SR3335_LCMS_07539_MedChemExpress
实验室常用耗材、试剂订购电话

序号试剂/耗材公司联系人电话1枪头、离心管、一次性手套(国产)海门殷伟建137709976642玻璃瓶、烧杯、三角瓶农科院内佘凯158********卓越(农科院内)陈道荣133********南京寿德(卫岗)84397881枪头、离心管(进口)琼脂糖、酵母粉、二抗、NC 膜、蛋白Marker 、Trizol 、质粒提取(康为)、感受态(Trans )、Taq 酶、dNTP 、反转录酶(Fermentas)圣比奥尤宝明实验室常用耗材、试剂订购电话常规无机、有机试剂;培养皿、洗瓶、烧杯;琼脂条、橡皮筋、脱脂棉、纱布等150********345感受态、pEASY-T3载体百斯凯吕群香139138619466TAKARA 载体、酶、DNA Marker 、随机引物等皓嘉薛红梅139139872447引物合成、PCR管上海生工南京办83434586DNA Marker (东盛)南京华普张迎来158********DNA Marker (生兴)生兴生物400-004-88019Trizol (Invitrogen )上海普飞1865185505710反转录酶(Promega)徐乐意139********陆(姓)158********生兴生物400-004-8801;136********麦生物李端阳150********上海捷瑞王宁乐150********汪伟158********鲁威137********南京金斯瑞杨波158********上海生工南京办8343458614荧光定量PCR (Bio-rad )Bio-rad 崔佩茹150********质粒提取、凝胶回收(Axygen )、RNAse 抑制剂RNAse 抑制剂DNA测序811121315植物激素、HPLC 级化学试剂、抗体等Sigma 中国800-819-3336尧化门花卉市场1300257742317MS 培养基一基生化-南京江宁158********18组培瓶、枪状镊上海稼丰园艺用品021-6453199619液氮农科院内1381408215520X-Gluc 南京生兴生物400-004-880121bluepard 光照培养箱上海一恒021-********; 56632802营养土、花肥、蛭石、盆钵16。
T0070907_LCMS_04465_MedChemExpress
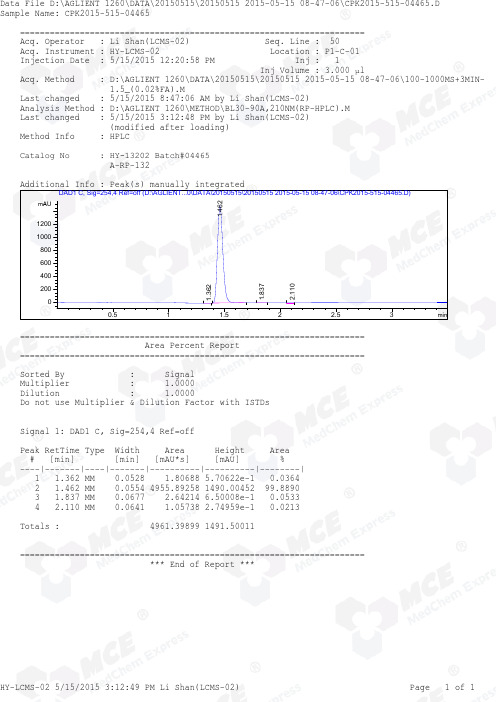
=====================================================================Acq. Operator : Li Shan(LCMS-02) Seq. Line : 50Acq. Instrument : HY-LCMS-02 Location : P1-C-01Injection Date : 5/15/2015 12:20:58 PM Inj : 1Inj Volume : 3.000 µlAcq. Method : D:\AGLIENT 1260\DATA\20150515\20150515 2015-05-15 08-47-06\100-1000MS+3MIN-1.5_(0.02%FA).MLast changed : 5/15/2015 8:47:06 AM by Li Shan(LCMS-02)Analysis Method : D:\AGLIENT 1260\METHOD\BL30-90A,210NM(RP-HPLC).MLast changed : 5/15/2015 3:12:48 PM by Li Shan(LCMS-02)(modified after loading)M ethod Info : HPLC Catalog No : HY-13202 Batch#04465 A-RP-132Additional Info : Peak(s) manually integratedmin0.51 1.52 2.53mAU20040060080010001200DAD1 C, Sig=254,4 Ref=off (D:\AGLIENT...0\DATA\20150515\20150515 2015-05-15 08-47-06\CPK2015-515-04465.D)1.362 1.462 1.8372.110=====================================================================Area Percent Report=====================================================================Sorted By : SignalMultiplier : 1.0000Dilution : 1.0000Do not use Multiplier & Dilution Factor with ISTDsSignal 1: DAD1 C, Sig=254,4 Ref=offPeak RetTime Type Width Area Height Area# [min] [min] [mAU*s] [mAU] %----|-------|----|-------|----------|----------|--------|1 1.362 MM 0.0528 1.80688 5.70622e-1 0.03642 1.462 MM 0.0554 4955.89258 1490.00452 99.88903 1.837 MM 0.0677 2.64214 6.50008e-1 0.05334 2.110 MM 0.0641 1.05738 2.74959e-1 0.0213Totals : 4961.39899 1491.50011=====================================================================*** End of Report ***===========================================================Acq. Operator : Li Shan(LCMS-02) Seq. Line : 50Acq. Instrument : HY-LCMS-02 Location : P1-C-01Injection Date : 5/15/2015 12:20:58 PM Inj : 1Inj Volume : 3.000 µlAcq. Method : D:\AGLIENT 1260\DATA\20150515\20150515 2015-05-15 08-47-06\100-1000MS+3MIN-1.5_(0.02%FA).MLast changed : 5/15/2015 8:47:06 AM by Li Shan(LCMS-02)Analysis Method : D:\AGLIENT 1260\METHOD\BL30-90A,210NM(RP-HPLC).MLast changed : 5/15/2015 3:14:15 PM by Li Shan(LCMS-02)(modified after loading)M ethod Info : HPLC