PD98059_167869-21-8_DataSheet_MedChemExpress
高效液相色谱法检测(S)-2-氨基丁酰胺盐酸盐的纯度

高效液相色谱法检测(S)-2-氨基丁酰胺盐酸盐的纯度郝玉红;朱力敏;薛韧婕【摘要】用高效液相色谱法定量分析(S)-2-氨基丁酰胺盐酸盐的纯度。
用Agilent 1100型高效液相色谱仪,色谱柱Xterra C18(5µm,4.6 mm×250 mm),流动相为20%乙腈-80%,缓冲液:1000 mL水+5.0 g庚烷磺酸钠;检测波长为210 nm,流量为0.7 mL•min-1,柱温30℃,对(S)-2-氨基丁酰胺盐酸盐纯度进行定量测定。
相对标准偏差0.15%,标准回收率99.8%~100.2%,方法重复性好,定量准确度高。
%Purity analysis of (S)-2-Aminobutyramide hydrochloride was established by high performance liquid chromatography with Agilent 1100, Xterra C18(5 μm, 4.6 mm×250 mm)column, m obile phase was acetonitrile-water, detection wavelength was 210 nm, flow rate was 0.7 mL·min-1, column temperature was 30℃. The method showed repeatability and relative standard deviation was 0.15%, and the standard recoveries were 99.8%-100.2%. These quantitative results showed high accuracy.【期刊名称】《上海计量测试》【年(卷),期】2014(000)002【总页数】2页(P40-41)【关键词】高效液相色谱法;(S )-2-氨基丁酰胺盐酸盐【作者】郝玉红;朱力敏;薛韧婕【作者单位】上海市计量测试技术研究院;上海市计量测试技术研究院;上海市计量测试技术研究院【正文语种】中文(S)-2-氨基丁酰胺盐酸盐(简称ABAH),为白色或类白色固体粉末,主要用于抗癫痫、抗惊厥药物中间体。
Lumoxiti (moxetumomab pasudotox-tdfk) 产品说明书

Lumoxiti™ (moxetumomab pasudotox-tdfk)(Intravenous)Document Number: IC-0393 Last Review Date: 10/02/2018Date of Origin: 10/02/2018Dates Reviewed: 10/2018I.Length of AuthorizationCoverage is provided for six months (6 cycles) and may not be renewed.II.Dosing LimitsA.Quantity Limit (max daily dose) [Pharmacy Benefit]:-Lumoxiti 1 mg SDV: 15 vials per 28 day cycleB.Max Units (per dose and over time) [Medical Benefit]:∙ 5 mg on days 1, 3 and 5 of a 28-day cycleIII.Initial Approval CriteriaCoverage is provided in the following conditions:∙Patient is at least 18 years or older; AND∙Patient is pseudomonas-immunotoxin naïve (e.g., moxetumomab pasudotox, etc.); AND∙Patient does not have severe renal impairment defined as CrCl ≤ 29 mL/min; ANDHairy Cell Leukemia (HCL)†∙Patient has a confirmed diagnosis of Hairy Cell Leukemia or a HCL variant; AND∙Patient must has relapsed or refractory disease; AND∙Patient has previously failed at least TWO prior systemic therapies as one of the following: o Failure to two courses of purine analog therapy (e.g., cladribine, pentostatin, etc.); ORo Failure to at least one purine analog therapy AND one course of rituximab or a BRAF-inhibitor (e.g., vemurafenib, etc.)†FDA Approved Indication(s); ‡ Compendia recommended indication(s)IV.Renewal CriteriaCoverage cannot be renewed.V.Dosage/AdministrationVI.Billing Code/Availability InformationJcode:J9999: Not otherwise classified, antineoplastic drugsNDC:Lumoxiti 1 mg single-dose vial: 00310-4700-xxo IV solution stabilizer for use during administration: 00310-4715-xxVII.References1.Lumoxiti [package insert]. Wilmington, DE; Astra Zeneca Pharmaceuticals; September2018. Accessed September 2018.2.Referenced with permission from the NCCN Drugs & Biologics Compendium (NCCNCompendium®) for moxetumomab pasudotox. National Comprehensive Cancer Network,2018. The NCCN Compendium® is a derivative work of the NCCN Guidelines®. NATIONALCOMPREHENSIVE CANCER NETWORK®, NCCN®, and NCCN GUIDELINES® aretrademarks owned by the National Comprehensive Cancer Network, Inc.” To view the mostrecent and complete version of the Compendium, go online to . AccessedSeptember 2018.3.Kreitman RJ, Dearden C, Zingani PL, et al. Moxetumomab pasudotox inrelapsed/refractory hairy cell leukemia. Leukemia. 2018; 32(8): 1768–1777.4.Robbins BA, Ellison DJ, Spinosa JC, et al. Diagnostic application of two-color flowcytometry in 161 cases of hairy cell leukemia. Blood 1993;82:1277-1287.Appendix 1 – Covered Diagnosis CodesAppendix 2 – Centers for Medicare and Medicaid Services (CMS)Medicare coverage for outpatient (Part B) drugs is outlined in the Medicare Benefit Policy Manual (Pub. 100-2), Chapter 15, §50 Drugs and Biologicals. In addition, National Coverage Determination (NCD) and Local Coverage Determinations (LCDs) may exist and compliance with these policies is required where applicable. They can be found at: /medicare-coverage-database/search/advanced-search.aspx. Additional indications may be covered at the discretion of the health plan.Medicare Part B Covered Diagnosis Codes (applicable to existing NCD/LCD): N/A。
超高效液相色谱-串联质谱法测定人血浆中精氨酸及衍生物含量

