Dexmedetomidine attenuates sympathoadrenal responses to tracheal intubation and reduces the need
右美托咪啶的临床应用 (2)

林军 南方医科大学南海医院
2019.04
内容
药物作用机制 在围术期、ICU、神经外科及其他应用 临床常见不良反应及处理
前言
1999 年,一种新型高选择 性α 2肾上腺素能受体激动剂 (α 2AR)右美托咪啶 ( dexmedetomidine,DEX) 在 美国引入临床实践中。2009年6 月,该药在我国上市。DEX具有 较强的抗焦虑、镇静、镇痛效应。 由于其独特的药理学特性,许多 研究已将它广泛应用于临床领域。
抗心律失常作用 最近一项研究发现,DEX对控制先天性 心脏病围术期急性房性和交界性快速性 心律失常很有潜力。在心脏外科监护病 房中14 例出现快速性心律失常的先天 性心脏病手术患者应用DEX 后,13 例 ( 13 /14) 的心率下降或转为窦性心率, 从而改善血流动力学。由于先天性心脏 病围术期快速性心律失常的处理常常无 效、患者很难耐受且具有很大的不良反 应( 如最常用的抗心律失常药物之一胺 碘酮,在此类病例中应用,被报道有 87%的不良反应,包括死亡、低血压、 房室阻滞等) ,所以,DEX 在心律失常 的应用前景值得期待。
防治心肌缺血的作用 手术及术后应激引起交感神经刺激导 致血压上升及心跳加剧,增加心肌耗 氧量和术后心肌缺血并发症。特别是 冠心病和冠状动脉血流储备下降的患 者。欧洲围术期心肌缺血多中心研究 组首次评价了α 2AR的心脏保护作用。 心血管手术患者术前1 h 至术后48 h 使用DEX,明显减少心肌缺血性发生 率。所有缺血性事件都与心率、收缩 压显著增加( > 40%) 和持续时间在 1 ~ 5 min有关。心血管手术应用 DEX的患者病死率下降2%。
在区域麻醉中的应用 研究认为,硬膜外或蛛网膜下腔应用 DEX产生镇痛作用的机制是通过刺激脊 髓后角突触后膜的α 2AR,引起神经细胞 膜超极化的结果,可产生剂量依赖性镇 痛作用。 DEX经硬膜外给药可 明显逆转炎症性 痛觉过敏,可望 成为治疗神经性 疼痛的有效药物。
右美托咪定防治止血带引起的高血压
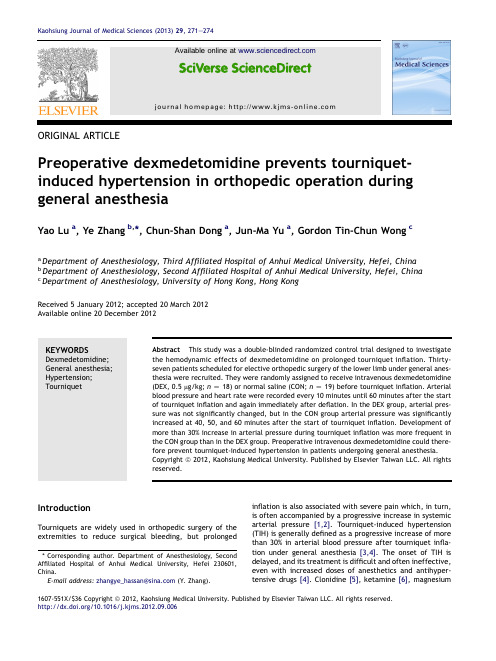
ORIGINAL ARTICLEPreoperative dexmedetomidine prevents tourniquet-induced hypertension in orthopedic operation during general anesthesiaYao Lu a ,Ye Zhang b ,*,Chun-Shan Dong a ,Jun-Ma Yu a ,Gordon Tin-Chun Wong caDepartment of Anesthesiology,Third Affiliated Hospital of Anhui Medical University,Hefei,China bDepartment of Anesthesiology,Second Affiliated Hospital of Anhui Medical University,Hefei,China cDepartment of Anesthesiology,University of Hong Kong,Hong KongReceived 5January 2012;accepted 20March 2012Available online 20December 2012KEYWORDSDexmedetomidine;General anesthesia;Hypertension;TourniquetAbstract This study was a double-blinded randomized control trial designed to investigate the hemodynamic effects of dexmedetomidine on prolonged tourniquet inflation.Thirty-seven patients scheduled for elective orthopedic surgery of the lower limb under general anes-thesia were recruited.They were randomly assigned to receive intravenous dexmedetomidine (DEX,0.5m g/kg;n Z 18)or normal saline (CON;n Z 19)before tourniquet inflation.Arterial blood pressure and heart rate were recorded every 10minutes until 60minutes after the start of tourniquet inflation and again immediately after deflation.In the DEX group,arterial pres-sure was not significantly changed,but in the CON group arterial pressure was significantly increased at 40,50,and 60minutes after the start of tourniquet inflation.Development of more than 30%increase in arterial pressure during tourniquet inflation was more frequent in the CON group than in the DEX group.Preoperative intravenous dexmedetomidine could there-fore prevent tourniquet-induced hypertension in patients undergoing general anesthesia.Copyright ª2012,Kaohsiung Medical University.Published by Elsevier Taiwan LLC.All rights reserved.IntroductionTourniquets are widely used in orthopedic surgery of the extremities to reduce surgical bleeding,but prolongedinflation is also associated with severe pain which,in turn,is often accompanied by a progressive increase in systemic arterial pressure [1,2].Tourniquet-induced hypertension (TIH)is generally defined as a progressive increase of more than 30%in arterial blood pressure after tourniquet infla-tion under general anesthesia [3,4].The onset of TIH is delayed,and its treatment is difficult and often ineffective,even with increased doses of anesthetics and antihyper-tensive drugs [4].Clonidine [5],ketamine [6],magnesium*Corresponding author.Department of Anesthesiology,Second Affiliated Hospital of Anhui Medical University,Hefei 230601,China.E-mail address:zhangye_hassan@ (Y.Zhang).1607-551X/$36Copyright ª2012,Kaohsiung Medical University.Published by Elsevier Taiwan LLC.All rights reserved./10.1016/j.kjms.2012.09.006Available online atjournal homepage:Kaohsiung Journal of Medical Sciences (2013)29,271e 274[7],and stellate ganglion block[8]have been used prophylactically to prevent TIH.Although the mechanism of TIH is unknown,the autonomic nervous system is involved [1].Dexmedetomidine is a potent a2-adrenoceptor agonist with eight times higher affinity for the a2-adrenoceptor than clonidine,and it has shown sedative,analgesic,and anxiolytic effects after intravenous administration[9].Dexmedetomi-dine attenuates hyperadrenergic responses and therefore may be of therapeutic or prophylactic value for TIH.In this study,we investigated the effect of preoperative intravenous dexmedetomidine on arterial blood pressure and heart rate in patients undergoing general anesthesia for orthopedic surgery of the lower limbs with a tourniquet. MethodsThis study was randomized,double-blinded,and placebo-controlled.After approval was obtained from the ethics committee of the Third Affiliated Hospital of Anhui Medical University and written informed consent was received from each patient,37ASA(American Society of Anesthesiologists) physical status class I and II patients scheduled for ortho-pedic operation requiring tourniquet inflation of the lower limbs under general anesthesia were enrolled.Patients with known contraindications to dexmedetomidine;who had ischemic heart disease,hypertension,kidney dysfunction,or diabetes mellitus;and with expected tourniquet inflation time shorter than60minutes were excluded.All patients received intramuscular phenobarbital (0.5mg)30minutes before induction of anesthesia. Patients in the dexmedetomidine group(DEX;n Z18) received intravenous dexmedetomidine(0.5m g/kg)diluted in10mL normal saline infused over10minutes,immedi-ately before induction of anesthesia.Patients in the control group(CON;n Z19)received the same volume of normal saline infused over the same period.The infusions were prepared by a nurse anesthetist not involved with the case according to a computer-generated sequence.Anesthesia was induced with fentanyl(2e4m g/kg)and propofol(2mg/ kg),and vecuronium(0.1mg/kg)was given intravenously to facilitate intubation of the trachea.Arterial pressure before induction of anesthesia was measured noninvasively. After anesthesia induction,a radial artery catheter was inserted for invasive blood pressure measurements.Anes-thesia was maintained with propofol(75m g/kg/min)and remifentanil(0.1m g/kg/min).Muscle paralysis was main-tained with vecuronium.Tidal volume was adjusted to keep the end-tidal CO2concentration35e45mmHg.After skin incision,the tourniquet was inflated to a pressure of 300mmHg.If systolic arterial pressure exceeded180mmHg despite increased doses of propofol and remifentanil,an additional bolus of nitroglycerol was given intravenously.Blood pressure and heart rate were recorded at0,10, 20,30,40,50,and60minutes after the start of tourniquet inflation and immediately after tourniquet deflation.The number of patients who developed TIH,as defined by an increase in arterial blood pressure greater than30%of the baseline value,was recorded.The patients were extubated at the end of surgery after reversal with atropine(0.5mg) and neostigmine(1mg).Statistical analysis was performed using the SPSS10.0 program(SPSS Inc.,Chicago,IL,USA).All data are presented as meanÆstandard deviation.Demographic data for the patients were compared among the groups using one-way analysis of variance with the post hoc Scheffe´test.The hemodynamic changes were compared with the baseline values and among the groups by using two-way repeated-measures analysis of variance with Tukey’s post hoc test.The c2test was used to compare the number of patients with TIH. Statistical differences were considered significant if the p value was less than0.05.ResultsThere were no statistically significant differences between the groups with respect to the patients’demographic characteristics,dose of propofol and remifentanil,time for duration of tourniquet inflation,and anesthesia(Table1).In the DEX group,systolic and diastolic arterial pressures were not changed during the study period,but in the CON group,systolic arterial pressure and diastolic arterial pressure were significantly increased at40,50,and60 minutes after the start of tourniquet inflation.In all patients,the heart rate did not change significantly during tourniquet inflation,but there was a significant increase immediately after deflation in the CON group(Fig.1).The CON group had a greater percentage of patients who developed TIH when compared with the DEX group. DiscussionThe results from this study showed that preoperative intravenous dexmedetomidine significantly prevented a systemic arterial pressure increase during prolonged tourniquet inflation in patients under general anesthesia.Perioperative hypertension may be associated with serious cardiac complications[10e12].Furthermore,the level of hypertension is correlated with the occurrence of postoperative silent myocardial ischemia[12].The intra-operative hypertension induced by prolonged tourniquet inflation of the lower limbs is often unresponsive to increased doses of anesthetics and antihypertensive drugs272Y.Lu et al.[4].Once tourniquet-induced arterial pressure increase develops,it is often difficult to control.In these patients,intravenous dexmedetomidine before tourniquet inflation may have a role in attenuating these blood pressure increases.Tetzlaff et al.[1]showed that tourniquet-induced arterial pressure increases correlate with the activation of the sympathetic nervous system,as measured by power spectral heart rate analysis.An increase in plasma norepinephrine levels was related to the tourniquet-induced arterial pressure increase under general anes-thesia [13].Catecholamine release after the activation of the sympathetic nervous system may contribute to the increase in systemic arterial pressure during prolonged tourniquet inflation.Dexmedetomidine is an a 2-receptor agonist with both sedative and analgesic properties that reduce the sedation,anxiolytic,and analgesic require-ments in the perioperative setting [9].Dexmedetomidine improves hemodynamic stability in the perioperative period by exerting sympatholytic effects via activation of the inhibitory a 2-receptors both in the central nervous system and on peripheral sympathetic nerve endings [14,15],and reduces plasma epinephrine and norepinephrine levels [16,17].Dexmedetomidine has been reported to be useful in attenuating hemodynamic stress secondary to hyper-adrenergic over-reactivity [18].In awake patients,the addition of dexmedetomidine to the local anesthetic solution in intravenous regional anes-thesia decreases tourniquet pain [19].Preoperative intra-venous clonidine blunts both the increase in sympathetic out flow and arterial hypertension associated with tourni-quet inflation under general anesthesia [5].In this study,we have shown that preoperative intravenous dexmedeto-midine could also prevent TIH.The use of dexmedetomi-dine may have added benefits such as attenuating the cardiovascular and sympathoadrenal response to intubation and extubation [20]and reducing opioid requirements during and after surgery [20,21].Dexmedetomidine decreases the incidence and frequency of delirium in chil-dren [22]and adults [23].There are several limitations in this study.First,we did not perform a dose e response study having only used one dose.Future studies could evaluate whether smaller doses can achieve the same benefit or whether larger doses can reduce TIH to a greater extent.Second,the effect of dexmedetomidine on the relationship between tourniquet-induced pain and hypertension was not evaluated,because this study was performed in patients receiving general stly,the depth of anesthesia might have been different in the two groups as we did not use any depth of anesthesia monitoring However,there were no significant differences in propofol and remifentanil consumption during the study period and arterial pressure before tour-niquet inflation between the groups.In conclusion,preoperative intravenous dexmedetomi-dine significantly prevents hemodynamic responses to pro-longed tourniquet inflation of the lower limbs under general anesthesia in patients.On the basis of the results of this study,further investigations are needed to show whether perioperative outcome in patients with arterial hyperten-sion or cardiovascular disease is improved by dexmedeto-midine treatment.References[1]Tetzlaff JE,O’Hara J,Yoon HJ,Schubert A.Tourniquet-induced hypertension correlates with autonomic nervous system changes detected by power spectral heart rate anal-ysis.J Clin Anesth 1997;9:138e 42.[2]Hagenouw RR,Bridenbaugh PO,van Egmond J,Stuebing R.Tourniquet pain:a volunteer study.Anesth Analg 1986;65:1175e80.Figure 1.Hemodynamic changes in the two groups.Values are presented as mean ÆSD.After Z immediately after tourniquet deflation;Baseline Z immediately before anes-thesia induction;Before Z after anesthesia induction and immediately before tourniquet inflation;CON Z group receiving normal saline;DBP Z diastolic blood pressure;DEX Z dexmedetomidine;HR Z heart rate;SBP Z systolic blood pressure.Dexmedetomidine prevents hypertension 273[3]Valli H,Rosenberg PH,Kytta J,Nurminen M.Arterial hyper-tension associated with the use of a tourniquet with either general or regional anaesthesia.Acta Anaesthesiol Scand 1987;31:279e83.[4]Valli H,Rosenberg PH.Effects of three anaesthesia methodson haemodynamic responses connected with the use of thigh tourniquet in orthopaedic patients.Acta Anaesthesiol Scand 1985;29:142e7.[5]Zalunardo MP,Serafino D,Szelloe P,Weisser F,Zollinger A,Seifert B,et al.Preoperative clonidine blunts hyperadrenergic and hyperdynamic responses to prolonged tourniquet pressure during general anesthesia.Anesth Analg2002;94:615e8. [6]Satsumae T,Yamaguchi H,Sakaguchi M,Yasunaga T,Yamashita S,Yamamoto S,et al.Preoperative small-dose ketamine prevented tourniquet-induced arterial pressure increase in orthopedic patients under general anesthesia.Anesth Analg2001;92:1286e9.[7]Lee DH,Jee DL,Kim SY,Kim JM,Lee HM.Magnesium sulphateattenuates tourniquet-induced hypertension and spinal c-fos mRNA expression:a comparison with ketamine.J Int Med Res 2006;34:573e84.[8]Arai YC,Ogata J,Matsumoto Y,Yonemura H,Kido K,Uchida T,et al.Preoperative stellate ganglion blockade prevents tourniquet-induced hypertension during general anesthesia.Acta Anaesthesiol Scand2004;48:613e8.[9]Bhana N,Goa KL,McClellan KJ.Dexmedetomidine.Drugs2000;59:263e8.[10]Seltzer JL,Gerson JI,Grogono AW.Hypertension in peri-operative period.N Y State J Med1980;80:29e31.[11]Howell SJ,Hemming AE,Allman KG,Glover L,Sear JW,Foex P.Predictors of postoperative myocardial ischaemia.The role of intercurrent arterial hypertension and other cardiovascular risk factors.Anaesthesia1997;52:107e11.[12]Forrest JB,Rehder K,Cahalan MK,Goldsmith CH.Multicenterstudy of general anesthesia:III.Predictors of severe peri-operative adverse outcomes.Anesthesiology1992;76:3e15.[13]Crews JC,Sehlhorst CS.Response to maintenance of tourni-quet inflation in a primate model.Reg Anesth1991;16:195e8.[14]Schmelling W,Farber N.The effects of alpha-2adrenergicagonists on cardiovascular system.Semin Cardiothor Vasc Anesth1997;1:1e20.[15]Lawrence C,DeLange S.Effects of a single pre-operativedexmedetomidine dose on isoflurane requirements and peri-operative haemodynamic stability.Anaesthesia1997;52: 736e44.[16]Ebert TJ,Hall JE,Barney JA,Uhrich TD,Colinco MD.Theeffects of increasing plasma concentrations of dexmedeto-midine in humans.Anesthesiology2000;93:382e94.[17]Talke P,Chen R,Thomas B,Aggarwall A,Gottlieb A,Thorberg P,et al.The hemodynamic and adrenergic effects of perioperative dexmedetomidine infusion after vascular surgery.Anesth Analg2000;90:834e9.[18]Kulkarni A,Price G,Saxena M,Skowronski G.Difficult extu-bation:calming the sympathetic storm.Anaesth Intensive Care2004;32:413e6.[19]Ramadhyani U,Park JL,Carollo DS,Waterman RS,Nossaman BD.Dexmedetomidine:clinical application as an adjunct for intravenous regional anesthesia.Anesthesiol Clin 2010;28:709e22.[20]Scheinin B,Lindgren L,Randell T,Scheinin H,Scheinin M.Dexmedetomidine attenuates sympathoadrenal responses to tracheal intubation and reduces the need for thiopentone and peroperative fentanyl.Br J Anesthesia1992;68:126e31. [21]Lin TF,Yeh YC,Lin FS,Wang YP,Lin CJ,Sun WZ,et al.Effect ofcombining dexmedetomidine and morphine for intravenous patient-controlled analgesia.Br J Anesthesia2009;102:117e22.[22]Shukry M,Clyde MC,Kalarickal PL,Ramadhyani U.Does dex-medetomidine prevent emergence delirium in children after sevoflurane-based general anesthesia?Pediatr Anaesth2005;15:1098e104.[23]Shehabi Y,Grant P,Wolfenden H,Hammond N,Bass F,Campbell M,et al.Prevalence of delirium with dexmedeto-midine compared with morphine based therapy after cardiac surgery:a randomized controlled trial(DEXmedetomidine COmpared to Morphine-DEXCOM Study).Br J Anesthesia2009;111:1075e84.274Y.Lu et al.。
Does dexmedetomidine prevent emergence delirium 是否美托咪啶防止儿童出现谵妄七氟醚后的全身麻醉

