RTOG 0617 lancet oncology 2015
榄香烯围手术期干预对胃癌患者外周血淋巴细胞细胞周期蛋白cyclinD1、p16、p27的影响

淋巴 细胞;
榄香烯;
ห้องสมุดไป่ตู้
细胞周期蛋白 D1 ;
基因 p1 6 ;
周期 素依赖激酶
p1 6, p2 7 单克隆抗体 ) � 二抗 � � (FT TC IgG 抗体 ) , 加入 1 m l PBS 缓冲液, 并设阴性 对照�样品放 在 0 4 ( F I 值) �
� 3 2 9 4�
中华临床医师杂志 ( 电子版 ) 2 01 1 年 6 月第 5 卷第 1 1 期
C hin JCl i n i ci a n s( E l ec t ro n i c E d i t i o n ), J un e1, 2 01 1 , Vo l .5 , N o .1 1
� 短篇论著 �
回顾性分析新入院确诊且准备手术同意围手术期输注 榄香烯的进展期胃癌患者 1 8 例�
用流式细胞仪检测榄香烯干预治 疗者术前 , 后 外周血 淋巴细 胞 c yc l in D1 , p1 6 , p2 7 的 表达�结果
in D 1, p2 7 的 表达 在术 前, 香烯 干预 治疗 者外 周血 淋巴 细胞 c yc l 后 存在 差异 性( P < 0. 05 ) , 其中 c ycl in D1 的表达术后低于 术前, p2 7 的表 达术 后 高于 术前, 而 p1 6 的表 达 术前 , 后差 异 无 统计 学 意义 ( P > 0 . 0 5 ) �结论 c yc l in D1 的表达明显下调, p1 6 的下调被扼制, p2 7 的表达上 调, 榄香烯干预后, 癌 基因表达下调, 抑癌基因表达增高, 榄香烯对细胞周 期蛋白质 具有调控作 用, 且这种调 控模式 是否就 是扼制手术后远处转移和复发的标志还需进一步的研究与认 证� � 关键 词� 胃肿瘤; 抑制剂 p2 7 胃癌的发病率居消化道肿瘤的首位 , 居全球肿瘤发病 和癌症 死亡率 的第二位 , 手 术是目 前惟一 有可能 根治胃癌 的手段 , 但根治术后仍有很多进展期的胃癌患者出现局部复发或转移 �故临床常尽可能地采用切除病灶, 再 结合化疗 和( 或 ) 放疗, 尤 其是采用围手术期治疗, 可以降低病期, 使原先预后较 差的中 后期患 者变为早 中期, 不但 创造手 术机会, 提高 手术疗 效, 更主 要的目的是控制局部复发和消灭微小转移 , 提高术后的无病生存和总生存[1 -7]� 榄香烯抗恶性肿瘤疗效确切, 可以抑制肿瘤细胞分裂使其停滞在 G 1 期, 诱发肿瘤细胞凋亡, 并作为一种 可通过血脑 屏障 的小分子类中药物质, 广谱抗肿瘤, 与化疗药物联合使 用时, 可增 强恶性肿 瘤尤其 是肝癌, 胃 癌的疗 效, 围 手术期 用药对 术后 生存质量的提高, 癌性胸腹水的减少, 机体免疫力的提高 及远处转移率的降低具有深远的临床意义� 本实验通过观察榄香烯对胃癌手术前后外周血淋巴细胞 c yc l i n D 1, p1 6 , p2 7 的变化探讨其抑制胃癌的作用点及临床意义 , 报道如下� 一, 资料与方法 1 . 一般资料: 病例组为 2 009 年 7 月至 2 0 1 0 年 1 2 月山西省肿瘤医院病理组织或细胞学 确诊的进展 期胃癌患者 共 1 8 例 , 男 1 0 例, 女 8 例, 男女比例 1 . 2 5 :1 ; 年龄 37 81 岁, 中位年龄 5 4 岁�根据患者 意愿给予患 者围手术期 榄香烯干预 治疗, 并征 求患者同意检查 cyc li n D1 , p1 6 , p2 7 指 标( 因患者经济和自身问 题, 故有些 指标数据 减少) �胃癌患 者均排 除影响 外周血 淋巴 细胞水平的疾病( 如炎症, 过敏, 自身免疫疾病等) � 2 . 治疗方法: 榄香烯注 射液( 1 00 m g/支, 大连金港制药有限公 司) 干预治疗: 术前输 注榄香烯 0 . 6 g/d , 连续 5 d , 休息 1 d , 后手术, 术中榄香烯 1 00 m g +0. 9% 生理盐水 1 00 m l, 病灶局 部或胸腹腔内淋洒, 术后 4 d开始 再次输注榄 香烯 0. 6 g/d 连续 5 d 后观察� 3. 主要试剂和仪器: 鼠抗人 c ycli n D 1, p1 6 , p2 7 单克隆抗体( 1 :1 00 ) 购自福州 迈新生物 技术开发 有限公司 , 异 硫氰酸 荧光 素( F TTC ) 羊抗鼠 IgG ( 1 :400 ) 购自北京华美生物技术开发有限公司, 流式细胞 仪为美国 BD 公司 F AC S42 0 型� 4. 实验方法: ( 1 ) 取样: 在清晨, 空腹, 静卧条件下, 取外周血 2 m l, 注入盛有 0. 01 m l0 . 1 % 乙二胺四乙酸二钠抗凝剂的试 管中混匀�( 2 ) 实验步骤: 取抗凝外周血 2 m l , 用国产淋巴细胞分离 液分离人 抗凝外周血 中的淋巴细 胞, 用 PBS 洗 涤, 洗 涤后
III期非小细胞肺癌的最新治疗选择
既往研究入组的“局部晚期”患者实为IV期胸腔积液患者
IPASS研究是一项随机、开放、多中心、III期临床试验,共纳入共纳入1217例来自东亚地区初治晚期 肺腺癌患者(IIIB\IV期),目的是比较晚期肺癌患者中吉非替尼与常规含铂双药化疗的疗效
纳入标准
1 提供知情同意书
2 18岁以上男性或女性
3
组织学或细胞学证实的NSCLC伴腺癌组织学(包括支气管肺泡)。 注意:不接受腺鳞癌组织学标本,不接受仅痰细胞学检查的标本。
为什么III期肺癌分期受到重视?
1. NCCN Guidelines Version 4.2019 Non-Small Cell Lung Cancer
IIIA IIIB IIIC
分期调整原因:不同T、N,预后大不相同
按新的分期对肿瘤患者的生存期进行研究时,生存曲线分离良好,无交叉或重叠 究其原因,不同T,N患者的预后不同
5-Year Estimate
90% 83% 76% 66% 56% 48% 38%
0%
1
2
3
4
5
6
术后(年)
60%
40% 20%
0%
N1 Single
N1 Muftiple
N2 Single N2 Single
+N1 N2 Muftiple
N2
Events/N 438/1135 153/325 261/602 304/582
标准放疗剂量组 ( 60Gy ) 的 5 年 PFS 率 达到18.3%(vs 高剂量 组13%,P = 0.055)2
1. Bradley, J. D et al, The Lancet Oncology, 16(2), 187–199. 2. Bradley, J.D. et al. ASTRO 2017.
贝伐珠单抗在宫颈癌中的应用

RR (%)
36(CR=14)
48(CR=28)
双侧p=0.00607
5.9
8.2
贝伐珠单抗在宫颈癌中的应用
第12页
GOG 240: 顺铂/紫杉醇贝伐-OS
Tewari KS, et al. ASCO Abstract 3.
CP(n=114)
贝伐珠单抗在宫颈癌中的应用
第21页
GOG 240: 结论
贝伐珠单抗联合化疗显著改进IVB期/复发/顽固宫颈癌OSOS改进3.5个月含有临床显著性意义中位PFS和ORR也得到改进顺铂+紫杉醇是当前标准方案, 联合贝伐珠单抗获益更大贝伐珠单抗组不良事件发生率更高, 但并无新安全顾虑贝伐珠单抗组延长OS同时, 并不降低健康相关生活质量
12.7
16.2
贝伐珠单抗在宫颈癌中的应用
第14页
GOG 240: OS与预后原因
Tewari KS, et al. ASCO Abstract 3.
