14MG+abnormal+issue+report
SCAR 不合格品通知单

SCAR - SMPCD - 14005 采购部
发件人 发件日期 索赔金额
缺陷描述
处理决定 不合格等级
报废 √ 重大 有 有 返工处理, 让步接收(打折), 选用, √ √ 轻度 否 否 送出日期: 验查日期
样品提供给供应商 供应商现场核查
处理决定:退货,
3. 根本原因/可能的原因( 供应商要在样品验证后的三天内详细描述根本原因) Root Cause/Possible Cause( To be completed by Supplier in detail within 3 working days upon sample verification)
接受
拒绝
不合格的处置 (由供应商核实)
退货
在SMP厂报废并由供方赔偿
选用, 报废并由供方核实后决定赔偿事项
同意索赔金额
供方代表 职位 7. SMP 公司核定和认证 Close & Sign off by SMP Plant 验证纠正措施 (根据改进后的批号/批次) 满意 不适用
索赔日期
签名 日期
不满意
实施后的产品批号
6. 验证状况(必须由供应商完成的-强制性) Verification Status(Must be completed by Supplier-mandatory) 纠正及预防措施是否有应用到其他类似的产品或供应商的其它生产线过程工序? 有 否 如有,请鉴定产品的尺寸和生产线号
投诉验证
SCAR的状态完结Biblioteka 有条件完结拒绝。再重发SCAR
品管经理审查 总经理批准 索赔(由采购部处理) 向供应商索赔? 如是, 赔款收到? 是 是
签名 签名
日期 日期
港口国监督检查缺陷代码表

01139 Maritime Labour Certificate 01140 Declaration of Maritime Labour Compliance (part I and II) 01201 Certificates for master and officers 01202 Certificate for rating for watchkeeping 01203 Certificates for radio personnel 01204 Certificate for personnel on tankers 01205 Certificate for personnel on fast rescue boats 01206 Certificate for advanced fire-fighting 01209 Manning specified by the minimum safe manning doc 01210 Certificate for medical first aid 01211 Cert for personnel on survival craft & rescue boat 01212 Certificate for medical care 01213 Evidence of Basic Training 01214 Endorsement by flagstate 01215 Application for Endorsement by flagstate 01299 Other (STCW) 01217 Ship Security Officer Certificate 01218 Medical certificate 01219 Training and qualification MLC - Personal safety training 01220 Seafarers' employment agreement (SEA) 01221 Record of employment 01222 Doc evidence for personnel on passenger ships 01223 Security awareness training 01301 Cargo Gear Record Book 01302 SAR Co-operation plan for pass.ships trad on fixe 01303 Unattended Machinery Spaces (UMS) Evidence 01304 Declaration of AFS compliance 01305 Log-books/compulsory entries 01306 Schedules for watchkeeping personnel 01307 Tables of working hours 01308 Records of rest 01309 Fire control plan - all 01310 Signs, indications 01311 Survey report file 01312 Thickness measurement report 01313 Booklet for bulk cargo loading/unloading/stowage 01314 SOPEP 01315 Oil record book 01316 Cargo information 01317 Cargo record book
案例学习 ——哪些不良事件和市场纠正措施需要报告给欧盟主管当局?

为了帮助大家更好的理解欧盟法规要求,本文根据MEDDEV2.12∙1rev8医疗器械警戒系统指南,翻译整理了不良事件和市场纠正措施(FSCA)的案例,供大家学习使用。
备注:如下案例仅作示例之用,为制造商确定是否需要报告给主管当局提供指南。
示例目的是说明报告决定应基于相当多的考量因素。
如有歧义,请以英文指南为准。
不良事件示例O1病患在使用除颤器后死亡,且有证据表明除颤器存在问题。
A.需要报告B.不需要报告C.视情况而定A02按照制造商的使用说明,病患接受外科透热治疗,在使用中产生了灼伤。
A.需要报告B.不需要报告C.视情况而定C如果灼伤明显,应报告。
因为这是正常预期外的严重健康损伤。
03由于输液泵故障,输液泵停止了工作,但未能发出适当的报警,无病患因此而受伤。
A.需要报告B.不需要报告C.视情况而定A因为如果相同的场景再次发生,此问题可能会引发了严重的健康损害。
04因输液泵与使用的输液器间的不匹配,导致输注剂量错误。
A.需要报告B.不需要报告C.视情况而定C若此组合使用符合输液泵或输液器(两者任一)的使用说明,则此事件应报告。
05因对器械的不当操作导致主动脉球囊导管泄漏,使患者处于潜在的危险处境之中。
A.需要报告B.不需要报告C.视情况而定C如果该不当操作是由标签信息不充分导致的,此事件应报告。
06导管在插入过程中断裂,没有操作不当的迹象。
断裂发生的位置使断裂的部分很容易取出。
A.需要报告B.不需要报告C.视情况而定A因为这显然是一个幸运的情况,如果导管在一个稍微不同的位置断裂,就需要外科手术来取回断裂的一端。
07用户发现装隐形眼镜的小瓶中有玻璃颗粒。
A.需要报告B.不需要报告C.视情况而定A08起搏器寿命到期后感应失效。
择期更换指数(ER1)没有及时显示,根据设备规格说明它应该显示。
A.需要报告B.不需要报告C.视情况而定A09病患在接受X射线血管造影系统检查中,X射线血管造影系统的C形臂运动不受控制,病人被影像增强器击中,鼻子骨折。
dnv 201.nor certificate of conformity 2014 90 eu f