Pediatric Anesthesia200515:1098–1104doi:10.1111/j.1460-9592.2005.01660.x Does dexmedetomidine prevent emergence deliriumin children after sevoflurane-based general anesthesia?MOHANAD SHUKRY M D*,MATHISON C.CLYDE B S†,PHILIP L.KALARICKAL M D†AND USHARAMADHYANI M B B S†*Department of Anesthesiology,Tulane University,New Orleans,LA,USASummaryBackground:Emergence agitation or delirium(ED)is a frequentphenomenon in children recovering from general anesthesia(GA).Dexmedetomidine,an alpha2receptor agonist,has analgesic andsedative properties that might be helpful in the management of ED.We studied the effects of a continuous perioperative infusion of0.2l gÆkg)1Æh)1dexmedetomidine on the incidence of ED in50children aged1–10years scheduled for sevoflurane-based GA.Methods:Following inhalation induction of GA,the children wererandomly assigned into dexmedetomidine or placebo Groups D and S,respectively.The infusion of0.2l gÆkg)1Æh)1dexmedetomidine orequal volume of saline was started after securing the airway.Depth ofanesthesia was maintained by adjusting the concentration of sevo-flurane to achieve a Bispectral Index Score of40–60.Intraoperativehemodynamics were recorded every5min and the trachea wasextubated at the end of the procedure.Perioperative pain manage-ment was determined by the blinded anesthesia team,and the studydrug infusion was maintained for15min following the postanesthesiacare unit(PACU)admission.ED and pain scores were evaluated by ablinded observer.Results:The incidence of ED was statistically significantly differentbetween the two groups,26%in Group D Vs60.8%in Group S(P¼0.036).Additionally,the number of episodes of ED was lower inGroup D(P<0.017).Pain scores and the times to extubate anddischarge from PACU were the same.Conclusions:The perioperative infusion of0.2l gÆkg)1Æh)1dexmede-tomidine decreases the incidence and frequency of ED in childrenafter sevoflurane-based GA without prolonging the time to extubate ordischarge.Keywords:dexmedetomidine;emergence delerium;sevoflurane Correspondence to:Dr Mohanad Shukry,Department of Anesthesiology,Oklahoma University,Children’s Hospital of Oklahoma,940NE 13th St.,4B4200,Oklahoma City,OK73104,USA(email:mohanad-shukry@).1098Ó2005Blackwell Publishing LtdIntroductionEmergence agitation or delirium(ED)is a frequent phenomenon in children recovering from general anesthesia(GA)that can create a challenging situ-ation to the health care providers(1,2).ED has been studied in several clinical trials and different med-ications have been suggested for either prophylaxis or treatment with varying degrees of success(3–7). Dexmedetomidine,an alpha2receptor agonist,has been described to reduce ED when administered as a single dose intraoperatively(7).This double-blind randomized prospective study was conducted to test the hypothesis that continuous perioperative infusion of0.2l gÆkg)1Æh)1dexmede-tomidine reduces the incidence and frequency of ED in children recovering from sevoflurane-based GA. MethodologyFollowing Internal Review Board approval and parental informed consent,50ASA physical status I-II children(1–10years old)who were scheduled to undergo GA for elective outpatient surgical procedures were enrolled.Exclusion criteria inclu-ded lack of consent,known adverse effects to dexmedetomidine,mental retardation,developmen-tal delay,or neurological or psychiatric illness that may associate with agitation(cerebral palsy,sei-zure,etc).In the preoperative holding area,the children were enrolled in a preoperative preparation pro-gram conducted by a child-care specialist.In this program,the children were introduced to informa-tion about GA,surgery,and the operating room (OR).This information included pictures of the staff and the OR,facemask,blood pressure cuff,etc.The children were then transported to the OR accom-panied by the child-care specialist,and GA was induced with8%sevoflurane in oxygen and spon-taneous ventilation.An intravenous(i.v.)catheter was inserted,and the airway was secured with a tracheal tube(76%)or laryngeal mask airway(24%). At T0(5min following securing the airway),the children were randomized by a computer-generated program to receive either dexmedetomidine(Group D)or placebo(Group S).The study drug,either dexmedetomidine in a concentration of4l gÆml)1or normal saline(placebo),was infused at a dose that was determined in a pilot study(0.2l gÆkg)1Æh)1). GA was maintained with sevoflurane by adjusting its concentration to achieve a Bispectral Index Score (BIS)of40–60.Intraoperative opioid requirements (fentanyl)were decided by the blinded anesthesia team(an anesthesiologist with a resident or a certified registered nurse anesthetist)as they found appropriate.At the end of the procedure,sevoflurane admin-istration was discontinued while the study drug infusion was continued at the same rate of infusion. The trachea was extubated and the patient was transferred to the postanesthesia care unit(PACU). The study drug infusion was discontinued15min following the PACU admission.As per the PACU protocol,parents were allowed in the PACU,and pain and ED were treated with i.v.morphine (0.1mgÆkg)1up to0.3mgÆkg)1).Additionally,i.v. midazolam(0.1mgÆkg)1)was used as a rescue medicine for severe ED(score of four that will not respond to morphine).Children were discharged from the PACU when they were calm,had no complaints of pain or nausea,and had an Aldrete score of9–10.Children’s cooperation during the inhalation induction of GA was rated on a four-point scale (Table1)(8).For purpose of analysis,scores one to two were considered satisfactory,and scores three to four were considered unsatisfactory.Intraoperative-ly,heart rate(HR),mean arterial pressure(MAP), hemoglobin oxygen saturation(SpO2),BIS,and endtidal sevoflurane concentration(P E sevo)were documented at the time of securing the airway, starting study drug infusion(T0),T30(T0+30min), T60(T0+60min),then every hour,and at extubation. In the PACU,HR,MAP,SpO2,objective pain scale (OPS)and ED score were documented upon P0 (admission),P5(P0+5min),P15(P0+15min),and then every15min.The OPS described by Hannallah et al.(9,10)(Table2)was used to assess pain.ED Table1Cooperation on Induction ScaleScore Description1Excellent(unafraid,cooperative,accepts mask readily) 2Good(slight fear of mask,easily calmed)3Fair(moderate fear,not calmed with reassurance)4Poor(terrified,crying,agitated)DEXMEDETOMIDINE AND EMERGENCE DELIRIUM1099Ó2005Blackwell Publishing Ltd,Pediatric Anesthesia,15,1098–1104was assessed continuously and rated on the scale described by Watcha et al.(11)(0¼child is asleep. 1¼calm.2¼crying,but can be consoled.3¼cry-ing and cannot be consoled.4¼agitated and thrashing around).Children with scores three or four were considered to have an ED episode,and the frequency of episodes was documented where3min of continuous ED were considered an episode(12);if the time between two episodes(scores three or four) was less than1min,it was considered to be the same episode.ED treatments and medication doses were recorded along with duration of anesthesia and surgery,time to see family,time to extubate,and time for readiness to be discharged from PACU.A single blinded investigator(MS)performed the documentation and assessment,but a different clinician prepared syringes with the study drug. Statistical analysisThe primary outcome of this study was the inci-dence of postsevoflurane ED in the PACU.The sample size was designed to evaluate the difference in the primary outcome between the two groups. Murray et al.(5)reported the incidence of postsevo-flurane ED in children to be48%.A sample size of 22subjects per group was calculated to have at least 80%power to detect a60%reduction in the incidence of ED.All data analysis was carried out according to a preestablished analysis plan.For purpose of analysis,grades zero,one,and two on the ED scale were considered‘no ED’while grades three and four were considered‘presence of ED’.The incidence of ED between the two groups was compared using a two-side Fisher’s exact test.A two-tail unpaired t-test was performed to compare mean values of quantitative data.A P-value of0.05 was used for statistical significance.ResultsAge-eligible children were recruited at Tulane Uni-versity Health Sciences Center from January to June of2004.Eighty-eight children and their familiesTable2Hannallah et al.’s Objective Pain Scale(OPS)Observation Criteria Points Blood pressure±10%of preoperative value0>20%of preoperative value1>30%of preoperative value2 Crying Not crying0Crying,but stops withtender,loving care1Crying without stopping,does not respond totender,loving care2Movement None0Restless1Thrashing around2 Agitation Asleep or calm0Mild agitation1Hysterical2 Verbalization of pain Asleep or states no pain0States there is pain butcannot localize1 Can localize pain2Table3General dataVariable Group D Group S P-value Number of patients2323NS Age(month)59±25.448±33.4NS Weight(kg)21±10.318±10.6NS Gender(male/female)16/713/10NS ASA I(%)/ASA II(%)87/1386/14NS Race(AA/C/other)11/11/112/10/1NS History of previoussurgery(%)60.852.1NSDuration of Anesthesia(min)97±47.757±26.50.001Duration of Surgery(min)66±39.933±19.10.0007 Time to extubation(min)10.3±4.410.4±4.9NSTime to see family(min)10.6±12.69.5±13.6NS Time to discharge fromPACU(min)49.9±17.644.9±14.2NSFentnyl in OR(l gÆkg)1Æh)1)1.4±0.7 1.3±1.1NS Morphine in PACU(mgÆkg)1)0.06±0.080.11±0.19NSSaturation on arrival toPACU(%)97.7±2.797.4±3.9NS Cooperation score atinduction1.5±0.7 1.9±1.1NS Type of surgeryOtorhinolaryngological(%)30.430.4NSOphthalmological(%)8.626NS Orthopedic(%)30.4 4.3NS General surgery(%)266NS Endoscopy(%) 4.313NS ASA,American Society of Anesthesiology class;AA,African American;C,Caucasian.1100M.SHUKRY ET AL.Ó2005Blackwell Publishing Ltd,Pediatric Anesthesia,15,1098–1104were approached;50agreed to participate andcompleted the study.However,four children hadto be excluded from the statistical analysis;child #8(D)inadvertently received an overdose of 5l g Ækg )1fentanyl;child #12(S),the i.v.access was dislodged afew minutes after starting the infusion;child #18(S)was later discovered to be known by the PACUnurses for his previous history of severe ED;child#22(D)was later found to have a previous history ofseizures.All anesthetics were performed by a groupof 11-blinded anesthesiologists.There were no significant differences between thetwo groups regarding general data (Table 3).How-ever,duration of surgery and anesthesia were longerin Group D.Hemodynamic instability did not occurin any of the patients and vital signs remainedwithin 20%of baseline in all patients.Additionally,HR,MAP,SpO 2,endtidal sevo,and BIS were thesame in both groups (Figures 1and 2).The incidence of ED was 26%in Group D and60.8%in Group S (P ¼0.036),with a relative riskof 0.42and 95%confidence interval of 0.2–0.9(Table 4).The number of ED episodes was statis-tically significantly less in Group D (12episodes inGroups D Vs 36episodes in Group S,P ¼0.017).ED scores were highest on admission to PACU(P0)in Group S,while they were highest in P30(15min after discontinuing the infusion)in GroupD (Figure 3).None of the children included in theanalysis received midazolam as a rescue medicine.OPS score,time to extubation,opioid require-ments,and PACU discharge time were the same(Table 3).Discussion The results of this study show that continuous perioperative infusion of 0.2l g Ækg )1Æh )1dexmede-tomidine reduces the incidence of postsevoflurane ED in children and decreases the frequencies of ED episodes.Table 4ED and OPS in PACU Variable Group D Group S P -value Patients assessed to be agitated (%)2660.80.036Number of episodes of agitation 0.5±1.1 1.6±1.70.017OPS in PACU 2(0–8)3(1–7)Valuesare mean ±SD ,(%),or median (range).ED,emergence delirium;OPS,objective pain score;PACU,postanesthesia care unit.DEXMEDETOMIDINE AND EMERGENCE DELIRIUM 1101Ó2005Blackwell Publishing Ltd,Pediatric Anesthesia ,15,1098–1104Although there is no clinical evidence that ED has any impact on the patient’s long-term outcome,it creates a challenging situation to the postanesthetic care providers.Moreover,it may lead to higher complication rates:increased bleeding from opera-tive sites,pulling out a surgical drain or i.v.catheter, pulling of surgical dressings,unhappy parents,and disturbances to the other recovering patients(13). Sevoflurane-based GA,in addition to many other factors,has been identified to contribute to ED with an incidence as high as67%(1–3,13,14).On the other hand,several modalities have been shown to improve