GW788388_DataSheet_MedChemExpress
磁力架说明书_Magnetic Stand Manual_MCE
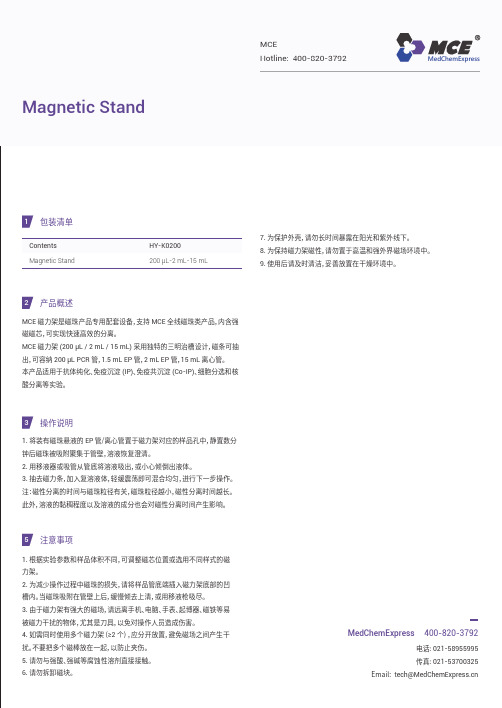
1包装清单产品概述MCE 磁力架是磁珠产品专用配套设备,支持 MCE 全线磁珠类产品,内含强磁磁芯,可实现快速高效的分离。
MCE 磁力架 (200 μL / 2 mL / 15 mL ) 采用独特的三明治槽设计,磁条可抽出,可容纳 200 μL PCR 管,1.5 mL EP 管,2 mL EP 管,15 mL 离心管。
本产品适用于抗体纯化、免疫沉淀 (IP )、免疫共沉淀 (Co-IP )、细胞分选和核酸分离等实验。
2操作说明31. 将装有磁珠悬液的 EP 管/离心管置于磁力架对应的样品孔中,静置数分钟后磁珠被吸附聚集于管壁,溶液恢复澄清。
2. 用移液器或吸管从管底将溶液吸出,或小心倾倒出液体。
3. 抽去磁力条,加入复溶液体,轻缓震荡即可混合均匀,进行下一步操作。
注:磁性分离的时间与磁珠粒径有关,磁珠粒径越小,磁性分离时间越长。
此外,溶液的黏稠程度以及溶液的成分也会对磁性分离时间产生影响。
5注意事项1. 根据实验参数和样品体积不同,可调整磁芯位置或选用不同样式的磁力架。
2. 为减少操作过程中磁珠的损失,请将样品管底端插入磁力架底部的凹槽内。
当磁珠吸附在管壁上后,缓慢倾去上清,或用移液枪吸尽。
3. 由于磁力架有强大的磁场,请远离手机、电脑、手表、起博器、磁铁等易被磁力干扰的物体,尤其是刀具,以免对操作人员造成伤害。
4. 如需同时使用多个磁力架 (≥2 个) ,应分开放置,避免磁场之间产生干扰。
不要把多个磁棒放在一起,以防止夹伤。
5. 请勿与强酸、强碱等腐蚀性溶剂直接接触。
6. 请勿拆卸磁块。
7. 为保护外壳,请勿长时间暴露在阳光和紫外线下。
8. 为保持磁力架磁性,请勿置于高温和强外界磁场环境中。
9. 使用后请及时清洁,妥善放置在干燥环境中。
Magnetic StandMedChemExpress MedChemExpress 400-820-3792 电话: ************ 传真: ************Email: t *********************MCE Hotline: 400-820-3792Contents HY-K0200Magnetic Stand 200 μL-2 mL-15 mL。
BFH772_DataSheet_MedChemExpress

Inhibitors, Agonists, Screening Libraries Data SheetBIOLOGICAL ACTIVITY:BFH772 is a potent oral VEGFR2 inhibitor, which is highly effective at targeting VEGFR2 kinase with an IC 50 value of 3 nM.IC50 & Target: IC50: 2.7±0.9 nM (hVEGFR2), 1.5±0.53 μM (mVEGFR2), 1.7±0.36 μM (hVEGFR1), 1.1±0.29 μM (hVEGFR3)[1]In Vitro: BFH772 is highly selective; apart from inhibiting VEGFR2 at 3 nM IC 50, it also targets B–RAF, RET, and TIE–2, albeit with atleast 40–fold lower potency. BFH772 is inactive (IC 50>10 μM; >2 μM for cKIT) against all other tyrosine specific– andserine/threonine–specific protein kinases tested. BFH772 inhibits VEGFR2 with IC 50 of 4.6±0.6 nM in CHO cells. BFH772 inhibits VEGFR2 with IC 50 of 3 nM in HUVEC cells. BFH772 inhibits the ligand induced autophosphorylation of RET, PDGFR, and KIT kinases,with IC 50 values ranging between 30 and 160 nM. BFH772 is selective (IC 50 values >0.5 μM) against the kinases of EGFR, ERBB2,INS–R, and IGF–1R and against the cytoplasmic BCR–ABL kinase. IC 50 of BFH772 (<0.01 nM, n=2) demonstrates that they abrogated VEGF induced proliferation at remarkably low nM concentrations [1].In Vivo: BFH772 at 3 mg/kg orally dosed once per day potently inhibits melanoma growth (by 54–90% for primary tumor and71–96% for metastasis growth) as depicted by treatment to control ratios. Dose–response curves of BFH772 at 0.3, 1, and 3 mg/kg demonstrate that even at the lowest concentrations, this naphthalene–1–carboxamide inhibits VEGF induced tissue weight and TIE–2 levels but only reaches statistical significance at 1 mg/kg and above [1].PROTOCOL (Extracted from published papers and Only for reference)Kinase Assay:[1]In vitro kinase assay is based on a filter binding assay, using the recombinant GST–fused kinase domainsexpressed in baculovirus and purified over glutathione–sepharose, γ–[33P]ATP as the phosphate donor, and poly(Glu:Tyr 4:1) peptide as the acceptor. Each GST–fused kinase is incubated under optimized buffer conditions [20 mM Tris–HCl buffer (pH 7.5), 1–3 mM MnCl 2, 3–10 mM MgCl 2, 3–8 μg/mL poly(Glu:Tyr 4:1), 0.25 mg/mL polyethylene glycol 20000, 8 μM ATP, 10 μM sodium vanadate, 1mM DTT] and 0.2 μCi γ–33P ATP in a total volume of 30 μL in the presence or absence of a test substance for 10 min at ambient temperature. The reaction is stopped by adding 10 mL of 250 mM EDTA. Using a 384–well filter system, half the volume istransferred onto an Immobilon–polyvinylidene difluoride membrane. The membrane is then washed extensively and dried, and scintillation counting is performed. IC 50s for compounds are calculated by linear regression analysis of the percentage inhibition [1].Cell Assay: BFH772 is dissolved in DMSO (10 mM) and stored, and then diluted with appropriate medium before use [1]. [1]DifferentBa/F3 cell lines rendered IL–3 independent by transduction with various constitutively active tyrosine kinases are grown in RPMI 1640 medium containing 10% fetal calf serum. For maintenance of parental Ba/F3 cells, the medium is additionally supplemented with 10 ng/mL interleukin–3 (IL–3). For proliferation assays, Ba/F3 cells are seeded on 96–well plates in triplicates at 10000 cells per well and incubated with various concentrations of compounds for 72 h followed by quantification of viable cells using a resazurin sodium salt dye reduction readout (commercially known as Alamar Blue assay). IC 50s are determined with the XLFit Excel Add–In using a four–parameter dose response model [1].Animal Administration: BFH772 is prepared in PEG200 100% (Mice)[1].Product Name:BFH772Cat. No.:HY-100419CAS No.:890128-81-1Molecular Formula:C 23H 16F 3N 3O 3Molecular Weight:439.39Target:VEGFR Pathway:Protein Tyrosine Kinase/RTK Solubility:DMSO: 7.75 mg/mLBFH772 is dissolved in N–methyl pyrrolidone/polyethylene glycol200 (30:70, v/v) (Rat)[1].[1]Mice[1]Female FVB mice weighing between 18 and 20 g are housed in groups of six. Porous chambers containing VEGF (2 μg/mL) in 0.5 mL of 0.8% w/v agar (containing heparin, 20 U/mL) are implanted subcutaneously in the flank of the mice (n=6 per group). VEGF induces the growth of vascularized tissue around the chamber. This response is dose–dependent and can be quantified by measuring the weight and TIE–2 levels of the tissue. Mice are treated either orally once daily with compounds or vehicle (PEG200 100%, 5 mL/kg) starting4–6 h before implantation of the chambers and continuing for 4 days. The animals are sacrificed for measurement of the vascularized tissues 24 h after the last dose. Tissue weight is taken and then a lysate prepared for TIE–2 ELISA analysis .Rat[1]Catheters are implanted into the femoral artery and vein of na?ve female rats strain OFA for BFH772, and BAW2881, or in the jugular vein and femoral artery in female Sprague–Dawley rats for compounds 4, 9, and 10. Animals are allowed to recover for 96 h and are housed in single cages with free access to food and water throughout the experiment. Female OFA rats received 2.5 mg/kg ofBAW2881 dissolved in ethanol/dimethylisosorbide/polyethylene glycol400/D5W (10/15/35/40 v/v) or 1 mg/kg of BFH772 dissolved in N–methyl pyrrolidone/polyethylene glycol200 (30:70, v/v) via injection into the femoral vein. D5W is glucose 5%/water (v/v). Oral administration: BAW2881 and BFH772 are formulated as a micronized suspension (dissolved/suspended in 0.5% carboxymethyl cellulose in distilled water) and administered by gavage to female OFA rats to deliver a dose of 25 mg/kg for BAW2881 or 3 mg/kg BFH772 (n=4 rats per group). For compounds 4, 9, and 10, female Sprague–Dawley rats at 8 weeks of age received an intraveno References:[1]. Bold G, et al. A Novel Potent Oral Series of VEGFR2 Inhibitors Abrogate Tumor Growth by Inhibiting Angiogenesis. J Med Chem. 2016 Jan 14;59(1):132–46.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
PHA-793887_DataSheet_MedChemExpress

Inhibitors, Agonists, Screening Libraries Data SheetBIOLOGICAL ACTIVITY:PHA–793887 is a novel pan–cdk inhibitor, including cdk1, cdk2, cdk4, cdk5, cdk7, and cdk9 with IC50 in the 5 to 140 nM range.IC50 Value: 8 nM (CDK2), 5 nM(CDK5), 10 nM (CDK7)Target: CDKsin vitro: PHA–793887 is cytotoxic for leukemic cell lines, including K562, KU812, KCL22, and TOM1, with IC50 of 0.3–7 μM, but it is not cytotoxic for normal unstimulated peripheral blood mononuclear cells or CD34+ hematopoietic stem cells. In colony assays,PHA–793887 shows very high activity against leukemia cell lines with IC50 less than 0.1 μM. PHA–793887 induces cell–cycle arrest,inhibits Rb and nucleophosmin phosphorylation, and modulates cyclin E and cdc6 expression at 0.2–1 μM and induces apoptosis at 5μM. PHA–793887 has low activity against CDK1, CDK4, CDK9 and GSK3β with IC50 of 60 nM, 62 nM, 138 nM and 79 nM, respectively.PHA–793887 inhibits cell proliferation of many tumor cell lines, including A2780, HCT–116, COLO–205, C–433, DU–145, A375, PC3,MCF–7, and BX–PC3, with IC50 of 88 nM–3.4 μM. PHA–793887 (1 μM) shows a decrease in the S phase, a subsequent increase of the G1 phase and a slight accumulation of G2/M phase in A2780 cells. PHA–793887 (3 μM) significantly increases G2/M phase and reduces DNA synthsis.in vivo: PHA–793887 (20 mg/kg) is effective in xenograft models of K562 and HL60 cells, primary leukemic disseminated model, and a high–burden disseminated ALL–2 model derived from a relapsed Philadelphia–positive acute lymphoid leukemia patient. PHA–793887(10–30 mg/kg) shows good efficacy in the human ovarian A2780, colon HCT–116, and pancreatic BX–PC3 carcinoma xenograft models.PROTOCOL (Extracted from published papers and Only for reference)Animal administration [1]A2780 human ovarian adenocarcinoma–bearing CD–1 nude mice (Charles River Breeding Laboratories) were treated with 15 or 30mg/kg of PHA–793887 once a day by i.v. route for 2 days. Animals were sacrificed at 1.5, 6, and 24 hours after the last treatment, and tumor slices from mice treated with compound or vehicle (n = 9 animals per group) were collected and immediately soaked inRNAlater solution (Qiagen) for gene expression analysis. For pharmacokinetic analysis, plasma samples were collected from six animals at each time interval and analyzed by liquid chromatography–tandem mass spectrometry. Data were evaluated by nonlinear mixed effect modeling. Plasma levels of the compound were fitted using a two–compartment model with bolus input and first–order output mean, and random parameters were considered in the following equation: Pi = P.exp (η). The random effects were considered normal distributed with zero mean and variance ω2.References:[1]. Locatelli G et al Transcriptional analysis of an E2F gene signature as a biomarker of activity of the cyclin–dependent kinase inhibitor PHA–793887 inProduct Name:PHA–793887Cat. No.:HY-11001CAS No.:718630-59-2Molecular Formula:C 19H 31N 5O 2Molecular Weight:361.48Target:CDK Pathway:Cell Cycle/DNA Damage Solubility:10 mM in DMSOtumor and skin biopsies from a phase I clinical study. Mol Cancer Ther. 2010 May;9(5):1265–73.[2]. Massard C, Soria JC, Anthoney DA, Proctor A, Scaburri A, Pacciarini MA, Laffranchi B, Pellizzoni C, Kroemer G, Armand JP, Balheda R, Twelves CJ.A first in man, phase I dose–escalation study of PHA–793887, an inhibitor of multiple cyclin–dependent kinases (CDK2, 1 and 4) reveals unexpected hepatotoxicity in patients with solid tumors.Cell Cycle. 2011 Mar 15;10(6):963–70. Epub 2011 Mar 15.[3]. Alzani R, Pedrini O, Albanese C, Ceruti R, Casolaro A, Patton V, Colotta F, Rambaldi A, Introna M, Pesenti E, Ciomei M, Golay J.Therapeutic efficacy of the pan–cdk inhibitor PHA–793887 in vitro and in vivo in engraftment and high–burden leukemia models.Exp Hematol. 2010 Apr;38(4):259–269.e2. Epub 2010 Feb 16.[4]. Brasca MG, Albanese C, Alzani R, Amici R, Avanzi N, Ballinari D, Bischoff J, Borghi D, Casale E, Croci V, Fiorentini F, Isacchi A, Mercurio C, Nesi M, Orsini P, Pastori W, Pesenti E, Pevarello P, Roussel P, Varasi M, Volpi D, Vulpetti A, Ciomei M.Optimization of 6,6–dimethyl pyrrolo[3,4–c]pyrazoles: Identification of PHA–793887, a potent CDK inhibitor suitable for intravenous dosing.Bioorg Med Chem. 2010 Mar 1;18(5):1844–53. Epub 2010 Jan 25.[5]. Zoubir M, Flament C, Gdoura A, Bahleda R, Litvinova E, Soumelis V, Conforti R, Viaud S, Soria JC, Kroemer G, Zitvogel L, Chaput N.An inhibitor of cyclin–dependent kinases suppresses TLR signaling and increases the susceptibility of cancer patients to herpesviridae.Cell Cycle. 2011 Jan 1;10(1):118–26. Epub 2011 Jan 1.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
