USP1227-VALIDATION OF MICROBIAL RECOVERY FROM PHARMACOPEIAL ARTICLES美国药典微生物回收率验证
USP40 1226 药典的确认中英文对照

1226 VERIFICATION OF COMPENDIAL PROCEDURES药典方法的确认The intent of this general information chapter is to provide general information on the verification of compendial procedures that are being performed for the first time to yield acceptable results utilizing the personnel, equipment, and reagents available.此章节的意图是对药典方法的确认提供基本资料,使用人员,设备和试剂使第一次进行运用药典方法以产生可接受的结果。
This chapter is not intended for retroactive application to already successfully established laboratory procedures. The chapter Validation of Compendial Procedures <1225>provides general information on characteristics that should be considered for various test categories and on the documentation that should accompany analytical procedures submitted for inclusion in USP–NF.Verification consists of assessing selected analytical performance characteristics, such as those that are described in chapter <1225>to generate appropriate, relevant data rather than repeating the validation process.此章节并不旨在对已经成功建立的实验室方法进行回顾性运用。
USP微生物试验说明

美國藥典微生物試驗說明Essentials of USP Microbiological Testing研討會大綱I.基礎微生物學II.良好微生物實驗室規範III.藥典協同之改變IV.IV.滅菌滅菌滅菌與無菌保證與無菌保證V.微生物方法之確效微生物學研究微小生命形式的生物學分支研究微小生命形式的生物學分支。
典型的微小生命形式是單細胞的生命形式的微小生命形式是單細胞的生命形式,,也包括非常少也包括非常少量量的多細胞生物體 新陳代謝基本需求能量(如光、硫、一氧化碳或氨一氧化碳或氨))碳、氮、鈣、磷、硫、鎂、鉀和鈉 水(即使真菌也需要0.6 Aw 以上的游以上的游離離水)分類學生物環境多樣性嗜低溫菌嗜中溫菌嗜高溫菌生物環境多樣性嗜酸性菌嗜中性菌嗜鹼性菌依DNA在細胞中的情況可將細胞分為兩大類:真核細胞Eukaryotic cells原核細胞Prokaryotic cells原核生物Prokaryotic cells大部分細菌對人體無害如: 乳酸菌但, 也有微生物會致病細菌(分2界) 古細菌 真細菌原核生物革原核生物革蘭蘭氏分類氏分類法法Hans C.J. Gram –丹麥細菌學家(1853-1938).發現發現了了細胞學上的染色和染色技術細胞學上的染色和染色技術((革蘭氏染色染色),),),用於區分細菌的分用於區分細菌的分用於區分細菌的分類類學組群學組群。
脂多糖(相對耐熱)如果進入人體,能導致發燒、白血球減少、腹瀉、休克(熱原Pyrogen)(內毒素Endotoxin)革蘭氏陽性結構革蘭氏陰性結構什麼是芽孢?在惡劣惡劣的環境條件下產生的有再生能的環境條件下產生的有再生能的環境條件下產生的有再生能力力的細胞或細胞群的細胞或細胞群。
特徵特徵::–厚壁–耐惡劣惡劣環境環境–非常緩慢的代謝速非常緩慢的代謝速度度分4界原生動物 動物植物真菌真菌-特徵皆為化學異異營菌皆為化學具強大的酵素 (腐生或寄生腐生或寄生) ) ) 具強大的酵素pH5))酸性的生活環境((pH5 比細菌比細菌更更酸性的生活環境高鹽、、高糖環境中生長高鹽黴菌和蕈是多細胞酵母是單細胞新陳代謝Metabolism 依據分解代謝是否能夠使用O2為什麼了解微生物對新陳代謝的需求很重要?為的是想培養微生物生長更為的是防止微生物生長USP微生物相關務必遵守章節<51> 防腐效力/Antimicrobial Preservatives –Effectiveness耐受力力測試<55> 生物指示劑–耐受Biological Indicators –Resistance Performance Tests微生物計數數試驗非無菌產品的微生物檢驗::微生物計<61> 非無菌產品的微生物檢驗Microbiological Examination of Nonsterile Products:Microbial Enumeration Tests<62> 特定菌的微生物檢查/ Microbiological Proceduresfor Absence of Specified Microorganisms<71> 無菌試驗/ Sterility Tests<85> 細菌內毒素試驗Bacterial Endotoxins TestUSP微生物相關章節建議遵循<1035> 滅菌生物指示劑<1072> 消毒劑與殺菌劑<1111> 非無菌產品的微生物評估<1112> 水活性應用<1116> 清淨室和其他管制環境的微生物評估<1117> 微生物實驗室規範 <1207> 無菌產品包裝–完整性評估 <1209> 滅菌–化學和物理化學指示劑,及綜合指示劑<1211> 滅菌與無菌保證<1222> 最終滅菌藥品–參數放行 <1223> 微生物方法確效<1227> 藥物的微生物回收率確效 <1231> 製藥用水<2021> 微生物計數試驗–營養和膳食補充劑<2022> 特定菌的微生物檢查–營養和膳食補充劑<2023> 非無菌營養和膳食補充劑的微生物評估EP & USP & JP 藥典方法的國際協合International Harmonization of Pharmacopoeia時限–現行的EP 只維持到2008年12月31日–USP 將維持至2009年4月影響行適用性試驗並需重新進行–各藥廠將必需提早因應,並需重新進批產品來來測試其一致性.( Suitability Test),以至少3 批產品此協合後的方法不不僅在產品( non-sterile)的稀釋液此協合後的方法及預試驗,生長試驗上有改變,在部份培養基的組成有所改變,甚至新增一些檢驗項目與培養基,在有些品管菌株及接種量量都有改變品管菌株及接種微生物限量(USP <61> & EP 2.6.12) Microbial Enumeration TestTAMC= Total aerobic microbial count (on TSA, incl. fungi)TYMC= Total combined yeast/mould count (on Sab.4%-Dextrose Agar, incl. bacteria)特定微生物USP <62> & EP 2.6.13 Tests for Specific Microorganisms目前–Escherichia coli–Salmonella–Pseudomonasaeruginosa–Staphylococcusaureus 協調後–Escherichia coli–Salmonella–Pseudomonas aeruginosa–Staphylococcus aureus–Clostridia 產芽孢梭菌–Bile-tolerant Gram-negative bacteria 耐膽鹽革耐膽鹽革蘭蘭氏陰性菌–Candida albicans白色白色念念珠菌Test for Escherichia coli:Presence/Absence (Current USP Method)Test for Escherichia coli:Presence/Absence (Harmonized Method)<1112> 水活性應用水活性是水活性是什什麼?–產品中水的蒸氣壓P 與相同溫與相同溫度度下純水中的蒸氣壓Po 的比的比率率。
USP401225药典的验证中英文对照

VALIDATION OF COMPENDIAL PROCEDURES药典方法的验证Test procedures for assessment of the quality levels of pharmaceutical articles are subject to various requirements. According to Section 501 of the Federal Food, Drug, and Cosmetic Act, assays and specifications in monographs of the United States Pharmacopeia and the National Formulary constitute legal standards. The Current Good Manufacturing Practice regulations [21 CFR 211.194(a)] require that test methods, which are used for assessing compliance of pharmaceutical articles with established specifications, must meet proper standards of accuracy and reliability. Also, according to these regulations [21 CFR 211.194(a)(2)], users of analytical methods described in USP NF are not required to validate the accuracy and reliability of these methods, but merely verify their suitability under actual conditions of use. Recognizing the legal status of USP and NF standards, it is essential, therefore, that proposals for adoption of new or revised compendial analytical procedures be supported by sufficient laboratory data to document their validity.用于评估药品质量的检验方法需要满足不同的要求。
注射用盐酸吉西他滨无菌方法验证

注射用盐酸吉西他滨无菌方法验证目的对注射用盐酸吉西他滨的无菌检查方法进行探讨,建立其无菌检查方法。
方法按中国药典2010附录ⅪH无菌检查方法进行。
结果在验证条件下,该方法能消除对微生物生长的抑制作用。
结论可以采用此法对本品进行无菌检查。
标签:注射用盐酸吉西他滨;无菌方法;验证盐酸吉西他滨(gemcitabine hydrochloride)化学名2-脱氧-2,2-盐酸二氟脱氧胞苷(β-异构体),是一种阿糖胞苷类似物,由美国礼来公司研发的核苷类抗代谢抗癌药[1]。
属于新型抗嘧啶核苷酸代谢化疗药物,属细胞周期特异性抗代谢类药物,主要作用于DNA合成期的肿瘤细胞,即S期细胞。
在一定条件下,可以阻止G1期向S期的进展;具有抗瘤谱广、作用机制独特、毒性反应低、与其他化疗药物无交叉耐药且毒性反应无叠加等特点。
在过去的临床工作中,观察到盐酸吉西他滨是疗效较好而毒副反应较少的化疗药物之一[2-3]。
无菌检查、细菌内毒素、微生物限度检查是药品安全性检查的重要项目[4],本品为冻干粉针按照中国药典2010版附录ⅠB[5]的要求,要进行细菌内毒素检查。
1 材料与方法1.1 仪器HTY-2000A全封闭集菌仪和NKF集菌培养器(杭州高德泰林有限公司),LS-B50L立式压力蒸汽灭菌锅,SP120水夹套恒温培养箱,PYX-DHS-50X65-BS 隔水式电热恒温培养箱,MJX-160B霉菌培养箱。
AKL1500净化工作台。
1.2 试剂培养基:硫乙醇酸盐流体培养基,改良马丁培养基,营养琼脂培养基,玫瑰红钠琼脂培养基,0.1%无菌蛋白胨水溶液,0.9%无菌氯化钠溶液。
实验菌株:白色念珠菌[CMCC(F)98 001],黑曲霉[CMCC(F)98 003],铜绿假单胞菌[CMCC (B)10 104],金黄色葡萄球菌[CMCC(B)26 003],枯草芽孢杆菌[CMCC(B)63 501],生孢梭菌[CMCC(B)64 941],大肠埃希菌[CMCC(B)44 102]以上菌种均为江苏省康华医药科技实业中心。
USP 1223 VALIDATION OF ALTERNATIVE MICROBIOLOGICAL METHODS

