AHU-377_hemicalcium_salt_SDS_MedChemExpress
高效液相色谱-质谱法测定特定用途保健药品中的褪黑激素

卷第 期 年 月
色
谱
高效液相色谱质谱法测定特定用途保健药品中的褪黑激素
陈文锐
提 要 采用
Ξ
陈永红
胡国昌
广州
国家进口食品卫生监督检验中心
联用法测定了具有改善睡眠等特定功能的保健药品中的褪黑激素 ∀ 利用质谱谱库检索
Β
和 二 极 管 阵 列 检 测 光 谱 图 鉴 定 松 果 体 素 药 品 中 的 褪 黑 激 素 以 甲 醇 水
Ù 质量浓度范围内
精密度
表
对一个样品进行
精密度测定 ν
ετηοδ ν Σ∆
次测定的结果
Ù Ù
见表 ∀
Ταβλ ε Πρεχισιον οφ τηε
作标准曲线 进样 Λ 见图 回归方程为 Ψ
测得相关系数为
Ξ ∀
测定值
平均值
Ξ
标准偏差
变异系数
Χς
图
Φιγ
为褪黑激素标准品和样品的在线紫外光谱图 在 和 和 附近各有一个吸收峰 与文献值 相符 而且样品和标准品的在 为样品组分的质谱图
线紫外光谱图完全一致 ∀ 图
杂质
褪黑激素组分的鉴定
及从谱库检索到的对照图 ∀ Ù 为分子离子峰 ζ 为主要的碎片离子峰 ∀ Ù ζ
图
Φιγ
褪黑激素标准品 α 和样品 β 的在线 Υ ς 谱图
联用技术的成熟 则使非挥发性化合 分别
物的分离和鉴定变得简单 我们将这两者结合起来对 样品中的褪黑激素组分进行确认∀图 的 和
图 褪黑激素标准品 α 和样品 β 色谱图
Φιγ 褪黑激素 Χηρο ατογ ρα σ οφ στανδαρδ α ανδ σ α ∀ ελ ατονιν λ ε β
文蛤多肽的生物提纯及抗肿瘤活性研究

文蛤多肽的生物提纯及抗肿瘤活性研究众所周知,癌症已经成为威胁人类健康的疾病之一,仅次于心血管疾病位居第二。
我们知道,传统的肿瘤治疗方法对人体有强大的副作用,寻找合理的方法和高效低毒的药物来预防和治疗癌症的发生是国内外医学界专家研究的热点内容,也是药学界的主流话题。
世界上海洋资源无比丰富,物种多样性为抗肿瘤新药的研究提供了物质基础。
最近几十年,已经有多种抗肿瘤活性物质被发现并应用于临床中,其中,文蛤用于治疗癌症已经有悠久的历史了。
在前人的基础上,本文就文蛤抗肿瘤作用进行了介绍。
我们从其体液中分离得到一种新的活性物质,并对其抗肿瘤活性进行了研究。
本课题组采用了一系列的生物分离技术,主要有硫酸铵分级沉淀、离子交换层析、疏水层析、凝胶层析等,对文蛤体液进行分离纯化,并得到了一种新的活性物质,对肿瘤具有较好的抑制作用。
SDS-PAGE凝胶电泳检测到该多肽的分子量大小为15 KDa,并将其命名为MM-15。
用BCA法检测蛋白浓度,采用MTT法检测MM-15对肿瘤细胞株(人肺癌A549、人肝癌BEL-7402、人结直肠癌HCT-116、人卵巢癌HeLa细胞、人胰腺癌Aspc-1)和正常细胞株(乳腺细胞MCF-10A和小鼠胚胎成纤维细胞NIH 3T3)的生长抑制活性。
按照上述排列顺序,MM-15对上述细胞株的IC<sub>50</sub>值依次为31.80μg/mL、47.75μg/mL、64.3μg/mL、46.00μg/mL、45.13μg/mL、149.51μg/mL和123.13μg/mL。
研究结果表明,MM-15具有广谱抗肿瘤活性,其中对人肺癌A549的抑制效果最好,而对于两种正常细胞无明显作用。
后续以人肺癌细胞A549作为研究对象,探究了MM-15的浓度及作用时间对肿瘤细胞增殖的影响。
结果表明,随着MM-15浓度的增加对A549细胞抑制作用也逐渐增强,IC<sub>50</sub>为30.30μg/mL;MM-15作用于A549两天后效果达到最佳,且药效可持续至三天以上。
嗜热栖热菌发酵液延缓衰老及促进修复功效评价研究

SUBJECT R ESE AR C H丨学科研究嗜热栖热菌发酵液延缓衰老及促进修复功效评价研究嗜热栖热菌具有促进细胞生长、延缓衰老、阻抗日光对皮肤的损害和修复皮肤的能力。
本文采用高温发酵制备嗜热栖热菌发酵液(Thermus thermophilus fermentation broth),从细胞层面对其安全性、延缓衰老、防晒和创伤修复能力进行评价。
结果表明,积分数<10%的嗜热栖热菌发酵液对人永生化表皮细胞(HAC AT)和人皮肤成纤维细胞(H SF)都是安全无毒的,并具有促进细胞增殖的作用;UVB光紫外照射损伤修复模型中,体积分数为1 %〜10 %的嗜热栖热菌发酵液均有较强的防晒能力和促进细胞生长能力,且具有延缓细胞衰老的功效;在细胞创伤修复模型中,体积分数为1 %~ 10 %的嗜热栖热菌发酵液具有不同程度的创伤修复能力。
上述结果表明嗜热栖热菌发酵液安全无毒,并具有一定的延缓衰老、防晒和创伤修复功效。
文/郑雅方颜贵卉姚雨辰章鹏坤编辑/姚丽China Cosmetic's Rev嗜热栖热菌中较为独特的“生长因子”以核酸、维生素 A、维生素B2、烟酰胺、P类胡萝卜素、谷胱甘肽、聚多氨(多聚氨基酸)等为主要成分,近年来,研究发现其还含有烟酰 胺单核苷酸(Nicotinamide Mononucleotide)等延缓衰老 的活性物质。
核酸是细胞代谢的必需物质,有助于表皮细胞 基因的营养及其损伤的修复,对皮肤进行深层滋润,使皮肤 柔软。
维生素、谷胱甘肽、多聚氨为细胞生长因子,能够恢复 和促进细胞生理机能,在慢性、非治疗伤口(如糖尿病溃疡 的伤口)修复时能够促进细胞生长,还可以减轻皮肤炎症反 应和抵抗日光的损害。
耐热活性酶使嗜热栖热菌在高温条 件下仍具备超强的D N A保护与修复能力。
利用该耐热特性,K.B.Mullis将嗜热菌中的t a q D N A聚合酶应用开发了多聚酶 链式反应,获得了 1993年的诺贝尔奖,基因工程研究也因此 技术得以飞速发展141。
低氘水对人黑色素瘤A375细胞增殖、迁移、凋亡的调控作用及其机制探讨

低氘水对人黑色素瘤A375细胞增殖、迁移、凋亡的调控作用及其机制探讨吴斯敏;林贤桂;杜歆玥;杨慧龄【期刊名称】《山东医药》【年(卷),期】2022(62)8【摘要】目的观察低氘水(DDW)对人黑色素瘤A375细胞增殖、迁移、凋亡的调控作用,并探讨相关机制。
方法A375细胞与人角质形成细胞HaCaT分别用氘体积分数为0.0025%、0.0050%、0.0100%的DDW和超纯水(氘体积分数0.0150%)配成的高糖DMEM培养基培养。
采用细胞增殖实验和平板克隆形成实验检测A375细胞和Ha⁃CaT细胞的增殖能力,采用划痕实验检测两种细胞迁移能力,DAPI 染色观察两种细胞形态变化。
采用流式细胞仪检测A375细胞凋亡和细胞周期分布情况,ATP酶测试盒检测A375细胞Na^(+)-K^(+)-ATP酶、Ca^(2+)-Mg^(2+)-ATP酶、总ATP酶活性,采用Westernblotting法检测A375细胞中的凋亡相关蛋白(Bcl-2、Bax)及Caspase通路蛋白(Caspase-3、cleaved Caspase-3、Caspase-9、cleavedCaspase-9、PARP、cleavedPARP)。
结果与超纯水处理相比,0.0100%、0.0050%、0.0025%氘体积分数的DDW处理的A375细胞增殖抑制率依次增高,克隆形成率和细胞迁移率依次降低(P均<0.05);DDW处理的A375细胞出现核染色质固缩、凝聚、核碎裂等现象,细胞核中凋亡小体增多。
DDW对HaCat细胞的增殖能力、集落形成能力、细胞迁移能力及细胞形态没有明显影响。
与超纯水处理相比,0.0100%、0.0050%、0.0025%氘体积分数的DDW处理的A375细胞凋亡率及G_(0)/G_(1)期细胞比例依次增高,G_(2)/M期细胞比例依次降低(P均<0.05)。
与超纯水处理相比,0.0100%、0.0050%、0.0025%氘体积分数的DDW处理的A375细胞Na^(+)-K^(+)-ATP酶、Ca^(2+)-Mg^(2+)-ATP酶、总ATP酶活性及Bcl-2、Caspase-9、PARP蛋白表达依次降低,Bax、cleaved Caspase-3/9、cleavedPARP蛋白表达依次增高(P均<0.05)。
