263202128_1_carbohydrate
2羟基2甲基丙胺磷酸盐

2羟基2甲基丙胺磷酸盐英文回答:2-Hydroxy-2-methylpropionamide (calcium salt) is a calcium salt of 2-hydroxy-2-methylpropionic acid, widely known as lactic acid. Lactic acid is a colorless, syrupy liquid with a sour taste and odor. It is produced by the fermentation of carbohydrates by bacteria, yeast, and molds. Lactic acid is used as a food preservative, a flavoring agent, and a humectant. It is also used in the productionof pharmaceuticals, cosmetics, and other products.The calcium salt of lactic acid is a white, crystalline powder. It is soluble in water and has a slightly bitter taste. Calcium lactate is used as a food supplement, a calcium source, and a stabilizer. It is also used in the production of pharmaceuticals, cosmetics, and other products.中文回答:2-羟基-2-甲基丙酰胺(钙盐)是 2-羟基-2-甲基丙酸的钙盐,通常称为乳酸。
乳酸是一种无色糖浆状液体,具有酸味和气味。
carbohydrate polymers影响因子

carbohydrate polymers影响因子Carbohydrate polymers, commonly referred to as “starch,” is an important macronutrient found in plants. The macromolecules of carbohydrate polymers play a vital role in human health and nutrition as an energy source and as a structural component of plant cells. Additionally, carbohydrate polymers are also utilized in various industries for a variety of uses.The primary factor that affects the macromolecular structure of carbohydrate polymers is the source of the raw material. Starch can be derived from a variety of plant sources such as corn, wheat, potatoes, and rice and the macromolecular structure of the starch molecules differs accordingly. The size, shape, and arrangement of the macromolecules are of distinct importance since they are intimately associated with the biochemical and nutraceutical properties of starch molecules.In general, starch molecules are primarily based on two distinct types of macromolecules, amylose and amylopectin. Amylose is composed of straight-chain glucose molecules, while amylopectin is composed of branched glucose molecules. The ratio of amylose to amylopectin can vary significantly between different sources which may have an influence on properties such as solubility, viscosity, swelling, and water absorption and retention capacities.Another factor that affects the macromolecular structure of carbohydrate polymers is the chemical processing methods employed. Starch molecules can be modified by enzymatic processing and other chemical treatments resulting in different physical characteristics such as increased solubility and stability, improved sensitivity to water and lower viscosity. Chemical processing can also be used to produce a gelling agent when carbohydrates are treated with anionic detergents.Lastly, the environment in which starch molecules are processed can also have an impact on their macromolecular structure. For instance, changes in temperature and pH levels can alter the structure of the molecules. In addition, other environmental conditions such as the presence of light, oxygen, and ultraviolet radiation can also affect the conformation of the molecules.In conclusion, there are a variety of different factors which can influence the macromolecular structure of carbohydrate polymers. Depending on the source of starch, the chemical processing methods employed and the environment in which it is processed, the physical properties and properties associated with nutritional and functional features ofstarch can be altered. Therefore, it is important to understand the interactions between these factors in order to develop novel products with appropriate qualities.。
胃食管反流患者应用富马酸伏诺拉生片与雷贝拉唑肠溶胶囊的疗效及药物经济学评价

doi :10.3969/j.issn.1002-7386.2024.02.024·论著·胃食管反流患者应用富马酸伏诺拉生片与雷贝拉唑肠溶胶囊的疗效及药物经济学评价朱怀平 刘守珠 王健 刘娜作者单位:257000 山东省东营市东营区人民医院消化内科通信作者:刘守珠 E⁃mail:185********@ 【摘要】 目的 探讨富马酸伏诺拉生片与雷贝拉唑肠溶胶囊治疗胃食管反流的效果及药物经济学情况。
方法 选取2018年2月至2021年10月进行胃食管反流症治疗的2000例患者,等分法将患者分为对照组和研究组,每组1000例。
对照组采用雷贝拉唑肠溶胶囊治疗,研究组采用富马酸伏诺拉生片治疗。
比较2组患者胃食管反流治疗效果、基础酸排出量及不良反应发生率,2组成本⁃效果比等指标。
结果 研究组患者治疗有效率显著高于对照组(P <0.05);研究组基础酸排出量显著小于对照组(P <0.05);研究组临床症状包括反流、腹痛、反酸、嗳气消失时间较对照组短(P <0.05),研究组不良反应发生率显著小于对照组(P <0.05);雷贝拉唑肠溶胶囊的经济性高于富马酸伏诺拉生片。
结论 富马酸伏诺拉生片在治疗胃食管反流中的疗效、基础酸排出量及不良反应发生率等方面的效果均优异于雷贝拉唑肠溶胶囊,但根据药物经济学成本⁃效果分析显示雷贝拉唑肠溶胶囊的药物经济学价值更高。
【关键词】 富马酸伏诺拉生片;雷贝拉唑肠溶胶囊;胃食管反流;药物经济学【中图分类号】 R 571 【文献标识码】 A 【文章编号】 1002-7386(2024)02-0264-03Pharmacoeconomics and efficacy of Vonoprazan Fumarate tablets versus Rabeprazole enteric⁃coated capsules in patients with gastroesophageal reflux ZHU Huaiping ,LIU Shouzhu ,WANG Jian ,et al.Department of Gastroenterology ,Dongying District People ’s Hospital ,Shandong ,Dongying 257000,China【Abstract 】 Objective To compare the efficacy and pharmacoeconomics of Vonoprazan Fumarate tablets versus Rabeprazole enteric⁃coated capsules on the treatment of gastroesophageal reflux.Methods A total of 2,000patients with gastroesophageal reflux disease in our hospital from February 2018to October 2021were selected.They were randomly divided into control group and study group ,with 1,000cases per group.Patients in the control group were medicated by Rabeprazole enteric⁃coated capsules ,and those in the study group were medicated by Vonoprazan Fumarate tablets.The therapeutic effect on gastroesophageal reflux ,basal acid output ,incidence of adverse events ,cost⁃effectiveness ratio of the two groups were compared.Results The treatment effective rate in the study group was significantly higher than that of the control group (P <0.05).The basal acid output in the study group was significantly lower than that of the control group (P <0.05).The disappearance time of regurgitation ,abdominal pain ,acid regurgitation and belching in the study group was significantly shorter than that of the control group (P <0.05).The incidence of adverse events in the study group was significantly lower than that of the control group (P <0.05).The pharmacoeconomic cost⁃effectiveness of Rabeprazole enteric⁃coated capsules was significantly higher than that of Vonoprazan Fumarate tablets.Conclusion Vonoprazan Fumarate tablets are superior to Rabeprazole enteric⁃coated capsules on the treatment of gastroesophageal reflux in the therapeutic efficacy ,basal acid output and lower incidence of adverse events.However ,the pharmacoeconomic cost⁃effectiveness of Rabeprazole enteric⁃coated capsules is significantly higher than that of Vonoprazan Fumarate tablets.【Key words 】 Vonoprazan Fumarate tablet ;Rabeprazole enteric⁃coated capsule ;gastroesophageal reflux ;pharmacoeconomics 胃食管反流病为临床中较为常见的上消化道疾病,直接病因为患者胃食管腔过度接触(或暴露于)胃液导致患者出现食管黏膜损伤[1]。
不同亚型CHO_宿主细胞对抗体表达的影响

生物技术进展 2023 年 第 13 卷 第 5 期 698 ~ 703Current Biotechnology ISSN 2095‑2341进展评述Reviews不同亚型CHO 宿主细胞对抗体表达的影响曹辉 , 董静 , 贾宇 , 江一帆*华北制药集团新药研究开发有限责任公司,抗体药物河北省工程研究中心,抗体药物研究国家重点实验室,石家庄 050015摘要:CHO 细胞作为宿主细胞广泛应用于生物药工业化生产中。
其中,CHO -K1、CHO -DG44和CHO -S 是最常见的3种亚型。
虽然这些亚型是从共同的原始CHO 细胞分离出来的,但在不同的实验室或生物医药公司、研究人员、培养基或培养方式下连续传代、驯化和保存,使得CHO 细胞积累了大量变异,导致宿主细胞应用于抗体药生产时会在细胞生长状态、抗体表达量及以糖型为代表的质量属性方面表现出较大差异。
综述了CHO 细胞不同亚型的染色体差异、生长状态、表达差异以及糖型差异,以期为抗体药物研发中宿主细胞的选择提供参考。
关键词:CHO 细胞;抗体;表达量;糖型DOI :10.19586/j.2095‑2341.2023.0064中图分类号:Q28, R392-33 文献标志码:AEffects of Different Sources of CHO Host Cells on Antibody ExpressionCAO Hui , DONG Jing , JIA Yu , JIANG Yifan *State Key Laboratory of Antibody Drug Development , Hebei Engineering Research Center of Antibody Medicine , New Drug Research and Development Co. Ltd , North China Pharmaceutical Corporation , Shijiazhuang 050015, ChinaAbstract :CHO cells comprise a variety of lineages including CHO -K1, CHO -DG44 and CHO -S , which have been widely used in the industrial production of biological drugs. All CHO cell lines share a common ancestor , however , during the process of cell passage cultivation , cell domesticated , and preservation by different laboratories or companies , substantial genetic heterogeneity among them has been produced , that showed great differences in cell growth state , antibody titer , glycosylation and other product quality attributes. This article reviewed the difference in chromosome , growing status and expression , and glycoform in different sources ofCHO host cells , which was expected to be helpful in host cell selection during antibody drug research and development process.Key words :CHO cells ; monoclonal antibody ; antibody titer ; glycosylation生物药物在国际医药市场中占据主导地位,截至2023年,全球范围内已有100多个抗体药物被批准上市,近1 200个抗体药物处于不同临床试验阶段[1]。
分子生物学词汇(中英文对照表 )

