纳米颗粒物在肾上皮细胞的转化通路
TGF-β1介导的Smad和ERK信号通路在肾纤维化中的研究进展

中国免疫学杂志2022年第38卷TGF -β1介导的Smad 和ERK 信号通路在肾纤维化中的研究进展郭帅方敬陈志强(河北中医学院,石家庄050000)中图分类号R392.11文献标志码A文章编号1000-484X (2022)06-0766-05[摘要]肾纤维化的发生发展受到生长因子、细胞因子、趋化因子等多种因素的调控。
TGF -β1是目前已知的最重要的致纤维化因子。
TGF -β1/Smad 和TGF -β1/ERK 信号通路是传导TGF -β1的主要信号通路,在肾纤维化中发挥着重要作用。
因此,本文结合最新研究成果就TGF -β1/Smad 和TGF -β1/ERK 信号通路在肾纤维化中的作用及其二者之间的相互关系进行综述。
[关键词]肾纤维化;TGF -β1/Smad 信号通路;TGF -β1/ERK 信号通路;作用机制Research progress of Smad and ERK signaling pathway mediated by TGF -β1in renal fibrosisGUO Shuai ,FANG Jing ,CHEN Zhiqiang.Hebei University of Chinese Medicine ,Shijiazhuang 050000,China[Abstract ]The occurrence and development of renal fibrosis is regulated by growth factors ,cytokines ,chemokines and otherfactors.TGF -β1is the most important fibrogenic factor at present.TGF -β1/Smad and TGF -β1/ERK signaling pathways are the main signaling pathways of TGF -β1,which play an important role in renal fibrosis.Therefore ,combined with the latest research results ,thefunction of TGF -β1/Smad and TGF -β1/ERK signaling pathway in renal fibrosis and the relationship between them was reviewed in this paper.[Key words ]Renal fibrosis ;TGF -β1/Smad signaling pathway ;TGF -β1/ERK signaling pathway ;Mechanism肾纤维化是慢性肾脏疾病的最终共同途径,以细胞外基质(extracellular matrix ,ECM )的过度沉积为主要特征,与患者的长期预后密切相关。
纳米微粒跨细胞膜转运途径及机制的研究进展

纳米微粒跨细胞膜转运途径及机制的研究进展孙宏晨;徐晓薇;张恺;史册;金晗;袁安亮【摘要】纳米材料通过有效转运药物、生物分子或显像剂到病变部位的靶细胞,实现疾病的诊断和治疗.这种应用于诊断和治疗的纳米材料,通常需要进入细胞的特定部位,将其负载物转运至亚细胞中.目前普遍认为纳米微粒主要是通过胞吞作用入胞,根据形成囊泡大小或内容成分的不同可将胞吞作用分为吞噬作用和胞饮作用.纳米微粒的尺寸、形状、化学组成、表面电荷等理化性质对其入胞途径均有影响;此外,对于同一纳米微粒,所选细胞系不同时,其入胞途径也不相同.通过研究纳米微粒与细胞间的相互作用了解其转运机制,对于提高转运效率将产生重大帮助.本综述以纳米微粒跨细胞膜转运途径为基础,着重介绍了纳米载体跨细胞膜转运的机制,包括纳米载体如何进入细胞及不同途径的特点,影响纳米材料进入细胞的因素,以及提高转运效率的方法等方面的进展.【期刊名称】《吉林大学学报(医学版)》【年(卷),期】2011(037)006【总页数】4页(P1157-1160)【关键词】纳米微粒;跨膜转运;胞吞作用;理化性质【作者】孙宏晨;徐晓薇;张恺;史册;金晗;袁安亮【作者单位】吉林大学口腔医院病理科,吉林长春130021;吉林大学口腔医院病理科,吉林长春130021;吉林大学超分子结构与材料国家重点实验室,吉林长春130012;吉林大学口腔医院病理科,吉林长春130021;吉林大学口腔医院病理科,吉林长春130021;吉林大学口腔医院病理科,吉林长春130021【正文语种】中文【中图分类】R318.08纳米技术被认为是对21世纪一系列高新技术的产生与发展有重要影响的一门热点科学。
人们期待通过将纳米技术应用于药物转运来改变药理学和生物技术的现状。
利用纳米技术,将可能实现:①改善水溶性差的药物的转运;②靶向转运药物到特定细胞或组织[1];③药物跨细胞膜转运穿过上皮细胞和血管内皮障碍;④转运大的高分子药物到细胞内的作用位点;⑤两种或多种药物/治疗方法同时转运,实现联合治疗;⑥将治疗药物与显像方法结合来观察药物转运[2];⑦对治疗药物体内效能的实时监测[3]。
TAK1通过调控p38 MAPK信号通路影响肾小管上皮细胞纤维化

TAK1通过调控p38 MAPK信号通路影响肾小管上皮细胞纤维化DING Wen-fei;SONG Lei;LIU Hai-yun;QIN Jian-hua;CAO Ling;GAO Li-chao;OU San-tao;WU Wei-hua【摘要】目的:探讨转化生长因子β激活激酶1(TAK1)对肾小管上皮细胞纤维化的影响及机制.方法:以肾小管上皮细胞HK-2作为研究对象,用转化生长因子β1(TGF-β1)诱导肾小管上皮细胞纤维化,以real-time PCR和Western blot检测细胞中TAK1表达的变化.用TAK1 shRNA慢病毒感染肾小管上皮细胞,以real-time PCR 和Western blot检测其对TGF-β1刺激下肾小管上皮细胞中TAK1表达的影响以检测干扰效果.ELISA法检测细胞分泌的I型胶原和III型胶原水平,Western blot法检测细胞中α-平滑肌肌动蛋白(α-SMA)、结缔组织生长因子(CT-GF)和磷酸化的p38 MAPK(p-p38 MAPKThr 180/Tyr 182)的蛋白水平.用p38 MAPK激活剂处理已敲减TAK1表达的肾小管上皮细胞,检测其对细胞分泌I型胶原和III型胶原的影响及对细胞中α-SMA、CTGF和p-p38 MAPKThr 180/Tyr 182蛋白水平的影响.结果:TGF-β1可以明显上调肾小管上皮细胞中TAK1的表达水平.TAK1 shRNA 可明显下调TGF-β1刺激下的肾小管上皮细胞中TAK1的表达水平.TGF-β1处理后的肾小管上皮细胞分泌I型胶原和III型胶原增多,细胞中α-SMA、CTGF和p-p38 MAPKThr 180/Tyr 182蛋白水平升高.敲减TAK1表达可以明显抑制TGF-β1诱导的肾小管上皮细胞分泌I型胶原和III型胶原,减少细胞中α-SMA、CTGF和p-p38 MAPKThr 180/Tyr 182的蛋白水平(P<0.05).p38 MAPK激活剂处理可以逆转敲减TAK1表达对肾小管上皮细胞分泌I型胶原和III型胶原及α-SMA、CTGF和p-p38 MAPKThr 180/Tyr 182蛋白水平的抑制作用.结论:敲减TAK1表达能够通过抑制p38 MAPK信号通路而降低TGF-β1诱导的肾小管上皮细胞纤维化水平.【期刊名称】《中国病理生理杂志》【年(卷),期】2019(035)007【总页数】6页(P1248-1253)【关键词】肾小管上皮细胞;转化生长因子β激活激酶1;p38MAPK信号通路;纤维化【作者】DING Wen-fei;SONG Lei;LIU Hai-yun;QIN Jian-hua;CAO Ling;GAO Li-chao;OU San-tao;WU Wei-hua【作者单位】;;;;;;;【正文语种】中文【中图分类】R692.9;R363.2肾组织纤维化是常见慢性肾脏疾病发生的基础,其主要以肾小管间质纤维化和肾小球硬化为主要特征[1]。
肾小管上皮细胞病及其发病机理ppt课件

终末尿与原尿不仅在量上而且在溶质成分上
都有很大区别。只是当原尿流经肾小管的过
程中,有99%以上的水、电解质、葡萄糖、
氨基酸和碳酸氢根被肾小管上皮细胞重吸收
入血液;同时有大量的钾离子、氢离子、氨
离子、和有机酸、有机碱等都分泌到肾小管
液中,这样肾脏就起到了一个重吸收对机体
可利用的物质,并排出体内代谢的产物的作
(8)继发于间质性肾炎中的炎症反应与促纤维 化因子的刺激均可损伤肾小管上皮细胞。
ppt课件
2、肾小管上皮细胞受损伤后的功能变化
(1)肾小管上皮细胞受损伤被激活致使肾小管上皮 细胞分泌异常
①肾小管上皮细胞受损后,释放多种炎症性因子,如: 白介素-1(IL-1),结缔组织生长因子(CTGF), 碱性成纤维细胞生长因子(bFGF)以及释放促纤 维化生长因子TGF-β、PDGF,该系列因子在纤维 化早期具有促炎症反应作用,而在晚期具有促进胶 原合成导致间质纤维化形成。
②肾小管受损被激活后,还可释放各种趋化因子 (MCP-1),吸引炎症细胞进入肾小管和肾间质, 造成间质炎症性反应。
③肾小管上皮细胞受损被激活后,合成与分泌血管活 性因子,如内皮素-1、血管紧张素Ⅱ,促进肾血管 以及肾小管周围毛细血管收缩,进一步加重肾缺血。
ppt课件
(2)肾小管上皮细胞受损被激活后出现细胞增殖, 凋亡异常,导致肾小管肥大或萎缩,促进肾间质纤 维化进展 肾小管上皮细胞受损后出现的肾小管肥大,萎 缩这种生长特征变化既是肾间质损伤结果又是促进 间质纤维化发展的原因。肾小管上皮细胞受损后的 异常增殖,对肾间质纤维的形成有双重作用,一是 自我修复过程,二是病理损伤过程。
用,从而保持体内水、电解质和酸碱平衡状
态,对维持体内各脏器功能正常运行起了关
上皮间质转化和内皮间质转化在肾纤维化中的研究进展