GW788388_LCMS_16713_MedChemExpress

=====================================================================Acq. Operator : Li Shan(LCMS-02) Seq. Line : 21Acq. Instrument : HY-LCMS-02 Location : P1-C-03Injection Date : 6/24/2015 12:08:32 PM Inj : 1Inj Volume : 3.000 µlAcq. Method : D:\AGLIENT 1260\DATA\20150611\20150611 2015-06-24 10-40-08\100-1000MS+3MIN- 1.5_(0.02%FA).MLast changed : 6/24/2015 10:40:08 AM by Li Shan(LCMS-02)Analysis Method : D:\AGLIENT 1260\METHOD\5-80A,15MIN(210NM).M Last changed : 6/25/2015 10:28:27 AM by Li Shan(LCMS-02) (modified after loading)Method Info : 5-50A(RP-HPLC)Catalog No : HY-10326 Batch#16713 A-RP-132Additional Info : Peak(s) manually integratedmin0.511.52 2.53mAU 020040060080010001200 DAD1 C, Sig=254,4 Ref=off (D:\AGLIENT...60\DATA\20150611\20150611 2015-06-24 10-40-08\BIZ2015-624-DJL1.D)1.5081.6111.719 1.7931.891===================================================================== Area Percent Report =====================================================================Sorted By : Signal Multiplier : 1.0000Dilution : 1.0000Do not use Multiplier & Dilution Factor with ISTDsSignal 1: DAD1 C, Sig=254,4 Ref=offPeak RetTime Type Width Area Height Area # [min] [min] [mAU*s] [mAU] %----|-------|----|-------|----------|----------|--------| 1 1.508 MM 0.0432 3764.56860 1451.88440 98.2121 2 1.611 MM 0.0364 23.24872 10.65138 0.6065 3 1.719 MM 0.0292 7.06897e-1 4.03378e-1 0.0184 4 1.793 MM 0.0436 43.75251 16.72953 1.1414 5 1.891 MM 0.0326 8.22000e-1 4.19868e-1 0.0214Totals : 3833.09873 1480.08856===================================================================== *** End of Report ***=====================================================================Acq. Operator : Li Shan(LCMS-02) Seq. Line : 21Acq. Instrument : HY-LCMS-02 Location : P1-C-03Injection Date : 6/24/2015 12:08:32 PM Inj : 1Inj Volume : 3.000 µlAcq. Method : D:\AGLIENT 1260\DATA\20150611\20150611 2015-06-24 10-40-08\100-1000MS+3MIN- 1.5_(0.02%FA).MLast changed : 6/24/2015 10:40:08 AM by Li Shan(LCMS-02)Analysis Method : D:\AGLIENT 1260\METHOD\30-90A,210NM(RP-HPLC).M Last changed : 6/24/2015 1:37:51 PM by Li Shan(LCMS-02) (modified after loading) M ethod Info : HPLCCatalog No : HY-10326 Batch#16713 A-RP-132Additional Info : Peak(s) manually integratedmin0.511.522.53100000200000300000400000500000600000700000800000 MSD1 TIC, MS File (D:\AGLIENT 1260\DATA\20150611\20150611 2015-06-24 10-40-08\BIZ2015-624-DJL1.D) ES-API, Pos, Sca1.512 1.5851.7951.8852.476MS Signal: MSD1 TIC, MS File, ES-API, Pos, Scan, Frag: 50 Spectra averaged over upper half of peaks.Noise Cutoff: 1000 counts.Reportable Ion Abundance: > 10%.Retention Mol. WeightTime (MS) MS Area or Ion1.512 3226000 427.20 I426.20 I213.60 I1.585 205114 455.20 I454.20 I427.15 I426.15 I425.30 I365.05 I275.95 I227.60 I213.60 I102.20 I100.10 I1.795 203891 646.30 I498.20 I365.15 I323.70 I100.10 I1.885 115670 426.20 I387.10 I365.10 I340.10 I334.25 I323.70 I318.30 I290.25 I275.90 I274.25 I230.30 I219.65 I105.10 I100.10 I2.476 172869 365.10 I340.10 I282.25 I275.95 I105.10 I102.15 I100.15 Im/z10020030040050060070080020406080100Max: 418155427.2213.6426.2m/z10020030040050060070080000.511.522.5*MSD1 SPC, time=1.580:1.635 of D:\AGLIENT 1260\DATA\20150611\20150611 2015-06-24 10-40-08\BIZ2015-624-DJL1.D ES-API,199.6323.0283.7342.2105.0276.0227.6455.2 365.0427.1 100.1425.3454.2213.6 426.1m/z1002003004005006007008000123456*MSD1 SPC, time=1.780:1.817 of D:\AGLIENT 1260\DATA\20150611\20150611 2015-06-24 10-40-08\BIZ2015-624-DJL1.D ES-API,213.6426.2276.0365.1100.1498.2646.3323.7m/z10020030040050060070080000.10.20.30.40.50.60.70.80.9*MSD1 SPC, time=1.835:1.908 of D:\AGLIENT 1260\DATA\20150611\20150611 2015-06-24 10-40-08\BIZ2015-624-DJL1.D ES-API,338.0388.0499.3 245.4201.9 130.1231.1 186.2214.3 171.0111.1439.3371.1282.0646.3323.3 498.3427.1 290.3219.6213.8105.1230.3275.9334.3387.1426.2318.3365.1274.3100.1m/z10020030040050060070080000.20.40.60.811.21.4 324.0 171.0352.2426.0 111.0 159.0 123.2438.3145.1 243.1279.1 387.0336.1282.3102.2365.1340.1105.1 100.2*** End of Report ***。
Y-27632_DataSheet_MedChemExpress

Inhibitors, Agonists, Screening Libraries Data SheetBIOLOGICAL ACTIVITY:Y–27632 is an ATP–competitive inhibitor of ROCK–I and ROCK–II , with K i of 220 nM and 300 nM for ROCK–I and ROCK–II , respectively.IC50 & Target: Ki: 220/300 nM (ROCK–I/II)[1]In Vitro: Y–27632 inhibits the ROCK family of kinases 100 times more potently than other kinases including protein kinase C,cAMP–dependent kinase and myosin light chain kinase. Y–27632 prolongs the lag time and delays the appearance of BrdU–labeled cells in a concentration–dependent manner, delays of about 1 and 4 h are noticed in the Swiss 3T3 cells treated with 10 and 100 μM Y–27632, respectively [1]. Y–27632 promotes neuronal differentiation of adipose tissue–derived stem cells (ADSCs). Compared to 1.0and 2.5 μM Y–27632 induced groups, percentages of neuroal–like cells achieved a peak in the 5.0 μM Y–27632 induced group [2].In Vivo: Y–27632 (5 and 10 mg/kg) significantly prolongs the onset time of myoclonic jerks when compare with saline group.Y–27632 (5 and 10 mg/kg) significantly prolongs the onset time of clonic convulsions when compare with saline group [3].Treatment with Dimethylnitrosamine (DMN) causes a significant decrease in rat body and liver weight (DMN–S group) compared with control animals (S–S group). Oral Y27632 (30 mg/kg) essentially prevents this DMN–induced rat body and liver weight loss (DMN–Y group)[4].PROTOCOL (Extracted from published papers and Only for reference)Kinase Assay:[1]Recombinant ROCK–I, ROCK–II, PKN, or citron kinase is expressed in HeLa cells as Myc–tagged proteins by transfection using Lipofectamine, and is precipitated from the cell lysates by the use of 9E10 monoclonal anti–Myc antibodycoupled to G protein–Sepharose. Recovered immunocomplexes are incubated with various concentrations of [32P]ATP and 10 mg of histone type 2 as substrates in the absence or presence of various concentrations of either Y–27632 or Y–30141 at 30°C for 30 min in a total volume of 30 μL of the kinase buffer containing 50 mM HEPES–NaOH, pH 7.4, 10 mM MgCl 2, 5 mM MnCl 2, 0.02% Briji 35, and 2 mM dithiothreitol. PKCa is incubated with 5 μM [32P]ATP and 200 μg/mL histone type 2 as substrates in the absence or presence of various concentrations of either Y–27632 or Y–30141 at 30°C for 10 min in a kinase buffer containing 50 mM Tris–HCl,pH 7.5, 0.5 mM CaCl 2, 5 mM magnesium acetate, 25 μg/mL phosphatidyl serine, 50 ng/mL 12–O–tetradecanoylphorbol–13–acetate and 0.001% leupeptin in a total volume of 30 μL. Incubation is terminated by the addition of 10 μL of 43 Laemmli sample buffer.After boiling for 5 min, the mixture is subjected to SDS–polyacrylamide gel electrophoresis on a 16% gel. The gel is stained withCoomassie Brilliant Blue, and then dried. The bands corresponding to histone type 2 are excised, and the radioactivity is measured [1]. Cell Assay: Y–27632 is dissolved in water and stored [1].[1]HeLa cells are plated at a density of 3×104 cells per 3.5–cm dish. The cells are cultured in DMEM containing 10% FBS in the presence of 10 mM Thymidine for 16 h. After the cells are washed with DMEM containing 10% FBS, they are cultured for an additional 8 h, and then 40 ng/mL of Nocodazole is added. After 11.5 h of theNocodazole treatment, various concentrations of Y–27632 (0–300 μM), Y–30141, or vehicle is added and the cells are incubated for another 30 min [1].Animal Administration: Y–27632 is dissolved in 0.9% NaCl (saline) (Mice)[3].Product Name:Y–27632Cat. No.:HY-10071CAS No.:146986-50-7Molecular Formula:C 14H 21N 3O Molecular Weight:247.34Target:ROCK; ROCK; ROCK Pathway:TGF–beta/Smad; Stem Cell/Wnt; Cell Cycle/DNA Damage Solubility:DMSO: ≥ 32 mg/mLY–27632 is dissolved in saline (final concentration 2%) (Rat)[4].[3][4]Mice[3]Male, inbred Swiss albino mice (2–3 months old) weighing 25–30 g are used. Mice are injected with a sub–convulsive dose of PTZ (35 mg/kg, i.p.) (on Mondays, Wednesdays and Fridays) of each week for a total of 11 injections. After each PTZ injection, mice are observed for 30 min and the occurrence of convulsive activity is recorded. After 30 min, the mice are then injected with either Fasudil (25 mg/kg, i.p.) or Y–27632 (5 mg/kg, i.p.) and returned to their home cages until the next injection. Control mice for Fasudil andY–27632 receives saline.Rat[4]Male Wistar Kind A rats (200–250 g) are used. DMN (1 g/mL) is diluted ten times with saline (final concentration 1%) and 10 mg/kg per day of DMN is injected intraperitoneally (i.p.) on the first 3 days of each week for 4 weeks. Y27632 is given orally once per day at a dose of 30 mg/kg for 4 weeks starting on the day of the first injection of DMN. The dose of 30 mg/kg corrects hypertension in several rat models without toxicity. Twenty rats are randomized into four experimental groups (n=5 in each group) as follows: (1) S–S (injection of saline i.p. and oral administration of saline); (2) S–Y (injection of saline i.p. and oral administration of Y27632); (3) DMN–S (DMN i.p. and oral administration of saline); (4) DMN–Y (DMN i.p. and oral administration of Y27632). The rats are weighed every week. They are sacrificed at the end of the fourth week and the liver is excised. In addition, a blood sample is taken immediately before the rats are sacrificed.References:[1]. Ishizaki T, et al. Pharmacological properties of Y–27632, a specific inhibitor of rho–associated kinases. Mol Pharmacol. 2000 May;57(5):976–83.[2]. Xue ZW, et al. Rho–associated coiled kinase inhibitor Y–27632 promotes neuronal–like differentiation of adult human adipose tissue–derived stem cells.Chin Med J (Engl). 2012 Sep;125(18):3332–5.[3]. Inan S, et al. Antiepileptic effects of two Rho–kinase inhibitors, Y–27632 and fasudil, in mice. Br J Pharmacol. 2008 Sep;155(1):44–51.[4]. Tada S, et al. A selective ROCK inhibitor, Y27632, prevents dimethylnitrosamine–induced hepatic fibrosis in rats. J Hepatol. 2001 Apr;34(4):529–36.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