贝伐珠单抗在宫颈癌中的应用
第15页
GOG 240: 健康相关生活质量
FACT-宫颈癌-TOI机体状态(7项);功效状态(7项);宫颈癌亚量表(15项);临床有意义改变: 4-5分;评分范围: 0-116分HRQOL问卷完成依从性为从第1周期前96%到第1周期后9个月63%,两组均衡贝伐珠单抗组汇报FACT-Cx-TOI评分比对照组平均低1.2分(P=0.3)
Monk BJ, et al.J Clin Oncol.NCCN Guidelines Version 2.Cervical Cancer.
GOG
方案
n
6m-PFS率(%)
mOS(月)
III期不可切NSCLC的治疗策略

III期NSCLC进行CRT后的巩固化疗:未显示临床获益
• 一项在III期不可切除的NSCLC接受同步放化疗(多西他赛/顺铂+放疗66Gy)后 • 随机予以多西他赛/顺铂3周期巩固治疗 vs 观察的III期亚洲临床研究
0.4
0.2
0.2
0 0 12 24 36 48 60 72 84
月
0 0 12 24 36 48 60 72 84 月
治疗组
历史对照组
历史对照组:RTOG88-08 (标准TRT vs 序贯化疗和TRT vs 序贯化疗和超分割TRT, 结果显示序贯化疗组的OS13.2个月为最优,研究进一步随访OS达到13.7个月)
? 诱导化疗
不可切除局部晚期
同步
NSCLC
放化疗
巩固化疗
?
10
患者生存期 (%)
III期NSCLC放疗与化疗不同的联合方式提示:OS基本一致
? 诱导化疗
不可切除局部晚期
同步
NSCLC
放化疗
8
患者生存期 (%)
III期NSCLC放疗与化疗不同的联合方式提示:OS基本一致
• III期不可切除的NSCLC: 接受不同的联合方式 vs 历史序贯治疗对照组 II期随机非对照临床研究
化疗 放疗(n=91)
化疗→放疗
1 yr 57%
100
2 yrs 30% 3 yrs 17%
OS
1.0
患者数 事件数 mOS (95% CI)
CCRT alone
肿瘤学最新进展
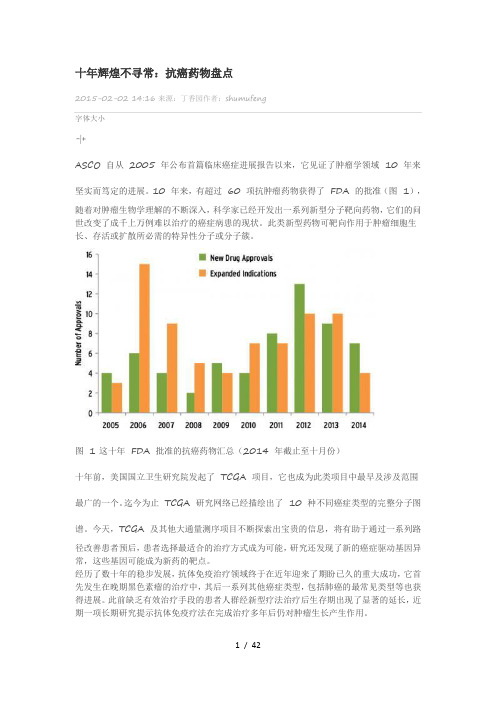
十年辉煌不寻常:抗癌药物盘点2015-02-02 14:16来源:丁香园作者:shumufeng字体大小-|+ASCO 自从2005 年公布首篇临床癌症进展报告以来,它见证了肿瘤学领域10 年来坚实而笃定的进展。
10 年来,有超过60 项抗肿瘤药物获得了FDA 的批准(图1),随着对肿瘤生物学理解的不断深入,科学家已经开发出一系列新型分子靶向药物,它们的问世改变了成千上万例难以治疗的癌症病患的现状。
此类新型药物可靶向作用于肿瘤细胞生长、存活或扩散所必需的特异性分子或分子簇。
图 1 这十年FDA 批准的抗癌药物汇总(2014 年截止至十月份)十年前,美国国立卫生研究院发起了TCGA 项目,它也成为此类项目中最早及涉及范围最广的一个。
迄今为止TCGA 研究网络已经描绘出了10 种不同癌症类型的完整分子图谱。
今天,TCGA 及其他大通量测序项目不断探索出宝贵的信息,将有助于通过一系列路径改善患者预后,患者选择最适合的治疗方式成为可能,研究还发现了新的癌症驱动基因异常,这些基因可能成为新药的靶点。
经历了数十年的稳步发展,抗体免疫治疗领域终于在近年迎来了期盼已久的重大成功,它首先发生在晚期黑色素瘤的治疗中,其后一系列其他癌症类型,包括肺癌的最常见类型等也获得进展。
此前缺乏有效治疗手段的患者人群经新型疗法治疗后生存期出现了显著的延长,近期一项长期研究提示抗体免疫疗法在完成治疗多年后仍对肿瘤生长产生作用。
另一种免疫疗法致力于重组自身的免疫细胞以攻击肿瘤细胞,它对于特定的血液肿瘤以及一系列实体肿瘤同样表现出色。
过去的十年间首款癌症疫苗也得以问世(宫颈癌Gardasil 疫苗)。
探索其他类型癌症疫苗的试验也正在进行中。
最后,大规模的筛查研究带来了新的重要证据,表明对于一些常见癌症如肺癌、乳腺癌以及前列腺癌可推进筛查实践。
靶向治疗迅速发展最近的十年间,我们看到由FDA 批准的新型靶向治疗药物有了稳步且迅猛的增加,远远超过新型化疗药物的研发速度(图2)。
国外常见英文医学杂志影响因子及网址

国外常见英文医学杂志影响因子及网址<综合>1、期刊名称:Nature Medicine影响因子:大约31网络版地址:.nature./nm/journal/v14/n5/index.html#af有提前公布的文摘,但是时间不定:.nature./nm/journal/vaop/ncurrent/index.html2、期刊名称:NEJM New England Journal of Medicine (新英格兰医学杂志)影响因子:44点多(05年)网络版地址:/3、期刊名称:British medical journal (BMJ)影响因子:9.245(2006)网络版地址:.bmj./4、期刊名称:PNAS影响因子:9.6(2006)网络版地址:/——也可以免费获得全文5、期刊名称:lancet影响因子:网络版地址:.thelancet./6、期刊名称:Science影响因子:网络版地址:/7、期刊名称: Nature影响因子:网络版地址:.nature./8、期刊名称:Nature Genetics网址:.nature./ng/index.htm<生命科学>1、期刊名称:《Cell》影响因子:大约28(2005),29(2006)网络版地址:.cell./Online First版本:.cell./content/current2、期刊名称:stem cells影响因子:7.924(2006)网络版地址:.stemcells./view/0/index.html——该期刊所有文章PDF全文全部可在上述网址获得,而且更新很快3、期刊名称:科学家影响因子:网络版地址:.the-scientist./<细胞生物>1、期刊名称:journal of cell Science影响因子:6.427网络版地址:/2、期刊名称:Current Biology影响因子:10.988网络版地址:.current-biology./3、期刊名称:Molecular Cell影响因子:14.033网络版地址:/4、期刊名称:Nature review molecular cell biology影响因子:31.354网络版地址:.nature./nrm/5、期刊:fertility sterility影响因子:3.220(3点多)网址:.sciencedirect./science/journal/00150282<消化>1、期刊名称:journal of hepatology影响因子:6.64网络版地址:.jhep-elsevier./2、期刊名称:gut影响因子:9.02网络版地址:gut.bmj./Online First版本(如果有,请列出):gut.bmj./onlinefirst.dtl3、期刊名称:hepatology影响因子:10.77网络版地址:.interscience.wiley./journal/hepatology4、期刊名称:gastroenterology影响因子:约13网络版地址:/<消化镜方向>1、杂志名称:GIEIF:5.8网址:/2、杂志名称:EndoscopyIF:4.1网址:.thieme.de/fz/index.html<呼吸>下面4本杂志比较偏重临床,可读性强——1、期刊名称:《American journal of respiratory and critical care medicine 》影响因子:大约8网络版地址:/Online First版本:/articlesinpress.shtml2、期刊名称:Thorax影响因子:网络版地址:thorax.bmj./content/vol52/suppl_2/3、期刊名称:ERJ欧洲呼吸杂志影响因子:网络版地址:erj.ersjournals./4、期刊名称:Chest(有中文版)影响因子:网络版地址:/下列杂志也比较出名,但偏重基础——5、期刊名称:American Journal of Respiratory Cell and Molecular Biology (AJRCMB )影响因子:网络版地址:/6、期刊名称: American Journal of Physiology-《Lung Cellular and Molecular Physiology 》影响因子:网络版地址:/以下两个杂志的哮喘文献很有影响——7、期刊名称:Allergy影响因子:网络版地址:.blackwellpublishing./journal.asp?ref=0105-45388、期刊名称:Clinical & Experimental Allergy影响因子:网络版地址:.blackwellpublishing./journal.asp?ref=0954-7894<危重症>1、期刊名称:《American Journal of Respiratory and Critical Care Medicine》影响因子:8.123网址:2、期刊名称:Critical Care Medicine影响因子:约7网络版地址:.ccmjournal.——创刊于1973年,为月刊,2006年IF=6.599。
二氯乙酸钠联合ONYX-015对结肠癌细胞株的杀伤性研究

0 引 言
在世 界范 围 内 , 肠癌 是人类 第 三大 常见 癌症 , 致 死率 也 位 于各 癌症 前 列 [ , 现 在 最 常见 的恶性 肿 结 其 1是 ]
瘤之一 。尽管针对结肠癌的传统疗法在过去的几十年里取得了重大的进步 , 但是结肠癌患者 的 5 年存活率 仍然 只有 4 ~6 , O O 因为有 超过一 半 的患者 最后 肿瘤都 发 生 了局 部反 弹或 远 端转 移 导致 病情 极 度 恶化并
最终致 使 患者死 亡E 。因此 迫切需 要更 为有 效 的治疗 方法 的诞生 , 中生物 疗法 越来 越受 到研究 者 的青 睐 。 其
紫购 自美 国 A rso公 司 ; 有 细胞都 在 5 C ,7 培养 条件 下 培养 , meec 所 O 3℃ 培养 液 为含 1 F S的 D M 0 B ME
( l e c ’ M o i e g eSM e i m ) Duh co S d f d Ea l’ i du 。
如肺 癌 、 乳腺癌 和脑 癌细 胞等 , 但对 健康 细胞 却没有 损 害 。二氯 乙酸通 过 激活 肿 瘤 细胞 内线粒 体 的功 能 , 从
而激活 细胞 内源性 凋亡通 路达 到杀 伤癌 细胞 的 目的 。二氯 乙酸ቤተ መጻሕፍቲ ባይዱ盐 对一 些 病人 来 说可 能会 引起 疼 痛 、 麻木 等 问题 , 但停 药后 这些 症状会 自行 消失 。
由美 国 ONYX药物 公 司研 究开 发 的肿 瘤 特异 性 增 殖 型病 毒 O X 0 5 通 过 缺 失 EI 5k NY - 1 , B 5 一 D基 因实
RTOG 0617-2013-WCLC

国内专家精彩视频解读 请关注微信公号
肿瘤资讯
关注肿瘤领域新进展,提供肿瘤专业信息
新浪微博:@肿瘤资讯 微信公众帐号:肿瘤资讯 (Oncology_news)
【肿瘤资讯】微信公众帐号添加办法: 1-搜索微信号:oncology_news; 2-查找公众帐号:肿瘤资讯; 3- 扫描右边的二维码。
谢 谢!
28.2%
4-5级不良事件
Bradley J, et al. WCLC abstract PL03.05.
RTOG 0617:比较标准剂量(60Gy)vs高剂量(74Gy) 放化疗±西妥昔单抗治疗III期NSCLC的随机III期研究
研究结论
• 对于III期不可切除的NSCLC患者,在标准放化疗基础上增加西妥昔单
研究设计
N=544 放疗技术:1. 3D-CRT; 2. IMRT 分 ZPS:1.0; 2.1 PET分期:1. 否;2. 是 组织学:1. 鳞癌;2. 非鳞癌 随 机 同步组 A组:同步化疗* RT to 60 Gy, 5次/w×6w B组:同步化疗* RT to 74 Gy, 5次/w×7.5w 巩固治疗 A组:巩固化疗* B组:巩固化疗* C组:巩固化疗*+西妥昔单抗 D组:巩固化疗*+西妥昔单抗
Bradley J, et al. WCLC abstract PL03.05.
RTOG 0617:比较标准剂量(60Gy)vs高剂量(74Gy) 放化疗±西妥昔单抗治疗III期NSCLC的随机III期研究
患者特征
结果 中位年龄 (岁) 西妥昔单抗 (n=237) 64 无西妥昔单抗 (n=228) 64 全组 (n=465) 64
91.8% (84.4-95.8) 78.4% (68.9-85.4) 67.9% (57.6-76.2)
利奈唑胺联合磷霉素对肠球菌的体外药动学-药效学研究

利奈唑胺联合磷霉素对肠球菌的体外药动学-药效学研究摘要:目的:研究利奈唑胺(LIN)与磷霉素(PM)联合对肠球菌的体外药动学/药效学特性。
方法:采用微量平板洗涤技术,应用CASP(药物组合作用研究)和min-inhibitory concentration(MIC)等实验方法,研究了不同浓度下LIN和PM联合对肠球菌的体外药动学/药效学,包括最小抑菌浓度(MIC)、最小杀菌浓度(MBC)、合作指数(CI)和检测时间(T)等指标。
结果:与单用LIN或PM相比,在各浓度下,LIN联合PM的MIC和MBC值均降低。
联合组的CI值也较单用组(CI=1)有所提高,并具有示踪效应。
此外,联合组所需时间(T)同样显著减少。
结论:LIN联合PM对肠球菌的体外药效明显优于单用LIN或PM,具有合作增强效果和时间缩短的双重优势,足以作为治疗此细菌感染的一种常见方法。