上皮间质转化和内皮间质转化在肾纤维化中的研究进展金芬;张忠寿;黄卫锋【摘要】上皮间质转化(EMT)和内皮间质转化(EndMT)参与各种纤维化疾病的发病机制,EMT和EndMT已经成为器官纤维化研究的一个重点课题。
EMT和EndMT在肾的纤维化过程中起到至关重要的作用。
越来越多细胞内外分子可控制EMT和EndMT的表达,尤其是microRNA (miRNA)在EMT和EndMT中的调控作用,已被确定可利用于开发治疗纤维化。
本文综述了EMT和EndMT在肾纤维化中的研究进展,了解EMT和EndMT参与肾病纤维化过程的机制,这将会给人类肾纤维化疾病的治疗提供一种新的靶点和方法。
【期刊名称】《海南医学》【年(卷),期】2014(000)018【总页数】3页(P2723-2725)【关键词】上皮间质转化;内皮间质转化;肾的纤维化;miRNA【作者】金芬;张忠寿;黄卫锋【作者单位】三峡大学医学院,湖北宜昌 443002;三峡大学医学院,湖北宜昌443002;三峡大学医学院,湖北宜昌 443002【正文语种】中文【中图分类】R692.1+2肾纤维化是肾长期损伤或正常伤口愈合过程中功能失调,导致过量的细胞外基质(ECM)沉积造成的。
EMT与EndMT过程中激活产生的成纤维细胞是肾成纤维细胞的主要来源。
在肾纤维化过程中,肾成纤维细胞发挥着重要的作用。
在EMT和EndMT过程中存在复杂的调控,最新的研究表明,miRNA在EMT和EndMT过程中发挥重要的调节作用。
miRNA是小的非编码RNA,通过抑制蛋白质翻译或诱导mRNA降解来抑制靶基因的表达。
miRNA可调控细胞的分化、增殖、死亡、代谢等多种病理生理机制的基本过程[1]。
因此,研究EMT和EndMT过程在纤维化中的作用,可以推进我们对常见发病机制的理解,也可能为治疗干预提供新的靶点。
这篇综述侧重于上皮间质转化和内皮间质转化在肾脏疾病中的生物学角色。
在上皮-间质转化过程中上皮细胞有一系列的变化,它的极性丧失,迁移和运动能力增强,同时获得间质细胞特性。
纳米颗粒溶酶体逃逸能力

纳米颗粒溶酶体逃逸能力引言纳米颗粒溶酶体逃逸是指纳米颗粒通过溶酶体逃逸进入细胞质,从而避免被溶酶体降解的过程。
这一现象在纳米医药领域具有重要的意义,因为它能够提高纳米药物的生物利用度和治疗效果。
本文将探讨纳米颗粒溶酶体逃逸的机制和影响因素。
一、纳米颗粒溶酶体逃逸的机制1. 溶酶体逃逸通路纳米颗粒溶酶体逃逸的机制主要包括溶酶体穿透和溶酶体融合两个通路。
在溶酶体穿透通路中,纳米颗粒能够通过溶酶体膜的破裂或溶酶体膜内小孔的形成,逃逸进入细胞质。
而在溶酶体融合通路中,纳米颗粒与溶酶体融合形成融合体,然后通过溶酶体与细胞膜的融合,释放到细胞质中。
2. 逃逸机制的调控因素纳米颗粒溶酶体逃逸的能力受多种因素的调控。
首先是纳米颗粒的物理化学性质,如大小、形状、表面电荷等。
研究发现,较小的纳米颗粒和表面带正电荷的纳米颗粒更容易逃逸。
其次是纳米颗粒与溶酶体的相互作用。
某些纳米颗粒能够破坏溶酶体膜的完整性,从而促进逃逸过程。
此外,细胞内信号通路也对纳米颗粒溶酶体逃逸起到重要作用。
研究表明,细胞内的自噬和内质网应激等信号通路可以调控纳米颗粒溶酶体逃逸。
二、纳米颗粒溶酶体逃逸的应用1. 提高药物生物利用度纳米颗粒溶酶体逃逸能够增加纳米药物在细胞内的稳定性和生物利用度,从而提高药物的疗效。
溶酶体是细胞内最重要的降解系统之一,能够降解各种细胞内外的物质。
然而,溶酶体对纳米颗粒的降解活性也很高,导致纳米药物的有效成分被降解,药效降低。
因此,纳米颗粒溶酶体逃逸能够避免这一问题,提高药物的生物利用度。
2. 增强抗肿瘤效果纳米颗粒溶酶体逃逸还可以增强抗肿瘤药物的疗效。
溶酶体逃逸使纳米药物能够逃避溶酶体的降解,从而在细胞内稳定存在,增加与靶点的相互作用,提高药物的抗肿瘤效果。
此外,纳米颗粒溶酶体逃逸还可以促进药物的内吞作用,增加药物在肿瘤细胞内的积累。
3. 促进基因传递纳米颗粒溶酶体逃逸还可以用于基因传递。
基因传递是一种将外源基因导入细胞内的技术,用于治疗遗传性疾病和癌症等疾病。
纳米颗粒作为药物载体在肿瘤治疗中应用前景

纳米颗粒作为药物载体在肿瘤治疗中应用前景随着科技的发展,纳米技术在各个领域都得到了广泛的应用,其中之一就是在肿瘤治疗中的应用。
纳米颗粒作为一种药物载体,具有小尺寸、高比表面积、稳定性好的特点,能够改善药物的溶解性、提高药物的生物利用度、降低药物的副作用,因此在肿瘤治疗中有着广阔的应用前景。
首先,纳米颗粒能够提高药物的溶解度和稳定性。
很多常用的抗肿瘤药物因其溶解度低而难以发挥药效,而通过将这些药物包裹在纳米颗粒中,可以有效提高其溶解度,并且保护药物不受外界环境的影响,提高药物的稳定性。
这样一来,患者在服药过程中就能够更好地吸收药物,从而提高治疗效果。
其次,纳米颗粒具有高比表面积,有利于药物的靶向输送。
纳米颗粒尺寸小,表面积大,这为药物的靶向输送提供了有利条件。
通过表面修饰纳米颗粒,可以使其选择性地与肿瘤细胞表面的分子结合,从而实现药物的靶向输送。
这种靶向输送的方式,既可以减少对正常细胞的毒副作用,又可以提高药物在肿瘤细胞中的浓度,进而增强治疗效果。
此外,纳米颗粒还可以延长药物在体内的循环时间。
常规的抗肿瘤药物往往在体内的循环时间很短,使得药物很难达到治疗的最佳浓度。
而将药物包裹在纳米颗粒中,既能够提高药物的稳定性,延长药物的循环时间,又能够减少药物在体内的代谢和排泄,从而增加药物对肿瘤的作用时间,进一步提高治疗效果。
同时,纳米颗粒还可以实现多药联合治疗。
肿瘤治疗往往需要采用多种不同的药物联合使用,以增强抗肿瘤效果。
然而,多药联合使用往往伴随着药物的相互干扰和毒副作用的增加。
而通过将多种药物同时封装在纳米颗粒中,可以实现不同药物的同时释放,避免了药物之间的相互干扰,并且能够减少毒副作用,提高抗肿瘤效果。
纳米颗粒作为药物载体在肿瘤治疗中的应用前景广阔,但也面临一些挑战。
首先,纳米颗粒的制备和表面修饰技术仍然存在一定的难度,需要不断的研发和改进。
其次,纳米颗粒在体内的分解和代谢途径尚不完全清楚,需要进一步深入研究。
马兜铃酸通过膜转运蛋白进入肾小管上皮细胞的研究的开题报告

