材料热力学》试题
材料热力学习题集

问:1)当混合气体(97%H2O + 3%H2, 体积)在 1000 K 是否能将 Ni 氧化? 2)现有 Ni-Au 固溶体(XNi = 0.1)。已知在 1000 K 时, 与此合金平衡的氢气体体积
例题 6-8 右图所示是铜和铜铝合金(18 at.% Al)在 700℃温度下扩散退火 38.4 天的浓度分
布曲线。求当 Al 的浓度为 4 at%时,Al 在 Cu 中的扩散系数。
18
Cu - Al
Cu
16
A
14
C , at.% Al
12
k
Matano interface
10
8 mark interface
.
材料热力学上课题目
例 1-1 已知液体铅在 1 个大气压下的热容量 Cp(l)为 Cp(l)=32.43-3.10×10-3T J/(mol·k),固 体铅的热容量 Cp(s)为 Cp(s)=23.56+9.75×10-3T J/(mol·k),已知液体铅在熔点(600 K)凝固为固 体时放热 4811.60 J/mol,求液体铅过冷至 590K 凝固为固体时焓的变化。
6
4
2
A
1
0 -10 -8 -6 -4 -2 0 2 4 6 8 10
x
六大 板块
第一章 第二章 第三章 第四章 第五章 第六章
物理化学复习纲要
热力学定律(热力学第一定律、热力学第二定律) 自由能(ΔF、ΔG) 热力学状态函数、关系式及应用 相变热力学 溶液 扩散
.
材料热力学习题集

材料热力学习题集液态铅在1个大气压下的热容量Cp(l)称为Cp(L)= 32.43-3.10×10-3TJ/(mol·k),固态铅的热容量Cp(s)为Cp(S)= 23.56+9.75×10-3TJ/(mol·k)。
众所周知,当液态铅的熔点(600 K)固化成固体时,液态铅释放4811.60 J/mol的热量,并计算了当液态铅过冷到590K并固化成固体时的焓变化。
液态铅固态铅600Kb恒温相变c温升590Ka初始状态相变d最终状态?H示意图实施例1-2众所周知,锡在505K(熔点)时的熔化热为7070.96焦耳/摩尔,厘泊(L) = 34.69-9.20×10-3TJ/(摩尔·K)厘泊(S) = 18.49+26.36×10-3TJ/(摩尔·K)用于计算锡过冷至495 K时自动凝固的比例505K恒温,放热b相变c最终状态吸收热上升温度吸收热相变放热495Ka初始状态1摩尔液体d x摩尔固体(1-x)摩尔液体?H图例1-3铅的熔点为600K,凝固热为4811.6 J/mol,计算了铅在600K 凝固时的熵值变化(在一个大气压下)。
例1-4已知在1个大气压下液态铅的比热为32.43-3.10×10-3tj/(mol·k)CP(s)= 23.56+9.75×10-3tj/(mol·k)液态铅在其熔点(600K)固化成固体时释放4811.6 J/mol的热量。
计算了液态铅过冷到590K凝固时(在一个大气压下)熵值的变化。
1液态铅固态铅恒温相变600Kbc冷却温升590Ka初始相变d最终状态计算?S示意图实施例2-1已知液态锌的Cp(l)为Cp(L)= 29.66+4.81×10-3TJ/(mol·k),固态锌的Cp(s)为Cp(S)= 22.13+11.05×10-3TJ/(mol·k),锌的熔点为692.6K,熔化热δH = 6589.8J/mol,自由能差δG(δ的实施例2-2使用第一章中的数据计算铅在590 K(过冷10 K)凝固时的自由能变化δg(590 K),并将其与简单近似计算的结果(铅在590K 凝固时δH =-4811.6J/mol)进行比较可以从第一章的计算中看出:当铅在590K凝固时,焓变化δH =-4722.56J/mol;熵变化δs =-8.0j/(Mol·k)例2-3已知γ-铁、δ-铁和液态铁的Cp为Cp(γ)= 7.70+19.50×10-3 TJ/Mol·kcp(d)= 43.93j/Mol·k(1674 ~ 1809k)Cp(L)= 41.84j/Mol·k(L)G亚稳态?相的理论熔点?第一阶段?1673年?L1809G?g?GLT/K?阶段225y = 246.65t-34.138 tlnt+9.75?10t 20-32y/100015y = 14861.57t = 1793.82k 105005001000t,k 1500200025003000| 286K时199例4-1,α-Sn β-Sn的δh = 2092j/mol,锡的= 118.7,πα-Sn = 5.75g/mL,ψ计算100个大气压下相变温度的变化值例4-2在95.5℃单斜硫菱形硫中,δV = 0.01395毫升/克,δH = 13.05焦耳/克,找出压力对相变温度的影响例4-3固体锌的蒸气压与温度的关系为:lgp(ATM)=-6850/T-0.755 gt+8.36液态锌的蒸气压与温度的关系为:lgp(ATM)=-6620/T-1.255 LGT+9.46q:1)液态锌在1个大气压下的沸点;2)三点温度;3)1 ATM沸点下的汽化热;4)三相点的熔化热;5)固体锌和液体锌之间的δCp 例4-4锌在610 K时的蒸气压为10 mmHg,镉的计算蒸气压也为10-5 mmHg杜林定律:当相似物质具有相同的蒸汽压时,T1/T2 =常数例4-5碳在1个大气压和25℃下以石墨为稳定相,并试图找出在25℃下将石墨转化为金刚石所需的压力实施例5-1实验测得的镉-镁的摩尔体积如下表所示Cd-镁合金-5实施例5-2已知三元溶液的摩尔体积为VM = 7x1+10 x2+12x 3-2x1x2+3x1x2x 3(cm3/mol)339解决方案:虚拟机∠X1 = x2 = x3 = 1/3 =869 X1 = 1-X2-X3,因为X1+X2+X3 = 1经过取代,我们可以得到:实施例5-3在1075℃下实验测得的氧在银中的溶解度如下表所示,我们可以找出:1)氧在银中的溶解度是否符合西沃特定律,我们可以找出溶解度常数;2)1075℃时空气中氧在银中的溶解度实施例5-4将0.567 g尿素(CON2H4)溶解在500 g水中,测量该水溶液的冰点为-0.0351℃,并计算尿素的分子量。
材料物理化学作业-热力学第一定律

材料物理化学作业第一章 热力学第一定律1.某体系在压力101.3kPa 下,恒压可逆膨胀,体积增大5L ,计算所做的功。
2. 在300K 的常压下,2mol 的某固体物质完全升华过程的体积功为多少?3.2mol H 2在00C ,压力为101.3kPa 下恒压可逆膨胀至100L ,求W 、Q 、ΔU 、ΔH 。
4.计算1mol 铅由250C 加热到3000C 时所吸收的热。
已知铅的C p =23.55+9.74×10-3T/K J ·K -1·mol -15.1mol 单原子理想气体,温度为250C ,压力为101.3kPa ,经两种过程达到同一末态:Ⅰ、恒压加热,温度上升到12170C ,然后再经恒容降温到250C 。
Ⅱ、恒温可逆膨胀到20.26kPa 。
分别计算两个过程的W 、Q 、ΔU 和ΔH 。
6.已知250C 时下列反应的热效应:2Pb+O 2=2PbO ΔH 1=-438.56kJ ·mol -1 S+O 2=SO 2 ΔH 2=-296.90kJ ·mol -1 2SO 2+ O 2=2SO 3 ΔH 3=-197.72kJ ·mol -1 Pb+S+2O 2=PbSO 4 ΔH 4=-918.39kJ ·mol -1 求反应PbO+SO 3= PbSO 4的热效应。
7.已知250C 时下列反应的热效应:Ag 2O+2HCl (g )=2Agl+H 2O (l ) ΔH 1=-324.71kJ ·mol -12 Ag+21O 2= Ag 2O ΔH 2=-30.57kJ ·mol -1 21H 2+21Cl 2=HCl (g ) ΔH 3=-92.31kJ ·mol -1 H 2+21O 2= H 2O (l ) ΔH 4=-285.84kJ ·mol -1 求AgCl 的生成热。
安徽工业大学研究生材料热力学考试题

安徽工业大学研究生材料热力学考试题
一、仔细阅读下列论述,判断正误,如果错误,请说明该论述违反了哪些热力学原理,并给出正确的论述。
(20分)
(1) 低压下不可能将石墨转变为金刚石。
(2) 在一炉10 吨的钢水(Fe-C 二元溶体)中加入12 克碳后,使钢水的吉布斯自由能的增加值即为Fe 的
化学位。
(3) 恒温恒压下,如果两相的吉布斯自由能相等,则两相彼此处于平衡状态。
(4) 纯金属中不存在空位时的吉布斯自由能最低。
(20分)
分)
三、(1)固态纯组元的G-T 曲线如下图所示,请判断哪条线正确,并解释原因。
(20
(2)A-B 二元系中,固相和液相的摩尔自由能-成分曲线如下图所示。
请在自由能-成分曲线上,图示出体系成分为X*处,固相纯A和液相纯B混合后的吉布斯自由能的变化量∆Gmix,并说明原因。
(20分)
四、试通过如图所示的A-B二元相图,判断A-B固溶体的性质、溶体组元间的相互作用能。
并请画出T1 温度下所存在的相的自由能-成分曲线。
(20分)。
材料热力学习题答案1

