Mifepristone_DataSheet_MedChemExpress
非索非那定片29页

上市信息
非索非那定fexofenadine,为一抗过敏药物, 于2019年上市后,相继在美国\澳大利亚、 澳地利、比利时、巴西、加拿大、丹麦、 芬兰、法国、德国、希腊、香港、印度、 日本、卢森堡、马来西亚、墨西哥、荷兰、 新西兰、挪威、葡萄牙、南非、西班牙、 瑞典、瑞士、泰国、英国等数十个国家和 地区上市,用于治疗过敏性鼻炎、荨麻疹 等过敏性疾病。
盐酸非索非那定片
----毕馨
【分子式】 C32H39NO4·HCl 【分子量】 538.13 【性状】 本品为薄膜衣片,除去包衣后显白色或类白色。
2020/3/29
上市信息
赫斯特公司开发,2019年首次在欧洲上市 2019年FDA批准了SANOFI AVENTIS的新 药申请 2019年10月FDA批准口服混悬剂
2020/3/29
适应症
季节性过敏性鼻炎 适用于缓解成人和6岁及6岁以上的儿童的 季节性过敏性鼻炎相关的症状。如打喷嚏, 流鼻涕,鼻、上腭、咽喉发痒,眼睛发痒、 潮湿、发红。
慢性特发性荨麻疹 适用于治疗成人和6岁及6岁以上儿童的慢 性特发性荨麻疹的皮肤症状,能够减轻瘙 痒和风团的数量。
2020/3/29
2020/3/29
毒性反应
急性毒性试验: 口服毒性低,小鼠和大鼠口服LD50均大于 5000mg/kg
亚急性毒性试验: 犬连续口服本品 100mg/kg28天,均未观 察到任何不良反应发生
2020/3/29
毒性反应
体内和体外试验结果表明,非索非那定没 有致癌性、致突变性
支物生殖毒性研究:当给予大鼠和家兔口 服特非那丁高达300mg/kg,它们产生的非 索非那定血浆AUC值分别相当于人体治疗值 (60mg,一日二次)的4倍和37倍,结果 均未发现有致畸的作用。
Teriflunomide_DataSheet_MedChemExpress

Inhibitors, Agonists, Screening Libraries Data SheetBIOLOGICAL ACTIVITY:Teriflunomide is the active metabolite of leflunomide, which inhibits pyrimidine de novo synthesis by blocking the enzyme dihydroorotate dehydrogenase, used as an immunomodulatory agent.In Vitro: Teriflunomide primarily acts as an inhibitor of dihydroorotate dehydrogenase (DHODH), a key mitochondrial enzymeinvolved in the de novo synthesis of pyrimidines in rapidly proliferating cells. By reducing the activity of high–avidity proliferating T lymphocytes and B lymphocytes, teriflunomide likely attenuates the inflammatory response to autoantigens in MS. Thus,teriflunomide can be considered a cytostatic rather than a cytotoxic drug to leukocytes [1].In Vivo: Teriflunomide has demonstrated beneficial effects in two independent animal models of demyelinating disease. In the dark agouti rat model of experimental autoimmune encephalitis (EAE), teriflunomide administration results in clinical, histopathological,and electrophysiological evidence of efficacy both as a prophylactic and therapeutic agent. Similarly, in the female Lewis rat model of EAE, teriflunomide administration results in beneficial prophylactic and therapeutic clinical effects, with a delay in disease onset and symptom severity [1].References:[1]. Oh J, et al. An update of teriflunomide for treatment of multiple sclerosis. Ther Clin Risk Manag. 2013;9:177–90.Product Name:Teriflunomide Cat. No.:HY-15405CAS No.:163451-81-8Molecular Formula:C 12H 9F 3N 2O 2Molecular Weight:270.21Target:Others Pathway:Others Solubility:DMSO: 26 mg/mLCaution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@ Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
非索非那定片

用药安全
【儿童用药】 儿童用药】 盐酸非索非那定对6 盐酸非索非那定对6岁以下儿童患者的安全 性和疗效尚未建立。 【老年患者用药】 老年患者用药】 尚不能确定老年患者与年轻患者的反应是 否有差异。但是由于该药物经肾脏充分排 泄,肾功能损伤的患者药物毒性反应发生 的危险性有可能增加。
第三代抗组胺药物
盐酸非索非那定片 ----毕馨
-------- 玉兴堂医药学术推广部
20122012-4-5
盐酸非索非那定片
----毕馨 ----毕馨
【分子式】 C32H39NO4HCl 【分子量】 538.13 【性状】 本品为薄膜衣片,除去包衣后显白色或类白色。
20122012-4-5
上市信息
20122012-4-5
用法用量
慢性特发性荨麻疹 成人、12岁及12岁以上儿童,盐酸非索非 成人、12岁及12岁以上儿童,盐酸非索非 那定的推荐剂量为60mg,一日2 那定的推荐剂量为60mg,一日2次。肾功 能不全的患者推荐起始剂量为60mg一日一 能不全的患者推荐起始剂量为60mg一日一 次。 6至11岁儿童,盐酸非索非那定的推荐剂量 11岁儿童,盐酸非索非那定的推荐剂量 为30mg,一日2次,肾功能不全的儿童患 30mg,一日2 者推荐起始剂量30mg,一日1 者推荐起始剂量30mg,一日1次。
20122012-4-5
优势特点
⑴作用机制明确; ⑵半衰期长,作用时间长,服用方便; ⑶不被肝脏代谢,能安全用于肝功能损害 的患者,对肝脏无损伤; ⑷作用特点明显:非索非那定对过敏性鼻 炎和慢性自发性寻麻疹有效且耐受性好, 对组胺引起的皮肤疹块和潮红有一定抑制 作用。 ⑸不良反应少轻,未见有心脏毒性。
上市信息
培非格司亭中英文介绍

王婕913103860408NEULASTA(PEGFILGRASTIM)|培非格司亭注射液1.Introduction(简介)【产地英文商品名】:NEULASTA-6mg/0.6ml/Syringe【原产地英文药品名】:PEGFILGRASTIM【中文参考商品译名】:纽拉思塔-6毫克/0.6毫升/支【中文参考药品译名】:培非格司亭【生产厂家中文参考译名】:安进【生产厂家英文名】:Amgen, IncAmgen Announces Novel Drugs for Antitumor Chemotherapy Side Effects of FGT (TM) (pegfilgrastim), a drug developed by the US Food and Drug Administration (FDA), has been approved by the US Food and Drug Administration (FDA) Approval. Amphetamycin, the chief executive of Amgen, says that pemetrexedin will make it easier for healthcare workers to prevent chemotherapy-induced neutropenia and its serious complications. The third drug approved by Amgen in the past six months will significantly improve the prognosis of chemotherapy patients and is expected to enter the market in early April.BUSINESS WIRE 2002年2月1日美国加州THOUSAND OAKS消息,安进公司宣布抗肿瘤化疗副作用新药培非格司亭(TM) (pegfilgrastim)通过美国食品与药品管理局(FDA)的审批。
AMI-1_DataSheet_MedChemExpress

