Folinic acid_58-05-9_DataSheet_MedChemExpress
HSF1A_DataSheet_MedChemExpress

Inhibitors, Agonists, Screening Libraries Data SheetBIOLOGICAL ACTIVITY:HSF1A is a cell–permeable activator of heat shock transcription factor 1 (HSF1).IC50 & Target: HSF1[1]In Vitro: HSF1A protects cells from stress–induced apoptosis, binds TRiC subunits and inhibits TRiC activity without perturbation of ATP hydrolysis. Genetic inactivation or depletion of the TRiC complex results in human HSF1 activation and HSF1A inhibits the direct interaction between purified TRiC and HSF1 in vitro. Moreover, fluorescence anisotropy experiments using FITC coupled to HSF1A demonstrates that HSF1A–FITC binds to a purified Tcp1 subunit of TRiC with an affinity of approximately 600 nM. This is validated qualitatively via titration of purified Tcp1 into binding reactions containing 500 nM Biotin or HSF1A–Biotin [1]. Quantification bycounting the number of cell containing aggregates as a function of the total number of cells reveals that at HSF1A concentrations as low as 2 μM, a reduced number of aggregate–containing cells are observed. The fraction of cells containing aggregates continued to decrease in a dose–dependent manner such that pretreatment with 12 μM HSF1A resulta in ~20% of the cells exhibiting aggregates visible by fluorescence microscopy [2].In Vivo: HSF1A enhances HSF1 activity, stabilizes HSF1 expression and minimizes Doxorubicin (DOX)–induced cardiac damage. WKY rats are challenged with DOX (accumulated dose: 30 mg/kgw), and DOX combined with HSF1A (100 mg/kgw/day). Supplementation with HSF1A significantly elevates cardiac functions back to the levels of the control group. HSF1A has been shown to stimulate human HSF1 nuclear translocation, elevate protein chaperone expression and ameliorate protein misfolding and cell death in aneurodegenerative disease model. The echocardiographic results show that HSF1A also alleviates DOX–induced failures in cardiac function [3].PROTOCOL (Extracted from published papers and Only for reference)Kinase Assay:[1]Protein extracts are generated from mammalian, yeast and E. coli cultures using biotin–binding buffer (20 mM HEPES,5 mM MgCl 2, 1 mM EDTA, 100 mM KCl, 0.03% NP–40) supplemented with 1% Trition–X100 and protease inhibitors. Approximately 0.5mg of protein extract is incubated with 100 μM HSF1A–Biotin for 4 h at 4°C and HSF1A–Biotin associated proteins captured by with NeutrAvidin Agarose Resin. After washing in biotin binding buffer proteins are eluted using 50 μL biotin elution buffer (100 mM Tris,150 mM NaCl, 0.1 mM EDTA, 2 mM D–biotin), resolved on a 4–20% SDS–PAGE, and immunoblotted. For purified TRiC and Hsp70analyses, 5 nM protein is incubated in biotin–binding buffer+0.5% Triton X–100 with 100 μM biotin or 100 μM HSF1A–Biotin for 4 h at 4°C and captured with NeutrAvidin Resin. For NiNTA purified yeast Tcp1, different concentrations of Tcp1 0.5 μM, 1 mM, 2 mM, 3 mM and 4 mM in 25 mM Hepes pH 7.5, 150 mM NaCl are incubated with 0.5 μM Biotin or HSF1A–Biotin for 4 h at 4°C and captured with NeutrAvidin Resin [1].Cell Assay:[2]PC12 cells seeded into a 96–well plate (5×104 cells/well) are treated with increasing concentrations of HSF1A (2, 4, 8 andProduct Name:HSF1A Cat. No.:HY-103000CAS No.:1196723-93-9Molecular Formula:C21H19N3O2S2Molecular Weight:409.52Target:HSP Pathway:Cell Cycle/DNA Damage; Metabolic Enzyme/Protease Solubility:DMSO: ≥ 150 mg/mL12 μM) for 15 h, at which time httQ74–GFP expression is stimulated by incubation in the presence of 1 μg/mL Doxycycline for 5 d. Cell viability is assessed via the XTT viability assay[2].Animal Administration:[3]Rat[3]Ten–week–old Wistar Kyoto rats (WKY) are used. The rats are housed at a constant temperature (22°C) on a 12–h light/dark cycle with food and tap water. The animals are arranged into three groups: WKY rats (the control group), DOX rats and DOX rats treated with HSF1A. Each group contain five animals. The DOX group is injected with DOX (5 mg/kg) for 6 consecutive weeks intraperitoneal injection to achieve a cumulative dose of 30 mg/kg, which has been well documented to achieve cardiotoxicity. The small molecular HSF1 activator HSF1A (100 mg/kg/day) is injected intraperitoneally.References:[1]. Neef DW, et al. A direct regulatory interaction between chaperonin TRiC and stress–responsive transcription factor HSF1. Cell Rep. 2014 Nov 6;9(3):955–66.[2]. Neef DW, et al. Modulation of heat shock transcription factor 1 as a therapeutic target for small molecule intervention in neurodegenerative disease. PLoS Biol. 2010 Jan 19;8(1):e1000291.[3]. Huang CY, et al. Doxorubicin attenuates CHIP–guarded HSF1 nuclear translocation and protein stability to trigger IGF–IIR–dependent cardiomyocyte death. Cell Death Dis. 2016 Nov 3;7(11):e2455.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
HIF-1α_纳米抗体的制备及其抑制黑素瘤生长的作用

山西农业科学 2023,51(12):1435-1441Journal of Shanxi Agricultural Sciences HIF-1α纳米抗体的制备及其抑制黑素瘤生长的作用李佳敏1,贾琼1,秦蓉芬1,迟志端1,王富明2,范瑞文1(1.山西农业大学动物医学学院,山西太谷 030801;2.晋中市庄子乡综合便民服务中心,山西晋中 030600)摘要:缺氧诱导因子1α(Hypoxia inducible factor 1α,HIF-1α)参与低氧微环境相关疾病的发生等过程,具有控制肿瘤生长和发展的功能。
黑色素瘤是一种发生于人和动物恶性程度较高的肿瘤。
为探明HIF-1α纳米抗体对黑色素瘤的影响,研究利用前期保存的羊驼源黑色素瘤细胞噬菌体文库筛选HIF-1α纳米抗体,经原核表达与纯化后,通过Western Blot和免疫组织化学验证HIF-1α纳米抗体与抗原的结合性;分别通过CCK-8法、划痕试验以及Western Blot法检测其对B16黑素瘤细胞的增殖和迁移能力及其相关分子表达的影响。
结果表明,经表达和纯化获得的HIF-1α纳米抗体分子质量约为16 ku,没有跨膜结构,具有亲水性。
通过Western Blot和免疫组织化学验证了其具有良好的抗原结合性。
在增殖试验和划痕试验中,与对照组相比,HIF-1α纳米抗体抑制了B16细胞的增殖和迁移,下调了靶基因VEGF的表达,并使细胞增殖和迁移相关蛋白Ras、ERK、RAC和RAF的表达量下调。
预测HIF-1α纳米抗体进入B16细胞内,与抗原结合,通过下游靶基因VEGF下调RAs、ERK、RAC、RAF的表达,从而对细胞增殖和迁移起抑制作用,可作为黑色素瘤治疗的新靶点。
关键词:HIF-1α;纳米抗体;B16细胞;Western Blot法;CCK-8法;细胞增殖;细胞迁移中图分类号:R739.5 文献标识码:A 文章编号:1002‒2481(2023)12‒1435‒07Effect on Preparation of HIF-1α Nano-Antibody and ItsInhibition of Melanoma GrowthLI Jiamin1,JIA Qiong1,QIN Rongfen1,CHI Zhiduan1,WANG Fuming2,FAN Ruiwen1(1.College of Veterinary Medicine,Shanxi Agricultural University,Taigu 030801,China;2.Jinzhong City Zhuangzi Integrated Convenient Service Center,Jinzhong 030600,China)Abstract:The hypoxia inducible factor 1α(HIF-1α) is involved in the occurrence of diseases related to hypoxia microenvironment and has the function of controlling tumor growth and development. As we known, melanoma is a highly malignant tumor occurring in animals and humans. To explore the effect of HIF-1α nano-antibody on melanoma, in this study, the phage library of alpaca-drived melanoma cells previously preserved in our laboratory was used to screen HIF-1α nano-antibodies. After prokaryotic expression and purification, the binding of HIF-1α nano-antibody and its antigen was verified by Western blot and immunohistochemistry. The effects of HIF-1α nano-antibody on the proliferation and migration of B16 melanoma cells and the expression of related molecules were detected by CCK-8, wound healing test, and Western blot methods. The results showed that HIF-1α nano-antibody obtained by expression and purification was hydrophilic protein without transmembrane structure and had a molecular weight of about 16 ku. Western blot and immunohistochemistry results showed that it had good antigenic binding. In the proliferation and wound healing experiments, HIF-1α nano-antibody inhibited the proliferation and migration of B16 cells, down-regulated the expression of target gene VEGF and the proliferation and migration related proteins Ras, ERK, RAC, and RAF, comparing with the control group. In Conclusion, it was predicted that HIF-1α nano-antibody entered B16 cells and combined with antigens and down-regulated the expression of RAs, ERK, RAC, RAF through the downstream target gene VEGF, which inhibited cell proliferation and migration, and could be used as a new target for melanoma treatment.Key words:HIF-1α; nano-antibody; B16 cells; Western Blot method; CCK-8 method; cell proliferation; cell migration氧是生命活动中所必需的物质,且在其中起重要作用[1]。
氟吗啉原药大鼠急性吸入毒性试验

氟吗啉原药大鼠急性吸入毒性试验作者:吴宗澄来源:《绿色科技》2017年第18期摘要:为测定氟吗啉原药对大鼠的急性吸入毒性,求出半数致死浓度(LC50),同时为亚慢性和其他毒理学研究中接触剂量的选择提供依据,参照《农药登记毒理学试验方法》,以中华人民共和国国家标准(GB15670-1995)为准则,用SD(Sprague Dawley)大鼠作为试验动物,对氟吗啉原药进行了急性吸入毒性试验。
结果表明:氟吗啉原药对SD雌雄大鼠急性吸入毒性试验LC50均大于2093±30 mg/m3。
根据GB15670-1995《农药登记毒理学试验方法》大鼠急性吸入毒性分类标准,氟吗啉原药大鼠急性吸入毒性为低毒,该结论可为安全使用提供依据。
关键词:氟吗啉原药;大鼠;急性吸入毒性;LC50中图分类号:X43文献标识码:A文章编号:16749944(2017)180219021引言氟吗啉原药是沈阳化工研究院自主研制的新型杀菌剂\[1\]。
该杀菌剂是一种内吸型杀菌剂,主要用于防治霜霉和疫霉属病害,由卵菌纲病原菌引起的霜霉病、晚疫病等重要植物病害,尤其用于防治抗性病害\[1,2\]。
氟吗啉是我国第一个真正实现工业化且具有自主知识产权的农药品种,也是第一个获得中国发明专利的农用杀菌剂\[3\]。
氟吗啉原药杀菌活性高,抗菌谱广,与环境相容性优于国外同类产品烯酰吗啉\[4\]。
但目前尚未见有关氟吗啉原药吸入毒性的报道。
本实验参照《农药登记毒理学试验方法》,以中华人民共和国国家标准(GB15670-1995)为准则[5],通过对SD大鼠的急性吸入毒性试验来检测氟吗啉原药的急性吸入毒性,了解其经呼吸道进入动物机体后对呼吸道及全身的损伤和危害程度,为该农药的毒理学安全评价提供资料。
2材料与方法2.1受试物与仪器氟吗啉原药为白色结晶固体,纯度为95%,由沈阳化工研究院有限公司提供。
称取 95.0 g 受试样品,加入 5.0 g 白炭黑用行星式球磨仪进行粉碎和研磨,配成 95%的受试样待用。
黄嘌呤氧化酶(XOD)活性检测试剂盒说明书

黄嘌呤氧化酶(XOD微量法注意:本产品试剂有所变动,请注意并严格按照该说明书操作。
货号:BC1095规格:100T/96S产品组成:使用前请认真核对试剂体积与瓶内体积是否一致,有疑问请及时联系索莱宝工作人员。
试剂名称规格保存条件提取液液体110 mL×1瓶4℃保存试剂一液体20mL×1瓶4℃保存试剂二粉剂×1瓶4℃保存溶液的配制:1、试剂二:粉剂置于试剂瓶内EP管中;2、工作液的配制:用时在试剂二中加入9.375 mL试剂一充分溶解,用不完的试剂4℃可保存2周;按需用蒸馏水稀释10倍后备用,现用现配。
产品说明:XOD(EC 1.17.3.2)催化黄嘌呤氧化生成尿酸和超氧阴离子,是活性氧主要来源之一;同时也是核苷酸代谢的关键酶之一。
XOD主要分布于哺乳动物的心、肺、肝脏等组织中,当肝功能受损时,XOD大量释放到血清中,对肝损害的诊断具有特异性的意义。
XOD催化黄嘌呤产生尿酸,尿酸在290nm下有特征吸收峰。
注意:实验之前建议选择2-3个预期差异大的样本做预实验。
如果样本吸光值不在测量范围内建议稀释或者增加样本量进行检测。
需自备的仪器和用品:紫外分光光度计/酶标仪、台式离心机、可调式移液器、微量石英比色皿/96孔UV板、匀浆器/研钵、冰和蒸馏水。
操作步骤:一、样本处理(可适当调整待测样本量,具体比例可以参考文献)1.细菌、细胞或组织样本的制备:细菌或细胞:先收集细菌或细胞到离心管内,离心后弃上清;按照细菌或细胞数量(104个):提取液体积(mL)为500~1000:1的比例(建议500万细菌或细胞加入1mL提取液),超声波破碎细菌或细胞(冰浴,功率20%或200W,超声3s,间隔10s,重复30 次);8000g 4℃离心10min,取上清,置冰上待测。
组织:按照组织质量(g):提取液体积(mL)为1:5~10的比例(建议称取约0.1g组织,加入1mL提取液),进行冰浴匀浆。
Pyrimethamine_58-14-0_DataSheet_MedChemExpress

