24h后,收集各组细胞至新EP管内㊂使用细胞核蛋白与细胞浆蛋白提取试剂盒分别提取HeLa细胞的膜蛋白核核蛋白,BCA 法测定蛋白浓度㊂蛋白变性后,取等质量总蛋白(30μg)进行SDS⁃PAGE 电泳㊂之后将凝胶上的蛋白转移至PVDF 膜㊂PVDF 膜使用封闭液封闭2h㊂对于提取的细胞质内的蛋白,在封闭后孵育抗体GAPDH(1∶5000)和Nrf2(1∶500),对于提取的细胞核内的蛋白,在封闭后孵育抗体LaminB1(1∶5000)和Nrf2(1∶500),4℃过夜后洗膜3次㊂HRP 标记的山羊抗兔IgG(1∶10000)孵育2h 后,再次洗膜3次㊂使用凝胶成像系统化学发光成像并保存印迹图片㊂1.9 免疫荧光法检测细胞Nrf2的核转位 6孔板放入细胞爬片,并接种HeLa 细胞,每组1块6孔板㊂用含有不同浓度顺铂(0㊁2.5㊁5㊁10μmol /L)的完全培养基孵育24h 后,4%多聚甲醛固定细胞30min,PBS 缓冲液清洗2次,加入Nrf2(1∶100)抗体4℃孵育过夜㊂PBS 缓冲液清洗2次,加入FITC 标记的山羊抗兔IgG(1∶500)孵育2h 后,再次使用PBS 清洗2次,加入DPAI 染细胞核5min㊂使用PBS 清洗细胞3次,取出细胞爬片,覆盖在载玻片上,使用荧光显微镜观察Nrf2的荧光强度并拍照㊂1.10 统计学方法 采用方差分析和q 检验㊂2 结果2.1 顺铂抑制HeLa 人宫颈癌细胞的生长及迁移 与对照组相比,2.5㊁5㊁10μmol /L 的顺铂均可抑制HeLa 细胞的活性及细胞迁移能力(P <0.05),且随着药物浓度增高抑制作用增强(P <0.05)(见图1㊁表1)㊂2.2 顺铂促进HeLa 人宫颈癌细胞的自噬和细胞凋亡 使用mCherry⁃GFP⁃LC3B 融合蛋白腺病毒转染HeLa 细胞,采用荧光显微镜观察自噬相关蛋白LC3B 的表达情况,结果显示,与对照组相比,2.5㊁5㊁10μmol /L 的顺铂均可促进HeLa 细胞中LC3B 的表达(P <0.05)(见图2㊁表2)㊂使用流式细胞术检测各组HeLa 细胞的凋亡情况,结果显示,与对照组相比,2.5㊁5㊁10μmol /L 的顺铂均可促进HeLa 细胞的凋亡(P <0.05)(见图2㊁表2)㊂2.3 顺铂促进HeLa 人宫颈癌细胞中Nrf2的入核 免疫蛋白印迹结果显示,与对照组相比,2.5㊁5㊁10μmol /L 的顺铂均可降低HeLa 细胞的细胞质中Nrf2的蛋白表达水平(P <0.05),并升高细胞核中Nrf2的蛋白表达水平(P <0.05)(见图3A㊁表3)㊂使用免疫荧光法观察各组细胞中Nrf2的入核情况,结果显示,与对照组相比,2.5㊁5㊁10μmol /L 的顺铂均可促进HeLa 人宫颈癌细胞的中Nrf2的核转位(P <0.05)(见图3B㊁表3)㊂ 表1 顺铂对HeLa人宫颈癌细胞的细胞活性及迁移能力的影响(x±s)分组细胞存活率/%(n i=10)迁移细胞数/个(n i=6)对照组100.0±5.6213.0±14.5顺铂2.5μmol/L组75.5±4.1*107.0±9.9*顺铂5.0μmol/L组63.0±4.3*#83.7±6.1*#▲顺铂10.0μmol/L组40.6±4.6*#▲59.5±7.1*#▲F281.72279.04P<0.01<0.01MS组内21.84798.391 q检验:与对照组比较*P<0.05;与顺铂2.5μmol/L组比较#P <0.05;与顺铂5.0μmol/L组比较▲P<0.053 讨论 研究[5-6]表明,癌细胞中Nef2的激活可促进癌症的进展和转移,并且会导致化疗和放疗敏感性的降低[7-8]㊂Nrf2在肿瘤中具有刺激肿瘤细胞增殖㊁抑制肿瘤细胞凋亡㊁增强肿瘤细胞复制潜力和肿瘤血管生产㊁增强肿瘤细胞对组织的侵袭和转移㊁促进炎症和增加基因突变等作用[9]㊂Nrf2在癌症中广泛的作用表明靶向Nrf2可能是治疗肿瘤的有效方法,如Nrf2的抑制剂可用于癌症治疗[9]㊂未来Nrf2可能会成为癌症的预后指标和治疗靶点㊂研究[10]显示Nrf2的高表达会增加肿瘤细胞的抗氧化能力,核内Nrf2高表达宫颈癌细胞具有增加的恶性潜能,Nrf2可能是宫颈癌病人预后不良的标志㊂有学者[11]报道,宫颈癌病变程度的升级增加Nrf2的核水平,并增强肿瘤细胞抗氧化反应中下游蛋白的表达㊂此外,有研究[12]证实辣椒素通过抑制宫颈癌细胞中Nrf2的表达抑制宫颈癌细胞的迁移与侵袭㊂本研究结果显示,顺铂作用于HeLa人宫颈癌细胞后,细胞核中的Nrf2蛋白水平升高,但降低了细胞质中的Nrf2水平㊂作为宫颈癌常用的化疗药物,顺铂对各类肿瘤的作用被广泛报道,包括其对宫颈癌细胞的抑制作用[13]㊂本研究结果还表明,顺铂对HeLa细胞的生长与迁移具有抑制作用,这些结果与前人的报道相一致㊂关于顺铂通过抑制Nrf2调节HeLa人宫颈癌细胞生长与迁移的潜在机制,本研究主要关注了顺铂对HeLa人宫颈癌细胞自噬依赖性的细胞凋亡的影响㊂据报道[14],自噬在化疗药物发挥作用中有重要的作用㊂有证据[15-16]显示,宫颈癌细胞中自噬作用的增强促进了宫颈癌细胞的凋亡,但也有研究[17-18]证实阻断自噬增加了耐药宫颈癌细胞对顺铂的敏感性㊂本研究结果显示,顺铂促进HeLa细胞的自噬并增加细胞凋亡㊂ 表2 顺铂对HeLa细胞自噬及凋亡的影响(n i=6;x±s)分组GFP⁃LC3B阳性面积/%mCherry⁃LC3B阳性面积/%细胞凋亡率/%对照组 5.4±1.5 3.2±1.87.3±0.2顺铂2.5μmol/L组22.0±1.4*20.4±1.5*20.6±0.2*顺铂5.0μmol/L组22.9±1.8*22.1±1.6*63.1±0.6*#顺铂10.0μmol/L组29.5±3.6*#▲28.1±3.2*#▲68.4±0.3*#▲F126.62150.2544835.04P<0.01<0.01<0.01MS组内 5.004 4.5520.124 q检验:与对照组比较*P<0.05;与顺铂2.5μmol/L组比较#P<0.05;与顺铂5.0μmol/L组比较▲P<0.05 表3 顺铂对HeLa细胞中Nrf2表达的影响(n i=6;x±s)分组细胞质Nrf2蛋白相对表达水平细胞质Nrf2蛋白相对表达水平细胞核内Nrf2荧光强度(总光密度)对照组 3.7±0.3 1.1±0.1411.0±30.3顺铂2.5μmol/L 2.2±0.1* 2.2±0.1*1438.7±69.0*顺铂5.0μmol/L 2.2±0.2* 3.9±0.2*#1730.5±37.5*#顺铂10.0μmol/L 1.1±0.1*#▲ 3.9±0.3*#2163.3±57.9*#▲F219.46351.791278.08P<0.01<0.01<0.01MS组内0.0310.0322607.613 q检验:与对照组比较*P<0.05;与顺铂2.5μmol/L组比较#P<0.05;与顺铂5.0μmol/L组比较▲P<0.05 综上,顺铂通过增加HeLa人宫颈癌细胞中Nrf2入核促进细胞自噬和细胞凋亡,并抑制细胞的活性和迁移能力㊂[参考文献][1] 许驰,何玉.宫颈癌筛查方法的现状及进展[J].蚌埠医学院学报,2018,43(11):1538.[2] 周莉,陆安伟.局部晚期宫颈癌治疗的争议与对策[J].中国实用妇科与产科杂志,2019,35(10):1116.[3] 何白云,王艳林,黄利鸣.细胞自噬与宫颈癌关系的研究进展[J].现代妇产科进展,2018,27(2):149.[4] 邹霞,郑建英,赖开发,等.RAC3激活Akt通路促进宫颈癌对顺铂耐药的实验研究[J].临床肿瘤学杂志,2020,25(6):516.[5] TAO S,ROJO DE LA VEGA M,CHAPMAN E,et al.The effects ofNRF2modulation on the initiation and progression of chemicallyand genetically induced lung cancer[J].Mol Carcinog,2018,57(2):182.[6] 周燕妮,章宏,张玉媛.Nrf2在镉诱导的氧化损伤和致癌中保护作用的研究进展[J].蚌埠医学院学报,2017,42(8):1149.[7] PADMANABHAN B,TONG KI,OHTA T,et al.Structural basis fordefects of Keap1activity provoked by its point mutations in lungcancer[J].Mol Cell,2006,21(5):689.[8] 邰宵辉,张玲芳,张旭霞,等.Nrf2/ARE信号通路及其在肿瘤发生发展中作用的研究进展[J].现代肿瘤医学,2021,29(17):3113.(下转第310页)Parkinson′s disease pathology[J].Neuroscientist,2020,27(4):340.[7] 史晓燕,李京涛.内质网应激及其对肝纤维化调控作用的研究进展[J].中华肝脏病杂志,2018,26(11):865. [8] PASQUALE ED,Condorelli G,PASQUALE,CHEVET E.Endoplasmic reticulum stress at the crossroads of progeria andatherosclerosis[J].Embo Molecular Medicine,2019,11(4):e10360.[9] LARA⁃GUZMAN OJ,GIL⁃IZQUIERDO A,MEDINA S,et al.Oxidized LDL triggers changes in oxidative stress andinflammatory biomarkers in human macrophages[J].Redox Biol,2018,15(1):11.[10] ZHOU AX,TABAS IRA.The UPR in atherosclerosis[J].SeminImmunopathol,2013,35(3):321.[11] YANG X,YIN M,YU L,et al.Simivastatin inhibited oxLDL⁃induced proatherogenic effects through calpain⁃1/PPARγ/CD36pathway[J].Can J Physiol Pharmacol,2016,94(12):1. [12] WEINSTOCK A,FISHER EA.Methods to study monocyte andmacrophage trafficking in atherosclerosis progression andresolution[J].Methods Mol Biol,2019,1951:153.[13] WANG D,YANG Y,LEI Y,et al.Targeting foam cell formation inatherosclerosis:therapeutic potential of natural products[J].Pharmacol Rev,2019,71(4):596.