Masitinib_COA_09866_MedChemExpress
新药Masitinib(马赛替尼)合成检索总结报告

新药Masitinib(马赛替尼)合成检索总结报告
一、Masitinib(马赛替尼)简介
Masitinib(马赛替尼)是一种特异性的Ckit抑制剂,主要用于治疗因为Ckit突变(gain of function)引起的胃肠道间质瘤。
但是因为其可以抑制肥大细胞的活性,现在也开始用于治疗哮喘,多发硬化症。
Masitinib(马赛替尼)分子结构式如下:
英文名称:Masitinib
中文名称:马赛替尼
本文主要对Masitinib(马赛替尼)的合成路线、关键中间体的合成方法及实验操作方法进行了文献检索并作出了总结。
二、Masitinib(马赛替尼)合成路线
三、Masitinib(马赛替尼)合成检索总结报告(一) Masitinib(马赛替尼)中间体3的合成
(二) Masitinib(马赛替尼)中间体5的合成方法一
(三) Masitinib(马赛替尼)中间体5的合成方法二
①Masitinib(马赛替尼)中间体6的合成
②Masitinib(马赛替尼)中间体5的合成
(四) Masitinib(马赛替尼)中间体8的合成。
线粒体靶向抗氧化剂Mito-TEMPO对氮芥诱导BEAS-2B细胞损伤的影响

收稿日期:2022-03-24;修订日期:2022-05-13基金项目:国家自然科学基金青年基金(31900892)作者简介:赵晨茜,E-mail:。
*通信作者,刘江正,E-mail:线粒体靶向抗氧化剂Mito-TEMPO对氮芥诱导BEAS-2B细胞损伤的影响赵晨茜1,徐安琦1,2,艾多1,2,孔德钦1,张晓迪1,李文丽1,海春旭1,刘江正1,*(1.空军军医大学军事预防医学系军事毒理学与防化医学教研室,陕西省自由基生物学与医学重点实验室,教育部特殊作业环境危害评估与防治重点实验室,陕西西安710032;2.空军军医大学基础医学院学员二大队,陕西西安710032)Effects of Mito-TEMPO treatmenton nitrogen mustard-inducedcytotoxicity in BEAS-2B cellsZHAO Chenqian1,XU Anqi1,2,AI Duo1,2,KONG Deqin1,ZHANG Xiaodi1,LI Wenli1,HAI Chunxu1,LIU Jiangzheng1,*(1.Department of Military Toxicology and Chemical Defense Medicine,School of Military Preventive Medicine,Air Force Medical University;KeyLaboratory of Free Radical Biology and Medicine of Shaanxi Province;KeyLaboratory of Environmental Hazard Assessment and Prevention of SpecialOperations of Ministry of Education,Xi an710032;2.The SecondBrigade of Basic Medical College,Air Force MedicalUniversity,Xi an710032,Shaanxi,China)【摘要】目的:探讨线粒体靶向抗氧化剂Mito-TEMPO对氮芥(HN2)诱导人支气管上皮细胞系BEAS-2B的影响。
稳定性英文版

HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationFLUOXETINE HClC17H18F3NO•HClM.W. = 345.79CAS — 59333-67-4STABILITY INDICATINGA S S A Y V A L I D A T I O NMethod is suitable for:ýIn-process controlþProduct ReleaseþStability indicating analysis (Suitability - US/EU Product) CAUTIONFLUOXETINE HYDROCHLORIDE IS A HAZARDOUS CHEMICAL AND SHOULD BE HANDLED ONLY UNDER CONDITIONS SUITABLE FOR HAZARDOUS WORK.IT IS HIGHLY PRESSURE SENSITIVE AND ADEQUATE PRECAUTIONS SHOULD BE TAKEN TO AVOID ANY MECHANICAL FORCE (SUCH AS GRINDING, CRUSHING, ETC.) ON THE POWDER.ED. N0: 04Effective Date:APPROVED::HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationTABLE OF CONTENTS INTRODUCTION........................................................................................................................ PRECISION............................................................................................................................... System Repeatability ................................................................................................................ Method Repeatability................................................................................................................. Intermediate Precision .............................................................................................................. LINEARITY................................................................................................................................ RANGE...................................................................................................................................... ACCURACY............................................................................................................................... Accuracy of Standard Injections................................................................................................ Accuracy of the Drug Product.................................................................................................... VALIDATION OF FLUOXETINE HCl AT LOW CONCENTRATION........................................... Linearity at Low Concentrations................................................................................................. Accuracy of Fluoxetine HCl at Low Concentration..................................................................... System Repeatability................................................................................................................. Quantitation Limit....................................................................................................................... Detection Limit........................................................................................................................... VALIDATION FOR META-FLUOXETINE HCl (POSSIBLE IMPURITIES).................................. Meta-Fluoxetine HCl linearity at 0.05% - 1.0%........................................................................... Detection Limit for Fluoxetine HCl.............................................................................................. Quantitation Limit for Meta Fluoxetine HCl................................................................................ Accuracy for Meta-Fluoxetine HCl ............................................................................................ Method Repeatability for Meta-Fluoxetine HCl........................................................................... Intermediate Precision for Meta-Fluoxetine HCl......................................................................... SPECIFICITY - STABILITY INDICATING EVALUATION OF THE METHOD............................. FORCED DEGRADATION OF FINISHED PRODUCT AND STANDARD..................................1. Unstressed analysis...............................................................................................................2. Acid Hydrolysis stressed analysis..........................................................................................3. Base hydrolysis stressed analysis.........................................................................................4. Oxidation stressed analysis...................................................................................................5. Sunlight stressed analysis.....................................................................................................6. Heat of solution stressed analysis.........................................................................................7. Heat of powder stressed analysis.......................................................................................... System Suitability stressed analysis.......................................................................................... Placebo...................................................................................................................................... STABILITY OF STANDARD AND SAMPLE SOLUTIONS......................................................... Standard Solution...................................................................................................................... Sample Solutions....................................................................................................................... ROBUSTNESS.......................................................................................................................... Extraction................................................................................................................................... Factorial Design......................................................................................................................... CONCLUSION...........................................................................................................................ED. N0: 04Effective Date:APPROVED::HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationBACKGROUNDTherapeutically, Fluoxetine hydrochloride is a classified as a selective serotonin-reuptake inhibitor. Effectively used for the treatment of various depressions. Fluoxetine hydrochloride has been shown to have comparable efficacy to tricyclic antidepressants but with fewer anticholinergic side effects. The patent expiry becomes effective in 2001 (US). INTRODUCTIONFluoxetine capsules were prepared in two dosage strengths: 10mg and 20mg dosage strengths with the same capsule weight. The formulas are essentially similar and geometrically equivalent with the same ingredients and proportions. Minor changes in non-active proportions account for the change in active ingredient amounts from the 10 and 20 mg strength.The following validation, for the method SI-IAG-206-02 , includes assay and determination of Meta-Fluoxetine by HPLC, is based on the analytical method validation SI-IAG-209-06. Currently the method is the in-house method performed for Stability Studies. The Validation was performed on the 20mg dosage samples, IAG-21-001 and IAG-21-002.In the forced degradation studies, the two placebo samples were also used. PRECISIONSYSTEM REPEATABILITYFive replicate injections of the standard solution at the concentration of 0.4242mg/mL as described in method SI-IAG-206-02 were made and the relative standard deviation (RSD) of the peak areas was calculated.SAMPLE PEAK AREA#15390#25406#35405#45405#55406Average5402.7SD 6.1% RSD0.1ED. N0: 04Effective Date:APPROVED::HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationED. N0: 04Effective Date:APPROVED::PRECISION - Method RepeatabilityThe full HPLC method as described in SI-IAG-206-02 was carried-out on the finished product IAG-21-001 for the 20mg dosage form. The method repeated six times and the relative standard deviation (RSD) was calculated.SAMPLENumber%ASSAYof labeled amountI 96.9II 97.8III 98.2IV 97.4V 97.7VI 98.5(%) Average97.7SD 0.6(%) RSD0.6PRECISION - Intermediate PrecisionThe full method as described in SI-IAG-206-02 was carried-out on the finished product IAG-21-001 for the 20mg dosage form. The method was repeated six times by a second analyst on a different day using a different HPLC instrument. The average assay and the relative standard deviation (RSD) were calculated.SAMPLENumber% ASSAYof labeled amountI 98.3II 96.3III 94.6IV 96.3V 97.8VI 93.3Average (%)96.1SD 2.0RSD (%)2.1The difference between the average results of method repeatability and the intermediate precision is 1.7%.HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationLINEARITYStandard solutions were prepared at 50% to 200% of the nominal concentration required by the assay procedure. Linear regression analysis demonstrated acceptability of the method for quantitative analysis over the concentration range required. Y-Intercept was found to be insignificant.RANGEDifferent concentrations of the sample (IAG-21-001) for the 20mg dosage form were prepared, covering between 50% - 200% of the nominal weight of the sample.Conc. (%)Conc. (mg/mL)Peak Area% Assayof labeled amount500.20116235096.7700.27935334099.21000.39734463296.61500.64480757797.52000.79448939497.9(%) Average97.6SD 