hcp说明书1
申科sf9 hcp残留检测试剂盒 (一步酶联免疫吸附法)说明书

SHENTEK®Sf9HCP残留检测试剂盒(一步酶联免疫吸附法)说明书货号:1301310在实验前请完整阅读本说明书,务必重视注意点和常见问题!版本:A/0仅供研究用湖州申科生物技术股份有限公司⏹产品名称通用名:Sf9HCP残留检测试剂盒(一步酶联免疫吸附法)。
⏹包装规格96测试/盒。
⏹预期用途Sf9HCP残留检测试剂盒(一步酶联免疫吸附法)适用于昆虫细胞Sf9来源的宿主蛋白残留定量检测,如基于昆虫细胞-杆状病毒表达系统的生物制品,包括但不限于重组蛋白、疫苗,基因治疗AAV载体等。
该试剂盒仅供研究使用,不可用于临床。
⏹检测原理本试剂盒基于固相酶联免疫吸附法(Enzyme-linked Immunosorbent Assay,ELISA),采用双抗体夹心的方式对样品中残留Sf9HCPs进行定量检测。
该试剂盒内的多克隆抗体是通过裂解sf9细胞所得HCPs作为抗原,免疫绵羊获得血清,进而通过亲合纯化方法得到高质量抗体。
抗体通过目前主流的覆盖率分析法规方法评估其覆盖率水平。
该分析方法通过在预包被抗Sf9HCPs多克隆抗体的酶标板中加入校准品或待测样品、HRP标记的抗Sf9HCPs多克隆抗体进行共孵育;洗涤后,利用加入的TMB底物进行显色反应,最后使用终止液终止酶催化反应。
利用酶标仪在450nm波长下测读吸光度值,其吸光度与校准品和样品中的HCPs浓度成正相关,通过剂量-反应曲线可计算得出样品中Sf9HCPs的浓度。
本试剂盒对实际样品无需进行特殊处理,仅需通过合适的稀释比例进行适用性验证即可直接使用。
本试剂盒检测步骤少,快速,专一性强,性能稳定可靠。
图1检测原理示意图试剂盒组分表1.试剂盒组分组分产品号规格说明Sf9HCP 校准品PNB0112瓶冻干粉。
精确量取500μL复溶液,溶解,静置约5分钟,溶液应该澄清透明,无肉眼可见不溶物。
具体浓度见瓶身标注。
抗Sf9HCP 预包被酶标板PNA0128孔×12条已包被适量的绵羊抗Sf9HCPs多克隆抗体,铝箔袋密封包装,含干燥剂。
Vero 细胞 HCP(宿主残留蛋白)ELISA试剂盒说明书

Vero 细胞HCP(宿主细胞蛋白)Vero细胞宿主蛋白酶联免疫检测目录#F500预期用途该试剂盒用于检测用Vero 细胞.生产的制品中是否有宿主蛋白残留。
仅供研究和工业生产,不能用于人和动物的诊断。
总结与说明病毒疫苗或其他治疗用蛋白在Vero细胞中表达使商业用大量生产药物原料产品的经济简便方法。
生产和纯化这些产品的过程中会残留Vero细胞中的一些蛋白质(称为宿主细胞蛋白,HCPs)而造成污染。
这种污染可以减少药物的疗效,引发毒副反应和免疫学反应,因此最好在实际生产制品过程中将HCPs降低到最低水平。
一些利用抗体来除HCPs的免疫学方法,如Western Blot 和ELISA被广泛使用。
虽然Western blot是一种检测HCPs的有效方法,但它受到了一些限制。
因为它过程复杂且技术依赖性强,需要操作者对结果进行分析解释。
而且,它在本质上是一种定性检测,不能定量分析。
Western blot的敏感性易受被检测样品量的影响,也受目的产品浓度的干扰。
因此Western blot可用来检测纯化上游的蛋白质,而对纯化下游或终产品的检测灵敏度与特异性较低。
本试剂盒中用到的ELISA方法克服了Western Blot的缺点,将敏感度提高了100倍。
ELISA操作简单、客观、可获得半定量的结果,是纯化工艺,过程控制,常规质量检测的最佳选择。
这个试剂盒可与纯化过程中残留的可独立污染产品的所有HCPs反应,就这个意义上来说,这个试剂盒是通用的。
用Vero细胞轻度裂解物获得抗体并经亲和纯化,得到的最终抗体可以与用于生产各种病毒疫苗和蛋白质产品的四种商用细胞系反应。
这一分析表明,绝大多数HCP存在于各种Vero细胞系和纯化过程中。
如果你需要一个更为敏感和特异性的方法去检测样品中的HCP量,Cygnus Technologies公司为你推荐一个优于2D Western blot的方法,我们将这个方法称为2D HPLC-ELISA。
日立投影机hcp 使用说明

投影机使用说明书.操作指南承蒙您购买本投影机,谨向您表示衷心的感谢。
•本说明书中的信息如有变更,恕不另行通知。
•本说明书中的插图用作图解。
与您的投影机可能有少许差异。
•制造商对本说明书中可能出现的任何错误概不负责。
•未经明确的书面许可,不得翻印、转载或复制本文档的全部或任何部分。
通知事项本说明书中使用了各种符号。
这些符号的意义说明如下。
本符号表示如果忽略这些信息,可能会因错误操作而导致人身伤害,甚至死亡。
本符号表示如果忽略这些信息,可能会因错误操作而导致人身伤害或实物损坏。
请参阅本符号后标明的页码。
商标承认•Mac ® 是 Apple Inc. 的注册商标。
• W indows ®, DirectDraw ® 和 Direct3D ® 是微软公司在美国和/或其它国家的㊟册商标。
•VESA 和 DDC 是 Video Electronics Standard Association 的商标。
• H DMI、HDMI 徽标以及 High-Definition Multimedia Interface 是 HDMI Licensing LLC. 在美国和其他国家的商标或注册商标。
• B lu-ray Disc TM 和Blu-ray TM 是Blu-ray Disc Association 的商标。
其他所有商标均为其各自所有者的财产。
HCP-635X通告本条目用于告知可能会导致故障。
介绍. . . . . . . . . . . . . . . . . . . . . . . . . .3特点. . . . . . . . . . . . . . .3关于电磁干扰. . . . . . . . . . .3包装箱中的物品. . . . . . . . . .3部件名称. . . . . . . . . . . . .4设置. . . . . . . . . . . . . . . . . . . . . . . . . .7安装. . . . . . . . . . . . . . .7连接设备. . . . . . . . . . . .10连接电源. . . . . . . . . . . .16固定适配器盖. . . . . . . . . .17使用防盗杆和防盗槽. . . . . . .17遥控器. . . . . . . . . . . . . . . . . . . . . . .18装入电池. . . . . . . . . . . .18关于遥控信号. . . . . . . . . .19改变遥控信号的频率. . . . . . .19用作简易电脑鼠标和键盘. . . . .20电源开/关. . . . . . . . . . . . . . . . . . . .21开启电源. . . . . . . . . . . .21关闭电源. . . . . . . . . . . .22操作. . . . . . . . . . . . . . . . . . . . . . . . .23调节音量. . . . . . . . . . . .23暂时静音. . . . . . . . . . . .23选择输入信号. . . . . . . . . .24搜索输入信号. . . . . . . . . .25选择宽高比. . . . . . . . . . .26调节投影机的支撑脚. . . . . . .27调节变焦和聚焦. . . . . . . . .27使用自动调节功能. . . . . . . .28调节位置. . . . . . . . . . . .28校正梯形失真. . . . . . . . . .29使用放大功能. . . . . . . . . .30静止画面. . . . . . . . . . . .31暂时遮屏. . . . . . . . . . . .31使用菜单功能. . . . . . . . . .32简易菜单. . . . . . . . . . . . . . . . . . . . . .34宽高比、自动梯形校正、梯形校正、图像模式、省电模式、安装、复位、.过滤器使用时间、语言、高级菜单、.关闭图像菜单. . . . . . . . . . . . . . . . . . . . . .36亮度、对比度、伽马、色温、彩色、.色调、清晰度、动态光圈、我的存储器影像菜单. . . . . . . . . . . . . . . . . . . . . .39宽高比、扫描度、垂直位置、水平位置、水平相位、水平尺寸、自动调节执行输入菜单. . . . . . . . . . . . . . . . . . . . . .42逐行、视频降噪、彩色空间、视频格式、HDMI.格式、HDMI范围、COMPUTER-IN、.帧锁定、分辨率设置菜单. . . . . . . . . . . . . . . . . . . . . .46自动梯形校正、梯形校正、自动省电模式、省电模式、安装、待机模式、显示器输出声音菜单. . . . . . . . . . . . . . . . . . . . . .49音量、扬声器、音频源、麦克风级别、麦克风音量屏幕菜单. . . . . . . . . . . . . . . . . . . . . .50语言、菜单位置、遮屏画面、启动画面、自选画面、自选画面锁定、消息、输入源名称、模板、C .C .(隐藏字幕)选项菜单. . . . . . . . . . . . . . . . . . . . . .56自动搜索、自动梯形校正、直接开启电源、自动关闭电源、USB.TYPE.B、灯泡使用时间、过滤器使用时间、快捷按钮、我的端口源、特殊设定网络菜单. . . . . . . . . . . . . . . . . . . . . .67无线设置、无线信息、有线设置、有线信息、投影机名称、我的图像、AMX.D .D .、演示、特殊设定安全菜单. . . . . . . . . . . . . . . . . . . . . .78变更安全密码、自选画面密码、身份识别号码锁、状态监视功能、个人文本密码、显示个人文本、填写个人文本、复制锁定演示工具. . . . . . . . . . . . . . . . . . . . . .84无需电脑演示. . . . . . . . . .84 USB显示(Windows电脑). . . . .94 USB显示(Mac电脑). . . . . . .98维护. . . . . . . . . . . . . . . . . . . . . . . . .100更换灯泡. . . . . . . . . . . .102清洁和更换空气过滤器. . . . . .104其他维护. . . . . . . . . . . .106故障诊断. . . . . . . . . . . . . . . . . . . . . .107相关消息. . . . . . . . . . . .107关于指示灯. . . . . . . . . . .109重设所有设置. . . . . . . . . .112容易误认为是机器故障的现象. . .112规格. . . . . . . . . . . . . . . . . . . . . . . . .117•请妥善保管原包装材料,以备日后重新装运。
第三篇:CHO细胞宿主蛋白(CHO-HCP)酶联免疫分析试剂盒使用说