the quality of ED,such as premedication with oral midazolam,intraoperative administration of i.v.ketorolac,i.v.clonidine,intranasal fentanyl, and i.v.dexmedetomidine(3,6,7,15,16). Dexmedetomidine is the pharmacologically active dextro-isomer of the imidazole compound,medetom-idine.It is a relatively selective alpha2-adrenergic agonist,possessing a differential specificity for the alpha2:alpha1receptors of1620:1compared with 220:1for clonidine(17).As dexmedetomidine has sedative,analgesic,and anxiolytic effects(18),its intraoperative administration reduces anesthetic requirements,speeds postoperative recovery,and blunts the sympathetic nervous system response to surgical stimulation(19).Although dexmedetomi-dine is currently approved by the FDA for short-term (24h)sedation of adults,new reports demonstrate its potential role in pediatric patients in the intensive care unit(ICU)and during GA(20,21).The advantages of dexmedetomidine in prevent-ing ED were anticipated as another alpha agonist, clonidine,has been shown to decrease the incidence of ED.Intraoperative administration of3l gÆkg)1i.v. clonidine reduced the incidence of postsevoflurane ED from39%in the control group to5%in the clonidine group with no delay infitness to discharge (22).Moreover,intraoperative administration of 2l gÆkg)1i.v.clonidine decreased the incidence of ED from80%in the control group to10%in the clonidine group(6).Dexmedetomidine reduces nor-epinephrine release and sympathetic activity,which could explain its role in achieving superior sedation and preventing ED(23).In a double-blind prospect-ive controlled study on children recovering from GA with sevoflurane and caudal block after undergoing superficial lower abdominal and genital surgery, intraoperative single i.v.dose of dexmedetomidine reduced the incidence of ED from37%in the control group(saline)to17and10%in the groups receiving 0.15l gÆkg)1and0.3l gÆkg)1,respectively(7). Although hemodynamic instability was not reported when dexmeditomdine was used as a single i.v. dose,the pharmacokinetics and pharmacodynamics of this method of administration have not been studied in adults or children.As rapid administra-tion of dexmedetomidine may cause bradycardia and hypertension,it is recommended to administer the loading dose of the drug(1l gÆkg)1)over10min. We determined this infusion dose(0.2l gÆkg)1Æh)1)in a pilot study where we perioperatively infused dexmedetomidine in different dosages and clinically observed the emergence characteristic of the children.We experienced moderate hypotension when the recommended1l gÆkg)1loading dose was administered to the eu/hypovolemic anesthetized children;afinding observed by Tobias and Berk-enbosch(20).Additionally,a prolongation of time to extubation without additional benefits in preventing ED was another concern with the reported loading dose.With the0.2l gÆkg)1Æh)1infusion rate,the hemodynamics(BP,HR.etc)were the same between both groups and no instability was observed during any time of the study.This low infusion dose could be the reason that anesthetic requirements(defined as the P E sevo titrated to BIS)were the same between the two groups.However,more studies are needed to determine both the efficacy and safety of dex-medetomidine in different dosing methods in chil-dren.Studies of ED are especially difficult to conduct and compare for many reasons.First,the variety of ED assessment tools and scales(24).Secondly,the different definitions about what behavior constitutes disturbance during a specific period of time.Thirdly, the difficulties in interpreting behavior in nonverbal populations with other influencing factors such as pain,hunger,or fear of strangers.Fourthly,the disagreeing opinions about the point at which a ‘normal’emergence transcends into the spectrum of ‘abnormal’.These observational differences results in a wide range of estimating the incidence of ED in children recovering from GA from as low as25%to as high as80%(1,16);the incidence of ED we experienced in Group S(60.8%)falls within this range.Sikich and Lerman(24)tried to solve part of the problem by developing a reliable and valid1102M.SHUKRY ET AL.Ó2005Blackwell Publishing Ltd,Pediatric Anesthesia,15,1098–1104rating scale that can be used to assess ED.This scale was developed at the same time we were conducting our study and we were unable to use it.However, the scale we used includes a measurement of several factors that need additional nursing personnel,and was used in different studies on ED which makes our outcome more comparable with their results(12, 16,25).A single-blinded observer performed all scoring in our study,which eliminates the issues of interpreter variability.Additionally,BIS monitor was used to standardize the dose of sevoflurane administered in both groups;BIS monitoring during sevoflurane anesthesia has been validated in pedi-atric patients(26).One important factor we meas-ured,which was not considered in most other studies on ED,is the frequency of agitation in the PACU.From our experience,a child who is agitated for a few minutes requires less‘one-on-one’atten-tion than a child who is agitated for several minutes or intermittently.Only50%of the agitated children in Group D had more than one episode of ED, compared with85%in Group S(Figure4).This is a significant reduction in the number of episodes. This study may be criticized for the possible effects of pain on the assessment of ED.Although pain assessment in children in the preverbal stage of development is difficult,the method we used[Nor-den et al.(10,27)OPS]is well accepted and has been validated in other studies.Norden et al.’s(10)OPS has been demonstrated to have interobserver reliab-ility in infants and children,and also to have concurrent validity with the Children’s Hospital of Eastern Ontario Pain Scale(CHEOPS),a behavioral pain scale with documented validity and reliability. Pain scores were the same in both groups,which suggest that the difference in the incidence of ED is unlikely to be related to pain.More interestingly,the amount of opioids administered were the same in both groups in spite of the analgesic effects of dexmedetomidine.However,the small infusion dose of dexmedetomidine without a loading dose could be the explanation.An objective measure of postoper-ative sedation was not used in the study which could have affected the blinding in the PACU.Another criticism of the study is the longer duration of anesthesia time in Group D,and the higher number of children who underwent ophthalmology proce-dures in Group S.Length of anesthesia or surgery is not a risk factor for ED;however ophthalmology procedure is a risk factor(13).Only one out of thefive children who underwent an ophthalmology proce-dure in Group S experienced ED,which indicates that ophthalmology procedure did not affect our results. Furthermore,Group D had more orthopedic proce-dures,which in our clinical experience could cause more discomfort in the PACU.ED in children is a problem for the anesthesiologist, the patient,and the family.Moreover,it is a problem for the nursing staff in the PACU.Perioperative infusion of0.2l gÆkg)1Æh)1dexmedetomidine decrea-ses the incidence and frequency of ED,which gives the PACU nurses the time to apply the monitors and measure vital signs while the child is quiet or sedated. Furthermore,it gives the family the time to speak to the surgeon and report to the PACU before their child wakes up in the presence of strangers. AcknowledgementsThe authors thank the residents,CRNAs,anesthesi-ologists(Department of Anesthesiology,Tulane Uni-versity),and PACU nurses(Tulane University Hospital)for their assistance in conducting this study; Bobby D.Nossaman(Associate Professor,Anesthes-iology,Tulane University)for reviewing the manu-script;Jessica McNulty(Child-Care Specialist,Tulane University)for her assistance in the preoperative program.References1Cravero J,Surgenor S.Emergence agitation in paediatric patients after sevoflurane anaesthesia and no surgery:a comparison with halothane.Paediatr Anaesth2000;10:419–424.DEXMEDETOMIDINE AND EMERGENCE DELIRIUM1103Ó2005Blackwell Publishing Ltd,Pediatric Anesthesia,15,1098–11042Beskow A,Western P.Sevoflurane causes more postoperative agitation in children than does halothane.Acta Anaesthsiol Scand1999;43:536–541.3Lapin SL,Auden SM,Goldsmith LJ et al.Effects of sevoflurane anaesthesia on recovery in children:a comparison with halothane.Paediatr Anaesth1999;9:299–304.4Cohen IT,Finkel JC,Hannallah RS et al.The effect of fentanyl on the emergence characteristics after desflurane or sevoflu-rane anesthesia in children.Anesth Analg2002;94:1178–1181. 5Murray DJ,Cole JW,Shrock CD et al.Sevoflurane versus halothane:effects of oxycodone premedication on emergence behaviour in children.Paediatr Anaesth2002;12:308–312.6Kulka PJ,Bressem M,Tryba M.Clonidine prevents sevo-flurane-induced agitation in children.Anesth Analg2001;93: 335–338.7Ibacache ME,Mun˜oz HR,Brandes V et al.Single-dose dex-medetomidine reduces agitation after sevoflurane anesthesia in children.Anesth Analg2004;98:60–63.8Weldon BC,Watch MF,White PF.Oral midazolam in children: effect of time and adjunctive therapy.Anesth Analg1992;75: 51–55.9Hanallah RS,Broadman LM,Belman AB et parison of caudal and ilioinguinal/iliohypogastric nerve blocks for the control of post-orchio-pexy pain in pediatric ambulatory sur-gery.Anesthesiology1987;66:832–835.10Norden J,Hannallah R,Geston P et al.Concurrent validation of an objective pain scale for infants and children.Anesthesio-logy1991;75:A934.11Watcha MF,Ramirez-Ruiz M,White PF et al.Perioperative effects of oral ketorlac and acetaminophen in children under-going bilateral myringectomy.Can J Anaesth1992;39:649–654. 12Aono J,Ueda W,Mamiya K et al.Greater incidence of delirium during recovery from sevoflurane anesthesia in preschool boys.Anesthesiology1997;87:1298–1300.13Voepel-Lewis T,Malviya S,Tait AR.A prospective cohort study of emergence agitation in the pediatric postanesthesia care unit.Anesth Analg2003;96:1625–1630.14Lerman J,Davis PF,Welborn LG et al.Induction,recovery,and safety characteristics of sevoflurane in children undergoing ambulatory surgery:a comparison with halothane.Anesthesi-ology1996;84:1332–1340.15Davis PJ,Greenberg JA,Gendelman M et al.Recovery char-acteristics of sevoflurane and halothane in preschool-aged children undergoing bilateral myringotomy and pressure equalization tube insertion.Anesth Analg1999;88:34–38.16Galinkin JL,Fazi LM,Cuy RM et e of intranasal fentanyl in children undergoing myringotomy and tube placement during halothane and sevoflurane anesthesia.Anesthesiology 2000;93:1378–1383.17Dyck JB,Shafer SL.Dexmedetomidine pharmacokinetics and pharmacodynamics.Anaesth Pharm Rev1993;1:238–245.18Hall JE,Uhrich T,Barney JA et al.Sedative,amnestic,and analgesic properties of small dose dexmedetomidine infusions.Anesth Analg2000;90:699–705.19Aantaa R,Jaakola ML,Kallio A et al.Reduction of the mini-mum alveolar concentration of isoflurane by dexmedeto-midine.Anesthesiology1997;86:1055–1060.20Tobias JD,Berkenbosch JW.Initial experience with dexmede-tomidine in paediatric-aged patients.Paediatr Anaesth2002;12: 171–175.21Tobias JD.Sedation during mechanical ventilation in infants and children:dexmedetomidine versus midazolam.South Med J2004;97:451–455.22Bock M,Kunz P,Schreckenberger R et parison of caudal and intravenous clonidine in the prevention of agita-tion after sevoflurane in children.Br J Anaesth2002;88:790–796.23Herr DL,Sum-Ping J,England M.ICU sedation after coronary artery bypass graft surgery:dexmedetomidine-based versus propofol-based sedation regimens.J Cardiothorac Vasc Anesth 2003;7:576–584.24Sikich N,Lerman J.Development and psychometric evalua-tion of the pediatric anesthesia emergence delirium scale.Anesthesiology2004;100:1138–1145.25Finkel JC,Cohen IT,Hannallah RS et al.The effect of intranasal fentanyl on the emergence characteristics after sevoflurane anesthesia in children undergoing surgery for bilateral my-ringotomy tube placement.Anesth Analg2001;92:1164–1168. 26Denman WT,Swanson EL,Rosow D et al.Pediatric evaluation of the bispectral index(BIS)monitor and correlation of BIS with end-tidal sevoflurane concentration in infants and chil-dren.Anesth Analg2000;90:872–877.27McGrath PA.An assessment of children’s pain:a review of behavioral,physiological and direct scaling techniques.Pain 1987;31:147–176.Accepted18February20051104M.SHUKRY ET AL.Ó2005Blackwell Publishing Ltd,Pediatric Anesthesia,15,1098–1104。
右美托咪啶脑保护作用的研究进展