第一页A band|A带A chromosome|A染色体[二倍体染色体组中的正常染色体(不同于B染色体)] A site|[核糖体]A部位ABA|脱落酸abasic site|脱碱基位点,无碱基位点abaxial|远轴的abequose|阿比可糖,beta脱氧岩藻糖aberrant splicing|异常剪接aberration|象差;畸变;失常abiogenesis|自然发生论,无生源论ablastin|抑殖素(抑制微生物细胞分裂或生殖的一种抗体)abnormal distrbution|非正态分布abnormality|异常,失常;畸形,畸变ABO blood group system|ABO血型系统aboriginal mouse|原生鼠abortin|流产素abortion|流产,败育abortive egg|败育卵abortive infection|流产(性)感染abortive transduction|流产(性)转导ABP|肌动蛋白结合蛋白abrin|相思豆毒蛋白abscisic acid|脱落酸abscission|脱落absolute|绝对的absolute configuration|绝对构型absolute counting|绝对测量absolute deviation|绝对偏差absolute error|绝对误差absorbance|吸收,吸光度absorbed dose|吸收剂量absorbent|吸收剂absorptiometer|吸光计absorptiometry|吸光测定法absorption|吸收absorption band|吸收谱带absorption cell|吸收池absorption coefficient|吸收系数absorption spectroscopy|吸收光谱法absorption spectrum|吸收光谱;吸收谱absorptive endocytosis|吸收(型)胞吞(作用) absorptive pinocytosis|吸收(型)胞饮(作用) absorptivity|吸光系数;吸收性abundance|丰度abundant|丰富的,高丰度的abundant mRNAs|高丰度mRNAabzyme|抗体酶acaricidin|杀螨剂accedent variation|偶然变异accelerated flow method|加速流动法accepting arm|[tRNA的]接纳臂acceptor|接纳体,(接)受体acceptor site|接纳位点,接受位点acceptor splicing site|剪接受体acceptor stem|[tRNA的]接纳茎accessible|可及的accessible promoter|可及启动子accessible surface|可及表面accessory|零件,附件;辅助的accessory cell|佐细胞accessory chromosome|副染色体accessory factor|辅助因子accessory nucleus|副核accessory pigment|辅助色素accessory protein|辅助蛋白(质)accommodation|顺应accumulation|积累,累积accuracy|准确度acenaphthene|二氢苊acene|并苯acentric|无着丝粒的acentric fragment|无着丝粒断片acentric ring|无着丝粒环acetal|缩醛acetaldehyde|乙醛acetalresin|缩醛树脂acetamidase|乙酰胺酶acetamide|乙酰胺acetate|乙酸盐acetic acid|乙酸,醋酸acetic acid bacteria|乙酸菌,醋酸菌acetic anhydride|乙酸酐acetification|乙酸化作用,醋化作用acetin|乙酸甘油酯,三乙酰甘油酯acetoacetic acid|乙酰乙酸Acetobacter|醋杆菌属acetogen|产乙酸菌acetogenic bacteria|产乙酸菌acetome body|酮体acetome powder|丙酮制粉[在-30度以下加丙酮制成的蛋白质匀浆物] acetomitrile|乙腈acetone|丙酮acetyl|乙酰基acetyl coenzyme A|乙酰辅酶Aacetylcholine|乙酰胆碱acetylcholine agonist|乙酰胆碱拮抗剂acetylcholine receptor|乙酰胆碱受体acetylcholinesterase|乙酰胆碱酯酶acetylene|乙炔acetylene reduction test|乙炔还原试验[检查生物体的固氮能力] acetylglucosaminidase|乙酰葡糖胺糖苷酶acetylglutamate synthetase|乙酰谷氨酸合成酶acetylsalicylate|乙酰水杨酸;乙酰水杨酸盐、酯、根acetylsalicylic acid|乙酰水杨酸acetylspiramycin|乙酰螺旋霉素AchE|乙酰胆碱酯酶achiral|非手性的acholeplasma|无胆甾原体AchR|乙酰胆碱受体achromatic|消色的;消色差的achromatic color|无色achromatic lens|消色差透镜achromatin|非染色质acid catalysis|酸催化acid fibroblast growth factor|酸性成纤维细胞生长因子acid fuchsin|酸性品红acid glycoprotein|酸性糖蛋白acid hydrolyzed casein|酸水解酪蛋白acid medium|酸性培养基acid mucopolysaccharide|酸性粘多糖acid phosphatase|酸性磷酸酶acid protease|酸性蛋白酶acid solvent|酸性溶剂acidic|酸性的acidic amino acid|酸性氨基酸acidic protein|酸性蛋白质[有时特指非组蛋白]acidic transactivator|酸性反式激活蛋白acidic transcription activator|酸性转录激活蛋白 acidification|酸化(作用)acidifying|酸化(作用)acidolysis|酸解acidophilia|嗜酸性acidophilic bacteria|嗜酸菌acidophilous milk|酸奶aclacinomycin|阿克拉霉素acoelomata|无体腔动物acomitic acid|乌头酸aconitase|顺乌头酸酶aconitate|乌头酸;乌头酸盐、酯、根aconitine|乌头碱aconitum alkaloid|乌头属生物碱ACP|酰基载体蛋白acquired character|获得性状acquired immunity|获得性免疫acridine|吖啶acridine alkaloid|吖啶(类)生物碱acridine dye|吖啶燃料acridine orange|吖啶橙acridine yellow|吖啶黄acriflavine|吖啶黄素acroblast|原顶体acrocentric chromosome|近端着丝染色体acrolein|丙烯醛acrolein polymer|丙烯醛类聚合物acrolein resin|丙烯醛树脂acropetal translocation|向顶运输acrosin|顶体蛋白acrosomal protease|顶体蛋白酶acrosomal reaction|顶体反应acrosome|顶体acrosome reaction|顶体反应acrosomic granule|原顶体acrosyndesis|端部联会acrylamide|丙烯酰胺acrylate|丙烯酸酯、盐acrylic acid|丙烯酸acrylic polymer|丙烯酸(酯)类聚合物acrylic resin|丙烯酸(酯)类树脂acrylketone|丙烯酮acrylonitrile|丙烯腈actidione|放线(菌)酮[即环己酰亚胺]actin|肌动蛋白actin filament|肌动蛋白丝actinin|辅肌动蛋白[分为alfa、beta两种,beta蛋白即加帽蛋白] actinmicrofilament|肌动蛋白微丝actinometer|化学光度计actinomorphy|辐射对称[用于描述植物的花]actinomycetes|放线菌actinomycin D|放线菌素Dactinospectacin|放线壮观素,壮观霉素,奇霉素action|作用action current|动作电流action potential|动作电位action spectrum|动作光谱activated sludge|活性污泥activated support|活化支持体activating group|活化基团activating transcription factor|转录激活因子activation|激活;活化activation analysis|活化分析activation energy|活化能activator|激活物,激活剂,激活蛋白activator protein|激活蛋白active absorption|主动吸收active biomass|活生物质active carbon|活性碳active center|活性中心active chromatin|活性染色质active dry yeast|活性干酵母active dydrogen compounds|活性氢化合物active ester of amino acid|氨基酸的活化酯active hydrogen|活性氢active immunity|主动免疫active oxygen|活性氧active site|活性部位,活性中心active transport|主动转运active uptake|主动吸收activin|活化素[由垂体合成并由睾丸和卵巢分泌的性激素]activity|活性,活度,(放射性)活度actomyosin|肌动球蛋白actophorin|载肌动蛋白[一种肌动蛋白结合蛋白]acute|急性的acute infection|急性感染acute phase|急性期acute phase protein|急性期蛋白,急相蛋白acute phase reaction|急性期反应,急相反应[炎症反应急性期机体的防御反应] acute phase reactive protein|急性期反应蛋白,急相反应蛋白acute phase response|急性期反应,急相反应acute toxicity|急性毒性ACV|无环鸟苷acyclic nucleotide|无环核苷酸acycloguanosine|无环鸟苷,9-(2-羟乙氧甲基)鸟嘌呤acyclovir|无环鸟苷acyl|酰基acyl carrier protein|酰基载体蛋白acyl cation|酰(基)正离子acyl chloride|酰氯acyl CoA|脂酰辅酶Aacyl coenzyem A|脂酰辅酶Aacyl fluoride|酰氟acyl halide|酰卤acylamino acid|酰基氨基酸acylase|酰基转移酶acylating agent|酰化剂acylation|酰化acylazide|酰叠氮acylbromide|酰溴acyloin|偶姻acyltransferase|酰基转移酶adamantanamine|金刚烷胺[曾用作抗病毒剂]adamantane|金刚烷adaptability|适应性adaptation|适应adapter|衔接头;衔接子adapter protein|衔接蛋白质adaptin|衔接蛋白[衔接网格蛋白与其他蛋白的胞质区]adaptive behavior|适应性行为adaptive enzyme|适应酶adaptive molecule|衔接分子adaptive response|适应反应[大肠杆菌中的DNA修复系统]adaptor|衔接头;衔接子adaxial|近轴的addition|加成addition compound|加成化合物addition haploid|附加单倍体addition line|附加系additive|添加物,添加剂additive effect|加性效应additive genetic variance|加性遗传方差additive recombination|插入重组,加插重组[因DNA插入而引起的基因重组] addressin|地址素[选择蛋白(selectin)的寡糖配体,与淋巴细胞归巢有关]adducin|内收蛋白[一种细胞膜骨架蛋白,可与钙调蛋白结合]adduct|加合物,加成化合物adduct ion|加合离子adenine|腺嘌呤adenine arabinoside|啊糖腺苷adenine phosphoribosyltransferase|腺嘌呤磷酸核糖转移酶adenoma|腺瘤adenosine|腺嘌呤核苷,腺苷adenosine deaminase|腺苷脱氨酶adenosine diphoshate|腺苷二磷酸adenosine monophosphate|腺苷(一磷)酸adenosine phosphosulfate|腺苷酰硫酸adenosine triphosphatase|腺苷三磷酸酶adenosine triphosphate|腺苷三磷酸adenovirus|腺病毒adenylate|腺苷酸;腺苷酸盐、酯、根adenylate cyclase|腺苷酸环化酶adenylate energy charge|腺苷酸能荷adenylate kinase|腺苷酸激酶adenylic acid|腺苷酸adenylyl cyclase|腺苷酸环化酶adenylylation|腺苷酰化adherence|粘着,粘附,粘连;贴壁adherent cell|贴壁赴 徽匙牛ㄐ裕┫赴 掣剑ㄐ裕┫赴?/P>adherent culture|贴壁培养adhering junction|粘着连接adhesin|粘附素[如见于大肠杆菌]adhesion|吸附,结合,粘合;粘着,粘附,粘连adhesion factor|粘着因子,粘附因子adhesion molecule|粘着分子,粘附分子adhesion plaque|粘着斑adhesion protein|粘着蛋白,吸附蛋白adhesion receptor|粘着受体adhesion zone|粘着带[如见于细菌壁膜之间]adhesive|粘合剂,胶粘剂adhesive glycoprotein|粘着糖蛋白adipic acid|己二酸,肥酸adipocyte|脂肪细胞adipokinetic hormone|脂动激素[见于昆虫]adipose tissue|脂肪组织adjust|[动]调节,调整;修正adjustable|可调的adjustable miropipettor|可调微量移液管adjustable spanner|活动扳手adjusted retention time|调整保留时间adjusted retention volume|调整保留体积adjuvant|佐剂adjuvant cytokine|佐剂细胞因子adjuvant peptide|佐剂肽adjuvanticity|佐剂(活)性adoptive immunity|过继免疫adoptive transfer|过继转移ADP ribosylation|ADP核糖基化ADP ribosylation factor|ADP核糖基化因子ADP ribosyltransferase|ADP核糖基转移酶adrenal cortical hormone|肾上腺皮质(激)素adrenaline|肾上腺素adrenergic receptor|肾上腺素能受体adrenocepter|肾上腺素受体adrenocorticotropic hormone|促肾上腺皮质(激)素adrenodoxin|肾上腺皮质铁氧还蛋白adriamycin|阿霉素,亚德里亚霉素adsorbent|吸附剂adsorption|吸附adsorption catalysis|吸附催化adsorption center|吸附中心adsorption chromatography|吸附层析adsorption film|吸附膜adsorption isobar|吸附等压线adsorption isotherm|吸附等温线adsorption layer|吸附层adsorption potential|吸附电势adsorption precipitation|吸附沉淀adsorption quantity|吸附量adult diarrhea rotavirus|成人腹泻轮状病毒advanced glycosylation|高级糖基化advanced glycosylation end product|高级糖基化终产物 adventitious|不定的,无定形的adverse effect|反效果,副作用aecidiospore|锈孢子,春孢子aeciospore|锈孢子,春孢子aequorin|水母蛋白,水母素aeration|通气aerator|加气仪,加气装置aerial mycelium|气生菌丝体aerobe|需氧菌[利用分子氧进行呼吸产能并维持正常生长繁殖的细菌] aerobic|需氧的aerobic bacteria|需氧(细)菌aerobic cultivation|需氧培养aerobic glycolysis|有氧酵解aerobic metabolism|有氧代谢aerobic respiration|需氧呼吸aerobic waste treatment|需氧废物处理aerobiosis|需氧生活aerogel|气凝胶aerogen|产气菌aerolysin|气单胞菌溶素Aeromonas|气单胞菌属aerosol|气溶胶aerosol gene delivery|气溶胶基因送递aerospray ionization|气喷射离子化作用aerotaxis|趋氧性[(细胞)随环境中氧浓度梯度进行定向运动]aerotolerant bacteria|耐氧菌[不受氧毒害的厌氧菌]aerotropism|向氧性aesculin|七叶苷,七叶灵aetiology|病原学B cell|B细胞B cell antigen receptor|B细胞抗原受体B cell differentiation factor|B细胞分化因子B cell growth factor|B细胞生长因子B cell proliferation|B细胞增殖B cell receptor|B细胞受体B cell transformation|B细胞转化B chromosome|B染色体[许多生物(如玉米)所具有的异染质染色体] B to Z transition|B-Z转换[B型DNA向Z型DNA转换]Bacillariophyta|硅藻门Bacillus|芽胞杆菌属Bacillus anthracis|炭疽杆菌属Bacillus subtillis|枯草芽胞杆菌bacitracin|杆菌肽back donation|反馈作用back flushing|反吹,反冲洗back mutation|回复突变[突变基因又突变为原由状态]backbone|主链;骨架backbone hydrogen bond|主链氢键backbone wire model|主链金属丝模型[主要反应主链走向的实体模型]backcross|回交backflushing chromatography|反吹层析,反冲层析background|背景,本底background absorption|背景吸收background absorption correction|背景吸收校正background correction|背景校正background gactor|背景因子background genotype|背景基因型[与所研究的表型直接相关的基因以外的全部基因]background hybridization|背景杂交background radiation|背景辐射,本底辐射backmixing|反向混合backside attack|背面进攻backward reaction|逆向反应backwashing|反洗bacmid|杆粒[带有杆状病毒基因组的质粒,可在细菌和昆虫细胞之间穿梭]bacteremia|菌血症bacteria|(复)细菌bacteria rhodopsin|细菌视紫红质bacterial adhesion|细菌粘附bacterial alkaline phosphatase|细菌碱性磷酸酶bacterial artificial chromosome|细菌人工染色体bacterial colony|(细菌)菌落bacterial colony counter|菌落计数器bacterial conjugation|细菌接合bacterial filter|滤菌器bacterial invasion|细菌浸染bacterial motility|细菌运动性bacterial rgodopsin|细菌视紫红质,细菌紫膜质bacterial vaccine|菌苗bacterial virulence|细菌毒力bactericidal reaction|杀(细)菌反应bactericide|杀(细)菌剂bactericidin|杀(细)菌素bactericin|杀(细)菌素bacteriochlorophyll|细菌叶绿素bacteriochlorophyll protein|细菌叶绿素蛋白bacteriocide|杀(细)菌剂bacteriocin|细菌素bacteriocin typing|细菌素分型[利用细菌素对细胞进行分型]bacterioerythrin|菌红素bacteriofluorescein|细菌荧光素bacteriology|细菌学bacteriolysin|溶菌素bacteriolysis|溶菌(作用)bacteriolytic reaction|溶菌反应bacteriophaeophytin|细菌叶褐素bacteriophage|噬菌体bacteriophage arm|噬菌体臂bacteriophage conversion|噬菌体转变bacteriophage head|噬菌体头部bacteriophage surface expression system|噬菌体表面表达系统bacteriophage tail|噬菌体尾部bacteriophage typing|噬菌体分型bacteriophagology|噬菌体学bacteriopurpurin|菌紫素bacteriorhodopsin|细菌视紫红质bacteriosome|细菌小体[昆虫体内一种含有细菌的结构]bacteriostasis|抑菌(作用)bacteriostat|抑菌剂bacteriotoxin|细菌毒素bacteriotropin|亲菌素bacterium|细菌bacteroid|类菌体baculovirus|杆状病毒bag sealer|封边机baking soda|小苏打BAL 31 nuclease|BAL 31核酸酶balance|天平balanced heterokaryon|平衡异核体balanced lethal|平衡致死balanced lethal gene|平衡致死基因balanced linkage|平衡连锁balanced pathogenicity|平衡致病性balanced polymorphism|平衡多态性balanced salt solution|平衡盐溶液balanced solution|平衡溶液balanced translocation|平衡易位balbaini ring|巴尔比亚尼环[由于RNA大量合成而显示特别膨大的胀泡,在多线染色体中形成独特的环]Balbiani chromosome|巴尔比亚尼染色体[具有染色带的多线染色体,1881年首先发现于双翅目摇蚊幼虫]ball mill|球磨ball mill pulverizer|球磨粉碎机ball milling|球磨研磨balloon catheter|气囊导管[可用于基因送递,如将DNA导入血管壁]banana bond|香蕉键band|条带,带[见于电泳、离心等]band broadening|条带加宽band sharpening|条带变细,条带锐化band width|带宽banding pattern|带型banding technique|显带技术,分带技术barbiturate|巴比妥酸盐barium|钡barly strip mosaic virus|大麦条纹花叶病毒barly yellow dwarf virus|大麦黄矮病毒barnase|芽胞杆菌RNA酶[见于解淀粉芽胞杆菌]barophilic baceria|嗜压菌baroreceptor|压力感受器barotaxis|趋压性barotropism|向压性barr body|巴氏小体barrel|桶,圆筒[可用于描述蛋白质立体结构,如beta折叠桶]barrier|屏障,垒barstar|芽胞杆菌RNA酶抑制剂[见于解淀粉芽胞杆菌]basal|基础的,基本的basal body|基粒basal body temperature|基础体温basal component|基本成分,基本组分basal expression|基础表达,基态表达basal granule|基粒basal heat producing rate|基础产热率basal lamina|基膜,基板basal level|基础水平,基态水平basal medium|基本培养基,基础培养基basal medium Eagle|Eagle基本培养基basal metabolic rate|基础代谢率basal metabolism|基础代谢basal promoter element|启动子基本元件basal transcription|基础转录,基态转录basal transcription factor|基础转录因子base|碱基;碱base analog|碱基类似物,类碱基base catalysis|碱基催化base composition|碱基组成base pairing|碱基配对base pairing rules|碱基配对法则,碱基配对规则base peak|基峰base pire|碱基对base ratio|碱基比base stacking|碱基堆积base substitution|碱基置换baseline|基线baseline drift|基线漂移baseline noise|基线噪声basement membrane|基底膜basement membrane link protein|基底膜连接蛋白basic amino acid|碱性氨基酸basic fibroblast growth factor|碱性成纤维细胞生长因子basic fuchsin|碱性品红basic medium|基础培养基basic number of chromosome|染色体基数basic protein|碱性蛋白质basic solvent|碱性溶剂basic taste sensation|基本味觉basidiocarp|担子果basidiomycetes|担子菌basidium|担子basipetal translocation|向基运输basket centrifuge|(吊)篮式离心机basket drier|篮式干燥机basket type evaporator|篮式蒸发器basonuclin|碱(性)核蛋白[见于角质形成细胞,含有多对锌指结构] basophil|嗜碱性细胞basophil degranulation|嗜碱性细胞脱粒basophilia|嗜碱性batch|分批;批,一批batch cultivation|分批培养batch culture|分批培养物batch digestor|分批消化器batch extraction|分批抽提,分批提取batch fermentation|分批发酵,(罐)批发酵batch filtration|分批过滤batch operation|分批操作batch process|分批工艺,分批法batch reactor|间歇反应器,分批反应器batch recycle cultivation|分批再循环培养batch recycle culture|分批再循环培养(物)bathochrome|向红基bathochromic shift|红移bathorhodopsin|红光视紫红质,前光视紫红质batrachotoxin|树蛙毒素[固醇类生物碱,作用于钠通道] baytex|倍硫磷BCG vaccine|卡介苗bead mill|玻珠研磨机bead mill homogenizer|玻珠研磨匀浆机bean sprouts medium|豆芽汁培养基beauvericin|白僵菌素becquerel|贝可(勒尔)bed volume|(柱)床体积bee venom|蜂毒beef broth|牛肉汁beef extract|牛肉膏,牛肉提取物beet yellows virus|甜菜黄化病毒Beggiatoa|贝日阿托菌属[属于硫细菌]behavior|行为;性质,性能behavioral control|行为控制behavioral isolation|行为隔离behavioral thermoregulation|行为性体温调节behenic acid|山yu酸,二十二(烷)酸belt desmosome|带状桥粒belt press|压带机belt press filter|压带(式)滤器bench scale|桌面规模,小试规模benchtop bioprocessing|桌面生物工艺[小试规模]benchtop microcentrifuge|台式微量离心机bend|弯曲;弯管;转折bending|弯曲;转折,回折beneficial element|有益元素bent bond|弯键bent DNA|弯曲DNA,转折DNAbenzene|苯benzhydrylamine resin|二苯甲基胺树脂benzidine|联苯胺benzilate|三苯乙醇酸(或盐或酯)benzimidazole|苯并咪唑benzodiazine|苯并二嗪,酞嗪benzoin|苯偶姻,安息香benzophenanthrene|苯并菲benzopyrene|苯并芘benzoyl|苯甲酰基benzoylglycine|苯甲酰甘氨酸benzyl|苄基benzyladenine|苄基腺嘌呤benzylaminopurine|苄基氨基嘌呤benzylisoquinoline|苄基异喹啉benzylisoquinoline alkaloid|苄基异喹啉(类)生物碱benzylpenicillin|苄基青霉素berberine|小檗碱Bertrand rule|贝特朗法则bestatin|苯丁抑制素[可抑制亮氨酸氨肽酶的一种亮氨酸类似物]C value|C值[单倍基因组DNA的量]C value paradox|C值悖理[物种的C值和它的进化复杂性之间无严格对应关系]C4 dicarboxylic acid cycle|C4二羧酸循环cachectin|恶液质素[即alfa肿瘤坏死因子]cadaverine|尸胺cadherin|钙粘着蛋白[介导依赖(于)钙的细胞间粘着作用的一类跨膜蛋白质,分为E-,N-,P-等若干种,E表示上皮(epithelia),N表示神经(neural),P表示胎盘(placental)] cadmium|镉caerulin|雨蛙肽cage|笼cage compound|笼形化合物cage coordination compound|笼形配合物cage effect|笼效应cage structure|笼形结构[非极性分子周围的水分子所形成的有序结构]calbindin|钙结合蛋白calciferol|麦角钙化(固)醇calcimedin|钙介蛋白[钙调蛋白拮抗剂]calcineurin|钙调磷酸酶[依赖于钙调蛋白的丝氨酸—苏氨酸磷酸酶]calcionin|降钙素calcium binding protein|钙结合蛋白(质)calcium binding site|钙结合部位calcium channel|钙通道calcium chloride|氯化钙calcium influx|钙流入calcium mediatory protein|钙中介蛋白(质)calcium phosphate|磷酸钙calcium phosphate precipitation|磷酸盐沉淀calcium pump|钙泵calcium sensor protein|钙传感蛋白(质)calcium sequestration|集钙(作用)calcyclin|钙(细胞)周边蛋白calcyphosine|钙磷蛋白[是依赖于cAMP的蛋白激酶的磷酸化底物]caldesmon|钙调(蛋白)结合蛋白[主要见于平滑肌,可与钙调蛋白及肌动蛋白结合] calelectrin|钙电蛋白[最初发现于鳗鱼电器官的一种钙结合蛋白]calf intestinal alkaline phosphatase|(小)牛小肠碱性磷酸酶calf serum|小牛血清calf thymus|小牛胸腺calgranulin|钙粒蛋白calibration|校准,标准calibration curve|校正曲线calibration filter|校准滤光片calibration protein|校准蛋白calicheamycin|刺孢霉素[来自刺孢小单胞菌的抗肿瘤抗生素,带有二炔烯官能团] calicivirus|杯状病毒calli|(复)胼胝体,愈伤组织[用于植物];胼胝[见于动物皮肤]callose|胼胝质,愈伤葡聚糖callose synthetase|愈伤葡聚糖合成酶callus|胼胝体,愈伤组织[用于植物];胼胝[见于动物皮肤]callus culture|愈伤组织培养calmodulin|钙调蛋白calnexin|钙联结蛋白[内质网的一种磷酸化的钙结合蛋白]calomel|甘汞calomel electrode|甘汞电极calorie|卡calpactin|依钙(结合)蛋白[全称为“依赖于钙的磷脂及肌动蛋白结合蛋白”]calpain|(需)钙蛋白酶calpain inhibitor|(需)钙蛋白酶抑制剂calpastatin|(需)钙蛋白酶抑制蛋白calphobindin|钙磷脂结合蛋白calphotin|钙感光蛋白[感光细胞的一种钙结合蛋白]calprotectin|(肌)钙网蛋白[骨骼肌肌质网膜上的钙结合蛋白]calretinin|钙(视)网膜蛋白calsequestrin|(肌)集钙蛋白calspectin|钙影蛋白calspermin|钙精蛋白[睾丸的一种钙调蛋白结合蛋白]caltractin|钙牵蛋白[一种与基粒相关的钙结合蛋白]Calvin cycle|卡尔文循环,光合碳还原环calyculin|花萼海绵诱癌素[取自花萼盘皮海绵的磷酸酶抑制剂]calyptra|根冠calyx|花萼cambium|形成层[见于植物]cAMP binding protein|cAMP结合蛋白cAMP receptor protein|cAMP受体蛋白cAMP response element|cAMP效应元件cAMP response element binding protein|cAMP效应元件结合蛋白Campbell model|坎贝尔模型camphane|莰烷camphane derivative|莰烷衍生物camphore|樟脑camptothecin|喜树碱Campylobacter|弯曲菌属Campylobacter fetus|胎儿弯曲菌属Canada balsam|加拿大香脂,枞香脂canaline|副刀豆氨酸canalization|[表型]限渠道化,发育稳态[尽管有遗传因素和环境条件的干扰,表型仍保持正常]canavanine|刀豆氨酸cancer|癌症cancer metastasis|癌症转移cancer suppressor gene|抑癌基因cancer suppressor protein|抑癌基因产物,抑癌蛋白(质)candicidin|杀假丝菌素candida|念珠菌属Candida albicans|白色念珠菌candle jar|烛罐cannabin|大麻苷;大麻碱canonical base|规范碱基canonical molecular orbital|正则分子轨道canonical partition function|正则配分函数canonical sequence|规范序列cantharidin|斑蝥素canthaxanthin|角黄素canyon|峡谷[常用于比喻某些生物大分子的主体结构特征]cap|帽,帽(结构)cap binding protein|帽结合蛋白cap site|加帽位点capacitation|获能[特指镜子在雌性生殖道中停留后获得使卵子受精的能力]capacity|容量capacity factor|容量因子capillarity|毛细现象capillary|毛细管;毛细血管capillary absorption|毛细吸收capillary action|毛细管作用capillary attraction|毛细吸力capillary column|毛细管柱capillary culture|毛细管培养capillary electrode|毛细管电极capillary electrophoresis|毛细管电泳capillary free electrophoresis|毛细管自由流动电泳capillary gas chromatography|毛细管气相层析capillary isoelectric focusing|毛细管等电聚焦capillary isotachophoresis|毛细管等速电泳capillary membrane module|毛细管膜包capillary transfer|毛细管转移[通过毛细管作用进行核酸的印迹转移] capillary tube|毛细管capillary tubing|毛细管capillary zone electrophoresis|毛细管区带电泳capillovirus|毛状病毒组capping|加帽,加帽反应;封闭反应;帽化,成帽capping enzyme|加帽酶capping protein|[肌动蛋白]加帽蛋白caprin|癸酸甘油酯caproin|己酸甘油酯capromycin|卷曲霉素,缠霉素caproyl|己酸基caprylin|辛酸甘油酯capsid|(病毒)衣壳,(病毒)壳体capsid protein|衣壳蛋白capsidation|衣壳化capsomer|(病毒)壳粒capsular polysaccharide|荚膜多糖capsulation|包囊化(作用),胶囊化(作用)capsule|荚膜capsule swelling reaction|荚膜肿胀反应capture|捕捉,俘获capture antigen|捕捉抗原[酶免疫测定中用于捕捉抗体的抗原]capture assay|捕捉试验carbamyl|氨甲酰基carbamyl ornithine|氨甲酰鸟氨酸carbamyl phosphate|氨甲酰磷酸carbamyl phosphate synthetase|氨甲酰磷酸合成酶carbamyl transferase|氨甲酰(基)转移酶carbamylation|氨甲酰化carbanion|碳负离子carbanyl group|羰基carbene|卡宾carbenicillin|羧苄青霉素carbenoid|卡宾体carbocation|碳正离子carbodiimide|碳二亚胺carbohydrate|糖类,碳水化合物carbohydrate fingerprinting|糖指纹分析carbohydrate mapping|糖作图,糖定位carbohydrate sequencing|糖测序carbol fuchsin|石炭酸品红carboline|咔啉,二氮芴carbon assimilation|碳同化carbon balance|碳平衡carbon cycling|碳循环carbon dioxide|二氧化碳carbon dioxide compensation|二氧化碳补偿点carbon dioxide fertilization|二氧化碳施肥carbon dioxide fixation|二氧化碳固定carbon dioxide tension|二氧化碳张力carbon fiber|碳纤维carbon fixation|碳固定carbon isotope|碳同位素carbon isotope analysis|碳同位素分析carbon isotope composition|碳同位素组成carbon monoxide|一氧化碳carbon source|碳源carbonate|碳酸盐,碳酸酯carbonate plant|碳化植物carbonic anhydrase|碳酸酐酶carbonium ion|碳正离子carbonyl|羰基carbonylation|羰基化carboxydismutase|羰基岐化酶,核酮糖二磷酸羧化酶 carboxydotrophic bacteria|一氧化碳营养菌carboxyglutamic acid|羧基谷氨酸carboxyl|羧基carboxyl protease|羧基蛋白酶carboxyl terminal|羧基端carboxyl transferase|羧基转移酶carboxylase|羧化酶carboxylation|羧(基)化carboxylic acid|羧酶carboxymethyl|羧甲基carboxymethyl cellulose|羧甲基纤维素carboxypeptidase|羧肽酶[包括羧肽酶A、B、N等]carcinogen|致癌剂carcinogenesis|致癌,癌的发生carcinogenicity|致癌性carcinoma|癌carcinostatin|制癌菌素cardenolide|强心苷cardiac aglycone|强心苷配基,强心苷元cardiac cycle|心动周期cardiac glycoside|强心苷cardiac receptor|心脏感受器cardiohepatid toxin|心肝毒素[如来自链球菌]cardiolipin|心磷脂cardiotoxin|心脏毒素cardiovascular center|心血管中枢cardiovascular disease|心血管疾病cardiovirus|心病毒属[模式成员是脑心肌炎病毒]carlavirus|香石竹潜病毒组carmine|洋红carminomycin|洋红霉素carmovirus|香石竹斑驳病毒组carnation latent virus|香石竹潜病毒carnation mottle virus|香石竹斑驳病毒carnation ringspot virus|香石竹环斑病毒carnitine|肉碱carnitine acyl transferase|肉碱脂酰转移酶carnosine|肌肽[即beta丙氨酰组氨酸]carotene|胡萝卜素carotene dioxygenase|胡萝卜素双加氧酶carotenoid|类胡萝卜素carotenoprotein|胡萝卜素蛋白carpel|[植物]心皮carrageen|角叉菜,鹿角菜carrageenin|角叉菜胶carrier|载体,运载体,携载体;携带者,带(病)毒者,带菌者 carrier ampholyte|载体两性电解质carrier catalysis|载体催化carrier coprecipitation|载体共沉淀carrier DNA|载体DNAcarrier free|无载体的carrier phage|载体噬菌体carrier precipitation|载体沉淀(作用)carrier state|携带状态carriomycin|腐霉素,开乐霉素cartridge|[萃取柱的]柱体;软片,胶卷;子弹,弹药筒casamino acid|(水解)酪蛋白氨基酸,酪蛋白水解物cascade|串联,级联,级联系统cascade amplification|级联放大cascade chromatography|级联层析cascade fermentation|级联发酵casein|酪蛋白,酪素casein kinase|酪蛋白激酶[分I、II两种]Casparian band|凯氏带[见于植物内表皮细胞]Casparian strip|凯氏带cassette|盒,弹夹[借指DNA序列组件]cassette mutagenesis|盒式诱变casting|铸,灌制CAT box|CAT框[真核生物结构基因上游的顺式作用元件]catabolism|分解代谢catabolite gene activator protein|分解代谢物基因激活蛋白 catabolite repression|分解代谢物阻抑,分解代谢产物阻遏catalase|过氧化氢酶catalytic active site|催化活性位catalytic activity|催化活性catalytic antibody|催化性抗体,具有催化活性的抗体catalytic constant|催化常数[符号Kcat]catalytic core|催化核心catalytic mechanism|催化机理catalytic RNA|催化性RNAcatalytic selectivity|催化选择性catalytic site|催化部位catalytic subunit|催化亚基cataphoresis|阳离子电泳cataract|白内障catechin|儿茶素catechol|儿茶酚,邻苯二酚catecholamine|儿茶酚胺catecholamine hormones|儿茶酚胺类激素catecholaminergic recptor|儿茶酚胺能受体catenane|连环(体),连锁,链条[如DNA连环体];索烃catenating|连环,连接catenation|连环,连锁,成链catenin|连环蛋白[一类细胞骨架蛋白,分alfa/beta/gama三种] catharanthus alkaloid|长春花属生物碱cathepsin|组织蛋白酶[分为A、B、C、D、E…H、L等多种]catheter|导管cathode layer enrichment method|阴极区富集法cathode ray polarograph|阴极射线极谱仪cation acid|阳离子酸cationic acid|阳离子酸cationic catalyst|正离子催化剂cationic detergent|阳离子(型)去污剂cationic initiator|正离子引发剂cationic polymerization|正离子聚合,阳离子聚合 cationic surfactant|阳离子(型)表面活性剂cationization|阳离子化cauliflower mosaic virus|花椰菜花叶病毒caulimovirus|花椰菜花叶病毒组caulobacteria|柄病毒Cavendish laboratory|(英国)卡文迪什实验室caveola|小窝,小凹caveolae|(复)小窝,小凹caveolin|小窝蛋白cavitation|空腔化(作用)cavity|沟槽,模槽,空腔dammarane|达玛烷dammarane type|达玛烷型Dane particle|丹氏粒[乙型肝炎病毒的完整毒粒]dansyl|丹(磺)酰,1-二甲氨基萘-5-磺酰dansyl chloride|丹磺酰氯dansyl method|丹磺酰法dantrolene|硝苯呋海因[肌肉松弛剂]dark current|暗电流dark field|暗视野,暗视场dark field microscope|暗视野显微镜,暗视场显微镜 dark field microscopy|暗视野显微术,暗视场显微术 dark reaction|暗反应dark repair|暗修复dark respiration|暗呼吸dark room|暗室,暗房dark seed|需暗种子data accumulation|数据积累data acquisition|数据获取data analysis|数据分析data bank|数据库data base|数据库data handling|数据处理data logger|数据记录器data logging|数据记录data output|数据输出data processing|数据处理data recording|数据记录dauermodification|持续饰变daughter cell|子代细胞daughter chromatid|子染色单体daughter chromosome|子染色体daughter colony|子菌落[由原生菌落续发生长的小菌落]daunomycin|道诺霉素daunorubicin|道诺红菌素de novo sequencing|从头测序de novo synthesis|从头合成deactivation|去活化(作用),失活(作用),钝化deacylated tRNA|脱酰tRNAdead time|死时间dead volume|死体积deadenylation|脱腺苷化DEAE Sephacel|[商]DEAE-葡聚糖纤维素,二乙氨乙基葡聚糖纤维素 dealkylation|脱烷基化deaminase|脱氨酶deamination|脱氨(基)death phase|死亡期[如见于细胞生长曲线]death point|死点deblocking|去封闭debranching enzyme|脱支酶,支链淀粉酶debris|碎片,残渣decahedron|十面体decane|癸烷decantation|倾析decanting|倾析decapacitation|去(获)能decarboxylase|脱羧酶decarboxylation|脱羧(作用)decay|原因不明腐败decay accelerating factor|衰变加速因子decay constant|衰变常数deceleration phase|减速期[如见于细胞生长曲线]dechlorination|脱氯作用deciduous leaf|落叶decline phase|[细胞生长曲线的]衰亡期decoagulant|抗凝剂decoding|译码,解码decomposer|分解者[可指具有分解动植物残体或其排泄物能力的微生物] decompression|降压,减压decondensation|解凝(聚)decontaminant|净化剂,去污剂decontaminating agent|净化剂,去污剂decontamination|净化,去污decorin|核心蛋白聚糖[一种基质蛋白聚糖,又称为PG-40]dedifferentiation|去分化,脱分化deep colony|深层菌落deep etching|深度蚀刻deep jet fermentor|深部喷注发酵罐deep refrigeration|深度冷冻deep shaft system|深井系统[如用于污水处理]defasciculation factor|解束因子[取自水蛭,可破坏神经束]defective|缺损的,缺陷的defective interfering|缺损干扰defective interfering particle|缺损干扰颗粒,干扰缺损颗粒defective interfering RNA|缺损干扰RNAdefective interfering virus|缺损干扰病毒defective mutant|缺损突变体,缺陷突变型,缺陷突变株defective phage|缺损噬菌体,缺陷噬菌体defective virus|缺损病毒,缺陷病毒defense|防御,防卫defense peptide|防卫肽defense response|防御反应,防卫反应defensin|防卫素[动物细胞的内源性抗菌肽]deficiency|缺乏,缺损,缺陷deficient|缺少的,缺损的,缺陷的defined|确定的defined medium|确定成分培养基,已知成分培养液defintion|定义defoliating agent|脱叶剂defoliation|脱叶deformylase|去甲酰酶[见于原核细胞,作用于甲酰甲硫氨酸]degasser|脱气装置degassing|脱气,除气degeneracy|简并;简并性,简并度degenerate|简并的degenerate codon|简并密码子degenerate oligonucleotide|简并寡核苷酸degenerate primer|简并引物degenerate sequence|简并序列degeneration|退化,变性degenerin|退化蛋白[与某些感觉神经元的退化有关]deglycosylation|去糖基化degradable polymer|降解性高分子degradation|降解degranulation|脱(颗)粒(作用)degree of acidity|酸度degree of dominance|显性度degree of polymerization|聚合度degron|降解决定子[决定某一蛋白发生降解或部分降解的序列要素] deguelin|鱼藤素dehalogenation|脱卤(作用)dehardening|解除锻炼dehumidifier|除湿器dehydratase|脱水酶dehydrated medium|干燥培养基dehydration|脱水(作用)dehydroepiandrosterone|脱氢表雄酮dehydrogenase|脱氢酶dehydrogenation|脱氢(作用)dehydroluciferin|脱氢萤光素deionization|去离子(作用)deionized|去离子的deionized water|去离子水deionizing|去离子(处理)delayed early transcription|(延)迟早期转录[可特指病毒]delayed fluorescence|延迟荧光delayed heat|延迟热delayed hypersensitivity|延迟(型)超敏反应delayed ingeritance|延迟遗传delayed type hypersensitivity|迟发型超敏反应deletant|缺失体deletion|缺失deletion mapping|缺失定位,缺失作图deletion mutagenesis|缺失诱变deletion mutant|缺失突变体deletion mutantion|缺失突变deletional recombination|缺失重组delignification|脱木质化(作用)deliquescence|潮解delivery flask|分液瓶delocalized bond|离域键。
医药用级聚丙烯酸树脂特征

