Generalized Matric Massey Products for Graded Modules
USP 通用章节目录

USP29-通用章节指导目录(附录)Guide to General Chapters 通用章节指导目录中此颜色并且带有“***”的为新增内容。
General Requirements for Test and Assays检查与含量分析的一般要求<1>INJECTIONS……2455注射剂<11>USP REFERENCE STANDARDS……2458USP对照品Apparatus for Test and Assays用于检查与含量分析的器具<16>AUTOMATED METHODS OF ANAL YSIS……2491自动化分析方法<21>THERMOMETERS……2497温度计<31>VOLUMETRIC APPARATUS……2497容量器具<41>WEIGHTS AND BALANCES……2499砝码与天平Microbiological Tests 微生物检查法<51>ANTIMICROBIAL EFFECTIVENESS TESTING……2499抗菌剂有效性检查法<55>BIOLOGICAL INDICATORS—RESISTANCE PERFORMANCE TESTS (2501)生物指示剂-耐药性实验<61>MICROBIAL LIMIT TESTS……2503微生物限度检查法<71>STERILITY TESTS……2508无菌检查法Biological tests and assays生物检查法与测定法<81>ANTIBIOTICS—MICROBIAL ASSAYS……2513抗生素-微生物测定<85>BACTERIAL ENDOTOXINS TEST……2521细菌内毒素检查法<87>BIOLOGICAL REACTIVITY TESTS, IN VITRO……2525体外的生物反应性检查法<88>BIOLOGICAL REACTIVITY TESTS, IN VIVO……2526体内的生物反应性检查法<91>CALCIUM PANTOTHENATE ASSAY……2530泛酸钙测定法<111>DESIGN AND ANAL YSIS OF BIOLOGICAL ASSAYS……2531 生物测定法的设计与分析<115>DEXPANTHENOL ASSAY……2543右泛醇(拟胆碱药)测定法<121>INSULIN ASSAYS……2544胰岛素测定法<141>PROTEIN—BIOLOGICAL ADEQUACY TEST……2546蛋白质-生物适应性试验<151>PYROGEN TEST……2546热原检查法<161>TRANSFUSION AND INFUSION ASSEMBLIES AND SIMILAR MEDICAL DEVICES (2547)输血输液用具以及相类似的医疗器械<171>VITAMIN B12 ACTIVITY ASSAY……2548维生素B12活性测定法Chemical Tests and assays化学实验与测定法<181>IDENTIFICATION—ORGANIC NITROGENOUS BASES (2549)鉴别-有机氮碱?<191>IDENTIFICATION TESTS—GENERAL……2550鉴别实验-通用<193>IDENTIFICATION—TETRACYCLINES……2551鉴别-四环素<197>SPECTROPHOTOMETRIC IDENTIFICATION TESTS......2552分光光度计鉴别实验<201>THIN-LAYER CHROMATOGRAPHIC IDENTIFICATION TEST.. (2553)薄层色谱鉴别实验Limit Test 限度检查法<206>ALUMINUM……2554铝<211>ARSENIC……2554砷<221>CHLORIDE AND SULFATE……2555氯和硫<223>DIMETHYLANILINE……2555二甲基苯胺<226>4-EPIANHYDRO-TETRACYCLINE……25564-?-四环素<231>HEA VY METALS……2556重金属<241>IRON……2557铁<251>LEAD……2558铅<261>MERCURY……2558汞<271>READIL Y CARBONIZABLE SUBSTANCES TEST……2560易碳化物检查法<281>RESIDUE ON IGNITION……2560灼烧残渣<291>SELENIUM……2560硒Other Tests and Assays 其它检查法与测定法<301>ACID-NEUTRALIZING CAPACITY……2561酸中和容量<311>ALGINATES ASSAY……2562藻酸盐测定法<331>AMPHETAMINE ASSAY……2562苯丙胺测定法<341> ANTIMICROBIAL AGENTS—CONTENT……2563 抗菌剂-含量<345> Assay for Citric Acid/Citrate and Phosphate……2565 柠檬酸/柠檬酸盐和磷酸盐的测定<351>ASSAY FOR STEROIDS……2565类固醇(甾类化合物)测定法<361> BARBITURATE ASSAY……2565 巴比妥类药物测定法<371>COBALAMIN RADIOTRACER ASSAY……2566钴铵素放射性跟踪剂测定法<381>ELASTOMERIC CLOSURES FOR INJECTIONS……2567 注射剂的弹性密封件<391>EPINEPHRINE ASSAY……2567肾上腺测定法<401>FATS AND FIXED OILS……2568脂肪与混合油<411>FOLIC ACID ASSAY……2571叶酸测定法<425>IODOMETRIC ASSAY—ANTIBIOTICS……2572碘量检查法-抗生素<429>LIGHT DIFFRACTION MEASUREMENT OF PARTICLE SIZE (2572)粒子尺寸的光衍射测量<431>METHOXY DETERMINA TION……2575甲氧基测定法<441>NIACIN OR NIACINAMIDE ASSAY……2576烟酰或烟酰胺测定法<451>NITRITE TITRATION……2578亚硝酸盐滴定<461>NITROGEN DETERMINA TION……2578氮测定法<466>ORDINARY IMPURITIES……2579一般杂质<467>ORGANIC VOLATILE IMPURITIES……2580有机的易挥发杂质<471>OXYGEN FLASK COMBUSTION……2590氧瓶燃烧法<481>RIBOFLAVIN ASSAY……2590核黄素测定法<501>SALTS OF ORGANIC NITROGENOUS BASES……2591有机氮盐<511>SINGLE-STEROID ASSAY……2591单一的类固醇测定法<521>SULFONAMIDES……2592磺胺制剂<531>THIAMINE ASSAY……2593硫胺素测定法<541>TITRIMETRY……2593滴定法<551>ALPHA TOCOPHEROL ASSAY……2596α-维生素E测定法<561>ARTICLES OF BOTANICAL ORIGIN……2596植物起源的药品<563>IDENTIFICATION OF ARTICLES OF BOTANICAL ORIGIN……2603植物药品的鉴别<565>BOTANICAL EXTRACTS……2609植物提取<571>VITAMIN A ASSAY……2611维生素A的测定法<581>VITAMIN D ASSAY……2612维生素D的测定法<591>ZINC DETERMINATION……2616锌的测定法Physical Test and Determinations物理检查与测定法INHALERS, AND DRY POWDER <601>AEROSOLS, NASAL SPRAYS,USP28METERED-DOSEINHALERS……2617气溶胶,鼻用喷雾剂,定量吸入器与干粉吸入器<611>ALCOHOL DETERMINATION……2637乙醇测定法<616>BULK DENSITY AND TAPPED DENSITY……2638堆密度与拍实密度<621>CHROMATOGRAPHY…….2639色谱法<631>COLOR AND ACHROMICITY……2651呈色与消色<641>COMPLETENESS OF SOLUTION……2652完全溶解<643>TOTAL ORGANIC CARBON……2652总有机碳<645>WA TER CONDUCTIVITY……2653水电导率<651>CONGEALING TEMPERA TURE……2654凝点温度<661>CONTAINERS……2655容器<671>CONTAINERS—PERMEATION……2663容器-渗透<691>COTTON……2664棉花<695>CRYSTALLINITY……2665结晶性<696>Crystallinity Determination By Solution Calorimetry……2666 通过溶液量热学测定结晶性<698>DELIVERABLE VOLUME……2667可转移的体积<699>DENSITY OF SOLIDS……2669固体密度<701>DISINTEGRATION……2670崩解时限***<701>Disintegration (Harmonized Chapter, Official April 1,2006)………..2671崩解时限(协调的章节,法定日期,2006.4.1)<711>DISSOLUTION……2673 溶出度***<711>Dissolution (Harmonized Chapter, Official April 1,2006)………..2675 溶出度(协调的章节,法定日期,2006.4.1)<721>DISTILLING RANGE……2682馏程<724>DRUG RELEASE……2682药物释放度***<724>Drug releasee (Harmonized Chapter, Official April 1,2006)………..2690药物释放度(协调的章节,法定日期,2006.4.1)<726>ELECTROPHORESIS……2694电泳<727>CAPILLARY ELECTROPHORESIS……2696毛细管电泳法***<730>Plasma Spectrochemistry….2700 血浆光谱化学<731>LOSS ON DRYING……2704干燥失重<733>LOSS ON IGNITION……2704灼烧失重<736>MASS SPECTROMETRY……2705 质谱<741>MELTING RANGE OR TEMPERATURE……2708熔距或熔点<751>METAL PARTICLES IN OPHTHALMIC OINTMENTS……2709眼用软膏中的金属粒子<755>MINIMUM FILL……2710最低装填量<761>NUCLEAR MAGNETIC RESONANCE……2710核磁共振<771>OPHTHALMIC OINTMENTS……2715眼用软膏<776>OPTICAL MICROSCOPY……2716光学显微镜<781>OPTICAL ROTATION……2718旋光<785>OSMOLALITY AND OSMOLARITY……2718同渗重摩与同渗容摩<786>PARTICLE SIZE DISTRIBUTION ESTIMATION BY ANAL YTICAL SIEVING (2720)通过筛分法估算粒子分布<788>PARTICULATE MATTER IN INJECTIONS……2722注射剂中的颗粒<789>PARTICULATE MATTER IN OPHTHALMIC SOLUTIONS……2729眼用溶液中的颗粒<791>pH (2730)<795>PHARMACEUTICAL COMPOUNDING—NONSTERILE PREPARATIONS (2731)药物混合-非无菌制剂<797>PHARMACEUTICAL COMPOUNDING—STERILE PREPARATIONS (2735)药物混合-无菌制剂<801>POLAROGRAPHY……2752极谱法<811>POWDER FINENESS……2754粉剂细度<821>RADIOACTIVITY……2755放射性<823>RADIOPHARMACEUTICALS FOR POSITRON EMISSION TOMOGRAPHY —COMPOUNDING……2763用于正电子发射断层摄影术的放射性药物<831>REFRACTIVE INDEX……2766折光率<841>SPECIFIC GRA VITY……2766比重<846>SPECIFIC SURFACE AREA……2767 比表面积<851>SPECTROPHOTOMETRY AND LIGHT-SCA TTERING……2770分光光度计与光散射<861>SUTURES—DIAMETER…2775缝线-直径<871>SUTURES—NEEDLE ATTACHMENT……2775缝线-穿孔实验<881>TENSILE STRENGTH…..2776张力<891>THERMAL ANAL YSIS……2776热分析<905>UNIFORMITY OF DOSAGE UNITS……2778制剂单位的含量均匀度<905>UNIFORMITY OF DOSAGE UNITS (Harmonized Chapter, Official April 1,2006)……2780制剂单位的含量均匀度(协调的章节2006.4.1)<911>VISCOSITY……2785粘度<921>WA TER DETERMINA TION……2785水测定法<941>X-RAY DIFFRACTION……2788X光衍射General Information通用信息<1010>ANAL YTICAL DATA—INTERPRETA TION AND TREATMENT (2790)分析数据-解释与处理<1015>AUTOMA TED RADIOCHEMICAL SYNTHESIS APPARATUS (2801)放射性自动合成装置<1031>THE BIOCOMPATIBILITY OF MATERIALS USED IN DRUG CONTAINERS, MEDICAL DEVICES, AND IMPLANTS (2802)用于药物容器、医疗设施和植入剂的材料的生物相容性<1035>BIOLOGICAL INDICATORS FOR STERILIZATION……2811灭菌用生物指示剂<1041>BIOLOGICS……2814生物制剂***<1043>Ancillary Material for Cell, Gene, and Tissue-Engineered Products…….2814 细胞,基因与组织设计产品的辅助材料<1045>BIOTECHNOLOGY-DERIVED ARTICLES……2821生物技术提取产品<1046>CELL AND GENE THERAPY PRODUCTS……2831细胞与基因治疗产品<1047>BIOTECHNOLOGY-DERIVED ARTICLES—TESTS……2858生物技术产品-检查法<1048>QUALITY OF BIOTECHNOLOGICAL PRODUCTS: ANAL YSIS OF THE EXPRESSION CONSTRUCT IN CELLS USED FOR PRODUCTION OF r-DNA DERIVED PROTEIN PRODUCTS1 (2883)生物产品质量:从蛋白质产品中提取的r-DNA产品在细胞中表达结构的分析<1049>QUALITY OF BIOTECHNOLOGICAL PRODUCTS: STABILITY TESTING OF BIOTECHNOLOGICAL/BIOLOGICAL PRODUCTS1 (2884)生物技术产品的质量:生物技术/生物产品的稳定性实验<1050>VIRAL SAFETY EV ALUA TION OF BIOTECHNOLOGY PRODUCTS DERIVED FROM CELL LINES OF HUMAN OR ANIMAL ORIGIN (2887)从人或动物细胞中提取的生物技术产品的病毒安全性评估<1051>CLEANING GLASS APPARATUS……2896玻璃容器的清洗<1061>COLOR—INSTRUMENTAL MEASUREMENT……2896显色-仪器测量***<1065>Ion Chromatography………2898 离子色谱法<1074>EXCIPIENT BIOLOGICAL SAFETY EV ALUA TION GUIDELINES (2900)赋形剂(辅料)生物安全性评估指导<1075>GOOD COMPOUNDING PRACTICES……2903好的混合操作<1078>GOOD MANUFACTURING PRACTICES FOR BULK PHARMACEUTICAL EXCIPIENTS (2906)批药品赋形剂的生产管理规范***<1079>Good Storage and Shipping Practices……2915 良好的贮存与船运规范<1081>GEL STRENGTH OF GELATIN……2920白凝胶的凝胶强度<1086>IMPURITIES IN OFFICIAL ARTICLES……2920药典物品中的杂质<1087>INTRINSIC DISSOLUTION……2923内部的溶出度<1088>IN VITRO AND IN VIVO EV ALUA TION OF DOSAGE FORMS (2924)体内与体外的剂型的评估<1090>IN VIVO BIOEQUIV ALENCE GUIDANCES……29291体内生物等效性指导<1091>LABELING OF INACTIVE INGREDIENTS……2968非活性成分的标示<1101>MEDICINE DROPPER……2969医用滴管<1111>MICROBIOLOGICAL ATTRIBUTES OF NONSTERILE PHARMACEUTICAL PRODUCTS (2969)非无菌药品中的微生物分布<1116>MICROBIOLOGICAL EV ALUA TION OF CLEAN ROOMS AND OTHER CONTROLLED ENVIRONMENTS……2969洁净的房间与其它可控环境的微生物评估<1118>MONITORING DEVICES—TIME, TEMPERATURE, AND HUMIDITY (2976)监控装置-时间、温度与湿度<1119>NEAR-INFRARED SPECTROPHOTOMETRY……2979近红外分光光度测定法***<1120>Raman Spectrophotometry……..2983 Raman分光光度测定法<1121>NOMENCLATURE……2988命名***<1136>Packaging-Unit-of-Use……2989包装-单元使用<1146>PACKAGING PRACTICE—REPACKAGING A SINGLE SOLID ORAL DRUG PRODUCT INTO A UNIT-DOSE CONTAINER……2990 包装操作-将单一固体口服药品产品再包装成单元剂量<1150>PHARMACEUTICAL STABILITY……2994药物稳定性<1151>PHARMACEUTICAL DOSAGE FORMS……2996药物剂型<1160>PHARMACEUTICAL CALCULATIONS IN PRESCRIPTION COMPOUNDING (3006)按处方混合的药物的计算<1171>PHASE-SOLUBILITY ANAL YSIS……3016相溶解分析***<1174>Powder Flow….3017 粉末流动性<1176>PRESCRIPTION BALANCES AND VOLUMETRIC APPARATUS….3020 处方天平与容量器具***<1177>Good Packaging Practices….3021 良好的包装操作***<1178>Good Repackaging Practices….3023 良好的再包装操作<1181>SCANNING ELECTRON MICROSCOPY……3025扫描电子显微镜<1191>STABILITY CONSIDERATIONS IN DISPENSING PRACTICE……3029 分装操作中稳定性考察<1196>PHARMACOPEIAL HARMONIZATION……3031药典的一致性<1207>STERILE PRODUCT PACKAGING—INTEGRITY EV ALUATION (3035)无菌产品包装-完整性评估<1208>STERILITY TESTING—V ALIDATION OF ISOLATOR SYSTEMS (3037)无菌实验-隔离系统的验证<1209>STERILIZATION—CHEMICAL AND PHYSICOCHEMICAL INDICATORS AND INTEGRATORS……3040灭菌-化学与物理化学的指示剂以及二者的综合<1211>STERILIZATION AND STERILITY ASSURANCE OF COMPENDIAL ARTICLES (3041)药典物品中的灭菌与灭菌保证<1216>TABLET FRIABILITY……3046片剂的脆碎度<1221>TEASPOON……3047茶匙<1222>TERMINALL Y STERILIZED PHARMACEUTICAL PRODUCTS—PARAMETRIC RELEASE……3047最终灭菌产品-放行参数<1225>V ALIDATION OF COMPENDIAL METHODS……3050药典方法的验证<1227>V ALIDATION OF MICROBIAL RECOVERY FROM PHARMACOPEIAL ARTICLES (3053)从药物中回收微生物的验证<1230>W ATER FOR HEALTH APPLICATIONS……3055健康用水<1231>W ATER FOR PHARMACEUTICAL PURPOSES……3056制药用水<1241>W ATER–SOLID INTERACTIONS IN PHARMACEUTICAL SYSTEMS (3074)在药物系统中水与固体的相互作用<1251>WEIGHING ON AN ANAL YTICAL BALANCE……3076关于分析天平的称重***<1265>Written Prescription Drug Information-Guidelines……….3078 书面的处方药信息-指南Dietary Supplements营养补充剂General Tests and Assays 一般检查法与测定法<2021>MICROBIAL ENUMERATION TESTS—NUTRITIONAL AND DIETARY SUPPLEMENTS (3080)微生物数量实验-营养与食品添加剂<2022>MICROBIOLOGICAL PROCEDURES FOR ABSENCE OF SPECIFIED MICROORGANISMS—NUTRITIONAL AND DIETARY SUPPLEMENTS (3083)不得检出特定微生物的程序-营养与营养补充剂<2023>MICROBIOLOGICAL A TTRIBUTES OF NONSTERILE NUTRITIONAL AND DIETARY SUPPLEMENTS……3087非无菌的营养与食品添加剂中的微生物分布<2040>DISINTEGRATION AND DISSOLUTION OF DIETARY SUPPLEMENTS (3089)食品添加剂的崩解与溶出<2091>WEIGHT VARIATION OF DIETARY SUPPLEMENTS……3092食品添加剂的重量差异<2750>MANUFACTURING PRACTICES FOR DIETARY SUPPLEMENTS (3093)食品添加剂的生产操作。
Thermo Scientific 2D和1D条形码读者兼容的试管条形码标记说明书