[14] HERNANDEZ⁃TRUJILLO Y,RODRIGUEZ⁃ESPARRAGON F,MACIAS⁃RERES A,et al.Rosiglitazone but not losartan preventsNrf⁃2dependent CD36gene expression up⁃regulation in an in vivoatherosclerosis model[J].Cardiovascular Diabetology,2008,7(1):3.[15] 胥亚.TLR4/NF⁃κB通路在oxLDL/β2GPI/anti⁃β2GPI复合物诱导小鼠巨噬细胞泡沫化中的作用探讨[D].镇江:江苏大学,2014.[16] ZHANG P,ZHOU H,HE C,et al.OxLDL/β2GPⅠ/β2GPⅠ⁃Abcomplex in regulating the phenotypic transformation of A7r5andthe expression of lipid transporters[J].Chin J Clin Lab Sci,2019,37(3):195.[17] 杨金伟,赵灿,刘秀,等.左归降糖舒心方含药血浆对ox⁃LDL诱导小鼠巨噬细胞泡沫化和凋亡的影响[J].南京中医药大学学报,2020,36(3):96.(本文编辑 赵素容)(上接第305页)[9] ROJO DE LA VEGA M,CHAPMAN E,ZHANG DD.NRF2andthe hallmarks of cancer[J].Cancer Cell,2018,34(1):21. [10] MA JQ,TUERSUN H,JIAO SJ,et al.Functional role of NRF2incervical carcinogenesis[J].PLoS One,2015,10(8):e0133876.[11] MA X,ZHANG J,LIU S,et al.Nrf2knockdown by shRNAinhibits tumor growth and increases efficacy of chemotherapy incervical cancer[J].Cancer Chemother Pharmacol,2012,69(2):485.[12] ZHANG Q,YANG D.Allicin suppresses the migration andinvasion in cervical cancer cells mainly by inhibiting NRF2[J].Exp Ther Med,2019,17(3):1523.[13] 毛万丽,李杰慧,冉立.宫颈癌顺铂耐药研究进展[J].现代肿瘤医学,2021,29(16):2927.[14] CHUDE CI,AMARAVADI RK.Targeting autophagy in cancer:update on clinical trials and novel inhibitors[J].Int J Mol Sci,2017,18(6):1279.[15] DAVIS MA,DELANEY JR,PATEL CB,et al.Nelfinavir iseffective against human cervical cancer cells in vivo:a potentialtreatment modality in resource⁃limited settings[J].Drug DesDevel Ther,2016,10:1837.[16] 阎臻,付晓瑞,李新敏,等.HRAS基因表达对宫颈癌细胞自噬及凋亡作用及机制[J].青岛大学学报(医学版),2021,57(2):240.[17] LI N,ZHANG W.Protein kinase C beta inhibits autophagy andsensitizes cervical cancer Hela cells to cisplatin[J].Biosci Rep,2017,37(2):BSR20160445.[18] LIN WM,LI ZG.Blockage of cisplatin⁃induced autophagysensitizes cervical cancer cells to cisplatin[J].Genet Mol Res,2015,14(4):16905.(本文编辑 赵素容)。
泽泻醇在小胶质细胞中对基质金属蛋白酶3与一氧化氮的抑制作用

泽泻醇在小胶质细胞中对基质金属蛋白酶3与一氧化氮的抑制作用刘瑜【摘要】This paper investigates the inhibitory effect and mechanism of alismol on neuroinflamination in the activated BV2 microglial cells which are stimulated by lipopolysaccharides(LPS). NO was measured by using Griess reagent. RT-PCR and Western blot are used to analyse ERK, JNK, Akt, and MMP3 . Alismol can significantly inhibit LPS-induced NO production and the MMP3 expression. The mechanism is involved to its inhibition of PI3K/Akt pathway.%利用脂多糖(LPS)刺激小鼠小胶质细胞BV2,研究泽泻醇对炎症相关分子的抑制及机制.Griess法测定一氧化氮(NO)浓度,RT-PCR和Western blot法检测细胞外调节蛋白激酶(ERK)、p38、c-Jun氨基末端激酶(JNK)、蛋白激酶B(Akt)、基质金属蛋白酶3(MMP3)的变化.研究结果表明,泽泻醇不仅对LPS刺激小胶质细胞产生的NO有明显抑制作用,还能在mRNA与蛋白质水平抑制MMP3的表达,这种抑制与其对PI3K/Akt通路的干预相关.阐述了泽泻醇对小胶质细胞的抑制与PI3K/Akt通路的相关机制.【期刊名称】《实验技术与管理》【年(卷),期】2012(029)010【总页数】4页(P47-50)【关键词】小胶质细胞;泽泻醇;一氧化氮;基质金属蛋白酶3【作者】刘瑜【作者单位】南开大学医学院,天津 300071【正文语种】中文【中图分类】R914Abstract:This paper investigates the inhibitory effect and mechanism of alismol on neuroinflammation in the activated BV2microglial cells whichare stimulated by lipopolysaccharides(LPS).NO was measured by using Griess reagent.RT-PCR and Western blot are used to analyse ERK,JNK,Akt,and MMP3 .Alismol can significantly inhibit LPS-induced NO production and the MMP3expression.The mechanism is involved to its inhibition of PI3K/Akt pathway.Key words:microglia;alismol;NO;MMP3小胶质细胞是中枢神经系统内的免疫细胞,长期激活而形成中枢神经系统的慢性炎症,其释放的大量氧自由基、炎症介质细胞因子以及基质金属蛋白酶(matrix metalloproteinases,MMP)是神经元损伤的重要原因之一,也是阿尔茨海默病(Alzheimer’s disease,AD)与帕金森病(Parkinson’s disease,PD)等许多中枢神经退行性疾病发生与发展的重要因素之一[1-2]。
2-c-甲基-d-赤藓糖醇4-磷酸胞苷酰转移酶作用

2-c-甲基-d-赤藓糖醇4-磷酸胞苷酰转移酶作用引言是一篇文章的开头部分,用于介绍研究主题、概述研究背景,并阐明研究的目的和意义。
本文的主题是关于2-c-甲基-d-赤藓糖醇4-磷酸胞苷酰转移酶作用的研究,下面将详细说明引言部分的内容。
1.1 概述在本部分,将对整篇文章要涉及的主题进行简单概括。
首先可以介绍2-c-甲基-d-赤藓糖醇4-磷酸胞苷酰转移酶(以下称为XMT)这个酶类以及其在生物体内中起到的重要作用。
也可以简要描述该酶与底物之间发生转化反应的过程。
1.2 研究背景在此部分,需要回顾现有文献和相关实践工作,探讨当前对于XMT已有的了解以及相关领域中可能存在的问题或待解决的挑战。
可以描述近年来该领域内XMT相关研究成果,提出已知问题或待解答之难点,并指明目前对于XMT催化机制和功能特点等方面的研究现状。
1.3 目的和意义本部分应明确本文的研究目标以及对于科学领域和应用领域的重要意义。
可以论述研究XMT催化机制相关问题的重要性,以及深入理解XMT酶类活性对于生物医药、工业生产等方面带来的潜在益处。
同时,也可以展望XMT酶类相关研究在未来可持续发展领域中所能发挥的作用,包括但不限于新药开发、底物转化等方面。
以上内容可以根据实际情况进行适当调整和补充,以完整而清晰地介绍2-c-甲基-d-赤藓糖醇4-磷酸胞苷酰转移酶作用这一主题,并为读者提供足够的背景信息和动机理解接下来文章将涉及到的内容。
2. 2-c-甲基-d-赤藓糖醇4-磷酸胞苷酰转移酶的结构特点2-c-甲基-d-赤藓糖醇4-磷酸胞苷酰转移酶(简称MTDGP)是一种关键酶,参与细菌中碳代谢和能量产生的过程。
该酶具有特定的结构特点,决定了其催化活性和底物特异性。
结构分析表明,MTDGP是一个多亚基复合物,由多个蛋白质亚基组成。
每个亚基都包含一个活性位点,其中存在着底物结合和催化反应所需的氨基酸残基。
这些亚基通过非共价相互作用保持在一起,并形成一个稳定的三维空间结构。
Febuxostat_SDS_MedChemExpress