The problems of the first law1. a lead bullet is fired at a frigid surface. At what speed must it travel to melt on impact, if its initial temperature is 25℃ and heating of the rigid surface of the rigid surface is neglected? The melting point of lead is 327℃. The molar heat of fusion of the lead is 4.8kJ/mol. The molar heat capacity C P of lead may be taken as 29.3J/(mol K) (1.1)Solution: )/(5.112.20721]108.4)25327(3.29[2121)(2322s m V v n n WQ nMv mv W H T C n Q Q Q absorb melting p melt increase absorb ==⨯+-⨯===∆+∆=+=2. what is the average power production in watts of a person who burns 2500 kcal of food in a day? Estimate the average additional powder production of 75Kg man who is climbing a mountain at eh rate of 20 m/min (1.2)Solution )/(24560208.975)/(12160602410467000//)(104670001868.4102500sin 3S J t h mg P S J t Q t W P J Q gincrea Burning Burning =⨯⨯=∆==⨯⨯====⨯⨯=3 One cubic decimeter (1 dm 3) of water is broken into droplets having a diameter of one micrometer (1 um) at 20℃. (1.3)(a) what is the total area of the droplets?(b) Calculate the minimum work required to produce the droplets. Assume that the droplets arerest (have zero velocity)Water have a surface tension of 72.75 dyn/cm at 20℃ (NOTES: the term surface energy (ene/cm 2) is also used for surface tension dyn/cm)Solution)(25.218)106103(1075.72)(103)101(4)101(34)101(232523263631J S W m nS S Single total =⨯-⨯⨯⨯=∆=⨯=⨯⨯⨯⨯⨯⨯⨯⨯==-+----σππ4.Gaseous helium is to be used to quench a hot piece of metal. The helium is in storage in aninsulated tank with a volume of 50 L and a temperature of 25℃, the pressure is 10 atm. Assume that helium is an ideal gas.(a) when the valve is opened and the gas escapes into the quench chamber (pressure=1 atm), whatwill be the temperature of the first gas to hit the specimen?(b) As the helium flows, the pressure in the tank drops. What will be the temperature of thehelium entering the quench chamber when the pressure in the tank has fallen to 1 atm? (1.4)Solution: )(180118298)(1185.229810101325501010101325)5500(1)()(118)101(298)()(0334.0/00K T T T K RR nC W T b K T P PT T Adiabatic a p C R P=-=∆-==⨯⨯⨯⨯⨯⨯⨯-⨯==∆=⨯==--5 An evacuated (P=0), insulted tank is surrounded by a very large volume (assume infinite volume) of an ideal gas at a temperature T 0. The valve on the tank is opened and the surrounding gas is allowed to flow quickly into the tank until the pressure inside the tank is equals the pressure outside. Assume that no heat flow takes place. What is the final tempeture of the gas in the tank? The heat capacity of the gas, C p and C v each may be assumed to be constant over the temperature rang spanned by the experiment. You answer may be left in terms of C p and C vhint: one way to approach the problem is to define the system as the gas ends up in the tank. (1.5)solution 0/000/00)0()(T P P T T P PT T Adiabatic PPC R C R ≈-==6. Calculate the heat of reaction of methane with oxygen at 298K, assuming that the products of reaction are CO 2 and CH 4 (gas)[This heat of reaction is also called the low calorific power of methane] convert the answer into unites of Btu/1000 SCF of methane. SCF means standard cubic feet, taken at 298 and 1atmNOTE: this value is a good approximation for the low calorific powder of natural gas (1.6)DA TA:)()()(224g O H g CO g CH FOR80.5705.9489.17]/[0298---•∆mol g Kcal Hsolution)1000/(9.2610252103048.01101076.191)/(76.191)89.1780.57205.94()2(22333332982982224422SCF Btu mol g Kcal H H H H H OH CO O CH CH O H CO =⨯⨯⨯⨯⨯=•=∆+⨯---=∆-∆+∆-=∆+=+-7. Methane is delivered at 298 K to a glass factory, which operates a melting furnace at 1600 K. The fuel is mixed with a quantity of air, also at 298 K, which is 10% in excess of the amount theoretically needed for complete combustion (air is approximately 21% O 2 and 79% N 2) (1.7) (a) Assuming complete combustion, what is the composition of the flue gas (the gas followingcombustion)?(b) What is the temperature of the gas, assuming no heat loss?(c) The furnace processes 2000kg of glass hourly, and its heat losses to the surroundings average400000 kJ/h. calculate the fuel consumption at STP (in m 3/h) assuming that for gas H 1600-H 298=1200KJ/KG(d) A heat exchanger is installed to transfer some of the sensible heat of the flue gas to thecombustion air. Calculate the decrease in fuel consumption if the combustion air is heated to 800KDA TA STP means T=298K, P=1atm22224O N O H CO CH for 2.82.89.117.1316)/(C mol cal C P •Solution)(210448.1125.9100076.191298)/(25.9)]87.012.72(2.843.179.1171.87.13[01.0)(%87.0%%12.72%%43.17%2%%71.8)11.1(221791.1231%22)(0,,222222224K T T T C mol cal X C C b O N CO O H CO O H CO O CH a i i p p p =⨯⨯+=∆+=•=+⨯+⨯+⨯=======-⨯+⨯⨯+=+=+∑)/(1644)0224.011868.448.11)8001600(48.1125.9189570(102800000)/(189570)298800)](48.1187.8)48.1125.9[(100076.191)()/(87.848.11/]211002.22.816[)()/(3214)0224.011868.448.11)2981600(48.1125.9100076.191(102800000)/(280000040000020001200)(33min ,,,,298,,33min h m V mol g cal dTn C n C H H C mol cal X C C d h m V h KJ P C gConsu i i r p i i p p i i p r p g Consu =⨯⨯-⨯-⨯=•=-⨯-⨯-⨯=--∆=∆•=⨯⨯+===⨯⨯-⨯-⨯⨯==+⨯=⎰∑∑∑8.In an investigation of the thermodynamic properties of a-manganese, the following heat contents were determined:H 700-H 298=12113 J/(g atom) H 1000-H 298=22803 J/(g atom)Find a suitable equation for H T -H 298 and also for C P as a function of temperature in the form (a+bT) Assume that no structure transformation takes place in the given tempeture rang. (1.8)Solution )298(0055.0)298(62.35011.062.35011.062.3522803)2981000(2)2981000(12113)298700(2)298700(]2[2229822222982---=∆-=-===-+-=-+-+=+==∆⎰⎰T T H TC b a ba ba T baT bTdT a dT C H TP T P9.A fuel gas containing 40% CO, 10% CO 2, and the rest N 2 (by volume) is burnt completely with air in a furnace. The incoming and ongoing temperatures of the gases in the furnace are 773K and 1250K,respectively. Calculate (a) the maximum flame temperature and (b) heat supplied to the furnace per cu. ft of exhaust gas (1.9)molJ Hmol J H CO f CO f /393296/1104580,298,0,298,2-=∆-=∆)/(10184.403.29)/(1067.11010.492.19)/(1037.81020.935.44)/(1042.01097.345.283,253,253,253,222molK J T C molK J T T C molK J T T C molK J T T C N P O P CO P CO P -------⨯+=⨯-⨯+=⨯-⨯+=⨯-⨯+=Solution?0)499.0321.018.1()1067.01019.277.28(28.282831067.01038.477.289.0)1019.01058.528.33(2.0282838)()/(1019.01058.528.33722.0278.0)/(1067.01038.477.281.065.005.02.0)()/(282838110458393296%2.72%8.27%10%65%5%20)4/(1122298127332981523733253253298,,,,298,253,,,,,253,,,,,,,0,298,0,298,298,22222222222222==+--⨯+⨯++⨯=⨯-⨯++⨯⨯-⨯+-⨯=--∆=∆⨯-⨯+=+==⨯-⨯+=+++===-=∆-∆=∆========+-----------⎰⎰⎰∑∑⎰∑∑∑∑T T T T T T T dTT T dTT T dT n C n C n H H molK J T T C C n C C molK J T T C C C C n C C a mol J n H n H H N CO production O N CO CO reation then O N air mole need fuel mole when CO O CO T TT i i r p i i p p i i N P CO P i i p p r p O P N P CO P CO P i i p p r p i p f i r f idTT T Q dT T T Q b T T T T T T T dT T T dTT T dT n C n C n H H T TT i i r p i i p p i i 9.0)1019.01058.528.33(2.02828389.0)1019.01058.528.33(2.0282838)(0)499.0321.018.1()1067.01019.277.28(28.282831067.01038.477.289.0)1019.01058.528.33(2.0282838)(253125029812502982531250298125029829812125029815231250253253298,,,,298,⨯⨯-⨯++⨯-=⨯⨯-⨯++⨯-===+--⨯+⨯++⨯=⨯-⨯++⨯⨯-⨯+-⨯=--∆=∆-----------⎰⎰⎰⎰⎰∑∑⎰10. (a) for the reaction 2221CO O CO →+,what is the enthalpy of reaction (0H ∆) at 298 K ? (b) a fuel gas, with composition 50% CO, 50% N 2 is burned using the stoichiometric amount of air. What is the composition of the flue gas?(c) If the fuel gas and the air enter there burner at 298 K, what is the highest temperature theflame may attain (adiabatic flame temperature)? DA TA :standard heats of formation f H ∆ at 298 K (1.10))/(393000)/(1100002mol J CO mol J CO -=-=Heat capacities [J/(mol K)] to be used for this problem N 2=33, O 2=33, CO=34, CO 2=57 Solution)(21100)298)(39889.0(222.02830000)/(3975.03325.057)/(33111.034222.033666.033)(%,75%%,251.111002.22%%1.11%%,6.66%%,2.222.0/25.015.0%)()/(283000393000110000)(,0,,,,,,22220,298,0,298,0K T T dT C n H H K mol J X C C K mol J X C C C N CO product O N CO fuel b mol J n H n H H a P p p i P r i P r i P p i P p i P f i r f ==-⨯-⨯=-∆=∆•=⨯+⨯==•=⨯+⨯+⨯====-====+==+-=∆-∆=∆⎰∑∑∑∑11.a particular blast furnace gas has the following composition by (volume): N 2=60%, H 2=4, CO=12%, CO 2=24%(a) if the gas at 298K is burned with the stochiometric amount of dry air at 298 K, what is the composition of the flue gas? What is the adiabatic flame temperature? (b) repeat the calculation for 30% excess combustion air at 298K(C)what is the adiabatic flame temperature when the blast furnace gas is preheated to 700K (the dry air is at 298K)(d) suppose the combustion air is not dry ( has partial pressure of water 15 mm Hg and a total pressure of 760 mm Hg) how will the flame temperature be affected? DA TA(k J/mol) (1.11)2CO CO FOR513.393523.110)/(--∆mol kJ H f 2222,)(O N g O H CO CO FOR34505733]/[K mol J C P •Solution)(1052)(75438286370])295.03450(241604[026.0])335.03457(110523393513[079.0])([%8.66%%,8.6%%,6.2%%,8.15%%,9.72.0/83.110012%)()(1122)(82538313430])295.03450(241604[029.0])335.03457(110523393513[086.0])([%7.65%%,7.5%%,9.2%%,1.17%%,6.82.0/810012%2121)(,,,,,,,02222,,,,,,,0222222222K T K T T n C T T X C dT n C n C H x H N O H CO CO b K T K T T n C T T X C dT n C n C H x H N O H CO CO OH O H CO O CO a i i r P ii P i i r P i i p P i i i i r P ii P i i r P i i p P i i ===∆=∆-∆-⨯--+∆-⨯---=+--∆=∆=====⨯+====∆=∆-∆-⨯--+∆-⨯---=+--∆=∆=====+=→+→+∑∑∑⎰∑∑∑∑∑⎰∑∑)(1419),(11213842594034286.0)402(2.39714.0])295.03450(241604[029.0])335.03457(110523393513[086.0)3(K T K T T T T T H ===∆=∆⨯--∆⨯-∆-⨯--+∆-⨯---=∆12.A bath of molten copper is super cooled to 5℃ below its true melting point. Nucleation of solid copper then takes place, and the solidification proceeds under adiabatic conditions. What percentage of the bath solidifies?DA TA: Heat of fusion for copper is 3100 cal/mol at 1803℃(the melting point of copper) C P,L =7.5(cal/mol ℃), C P,S =5.41+(1.5*10-3T )(cal/mol ℃) (1.12) Solution)/(310355.75.0)17981803(105.1541.5310002231798,1798,17981803,18031798,1803,mol cal H H dT C dT C H L S SL L P S P L S =⨯-⨯-⨯+⨯+==+++-⎰⎰13.Cuprous oxide (Cu 2O) is being reduced by hydrogen in a furnace at 1000K, (a)write the chemical reaction for the reduced one mole of Cu 2O(b)how much heat is release or absorbed per mole reacted? Given the quantity of heat and state whether heat is evolved (exothermic reaction) or absorbed (endothermic reaction)DA TA: heat of formation of 1000K in cal/mol Cu 2O=-41900 H 2O=-59210 (1.13) solution)/(173104190059210222mol cal H OH Cu H O Cu =-=∆+=+,exothermic reaction14. (a) what is the enthalpy of pure, liquid aluminum at 1000K?(b) an electric resistance furnace is used to melt pure aluminum at the rate of 100kg/h. the furnace is fed with solid aluminum at 298K. The liquid aluminum leaves the furnace at 1000K. what is the minimum electric powder rating (kW) of furnace.DA TA : For aluminum : atomic weight=27g/mol, C p,s =26(J/molK), C p,L =29(J/molK), Melting point=932K, Heat of fusion=10700J/mol (1.14)Solution )(28.0)(7.2793600110002727184)/(2718410700)9321000(29)298932(261000932,932298,1000,kW W P mol J H dT C dT C H SLL P S P l ==⨯⨯==+-⨯+-⨯=++=⎰⎰15 A waste material (dross from the melting of aluminum) is found to contain 1 wt% metallic aluminum. The rest may be assumed to aluminum oxide. The aluminum is finely divided and dispersed in the aluminum oxide; that is the two material are thermally connected.If the waster material is stored at 298K. what is the maximum temperature to which it may rise if all the metallic aluminum is oxidized by air/ the entire mass may be assumed to rise to the same temperature. Data : atomic weight Al=27g/mol, O=16g/mol, C p,s,Al =26(J/molK), C p,s,Al2O3=104J/mol, heat formation of Al 2O 3=-1676000J/mol (1.15)Solution;)(600)(3021041029927275.116122711676000K T K T T ==∆∆⨯⨯++⨯⨯=⨯⨯16 Metals exhibit some interesting properties when they are rapidly solidified from the liquid state. An apparatus for the rapid solidification of copper is cooled by water. In the apparatus, liquid copper at its melting point (1356K) is sprayed on a cooling surface, where it solidified and cools to 400K. The copper is supplied to the apparatus at the rate of one kilogram per minute. Cooling water is available at 20℃, and is not allowed to raise above 80℃. What is the minimum flow rate of water in the apparatus, in cubic meters per minute?DA TA; for water: C p =4.184J/g k, Density=1g/cm 3; for copper: molecular weight=63.54g/mol C p =7cal/mol k, heat of fusion=3120 cal/mol (1.16)Solution:min)/(10573.2)2080(1min /min54.631000)]4001356(73120[min /33m V VQ Q Water Copper -⨯=-=⨯⨯-⨯+=17 water flowing through an insulated pipe at the rate of 5L/min is to be heated from 20℃ to 60℃ b an electrical resistance heater. Calculate the minimum power rating of the resistance heater in watts. Specify the system and basis for you calculation. DA TA; For water C p =4.184J/g k, Density=1g/cm 3 (1.17)Solution: )(139476010005)2060(184.4W W =⨯⨯-⨯=18 The heat of evaporation of water at 100℃ and 1 atm is 2261J/mol (a) what percentage of that energy is used as work done by the vapor?(b)if the density of water vapor at 100℃ and 1 atm is 0.597kg/m 3 what is the internal energy change for the evaporation of water? (1.18)Solution: )/(375971822613101%6.71822613101%)/(31010224.0273373101325mol J Q W U mol J V P =⨯+-=+=∆=⨯==⨯⨯=∆19 water is the minimum amount of steam (at 100℃ and 1 atm pressure) required to melt a kilogram of ice (at 0℃)? Use data for problem 1.20 (1.19) Solution )(125,3341000)10018.42261(g m m =⨯=⨯+20 in certain parts of the world pressurized water from beneath the surface of the earth is available as a source of thermal energy. To make steam, the geothermal water at 180℃is passed through a flash evaporator that operates at 1atm pressure. Two streams come out of the evaporator, liquid water and water vapor. How much water vapor is formed per kilogram of geothermal water? Is the process reversible? Assume that water is incompressible. The vapor pressure of water at 180℃is1.0021 Mpa( about 10 atm) Data: C P,L=4.18J/(g k), C P,v=2.00J/(g k), △H V=2261J/g, △H m=334 J/g (1.20)Solution:leirreversibgxxx)(138),1000(8018.4)8018.48022261(=-⨯⨯=⨯-⨯+。
材料热力学习题解答