Form code: MED 201.NORRevision: 2017-07Page 1 of 4Certificate No: MEDB00005BKApplication of: Directive 2014/90/EU of 23 July 2014 on marine equipment (MED), issued as "Forskrift om Skipsutstyr" by the Norwegian Maritime Authority. This Certificate is issued by DNV GL AS under the authority of the Government of Norway.This is to certify:That the Equivalent fixed gas fire extinguishing systems components (extinguishing medium, head valves and nozzles) for machinery spaces and cargo pump roomswith type designation(s) NOVEC ECS 500Issued toKidde-Fenwal, Inc.Ashland, MA , USAis found to comply with the requirements in the following Regulations/Standards: item No. MED/3.45. SOLAS 74 as amended Regulation II-2/10 & X/3, IMO MSC/Circ. 848, IMO MSC.1/Circ.1313, FSS Code 5 and 2000 HSC Code 7Further details of the equipment and conditions for certification are given overleaf.This Certificate is valid until 2025-09-10.Issued at Høvik on 2020-09-11DNV GL local station:Certification & Inspection ServicesApproval Engineer: Helge BjørnaråNotified Body No.:0575for DNV GL ASRoald Vårheim Head of Notified BodyThe mark of conformity may only be affixed to the above type approved equipment and a Manufacturer’s Declaration of Conformit y issued when the production-surveillance module (D, E or F) of Annex B of the MED is fully complied with and controlled by a written inspection agreement with a Notified Body. The product liability rests with the manufacturer or his representative in accordance with Directive 2014/90/EU.This certificate is valid for equipment, which is conform to the approved type. The manufacturer shall inform DNV GL AS of any changes to the approved equipment. This certificate remains valid unless suspended, withdrawn, recalled or cancelled.Should the specified regulations or standards be amended during the validity of this certificate, the product is to be re-approved before being placed on board a vessel to which the amended regulations or standards apply.Digitally Signed By: Hoff, Øyvind Location: DNV GL Høvik, Norwayon behalf ofJob Id: 344.1-009310-1Certificate No: MEDB00005BK Product description“Novec ECS 500”,is a fixed gas fire extinguishing system using fire extinguishing agent Novec 1230 stored in steel cylinders as liquid and pressurized with nitrogen and distributed through pipes and nozzles.The extinguishing concentration and nozzles are covered by this type approval certificate. Documentation for the other system components shall be submitted and approved for each project. The system is to be designed in accordance with IMO MSC/Circ.848 as amended by IMO MSC.1/Circ.1267 and IMO MSC.1/Circ.1316.The extinguishing agent, Novec 1230, is produced by 3M, Cordova, Illinois, USA.2)When calculated at 20°C. Ambient temperature to be determined case by case for each project3)NFPA 2001 (2008 Edition)The following associated companies are authorised by Kidde-Fenwal to apply this certificate: -Kidde-Fenwal Inc., Ashland, USA-Kidde Fire Protection, Stokenchurch, UKApplication/LimitationThe design gas concentration (diesel) shall be minimum 5.85% (applied on a net volume) and the maximum agent discharge time shall be 10 seconds. The extinguishing system shall be designed and installed according to SOLAS Ch. II-2, IMO MSC/Circ.848 as amended by IMO MSC.1/Circ.1267, IMO MSC.1/Circ.1316 and the Kidde manual.The following additional limitations will apply:A.Novec ESC 500 systems are not suitable for the ship’s cargo holds. If Novec ESC 500 systemsare installed inside cargo pump rooms, all components shall be certified for use in hazardousareas, the design gas concentration shall be increased and the system is subjected to case bycase approval.B.If Novec 1230 agent is used above its NOAEL (calculated on net volume at max expectedambient temperature), means should be provided to limit exposure (IMO MSC.1/Circ.1267, 6).In no case should Novec 1230 be used in concentrations above its LOAEL.C.Steel storage cylinders of size 10 lb (4.5 kg) to 900 lb (408 kg). Cylinders being 81 L or larger isonly accepted when arrangements are provided on board to ensure that cylinders can be easily