1223VALIDATION OF ALTERNATIVE MICROBIOLOGICAL METHODSINTRODUCTIONThe purpose of this chapter is to provide guidance for validating methods for use as alternatives to the official compendial microbiological methods. For microbial recovery and identification, microbiological testing laboratories sometimes use alternative test methods to those described in the general chapters for a variety of reasons, such as economics, throughput, and convenience. Validation of these methods is required. Some guidance on validation of the use of alternate methods is provided in the Tests and Assays section in the General Notices and Requirements. This section also notes that in the event of a dispute, only the result obtained by the compendial test is conclusive.Validation studies of alternate microbiological methods should take a large degree of variability into account. When conducting microbiological testing by conventional plate count, for example, one frequently encounters a range of results that is broader (%RSD 15 to 35) than ranges in commonly used chemical assays (%RSD 1 to 3). Many conventional microbiological methods are subject to sampling error, dilution error, plating error, incubation error, and operator error.Validation of Compendial Procedures 1225defines characteristics such as accuracy, precision, specificity, detection limit, quantification limit, linearity, range, ruggedness, and robustness in their application to analytical methods. These definitions are less appropriate for alternate microbiological m ethod validation as ―at least equivalent to the compendial method‖ given the comparative nature of the question (see the Tests and Assays—Procedures section in General Notices and Requirements). The critical question is whether or not the alternate method will yield results equivalent to, or better than, the results generated by the conventional method.Other industry organizations have provided guidance for the validation of alternate microbiological methods.* The suitability of a new or modified method should be demonstrated in a comparison study between the USP compendial method and the alternate method. The characteristics defined in this chapter may be used to establish this comparison.TYPES OF MICROBIOLOGICAL TESTSIt is critical to the validation effort to identify the portion of the test addressed by an alternate technology. For example, there is a variety of technologies available to detect the presence of viable cells. These techniques may have application in a variety of tests (e.g., bioburden, sterility test) but may not, in fact, replace the critical aspects of the test entirely. For example, a sterility test by membrane filtration may be performed according to the compendial procedure up to the point of combining the processed filter with therecovery media, and after that the presence of viable cells might then be demonstrated by use of some of the available technologies. Validation of this application would, therefore, require validation of the recovery system employed rather than the entire test.There are three major types of determinations specific to microbiological tests. These include tests to determine whether microorganisms are present in a sample, tests to quantify the number of microorganisms (or to enumerate a specific subpopulation of the sample), and tests designed to identify microorganisms. This chapter does not address microbial identification.Qualitative Tests for the Presence or Absence of MicroorganismsThis type of test is characterized by the use of turbidity in a liquid growth medium as evidence of the presence of viable microorganisms in the test sample. The most common example of this test is the sterility test. Other examples of this type of testing are those tests designed to evaluate the presence or absence of a particular type of microorganism in a sample (e.g., coliforms in potable water and E. coli in oral dosage forms).Quantitative Tests for MicroorganismsThe plate count method is the most common example of this class of tests used to estimate the number of viable microorganisms present in a sample. The membrane filtration and Most Probable Number (MPN) multiple-tube methods are other examples of these tests. The latter was developed as a means to estimate the number of viable microorganisms present in a sample not amenable to direct plating or membrane filtration.General ConcernsValidation of a microbiological method is the process by which it is experimentally established that the performance characteristics of the method meet the requirements for the intended application, in comparison to the traditional method. For example, it may not be necessary to fully validate the equivalence of a new quantitative method for use in the antimicrobial efficacy test by comparative studies, as the critical comparison is between the new method of enumeration and the plate count method (the current method for enumeration). As quantitative tests, by their nature, yield numerical data, they allow for the use of parametric statistical techniques. In contrast, qualitative microbial assays, e.g., the sterility test in the example above, may require analysis by nonparametric statistical methods. The validation of analytical methods for chemical assays followswell-established parameters as described in Validation of Compendial Procedures 1225. Validation of microbiological methods shares some of the same concerns, although consideration must be given to the unique nature of microbiological assays (see Table 1).Table 1. Validation Parameters by Type of Microbiological TestVALIDATION OF QUALITATIVE TESTS FOR DEMONSTRATION OF VIABLEMICROORGANISMS IN A SAMPLESpecificityThe specificity of an alternate qualitative microbiological method is its ability to detect a range of microorganisms that may be present in the test article. This concern is adequately addressed by growth promotion of the media for qualitative methods that rely upon growth to demonstrate presence or absence of microorganisms. However, for those methods that do not require growth as an indicator of microbial presence, the specificity of the assay for microbes assures that extraneous matter in the test system does not interfere with the test.Limit of DetectionThe limit of detection is the lowest number of microorganisms in a sample that can be detected under the stated experimental conditions. A microbiological limit test determines the presence or absence of microorganisms, e.g., absence of Salmonella spp. in 10 g. Due to the nature of microbiology, the limit of detection refers to the number of organisms present in the original sample before any dilution or incubation steps; it does not refer to the number of organisms present at the point of assay.One method to demonstrate the limit of detection for a quantitative assay would be to evaluate the two methods (alternative and compendial) by inoculation with a low number of challenge microorganisms (not more than 5 cfu per unit) followed by a measurement of recovery. The level of inoculation should be adjusted until at least 50% of the samples show growth in the compendial test. It is necessary to repeat this determination several times, as the limit of detection of an assay is determined from a number of replicates (notless than 5). The ability of the two methods to detect the presence of low numbers of microorganisms can be demonstrated using the Chi square test. A second method to demonstrate equivalence between the two quantitative methods could be through the use of the Most Probable Number technique. In this method, a 5-tube design in a ten-fold dilution series could be used for both methods. These would then be challenged with equivalent inoculums (for example, a 10–1, 10–2, and 10–3 dilution from a stock suspension of approximately 50 cfu per mL to yield target inocula of 5, 0.5, and 0.05 cfu per tube) and the MPN of the original stock determined by each method. If the 95% confidence intervals overlapped, then the methods would be considered equivalent.RuggednessThe ruggedness of a qualitative microbiological method is the degree of precision of test results obtained by analysis of the same samples under a variety of normal test conditions, such as different analysts, instruments, reagent lots, and laboratories. Ruggedness can be defined as the intrinsic resistance to the influences exerted by operational and environmental variables on the results of the microbiological method. Ruggedness is a validation parameter best suited to determination by the supplier of the test method who has easy access to multiple instruments and batches of components.RobustnessThe robustness of a qualitative microbiological method is a measure of its capacity to remain unaffected by small but deliberate variations in method parameters, and provides an indication of its reliability during normal usage. Robustness is a validation parameter best suited to determination by the supplier of the test method. As there are no agreed upon standards for current methods, acceptance criteria are problematic and must be tailored to the specific technique. It is essential, however, that an estimate of the ruggedness of the alternate procedure be developed. The measure of robustness is not necessarily a comparison between the alternate method and the traditional, but rather a necessary component of validation of the alternate method so that the user knows the operating parameters of the method.VALIDATION OF QUANTITATIVE ESTIMATION OF VIABLE MICROORGANISMS IN ASAMPLEAs colony-forming units follow a Poisson distribution, the use of statistical tools appropriate to the Poisson rather than those used to analyze normal distributions is encouraged. If the user is more comfortable using tools geared towards normally distributed data, the use of a data transformation is frequently useful. Two techniques are available and convenient for microbiological data. Raw counts can be transformed to normally distributed data either by taking the log10 unit value for that count, or by takingthe square root of count +1. The latter transformation is especially helpful if the data contain zero counts.AccuracyThe accuracy of this type of microbiological method is the closeness of the test results obtained by the alternate test method to the value obtained by the traditional method. It should be demonstrated across the operational range of the test. Accuracy is usually expressed as the percentage of recovery of microorganisms by the assay method. Accuracy in a quantitative microbiological test may be shown by preparing a suspension of microorganisms at the upper end of the range of the test, that has been serially diluted down to the lower end of the range of the test. The operational range of the alternate method should overlap that of the traditional method. For example, if the alternate method is meant to replace the traditional plate count method for viable counts, then a reasonable range might be from 100 to 106 cfu per mL. At least 5 suspensions across the range of the test should be analyzed for each challenge organism. The alternate method should provide an estimate of viable microorganisms not less than 70% of the estimate provided by the traditional method, or the new method should be shown to recover at least as many organisms as the traditional method by appropriate statistical analysis, an example being an ANOVA analysis of the log10 unit transforms of the data points. Note that the possibility exists that an alternate method may recover an apparent higher number of microorganisms if it is not dependent on the growth of the microorganisms to form colonies or develop turbidity. This is determined in the Specificity evaluation.PrecisionThe precision of a quantitative microbiological method is the degree of agreement among individual test results when the procedure is applied repeatedly to multiple samplings of suspensions of laboratory microorganisms across the range of the test. The precision of a microbiological method is usually expressed as the standard deviation or relative standard deviation (coefficient of variation). However, other appropriate measures may be applied. One method to demonstrate precision uses a suspension of microorganisms at the upper end of the range of the test that has been serially diluted down to the lower end of the range of the test. At least 5 suspensions across the range of the test should be analyzed. For each suspension at least 10 replicates should be assayed in order to be able to calculate statistically significant estimates of the standard deviation or relative standard deviation (coefficient of variation). Generally, a RSD in the 15% to 35% range would be acceptable. Irrespective of the specific results, the alternate method should have a coefficient of variation that is not larger than that of the traditional method. For example, a plate count method might have the RSD ranges as shown in the following table.Table 2. Expected RSD as a Function of cfu per PlateThe specificity of a quantitative microbiological method is its ability to detect a panel of microorganisms suitable to demonstrate that the method is fit for its intended purpose. This is demonstrated using the organisms appropriate for the purpose of the alternate method. It is important to challenge the alternate technology in a manner that would encourage false positive results (specific to that alternate technology) to demonstrate the suitability of the alternate method in comparison to the traditional method. This is especially important with those alternate methods that do not require growth for microbial enumeration (for example, any that do not require enrichment or can enumerate microorganisms into the range of 1–50 cells).Limit of QuantificationThe limit of quantification is the lowest number of microorganisms that can be accurately counted. As it is not possible to obtain a reliable sample containing a known number of microorganisms, it is essential that the limit of quantification of an assay is determined from a number of replicates (n > 5) at each of at least 5 different points across the operational range of the assay. The limit of quantification should not be a number greater than that of the traditional method. Note that this may have an inherent limit due to the nature of bacterial enumeration and the Poisson distribution of bacterial counts (see Validation of Microbial Recovery from Pharmacopeial Articles 1227). Therefore, the alternate method need only demonstrate that it is at least as sensitive as the traditional method to similar lower limits.LinearityThe linearity of a quantitative microbiological test is its ability to produce results that are proportional to the concentration of microorganisms present in the sample within a given range. The linearity should be determined over the range of the test. A method to determine this would be to select at least 5 concentrations of each standard challenge microorganism and conduct at least 5 replicate readings of each concentration. An appropriate measure would be to calculate the square of the correlation coefficient, r2, from a linear regression analysis of the data generated above. While the correlation coefficient does not provide an estimate of linearity, it is a convenient and commonly applied measure to approximate the relationship. The alternate method should not have an r2 value less than 0.95.Limit of DetectionSee Limit of Detection under Validation of Qualitative Tests for Demonstration of Viable Microorganisms in a Sample.RangeThe operational range of a quantitative microbiological method is the interval between the upper and lower levels of microorganisms that have been demonstrated to be determined with precision, accuracy, and linearity.RuggednessSee Ruggedness under Validation of Qualitative Tests for Demonstration of Viable Microorganisms in a Sample.RobustnessSee Robustness under Validation of Qualitative Tests for Demonstration of Viable Microorganisms in a Sample.* PDA Technical Report No. 33. The Evaluation, Validation and Implementation of New Microbiological Testing Methods. PDA Journal of Pharmaceutical Science & Technology.54 Supplement TR#33 (3) 2000 and Official Methods Programs of AOAC International.。
微生物检测美国药典
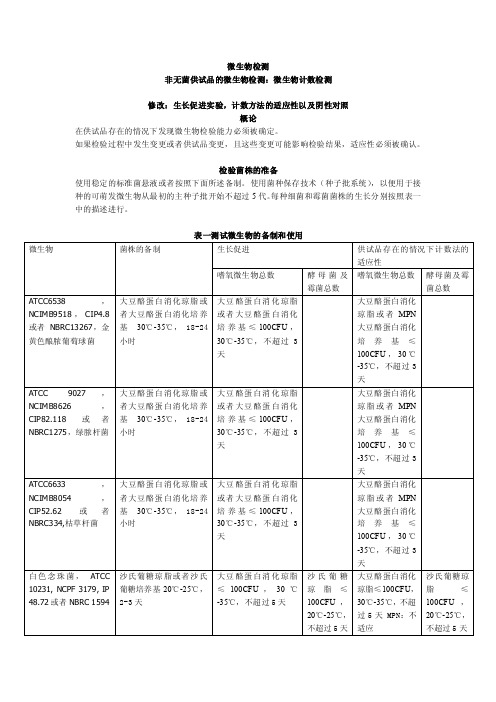
微生物检测非无菌供试品的微生物检测:微生物计数检测修改:生长促进实验,计数方法的适应性以及阴性对照概论在供试品存在的情况下发现微生物检验能力必须被确定。
如果检验过程中发生变更或者供试品变更,且这些变更可能影响检验结果,适应性必须被确认。
检验菌株的准备使用稳定的标准菌悬液或者按照下面所述备制。
使用菌种保存技术(种子批系统),以便用于接种的可萌发微生物从最初的主种子批开始不超过5代。
每种细菌和霉菌菌株的生长分别按照表一中的描述进行。
表一测试微生物的备制和使用使用pH7.0的缓冲氯化钠蛋白胨溶液或者pH7.2的磷酸盐缓冲溶液备制测试菌悬液;备制黑曲霉孢子悬浮液时,0.05%的聚山梨脂80可以被添加到缓冲液中。
测试菌悬液应在两小时内使用,或者在2-8℃的条件下24小时内使用。
也可以通过制备并稀释枯草芽胞杆菌营养细胞的新鲜悬液进行替代,制备稳定的胞子悬液,在接种测试中使用适当体积的胞子菌悬液,稳定的胞子悬液在2-8℃保存,保存期是经过验证的。
阴性对照为了确定检测条件,用选择好的稀释液代替测试备制来进行阴性对照。
必须没有微生物的生长。
当按照供试品检测中的描述进行检验时,也需要进行阴性对照。
如果阴性对照不合格需要进行调查。
培养基的生长促进检测每个批次的已经备制好的培养基以及通过脱水培养基或者描述的配料备制的每个批次的培养基。
在部分/盘大豆酪蛋白消化肉汤培养基以及大豆酪蛋白消化琼脂上接种少量(不超过100CFU)微生物,按表一中所示,每一种微生物应使用单独一部分/一盘培养基。
在沙氏葡萄糖琼脂上接种少量(不超过100CFU)微生物,按表一中所示,每一种微生物应使用单独的一盘培养基。
按照表一中所述的条件进行接种。
固体培养基的生长通过一个不大于2的系数的调节必须不能与标准接种体计算得到的数值有区别。
新鲜备制的接种体微生物的生长应与上一批通过检测的培养基的生长情形相同。
如果肉眼能清晰看到的微生物的生长与上一批通过检测的培养基的生长情形相同的话,液体培养基是适合的。
美国药典2011-5-3