Catalog No : HY-13202 Batch#04465 A-RP-132Additional Info : Peak(s) manually integratedmin 0.51 1.52 2.530100000200000300000400000500000600000700000800000MSD1 TIC, MS File (D:\AGLIENT 1260\DATA\20150515\20150515 2015-05-15 08-47-06\CPK2015-515-04465.D) ES-API, Pos, Sc1.466MS Signal: MSD1 TIC, MS File, ES-API, Pos, Scan, Frag: 50 Spectra averaged over upper half of peaks. Noise Cutoff: 1000 counts. Reportable Ion Abundance: > 10%.Retention Mol. WeightTime (MS) MS Area or Ion1.466 4473754 280.00 I279.00 I278.00 Im/z 100200300400500600020406080100*MSD1 SPC, time=1.434:1.507 of D:\AGLIENT 1260\DATA\20150515\20150515 2015-05-15 08-47-06\CPK2015-515-04465.D ES-API Max: 432781279.0 278.0 *** End of Report ***。
Calcipotriol_SDS_MedChemExpress
Inhibitors, Agonists, Screening LibrariesSafety Data Sheet Revision Date:Sep.-13-2017Print Date:Sep.-13-20171. PRODUCT AND COMPANY IDENTIFICATION1.1 Product identifierProduct name :CalcipotriolCatalog No. :HY-10001CAS No. :112965-21-61.2 Relevant identified uses of the substance or mixture and uses advised againstIdentified uses :Laboratory chemicals, manufacture of substances.1.3 Details of the supplier of the safety data sheetCompany:MedChemExpress USATel:609-228-6898Fax:609-228-5909E-mail:sales@1.4 Emergency telephone numberEmergency Phone #:609-228-68982. HAZARDS IDENTIFICATION2.1 Classification of the substance or mixtureNot a hazardous substance or mixture.2.2 GHS Label elements, including precautionary statementsNot a hazardous substance or mixture.2.3 Other hazardsNone.3. COMPOSITION/INFORMATION ON INGREDIENTS3.1 SubstancesSynonyms:MC 903; CalcipotrieneFormula:C27H40O3Molecular Weight:412.60CAS No. :112965-21-64. FIRST AID MEASURES4.1 Description of first aid measuresEye contactRemove any contact lenses, locate eye-wash station, and flush eyes immediately with large amounts of water. Separate eyelids with fingers to ensure adequate flushing. Promptly call a physician.Skin contactRinse skin thoroughly with large amounts of water. Remove contaminated clothing and shoes and call a physician.InhalationImmediately relocate self or casualty to fresh air. If breathing is difficult, give cardiopulmonary resuscitation (CPR). Avoid mouth-to-mouth resuscitation.IngestionWash out mouth with water; Do NOT induce vomiting; call a physician.4.2 Most important symptoms and effects, both acute and delayedThe most important known symptoms and effects are described in the labelling (see section 2.2).4.3 Indication of any immediate medical attention and special treatment neededTreat symptomatically.5. FIRE FIGHTING MEASURES5.1 Extinguishing mediaSuitable extinguishing mediaUse water spray, dry chemical, foam, and carbon dioxide fire extinguisher.5.2 Special hazards arising from the substance or mixtureDuring combustion, may emit irritant fumes.5.3 Advice for firefightersWear self-contained breathing apparatus and protective clothing.6. ACCIDENTAL RELEASE MEASURES6.1 Personal precautions, protective equipment and emergency proceduresUse full personal protective equipment. Avoid breathing vapors, mist, dust or gas. Ensure adequate ventilation. Evacuate personnel to safe