任研究发现在右美托咪陡脑保护作用的研究进展长和△(综述),欧册华※(审校)(洲州医学院附属医院麻醉科,四 洲州646000)中日分类号:R971.3 文献标识码:A 文童编号:1006⁃2084(2012)05⁃0721⁃03 摘要:右美托咪嚏(DEX )是α2肾上腺素受体激动剂美托咪嚏的右旋异构体,高选择性激动α2肾上腺素受体,抑制交感神经兴奋,产生镇静、镇痛和抗焦虑作用,无呼吸抑制和不于扰脑电生理活动,不良反应少。
因此,DEX 被广泛应用于临床。
研究发现,DEX 对心脏、大脑、肝脏、肾脏、肺等器官具有一定的保护作用。
关键词:右美托咪嚏;脑保护;作用机制Research Progress of Brain Protective Effect of Dexmedetomidine REN Chang⁃he ,OU Ce⁃hua.(De⁃partment of Anesthesiology ,the First Affiliated Hospital of Luzhou Medical College ,Luzhou 646000,China )Abstract :Dexmedetomidine (DEX )is a dextroisomer of the α2⁃adrenergic receptor agonist.It is a highly selective α2⁃adrenergic agonist ,inhibiting sympatholytic excitement ,producingsedation ,analgesiaia ,antianxiety function without respiratory depression or interference of the cerebral electrophysiologic activi⁃ties ,with less adverse effects.So ,it′s widely used in clinic.The studies have found that DEX has a certain protective effects for organs including heart ,brain ,livers ,kidneys ,and lungs etc..Key words :Dexmedetomidine ;Brain protection ;Mechanism 盐酸右美托咪陡(dexmedetomidine ,DEX )是一种韶型的高选择性α2肾k腺素受体激动剂,是美托咪陡的右旋异构体,选择性地与α2∶α1肾k腺素受体结合的比例为1620∶1;与α2肾k腺素受体的亲和力为可乐定的8倍[1],并巨其半衰期也较可乐定短,分布半衰期约为6min ,消除半衰期约为2h ,在药动学方面的可预测性更强。
dexmedetomidine