CHO细胞宿主蛋白(CHO-HCP)酶联免疫分析试剂盒使用说明书本试剂盒仅供研究使用。
96T使用目的:本试剂盒用于测定血清、血浆及相关液体样本中CHO细胞宿主蛋白(CHO-HCP)表达。
实验原理本试剂盒应用双抗体夹心法测定标本中CHO细胞宿主蛋白(CHO-HCP)表达。
用纯化的CHO细胞宿主蛋白(CHO-HCP)抗体包被微孔板,制成固相抗体,可与样品中CHO细胞宿主蛋白(CHO-HCP)相结合,经洗涤除去未结合的抗原和其他成分后再与HRP标记的CHO细胞宿主蛋白(CHO-HCP)抗体结合,形成抗体-抗原-酶标抗体复合物,经过彻底洗涤后加底物TMB显色。
TMB在HRP酶的催化下转化成蓝色,并在酸的作用下转化成最终的黄色。
用酶标仪在450nm波长下测定吸光度(OD值),与CUTOFF值相比较,从而判定标本中CHO细胞宿主蛋白(CHO-HCP)的存在与否。
试剂盒组成1.标本采集后尽早进行提取,提取按相关文献进行,提取后应尽快进行实验。
若不能马上进行试验,可将标本放于-20℃保存,但应避免反复冻融2.不能检测含NaN3的样品,因NaN3抑制辣根过氧化物酶的(HRP)活性。
操作步骤1.编号:将样品对应微孔按序编号,每板应设阴性对照2孔、阳性对照2孔、空白对照1孔(空白对照孔不加样品及酶标试剂,其余各步操作相同)2.加样:分别在阴、阳性对照孔中加入阴性对照、阳性对照50μl。
然后在待测样品孔先加样品稀释液40μl,然后再加待测样品10μl。
加样将样品加于酶标板孔底部,尽量不触及孔壁,轻轻晃动混匀,3.温育:用封板膜封板后置37℃温育30分钟。
4.配液:将30倍浓缩洗涤液用蒸馏水30倍稀释后备用5.洗涤:小心揭掉封板膜,弃去液体,甩干,每孔加满洗涤液,静置30秒后弃去,如此重复5次,拍干。
6.加酶:每孔加入酶标试剂50μl,空白孔除外。
7.温育:操作同3。
8.洗涤:操作同5。
9.显色:每孔先加入显色剂A50μl,再加入显色剂B50μl,轻轻震荡混匀,37℃避光显色15分钟.10.终止:每孔加终止液50μl,终止反应(此时蓝色立转黄色)。
移动式滤油机使用手册 HCP100A

前言此手册是本公司按照客户采购单的要求而制造的设备的安装、操作及保养指南。
在安装、操作及保养此设备之前,必须阅读此手册的有关内容。
此设备和选购部件将分别包装,并由客户装配。
拆开包装箱时,要细心谨慎,并按发货单核查所收到的部件。
此设备已按颇尔公司标准程序进行了试验和质量检验,而且也根据合同的要求进行必要的试验。
然而,在试验后,为便于运输,设备可能已打开或解体,并已将其放空和清洗等。
客户应检查并确保运输时,螺母、螺栓、软管或其它任何部件均未松动,必要时将其紧固。
铭牌已固定在设备上,如需要参数、服务或备件时,可查阅铭牌提供的信息。
用户有责任检查实际的操作条件,并确保过滤部件、滤芯、容器及密封材料与实际应用条件相匹配并符合地方安全法规。
目录概述 3 选型 4 技术参数 5 报警条件7 储存与安装9储存9安装前检验9HCP与用户系统的连接10HCP与电源的连接12 操作屏幕14操作屏幕概述14主菜单15 设备启动19脱水循环19过滤循环20设备排空20 维护22日常维护22滤芯更换22故障诊断23 附录25备件清单25PALL图纸HCP100A INST 共2张PALL图纸H0473.E 共5张概述此设备是颇尔公司专为汽轮机油设计的过滤脱水系统,它集油液的精密过滤及高效脱水于一体,能有效清除油液中的颗粒及乳化水、游离水。
处理完成后油液清洁度可达NAS1638 6级,自由水含量可低于100 PPM。
本设备的功能包括:1.过滤循环:可单独作为颗粒过滤设备使用。
2.脱水循环:可去除汽轮机油中乳化水、游离水。
本设备具备如下特点:1.无人值守运行:经调试合格进入正常运行后,无须值守;2.故障自动报警:运行异常需维护时可自动报警并提示操作方法;3.遇险自动停机:运行中出现危急情况时可自动停机关闭系统;4.系统状态显示:设备操作屏幕可显示设备当前及历史操作数据;5.远程工况监视:预留设备状态信号远传接口,用户可根据需要连接。
Emotron HCP 2.0 手持式控制面板 使用说明书

Emotron HCP 2.0 Handheld control panel for Emotron FDU/VFX 2.0Emotron CDU/CDX 2.0Instruction manualEnglishHandheld control panel for Emotron FDU/VFX 2.0 and Emotron CDU/CDX 2.0The handheld control panel - HCP is a complete control panel, easy to connect to the variable speed drive, for temporary use when e.g. commissioning, servicing and so on.The HCP has full functionality including memory. It is possible to set parameters, view signals, actual values, fault logger information and so on. It is also possible to use the memory to copy all data (such as parameter set data and motor data) from one VSD (Variable Speed Drive) to the HCP and then load this data to other VSDs. For further information see Instruction manual for Emotron FDU/VFX 2.0 or Emotron CDU/CDX 2.0.Following cable kits are available:A.HCP cable for FDU/VFX Standard drives. 2 x 9-pin D-sub connectors, length 3 meters.B.HCP cable for CDU/CDX Compact drives.9-pin D-sub and RJ12 connectors (for connection to internal control board), length 3.3 meters.C.HCP cables for both CDU/CDX and FDU/VFX drives. One cable with 9-pin D-sub and RJ12 connectors + one cable with 2 x D-sub connectors.Connect the HCP to Emotron FDU/VFX 2.0It is easy to connect the HCP instead of the control panel or the blank panel in following way.1.Remove the control panel or the blank panel on theVSD. Then the RS232 connector will be visible.Fig. 1When you remove the blank panel or control panel you will be able to connect the HCP .B.Connect the standard RS232 cable, to the female connector on the VSD and to the connector on the HCP .Fig. 2RS232 cable for connecting the HCP to Emotron FDU/VFX 2.0C.Now it is possible to use the HCP .Part number Description01-5039-00Handheld Control Panel complete for FDU/VFX2.0 or CDU/CDX 2.0NOTE: When ordering the HCP it is important to specify if you are going to connect the HCP to Emotron FDU/VFX 2.0 or Emotron CDU/CDX2.0 so the correct cable kit is delivered.NOTE: It is important that only one control panel isconnected at the same time. Always remove/disconnect the existing control panel before connecting the HCP.Connect the HCP to Emotron CDU/CDX 2.0Fig. 3Adapter and cable for connecting the HCP toEmotron CDU/CDX 2.0.1.Mount the adapter to the RS232 connector on the HCP.B.Switch off the mains power supply.C.On Emotron CDU/CDX 008 - 018, remove the frontcover in order to expose the control board.Fig. 4Remove the front cover on EmotronCDU/CDX 008 - 018D.On Emotron CDU/CDX 026 - 046, remove the sidecover in order to expose the control board .Fig. 5Remove the side cover on EmotronCDU/CDX 026 - 046E.Disconnect the RJ12 cable from the built in controlpanel and connect the RJ12 cable from the HCPFig. 6Connect the HCP cable to the RJ12 connector on the control board.WARNING!Always switch off the mains voltage if it isnecessary to open the VSD and wait at least5 minutes to allow the capacitors todischarge.Remove the front coverRemove the side coverConnect theHCPcable to theRJ12 connector.F.Now it is possible to switch on the mains power supplyand use the HCP.WARNING!Be careful as the cover is open. Although theconnections for the control signals and theswitches are isolated from the main voltage, do not touch the control board when the variable speed drive is switched on.After having used the HCP, turn off the main power supply. Wait at least 5 minutes to allow the capacitors to discharge.1.Disconnect the cable from the control board. Reconnectthe cable from the built in control panel again.B.Mount the cover.C GD r i v e s & A u t o m a t i o n , 01-5925-01r 1 2015-04-20CG Drives & Automation Sweden ABMörsaregatan 12Box 222 25SE-250 24 Helsingborg SwedenT +46 42 16 99 00F +46 42 16 99 49/。
奈西立肽 说明书natrecor_hcp