areas.Refer to protective measures listed in sections 8.6.2 Environmental precautionsTry to prevent further leakage or spillage. Keep the product away from drains or water courses.6.3 Methods and materials for containment and cleaning upAbsorb solutions with finely-powdered liquid-binding material (diatomite, universal binders); Decontaminate surfaces and equipment by scrubbing with alcohol; Dispose of contaminated material according to Section 13.7. HANDLING AND STORAGE7.1 Precautions for safe handlingAvoid inhalation, contact with eyes and skin. Avoid dust and aerosol formation. Use only in areas with appropriate exhaust ventilation.7.2 Conditions for safe storage, including any incompatibilitiesKeep container tightly sealed in cool, well-ventilated area. Keep away from direct sunlight and sources of ignition.Recommended storage temperature: 4°C, protect from light, stored under nitrogenShipping at room temperature if less than 2 weeks.7.3 Specific end use(s)No data available.8. EXPOSURE CONTROLS/PERSONAL PROTECTION8.1 Control parametersComponents with workplace control parametersThis product contains no substances with occupational exposure limit values.8.2 Exposure controlsEngineering controlsEnsure adequate ventilation. Provide accessible safety shower and eye wash station.Personal protective equipmentEye protection Safety goggles with side-shields.Hand protection Protective gloves.Skin and body protection Impervious clothing.Respiratory protection Suitable respirator.Environmental exposure controls Keep the product away from drains, water courses or the soil. Cleanspillages in a safe way as soon as possible.9. PHYSICAL AND CHEMICAL PROPERTIES9.1 Information on basic physical and chemical propertiesAppearance White to off-white (Solid)Odor No data availableOdor threshold No data availablepH No data availableMelting/freezing point No data availableBoiling point/range No data availableFlash point No data availableEvaporation rate No data availableFlammability (solid, gas)No data availableUpper/lower flammability or explosive limits No data availableVapor pressure No data availableVapor density No data availableRelative density No data availableWater Solubility No data availablePartition coefficient No data availableAuto-ignition temperature No data availableDecomposition temperature No data availableViscosity No data availableExplosive properties No data availableOxidizing properties No data available9.2 Other safety informationNo data available.10. STABILITY AND REACTIVITY10.1 ReactivityNo data available.10.2 Chemical stabilityStable under recommended storage conditions.10.3 Possibility of hazardous reactionsNo data available.10.4 Conditions to avoidNo data available.10.5 Incompatible materialsStrong acids/alkalis, strong oxidising/reducing agents.10.6 Hazardous decomposition productsUnder fire conditions, may decompose and emit toxic fumes.Other decomposition products - no data available.11.TOXICOLOGICAL INFORMATION11.1 Information on toxicological effectsAcute toxicityClassified based on available data. For more details, see section 2Skin corrosion/irritationClassified based on available