Ginsenoside_F1_DataSheet_MedChemExpress
INVSc1酵母感受态细胞

RC321 50ulRC321 INVSc1酵母感受态细胞50ul 50支RCC12 INVSc1酵母感受态细胞50ul 100支毕赤酵母感受态细胞试剂盒1 产品介绍本试剂盒包括酵母感受态细胞、Solution 1和Solution 2。
其中酵母感受态细胞是用于转化的细胞,需-70度低温保存;Solution 1和Solution2为转化时使用的试剂,均为无菌,需-20度保存,使用时解冻即可。
本酵母感受态细胞采用专利技术制备的感受态,有别于传统山梨醇制备的感受态细胞,并配有Solution 1和Solution 2,可直接用于热击法转化,而不需要使用电转仪和电击杯。
本方法是电击法的替代方法,也避免了LiCl方法需要现制备感受细胞的繁琐。
转化效率可以达到102-103cfu/ug线性化DNA,经本公司反复验证-80保存6个月不影响转化效率。
2 转化步骤2.1 线性化质粒片段的制备取过夜培养的质粒菌种3ml,使用质粒抽提试剂盒抽提质粒,最后溶解于50ul 的TE或水中。
将所有的质粒片段在200μl的体系中酶切线性化。
用酚氯仿试剂去除蛋白并且浓度片段至20ul体系中(保证有100μg的质粒片段)。
2.2 转化2.2.1直接加于冻结的酵母细胞中,以获得最大转化率;(注意加入至冻结的细胞中,而不是解冻的细胞中);2.2.2. 加入后迅速放入37℃水浴孵育5min,中间混合样品1~2次;2.2.3. 取出离心管,加入1.5ml Solution 1,彻底混匀;(注意可以弹或移液器轻轻混合,不可用移液器剧烈来回混合也不可用涡旋振荡器) ;北京华越洋生物2.2.4. 30℃水浴孵育1h;2.2.5. 室温下2000g离心10min,去除上清液,菌体沉淀重悬于1.5ml Solution 2中;2.2.6. 离心样品,去除上清液,轻微操作将样品重悬于0.2ml Solution 2中;2.2.7. 将所有转化液铺于选择性平板(例如MD板),于30℃孵育3~4天后,鉴定;3 注意事项1.转化步骤1中DNA要加入至冻结的细胞中,注意不可解冻细胞后再加入DNA;2.转化步骤2中混合样品是必需的;3.转化步骤3中要充分混匀,但不可剧烈混合;4.所有混合细胞均要轻柔;。
Trigonox B(滴苷但羊水)产品数据表单说明书

Product Data SheetTrigonox BDi-tert-butyl peroxideTrigonox® B is a pure peroxide in liquid form.CAS number110-05-4EINECS/ELINCS No. 203-733-6TSCA statuslisted on inventory Molecular weight 146.2Active oxygen contentperoxide10.94%SpecificationsAppearance Clear liquidAssay≥ 99.0 %ApplicationsTrigonox® B (Di-tert-butyl peroxide) can be used for the market segments: polymer production, polymer crosslinking and acrylics production with their different applications/functions. For more information please check our website and/or contact us.Half-life dataThe reactivity of an organic peroxide is usually given by its half-life (t½) at various temperatures. For Trigonox® B in chlorobenzene half-life at other temperatures can be calculated by using the equations and constants mentioned below:0.1 hr at 164°C (327°F)1 hr at 141°C (286°F)10 hr at 121°C (250°F)Formula 1kd = A·e-Ea/RTFormula 2t½ = (ln2)/kdEa153.46 kJ/moleA 4.20E+15 s-1R8.3142 J/mole·KT(273.15+°C) KThermal stabilityOrganic peroxides are thermally unstable substances which may undergo self-accelerating decomposition. The lowest temperature at which self-accelerating decomposition may occur with a substance in the packaging as used for transport is the Self-Accelerating Decomposition Temperature (SADT). The SADT is determined on the basis of the Heat Accumulation Storage Test.SADT80°C (176°F)Method The Heat Accumulation Storage Test is a recognized test method for thedetermination of the SADT of organic peroxides (see Recommendations on theTransport of Dangerous Goods, Manual of Tests and Criteria - United Nations, NewYork and Geneva).StorageDue to the relatively unstable nature of organic peroxides, a loss of quality will occur over a period of time. To minimize the loss of quality, Nouryon recommends a maximum storage temperature (Ts max. ) for each organic peroxide product.Ts Max.40°C (104°F) andTs Min.-30°C (-22°F) to prevent crystallizationNote When stored according to these recommended storage conditions, Trigonox® Bwill remain within the Nouryon specifications for a period of at least 6 months afterdelivery.Packaging and transportIn North America Trigonox® B is packed in non-returnable, five gallon polyethylene containers of 30 lb net weight and steel drums of 100 or 340 lb net weight. In other regions the standard packaging is a 30-liter HDPE can (Nourytainer®) for 20 kg peroxide. Delivery in a 200 l steel drum for 150 kg peroxide is also possible in a number of countries. Both packaging and transport meet the international regulations. For the availability of other packed quantities consult your Nouryon representative. Trigonox® B is classified as Organic peroxide type E; liquid, Division 5. 2; UN 3107.Safety and handlingKeep containers tightly closed. Store and handle Trigonox® B in a dry well-ventilated place away from sources of heat or ignition and direct sunlight. Never weigh out in the storage room. Avoid contact with reducing agents (e. g. amines), acids, alkalis and heavy metal compounds (e. g. accelerators, driers and metal soaps). Please refer to the Safety Data Sheet (SDS) for detailed information on the safe storage, use and handling of Trigonox® B. This information should be thoroughly reviewed prior to acceptance of this product. The SDS is available at /sds-search.Major decomposition productsAcetone, Methane, tert-ButanolAll information concerning this product and/or suggestions for handling and use contained herein are offered in good faith and are believed to be reliable.Nouryon, however, makes no warranty as to accuracy and/or sufficiency of such information and/or suggestions, as to the product's merchantability or fitness for any particular purpose, or that any suggested use will not infringe any patent. Nouryon does not accept any liability whatsoever arising out of the use of or reliance on this information, or out of the use or the performance of the product. Nothing contained herein shall be construed as granting or extending any license under any patent. Customer must determine for himself, by preliminary tests or otherwise, the suitability of this product for his purposes.The information contained herein supersedes all previously issued information on the subject matter covered. The customer may forward, distribute, and/or photocopy this document only if unaltered and complete, including all of its headers and footers, and should refrain from any unauthorized use. Don’t copythis document to a website.Trigonox® and Nourytainer are registered trademarks of Nouryon Functional Chemicals B.V. or affiliates in one or more territories.Contact UsPolymer Specialties Americas************************Polymer Specialties Europe, Middle East, India and Africa*************************Polymer Specialties Asia Pacific************************2022-6-30© 2022Polymer crosslinking Trigonox B。
高通量药物筛选利器——HTRF,在激酶研究(kinase)中的应用