Inhibitors, Agonists, Screening Libraries Data SheetBIOLOGICAL ACTIVITY:AMI–1 is a potent, cell–permeable compound which inhibits protein arginine N–methyltransferases (PRMTs), including human PRMT1(IC50 = 8.8μM) and yeast–Hmt1p (IC50 = 3.0μM), by blocking peptide–substrate binding.IC50 value: 8.8μM (human PRMT1), 3.0μM (yeast–Hmt1p)Target: human PRMT1, yeast–Hmt1pin vitro: AMI–1 suppresses the transcriptional coactivator activity of PRMT1 and PRMT4 and it inhibits HIV–1 RT polymerase (IC50 =5.0μM). PRMT1 methylates histone H4, and is essential for other subsequent histone modifications.[1] AMI–1 is the most active nonpeptidic inhibitor reported to be selective against PRMT1. AMI–1 is a selective PRMT inhibitor with a bisanionic structure that is related to compounds known to generate pleiotropic interactions with many proteins, should be further optimized before exploring additional binding pockets. [2]in vivo: AMI–1 is administered intranasally to chronic AIPI rats to determine PRMT effects on asthmatic parameters. AMI–1 inhibited the expression of COX2 in TGF–β–stimulated cells. AMI–1 administered to AIPI rats reduced COX2 production and humoral immune response, and it abrogated mucus secretion and collagen generation.[1]PROTOCOL (Extracted from published papers and Only for reference)Cell assay [3]INS–1 cells were grown in a humidified atmosphere containing 95 % air and 5 % CO2 in RPMI–1640 medium containing 11.1 mM glucose, 10 % FBS, 1 mM pyruvate, 10 mM HEPES, 50 lM 2–mercaptoethanol, 100 U/mL penicillin, and 100 lg/mL streptomycin. Cells were then transfected with siPRMT1 or the indicated plasmid, and subsequently cultured in RPMI–1640 medium containing 5.6 or 25mM glucose (5.6 G and 25 G, respectively) and/ or 100 lM AMI–1. For transfection with siPRMT1 (target sequence50–CCAACGCCTGCCTCATAAA–30) or pALTER– FOXO1, INS–1 cells grown in 6–well plates were transfected using Lipofectamine 2000,and the media were replaced 6 h after transfection. Seventy–two hours after transfection, the cells were treated with 25 mM glucose and/or AMI–1 (100 lM) for an additional 48 h, and then harvested for the assays described below.Animal administration [1]For the AMI–1 treatment experiment, 24 rats were divided into three groups: control group, chronic AIPI group, and AMI–1 group.Two weeks after sensitization, control group rats were sham sensitized and exposed to the same volume of PBS. In the AMI–1 group,rats were administered 50μl AMI–1 at a concentration of 0.1 mg/ml in PBS 2 h before OVA challenge. The asthma index included serum levels of OVA–specific IgG1 and total serum IgE, which were determined by ELISA as described in previous studies.References:Product Name:AMI–1Cat. No.:HY-18962CAS No.:20324-87-2Molecular Formula:C 21H 14N 2Na 2O 9S 2Molecular Weight:548.45Target:Histone Methyltransferase Pathway:Epigenetics Solubility:DMSO: ≥ 46 mg/mL[1]. Sun Q, et al. PRMT1 Upregulated by Epithelial Proinflammatory Cytokines Participates in COX2 Expression in Fibroblasts and Chronic Antigen–Induced Pulmonary Inflammation. J Immunol. 2015 Jul 1;195(1):298–306.[2]. Castellano S, et al. Design, synthesis and biological evaluation of carboxy analogues of arginine methyltransferase inhibitor 1 (AMI–1). ChemMedChem. 2010 Mar 1;5(3):398–414.[3]. Lv L, et al. PRMT1 promotes glucose toxicity–induced β cell dysfunction by regulating the nucleo–cytoplasmic trafficking of PDX–1 in aFOXO1–dependent manner in INS–1 cells. Endocrine. 2015 Aug;49(3):669–682.[4]. Wang J, et al. Pharmacophore–based virtual screening and biological evaluation of small molecule inhibitors for protein arginine methylation. J Med Chem. 2012 Sep 27;55(18):7978–7987.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
西咪替丁的化学结构式

西咪替丁的化学结构式1. 西咪替丁的概述西咪替丁(Simvastatin)是一种用于降低胆固醇和脂蛋白水平的药物,属于他汀类药物。
它通过抑制胆固醇合成的关键酶HMG-CoA还原酶,从而减少胆固醇在体内的合成。
西咪替丁是一种处方药,常用于治疗高胆固醇和高脂蛋白血症,预防心血管疾病的发生。
2. 西咪替丁的化学结构式西咪替丁的化学名为(1S,3R,7S,8S,8aR)-8-{2-[(2R,4R)-4-羟基-6-氧代-3,5-二甲基-4-甲硫基-4-氧代-5-氮-6-甲基-1,2,3,4-四氢-2-吡啶基]乙基}-3,7-二甲基-1,2,3,7,8,8a-六氢-1-萘酮。
西咪替丁的化学式为C25H38O5S,分子量为418.57 g/mol。
西咪替丁的结构式如下所示:3. 西咪替丁的合成途径西咪替丁的合成途径相对复杂,主要包括以下几个步骤:3.1 邻氨基苯甲酸的合成首先,通过邻氨基苯甲酸的合成作为起始原料。
邻氨基苯甲酸是通过对硝基苯甲酸的氢化还原得到的。
3.2 吡咯的合成邻氨基苯甲酸与乙酰乙酸乙酯反应生成吡咯化合物。
该反应需要碱催化。
3.3 吡咯的环化吡咯化合物通过烷基化反应得到环化产物。
该反应需要环化试剂和酸催化。
3.4 吡咯的氧化环化产物经氧化反应生成相应的醛。
该反应需要氧化剂。
3.5 醛的还原醛经还原反应生成相应的醇。
该反应需要还原试剂。
3.6 醇的酯化醇经酯化反应生成相应的酯。
该反应需要酯化试剂和酸催化。
3.7 酯的水解酯经水解反应生成相应的酸。
该反应需要水解试剂。
最终,通过以上合成步骤,得到西咪替丁。
4. 西咪替丁的药理作用西咪替丁通过抑制HMG-CoA还原酶的活性,阻断胆固醇的合成途径,从而降低体内胆固醇水平。
此外,西咪替丁还可以增加低密度脂蛋白受体的表达,促进低密度脂蛋白的清除,进一步降低胆固醇水平。
西咪替丁的主要药理作用包括:4.1 降低胆固醇水平西咪替丁通过抑制胆固醇的合成,可以显著降低总胆固醇、低密度脂蛋白胆固醇和甘油三酯的水平。
美国Medchemexpress化合物库(小分子库)_Medchemexpress_(MCE中国)

美国Medchemexpress化合物库(小分子库)-原装进口,现货供
应,提供组合定制服务
品牌:Medchemexpress (MCE)
保存条件:-20℃
供应商:MCE中国
数量:大量
保质期:2年
Size:
Pre-dissolved DMSO/Solid(Or dry solid)
100 uL/well (10 mM solution)
200 uL/well (10 mM solution)
MedChemExpress (MCE)专注于各种抑制剂、调节剂、API、天然产物及化合物库,总部位于美国新泽西。
MCE经过十年努力已成为全球生物活性小分子领域的一流供应商。
MedChemExpress(MCE)产品涵盖近20个热门研究领域,1000多个细分靶点,超过3000个现货抑制剂、拮抗剂和激动剂。
相关的应用成果已发表于Nature、Cell等国际知名杂志,在全球20余个国家地区设有代理机构。
上海皓元生物医药科技有限公司(MCE 中国) 是MedChemExpress (MCE) 亚洲总代理。
MCE化合物库涵盖20余种不同的类型,超过2500个化合物,进口原装,
现货供应,提供详实的生物活性信息、化学结构信息、质控图谱(NMR和HPLC 等)。
还可根据您的实际研究需要,为您度身定制任意组合、规格、布板的特殊化合物库。
/screening-libraries.html
现有特色化合物库有:。
美诺芬成分