moved (even to shore) for service and recharging. All cylinders shall be of the same size.D.Gas cylinders shall be delivered on board with a product certificate of the Society or with acertificate issued by a recognized certification authority according to national regulations based on the requirements of the design standard and marked accordingly π, UN or DOT.E.Cylinders are topped up with nitrogen to 34.5 bar (500 psi) at 21°C. The fill density shall bemaximum 1.12 kg/L. Cylinders are to be delivered with DNV product certificate or equivalentcertificates acceptable to the flag administration and class.F.Cylinders to be located in a separate room in accordance with SOLAS Ch. II-2 Reg. 10.4.3, ordistributed throughout the protected space in accordance with the requirements in IMOMSC/Circ.848 item 11 as amended by IMO MSC.1/Circ.1267. When distributed within theprotected space, the min extinguishing concentration (after any single failure) shall be 4.5 %.Job Id: 344.1-009310-1Certificate No: MEDB00005BKponents in the system will be regarded under pressure class II with a maximum designpressure of 40 bar (at 54 °C). Consideration will though be made for piping and couplings inside the protected space.H.The nozzles are to be located in accordance with the Kidde manual. A basic rule is that onenozzle can as a maximum cover an area of 5 m x 10 m. A 360° nozzle shall be located centrally in this area, the 180° nozzles on the sides (as applicable). The maximum cover height is 5 m.The minimum average nozzle pressure is 4.2 bar.I.Bilges (except open bilges in small volume engine rooms) are to be protected with a dedicatednozzle network.The following documentation is to be submitted to the flag administration in each case:1.Plans showing location of cylinders, piping, nozzles and release stations as well as the assembledsystem2.Capacity calculations, including hydraulic flow calculations.3.Plans defining release lines and alarm system.4.Material specification and dimensions for piping and specifications for all other components.5.Ship specific release procedures and post discharge ventilation procedures.6.Manual containing design, inspection, operation and maintenance procedures.7.Control arrangements for closure of openings and stop of fans and any pressure relief devices asper IMO MSC/Circ. 848, 13. These plans can also be supplied by yard.Testing at installations and periodical surveys-The system shall be tested as per maker’s manual both at installations and at periodical surveys, except that DNV do not require monthly content check of cylinders. The minimum test pressure is minimum 53 bar for any closed sections, whereas open section shall be tightness tested atminimum 7 bar.-The system is subject to biennual (every 2nd year) inspections by an approved service supplier.The attending surveyor will also apply requirement relevant for flag administration and / or class on newbuilding and ship in operation surveys.Type Examination documentationDesign, Installation, Operation and Maintenance Manual – Novec ECS 500, No. P/N 06-237589-001, dated May 2017 from Kidde.Report No. HAI Project #5087, dated 28 June 2002, from Hughes Associates, Inc., Baltimore, USA. (tested on U.S. Coast Guard’s Fire & Safety Test Detachment in Mobile, AL).Report No. 04-CRADA-RDC-001, dated 16 November 2004, from Kidde-Fenwal Inc., Massachusetts, USA. (tested on U.S. Coast Guard’s Fire & Safety Test Detachment in Mobile, AL, witnessed by UL).Test Report File EX4674, Project 04NK23160, dated 1 February 2005, from UL, Northbook, USA.Report No. 3026502, dated 24 March 2006, from FM Approvals, Norwood, USA.Test report File EX4674, project 4788267101, dated 30 March 2018, from UL, Northbook, USAKidde Fenwal component sheets, stamped July 2005.Tests carried outTested in accordance with IMO MSC/Circ.848 as amended by IMO MSC.1/Circ.1267 and MSC.1/Circ.1316.Job Id: 344.1-009310-1Certificate No: MEDB00005BK Marking of productMain components in the system are to be marked with name and address of manufacturer, type designation and Mark of Conformity (see first page).。
9101-Form-4-14-Feb-2022 AS 9101 表格 4 – 不合格报告(NCR)