Inhibitors, Agonists, Screening LibrariesSafety Data Sheet Revision Date:Jul.-04-2017Print Date:Jul.-04-20171. PRODUCT AND COMPANY IDENTIFICATION1.1 Product identifierProduct name :FebuxostatCatalog No. :HY-14268CAS No. :144060-53-71.2 Relevant identified uses of the substance or mixture and uses advised againstIdentified uses :Laboratory chemicals, manufacture of substances.1.3 Details of the supplier of the safety data sheetCompany:MedChemExpress USATel:609-228-6898Fax:609-228-5909E-mail:sales@1.4 Emergency telephone numberEmergency Phone #:609-228-68982. HAZARDS IDENTIFICATION2.1 Classification of the substance or mixtureGHS Classification in accordance with 29 CFR 1910 (OSHA HCS)Acute toxicity, Oral (Category 4), H302Acute aquatic toxicity (Category 1), H400Chronic aquatic toxicity (Category 1), H4102.2 GHS Label elements, including precautionary statementsPictogramSignal word WarningHazard statement(s)H302 Harmful if swallowed.H410 Very toxic to aquatic life with long lasting effects.Precautionary statement(s)P264 Wash skin thoroughly after handling.P270 Do not eat, drink or smoke when using this product.P273 Avoid release to the environment.P301 + P312 IF SWALLOWED: Call a POISON CENTER or doctor/ physician if you feel unwell.P330 Rinse mouth.P391 Collect spillage.P501 Dispose of contents/ container to an approved waste disposal plant.2.3 Other hazardsNone.3. COMPOSITION/INFORMATION ON INGREDIENTS3.1 SubstancesSynonyms:TEI 6720; TMX 67Formula:C16H16N2O3SMolecular Weight:316.37CAS No. :144060-53-74. FIRST AID MEASURES4.1 Description of first aid measuresEye contactRemove any contact lenses, locate eye-wash station, and flush eyes immediately with large amounts of water. Separate eyelids with fingers to ensure adequate flushing. Promptly call a physician.Skin contactRinse skin thoroughly with large amounts of water. Remove contaminated clothing and shoes and call a physician.InhalationImmediately relocate self or casualty to fresh air. If breathing is difficult, give cardiopulmonary resuscitation (CPR). Avoid mouth-to-mouth resuscitation.IngestionWash out mouth with water; Do NOT induce vomiting; call a physician.4.2 Most important symptoms and effects, both acute and delayedThe most important known symptoms and effects are described in the labelling (see section 2.2).4.3 Indication of any immediate medical attention and special treatment neededTreat symptomatically.5. FIRE FIGHTING MEASURES5.1 Extinguishing mediaSuitable extinguishing mediaUse water spray, dry chemical, foam, and carbon dioxide fire extinguisher.5.2 Special hazards arising from the substance or mixtureDuring combustion, may emit irritant fumes.5.3 Advice for firefightersWear self-contained breathing apparatus and protective clothing.6. ACCIDENTAL RELEASE MEASURES6.1 Personal precautions, protective equipment and emergency proceduresUse full personal protective equipment. Avoid breathing vapors, mist, dust or gas. Ensure adequate ventilation. Evacuate personnel to safe areas.Refer to protective measures listed in sections 8.6.2 Environmental precautionsTry to prevent further leakage or spillage. Keep the product away from drains or water courses.6.3 Methods and materials for containment and cleaning upAbsorb solutions with finely-powdered liquid-binding material (diatomite, universal binders); Decontaminate surfaces and equipment by scrubbing with alcohol; Dispose of contaminated material according to Section 13.7. HANDLING AND STORAGE7.1 Precautions for safe handlingAvoid inhalation, contact with eyes and skin. Avoid dust and aerosol formation. Use only in areas with appropriate exhaust ventilation.7.2 Conditions for safe storage, including any incompatibilitiesKeep container tightly sealed in cool, well-ventilated area. Keep away from direct sunlight and sources of ignition.Recommended storage temperature:Powder-20°C 3 years4°C 2 yearsIn solvent-80°C 6 months-20°C 1 monthShipping at room temperature if less than 2 weeks.7.3 Specific end use(s)No data available.8. EXPOSURE CONTROLS/PERSONAL PROTECTION8.1 Control parametersComponents with workplace control parametersThis product contains no substances with occupational exposure limit values.8.2 Exposure controlsEngineering controlsEnsure adequate ventilation. Provide accessible safety shower and eye wash station.Personal protective equipmentEye protection Safety goggles with side-shields.Hand protection Protective gloves.Skin and body protection Impervious clothing.Respiratory protection Suitable respirator.Environmental exposure controls Keep the product away from drains, water courses or the soil. Cleanspillages in a safe way as soon as possible.9. PHYSICAL AND CHEMICAL PROPERTIES9.1 Information on basic physical and chemical propertiesAppearance White to off-white (Solid)Odor No data availableOdor threshold No data availablepH No data availableMelting/freezing point No data availableBoiling point/range No data availableFlash point No