美诺芬成分
(原创版)
目录
1.美诺芬的定义与背景
2.美诺芬的主要成分
3.美诺芬的药理作用与应用
4.美诺芬的副作用与注意事项
5.美诺芬的未来发展前景
正文
【美诺芬的定义与背景】
美诺芬(Minoxidil)是一种用于治疗高血压和心衰的药物,由美国辉瑞制药公司研发并在 1979 年首次上市。
美诺芬属于一类称为“ACE 抑制剂”的药物,其作用机制是通过抑制血管紧张素转换酶(ACE)的活性,从而降低血压和减轻心脏负担。
【美诺芬的主要成分】
美诺芬的主要成分是 Minoxidil,化学名为 3-[[2-[(2-甲基 -3-硝基 -4-羟基苯基) 甲基]-1H-咪唑 -1-基] 甲基]-2-硝基 -4-羟基苯酚。
【美诺芬的药理作用与应用】
美诺芬的药理作用主要是通过抑制 ACE 的活性,减少血管紧张素Ⅱ的生成,从而降低血压和减轻心脏负担。
此外,美诺芬还可以减少醛固酮的分泌,减少水钠潴留,进一步降低血压。
美诺芬主要用于治疗高血压和心衰,也可用于降低糖尿病和肾脏疾病患者的高血压。
【美诺芬的副作用与注意事项】
美诺芬的常见副作用包括头痛、乏力、低血压、皮疹、瘙痒等。
在服
用美诺芬期间,应注意监测血压,避免出现低血压的情况。
此外,孕妇、哺乳期妇女、儿童以及对美诺芬过敏的患者应慎用或禁用。
【美诺芬的未来发展前景】
随着对美诺芬研究的深入,科学家们发现美诺芬除了具有降压作用外,还具有其他多种生物活性。
例如,美诺芬可以通过抗氧化、抗炎等作用保护心血管系统,降低心血管疾病的发生风险。
此外,美诺芬在神经保护、抗肿瘤等方面也具有一定的潜力。
Mifepristone_SDS_MedChemExpress

Inhibitors, Agonists, Screening LibrariesSafety Data Sheet Revision Date:May-24-2017Print Date:May-24-20171. PRODUCT AND COMPANY IDENTIFICATION1.1 Product identifierProduct name :MifepristoneCatalog No. :HY-13683CAS No. :84371-65-31.2 Relevant identified uses of the substance or mixture and uses advised againstIdentified uses :Laboratory chemicals, manufacture of substances.1.3 Details of the supplier of the safety data sheetCompany:MedChemExpress USATel:609-228-6898Fax:609-228-5909E-mail:sales@1.4 Emergency telephone numberEmergency Phone #:609-228-68982. HAZARDS IDENTIFICATION2.1 Classification of the substance or mixtureGHS Classification in accordance with 29 CFR 1910 (OSHA HCS)Acute toxicity, Oral (Category 4),H302Acute aquatic toxicity (Category 1),H400Chronic aquatic toxicity (Category 1),H4102.2 GHS Label elements, including precautionary statementsPictogramSignal word WarningHazard statement(s)H302 Harmful if swallowed.H410 Very toxic to aquatic life with long lasting effects.Precautionary statement(s)P264 Wash skin thoroughly after handling.P270 Do not eat, drink or smoke when using this product.P273 Avoid release to the environment.P301 + P312 IF SWALLOWED: Call a POISON CENTER or doctor/ physician if you feel unwell.P330 Rinse mouth.P391 Collect spillage.P501 Dispose of contents/ container to an approved waste disposal plant.2.3 Other hazardsNone.3. COMPOSITION/INFORMATION ON INGREDIENTS3.1 SubstancesSynonyms:RU⁻38486; RU⁻486; RU 486; RU 38486; RU486; RU38486Formula:C29H35NO2Molecular Weight:429.59CAS No. :84371-65-34. FIRST AID MEASURES4.1 Description of first aid measuresEye contactRemove any contact lenses, locate eye-wash station, and flush eyes immediately with large amounts of water. Separate eyelids with fingers to ensure adequate flushing. Promptly call a physician.Skin contactRinse skin thoroughly with large amounts of water. Remove contaminated clothing and shoes and call a physician.InhalationImmediately relocate self or casualty to fresh air. If breathing is difficult, give cardiopulmonary resuscitation (CPR). Avoid mouth-to-mouth resuscitation.IngestionWash out mouth with water; Do NOT induce vomiting; call a physician.4.2 Most important symptoms and effects, both acute and delayedThe most important known symptoms and effects are described in the labelling (see section 2.2).4.3 Indication of any immediate medical attention and special treatment neededTreat symptomatically.5. FIRE FIGHTING MEASURES5.1 Extinguishing mediaSuitable extinguishing mediaUse water spray, dry chemical, foam, and carbon dioxide fire extinguisher.5.2 Special hazards arising from the substance or mixtureDuring combustion, may emit irritant fumes.5.3 Advice for firefightersWear self-contained breathing apparatus and protective clothing.6. ACCIDENTAL RELEASE MEASURES6.1 Personal precautions, protective equipment and emergency proceduresUse full personal protective equipment. Avoid breathing vapors, mist, dust or gas. Ensure adequate ventilation. Evacuate personnel to safe areas.Refer to protective measures listed in sections 8.6.2 Environmental precautionsTry to prevent further leakage or spillage. Keep the product away from drains or water courses.6.3 Methods and materials for containment and cleaning upAbsorb solutions with finely-powdered liquid-binding material (diatomite, universal binders); Decontaminate surfaces and equipment by scrubbing with alcohol; Dispose of contaminated material according to Section 13.7. HANDLING AND STORAGE7.1 Precautions for safe handlingAvoid inhalation, contact with eyes and skin. Avoid dust and aerosol formation. Use only in areas with appropriate exhaust ventilation.7.2 Conditions for safe storage, including any incompatibilitiesKeep container tightly sealed in cool, well-ventilated area. Keep away from direct sunlight and sources of ignition.Recommended storage temperature:Powder-20°C 3 years4°C 2 yearsIn solvent-80°C 6 months-20°C 1 monthShipping at room temperature if less than 2 weeks.7.3 Specific end use(s)No data available.8. EXPOSURE CONTROLS/PERSONAL PROTECTION8.1 Control parametersComponents with workplace control parametersThis product contains no substances with occupational exposure limit values.8.2 Exposure controlsEngineering controlsEnsure adequate ventilation. Provide accessible safety shower and eye wash station.Personal protective equipmentEye protection Safety goggles with side-shields.Hand protection Protective gloves.Skin and body protection Impervious clothing.Respiratory protection Suitable respirator.Environmental exposure controls Keep the product away from drains, water courses or the soil. Cleanspillages in a safe way as soon as possible.9. PHYSICAL AND CHEMICAL PROPERTIES9.1 Information on basic physical and chemical propertiesAppearance White to off-white (Solid)Odor No data availableOdor threshold No data availablepH No data availableMelting/freezing point No data availableBoiling point/range No data availableFlash point No data availableEvaporation rate No data availableFlammability (solid, gas)No data availableUpper/lower flammability or explosive limits No data availableVapor pressure No data availableVapor density No data availableRelative density No data availableWater Solubility No data availablePartition coefficient No data availableAuto-ignition temperature No data availableDecomposition temperature No data availableViscosity No data availableExplosive properties No data availableOxidizing properties No data available9.2 Other safety informationNo data available.10. STABILITY AND REACTIVITY10.1 ReactivityNo data available.10.2 Chemical stabilityStable under recommended storage conditions.10.3 Possibility of hazardous reactionsNo data available.10.4 Conditions to avoidNo data available.10.5 Incompatible materialsStrong acids/alkalis, strong oxidising/reducing agents.10.6 Hazardous decomposition productsUnder fire conditions, may decompose and emit toxic fumes.Other decomposition products - no data available.11.TOXICOLOGICAL INFORMATION11.1 Information on toxicological effectsAcute toxicityClassified based on available data. For more details, see section 2Skin corrosion/irritationClassified based on available data. For more details, see section 2Serious eye damage/irritationClassified based on available data. For more details, see section 2Respiratory or skin sensitizationClassified based on available data. For more details, see section 2Germ cell mutagenicityClassified based on available data. For more details, see section 2CarcinogenicityIARC: No component of this product present at a level equal to or greater than 0.1% is identified as probable, possible or confirmed human carcinogen by IARC.ACGIH: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by ACGIH.NTP: No component of this product present at a level equal to or greater than 0.1% is identified as a anticipated or confirmed carcinogen by NTP.OSHA: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by OSHA.Reproductive toxicityClassified based on available data. For more details, see section 2Specific target organ toxicity - single exposureClassified based on available data. For more details, see section 2Specific target organ toxicity - repeated exposureClassified based on available data. For more details, see section 2Aspiration hazardClassified based on available data. For more details, see section 212. ECOLOGICAL INFORMATION12.1 ToxicityNo data available.12.2 Persistence and degradabilityNo data available.12.3 Bioaccumlative potentialNo data available.12.4 Mobility in soilNo data available.12.5 Results of PBT and vPvB assessmentPBT/vPvB assessment unavailable as chemical safety assessment not required or not conducted.12.6 Other adverse effectsNo data available.13. DISPOSAL CONSIDERATIONS13.1 Waste treatment methodsProductDispose substance in accordance with prevailing country, federal, state and local regulations.Contaminated packagingConduct recycling or disposal in accordance with prevailing country, federal, state and local regulations.14. TRANSPORT INFORMATIONDOT (US)This substance is considered to be non-hazardous for transport.IMDGUN number: 3077Class: 9Packing group: IIIEMS-No: F-A, S-FProper shipping name: ENVIRONMENTALLY HAZARDOUS SUBSTANCE, SOLID, N.O.S.Marine pollutant: Marine pollutant.IATAUN number: 3077Class: 9Packing group: IIIProper shipping name: Environmentally hazardous substance, solid, n.o.s.15. REGULATORY INFORMATIONSARA 302 Components:No chemicals in this material are subject to the reporting requirements of SARA Title III, Section 302.SARA 313 Components:This material does not contain any chemical components with known CAS numbers that exceed the threshold (De Minimis) reporting levels established by SARA Title III, Section 313.SARA 311/312 Hazards:No SARA Hazards.Massachusetts Right To Know Components:No components are subject to the Massachusetts Right to Know Act.Pennsylvania Right To Know Components:No components are subject to the Pennsylvania Right to Know Act.New Jersey Right To Know Components:No components are subject to the New Jersey Right to Know Act.California Prop. 65 Components:This product does not contain any chemicals known to State of California to cause cancer, birth defects, or anyother reproductive harm.16. OTHER INFORMATIONCopyright 2017 MedChemExpress. The above information is correct to the best of our present knowledge but does not purport to be all inclusive and should be used only as a guide. The product is for research use only and for experienced personnel. It must only be handled by suitably qualified experienced scientists in appropriately equipped and authorized facilities. The burden of safe use of this material rests entirely with the user. MedChemExpress disclaims all liability for any damage resulting from handling or from contact with this product.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
西咪替丁结构式