通过直接激发使镧系元素离子产生荧光是 不容易的,因为这些离子很难吸收光子。镧系 元素必须首先与有机分子形成复合物,有机分子收集光子并通过分子内非放射过程转移到 镧系元素上。稀土元素螯合物和穴状化合物是能量收集装置的典型代表,它们收集能量并 转移到镧系元素离子上,后者则发出其特征性的长寿命的荧光。
为了能够成功应用于生物学检测中,稀土元素复合物应该具有特定的性质,包括稳定 性、较高的发射光产率,并且能够与生物分子连接。除此之外,当直接在生物溶液中反应 时,能够耐受荧光淬灭就显得尤为重要。稀土元素螯合物稳定性较差,而且有的化合物可 竞争螯合物活性基团,当与 FRET 技术结合在一起时其灵敏度也受到限制。如果稀土元素 与穴状化合物结合,许多限制因素都可去除。
一般地,在 FRET 实验中使用的供体和受体是快速荧光基团,半衰期非常短。传统 FRET 技术的限制因素是由背景荧光引起的,其来自于样品成分,包括缓冲液、蛋白质、 化合物和细胞裂解液。检测到的荧光强度必须对这些自发荧光进行校正,极大地影响了实 验灵敏度,并使数据分析变得复杂。背景荧光非常短暂(寿命为纳秒级),可以利用时间 分辨荧光方法将其去除。
图 2 :试剂盒包装
产品特点 应用单克隆抗体保证批次的一致性 在 100 多种丝氨酸/苏氨酸激酶和 60 多种酪氨酸激酶上验证过 酶用量很少 ATP 浓度没有限制,因此可以用 ATP Km 进行筛选药物 每一个底物只有一个位点可以被磷酸化 针对酪氨酸激酶有特殊的试剂可以增强底物的稳定性 整个实验少于 2 小时 反应体系可以减少到 4ul
人参皂苷治疗骨性关节炎的研究进展

特产研究163Special Wild Economic Animal and Plant ResearchDOI:10.16720/ki.tcyj.2023.093人参皂苷治疗骨性关节炎的研究进展郭校妍1,张伟东1,张扬1※(吉林大学药学院,吉林长春130021)摘要:人参在防治关节软骨损伤退变及参与体外培养软骨细胞修复关节软骨缺损中具有较好治疗前景。
人参皂苷作为人参的主要药理活性成分,在治疗骨性关节炎的进程中发挥关键作用。
人参皂苷根据不同的结构被分为不同的类型,各类型均含有多种人参皂苷单体成分,其治疗骨性关节炎的机制也各不相同。
本文对不同人参皂苷单体治疗骨性关节炎的研究进行梳理和总结,探讨其治疗骨性关节炎的潜在可能性和作用机制,为后期临床应用提供依据。
关键词:骨性关节炎;人参皂苷;信号通路中图分类号:R285文献标识码:A文章编号:1001-4721(2023)03-0163-06Research Progress of Ginsenosides in the Treatment of OsteoarthritisGUO Xiaoyan1,ZHANG Weidong1,ZHANG Yang1※(School of Pharmaceutical Sciences,Jilin University,Changchun130021,China)Abstract:Ginseng has pharmacological effects such as anti-inflammatory,antioxidant,antidepressant,anti-Alzheimer's and anti-athero-sclerosis.Current studies have found that it has good therapeutic prospects in preventing degeneration of articular cartilage damage and parti-cipating in in vitro culture of chondrocytes to repair articular cartilage defects.Ginsenosides,as the main pharmacological active component of ginseng,also play an important role in the process of treating osteoarthritis.Ginsenosides can be classified into different types because of their different structures,and each type contains a variety of ginsenoside monomer components with different mechanisms for the treatment of osteoarthritis.In this paper,we review the research progress of different ginsenoside monomers in the treatment of osteoarthritis,and ex-plore their potential possibilities and mechanisms for the treatment of osteoarthritis,so as to provide a basis for later clinical application. Key words:osteoarthritis;ginsenosides;signaling pathway骨性关节炎(Osteoarthritis,OA)是一种退行性病变,系由于增龄、肥胖、遗传、劳损、创伤、关节先天性异常和关节畸形等诸多因素引起的关节软骨退化损伤、关节边缘和软骨下骨反应性增生。
CGF的应用(脂肪和注射、抗衰)LPCGF标准操作程序

北京瑞徳喜医疗器材有限公司编号: ST/S01 LPCGF 制备标准操作程序1. 目的• 此SOP 的目的是抽取求美者血液,通过Silfradent 系统,制备LPCGF 。
2. 范围 • 此SOP 适用于利用意大利Silfradent 塞法登特高浓缩生长因子变速离心系列产品来准备LPCGF 用以作中胚层注射、穴位注射、涂抹等临床用途; • 此SOP 建议的耗材数量仅提供提取2.5ml LPCGF 使用; • 此SOP 仅适用于利用意大利Silfradent 塞法登特高浓缩生长因子变速离心系列产品以下型号和耗材:a) 变速离心机 (MF200)b) Silfradent 9ml 专用负压试管 (绿色) • 此SOP 仅提供制备程序,不包括临床使用的程序。
3. 负责人员• 负责操作的人员必须具备以下条件:1. 有资质的执业医师(主要负责人)和护士2. 接受Silfradent 经销商的有关操作培训并考核合格4. 需要准备的仪器,材料和工具1. 变速离心机 (MF200) x 12. Silfradent 9ml 专用负压试管(绿色) x 23. 21G 采血针x 14. 5ml 注射器 x 25. 70mm-80mm/9#-12#针头 x 1(以下简称长针头)6. 二通或者三通管 x 17. 试管架8. 其他医疗美容项目需要的常规工具和耗材 <适量> 9. “求美者名字“,“PPP”“CD34+”和 “αPRP”标签疗器材有限公司SN¬t^_³UœS ;疗器材有限公司5. 操作程序 5.1 采血5.1.1 启动MF200消毒模式,把两支贴好求美者名字的9ml 的绿色负压试管两两相对放入转子内,5分钟后取出备用;5.1.2 利用碘伏为求美者采血部位(一般为肘正中静脉)进行消毒,然后绑上止血带,止血带位置在进针点以上约5cm 左右;5.1.3 采血针刺入静脉血管,采血针另一端插进Silfradent 9ml 专用负压试管(绿色), 分别注满,整个过程负压试管轻拿轻放勿摇晃;5.1.4 与求美者核对名字,如有错误,马上更改,并重新核对。
difene_英文说明书