171 VITAMIN B12 ACTIVITY ASSAY
Chemical Tests and Assays
Identification Tests
18BASES 191 IDENTIFICATION TESTS-GENERAL 194 IDENTIFICATION-TETRACYCLINES 197 SPECTROPHOTOMETRIC IDENTIFICATION TESTS 201 THIN-LAYER CHROMATOGRAPHIC IDENTIFICATION TEST
651 CONGEALING TEMPERATURE 661 CONTAINERS 671 CONTAINERS-PERMEATION 691 COTTON 695 CRYSTALLINITY 698 DELIVERABLE VOLUME 699 DENSITY OF SOLIDS 701 DISINTEGRATION 711 DISSOLUTION 721 DISTILLING RANGE 724 DRUG RELEASE
)尚未收入的新药和新制剂。 美国药典最新版为USP34-NF29,2010 年12 月出版,2011年5月1日生效。
Introduction
Front Matter
General Notices General Chapters Dietary Supplements Chapters Reagents
Chart 9 Chart 10 Chart 11 Chart 12 Chart 13
General Tests and Assays
Physical Tests and Determinations
601 AEROSOLS, NASAL SPRAYS, METEREDDOSE INHALERS,AND DRY POWDER INHALERS 616 BULK DENSITY AND TAPPED DENSITY 621 CHROMATOGRAPHY 631 COLOR AND ACHROMICITY 641 COMPLETENESS OF SOLUTION 643 TOTAL ORGANIC CARBON 645 WATER CONDUCTIVITY
化妆品微生物标准检验方

如有均质器,则采用无菌样品袋,将上述水溶性膏、霜、粉剂等,称10g样品加入90mL灭菌生理盐水,均质1min~2min;疏水性膏、霜及眉笔、口红等,称10g样品,加10mL灭菌液体石蜡,10mL吐温80,70mL灭菌生理盐水,均质3min~5min。
菌落总数
1范围
本标准规定了化妆品中菌落总数的检验方法。
本标准适用于化妆品菌落总数的测定。
2术语和定义
下列术语和定义适用于本标准:
菌落总数aerobic bacterial count化妆品检样经过处理,在一定条件下培养后(如培养基成分、培养温度、培养时间、pH值、需氧性质等),1g(1mL)检样中所含菌落的总数。所得结果只包括一群本方法规定的
条件下生长的嗜中温的需氧性和兼性厌氧菌落总数。测定菌落总数便于判明样品被细菌污染
10-1 10-2 10-3两稀释度
菌数之比
菌落总数
(CFU/mL或CFU/g)
报告方式
(CFU/mL或CFU/g)
1 1365 164 20─16400 16000或1.6×104 2 2760 295 46 1.6 38000 38000或3.8×104 3 2890 271 60 2.2 27100 27000或2.7×104 4不可计4650 513─513000 510000或5.1×105 5 27 11 5─270 270或2.7×102 6不可计305 12─30500 31000或3.1×104 7 0 0 0─<1×10
3仪器
3.1恒温水浴箱或隔水式恒温箱:44℃±0.5℃。
3.2温度计。
3.3显微镜。
3.4载玻片。
3.5接种环。
3.6电磁炉。
3.7三角瓶,250mL。
美国药典检验方法确认

Verification :确认。
针对使用法定方法(注意: 需要严格遵守方法要求,参数, 试剂, 标准品, 步骤等等), 需要在目前实验室的条件下确认是否可以获得可靠结果. 不一定是全部项目的验证,FDA针对常州SPL肝素钠的警告信中就有关于方法确认的内容. 可以参考。
<1226> VERIFICATION OF COMPENDIAL PROCEDURES药典规程的确认The intent of this chapter is to provide general information on the verification of compendial procedures that are being performed for the first time to yield acceptable results utilizing the personnel, equipment, and reagents available. This chapter is not intended for retroactive application to already successfully established laboratory procedures. The chapter Validation of Compendial Procedures <1225> provides general information on characteristics that should be considered for various test categories and on the documentation that should accompany analytical procedures submitted for inclusion in USP-NF. Verification consists of assessing selected analytical performance characteristics, such as those that are described in chapter <1225>, to generate appropriate, relevant data rather thanrepeating the validation process.本章节的目的是提供关于药典规程确认的信息,这些规程是第一次执行,利用可用的人员、设备和试剂,来生成可接受的结果。
《美国药典》(USP42-NF372S)通则1227药品微生物回收的验证

第一作者简介:解慧,硕士研究生;研究方向:微生物学。
Tel:022 23513983;13820255336;E mail:xiehui05qsd@163 com《美国药典》(USP42 NF372S)通则<1227>药品微生物回收的验证解慧,刘洪祥,杨倩,曹晓云(天津市药品检验研究院,天津300070)摘要:《中华人民共和国药典》、美国药典(USP)及欧洲药典(EP)等均指出具有抑菌性产品的微生物计数、检测、抑菌效力检查或无菌检查,应首先中和其抑菌性,并需对中和抑菌性的方法进行验证。
《美国药典》通则<1227>药品微生物回收的验证首次详细阐释了中和方法验证方案的设计和试验结果的判定,对于完善药品微生物检查方法、保证微生物检测结果准确,具有指导意义。
本文将《美国药典》(USP42 NF372S)通则<1227>章节译出,以期为医药界的科研工作者提供参考。
关键词:美国药典;微生物回收验证;抑菌性中图分类号:R921 2 文献标识码:A 文章编号:1009-3656(2020)-2-0146-4doi:10 19778/j chp 2020 02 011Validationofmicrobialrecoveryfrompharmacopoeiaarticlesingeneralnotice<1227>ofUSP42 NF372SXIEHui,LIUHongxiang,YANGQian,CAOXiaoyun(TianjinInstituteforDrugControl,Tianjin300070,China)Abstract:Theantimicrobialpropertyshouldbeneutralizedfirstlyandtheneutralizationmethodmustbevalidatedwhenthetestsofmicrobialenumeration,specifiedmicroorganisms,antimicrobialeffectivenessorsterilityareper formedforantibacterialproductsasperChP,USPandEP Thedesignofvalidationprotocolofneutralizationmeth odandtheresultsjudgementaredescribedindetailsinthegeneralnotice<1227>(ValidationofMicrobialRe coveryfromPharmacopoeiaArticles)ofUSPforthefirsttime Thathastheguidancesignificanceonimprovingthemicrobialtestmethodandensuringtheaccuracyofresults Thegeneralnotice<1227>ofUSP42 NF372Sistranslatedtoprovidethereferencefortheresearchersinpharmaceuticalindustry Keywords:USP;validationofmicrobialrecovery;antimicrobialproperty1 概述本章节为以下检查方法的验证提供指导:制药产品的存活微生物计数法、指示菌或者控制菌检查法及无菌检查法。
美国药典(USP)沿革及2007年版简介

美国药典(USP)沿革及2007年版简介1820年1月1日,11位医生在美国国会大厦的参议院聚会,商讨创作USP。
他们意图编出一部最佳治疗药品的汇编,给出适用的药名,并提供制剂的处方。
经过不到一年的时间,USP第一版于1820年12月15日出版。
它的前言提出,刊印药典的目的是从具有治疗效力的物质中,选择那些功能充分证实、作用明确了解的药物,并由此做出制剂,使其效力得到最大的发挥。
它也要给采用的各种药物提出一个合适而确切的名称,以防止医师与药师间交流的麻烦与不确定性。
这一要求在今天的药典中仍然如此。
随着时间的推移,USP的性质从处方汇编改变为药品标准的汇编。
它的出版周期也改变了,从1840年到1942年,每10年一版;1942到2000年,每5年一版;从2002年开始每年一版。
1888年,美国药学会出版了第一部国家处方集,名称叫非法定制剂的国家处方集,简称NF。
USP和NF是被1906年美国食品药品法和1938年的食品药品和化妆品法所认可的。
1975年USP与NF合并出版,叫USP-NF。
现在,USP根据分析和计量科学以及其他相关学科的进展,继续发展USP-NF成为提供药品标准的汇编。
USP30版与NF25版于2007年5月1日实施。
它收载了药物、生物制品、食品增补剂和赋形剂的科学标准,可用于生产各种剂型和成品。
USP30-NF25各论中所提供的所有物品(除极少数外)在美国都是法定上市的或者含在法定上市的物品中。
在USP-NP的各论中,一个物质(原料)或制品(制剂)列有该物品的定义、包装、储存、其他项以及技术要求。
技术要求包括一系列的常用试验(性状、鉴别、杂质、含量测定)和特殊试验,每项试验用一种或多种分析方法及其判定标准。
组分是指药物或赋形剂。
赋形剂是指有意加入到剂型的组方中,除了活性物质以外的任何成分,但它不一定是无活力的。
药物和赋形剂可以是合成的、半合成的、来自自然界的或用重组技术生产的。
需要效价测定的大分子和混合物通常叫做生物制品或生物技术物品。
USP微生物限度检查 中文

USP微生物限度检查中文61)微生物限度检测(MICROBIAL LIMIT TESTS)此章提供方法来检测可能存在的好氧微生物其他制药过程中可能出现的微生物的数量,包括原材料和成品中的。
如果经过验证确认可以得到相同或更好的检测结论,也允许采用自动化的检测方法。
在样品检测过程中须进行无菌操作。
若无特别说明,则“培养(incubate)”一词指在30—35℃的培养箱内培养24至48小时;“生长(growth)”一词用于专门的判定,说明“存在和可能存在活的微生物”。
准备实验 (Preparatory Testing)本章涉及实验结果的有效性取决于:提供的被检测样品本身在实验条件下,被充分证明不会抑制可能存在的微生物的生长。
因此,在准备样品时,需要正规的实验操作和符合要求的实验条件,接种稀释样品到含有以下(微生物)培养物的培养基:金黄色(奥里斯)葡萄球菌(Staphylococcus aureus),大肠埃希氏菌(Escherichia coli), 铜绿假单胞菌(Pseudomonas aeruginosa), 和沙门氏菌(Salmonella)。
方法如下:将用肉汤培养基培养24小时后的(微生物)不小于10-3稀释的微生物培养物,加1 ml(微生物)培养液到磷酸(盐)缓冲液(pH 7.2),液体大豆酪蛋白消化物培养基(Fluid Soybean-Casein Digest Medium),或者液体乳糖培养基(Fluid Lactose Medium)。
相应培养基培养失败则需要采取以下方法更改检测程序:(1)增加稀释液体积,检测样品加入量仍维持不变;或者(2)中和一定数量的干扰因子;或者(3)结合(1)、(2)得出适当条件,使接种物得以生长。
以下是一些物质的成分和浓度,该物质及浓度可用于加入培养基、阻止物质发挥抑菌作用:大豆卵磷脂(soy lecithin, 0.5%)或者聚山梨醇酯20(polysorbate 20, 4.0%)。
美国药典微生物试验说明

實驗數據判讀
不合格原因
–實驗室錯誤 –產品失敗 無論上述何種情況,管理階層必須立即被告知
OOS調查
–數據偏離的程度(嚴重性?) –實驗室環境 –取樣方法 –物料的特性(存活的污染微生物?) –留樣的評估
32/9
<1211>滅菌和無菌保證
每批產品不是通過<71>無菌試驗就可得到無 菌保證。
<1209> 滅菌–化學和物理化學指 示劑,及綜合指示劑
<1211> 滅菌與無菌保證
<1222> 最終滅菌藥品–參數放行
<1223> 微生物方法確效
<1227> 藥物的微生物回收率確效
<1231> 製藥用水
<2021> 微生物計數試驗–營養和 膳食補充劑
<2022> 特定菌的微生物檢查–營 養和膳食補充劑
Microbiological Examination of Nonsterile Products: Microbial Enumeration Tests <62> 特定菌的微生物檢查 / Microbiological Procedures for Absence of Specified Microorganisms <71> 無菌試驗 / Sterility Tests <85> 細菌內毒素試驗 Bacterial Endotoxins Test
劑型 (投藥途徑) 口服固體 口服液劑
外用或鼻腔用藥
陰道用藥
直腸用藥 藥品主成分&賦型劑
<61> TAMC [CFU/g or CFU/ml]
细胞治疗产品药典参考目录(Gene and Cell Therapy Products)