Product Name:Pyrimethamine CAS No.:58-14-0Cat. No.:HY-18062Product Data SheetMWt:248.71Formula:C12H13ClN4Purity :>98%Solubility:DMSO 10 mg/mL; Water <1Mechanisms:Biological Activity:Pyrimethamine(RP4753)is a medication used for protozoal infections;interferes with tetrahydrofolicPathways:Cell Cycle/DNA Damage; Target:AntifolatePathways:Anti-infection; Target:Antiparasitic mg/mL; Ethanol <1 mg/mLPyrimethamine(RP4753) is a medication used for protozoal infections; interferes with tetrahydrofolicacid synthesis from folic acid by inhibiting the enzyme dihydrofolate reductase (DHFR).IC50 Value: 15.4 nM (Plasmodium falciparum) [1]Target: DHFR; antifolate in vitro: Three susceptibility levels (susceptible, intermediate, and resistant) were observed in the response of culture-adapted clones and strains to pyrimethamine (50% inhibitory concentration[IC50]) < 100, 100-2,000, and > 2,000 nM) and cycloguanil (IC50 < 50, 50-500, and > 500 nM).Based on these susceptibility levels, 73 and 68 of 96 fresh clinical isolates were susceptible to pyrimethamine (mean IC50 15.4 nM) and cycloguanil (mean IC50 11.1 nM), respectively [1]. We References:[1]. Basco LK, et al. In vitro activity of pyrimethamine, cycloguanil, and other antimalarial drugs against African isolates and clones of Plasmodium falciparum. Am J Trop Med Hyg. 1994 Feb;50(2):193-9.tested pyrimethamine(previously reported to suppress SOD1 expression), several compounds currently in trials in human and murine ALS, and a...()[2]. Inceboz T, et al. Preparation of (131)I-Pyrimethamine and evaluation for scintigraphy ofexperimentally Toxoplasma gondii-infectedrats. J Drug Target. 2013 Feb;21(2):175-9.[3]. Wright PD, et al. Screening for inhibitors of the SOD1 gene promoter: pyrimethamine does notreduce SOD1 levels in cell and animal models. Neurosci Lett. 2010 Oct 4;482(3):188-92.[4]. Martins-Duarte ?S, et al. Toxoplasma gondii: the effect of fluconazole combined withsulfadiazine and pyrimethamine against acute toxoplasmosis in murine model. Exp Parasitol. 2013Mar;133(3):294-9.[5]. Taylor WR, et al. Antimalarial drug toxicity: a review. Drug Saf. 2004;27(1):25-61.Caution: Not fully tested. For research purposes onlyMedchemexpress LLC...18 W i l k i n s o n W a y , P r i n c e t o n , N J 08540,U S AE m a i l : i n f o @m e d c h e m e x p r e s s .c o m W e b : w w w .m e d c h e m e x p r e s s .c o m。
黄素腺嘌呤二核苷酸(FAD)酶联免疫吸附测定试剂盒

5th Edition, revised in Dec, 2013(本试剂盒仅供体外研究使用,不用于临床诊断!)去甲肾上腺素(NA/NE)酶联免疫吸附测定试剂盒 使用说明书NA/NE (Noradrenaline/Norepinephrine) ELISA Kit 产品货号:E-EL-0047c使用前请仔细阅读说明书。
如果有任何问题,请通过以下方式联系我们:全国免费电话400-660-4808 销售部电话************技术部电话************电子邮箱(销售)********************电子邮箱(技术) **************************QQ 客服1037150941 网址 联系时请提供产品货号(见试剂盒标签),以便我们更高效地为您服务。
去甲肾上腺素(NA/NE)酶联免疫吸附测定试剂盒使用说明书产品货号:E-EL-0047c(本试剂盒仅供体外研究使用、不用于临床诊断!)声明:尊敬的客户,感谢您选用本公司的产品。
本产品适用于体外定量检测血清、血浆或其它相关生物液体中天然和重组NA/NE浓度。
使用前请仔细阅读说明书并检查试剂组分!如有疑问,请及时联系伊莱瑞特生物科技有限公司。
试剂盒组成:特别说明:*: [96T/48T](打开包装后请及时检查所有物品是否齐全完整)#:一周内使用可存于4℃,需长时间存放或多次使用建议存于-20℃.相关试剂在分装时会比标签上标明的体积稍多一些,请在使用时量取而非直接倒出!检测原理:本试剂盒采用竞争ELISA法。
用NA/NE抗原包被于酶标板上,实验时样品或标准品中的NA/NE 与包被的NA/NE竞争生物素标记的抗NA/NE单抗上的结合位点,游离的成分被洗去。
加入辣根过氧化物酶标记的亲和素,生物素与亲和素特异性结合而形成免疫复合物,游离的成分被洗去。
加入显色底物(TMB),TMB在辣根过氧化物酶的催化下呈现蓝色,加终止液后变成黄色。
Medlife,58-97-9,尿苷单磷酸,技术规格说明书(SDS)

58-97-9
分子式:
C9H13N2O9P
分子量:
324.18
详细描述:
创赛优选提供58-97-9,尿苷單磷酸,UMP,Medlife,上海现货。
Medlife,致力于提供高品质、高性价比小分子化合物的产品。
Medlife小分子化合物大量库存,提供超过2万种的抑制剂、激动剂、拮抗剂等产品,是药物及疾病研究的重要原料供应商。
沸点
700.1±70.0 °C at 760 mmHg
熔点
202 ºC (decomp)
分子式
C9H13N2O9P
分子量
324.181ห้องสมุดไป่ตู้
闪点
377.2±35.7 °C
精确质量
324.035858
PSA
181.12
LogP
-1.8
外观性状
solid
蒸汽压
0.0±5.0 mmHg at 25°C
折射率
1.754
英文名称:
Uridine-5'-monophosphate
英文别名:
5'-Uridylic acid;5'-UMP;UMP;Uridine 5′-monophosphate;Uridine 5'-Monophosphate;IMP;U 5'-P;Uridine 5`-monophosphate;Uridine-5'-phosphate;uridinemonophosphate;uridinephosphate;Uridylate;Uridylic acid;U 5′-P;Uridine 5'-phosphoric acid;Uridine monophosphate;uridine-5'-monophosphate;Uridine phosphate;Uridine 5'-phosphate;UMP (nucleic acid);Uridine 5'-(dihydrogen phosphate);5'Uridylic acid;E2OU15WN0N;2,4(1H,3H)-pyrimidinedione, 1-(5-O-phosphono-beta-D-ribofuranosyl)-;Polyuridylic acids;{[(2R,3S,4R,5R)-5-(2,4-dioxo-1,2,3
嘌呤霉素盐酸盐58-58-2

14.3 运输危险类别
欧洲陆运危规 : 6.1
国际海运危规 : 6.1
国际空运危规 : 6.1
14.4 包裹组
欧洲陆运危规 : III
国际海运危规 : III
国际空运危规 : III
14.5 环境危害
欧洲陆运危规 :否
国际海运危规 海运污染物 :否
国际空运危规 : 否
14.6 对使用者的特别预防
无数据资料
6.2 环境预防措施
丢弃处理请参阅第160节
6.3 抑制和清除溢出物的方法和材料
避免接触皮肤和眼睛。避免形成粉尘和气溶胶。在有粉尘生成的地方,提供合适的排风设备。
7 安全操作与储存
7.1 安全操作的注意事项
无数据资料
7.2 安全储存的条件,包括任何不兼容性
无数据资料
7.3 特定用途
避免与皮肤、眼睛和衣服接触。休息前和操作本品后立即洗手。Aldrich-D60400页码4的7
4.2 最重要的症状和影响,急性的和滞后的
主要症状和影响,急性和迟发效应
4.3 及时的医疗处理和所需的特殊处理的说明和指示
如必要的话,戴自给式呼吸器去救火。
5 消防措施
5.1 灭火介质
火灾特征
https:// 1/4
化学品安全技术说明书
无数据资料 灭火方法及灭火剂 碳氧化物,氮氧化物,氯化氢气体,氢氰酸
吸入 可能引起眼睛刺激。 吞咽 无数据资料 皮肤 无数据资料 眼睛 无数据资料 接触后的征兆和症状 无数据资料 附加说明 无数据资料
https:// 3/4
化学品安全技术说明书
12 生态学资料
12.1 毒性
无数据资料
12.2 持久存留性和降解性
AA-0055_2010.6耐化学试剂(碱)第7页开始

This document is valid for the BMW Group Technical Laboratory.
3
3.1
Procedure and Responsibilities
Terms
Abbreviations, terms and symbol explanations are included in the MPM DMS System and are available online. Further explanation of abbreviations is possible through the corresponding link on the QualityHomepage of the Labortechnik.
June 2010 AA-0055
Page 7 of 12
This work instruction describes the test media and methods used in the chemical resistance test of surfaces.
2
Scope of Application
3.4 Test
The tests are carried out in a normal climate DIN 50 014 – 23/50-2. Allocation of test material / test media / test method see table 2 – 4 .
Author / Document Owner:
Verifier:
Release Owner:
signed Blazenka Gido
Revision: „-- “
黄芩素通过调节HIF-1α

黄芩素通过调节HIF -1α/VEGF 信号通路抑制类风湿关节炎大鼠的炎症反应和病理性血管生成*杜红丽1,张晨宇1,赵清2△[1河南中医药大学第五临床医学院(郑州人民医院)风湿免疫科,河南郑州450053;2河南大学淮河医院风湿免疫科,河南开封475099][摘要]目的:探讨黄芩素(BA )调节缺氧诱导因子1α(HIF -1α)/血管内皮生长因子(VEGF )信号通路对类风湿关节炎(RA )大鼠炎症反应和病理性血管生成的影响。
方法:按照随机数字表法将SD 大鼠分为对照(control )组、模型(model )组、低剂量(10mg/kg )BA (BA -L )组、高剂量(30mg/kg )BA (BA -H )组、雷公藤多苷片(TWP ;6.25mg/kg )组和BA -H+HIF -1α激动剂二甲基草酰甘氨酸(DMOG ;40mg/kg )组,每组12只。
除control 组外,其它组大鼠均采用II 型胶原蛋白-完全弗氏佐剂法诱导RA 大鼠模型。
第2次免疫24h 后开始给药处理,每天给药一次,持续4周。
检测大鼠在给药第0、7、14和28天时的足趾肿胀度,计算关节炎指数;计算大鼠胸腺和脾脏指数;HE 染色检测大鼠踝关节滑膜组织病理损伤;ELISA 法检测大鼠踝关节滑膜组织中肿瘤坏死因子α(TNF -α)和白细胞介素6(IL -6)水平;免疫组化检测大鼠踝关节滑膜组织中VEGF 和VEGF 受体2(又称激酶插入域受体,KDR )表达;Western blot 检测各组大鼠踝关节滑膜组织中HIF -1α和VEGF 蛋白表达。
结果:与control 组比较,model 组大鼠踝关节滑膜组织病理损伤严重,足趾肿胀度、关节炎指数、胸腺和脾脏指数,以及滑膜组织TNF -α、IL -6、VEGF 、KDR 、HIF -1α和VEGF 水平均显著升高(P <0.05);与model 组比较,BA -L 组、BA -H 组和TWP 组对应指标变化趋势与上述相反(P <0.05);BA -H 组与TWP 组比较,上述指标变化差异无统计学意义(P >0.05);DMOG 减弱了BA -H 对RA 大鼠炎症反应和病理性血管生成的抑制作用。
53-84-9,辅酶I,技术规格说明书(SDS)

53-84-9|辅酶I,技术规格说明书(SDS)简介:辅酶I,NAD+ 即烟酰胺腺嘌呤二核苷酸,是一种转递氢离子的辅酶。
NAD+是NADH的氧化形式。
辅酶I物理化学性质:辅酶I详细介绍:Less Hazardous Chemical SynthesesSafer Solvents and AuxiliariesDesign for Energy EfficiencyUse of Renewable FeedstocksInherently Safer Chemistry for Accident PreventionLearn more about the Principles of Green Chemistry.颜色:white to off-white环保替代产品分类:Re-engineered储存温度:−20°CSMILESstring:NC1=NC=NC2=C1N=CN2.O[C@@H]3[C@@H](COP(O)(O P(OC[C@@H](O4)[C@@H](O)[C@@H](O)[C@H]4[N+]5=CC=CC( C(N)=O)=C5)([O-])=O)=O)OC[C@@H]3O一般描述:烟酰胺腺嘌呤二核苷酸水合物是NAD的常见形式,主要用作电子受体。
应用:β-烟酰胺腺嘌呤二核苷酸(β-NAD)是乙醇脱氢酶的辅因子,在内脏平滑肌中起到抑制性神经递质的作用。
NAD/NADH比值对细胞内氧还电势具有调节作用,进而影响体内的代谢反应。
包装:250 mg in poly bottle1, 5, 10, 25 g in poly bottle根据固体质量包装。
生化/生理作用:电子受体其他说明:本品是普通型 NAD。
辅酶I参考文献:[1]. Viollet, B., et al., Cellular and molecular mechanisms of metformin: an overview. Clin Sci (Lond), 2012. 122(6): p. 253-70.[2]. Brandt, U., Energy converting NADH:quinone oxidoreductase (complex I). Annu Rev Biochem, 2006. 75: p. 69-92.[3]. Kussmaul, L. and J. Hirst, The mechanism of superoxide production by NADH:ubiquinone oxidoreductase (complex I) from bovine heart mitochondria. Proc Natl Acad Sci U S A, 2006. 103(20): p. 7607-12.产品技术规格说明书由上海创赛科技有限公司收集整理,仅做参考使用。
希森美康血凝仪

CS-2100i/2000i根据SYSMEX在血栓与止血领域多年积累的经验,为全面满足各类实验室的需求率先开发CS-2100i/2000i。
四种方法学:凝固法/发色底物法/免疫比浊法/聚集法unique!多波长高精度光学检测:5种波长检测提供可靠数据新型智能监测:特有的HIL check 功能排除溶血/黄疸/脂血干扰更多新检测项目的选择:FXIII,vWF:Rco等更可靠的实验室质量保证:SNCS实时在线质控全面满足血栓与止血检测需求!CA-7000以全球最快的分析速度提供全面和高精准的止凝血项目检测结果,仪器集中了凝固法,发色底物法和免疫法于一体,设计高度人性化和智能化,操作简便,成为大规模实验室的首选。
● 多参数测试,500测试/小时高速分析能力● 操作简便,灵活对待各种需求● 优秀的试剂管理系统(SRS)● 安全、实用的系统设计● 大容量的数据管理能力,完整的质控系统CA-1500汇集了当今血栓/止血分析仪最新的各种先进功能于一身,是市场上少见的性能/价格比极高的一台仪器,是大型教学医院,综合医院实验室的首选。
它具有快速处理能力,最快180测试/小时,集多种检测功能于一身:凝固法、发色底物法、免疫法。
具有全能随机组合能力,两种方法测定纤维蛋白质,适合常规大量和急诊使用。
● 拥有高速处理能力、随机测试功能和自动再检查功能● 三种分析方式,包括多规则监视的广泛质控文件和平行线生物分析功能● 卓越的性能可以灵活适应实验室的多样化需求CA-500系列CA-500系列包含了六款机型,设计新颖、符合经济原则,是各中小型实验室开展血栓/止血实验的最佳选择,也是半自动升级到全自动的理想机型。
小型台式仪实用可靠,具备三种检测系统即凝固法、发色底物法、免疫法的自由组合用户可根据需要选择相应机型。
CA-50设计上完全沿用了全自动CA系列的检测原理,锁定人为误差因素的设计确保它有别于其他半自动血凝仪,达到全自动仪器的准确性与重复性效果,四通道即可批量检测又可单独检测,内置质控文件,适用于小标本量实验室使用。
BD OptiBuild