AHU-377_hemicalcium_salt_NP-HPLC_15078_MedChemExpress
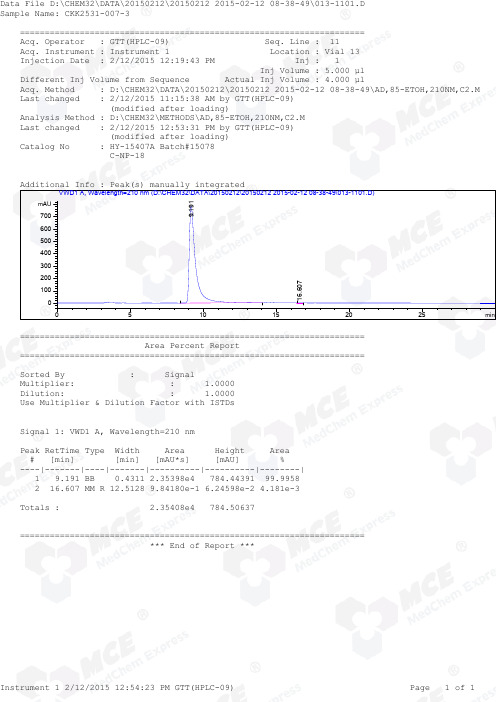
Different Inj Volume from Sequence Actual Inj Volume : 4.000 µlAcq. Method : D:\CHEM32\DATA\20150212\20150212 2015-02-12 08-38-49\AD,85-ETOH,210NM,C2.M Last changed : 2/12/2015 11:15:38 AM by GTT(HPLC-09) (modified after loading)Analysis Method : D:\CHEM32\METHODS\AD,85-ETOH,210NM,C2.M Last changed : 2/12/2015 12:53:31 PM by GTT(HPLC-09) (modified after loading)Catalog No : HY-15407A Batch#15078 C-NP-18Additional Info : Peak(s) manually integratedmin510152025mAU 010*******400500600700 VWD1 A, Wavelength=210 nm (D:\CHEM32\DATA\20150212\20150212 2015-02-12 08-38-49\013-1101.D)9.19116.607===================================================================== Area Percent Report =====================================================================Sorted By : SignalMultiplier: : 1.0000Dilution: : 1.0000Use Multiplier & Dilution Factor with ISTDsSignal 1: VWD1 A, Wavelength=210 nmPeak RetTime Type Width Area Height Area # [min] [min] [mAU*s] [mAU] %----|-------|----|-------|----------|----------|--------| 1 9.191 BB 0.4311 2.35398e4 784.44391 99.9958 2 16.607 MM R 12.5128 9.84180e-1 6.24598e-2 4.181e-3Totals : 2.35408e4 784.50637===================================================================== *** End of Report ***Different Inj Volume from Sequence Actual Inj Volume : 3.000 µlAcq. Method : D:\CHEM32\DATA\20150212\20150212 2015-02-12 08-38-49\AD,85-ETOH,210NM,C2.M Last changed : 2/12/2015 11:15:38 AM by GTT(HPLC-09) (modified after loading)Analysis Method : D:\CHEM32\METHODS\AD,85-ETOH,210NM,C2.M Last changed : 2/12/2015 12:53:31 PM by GTT(HPLC-09) (modified after loading)Catalog No : HY-15407A Batch#15078 C-NP-18Additional Info : Peak(s) manually integratedmin510152025mAU 0100200300400500600 VWD1 A, Wavelength=210 nm (D:\CHEM32\DATA\20150212\20150212 2015-02-12 08-38-49\011-0901.D)9.12516.788===================================================================== Area Percent Report =====================================================================Sorted By : SignalMultiplier: : 1.0000Dilution: : 1.0000Use Multiplier & Dilution Factor with ISTDsSignal 1: VWD1 A, Wavelength=210 nmPeak RetTime Type Width Area Height Area # [min] [min] [mAU*s] [mAU] %----|-------|----|-------|----------|----------|--------| 1 9.125 VB 0.4157 1.91409e4 659.32562 45.8477 2 16.788 MM R 1.1376 2.26081e4 473.77341 54.1523Totals : 4.17490e4 1133.09903===================================================================== *** End of Report ***Different Inj Volume from Sequence Actual Inj Volume : 4.000 µlAcq. Method : D:\CHEM32\DATA\20150212\20150212 2015-02-12 08-38-49\AD,85-ETOH,210NM,C2.M Last changed : 2/12/2015 11:15:38 AM by GTT(HPLC-09) (modified after loading)Analysis Method : D:\CHEM32\METHODS\AD,85-ETOH,210NM,C2.M Last changed : 2/12/2015 12:55:33 PM by GTT(HPLC-09) (modified after loading)Catalog No : HY-15407A Batch#15078 C-NP-18Additional Info : Peak(s) manually integratedmin510152025mAU -1000100200300400500600700 VWD1 A, Wavelength=210 nm (D:\CHEM32\DATA\20150212\20150212 2015-02-12 08-38-49\012-1001.D)===================================================================== Area Percent Report =====================================================================Sorted By : SignalMultiplier: : 1.0000Dilution: : 1.0000Use Multiplier & Dilution Factor with ISTDsNo peaks found===================================================================== *** End of Report ***。