《材料热力学》复习思考题解答3. 在1560℃时,C 在液态铁中的活度系数和偏摩尔超额焓由下列式表示: 2l n 0.37711.7c C C X X γ=-++25.415.017.25E C C C H X X =++(K Cal) 其标准态为纯石墨,计算1560℃时液相与石墨平衡的相线的斜率。
解:以石墨为标准态时,C 在液态铁中的化学位为:l n (1)LC CC R T a μμ=+ 石墨 当液相与石墨平衡时,L C Cμμ=石墨。
即ln 0C α=。
又ln ln ln C C C X αγ=+ln ln 0(2)C C X γ∴+=由(2)式得:平衡时0.2067C X =两边取微分得:(ln )(ln )1[](1/)[]0(1/)C C C X T C C C C d T dX dX T X X γγ∂∂++=∂∂ (ln )[](1/)ln ln 1(1/)[()]1()CC X EC C C C C T C TC C CdX H X T d T R X X X X γγγ∂-∂∴==⋅∂∂-++∂∂2(5.415.017.25) 4.1810000.20678.311(723.4)278.6C C CC X X X X ++⨯⨯=-⋅++=- 2C dX T dT=-CdX 又d(1/T)5221278.68.310(1560273)C dX dT T -∴=-==⨯+C dX d(1/T) 1()K - 4. 在1000K 时,A-B 二元溶液中,当0.01B X =时,0.1B a =。
在盛有大量A 的量热计中加入少量的B 组元时,测得吸热7000Cal/mol ,假定2ln ln B A B X γγ=。
求1500K 时,当0.02B X =时,B 组元的活度。
解:在1000K 时,当0.01B X =时,0.1B a =0.1100.01B γ∴== 又022ln ln10ln 2.3490.99B B A X γγ=== 又ln [](1/)ii P H R T γ∂∆=∂15001500010001000l n (1/)BBH d d T Rγ∆∴=⎰⎰1500100011[ln ][ln ]()15001000B B B H R γγ∆∴=+-7000 4.18112.349()8.31150010001.175⨯=+-= 202l n (l n )0.981.175B A B X γγ∴==⨯ 1.128= l n 3.09B γ∴= 3.090.020.0B B B a X γ==⨯=7. 若A-B 二元合金系在液、固态两组元均能无限互溶,且均为理想溶液。
材料热力学考试习题

材料热力学考试习题6、10个小球分配在4个完全相同的容积中,试求4个小容积中各分得3、2、0、5个小球的微观状态数为多少?7、由5个粒子所组成的体系,其能级分别为0、、2及3,体系的总能量为3试分析5个粒子可能出现的分布方式;求出各种分布方式的微观状态数及总微观状态数。
8、有6个可别粒子,分布在4个不同的能级上(、2、3及4),总能量为10,各能级的简并度分别为2、2、2、1,计算各类分布的j及总。
9、振动频率为的双原子分子的简谐振动服从量子化的能级规律。
有N个分子组成玻耳兹曼分布的体系。
求在温度T时,最低能级上分子数的计算式。
10、气体N2的转动惯量I=1.39410-46kgm2,计算300K时的qJ。
11、已知NO分子的=2696K,试求300K时的q~如12、已知下列各双原子分子在基态时的平均核间距r0及振动波数下:分子H2O2COHIr0/10-10m0.7511.2111.1311.615~/(0.01m)-14395158021702310计算各分子的转动惯量、J及13、计算300K时,1molHI振动时对内能和熵的贡献。
14、在298K及101.3kPa条件下,1molN2的qt等于多少?15、在300K时,计算CO按转动能级的分布,并画出分子在转动能级间的分布曲线。
16、计算H2及CO在1000K时按振动能级的分布,并画出分子在振动能间的分布曲线;再求出分子占基态振动能级的几率。
17、已知HCl在基态时的平均核间距为1.26410-10m,振动波数~=2990m。
计算298K时的Sm-118、证明1mol理想气体在101.3kPa压力下qt=bLM3/2(T/K)5/2(b为常数)19、计算1molO2在25C及101.3kPa条件下的Gm、Sm及Hm。
设U0等于零。
20、已知300K时金刚石的定容摩尔热容CV,m=5.65Jmol-1K-1,求E及21.已知300K时硼的定容摩尔热容CV,m=10.46Jmol-1K-1,求(1)D;(2)温度分别为30K、50K、100K、700K、1000K时的CV,m值;(3)作CV,m值T图形。
材料热力学:热力学第一定律单元测验

一、单选题1、下列过程中,系统内能变化不为零的是()A.两种理想气体的等温混合过程B.可逆循环过程C. 纯液体的真空蒸发过程D.不可逆循环过程正确答案:C2、在实际气体的节流膨胀过程中,哪一组描述是正确的()A.Q =0, DH =0, Dp <0B.Q <0, DH =0, Dp <0C.Q >0, DH =0, Dp < 0D.Q =0, DH <0, Dp >0正确答案:A3、关于热平衡,下列说法中正确的是()A.并不是所有热力学平衡系统都必须满足热平衡的条件B.系统处于热平衡时,系统的温度一定等于环境的温度C.在等温过程中系统始终处于热平衡D. 若系统A与B成热平衡,B与C成热平衡,则A与C直接接触时也一定成热平衡正确答案:D4、将1 mol 373 K,标准压力下的水,分别经历:(1) 等温等压可逆蒸发,(2) 真空蒸发,变成373 K,标准压力下的水气。
这两种过程的功和热的关系为:A. W1>W2 Q1<Q2B. W1=W2 Q1=Q2C.W1<W2 Q1>Q2D.W1<W2 Q1<Q2正确答案:C5、对有分子间相互作用的实际气体绝热自由膨胀过程,描述错误的是()A.一定是热力学能不变的过程B.不一定是温度降低的过程C.一定是温度降低的过程D.一定是体积增大的过程正确答案:B6、下面的说法符合热力学第一定律的是()A.气体在绝热膨胀或绝热压缩过程中, 其内能的变化值与过程完成的方式无关B.在无功过程中, 内能变化等于过程热, 这表明内能增量不一定与热力学过程无关C.封闭系统在指定的两个平衡态之间经历绝热变化时, 系统所做的功与途径无关D.在一完全绝热且边界为刚性的密闭容器中发生化学反应时,其内能一定变化正确答案:C7、下列过程中, 系统内能变化不为零的是()A.两种理想气体的混合过程B.纯液体的真空蒸发过程C.可逆循环过程D.不可逆循环过程正确答案:B8、关于焓的性质, 下列说法中正确的是()A. 焓是能量, 它遵守热力学第一定律B.焓是系统内含的热能, 所以常称它为热焓C. 系统的焓值等于内能加体积功D.焓的增量只与系统的始末态有关正确答案:D9、下列哪个封闭体系的内能和焓仅是温度的函数?()A.理想气体B.理想溶液C.所有气体D.稀溶液正确答案:A10、关于节流膨胀, 下列说法正确的是()A.节流膨胀中系统的焓值改变B.节流过程中多孔塞两边的压力不断变化C.节流膨胀中系统的内能变化D.节流膨胀是绝热可逆过程正确答案:C11、关于热力学可逆过程,下面的说法中不正确的是()A.在等温可逆过程中,系统做功时,系统损失的能量最小B.可逆过程中的任何一个中间态都可从正逆两个方向到达C.在等温可逆过程中,环境做功时,系统得到的功最小D.可逆过程不一定是循环过程正确答案:A12、一定量的理想气体,从同一初态分别经历等温可逆膨胀、绝热可逆膨胀到具有相同压力的终态,终态体积分别为V1、V2。
2012硕士《材料热力学与动力学》复习练习题

Question 16
1) 指出各水平线的三相平衡反应 2) w(SiO2)=0.40 的系统(图中 R 点)从 1700C 冷却到 1000C 时的冷却曲线示意图。 注明每一阶段系统有哪些相?发生哪些 变化?指出各阶段的自由度数? 3) w(SiO2)=0.10 的系统 12 kg,冷却到 1400C 时,液相中含 MnO 多少 kg? 4) w(SiO2)=0.60 的系统 1500C 以哪些相存在?计算其相对 量。
4
2012 研究生《材料热力学与动力学》复习练习题(10 月 8 日交,手写完成)
Question 1 进行下述过程时,系统的ΔU、ΔH、ΔS和ΔG何者为零? 1.1 非理想气体的卡诺循环; 1.2 隔离系统中的任意过程; 1.3 在100C,1大气压下1mol水蒸发成水蒸汽; 1.4 绝热可逆过程。 Question 2 1mol 理想气体等容升温到状态 3,求 Q,W,ΔU,ΔH。 若将理想气体先等压膨胀到状态 2,然后再等温(可 逆)压缩到状态 3,求 Q,W,ΔU,ΔH,并与直接从 1 到 3 的途径相比较。
Question 11 导出液相中 Bi 的活度系数的估算公式。
H m T a Bi exp ( 1) RT Tm
其中,熔化热为 H m 纯 Bi 的熔点为 Tm,R 为气体常数。
Question 12 对下列二元相图,指出其中的错误 (用相律说明原因)
2
Question 13
Trouton's定律为表示为:
1 (V1,T1) 2 (V2,T2) V
H vap 90Tb
单位J/mol, 其中Tb为沸点(K), 汞的沸点为630 K. 计算在
298K液态汞的分压. 用Troutons定律估算汞的汽化热.
热力学考试题库及答案

热力学考试题库及答案一、选择题(每题2分,共20分)1. 热力学第一定律表明能量守恒,下列哪项描述是错误的?A. 能量不能被创造或消灭B. 能量可以从一种形式转换为另一种形式C. 能量可以在系统和周围环境之间转移D. 能量可以在系统中无限增加或减少答案:D2. 根据热力学第二定律,下列哪项描述是正确的?A. 热能自发地从低温物体传递到高温物体B. 热能自发地从高温物体传递到低温物体C. 热能自发地从低温物体传递到高温物体,但需要外部工作D. 热能不能自发地从低温物体传递到高温物体答案:B3. 熵是一个状态函数,它表示系统的哪种属性?A. 能量B. 温度C. 混乱程度D. 压力答案:C4. 在理想气体的等温过程中,下列哪项是正确的?A. 体积和压力成正比B. 体积和压力成反比C. 体积和温度成正比D. 体积和温度成反比答案:B5. 热力学第三定律指出,当温度趋近于绝对零度时,下列哪项属性趋近于零?A. 熵B. 内能C. 压力D. 体积答案:A6. 卡诺循环的效率与哪些因素有关?A. 热源和冷源的温度B. 热源的温度C. 冷源的温度D. 工作介质的种类答案:A7. 热力学中,一个系统经历可逆过程时,下列哪项是正确的?A. 系统和周围环境之间没有能量交换B. 系统和周围环境之间有能量交换,但系统状态可以完全恢复C. 系统和周围环境之间有能量交换,且系统状态不能恢复D. 系统和周围环境之间没有能量交换,且系统状态不能恢复答案:B8. 绝热过程是指系统与外界没有热量交换的过程,下列哪项描述是正确的?A. 系统和周围环境之间有热量交换B. 系统和周围环境之间没有热量交换C. 系统和周围环境之间有做功D. 系统和周围环境之间没有做功答案:B9. 理想气体状态方程为PV=nRT,其中R是?A. 气体常数B. 普朗克常数C. 玻尔兹曼常数D. 阿伏伽德罗常数答案:A10. 根据热力学第一定律,下列哪项描述是错误的?A. 系统内能的增加等于系统吸收的热量和对外做的功之和B. 系统内能的减少等于系统放出的热量和对外做的功之差C. 系统内能的增加等于系统吸收的热量和对外做的功之差D. 系统内能的减少等于系统放出的热量和对外做的功之和答案:C二、填空题(每题2分,共20分)1. 热力学第一定律也称为______定律。
材料热力学 习题答案