BD OptiBuild™Technical Data SheetBB700 Rat Anti-Mouse CD8bProduct InformationMaterial Number:742198Size:50 µgClone:H35-17.2Alternative Name:Ly-3; Lyt-3; Lymphocyte antigen 3; Ly-C; CD8b1Reactivity:Tested in Development:MouseIsotype:Rat IgG2b, κImmunogen:5-day MLR, C57BL/6 anti-BALB/cApplication:Flow cytometry(Qualified)Concentration:0.2 mg/mlEntrez Gene ID:12526Storage Buffer:Aqueous buffered solution containing ≤0.09% sodium azide. Regulatory Status:RUODescriptionThe H35-17.2 monoclonal antibody specifically binds to both alloantigeneic forms of the β chain of the CD8differentiation antigen (Ly-3 or Lyt- 3). The CD8 α and α' chains (CD8a) form heterodimers with the CD8 β chain (CD8b, Ly-3, or Lyt-3) on the surface of most thymocytes. A subpopulation of mature T lymphocytes (i.e., MHC class I-restrictedT cells, including most T suppressor/cytotoxic cells) expresses almost exclusively the CD8 αβ heterodimer (the α' chain is absent). Subsets of γδ TCR-bearing T cells, intestinal intraepithelial lymphocytes, and dendritic cells express CD8a without CD8b. It has been suggested that the expression of the CD8a/CD8b heterodimer is restricted to T lymphocytes which matured in the thymus or in an extrathymic environment that had been influenced by thymus- initiated neuroendocrine signals. CD8 is an antigen coreceptor on the T-cell surface which interacts with MHC class I molecules on antigen-presenting cells. It participates in T-cell activation through its association with the T-cell receptor complex and protein tyrosine kinase lck (p56lck). The H35-17.2 mAb blocks T-cell-mediated cytolysis of allogeneic lymphoma cells.The antibody was conjugated to BD Horizon™ BB700, which is part of the BD Horizon Brilliant™ Blue family of dyes. It is a polymer-based tandem dye developed exclusively by BD Biosciences. With an excitation max of 485 nm and an emission max of 693 nm, BD Horizon BB700 can be excited by the 488 nm laser and detected in a standard PerCP-Cy™5.5 set (eg, 695/40-nm filter). This dye provides a much brighter alternative to PerCP-Cy5.5 with less cross laser excitation off the 405 nm and 355 nm lasers.Preparation and StorageStore undiluted at 4°C and protected from prolonged exposure to light. Do not freeze. The monoclonal antibody waspurified from tissue culture supernatant or ascites by affinity chromatography. The antibody was conjugated with BD Horizon BB700 under optimal conditions that minimize unconjugated dye and antibody.Recommended Assay ProcedureFor optimal and reproducible results, BD Horizon Brilliant Stain Buffer should be used anytime two or more BD Horizon Brilliant dyes are used in the same experiment. Fluorescent dye interactions may cause staining artifacts which may affect data interpretation. The BD Horizon Brilliant Stain Buffer was designed to minimize these interactions. More information can be found in the Technical Data Sheet of the BD Horizon Brilliant Stain Buffer (Cat. No. 563794 or 566349).When setting up compensation, it is recommended to compare spillover values obtained from cells and BD™ CompBeads to ensure that beads will provide sufficiently accurate spillover values.For optimal results, it is recommended to perform two washes after staining with antibodies. Cells may be prepared, stained with antibodies and washed twice with wash buffer per established protocols for immunofluorescent staining prior to acquisition on a flow cytometer. Performing fewer than the recommended wash steps may lead to increased spread of the negative population.Suggested Companion ProductsCatalog Number Name Size Clone553141Purified Rat Anti-Mouse CD16/CD32 (Mouse BD Fc Block™)0.1 mg 2.4G2 554656Stain Buffer (FBS)500 mL554657Stain Buffer (BSA)500 mL563794Brilliant Stain Buffer100 Tests555899Lysing Buffer100 mLProduct Notices1.This antibody was developed for use in flow cytometry.2.The production process underwent stringent testing and validation to assure that it generates a high-qualityconjugate with consistent performance and specific binding activity. However, verification testing has not been performed on all conjugate lots.3.Researchers should determine the optimal concentration of this reagent for their individual applications.4.An isotype control should be used at the same concentration as the antibody of interest.5.Caution: Sodium azide yields highly toxic hydrazoic acid under acidic conditions. Dilute azide compounds in runningwater before discarding to avoid accumulation of potentially explosive deposits in plumbing.6.For fluorochrome spectra and suitable instrument settings, please refer to our Multicolor Flow Cytometry web page at/colors.7.Please refer to /us/s/resources for technical protocols.8.BD Horizon Brilliant Stain Buffer is covered by one or more of the following US patents: 8,110,673; 8,158,444;8,575,303; 8,354,239.9.BD Horizon Brilliant Blue 700 is covered by one or more of the following US patents: 8,455,613 and 8,575,303.10.Cy is a trademark of GE Healthcare.ReferencesGolstein P, Goridis C, Schmitt-Verhulst AM . Lymphoid cell surface interaction structures detected using cytolysis-inhibiting monoclonal antibodies. Immunol Rev. 1982; 68:5-42. (Immunogen: Cytotoxicity, Immunoprecipitation, Inhibition). Lefrancois L. Phenotypic complexity of intraepithelial lymphocytes of the small intestine. J Immunol. 1991;147(6):1746-1751. (Biology).Ledbetter JA, Seaman WE, Tsu TT, Herzenberg LA. Lyt-2 and Lyt-3 antigens are on two different polypeptide subunits linked by disulfide bonds. Relationship of subunits to T cell cytolytic activity. J Exp Med. 1981; 153:1503-1516. (Biology). Walker ID, Murray BJ, Hogarth PM, Kelso A, McKenzie IF. Comparison of thymic and peripheral T cell Ly-2/3 antigens. Eur J Immunol. 1984; 14(10):906-910. (Biology).Nakayama K, Nakayama K, Negishi I, et al. Requirement for CD8 beta chain in positive selection of CD8-lineage T cells. Science. 1994; 263(5150):1131-1133. (Biology).MacDonald HR, Schreyer M, Howe RC, Bron C. Selective expression of CD8 alpha (Ly-2) subunit on activated thymic gamma/delta cells. Eur J Immunol. 1990; 20(4):927-930. (Biology).Rocha B, Vassalli P, Guy-Grand D. The extrathymic T-cell development pathway. Immunol Today. 1992; 14(3):140-141. (Biology).Murosaki S, Yoshikai Y, Ishida A, et al. Failure of T cell receptor V beta negative selection in murine intestinal intra-epithelial lymphocytes. Int Immunol. 1991; 3(10):1005-1013. (Biology).Wang J, Klein JR. Thymus-neuroendocrine interactions in extrathymic T cell development. Science. 1994;265(5180):1860-1862. (Biology).Sydora BC, Brossay L, Hagenbaugh A, Kronenberg M, Cheroutre H. TAP-independent selection of CD8+ intestinal intraepithelial lymphocytes. J Immunol. 1996; 156(11):4209-4216. (Biology).Vremec D, Zorbas M, Scollay R, et al. The surface phenotype of dendritic cells purified from mouse thymus and spleen: investigation of the CD8 expression by a subpopulation of dendritic cells. J Exp Med. 1992; 176(1):47-58. (Biology).Wu L, Vremec D, Ardavin C, et al. Mouse thymus dendritic cells: kinetics of development and changes in surface markers during maturation. Eur J Immunol. 1995; 25(2):418-425. (Biology).Süss G, Shortman K. A subclass of dendritic cells kills CD4 T cells via Fas/Fas-ligand-induced apoptosis. J Exp Med. 1996; 183(4):1789-1796. (Biology).Fujiura Y, Kawaguchi M, Kondo Y, et al. Development of CD8 alpha alpha+ intestinal intraepithelial T cells in beta 2-microglobulin- and/or TAP1-deficient mice. J Immunol. 1996; 156(8):2710-2715. (Biology).Bierer BE, Sleckman BP, Ratnofsky SE, Burakoff SJ. The biologic roles of CD2, CD4, and CD8 in T-cell activation. Annu Rev Immunol. 1989; 7:579-599. (Biology).Janeway CA Jr. The T cell receptor as a multicomponent signalling machine: CD4/CD8 coreceptors and CD45 in T cell activation. Annu Rev Immunol. 1992; 10:645-674. (Biology).Zamoyska R. The CD8 coreceptor revisited: one chain good, two chains better. Immunity. 1994; 1(4):243-246. (Biology). LeFrancois L. Extrathymic differentiation of intraepithelial lymphocytes: generation of a separate and unequal T-cell repertoire. Immunol Today. 1991; 12(12):436-438. (Biology).O'Rourke AM, Mescher MF. The roles of CD8 in cytotoxic T lymphocyte function. Immunol Today. 1993; 14(4):183-188. (Biology).Ledbetter JA, Rouse RV, Micklem HS, Herzenberg LA. T cell subsets defined by expression of Lyt-1,2,3 and Thy-1 antigens.Two-parameter immunofluorescence and cytotoxicity analysis with monoclonal antibodies modifies current views. J ExpMed. 1980; 152(2):280-295. (Biology).BD BiosciencesUnited States Canada Europe Japan Asia Pacific Latin America/Caribbearn877.232.8995888.268.543032.53.720.5500120.8555.9065.6861.06330800.771.7157For country contact information, visit /contactConditions: The information disclosed herein is not to be construed as a recommendation to use the above product in violation of any patents. BD Biosciences will not be held responsible for a patent infringement or other v ©2020 BD. All rights reserved. Unless otherwise noted, BD, the BD Logo and all other trademarks are the property of Becton, Dickinson and Company or its affiliates.。
IPI549-SDS-MedChemExpress

Inhibitors, Agonists, Screening LibrariesSafety Data Sheet Revision Date:Oct.-12-2018Print Date:Oct.-12-20181. PRODUCT AND COMPANY IDENTIFICATION1.1 Product identifierProduct name :IPI549Catalog No. :HY-100716CAS No. :1693758-51-81.2 Relevant identified uses of the substance or mixture and uses advised againstIdentified uses :Laboratory chemicals, manufacture of substances.1.3 Details of the supplier of the safety data sheetCompany:MedChemExpress USATel:609-228-6898Fax:609-228-5909E-mail:sales@1.4 Emergency telephone numberEmergency Phone #:609-228-68982. HAZARDS IDENTIFICATION2.1 Classification of the substance or mixtureGHS Classification in accordance with 29 CFR 1910 (OSHA HCS)Acute toxicity, Oral (Category 4), H302Acute aquatic toxicity (Category 1), H400Chronic aquatic toxicity (Category 1), H4132.2 GHS Label elements, including precautionary statementsPictogramSignal word No data availableHazard statement(s)H302 Harmful if swallowed.H413 Very toxic to aquatic life with long lasting effects.Precautionary statement(s)P264 Wash skin thoroughly after handling.P270 Do not eat, drink or smoke when using this product.P273 Avoid release to the environment.P301 + P312 IF SWALLOWED: Call a POISON CENTER or doctor ⁄physician if you feel unwell.P333 Rinse mouth.2.3 Other hazardsNone.3. COMPOSITION/INFORMATION ON INGREDIENTS3.1 SubstancesSynonyms:IPI-549;IPI 549Formula:C30H24N8O2Molecular Weight:528.56CAS No. :1693758-51-84. FIRST AID MEASURES4.1 Description of first aid measuresEye contactRemove any contact lenses, locate eye-wash station, and flush eyes immediately with large amounts of water. Separate eyelids with fingers to ensure adequate flushing. Promptly call a physician.Skin contactRinse skin thoroughly with large amounts of water. Remove contaminated clothing and shoes and call a physician.InhalationImmediately relocate self or casualty to fresh air. If breathing is difficult, give cardiopulmonary resuscitation (CPR). Avoid mouth-to-mouth resuscitation.IngestionWash out mouth with water; Do NOT induce vomiting; call a physician.4.2 Most important symptoms and effects, both acute and delayedThe most important known symptoms and effects are described in the labelling (see section 2.2).4.3 Indication of any immediate medical attention and special treatment neededTreat symptomatically.5. FIRE FIGHTING MEASURES5.1 Extinguishing mediaSuitable extinguishing mediaUse water spray, dry chemical, foam, and carbon dioxide fire extinguisher.5.2 Special hazards arising from the substance or mixtureDuring combustion, may emit irritant fumes.5.3 Advice for firefightersWear self-contained breathing apparatus and protective clothing.6. ACCIDENTAL RELEASE MEASURES6.1 Personal precautions, protective equipment and emergency proceduresUse full personal protective equipment. Avoid breathing vapors, mist, dust or gas. Ensure adequate ventilation. Evacuate personnel to safe areas.Refer to protective measures listed in sections 8.6.2 Environmental precautionsTry to prevent further leakage or spillage. Keep the product away from drains or water courses.6.3 Methods and materials for containment and cleaning upAbsorb solutions with finely-powdered liquid-binding material (diatomite, universal binders); Decontaminate surfaces and equipment by scrubbing with alcohol; Dispose of contaminated material according to Section 13.7. HANDLING AND STORAGE7.1 Precautions for safe handlingAvoid inhalation, contact with eyes and skin. Avoid dust and aerosol formation. Use only in areas with appropriate exhaust ventilation.7.2 Conditions for safe storage, including any incompatibilitiesKeep container tightly sealed in cool, well-ventilated area. Keep away from direct sunlight and sources of ignition.Recommended storage temperature:Powder-20°C 3 years4°C 2 yearsIn solvent-80°C 6 months-20°C 1 monthShipping at room temperature if less than 2 weeks.7.3 Specific end use(s)No data available.8. EXPOSURE CONTROLS/PERSONAL PROTECTION8.1 Control parametersComponents with workplace control parametersThis product contains no substances with occupational exposure limit values.8.2 Exposure controlsEngineering controlsEnsure adequate ventilation. Provide accessible safety shower and eye wash station.Personal protective equipmentEye protection Safety goggles with side-shields.Hand protection Protective gloves.Skin and body protection Impervious clothing.Respiratory protection Suitable respirator.Environmental exposure controls Keep the product away from drains, water courses or the soil. Cleanspillages in a safe way as soon as possible.9. PHYSICAL AND CHEMICAL PROPERTIES9.1 Information on basic physical and chemical propertiesAppearance White to yellow (Solid)Odor No data availableOdor threshold No data availablepH No data availableMelting/freezing point No data availableBoiling point/range No data availableFlash point No data availableEvaporation rate No data availableFlammability (solid, gas)No data availableUpper/lower flammability or explosive limits No data availableVapor pressure No data availableVapor density No data availableRelative density No data availableWater Solubility No data availablePartition coefficient No data availableAuto-ignition temperature No data availableDecomposition temperature No data availableViscosity No data availableExplosive properties No data availableOxidizing properties No data available9.2 Other safety informationNo data available.10. STABILITY AND REACTIVITY10.1 ReactivityNo data available.10.2 Chemical stabilityStable under recommended storage conditions.10.3 Possibility of hazardous reactionsNo data available.10.4 Conditions to avoidNo data available.10.5 Incompatible materialsStrong acids/alkalis, strong oxidising/reducing agents.10.6 Hazardous decomposition productsUnder fire conditions, may decompose and emit toxic fumes.Other decomposition products - no data available.11.TOXICOLOGICAL INFORMATION11.1 Information on toxicological effectsAcute toxicityClassified based on available data. For more details, see section 2Skin corrosion/irritationClassified based on available data. For more details, see section 2Serious eye damage/irritationClassified based on available data. For more details, see section 2Respiratory or skin sensitizationClassified based on available data. For more details, see section 2Germ cell mutagenicityClassified based on available data. For more details, see section 2CarcinogenicityIARC: No component of this product present at a level equal to or greater than 0.1% is identified as probable, possible or confirmed human carcinogen by IARC.ACGIH: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by ACGIH.NTP: No component of this product present at a level equal to or greater than 0.1% is identified as a anticipated or confirmed carcinogen by NTP.OSHA: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by OSHA.Reproductive toxicityClassified based on available data. For more details, see section 2Specific target organ toxicity - single exposureClassified based on available data. For more details, see section 2Specific target organ toxicity - repeated exposureClassified based on available data. For more details, see section 2Aspiration hazardClassified based on available data. For more details, see section 212. ECOLOGICAL INFORMATION12.1 ToxicityNo data available.12.2 Persistence and degradabilityNo data available.12.3 Bioaccumlative potentialNo data available.12.4 Mobility in soilNo data available.12.5 Results of PBT and vPvB assessmentPBT/vPvB assessment unavailable as chemical safety assessment not required or not conducted.12.6 Other adverse effectsNo data available.13. DISPOSAL CONSIDERATIONS13.1 Waste treatment methodsProductDispose substance in accordance with prevailing country, federal, state and local regulations.Contaminated packagingConduct recycling or disposal in accordance with prevailing country, federal, state and local regulations.14. TRANSPORT INFORMATIONDOT (US)This substance is considered to be non-hazardous for transport.IMDGUN number: 3077Class: 12Packing group: IIIEMS-No: F-A, S-FProper shipping name: ENVIRONMENTALLY HAZARDOUS SUBSTANCE, SOLID, N.O.S.Marine pollutant: Marine pollutantIATAUN number: 3077Class: 12Packing group: IIIProper shipping name: Environmentally hazardous substance, solid, n.o.s.15. REGULATORY INFORMATIONSARA 302 Components:No chemicals in this material are subject to the reporting requirements of SARA Title III, Section 302.SARA 316 Components:This material does not contain any chemical components with known CAS numbers that exceed thethres33&33U_HKSCS33&MingLiU_HKSCS3333333333316. OTHER INFORMATIONCopyright 2018 MedChemExpress. The above information is correct to the best of our present knowledge but does not purport to be all inclusive and should be used only as a guide. The product is for research use only and for experienced personnel. It must only be handled by suitably qualified experienced scientists in appropriately equipped and authorized facilities. The burden of safe use of this material rests entirely with the user. MedChemExpress disclaims all liability for any damage resulting from handling or from contact with this product.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
MedBio_80557-12-6_Grifolic acid技术材料
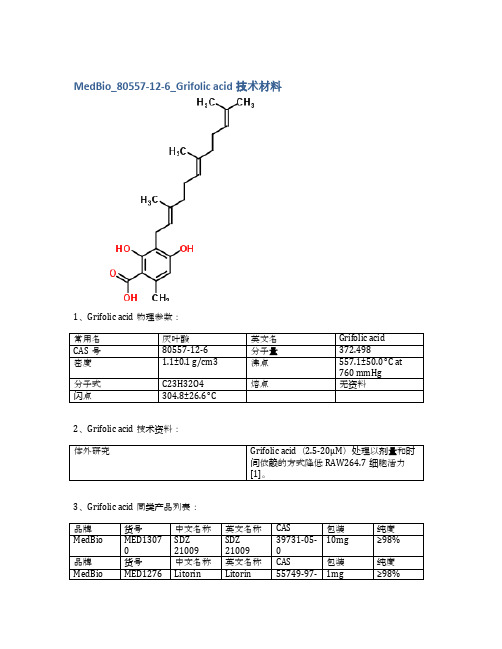
1、Grifolic acid物理参数:
常用名
灰叶酸
英文名
Grifolic acid
CAS号
80557-12-6
分子量
372.498
密度
1.1±0.1 g/cm3
沸点
557.1±50.0 °C at 760 mmHg
分子式
C23H32O4
熔点
无资料
闪点
304.8±26.6 °C
2、Grifolic acid技术资料:
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
MED12763
Litorin
Litorin
55749-97-8
1mg
≥98%
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
MED13068
CL 316243 disodium salt
CL 316243 disodium salt
151126-84-0
50mg
≥98%
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
MED13230
UTPγS trisodium salt
UTPγS trisodium salt
1266569-94-1
1mg
≥98%
体外研究
Grifolic acid(2.5-20µM)处理以剂量和时间依赖的方式降低RAW264.7细胞活力[1]。
3、Grifolic acid同类产品列表:
品牌
货号
中文名称
英文名称
Incucyte