Scios Inc.6500 Paseo Padre ParkwayFremont, CA 94555Telephone 510 248 2500Telefax 510 248 2389May 6, 2005Dear Physician/Patient Advocate:Recently, there have been several published reports about NATRECOR® (nesiritide), a treatment for patients with acutely decompensated congestive heart failure (ADHF) with dyspnea. These reports raise the question of whether NATRECOR® may have adverse effects on survival and kidney function compared to control agents (generally nitroglycerin and diuretics). We would like to provide you information on the following:• Information Scios has added to the Prescribing Information for NATRECOR®• Meta-analyses published in the April 20, 2005 issue of The Journal of the American Medical Association (JAMA) and in the March 29, 2005 issue of Circulation• An independent panel of experts, led by a distinguished cardiologist, that will convene at the company’s request to assess clinical data and review the clinical development program forNATRECOR®NATRECOR® is an effective drug with a well-described safety profile for patients requiring intravenous treatment for acutely decompensated congestive heart failure with dyspnea at rest or with minimal activity. Heart failure affects about 5 million Americans, and ADHF is a life-threatening condition for which there are limited treatment options. Advanced ADHF patients have a 30% risk of mortality within one year.1 NATRECOR® was approved for the treatment of advanced ADHF on the basis of its ability to improve dyspnea and reduce pulmonary capillary wedge pressure. In these studies the mortality was somewhat greater in the NATRECOR® group than in the comparator groups and this has been noted in labeling since NATRECOR® was first marketed. It was not clear that the small increase was drug related. Updated labeling for NATRECOR® was approved recently based on ongoing discussions with the U.S. Food and Drug Administration. The revised label language is based on the analyses of survival data from seven controlled studies, including the three studies used in the meta-analysis that recently appeared in JAMA. The label now includes 30-day mortality data in addition to the 180-day data that previously appeared in our label. The analyses show a nominal increase in mortality, but the increases are not statistically significant, and thus remain of uncertain clinical significance.In seven NATRECOR® clinical trials (1700 patients), the 30-day mortality rate is 5.3% in the NATRECOR®treatment group as compared with 4.3% in the group treated with other standard medications. In four out of these seven clinical trials where it was measured, the 180-day mortality rate is 21.7% in the NATRECOR® treatment group as compared with 21.5% in the group treated with other standard medications. None of these mortality differences reached statistical significance. As described in the revised labeling, “There were few deaths in these studies, so the confidence limits around the hazard ratios for mortality are wide. The studies are also small, so some potentially important baseline imbalances exist among the treatment groups, the effects of which cannot be ascertained.”A copy of the full Prescribing Information for NATRECOR®, including the expanded “Effect on Mortality,” section is attached for your reference.Recent news focused on a meta-analysis published in JAMA regarding the potential risk of mortality associated with the use of NATRECOR®. The same author published another meta-analysis that focused © 2005 Scios Inc. Please see important safety information on reverse side. P0507000on the renal effects of NATRECOR® in the March 29th issue of Circulation. We take seriously the questions raised by the authors.The JAMA meta-analysis is a three-study subset of the original clinical research on NATRECOR®. The authors state that this meta-analysis is hypothesis generating only, and the observed differences were not statistically significant.Eugene Braunwald, MD, distinguished Hersey Professor of Medicine, Harvard Medical School, and Chairman of TIMI Study Group at Brigham and Women’s Hospital in Boston, has agreed, at the company’s request, to convene a panel of external experts to review all available studies and advise the company on our clinical development program. Information will be available following the panel’s review.We remain confident in the safety profile of NATRECOR® and the benefits it offers to patients with ADHF. With the best interests of your patients in mind, we are committed to answering any questions that arise as a result of these recent reports.If you have questions or need additional information, please contact Scios Medical Information at 877-4-NATRECOR (877-462-8732). Additionally, a summary of the 13 company-sponsored nesiritide clinical trials can be accessed at .Sincerely,Darlene P. Horton, MDSenior Vice President, Clinical Research and Medical Affairs/attIMPORTANT SAFETY INFORMATIONNATRECOR® (nesiritide) may cause hypotension. If hypotension occurs during administration of NATRECOR®, the dose should be reduced or discontinued, and blood pressure should be monitored closely. At the recommended dose of NATRECOR®, the incidence of symptomatic hypotension (4%) was similar to that of IV nitroglycerin (5%). Asymptomatic hypotension occurred in 8% of patients treated with either drug. The mean duration of symptomatic hypotension was longer with NATRECOR® than IV nitroglycerin (2.2 versus 0.7 hours, respectively).NATRECOR® may affect renal function in susceptible individuals. In patients with severe heart failure whose renal function may depend on the activity of the renin-angiotensin-aldosterone system, treatment with NATRECOR® may be associated with azotemia. Other adverse events reported at a rate of at least 5% during the first 24 hours of infusion with either NATRECOR® plus standard care or IV nitroglycerin plus standard care therapy, included, respectively: ventricular tachycardia (3%, 5%), nonsustained ventricular tachycardia (3%, 5%), headache (8%, 20%), abdominal pain (1%, 5%), and nausea (4%, 6%).Higher doses of NATRECOR® increased the risk of hypotension and elevated creatinine. NATRECOR® should not be used in patients with systolic blood pressure <90 mm Hg or as primary therapy in patients with cardiogenic shock. NATRECOR® is not recommended for patients for whom vasodilating agents are not appropriate and should be avoided in patients with low cardiac filling pressures.In seven Natrecor clinical trials, at 30 days, 5.3% in the Natrecor treatment group died as compared with 4.3% in the group treated with other standard medications. In four clinical trials, at 180 days, 21.7% in the Natrecor treatment group died as compared with 21.5% in the group treated with other medications. There is not enough information to know if there is an increased risk of death after treatment with Natrecor.Please see accompanying full prescribing information for NATRECOR®.1Lee DS, Austin PC, Rouleau JL et al. Predicting mortality among patients hospitalized for heart failure: derivation and validation of a clinical model. JAMA. 2003;290:2581-2587.race/ethnicity, baseline endogenous hBNP concentration, severity of CHF (as indicated by baseline PCWP , baseline Cl, or New York Heart Association [NYHA] classification), or concomitant administration of an ACE inhibitor.Effects of Concomitant MedicationsThe co-administration of Natrecor with enalapril did not have significant effects on the PK of Natrecor. The PK effect of co-administration of Natrecor with other IV vasodilators such as nitroglycerin, nitroprusside, milrinone, or IV ACE inhibitors has not been evaluated. During clinical studies, Natrecor was administered concomitantly with other medications, including: diuretics,digoxin, oral ACE inhibitors, anticoagulants, oral nitrates, statins, class III antiarrhythmic agents, beta-blockers, dobutamine, calcium channel blockers,angiotensin II receptor antagonists, and dopamine. Although no PKinteractions were specifically assessed, there did not appear to be evidence suggesting any clinically significant PK interaction.PharmacodynamicsThe recommended dosing regimen of Natrecor is a 2 mcg/kg IV bolusfollowed by an intravenous infusion dose of 0.01 mcg/kg/min. With this dosing regimen, 60% of the 3-hour effect on PCWP reduction is achieved within 15minutes after the bolus, reaching 95% of the 3-hour effect within 1 hour.Approximately seventy percent of the 3-hour effect on SBP reduction is reached within 15 minutes. The pharmacodynamic (PD) half-life of the onset and offset of the hemodynamic effect of Natrecor is longer than what the PK half-life of 18 minutes would predict. For example, in patients who developed symptomatic hypotension in the VMAC (Vasodilation in the Management of Acute Congestive Heart Failure) trial, half of the recovery of SBP toward the baseline value after discontinuation or reduction of the dose of Natrecor was observed in about 60 minutes. When higher doses of Natrecor were infused,the duration of hypotension was sometimes several hours. Clinical TrialsNatrecor has been studied in 10 clinical trials including 941 patients with CHF (NYHA class II-III 61%, NYHA class IV 36%; mean age 60 years, women 28%).There were five randomized, multi-center, placebo- or active-controlled studies (comparative agents included nitroglycerin, dobutamine, milrinone,nitroprusside, or dopamine) in which 772 patients with decompensated CHF received continuous infusions of Natrecor at doses ranging from 0.01 to 0.03mcg/kg/min. (See the ADVERSE REACTIONS section for relativefrequency of adverse events at doses ranging from the recommended dose up to 0.03 mcg/kg/min). Of these patients, the majority (n = 541, 70%) received the Natrecor infusion for at least 24 hours; 371 (48%) received Natrecor for 24–48 hours, and 170 (22%) received Natrecor for greater than 48 hours.In controlled trials, Natrecor has been used alone or in conjunction with other standard therapies, including diuretics (79%), digoxin (62%), oral ACE inhibitors (55%), anticoagulants (38%), oral nitrates (32%), statins (18%), class III antiarrhythmic agents (16%), beta-blockers (15%), dobutamine (15%),calcium channel blockers (11%), angiotensin II receptor antagonists (6%),and dopamine (4%). Natrecor has been studied in a broad range of patients,including the elderly (42% >65 years of age), women (30%), minorities (26%black), and patients with a history of significant morbidities such as hypertension (67%), previous myocardial infarction (50%), diabetes (44%),atrial fibrillation/flutter (34%), nonsustained ventricular tachycardia (25%),ventricular tachycardia/fibrillation (12%), preserved systolic function (9%), and acute coronary syndromes less than 7 days before the start of Natrecor (4%).The VMAC (Vasodilation in the Management of Acute Congestive Heart Failure) trial was a randomized, double-blind study of 489 patients (246patients requiring a right heart catheter, 243 patients without a right heart catheter) who required hospitalization for management of shortness of breath at rest due to acutely decompensated CHF. The study compared the effects of Natrecor, placebo, and IV nitroglycerin when added to background therapy (IV and oral diuretics, non-IV cardiac medications, dobutamine, and dopamine). Patients with acute coronary syndrome, preserved systolicfunction, arrhythmia, and renal insufficiency were not excluded. The primary endpoints of the study were the change from baseline in PCWP and the change from baseline in patients’ dyspnea, evaluated after three hours. Close attention was also paid to the occurrence and persistence of hypotension, given nesiritide’s relatively long (compared to nitroglycerin) PK and PD half-life.Natrecor was administered as a 2 mcg/kg bolus over approximately60seconds, followed by a continuous fixed dose infusion of 0.01 mcg/kg/min.After the 3-hour placebo-controlled period, patients receiving placebo crossed over to double-blinded active therapy with either Natrecor or FOR INTRAVENOUS INFUSION ONLYDESCRIPTIONNatrecor ®(nesiritide) is a sterile, purified preparation of a new drug class,human B-type natriuretic peptide (hBNP), and is manufactured from E.coli using recombinant DNA technology.Nesiritide has a molecular weight of 3464 g/mol and an empirical formula of C 143H 244N 50O 42S 4. Nesiritide has thesame 32 amino acid sequence as the endogenous peptide, which is produced by the ventricular myocardium.Natrecor is formulated as the citrate salt of rhBNP , and is provided in asterile, single-use vial. Each 1.5 mg vialcontains a white- to off-white lyophilized powder for intravenous (IV)administration after reconstitution. The quantitative composition of the lyophilized drug per vial is: nesiritide 1.58mg, mannitol 20.0 mg, citric acid monohydrate 2.1 mg, and sodium citrate dihydrate 2.94 mg.Mechanism of ActionHuman BNP binds to the particulate guanylate cyclase receptor of vascular smooth muscle and endothelial cells, leading to increased intracellularconcentrations of guanosine 3'5'-cyclic monophosphate (cGMP) and smooth muscle cell relaxation. Cyclic GMP serves as a second messenger to dilate veins and arteries. Nesiritide has been shown to relax isolated human arterial and venous tissue preparations that were precontracted with either endothelin-1 or the alpha-adrenergic agonist, phenylephrine.In human studies, nesiritide produced dose-dependent reductions inpulmonary capillary wedge pressure (PCWP) and systemic arterial pressure in patients with heart failure.In animals, nesiritide had no effects on cardiac contractility or on measures of cardiac electrophysiology such as atrial and ventricular effective refractory times or atrioventricular node conduction.Naturally occurring atrial natriuretic peptide (ANP), a related peptide, increases vascular permeability in animals and humans and may reduce intravascular volume. The effect of nesiritide on vascular permeability has not been studied.PharmacokineticsIn patients with congestive heart failure (CHF), Natrecor administered intravenously by infusion or bolus exhibits biphasic disposition from the plasma. The mean terminal elimination half-life (t 1/2) of Natrecor isapproximately 18 minutes and was associated with approximately 2/3 of the area-under-the-curve (AUC). The mean initial elimination phase wasestimated to be approximately 2 minutes. In these patients, the mean volume of distribution of the central compartment (Vc) of Natrecor was estimated to be 0.073 L/kg, the mean steady-state volume of distribution (Vss) was0.19L/kg, and the mean clearance (CL) was approximately 9.2 mL/min/kg. At steady state, plasma BNP levels increase from baseline endogenous levels by approximately 3-fold to 6-fold with Natrecor infusion doses ranging from 0.01 to 0.03 mcg/kg/min.EliminationHuman BNP is cleared from the circulation via the following three independent mechanisms, in order of decreasing importance: 1) binding to cell surface clearance receptors with subsequent cellular internalization and lysosomal proteolysis; 2) proteolytic cleavage of the peptide by endopeptidases, such as neutral endopeptidase, which are present on the vascular lumenal surface;and 3) renal filtration.Special PopulationsAlthough Natrecor is eliminated, in part, through renal clearance, clinical data suggest that dose adjustment is not required in patients with renal insufficiency. The effects of Natrecor on PCWP , cardiac index (CI), andsystolic blood pressure (SBP) were not significantly different in patients with chronic renal insufficiency (baseline serum creatinine ranging from 2 mg/dL to 4.3 mg/dL), and patients with normal renal function. The population pharmacokinetic (PK) analyses carried out to determine the effects of demographics and clinical variables on PK parameters showed that clearance of Natrecor is proportional to body weight, supporting theadministration of weight-adjusted dosing of Natrecor (i.e., administration on a mcg/kg/min basis). Clearance was not influenced significantly by age, gender,rhBNPNatrecor ®(nesiritide) for InjectionNatrecor ®(nesiritide) for InjectionOnlynitroglycerin. The nitroglycerin dose was titrated at the physician’s discretion.A subset of patients in the VMAC trial with central hemodynamic monitoring who were treated with Natrecor (62 of 124 patients) were allowed dose increases of Natrecor after the first 3 hours of treatment if the PCWP was ≥20mm Hg and the SBP was ≥100 mm Hg. Dose increases of a 1 mcg/kg bolus followed by an increase of the infusion dose by 0.005 mcg/kg/min were allowed every 3 hours, up to a maximum dose of 0.03 mcg/kg/min. Overall,23patients in this subset had the dose of Natrecor increased in the VMAC trial.In a second double-blind study, 127 patients requiring hospitalization for symptomatic CHF were randomized to placebo or to one of two doses of Natrecor (0.015 mcg/kg/min preceded by an IV bolus of 0.3 mcg/kg, and0.03mcg/kg/min preceded by an IV bolus of 0.6 mcg/kg). The primary endpoint of the trial was the change in PCWP from baseline to 6 hours, but the effect on symptoms also was examined.Effects on SymptomsIn the VMAC study, patients receiving Natrecor reported greater improvement in their dyspnea at 3 hours than patients receiving placebo (p = 0.034).In the dose-response study, patients receiving both doses of Natrecor reported greater improvement in dyspnea at 6 hours than patients receiving placebo.Effects on HemodynamicsThe PCWP , right atrial pressure (RAP), CI, and other hemodynamic variables were monitored in 246 of the patients in the VMAC trial. There was areduction in mean PCWP within 15 minutes of starting the Natrecor infusion,with most of the effect seen at 3 hours being achieved within the first 60minutes of the infusion (see Pharmacodynamics).In several studies, hemodynamic parameters were measured after Natrecor withdrawal. Following discontinuation of Natrecor, PCWP returns to within 10% of baseline within 2 hours, but no rebound increase to levels abovebaseline state was observed. There was also no evidence of tachyphylaxis to the hemodynamic effects of Natrecor in the clinical trials.The following table and graph summarize the changes in the VMAC trial in PCWP and other measures during the first 3 hours.The VMAC study does not constitute an adequate effectiveness comparison with nitroglycerin. In this trial, the nitroglycerin group provides a rough landmark using a familiar therapy and regimen.Effect on Urine Output In the VMAC trial, in which the use of diuretics was not restricted, the mean change in volume status (output minus input) during the first 24 hours in the nitroglycerin and Natrecor groups was similar: 1279 ±1455 mL and 1257±1657 mL,respectively.INDICATIONS AND USAGENatrecor (nesiritide) is indicated for the intravenous treatment of patients with acutely decompensated congestive heart failure who have dyspnea at rest or with minimal activity. In this population, the use of Natrecor reduced pulmonary capillary wedge pressure and improved dyspnea.CONTRAINDICATIONSNatrecor is contraindicated in patients who are hypersensitive to any of its components. Natrecor should not be used as primary therapy for patients with cardiogenic shock or in patients with a systolic blood pressure <90 mm Hg.WARNINGSAdministration of Natrecor should be avoided in patients suspected of having,or known to have, low cardiac filling pressures.PRECAUTIONSGeneral:Parenteral administration of protein pharmaceuticals or E . coli-derived products should be attended by appropriate precautions in case of an allergic or untoward reaction. No serious allergic or anaphylactic reactions have been reported with Natrecor.Natrecor is not recommended for patients for whom vasodilating agents are not appropriate, such as patients with significant valvular stenosis, restrictive or obstructive cardiomyopathy, constrictive pericarditis, pericardialtamponade, or other conditions in which cardiac output is dependent upon venous return, or for patients suspected to have low cardiac filling pressures.(See CONTRAINDICATIONS.)Renal:Natrecor may affect renal function in susceptible individuals. In patients with severe heart failure whose renal function may depend on the activity of the renin-angiotensin-aldosterone system, treatment with Natrecor may be associated with azotemia. When Natrecor was initiated at doses higher than 0.01 mcg/kg/min (0.015 and 0.03 mcg/kg/min), there was an increased rate of elevated serum creatinine over baseline compared with standard therapies, although the rate of acute renal failure and need for dialysis was not increased. In the 30-day follow-up period in the VMAC trial,5patients in the nitroglycerin group (2%) and 9 patients in the Natrecor group (3%) required first-time dialysis.Cardiovascular:Natrecor may cause hypotension. In the VMAC trial, in patients given the recommended dose (2 mcg/kg bolus followed by a 0.01mcg/kg/min infusion) or the adjustable dose, the incidence of symptomatic hypotension in the first 24 hours was similar for Natrecor (4%)and IV nitroglycerin (5%). When hypotension occurred, however, the durationof symptomatic hypotension was longer with Natrecor (mean duration was2.2 hours) than with nitroglycerin (mean duration was 0.7 hours). In earlier trials, when Natrecor was initiated at doses higher than the 2 mcg/kg bolusfollowed by a 0.01 mcg/kg/min infusion (i.e., 0.015 and 0.03 mcg/kg/minpreceded by a small bolus), there were more hypotensive episodes and theseepisodes were of greater intensity and duration. They were also more often symptomatic and/or more likely to require medical intervention (see ADVERSE REACTIONS). Natrecor should be administered only in settings where blood pressure can be monitored closely, and the dose of Natrecor should be reduced or the drug discontinued in patients who develop hypotension (see Dosing Instructions). The rate of symptomatic hypotension may be increased in patients with a blood pressure <100 mm Hg at baseline, and Natrecor should be used cautiously in these patients. The potential for hypotensionmay be increased by combining Natrecor with other drugs that may causehypotension. For example, in the VMAC trial in patients treated with either Natrecor or nitroglycerin therapy, the frequency of symptomatic hypotension in patients who received an oral ACE inhibitor was 6%, compared to a frequency of symptomatic hypotension of 1% in patients who did not receive an oral ACE inhibitor.Drug Interactions:No trials specifically examining potential drug interactions with Natrecor were conducted, although many concomitant drugs were usedin clinical trials. No drug interactions were detected except for an increase insymptomatic hypotension in patients receiving oral ACE inhibitors (seePRECAUTIONS, Cardiovascular).The co-administration of Natrecor with IV vasodilators such as nitroglycerin,nitroprusside, milrinone, or IV ACE inhibitors has not been evaluated (thesedrugs were not co-administered with Natrecor in clinical trials).Carcinogenesis, Mutagenesis, Impairment of Fertility:Long-term studies inanimals have not been performed to evaluate the carcinogenic potential or the effect on fertility of nesiritide. Nesiritide did not increase the frequency of mutations when used in an in vitro bacterial cell assay (Ames test). No other genotoxicity studies were performed.Pregnancy: Category C:Animal developmental and reproductive toxicity studies have not been conducted with nesiritide. It is also not known whether Natrecor can cause fetal harm when administered to pregnant women or can affect reproductive capacity. Natrecor should be used during pregnancy onlyif the potential benefit justifies any possible risk to the fetus.Mean Hemodynamic Change from BaselinePlacebo Nitroglycerin NatrecorEffects at 3 Hours (n = 62) (n = 60) (n = 124)Pulmonary capillary wedge pressure (mm Hg) -2.0 -3.8 -5.8‡Right atrial pressure (mm Hg) 0.0 -2.6 -3.1‡Cardiac index (L/min/M 2) 0.0 0.2 0.1Mean pulmonary artery pressure (mm Hg) -1.1 -2.5 -5.4‡Systemic vascular resistance (dynes*sec*cm -5) -44 -105 -144Systolic blood pressure † (mm Hg) -2.5 -5.7‡ -5.6‡†Based on all treated subjects: placebo n = 142, nitroglycerin n = 143, Natrecor n = 204‡p<0.05 compared to placeboPCWP through 3 Hours#p<0.05 compa r ed to placebo Time Since Start of Study Drug M e a n C h a n g e (m m H g )Effect on MortalityData from all seven studies in which 30-day data were collected arepresented in the chart below. The data depict hazard ratios and confidence intervals of mortality data for randomized and treated patients with Natrecor ®relative to active controls through day 30 for each of the 7 individual studies (Studies 311, 325, 326, 329 [PRECEDENT], 339 [VMAC], 341 [PROACTION], and 348 [FUSION I]).The figure (on logarithmic scale) also contains a plot for the six studies involving hospitalized or Emergency Department patients combined(n =1507), and for all 7 studies combined (n =1717). The percentage is the Kaplan-Meier estimate.The figure below represents 180-day mortality hazard ratios for randomized and treated patients from all four individual studies where 180-day data were collected, 16 week hazard ratios for Study 348 (180-day data were not collected), and the four studies with 180-day data pooled (n =1167).There were few deaths in these studies, so the confidence limits around the hazard ratios for mortality are wide. The studies are also small, so some potentially important baseline imbalances exist among the treatment groups,the effects of which cannot be ascertained.OVERDOSAGENo data are available with respect to overdosage in humans. The expected reaction would be excessive hypotension, which should be treated with drug discontinuation or reduction (see PRECAUTIONS) and appropriate measures.DOSAGE AND ADMINISTRATIONThe Natrecor bolus must be drawn from the prepared infusion bag.Natrecor (nesiritide) is for intravenous use only. There is limited experience with administering Natrecor for longer than 48 hours. Blood pressure should be monitored closely during Natrecor administration.If hypotension occurs during the administration of Natrecor, the dose should be reduced or discontinued and other measures to support blood pressure should be started (IV fluids, changes in body position). In the VMAC trial,when symptomatic hypotension occurred, Natrecor was discontinued and subsequently could be restarted at a dose that was reduced by 30% (with no bolus administration) once the patient was stabilized. Because hypotension caused by Natrecor may be prolonged (up to hours), a period of observation may be necessary before restarting the drug.19/85 (23.1%)42/203 (20.8%)26/163 (16.3%)67/273 (25.1%)13/141 (9.4%)154/724 (21.7%)Nat r eco r ®N (Pe r ce n tage)Pooled (4 S tudies)8/42 (19.3%)24/102 (23.5%)18/83 (22.2%)44/216 (20.8%)9/69 (13.5%)94/443 (21.5%)Co n t r ol N (Pe r ce n tage)Haza r d Ratio (95% C onfiden c e Inte r val)180-Day Haza r d Ratios*Data c olle c ted th r ough week 16†S tudies 704.325, 704.326, 704.329, and 704.339Nat r eco r ®N (Pe r ce n tage)Pooled (6 S Pooled (7 S tudies)2/29 (7.5%)2/42 (4.8%)5/102 (4.9%)5/83 (6.1%)11/216 (5.1%)1/117 (0.9%)2/69 (2.9%)26/589 (4.4%)28/658 (4.3%)Co n t r olN (Pe r ce n tage)Haza r d Ratio (95% C onfiden c e Inte r val)30-Day Haza r d Ratios0.1110*S tudies 704.311, 704.325, 704.326, 704.329, 704.339, and 704.341†S tudies 704.311, 704.325, 704.326, 704.329, 704.339, 704.341, and 704.348Nursing Mothers:It is not known whether this drug is excreted in human milk.Therefore, caution should be exercised when Natrecor is administered to a nursing woman.Pediatric Use:The safety and effectiveness of Natrecor in pediatric patients has not been established.Geriatric Use:Of the total number of subjects in clinical trials treated with Natrecor (n = 941), 38% were 65 years or older and 16% were 75 years or older. No overall differences in effectiveness were observed between these subjects and younger subjects, and other reported clinical experience has not identified differences in responses between the elderly and younger patients. Some older individuals may be more sensitive to the effect of Natrecor than younger individuals.ADVERSE REACTIONSAdverse events that occurred with at least a 3% frequency during the first 24hours of Natrecor infusion are shown in the following table.Adverse events that are not listed in the above table that occurred in at least 1% of patients who received any of the above Natrecor doses included:Tachycardia, atrial fibrillation, AV node conduction abnormalities, catheter pain, fever, injection site reaction, confusion, paresthesia, somnolence,tremor, increased cough, hemoptysis, apnea, increased creatinine, sweating,pruritus, rash, leg cramps, amblyopia, anemia. All reported events (at least 1%) are included except those already listed, those too general to be informative, and those not reasonably associated with the use of the drug because they were associated with the condition being treated or are very common in the treated population.In placebo and active-controlled clinical trials, Natrecor has not been associated with an increase in atrial or ventricular tachyarrhythmias. In placebo-controlled trials, the incidence of VT in both Natrecor and placebo patients was 2%. In the PRECEDENT (Prospective Randomized Evaluation of Cardiac Ectopy with Dobutamine or Natrecor Therapy) trial, the effects of Natrecor (n = 163) and dobutamine (n = 83) on the provocation or aggravation of existing ventricular arrhythmias in patients with decompensated CHF was compared using Holter monitoring. Treatment with Natrecor (0.015 and 0.03mcg/kg/min without an initial bolus) for 24 hours did not aggravate pre-existing VT or the frequency of premature ventricular beats, compared to a baseline 24-hour Holter tape.Clinical LaboratoryIn the PRECEDENT trial, the incidence of elevations in serum creatinine to >0.5 mg/dL above baseline through day 14 was higher in the Natrecor0.015mcg/kg/min group (17%) and the Natrecor 0.03 mcg/kg/min group (19%)than with standard therapy (11%). In the VMAC trial, through day 30, the incidence of elevations in creatinine to >0.5 mg/dL above baseline was 28%and 21% in the Natrecor (2 mcg/kg bolus followed by 0.01 mcg/kg/min) and nitroglycerin groups, respectively.VMAC TrialOther Long Infusion TrialsNatrecor Natrecor mcg/kg/minRecommendedNitroglycerin Dose Control*0.0150.03Adverse Events(n = 216)(n = 273)(n = 256)(n = 253)(n = 246)Cardiovascular Hypotension 25 (12%)31 (11%)20 (8%)56 (22%)87 (35%)Symptomatic Hypotension 10 (5%)12 (4%)8 (3%)28 (11%)42 (17%)Asymptomatic Hypotension 17 (8%)23 (8%)13 (5%)31 (12%)49 (20%)Ventricular Tachycardia (VT)11 (5%)9 (3%)25 (10%)25 (10%)10 (4%)Non-sustained VT 11 (5%)9 (3%)23 (9%)24 (9%)9 (4%)Ventricular Extrasystoles 2 (1%)7 (3%)15 (6%)10 (4%)9 (4%)Angina Pectoris 5 (2%) 5 (2%) 6 (2%)14 (6%) 6 (2%)Bradycardia1 (<1%)3 (1%)1 (<1%)8 (3%)13 (5%)Body as a Whole Headache 44 (20%)21 (8%)23 (9%)23 (9%)17 (7%)Abdominal Pain 11 (5%) 4 (1%)10 (4%) 6 (2%)8 (3%)Back Pain7 (3%)10 (4%)4 (2%)5 (2%)3 (1%)Nervous Insomnia 9 (4%) 6 (2%)7 (3%)15 (6%)15 (6%)Dizziness 4 (2%)7 (3%)7 (3%)16 (6%)12 (5%)Anxiety6 (3%)8 (3%)2 (1%)8 (3%)4 (2%)Digestive Nausea 13 (6%)10 (4%)12 (5%)24 (9%)33 (13%)Vomiting4 (2%)4 (1%)2 (1%)6 (2%)10 (4%)*Includes dobutamine, milrinone, nitroglycerin, placebo, dopamine, nitroprusside, or amrinone.。
GL-1 HCP Injector Models说明书
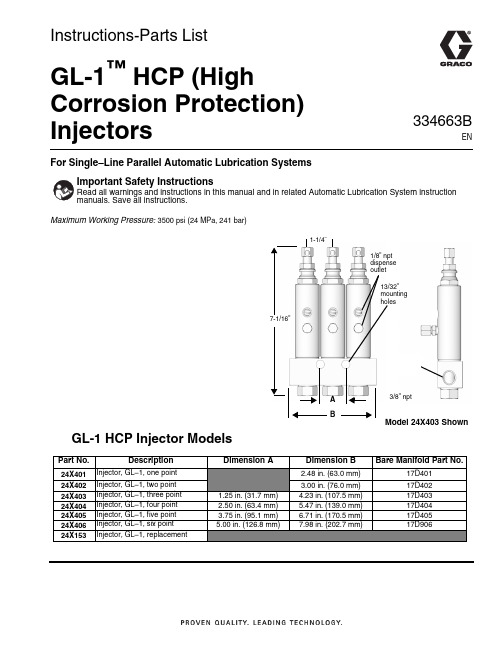
For Single–Line Parallel Automatic Lubrication SystemsMaximum Working Pressure: 3500 psi (24 MPa, 241 bar)GL-1 HCP Injector ModelsImportant Safety InstructionsRead all warnings and instructions in this manual and in related Automatic Lubrication System instruction manuals. Save all instructions.1-1/4”7-1/16”A B1/8” npt dispense outlet13/32”mounting holes3/8” nptModel 24X403 ShownPart No.DescriptionDimension A Dimension BBare Manifold Part No.24X401Injector, GL–1, one point 2.48 in. (63.0 mm)17D40124X402Injector, GL–1, two point 3.00 in. (76.0 mm)17D40224X403Injector, GL–1, three point 1.25 in. (31.7 mm) 4.23 in. (107.5 mm)17D40324X404Injector, GL–1, four point 2.50 in. (63.4 mm) 5.47 in. (139.0 mm)17D40424X405Injector, GL–1, five point 3.75 in. (95.1 mm) 6.71 in. (170.5 mm)17D40524X406Injector, GL–1, six point 5.00 in. (126.8 mm)7.98 in. (202.7 mm)17D90624X153Injector, GL–1, replacementInstructions-Parts ListGL-1™HCP (HighCorrosion Protection) Injectors334663BENWarningsWarningsThe following warnings are for the setup, use, grounding, maintenance, and repair of this equipment. The exclamation point symbol alerts you to a general warning and the hazard symbols refer to procedure-specific risks. When these symbols appear in the body of this manual or on warning labels, refer back to these Warnings. Product-specific hazard symbols and warnings not covered in this section may appear throughout the body of this manual where applicable.2334663BWarnings334663B 3Installation InstructionsReference letters used in the following instructions, refer to F IG . 1.•Group injectors to minimize feed line length.•Install injectors in locations that allow easy and safe servicing access.•Install injectors in areas that minimize accidental injector damage by moving equipment.•Injector outputs can be combined for a common bearing point with a large grease requirement but the output for a single injector cannot be split into multiple bearing points.•Graco recommends using steel tubing instead of pipe and hose for supply lines when possible. Pipe is often contaminated with scale and requires proper cleaning prior to use. Hose lines expand under pressure which leads to longer pump cycle time.NOTE: The equipment may be pressurized by an auto-matic lube cycle initiated by a lubrication controller (timer).1.Before installing the injectors, disconnect the powersupplies to the lubrication controller and to the pump.2.Relieve pump pressure. See the pressure relief pro-cedure provided in the pump manual for your sys-tem. 3.Install injectors on a flat, hard surface using holes(a) (F IG . 1) in manifold.4.Connect fluid supply line to injectors.5.Connect lube point feed lines (b).6.Flush the system with low viscosity oil or mineralspirits to remove contamination introduced during e a purge gun or run the pump until clean lubri-cant is dispensed at the end of each feed line to purge the system of flushing fluid or air.8.Run the system at full output and verify that all injec-tors are cycling.9.Adjust injector volume output. (See Volume Adjust-ment page 5.)10.Connect feed lines to lubrication points.F IG. 1aabInjector Parts4334663BInjector PartsModel 24X401 ShownAvailable KitsUse Only Genuine Graco Repair PartsGraco Part No. 17L754 .Clear Polycarbonate Injector Cover (see page 3 for installation instructions)Graco Part No. 128139 . . . . . . . . . . . . .Crossover Kit(for connecting the outlets of injectorsfor increased output)Injector Cover Kit 17L754Installation Instructions1.Apply a light coating of transparent lubricant to theinside of cap (21).2.Slide the o-ring (22) down over the indicator stem tothe groove in the piston plug.3.Slide cap (21) over the indicator stem of the injectorfar enough to cover the groove in the piston plug.Item DescriptionQty 1Injector body 12Adjusting screw 13Lock nut15Zerk fitting and cap assembly 16Gasket17Adapter bolt 18Indicator pin 19Gasket22916Manifold97531Torque to 50-55 ft-lbs (68-74.5 N.m)18Item DescriptionQty 2SCREW, adjusting 13NUT, lock 121CAP 122O-RING12122groove23334663B 5Volume Adjustment*Maximum adjustment setting is when adjusting screw (2) is just making contact with the indicator pin (8) with no inlet pressure. Turn adjusting screw clockwise (in), to reduce output. To adjust, loosen lock nut (3) and turn adjusting screw (2) the number of turns indicated on the GL-1 Volume Adjustment Table to obtain the desired volume. Tighten lock nut (3) when desired volume setting is reached .Technical DataGL-1 HCP Volume Adjustment Table DescriptionNumber of TurnsVolumein.3cc Maximum Adjustment*00.080 1.31360° Clockwise Turn 10.071 1.16360° Clockwise Turn 20.062 1.02360° Clockwise Turn 30.0530.87360° Clockwise Turn 40.0440.72360° Clockwise Turn 50.0350.57360° Clockwise Turn 60.0260.43360° Clockwise Turn 70.0170.28Minimum Adjustment80.0080.13Maximum operating pressure 3500 psi (24 MPa, 241 bar)Suggested operating pressure 2500 psi (17 MPa, 172 bar)Reset pressure600 psi (4.1 MPa, 41 bar)Output volume per cycle adjustable*: 0.008 to 0.08 in.3Wetted parts carbon steel, stainless steel, fluoroelastomer Recommended fluidsN.L.G.I. #2 grease down to 32° F (0° C)All written and visual data contained in this document reflects the latest product information available at the time of publication.Graco reserves the right to make changes at any time without notice.Original instructions. This manual contains English. MM 334663Graco Headquarters: MinneapolisInternational Offices: Belgium, China, Japan, KoreaGRACO INC. AND SUBSIDIARIES • P.O. BOX 1441 • MINNEAPOLIS MN 55440-1441 • USA Copyright 2015, Graco Inc. All Graco manufacturing locations are registered to ISO 9001. November 2017Graco Standard WarrantyGraco warrants all equipment referenced in this document which is manufactured by Graco and bearing its name to be free from defects in material and workmanship on the date of sale to the original purchaser for use. With the exception of any special, extended, or limited warranty published by Graco, Graco will, for a period of twelve months from the date of sale, repair or replace any part of the equipment determined by Graco to be defective. This warranty applies only when the equipment is installed, operated and maintained in accordance with Graco’s written recommendations.This warranty does not cover, and Graco shall not be liable for general wear and tear, or any malfunction, damage or wear caused by faulty installation, misapplication, abrasion, corrosion, inadequate or improper maintenance, negligence, accident, tampering, or substitution ofnon-Graco component parts. Nor shall Graco be liable for malfunction, damage or wear caused by the incompatibility of Graco equipment with structures, accessories, equipment or materials not supplied by Graco, or the improper design, manufacture, installation, operation or maintenance of structures, accessories, equipment or materials not supplied by Graco.This warranty is conditioned upon the prepaid return of the equipment claimed to be defective to an authorized Graco distributor for verification of the claimed defect. If the claimed defect is verified, Graco will repair or replace free of charge any defective parts. The equipment will be returned to the original purchaser transportation prepaid. If inspection of the equipment does not disclose any defect in material or workmanship, repairs will be made at a reasonable charge, which charges may include the costs of parts, labor, and transportation.THIS WARRANTY IS EXCLUSIVE, AND IS IN LIEU OF ANY OTHER WARRANTIES, EXPRESS OR IMPLIED, INCLUDING BUT NOT LIMITED TO WARRANTY OF MERCHANTABILITY OR WARRANTY OF FITNESS FOR A PARTICULAR PURPOSE .Graco’s sole obligation and buyer’s sole remedy for any breach of warranty shall be as set forth above. The buyer agrees that no other remedy (including, but not limited to, incidental or consequential damages for lost profits, lost sales, injury to person or property, or any other incidental or consequential loss) shall be available. Any action for breach of warranty must be brought within two (2) years of the date of sale.GRACO MAKES NO WARRANTY, AND DISCLAIMS ALL IMPLIED WARRANTIES OF MERCHANTABILITY AND FITNESS FOR A PARTICULAR PURPOSE, IN CONNECTION WITH ACCESSORIES, EQUIPMENT, MATERIALS OR COMPONENTS SOLD BUT NOTMANUFACTURED BY GRACO . These items sold, but not manufactured by Graco (such as electric motors, switches, hose, etc.), are subject to the warranty, if any, of their manufacturer. Graco will provide purchaser with reasonable assistance in making any claim for breach of these warranties.In no event will Graco be liable for indirect, incidental, special or consequential damages resulting from Graco supplying equipment hereunder, or the furnishing, performance, or use of any products or other goods sold hereto, whether due to a breach of contract, breach of warranty, the negligence of Graco, or otherwise.FOR GRACO CANADA CUSTOMERSThe Parties acknowledge that they have required that the present document, as well as all documents, notices and legal proceedings entered into, given or instituted pursuant hereto or relating directly or indirectly hereto, be drawn up in English. Les parties reconnaissent avoir convenu que la rédaction du présente document sera en Anglais, ainsi que tous documents, avis et procédures judiciaires exécutés, donnés ou intentés, à la suite de ou en rapport, directement ou indirectement, avec les procédures concernées.Graco InformationFor the latest information about Graco products, visit .For patent information, see /patents .TO PLACE AN ORDER, contact your Graco distributor or call to identify the nearest distributor.Phone: 612-623-6928 or Toll Free: 1-800-533-9655, Fax: 612-378-3590。
HCP8000 系列高频电流探头说明书