data. For more details, see section 2Serious eye damage/irritationClassified based on available data. For more details, see section 2Respiratory or skin sensitizationClassified based on available data. For more details, see section 2Germ cell mutagenicityClassified based on available data. For more details, see section 2CarcinogenicityIARC: No component of this product present at a level equal to or greater than 0.1% is identified as probable, possible or confirmed human carcinogen by IARC.ACGIH: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by ACGIH.NTP: No component of this product present at a level equal to or greater than 0.1% is identified as a anticipated or confirmed carcinogen by NTP.OSHA: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by OSHA.Reproductive toxicityClassified based on available data. For more details, see section 2Specific target organ toxicity - single exposureClassified based on available data. For more details, see section 2Specific target organ toxicity - repeated exposureClassified based on available data. For more details, see section 2Aspiration hazardClassified based on available data. For more details, see section 212. ECOLOGICAL INFORMATION12.1 ToxicityNo data available.12.2 Persistence and degradabilityNo data available.12.3 Bioaccumlative potentialNo data available.12.4 Mobility in soilNo data available.12.5 Results of PBT and vPvB assessmentPBT/vPvB assessment unavailable as chemical safety assessment not required or not conducted.12.6 Other adverse effectsNo data available.13. DISPOSAL CONSIDERATIONS13.1 Waste treatment methodsProductDispose substance in accordance with prevailing country, federal, state and local regulations.Contaminated packagingConduct recycling or disposal in accordance with prevailing country, federal, state and local regulations.14. TRANSPORT INFORMATIONDOT (US)This substance is considered to be non-hazardous for transport.IMDGThis substance is considered to be non-hazardous for transport.IATAThis substance is considered to be non-hazardous for transport.15. REGULATORY INFORMATIONSARA 302 Components:No chemicals in this material are subject to the reporting requirements of SARA Title III, Section 302.SARA 313 Components:This material does not contain any chemical components with known CAS numbers that exceed the threshold (De Minimis) reporting levels established by SARA Title III, Section 313.SARA 311/312 Hazards:No SARA Hazards.Massachusetts Right To Know Components:No components are subject to the Massachusetts Right to Know Act.Pennsylvania Right To Know Components:No components are subject to the Pennsylvania Right to Know Act.New Jersey Right To Know Components:No components are subject to the New Jersey Right to Know Act.California Prop. 65 Components:This product does not contain any chemicals known to State of California to cause cancer, birth defects, or anyother reproductive harm.16. OTHER INFORMATIONCopyright 2017 MedChemExpress. The above information is correct to the best of our present knowledge but does not purport to be all inclusive and should be used only as a guide. The product is for research use only and for experienced personnel. It must only be handled by suitably qualified experienced scientists in appropriately equipped and authorized facilities. The burden of safe use of this material rests entirely with the user. MedChemExpress disclaims all liability for any damage resulting from handling or from contact with this product.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
