MolBio_Sp04_pres_20
cas638132-34-0_ONO-7300243_MedBio使用方法

品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
MED13195
L-365,260
L-365,260
118101-09-0
50mg
≥98%
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
MED13205
Atipamezole hydrochloride
Atipamezole hydrochloride
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
MED13078
Mesulergine hydrochloride
Mesulergine hydrochloride
72786-12-0
50mg
≥98%
Kinetensin (human)
103131-69-7
1mg
≥98%
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
MED12631
Montelukast Sodium
Montelukast Sodium
151767-02-1
10mM (in 1mL DMSO)
≥98%
品牌
货号
中文名称
英文名称
104075-48-1
10mg
≥98%
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
MED12896
DV 7028 hydrochloride
大孔合成吸附树脂介绍

大孔合成吸附树脂介绍><: 提纯介质大孔树脂吸附技术是上世纪七十年代发展起来的一种新工艺。
这是一种纯化精制药的有效方法,其工艺程序是药液通过大孔树脂吸附,其中的有效成分吸附在树脂上,再经洗脱回收,除掉药液中杂质。
当然,根据药液成分和提取物的不同,可选择不同型号的树脂。
非极性吸附树脂在吸附药液中成分时,主要依靠物理结构(如比表面、孔径等)起作用,不同的树脂有不同的针对性。
其操作的基本程度大多是:提取液-通过大孔树脂-吸附上有效成分的树脂-洗脱-洗脱液回收-洗脱液干燥-半成品。
该技术目前已广泛应用于新药的开发和生产中,主要用于分离和提纯。
1.(1)适合中等程度的水溶性化合物:中药、天然色素、从发酵液中提取抗生素(青霉素、先锋霉素、螺旋霉素)、蛋白质(胰岛、肽系抗生素)、功能性食品添加剂(维生素)等。
(2)聚苯乙烯合成吸附树脂:吸附含有π电子的合化物,如含有苯环和共轭双键的化合物。
(3)甲基丙烯酸甲酯类吸附剂:吸附含羧基、酯基、氨基、酰胺基等与H可结合的官能团的化合物。
合成吸附树脂的选择标准必须以其吸附能力、吸附速度、选择性、树脂寿命等为主要决定因素,其中树脂的微孔结构影响最大,因为它决定了树脂吸附能力的高低。
此外,在有机溶剂中的膨胀程度、耐压性能和比重也是考滤选用的重要因素。
(1)水溶性较高的化合物应采用离子交换或分子尺寸排除模式提取。
(2)水不溶化合物应使用溶剂提取或正相色谱等提取。
2.(1)同一类药采用大孔树脂提纯后,药效得到显著提高。
这一结论已经通过药效学试验和临床观察得以证实。
该工艺一次完成了除杂和浓缩两道工序,如人参茎叶中也含人参皂甙,可以提取出来作为药用,但含量低,用一般方法提取麻烦,而用大孔树脂吸附技术提纯后,人参皂甙含量可达70%以上,提取方法简便。
(2)减小产品的吸潮性。
传统工艺制备的中成药大部分都有较强的吸潮性,是中药生产及贮藏中长期存在的难题。
经大孔树脂吸附技术处理后,有效地去除了水煎液中大量的糖类、无机盐、黏液质等吸潮成分,有利于多种中药剂型的生产、增强产品的稳定性。
谷胱甘肽还原酶测定试剂盒 (谷胱甘肽底物法)产品技术要求北京世纪沃德生物
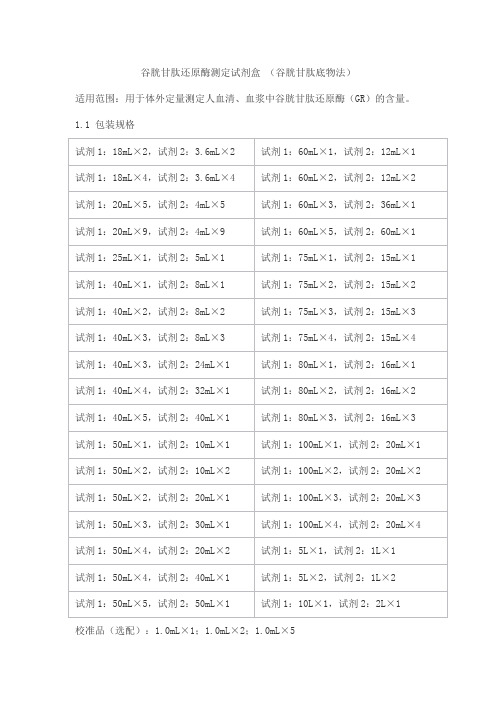
谷胱甘肽还原酶测定试剂盒(谷胱甘肽底物法)适用范围:用于体外定量测定人血清、血浆中谷胱甘肽还原酶(GR)的含量。
1.1 包装规格校准品(选配):1.0mL×1;1.0mL×2;1.0mL×5质控品(选配):水平1: 1.0mL×1;1.0mL×2;1.0mL×5水平2: 1.0mL×1;1.0mL×2;1.0mL×5 1.2主要组成成分校准品靶值批特异,详见校准品瓶签;质控品质控范围批特异,详见质控品瓶签。
2.1 外观试剂1为无色澄清液体;试剂2为无色澄清液体;校准品、质控品为白色至黄色冻干粉,复溶后为无色至黄色液体;试剂盒标签标识清晰,外包装完整无损。
2.2 装量试剂液体成分的装量不少于瓶签标示量。
2.3 试剂空白2.3.1试剂空白吸光度在340nm处测定试剂空白吸光度,应≤1.5。
2.3.2试剂空白吸光度变化率在340nm处测定试剂空白吸光度变化率(ΔA/min)应≤0.01。
2.4 分析灵敏度测试70U/L的被测物时,吸光度变化率(ΔA/min)的绝对值应≤0.5。
2.5准确度待检系统与已上市比对系统测值的相关系数r≥0.975;在[10,30]U/L区间内,绝对偏差应不超过±4.5U/L;在(30,184]U/L区间内,相对偏差应不超过±15%。
2.6 线性2.6.1在[10,184]U/L区间内,线性相关系数r≥0.990;2.6.2在[10,30]U/L区间内,线性绝对偏差应不超过±4.5U/L;在(30,184]U/L 区间内,线性相对偏差应不超过±15%。
2.7 精密度2.7.1重复性测试高、低两浓度样本,其结果的变异系数CV应≤10%。
2.7.2 批间差随机抽取三批试剂盒,测试同一份样本,批间相对极差(R)应≤10%。
2.7.3瓶间均匀性校准品、质控品瓶间变异系数CV≤10%。
自噬研究方法

MDC:取12 mg粉末溶于720 nl DMSO使其浓度为50 mmol/L,分装后-20冰箱保存。
临用前用MEM稀释到终浓度50 umol/L;Rapamycin:用MEM培养基配成终浓度为1 umol/L,现用现配;400ng/ml喹乙醇:称取4 mg喹乙醇,DMSO预溶(体积<0.1%)后加入10 ml MEM培养液至完全溶解,现用现配,避光保存;3-MA:首先用PBS溶解粉末,临用前加热至完全溶解后再加入MEM培养基至终浓度10mmol/L; PI3K抑制剂(3-MA,Wortmannin)可干扰或阻断自噬体的形成用RAPAMYCIN诱导自噬我也查过一部分文献,有用无血清的,也有用,一般培养基的,浓度从25nM到100nM都有,用的是50nM的雷帕霉素,加入一般的培养基中,目的是排除无血清所诱导出来的自噬。
文献说饥饿初期激活的是大分子自噬,在4-6小时活力达到最大,24h后以CMA途径为主Earle's balanced salts solution (EBSS) for 48 hsigma的EBSS,货号E2888,有碳酸氢钠,有酚红的,酚红到不是很必须,只是一个PH指示作用,好看些无血清诱导自噬:EBSS 诱导6个小时就可以了。
EBSS一定可以诱导出来,只是需要说明的是时间点的设置,因为从饥饿诱导开始半个小时就可能开始自噬了,一直到24小时都持续,所以应该设置不同的时间点观察这个作用。
另外一个很大的问题是,饥饿诱导的一个很大的弊端是细胞死亡,这也是我面临的问题,就是在细胞收养的时候蛋白浓度太小了。
24小时就很少了,更不要说48小时和72小时了Hank's诱导,也就是通常所说的饥饿诱导,细胞培养到对数生长期后以Hank's替代常规完全培养基,3h后就可诱导出自噬。
我用Hank's诱导了3h后电镜观察有30%细胞都有自噬这种现象,但不如国外报道的高。
泊洛沙姆的型号

407-0 泊洛沙姆 Poloxamer[别名] 普流罗尼克:Poloxalkol: Monolan;Supronic'PolyvethylenePropylene Glycol;Pluronic.[来源与制法] 本品为合成品,其合成方法是先将氧化丙烯缩合到丙二醇基上,再将氧化乙烯缩合到聚(氧丙烯)基的两端而制得的氧乙烯、氧丙烯嵌段聚合物,共聚物分子中聚乙烯亲水链占10~80%,余下的则为聚氧丙烯亲脂链,不同的规格型号,所占比例各不相同。
[性状] 本品规格型号有多种,随聚合度增大,物态从液体、半固本至蜡状固体,从难溶于水的液体到易溶于水的固体。
均有较高的HLB值。
有些型号的产品如泊洛沙姆 124、188等,在通常使用的浓度,实际上是无色、无臭、无味的。
溶液可以高压蒸气灭菌不会分解破坏。
分子中存在众多醚键,能与水的质子形成氢键,温度升高到一定程度对氢键破坏而出现起昙现象,10%水溶液的昙点在80~90℃之间。
有些型号的产品如泊洛沙姆108、188、124,l%浓度昙点在l00℃以上。
多数型号的产品在水中易溶,溶解度随分子中氧乙烯含量的增加而增加,在矿物油中不溶,在乙醚和石油醚中几乎不溶,溶于无水乙醇、乙酸乙酯、氯仿。
2.5%水溶液的,H在5.0~7.5之间,注射用者pH在6.0~ 7.0。
本品有一定的起泡性,1%水溶液,在 40℃时,400ml/min的流速,泡沫高度为 600mm。
水溶液在空气中较稳定,遇光则使 pH值下降。
本品属于非离子表面活性剂,具有良好的乳化性,同系物中,聚氧丙烯含量较高,乳化性越好。
对矿物油和烷烃类的乳化性比对脂肪油的乳化性好。
临界胶团浓度(CMC值)约为0.2%,胶团结构的分子数在8个以下。
分子量大的同系物具有在水溶液中形成凝胶(Gels)的性质。
本品对酸、碱水溶液和金属离子稳定。
泊洛沙姆部分型号产品的理化参数泊洛沙姆型号物理形态氧乙烯含量(%) 不饱和值mmol 熔点℃粘度(>7℃)cp HLB 最低凝胶浓度浓%W/W124 液体46.7±1.9 0.020±0.008 16 440 16188 固体81.1±0.63 0.26±0.004 52 100 29 60237 固体72.4±1.9 0.034±0.008 49 700 24 65338 固体83.1±1.7 0.031±0.008 57 2800 27 30407 固体73.2±1.7 2.048±0.017 56 3100 22 20[作用与用途] Poloxamers(Px)为一大类非离子表面活性剂,具有上述各种优良性质,现已广泛用于制药工业,是一类优良的药物制剂新辅料,其作用与用途如下;1.作乳化剂和稳定剂用于制备乳剂, Pxl88是制备静脉脂肪乳剂良好的乳化剂,形成O/W型乳剂,用量0.1~5%。
Rink Amide Resin_0_13653-84-4_Apexbio

For obtaining a higher solubility , please warm the tube at 37°C and shake it in the ultrasonic bath for a while.Stock solution can be stored below -20°C for several months. Evaluation sample solution : ship with blue ice All other available size: ship with RT , or blue ice upon request
产品说明书
化学性质
产品名: Cas No.: 分子量: 分子式:
Rink Amide Resin 13653-84-4 318.32 C20H14O4
【脉铂医药】MedBio143360-00-3SMIP004技术资料
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
MED11190
2-[(2,6-dioxocyclohexyl)methyl]cyclohexane-1,3-dione
Apoptosis Inhibitor
54135-60-3
25mg
≥98%
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
MED11328
CAS
包装
纯度
MedBio
MED11270
VU0661013
VU661013
2131184-57-9
10mg
≥98%
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
MED11183
(3S)-3-[[叔丁氧羰基]氨基]-5-氟-4-氧代-戊酸甲酯
Boc-D-FMK
187389-53-3
10mg
≥98%
【脉铂医药】MedBio143360-00-3SMIP004技术资料
1、SMIP004物理参数:
常用名
SMIP004
英文名
SMIP004
CAS号
143360-00-3
分子量
205.29600
密度
1.003g/cm3
沸点
343ºC at 760mmHg
分子式
C13H19NO
熔点
无资料
闪点
206.1ºC
2、SMIP004物理化学性质:
密度
1.003g/cm3
沸点
343ºC at 760mmHg
生物指示剂

生物指示剂LT另外根据客户要求可提供:生孢梭菌(Clostridium sporogenes),枯草芽孢杆菌(B. subtilis),巨大芽孢杆菌(B. megaterium),蜡状芽孢杆菌(B. cereus)等的芽孢悬液。
2.工业生物指示剂工业生物指示剂是根据工业企业实际情况,按照标准要求定制加工而成,以满足企业的特殊需求,通常采用不同的载体材料和包装形式,例如钢片、钢线、纸片、棉线、塑料片和滑石粉等,它与芽孢条、自含式等标准生物指示剂相比更具优势。
CICC可按照客户要求提供定制工业生物指示剂。