深圳市知用电子有限公司高频电流探头HCP8000系列HCP8030 30A/DC ~ 50 MHz HCP8030D 30A/DC ~100 MHz HCP8050 50A/DC ~ 50 MHz HCP8150 150A/DC ~12 MHz HCP8300 300A/DC ~ 6 MHz HCP8500 500A/DC ~ 5 MHz深圳市知用电子有限公司CY BE R TE K前 言首先,感谢您购买该产品。
为了你安全正确地使用本产品,请先仔细阅读说明书。
这份产品使用说明书是关于该产品的功能、使用方法、操作注意事项等方面的介绍。
说明书中,注释将用以下的符号进行区分。
该符号表示对人体和机器有危害,必须参照说明书操作。
在错误操作的情况下,用户有受伤的威胁,为避免此类危险,记载了相关的注意事项。
错误操作时,用户有受轻伤和物质损害的可能,为避免此类情况,记载的注意事项。
记载着使用该机器时的重要说明。
为安全使用本机器必须严格遵守以下安全注意事项。
如果不按照该说明书使用的话,有可能会损害机器的保护功能。
此外,因违反注意事项进行操作所产生的问题,本公司概不负责。
◆ 为避免短路及人身事故,被测电路要求300V 以下。
◆ 请避免接触裸导体。
因为核心和屏蔽盖没有绝缘,有危险。
◆ 测量时请不要接触被测导体和传感器头。
◆ 连接本机器的输出端子BNC 的示波器,也请使用带有保护接地的双重绝缘结构。
◆ 当示波器连接其它测试终端时,该测试终端会因为连接其他输入部分,使得本机器的连接端子和内部线路变成某种隐患,此时必须注意以下几点:✧ 连接本机器的测试终端和其他测试终端间,使用带有符合过电压范畴及污染度的基础绝缘设备。
✧ 若测试终端的基本绝缘无法满足的话,请不要输入超出安全的电压。
✧ 请参照连接电器的触电等安全性相关的注意事项,进行使用。
◆ 机器潮湿,或用湿手测定的话,会发生触电事故,请注意。
Y BE R TE K◆ 搬运和操作时,避免振动、冲击。
HCP-4050X_NET_CHI
7. 其它功能 ......................................................................................... 56
7.1 7.2 7.3 7.4 7.5 电子邮件警报 ................................................. 使用 SNMP 进行投影机管理 ..................................... 日程安排 . .................................................... 通过网络进行命令控制 ......................................... .......................................... Crestron RoomView® . 56 58 59 62 67
投影机
HCP-4050X
使用说明书 网络指南
承蒙您购买本产品,谨向您表示衷心的感谢。 本说明书仅对网络功能进行说明。请参阅本说明书及本产品的其他说明书,了解本 产品的正确使用方法。 警告 ► 使用本产品前,务必阅读本产品的所有说明书。 阅读后,请妥善保管以备日后参考。
功能
本投影机具有下主要的网络功能。 ü ü ü ü ü 网络演示 : 可以投射通过网络传输的电脑图像。(&15) 络控制 : 网 可通过网络监控投影机。(&16) 我的图像 : 可存储并投射最多 4 张静态图像。(&50) 信差 : 可显示通过网络发送的文本。(&51) 网络桥 : 可通过投影机控制外部设备。(&52)
1.1 系统要求 . ..................................................... 5
hcp说明书1