Eliminate Secondary Labeling Steps. Matrix 2D Barcoded ScrewTop Storage tubes are available pre-printed with 1D and human-readable codes that match the tube’s 2D code. Laser etched 2D, linear and human readable barcodes are also included on the sides of the standard latch racks. The additional barcoding on tube and rack eliminates the need for the secondary application of labels, streamlining storage procedures.Enhanced Secure Tracking. A permanently bonded, unique 2D barcode is laser etched onto the base of every tube to securely identify and track samples. The linear and human readable codes match this unique 2D barcode, allowing 1D scanning or visual identification of samples across satellite or collaborative sites without2D readers. Complementary Thermo Scientific 2D and 1D barcode readers scan and instantly decode each tube’s barcode into any application or database. Laser etched 2D, linear, and human readable barcodes are included on three sides of the standard latch rack, providing traceability at the rack level and providing readability to laboratories with or without barcode decoding equipment. The 2D barcode included on the bottom of the rack serves as both an orientation and identification feature for automated and benchtop storage equipment.Superior Storage Format. Matrix 2D Barcoded ScrewTop storage tubes are available in specially designed, barcoded, stackable, microplate-footprint Latch Racks to save precious space in storage and on the bench top while maintaining traceability. Lid canbe positioned to rest on the rack, preventing contamination during manual pipetting. Or remove lid completely for robotic handling. Flexibility. Side-printed Matrix ScrewTop tubes and barcoded latch racks maintain compatibility with existing ScrewTop tube storage systems and accessories. ScrewTop Removal Tool caps/ decaps tubes individually to enable one-handed pipetting. The Thermo Scientific ScrewTop Cap Tray can be used to cap/decap several tubes at once with automated capping systems or to fill tubes on an automated liquid handling platform, then cap an entire rack at once. Seven color cap options allow easy identification of samples at a glance.Stringent Quality Control. Every 2D barcoded storage tube is scanned to ensure readability. All barcodes are checked against our database of previously assigned codes to prevent duplicates.S A M P L E S T O R A G EThermo Scientific™ Matrix™2D Barcoded ScrewTop Tubes in Barcoded RacksMatrix 2D Barcoded ScrewTop Storage tubes are now available in a standard barcoded latch rack. The barcoded latch rack provides permanent 2D, linear,and human readable codes on 3 sidesof the rack. A 2D barcode on the rack bottom enables orientation detection and identification when using automated storage equipment. These additions to the Matrix ScrewTop Storage tube portfolio allow visual sample identification ina range of laboratory procedures and eliminate the need for secondary labeling. Identify and track your samplesfor optimal sample storageProduct SpecificationsThermo Scientific MatrixBarcoded Tubes and RacksDescription 500 µl ScrewTop Storage Tubes 1.0 ml ScrewTop Storage Tubes Item Number3743, 3744, 37453744-WP; 3744-WP1D; 3745-WP; 3745-WP1D 3740, 3741, 37423741-WP; 3741-WP1D; 3742-WP; 3742-WP1D Figure 1A: Tube Side ViewWidth (1A)7.40±0.10 [0.290±0.002]7.40±0.10 [0.290±0.002]Height (1A)30.60±0.20 [1.204±0.007]44.00±0.10 [1.732±0.003]Figure 1B: Tube Side View w/ ScrewTop Width (1B)8.70±0.10 [0.344±0.003]8.70±0.10 [0.344±0.003]Height (1B)39.20 [1.543]52.60 [2.071]Figure 2: Rack Side ViewHeight (2A)29.50±0.10 [1.160±0.005]38.10±0.10 [1.500±0.005]Height (2B)29.10±0.10 [1.145±0.005]42.50±0.20 [1.673±0.008]Height (2C)32.60±0.30 [1.282±0.012]46.00±0.20 [1.809±0.008]Height (2D)41.30±0.40 [1.626±0.017]54.60±0.30 [2.149±0.110]Figure 3: Rack Top View Length 127.75±0.13 [5.029±0.005]127.75±0.13 [5.029±0.005]Width85.46±0.13 [3.364±0.005]85.46±0.13 [3.364±0.005]Figure 4: Stacked Rack ViewSingle Height (4A)44.32±0.24 [1.745±0.010]58.42±0.25 [2.300±0.010]Incremental Height (4B)43.31±0.25 [1.705±0.010]57.30±0.25 [2.220±0.010]Stacked Height (4C)87.63±0.51 [3.450±0.020]114.81±0.51 [4.520±0.020]Description Storage Tubes Barcoded RackItem Number3743-BR, 3744-BR, 3745-BR, 3744-WP-BR, 3744-WP1D-BR, 3745-WP-BR, 3745-WP1D-BR, 3740-BR, 3741-BR, 3742-BR, 3741-WP-BR, 3741-WP1D-BR, 3742-WP-BR, 3742-WP1D-BR, 3748-BR Figure 5: Rack Bottom ViewDistance to barcode from short side (5A)120.48 ± 0.254 [4.74 ± 0.010]Distance to barcode from short side (5B) 1.443 ± 0.203 [0.057 ± 0.008]Barcode length5.83 ± 0.127 [0.230 ± 0.005]Distance to barcode from long side (5C)71.98 ± 0.254 [2.834 ± 0.010]Distance to barcode from long side (5D)8.18 ± 0.203 [0.322 ± 0.008]Barcode width5.30 ± 0.127 [0.209 ± 0.005]Description ScrewTop Cap Tray Item Number 4906, 4477Figure 6: Tray Top View Length 127.75±0.25 [5.03±0.010]Width85.58±0.25 [3.37±0.010]Figure 7: Stacked Tray ViewSingle Height (2A)19.81±0.13 [0.78±0.005]Incremental Height (2B)18.29±0.25 [0.72±0.010]Stacked Height (2C)38.10±0.25 [1.50±0.010]Tube Material Virgin Class VI Medical Grade Polypropylene Rack Material Polycarbonate with acetal latchesCap MaterialVirgin Class VI Medical Grade Polypropylene with Santoprene™ gasket ScrewTop Cap Tray Material Acrylonitrile Butadiene Styrene (ABS)Contaminant-free All tubes and tray are supplied free from DNA, RNAse, DNAse and endotoxinsAutoclavable Racked and unracked tubes are autoclavable with the caps loosened; ScrewTop trays are not autoclavable 2D CodeNon-proprietary, 12x12 Data-matrix with ECC200 Built-in Error Correction Tube/Cap Temp Range -196°C to 121°C; Autoclaving and boiling to vapor phase liquid nitrogen storage Latch Rack Coding 2D Datamatrix; Linear EAN Code 128; Human Readable Matrix Barcoded ScrewTop TubesProduct SpecificationsBarcode WidthDistance to barcodefrom short side (5B)Barcode LengthDistance to barcode from short side (5A)Distance to barcode from long side (5C)s Figure1: Tube Side View Width (1B)s Figure 2: Rack Side ViewSSSST2DTUBES 0615© 2015 Thermo Fisher Scientific Inc. All rights reserved. Santoprene is a registered trademark of Exxon Mobil Corporation. All other trademarks are the property of Thermo Fisher Scientific Inc. and its subsidiaries./samplestorageCat. No.Non-Barcoded Rack Cat. No.Barcoded Rack DescriptionMax Working Volume, µL Cap Color Qty37443744-BR Tube, ScrewTop, 500 µL 2D, V-Bottom 590Colorless 5 racks of 96/case Sterile3744RED 3744RED-BR Tube, ScrewTop, 500 µL 2D, V-Bottom 590Red 5 racks of 96/case Sterile 3744YEL 3744YEL-BR Tube, ScrewTop, 500 µL 2D, V-Bottom 590Yellow 5 racks of 96/case Sterile 3744BLU 3744BLU-BR Tube, ScrewTop, 500 µL 2D, V-Bottom 590Blue 5 racks of 96/case Sterile 3744GRE 3744GRE-BR Tube, ScrewTop, 500 µL 2D, V-Bottom 590Green 5 racks of 96/case Sterile 3744WHI 3744WHI-BR Tube, ScrewTop, 500 µL 2D, V-Bottom 590White 5 racks of 96/case Sterile 3744PUR 3744PUR-BR Tube, ScrewTop, 500 µL 2D, V-Bottom 590Purple 5 racks of 96/case Sterile 3744AMB 3744AMB-BR Amber Tube, ScrewTop, 500µL 2D, V-bottom 590 5 racks of 96/case Sterile 3743AMB 3743AMB-BR Amber Tube, ScrewTop, 500µL 2D, V-bottom 590Red5 racks of 96/case Sterile 37453745-BR Tube, ScrewTop, 500 µL 2D, V-Bottom, No Caps 590 5 racks of 96/caseSterile 3744-WP 3744-WP-BR Tube, ScrewTop, 500 µL 2D, WP, V-Bottom 590Colorless 5 racks of 96/case Sterile 3744-WP1D 3744-WP1D-BR Tube, ScrewTop, 500 µL 2D, WP/1D, V-Bottom 590Colorless5 racks of 96/case Sterile 3745-WP 3745-WP-BR Tube, ScrewTop, 500 µL 2D, WP, V-Bottom, No caps 590 5 racks of 96/case Sterile 3745-WP1D 3745-WP1D-BRTube, ScrewTop, 500 µL 2D, WP/1D, V-Bottom, No caps 590 5 racks of 96/caseSterile 3743Tube, ScrewTop, 500 µL 2D, V-Bottom 590Colorless Bulk, 480/case Sterile 3743RED Tube, ScrewTop, 500 µL 2D, V-Bottom 590Red Bulk, 480/case Sterile 3743YEL Tube, ScrewTop, 500 µL 2D, V-Bottom 590Yellow Bulk, 480/case Sterile 3743BLU Tube, ScrewTop, 500 µL 2D, V-Bottom 590Blue Bulk, 480/case Sterile 3743GRE Tube, ScrewTop, 500 µL 2D, V-Bottom 590Green Bulk, 480/case Sterile 3743WHI Tube, ScrewTop, 500 µL 2D, V-Bottom 590White Bulk, 480/case Sterile 3743PUR Tube, ScrewTop, 500 µL 2D, V-Bottom590Purple Bulk, 480/case Sterile 37413741-BR Tube, ScrewTop, 1.0 mL 2D, V-Bottom 940Colorless 5 racks of 96/case Sterile 3741RED 3741RED-BR Tube, ScrewTop, 1.0 mL 2D, V-Bottom 940Red 5 racks of 96/case Sterile 3741YEL 3741YEL-BR Tube, ScrewTop, 1.0 mL 2D, V-Bottom 940Yellow 5 racks of 96/case Sterile 3741BLU 3741BLU-BR Tube, ScrewTop, 1.0 mL 2D, V-Bottom 940Blue 5 racks of 96/case Sterile 3741GRE 3741GRE-BR Tube, ScrewTop, 1.0 mL 2D, V-Bottom 940Green 5 racks of 96/case Sterile 3741WHI 3741WHI-BR Tube, ScrewTop, 1.0 mL 2D, V-Bottom 940White 5 racks of 96/case Sterile 3741PUR 3741PUR-BR Tube, ScrewTop, 1.0 mL 2D, V-Bottom 940Purple 5 racks of 96/case Sterile 3741AMB 3741AMB-BR Amber Tube, ScrewTop, 1.0mL 2D, V-Bottom 940Red5 racks of 96/case Sterile 3742AMB 3742AMB-BR Amber Tube, ScrewTop, 1.0mL 2D, V-Bottom 940 5 racks of 96/case 37423742-BR Tube, ScrewTop, 1.0 mL 2D, V-Bottom, No Caps 940 5 racks of 96/caseSterile 3741-WP 3741-WP-BR Tube, ScrewTop, 1.0 mL 2D, WP, V-Bottom 940Colorless 5 racks of 96/case Sterile 3741-WP1D 3741-WP1D-BR Tube, ScrewTop, 1.0 mL 2D, WP/1D, V-Bottom 940Colorless5 racks of 96/case Sterile 3742-WP 3742-WP-BR Tube, ScrewTop, 1.0 mL 2D, WP, V-Bottom, No caps 940 5 racks of 96/case Sterile 3742-WP1D 3742-WP1D-BRTube, ScrewTop, 1.0 mL 2D, WP/1D, V-Bottom, No caps 940 5 racks of 96/caseSterile 3740Tube, ScrewTop, 1.0 mL 2D, V-Bottom 940Colorless Bulk, 480/case Sterile 3740RED Tube, ScrewTop, 1.0 mL 2D, V-Bottom 940Red Bulk, 480/case Sterile 3740YEL Tube, ScrewTop, 1.0 mL 2D, V-Bottom 940Yellow Bulk, 480/case Sterile 3740BLU Tube, ScrewTop, 1.0 mL 2D, V-Bottom 940Blue Bulk, 480/case Sterile 3740GRE Tube, ScrewTop, 1.0 mL 2D, V-Bottom 940Green Bulk, 480/case Sterile 3740WHI Tube, ScrewTop, 1.0 mL 2D, V-Bottom 940White Bulk, 480/case Sterile 3740PURTube, ScrewTop, 1.0 mL 2D, V-Bottom940PurpleBulk, 480/caseSterileORDERING GUIDEAsia: Australia: 1300-735-292; New Zealand: 0800-933-966; China +86-21-6865-4588 or +86-10-8419-3588;China Toll-free: 800-810-5118 or 400-650-5118; Singapore +65-6872-9718; Japan: +81-3-5826-1616; Korea +82-2-2023-0640; Taiwan + 886-2-87516655; India: +91-22-6680-3000 Europe: Austria: +43-1-801-40-0; Belgium: +32-2-482-30-30; Denmark: +45-4631-2000; France: +33-2-2803-2180; Germany: +49-6184-90-6000; Germany Toll-free: 0800-1-536-376;Italy: +39-02-95059-554; Netherlands: +31-76-571-4440; Nordic/Baltic/CIS countries: +358-10-329-2200; Russia: +7-(812)-703-42-15; Spain/Portugal: +34-93-223-09-18; Switzerland: +41-44-454-12-12; UK/Ireland: +44-870-609-9203 North America: USA/Canada +1-585-586-8800; USA Toll-free: 800-625-4327South America: USA sales support: +1-585-586-8800 Countries not listed: +49-6184-90-6000 or +33-2-2803-2000Sterile ScrewTop Tube Caps Cat. No.DescriptionCap Color Qty/Case Cat. No.Description Cap Color Qty/Case 4906Tray, ScrewTop Cap, Empty 5 trays4470Caps, ScrewTop Tube Colorless Bulk, 5004477Tray, ScrewTop Cap Coloreless 5 trays of 96 caps 4470RED Caps, ScrewTop Tube Red Bulk, 5004477RED Tray, ScrewTop Cap Red 5 trays of 96 caps 4470YEL Caps, ScrewTop Tube Yellow Bulk, 5004477YEL Tray, ScrewTop Cap Yellow 5 trays of 96 caps 4470BLU Caps, ScrewTop Tube Blue Bulk, 5004477BLU Tray, ScrewTop Cap Blue 5 trays of 96 caps 4470GRE Caps, ScrewTop Tube Green Bulk, 5004477GRE Tray, ScrewTop Cap Green 5 trays of 96 caps 4470WHI Caps, ScrewTop Tube White Bulk, 5004477WHI Tray, ScrewTop Cap White 5 trays of 96 caps 4470PURCaps, ScrewTop TubePurpleBulk, 5004477PURTray, ScrewTop CapPurple5 trays of 96 caps。
_矩阵的Kronecker乘积的性质与应用