1 Chapter Guide: Gene and Cell Therapy ProductsTo return to the Chapter Charts main list,click here.Universal TestsIdentification• 11 USP Reference Standards• 1030 Biological Assay Chapters—Overview and Glossary• 1032 Design and Development of Biological Assays• 1033 Biological Assay Validation• 1034 Analysis of Biological Assays• 1044 Cryopreservation of Cells• 1102 Immunological Test Methods—General Considerations• 1103 Immunological Test Methods—Enzyme-Linked Immunosorbent Assay(ELISA)• 1104 Immunological Test Methods—Immunoblot Analysis• 1285 Preparation of Biological Specimens for Histologic and Immunohistochemical Analysis• 1285.1 Hematoxylin and Eosin Staining of Sectioned Tissue for Microscopic ExaminationAssay• 621 Chromatography• 1030 Biological Assay Chapters—Overview and Glossary• 1032 Design and Development of Biological Assays• 1033 Biological Assay Validation• 1034 Analysis of Biological Assays• 1044 Cryopreservation of Cells• 1102 Immunological Test Methods—General Considerations• 1103 Immunological Test Methods—Enzyme-Linked Immunosorbent Assay(ELISA)• 1430.1 Analytical Methodologies Based on Scattering Phenomena—Static Light Scattering Specific Tests1 , 2Biocompatibility• 87 Biological Reactivity Tests,In Vitro• 88 Biological Reactivity Tests,In Vivo• 1031 The Biocompatibility of Materials Used in Drug Containers, Medical Devices, and Implants• 1184 Sensitization TestingMicrobial/Sterility Issues• 61 Microbiological Examination of Nonsterile Products:Microbial Enumeration Tests• 62 Microbiological Examination of Nonsterile Products:Tests for Specified Microorganisms• 63 Mycoplasma Tests• 71 Sterility Tests• 85 Bacterial Endotoxins Test• 151 Pyrogen Test• 161 Medical Devices—Bacterial Endotoxin and Pyrogen Tests• 1050 Viral Safety Evaluation of Biotechnology Products Derived from Cell Lines of Human or Animal Origin• 1071 Rapid Microbial Tests for Release of Sterile Short-Life Products:A Risk-Based Approach• 1085 Guidelines on Endotoxins Test• 1113 Microbial Characterization,Identification,and Strain Typing• 1116 Microbiological Control and Monitoring of Aseptic Processing Environments• 1208 Sterility Testing—Validation of Isolator Systems• 1211 Sterility Assurance• 1227 Validation of Microbial Recovery from Pharmacopeial Articles• 1228 Depyrogenation• 1228.1 Dry Heat Depyrogenation• 1228.3 Depyrogenation by Filtration• 1228.4 Depyrogenation by Rinsing• 1228.5 Endotoxin Indicators for Depyrogenation• 1229 Sterilization of Compendial Articles• 1229.3 Monitoring of Bioburden• 1229.4 Sterilizing Filtration of Liquids2• 1229.14 Sterilization Cycle Development• 1229.15 Sterilizing Filtration of Gases• 1229.17 Mycoplasma Sterilization• 1229.18 Viral Clearance MethodsProduction Issues• 1 Injections and Implanted Drug Products (Parenterals)—Product Quality Tests• 90 Fetal Bovine Serum—Quality Attributes and Functionality Tests• 92 Growth Factors and Cytokines Used in Cell Therapy Manufacturing• 797 Pharmaceutical Compounding—Sterile Preparations• 1024 Bovine Serum• 1041 Biologics• 1043 Ancillary Materials for Cell,Gene,and Tissue-Engineered Products• 1044 Cryopreservation of Cells• 1046 Cell-based Advanced Therapies and Tissue-Based Products• 1047 Gene Therapy Products• 1074 Excipient Biological Safety Evaluation Guidelines• 1126 Nucleic Acid-Based Techniques—Extraction, Detection, and Sequencing• 1127 Nucleic Acid-Based Techniques—Amplification• 1229.16 Prion Inactivation• 1229.17 Mycoplasma Sterilization• 1229.18 Viral Clearance Methods• 1237 Virology Test Methods• 1285 Preparation of Biological Specimens for Histologic and Immunohistochemical Analysis• 1285.1 Hematoxylin and Eosin Staining of Sectioned Tissue for Microscopic Examination Product Issues• 381 Elastomeric Components in Injectable Pharmaceutical Product Packaging/Delivery Systems• 382 Elastomeric Component Functional Suitability in Parenteral Product Packaging/Delivery Systems• 1046 Cell-based Advanced Therapies and Tissue-Based Products• 1086 Impurities in Drug Substances and Drug Products• 1121 Nomenclature• 1151 Pharmaceutical Dosage Forms• 1229.17 Mycoplasma SterilizationEquipment• 31 Volumetric Apparatus• 41 Balances• 1051 Cleaning Glass Apparatus• 1228.4 Depyrogenation by Rinsing• 1229.13 Sterilization-in-Place• 1229.15 Sterilizing Filtration of Gases• 1229.16 Prion Inactivation• 1251 Weighing on an Analytical BalanceCharacterization• 111 Design and Analysis of Biological Assays• 507 Protein Determination Procedures• 621 Chromatography• 785 Osmolality and Osmolarity• 787 Subvisible Particulate Matter in Therapeutic Protein Injections• 788 Particulate Matter in Injections• 791 pH• 905 Uniformity of Dosage Units• 911 Viscosity—Capillary Methods• 912 Viscosity—Rotational Methods• 913 Viscosity—Rolling Ball Method• 1027 Flow Cytometry• 1030 Biological Assay Chapters—Overview and Glossary• 1032 Design and Development of Biological Assays• 1033 Biological Assay Validation• 1034 Analysis of Biological Assays• 1044 Cryopreservation of Cells• 1046 Cell-based Advanced Therapies and Tissue-Based Products3• 1048 Quality of Biotechnology Products: Analysis of the Expression Construct in Cells Used for Production of r-DNA Derived Protein Products• 1049 Quality of Biotechnological Products:Stability Testing of Biotechnological/Biological Products• 1052 Biotechnology Derived Articles—Amino Acid Analysis• 1053 Capillary Electrophoresis• 1054 Biotechnology Derived Articles—Isoelectric Focusing• 1055 Biotechnology Derived Articles—Peptide Mapping• 1056 Biotechnology Derived Articles—Polyacrylamide Gel Electrophoresis• 1057 Biotechnology Derived Articles—Total Protein Assay• 1084 Glycoprotein and Glycan Analysis—General Considerations• 1102 Immunological Test Methods—General Considerations• 1103 Immunological Test Methods—Enzyme-Linked Immunosorbent Assay(ELISA)• 1104 Immunological Test Methods—Immunoblot Analysis• 1126 Nucleic Acid-Based Techniques—Extraction, Detection, and Sequencing• 1127 Nucleic Acid-Based Techniques—Amplification• 1128 Nucleic Acid-Based Techniques—Microarray• 1129 Nucleic Acid-Based Techniques—Genotyping• 1130 Nucleic Acid-Based Techniques—Approaches for Detecting Trace Nucleic Acids (Residual DNA Testing)• 1237 Virology Test Methods• 1285 Preparation of Biological Specimens for Histologic and Immunohistochemical Analysis• 1285.1 Hematoxylin and Eosin Staining of Sectioned Tissue for Microscopic Examination• 1430.1 Analytical Methodologies Based on Scattering Phenomena—Static Light Scattering• 1776 Image Analysis of Pharmaceutical Systems• 1787 Measurement of Subvisible Particulate Matter in Therapeutic Protein Injections• 1788 Methods for the Determination of Subvisible Particulate Matter• 1788.1 Light Obscuration Method for the Determination of Subvisible Particulate Matter• 1788.2 Membrane Microscope Method for the Determination of Subvisible Particulate Matter• 1788.3 Flow Imaging Method for the Determination of Subvisible Particulate Matter。
白藜芦醇的检测解决方案
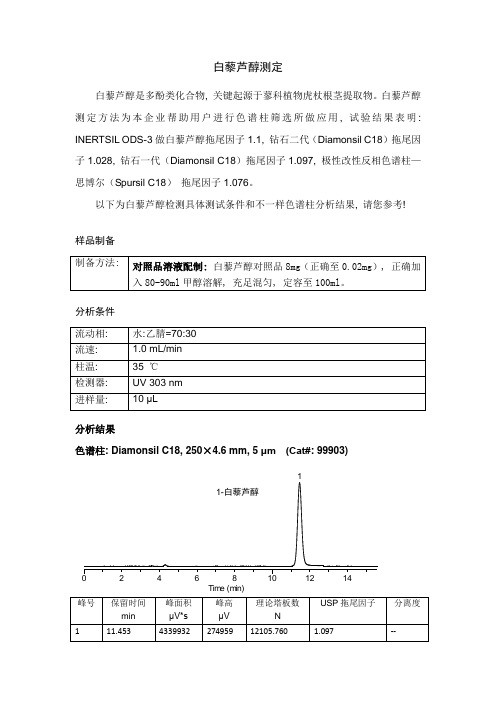
白藜芦醇测定
白藜芦醇是多酚类化合物, 关键起源于蓼科植物虎杖根茎提取物。
白藜芦醇测定方法为本企业帮助用户进行色谱柱筛选所做应用, 试验结果表明: INERTSIL ODS-3做白藜芦醇拖尾因子1.1, 钻石二代(Diamonsil C18)拖尾因子1.028, 钻石一代(Diamonsil C18)拖尾因子1.097, 极性改性反相色谱柱—思博尔(Spursil C18)拖尾因子1.076。
以下为白藜芦醇检测具体测试条件和不一样色谱柱分析结果, 请您参考!
样品制备
分析条件
分析结果
色谱柱: Diamonsil C18, 250×4.6 mm, 5 μm (Cat#: 99903)
1
1-白藜芦醇
024********
Tim e (m in)
色谱柱: Spursil C18, 250×4.6 mm, 5 μm (Cat#: 8)
1
1-白藜芦醇024681012141618
Tim e (m in)
色谱柱: Diamonsil C18(2), 250×4.6 mm, 5 μm (Cat#: 99603)
1
1-白藜芦醇0246810121416
Tim e (m in)
白藜芦醇检测相关色谱消耗品(现货)。
USP40-NF35通用章节目录