西咪替丁结构式西咪替丁(Simvastatin)是一种常用的降低胆固醇的药物,属于一种HMG-CoA还原酶抑制剂,它的化学名为(1S,3R,7S,8S,8aR)-8-[2-[(2R,4R)-4-hydroxy-6-oxooxan-2-yl]ethyl]-3,7-dimethyl-1,2,3,7,8,8a-hexahydronaphthalen-1-yl 2,2-dimethylbutanoate,其分子式为C25H38O5,分子量为418.57 g/mol。
西咪替丁是一种无色晶体,熔点为137-139℃,它的水溶性较差,在水中难以溶解,但是在乙醇、丙酮、二甲基亚砜等有机溶剂中容易溶解。
西咪替丁最初是在1980年由美国药物制剂公司密歇根大学药学院自然产物实验室第一次合成的。
它是一种世界范围内广泛使用的药物,被公认是降低胆固醇的最有效药物之一。
它的药效一般在服用后的4-8周之内开始显现。
西咪替丁通过抑制HMG-CoA还原酶的活性来抑制胆固醇的合成。
HMG-CoA还原酶是迄今为止发现的最主要的胆固醇合成途径中的限速酶之一,它参与了胆固醇、同型半萜、甾体激素的合成以及细胞信号传递等多种生物过程。
西咪替丁可以通过降低体内胆固醇水平来预防冠心病、中风和其他心血管疾病,并通过降低胆固醇水平来防止动脉粥样硬化的进一步发展。
从化学结构上来看,西咪替丁是由一系列的环和链组成的。
其中,环是由七个碳原子和一个1,2,3,7,8,8a-六氢萘基组成,六环上连接有一个羧酸基,与一个2,2-二甲基丁酸乙酯基相连。
链是由一个来自D-半乳糖的六元环和一个1,2,3-三氢-1-羟基环丙基基组成,链上还连接有一个加分子的2,4,6-苯三酚环。
总之,西咪替丁结构式的研究对于了解它的化学性质、药效机理等方面含义深远。
未来,结构式的研究将有助于优化西咪替丁的疗效,为人体健康做出更大的贡献。
如何安全使用Meperidine