Product Information Presentation, Storage and StabilityThe Incucyte® Fabfluor-pH Antibody Labeling Reagents for antibody internalization are supplied as lyophilized solids in sufficient quantity to label 50 μg of test antibody, when used at the suggested molar ratio (1:3 of test antibody to labeling Fab). The lyophilized solid can be stored at 2-8° C for one year. Once re-hydrated, any unused reagent should be aliquoted and stored at -80° C for up to one year. Avoid repeated freeze-thaw cycles.Incucyte® Fabfluor-pH Antibody Labeling ReagentsFor Antibody Internalization AssaysAntibody Labeling Reagent Rehydrated: -80° C *Excitation and Emission maxima were determined at a pH of 4.5.Fabfluor_quick_guideBackgroundIncucyte ® Fabfluor-pH Antibody Labeling Reagents are designed for quick, easy labeling of Fc-containing test antibodies with a Fab fragment-conjugated pH-sensitive fluorophore. The pH-sensitive dye based system exploits the acidic environment of the lysosomes to quantify in-ternalization of the labeled antibody. As Fabfluor labeled antibodies reside in the neutral extracellular solution (pH 7.4), they interact with cell surface specific antigens and are internalized. Once in the lysosomes, they enter an acidic environment (pH 4.5–5.5) and a substantial in-crease in fluorescence is observed. In the absence of ex-pression of the specific antigen, no internalization occurs and the fluorescence intensity of the labeled antibodies remains low. With the Incucyte ® integrated analysis soft-ware, background fluorescence is minimized. These reagents have been validated for use with a number of different antibodies in a range of cell types. The Incucyte ® Live-Cell Analysis System enables real-time, kinetic eval -uation of antibody internalization.Recommended UseWe recommend that the Incucyte ® Fabfluor-pH Antibody Labeling Reagents are prepared at a stock concentration of 0.5 mg/mL by the addition of 100 μL of sterile water and triturated (centrifuge if solution not clear). The reagent may then be diluted directly into the labeling mixture with test antibody. Do NOT sonicate the solution.Additional InformationThe Fab antibody was purified from antisera by a combination of papain digestion and immunoaffinity chromatography using antigens coupled to agarose beads. Fc fragments and whole IgG molecules have been removed.Human Red (Cat. No. 4722) or Human Orange (Cat. No. 4812)—Based on immunoelectrophoresis and/ or ELISA, the antibody reacts with the Fc portion of human IgG heavy chain but not the Fab portion of human IgG. No antibody was detected against human IgM, IgA or against non-immunoglobulin serum proteins. The anti-body may cross-react with other immunoglobulins from other species.Mouse IgG1 (Cat. No. 4723), IgG2a (Cat. No. 4750) or IgG2b (Cat. No. 4751)—Based on antigen-binding assay and/or ELISA, the antibody reacts with the Fc portion of mouse IgG, IgG2a or IgG2b, respectively, but not the Fab portion of mouse immunoglobulins. No antibody was detected against mouse IgM or against non–immunoglobulin serum proteins. The antibody may cross-react with other mouse IgG subclasses or with immunoglobulins from other species.Rat (Cat. No. 4737)—Based on immunoelectrophoresis and/or ELISA, the antibody reacts with the Fc portion of rat IgG heavy chain but not the Fab portion of rat IgG. No antibody was detected against rat IgM, IgA or against non-immunoglobulin serum proteins. The antibody may cross-react with other immunoglobulins from other species.A.B.C.D.R e d O b j e c t A r e a (x 105 μm 2 p e r w e l l )Time (hours)A U C x 106 (0–12 h )log [α–CD71] (g/mL)Example DataFigure 1: Concentration-dependent increase in antibody internalization of Incucyte ® Fabfluor labeled-α-CD71 in HT1080 cells. α-CD71 and mouse IgG1 isotype control were labeled with Incucyte ® Mouse IgG1 Fabfluor-pH Red Antibody Labeling Reagent. HT1080 cells were treated with either Fabfluor-α-CD71 or Fabfluor-IgG1 (4 μg/mL); HD phase and red fluorescence images were captured every 30 minutes over 12 hours using a 10X magnification. (A) Images of cells treated with Fabfluor-α-CD71 display red fluorescence in the cytoplasm (images shown at 6 h). (B) Cells treated with labeled isotype control display no cellular fluorescence. (C) Time-course of Fabfluor-α-CD71 internalization with increasing concentrations of Fabfluor-α-CD71 (progressively darker symbols). Internalization has been quantified as the red object area for each time-point. (D) Concentration response curve to Fabfluor-α-CD71. Area under the curve (AUC) values have been determined from the time-course shown in panel C (0-12 hours) and are presented as the mean ± SEM, n=3 wells.CD71-FabfluorIgG-FabfluorProtocols and ProceduresMaterialsIncucyte® Fabfluor-pH Antibody Labeling ReagentTest antibody of interest containing human, mouse, or rat IgG Fc region (at known concentration)Target cells of interestTarget cell growth mediaSterile distilled water96-well flat bottom microplate (e.g. Corning Cat. No. 3595) for imaging96-well round black round bottom ULA plate (e.g. Corning Cat. No. 45913799) or amber microtube (e.g. Cole Parmer Cat. No. MCT-150-X, autoclaved) for conjugation step0.01% Poly-L-Ornithine (PLO) solution (e.g. Sigma Cat. No. P4957), optional for non-adherent cells Recommended control antibodiesIt is strongly recommended that a positive and negative control is run alongside test antibodies and cell lines. For example, CD71, which is a mouse anti-human antibody, is recommended as a positive control for the mouse Fab.Anti-CD71, clone MEM-189, IgG1 e.g. Sigma Cat. No. SAB4700520-100UGAnti-CD71, clone CYG4, IgG2a e.g. BioLegend Cat. No. 334102Isotype controls, depending on isotype being studied—Mouse IgG1, e.g. BioLegend Cat. No. 400124, Mouse IgG2a e.g. BioLegend Cat. No. 401501Preparation of Incucyte® Antibody Internalization Assay 1. Seed target cells of interest1.1 Harvest cells of interest and determine cell concentra-tion (e.g. trypan blue + hemocytometer).1.2 Prepare cell seeding stock in target cell growth mediawith a cell density to achieve 40–50% confluence be-fore the addition of labeled antibodies. The suggested starting range is 5,000–30,000 cells/well, although the seeding density will need to be optimized for each cell type.Note: For non-adherent cell types, a well coating may be required to maintain even cell distribution in the well. For a 96-well flat bottom plate, we recommend coating with 50 μL of either 0.01% Poly-L-Or-nithine (PLO) solution or 5 μg/mL fibronectin diluted in 0.1% BSA.Coat plates for 1 hour at ambient temperature, remove solution from wells and then allow the plates to dry for 30-60 minutes prior to cell addition.1.3 Using a multi-channel pipette, seed cells (50 µL perwell) into a 96-well flat bottom microplate. Lightly tapplate side to ensure even liquid distribution in well. Toensure uniform distribution of cells in each well, allowthe covered plate sit on a level surface undisturbed at room temperature in the tissue culture hood for 30minutes. After cells are settled, place the plate insidethe Incucyte® Live-Cell Analysis System to monitor cell confluence.Note: Depending on cell type, plates can be used in assay once cells have adhered to plastic and achieved normal cell morphology e.g.2-3 hours for HT1080 or 1-2 hours for non-adherent cell types. Some cell types may require overnight incubation.2. Label Test Antibody2.1 Rehydrate the Incucyte® Fabfluor-pH Antibody Label-ing Reagent with 100 µL sterile water to result in a final concentration of 0.5 mg/mL. Triturate to mix (centrifuge if solution is not clear).Note: The reagent is light sensitive and should be protected fromlight. Rehydrated reagent can be aliquoted into amber or foilwrapped tubes and stored at -80° C for up to 1 year (avoid freezing and thawing).2.2 Mix test antibody with rehydrated Incucyte® Fabfluor–pH Antibody Labeling Reagent and target cell growth media in a black round bottom microplate or ambertube to protect from light (50 µL/well).a. Add test antibody and Incucyte® Fabfluor–pH Anti-body Labeling Reagent at 2X the final concentration.We suggest optimizing the assay by starting with afinal concentration of 4 µg/mL of test antibody or theFabfluor-pH Antibody Labeling Reagent (i.e. 2Xworking concentration = 8 µg/mL).Note: A 1:3 molar ratio of test antibody to Incucyte® Fabfluor-pHAntibody Labeling Reagent is recommended. The labeling re-agent is a third of the size of a standard antibody (50 and 150KDa, respectively). Therefore, labeling equal quantities will pro-duce a 1:3 molar ratio of test antibody to labeling Fab.b. Make sufficient volume of 2X labeling solution for50 µL/well for each sample. Triturate to mix.c. Incubate at 37° C for 15 minutes protected from light.Note: If performing a range of concentrations of test antibody,e.g. concentration response-curve, it is recommended to createthe dilution series post the conjugation step to ensure consistentmolar ratio. We strongly recommend the use of both a negativeand positive control antibody in the same plate.3. Add labeled antibody to cells3.1 Remove cell plate from incubator.3.2 Using a multi-channel pipette, add 50 µL of 2X labeledantibody and control solutions to designated wells.Remove any bubbles and immediately place plate in the Incucyte® Live-Cell Analysis System and start scanning.Note: To reduce the risk of condensation formation on the lid priorto first image acquisition, maintain all reagents at 37° C prior toplate addition.4. Acquire images and analyze4.1 In the Incucyte® Software, schedule to image every15-30 minutes, depending on the speed of the specific antibody internalization.a Scan on schedule, standard. If the Incucyte® Cell-by-Cell Analysis Software Module (Cat. No. 9600-0031)is available, adherent cell-by-cell or non-adherentcell-by-cell scan types can be selected.b Channel selection: select “phase” and “red” or“phase” and "orange” (depending on reagent used).c Objective: 10X or 20X depending on cell types used,generally 10X is recommended for adherent cells,and 20X for non-adherent or smaller cells.NOTE: The optional Incucyte® Cell-by-Cell Analysis SoftwareModule enables the classification of cells into sub-populationsbased on properties including fluorescence intensity, size andshape. For further details on this analysis module and its appli-cation, please see: /cell-by-cell.4.2 To generate the metrics, user must create an AnalysisDefinition suited to the cell type, assay conditions andmagnification selected.4.3 Select images from a well containing a positiveinternalization signal and an isotype control well(negative signal) at a time point where internalizationis visible.4.4 In the Analysis Definition:Basic Analyzer:a. Set up the mask for the phase confluence measurewith fluorescence channel turned off.b. Once the phase mask is determined, turn the fluores-cence channel on: Exclude background fluorescencefrom the mask using the background subtractionfeature. The feature “Top-Hat” will subtract localbackground from brightly fluorescent objects withina given radius; this is a useful tool for analyzing ob-jects which change in fluorescence intensity overtime.i The radius chosen should reflect the size of thefluorescent object but contain enough backgroundto reliably estimate background fluorescence inthe image; 20-30 μm is often a useful startingpoint.ii The threshold chosen will ensure that objectsbelow a fluorescence threshold will not bemasked.iii Choose a threshold in which red or orange objectsare masked in the positive response image but lownumbers in the isotype control, negative responsewell. For a very sensitive measurement, for example,if interested in early responses, we suggest athreshold of 0.2.NOTE: The Adaptive feature can be used for analysis but maynot be as sensitive and may miss early responses. If interestedin rate of response, Top-Hat may be preferable.Cell-by-Cell (if available):a. Create a Cell-by-Cell mask following the softwaremanual.b. There is no need to separate phase and fluorescencemasks. The default setting of Top-Hat No Mask forthe fluorescence channel will enable backgroundsubtraction without generation of a mask. Ensurethat the Top-Hat radius is set to a value higher thanthe radius of the larger clusters to avoid excess back-ground subtraction.c. The threshold of fluorescence can be determined inCell-by-Cell Classification.Specifications subject to change without notice.© 2020. All rights reserved. Incucyte, Essen BioScience, and all names of Essen BioScience prod -ucts are registered trademarks and the property of Essen BioScience unless otherwise specified. Essen BioScience is a Sartorius Company. Publication No.: 8000-0728-A00Version 1 | 2020 | 04Sales and Service ContactsFor further contacts, visit Essen BioScience, A Sartorius Company /incucyte Sartorius Lab Instruments GmbH & Co. KGOtto-Brenner-Strasse 20 37079 Goettingen, Germany Phone +49 551 308 0North AmericaEssen BioScience Inc. 300 West Morgan Road Ann Arbor, Michigan, 48108USATelephone +1 734 769 1600E-Mail:***************************EuropeEssen BioScience Ltd.Units 2 & 3 The Quadrant Newark CloseRoyston Hertfordshire SG8 5HLUnited KingdomTelephone +44 (0) 1763 227400E-Mail:***************************APACEssen BioScience K.K.4th floor Daiwa Shinagawa North Bldg.1-8-11 Kita-Shinagawa Shinagawa-ku, Tokyo 140-0001 JapanTelephone: +81 3 6478 5202E-Mail:*************************5. Analysis GuidelinesAs the labeled antibody is internalized into the acidic environment of the lysosome, the area of fluorescence intensity inside the cells increases.This can be reported in two ways:Ways to Report Basic AnalyzerCell-by-Cell Analysis* To correct for cell proliferation, it is advisable to normalize the fluorescence area to the total cell area using User Defined Metrics.For Research Use Only. Not For Therapeutic or Diagnostic Use.LicensesFor non-commercial research use only. Not for therapeutic or in vivo applications. Other license needs contact Essen BioS cience.Fabfluor-pH Red Antibody Labeling Reagent: This product or portions thereof is manufactured under license from Carnegie Mellon University and U.S. patent numbers 7615646 and 8044203 and related patents. This product is licensed for sale only for research. It is not licensed for any other use. There is no implied license hereunder for any commercial use.Fabfluor-pH Orange Antibody Labeling Reagent: This product or portions thereof is manufactured under a license from Tokyo University and is covered by issued patents EP2098529B1, JP5636080B2, US8258171, and US9784732 and related patent applications. This product and related products are trademarks of Goryo Chemical. Any application of above mentioned technology for commercial purpose requires a separate li -cense from: Goryo Chemical, EAREE Bldg., SF Kita 8 Nishi 18-35-100, Chuo-Ku, Sapporo, 060-0008 Japan.SupportA complete suite of cell health applications is available to fit your experimental needs. Find more information at /incucyte Foradditionalproductortechnicalinformation,************************************************************/incucyte。
ICH术语表