data availableEvaporation rate No data availableFlammability (solid, gas)No data availableUpper/lower flammability or explosive limits No data availableVapor pressure No data availableVapor density No data availableRelative density No data availableWater Solubility No data availablePartition coefficient No data availableAuto-ignition temperature No data availableDecomposition temperature No data availableViscosity No data availableExplosive properties No data availableOxidizing properties No data available9.2 Other safety informationNo data available.10. STABILITY AND REACTIVITY10.1 ReactivityNo data available.10.2 Chemical stabilityStable under recommended storage conditions.10.3 Possibility of hazardous reactionsNo data available.10.4 Conditions to avoidNo data available.10.5 Incompatible materialsStrong acids/alkalis, strong oxidising/reducing agents.10.6 Hazardous decomposition productsUnder fire conditions, may decompose and emit toxic fumes.Other decomposition products - no data available.11.TOXICOLOGICAL INFORMATION11.1 Information on toxicological effectsAcute toxicityClassified based on available data. For more details, see section 2Skin corrosion/irritationClassified based on available data. For more details, see section 2Serious eye damage/irritationClassified based on available data. For more details, see section 2Respiratory or skin sensitizationClassified based on available data. For more details, see section 2Germ cell mutagenicityClassified based on available data. For more details, see section 2CarcinogenicityIARC: No component of this product present at a level equal to or greater than 0.1% is identified as probable, possible or confirmed human carcinogen by IARC.ACGIH: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by ACGIH.NTP: No component of this product present at a level equal to or greater than 0.1% is identified as a anticipated or confirmed carcinogen by NTP.OSHA: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by OSHA.Reproductive toxicityClassified based on available data. For more details, see section 2Specific target organ toxicity - single exposureClassified based on available data. For more details, see section 2Specific target organ toxicity - repeated exposureClassified based on available data. For more details, see section 2Aspiration hazardClassified based on available data. For more details, see section 212. ECOLOGICAL INFORMATION12.1 ToxicityNo data available.12.2 Persistence and degradabilityNo data available.12.3 Bioaccumlative potentialNo data available.12.4 Mobility in soilNo data available.12.5 Results of PBT and vPvB assessmentPBT/vPvB assessment unavailable as chemical safety assessment not required or not conducted.12.6 Other adverse effectsNo data available.13. DISPOSAL CONSIDERATIONS13.1 Waste treatment methodsProductDispose substance in accordance with prevailing country, federal, state and local regulations.Contaminated packagingConduct recycling or disposal in accordance with prevailing country, federal, state and local regulations.14. TRANSPORT INFORMATIONDOT (US)This substance is considered to be non-hazardous for transport.IMDGUN number: 3077Class: 9Packing group: IIIEMS-No: F-A, S-FProper shipping name: ENVIRONMENTALLY HAZARDOUS SUBSTANCE, SOLID, N.O.S.Marine pollutant: Marine pollutantIATAUN number: 3077Class: 9Packing group: IIIProper shipping name: Environmentally hazardous substance, solid, n.o.s.15. REGULATORY INFORMATIONSARA 302 Components:No chemicals in this material are subject to the reporting requirements of SARA Title III, Section 302.SARA 313 Components:This material does not contain any chemical components with known CAS numbers that exceed the threshold (De Minimis) reporting levels established by SARA Title III, Section 313.SARA 311/312 Hazards:No SARA Hazards.Massachusetts Right To Know Components:No components are subject to the Massachusetts Right to Know Act.Pennsylvania Right To Know Components:No components are subject to the Pennsylvania Right to Know Act.New Jersey Right To Know Components:No components are subject to the New Jersey Right to Know Act.California Prop. 65 Components:This product does not contain any chemicals known to State of California to cause cancer, birth defects, or anyother reproductive harm.16. OTHER INFORMATIONCopyright 2017 MedChemExpress. The above information is correct to the best of our present knowledge but does not purport to be all inclusive and should be used only as a guide. The product is for research use only and for experienced personnel. It must only be handled by suitably qualified experienced scientists in appropriately equipped and authorized facilities. The burden of safe use of this material rests entirely with the user. MedChemExpress disclaims all liability for any damage resulting from handling or from contact with this product.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