阿尔茨海默病患者血清及尿液中外泌体的提取鉴定

阿尔茨海默病患者血清及尿液中外泌体的提取鉴定周颖;王周凡;朱焰;王爱民;陈娟;洪辉;陆微微【摘要】目的从阿尔茨海默病(AD)患者血清及尿液中提取外泌体.方法用ExoQuick试剂盒提取纯化AD患者血清及尿液中的外泌体,透射电镜观察其形态特征,Western blotting测定外泌体表面标志物CD63.结果 3例AD患者的血清样本中外泌体蛋白浓度分别为5.206、3.955、4.268 mg/mL,3例AD患者的尿液样本中外泌体蛋白浓度分别为1.811、1.348、1.133 mg/mL.透射电镜下观察患者血清及尿液中外泌体呈圆形或椭圆形的囊泡结构,直径30~150 nm,有完整脂质包膜.AD患者血清及尿液外泌体的CD63均呈阳性表达.结论采用ExoQuick试剂成功从AD患者的外周血及尿液中分离出外泌体.【期刊名称】《山东医药》【年(卷),期】2018(058)035【总页数】3页(P58-60)【关键词】外泌体;外泌体表面标志物CD63;阿尔茨海默病【作者】周颖;王周凡;朱焰;王爱民;陈娟;洪辉;陆微微【作者单位】南华大学附属长沙医院/长沙市第一医院,长沙410005;湘潭市中心医院;湘潭市中心医院;南华大学附属长沙医院/长沙市第一医院,长沙410005;南华大学附属长沙医院/长沙市第一医院,长沙410005;南华大学附属长沙医院/长沙市第一医院,长沙410005;南华大学附属长沙医院/长沙市第一医院,长沙410005【正文语种】中文【中图分类】R741阿尔茨海默病(Alzheimer′s disease,AD)是一种以进行性记忆力减退、认知功能障碍及人格改变为主要特征的中枢神经系统退行性疾病。
外泌体是一种由体内多种类型细胞主动分泌的大小均一、直径30~150 nm的脂质双分子层结构囊泡,含有丰富的蛋白质、脂类以及RNA,可在大多数体液中检测到[1]。
外泌体是一种细胞间信息传递媒介,可运载的生物活性分子种类繁多,涵盖了蛋白质、mRNA、microRNA、细胞因子、转录因子受体等多种生物活性物质,参与细胞的生理及病理过程。
优质护理在脑梗死患者护理中的应用效果评价

review[J].Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub,2012,156:186-199.[12] Lubiński J,Lener MR,Marciniak W,et al.Serum essential elements andsurvival after cancer diagnosis[J].Nutrients,2023,15(11):2611.[13] Schenk JM,Till C,Neuhouser M,et al.Differential biopsy patternsinfluence associations between multivitamin use and prostate cancer risk in the selenium and vitamin e cancer prevention trial[J].Cancer Epidemiol Biomarkers Prev,2022,31:2063-2069.[14] Crowe FL,Appleby PN,Travis RC,et al.Endogenous hormones,nutritionalbiomarkers and prostate cancer collaborative group. Circulating fatty acids and prostate cancer risk: Individual participant meta-analysis of prospective studies[J].J Natl Cancer Inst,2014,106(9):dju240.[15] Parra-SS,Ahumada D,Petermann-Rocha F,et al.Association ofmeat,vegetarian,pescatarian and fish-poultry diets with risk of 19 cancer sites and all cancer:findings from the UK Biobank prospective cohort study and meta-analysis[J].BMC Med,2022,20:79.[16] Birney E.Mendelian randomization[J].Cold Spring Harb PerspectMed,2022,12(4):a041302.[17] Davey SG,Hemani G.Mendelian randomization:genetic anchors for causalinference in epidemiological studies[J].Hum Mol Genet,2014,23:89-98.[18] Dong H,Kong X,Wang X,et al.The causal effect of dietary compositionon the risk of breast cancer: A mendelian randomization study[J].Nutrients,2023,15(11):2586.[19] Yan H,Jin X,Zhang C,et al.Associations between diet and incidencerisk of lung cancer: A Mendelian randomization study[J].Front Nutr,2023,10:1149317.[20] Yin L,Yan H,Chen K,et al.Diet-derived circulating antioxidants andrisk of digestive system tumors: A mendelian randomization study[J].Nutrients,2022,14(16):3274.[21] Brasky TM,Darke AK,Song X,et al.Plasma phospholipid fatty acidsand prostate cancer risk in the SELECT trial[J].J Natl Cancer Inst,2013,105:1132-1141.[22] Outzen M,Tj ønneland A,Christensen J,et al.Fish consumption andprostate cancer risk and mortality in a Danish cohort study[J].Eur J Cancer Prev,2018,27:355-360.[23] Fu YQ,Zheng JS,Yang B,et al.Effect of individual omega-3 fatty acids onthe risk of prostate cancer: A systematic review and dose-response meta-analysis of prospective cohort studies[J].J Epidemiol,2015,25:261-274.[24] Burgess S,Swanson SA,Labrecque JA.are mendelian randomizationinvestigations immune from bias due to reverse causation[J].Eur J Epidemiol,2021,36:253-257.[25] Guo JZ,Xiao Q,Gao S,et al.Review of Mendelian Randomization Studieson Ovarian Cancer[J].Front Oncol,2021,11:681396.[2024-01-07收稿]脑血管疾病是指脑血管病变所引起的脑功能障碍。