关键词:利奈唑胺、磷霉素、肠球菌、药动学、药效学Abstract:Purpose: To study the in vitro pharmacokinetics and pharmacodynamics of combined use of linezolid (LIN) and phosphomycin (PM) against enterococcus.Methods: Micro-plate washing technique, CASP (drug combination action research) and min-inhibitory concentration (MIC) were used to study the in vitro pharmacokinetics and pharmacodynamics of different concentrations of LIN and PM in combination against enterococcus. The indicators included minimuminhibitory concentration (MIC), minimum bactericidal concentration (MBC), Cooperating index (CI), and detection time (T).Results: Compared with single use of LIN or PM, the MIC and MBC values of LIN in combination with PM decreased at various concentrations. The CI value of the combination group was also higher than that of the single use group (CI=1), and had a tracer effect. In addition, the time required for the combination group also decreased significantly.Conclusion: The in vitro pharmacological effect of LIN in combination with PM against enterococcus was significantly better than that of single use of LIN or PM, with the dual advantages of enhanced cooperationand shortened time, which can be used as a common method for the treatment of this bacterial infection.Keywords: linezolid, phosphomycin, enterococcus, pharmacokinetics, pharmacodynamicIntroductionEnterococcus is a type of gram-positive bacteria that can cause various infections, including urinary tract infections, endocarditis, and bacteremia. The emergence of antibiotic-resistant strains of enterococcus has become a major problem in clinical practice. Linezolid (LIN) and phosphomycin (PM) are two antibiotics commonly used to treat enterococcus infections. However, the single use of one antibiotic may lead to the development of drug resistance and the failure of treatment. Therefore, studying the combined use of antibiotics is an important strategy for treating enterococcus infections. In this study, we investigated the in vitro pharmacological effect of LIN in combination with PM against enterococcus.MethodsEnterococcus strains were isolated from clinical samples and identified by standard methods. Theminimum inhibitory concentration (MIC) of LIN and PM against the test strains was determined by broth microdilution. The pharmacokinetic (PK) parameters of LIN and PM were determined by high-performance liquid chromatography (HPLC). The pharmacodynamic (PD) index was calculated as 24-hour area under the concentration-time curve (AUC24)/MIC ratio. The in vitro interaction of LIN and PM was evaluated by checkerboard assay and time-kill assay.ResultsThe MICs of LIN and PM against enterococcus strains ranged from 4 to 8 μg/ml and from 16 to 32 μg/ml, respectively. The PK parameters of LIN and PM were as follows: Cmax, 23.47±1.43 μg/ml and 101.34±5.25μg/ml; Tmax, 0.5 h and 1 h; T1/2, 3.98±0.12 h and 3.81±0.14 h; AUC24, 86.44±6.38 μg·h/ml and 1482.43±56.99 μg·h/ml, respectively. The PD index of LIN and PM was 10.81±0.80 and 46.32±1.78, respectively. The checkerboard assay showed that the combination of LIN and PM had a synergistic effect against enterococcus strains. The time-kill assay showed that the combination group had a significant bactericidal effect compared to the single use of LIN or PM. The time required for the combination group to achieve bactericidal activity was also significantlyshortened.ConclusionThe in vitro pharmacological effect of LIN in combination with PM against enterococcus was significantly better than that of single use of LIN or PM. The dual advantages of enhanced cooperation and shortened time make this combination a promising method for the treatment of enterococcus infectionsOverall, the emergence of antibiotic resistance among enterococcal strains has become a serious health issue. Finding effective treatment options for enterococcal infections has become a priority in the medical field. Combination therapy has been increasingly explored as a strategy for improving the efficacy of antibiotics against enterococcal infections.This study demonstrated that the combination of LIN with PM can produce a synergistic effect against enterococcus. This result is consistent with previous studies that have explored combinations of antibiotics against enterococcal infections. The synergisticeffect observed in this study may be due to the mechanism of action of LIN and PM on bacterial cell walls.LIN is known to bind to the peptidoglycan layer of bacterial cell walls and inhibit the synthesis of the peptidoglycan layer. This inhibition weakens the bacterial cell wall and increases its permeability, making it easier for PM to penetrate into thebacterial cell and exert its bactericidal effect. PM is a membrane-active antibiotic that binds to thelipid bilayer of bacterial cell membranes, causing membrane depolarization and damage to the cell wall.The combination of LIN and PM may also benefitpatients by shortening the duration of the treatment period. This study found that the dual use of LIN and PM achieved bactericidal activity against enterococcus in a shorter time period than the single use of the two drugs individually. Shortening the duration of antibiotic treatment can reduce the incidence of antibiotic resistance and the cost of treatment for patients.In conclusion, combination therapy of LIN with PM may be a promising option for the treatment of enterococcal infections. However, further studies are still required to investigate the pharmacokinetics, safety and clinical efficacy of this combination in humans. With the increasing prevalence of antibioticresistance, the development of effective combination therapies against enterococcus is critical for improving patient outcomes and reducing the burden of enterococcal infections on the healthcare systemEnterococcal infections continue to be a challenge for healthcare professionals worldwide, as they are notorious for their ability to develop multidrug resistance to many commonly used antibiotics. This has led to an urgent need for alternative treatment options against these bacteria, and combination therapies have emerged as a promising approach. One promising combination therapy that has shown potential in preclinical studies is the use of linezolid (LIN) and polymyxin (PM) against enterococcus.Linezolid is a synthetic antibiotic that belongs to the oxazolidinone class, and it works by inhibiting bacterial protein synthesis. It has been approved by the US Food and Drug Administration (FDA) for the treatment of various infections, including those caused by enterococcus. Although enterococcal infections are generally susceptible to LIN, the increasing prevalence of resistance to this antibiotic has become a growing concern in recent years.Polymyxins are another class of antibiotics that havebeen used to treat life-threatening infections caused by multidrug-resistant Gram-negative bacteria, such as Pseudomonas aeruginosa and Acinetobacter baumannii. Polymyxins target the bacterial cell membrane, causing damage to its structure and leading to bacterial death. Although they are not typically used against enterococcal infections, recent