1.0(%) RSD 1.0ED. N0: 04Effective Date:APPROVED::HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationED. N0: 04Effective Date:APPROVED::RANGE (cont.)The results demonstrate linearity as well over the specified range.Correlation coefficient (RSQ)0.99981 Slope11808.3Y -Interceptresponse at 100%* 100 (%) 0.3%ACCURACYACCURACY OF STANDARD INJECTIONSFive (5) replicate injections of the working standard solution at concentration of 0.4242mg/mL, as described in method SI-IAG-206-02 were made.INJECTIONNO.PEAK AREA%ACCURACYI 539299.7II 540599.9III 540499.9IV 5406100.0V 5407100.0Average 5402.899.9%SD 6.10.1RSD, (%)0.10.1The percent deviation from the true value wasdetermined from the linear regression lineHPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationED. N0: 04Effective Date:APPROVED::ACCURACY OF THE DRUG PRODUCTAdmixtures of non-actives (placebo, batch IAG-21-001 ) with Fluoxetine HCl were prepared at the same proportion as in a capsule (70%-180% of the nominal concentration).Three preparations were made for each concentration and the recovery was calculated.Conc.(%)Placebo Wt.(mg)Fluoxetine HCl Wt.(mg)Peak Area%Accuracy Average (%)70%7079.477.843465102.27079.687.873427100.77079.618.013465100.0101.0100%10079.6211.25476397.910080.8011.42491799.610079.6011.42485498.398.6130%13079.7214.90640599.413080.3114.75632899.213081.3314.766402100.399.618079.9920.10863699.318079.3820.45879499.418080.0820.32874899.599.4Placebo, Batch Lot IAG-21-001HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationED. N0: 04Effective Date:APPROVED::VALIDATION OF FLUOXETINE HClAT LOW CONCENTRATIONLINEARITY AT LOW CONCENTRATIONSStandard solution of Fluoxetine were prepared at approximately 0.02%-1.0% of the working concentration required by the method SI-IAG-206-02. Linear regression analysis demonstrated acceptability of the method for quantitative analysis over this range.ACCURACY OF FLUOXETINE HCl AT LOW CONCENTRATIONThe peak areas of the standard solution at the working concentration were measured and the percent deviation from the true value, as determined from the linear regression was calculated.SAMPLECONC.µg/100mLAREA FOUND%ACCURACYI 470.56258499.7II 470.56359098.1III 470.561585101.3IV 470.561940100.7V 470.56252599.8VI 470.56271599.5(%) AverageSlope = 132.7395299.9SD Y-Intercept = -65.872371.1(%) RSD1.1HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationSystem RepeatabilitySix replicate injections of standard solution at 0.02% and 0.05% of working concentration as described in method SI-IAG-206-02 were made and the relative standard deviation was calculated.SAMPLE FLUOXETINE HCl AREA0.02%0.05%I10173623II11503731III10103475IV10623390V10393315VI10953235Average10623462RSD, (%) 5.0 5.4Quantitation Limit - QLThe quantitation limit ( QL) was established by determining the minimum level at which the analyte was quantified. The quantitation limit for Fluoxetine HCl is 0.02% of the working standard concentration with resulting RSD (for six injections) of 5.0%. Detection Limit - DLThe detection limit (DL) was established by determining the minimum level at which the analyte was reliably detected. The detection limit of Fluoxetine HCl is about 0.01% of the working standard concentration.ED. N0: 04Effective Date:APPROVED::HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationED. N0: 04Effective Date:APPROVED::VALIDATION FOR META-FLUOXETINE HCl(EVALUATING POSSIBLE IMPURITIES)Meta-Fluoxetine HCl linearity at 0.05% - 1.0%Relative Response Factor (F)Relative response factor for Meta-Fluoxetine HCl was determined as slope of Fluoxetine HCl divided by the slope of Meta-Fluoxetine HCl from the linearity graphs (analysed at the same time).F =132.7395274.859534= 1.8Detection Limit (DL) for Fluoxetine HClThe detection limit (DL) was established by determining the minimum level at which the analyte was reliably detected.Detection limit for Meta Fluoxetine HCl is about 0.02%.Quantitation Limit (QL) for Meta-Fluoxetine HClThe QL is determined by the analysis of samples with known concentration of Meta-Fluoxetine HCl and by establishing the minimum level at which the Meta-Fluoxetine HCl can be quantified with acceptable accuracy and precision.Six individual preparations of standard and placebo spiked with Meta-Fluoxetine HCl solution to give solution with 0.05% of Meta Fluoxetine HCl, were injected into the HPLC and the recovery was calculated.HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationED. N0: 04Effective Date:APPROVED::META-FLUOXETINE HCl[RECOVERY IN SPIKED SAMPLES].Approx.Conc.(%)Known Conc.(µg/100ml)Area in SpikedSampleFound Conc.(µg/100mL)Recovery (%)0.0521.783326125.735118.10.0521.783326825.821118.50.0521.783292021.55799.00.0521.783324125.490117.00.0521.783287220.96996.30.0521.783328526.030119.5(%) AVERAGE111.4SD The recovery result of 6 samples is between 80%-120%.10.7(%) RSDQL for Meta Fluoxetine HCl is 0.05%.9.6Accuracy for Meta Fluoxetine HClDetermination of Accuracy for Meta-Fluoxetine HCl impurity was assessed using triplicate samples (of the drug product) spiked with known quantities of Meta Fluoxetine HCl impurity at three concentrations levels (namely 80%, 100% and 120% of the specified limit - 0.05%).The results are within specifications:For 0.4% and 0.5% recovery of 85% -115%For 0.6% recovery of 90%-110%HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationED. N0: 04Effective Date:APPROVED::META-FLUOXETINE HCl[RECOVERY IN SPIKED SAMPLES]Approx.Conc.