【生化】Chem.Sci.:一种用于泛酰巯基乙胺酶检测的近红外荧光探针

【生化】Chem.Sci.:一种用于泛酰巯基乙胺酶检测的近红外荧光探针泛酰巯基乙胺酶是一种酰胺水解酶,常被称为血管非炎性分子vanin-1,可将泛酰巯基乙胺分解为泛酸和半胱胺,在肝、肠、肾和肺等多种器官都有着高表达。
近年来,许多研究表明泛酰巯基乙胺酶在炎症以及其他疾病方面有着潜在的作用。
例如,vanin-1基因敲除小鼠对药物引起的肝损伤表现出更高的敏感性,表明泛酰巯基乙胺酶具有一定的酶促保护作用。
此外,泛酰巯基乙胺酶在肾脏损伤和炎症性肠病早期均表达上调,且其抑制剂可以减轻急性和慢性肠道疾病中的氧化应激和炎症,这表明泛酰巯基乙胺酶是促炎因子。
因此,寻求一种合适的方式用于泛酰巯基乙胺酶的检测显得尤为重要。
基于此,北京中国科学院化学研究所的史文副研究员和马会民研究员设计合成了一种新型近红外荧光探针CYLP,以自分解的长链作为linker将荧光团和识别基团泛酸相连接,用于泛酰巯基乙胺酶的生物成像。
相关成果以“A near-infrared fluorescence probe for imaging of pantetheinase incells and mice in vivo”为题发表在Chemical Science上(DOI: 10.1039/d0sc04537c)。
首先,作者将泛酸直接偶联至氨基半菁荧光团上构建了一个荧光探针(control,Scheme 1),期望在泛酰巯基乙胺酶的作用下释放NIR荧光,但结果并不理想,这可能是因为荧光团和催化位点的空间位阻影响了底物进入活性中心。
为了解决这一难题,作者将自分解的长链作为linker连接大分子荧光团和泛酸,得到探针CYLP(Scheme 1),其在近红外波段(>700 nm)下对泛酰巯基乙胺酶表现出优异的选择性和灵敏性。
(来源:Chemical Science)随后,作者探究了CYLP对泛酰巯基乙胺酶的响应性。
CYLP(10 mM)在NIR区域几乎不显示荧光(量子产率<0.1%);而在泛酰巯基乙胺酶(50 ng/ml)的作用下,其在710 nm处的荧光增强约40倍(Fig. 1A)。
分子生物色谱技术中生物大分子在中药研究中的应用进展

分子生物色谱技术中生物大分子在中药研究中的应用进展刘珏玲; 安琪; 陈素娥; 赵龙山; 刘亮亮【期刊名称】《《药学研究》》【年(卷),期】2019(038)011【总页数】5页(P652-656)【关键词】分子生物色谱; 中药; 活性成分; 筛选【作者】刘珏玲; 安琪; 陈素娥; 赵龙山; 刘亮亮【作者单位】山西卫生健康职业学院山西生物样品分析检测中心山西晋中030619; 山西省中医学校山西晋中030619; 沈阳药科大学药学院辽宁沈阳110013【正文语种】中文【中图分类】R284中药的活性物质和作用机理的研究,是中药发展的瓶颈问题。
中药及复方具有多组分、多靶点的特点[1],从而具有复杂性、多效性和某些成分的双向调节性。
如果单纯依靠传统分离分析技术研究中药及复方中的药效物质,过程复杂且难度较大,限制了中医药的发展进程[2]。
分子生物色谱(molecular biochromatography,MBC)是一种以生物大分子为固定相配基的新型液相色谱技术,其原理是分子特异性识别,具有重现性好、分析速度快、具有药理学意义等特点。
由于不同药物组分与生物大分子结合度及亲和性不同,使得其在色谱柱上的保留时间不同,由此可将活性物质快速筛选出来。
同时MBC与其他分析检测技术结合,能进一步阐明与机体有关的药效组分,将其应用于中药复方活性成分的筛选,可有效地排除无生物活性成分的干扰,为中药药效物质基础研究提供新的解决途径[3-6]。
本文对近10年国内外分子生物色谱技术在中药物质基础分析方面的应用和研究进展进行了归纳总结,旨在为药学工作者更好地掌握和应用分子生物色谱技术提供参考。
1 MBC常用大分子1.1 血浆蛋白血浆蛋白能与药物发生可逆结合成为“血浆蛋白-药物复合物”,到达作用部位发生药理作用。
将血浆蛋白固载于载体上作为生物色谱填料,形成一种可以模拟体内环境中药物与血浆蛋白间相互作用的色谱系统,由于不同的药物分子与血浆蛋白的结合率具有差异,它在固定相的保留行为也不同,结合色谱技术中的各种参数计算,一方面可以筛选中药中的活性物质,还能进行药物与血浆蛋白的作用关系的研究[7-8]。
超高效液相色谱-质谱法测定大鼠血浆中的姜黄素

超高效液相色谱-质谱法测定大鼠血浆中的姜黄素李智宁;魏悦;朱杰;范毅;于立芹;李晓;李飞飞【摘要】建立一个测定大鼠血浆中姜黄素含量的液相色谱串联三重四级杆质谱法(UPLC-MS/MS).血浆样品用甲醇沉淀蛋白,采用Agilent Extend-C 18色谱柱(RRHD 1.8 μm,2.1 mm×50 mm),以乙腈(A)-0.1%甲酸水(B)为流动相梯度洗脱,流速为0.2 mL/min,柱温30℃,质谱条件为电喷雾离子源(ESI),正离子模式,内标为沙丁胺醇,采用多反应监测(MRM)模式测定,用于定量分析的离子对:姜黄素和内标分别为369.1→177.1和240.1→148.姜黄素在2.5~400 ng/mL范围内线性关系良好,最低检出限为0.084 ng/mL,提取回收率在84.66%~95.71%,日内及日间精密度RSD均小于7%(n=3),稳定性较好.该方法准确、高效、专属性强、灵敏度高、测定结果准确可靠,可用于实际大鼠血浆中姜黄素的含量测定,为监测姜黄素口服给药后体内的血药浓度和药代动力学特征提供参考,也为其新制剂的设计和临床应用提供数据基础.%A method for the determination of curcumin in rat plasma was established by liquid chromatographytandem quadrupole mass spectrometry (UPLC-MS/MS).Plasma samples were precipitated with methanol and eluted with an Agilent Extend-C18 column (RRHD 1.8 μm,2.1 mm×50 mm) and during with acetonitrile (A)-0.1% formic acid (B) as the mobile phase gradient at a flow rate of 0.2 mL/min,and the column temperature was 30 ℃.The mass spectrometric conditions were electrospray ionization (ESI),positive ion mode,and the internal standard (IS) was salbutamol.Detection was carried out by multiple reaction monitoring (MRM) of the transitions (precursor ion to product ion) at m/z 369.1 to 177.1 for curcumin,m/z 240.1 to 148 for IS.The linear range was 2.5 ng/mLto 400 ng/mL,the limit of detection of curcumin was 0.084 ng/mL,the recoveries were 84.66% to 95.71%,and the RSD of intra and inter-day precisions were less than 7%(n=3).The stability was better.The method was accurate,efficient,specific and high sensitive.The determination result was accurate and reliable.It could be used for the determination of curcumin in rat plasma and to provide reference for the monitoring concentration and pharmacokinetic characteristics of curcumin in rat plasma after oral administration,and to provide a theoretical basis for the design and clinical application of the new formulations.【期刊名称】《河南科学》【年(卷),期】2017(035)008【总页数】6页(P1252-1257)【关键词】超高效液相色谱-质谱;姜黄素;药动学【作者】李智宁;魏悦;朱杰;范毅;于立芹;李晓;李飞飞【作者单位】河南省生物技术开发中心,河南省科高植物天然产物开发工程技术研究中心,郑州450000;河南省生物技术开发中心,河南省科高植物天然产物开发工程技术研究中心,郑州450000;河南省生物技术开发中心,河南省科高植物天然产物开发工程技术研究中心,郑州450000;河南省生物技术开发中心,河南省科高植物天然产物开发工程技术研究中心,郑州450000;河南省生物技术开发中心,河南省科高植物天然产物开发工程技术研究中心,郑州450000;河南省生物技术开发中心,河南省科高植物天然产物开发工程技术研究中心,郑州450000;河南省生物技术开发中心,河南省科高植物天然产物开发工程技术研究中心,郑州450000【正文语种】中文【中图分类】R284.1姜黄[1-2]为传统常用中药,临床应用历史悠久,疗效肯定,其味苦、辛,性温,有破血行气、通经止痛、祛风疗痹的功能,广泛分布在我国广东、广西、云南、福建等地区.姜黄素是从姜科植物姜黄等根茎中提取出来的一种多酚类化合物,是姜黄中的主要有效成分.许多研究[3-4]表明,姜黄素具有广泛药理活性,如抗炎、抗氧化、降血脂、抗肿瘤等,且大剂量使用时几乎不产生毒副作用,由于以上优点,使姜黄素在各领域具有广泛的应用前景.但是口服给药后血药浓度很低,给检测带来困难,几种常见的检测方法比如高效液相色谱法[5]、光谱分析法虽然能够测定姜黄素,但是这些分析方法都普遍存在分析时间长、血浆样品用量大、提取步骤繁琐、灵敏度低、干扰因素较多,选择性差等缺点,而UPLC-MS/MS技术综合了色谱的高分离效能以及质谱的高灵敏度和选择性,同时笔者发现有关UPLC-MS/MS方法检测血浆中姜黄素的报道较少,因此有必要建立基于此技术测定血浆中姜黄素的方法.