染菌滑石粉CICC研制的标准专用染菌滑石粉,以符合标准要求的滑石粉为载体,选用CICC 自行生产的微生物材料萎缩芽孢杆菌ATCC 9372芽孢悬液,按标准要求精制而成,每批染菌滑石粉产品均经过芽孢含量检验,确保质量稳定。
该产品适用于评定屏障材料对携菌微粒阻穿透性的实验方法,适用于国家医药行业手术衣标准《病人、医护人员和器械用手术单、手术衣和洁净服》(YY/T 0506-2009)、国际标准ISO 22612:2005 Clothing for protection against infectious agents -- Test method for resistance to dry microbial penetration(传染介质防护服--防干微生物渗入的试验方法)。
产品参数:产品名称染菌滑石粉微生物材料萎缩芽孢杆菌(B. atrophaeus)ATCC 9372芽孢含量108 CFU/g规格0.5g+0.1 g /瓶粒度<800目(95%<15μm)贮藏条件2~8℃,避免阳光直射和接触灭菌剂。
有效期生产日起12个月染菌石英粉CICC研制的标准专用染菌石英粉,以符合标准要求的石英粉为载体,选用CICC 自行生产的微生物材料萎缩芽孢杆菌ATCC 9372芽孢悬液,按标准要求精制而成,每批染菌石英粉产品均经过芽孢含量检验,产品质量稳定。
IFCC Aspartate Aminotransferase 检测手册说明书

ASTAspartate Aminotransferase IFCCMANUAL RX MONZAINTENDED USEFor the quantitative in vitro determination of AspartateAminotransferase (AST) in serum and plasma. This product is suitable for manual use and on the Rx Monza analyser.Cat. No. AS 1202 R1a. Buffer/Substrate 1 x 70 ml 20 x 2 ml R1b. Enzyme/Coenzyme/ 20 x 2 ml α-oxoglutarate GTIN: 05055273200416AS 1204 R1a. Buffer/Substrate 1 x 105 ml 10 x 10 ml R1b. Enzyme/Coenzyme/ 10 x 10 ml α-oxoglutarate GTIN: 05055273200423AS 1267 R1a. Buffer/Substrate 1 x 105 ml 5 x 20 ml R1b. Enzyme/Coenzyme/ 5 x 20 ml α-oxoglutarate GTIN: 05055273200430AS 2359 R1a. Buffer/Substrate 5 x 100 ml 5 x 100 ml R1b. Enzyme/Coenzyme/ 5 x 100 ml α-oxoglutarate GTIN: 05055273200454UV METHODThis is an optimised standard method according to the concentrations recommended by the IFCC.CLINICAL SIGNIFICANCE (1,2,3,4)The aminotransferases are a group of enzymes that catalyse the inter conversions of amino acids and α-oxoacids by transfer of amino groups. AST (aspartate aminotransferase or glutamate oxaloacetatetransaminase) has been found in the cytoplasm and the mitochondria of cells that have been studied. In cases of mild tissue damage, e.g. liver, the predominant form of serum AST is that from the cytoplasm, with a smaller amount coming from the mitochondria. Severe tissue damage will result in more mitochondrial enzyme being released. Elevated levels of AST can signal myocardial infarction, hepatic disease, muscular dystrophy and organ damage.Although heart muscle is found to have the most activity of the enzyme, significant activity has also been seen in the brain, liver, gastric mucosa, adipose tissue and kidneys of humans.The IFCC has now recommended (1980) standardised procedures for AST determinations including:-1. optimization of substrate concentrations.2. Employment of Tris buffers (instead of phosphate, which has beenshown to inhibit recombination of the apoenzyme with pyridoxal phosphate).3. Pre-incubation of combined buffer and serum to allow sidereactions with NADH to occur. 4. Substrate start (α-oxoglutarate)5. Optional pyridoxal phosphate activation.This is an optimised standard method according to the recommendations of the IFCC.PRINCIPLEα-oxoglutarate reacts with L-aspartate in the presence of AST to form L-glutamate plus oxaloacetate. The indicator reaction utilises the oxaloacetate for a kinetic determination of NADH consumption. AST -oxoglutarate + L-aspartate L-glutamate + oxaloacetate MDH oxaloacetate + NADH + H + L-malate + NAD +SPECIMEN COLLECTION AND PREPARATION (5) Serum:- Use serum free from haemolysis.Plasma:- EDTA or heparin can be used as the anticoagulant.Plasma should be separated from cells within one hour after collection.Specimens should be refrigerated if not used immediately:-Specimens stored longer than 3 days should be frozen at -20︒C.REAGENT COMPOSITIONContents Concentrations in the TestR1a. Buffer/Substrate Tris buffer 80 mmol/l, pH 7.5 L-aspartate 240 mmol/l R1b. Enzyme/Coenzyme/α-oxoglutarate α-oxoglutarate 12 mmol/l MDH ≥420 U/l LD ≥600 U/l NADH 0.18 mmol/lSAFETY PRECAUTIONS AND WARNINGS For in vitro diagnostic use only. Do not pipette by mouth.Exercise the normal precautions required for handling laboratory reagents.Solution R1a contains Sodium Azide. Avoid ingestion or contact with skin or mucous membranes. In case of skin contact, flush affected area with copious amounts of water. In case of contact with eyes or if ingested, seek immediate medical attention.Sodium Azide reacts with lead and copper plumbing, to form potentially explosive azides. When disposing of such reagents flush with large volumes of water to prevent azide build up. Exposed metal surfaces should be cleaned with 10% sodium hydroxide.Health and Safety data sheets available on request.The reagents must be used only for the purpose intended by suitably qualified laboratory personnel, under appropriate laboratory conditions.STABILITY AND PREPARATION OF REAGENTS R1a. Buffer/SubstrateContents ready for use. Stable up to the expiry date when stored at +2 to +8︒C.R1b. Enzyme/Coenzyme/α-oxoglutarate Reconstitute one vial of Enzyme/Coenzyme/α-oxoglutarate R1b with the appropriate volume of Buffer/Substrate R1a: 2 ml for the 20 x 2 ml kit (AS 1202) 10 ml for the 10 x 10 ml kit (AS 1204) 20 ml for the 5 x 20 ml kit (AS 1267) Stable for 14 days at +2 to +8︒C or 24 hours at +15 to +25︒C. Cat. AS 2359 5 x 100 mlReconstitute one vial of Enzyme/Coenzyme/α-oxoglutarate R1b with a portion of Buffer/Substrate R1a and then transfer the entire contents to bottle R1a rinsing bottle R1b several times. Stable for 14 days at +2 to +8︒C or 24 hours at +15 to +25︒C.MATERIALS PROVIDED Buffer/SubstrateEnzyme/Coenzyme/ -oxoglutarateMATERIALS REQUIRED BUT NOT PROVIDEDRandox Assayed Multisera Level 2 (Cat. No. HN 1530) and Level 3 (Cat. No. HE 1532)Randox Calibration Serum Level 3 (Cat. No. CAL 2351) RX series Saline (Cat. No. SA 3854)PROCEDUREAspirate fresh ddH 2O and perform a new Gain Calibration in flow cell mode. Select AST in the Run Test screen and carry out a water blank as instructed.Pipette into a test tube:Sample 0.05 ml Reagent 0.5 mlMix and aspirate into the Rx Monza.CALIBRATION FOR RX MONZAThe use of Saline