Package: Blister Pack (10 tablets per blister)Ingredients:- Active Ingredient: Ibuprofen (200 mg)- Inactive Ingredients: Magnesium stearate, cellulose, lactose monohydrate, pregelatinized starch, croscarmellose sodium, hypromellose, titanium dioxide, and iron oxide.Indications:Difene Tablets are a nonsteroidal anti-inflammatory drug (NSAID) used to relieve symptoms of various conditions, including:- Pain: such as headache, dental pain, menstrual cramps, arthritis, backache, and muscular pain.- Fever: to reduce feverish symptoms.- Inflammation: to reduce inflammation in conditions such as arthritis, ankylosing spondylitis, and tendinitis.Contraindications:Do not use Difene Tablets if you have any of the following conditions:- Hypersensitivity to ibuprofen or any other NSAID.- Severe kidney disease.- Active peptic ulcer or gastrointestinal bleeding.- Third trimester of pregnancy.- Severe liver disease.- Hemorrhagic diathesis.- Children under 12 years of age (unless directed by a physician).Warnings and Precautions:- Consult a healthcare professional before taking Difene Tablets if you have a history of stomach ulcers, gastrointestinal bleeding, heart disease, high blood pressure, liver or kidney disease, asthma, or if you are taking any other medications.- Do not exceed the recommended dose to avoid potential side effects.- Do not use Difene Tablets for more than 10 days unless directed by a healthcare professional.- Avoid alcohol consumption while taking Difene Tablets.- Use caution when driving or operating machinery as Difene Tablets may cause drowsiness or dizziness in some individuals.- Difene Tablets may interact with certain medications, including anticoagulants, corticosteroids, and diuretics. Consult a healthcare professional if you are taking any of these medications.Dosage:The recommended dosage of Difene Tablets is as follows:- Adults and children over 12 years of age: 1 tablet every 4 to 6 hours as needed, not to exceed 6 tablets in 24 hours.- Elderly patients: Adjust dosage based on renal function and healthcare professional advice.How to Use:- Swallow the tablet whole with a glass of water.- Do not chew or crush the tablet.- Take Difene Tablets with or without food.Side Effects:The following side effects may occur while taking Difene Tablets:- Gastrointestinal: Nausea, vomiting, stomach pain, heartburn, indigestion, diarrhea, constipation, and ulcers.- Hematologic: Increased risk of bleeding and bruising.- Dermatologic: Skin rash, itching, and hives.- Cardiovascular: Increased blood pressure, heart failure, and myocardial infarction.- Central nervous system: Dizziness, headache, and drowsiness.Overdose:If an overdose is suspected, contact a healthcare professional immediately. Symptoms of an overdose may include severe stomach pain, vomiting, bleeding, and kidney damage.Storage:- Store Difene Tablets at room temperature (15°C to 30°C or 59°F to 86°F).- Keep the blister pack tightly closed when not in use.- Protect from light and moisture.- Do not use after the expiration date.Manufactured by:[Manufacturing Company Name][Address][City, State, ZIP Code]Please read this leaflet carefully before taking Difene Tablets. If you have any questions or concerns, consult your healthcare professional.---Note: This is a fictional product and the information provided here is for illustrative purposes only. The actual dosage, contraindications, warnings, and side effects may vary based on the specific product andits labeling. Always consult the product's official labeling and a healthcare professional before use.。
Ginsenoside_F1_SDS_MedChemExpress

Inhibitors, Agonists, Screening LibrariesSafety Data Sheet Revision Date:May-24-2017Print Date:May-24-20171. PRODUCT AND COMPANY IDENTIFICATION1.1 Product identifierProduct name :Ginsenoside F1Catalog No. :HY-N0598CAS No. :53963-43-21.2 Relevant identified uses of the substance or mixture and uses advised againstIdentified uses :Laboratory chemicals, manufacture of substances.1.3 Details of the supplier of the safety data sheetCompany:MedChemExpress USATel:609-228-6898Fax:609-228-5909E-mail:sales@1.4 Emergency telephone numberEmergency Phone #:609-228-68982. HAZARDS IDENTIFICATION2.1 Classification of the substance or mixtureNot a hazardous substance or mixture.2.2 GHS Label elements, including precautionary statementsNot a hazardous substance or mixture.2.3 Other hazardsNone.3. COMPOSITION/INFORMATION ON INGREDIENTS3.1 SubstancesSynonyms:20(S)⁻Ginsenoside F1Formula:C36H62O9Molecular Weight:638.87CAS No. :53963-43-24. FIRST AID MEASURES4.1 Description of first aid measuresEye contactRemove any contact lenses, locate eye-wash station, and flush eyes immediately with large amounts of water. Separate eyelids with fingers to ensure adequate flushing. Promptly call a physician.Skin contactRinse skin thoroughly with large amounts of water. Remove contaminated clothing and shoes and call a physician.InhalationImmediately relocate self or casualty to fresh air. If breathing is difficult, give cardiopulmonary resuscitation (CPR). Avoid mouth-to-mouth resuscitation.IngestionWash out mouth with water; Do NOT induce vomiting; call a physician.4.2 Most important symptoms and effects, both acute and delayedThe most important known symptoms and effects are described in the labelling (see section 2.2).4.3 Indication of any immediate medical attention and special treatment neededTreat symptomatically.5. FIRE FIGHTING MEASURES5.1 Extinguishing mediaSuitable extinguishing mediaUse water spray, dry chemical, foam, and carbon dioxide fire extinguisher.5.2 Special hazards arising from the substance or mixtureDuring combustion, may emit irritant fumes.5.3 Advice for firefightersWear self-contained breathing apparatus and protective clothing.6. ACCIDENTAL RELEASE MEASURES6.1 Personal precautions, protective equipment and emergency proceduresUse full personal protective equipment. Avoid breathing vapors, mist, dust or gas. Ensure adequate ventilation. Evacuate personnel to safe areas.Refer to protective measures listed in sections 8.6.2 Environmental precautionsTry to prevent further leakage or spillage. Keep the product away from drains or water courses.6.3 Methods and materials for containment and cleaning upAbsorb solutions with finely-powdered liquid-binding material (diatomite, universal binders); Decontaminate surfaces and equipment by scrubbing with alcohol; Dispose of contaminated material according to Section 13.7. HANDLING AND STORAGE7.1 Precautions for safe handlingAvoid inhalation, contact with eyes and skin. Avoid dust and aerosol formation. Use only in areas with appropriate exhaust ventilation.7.2 Conditions for safe storage, including any incompatibilitiesKeep container tightly sealed in cool, well-ventilated area. Keep away from direct sunlight and sources of ignition.Recommended storage temperature:Powder-20°C 3 years4°C 2 yearsIn solvent-80°C 6 months-20°C 1 monthShipping at room temperature if less than 2 weeks.7.3 Specific end use(s)No data available.8. EXPOSURE CONTROLS/PERSONAL PROTECTION8.1 Control parametersComponents with workplace control parametersThis product contains no substances with occupational exposure limit values.8.2 Exposure controlsEngineering controlsEnsure adequate ventilation. Provide accessible safety shower and eye wash station.Personal protective equipmentEye protection Safety goggles with side-shields.Hand protection Protective gloves.Skin and body protection Impervious clothing.Respiratory protection Suitable respirator.Environmental exposure controls Keep the product away from drains, water courses or the soil. Cleanspillages in a safe way as soon as possible.9. PHYSICAL AND CHEMICAL PROPERTIES9.1 Information on basic physical and chemical propertiesAppearance White to off-white (Solid)Odor No data availableOdor threshold No data availablepH No data availableMelting/freezing point No data availableBoiling point/range No data availableFlash point No data availableEvaporation rate No data availableFlammability (solid, gas)No data availableUpper/lower flammability or explosive limits No data availableVapor pressure No data availableVapor density No data availableRelative density No data availableWater Solubility No data availablePartition coefficient No data availableAuto-ignition temperature No data availableDecomposition temperature No data availableViscosity No data availableExplosive properties No data availableOxidizing properties No data available9.2 Other safety informationNo data available.10. STABILITY AND REACTIVITY10.1 ReactivityNo data available.10.2 Chemical stabilityStable under recommended storage conditions.10.3 Possibility of hazardous reactionsNo data available.10.4 Conditions to avoidNo data available.10.5 Incompatible materialsStrong acids/alkalis, strong oxidising/reducing agents.10.6 Hazardous decomposition productsUnder fire conditions, may decompose and emit toxic fumes.Other decomposition products - no data available.11.TOXICOLOGICAL INFORMATION11.1 Information on toxicological effectsAcute toxicityClassified based on available data. For more details, see section 2Skin corrosion/irritationClassified based on available data. For more details, see section 2Serious eye damage/irritationClassified based on available data. For more details, see section 2Respiratory or skin sensitizationClassified based on available data. For more details, see section 2Germ cell mutagenicityClassified based on available data. For more details, see section 2CarcinogenicityIARC: No component of this product present at a level equal to or greater than 0.1% is identified as probable, possible or confirmed human carcinogen by IARC.ACGIH: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by ACGIH.NTP: No component of this product present at a level equal to or greater than 0.1% is identified as a anticipated or confirmed carcinogen by NTP.OSHA: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by OSHA.Reproductive toxicityClassified based on available data. For more details, see section 2Specific target organ toxicity - single exposureClassified based on available data. For more details, see section 2Specific target organ toxicity - repeated exposureClassified based on available data. For more details, see section 2Aspiration hazardClassified based on available data. For more details, see section 212. ECOLOGICAL INFORMATION12.1 ToxicityNo data available.12.2 Persistence and degradabilityNo data available.12.3 Bioaccumlative potentialNo data available.12.4 Mobility in soilNo data available.12.5 Results of PBT and vPvB assessmentPBT/vPvB assessment unavailable as chemical safety assessment not required or not conducted.12.6 Other adverse effectsNo data available.13. DISPOSAL CONSIDERATIONS13.1 Waste treatment methodsProductDispose substance in accordance with prevailing country, federal, state and local regulations.Contaminated packagingConduct recycling or disposal in accordance with prevailing country, federal, state and local regulations.14. TRANSPORT INFORMATIONDOT (US)This substance is considered to be non-hazardous for transport.IMDGThis substance is considered to be non-hazardous for transport.IATAThis substance is considered to be non-hazardous for transport.15. REGULATORY INFORMATIONSARA 302 Components:No chemicals in this material are subject to the reporting requirements of SARA Title III, Section 302.SARA 313 Components:This material does not contain any chemical components with known CAS numbers that exceed the threshold (De Minimis) reporting levels established by SARA Title III, Section 313.SARA 311/312 Hazards:No SARA Hazards.Massachusetts Right To Know Components:No components are subject to the Massachusetts Right to Know Act.Pennsylvania Right To Know Components:No components are subject to the Pennsylvania Right to Know Act.New Jersey Right To Know Components:No components are subject to the New Jersey Right to Know Act.California Prop. 65 Components:This product does not contain any chemicals known to State of California to cause cancer, birth defects, or anyother reproductive harm.16. OTHER INFORMATIONCopyright 2017 MedChemExpress. The above information is correct to the best of our present knowledge but does not purport to be all inclusive and should be used only as a guide. The product is for research use only and for experienced personnel. It must only be handled by suitably qualified experienced scientists in appropriately equipped and authorized facilities. The burden of safe use of this material rests entirely with the user. MedChemExpress disclaims all liability for any damage resulting from handling or from contact with this product.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
高效液相色谱法快速测定保健品中6种人参皂苷的含量