studies have shownthat polymyxins may also be effective against these bacteria, especially in combination therapy with other antibiotics.When used in combination, LIN and PM have been shownto have a synergistic effect against enterococcus in vitro and in animal models of infection. The mechanism of this synergy is believed to be due to the abilityof PM to disrupt the bacterial cell membrane, which enhances the activity of LIN in inhibiting protein synthesis. This combination has also been found to be effective against LIN-resistant strains of enterococcus, which is a promising finding in the face of the increasing prevalence of antibiotic resistance.Despite the promising results of preclinical studies, further research is still needed to determine the safety, pharmacokinetics, and clinical efficacy ofthis combination therapy in humans. One potential concern is the risk of nephrotoxicity associated withPM, which may limit its clinical use in certainpatient populations. However, recent studies have shown that this risk can be minimized by adjusting the dose and duration of treatment, as well as monitoring renal function.In conclusion, the combination of LIN and PM may offer a promising alternative treatment option for enterococcal infections, especially in cases where resistance to other antibiotics is present. However, further studies are necessary to determine the optimal dosing and duration of treatment, as well as to evaluate the safety and efficacy of this combinationin clinical settings. The development of effective combination therapies against enterococcus is crucial in the fight against antibiotic resistance and the improvement of patient outcomesIn conclusion, the combination of daptomycin and tedizolid holds promise as an alternative treatment option for enterococcal infections, particularly in cases where resistance to other antibiotics is present. However, more research is needed to determine the optimal dosing and duration of treatment, as well asto assess the safety and efficacy of this combination in clinical settings. Developing effective combinationtherapies is essential in combating antibiotic resistance and improving patient outcomes。
用于治疗化疗抗性癌症起始细胞的方法[发明专利]
![用于治疗化疗抗性癌症起始细胞的方法[发明专利]](https://img.taocdn.com/s3/m/5b1a2ec6dd36a32d72758188.png)
专利名称:用于治疗化疗抗性癌症起始细胞的方法
专利类型:发明专利
发明人:R·卡斯,L·李,路秀玲,J·M·佩里,G·S·斯塔姆帕拉姆,A·罗伊,X·C·何
申请号:CN201680043900.1
申请日:20160728
公开号:CN109310767A
公开日:
20190205
专利内容由知识产权出版社提供
摘要:本公开提供了通过选择性抑制p‑S‑β‑链蛋白,p‑T‑β‑链蛋白,p‑T‑β‑链蛋白和/或
p‑S‑β‑链蛋白的产生和/或活性而治疗癌症的方法。
这样的方法还减少和/或限制癌症起始细胞。
申请人:康涅狄格大学,美国斯托瓦斯医学研究所,堪萨斯大学
地址:美国康涅狄格州
国籍:US
代理机构:北京市中伦律师事务所
更多信息请下载全文后查看。
胸部放疗危及器官图谱PPT精选课件

AA=升主动脉, PA=肺动脉, DA=降主动脉, SVC=上腔静脉
食管、肺、脊髓、大血管和近端气管树
AA=升主动脉, PA=肺动脉, DA=降主动脉, SVC=上腔静脉
食管、肺、脊髓、大血管和近端气管树
AA=升主动脉, PA=肺动脉, DA=降主动脉, SVC=上腔静脉
食管、肺、脊髓、大血管和近端气管树(续)
这与正常进行的RTOG研究不同点在于其他研究一 律要求画到PTV上下10cm 不应用10cm外扩的原因是它通常外扩到CT扫描的 肺癌计划外,在肺下叶肿瘤中它可能跑到脊髓结束 的下方或到了CT扫描范围的上界之上 因为脊髓本身在CT扫描上通常 是看不见的,而邻近 脊髓肿瘤病人行SBRT时,MRI可以用于确认真正的 脊髓所在
食管
食管应该在CT 的纵隔窗上勾画,包括粘膜、粘膜下和所有肌层乃至脂肪外 膜,推荐从环状软骨水平开始,逐层勾画到食管胃连接部最后至胃才结束 除非肿块位于食管周围,不然我们不常规推荐口服对比剂,因为这将影响剂 量计算和食管的解剖形态
食管是胸部放疗的危及器官
多个体积剂量因素,包括V15, V35, V50, V65, V80,和最大剂量、平均剂量,已有报导与食管炎显 著相关
有些报导周长或轴向面积与放射性食管炎相关
所有这些参数与绝对剂量和解剖位置高度相关
然而没有就食管的勾画达成共识,特别是尾侧(食管 胃连接部)和头侧(包括颈侧食管)长度 当前的数据仍无法确定发生食管炎危险的单因素阈 值
脊髓
在肺部肿瘤治疗中,我们推荐脊髓按脊髓腔的骨性 标志来勾画。脊髓的勾画头侧从环状软骨开始到L2 下缘或脊髓结束的位置终止
已被广泛接受的OAR 限制剂量
OAR勾画的问题
在现在的软件中多数能自勾画肺组织,但因为阈值和感兴趣区的不同,可能会捕 捉到不需要的区域(不是肺),如气道、肺不张区、支气管扩张、瘢痕和大血管 多个因素会影响到最后的勾画效果,包括阈值的设置、对气管支气管和小血管的 修改、呼吸时相、排除或包含如PTV/CTV/GTV等靶区,图1 显示的是自动勾画 失败和勾画变异会影响到肺的DVH。
参与金黄色葡萄球菌杀白细胞素的细胞毒性的细胞因素:新型治疗靶点[发明专利]
![参与金黄色葡萄球菌杀白细胞素的细胞毒性的细胞因素:新型治疗靶点[发明专利]](https://img.taocdn.com/s3/m/1c722a16910ef12d2bf9e7dc.png)
专利名称:参与金黄色葡萄球菌杀白细胞素的细胞毒性的细胞因素:新型治疗靶点
专利类型:发明专利
发明人:V·J·托雷斯,T·雷耶斯-罗布莱斯,F·阿朗佐三世
申请号:CN201480045457.2
申请日:20140618
公开号:CN105722972A
公开日:
20160629
专利内容由知识产权出版社提供
摘要:本发明涉及在对象中治疗和预防金黄色葡萄球菌(Staphylococcus aureus)感染和/或由金黄色葡萄球菌感染导致的状况的方法,所述方法包括施用抑制金黄色葡萄球菌与CXCR1/CXCR2和DARC细胞受体相互作用的组合物。
本发明还涉及用于实现这些和其他方法的新型组合物。
申请人:纽约大学
地址:美国纽约州
国籍:US
代理机构:北京商专永信知识产权代理事务所(普通合伙)
更多信息请下载全文后查看。
一种筛选前列腺癌潜在生物标志物的方法及其应用[发明专利]
![一种筛选前列腺癌潜在生物标志物的方法及其应用[发明专利]](https://img.taocdn.com/s3/m/a18406ce5ff7ba0d4a7302768e9951e79b896968.png)
专利名称:一种筛选前列腺癌潜在生物标志物的方法及其应用专利类型:发明专利
发明人:李宇同,陈果,高白云,潘斌
申请号:CN202111512080.5
申请日:20211207
公开号:CN114252611A
公开日:
20220329
专利内容由知识产权出版社提供
摘要:本发明涉及一种筛选前列腺癌潜在生物标志物的方法和应用。
本发明对前列腺癌进行了深入和全面的生物信息学分析,建立了筛选前列腺癌潜在生物标志物的方法,能够快速、准确、高效地确定前列腺癌潜在的靶标。
本发明基于几个大型公共数据库评估了作为治疗靶点和预后生物标志物的潜力,寻找在前列腺癌肿瘤微环境中与免疫相关的因子,通过进一步网络预测靶标,寻找其上游miRNA,进一步明确其在前列腺癌中的调节机制。
为前列腺癌后续的药物研发、临床治疗等提供切实的理论基础和科学依据。
申请人:暨南大学附属第一医院(广州华侨医院)
地址:510630 广东省广州市黄埔大道西613号
国籍:CN
代理机构:广州艾维专利商标代理事务所(普通合伙)
代理人:黄强
更多信息请下载全文后查看。
标准剂量放疗比高剂量放疗更安全有效

标准剂量放疗比高剂量放疗更安全有效美国临床肿瘤学会(ASCO)一项针对晚期肺癌(3期)的研究得到的结果证实:标准剂量放疗比高剂量放疗更安全有效。
这一结论与当初的预期结果相反,高于标准剂量的放疗并不会改善疾病。
这项大型的3期RTOG 0617试验的最终结果将在2013年美国临床肿瘤学会(ASCO)年会中公布, RTOG 0617之前的结果曾在2011年公布。
来自于华盛顿肿瘤研究所的Sandra Swain医学博士介绍“这项研究对于肿瘤放射学是具有决定性意义的。
在经历了10年的研究之后,我们最终为肺癌中高剂量和标准剂量放疗的争论画上了一个句号。
”研究结果令人惊讶RTOG 0617试验纳入了464例3期非小细胞肺癌(NSCLC)患者,比较了高剂量(74 Gy)和标准剂量(60Gy)放疗。
所有的患者也接受了紫杉醇和卡铂放疗。
这项研究同时把病人随机分配接受靶向药物西妥昔单抗(爱必妥)治疗,但是目前还未得出相应结果。
在中期分析发现高剂量放疗没有体现出相对于标准剂量的优越性时,该研究团体就中断了研究。
来自圣路易斯华盛顿医学院的首席作者Jeffrey Bradley医学博士解释道“我们起初认为高剂量放疗会使结果改善,但是,标准放疗剂量的患者结果都比较好,而且两种剂量的差别具有统计学意义。
”标准剂量和高剂量放疗的结果对比Bradley博士写道“我们很高兴发现较缓和的治疗能够更好的控制肿瘤的发展和扩散,甚至能改善总体的存活情况”。
他报道称2组患者的生存率都比以往见过的要高,但是2组的差异是有统计学意义的。
高剂量组患者死亡风险比标准剂量组患者高出56%,而肿瘤局部发展的风险要高出37%。
标准剂量组的生存优势“与西妥昔单抗无关”。
高剂量组结果较差的原因犹未可知,传统思维认为如果给予的放疗剂量越大,就越能杀伤肿瘤并能更好地延长生存期。
原因可能是高剂量放疗增加了对心脏的辐射、过度治疗或者引起尚未报道的中毒反应,或者是上述这些因素的结合。
肿瘤有效性评估解析

- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