and Randox Calibration Serum Level 3 isrecommended for calibration. Calibration is recommended with change of reagent lot or as indicated by quality control procedures.FOR MANUAL USEWavelength: 340 nm (Hg 334 nm or Hg 365 nm) Cuvette: 1 cm light path Temperature: 25/30/37︒C Measurement: against airPipette into cuvette: Macro MicroSample 0.2 ml 0.1 ml Enzyme/Coenzyme/ α-oxoglutarate R1 2.0 ml 1.0 mlMix, read initial absorbance after 1 minute. Read again after 1, 2 and 3 minutes. Note: If the absorbance change per minute is between 0.11 and 0.16 at 340/Hg 334 nm 0.06 and 0.08 at Hg 365 nmuse only the values for the first 2 minutes for the calculation.MANUAL CALCULATIONTo calculate the AST activity, use the following formulae:U/l = 1746 x A 340 nm/min U/l = 1780 x A Hg 334 nm/min U/l = 3235 x A Hg 365 nm/minSTANDARDISATIONRandox Calibration Serum Level 3 is traceable to AST reference material JSCC TS01.QUALITY CONTROLRandox Assayed Multisera, Level 2 and Level 3 are recommended for daily quality control. Two levels of controls should be assayed at least once a day. Values obtained should fall within a specified range. If these values fall outside the range and repetition excludes error the following steps should be taken:1. Check instrument settings and light source.2. Check cleanliness of all equipment in use.3. Check water. Contaminants, i.e. bacterial growth, maycontribute to inaccurate results. 4. Check reaction temperature.5. Check expiry date of kit and contents.6. Contact Randox Laboratories Customer Technical Services, Northern Ireland +44 (0) 28 9445 1070.SPECIFICITY/INTERFERENCE (6,7)Gross haemolysis will produce falsely elevated test results. The effects of various drugs on AST activity should be taken intoconsideration in the case of patients receiving large doses of drugs.The analytes below were tested up to the following levels and were found not to interfere: Haemoglobin 250 mg/dl Free Bilirubin 25 mg/dl Conjugate Bilirubin 25 mg/dl Triglycerides 1000 mg/dlIntralipid ® 200 mg/dlA list of substances and conditions known to effect AST activity in vivo is given by both Young et al and Friedman et al. Norepresentation is made by Randox Laboratories Ltd regarding the completeness of these lists and the accuracy of the information contained therein.NORMAL VALUES IN SERUM (8,9) +25︒C +30︒C +37︒C Men up to 18 U/l up to 25 U/l up to 37 U/l Women up to 15 U/l up to 21 U/l up to 31 U/lIt is recommended that each laboratory establish its own reference range to reflect the age, sex, diet and geographical location of the population.SPECIFIC PERFORMANCE CHARACTERISTICS The following performance data were obtained using an Rx Monza analyser running at +37o C.LINEARITYThis method is linear up to 562 U/l. If the sample concentration exceeds this value, dilute the sample 1+9 with 0.9% NaCl solution and re-assay. Multiply the result by 10.SENSITIVITYThe minimum detectable concentration of AST with an acceptable level of precision was determined as 9.3 U/l.PRECISIONIntra AssayLevel 2 Level 3Mean (U/l) 35.6 153SD 1.66 1.47CV(%) 4.65 0.96n 20 20Inter AssayLevel 2 Level 3Mean (U/l) 35.6 153SD 1.77 7.10CV(%) 4.96 4.63n 20 20CORRELATIONThis method (Y) was compared with another commerciallyavailable method (X) and the following linear regression equationobtained:Y = 1.07X + 4.9and a correlation coefficient of r = 0.997543 patient samples were analysed spanning the range 28 to 559U/l.REFERENCES1. Wroblewski F, La Due J.S: Ann Intern Med. 1956; 45: 801.2. Wroblewski F, La Due J.S: Proc Soc Exp Biol Med 1956;91: 569.3. Bergmeyer HU, Bowers GN Jr, et al: Clin Chem 1977; 23:887.4. Bergmeyer HU, Bowers GN Jr, et al: J.Clin Chem ClinBiochem 1980; 18: 521-534.5. Tietz N W: Fundamentals of Clinical Chemistry ed 3.Philadelphia, WB Saunders Co. 1987, pg 372.6. Young D S, et al: Clin Chem 1975, 21; No5.7. Friedman RB, et al: Clin Chem 1980, 26; No4.8. Wallnofer H, Schmidt.E, Schmidt FW, eds: Synopsis derLeberkrankheiten Stuttgart, Georg Thieme Verlag, 1974.9. Thefeld W, et al: Dtsch Med Wschr 1974; 99: 343.Revised 26 Apr 16 biRev. 003THIS PAGE IS INTENTIONALLY BLANK。
细胞粘附的检测方法
包被: 1、 96 孔板每孔加入 100ul 包被液。 2、 将培养板置 2-8°C 过夜。 3、 移除包被液,用洗涤液洗涤 2-3 次。
细胞接种: 1、 待测定细胞处理好后,用胰酶消化,PBS 洗涤,然后用相应培养基重悬,制成细胞
悬液。 2、 按 5×104 细胞/孔接种 96 孔板,建议设 3-5 个复孔。同时设立对照组,即孵育后不
体损失导致试剂量不够用。 ● 细胞处理需要小心操作,尽量避免人为的损伤细胞。离心力在不损失细胞的前提下尽可能小,重悬
细胞是动作要轻柔,避免多次反复的激烈吹打。不用涡旋振荡器。 ● 染色培养时间根据细胞种类的不同和每孔内细胞数量的多少而异。一般情况下,白细胞较难染色,
因此需要较长的培养时间。培养时间一般为 1- 4 小时,但在培养 30 分钟左右即可取出肉眼观察 染色程度 (根据细胞种类而定,需要摸索一下条件)。当使用标准 96 孔板时,贴壁细胞的最小接 种量至少为 1,000 个/孔 (100 μl 培养基)。检测白细胞时的灵敏度相对较低,因此推荐接种量不 低于 2,500 个/孔 (100 μl 培养基)。如果要使用 24 孔板或 6 孔板实验,请先计算每孔相应的接 种量,并按照每孔培养基总体积的 10%加入染色液 B。 ● 有条件的情况下建议采用多通道移液器,可以减少平行孔间的差异。加染色液 B 试剂时,建议斜
产品说明书
细胞粘附检测试剂盒
货号:BB-48120
V2.16
试剂盒储存条件: 2-8℃保存。
开盖后组份按要求条件保存。 Nhomakorabea试剂盒组成:
产品组成
BB-48120-1
BB-48120-2
规格
100 T
250 T
试剂 A:包被液 A
FDA兽药管理目录4

Section 2.0 - Active IngredientsApplicationActive Ingredients Trade NameNumber005-2362-Mercaptobenzothiazole Sulfodene® Medication for Dogs015-030Acepromazine Maleate PromAce® Injectable032-702Acepromazine Maleate PromAce® Tablets117-531Acepromazine Maleate Acepromazine Maleate Injection117-532Acepromazine Maleate Acepromazine Maleate Tablets200-319Acepromazine Maleate Acepromazine Maleate Injection200-361Acepromazine Maleate Acepromazine Maleate Injection011-582Acetazolamide Sodium Vetamox Soluble Powder011-700Acetylsalicylic Acid, Methylprednisolone Cortaba® Tablets141-406Afoxolaner NexGard™014-250Aklomide, Sulfanitran Novastat Type A Medicated Premix034-536Aklomide, Roxarsone Aklomix Type A Medicated Article, Aklomix-3034-537Aklomide, Roxarsone, Sulfanitran Novastat-3 Type A Medicated Article035-388Aklomide, Sulfanitran Novastat-W110-048Albendazole Valbazen®128-070Albendazole Valbazen®140-934Albendazole Valbazen®141-180Albuterol Sulfate