超高效液相色谱-串联质谱法测定人血浆中精氨酸及衍生物含量田晔;江骥;胡蓓;薛金萍;王洪允【摘要】建立了超高效液相色谱-串联质谱(UPLC-MS/MS)法同时测定使用艾普拉唑后人血浆中二甲基精氨酸(ADMA)、对称二甲基精氨酸(SDMA)、单甲基精氨酸(NMMA)、瓜氨酸(Cit)和L-精氨酸(L-Arg)的浓度.采用HILIC亲水相互作用色谱和非衍生化的蛋白沉淀法进行分离分析,色谱柱选取Waters Atlantic HILIC柱(2.1 mm×50 mm×3μm),流动相由乙腈(含0.5%乙酸和0.025%三氟乙酸)-水(含0.5%乙酸和0.025%三氟乙酸)(85:15,v/V)组成,流速0.25 mL/min.采用多反应离子监测(MRM)模式,以电喷雾离子源(ESI)正离子方式检测.结果显示,ADMA、SDMA、NMMA、L-Arg和Cit的线性关系良好,相关系数r均大于0.994 0;ADMA、SDMA和NMMA的线性范围为0.1~5 mmol/L,L-Arg和Cit的线性范围为10~250 mmol/L;5种氨基酸的日内、日间精密度均小于15%,准确度在85%~115%之间.该方法快速、简便、灵敏,可为相关疾病的临床诊断提供一种高效的检测手段.【期刊名称】《质谱学报》【年(卷),期】2016(037)005【总页数】7页(P446-452)【关键词】超高效液相色谱-串联质谱(UPLC-MS/MS);艾普拉唑;蛋白沉淀法;亲水性色谱【作者】田晔;江骥;胡蓓;薛金萍;王洪允【作者单位】福州大学化学学院,福建省功能材料工程研究中心,福建省光动力治疗药物与诊疗工程技术研究中心,福建福州350108;中国医学科学院北京协和医院临床药理中心,北京100730;中国医学科学院北京协和医院临床药理中心,北京100730;中国医学科学院北京协和医院临床药理中心,北京100730;福州大学化学学院,福建省功能材料工程研究中心,福建省光动力治疗药物与诊疗工程技术研究中心,福建福州350108;中国医学科学院北京协和医院临床药理中心,北京100730【正文语种】中文【中图分类】O657.63一氧化氮是人体重要的信使分子,L-精氨酸(L-Arg)在一氧化氮全酶(NOS)的催化下,产生一氧化氮(NO)和瓜氨酸(Cit)[1-2]。
常用多肽缩合试剂
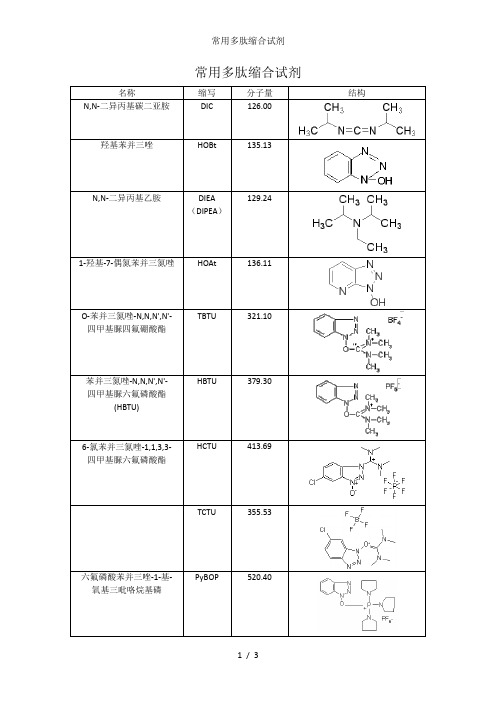
名称缩写分子量结构N,N-二异丙基碳二亚胺DIC 126.00羟基苯并三唑HOBt 135.13129.24N,N-二异丙基乙胺DIEA(DIPEA)1-羟基-7-偶氮苯并三氮唑HOAt 136.11TBTU 321.10O-苯并三氮唑-N,N,N',N'-四甲基脲四氟硼酸酯HBTU 379.30苯并三氮唑-N,N,N',N'-四甲基脲六氟磷酸酯(HBTU)HCTU 413.696-氯苯并三氮唑-1,1,3,3-四甲基脲六氟磷酸酯TCTU 355.53PyBOP 520.40六氟磷酸苯并三唑-1-基-氧基三吡咯烷基磷名称缩写分子量结构PyAOP 521.38(3H-1,2,3-三唑并[4,5-b]吡啶-3-氧基)三-1-吡咯烷基鏻六氟磷酸盐DCC 206.33N,N'-二环己基碳二亚胺4-二甲氨基吡啶DMAP 122.17DBU 152.241,8-二氮杂双环[5.4.0]十一碳-7-烯1,1’-羰基二咪唑CDI 162.15HATU 380.232-(7-偶氮苯并三氮唑)-N,N,N',N'-四甲基脲六氟磷酸酯HOOBt 163.103-羟基-1,2,3-苯并三嗪-4(3H)-酮Cl-HOBt 169.576-氯-1-羟基苯并三氮唑EDC.HCl 191.71-(3-二甲氨基丙基)-3-乙基碳二亚胺盐酸盐TATU 322.1O-(7-氮杂苯并三氮唑)-N,N,N',N'-四甲基脲四氟硼酸盐名称缩写分子量结构O-(1,2-二氢-2-氧-吡啶基)--1,1,3,3-四甲基脲四氟硼酸盐TPTU 297.10O-(N-琥珀酰亚胺基)-N NN'N'-四甲基四氟硼酸脲TSTU 301.10三吡咯烷基溴化鏻六氟磷酸盐PyBrOP 466.20N,N,N',N'-四甲基氯甲脒六氟磷酸盐TCFH 280.583 -二乙氧基磷酰基-1,2,3-苯唑4(3H)-酮DEPBT 299.23O-[(乙氧基羰基)氰基甲胺]-N,N,N',N'-四甲基硫尿四氟硼酸TOTU 328.1苯并三氮唑-1-基氧基三(二甲基氨基)磷鎓六氟磷酸盐BOP(卡特缩合剂)442.50N,N,N',N'-四甲基-O-(3,4-二氢-4-氧代-1,2,3-苯并三嗪-3-基)脲四氟硼酸盐TDBTU 349.09。
Amtech Tacky 助焊膏系列安全数据表说明书