ArticlesLancet Oncol 2015Published Online January 16, 2015/10.1016/S1470-2045(14)71207-0See Online/Comment//10.1016/S1470-2045(15)70001-X Washington University, St Louis, MO, USA (Prof J D Bradley MD,C Robinson MD); Radiation Therapy Oncology Group-Statistical Center, Philadelphia, PA, USA(R Paulus BS, C Hu PhD); The University of Texas MD Anderson Cancer Center, Houston, TX, USA (Prof R Komaki MD,Prof G Blumenschein MD);Christiana Care/Helen Graham Medical Center, Newark, DE, USA (G Masters MD); Mayo Clinic , Scottsdale, AZ, USA (Prof S Schild MD); Mayo Clinic, Rochester, MN, USA(Y I Garces MD); SUNY Upstate Medical University, Syracuse NY, USA (Prof J Bogart MD); H Lee Moffi tt Cancer Center, Tampa, FL, USA (K Forster PhD, Prof A Magliocco MD); USON- Texas Oncology-Sugarland, Sugarland, TX, USA (V Kavadi MD); Michigan Cancer Research Consortium CCOP, Ypsilanti, MI, USA(S Narayan MD); University of Texas Southwestern, Dallas, TX, USA (P Iyengar MD, Prof H Choy MD);UPMC-Shadyside Hospital, Pittsburgh, PA, USA(Prof R B Wynn MD); Christiana Care Health CCOP, Newark, DE, USA (C Koprowski MD); The Ottawa Hospital Cancer Centre, Ottawa, Canada (J Meng MD); EmoryUniversity, Atlanta, GA, USAStandard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial phase 3 studyJeff rey D Bradley, Rebecca Paulus, Ritsuko Komaki, Gregory Masters, George Blumenschein, Steven Schild, Jeff rey Bogart, Chen Hu, Kenneth Forster, Anthony Magliocco, Vivek Kavadi, Yolanda I Garces, Samir Narayan, Puneeth Iyengar, Cliff Robinson, Raymond B Wynn, Christopher Koprowski, Joanne Meng, Jonathan Beitler, Rakesh Gaur, Walter Curran Jr, Hak ChoySummaryBackground We aimed to compare overall survival after standard-dose versus high-dose conformal radiotherapy with concurrent chemotherapy and the addition of cetuximab to concurrent chemoradiation for patients with inoperable stage III non-small-cell lung cancer.Methods In this open-label randomised, two-by-two factorial phase 3 study in 185 institutions in the USA and Canada, we enrolled patients (aged ≥18 years) with unresectable stage III non-small-cell lung cancer, a Zubrod performance status of 0–1, adequate pulmonary function, and no evidence of supraclavicular or contralateral hilar adenopathy. We randomly assigned (1:1:1:1) patients to receive either 60 Gy (standard dose), 74 Gy (high dose), 60 Gy plus cetuximab, or 74 Gy plus cetuximab. All patients also received concurrent chemotherapy with 45 mg/m² paclitaxel and carboplatin once a week (AUC 2); 2 weeks after chemoradiation, two cycles of consolidation chemotherapy separated by 3 weeks were given consisting of paclitaxel (200 mg/m²) and carboplatin (AUC 6). Randomisation was done with permuted block randomisation methods, stratifi ed by radiotherapy technique, Zubrod performance status, use of PET during staging, and histology; treatment group assignments were not masked. Radiation dose was prescribed to the planning target volume and was given in 2 Gy daily fractions with either intensity-modulated radiation therapy or three-dimensional conformal radiation therapy. The use of four-dimensional CT and image-guided radiation therapy were encouraged but not necessary. For patients assigned to receive cetuximab, 400 mg/m² cetuximab was given on day 1 followed by weekly doses of 250 mg/m², and was continued through consolidation therapy. The primary endpoint was overall survival. All analyses were done by modifi ed intention-to-treat. The study is registered with , number NCT00533949.Findings Between N ov 27, 2007, and N ov 22, 2011, 166 patients were randomly assigned to receive standard-dose chemoradiotherapy, 121 to high-dose chemoradiotherapy, 147 to standard-dose chemoradiotherapy and cetuximab, and 110 to high-dose chemoradiotherapy and cetuximab. Median follow-up for the radiotherapy comparison was 22·9 months (IQR 27·5–33·3). Median overall survival was 28·7 months (95% CI 24·1–36·9) for patients who received standard-dose radiotherapy and 20·3 months (17·7–25·0) for those who received high-dose radiotherapy (hazard ratio [HR] 1·38, 95% CI 1·09–1·76; p=0·004). Median follow-up for the cetuximab comparison was 21·3 months (IQR 23·5–29·8). Median overall survival in patients who received cetuximab was 25·0 months (95% CI 20·2–30·5) compared with 24·0 months (19·8–28·6) in those who did not (HR 1·07, 95% CI 0·84–1·35; p=0·29). Both the radiation-dose and cetuximab results crossed protocol-specifi ed futility boundaries. We recorded no statistical diff erences in grade 3 or worse toxic eff ects between radiotherapy groups. By contrast, the use of cetuximab was associated with a higher rate of grade 3 or worse toxic eff ects (205 [86%] of 237 vs 160 [70%] of 228 patients; p<0·0001). There were more treatment-related deaths in the high-dose chemoradiotherapy and cetuximab groups (radiotherapy comparison: eight vs three patients; cetuximab comparison: ten vs fi ve patients). There were no diff erences in severe pulmonary events between treatment groups. Severe oesophagitis was more common in patients who received high-dose chemoradiotherapy than in those who received standard-dose treatment (43 [21%] of 207 patients vs 16 [7%] of 217 patients; p<0·0001).Interpretation 74 Gy radiation given in 2 Gy fractions with concurrent chemotherapy was not better than 60 Gy plus concurrent chemotherapy for patients with stage III non-small-cell lung cancer, and might be potentially harmful.Addition of cetuximab to concurrent chemoradiation and consolidation treatment provided no benefit in overall survival for these patients.Funding National Cancer Institute and Bristol-Myers Squibb.Articles(Prof J Beitler MD,Prof W Curran Jr MD); and Kansas City CCOP, Kansas City,MO, USA (R Gaur MD)Correspondence to:Prof J D Bradley, Washington University, 63110 St Louis, MO,USAjbradley@IntroductionThe commonly accepted radiation therapy dose (60–63 Gy in 1·8–2·0 Gy fraction sizes) for patients with stage III non-small-cell lung cancer was established by the Radiation Therapy Oncology Group (RTOG) 7301 trial and has remained unchanged for more than 30 years.1 With the idea that increasing radiation dose would improve both local-regional control and overall survival, the RTOG and other investigators did separate prospective phase 1 and 2trials to establish the safety and effi cacy of increasing the total radiation dose in the setting of concurrent chemotherapy while reducing irradiated volumes by use of image guidance and either three-dimensionalconformal or intensity-modulated radiation therapy for locally advanced non-small-cell lung cancer.2–7 Findings from these trials were similar, showing that a maximum tumour dose of 74 Gy given with concurrent weekly paclitaxel and carboplatin was safe and resulted in a median overall survival of roughly 24 months 3–6 versus a median overall survival of around 17·1 months in patients given a 60 Gy dose in RTOG 9410.8Our trial (RTOG 0617) was designed to establish whether a 74 Gy dose was better than a 60 Gy dose and whether adding cetuximab to concurrent chemo-radiation would confer an overall survival benefi t. Cetuximab is a chimerised antibody of the immuno-globulin G1 subclass that blocks binding of EGF and TGF α to EGFR.9 The use of cetuximab in this setting was tested in RTOG 0324, a phase 2 study combining chemoradiation with cetuximab in patients with unresectable stage III non-small-cell lung cancer.10 The trial enrolled 93 