医药用级聚丙烯酸树脂特征医药用级聚丙烯酸树脂特征中文名称:聚丙烯酸树脂英文名称: CARBOMER别名:卡波树脂,丙烯酸聚合物;Carboxy vinyl polymer性状:松散白色,微酸性粉末状,是丙烯酸和长链的烷基甲基丙烯酸通过蔗糖烯丙基醚或交联而成的高分子量共聚物,并含有肯定量的表面活性剂,为一族水溶性树脂,具有增稠、悬浮、稳定功能,能产生肯定范围的粘度和流动性,使不溶性组分及固体颗粒悬浮于体系中,使所制备乳剂的耐热和耐寒性能。
虽然重要用于经中和的水介质中,也可用于经中和或不中和的有机溶剂水介质中。
溶解度:不溶于水,溶于极性有机溶剂如乙醇、异丙醇等。
用途:本片重要用作片剂、丸剂、颗粒剂的包衣料子和粘合剂。
也可用于胶囊剂、膜剂等的制造,调整药物的释放部位和速度,常用85—95%乙醇作溶剂,配成5—8%溶液作包衣用。
醋酸钠CAS号: 6131—90—4分子式: C2H9NaO5分子量:136.08EINECS号: 204—823—8氨丁三醇分子式: C57H110O6 分子量: 891.48CAS号:68334—00—9DL酒石酸CAS号: 133—37—9分子式: C4H6O6分子量:150.09EINECS号: 205—105—7聚丙烯酸树脂CAS号:24938—16—7分子式:(C8H15NO2·C8H14O2.·C5H8O2)x山梨酸钾CAS号: 590—00—1分子式: C6H7KO2分子量:150.22三氯蔗糖Sucralose蔗糖素分子式 C12H19Cl3O8分子量397.6335三乙醇胺CAS号: 102—71—6分子式: C6H15NO3分子量: 149.19松节油CAS号: 8006—64—2分子式: C12H20O7分子量:276.283司盘80分子式与分子量C24H44O6 428.60CAS号[1338—43—8] 香兰素分子式: C8H8O3分子量: 152.15微晶纤维素CAS号: 9004—34—6分子式: H2分子量: 2.01588 乙基纤维素CAS号: 9004—57—3分子式: N/A分子量: 0。
大口黑鲈的营养需要研究进展____

动物科学现代农业科技2011年第21期大口黑鲈(Micropterus salmoides ),俗称加州鲈,原产于美国加利福尼亚州,隶属鲈形目(Perciformes ),太阳鱼科(Ceutrarchidae )。
20世纪80年代初引入我国,由于其生长快、病害少、耐低温、肉多刺少、味道鲜美及营养丰富等优点,已成为我国养殖的主要淡水鱼品种之一。
大口黑鲈属典型淡水肉食性鱼,迄今尚未成功开发出营养平衡的全价专用饲料,尤其全程使用饲料一直是业界的一大难题,表现在中后期经常出现生长慢、厌食、肝脏疾病等问题[1]。
虽然大口黑鲈的养殖在国内外均有一定的规模,而且饲料成本占养殖成本的比例较高,但有关大口黑鲈营养需要的研究仍十分缺乏[2]。
在国外,大部分大口黑鲈的养殖,均采用比较容易获得的其他肉食性鱼类如鲑鱼和鳟鱼的饲料,而非采用针对大口黑鲈自身营养需要配制的专用饲料[3]。
在国内,养殖户投喂的饲料多以冰鲜下杂鱼和其他动物性饲料为主,这对海洋资源无疑是一种浪费,同时对养殖环境的污染也十分明显,容易引起各种疾病的暴发[4]。
按大口黑鲈2010年的产量测算,我国潜在的鲈鱼专用饲料需求可达20万t/年[1]。
对配合饲料的需要日益增加,亟待进一步全面开展其营养需要的研究。
因此,该文综述了国外内大口黑鲈营养需要的研究进展,并参考其他鱼类的营养需要,比较全面地总结了大口黑鲈对饲料中各营养素的需要量,以期为大口黑鲈专用饲料的研发和配制提供参考。
1大黑鲈对各种营养成分的需要量1.1蛋白质和氨基酸由于没有专门为大口黑鲈开发的商用饲料,目前在国外均采用其他肉性鱼类的饲料(蛋白质含量>40%,鱼粉含量50%~70%)[5-8]。
最早关于大口黑鲈饲料蛋白质营养需要的研究见于1981年[5]。
研究发现,0~1龄的大口黑鲈对饲料中蛋白质的需要量为39.9%~40.8%(基于饲料干物质)。
以饲料中水分含量为10%来计算的话,蛋白质含量为36%~37%(饲料湿重)即可满足1龄及之前的大口黑鲈鱼的生长。
Carbohydrates

CarbohydratesCarbohydrates, empirical formula CH2O, are the main energy source for our bodies and are vi tal to the synthesis of cells. They serve as food sources for living organisms and provide the struct ural support for plants. Many of the carbohydrates are large polymeric molecules made of simple s ugars. Only plants synthesise carbohydrates. Important carbohydrates are: starch (a polysaccharid e), lactose and sucrose (disaccharides) and glucose and fructose (monosaccharides). Glucose, mole cular formula C6H12O6, is found in all body cells and fructose, which has the same molecular form ula as glucose, is found in fruits and honey.Most carbohydrates (but not cellulose in humans) are changed to glucose, a simple sugar, as a result of digestion. Glucose is then carried by the blood to body cells, where it is oxidised during r espiration. The energy available goes to physical activities, keeps the body warm and is used for re pair and growth of cells. Excess carbohydrates are converted to fats and stored in the body.Cellulose, another polysaccharide, is the major component of plant cells. It cannot be digested by humans who lack the enzyme cellulase to hydrolyse it. Cellulose though not a source of energy , is an important part of diet and is called fibre or roughage.Compare the structural properties of starch and cellulose andexplain why humans can digest starch but not cellulose.Starch exists in two forms: amylose, which is a straight-chain polymer (α-1,4 linkage), and amylopectin, which is a branched structure with both α-1,4 and α-1,6 linkages. Cellulose on the other hand contains only β-1,4 linkage, which can be hydrolysed to glucose by the enzyme cellulase, which is absent in most animals, including mammals. Thus humans cannot digest cellulose. Cows and many other animals are able to digest cellulose in plants such as grass to produce glucose as a source of energy since they have bacteria in their alimentary canal that produce the cellulase enzyme.State what is meant by the term dietary fibre?Dietary fibre is mainly plant material that is part of fruits, grains and vegetables that the human bo dy cannot digest as it is not hydrolysed by enzymes produced by the human digestive tract (but ma y be digested by bacteria in the gut). Examples include cellulose, hemicellulose (made of different sugar monomers unlike cellulose) lignin and pectin.Constipation便秘 and diverticulosis肠憩室病The large intestine (肠) makes, stores and eliminates stool (大便). Low fibre diets lead to constipation due to the presence of hard stool that does not pass easily or frequently through the colon an d requires effort. Pressure applied to move a stool along causes diverticulosis, the presence of bulg es凸出 in the colon at weak places leading to abdominal腹 pain. Diverticulosis is quite common i n the western world where some diets consist of too much processed foods, which often lack fibre. Irritable应激性 Bowel肠 syndrome综合症Irritable Bowel Syndrome (IBS) refers to symptomsarising from the bowel not working as it normally should and includes constipation, bloating (feeli ng full), abdominal pain, etc. One way to decrease symptoms of IBS is to include more dietary fibr e in the diet.Obesity肥胖Regular intake of excess food leads to storage of energy in the fatty tissues. Obesity is excess body mass and leads to problems such as cardiovascular disease (involving heart and/or blood vessels), obesity related diabetes, breathing difficulties during sleep, etc. A high fibre diet leads to feeling fu ll on a diet with reduced carbohydrates and fats, which then reduces weight gain.Crohn’s disease克罗恩病This is an inflammatory发炎 bowel disease of the lower part small intestine and/or the large intest ine. The cause of the disease is unknown; dietary fibre may be helpful in its prevention. Haemorrhoids痔疮In this condition there are enlarged blood vessels in and around the rectum and anus that are swoll en at weak points and can burst causing bleeding; these can also occur when the blood vessels ge t infected. Haemorrhoids can be caused by pressure in the abdomen as a result of constipation whi ch can cause strain during bowel movements and by being obese or overweight. High fibre diet m akes the bulk move through the large intestine more easily.实验:1. Molish反应(α-萘酚反应):在试管中加入1 mL 2%的样品水溶液,滴入4滴10%的α-萘酚乙醇溶液,混合均匀后将试管倾斜约45度,沿管壁慢慢加入1 mL浓硫酸(勿摇动)。
巴斯夫露保康

安全技术说明书页: 1/14 巴斯夫安全技术说明书按照GB/T 16483编制日期 / 本次修订: 07.11.2022版本: 7.0日期/上次修订: 18.12.2021上次版本: 6.1日期 / 首次编制: 29.12.2005产品: 露保康®Product: Lupro-Grain®(30062123/SDS_GEN_CN/ZH)印刷日期 10.09.20231. 化学品及企业标识露保康®Lupro-Grain®推荐用途和限制用途: 饲料添加剂公司:巴斯夫(中国)有限公司中国上海浦东江心沙路300号邮政编码 200137电话: +86 21 20391000传真号: +86 21 20394800E-mail地址: **********************紧急联络信息:巴斯夫紧急热线中心(中国)+86 21 5861-1199巴斯夫紧急热线中心(国际):电话: +49 180 2273-112Company:BASF (China) Co., Ltd.300 Jiang Xin Sha RoadPu Dong Shanghai 200137, CHINA Telephone: +86 21 20391000Telefax number: +86 21 20394800E-mail address: ********************** Emergency information:Emergency Call Center (China):+86 21 5861-1199International emergency number: Telephone: +49 180 2273-1122. 危险性概述纯物质和混合物的分类:皮肤腐蚀/刺激: 分类2巴斯夫安全技术说明书日期 / 本次修订: 07.11.2022版本: 7.0产品: 露保康®Product: Lupro-Grain®(30062123/SDS_GEN_CN/ZH)印刷日期 10.09.2023易燃液体: 分类3急性毒性: 分类5 (口服)急性毒性: 分类5 (皮肤接触)严重损伤/刺激眼睛: 分类1特异性靶器官毒性-一次接触: 分类3 (对呼吸道系统有刺激性)标签要素和警示性说明:图形符号警示词:危险危险性说明:H226易燃液体和蒸气。
Comparedrugreleaseprofiles