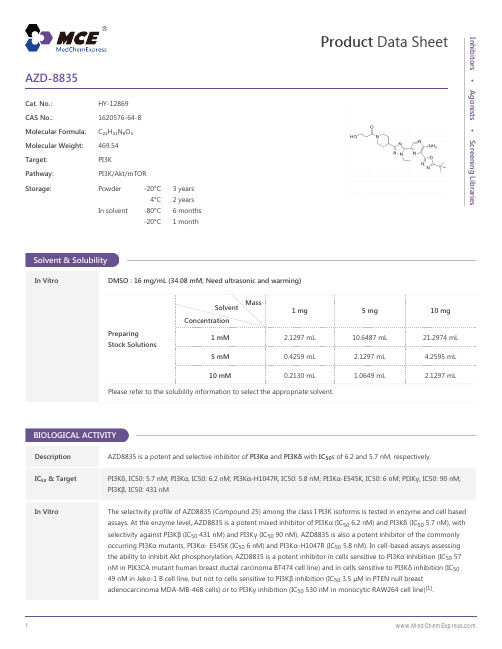
AZD-8835-DataSheet-MedChemExpress
AZD-8835In Vivo AZD8835 (Compound 25) displays good solubility, good permeability and low turnover in hepatocytes from various species. As expected from the in vitro data, low in vivo clearance associated with high bioavailability is seen in bothrat and dog. AZD8835 shows high exposure following oral administration to SCID mice (AUC: 137 μM.h and C max 34μM at 50 mg/kg p.o.) and is selected for further in vivo evaluation. In a pharmacodynamic experiment followingchronic oral dosing (25 mg/kg b.i.d. or 6 mg/kg b.i.d. of AZD8835) in nude mice bearing mutant H1047R PI3KαSKOV-3 tumour xenografts, target modulation is assessed by measuring Akt phosphorylation levels at Ser473 at 30minutes and 8 hours. At both doses, strong inhibition of Akt phosphorylation is observed at the 30 minute timepoint.At 8 hours, significant inhibition is still seen at the 25 mg/kg dose, whereas no inhibition is seen at the lower dose of6 mg/kg, consistent with the lower plasma concentrations observed[1].REFERENCES[1]. Barlaam B, Discovery of 1-(4-(5-(5-amino-6-(5-tert-butyl-1,3,4-oxadiazol-2-yl)pyrazin-2-yl)-1-ethyl-1,2,4-triazol-3-yl)piperidin-1-yl)-3-hydroxypropan-1-one (AZD8835): A potent and selective inhibitor of PI3Kα and PI3Kδ for the treatment of cancers. Bioorg Med Chem Lett. 2015 Nov 15;25(22):5155-62.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
GW788388_452342-67-5_MSDS_MedChemExpress

MSDS1 Composition7 Accident Release MeasureProduct Name:GW788388Chemical Name:PROCEDURE(S) OF PERSONAL PRECAUTION(S)-Wear respirator, chemical safety goggles, rubber boots, and heavyrubber gloves.METHODS FOR CLEANING UP-Sweep up, place in a bag and hold for waste disposal. Avoid raising dust. Ventilate area andwash spill site after material pickup is complete.Benzamide, 4-[4-[3-(2-pyridinyl)-1H-pyrazol-4-yl]-2-pyridinyl]-N-(tetrahydro-2H-pyran-4-yl)-CAS No.:452342-67-58 Accident Release MeasureAppearance:White to off-white(solid)Formula:C25H23N5O29 Toxicological InformationSolubility:To the best of our knowledge, the chemical, physical, andtoxicological properties have not been thoroughly investigated.No data available.p p p p DMSO2 Handling and Storage10 Regulary Information3 Stability and Reactivity11Disposal ConsiderationsCLASSIFICATION- Substance not yet fully tested.SAFETY PHASES- 26-36 (In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. Wear suitable protective clothing.) 36/37/38 (Irritating to eyes,respiratory system and skin.)STABILITY- Stable under normal handling conditions.HANDLING- Do not breathe dust. Avoid contact with eyes,skin,and clothing.Avoid prolonged or repeated exposure.STORAGE- Store in a properly sealed container store at -20℃,shelflife is 2 years.11 Disposal Considerations 4 Hazards Identification12 Transport Information5First Aid RID/ADR- Non-hazardous for road transport. IMDG- Non-hazardous for sea transport.IATA - Non-hazardous for air transport.As specific country, federal, state and local environmentalregulations vary and change frequently we suggest you contact a local, authorized waste disposal contractor for adequate disposal.Special indication of hazards to humans and the environment.Irritating to eyes, respiratory system and skin.MATERIALS TO AVOID- Strong oxidizing agents.REACTIVITY- May emit toxic gasses like Carbon monoxide,Carbon dioxide, Nitrogen oxides upon thermal decomposition.5 First Aid13 Other InformationThe above information is believed to be correct but does not purport to be all inclusive and shall be used only as a guide. The information in this document is based on the present state of our knowledge and is applicable to the product with regard to appropriate safety precautions. It does not represent any guarantee of the properties of the product. Medchemexpress LLC shall not be held liable for any damage resulting from h dli f t t ith th b d tINHALATION- If inhaled, remove to fresh air. If not breathing give, artificial respiration. If breathing is difficult, give oxygen.SKIN CONTACT- In case of contact, immediately wash skin withsoap and copious amounts of water.EYE CONTACT- In case of contact, immediately flush eyes withcopious amounts of water for at least 15 minutes.INGESTION- If swallowed, wash out mouth with water provided person is conscious. Call a physician.6 Fire Fighting Measureshandling or from contact with the above product.EXTINGUISHING MEDIA Water spray- Carbon dioxide, dry chemical powder, or appropriate foam.SPECIAL RISKS Specific Hazard(s)- Emits toxic fumes under fire conditions. SPECIAL PROTECTIVE EQUIPMENT FOR FIREFIGHTERS Wear self-contained breathing apparatus and protective clothing Caution: Not fully tested. For research purposes onlyMedchemexpress LLCto prevent contact with skin and eyes.18 W i l k i n s o n W a y , P r i n c e t o n , N J 08540,U S AE m a i l : i n f o @m e d c h e m e x p r e s s .c o m W e b : w w w .m e d c h e m e x p r e s s .c o m。
Hoechst_33342_trihydrochloride_DataSheet_MedChemExpress

Inhibitors, Agonists, Screening Libraries Data SheetBIOLOGICAL ACTIVITY:Hoechst 33342 trihydrochloride is an AT–specific DNA minor groove ligand used fluorochrome for visualizing cellular DNA .IC50 & Target: Dye reagent [1]DNA Stain [1]In Vitro: Hoechst 33342 binds to adenine–thymine–rich regions of DNA in the minor groove. On binding to DNA, the fluorescence greatly increases. This protocol describes the use of Hoechst 33342 to label nuclear DNA of cells grown in culture. Hoechst 33342can also be used to stain fixed cells by substituting Hoechst 33342 for DAPI [1].PROTOCOL (Extracted from published papers and Only for reference)Cell Assay: Hoechst 33342 (10 mg/mL in H 2O stock solution; protected from light)[1].[1]Labeling Nuclear DNA with Hoechst 33342[1]Step 1, Dilute the Hoechst stock solution 1:100 in H 2O for use in labeling. Step 2, Aspirate the cell medium from cells grown on coverslips. Rinse the cells three times with PBS +. Step 3, Incubate the cells in the Hoechst labeling solution (from Step 1) for 10–30min at room temperature. Step 4, Aspirate the labeling solution. Rinse the cells three times in PBS +. Step 5, Mount the coverslips.Step 6, Image the cells (λex ~353 nm, λem ~483 nm for Hoechst 33342)[1].References:[1]. Chazotte B. Labeling nuclear DNA with hoechst 33342. Cold Spring Harb Protoc. 2011 Jan 1;2011(1):pdb.prot5557.Product Name:Hoechst 33342 (trihydrochloride)Cat. No.:HY-15559A CAS No.:875756-97-1Molecular Formula:C 27H 31Cl 3N 6O Molecular Weight:561.93Target:DNA Stain; Autophagy Pathway:Cell Cycle/DNA Damage; Autophagy Solubility:DMSO: ≥ 46 mg/mL; H 2O: ≥ 5.6 mg/mLCaution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@ Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
AZD7545_SDS_MedChemExpress
Inhibitors, Agonists, Screening LibrariesSafety Data Sheet Revision Date:May-24-2017Print Date:May-24-20171. PRODUCT AND COMPANY IDENTIFICATION1.1 Product identifierProduct name :AZD7545Catalog No. :HY-16082CAS No. :252017-04-21.2 Relevant identified uses of the substance or mixture and uses advised againstIdentified uses :Laboratory chemicals, manufacture of substances.1.3 Details of the supplier of the safety data sheetCompany:MedChemExpress USATel:609-228-6898Fax:609-228-5909E-mail:sales@1.4 Emergency telephone numberEmergency Phone #:609-228-68982. HAZARDS IDENTIFICATION2.1 Classification of the substance or mixtureGHS Classification in accordance with 29 CFR 1910 (OSHA HCS)Acute toxicity, Oral (Category 4),H302Acute aquatic toxicity (Category 1),H400Chronic aquatic toxicity (Category 1),H4102.2 GHS Label elements, including precautionary statementsPictogramSignal word WarningHazard statement(s)H302 Harmful if swallowed.H410 Very toxic to aquatic life with long lasting effects.Precautionary statement(s)P264 Wash skin thoroughly after handling.P270 Do not eat, drink or smoke when using this product.P273 Avoid release to the environment.P301 + P312 IF SWALLOWED: Call a POISON CENTER or doctor/ physician if you feel unwell.P330 Rinse mouth.P391 Collect spillage.P501 Dispose of contents/ container to an approved waste disposal plant.2.3 Other hazardsNone.3. COMPOSITION/INFORMATION ON INGREDIENTS3.1 SubstancesSynonyms:AZD 7545; AZD⁻7545Formula:C19H18ClF3N2O5SMolecular Weight:478.87CAS No. :252017-04-24. FIRST AID MEASURES4.1 Description of first aid measuresEye contactRemove any contact lenses, locate eye-wash station, and flush eyes immediately with large amounts of water. Separate eyelids with fingers to ensure adequate flushing. Promptly call a physician.Skin contactRinse skin thoroughly with large amounts of water. Remove contaminated clothing and shoes and call a physician.InhalationImmediately relocate self or casualty to fresh air. If breathing is difficult, give cardiopulmonary resuscitation (CPR). Avoid mouth-to-mouth resuscitation.IngestionWash