Dex
起始 10 分钟负荷剂量 1μ g/kg 维持 0.4 ~ 0.7μ g/(kg·min)
结果:
• • • •
Propofol 组达到镇静要求的速度显著快于 Dex 组
两组术后复苏的精神运动表现及呼吸频率无明显差异
预先使用 Dex 在复苏期能有更强的镇静效应 Dex 降低血压, 改善镇痛(减少吗啡的用量)
持续静脉注射会导致低血压和心动过缓 —— 补液、麻黄素、阿托品
呼吸系统
无明显的呼吸抑制
Dex 0.2μ
g/(kg·h)
(▲)
0.6μ g/(kg·h) (○)
Placebo
(□)
Hall JE, Uhrich TD, Barney JA, Arain SR, Ebert TJ. Anesth Analg 2000;90:699–705.
减少兴奋性神经递质(例如谷氨酸)的释放 抑制钙离子内流
Ma D, Hossain M, etal Eur J Pharmacol. 2004 Oct 11;502(1-2):87-97.
术后
镇静
镇痛
抗寒颤
抗谵妄
术后镇痛
Group M:100 ㎎ morphine Group D:100 ㎎ morphine + 500 g Dex
Moore TA, Markert JM,etal [J].J Neurosurg Anesthesiol,2007,19:38-44
神经保护作用
动物实验研究表明:
Dex具有神经保护的功能,减轻实验动物短暂性整体 或局部脑缺血后的神经损伤 调节凋亡前蛋白和抗凋亡蛋白的平衡
右美托咪定的临床应用