Guide to General Chapters 通用章节指导(标注底色部分为USP40-NF35新增章节)General Requirements for Test and Assays 检查与含量分析的一般要求<1>INJECTIONS AND IMPLANTED DRUG PRODUCTS (PARENTERALS)—PRODUCT QUALITY TESTS 注射和植入药物产品(注射用) —产品质量测试<1>INJECTIONS 注射剂<2>ORAL DRUG PRODUCTS—PRODUCT QUALITY TESTS 口服药物产品质量测试<3>TOPICAL AND TRANSDERMAL DRUG PRODUCTS—PRODUCT QUALITY TESTS局部和透皮药物产品—产品质量测试<4>MUCOSAL DRUG PRODUCTS—PRODUCT QUALITY TESTS 粘膜药物产品质量测试<5>INHALATION AND NASAL DRUG PRODUCTS—GENERAL INFORMATION AND PRODUCT QUALITY TESTS 吸入剂产品—产品质量测试<7>LABELING 标签<11>USP REFERENCE STANDARDS USP标准品Apparatus for Test and Assays 用于检查与含量分析的器具<17>PRESCRIPTION CONTAINER LABELING 处方容器标签<21>THERMOMETERS 温度计(本版本已删除)<31>VOLUMETRIC APPARATUS 容量器具<41>BALANCES 天平Microbiological Tests 微生物检查法<51>ANTIMICROBIAL EFFECTIVENESS TESTING 抗菌剂有效性检查法<55>BIOLOGICAL INDICATORS—RESISTANCE PERFORMANCE TESTS生物指示剂-耐药性实验<61>MICROBIOLOGICAL EXAMINATION OF NONSTERILE PRODUCTS: MICROBIAL ENUMERATION TESTS 非无菌产品的微生物限度检查:微生物列举检查法<62>MICROBIOLOGICAL EXAMINATION OF NONSTERILE PRODUCTS: TESTS FOR SPECIFIED MICROORGANISMS 非无菌产品的微生物限度检查:特定微生物检查法<63>MYCOPLASMA TESTS 支原体检查法<71>STERILITY TESTS 无菌检查法Biological tests and assays 生物检查法与测定法<81>ANTIBIOTICS—MICROBIAL ASSAYS 抗生素-微生物测定<85>BACTERIAL ENDOTOXINS TEST 细菌内毒素检查法<87>BIOLOGICAL REACTIVITY TESTS, IN VITRO 体外的生物反应性检查法<88>BIOLOGICAL REACTIVITY TESTS, IN VIVO 体内的生物反应性检查法<89>ENZYMES USED AS ANCILLARY MATERIALS IN PHARMACEUTICAL MANUFACTURING药品生产中酶作为辅料所使用<89.1> COLLAGENASEⅠ胶原酶Ⅰ<89.2> COLLAGENASEⅡ胶原酶Ⅱ<90>FETAL BOVINE SERUM—QUALITY ATTRIBUTES AND FUNCTIONALITY TESTS牛胎儿血清-质量品质和功能检查法<91>CALCIUM PANTOTHENATE ASSAY 泛酸钙测定法<92>GROWTH FACTORS AND CYTOKINES USED IN CELL THERAPY MANUFACTURING在细胞疗法中使用生长因子和细胞因子<111>DESIGN AND ANALYSIS OF BIOLOGICAL ASSAYS 生物测定法的设计与分析<115>DEXPANTHENOL ASSAY右泛醇(拟胆碱药)测定法<121>INSULIN ASSAYS 胰岛素测定法<121.1>PHYSICOCHEMICAL ANALYTICAL PROCEDURES FORINSULINS胰岛素的物理化学分析程序<123>GLUCAGON BIOIDENTITY TESTS 高血糖素的生物鉴别检查法<124>ERYTHROPOIETIN BIOASSAYS 红细胞生成素的微生物测定<126>SOMATROPIN BIOIDENTITY TESTS 生长激素的生物鉴别检查法<127>FLOW CYTOMETRIC ENUMERATION OF CD34+ CELL 流式细胞术计术34阳性细胞<129>ANALYTICAL PROCEDURES FOR RECOMBINANT THERAPEUTIC MONOCLONAL ANTIBODIES重组治疗性单克隆抗体的分析方法<130>PROTEIN A QUALITY ATTRIBUTES 蛋白质A的质量特征<151>PYROGEN TEST 热原检查法<161>TRANSFUSION AND INFUSION ASSEMBLIES AND SIMILAR MEDICAL DEVICES输血输液用具以及相类似的医疗器械<162>DIPHTHERIA ANTITOXIN POTENCY TESTING FOR HUMAN IMMUNE GLOBULINS 人免疫球蛋白的白喉抗毒素效力检测<165>PREKALLIKREIN ACTIVATOR前激肽释放酶原激活剂<171>VITAMIN B12 ACTIVITY ASSAY……2548 维生素B12活性测定法Chemical Tests and assays 化学实验检查与测定法鉴别检查<181>IDENTIFICATION—ORGANIC NITROGENOUS BASES鉴别-有机氮碱化合物<191>IDENTIFICATION TESTS—GENERAL 鉴别实验-通用<193>IDENTIFICATION—TETRACYCLINES 鉴别-四环素类<197>SPECTROPHOTOMETRIC IDENTIFICATION TESTS 分光光度计鉴别实验<201>THIN-LAYER CHROMATOGRAPHIC IDENTIFICATION TEST 薄层色谱鉴别实验<202>IDENTIFICATION OF FIXED OILS BY THIN-LAYER CHROMATOGRAPHY 薄层色谱法对非挥发性油的鉴别<203>HIGH-PERFORMANCE THIN-LAYER CHROMATOGRAPHY PROCEDURE FOR IDENTIFICATION OF ARTICLES OF BOTANICAL ORIGIN 高性能薄层色谱法对植物的鉴别Limit Tests 限度检查法<206>ALUMINUM 铝<207>TEST FOR 1,6-ANHYDRO DERIVATIVE FOR ENOXAPARIN SODIUM依诺肝素钠的酐类衍生物实验<208>ANTI-FACTOR Xa AND ANTI-FACTOR IIa ASSAYS FORUNFRACTIONATED AND LOW MOLECULAR WEIGHT HEPARINS 普通肝素和低分子肝素产品中抗体Xa和抗体IIa测定<209>LOW MOLECULAR WEIGHT HEPARIN MOLECULAR WEIGHT DETERMINATIONS低分子肝素钠分子量测定<211>ARSENIC 砷<212>OLIGOSACCHARIDE ANALYSIS 低聚糖的分析<221>CHLORIDE AND SULFATE 氯和硫<223>DIMETHYLANILINE 二甲基苯胺<226>4-EPIANHYDRO-TETRACYCLINE 4-?-四环素<227>4-AMINOPHENOL IN ACETAMINOPHEN-CONTAINING DRUG PRODUCTS对乙酰氨酚药物产品中氨基酚<228>ETHYLENE OXIDE AND DIOXANE 环氧乙烷和二氧六环<231>HEAVY METALS 重金属(删除)<232>ELEMENTAL IMPURITIES—LIMITS 元素杂质-限度<233>ELEMENTAL IMPURITIES—PROCEDURES 元素杂质-规程<241>IRON 铁<251>LEAD 铅<261>MERCURY 汞<267>POROSIMETRY BY MERCURY INTRUSION 水银孔隙仪<268>POROSITY BY NITROGEN ADSORPTION–DESORPTION 氮吸附-解吸测定孔隙率<271>READILY CARBONIZABLE SUBSTANCES TEST 易碳化物检查法<281>RESIDUE ON IGNITION 炽灼残渣<291>SELENIUM 硒Other Tests and Assays 其它检查法与测定法<301>ACID-NEUTRALIZING CAPACITY 酸中和容量<311>ALGINATES ASSAY 藻酸盐测定法<341>ANTIMICROBIAL AGENTS—CONTENT 抗菌剂-含量<345>Assay for Citric Acid/Citrate and Phosphate 柠檬酸/柠檬酸盐和磷酸盐的测定<351>ASSAY FOR STEROIDS 类固醇(甾类化合物)测定法<361> BARBITURATE ASSAY 巴比妥类药物测定法(本版本已删除)<371>COBALAMIN RADIOTRACER ASSAY 钴铵素放射性跟踪剂测定法<381>ELASTOMERIC CLOSURES FOR INJECTIONS 注射剂的弹性密封件<391>EPINEPHRINE ASSAY 肾上腺素测定法<401>FATS AND FIXED OILS 脂肪与混合油<411>FOLIC ACID ASSAY 叶酸测定法<413>IMPURITIES TESTING IN MEDICAL GASES 医用气体杂质检查<415>MEDICAL GASES ASSAY 医用气体含量检查<425>IODOMETRIC ASSAY—ANTIBIOTICS 碘量检查法-抗生素<429>LIGHT DIFFRACTION MEASUREMENT OF PARTICLE SIZE 粒径的光衍射测量法<431>METHOXY DETERMINATION 甲氧基测定法<441>NIACIN OR NIACINAMIDE ASSAY 烟酰或烟酰胺测定法<451>NITRITE TITRATION 亚硝酸盐滴定<461>NITROGEN DETERMINATION 氮测定法<466>ORDINARY IMPURITIES 一般杂质<467>RESIDUAL SOLVENTS 残留溶剂<469>ETHYLENE GLYCOL, DIETHYLENE GLYCOL, AND TRIETHYLENE GLYCOL IN ETHOXYLATED SUBSTANCES乙氧基物质中乙二醇、二甘醇、三甘醇测定<471>OXYGEN FLASK COMBUSTION 氧瓶燃烧法<481>RIBOFLAVIN ASSAY 核黄素(维生素B2)测定法<501>SALTS OF ORGANIC NITROGENOUS BASES 有机氮盐<503>ACETIC ACID IN PEPTIDES 多肽类中乙酸测定<503.1>TRIFLUOROACETIC ACID(TFA)IN PEPTIDES 肽中三氟乙酸<507>PROTEIN DETERMINATION PROCEDURES 蛋白质测定程序<511>SINGLE-STEROID ASSAY 单一的类固醇测定法<525>SULFUR DIOXIDE 二氧化硫<531>THIAMINE ASSAY 硫胺素测定法<541>TITRIMETRY 滴定法<551>VITAMIN E ASSAY 维生素E测定法<561>ARTICLES OF BOTANICAL ORIGIN 植物起源的药品<563>IDENTIFICATION OF ARTICLES OF BOTANICAL ORIGIN 植物药品的鉴别<565>BOTANICAL EXTRACTS 植物提取<571>VITAMIN A ASSAY 维生素A测定法<580>VITAMIN C ASSAY 维生素C的测定法<581>VITAMIN D ASSAY 维生素D测定法<591>ZINC DETERMINATION 锌的测定法Physical Test and Determinations 物理检查与测定法<601>INHALATION AND NASAL DRUG PRODUCTS: AEROSOLS, SPRAYS, AND POWDERS—PERFORMANCE QUALITY TESTS吸入剂、鼻雾剂:气溶胶,喷雾,干粉-质量通则<602>PROPELLANTS 推进剂<603>TOPICAL AEROSOLS 局部喷雾剂<604>LEAK RATE 渗漏率<610>ALTERNATIVE MICROBIOLOGICAL SAMPLING METHODS FOR NONSTERILE INHALED AND NASAL PRODUCTS非无菌吸入和鼻雾剂可供选择的微生物取样方法<611>ALCOHOL DETERMINATION 乙醇测定法<616>BULK DENSITY AND TAPPED DENSITY 堆密度与振实密度<621>CHROMATOGRAPHY 色谱法<631>COLOR AND ACHROMICITY 呈色与消色<641>COMPLETENESS OF SOLUTION 溶解度<643>TOTAL ORGANIC CARBON 总有机碳<645>WATER CONDUCTIVITY 水电导率<651>CONGEALING TEMPERATURE 凝点温度<659>PACKAGING AND STORAGE REQUIREMENTS 包装和储藏要求<660>CONTAINERS—GLASS 容器-玻璃<661>CONTAINERS—PLASTICS容器-塑料<661.1>PLASTIC MATERIALS OF CONSTRUCTION塑料包装材料<661.2>PLASTIC PACKAGING SYSTEMS FOR PHARMACEUTICAL USE药用塑料包装系统<670>AUXILIARY PACKAGING COMPONENTS 辅助包装部件<671>CONTAINERS—PERFORMANCE TESTING 容器-性能测试<691>COTTON 棉花<695>CRYSTALLINITY 结晶度<696>CHARACTERIZATION OF CRYSTALLINE SOLIDS BY MICROCALORIMETRY AND SOLUTION CALORIMETRY 通过溶液量热学测定结晶性<697>CONTAINER CONTENT FOR INJECTIONS 注射剂容器容积<698>DELIVERABLE VOLUME 抽取体积<699>DENSITY OF SOLIDS 固体密度<701>DISINTEGRATION 崩解时限<705>QUALITY ATTRIBUTES OF TABLETS LABELED AS HAVING A FUNCTIONAL SCORE?<711>DISSOLUTION 溶出度<721>DISTILLING RANGE 馏程<724>DRUG RELEASE 药物释放度<729>GLOBULE SIZE DISTRIBUTION IN LIPID INJECTABLE EMULSIONS脂类可注射的乳剂的粒径分布<730>Plasma Spectrochemistry 血浆光谱化学?<731>LOSS ON DRYING4 干燥失重<733>LOSS ON IGNITION 灼烧失重<735>X-RAY FLUORESCENCE SPECTROMETRY X射线光谱<736>MASS SPECTROMETRY 质谱<741>MELTING RANGE OR TEMPERATURE 熔距或熔点<751>METAL PARTICLES IN OPHTHALMIC OINTMENTS 眼用软膏中的金属粒子<755>MINIMUM FILL 最低装量<761>NUCLEAR MAGNETIC RESONANCE 核磁共振<771>OPHTHALMIC OINTMENTS 眼用软膏<776>OPTICAL MICROSCOPY 光学显微镜<781>OPTICAL ROTATION 旋光度<782>VIBRATIONAL CIRCULAR DICHROISM SPECTROSCOPY 振动圆二色谱<785>OSMOLALITY AND OSMOLARITY 渗透压<786>PARTICLE SIZE DISTRIBUTION ESTIMATION BY ANALYTICAL SIEVING筛分法估算粒径分布<787>SUBVISIBLE PARTICULATE MATTER IN THERAPEUTIC PROTEIN INJECTIONS显微计数法在治疗性蛋白注射剂中应用<788>PARTICULATE MATTER IN INJECTIONS 注射剂中的不溶性微粒<789>PARTICULATE MATTER IN OPHTHALMIC SOLUTIONS 眼用溶液中的不溶性微粒<790>VISIBLE PARTICULATES IN INJECTIONS 注射剂中可见异物<791>pH<795>PHARMACEUTICAL COMPOUNDING—NONSTERILE PREPARATIONS药物混合-非无菌制剂<797>PHARMACEUTICAL COMPOUNDING—STERILE PREPARATIONS药物混合-无菌制剂<800>HAZARDOUS DRUGS—HANDLING IN HEALTHCARE SETTINGS 在医疗环境中危险药品的处理<801>POLAROGRAPHY 极谱法<811>POWDER FINENESS 粉剂细度<821>RADIOACTIVITY 放射性<823>POSITRON EMISSION TOMOGRAPHY DRUGS FOR COMPOUNDING, INVESTIGATIONAL, AND RESEARCH USES用于正电子发射断层造影术的放射性药物<831>REFRACTIVE INDEX 折光率<841>SPECIFIC GRAVITY 比重<846>SPECIFIC SURFACE AREA 比表面积<851>SPECTROPHOTOMETRY AND LIGHT-SCATTERING 分光光度计与光散射(本版本已删除)<852>ATOMIC ABSORPTION SPECTROSCOPY 原子吸收光谱<853>FLUORESCENCE SPECTROSCOPY 荧光光谱<854>MID-INFRARED SPECTROSCOPY 中红外光谱<855>NEPHELOMETRY, TURBIDIMETRY, AND VISUAL COMPARISO 浊度测定、比浊法、视觉比较<857>ULTRAVIOLET-VISIBLE SPECTROSCOPY 紫外可见光谱<861>SUTURES—DIAMETER 缝线-直径?<871>SUTURES—NEEDLE ATTACHMENT 缝线-穿孔实验<881>TENSILE STRENGTH 张力<891>THERMAL ANALYSIS 热分析<905>UNIFORMITY OF DOSAGE UNITS 制剂单位的含量均匀度<911>VISCOSITY—CAPILLARY METHODS 黏度-毛细管法<912>VISCOSITY—ROTATIONAL METHODS 黏度-旋转法<913>VISCOSITY—ROLLING BALL METHOD 黏度-球法<914>VISCOSITY—PRESSURE DRIVEN METHODS 黏度-压力驱动方法<921>WATER DETERMINATION 水分测定<941>CHARACTERIZATION OF CRYSTALLINE AND PARTIALLY CRYSTALLINE SOLIDS BY X-RAY POWDER DIFFRACTION(XRPD) X光衍射General Information 通用信息<1004>MUCOSAL DRUG PRODUCTS—PERFORMANCE TESTS粘膜药物产品性能测试<1005>ACOUSTIC EMISSION 声频发射<1010>ANALYTICAL DATA—INTERPRETATION AND TREATMENT 分析数据-解释与处理<1015>AUTOMATED RADIOCHEMICAL SYNTHESIS APPARATUS 放射性自动合成装置<1024>BOVINE SERUM 牛血清<1025>PANCREATIN胰液素<1027>FLOW CYTOMETRY 流式细胞仪<1029>GOOD DOCUMENTATION GUIDELINES 好的文件指南<1030>BIOLOGICAL ASSAY CHAPTERS—OVERVIEW AND GLOSSARY生物测定章节-综述和术语<1031>THE BIOCOMPATIBILITY OF MATERIALS USED IN DRUG CONTAINERS, MEDICAL DEVICES, AND IMPLANTS用于药物容器、医疗设施和植入剂的材料的生物相容性<1032>DESIGN AND DEVELOPMENT OF BIOLOGICAL ASSAYS 生物试验的设计和开发<1033>BIOLOGICAL ASSAY VALIDATION 生物试验的验证<1034>ANALYSIS OF BIOLOGICAL ASSAYS 生物测定分析<1035>BIOLOGICAL INDICATORS FOR STERILIZATION 灭菌用生物指示剂(本版本已删除)<1039>CHEMOMETRICS 化学<1041>BIOLOGICS 生物制剂<1043>Ancillary Material for Cell, Gene, and Tissue-Engineered Products细胞,基因与组织设计产品的辅助材料<1044>CRYOPRESERVATION OF CELLS 细胞低温保存<1045>BIOTECHNOLOGY-DERIVED ARTICLES 生物技术提取产品(本版本已删除)<1046>CELLULAR AND TISSUE-BASED PRODUCTS 细胞与组织产品<1047>GENE THERAPY PRODUCTS 基因治疗产品<1048>QUALITY OF BIOTECHNOLOGICAL PRODUCTS: ANALYSIS OF THE EXPRESSION CONSTRUCT IN CELLS USED FOR PRODUCTION OF r-DNA DERIVED PROTEIN PRODUCTS生物技术产品的质量:从蛋白质产品中提取的r-DNA产品在细胞中表达结构的分析<1049>QUALITY OF BIOTECHNOLOGICAL PRODUCTS: STABILITY TESTING OF BIOTECHNOLOGICAL/BIOLOGICALPRODUCTS生物技术产品的质量:生物技术/生物产品的稳定性实验<1050>VIRAL SAFETY EVALUATION OF BIOTECHNOLOGY PRODUCTS DERIVED FROM CELL LINES OF HUMAN OR ANIMALORIGIN从人或动物细胞中提取的生物技术产品的病毒安全性评估<1050.1>DESIGN, EVALUATION,AND CHARACTERIZATION OF VIRAL CLEARANCE PROCEDURES 病毒清除过程的设计、评估和鉴定。
【管理精品】USP-微生物限度检查