Inventec Performance Chemicals USA, LLCSAFETY DATA SHEET (SDS)SECTION 1: PRODUCT AND COMPANY IDENTIFICATIONPRODUCT NAME: Amtech Tacky Paste Flux Series: 200, 400, 500, 600, 4000, SynTECH, WSFC-305L and #61 SYNONYMS:Tacky FluxMANUFACTURER: Inventec Performance Chemicals USA, LLCADDRESS:PO Box 989 Deep River, CT 06417 USAPHONE:860-526-8300FAX:860-526-8243EMERGENCY:Infotrac-(800)535-5035REVISION DATE:December 19, 2014REVISION DATE: 3DOCUMENT NAME:SDS-Tacky Flux-008PRODUCT USE:Bonding solder joints in production and repair of circuit boardsSECTION 2: HAZARDS IDENTIFICATIONCHEMICAL NAME:N/ACHEMICAL FAMILY:MixtureCHEMICAL FORMULA:N/AROUTES OF ENTRY: Inhalation, Ingestion, Skin/Eye ContactGHS:Signal Word: WarningHazard statement(s)H302 Harmful if swallowedH317 May cause an allergic skin reactionH320 Causes eye irritationH335 May cause respiratory irritationPrecautionary statement(s)P102 Keep out of reach of childrenP233 Keep container tightly closedP264 Wash hands thoroughly after handlingP270 Do not eat, drink or smoke when using this productP280 Wear protective gloves/protective clothing/eye protection/face protectionP302+P352 IF ON SKIN: Wash with plenty of soap and waterP305+P351 IF IN EYES: Rinse continuously with water for several minutesP404 Store in a closed containerP501 Dispose of contents/containers in accordance with Federal, State/Provincial, and/or local regulations POTENTIAL HEALTH EFFECTS:EYE CONTACT: May cause moderate irritation. Do not allow material to come in contact with eyes.SKIN CONTACT: May cause moderate skin irritation.INHALATION: May cause irritation to the respiratory tract.INGESTION: Harmful if swallowed. May cause irritation to the mouth, throat, and stomach. May cause abdominal discomfort, nausea, vomiting, and/or diarrhea.CHRONIC: Not established.SECTION 2 NOTES:Inventec Performance Chemicals USA, LLC does not recommend, manufacture, market, or endorse any of its products for human consumption.SECTION 3: COMPOSITION/INFORMATION ON INGREDIENTSIngredient CAS Number Exposure LimitsModified Rosins N/A N/APine Oil Derivatives 8000-41-7 N/AProprietary Ingredients N/A N/AMixed Carboxylic Acids N/A N/ASECTION 3 NOTES:Percentages of individual components are not listed as this information is considered a trade secret.SECTION 4: FIRST AID MEASURESEYES: Flush with plenty of water, contact a physician. If contact lenses can be removed easily, flush eyes without contact lenses. SKIN: Wash affected area with plenty of warm, soapy water. If irritation persists, seek medical attention.INGESTION: Call a physician or Poison Control Center immediately. Do not induce vomiting.INHALATION: Remove to fresh air. If not breathing, seek immediate medical attention.SECTION 5: FIRE-FIGHTING MEASURESEXTINGUISHING MEDIA: Dry chemical, foamSPECIAL FIRE FIGHTING PROCEDURES: Do not use water. Use NIOSH-approved self-contained Breathing Apparatusand full protective clothing if involved in a fire.UNUSUAL FIRE AND EXPLOSION HAZARDS:This product does not present any unusual fire and explosion hazards. SECTION 6: ACCIDENTAL RELEASE MEASURESACCIDENTAL RELEASE MEASURES: If material spills or leaks, collect and place into a properly labeled waste container. Remove traces of tacky flux using cloth rags or paper towels moistened with Isopropyl Alcohol. Follow on-site personal protective equipment recommendations.SECTION 6 NOTES:See Sections 2, 4, and 7 for additional information.SECTION 7: HANDLING AND STORAGEHANDLING/STORAGE: Keep containers tightly closed when not in use. Use care to avoid spills. Avoid inhalation of fumes or dust. Avoid contact with eyes, skin, and clothing.OTHER PRECAUTIONS: Empty containers may retain product residues in vapor, liquid, and/or solid form. All labeled hazard precautions should be observed.WORK HYGIENIC PRACTICES: Cosmetics/Food/Drink/Tobacco should not be consumed or used in work areas. Always wash hands after handling material and before applying or using cosmetics/food/drink/tobacco.SECTION 7 NOTES:For industrial use only.SECTION 8: EXPOSURE CONTROLS/PERSONAL PROTECTIONVENTILATION: Provide sufficient mechanical (general and/or local exhaust) ventilation to maintain exposure below TLVs. RESPIRATORY PROTECTION: Use with adequate ventilation.EYE PROTECTION: Use with appropriate safety glasses.SKIN PROTECTION: Protective gloves and clothing should be worn when handling material. Wash hands thoroughly with soap and water upon leaving the work area.SECTION 9: PHYSICAL AND CHEMICAL PROPERTIESAPPEARANCE: Clear, White, or Yellow to Dark Amber gelODOR: Mild odorODOR THRESHOLD: Not establishedpH as SUPPLIED: N/ASECTION 9: PHYSICAL AND CHEMICAL PROPERTIES (continued)MELTING POINT: Not establishedFREEZING POINT: Not establishedINITIAL BOILING POINT: Not establishedBOILING RANGE: Not establishedFLASH POINT: Not establishedEVAPORATION RATE: Not establishedFLAMMABILITY (solid): Not establishedUPPER/LOWER FLAMMABILITY: Not establishedUPPER/LOWER EXPLOSIVE LIMITS:Not establishedVAPOR PRESSURE (mmHg): N/A (°F/°C)VAPOR DENSITY (AIR = 1): N/A (°F/°C)RELATIVE DENSITY: Not establishedSOLUBILITY IN WATER: PartiallyPARTITION COEFFICIENT (n-octanol/water): Not establishedAUTOIGNITION TEMPERATURE: Not establishedDECOMPOSITION TEMPERATURE: Not establishedVISCOSITY: N/A (°F/°C)SECTION 10: STABILITY AND REACTIVITYSTABILITY: StableCONDITIONS TO AVOID (STABILITY): Freezing temperatures. High temperatures. INCOMPATIBILITY (MATERIAL TO AVOID): Strong oxidizing materialsHAZARDOUS DECOMPOSITION/BY-PRODUCTS: Harmful organic fumes and toxic oxide fumes may form at elevatedtemperatures.POSSIBILITY OF HAZARDOUS REACTIONS: Will not occurSECTION 11: TOXICOLOGICAL INFORMATIONACUTE TOXICITY: Not availableSKIN CORRISION/IRRITATION: Not establishedSERIOUS EYE DAMAGE/IRRITATION: Not availableRESPIRATORY OR SKIN SENSITIZATION: Not establishedGERM CELL MUTAGENICITY: Not availableCARCINOGENICITY: Not availableREPRODUCTIVE TOXICITY: Not availableSTOT-SINGLE EXPOSURE: Not availableSTOT-REPEATED EXPOSURE: Not availableASPIRATION HAZARD: Not availableSECTION 12: ECOLOGICAL INFORMATIONTOXICITY: Product not testedPERSISTENCE AND DEGRADIBILITY: Product not testedBIOACCUMULATIVE POTENTIAL: Product not testedMOBILITY IN SOIL: Product not testedOTHER ADVERSE EFFECTS: Product not testedSECTION 13: DISPOSAL CONSIDERATIONSWASTE DISPOSAL METHOD: Scrap and waste solder should be stored in a dry, sealed container for later disposal. Disposal must be in accordance with Federal, State/Provincial, and Local Regulations.SECTION 14: TRANSPORT INFORMATIONTransport in accordance with applicable regulations and requirements.UN Number: Not availableUN Proper Shipping Name: Not availablePackaging Group:Not applicableEnvironmental Hazards:NoneTRANSPORT HAZARD CLASSES:US DOT Hazardous Material Classification: Tacky Flux is not listed as a DOT hazardous materialWater Transportation: Tacky Flux is not listed as a hazardous materialIATA Hazardous Material Classification: Tacky Flux is not listed as IATA hazardous materialSECTION 15: REGULATORY INFORMATIONAll ingredients used to manufacture this product are listed on the EPA TSCA Inventory.U.S. FEDERAL REGULATIONS: Not regulatedSTATE REGULATIONS: Not regulatedINTERNATIONAL REGULATIONS: Not regulatedSECTION 16: OTHER INFORMATIONHMIS Rating: Health=1 Flammability=1 Physical Hazard=0 Personal Protection=X KEY:N/A: Not applicableGHS: Global Harmonized SystemOSHA: Occupational Safety and Health AdministrationACGIH: American Conference of Governmental Industrial HygienistsNTP: National Toxicology ProgramIARC: International Agency for Research on CancerCAS: Chemical Abstract ServiceNIOSH: National Institute for Occupational Safety & HealthSTOT: Specific target organ toxicityTLV: Threshold limit valueUS DOT: United States Department of TransportationDOT: Department of TransportationIATA: International Air Transport AssociationEPA:Environmental Protection AgencyTSCA:Toxic Substance Control ActHMIS:Hazardous Material Identification SystemPREPARATION INFORMATION:This update supersedes all previously released documents.PREPARED BY: Wendy W. GesickAPPROVED BY: Leigh W. GesickDISCLAIMER:The information contained herein is based on data considered to be accurate but does not purport to be all-inclusive and shall be used only as a guide. No warranty is expressed or implied regarding the accuracy of this data and Inventec Performance Chemicals USA, LLC shall not be held liable for any damage resulting from any handling or contact with the above product. Liability is expressly disclaimed for loss or injury arising out of use of this information or the use of any materials designated. This material is not for resale, unauthorized distribution, or personal use.。
封闭添加试剂(按字母顺序排列)