法瑞西单抗结构式

法瑞西单抗结构式法瑞西单抗是一种被广泛用于治疗多种恶性肿瘤的免疫调节药物。
它是一种被称为单克隆抗体的生物制剂,具有特异的抗肿瘤效果。
下面我们将详细介绍法瑞西单抗的结构式,并讨论其在临床上的应用及研究进展。
法瑞西单抗的化学结构是由一个特定的抗体蛋白分子构成,该抗体蛋白分子是通过重组DNA技术人工合成的。
它的结构式主要包括抗体的变量区和恒定区。
抗体的变量区是由抗原结合部位(Fab)和抗体可变区(VH和VL)组成。
抗体可变区存在于抗体的两个轻链和两个重链之间,它是由高度变异的氨基酸序列组成,通过这些变异性序列,抗体能够识别和结合不同的抗原。
这使得法瑞西单抗能够特异性地结合到肿瘤细胞上,从而发挥抗肿瘤作用。
抗体的恒定区(Fc)是抗体分子的结构上一个高度保守的部分,它通过与细胞表面的Fc受体相互作用,介导了抗体免疫效应,包括抗原结合后的细胞毒性作用和对肿瘤细胞的免疫调节作用。
法瑞西单抗的Fc区具有更强的细胞毒性和免疫调节效应,这使得它在治疗肿瘤时更加有效。
在临床上,法瑞西单抗主要被用于治疗乳腺癌、结直肠癌、肾细胞癌等多种恶性肿瘤。
它通常与化疗药物或放疗联合应用,能够显著提高肿瘤患者的治疗效果,延长生存期。
法瑞西单抗也被用于预防器官移植排斥反应,并在自身免疫性疾病的治疗中取得了一定的成效。
除了在临床上的应用,法瑞西单抗在科研领域也有着广泛的应用。
科研人员正在不断尝试利用法瑞西单抗的特异结构和作用机制,开发新的肿瘤治疗方法。
一些研究表明,法瑞西单抗可以通过激活免疫细胞,增强宿主的免疫应答,从而加强对肿瘤的抗击能力。
还有研究利用法瑞西单抗来开发靶向药物输送系统,将药物精确地传递到肿瘤部位,减少对正常细胞的伤害。
在未来,法瑞西单抗有望成为肿瘤治疗的重要利器。
随着对其结构和功能的进一步探索,相信会有更多的临床应用和治疗方法得以开发,从而更好地服务于肿瘤患者的治疗需求。
LCMS检测西他沙星原料中基因毒性杂质的含量

LC-MS检测西他沙星原料中基因毒性杂质的含量石莹1宋雪洁3李浩冬2路显锋2*1药物研究院分析所,扬子江药业集团,泰州2253212药物制剂新技术国家重点实验室,扬子江药业集团,泰州2253213质量管理部,扬子江药业集团,泰州225321摘要建立了LC-MS 法测定西他沙星中基因毒性杂质对甲苯磺酸甲酯和对甲苯磺酸乙酯含量的方法。
方法:采用Agilent Poroshell 120 EC-C18色谱柱;流动相为纯水(0.1%甲酸):甲醇(V/V)=60:40;稀释剂为乙腈(0.1%甲酸):纯水(V/V)=50:10;柱温为40℃;进样体积为5µl;流速为0.4ml/min;采用正离子模式进行扫描。
对甲苯磺酸甲酯测定浓度在0.76ng/ml~15.27ng/ml范围内,线性关系良好;对甲苯磺酸乙酯测定浓度在0.75ng/ml~15.01ng/ml范围内,线性关系良好。
对甲苯磺酸甲酯的定量限为0.0038ng;对甲苯磺酸乙酯的定量限为0.0038ng。
杂质回收率在限度浓度80%、100%和160%三个浓度水平均在90~110%之间,该方法准确度良好。
该方法适用于西他沙星原料中对甲苯磺酸甲酯和对甲苯磺酸乙酯的检测。
西他沙星(sitafloxacin)是日本第一制药有限公司继左氧氟沙星后开发出的一种强力广谱新氟喹诺酮类抗菌剂,该药对革兰氏阳性球菌,革兰氏阴性菌以及厌氧菌的抗菌活性是左氧氟沙星的4~32倍,同时对肺炎球菌DNA 促旋酶和拓扑同功酶有双重抑制作用。
临床表现有极广的抗菌谱,特别是对呼吸道的病菌有极强的抗菌活性。
因西他沙星的一个起始物料为对甲苯磺酸盐,在后续反应中对甲苯磺酸若有残留,可能会与溶剂甲醇、乙醇反应生成具有基因毒性的杂质—对甲苯磺酸甲酯和对甲苯磺酸乙酯,故采用LC-MS法对产品中的对甲苯磺酸甲酯/乙酯进行控制。
1、实验部分1.1仪器与试药Agilent 1200液相色谱仪(美国安捷伦公司);Agilent 6460三重串联四极杆质谱仪(美国安捷伦公司);XP205型电子天平(瑞士梅特勒托利多公司)。
基于miR-139

郭瑾,王梓仪,张倩,孟骊冲,胡志希*湖南中医药大学,湖南长沙410208〔收稿日期〕2023-07-12〔基金项目〕国家自然科学基金项目(82274412);广东省重点领域研发项目(2020B1111100001);湖南省教育厅项目(21A0230,21B0361)。
〔通信作者〕*胡志希,男,医学博士,教授,博士研究生导师,E-mail:****************。
〔摘要〕目的探讨参附注射液对盐酸异丙肾上腺素(isoprenaline hydrochloride ,ISO )诱导的慢性心力衰竭(chronic heart fail⁃ure ,CHF )大鼠心肌纤维化的作用与机制。
方法采用随机数字表法将45只SD 大鼠随机分为正常组9只,造模组36只,采用ISO 背部皮下多点注射14天制备CHF 大鼠模型,再正常饲养14d 通过模型验证和评价,期间死亡大鼠5只,未成模大鼠4只。
将造模成功的27只大鼠按随机数字表法分为3组:模型组(腹腔注射6mL ·kg -1氯化钠注射液+灌胃10mL ·kg -1蒸馏水)、参附注射液组(腹腔注射6mL ·kg -1参附注射液+灌胃10mL ·kg -1蒸馏水)、卡托普利组(腹腔注射6mL ·kg -1生理盐水+灌胃10mL ·kg -1蒸馏水,蒸馏水含8.8mg ·kg -1卡托普利,相当于临床等效量)。
药物干预15d 后,检测各组大鼠心功能及心肌纤维化的相关指标:超声心动图、体质量、心脏质量指数及左心室质量指数;ELISA 法检测氨基末端脑钠肽前体(N-terminal pro brain natriuretic peptide ,NT-proBNP );HE 染色、Masson 染色检测心肌组织病理形态及纤维化状态;RT-qPCR 检测心肌组织中miR-139、Wnt 家族成员3a (Wnt family member 3a ,Wnt3a )、β-连环蛋白(β-catenin )、糖原合成酶激酶3β(glycogen synthase kinase 3β,GSK3β)、Ⅰ型胶原蛋白(typeⅠcollagen ,Col-Ⅰ)、Ⅲ型胶原蛋白(typeⅢcollagen ,Col-Ⅲ)及α-平滑肌肌动蛋白(α-smooth muscle actin ,α-SMA)、基质金属蛋白酶-2(matrix metalloproteinase-2,MMP-2)、基质金属蛋白酶-9(matrix metalloproteinase-9,MMP-9)mRNA 的表达;Western blot 检测心肌组织中Wnt3a 、β-catenin 、GSK3β、Col-Ⅰ、Col-Ⅲ、α-SMA 、MMP-2、MMP-9蛋白表达。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