如何安全使用Meperidine蘇麗如藥師、莊美華主任大林慈濟醫院藥劑科前言有效且安全的疼痛控制是疼痛治療的終極目標,鴉片類止痛藥於臨床普遍應用於急性或慢性疼痛之控制,然而並非所有的鴉片類止痛藥其藥理特性都是相同的,尤其以meperidine有許多使用時應特別考量之風險。
Meperidine簡介Meperidine於1942年於美國上市,是一種合成的鴉片類止痛藥,若經由靜脈給藥,約1-5分鐘即可止痛,口服、皮下或肌肉注射則約10-15分鐘發揮止痛作用,由於具有如此快的疼痛緩解作用,因此廣泛應用於疼痛的控制。
Meperidine於人體之代謝主要經由兩種途徑,其一是經由肝臟的carboxylesterase代謝成meperidinic acid (非活性代謝物),另一種途徑則是經由肝臟的CYP-450(cytochrome P-450)酵素系統代謝成normeperidine,而normeperidine是一種非鴉片類具有神經毒性的活性代謝物,於體內代謝後經由腎臟排出,它亦如同meperidine具有止痛效果(止痛強度為meperidine之一半),另具有強效的中樞神經興奮作用1,2。
因此meperidine於臨床使用上須特別注意劑量之調整,CLcr 10-50 ml/mins的患者,meperidine之建議劑量為一般正常人之75 %,若CLcr < 10 ml/mins的患者,則建議投與劑量減少為一般正常人之50 %。
Meperidine使用之風險由於meperidine於肝臟經由CYP-450代謝後,產生的活性代謝物normeperidine,會刺激中樞神經系統產生焦慮、震顫、肌肉痙攣,甚至可能發生癲癇,而且normeperidine是經由腎臟排出體外,對於腎功能不良、老年人、癌症患者、鎌狀細胞性貧血的患者,在使用meperidine以後更容易因為normeperidine 蓄積於體內,而發生前述中樞神經系統方面的副作用3,4。
Mifepristone_84371-65-3_DataSheet_MedChemExpress
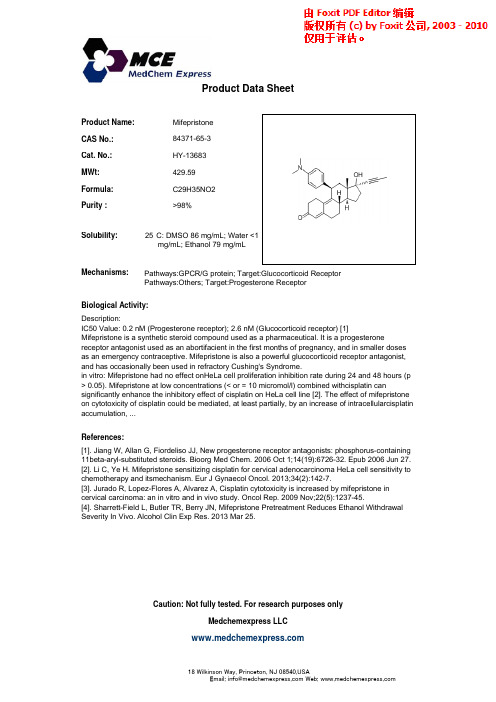
Product Name:Mifepristone CAS No.:84371-65-3Cat.No.:HY-13683Product Data SheetCat. No.:HY 13683MWt:429.59Formula:C29H35NO2Purity :>98%25°C:DMSO 86mg/mL;Water<1Solubility:Mechanisms:Biological Activity:D i tiPathways:GPCR/G protein; Target:Glucocorticoid ReceptorPathways:Others; Target:Progesterone Receptor 25C: DMSO 86 mg/mL; Water 1mg/mL; Ethanol 79 mg/mLDescription:IC50 Value: 0.2 nM (Progesterone receptor); 2.6 nM (Glucocorticoid receptor) [1]Mifepristone is a synthetic steroid compound used as a pharmaceutical. It is a progesteronereceptor antagonist used as an abortifacient in the first months of pregnancy, and in smaller doses as an emergency contraceptive. Mifepristone is also a powerful glucocorticoid receptor antagonist,and has occasionally been used in refractory Cushing's Syndrome.in vitro: Mifepristone had no effect onHeLa cell proliferation inhibition rate during 24 and 48 hours (p > 0.05). Mifepristone at low concentrations (< or = 10 micromol/l) combined withcisplatin cansignificantly enhance the inhibitory effect of cisplatin on HeLa cell line [2]The effect of mifepristone References:[1]. Jiang W, Allan G, Fiordeliso JJ, New progesterone receptor antagonists: phosphorus-containing11beta-aryl-substituted steroids. Bioorg Med Chem. 2006 Oct 1;14(19):6726-32. Epub 2006 Jun 27.[2]. Li C, Ye H. Mifepristone sensitizing cisplatin for cervical adenocarcinoma HeLa cell sensitivity to significantly enhance the inhibitory effect of cisplatin on HeLa cell line [2]. The effect of mifepristone on cytotoxicity of cisplatin could be mediated, at least partially, by an increase of intracellularcisplatin accumulation, ...chemotherapy and itsmechanism. Eur J Gynaecol Oncol. 2013;34(2):142-7.[3]. Jurado R, Lopez-Flores A, Alvarez A, Cisplatin cytotoxicity is increased by mifepristone incervical carcinoma: an in vitro and in vivo study. Oncol Rep. 2009 Nov;22(5):1237-45.[4]. Sharrett-Field L, Butler TR, Berry JN, Mifepristone Pretreatment Reduces Ethanol WithdrawalSeverity In Vivo. Alcohol Clin Exp Res. 2013 Mar 25.Caution: Not fully tested. For research purposes onlyMedchemexpress LLC18W i l k i n s o n W a y , P r i n c e t o n , N J 08540,U S AE m a i l : i n f o @m e d c h e m e x p r e s s .c o m W e b : w w w .m e d c h e m e x p r e s s .c om。
人巨噬细胞移动抑制因子(MIF)试剂盒使用方法

人巨噬细胞移动抑制因子(MIF)试剂盒使用方法检测范围:96T4.25μg/L -100μg/L使用目的:本试剂盒用于测定人血清、血浆及相关液体样本中巨噬细胞移动抑制因子(MIF)含量。
实验原理本试剂盒应用双抗体夹心法测定标本中人巨噬细胞移动抑制因子(MIF)水平。
用纯化的人巨噬细胞移动抑制因子(MIF)抗体包被微孔板,制成固相抗体,往包被单抗的微孔中依次加入巨噬细胞移动抑制因子(MIF),再与HRP标记的巨噬细胞移动抑制因子(MIF)抗体结合,形成抗体-抗原-酶标抗体复合物,经过彻底洗涤后加底物TMB显色。
TMB在HRP酶的催化下转化成蓝色,并在酸的作用下转化成最终的黄色。
颜色的深浅和样品中的巨噬细胞移动抑制因子(MIF)呈正相关。
用酶标仪在450nm波长下测定吸光度(OD值),通过标准曲线计算样品中人巨噬细胞移动抑制因子(MIF)浓度。
试剂盒组成1 30倍浓缩洗涤液20ml×1瓶7 终止液6ml×1瓶2 酶标试剂6ml×1瓶8 标准品(200μg/L)0.5ml×1瓶3 酶标包被板12孔×8条9 标准品稀释液 1.5ml×1瓶4 样品稀释液6ml×1瓶10 说明书1份5 显色剂A液6ml×1瓶11 封板膜2张6 显色剂B液6ml×1/瓶12 密封袋1个标本要求1.标本采集后尽早进行提取,提取按相关文献进行,提取后应尽快进行实验。
若不能马上进行试验,可将标本放于-20℃保存,但应避免反复冻融2.不能检测含NaN3的样品,因NaN3抑制辣根过氧化物酶的(HRP)活性。
操作步骤1.标准品的稀释:本试剂盒提供原倍标准品一支,用户可按照下列图表在小试管中进行稀释。
100μg/L 5号标准品150μl的原倍标准品加入150μl标准品稀释液50μg/L 4号标准品150μl的5号标准品加入150μl标准品稀释液25μg/L 3号标准品150μl的4号标准品加入150μl标准品稀释液12.5μg/L 2号标准品150μl的3号标准品加入150μl标准品稀释液6.25μg/L 1号标准品150μl的2号标准品加入150μl标准品稀释液2.加样:分别设空白孔(空白对照孔不加样品及酶标试剂,其余各步操作相同)、标准孔、待测样品孔。
雷美替胺结构编号