化妆品刺激性评价HaCaT_细胞模型的构建

◼引言2021年4月,国家药监局发布的《化妆品功效宣称评价规范》中明确要求化妆品的功效宣称必须出具有力的科学依据[1]。
然而,现阶段我国并无化妆品刺激性评价的统一标准与方法[2]。
人永生化表皮细胞系HaCaT 是1988年由Boukamp 等人使用腹部表皮细胞衍化而来的一种人正常皮肤永生化角质形成细胞[3],目前已被广泛应用于化妆品安全、保湿、抗氧化等相关研究之中[4]。
孙静秋等[5]使用重组人皮肤模型提出了一种可用于护肤类和彩妆类化妆品刺激性的评价方法,但该方法只能将化妆品区分为有无刺激性,缺乏更加细致的刺激性分级评价标准,不利于企业对产品进行改进[6-7]。
本研究拟构建一种基于HaCaT 细胞的化妆品温和刺激性分级评价方法,为建立化妆品温和刺激性评价的相关标准提供实验依据与思路。
同时,传播并行优化相关科学知识。
◼1 材料和方法1.1 材料和试剂HaCaT 细胞株购自无锡欣润生物科技有限公司,化妆品样品及原料由广州蜜妆生物科技有限公司提供。
DMEM 、胎牛血清(FBS )、0.25%胰蛋白酶(含EDTA )、噻唑蓝(MTT )、二甲基亚砜(DMSO )等均为国产分析纯试剂。
1.2 方法1�2�1 HaCaT 细胞的培养HaCaT 细胞使用含10% FBS 的DMEM 培养基培养,置于37 ℃、5% CO 2、95%相对湿度的CO 2培养箱内培作者简介:李梓充,福建人,华南农业大学生命科学学院2023级硕士研究生。
通信作者:黄九九,博士,高级实验师,研究方向为植物有效成分分析与应用、化妆品功效评价。
基金项目:华南农业大学横向技术开发合作项目“体外细胞模型构建及在化妆品功效评价中的应用”(2021440002000883)。
化妆品刺激性评价HaCaT细胞模型的构建 "李梓充1 田贵丰2 廖思艺3 刘伟1 黄九九1*(1.华南农业大学生命科学学院,广东 广州 510642; 2.广州蜜妆生物科技有限公司,广东 广州 511450; 3.广州市博藤贸易有限公司,广东 广州 511458)摘要:基于HaCaT 细胞建立化妆品刺激性分级评价细胞模型。
山茱萸水提取物对Hela细胞体外增殖的影响

限 公 司
13 3 形 态学 观察 ..
取 对 数 生长 期 的 H l ea细胞 接
种于 4 L 8孑 细胞 培 养 板 内 , 养 2 , 培 4h 实验 组 每孔 加。
科 学 院 提 供 , 1 g相 当 于 原 生 药 2 g 每 。制 成 5 0 0 mg m 的溶 液 , /l 高压 灭菌 4℃ 保存 , 生药 购 自河北 原 安 围 。人 宫颈癌 细胞 株 H l ea南河 北 农 业 大学 生命 科 学 院提供 。
剂 量 的 山茱 萸 水 提 取 物 , 别 为 每 孑 0 2 、0、0 分 L1 、O 4 8
1 材料 与方 法
13 方 法 .
13 1 细 胞培 养 ..
将 Hl ea细胞 贴壁 生 长 于 D M ME
完 全培 养 液体 系 ( 1 % 胎 牛 血 清 , 霉 素 和 链 霉 含 0 青
素各 1 0U m1 , 0 / ) 置于 3 ℃ 、 和 湿度 、% C 的 培 7 饱 5 O
1 1 材料 .
山茱 萸水 提 取 物 南河 北 农 业 大 学 生命
l a细胞 接 种 到 9 6孔 培 养 板 中 , 体 积 为 每 孔 20 终 0
1 。实 验分 3组 , 阴性 对 照组 加入 生 理盐 水 , 阳性 对 照组加 入顺 铂 ( 浓度 为 2 g m1 , 0 / ) 实验 组 加入 不 同
( D值 ) 测 定 波 长 入=5 0 t 参 考 波 长 入=6 0 O , 7 i m, 3 n 计算 抑 制率 :( m, 对照 组 O D值 ~ 验组 O 实 D值 ) /
山东省海马产业现状及问题建议

◆海洋中药古今应用研究◆山东省海马产业现状及问题建议彭晓娟1,付先军1,2,渠立群1,2,宋 鹏3,李 卉1,俞兰良4,白雪松5(1.山东中医药大学,山东 济南 250355; 2.山东中医药大学青岛中医药科学院,山东 青岛 266114;3.澳门科技大学,澳门 999078;4.威海银泽生物科技股份有限公司,山东 威海 264400;5.东营市阔海水产科技有限公司,山东 东营 257500)[摘要] 海马是我国名贵海洋动物中药材,具有极高的药用价值及经济价值。
野生海马因生态资源急剧下降等原因,面临供应限制等问题,故海马养殖产业发展迅速。
目前,山东省已经成为我国海马养殖的重要基地之一,推动山东省海马有序规范养殖,构建海马养殖、产品研发、生产和销售完整产业链,将提升山东省海马产业的社会经济效益和生态效益。
对山东省海马养殖企业分布、养殖规模、产值效益和海马产品及专利进行全面调研和分析,挖掘海马产业情况和产业发展存在的主要问题并提出相应建议,为山东省海马产业的发展决策提供参考,以推进山东省海洋生物医药的快速发展。
[关键词] 海马;海洋中药;海洋生物医药;产业;山东省;生态效益[中图分类号] R282.74 [文献标志码] A [文章编号] 1007-659X (2024)01-0001-009DOI :10.16294/ki.1007-659x.2024.01.001Current Situation and Suggestion of Haima (Hippocampus ) Industry in Shandong ProvincePENG Xiaojuan 1,FU Xianjun 1,2,QU Liqun 1,2,SONG Peng 3,LI Hui 1,YU Lanliang 4,BAI Xuesong 5(1.Shandong University of Traditional Chinese Medicine ,Jinan 250355,China ;2.Qingdao Academy of Chinese Medical Sciences ,Shandong University of Traditional Chinese Medicine ,Qingdao 266114,China ;3.Macau Univer⁃sity of Science and Technology ,Macau 999078,China ;4.Weihai Yinze Biotechnology Co.,LTD.,Weihai264400,China ;5.Dongying Kuohai Aquatic Technology Co.,LTD.,Dongying 257500,China )Abstract Haima (Hippocampus ) is one of the most valuable marine animal medicinal materials in China. It has high medicinal value and economic value. Due to the sharp decline of ecological resources and otherreasons ,the supply of wild Haima is limited ,thus the industry of Haima breeding has developed rapidly.At present ,Shandong Province has become one of the important bases of Haima breeding in China. Promoting the orderly and standardized cultivation of Haima in Shandong Province and building a complete industrial chain of Haima breeding ,productresearch and development ,production and sales will enhance the social and economic benefits and ecological benefits of the Haima industry in Shan⁃dong Province. The distribution ,scale ,output value[收稿日期] 2023-12-04 [基金项目] 山东省自然科学基金(创新发展联合基金)重点项目(编号:ZR2022LZY026);国家中医药管理局高水平中医药重点学科建设项目(编号:zyyzdxk -2023124);山东省中医药重点学科建设项目(批文号:鲁卫中医药科教字〔2022〕4号) [作者简介] 彭晓娟(1994—),女,四川自贡人,2023年级博士研究生,研究方向:海洋中药研究。