patients, showed a median survival of 22·7 months, and 24-month overall survival of 49·3%. On the basis of these encouraging data, we investigated cetuximab in this trial.MethodsStudy design and participantsIn this randomised phase 3 study, we recruited patients aged 18 years and older with stage IIIA/IIIB non-small cell-lung cancer from 185 institutions in the USA and Canada. Eligibility criteria included having stage IIIA or IIIB non-small-cell lung cancer, no previous invasive cancer during the previous 3 years, Zubrod performance status score of 0–1, less than 10% weight loss (in the month before study entry), and pulmonary function (before or after broncho d ilation) of 1·2 L per s or higher. Tumour histology was classifi ed as squamous cell, adeno-carcinoma, large-cell carcinoma, or non-small-cell lung cancer not otherwise specifi ed. Specifi c mutational analyses were not necessary for trial entry. Patients with contralateral hilar or supraclavicular adenopathy or Pancoast tumours were excluded because of the risk of lung or brachial plexus toxic eff ects. Minimum pleural eff usions were allowed if they were transudative and cytologically negative by thoracentesis. CT of the lungand upper abdomen and brain MRI with contrast wasneeded within 6 weeks of registration. Laboratory investigation requirements included the following: absolute neutrophil count of 1800 cells per μL or higher,platelets 100000 cells per μL or higher, haemoglobin 10 g/dL or higher, normal serum creatinine and bilirubin,and aspartate aminot ransferase and alanine amino-transferase concent rations 2·5 times or lower the upper institutional normal limit.The institutional review board of each participating institution approved the study protocol. All patients were required to read and sign an institutional review board approved informed consent document.Randomisation and maskingWe randomly assigned (1:1:1:1) eligible patients to one of four treatment groups: 60 Gy versus 74 Gy with concurrent and consolidation chemotherapy, with or without cetuximab. Treatment groups were assigned with the permuted block randomisation scheme described by Zelen 11 and stratifi ed by radiotherapytechnique (three dimensional conformal radiotherapy vs intensity-modulated radiation therapy), Zubrod performance status at the time of enrollment (0 vs 1), use of PET during tumour staging (no vs yes), and histology (squamous vs non-squamous). Allocation sequences were generated algorithmically at the RTOG statistics and data management centre, and access to these sequences by participating centres and statistics and data management was prohibited. Participating centres enrolled patients initially via the RTOG website, and then beginning on June 2, 2011, via the National Cancer Institute’s Oncology Patient Enrollment Network (OPEN) enrolment system, which remotely accessed the allocation sequence as necessary througha secure connection. Treatment group assignments, once allocated, were not masked.ProceduresRadiation therapy was given 5 days per week (ie, Monday to Friday with the weekend off ) in 2 Gy fractions daily by use of 6–18 MV x-rays. Use of image-guided radiation therapy was encouraged. Both three-dimensional conformal and intensity-modulated radiation therapy were allowed. Compliance with normal tissue dose constraints was encouraged but not neccessary (appendix p 1). Radiation doses were prescribed to the planning target volume. Motion management was required, and internal target volumes, clinical target volumes, and planning target volumes depended on which motion management method was used. Use of PET or CT and four-dimensional CT for radiation therapy planning was encouraged. Elective nodal irradiation was not permitted. The gross tumour volume was defi ned as the primary tumour and any regionally involved nodes on CT (>1 cm on short axis) or pretreatment PET scan (standardised uptake value >3). The internal target volume was defi nedSee Online for appendixFor the trial protocol see http:///ClinicalTrials/ProtocolTable/StudyDetails.aspx?study=0617Articlesas the envelope that encompasses the gross tumour volume plus ventilatory motion. Clinical target volume margins were 0·5–1·0 cm beyond the internal target volume. Planning target volume margins were 0·5–1·5 cm beyond the clinical target volume, depending on the use of four-dimensional CT for planning and image-guided radiation therapy for delivery. The appendix shows institutional credentialing protocol compliance definitions for both radiation therapy and chemotherapy (appendix p 2). Radiation therapy plans were reviewed centrally and scored for both target delineation and dose and normal tissue delineation and dose on submitted plans. Per-protocol planning target volume coverage was achieved when more than 99% of the planning target volume received 93% or more of the prescribed dose and when minimum margin values for both clinical target volume and planning target volume were achieved.Chemotherapy consisted of weekly paclitaxel (45 mg/m² per week) and carboplatin (area under the curve [AUC] 2 per week) during radiation therapy.2 weeks after chemoradiation, two cycles of consolidation chemotherapy separated by3 weeks were given consisting of paclitaxel (200 mg/m²) and carboplatin (AUC 6). Paclitaxel was given for 3 h 30 min after diphenhydramine (25–50 mg), followed by an H2 blocker, and dexamethasone (oral or intravenous administration allowed). Carboplatin was given for 30 min with standard anti-emetics after paclitaxel. Patients in the cetuximab groups received the agent during both concurrent and consolidative phases. Cetuximab was given at 400 mg/m² intravenously on day 1, with concurrent chemoradiation starting on day 8. Weekly cetuximab dosing was 250 mg/m², given before chemotherapy and radiation therapy that day. Consolidation cetuximab (250 mg/m²per week) was given weekly during consolidation.We did follow-up assessments every 3 months for the fi rst year, every 4 months for year 2, every 6 months for years 3–5, then every year. Routine follow-up assessments included assessment of vital signs, Zubrod performance status, and any adverse events. CT scans were done every 6 months for the fi rst 2 years, and then once a year. Pulmonary functioning was assessed at 6 months and then 1 year after completion of treatment. All data were collected by the enrolling site and then reported to RTOG via standard case report forms. All adverse events were graded with the CTCAE version 