Torpex™141-342Alfaxalone Alfaxan®131-310Altrenogest Regu-Mate®141-222Altrenogest Matrix®127-892Amikacin Sulfate Amiglyde-V200-178Amikacin Sulfate Amikacin Sulfate Injection200-181Amikacin Sulfate AmiMax™ E Solution043-078Aminopentamide Hydrogen Sulfate Centrine Oral Tablets043-079Aminopentamide Hydrogen Sulfate Centrine Injectable092-116Aminopentamide Hydrogen Sulfate, Ketamine Ketaset® PlusHydrochloride, Promazine Hydrochloride011-877Aminopropazine Fumarate Jenotone Tablets013-181Aminopropazine Fumarate, Neomycin Sulfate Jenomycin Tablets034-477Aminopropazine Fumarate Jenotone Solution120-299Amitraz Mitaban® Liquid Concentrate009-339Ammonium Chloride, Caramiphen Edisylate Carafen Cough Syrup, Carafen Cough Tablets055-078Amoxicillin Trihydrate Amoxi-Tabs055-080Amoxicillin Trihydrate Amoxi-DoserFDA APPROVED PRODUCTS January 2014Section 2.0 - Active Ingredients Page 1 of 70Number055-081Amoxicillin Trihydrate Amoxi-Tabs055-085Amoxicillin Trihydrate Amoxi-Drop® Oral Suspension055-087Amoxicillin Trihydrate Amoxi-Bol055-088Amoxicillin Trihydrate Amoxi-Sol055-089Amoxicillin Trihydrate Amoxi-Inject (Cattle)055-091Amoxicillin Trihydrate Amoxi-Inject (Dogs and Cats)055-099Amoxicillin Trihydrate, Clavulanate Potassium Clavamox® Tablets055-100Amoxicillin Trihydrate Amoxi-Mast055-101Amoxicillin Trihydrate, Clavulanate Potassium Clavamox® Drops065-492Amoxicillin Trihydrate Biomox065-495Amoxicillin Trihydrate Biomox®141-004Amoxicillin Trihydrate Robamox®-V141-005Amoxicillin Trihydrate Robamox®-V TabletsKanfosone Ointment043-784Amphomycin Calcium, Hydrocortisone Acetate,Kanamycin Sulfate047-997Amphomycin Calcium, Hydrocortisone Acetate,Amphoderm OintmentKanamycin Sulfate055-013Ampicillin Anhydrous Omnipen 250 mg055-084Ampicillin Sodium Amp-Equine200-335Ampicillin sodium Ampicillin Sodium055-030Ampicillin Trihydrate Polyflex®055-036Ampicillin Trihydrate Princillin Capsules 125 mg, Princillin Capsules 250 mg,Princillin Capsules 500 mg055-042Ampicillin Trihydrate Ampi-Tab055-050Ampicillin Trihydrate Princillin Soluble Powder055-056Ampicillin Trihydrate Princillin Bolus055-061Ampicillin Trihydrate Princillin "125" For Oral Suspension055-064Ampicillin Trihydrate Princillin Injection055-066Ampicillin Trihydrate Princillin Injection055-071Ampicillin Trihydrate Princillin Injection 200 mg055-074Ampicillin Trihydrate Ampi-Bol055-079Ampicillin Trihydrate Ampi-Ject200-180Ampicillin Trihydrate Ampicillin Trihydrate012-350Amprolium Amprovine 25%, CORID® 25% Type A Medicated Article 013-149Amprolium Amprovine 9.6% Solution013-461Amprolium, Ethopabate, Roxarsone Broiler PMX No.1620, Amprol Plus®, Amprol Hi-E®, AmprolPlus + Nitro®013-663Amprolium Purina® Liquid Amprol033-165Amprolium Amprovine 20% Soluble Powder, Corid 20% Soluble Powder FDA APPROVED PRODUCTS January 2014Section 2.0 - Active Ingredients Page 2 of 70NumberAmprol HI-E® Plus036-304Amprolium, Bacitracin Methylene Disalicylate,EthopabateAmp Ethopabate CTC® Sodium Sulfate 036-361Amprolium, Chlortetracycline Calcium Complex,Ethopabate, Sodium SulfateErythro® (Low Lev) / Amp plus Etho 038-242Amprolium, Arsanilic Acid, ErythromycinThiocyanate, Ethopabate041-178Amprolium, Ethopabate, LincomycinAmprol® Plus / Lincomix® / RoxarsoneHydrochloride Monohydrate, RoxarsoneLincomix® / Amprol Plus044-820Amprolium, Ethopabate, LincomycinHydrochloride Monohydrate049-179Amprolium, Ethopabate, Roxarsone Amprol HI-E® / RoxarsoneAmprol HI-E® / BMD® / Roxarsone 049-180Amprolium, Bacitracin Methylene Disalicylate,Ethopabate, Roxarsone095-543Amprolium, Bambermycins, Ethopabate Amprol HI-E® & Bambermycins, Amprol HI-E® / Flavomycin®3-Nitro® / Amprol HI-E® / Flavomycin®095-547Amprolium, Bambermycins, Ethopabate,Roxarsone095-548Amprolium, Bambermycins, Roxarsone3-Nitro® / Amprol / Flavomycin®095-549Amprolium, Bambermycins, Ethopabate,3-Nitro® / Amprol / Flavomycin®RoxarsoneAmprol HI-E® / Baciferm® / 3-Nitro®105-758Amprolium, Bacitracin Zinc, Ethopabate,Roxarsone114-794Amprolium, Bacitracin Zinc, Ethopabate Baciferm® / Amprol HI-E® Premix118-507Amprolium, Carbarsone Amprol® / Carb-O-Sep®122-822Amprolium, Ethopabate, Virginiamycin Amprol HI-E® / Stafac®130-185Amprolium, Bambermycins Flavomycin® / Amprolium3-Nitro® / Amprol® / BMD®141-142Amprolium, Bacitracin Methylene Disalicylate,Roxarsone141-156Amprolium, Bacitracin Methylene Disalicylate Amprol® / BMD®200-205Amprolium, Bacitracin Zinc, Ethopabate Albac® / Amprol Hi-E®3-Nitro® / Albac® / Amprol Hi-E®200-214Amprolium, Bacitracin Zinc, Ethopabate,Roxarsone3-Nitro® / Albac® / Amprol Hi-E®200-217Amprolium, Bacitracin Zinc, Ethopabate,Roxarsone200-389Amprolium Amprolium 9.6% Oral Solution200-463Amprolium Amprolium 9.6% Oral Solution200-464Amprolium Ampromed™ for Calves200-482Amprolium AmproMed™ for Calves200-488Amprolium Ampromed™ P for Poultry200-496Amprolium AmproMed™ P for Poultry200-514Amprolium Boviprol™ 9.6% Oral Solution106-964Apramycin Sulfate Apralan® Soluble Powder126-050Apramycin Sulfate Apralan® 75 Soluble PowderErythro® (High Lev) / Zoalene plus Arsanilic Acid 038-241Arsanilic Acid, Erythromycin Thiocyanate,ZoaleneFDA APPROVED PRODUCTS January 2014Section 2.0 - Active Ingredients Page 3 of 70Number038-242Amprolium, Arsanilic Acid, ErythromycinErythro® (Low Lev) / Amp plus EthoThiocyanate, Ethopabate038-624Arsanilic Acid, Erythromycin Thiocyanate Pro-Gallimycin-10006-623Arsenamide Sodium Caparsolate Sodium141-033Atipamezole Hydrochloride Antisedan®013-248Atropine, Trichlorfon Freed No. 10, Freed No. 25035-650Atropine, Trichlorfon Dyrex Powder042-548Attapulgite, Bismuth Subcarbonate, KanamycinAmforol® SuspensionSulfateAmforol® Veterinary Oral Tablets042-841Attapulgite, Bismuth Subcarbonate, KanamycinSulfate115-732Azaperone Stresnil InjectionAmprol HI-E® Plus036-304Amprolium, Bacitracin Methylene Disalicylate,Ethopabate039-646Bacitracin Methylene