(%)Known Conc.(µg/100mL)Area in spikedSample Found Conc.(µg/100mL)Recovery (%)[0.4%]0.4174.2614283182.66104.820.4174.2614606187.11107.370.4174.2614351183.59105.36[0.5%]0.5217.8317344224.85103.220.5217.8316713216.1599.230.5217.8317341224.81103.20[0.6%]0.6261.3918367238.9591.420.6261.3920606269.81103.220.6261.3920237264.73101.28RECOVERY DATA DETERMINED IN SPIKED SAMPLESHPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationED. N0: 04Effective Date:APPROVED::REPEATABILITYMethod Repeatability - Meta Fluoxetine HClThe full method (as described in SI-IAG-206-02) was carried out on the finished drug product representing lot number IAG-21-001-(1). The HPLC method repeated serially, six times and the relative standard deviation (RSD) was calculated.IAG-21-001 20mg CAPSULES - FLUOXETINESample% Meta Fluoxetine % Meta-Fluoxetine 1 in Spiked Solution10.0260.09520.0270.08630.0320.07740.0300.07450.0240.09060.0280.063AVERAGE (%)0.0280.081SD 0.0030.012RSD, (%)10.314.51NOTE :All results are less than QL (0.05%) therefore spiked samples with 0.05% Meta Fluoxetine HCl were injected.HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationED. N0: 04Effective Date:APPROVED::Intermediate Precision - Meta-Fluoxetine HClThe full method as described in SI-IAG-206-02 was applied on the finished product IAG-21-001-(1) .It was repeated six times, with a different analyst on a different day using a different HPLC instrument.The difference between the average results obtained by the method repeatability and the intermediate precision was less than 30.0%, (11.4% for Meta-Fluoxetine HCl as is and 28.5% for spiked solution).IAG-21-001 20mg - CAPSULES FLUOXETINESample N o:Percentage Meta-fluoxetine% Meta-fluoxetine 1 in spiked solution10.0260.06920.0270.05730.0120.06140.0210.05850.0360.05560.0270.079(%) AVERAGE0.0250.063SD 0.0080.009(%) RSD31.514.51NOTE:All results obtained were well below the QL (0.05%) thus spiked samples slightly greater than 0.05% Meta-Fluoxetine HCl were injected. The RSD at the QL of the spiked solution was 14.5%HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationSPECIFICITY - STABILITY INDICATING EVALUATIONDemonstration of the Stability Indicating parameters of the HPLC assay method [SI-IAG-206-02] for Fluoxetine 10 & 20mg capsules, a suitable photo-diode array detector was incorporated utilizing a commercial chromatography software managing system2, and applied to analyze a range of stressed samples of the finished drug product.GLOSSARY of PEAK PURITY RESULT NOTATION (as reported2):Purity Angle-is a measure of spectral non-homogeneity across a peak, i.e. the weighed average of all spectral contrast angles calculated by comparing all spectra in the integrated peak against the peak apex spectrum.Purity Threshold-is the sum of noise angle3 and solvent angle4. It is the limit of detection of shape differences between two spectra.Match Angle-is a comparison of the spectrum at the peak apex against a library spectrum.Match Threshold-is the sum of the match noise angle3 and match solvent angle4.3Noise Angle-is a measure of spectral non-homogeneity caused by system noise.4Solvent Angle-is a measure of spectral non-homogeneity caused by solvent composition.OVERVIEWT he assay of the main peak in each stressed solution is calculated according to the assay method SI-IAG-206-02, against the Standard Solution, injected on the same day.I f the Purity Angle is smaller than the Purity Threshold and the Match Angle is smaller than the Match Threshold, no significant differences between spectra can be detected. As a result no spectroscopic evidence for co-elution is evident and the peak is considered to be pure.T he stressed condition study indicated that the Fluoxetine peak is free from any appreciable degradation interference under the stressed conditions tested. Observed degradation products peaks were well separated from the main peak.1® PDA-996 Waters™ ; 2[Millennium 2010]ED. N0: 04Effective Date:APPROVED::HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationFORCED DEGRADATION OF FINISHED PRODUCT & STANDARD 1.UNSTRESSED SAMPLE1.1.Sample IAG-21-001 (2) (20mg/capsule) was prepared as stated in SI-IAG-206-02 and injected into the HPLC system. The calculated assay is 98.5%.SAMPLE - UNSTRESSEDFluoxetine:Purity Angle:0.075Match Angle:0.407Purity Threshold:0.142Match Threshold:0.4251.2.Standard solution was prepared as stated in method SI-IAG-206-02 and injected into the HPLC system. The calculated assay is 100.0%.Fluoxetine:Purity Angle:0.078Match Angle:0.379Purity Threshold:0.146Match Threshold:0.4272.ACID HYDROLYSIS2.1.Sample solution of IAG-21-001 (2) (20mg/capsule) was prepared as in method SI-IAG-206-02 : An amount equivalent to 20mg Fluoxetine was weighed into a 50mL volumetric flask. 20mL Diluent was added and the solution sonicated for 10 minutes. 1mL of conc. HCl was added to this solution The solution was allowed to stand for 18 hours, then adjusted to about pH = 5.5 with NaOH 10N, made up to volume with Diluent and injected into the HPLC system after filtration.Fluoxetine peak intensity did NOT decrease. Assay result obtained - 98.8%.SAMPLE- ACID HYDROLYSISFluoxetine peak:Purity Angle:0.055Match Angle:0.143Purity Threshold:0.096Match Threshold:0.3712.2.Standard solution was prepared as in method SI-IAG-206-02 : about 22mg Fluoxetine HCl were weighed into a 50mL volumetric flask. 