out mouth with water; Do NOT induce vomiting; call a physician.4.2 Most important symptoms and effects, both acute and delayedThe most important known symptoms and effects are described in the labelling (see section 2.2).4.3 Indication of any immediate medical attention and special treatment neededTreat symptomatically.5. FIRE FIGHTING MEASURES5.1 Extinguishing mediaSuitable extinguishing mediaUse water spray, dry chemical, foam, and carbon dioxide fire extinguisher.5.2 Special hazards arising from the substance or mixtureDuring combustion, may emit irritant fumes.5.3 Advice for firefightersWear self-contained breathing apparatus and protective clothing.6. ACCIDENTAL RELEASE MEASURES6.1 Personal precautions, protective equipment and emergency proceduresUse full personal protective equipment. Avoid breathing vapors, mist, dust or gas. Ensure adequate ventilation. Evacuate personnel to safe areas.Refer to protective measures listed in sections 8.6.2 Environmental precautionsTry to prevent further leakage or spillage. Keep the product away from drains or water courses.6.3 Methods and materials for containment and cleaning upAbsorb solutions with finely-powdered liquid-binding material (diatomite, universal binders); Decontaminate surfaces and equipment by scrubbing with alcohol; Dispose of contaminated material according to Section 13.7. HANDLING AND STORAGE7.1 Precautions for safe handlingAvoid inhalation, contact with eyes and skin. Avoid dust and aerosol formation. Use only in areas with appropriate exhaust ventilation.7.2 Conditions for safe storage, including any incompatibilitiesKeep container tightly sealed in cool, well-ventilated area. Keep away from direct sunlight and sources of ignition.Recommended storage temperature:Powder-20°C 3 years4°C 2 yearsIn solvent-80°C 6 months-20°C 1 monthShipping at room temperature if less than 2 weeks.7.3 Specific end use(s)No data available.8. EXPOSURE CONTROLS/PERSONAL PROTECTION8.1 Control parametersComponents with workplace control parametersThis product contains no substances with occupational exposure limit values.8.2 Exposure controlsEngineering controlsEnsure adequate ventilation. Provide accessible safety shower and eye wash station.Personal protective equipmentEye protection Safety goggles with side-shields.Hand protection Protective gloves.Skin and body protection Impervious clothing.Respiratory protection Suitable respirator.Environmental exposure controls Keep the product away from drains, water courses or the soil. Cleanspillages in a safe way as soon as possible.9. PHYSICAL AND CHEMICAL PROPERTIES9.1 Information on basic physical and chemical propertiesAppearance White to off-white (Solid)Odor No data availableOdor threshold No data availablepH No data availableMelting/freezing point No data availableBoiling point/range No data availableFlash point No data availableEvaporation rate No data availableFlammability (solid, gas)No data availableUpper/lower flammability or explosive limits No data availableVapor pressure No data availableVapor density No data availableRelative density No data availableWater Solubility No data availablePartition coefficient No data availableAuto-ignition temperature No data availableDecomposition temperature No data availableViscosity No data availableExplosive properties No data availableOxidizing properties No data available9.2 Other safety informationNo data available.10. STABILITY AND REACTIVITY10.1 ReactivityNo data available.10.2 Chemical stabilityStable under recommended storage conditions.10.3 Possibility of hazardous reactionsNo data available.10.4 Conditions to avoidNo data available.10.5 Incompatible materialsStrong acids/alkalis, strong oxidising/reducing agents.10.6 Hazardous decomposition productsUnder fire conditions, may decompose and emit toxic fumes.Other decomposition products - no data available.11.TOXICOLOGICAL INFORMATION11.1 Information on toxicological effectsAcute toxicityClassified based on available data. For more details, see section 2Skin corrosion/irritationClassified based on available data. For more details, see section 2Serious eye damage/irritationClassified based on available data. For more details, see section 2Respiratory or skin sensitizationClassified based on available data. For more details, see section 2Germ cell mutagenicityClassified based on available data. For more details, see section 2CarcinogenicityIARC: No component of this product present at a level equal to or greater than 0.1% is identified as probable, possible or confirmed human carcinogen by IARC.ACGIH: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by ACGIH.NTP: No component of this product present at a level equal to or greater than 0.1% is identified as a anticipated or confirmed carcinogen by NTP.OSHA: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by OSHA.Reproductive toxicityClassified based on available data. For more details, see section 2Specific target organ toxicity - single exposureClassified based on available data. For more details, see section 2Specific target organ toxicity - repeated exposureClassified based on available data. For more details, see section 2Aspiration hazardClassified based on available data. For more details, see section 212. ECOLOGICAL INFORMATION12.1 ToxicityNo data available.12.2 Persistence and degradabilityNo data available.12.3 Bioaccumlative potentialNo data available.12.4 Mobility in soilNo data available.12.5 Results of PBT and vPvB assessmentPBT/vPvB assessment unavailable as chemical safety assessment not required or not conducted.12.6 Other adverse effectsNo data available.13. DISPOSAL CONSIDERATIONS13.1 Waste treatment methodsProductDispose substance in accordance with prevailing country, federal, state and local regulations.Contaminated packagingConduct recycling or disposal in accordance with prevailing country, federal, state and local regulations.14. TRANSPORT INFORMATIONDOT (US)This substance is considered to be non-hazardous for transport.IMDGUN number: 3077Class: 9Packing group: IIIEMS-No: F-A, S-FProper shipping name: ENVIRONMENTALLY HAZARDOUS SUBSTANCE, SOLID, N.O.S.Marine pollutant: Marine pollutantIATAUN number: 3077Class: 9Packing group: IIIProper shipping name: Environmentally hazardous substance, solid, n.o.s.15. REGULATORY INFORMATIONSARA 302 Components:No chemicals in this material are subject to the reporting requirements of SARA Title III, Section 302.SARA 313 Components:This material does not contain any chemical components with known CAS numbers that exceed the threshold (De Minimis) reporting levels established by SARA Title III, Section 313.SARA 311/312 Hazards:No SARA Hazards.Massachusetts Right To Know Components:No components are subject to the Massachusetts Right to Know Act.Pennsylvania Right To Know Components:No components are subject to the Pennsylvania Right to Know Act.New Jersey Right To Know Components:No components are subject to the New Jersey Right to Know Act.California Prop. 65 Components:This product does not contain any chemicals known to State of California to cause cancer, birth defects, or anyother reproductive harm.16. OTHER INFORMATIONCopyright 2017 MedChemExpress. The above information is correct to the best of our present knowledge but does not purport to be all inclusive and should be used only as a guide. The product is for research use only and for experienced personnel. It must only be handled by suitably qualified experienced scientists in appropriately equipped and authorized facilities. The burden of safe use of this material rests entirely with the user. MedChemExpress disclaims all liability for any damage resulting from handling or from contact with this product.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
JNJ-678-SDS-MedChemExpress

Inhibitors, Agonists, Screening LibrariesSafety Data Sheet Revision Date:Feb.-16-2019Print Date:Feb.-16-20191. PRODUCT AND COMPANY IDENTIFICATION1.1 Product identifierProduct name :JNJ-678Catalog No. :HY-112180CAS No. :1383450-81-41.2 Relevant identified uses of the substance or mixture and uses advised againstIdentified uses :Laboratory chemicals, manufacture of substances.1.3 Details of the supplier of the safety data sheetCompany:MedChemExpress USATel:609-228-6898Fax:609-228-5909E-mail:sales@1.4 Emergency telephone numberEmergency Phone #:609-228-68982. HAZARDS IDENTIFICATION2.1 Classification of the substance or mixtureNot a hazardous substance or mixture.2.2 GHS Label elements, including precautionary statementsNot a hazardous substance or mixture.2.3 Other hazardsNone.3. COMPOSITION/INFORMATION ON INGREDIENTS3.1 SubstancesSynonyms:JNJ-53718678Formula:C21H20ClF3N4O3SMolecular Weight:500.92CAS No. :1383450-81-44. FIRST AID MEASURES4.1 Description of first aid measuresEye contactRemove any contact lenses, locate eye-wash station, and flush eyes immediately with large amounts of water. Separate eyelids with fingers to ensure adequate flushing. Promptly call a physician.Skin contactRinse skin thoroughly with large amounts of water. Remove contaminated clothing and shoes and call a physician.InhalationImmediately relocate self or casualty to fresh air. If breathing is difficult, give cardiopulmonary resuscitation (CPR). Avoid mouth-to-mouth resuscitation.IngestionWash out mouth with water; Do NOT induce vomiting; call a physician.4.2 Most important symptoms and effects, both acute and delayedThe most important known symptoms and effects are described in the labelling (see section 2.2).4.3 Indication of any immediate medical attention and special treatment neededTreat symptomatically.5. FIRE FIGHTING MEASURES5.1 Extinguishing mediaSuitable extinguishing mediaUse water spray, dry chemical, foam, and carbon dioxide fire extinguisher.5.2 Special hazards arising from the substance or mixtureDuring combustion, may emit irritant fumes.5.3 Advice for firefightersWear self-contained breathing apparatus and protective clothing.6. ACCIDENTAL RELEASE MEASURES6.1 Personal precautions, protective equipment and emergency proceduresUse full personal protective equipment. Avoid breathing vapors, mist, dust or gas. Ensure adequate ventilation. Evacuate personnel to