Disalicylate, Carbarsone Carb-O-GainBMD® 15 / Coyden 25®041-541Bacitracin Methylene Disalicylate, Clopidol,Roxarsone046-592Bacitracin Methylene Disalicylate BMD® 50 Granular A Type A Medicated Article, BMD® 30Type A Medicated ArticleAmprol HI-E® / BMD® / Roxarsone 049-180Amprolium, Bacitracin Methylene Disalicylate,Ethopabate, Roxarsone049-463Bacitracin Methylene Disalicylate, Monensin USP Coban® plus BMD®Coban® plus 3-NITRO® plus BMD®049-464Bacitracin Methylene Disalicylate, MonensinUSP, RoxarsoneEntromycin Powder065-107Bacitracin Methylene Disalicylate, StreptomycinSulfate065-280Bacitracin Methylene Disalicylate Soluble Fortracin Concentrate065-470Bacitracin Methylene Disalicylate BMD® Soluble, BMD® Soluble 50%, Solu-tracin 200, Solu-tracin 50Bac MD / Robenz®097-085Bacitracin Methylene Disalicylate, RobenidineHydrochloride098-378Bacitracin Methylene Disalicylate, Nicarbazin Nicarb® 25% and bacitracin methylene disalicylate099-150Bacitracin Methylene Disalicylate, Clopidol BMD® / Coyden 25®107-996Bacitracin Methylene Disalicylate, Lasalocid Avatec® / Fortracin Premix3-Nitro® / Avatec® / BMD®116-082Bacitracin Methylene Disalicylate, Lasalocid,Roxarsone116-088Bacitracin Methylene Disalicylate, Monensin3-Nitro® / BMD® / Coban®, 3-Nitro® / Coban® / Fortracin Sodium, Roxarsone3-Nitro® / Avatec® / Fortracin Broiler Premix 131-894Bacitracin Methylene Disalicylate, Lasalocid,Roxarsone3-Nitro® / Bio-Cox® / BMD®135-321Bacitracin Methylene Disalicylate, Roxarsone,Salinomycin Sodium135-746Bacitracin Methylene Disalicylate, SalinomycinBio-Cox® / BMD®SodiumFDA APPROVED PRODUCTS January 2014Section 2.0 - Active Ingredients Page 4 of 70Number138-456Bacitracin Methylene Disalicylate, MonensinSodiumCoban® / BMD®140-533Bacitracin Methylene Disalicylate, HalofuginoneHydrobromide, Roxarsone3-Nitro® / BMD® / Stenorol®140-584Bacitracin Methylene Disalicylate, HalofuginoneHydrobromideBMD / Stenorol®140-852Bacitracin Methylene Disalicylate, Narasin,Roxarsone3-Nitro® / BMD® / Monteban®140-853Bacitracin Methylene Disalicylate, Narasin BMD® / Monteban®140-919Bacitracin Methylene Disalicylate, HalofuginoneHydrobromideBMD® / Stenorol®140-926Bacitracin Methylene Disalicylate, Narasin,NicarbazinBMD® / Maxiban®140-937Bacitracin Methylene Disalicylate, MonensinSodiumBMD® / Coban®141-058Bacitracin Methylene Disalicylate, Roxarsone,Semduramicin Sodium3-Nitro® / Aviax™ / BMD®141-059Bacitracin Methylene Disalicylate,Chlortetracycline BMD® 10, 25, 30, 40, 50, 60, 75 / CTC® 50, 65, 70, BMD® 25, 30, 40, 50, 60, 75 / ChlorMax™®- 50, 60, 70, MICRO-CTC® 100141-065Bacitracin Methylene Disalicylate, SemduramicinSodiumAviax™ / BMD®141-085Bacitracin Methylene Disalicylate, Zoalene BMD® / Zoamix®141-088Bacitracin Methylene Disalicylate, Nitarsone BMD® / Histostat®141-097Bacitracin Methylene Disalicylate, Ivermectin BMD® / Ivomec® Premix for Swine141-100Bacitracin Methylene Disalicylate, Decoquinate,Roxarsone3-Nitro® / BMD® / Deccox®141-102Bacitracin Methylene Disalicylate, Decoquinate BMD® / Deccox®141-112Bacitracin Methylene Disalicylate, Narasin,Nicarbazin, Roxarsone3-Nitro® / BMD® / Maxiban®141-121Bacitracin Methylene Disalicylate, Roxarsone,Salinomycin Sodium3-Nitro® / Bio-Cox® / BMD®141-124Bacitracin Methylene Disalicylate, Narasin,NicarbazinBMD® / Maxiban®141-136Bacitracin Methylene Disalicylate, SalinomycinSodiumBio-Cox® / BMD®141-138Bacitracin Methylene Disalicylate, Monensin,Roxarsone3-Nitro® / BMD® / Coban®141-140Bacitracin Methylene Disalicylate, Monensin BMD® / Coban®141-142Amprolium, Bacitracin Methylene Disalicylate,Roxarsone3-Nitro® / Amprol® / BMD®141-144Bacitracin Methylene Disalicylate, Fenbendazole BMD® / Safe-Guard®141-153Bacitracin Methylene Disalicylate, Diclazuril BMD® / Clinacox™141-154Bacitracin Methylene Disalicylate, RobenidineHydrochlorideBMD® / Robenz®FDA APPROVED PRODUCTS January 2014Section 2.0 - Active Ingredients Page 5 of 70Number141-155Bacitracin Methylene Disalicylate, RobenidineHydrochloride, Roxarsone3-Nitro® / BMD® / Robenz®141-156Amprolium, Bacitracin Methylene Disalicylate Amprol® / BMD®141-179Bacitracin Methylene Disalicylate, Lasalocid Avatec® / BMD®141-190Bacitracin Methylene Disalicylate, Diclazuril,Roxarsone3-Nitro® / BMD® / Clinacox™141-194Bacitracin Methylene Disalicylate, Diclazuril BMD® / Clincox®141-195Bacitracin Methylene Disalicylate, Diclazuril Clinacox™/ Flavomycin®141-279Nicarbazin, Bacitracin methylene disalicylate Nicarb® 25% plus BMD®200-081Bacitracin Methylene Disalicylate, Roxarsone,Salinomycin Sodium3-Nitro® / BMD® / Sacox®200-082Bacitracin Methylene Disalicylate, SalinomycinSodiumBMD® / Sacox®200-164Bacitracin Methylene Disalicylate, Nicarbazin BMD® / Nicarmix 25®200-242Bacitracin Methylene Disalicylate,Chlortetracycline Calcium Complex BMD®-25, -30, -40, -50, -60, or -75 / Aureomycin® -50, -70, -80, -90 or -100200-358Bacitracin Methylene Disalicylate,Chlortetracycline HydrochloridePennchlor/ BMD044-016Bacitracin Zinc, Clopidol, Roxarsone Coyden 25® with Roxarsone and Bacitracin Zinc045-348Bacitracin Zinc, Decoquinate Albac® / Deccox®, Broiler Finisher Medicated046-920Bacitracin Zinc Baciferm® - 10, Baciferm® - 25, Baciferm® - 40, Baciferm® -50047-933Bacitracin Zinc, Monensin, USP Coban® plus Baciferm®049-934Bacitracin Zinc, Clopidol Coyden 25® with Bacitracin Zinc065-015Bacitracin Zinc, Hydrocortisone Acetate,Neomycin Sulfate, Polymyxin B Sulfate Bacitracin-Neomycin-Polymyxin with Hydrocortisone Acetate Ophthalmic Ointment, Vetropolycin HC Ophthalmic Ointment065-016Bacitracin Zinc, Neomycin Sulfate, Polymyxin B Sulfate Bac-Neo-Poly Ophthalmic Ointment, Vetropolycin Ophthalmic Ointment065-114Bacitracin Zinc, Neomycin Sulfate, Polymyxin BSulfateMycitracin® Sterile Ophthalmic Ointment065-313Bacitracin Zinc Baciferm® Soluble 50065-476Bacitracin Zinc, Hydrocortisone Acetate,Neomycin Sulfate, Polymyxin B