20mL Diluent were added. 2mL of conc. HCl were added to this solution. The solution was allowed to stand for 18 hours, then adjusted to about pH = 5.5 with NaOH 10N, made up to volume with Diluent and injected into the HPLC system.Fluoxetine peak intensity did NOT decrease. Assay result obtained - 97.2%.ED. N0: 04Effective Date:APPROVED::HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationSTANDARD - ACID HYDROLYSISFluoxetine peak:Purity Angle:0.060Match Angle:0.060Purity Threshold:0.099Match Threshold:0.3713.BASE HYDROLYSIS3.1.Sample solution of IAG-21-001 (2) (20mg/capsule) was prepared as per method SI-IAG-206-02 : An amount equivalent to 20mg Fluoxetine was weight into a 50mL volumetric flask. 20mL Diluent was added and the solution sonicated for 10 minutes. 1mL of 5N NaOH was added to this solution. The solution was allowed to stand for 18 hours, then adjusted to about pH = 5.5 with 5N HCl, made up to volume with Diluent and injected into the HPLC system.Fluoxetine peak intensity did NOT decrease. Assay result obtained - 99.3%.SAMPLE - BASE HYDROLYSISFluoxetine peak:Purity Angle:0.063Match Angle:0.065Purity Threshold:0.099Match Threshold:0.3623.2.Standard stock solution was prepared as per method SI-IAG-206-02 : About 22mg Fluoxetine HCl was weighed into a 50mL volumetric flask. 20mL Diluent was added. 2mL of 5N NaOH was added to this solution. The solution was allowed to stand for 18 hours, then adjusted to about pH=5.5 with 5N HCl, made up to volume with Diluent and injected into the HPLC system.Fluoxetine peak intensity did NOT decrease - 99.5%.STANDARD - BASE HYDROLYSISFluoxetine peak:Purity Angle:0.081Match Angle:0.096Purity Threshold:0.103Match Threshold:0.3634.OXIDATION4.1.Sample solution of IAG-21-001 (2) (20mg/capsule) was prepared as per method SI-IAG-206-02. An equivalent to 20mg Fluoxetine was weighed into a 50mL volumetric flask. 20mL Diluent added and the solution sonicated for 10 minutes.1.0mL of 30% H2O2 was added to the solution and allowed to stand for 5 hours, then made up to volume with Diluent, filtered and injected into HPLC system.Fluoxetine peak intensity decreased to 95.2%.ED. N0: 04Effective Date:APPROVED::HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationSAMPLE - OXIDATIONFluoxetine peak:Purity Angle:0.090Match Angle:0.400Purity Threshold:0.154Match Threshold:0.4294.2.Standard solution was prepared as in method SI-IAG-206-02 : about 22mg Fluoxetine HCl were weighed into a 50mL volumetric flask and 25mL Diluent were added. 2mL of 30% H2O2 were added to this solution which was standing for 5 hours, made up to volume with Diluent and injected into the HPLC system.Fluoxetine peak intensity decreased to 95.8%.STANDARD - OXIDATIONFluoxetine peak:Purity Angle:0.083Match Angle:0.416Purity Threshold:0.153Match Threshold:0.4295.SUNLIGHT5.1.Sample solution of IAG-21-001 (2) (20mg/capsule) was prepared as in method SI-IAG-206-02 . The solution was exposed to 500w/hr. cell sunlight for 1hour. The BST was set to 35°C and the ACT was 45°C. The vials were placed in a horizontal position (4mm vials, National + Septum were used). A Dark control solution was tested. A 2%w/v quinine solution was used as the reference absorbance solution.Fluoxetine peak decreased to 91.2% and the dark control solution showed assay of 97.0%. The difference in the absorbance in the quinine solution is 0.4227AU.Additional peak was observed at RRT of 1.5 (2.7%).The total percent of Fluoxetine peak with the degradation peak is about 93.9%.SAMPLE - SUNLIGHTFluoxetine peak:Purity Angle:0.093Match Angle:0.583Purity Threshold:0.148Match Threshold:0.825 ED. N0: 04Effective Date:APPROVED::HPLC ASSAY with DETERMINATION OF META-FLUOXETINE HCl.ANALYTICAL METHOD VALIDATION10 and 20mg Fluoxetine Capsules HPLC DeterminationSUNLIGHT (Cont.)5.2.Working standard solution was prepared as in method SI-IAG-206-02 . The solution was exposed to 500w/hr. cell sunlight for 1.5 hour. The BST was set to 35°C and the ACT was 42°C. The vials were placed in a horizontal position (4mm vials, National + Septum were used). A Dark control solution was tested. A 2%w/v quinine solution was used as the reference absorbance solution.Fluoxetine peak was decreased to 95.2% and the dark control solution showed assay of 99.5%.The difference in the absorbance in the quinine solution is 0.4227AU.Additional peak were observed at RRT of 1.5 (2.3).The total percent of Fluoxetine peak with the degradation peak is about 97.5%. STANDARD - SUNLIGHTFluoxetine peak:Purity Angle:0.067Match Angle:0.389Purity Threshold:0.134Match Threshold:0.8196.HEAT OF SOLUTION6.1.Sample solution of IAG-21-001-(2) (20 mg/capsule) was prepared as in method SI-IAG-206-02 . Equivalent to 20mg Fluoxetine was weighed into a 50mL volumetric flask. 20mL Diluent was added and the solution was sonicated for 10 minutes and made up to volume with Diluent. 4mL solution was transferred into a suitable crucible, heated at 105°C in an oven for 2 hours. The sample was cooled to ambient temperature, filtered and injected into the HPLC system.Fluoxetine peak was decreased to 93.3%.SAMPLE - HEAT OF SOLUTION [105o C]Fluoxetine peak:Purity Angle:0.062Match Angle:0.460Purity Threshold:0.131Match Threshold:0.8186.2.Standard Working Solution (WS) was prepared under method SI-IAG-206-02 . 4mL of the working solution was transferred into a suitable crucible, placed in an oven at 105°C for 2 hours, cooled to ambient temperature and injected into the HPLC system.Fluoxetine peak intensity did not decrease - 100.5%.ED. N0: 04Effective Date:APPROVED::。