询价
4、BMPA
改变巯基基团为羧基基团,用于制备肽段-蛋白质交联物。
产品特点:
•反应基团:马来酰亚胺和羧基
•反应指向:巯基基团和胺(当与EDC结合使用时)
参考文献
Hermanson, G.T. (2008). Bioconjugate Techniques, 2nd ed., Elsevier Inc., pp.111-113. (Product # 20036)
25g
473
相关产品:
产品货号
产品名称
价格¥
24510
Sulfo-NHS
1848
22980
EDC
1005
20320
DCC
488
14、STAT,SATP和巯基添加试剂盒
添加保护性巯基防止二肽形成。
产品特点:
•与伯胺反应添加保护性巯基
•简单的脱保护步骤即可产生游离的巯基
•Thermo Scientific巯基添加试剂盒包含硫醇化作用,脱保护,纯化和定量所有需要的组分
订购信息:
产品货号
产品名称
包装
价格¥
23031
二盐酸乙二胺
10g
399
8、碘乙酰胺(Iodoacetamide),一次性使用
质谱前可靠的烷基化试剂。产品ຫໍສະໝຸດ 点:•与巯基基团形成共价键
•质谱级别(高纯度)
•方便,一次性使用形式(3个微型管,每管含有9.4mg)
•当用132μl碳酸氢铵(ammonium bicarbonate)溶解时,每管产生375mM的溶液
500mg
620
10、MMTS
可与巯基反应的可逆试剂。
产品特点:
•将-SH基团转化为-S-S-CH3
QuEChERS

第43 卷第 2 期2024 年2 月Vol.43 No.2254~260分析测试学报FENXI CESHI XUEBAO(Journal of Instrumental Analysis)QuEChERS/超高效液相色谱-串联质谱法同时测定大米中16种香豆素和香兰素及其衍生物邓楠1,尹芳平1,刘川1,汪辉1,2*,向芬1,陈娅娅3(1.长沙市食品药品检验所,湖南长沙410036;2.国家酒类产品质量检验检测中心(湖南),湖南长沙410036;3.张家界市高级技工学校,湖南张家界427000)摘要:建立了大米中16种香豆素和香兰素及其衍生物的超高效液相色谱-串联质谱(UPLC-MS/MS)检测方法。
大米样品采用1%甲酸乙腈提取,150 mg C18与450 mg无水MgSO4吸附剂净化,采用Agilent SB-C18色谱柱(2.1 mm×50 mm,1.8 μm)分离,0.2%甲酸水溶液(含5 mmol/L甲酸铵)和乙腈为流动相梯度洗脱,在电喷雾离子源正负离子模式下采用多反应监测模式(MRM)进行测定,以基质匹配标准溶液内标法定量。
结果显示,16种待测组分在各自质量浓度范围内线性关系良好(r≥0.998),方法检出限为0.001~0.400 mg/kg,定量下限为0.004~0.600 mg/kg,平均回收率为71.3%~120%,相对标准偏差(RSD,n=6)为1.6%~6.5%,日内RSD(n= 6)为1.0%~8.8%,日间RSD(n=3)为0.60%~14%。
该法前处理简单易操作、灵敏度高、准确性好,适用于大米中香豆素与香兰素及其衍生物的同时检测。
关键词:QuEChERS;超高效液相色谱-串联质谱;大米;香豆素;香兰素;衍生物;同位素内标中图分类号:O657.7;TS207.3文献标识码:A 文章编号:1004-4957(2024)02-0254-07Simultaneous Determination of 16 Coumarins and Vanillins andTheir Derivatives in Rice by QuEChERS/Ultra-high Performance Liq⁃uid Chromatography-Tandem Mass SpectrometryDENG Nan1,YIN Fang-ping1,LIU Chuan1,WANG Hui1,2*,XIANG Fen1,CHEN Ya-ya3(1.Changsha Institute for Food and Drug Control,Changsha 410036,China;2.National Liquor ProductQuality Supervision and Inspection Center(Hunan),Changsha 410036,China;3.Zhangjiajie Senior Technical School,Zhangjiajie 427000,China)Abstract:A ultra-high performance liquid chromatography-tandem mass spectrometry(UPLC-MS/ MS) method was established for the determination of 16 coumarins and vanillins and their derivatives in rice. The rice sample was extracted by acetonitrile with 1% formic acid,purified with a combined dadsorbent with 150 mg C18 and 450 mg anhydrous MgSO4. The chromatography separation for the an⁃alytes was achieved on an Agilent SB-C18 column(2.1 mm×50 mm,1.8 μm),and gradient elution was performed using 0.2% formic acid solution(containing 5 mmol/L ammonium formate) and acetoni⁃trile. The analytes were determined by multiple reaction monitoring(MRM) of electrospray ionization in positive and negative ion modes,and quantified by matrix-matched internal standard method. The 16 components measured had good linear relationships within their respective mass concentration ranges(r≥0.998). The limits of detection were 0.001-0.400 mg/kg,and the limits of quantitation were 0.004-0.600 mg/kg.Average recoveries of the 16 components ranged from 71.3%to 120%,the relative standard deviations(RSDs,n=6) ranged from 1.6% to 6.5%,the intra-day RSDs(n=6)ranged from 1.0% to 8.8%,and the inter-day RSDs(n=3) ranged from 0.60% to 14%. The method is simple and easy to operate,with high sensitivity and accuracy,and is suitable for the simultaneous determination of coumarins and vanillins and their derivatives in rice.Key words:QuEChERS;UPLC-MS/MS;rice;coumarins;vanillins;derivative;isotope inter⁃nal standard收稿日期:2023-08-16;修回日期:2023-10-22基金项目:湖南省自然科学科药联合基金项目(2022JJ80037)∗通讯作者:汪辉,硕士,高级工程师,研究方向:食品和化妆品成分分析,E-mail:wanghuei158@doi:10.12452/j.fxcsxb.23081602255第 2 期邓楠等:QuEChERS/超高效液相色谱-串联质谱法同时测定大米中16种香豆素和香兰素及其衍生物大米是中国人饮食结构中必不可缺的食材,其质量安全关乎人民的身体健康。
MSDS合集(16):248种3.3类高闪点易燃液体(五)