• 泌尿系统
– 利尿
• 肾素-血管紧张素 • 抗利尿激素 • 心房利钠肽
• 内分泌系统
– 去甲肾上腺素释放减少 – 胰岛素释放减少
– 皮质醇释放减少
– 生长激素释放增加
• 胃肠道
– 唾液腺分泌减少 – 肠道运动减弱
低剂量
•
• • •
阻断交感活性
主要作用: 低血压 降低循环中儿茶酚胺的含量 降低外周神经节神经递质的传递
a2-调节镇痛的部位
脊髓上的很多位点 脊髓α2 受体也可能参与 最重要的作用位点可能为脊髓 外周确切的机制和通路尚不清楚
皮层 丘脑
a2 激动剂
中脑 延髓
硬膜外或鞘内注射
投射神经元
脊髓 初级传入纤维
α2肾上腺素能受体激动剂—术后多模式镇痛的选择之一
术后多模式镇痛的优势
1. 联合应用作用机制不同的镇痛药物,实施多靶 点镇痛 阿片类 局麻药、β受体阻滞剂等 NSAIDs类 α2受体激动剂 2. 作用机制不同而互补,镇痛作用相加或协同
右美在局麻中的临床常用剂量和方法
局麻手术中: 补液结束后,手术开始前10-20min,给予1-1.5μg/kg/h的右美 ,静脉、泵注,达到满意镇静程度后,逐渐减量到0.3-0.5 μg/kg/h,维持到手术结束。
——中山大学附属第一医院麻醉科
局麻手术中: 患者进入手术室补液时开始给予右美,剂量从 1.5μg/kg/h逐渐减 量至0.5μg/kg/h,术中根据患者的个体差异及镇静深度调整剂量
• 研究方法:取培养的海马脑片,造成局部机械创伤,随后暴露于不同浓度 的右美托咪定。72 h后观察细胞损伤情况。
入选54例行幕上脑肿瘤切除术患者,麻醉前20min, 静脉注射 0.2 或 0.4μg/L 右美托咪定 (Dex) 或安慰剂, 直至皮肤缝合,评估各组的拔管时间
右美托咪定在焦虑、抑郁、睡眠障碍中的研究进展2023

右美托咪定在焦虑、抑郁、睡眠障碍中的研究进展2023右美托咪定(dexmedetomidine,Dex)是一种新型镇静药物,主要通过高选择性激活《2肾上腺素能受侬alpha2adrenergicreceptor,α2-AR)发挥镇静、镇痛、中枢性抗交感等作用,被广泛应用于临床麻醉和重症监护治疗病房(intensivecareunit,ICU\与其他镇静类药物不同,Dex可以通过内源性促进睡眠通路,产生类似非快速眼动睡眠(non-rapideyemovementsleep,NREM),并且在一定剂量范围内保留机体的唤醒功能。
近年来的研究发现,Dex可以快速调节焦虑、抑郁、狂躁等负面情绪,同时具有神经保护作用、成瘾性低等特征,在治疗精神障碍方面具有独特的优越性。
因此,本文将重点讨论Dex在焦虑、抑郁、睡眠障碍中的应用,探讨其在精神病学领域中的潜在价值,为临床精神障碍的治疗提供新的思路。
1DeX在焦虑中的应用焦虑是一种以恐惧、紧张、忧虑和烦躁为主的情绪反应,当其症状强烈或持续存在时,可能发展为病理性的〃焦虑状态〃或〃焦虑障碍〃,导致心率加快、头晕、头痛、肌肉紧张等躯体不适,甚至影响患者的日常功能。
病理性焦虑通常需要接受临床治疗,一线治疗主要包括选择性5混色胺(5・hydroxytryptamine,5-HT)再摄取抑制剂、5∙HT去甲肾上腺素再摄取抑制剂以及认知行为疗法。
然而,这些治疗方案的起效时间长达数周,患者往往因神经敏感性增加在治疗初期便停止治疗,导致疗效减弱。
此外,对于急性焦虑的患者,常使用苯二氮类药物进行治疗,但可能带来嗜睡、记忆受损、运动协调障碍以及成瘾性等风险。
因此,寻找一种快速起效且副作用小的抗焦虑治疗方案具有重要的临床意义。
1.1DeX抗焦虑的机制目前,焦虑产生的确切机制尚未明确,有研究认为蓝斑去甲肾上腺素能神经系统(locuscoeruleus-noradrenergicsystem,LC-NE)的过度激活是急性应激引发焦虑的关键因素,而选择性抑制LCNE神经元活动可以预防焦虑的产生。
右美托咪定对肿瘤作用的研究进展

第30卷 第15期 中国现代医学杂志 Vol. 30 No.15 2020年 8月 China Journal of Modern Medicine Aug. 2020收稿日期:2019-06-14[通信作者] 邵东华,E-mail :138****************;Tel :138****1211DOI: 10.3969/j.issn.1005-8982.2020.15.009文章编号: 1005-8982(2020)15-0050-05右美托咪定对肿瘤作用的研究进展刘晓田,郑永锋,马晓冬,谢云斌,程应湘,邵东华(江苏大学附属人民医院 麻醉科,江苏 镇江 212002)摘要: 手术是目前治疗多数实体肿瘤的首选方法,围手术期麻醉药的使用对肿瘤发展及预后产生极大的影响。
右美托咪定是目前临床常用的麻醉药物之一,广泛应用于全身麻醉和区域阻滞。
研究发现,右美托咪定在体内外可促进乳腺癌、肺癌和胃肠道肿瘤的进展,也有数据提示右美托咪定可抑制卵巢癌、骨肉瘤的生长。
探究右美托咪定对肿瘤的作用可以指导临床用药,规避临床风险。
该文对右美托咪定对肿瘤的影响及机制作一综述。
关键词: 右美托咪定;肿瘤;α2肾上腺素受体;免疫中图分类号: R614文献标识码: AResearch progress of dexmedetomidine on cancerXiao-tian Liu, Yong-feng Zheng, Xiao-dong Ma, Yun-bin Xie, Ying-xiang Cheng, Dong-hua Shao(Department of Anesthesiology, Affiliated People’s Hospital of Jiangsu University,Zhenjiang, Jiangsu 212002, China)Abstract: Surgery is currently the preferred treatment for most solid tumors. Many factors in the perioperative period, such as the use of anesthetics, would have a great impact on the development and prognosis of tumor. Dexmedetomidine is one of the most common anesthetics used in clinical general anesthesia and regional block. Studies found that dexmedetomidine might promote the progression of breast, lung and gastrointestinal cancer in vitro and in vivo, yet data also suggested that dexmedetomidine could inhibit the growth of ovarian cancer and osteosarcoma. Exploring the effect of dexmedetomidine on cancer can better guide clinical medications and circumvent risks. This review would briefly introduce the clinical pharmacology of dexmedetomidine and summarize the recent trends concerning the effect of dexmedetomidine on tumor.Keywords: dexmedetomidine; neoplasms; receptors, adrenergic; immunity近年来,肿瘤的发病率和病死率均呈上升趋势。
右美托咪定复合瑞芬太尼和七氟醚在老年全身麻醉患者中实施对血流

3 讨论肾病综合征是由于多种疾病引起的, 它的临床表现为:大量蛋白尿、低蛋白血症、水肿、高脂血症、血尿等一种慢性疾病[4-6]。
它可以分为原发性、继发性和遗传性三大类, 其最主要的临床表现为尿病理检验中有大量的蛋白尿, 同时也是其最基本的病理生理机制。
肾病综合征对患者的影响较大, 可以引起较多的肾脏疾病和其他器官功能脏器疾病, 若不能很好地治疗, 严重时则会对患者的生命造成威胁[7]。
对肾病综合征患者给予还原型谷胱甘肽治疗, 对患者的血清蛋白水平可以显著提高, 也可以有效降低患者的三酰甘油水平[8]。
还原型谷胱甘肽是由于半氨酸、半胱氨酸与谷氨酸等组成, 能够对细胞的代谢具有较好的阻碍效果, 有较好的还原性;在人体内存在着较多的还原型谷胱甘肽, 能够激活身体的多种酶, 可以较好地维持患者细胞的生物功能, 还可以促进酸类物质进行转化, 使得自由基的排泄加快;还原型谷胱甘肽还能够对患者的炎症状态具有一定的抑制作用, 对自由基的产生有阻碍作用, 有效促进患者肾功能的恢复;对肝脏也具有一定的保护作用, 显著改善患者的临床症状[9, 10]。
综上所述, 还原型谷胱甘肽可以有效缓解患者肾病综合征的临床症状, 促进自由基的排泄, 还可以保护肾功能和肝功能, 效果明显, 值得临床推广。
参考文献[1] 孙薇, 杜娟, 郭颖, 等.还原型谷胱甘肽在肾病综合征临床治疗中应用价值分析.中国现代药物应用, 2016, 10(12):224-225.[2] 王双. 瑞替普酶联合还原型谷胱甘肽在急性ST 段抬高型心肌梗死治疗中的应用价值研究. 中国疗养医学, 2016, 25(5):524-525.[3] 张文玉, 常文秀, 韩鹦赢.肾炎康复片联合还原型谷胱甘肽治疗糖尿病肾病的疗效观察.现代药物与临床, 2016, 31(5):683-686.[4] 蒋红莲, 梁劲松, 潘家萍.还原型谷胱甘肽治疗肾病综合征的临床疗效.临床合理用药杂志, 2015, 8(24):63-64.[5] 王丽雅, 王少渠, 张鹏.还原型谷胱甘肽辅助治疗难治性肾病综合征的临床观察.社区医学杂志, 2012, 10(6):3-4.[6] 陈逸心. 还原型谷胱甘肽在肾病综合征治疗中的效果观察. 医学信息, 2015(3):316.[7] 王锋, 范亚平, 俞燕, 等. 还原型谷胱甘肽对原发性肾病综合征并发急性肾衰竭微炎症状态的影响. 山东医药, 2011, 51(49): 76-77.[8] 张成, 李彬, 张晓荣, 等. 小儿肾病综合征还原型谷胱甘肽和总抗氧化能力的变化. 中国实用医刊, 2009, 36(24):20-21.[9] 单娟萍. 还原型谷胱甘肽治疗原发性肾病综合征的临床观察.现代中西医结合杂志, 2006, 15(8):1024-1025.[10] 孟小芹, 曹绍岐, 叶华茂. 还原型谷胱甘肽治疗肾病综合征临床观察. 山东医药, 2007, 47(1):48.[收稿日期:2017-03-23]右美托咪定复合瑞芬太尼和七氟醚在老年全身麻醉患者中实施对血流动力学的影响徐建武 林毅瑜【摘要】 目的 探究在老年患者中应用右美托咪定复合瑞芬太尼和七氟醚实施全身麻醉对血流动力学的影响。
右美托咪定对免疫功能的影响及机制回顾