SulfateCortisporin Veterinary Ophthalmic Ointment065-485Bacitracin Zinc, Neomycin Sulfate, Polymyxin BSulfateNeosporin Ophthalmic Ointment091-326Bacitracin Zinc, Decoquinate, Roxarsone3-Nitro® / Deccox® / Albac®096-933Bacitracin Zinc, Robenidine Hydrochloride Robenz® Plus Zn Bacitracin098-452Bacitracin Zinc Albac® 50 Type A Medicated Article105-758Amprolium, Bacitracin Zinc, Ethopabate,RoxarsoneAmprol HI-E® / Baciferm® / 3-Nitro®114-794Amprolium, Bacitracin Zinc, Ethopabate Baciferm® / Amprol HI-E® Premix123-154Bacitracin Zinc, Monensin Sodium, Roxarsone3-Nitro®-10 / Baciferm® / Coban® Premix126-052Bacitracin Zinc, Lasalocid, Roxarsone3-Nitro® / Avatec® / Baciferm®134-830Bacitracin Zinc, Monensin Sodium Albac® / Coban®FDA APPROVED PRODUCTS January 2014Section 2.0 - Active Ingredients Page 6 of 70Number136-484Bacitracin Zinc, Carbarsone Baciferm® / Carb-O-Sep®137-536Bacitracin Zinc, Roxarsone, Salinomycin Sodium3-Nitro® / Albac® / Bio-Cox®138-703Bacitracin Zinc, Monensin Sodium, Roxarsone3-Nitro® / Albac® / Coban®139-190Bacitracin Zinc, Roxarsone, Salinomycin Sodium3-Nitro® / Bio-Cox® / Baciferm®139-235Bacitracin Zinc, Salinomycin Sodium Baciferm® / Bio-Cox®140-865Bacitracin Zinc, Narasin Baciferm® / Albac®, Baciferm® / Monteban®141-083Bacitracin Zinc, Lasalocid Avatec® / Baciferm®141-109Bacitracin Zinc, Lasalocid Avatec® / Baciferm®141-146Bacitracin Zinc, Nicarbazin Nicarb® / Baciferm®141-181Bacitracin Zinc, Lasalocid Avatec® / Albac®200-086Bacitracin Zinc, Roxarsone, Salinomycin Sodium3-Nitro® / Albac® / Sacox®200-089Bacitracin Zinc, Salinomycin Sodium Baciferm® / Sacox®200-143Bacitracin Zinc, Roxarsone, Salinomycin Sodium3-Nitro® / Baciferm® / Sacox®200-203Bacitracin Zinc, Carbarsone Albac® / Carb-O-Sep®200-204Bacitracin Zinc, Salinomycin Sodium Albac® / Bio-Cox®200-205Amprolium, Bacitracin Zinc, Ethopabate Albac® / Amprol Hi-E®200-206Bacitracin Zinc, Decoquinate, Roxarsone3-Nitro® / Albac® / Deccox®200-207Bacitracin Zinc, Clopidol, Roxarsone3-Nitro® / Albac® / Coyden 25®200-208Bacitracin Zinc, Lasalocid, Roxarsone Avatec® / 3-Nitro® / Albac®200-209Bacitracin Zinc, Roxarsone, Salinomycin Sodium3-Nitro® / Albac® / Sacox®200-210Bacitracin Zinc, Salinomycin Sodium Albac® / Sacox®200-211Bacitracin Zinc, Monensin, Roxarsone3-Nitro® / Albac® / Coban®200-212Bacitracin Zinc, Robenidine Hydrochloride Albac® / Robenz®200-213Bacitracin Zinc, Decoquinate Albac® / Deccox®3-Nitro® / Albac® / Amprol Hi-E®200-214Amprolium, Bacitracin Zinc, Ethopabate,Roxarsone200-215Bacitracin Zinc, Roxarsone, Salinomycin Sodium3-Nitro® / Albac® / Bio-Cox®200-217Amprolium, Bacitracin Zinc, Ethopabate,3-Nitro® / Albac® / Amprol Hi-E®Roxarsone200-218Bacitracin Zinc, Clopidol Albac® / Coyden 25® / Albac®200-223Bacitracin Zinc Albac® 50 Type A Medicated Article031-555Balsam Peru Oil, Castor Oil, Trypsin Trypzyme® Aerosol039-583Balsam Peru Oil, Castor Oil, Trypsin Granulex Aerosol Spray044-759Bambermycins Flavomycin® 4095-543Amprolium, Bambermycins, Ethopabate Amprol HI-E® & Bambermycins, Amprol HI-E® / Flavomycin®3-Nitro® / Amprol HI-E® / Flavomycin®095-547Amprolium, Bambermycins, Ethopabate,Roxarsone095-548Amprolium, Bambermycins, Roxarsone3-Nitro® / Amprol / Flavomycin®3-Nitro® / Amprol / Flavomycin®095-549Amprolium, Bambermycins, Ethopabate,RoxarsoneFDA APPROVED PRODUCTS January 2014Section 2.0 - Active Ingredients Page 7 of 70Number098-340Bambermycins, Monensin Sodium Flavomycin® / Monensin098-341Bambermycins, Monensin, Roxarsone3-Nitro® / Coban® / Flavomycin®101-628Bambermycins, Roxarsone, Zoalene3-Nitro® / Flavomycin® / Zoalene101-629Bambermycins, Zoalene Flavomycin® / Zoalene112-687Bambermycins, Lasalocid, Roxarsone3-Nitro® / Avatec® / Flavomycin®130-185Amprolium, Bambermycins Flavomycin® / Amprolium130-661Bambermycins, Carbarsone Carb-O-Sep / Flavomycin®131-413Bambermycins Flavomycin® 0.4, Flavomycin® 2132-448Bambermycins Flavomycin®134-185Bambermycins, Roxarsone, Salinomycin Sodium3-Nitro® / Bio-Cox® / Flavomycin®134-284Bambermycins, Salinomycin Sodium Bio-Cox® / Flavomycin®137-483Bambermycins, Halofuginone Hydrobromide Flavomycin® / Stenorol®140-339Bambermycins, Nicarbazin Flavomycin®, Nicarb®140-843Bambermycins, Narasin, Roxarsone3-Nitro® / Flavomycin® / Monteban®140-845Bambermycins, Narasin Flavomycin® / Monteban®140-918Bambermycins, Halofuginone Hydrobromide Flavomycin® / Stenorol®140-942Bambermycins, Narasin, Nicarbazin Flavomycin® / Maxiban®141-034Bambermycins Gainpro® Type A Medicated Article141-129Bambermycins, Lasalocid Avatec® / Flavomycin®141-158Bambermycins, Diclazuril Clinacox™ / Flavomycin®200-080Bambermycins, Roxarsone, Salinomycin Sodium3-Nitro® / Flavomycin® / Sacox®200-080Bambermycins, Roxarsone, Salinomycin Sodium3-Nitro® / Flavomycin® / Sacox®200-083Bambermycins, Salinomycin Sodium Flavomycin® / Sacox®200-388Gentamicin Sulfate, Betamethason Valerate GB Topical Spray034-010Betamethasone Acetate, Betamethasone SodiumBetavet Soluspan SuspensionPhosphate034-267Betamethasone Acetate, Gentamicin Sulfate Gentocin® Durafilm Ophthalmic SolutionBetasone Aqueous Suspension049-185Betamethasone Dipropionate, BetamethasoneSodium Phosphate034-010Betamethasone Acetate, Betamethasone SodiumBetavet Soluspan SuspensionPhosphateBetasone Aqueous Suspension049-185Betamethasone Dipropionate, BetamethasoneSodium Phosphate046-821Betamethasone Valerate, Gentamicin Sulfate Gentocin® Otic Solution113-231Betamethasone Valerate, Gentamicin Sulfate Topagen® Ointment132-338Betamethasone Valerate, Gentamicin Sulfate Gentocin® Topical SprayOtomax®140-896Betamethasone Valerate, Clotrimazole,Gentamicin Sulfate200-183Betamethasone Valerate, Gentamicin Sulfate Gentavet® Otic Solution200-188Betamethasone Valerate, Gentamicin Sulfate Betagen™ Topical SprayFDA APPROVED PRODUCTS January 2014Section 2.0 - Active Ingredients Page 8 of 70NumberTri-Otic Ointment200-229Betamethasone