目录化学品安全技术说明书-碳酸二乙酯;碳酸乙酯 (1)化学品安全技术说明书-正硅酸乙酯;硅酸四乙酯;四乙氧基硅烷 (6)化学品安全技术说明书-亚磷酸二丁酯;二正丁基亚磷酸酯 (11)化学品安全技术说明书-亚磷酸三甲酯 (16)化学品安全技术说明书-亚磷酸三乙酯 (22)化学品安全技术说明书-4,4'-二甲基1,3-二恶烷;二甲基二恶烷 (27)化学品安全技术说明书-吡咯;一氮二烯五环;氮(杂)茂 (32)化学品安全技术说明书-2-甲基吡啶;α-皮考林;α-甲基吡啶 (37)化学品安全技术说明书-3-甲基吡啶;β-皮考林 (43)化学品安全技术说明书-2,4-二甲基吡啶;2,4-卢剔啶 (48)化学品安全技术说明书-2,5-二甲基吡啶;2,5-二甲基氮杂苯 (54)化学品安全技术说明书-2,6-二甲基吡啶;2,6-卢剔啶 (59)化学品安全技术说明书-3,4-二甲基吡啶;3,4-二甲基氮杂苯 (64)化学品安全技术说明书-3,5-二甲基吡啶;3,5-卢剔啶 (70)化学品安全技术说明书-1,4-二甲基哌嗪;N,N'-二甲基哌嗪 (75)化学品安全技术说明书-2,6-二甲基吗啡啉;2,6-二甲基吗啉 (80)化学品安全技术说明书- N-乙基吗啉;N-乙基四氢-1,4-噁嗪 (86)化学品安全技术说明书-吗啉;1,4-氧氮杂环己烷 (91)化学品安全技术说明书-三正丙胺 (97)化学品安全技术说明书-二异丁胺 (102)化学品安全技术说明书-正己胺;1-氨基己烷 (108)化学品安全技术说明书-叔辛胺 (113)化学品安全技术说明书-三烯丙基胺;三(2-丙烯基)胺;三烯丙胺 (118)化学品安全技术说明书-3-二甲氨基-1-丙胺;N,N-二甲基-1,3-丙二胺;二甲氨基丙胺 (124)化学品安全技术说明书- N,N-二甲基-1,3-丙二胺;N,N-二甲氨基丙胺;3-二甲胺基-1-丙烷 (130)化学品安全技术说明书- N,N-二甲基乙醇胺;2-二甲基氨基乙醇 (135)化学品安全技术说明书- N,N-二甲基丙醇胺;3-(二甲胺基)-1-丙醇 (140)化学品安全技术说明书- N,N-二甲基异丙醇胺;1-(二甲胺基)-2-丙醇 (146)化学品安全技术说明书- N,N-二乙基乙醇胺;2-二乙氨基乙醇 (151)化学品安全技术说明书- N,N-二甲基甲酰胺;甲酰二甲胺 (156)化学品安全技术说明书-乙醛肟;亚乙基羟胺;亚乙基胲 (162)化学品安全技术说明书-丁醛肟;丁缩醛肟 (167)化学品安全技术说明书- N,N-二甲基氨基乙腈;2-(二甲胺基)乙腈 (172)化学品安全技术说明书-无水肼;无水联胺 (178)化学品安全技术说明书- N,N-二乙肼;二乙基肼;二乙肼 (185)化学品安全技术说明书-糠胺;2-呋喃甲胺;麸胺 (191)化学品安全技术说明书-四氢糠胺 (196)化学品安全技术说明书-乙基三乙氧基硅烷;三乙氧基乙基硅烷 (201)化学品安全技术说明书-乙烯三乙氧基硅烷;三乙氧基乙烯硅烷 (206)化学品安全技术说明书-乳香油 (212)化学品安全技术说明书-松节油 (217)化学品安全技术说明书-1,8-萜二烯;苎烯;双戊烯;二聚戊烯 (223)化学品安全技术说明书-氧化环己烯 (228)化学品安全技术说明书-萜品油烯;异松油烯;△1-2,4-8萜二烯 (233)化学品安全技术说明书-α-蒎烯 (239)化学品安全技术说明书-β-蒎烯 (244)化学品安全技术说明书-十一烷;十一碳烷 (249)化学品安全技术说明书-桉叶油醇;桉树脑 (254)化学品安全技术说明书-迷迭香油;迷叠香油 (260)化学品安全技术说明书-碳酸二乙酯;碳酸乙酯第一部分化学品及企业标识化学品中文名:碳酸二乙酯;碳酸乙酯化学品英文名:diethyl carbonate;ethyl carbonate企业名称:生产企业地址:邮编: 传真:企业应急电话:电子邮件地址:技术说明书编码:第二部分成分/组成信息√纯品混合物有害物成分浓度CAS No.碳酸二乙酯105-58-8第三部分危险性概述危险性类别:第3.3类高闪点液体侵入途径:吸入、食入、经皮吸收健康危害:本品为轻度刺激剂和麻醉剂。
琥珀酸脱氢酶(SDH)活性检测试剂盒说明书

琥珀酸脱氢酶(SDH )活性检测试剂盒说明书微量法注意:本产品试剂有所变动,请注意并严格按照该说明书操作。
货号:BC0955 规格:100T/96S产品组成:使用前请认真核对试剂体积与瓶内体积是否一致,有疑问请及时联系索莱宝工作人员。
试剂名称 规格 保存条件 试剂一 液体110 mL×1瓶 -20℃保存 试剂二 液体0.6mL×2支 -20℃保存 试剂三 液体18 mL×1瓶 2-8℃保存 试剂四 液体3mL×1瓶 2-8℃保存 试剂五液体3mL×1瓶2-8℃保存溶液的配制:1、 试剂二:为易挥发试剂,用完后尽快密封,-20℃保存; 产品说明:SDH (EC 1.3.5.1)广泛存在于动物、植物、微生物和培养细胞中。
SDH 是线粒体的一种标志酶,位于线粒体内膜上的一种膜结合酶,是连接呼吸电子传递和氧化磷酸化的枢纽之一。
此外,为多种原核细胞产能的呼吸链提供电子。
SDH 催化琥珀酸脱氢生成延胡索酸,脱下的氢通过吩嗪二甲酯硫酸(PMS )传递还原2,6-二氯酚靛酚(DCPIP ),并且在600nm 处具有特征吸收峰,通过600nm 吸光度的变化,测定2,6-DCPIP 的还原速度,代表SDH 酶活性。
Succinic Acid + FAD Fumaric Acid + FADHFADH + PMS FAD + PMSH 2PMSH 2 + Dichlorophenolindophenol(600nm) PMS + Reduced Dichlorophenolindophenol 注意:实验之前建议选择2-3个预期差异大的样本做预实验。
如果样本吸光值不在测量范围内建议稀释或者增加样本量进行检测。
需自备的仪器和用品:可见分光光度计/酶标仪、水浴锅、台式离心机、可调式移液器、微量玻璃比色皿/96孔板、研钵/匀浆器、冰和蒸馏水。
操作步骤:一、样本处理(可适当调整待测样本量,具体比例可以参考文献)1. 组织样本:称取约 0.1g 组织,加入1mL 试剂一和 10μL 试剂二,用冰浴匀浆器或研钵匀浆充分研磨,4℃11000g 离心10min ,取上清,置冰上待测。
PD98059抑制卵巢癌细胞株HO-8910活性的研究