safe areas.Refer to protective measures listed in sections 8.6.2 Environmental precautionsTry to prevent further leakage or spillage. Keep the product away from drains or water courses.6.3 Methods and materials for containment and cleaning upAbsorb solutions with finely-powdered liquid-binding material (diatomite, universal binders); Decontaminate surfaces and equipment by scrubbing with alcohol; Dispose of contaminated material according to Section 13.7. HANDLING AND STORAGE7.1 Precautions for safe handlingAvoid inhalation, contact with eyes and skin. Avoid dust and aerosol formation. Use only in areas with appropriate exhaust ventilation.7.2 Conditions for safe storage, including any incompatibilitiesKeep container tightly sealed in cool, well-ventilated area. Keep away from direct sunlight and sources of ignition.Recommended storage temperature:Powder-20°C 3 years4°C 2 yearsIn solvent-80°C 6 months-20°C 1 monthShipping at room temperature if less than 2 weeks.7.3 Specific end use(s)No data available.8. EXPOSURE CONTROLS/PERSONAL PROTECTION8.1 Control parametersComponents with workplace control parametersThis product contains no substances with occupational exposure limit values.8.2 Exposure controlsEngineering controlsEnsure adequate ventilation. Provide accessible safety shower and eye wash station.Personal protective equipmentEye protection Safety goggles with side-shields.Hand protection Protective gloves.Skin and body protection Impervious clothing.Respiratory protection Suitable respirator.Environmental exposure controls Keep the product away from drains, water courses or the soil. Cleanspillages in a safe way as soon as possible.9. PHYSICAL AND CHEMICAL PROPERTIES9.1 Information on basic physical and chemical propertiesAppearance White to off-white (Solid)Odor No data availableOdor threshold No data availablepH No data availableMelting/freezing point No data availableBoiling point/range No data availableFlash point No data availableEvaporation rate No data availableFlammability (solid, gas)No data availableUpper/lower flammability or explosive limits No data availableVapor pressure No data availableVapor density No data availableRelative density No data availableWater Solubility No data availablePartition coefficient No data availableAuto-ignition temperature No data availableDecomposition temperature No data availableViscosity No data availableExplosive properties No data availableOxidizing properties No data available9.2 Other safety informationNo data available.10. STABILITY AND REACTIVITY10.1 ReactivityNo data available.10.2 Chemical stabilityStable under recommended storage conditions.10.3 Possibility of hazardous reactionsNo data available.10.4 Conditions to avoidNo data available.10.5 Incompatible materialsStrong acids/alkalis, strong oxidising/reducing agents.10.6 Hazardous decomposition productsUnder fire conditions, may decompose and emit toxic fumes.Other decomposition products - no data available.11.TOXICOLOGICAL INFORMATION11.1 Information on toxicological effectsAcute toxicityClassified based on available data. For more details, see section 2Skin corrosion/irritationClassified based on available data. For more details, see section 2Serious eye damage/irritationClassified based on available data. For more details, see section 2Respiratory or skin sensitizationClassified based on available data. For more details, see section 2Germ cell mutagenicityClassified based on available data. For more details, see section 2CarcinogenicityIARC: No component of this product present at a level equal to or greater than 0.1% is identified as probable, possible or confirmed human carcinogen by IARC.ACGIH: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by ACGIH.NTP: No component of this product present at a level equal to or greater than 0.1% is identified as a anticipated or confirmed carcinogen by NTP.OSHA: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by OSHA.Reproductive toxicityClassified based on available data. For more details, see section 2Specific target organ toxicity - single exposureClassified based on available data. For more details, see section 2Specific target organ toxicity - repeated exposureClassified based on available data. For more details, see section 2Aspiration hazardClassified based on available data. For more details, see section 212. ECOLOGICAL INFORMATION12.1 ToxicityNo data available.12.2 Persistence and degradabilityNo data available.12.3 Bioaccumlative potentialNo data available.12.4 Mobility in soilNo data available.12.5 Results of PBT and vPvB assessmentPBT/vPvB assessment unavailable as chemical safety assessment not required or not conducted.12.6 Other adverse effectsNo data available.13. DISPOSAL CONSIDERATIONS13.1 Waste treatment methodsProductDispose substance in accordance with prevailing country, federal, state and local regulations.Contaminated packagingConduct recycling or disposal in accordance with prevailing country, federal, state and local regulations.14. TRANSPORT INFORMATIONDOT (US)This substance is considered to be non-hazardous for transport.IMDGThis substance is considered to be non-hazardous for transport.IATAThis substance is considered to be non-hazardous for transport.15. REGULATORY INFORMATIONSARA 302 Components:No chemicals in this material are subject to the reporting requirements of SARA Title III, Section 302.SARA 313 Components:This material does not contain any chemical components with known CAS numbers that exceed the threshold (De Minimis) reporting levels established by SARA Title III, Section 313.SARA 311/312 Hazards:No SARA Hazards.Massachusetts Right To Know Components:No components are subject to the Massachusetts Right to Know Act.Pennsylvania Right To Know Components:No components are subject to the Pennsylvania Right to Know Act.New Jersey Right To Know Components:No components are subject to the New Jersey Right to Know Act.California Prop. 65 Components:This product does not contain any chemicals known to State of California to cause cancer, birth defects, or anyother reproductive harm.16. OTHER INFORMATIONCopyright 2019 MedChemExpress. The above information is correct to the best of our present knowledge but does not purport to be all inclusive and should be used only as a guide. The product is for research use only and for experienced personnel. It must only be handled by suitably qualified experienced scientists in appropriately equipped and authorized facilities. The burden of safe use of this material rests entirely with the user. MedChemExpress disclaims all liability for any damage resulting from handling or from contact with this product.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
MK-0773_LCMS_09383_MedChemExpress

=====================================================================Acq. Operator : Li Shan(LCMS-02) Seq. Line : 63Acq. Instrument : HY-LCMS-02 Location : P1-F-05Injection Date : 5/28/2015 4:37:57 PM Inj : 1 Inj Volume : 3.000 µl Acq. Method : D:\AGLIENT 1260\DATA\20150528\20150528 2015-05-28 09-07-25\100-1000MS+3MIN- 1.5_(0.02%FA).M Last changed : 5/28/2015 9:07:25 AM by Li Shan(LCMS-02)Analysis Method : D:\AGLIENT 1260\METHOD\50-90A,15MIN(RP-HPLC).M Last changed : 5/28/2015 4:51:43 PM by Li Shan(LCMS-02) (modified after loading)Method Info : 10-80A,16MIN Catalog No : HY-11027 Batch#09383 A-RP-132 Additional Info : Peak(s) manually integrated min0.51 1.52 2.53mAU 0200400600800100012001400 DAD1 B, Sig=214,4 Ref=off (D:\AGLIENT...0\DATA\20150528\20150528 2015-05-28 09-07-25\CPK2015-528-09383.D)1.251 1.7082.733 ===================================================================== Area Percent Report ===================================================================== Sorted By : Signal Multiplier : 1.0000Dilution : 1.0000Do not use Multiplier & Dilution Factor with ISTDs Signal 1: DAD1 B, Sig=214,4 Ref=off Peak RetTime Type Width Area Height Area # [min] [min] [mAU*s] [mAU] %----|-------|----|-------|----------|----------|--------| 1 1.251 MM 0.0457 18.53222 6.75743 0.4440 2 1.708 MM 0.0448 4153.08350 1543.88892 99.5063 3 2.733 MM 0.0557 2.07316 6.20486e-1 0.0497 Totals : 4173.68888 1551.26683 ===================================================================== *** End of Report ***==========================================================Acq. Operator : Li Shan(LCMS-02) Seq. Line : 63Acq. Instrument : HY-LCMS-02 Location : P1-F-05Injection Date : 5/28/2015 4:37:57 PM Inj : 1 Inj Volume : 3.000 µl Acq. Method : D:\AGLIENT 1260\DATA\20150528\20150528 2015-05-28 09-07-25\100-1000MS+3MIN- 1.5_(0.02%FA).M Last changed : 5/28/2015 9:07:25 AM by Li Shan(LCMS-02)Analysis Method : D:\AGLIENT 1260\METHOD\50-90A,15MIN(RP-HPLC).M Last changed : 5/28/2015 4:54:18 PM by Li Shan(LCMS-02) (modified after loading)Method Info : 10-80A,16MIN Catalog No : HY-11027 Batch#09383 A-RP-132 Additional Info : Peak(s) manually integrated min0.51 1.52 2.530200000400000600000800000MSD1 TIC, MS File (D:\AGLIENT 1260\DATA\20150528\20150528 2015-05-28 09-07-25\CPK2015-528-09383.D) ES-API, Pos, Sc1.712MS Signal: MSD1 TIC, MS File, ES-API, Pos, Scan, Frag: 50 Spectra averaged over upper half of peaks. Noise Cutoff: 1000 counts. Reportable Ion Abundance: > 10%. Retention Mol. Weight Time (MS) MS Area or Ion 1.712 4599394 481.30 I 480.30 Im/z200400600800020406080100*MSD1 SPC, time=1.689:1.744 of D:\AGLIENT 1260\DATA\20150528\20150528 2015-05-28 09-07-25\CPK2015-528-09383.D ES-API Max: 525296 482.3 240.6 481.3 480.3 *** End of Report ***。
GW0742_SDS_MedChemExpress