通过查阅相关文献[6-12],姜黄素的分析方法多采用液相方法,液质方法相对较少,比较国内外关于姜黄素类化合物检测方法的优缺点,结合姜黄素的理化性质以及实验室的现有条件,建立了UPLC-MS/MS法测定大鼠血浆中姜黄素的含量,并进行了分析验证.姜黄素结构式如下所示.标准品:姜黄素(批号:151022,纯度98%),购于成都普菲德生物技术有限公司;内标物:沙丁胺醇(批号:100204-201103,按C13H21NO3计含量为99.7%),购于河南省食品药品检验所;甲醇、乙腈(LC/MS级别,美国ACS恩科化学);甲酸(LC/MS级,Fisher Scientific);超纯水(娃哈哈矿泉水). Agilent G6460C UPLC-MS/MS三重四级杆液质串联系统(美国安捷伦科技有限公司);Dura-CYL型杜瓦罐及氮气瓶(CHART)(河南源正科技发展有限公司);AUW220D型电子分析天平(日本Shimadzu公司);QL-901型涡旋混合器(海门市其林贝尔仪器制造有限公司);HSC-12B型氮吹仪(天津市恒奥科技发展有限公司);H1850r型台式冷冻离心机(湖南长沙湘仪离心机仪器有限公司);NBS Premium u410型超低温冰箱(美国Eppendorf公司);KQ-500E型超声波萃取机(昆山市超声仪器有限公司).健康雄性SD大鼠,6只,体重(250±20)g,洁净度SPF级,由河南省实验动物中心提供;质量检测单位为河南省实验动物质量监督检测站.动物使用许可证号SC XK(豫)2015-0004.实验前适应性饲养7 d,自由饮水.色谱柱:Agilent Extend-C18分析柱(RRHD 2.1 mm×50 mm,1.8 μm);流动相:乙腈(A)和0.1%甲酸水(B),按下列程序进行梯度洗脱:0 min,5%A;0.5 min,5%A;1.5 min,95%A;5 min,95%A;6 min,5%A;10 min,5%A;流速0.2 mL/min.进样量5 μL.柱温30℃.离子源:ESI;模式:MRM-Positive;喷雾干燥气温度:350℃;喷雾气压力:275.8 kPa;干燥气流速:10 L/min;毛细管电压:4 kV.用于定量分析的离子对参数见表1,MRM谱图见图1.准确称取约2.0 mg姜黄素标准品(精确至0.000 01 g)置于10 mL容量瓶内,用甲醇定容至刻度,作为标准储备液(200 μg/mL).精密量取储备液适量,用初始流动相逐级稀释成5、10、80、200、400、800 ng/mL一系列浓度标准工作溶液;内标沙丁胺醇同法用甲醇配制质量浓度为100 μg/mL的储备液,临用时取适量用初始流动相稀释至质量浓度为100 ng/mL.上述溶液均置于-4℃冰箱中保存备用.SD雄性大鼠6只,给药前禁食12 h,全程不禁水,灌胃给药相当于纯姜黄素250 mg/kg.分别在给药前和给药后15、30、45 min及1、1.25、1.5、1.75、2、3、4 h在大鼠眼眶静脉丛取血0.5 mL.将全血样本置于含有肝素的EP管中,5000 r/min离心10 min,取上层血浆样品,置于-80℃超低温冷冻冰箱中保存.取大鼠血浆样品25 μL,置于1.5 mL EP管中,依次加入50 μL甲醇、10 μL内标溶液,然后加入100 μL甲醇沉淀蛋白,提取姜黄素,涡混2 min后,4℃下,以12 000 r/min的转速离心10 min,取上清液于2 mL EP管中,35℃条件下氮气吹干,100 μL初始流动相复容,涡混1 min;同样在4℃下,以12 000 r/min的转速离心10 min,取上清液上机检测.2.6.1 专属性考察在选定的色谱条件和质谱条件下,空白血浆加姜黄素和沙丁胺醇(内标)以及大鼠给药后血浆样品色谱图见图1.由图可见,姜黄素和沙丁胺醇的保留时间分别为4.2 min和1.7 min,各成分分离良好,未见血浆中内源性物质的干扰,本方法具有较好的专属性.2.6.2 线性范围及检出限考察姜黄素在大鼠血浆中的线性范围,分别吸取定量上述2.3中系列标准溶液按2.4方法进行测定,以姜黄素血浆浓度为横坐标,姜黄素与内标沙丁胺醇峰面积的比值为纵坐标作线性回归分析,大鼠血浆中姜黄素在2.5~400 ng/mL浓度范围内标准曲线方程为:y=0.053 2x+0.018 4(1/x2),相关系数为R2=0.990 8:结果表明:血浆中姜黄素在2.5~400 ng/mL浓度范围线性关系良好,血浆姜黄素最低检出限为:0.084 ng/mL(S/N≥3).2.6.3 提取回收率与精密度分别制备低、中、高浓度(5、100、400 ng/mL)血浆质控样品,每个浓度各3份,连续3天分别配制,并按血浆样品处理项下方法进行处理后进样分析,每天做随行标准曲线,由标准曲线反算实测浓度,计算3个浓度的日内、日间精密度,并与实测浓度相比计算回收率,不同浓度质控样品回收率及精密度结果见表2,数据显示该方法日内及日间RSD在7%以内,方法回收率为84.66%~95.71%.结果表明该试验条件下姜黄素在血浆中回收率符合要求,方法精密度良好.2.6.4 基质效应考察用来自1份不同个体的空白血浆同法萃取后的上清配制不同浓度溶液各1份,质量浓度分别为5、100、400 ng/mL,进UPLC-MS/MS分析,记录峰面积A1,同法配制相同浓度的低、中、高浓度对照品溶液进UPLC-MS/MS分析,记录峰面积A2,以相同浓度平均峰面积比值A1/A2计算不同浓度下基质效应.姜黄素低、中、高三个浓度下基质效应见表2,结果表明该法无明显的基质效应.2.6.5 稳定性实验按上述方法制成含姜黄素药物的低、中、高三个浓度的样品,每个浓度平行制备3份,制作3个分析批,分别在室温(25℃)下放置8 h,置-80℃条件下反复冻融3次,处理后的进样液在室温(25℃)放置8 h,详细结果见表3,由低到高不同浓度的血浆样品在上述条件下所测定值RSD均低于7%,进样液在室温下放置8 h后姜黄素浓度有少量下降,虽然对实验结果影响不大,但是为了更准确地测定样品,尽量保证处理后的样品及时进样分析.大鼠进行灌胃给药姜黄素(经0.5%CMC-Na混悬液处理)后,采用DAS软件对血药浓度(C)与时间(t)数据进行处理分析,其中药-时曲线下面积(AUC(0-t)和AUC(0-∞))采用梯形法计算,半衰期(t1/2)利用药-时曲线末端相计算,达峰浓度(Cmax)和达峰时间(tmax)为实测值.平均血药浓度-时间曲线(C-t曲线)见图2,药动学参数详见表4,结果表明,姜黄素在体内血药浓度较低,入血吸收较差,体内存留时间长,可能是姜黄素药物进入机体内后代谢较快、分布较广,并且较多地分布在特定的组织或器官中.由于本研究旨在验证该方法应用于测定大鼠血浆中姜黄素含量的可行性,未做过多的比较研究,如不同剂量、联合其他药物(中药或西药)同时给药对其药动学过程产生的影响等,此外,姜黄素在大鼠体内其他组织当中的代谢分布情况也有待进一步研究和评价.本研究建立并验证了一个具有较高专属性且更加灵敏,高效,准确快速地检测大鼠血浆中姜黄素的UPLC-MS/MS方法,姜黄素在2.5~400 ng/mL范围内线性关系良好,定量限为2.5 ng/mL,提取回收率在84.66%~95.71%,日内及日间精密度RSD均小于7%(n=3),基质效应不明显,室温,冻融条件下放置稳定性较好,进样液室温放置8 h内稳定性有稍许降低,这就要求制备好的进样液应及时进样分析,以免使药物降解对结果造成影响.本研究还比较了液-液萃取法和蛋白沉淀法提取待测物,结果表明选用乙酸乙酯和二氯甲烷液-液萃取时对待测物的提取率一般,特别是乙酸乙酯易挥发,定量不准确,而用甲醇、乙腈和叔丁基甲醚的蛋白沉淀法测定姜黄素的提取率时乙腈和甲醇较高,但使用甲醇作为沉淀剂所得到的峰形更好,干扰更少,故最终采用以甲醇为沉淀剂的蛋白沉淀法.另外,本研究筛选内标物时,采用沙丁胺醇做内标相比使用大黄素做内标更稳定,峰形更尖锐,出峰时间更合适.总之,该法的线性范围适合实际样品血药浓度的测定,定量限低于最低血药浓度,并具有较好的准确度与精密度,研究采用内标法定量,尤其是在生物样本的分析中,可较好地防止和降低前处理过程中因操作的失误、溶剂的挥发、残渣的复溶等因素对实验造成的误差,相比其他文献[13-14]报道的前处理方法更简单、快速、样品用量更少,而且使用液质分析提取姜黄素和内标的选择离子流灵敏度更高、选择性更好.通过测定大鼠血浆中血药含量,验证了该方法在大鼠血浆中姜黄素的药代动力学应用的可行性,同时反映出灌胃给药后血药浓度较低,入血吸收较差,体内存留时间长,可能是因为姜黄素在平衡前被总血池快速稀释或被组织迅速摄取,而代谢转化后却较长时间滞留于各组织中的缘故,可见其具有代谢较快、分布较广的特点,与有关报道[15-18]一致.相比在家兔[19]和Beagle犬[20]血浆中的药动学应用,得出的一些药动学参数不同,呈单峰吸收现象,这就反映出不同种属类别之间以及不同剂型姜黄素在体内的吸收和分布规律是有差异的,故本研究可以为大鼠血浆中姜黄素血药浓度测定提供依据,为更多使用液质分析姜黄素提供方法参考,为监测姜黄素口服给药后体内的血药浓度和药代动力学特征提供参考,也为其新制剂的设计和临床应用提供一定的数据基础.【相关文献】[1]刘安昌,姿红祥,赵丽霞.姜黄素药理活性及体内代谢[J].国外医药·植物药分册,2004,19(1):1-5.[2]汪海慧,成扬.姜黄素药理作用的研究进展[J].上海中医药大学学报,2007,21(6):73-76.[3]仲明远,全山丛,胡晋红.姜黄素制剂学研究进展[J].中成药,2007,29(2):255-258. [4]程旸,卞勇,许立,等.姜黄素体内药动学研究概况[J].安徽医药,2011,15(1):4-6. [5]李占彬,杨昌彪,谭红,等.高效液相色谱仪串联三重四级杆质谱法快速测定姜茶中的姜黄素[J].分析仪器,2014(2):65-68.[6]彭文兴,王佳斌.HPLC-MS/MS法测定人血浆中姜黄素及其代谢产物四氢姜黄素[C]//湖南省药学会2007年学术年会论文集,2007:52-56.[7]林观样,王贤亲,胡卢丰.LC-ESI-MS测定人血浆中的姜黄素[J].中国卫生检验,2010,20(4):756-757.[8]王凤飞,章晓茜,曹聪,等.高效液相色谱-质谱联用测定大鼠全血中二甲基姜黄素的浓度[J].中国药学,2015,50(22):1996-1999.[9]MA W Z,WANG J L,GUO Q,et al.Simultaneous determination of doxorubicin and curcumin in rat plasma by LC-MS/MS and its application to pharmacokinetic study [J].Journal of Pharmaceutical and Biomedical Analysis,2015,111:215-221.