矩阵Kronecker乘积的性质与应用摘要按照矩阵乘法的定义,我们知道要计算矩阵的乘积AB,就要求矩阵A的列数和矩阵B的行数相等,否则乘积AB是没有意义的。
那是不是两个矩阵不满足这个条件就不能计算它们的乘积呢?本文将介绍矩阵的一种特殊乘积BA ,它对矩阵的行数和列数的并没有具体的要求,它叫做矩阵的Kronecker积(也叫直积或张量积)。
本文将从矩阵的Kronecker积的定义出发,对矩阵的Kronecker 积进行介绍和必要的说明。
之后,对Kronecker积的运算规律,可逆性,秩,特征值,特征向量等性质进行了具体的探究,得出结论并加以证明。
此外,还对矩阵的拉直以及矩阵的拉直的性质进行了说明和必要的证明。
矩阵的Kronecker积是一种非常重要的矩阵乘积,它应用很广,理论方面在诸如矩阵方程的求解,矩阵微分方程的求解等矩阵理论的研究中有着广泛的应用,实际应用方面在诸如图像处理,信息处理等方面也起到重要的作用。
本文讨论矩阵的Kronecker积的性质之后还会具体介绍它在矩阵方程中的一些应用。
关键词:矩阵;Kronecker积;矩阵的拉直;矩阵方程;矩阵微分方程Properties and Applications of matrix KroneckerproductAbstractAccording to the definition of matrix multiplication, we know that to calculate the matrix product AB, requires the number of columns of the matrix A and matrix B is equal to the number of rows, otherwise the product AB makes no sense.That is not two matrices not satisfy this condition will not be able to calculate their product do?This article will describe a special matrix product BA , the number of rows and columns of a matrix and its no specific requirements, it is called the matrix Kronecker product (also called direct product or tensor product).This paper will define the matrix Kronecker product of view, the Kronecker product matrix are introduced and the necessary instructions. Thereafter, the operation rules Kronecker product, the nature of reversibility, rank, eigenvalues, eigenvectors, etc. specific inquiry, draw conclusions and to prove it. In addition, the properties of the stretch of matrix and its nature have been described and the necessary proof.Kronecker product matrix is a very important matrix product, its use is very broad, theoretical research, and other matrix solving differential equations, such as solving the matrix equation matrix theory has been widely applied in practical applications such as image processing aspects of information processing, also play an important role. After the article discusses the nature of the matrix Kronecker product it will introduce a number of specific applications in the matrix equation. Keywords:Matrix; Kronecker product; Stretch of matrix; Matrix equation; Matrix Differential Equations目录摘要 .................................................................................................................................................. I Abstract ........................................................................................................................................... II 第一章 矩阵的Kronecker 积 (1)1.1 矩阵的Kronecker 积的定义 ........................................................................................... 1 1.2 矩阵的Kronecker 积的性质 ........................................................................................... 1 第二章 Kronecker 积的有关定理及推论 ...................................................................................... 6 第三章 矩阵的拉直 . (9)3.1矩阵的拉直的定义 ............................................................................................................ 9 3.2矩阵的拉直的性质 ............................................................................................................ 9 第四章 矩阵的Kronecker 积与矩阵方程 .. (11)4.1矩阵的Kronecker 积与Lyapunov 矩阵方程 ................................................................ 11 4.2矩阵的Kronecker 积与一般线性矩阵方程 .................................................................. 13 4.3矩阵的Kronecker 积与矩阵微分方程 .......................................................................... 14 参考文献......................................................................................................................................... 16 致谢 (18)符号说明W a W a 属于集合元素nm ij a A ⨯=)( 矩阵的记法列元素的行为以n m j i a ij⨯ij A )( 列的元素行的矩阵j i AT A 的转置矩阵A H A 的共轭转置矩阵A 1-A 的逆矩阵矩阵A→A 按行拉直得到的列向量矩阵AA det 的行列式方阵AtrA 的主对角元素之和的迹,方阵A A)(A rank 的秩矩阵A)(A λ 的特征值方阵An I 阶单位矩阵nR 实数域 C 复数域n C 维复向量的全体n n m C ⨯ 复矩阵全体n m ⨯O 零矩阵B A ⊗ 的和矩阵B A Kronecker 积第一章 矩阵的Kronecker 积1.1 矩阵的Kronecker 积的定义定义1.1设矩阵n m C A ⨯∈,矩阵q p C B ⨯∈,定义A 和B 的Kronecker 积(或直积,张量积)B A ⊗为:⎥⎥⎥⎥⎦⎤⎢⎢⎢⎢⎣⎡=⊗B a B a B a B a B a B a B a B a B a B A mn m m n n 212222111211 可以看出,其结果是一个)()(nq mp ⨯矩阵,同时也是一个以B a ij 为子块的分块矩阵.例1.1 设⎥⎦⎤⎢⎣⎡-=1201A ,[]31-=B ,则 ⎥⎦⎤⎢⎣⎡---=⎥⎦⎤⎢⎣⎡-=⊗316200312B B O BB A []⎥⎦⎤⎢⎣⎡---=-=⊗361203013A A A B 由此可见,B A ⊗与A B ⊗具有相同的阶数,但是它们并不相等,也就是说,Kronecker 积不满足交换律.1.2 矩阵的Kronecker 积的性质虽然Kronecker 积不满足交换律,但是具有以下一些性质: 性质1.2.1 设矩阵n m C A ⨯∈,矩阵q p C O ⨯∈,则O O A A O =⊗=⊗(这个O 为)()(nq mp ⨯矩阵).证明:略.性质1.2.2 设k 为任一常数,矩阵n m C A ⨯∈,矩阵q p C B ⨯∈,则)()()(B A k kB A B kA ⊗=⊗=⊗.证明:不失一般性,设⎥⎥⎥⎥⎦⎤⎢⎢⎢⎢⎣⎡=mn m m n n a a a a a aa a a A 212222111211,则:⎥⎥⎥⎥⎦⎤⎢⎢⎢⎢⎣⎡=mn m m n n ka ka ka ka ka ka ka ka ka kA 212222111211,根据Kronecker 积的定义可以得到:⎥⎥⎥⎥⎦⎤⎢⎢⎢⎢⎣⎡=⎥⎥⎥⎥⎦⎤⎢⎢⎢⎢⎣⎡=⊗B ka B ka B ka B ka B ka B ka B ka B ka B ka B ka B ka B ka B ka B ka B ka B ka B ka B ka B kA mn m m n n mn m m n n 212222111211212222111211)()()()()()()()()()(, ⎥⎥⎥⎥⎦⎤⎢⎢⎢⎢⎣⎡=⎥⎥⎥⎥⎦⎤⎢⎢⎢⎢⎣⎡=⊗B ka B ka B ka B ka B ka B ka B ka B ka B ka kB a kB a kB a kB a kB a kB a kB a kB a kB a kB A mn m m n n mn m m n n 212222111211212222111211)()()()()()()()()()(, 即)(B A k B kA ⊗=⊗,)()(B A k kB A ⊗=⊗. 所以)()()(B A k kB A B kA ⊗=⊗=⊗.性质1.2.3 设A ,B 为同阶矩阵(同阶是为了可以做加法),则C B C A C B A ⊗+⊗=⊗+)(,B C A C B A C ⊗+⊗=+⊗)(.证明:不失一般性,设⎥⎥⎥⎥⎦⎤⎢⎢⎢⎢⎣⎡=mn m m n n a a a a a aa a a A 212222111211,⎥⎥⎥⎥⎦⎤⎢⎢⎢⎢⎣⎡=mn m m n n b b b b b b b b b B 212222111211,则:⎥⎥⎥⎥⎦⎤⎢⎢⎢⎢⎣⎡+++++++++=+mn mn m m m m n n n n b a b a b a b a b a b a b a b a b a B A221122222221211112121111,根据Kronecker 积的定义可以得到:⎥⎥⎥⎥⎦⎤⎢⎢⎢⎢⎣⎡+++++++++=⊗+C b a C b a C b a C b a C b aC b a C b a Cb a C b a C B A mn mn m m m m n n n n )()()()()()()()()()(221122222221211112121111(1.1)*,⎥⎥⎥⎥⎦⎤⎢⎢⎢⎢⎣⎡=⊗C a C a C a C a C a C a C a C a C a C A mn m m n n 212222111211 (1.2)*, ⎥⎥⎥⎥⎦⎤⎢⎢⎢⎢⎣⎡=⊗C b C b C b C b C b C b C b C b C b C B mn m m n n 212222111211 (1.3)*,由(1.2)*,(1.3)*得:⎥⎥⎥⎥⎦⎤⎢⎢⎢⎢⎣⎡+++++++++=⊗C b C a C b C a C b C a C b C a C b C a C b C a C b C a C b C a C b C a C A mn mn m m m m n n n n 221122222221211112121111 (1.4)*, 由(1.1)*,(1.4)*可得:C B C A C B A ⊗+⊗=⊗+)(.同理设⎥⎥⎥⎥⎦⎤⎢⎢⎢⎢⎣⎡=mn m m n n c c c c c cc c c C 212222111211可证:B C A C B A C ⊗+⊗=+⊗)(.性质1.2.4 设矩阵n m C A ⨯∈,矩阵q p C B ⨯∈,矩阵s r C F ⨯∈,则)()(F B A F B A ⊗⊗=⊗⊗证明:不失一般性,设⎥⎥⎥⎥⎦⎤⎢⎢⎢⎢⎣⎡=mn m m n n a a a a a aa a a A 212222111211,则:⎥⎥⎥⎥⎦⎤⎢⎢⎢⎢⎣⎡⊗⊗⊗⊗⊗⊗⊗⊗⊗=⊗⊗)()()()()()()()()()(212222111211F B a F B a F B a F B a F B a F B a F B a F B a F B a F B A mn m m n n)(212222111211F B A F B a B a B a B a B a B a B a B a B a mn m m n n ⊗⊗=⊗⎥⎥⎥⎥⎦⎤⎢⎢⎢⎢⎣⎡= 得证.性质1.2.5设矩阵n m C A ⨯∈,矩阵q p C B ⨯∈,矩阵s n C F ⨯∈,矩阵t q C D ⨯∈,则)()())((BD AF D F B A ⊗=⊗⊗证明:不失一般性,设⎥⎥⎥⎥⎦⎤⎢⎢⎢⎢⎣⎡=mn m m n n a a a a a aa a a A 212222111211,⎥⎥⎥⎥⎦⎤⎢⎢⎢⎢⎣⎡=ns n n s s f f f f f f f f f F212222111211, 则:⎥⎥⎥⎥⎦⎤⎢⎢⎢⎢⎣⎡⎥⎥⎥⎥⎦⎤⎢⎢⎢⎢⎣⎡=⊗⊗D f D f D f D f D f Df D f D f D f B a B a B a B a B a B a B a B a B a D F B A ns n n s s mn m m n n212222111211212222111211))(()()()()()()()()()()()(112111112211211121111BD AF BD f a BD f a BD f a BD c a BD f a BD f a BD f a BD f a BD f a nk ks mk n k k mk n k k mk nk ks k n k k k n k k k n k ks k n k k k n k k k ⊗=⎥⎥⎥⎥⎥⎦⎤⎢⎢⎢⎢⎢⎣⎡=∑∑∑∑∑∑∑∑∑=========得证.性质1.2.6 设矩阵m m C A ⨯∈可逆, 且矩阵n n C B ⨯∈可逆,则B A ⊗可逆,且111)(---⊗=⊗B A B A .证明:mn n m I I I BB AA B A B A =⊗=⊗=⊗⊗----)()())((1111(这里I n 与数的乘法中的1起到相同的作用), 故111)(---⊗=⊗B A B A .性质1.2.7 设矩阵n m C A ⨯∈,矩阵q p C B ⨯∈,则T T T B A B A ⊗=⊗)(H H H B A B A ⊗=⊗)(证明: ij T T T ji ij T B A B a B A ][])[(⊗==⊗ 得证.同理可证:H H H B A B A ⊗=⊗)(.性质1.2.8 两个正交(酉)矩阵的Kronecker 积还是正交(酉)矩阵. 证明:设矩阵m m C A ⨯∈,矩阵n n C B ⨯∈.因为A ,B 都是正交(酉)矩阵,所以有m T T I A A AA ==,n T T I B B BB ==. 由性质1.2.7和性质1.2.5可得:mn n m T T T T T I I I BB AA B A B A B A B A =⊗=⊗=⊗⊗=⊗⊗))(())((. mn m n T T T T T I I I B B A A B A B A B A B A =⊗=⊗=⊗⊗=⊗⊗))(()()(.故mn T T I B A B A B A B A =⊗⊗=⊗⊗)()())((. 得证.第二章 Kronecker 积的有关定理及推论定理2.2.2 设矩阵n m C A ⨯∈,矩阵q p C B ⨯∈,则)()()(B rank A rank B A rank =⊗.证明:设rank A =r ,rank B=s ,A ,B 的标准形分别为:1111--⎥⎦⎤⎢⎣⎡=Q O O O I P A r ,1212--⎥⎦⎤⎢⎣⎡=Q O O O I P B s其中i P ,i Q =i (1,2)均为非奇异矩阵,则由性质1.2.5和1.2.6可以得:`1211211211121112121111)()()()(----------⊗⎥⎦⎤⎢⎣⎡⊗=⊗⎪⎪⎭⎫ ⎝⎛⎥⎦⎤⎢⎣⎡⎥⎦⎤⎢⎣⎡⊗=⎪⎪⎭⎫⎝⎛⎥⎦⎤⎢⎣⎡⊗⎪⎪⎭⎫ ⎝⎛⎥⎦⎤⎢⎣⎡=⊗Q Q O OO I P P Q Q O O O I O OO I P P Q O O O I P Q O OO I P B A rssrsr所以)()()(B rank A rank s r B A rank =•=⊗ 得证.定理2.2.3 设矩阵m m C A ⨯∈,矩阵n n C B ⨯∈,对于向量m C x ∈和n C y ∈,若x 是A 关于特征值λ的一个特征向量,y 是A 关于特征值μ的一个特征向量,则y x ⊗是B A ⊗对应特征值λμ的一个特征向量.证明:因为x ,y 都是非零向量,所以x ⊗y 也是非零向量,由性质1.2.2和性质1.2.5可得:)()()()()())((y x y x By Ax y x B A ⊗=⊗=⊗=⊗⊗λμμλ.所以,y x ⊗是B A ⊗对应特征值λμ的一个特征向量.推论2.2.4 设矩阵m m C A ⨯∈,矩阵n n C B ⨯∈,对于向量m C x ∈和n C y ∈,若A 的特征值是1λ,2λ,…,m λ;B 的特征值是1μ,2μ,…,n μ,则B A ⊗的特征值为t s μλ,m s ≤≤1,n t ≤≤1(k 重根算k 个).定理2.2.5 设矩阵m m C A ⨯∈,矩阵n n C B ⨯∈,对于向量m C x ∈和n C y ∈,若x 是A 关于特征值λ的一个特征向量,y 是A 关于特征值μ的一个特征向量,则y x ⊗是B I I A m n ⊗+⊗对应特征值μλ+的一个特征向量.证明:由性质1.2.3,性质1.2.5可以得到:)()()()())((y x y x y I Ax y x I A n n ⊗=⊗=⊗=⊗⊗λλ, )()()()())((y x y x By x I y x B I m m ⊗=⊗=⊗=⊗⊗μμ,故))(())(())(())((y x y x B I y x I A y x B I I A m n m n ⊗+=⊗⊗+⊗⊗=⊗⊗+⊗μλ.所以,y x ⊗是B I I A m n ⊗+⊗对应特征值μλ+的一个特征向量.推论2.2.6 设矩阵m m C A ⨯∈,矩阵n n C B ⨯∈,对于向量m s C x ∈和n t C y ∈,若1x ,2x ,…,m x 是A 关于特征值1λ,2λ,…,m λ的特征向量,1y ,2y ,…,n y 是B 关于特征值1μ,2μ,…,n μ的特征向量,则B I I A m n ⊗+⊗的n m •个特征值为{t s μλ+}.(s=1,2,…,m ;t=1,2,…,n ).例2.2 设矩阵m m C A ⨯∈,矩阵n n C B ⨯∈,对于向量m i C x ∈和n j C y ∈,若1x ,2x ,…,m x 是A 关于特征值1λ,2λ,…,m λ的特征向量,1y , 2y ,…,n y 是B 关于特征值1μ,2μ,…,n μ的特征向量,证明:矩阵)()(B A I I n m ⊗-⊗的特征值是j i μλ-1,对应的特征向量为j i y x ⊗.(i=1,2,…,m ;j=1,2,…,n ).证明:由性质1.2.3和性质1.2.5可得:))(()()()()())((j i j i j j i i j i j i y x y x By Ax y x B A ⊗=⊗=⊗=⊗⊗μλμλ,故有:))(1())(()())(()())(())(())](()[(j i j i j i j i j i j i j i j i mn j i j i n m j i n m y x y x y x y x y x I y x B A y x I I y x B A I I ⊗-=⊗-⊗=⊗-⊗=⊗⊗-⊗⊗=⊗⊗-⊗μλμλμλ所以,矩阵)()(B A I I n m ⊗-⊗的特征值是j i μλ-1,对应的特征向量j i y x ⊗. 定理2.2.7 设矩阵m m C A ⨯∈,矩阵n n C B ⨯∈,则trB trA B A tr •=⊗)(证明:由Kronecker 积和迹的定义可得:trBtrA trB a trB a trB a B a tr B a tr B a tr B A tr nn nn •=+++=+++=⊗ 22112211)()()()(得证.定理2.2.8 设矩阵m m C A ⨯∈,矩阵n n C B ⨯∈,则m n B A B A )(det )(det )det(=⊗证明:设A 的特征值为1λ,2λ,…,m λ,B 的特征值为1μ,2μ,…,n μ, 由推论2.2.4可得:mn m n n m n m m n n nj j m nj j mnji nj j j i B A B A )(det )(det )()()())(())(()()()()()det(21211212111112,11=====⊗∏∏∏∏===μμμλλλμλμλμλμλμλμλμλμλμλμλ得证.第三章 矩阵的拉直3.1矩阵的拉直的定义定义3.1 设n m ij a A ⨯=)(,定义矩阵A 的按行拉直为:T mn m n n a a a a a a A A vec )()(1221111,,,,,,,,, ==→即矩阵A 的拉直是一个mn 元的列向量,它是由矩阵A 所有元素按行顺序依次排成一列得到的.例如:⎥⎦⎤⎢⎣⎡=d c b a A ,则矩阵A 的拉直为T d c b a A )(,,,=→.3.2矩阵的拉直的性质矩阵的拉直具有以下性质:性质 3.2.1 设矩阵n m C A ⨯∈,矩阵n m C B ⨯∈,k 和l 是常数,则)(lB kA +=→→+B l A k .证明:略.性质3.2.2 设n m ij t a t A ⨯=))(()(,则dtt dA )(=dt d)(t A . 证明:左边==))((dtt dA vet ij a vet ((′)))(n m t ⨯ = [(a 11′(t ),…,a n 1′(t ),a 21′(t ),…,a n 2′(t ),…,a 1m ′(t ),…,a mn ′(t ) ]T =[(a 11(t ),…,a n 1(t ),a 21(t ),…,a n 2(t ),…,a 1m (t ),…,a mn (t ) )T ]′ = ))](([t A vet ′=))](([t A vec dtd=右边,得证. 性质 3.2.3设矩阵n m C A ⨯∈,矩阵p n C X ⨯∈,矩阵q p C B ⨯∈,则AXB →⊗=X B A T)(.证明:设⎥⎥⎥⎥⎦⎤⎢⎢⎢⎢⎣⎡=mn m m n n a a a a a aa a a A 212222111211,T n x x X )(1,, =→,其中,T i x 是X 的第i 行=i (1,2,…,)n ,则⎥⎥⎥⎦⎤⎢⎢⎢⎣⎡++++=B x a x a B x a x a AXB T n mn T m Tn n T )()(111111 ,⎥⎥⎥⎦⎤⎢⎢⎢⎣⎡=→n x x X 1 所以AXB T Tn mn T m T n n T B x a x a B x a x a ])()[(111111++++= ,, →⊗=⎥⎥⎥⎦⎤⎢⎢⎢⎣⎡⎥⎥⎥⎦⎤⎢⎢⎢⎣⎡++++=⎥⎥⎥⎦⎤⎢⎢⎢⎣⎡++++=X B A x x B a B a B a B a x a x a B x a x a B n T mn T m T n T n mn m T n n T )()()()()(11111111111 得证. 推论3.2.4 设矩阵m m C A ⨯∈,矩阵n m C X ⨯∈,矩阵n n C B ⨯∈,则有1.AX →⊗=X I A n )( 2.XB →⊗=X B I Tm )(.3(AX +XB )→⊗+⊗=X B I I A Tm n )(.第四章 矩阵的Kronecker 积与矩阵方程4.1矩阵的Kronecker 积与Lyapunov 矩阵方程设矩阵m m C A ⨯∈,矩阵n n C B ⨯∈,矩阵n m C F ⨯∈,解Lyapunov 矩阵方程: AX+XB=F .第一步:将方程两边拉直,由推论3.2.4可得:→→=⊗+⊗C X B I I A Tm n )(. (4.1) 第二步:判断是否有解,根据线性方程组是否有解的判别条件可得:矩阵方程(4.1)有解的充要条件是:Tm n B I I A rank ⊗+⊗(┊)()T m n B I I A rank C ⊗+⊗=→,:有唯一解的充要条件是det(A ⊗I n + I m ⊗B T )≠0,即A 和(-B )没有公共的特征值或者说A 和B 无互为相反数的特征值.例4.1 分别在下2列条件下解矩阵方程AX+XB=C.(1) ⎥⎦⎤⎢⎣⎡-=0112A ,⎥⎦⎤⎢⎣⎡=42-1-3B ,⎥⎦⎤⎢⎣⎡--=1081710C (2) ⎥⎦⎤⎢⎣⎡=3201A ,⎥⎦⎤⎢⎣⎡--=1052B ,⎥⎦⎤⎢⎣⎡--=11353C 解:(1) 首先计算A 和B 的特征值,解0=-A I λ得:121==λλ,解0=-B I μ得:5221==μμ,.观察有无互为相反数的特征值发现,A 和B 没有互为相反数的特征值,所以矩阵方程有唯一解. 将矩阵方程两边拉直,得到:→→=⊗+⊗C X B I I A Tm n )(. (4.1)设⎥⎦⎤⎢⎣⎡=4321x x x x X ,计算⎥⎦⎤⎢⎣⎡--=4123TB ,将A ,T B ,X ,C 代入(4.1)得: ⎥⎥⎥⎥⎦⎤⎢⎢⎢⎢⎣⎡--=⎥⎥⎥⎥⎦⎤⎢⎢⎢⎢⎣⎡⎪⎪⎭⎫ ⎝⎛⎥⎦⎤⎢⎣⎡--⊗⎥⎦⎤⎢⎣⎡+⎥⎦⎤⎢⎣⎡⊗⎥⎦⎤⎢⎣⎡-108171041231001100101124321x x x x ,计算得到:⎥⎥⎥⎥⎦⎤⎢⎢⎢⎢⎣⎡--=⎥⎥⎥⎥⎦⎤⎢⎢⎢⎢⎣⎡⎥⎥⎥⎥⎦⎤⎢⎢⎢⎢⎣⎡------108171041102301106101254321x x x x , 根据矩阵的乘法的定义可以求得:21314321-===-=x x x x ,,,. 故矩阵方程AX+XB=C 的唯一解为:⎥⎦⎤⎢⎣⎡--=2131X . (2) 同样先计算A 和B 的特征值,解0=-A I λ得:3121==λλ,, 解0=-B I μ得:1221-==μμ,.通过观察可知:021=+μλ. 一所以矩阵方程的解不唯,即存在通解. 将矩阵方程两边拉直,得到:→→=⊗+⊗C X B I I A Tm n )(. (4.1)设⎥⎦⎤⎢⎣⎡=4321x x x xX ,计算⎥⎦⎤⎢⎣⎡--=1502TB ,将A ,T B ,X ,C 代入(4.1)得: ⎥⎥⎥⎥⎦⎤⎢⎢⎢⎢⎣⎡--=⎥⎥⎥⎥⎦⎤⎢⎢⎢⎢⎣⎡⎪⎪⎭⎫ ⎝⎛⎥⎦⎤⎢⎣⎡--⊗⎥⎦⎤⎢⎣⎡+⎥⎦⎤⎢⎣⎡⊗⎥⎦⎤⎢⎣⎡1135315021001100132014321x x x x , - 计算得到:⎥⎥⎥⎥⎦⎤⎢⎢⎢⎢⎣⎡--=⎥⎥⎥⎥⎦⎤⎢⎢⎢⎢⎣⎡⎥⎥⎥⎥⎦⎤⎢⎢⎢⎢⎣⎡--113532520050200050034321x x x x ,根据矩阵的乘法的定义可以求得:c x x c x x -=-===3114321,,,. 故矩阵方程AX+XB=C 的通解为:⎥⎦⎤⎢⎣⎡--=c c X 311(c 为任意常数).4.2矩阵的Kronecker 积与一般线性矩阵方程设矩阵n m k C A ⨯∈,矩阵q p C B ⨯∈,矩阵q m C F ⨯=,解一般线性矩阵方程:F XB Ark k k=∑=1(r = 1,2,…).第一步,将矩阵方程两边拉直,由性质3.2.3可以得到:∑=→→=⊗rk T kkF X B A1)][(. (4.2)第二步:判断是否有解,根据线性方程组是否有解的判别条件可得:矩阵方程(4.2)有解的充要条件是:∑⊗)((Tkk B A rank ┊))(()1∑=→⊗=rk Tkk B A rank F . 即∑=⊗rk Tkk B A 1)(的所有特征值均不为0. 例4.2 设A 和C 都是n ⨯n 矩阵,A 的特征值λi (i=0,1,2,…,n )R ∈(实数),求证:矩阵方程C XA A AXA X =++22有唯一解.证明:将两边方程拉直得到:→→=⊗+⊗+⊗C X A A A A I I T T n n ])([(22,化简得到:→→=⊗+⊗+C X A A A A I TTn ])()([22.由定义3.1可知:T A A ⊗的2n 个特征值是=j i j i ,(λλ0,1,2,…,n ). 故:2)()(2T T n A A A A I ⊗+⊗+的2n 个特征值是:22)21(43)()(1j i j i j i λλλλλλ++=++>00(=j i ,,1,2,…,n ). 即2)()(2T T n A A A A I ⊗+⊗+是可逆的,由唯一解的判断方法可知:矩阵方程C XA A AXA X =++22有唯一解.例4.3 在下列条件下解矩阵方程C XB A XB A =+2211.已知:⎥⎦⎤⎢⎣⎡-=20311A ,⎥⎦⎤⎢⎣⎡-=13101B ,⎥⎦⎤⎢⎣⎡-=11022A ,⎥⎦⎤⎢⎣⎡-=01232B ,⎥⎦⎤⎢⎣⎡--=48213C . 解:将矩阵方程两边拉直得到:→→=⊗+⊗C X B A B A T T)(2211. (4.3)*设⎥⎦⎤⎢⎣⎡=4321x x x xX ,计算⎥⎦⎤⎢⎣⎡-=11301T B 和 ⎥⎦⎤⎢⎣⎡-=02132TB 代入(4.3)*得到:⎥⎥⎥⎥⎦⎤⎢⎢⎢⎢⎣⎡--=⎥⎥⎥⎥⎦⎤⎢⎢⎢⎢⎣⎡⎪⎪⎭⎫ ⎝⎛⎥⎦⎤⎢⎣⎡-⊗⎥⎦⎤⎢⎣⎡-+⎥⎦⎤⎢⎣⎡-⊗⎥⎦⎤⎢⎣⎡-4821302131102113020314321x x x x .计算化简得:⎥⎥⎥⎥⎦⎤⎢⎢⎢⎢⎣⎡--=⎥⎥⎥⎥⎦⎤⎢⎢⎢⎢⎣⎡⎥⎥⎥⎥⎦⎤⎢⎢⎢⎢⎣⎡------4821320027313331390564321x x x x . 根据矩阵的乘法的定义可以求得:10214321===-=x x x x ,,,.计算T T B A B A rank 2211(⊗+⊗┊4)()2211=⊗+⊗=TT B A B A rank C , 所以方程有唯一解:⎥⎦⎤⎢⎣⎡-=1021X . 4.3矩阵的Kronecker 积与矩阵微分方程设m m C A ⨯∈矩阵,n n C B ⨯∈矩阵,n m C t X ⨯∈)(,求下列矩阵微分方程初值问题的解:⎪⎩⎪⎨⎧=+=0)0()()()(X X B t X t AX dt t dX (4.3)引理:设m m C A ⨯∈矩阵A ,矩阵n m C B ⨯∈,则n A I A I e e n ⊗=⊗,B m B I e I e m ⊗=⊗. 证明:因为性质1.2.5可得:∑∑∞=∞=⊗⊗=⊗=11)(!1)(!1k k k k kI A I A k I A k enn A k kI e I A k ⊗=⊗=∑∞=1)!1(. 同理可证:B m B I e I e m ⊗=⊗.将矩阵微分方程(4.3)两边拉直,由推论3.2.4可以得到:⎪⎩⎪⎨⎧=⊗+⊗=→0)0()()()(X X t X B I I A dt t X d T m n (4.4)由引理可得:T t B At tB AtB I I A t TT m n e X e X ee X et X )()()(000)(=⊗==→→⊗+⊗,又因为∑∑∞=∞====11!1))(!1()(k Bt k k T k k k T Tt B e t B k t B k eT ,故Bt At e X e t X 0)(= (4.5) 这就是微分方程(4.3)的解.例4.4 求解下列矩阵微分方程的初值问题:⎪⎩⎪⎨⎧=+=0)0()()()(X X B t X t AX dt t dX (4.6)已知:⎥⎦⎤⎢⎣⎡=0011A ,⎥⎦⎤⎢⎣⎡-=0011B ,⎥⎦⎤⎢⎣⎡=10010X . 解:可计算得到:⎥⎦⎤⎢⎣⎡-=101t tAte e e,⎥⎦⎤⎢⎣⎡-=101t t Bte e e .由(4.5)式可以得到: ⎥⎦⎤⎢⎣⎡--==10)1()(220t tBtAt e e eX e t X . 即(4.6)的解为⎥⎦⎤⎢⎣⎡--=10)1()(22t te e t X . 通过本章的学习,我们知道矩阵的Kronecker 积在解矩阵方程领域有很大的作用,利用Kronecker 积的性质,我们可以解决Lyapunov 矩阵方程,一般矩阵方程,矩阵微分方程的初值问题等问题.参考文献[1]矩阵论简明教程(第三版).徐仲等编.北京:科学出版社.2014.1.[2]矩阵论教程(第2版).张绍飞,赵迪编.北京:机械工业出版社.2012.5.[3]矩阵论引论(第2版).陈祖明,周家胜编.北京:北京航空航天大学出版社.2012.10.[4]矩阵论十讲.李乔,张晓东编.合肥:中国科学技术大学出版社.2015.3.[5]矩阵理论及方法.谢冬秀,雷纪刚,陈桂芝编.北京:科学出版社.2012.[6]H-矩阵类的理论及应用.徐仲等编.北京:科学出版社.2013.[7]高等代数教程(上).王萼芳编.北京:清华大学出版社.1997(2008重印).[8]常微分方程(第二版).东北师范大学微分方程教研室.北京:高等教育出版社.2005.4(2012.12重印).[9]矩阵分析与应用(第2版).张贤达编.北京:清华大学出版社.2013(2014.6重印).[10]线性代数及其应用.毛立新,咸美新编.北京:高等教育出版社.2015.8.[11]线性代数(第2版).钟玉泉,周建编.北京:科学出版社.2015.1.[12]矩阵理论与方法(第2版).吴昌悫,魏洪增编.北京:电子工业出版社.2013.8.[13]线性代数学习指导.赵春燕,单净,王麟编.哈尔滨:哈尔滨工程大学出版社.2012.2.[14]矩阵论.张凯院等编.北京:科学出版社.2013.[15]矩阵论导教·导学·导考.张凯院,徐仲编.西安:西北工业大学出版社.2014.8.[16]矩阵函数与矩阵方程.柏兆俊,高卫国,苏仰锋编.北京:高等教育出版社.2015.5.[17]矩阵分析.姜志侠,孟品超,李延忠编.北京:清华大学出版社.2015.[18]矩阵论札论.梁昌洪编.北京:科学出版社.2014.[19]线性代数及其应用.马新顺,王涛,郭燕编.北京:高等教育出版社.2014.7.[20]矩阵论引论.田振际,王永铎,吴德军编.北京:科学出版社.2013.[21]线性代数及其应用(第2版).河北农业大学理学院编.北京:高等教育出版社.2006.11.(2015.2重印).[22]线性代数及其应用.王坤龙编.北京:电子工业出版社.2014.10.[23]线性代数(第2版).许峰,范爱华编.合肥:中国科学技术大学出版社.2013.4.[24]线性代数及其应用.俞方元编.上海:同济大学出版社.2014.8.[25]线性代数学习指导.谢政,陈挚编.北京:清华大学出版社.2012.10.[26]高等线性代数学.黎景辉,白正简,周国晖编.北京:高等教育出版社.2014.9.[27]线性代数讲义.江惠坤,邵荣,范红军编.北京:科学出版社.2013.[28]线性代数.贾屹峰编.上海:上海交通大学出版社.2012.[29]线性代数.侯亚君,艾玲,沙萍,林洪娟编.北京:机械工业出版社.2012.1(2012.7重印).[30]线性代数.郝秀敏,姜庆华编.北京:经济科学出版社.2013.7.[31]线性代数.韩旸,王静宇,周莉编.北京:化学工业出版社.2013.8.[32]线性代数重点难点考点辅导与精析.高淑萍,张剑湖编.西安:西北工业大学出版社.2014.5.[33]线性代数.傅媛编.武汉:武汉大学出版社.2013.2(2013.11重印).[34]跟我学线性代数:导学与习题精解.董晓波编.北京:机械工业出版社.2014.1.[35]线性代数同步学习辅导.陈绍林,唐道远编.北京:科学出版社,2014.7.[36]线性代数及应用.刘三明编.南京:南京大学出版社.2012.8.[37]线性代数.谭福锦,黎进香编.北京.人民邮电出版社.2012.8.[38]工程数学.线性代数(第6版).同济大学数学系编.北京:高等教育出版社.2014.6.[39]矩阵分析与计算.李继根,张新发编.武汉:武汉大学出版社.2013.10.[40]矩阵计算的理论与方法.徐树方编.北京:北京大学出版社.1995.8.[41]矩阵分析及其应用.曾祥金,吴华安编.武汉:武汉大学出版社.2007.8.[42]矩阵理论与应用.张跃辉编.北京:科学出版社.2011.8.致谢通过一个月来不断的努力,终于完成了这篇毕业论文。
ARTISAN技术集团设备购买与售卖说明书