维普资讯
・
2 0・ 3
用癌症杂志 20 0 8年 5 月第 2 卷第 3 3 期
. e r t a J r f acrM y 0 8 v l 3 N . 1 a i l o n o Cn e。 a 2o 。 0 2 . o3 1 P cc u a l l
t t n o 1 0 I o Lc n s nf a t hb e ib i eo a a a io acl P< . 1 ; D 8 5 a l hb r i 5 m l i ic nl i ii t a it o t v r nc cn m e s( 0 0 ) P 9 0 9 c la oi ii ao f  ̄ / a g i y n t h v lyf h i r l l s n t
细 胞 外 调节 蛋 白激 酶 (xr eua glt r- etcl l r ua dpo a l re e ti knssE K) e iae,R 分为 E K n R 1和 E K 统称 为 E K1 R 2, R / 2是 由 Bu o … 于 9 , ohn等 0年代 初期 先后 分离 鉴定 的 1 种 蛋 白激 酶 , 相对 分子量 分别 为 4 D和 4 D, 4k 2k 它们
w s r lt 免疫 细胞化学检测 细胞 内磷酸化 cF s 白的表 达。结果 et nbo和 e —o 蛋
而抑 制 卵 巢癌 细胞 的 生 长 。
与对照组相 比,5 m lLP 90 9组可显著 10i o D 8 5 x / P 90 9可能通过 降低 cFs 白的表达 D85 .o 蛋
MTF w s u e o me s r h v r n c r i o el v a i t a d t e w se l t n mmu o yo c e sr e eu e e a sd t a u e t e o a i a cn ma c l ibl y, n h e tr b o d i a s i n a n c t —h mit w rபைடு நூலகம் s d t d - y o tc h x rs in o — o n t eo a in c ri o el, s l C mp r d wi h o t l ru . D 8 5 t h o c n e t e e p e s fc F s i v r ac n ma c l Re u t t o h a s s o a e t te c n r o p P 9 0 9 wi te c n e - h og h
硫辛酸注射液联合胰激肽原酶肠溶片对DPN_的临床疗效及生存质量的影响

DOI:10.16658/ki.1672-4062.2024.01.174硫辛酸注射液联合胰激肽原酶肠溶片对DPN的临床疗效及生存质量的影响王莉,朱海峰濉溪县中医医院内分泌科,安徽淮北235100[摘要]目的探讨硫辛酸注射液联合胰激肽原酶肠溶片对2型糖尿病周围神经病变(Diabetic Peripheral Neu⁃ropathy, DPN)患者的临床疗效、生存质量及安全性的影响。
方法选取2021年2月—2022年4月濉溪县中医医院60名DPN患者作为研究对象。
通过随机数表法分为两组,每组30例。
对照组采用常规治疗,观察组在对照组基础上加用硫辛酸注射液和胰激肽原酶肠溶片治疗。
比较两组患者的神经病变评分、神经电生理指标、生存质量评分、安全性指标和不良反应发生率。
结果治疗后,观察组神经病变评分(6.2±0.9)分低于对照组(7.6±1.1)分,差异有统计学意义(t=5.438,P<0.05);观察组神经电生理指标、生存质量评分、安全性指标均优于对照组,差异有统计学意义(P均<0.05);两组患者不良反应发生率比较,差异无统计学意义(P>0.05)。
结论硫辛酸注射液联合胰激肽原酶肠溶片对DPN患者有良好的临床疗效,能够改善神经功能、改善神经电生理指标、提高生存质量,且安全性高。
[关键词] 硫辛酸注射液;胰激肽原酶肠溶片;2型糖尿病周围神经病变;临床疗效[中图分类号] R587.2 [文献标识码] A [文章编号] 1672-4062(2024)01(a)-0174-05Effect of Lipoic Acid Injection Combined with Pancreatic Kininogenase Enteric-coated Tablets on Clinical Efficacy and Quality of Survival in DPN WANG Li, ZHU HaifengDepartment of Endocrinology, Suixi County Hospital of Traditional Chinese Medicine, Huaibei, Anhui Province, 235100 China[Abstract] Objective To investigate the effects of lipoic acid injection combined with pancreatic kininogenase enteric-coated tablets on the clinical efficacy, quality of survival and safety of patients with type 2 diabetic peripheral neuropathy (DPN). Methods 60 DPN patients admitted to Suixi County Hospital of Traditional Chinese Medicine from February 2021 to April 2022 were selected as the study objects. They were divided into two groups with 30 cases in each group by random number table method. The control group received conventional treatment, and the observation group was treated with lipoic acid injection and pancreatic kininogenase enteric-coated tablets on the basis of control group. Neuropathy score, neuroelectrophysiological index, quality of life score, safety index and incidence of adverse reactions were compared between the two groups. Results After treatment, the neuropathy score of observation group (6.2±0.9) points was lower than that of control group (7.6±1.1) points, and the difference was statistically significant (t= 5.438, P<0.05). Neuroelectrophysiological indexes, quality of survival scores and safety indexes of the observation group were better than those of the control group, and the differences were statistically significant (all P<0.05). There was no significant difference in the incidence of adverse reactions between the two groups (P>0.05). Conclusion Li⁃[作者简介]王莉(1982-),女,本科,主治医生,研究方向为糖尿病周围神经病变。
PfuTurbo Cx Hotstart DNA Polymerase 说明书

PfuTurbo Cx Hotstart DNA Polymerase, Part Number 600410*************(24小时)化学品安全技术说明书GHS product identifier 应急咨询电话(带值班时间)::供应商/ 制造商:安捷伦科技贸易(上海)有限公司中国(上海)外高桥自由贸易试验区英伦路412号(邮编:200131)电话号码: 800-820-3278传真号码: 0086 (21) 5048 2818PfuTurbo Cx Hotstart DNA Polymerase, Part Number 600410化学品的推荐用途和限制用途DMSO 600260-53PfuTurbo Cx Hotstart DNA Polymerase 600410-5110X PfuTurbo Cx Reaction Buffer 600410-52部件号:部件号(化学品试剂盒):600410安全技术说明书根据 GB/ T 16483-2008 和 GB/ T 17519-2013GHS化学品标识:PfuTurbo Cx 热启动 DNA 聚合酶,货号 600410推荐用途DMSO 1 mlPfuTurbo Cx Hotstart DNA Polymerase40 μl (100 U 2.5 U/µl)600410-5210X PfuTurbo Cx Reaction Buffer4 x 1 ml:紧急情况概述DMSO 液体。
[透明。
]PfuTurbo Cx Hotstart DNA Polymerase液体。
10X PfuTurbo Cx Reaction Buffer 液体。
DMSO 无色。
PfuTurbo Cx Hotstart DNA Polymerase无资料。
10X PfuTurbo Cx Reaction Buffer 无资料。
DMSO 无气味的。
[轻微]PfuTurbo Cx Hotstart DNA Polymerase无资料。
乙基己基三嗪酮 结构式 分子量 透皮