第30卷 第8期 中国现代医学杂志 Vol. 30 No.8 2020年4月 China Journal of Modern Medicine Apr . 2020收稿日期:2019-10-19*基金项目:甘肃省自然科学基金(No :17JR5RA262)[通信作者] 李玉兰,E-mail :*************DOI: 10.3969/j.issn.1005-8982.2020.08.010文章编号: 1005-8982(2020)08-0057-05右美托咪定对免疫功能的影响及机制回顾*李春兰,李玉兰,李霞霞,王永琦(兰州大学第一医院 麻醉科,甘肃 兰州 730000)摘要: 大多数镇静药对机体免疫功能有抑制作用,这与患者围手术期感染、肿瘤转移和复发等有关。
由于ɑ2受体激动剂右美托咪定(DEX)对免疫系统的特殊影响,其用于围手术期、脓毒症及肿瘤等具有独特的临床优势。
该文就近10年来DEX 在动物实验和临床研究中对固有免疫、特异性免疫反应的研究进展作一综述,包括对巨噬细胞、树突状细胞、NK 细胞、炎症因子、补体及抗体的影响。
关键词: 免疫;右美托咪定/处方药;炎症中图分类号: R593 文献标识码: AEffects of dexmedetomidine on immune functionand its mechanism reviews *Chun-lan Li, Yu-lan Li, Xia-xia Li, Yong-qi Wang(Department of Anesthesiology, The First Hospital of Lanzhou University,Lanzhou, Gansu 730000, China)Abstract: Most sedatives inhibit the function of immune system of the body, and it is closely related to the perioperative infections, tumor metastasis and recurrence. Dexmedetomidine (Dex), as a ɑ2 receptor agonist, due to its effects on the immune system, it has unique clinical advantages in perioperative, sepsis, tumor and other patients. This article reviews the research progress of Dex on the innate immunity and specific immune response of the experimental animals and clinical researches in the past 10 years, including the effects on macrophages, dendritic cells, NK cells, inflammatory cytokines, complement and some antibodies.Keywords: immune; dexmedetomidine / prescription drugs; inflammation大多数全身麻醉药具有免疫抑制作用,如七氟醚会引起肺切除患者血浆致炎因子IL-6浓度升高、抗炎因子IL-10浓度下降[1];使用丙泊酚或依托咪酯的患者术后24 h CD4+细胞减少[2]。
依托咪酯上瘾后的症状作文

依托咪酯上瘾后的症状作文英文回答:Addiction to Methylphenidate, commonly known as Ritalin, can have a range of symptoms. Methylphenidate is a central nervous system stimulant that is primarily used to treat attention deficit hyperactivity disorder (ADHD). However, when misused or taken without a prescription, it can leadto addiction.One of the most common symptoms of methylphenidate addiction is an intense craving for the drug. Individuals may feel an overwhelming need to use the drug regularly and may find it difficult to control or stop their use. This craving can lead to a preoccupation with obtaining andusing the drug, which can interfere with daily activities and relationships.Another symptom of methylphenidate addiction is tolerance. Over time, individuals may find that they needhigher doses of the drug to achieve the desired effects. This can lead to increased use and potential health risks.Withdrawal symptoms can also occur when a person stops using methylphenidate after prolonged use. These symptoms can include fatigue, depression, irritability, anddifficulty concentrating. Withdrawal symptoms can beintense and may drive individuals to continue using thedrug to avoid these uncomfortable experiences.In addition, individuals addicted to methylphenidate may experience negative consequences in their personal and professional lives. They may neglect responsibilities, experience financial difficulties, and have strained relationships with loved ones. The drug can become the central focus of their lives, leading to a decline inoverall well-being.中文回答:对咪酯(俗称利他林)的上瘾会出现一系列症状。
抗抑郁药物治疗英语

抗抑郁药物治疗英语抗抑郁药物治疗(Antidepressant Medication Treatment)是一种用于治疗抑郁症和其他情感障碍的医疗方法。
以下是有关抗抑郁药物治疗的英语相关表达和信息:1.Antidepressant Medications: This term refers to themedications prescribed to treat depression. These medications work by altering the levels of neurotransmitters in the brain to improve mood and reduce depressive symptoms.2.Types of Antidepressants: There are several classes ofantidepressant medications, including selective serotonin reuptake inhibitors (SSRIs), serotonin-norepinephrine reuptake inhibitors (SNRIs), tricyclic antidepressants (TCAs), and monoamine oxidase inhibitors (MAOIs).3.Prescription and Dosage: Antidepressants are typicallyprescribed by a healthcare provider, such as a psychiatrist or general practitioner. The specific medication and dosage will depend on the individual's symptoms and needs.4.Side Effects: Like all medications, antidepressants may have sideeffects. Common side effects include nausea, dizziness, sleep disturbances, and changes in appetite. It's essential to discuss any side effects with your healthcare provider.5.Duration of Treatment: The duration of antidepressanttreatment varies from person to person. Some individuals may need short-term treatment, while others may require long-term use of medication to manage their depression.6.Monitoring and Follow-up: It is crucial for patients to haveregular check-ins with their healthcare provider while taking antidepressants to monitor their progress and address any concerns.bination Therapy: In some cases, a healthcare providermay recommend a combination of medication and psychotherapy (talk therapy) for the most effective treatment of depression.8.Caution and Precautions: It's essential to follow yourhealthcare provider's guidance on medication use, including taking the prescribed dose and not abruptly discontinuing the medication. Stopping medication suddenly can lead to withdrawal symptoms.9.Risk of Suicidal Thoughts: Some antidepressants may carry arisk of increasing suicidal thoughts in certain individuals, especially when first starting treatment. It's crucial to communicate any such thoughts to your healthcare provider immediately.10.E fficacy: Antidepressant medications can be highly effective inmanaging depression, but their efficacy can vary among individuals. It may take some time to find the right medication and dosage that works best for you.Remember that the decision to start, continue, or change antidepressant medication should always be made in consultation with a qualified healthcare professional. Depression is a serious medical condition, and seeking professional help is crucial for effective treatment.。
右美托咪定的临床麻醉应用进展

右美托咪定的临床麻醉应用进展杨玥1,2),陶建平1)(1)昆明医科大学第二附属医院麻醉科,云南昆明650101;2)昆明医科大学第四附属医院麻醉科,云南昆明650021)[摘要]右美托咪定(DEX )是一种高选择性的α2肾上腺素能受体激动剂,广泛的应用于各类临床手术中,主要得益于其镇痛、抗焦虑、镇静的效果明显以及降低应激反应、稳定血流动力学、不会产生呼吸抑制等独特的药理特性。
右美托咪定与其它的镇静、镇痛药物合用具有良好的协同作用,不仅能够降低麻醉药物的使用剂量,而且在很大程度上也能够降低麻醉药物所带来的不良反应的发生率。
在麻醉手术中,气管插管、拔管时使用右美托咪定,可以明显降低患者的不良反应。
就右美托咪定的临床应用情况作一综述。
[关键词]右美托咪定;麻醉;临床应用[中图分类号]R614[文献标志码]A [文章编号]2095-610X (2019)12-0135-05The Advancement of Clinical Anesthesia Application of DexmedetomidineYANG Yue 1,2),TAO Jian-ping 1)(1)Dept.of Anesthesiology ,The 2nd Affiliated Hospital of Kunming Medical University ,Kunming Yunnan 650101;2)Dept.of Anesthesiology ,The 4th Affiliated Hospital of Kunming Medical University ,KunmingYunnan 650021,China )[Abstract ]Dexmedetomidine (DEX )is a highly selective α2adrenergic receptor agonist ,it is widely usedin various clinical operations.This is mainly due to the obvious effect of analgesia ,anti-anxiety and sedation ,and unique pharmacological properties such as reducing stress response ,stabilizing hemodynamics ,and not producing respiratory depression.The combination of dexmetomide and other sedation or analgesic drugs have a well synergistic effect ,which can not only reduce the dose of anesthetic drugs ,but also greatly reduce the incidence of adverse reactions caused by anesthetics.In anesthesia surgery ,dexmedetomidine is used for intubation and extubation ,which can significantly reduce adverse reactions in patients.This article reviews the clinical application of dexmedetomidine for clinical reference.[Key words ]Dexmedetomidine (DEX);Anesthesia ;Clinical Application Journal of Kunming Medical UniversityCN 53-1221R[收稿日期]2019-10-19收稿[基金项目]国家自然科学基金资助项目(81060033)[作者简介]杨玥(1991~),女,云南迪庆人,在读硕士研究生,主要从事临床麻醉相关工作。
右美托咪啶对离体大鼠肠系膜动脉的作用