The problems of the first law1. a lead bullet is fired at a frigid surface. At what speed must it travel to melt on impact, if its initial temperature is 25℃ and heating of the rigid surface of the rigid surface is neglected? The melting point of lead is 327℃. The molar heat of fusion of the lead is 4.8kJ/mol. The molar heat capacity C P of lead may be taken as 29.3J/(mol K) (1.1)Solution: )/(5.112.20721]108.4)25327(3.29[2121)(2322s m V v n n WQ nMv mv W H T C n Q Q Q absorb melting p melt increase absorb ==⨯+-⨯===∆+∆=+=2. what is the average power production in watts of a person who burns 2500 kcal of food in a day? Estimate the average additional powder production of 75Kg man who is climbing a mountain at eh rate of 20 m/min (1.2)Solution )/(24560208.975)/(12160602410467000//)(104670001868.4102500sin 3S J t h mg P S J t Q t W P J Q gincrea Burning Burning =⨯⨯=∆==⨯⨯====⨯⨯=3 One cubic decimeter (1 dm 3) of water is broken into droplets having a diameter of onemicrometer (1 um) at 20℃. (1.3)(a) what is the total area of the droplets?(b) Calculate the minimum work required to produce the droplets. Assume that the dropletsare rest (have zero velocity)Water have a surface tension of 72.75 dyn/cm at 20℃ (NOTES: the term surface energy (ene/cm 2) is also used for surface tension dyn/cm)Solution)(25.218)106103(1075.72)(103)101(4)101(34)101(232523263631J S W m nS S Single total =⨯-⨯⨯⨯=∆=⨯=⨯⨯⨯⨯⨯⨯⨯⨯==-+----σππ4.Gaseous helium is to be used to quench a hot piece of metal. The helium is in storage in an insulated tank with a volume of 50 L and a temperature of 25℃, the pressure is 10 atm. Assume that helium is an ideal gas.(a) when the valve is opened and the gas escapes into the quench chamber (pressure=1 atm),what will be the temperature of the first gas to hit the specimen?(b) As the helium flows, the pressure in the tank drops. What will be the temperature of thehelium entering the quench chamber when the pressure in the tank has fallen to 1 atm? (1.4)Solution: )(180118298)(1185.229810101325501010101325)5500(1)()(118)101(298)()(0334.0/00K T T T K RR nC W T b K T P PT T Adiabatic a p C R P=-=∆-==⨯⨯⨯⨯⨯⨯⨯-⨯==∆=⨯==--5 An evacuated (P=0), insulted tank is surrounded by a very large volume (assume infinite volume) of an ideal gas at a temperature T 0. The valve on the tank is opened and the surrounding gas is allowed to flow quickly into the tank until the pressure inside the tank is equals the pressure outside. Assume that no heat flow takes place. What is the final tempeture of the gas in the tank? The heat capacity of the gas, C p and C v each may be assumed to be constant over the temperature rang spanned by the experiment. You answer may be left in terms of C p and C vhint: one way to approach the problem is to define the system as the gas ends up in the tank. (1.5)solution 0/000/00)()(T P P T T P PT T Adiabatic PPC R C R ≈-==6. Calculate the heat of reaction of methane with oxygen at 298K, assuming that the products of reaction are CO 2 and CH 4 (gas)[This heat of reaction is also called the low calorific power of methane] convert the answer into unites of Btu/1000 SCF of methane. SCF means standard cubic feet, taken at 298 and 1atmNOTE: this value is a good approximation for the low calorific powder of natural gas (1.6)DA TA:)()()(224g O H g CO g CH FOR80.5705.9489.17]/[0298---∙∆mol g Kcal Hsolution)1000/(9.2610252103048.01101076.191)/(76.191)89.1780.57205.94()2(22333332982982224422SCF Btu mol g Kcal H H H H H OH CO O CH CH O H CO =⨯⨯⨯⨯⨯=∙=∆+⨯---=∆-∆+∆-=∆+=+-7. Methane is delivered at 298 K to a glass factory, which operates a melting furnace at 1600 K. The fuel is mixed with a quantity of air, also at 298 K, which is 10% in excess of the amount theoretically needed for complete combustion (air is approximately 21% O 2 and 79% N 2) (1.7)(a) Assuming complete combustion, what is the composition of the flue gas (the gasfollowing combustion)?(b) What is the temperature of the gas, assuming no heat loss?(c) The furnace processes 2000kg of glass hourly, and its heat losses to the surroundingsaverage 400000 kJ/h. calculate the fuel consumption at STP (in m 3/h) assuming that for gas H 1600-H 298=1200KJ/KG(d) A heat exchanger is installed to transfer some of the sensible heat of the flue gas to thecombustion air. Calculate the decrease in fuel consumption if the combustion air is heated to 800KDA TA STP means T=298K, P=1atm22224O N O H CO CH for 2.82.89.117.1316)/(C mol cal C P ∙Solution)(210448.1125.9100076.191298)/(25.9)]87.012.72(2.843.179.1171.87.13[01.0)(%87.0%%12.72%%43.17%2%%71.8)11.1(221791.1231%22)(0,,222222224K T T T C mol cal X C C b O N CO O H CO O H CO O CH a i i p p p =⨯⨯+=∆+=∙=+⨯+⨯+⨯=======-⨯+⨯⨯+=+=+∑)/(1644)0224.011868.448.11)8001600(48.1125.9189570(102800000)/(189570)298800)](48.1187.8)48.1125.9[(100076.191)()/(87.848.11/]211002.22.816[)()/(3214)0224.011868.448.11)2981600(48.1125.9100076.191(102800000)/(280000040000020001200)(33min ,,,,298,,33min h m V mol g cal dTn C n C H H C mol cal X C C d h m V h KJ P C gConsu i i r p i i p p i i p r p g Consu =⨯⨯-⨯-⨯=∙=-⨯-⨯-⨯=--∆=∆∙=⨯⨯+===⨯⨯-⨯-⨯⨯==+⨯=⎰∑∑∑8.In an investigation of the thermodynamic properties of a-manganese, the following heat contents were determined: H 700-H 298=12113 J/(g atom) H 1000-H 298=22803 J/(g atom)Find a suitable equation for H T -H 298 and also for C P as a function of temperature in the form (a+bT) Assume that no structure transformation takes place in the given tempeture rang. (1.8)Solution )298(0055.0)298(62.35011.062.35011.062.3522803)2981000(2)2981000(12113)298700(2)298700(]2[2229822222982---=∆-=-===-+-=-+-+=+==∆⎰⎰T T H TC b a ba ba T baT bTdT a dT C H TP T P9.A fuel gas containing 40% CO, 10% CO 2, and the rest N 2 (by volume) is burnt completely with air in a furnace. The incoming and ongoing temperatures of the gases in the furnace are 773K and 1250K,respectively. Calculate (a) the maximum flame temperature and (b) heat supplied to the furnace per cu. ft of exhaust gas (1.9)molJ Hmol J H CO f CO f /393296/1104580,298,0,298,2-=∆-=∆)/(10184.403.29)/(1067.11010.492.19)/(1037.81020.935.44)/(1042.01097.345.283,253,253,253,222molK J T C molK J T T C molK J T T C molK J T T C N P O P CO P CO P -------⨯+=⨯-⨯+=⨯-⨯+=⨯-⨯+=Solution?0)499.0321.018.1()1067.01019.277.28(28.282831067.01038.477.289.0)1019.01058.528.33(2.0282838)()/(1019.01058.528.33722.0278.0)/(1067.01038.477.281.065.005.02.0)()/(282838110458393296%2.72%8.27%10%65%5%20)4/(1122298127332981523733253253298,,,,298,253,,,,,253,,,,,,,0,298,0,298,298,22222222222222==+--⨯+⨯++⨯=⨯-⨯++⨯⨯-⨯+-⨯=--∆=∆⨯-⨯+=+==⨯-⨯+=+++===-=∆-∆=∆========+-----------⎰⎰⎰∑∑⎰∑∑∑∑T T T T T T T dT T T dTT T dT n C n C n H H molK J T T C C n C C molK J T T C C C C n C C a mol J n Hn H H N CO production O N CO CO reation then O N air mole need fuel mole when CO O CO T TT i i r p i i p p i i N P CO P i i p p r p O P N P CO P CO P i i p p r p i pf i rf idTT T Q dT T T Q b T T T T T T T dT T T dTT T dT n C n C n H H T TT i i r p i i p p i i 9.0)1019.01058.528.33(2.02828389.0)1019.01058.528.33(2.0282838)(0)499.0321.018.1()1067.01019.277.28(28.282831067.01038.477.289.0)1019.01058.528.33(2.0282838)(253125029812502982531250298125029829812125029815231250253253298,,,,298,⨯⨯-⨯++⨯-=⨯⨯-⨯++⨯-===+--⨯+⨯++⨯=⨯-⨯++⨯⨯-⨯+-⨯=--∆=∆-----------⎰⎰⎰⎰⎰∑∑⎰10. (a) for the reaction 2221CO O CO →+,what is the enthalpy of reaction (0H ∆) at 298 K ?(b) a fuel gas, with composition 50% CO, 50% N 2 is burned using the stoichiometric amount of air. What is the composition of the flue gas?(c) If the fuel gas and the air enter there burner at 298 K, what is the highest temperaturethe flame may attain (adiabatic flame temperature)? DA TA :standard heats of formation f H ∆ at 298 K (1.10))/(393000)/(1100002mol J CO mol J CO -=-=Heat capacities [J/(mol K)] to be used for this problem N 2=33, O 2=33, CO=34, CO 2=57 Solution)(21100)298)(39889.0(222.02830000)/(3975.03325.057)/(33111.034222.033666.033)(%,75%%,251.111002.22%%1.11%%,6.66%%,2.222.0/25.015.0%)()/(283000393000110000)(,0,,,,,,22220,298,0,298,0K T T dT C n H H K mol J X C C K mol J X C C C N CO product O N CO fuel b mol J n H n H H a P p p i P r i P r i P p i P p i P f i r f ==-⨯-⨯=-∆=∆∙=⨯+⨯==∙=⨯+⨯+⨯====-====+==+-=∆-∆=∆⎰∑∑∑∑11.a particular blast furnace gas has the following composition by (volume): N 2=60%, H 2=4, CO=12%, CO 2=24%(a) if the gas at 298K is burned with the stochiometric amount of dry air at 298 K, what is the composition of the flue gas? What is the adiabatic flame temperature? (b) repeat the calculation for 30% excess combustion air at 298K(C)what is the adiabatic flame temperature when the blast furnace gas is preheated to 700K (the dry air is at 298K)(d) suppose the combustion air is not dry ( has partial pressure of water 15 mm Hg and a total pressure of 760 mm Hg) how will the flame temperature be affected? DA TA(k J/mol) (1.11)2CO CO FOR513.393523.110)/(--∆m o lkJ H f 2222,)(O N g O H CO CO FOR34505733]/[K mol J C P ∙Solution)(1052)(75438286370])295.03450(241604[026.0])335.03457(110523393513[079.0])([%8.66%%,8.6%%,6.2%%,8.15%%,9.72.0/83.110012%)()(1122)(82538313430])295.03450(241604[029.0])335.03457(110523393513[086.0])([%7.65%%,7.5%%,9.2%%,1.17%%,6.82.0/810012%2121)(,,,,,,,02222,,,,,,,0222222222K T K T T n C T T X C dT n C n C H x H N O H CO CO b K T K T T n C T T X C dT n C n C H x H N O H CO CO OH O H CO O CO a i i r P ii P i i r P i i p P i i i i r P ii P i i r P i i p P i i ===∆=∆-∆-⨯--+∆-⨯---=+--∆=∆=====⨯+====∆=∆-∆-⨯--+∆-⨯---=+--∆=∆=====+=→+→+∑∑∑⎰∑∑∑∑∑⎰∑∑)(1419),(11213842594034286.0)402(2.39714.0])295.03450(241604[029.0])335.03457(110523393513[086.0)3(K T K T T T T T H ===∆=∆⨯--∆⨯-∆-⨯--+∆-⨯---=∆12.A bath of molten copper is super cooled to 5℃ below its true melting point. Nucleation of solid copper then takes place, and the solidification proceeds under adiabatic conditions. What percentage of the bath solidifies?DATA: Heat of fusion for copper is 3100 cal/mol at 1803℃(the melting point of copper) C P,L =7.5(cal/mol ℃), C P,S =5.41+(1.5*10-3T )(cal/mol ℃) (1.12) Solution)/(310355.75.0)17981803(105.1541.5310002231798,1798,17981803,18031798,1803,mol cal H H dT C dT C HL S SL L P S P LS =⨯-⨯-⨯+⨯+==+++-⎰⎰13.Cuprous oxide (Cu 2O) is being reduced by hydrogen in a furnace at 1000K, (a)write the chemical reaction for the reduced one mole of Cu 2O(b)how much heat is release or absorbed per mole reacted? Given the quantity of heat and state whether heat is evolved (exothermic reaction) or absorbed (endothermic reaction) DATA: heat of formation of 1000K in cal/mol Cu 2O=-41900 H 2O=-59210 (1.13) solution)/(173104190059210222mol cal H OH Cu H O Cu =-=∆+=+,exothermic reaction14. (a) what is the enthalpy of pure, liquid aluminum at 1000K?(b) an electric resistance furnace is used to melt pure aluminum at the rate of 100kg/h. the furnace is fed with solid aluminum at 298K. The liquid aluminum leaves the furnace at 1000K. what is the minimum electric powder rating (kW) of furnace.DATA : For aluminum : atomic weight=27g/mol, C p,s =26(J/molK), C p,L =29(J/molK), Melting point=932K, Heat of fusion=10700J/mol (1.14)Solution )(28.0)(7.2793600110002727184)/(2718410700)9321000(29)298932(261000932,932298,1000,kW W P mol J H dT C dT C H SLL P S P l ==⨯⨯==+-⨯+-⨯=++=⎰⎰15 A waste material (dross from the melting of aluminum) is found to contain 1 wt% metallic aluminum. The rest may be assumed to aluminum oxide. The aluminum is finely divided and dispersed in the aluminum oxide; that is the two material are thermally connected.If the waster material is stored at 298K. what is the maximum temperature to which it may rise if all the metallic aluminum is oxidized by air/ the entire mass may be assumed to rise to the same temperature. Data : atomic weight Al=27g/mol, O=16g/mol, C p,s,Al =26(J/molK), C p,s,Al2O3=104J/mol, heat formation of Al 2O 3=-1676000J/mol(1.15)Solution;)(600)(3021041029927275.116122711676000K T K T T ==∆∆⨯⨯++⨯⨯=⨯⨯16 Metals exhibit some interesting properties when they are rapidly solidified from the liquid state. An apparatus for the rapid solidification of copper is cooled by water. In the apparatus, liquid copper at its melting point (1356K) is sprayed on a cooling surface, where it solidified and cools to 400K. The copper is supplied to the apparatus at the rate of one kilogram per minute. Cooling water is available at 20℃, and is not allowed to raise above 80℃. What is the minimum flow rate of water in the apparatus, in cubic meters per minute? DATA; for water: C p =4.184J/g k, Density=1g/cm 3; for copper: molecular weight=63.54g/mol C p =7cal/mol k, heat of fusion=3120 cal/mol (1.16)Solution:min)/(10573.2)2080(1min /min54.631000)]4001356(73120[min /33m V VQ Q Water Copper -⨯=-=⨯⨯-⨯+=17 water flowing through an insulated pipe at the rate of 5L/min is to be heated from 20℃ to 60℃ b an electrical resistance heater. Calculate the minimum power rating of the resistance heater in watts. Specify the system and basis for you calculation. DATA; For water C p =4.184J/g k, Density=1g/cm 3 (1.17) Solution: )(139476010005)2060(184.4W W =⨯⨯-⨯=18 The heat of evaporation of water at 100℃ and 1 atm is 2261J/mol (a) what percentage of that energy is used as work done by the vapor?