高效液相色谱法快速测定保健品中6种人参皂苷的含量居广琳(福建省产品质量检验研究院,福建福州 350002)摘 要:目的:快速高效检测非人参及其加工品为主要原料的保健品中6种人参皂苷Rb1、Rb2、Rc、Rd、Re和Rg1的含量。
方法:以75%甲醇为提取剂超声提取,采用高效液相色谱法测定。
结果:6种人参皂苷在0.1~1.0 mg·mL-1线性关系良好;人参皂苷 Rb1、Rb2、Rc、Rd、Re和Rg1在阿拉伯糖粉中的加标回收率分别为93.1%、95.3%、94.8%、94.1%、98.6%和93.9%;在力保健牛磺酸功能饮料中的加标回收率分别为90.8%、94.6%、93.0%、91.7%、98.0%和92.1%;在蜂胶软胶囊中的加标回收率分别为91.1%、95.0%、92.3%、92.7%、98.4%和92.5%。
结论:本法能快速高效提取并测定非人参及其加工制品为主要材料的保健品中6种人参皂苷的含量。
关键词:保健品;人参皂苷;液相色谱Rapid Determination of Six Ginsenosides in Health Productsby HPLCJU Guanglin(Fujian Inspection and Research Institute for Product Quality, Fuzhou 350002, China) Abstract: Objective: To quickly and efficiently detect the content of six ginsenosides Rb1, Rb2, Rc, Rd, Re, and Rg1 in health products mainly made from non ginseng and its processed products. Method: The extracts were ultrasonically extracted with 75% methanol as the extractant and determined by high performance liquid chromatography (HPLC). Result: The six ginsenosides exhibited good linear relationships within the range of 0.1 mg·mL-1 to 1.0 mg·mL-1; the recovery rates of ginsenosides Rb1, Rb2, Rc, Rd, Re, and Rg1 in arabinose powder were 93.1%, 95.3%, 94.8%, 94.1%, 98.6%, and 93.9%; the recovery rates of Lipovitan taurine functional beverages were 90.8%, 94.6%, 93.0%, 91.7%, 98.0%, and 92.1%; the recovery rates of propolis soft capsules were 91.1%, 95.0%, 92.3%, 92.7%, 98.4%, and 92.5%. Conclusion: This method can quickly and efficiently extract and determine the content of six ginsenosides in health products mainly made from non ginseng and its processed products the main materials.Keywords: health products; ginsenoside; high performance liquid chromatography人参皂苷是人参、西洋参等人参属植物中被证实具有增强体质、调节神经、延缓衰老及抗肿瘤等功效的有效成分[1-2]。
美国药典 阿司匹林 中英文对照

About 143 °C (instantaneous method).
IDENTIFICATION
First identification A, B.
Second identification B, C, D.
A.Infrared absorption spectrophotometry {2.2.24).
Reference solution (a) Dissolve 50.0 mg of salicylic acid R in the mobile phase and dilute to 50.0 ml with the mobile phase.Dilute 1.0 ml of this solution to 100.0 ml with the mobile phase.
Limits:
--any impurity: for each impurity, not more than the area of the principal peak in the chromatogramobtained with reference solution (a) (0.1 per cent);
ASSAY
In a flask with a ground-glass stopper, dissolve 1.000 g in 10 ml of ethanol (96 per cent) R. Add 50.0 ml of 0.5 M sodium hydroxide. Close the flask and allow to stand for 1 h.
Comparison acetylsalicyiic acid CRS.
B.To 0.2 g add 4 ml of dilute sodium hydroxide solution R and boil for 3 min. Cool and add 5 ml of dilute sulphuric add R
多巴丝肼片日本药品说明书英文

多巴丝肼片日本药品说明书英文Doripenem Tablets: Japanese Medication Guide (English Version)Introduction:Doripenem is a commonly used medication in Japan for the treatment of various bacterial infections. This medication guide aims to provide users with comprehensive information about Doripenem tablets, including usage, dosage, precautions, side effects, and other important details. Please read this guide carefully before using Doripenem and consult your healthcare professional if you have any questions or concerns.Product Overview:- Name: Doripenem Tablets- Active Ingredient: Doripenem- Strength: 250 mg, 500 mg- Dosage Form: Tablets- Manufacturer: [Name of Manufacturer]- Approved by: [Name of Regulatory Authority]Usage:Doripenem is indicated for the treatment of the following infections caused by susceptible bacteria:- Community-acquired pneumonia- Hospital-acquired pneumonia- Complicated urinary tract infections, including pyelonephritis- Complicated intra-abdominal infections- Septicemia (bloodstream infections)- Febrile neutropenia (fever due to low white blood cell count)Dosage and Administration:- The recommended dosage for adults is [Dosage]. Please follow the instructions provided by your healthcare professional.- Doripenem tablets should be taken [Instructions on how to take the tablets, e.g., with or without food, with water].- The duration of treatment may vary depending on the type and severity of the infection. Please consult your healthcare professional for the recommended duration.Precautions:- Before using Doripenem, inform your healthcare professional about any allergies, medical conditions, or medications you are currently taking.- It is important to complete the full course of Doripenem treatment as prescribed by your healthcare professional, even if you start feeling better.- Do not share Doripenem tablets with others or use leftover medication for future infections.- Driving or operating machinery while under the influence of Doripenem may impair your abilities. Use caution until you know how Doripenem affects you.Side Effects:Doripenem may cause side effects in some individuals. Common side effects include:- Nausea- Diarrhea- Headache- Rash- Injection site reactions (if administered intravenously)If you experience severe or persistent side effects, contact your healthcare professional immediately. They will assess the risks and benefits of continuing Doripenem treatment.Storage:- Store Doripenem tablets at room temperature (between [temperature range]) in a dry place.- Keep the medication out of reach of children and pets.- Do not use Doripenem tablets beyond the expiration date mentioned on the packaging.Additional Information:- Doripenem may interact with certain medications. Inform your healthcare professional about all the medications you are taking to avoid potential drug interactions.- In case of an overdose, seek medical attention immediately or contact your local poison control center.- If you miss a dose of Doripenem, take it as soon as you remember. However, if it is close to the time for your next dose, skip the missed dose and continue with your regular dosing schedule.Conclusion:This medication guide provides essential information about Doripenem tablets, their proper usage, dosage, precautions, and potential side effects. It is crucial to consult with your healthcare professional and follow their instructions for the safe and effective use of Doripenem. If you have any additional questions or concerns, seek medical advice for further assistance.。
蛋白酶磷酸酶抑制剂混合物(细菌抽提用, 50X)