19Containment Action⑸:
^Organization Representative:
Date:
21Auditor Acceptance:
Date:
及Corrections):
23Planned Completion Date:
24Actual Completion Date:
3
Enter the name of organization audited.
4
Enter the name of the site and the Online Aerospace Supplier Information System (OASIS) Identification Number (OIN) of the organization audited.
18
Auditor enters an applicable due date for gaining a response from the organization relating to correction, root cause, corrective action and planned completion dates (see 9101 clause 5.5.2).
13Objective Evidence:
14Containment Required:
□ Yes
□ No
15Containment Due Date:
16Auditor:
^Organization Representative:
SECTION 2 - ORGANIZATIONS PLANNED ACTIONS
SCAR格式

供应商原因分析(Root Cause by Vendor):
供应商短期措施(Short-term countermeasures by Vendor):
供应商改善措施(Long-term corrective actions by Vendor):
Rej. Rate(%)
异常类别Abnormal type:
■进料检查 Incoming;□生产过程中In-process□其它情况Others
不合格严重性评价(Seriousness):
□致命CRI■主缺MAJ□次缺MIN
异常描述(Description of Symptom):
原材料进料检验报告
供应商纠正行动请求(SCAR)
发出日期(Issue date):2014-8-5编号(No.):
供 应 商
Vendor
物料名称
Description
物料名称
Description
不良数量
Rej.Qty
交付日期Delivery date
批 号
Lot No.
交付数量
Shipment qty
不 良 率100%
抽样标准
所在车间
供应商
来料日期
物料名称
物料批次
物料规格
来料数量
抽样数
不良数
不良率
不良描述
结果判定
力象
铭牌
铭牌logo粗细与封样不符
检验日期
检验员
备注
MRB结论(M□挑选使用 SCREENING □让步接受 UAI □ 报废 SCRAP □其它 Others
Non-Conformance Report-不 合 格 品 报 告 单

Position 职位:_________________ Signature 签署:________________________ Position 职位:__________________ Position 职位:_____________________
FORM NO. GHHJV-NCR-
Signature 签名:________________ Name
姓名:_________________________ Signature 签名:_________________ Signature 签名:_____________________
姓名:_________________ Position 职位:________________________ Name 日期:_________________ Date 日期:_________________________ Date
批注本地保存成功开通会员云端永久保存去开通
Non-Conformance Report 不 合 格 品 报 告 单
Client :China Harbour Engineering Company Limited Project Name :PROVISION OF BARRIER-FREE ACCESS FACILITIES FOR HIGHWAY STRUCTURES-PHASE 3 CONTRACT 2 Record No.记录编号: Production No.工单号: Location: 地点 □ Fabrication Yard 工厂 □ Erection Site 地盘 Shop Drawing No.图号:
Date日期:
Agreed by批准人(HC): Prepared by HC: Name Date Checked By QC engineer(HC):
医疗器械体外诊断试剂临床试验严重不良事件报告表SAE

SAE分类
口导致死亡年 月 日;
口致命的疾病或者伤害;
口身体结构或者身体功能的永久性缺陷;
口需要住院治疗或者延长住院时间
口需要采取医疗措施以避免对身体结构或者身体功能造成永久性缺陷; 口导致胎儿窘迫、胎儿死亡或者先天性异常、先天缺损;
口其它
SAE发生时间:年—月—日
研究者获知SAE时间:一年—月—日
附件
医疗器械/体外诊断试剂临床试验严重不良事件报告表
医疗器械临床试验备案号:
基本情况
报告类型
□首次报告 □随访报告 □总结报告报告时间:一年一月—日
临床试验名称
临床试验机构
临床试验专业
主要研究者
职称:
联系人
电话
申报单位名称
联系人
电话:
试验医疗器械情况
试验医疗器械名 称
规格型号/包 装规格
试验医疗器械分 类
与试验医疗器械无关:(1)两者不存在合理时间关系;(2)该不良事件为该试验医疗器械不 可能导致的事件类型;(3)该不良事件可用合并用械/药、患者病情进展、其他治疗影响来 解释。同时满足三条判断为“肯定无关”;满足其中一条判断为“可能无关”。
报告单位名称
报告人职务/ 职称
报告人签名
口是 □否
是否大范围严重不良事 件或其他重大安全性问 题
口是 □否
SAE发生及处理的详细情况:
详细处理情况除包含常规内容外,必须明确以下内容:
1、描述受试者参加医疗器械临床试验情况;
2、描述试验医疗器械使用情况,对有源和无源医疗器械应当描述器械具体操作使用情况,出现 的非预期结果,(可能)对受试者造成的伤害,采取的救治措施以及结果等。对体外诊断医 疗器械,应当描述患者诊疗信息(如疾病情况、用药情况等)、样本检测过程与结果、发现 的异常情况、采取的措施、最终结果判定、对临床诊疗的影响等;
境外不良反应报告规范与使用指南

境外不良反应报告规范与使用指南下载提示:该文档是本店铺精心编制而成的,希望大家下载后,能够帮助大家解决实际问题。
文档下载后可定制修改,请根据实际需要进行调整和使用,谢谢!本店铺为大家提供各种类型的实用资料,如教育随笔、日记赏析、句子摘抄、古诗大全、经典美文、话题作文、工作总结、词语解析、文案摘录、其他资料等等,想了解不同资料格式和写法,敬请关注!Download tips: This document is carefully compiled by this editor. I hope that after you download it, it can help you solve practical problems. The document can be customized and modified after downloading, please adjust and use it according to actual needs, thank you! In addition, this shop provides you with various types of practical materials, such as educational essays, diary appreciation, sentence excerpts, ancient poems, classic articles, topic composition, work summary, word parsing, copy excerpts, other materials and so on, want to know different data formats and writing methods, please pay attention!I. 简介在药品上市后,为了确保患者用药的安全性和有效性,需要对药品的不良反应进行监测和报告。
质量处罚通报模板范文