3.0 criteria; response was assessed per the RECIST criteria.12Pathological biomarker analysis was based on the FLEX trial,13 which suggested that the use of cetuximab in patients with EGFR expressing non-small-cell lung cancer conferred a survival benefit. We assessed the association between EGFR status in tumour samples that were available and the effect of cetuximab on patient outcomes. EGFR status established centrally was reported as H scores on the basis of EGFR immunohistochemical staining, with a score of 200 or more defi ned as a positive score.11OutcomesThe coprimary objectives of this trial were to compare the overall survival of patients given 74 Gy with those given 60 Gy conformal radiation therapy with concurrent chemotherapy and to compare the overall survival of patients given cetuximab with those not given cetuximab. There were several secondary objectives including a comparison of progression-free survival and local regional tumour control, comparison of toxic eff ects between 74 Gy versus 60 Gy, and between cetuximab versus without cetuximab, to assess patient-reported quality of life in each group of the trial (Movsas et al, unpublished data), and to explore biological markers that might predict clinical outcome (ie, EGFR expression by use of H score).Statistical analysisThe trial was a two-by-two factorial design, with radiation therapy dose as one treatment factor and cetuximab as the other. A log-rank test for each factor at one-sided α of 0·0125 (α of 0·0250 for both factors to account for multiple comparisons) would yield statistical power of 80% to detect an improvement in median overall survival from 17·1 months to 24 months after 339 deaths were reported from a sample of 500 patients. Three interim analyses with early stopping criteria with H aybittle14 and Peto15 boundaries for efficacy and Freidlin and Korn16 methods for futility were planned after 85, 170, and 225 events, and overseen by the independent RTOG data monitoring committee. Results are reported on a modifi ed intent-to-treat basis with all patients included in the assigned group, irrespective of treatment received, but excluding those patients who were found not to meet the pre-defi ned eligibility criteria. Endpoints of overall survival, progression-free survival, local failure, and distant metastasis were measured from the date of randomisation. We estimated overall survival and progression-free survival with the Kaplan-Meier method,17 compared with the log-rank test,18 and modelled with the Cox proportional hazards method.19 We used the cumulative incidence method20 to estimate local failure and distant metastasis rates, which were compared with the Gray’s test,21 and that were modelled with the Fine-Gray method.22 We compared categorical data with χ² test statistics; continuous data were compared with t tests or Wilcoxon rank sum tests as appropriate. All analyses were done with SAS version 9.2 except for Fine-Gray modelling, which was done with R (version 2.11.1). The appendix shows additional methods (appendix p 8).At the first interim analysis in June, 2011, the monitoring committee established that the trial hadArticlesArticles crossed the futility boundary with respect to high-dose Arrayradiation. The high-dose radiation groups were thenclosed, and the trial continued accruing patients to the60 Gy with and without cetuximab groups. At the thirdinterim analysis in June, 2013, it was likewiseestablished that a futility boundary with respect tocetuximab had been crossed.The study is registered with ,number NCT00533949.Role of the funding sourceThe trial design, data collection, analysis, interpretationof the data, and writing of the report was theresponsibility of the authors. The NCI approved thetrial design, monitored trial progress, and received thetwo interim futility analyses of both the radiotherapyand cetuximab endpoints. Bristol-Myers Squibb agreedto the initial trial design and received the data reportsfor both the radiotherapy and cetuximab endpoints.RTOG statisticians (RP and CH) had access to the rawdata. The corresponding author had full access to all ofthe data and the final responsibility to submit forpublication.ResultsBetween Nov 27, 2007, and Nov 22, 2011, the trialaccrued 544 patients from 185 institutions (hospital andoutpatient centres; median two per institution, range1–18), 464 while the radiotherapy dose randomisationwas still in effect, and 514 while the cetuximabrandomisation was in effrandomisation was closed early because of futility, butthe cetuximab randomisation met targeted accrualgoals. This report includes all data reported as of Oct 24,(IQR 10·5–30·3). Figure 1 shows the trial profi le. Afterexclusions, 495 patients were available for analysis(424 for the radiation therapy endpoint and 465 for the(IQR 57–70), and most patients were white men.Treatment technique was equally distributed betweenthree-dimensional conformal radiation therapy andintensity-modulated radiation therapy. 449 (91%)patients underwent diagnostic PET staging.Protocol compliance reviews were done for both(appendix pp 3–7). Rates of protocol non-complianceFigure 1: Trial profi leThe trial was initially opened only to the radiation dose groups. After the initial30 patients were enrolled, the cetuximab groups were opened to accrual. Afterthe 464th patient was enrolled, the 74 Gy and 74 Gy plus cetuximab groupswere closed to accrual because the futility boundary relating to the radiationtherapy endpoint had been crossed. We then continued to enrol to the 60 Gyand 60 Gy plus cetuximab groups. Thus the numbers of patients enrolled to theradiation therapy and cetuximab endpoints diff er according to these threeenrolment periods. CBC=complete blood count.Articleswith radiation therapy were greater in the high-dose group than in the standard-dose group (54 [26%] of 207 patients vs 37 [17%] of 217 patients; p=0·02), as were treatment delays. Contouring scores were poorer in the high-dose group than in the low-dose group for the planning target volume, heart, and brachial plexus.13 patients did not receive any radiation therapy. Most of the remaining patients received full dose with no interruptions in radiation therapy. There were no differences in receipt of radiation therapy in the cetuximab comparison. We recorded no diff erence in chemotherapy delivery between groups. Concurrent chemotherapy delivery was per-protocol in 192 (88%) of 217 patients in the 60 Gy group and 175 (85%) of 207 patients