Inhibitors, Agonists, Screening LibrariesSafety Data Sheet Revision Date:May-24-2017Print Date:May-24-20171. PRODUCT AND COMPANY IDENTIFICATION1.1 Product identifierProduct name :GW0742Catalog No. :HY-13928CAS No. :317318-84-61.2 Relevant identified uses of the substance or mixture and uses advised againstIdentified uses :Laboratory chemicals, manufacture of substances.1.3 Details of the supplier of the safety data sheetCompany:MedChemExpress USATel:609-228-6898Fax:609-228-5909E-mail:sales@1.4 Emergency telephone numberEmergency Phone #:609-228-68982. HAZARDS IDENTIFICATION2.1 Classification of the substance or mixtureGHS Classification in accordance with 29 CFR 1910 (OSHA HCS)Acute toxicity, Oral (Category 4),H302Acute aquatic toxicity (Category 1),H400Chronic aquatic toxicity (Category 1),H4102.2 GHS Label elements, including precautionary statementsPictogramSignal word WarningHazard statement(s)H302 Harmful if swallowed.H410 Very toxic to aquatic life with long lasting effects.Precautionary statement(s)P264 Wash skin thoroughly after handling.P270 Do not eat, drink or smoke when using this product.P273 Avoid release to the environment.P301 + P312 IF SWALLOWED: Call a POISON CENTER or doctor/ physician if you feel unwell.P330 Rinse mouth.P391 Collect spillage.P501 Dispose of contents/ container to an approved waste disposal plant.2.3 Other hazardsNone.3. COMPOSITION/INFORMATION ON INGREDIENTS3.1 SubstancesSynonyms:GW610742; GW 0742; GW⁻0742; GW 610742; GW⁻610742Formula:C21H17F4NO3S2Molecular Weight:471.49CAS No. :317318-84-64. FIRST AID MEASURES4.1 Description of first aid measuresEye contactRemove any contact lenses, locate eye-wash station, and flush eyes immediately with large amounts of water. Separate eyelids with fingers to ensure adequate flushing. Promptly call a physician.Skin contactRinse skin thoroughly with large amounts of water. Remove contaminated clothing and shoes and call a physician.InhalationImmediately relocate self or casualty to fresh air. If breathing is difficult, give cardiopulmonary resuscitation (CPR). Avoid mouth-to-mouth resuscitation.IngestionWash out mouth with water; Do NOT induce vomiting; call a physician.4.2 Most important symptoms and effects, both acute and delayedThe most important known symptoms and effects are described in the labelling (see section 2.2).4.3 Indication of any immediate medical attention and special treatment neededTreat symptomatically.5. FIRE FIGHTING MEASURES5.1 Extinguishing mediaSuitable extinguishing mediaUse water spray, dry chemical, foam, and carbon dioxide fire extinguisher.5.2 Special hazards arising from the substance or mixtureDuring combustion, may emit irritant fumes.5.3 Advice for firefightersWear self-contained breathing apparatus and protective clothing.6. ACCIDENTAL RELEASE MEASURES6.1 Personal precautions, protective equipment and emergency proceduresUse full personal protective equipment. Avoid breathing vapors, mist, dust or gas. Ensure adequate ventilation. Evacuate personnel to safe areas.Refer to protective measures listed in sections 8.6.2 Environmental precautionsTry to prevent further leakage or spillage. Keep the product away from drains or water courses.6.3 Methods and materials for containment and cleaning upAbsorb solutions with finely-powdered liquid-binding material (diatomite, universal binders); Decontaminate surfaces and equipment by scrubbing with alcohol; Dispose of contaminated material according to Section 13.7. HANDLING AND STORAGE7.1 Precautions for safe handlingAvoid inhalation, contact with eyes and skin. Avoid dust and aerosol formation. Use only in areas with appropriate exhaust ventilation.7.2 Conditions for safe storage, including any incompatibilitiesKeep container tightly sealed in cool, well-ventilated area. Keep away from direct sunlight and sources of ignition.Recommended storage temperature:Powder-20°C 3 years4°C 2 yearsIn solvent-80°C 6 months-20°C 1 monthShipping at room temperature if less than 2 weeks.7.3 Specific end use(s)No data available.8. EXPOSURE CONTROLS/PERSONAL PROTECTION8.1 Control parametersComponents with workplace control parametersThis product contains no substances with occupational exposure limit values.8.2 Exposure controlsEngineering controlsEnsure adequate ventilation. Provide accessible safety shower and eye wash station.Personal protective equipmentEye protection Safety goggles with side-shields.Hand protection Protective gloves.Skin and body protection Impervious clothing.Respiratory protection Suitable respirator.Environmental exposure controls Keep the product away from drains, water courses or the soil. Cleanspillages in a safe way as soon as possible.9. PHYSICAL AND CHEMICAL PROPERTIES9.1 Information on basic physical and chemical propertiesAppearance White to off-white (Solid)Odor No data availableOdor threshold No data availablepH No data availableMelting/freezing point No data availableBoiling point/range No data availableFlash point No data availableEvaporation rate No data availableFlammability (solid, gas)No data availableUpper/lower flammability or explosive limits No data availableVapor pressure No data availableVapor density No data availableRelative density No data availableWater Solubility No data availablePartition coefficient No data availableAuto-ignition temperature No data availableDecomposition temperature No data availableViscosity No data availableExplosive properties No data availableOxidizing properties No data available9.2 Other safety informationNo data available.10. STABILITY AND REACTIVITY10.1 ReactivityNo data available.10.2 Chemical stabilityStable under recommended storage conditions.10.3 Possibility of hazardous reactionsNo data available.10.4 Conditions to avoidNo data available.10.5 Incompatible materialsStrong acids/alkalis, strong oxidising/reducing agents.10.6 Hazardous decomposition productsUnder fire conditions, may decompose and emit toxic fumes.Other decomposition products - no data available.11.TOXICOLOGICAL INFORMATION11.1 Information on toxicological effectsAcute toxicityClassified based on available data. For more details, see section 2Skin corrosion/irritationClassified based on available data. For more details, see section 2Serious eye damage/irritationClassified based on available data. For more details, see section 2Respiratory or skin sensitizationClassified based on available data. For more details, see section 2Germ cell mutagenicityClassified based on available data. For more details, see section 2CarcinogenicityIARC: No component of this product present at a level equal to or greater than 0.1% is identified as probable, possible or confirmed human carcinogen by IARC.ACGIH: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by ACGIH.NTP: No component of this product present at a level equal to or greater than 0.1% is identified as a anticipated or confirmed carcinogen by NTP.OSHA: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by OSHA.Reproductive toxicityClassified based on available data. For more details, see section 2Specific target organ toxicity - single exposureClassified based on available data. For more details, see section 2Specific target organ toxicity - repeated exposureClassified based on available data. For more details, see section 2Aspiration hazardClassified based on available data. For more details, see section 212. ECOLOGICAL INFORMATION12.1 ToxicityNo data available.12.2 Persistence and degradabilityNo data available.12.3 Bioaccumlative potentialNo data available.12.4 Mobility in soilNo data available.12.5 Results of PBT and vPvB assessmentPBT/vPvB assessment unavailable as chemical safety assessment not required or not conducted.12.6 Other adverse effectsNo data available.13. DISPOSAL CONSIDERATIONS13.1 Waste treatment methodsProductDispose substance in accordance with prevailing country, federal, state and local regulations.Contaminated packagingConduct recycling or disposal in accordance with prevailing country, federal, state and local regulations.14. TRANSPORT INFORMATIONDOT (US)This substance is considered to be non-hazardous for transport.IMDGUN number: 3077Class: 9Packing group: IIIEMS-No: F-A, S-FProper shipping name: ENVIRONMENTALLY HAZARDOUS SUBSTANCE, SOLID, N.O.S.Marine pollutant: Marine pollutant.IATAUN number: 3077Class: 9Packing group: IIIProper shipping name: Environmentally hazardous substance, solid, n.o.s.15. REGULATORY INFORMATIONSARA 302 Components:No chemicals in this material are subject to the reporting requirements of SARA Title III, Section 302.SARA 313 Components:This material does not contain any chemical components with known CAS numbers that exceed the threshold (De Minimis) reporting levels established by SARA Title III, Section 313.SARA 311/312 Hazards:No SARA Hazards.Massachusetts Right To Know Components:No components are subject to the Massachusetts Right to Know Act.Pennsylvania Right To Know Components:No components are subject to the Pennsylvania Right to Know Act.New Jersey Right To Know Components:No components are subject to the New Jersey Right to Know Act.California Prop. 65 Components:This product does not contain any chemicals known to State of California to cause cancer, birth defects, or anyother reproductive harm.16. OTHER INFORMATIONCopyright 2017 MedChemExpress. The above information is correct to the best of our present knowledge but does not purport to be all inclusive and should be used only as a guide. The product is for research use only and for experienced personnel. It must only be handled by suitably qualified experienced scientists in appropriately equipped and authorized facilities. The burden of safe use of this material rests entirely with the user. MedChemExpress disclaims all liability for any damage resulting from handling or from contact with this product.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
Hoechst-33258-analog-SDS-MedChemExpress