=====================================================================Acq. Operator : Li Shan(LCMS-02) Seq. Line : 58
Acq. Instrument : HY-LCMS-02 Location : Vial 53Injection Date : 1/9/2015 2:09:10 PM Inj : 1
Inj Volume : 3.000 µl
Acq. Method : D:\AGLIENT 1260\DATA\20150109\20150109 2015-01-09 09-16-10\100-1000MS+3MIN( 0.02%FA).M
Last changed : 1/9/2015 9:16:10 AM by Li Shan(LCMS-02)
Analysis Method : D:\AGLIENT 1260\DATA\20150108\20150108 2015-01-08 09-25-26\100-1000MS+3MIN( 0.02%FA).M (Sequence Method)
Last changed : 1/9/2015 3:04:11 PM by Li Shan(LCMS-02) (modified after loading)
Method Info : Postive,MS:100-1000,Column ID:A-RP-102,40℃
Catalog No : HY-14413 Batch#07539 A-RP-32
Additional Info : Peak(s) manually integrated
min
0.5
1
1.5
2 2.53mAU 0
100200300400500600 DAD1 C, Sig=254,4 Ref=off (D:\AGLIENT...0\DATA\20150109\20150109 2015-01-09 09-16-10\CPK2015-109-07539.D)
1.844
1.999
===================================================================== Area Percent Report =====================================================================
Sorted By : Signal Multiplier : 1.0000Dilution : 1.0000
Do not use Multiplier & Dilution Factor with ISTDs
Signal 1: DAD1 C, Sig=254,4 Ref=off
Peak RetTime Type Width Area Height Area # [min] [min] [mAU*s] [mAU] %
----|-------|----|-------|----------|----------|--------| 1 1.844 MM 0.0485 1927.52087 662.44214 98.3472 2 1.999 MM 0.0478 32.39330 11.29737 1.6528
Totals : 1959.91418 673.73951
===================================================================== *** End of Report ***
=====================================================================Acq. Operator : Li Shan(LCMS-02) Seq. Line : 58
Acq. Instrument : HY-LCMS-02 Location : Vial 53Injection Date : 1/9/2015 2:09:10 PM Inj : 1
Inj Volume : 3.000 µl
Acq. Method : D:\AGLIENT 1260\DATA\20150109\20150109 2015-01-09 09-16-10\100-1000MS+3MIN( 0.02%FA).M
Last changed : 1/9/2015 9:16:10 AM by Li Shan(LCMS-02)
Analysis Method : D:\AGLIENT 1260\DATA\20150108\20150108 2015-01-08 09-25-26\100-1000MS+3MIN( 0.02%FA).M (Sequence Method)
Last changed : 1/9/2015 3:07:13 PM by Li Shan(LCMS-02) (modified after loading)
Method Info : Postive,MS:100-1000,Column ID:A-RP-102,40℃
Catalog No : HY-14413 Batch#07539 A-RP-32
Additional Info : Peak(s) manually integrated
min
0.5
1
1.5
2
2.5
3
25000
5000075000100000125000150000175000200000 MSD1 TIC, MS File (D:\AGLIENT 1260\DATA\20150109\20150109 2015-01-09 09-16-10\CPK2015-109-07539.D) ES-API, Pos, Sc
1.853
MS Signal: MSD1 TIC, MS File, ES-API, Pos, Scan, Frag: 50 Spectra averaged over upper half of peaks. Noise Cutoff: 1000 counts.
Reportable Ion Abundance: > 10%.
Retention Mol. Weight Time (MS) MS Area or Ion
1.853 192020 653.50 I 465.30 I 406.00 I 327.30 I
m/z
1002003004005006007000
2040
6080100*MSD1 SPC, time=1.835:1.871 of D:\AGLIENT 1260\DATA\20150109\20150109 2015-01-09 09-16-10\CPK2015-109-07539.D ES-API Max: 54019
211.1
654.5
466.3
423.0
406.0 302.8
105.1
327.3
*** End of Report ***。