马兜铃酸通过膜转运蛋白进入肾小管上皮细胞的研
究的开题报告
马兜铃酸是一种广泛存在于多种植物中的天然毒素,其有效成分为马兜铃酸。
常常被广泛用于中草药材的治疗,但是其对人体健康的副作用也发生了极多的研究。
其中最为重要的是对肾毒性的影响,从临床上可以看到马兜铃酸引起的肾病和肾衰竭的发生率不断上升。
因此,在深入研究马兜铃酸的肾毒性机制中,着重探讨其如何通过膜转运蛋白进入肾小管上皮细胞,对于揭示马兜铃酸毒性的发生机制具有重要意义。
众所周知,肾小管上皮细胞是肾脏中主要的充电器,通过细胞膜上的转运蛋白对其内外物质进行分离与转运。
相关研究表明,马兜铃酸在被吸收入体后主要通过尿液排出,其中的倍半萜类有毒成分对肾脏造成毒性作用。
马兜铃酸与尿液的排泄是通过肾小管上皮细胞的转运蛋白介导的,因此,探究马兜铃酸进入肾小管上皮细胞的机制和相关膜转运蛋白的功能具有一定的临床意义。
本研究将利用不同培养液中的肾小管上皮细胞的单层细胞膜作为研究对象,利用电生理技术和荧光探针法研究马兜铃酸的转运,并进一步挖掘其与膜转运蛋白的相互作用模式。
本研究的目的是阐述马兜铃酸的肾毒性作用机制,探究其进入肾小管上皮细胞的途径和相关膜转运蛋白的协同作用模式,从而为临床上的肾毒性预防和治疗提供理论依据。
NLRP3炎症小体与相关炎症信号通路在肾缺血-再灌注损伤中的作用
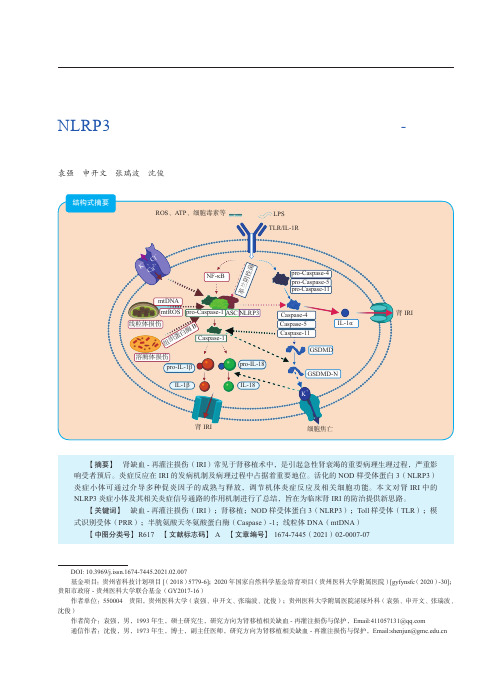
第12卷 第2期2021年3月Vol. 12 No.2Mar. 2021器官移植Organ TransplantationNLRP3炎症小体与相关炎症信号通路在肾缺血-再灌注损伤中的作用袁强 申开文 张瑞波 沈俊·移植前沿·DOI: 10.3969/j.issn.1674-7445.2021.02.007基金项目:贵州省科技计划项目[(2018)5779-6];2020年国家自然科学基金培育项目(贵州医科大学附属医院)[gyfynsfc (2020)-30];贵阳市政府-贵州医科大学联合基金(GY2017-16)作者单位:550004 贵阳,贵州医科大学(袁强、申开文、张瑞波、沈俊);贵州医科大学附属医院泌尿外科(袁强、申开文、张瑞波、沈俊)作者简介:袁强,男,1993年生,硕士研究生,研究方向为肾移植相关缺血-再灌注损伤与保护,Email:****************通信作者:沈俊,男,1973年生,博士,副主任医师,研究方向为肾移植相关缺血-再灌注损伤与保护,Email:***************.cn【摘要】 肾缺血-再灌注损伤(IRI )常见于肾移植术中,是引起急性肾衰竭的重要病理生理过程,严重影响受者预后。
炎症反应在IRI 的发病机制及病理过程中占据着重要地位。
活化的NOD 样受体蛋白3(NLRP3)炎症小体可通过介导多种促炎因子的成熟与释放,调节机体炎症反应及相关细胞功能。
本文对肾IRI 中的NLRP3炎症小体及其相关炎症信号通路的作用机制进行了总结,旨在为临床肾IRI 的防治提供新思路。
【关键词】 缺血-再灌注损伤(IRI );肾移植;NOD 样受体蛋白3(NLRP3);Toll 样受体(TLR );模式识别受体(PRR );半胱氨酸天冬氨酸蛋白酶(Caspase )-1;线粒体DNA (mtDNA )【中图分类号】 R617 【文献标志码】A 【文章编号】1674-7445(2021)02-0007-07结构式摘要LPS TLR/IL-1RROS 、ATP 、细胞毒素等NF-κB线粒体损伤mtDNAmtROS 溶酶体损伤组织蛋白酶Bpro-Caspase-4pro-Caspase-5pro-Caspase-11Caspase-5Caspase-11Caspase-4肾IRI肾IRI细胞焦亡ASC Caspase-1pro-IL-18IL-18GSDMD-N革兰阴性菌pro-Caspase-1NLRP3pro-IL-1βIL-1βGSDMDIL-1αK +、C l -Ca 2+K +·178·第12卷器官移植现阶段,全世界有10%的人口患有慢性肾病。
肾小管上皮细胞颗粒变性怎么办

如对您有帮助,可购买打赏,谢谢
生活常识分享肾小管上皮细胞颗粒变性怎么办
导语:有许多得了肾小管上皮细胞颗粒变性这个病之后,变得憔悴不堪,被病痛折磨的是不成样子。
有许多病人和家属都感到很着急和无助,他们想知道在
有许多得了肾小管上皮细胞颗粒变性这个病之后,变得憔悴不堪,被病痛折磨的是不成样子。
有许多病人和家属都感到很着急和无助,他们想知道在平常在家里的时候肾小管上皮细胞颗粒变性后自己该怎么办才好。
因为这件事情和问题,小编特地请教了当地的一些专家来为大家解答这个问题。
那么接下来就让我们一起看看肾小管上皮细胞颗粒变性怎么办。
1:肾炎病人在选择治疗方法时一定要慎重. 一般来说应注意休息,低盐饮食,若感染一般注射抗生素,利尿消肿,降血压,预防心脑合并症的发生.中医治疗往往采用祛风利水,清热解毒,凉水止血等治疗原则.本病具有自愈倾向,肾功能多可逐渐恢复,一般不需要长期维持透析。
本病治疗以休息及对症为主,少数急性肾功能衰竭病例应予透析,待其自然恢复。
2:肾小管具有重吸收,分泌和排泄的功能.在肾缺血,缺氧,感染及毒物作用下,可以发生肾小管上皮细胞变性甚至坏死,从而导致泌尿功能障碍.此外,在醛固酮,抗利尿激素利钠激素及甲状旁腺激素作用下,也会发生肾小管的功能改变.由于肾小管各段的结构和功能不同,故各段受损时所出现的功能障碍亦各异.
肾小管上皮细胞颗粒变性怎么办?上面的内容就是这个问题的答案了,平常患有肾小管上皮细胞颗粒变性的患者们在家里也可以做一些有助于病情痊愈的事情了,也不用再担心自己在家里会没有事情做了。
希望上面所说的关于肾小管上皮细胞颗粒变性怎么办的一些建议和方。
肾小管上皮细胞转分化的信号转导途径

泸 州 医学 院 学报
20 0 8年
第 3 卷 1
第l 期
J un lo u h u Me ia olg Vo. o r a f z o dc lC l e L e 11 3 No1 2 0 . 08
肾小管上皮细胞 转分化 的信 号转导途径
2 E T发 生 的主 要 过 程 M
目前 认 为 T F 1 S c 途 径 是 肾 小 管 上 皮 细 胞 转 分 化 G 6一m k a s 中最 重 要 的信 号 转 导 途 径 之 一 。TG 一6 F 1可 与 胞 浆 受体 结 合 而 发挥 生 物 学 效 应 ,与 T 一 1结 合 的 受 体 是 具 有 丝 氨 GF 6
蛋 白(met ) 些 改 变 为 转 分 化 细胞 的迁 移 、 袭 、 缩 能 v ni 。这 i n 侵 收
力 的增 强 提 供 了结 构 基 础 。
T MT 是 肾小 管 间质 纤 维 化 发 生和 进 展 的重 要 机 制 。多种 生 E )
长 因子 、 胞 因子 、 素 和 细 胞 外 某 些 因 素 , 细 激 如转 化 生 长 因 子 B (as r iggo t c r B , G — 1、 皮 生 长 因 子 1t nf m n rw hf t — 1 T F B )表 r o ao (pdr l rwhf tr,G )白 介 素 - ( tr u i- , 一 eie o t c ma g a o E F、 8 i e ekn 8I n l L 8 、 型 胶 原 等 通 过 不 同 方 式 参 与 肾小 管 上 皮 细 胞转 分 化 的 )I
肌 成 纤 维 细 胞 表 达 一 滑 肌 肌 动 蛋 白并 且 具 有 收 缩 能 力 , 平 说 明 收 缩 能 力的 获 得 是转 分化 细胞 进 入 间质 的另 一 种 途径 。
上转换纳米颗粒