in the 74 Gy group, with corresponding acceptable variation in 14 (6%) of 217 and 12 (6%) of 207 patients. Consolidation chemotherapy delivery did not differ between groups. H owever, fewer patients completed this treatment: per-protocol consolidation chemotherapy was completed by 151 (70%) of 217 patients in the 60 Gy group and 133 (64%) of 207 patients in the 74 Gy group, with acceptable variations noted of 11 (5%) of 217 and 11 (5%) of 207 patients. Concurrent chemotherapy delivery was per protocol in 198 (84%) of 237 patients in the cetuximab group and 203 (89%) of 228 patients in the no cetuximab group, with corresponding acceptable variation in 19 (8%) of 237 and 10 (4%) of 228 patients. Consolidation chemotherapy was completed by 159 (67%) of 237 patients in the cetuximab group and 153 (67%) of 228 patients in the no cetuximab group, with acceptable variations noted of 9 (4%) of 237 and 15 (7%) of 208 patients.Cetuximab compliance in patients assigned to cetuximab was not diff erent between the standard-dose and high-dose groups (131 [96%] of 137 patients in the standard-dose group vs 90 (90%) of 100 patients in the high-dose group) during the concurrent phase or in the consolidation phase (107 [78%] of 137 vs 73 [74%] of 99 patients).Median follow-up for the 419 analysable patients randomly assigned to the radiation therapy dose question was 22·9 months (IQR 27·5–33·3). Table 2 shows overall survival, progression-free survival, and failure rates. Median overall survival for the standard-dose group was 28·7 months (95% CI 24·1–36·9) and that for the high-dose group was 20·3 months (95% CI 17·7–25·0; one-sided p=0·996 for superiority of 74 Gy; two-sided p=0·008). The hazard ratio for radiation dose (74 Gy vs 60 Gy) on overall survival was 1·38 (95% CI 1·09–1·76; fi gure 2). 116 (58%) of 217 patients were alive at 2 years in the standard-dose group compared with 87 (45%) of 207 patients in the high-dose group. The primary cause of death was lung cancer (96 [76%] of 127 patients in the 60 Gy group and 105 [75%] of 140 patients in the 74 Gy group).Planning target volumes were similar in the two groups (mean 494·8 cm³ [SD 287·3] for standard-dose group vs 509·9 cm³ [274·7] for high-dose group). The percentages of the planning target volume covered by either 95% of the prescription dose (median 99·8% [IQR 98·9–100·0] in the standard-dose group vs 99·3% [97·1–99·9] in the high-dose group) or 100% of the prescription dose (median 95·3% [IQR 92·8–97·0] in the standard-dose group vs 94·3% [77·8–96·3] in the high-dose group) were significantly higher in the standard-dose group (p<0·0001). Mean lung dose was significantly higher in the high-dose group (mean 16·5 Gy vs 18·9 Gy; p<0·0001). We noted no diff erence in lung V5 distribution (mean 57·7% [SD 16·2] in the standard-dose group vs 58·0% [15·0] in the high-dose group), but lung V20 was higher in the high-dose group (mean 28·7% [7·9] vs 30·9% [7·8]; p=0·0012). The mean and maximum margin between planning target volume and clinical target volume were similar in both groups, but the minimum margin was smaller in the high-dose group (mean 4·5 mm [2·9] in the standard-dose group vsArticles 3·9 mm [3·0] in the high-dose group; p=0·0047).Oesophageal doses were signifi cantly higher in the high-dose group. Heart dose was also signifi cantly higher inthe high-dose group. The appendix shows a completesummary of dosimetric variables (appendix p 9–10).On univariate analysis, increasing values of grosstumour volume, planning target volume, lung V5, heartV5, and heart V30 were associated with increased riskof death. On multivariate analyses, factors predictingoverall survival were radiation dose (60 Gy), maximumoesophagitis grade, planning target volume, and heartV5 and V30. Notably, neither radiotherapy compliancenor technique (three-dimensional conformal radiationtherapy or intensity-modulated radiation therapy) weresignifi cant in these analyses. The appendix shows themultivariate analyses for both the radiation therapy andcetuximab endpoints (appendix pp 10–11).compromised overall survival or local failure within thetrial, we did separate analyses limiting the dataset tocases compliant on two measures—physician radiationtherapy review and 95% of the dose covering 90% ormore of the planning target volume. Signifi cant overallsurvival benefi ts were maintained for the 60 Gy group(appendix p 12). Although this unplanned subsetanalysis excludes patients who did not complete theradiation therapy course, it strongly suggests thatradiation therapy compliance was not the cause for thepoorer performance of the high-dose group.Additionally, we assessed the interaction of radiationtherapy dose and cetuximab use to establish whether theuse of cetuximab aff ected the dose results. We recordedno interactive eff ect (pinteraction=0·3984; appendix p 13).Median follow-up for the 465 analysable patientsrandomly assigned to the cetuximab question was21·3 months (IQR 23·5–29·8). Table 2 shows survivaland failure endpoints. Cetuximab showed no evidenceof benefit in terms of overall survival (25·0 months[95% CI 20·2–30·5] with cetuximab vs 24·0 months[19·8–28·6] without; one-sided p=0·29 for superiority;two-sided p=0·58; HR 0·94 [95% CI 0·74–1·19]; table 2).103 (52%) of 237 patients who received cetuximab and96 (50%) of 228 patients who did not were alive at2 years. The primary cause of death in both cohorts waslung cancer (107 [76%] of 140 patients in the cetuximabgroup and 103 [74%] of 139 patients in the no-cetuximabgroup). There was no diff erence in survival with the useof cetuximab based on histological subsets (data notshown).We did a separate planned retrospective analysis ofthe association of EGFR expression and outcome withcetuximab by use of prospectively obtained specimen.There were 203 patients (101 given cetuximab and 102not given cetuximab) with usable samples (table 1). AnEGFR H-score less than 200 (low EGFR expression) was noted more commonly in patients with non-squamous histology whereas an EGFR H score of 200 or more (EGFR-overexpression) was more common inthose with squamous histology (p=0·0003). We notedno diff erences in outcomes between H-score groups.Figure 2: Kaplan-Meier overall survival curves for radiation dose (A) and the use of cetuximab (B) (A) One-sided log-rank p=0·0042. (B) one-sided lo g-rank, p=0·2938.Figure 3: Kaplan-Meier overall survival curves for patients with high EGFR expression (H score ≥200) Two-sided log-rank, p=0·0325.。