质量处罚通报模板范文Mass Penalty Report Template.Purpose: To document the mass penalty issued to a vendor for failing to meet the contractual weight requirements.Reporting Party: [Your Name]Date: [Date]Vendor Name: [Vendor Name]Contract Number: [Contract Number]Item Description: [Item Description]Contractual Weight Requirement: [Contractual Weight Requirement]Actual Weight: [Actual Weight]Mass Penalty: [Mass Penalty]Penalty Calculation:The mass penalty was calculated based on the following formula:Mass Penalty = (Contractual Weight Actual Weight) Unit Price Penalty Factor.Unit Price: [Unit Price]Penalty Factor: [Penalty Factor]Total Penalty Amount: [Total Penalty Amount]Rationale:The vendor failed to meet the contractual weight requirement for the specified item. As a result, the vendoris subject to a mass penalty as per the terms of the contract.Supporting Documentation:The following supporting documentation is attached to this report:Copy of the contract.Weighing ticket.Inspection report.Actions Required:The vendor is required to take the following actions:Review the mass penalty and ensure that the weight discrepancy is corrected.Submit a corrective action plan to prevent futureweight discrepancies.Pay the mass penalty in full by [Due Date].Consequences of Non-Compliance:Failure to comply with the requirements of this mass penalty report may result in additional penalties, including termination of the contract.中文回答:质量处罚通报模板范文。
RoHS承诺书两篇

RoHS承诺书两篇篇一:RoHS承诺书尊敬的客户:我司郑重声明:自年月日起,所有由我司提供给贵司的产品,符合欧盟RoHS环保指令的要求。
我司承诺,在未得到贵司采购部门的书面同意之前,绝不提供不符合欧盟RoHS 指令要求的产品,若我司私自将不符合欧盟RoHS指令要求的产品发给贵司,我司将立即以书面形式通知贵司,并在贵司要求时间内补交符合指令要求的产品进行更换,并承担相应的责任,包括贵司客户方的索赔。
作为贵司的供应商,我司乐于提供必要的文件或信息以证明我们的产品符合上述条款,若我们发现产品有违反上述内容,将停止对贵司的供货。
RoHS六种有害物质的建议含量要求为,铅 (Pb) 0.1%(1000ppm)镉(Cd) 0.01% (100ppm)汞(Hg) 0.1%(1000ppm)六价铬 0.1%(1000ppm)有机溴化物多溴联苯类PBB 0.1%(1000ppm)多溴二苯醚PBDE 0.1%(1000ppm)若指令有变化,我司将提供满足新要求的产品给贵司。
公司名称:公司地址:公司授权代表签字(加盖公章):承诺日期:篇二:热缩管等绝缘套管ROHS2.0承诺书为适应市场和客户的环保要求,加强对环保物料的品质控制和过程控制,规范供货行为,保证向客户供应的产品、部件或材料等均符合本承诺书中的要求,任何未达到承诺书中要求而造成的损失均由供应商负全部责任并进行相应的赔偿,特此制定此承诺书。
1.总则自承诺书签定日开始所提热缩管呢等绝缘套管产品,保证满足以下要求。
我方保证产品中环保控制指标为:a 镉(Cd)含量小于70ppm;b 铅(Pb)、汞(Hg)、六价铬(Cr(VI))、多溴联苯(PBBs)、多溴二苯醚(PBDEs)、邻苯二甲酸二丁酯(DBP)、邻苯二甲酸丁苄酯(BBP)、邻苯二甲酸二(2-乙基己基)酯(DEHP)、邻苯二甲酸二异丁酯(DIBP)含量小于800ppm;c 豁免材料中:铜合金中的铅含量小于38000ppm;2.具体条款供应的所有物料均满足ROHS 2.0(修正指令EU 2015/863)要求并保证满足以下条款:2.1样品承认阶段,供方需提交:有效期内的RoHS 2.0(修正指令EU 2015/863)第三方(SGS或CTI)测试报告(中、英文版)。
不符合报告中英文版

Audit Criteria reference#: Management system documentation reference#:
审参考标准确相关的管理体系文件编号
Findings审核发现
Requirement要求:
Findings审核发现:
Auditor signature: Date:
审核员签字:受审核部门代表签字:日期:
Root Cause原因分析
Corrective& Prevention Action纠正预防措施
Date for completion:
完成的时间
Signature:
签字:
Date:
日期:
AUDITOR USE ONLY仅供审核员使用
Nonconformity closed: Corrective action(s) verified to be implemented.
纠正措施实施完成:
Minor Nonconformity轻微不符合Corrective action plan to be submitted within 30 calendar days:
纠正措施计划须在15天内提交:
Opportunityfor improvement No response required
不符合关闭:纠正措施经验证实施有效
Auditor signature :
审核员签字:
Date:
日期:
Form_PR_005_005版本A0
CAR REPORT不符合报告
Finding#: This page may be duplicated, and extras may be removed.
品质异常通知单模板