机械制造技术基础课程设计说明书设计题目设计“传动箱壳体”零件的机械加工工艺及工艺设备设计者胡春鹏指导教师黑龙江东方学院年月日目录设计任务书一.零件的分析--------------------------------- 3二.工艺过程设计------------------------------- 3(一)确定毛坯的制造形式---------------------3 (二)基面的选择---------------------------- 3 (三)制订工艺路线-------------------------- 4 (四)机械加工余量、工序尺寸及毛坯尺寸的确定-8 (五)确定切削用量及基本工时-----------------10 三.夹具设计------------------------------------14定位分析----------------------------------14夹紧装置设计------------------------------15夹具的平衡设计----------------------------15公差确定----------------------------------16夹紧力分析--------------------------------16 四.参考文献------------------------------------17 附录相关机械图1零件的分析1.1零件的作用题目所给零件是CA6140车床的上刀架,它位于车床床身的上半部,与夹持刀具的分刀架相连,通过一系列的构件相互作用实现刀具的快速更换。
零件上部有Φ82凸圆,用以安装方刀架并将其固定。
1.2零件的工艺分析上刀架属于车床床身上比较精密的部件,所以有一系列的形状与位置要求,现分析如下:1.上表面A对下表面的平行度公差为0.02㎜2.Φ82H7对上表面A的垂直度公差为0.02㎜2工艺规程设计2.1确定毛胚的制造形式零件材料为HT200考虑到车床在使用过程中零件的冲击载荷比较稳定;选用铸件即可满足使用要求;且零件为CA6140车床标准件,符合大批生产要求。
Hitachi Content Platform (HCP) 产品说明书