Inhibitors, Agonists, Screening LibrariesSafety Data Sheet Revision Date:Oct.-06-2018Print Date:Oct.-06-20181. PRODUCT AND COMPANY IDENTIFICATION1.1 Product identifierProduct name :Hoechst 33258 analogCatalog No. :HY-15623CAS No. :258843-62-81.2 Relevant identified uses of the substance or mixture and uses advised againstIdentified uses :Laboratory chemicals, manufacture of substances.1.3 Details of the supplier of the safety data sheetCompany:MedChemExpress USATel:609-228-6898Fax:609-228-5909E-mail:sales@1.4 Emergency telephone numberEmergency Phone #:609-228-68982. HAZARDS IDENTIFICATION2.1 Classification of the substance or mixtureNot a hazardous substance or mixture.2.2 GHS Label elements, including precautionary statementsNot a hazardous substance or mixture.2.3 Other hazardsNone.3. COMPOSITION/INFORMATION ON INGREDIENTS3.1 SubstancesSynonyms:NoneFormula:C29H30N6O3Molecular Weight:510.59CAS No. :258843-62-84. FIRST AID MEASURES4.1 Description of first aid measuresEye contactRemove any contact lenses, locate eye-wash station, and flush eyes immediately with large amounts of water. Separate eyelids with fingers to ensure adequate flushing. Promptly call a physician.Skin contactRinse skin thoroughly with large amounts of water. Remove contaminated clothing and shoes and call a physician.InhalationImmediately relocate self or casualty to fresh air. If breathing is difficult, give cardiopulmonary resuscitation (CPR). Avoid mouth-to-mouth resuscitation.IngestionWash out mouth with water; Do NOT induce vomiting; call a physician.4.2 Most important symptoms and effects, both acute and delayedThe most important known symptoms and effects are described in the labelling (see section 2.2).4.3 Indication of any immediate medical attention and special treatment neededTreat symptomatically.5. FIRE FIGHTING MEASURES5.1 Extinguishing mediaSuitable extinguishing mediaUse water spray, dry chemical, foam, and carbon dioxide fire extinguisher.5.2 Special hazards arising from the substance or mixtureDuring combustion, may emit irritant fumes.5.3 Advice for firefightersWear self-contained breathing apparatus and protective clothing.6. ACCIDENTAL RELEASE MEASURES6.1 Personal precautions, protective equipment and emergency proceduresUse full personal protective equipment. Avoid breathing vapors, mist, dust or gas. Ensure adequate ventilation. Evacuate personnel to safe areas.Refer to protective measures listed in sections 8.6.2 Environmental precautionsTry to prevent further leakage or spillage. Keep the product away from drains or water courses.6.3 Methods and materials for containment and cleaning upAbsorb solutions with finely-powdered liquid-binding material (diatomite, universal binders); Decontaminate surfaces and equipment by scrubbing with alcohol; Dispose of contaminated material according to Section 13.7. HANDLING AND STORAGE7.1 Precautions for safe handlingAvoid inhalation, contact with eyes and skin. Avoid dust and aerosol formation. Use only in areas with appropriate exhaust ventilation.7.2 Conditions for safe storage, including any incompatibilitiesKeep container tightly sealed in cool, well-ventilated area. Keep away from direct sunlight and sources of ignition.Recommended storage temperature:Powder-20°C 3 years4°C 2 yearsIn solvent-80°C 6 months-20°C 1 monthShipping at room temperature if less than 2 weeks.7.3 Specific end use(s)No data available.8. EXPOSURE CONTROLS/PERSONAL PROTECTION8.1 Control parametersComponents with workplace control parametersThis product contains no substances with occupational exposure limit values.8.2 Exposure controlsEngineering controlsEnsure adequate ventilation. Provide accessible safety shower and eye wash station.Personal protective equipmentEye protection Safety goggles with side-shields.Hand protection Protective gloves.Skin and body protection Impervious clothing.Respiratory protection Suitable respirator.Environmental exposure controls Keep the product away from drains, water courses or the soil. Cleanspillages in a safe way as soon as possible.9. PHYSICAL AND CHEMICAL PROPERTIES9.1 Information on basic physical and chemical propertiesAppearance Light yellow to yellow (Solid)Odor No data availableOdor threshold No data availablepH No data availableMelting/freezing point No data availableBoiling point/range No data availableFlash point No data availableEvaporation rate No data availableFlammability (solid, gas)No data availableUpper/lower flammability or explosive limits No data availableVapor pressure No data availableVapor density No data availableRelative density No data availableWater Solubility No data availablePartition coefficient No data availableAuto-ignition temperature No data availableDecomposition temperature No data availableViscosity No data availableExplosive properties No data availableOxidizing properties No data available9.2 Other safety informationNo data available.10. STABILITY AND REACTIVITY10.1 ReactivityNo data available.10.2 Chemical stabilityStable under recommended storage conditions.10.3 Possibility of hazardous reactionsNo data available.10.4 Conditions to avoidNo data available.10.5 Incompatible materialsStrong acids/alkalis, strong oxidising/reducing agents.10.6 Hazardous decomposition productsUnder fire conditions, may decompose and emit toxic fumes.Other decomposition products - no data available.11.TOXICOLOGICAL INFORMATION11.1 Information on toxicological effectsAcute toxicityClassified based on available data. For more details, see section 2Skin corrosion/irritationClassified based on available data. For more details, see section 2Serious eye damage/irritationClassified based on available data. For more details, see section 2Respiratory or skin sensitizationClassified based on available data. For more details, see section 2Germ cell mutagenicityClassified based on available data. For more details, see section 2CarcinogenicityIARC: No component of this product present at a level equal to or greater than 0.1% is identified as probable, possible or confirmed human carcinogen by IARC.ACGIH: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by ACGIH.NTP: No component of this product present at a level equal to or greater than 0.1% is identified as a anticipated or confirmed carcinogen by NTP.OSHA: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by OSHA.Reproductive toxicityClassified based on available data. For more details, see section 2Specific target organ toxicity - single exposureClassified based on available data. For more details, see section 2Specific target organ toxicity - repeated exposureClassified based on available data. For more details, see section 2Aspiration hazardClassified based on available data. For more details, see section 212. ECOLOGICAL INFORMATION12.1 ToxicityNo data available.12.2 Persistence and degradabilityNo data available.12.3 Bioaccumlative potentialNo data available.12.4 Mobility in soilNo data available.12.5 Results of PBT and vPvB assessmentPBT/vPvB assessment unavailable as chemical safety assessment not required or not conducted.12.6 Other adverse effectsNo data available.13. DISPOSAL CONSIDERATIONS13.1 Waste treatment methodsProductDispose substance in accordance with prevailing country, federal, state and local regulations.Contaminated packagingConduct recycling or disposal in accordance with prevailing country, federal, state and local regulations.14. TRANSPORT INFORMATIONDOT (US)This substance is considered to be non-hazardous for transport.IMDGThis substance is considered to be non-hazardous for transport.IATAThis substance is considered to be non-hazardous for transport.15. REGULATORY INFORMATIONSARA 302 Components:No chemicals in this material are subject to the reporting requirements of SARA Title III, Section 302.SARA 313 Components:This material does not contain any chemical components with known CAS numbers that exceed the threshold (De Minimis) reporting levels established by SARA Title III, Section 313.SARA 311/312 Hazards:No SARA Hazards.Massachusetts Right To Know Components:No components are subject to the Massachusetts Right to Know Act.Pennsylvania Right To Know Components:No components are subject to the Pennsylvania Right to Know Act.New Jersey Right To Know Components:No components are subject to the New Jersey Right to Know Act.California Prop. 65 Components:This product does not contain any chemicals known to State of California to cause cancer, birth defects, or anyother reproductive harm.16. OTHER INFORMATIONCopyright 2018 MedChemExpress. The above information is correct to the best of our present knowledge but does not purport to be all inclusive and should be used only as a guide. The product is for research use only and for experienced personnel. It must only be handled by suitably qualified experienced scientists in appropriately equipped and authorized facilities. The burden of safe use of this material rests entirely with the user. MedChemExpress disclaims all liability for any damage resulting from handling or from contact with this product.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
PHA-793887-SDS-MedChemExpress

Inhibitors, Agonists, Screening LibrariesSafety Data Sheet Revision Date:Oct.-05-2018Print Date:Oct.-05-20181. PRODUCT AND COMPANY IDENTIFICATION1.1 Product identifierProduct name :PHA-793887Catalog No. :HY-11001CAS No. :718630-59-21.2 Relevant identified uses of the substance or mixture and uses advised againstIdentified uses :Laboratory chemicals, manufacture of substances.1.3 Details of the supplier of the safety data sheetCompany:MedChemExpress USATel:609-228-6898Fax:609-228-5909E-mail:sales@1.4 Emergency telephone numberEmergency Phone #:609-228-68982. HAZARDS IDENTIFICATION2.1 Classification of the substance or mixtureGHS Classification in accordance with 29 CFR 1910 (OSHA HCS)Acute Toxicity, Oral (Category 4)2.2 GHS Label elements, including precautionary statementsPictogramSignal word WarningHazard statement(s)H302 Harmful if swallowed.Precautionary statement(s)P264 Wash {hands} thoroughly after handling.P301+312 IF SWALLOWED: Call a POISON CENTER or doctor ⁄physician if you feel unwell.P330 Rinse mouth.2.3 Other hazardsNone.3. COMPOSITION/INFORMATION ON INGREDIENTS3.1 SubstancesSynonyms:PHA 793887;PHA793887Formula:C19H31N5O2Molecular Weight:361.48CAS No. :718630-59-24. FIRST AID MEASURES4.1 Description of first aid measuresEye contactRemove any contact lenses, locate eye-wash station, and flush eyes immediately with large amounts of water. Separate eyelids with fingers to ensure adequate flushing. Promptly call a physician.Skin contactRinse skin thoroughly with large amounts of water. Remove contaminated clothing and shoes and call a physician.InhalationImmediately relocate self or casualty to fresh air. If breathing is difficult, give cardiopulmonary resuscitation (CPR). Avoid mouth-to-mouth resuscitation.IngestionWash out mouth with water; Do NOT induce vomiting; call a physician.4.2 Most important symptoms and effects, both acute and delayedThe most important known symptoms and effects are described in the labelling (see section 2.2).4.3 Indication