Company LOGO
2.1组成及晶体结构
上转换纳米颗粒:无机基质+稀土掺杂离子
掺杂离子:发光中心+敏化剂
发光中心:Er3+ Ho3+ Tm3(+ 常用)
(均匀分立的能级+较长的亚稳态寿命)
敏化剂: Yb3+
(对激发光吸收能力较强,将能量传给发光中心)
2.2光学性质
寿命长
电子跃迁禁阻 (磷光而非荧光)
(整体)无发光闪烁
Company LOGO
2.3表面化学
水/溶剂热法制备UC纳米颗粒时通过配体控制晶体生 长(尺寸、形貌) 填补表面缺陷;保护颗粒不受外界影响。提高发光效率
改变纳米颗粒的亲疏水油性(主要是亲油变亲水)
光 子 雪 崩
Company LOGO
2.2光学性质
光学稳定性好 发射峰窄
4f层电子跃迁,受外电子层屏蔽
Company LOGO
2.2光学性质
光色可调:掺杂物种、掺杂比例(浓度)、掺杂位置 基质类型、混色方式
Company LOGO
以其独特的光频“上转换”能力,在 生物领域具有重要的应用价值
Company LOGO
1 简介
Company LOGO
上转换发光材料 Vs 传统发光材料
作为生物成像造影剂
有机荧光染料
量子点
宽发射谱 光漂白
消光系数大 高量子产率 窄发射带宽 发光易调控 高光学稳定性
锐,在受体发射谱波长范围内没有探测到
供体的发射光
Company LOGO
3.2均相检测
Analyst, 2009, 134, 1713–1716
纳米粒子在肠上皮的转运机制_概述说明以及解释

纳米粒子在肠上皮的转运机制概述说明以及解释1. 引言1.1 概述纳米粒子在生物医学领域中引发了广泛的关注,其在药物传递和治疗等方面具有巨大的潜力。
肠道作为人体最重要的吸收器官之一,对于纳米粒子的转运机制起着关键作用。
本文旨在总结和探讨纳米粒子在肠上皮的转运机制,以阐明其在肠道内部吸收过程中所扮演的角色。
1.2 文章结构本文将从三个方面对纳米粒子在肠上皮的转运机制进行全面阐述。
第二节将介绍纳米粒子的定义、特性以及肠上皮结构与功能。
第三节将详细探讨纳米粒子与肠上皮细胞相互作用机制。
第四节将重点讨论环境因素对纳米粒子在肠上皮转运过程中的影响。
最后一节将总结这些研究结果并展望未来可能的应用和风险评估。
1.3 目的通过对纳米粒子在肠道内部吸收过程中所涉及的各个环节进行系统性概述和解释,旨在提供对纳米粒子转运机制的全面理解和深入认识。
这将有助于我们更好地把握纳米药物传递系统的设计原则以及潜在应用和风险评估方面的考虑,从而推动相关领域的研究和发展。
2. 纳米粒子的转运机制2.1 纳米粒子的定义和特性纳米粒子是指尺寸在1到100纳米之间的颗粒或聚集物。
由于其特殊的体积、表面活性及量子效应等独特属性,纳米粒子被广泛应用于医药、食品、化工等领域。
常见的纳米材料包括金属纳米颗粒、生物材料纳米载体和聚合物纳米粒子等。
2.2 肠上皮的结构与功能肠上皮是小肠黏膜内最外层的一层细胞组织,具有吸收和分泌等重要功能。
其表面覆盖着微细的绒毛和深沟,增加了吸收表面积,并且形成了一个相对隔离环境来控制物质从肠腔进入血液循环系统。
2.3 纳米粒子在肠上皮的吸收途径目前已知纳米粒子主要通过两种途径在肠上皮进行吸收:穿透型转运途径和胞饮作用。
穿透型转运途径是指纳米粒子穿过肠上皮细胞而进入血液循环系统。
胞饮作用则是指纳米粒子被肠上皮细胞吞噬形成内泡,然后通过内吞囊泡运输到细胞的其他区域。
穿透型转运途径主要受到纳米粒子的尺寸、形态和表面性质等因素的影响。
超小纳米点 肾代谢

超小纳米点肾代谢
超小纳米点(ultra-small nanoparticles)是指直径小于10纳米的纳米颗粒。
由于其小尺寸和大比表面积,超小纳米点具有独特的物理、化学和生物学特性,广泛应用于生物医学领域。
肾代谢(renal metabolism)是指在肾脏中发生的代谢过程。
肾脏是身体的重要排泄器官,通过肾小球滤过和肾小管排泄等过程,将代谢产物、药物代谢产物和其他体内废物排出体外。
肾代谢对于维持体内内环境的稳定和药物的排泄起着重要作用。
超小纳米点在肾代谢研究中具有潜在的应用价值。
其小尺寸和大比表面积使其能够更容易穿过肾小球滤过膜,进入肾小管,与肾脏进行更直接的相互作用。
通过荧光标记等方法,可以追踪超小纳米点在肾脏中的代谢和排泄过程,进一步了解肾脏的功能和药物在肾脏中的代谢途径。
超小纳米点还可以用于肾脏疾病的诊断和治疗。
通过改变超小纳米点的表面性质和载药能力,可以将其用作靶向性药物传递系统,将药物直接运送到肾脏病灶,提高治疗效果并减少药物的副作用。
超小纳米点在肾代谢研究和肾脏疾病治疗中具有广阔的应用前景,可以为肾脏相关疾病的治疗和监测提供新的工具和方法。
纳米颗粒在生物体内的输运与排泄研究

纳米颗粒在生物体内的输运与排泄研究纳米颗粒作为一种新型的材料,具有生物医学应用的广阔前景。
在临床应用中,纳米颗粒的输运和排泄是一个极其重要的问题,直接影响着其生物安全性和药效效力。
因此,纳米颗粒在生物体内的输运与排泄研究备受关注。
一、纳米颗粒输运的基本原理纳米颗粒在体内输运的为主要模式有两种:第一种是被细胞摄取进入细胞内进行药物释放,此类纳米颗粒主要是通过内吞饮食作用和随机扫描作用进入细胞;第二种是在体液中直接运输至目标部位或组织,此类纳米颗粒主要是通过扫描、透过和稳定性保持等作用实现,多通过药物载体输送至靶部位,如肿瘤细胞。
在纳米颗粒输运的过程中,重要的因素有纳米颗粒的大小、形态、表面电荷,以及生物体内环境如生物分泌物(免疫球蛋白)、组织尺寸、生理温度、物理与化学性质等。
在设计纳米颗粒药物时,必须充分考虑这些因素,以尽可能降低输运过程中的副作用和毒副反应。
二、纳米颗粒在体内排泄的研究在选择纳米颗粒输送药物时,其排泄途径和能力是重要的考虑因素。
一般来说,药物在体内的代谢和排泄途径决定了其药效和毒性。
对于低毒性的纳米颗粒而言,排泄途径多种多样,包括肝脏、肾脏、肠道等。
肝脏是纳米颗粒以肝素或草酸盐等形式代谢并改变其形态的主要器官,而肾脏主要是将纳米颗粒或其明显大小的代谢产物分泌出体外,并在输送过程中进行过滤、重吸收等过程。
此外,纳米颗粒在肺泡和肺淋巴上皮细胞上也可发生吸附和代谢反应,而器官间的相互作用常常是一个综合的过程。
三、纳米颗粒的毒性分析随着纳米技术的快速发展,人们对于纳米颗粒是否具有安全性问题尤为关注。
目前已有许多研究表明,纳米颗粒对于肺部、心血管系统、免疫系统、神经系统等器官具有毒性作用。
此外,纳米颗粒还有可能造成对矿物质、氧化等物质的诱发作用,从而导致环境污染和生态危机的产生。
在评估纳米颗粒的毒性时,需要考虑其组成、形态、表面性质、生化反应、慢性毒性、生物相容性等多个因素。
现代毒理学研究已经有了一些成熟的方法,但是针对于纳米颗粒的毒性评估还需要进一步的深入研究。
纳米颗粒在药物研发中的应用

纳米颗粒在药物研发中的应用随着现代科技的不断发展,纳米颗粒技术被广泛应用于各个领域,而医药领域也不例外。
纳米颗粒技术在药物研发中的应用,可以提高药物的生物利用度和稳定性,增强药物的疗效,改善药物的溶解度和药物的流动性,从而更好地满足患者的治疗需求。
本文将从四个方面介绍纳米颗粒在药物研发中的应用情况。
一、纳米颗粒在药物的载体中的应用纳米颗粒作为一种天然的、可自组装的药物载体,具有很好的细胞渗透性和生物相容性,可以高效地携带和运输药物。
在治疗癌症等疾病时,药物载体的选择尤为重要。
临床研究表明,纳米颗粒载体可以在肿瘤组织中积累,通过被肿瘤细胞吞噬后释放药物,增加药物的疗效,并且减少对正常细胞的损伤。
纳米颗粒还可以作为自由基清除剂,减少它们对细胞的损伤。
同时,纳米颗粒载体具有更长的生物半衰期,在排除代谢产物时维持药物在体内的时间更长,增加药物的持续性和稳定性。
二、纳米颗粒在靶向药物递送中的应用纳米颗粒载体还可以加上不同的靶向分子,如抗体和肽,以便将药物输送到特定的细胞或组织。
这种药物靶向递送的方法可以提高药物的生物利用度,同时减少副作用和药物的毒性。
比如,聚乙二醇(PEG)修饰的纳米颗粒,具有对血液的亲和性,可以延长药物半衰期,增强抗体药物的抗体依赖性细胞毒性(ADCC)效应,提高治疗癌症的疗效。
三、纳米颗粒在药物制剂中的应用纳米颗粒技术也可以用于改善药物的溶解度和稳定性。
一些药物由于水溶性较差或易分解或不稳定,会导致生物利用度低和疗效不佳。
纳米颗粒制剂可以通过降解药物粒度,使药物溶解度提高,从而提高生物利用度。
如替格瑞洛、伊曲康唑、卡淇卡单抗等药物都已经通过纳米颗粒技术制定,提高了药物疗效。
四、纳米颗粒在治疗疫苗中的应用疫苗可以干预疾病的穿透、传播和复原。
现今研究疫苗技术面临的一个主要挑战是如何提高疫苗的免疫反应和生物利用度,特别是提高疫苗在靶组织中的抗原特异性。
纳米颗粒制剂可以延长疫苗的保留时间和缩小疫苗的粒子大小,增强其对细胞和组织的渗透。
药物制剂中纳米颗粒的体内代谢研究