次品处理方法
PDN 生产
品质
FOLLOW 跟踪:
1. Return to related QC Inspection Group (请将表退还给相关QC检查组统筹/并CC与会相关生产门)
第一联/生产PD/二联QA/三联ENG
FHale Waihona Puke -2808-02--------------------有限公司
NON-CONFORMING MATERIAL REPORT 品质异常通知单
Dept Issue 相关部门 Machine No: 机器号 Process Name 工序号或名称
Item no. 排号 Qty D/F Defect Re'jd/ Rate Code Sple size 不良数/ 不良率 不良 抽量 代码
a000000xdescription检查結果should检查标准要求不良数检查纠正yes是no否s签名yes是no否s签名yes是no否s签名yes是no否s签名yes是remarks备注yes是remarks备注yes是remarks备注报废yes是remarks备注生产代班人请将表退还给相关qc检查组统筹并cc与会相关生产门第一联生产pd二联qa三联eng
Issued By 发出者 Date 日期
Cavity Insp No Method 模号 检查 方法
Lot number Ref 批号 序号 Time: Part number 时间 产品编号 Lot size 批量 Description of Non-Conformity (异常情况) IS (检查結果)
NCM:A000000X
Immed Act'n 即时 纠正
Yes(是)/NO(否)
SHOULD BE (检查标准要求)
可疑且非预期严重不良反应审查

⑥此安全性事件是否应报告成SUSAR;
⑦采取了保护受试者人身安全和健康权益措施。
【否】:以上七要点的任意一点不合理时,应判断为【此安全性信息报告中的内容不合理】。
【不适用】:一般无此情况。
10、是否导致受试者继续参与试验的风险大于获益
风险程度建议考虑:
目前SUSAR的转归状态;
可疑且非预期严重不良反应审查
可疑且非预期严重不良反应审查报告表
项目类别
□药物□医疗器械□IIT□医疗新技术
项目名称
申办者/项目来源
研究专业
我院主要研究者
一、可疑且非预期的不良反应的情况
报告类型
□首次□随访□总结
SUSAR诊断
发生时间
报告时间
预期的判断
□预期□非预期
是否为SUSAR
口是口否
SUSAR程度
【否】;
导致医疗事故的情况。
【不适用】:一般无此情况。
8、安全性信息报告内容是否完整
【是】:法规要求SUSAR报告需包含七要素:
①患者详细资料,如姓名缩写等;
②可疑的药物,包括药物名称等;
③其他治疗,如合并用药等;
④可疑的药物不良反应的详细资料:如ADR严重程度等;
⑤事件报告人信息;
⑥申办者公司信息;
【否】指当前相关资料(如IB)所描述预期风险。
【否】指当前相关资料(如IB)所描述预期风险。
【不适用】:一般无此情况。
3、安全性事件是否与试验药物有关
【是】:指判断SUSAR与试验药物肯定有关、可能有关、无法评价。
【否】:指判断SUSAR与试验药物可能无关、肯定无关。
【不适用】:待评价情况(因资料)。
说明书包装袋客户投诉案例
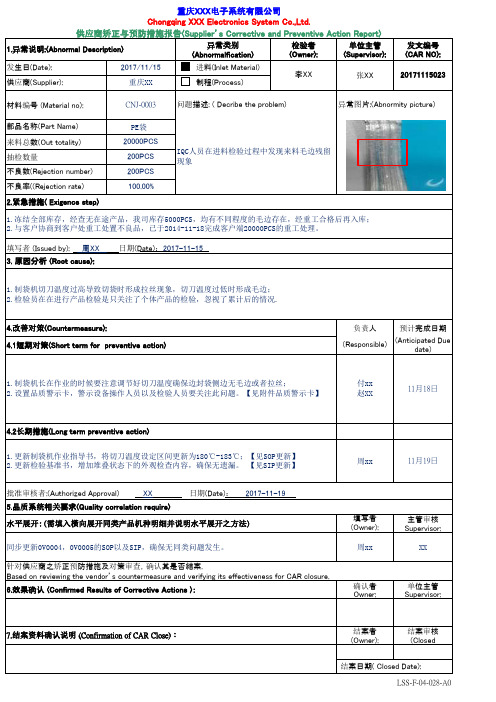
异常类别(Abnormalfication)检验者(Owner):单位主管(Supervisor):发文编号(CAR NO):发生日(Date):2017/11/15 进料(Inlet Material)供应商(Supplier):重庆XX 制程(Process)材料编号 (Material no):CNJ-0003部品名称(Part Name)PE袋 来料总数(Out totality)20000PCS 抽检数量200PCS 不良数(Rejection number)200PCS 不良率((Rejection rate)100.00%负责人预计完成日期(Responsible)(Anticipated Duedate)填写者(Owner):主管审核Supervisor:确认者Owner:单位主管Supervisor:结案者(Owner):结案审核(ClosedLSS-F-04-028-A04.改善对策(Countermeasure):7.结案资料确认说明 (Confirmation of CAR Close):结案日期( Closed Date):针对供应商之矫正预防措施及对策审查,确认其是否結案.Based on reviewing the vendor’s countermeasure and verifying its effectiveness for CAR closure.6.效果确认 (Confirmed Results of Corrective Actions ):周xx 1.更新制袋机作业指导书,将切刀温度设定区间更新为180℃-183℃;【见SOP更新】2.更新检验基准书,增加堆叠状态下的外观检查内容,确保无遗漏。
【见SIP更新】1.冻结全部库存,经查无在途产品,我司库存5000PCS,均有不同程度的毛边存在,经重工合格后再入库;2.与客户协商到客户处重工处置不良品,已于2014-11-18完成客户端20000PCS的重工处理。
低钾血症1例病例讨论