ICH领域专业术语表(质量、安全性)序号英文中文1"relevant" viruses and "model" viruses“相关”病毒和“模型”病毒225-fold AUC radio25倍的AUC比值3 a single 2 generation study单项包括两代(生殖毒性)的研究4abbreviated or abridged application简略申请5abnormal karyology异常核形6abortions流产7absorbed moisture吸附水8absorption吸收9acceptable daily intake可接受的日摄入量10acceptable test加速试验11acceptance criteia认可标准12accuracy准确性13accuracy准确度14acelerated/stress stability studies加速/强力破坏稳定性研究15acentric fragment无着丝点片段16acetylation 乙酰化作用17achiral assay非手性测定18achlorhydric eldderly老年性胃酸缺乏症19acridine orange吖啶橙20action limits内控限值21active components/compound/moiety活性成分22active ingredient活性组分23active metabolite活性代谢产物24adaption to specific culture conditions特定培养条件的适应25additional test 附加实验26additions添加剂27adduct加合物28adequate exposure充分暴露29adjuvant 佐剂30ADME吸收、分布、代谢、排泄31administration period给药期32adventitious agents外源性因子33adventitious contaminants外来污染物34adventitious viral or mycoplasma contamination外源性病毒或支原体污染35adventitious viruses外源病毒36advers effect不良反应37adverse reaction不良反应38aerobic microorganisms需氧微生物39affinity亲和力40affinity chromatography亲和层析41affinity column亲和柱42against humanised proteins serum antibodies抗人源蛋白血清抗体 43agar and broth琼脂和肉汤44aggregates 聚合体45aggregation聚集46aginal smear阴道涂片 47air ighting reflex空中翻正反射48alkylating electrophilic center烷化亲电子中心49allele基因突变产生的遗传因子50allergenic/allergic extracts过敏原抽提物51allergic reactions过敏性反应(变应性反应)52altenative validated test有效替代试验53altered conjugated forms改变的结合物形式54altered growth 生长改变55ambient condition自然条件56amino acid composition氨基酸组成57amino acid sequence氨基酸顺序58amino acids氨基酸59amino sugars氨基糖60amino-terminal amino acids氨基端氨基酸61ammonia production Rates产氨率62ammoniun sulphide staining of the uterus子宫硫化胺染色 63analogue类似物(同系物)64analogue series of substances同系物65analyte 被测物66analytical method 分析方法67analytical procedure分析方法68anaphase分裂后期69aneuploidy非整倍体70aneuploidy inducer非整倍体诱导剂71animal cell lines动物细胞系72animal tissues or organs动物组织或器官73antennary profile 触角形状74antibiotic resistance genes抗生素耐药基因75antibiotics抗生素76antibody抗体77antibody production tests抗体产生试验78antigenic specificity抗原特异性79antisera抗血清80apoptosis凋亡81applicant申报者82art and ethical standards技术和伦理标准83ascites腹水84assay含量测定85assay procedure定量方法86assessment of genotoxicity遗传毒性评价87attainment of full sexual function达到性成熟 88AUC曲线下面积89auditory startle relex惊愕反射(听觉惊跳反射)90autoimmune自身免疫91autoradiographic assessment放射自显影评价92autoradiography放射自显影93avian鸟类94avidity亲和性95background 背景96bacteria细菌97bacterial mutagenicity test细菌致变突试验98bacterial reverse mutation test细菌回复突变试验99bacterial strains菌株100bacterial test organisms微生物试验菌101base pairs碱基对102base set of strains基本菌株103base substitution碱基置换104batches批次105batch-to-batch逐批106between-assay variation试验间变异107binary fission双数分裂108binding assays结合试验109bioanalytical method生物学分析方法110bioavaiability生物利用度111bioburden生长量/生物负荷112biochemical methods生化方法113bioequivalency生物等效性114biohazard enformation生物有害信息115biological activity生物活性116biological products生物制品117biological relevance生物学意义118bioreactor生物反应器119biotechnological products生物技术产品120biotechnological/biological products生物技术/生物制品121biotechnology-derived pharmaceuticals生物技术药物122biphasic curve双相曲线123birth出生124blood plasma factors血浆因子125body burden机体负担126body fluids体液127bone marrow cell骨髓细胞128bouin's fixation包氏液固定129bovine牛130bovine spongiform encephalopathy(BSE)疯牛病131bracketing括号法132breakage of chromatid染色单体断裂133breakage of chromosome染色体断裂134breeding conditions饲养条件135bridging character桥梁作用136by-products副产物137C(time)一定剂量、某一时间的浓度138calibrate标化139canine犬140cap liner瓶帽内垫141capillary electrophoresis毛细管电泳142carbohydrate碳水化合物143carboxy-terminal amino acids羧基端氨基酸144carcinogen致癌物质145carcinogenesis致癌性146carcinogenic hazard致癌性危害147carcinogenicity bioassay致癌性生物检测148carcinogenicity potential of chemical化合物的潜在致癌性149carcinoginicity(oncogenicity)致癌(致瘤)150cardiovascular心血管151carrier载体/担体152case-by-case个例153catalysts催化剂154cell bank 细胞库155cell bank system细胞库系统156cell banking procedures细胞建库过程157cell banking system细胞库系统158cell culture-derived impurities来源于细胞培养基的杂质159cell cultures 细胞培养物160cell cultures 细胞培养161cell expansion细胞扩增162cell fusion细胞融合163cell line细胞系164cell lines 细胞系165cell membrane lipid细胞膜脂质层166cell metabolites细胞代谢物167cell pooling细胞混合168cell proliferation细胞增植169cell replication system细胞复制系统170cell substrate-derived impurities 来源于细胞基质的杂质171cell substrates细胞基质172cell suspension细胞悬液173cell viability细胞活力174cell-derived biological products细胞来源的生物制品175cell-mediated immunity细胞介导的免疫176cellular blood components血细胞成分177cellular therapy细胞治疗178cemadsorbing viruses红细胞吸附病毒179central nervous systems中枢神经系统180cerbral spinal fluid脑脊液181characterization and testing of cell banks细胞库鉴定及检测182charcoal活性炭183charge电荷184chemical actionmertric system化学光化线强度系统185chemical nature化学性质186chemical reactivity 化学反应性187chemical syntheses化学合成188chemically inert化学惰性189chewable tablets咀嚼片190childbeering potential生育可能性191chinese hamster V79 cell中国仓鼠V79细胞192chiral impurities手性杂质193CHL cell中国仓鼠肺细胞194CHO cell中国仓鼠卵巢细胞195chromatide染色单体196chromatograms色谱图197chromatographic behavior色谱行为198chromatographic procedures色谱方法199chromatography columns色谱分离柱200chromosomal aberration染色体畸变201chromosomal damage染色体损伤202chromosomal integrity染色体完整性203chronic toxicity testing 慢性毒性试验204circular dichroism圆二色性205classfical biotransformation studies经典的生物转化试验206clastogen染色体断裂剂207clastogenic致染色体断裂的208clearance studies清除研究209cleavage of the balanopreputial gland 龟头包皮腺裂开210climatic zones气候带211clinical indication临床适应证212clinical research临床研究213clinical trial application 临床试验申请214clisure闭塞物215cloning 克隆216cloning efficiency克隆形成率217closure of hard palate硬腭闭合218C max峰浓度219coat growth毛发生长220code number编号221coding sequence编码序列222coefficient of variance变异系数223collaborative studies协作实验研究224colony isolation菌落分离225colony sizing集落大小226colony-stimulating factors集落刺激因子227combination product复方制剂228comparative trial对比试验229complement binding补体结合230completely novel compound全新化合物231components成分232compound bearing stuctural alerts结构可疑化合物233concentration threshold阈浓度234conception受孕235concomitant toxicokinetics相伴毒代动力学236confidence interval置信区间237confidence limits可信限238confirmatory studies确认研究239conformance to specifcations符合规范240conformation构型241conjugated product连接产物242conjugation连接243consistency一致性244container容器245container/closure容器/闭塞物246container/closure integrity testing 容器/密封完整性试验247contaminants污染物248contaminated cell substrate污染的细胞基质249content uniformity含量均匀度250continuous treatment 连续接触251control methodology控制方法学252controlled released product控释制剂253conventional live virus vaccines传统的活病毒疫苗254conventional vaccines传统疫苗255cool white fluorescent冷白荧光灯256corpora lutea黄体257corpora lutea count黄体数258correction factor校正因子259correlation coefficient相关系数260covalent or noncovalent共价或非共价261creams霜剂262cross-contamination交叉污染263cross-linking agent交联剂264cross-reactivity交叉反应265cryopreservation冷冻保存266cryoprotectants防冻剂267crystals晶体268culture components 培养基成分269culture condiction培养条件270culture confluency培养克隆率271culture confluenty培养融合272culture media/medium培养基273culture medium培养基274cyanogen bromide溴化氰275cytogenetic细胞遗传学的276cytogenetic change细胞遗传学改变277cytogenetic evaluation细胞遗传学评价278cytokines细胞因子279cytopathic细胞病的280cytoplasmic A-and R-type particles细胞浆a型和r型颗粒281cytotoxicity细胞毒282dark control暗度控制283dead offspring at birth 出生时死亡的子代284deamidation去氨基285deaminated去酰胺化的286deamination脱氨基287decision flow chart/tree判断图288definable and measurable biological activity明确和可测定的生物学活性289degradant降解产物290degradation降解291degradation pathway降解途径292degradation product降解产物293degradation profile降解概况294degree of aggregation 凝集度295degree of scatter离散程度296delay of parturition分娩延迟297delayed-release延迟释放298deleterious有害的299deletion缺失300delivery systems给药体系301derivatives衍生物302description 性状303descriptive statistics描述性统计304detection limit检测限度305detection of bacterial mutagen细菌诱变剂检测306detection of clastogen染色体断裂剂检测307determination of metabolites测定代谢产物308development of the offspring 子代发育309developmental toxicity发育毒性310dilivery systems释放系统311dilution ratio释放倍数312dimers二聚体313diminution of the background lawn背景减少314diode array二极管阵列315diploid cells二倍体细胞316direct genetic damage 直接遗传损伤317dissociation解离318dissolution testing溶出试验319dissolution time溶出时间320distribution分布321DNA adduct DNA加合物322DNA damage DNA损伤323DNA repair DNA修复324DNA strand breaks DNA链断裂325dosage form剂型326dose dependence剂量依赖关系327dose escalation剂量递增328dose level剂量水平329dose -liming toxicity剂量限制性毒性330dose-ranging studies剂量范围研究331dose-related剂量相关 332dose-relatived cytotoxicity剂量相关性细胞毒性333dose-relatived genotoxic activity剂量相关性遗传毒性334dose-relatived mutagenicity剂量相关性诱变性335dose-response curve剂量-反应曲线336dosing route给药途径337downstream purification下游纯化338drug product制剂339drug product components制剂组方340drug substances原料药341duration周期342duration of pregnancy妊娠周期343eaning断奶344earlier physical malformation早期身体畸形345early embryonic development早期胚胎发育346early embryonic development to implantation着床早期的胚胎发育347ectromelia virus脱脚病病毒348elastomeric closures橡皮塞349electro ejaculation电射精350electron microscopy(EM)电镜351electrophoresis电泳352electrophoretic pattern电泳图谱353elimination消除354elution profile洗脱方案355embryofetal deaths胚胎和胎仔死亡356embryo-fetal development 胚胎-胎仔发育357embryo-fetal toxicity胚胎-胎仔毒性358embryonated eggs鸡胚359embryonic death胚胎死亡360embryonic development胚胎发育361embryonic period胚胎期362embryos胚胎 363embryotoxicity胚胎毒性364enantiomer对映体365enantiomer对映异构体366enantiomeric镜像异构体367enantioselective对映体选择性368encephalomyocarditis virus(EMC)脑心肌炎病毒369end of pregnancy怀孕终止370endocytic 内吞噬(胞饮)371endocytic activity内吞噬活性372endogenous agents内源性因子373endogenous components内源性物质374endogenous gene内源性基因375endogenous proteins内源性蛋白376endogenous retrovirus内源性逆转录病毒377endonuclease核酸内切酶378endonuclease release form lysosomes溶酶体释放核酸内切酶379endotoxins内毒素380end-point终点381end-product sterility test-ing最终产品的无菌试验382enhancers增强子383enveloped RNA viruses包膜RNA病毒384environmental factors环境因素385enzymatic reaction rates酶反应速率386enzyme酶387epididymal sperm maturation附睾精子成熟性388epitope表位389epitope抗原决定部位390Epstein-Barr virus (EBV)EB病毒391equine马392error prone repair易错性修复393erythropoietins促红细胞生成素394escalation递增395escherichia coli starn大肠杆菌菌株396esscherichia coli 大肠杆菌397ethnic origin种族起源398eukaryotic cell真核细胞399evaluation of test result试验结果评价400ex vivo体外401exaggerated pharmacological response超常增强的药理作用402excipient赋形剂403excipient specifications赋形剂规范404excretion排泄(消除)405expiration date/dating失效日期406exposure assessment 接触剂量评价407exposure level暴露程度408exposure period光照时间409exposure period接触期410expression constract表达构建体411expression system表达系统412expression vector表达载体413extended-release延时释放414extent of the virus test病毒测试的程度415external metabolising system体外代谢系统416extinction coefficient消光系数417extrachromosomal染色体外418extraneous contaminants外源性污染物419extrapolation 外推法420F1-animals子一代动物421false negative result假阴性结果422false positive result假阳性结果423fecundity多产424feed-back反馈425fermentation发酵426fermentation products发酵产品427fertilisation受精428fertility生育力429fertility studies生育力研究430fetal abnormalities胎仔异常431fetal and neonatal parameters胎仔和仔鼠的生长发育参数432fetal development and growth胎仔发育和生长433fetal period 胎仔期434fetotoxicity胎仔毒性435fill volume装量436filter aids 过滤介质437final manufacturing最终生产438finished product成品439first pass testing 一期试验440flanking region侧翼区441fluorescence in situ hybridisation (FISH)原位荧光分子杂文442foetuses胎仔443forced degradation testing强制降解试验444foreign matter异质性物质445formal labeling正式标签446formal stability studies正式的稳定性研究447formulation 处方/配方448formulation 制剂449fragmentation片段化450frameshift mutation移码突变451frameshift point mutation移码点突变452free-standing独立453freeze-dried product冻干产品454fresh dissection technique新鲜切片技术455friability脆碎度456functional deficits功能试验457functional test功能性指标458funetional indices融合蛋白459fungi真菌460fusion partners融合伴侣461fusion protein融合蛋白462fusion proteins配子463gametes动物性别464gel filtration 凝胶过滤465gender of animals性别专一性药物466gender-specific drug基因剔除467gene amplification基因扩增468gene knockout基因治疗469gene mutation基因突变470gene therapy基因疗法471generation of the cell substrate细胞基质的产生472genetic遗传473genetic change 遗传学改变474genetic damage遗传学损伤475genetic endpoint遗传终点476genetic manipulation基因操作477genetic toxicity遗传毒性478genomic dinucleotide repeats基因组双核苷酸重复数479genomic DNA基因组DNA480genomic polymorphism pattern基因组形态类型481genotoxic activity遗传毒性作用482genotoxic carcinogen遗传毒性致癌剂483genotoxic effect 遗传毒性效应484genotoxic hazard遗传毒性危害485genotoxic potential潜在遗传毒性486genotoxic rodent carcinogen啮齿类动物遗传毒性致癌剂487genotoxicity 遗传毒性488genotoxicity evaluation遗传毒性评价489genotoxicity test遗传毒性试验490genotoxicity test battery遗传毒性试验组合491genotypic 基因型492germ cell mutagen生殖细胞诱变剂493germ line mutation生殖系统突变494GLP临床前研究质量管理规范495glucose consumption rates耗糖率496glycoforms糖化形式497glycosylation糖基化498goegrapgical origin 地理起源499gross chromosomal damage 染色体大损伤500gross evaluation of placenta 胎盘的大体评价501growth factors生长因子502growth hormones 生长激素503guanidine胍504haematoxylin staining苏木素染色505half-life半衰期506hamster antibody production(HAP) test仓鼠抗体产生实验507Hantaan virus汉坦病毒508hardness硬度509heavy