Valerate, Clotrimazole,Gentamicin SulfateVetro-Max®200-283Betamethasone Valerate, Clotrimazole,Gentamicin SulfateGBC Ointment™200-287Betamethasone Valerate, Clotrimazole,Gentamicin Sulfate200-415Betamethasone Valerate, Gentamicin Sulfate Gentamicin Sulfate Topical Spray200-416Gentamicin sulfate and Betamethasone valerate Gentamicin Topical SprayAmforol® Suspension042-548Attapulgite, Bismuth Subcarbonate, KanamycinSulfate042-841Attapulgite, Bismuth Subcarbonate, KanamycinAmforol® Veterinary Oral TabletsSulfate034-705Boldenone Undecylenate Equipoise®140-872Bovine Somatotropin (Sometribove Zinc)Posilac 1 Step®035-016Bunamidine Hydrochloride Scolaban 400045-738Buquinolate, Lincomycin HydrochlorideLincomix® / Bonaid, Lincomycin & BuquinolateMonohydrate130-872Butacaine Sulfate, Nitrofurazone Nitrofurazone Anesthetic Dress.104-184Butamisole Hydrochloride Styquin132-533Butamisole Hydrochloride Styquin Parenteral 1.1%102-990Butorphanol Tartrate Torbutrol® Injection103-390Butorphanol Tartrate Torbutrol® Tablets135-780Butorphanol Tartrate Torbugesic®141-047Butorphanol Tartrate Torbugesic-SA®200-239Butorphanol Tartrate Dolorex®200-322Butorphanol Tartrate Butorphanol Tartrate Injection200-332Butorphanol Tartrate Butorphic™ Injection200-408Butorphanol Tartrate Butorphanol Tartrate Injection200-448Butorphanol Tartrate, Melengestrol Acetate,Heifermax® plus Optaflexx® and Rumensin®Monensin Sodium, Ractopamine Hydrochloride094-642Cambendazole Camvet Suspension Horse Wormer096-506Cambendazole Camvet Horse Wormer Pellets096-731Cambendazole Camvet Horse Wormer Paste 45%009-339Ammonium Chloride, Caramiphen Edisylate Carafen Cough Syrup, Carafen Cough Tablets041-061Carbadox Mecadox® Premix 10092-955Carbadox, Pyrantel Tartrate Banminth® / Mecadox®141-211Carbadox, Oxytetracycline Dihydrate Mecadox® 10 / Terramycin® 100, Mecadox® 10 / Terramycin®200, Mecadox® 10 / Terramycin® 50 039-646Bacitracin Methylene Disalicylate, Carbarsone Carb-O-Gain118-507Amprolium, Carbarsone Amprol® / Carb-O-Sep®130-661Bambermycins, Carbarsone Carb-O-Sep / Flavomycin®136-484Bacitracin Zinc, Carbarsone Baciferm® / Carb-O-Sep®FDA APPROVED PRODUCTS January 2014Section 2.0 - Active Ingredients Page 9 of 70Number200-203Bacitracin Zinc, Carbarsone Albac® / Carb-O-Sep®038-879Carbarsone, Zoalene Carb-O-Sep® / Zoamix®038-879Carbarsone, Zoalene Carb-O-Sep® / Zoamix®032-946Carbomycin, Oxytetracycline Hydrochloride Magna Terramycin® Soluble Powder139-633Carfentanil Citrate Wildnil®139-879Carnidazole Carnidazole, Spartrix®141-053Carprofen Rimadyl® Caplets for Dogs141-111Carprofen Rimadyl® Chewable Tablets141-199Carprofen Rimadyl® Injectable200-366Carprofen Novocox Caplets200-397Carprofen Vetprofen™200-498Carprofen Carprieve™200-555Carprofen Librevia™031-555Balsam Peru Oil, Castor Oil, Trypsin Trypzyme® Aerosol039-583Balsam Peru Oil, Castor Oil, Trypsin Granulex Aerosol Spray119-688Cefadroxil Cefa-Tabs®140-684Cefadroxil Cefa-Drops®141-285Cefovecin sodium Convenia®141-232Cefpodoxime proxetil Simplicef®200-543Cefpodoxime proxetil Cefpodoxime Proxetil Tablets141-209Ceftiofur Crystalline Free Acid Excede™ Sterile Suspension141-235Ceftiofur Crystalline Free Acid Excede™ For Swine140-890Ceftiofur Hydrochloride Excenel Sterile Suspension, Excenel® RTU, Excenel® SterilePowder141-238Ceftiofur Hydrochloride Spectramast™ LC Sterile Suspension141-239Ceftiofur Hydrochloride Spectramast™ DC Sterile Suspension141-288Ceftiofur Hydrochloride Excenel® RTU EZ140-338Ceftiofur Sodium Naxcel® Sterile Powder200-420Ceftiofur Sodium Ceftiofur Sodium Sterile Powder200-421Ceftiofur sodium Ceftiofur for Injection141-326Cephalexin Rilexine® Chewable Tablets108-114Cephapirin Benzathine Cefa-Dri®, Tomorrow® Infusion097-222Cephapirin Sodium Cefa-Lak®, Today® Intramammary Infusion046-789Chloral Hydrate, Magnesium Sulfate, ChloropentPentobarbital055-002Chloramphenicol Tevcocin055-051Chloramphenicol Chloromycetin Tablets 100 mg, Chloromycetin Tablets 250 mg,Chloromycetin Tablets 500 mg055-059Chloramphenicol Viceton® Tablets065-137Chloramphenicol Amphicol-VFDA APPROVED PRODUCTS January 2014Section 2.0 - Active Ingredients Page 10 of 70Number065-149Chloramphenicol Chloromycetin Ophthalmic Ointment065-150Chloramphenicol Chloramphenicol Capsules065-241Chloramphenicol Mychel-Vet Capsules (50 mg)065-460Chloramphenicol Chloramphenicol 1% Ophthalmic, Vetrocloricin OphthalmicOintment065-461Chloramphenicol Anacetin Tablets065-463Chloramphenicol Mychel-Vet Injection065-489Chloramphenicol Mychel-Vet Tabs065-491Chloramphenicol Medichol Tablets055-047Chloramphenicol Palmitate Chloromycetin Palmitate Oral Suspension009-782Chlorhexidine Acetate Nolvasan® Antiseptic Ointment200-301Chlorhexidine Acetate Privasan™ Antiseptic Ointment009-809Chlorhexidine Hydrochloride Nolvasan® Cap-Tabs®010-434Chlorhexidine Hydrochloride Nolvasan® Suspension006-983Chlorobutanol, Doxylamine Succinate A-H Injection141-245Chloroquine Phosphate, Embutramid, Lidocaine Tributame™ Euthanasia Solution011-678Chlorothiazide Diuril® Tablet012-734Chlorothiazide Diuril®038-160Chlorphenesin Carbamate Maolate® Tablets, Maolate® Veterinary048-761Chlortetracycline Aureomycin® 100 Granular, Aureomycin® 50 Granular,Aureomycin® 90 Granular, Aureomycin® 90 Meal138-935Chlortetracycline Pennchlor™ 100MR, Pennchlor™ 50, Pennchlor™ 50-G,Pennchlor™ 90, Pennchlor™ 90-G, Pennchlor™ 100-G,Pennchlor™ 100 Hi-Flo141-059Bacitracin Methylene Disalicylate,Chlortetracycline BMD® 10, 25, 30, 40, 50, 60, 75 / CTC® 50, 65, 70, BMD® 25, 30, 40, 50, 60, 75 / ChlorMax™®- 50, 60, 70, MICRO-CTC® 100141-147Chlortetracycline, Decoquinate Chloromax® / Deccox®141-185Chlortetracycline, Decoquinate Aureomycin® / Deccox®141-201Chlortetracycline, Laidlomycin PropionatePotassiumAureomycin® / Cattlyst®141-250Chlortetracycline, Lasalocid Aureomycin® and Bovatec®200-259Chlortetracycline, Roxarsone, SalinomycinSodium3-Nitro® / ChlorMax™ / Sacox®200-260Chlortetracycline, Roxarsone, SalinomycinSodium3-Nitro® / Bio-Cox® / ChlorMax™200-261Chlortetracycline, Salinomycin Sodium Bio-Cox® / ChlorMax™200-262Chlortetracycline, Salinomycin Sodium ChlorMax™ / Sacox®200-263Chlortetracycline, Monensin Sodium ChlorMax™ / Coban®200-314Chlortetracycline, Sulfamethazine Pennchlor S®200-355Chlortetracycline, Roxarsone, SalinomycinSodiumPennchlor™ / Bio-Cox® / 3-Nitro®FDA APPROVED PRODUCTS January 2014Section 2.0 - Active Ingredients Page 11 of 70。