〈61〉MICROBIAL LIMIT TESTSThis chapter provides tests for the estimation of the number of viable aerobic microorganisms present and for freedom from designated microbial species in pharmaceutical articles of all kinds, from raw materials to the finished forms. An automated method may be substituted for the tests presented here, provided it has been properly validated as giving equivalent or better results. In preparing for and in applying the tests, observe aseptic precautions in handling the specimens. Unless otherwise directed, where the procedure specifies simply “incubate,” hold the container in air that is thermostatically controlled at a temperature between 30and 35,for a period of 24to 48hours.The term “growth” is used in a special sense herein, i.e., to designate the presence and presumed proliferation of viable microorganisms.PREPARATORY TESTINGThe validity of the results of the tests set forth in this chapter rests largely upon the adequacy of a demonstration that the test specimens to which they are applied do not, of themselves, inhibit the multiplication, under the test conditions, of microorganisms that may be present. Therefore, preparatory to conducting the tests on a regular basis and as circumstances require subsequently, inoculate diluted specimens of the material to be tested with separate viable cultures of Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa and Salmonella. This can be done by adding 1mLof not less than 10-3dilution of a 24-hour broth culture of the microorganism to the first dilution (in pH7.2Phosphate Buffer, Fluid Soybean–Casein Digest Medium, or Fluid Lactose Medium)of the test material and following the test procedure. Failure of the organism(s)to grow in the relevant medium invalidates that portion of the examination and necessitates a modification of the procedure by (1)an increase in the volume of diluent, the quantity of testmaterial remaining the same, or by (2)the incorporation of a sufficient quantity of suitable inactivating agent(s)in the diluents, or by (3)an appropriate combination of modifications (1)and (2)so as to permit growth of the inocula. The following are examples of ingredients and their concentrations that may be added to the culture medium to neutralize inhibitory substances present in the sample: soy lecithin,0.5%;and polysorbate 20,4.0%. Alternatively, repeat the test as described in the preceding paragraph, using Fluid Casein Digest–Soy Lecithin–Polysorbate 20 Medium to demonstrate neutralization of preservatives or other antimicrobial agents in the test material. Where inhibitory substances are contained in the product and the latter is soluble, a suitable, validated adaptation of a procedure set forth in the section Membrane Filtration under Test for Sterility of the Product to be Examined under Sterility Tests 〈71〉,may be used.If in spite of the incorporation of suitable inactivating agents and a substantial increase in the volume of diluent, it is still not possible to recover the viable cultures described above and where the article is not suitable for employment of membrane filtration, it can be assumed that the failure to isolate the inoculated organism is attributable to the bactericidal activity of the product. This information serves to indicate that the article is not likely to be contaminated with the given species of microorganism. Monitoring should be continued in order to establish the spectrum of inhibition and bactericidal activity of the article.BUFFER SOLUTION AND MEDIACulture media may be prepared as follows, or dehydrated culture media may be used provided that, when reconstituted as directed by the manufacturer or distributor, they have similar ingredients and/or yield media comparable to those obtained from the formulas given herein.In preparing media by the formulas set forth herein, dissolve the soluble solids in the water, using heat, if necessary, to effect complete solution, and addsolutions of hydrochloric acid or sodium hydroxide in quantities sufficient to yield the desired pH in the medium when it is ready for use. Determine the pH at 25±2.Where agar is called for in a formula, use agar that has a moisture content of not more than 15%.Where water is called for in a formula, use Purified Water.PH7.2Phosphate BufferStock Solution— Dissolve 34g of monobasic potassium phosphate in about 500mLof water contained in a 1000-mLvolumetric flask. Adjust to pH7.2±0.1by the addition of sodium hydroxide TS(about 175mL),add water to volume, and mix. Dispense and sterilize. Store under refrigeration.For use, dilute the Stock Solution with water in the ratio of 1to 800,and sterilize.MediaUnless otherwise indicated, the media should be sterilized by heating in an autoclave (see Steam Sterilization under Sterilization 〈1211〉),the exposure time depending on the volume to be sterilized.I.Fluid Casein Digest–Soy Lecithin–Polysorbate 20MediumDissolve the pancreatic digest of casein and soy lecithin in 960mLof water, heating in a water bath at 48to 50for about 30minutes to effect solution. Add 40mLof polysorbate 20.Mix,and dispense as desired.II.Soybean–Casein Digest Agar MediumpHafter sterilization:7.3±0.2.III.Fluid Soybean–Casein Digest MediumIV.Mannitol–Salt Agar MediumMix,then heat with frequent agitation, and boil for 1minute to effect solution. pHafter sterilization:7.4±0.2.Heat with frequent agitation,and boil for 1minute.Sterilize,cool to between45and 50,and add 10mLof sterile potassium tellurite solution (1in 100)and50mLof egg-yolk emulsion. Mix intimately but gently, and pour into plates. (Prepare the egg-yolk emulsion by disinfecting the surface of whole shell eggs, aseptically cracking the eggs, and separating out intact yolks into a sterile graduated cylinder. Add sterile saline TS to obtain a 3to 7ratio of egg yolk to saline. Add to a sterile blender cup, and mix at high speed for 5seconds.) pHafter sterilization:6.8±0.2.VI.Vogel–Johnson Agar MediumBoil the solution of solids for 1minute.Sterilize,cool to between 45and 50,and add 20mLof sterile potassium tellurite solution (1in 100).pHafter sterilization:7.2±0.2.VII.Cetrimide Agar MediumDissolve all solid components in the water, and add the glycerin. Heat,with frequent agitation, and boil for 1minute to effect solution.pHafter sterilization:7.2±0.2.VIII. Pseudomonas Agar Medium for Detection of FluorescinDissolve the solid components in the water before adding theglycerin.Heat,with frequent agitation,and boil for 1minute to effect solution. pHafter sterilization:7.2±0.2.IX.Pseudomonas Agar Medium for Detection of PyocyaninDissolve the solid components in the water before adding theglycerin.Heat,with frequent agitation,and boil for 1minute to effect solution. pHafter sterilization:7.2±0.2.X.Fluid Lactose MediumCool as quickly as possible after sterilization.pHafter sterilization:6.9±0.2.XI.Fluid Selenite–Cystine MediumFinal pH:7.0±0.2.Mix,and heat to effect solution.Heat in flowing steam for 15minutes.Do not sterilize.XII.Fluid Tetrathionate MediumHeat the solution of solids to boiling. On the day of use, add a solution prepared by dissolving 5g of potassium iodide and 6g of iodine in 20mLofwater. Then add 10mLof a solution of brilliant green (1in 1000),and mix.Do not heat the medium after adding the brilliant green solution.XIII.Brilliant Green Agar MediumBoil the solution of solids for 1minute.Sterilize just prior to use, melt the medium, pour into petri dishes, and allow to cool.pHafter sterilization:6.9±0.2.XIV. Xylose–Lysine–Desoxycholate Agar MediumFinal pH:7.4±0.2.Heat the mixture of solids and water, with swirling, just to the boiling point. Do not overheat or sterilize. Transfer at once to a water bath maintained at about 50,and pour into plates as soon as the medium has cooled.XV.Bismuth Sulfite Agar MediumFinal pH:7.6±0.2.Heat the mixture of solids and water,with swirling,just to the boiling point.Do not overheat or sterilize.Transfer at once to a water bath maintained at about 50,and pour into plates as soon as the medium has cooled.XVI.Triple Sugar–Iron–Agar MediumpHafter sterilization:7.3±0.2.XVII.MacConkey Agar MediumBoil the mixture of solids and water for 1minute to effect solution.pHafter sterilization:7.1±0.2.XVIII.Levine Eosin–Methylene Blue Agar MediumDissolve the pancreatic digest of gelatin, the dibasic potassium phosphate, and the agar in the water, with warming, and allow to cool. Just prior to use, liquefy the gelled agar solution, add the remaining ingredients, as solutions, in the following amounts, and mix: for each 100mLof the liquefied agar solution—5mLof lactose solution (1in 5),2mLof the eosin Ysolution (1in 50),and 2mLof methylene blue solution (1in 300).The finished medium may not be clear.pHafter sterilization:7.1±0.2.XIX. Sabouraud Dextrose Agar MediumMix,and boil to effect solution.pHafter sterilization:5.6±0.2.XX.Potato Dextrose Agar MediumDissolve by heating,and sterilize.pHafter sterilization:5.6±0.2.For use, just prior to pouring the plates, adjust the melted and cooled to45medium with sterile tartaric acid solution (1in 10)to a pHof 3.5±0.1.Do not reheat the pH3.5medium.SAMPLINGProvide separate 10-mLor 10-g specimens for each of the tests called for in the individual monograph.PROCEDUREPrepare the specimen to be tested by treatment that is appropriate to its physical characteristics and that does not alter the number and kind of microorganisms originally present, in order to obtain a solution or suspension of all or part of it in a form suitable for the test procedure(s)to be carried out. For a solid that dissolves to an appreciable extent but not completely, reduce the substance to a moderately fine powder, suspend it in the vehicle specified, and proceed as directed under Total Aerobic Microbial Count, and under Test for Staphylococcus aureus and Pseudomonas aeruginosa and Test for Salmonella species and Escherichia coli.For a fluid specimen that consists of a true solution, or a suspension in water or a hydroalcoholic vehicle containing less than 30percent of alcohol, and for a solid that dissolves readily and practically completely in 90mLofpH7.2Phosphate Buffer or the media specified, proceed as directed under Total Aerobic Microbial Count,and under Test for Staphylococcus aureus and Pseudomonas aeruginosa and Test for Salmonella species and Escherichia coli.For water-immiscible fluids, ointments, creams, and waxes, prepare a suspension with the aid of a minimal quantity of a suitable, sterile emulsifying agent (such as one of the polysorbates), using a mechanical blender and warming to a temperature not exceeding 45,if necessary, and proceed with the suspension as directed under Total Aerobic Microbial Count,and under Test for Staphylococcus aureus and Pseudomonas aeruginosa and Test for Salmonella species and Escherichia coli.For a fluid specimen in aerosol form, chill the container in an alcohol-dry ice mixture for approximately 1hour,cut open the container, allow it to reach room temperature, permit the propellant to escape, or warm to drive off the propellant if feasible, and transfer the quantity of test material required for the procedures specified in one of the two preceding paragraphs, as appropriate. Where 10.0g or 10.0mLof the specimen, whichever is applicable, cannot be obtained from 10containers in aerosol form, transfer the entire contents from 10chilled containers to the culture medium, permit the propellant to escape, and proceed with the test on the residues. If the results of the test are inconclusive or doubtful, repeat the test with a specimen from 20more containers.Total Aerobic Microbial CountFor specimens that are sufficiently soluble or translucent to permit use of the Plate Method, use that method; otherwise, use the Multiple-Tube Method. With either method, first dissolve or suspend 10.0g of the specimen if it is a solid, or 10mL,accurately measured, if the specimen is a liquid, in pH7.2PhosphateBuffer, Fluid Soybean–Casein Digest Medium,or Fluid Casein Digest–Soy Lecithin-Polysorbate 20Medium to make 100mL.For viscous specimens that cannot be pipeted at this initial 1:10dilution,dilute the specimen until a suspension is obtained,i.e.,1:50or 1:100,etc.,that can be pipeted. Perform the test for absence of inhibitory (antimicrobial)properties as described under Preparatory Testing before the determination of Total Aerobic Microbial Count.Add the specimen to the medium not more than 1hour after preparing the appropriate dilutions for inoculation.PLATE METHODDilute further, if necessary, the fluid so that 1mLwill be expected to yield between 30and 300colonies.Pipet 1mLof the final dilution onto each of two sterile petri dishes. Promptly add to each dish 15to 20mLof Soybean–Casein Digest Agar Medium that previously has been melted and cooled to approximately 45.Cover the petri dishes, mix the sample with the agar by tilting or rotating the dishes, and allow the contents to solidify at room temperature. Invert the petri dishes, and incubate for 48to 72hours.Following incubation, examine the plates for growth, count the number of colonies, and express the average for the two plates in terms of the number of microorganisms per g or per mLof specimen.If no microbial colonies are recovered from the dishes representing the initial 1:10dilution of the specimen,express the results as “less than 10microorganisms per g or per mLof specimen.”MULTIPLE-TUBE METHODInto each of fourteen test tubes of similar size place 9.0mLof sterile Fluid Soybean–Casein Digest Medium. Arrange twelve of the tubes in four sets of three tubes each. Put aside one set of three tubes to serve as the controls. Into each of three tubes of one set (“100”)and into a fourth tube (A)pipet 1mLof the solution or suspension of the specimen, and mix. From tube A,pipet 1mLof its contents into the one remaining tube (B)not included in a set, and mix. These two tubes contain 100mg (or 100µL)and 10mg (or 10µL)of the specimen, respectively. Into each of the second set (“10”)of three tubes pipet 1mLfromtube A,and into each tube of the third set (“1”)pipet 1mLfrom tube B.Discard the unused contents of tubes A and B.Close well,and incubate all of the tubes. Following the incubation period, examine the tubes for growth: the three control tubes remain clear and the observations in the tubes containing the specimen, when interpreted by reference to Table 1,indicate the most probable number of microorganisms per g or per mLof specimen.Table 1.Most Probable Total Count by Multiple-Tube MethodTest for Staphylococcus aureus and Pseudomonas aeruginosaTo the specimen add Fluid Soybean–Casein Digest Medium to make100mL,mix,and incubate. Examine the medium for growth, and if growth ispresent, use an inoculating loop to streak a portion of the medium on the surface of Vogel–Johnson Agar Medium(or Baird–Parker Agar Medium,or Mannitol–Salt Agar Medium)and of Cetrimide Agar Medium, each plated on petri dishes. Cover and invert the dishes, and incubate. If, upon examination, none of the plates contains colonies having the characteristics listed in Tables 2and 3for the media used, the test specimen meets the requirements for freedom from Staphylococcus aureus and Pseudomonas aeruginosa.Table 2.Morphologic Characteristics of Staphylococcus aureus on SelectiveAgar MediaTable 3.Morphologic Characteristics of Pseudomonas aeruginosa onSelective and Diagnostic Agar MediaCoagulase Test (for Staphylococcus aureus)— With the aid of an inoculating loop, transfer representative suspect colonies from the agar surfaces of the Vogel–Johnson Agar Medium (or Baird–Parker Agar Medium, or Mannitol–Salt Agar Medium)to individual tubes, each containing 0.5mLof mammalian, preferably rabbit or horse, plasma with or without suitable additives. Incubate in a water bath at 37,examining the tubes at 3hours and subsequently at suitable intervals up to 24hours.Test positive and negative controls simultaneously with the unknown specimens. If no coagulation in any degree is observed, the specimen meets the requirements of the test for absence of Staphylococcus aureus.Oxidase and Pigment Tests (for Pseudomonas aeruginosa)— With the aid of an inoculating loop, streak representative suspect colonies from the agar surface of Cetrimide Agar Medium on the agar surfaces of Pseudomonas Agar Medium for Detection of Fluorescin and Pseudomonas Agar Medium for Detection of Pyocyanin contained in petri dishes. If numerous colonies are to be transferred, divide the surface of each plate into quadrants, each of which may be inoculated from a separate colony. Cover and invert the inoculated media, and incubate at 35±2for not less than three days.Examine the streaked surfaces under UVlight. Examine the plates to determine whether colonies having the characteristics listed in Table 3are present.Confirm any suspect colonial growth on one or more of the media as Pseudomonas aeruginosa by means of the oxidase test. Upon the colonial growth place or transfer colonies to strips or disks of filter paper that previously has been impregnated with N,N-dimethyl-p-phenylenediamine dihydrochloride: if there is no development of a pink color, changing to purple, the specimenmeets the requirements of the test for the absence of Pseudomonas aeruginosa. The presence of Pseudomonas aeruginosa may be confirmed by other suitable cultural and biochemical tests, if necessary.Test for Salmonella species and Escherichia coliTo the specimen, contained in a suitable vessel, add a volume of Fluid Lactose Medium to make 100mL,and incubate. Examine the medium for growth, and if growth is present, mix by gently shaking. Pipet 1-mLportions into vessels containing,respectively,10mLof Fluid Selenite–Cystine Medium and Fluid Tetrathionate Medium, mix, and incubate for 12to 24hours.(Retain the remainder of the Fluid Lactose Medium.)Test for Salmonella Species— By means of an inoculating loop, streak portions from both the selenite-cystine and tetrathionate media on the surface of Brilliant Green Agar Medium, Xylose–Lysine–Desoxycholate Agar Medium, and Bismuth Sulfite Agar Medium contained in petri dishes. Cover and invert the dishes, and incubate. Upon examination, if none of the colonies conforms to the description given in Table 4,the specimen meets the requirements of the test for absence of the genus Salmonella.Table 4.Morphologic Characteristics of Salmonella Species on Selective AgarMediaIf colonies of Gram-negative rods matching the description in Table 4are found, proceed with further identification by transferring representative suspect colonies individually, by means of an inoculating wire, to a butt-slant tube of Triple Sugar–Iron–Agar Medium by first streaking the surface of the slant andthen stabbing the wire well beneath the surface. Incubate. If examination discloses no evidence of tubes having alkaline (red)slants and acid (yellow)butts (with or without concomitant blackening of the butt from hydrogen sulfide production),the specimen meets the requirements of the test for the absence of the genus Salmonella.*Test for Escherichia coli— By means of an inoculating loop, streak a portion from the remaining Fluid Lactose Medium on the surface of MacConkey Agar Medium. Cover and invert the dishes, and incubate. Upon examination, if none of the colonies conforms to the description given in Table 5for this medium, the specimen meets the requirements of the test for absence of Escherichia coli. Table 5.Morphologic Characteristics of Escherichia coli on MacConkey AgarMediumIf colonies matching the description in Table 5are found, proceed with further identification by transferring the suspect colonies individually, by means of an inoculating loop, to the surface of Levine Eosin–Methylene Blue Agar Medium, plated on petri dishes. If numerous colonies are to be transferred, divide the surface of each plate into quadrants, each of which may be seeded from a separate colony. Cover and invert the plates, and incubate. Upon examination, if none of the colonies exhibits both a characteristic metallic sheen under reflected light and a blue-black appearance under transmitted light, the specimen meets the requirements of the test for the absence of Escherichia coli. The presence of Escherichia coli may be confirmed by further suitable cultural and biochemical tests.Total Combined Molds and Yeasts CountProceed as for the Plate Method under Total Aerobic Microbial Count, except for using the same amount of Sabouraud Dextrose Agar Medium or PotatoDextrose Agar Medium, instead of Soybean Casein Digest Medium, and except for incubating the inverted petri dishes for 5to 7days at 20to 25.RetestFor the purpose of confirming a doubtful result by any of the procedures outlined in the foregoing tests following their application to a 10.0-g specimen, a retest on a 25-g specimen of the product may be conducted. Proceed as directed for Procedure, but make allowance for the larger specimen size.微生物限度检查本章节提供了用于评估存在制药过程中,从原料到制剂的需氧微生物和其他指定微生物菌种的数量的检查方法。
USP微生物试验