一种AHU377钙盐的合成方法[发明专利]
![一种AHU377钙盐的合成方法[发明专利]](https://img.taocdn.com/s3/m/47db6b8e25c52cc58ad6be8b.png)
专利名称:一种AHU377钙盐的合成方法专利类型:发明专利
发明人:吴法浩,李钢,高仰哲
申请号:CN201710023329.3
申请日:20170112
公开号:CN108299226A
公开日:
20180720
专利内容由知识产权出版社提供
摘要:本发明公开了一种AHU377钙盐的合成方法,将4‑溴‑D‑苯丙氨酸与氯化亚砜反应,所得的4‑溴‑D‑苯丙氨酸甲酯盐酸盐与BOC酸酐反应后,再与苯基溴化镁反应得到的 N‑叔丁基氧基羰基‑氨基‑4,4‑联苯‑R‑丙氨酸甲酯,其与硼氢化钠反应后,再与乙氧甲酰基亚乙基三苯基膦反应得
(4R)‑5‑[1,1'‑联苯]‑4‑基‑4‑[[叔丁氧羰基]氨基]‑2‑甲基‑2‑戊烯酸乙酯,再与氢氧化锂反应,加氢催化后与氯化亚砜反应得到(2R,4S)‑5‑([1,1‑联苯基)‑4‑氨基‑2‑甲基戊酸乙酯盐酸盐,再将其与氯化钙和丁二酸酐搅拌反应得到本产品。
步骤相对简单,反应条件比较温和,纯度高,收率高。
申请人:南京红杉生物科技有限公司
地址:210000 江苏省南京市栖霞区纬地路9号F6栋202室
国籍:CN
代理机构:南京众联专利代理有限公司
代理人:顾进
更多信息请下载全文后查看。
A-317491 sodium salt hydrate__MSDS_MedChemExpress

Version 2.1 Revision Date: 07/08/2013Print Date: 11/21/2013MSDS1 Composition7 Accident Release MeasureProduct Name:A-317491 sodium salt hydrateChemical Name:PROCEDURE(S) OF PERSONAL PRECAUTION(S)-Wear respirator, chemical safety goggles, rubber boots, and heavy rubber gloves.METHODS FOR CLEANING UP-Sweep up, place in a bag and hold for waste disposal. Avoid raising dust. Ventilate area andwash spill site after material pickup is complete.(S)-5-((3-phenoxybenzyl)(1,2,3,4-tetrahydronaphthalen-1-yl)carbamoyl)benzene-1,2,4-tricarboxylic acid, sodium salthydrateCAS No.:8 Accident Release MeasureAppearance:White to off-white(solid)Formula:C33H29NNaO9+9 Toxicological InformationSolubility:To the best of our knowledge, the chemical, physical, andtoxicological properties have not been thoroughly investigated.No data available.p p p p water >50 mg/ml2 Handling and Storage10 Regulary Information3 Stability and ReactivityDisposal CLASSIFICATION- Substance not yet fully tested.SAFETY PHASES- 26-36 (In case of contact with eyes, rinse immediately with plenty of water and seek medical advice. Wear suitable protective clothing.) 36/37/38 (Irritating to eyes,respiratory system and skin.)STABILITY- Stable under normal handling conditions.HANDLING- Do not breathe dust. Avoid contact with eyes,skin,and clothing.Avoid prolonged or repeated exposure.STORAGE- Please store the product under the recommended conditions in the Certificate of Analysis.11Considerations4 Hazards Identification12 Transport InformationFirst AidRID/ADR- Non-hazardous for road transport.IMDG- Non-hazardous for sea transport.IATA - Non-hazardous for air transport.As specific country, federal, state and local environmentalregulations vary and change frequently we suggest you contact a local, authorized waste disposal contractor for adequate disposal.Special indication of hazards to humans and the environment.Irritating to eyes, respiratory system and skin.MATERIALS TO AVOID- Strong oxidizing agents.REACTIVITY- May emit toxic gasses like Carbon monoxide,Carbon dioxide, Nitrogen oxides upon thermal decomposition.513 Other InformationThe above information is believed to be correct but does not purport to be all inclusive and shall be used only as a guide. The information in this document is based on the present state of our knowledge and is applicable to the product with regard to appropriate safety precautions. It does not