• • • • • DATA SHEE TUnstructured data growth and application proliferation continue to accelerate. These developments lead to increased server and storage sprawl, with numerous silos of infrastructure supporting traditional and new workloads.Hitachi Content Platform (HCP) is an object storage software solution that connects data producers, users, applications and devices into a central cloud storage plat-form. It enables users to better understand, govern and control the degree of mobility of their data, as well as to identify insights and extract value for data-driven decisions and faster time to market.HCP lets IT organizations and cloud service providers securely and cost-effectively store, share, protect, preserve and analyze data. Beyond its efficiency, ease of use and ability to store data at massive scale, HCP auto-mates day-to-day IT operations like data governance and protection. This approach readily evolves to changes in scale, scope, regulatory compliance, applications, storage, server and cloud technologies over the life of data. HCP also automates the governance of data to ensure proper retention, access control, encryption and disposal of data, while simplifying e-discovery and search. In IT environments where data grows quickly or must live for years or even indefinitely, these capabilities are invaluable.Hitachi Content Platform eliminates the need for a siloed approach to storing unstructured content. The platform provides massive scale, multiple storage tiers, powerfulHitachi Content Platform: Enterprise-Class, Backup-Free Cloud and Archivesecurity, Hitachi reliability, cloud capabilities, broad protocol support, multitenancy and configurable attributes for each tenant. It can support a wide range of applications on a single physical cluster and is backed by a thriving community of third-party software partners. With access to a robust ecosys-tem of cloud applications, Hitachi Content Platform can solve a wide range of current problems and adapt to meet future needs.Flexible, Enterprise-Class CloudHitachi Content Platform multitenancy divides the physical cluster into a variety of tenants. These tenants can be assigned to different IP networks and further subdivided into thousands of namespaces for additional organization of content, more refined poli-cies, and robust access control. Openness is also a hallmark of HCP . It has powerful native REST and Amazon Simple Storage Service (S3) based interfaces, permitting seamless WAN or LAN access for new and existing Web 2.0 and mobile applications. Further, it supports the NFS, SMB, SMTP and WebDAV protocols, and offers dual-stack support for IPV4 and IPV6.The platform can handle all kinds of data and almost any application. It offers high reliability, massive scale, seamless data mobility and storage across private clouds and public cloud services, encryption, access control, easy provisioning, charge-back measurement and more. The HCP G series access nodes allow organizations greater flexibility to support mixed work-loads with varying performance and scalerequirements. These nodes virtualize capac-ity from Hitachi Content Platform S series nodes, local drives, Fibre Channel storage arrays, NFS shares and leading public cloud providers. HCP drastically reduces total cost of ownership and provides cost-effective storage with geographically dispersed era-sure coding data protection for content that must remain behind the firewall. Such attri-butes enable IT to take advantage of cloud and deliver a whole new range of IT services, without compromising security and control of information.Efficient, Backup-Free ArchiveHitachi Content Platform enables your IT organization to protect, preserve and retrieve data in a more efficient manner, without the need for tape-based backups. The high density of HCP storage is enhanced with built-in compression, single instancing and support for a variety of media to keep storage costs in control. With dynamic data protection, data integrity checks, data reten-tion enforcement, erasure coding and many other technologies to preserve and protect content, HCP delivers compliance-quality data protection. It eliminates the need for tape-based backups.Intelligent Structure for Unstructured File DataHitachi Content Platform enables trusted content mobility with full visibility of all the control points where data enters, exits and exists across a global ITlandscape. It optimizes cost by providingWith HCP , you have access to metadata and content search tools that enable more elegant and automated queries for faster, more accu-rate results. Through these features you can gain a better understanding of the content of stored files, how content is used and how objects may be related to one another. This understanding can help you to enable more intelligent automation, along with big data analytics based on best-in-class metadata architecture.Hitachi Content Platform provides morecapabilities, flexibility, configurability and input options for you to take advantage of cloud in your own way. It simplifies management via automation to ensure efficiency, reliability, data mobility and accessibility of your organizations’ data. With HCP you can not only address today’s challenges around storing and protect-ing data, but also set yourself up for the next big thing.provide erasure coding to deliver long-term compliance and protection at the lowest cost. There’s no need to learn any new datamovement procedures or processes. Using the HCP interface, you can orchestrateseamless and policy-based data movement to the HCP S series nodes to optimize agility and efficiency.HCP for Cloud ScaleHitachi Content Platform for cloud scale is designed for applications requiring hyperscale, high-performance and comprehensive S3 API compliance. Its novel microservicedesign enables massive scalability to support hundreds of nodes and trillions of objects, and also features rich, policy-driven management and data enrichment tools. Its globalnamespace allows for unified management across multiple on-premises and cloud deployments. Offering broad flexibility, HCP for cloud scale’s architecture is hardware agnostic. It can be deployed on “white box” servers, leading cloud platforms such as Amazon Elastic Compute Cloud (EC2), or Hitachi Unified Compute Platform using a variety of Linux distributions. HCP S series nodes provide ideal on-premises storage for HCP for cloud scale. HCP for cloud scale also supports any S3 storage endpoint, including Amazon ECS.the flexibility to maintain your critical data securely, on-premises or in public clouds. It automatically moves content based onbusiness value or your storage-related service level agreement to the most appropriate storage tier. For example, content can be moved to Hitachi Content Platform S series nodes or cloud storage services, including Amazon S3, Microsoft Azure, Google Cloud Storage or any other S3-enabled cloud.As shown in Figure 1:●●HCP G series nodes are access nodes.●●HCP S series nodes are optional and pro-vide massive scale.●●Fibre Channel storage and cloud are optional.Performance and Scale To Power Next-Gen Hybrid Data CentersHCP S Series NodesHitachi Content Platform S series nodes provide economical deep data storage and protection in a small footprint. They seamlessly extend your private or hybrid cloud and offload infrequently accessed content from valuable primary storage. These nodes deliver the scale and economics of the public cloud locally with large capacity drives and always-on, self-optimization processes to maintain data integrity, availability and durability. They alsoFigure 1. Flexibility of Hitachi Content PlatformVM = virtual machine, RDM = remote device management, KVM = kernel-based virtual machine, EB = exabyte All capacities raw. Usable capacity will vary based on data protection choices.TABLE 1. HITACHI CONTENT PLATFORM OFFERINGSHITACHI is a registered trademark of Hitachi, Ltd. Microsoft and Azure are trademarks or registered trademarks of Microsoft Corporation. All other trademarks, service marks and company names are properties of their respective owners.DS-125-U BTD March 2020Hitachi VantaraCorporate Headquarters 2535 Augustine DriveSanta Clara, CA 95054 USA | Contact Information USA: 1-800-446-0744Global: /contactHITACHI CONTENT PLATFORM IMPLEMENTATION SERVICEHitachi Vantara provides consulting, implementation, migration and replication services to help you bring the benefits of Hitachi content solutions to your business-driven IT environment. In this implementation service, we can help you address data growth challenges, manage unstructured data throughout its life cycle, enable mobility, cloud and converged infrastructure strategies, and harness the value of your data with sophisticated search and analytics. Complementary services include Hitachi Content Platform Replication Service and Hitachi Content Platform Migration Service.SSD = solid state disk, GbE = gigabit Ethernet, HDD = hard disk drive, RU = rack unit All capacities raw. Usable capacity will vary based on data protection choices. 1Total for both controllersTABLE 2. HITACHI CONTENT PLATFORM HARDWAREWe Are Hitachi VantaraWe guide our customers from what’s now to what’s next by solving their digital challenges. Working alongside each customer, we apply our unmatched industrial and digital capabilities to their data and applications to benefit both business and society.。
弗莱马斯特 HCP 保温柜操作手册说明书