白酶、金属蛋白酶等)和磷酸酶(如丝氨酸/苏氨酸、酪氨酸、酸性及碱性磷酸酶等)。适用于 Western Blot 和免疫共沉淀检测磷酸化蛋白质、蛋白激酶活性测定等。
使用方法: 1、蛋白酶抑制剂混合物和磷酸酶抑制剂混合物(50X),使用时分别按照 1:50 的比例加入到裂解液中,混 匀后即可使用。含有蛋白酶磷酸酶抑制剂混合物的裂解液宜现用现配,不宜配制后冻存待后续使用。 2、根据需要,0.05M 的 EDTA 也按照 1:50 的比例加入到裂解液中。
蛋白酶磷酸酶抑制剂混合物(细菌抽提ห้องสมุดไป่ตู้, 50X) 货号:P1264 规格:100T 保存:-20ºC 保存,有效期 12 个月。
产品组成:
产品名称 蛋白酶抑制剂混合物(细菌抽提用,50X)
磷酸酶抑制剂混合物(50X) 0.05M EDTA, pH 8.0
规格
2×1mL 2×1mL 2×1mL
北京索莱宝科技有限公司
保存 -20ºC 避光
-20ºC RT
产品说明: 细菌提取物中含有许多内源性的蛋白酶、磷酸酶等,容易导致提取物中的蛋白降解或去修饰,从而
影响后续的蛋白检测。因此在提取物中添加适当的蛋白酶、磷酸酶等抑制剂是防止蛋白降解和去修饰的 有效方法。
本产品用于细菌蛋白提取的蛋白酶和磷酸酶抑制剂混合物,包含了广谱的丝氨酸、半胱氨酸和酸性蛋 白酶抑制剂/氨基肽酶抑制剂,以及丝氨酸/苏氨酸、酪氨酸、酸性及碱性磷酸酶抑制剂。如用于检测金属 蛋白酶活性,则不宜添加 EDTA。以 1:50 的比例分别把蛋白酶磷酸酶抑制剂混合物(细菌抽提用, 50X)和 0.05M EDTA 加入裂解液中,即可用于细菌蛋白的提取,并有效抑制蛋白降解。
OXOID药敏纸片中英文对照
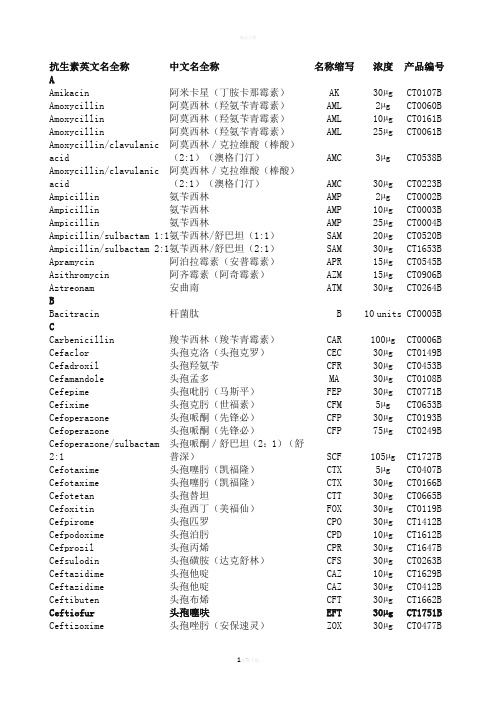
AAmikacin 阿米卡星(丁胺卡那霉素)AK 30µg CT0107B Amoxycillin 阿莫西林(羟氨苄青霉素)AML 2µg CT0060B Amoxycillin 阿莫西林(羟氨苄青霉素)AML 10µg CT0161B Amoxycillin 阿莫西林(羟氨苄青霉素)AML 25µg CT0061BAmoxycillin/clavulanic acid 阿莫西林/克拉维酸(棒酸)(2:1)(澳格门汀)AMC 3µg CT0538BAmoxycillin/clavulanic acid 阿莫西林/克拉维酸(棒酸)(2:1)(澳格门汀)AMC 30µg CT0223BAmpicillin 氨苄西林AMP 2µg CT0002B Ampicillin 氨苄西林AMP 10µg CT0003B Ampicillin 氨苄西林AMP 25µg CT0004B Ampicillin/sulbactam 1:1 氨苄西林/舒巴坦(1:1)SAM 20µg CT0520B Ampicillin/sulbactam 2:1 氨苄西林/舒巴坦(2:1)SAM 30µg CT1653B Apramycin 阿泊拉霉素(安普霉素)APR 15µg CT0545B Azithromycin 阿齐霉素(阿奇霉素)AZM 15µg CT0906B Aztreonam 安曲南ATM 30µg CT0264B BBacitracin 杆菌肽 B 10 units CT0005B CCarbenicillin 羧苄西林(羧苄青霉素)CAR 100µg CT0006B Cefaclor 头孢克洛(头孢克罗)CEC 30µg CT0149B Cefadroxil 头孢羟氨苄CFR 30µg CT0453B Cefamandole 头孢孟多MA 30µg CT0108B Cefepime 头孢吡肟(马斯平)FEP 30µg CT0771B Cefixime 头孢克肟(世福素)CFM 5µg CT0653B Cefoperazone 头孢哌酮(先锋必)CFP 30µg CT0193B Cefoperazone 头孢哌酮(先锋必)CFP 75µg CT0249BCefoperazone/sulbactam 2:1 头孢哌酮/舒巴坦(2:1)(舒普深)SCF 105µg CT1727BCefotaxime 头孢噻肟(凯福隆)CTX 5µg CT0407B Cefotaxime 头孢噻肟(凯福隆)CTX 30µg CT0166B Cefotetan 头孢替坦CTT 30µg CT0665B Cefoxitin 头孢西丁(美福仙)FOX 30µg CT0119B Cefpirome 头孢匹罗CPO 30µg CT1412B Cefpodoxime 头孢泊肟CPD 10µg CT1612B Cefprozil 头孢丙烯CPR 30µg CT1647B Cefsulodin 头孢磺胺(达克舒林)CFS 30µg CT0263B Ceftazidime 头孢他啶CAZ 10µg CT1629B Ceftazidime 头孢他啶CAZ 30µg CT0412B Ceftibuten 头孢布烯CFT 30µg CT1662B Ceftiofur 头孢噻呋EFT 30µg CT1751B Ceftizoxime 头孢唑肟(安保速灵)ZOX 30µg CT0477BCeftriaxone 头孢曲松(头孢三嗪)CRO 30µg CT0417B Cefuroxime sodium 头孢呋新钠CXM 5µg CT0406B Cefuroxime sodium 头孢呋新钠CXM 30µg CT0127B Cephalexin 头孢氨苄(头孢力新,先锋IV)CL 30µg CT0007B Cephalothin 头孢噻吩(头孢菌素,先锋I )KF 30µg CT0010B Cephazolin 头孢唑啉(先锋V)KZ 30µg CT0011B Cephradine 头孢拉定(先锋VI)CE 30µg CT0063B Chloramphenicol 氯霉素 C 10µg CT0012B Chloramphenicol 氯霉素 C 30µg CT0013B Chloramphenicol 氯霉素 C 50µg CT0014B Cinoxacin 西诺沙星CIN 100µg CT0162B Ciprofloxacin 环丙沙星(悉复欢)CIP 1µg CT0623B Ciprofloxacin 环丙沙星(悉复欢)CIP 5µg CT0425B Ciprofloxacin 环丙沙星(悉复欢)CIP 10µg CT1615B Clarithromycin 克拉霉素CLR 2µg CT1599B Clarithromycin 克拉霉素CLR 5µg CT1623B Clarithromycin 克拉霉素CLR 15µg CT0693BClindamycin 克林霉素(氯林可霉素,氯洁霉素)DA 2µg CT0064BClindamycin 克林霉素(氯林可霉素,氯洁霉素)DA 10µg CT0015BCloxacillin 氯唑西林(邻氯青霉素)OB 5µg CT0016B Colistin sulphate 多粘菌素E(硫酸粘杆菌素)CT 10µg CT0017B Colistin sulphate 多粘菌素E(硫酸粘杆菌素)CT 25µg CT0065B Colistin sulphate 多粘菌素E(硫酸粘杆菌素)CT 50µg CT0664B Compound sulphonamides 磺胺复合物S3 300µg CT0059B 抗生素英文名全称中文名全称名称缩写浓度产品编号DDoxycycline 强力霉素DO 30µg CT0018B EEnrofloxacin 恩诺沙星ENR 5µg CT0639B Ertapenem 厄他培南ETP 10µg CT1761B Erythromycin 红霉素 E 5µg CT0066B Erythromycin 红霉素 E 10µg CT0019B Erythromycin 红霉素 E 15µg CT0020B Erythromycin 红霉素 E 30µg CT0021B抗生素英文名全称中文名全称名称缩写浓度产品编号FFlorfenicol 氟苯尼考FFC 30µg CT1754BFluconazole 氟康唑FCA 25µg CT1806B Flumequine 氟甲喹UB 30µg CT0666B Fosfomycin 磷霉素FOS 50µg CT0183B 抗生素英文名全称中文名全称名称缩写浓度产品编号Framycetin 新霉素B FY 100µg CT0071B Fusidic acid 褐霉素(夫西地酸)FD 5µg CT0493B Fusidic acid 褐霉素(夫西地酸)FD 10µg CT0023B Fusidic acid 褐霉素(夫西地酸)FD 50µg CT1617B GGentamicin 庆大霉素CN 10µg CT0024B Gentamicin 庆大霉素CN 30µg CT0072B Gentamicin 庆大霉素CN 120µg CT0794B Gentamicin 庆大霉素CN 200µg CT0695B IImipenem 亚胺培南(配能)IPM 10µg CT0455B KKanamycin 卡那霉素K 5µg CT0025B Kanamycin 卡那霉素K 30µg CT0026B LLatamoxef 拉氧头孢MOX 30µg CT0302B Levofloxacin 左氧氟沙星(可乐必妥)LEV 1µg CT1586B Levofloxacin 