4×非变性蛋白上样缓冲液

北京索莱宝科技有限公司
4×非变性蛋白上样缓冲液
货号:P1017
规格:10ml
保存:-20℃保存,有效期1年。
产品简介:
4×非变性蛋白上样缓冲液中不含SDS及DTT或者β-巯基乙醇,适合于常规的非变性PAGE蛋白样品的电泳。
使用说明:
1.请按每30微升蛋白样品加入10微升蛋白上样缓冲液的比例(4倍稀释)来使用。
如果蛋白浓度过高,可用双蒸水稀释。
混匀后直接上样到PAGE胶加样孔内即可。
2.通常电泳时蓝色染料到达胶的底端处附近即可停止电泳。
注意事项:
1、为了您的安全和健康,请穿实验服并戴一次性手套操作。
2、蛋白上样缓冲液含有溴酚蓝指示剂,PH值受保存温度影响,在低温冻存状态下,溶液可能会呈现深棕色,不影响产品使用。
第1页,共1页。
苏州蓝晓生物科技有限公司SP Seplife HP层析介质说明书

SP Seplife HP说明书1.产品介绍SP Seplife HP层析介质是蓝晓科技自主研发的一种新型高度交联的琼脂糖层析介质,是将磺酸基丙基键合在琼脂糖凝胶过滤层析介质上形成的一种强阳离子交换层析介质,具有高流速、低反压、高动态载量、良好的化学稳定性和机械性能,非特异性吸附低,回收率高,方便进行规模放大,可缩短生产时间,提高生产效率。
广泛用于生物制药和生物工程下游蛋白质、核酸及多肽的离子交换分离。
2.性能介绍产品牌号SP Seplife HP外观白色球状凝胶,无臭无味种类强阳离子交换填料基质Seplife 6FF配基磺酸基丙基粒径(μm)24~44pH稳定性4~13(长期),3~14(短期,在位清洗[CIP])在以下液体中稳定:所有常用的水相缓冲液;1mol/L 氢氧化钠;化学稳定性8mol/L 尿素;8mol/L 盐酸胍;70% 乙醇吸附量(mg /ml)>60mg/ml (溶菌酶)离子交换量(mmol /ml)0.15~0.20工作温度4~40℃耐热性121℃,水中30min最高流速*(cm/h)>150耐压(MPa)0.3应用用于生物制药和生物工程下游蛋白质、核酸及多肽的离子交换层析纯化*检测条件:层析柱16mm×300mm;*柱床高10cm;温度25℃;流动相为0.1mol/L NaCl。
3.使用方法3.1 装柱装柱按照标准操作规程操作。
必须保证每种材料都处于工作温度,凝胶装柱前需要脱气。
3.2平衡使用2~5倍柱床体积的上样平衡液平衡柱子,务必使流出液的电导和pH同上样缓冲液的电导和pH完全一致。
平衡液是低浓度的缓冲溶液,如NaOAC、PBS等。
常用的平衡液是0.1mol/L 醋酸缓冲液,pH5.0。
3.3上样(1)样品用平衡液配制,浑浊的样品要离心和过滤后上样。
盐浓度太大的样品处理后再配。
(2)一般情况是让目标产品结合在柱子上,用平衡液洗去杂质,再选择一种洗脱液洗下目标产品。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Proto-oncogene to Oncogene
Retrovirus induced cancers are rare in humans Most cancers involve mutation in cellular proto-oncogene to produce an oncogene Three ways to produce oncogene
Point mutation to alter regulation of gene or gene product Localized amplification of gene to cause overexpression of gene product nslocation of gene into new location leading to loss of regulation
Amplification of Oncogenes
Point mutations and translocations convert proto-oncogenes to oncogenes Conversion can also result from amplification Myc oncogene is amplified in neuroblastoma ErbB oncogene is amplified in many breast tumors
Molecular Basis of Cancer
Transformation of a normal cell into a malignant cancer cell requires DNA damage The DNA damage events alter genes involved in controlling cell growth Much is known about specific molecular events that lead to cancer Research dates to 1911 when Peyton Rous discovered the first oncogenic virus
Won’t respond to GAP
MAP kinase pathway is always “on” Cell cycle is always running
Several Proto-Oncogenes in MAP Kinase Pathway
Class II, growth factor receptor is ErbB Normally binds epidermal growth factor (EGF) to activate the cell cycle Deletion causes loss of growth factor dependence
Src is a protein-tyrosine kinase (class III, intracellular transducer oncogene) Phosphorylates proteins that activate the cell cycle So, when RSV genome inserts into host chromosome, src gene is expressed Src kinase activates cell cycle Cell loses control of division
Rous Sarcoma Virus (RSV)
First demonstration of a cause for cancer Infect chickens with RSV Sarcoma develops within 2 weeks RSV is a retrovirus Nobel for Rous in 1966
Several Class III Oncogenes
Intracellular transducers in MAP kinase pathway are all oncogenes Ras, raf, mek, erk, elk Mutations in any of these genes can cause loss of cell cycle regulation
MAP Kinase Pathway 15.26
Ras is a signaling molecule (kinase transducer) in the MAP kinase pathway Long signal transduction pathway that ends in production of Fos Fos is a transcription factor that activates genes involved in the G1 to S transition
Oncogenes are also in Genome
Key experimental finding: Genes that cause cancer (oncogenes) are also found in normal genomes They are normal cellular genes They have normal cellular functions In the cell they are called proto-oncogenes There are several ways to convert a protooncogene into an oncogene
Retrovirus Genome 15.17
Only 3 genes, gag pol and env
Oncogenic Retrovirus Genome
Oncogenic retroviruses like RSV have an extra gene Not required for viral multiplication Causes transformation to cancer cell 15.19 Called oncogene Activates cell cycle (proliferation) Over 40 different viral oncogenes see table 15.3 Src in RSV
For Example, Src
Src gene is in cellular genome When expressed, src gene product (kinase) activates cell cycle Expression and activity of src is regulated Cellular src protein is kept inactive by a key phosphorylation Viral src does not have phosphorylation target region Viral version is not regulated at either expression or activity
Ras is a G-Protein
GDP bound form is inactive Guanine nucleotide exchange factor (GEF) stimulates exchange to GTP form GTPase activating protein (GAP) stimulates GTP hydrolysis
Why so long?
Retrovirus Replication Cycle
Retroviruses convert an RNA genome into double-stranded DNA The DNA copy then integrates into the host chromosome Genome has only 3 genes
Ras 15.23
Large number of human cancers have mutated ras gene Normal product is an intracellular transducer Some tumor initiating mutations in ras can be as simple as a single nucleotide change Mutated gene product cannot be regulated Constant activation of growth factor pathway
Ras-GTP Activates Raf
Begins phosphorylation cascade Cascade ends in the nucleus with new gene expression
Chemical Carcinogens Mutate Ras
Several chemical carcinogens have been shown to induce point mutations in ras Converts proto-oncogene to oncogene Mutant protein does not hydrolyze GTP
Myc Oncogene 15-24