乙基己基三嗪酮的结构式是C18H26N2O,分子量为298.42 g/mol。
透皮是指物质穿过皮肤的过程,它可以通过皮肤表面透过皮肤下层进入血液循环系统。
乙基己基三嗪酮作为一种透皮增透剂,在化妆品和药物中起着重要作用。
1. 引言乙基己基三嗪酮是一种常用的化学成分,它在化妆品和药物中被广泛使用。
乙基己基三嗪酮的分子结构如下所示(图1):(图1: 乙基己基三嗪酮结构式)2. 乙基己基三嗪酮的分子量及作用乙基己基三嗪酮的分子量为298.42 g/mol,这意味着它的分子在摩尔质量方面比例为298.42克。
这一特性使得乙基己基三嗪酮在化妆品和药物中的使用非常方便,可以精确地控制用量,从而达到理想的效果。
乙基己基三嗪酮还具有良好的透皮性,能够快速透过皮肤进入到血液循环系统。
这种特性使其成为一种理想的透皮增透剂,在许多药物和化妆品中得到广泛应用。
3. 乙基己基三嗪酮在化妆品中的应用在化妆品中,乙基己基三嗪酮通常被添加到护肤品和化妆品中,以提高其透皮性,增加功效成分的渗透效果。
乙基己基三嗪酮可以帮助有效成分更好地渗透到皮肤深层,从而达到更好的护肤和美容效果。
4. 乙基己基三嗪酮在药物中的应用在药物中,乙基己基三嗪酮常常被用作透皮贴剂的增透剂,帮助药物有效成分透过皮肤层进入体内,从而实现缓慢释放和持续治疗的效果。
这种用法不仅能够提高药物的吸收效率,还可以降低药物对胃肠道的刺激,减轻药物的不良反应。
5. 个人观点和理解乙基己基三嗪酮作为一种透皮增透剂,在化妆品和药物领域发挥着重要作用。
它的分子结构和透皮性使得其在这两个领域具有广泛的应用前景。
然而,对于乙基己基三嗪酮的安全性和潜在的副作用问题,还需要更多的研究和探讨,以确保其在产品中的安全性和可靠性。
6. 总结与回顾本文通过介绍乙基己基三嗪酮的分子结构、分子量、透皮作用以及在化妆品和药物中的应用,深入探讨了这一化学成分的重要性和作用机制。
通过这些内容的讨论,读者不仅可以了解乙基己基三嗪酮的基本特性,还可以深入了解其在实际应用中的作用和潜在问题。
百灵威核磁耗材产品

NMR Consumables and Accessories
客服热线:400-666-7788
全球 NMR 耗材 引领 60 年
百灵威
百灵威科技有限公司成立于 1992 年,始终以“为科研和生产提供世界一流的产品和服务”为宗旨,致力于超精细化学品的研发与 制造。经过近二十年的发展,百灵威已具备为化学、分析、生物、材料、物理及药物研发等领域提供近五十万种产品和专业服务 的能力。 百灵威拥有一支强大的具有丰富经验和创新能力的研发团队,在江苏、河北设立的两个研发中心可迅速研发出毫克至数百公斤级 的医药、生化、材料等中间体及特殊高端化学品,并可为客户定制合成各类产品,尤其擅长小分子药物中间体以及催化剂配体的 合成。 百灵威人坚信“发展民族科技”的理念,坚持依靠中国人自己的智慧和力量, 不断建设和发展位于潮白河畔的现代化工业生产基地,发挥百灵威在尖端技术研究、敏捷制造和系统性物流管理等方面的突出优 势,积极地将中国的各种高端化合物推荐给国际同行,为促进中国化学事业发展,推动世界文明与和谐进步而奋斗不息。 百灵威的使命 促进科技和工业发展,造福人类……
管壁厚度(mm) 平均凸度(µm)
包装
0.27
<60>
50只/塑料筒装
0.27
<60>
50只/塑料筒装
0.43
<60>
50只/塑料筒装
0.43
<60>
100只/纸盒装
0.43
<60>
50只/塑料筒装
0.43
<60>
100只/纸盒装
0.60
<60>
50只/塑料筒装
SampleJet®核磁管
Chemlok 6150 胶合剂技术数据表说明书

Chemlok® 6150 Adhesive Technical Data SheetChemlok® 6150 adhesive is a one-coat adhesive usedto bond a variety of elastomers to various metals. It is composed of a mixture of polymers, organic compounds and mineral fillers dissolved or dispersed in an organic solvent system.Chemlok 6150 adhesive provides strong adhesion and environmental resistance to harsh environments. It is anon-chlorinated solvent adhesive that can be applied as a one- or two-coat system.Features and Benefits:Versatile – bonds a wide variety of elastomers to metals, plastics and fabrics; suitable for existing production lines; tolerates a wide variety of stock formulations.Convenient – requires only a single coat for most applications, reducing labor, solvent usage, inventory and shipping costs.Durable – provides rubber tearing bonds; provides superior adhesion to plated metals, lowering scrap rates. Elastomers:• Natural Rubber (NR) • Nitrile (NBR)• Polyisoprene (IR) • Butyl (IIR)• Styrene-butadiene (SBR) • EPDM Polymers• Polybutadiene (BR) • Polyepichlorohydrin (ECO)• Polychloroprene (CR)Application:Surface Preparation – Thoroughly clean metal surfaces prior to application. Remove protective oils, cutting oilsand greases by solvent degreasing or alkaline cleaning. Remove rust, scale or oxide coatings by suitable chemical or mechanical cleaning methods.Apply Chemlok 6150 adhesive to stainless steel, aluminum, brass and other nonferrous substrates within one-half hour after cleaning. For ferrous substrates such as steel, a long layover can be tolerated if no if no rust is formed.For further detailed information on surface preparation of specific substrates, refer to Chemlok Adhesives application guide.Mixing – Thoroughly stir adhesive before use, and agitate sufficiently during use to keep dispersed solids uniformly suspended. If needed, proper dilution for the various application methods is best achieved by experience. Give careful attention to agitation since dilution will accelerate settling.Applying – Apply adhesive by brush, dip, roll coat, spray or any other method that gives a uniform coating and avoids excessive runs and tears.When using Chemlok 6150 adhesive as a one-coat adhesive, the dry film thickness should be 17.8-30.5 micron (0.7-1.2 mil). When used as a covercoat over a primer, the dry film thickness of Chemlok 6150 adhesive should be15.2-20.3 micron (0.6-0.8 mil).Parker LORDEngineered Materials Group 111 LORD DriveCary, NC 27511-7923USAphone +1 877 ASK LORD (275 5673)Values stated in this document represent typical values as not all tests are run on each lot of material produced. For formalized product specifications for specific product end uses, contact the Customer Support Center.Information provided herein is based upon tests believed to be reliable. In as much as Parker LORD has no control over the manner in which others may use this information, it does not guarantee the results to be obtained. In addition, Parker LORD does not guarantee the performance of the product or the results obtained from the use of the product or this information where the product has been repackaged by any third party, including but not limited to any product end-user. Nor does the company make any express or implied warranty of merchantability or fitness for a particular purpose concerning the effects or results of such use.WARNING — USER RESPONSIBILITY . FAILURE OR IMPROPER SELECTION OR IMPROPER USE OF THE PRODUCTS DESCRIBED HEREIN OR RELATED ITEMS CAN CAUSE DEATH, PERSONAL INJURY AND PROPERTY DAMAGE.This document and other information from Parker-Hannifin Corporation, its subsidiaries and authorized distributors provide product or system options for further investigation by users having technical expertise.The user, through its own analysis and testing, is solely responsible for making the final selection of the system and components and assuring that all performance, endurance, maintenance, safety and warning requirements of the application are met. The user must analyze all aspects of the application, follow applicable industry standards, and follow the information concerning the product in the current product catalog and in any other materials provided from Parker or its subsidiaries or authorized distributors.To the extent that Parker or its subsidiaries or authorized distributors provide component or system options based upon data or specifications provided by the user, the user is responsible for determining that such data and specifications are suitable and sufficient for all applications and reasonably foreseeable uses of the components or systems.©2021 Parker Hannifin - All Rights ReservedInformation and specifications subject to change without notice and without liability therefor. Trademarks used herein are the property of their respective owners.Chemlok 6150 Adhesive — Technical Data SheetOD DS4428 07/21 Rev.2Drying/Curing – Allow the applied adhesive to dry until visual examination of the film has shown that all solvent has evaporated. This will take approximately 20-40 minutes at room temperature. Drying time can be shortened by either preheating the metal inserts or oven drying after application. Metal parts may be preheated to a maximum of 65°C (150°F) prior to adhesive application. For coated parts, moderate drying temperatures should be used, but temperatures as high as 149°C (300°F) may be used for very short periods of time. Maximum air flow at minimum temperatures will give the best results.Cleanup – Use solvents such as xylene and MEK to remove adhesive before heat is applied. Once cured, removal by solvent is not possible.Shelf Life/Storage:Shelf life is one year from date of shipment when stored by the recipient in a well ventilated area at 21-27°C (70-80°F) in original, unopened container. Do not store or use near heat, sparks or open flame. Keep container tightly closed when not in use.Chemlok 6150 adhesive is moisture sensitive. Minimizeexposure of the adhesive to moisture during application and storage. Avoid excessive exposure to high humidity.Cautionary Information:Before using this or any Parker LORD product, refer to the Safety Data Sheet (SDS) and label for safe use and handling instructions.For industrial/commercial use only. Must be applied by trained personnel only. Not to be used in household applications. Not for consumer use.。
人蛋白质二硫键异构酶(PDI)说明书