Compare drug release profiles of water poor soluble drugs from a novel chitosan and polycarbophil interpolyelectrolytes complexation (PCC) and hydroxylpropyl - methylcellulose (HPMC) based matrix tabletsZhilei Lu*, Weiyang Chen, Eugene Olivier, Josias H., HammanDepartment of Pharmaceutical Sciences, Tshwane University of Technology, Private Bag X680, P retoria, 0001, South Africa资料个人收集整理,勿做商业用途*Corresponding author:Zhilei Lu (Dr.)Department of Pharmaceutical Sciences,Tshwane University of Technology,Private Bag X680,Pretoria, 0001,South Africa (e-mail: luzj@tut.ac.za)AbstractThe aim of this study was to compare the drug release behaviours of water poor soluble drugs from an interpolyelectrolyte complex (IPEC) of chitosan with polycarbophil (PCC) and hydroxylpropylmethylcellulose (HPMC) based matrix tablets. A novel interpoly - electrolyte complex (IPEC) of chitosan with polycarbophil (PCC) was synthesized and characterized. Water poor soluble drugsHydrochlorothiazide and Ketoprofen were used in this study as model drugs.Polymers (including PCC, HPMC K100M and HPMC K100LV) based matrix tablets drug controlled release system were prepared using direct compression method.The results illustrate PCC based-matrix tablets offer a swelling controlled release system for water poor soluble drug and drug release mechanism from this matrix drug delivery system can be improved by addition of microcrystalline cellulose (Avicel).Analysis of the in vitro release kinetic parametersof the matrix tablets, PCC based matrix tablets exhibited similar or higher drug release exponent (n) and mean dissolution time (MDT) values compared to the HPMC based matrix tablets. It demonstrated that PCC polymer can be successfully used as a matrixcontrolled release system for the water poor soluble model drugs such as hydroxylpropylmethylcellulose (HPMC). 资料个人收集整理,勿做商业用途1 IntroductionOver the last three decades years, as the expense and complications involved in marketing new drug entities have increased, with concomitant recognition of the therapeutic advantages of controlled drug delivery, greater attention has been focused on the development of novel and controlled release drug delivery systemsto provide a long-term therapeutic of drugs at the site of action following a single dose (Mandal, 2000; Jantzen and Robinson, 2002). Many formulation techniques have been used tobuild t”he barrier into the peroral dosage form to provide slow release of the maintenance dose. These techniques include the use of coatings, embedding of the drug in a wax, polymeric or plastic matrix, microencapsulation, chemical bindingto ion-exchange resins and incorporation into an osmotic pump (Collett and Moreton, 2002:293). Among different technologies used in controlled drug delivery, polymeric matrix systems are the most majority because of the simplicity of formulation, ease of manufacturing, low cost and applicability to drugs with wide range of solubility (Colombo, et al., 2000; Jamzad and Fassihi, 2006). 资料个人收集整理,勿做商业用途Drugs release profiles from polymeric matrix system can influence by different factors, but the type, amount, and physicochemical properties of the polymers used play a primary role (Jamzad and Fassihi, 2006). Hydroxylpropyl-methylcellulose (HPMC) is the most important hydrophilic carrier material used for oral drug sustained delivery systems (Colombo, 1993; Siepmann and Peppas, 2001). BecauseHPMC is water soluble polymer, it is generally recognized that drug release fromHPMC matrices follows two mechanisms, drug diffusion through the swelling gellayer and release by matrix erosion of the swollen layer (Ford et al., 1987; Raoet al., 1990; Colombo, 1993; Tahara et al., 1995; Reynolds, Gehrke et al., 1998; Siepmann et al., 1999; Siepmann and Peppas, 2001). However, diffusion, swelling and erosion are most important rate-controlling mechanisms of commercial available controlled release products (Langer and Peppas, 1983), the major advantages of swelling/erosionHPMC based matrix drug delivery system are: (i) minimum the drug burst release; (ii) the different physicochemical drugs release rate approach a constant; (iii) the possibility to predict the effect of the device design parameters (e.g. shape, size and composition of HPMC-based matrix tablets) on the resulting drug release rate, thus facilitating the development of new pharmaceutical products (Colombo, 1993;Siepmann and Peppas, 2001资).料个人收集整理,勿做商业用途Interpolyelectrolyte complexes (IPEC) are formed as precipitates by two oppositely charged polyelectrolytes in an aqueous solution, have been reported as a new class of polymer carriers, which play an important role in creating new oral drug delivery systems (Peppas and Khare, 1993; Berger et al., 2004). A variety chemical structure and stoichiometry of both components in interpolyelectrolyte complexes depends onthe pH values of the media, ionic strength, concentration, mixing ratio, and temperature (Peppas, 1986; Dumitriu and Chornet, 1998; Berger et al., 2004;Moustafine et al., 2005a). Chitosan is a positively charged (amino groups) deacetylated derivative of the natural polysaccharide, chitin (Paul and Sharma, 2000).Chitosan has already been successfully used to form complexes with natural anionic polymers such as carboxymethylcellulose, alginic acid, dextran sulfate,carboxymethyl dextran, heparin, carrageenan,pectin methacrylic acid copolymers ? (Eudragit polymers) and xanthan (Dumitriu and Chornet, 1998, Berger et al., 2004,Sankalia et al., 2007, Margulis and Moustafine, 2006)资. 料个人收集整理,勿做商业用途In this study, a novel polymer - IPEC between chitosan and polycarbophil (PCC) was synthesized, characterized and used as direct compressedexcipients in the matrix tablet. Although it have been well known that various IPEC have been used as a polymer carriers in drug controlled release system (Peppas and Khare, 1993, Garcia and Ghaly, 1996, Lorenzo-Lamoza et al., 1998, Soppirnath and Aminabhavi, 2002,Chen et al., 2004, Nam et al., 2004, Moustafine et al., 2005b), IPEC chitosan and polycarbophil was used as a polymer carriers have been investigated by Lu et al., (2006, 2007a, 2007 b, 2008a; 2008b资料个人收集整理,勿做商业用途The aim of this study was to comparein vitro water poor soluble drugs release profile of HPMC based matrices system to PCC based matrices system at same formulation.Water poor soluble model drugs Hydrochlorothiazide and ketoprofen were used in thisstudy. Two types HPMC (K100M and K100LV) and PCC polymers were used indirect compressedpolymers based matrix drug release system. The results of the hydration and erosion studies showed PCC based matrix systems have superior swelling properties. Drug release exponent (n) of each formulation PCC based matrices tablets are higher than HPMC based matrices tablets at pH 7.4 buffer solutions. It demonstrated that PCC has high potential to use in polymer based matrix drug con trolled released delivery for water poor soluble drugs资料个人收集整理,勿做商业用途2. Materials and methods2.1 MaterialsChitosan (Warren Chem Specialities, South Africa, Deacetylation Degree =91.25%),Polycarbophil (Noveon, Cleveland, USA), Hydroxylpropylmethylcellulose (MethocelK100M, K100LV Premium, Colorcon Limited, Kent, England), Ketoprofen (Changzhou Siyao Pharma. China), Hydrochlorothiazide (Huzhou Konch Pharmaceutical Co., Ltd. China), Microcrystalline cellulose (Avicel, pH101, FMC corporation NV, Brussels, Belgium), Sodium carboxymethyl starch (Explotab, Edward Mendell Co., Inc New York, USA). All other chemicals were of analytical grade and used as receive资料个人收集整理,勿做商业用途2.2 Preparation of interpolyelectrolytes complexation between chitosan and P olycarb op hil (PCC)资料个人收集整理,勿做商业用途Chitosan (30 g) was dissolved in 1000 ml of a 2% v/v acetic acid solution andpolycarbophil (30 g) was dissolved in 1000 ml of a 2% v/v acetic acid solution. Thechitosan solution was slowly added to the polycarbophil solution underhomogenisation (5200 rpm, ZKA , Germany) over a period of 20 minutes. Themixture was then mechanically stirred for a period of 1 hour at a speed of 1200 rpm(Heidolph RZR2021, Germany). The gel formed was separated by centrifuging for 5 min at 3000 rpm and then washed several times with a 2% v/v acetic acidsolution toremove any unreacted polymeric material. The gel was freeze dried for a period of 48 hours (Jouan LP3, France) and the lyophilised powder was screened through a 300prn sieve资料个人收集整理,勿做商业用途2.3Differential scanning calorimetry (DSC)DSC thernograns of the PCC were recorded with a Shinadzu DSC50 (Kyoto, Japan) instrument. The thermal behaviour was studied by sealing 2 mg samples of the material in aluminium crimp cells and heating it at a heating rate of 10o C per min under the flow of nitrogen at a flow rate of 20 ml/min. The calorimeter was calibrated with 2 mg of indium (Kyoto, Japan, melting point 156.4o C) at a heating rate of 10o C per min.资料个人收集整理,勿做商业用途2.4Fourier transforn infrared (FT-IR)Fourier transforn infrared (FT-IR) spectral data of the PCC polyner was obtained on a FTS-175C spectrophotoneter (BIO-RAD, USA) using the KBr disk nethod. 资料个人收集整理,勿做商业用途2.5Preparation of the natrix tabletsIn order to conpare the release profiles of water poor soluble drugs fron polyner based natrix tablets, nonolithic natrix type tablets containing hydrochlorothiazide or ketoprofen were prepared by conpressing a nixture of the ingredients with varying concentrations of the PCC, HPMC K100M and HPMC K100LV as indicated in Table 1. The ingredients of the different fornulationswere nanually pre-nixed by stirring in a 1000 nl glass beaker for 30 ninutes with a spatula. After the addition of 0.05 g of nagnesiun stearate (0.5% w/w), the powder nass was nixed for 10 nin. The powdernixture was conpressed using a rotating tablet press (Cadnach, India) fitted with round, shallow pun ches to p roduce matrix type tablets with a 6 mm diameter资料个人收集整理,勿做商业用途26 Weight, hard ness, thick ness and friability of tablets资料个人收集整理,勿做商业用途Weight variation was tested by weighing 20 randomly selected tablets individually, the n calculati ng the average weight and comparing the in dividual tablet weights to the average. The specification for weight variation is 10% from the average weight if the average weight < 0.08 g (USP 2006资料个人收集整理,勿做商业用途The hardnessof ten randomly selected matrix type tablets of each formulation was determined using a hardness tester (TBH 220 ERWE K A, Germany). The force (N) n eeded to break each tablet was recorded料个人收集整理,勿做商业用途The thick ness of each of 10 ran domly selected matrix type tablets were measured witha vernier calliper (accuracy = 0.02 mm). The thickness of the tablet should be within 5% variation of the average value资料个人收集整理,勿做商业用途A friability test was con ducted on the tablets using an Erweka Friabilator (TA3R,Germany). Twenty matrices were randomly selected from each formulation and any loose dust was removed with the aid of a soft brush. The selected tablets were weighed accurately and p laced in the drum of the friabilator. The drum was rotated at 25 rpm for 4 minu tes after which the matrices were removed. Any loose dust was removed from the matrices before they were weighed again. The friability maximal limit is 1% (USP 2006) was calculated using the following equation资料个人收集整理,勿做商业用途F (%) = W before (g)「W曲(g)X 100%(1)W after (g)Where F is the friability, W before is the initial weight of the matrices and W after is the weight of the matrices after test ing资料个人收集整理,勿做商业用途2.7 Swelli ng and erosi on studiesSwelling and erosion studies were carried out for all formulations matrix tablets. The matrices were weighed in dividually before they were pl aced in 900 ml p hos phate buffer (pH 7.4) at 37.0 0.寸C.± The medium was stirred with a paddle at a rotation speed of 50 rpm in a USP dissolution flask. At each time point, three tablets of each formulatio n were removed from the dissoluti on flask and gen tly wiped with a tissue toremove surface water, weighed and then placed into a plastic bowel. The matrix tablets were dried at 60°C until constant weight was achieved. The mean weights were determ ined for the three tablets at each time in terval. The data obta ined from this exp erime nt was used to calculate the swelli ng in dex and p erce ntage mass loss.料个人收集整理,勿做商业用途2.7.1Swelli ng indexThe swelli ng in dex (or degree of swelli ng) was calculated accord ing to the followi ngequati on资料个人收集整理,勿做商业用途s,=WJ—00W dWhere SI is the swelling index, W s and W d are the swollen and dry matrix weights, resp ectively, at immersio n timet in the buffer soluti on.资料个人收集整理,勿做商业用途2.7.2P erce ntage of matrix erosi onThe p erce ntage of matrix erosi on is calculated in relatio n to the in itial dry weight of the matrices, accord ing to the followi ng equation 资料个人收集整理,勿做商业用途Erow 册件"00%Where: dry weight (t) is the weight at time t.28 Assay of hydrochlorothiazide and ket oprofen in matrix tablets.料个人收集整理,勿做商业用途The drug content of the matrix type tablets was determ ined by crush ing 10 ran domly selected tablets from each formulatio n in a mortar and p estle. App roximately 80 mg po wder from the hydrochlorothiazide or ket oprofen containing matrices were weighed accurately and individually transferred into a 200 ml volumetric flasks, which were then made up to volume with p hos phate buffer soluti on (pH 7.4). This mixture was stirred for 30 minutes to allow compiete release of the drug. After filtration through a 0.45 阿filter membrane, the solution was assayed using ultraviolet (UV) spectrophotometry (Helios a Thermo , England) at a wavelength of 271 nm for hydrochlorothiazide and 261 nm for ketoprofen. The assay for drug content wasperformed in triplicate for each formulation. The percentage drug content of the tablets was calculated by mea ns of the followi ng equation:料个人收集整理,勿做商业用途DC (% w/w^W dru^x100%WmtWhere DC is the drug content, W drug is the weight of the drug and W mt is the weight of the matrix tablet .资料个人收集整理,勿做商业用途2.9 Release an alysisThe USP (2006) dissoluti on app aratus 2 (i.e. p addle) was used to determ ine the in vitro drug release from the different polymers based matrix tablets. The dissolution medium (900 ml) consisted of phosphate buffer solution (pH 7.4) at 37 0.5 o C and a ± rotation speed of 50 rpm was used. Three hydrochlorothiazide or ketoprofen matrix tablets of each formulatio n were in troduced into each of three dissoluti on vessels (i.e.?in triplicate) in a six station dissolution apparatus (TDT-08L, Electrolab , India).Samp les (5 ml) were withdraw n at sp ecially in tervals, and 5 ml of p reheated dissolution medium was replaced immediately. Sink conditions were maintained throughout the study. The samp les were filtered through a 0.45 阿membra ne, hydrochlorothiazide or ketoprofen content in the solution was determined using ultraviolet (UV) spectrop hotometry at a wavele ngth of 271 or 261 nm, res pectively.An alyses were p erformed in tripli cate资料个人收集整理,勿做商业用途2.9.1 Kin eticCon trolled release drug delivery systems may be classified accord ing to their mecha ni sms of drug release, which in cludes diffusi on-con trolled, dissoluti on con trolled, swelli ng con trolled and chemically con trolled systems (La nger et al., 1983). Drug release from sim pie swellable and erosi on systems may be described by the well-known power law expression and is defined by the following equation(Ritger and Pepp as, 1987; P illay and Fassihi, 1999资料个人收集整理,勿做商业用途Where M t is the amount of drug released at time t, M is the overall amount of drug released, K is the release con sta nt; n is the release or diffusi onal exponent; M/M is the cumulative drug concen trati on released at time t (or fractio nal drug release)料个人收集整理,勿做商业用途The release exponent (n) is used for the in terpretati on of the release mecha nism from poly meric matrix con trolled drug release systems (Peppas 1985). For the case of < 0.45 corrosFickdantdiffusi on release (Case I<an89homalous (non-Fickia n) transport, n = 0.89 toa zero-order (Case II) release kin etics, and n > 0.89to a super Case II transport (Ritger and Pepp as, 1987资料个人收集整理,勿做商业用途 The dissoluti on data were modelled by using the Po wer law equati on (Eq 7) withgraphs analysis software (Origin Scientific Graphing and Analysis software, Version 7, Origi nLab Corpo rati on, USA) using the Gaussia n-Newt on (Leve nberg-Hartely) app roach 资料个人收集整理,勿做商业用途2.9.2 Mea n dissolutio n time (MDT)MDT is a statistical moment that describes the cumulative dissolution process and provides a quantitative estimation of the drug release rate. It is defined by the following equation (Reppas and Nicolaides, 2000; Sousa^t al., 2002):资料个人收集整理,勿 做商业用途nMDTt i M t/M^ i =±Where MDT is the mean dissolution time, M t is the amount of the drug released at time t; t i is the time (min) at the midpoint between i and i-1 and M 乂 is the overall amount of the drug released.料个人收集整理,勿做商业用途cyli ndrical, i n sp ecially, n diffusi on al), 0.45 < n2.9.3 Differe nt factor f i and Similarityfactor f 2 The different factor f i is a measure of the relative error between two dissoluti on curves and the similarity factor f 2 is a measure of similarity in the p erce ntage dissoluti on betwee n two dissoluti on curves (Moore and Fla nn er, 1996). Assu ming that the p erce ntage dissolved values for two p rofiles cannot be higher tha n 100, the differe nt factor f 1 can have values from 0 (whe n no differe nee the two curves exists) to 100 (when maximum differenee exists). With the same assumption holding, the similarity factor f 2 can have values from 100 (when no differenee between the two curves exists) to 0 (when maximum differenee exists) (Pillay and Fassihi, 1999;Moore and Fla nn er, 1996; Re ppas and Nicolaides, 2000). In this study, these factors were used to confirm the relative of release p rofiles of water poor soluble model drugs from poly mers based matrix tablets of the same formulati ons. They are defi ned bythe followi ng equati ons:资料个人收集整理,勿做商业用途 f^100xn Z |Rt —Tt t 吕 n z R f2"0^「100hXG (Rt -T , I V ny 丿]Where n is the number of sample withdrawal points, R t is the percentage of the refere nee dissolved at time t, T is the p erce ntage of the test dissolved at time 资料个人 收集整理,勿做商业用途 3 Results and discussion 3.1 Prep arati on and characterisati on ofPCC The ion ic bond of the interpo "electrolyte comp lex (IP EC) betwee n chitosa n andpo lycarb op hil was con firmed by means p reviously p ublished differe ntial sea nning calorimetry (DSC) (Lu et al., 2007b) and Fourier tran sform in frared (FT-IR). Fig.1shows the FT-IR sp ectra of chitosa n, po lycarb op hil and the PCC poly mer.资料个人收集整理,勿做商业用途-1A peak that appears at 1561 cm on the IR spectrum of the PCC, which might be assigned to the carboxylate groups that formed ionic bonds with the protonated amino groups of chitosan as previously illustrated for the interaction between Eudragit E andEudragit L (Moustafine at al., 2005). This ionic bond seems to be the primary bin di ng force for the formatio n of a comp lex betwee n chitosa n and po lycarb op hil.资料个人收集整理,勿做商业用途Chitosan is a cationic polymer of natural origin with excellent gel and film forming properties. Polycarbophil can also be considered as polyanions with negatively charged carboxylate groups. Mixing chitosan and polycarbophil in acidic solution (2% acetic acid solution was used in the study), ionic bonds should form between the protonated free amino groups of chitosan and carboxylate groups of polycarbophil.According to the results obtained from DSC and FT-IR, the possible process of formatio n of interpo "electrolyte comp lexes may be described as illustrated in Fig.2资料个人收集整理,勿做商业用途3.2Physical characteristics and drug content of poly mers based matrix tablets^ 料个人收集整理,勿做商业用途As summarised in Table 2, the physical characteristics of matrix tablets showed the good thickness uniformity, as ranged from 3.40 0.04 to 4.12± 0.0±4mm, a variationof matrix tablets weight from 73.3 2.4 mg to±87.9 4.0±mg, furthermore the weight variation of all formulation tablets is very low (< 10% from the average weight) (USP 2006). Hardness of the matrix tablets shows a range from 68 ±14 to 94 ±12N.The tablets also pasted the friability test (<1%), confirm that all formulations tablets are within USP (2006) limits. Drugs content of all formulations ranged from 4.60 0.65 to 5.01 0.1±1%.资料个人收集整理,勿做商业用途3.3Swelli ng and erosi on prop erties of the poly mers based matrices tablets资料个人收集整理,勿做商业用途Investigation of matrix hydration and erosion by gravimetrical analysis is a valuable exercise to better understand the mechanism of release and the relative importance of participating parameters (Jamzad and Fassihi, 2006). Fig.3 and Fig.4 illustrate the water uptake profiles and Fig.5, Fig.6 illustrate percentage of matrix erosion of all formulation tablets, respectively. Swelling properties of the all formulation matrix tablets based on the content of PCC, HPMC K100M and HPMC K100LV in the matrices tablets. Water uptake and percentage of matrix erosion values of these matrix tablets show superior swelling characteristics either HPMC K100LV based matrix tablets, or PCC based matrix tablets. 资料个人收集整理,勿做商业用途IPEC betwee n chitosa n and po lycarb op hil is a three -dime nsional n etworks water insoluble poly (acrylic acid) polymer with free hydroxy groups. Hydroxy groups ofPCC contribute hydrophilic capacity significantly and polymer erosion characteristics depend on the reaction ratio of chitosan and polycarbophil while polymer synthesis.While the PCC based matrixes were put into the buffer solution, the electrostatic repulsion between fixed charges (hydroxy groups) uncoiled the polymers chains.The counterion diffusion inside the PCC gel creates an additional osmotic pressure difference across the gel, consequently lead to higher water uptake (Peppas and Khare, 1993; Lu, et al., 2007b). During the matrix erosion, the ionic bonds between chitosan and polycarbophil were not broken by the matrix swelling. PCC based matrix tablets (F1 and F7 formulation) have superior swelling behaviors compare to the HPMC based matrices. Swelling index values of F1 and F7 formulation matrix tablets are 1599.62±216.68 % and 1579.82 ±118.05 % at 12 hours, respectively.Furthermore, addition of microcrystalline cellulose (Avicel) can increase matrices erosion significantly. Compare the erosion behaviors of F1, F7 and F2, F8formulation (containing 20% Avicel), F1 and F7 matrix tablets erode 5.74 1.62 % and 6.59 1±.18 % on 12 hours only, cont rary F2 and F8 matrix tablets erode 55.59 1.43 and 100 % respectively. Microcrystalline cellulose (Avicel) is widely used in pharmaceutical, primarily as a binder/diluent, also has some disintegrant properties on oral tablet and capsule formulations where it is used in both wet granulation and direct-compression process (Wheatley, 2000). In this study, matrix erosion behaviours were act by microcrystalline cellulose facilitating the transport of liquid into the pore of matrix tablets. It demonstrates that PCC polymer have capacity to form swelli ng only or swelli ng-erosi on matrix drug delivery system.资料个人收集整理,勿做商业用途It also was confirmed that PCC based matrix tablets have much better swelling behaviors than HPMC based matrix tablets by comparing swelling curves in Fig.3 andFig.4. Swelling index values of F1 and F7 formulation matrix tablets are 1599.62216.68 % and 1579.82 11±8.05 % at 12 hours, contrary F3 and F9 matrix tablets are545.96 ±4.32% and 547.72 2±6.27%. HPMC K100LV based matrix tablets have excellence erosion curves in this study, F5, F6, F11 and F12 formulation matrixtablets eroded 100% on 12 hours, but F2 and F8 (PCC based tablets) formulation matrix tablets can eroded 55.59 ±1.43 and 100 % with microcrystalline cellulosefacilitating. 资料个人收集整理,勿做商业用途3.4Drug releaseIn vitro drug release was performed in pH 7.4 phosphate buffer solution for 12 hours.Results of percentage drug release versus time for hydrochlorothiazide and ketoprofen in different formulations matrices tablets are presented in Fig.7 and Fig. 8, while theMDT and drug release kinetics values were present in Table 3资.料个人收集整理,勿做商业用In this study, water poor soluble model drugs hydrochlorothiazide and ketoprofen release from polymers based matrix tablets was controlled by the polymer matrices swelling or swelling combination with erosion. Percentage of drug release, matrix swelling and erosion of F7 were summarised in Fig 9. The percentageketoprofen release curve follows the percentagematrix tablets swelling curve, it demonstrates that PCC based matrix drug delivery system is the swelling dependent drug release system for water poor soluble model drugs. Same as F7 matrix tablets, F1 matrix tablets is also a swelling only drug delivery system, in these matrix systems drugs release behaviour primarily depend on the matrix swelling characteristics. Because as the superior swelling capacity of PCC based matrix tablets, liquid environments inside of the matrix provide that the model drugs release are zero order drug release.As described in Table 3, release exponentsn)( of F1 and F7 are 0.83 0.03 a±nd 0.99 ± 0.02 during the experimental time, respectively. 资料个人收集整理,勿做商业用途Addition of microcrystalline cellulose (Avicel) influence the model drugs release profiles from PCC based matrix tablets significantly. Cumulative drug release of F2 and F8 formulation tablets is 93.7 4.13 % a±nd 99.6 4.2±5% at 12 hours, relativelyF1 and F7 formulation tablets is 73.8 1.13 % an±d 47.2 4.5±3 % only. This can be explained by drugs release mechanism were swelling and erosion instead of swelling only, consequently accelerate the drugs release. The adjustable capacity of PCC based matrix drug delivery system by addition of microcrystalline cellulose (Avicel) dem on strates the poten tial useful of PCC poly mer in drug con trolled release field 资料个人收集整理,勿做商业用途Compare to the PCC based matrix tablets, the drugs release profiles of HPMC based matrix tablets were adjusted difficultly. The relatives f1 and f2 values of difference polymers including PCC, HPMC 100M, HPMC 100LV based matrix tablets containing hydrochlorothiazide under same formulation were show in Table 4. As describedf1 and f2 values in Table 4, F3 and F4, F5 and F6 formulation tablets have similar drug release behaviours, but F1 and F2 formulation tablets illustrate different drug release behaviours. This phenomenacan be explained by the superior water uptake capacity of PCC polymer, more water containing can easier broken the physical tensility between the polymer particles. 资料个人收集整理,勿做商业用途However, HPMC 100LV polymer has excellence erosion characteristics, in this study model drugs release from HPMC 100LV based matrix tablets illustrate matrix erosion dependent properties. In generally, drug release from swelling and erosion matrix system shows zero order release pare the drug release exponentsn(), release constant (k1), and mean dissolution time (MDT) of F2 to F5, F6, they have not significantly different as described in Tablet 3, furthermore the relatives f1 and f2 values between F2 and F5, F6 in Table 4 show they are similar release profiles. It imply PCC based matrix tablets can become a swelling and erosion drug delivery system by the addition of microcrystalline cellulose (Avicel), this drug delivery system illustrate similar drug release p rofiles as HPMC 100LV based matrix tablets资料个人收集整理,勿做商业用途Although it is very complex process that the model drugs release from swelling and。
二苯甲酰酒石酸