metals重金属510hematopoietic cells造血细胞511heparins肝素512heptachlor七氯化合物513herbal products草药514heritable遗传515heritable defect遗传缺陷516heritable disease遗传性疾病517heritable effect 遗传效应518herpes virus 疱疹病毒519heterogeneities异质性520heterohybrid cell lines异种杂交细胞系521high concentration高浓度522high-resolution chromatography高分辨色谱523histologic appearance of reproductive organ生殖器官的组织学表现524histopathological chang组织病理学改变525homogeneity均一性526homologous proteins同系蛋白527homologous series同系528host cell 宿主细胞529host cell banks宿主细胞库530host cell DNA宿主细胞DNA531host cell proteins宿主细胞蛋白质532hot-stage microscopy热价显微镜533human carcinogen人类致癌剂534human cell lines人细胞系535human diploid fibroblasts人二倍体成纤维细胞536human lymphoblastoid TK6 cell 人成淋巴TK6细胞537human mutagen人类致突变剂538human polio virus人脊髓灰质炎病毒539human subjects人体540human tropism人向性541humidity湿度542humidity-protecting containers防湿容器543humoral immunity 体液免疫544hybridization techniques杂交技术545hybridoma cell杂交瘤细胞546hybridomas杂交瘤547hydrolysates水解物548hydrolytic enzymes水解酶549hydrophobicity疏水性550hygroscopic吸湿性551identification/identity鉴别552immature erythrocyte未成熟红细胞553immediate and latent effect速发和迟发效应554immediate container/closure直接接触的容器/密闭物555immediate pack内包装556immediate release立即释放557immortalization激活558immune spleen cells免疫脾细胞559immunoassay免疫检测560immunochemical methods免疫化学方法561immunochemical properties免疫化学性质562immunoelectrophoresis免疫电泳563immunogenicity免疫原性564immunological interations免疫相互作用565immunopathological effects免疫病理反应566immunoreactivity免疫反应性567immunotoxicity免疫毒性568implantation着床569implantation sites着床部位570impurity profile杂质概况571in vitro体外572in vitro and in vivo inoculation tests体内和体外接种试验573in vitro assay体外检测574in vitro cell age体外细胞传代期575in vitro lifespan体外生命周期576in vitro test体外试验577in vitro tests体外试验578in vitro/in vivo correlation体内体外相关性579in vivo体内580in vivo assays体内检测581in vivo test体内试验582inactivated vaccine 灭活疫苗583incidence of polyploid cell 多倍体细胞发生率584incisor eruption门齿萌出585independent test独立试验586indicator cell指示细胞587indicator organisms指示菌588individual fetal body weight单个胎仔体重589indoor indirect daylight室内间接日光590induced and spontaneous models of disease诱发或自发的疾病模型591inducer of micronuclei微核诱导剂592inducers 诱导剂593inedntification test鉴别试验594infectious agents感染性因子595influenza virus流感病毒596inhalation吸入597inhalation dosage forms 吸入剂型598inhibitor of DNA metabolism DNA代谢抑制剂599in-house内部的600in-house criterea内控标准601in-house primary reference material内部一级参比物质602in-house reference materials内部参比物质603in-house working reference material内部工作参比物质604initial filing原始文件605initial submission最初申报606initial text最初文本607inoculation接种608inorganic impurities无机杂质609inorganic mineral无机矿物质610inorganic salts无机盐611in-process acceptance criteia生产过程认可标准612in-process controls生产过程中控制613in-process testing生产过程中检测614insect昆虫615insulins胰岛素616intact animals完整动物(整体动物)617intake摄入618intended effect预期效果619intended storage period 预期的贮藏期620intentional degradation人为降解621interactions相互作用622interferon干扰素623interleukins白细胞介素624intermediate中间体625intermediate precision中间精密度626intermediates半成品627internal control内对照628international reference standards国际参比标准品629interphase muclei分裂间期细胞核630intra-and inter-individual个体与个体间631intra-assay precision间隙含量精密度632intracytoplasmic细胞浆内633introduction of virus病毒介入634inverted or horizontal position倒立或水平位置635ion-exchange离子交换636ionic content离子含量637isoelectric focusing/isoelectrofocusing等电聚焦638isoenzyme analysis同工酶分析639isoform pattern异构体类型640isolated organs离体器官641isomerized 异构化的642Jp/Ph.Eur./Usp.日本药局方/欧洲药典/美国药典643juvenile animal studies未成年动物研究644K virus K病毒645karyology胞核学646Kinetic profile动力学特点647Kinetics 动力学648laboratory scale实验室规模649lactate production rates乳糖产生速率650Lactating授乳、哺乳651lactic dehydrogenase virus (LDM)乳酸脱氢酶病毒652Large deletion event大缺失事件653Late embryo loss后期胚胎丢失654leachables沥出物655Level of safety安全水平656Libido性欲657Life threatering危及生命658ligand 配位体/配体659light光照660light resistant packaging避光包装661limit for in vitro cell age 细胞体外传代限度662limit of acceptance可接受的限度663limit of in vitro cell age 体外细胞代次664limit test限度试验665limulus amoebocyte lysate鲎试剂666linear relation ship 线性关系667linearity线性668Lipophilic compound亲脂性化合物669liquid nitrogen 液氮670liquid oral dosage forms 液体口服制剂671Litter size每窝胎仔数目672Live and dead conceptuese活胎和死胎673Live offspring at birth出生时存活的子代674live vaccine 活疫苗675living cells活细胞676Local toxicity局部毒性677Lockl tolerance studies 局部耐受性研究678Locu位点679logarithmic scale:对数级680long term test长期试验681Long-term carcinogenicity study长期致癌性试验682long-time and accelerated stability长期和加速稳定性试验683Loss of the tk gene tk 基因丢失684losses of activity活性丧失685lot release 批签发686low molecular weight subsances低分子量物质687lower-observed effect level (LOEL)能观察到反应的最低量688lymphocytic choriomeningitis virus (LCM)淋巴细胞性脉络丛脑膜炎病毒689lyophilised cakes冻干粉饼690lysate of cells 细胞溶解物691Major organ fomeation主要器官形成692Male fertility雄性生育力693Male fertility assessment雄性生育力评价694mammalian哺乳类695Mammalian cell mutation test哺乳动物细胞致突变试验696Mammalian cells哺乳动物细胞697Mammalian species哺乳类动物698manufacturing scale生产规模699marieting pack 上市包装700marker chromosome 标志染色体701marketing approval批准上市702Marketing approval上市许可703mass 重量704mass balance质量平衡705mass spectrometry质谱706master cell bank (MCB)主细胞库707Matemal animal亲代动物708material balance物质平衡709Mating behaviour交配行为710Mating period交配期711Mating ratio交配比例712Matrices基质713matrix基质、矩阵714matrix system矩阵化设计715matrixing每日最大剂量716maximum daily dose平均动力学温度717Maximum tolerated dese(MTD)最大耐受剂量718mean kinetic temperature后生动物细胞培养719Mechanism of genotoxicity遗传毒性机制720Mechanistic activation代谢活化721Mechanistic activation pathway代谢活化途径722Mechanistic activation system代谢活化系统723Mechanistic investigation机制研究724Metabolism代谢725Metabolites profile代谢物的概况726Metaphase中期727Metaphase analysis分裂中期相分析728Metaphase cell分裂中期细胞729metazoan cell culture微生物细胞培养730microbial cells微生物细胞731microbial contamunation 微生物污染732microbial expression system微生物表达系统733microbial limits微生物限度734microbial metabolites微生物代谢物735microbial proteases微生物蛋白酶736microbial vaccine antigens微生物疫苗抗原737microbiological testing 微生物学试验738Micronucleus微核739Micronucleus formation微核形成740Microtitre微滴定741Microtitre method微滴定法742Mimicking模拟743minimum exposure time最低作用时间744minimum of pilot plant试产规模745minute virus of mice小鼠小病毒746mirror image 镜像747mismached S-S linked错连的S-S键748Mitotic index有丝分裂指数749modified-/modifying release修饰释放750modifying factor修正因子751moisture level水分752molar absorptivity克分子吸收753Molecular characterisation分子特性754molecular characteristics分子特性755molecular confirmation分子构型756molecular entities/entity分子实体757molecular size分子大小758Molecular technique分子技术759Monitor监测760Monoclonal antibodies单克隆抗体761monoclonal antibody单克隆抗体762mork run空白对照试验763morphological analysis形态学分析764mouse antibody production (MAP) test小鼠抗体产生试验765mouse cytomegalovirus (MCMV)小鼠巨细胞病毒766mouse encephalomyelitis virus (GDVII)小鼠脑脊髓炎病毒767mouse hepatitis virus (MHV)小鼠肝炎病毒768Mouse lymphoma tk assay小鼠淋巴瘤tk检测769Mouse lymphoma L5178Y cell小鼠淋巴瘤L5178Y细胞770mouse rotavirus (EDIM)小鼠小轮状病毒771MuLV murine leukemia virus鼠白血病病毒772murine hybridoma cell lines鼠杂交瘤细胞系773Mutagen诱变原774Mutagen carcinogen诱变性致癌剂775Mutagen potential of chemical化合物的潜在致突变性776Mutant colony突变体集落777Mutation突变778Mutation induction in transgenes转基因诱导突变779mutations 突变780mycoplasma支原体781myeloma cell line骨髓瘤细胞系782Naked eye肉眼783national or international reference material国家或国际参比物质784national reference standards国家参比标准品785near ultraviolet lamp近紫外灯786Necropsy(macroscopic examination)解剖(大体检查)787Negative control阴性对照788Negative result阴性结果789Neonate adaptation to extrautenrine life新生仔宫外生活的适应性790neural sugars中性糖791new chemical entity新化学体792new dosage form新剂型793new drug products/produce新药制剂794new drug substance新原料药795new molecular entities新分子体796Newbom新生仔797Newcleated有核798no effect level不产生反应的量799Non rodent非啮齿类800Non-clinical非临床801noncovalent/convalent forces非共价/共价键802non-enveloped viruses非包膜病毒803Non-genotoxic carcinogen非遗传毒性致癌剂804Non-genotoxic mechanism非遗传毒性机制805Non-human primate非人灵长类806Non-linear非线性807non-mammalian animal cell lines非哺乳动物细胞系808non-recombinant cell-cul-ture expression systems非重组细胞培养表达系统809non-recombinant products/vaccines非重组制品/疫苗810non-specific model virus非特异模型病毒811Non-toxic compound无毒化合物812Non-toxic-effect dose level无毒性反应剂量水平813no-observed effect level不能观察到反应的量814N-terminal sequencing N端测序815nuclear magnetic resonance 核磁共振816Nucleated bone marrow cell有核骨髓细胞817nucleic acid核酸818Nucleoside analogue核苷酸同系物819nucleotide sequences 核苷酸序列820Number of live and dead implantation宫内活胎和死胎数821Numerical chromosmal aberration染色体数目畸变822Numerical chromosome changes染色体数目改变823Oestrous cycle动情周期824official procedure正式方法825ointments软膏826oligonucleotide低聚核苷酸827Oligonucleotide grugs寡核苷酸药物828oligosaccharide pattern寡糖类型829One,two,three generation studies一、二、三子代研究830opacity浊度831Organ development器官发育832organic impurities有机杂质833origins of replication复制起点834osmolality摩尔渗透压浓度835outdoor daylight室外日光836Ovulation rate排卵率837oxidation氧化838oxygen consumption rates耗氧量839package包装840Paraffine embedding石蜡包埋841parainfluenza virus副流感病毒842parallel control assays 平行对照分析843Parameter参数844Parent compound母体化合物845parent stability Guideline稳定性试验总指导原则846parental cell line母细胞系847Parenteral非肠道848parenterals非肠道制剂849particle size粒度850Particulate material颗粒物851particulate matter微粒852Parturition分娩延迟853parvoviruses细小病毒854passage history of the cell line细胞系的传代史855pathogenic agents致病因子856pathogenicity致病性857patterns of degradation降解方式858Pediatric populations小儿人群859peptide肽860peptide map 肽图861percent recovery回收率862periodic/skip testing定期检验/抽验863Peripheral blood erythrocyte外周血红细胞864permitted daily exposure允许的日接触量865Perpoductive competence生殖能力866phage typing噬菌体分型867pharcodynamic studies药效学研究868Pharmacodinetic药代动力学869Pharmacodynamic effects药效作用870Pharmacodynamics药效学(药效动力学)871pharmacopoeial药典872pharmacopoeial pharmacoppeial specifications药典规范873pharmacopoeial standards药典标准874phenotypic 表型875Phenylene diamine苯二胺876phosphorylation磷酸化作用877photostability testing光稳定性试验878Physical development身体发育879physicochemical changes理化改变880physicochemical methods物理化学方法881physico-chemical properties物理化学特性882Physiological stress生理应激883Pilot studies 前期研究884pilot-plant scale试生产规模/中试规模885Pinna unfolding耳廓张开886piston release force活塞释放力887piston travel force活塞移动力888pivotal stability studies关键的稳定性研究889plaque assays菌斑测定890plasmid质粒891Plasmid质粒892plasmid banks质粒库893plasminogen activators纤溶酶原激活素894Plasminogen activators纤维蛋白溶解酶原激活因子895Ploidy整倍体896pneumonia virus of mice小鼠肺炎病毒897Point mutation点突变898poisson distribution泊松分布899Polychromatic erythrocyte嗜多染红细胞900polyclonal antibody多克隆抗体901Polycyclic hydrocarbon多环芳烃902Polymer聚合物903polymerase chain reaction (PCR)聚合酶链式反应904polymorphic form多晶性型905polymorphs多晶型906polyoma virus多瘤病毒907polypeptides多肽908Polyploid cell多倍体细胞909Polyploidy多倍体910Polyploidy induction多倍体诱导911pooled havest集中回收912Poorly soluble compound难溶化合物913population doubling细胞数倍增/群体倍增914porcine猪915Positive control阳性对照916Positive result阳性结果917Post meiotic stages减数分裂后期918Post-approval批准后919Postcoital time frame交配后日期920Postimplantation deaths着床后死亡921Postnatal deaths出生后死亡922post-translational modifications批准后923post-translationally modified forms翻译后修饰924Postweaning development and growth断奶后发育和生长925potency效价926potent功效927Potential 潜在性928potential adverse consequences潜在的不良后果929potential excipients准赋形剂930Potential immunogenecity潜在免疫原性931potential impurity潜在杂质932potential new drug products准新药制剂933potential new drug substances准新药原料934Potentialtarget organs for toxicity潜在毒性靶器官935potentiometric titrimetry电位滴定936powders粉剂937power outages and human error断电和人为错误938preamble引言939Pre-and post-natal development study围产期的发育研究940Pre-and postweaning survival and growth断奶前后的存活和生长941pre-approval or pre-liscense stage批准前或发证前阶段942Precipitate沉淀物943precision精密度944preclinical and clinical studies临床前和临床研究945Preclinical safety evaluation临床前安全性评价946precursors前体947Predetermined criteria预定标准948Prediction of carcinogenicity致癌性预测949Pregnant怀孕950Pregnant and lactating animals怀孕与哺乳期动物951Preimplantation development着床前发育952Preimplantation stages of the embryo胚胎着床前期953preliminary assessment初步评估954preliminary cell bank初级细胞库955Preliminary studies预试验956Premating交配前957Premating treatment交配前给药958preparation制剂959Pre-screening预筛选960preservative防腐剂961Prevalence of abnormalities异常情况的普遍程度962Preweaning断奶前963Primary active entity主要活性实体964primary cells原代细胞965primary stability data主要稳定性数据966primary stability study/formal study/formal stability study主要稳定性研究/正式研究/正式稳定性研究967primary structure一级结构968primer引物969priming regimen接种方案970Priority selection优先选择971probability概率972process characterisation studies工艺鉴定研究973process controls工艺控制974process optimisation工艺优化975process parameters工艺参数976process validation工艺确证977process-related impurities工艺相关杂质978Pro-drug前体药物979product-related imputies产品相关杂质980progenitor祖细胞981prokaryotic cell原核细胞982Prolongation of parturition产程延长983promoters启动子984proposed commercial process模拟上市985protected samples避光样品。
OECD Test No. 460 Fluorescein Leakage Test Method for Identifying Ocular Corrosives and Severe Irrit