Frymaster 是商业食品设备服务协会 (Commercial Food Equipment Service Association, CFESA) 的成员,推荐使用 CFESA 认证技师。
价格:$6.00*8196437*24 小时服务热线1-800-551-8633819-64374 月 8 日CHINESEHoldMaster ® HCP安装与操作手册由基于 Palm 的掌上电脑来操控的 2 层和 4 层型号注意在保修期内,如果顾客在此 Enodis 设备中使用除直接从 Frymaster/Dean 或任意其授权服务中心购买的未经改造的新的或再循环零部件以外的零部件,和/或所用零部件的原始结构遭到改造,本保修将无效。
此外,对于直接或间接、全部或部分由于安装任何改造零部件和/或未经授权的服务中心提供的零部件而产生的任何顾客索赔、损坏或费用,Frymaster/Dean 及其附属机构概不负责。
HCP 不适于户外使用。
操作此设备时,必须将其置于水平面上。
HCP 不适合安装在可以使用喷水的地方。
决不可用喷水来清洗此设备。
安全起见请勿在本设备或任何其他设备附近存储或使用汽油或其他易燃蒸汽或液体。
未阅读本手册前请勿操作或维修 HCP。
除非它安装正确并经过检查,否则请勿操作 HCP。
除非所有的维护和通道面板都到位并被适当保护着,否则请勿操作 HCP。
除非该设备的所有电源已经断开,否则请勿试图修理或替换 HCP 的任何组件。
安装、操作或清洗 HCP 时请多加小心,避免接触到加热的表面。
目录安装、保修和零部件 1-1HCP 软件快速入门 2-1 加电 3-1操作概述 4-1 编程概述 4-4输入密码添加菜单条目5-1编程 6-1 从 4 层更改到 2 层操作 6-2 将产品从食品库移动到菜单 6-2 在 Palm 掌上电脑上设置保温柜 6-2保存保温柜 7-1 加载保温柜 7-2温度显示8-1上传保温柜配置和每日活动到 Palm 掌上电脑 9-1加载 Palm 掌上电脑的软件 10-1 检查 Palm 掌上电脑的操作系统版本号 10-1 在 Palm 掌上电脑上加载软件 10-3 在 Palm 掌上电脑之间传输 Palm 软件10-6HCP1 HCPFrymaster HCP 是用于保鲜各种熟食制品的短期贮藏设备。
知识普及详解HCP水解胶原蛋白