北京L()二苯甲酰酒石酸一水重庆L()二苯甲酰酒石酸一水甘肃L()二苯甲酰酒石酸一水贵州L() 二苯甲酰酒石酸一水河南L()二苯甲酰酒石酸一水上海L()二苯甲酰酒石酸一水安徽L()二苯甲酰酒石酸一水广东L()二苯甲酰酒石酸一水海南L()二苯甲酰酒石酸一水黑龙江L()二苯甲酰酒石酸一水天津L()二苯甲酰酒石酸一水福建L()二苯甲酰酒石酸一水广西L()二苯甲酰酒石酸一水河北L() 二苯甲酰酒石酸一水湖北L() 二苯甲酰酒石酸一水湖南L() 二苯甲酰酒石酸一水吉林L()二江苏L()二苯甲酰酒石酸一内蒙古L() 二苯甲酰酒石酸青海L() 二苯甲酰酒石酸一水陕西L() 二苯甲酰酒石酸一水新疆L()二苯甲酰酒石酸一水香港L()二苯甲酰酒石酸一水山东L() 二苯甲酰酒石酸四川L()二苯甲酰酒石酸云南L()二苯甲酰酒石酸澳门L()二苯甲酰酒石酸L-(-)- 二苯甲酰酒石酸无水物福建泉州龙岩厂家现货| 福建福州L-(-)- 二苯甲酰酒石酸一水物| 福建厦门L-(-)- 二苯甲酰酒石酸一水物| 福建漳州L-(-)-二苯甲酰酒石酸一水物|福建泉州L-(-)-二苯甲酰酒石酸一水物|福建三明L-(-)- 二苯甲酰酒石酸一水物| 福建莆田L-(-)- 二苯甲酰酒石酸一水物| 福建南平L-(-)- 二苯甲酰酒石酸一水物| 福建龙岩L-(-)- 二苯甲酰酒石酸一水物| 福建宁德L-(-)- 二苯甲酰酒石酸一水物| 福建平潭L-(-)- 二苯甲酰酒石酸一水物|中文名称(-)- 二苯甲酰-L- 酒石酸英文名称(2R,3R)-(-)-dibenzoyl-L-tartaric acid anhydrous中文别名二苯基甲酰基-L-酒石酸;无水(-)-二苯甲酰-L-酒石酸;L-(-)-二苯甲酰酒石酸;L-二苯甲酰酒石酸;L-DBTA;L-(-)- 二苯甲酰酒石酸(无水物)CAS RN 2743-38-6EINECSt 220-374-0分子式C18H16O9分子量376.3142物化性质用途:用作医药原料, 手性拆分剂销售指导价:103.5 元/公斤水江西L()二苯甲酰酒石酸一水辽宁L()二苯甲酰酒石酸一水一水宁夏L()二苯甲酰酒石酸一水一水山西L() 二苯甲酰酒石酸一水一水西藏L()二苯甲酰酒石酸一水一水浙江L() 二苯甲酰酒石酸一水一水台湾L()二苯甲酰酒石酸一水联系电话:座机:QQ:20 邮箱:生产方法:L-二苯甲酰酒石酸一水物的合成方法大同小异,都是采用L-酒石酸、苯甲酸/苯甲酰氯、亚硫酰氯合成L- 二苯甲酰酒石酸酐,再水解生成L- 二苯甲酰酒石酸/L- 二苯甲酰酒石酸一水物。
水落坡药品基本信息
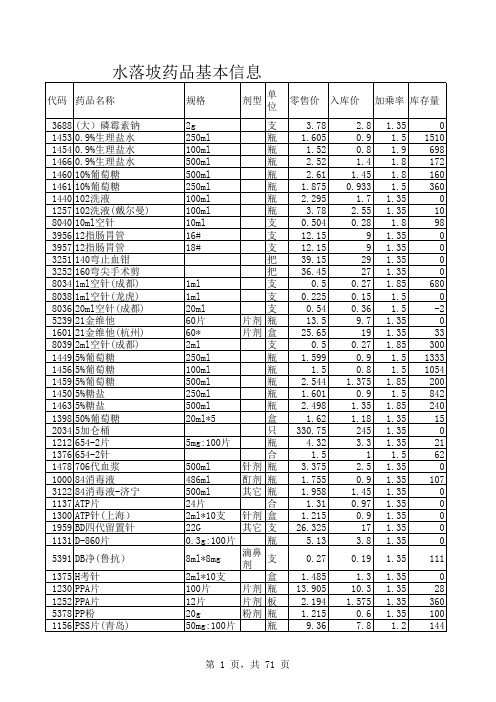
0.38 31.185 5.4 4.455 7.56 1.148 1.58 1.32 6.75 1.431 1.89 3.105 3.915 3.713 21.195 15.525 3.105 2.768 2.7 15.755 4.72 3.375 4.59 8.37 1.89 9.18 3.105 3.375 2.16 10.598 5.4 14.85 19.575 3.78 3.105 2.835 6.075 4.05 4.995 3.51 6.075 1.823 1.98 1.08 1.013 2.025 2.565
10支 1ml*10mg 0.25g*12t 20李*0.25g 0.25g*10粒 250mg*10粒 0.25g*10粒
0.25g*10s*2b
针剂 片剂 胶囊 胶囊 胶囊
颗粒 125mg*12代 0.6 0.6g-海口 0.6g 0.2285g6袋 20t 1g 1g 0.5g*12s 100片 6t*0.25g 0.1g*6 0.1g*4d 0.25g 0.1g*3包 0.25g*6t
粉剂
片剂 片剂 粉剂
针剂 粉剂 片剂
6粒* 0.25g*6 0.25G*10粒 0.1g*18d 0.1G*6D 0.1g*6包 粉剂 0.1g*6d 0.25g 25mg*6 0.25g*6t 250 0.25g 0.25g 25mg*100片 25mg 25mg*60t
片剂 片剂 针剂
片剂
g 盒 盒 盒 盒 板 板 板 盒 板 盒 合 支 支 支 盒 合 支 支 盒 瓶 盒 盒 盒 包 支 合 盒 盒 盒 盒 盒 盒 盒 盒 盒 盒 支 盒 合 瓶 支 支 瓶 瓶 瓶 瓶
3.78 3.78 3.78 1.215 1.62 1.62 1.02 1.208 1.17 0.81 1.2 0.945 1.971 1.08 0.878 2.16 0.9 0.945 0.975 2.1 1.013 1.92 2.025 1.89 4.32 2.16 2.295 26.865 8.64 2.12 4.32 1.688 1.89 2.4 2.805 2.43 1.188 8.033 2.295 10.125 6.75 7.425 2.538 1.418 5.13 607.5 297
泊沙康唑化学结构式

泊沙康唑化学结构式
泊沙康唑是一种广谱的抗真菌药物,其化学结构式如下:
C18H14Cl4N2O
\
N
/
C3H3Cl2
泊沙康唑是一种强效的抗真菌药物,被广泛用于治疗各种真菌感染疾病。
其化学结构式中,包含了多个氯原子和氮原子,这些原子的排列和连接方式共同构成了泊沙康唑的分子结构。
泊沙康唑的分子结构中,有一个环状结构和一个侧链结构。
环状结构是由碳原子和氮原子构成的,而侧链结构则由碳原子和氢原子构成。
这种分子结构使得泊沙康唑具有良好的抗真菌活性。
泊沙康唑的分子结构中的氯原子和氮原子与真菌细胞内的特定酶相互作用,抑制了真菌细胞内酶的活性,从而阻断了真菌的生长和繁殖。
泊沙康唑还可以改变真菌细胞膜的结构,使其失去完整性,从而进一步抑制真菌的生长。
泊沙康唑的分子结构使其具有较好的生物利用度和药代动力学特性,可以通过口服或静脉注射等途径给药。
泊沙康唑在体内经过代谢后形成的代谢产物具有较长的半衰期,从而保持了其较长的药效持续
时间。
泊沙康唑的广谱抗真菌活性使其成为治疗多种真菌感染疾病的首选药物之一。
它被广泛用于治疗念珠菌感染、皮肤真菌感染、口腔念珠菌感染等。
在临床应用中,泊沙康唑常常与其他抗真菌药物联合使用,以增强疗效。
泊沙康唑是一种重要的抗真菌药物,其化学结构为C18H14Cl4N2O,具有广谱的抗真菌活性,可用于治疗多种真菌感染疾病。
通过与真菌细胞内的特定酶相互作用,泊沙康唑能够抑制真菌的生长和繁殖,从而发挥治疗作用。
在临床应用中,泊沙康唑常与其他抗真菌药物联合使用,以提高疗效。
AOAC 30.1.23A AOAC Official Method 995.13-国外标准规范

30.1.23AAOAC Official Method995.13Carbohydrates in Soluble(Instant)CoffeeAnion-Exchange Chromatographic Methodwith Pulsed Amperometric DetectionFirst Action1995(Applicable for determination of free and total carbohydrates[ex-cept total fructose,which is degraded]in soluble[instant]coffee.) See Tables995.13A–J for the results of the interlaboratory study supporting the acceptance of the method.A.PrincipleFree carbohydrates.—Coffee is dissolved in H2O.Solution is fil-tered through C18disposable cartridge,and then through0.2µm membrane filter.Filtered solution is injected onto LC system.Car-bohydrates are separated on pellicular anion-exchange column and measured by pulsed amperometric detector.Total carbohydrates.—Coffee is hydrolyzed with1M HCl.Solu-tion is filtered and then passed through cation-exchange disposable cartridge in the Ag form to neutralize solution and to eliminate the Cl anion prior to injection onto LC system.B.Apparatus(a)Balance.—Analytical,weighing to0.1mg.(b)Flasks.—250mL round-bottom.(c)Volumetric flasks.—100and1000mL.(d)Pipets.—Delivering200–1000µL and5µL;with disposable tips.(e)Cylinders.—50and1000mL,tall-form,graduated.(f)Funnels.—Analytical,60°C.(g)Vacuum filtering system.—Aspirator with regulating device. System should include:heavy-walled filtering flask with ground cone neck,1L;funnel with ground glass joint,300mL;aluminum assembly clip;connection with vacuum outlet;filter holder,47mm id;and low-water extractable membrane filters,0.2µm porosity, 47mm diameter.(h)Filter papers.—Qualitative,folded,medium fast.(i)C18cartridges.—Octadecylated silica(ca10%C);6mL car-tridge volume;capable of holding500mg test portion;disposable.Con-dition and use cartridges according to manufacturer’s instructions. (j)Cation-exchange cartridges.—Styrene-based resin,Ag form, 1.8–2.0milliequivalent capacity/cartridge;disposable.Condition and use cartridges according to manufacturer’s instructions. (k)Membrane filters.—0.2µm porosity,25mm diameter;dis-posable;polypropylene.(l)Water bath.—Maintaining98±2°C.(m)Liquid chromatograph(LC).—Metal-free,compatible with 300mM NaOH.Operating conditions:mobile phase,isocratic(see Table995.13for mobile phase conditions);column temperature, ambient;flow rate,1.0mL/min;post-column solvent,300mM NaOH at0.6mL/min;detector settings,optimum parameters as pro-vided by e with computing integrator.(n)LC column.—250×4mm id;packed with polystyrene divinylbenzene substrate(10µm diameter)agglomerated with microbead quaternary amine functionalized latex(350nm diame-ter);5%cross-linked.(o)Pulsed amperometric detector.—With gold electrode.Fill reference cell with300mM NaOH.Select the detector range to avoid saturation of the major peak in chromatogram.(p)Guard column.—50×4mm id,packed with the same mate-rial as analytical column,(n).(q)Post-column solvent delivery system.—Compatible with 300mM NaOH.C.ReagentsUse18MΩ⋅cm demineralized H2O throughout.(a)Sodium hydroxide.—50%(w/w)aqueous solution,contain-ing minimum amount of Na2CO3and Hg.Do not shake or stir solu-tion before use.(b)Hydrochloric acid solution.—1.00M standard volumetric so-lution(83.3mL HCl/L).(c)Eluent A.—18MΩ⋅cm demineralized water.Filter through0.2µm membrane filter.Degas by sparging with He20–30min.Pre-pare fresh eluent A daily.(d)Eluent B.—300mM NaOH.Pipet15.6mL50%NaOH into 985mL eluent A.Degas by sparging with He20–30min.Eluent B is stable2days if stored at room temperature under He.(Note:It is crit-ical to remove dissolved CO2from eluents.Carbonate acts as strong “pusher”on LC column,resulting in drastic reduction in resolution.)(e)Carbohydrates standard solutions.—(1)Standard stock solu-tions.—1mg/mL aqueous stock solutions of arabinose,fructose, fucose,galactose,glucose,mannose,rhamnose monohydrate, ribose,xylose,sucrose,and mannitol.Weigh100mg each carbohy-drate to the nearest0.1mg into separate100mL volumetric flasks, dissolve in H2O,and dilute to volume with H2O.(2)Mixed carbohy-drates standard solution.—Further dilute and mix carbohydrates stock solutions to reach carbohydrate concentrations similar to those found in nonhydrolyzed or hydrolyzed soluble coffee test solutions. Pass diluted carbohydrates standard solution through0.2µm mem-brane filter prior to injection onto LC column.(Note:If resolution of rhamnose from arabinose is difficult to achieve,do not add rhamnose to mixed standard solution.)D.Isolation of CarbohydratesUse soluble coffee as is without grinding or homogenization.(a)Free carbohydrates.—Weigh300mg coffee to the nearest0.1mg into100mL volumetric flask.Add70mL H2O and shake until dissolution is complete.Dilute solution to volume with H2O.Filter 5–10mL solution through C18cartridge.Discard the first1mL.Pass fil-trate through0.2µm membrane filter prior to LC injection.(b)Total carbohydrates.—Weigh300mg coffee to the nearest0.1mg into100mL volumetric flask.Add50mL1.00M HCl and swirl.Place flask in boiling water bath for2.5h.(Note:Always keep the level of solution in flask below that of H2O in bath.)Swirl flaskTable995.13Conditions of mobile phase for determinationof free and total carbohydrates in solublecoffee by anion-exchange chromatographicmethod with pulsed amperometric detection Time,min Eluent A,%Eluent B,%01000(start acquisition) 50.01000(stop acquisition) 50.10100(start cleanup) 65.00100(stop cleanup) 65.11000(start re-equilibrium) 80.01000(stop re-equilibrium)Test sample N a Mean,%RSD r RSD R r R 17(2)0.024 1.6590.0010.041 1′11(0)0.1797.0500.0350.021 211(1)0.0600.91580.0020.10 2′11(0)0.1517.6460.0320.20 311(0) 1.5 2.89.90.120.44 3′11(1) 1.85 2.2180.120.94 411(0)0.619 3.6240.0630.42 4′11(0)0.782 4.6210.100.45 59(1)0.1928.2340.0450.18 5′11(0)0.30011370.0910.32 68(1)0.0597.1490.0120.083 6′10(0)0.1799.8500.050.25 a N=No.of laboratories after removal of outliers(in parentheses).Table995.13B Interlaboratory study results of the determination of free arabinose in soluble coffeeTest sample N a Mean,%RSD r RSD R r R 111(0)0.891 3.7140.0920.035 1′11(0) 3.54 6.6210.66 2.1 29(2) 1.320 1.6 5.10.0600.19 2′11(0) 4.83 3.3170.45 2.4 311(0)0.464 3.8110.0490.14 3′11(0) 4.76 3.1130.42 1.7 411(0)0.7477.3120.150.25 4′11(0) 4.54 4.618.40.59 2.4 510(1)0.505 4.47.70.0630.11 5′9(2) 4.08 3.0 4.90.340.56 611(0)0.629 4.18.90.0730.16 6′11(0) 3.79 5.7200.61 2.2a N=No.of laboratories after removal of outliers(in parentheses).Table995.13C Interlaboratory study results of the determination of free galactose in soluble coffeeTest sample N Mean,%RSD r RSD R r R 111(0)0.562 3.0 5.30.0470.084 1′10(1)17.88.18.9 4.1 4.8 211(0)0.339 4.18.00.0390.077 2′10(1)18.5 2.312 1.2 6.2 311(0)0.1919.8130.0530.070 3′11(0)8.08 2.78.00.6208.0 411(0)0.438 5.98.30.0740.10 4′11(0)13.3 3.913 1.8 5.9 59(2)0.475 3.1 4.10.0410.055 5′9(2)18.40 1.77.50.87 3.9 611(0)0.362 5.0120.0510.13 6′10(1)17.7 4.38.5 2.2 4.3a N=No.of laboratories after removal of outliers(in parentheses).Test sample N a Mean,%RSD r RSD R r R 111(0)0.1059.9210.0290.062 1′11(0)0.6848.7170.170.32 29(1)0.0421020.00.0120.024 2′10(1)0.8267.4220.170.50 310(1) 2.04 2.5 6.20.140.360 3′11(0)16.6 5.924.0 2.811.0 410(1) 1.66 4.1 6.10.190.29 4′11(0) 4.38 3.8240.47 2.9 510(1)0.18610210.0530.11 5′11(0) 1.95 5.7130.310.69 611(0)0.18610240.0520.12 6′11(0) 1.027.9140.230.40 a N=No.of laboratories after removal of outliers(in parentheses).Table995.13E Interlaboratory study results of the determination of free mannose in soluble coffeeTest sample N a Mean,%RSD r RSD R r R 111(0)0.583 4.9240.0800.40 1′10(1)17.9 5.811 2.9 5.7 210(0)0.1558.2360.0130.16 2′11(0)14.4 2.615 1.1 6.2 311(0)0.470 4.2180.0560.23 3′11(0) 2.60 2.0140.15 1.0 411(0)0.3297.0190.06517 4′11(0) 5.60 3.0150.48 2.4 510(1)0.277 4.2400.0330.31 5′11(0)7.65 2.8110.60 2.3 611(0)0.991 3.8170.110.48 6′10(0)19.1 2.222 1.212.00 a N=No.of laboratories after removal of outliers(in parentheses).Table995.13F Interlaboratory study results of the determination of free fructose in soluble coffeeTest sample N a Mean,%RSD r RSD R r R 19(0)0.17117310.0820.15 1′6(0)0.18924680.130.37 28(1)0.05421340.0320.052 39(2) 3.62 2.9180.30 1.9 3′9(0) 2.01 5.7720.32 4.1 410(1) 3.12 2.9180.26 1.6 4′7(1) 1.37 5.5700.21 2.7 510(0)0.2829.0450.0720.36 5′7(0)0.24420590.140.41 68(2)0.460 5.6160.0670.2 6′9(1)0.3637.3680.0750.70 a N=No.of laboratories after removal of outliers(in parentheses).Test sample N a Mean,%RSD r RSD R r R 17(2)0.07315740.0310.15 27(l)0.04528600.0350.077 58(0)0.10215980.0430.28 55(1)0.08016180.0360.041 67(l)0.1209.1810.0310.28 a N=No.of laboratories after removal of outliers(in parentheses).Table995.13H Interlaboratory study results of the determination of free xylose in soluble coffeeTest sample N a Mean,%RSD r RSD R r R 18(2)0.09723380.0630.10 29(0)0.1469.8200.0400.084 311(0) 1.86 4.6230.240.42 411(0)0.736 3.7280.0770.56 57(0)0.02925230.0200.023 511(0) 1.837.4220.38 1.2 69(0)0.13314240.0530.090 a N=No.of laboratories after removal of outliers(in parentheses).Table995.13I Interlaboratory study results of the determination of free sucrose in soluble coffeeTest sample N a Mean,%RSD r RSD R r R 210(0)0.149 4.8380.0200.16 310(1) 1.32 1.8100.0660.370 410(1)0.746 6.8120.140.25 511(0)0.18115420.0770.21 69(1)0.158 3.4330.0150.15 a N=No.of laboratories after removal of outliers(in parentheses).Table995.13J Interlaboratory study results of the determination of free fucose in soluble coffeeTest sample N a Mean,%RSD r RSD R r R 27(1)0.01620.0460.0090.021 38(0)0.0547.0670.0110.10 47(1)0.03623770.0230.074 57(1)0.011 5.4450.0020.014 66(1)0.02510360.0070.025 a N=No.of laboratories after removal of outliers(in parentheses).by hand every30min.Cool to room temperature under tap water. Dilute solution to100mL with H2O and filter through folded filter paper.Pass3mL filtrate through cation-exchange cartridge.Discard the first1mL.Filter neutralized solution through0.2µm membrane filter prior to LC injection.E.LC DeterminationInject equal volumes(10–20µL)of standard,C(e),and test solu-tions from D(a)or(b)onto LC column.[Note:Retention times and resolution tend to vary from column to column.Start clean-up and re-equilibration sequence only when the last monosaccharide (ribose)has been eluted.It may be necessary to perform2–3injec-tions of carbohydrates standard solution or to increase the re-equilibrium time in order to achieve a good separation of glucose, sucrose,and xylose.]Under normal conditions,approximate retention times of carbo-hydrates are:mannitol,4min;fucose,7min;rhamnose,15min;arabinose,16min;galactose,22min;glucose,25min;sucrose, 27min;xylose,30min;mannose,32min;fructose,40min;and ribose,43min.See Figure995.13for chromatogram of mixed carbo-hydrates standard solution.F.CalculationsCalculate concentration of carbohydrate in sample solution as follows:Carbohydrate,%=(R1/R2)×(W0/V0)×(V/W)×100 where R1=peak response of carbohydrate in test solution;R2=peak response of carbohydrate in carbohydrate standard solution;W0= mass of carbohydrate in the standard solution,mg;V0=total volume of the standard solution,mL;V=volume of standard solution,mL; and W=weight of test portion,mg.Express results as percent free or percent total carbohydrates (as is).References:J.AOAC Int.78,768(1995);79,1400(1996). Revised:June2000Figure995.13—HPAE-PAD chromatogram of mixed carbohydrates standard solution:mannitol,15m g;fucose,15m g/mL;rhamnose,35m g/mL;arabinose,40m g/mL;galactose,50m g/mL;glucose,55m g/mL;sucrose,45m g/mL; xylose,55m g/mL;mannose,45m g/mL;fructose,90m g/mL;and ribose,90m g/mL.。
盐酸拓扑替康