奥贝胆酸联合白藜芦醇对小鼠非酒精性脂肪性肝病的治疗作用

奥贝胆酸联合白藜芦醇对小鼠非酒精性脂肪性肝病的治疗作用高川;姚宜山;周奎臣;付海洋;王金柱;梁霞霞;王瑜【期刊名称】《中国药理学与毒理学杂志》【年(卷),期】2022(36)5【摘要】目的探索奥贝胆酸(OCA)和白藜芦醇(RSV)联合应用对非酒精性脂肪性肝病(NAFLD)模型小鼠的治疗作用和机制。
方法雄性C57BL/6N小鼠42只,除正常对照组8只外,其余用1%四氯化碳(CCl_(4))按5 mL·kg^(-1)每周ip注射1次联合高脂饲料喂食4周诱导NAFLD模型。
建模小鼠分为模型对照组、模型+OCA组、模型+RSV组和模型+OCA+RSV组,OCA和RSV均30 mg·kg^(-1) ig给药连续28 d。
小鼠处死取血制备血清,取肝称重计算肝指数,HE染色观察肝组织病理形态,油红O染色检测肝细胞脂质沉积并统计脂滴面积百分比;全自动生化分析仪和检测试剂盒检测血清谷丙转氨酶(GPT)、谷草转氨酶(GOT)、总胆固醇(TC)、甘油三酯(TG)、低密度脂蛋白(LDL)、高密度脂蛋白(HDL)、超氧化物歧化酶(SOD)活性、丙二醛(MDA)、白细胞介素1β(IL-1β)、IL-6和肿瘤坏死因子α(TNF-α)水平;Western印迹法检测肝组织沉默信息调节因子1(Sirt1)、NF-κB和p-NF-κB蛋白表达水平。
结果与正常对照组相比,模型组小鼠肝指数显著升高(P<0.01);与模型组相比,各给药组肝指数显著降低(P<0.01);与模型+RSV相比,模型+OCA+RSV组肝指数降低更加明显(P<0.05)。
与正常对照组相比,模型组小鼠肝组织有大量脂肪空泡和炎症损伤,油红O染色后细胞内有大量红色脂滴,血清GPT,GOT,TC,LDL,MDA,IL-1β,IL-6和TNF-α水平及肝组织NF-κB蛋白表达和磷酸化水平显著升高(P<0.01),血清HDL水平和肝组织Sirt1表达水平显著降低(P<0.05)。
超声引导下微波消融联合贝伐珠单抗治疗晚期结肠癌伴肝转移的临床价值

·临床研究·超声引导下微波消融联合贝伐珠单抗治疗晚期结肠癌伴肝转移的临床价值韩小军袁理郭道宁摘要目的探讨超声引导下微波消融联合贝伐珠单抗治疗晚期结肠癌伴肝转移的临床应用价值。
方法选取在我院就诊的102例晚期结肠癌伴肝转移患者,按随机数字表法分为观察组和对照组各51例,对照组采用贝伐珠单抗联合常规化疗治疗,观察组在此基础上采用超声引导下微波消融治疗;比较两组患者治疗后疗效、免疫功能、不良反应及预后情况。
结果治疗后,观察组客观缓解率(ORR)、疾病控制率(DCR)均高于对照组(均P<0.05);两组CD3+、CD4+、CD8+均较治疗前下降,且观察组CD3+、CD4+、CD4+/CD8+均高于对照组,CD8+低于对照组,差异均有统计学意义(均P<0.05)。
治疗后,两组胃肠道反应、食欲减退、疲劳乏力等不良反应比较差异均无统计学意义;观察组累积无复发生存率及累积总生存率分别为78.77%、57.45%,均高于对照组(49.32%、34.23%),差异均有统计学意义(χ2=10.086、4.536,P=0.001、0.033)。
结论超声引导下微波消融联合贝伐珠单抗能提高晚期结肠癌伴肝转移患者的治疗效果,缓解免疫功能抑制,改善生存状况,具有较好的临床应用价值。
关键词超声引导;微波消融;结肠癌,晚期;肝转移;贝伐珠单抗[中图法分类号]R445.1[文献标识码]AClinical value of ultrasound-guided microwave ablation combined withbevacizumab in the treatment of advanced colonadenocarcinoma with liver metastasisHAN Xiaojun,YUAN Li,GUO DaoningDepartment of Ultrasound Medicine,Mianyang Hospital Affiliated to School of Medicine,University of Electronic Science andTechnology of China,Sichuan621000,ChinaABSTRACT Objective To explore the application clinical value of ultrasound-guided microwave ablation combined with bevacizumab in the treatment of advanced colon adenocarcinoma(COAD)with liver metastasis.Methods A total of102 patients with advanced COAD with liver metastasis treated in our hospital were selected,and divided into the observation group and the control group by random number table method,with51cases in each group.The control group was treated with bevacizumab combined with conventional chemotherapy.On this basis,the observation group was treated with ultrasound-guided microwave thermal ablation.The curative effect,immune function,adverse reactions and prognosis after treatment of the two groups were compared.Results After treatment,the objective remission rate(ORR)and disease control rate(DCR)in the observation group were higher than those in the control group(both P<0.05).After treatment,the CD3+,CD4+and CD4/CD8+in the observation group were higher than those in the control group,and CD8+was lower than that in the control group,the differences were statistically significant(all P<0.05).After treatment,there were no statistically significant difference in the incidence rates of adverse reactions such as gastrointestinal reactions,loss of appetite and fatigue between the two groups.The cumulative recurrence-free survival rate and cumulative overall survival rate in observation group were78.77%and57.45% respectively,which were significantly higher than those in control group(49.32%and34.23%),the differences were statistically significant(χ2=10.086,4.536,P=0.001,0.033).Conclusion Ultrasound-guided microwave ablation combined with作者单位:621000四川省绵阳市,电子科技大学医学院附属绵阳医院绵阳市中心医院超声医学科(韩小军、郭道宁),肿瘤科(袁理)通讯作者:郭道宁,Email:******************结肠癌是常见的消化道肿瘤,近年来其发病率和死亡率均逐渐升高。
15704784_酸性鞘磷脂酶在非酒精性脂肪性肝病中的作用及应用前景

·综述·酸性鞘磷脂酶在非酒精性脂肪性肝病中的作用及应用前景齐 雪 韩海静 牛春燕 【摘要】 酸性鞘磷脂酶(ASMase)与多种肝脏疾病的发病机制相关。
近年来研究发现,ASMase在非酒精性脂肪性肝病(NAFLD)患者及动物肝脏中表达增加,且可导致氧化应激、脂质沉积、脂毒性、炎性反应、纤维化等改变。
此文综述了ASMase在NAFLD发病机制中的作用,并评价其在NAFLD的预测、诊断、靶向治疗以及预后判断方面的潜在应用价值。
【关键词】 酸性鞘磷脂酶;非酒精性脂肪性肝病;发病机制;应用价值DOI:10.3969/j.issn.1673 534X.2017.06.008 基金项目:陕西省普通高等学校优势学科建设[陕教位(2014)3号];西安医学院第一附属医院院级科研基金(XYFY2016 12);陕西省科学技术厅文件[陕科发(2017)13号] 作者单位:710068 西安医学院(齐雪,韩海静);710077 西安医学院第一附属医院消化内科(牛春燕) 通信作者:牛春燕,Email:nchy69@163.com 近年来,非酒精性脂肪性肝病(NAFLD)在发达国家和发展中国家的发病率均呈逐年升高趋势,但NAFLD的发病机制迄今尚未阐明,普遍认为在经典的“二次打击”的基础上,胰岛素抵抗(IR)、氧化应激/脂质过氧化、内质网应激、游离脂肪酸及线粒体功能障碍是其可能的发病机制[1]。
近年有研究发现,NAFLD的发生发展可能与酸性鞘磷脂酶(ASMase)/神经酰胺(Cer)代谢通路有关[2]。
ASMase能够催化鞘磷脂产生Cer,是鞘磷脂物质代谢的关键酶。
鞘磷脂由Cer和磷酰胆碱在鞘磷脂合成酶的作用下生成,能够在数种鞘磷脂酶(SMase)的催化作用下水解为Cer和磷酰胆碱[3]。
Cer及其代谢物可影响细胞的凋亡、衰老、分化和迁移过程[4]。
SMase作为一种糖蛋白,可通过调控Cer的生成而间接调节机体的各种生物化学反应,因此是调控Cer合成、分泌的关键酶[3]。
尿微量白蛋白联合血清胱抑素C在糖尿病肾病诊断中的应用

DOI:10.19368/ki.2096-1782.2023.09.018尿微量白蛋白联合血清胱抑素C在糖尿病肾病诊断中的应用孙前进江苏省徐州市中医院检验科,江苏徐州221000[摘要]目的探讨临床诊断糖尿病肾病时联合检测尿微量白蛋白(urine microalbumin, U-mAlb)、血清胱抑素C (serum cystatin C, CysC)的作用与效能。
方法随机抽取2021年7月—2022年10月江苏省徐州市中医院检验科收治的41例2型糖尿病合并肾病患者,另择取同期在本院进行体检的健康志愿者45例,两组受检者均进行U-mAlb、CysC水平检测,并比较两组检测结果,同时根据临床结果,分析U-mAlb、CysC单一检测和联合检测的诊断效能。