ErrataTitle & Document Type:Manual Part Number:Revision Date:HP References in this ManualThis manual may contain references to HP or Hewlett-Packard. Please note that Hewlett-Packard's former test and measurement, semiconductor products an d chemical analysisbusinesses are now part of Agilent Technologies. We have made no changes to thismanual copy. The HP XXXX referred to in this document is now the Agilent XXXX.For example, model number HP8648A is now model number Agilent 8648A.About this ManualWe’ve added this manual to the Agilent website in an effort to help you support yourproduct. This manual provides the best information we could find. It may be incomplete or contain dated information, and the scan quality may not be ideal. If we find a bettercopy in the future, we will add it to the Agilent website.Support for Your ProductAgilent no longer sells or supports this product. You will find any other availableproduct information on the Agilent Test & Measurement website:Search for the model number of this product, and the resulting product page will guideyou to any available information. Our service centers may be able to perform calibrationif no repair parts are needed, but no other support from Agilent is available.3562A Programming Manual03562-90031October 1985Artisan Technology Group - Quality Instrumentation ... Guaranteed | (888) 88-SOURCE | Artisan Technology Group - Quality Instrumentation ... Guaranteed | (888) 88-SOURCE | Artisan Technology Group - Quality Instrumentation ... Guaranteed | (888) 88-SOURCE | Artisan Technology Group - Quality Instrumentation ... Guaranteed | (888) 88-SOURCE | Artisan Technology Group - Quality Instrumentation ... Guaranteed | (888) 88-SOURCE | Artisan Technology Group - Quality Instrumentation ... Guaranteed | (888) 88-SOURCE | Artisan Technology Group - Quality Instrumentation ... Guaranteed | (888) 88-SOURCE | Artisan Technology Group - Quality Instrumentation ... Guaranteed | (888) 88-SOURCE | Artisan Technology Group - Quality Instrumentation ... Guaranteed | (888) 88-SOURCE | 。
1-丙醇 msds - sigma-aldrich说明书

SIGMA-ALDRICHMaterial Safety Data SheetVersion 4.2Revision Date 12/18/2012Print Date 05/07/20131. PRODUCT AND COMPANY IDENTIFICATIONProduct name :1-PropanolProduct Number : 402893Brand : Sigma-AldrichSupplier: Sigma-Aldrich3050 Spruce StreetSAINT LOUIS MO 63103 USATelephone : +1 800-325-5832 Fax: +1 800-325-5052 Emergency Phone # (For both supplier and manufacturer): (314) 776-6555Preparation Information: Sigma-Aldrich CorporationProduct Safety - Americas Region 1-800-521-89562. HAZARDS IDENTIFICATIONEmergency OverviewOSHA HazardsFlammable liquid, Target Organ Effect, Irritant Target Organs Nerves., LiverGHS ClassificationFlammable liquids (Category 2)Acute toxicity, Inhalation (Category 5) Skin irritation (Category 3)Serious eye damage (Category 1)Specific target organ toxicity - single exposure (Category 3)GHS Label elements, including precautionary statements PictogramSignal word DangerHazard statement(s) H225 Highly flammable liquid and vapour. H316 Causes mild skin irritation. H318 Causes serious eye damage. H333 May be harmful if inhaled. H336 May cause drowsiness or dizziness.Precautionary statement(s) P210 Keep away from heat/sparks/open flames/hot surfaces. - No smoking. P261 Avoid breathing dust/ fume/ gas/ mist/ vapours/ spray. P280 Wear protective gloves/ eye protection/ face protection. P305 + P351 + P338 IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, ifpresent and easy to do. Continue rinsing.HMIS ClassificationHealth hazard:2Chronic Health Hazard:*Flammability:3Physical hazards:0NFPA RatingHealth hazard:2Fire:3Reactivity Hazard:0Potential Health EffectsInhalation May be harmful if inhaled. Causes respiratory tract irritation.Vapours may causedrowsiness and dizziness.Skin May be harmful if absorbed through skin. Causes skin irritation.Eyes Causes eye irritation.Ingestion May be harmful if swallowed.3. COMPOSITION/INFORMATION ON INGREDIENTSSynonyms : Propyl alcoholFormula : C3H8OMolecular Weight : 60.10 g/mol4. FIRST AID MEASURESGeneral adviceConsult a physician. Show this safety data sheet to the doctor in attendance.Move out of dangerous area.If inhaledIf breathed in, move person into fresh air. If not breathing, give artificial respiration. Consult a physician.In case of skin contactWash off with soap and plenty of water. Consult a physician.In case of eye contactRinse thoroughly with plenty of water for at least 15 minutes and consult a physician.If swallowedDo NOT induce vomiting. Never give anything by mouth to an unconscious person. Rinse mouth with water. Consult a physician.5. FIREFIGHTING MEASURESConditions of flammabilityFlammable in the presence of a source of ignition when the temperature is above the flash point. Keep away from heat/sparks/open flame/hot surface. No smoking.Suitable extinguishing mediaUse water spray, alcohol-resistant foam, dry chemical or carbon dioxide.Special protective equipment for firefightersWear self contained breathing apparatus for fire fighting if necessary.Hazardous combustion productsHazardous decomposition products formed under fire conditions. - Carbon oxidesFurther informationUse water spray to cool unopened containers.6. ACCIDENTAL RELEASE MEASURESPersonal precautionsUse personal protective equipment. Avoid breathing vapors, mist or gas. Ensure adequate ventilation. Remove allsources of ignition. Evacuate personnel to safe areas. Beware of vapours accumulating to form explosiveconcentrations. Vapours can accumulate in low areas.Environmental precautionsPrevent further leakage or spillage if safe to do so. Do not let product enter drains.Methods and materials for containment and cleaning upContain spillage, and then collect with an electrically protected vacuum cleaner or by wet-brushing and place incontainer for disposal according to local regulations (see section 13).7. HANDLING AND STORAGEPrecautions for safe handlingAvoid inhalation of vapour or mist.Use explosion-proof equipment. Keep away from sources of ignition - No smoking. Take measures to prevent the build up of electrostatic charge.Conditions for safe storageKeep container tightly closed in a dry and well-ventilated place. Containers which are opened must be carefully resealed and kept upright to prevent leakage.8. EXPOSURE CONTROLS/PERSONAL PROTECTIONComponents with workplace control parametersPersonal protective equipmentRespiratory protectionWhere risk assessment shows air-purifying respirators are appropriate use a full-face respirator with multi-purpose combination (US) or type ABEK (EN 14387) respirator cartridges as a backup to engineering controls. If therespirator is the sole means of protection, use a full-face supplied air respirator. Use respirators and componentstested and approved under appropriate government standards such as NIOSH (US) or CEN (EU).Hand protectionHandle with gloves. Gloves must be inspected prior to use. Use proper glove removal technique (without touching glove's outer surface) to avoid skin contact with this product. Dispose of contaminated gloves after use inaccordance with applicable laws and good laboratory practices. Wash and dry hands.Full contactMaterial: Nitrile rubberMinimum layer thickness: 0.4 mmBreak through time: > 480 minMaterial tested:Camatril® (KCL 730 / Aldrich Z677442, Size M)Splash protectionMaterial: Nitrile rubberMinimum layer thickness: 0.2 mmBreak through time: 72 minMaterial tested:Dermatril® P (KCL 743 / Aldrich Z677388, Size M)datasource:KCLGmbH,D-36124Eichenzell,phone+49(0)665987300,******************,testmethod:EN374 If used in solution, or mixed with other substances, and under conditions which differ from EN 374, contact thesupplier of the CE approved gloves. This recommendation is advisory only and must be evaluated by an Industrial Hygienist familiar with the specific situation of anticipated use by our customers. It should not be construed asoffering an approval for any specific use scenario.Eye protectionTightly fitting safety goggles. Faceshield (8-inch minimum). Use equipment for eye protection tested and approved under appropriate government standards such as NIOSH (US) or EN 166(EU).Skin and body protectionComplete suit protecting against chemicals, Flame retardant antistatic protective clothing, The type of protectiveequipment must be selected according to the concentration and amount of the dangerous substance at the specific workplace.Hygiene measuresHandle in accordance with good industrial hygiene and safety practice. Wash hands before breaks and at the end of workday.9. PHYSICAL AND CHEMICAL PROPERTIESAppearanceForm clear, liquidColour colourlessSafety datapH 8.5 at 200 g/l at 20 °C (68 °F)Melting point/range: -127 °C (-197 °F) - lit.Meltingpoint/freezing pointBoiling point 97 °C (207 °F) - lit.Flash point 22 °C (72 °F) - closed cupIgnition temperature 395 °C (743 °F)Auto-ignitionno data availabletemperatureLower explosion limit 2.1 %(V)Upper explosion limit 13.7 %(V)Vapour pressure 19.3 hPa (14.5 mmHg) at 20 °C (68 °F) Density 0.804 g/cm3 at 25 °C (77 °F)Water solubility completely solublePartition coefficient:n-octanol/waterlog Pow: 0.25 - 0.34Relative vapor density 2.07- (Air = 1.0)Odour no data availableOdour Threshold no data availableEvaporation rate 110. STABILITY AND REACTIVITYChemical stabilityStable under recommended storage conditions.Possibility of hazardous reactionsVapours may form explosive mixture with air.Conditions to avoidHeat, flames and sparks. Extremes of temperature and direct sunlight.Materials to avoidStrong oxidizing agentsHazardous decomposition productsHazardous decomposition products formed under fire conditions. - Carbon oxidesOther decomposition products - no data available11. TOXICOLOGICAL INFORMATIONAcute toxicityOral LD50LD50Oral - rat - 8,038 mg/kgInhalation LC50LC50Inhalation - rat - 1 h - 20000 ppmDermal LD50LC50Dermal - rabbit - 4,000 mg/kgOther information on acute toxicityno data availableSkin corrosion/irritationSkin - rabbit - Mild skin irritationSerious eye damage/eye irritationEyes - rabbit - Severe eye irritationRespiratory or skin sensitizationno data availableGerm cell mutagenicityno data availableCarcinogenicityIARC: No component of this product present at levels greater than or equal to 0.1% is identified as probable, possible or confirmed human carcinogen by IARC.NTP: No component of this product present at levels greater than or equal to 0.1% is identified as aknown or anticipated carcinogen by NTP.OSHA: No component of this product present at levels greater than or equal to 0.1% is identified as a carcinogen or potential carcinogen by OSHA.Reproductive toxicityno data availableTeratogenicityno data availableSpecific target organ toxicity - single exposure (Globally Harmonized System)May cause drowsiness or dizziness.Specific target organ toxicity - repeated exposure (Globally Harmonized System)no data availableAspiration hazardno data availablePotential health effectsInhalation May be harmful if inhaled. Causes respiratory tract irritation.Vapours may causedrowsiness and dizziness.Ingestion May be harmful if swallowed.Skin May be harmful if absorbed through skin. Causes skin irritation.Eyes Causes eye irritation.Signs and Symptoms of ExposureCentral nervous system depression, prolonged or repeated exposure can cause:, narcosis, Skin irritation Synergistic effectsno data availableAdditional InformationRTECS: UH822500012. ECOLOGICAL INFORMATIONToxicityToxicity to fish LC50 - Pimephales promelas (fathead minnow) - 1,000 mg/l - 96 hToxicity to daphniaEC50 - Daphnia magna (Water flea) - 3,642 mg/l - 48 hand other aquaticinvertebratesPersistence and degradabilityBiodegradabilityBioaccumulative potentialno data availableMobility in soilno data availablePBT and vPvB assessmentno data availableOther adverse effectsno data available13. DISPOSAL CONSIDERATIONSProductBurn in a chemical incinerator equipped with an afterburner and scrubber but exert extra care in igniting as this material is highly flammable. Offer surplus and non-recyclable solutions to a licensed disposal company. Contact a licensed professional waste disposal service to dispose of this material.Contaminated packagingDispose of as unused product.14. TRANSPORT INFORMATIONDOT (US)UN number:1274 Class: 3 Packing group: IIProper shipping name: n-PropanolMarine Pollutant: NoPoison Inhalation Hazard: NoIMDGUN number: 1274 Class: 3 Packing group: II EMS-No: F-E, S-DProper shipping name: n-PROPANOLMarine Pollutant: NoIATAUN number:1274 Class: 3 Packing group: IIProper shipping name: n-Propanol15. REGULATORY INFORMATIONOSHA HazardsFlammable liquid, Target Organ Effect, IrritantSARA 302 ComponentsSARA 302: No chemicals in this material are subject to the reporting requirements of SARA Title III, Section 302.SARA 313 ComponentsSARA 313: This material does not contain any chemical components with known CAS numbers that exceed the threshold (De Minimis) reporting levels established by SARA Title III, Section 313.SARA 311/312HazardsFire Hazard, Acute Health Hazard, Chronic Health HazardMassachusetts Right To Know Componentsn-Propanol CAS-No.71-23-8Revision Date1993-04-24Pennsylvania Right To Know Componentsn-Propanol CAS-No.71-23-8Revision Date1993-04-24New Jersey Right To Know Componentsn-Propanol CAS-No.71-23-8Revision Date1993-04-24California Prop. 65 ComponentsThis product does not contain any chemicals known to State of California to cause cancer, birth defects, or any other reproductive harm.16. OTHER INFORMATIONFurther informationCopyright 2012 Sigma-Aldrich Co. LLC. License granted to make unlimited paper copies for internal use only.The above information is believed to be correct but does not purport to be all inclusive and shall be used only as a guide. The information in this document is based on the present state of our knowledge and is applicable to theproduct with regard to appropriate safety precautions. It does not represent any guarantee of the properties of the product. Sigma-Aldrich Corporation and its Affiliates shall not be held liable for any damage resulting from handling or from contact with the above product. See and/or the reverse side of invoice or packing slip for additional terms and conditions of sale.。
分析化学英文文献(精品资料)