人蛋白质二硫键异构酶 (PDI)试剂盒使用说明书--上海谷研生物公司专业代理销售本试剂盒仅供研究使用。
检测范围:1.5pg/ml-40pg/ml使用目的:96T本试剂盒用于测定人血清、血浆及相关液体样本中蛋白质二硫键异构酶(PDI)含量。
实验原理本试剂盒应用双抗体夹心法测定标本中人蛋白质二硫键异构酶(PDI)水平。
用纯化的人蛋白质二硫键异构酶(PDI)抗体包被微孔板,制成固相抗体,往包被单抗的微孔中依次加入 蛋白质二硫键异构酶(PDI),再与 HRP 标记的蛋白质二硫键异构酶(PDI)抗体结合,形成抗体 -抗原-酶标抗体复合物,经过彻底洗涤后加底物 TMB 显色。
TMB 在 HRP 酶的催化下转化 成蓝色,并在酸的作用下转化成最终的黄色。
颜色的深浅和样品中的蛋白质二硫键异构酶 (PDI)呈正相关。
用酶标仪在 450nm 波长下测定吸光度(OD 值),通过标准曲线计算样品中人蛋白质二硫键异构酶(PDI)浓度。
试剂盒组成1 30 倍浓缩洗涤液 20ml×1 瓶 7 终止液 6ml×1 瓶 马上进行试验,可将标本放于-20℃保存,但应避免反复冻融2.不能检测含 NaN3 的样品,因 NaN3 抑制辣根过氧化物酶的(HRP )活性。
操作步骤1.标准品的稀释:本试剂盒提供原倍标准品一支,用户可按照下列图表在小试管中进行稀释。
40pg/ml5 号标准品 150µl 的原倍标准品加入 150µl 标准品稀释液 2. 加样:分别设空白孔(空白对照孔不加样品及酶标试剂,其余各步操作相同)、标准孔、待测样品孔。
在酶标包被板上标准品准确加样50µl,待测样品孔中先加样品稀释液40µl,然后再加待测样品10µl(样品最终稀释度为5 倍)。
加样将样品加于酶标板孔底部,尽量不触及孔壁,轻轻晃动混匀。
3. 温育:用封板膜封板后置37℃温育30 分钟。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Product Name:
PD98059CAS No.:
167869-21-8Cat. No.:
HY-12028Product Data Sheet
MWt:
267.28Formula:
C16H13NO3Purity :>98%
Solubility:DMSO ≥
30mg/mL Water
Mechanisms:
Biological Activity:
PD98059is a non-ATP competitive MEK inhibitor with IC50of 2M specifically inhibits MEK-1-
Pathways:MAPK ; Target:MEK <1.2mg/mL Ethanol ≥2.6mg/mL
PD98059 is a non ATP competitive MEK inhibitor with IC50 of 2 μM, specifically inhibits MEK 1
mediated activation of MAPK; does not directly inhibit ERK1 or ERK2.
IC50 value: 2 uM
Target: MEK in vitro: PD98059 inhibits either basal MEK1 or a partially activated MEK produced by mutation of serine at residues 218 and 222 to glutamate (MEK-2E) with IC50 of 2 μM. PD98059 does not inhibit the MAPK homologues JNK and P38. PD98059 is highly selective against MEK, as it does not inhibit a number of other kinase activities including Raf kinase, cAMP-dependent kinase, protein kinase C, v-Src, epidermal growth factor (EGF) receptor kinase, insulin receptor kinase, PDGF
References:
[1]. Dudley DT, et al. A synthetic inhibitor of the mitogen-activated protein kinase cascade. Proc Natl
Acad Sci U S A, 1995, 92(17), 7686-7689.[2].Alessi DR,et al.PD 098059is a specific inhibitor of the activation of mitogen-activated protein receptor kinase, and phosphatidylinositol 3-kinase. PD98059 inhibits PDGF-stimulated activation of MAPK and thymidine incorporation into 3T3 cells with IC50 of ~10 μM and ~7 μM, respectively [1].PD98059 potently ...
[2]. Alessi DR, et al. PD 098059 is a specific inhibitor of the activation of mitogen activated protein
kinase kinase in vitro and in vivo. J Biol Chem, 1995, 270(46), 27489-27494.[3]. Alessandrini A, et al. MEK1 protein kinase inhibition protects against damage resulting from
focal cerebral ischemia. Proc Natl Acad Sci U S A, 1999, 96(22), 12866-12869.[4]. Clemons AP, et al. Cerulein-induced acute pancreatitis in the rat is significantly ameliorated by
treatment with MEK1/2 inhibitors U0126 and PD98059. Pancreas, 2002, 25(3), 251-259.Caution: Not fully tested. For research purposes only
Medchemexpress LLC
18W i l k i n s o n W a y , P r i n c e t o n , N J 08540,U S A
E m a i l : i n f o @m e d c h e m e x p r e s s .c o m W e b : w w w .m e d c h e m e x p r e s s .c o m。