盐酸拓扑替康一、 基本信息【类 型】: 抗肿瘤药【剂 型】:粉针,胶囊【规 格】:粉针:0.2mg/瓶;胶囊:0.25mg/粒;【适 应 症】:经一线化疗失败小细胞肺癌。
晚期转移性卵巢癌。
【用法用量】:1.剂量:推荐剂量为1.2mg/m2/日,静脉输注30分钟。
持续5天,21天为一疗程,治疗中严重的中性粒细胞减少症患者,在其后的疗程中剂量减少0.2mg/m 2或与G-CSF同时使用。
使用从第6天开始,即在持续5天使用本品后24小时后再用G-CSF 。
2.注射液配制:用无菌注射用水1ml 溶解本品1mg 比例溶解本品,按1.2mg/m 2二、 国内注册情况(截止2009.9.14)/日剂量抽取药液,用0.9%氯化钠或5%葡萄糖注射液稀释后静脉输注。
【原 研 厂】: 英国史克-比彻姆(Smith Kline Beecham )公司研制开发【国外上市】:在美国,欧洲等30多个国家上市【注册分类】:6类【制剂工艺】: 制备工艺可行性高,不需要特殊生产设备;在中国上市和注册情况列表A.已上市国产原料6家,制剂6家(胶囊1家,粉针:6家)1.盐酸拓扑替康(国药准字H20052425 黄石飞云制药有限公司)2.盐酸拓扑替康(国药准字H20000435 扬州奥赛康药业有限公司)3.盐酸拓扑替康(国药准字H20000427 贵州汉方制药有限公司)4.盐酸拓扑替康(国药准字H20000433 扬子江药业集团有限公司)5.盐酸拓扑替康(国药准字H20000437 江苏恒瑞医药股份有限公司)6.盐酸拓扑替康(国药准字H20000439 重庆润康药业有限公司)制剂1.盐酸拓扑替康胶囊(国药准字H20070203南京瑞年百思特制药有限公司)2.注射用盐酸拓扑替康(国药准字H20060891 江苏奥赛康药业有限公司3.注射用盐酸托泊替康(国药准字H20052432 黄石飞云制药有限公司)4.注射用盐酸拓扑替康(国药准字H20000429 贵州汉方制药有限公司)5.注射用盐酸拓扑替康(国药准字H20000434 扬子江药业集团有限公司)6.注射用盐酸拓扑替康(国药准字H20000438 江苏恒瑞医药有限公司)进口1家1.注射用盐酸托泊替康(H20080385 SmithKline Beecham)B.正在申报盐酸拓扑替康 CYHS0900251川四川科瑞德凯华制药有限公司三、立题分析【英文名】:Topotecan Hydrochloride【化学名】:【分子式】:C23H23N3O5.HCl【分子量】:457.91【CAS号码】:123948-87-8【结构式】:盐酸拓扑替康是英国史克-比彻姆(SmithKline Beecham)公司研制开发的水溶性喜树碱衍生物。
Ligusticumwallichii

Alternative Medicine Review Monographs Page 249C o p y r i g h t © 2002 T h o r n e R e s e a r c h , I n c . A l l r i g h t s r e s e r v e d . A l t e r n a t i v e M e d i c i n e R e v i e w M o n o g r a p h sLigusticum wallichiiDescriptionA member of the Umbelliferae family, Ligusticum wallichii is used in Chinese medicine for a variety of hematological disorders, including ischemia and thrombosis. When combined with Astragalus, Ligusticum has demonstrated a notable immunopotentiating effect. Included in many classic Chinese formulations, it is also part of the Japanese and Korean herbal formu-laries. Classically, it is prescribed for headaches, abdominal pain, arthralgias, and menstrual disorders due to blood stasis.1 Ligusticum’s active ingredients include tetramethylpyrazine, ferulic acid, chrysophanol, sedanoic acid, and 1-2 percent essential oils.Clinical Indications IschemiaOne-hundred-and-fifty-eight subjects with transient ischemic attacks were randomly divided into a Ligusticum group (111 cases) and an aspirin group (47 cases). The total effec-tive rate in the Ligusticum group was 89.2 percent, compared to 61.7 percent in the aspirin group (P<0.01). Ligusticum increased cerebral blood flow, accelerated the velocity of blood flow, dilated the spastic artery, and decreased peripheral arterial resistance.2 In another study, Ligusticum was evaluated in the treatment of ischemic stroke. Injectable preparations were shown to improve brain microcirculation through inhibiting thrombus formation, decreasing platelet aggregation, and improving blood viscosity. The effect of Ligusticum was the same or better than controls using papaverine, dextran, and aspirin-persantin.3Antibacterial/AntifungalLigusticum has demonstrated in vitro antibacterial activity against several strains of pathogenic bacteria including Pseudomonas aeruginosa, Shigella sonnei, Salmonella typhi and Vibrio cholera , as well as many dermatomycoses .4InflammationWhen given to guinea pigs with histamine/acetylcholine-induced bronchospasm, Ligusti-cum decreased plasma levels of thromboxane B2, relaxed tracheal muscle, increased the forced expiratory volume, and inhibited synthesis and release of thromboxane A2, with no adverse side effects. The total effective rate was 92 percent, compared with 62 percent in the control group (p <0.01).5 In a Japanese study, the active ingredients in Ligusticum, tetramethylpyrazine and ferulic acid, were found to have both significant anti-inflammatory and analgesic effects.6Page 250 Alternative Medicine Review MonographsC o p y r i g h t © 2002 T h o r n e R e s e a r c h , I n c . A l l r i g h t s r e s e r v e d . A l t e r n a t i v e M e d i c i n e R e v i e w M o n o g r a p h sDosage and ToxicityLigusticum is prescribed in traditional Chinese decoctions at dosages up to 9 grams, administered over several days. Overdose symptoms may include vomiting and dizziness.1References1. Hong YH. Oriental Materia Medica: A Concise Guide. Long Beach, CA: Oriental Healing Arts Institute; 1986.2. Chen DR. Clinical and experimental study of Ligusticum wallichii and aspirin in the treatment of transient ischemic attack. Zhongguo Zhong Xi Yi Jie He Za Zhi 1992;12:672-674. [article in Chinese]3. Chen KJ, Chen K. Ischemic stroke treated with Ligusticum chuanxiong . Chin Med J (Engl) 1992;105:870-873.4. Bensky D, Gamble A. Chinese Herbal Medicine : Materia Medica, Revised Edition. Seattle, WA: Eastland Press; 1993.5. Shao CR, Chen FM, Tang YX. Clinical and experimental study on Ligusticum wallichi mixture in prevent-ing and treating bronchial asthma. Zhongguo Zhong Xi Yi Jie He Za Zhi 1994;14:465-468. [article in Chinese]6.Ozaki Y . Anti-inflammatory effect of tetramethylpyrazine and ferulic acid. Chem Pharm Bull (Tokyo) 1992;40:954-956.。
生物学变异系数

3.7
9.1
载脂蛋白A1
49
血清
Apolipoprotein B
9
6.9
22.8
3.5
6
11.6
载脂蛋白B
50
血浆
Arginine
1
19.3
34.1
9.7
9.8
25.7
精氨酸
51
血清
Arilestearase activity, non inhibited
1
3.8
37.2
1.9
9.3
12.5
Arilestearase活动,非抑制
13
12.3
23.1
6.15
6.54
16.69
天冬氨酸转氨酶(AST)
56
血浆
Aspartic acid
1
31.2
55.1
15.6
15.8
41.6
天冬氨酸
57
血清
β-2-Microglobulin
1
5.9
15.5
3
4.1
9
β-2-Microglobulin
58
血浆
β-Carotene
1
18
48
9
12.8
5.1
16.5
α生育酚
24
血清
Acid phosphatase
2
8.9
8
4.5
3
10.3
酸性磷酸酶
25
血清
Acid phosphatase tartrate-resistant (TR-ACP)
2
8
13.3
4
3.9
Vitamin B12 (cobalamin) deficiency in elderly patients