I. vocabularyabsorbance吸光度acetic acid 乙酸acetone 丙酮acetonitrile 乙腈aliquot 等份(试液)aluminum foil 铝箔analytical chemistry 分析化学American Chemical Society (缩写ACS) 美国化学会autosampler 自动进样器beaker 烧杯bibliography 参考书目blender 混合器,搅拌机buffer solution 缓冲溶液burette 滴定管cartridge 柱管centrifugation 离心Chemical Abstracts (缩写CA) 化学文摘chemical analysis 化学分析chromatograph 色谱仪chromatogram色谱图cloud point extraction(缩写CPE)浊点萃取confidence level 置信水平conical flask 锥形瓶daughter ion 子离子dichloromethane 二氯甲烷Diode array detector (缩写DAD)二极管阵列检测器dilution 稀释(n.)disperser solvent 分散剂dispersive liquid–liquidmicroextraction 分散液液微萃取distilled water 蒸馏水dropping pipet 滴管electrochemical analysis电化学分析electrode 电极electrolyte 电解质electromagnetic spectrum 电磁波谱electrospray ionization (缩写ESI ) 电喷雾离子化eliminate 消除(v.)eluate 洗出液eluent 洗脱剂elute 洗脱(v.)elution 洗脱(n.)Encyclopedia of analytical chemistry分析化学百科全书The Engineering Index (缩写EI )工程索引enrichment factor 富集因子Evaporative Light Scattering Detector(缩写ELSD) 蒸发光散射检测器extract 萃取(v.)、萃取物(n.)extraction efficiency 萃取效率filter 过滤(v.)、过滤器(n.) filtrate 滤出液filtration 过滤fluorescence荧光fluorometry荧光分析法formic acid 甲酸funnel 漏斗gas chromatography–mass spectrometry (缩写GC–MS) 气相色谱-质谱gas chromatography coupled to tandem mass spectrometry (缩写GC–MS/MS)气相色谱-串联质谱gel filtration chromatography凝胶过滤色谱法gel permeation chromatography凝胶渗透色谱法graduated cylinder 量筒high performance liquid chromatography (缩写HPLC) 高效液相色谱homogenate 匀浆(n.) homogenize 使均质,将……打成匀浆hydrophobic 疏水的identification 鉴定Impact Factor影响因子incubation time 温育时间Index to Scientific Technical Proceedings (缩写ISTP)科技会议录索引indicator 指示剂instrumental analysis 仪器分析interference 干扰ion enhancement 离子加强ion exchange chromatography离子交换色谱法ion source 离子源ion suppression 离子抑制limit of detection (缩写LOD)检出限limit of quantitation (缩写LOQ)定量限linearity 线性linear range 线性范围linear regression equation 线性回归方程liquid chromatography tandem massspectrometry (缩写LC-MS/MS)液相色谱串联质谱liquid chromatography withelectrospray ionizationtandem mass spectrometry (缩写LC-ESI-MS/MS)液相色谱电喷雾串联质谱liquid-liquid partition chromatography液液分配色谱法liquid-solid adsorptionchromatography 液固吸附色谱法mass analyzer 质量分析器Mass Spectrometer 质谱仪mass spectrum 质谱图mass-to-charge ratio 质荷比matrix effect 基质效应maximum absorption 最大吸收maximum value 最大值measuring pipet 吸量管methanol 甲醇micelle 胶束microwave assisted extraction 微波辅助提取minimum value 最小值mobile phase 流动相molarity 摩尔浓度monograph专著Multiple-reaction monitoring 多反应监测(缩写MRM)normal phase liquid chromatography正相液相色谱法nominal concentration 标示浓度optimization 优化outlier 离群值parent ion 母离子pipette 移液管polycyclic aromatic hydrocarbons 多环芳烃potentiometry电位法preconcentration 预浓缩primary literature一次文献quadrupole-time- of-flight massspectrometry 四极杆-飞行时间质谱(缩写Q-TOF MS)qualitative analysis 定性分析quality assurance and quality control(缩写QA/QC)质量保证和质量控制quantification 定量quantitative analysis 定量分析reconstitute 重组、复溶(v.)recovery 回收率refractive index detector 折光指数检测器,示差折光检测器relative abundance 相对丰度relative standard deviation (缩写RSD)相对标准偏差reproducibility 重现性reversed phase liquid chromatography(缩写RPLC)反相液相色谱法Royal Society of Chemistry(缩写RSC)英国皇家化学会Science Citation Index (缩写SCI )科学引文索引Science Citation Index Expanded (缩写SCIE) 科学引文索引扩展版Scientific notation 科学计数法signal to noise ratio (缩写S/N)信噪比size exclusion chromatography尺寸排除色谱法secondary literature二次文献solid-phase extraction (缩写SPE)固相萃取solid-phase microextraction (缩写SPME)固相微萃取spike 添加(v.)standard solution标准溶液stationary phase 固定相stirring bar 搅拌棒stoichiometric point化学计量点surfactant 表面活性剂supernatant 上清液syringe 注射器tap water 自来水Teflon 聚四氟乙烯tetrahydrofuran 四氢呋喃titrant 滴定剂titration滴定Ultra performance liquidchromatography (缩写UPLC) 超高效液相色谱Ultraviolet/VisibleSpectrophotometry 紫外/可见分光光度法vacuum 真空vessel 容器volumetric flask 容量瓶volumetric analysis容量分析法voltammetry 伏安法II. T erms and their definitionsAccuracy 准确度A measure of the agreement between an experimental result and its expected value.Analysis 分析A process that provides chemical or physical information about the constituents in the sample or the sampleitselfAnalyte 被测物,被分析物The constituent of interest in sampleCalibration curve 校准曲线The result of a standardization showing gr aphically how a method’s signal changes with respectto the amount of analyte.Calibration method 校准方法The basis of quantitative analysis: magnitude of measured property is proportional toconcentration of analyteChromophore 生色团A functional group which absorbs a characteristic ultraviolet or visible wavelengthGradient elution 梯度洗脱T he process of changing the mobile phase’s solvent strength to enhance the separation of bothearly and late eluting solutes.Gravimetric analysis重量分析A type of quantitative analysis in which the amount of a species in a material is determined by converting the species into a product that can be isolated and weighed.Isocratic elution 等度洗脱the use of a mobile phase whose composition remains constant throughout theseparation.Matrix 基质All other constituents in a sample except for the analytesMethod blank方法空白A sample that contains all components of the matrix except the analyte.Outlier 离群值Data point whose value is much larger or smaller than the remaining data.Precision精密度An indication of the reproducibility of a measurement or resultQuantitative analysis 定量分析The determination of the amount of a substance or species present in a material. Quantitative transfer 定量转移The process of moving a sample from one container to another in a manner that ensures allmaterial is transferred.Selectivity选择性A measure of a method’s freedom from interferences as defined by the method’s selectivity coefficient. Significant figures有效数字The digits in a measured quantity, including all digits known exactly and one digit (the last) whosequantity is uncertain.Spectrophotometry分光光度法. An analytical method that involves how light interacts with a substanceStock solution储备液A solution of known concentration from which other solutions are prepared.Titration curve滴定曲线A graph showing the progress of a titration as a function of the volume of titrant added.V alidation(方法)确证,验证The process of verifying that a procedure yields acceptable results.Titration error滴定误差The determinate error in a titration due to the difference between the end point and the equivalencepoint.III. Common knowledges1.Some key journals in Analytical Chemistry: Analytical ChemistryTrends in Analytical ChemistryJournal of Chromatography AJournal of Chromatography BAnalystAnalytica Chimica ActaTALANTACritical Reviews in Analytical Chemistry Analytical and Bioanalytical ChemistryELECTROPHORESIS2. T ypes of articles published in scientific journals:Full Length Research PapersRapid CommunicationsReviewsShort CommunicationsDiscussions or Letters to the Editor(Some journals publish all types of articles, while others are devoted to only a single type.)3. The structure of a scientific paper:•Title•Authors (with affiliations and addresses) • Abstract (summary)• Key words•Introduction•Experimental•Results and discussion•Conclusion•Acknowledgement•References4. How to Read a Scientific Paper:Five Helpful Questions•1) WHY did they do this set of experiments?•2) HOW were the experiments actually done?•3) WHA T are the results?•4) WHA T can be concluded from the results?•5) Did they do everything correctly?5. Five-step analyzing process1) Identify and define the problem.2) Design the experimental procedure.3) Conduct an experiment and gather data.4) Analyze the experimental data.5) Report and suggestionIV. T ranslation exercises1. 用分散液- 液微萃取法对杀菌剂的水样品中的测定(杀真菌剂)开发的。
KraljicMatrix包的中文名字:克拉尔吉矩阵策略分析包说明书

Package‘KraljicMatrix’October12,2022Type PackageTitle A Quantified Implementation of the Kraljic MatrixVersion0.2.1Maintainer Bradley Boehmke<************************>Date2017-11-01Description Implements a quantified approach to the Kraljic Matrix(Kraljic,1983,<https: ///1983/09/purchasing-must-become-supply-management>)for strategically analyzing afirm’s purchasing portfolio.It combines multi-objective decision analysis to measure purchasing characteristics anduses this information to place products and services within the Kraljic Matrix.URL https:///koalaverse/KraljicMatrixBugReports https:///koalaverse/KraljicMatrix/issuesLicense MIT+file LICENSEEncoding UTF-8LazyData trueDepends R(>=2.10)Imports ggplot2,dplyr,tibble,magrittrSuggests knitr,rmarkdown,testthatVignetteBuilder knitrRoxygenNote6.0.1NeedsCompilation noAuthor Bradley Boehmke[aut,cre],Brandon Greenwell[aut],Andrew McCarthy[aut],Robert Montgomery[ctb]Repository CRANDate/Publication2018-03-0622:49:03UTC1R topics documented:geom_frontier (2)get_frontier (3)kraljic_matrix (4)kraljic_quadrant (5)MA VF_score (6)MA VF_sensitivity (7)psc (8)SA VF_plot (9)SA VF_plot_rho_error (10)SA VF_preferred_rho (11)SA VF_score (12)%>% (13)Index14 geom_frontier Plotting the Pareto Optimal FrontierDescriptionThe frontier geom is used to overlay the efficient frontier on a scatterplot.Usagegeom_frontier(mapping=NULL,data=NULL,position="identity",direction="vh",na.rm=FALSE,show.legend=NA,inherit.aes=TRUE,...)stat_frontier(mapping=NULL,data=NULL,geom="step",position="identity",direction="vh",na.rm=FALSE,show.legend=NA,inherit.aes=TRUE,quadrant="top.right",...) Argumentsmapping Set of aesthetic mappings created by aes or aes_.If specified and inherit.aes =TRUE(the default),it is combined with the default mapping at the top level ofthe plot.You must supply mapping if there is no plot mapping.data The data to be displayed in this layer.position Position adjustment,either as a string,or the result of a call to a position adjust-ment function.direction Direction of stairs:’vh’for vertical then horizontal,or’hv’for horizontal then vertical.na.rm If FALSE,the default,missing values are removed with a warning.If TRUE, missing values are silently removed.show.legend Logical.Should this layer be included in the legends?NA,the default,includesif any aesthetics are mapped.FALSE never includes,and TRUE always includes.inherit.aes If FALSE,overrides the default aesthetics,rather than combining with them.This is most useful for helper functions that define both data and aesthetics andshouldn’t inherit behaviour from the default plot specification,e.g.borders....Other arguments passed on to layer.These are often aesthetics,used to set anaesthetic to afixed value,like color="red"or size=3.They may also beparameters to the paired geom/stat.geom Use to override the default connection between geom_frontier and stat_frontier.quadrant See get_frontier.Examples##Not run:#default will find the efficient front in top right quadrantggplot(mtcars,aes(mpg,wt))+geom_point()+geom_frontier()#change the direction of the stepsggplot(mtcars,aes(mpg,wt))+geom_point()+geom_frontier(direction= hv )#use quadrant parameter to change how you define the efficient frontierggplot(airquality,aes(Ozone,Temp))+geom_point()+geom_frontier(quadrant= top.left )ggplot(airquality,aes(Ozone,Temp))+geom_point()+geom_frontier(quadrant= bottom.right )##End(Not run)get_frontier Compute the Pareto Optimal FrontierDescriptionExtract the points that make up the Pareto frontier from a set of data.Usageget_frontier(data,x,y,quadrant=c("top.right","bottom.right","bottom.left","top.left"),decreasing=TRUE)4kraljic_matrixArgumentsdata A data frame.x A numeric vector.y A numeric vector.quadrant Chararacter string specifying which quadrant the frontier should appear in.De-fault is"top.right".decreasing Logical value indicating whether the data returned is in decreasing or ascending order(ordered by x and then y).Default is decreasing order.ValueA data frame containing the data points that make up the efficient frontier.See Alsogeom_frontier for plotting the Pareto frontExamples#default will find the Pareto optimal observations in top right quadrantget_frontier(mtcars,mpg,wt)#the output can be in descending or ascending orderget_frontier(mtcars,mpg,wt,decreasing=FALSE)#use quadrant parameter to change how you define the efficient frontierget_frontier(airquality,Ozone,Temp,quadrant= top.left )get_frontier(airquality,Ozone,Temp,quadrant= bottom.right )kraljic_matrix Kraljic matrix plotting functionDescriptionkraljic_matrix plots each product or service in the Kraljic purchasing matrix based on the at-tribute value score of x and yUsagekraljic_matrix(data,x,y)kraljic_quadrant5Argumentsdata A data framex Numeric vector of valuesy Numeric vector of values with compatible dimensions to xValueA Kraljic purchasing matrix plotSee AlsoSAVF_score for computing the exponential single attribute value score for x and yExamples#Given the following\code{x}and\code{y}attribute values we can plot each#product or service in the purchasing matrix:#to add a new variable while preserving existing datalibrary(dplyr)psc2<-psc%>%mutate(x_SAVF_score=SAVF_score(x_attribute,1,5,.653),y_SAVF_score=SAVF_score(y_attribute,1,10,.7))kraljic_matrix(psc2,x_SAVF_score,y_SAVF_score)kraljic_quadrant Kraljic quadrant assignment functionDescriptionkraljic_quadrant assigns the Kraljic purchasing matrix quadrant based on the attribute value score of x and yUsagekraljic_quadrant(x,y)Argumentsx Numeric vector of valuesy Numeric vector of values with compatible dimensions to x6MA VF_score ValueA vector of the same length as x and y with the relevant Kraljic quadrant nameSee AlsoSAVF_score for computing the exponential single attribute value score for x and yExamples#Given the following\code{x}and\code{y}attribute values we can determine#which quadrant each product or service falls in:#to add a new variable while preserving existing datalibrary(dplyr)psc2<-psc%>%mutate(x_SAVF_score=SAVF_score(x_attribute,1,5,.653),y_SAVF_score=SAVF_score(y_attribute,1,10,.7))psc2%>%mutate(quadrant=kraljic_quadrant(x_SAVF_score,y_SAVF_score))MAVF_score Multi-attribute value functionDescriptionMAVF_score computes the multi-attribute value score of x and y given their respective weights UsageMAVF_score(x,y,x_wt,y_wt)Argumentsx Numeric vector of valuesy Numeric vector of values with compatible dimensions to xx_wt Swing weight for xy_wt Swing weight for yValueA vector of the same length as x and y with the multi-attribute value scoresMA VF_sensitivity7See AlsoMAVF_sensitivity to perform sensitivity analysis with a range of x and y swing weightsSAVF_score for computing the exponential single attribute value scoreExamples#Given the following\code{x}and\code{y}attribute values with\code{x}and#\code{y}swing weight values of0.65and0.35respectively,we can compute#the multi-attribute utility score:x_attribute<-c(0.92,0.79,1.00,0.39,0.68,0.55,0.73,0.76,1.00,0.74)y_attribute<-c(0.52,0.19,0.62,1.00,0.55,0.52,0.53,0.46,0.61,0.84)MAVF_score(x_attribute,y_attribute,x_wt=.65,y_wt=.35)MAVF_sensitivity Multi-attribute value function sensitivity analysisDescriptionMAVF_sensitivity computes summary statistics for multi-attribute value scores of x and y given a range of swing weights for each attributeUsageMAVF_sensitivity(data,x,y,x_wt_min,x_wt_max,y_wt_min,y_wt_max) Argumentsdata A data framex Variable from data frame to represent x attribute valuesy Variable from data frame to represent y attribute valuesx_wt_min Lower bound anchor point for x attribute swing weightx_wt_max Upper bound anchor point for x attribute swing weighty_wt_min Lower bound anchor point for y attribute swing weighty_wt_max Upper bound anchor point for y attribute swing weightDetailsThe sensitivity analysis performs a Monte Carlo simulation with1000trials for each product or service(row).Each trial randomly selects a weight from a uniform distribution between the lower and upper bound weight parameters and calculates the mult-attribute utility score.From these trials, summary statistics for each product or service(row)are calculated and reported for thefinal output.8pscValueA data frame with added variables consisting of sensitivity analysis summary statistics for eachproduct or service(row).See AlsoMAVF_score for computing the multi-attribute value score of x and y given their respective weights SAVF_score for computing the exponential single attribute value scoreExamples#Given the following data frame that contains\code{x}and\code{y}attribute#values for each product or service contract,we can compute how the range of#swing weights for each\code{x}and\code{y}attribute influences the multi-#attribute value score.df<-data.frame(contract=1:10,x_attribute=c(0.92,0.79,1.00,0.39,0.68,0.55,0.73,0.76,1.00,0.74),y_attribute=c(0.52,0.19,0.62,1.00,0.55,0.52,0.53,0.46,0.61,0.84)) MAVF_sensitivity(df,x_attribute,y_attribute,.55,.75,.25,.45)psc Product and service contractsDescriptionA dataset containing a single value score for the x attribute(i.e.supply risk)and y attribute(i.e.profit impact)of200product and service contracts(PSC).The variables are as follows:UsagepscFormatA tibble with200rows and3variables:PSC Contract identifier for each product and servicex_attribute x attribute score,from1(worst)to5(best)in.01incrementsy_attribute y attribute score,from1(worst)to10(best)in.01incrementsSA VF_plot9 SAVF_plot Plot the single attribute value curveDescriptionSAVF_plot plots the single attribute value curve along with the subject matter desired values for comparisonUsageSAVF_plot(desired_x,desired_v,x_low,x_high,rho)Argumentsdesired_x Elicited input x value(s)desired_v Elicited value score related to elicited input value(s)x_low Lower bound anchor point(can be different than min(x))x_high Upper bound anchor point(can be different than max(x))rho Exponential constant for the value functionValueA plot that visualizes the single attribute value curve along with the subject matter desired valuesfor comparisonSee AlsoSAVF_plot_rho_error for plotting the rho squared error termsSAVF_score for computing the exponential single attribute value scoreExamples#Given the single attribute x is bounded between1and5and the subject matter experts #prefer x values of3,4,&5provide a utility score of.75,.90&1.0respectively, #the preferred rho is0.54.We can visualize this value function:SAVF_plot(desired_x=c(3,4,5),desired_v=c(.75,.9,1),x_low=1,x_high=5,rho=0.54)10SA VF_plot_rho_error SAVF_plot_rho_error Plot the rho squared error termsDescriptionSAVF_plot_rho_error plots the squared error terms for the rho search space to illustrate the pre-ferred rho that minimizes the squared error between subject matter desired values and exponentially fitted scoresUsageSAVF_plot_rho_error(desired_x,desired_v,x_low,x_high,rho_low=0,rho_high=1)Argumentsdesired_x Elicited input x value(s)desired_v Elicited value score related to elicited input value(s)x_low Lower bound anchor point(can be different than min(x))x_high Upper bound anchor point(can be different than max(x))rho_low Lower bound of the exponential constant search space for a bestfit value func-tionrho_high Upper bound of the exponential constant search space for a bestfit value func-tionValueA plot that visualizes the squared error terms for the rho search spaceSee AlsoSAVF_preferred_rho for identifying the preferred rho valueSAVF_score for computing the exponential single attribute value scoreExamples#Given the single attribute x is bounded between1and5and the subject matter experts #prefer x values of3,4,&5provide a utility score of.75,.90&1.0respectively,we #can visualize the error terms for rho values between0-1:SAVF_plot_rho_error(desired_x=c(3,4,5),desired_v=c(.75,.9,1),x_low=1,x_high=5,rho_low=0,rho_high=1)SA VF_preferred_rho11 SAVF_preferred_rho Identify preferred rhoDescriptionSAVF_preferred_rho computes the preferred rho that minimizes the squared error between subject matter input desired values and exponentiallyfitted scoresUsageSAVF_preferred_rho(desired_x,desired_v,x_low,x_high,rho_low=0,rho_high=1)Argumentsdesired_x Elicited input x value(s)desired_v Elicited value score related to elicited input value(s)x_low Lower bound anchor point(can be different than min(x))x_high Upper bound anchor point(can be different than max(x))rho_low Lower bound of the exponential constant search space for a bestfit value func-tionrho_high Upper bound of the exponential constant search space for a bestfit value func-tionValueA single element vector that represents the rho value that bestfits the exponential utility function tothe desired inputsSee AlsoSAVF_plot_rho_error for plotting the rho squared error termsSAVF_score for computing the exponential single attribute value scoreExamples#Given the single attribute x is bounded between1and5and the subject matter experts #prefer x values of3,4,&5provide a utility score of.75,.90&1.0respectively,we #can search for a rho value between0-1that provides the best fit utility function: SAVF_preferred_rho(desired_x=c(3,4,5),desired_v=c(.75,.9,1),x_low=1,x_high=5,rho_low=0,rho_high=1)12SA VF_score SAVF_score Single attribute value functionDescriptionSAVF_score computes the exponential single attribute value score of xUsageSAVF_score(x,x_low,x_high,rho)Argumentsx Numeric vector of values to scorex_low Lower bound anchor point(can be different than min(x))x_high Upper bound anchor point(can be different than max(x))rho Exponential constant for the value functionValueA vector of the same length as x with the exponential single attribute value scoresSee AlsoSAVF_plot for plotting single attribute scoresSAVF_preferred_rho for identifying the preferred rhoExamples#The single attribute x is bounded between1and5and follows an exponential#utility curve with rho=.653x<-runif(10,1,5)x##[1]2.9648531.9631821.2239491.5620254.3814672.2860303.071066##[8]4.4708753.9209134.314907SAVF_score(x,x_low=1,x_high=5,rho=.653)##[1]0.78005560.50382750.14682340.33152170.96058560.61319440.8001003##[8]0.96731240.91896850.9553165%>%13 %>%Pipe functionsDescriptionLike dplyr,KraljicMatrix also uses the pipe function,%>%to turn function composition into a series of imperative statements.Argumentslhs,rhs An R object and a function to apply to itExamples#given the following\code{psc2}data setpsc2<-dplyr::mutate(psc,x_SAVF_score=SAVF_score(x_attribute,1,5,.653),y_SAVF_score=SAVF_score(y_attribute,1,10,.7))#you can use the pipe operator to re-write the following:kraljic_matrix(psc2,x_SAVF_score,y_SAVF_score)#aspsc2%>%kraljic_matrix(x_SAVF_score,y_SAVF_score)Index∗datasetspsc,8%>%,13geom_frontier,2,4get_frontier,3,3kraljic_matrix,4kraljic_quadrant,5MAVF_score,6,8MAVF_sensitivity,7,7psc,8SAVF_plot,9,12SAVF_plot_rho_error,9,10,11SAVF_preferred_rho,10,11,12SAVF_score,5–11,12stat_frontier(geom_frontier),214。
label matrix

label matrixLabel Matrix: An Ultimate GuideIntroductionIn the world of data analysis and machine learning, label matrix is an essential concept that plays a crucial role in various tasks such as classification, clustering, and supervised learning. This guide aims to delve into the depths of label matrix, explaining its definition, properties, and applications.What is a Label Matrix?A label matrix, also known as a target matrix or classification matrix, is a table-like data structure that represents the labels or categories to which data points belong. It is often used in supervised machine learning tasks, where the objective is to predict the label or category of unseen data based on a trained model.Properties of a Label Matrix1. Dimensions: A label matrix has two dimensions - rows and columns. The rows represent the unique data points or instances, while the columns represent the distinct labels or categories.2. Binary or Multiclass: A label matrix can be binary or multiclass, depending on the nature of the classification task. In binary classification, there are only two possible labels, often denoted as 0 or 1. On the other hand, multiclass classification involves more than two labels.3. Sparse or Dense: Label matrices can be sparse, meaning that a majority of the entries are empty or zero, or dense, where most of the entries have non-zero values. The sparsity of a label matrix depends on the distribution of the labels in the dataset.4. Class Imbalance: Class imbalance refers to the scenario where one or more labels have significantly more instances compared to others. This property is common in real-world datasets and can affect the model's performance. Handling class imbalance is crucial in machine learning tasks.Applications of Label Matrix1. Classification: The primary application of label matrix is in classification tasks. Given a labeled dataset, a model is trained using an algorithm such as logistic regression, support vector machines, or deep learning techniques. The label matrix is used during model training and evaluation, allowing the model to learn the relationship between the input features and the corresponding labels.2. Evaluation Metrics: Label matrix is essential in evaluating the performance of a classification model. Various evaluation metrics such as accuracy, precision, recall, and F1 score are calculated based on the values in the label matrix. These metrics provide insights into the model's predictive power and its ability to correctly classify different labels.3. Imbalanced Data Analysis: As mentioned earlier, label matrices often exhibit class imbalance. This property requires special attention to ensure the model performs well on minority classes. Various techniques such as oversampling, undersampling, and cost-sensitive learning can be applied to tackle class imbalance.4. Interpretation and Visualization: Label matrices can also be used for visualization and interpretation purposes.Techniques such as confusion matrices, heatmaps, and precision-recall curves can provide insights into the model's strengths and weaknesses in classifying different labels. These visualizations aid in identifying patterns and making informed decisions.Tips for Working with Label Matrices1. Preprocessing: Before working with label matrices, it is essential to preprocess the data. This may involve handling missing values, encoding categorical variables, and scaling numerical features. The quality of the label matrix greatly depends on the preprocessing steps performed.2. Model Selection: Choosing an appropriate model for a classification task is critical. Consider factors such as the dataset size, label imbalance, and the complexity of the problem. Different algorithms have their strengths and weaknesses, and it is crucial to select the one that suits the problem at hand.3. Cross-validation: To ensure the robustness of the model, it is advisable to use techniques like cross-validation. Cross-validation involves splitting the data into training and validation sets, allowing the model's performance to beevaluated on multiple partitions of the dataset. This technique helps in estimating the model's ability to generalize to unseen data.ConclusionLabel matrix is a fundamental concept in the field of data analysis and machine learning. It provides a structured representation of labels or categories associated with data points. Understanding the properties and applications of a label matrix is crucial for successful classification tasks, model evaluation, and handling class imbalance. By utilizing label matrix techniques, practitioners and researchers can enhance the accuracy and effectiveness of their machine learning models.。
高纯镁质量标准说明书