药物制剂中纳米颗粒的体内代谢研究在药物制剂和纳米技术的不断发展下,纳米颗粒作为一种传递药物的载体逐渐被广泛应用于药物研究和治疗领域。
然而,作为药物制剂中的一种新型材料,纳米颗粒的体内代谢机制及其安全性问题仍然不完全清楚。
因此,对药物制剂中纳米颗粒的体内代谢研究具有重要意义。
一、纳米颗粒的定义和制备方法纳米颗粒属于纳米尺度的粒子,其直径通常在1到100纳米之间。
制备纳米颗粒的常见方法包括物理法、化学法和生物法等。
物理法常用的有球磨法、气溶胶法和激光热解法等;化学法包括共沉淀法、溶剂挥发法和热分解法等;生物法主要通过生物合成或生物模板法来获得纳米颗粒。
二、纳米颗粒在体内的代谢途径纳米颗粒在体内会经历一系列的代谢途径,其中包括吸收、分布、代谢和排泄等过程。
这些过程受到多种因素的影响,如纳米颗粒的物理化学性质、表面修饰、剂量和给药途径等。
纳米颗粒一般通过口服、注射、吸入和局部给药等途径进入体内,然后通过血液和淋巴系统被分布到不同的器官和组织。
三、纳米颗粒的体内代谢途径研究方法为了深入研究纳米颗粒在体内的代谢途径,科学家们采用了多种研究方法。
其中,常见的方法包括动态荧光成像、放射性同位素示踪技术和质荷比分析等。
动态荧光成像技术可以实时观察纳米颗粒在体内的路径和分布情况;放射性同位素示踪技术可以追踪纳米颗粒的代谢和排泄过程;质荷比分析则可通过质谱技术来确定纳米颗粒在不同器官和组织中的代谢产物。
四、纳米颗粒体内代谢的影响因素纳米颗粒在体内的代谢受到多种因素的影响,如纳米颗粒的物理化学性质、表面修饰、剂量和给药途径等。
物理化学性质包括大小、形状、表面电荷和表面修饰等,这些性质会影响纳米颗粒在体内的吸收、分布和代谢。
剂量和给药途径也是影响纳米颗粒代谢的重要因素,不同给药途径和剂量会导致纳米颗粒在体内的代谢途径和速度发生变化。
五、纳米颗粒的体内安全性评价纳米颗粒的体内安全性评价对于药物制剂的临床应用至关重要。
常用的评估指标包括纳米颗粒的生物分布、组织损伤和生物毒性等。
清肾颗粒含药血清通过miR-23b-5p靶向Nrf2通路减轻NRK-52E细胞转分化

Qingshen Granules-medicated serum reduces transdifferentiation of NRK-52E cells by miR-23b-5p-mediated activation of the Nrf2pathwayLIU Min 1,JIN Hua 2,3,HU Qin 1,CHEN Nuo 1,ZHANG Yeqing 1,WANG Yiping 21The First Clinical Medical College of Anhui University of Traditional Chinese Medicine,Hefei 230000,China;2Department of Nephrology,First Affiliated Hospital of Anhui University of Traditional Chinese Medicine,Hefei 230000,China;3Center for Xin'an Medicine and Modernization of Traditional Chinese Medicine of IHM,Anhui University of Traditional Chinese Medicine,Hefei 230000,China摘要:目的探讨转化生长因子β1(TGF-β1)诱导NRK-52E (大鼠肾小管上皮细胞)细胞转分化模型中miR-23b-5p 是否能对Nrf2通路靶向调节,并阐明清肾颗粒含药血清减轻NRK-52E 细胞转分化的机制。
方法构建TGF-β1诱导的NRK-52E 细胞转分化模型,分别使用miR-23b-5p mimic 、inhibitor 以及阴性对照(NC )siRNA 转染细胞;采用清肾颗粒含药血清进行干预,分为正常组、TGF-β1组、清肾颗粒组、miR-23b-mimic-NC 组、miR-23b-mimic 组、miR-23b-mimic+清肾颗粒组。
纳米颗粒 胞吐 高尔基体

纳米颗粒胞吐高尔基体
纳米颗粒、胞吐和高尔基体是生物学和医学领域中的重要概念。
以下是这些概念的简要解释:
1.纳米颗粒:纳米颗粒是指尺寸在纳米级别(1-100纳米)的颗粒。
由于其
极小的尺寸,纳米颗粒具有许多独特的物理和化学性质,因此在许多领域中都有应用,例如药物传递、医学诊断和癌症治疗等。
2.胞吐:胞吐是细胞生物学中的一个过程,指细胞通过胞吐作用将胞吞或胞
饮所摄取的物质传递到细胞外。
这个过程涉及到膜的动态变化和物质的释放,对于细胞通讯、信号转导和物质转运等有重要作用。
3.高尔基体:高尔基体是细胞内的一个复杂的膜性囊泡结构,参与蛋白质的
修饰、加工和转运。
高尔基体在细胞的分泌活动、膜泡运输和细胞信号转导等方面起着关键作用。
它是细胞内重要的分泌器官之一,能够将蛋白质从内质网转运到溶酶体、质膜或胞吐泡,并对蛋白质进行糖基化等修饰。
综上所述,纳米颗粒、胞吐和高尔基体分别涉及到尺寸在纳米级别的物质、细胞内物质释放的过程以及细胞内复杂的膜性结构等概念。
这些概念在药物传递、医学诊断和治疗、细胞生物学和膜泡运输等方面有重要的应用和研究价值。
ldlr循环机制和纳米粒