结果糖尿病肾病患者的U-mAlb(51.48±5.46)mg/L、CysC(1.57±0.38)mg/L与健康者的U-mAlb (9.35±1.55)mg/L、CysC(0.70±0.11)mg/L比较,差异有统计学意义(t=47.688、14.130,P<0.05)。
对糖尿病肾病患者进行U-mAlb以及CysC联合检测的诊断准确度93.02%、敏感度95.12%、特异度93.33%,阳性预测度90.48%与阴性预测度95.45%均比各指标单一检测的诊断效能高,差异有统计学意义(P<0.05)。
结论在糖尿病肾病的诊断中联合检测U-mAlb以及CysC指标可获得较高的敏感度、特异度,可为疾病的诊疗提供有效依据,因此可在临床中进行推广应用。
[关键词]尿微量白蛋白;血清胱抑素C;糖尿病肾病;临床诊断[中图分类号]R59 [文献标识码]A [文章编号]2096-1782(2023)05(a)-0018-04Urine Microalbumin Combined with Serum Cystatin C in the Diagnosis of Diabetic NephropathySUN QianjinDepartment of Laboratory, Xuzhou Hospital of Traditional Chinese Medicine, Xuzhou, Jiangsu Province, 221000 China [Abstract] Objective To investigate the role and efficacy of combined urine microalbumin (U-mAlb) and serum cys‐tatin C (CysC) in the clinical diagnosis of diabetic nephropathy. Methods From July 2021 to October 2022, 41 pa‐tients with type 2 diabetes complicated with kidney disease were randomly selected from the Department of Laboratory Medicine of Xuzhou Hospital of Traditional Chinese Medicine in Jiangsu Province, and 45 healthy volunteers who had physical examination in this hospital at the same time were selected. The levels of U-mAlb and CysC were tested in both groups, and the results of the two groups were compared. At the same time, according to the clinical results, the diagnostic efficacy of single and joint U-mAlb and CysC tests was analyzed. Results The U-mAlb (51.48±5.46) mg/L, CysC (1.57±0.38) mg/L in patients with diabetes nephropathy were compared with those in healthy subjects (9.35±1.55) mg/L, CysC (0.70±0.11) mg/L, and the difference was statistically significant (t=47.688, 14.130, P<0.05). The combined detection of U-mAlb and CysC in patients with diabetes nephropathy had a diagnostic accuracy of 93.02%,a sensitivity of 95.12%, a specificity of 93.33%, a positive predictive rate of 90.48% and a negative predictive rate of95.45%, which were higher than the diagnostic efficacy of single detection of each index, and the difference was statis‐tically significant (P<0.05). Conclusion Joint detection of U-mAlb and CysC in the diagnosis of diabetes nephropathy can obtain high sensitivity and specificity, which can provide effective basis for disease diagnosis and treatment, so it can be popularized in clinical application.[作者简介] 孙前进(1983-),男,本科,副主任检验师,研究方向为输血与凝血。
血清肌钙蛋白、肌红蛋白及肌酸激酶同工酶联合检测早期诊断急性心肌梗死的临床价值

DOI:10.19368/ki.2096-1782.2023.11.027血清肌钙蛋白、肌红蛋白及肌酸激酶同工酶联合检测早期诊断急性心肌梗死的临床价值施磊,何丽,徐璐,赵玲张家港市中医医院检验科,江苏苏州215600[摘要]目的探讨血清肌钙蛋白(cardiac troponin I, cTnI)、肌红蛋白(myoglobin, Mb)及肌酸激酶同工酶(cre⁃atine kinase isoenzyme, CK-MB)联合检测早期诊断急性心肌梗死的价值。
方法选择张家港市中医医院2021年1月—2022年12月收治的急性心肌梗死患者46例设为观察组,另取同期健康体检者45例设为对照组,比较两组cTnI、Mb、CK-MB水平,评估联合检测对急性心肌梗死的诊断效能,另根据发病时间差异将患者分为0~<6 h与6~12 h两个亚组,比较两亚组cTnI、Mb、CK-MB水平的差异。
结果观察组cTnI(21.16±5.20)ng/mL、Mb(156.47±25.19)ng/mL、CK-MB(51.92±10.33)U/L,均高于对照组,差异有统计学意义(t=25.915、33.263、28.054,P<0.05)。
联合诊断准确率、灵敏度分别为93.41%、95.65%,高于CTnI、Mb、CK-MB单独检测的81.32%、69.57%;82.42%、73.91%;81.32%、67.39%,差异有统计学意义(P<0.05)。
联合检测诊断特异度为91.11%,与CTnI、Mb、CK-MB单独检测的93.33%、91.11%、95.56%对比,差异无统计学意义(P>0.05)。
6~12 h组cTnI、CK-MB水平均高于0~6 h组,Mb水平低于0~<6 h组,差异有统计学意义(P<0.05)。
结论急性心肌梗死患者cTnI、Mb、CK-MB水平均明显升高,联合检测对诊断急性心肌梗死诊断效能更高。
基于人工智能的冠状动脉易损斑块腔内影像学研究进展

基于人工智能的冠状动脉易损斑块腔内影像学研究进展陈远兴综述韩韦钰,赵然尊审校遵义医科大学附属医院心血管内科,贵州遵义563000【摘要】斑块的不稳定导致冠状动脉的血栓性闭塞是大多数急性冠脉综合征(ACS)的原因。
尽管罪犯血管得以及时开通,但非罪犯血管的易损斑块对患者远期预后仍存在较大威胁。
因此,动态评估易损斑块的变化,对冠心病患者格外重要。
冠状动脉血管腔内成像技术,如血管内超声(IVUS)、光学相干断层扫描(OCT)、近红外光谱(NIRS)以及其多模态融合技术等,因其可视化、准确度高,可以揭示易损斑块的不同特征,常用于检测易损斑块。
而IVUS 、OCT 等图像解释需有经验的心血管临床医生逐帧判断,需要大量的时间成本,且图像的解读存在的观察者内及观察者间的差异,这推动了人工智能(AI)在冠状动脉血管腔内影像学应用的发展。
由于电子医疗系统的广泛应用、临床大数据的日益暴增,AI 已在医疗行业获得了极大的进展。
人工智能结合腔内影像学在斑块的识别、干预、预后等诸多方面广泛应用,未来将不断优化诊疗系统,提高精准医疗水平,实现对易损斑块的早期诊断及合理干预。
【关键词】动脉粥样硬化;急性冠脉综合征;腔内成像;易损斑块;人工智能【中图分类号】R541.4【文献标识码】A【文章编号】1003—6350(2023)03—0445—05Research progress of intravascular imaging of vulnerable coronary plaque based on artificial intelligence.CHEN Yuan-xing,HAN Wei-yu,ZHAO Ran-zun.Department of Cardiovascular Medicine,Affiliated Hospital of Zunyi Medical University,Zunyi 563000,Guizhou,CHINA【Abstract 】Plaque vulnerability leading to thrombotic occlusion of coronary arteries is the main cause of majori-ty of acute coronary syndrome (ACS).Despite the criminal vessels can be opened in time,the vulnerable plaques of non-criminal vessels still cause a great threat to the long-term prognosis of patients.Thus,dynamic assessment of vulner-able plaque changes is particularly important for patients with coronary heart disease.Intravascular imaging techniques in coronary arteries,such as intravascular ultrasound (IVUS),Optical Coherence Tomography (OCT),Near Infrared Spectrum Instrument (NIRS),and its multi-mode fusion technology,are often used to detect vulnerable plaques due to their high visualization and accuracy,which can reveal different characteristics of vulnerable plaques.However,IVUS,OCT and other image interpretation requires experienced cardiovascular clinicians to judge frame by frame,which re-quires a large amount of time cost,and there are intra-observer and inter-observer differences in image interpretation,which all promotes the development of AI in the application of intravascular coronary imaging.Artificial intelligence (AI)has made great progress in the medical field due to the wide application of electronic medical information system and the increasing explosion of clinical big data.Artificial intelligence combined with intravascular imaging has been widely ap- ·综述·doi:10.3969/j.issn.1003-6350.2023.03.035第一作者:陈远兴(1995—),男,住院医师,主要研究方向为冠状动脉粥样硬化性心脏病腔内影像学图像分析。