Page 1 of 2Product No.: 013841 Germanium Plasma StandardCertified Concentration of Ge: 1000 ± 6 µg/mL (978.0 ± 6.0 µg/g) Lot No.: 1245948Matrix: 5% HNO 3/tr. HFExpiry Date: November 30, 2024Intended Use: This solution is intended for use as a certified reference material or calibration standard for inductively coupled plasma optical emission spectroscopy (ICP-OES), inductively coupled plasma mass spectrometry (ICP-MS), flame or furnace atomic absorption spectroscopy (AA or GFAA), x-ray fluorescence spectroscopy (XRF), and other techniques for elemental analysis.Certification & Traceability: This CRM was manufactured and certified under an ISO 9001, ISO/IEC 17025, and ISO 17034 quality management system. This CRM was prepared to a nominal concentration of 1000 µg/mL by gravimetric methods using 99.999% pure germanium (Ge) dissolved in high purity nitric acid (HNO 3), trace hydrofluoric acid (HF) and diluted with filtered (0.22µm), 18 M-ohm deionized water. The balances used in the preparation of this CRM are calibrated regularly with traceability to NIST. All volumetric dilutions are performed in Class A calibrated glassware. The certified concentration and uncertainty were determined using the “High Performance ICP-OES” protocol developed by NIST and both the certified concentration and uncertainty values are traceable to NIST SRM 3120a, lot #151115. The uncertainty associated with the certified concentration represents the expanded uncertainty at the 95% confidence level using a coverage factor of k=2.Uncertified Values: ICP-MS was used to determine trace metal concentrations for this product (nd = not determined).Trace Concentrations (µg/L)Ag <0.5 Co <1 Ge MAJOR Lu <0.2 P <100 Sb 0.7 Te <1 Al <2 Cs <0.5 Hf 0.5 Mg <5 Pb <1 Sc <5 Ti 2 As 19 Cr <0.5 Hg <0.5 Mn <1 Pd 11 Se 2 Tl <0.5 Au <0.5 Cu 3 Ho <0.2 Mo 0.9 Pr <0.2 Si <100 Tm <0.2 B <5 Dy <0.2 In nd Na <25 Pt <0.5 Sm <0.2V <1 Ba <1 Er <0.2 Ir <0.2 Nb 2 Rb <0.5 Sn 5 W <0.5 Bi <0.2 Eu <0.2 K <25 Nd <0.2 Re <0.2 Sr 4Y 3 Ca <25 Fe <10 La <0.5 Ni <2 Rh <0.5 Ta 5 Yb <0.2 Cd <0.5 Ga <0.5 Li<2Os <0.5Ru <0.5Tb <0.5Zn 2Ce <0.2Gd <0.2Instructions for Use: We recommend that the solution be thoroughly mixed by repeated shaking or swirling of the bottle immediatelyprior to use. To achieve the highest accuracy the analyst should: (1) use only pre-cleaned containers and transferware, (2) not pipette directly from the CRM’s original container, (3) use a minimum sub-sample size of 500µL, (4) make dilutions using calibrated balances or certified volumetric class A flasks and pipettes, (5) dilute with the same matrix as the original CRM, and (6) never pour used product back into the original container. The solution should be kept tightly capped and stored under normal laboratory conditions. Do not freeze, heat, or expose to direct sunlight. Minimize exposure to moisture or high humidity.Period of Validity: Thermo Fisher Scientific guarantees the accuracy of this Specpure® solution until the expiry date shown above, provided the instructions for use are followed. During the period of validity, the purchaser will be notified if this product is recalled due to any significant changes in the stability of the solution.______9/23/2022______ Certification DateOrder our products online /chemicalsThis document has been electronically generated and does not require a signature.Page 2 of 2Hazard Information: Refer to the Material Safety Data Sheet (MSDS).Homogeneity: This solution was determined to be homogeneous by procedures consistent with the requirements of ISO 17034 and ISO Guide 35. Replicate samples of the finished solution were analyzed to confirm its homogeneity, in accordance with QSP 6-13 Assessment of Homogeneity and Stability. To ensure homogeneity, users should not take a smaller sub-sample than specified in the Instructions for Use, as doing so will invalidate the certified values and uncertainties.Further Information: Please contact Thermo Fisher Scientific for further information about this CRM.Quality Certifications: This CRM was prepared under a quality management system that is:• Registered to ISO 9001 – Quality Management Systems – Requirements (TUV NORD Cert. No. 44 100 16560231)• Accredited to ISO 17034 – General Requirements for the Competence of Reference Material Producers (A2LA Cert. No.2848.02)o ISO 17034 references additional requirements specified in ISO Guide 31 and ISO Guide 35• Accredited ISO/IEC 17025 – General Requirements for the Competence of Testing and Calibration Laboratories (A2LA Cert.No. 2848.01)Order our products online /chemicalsThis document has been electronically generated and does not require a signature.。
开启片剂完整性的窗户(中英文对照)
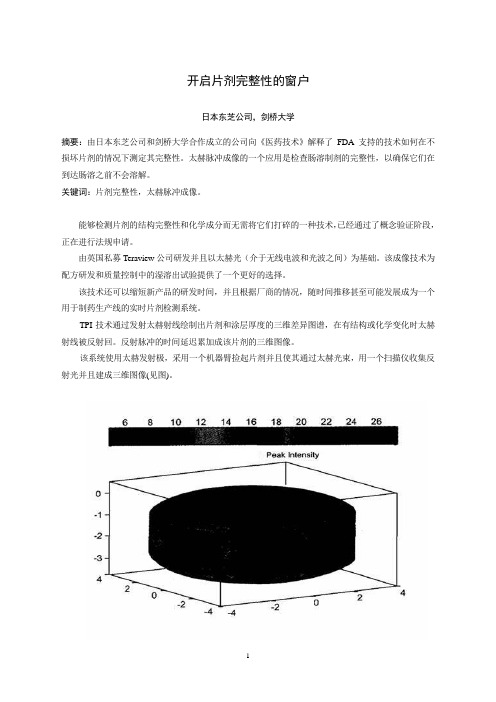
开启片剂完整性的窗户日本东芝公司,剑桥大学摘要:由日本东芝公司和剑桥大学合作成立的公司向《医药技术》解释了FDA支持的技术如何在不损坏片剂的情况下测定其完整性。
太赫脉冲成像的一个应用是检查肠溶制剂的完整性,以确保它们在到达肠溶之前不会溶解。
关键词:片剂完整性,太赫脉冲成像。
能够检测片剂的结构完整性和化学成分而无需将它们打碎的一种技术,已经通过了概念验证阶段,正在进行法规申请。
由英国私募Teraview公司研发并且以太赫光(介于无线电波和光波之间)为基础。
该成像技术为配方研发和质量控制中的湿溶出试验提供了一个更好的选择。
该技术还可以缩短新产品的研发时间,并且根据厂商的情况,随时间推移甚至可能发展成为一个用于制药生产线的实时片剂检测系统。
TPI技术通过发射太赫射线绘制出片剂和涂层厚度的三维差异图谱,在有结构或化学变化时太赫射线被反射回。
反射脉冲的时间延迟累加成该片剂的三维图像。
该系统使用太赫发射极,采用一个机器臂捡起片剂并且使其通过太赫光束,用一个扫描仪收集反射光并且建成三维图像(见图)。
技术研发太赫技术发源于二十世纪九十年代中期13本东芝公司位于英国的东芝欧洲研究中心,该中心与剑桥大学的物理学系有着密切的联系。
日本东芝公司当时正在研究新一代的半导体,研究的副产品是发现了这些半导体实际上是太赫光非常好的发射源和检测器。
二十世纪九十年代后期,日本东芝公司授权研究小组寻求该技术可能的应用,包括成像和化学传感光谱学,并与葛兰素史克和辉瑞以及其它公司建立了关系,以探讨其在制药业的应用。
虽然早期的结果表明该技术有前景,但日本东芝公司却不愿深入研究下去,原因是此应用与日本东芝公司在消费电子行业的任何业务兴趣都没有交叉。
这一决定的结果是研究中心的首席执行官DonArnone和剑桥桥大学物理学系的教授Michael Pepper先生于2001年成立了Teraview公司一作为研究中心的子公司。
TPI imaga 2000是第一个商品化太赫成像系统,该系统经优化用于成品片剂及其核心完整性和性能的无破坏检测。
MATRIX 120

MATRIX 120™APPLICATION• Electronics: Track and trace PCB board manufacturing• Factory Automation: Print & Apply – label verification• Factory Automation: Food & Beverage – traceability• OEM: Kiosks: ticketing machine• Healthcare: Clinical Lab – vials identification • Chemical and biomedical analysis machineHIGHLIGHTS• Ultra compact dimensions for easy integration • WVGA – 1.2 MP and wide angle models• Smart user selectable focus for high application flexibility• Digimarc Barcode reading technology for added value decoding applications • Embedded Ethernet connectivity• Serial and USB options on the same model • ESD versions for electronic applications• Polarized Version for 90° mounting and reflecting surfaces• Models equipped with shorter output cables (100mm of length) for easier integration in OEM machines • Top industrial grade: IP65; operating temperatures: 0-45 ºC / 32 – 133 ºF • DL.CODE software configurator for outstanding ease of setup• Xpress, Datalogic’s ‘Green Spot’ technology and intuitive HMI for top ease of useThe Matrix 120 is the smallest ultra-compact industrial 2D imager range in the market to fit any integration space and the smallest compact 2D imager with embedded Ethernet connectivity.The Matrix 120 is available in different models, including a WVGA sensor for standard applications or a 1.2 MP sensor for high resolution bar codes. Moreover, a wide angle version makes the Matrix 120 the perfect solution for proximity reading.The Matrix 120 with the red light model is the first stationaryindustrial scanner in the market able to read Digimarc Barcode for added value decoding applications.The Matrix 120 features the top industrial grade parts in its class (IP65 and 0-45 ºC / 32 – 133 ºF), with ESD safe models for applications in the electronic industry and a glass-free reading window, suitable for the Food and Beverage environment.Sulfur gas protection allows the use of Matrix 120 in tires applications through rough manufacturing, final finishing and inspection stations.As part of the full Matrix series, the Matrix 120 leads the market for customer ease of use because of DL.CODE™ configuration software, X-Press™ button and intuitive HMI.The Matrix 120 is the entry level model of the best-in-class Matrix family of high performance industrial 2D imagers.The Matrix 120 is the perfect solution when small dimension, simple integration and performance are the key drivers. The Matrix 120 models with shorter output cables (only 100 mm of length) allow an easier integration in OEM machines, making this product ideal for customers belonging to Chemical/Biomedical Industry and Print & Apply applications. Additionally, this imager is perfect for entry level applications in the Factory Automation arena: Electronics, Packaging and Food/Beverage.MATRIX 120™TECHNICAL DATAOPTICAL FEATURESSensor CMOS sensor with Global Shutter/WVGA - 752x480 pxCMOS sensor with Global Shutter/1.2 MP - 1280x960 pxFrame Rate up to 57 full-frame/s (WVGA model) , up to 36 full-frame/s(1.2 MP model)Illumination White Internal IlluminatorFocusing System Manual adjustment in three precalibrated positions(45, 70, 125mm - WVGA ; 45, 80, 125mm - 1.2 MP)DATALOGIC PRODUCT OFFERINGSafety Light Safety Laser Laser Marking Mobile ComputersVision SensorsHand Held MODELSCODE 937800000937800001937800002937800003937800004937800005。
TS16949英文版