ldlr循环机制和纳米粒
LDLR循环机制(低密度脂蛋白受体循环):
通俗来说,人体内的低密度脂蛋白(LDL)就像一辆辆“货车”,它们装载着胆固醇,在血液中运输。
而低密度脂蛋白受体(LDLR)就像是分布在细胞表面的“卸货站”。
当这些“货车”LDL 与“卸货站”LDLR结合后,细胞就能够吸收并利用其中的胆固醇进行各种生物合成和代谢活动。
具体过程如下:
1.LDL从肝脏等器官释放到血液中,携带胆固醇。
2.血液中的LDL通过血液循环到达全身各组织细胞表面。
3.细胞膜上的LDLR识别并结合LDL。
4.结合后的复合物被内吞进入细胞内部形成内体(endosome)。
5.在内体中,LDL和LDLR分离,胆固醇被释放出来供细胞使
用,而LDLR则被再循环回到细胞膜上,准备与新的LDL结
合。
纳米粒在体内长循环机制的通俗解释:
在药物传输领域,科学家们设计了一种名为纳米粒的小型颗粒,大小约为几十到几百纳米,可以负载药物或其他治疗物质。
为了让这些纳米粒在体内停留更长时间以便于药物有效传递到病灶部
位,科研人员通常会在其表面修饰一层或多层特殊材料,如聚乙二醇(PEG),这种技术被称为"PEGylation"。
修饰后的纳米粒能够在血液中“隐身”,避免被免疫系统快速清除,从而延长在血液循环中的时间,提高药物在目标部位的积累量,这就是所谓的“长循环”机制。
简单地说,就是给纳米药物穿上一件“隐形衣”,让它能在人体内“悄悄地”、持久地行动,最终将所载药物送达需要治疗的地方。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
The transport pathways of polymer nanoparticles in MDCK epithelial cellsBing He,Zengrong Jia,Wenwen Du,Chao Yu,Yuchen Fan,Wenbing Dai,Lan Yuan,Hua Zhang,Xueqing Wang,Jiancheng Wang,Xuan Zhang,Qiang Zhang *State Key Laboratory of Natural and Biomimetic Drugs,School of Pharmaceutical Sciences,Peking University,Beijing 100191,Chinaa r t i c l e i n f oArticle history:Received 10January 2013Accepted 30January 2013Available online 7March 2013Keywords:Polymer nanoparticles Epithelial cellsTransport mechanism Endocytosis Exocytosis Transcytosisa b s t r a c tEpithelial cell membranes as the typical biological barrier constitute the prime obstacle for the transport of therapeutic agents including nanomedicines.The previous studies on the interaction between nanomedicines and cells are mostly emphasized on cellular uptake and intracellular traf ficking,but seldom on epithelial cells,although more and more oral nanomedicines are available now.In an attempt to clarify the transport pathways of nanomedicines in epithelial cells,the different molecular mecha-nisms among endocytosis,exocytosis and transcytosis processes were carefully studied and compared here using a kind of polymer nanoparticles (PNs)and MDCK epithelial cells as models.As the result,their similarity and difference were demonstrated.The similarities among all the three processes included the mediation of lipid rafts,the involvement of some protein kinases such as protein tyrosine kinase (PTK),protein kinase C (PKC)and phosphatidylinositol 3-kinase (PI3K),and the existence of multiple pathways.However,the difference among these processes was very signi ficant,including different pathways,and especially the disparate effects of lipid rafts and protein kinases for different processes.The endocytosis involved both lipid raft and clathrin mechanisms but no macropinocytosis,via the invagination of membrane but no pore formation,the exocytosis contained ER/Golgi and Golgi/PM pathways,and transcytosis included AEE/CE/BSE and Golgi/BSE pathways.The roles of lipid rafts on endocytosis were positive but that on exocytosis and transcytosis was negative.The impacts of PTK and PKC on endocytosis were positive,while the in fluences of PTK,PKC and P13K on AEE/CE/BSE,as well as PTK and P13K on Golgi/BSE transcytosis pathways were negative.Moreover,the discrepancy between inward and outward transport of PNs elucidated an interesting fact that the endocytosis was rather easy and outward transport including exocytosis and transcytosis was rather dif ficult.Finally,it was indicated by com-parison with previous reports that the molecular mechanisms between PNs and macromolecules such as proteins were also dissimilar.Ó2013Elsevier Ltd.All rights reserved.1.IntroductionBiological barrier,mainly represented by epithelial tissue,con-trols the absorption of exogenous substances and protects against the invasion of pathogenic microorganisms.The prime character-istic of epithelial cells is the polarity.Because of diversities on lipid composition and protein distribution,the plasma membranes (PM)of epithelial cells are distinguished as apical and basolateral membrane respectively,selectively controlling the transportation of different substances [1].Furthermore,the existence of tight junction makes epithelial cell membranes into a compact barrier[2].In clinic,most medicines with different administration routes suffer the hindrance from epithelial cell monolayer because of the throughout distribution of epithelial cells in our body.Especially for the drugs with poor water-solubility or membrane permeability,their oral bioavailability and therapeutic ef ficacy are often reduced signi ficantly due to the block effect of epithelial cells on the drug absorption in gastrointestinal tract [3].How to conquer the epi-thelium barrier and accelerate the transport of drugs through are the great challenges for pharmaceutical science for a long time.Over the last couple of decades,the application of nano-technology has been one of the main approaches to overcome the epithelial barrier [4].Compared with free drug,polymer nano-particles (PNs)alter the pathways of cell uptake and promote the transport of medicines loaded in PNs through epithelial cell mon-olayer [5].As a representative of PNs,polylactic-co-glycolic acid*Corresponding author.Tel./fax:þ861082802791.E-mail address:zqdodo@ (Q.Zhang).Contents lists available at SciVerse ScienceDirectBiomaterialsjournal homepage:w ww.elsevi/locate/biomaterials0142-9612/$e see front matter Ó2013Elsevier Ltd.All rights reserved./10.1016/j.biomaterials.2013.01.100Biomaterials 34(2013)4309e 4326(PLGA)nanoparticles are frequently reported as the drug delivery systems because of its biocompatibility and biodegradability[6]. Via different fabrication methods,this kind of PNs can be obtained with desired particle size from50nm to300nm[7].Drugs with different solubility characteristics,water-soluble or water-insoluble,can all be loaded into this kind of nanoparticles by dif-ferent techniques to improve their bioavailability[8,9].Various surface and structure modifications are also reported to facilitate the uptake and transport efficiency of PLGA nanoparticles in cells [10].Generally,to improve the transport of nanoparticles across the epithelial cell membrane,it is vital to comprehend the mechanisms related,which has caused increasing interesting in recent years. Currently,studies on the transport mechanism are mostly focused on the uptake pathway and partially on intracellular trafficking of nanoparticles in different cells,but mostly tumor cells.In fact, during the transport across the epithelial barrier,the nanoparticles need to undergo the whole process from the apical membrane to the basolateral ly,the transcytosis of PNs may include their entry into cytoplasma via the endocytosis,transport intra-cellularly,andfinal exit from apical and basolateral membrane via exocytosis.So,the comprehensive understanding of the whole trans-cellular process is desperately needed.On the other hand,the roles and pathways in different steps of whole process may be different.Therefore,the careful comparison among the endocy-tosis,exocytosis and transcytosis of PNs should be significant for the further comprehension of the molecular mechanisms related during the transport of nanoparticles across epithelial cells.In order to study the whole trans-cellular process of PNs and the mechanism diversity among endocytosis,exocytosis and trans-cytosis,MDCK cell line is chosen as the model of epithelial barrier and PLGA nanoparticles as the representative of PNs in this work. Due to the polarity and tight junctions,MDCK epithelial cells are often utilized as the simulation of gastrointestinal tract[11],and their similarity with Caco-2cells is already demonstrated[12]. Various models of endocytosis(uptake in cells in12-well plate), exocytosis(exit upward from cells in12-well plate)and trans-cytosis(across cell monolayer on porous polycarbonate membrane) were established ly,exocytosis model only involved the exit from apical side in order to simply the situation.Plenty of techniques or systems were applied here,including confocal laser scanning microscope(CLSM)with different scanning models,near-infraredfluorescence(NIRF)imaging system,flow cytometry sys-tem(FCS),quantitative colocalization and so on.Regulators of protein kinases and transport pathways were also utilized to illustrate the molecular mechanisms related.2.Materials and methods2.1.MaterialsMDCK cell lines were obtained from National Platform of Experimental Cell Resources for Sci-Tech(Beijing,China).Fetal bovine serum(FBS)was purchased from Gibco(Grand Island,NY,USA).Poly(D,L-lactic-co-glycolic acid)(PLGA,50:50,Av. MW15000)was from Shandong Daigang Institute of Medical Instrument(Jinan, Shandong,China).LysoTracker@Red DND-99dye,ER-TrackerÔRed dye and Rhodamine-phalloidine were all supplied by Invitrogen(Eugene,Oregon,USA). Rabbit anti-Rab5and mouse anti-Rab7antibodys were obtained from Abcam (Cambridge,MA,USA).Coumarin-6,poloxamer188(Pluronic F68),methyl-b-cyclodextran(M b CD),fillipin,nystatin,5-(N-ethyl-N-isopropyl)-amiloride(EIPA) and genistein were purchased from Sigma e Aldrich(St.Louis,MO,USA).Wort-mannin,LY294002,staurosporine,brefeldinA,monensin,phorbol12-myristate13-acetate(PMA),1,10-dioctadecyl-3,3,30,30-tetramethylindocarbocyanine perchlorate (DiI),1,10-dioctadecyl-3,3,30,30-tetramethyl indotricarbocyanine iodide(DiR)and hoechst33258were obtained from Beyotime(Haimen,Jiangsu,China).Texas Red-conjugated goat anti-rabbit IgG,Texas Red-conjugated goat anti-mouse IgG, DMEM(4.5g/L glucose)culture solution,penicillin,streptomycin,trypsin,EDTA, phosphate buffered saline(PBS)and sucrose were supplied by Mcgene Co(Beijing, China).All other chemical reagents were analytical grade or better.2.2.Preparation and characterization of PNsPNs were prepared by a modified emulsion solvent-evaporation method[13].In brief,5mg PLGA was dissolved in2ml acetone.Under the2000rpm stirring at the room temperature,the organic phase was slowly poured into5ml water phase with 2mg F68.After stirring for15min,the initial emulsion was transferred into a50ml flask followed by evaporation for20min at60 C.The nanoparticle dispersion was then centrifuged at10,000g for20min,and the obtained pellets were dispersed again in certain mediums.The preparation of C6-PNs was similar and the only exception was the addition of5m g coumarin-6in acetone.The size and surface charge of PNs dispersed in different mediums were measured by a dynamic light scattering(DLS)analyzer(Malvern,Zetasizer Nano ZS,UK).In addition,PNs were also detected by transmission electron microscopy(TEM).After negatively stained by phosphotungstic