represent any guarantee of the properties of the product. Medchemexpress LLC shall not be held liable for any damage resulting from h dli f t t ith th b d tINHALATION- If inhaled, remove to fresh air. If not breathing give, artificial respiration. If breathing is difficult, give oxygen.SKIN CONTACT- In case of contact, immediately wash skin with soap and copious amounts of water.EYE CONTACT- In case of contact, immediately flush eyes with copious amounts of water for at least 15 minutes.INGESTION- If swallowed, wash out mouth with water provided person is conscious. Call a physician.6 Fire Fighting Measureshandling or from contact with the above product.EXTINGUISHING MEDIAWater spray- Carbon dioxide, dry chemical powder, or appropriate foam.SPECIAL RISKSSpecific Hazard(s)- Emits toxic fumes under fire conditions.SPECIAL PROTECTIVE EQUIPMENT FOR FIREFIGHTERS Wear self-contained breathing apparatus and protective clothing Caution: Not fully tested. For research purposes onlyMedchemexpress LLCto prevent contact with skin and eyes.㪈㪏㪮㫀㫃㫂㫀㫅㫊㫆㫅㩷㪮㪸㫐㪃㩷㪧㫉㫀㫅㪺㪼㫋㫆㫅㪃㩷㪥㪡㩷㪇㪏㪌㪋㪇㪃㪬㪪㪘㪜㫄㪸㫀㫃㪑㩷㫀㫅㪽㫆㪗㫄㪼㪻㪺㪿㪼㫄㪼㫏㫇㫉㪼㫊㫊㪅㪺㫆㫄㩷㪮㪼㪹㪑㩷㫎㫎㫎㪅㫄㪼㪻㪺㪿㪼㫄㪼㫏㫇㫉㪼㫊㫊㪅㪺㫆㫄。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Inhibitors, Agonists, Screening LibrariesSafety Data Sheet Revision Date:May-24-2017Print Date:May-24-20171. PRODUCT AND COMPANY IDENTIFICATION1.1 Product identifierProduct name :AHU-377 (hemicalcium salt)Catalog No. :HY-15407ACAS No. :1369773-39-61.2 Relevant identified uses of the substance or mixture and uses advised againstIdentified uses :Laboratory chemicals, manufacture of substances.1.3 Details of the supplier of the safety data sheetCompany:MedChemExpress USATel:609-228-6898Fax:609-228-5909E-mail:sales@1.4 Emergency telephone numberEmergency Phone #:609-228-68982. HAZARDS IDENTIFICATION2.1 Classification of the substance or mixtureNot a hazardous substance or mixture.2.2 GHS Label elements, including precautionary statementsNot a hazardous substance or mixture.2.3 Other hazardsNone.3. COMPOSITION/INFORMATION ON INGREDIENTS3.1 SubstancesSynonyms:Sacubitril hemicalcium salt; AHU377 hemicalcium salt; AHU 377 hemicalcium saltFormula:C24H28Ca0.5NO5Molecular Weight:430.52CAS No. :1369773-39-64. FIRST AID MEASURES4.1 Description of first aid measuresEye contactRemove any contact lenses, locate eye-wash station, and flush eyes immediately with large amounts of water. Separate eyelids with fingers to ensure adequate flushing. Promptly call a physician.Skin contactRinse skin thoroughly with large amounts of water. Remove contaminated clothing and shoes and call a physician.InhalationImmediately relocate self or casualty to fresh air. If breathing is difficult, give cardiopulmonary resuscitation (CPR). Avoid mouth-to-mouth resuscitation.IngestionWash out mouth with water; Do NOT induce vomiting; call a physician.4.2 Most important symptoms and effects, both acute and delayedThe most important known symptoms and effects are described in the labelling (see section 2.2).4.3 Indication of any immediate medical attention and special treatment neededTreat symptomatically.5. FIRE FIGHTING MEASURES5.1 Extinguishing mediaSuitable extinguishing mediaUse water spray, dry chemical, foam, and carbon dioxide fire extinguisher.5.2 Special hazards arising from the substance or mixtureDuring combustion, may emit irritant fumes.5.3 Advice for firefightersWear self-contained breathing apparatus and protective clothing.6. ACCIDENTAL RELEASE MEASURES6.1 