左氧氟沙星(可乐必妥)LEV 5µg CT1587B Lincomycin 林可霉素(洁霉素)MY 2µg CT0027B Lincomycin 林可霉素(洁霉素)MY 10µg CT0123B Lincomycin 林可霉素(洁霉素)MY 15µg CT0028BLincomycin/neomycin 林可霉素(洁霉素)/新霉素LN 75µg CT1757BLincomycin/spectinomycin 林可霉素/壮观霉素LS 109µg CT1758B Linezolid 利奈唑胺LZD 10µg CT1649B Linezolid 利奈唑胺LZD 30µg CT1650B Lomefloxacin 洛美沙星LOM 10µg CT1661B MMecillinam 美西林MEL 10µg CT0096B Mecillinam 美西林MEL 25µg CT0091B Meropenem 美罗培南(美平)MEM 10µg CT0774B Metronidazole 甲硝唑(灭滴灵)MTZ 5µg CT0067B Metronidazole 甲硝唑(灭滴灵)MTZ 50µg CT0466B Mezlocillin 美洛西林MEZ 30µg CT0174B Mezlocillin 美洛西林MEZ 75µg CT0192BMinocycline 米诺环素(二甲胺四环素)MH 30µg CT0030BMoxalactam 拉氧头孢MOX 30µg CT0302B Moxifloxacin 莫西沙星MXF 1µg CT1683B Moxifloxacin 莫西沙星MXF 5µg CT1633BMupirocin 莫匹罗星MUP 5µg CT0522B Mupirocin 莫匹罗星MUP 20µg CT1826B Mupirocin 莫匹罗星MUP 200µg CT0523B NNalidixic acid 萘啶酸NA 30µg CT0031B 抗生素英文名全称中文名全称名称缩写浓度产品编号Neomycin 新霉素N 30µg CT0033BNetilmicin 奈替米星(乙基西梭霉素)NET 10µg CT0424BNetilmicin 奈替米星(乙基西梭霉素)NET 30µg CT0225BNitrofurantoin 呋喃妥因(呋喃妥英) F 50µg CT0069B Nitrofurantoin 呋喃妥因(呋喃妥英) F 100µg CT0034B Nitrofurantoin 呋喃妥因(呋喃妥英) F 200µg CT0035B Nitrofurantoin 呋喃妥因(呋喃妥英) F 300µg CT0036B Norfloxacin 诺氟沙星(氟哌酸)NOR 2µg CT0687B Norfloxacin 诺氟沙星(氟哌酸)NOR 5µg CT0668B Norfloxacin 诺氟沙星(氟哌酸)NOR 10µg CT0434B Novobiocin 新生霉素NV 5µg CT0037B Novobiocin 新生霉素NV 30µg CT0038B Nystatin 制霉菌素NS 100units CT0073B OOfloxacin 氧氟沙星(泰利必妥)OFX 5µg CT0446B Oleandomycin 竹桃霉素OL 15µg CT0039B Oxacillin 苯唑西林OX 1µg CT0159B Oxacillin 苯唑西林OX 5µg CT0040B Oxolinic acid 奥索利酸(恶喹酸)OA 2µg CT0181BOxytetracycline 土霉素(氧四环素,地霉素)OT 30µg CT0041BPPefloxacin 培氟沙星(甲氟哌酸)PEF 5µg CT0661B Penicillin G 青霉素G P 1unit CT0152B Penicillin G 青霉素G P 1.5unit CT0042B Penicillin G 青霉素G P 2units CT0088B Penicillin G 青霉素G P 5units CT0124B Penicllin G 青霉素G P 10units CT0043B Penicillin/novobiocin 青霉素/新生霉素PNV 40 CT1755B Pipemidic acid 吡哌酸PIP 20µg CT0180BPiperacillin 哌拉西林(氧哌嗪青霉素)PRL 30µg CT1619BPiperacillin 哌拉西林(氧哌嗪青霉素)PRL 75µg CT0261BPiperacillin 哌拉西林(氧哌嗪青霉素)PRL 100µg CT0199BPiperacillin/tazobactam 哌拉西林/他唑巴坦(特治星)TZP 36µg CT1616B Piperacillin/tazobactam 哌拉西林/他唑巴坦(特治星)TZP 40µg CT1628B Piperacillin/tazobactam 哌拉西林/他唑巴坦(特治星)TZP 85µg CT0720B Piperacillin/tazobactam 哌拉西林/他唑巴坦(特治星) TZP 110µg CT0725B Pirlimycin 吡利霉素 PIR 2µg CT1668B Polymyxin B多粘菌素BPB300units CT0044BQQuinupristin/dalfopristin 喹奴普汀/达福普汀 QD 15µg CT1644BR抗生素英文名全称 中文名全称 名称缩写 浓度 产品编号 Rifampicin 利福平 RD 2µg CT0078B Rifampicin 利福平 RD 5µg CT0207B Rifampicin 利福平 RD 30µg CT0104BSSpectinomycin 大观霉素(壮观霉素) SH 10µg CT0046B Spectinomycin 大观霉素(壮观霉素) SH 25µg CT0411B Spectinomycin 大观霉素(壮观霉素) SH 100µg CT0823B Spiramycin 螺旋霉素 SP 100µg CT0232B Streptomycin 链霉素 S 10µg CT0047B Streptomycin链霉素 S 25µg CT0048B Sulbactam/ampicillin 1 : 1舒巴坦/氨苄西林 SAM 20µg CT0520BSulbactam/ampicillin 1 : 2 舒巴坦/氨苄西林(优立新) SAM 30µg CT1653B Sulphafurazole 磺胺异恶唑 SF300µg CT0075B Sulphamethoxazole 磺胺甲基异恶唑(新诺明) RL25µg CT0051B Sulphamethoxazole 磺胺甲基异恶唑(新诺明) RL 100µg CT0074B Sulphamethoxazole/trimethoprim 19 : 1 磺胺甲基异恶唑(新诺明)/甲氧苄氨嘧啶 SXT 25µg CT0052B Sulphonamides compound 磺胺复合物 S3 300µg CT0059BT Teicoplanin 替考拉宁(壁霉素) TEC 30µg CT0647B Telithromycin 泰利霉素 TEL 15µg CT1714B Tetracycline 四环素 TE 10µg CT0053B Tetracycline 四环素 TE 30µg CT0054B Ticarcillin 替卡西林(羧噻吩青霉TIC75µgCT0167B素)Ticarcillin/clavulanic acid 7.5 : 1 替卡西林/克拉维酸(7.5:1) TIM 85µg CT0449B Tigecycline 替加环素 TGC 15µg CT1841B Tilmicosin 替米考星 TIL 15µg CT1756B Tobramycin 妥布霉素(托普霉素) TOB 10µg CT0056B Tobramycin 妥布霉素(托普霉素) TOB 30µg CT1618B Trimethoprim 甲氧苄氨嘧啶 W 1.25µg CT0057B Trimethoprim 甲氧苄氨嘧啶 W 2.5µg CT0070B Trimethoprim 甲氧苄氨嘧啶 W 5µg CT0076B Trimethoprim/sulphamethoxazole 1:19 甲氧苄氨嘧啶/磺胺甲基异恶唑(复方新诺明) SXT 25µg CT0052B V Vancomycin 万古霉素(稳可信) VA 5µg CT0188B Vancomycin 万古霉素(稳可信) VA 30µg CT0058B Voriconazole 优立康唑 VOR 1µg CT1807B 注释: CLSI: Clinical and Laboratory Standards Institute 美国临床实验室标准化研究所DIN: Deutsches Institut f ür Normung 德国标准化学会 BSAC: British Society for Antimicrobial Chemotherapy 英国抗生素化疗协会SRGA: Swedish Reference Group for Antibiotics 瑞典抗生素委员会 SFM: Soci ét é Fran çaise de Microbiologie 法国微生物学会注:红色为兽禽用抗生素,绿色为农作物用抗生素,其余为人用抗生素。
牛的神经生长因子(NGF)酶联免疫吸附测定试剂盒 说明书