OECD/OCDE460Adopted: 2 October 2012© OECD, (2012)You are free to use this material for personal, non-commercial purposes without seeking prior consent from the OECD, provided the source is duly mentioned. Any commercial use of this material is subject to written permission from the OECD.OECD GUIDELINE FOR THE TESTING OF CHEMICALS Fluorescein Leakage Test Method for Identifying Ocular Corrosives and Severe IrritantsINTRODUCTION1. The Fluorescein Leakage (FL) test method is an in vitro test method that can be used under certain circumstances and with specific limitations to classify chemicals (substances and mixtures) as ocular corrosives and severe irritants, as defined by the United Nations (UN) Globally Harmonized System of Classification and Labelling of Chemicals (GHS) (Category 1), the European Union (EU) Regulation on Classification, Labelling and Packaging of Substances and Mixtures (CLP) (Category 1), and the U.S. Environmental Protection Agency (EPA) (Category I) (1) (2) (3). For the purpose of this Test Guideline, severe irritants are defined as chemicals that cause tissue damage in the eye following test substance administration that is not reversible within 21 days or causes serious physical decay of vision, while ocular corrosives are chemicals that cause irreversible tissue damage to the eye. These chemicals are classified as UN GHS Category 1, EU CLP Category 1, or U.S. EPA Category I.2. While the FL test method is not considered valid as a complete replacement for the in vivo rabbit eye test, the FL is recommended for use as part of a tiered testing strategy for regulatory classification and labelling. Thus, the FL is recommended as an initial step within a Top-Down approach to identify ocular corrosives/severe irritants, specifically for limited types of chemicals (i.e. water soluble substances and mixtures) (4)(5).3. It is currently generally accepted that, in the foreseeable future, no single in vitro eye irritation test will be able to replace the in vivo eye test (TG 405 (6)) to predict across the full range of irritation for different chemical classes. However, strategic combinations of several alternative test methods within a (tiered) testing strategy may be able to replace the in vivo eye test (5). The Top-Down approach (5) is designed to be used when, based on existing information, a chemical is expected to have high irritancy potential.Based on the prediction model detailed in paragraph 35, the FL test method can identify Category 1; EU CLP Category 1; U.S. EPA Category I) without any further testing. The same is assumed for mixtures although mixtures were not used in the validation. Therefore, the FL test method may be used to determine the eye irritancy/corrosivity of chemicals, following the460OECD/OCDEsequential testing strategy of TG 405 (6). However, a chemical that is not predicted as ocular corrosive or severe irritant with the FL test method would need to be tested in one or more additional test methods (in vitro and/or in vivo) that are capable of accurately identifying i) chemicals that are in vitro false negative ocular corrosives/severe irritants in the FL (UN GHS Category 1; EU CLP Category 1; U.S. EPA Category I); ii) chemicals that are not classified for eye corrosion/irritation (UN GHS No Category; EU CLP No Category; U.S. EPA Category IV); and/or iii) chemicals that are moderate/mild eye irritants (UN GHS Categories 2A and 2B; EU CLP Category 2; U.S. EPA Categories II and III).5. The purpose of this Test Guideline is to describe the procedures used to evaluate the potential ocular corrosivity or severe irritancy of a test substance as measured by its ability to induce damage to an impermeable confluent epithelial monolayer. The integrity of trans-epithelial permeability is a major function of an epithelium such as that found in the conjunctiva and the cornea. Trans-epithelial permeability is controlled by various tight junctions. Increasing the permeability of the corneal epithelium in vivo has been shown to correlate with the level of inflammation and surface damage observed as eye irritation develops.6. In the FL test method, toxic effects after a short exposure time to the test substance are measured by an increase in permeability of sodium fluorescein through the epithelial monolayer of Madin-Darby Canine Kidney (MDCK) cells cultured on permeable inserts. The amount of fluorescein leakage that occurs is proportional to the chemical-induced damage to the tight junctions, desmosomal junctions and cell membranes, and can be used to estimate the ocular toxicity potential of a test substance. Annex I provides a diagram of MDCK cells grown on an insert membrane for the FL test method.7. Definitions are provided in Annex II.INITIAL CONSIDERATIONS AND LIMITATIONS8. This Test Guideline is based on the INVITTOX protocol No. 71 (7) that has been evaluated in an international validation study by the European Centre for the Validation of Alternative Methods (ECVAM) (8), in collaboration with the US Interagency Coordinating Committee on the Validation of Alternative Methods (ICCVAM) and the Japanese Center for the Validation of Alternative Methods (JaCVAM).9. The FL test method is not recommended for the identification of chemicals which should be classified as mild/moderate irritants or of chemicals which should not be classified for ocular irritation (substances and mixtures) (i.e. GHS Cat. 2A/2B, no category; EU CLP Cat. 2, no category; US EPA Cat. II/III/IV), as demonstrated by the validation study (4) (8).10. The test method is only applicable to water soluble chemicals (substances and mixtures). The ocular severe irritation potential of chemicals that are water soluble and/or where the toxic effect is not affected by dilution is generally predicted accurately using the FL test method (8). To categorise a chemical as water soluble, under experimental conditions, it should be soluble in sterile calcium-containing (at a concentration of 1.0-1.8 mM), phenol red-free, Hanks’ Buffered Salt Solution (HBSS) at a concentration ≥ 250 mg/mL (one dose above the cut-off of 100 mg/mL). However, if the test substance is soluble below the concentration 100 mg/mL,2© OECD, (2012)OECD/OCDE 460 but already induces a FL induction of 20 % at that concentration (meaning FL20 < 100 mg/mL), it can still be classified as GHS Cat. 1 or EPA Cat. 1.11. The identified limitations for this test method exclude strong acids and bases, cell fixatives and highly volatile chemicals from the applicability domain. These chemicals have mechanisms that are not measured by the FL test method, e.g. extensive coagulation, saponification or specific reactive chemistries. Other identified limitations for this method are based upon the results for the predictive capacity for coloured and viscous test substance (8). It is suggested that both types of chemicals are difficult to remove from the monolayer following the short exposure period and that predictivity of the test method could be improved if a higher number of washing steps was used. Solid chemicals suspended in liquid have the propensity to precipitate out and the final concentration to cells can be difficult to determine. When substances within these chemical and physical classes are excluded from the database, the accuracy of FL across the EU, EPA, and GHS classification systems is substantially improved (8).12. Based on the purpose of this test method (i.e. to identify ocular corrosives/severe irritants only), false negative rates (see Paragraph 13) are not critical since such substances would be subsequently tested with other adequately validated in vitro tests or in rabbits, depending on regulatory requirements, using a sequential testing strategy in a weight of evidence approach (6) (see also paragraphs 3 and 4).13. Other identified limitations of the FL test method are based on false negative and false positive rates. When used as an initial step within a Top-Down approach to identify water soluble ocular corrosive/severe irritant substances and mixtures (UN GHS Category 1; EU CLP Category 1; U.S. EPA Category I), the false positive rate for the FL test method ranged from 7% (7/103; UN GHS and EU CLP) to 9% (9/99; U.S. EPA) and the false negative rate ranged from 54% (15/28; U.S. EPA) to 56% (27/48; UN GHS and EU CLP) when compared to in vivo results. Chemical groups showing false positive and/or false negative results in the FL test method are not defined here.14. Certain technical limitations are specific to the MDCK cell culture. The tight junctions that block the passage of the sodium-fluorescein dye through the monolayer are increasingly compromised with increasing cell passage number. Incomplete formation of the tight junctions results in increased FL in the non-treated control. Therefore, a defined permissible maximal leakage in the non-treated controls is important (see paragraph 38: 0% leakage). As with all in vitro assays there is the potential for the cells to become transformed over time, thus it is vital that passage number ranges for the assays are stated.15. The current applicability domain might be increased in some cases, but only after analyzing an expanded data set of studied test substances, preferably acquired through testing (4). This Test Guideline will be updated accordingly as new information and data are considered.16. For any laboratory initially establishing this assay, the proficiency chemicals provided in Annex III should be used. Laboratories can use these chemicals to demonstrate their technical competence in performing the FL test method prior to submitting FL assay data for regulatory hazard classification purposes.PRINCIPLE OF THE TEST3© OECD, (2012)460OECD/OCDE17. The FL test method is a cytotoxicity and cell-function based in vitro assay that is performed on a confluent monolayer of MDCK CB997 tubular epithelial cells that are grown on semi-permeable inserts and model the non-proliferating state of the in vivo corneal epithelium. The MDCK cell line is well established and forms tight junctions and desmosomal junctions similar to those found on the apical side of conjunctival and corneal epithelia. Tight and desmosomal junctions in vivo prevent solutes and foreign materials penetrating the corneal epithelium. Loss of trans-epithelial impermeability, due to damaged tight junctions and desmosomal junctions, is one of the early events in chemical-induced ocular irritation.18. The test substance is applied to the confluent layer of cells grown on the apical side of the insert. A short 1 min exposure is routinely used to reflect the normal clearance rate in human exposures. An advantage of the short exposure period is that water-based substances and mixtures can be tested neat, if they can be easily removed after the exposure period. This allows more direct comparisons of the results with the chemical effects in humans. The test substance is then removed and the non-toxic, highly fluorescent sodium-fluorescein dye is added to the apical side of the monolayer for 30 minutes. The damage caused by the test substance to the tight junctions is determined by the amount of fluorescein which leaks through the cell layer within a defined period of time.19. The amount of sodium-fluorescein dye that passes through the monolayer and the insert membrane into a set volume of solution present in the well (to which the sodium-fluorescein dye leaks in) is determined by measuring spectrofluorometrically the fluorescein concentration in the well. The amount of fluorescein leakage (FL) is calculated with reference to fluoresence intensity (FI) readings from two controls: a blank control, and a maximum leakage control. The percentage of leakage and therefore amount of damage to the tight junctions is expressed, relative to these controls, for each of the set concentrations of the test substance. Then the FL20 (i.e. concentration that causes 20% FL relative to the value recorded for the untreated confluent monolayer and inserts without cells), is calculated. The FL20 (mg/mL) value is used in the prediction model for identification of ocular corrosives and severe irritants (see paragraph 35).20. Recovery is an important part of a test substance’s toxicity profile that is also assessed by the in vivo ocular irritation test. Preliminary analyses indicated that recovery data (up to 72 h following the chemical exposure) could potentially increase the predictive capacity of INVITTOX Protocol 71 but further evaluation is needed and would benefit from additional data, preferably acquired by further testing (7). This Test Guideline will be updated accordingly as new information and data are considered.PROCEDUREPreparation of the cellular monolayer21. The monolayer of MDCK CB997 cells is prepared using sub-confluent cells growing in cell culture flasks in DMEM/Nutrient Mix F12 (1x concentrate with L-glutamine, 15 mM HEPES, calcium (at a concentration of 1.0-1.8 mM) and 10% heat-inactivated FCS/FBS). Importantly, all media/solutions used throughout the FL assay should contain calcium at a concentration between 1.8 mM (200 mg/L) and 1.0 mM (111 mg/L) to ensure tight junction formation and integrity. Cell passage number range should be controlled to ensure even and4© OECD, (2012)OECD/OCDE 460 reproducible tight junctions formation. Preferably, the cells should be within the passage range 3-30 from thawing because cells within this passage range have similar functionality, which aids assay results to be reproducible.22. Prior to performing the FL test method, the cells are detached from the flask by trypsinisation, centrifuged and an appropriate amount of cells is seeded into the inserts placed in 24-well plates (see Annex I). Twelve mm diameter inserts with membrane of mixed cellulose esters, a thickness of 80-150 µm and a pore size of 0.45 µm, should be used to seed the cells. In the validation study, Millicell-HA 12 mm inserts were used. The properties of the insert and membrane type are important as these may affect cell growth and chemical binding. Certain types of chemicals may bind to the Millicell-HA insert membrane, which could affect the interpretation of results. Proficiency chemicals (see Annex III) should be used to demonstrate equivalency if other membranes are used.23. Chemical binding to the insert membrane is more common for cationic chemicals, such as benzalkonium chloride, which are attracted to the positively charged membrane (8). Chemical binding to the insert membrane may increase the chemical exposure period, leading to an over-estimation of the toxic potential of the chemical, but can also physically reduce the leakage of fluorescein through the insert by binding of the dye to the cationic chemical bound to the insert membrane, leading to an under-estimation of the toxic potential of the chemical. This can be readily monitored by exposing the membrane alone to the top concentration of the chemical tested and then adding sodium-fluorescein dye at the normal concentration for the standard time (no cell control). If binding of the sodium-fluorescein dye occurs, the insert membrane appears yellow after the test material has been washed-off. Thus, it is essential to know the binding properties of the test substance in order to be able to interpret the effect of the chemical on the cells.24. Cell seeding on inserts should produce a confluent monolayer at the time of chemical exposure. 