雷美替胺结构编号1. 介绍雷美替胺(Levetiracetam)是一种常用的抗癫痫药物,属于嘌呤类化合物。
它的结构编号是2S-(2α,5α,6β)-2-(2-氨基-4-氧代-5-苯基-6H-[1,2,4]三嗪-6-基)乙酰胆碱。
雷美替胺通过调节神经递质的释放和抑制异常兴奋,从而发挥抗癫痫作用。
2. 结构式雷美替胺的结构式如下所示:3. 结构解析根据结构式,我们可以对雷美替胺的结构进行解析:首先,在化合物名称中,“2S”表示该分子中有一个手性中心,其中S表示“sinister”(左旋)的意思。
这意味着雷美替胺存在两个立体异构体,即左旋和右旋形式。
接下来,“(2α,5α,6β)”表示了分子中其他三个手性中心的相对配置。
这些配置通过它们与基团的连接方式来确定。
然后,我们看到“2-(2-氨基-4-氧代-5-苯基-6H-[1,2,4]三嗪-6-基)乙酰胆碱”。
这部分描述了雷美替胺的化学成分。
最后,“乙酰胆碱”表示该化合物是乙酰胆碱的衍生物,即其结构中含有乙酰胆碱基团。
4. 化学性质雷美替胺是一种白色结晶性粉末,在水中溶解度较高。
它在酸性条件下比较稳定,但在碱性条件下会发生水解反应。
雷美替胺的熔点约为115°C。
5. 药理作用雷美替胺通过调节神经递质(如谷氨酸和GABA)的释放来发挥抗癫痫作用。
它主要通过与突触囊泡蛋白SV2A结合,抑制谷氨酸释放,并减少突触前膜上钙离子通道的活动。
此外,雷美替胺还可增加GABA能神经元的抑制效应,从而抑制异常兴奋。
6. 临床应用雷美替胺是一种广泛用于治疗癫痫的药物。
它可用于单纯性癫痫、复杂性部分发作以及全身性发作的治疗。
雷美替胺也可以作为辅助治疗用于其他抗癫痫药物无效或不耐受的患者。
7. 药代动力学雷美替胺经口服后,快速吸收并达到峰值浓度。
它在体内通过非酶催化的水解反应代谢,主要由肾脏排泄。
由于雷美替胺的半衰期较长,通常需要进行两次或三次每日剂量。
8. 不良反应雷美替胺在临床使用中常见的不良反应包括头晕、嗜睡、乏力和消化系统不适等。
【VIP专享】非索非那定片42

盐酸非索非那定片 ----毕馨
-------- 玉兴堂医药学术推广部
2020/5/19
盐酸ቤተ መጻሕፍቲ ባይዱ索非那定片
----毕馨
【分子式】 C32H39NO4·HCl 【分子量】 538.13 【性状】 本品为薄膜衣片,除去包衣后显白色或类白色。
2020/5/19
上市信息
赫斯特公司开发,1996年首次在欧洲上市 1996年FDA批准了SANOFI AVENTIS的新 药申请 2006年10月FDA批准口服混悬剂
1]和黏附到内皮细胞上的部分嗜酸性化 学介质的释放
2020/5/19
药理作用
非索非那定可以抑制人肥大细胞和嗜碱粒细胞释 放及贮存新合成的炎症介质,与嗜酸粒细胞共孵 育后,在较低浓度即能下调嗜酸粒细胞分泌嗜酸 粒细胞阳离子蛋白
2020/5/19
药理作用
临床研究表明非索非那定即使在较低的剂 量(20 mg,每日2次)下也能显著控制瘙 痒症状和减少风团数量,60 mg每日2次能 够显著改善慢性荨麻疹患者的生活质量, 安全性较好,副作用发生率与剂量无依赖 性
2020/5/19
药物代谢
药代特点 非索非那定达峰时间1.5小时,半衰期14~ 15小时,作用维持时间16小时。
2020/5/19
药物相互作用
与红霉素和酮康唑的药物间相互作用
盐酸非索非那定虽然表现出较小的肝脏代 谢率(5%),但当与红霉素、酮康唑作用时 会导致盐酸非索非那定的血药浓度升高。 盐酸非索非那定对红霉素和酮康唑的药代 动力学没有影响。
非索非那定不被肝脏代谢,能安全用于肝 功能损害的患者
2020/5/19
药物相互作用
非索非那定不经过肝脏的生物转化,因此 它与那些依赖于肝代谢的药物之间不存在 相互作用。
Etomoxir_DataSheet_MedChemExpress