右美托咪啶对离体大鼠肠系膜动脉的作用马正敏;景桂霞;李小刚;周荣胜【摘要】Objective To investigate the dilating effect of dexmedetomidine (DEX)on isolated vascular smooth muscles and to explore the mechanism.Methods Tension of isolated mesenteric arterial rings of male Sprague-Dawley rats was recorded.The effects of DEX on the rings and the effects of DEX on vascular reaction induced by various drugs were recorded. Results DEX completely relaxed the contraction induced by phenylephrine (PE)and KCl in a concentration-dependent manner in endothelium intact mesenteric arterial rings in rats.The vasodilating effectof DEX was increased by sodium nitroprusside.In phenylephrine (10-5mol/L)based on pre-vasoconstriction,adding acetylcholine could not suppress DEX’s vasodilating effect.Vasodilation was not related to the endothelial cells.In physiological saline solution without calcium,DEX significantly inhibited the contraction induced by addition ofCaCl2 .Conclusion DEX can induce vasodilation in a concentration-dependent manner,which is not dependent on the endothelial cells.%目的:研究右美托咪啶(dexmedetomidine,DEX)对血管平滑肌旳舒张作用,并探讨其作用机制。
右美托咪定的神经保护作用及其相关机制

右美托咪定的神经保护作用及其相关机制陈丽娇;薛庆生;于布为【期刊名称】《中国药理学通报》【年(卷),期】2015(31)11【摘要】Dexmedetomidine( DEX) is a pure potent, highly se-lective and highly specific agonist ofα2-adrenergic receptors with sedative, analgesic and sympatholytic properties. The sedative effect mimics natural sleepof“arousable” and“cooperative” se-dation without respiratory depression. Due to the above properties and advantages, DEX has received adequate attention in clinical practice and its spectrum of application is also expanding. In re-cent years, it is proved that DEX is neuroprotective not only in animal researches but also in clinical studies. The neuroprotec-tion of DEX and its related mechanism will be briefly reviewed in this paper.%右美托咪定( dexmedetomidine,DEX)是一种新型的高选择性、高特异性α2-肾上腺素受体激动剂,具有镇静、镇痛和抗交感的作用。
其镇静作用模拟了自然睡眠的“可唤醒”、“合作”状态,且不伴呼吸抑制。
胃泌素瘤用药——法莫替丁

胃泌素瘤用药——法莫替丁……法莫替丁Famotidine【别名】信法丁、保维坚、保胃健、高舒达、噻唑咪胺、胃舒达、愈疡宁、卡玛特一非溃、卡玛特。
【作用与用途】本品为组织胺H2受体拮抗剂。
对胃酸分泌具有明显的抑制作用,其作用强度比西咪替丁强30多倍,比雷尼替丁强6-10倍。
健康人及消化性溃疡患者口服本品20mg对基础分泌及因给予各种刺激而引起的胃酸及胃蛋白酶分泌增加有抑制作用。
临床用于胃及十二指肠溃疡、吻合口溃疡、应激性溃疡、急性胃粘膜出血、胃泌素瘤以及反流性食道炎等。
【剂量与用法】成人口服:每次20mg,一日2次早、晚餐后,或一次40mg,临睡前服用,4-6周为一疗程。
溃疡愈后的维持量可减半。
上消化道缓慢静注或静滴20mg(溶于等渗盐水或葡萄糖注射液20ml中),1日2次。
病人能口服时,静注应改口服。
【不良反应】少数患者可有口干、头痛、头晕、失眠、便秘、腹泻、面部潮红。
罕见有腹部胀满感、食欲不振及心率增加、血压上升、月经不调、泌乳、脱发等。
过敏反应:胸闷、气急、大汗、寒战、皮疹、荨麻疹。
偶有白细胞减少、轻度转氨酶增高、等。
精神症状。
【注意事项】对本品过敏者禁用。
发生皮疹、荨麻疹应停药。
肝、肾功能不全者及婴幼儿慎用,肾功能不全者应遵医嘱调整剂量。
严重肾功能不全禁用。
孕妇、哺乳期妇女禁用。
哺乳妇女必须使用时应停止哺乳。
对小儿的安全性尚未确立。
注意应排除胃癌后才能使用本品。
【制剂】片剂:每片20mg。
散剂:10%(100mg/g)。
注射液:每支20mg(2ml)。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
出版日期 : Feb
语言 : eng
出版类型 : Clinical Trial;Journal Article;Randomized Controlled Trial
ISBN/ISSN : 0007-09120007-0912
索取号 : 1347229
摘要 : Dexmedetomidine is a relatively new, highly selective, short-acting central alpha 2 agonist. Although not yet officially introduced for clinical use in Israel, it has become increasingly popular among anesthesiologists and intensive care physicians abroad when used as an adjuvant to the classical regimen of anesthesia techniques. Its administration potentiates the effect of other sedative and hypnotic agents while causing minimal respiratory depression. It also blunts the sympathetic response--thus minimizing changes in blood pressure and heart rate--during critical moments such as laryngoscopy and intubation. However, bradycardia and hypotension may ensue. DXM minimizes opioid-induced muscle rigidity and attenuates postoperative shivering. These pro-anesthesia effects are attributed to the capability of DXM to reduce central adrenergic outflow. Although its precise mechanism(s) of action are still debatable, DXM will undoubtedly find an increasing role in clinical peri-operative anesthesia.
作者 : Scheinin B;Lindgren L;Randell T;Scheinin H;Scheinin M
作者地址 : Department of Anaesthesia, University Central Hospital, Helsinki, Finland.
刊名 : Br J Anaesth
刊名翻译 : British journal of anaesthesia
出版年 : 1992
卷 : 68
期 : 2
页码 : 126-31
出版地 : ENGLAND
索取号 : 1351736
标签 : (Print) (Linking)
文献类型 : 期刊
标题 : Dexmedetomidine: a promising agent for anesthesia and perioperative care.
作者 : Jaakola ML;Ali-Melkkila T;Kanto J;Kallio A;Scheinin H;Scheinin M
作者地址 : Department of Anaesthesiology, Turku University Hospital, Finland.
文献类型 : 期刊
标题 : Dexmedetomidine attenuates sympathoadrenal responses to trachea thiopentone and peroperative fentanyl.
刊名 : Br J Anaesth
刊名翻译 : British journal of anaesthesia
出版年 : 1992
卷 : 68
期 : 6
页码 : 570-5
出版地 : ENGLAND
摘要 : We studied the effects of a single i.v. dose of dexmedetomidine, a highly selective and specific alpha 2 adrenoceptor agonist, on intraocular pressure (IOP), haemodynamic and sympathoadrenal responses to laryngoscopy and tracheal intubation, and on anaesthetic requirements in ophthalmic surgery. Thirty ASA I-II patients undergoing cataract surgery were allocated randomly to receive either dexmedetomidine 0.6 microgram kg-1 or saline placebo i.v. 10 min before induction of anaesthesia in a double-blind design. After dexmedetomidine there was a 34% (95% confidence interval (CI) 27-43%) reduction in IOP (P less than 0.001) and 62% (CI 57-68%) decrease in plasma noradrenaline concentrations (P less than 0.001). After intubation, maximum heart rate was 18% (CI 3-33%, P = 0.036) and the maximum IOP 27% (CI 11-43%, P = 0.005) less in the dexmedetomidine group compared with the patients treated with placebo. Within 10 min after intubation, maximum systolic and diastolic arterial pressures were also significantly (P = 0.013 and P = 0.020) smaller in the dexmedetomidine group. The induction dose of thiopentone was smaller (23% (CI 20-26%) P = 0.012), and the use of isoflurane or fentanyl supplements during anaesthesia was less frequent in the dexmedetomidine group. The patients premedicated with dexmedetomidine recovered faster from anaesthesia (P = 0.042). These results suggest that dexmedetomidine may be a useful anaesthetic adjunct in ophthalmic surgery.
摘要 : The effects of the new, highly selective alpha 2-adrenergic agonist, dexmedetomidine, were studied in a randomized, placebo-controlled, double-blind trial in 24 ASA I patients. Dexmedetomidine 0.6 micrograms kg-1 or saline was given i.v. 10 min before induction of anaesthesia. The required dose of thiopentone was significantly (P less than 0.001) smaller in the dexmedetomidine group (mean 4.4 (sd 0.9) mg kg-1) than in the control group (6.9 (1.6) mg kg-1), and the drug attenuated the cardiovascular responses to laryngoscopy and tracheal intubation. The concentration of noradrenaline in mixed venous plasma was smaller in the dexmedetomidine group during all phases of induction (P less than 0.01). During surgery, fentanyl was required in a dose of 0.5 (0.6) mg kg-1 and 2.8 (2.6) mg kg-1 in the dexmedetomidine and control groups, respectively (P less than 0.001). During 2 h postoperative follow-up, oxycodone 0.06 (0.06) mg kg-1 and 0.16 (0.1) mg kg-1 (P less than 0.05) was given to the two groups respectively.