CMAJ • AUG. 3, 2004; 171 (3)251© 2004 Canadian Medical Association or its licensorsVitamin B 12(cobalamin) deficiency in elderly patientsEmmanuel Andrès, Noureddine H. Loukili, Esther Noel, Georges Kaltenbach, Maher Ben Abdelgheni, Anne E. Perrin, Marie Noblet-Dick, Frédéric Maloisel, Jean-Louis Schlienger, Jean-Frédéric BlickléD O I :10.1503/c m a j .1031155252JAMC • 3 AO ÛT 2004; 171 (3)Salivary R-proteinDietary cobalamin bound to animal proteinSalivary glandsDietaryintakeHCl PepsinR-protein from parietal cellsIntrinsic factorJejunumMethyl-cobalaminAdenosyl-cobalaminDistal 80 cm of ileumCobalamin transported via portal system bound to transcobalamin I, II and IIIIleal mucosal cellCubilinPancreatic proteaseGastric secretionCbl –R-protein complexes secreted in bile (5–10 µg/d)Gall bladderDietary deficiencyNitrous oxide*Achlorhydriaresulting in inability to sever the animal protein from the cobalaminLack of IF with totalgastrectomy or pernicious anemia (idiopathic atrophy of gastric mucosa inassociation with antibodies to parietal cells and IF)Exocrine failure leads to cobalaminmalabsorption (inability to degrade Cbl –R-protein complexes)Genetic disorders involving plasma transportResection or disease of the distal 80 cm of the ileum Genetic disorders involving conversion to coenzyme formsHigh concentration of bacteria and certain parasites in small intestine can absorb cobalamin123456C h r i s t i n e K e n n e yselected by the authors. Using the definitions shown in Box 1, we found a prevalence of greater than 4.8% in a large group of patients in hospital between the ages of 65 and 98(data submitted to the 47th Congress of the French Na-tional Society for Internal Medicine in Bordeaux, June 11–13, 1998).Cobalamin metabolism and functionCobalamin metabolism is complex and requires many processes, any one of which, if not present, may lead to cobalamin deficiency.4,13–15The causes of cobalamin defi-ciency are shown in Fig.1and listed in Table 1.Once metabolized, cobalamin is a cofactor and co-enzyme in many biochemical reactions, including DNA synthesis, methionine synthesis from homocysteine and conversion of propionyl into succinyl coenzyme A from methylmalonate, as shown in Fig. 2.A typical Western diet contributes 3–30 µg of cobalamin per day toward the estimated daily requirement of 2–5 µg that is recommended by the American Society of Geri-atry,16the US Food and Drug Administration and the As-sociation Française de Sécurité Sanitaire des Aliments.Reserves, which are primarily hepatic, are significant (>1.5mg). The 5- to 10-year delay between the onset of in-sufficient intake and the development of clinical illness is a direct result of the hepatic stores and the enterohepatic cy-cle, whereby cobalamin is excreted in bile and then reab-sorbed in the small intestine (see Fig.1).4,13Between 1%and 5% of free cobalamin (and crystalline cobalamin) is absorbed along the entire intestine by passive diffusion,which explains the mechanism underlying oral treatment of deficiencies associated with pernicious anemia and food-cobalamin malabsorption.4,17,18In a clinical setting, cobalamin absorption is examined (imperfectly) by the Schilling test.4,8 The test is currently performed as follows: patients are given 1000 µg of cyano-cobalamin intramuscularly at day 1 to saturate the intestinal mucosal cells, followed by 1000 µg of free 58Cocyanocobal-amin orally on day 2. Excess cobalamin, which is not ab-sorbed, is excreted, and the patient’s urine is collected for 24 hours (from day 2 to day 3) and the percentage of la-belled cyanocobalamin is determined. Abnormally low lev-els of cobalamin in the collected urine indicate cases of malabsorption or pernicious anemia; normal levels indicate dietary deficiency, food-cobalamin malabsorption or hereditary cobalamin metabolism deficiencies.Causes of cobalamin deficiencyIn elderly patients, cobalamin deficiency is caused pri-marily by food-cobalamin malabsorption and pernicious anemia. Cobalamin deficiency caused by dietary deficiency or malabsorption (Fig. 1, Table 1) is rarer.1,8,11In our stud-ies, in which we followed a total of more than 200 patients with a proven cobalamin deficiency, food-cobalamin mal-CMAJ • AUG. 3, 2004; 171 (3)253Table 1: Stages of cobalamin metabolism and correspondingcauses of cobalamin deficiency13,15Stage of cobalamin metabolism Cause of cobalamin deficiency Intake solely through foodStrict vegetarianism without vitamin supplementationDigestion brings into play haptocorrin, gastric secretions (HCl and pepsin), intrinsic factor,pancreatic and biliary secretions,enterohepatic cycle Gastrectomy; pernicious anemia (Biermer ’s disease);* food-cobalamin malabsorption*Absorption brings into play intrinsic factor and cubilin Ileal resection; malabsorption;pernicious anemia;* Imerslund syndrome †Transport by transcobalaminsCongenital deficiency in transcobalamin II †Intracellular metabolism based on various intracellular enzymes Congenital deficiency in various intracellular enzymes †Note: HCl = hydrochloric acid.*Very frequent cause among elderly people.†Rare cause among adults, even more so among elderly patients.Fig. 1: Cobalamin metabolism and corresponding causes of deficiency. Causes of cobalamin deficiency are shown in blue.The metabolic pathway starts when (1) dietary cobalamin (Cbl),obtained through animal foods, enters the stomach bound to animal proteins (P). (2) Pepsin and hydrochloric acid (HCl) in the stomach sever the animal protein, releasing free cobalamin.Most of the free cobalamin is then bound to R-protein (R),which is released from the parietal and salivary cells. Intrinsic factor (IF) is also secreted in the stomach, but its binding to cobalamin is weak in the presence of gastric and salivary R-protein. (3) In the duodenum, dietary cobalamin bound to R-protein is joined by cobalamin –R-protein complexes that have been secreted in the bile. Pancreatic enzymes degrade both biliary and dietary cobalamin –R-protein complexes, releasing free cobalamin. (4) The cobalamin then binds with intrinsic factor. The cobalamin –intrinsic factor complex remains undis-turbed until the distal 80 cm of the ileum, where (5) it attaches to mucosal cell receptors (cubilin) and the cobalamin is bound to transport proteins known as transcobalamin I, II and III (TCI,TCII and TCIII). Transcobalamin II, although it represents only a small fraction (about 10%) of the transcobalamins, is the most important because it is able to deliver cobalamin to all cells in the body. The cobalamin is subsequently transported systemi-cally via the portal system. (6) Within each cell, the transcobal-amin II –cobalamin complex is taken up by means of endocyto-sis and the cobalamin is liberated and then converted enzymatically into its 2 coenzyme forms, methylcobalamin and adenosylcobalamin (this process is shown in greater detail in Fig. 2).*Nitrous oxide, a general anesthetic, causes multiple defects in cobalamin use, most of which are intracellular and clinically relevant only in people who have low or borderline-low serum cobalamin levels.254JAMC • 3 AO ÛT 2004; 171 (3)Methionine S-Adenosyl methionineDimethyl-glycine BetaineS-Adenosyl homocysteineHomocysteine5-Methyl 5,10-Methylene tetrahydrofolateMethyltetrahydrofolatereductase RiboflavinTetrahydrofolateNucleic acidsynthesisMethionine synthase Cob(I)alaminBHMTPlasma membraneCbl III ITCIIT II CReductaseMitochondria drM et MetMethionineMeCblH 4PteGlu 5-CH H 4PteGluHomocysteineMethionine synthaseoAabsorption accounted for about 60%–70% of the cases among elderly patients, and pernicious anemia accounted for 15%–20% of the cases.14,19,20,21Other causes included di-etary deficiency (< 5%), malabsorption (< 5%) and heredi-tary cobalamin metabolism diseases (< 1%).Food-cobalamin malabsorptionFirst described by Carmel in 1995,22food-cobalamin mal-absorption syndrome is characterized by the inability to release cobalamin from food or from intestinal transport pro-teins (Table 1), particularly in the presence of hypo-chlorhydria, where the absorption of “unbound” cobalamin remains normal. As various studies have shown,14,22,23this syn-drome is defined by cobalamin deficiency in the presence of sufficient food-cobalamin intake and a negative Schilling test, where the latter rules out malabsorption or pernicious anemia (Box 2). In theory — because the test is rarely practical in clinical settings —the indisputable evidence of food-cobal-amin malabsorption comes from using a modified Schilling test, which uses radioactive cobalamin bound to animal pro-teins (e.g., salmon, trout) and reveals malabsorption when the results of a standard Schilling test are normal.4,22Food-cobalamin malabsorption is caused primarily by gastric atrophy. Over 40% of patients older than 80 years have gastric atrophy that may or may not be related to He-licobacter pylori infection.11,24Other factors that contribute to food-cobalamin malabsorption in elderly people include chronic carriage of H. pylori and intestinal microbial prolif-eration (which can be caused by antibiotic treatment);25 long-term ingestion of biguanides (metformin)26,27and antacids, including H2-receptor antagonists and proton pump inhibitors28,29(particularly among patients with Zollinger–Ellison syndrome30);chronic alcoholism; surgery or gastric reconstruction (e.g., bypass surgery for obesity); partial pancreatic exocrine failure;4,14and Sjögren’s syn-drome31(Box 2).Pernicious anemiaPernicious anemia, or Biermer’s disease, is a classic cause of cobalamin deficiency and one of the most frequent among elderly patients: 20%–50% of cases according to 2 studies32,33and more than 15% in our patient series.14,19,20,21 Pernicious anemia is an autoimmune disease characterized by the destruction of the gastric mucosa, especially fundal mucosa, by a primarily cell-mediated process.34Gastric se-cretions are neutral to slightly acidic even in the presence of gastrin (which normally increases acidity) and contain little or no intrinsic factor.13,32,34The disease is also charac-terized by the presence of 2 antibodies, particularly in plas-ma and gastric secretions: few people who do not have the disease have antibodies (specificity 98%). However, only about 50% of patients with pernicious anemia will have anti-intrinsic factor antibodies (sensitivity 50%). Anti-gastric parietal cell antibodies, which target the H+/K+adenosine triphosphatase alpha and beta subunits, can also be measured in the serum; sensitivity is higher (> 90%), but specificity is much lower (50%).32,34Moderate hypergas-trinemia, and sometimes major hypergastrinemia (levels of up to 4 to 8 times above normal), has also been associated with pernicious anemia. Owing to gastric atrophy with hypochlorhydria, patients have a feedback hypergastrine-mia with hyperplasia of antral G cells. Hypergastrinemia is suggestive but not pathognomonic of pernicious anemia (sensitivity > 80%, specificity < 50%).32,33A positive Schilling test (with the addition of a test for anti-intrinsic factor antibodies) virtually confirms the diagnosis (speci-ficity > 99%).21,32From a clinical perspective, pernicious anemia is asso-ciated with many autoimmune disorders, including vi-tiligo, dysthyroidia, Addison’s disease and Sjögren’s syn-drome.21,32It is also associated with an increased frequency of gastric neoplasms: adenocarcinomas, lymphomas and carcinoid tumours.32,33Most experts recommend that pa-tients with pernicious anemia undergo endoscopic surveil-lance every 3 to 5 years with multiple biopsies, even in the absence of macroscopic lesions.21This practice has re-cently revealed the near absence of mucosal H. pylori in patients with the disease.21CMAJ • AUG. 3, 2004; 171 (3)255 Box 2: Indications of food-cobalaminmalabsorption syndrome*14,19•Serum cobalamin level < 150 pmol/L•Result of standard Schilling test (with freecyanocobalamin marked with cobalt-58) is normal,or result of modified Schilling test (using radioactivecobalamin bound to food protein) is abnormal†•No dietary cobalamin deficiency (intake > 2 µg perday)•Existence of a predisposing factor in cobalamindeficiency:-Atrophic gastritis, chronic Helicobacter pyloriinfection-Microbial proliferation, AIDS-Long-term ingestion of antacids (H2-receptorantagonists or proton pump inhibitors) orbiguanides-Chronic alcoholism-Gastrectomy, gastric bypass surgery-Pancreatic exocrine failure-Idiopathic (age-related)*The first 3 items are required for a diagnosis of food-cobalaminmalabsorption.†The modified Schilling test uses cobalamin bound to egg, chicken orfish proteins.4Dietary deficiencyIntake or nutritional deficiency of cobalamin is rare among healthy adults in industrialized countries, even among elderly people: less than 5% in our experience.14It is limited to rare instances of patients on strict vegetarian diets and people who are already malnourished, such as elderly patients, patients in institutions or patients in psychiatric hospitals.16,35Studies of the dietary intake of elderly peoplein the United States have shown that up to 50% may have an insufficient intake of cobalamin (< 2 µg/d).16Such studies,however, are extremely difficult to conduct because they rely mainly on dietary histories.8Moreover, even if present,dietary deficiency does not result in symptomatic cobalamin deficiency until hepatic reserves are exhausted.Cobalamin malabsorptionGastrectomy and surgical resection of the terminal small intestine have been the most common causes of cobalamin malabsorption in elderly people.4,9However, as we have shown, these causes have become rare (< 5%),14owing mainly to the decreasing frequency of the operations. Total gastrectomies and most partial gastrectomies eliminate both the only source of intrinsic factor and, especially, gas-tric acidity. In the absence of gastric acidity, cobalamin malabsorption is associated with intraluminal microbial proliferation (or “blind loop syndrome”)13,14and can be cor-rected with antibiotics.25Other causes of cobalamin malabsorption that are rarely encountered in elderly people (< 2% in our practice)14in-clude disorders that result in damage to the last 80 cm of the small intestine mucosa, which is the site of elective cobal-amin absorption: Crohn’s disease, lymphomas, tuberculosis,amyloidosis, scleroderma, Whipple’s disease, and even celiac disease,4,7or ingestion of colchicine or cholestyra-mine.7,36Agammaglobulinemia, AIDS (because of the associ-ated microbial proliferation) and Diphyllobothrium infections may also cause cobalamin deficiency in elderly people.4Even rarer, as we have reported,14is deficiency in the ex-ocrine function of the pancreas following chronic pancre-atitis (which is usually caused by alcoholism) or a pancrea-tectomy.11,19,36Hereditary cobalamin metabolism diseasesThese hereditary diseases may cause deficiency in cu-bilin (as with Imerslund syndrome) or transcobalamin II and, more rarely, deficiency in intracellular enzymes that are involved in the signal transduction chain in cells, for ex-ample in methylating pathways.4,9These deficiencies appear in newborns and therefore do not involve elderly patients.Clinical manifestationsThe primary clinical manifestations of cobalamin defi-ciency are described in Table 2.They are highly polymor-phic and of varying severity, ranging from milder conditions,such as the common sensory neuropathy and isolated anom-alies of macrocytosis and hypersegmentation of neutrophils,to severe disorders, including combined sclerosis of the spinal cord, hemolytic anemia and even pancytopenia.2,4,14,37Among the classic manifestations are Hunter’s glossitis,which causes the lingual papillae to atrophy, making the tongue look smooth and shiny, and neuroanemic syndrome.256JAMC • 3 AO ÛT 2004; 171 (3)Table 2: Major clinical manifestations of cobalamindeficiency2,4,14,15,32,36,37System ManifestationComment Macrocytosis; hypersegmentation of the neutrophils; aregenerative macrocytary anemia; medullary megaloblastosis (“blue spinal cord ”)FrequentIsolated thrombocytopenia and neutropenia; pancytopenia Rare HematologicalHemolytic anemia; thrombotic microangiopathy (presence of schistocytes)Very rareCombined sclerosis of the spinalcordClassic Polyneurites (especially sensitive ones); ataxia; Babinski ’s phenomenonFrequentCerebellar syndromes affecting the cranial nerves, including optic neuritis, optic atrophy, urinary or fecal incontinenceRare Neuropsychiatric Changes in the higher functions,even dementia, stroke and atherosclerosis(hyperhomocysteinemia);Parkinsonian syndromes;depressionUnder studyHunter ’s glossitis; jaundice; lactate dehydrogenase and bilirubin elevation (“intramedullary destruction ”)Classic Resistant and recurring mucocutaneous ulcers Rare DigestiveAbdominal pain; dyspepsia;nausea; vomiting; diarrhea;disturbances in intestinal functioningDebatableGynecologicalAtrophy of the vaginal mucosa and chronic vaginal and urinary infections (especially mycosis);hypofertility and repeated miscarriagesUnder study OtherVenous thromboembolic disease;angina (hyperhomocysteinemia)Under studyCMAJ • AUG. 3, 2004; 171 (3)257similar results.18,39At this time a causal role of cobalamin in these conditions remains speculative.Cobalamin deficiency can go undetected for several years, during which time the neuropsychiatric manifesta-tions may become irreversible (and worsen with folate sup-plementation).2,4,15,32,36Diagnostic processThe diagnostic process for cobalamin deficiency is de-tailed in Fig. 3. This process is intended to avoid invasive, systematic or unnecessary explorations, such as a myelo-gram, which in occasional cases is the only way to rule out hemopathies. In the presence of megaloblastosis on the blood smear and equivocal cobalamin serum levels, the di-agnosis can be securely made only by showing degenera-tion of the spinal cord.14All people over 65 years of age who are malnourished, all people in institutions or psychiatric hospitals, and all people with hematological or neuropsychiatric manifesta-tions of cobalamin deficiency as reported in Table 2should have their serum cobalamin levels measured. Therapeutic managementThe classic treatment of cobalamin deficiency, particu-larly when the cause is not dietary deficiency, has been par-enteral administration — usually by intramuscular injection — of the vitamin (in the form of cyanocobalamin and, more rarely, hydroxocobalamin).1,17,18,32In France, the rec-ommended practice to build up the tissue stores of the vita-min quickly and correct serum cobalamin hypovitaminosis, particularly in the case of pernicious anemia, involves ad-ministration of 1000 µg of cobalamin per day for 1week, followed by 1000 µg per week for 1month and then by 1injection of the same dose once per month, normally for the rest of the patient’s life (Table 3).32,33In other Western countries, dosages of 100–1000 µg per day are used.17 In cases other than those caused by nutritional defi-ciency, alternative routes of administration have recently been proposed: oral17,18,39–42or nasal.43,44Our working group has developed an effective oral treatment of food-cobalamin malabsorption40,41and pernicious anemia45using crystalline cobalamin (cyanocobalamin). However, until the procedure is validated, the following may be proposed: on-going supplementation until associated disorders are cor-rected (e.g., by halting the ingestion of the offending med-ication or exogenosis, or by treating H. pylori infection or pancreatic exocrine failure), lifelong administration or, where applicable, sequential administration as shown in Table 3.46References1.Matthews JH. Cobalamin and folate deficiency in the elderly. Baillères ClinHaematol1995;54:245-53.2.Stabler SP, Allen RH, Savage DG, Lindenbaum J. Clinical spectrum and di-agnosis of cobalamin deficiency. Blood1990;76:871-81.3.Reynolds EH. Neurological aspects of folate and vitamin B12metabolism. Clin Haematol1976;5:661-96.4.Carmel R. Current concepts in cobalamin deficiency. Annu Rev Med2000;51:357-75.5.Snow C. Laboratory Diagnosis of vitamin B12and folate deficiency. A guide for the primary care physician. Arch Intern Med1999;159:1289-98.6.Zittoun J, Zittoun R. Modern clinical testing strategies in cobalamin and fo-late deficiency. Semin Hematol1999;36:35-46.7.Klee GG. Cobalamin and folate evaluation: measurements of methylmalonicacid and homocystein vs vitamin B12and folate. Clin Chem2000;46:1277-83. 8.Andrès E, Perrin AE, Kraemer JP, Goichot B, Demangeat C, Ruellan A, et al.Anémies par carence en vitamine B12chez le sujet âgé de plus de 75 ans: nou-veaux concepts. À propos de 20 observations. Rev Med Interne2000;21:946-55.9.Markle HV. Cobalamin. Crit Rev Clin Lab Sci1996;33:247-356.10.Lindenbaum J, Rosenberg IH, Wilson PWF, Stabler SP, Allen RH. Preva-lence of cobalamin deficiency in the Framingham elderly population. Am J Clin Nutr1994;60:2-11.11.Pautas E, Chérin P, De Jaeger C, Godeau P. Carence en vitamine B12chez le sujet âgé. Presse Med1999;28:1767-70.12.Van Asselt DZ, Blom HJ, Zuiderent R, Wevers RA, Jakobs C, van den BroekWJ, et al. Clinical significance of low cobalamin levels in older hospital pa-tients. Neth J Med2000;57:41-9.13.Nicolas JP, Guéant JL. Absorption, distribution et excrétion de la vitamineB12. Ann Gastroenterol Hepatol (Paris)1994;30:270-6,281-2.14.Andrès E, Perrin AE, Demangeat C, Kurtz JE, Vinzio S, Grunenberger F, etal. The syndrome of food-cobalamin malabsorption revisited in a department of internal medicine. A monocentric cohort study of 80 patients. Eur J Intern Med2003;14:221-6.15.Swain R. An update of vitamin B12metabolism and deficiency states. J Fam Pract1995;41:595-600.16.Russel RM. Vitamin requirements in old age. Age Nutrition1992;3:20-3.17.Kuzminski AM, Del Giacco EJ, Allen RH, Stabler SP, Lindenbaum J. Effectivetreatment of cobalamin deficiency with oral cobalamin. Blood1998;92:1191-8. ne LA, Rojas-Fernandez C. Treatment of vitamin B12deficiency anemia: oral versus parenteral therapy. Ann Pharmacother2002;36:1268-72.19.Andrès E, Goichot B, Schlienger JL. Food-cobalamin malabsorption: a usualcause of vitamin B12deficiency. Arch Intern Med2000;160:2061-2.20.Andrès E, Kaltenbach G, Perrin AE, Kurtz JE, Schlienger JL. Food-cobal-amin malabsorption in the elderly. Am J Med2002;113:351-2.258JAMC • 3 AOÛT 2004; 171 (3)This article has been peer reviewed.Competing interests:None declared.Contributors:Emmanuel Andrès and Noureddine H. Loukili contributed to the conception and design of the study, collection, assembly of data and Internet search, interpretation and analysis of data and drafting and revisions of the article. Emmanuel Andrès and Anne E. Perrin conducted the statistical analysis of the studies we have conducted. All authors gave final approval of the version submitted to be published.From the Departments of Internal Medicine and of Diabetes and Metabolic Dis-eases, Medical Clinic B (Andrès, Loukili, Noel, Ben Abdelgheni, Blicklé), Internal Medicine and Geriatrics (Kaltenbach, Noblet-Dick),Internal Medicine and Nutri-tion (Perrin, Schlienger), and the Oncology and Hematology Department (Mal-oisel), Strasbourg University Hospitals, Strasbourg, France.Table 3: Therapeutic management of cobalamin deficiency using cobalamin treatmentRoute ofadministration Initial treatment Maintenance treatmentParenteral*1000 µg/d for 1 wk,then 1000 µg/wk for1 mo 1000 µg/mo until the cause of deficiency is corrected, or for life in the case of pernicious anemiaOral†1000 µg/d for 1 mo125–500 µg/d for intakedeficiency and food-cobalamin malabsorption;1000 µg/d for perniciousanemia*Regardless of the cause of the vitamin deficiency.†For intake deficiency, food-cobalamin malabsorption and pernicious anemia.CMAJ s E d i t o r i a l F e l l o w s h i p“You will be exposed to aspects of medicine that you never hadoccasion to ponder before. And, perhaps, you will betterunderstand why we physicians do what we do.”CMAJ • AUG. 3, 2004; 171 (3)。
OECD Test No. 460 Fluorescein Leakage Test Method for Identifying Ocular Corrosives and Severe Irrit