(b)if the density of water vapor at 100℃ and 1 atm is 0.597kg/m 3 what is the internal energy change for the evaporation of water? (1.18)Solution: )/(375971822613101%6.71822613101%)/(31010224.0273373101325mol J Q W U mol J V P =⨯+-=+=∆=⨯==⨯⨯=∆19 water is the minimum amount of steam (at 100℃ and 1 atm pressure) required to melt a kilogram of ice (at 0℃)? Use data for problem 1.20 (1.19) Solution )(125,3341000)10018.42261(g m m =⨯=⨯+20 in certain parts of the world pressurized water from beneath the surface of the earth is available as a source of thermal energy. To make steam, the geothermal water at 180℃ is passed through a flash evaporator that operates at 1atm pressure. Two streams come out of the evaporator, liquid water and water vapor. How much water vapor is formed per kilogram of geothermal water? Is the process reversible? Assume that water is incompressible. The vapor pressure of water at 180℃ is 1.0021 Mpa( about 10 atm) Data: C P,L =4.18J/(g k), C P,v =2.00J/(g k), △H V =2261J/g, △H m =334 J/g (1.20) Solution:leirreversib g x x x )(138),1000(8018.4)8018.48022261(=-⨯⨯=⨯-⨯+The problems of the second law1 The solar energy flux is about 4J cm 2/min. in no focusing collector the surface temperature can reach a value of about 900℃. If we operate a heat engine using the collector as the heat source and a low temperature reservoir at 25℃, calculate the area of collector needed if the heat engine is to produce 1 horse power. Assume the engine operates at maximum efficiency. (2.1)Solution )(664.0)(74660104273900)25900(24m S W tWP StQ T T T W H H L H ===⨯⨯+-=-=2 A refrigerator is operated by 0.25 hp motor. If the interior of the box is to be maintained at -20℃ ganister a maximum exterior temperature of 35℃, what the maximum heat leak (in watts) into the box that can be tolerated if the motor runs continuously? Assume the coefficient of performance is 75% of the value for a reversible engine. (2.2)Solution:)(114474625.02035202733475.0%75W P P T T T P Q T T T W L LLLH HHLH =⨯⨯+-⨯=-=-=3 suppose an electrical motor supplies the work to operate a Carnot refrigerator. The interior of the refrigerator is at 0℃. Liquid water is taken in at 0℃ and converted to ice at 0℃. To convert 1 g of ice to 1 g liquid. △H=334J/g is required. If the temperature outside the box is 20℃, what mass of ice can be produced in one minute by a 0.25 hp motor runningcontinuously? Assume that the refrigerator is perfectly insulated and that the efficiencies involved have their largest possible value. (2.3)Solution: )(4576033474625.020273g m M m P P T T T P L LLLH ===⨯⨯=-=4 under 1 atm pressure, helium boils at 4.126K. The heat of vaporization is 84 J/mol what size motor (in hp) is needed to run a refrigerator that must condense 2 mol of gaseous helium at 4.126k to liquid at the same temperature in one minute? Assume that the ambient temperature is 300K and that the coefficient of performance of the refrigerator is 50% of the maximum possible. (2.4)Solution: )(52.0)(393'60284216.4216.4300'5.0%50hp W P P T T T P P Q T T T W L L L H LLLH ==⨯⨯-=-==-= 5 if a fossil fuel power plant operating between 540 and 50℃ provides the electrical powerto run a heat pump that works between 25 and 5℃, what is the amount of heat pumped into the house per unit amount of heat extracted from the power plant boiler. (a) assume that the efficiencies are equal to the theoretical maximum values(b) assume the power plant efficiency is 70% of maximum and that coefficient ofperformance of the heat pump is 10% of maximum(c) if a furnace can use 80% of the energy in fossil foe to heat the house would it be moreeconomical in terms of overall fissile fuel consumption to use a heat pump or a furnace ? do the calculations for cases a and b (2.5)solution:1,2,2,1,212,2,2,2,21,1,1,1,198.82527352527354050540)(H H H H H H L H H H L H P P P P P P P T T T P P T T T P a =+-=+-=-=-=.,)(6286.0)(1,2,not is b ok is a c P P b H H =6 calculate △U and △S when 0.5 mole of liquid water at 273 K is mixed with 0.5 mol of liquid water at 373 K and the system is allowed to reach equilibrium in an adiabaticenclosure. Assume that C p is 77J /(mol K) from 273K to 373K (2.6) Solution:)/(933.0)273323ln(5.0)373323ln(5.0)ln()ln()(02211K J C C T T C n T T C n S J U P P E P E P =+=+=∆=∆ 7 A modern coal burning power plant operates with a steam out let from the boiler at 540℃and a condensate temperature of 30℃.(a) what is the maximum electrical work that can be produced by the plant per joule of heatprovided to the boiler?(b) How many metric tons (1000kg) of coal per hour is required if the plant out put is to be500MW (megawatts). Assume the maximum efficiency for the plant. The heat of combustion of coal is 29.0 MJ/k g(c) Electricity is used to heat a home at 25℃ when the out door temperature is 10℃ bypassing a current through resistors. What is the maximum amount of heat that can be added to the home per kilowatt-hour of electrical energy supplied? (2.7)Solution:)(3.69)(6937136005000.29)()(89.013054030540)(ton kg m T T T mb J Q T T T W a LH LH H L H ==⨯=-=+-=-=)(9.191102525273)(J Q Q T T T W c H HHLH =-+=-=8 an electrical resistor is immersed in water at the boiling temperature of water (100℃) the electrical energy input into the resistor is at the rate of one kilowatt(a) calculate the rate of evaporation of the water in grams per second if the water containeris insulated that is no heat is allowed to flow to or from the water except for that provided by the resistor(b) at what rate could water could be evaporated if electrical energy were supplied at therate of 1 kw to a heat pump operating between 25 and 100℃data for water enthalpy of evaporation is 40000 J/mol at 100℃; molecular weight is 18g/mol; density is 1g/cm 3 (2.8)solution:)(23.2,2510027310010004000018)()(45.0,10004000018)(g m m b g m ma =-+===9 some aluminum parts are being quenched (cooled rapidly ) from 480℃ to -20℃ byimmersing them in a brine , which is maintained at -20℃ by a refrigerator. The aluminum is being fed into the brine at a rate of one kilogram per minute. The refrigerator operates in an environment at 30℃; that is the refrigerator may reject heat at 30℃. what is them minuspower rating in kilowatts, of motor required to operate the refrigerator? Data for aluminum heat capacity is 28J/mol K; Molecular weight 27g/mol (2.9)Solution:)(5.102)(102474202732030)20480(28271000kW W P P T T T P P L L L L H W L ==---=-=--⨯=10 an electric power generating plant has a rated output of 100MW. The boiler of the plantoperates at 300℃. The condenser operates at 40℃(a) at what rate (joules per hour) must heat be supplied to the boiler?(b) The condenser is cooled by water, which may under go a temperature rise of no morethan 10℃. What volume of cooling water in cubic meters per hour, is require to operate the plant?(c) The boiler tempeture is to be raised to 540℃,but the condensed temperature and electricoutput will remain the same. Will the cooling water requirement be increased, decreased, or remain the same?Data heat capacity 4.184, density 1g/cm 3 (2.10)Solution: )(109.7)(102.21040300273300)(1188J t P Q W P T T T P a H H L H H H ⨯==⨯=-+=-=)(1003.1184.41010)(103.4)(34611m V Q V J Q b L L ⨯==⨯⨯⨯⨯=noW P T T T P c L H H H )(10626.11040540273540)(88⨯=-+=-=11 (a) Heat engines convert heat that is available at different temperature to work. Theyhave been several proposals to generate electricity y using a heat engine that operate on the temperature differences available at different depths in the oceans. Assume that surface water is at 20℃, that water at a great depth is at 4℃, and that both may be considered to be infinite in extent. How many joules of electrical energy may be generated for each joule of energy absorbed from surface water? (b) the hydroelectric generation of electricity use the drop height of water as the energy source. in a particular region the level of river drops from 100m above sea level to 70m above the sea level . what fraction of the potential energy change between those two levels may be converted into electrical energy? how much electrical energy ,in kilowatt-hours, may be generated per cubic meter of water that undergoes such a drop? (2.11)Solution:)/(1006.136001000)()(055.0127320420)(6h kW hmg P b J Q T T T W a H H L H ⨯=⨯∆==+-=-=12 a sports facility has both an ice rink and a swimming pool. to keep the ice frozen during the summer requires the removal form the rink of 105 KJ of thermal energy per hour. It has been suggested that this task be performed by a thermodynamic machine, which would be use the swimming pool as the high temperature reservoir. The ice in the rink is to be maintain at a temperature of –15℃, and the swimming pool operates at 20℃, (a) what is the theoretical minimum power, in kilowatts, required to run the machine? (b) how much heat , in joule per hour , would be supplied t the pool by this machine? (2.12)Solution:)(1014.1101527320273)()(77.33600/10152731520)(555kJ Q b kW P T T T P a H L L L H ⨯=-+==-+=-=13solution:)/(81.6810ln 314.877.45277.6282.4)/(152940)()/(67.4977.45277.6282.4)()/(152940)(22)(2molK cal S mol cal H d molK cal S c mol cal H b AlNN Al a -=+-⨯-⨯=∆=∆-=-⨯-⨯=∆=∆=+14solution:)/(2257412000)27340273ln 184.4273336263273ln1.2()(40,010,K J dT T C T H dT T C m S WATER P m mICE P =+++=+∆+=∆⎰⎰- 15)(70428)(2896100077773002J W J Q T T T W L L L H ==-=-=16)(4.3719))2.4300(314.85.13.83(3002.4300)(7.58663.832.42.4300J Q T T T W J Q T T T W H H L H L L L H =-⨯+-=-==-=-=17yesd Q c K J PPnR S b J pdV n W Q OU T a )(0)()/(1.1910ln 314.81ln )()(570410ln 298314.810)(0==⨯⨯==∆=⨯⨯=-=-==∆=∆⎰18)(122233527302033560500g m m m T T T L L H =-=-=⨯教材各章习题参考答案 (魏)3.2 ΔG = -108.9 J/mol; ΔS = -21.42 J/(mol.K)3.6 (a ) 22.09/(.)S J mol K ∆=;(b) At 0︒C, ∆G =0; (c) ∆H = 5841.9 J;(d) ∆S =21.39J /(mol.K),∆G = 109.38 J/mol4.1 (a ) 2898.28J/mol; ( b ) No; ( c ) 345 J/mol; ( d ) 14939 atm; ( e )4921 J/mol4.2 ( a ) 272.8K; ( b ) Pa P 610345⨯≈∆ ; ( c ) 249.46K 4.3 1202K4.4 P=5.73⨯10-6 atm 4.5 0.16P4.7 08.10430685ln +-=TP 4.8 ( a ) 1180K; ( b ) 695.3K; ( c ) 114.4kJ/mol; ( d ) 7123 J/mol; ( e )4.2J/mol4.9 In the initial state: 4.06 mol %; in the final state:5.3 mol% 4.10 ( a )348 kJ; ( b ) 2.3×10-3Pa ;( c ) “ solution not possible ”; (d ) “solution not possible ”5.1 atm p H 0005.0= 5.2、atmp o 1221007.1-⨯=If the error in enthalpy is 500cal, the uncertainty in the pressure calculated is 28.6%, and if the error in enthalpy is -500cal, the uncertainty is -22.1%5.3、(a) T =462K; (b) T = 420K5.4 (a) atm P O 2621014.1-⨯=, (b) P O2 =2.28⨯10-10 atm., (c) The equilibriumoxygen pressure remains the same when the total pressure increases, which means a higher purity level of N 2 .5.5 (a) 略; (b) Pa atm P H 8.181013056.1800019.0)('2=⨯==; (c) 21.5L Ar isneeded to be bubbled into the melt.5.6(a )l n K a1/T, 10-31/K=∆-=∆o o G kJ H 1000;50- 66.6kJ(b) Ja = 3 < Ka, the reaction will proceed from left to right, and theatmosphere will not oxidize Ni. 5.7 略5.8. (a) P SiO = 8.1⨯10-8 (atm) (b) ∆H o = 639500J; ∆So =334.9J/K (c ) PO2 =10-30 atm 5.9 5.10.J H o72250=∆,the reaction is an endothermic one.5.11. (a),166528J H o =∆ the reaction is an endothermic one.; (b) At 1168K, the equilibrium pressure of CO2 equals one atmosphere.)(106.08)(atm Pg u -⨯=5.12 (a) 略 , (b) Mg CO P P =; (c) T = 2037 K 5.13 (a) 略; (b) 13109.2⨯=K ; (c) ppm 186.0 5.14 (a) 略; (b) kJ H 52.267=∆; (c) K T 1592= 5.15 (a) )(106.13atm -⨯≈; (b) )(1028.210)(2atm P g O H -⨯=5.16 (a) 97.9=K ; (b) atm x 14.4=; (c) if the temperature is increased, the fraction of water reacted will increase since the equilibria constant increases with increasing temperature.6.2 (a )1.287V;(b) When the water impure, the voltage will go higher; (c) 1.219V 6.4 (a) 145.3kJ;(b) The maximum work that could be derived is 702.36kJ; (c) In this case, the maximum work that could be derived is696.56kJ.6.5 (a) -6252J/mol; (b) 370.0)(=II Cd a ; (c) )(42.3mmHg P Cd =; 6.67.87⨯10-4 V 6.7 (a))(22g Cl Mg MgCl +=(b) Pa P Cl 21'1086.82-⨯=;(c) 2.485V6.8 (a) Pa P O 11'2105.5-⨯=;(b) Anode: e Ni Ni 2+→Cathode: -→+2222/1O e O ;(c) 0.757V; (d) 0.261V6.10 (a) )(509.3V E o=;(b) 0.074kJ;(c) 4.1⨯106J;(d) Yes. In this case, the open circuit voltage is 3.648V;(e) In this case, to keep the temperature constant, 3.92⨯106J heatshould be removed from the battery per hour. 6.11(a) TG CO Al C O Al o 26.3211008.12/322/36232-⨯=+=+Δ(b) The minimum voltage at which the electrolysis may be carriedout at 1250K is 1.172V .7.1 0.117 atm 7.5 ( a ) ,82.5 2.5 2.5B A BA BB T PV V V x x x x x ⎛⎫∂=+=--⎪∂⎝⎭ ,102.5 2.5 2.5A B A A B A T PV V V x x x x x ⎛⎫∂=+=-- ⎪∂⎝⎭( b) B A M x x V 5.2=7.7 2)1(736.0ln Sn Sn x --=γ7.8 The maximum solubility of MgF2 in liquid MgCl at 900︒C is 19。
材料热力学习题