1.6 x 105 cells should be added per insert (400 µL of a cell suspension with a density of 4 x 105 cells / mL). Under these conditions, a confluent monolayer is usually obtained after 96 hours in culture. Inserts should be examined visually prior to seeding, so as to ensure that any damages recorded at the visual control described at paragraph 30 is due to handling.25. The MDCK cell cultures should be kept in incubators in a humidified atmosphere, at 5% ± 1% CO2and 37 ± 1 ºC. The cells should be free of contamination by bacteria, viruses, mycoplasma and fungi.Application of the Test and Control Chemicals26. A fresh stock solution of test substance should be prepared for each experimental run and used within 30 minutes of preparation. Test substances should be prepared in calcium-containing (at a concentration of 1.0-1.8 mM), phenol red-free, HBSS to avoid serum protein binding. Solubility of the chemical at 250 mg/mL in HBSS should be assessed prior to testing. If at this concentration the chemical forms a stable suspension or emulsion (i.e.maintains uniformity and does not settle or separate into more than one phase) over 30 minutes, HBSS can still be used as solvent. However, if the chemical is found to be insoluble in HBSS at this concentration, the use of other test methods instead of FL should be considered. The use of light mineral oil as a solvent, in cases where the chemical is found to be insoluble in HBSS, should be5© OECD, (2012)460OECD/OCDEconsidered with caution as there is not enough data available to conclude on the performance of the FL assay under such conditions.27. All chemicals to be tested are prepared in sterile calcium-containing (at a concentration of 1.0-1.8 mM), phenol red-free, HBSS from the stock solution, at five fixed concentrations diluted on a weight per volume basis: 1, 25, 100, 250 mg/mL and a neat or a saturated solution. When testing a solid chemical, a very high concentration of 750 mg/mL should be included. This concentration of chemical may have to be applied on the cells using a positive displacement pipette. If the toxicity is found to be between 25 and 100 mg/mL, the following additional concentrations should be tested twice: 1, 25, 50, 75, 100 mg/mL. The FL20value should be derived from these concentrations provided the acceptance criteria were met.28. The test substances are applied to the confluent cell monolayers after removal of the cell culture medium and washing twice with sterile, warm (37ºC), calcium-containing (at a concentration of 1.0-1.8 mM), phenol red-free, HBSS. Previously, the filters have been visually checked for any pre-existing damages that could be falsely attributed to potential incompatibilities with test chemicals. At least three replicates should be used for each concentration of the test substance and for the controls in each run. After 1 min of exposure at room temperature, the test substance should be carefully removed by aspiration, the monolayer should be washed twice with sterile, warm (37ºC), calcium-containing (at a concentration of 1.0-1.8 mM), phenol red-free, HBSS, and the fluorescein leakage should be immediately measured. 29. Concurrent negative (NC) and positive controls (PC) should be used in each run to demonstrate that monolayer integrity (NC) and sensitivity of the cells (PC) are within a defined historical acceptance range. The suggested PC chemical is Brij 35 (CAS No. 9002-92-0) at 100 mg/mL. This concentration should give approximately 30% fluorescein leakage (acceptable range 20-40% fluorescein leakage, i.e. damage to cell layer). The suggested NC chemical is calcium-containing (at a concentration of 1.0-1.8 mM), phenol red-free, HBSS (untreated, blank control).A maximum leakage control should also be included in each run to allow for the calculation of FL20 values. Maximum leakage is determined using a control insert without cells.Determination of fluorescein permeability30. Immediately after removal of the test and control substances, 400μL of 0.1 mg/mL sodium-fluorescein solution (0.01% (w/v) in calcium-containing [at a concentration of 1.0-1.8 mM], phenol red-free, HBSS) is added to the Millicell-HA inserts. The cultures are kept for 30 minutes at room temperature. At the end of the incubation with fluorescein, the inserts are carefully removed from each well. Visual check is performed on each filter and any damage which may have occurred during handling is recorded.31. The amount of fluorescein that leaked through the monolayer and the insert is quantified in the solution which remained in the wells after removal of the inserts. Measurements are done in a spectrofluorometer at excitation and emission wavelengths of 485 nm and 530 nm, respectively. The sensitivity of the spectrofluorometer should be set so that there is the highest numerical difference between the maximum FL (insert with no cells) and the minimum FL (insert with confluent monolayer treated with NC). Because of the differences in the used spectrofluorometer, it is suggested that a sensitivity is used which will give fluorescence intensity > 4000 at the maximum fluorescein leakage control. The maximum FL value should not be6© OECD, (2012)OECD/OCDE 460 greater than 9999. The maximum fluorescence leakage intensity should fall within the linear range of the spectrofluorometer used.Interpretation of results and Prediction model32. The amount of FL is proportional to the chemical-induced damage to the tight junctions. The percentage of FL for each tested concentration of chemical is calculated from the FL values obtained for the test substance with reference to FL values from the NC (reading from the confluent monolayer of cells treated with the NC) and a maximum leakage control (reading for the amount of FL through an insert without cells).The mean maximum leakage fluorescence intensity = xThe mean 0% leakage fluorescence intensity (NC) = yThe mean 100% leakage is obtained by subtracting the mean 0% leakage from the mean maximum leakage,i.e. x - y = z33. The percentage leakage for each fixed dose is obtained by subtracting the 0% leakage to the mean fluorescence intensity of the three replicate readings (m), and dividing this value by the 100% leakage, i.e. %FL = [(m-y) / z] x 100%, where:m = the mean fluorescence intensity of the three replicate measurements for the concentration involved% FL = the percent of the fluorescein which leaks through the cell layer34. The following equation for the calculation of the chemical concentration causing 20% FL should be applied:FL D = [(A-B) / (C-B)] x (M C –M B) + M BWhere:D = % of inhibitionA = % damage (20% fluorescein leakage)B = % fluorescein leakage < AC = % fluorescein leakage > AM C = Concentration (mg/mL) of CM B = Concentration (mg/mL) of B35. The cut-off value of FL20 for predicting chemicals as ocular corrosives/severe irritants is given below:7© OECD, (2012)460OECD/OCDE36. The FL test method is recommended only for the identification of water soluble ocular corrosives and severe irritants (UN GHS Category 1, EU CLP Category 1, U.S. EPA Category I) (see paragraphs 1 and 10).37. In order to identify water soluble chemicals (substances and mixtures) (4) (7) (8) as "inducing serious eye damage" (UN GHS/EU CLP Category 1) or as an "ocular corrosive or severe irritant" (U.S. EPA Category I), the test substance should induce an FL20 value of ≤ 100 mg/mL.Acceptance of results38. The mean maximum fluorescein leakage value (x) should be higher than 4000 (see paragraph 31), the mean 0% leakage (y) should be equal or lower than 300, and the mean 100% leakage (z) should fall between 3700 and 6000.39. A test is considered acceptable if the positive control produced 20% to 40% damage to the cell layer (measure as % fluorescein leakage).DATA AND REPORTINGData40. For each run, data from individual replicate wells (e.g. fluorescence intensity values and calculated percentage FL data for each test substance, including classification) should be reported in tabular form. In addition, means ± SD of individual replicate measurements in each run should be reported.Test Report41. The test report should include the following information:Test and Control Substances-Chemical name(s) such as the structural name used by the Chemical Abstracts Service (CAS), followed by other names, if known;-Chemical CAS number, if known;-Purity and composition of the substance or mixture (in percentage(s) by weight), to the extent this information is available;-Physical-chemical properties relevant to the conduct of the study (e.g. physical state, volatility, pH, stability, water solubility, chemical class);-Treatment of the test/control substance prior to testing, if applicable (e.g. warming, grinding);-Storage conditions;Justification of the Test Method and Protocol Used-Should include considerations regarding applicability domain and limitations of the test method;Test Conditions8© OECD, (2012)OECD/OCDE 460 -Description of cell system used, including certificate of authenticity and the mycoplasma status of the cell line;-Details of test procedure used;-Test substance concentration(s) used;-Duration of exposure to the test substance;-Duration of incubation with fluorescein;-Description of any modifications of the test procedure;-Description of evaluation criteria used;-Reference to historical data of the model (e.g. negative and positive controls, benchmark chemicals, if applicable);-Information on the technical proficiency demonstrated by the laboratory;Results-Tabulation of data from individual test substances and controls for each run and each replicate measurement (including individual results, means and SDs);-The derived classification(s) with reference to the prediction model and/or decision criteria used;-Description of other effects observed;Discussion of the Results-Should include considerations regarding a non-conclusive outcome (paragraph 35: FL20 > 100 mg/mL) and further testing;Conclusions9© OECD, (2012)460OECD/OCDELITERATURE1.UN (2009), United Nations Globally Harmonized System of Classification and Labelling ofChemicals (GHS), Third revised edition, New York & Geneva: United Nations Publications.ISBN: 978-92-1-117006-1. Available at:[/trans/danger/publi/ghs/ghs_rev03/03files_e.html]2.EC (2008), Regulation (EC) No 1272/2008 of the European Parliament and of the Council of16 December 2008 on classification, labelling and packaging of substances and mixtures,amending and repealing Directives 67/548/EEC and 1999/45/EC, and amending Regulation (EC) No 1907/2006, Official Journal of the European Union L353, 1-1355.3.U.S. EPA (1996), Label Review Manual: 2nd Edition, EPA737-B-96-001, Washington DC:U.S. Environmental Protection Agency.4.EC-ECVAM (2009), Statement on the scientific validity of cytotoxicity/cell-function based invitro assays for eye irritation testing. Available under Publications at: [http://ecvam.jrc.it/index.htm]5.Scott, L. et al. (2010), A proposed eye irritation testing strategy to reduce and replace in vivostudies using Bottom-Up and Top-Down approaches, Toxicol. In Vitro 24, 1-9.6.OECD (2002), Test No. 405: Acute Eye Irritation/Corrosion, OECD Guidelines for theTesting of Chemicals, Section 4, OECD Publishing. doi: 10.1787/9789264070646-en7.EC-ECVAM (1999), INVITOX Protocol 71: Fluorescein Leakage Test, Ispra, Italy:European Centre for the Validation of Alternative Methods (ECVAM). Available at: [http://ecvam-dbalm.jrc.ec.europa.eu]8.EC-ECVAM (2008), Fluorescein Leakage Assay Background Review Document as anAlternative Method for Eye Irritation Testing. Available under Validation Study Documents, Section Eye Irritation at: [http://ecvam.jrc.it/index.htm]9.OECD (2005), Guidance Document on the Validation and International Acceptance of Newor Updated Test Methods for Hazard Assessment, OECD Series on Testing and Assessment No. 34. OECD, Paris. Available at: [/env/testguidelines]10© OECD, (2012)。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Product Name:
Folinic acid CAS No.:
58-05-9Cat. No.:
HY-17556Product Data Sheet
MWt:
473.44Formula:
C20H23N7O7Purity :>98%
Solubility:
DMSO
y Mechanisms:
Biological Activity:
Folinic acid is an adjuvant used in cancer chemotherapy involving the drug methotrexate Pathways:Cell Cycle/DNA Damage; Target:Antifolate Folinic acid is an adjuvant used in cancer chemotherapy involving the drug methotrexate.
Target: Antifolate Folinic acid is a 5-formyl derivative of tetrahydrofolic acid. It is readily converted to other reduced folic acid derivatives (e.g., tetrahydrofolate), and, thus, has vitamin activity that is equivalent to that of folic acid. Since it does not require the action of dihydrofolate reductase for its conversion, its function as a vitamin is unaffected by inhibition of this enzyme by drugs such as methotrexate. In 1980s, however, folinic acid was found to reactivate the dihydrofolate reductase itself even when methotrexate exists. Although the mechanism is not very clear, the polyglutamylation of
methotrexate and dihydrofolate in malignant cells is considered to play an important role in the
References:
[1]. Goldman, I.D. and L.H. Matherly, Biochemical factors in the selectivity of leucovorin rescue:selective inhibition of leucovorin reactivation of dihydrofolate reductase and leucovorin utilization in purine and pyrimidine biosynthesis by methotrexate and dihydrofolate polyglutamates. NCI Monogr,y g p y p selective reactivation of dihydrofolate reductase by folinic acid in normal ...
1987(5): p. 17-26.[2]. Keshava, C., et al., Inhibition of methotrexate-induced chromosomal damage by folinic acid in
V79 cells. Mutat Res, 1998. 397(2): p. 221-8.Caution: Not fully tested. For research purposes only
Medchemexpress LLC
18W i l k i n s o n W a y , P r i n c e t o n , N J 08540,U S A
E m a i l : i n f o @m e d c h e m e x p r e s s .c o m W e b : w w w .m e d c h e m e x p r e s s .c o m。