E. coli
10倍稀釋檢液( TSB )
預備試驗
檢測試驗
將稀釋檢液加菌培養在 30~35℃,18 ~ 24小時
稀釋檢液培養在30~35℃ ,18 ~ 24小時
取1 mL稀釋液,接種於100 mL MacConkey Broth,於42~ 44℃培養24 ~ 48小時
劃線接種到MacConkey Agar 上 ,30~35℃培養18 ~ 72小時。
生長及區分
綠膿桿菌 Cetrimide Agar
P. aeruginosa:生長及區分 E. coli:抑制
Pseudomonas agar P 金黃色葡萄球菌 Mannitol Salt Agar
P. aeruginosa:生長及區分 E. coli:抑制
S. aureus:生長及區分 E. coli:抑制
Growth promotion and Inhibitory properties of the media
測試培養基
測試菌種及特性
大腸桿菌
MacConkey Broth
E. coli:生長、S. aureus:抑制
MacConkey Agar 沙門氏菌
E. coli:生長及區分
Rappaport-Vassiliadis Broth
新USP/EP/JP 藥典微生 物檢驗方法
USP Microbial Limits
Microbial Limit Tests” into two chapters:
<61> Microbial Enumeration Tests: Total aerobic microbial count (TAMC)-嗜氧性總生菌數 Total combined yeasts and molds count (TYMC)- 總黴菌及總酵母菌數
美国药典微生物试验说明