材料热力学习题
1熟悉的条件、表达式及意义()。
2. 熟悉配分函数的条件、表达式及意义()。
3.三大分布是什么?,表达式?
4、热力学函数的微分表达式;热力学意义定律,第二定律;
5、固溶体中配置熵的计算;高分子模型中配置熵的计算;
6、分析铁基马氏体与有色金属中马氏体相变时的动力与阻力。
7、交互作用参数意义,各参数变化对其影响;
8、正规熔体及热函数表达式
9、在定压热容C p的经验表达式中,,试导出这时的焓(H)、熵(S)和Gibbs自由能(G)的表达式。
10、利用讨论偏析引起的自由能的变化。
材料热力学习题答案

材料热力学习题答案材料热力学习题答案热力学是研究物质的能量转化和能量传递规律的科学。
在材料科学中,热力学是一个重要的分支,它可以帮助我们理解材料在不同条件下的性质和行为。
在学习热力学的过程中,我们经常会遇到一些习题,下面我将给出一些常见材料热力学习题的答案。
1. 问题:在常压下,将1mol的水从25℃加热到100℃,需要吸收多少热量?答案:要计算这个问题,我们可以使用热容的概念。
热容是物质在单位温度变化下吸收或释放的热量。
对于水来说,其热容为4.18J/(g℃)。
首先,我们需要知道水的质量,由于1mol的水的摩尔质量为18g/mol,因此1mol的水的质量为18g。
接下来,我们需要计算水的温度变化,即100℃-25℃=75℃。
最后,我们可以使用公式Q=mCΔT来计算所需吸收的热量,其中Q是热量,m是质量,C是热容,ΔT是温度变化。
代入数值得到Q=18g×4.18J/(g℃)×75℃=5613J。
2. 问题:在恒定温度下,气体的体积与压力之间的关系是什么?答案:根据热力学的理论,理想气体的体积与压力成反比。
这可以用理想气体状态方程PV=nRT来解释,其中P是压力,V是体积,n是物质的摩尔数,R是气体常数,T是温度。
根据这个方程,当温度保持不变时,如果压力增加,体积将减小,反之亦然。
这种关系被称为波义尔定律。
3. 问题:在材料科学中,什么是熵?答案:熵是热力学中的一个重要概念,它用于描述物质的无序程度。
熵可以理解为系统的混乱程度或无序程度。
根据热力学的第二定律,系统的熵总是趋向于增加,即系统总是朝着更高的熵状态发展。
当物质从有序状态转变为无序状态时,熵会增加。
例如,当固体融化成液体,或者液体蒸发成气体时,系统的熵会增加。
熵在材料科学中起着重要的作用,可以帮助我们理解材料的相变行为和稳定性。
4. 问题:什么是自由能?答案:自由能是热力学中另一个重要的概念,它用于描述系统的稳定性和可逆性。
材料热力学习题及答案
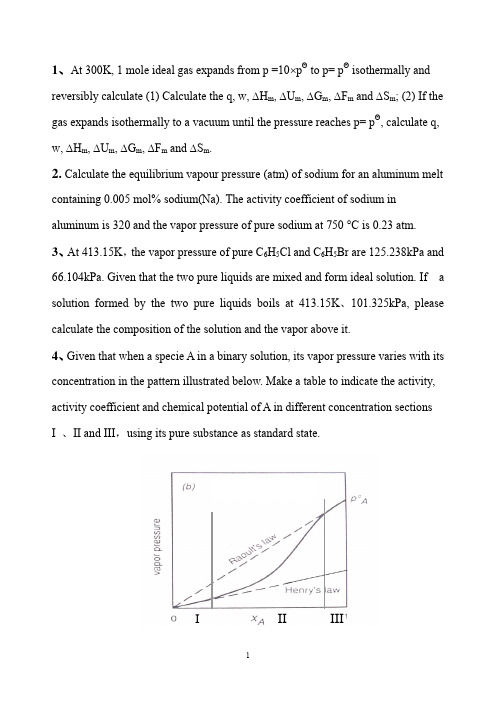
1、At 300K, 1 mole ideal gas expands from p =10⨯pΘ to p= pΘ isothermally and reversibly calculate (1) Calculate the q, w, ∆H m, ∆U m, ∆G m, ∆F m and ∆S m; (2) If the gas expands isothermally to a vacuum until the pressure reaches p= pΘ, calculate q, w, ∆H m, ∆U m, ∆G m, ∆F m and ∆S m.2. Calculate the equilibrium vapour pressure (atm) of sodium for an aluminum melt containing 0.005 mol% sodium(Na). The activity coefficient of sodium in aluminum is 320 and the vapor pressure of pure sodium at 750 °C is 0.23 atm.3、At 413.15K,the vapor pressure of pure C6H5Cl and C6H5Br are 125.238kPa and 66.104kPa. Given that the two pure liquids are mixed and form ideal solution. If a solution formed by the two pure liquids boils at 413.15K、101.325kPa, please calculate the composition of the solution and the vapor above it.4、Given that when a specie A in a binary solution, its vapor pressure varies with its concentration in the pattern illustrated below. Make a table to indicate the activity, activity coefficient and chemical potential of A in different concentration sectionsI 、II and III,using its pure substance as standard state.III III5、At 300K, the vapor pressure of liquid A and liquid B are 37.33kPa and 22.66kPa.When 2 moles of A and 2 moles of B are mixed to form a solution, the vapor pressure above the solution is 50.66kPa, and the molar fraction of A in the vapor is 0.60. Given that vapors can be taken as ideal gases. ①Calculate a A( R )and a B( R) in the solution, ②γA and γB , ③∆mix G , ④ If the solution is an ideal solution, what is the value of ∆mix G id ? ⑤ What is the value of ∆mix G ex of this solution?6、The variation, with composition, of G E for Fe-Mn alloys at 1863K is listedbelow:X Mn 0.1 0.2 0.3 0.4 0.5 0.6 0.7 0.8 0.9 G m E ,Joules 395703 925 1054 1100 1054925 703 395a 、Is the process to form Fe-Mn alloy at 1863K an exothermic one or an endothermic one ?b. Does the system exhibit regular solution behavior?c. Calculate E Feμ and EMn μ at X Mn = 0.6; d. Calculate m mix G ∆ atX Mn = 0.4; e. Calculate the partial pressures of Mn and Fe exerted by the alloy of X Mn = 0.27、Melts in the system Pb-Sn exhibit regular solution behavior. At 473︒C, a Pb =0.055 in a liquid solution of X Pb = 0.1. Calculate the value of PbSn ωfor the system and calculate the activity of Sn in the liquid solution of X Sn = 0.5 at 500︒C.8、With respect to the Ellingham diagram, answer the following questions:93.23ln 27.145390)(ln *+--=T Tatm pFe68.37ln 02.333440)(ln *+--=T Tatm p Mna) Explain the slope changes for the reaction 2Mg + O 2 = 2MgO; b) You want to heat up and a piece of silicon metal to 1600︒C, decide on a suitable crucible material;c) What is the value of ∆H Θ of formation of TiO 2 ? d) Find ∆G Θfor the reaction Fe + 0.5O 2 =FeO at 1200 ︒C;e) Find ∆G Θ for the reaction 3Mg + AlO 3 = 3MgO + 2A1 at 1500 ︒C; f) What is the equilibrium oxygen pressure when metallic titanium is in equilibrium with TiO 2 at 1000 ︒C?g) If you want to reduce pure TiO 2 to pure metallic titanium at 1000︒C using a CO/CO 2 gas mixture, what is the minimum CO/CO 2 ratio that can achieve such a reduction.9、Answer the following questions according to Ellingham diagram:① At what temperature(s) C can reduce SnO 2(s)、Cr 2O 3(s) and SiO 2(s) ? ② At what temperature, the decomposition pressure of CuO reaches 1.01325⨯105 Pa ?③ The temperature(s) at which Fe 3O 4 can be reduced to FeO by H 2 ? ④ ∆G Θ when Mg reduces Al 2O 3 at 1000︒C,⑤ At what temperature, for the reaction )(322)(3234S S O Cr O Cr =+,Pa 1019'2-(平)is p O ⑥ Calculate the ∆G when Fe reacts with O 2 at 10-5Pa and 10-10Pa respectivelyat1000︒C, and '(2平)O p as well. ⑦ Calculate the equilibrium constant of reaction 2)()(CO Mn CO MnO s s +=+ at1100︒C (CO CO p p K /2=)⑧ At what temperature, for reaction )(2)(2)(g s s O H Mn H MnO +=+, the(平))/(22O H H is 104/1 ? 10、The standard Gibbs free energy change for reaction I:Ni (s ) + 1/2 O2 == NiO (s )is -244560 + 98.53TlnT J/ mol , question: a) How much is the standard Gibbs free energy change for reaction II : 2Ni (s ) + O2 == 2 NiO (s )b) Calculate the equilibrium constants for reaction I and reaction II respectively at 1000︒ C.c) At 1000︒ C, when oxygen pressure is maintained at 10-4 atm, how much is theGibbs free energy change for reaction I ? Can reaction I proceed forward ? Is Ni stable under this condition ? Is NiO stable under this condition ? d) At 1000︒ C, how much should be the oxygen pressure if we want the Gibbs free energy change for reaction I to be 0, and how much should be the oxygen pressure if we want a Ni-NiO-O 2 system to be at equilibrium ?e) At 1000 C, what is the condition to prevent Ni from being oxidized ? and whatis the condition to reduce NiO ?11、Liquid FeO is reduced to metallic iron at 1600 °C with CO(gas) accordingto the following reaction:FeO(liquid) + CO(g) = Fe(liquid) + CO 2a) Calculate ∆G Θ at 1600 °C for this reactionb) Detennine the minimum CO/C02 ratio required to reduce pure liquid FeO topure metallic iron at 1600 °C.c) Determine the minimum CO/CO2 ratio required to reduce FeO dissolved in a liquid slag to metallic iron at 1600 °C. The metallic iron formed has a purity of 96 mole % iron. The activity of FeO in the liquid slag is 0.3.CO(g) at 1600 °C: ∆GΘ= -274.9 kJ/molCO2(g) at 1600 °C: ∆GΘ = -396.3 kJ/molFeO at 1600 °C: ∆GΘ = -144.6 kJ/molR= 8.314 J/ mol.K= 1.987 ca1/mol.K12、In an experiment, it was found that the Ar was not pure enough. So a setup was devised in an attempt to purify the Ar, as illustrated below. Ar which was at 2 atm was let to flow through a glass tube and the Cu powder pile in it. Given that the temperature in the glass tube is 600︒C and gas pressure is constant at 2 atm.. Calculate the purity of the outgoing Ar in percentage.Ellingham Diagram习题参考答案3.2 ΔG = -108.9 J/mol; ΔS = -21.42 J/(mol.K)3.6 (a ) 22.09/(.)S J mol K ∆=;(b) At 0︒C, ∆G =0; (c) ∆H = 5841.9 J;(d) ∆S =21.39J /(mol.K),∆G = 109.38 J/mol4.1 (a ) 2898.28J/mol; ( b ) No; ( c ) 345 J/mol; ( d ) 14939 atm; ( e ) 4921 J/mol 4.2 ( a ) 272.8K; ( b ) Pa P 610345⨯≈∆ ; ( c ) 249.46K 4.3 1202K4.4 P=5.73⨯10-6 atm 4.5 0.16P4.7 08.10430685ln +-=TP 4.8 ( a ) 1180K; ( b ) 695.3K; ( c ) 114.4kJ/mol; ( d ) 7123 J/mol; ( e ) 4.2J/mol 4.9 In the initial state: 4.06 mol %; in the final state: 5.3 mol% 4.10 ( a )348 kJ; ( b ) 2.3×10-3Pa ;( c ) “ solution not possible”; (d ) “solution not possible”5.1 atm p H 0005.0=5.2、atm p o 1221007.1-⨯= If the error in enthalpy is 500cal, the uncertainty in the pressure calculated is 28.6%, and if the error in enthalpy is -500cal, the uncertainty is -22.1%5.3、(a) T =462K; (b) T = 420K5.4 (a) atm P O 2621014.1-⨯=, (b) P O2 =2.28⨯10-10 atm., (c) The equilibrium oxygen pressureremains the same when the total pressure increases, which means a higher purity level of N 2 .5.5 (a) 略; (b) Pa atm P H 8.181013056.1800019.0)('2=⨯==; (c) 21.5L Ar is needed to bebubbled into the melt.5.6(a )l n K a1/T, 10-31/K=∆-=∆ooG kJ H 1000;50- 66.6kJ(b) Ja = 3 < Ka, the reaction will proceed from left to right, and the atmosphere willnot oxidize Ni.5.7 略5.8. (a) P SiO = 8.1⨯10-8 (atm) (b) ∆H o = 639500J; ∆So =334.9J/K (c ) PO2 =10-30 atm5.9 5.10.J H o72250=∆,the reaction is an endothermic one. )(106.08)(atm P g u -⨯=5.11. (a),166528J H o =∆ the reaction is an endothermic one.; (b) At 1168K, the equilibrium pressure of CO2 equals one atmosphere. 5.12 (a) 略 , (b) Mg CO P P =; (c) T = 2037 K 5.13 (a) 略; (b) 13109.2⨯=K ; (c) ppm 186.0 5.14 (a) 略; (b) kJ H 52.267=∆; (c) K T 1592= 5.15 (a) )(106.13atm -⨯≈; (b) )(1028.210)(2atm P g O H -⨯=5.16 (a) 97.9=K ; (b) atm x 14.4=; (c) if the temperature is increased, the fraction of water reacted will increase since the equilibria constant increases with increasing temperature.6.2 (a )1.287V;(b) When the water impure, the voltage will go higher; (c) 1.219V 6.4 (a) 145.3kJ;(b) The maximum work that could be derived is 702.36kJ;(c) In this case, the maximum work that could be derived is 696.56kJ. 6.5 (a) -6252J/mol; (b) 370.0)(=II Cd a ; (c) )(42.3mmHg P Cd =; 6.6 7.87⨯10-4 V 6.7 (a))(22g Cl Mg MgCl +=(b) Pa P Cl21'1086.82-⨯=; (c) 2.485V6.8 (a) Pa P O 11'2105.5-⨯=;(b) Anode:e NiNi 2+→Cathode:-→+2222/1O e O ; (c) 0.757V; (d) 0.261V6.10 (a) )(509.3V E o=;(b) 0.074kJ;(c) 4.1⨯106J;(d) Yes. In this case, the open circuit voltage is 3.648V;(e) In this case, to keep the temperature constant, 3.92⨯106J heat should beremoved from the battery per hour.6.11(a) TG CO Al C O Al o 26.3211008.12/322/36232-⨯=+=+Δ(b) The minimum voltage at which the electrolysis may be carried out at1250K is 1.172V .7.1 0.117 atm 7.5 ( a ) ,82.5 2.5 2.5B A B A B B T PV V V x x x x x ⎛⎫∂=+=--⎪∂⎝⎭ ,102.5 2.5 2.5A B A A B A T PV V V x x x x x ⎛⎫∂=+=-- ⎪∂⎝⎭( b) B A M x x V 5.2=7.7 2)1(736.0ln Sn Sn x --=γ7.8 The maximum solubility of MgF2 in liquid MgCl at 900 C is 19 mol% .7.9 ( a ) 1121K; ( b ) 1. 8 cal/K9.6Temperature(ºC ) Phase Composition Fraction1300 Liquid 0.59 0.64 α 0.06 0.36 β ---- 01000+Liquid 0.8 0.43 α 0.1 0.57 β ---- 01000-Liquid ---- 0α 0.1 0.65 β 0.95 0.359.8 Solution:(a) 90 mol%B is the composition of the first solid to form;10 mol % is the composition of the last liquid drop.(b) solid (60 mol%B is the composition) is about 77% ; liquid (15 mol%B is the composition) is 23%9.9 (a) 2900℃, α(12%) (b) 2300℃, liq(95%) (c) 8.2%α(composition is24% )+91.8%β(85%)习题参考答案1.ΔS m =19.1J/mol.K, ΔG m = -5740 J/mol, ΔF m = -5740 J/molIsothermally expands to a vacuum: w = 0, ΔH m =0 , ΔU m = 0,ΔS m =19.1J/mol.K, ΔG m = -5740 J/mol, ΔF m = -5740 J/mol2. 3.68 × 10-3 atm3、Pa x x Br H C Cl H C 406.0;594.05556==Pa p Pa p Br H C Cl H C 26838;744445556==4.5、JG J G J G a a ex mix id mix mix B A R B R A 5302)5(;6912)4(;1610)3(;788.1;62.1)2(;894.0;81.0)1()()(=∆-=∆-=∆====γγ 6. a endothermic one; b. Yes; c J J EMn E Fe 704;1584==μμd ;/9363mol J G m mix -=∆e Pa p Pa p Fe Mn 4;1198==7. J SnPb 4578-=ω; 418.0=Sn aPure Substance as Stand ard Statepq(b )I 、II 、IIIAAA AAA A AAAA x R T T p x k R T T p p R T T T 0'ln )(ln)(ln )()(γμμμμ+=+=+=*****III:I:II:AA AAAA a RT T p p RT T T ln )(ln )()('+=+=***μμμAA AA AA x RT T p p RT T T ln )(ln)()(*'*+=+=*μμμk A8. a) Mg boils and which makes o S ∆more negative, so the slope changes for larger; b) Firstly, we should avoid using metallic material for this purpose since the melting points of metals are mostly too low. Ceramic materials, usually composed of oxides and having high melting points can be chosen The material should not be reduced by pure silicon at 1600ºC. By examing Ellingham diagram, crucibles (坩埚) made of Al 2O 3 .c ) -890kJ /molO2;d ) -170kJ /molFeO; e) -30kJ; f) Pa 2110-; g)721063.0/⨯=pco p CO 9、⑨ 650ºC ,1220 ºC and 1520 ºC ; ⑩ 1480 ºC ;⑪ When the temperature is equal to or higher that 710 ºC ; ⑫ 2/100molO kJ G o -=∆ ⑬ 900 ºC; ⑭0,102/112,1010'25'2=∆=-=∆=--G Pa P molO kJ G Pa P O O , Pa p e O 10')(210-= ⑮ 510-=K ;⑯ 1220ºC10、a) -489120+197.06TlnT J/mol;b) 2.89×10-54 ; c) J G 749429=∆; Ni is stable under this condition, and NiO is not stable; d) Pa p e o 58')(21046.3⨯= e) from the calculation, we found that at 1000ºC,Pa p e o 58')(21046.3⨯=.So at 1000ºC, when theoxygen pressure is less than 3.46×1058Pa, Ni is stable and can not be oxidized, and NiO will be reduced to Ni under this condition.11. a) mol kJ G o /2.23=∆; b)43.42=⎪⎭⎫ ⎝⎛eCO COp p. This is the minimumCO/CO2 ratio required to reduce pure FeO to Fe at 1600ºC. c)2.142=⎪⎭⎫ ⎝⎛eCO CO p p . This is the minimum CO/CO2 ratio required to reduce FeO in a slag( 炉渣) to Fe in a metallic iron melt under the given conditions at 1600ºC.12.%100)1015.3%10⨯⨯-=(Ar。
SYU材料热力学复习题