Personal precautions, protective equipment and emergency proceduresUse full personal protective equipment. Avoid breathing vapors, mist, dust or gas. Ensure adequate ventilation. Evacuate personnel to safe areas.Refer to protective measures listed in sections 8.6.2 Environmental precautionsTry to prevent further leakage or spillage. Keep the product away from drains or water courses.6.3 Methods and materials for containment and cleaning upAbsorb solutions with finely-powdered liquid-binding material (diatomite, universal binders); Decontaminate surfaces and equipment by scrubbing with alcohol; Dispose of contaminated material according to Section 13.7. HANDLING AND STORAGE7.1 Precautions for safe handlingAvoid inhalation, contact with eyes and skin. Avoid dust and aerosol formation. Use only in areas with appropriate exhaust ventilation.7.2 Conditions for safe storage, including any incompatibilitiesKeep container tightly sealed in cool, well-ventilated area. Keep away from direct sunlight and sources of ignition.Recommended storage temperature:Powder-20°C 3 years4°C 2 yearsIn solvent-80°C 6 months-20°C 1 monthShipping at room temperature if less than 2 weeks.7.3 Specific end use(s)No data available.8. EXPOSURE CONTROLS/PERSONAL PROTECTION8.1 Control parametersComponents with workplace control parametersThis product contains no substances with occupational exposure limit values.8.2 Exposure controlsEngineering controlsEnsure adequate ventilation. Provide accessible safety shower and eye wash station.Personal protective equipmentEye protection Safety goggles with side-shields.Hand protection Protective gloves.Skin and body protection Impervious clothing.Respiratory protection Suitable respirator.Environmental exposure controls Keep the product away from drains, water courses or the soil. Cleanspillages in a safe way as soon as possible.9. PHYSICAL AND CHEMICAL PROPERTIES9.1 Information on basic physical and chemical propertiesAppearance White to off-white (Solid)Odor No data availableOdor threshold No data availablepH No data availableMelting/freezing point No data availableBoiling point/range No data availableFlash point No data availableEvaporation rate No data availableFlammability (solid, gas)No data availableUpper/lower flammability or explosive limits No data availableVapor pressure No data availableVapor density No data availableRelative density No data availableWater Solubility No data availablePartition coefficient No data availableAuto-ignition temperature No data availableDecomposition temperature No data availableViscosity No data availableExplosive properties No data availableOxidizing properties No data available9.2 Other safety informationNo data available.10. STABILITY AND REACTIVITY10.1 ReactivityNo data available.10.2 Chemical stabilityStable under recommended storage conditions.10.3 Possibility of hazardous reactionsNo data available.10.4 Conditions to avoidNo data available.10.5 Incompatible materialsStrong acids/alkalis, strong oxidising/reducing agents.10.6 Hazardous decomposition productsUnder fire conditions, may decompose and emit toxic fumes.Other decomposition products - no data available.11.TOXICOLOGICAL INFORMATION11.1 Information on toxicological effectsAcute toxicityClassified based on available data. For more details, see section 2Skin corrosion/irritationClassified based on available data. For more details, see section 2Serious eye damage/irritationClassified