更為的是防止微生物生長
17/9
USP微生物相關
務必遵守章節
<51> 防腐效力/Antimicrobial Preservatives – Effectiveness <55> 生物指示劑–耐受力測試
Biological Indicators – Resistance Performance Tests <61> 非無菌產品的微生物檢驗:微生物計數試驗
而是通過適當滅菌工程或無菌充填過程確效, 並嚴格執行製程規定(cGMP)才可得到的。
–環境的微生物控制 –原料和組成分的微生物控制 –所有微生物控制的整合 –人員的微生物控制 –製程的微生物控制 –最終成品的微生物控制
33/9
無菌的概率possibility
每批產品的無菌性是根據概率推定的,即有產 品被污染的可能性也是微乎其微的
通常被接受的無菌保證度(SAL)是 10-6 的 微生物存活率或更低,即保證經滅菌的物品或 劑型中有活的微生物的可能性不超過百萬分之 一。
我們能做到比 10-6 更好嗎?
34/9
滅菌前的生物負荷
對熱不安定的物品,透過了解滅菌前物品的生物負 荷,經過 適當的時間週期,及足夠的滅菌前產品批 數,才足以建立滅 菌製程。
<2023> 非無菌營養和膳食補充劑 的微生物評估
19/9
EP & USP & JP 藥典方法的國際協合
International Harmonization of Pharmacopoeia
United States Pharmacopeia
European Pharmacopoeia
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
1227VALIDATION OF MICROBIAL RECOVERY FROM PHARMACOPEIAL ARTICLESThis chapter provides guidelines for the validation of methods for the estimation of the number of viable microorganisms, for the detection of indicators or objectionable microorganisms, for the validation of microbiological methods used in antimicrobial effectiveness testing, and for the sterility testing of Pharmacopeial articles. It is generally understood that if a product possesses antimicrobial properties because of the presence of a specific preservative or because of its formulation, this antimicrobial property must be neutralized to recover viable microorganisms. This neutralization may be achieved by the use of a specific neutralizer, by dilution, by a combination of washing and dilution, or by any combination of these methods.The tests under Antimicrobial Effectiveness Testing 51, Sterility Tests 71, and Microbial Limit Tests 61require the validation of recovery methods. To ensure that the results of the tests are credible, neutralization of antimicrobial properties of the test solution is required before estimating the number of viable microorganisms.INFLUENTIAL FACTORSSeveral factors affect the measurement of a test solution's antimicrobial activity, and these must be considered in the validation design. They include the nature of the microorganisms used as challenge organisms, the preparation of the inoculum of challenge organisms, the specific conditions of the test, and the conditions of recovery. These factors also affect the validation of recovery methods for aqueous or nonaqueous products, irrespective of their antimicrobial properties; thus, all test methods should be validated with these factors in mind.The nature of the challenge microorganism exerts a strong effect upon the response to the antimicrobial agent, and so upon the neutralization required for recovery. Represented among these organisms in compendial tests are Gram-positive bacteria, Gram-negative bacteria, yeasts, and molds. Each organism to be used in the test must be included in the validation.The preparation of the inoculum of challenge microorganisms also affects the testing of products having antimicrobial properties. The growth and preparation of the challenge organism determines the physiological state of the cell. This state has a direct influence on the results of any test of antimicrobial efficacy. Microbial tests do not use individual cells; rather, populations of cells are harvested for study. The data generated from these studies are less variable if the cell populations are homogeneous. Liquid cultures or confluent growths on solid medium are best suited for reproducible culture preparation. The conditions of organism preparation and storage must be standardized for the neutralizer evaluation and should reflect the conditions of the antimicrobial assay.The specific conditions of the test, including buffers used, water, light conditions, and temperature, must be reproduced in the validation study. All test conditions also should be standardized and performed in the validation study exactly as performed in the test.The conditions of microbial recovery are among the most crucial in accurately estimating the number of microorganisms present in a test solution. The first consideration is the recovery medium used to support the growth of survivors. This concern is discussed in detail below. The second consideration is the incubation conditions. Optimal conditions for growth must be present to ensure complete growth and reproducible results.METHODS OF NEUTRALIZING ANTIMICROBIAL PROPERTIESThree common methods are used to neutralize antimicrobial properties of a product: (1) chemical inhibition, (2) dilution, and (3) filtration and washing.Chemical InhibitionTable 1 shows known neutralizers for a variety of chemical antimicrobial agents and the reported toxicity of some chemical neutralizers to specific microorganisms. However, despite potential toxicity, the convenience and quick action of chemical inhibitors encourage their use. Chemical inhibition of bactericides is the preferred method for the antimicrobial efficacy test. The potential of chemical inhibitors should be considered in the membrane filtration and the direct transfer sterility tests. Antibiotics may not be susceptible to neutralization by chemical means, but rather by enzymatic treatment (e.g., penicillinase). These enzymes may be used where required.Table 1. Some Common Neutralizers for Chemical BiocidesDilutionA second approach to neutralizing antimicrobial properties of a product is by dilution, because the concentration of a chemical bactericide exerts a large effect on its potency. The relationship between concentration and antimicrobial effect differs among bactericidal agents but is constant for a particular antimicrobial agent. This relationship is exponential in nature, with the general formula:C t = k,in which C is the concentration; t is the time required to kill a standard inoculum; k is a constant; and the concentration exponent, , is the slope of the plot of log t versus log C. Antimicrobial agents with high values are rapidly neutralized by dilution, whereas those with low values are not good candidates for neutralization by dilution.Membrane FiltrationAn approach that is often used, especially in sterility testing, is neutralization by membrane filtration. This approach relies upon the physical retention of the microorganism on the membrane filter, with the antimicrobial agent passing through the filter into the filtrate. The filter is then incubated for recovery of viable microorganisms. However, filtration alone may not remove sufficient quantities of the bactericidal agent toallow growth of surviving microorganisms. Adherence of residual antimicrobial agents to the filter membrane may cause growth inhibition. Filtration through a low-binding filter material, such as polyvinylidene difluoride, helps to minimize this growth inhibition. Additionally, the preservative may be diluted or flushed from the filter by rinsing with a benign fluid, such as diluting Fluid A(see Diluting and Rinsing Fluids for Membrane Filtration under Sterility Tests 71for diluting fluid compositions). Chemical neutralizers in the rinsing fluid can ensure that any antimicrobial residue on the membrane does not interfere with the recovery of viable microorganisms.VALIDATION OF NEUTRALIZATION METHODS—RECOVERY COMPARISONSA validated method for neutralizing the antimicrobial properties of a product must meet two criteria: neutralizer efficacy and neutralizer toxicity. The validation study documents that the neutralization method employed is effective in inhibiting the antimicrobial properties of the product (neutralizer efficacy) without impairing the recovery of viable microorganisms (neutralizer toxicity). Validation protocols may meet these two criteria by comparing recovery results for treatment groups.The first is the test group, in which the product is subjected to the neutralization method, then a low level of challenge microorganism [less than 100 colony-forming units (cfu)] is inoculated for recovery. The second is the peptone control group, in which the neutralization method is used with peptone, or diluting Fluid A (see Sterility Tests 71), as the test solution. The third is the viability group, in which the actual inoculum is used without exposure to the neutralization scheme. Similar recovery between the test group and the peptone group demonstrates adequate neutralizer efficacy; similar recovery between the peptone group and the viability group demostrates adequate neutralizer toxicity.In principle, the protocol must show that recovery of a low inoculum (less than 100 cfu) is not inhibited by the test sample and the neutralization method. Validation protocols may meet these two criteria by comparing recovery among three distinct test groups: (1) neutralized product with inoculum, (2) challenge inoculum control in buffered solution, and(3) inoculum in the absence of product or neutralizer. This can be established by directly comparing the result in the treated solution (1) to the inoculum (3) above. If the growth on the treated solution is not comparable to the growth on the inoculum group, it should be determined whether the neutralization method itself is toxic to the microorganisms. Recovery on Agar MediumIn the tests under Antimicrobial Effectiveness Testing 51and Microbial Limit Tests 61, the number of viable challenge microorganisms in the product is estimated at various time intervals by calculating the concentration of cfu per mL by the plate count method. A design for validating neutralization would incorporate the treatment groups as described under Validation of Neutralization Methods—Recovery Comparisons. At least three independent replicates of the experiment should be performed, and each should demonstrate that the average number of cfu recovered from the challenge product is not less than 70% of that recovered from the inoculum control.If a greater number of replicates is required in the validation study, the comparisons may be evaluated by transforming the numbers of cfu to their logarithmic values and analyzing the data statistically by the Student t test (pairwise comparisons) or by analysis of variance (ANOVA) (for comparing all groups). If ANOVA is used, and significant differences among the populations are determined, a test such as Dunnett's test may be used, with the peptone group used as the control group.Recovery by Membrane FiltrationThis validation follows the procedure described for Validation Test under Sterility Tests 71, with the exception of plating on solid medium to quantitate recovery. Three 100-mL rinses are assumed, but the volume and number of rinses are subject to validation. Each validation run should be performed independently at least three times.In the test solution group, the product is filtered through the membrane filter, followed by two 100-mL portions of diluting-neutralizing fluid. After the second rinse has been filtered, a final 100-mL portion containing less than 100 cfu of the specific challengemicroorganism is passed through the filter. This filter is then placed on the appropriate agar recovery medium and incubated for recovery.The inoculum is directly plated onto the solid medium. It is possible that filtration will lead to reduced recovery of the challenge microorganism, either through inherent toxicity of the membrane or by adherence of the microrganism to the filtration vessel walls. A control group can be used to evaluate this component of membrane filtration validation. Diluting Fluid A is used as the dilution medium without exposing the filter to the product. After addition of the low-level inoculum to the final rinse, the filter is plated as above. Technique-specific loss of microorganisms can be estimated by comparing the recovery in the diluting Fluid A group to the inoculum count.It is assumed in this discussion that the test sample can be filtered. If it is necessary to solubilize the test sample, the effects of the solubilization method on viable microorganisms must be determined. This situation can occur when testing ointments, suspensions, or other articles.The method can be considered validated if the recovery rate in the three independent replicates is similar for the test solution and the diluting Fluid A control.Recovery in Liquid MediumIt is assumed in Direct Inoculation of the Culture Medium in the section Test for Sterility of the Product to be Examined under Sterility Tests 71that the recovery medium will allow for growth of all surviving microorganisms. The broth in that test must serve both to neutralize any antimicrobial properties of the test solution and to support the growth of the microorganisms. The treatment groups described under Validation of Neutralization Methods—Recovery Comparisons above can be used for validation of the recovery method, with the proportions of product and recovery medium varied to achieve adequate neutralization. The method can be considered validated if all groups show copious growth within 7 days for all microorganisms.RECOVERY OF INJURED MICROORGANISMSThe validation studies described above use challenge microorganisms that have never been exposed to antimicrobial agents, and thus are not identical to organisms seen in antimicrobial effectiveness testing or when a sterility test is performed on a preserved product. If the use of alternative media is desired, the recovery of injured microorganisms should be addressed in the validation study. This may be done by directly comparing the recovery of each challenge microorganism on the preferred medium and on the alternative medium, after exposure to the product. This exposure should include at least two time periods showing survival of less than 100 cfu per mL, unless the rate of kill of the antimicrobial agent is such that no recovery is possible even if the microorganism is plated within minutes of exposure. This comparison should be performed at least three times. The alternative medium is validated if the recovery seen on that medium is no less than that seen on the preferred medium, within an error of 0.5 log units.ESTIMATING THE NUMBER OF COLONY-FORMING UNITSThe accuracy of any estimate of viable cfu is affected by the number plated. As the number of viable cells plated increases, crowding effects decrease the accuracy of the count, reducing the estimate. As the number decreases, random error plays an increasing role in the estimate.The accepted range for countable colonies on a standard agar plate is between 25 and 250 for most bacteria and Candida albicans.This range was established in the food industry for counting coliform bacteria in milk. This range is acceptable for compendial organisms, except for fungi. It is not optimal for counting all environmental isolates. The recommended counting range for Aspergillus niger is between 8 and 80 cfu per plate. The use of membrane filtration to recover challenge microorganisms, or the use of environmental isolates as challenge microorganisms in antimicrobial effectiveness testing, requires validation of the countable range. This validation may be performed by statistical comparison of estimated cfu from successive pairs in a dilution series. Prepare a suspension so that plating will provide approximately 1000 cfu per plate, and then dilute twofold to a theoretical concentration of approximately 1 cfu per plate. Plate all dilutions in the series in duplicate, and incubate for recovery under the conditions of the AntimicrobialEffectiveness Testing 51. Compare the estimates of cfu per mL from paired tubes in the dilution series by the formula:in which L cfu is the number of colonies on the plate with the lower count (greater dilution), and H cfu is the number of colonies on the plate with the higher count (lesser dilution). The estimates of the cfu per mL provided by L cfu and H cfu should agree within the limits of the formula with a critical value of 1.96. The upper limit of plate counts is then defined as the number (H cfu) that reproducibly passes this test. This study should be independently repeated a sufficient number of times to establish an upper limit of cfu for the particular plating conditions.There is a lower limit at which the ability of the antimicrobial effectiveness test to recover microorganisms becomes untenable. If the first plating is performed with 1 mL of a 10–1 dilution, cfu in the range of 1 to 10 per mL would not be seen. On this dilution plating, only the lower number of cfu may be reduced to 3, allowing as few survivors as 30 cfu per mL to be reported.Lower counting thresholds for the greatest dilution plating in series must be justified. Numbers of colonies on a plate follow the Poisson distribution, so the variance of the mean value equals the mean value of counts. Therefore, as the mean number of cfu per plate becomes lower, the percentage error of the estimate increases (see Table 2). Three cfu per plate at the 10–1 dilution provide an estimate of 30 cfu per mL, with an error of 58% of the estimate.Table 2. Error as a Percentage of Mean for Plate Counts。