acid,10m g/ml nanoparticle water dispersion was dropped on the copper grid and examined by a transmission electron microscope(JEM1230, JEOL,Japan).2.3.In vitro leakage of coumarin-6from C6-PNsBriefly,2mg C6-PNs were dispersed in2ml PBS or serum free medium(SFM) with pH7.2and pH5.5,respectively.Then the nanoparticle dispersion was located in a dialysis bag(MW cut off12kD)and dialyzed against48ml release medium same as the dispersion solution within the dialysis bag.Under the100rpm at37 C,0.5ml release medium was taken out and detected by a high performance liquid chro-matography(HPLC)system(Shimadzu,Japan)equipped with afluorescence de-tector(Model RF-10AXL)and a pump(Model LC-10AT).The excitation wavelength was set at467nm and that of emission at502nm.Under the column temperature of 35 C,20m l release medium was injected into the HPLC system and eluted via the mobile phase consist of methanol and water(95:5,v/v)at aflow rate of1ml/min. The peak area of C6was recorded at the retention time of5min.2.4.MDCK cell cultureFor endocytosis and exocytosis studies,Madin e Darby canine kidney(MDCK) cells were grown in cultureflasks containing Dulbecco modified Eagle’s minimal essential medium(DMEM,25m M glucose)supplemented with10%(v/v)fetal bovine serum,1%(v/v)L-glutamine,100UI/ml penicillin and100m g/ml streptomycin.After culture at37 C with5%CO2for6days,MDCK cells were digested with0.25% trypsin/0.02%EDTA and seeded in12-well sterile plate at1Â105/ml.For transcytosis studies,MDCK cells were cultured on a polycarbonate membrane(Transwell@,12wells,CORNING)with pores of3m m in diameter. 500m l DMEM was added into the upper compartment of transwell insert and the medium volume in basolateral side was set as1.5ml.During the culture of7days, mediums in both upper and basilar compartments were changed every other day and the transepithelial electrical resistances(TEER)were measured by an epi-thelial volt-U m(Millicell@ERS-2,Millipore).At the end of one week culture,the cell monolayers with TEER value above180U/cm2were selected for the subse-quent studies.2.5.Endocytosis characterization of C6-PNs by MDCK cellsctate dehydrogenase(LDH)release assayMDCK cells were seeded into96-well plate at5Â104/ml in200m l culture medium until80%cells confluence.Prior to the detection,cells were incubated with 1mg/ml PNs in SFM dispersion at37 C for1h.After the incubation,20m l lactate dehydrogenase(LDH)release solution was added into each well for subsequent1h incubation at37 C.Then,120m l obtained cell supernatant was aspirated and transferred into another plate according to the instruction.Finally,the absorbance of supernatant was recorded at490nm by a multiskan FC(Thermo Scientific,USA).2.5.2.Confocal image series of cellular uptake of C6-PNsThe internalization of C6-PNs by MDCK cells was monitored by a confocal laser scanning microscope(CLSM,LEICA TCS SP5,Germany).When MDCK cells cultured in sterile cover slips met the requirement of80%confluence,1mg/ml SFM dispersion of C6-PNs was added and incubated for5min,10min,30min and60min, respectively.In the end of incubation,cells were rinsed with cold PBS,fixed by3.7% paraformaldehyde and sealed with glycine/PBS(v/v¼1:1).Finally,the obtained cover slips containing cell monolayer were detected by CLSM under488nm exci-tation condition.Real-time imaging of MDCK cells in endocytosis study was also conducted via CLSM(LEICA TCS SP2,Germany).MDCK cells were cultured in a sterile glass bottom dish and incubated with1m M DiI at37 C for1h to label the lipid membranes.After rinsed by warming PBS,the living MDCK cells were incubated with1mg/ml SFM dispersion of C6-PNs for1h at37 C.The excitation wavelength for C6-PNs detection was set at488nm and adjusted to561nm for lipid membrane.The merged images between C6-PNs and lipid membrane were obtained by image acquisition software of LEICA.B.He et al./Biomaterials34(2013)4309e4326 43102.5.3.Flow cytometry detection of endocytosisThe influence of temperature on cellular uptake of PNs was quantitatively detected by aflow cytometry system(FCS,FACS Calibur BD,USA).MDCK cells were grown in12-well plate and incubated with1mg/ml SFM dispersion of C6-PNs at 37 C and4 C for5min,15min,30min and60min,respectively.After that,cells were rinsed with cold PBS for three times to abort endocytosis.Digested from plate wells,cells were then collected to form single cell suspension.Then,the mean intracellularfluorescence intensity of cells was measured by FCS with excitation of 488nm.2.6.Exocytosis demonstration of C6-PNsFirstly,MDCK cell monolayer that cultured in12-well plate with more than80% cell confluence was incubated with1mg/ml SFM dispersion of C6-PNs at37 C for 1h.After the aspiration of nanoparticle dispersion,cell monolayer was rinsed by cold PBS for three times and subsequently incubated with only fresh culture me-dium for determined time.The following detection was exactly the same as that in endocytosis study.2.7.Mechanisms detection of endocytosis and exocytosis2.7.1.Cell counting kit-8(CCK-8)assay of multiple pharmacological regulatorsDuring the mechanism studies of endocytosis and exocytosis,different phar-macological agents were chosen(Table1).For the cell viability detection by cell counting kit-8(CCK-8)assay,MDCK cells were cultured in96-well plate at5Â104/ ml in200m l culture medium under37 C and5%CO2.After the adherence of cells, different agents were added under the given concentrations listed in Table1and incubated with cells for6h.Then these regulators were constituted by the CCK-8 solutions as the instruction and cells were further incubated for2h.Finally,the OD value in each well was measured at450nm and the data in at least three wells were detected for each agent.2.7.2.Endocytosis pathway investigationFor the endocytosis pathway detection,various agents at the given concentra-tions listed in Table1were pre-incubated with MDCK cells that seeded in12-well plate for30min.After the aspiration of pre-incubation solutions,1mg/ml C6-PNs SFM dispersion and different agents with the same concentrations were simulta-neously added and further incubated at37 C for1h.The test was aborted by aspirating the nanoparticle/agent dispersions and rinsing cells with cold PBS for three times.Then cells were digested and centrifuged.The cell lysis buffer RIPA was added into the cell pellets to release C6-PNs in cells.By adding methanol to pre-cipitate impurities and dissolve C6from C6-PNs,the supernatant sample was measured by HPLC described above.2.7.3.Intracellular transportation and location of C6-PNs during endocytosisFor the detection of PNs in various types of endosomes,the cover-slips seeded with MDCK cells were treated with SFM dispersion containing1mg/ml C6-PNs for 10min and60min respectively.After washing with PBS andfixation by3.7%par-aformaldehyde,cells were treated with PBS containing0.1%Triton X-100and5%BSA for1h,incubated with rabbit anti-Rab5antibody and mouse anti-Rab7antibody at 37 C for2h,respectively,and then treated with the matchingfluorescence labeled IgG in dark environment for1h.The stained cells were then counterstained to visualize nucleus by hoechst33258and investigated by CLSM.The colocalization analysis was performed via the internal LEICA quantification software.By choosing images under488nm and561nm channels as image pair,the colocalization rates, Pearson’s Correlation(R pc)and Overlap Coefficient(R oc)were obtained.For the detection of C6-PNs in lysosomes,the cells on the glass bottom dish were firstly stained by LysoTracker.Then1mg/ml C6-PNs was added and the incubation was conducted in dark environment at37 C for10min and60min,respectively. After that,cold PBS buffer were added to abort the uptake and cells were further incubated with SFM.The stained living cells were immediately detected by CLSM.2.7.4.Exocytosis characterization of C6-PNsLiving MDCK cell monolayer wasfirstly stained by LysoTracker to mark lyso-somes.After the incubation with1mg/ml C6-PNs,nanoparticles were immediately aspirated and replaced by fresh culture medium for following re-incubation at37 C. Confocal images of cell monolayer were simultaneously recorded as soon as the substitution of medium.Brightfield andfluorescence images under488nm and 561nm excitations were all monitored during the exocytosis studies.To evaluate the effects of lipid raft and Golgi complex on exocytosis,M b CD, brefeldinA and monensin were utilized.After MDCK cell monolayer was incubated with SFM dispersion containing1mg/ml C6-PNs at37 C for1h,culture mediums containing M b CD,brefeldinA or monensin at the given concentrations(Table1) were added to re-incubate with cells for following2h at37 C during exocytosis process.The intracellular intensities of C6-PNs were measured viafluorescence HPLC as described above.parison of molecular mechanisms between endocytosis and exocytosisVarious regulators of signaling related kinases as listed in Table1were utilized to detect their molecular mechanisms during endocytosis and exocytosis.For endocytosis,MDCK cell monolayer wasfirstly pre-incubated with regulators for 30min.Then,it was co-incubated with1mg/ml C6-PNs and regulators at37 C for 1h.For exocytosis,MDCK cell monolayer wasfirstly incubated with1mg/ml C6-PNs 37 C for1h.Then,different regulators were added into culture medium for re-incubation at37 C for2h.The intracellularfluorescence intensities of C6-PNs were all detected by HPLC described above.2.8.Trans-cellular demonstration of C6-PNs across MDCK cell monolayer2.8.1.Confocal image series of the transportation of C6-PNs through MDCK cell monolayerAfter the verification by TEER values,MDCK cell monolayer grown on the porous transwell membrane was incubated with1mg/ml C6-PNs at37 C for3h.The polycarbonate membrane with cells on was carefully taken from Transwell@insert (12-well,CORNING)after the incubation.Washed with PBS buffer andfixed by3.7% paraformaldehyde,the membrane was then stained with165n M rodamine-phalloidine and hoechst33258to label F-actin and cell nuclei,respectively.Finally the membrane was placed in a glass slide and examined by CLSM in x-y-z scanning mode.To investigate the contribution of lysosomes to the trans-cellular process of C6-PNs,MDCK cell monolayer on porous membrane was stained by LysoTracker.Briefly, 1mg/ml SFM dispersion of C6-PNs was added into the upper insert of transwell plate to incubate with stained cell monolayer at37 C for2h.Finally,the porous