based on available data. For more details, see section 2Respiratory or skin sensitizationClassified based on available data. For more details, see section 2Germ cell mutagenicityClassified based on available data. For more details, see section 2CarcinogenicityIARC: No component of this product present at a level equal to or greater than 0.1% is identified as probable, possible or confirmed human carcinogen by IARC.ACGIH: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by ACGIH.NTP: No component of this product present at a level equal to or greater than 0.1% is identified as a anticipated or confirmed carcinogen by NTP.OSHA: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by OSHA.Reproductive toxicityClassified based on available data. For more details, see section 2Specific target organ toxicity - single exposureClassified based on available data. For more details, see section 2Specific target organ toxicity - repeated exposureClassified based on available data. For more details, see section 2Aspiration hazardClassified based on available data. For more details, see section 212. ECOLOGICAL INFORMATION12.1 ToxicityNo data available.12.2 Persistence and degradabilityNo data available.12.3 Bioaccumlative potentialNo data available.12.4 Mobility in soilNo data available.12.5 Results of PBT and vPvB assessmentPBT/vPvB assessment unavailable as chemical safety assessment not required or not conducted.12.6 Other adverse effectsNo data available.13. DISPOSAL CONSIDERATIONS13.1 Waste treatment methodsProductDispose substance in accordance with prevailing country, federal, state and local regulations.Contaminated packagingConduct recycling or disposal in accordance with prevailing country, federal, state and local regulations.14. TRANSPORT INFORMATIONDOT (US)This substance is considered to be non-hazardous for transport.IMDGThis substance is considered to be non-hazardous for transport.IATAThis substance is considered to be non-hazardous for transport.15. REGULATORY INFORMATIONSARA 302 Components:No chemicals in this material are subject to the reporting requirements of SARA Title III, Section 302.SARA 313 Components:This material does not contain any chemical components with known CAS numbers that exceed the threshold (De Minimis) reporting levels established by SARA Title III, Section 313.SARA 311/312 Hazards:No SARA Hazards.Massachusetts Right To Know Components:No components are subject to the Massachusetts Right to Know Act.Pennsylvania Right To Know Components:No components are subject to the Pennsylvania Right to Know Act.New Jersey Right To Know Components:No components are subject to the New Jersey Right to Know Act.California Prop. 65 Components:This product does not contain any chemicals known to State of California to cause cancer, birth defects, or anyother reproductive harm.16. OTHER INFORMATIONCopyright 2017 MedChemExpress. The above information is correct to the best of our present knowledge but does not purport to be all inclusive and should be used only as a guide. The product is for research use only and for experienced personnel. It must only be handled by suitably qualified experienced scientists in appropriately equipped and authorized facilities. The burden of safe use of this material rests entirely with the user. MedChemExpress disclaims all liability for any damage resulting from handling or from contact with this product.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