荧光PCR探针熔解曲线法与微孔板法检测MTB耐药性的临床应用比较

• 132 •中国防痨杂志 2021 年 2 月第 43 卷第 2 期 Chin J Am ituberc ,Fc»bruary 2021,V 〇U 3,N 〇. 2•论著•荧光P C R 探针熔解曲线法与微孔板法检测M T B 耐药性的临床应用比较王佩赵国连雷倩郑丹崔晓利周俊【摘要】目的分析荧光P C K 探针熔解曲线法与微孔板法药物敏感性试验(简称“药敏试验”)检测结核分枝 杆菌(M TB )对抗结核药品耐药性结果的一致性及M T B 基因突变与耐药的相关性,为临床诊疗优化提供参考。
方法搜集2019年1 -12月分离自西安市胸科医院就诊患者并经鉴定确认的343株M T B 临床分离株,菌株均进 行了微孔板法药敏试验和荧光P C R 探针熔解曲线法检测。
以微孔板法药敏试验结果为参照.评价荧光P C R 探针 熔解曲线法检测M T B 对异烟肼、利福平、链霉素、乙胺丁醇、莫西沙星和左氧氟沙星耐药性的检测效能,并分析荧 光P C R 探针培解曲线法检测的M T B 基因突变与微孔板法药敏试验最低抑菌浓度(minimum inhibitory concentre - tio n ,M IC )的相关性。
结果以微孔板法药敏试验结果为参照,荧光P C R 探针熔解曲线法检测M T B 对异烟肼、利福平、链霉素、乙胺丁醇、莫西沙星和左氧氟沙星耐药性的敏感度、特异度、沖《值分别为:96. 20% (76/79)、 95. 28%(242/254)、0. 88:93. 62%(44/47)、94. 58% (279/295)、0. 79; 96. 88% (62/64 )、94. 96% (264/278)、0. 86; 93. 33%(14/15),95. 37% (309/324 ),0. 61;92. 31%(24/26),97. 16%(308/317),0. 80s 91. 18% (31/34) ,99. 35% (307/309)、0. 92。
细胞蛇的研究进展

2007年,英国牛津大学的刘骥陇等在研究果蝇U 小体和P 小体(U 小体和P 小体是真核生物细胞质中的无膜细胞器)的功能关系时,用4种针对Cup (P 小体中的一种蛋白质)的抗体,对雌性果蝇的卵巢组织进行免疫组织化学染色,染色结果除了预期标记上的P 小体外,还标记出了长条形的丝状结构[1]。
这种结构的形状和数量与纤毛很相似,导致当时以为在果蝇中找到了有纤毛的新细胞类型。
但后来的一系列实验表明,该结构与纤毛没有关系,于是将其命名为“细胞蛇”。
最初是抗Cup 抗体不纯产生假象,意外发现的细胞蛇,而采用亲和层析纯化后的抗Cup 抗体无法再DOI:10.16605/ki.1007-7847.2020.10.0258细胞蛇的研究进展收稿日期:2020-10-22;修回日期:2020-11-19;网络首发日期:2021-07-27基金项目:宁夏自然科学基金项目(2020AAC03179);国家自然科学基金资助项目(31560329)作者简介:李欣玲(1999—),女,广西贵港人,学生;*通信作者:俞晓丽(1984—),女,宁夏银川人,博士,副教授,主要从事干细胞与生殖生物学研究,E-mail:********************。
李欣玲,张樱馨,李进兰,潘文鑫,王彦凤,杨丽蓉,王通,俞晓丽*(宁夏医科大学生育力保持教育部重点实验室临床医学院基础医学院,中国宁夏银川750000)摘要:细胞蛇是近年来细胞生物学研究的热门方向之一,由于其在细胞的增殖、代谢和发育上具有一定的生物学功能,因此,对一些疾病如癌症等的临床诊断或治疗具有一定的指导意义。
细胞蛇是由三磷酸胞苷合成酶(cytidine triphosphate synthetase,CTPS)聚合而成的无膜细胞器,其形成过程及功能在不同类型的细胞中不尽相同。
例如:细胞蛇能促进癌细胞增殖,并使患者病情恶化;过表达的细胞蛇可抑制神经干细胞增殖,影响大脑皮层发育;在卵泡细胞中,细胞蛇相当于CTPS 的存储库,在卵子发生过程起到促进细胞增殖和代谢的作用。
一种马铃薯腐烂茎线虫脂肪酸去饱和酶基因序列[发明专利]
![一种马铃薯腐烂茎线虫脂肪酸去饱和酶基因序列[发明专利]](https://img.taocdn.com/s3/m/97ccb85065ce050877321399.png)
专利名称:一种马铃薯腐烂茎线虫脂肪酸去饱和酶基因序列专利类型:发明专利
发明人:魏利辉,邵颖,万景旺,赵雷,张红艳
申请号:CN201210075653.7
申请日:20120321
公开号:CN102634526A
公开日:
20120815
专利内容由知识产权出版社提供
摘要:本发明公开了一种马铃薯腐烂茎线虫脂肪酸去饱和酶基因序列,该基因cDNA全长
1183bp,自110bp至1108bp区段为其开放阅读框,编码333个氨基酸,5’非编码区长
109bp,3’非编码区长75bp,有多聚核苷酸尾巴,该基因在线虫耐寒作用机制中起作用,同时还在抗线虫转基因作物方面应用。
申请人:江苏省农业科学院
地址:210014 江苏省南京市玄武区钟灵街50号
国籍:CN
更多信息请下载全文后查看。
Masitinib_LCMS_09866_MedChemExpress
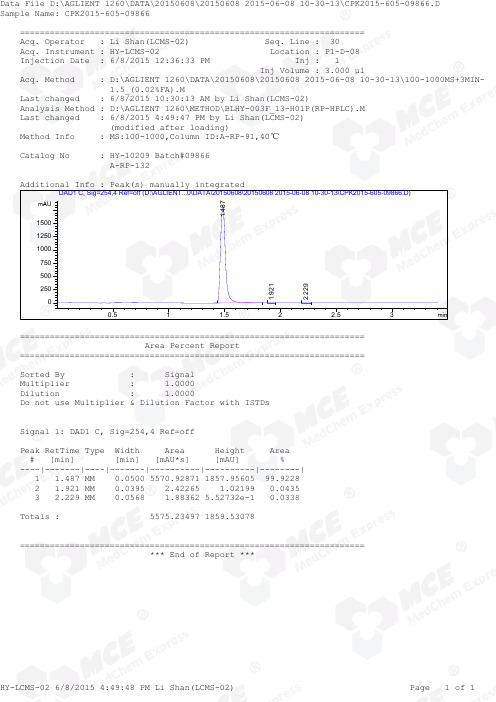
=====================================================================Acq. Operator : Li Shan(LCMS-02) Seq. Line : 30Acq. Instrument : HY-LCMS-02 Location : P1-D-08Injection Date : 6/8/2015 12:36:33 PM Inj : 1 Inj Volume : 3.000 µl Acq. Method : D:\AGLIENT 1260\DATA\20150608\20150608 2015-06-08 10-30-13\100-1000MS+3MIN- 1.5_(0.02%FA).M Last changed : 6/8/2015 10:30:13 AM by Li Shan(LCMS-02)Analysis Method : D:\AGLIENT 1260\METHOD\BLHY-003F_13-H01P(RP-HPLC).M Last changed : 6/8/2015 4:49:47 PM by Li Shan(LCMS-02) (modified after loading)Method Info : MS:100-1000,Column ID:A-RP-91,40℃ Catalog No : HY-10209 Batch#09866 A-RP-132 Additional Info : Peak(s) manually integrated min0.51 1.52 2.53mAU250500750100012501500DAD1 C, Sig=254,4 Ref=off (D:\AGLIENT...0\DATA\20150608\20150608 2015-06-08 10-30-13\CPK2015-605-09866.D)1.487 1.9212.229 ===================================================================== Area Percent Report ===================================================================== Sorted By : Signal Multiplier : 1.0000Dilution : 1.0000Do not use Multiplier & Dilution Factor with ISTDs Signal 1: DAD1 C, Sig=254,4 Ref=off Peak RetTime Type Width Area Height Area # [min] [min] [mAU*s] [mAU] %----|-------|----|-------|----------|----------|--------| 1 1.487 MM 0.0500 5570.92871 1857.95605 99.9228 2 1.921 MM 0.0395 2.42265 1.02199 0.0435 3 2.229 MM 0.0568 1.88362 5.52732e-1 0.0338 Totals : 5575.23497 1859.53078 ===================================================================== *** End of Report ***=====================================================================Acq. Operator : Li Shan(LCMS-02) Seq. Line : 30Acq. Instrument : HY-LCMS-02 Location : P1-D-08Injection Date : 6/8/2015 12:36:33 PM Inj : 1 Inj Volume : 3.000 µl Acq. Method : D:\AGLIENT 1260\DATA\20150608\20150608 2015-06-08 10-30-13\100-1000MS+3MIN- 1.5_(0.02%FA).M Last changed : 6/8/2015 10:30:13 AM by Li Shan(LCMS-02)Analysis Method : D:\AGLIENT 1260\METHOD\BLHY-003F_13-H01P(RP-HPLC).M Last changed : 6/8/2015 4:51:25 PM by Li Shan(LCMS-02) (modified after loading)Method Info : MS:100-1000,Column ID:A-RP-91,40℃ Catalog No : HY-10209 Batch#09866 A-RP-132 Additional Info : Peak(s) manually integrated min0.51 1.52 2.5302000004000006000008000001000000MSD1 TIC, MS File (D:\AGLIENT 1260\DATA\20150608\20150608 2015-06-08 10-30-13\CPK2015-605-09866.D) ES-API, Pos, Sc1.496MS Signal: MSD1 TIC, MS File, ES-API, Pos, Scan, Frag: 50 Spectra averaged over upper half of peaks. Noise Cutoff: 1000 counts. Reportable Ion Abundance: > 10%. Retention Mol. Weight Time (MS) MS Area or Ion 1.496 6525681 499.20 I 250.20 Im/z 200400600800020406080100*MSD1 SPC, time=1.470:1.525 of D:\AGLIENT 1260\DATA\20150608\20150608 2015-06-08 10-30-13\CPK2015-605-09866.D ES-API Max: 728592500.2 250.2 *** End of Report ***。