材料热力学复习题一、填空:1、系统的平衡态是在(系统不受外界作用)的条件下,系统的(宏观物理性质)不随时间变化的状态。
2、逆卡诺循环是由两个(等温)过程、两个(等熵)过程所组成的。
3、G-T关系曲线反映了在(定压)条件下,G函数与温度T之间的关系。
4、dG=dU+PdV- TdS 成立的条件是(等温)。
5、亨利定律和拉乌尔定律分别从(溶质)和(溶剂)的角度描述了溶液的蒸气压。
6、液相或固相与其气相形成(相平衡)时的压强称为饱和蒸汽压。
7、表面能是指(产生单位表面面积所做的可逆功)。
8、两个组元的体系最多可以存在( 4 )相平衡。
9、二元系(组元设为i, j)中α,β和γ三相形成平衡的条件是( 3 )。
10、G-T 关系曲线反映了定压条件下,G 函数与温度T 之间的关系,其斜率的负值表示了体系的(熵)。
二、判断:1、闭口体系的绝热膨胀过程是等熵过程。
(×)2、在等压条件下,闭口体系的熵不变。
(×)3、闭口体系的可逆绝热过程是否为等熵过程。
(√)4、闭口体系的的自由能只能减少不能增加。
( )5、孤立系统的熵的变化不可能通过外界的加热来实现(√)。
6、闭口体系发生相变的方向不一定向着体系自由能减少的方向进行( )7、对于铁在一定温度、一定压力下的固——液平衡,若单一增大压力或提高温度,还有可能保持平衡( )。
8、体系发生相变,则体系的自由能减少。
( )9、三组元的体系中可能存在两相平衡。
( )10混合相的mol 自由能是构成它的两个相互平衡的相的mol 自由能之和( )11、混合相mol自由能绝对不是构成它的两个相互平衡的相mol自由能之和( )12、体系发生相变,则体系的自由能减少。
( )13、母相中形成新相,新相的mol自由能一定要低于原来母相的mol自由能( )14、在等温等压条件下,闭口体系发生平衡相变,则体系的自由能减少。
( )15对于一个体系的平衡相而言亚稳相由于摩尔自由能更高而不能够先出现( )三、简答:1、一个绝热开口体系的熵能减少吗?能增加吗?试举例说明。
材料热力学试题_参考答案 (2007年)