患者女,63岁,聋哑人,因双下肢无力1月,加重2 天,于2012年2月2日来急诊 现病史:患者近一月食欲减退,进食减少,双下肢无力,无 恶心、呕吐,未引起患者及家人重视,未诊治,近2天上 述症状加重,周身软瘫,行走不能,无明显呼吸困难及 吞咽困难,无意识障碍及抽搐,于当地医院就诊,查血 钾1.4mmol/L,心电图示频发室早,心肌酶高。给予补钾 治疗,症状略有好转,为进一步诊治,来诊。
病例报告
既往史:先天性聋哑,慢支病史10余年,高血压 病史4年,血压最高180/100mmHg,间断服用 非洛地平,未监测血压1年前诊断甲状腺囊 肿;否认糖尿病、精神疾病史,否认食物药物过敏史。 家族史:无特殊病史。 查体:T:36.0℃,P:78次/分,R:15次/分,BP: 131/78mmHg,神志清醒,自动体位,呼吸正 常,甲状腺II度肿大,质地中等,无压痛,心、肺、 腹部检查无明显异常,双下肢肌力III级,肌张力 可,双侧病理征阴性。
低钾血症判定程序
17-a 羟化酶缺乏症
患者女,24 岁,因“发作性四肢无力13 年加重2 个月伴原发性闭经”入院 现病史:患者11 岁起反复出现四肢麻木无力,双手持物落地,双下肢不能站立, 伴头痛、短暂意识丧失,无发热、呕吐、抽搐、大小便失禁。至18 岁 未来过月经,腋毛、阴毛缺如,乳房不发育。曾行染色体检查为46XX 。 查促卵泡生成激素(FSH )178.3U/L,促黄体生成激素(LH): 98.6U/L,泌乳素(PRL):29mg/L,雌二醇(E2)51.03pmol/L。 曾诊断单纯性发育不良,口服乙芪酚、安宫黄体酮有月经来潮,停药则 无月经或周期性腹痛。
辅助检查:血皮质醇:上午8 时为11.04nmol/L,下午4 时为0.28nmol/L,半夜12 时为0.0nmol/L。 促肾上腺皮质激素(ACTH):27.73pmol/L, 血醛固酮:4235.9pmol/L, 肾素和血管紧张素基础与激化分别为:0.01mg·L-1/h 与 0.01mg·L-1/h 和65ng/L(正常18~103ng/L)与 68ng/L(正常26~208ng/L)。 尿醛固酮:8.6nmol/24h, 尿儿茶酚胺:110.54nmol/24h, FSH:51.8U/L, LH:43.3U/L, PRL:14mg/L E2:3.67pmol/L, 孕酮(P)12.4nmol/L, 睾酮(T)0.35nmol/L, 17-羟孕酮1.42nmol/L(正常1.85~10.12nmol/L)。 B 超示:左肾上腺见18mm × 1 3 m m 低回声区,边界欠清,双肾肾盂轻 度分离,子宫附件未见异常。 肾上腺CT 未见异常。
MGB麦德龙验厂标准