membrane with cells on was rinsed by PBS,fixed with paraformaldehyde and monitored by CLSM in x-z scanning mode.2.8.2.Trans-cellular demonstration of PNs via NIRF imaging systemNear-infraredfluorescence(NIRF)imaging system(In-Vivo FX PRO,KODAK, USA)was utilized to evaluate the trans-cellular efficiency of PNs through the cell monolayer on porous membrane.DiR loaded PNs were prepared with the same method as C6-PNs,and the mass ratio of DiR to PLGA was also defined as1:1000. After the addition of1mg/ml DiR-PNs in the upper insert of transwell plate for determined times,the cellular uptake was aborted and the transwell was placed in the sample room of imaging system to obtain X-ray images.Then cell monolayer on transwell was carefully scraped with cell scraper,and the porous polycarbonate membrane was taken down.Under near-infraredfluorescence mode with EX 748nm and EM780nm condition,the porous membrane and medium in basolateral compartment of transwell were all detected.For the comparison,the same amount of DiR dispersed in medium was incubated with cells monolayer in transwell.2.8.3.Quantitative detection of trans-cellular of C6-PNsThe MDCK cells cultured on transwell membrane were incubated with1mg/ml SFM dispersion of C6-PNs.At the predetermined time points,100m l solution in basolateral compartment was sampled with and measured byfluorescence HPLC. Meanwhile,the porous membrane was taken down from the plate and the cells on membrane were scraped by cell scraper.After washing with PBS buffer,the obtained porous membrane was put in methanol to dissolve coumarin-6in C6-PNs that adhered in the membrane pores.Thus the total amount of the C6-PNs in basolateral compartment and transwell membrane was defined as the transcytosed C6-PNs. 2.8.4.Trans-cellular pathway detection of C6-PNsWhether PNs were transported through cell monolayer in paracytosis pattern was evaluated by the TEER measurement of cell monolayer.When MDCK cells cultured on porous membrane met the TEER requirement of180U/cm2for trans-cellular detection,1mg/ml PNs was added into insert.TEER values were meas-ured by an epithelial volt-U m(Millicell@ERS-2,Millipore)at determined time points during the incubation.SFM without PNs was also set as control to investigate its influence on TEER.2.9.Mechanism detection of the trans-cellular process of PNsMDCK cells werefirstly cultured on the transwell membrane until TEER was appropriate.Then,the mechanism investigations were conducted under two dif-ferent conditions.For the normal condition,namely the coexistence of endocytosis,Table1Regulators and their concentrations used in mechanism study.Regulators Finalconcentration Regulators FinalconcentrationM b CD10m M BrefeldinA25m g/mlFilipin0.5m g/ml Monensin32.5m g/mlEIPA20m M LY29400250m MNystatin30m M Wortmannin10m MHypertonicSucrose0.4M PMA10m MGenistein100m M Staurosporine1m MB.He et al./Biomaterials34(2013)4309e43264311exocytosis and transcytosis process,cells on the transwell membrane were pre-incubated with different agents for30min,respectively.Afterwards,the incuba-tion medium was aspirated and the SFM containing1mg/ml C6-PNs and different agents were added to the upper compartments to incubate with cells for another4h. After that,the total amount of C6-PNs in both the basolateral compartment and porous membrane was detected byfluorescence HPLC described above.For the condition without endocytosis,cells were cultured with the SFM containing1mg/ml C6-PNs for2h.Then the nanoparticle dispersions were replaced by the SFM con-taining different agents to incubate with cells for another2h.Finally,the trans-cytosed C6-PNs was determined by the same HPLC.2.10.StatisticsAll data shown as mean valueÆSD were obtained via at least three independent experiments and analyzed by ANOVA.A p-value less than0.05was considered to be statistically significant.3.Results and discussion3.1.Preparation and characterization of C6-PNsThe PNs were prepared by classical emulsifying solvent-evaporation method[13].Less than0.1%of coumarin-6(C6),a lip-ophilic dye with strongfluorescence at502nm emission[14],was loaded into PLGA nanoparticles in order to trace PNs in this study. Fig.1shows the characterization of C6-PNs.Via the dynamic light scattering(DLS)detection,the z-average diameter of C6-PNs was 78.82nm(Fig.1A),and the poly distribution index(PDI)was0.089 (<0.1).The sizes observed by Transmission electron microscope (TEM)were also less than100nm with uniform distribution,in agreement with DLS analysis.The uniform size distribution of PNs was favorable in eliminating the influence of sizes on their cellular uptake[15].TEM graph of C6-PNs(Fig.1B)displayed the spherical morphology of PNs.To evaluate the qualification of C6as the marker of PNs,in vitro leakage of C6-PNs was conducted in phos-phate buffer solution(PBS)and serum free medium(SFM)at pH7.2 (simulating newly endocytosed vesicles and caveosomes)and pH 5.5(simulating late endosomes and lysosomes),respectively.As seen in Fig.1C and D,less than4%of C6released under various test conditions up to8h,very tiny compared to the total C6loaded in PNs,indicating that C6could be utilized as the effective marker of PNs for the whole intracellular trafficking pathways among or-ganelles with different pH environments.3.2.Endocytosis of C6-PNs by MDCK epithelial cells3.2.1.LDH release assay of cellsTo evaluate the internalization pattern of PNs by plasma mem-brane of MDCK cells,the LDH assay was conducted.Fig.2A shows the comparison of LDH released between PNs and SFM.There was no significant difference between them,indicating that PNs did not trigger the extra increase of LDH from cytoplasm.The leakage of LDH correlates with the pore formulation of plasma membrane [16].The positive charge and small size less than20nm always make nanoparticles easier to cause the formulation of pores in cell membrane[14,17].As demonstrated above,PNs used here were negatively charged and much larger than20nm.So the cellular uptake of PNs might not be attributed to the pore formation,but likely to the invagination and wrapping of membrane.3.2.2.Confocal image series of endocytosisFig.2B shows the confocal images of MDCK cells incubated with C6-PNs at37 C for different time.At5min,fluorescence spots were only observed at cell junctions or on the surface of cell membranes, and little C6-PNs were found in cytoplasm.With the prolongation of incubation,more C6-PNs internalized into cells in a time-dependent manner,and most C6-PNs were found in cytoplasm at 60min.Meanwhile,living MDCK cells were stained by lipid membrane specific dye DiI(red color)and followed by the incu-bation with C6-PNs at37 C for1h(Fig.2C).Lots of vesicle struc-tures containing C6-PNs(green color)were found in cytoplasm, and obvious colocalization(yellow color)between C6-PNs and DiI was also observed in merged confocal micrograph.This illustrated that C6-PNs were always packed by lipid membrane during and after endocytosis.As a result,it was confirmed that the internal-ization and intracellular transport of C6-PNs were all mediated by lipid vesicles.3.2.3.Flow cytometry detection of endocytosisVia FCS assay,the differences in cellular uptake at37 C and4 C at different incubation times were quantitatively compared (Fig.2D).At all determined time points,the relative intracellular fluorescence percentages of C6-PNs at37 C were always sig-nificantly greater than that at4 C.More concretely,from5min to 60min,the cellular uptake of PNs at37 C was about three times higher than that at4 C.It is reported that the active transportation on cell membranes will significantly reduce under4 C condition [18].The influence of temperature on cellular uptake is often uti-lized to evaluate the active transportation of macromolecules[19]. Therefore,the study here indicated that the endocytosis of PNs by MDCK cells was energy-dependent,involving active process.3.2.4.Endocytosis pathways of PNsDuring the incubation of C6-PNs with MDCK cells,various endocytosis inhibitors were utilized to identify the endocytosis pathways.First,the cell counting kit-8(CCK-8)assay showed that the addition of these inhibitors caused little change in cell viability compared to SFM control(Supplemental Fig.S1),confirming the qualification of those inhibitors for endocytosis investigation.As shown in Fig.3,cholesterol binding agents,M b CD andfilipin, known as inhibitors of caveolae/lipid raft-mediated endocytosis, reduced the cell uptake of PNs significantly.The cholesterol sequestering agent nystatin,which triggers the absence of lipid rafts and caveolaes in cells,also inhibited the internalization of nanoparticles.The hyperosmotic sucrose,inhibiting the clathrin-dependent endocytosis by Kþdepletion effect,reduced the endo-cytosis by55.4%.However,macropinocytosis inhibitor EIPA had no effect on endocytosis.The inhibition effect of M b CD on cellular uptake of C6-PNs was most obvious compared to other inhibitors used in this study, reducing the cellular uptake by96.8%.It is reported that,lots of cholesterol-rich microdomains named as lipid rafts andflask-shaped structures rich in proteins and lipids including cholesterol called caveolaes,distribute with different extents in various cell membranes[20].The internalization of many extracellular macro-molecules is reported to be mediated by lipid rafts or caveolaes via specific or non-specific interactions[21].After pinched off from plasma membrane,the internalized vesicle containing extracellular cargos forms a special structure named as caveosome,which is about50e80nm in diameter[22].The endocytosis process based on lipid raft/caveolae can be effectively blocked via the depletion of cholesterol of M b CD[23].In terms of polar MDCK epithelial cells,it has been verified that the apical membranes of cells are devoid of caveolaes but distributed with lipid rafts[24].So we actually demonstrated that the endocytosis of PNs by MDCK cell monolayer was mediated by lipid rafts in plasma membrane.Although the sequestering effects offilipin and nystatin on cholesterol were more moderate than M b CD[25],their inhibitions on cellular uptake of PNs further confirmed the lipid raft dependency of endocytosis.Clathrin-mediated endocytosis is the classical endocyotis path-way of macromolecules and particles.Via the formulation of clathrin-coated pits,extracellular substances are loaded into cellB.He et al./Biomaterials34(2013)4309e4326 4312。