材料热力学试题_参考答案 (2007年)一、填空题(每题5分,共10分)1. 0≥m dS 作为过程的可逆性判据,其适用条件是 绝热或隔离体系 。
2. 热力学基本方程Vdp SdT dG +-=的适用条件是 只作体积功的封闭体系 。
二、简答题 (本题20分,每小题10分)1. 平衡态的判据(即判断某一过程是否能自发进行)有哪几个?哪一个最广泛地在实践中被运用?为什么?(10分)[答] (1) 熵(S)判据( dS ≥0 );(2) 恒温恒容且只作体积功的亥姆霍兹(Helmholtz)自由能判据:自发过程dF <0,平衡过程dF=0 ;(3) 恒温恒压且只作体积功的的吉布斯(Gibbs)自由能判据:自发过程dG <0,平衡过程dG=0。
吉布斯(Gibbs)自由能判据最广泛地被运用。
因为绝大多数的实际过程发生在恒温恒压的条件下。
2. 简述马氏体相变的特点。
并举一实例说明其在具体实践中应用。
(10分)[答] 马氏体相变的特点有:马氏体相变是无扩散相变之一,原子只做有规则的重排(集团式变位)而不进行扩散;(2) 马氏体沿惯习(析)面生长并与奥氏体母相保持一定的取向关系,形成共格晶界;(3) 马氏体相变发生在一定的温度区间内(M s ~M f );(4) 马氏体相变的可逆性;(5) 快速长大;(6) 马氏体转变的不完全性。
实例1:刀具通过锋刄的高温局部淬火,可达到强度和韧性的结合;实例2:通过高温淬火生产弹簧。
三、计算题(共40分)1. Al 在Fcc 结构时的摩尔吉布斯自由能为G Fcc_A1:-7976.15 + 137.093T - 24.3672 T lnT - 1.88466 T 2×10–3 - 8.7766 T 3×10–7 + 74092 T –1 (298.15<T<700) 计算300K ,500K 时Al 的Cp 值,S 值以及H 值。
最新《材料热力学与动力学》复习题

最新《材料热力学与动力学》复习题最新《材料热力学与动力学》复习题篇一:《材料热力学与动力学》复习题一、常压时纯Al的密度为= 2.7 g/cm3,熔点Tm = 660.28 C,熔化时体积增加5%。
用理查德规则和克劳修斯-克拉佩龙方程估计一下,当压力增加等1 GPa时其熔点大约是多少。
二、热力学平衡包含哪些内容,如何判断热力学平衡?三、试比较理想熔体模型与规则熔体模型的异同点。
四、固溶体的亚规则溶体模型中,自由能表示为Gmxi0GiRTxilnxiEGmii其中过剩自由能表示为EGmxAxBLAB(xAxB)实际测得某相中0LAB和1LAB,请分别给出组元A和B的化学位表达式五、向Fe中加入形成元素会使区缩小,但无论加入什么元素也不能使两相区缩小到0.6 at%以内,请说明原因。
六、今有Fe-18Cr-9Ni和Ni80-Cr20两种合金,设其中含碳量为0.1wt%,求T=1273C时碳在这两种合金中活度。
七、假如白口铁中含有3.96%C及2.2%Si,计算在900C时发生石墨化的驱动力,以铸铁分别处于+渗碳体两相状态与+石墨两相状态时碳的活度差来表示此驱动力。
由于Si不进入Fe3C中,所以有KSiCem/= 0。
在Fe-C二元合金中,已知900C时+渗碳体两相状态碳的活度为二aC= 1.04;当与石墨平衡时aC= 1。
八、通过相图如何计算溶体的热力学量如熔化热、组元活度。
九、请说明相图要满足那些基本原理和规则。
十、请说明表面张力产生的原因?十一、已知温度为608 K时,Bi的表面张力为371 mJ/m2,Sn 的表面张力为560 mJ/m2,Bi的摩尔原子面积为6.95104 m2/mol,Sn的摩尔原子面积为6.00104 m2/mol。
试Bi-Sn二元合金的表面张力。
十二、以二元合金为例,分析析出相表面张力对相变的影响。
十三、请解释钢中淬火马氏体低温回火时为什么先析出亚稳化合物而不是稳定的渗碳体(Fe3C)十四、通过原子的热运动,分析影响扩散系数的因素。
材料热力学与动力学复习资料+课后习题

材料热力学与动力学(复习资料)一、 概念•热力学基本概念和基本定律1. 热0:一切互为热平衡的物体,具有相同的温度。
2. 热1: - 焓:恒压体系→吸收的热量=焓的增加→焓变等于等压热效应 - 变化的可能性→过程的方向;限度→平衡3. 热2:任何不受外界影响体系总是单向地趋向平衡状态→熵+自发过程+可逆过程→隔绝体系的熵值在平衡时为最大→熵增原理(隔离体系)→Gibbs 自由能:dG<0,自发进行(同T ,p : )4. 热3:- (H.W.Nernst ,1906): - (M .Plank ,1912):假定在绝对零度时,任何纯物质凝聚态的熵值为零S*(0K)=0 - (Lewis ,Gibson ,1920):对于过冷溶体或内部运动未达平衡的纯物质,即使在0K 时,其熵值也不等于零,而是存在所谓的“残余熵” - Final :在OK 时任何纯物质的完美晶体的熵值等于零• 单组元材料热力学1. 纯金属固态相变的体积效应- 除非特殊理由,所有纯金属加热固态相变都是由密排结构(fcc )向疏排结构(bcc )的转变→加热过程发生的相变要引起体积的膨胀→BCC 结构相在高温将变得比其他典型金属结构(如FCC 和HCP 结构)更稳定(除了Fe )- 热力学解释1→G :温度相同时,疏排结构的熵大于密排结构;疏排结构的焓大于密排结构→低温:H ;高温:TS - 热力学解释2→ Maxwell 方程: - α-Fe →γ-Fe :磁性转变自由能- Richard 规则:熔化熵-Trouton 规则:蒸发熵 (估算熔沸点)2. 晶体中平衡状态下的热空位- 实际金属晶体中空位随着温度升高浓度增加,大多数常用金属(Cu 、Al 、Pb 、W 、Ag …)在接近熔点时,其空位平衡浓度约为10-4;把高温时金属中存在的平衡空位通过淬火固定下来,形成过饱和空位状态,对金属中的许多物理过程(例如扩散、时效、回复、位错攀移等)产生重要影响3. 晶体的热容- Dulong-Petit :线性谐振动子+能量均分定律→适应于较高温度及室温附近,低温时与实验不符U Q W∆=-dH PV U d Q =+=)(δRd Q S Tδ=()d dH TdS G H d TS =--=00lim()lim()0p T T T GS T→→∂∆-=∆=∂()()V T T P V V S ∂∂=∂∂//()()()T T T V P V V S T V H ∂∂+∂∂=∂∂///RK mol J T H S mm m ≈⋅≈∆=∆/3.8/K mol J T H S b v v ⋅≈∆=∆/9.87/3V V VQ dU C RdT dT δ⎛⎫⎛⎫=== ⎪ ⎪⎝⎭⎝⎭-Einstein(固体振动热容理论):晶体总共吸收了n 个声子,被分配到3N 个谐振子中;不适用于极低温度,无法说明在极低温度时定容热容的实验值与绝对温度的3次方成比例。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
2. 已知纯钛α/β平衡相变温度为882℃,相变焓为
14.65kJ.mol-1,试求将βTi过冷到800℃时,β→α的相变 驱动力。
3.试用正规溶体模型计算一个IAB=16.7kJ.mol-1成分为XB
=0.4的二元固溶体,其发生Spinodal分解的上限温度
是多少?
4.试根据AI-Mg二元相图中Mg17Al12在Mg基固溶体(β)中的
《材料热力学》试题
2007级
一、计算题
1.已知液体锌的Cp(l)为
Cp(l)=29.66+4.81×10-3T J/mol.K 固体密排六方锌的Cp(l)为 Cp(l)=22.13+11.05×10-3T J/mol.K 锌的熔点692.6K,熔化热ΔH=6589.9J/mol,求固、 液相之间随温度变化自由能差值ΔG(T)。
(模型的建立、主要结论、适用对象)
3. 试画出如下共晶相图T2、T3、T5温度 下各相的自由能-成分曲线。
4. 试用摩尔自由能-成分图说明,为什么碳 素钢在淬火之后回火时,渗碳体的粒子越 细,其周围的铁素体中的含碳量越高?
三. 问答题
结合自己的研究课题,试述热力学
在材料中的应用。
谈谈本课程的学习体会以及对本课程
溶解度曲线数据(见下表),求Mg17Al12的生成自由能。 温度/℃ 400 11 350 8.2 300 6.1 250 4.3 200 2.9 150 1.7
溶解度(Al)/at.%
二、简答题
1. 用正规溶体近似解释二元合金固溶体的illiams近似和双亚点阵模型。
的建议。