OECD/OCDE460Adopted: 2 October 2012© OECD, (2012)You are free to use this material for personal, non-commercial purposes without seeking prior consent from the OECD, provided the source is duly mentioned. Any commercial use of this material is subject to written permission from the OECD.OECD GUIDELINE FOR THE TESTING OF CHEMICALS Fluorescein Leakage Test Method for Identifying Ocular Corrosives and Severe IrritantsINTRODUCTION1. The Fluorescein Leakage (FL) test method is an in vitro test method that can be used under certain circumstances and with specific limitations to classify chemicals (substances and mixtures) as ocular corrosives and severe irritants, as defined by the United Nations (UN) Globally Harmonized System of Classification and Labelling of Chemicals (GHS) (Category 1), the European Union (EU) Regulation on Classification, Labelling and Packaging of Substances and Mixtures (CLP) (Category 1), and the U.S. Environmental Protection Agency (EPA) (Category I) (1) (2) (3). For the purpose of this Test Guideline, severe irritants are defined as chemicals that cause tissue damage in the eye following test substance administration that is not reversible within 21 days or causes serious physical decay of vision, while ocular corrosives are chemicals that cause irreversible tissue damage to the eye. These chemicals are classified as UN GHS Category 1, EU CLP Category 1, or U.S. EPA Category I.2. While the FL test method is not considered valid as a complete replacement for the in vivo rabbit eye test, the FL is recommended for use as part of a tiered testing strategy for regulatory classification and labelling. Thus, the FL is recommended as an initial step within a Top-Down approach to identify ocular corrosives/severe irritants, specifically for limited types of chemicals (i.e. water soluble substances and mixtures) (4)(5).3. It is currently generally accepted that, in the foreseeable future, no single in vitro eye irritation test will be able to replace the in vivo eye test (TG 405 (6)) to predict across the full range of irritation for different chemical classes. However, strategic combinations of several alternative test methods within a (tiered) testing strategy may be able to replace the in vivo eye test (5). The Top-Down approach (5) is designed to be used when, based on existing information, a chemical is expected to have high irritancy potential.Based on the prediction model detailed in paragraph 35, the FL test method can identify Category 1; EU CLP Category 1; U.S. EPA Category I) without any further testing. The same is assumed for mixtures although mixtures were not used in the validation. Therefore, the FL test method may be used to determine the eye irritancy/corrosivity of chemicals, following the460OECD/OCDEsequential testing strategy of TG 405 (6). However, a chemical that is not predicted as ocular corrosive or severe irritant with the FL test method would need to be tested in one or more additional test methods (in vitro and/or in vivo) that are capable of accurately identifying i) chemicals that are in vitro false negative ocular corrosives/severe irritants in the FL (UN GHS Category 1; EU CLP Category 1; U.S. EPA Category I); ii) chemicals that are not classified for eye corrosion/irritation (UN GHS No Category; EU CLP No Category; U.S. EPA Category IV); and/or iii) chemicals that are moderate/mild eye irritants (UN GHS Categories 2A and 2B; EU CLP Category 2; U.S. EPA Categories II and III).5. The purpose of this Test Guideline is to describe the procedures used to evaluate the potential ocular corrosivity or severe irritancy of a test substance as measured by its ability to induce damage to an impermeable confluent epithelial monolayer. The integrity of trans-epithelial permeability is a major function of an epithelium such as that found in the conjunctiva and the cornea. Trans-epithelial permeability is controlled by various tight junctions. Increasing the permeability of the corneal epithelium in vivo has been shown to correlate with the level of inflammation and surface damage observed as eye irritation develops.6. In the FL test method, toxic effects after a short exposure time to the test substance are measured by an increase in permeability of sodium fluorescein through the epithelial monolayer of Madin-Darby Canine Kidney (MDCK) cells cultured on permeable inserts. The amount of fluorescein leakage that occurs is proportional to the chemical-induced damage to the tight junctions, desmosomal junctions and cell membranes, and can be used to estimate the ocular toxicity potential of a test substance. Annex I provides a diagram of MDCK cells grown on an insert membrane for the FL test method.7. Definitions are provided in Annex II.INITIAL CONSIDERATIONS AND LIMITATIONS8. This Test Guideline is based on the INVITTOX protocol No. 71 (7) that has been evaluated in an international validation study by the European Centre for the Validation of Alternative Methods (ECVAM) (8), in collaboration with the US Interagency Coordinating Committee on the Validation of Alternative Methods (ICCVAM) and the Japanese Center for the Validation of Alternative Methods (JaCVAM).9. The FL test method is not recommended for the identification of chemicals which should be classified as mild/moderate irritants or of chemicals which should not be classified for ocular irritation (substances and mixtures) (i.e. GHS Cat. 2A/2B, no category; EU CLP Cat. 2, no category; US EPA Cat. II/III/IV), as demonstrated by the validation study (4) (8).10. The test method is only applicable to water soluble chemicals (substances and mixtures). The ocular severe irritation potential of chemicals that are water soluble and/or where the toxic effect is not affected by dilution is generally predicted accurately using the FL test method (8). To categorise a chemical as water soluble, under experimental conditions, it should be soluble in sterile calcium-containing (at a concentration of 1.0-1.8 mM), phenol red-free, Hanks’ Buffered Salt Solution (HBSS) at a concentration ≥ 250 mg/mL (one dose above the cut-off of 100 mg/mL). However, if the test substance is soluble below the concentration 100 mg/mL,2© OECD, (2012)OECD/OCDE 460 but already induces a FL induction of 20 % at that concentration (meaning FL20 < 100 mg/mL), it can still be classified as GHS Cat. 1 or EPA Cat. 1.11. The identified limitations for this test method exclude strong acids and bases, cell fixatives and highly volatile chemicals from the applicability domain. These chemicals have mechanisms that are not measured by the FL test method, e.g. extensive coagulation, saponification or specific reactive chemistries. Other identified limitations for this method are based upon the results for the predictive capacity for coloured and viscous test substance (8). It is suggested that both types of chemicals are difficult to remove from the monolayer following the short exposure period and that predictivity of the test method could be improved if a higher number of washing steps was used. Solid chemicals suspended in liquid have the propensity to precipitate out and the final concentration to cells can be difficult to determine. When substances within these chemical and physical classes are excluded from the database, the accuracy of FL across the EU, EPA, and GHS classification systems is substantially improved (8).12. Based on the purpose of this test method (i.e. to identify ocular corrosives/severe irritants only), false negative rates (see Paragraph 13) are not critical since such substances would be subsequently tested with other adequately validated in vitro tests or in rabbits, depending on regulatory requirements, using a sequential testing strategy in a weight of evidence approach (6) (see also paragraphs 3 and 4).13. Other identified limitations of the FL test method are based on false negative and false positive rates. When used as an initial step within a Top-Down approach to identify water soluble ocular corrosive/severe irritant substances and mixtures (UN GHS Category 1; EU CLP Category 1; U.S. EPA Category I), the false positive rate for the FL test method ranged from 7% (7/103; UN GHS and EU CLP) to 9% (9/99; U.S. EPA) and the false negative rate ranged from 54% (15/28; U.S. EPA) to 56% (27/48; UN GHS and EU CLP) when compared to in vivo results. Chemical groups showing false positive and/or false negative results in the FL test method are not defined here.14. Certain technical limitations are specific to the MDCK cell culture. The tight junctions that block the passage of the sodium-fluorescein dye through the monolayer are increasingly compromised with increasing cell passage number. Incomplete formation of the tight junctions results in increased FL in the non-treated control. Therefore, a defined permissible maximal leakage in the non-treated controls is important (see paragraph 38: 0% leakage). As with all in vitro assays there is the potential for the cells to become transformed over time, thus it is vital that passage number ranges for the assays are stated.15. The current applicability domain might be increased in some cases, but only after analyzing an expanded data set of studied test substances, preferably acquired through testing (4). This Test Guideline will be updated accordingly as new information and data are considered.16. For any laboratory initially establishing this assay, the proficiency chemicals provided in Annex III should be used. Laboratories can use these chemicals to demonstrate their technical competence in performing the FL test method prior to submitting FL assay data for regulatory hazard classification purposes.PRINCIPLE OF THE TEST3© OECD, (2012)460OECD/OCDE17. The FL test method is a cytotoxicity and cell-function based in vitro assay that is performed on a confluent monolayer of MDCK CB997 tubular epithelial cells that are grown on semi-permeable inserts and model the non-proliferating state of the in vivo corneal epithelium. The MDCK cell line is well established and forms tight junctions and desmosomal junctions similar to those found on the apical side of conjunctival and corneal epithelia. Tight and desmosomal junctions in vivo prevent solutes and foreign materials penetrating the corneal epithelium. Loss of trans-epithelial impermeability, due to damaged tight junctions and desmosomal junctions, is one of the early events in chemical-induced ocular irritation.18. The test substance is applied to the confluent layer of cells grown on the apical side of the insert. A short 1 min exposure is routinely used to reflect the normal clearance rate in human exposures. An advantage of the short exposure period is that water-based substances and mixtures can be tested neat, if they can be easily removed after the exposure period. This allows more direct comparisons of the results with the chemical effects in humans. The test substance is then removed and the non-toxic, highly fluorescent sodium-fluorescein dye is added to the apical side of the monolayer for 30 minutes. The damage caused by the test substance to the tight junctions is determined by the amount of fluorescein which leaks through the cell layer within a defined period of time.19. The amount of sodium-fluorescein dye that passes through the monolayer and the insert membrane into a set volume of solution present in the well (to which the sodium-fluorescein dye leaks in) is determined by measuring spectrofluorometrically the fluorescein concentration in the well. The amount of fluorescein leakage (FL) is calculated with reference to fluoresence intensity (FI) readings from two controls: a blank control, and a maximum leakage control. The percentage of leakage and therefore amount of damage to the tight junctions is expressed, relative to these controls, for each of the set concentrations of the test substance. Then the FL20 (i.e. concentration that causes 20% FL relative to the value recorded for the untreated confluent monolayer and inserts without cells), is calculated. The FL20 (mg/mL) value is used in the prediction model for identification of ocular corrosives and severe irritants (see paragraph 35).20. Recovery is an important part of a test substance’s toxicity profile that is also assessed by the in vivo ocular irritation test. Preliminary analyses indicated that recovery data (up to 72 h following the chemical exposure) could potentially increase the predictive capacity of INVITTOX Protocol 71 but further evaluation is needed and would benefit from additional data, preferably acquired by further testing (7). This Test Guideline will be updated accordingly as new information and data are considered.PROCEDUREPreparation of the cellular monolayer21. The monolayer of MDCK CB997 cells is prepared using sub-confluent cells growing in cell culture flasks in DMEM/Nutrient Mix F12 (1x concentrate with L-glutamine, 15 mM HEPES, calcium (at a concentration of 1.0-1.8 mM) and 10% heat-inactivated FCS/FBS). Importantly, all media/solutions used throughout the FL assay should contain calcium at a concentration between 1.8 mM (200 mg/L) and 1.0 mM (111 mg/L) to ensure tight junction formation and integrity. Cell passage number range should be controlled to ensure even and4© OECD, (2012)OECD/OCDE 460 reproducible tight junctions formation. Preferably, the cells should be within the passage range 3-30 from thawing because cells within this passage range have similar functionality, which aids assay results to be reproducible.22. Prior to performing the FL test method, the cells are detached from the flask by trypsinisation, centrifuged and an appropriate amount of cells is seeded into the inserts placed in 24-well plates (see Annex I). Twelve mm diameter inserts with membrane of mixed cellulose esters, a thickness of 80-150 µm and a pore size of 0.45 µm, should be used to seed the cells. In the validation study, Millicell-HA 12 mm inserts were used. The properties of the insert and membrane type are important as these may affect cell growth and chemical binding. Certain types of chemicals may bind to the Millicell-HA insert membrane, which could affect the interpretation of results. Proficiency chemicals (see Annex III) should be used to demonstrate equivalency if other membranes are used.23. Chemical binding to the insert membrane is more common for cationic chemicals, such as benzalkonium chloride, which are attracted to the positively charged membrane (8). Chemical binding to the insert membrane may increase the chemical exposure period, leading to an over-estimation of the toxic potential of the chemical, but can also physically reduce the leakage of fluorescein through the insert by binding of the dye to the cationic chemical bound to the insert membrane, leading to an under-estimation of the toxic potential of the chemical. This can be readily monitored by exposing the membrane alone to the top concentration of the chemical tested and then adding sodium-fluorescein dye at the normal concentration for the standard time (no cell control). If binding of the sodium-fluorescein dye occurs, the insert membrane appears yellow after the test material has been washed-off. Thus, it is essential to know the binding properties of the test substance in order to be able to interpret the effect of the chemical on the cells.24. Cell seeding on inserts should produce a confluent monolayer at the time of chemical exposure. 1.6 x 105 cells should be added per insert (400 µL of a cell suspension with a density of 4 x 105 cells / mL). Under these conditions, a confluent monolayer is usually obtained after 96 hours in culture. Inserts should be examined visually prior to seeding, so as to ensure that any damages recorded at the visual control described at paragraph 30 is due to handling.25. The MDCK cell cultures should be kept in incubators in a humidified atmosphere, at 5% ± 1% CO2and 37 ± 1 ºC. The cells should be free of contamination by bacteria, viruses, mycoplasma and fungi.Application of the Test and Control Chemicals26. A fresh stock solution of test substance should be prepared for each experimental run and used within 30 minutes of preparation. Test substances should be prepared in calcium-containing (at a concentration of 1.0-1.8 mM), phenol red-free, HBSS to avoid serum protein binding. Solubility of the chemical at 250 mg/mL in HBSS should be assessed prior to testing. If at this concentration the chemical forms a stable suspension or emulsion (i.e.maintains uniformity and does not settle or separate into more than one phase) over 30 minutes, HBSS can still be used as solvent. However, if the chemical is found to be insoluble in HBSS at this concentration, the use of other test methods instead of FL should be considered. The use of light mineral oil as a solvent, in cases where the chemical is found to be insoluble in HBSS, should be5© OECD, (2012)460OECD/OCDEconsidered with caution as there is not enough data available to conclude on the performance of the FL assay under such conditions.27. All chemicals to be tested are prepared in sterile calcium-containing (at a concentration of 1.0-1.8 mM), phenol red-free, HBSS from the stock solution, at five fixed concentrations diluted on a weight per volume basis: 1, 25, 100, 250 mg/mL and a neat or a saturated solution. When testing a solid chemical, a very high concentration of 750 mg/mL should be included. This concentration of chemical may have to be applied on the cells using a positive displacement pipette. If the toxicity is found to be between 25 and 100 mg/mL, the following additional concentrations should be tested twice: 1, 25, 50, 75, 100 mg/mL. The FL20value should be derived from these concentrations provided the acceptance criteria were met.28. The test substances are applied to the confluent cell monolayers after removal of the cell culture medium and washing twice with sterile, warm (37ºC), calcium-containing (at a concentration of 1.0-1.8 mM), phenol red-free, HBSS. Previously, the filters have been visually checked for any pre-existing damages that could be falsely attributed to potential incompatibilities with test chemicals. At least three replicates should be used for each concentration of the test substance and for the controls in each run. After 1 min of exposure at room temperature, the test substance should be carefully removed by aspiration, the monolayer should be washed twice with sterile, warm (37ºC), calcium-containing (at a concentration of 1.0-1.8 mM), phenol red-free, HBSS, and the fluorescein leakage should be immediately measured. 29. Concurrent negative (NC) and positive controls (PC) should be used in each run to demonstrate that monolayer integrity (NC) and sensitivity of the cells (PC) are within a defined historical acceptance range. The suggested PC chemical is Brij 35 (CAS No. 9002-92-0) at 100 mg/mL. This concentration should give approximately 30% fluorescein leakage (acceptable range 20-40% fluorescein leakage, i.e. damage to cell layer). The suggested NC chemical is calcium-containing (at a concentration of 1.0-1.8 mM), phenol red-free, HBSS (untreated, blank control).A maximum leakage control should also be included in each run to allow for the calculation of FL20 values. Maximum leakage is determined using a control insert without cells.Determination of fluorescein permeability30. Immediately after removal of the test and control substances, 400μL of 0.1 mg/mL sodium-fluorescein solution (0.01% (w/v) in calcium-containing [at a concentration of 1.0-1.8 mM], phenol red-free, HBSS) is added to the Millicell-HA inserts. The cultures are kept for 30 minutes at room temperature. At the end of the incubation with fluorescein, the inserts are carefully removed from each well. Visual check is performed on each filter and any damage which may have occurred during handling is recorded.31. The amount of fluorescein that leaked through the monolayer and the insert is quantified in the solution which remained in the wells after removal of the inserts. Measurements are done in a spectrofluorometer at excitation and emission wavelengths of 485 nm and 530 nm, respectively. The sensitivity of the spectrofluorometer should be set so that there is the highest numerical difference between the maximum FL (insert with no cells) and the minimum FL (insert with confluent monolayer treated with NC). Because of the differences in the used spectrofluorometer, it is suggested that a sensitivity is used which will give fluorescence intensity > 4000 at the maximum fluorescein leakage control. The maximum FL value should not be6© OECD, (2012)OECD/OCDE 460 greater than 9999. The maximum fluorescence leakage intensity should fall within the linear range of the spectrofluorometer used.Interpretation of results and Prediction model32. The amount of FL is proportional to the chemical-induced damage to the tight junctions. The percentage of FL for each tested concentration of chemical is calculated from the FL values obtained for the test substance with reference to FL values from the NC (reading from the confluent monolayer of cells treated with the NC) and a maximum leakage control (reading for the amount of FL through an insert without cells).The mean maximum leakage fluorescence intensity = xThe mean 0% leakage fluorescence intensity (NC) = yThe mean 100% leakage is obtained by subtracting the mean 0% leakage from the mean maximum leakage,i.e. x - y = z33. The percentage leakage for each fixed dose is obtained by subtracting the 0% leakage to the mean fluorescence intensity of the three replicate readings (m), and dividing this value by the 100% leakage, i.e. %FL = [(m-y) / z] x 100%, where:m = the mean fluorescence intensity of the three replicate measurements for the concentration involved% FL = the percent of the fluorescein which leaks through the cell layer34. The following equation for the calculation of the chemical concentration causing 20% FL should be applied:FL D = [(A-B) / (C-B)] x (M C –M B) + M BWhere:D = % of inhibitionA = % damage (20% fluorescein leakage)B = % fluorescein leakage < AC = % fluorescein leakage > AM C = Concentration (mg/mL) of CM B = Concentration (mg/mL) of B35. The cut-off value of FL20 for predicting chemicals as ocular corrosives/severe irritants is given below:7© OECD, (2012)460OECD/OCDE36. The FL test method is recommended only for the identification of water soluble ocular corrosives and severe irritants (UN GHS Category 1, EU CLP Category 1, U.S. EPA Category I) (see paragraphs 1 and 10).37. In order to identify water soluble chemicals (substances and mixtures) (4) (7) (8) as "inducing serious eye damage" (UN GHS/EU CLP Category 1) or as an "ocular corrosive or severe irritant" (U.S. EPA Category I), the test substance should induce an FL20 value of ≤ 100 mg/mL.Acceptance of results38. The mean maximum fluorescein leakage value (x) should be higher than 4000 (see paragraph 31), the mean 0% leakage (y) should be equal or lower than 300, and the mean 100% leakage (z) should fall between 3700 and 6000.39. A test is considered acceptable if the positive control produced 20% to 40% damage to the cell layer (measure as % fluorescein leakage).DATA AND REPORTINGData40. For each run, data from individual replicate wells (e.g. fluorescence intensity values and calculated percentage FL data for each test substance, including classification) should be reported in tabular form. In addition, means ± SD of individual replicate measurements in each run should be reported.Test Report41. The test report should include the following information:Test and Control Substances-Chemical name(s) such as the structural name used by the Chemical Abstracts Service (CAS), followed by other names, if known;-Chemical CAS number, if known;-Purity and composition of the substance or mixture (in percentage(s) by weight), to the extent this information is available;-Physical-chemical properties relevant to the conduct of the study (e.g. physical state, volatility, pH, stability, water solubility, chemical class);-Treatment of the test/control substance prior to testing, if applicable (e.g. warming, grinding);-Storage conditions;Justification of the Test Method and Protocol Used-Should include considerations regarding applicability domain and limitations of the test method;Test Conditions8© OECD, (2012)OECD/OCDE 460 -Description of cell system used, including certificate of authenticity and the mycoplasma status of the cell line;-Details of test procedure used;-Test substance concentration(s) used;-Duration of exposure to the test substance;-Duration of incubation with fluorescein;-Description of any modifications of the test procedure;-Description of evaluation criteria used;-Reference to historical data of the model (e.g. negative and positive controls, benchmark chemicals, if applicable);-Information on the technical proficiency demonstrated by the laboratory;Results-Tabulation of data from individual test substances and controls for each run and each replicate measurement (including individual results, means and SDs);-The derived classification(s) with reference to the prediction model and/or decision criteria used;-Description of other effects observed;Discussion of the Results-Should include considerations regarding a non-conclusive outcome (paragraph 35: FL20 > 100 mg/mL) and further testing;Conclusions9© OECD, (2012)460OECD/OCDELITERATURE1.UN (2009), United Nations Globally Harmonized System of Classification and Labelling ofChemicals (GHS), Third revised edition, New York & Geneva: United Nations Publications.ISBN: 978-92-1-117006-1. Available at:[/trans/danger/publi/ghs/ghs_rev03/03files_e.html]2.EC (2008), Regulation (EC) No 1272/2008 of the European Parliament and of the Council of16 December 2008 on classification, labelling and packaging of substances and mixtures,amending and repealing Directives 67/548/EEC and 1999/45/EC, and amending Regulation (EC) No 1907/2006, Official Journal of the European Union L353, 1-1355.3.U.S. EPA (1996), Label Review Manual: 2nd Edition, EPA737-B-96-001, Washington DC:U.S. Environmental Protection Agency.4.EC-ECVAM (2009), Statement on the scientific validity of cytotoxicity/cell-function based invitro assays for eye irritation testing. Available under Publications at: [http://ecvam.jrc.it/index.htm]5.Scott, L. et al. (2010), A proposed eye irritation testing strategy to reduce and replace in vivostudies using Bottom-Up and Top-Down approaches, Toxicol. In Vitro 24, 1-9.6.OECD (2002), Test No. 405: Acute Eye Irritation/Corrosion, OECD Guidelines for theTesting of Chemicals, Section 4, OECD Publishing. doi: 10.1787/9789264070646-en7.EC-ECVAM (1999), INVITOX Protocol 71: Fluorescein Leakage Test, Ispra, Italy:European Centre for the Validation of Alternative Methods (ECVAM). Available at: [http://ecvam-dbalm.jrc.ec.europa.eu]8.EC-ECVAM (2008), Fluorescein Leakage Assay Background Review Document as anAlternative Method for Eye Irritation Testing. Available under Validation Study Documents, Section Eye Irritation at: [http://ecvam.jrc.it/index.htm]9.OECD (2005), Guidance Document on the Validation and International Acceptance of Newor Updated Test Methods for Hazard Assessment, OECD Series on Testing and Assessment No. 34. OECD, Paris. Available at: [/env/testguidelines]10© OECD, (2012)。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
1.Why are monosaccharides appropriately named “carbo hydrates”?2.Why are monosaccharides soluble in water but not in nonpolar solvents?3.What are the naming conventions for monosaccharides?4.The word “chiral” is derived from the Greek word meaning “hand”. How does this relate to the structure of carbohydrates?5.How many D isomers would an aldopentose have? How many total isomers?6.What is a possible explanation for the observation that most of the hexoses found in living organisms are D isomers?7.Is galactose an epimer of mannose?8.In an aqueous solution of D-glucose, why will there always be a very small amount of the liner form of the monosaccharide?9.Does the discussion on conformations of cyclic forms of monosaccharides suggest an explanation for why the more common anomer of D-fructofuranose is the β anomer? 10.How many D isomers would a cyclized aldopentose have? How many total isomers? 11.What are some of the biologically more important monosaccharide derivatives?12.Why is phosphorylation of sugars beneficial to a cell?13.What is reduced and what is oxidized in the reaction between a monosaccharide and a ferric ion?14.Be able to draw and give the complete name of the disaccharides commonly known as maltose, lactose, and sucrose. Which of these is/are reducing sugars?15.What are the structural and functional differences between homopolysaccharides and heteropolysaccharides?16.Why are homo polysaccharides not useful as informational molecules?17.Suppose you had three polysaccharides, amylose, amylopection, and glycogen, each with the same number of monosaccharide subunits. Which would be degraded the fastest, assuming that the enzymes acting to degrade the polysaccharides all worked at the same rate?18.What are the similarities and differences between cellulose and chitin?19.What are the most important noncovalent bonds or interactions in cellulose?20.How does lysozyme act as a first defense against bacterial infection? How does penicillin combat bacterial infections?21.How does heparin work to inhibit blood coagulation?22.One of the distinctions between proteoglycans and glycoproteins is entirely relative. The second half of the name usually indicates the predominant species. Proteo glycans are primarily glycans (or polysaccharides), whereas glyco proteins usually contain more protein (by weight).23.How does the R-group of Ser make it a good amino acid for connecting to a carbohydrate moiety?24.What is the relationship among glycosaminoglycans, proteoglycans, fibrous proteins, integrins, adhesion proteins, and the extracellular matrix? It may be helpful to diagram the cellular locations of each of these components.25.How are the functions of glycoproteins different from those of glycosaminoglycans? 26.What types of linkages connect oligosaccharides to proteins?27.What kinds of biologically important information are encoded by the oligosaccharideportions of glycoproteins?28.How does the addition of carbohydrate moieties alter the chemistry of glycoproteins and glycolipids?29.How do glycolipids and lipopolysaccharides differ?30.How can so much distinguishing information be packed into an oligosaccharide of comparatively few monosaccharide units?31.Why is it important that there are signals for removal and destruction of “old”cells and hormones?32.Why do people of blood type O tend to have gastric ulcers more often than do people of blood type A or B?Do You Know the Facts?1. Which of the following is not a characteristic of carbohydrates in cells?A. They serve as energy stores in plants and animals.B. They are major structural components of plant tissues.C. They act as binding sites for proteins.D. They are organic catalysts.E. They play a role in cell-cell recognition.2. Which of the following contributes to the structural rigidity of cellulous?A. Adjacent glucose polymers are stabilized by hydrogen bonding.B. Glucose residues are joined by (α1——4) linkages.C. Cellulose is a highly branched molecule.D. The conformation of the glucose polymer is a coiled structure.E. Adjacent polymers are covalently linked by short peptides.3. Which of the following is an epimer of glucose?A. TaloseB. IdoseC. GuloseD. AltroseE. Allose4. Why are sugars usually found as phosphorylated derivatives in cells?A. Phosphorylated sugars are important in regulating cellular pH.B. Unphosphorylated sugars can be transported across cell membranes.C. Unphosphorylated sugars are rapidly degraded by cellular enzymes.D. Phosphorylated sugars encode genetic information.E. None of the above is a correct explanation.5. Which of the following disaccharides could be extended to form a cellulose polymer?A. SucroseB. ThrehaloseC. MaltoseD. LactoseE. None of the above.6. Which of the following is a heteropolysaccharide? What is its function?A. GlycogenB. HyaluronateC. StarchD. CelluloseE. Chitin。