Uscn Life Science Inc. Wuhan网址: 电话: +86 27 84259552传真: +86 27 84259551E-mail:***************牛的神经生长因子(NGF)酶联免疫吸附测定试剂盒使用说明书产品编号:E0105Bo规格:96T本试剂盒仅供体外研究使用,不用于临床诊断!预期应用本酶联免疫吸附测定试剂盒运用双抗体夹心ELISA法定量测定牛血清、血浆或其它相关生物液体中NGF含量。
本试剂盒已提供的试剂试剂名称数量96孔板(预包被) 1标准品(冻干) 2标准品稀释液 1 × 20ml检测溶液A 1 × 120μl检测溶液B 1 × 120μl检测稀释液A(2 x) 1 × 6ml检测稀释液B(2 x) 1 × 6mlTMB底物 1 × 9ml终止液 1 × 6ml浓洗涤液(30 x) 1 × 20ml96孔板覆膜 4使用说明书 1本试剂盒未提供但需自备的设备及试剂1、450±10nm滤光片的酶标仪(建议仪器使用前提前预热)2、单道和多道微量加液器及吸头3、稀释样品的EP管4、蒸馏水或去离子水5、吸水纸6、盛放洗液的容器试剂盒的储存及有效期所有试剂均按试剂瓶标签上所示保存。
请注意,收到试剂盒后请尽快将标准品、检测溶液A、检测溶液B以及96孔板保存于-20。
开封后的酶标板要密封加干燥剂后保存于-20,避免潮湿。
有效期为6个月。
实验原理将NGF抗体包被于96孔微孔板中,制成固相载体,向微孔中依次加入标准品和标本,其中的NGF与连接于固相载体上的抗体结合,洗板之后加入生物素化的NGF抗体,将未结合的生物素化抗体洗净后,加入HRP标记的亲和素,再次彻底洗涤后加入底物(TMB)显色。
TMB在过氧化物酶的催化下转化成蓝色,并在酸的作用下转化成最终的黄色。
颜色的深浅和样品中的NGF呈正相关。
CAS6902-77-8_Genipin_使用说明MedBio