MGB麦德龙验厂标准MGB METRO Group Buying HK LimitedSelf Assessment Report 自我评审报告MGB Reference No.: _____________________Supplier Name(供应商)Supplier Code(供应商编码)Factory Name(工厂)Factory Address(工厂地址)Property of MGB HK Limited. MGB HK 2010 All right reserved. Factory Self Assessment Template_Ver#100507R1 Page 1 of 17MGB METRO Group Buying HK LimitedSelf Assessment Report 自我评审报告MGB Reference No.: _____________________Factory Site Information(工厂信息)Ownership (private, public, belonging to group)所有关系(私营、集体所有、归属于集团)Year of establishment (in current legal document) 成立时间(以最近的法律文件为准)Organization Chart of Factory 工厂组织结构Products Manufactured (产品)Products for MGB (生产的MGB产品)Production Capacity (monthly/ annual Qty)产能(月产量)Manufacturing Premises (Sq M/ Sq Ft)生产场地(平方米/平方英尺) Machine/Equipment used (no. of units) 使用的机器设备(每款数量)Employees Details (no. of people) 员工信息(员工数量)Supervisors/Managers (管理者/经理)Administration (行政人员)Quality Control (品管)Engineering (技术人员)Design & Development (研发人员)Permanent Workers (固定员工人数)Temporary Workers (流动员工)Grand Total(总计)Working Hour (how many shift, from when to when?)工作时间(几班,具体从几点到几点)No. of subcontractors used (转包商数量)Property of MGB HK Limited. MGB HK 2010 All right reserved. Factory Self Assessment Template_Ver#100507R1 Page 2 of 17MGB METRO Group Buying HK LimitedSelf Assessment Report 自我评审报告MGB Reference No.: _____________________Name & process of main 无subcontractors)主要转包商的名字及具体工作)Property of MGB HK Limited. MGB HK 2010 All right reserved. Factory Self Assessment Template_Ver#100507R1 Page 3 of 17MGB METRO Group Buying HK LimitedSelf Assessment Report 自我评审报告MGB Reference No.: _____________________Section 1: General Organization StructureItem Question Result RemarkYes No N/A1.1 Does the factory have the up to dateorganizational chart? If yes, please attach a copy of organization chart.是否有最新的组织结构图,如有,请提供一份组织结构图。
RoHS新增禁用四项可塑剂不使用证明书 模版

EU RoHS新增禁用四項可塑劑不使用證明書
歐盟於2015年6月4日正式公告RoHS(recast)指令(2011/65/EU) 禁用物質清單(Annex II)新增下列四項鄰苯二甲酸酯:
•鄰苯二甲酸二(2-乙基己基)酯(DEHP) (0,1 %)
•鄰苯二甲酸丁酯苯甲酯(BBP) (0,1 %)
•鄰苯二甲酸二丁酯(DBP) (0,1 %)
•鄰苯二甲酸二異丁酯(DIBP) (0,1 %)
執行日期為2019年7月22日(醫療設備和監控儀器2021年7月22日)
自即日起為宣告期,但至2018年底止,本公司會強制要求所有有業務往來之供應商所供應給本公司之材料均須符合Rohs2的標準,確定符合Rohs2之材料,須提供第三方認證之檢測報告上傳至本公司GPM系統,以確保2019年起本公司出給客人的貨品是完全符合歐盟Rohs2的標準。
茲證明關於向貴公司交貨的零部件、輔助材料及裝置零部件的使用材料、包裝材料以及生產工程中的添加劑等,無EU RoHS新增禁用四項可塑劑之禁用物質。
公司名稱/廠商編號:
公司住址:
負責人姓名/職稱/簽章:
E-mail/Tel No:
產品名稱/全能料號:
保證提交日期:
備註:。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Z轴位置传感器
14#电机控制板
3. 由于手动控制电机运动正常,怀疑14#电机控制板损坏导致控制错误.更换14#板,但 系统报警连接错误.
Containment Action
5
机器软,硬件从新启动并复位正常,运行2小时过后,14#电机又开始报警,印刷头位 置超出极限位置,复位无效.
Containment Action
Containment Action
6 检查14#电机和编码器,没有发现异常现象.
பைடு நூலகம்
14#电机 编码器
Containment Action
7 检查控制14#电机运动的各主件器件的连接线,更换控制板连接卡,从新复位,机器运行正常.
14#电机控制板连接卡
Root Cause
1 2 14#电机控制板损坏或数据丢失. 14#电机控制板连接卡损坏.
Preventions
1 2 完善机台的零件储备. 进一步熟悉机台结构,提高解决问题的技能.
Thank
You
Print screen 14#MG abnormal issue report
Guangming.tang 2007/08/29
CONFIDENTIAL
Issue description
1. 8/27 丝网印刷一线二号印刷机报警, 14#电机(控制印刷头Z轴运动)报警,显示印 刷头位置错误.
Containment Action
4 检查线路正常,怀疑所跟换的14#本身已经损坏,第二次更换14#板,系统显示正确, 安装14#电机软件.
• • • • • • • • 从新启动电脑,在系统软件上输入密码,关闭系统软件,在桌面打开composer软件. 在弹出的窗口中选择“Open an Existing Application”按下. 在新窗口中选择“Tools→ open an existing application”. 在出现的新窗口中选择你要拷贝的电机号码,按下“change”. 在更新的窗口中选择“RS232”点击“properties”. 出现新窗口中选择“COM2”点击“connect”. 将回到选择电机号码的窗口, 点击“Download”. 电脑将会弹出一个小窗口, 输入“PP[13]=14”(这里的14为你所选的电机号码),按 回车键再输入“SV”再按回车键,这时如果输入正确,电脑会显示“0;”或“;”作为 回应.最后等待拷贝完成.