Inhibitors, Agonists, Screening Libraries Data SheetBIOLOGICAL ACTIVITY:Etomoxir is a potent inhibitor of carnitine palmitoyltransferase–I (CPT–1).IC50 & Target: CPT–1[1]In Vitro: Etomoxir binds irreversibly to the catalytic site of CPT–1 inhibiting its activity, but also upregulates fatty acid oxidation enzymes. Etomoxir is developed as an inhibitor of the mitochondrial carnitine palmitoyltransferase–1 (CPT–1) located on the outer mitochondrial membrane. Etomoxir, in the liver can act as peroxisomal proliferator, increasing DNA synthesis and liver growth.Thus, etomoxir, in addition of being a CPT1 inhibitor could be considered as a PPARalpha agonist [1]. Etomoxir is a member of the oxirane carboxylic acid carnitine palmitoyl transferase I inhibitors and has been suggested as a therapeutic agent for the treatment of heart failure. Acute Etomoxir treatment irreversibly inhibits the activity of carnitine palmitoyltransferase I. As a result, fatty acid import into the mitochondria and β–oxidation is reduced, whereas cytosolic fatty acid accumulates and glucose oxidation is elevated. Prolonged incubation (24 h) with Etomoxir produces diverse effects on the expression of several metabolic enzyme [2].In Vivo: Etomoxir is an inhibitor of free fatty acid (FFA) oxidation–related key enzyme CPT1. P53 interacts directly with Bax, which is inhibited by Etomoxir, further confirming the direct interaction of P53 and Bax, and the involvement of FAO–mediatedmitochondrial ROS generation in db/db mice [3]. Rats are injected daily with Etomoxir, a specific CPT–I inhibitor, for 8 days at 20mg/kg of body mass. Etomoxir–treated rats display a 44% reduced cardiac CPT–I activity. The treatment of Lewis rats for 8 days with 20 mg/kg Etomoxir does not alter blood glucose, which is in line with comparable etomoxir–feeding studies. Similarly, Etomoxir feeding does not affect general growth characteristics such as gain in body mass, nor does it affect hindlimb muscle mass.However, heart mass and liver mass are both significantly increased by 11% in Etomoxir–treated rats [4].PROTOCOL (Extracted from published papers and Only for reference)Cell Assay: Etomoxir is dissolved in DMSO and stored, and then diluted with appropriate medium before use [2]. [2]Rat heart H9c2 myoblastic cells are incubated in DMEM containing 10% fetal bovine serum until near confluence. In some experiments,cells are preincubated for 2 h with DMEM (serum–free) in the absence or presence of 1–80 μM Etomoxir and then incubated for 2 h with 0.1 mM [1–14C]oleic acid (10 μCi/dish, binds to BSA in a 1:1 molar ratio). In other experiments, cells arepreincubated for 2 h plus or minus 40 μM Etomoxir and then incubated for 2 h with 0.1 μM or 0.1 mM [1,3–3H]glycerol (10μCi/dish), 0.1 mM [1–14C]oleic acid (2 μCi/dish, binds to BSA in a 1:1 molar ratio), 0.1 mM [1–14C]palmitic acid (2μCi/dish, binds to BSA in a 1:1 molar ratio), 28 μM [3H]ethanolamine (2 μCi/dish), 28 μM [methyl–3H]choline (2 μCi/dish), 0.4mM [3H]serine (20 μCi/dish), or 40 μM myo–[3H]inositol (10 μCi/dish). The medium is removed and the cells washed twice withice–cold saline and then harvested from the dish with 2 mL methanol–water (1:1, v/v) for lipid extraction. An aliquot of thehomogenate is taken for the determination of total uptake of radioactivity into cells. Phospholipids are then isolated andradioactivity in these determined [2].Animal Administration: Etomoxir is dissolved in 0.9% (w/v) NaCl (Rat)[4].[3][4]Mice [3]Product Name:Etomoxir Cat. No.:HY-50202CAS No.:124083-20-1Molecular Formula:C 17H 23ClO 4Molecular Weight:326.82Target:Others Pathway:Others Solubility:10 mM in DMSO80 male C57BLKS/J lar–Lepr db/db mice and 20 wild type littermates (8 week) are used. db/db mice are randomly divided into four groups: db/db group, Etomoxir group, MitoQ group, and PFT–α group. In the Etomoxir group, mice are intraperitoneally injected with 1 mg/kg Etomoxir twice every week. In the MitoQ group, 50 μM MitoQ is given to the mice in water. Water bottles, containing either MitoQ, are covered with aluminum foil, and all bottles are refilled every 3 days. In the PFT–α group, mice are intraperitoneally injected with 1 mg/kg PFT–α twice every week. WT mice are administrated with vehicle instead. The experimental period is 8 weeks. At the end, peripheral blood samples and bone marrow cells are harvested for the assays.Rat[4]Male Lewis rats, weighing 150–200 g, are used in the present study. Animals are kept on a 12 h:12 h light/dark cycle and fed a Purina Chow diet and water ad libitum. The rats are divided into two groups: (1) control and (2) Etomoxir. Etomoxir (20 mg/kg of body weight) is dissolved in 0.9% (w/v) NaCl and administered intraperitoneally for 8 days. Control rats receive saline. The last injection is given 24 h before the experiment. Animals are anaesthetized with an intraperitoneal injection of a nembutal and heparin (3:1) mixture. Subsequently, the heart is removed for LCFA uptake studies and for analyses of transporter protein contents.References:[1]. Rupp H, et al. The use of partial fatty acid oxidation inhibitors for metabolic therapy of angina pectoris and heart failure. Herz. 2002 Nov;27(7):621–36.[2]. Xu FY, et al. Etomoxir mediates differential metabolic channeling of fatty acid and glycerol precursors into cardiolipin in H9c2 cells. J Lipid Res. 2003 Feb; 44(2):415–23.[3]. Li J, et al. FFA–ROS–P53–mediated mitochondrial apoptosis contributes to reduction of osteoblastogenesis and bone mass in type 2 diabetes mellitus. Sci Rep. 2015 Jul 31;5:12724.[4]. Luiken JJ, et al. Etomoxir–induced partial carnitine palmitoyltransferase–I (CPT–I) inhibition in vivo does not alter cardiac long–chain fatty acid uptake and oxidation rates. Biochem J. 2009 Apr 15;419(2):447–55.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
Alda-1_DataSheet_MedChemExpress