20mg
≥98%
品牌
货号
中文名称
英文名称
CAS
包装ห้องสมุดไป่ตู้
纯度
MedBio
MED16150
(+)- Praeruptorin C
(+)- Praeruptorin C
20mg
≥98%
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
MED15809
Bufalin
Bufalin
465-21-4
20mg
≥98%
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
MED16425
Synephrine
Synephrine
94-07-5
500mg
≥98%
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
MED16106
Berberine Sulfate
Berberine Sulfate
633-66-9
3、同类产品列表:
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
MED16240
Vinorelbine
Vinorelbine
71486-22-1
10mM (in 1mL DMSO)
≥98%
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
MED15788
10-Gingerol
Abbkine SuperKine

SuperKine™ 增强型抗体稀释液货号: BMU103-CN规格: 100 mL/500 mL草莓时刻:除了增强型抗体稀释液,提升免疫学实验的信号还可以用多种办法实现,比如在WB 实验中选择增强型ECL 发光底物(货号:BMU101-CN ),在IF 实验中选择Dylight 或IFKine™免疫荧光专用二抗,以及优质动物血清等都是不错的选择。
扫描右侧二维码,查看更多Abbkine 产品信息。
结果展示:产品描述: SuperKine™ 增强型抗体稀释液,用于稀释抗体 货号: BMU103-CN 批号: 见产品包装外标签形态: 即用型溶液应用试验:WB ,IF ,ICC ,IHC ,ELISA保存建议: 储存4°C ,保质期1年,-20度长期保存注意事项: 无产品应用应用原理:SuperKine™ 增强型抗体稀释液(SuperKine™ Enhanced Antibody Dilution Buffer )是一种即用的,适合WB ,IF ,ICC ,IHC ,ELISA 等免疫检测实验的通用型抗体稀释液。
通过添加专业的免疫信号增强组分,以及独特开发的免疫封闭和稳定组分,本抗体稀释液可用于各种一抗或二抗的稀释和配制,增强免疫实验的信号强度,降低反应背景,以获得最佳的免疫实验效果。
本产品不含有蛋白、磷酸盐、叠氮钠或硫柳汞类防腐剂,适合于大部分类型的抗体稀释。
独特优势 机理阐述应用体验提升灵敏度 添加独有的免疫信号增强组分 更容易获得检测信号,检测低丰度蛋白效果更佳 降低背景 添加独特开发的免疫封闭组分 排除更多干扰,获得更特异的目的蛋白检测信号 提升稳定性 包含优化的抗体稳定组分 提升稀释后抗体的保存时间,并可重复多次使用 应用广泛 通用的广谱试剂可应用于WB, IF, ELISA 等免疫实验,以及多种抗体应用建议:1. 参考待稀释抗体说明书,并结合实际免疫检测实验的稀释建议,直接用本产品按照适当比例稀释一抗或二抗。
抗原修复液柠檬酸(20X) ph9.0 说明书

******************* 400-8787-807抗原修复液柠檬酸(20X) pH9.0说明书【产品货号】YS0004【产品名称】1、通用名称:抗原修复液柠檬酸(20X) ph9.02、英文名称:Antigen Retrieval buffer(20X)Citrate buffer of pH9.0 【包装规格】100mL 250mL【预期用途】细胞或组织用多聚甲醛、甲醛或其它醛类试剂固定后,会导致蛋白之间的交联(cross-link),从而遮蔽样品的抗原位点,导致免疫染色时染色信号减弱,甚至出现一些假阳性染色结果。
本抗原修复液可以有效去除醛类固定试剂导致的蛋白之间的交联,充分暴露石蜡切片等样品中的抗原表位,从而更好地改善免疫染色效果。
通常石蜡切片都需进行抗原修复处理,而冰冻切片不进行抗原修复处理。
抗原修复会大大改善石蜡切片的免疫染色效果,但对于冰冻切片的染色效果很多文献资料表明也有显著改善。
特别是当冰冻切片免疫染色效果欠佳时,可以考虑尝试进行抗原修复。
从原理上来看,无论冰冻切片还是细胞爬片等,只要是用多聚甲醛、甲醛或其它醛类试剂固定的样品,进行抗原修复都会有效去除蛋白之间的交联,充分暴露抗原表位,从而大大改善免疫染色效果。
本产品适用于石蜡切片,也可以用于冰冻切片等其它样品。
【储存条件及有效期】1、储存要求:2℃-8℃密封保存。
2、有效期:二年。
【使用方法】1. 将切片置于以下染色缸中梯度脱蜡反应:二甲苯10分钟×2次二甲苯5分钟无水乙醇 5分钟×2次95%乙醇 5分钟85%乙醇 5分钟PBS清洗3分钟×3次2. 切片脱蜡后采用本修复液进行抗原修复。
本产品可使用微波法或高温高压法修复,通常使用微波法。
可根据需求量将三级水或一级水与20X浓缩液按体积比19:1比例进行混合。
2.1.对于微波法,将切片浸泡在抗原修复液(1X)中,95-100ºC加热约20分钟(加热时间可以控制在10-30分钟内,最佳的加热时间需根据不同的样品和目的蛋白自行摸索)。
CAS53963-43-2_Ginsenoside F1_MedBio合成方法

≥98%
体内研究
给ApoE - / - 小鼠喂食高脂肪饮食,并用人参皂苷F1(50mg / kg /天)口服治疗8周。与模型组小鼠相比,人参皂甙F1处理的小鼠显着减少了病变大小[2]。
激酶实验
用过表达的BSGT1酶和F1测定糖基化能力。反应混合物含有100μL的0.5mM F1和100μL的2.5mM UDP-葡萄糖和800μL纯化的酶(终浓度为0.1mg / mL)(pH7.0)。将混合物在30℃下孵育24小时。此外,在相同条件下孵育三组对照:(1)对照1(C1)由人参皂苷F1和BSGT1组成; (2)对照2(C2)由具有UDP-葡萄糖的BSGT1组成; (3)对照3(C3)由人参皂甙F1和UDP-葡萄糖组成[1]。
≥98%
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
MED16338
3,4-Dihydroxybenzoic acid
3,4-Dihydroxybenzoic acid
99-50-3
1g
≥98%
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
MED15952
Osthole
Osthole
484-12-8
3、同类产品列表:
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
MED16240
Vinorelbine
Vinorelbine
71486-22-1
10mg
≥98%
品牌
货号
中文名称
英文名称
CAS
包装
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Inhibitors, Agonists, Screening Libraries
Data Sheet
BIOLOGICAL ACTIVITY:
Ginsenoside F1, a metabolite of ginsenoside Rg1, is reported to be antiaging and antioxidative, and to have beneficial effects on skin.IC50 value:
Target:
In vitro: Ginsenoside F1 reduced α–melanocyte–stimulating hormone–induced melanin secretion in B16F10 cell culture media by 60%.Immunofluorescence assay showed that ginsenoside F1 significantly induced dendrite retraction. Pull–down assay demonstrated that ginsenoside F1 primarily modulates the Rho family GTPases resulting in dendrite retraction [1]. Ginsenoside F1 enhanced production of interleukin 13 (IL–13) from human epidermal γδ T cells. IL–13 significantly reduced the mRNA expression and protein amount of both tyrosinase and DCT and reduced melanin synthesis activities in NHEMs, resulting in visible brightening of NHEM pellet [2].
Ginsenoside F1 significantly reduced ultraviolet–B–induced cell death and protected HaCaT cells from apoptosis caused by ultraviolet
B irradiation. Ginsenoside F1 prevented ultraviolet–B–induced cleavage of poly(ADP–ribose) polymerase in HaCaT cells [3].
In vivo:
References:
[1]. Kim JH, et al. Ginsenoside F1 attenuates hyperpigmentation in B16F10 melanoma cells by inducing dendrite retraction and activating Rho signalling. Exp Dermatol. 2015 Feb;24(2):150–2.
[2]. Han J, et al. Role of epidermal γδ T–cell–derived interleukin 13 in the skin–whitening effect of Ginsenoside F1. Exp Dermatol. 2014 Nov;23(11):860–2.
[3]. Lee EH, et al. Ginsenoside F1 protects human HaCaT keratinocytes from ultraviolet–B–induced apoptosis by maintaining constant levels of Bcl–2. J Invest Dermatol. 2003 Sep;121(3):607–13.
Product Name:
Ginsenoside F1Cat. No.:
HY-N0598CAS No.:
53963-43-2Molecular Formula:
C 36H 62O 9Molecular Weight:
638.87Target:
NF–κB Pathway:
NF–κB Solubility:
10 mM in DMSO
Caution: Product has not been fully validated for medical applications. For research use only.
Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@ Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