General Motors Customer Specific Requirements - ISO/TS16949 1. ScopeISO/TS 16949:2002, Second Edition, March 1, 2002, “Quality management systems – Particular requirements for the application of ISO 9001:2000 for automotive production and relevant service part organizations,” and this document define General Motors fundamental quality system requirements for organizations where automotive customer-specified parts, for production and/or service are manufactured. To satisfy supplier quality system requirements, General Motors will accept, as optional to QS-9000, a third party certification to ISO/TS 16949 that meets the following conditions:• The certification scope must include both ISO/TS 16949 and the accompanying ISO/TS 16949 GM-Customer Specific Requirements,• The certification must be conducted in compliance with the IATF recognized automotive certification scheme by a certification body contracted and recognized by an IATFOversight office.NOTE: The Quality System Requirements, QS-9000, 3rd Edition (QS-9000:1998),expires on December 14, 2006.All ISO/TS 16949:2002 requirements and the requirements of this document shall be addressed in the organization’s quality management system.2. References2.1 DaimlerChrysler, Ford Motor, General Motors Quality System Requirements (QS-9000), Third Edition, March, 1998.2.2 DaimlerChrysler, Ford Motor, General Motors Production Part Approval ProcessPPAP), Third Edition, September, 1999.2.3 DaimlerChrysler, Ford Motor, General Motors Statistical Process Control (SPC),FirstEdition, 1992.2.4 DaimlerChrysler, Ford Motor, General Motors Advanced Product Quality Planning andControl Plan, June, 1994.2.5 DaimlerChrysler, Ford Motor, General Motors Measurement Systems Analysis, MSAThird Edition, March, 2002.2.6 DaimlerChrysler, Ford Motor, General Motors Potential Failure Mode and EffectsAnalysis, FMEA Third Edition, July 2001.2.7 IAF Guidance on the Application of ISO/IEC Guide 62:1996, December, 2001.2.8 IATF Guidance to ISO/TS 16949:2002, AIAG Edition, 2002.2.9 Automotivecertification scheme for ISO/TS 16949:2002, Rules for Achieving IATF Recognition, First Edition for ISO/TS 16949:2002, March, 2002.2.10 ISO/TS 16949:2002, 1st Edition, March 20022.11 ISO/TS 16949:1999, First Edition (2nd Printing), March, 1999.The latest edition of the reference documents listed applies unless otherwise specified by the GM Procuring Division. Copies of QS-9000, PPAP, APQP, FMEA, MSA, SPC, IATF Guidance, ISO/TS 16949 “Rules”, ISO/TS 16949 Checklist, ISO/TS 16949:1999, and other related manuals are available from AIAG at 1-248-358-3003. Copies of ISO documents are available from the American National Standards Institute (ANSI) at (212) 642-4980.The above references listed as requirements are described in section 4 of this document.3. DefinitionsWhere inconsistent terminology exists between ISO/TS 16949:2002 and this document, this document shall take precedence. Otherwise the definitions from ISO/TS 16949:2002 apply to this document.3.1 Accredited LaboratoryAccredited Laboratory is one that has been reviewed and approved by a nationally-recognized accreditation body, or as an alternative a customer recognized accreditation body, conforming to ISO/IEC Guide 58 for calibration or test laboratory accreditation to ISO/IEC Guide 17025, or national equivalent.NOTE: The above definition also applies to the QS-9000 reference manuals currently in effect.Part3.2 ActiveAn active part is one currently being supplied to the customer for original equipment or service applications. The part remains active until tooling scrap authorization is given by the appropriate customer activity. For parts with no customer-owned tooling or situations where multiple parts are made from the same tool, written confirmation from the customer Purchasing activity is required to deactivate a part.NOTE: For bulk material, “active part” refers to the bulk material contracted, not the parts that are subsequently produced from that material.Parts3.3 AftermarketReplacement parts not procured or released by OEM for service part applications which may or may not be produced to original equipment specifications.3.4 ConsultingFor the purposes of TS16949:2002, consulting is the provision of training, documentation development, or assistance with implementation of quality systems to a specific customer. If these activities are open to the public, advertised, and not customer specific, they are considered training rather than consulting. Other products, processes or services may be offered directly or indirectly, provided they do not compromise confidentiality or the objectivity or impartiality of its certification process or decisions (refer to IAF Guidance on the Application of ISO/IEC Guide 62, Issue 2, dated December, 2001.)]3.5 CustomerReferences to “customer” in ISO/TS 16949:2002 and this document shall be interpreted as the Procuring Division of General Motors for suppliers pursuing third party registration to ISO/TS 16949:2002 to satisfy General Motors sourcing requirements third party quality system assessment registration.3.6 ErgonomicsErgonomics is the evaluation of the design of a product or process to assure compatibility with the capabilities of human beings. Analysis of motion refers to capabilities of people with respect to tasks (e.g. lifting, twisting, reaching) to prevent or relieve problems of strain, stress, excessive fatigue, etc. Factors involved include anatomical dimensions of the worker, placement of products to be worked upon, placement of buttons/switches, physical loads imposed on the worker, and environmental effects such as noise, vibration, lighting and space.3.7 Initial Process StudyInitial Process Studies are short-term studies conducted to obtain early information on the performance of new or revised processes relative to internal or customer requirements. In many cases, preliminary studies should be conducted at several points in the evolution of new processes (e.g. at the equipment or tooling subcontractor’s plant, after installation at the supplier’s plant). These studies should be based on as many measures as possible. When utilizing X-Bar and R charts, at least twenty-five subgroups (minimum of four pieces per sub-group) are required to obtain sufficient data for decision-making. When this amount of data isnot available, control charts should be started with whatever data is available. See Production Part Approval Process manual.3.8 PPMPPM (parts per million) is a method of stating the performance of a process in terms of actual nonconforming material. PPM data can be used to prioritize corrective actions. Definition of defective units varies with customer (e.g. all sorted, only those found to be wrong, all in box). (Reference GP-5 Supplier Quality Processes and Measurements Procedure, GM1746 for additional PPM definition.)Indices3.9 QualitySee DaimlerChrysler, Ford, General Motors Statistical Process Control reference manual.3.10 OrganizationOrganizations are defined as providers of: a) production materials, b) production or service parts, or c) heat treating, plating, painting or other finishing services, directly to General Motors or other customers subscribing to this document.NOTE: In QS-9000, these providers are typically referred to as suppliers toDaimlerChrysler, Ford and General Motors however for the purpose of this documentthey are defined as the “organization” or “supply organization.” ISO/TS 16949:2002 (See also Section 3 Terms and definitions.)3.11 Service partsReplacement parts manufactured to OEM specifications, which are procured or released by the OEM for service part application.3.12SuppliersSuppliers (previously called subcontractors in QS-9000)are defined as providers of production materials, or production or service parts, directly to an organization provider of General Motors or other customers subscribing to this document. Also included are providers of heat-treating, painting, plating or other finishing services.3.13 Value-Added Production ProcessesActivities or operations for which a customer would be willing to pay, if given the option.See also ISO/TS 16949:2002, Second Edition (March, 2002), definition of “manufacturing” 3.1.6, “site” 3.1.11, and “remote location” 3.1.10.4. Requirements4.1 ISO TS 16949:2002 (Second Edition), March, 2002- Related RequirementsAll references to clauses in this section pertain to ISO/TS 16949:2002, unless otherwise stated. 4.1.1 Tooling ManagementThe requirements for tooling management (7.5.1.5) may not be applicable to warehouses or distributors as remote sites.4.1.2 Records RetentionProduction part approvals, tooling records, APQP records, purchase orders and amendments shall be maintained for the length of time that the part (or family of parts) is active (see Definitions 3.1) for production and service requirements plus one calendar year unless otherwise specified by the customer.NOTE: All customer purchase orders/amendments are included in this requirement.Organization purchase orders/amendments for customer-owned tooling are included in this requirement.Quality performance records (e.g. control charts, inspection and test results) shall be retained for one calendar year after the year in which they were created.Records of internal quality system audits and management review shall be retained for three years.Retention periods longer than those specified above may be specified by an organization in their procedures. The organization shall eventually dispose of records.These requirements do not supersede any regulatory requirements. All specified retention periods shall be considered “minimums”.4.1.3 Electronic CommunicationReference cl. 7.2.3.1NOTE: Examples of such systems for suppliers to GM’s North American Operations are:1) requirement planning information such as the Electronic Data Interchange (EDI) ANSIASC X12 830 transaction set or the EDIFACT DELFOR message, and 2) shippingschedules such as the ANSI ASC X12 862 or 866 transaction sets or the EDIFACTDELJIT message.4.1.4 Shipment Notification SystemReference cl. 7.2.3.1NOTE: Examples of such systems for suppliers to GM’s North American Operations are:1) the ANSI ASC X12 856 transaction set, or 2) the EDIFACT DESADV message. ForEDI assistance, contact 01-810-947-5566. For EDIFACT assistance, and confirmationof the required implementation date for a supplier, contact 01-248-265-9907.4.1.5 Special CharacteristicsThe supplier shall use General Motors Key Characteristic Designation System definitions and symbols to comply with ISO/TS 16949:2002 special characteristics requirements (e.g. cl.7.2.1.1), and as provided in 4.2.2, General Procedures and Other Requirements, and 4.2.2.11, Key Characteristic Designation System (KCDS), (GM 1805 QN) which defines GM’s approach to “special” characteristics.4.1.6 Design ChangesAll design changes, including those proposed by suppliers, shall have written customer approval, or waiver of such approval, prior to production implementation. See cl. 7.3.7 and 7.1.4. See also the Production Part Approval Process manual.For proprietary designs, impact on form, fit, function, performance, and/or durability shall be determined with the customer so that all effects can be properly evaluated.4.1.7 Official Language VersionThe English language version of ISO/TS 16949:2002 or QS-9000, 3rd Edition and related reference documents shall be the official version for purposes of third party registration. Sanctioned translations shall:• be for reference only,• reference the English language as the official version,• not contain ISO 9001:2000 text verbatim, and• include General Motors in the copyright statement.Any other language translations are not authorized.4.1.8 Part Approval ProcessThe supplier shall comply with the Chrysler, Ford, GM Production Part Approval Process (PPAP) manual to comply with cl. 7.3.6.31. PPAP-Vehicle Assembly Centers (Assembly Plants)Unless otherwise specified by the Customer, PPAP requirements for vehicle assemblycenters shall be taken from a specified production run of saleable pilot vehicles.4.1.9 Customer SatisfactionTrends in quality system performance and customer satisfaction (see Cl. 5.2, 5.6.1.1, 7.4.3.2, and 8.2.1.1) should be compared to those of competitors, or appropriate benchmarks, and reviewed by top management.4.1.10 Internal Auditor QualificationsInternal auditors should be qualified as recommended in ISO 19011, 1st Edition – Sections7.1-7.5, for Quality Management Systems application. In addition internal auditors should be competent in understanding and applying the Process Approach of Auditing (See “Process Approach”, Section 0.2 of ISO/TS 16949:2002), Core Tools (e.g. reference manuals including PPAP, APQP, MSA, SPC, and FMEA) as applicable, and GM Customer Specifics, as applicable.NOTE: A process and plan with implementation monitoring to assure qualified internal auditors is evidence of compliance.4.1.11 Supplier Quality Management System Development (cl. 7.4.1.2)Note: This supplier development clause, cl. 4.1.2, applies to suppliers of the organization who are providers of production materials, or production or service parts, directly to a supplier to Chrysler, Ford, General Motors or other customers subscribing to this document. Also included are providers of heat-treating, painting, plating or other finishing services.Indirect and service providers are not included in this requirement, e.g. distributors adding no manufacturing value, logistics, sequencers, parts packagers, tooling & equipment.Note: The use of customer-designated suppliers to the organization (subcontractors) does not relieve the supplier of the responsibility for ensuring the quality of subcontracted parts, materials and services.4.1.11.1 Customer acceptance of QS-9000:1998Registration to QS-9000:1998, (QS-9000, 3rd Edition) shall be accepted as an alternative to registration to ISO 9001:2000.4.1.11.2 Customer acceptance of 2nd Party Audits and Criteria for Approval General Motors Corporation will recognize 2nd Party audits as compliance to ISO/TS 16949:2002, Clause 7.4.1.2 and as an alternative to ISO 9001:2000 certification. The statement of authorization below provides the requirements and conditions for GM approval.A supply organization that utilizes 2nd party assessment to comply with clause 7.4.1.2 is required by General Motors to utilize second party assessors who satisfy all elements of the criteria specified as “GM approved 2nd Party requirements” stated below.GM-approved 2nd Party requirements:1. The supply organization (2nd Party) must be ISO/TS 16949 certified and registered bythe IATF.2. The supply organization (2nd Party) cannot be on ISO/TS 16949 probation or suspension.3. The supply organization (2nd Party) must utilize a qualified ISO Lead Auditor, or aqualified Internal Auditor with evidence of their successful completion of training, such as AIAG "Internal Auditing for ISO/TS 16949," or evidence of a minimum of five internal ISO/TS 16949 audits under the supervision of a qualified Lead Auditor.4. The supply organization (2nd Party) must audit annually each qualifying subcontractor for whom it has performed a 2nd Party assessment, and maintain records of these audits.5. The duration of these audits must conform to the full application of the Audit Day Requirements table of the current edition of “Automotive Certification Scheme for ISO/TS 16949:2002, Rules for achieving IATF recognition”.6. Any of the IATF recognized and currently approved auditors may perform such audits when contracted by the supply organization.4.1.11.3 Supplier Development of Specially Designated Small Suppliers When a supplier (subcontractor) to an organization is so small as to not have adequate resources to develop a system according to ISO/TS 16949:2002 or ISO 9001:2000 certain specified elements may be waived by the organization of their supplier. The organization shall have decision criteria in writing, approved by the customer and applied consistently to determine the specially designated suppliers for which this provision may apply.Note: ISO 9001:2000 and ISO/TS 16949:2002 contain fundamental quality system requirements of value to any size of provider of production/ service parts/ materials. There are a number of methods to implement a compliant system, so it is recognized that a simpler Quality Management System approach could be used for the smaller suppliers of organizations to which ISO/TS 16949, clause 7.4.1.2 applies.4.2 General Motors - Specific Requirements4.2.1 Third-Party Registration RequirementsProduction and Service Part Suppliers to General Motors, including GM Holdens, shall be third-party registered to ISO/TS 16949:2002, including the requirements in this document, by an IATF-recognized certification body using the current edition in effect of the automotive registration scheme, “Automotive Certification Scheme for ISO/TS 16949:2002, Rules for achieving IATF recognition.” In the alternative, supply organizations for which certification applies, may satisfy General Motors third party registration requirements by obtaining certification to ISO/TS 16949:1999 by an IATF recognized certification body in accordance with the appropriate and current “Rules” for certification until December 15, 2003, or to QS-9000:1998 by an automotive registration scheme recognized by General Motors until December 14, 2006. Such certification shall include the requirements in this document, or in the case of QS-9000:1998, the General Motors-Specific Requirements.NOTE 1: Supply organizations to General Motors certified to ISO/TS 16949:1999 may upgrade certification to ISO/TS 16949:2002 for the period of up to one year after 15 December 2003, consistent with the surveillance cycle.NOTE 2: Supply organizations to General Motors who fit the applicability requirements of ISO/TS 16949:2002 and are not certified to ISO/TS 16949:2002 by 14 December 2006, at a minimum, are subject to New Business Hold – Quality status. See also 4.2.3, ISO/TS 16949:2002 Applicability, and 4.2.8, Certification Body Notification and Certification – New Business Hold-Quality.NOTE 3: Waiver of supply organization certification for those organizations who meet the applicability requirements of ISO/TS 16949:2002 is not permitted unless approved in writing by the following: GM North America - General Motors Group Manager, Global Supplier Quality and Development, GM Europe - Exec. Dir Supplier Quality and Readiness, GM Asia Pacific – Director, Supplier Quality/Development, GM LAAM – Director of Supplier Quality Engineering. 4.2.2 General Procedures and Other RequirementsThe GM publications listed below contain additional requirements or guidance that shall be met, if applicable, by GM supply organizations, or unless otherwise specified by GM Procuring Divisions. Specific questions on the content of these publications should be directed to the appropriate contact at the GM Procuring Division. (The latest revisions for these documents can be found on the GM SupplyPower website.)GM Supply Organizations shall verify annually that they are using the latest version of these documents:4.2.2.1 Pre-Production/Pilot Material Shipping Procedures, (GM 1407).4.2.2.2 Supplier Submission of Match Check Material, (GM 1689)..4.2.2.3 Shipping Parts Identification Label Standard, (GM 1724).4.2.2.4 Component Verification & Traceability Procedure, (GM 1730).Note: APPLICABILITY OF GM 1730 IS LIMITED TO GM POWERTRAIN.4.2.2.5 Traceability Identifier Equipment (TIR 15-300), (GM 1731).4.2.2.6 Bar Code Standard for Part/Component/Module Identification and Traceability(GM 1737).4.2.2.7 Supplier Quality Processes and Measurements Procedure, (GM 1746).4.2.2.8 Continuous Improvement Procedure, (GM 1747).4.2.2.9 GP-10 Evaluation and Accreditation Test Facilities, (GM 1796/A).- See ISO/TS 16949:2002, cl., 7.6.34.2.2.10 Shipping and Delivery Performance Requirements, (GM 1797).4.2.2.11 Key Characteristic Designation System (KCDS),(GM 1805 QN).4.2.2.12 GP-11 General Procedure for Pre-Prototype and Prototype Material, (GM 1820).4.2.2.13 C4 Technology Program, GM - Supplier C4 Information, (GM 1825). .4.2.2.14 GP-12 Early Production Containment Procedure, (GM 1920).4.2.2.15 Run-at-Rate Procedure, (GM 1960).NOTE: Access the GM SupplyPower web-site for the current document version.4.2.3 ISO/TS 16949:2002 ApplicabilityISO/TS 16949:2002 with this document applies to all applicable contracted GM supply organizations (see Definitions 3.9) utilizing ISO/TS 16949 to satisfy General Motors third party certification requirements for quality system assessment.NOTE: QS-9000:1998 (3rd Edition) expires December 14, 2006, and QS-9000 certified supply organizations are strongly urged to upgrade to ISO/TS 16949:2002. In addition, supply organizations certified to ISO/TS 16949:1999 are strongly urged to upgrade to ISO/TS 16949:2002 before 15 December 2003, but no later than 15 December 2004 consistent with the surveillance cycle in effect or upon expiration of their current certificate whichever occurs first.4.2.4 UPC Labeling For Commercial Service ApplicationsGM Service Parts Operations (SPO) requires use of UPC labeling for certain commercial applications rather than AIAG labeling. Contact your SPO buyer for instructions.4.2.5 Layout Inspection and Functional TestUnless specified otherwise by a GM Procuring Division, there is no customer-established frequency for layout inspection after receiving production part approval (PPAP). Reference is made to ISO/TS 16949:2002, cl..8.2.4.14.2.6 Customer Signature on Control PlanGeneral Motors does not provide waivers to suppliers for control plan approval because General Motors signatures on the Control Plan are not required.4.2.7 GM Holdens-Specific RequirementsPreviously listed specific requirements for additional documents for GM Holdens in Australia are obsolete. GM Holdens operates in accordance with GM Customer Specifics.4.2.8 Certification Body Notification and Certification Status – “New Business Hold – Quality”The organization shall notify its Certification Body within 5 business days after being placed in GM New Business Hold – Quality. The status of “New Business Hold – Quality” shall be a violation of clause 8.2.1.1 Customer satisfaction – Supplemental.The certification of the organization shall be placed on immediate probation * by the certification body of record upon receiving notice of GM “New Business Hold – Quality.”*See Annex 4, Automotive Certification Scheme for ISO/TS 16949:2002, Rules forachieving IATF recognition.”1. In the event of certification probation as a result of an organization receiving notice ofGeneral Motors “New Business Hold – Quality,” the organization shall complete a corrective action plan. The supplier shall submit the corrective action plan to the Certification Body of record and to the affected customer(s) within 10 business days of the date of the letter ofnotification of probation. The corrective action plan of the organization shall be consistent with the affected customer(s) requirements including correction steps, responsibilities, timing information, and key metrics to identify effectiveness of the action plan.2. Before any probation can be lifted, the Certification Body of record will conduct an on-siteassessment of appropriate length to verify effective implementation of all corrective actions. 3. If probation is not lifted within four months of its issuance, the Certification Body of recordshall revoke the ISO/TS 16949 certificate of the organization. Exceptions to this revocation shall be justified in writing by the Certification Body based upon its on-site review of theorganization’s corrective action plan effectiveness and agreement obtained from the affected GM customer(s).NOTE 1: The permitted probation period for General Motors Europe (GME) is six (6) months.NOTE 2: The GM special supplier status conditions of CS I (Controlled Shipping – Level I), or CS II (Controlled Shipping – Level II) are performance indicators of organization product realization problems. Such status should have resolution, or credible resolution andcorrective plans in place, which are confirmed by the customer.4.2.9 Similar RequirementsWhere similar requirements are contained in both QS-9000:1998 and ISO/TS 16949:2002, the requirements in ISO/TS 16949:2002 take precedence for suppliers choosing to use ISO/TS 16949:2002 rather than QS-9000.4.2.10 Management ReviewManagement review of quality system performance (Cl. 5.6.1.1) at a minimum shall be conducted at planned intervals, but not less than annually.。
SNP说明书

Matrix Standard DS-02/3130 and 3100 Series SystemsProduct P/N 4323014Insert P/N 4363121 REV A Printed in USA For Research Use Only. Not for use in diagnostic procedures.Dye-labeled oligonucleotides included in the Matrix Standard Set DS-02 [dR110, dR6G, dTAMRA, dROX, and LIZ] are used to generate the "multicomponent matrix" required for the SNaPshot ® Multiplex Kit on the Applied Biosystems 3130 and 3100 Series Systems. The Data Collection software utilizes the multicomponent matrix to automatically correct for the spectral overlap in samples labeled with DS-02 dyes.Matrix standards do not need to be run with every set of sample injections. A set of five matrix standards only needs to be run once in order to generate a matrix file which is then applied to samples run under similar conditions. For more information on the use of matrix standards, refer to the instrument User's Manual or Getting Started Guide.The kit consists of one tube of matrix standard which is sufficient for eight 16-capillary array runs. This tube contains a mixture of DNA fragments of specific sizes, each labeled with a unique fluorescent dye within the DS-02 dye set. The standards are diluted in 1x TE buffer and are stable for one year when stored at 2°C to 8°C. (Do not freeze.)Preparing the Matrix Standard Set DS-02 for the Applied Biosystems 3130 and 3100 Series Systems:WARNING! CHEMICAL HAZARD. Hi-Di™ Formamide. Exposure causes eye, skin, and respiratory tract irritation. It is a possible developmental and birth defect hazard. Read the MSDS, and follow the handling instructions. Wear appropriate protective eyewear, clothing, and gloves.1. Thoroughly mix the contents of the Matrix Standard Set DS-02 tube and spin briefly in a microcentrifuge.2. Prepare the Matrix Standard by combining 5 µL of the tube labeled "Matrix Standard DS-02 for the 3130 and 3100 Series system" supplied in the kit and 195 µL of Hi-Di™ Formamide (P/N 4311320) in a 1.5 mL microcentrifuge tube.3. Mix thoroughly and spin briefly in a microcentrifuge.4. Denature:For convenience, we recommend dispensing the contents of the tube into a 96-well microtiter plate first, and then using a GeneAmp ® PCR System 9600 thermal cycler for denaturation.If a GeneAmp 9600/9700 thermal cycler is available for denaturation, follow steps A and B below.A) Dispense 10 µL of the Matrix Standard / Hi-Di™ Formamide mixture into two columns (16 wells) of a 96-well microtiter plate.B) Cover the plate and denature at 95°C for 5 minutes. Immediately place on ice.ORIf a GeneAmp 9600/9700 thermal cycler is not available, follow steps C and D below.C) Heat the mixture at 95°C for 5 minutes to denature, and immediately place on ice.D) Dispense 10 µL of the denatured mixture into two columns (16 wells) of a 96-well microtiter plate.5. Place the 96-well microtiter plate on the instrument.6. Refer to your User's Manual or Getting Started Guide for instructions on running samples.Note: In the spectral display window on some versions of Data Collection software, the LIZ dye is displayed in a light blue color instead of the orange color used in ABI P RISM GeneScan ® or Gene Mapper™ software. If the LIZ dye is displayed in light blue, the color will not cause any problems with data output or analysis. If you would like to change the color to orange, simply open the "Instrument" menu within the menu bar of the "Run View" window, select "Data Acquisition" and then select "Set Color." The "Edit Dye Display Information" window will automatically open. Click on the fifth dye color tile. Then select an orange color of your choice on the color wheel and click ok.©Copyright 2004. Applied Biosystems All rights reserved. ABI PRISM, Applied Biosystems, GeneScan, LIZ, and SNaPshot are registered trademarks and Gene Mapper, Hi-Di, ROX, and TAMRA aretrademarks of Applera Corporation or its subsidiaries in the U.S. and/or certain other countries.Product Insert 8/Dec/2004。
7500 1.4中文说明书 基因鉴定
Part Number 4347964 Rev. B 3/2004
ii
7300/7500 实时定ቤተ መጻሕፍቲ ባይዱ PCR 仪等位基因鉴别实验入门指南
3 30, 2004 8:25 œ¬ŒÁ, AD_GSG_FrontCover.fm
þæ¿″Œƒµµ
ķȉ
జ1
1 ˫ǎ 7300/7500 PCR ˫ǎķȉ ደ ˫ǎķȉ ደ ҔᏄ ˫ǎķȉ ደ 7ҔᏄ
ྜྷඡᒎฉ
等位基因鉴别实验
美国应用生物系统公司 7300/7500 实时定量 PCR 仪
简介和示例实验
设计等位基因 鉴别实验
设定反应板
读取等位基因鉴 别实验扩增前 信号
生成 扩增数据
读取等位基因鉴 别实验扩增后 信号
© Copyright 2004, Applied Biosystems. All rights reserved. For Internal Use Only. For Research Use Only. Not for use in diagnostic procedures.
第2章
设计等位基因鉴别实验
13
工作流程 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .13 使用 TaqMan® 探针法的序列检测化学试剂 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .14 选择探针和引物 . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . . .15
品牌活化矩阵 英语

品牌活化矩阵英语Brand Activation Matrix.In the dynamic and ever-evolving world of marketing, the concept of brand activation has become increasingly important. A brand, after all, is not just a logo or a slogan; it's a promise to consumers, a representation of values, and a reflection of the company's mission and vision. The Brand Activation Matrix is a strategic framework that helps organizations identify, prioritize, and execute on the various touchpoints that contribute to activating and rejuvenating a brand.At the heart of the Brand Activation Matrix lies the understanding that a brand exists not just in the minds of consumers but also in the experiences they have with it. These experiences can be physical, emotional, or cognitive, and they occur across a range of touchpoints, including advertising, packaging, product usage, customer service, and social media interactions.The matrix is typically structured around four key axes: reach, frequency, engagement, and impact. By plotting brand activations along these axes, companies can gain a holistic view of their branding efforts and identify areas wherethey might be falling short.Reach represents the number of people who are exposedto a brand activation. This could be through traditional media like television or radio, or digital channels like social media or search engines. The goal is to ensure that the brand message reaches as wide an audience as possible.Frequency refers to how often consumers are exposed toa brand. High frequency can build familiarity and trust,but it can also lead to burnout if not carefully managed. The key is to strike a balance between being omnipresentand overbearing.Engagement measures the level of interaction consumers have with a brand. This could be through likes, shares, comments, or even purchases. Engagement is crucial becauseit turns passive consumers into active brand ambassadors.Impact is a measure of how effectively a brand activation achieves its desired outcome. This could be increasing brand awareness, driving sales, or building brand loyalty. Impact is often the hardest to measure butis ultimately the most important metric.By plotting activations on this matrix, companies can identify which activations are most effective at reaching their target audience, engaging them, and driving the desired impact. They can also use the matrix to prioritize investments, focusing resources on those activations that offer the greatest potential for return.Moreover, the Brand Activation Matrix is not static; it evolves as consumer behavior and market conditions change. Companies must regularly review and update their matrix to ensure that their branding strategies remain relevant and effective.In conclusion, the Brand Activation Matrix is apowerful tool that helps companies activate and rejuvenate their brands. By understanding and leveraging the reach, frequency, engagement, and impact of their activations, companies can build stronger, more resilient brands that resonate with their target audience and drive business success.。
fabric-quality-manual