Inhibitors, Agonists, Screening Libraries Data SheetBIOLOGICAL ACTIVITY:Alda–1 is a potent ALDH2 agonist, which significantly improves ALDH2 activity.In Vivo: Alda–1 treatment results in a significant decrease of 4–HNE–protein content in the plasma of apoE -/- mice. Alda–1administration leads to a slight increase in gene expression related to neurogenesis (Nog ), mitochondrial biogenesis (CYTB , ND1),and apoptosis (Bax , Gsk3b ) in the Hp of apoE -/- mice. Alda–1 administration leads to 2 and 10 differentially expressed proteins in theFCx and Hp of apoE -/- mice, respectively [1]. Alda–1 (1.5 mg/kg, b.w., i.p.) administration significantly increases the climbing time,tends to reduce the immobility time and increases the swimming time of the prenatally stressed rats in the forced swim test.Moreover, treatment of prenatally stressed rats with Alda–1 significantly increases number of entries into the open arms of the maze and the time spent therein, as assessed by elevated plus–maze test [2]. Alda–1 (8.5 mg/kg, i.p.) with glucose significantly lowers 4–HNE and FJB–positive cells in the cerebral cortex of Alda–1–treated rats than in DMSO–treated rats 24 h after glucose administration [3]. Alda–1 (10 mg/kg per day) treatment prevents aldehydic overload, mitochondrial dysfunction and improves ventricular function in post–MI cardiomyopathy rats [4].PROTOCOL (Extracted from published papers and Only for reference)Cell Assay:[2]Spleen cells (4×106 cells/mL) are stimulated by optimal concentrations of concanavalin A (Con A; 2.5μg/mL and 0.6 μg/mL) and lipopolysaccharide (LPS, 5 μg/mL) and are incubated in 96–well plates at final volume of 0.2mL for 72 h. Cell proliferation is determined by adding 0.5 μCi of [3H]–thymidine per well at 16 h before the end of the incubation.The cultures are harvested with an automatic cell harvester, and [3H] thymidine incorporation is assessed using a liquid scintillationcounter.Animal Administration: Alda–1 is dissolved in 1 mL/kg b.w. DMSO/water 50/50.[2]After behavioral verification at three months of age,the animals are divided into the following four groups: control, control + Alda–1, prenatally stressed and prenatally stressed + Alda–1(6 animals per group). Alda–1 injections are given intraperitoneally (i.p.) once daily at a dose of 1.5 mg/kg b.w. (dissolved in 1 mL/kg b.w. DMSO/water 50/50) for 14 days. At the same time, the control and prenatally stressed rats receive 1 mL/kg b.w. DMSO/water 50/50. The injections of Alda–1 and vehicle are given between 10 a.m and 11 a.m. In the last five days of Alda–1 treatment the behavioral parameters in the elevated plus maze test and then in the forced swim test are measured.References:[1]. Stachowicz A, et al. Proteomic Analysis of Mitochondria–Enriched Fraction Isolated from the Frontal Cortex and Hippocampus of Apolipoprotein E Knockout Mice Treated with Alda–1, an Activator of Mitochondrial Aldehyde Dehydrogenase (ALDH2). Int J Mol Sci.[2]. Stachowicz A, et al. The impact of mitochondrial aldehyde dehydrogenase (ALDH2) activation by Alda–1 on the behavioral and biochemical disturbances in animal model of depression. Brain Behav Immun. 2016 Jan;51:144–53.Product Name:Alda–1Cat. No.:HY-18936CAS No.:349438-38-6Molecular Formula:C 15H 11Cl 2NO 3Molecular Weight:324.16Target:Aldehyde Dehydrogenase (ALDH)Pathway:Metabolic Enzyme/Protease Solubility:DMSO: ≥ 51 mg/mL[3]. Ikeda T, et al. Effects of Alda–1, an Aldehyde Dehydrogenase–2 Agonist, on Hypoglycemic Neuronal Death. PLoS One. 2015 Jun 17;10(6):e0128844.[4]. Gomes KM, et al. Aldehydic load and aldehyde dehydrogenase 2 profile during the progression of post–myocardial infarction cardiomyopathy: benefits of Alda–1. Int J Cardiol. 2015 Jan 20;179:129–138.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Inhibitors, Agonists, Screening Libraries Data SheetBIOLOGICAL ACTIVITY:Mifepristone is a progesterone receptor (PR ) antagonist (IC 50=0.2 nM) in a T47D cell–based assay, also is a glucocorticoid receptor (GR ) antagonist (IC 50=2.6 nM) in an A549 cell–based assay.IC50 & Target: IC50: 0.2 nM (progesterone receptor, in T47D cells), 2.6 nM (glucocorticoid receptor, in A549 cells)[1]In Vitro: The discovery of the first competitive progesterone antagonist, Mifepristone, has stimulated an intense search for more potent and more selective antiprogestins [1]. Cell growth is evaluated after 4 days of exposure to Mifepristone at 10 μM, aconcentration close to the plasma concentration achievable in humans. The antiproliferative effect of Cisplatin is potentiated when administered in combination with Mifepristone in HeLa cells. The IC 50 of Cisplatin in combination with Mifepristone is lower (14.2μM) than that of Cisplatin alone (34.2 μM) in HeLa cells with an approximately 2.5–fold difference. After treatment withMifepristone, the accumulation of intracellular Cisplatin in HeLa cells is 2–fold greater, representing a significant difference (p=0.009), compare with Cisplatin alone from 0.79 to 1.52 μg/mg of protein [2].In Vivo: The cervix tumor xenograft models are treated with Cisplatin alone, there is a tumor growth inhibition compare with control group. However, the tumor weight loss is even more significant (p<0.05) with the combination of Cisplatin and Mifepristone at the doses used, showing a decrease of ~50% compared with the treatments alone by the end of the study [2]. Adult maleSprague–Dawley rats are subjected to a 4–day binge–like EtOH administration regimen (3 to 5 g/kg/i.g. every 8 hours designed to produce peak blood EtOH levels (BELs) of <300 mg/dL). Subgroups of animals receive s.c. injection of Mifepristone (20 or 40 mg/kg in peanut oil). Although Mifepristone produces no significant changes in behavior of EtOH–na?ve animals, pretreatment with Mifepristone (40 mg/kg) significantly reducesthe severity of EtOH withdrawal. Asignificant interaction between diet and drug,F(5,55)=3.92, p<0.05, such that EtOH–treated animals receiving vehicle or 20 mg/kg of Mifepristone displayssignificantly more signs of EtOH withdrawal than does EtOH–na?ve animals receiving the same drug treatment. Importantly, treatment with 40 mg/kg of Mifepristone significantly reduces the severity of EtOH withdrawal, in a dose–dependent manner [3].PROTOCOL (Extracted from published papers and Only for reference)Kinase Assay:[1]T47D human breast cancer cells are plated in 96–well tissue culture plates at 10,000 cells per well in assay medium[RPMI medium without phenol red containing 5% (v/v) charcoal–treated FBS and 1% (v/v) penicillin–streptomycin]. Two days later,the medium is decanted and Mifepristone or control is added at a final concentration of 0.1% (v/v) dimethylsulfoxide in fresh assay medium. Twenty–four hours later, an alkaline phosphatase assay is performed using a SEAP kit. Briefly, the medium is decanted and the cells are fixed for 30 min at room temperature with 5% (v/v) formalin. The cells are washed once at room temperature with Hanks’ buffered saline solution. Equal volumes (0.05 mL) of 1× dilution buffer, assay buffer, and 1:20 substrate/enhancer mixture are then added. After a 1–h incubation at room temperature in the dark, the lysate is transferred to a white 96–well plate and luminescence is read using a LuminoSkan Ascent [1].Cell Assay: Mifepristone is reconstituted in absolute ethanol and stored (–20?C), and then diluted with appropriate mediaProduct Name:Mifepristone Cat. No.:HY-13683CAS No.:84371-65-3Molecular Formula:C 29H 35NO 2Molecular Weight:429.59Target:Progesterone Receptor; Glucocorticoid Receptor; Autophagy Pathway:Others; GPCR/G Protein; Autophagy Solubility:DMSO: ≥ 59 mg/mLbefore use[2].[2]The HeLa and CaSki human cervical cancer cell lines are used. The effect of Mifepristone on proliferation of cells exposed to Cisplatin is evaluated using the XTT assay. The assay is based on the cleavage of the yellow tetrazolium salt XTT to form an orange formazan dye by metabolically active cells. The procedure is as follows. Cells are seeded into 96–well plates; Costar at a density of 6×103 viable cells per well in 100 μL culture medium. At the end of treatment with Cisplatin alone or the combination of Cisplatin plus Mifepristone, 50 μL XTT is added to each well (final concentration 0.3 mg/mL), follow by incubation for 4 h in a humidified atmosphere containing 5% CO2 at 37?C. The absorbance of the samples is measured spectrophotometrically at 492 nm using a microtiter plate ELISA reader[2].Animal Administration: Mifepristone is suspended in peanut oil (Rat)[3].[2][3]Mice[2]Female Nude mice between 6–8 weeks of age are implanted subcutaneously with 6×106 HeLa cells in a flank. Once tumors are ~5×5 mm, the animals are pair–matched into treatment and control groups. Each group consist of 8 tumor–bearing mice. The intraperitoneal administration of drugs or vehicle begin on day 0. Cisplatin, as a single agent, is administered intraperitoneally at a dose of 3 mg/kg daily on days 1 through 3; the dose of Mifepristone, as a single agent, is 2 mg/kg/day subcutaneously for 3 days; in the combination study, the mice concurrently receive Cisplatin on the same schedule, and Mifepristone at the same dose 3 days previous to the administration of Cisplatin. The control animals receive only the vehicle. After administration of the drugs, mice are weighed and the tumors are measured with a caliper twice weekly. The tumor weight is calculated. Experiment is conducted for 74 days, after which time all animals are weighed and humanely euthanized.Rat[3]Adult male Sprague–Dawley rats, weighing between 224 and 245 g upon arrival, are used. Mifepristone (20 or 40 mg/kg) or vehicle (peanut oil) are administered subcutaneously (s.c.) once daily following the 0800 administration of EtOH or control diet. Mifepristone is suspended in peanut oil and sonicated for 30 minutes at least 24 hours prior to injection, it is then stored at 4°C until needed. Suspension is vortexed for 10 to 15 minutes prior to and as needed throughout dosing.References:[1]. Jiang W, et al. New progesterone receptor antagonists: phosphorus–containing 11beta–aryl–substituted steroids. Bioorg Med Chem. 2006 Oct 1;14(19):6726–32.[2]. Jurado R, et al. Cisplatin cytotoxicity is increased by mifepristone in cervical carcinoma: an in vitro and in vivo study. Oncol Rep. 2009 Nov;22(5):1237–45.[3]. Sharrett–Field L, et al. Mifepristone Pretreatment Reduces Ethanol Withdrawal Severity In Vivo. Alcohol Clin Exp Res. 2013 Aug;37(8):1417–23.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