[10]RAMALINGAM P,KO Y T.A validated LC-MS/MS method for quantitative analysis of curcumin in mouse plasma and brain tissue and its application in pharmacokinetic and brain distribution studies[J].Journal of Chromatography B,2014,969:101-108. [11]李好枝.体内药物分析[M].北京:中国医药科技出版社,2003:118-136.[12]韩刚,翟丽,赵琳琳,等.姜黄素固体分散体在大鼠体内的药动学研究[J].中国药学,2009,44(9):698-700.[13]曾晓会,陈玉兴,赵自明,等.姜黄素微囊在大鼠体内的药代动力学研究[J].中国实验方剂学,2010,16(2):107-110.[14]钟荣玲,夏智,武洁,等.姜黄素纳米混悬剂在大鼠体内药代动力学考察[J].中国实验方剂学,2013,19(20):137-139.[15]张聪聪,金若敏.HPLC法测定大鼠血浆中姜黄素的含量及其药动学研究[J].中国新药,2014,23(15):1829-1832.[16]BISHT S,MAITRA A.Systemic delivery of curcumin:21st century solutions for an ancient conundrum[J].Current Drug Discovery Technologies,2009,6(3):192-199. [17]SONG Z,FENG R,SUN M,et al.Curcumin-loaded PLGA-PEG-PLGA triblockcopolymeric micelles:preparation,pharmacokinetics and distribution in vivo[J].J Colloid Interface Sci,2011,354(1):116-123.[18]张继芬,侯世祥,刘惠莲,等.小鼠血浆中姜黄素的含量测定及药动学研究[J].中国药学,2007,42(4):288-290.[19]罗文汇,孙冬梅,高华宏,等.LC-MS/MS法测定血浆中姜黄素的血药浓度[J].中国药事,2012,26(11):1246-1248.[20]石磊,王汝上.姜黄素自微乳在Beagle犬体内的药动学分析[J].中国实验方剂学,2014,20(12):133-135.。
乳苣水提取物诱导肺癌细胞H1299和A549的凋亡

乳苣水提取物诱导肺癌细胞H1299和A549的凋亡作者:张彩艳刘小敏华子义贺燕云来源:《上海师范大学海报·自然版》2014年第02期摘要:为探讨乳苣水提取物对体外培养的肺癌细胞H1299和A549的影响,用不同浓度的乳苣水提取物分别作用于H1299和A549细胞,从细胞形态(显微镜观察)、细胞增殖活力(cck8法)、细胞凋亡(AnexinⅤFITC/PI染色)、线粒体膜电位(JC1染色)几个方面检测乳苣水提取物对H1299和A549细胞的影响.当处理浓度达到1.2 mg/mL,作用细胞48 h后,细胞出现明显皱缩的凋亡形态学特征,与对照组相比处理组细胞增殖活力显著下降(P关键词:乳苣水提取物;肺癌细胞;增殖;凋亡中图分类号: Q 28 文献标识码: A 文章编号: 10005137(2014)02019608据世界卫生组织(WHO)统计,全世界每年有1000万新发癌症患者,600万人死于癌症,癌症几乎是死亡的代名词.目前,治疗癌症的措施有手术、化疗、放疗和免疫治疗等方法,但其治疗效果都不理想.由于中药的毒副作用低,能提高机体的免疫力,所以越来越多的中晚期癌症患者选择中药治疗,许多报道也证明了中药在抗肿瘤复发、转移中的优势.因此寻找能预防癌症并能长期服用的高效无毒的中草药成为迫切的问题.药食两用植物乳苣(Mulgedium tataricum (Linn.) DC.)为菊科乳苣属植物,又名蒙山莴苣、紫花山莴苣、苦菜等,多年生草本植物,民间作为野菜采食,有抗菌、消炎、止痛等功效.已有的对乳苣的研究主要集中在对乳苣的总黄酮和多酚的提取和抗氧化方面的研究[1-4].天然的黄酮类化合物具有抗心脑血管疾病、抗炎镇痛、免疫调节、降血糖、治疗骨质疏松,它具有清除自由基、超氧化物和羟基自由基、抑制脂质过氧化及抗癌、抗病毒、抗氧化、抗衰老、抗辐射等作用 [5-6].王小雄等从乳苣全草中分离到41种化合物,其中3种物质莴苣素8O对甲氧基苯乙酸酯、莴苣素、山莴苣素被实验证明能够抑制体外培养的人的白血病细胞(HL60)和人肝癌细胞(SMMC7721)的生长[7-8].本文作者想要验证乳苣作为中草药对癌细胞的抑制作用,所以直接用沸水浸泡乳苣所得的水提取物作用于体外培养的人肺癌细胞株H1299和A549,探讨乳苣的水提取物对肿瘤细胞的生长抑制及诱导细胞凋亡的作用,为保健品和新药的研究开发提供线索.1材料与方法1.1实验材料1.1.1细胞株人肺癌细胞株H1299和A549;购自中国科学院上海细胞生物学研究院.1.1.2主要试剂乳苣(Mulgedium tataricum (Linn.) DC.)为菊科乳苣属植物,采于晋西北农田,由上海大学生命学院王伟博士鉴定.RPMI1640培养基(CORNING);胎牛血清(Hyclone);胰蛋白酶(Hyclone);PenicillinStreptomycin Solution(Hyclone);CCK8试剂盒(上海七海复泰生物公司);Annexin VFITC/PI双染法细胞凋亡检测试剂盒(上海七海复泰生物公司);线粒体膜电位检测试剂盒(Jc1)(碧云天生物公司).1.1.3主要仪器流式细胞仪为Moflow XDP;CO2培养箱;酶标仪为美国BioRad产品;倒置显微镜为LEICA 公司产品;冷冻干燥机为美国LABCONCO公司产品.1.2实验方法1.2.1乳苣水提取物制备采全草,阴干,-80 ℃保存.将8 g的干乳苣,粉碎,浸泡到100 mL沸水中,超声1 h,沸水浴30 min,过滤2次,分装原液经48 h冷冻干燥后制成冻干粉,-80℃保存,备用.使用时,用培养基重新溶解冻干粉,0.22 μm滤膜过滤后使用.1.2.2细胞培养以含10%新生牛血清、100 μg/mL青霉素、100 μg/mL链霉素的RPMI1640培养基培养H1299和A549,37 ℃、5 % CO2,恒温培养箱中常规消化传代.1.2.3细胞形态观察细胞均匀铺在24孔板中,待细胞贴壁生长至覆盖培养板底部50%~60%时,用不同浓度乳苣水提取物处理细胞48 h,另设不加药的空白对照,每个剂量设3个重复,在光学显微镜下观察细胞形态.1.2.4细胞增殖活力检测(CCK8法)将细胞均匀铺于96孔板中,待细胞贴壁生长至覆盖培养板底部50%~60%时,用不同浓度乳苣水提取物处理细胞48 h,检测前4 h先换无血清培养基培养,每孔加5 μL C CK8溶液,避光温育1~2 h,用酶标仪测450 nm下的吸光值,每个剂量重复4次,实验重复3次.1.2.5Annexin VFITC/PI法检测细胞凋亡不同浓度乳苣水提取物处理细胞48 h后收集到的细胞,取200 μL loading buffer悬浮细胞,2.5 μL FITC染色,室温避光处理15 min,再用5 μL PI染色,冰浴避光处理5 min后上流式细胞仪检测,用Summit 5.2 获取数据并分析.1.2.6细胞膜电位的检测不同浓度乳苣水提取物处理细胞48 h后收集到的细胞,加入Jc1染色工作液后,避光温育20 min.染色完成后,用1 mL Jc1(5X)与4 mL超纯水比例配制的Jc1(1X)洗涤缓冲液(冰浴)洗涤2遍后流式细胞仪检测,用试剂盒提供的CCCP作为诱导线粒体膜电位下降的阳性对照.用FL1(FITC)和FL2(RPETR)通道接收信号.1.2.7统计学处理所得数据采用GraphPad Prism5软件进行统计学分析,以P2结果2.1乳苣水提取物对H1299和A549细胞形态的影响如图1所示,不同浓度乳苣水提取物分别作用H1299和A549细胞48 h后,光镜下直接可见未给药对照组细胞贴壁状况良好,细胞透明,折光性好;浓度达到1.2 mg/mL的处理组密度明显降低,细胞贴壁能力减弱,细胞皱缩,出现了明显的凋亡形态学特征.2.4乳苣水提取物处理H1299和A549细胞后细胞膜电位下降线粒体膜电位的下降是细胞凋亡的一个标志性事件[9].在线粒体膜电位较高时,探针JC1聚集在线粒体的基质中,形成聚合物,产生红色荧光;在线粒体膜电位较低时,JC1不能聚集在线粒体的基质中,此时JC1为单体,产生绿色荧光.因此可通过荧光颜色的转变来检测线粒体膜电位的变化.如图5所示活细胞线粒体膜电位高,线粒体内JC1聚合物的浓度高,红色荧光很强,在流式图上表现为双阳性,而凋亡细胞则大多为FITC单阳性.用带红色荧光信号的细胞比例的下降来表示线粒体去极化程度,乳苣水提取物处理H1299和A549 48 h后,经JC1染色,双阳性细胞随着药物浓度的增加逐渐减少,表明乳苣水提取物处理细胞后,细胞的膜电位明显下降,结合前期的实验结果表明乳苣确实能诱导肺癌细胞H1299和A549凋亡.3讨论近年我国各类癌症发病率日益上升,迫切需要找出既能抑制癌细胞增殖又对人体危害较少的药物,因此开发天然抗癌药物成为研究热点.本研究中的菊科乳苣属植物乳苣(Mulgedium tataricum (Linn.) DC.)为药食两用野生植物,我国晋西北人长期采食,以其特有的苦寒属性及所含成份,煮熟放置后长期不馊、食后具有泄热宁神、清心明目、消炎解毒作用.由于苦菜的食用方法多为沸水煮食或阴干后泡食,所以实验材料用阴干的乳苣全草的水浸泡提取物作用于癌细胞,以此探索乳苣水溶状态下对癌细胞的作用.这样的研究为开发乳苣这种食用野菜,寻找预防和治疗癌症的新药物提供理论基础.本实验观察了乳苣水提取物对体外培养的肺癌细胞H1299和A549生长、增殖、凋亡的影响.虽然低浓度剂量组(0.4 mg/mL)在细胞形态和增殖活力检测的结果中与对照组相比差异不是很明显,但是流式的检测结果表明,当浓度低至0.4 mg/mL时,乳苣水提取物作用48 h对H1299细胞有诱导凋亡的作用,但当浓度达到1.2 mg/mL时才能明显地诱导A549细胞的凋亡.本实验中同时观察了另外一种十字花科芸薹属蔬菜青菜对这两株细胞生长的影响,用同样方法处理材料,高浓度(2 mg/mL)的青菜水提取物处理肺癌细胞H1299和A549 48 h后却能很明显地促进细胞的生长(结果未显示).由于乳苣这种食用植物中同样也含有有利于细胞生长的营养成分,低浓度时促进细胞生长,但高剂量的乳苣提取物,抑癌活性物质的浓度也会增加,这些活性物质诱导了细胞的凋亡,而青菜中并不含有抑制肺癌细胞生长的活性物质,所以较高浓度下并不能诱导癌细胞凋亡,说明乳苣这种食用野菜中确实含有能抑制癌细胞生长的活性物质.