Matrix SourcingMaterial Quality ManualThis manual is provided to understand Fabric inspection process and requirements to deliver the highest possible quality as well as report all test results as per Customer requirements.Although this manual describes Matrix Sourcing quality requirements and we also expects its partner suppliers to develop a positive culture of quality. This culture must begin with management commitment to prevention-based and continuous improvement- based quality systems including:∙Allocating appropriate resources to ensure quality systems are effective∙Encouraging active participation of all workers in improvement efforts∙Ensuring that processes are stable, capable, and centered through the application of statistical data analysis techniques∙Documenting systems, processes, procedures, etc. as necessary to maintain effective operationsQuality Mission Statement“Create consumer loyalty and lead brand integrity through an integratedQuality Strategy.”Matrix Sourcing Quality philosophy is based on the following principles:∙Establish clear, consumer-focused requirements∙Plan for Defect-Free performance∙Quality happens within every Process (each supplier, department, & individual owns quality)∙Ensure the Process is capable of meeting the Customer requirements∙Control the Process∙Continually reduce Variation and factory Defective RatePrevention System Definitions:Design QualificationSystems and procedures where Matrix’s product engineering team works with customer design/development groups to help identify and prevent failure modes in product design.Supplier QualificationProcess of evaluating and approving suppliers to ensure they are capable of achieving Matrix Sourcing requirements, and include a review of suppliers’ quality manufacturing and management systems against our requirements and world-class benchmarks.Materials QualificationProcess of testing, evaluating and approving raw material to ensure that the manufacturing process is capable of producing material that always meets World class customer specificationsProduct QualificationProcess of evaluating and approving finished goods to ensure that the manufacturing process is capable of producing product that always meet specifications.Metrics & Strategic ReportingProcess of collecting vendor product quality performance data to track performance and set priorities for corrective action.Greige Fabric Quality RequirementsIntroduction:We will provide a guideline to the factory for evaluating, grading and separating the incoming quality of fabric to be used for Matrix Sourcing Clients. This is important for the vendors to note on what are the expectations for incoming fabric quality. This process also assists factories in detecting defects and preventing substandard fabric. We have described widely recognized inspection procedure as a training framework for inspectors. Our ultimate goal is to promote communication and align procedures between the factory and the vendor to resolve quality problems.Inspection RequirementsAreaThe inspection area should be:∙Open, clean and dry ∙ Well litEquipment ∙ Inspection Frame: The frame should be equipped with a variable speed drive, a yard (or meter) counter, and an undercarriage light to see through the construction and an overhead light to inspect the face of the fabric.∙ Inspection Speed: The frame should be capable of running up to 30 yards per minute (27 meters per minute) and should have both forward and reverse controls.∙ Viewing Distance: The inspection should be performed from an observation distance of 2 to 4 feet (60 to 120 centimeters) so as to get full vision of the fabric width.∙Lighting: Overhead Customer required lighting is recommended for the inspection. The surface illumination level should be a minimum of 1075 lux (100 foot candles).Variable speed Undercarriage lighting with adjustableYard / MeterCalibration and Maintenance∙Inspection frame should be included in vendor’s periodic maintenance plan. ∙ All components of equipment which can output a quantifiably value needs to becalibrated annually e.g. Speed drive/ Counter/ Weight (if scales available on frame)Light Box and Color RoomEach Vendor should have an International light box equipped with:Light Source Light TypePrimary Light Source D 65 (6500K) Average North Sky DaylightSecondary Light Source CWF (4150K) Tertiary Light Source A (2856 K) TungstenThe Light box must be in a closed conditioned room with no interference from outside light and it must be calibrated from time to time as recommended by its manufacturer. The walls of the room should be painted neutral gray .Light box in a conditioned room, or protected area with black curtains.Note: Suggested Light Box brands are:∙Verivide CAC ∙MacBeth Judge II ∙MacBeth Spectra light III ∙Datacolor TruVueTools∙Measuring Tape∙Pick Glass∙Scissors∙Defect Stickers / Tags∙Selvedge Defect Flags∙Inspection Report FormsApproved Item SwatchThe vendor is required to use the Matrix Sourcing approved Fabric swatch in development stage as a quality reference sample for hand-feel/ Color and aesthetics verifications in bulk production.This approved item swatch must be properly stored for use in quality comparison. Keep a clean sample folder with appropriate information such as vendor name, item number, color name, date approved, Approved test report, Production SPEC, and any other relevant information.Inspection Procedure / ExecutionMatrix Sourcing expects the vendor to inspect 100% of all outgoing shipments, identify, label and records defects and ship only first quality materials.The vendor should be familiar with Matrix Sourcing inspection procedures and standards to ensure that vendor’s internal inspection is capable of at least meeting or better exceeding Matrix Sourcing expectations.Color and AestheticsCut a head-middle-end sample across the width of the fabric, preferably about 2 yards inside of each dye-lot. This sample will be used for color and aesthetic verification toensure lot to lot, head to end fabric consistency.Steel or reinforced fiberglass 1/4” tapemeasure (cloth not recommended)Scissors Defect stickersColor Evaluation1. Color Standard: Check the head-middle-end sample in the light box against theCustomer color standard and/or Matrix Sourcing approved lab-dip under primary & secondary light source. This process should be done once for each color inspected.2. Shading within the roll: Check for shading at the beginning, in the middle and atthe end of each roll inspected, using the head-middle-end sample for comparison.∙Side to center∙Side to side∙End to end3. Color Continuity Card: Check for color consistency between the dye-lots. Cut asmall sample (about 2 x 2 inches or 5 x 5 centimeters) from the left side, the center and the right side of the head-middle-end sample of each inspected dye-lot and attach to the color continuity card. Ensure that cards are labeled. Check the samples against the Customer color standard &/or Matrix Sourcing approvedlab-dip. Note any significant variation of shade from roll to roll.Aesthetics EvaluationInspect overall hand-feel and appearance against customer approved item swatch indevelopment stage for cleanliness, texture, recovery, drape, resilience, wrinkling, etc.If the aesthetics does not match the sample, Vendor will reprocess that yards or will submit to Matrix/ Customer for approval.It is the vendor’s responsibility to ensure that the reference sample provided to the factory is the original. This sample will be used by the factory for, hand-feel and aesthetics verification..The reference Sample size is 12 x 12 inches (30 x 30 centimeters) cut out from the approved batch. This reference sample must be properly stored for use in qualitycomparison. Keep a clean sample folder with appropriate information such as vendorname, item number, color name/code, date, batch number, and any other relevantinformation.Fabric Reference SampleVisual InspectionOnce the color and aesthetics have been checked and approved, begin the visual inspection for defects. Defects which are not desirable and are clearly detectable on the inspection frame will be assigned points as shown in the Four Point Table. The Four Point system will be applied to all fabrics, i.e. woven, circular knit and warp knit.Always inspect the side which will be used as the face in the finished garment. Mount the roll on the inspection frame with the face side up.Fabric WidthMeasure the fabric width at the beginning, in the middle, and at the end of every roll. If a variation in fabric width is found, the vendor will contact Matrix Sourcing to determine if the fabric is usable and if a replacement is necessary. At this point, the inspector should also check the condition of the selvedges. The selvedges should as per customer requirement, lay flat, be free of tension, and should not have a tendency to curl.Roll LengthMeasure and record the length of each roll inspected.Inspection for DefectsThe Vendor should train and/or conduct internal certification for the inspectors according to this Manual and Matrix Sourcing requirements. The Vendor inspectors should inspect each material type based on Four Point System before shipped.~ Four Point System ~A widely recognized inspection method frequently used in the textile industry. It is a technique issued by the American Society for Testing & Materials with reference to the designation ASTM D5 430-93Start the frame and inspect for defects. Run the frame at 20 ± 5 yards per minute (18 ± 5 meters per minute). If a defect is observed:1. Faults are classified and scored with penalty points of 1, 2, 3 and 4, according to theirsize and significance. Each defect should be counted as a separate defect even if the nature is the same and then based on the points allocated to each defect, total pointsshould be calculated.~ Four Point Table ~Vertical Defects Horizontal Defects(along the length) (along the width)Length Points Length Points0.1 to 3.0 in (0.1 to 8.0 cm) 10.1 to 3.0 in (0.1 to 8.0cm)13.1 to 6.0 in (8.1 to 15.0 cm) 23.1 to 6.0 in (8.1 to15.0 cm)26.1 to 9.0 in (15.1 to 23.0 cm) 36.1 to 9.0 in (15.1 to23.0 cm)39.1 to 36.0 in (23.1 to 92.0 cm) 4 9.1 to full width (above 23.1 cm) 4Note:∙ A maximum of 4 points may be assigned to any one linear yard), regardless of the number or size of the individual defects.∙For a continuous lengthwise running defect, 4 points and 1 defect will be assigned to each linear yard where the defect exceeds 9 inches (23 centimeters). Example: Short End/ barre effect on the full roll in 100linear yards: 400 points should be assigned with 100 defects2. Mark the defect at the selvedge and/or at the defect.3.Record the defect and the assigned points on the inspection worksheet.Inspection ResultsComplete the worksheet and calculate the total points of each inspected roll and the total of the inspected linear yards/meters. With this information use the appropriate formula below to calculate the Average Points (per 100 linear yards or linear meters).Points/100 yd2 = Total Points of inspected roll x 36 X 100Total Inspected Yards x Fabric Width in inchesEvaluation GuidelineFactory will evaluate a vendor’s quality performance based on the point count system, using the following guideline:Fabric Type Allowable Points per 100 linear yardsWoven Fabric 20Warp Knit Fabric 20Knit Fabrics (Open width Or Tubular) 20Note: This criterion is subject to change at Matrix Sourcing/Customer discretion.D.2 Accept / Reject ProcedureVendor should not ship any roll or shipment with defect points exceed the above evaluation guideline. The factory will determine how the shipment can best meet Matrix Sourcing/ Customer standards.However, vendor might communicate with Matrix Sourcing prior to the shipment if the average points per 100 linear yards (90 linear meters) of any roll shows a higher number than allowed on the Matrix Sourcing evaluation guideline above. In this case, the Matrix Sourcing & Customer may decide to use a shipment or the roll even if the average points per 100 linear yards (90 linear meters) shows a higher number than allowed. .D.3 ReportingThe inspection is intended to determine the shipment quality and to capture information for quality performance evaluation of the vendor. One inspection report should be completed for each Item / color inspected and send a copy of the Inspection Report to garment factory prior to or with shipment.Inspection Report information containing inspection date / shipped date / season year / buy month / garment factory / order number / item number / batch number / dye lot number / color code and color name / inspection points / inspection results / yardages / weight / width … etc. should be clearly visible on documentation with English language at least. Records should be kept at least 2 years and available to Matrix/ customer upon request.We expects all material suppliers to follow the same guidelines to ensure quality product delivered to the factories,In next section, we will continue the classification and description on fabric defects.C. Fabric Defect ClassificationsDefect Classifications for FabricDefect DescriptionSlub , Uneven Yarn (thick & thin) Thick uneven spot(s) in the fabric caused by lint or small lengths of yarn adhering to itBarré Flaw in fabric consisting of textured or color bars in thedirection of warp or filing which can be caused byimperfection in the yarn or in the construction or finishing ofthe fabric.Yarn contamination / Fly Foreign fibers or soil, woven or knitted in the fabric.Dead cotton Damaged cotton by the weather conditions, over or undermature cotton, which is difficult to process, leaving behindwhite or black spots.Missing Line Filling yarn broken when weaving / the harness misdrawresults in two ends weaving as one, caused by one end ofyarn missing from feed and machine continue to run. Holes Missing yarn, leaving behind a space, caused by brokenneedleReed mark Running lines in the warp direction, caused by bent reedwire causing warp ends to be held apart.Streaks Dark of light uneven lines, caused by faulty processing Stop marks Lines in the weft direction, caused when machine stopped ,the yarn elongates the tension results in making across thewidthKnots Uneven raised knot, two yarn ends are tied togetherMiss weave/miss-knit pattern Pattern that different to the other area/ stitches failed toform due to a malfunctioning needle or jackPuckered Selvage Uneven surface, caused by bent needle forming distortedstitches , usually a vertical lineSelvage Torn A break in the yarn of a knit fabric that causes the stitch to"run" along the needle line or a void caused by a missingwarp yarnPilling Fiber gathering in the form of a bead on the surface as aresult of friction caused by abrasion with other surfacecaused by yarn qualityShading (selvage to selvage) A change in shade either abrupt or gradual, caused by poorprocessingDye Streaks Uneven steaks occurred during dyeing or finishingColor smear Uneven color application as the result of color beingsmeared during printingCrease Streak Uneven marks showing light or dark lines as a results fromcreased fabric passing through squeeze rollers in dyeingprocessSlippage Uneven blotch marks caused due to improper dyeingprocessBowing Woven filling yarns lie in an arc across fabric width; in knitsthe course lines lie in an arch across width of goods. Skewing/Bias Condition where filling yarns are not square with warpyarns on woven fabrics or where courses are not squarewith walse lines on knitsDefect DescriptionCrease marks/Wrinkles/Fold Marks appears where creases are caused by fabric folds under pressure in the finishing processPin holes Holes along selvage caused by pins holding fabric while itprocesses through tender frame. – Major, if pin holesextend into body of fabric far enough to be visible in thefinished productSnagging A break, tear or pull in the fabric, caused by a pulled threadin the fabric/yarn pulled out from the fabricAbrasion Mark Improper scratch during finishingDirt/Soil/stain Fabric got dirty spots when finishingOil Spots / grease spots An oil spot on the fabricWater Spots / Water marks Light marks, usually caused by wet fabric being allowed toremain too long before drying; color migrates leavingblotchy spots.Yellowing of fabric This is a phenomenon which causes light colored fabric toyellow over timePrinter Machine stop Dye smudged along width of fabric as a result of stop theprinting machine stoppingPrint Out of register Print is out of fit, caused by print rollers not beingsynchronized properly; results in various colors of thedesign not printed in proper positionMiss print Missing color in the pattern caused by color feedingstoppage or faulty printingD: Summary of Production Fabric Testing & InspectionMaterial Quality Process is the process developed to measure vendor performance. In this process, both vendors and factories are required to take steps to ensure first quality fabric reaches the cutting table to manufacture ultimate garment products. However to emphasize the importance and responsibilities, we have developed a summary page as described below:II. Inspection Proceduresa. Inspection Sample∙must sample a minimum of 10% or 1 Roll, whichever is greater, of every incoming shipment by colorb. Check color∙make Color continuity card between rolls inspected∙check against color standard∙check against vendor’s reference cutting and Spectrophotometer readingc. Aesthetics∙check hand feel∙check fabric appearance of face sided. Visual Inspection∙measure fabric width & length∙inspection for Defects by using Four Point Systeme. Fill out Inspection worksheetIII. Inspection Resultsa. Evaluation guideline:∙Allowable Points per 100 yards (90 meters): 10 points for all fabric typesb. Result & Reporting∙Input Accept, Reject or Standard Exception result into MIS (Material Inspection System)IV. Record keeping (at least two years)a. Documents provided by Vendor:∙Seasonal Reference Sample∙Vendor Internal Inspection Report∙Vendor Internal Physical Test Report∙Reference cutting of each dye lot & Spectrophotometer Reading∙Vendor Letter for “Limited Approved” status and “Spectrophotometer non-readable valuesb. Documents prepared by factory:∙Color continuality card between rolls inspected∙Inspection Worksheet。
MAZATROL MATRIX维护资料_推荐

处理机密标题MAZATROL MATRIX 维护资料概 要 本资料作为服务窗口资料整理MAZATROL MATRIX/MATRIX NEXUS 的机型构成概要和按机械的部品构成等内容。
登録番号 頁MTSL0Y-D001/改 定 履 歴副番 日付 改定内容* 2006年12月8日 新建。
A 2007年2月23日 ·追加 “4. MAZATROL MATRIX NC系统图”。
·追加 “5. MAZATROL MATRIX NEXUS NC系统图”。
B 2007年10月18日 ·随着NC单元切换(FCU7-MA513-43/54)补记和修正。
·更新“6.MATRIX 按机械构成列表”。
C 2007年12月12日 ·追加“1.MAZATROL MATRIX/MATRIX NEXUS 构成列表”对象机型。
·更新 “2.MAZATROL MATRIX 服务部品列表”。
·更新 “3.MAZATROL MATRIX NEXUS 服务部品列表”。
·更新 “6.MATRIX 按机械构成列表”。
D 2008年5月1日 ·追加“1.MAZATROl MATRIX/MATRIX NEXUS 构成列表”中追加对象机型。
·更新“2.MAZATROL MATRIX 服务部品列表”。
·更新“6.MATRIX按机械构成一览”。
E 2008年7月2日 ·部分修正“1.MAZATROL MATRIX/MATRIX NEXUS 构成列表”对象机型。
·更新 “2.MAZATROL MATRIX 服务部品列表”。
·在“5.MAZATROL MATRIX NEXUS NC系统图”中追加注2。
·更新 “6.MATRIX 按机械构成列表”。
F 2008年12月2日 ·部分修改 “2.MAZATROL MATRIX 服务部品一览”的功能代码。
USP30-1211翻译-灭菌和无菌保证

1211STERILIZATION AND STERILITY ASSURANCE OF COMPENDIAL ARTICLES灭菌和无菌保证纲要条款This informational chapter provides a general description of the concepts and principles involved in the quality control of articles that must be sterile. Any modifications of or variations in sterility test procedures from those described under Sterility Tests 71should be validated in the context of the entire sterility assurance program and are not intended to be methods alternative to those described in that chapter.报告章节规定涉及到条款质量控制总的概念描述和原则是必须是无菌的.从无菌测试<71>的章节描述中,无菌保证程序的整个上下文中规定没有可选择的方法,任何有关无菌测试程序变更或修改应该得到验证.Within the strictest definition of sterility, a specimen would be deemed sterile only when there is complete absence of viable microorganisms from it. However, this absolute definition cannot currently be applied to an entire lot of finished compendial articles because of limitations in testing. Absolute sterility cannot be practically demonstrated without complete destruction of every finished article.在最严格的无菌定义里,仅当它不含有任何可存的微生物,样品才被认为无菌.然而, 因为测试的局限性,这个绝对的定义不能普遍应用到整个纲要条款中。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Keywords: Deformation theory, graded modules, obstruction theory, Generalized Matric Massey products, postulation Hilbert scheme.
1
Introduction
The theory of generalized matric Massey products (GMMP) for A-modules, A a k -algebra, is given by Laudal in [3], and applied to the theory of moduli of global and local modules in [6],[5]. This theory can obviously be applied also to the study of various Hilbert Schemes, leading to GMMP for graded R-modules M , R a graded k -algebra.
Abstract The theory of generalized matric Massey products has been applied for some time to A-modules M , A a k-algebra. The main application is to ˆ M , isomorphic to the local ring of the compute the local formal moduli H moduli of A-modules. This theory is also generalized to OX -modules M, X a k- scheme. In these notes we consider the definition of generalized Massey products and the relation algebra in any obstruction situation (a differential graded k-algebra with certain properties), and prove that this theory applies to the case of graded R-modules, R a graded k-algebra, k algebraically closed. When the relation algebra is algebraizable, that is the relations are polynomials rather than power series, this gives a combinatorial way to compute open (´ etale) subsets of the moduli of graded Rmodules. This also gives a sufficient condition for the corresponding point in the moduli of OProj(R) -modules to be singular. The computations are straight forward, algorithmic, and an example on the postulation Hilbert scheme is given.
∗ Mathematics
Subject Classification 14D15,13D10,13D02,13D07,14D22
1
Let (A• , d• ) be a differential graded k -algebra, and let α = {αe1 , . . . , αed } be a set of elements in H 1 (A• ). For n ∈ (N − {0})d , |n| = n1 + · · · + nd = 2 we have the ordinary cup-products α ⊗k α → h2 (A• ) given by < α; n >=
Hale Waihona Puke 2.2Homomorphisms
Classically, homomorphisms of graded k algebras R are homogeneous of degree 0. This is also the case with morphisms of graded R-modules. We might extend this definition by giving a grading to the homomorphisms: HomR ( ⊕ Md , ⊕ Nd ) = ⊕ HomR,d (M, N )
mi ∈(N∪{0})d
m1 +m2 =n
αm1 · αm2 For example
< α; (1, 0, . . . , 0, 1) >= αe1 · αed + αed · αe1 and < α; (1, 1, , 0 . . . , 0) >= αe1 · αe2 . By inductively adding elements αm ∈ A1 , m ∈ B ⊆ (N ∪ {0})d due to some relations, we define the higher order generalized matric Massey products < α; n >, n ∈ B ′ ⊆ (N ∪ {0})d , for some n of higher order |n|, provided A• satisfies certain properties. The inductive definition of GMMP is controlled at ˆ α constructed each step by the relations between the monomials in an algebra H in parallel. We call this algebra the relation algebra of α. It is interesting in it own right to study the GMMP structure and the relation algebra of various sets of α ∈ h1 (A• ). Deformation theory is introduced as a tool for studying local properties of various moduli spaces. It is well known that the prorepresenting hull of the deformation functor of a point M in moduli is the completion of the local ring in that point [4]. Consider a graded R-module M , R a graded k -algebra. Choose a minimal resolution 0 ← M ← L• of M and consider the degree zero • part Hom• R,0 (L• , L• ) of the Yoneda complex. Then (HomR,0 (L• , L• ), d• ) is a • ∗ 1 ∼ differential graded k -algebra. Let x∗ = {x∗ 1 , . . . , xd } ⊆ H (HomR,0 (L• , L• )) = 1 ˆ ExtR,0 (M, M ) be a k -basis. Then the relation algebra Hx∗ is isomorphic to ˆ M of the (graded) deformation functor Def M , i.e. the prorepresenting hull H ∼ ˆ ˆ ∗ Hx = HM . In addition to the definition of the graded GMMP, this is the main result of the paper, implying that general results about the GMMP gives local information about moduli. In addition, we get the following result, telling us how GMMP on R can be used to study the singular locus of sheaves on Proj(R): Proposition 2. Let M = Γ∗ (M) for M ∈ CR (Spec(k )). Then HM , the hull of Def R,M , is nonsingular if HM , the hull of Def R,M , is. We conclude the paper with an explicit example.
arXiv:math/0603425v1 [math.AG] 17 Mar 2006
Generalized Matric Massey Products for Graded Modules∗
Arvid Siqveland Buskerud University College PO. Box 251 N-3601 Kongsberg,Norway email:arvid.siqveland@hibu.no February 2, 2008
2
2.1
Classical graded theory