of any immediate medical attention and special treatment neededTreat symptomatically.5. FIRE FIGHTING MEASURES5.1 Extinguishing mediaSuitable extinguishing mediaUse water spray, dry chemical, foam, and carbon dioxide fire extinguisher.5.2 Special hazards arising from the substance or mixtureDuring combustion, may emit irritant fumes.5.3 Advice for firefightersWear self-contained breathing apparatus and protective clothing.6. ACCIDENTAL RELEASE MEASURES6.1 Personal precautions, protective equipment and emergency proceduresUse full personal protective equipment. Avoid breathing vapors, mist, dust or gas. Ensure adequate ventilation. Evacuate personnel to safe areas.Refer to protective measures listed in sections 8.6.2 Environmental precautionsTry to prevent further leakage or spillage. Keep the product away from drains or water courses.6.3 Methods and materials for containment and cleaning upAbsorb solutions with finely-powdered liquid-binding material (diatomite, universal binders); Decontaminate surfaces and equipment by scrubbing with alcohol; Dispose of contaminated material according to Section 13.7. HANDLING AND STORAGE7.1 Precautions for safe handlingAvoid inhalation, contact with eyes and skin. Avoid dust and aerosol formation. Use only in areas with appropriate exhaust ventilation.7.2 Conditions for safe storage, including any incompatibilitiesKeep container tightly sealed in cool, well-ventilated area. Keep away from direct sunlight and sources of ignition.Recommended storage temperature:Powder-20°C 3 years4°C 2 yearsIn solvent-80°C 6 months-20°C 1 monthShipping at room temperature if less than 2 weeks.7.3 Specific end use(s)No data available.8. EXPOSURE CONTROLS/PERSONAL PROTECTION8.1 Control parametersComponents with workplace control parametersThis product contains no substances with occupational exposure limit values.8.2 Exposure controlsEngineering controlsEnsure adequate ventilation. Provide accessible safety shower and eye wash station.Personal protective equipmentEye protection Safety goggles with side-shields.Hand protection Protective gloves.Skin and body protection Impervious clothing.Respiratory protection Suitable respirator.Environmental exposure controls Keep the product away from drains, water courses or the soil. Cleanspillages in a safe way as soon as possible.9. PHYSICAL AND CHEMICAL PROPERTIES9.1 Information on basic physical and chemical propertiesAppearance White to yellow (Solid)Odor No data availableOdor threshold No data availablepH No data availableMelting/freezing point No data availableBoiling point/range No data availableFlash point No data availableEvaporation rate No data availableFlammability (solid, gas)No data availableUpper/lower flammability or explosive limits No data availableVapor pressure No data availableVapor density No data availableRelative density No data availableWater Solubility No data availablePartition coefficient No data availableAuto-ignition temperature No data availableDecomposition temperature No data availableViscosity No data availableExplosive properties No data availableOxidizing properties No data available9.2 Other safety informationNo data available.10. STABILITY AND REACTIVITY10.1 ReactivityNo data available.10.2 Chemical stabilityStable under recommended storage conditions.10.3 Possibility of hazardous reactionsNo data available.10.4 Conditions to avoidNo data available.10.5 Incompatible materialsStrong acids/alkalis, strong oxidising/reducing agents.10.6 Hazardous decomposition productsUnder fire conditions, may decompose and emit toxic fumes.Other decomposition products - no data available.11.TOXICOLOGICAL INFORMATION11.1 Information on toxicological effectsAcute toxicityClassified based on available data. For more details, see section 2Skin corrosion/irritationClassified based on available data. For more details, see section 2Serious eye damage/irritationClassified based on available data. For more details, see section 2Respiratory or skin sensitizationClassified based on available data. For more details, see section 2Germ cell mutagenicityClassified based on available data. For more details, see section 2CarcinogenicityIARC: No component of this product present at a level equal to or greater than 0.1% is identified as probable, possible or confirmed human carcinogen by IARC.ACGIH: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by ACGIH.NTP: No component of this product present at a level equal to or greater than 0.1% is identified as a anticipated or confirmed carcinogen by NTP.OSHA: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by OSHA.Reproductive toxicityClassified based on available data. For more details, see section 2Specific target organ toxicity - single exposureClassified based on available data. For more details, see section 2Specific target organ toxicity - repeated exposureClassified based on available data. For more details, see section 2Aspiration hazardClassified based on available data. For more details, see section 212. ECOLOGICAL INFORMATION12.1 ToxicityNo data available.12.2 Persistence and degradabilityNo data available.12.3 Bioaccumlative potentialNo data available.12.4 Mobility in soilNo data available.12.5 Results of PBT and vPvB assessmentPBT/vPvB assessment unavailable as chemical safety assessment not required or not conducted.12.6 Other adverse effectsNo data available.13. DISPOSAL CONSIDERATIONS13.1 Waste treatment methodsProductDispose substance in accordance with prevailing country, federal, state and local regulations.Contaminated packagingConduct recycling or disposal in accordance with prevailing country, federal, state and local regulations.14. TRANSPORT INFORMATIONDOT (US)This substance is considered to be non-hazardous for transport.IMDGThis substance is considered to be non-hazardous for transport.IATAThis substance is considered to be non-hazardous for transport.15. REGULATORY INFORMATIONSARA 302 Components:No chemicals in this material are subject to the reporting requirements of SARA Title III, Section 302.SARA 313 Components:This material does not contain any chemical components with known CAS numbers that exceed the threshold (De Minimis) reporting levels established by SARA Title III, Section 313.SARA 311/312 Hazards:No SARA Hazards.Massachusetts Right To Know Components:No components are subject to the Massachusetts Right to Know Act.Pennsylvania Right To Know Components:No components are subject to the Pennsylvania Right to Know Act.New Jersey Right To Know Components:No components are subject to the New Jersey Right to Know Act.California Prop. 65 Components:This product does not contain any chemicals known to State of California to cause cancer, birth defects, or anyother reproductive harm.16. OTHER INFORMATIONCopyright 2018 MedChemExpress. The above information is correct to the best of our present knowledge but does not purport to be all inclusive and should be used only as a guide. The product is for research use only and for experienced personnel. It must only be handled by suitably qualified experienced scientists in appropriately equipped and authorized facilities. The burden of safe use of this material rests entirely with the user. MedChemExpress disclaims all liability for any damage resulting from handling or from contact with this product.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Inhibitors, Agonists, Screening Libraries
Data Sheet
BIOLOGICAL ACTIVITY:
GW788388 is a potent and selective inhibitor of ALK5 with IC 50 of 18 nM, and also inhibits TGF–β type II receptor and activin type II receptor activities, without inhibiting BMP type II receptor.
IC50 & Target: IC50: 18 nM (ALK5)
In Vivo: GW788388 given orally for 5 weeks significantly reduces renal fibrosis and decreased the mRNA levels of key mediators of extracellular matrix deposition in kidneys in db/db mice [1]. GW788388 (50 mg/kg/day, p.o.) significantly attenuates systolic
dysfunction in the MI animals, together with the attenuation of the activated (phosphorylated) Smad2 (P < 0.01), α–smooth muscle actin (P < 0.001), and collagen I (P < 0.05) in the noninfarct zone of MI rats [2]. GW788388 reduces the expression of collagen IA1 by
80% at a dose of 1 mg/kg twice a day (b.i.d.). GW788388 significantly reduces the expression of collagen IA1 mRNA when administered orally at 10 mg/kg once a day (u.i.d.) in a model of puromycin aminonucleoside–induced renal fibrosis [3]. PROTOCOL (Extracted from published papers and Only for reference)
Animal Administration: GW788388 is formulated in 1% carboxymethyl cellulose solution.[2]One week postsurgery, sham–operated (N=6) and infarcted animals (N=10) are randomized to treatment with the ALK5 inhibitor GW788388 (GSK) at a dosage of 50mg/kg/day by gavage, which has been shown to significantly attenuate collagen overexpression in a rodent model of
dimethylnitrosamine–induced liver disease. Untreated rats, that is, sham–operated (N=9) and MI animals (N=15), are gavaged with vehicle (1% carboxymethyl cellulose solution). Four animals with < 25% infarct size as determined postmortem by histology are excluded from further analyses.
References:
[1]. Petersen M, et al. Oral administration of GW788388, an inhibitor of TGF–beta type I and II receptor kinases, decreases renal fibrosis. Kidney Int, 2008,73(6), 705–715.
[2]. Tan SM, et al. Targeted inhibition of activin receptor–like kinase 5 signaling attenuates cardiac dysfunction following myocardial infarction. Am J Physiol Heart Circ Physiol, 2010, 298(5), H1415–1425.
[3]. Gellibert F, et al. Discovery of 4–<4–[3–(pyridin–2–yl)–1H–pyrazol–4–yl]pyridin–2–yl>–N–(tetrahydro–2H– pyran–4–yl)benzamide (GW788388): a potent,selective, and orally active transforming growth factor–beta type I receptor inhibitor. J Med Chem. 2006, 49
Product Name:
GW788388Cat. No.:
HY-10326CAS No.:
452342-67-5Molecular Formula:
C 25H 23N 5O 2Molecular Weight:
425.48Target:
TGF–β Receptor Pathway:
TGF–beta/Smad Solubility:
DMSO: ≥ 48 mg/mL
Caution: Product has not been fully validated for medical applications. For research use only.
Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@
Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