所有数据表明乳苣水提取物可以明显地抑制体外培养的肺癌细胞H1299和A549的增殖,而这种抑制作用可能是通过诱导细胞凋亡实现的,药物处理后细胞膜电位的下降说明这种凋亡可能是线粒体凋亡途径介导的.后期将对乳苣水提取物诱导肺癌细胞H1299和A549凋亡的机制和对体内生长的细胞的影响做进一步研究,这对临床应用将有十分重要的指导意义.参考文献:[1]GAO Y X,JANG H Y,JIANG Z J,et al.Optimization of the extraction technology of total flavonoids from Mulgedium tataricum using response surface analysis[J].Zhong Yao Cai,2010,Apr,33(4):621-624.[2]高义霞,周向军,杨声,等.不同溶剂提取乳苣的抗氧化作用研究[J].食品工业科技,2012,1:85-87.[3]高义霞,周向军,张继,等.乳苣总黄酮的提取及抗氧化作用研究[J].资源开发与市场,2010,26(3):207-209.[4]周向军,高义霞,李娟娟,等.乳苣多酚提取工艺及抗氧化研究[J].中国酿造,2011,9(234):118-121.[5]黄河胜.黄酮类化合物药理作用研究进展[J].中国中药杂志,2000,25(10):589-591.[6]曹纬国.黄酮类化合物药理作用的研究进展[J].西北植物学,2003,23(12):2241-2247.[7]WANG X X,LIU C J,JIA Z J.Triterpenoid and Sesquiterpenes from Mulgedium tataricum[J].Planta Med,2006,72:764-767.[8]王小雄.菊科和木贼科三种药用植物化学成分及其生物活性[D].兰州:兰州大学,2006.[9]LY J D,GRUBB D R,LAWEN A.The mitochondrial membrane potential(deltapsi (m)) in apoptosis;an update[J].Apoptosis,2003,8(2):115-128.Abstract: To illustrate the effects in human lung cancer H1299 and A549 cells treated with Mulgedium tataricum (Linn.) DC.(MTDC) aqueous extracts,assays were performed.These assays included morphologic changes observed by microscope,cell proliferation vitality tested by cell growth assay using cck8 kit,the apoptosis detected by Flow Cytometry,mitochondrial membrane potential assessed with the fluorescent probe pared with the control cells,cells treated with high dose(≥1.2 mg/mL)of MTDC were shrunken and their morphological characteristics of apoptosis was obvious under microscope,cell proliferation was significantly inhibited(PKey words: Mulgedium tataricum (Linn.) DC.; lung cancer cell; apoptosis;proliferation(责任编辑:顾浩然)。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
=====================================================================Acq. Operator : Su Xiao Ying(LCMS-02) Seq. Line : 84Acq. Instrument : HY-LCMS-02 Location : P1-D-03Injection Date : 5/16/2016 4:12:32 PM Inj : 1 Inj Volume : 3.000 µl Acq. Method : D:\AGLIENT 1260\DATA\20160516\20160516 2016-05-16 08-46-09\100-1000MS+3MIN- 1.5_(0.02%FA).M Last changed : 5/16/2016 8:46:09 AM by Su Xiao Ying(LCMS-02)Analysis Method : D:\AGLIENT 1260\DATA\20160516\20160516 2016-05-16 08-46-09\100-1000MS+3MIN- 1.5_(0.02%FA).M (Sequence Method)Last changed : 5/16/2016 4:24:16 PM by Su Xiao Ying(LCMS-02) (modified after loading) M ethod Info : HY-365_5HO1RS,M,A-RP-108,210nm,23min Catalog No : HY-N0148 Batch#20442 A-RP-134 Additional Info : Peak(s) manually integrated min
0.51 1.52 2.53mAU 0
250
500
750
1000
1250
1500
1750
DAD1 B, Sig=214,4 Ref=off (D:\AGLIENT 1260\DATA\20160516\20160516 2016-05-16 08-46-09\BIZ2016-516-WJ7.D)
1.442 1.720 ===================================================================== Area Percent Report ===================================================================== Sorted By : Signal Multiplier : 1.0000Dilution : 1.0000Do not use Multiplier & Dilution Factor with ISTDs Signal 1: DAD1 B, Sig=214,4 Ref=off Peak RetTime Type Width Area Height Area # [min] [min] [mAU*s] [mAU] %----|-------|----|-------|----------|----------|--------| 1 1.442 MM 0.0559 6429.84473 1915.65759 98.6707 2 1.720 MM 0.0511 86.62060 28.23907 1.3293 Totals : 6516.46532 1943.89666 ===================================================================== *** End of Report ***
=====================================================================Acq. Operator : Su Xiao Ying(LCMS-02) Seq. Line : 84Acq. Instrument : HY-LCMS-02 Location : P1-D-03Injection Date : 5/16/2016 4:12:32 PM Inj : 1 Inj Volume : 3.000 µl Acq. Method : D:\AGLIENT 1260\DATA\20160516\20160516 2016-05-16 08-46-09\100-1000MS+3MIN- 1.5_(0.02%FA).M Last changed : 5/16/2016 8:46:09 AM by Su Xiao Ying(LCMS-02)Analysis Method : D:\AGLIENT 1260\DATA\20160516\20160516 2016-05-16 08-46-09\100-1000MS+3MIN- 1.5_(0.02%FA).M (Sequence Method)Last changed : 5/16/2016 4:23:14 PM by Su Xiao Ying(LCMS-02) (modified after loading) M ethod Info : HY-365_5HO1RS,M,A-RP-108,210nm,23min Catalog No : HY-N0148 Batch#20442 A-RP-134 Additional Info : Peak(s) manually integrated min
0.51 1.52 2.5350000
60000
70000
80000
90000
100000
110000
MSD1 TIC, MS File (D:\AGLIENT 1260\DATA\20160516\20160516 2016-05-16 08-46-09\BIZ2016-516-WJ7.D) ES-API, Pos, Scan
1.445
MS Signal: MSD1 TIC, MS File, ES-API, Pos, Scan, Frag: 50 Spectra averaged over upper half of peaks. Noise Cutoff: 1000 counts. Reportable Ion Abundance: > 10%. Retention Mol. Weight Time (MS) MS Area or Ion 1.445 290145 611.20 I 465.10 I 303.10 I 130.20 I
m/z 1002003004005006007008009000
20
40
60
80
100
*MSD1 SPC, time=1.416:1.489 of D:\AGLIENT 1260\DATA\20160516\20160516 2016-05-16 08-46-09\BIZ2016-516-WJ7.D ES-API, Max: 44534 612.2 102.2 131.2 465.1
303.1 130.2 *** End of Report ***。