知识普及:详解HCP水解胶原蛋白一、HCP是什么HCP是什么?这个问题对于国内一些从事保健品多年的人来说都是一个难题。
然而当我们究其概念出处时,“大隐隐于市”的感觉油然而生。
如果说胶原蛋白对护肤、美容的功效已经为广大消费者所认可的话,那么在保健品原料行业,HCP立足高端,成为“健康、安全、专业”的代名词也是一个不争的事实。
在欧美一些国家的高端胶原蛋白类保健食品行业,HCP所提供的胶原蛋白原料可以占到其市场总量的70%,这样的市场占有率背后,体现着HCP产品及其供应商的专业、诚信的经营理念和先进、严谨的生产工艺。
HCP(Hydrolyzed Collagen Peptide)是德国海德森集团的专利产品,全称水解胶原蛋白肽,是一种高浓度天然的生物活性产品,具有独特的稳定纳米小分子结构。
因其出众的活性与稳定结构,HCP也被称为世界上唯一有“生命”的微肽胶原蛋白。
二、HCP原料来源HCP原料发现历史还要追溯到上世纪90年代初。
1992年,德国海德森集团首席研发专家施瓦茨博士在汉诺威以北的深海中,发现在天然、纯净、无污染的自然之水养育下,深海鱼皮和鱼鳞中蕴含着最优质的胶原蛋白。
他成功将原料中的胶原蛋白提炼而出,运用到产品研发工作,最终实现了高效的稳定量产,并将此类胶原蛋白命名为“HCP微肽胶原蛋白”。
至今为止,汉诺威以北的深海仍被誉为优质胶原蛋白的最佳产地。
HCP的品牌释义为H—High(高品质),C—Crude(天然的),P—Pure(纯净的),这恰好是德国海德森集团精神的体现。
对于每一款产品,海德森集团始终从源头确保其安全、天然。
HCP发展历史已超过20年,为满足不同客户的需求,海德森工作人员的足迹遍布世界各地,但时至今日,HCP的原料仅选择汉诺威以北深海中的深海鱼,以及德国吕纳堡农场的禽畜。
尽管这些地区的原料来源有限,提炼过程相对困难,制作成本比较高昂,但海德森集团秉承创始人——诺贝尔奖获得者里夏德·库恩博士的精神,立志做最好的胶原蛋白。
仓鼠CHO细胞宿主蛋白(CHO-HCP)说明书定性

操作步骤
1. 编号:将样品对应微孔按序编号,每板应设阴性对照 2 孔、阳性对照 2 孔、空白对照 1
孔(空白对照孔不加样品及酶标试剂,其余各步操作相同)
2. 加样:分别在阴、阳性对照孔中加入阴性对照、阳性对照 50µl。然后在待测样品孔先 加样品稀释液 40µl,然后再加待测样品 10µl。加样将样品加于酶标板孔底部,尽量不 触及孔壁,轻轻晃动混匀,
3. 温育:用封板膜封板后置 37℃温育 30 分钟。 4. 配液:将 30 倍浓缩洗涤液用蒸馏水 30 倍稀释后备用 5. 洗涤:小心揭掉封板膜,弃去液体,甩干,每孔加满洗涤液,静置 30 秒后弃去,如此
重复 5 次,拍干。
6. 加酶:每孔加入酶标试剂 50µl,空白孔除外。 7. 温育:操作同 3。 8. 洗涤:操作同 5。 9. 显色:每孔先加入显色剂 A50µl,再加入显色剂 B50µl,轻轻震荡混匀,37℃避光显色
阴性 阳性判定:样品 OD 值≥ 临界值(CUT OFF)者为 CHO 细胞宿主蛋白(CHO-HCP)
阳性 。
注意事项 1.操作严格按照说明书进行,本试剂不同批号组分不得混用。 2.试剂盒从冷藏环境中取出应在室温平衡 15-30 分钟后方可使用,酶标包被板开封后如未
用完,板条应装入密封袋中保存。 3.浓洗涤液可能会有结晶析出,稀释时可在水浴中加温助溶,洗涤时不影响结果。 4. 封板膜只限一次性使用,以避免交叉污染。 5.底物请避光保存。 6.试验结果判定必须以酶标仪读数为准,使用双波长检测时,参考波长为 630nm 7.所有样品,洗涤液和各种废弃物都应按传染物处理。终止液为 2M 的硫酸,使用时必须
6ml×1 瓶
HCP操作维护说明书D

Pall Corporation操作维修手册IINSTALLATION, OPERATIONANDMAINTENANCE MANUALHCP 聚结分离式净油机Pall Filter (Beijing) Ltd.目录1概述1.1产品适用范围1.2产品特点2结构和功能2.1结构说明2.2功能介绍2.2.1整机功能2.2.2过滤系统2.2.3聚结系统2.2.4分离系统2.2.5排水系统2.2.6排放系统2.2.7驱动2.2.8整体结构与管路2.2.9电器系统2.2.10与Purifier的配合使用3安全3.1设备的安全保护功能3.2用户需注意的安全事项4搬运与储存4.1搬运4.2储存5安装6启动运行6.1启动前检查6.2启动6.3关机6.4紧急停车6.5运行7维护7.1故障诊断和排除7.2日常维护规程7.2.1滤芯检查、清洗和更换7.2.2泵安全阀压力调节7.3检查/保养日程8技术参数9附件9.1油水界面仪数显表的设置9.2计时器的计时方法9.3循环过滤、脱水运行及排空容器时各阀启闭表:9.4产品图纸9.5备件清单如有疑问,请及时联系!1简介1.1产品适用范围本设备可高效率地去除液压油、润滑油、气轮机油等石油基油品中的游离水和固体污染物,也可以根据需要单独对油液进行旁路或在线净化处理。
1.2产品功能HCP 系列设备可以:A. 破除介质中的全部油水乳化结构。
B. 去除介质中的游离水。
处理后介质的游离水含量低于100PPM,极限时可低于50 PPM。
C.滤除介质中的固体颗粒。
控制油液清洁度,可以达到NAS 4级或更高。
2结构与功能2.1结构说明HCP分移动式和固定式两种结构。
移动式设备可以根据需要更换工作场所,其配备的万向制动脚轮,有助于将设备布置在比较狭窄或不平整的工作场地上面。
固定式结构用于长期不移动的工作场合。
2.2功能介绍2.2.1整机HCP具备脱水和颗粒过滤两种功能,使用者通过简单的操作可以随时切换上述两种功能。
hcp相关法规

hcp相关法规摘要:一、引言二、HCp 的定义和分类三、HCp 相关法规概述1.我国法律法规体系2.相关法律法规内容简介四、HCp 法规在实际操作中的应用1.企业合规要求2.监管部门职责与执法五、违反HCp 法规的法律责任六、结论正文:HCp,即危害化学品,是指在生产、储存、运输、使用和处置过程中,可能对人类健康和环境造成危害的化学品。
为了加强对HCp 的管理和监督,我国制定了一系列法律法规,旨在保障人民群众的生命安全和身体健康,维护生态环境的可持续发展。
一、引言危害化学品在我们的生活中无处不在,从生产、储存到运输、使用和处置,都涉及到人们的生命安全和生态环境的可持续发展。
因此,对HCp 的管理和监督显得尤为重要。
我国针对HCp 的法规旨在规范相关行为,降低潜在危害。
二、HCp 的定义和分类HCp 是指在生产、储存、运输、使用和处置过程中,可能对人类健康和环境造成危害的化学品。
根据其危害特性,HCp 可分为有毒化学品、易燃化学品、爆炸品等不同类别。
三、HCp 相关法规概述1.我国法律法规体系我国法律法规体系包括宪法、法律、行政法规、地方性法规、部门规章等。
在HCp 管理方面,涉及到《中华人民共和国安全生产法》、《中华人民共和国环境保护法》、《危险化学品安全管理条例》等多部法律法规。
2.相关法律法规内容简介《危险化学品安全管理条例》明确了HCp 的生产、储存、运输、使用和处置等环节的安全管理和监督责任。
同时,该条例还规定了HCp 企业的合规要求,如必须进行安全评价、建立安全管理制度等。
四、HCp 法规在实际操作中的应用1.企业合规要求HCp 企业在生产、储存、运输、使用和处置过程中,必须严格遵守相关法律法规,确保安全管理和监督责任落实到位。
企业应建立完善的安全管理制度,进行安全评价,并定期对员工进行安全培训。
2.监管部门职责与执法监管部门应加强对HCp 企业的监督管理,对违反法规的企业进行查处,确保法律法规得到有效执行。
飞兆半导体HCPL-M454-500E光耦隔离器说明书

Features• Function compatible with HCPL-4504• Surface mountable• Very small, low profile JEDEC registered package outline • Compatible with infrared vapor phase reflow and wave soldering processes • Short propagation delays for TTL and IPM applications • Very high common mode transient immunity: Guaranteed 15 kV/µs at V CM = 1500 V • High CTR: >25% at 25°C • Guaranteed specificationsforcommonIPMapplications• TTL compatible• Guaranteed ac and dc performanceovertemperature: 0°C to 70°C • Open collector output • Safety approval:UL Recognized 3750 Vac / 1 min. per UL 1577IEC/EN/DIN EN 60747-5-2Approved V IORM = 560 Vpeak for Option 060.CSA Approved • Lead free option “-000E”Applications• Inverter Circuits and Intelligent Power Module (IPM) Interfacing: Shorter propagation delays and guaranteed (t PLH - t PHL ) specifications. (See power inverter dead time section)• High speed logic ground isolation: TTL/TTL, TTL/LTTL, TTL/CMOS, TTL/LSTTL • Line Receivers: High common mode transient immunity (>15 kV/µs for a TTL load/drive) and low input-output capacitance (0.6 pF)• Replace pulse transformers: ave board space and weight • Analog signal ground isolation: Integrated photon detector provides improved linearity over phototransistorsCAUTION: The small junction sizes inherent to the design of this bipolar component increase the component's susceptibility to damage from electrostatic discharge (ESD). It is advised that normal static precautions be taken in handling and assembly of this component to prevent damage and/or degradation which may be induced by ESD.HCPL-M454Ultra High CMR, Small Outline, 5 Lead, High Speed OptocouplerDescriptionThe HCPL-M454 is similar to Avago’s other high speed transistor output optocouplers, but with shorter propa-gation delays and higher CTR. The HCPL-M454 also has a guaranteed propagation delay difference (t PLH - t PHL ). These features make the HCPL-M454 an excellent solu-tion to IPM inverter dead time and other switching prob-lems.The HCPL-M454 CTR, propagation delays, and CMR are specified both for TTL load and drive conditions and for IPM (Intelligent Power Module) load and drive condi-tions. Specifications and typical performance plots for both TTL and IPM conditions are provided for ease of ap-plication.This diode-transistor optocoupler uses an insulating lay-er between the light emitting diode and an integrated photon detector to provide electrical insulation between input and output. Separate connections for the photo-diode bias and output transistor collector increase the speed up to a hundred times over that of a conventional= 0.102 (0.004)DIMENSIONS IN MILLIMETERS (INCHES)* MAXIMUM MOLD FLASH ON EACH SIDE IS 0.15 mm (0.006)NOTE: FLOATING LEAD PROTRUSION IS 0.15 mm (6 mils) MAX.Outline Drawing (JEDEC MO-155)Ordering InformationHCPL-M454 is UL Recognized with 3750 Vrms for 1 minute per UL1577. Option Part RoHS non RoHSSurface Tape IEC/EN/DIN NumberCompliantCompliantPackageMount& ReelEN 60747-5-2QuantityHCPL-M454To order, choose a part number from the part number column and combine with the desired option from the option column to form an order entry. Example 1:HCPL-M454-560E to order product of SO-5 Surface Mount package in Tape and Reel packaging with IEC/EN/DIN EN 60747-5-2 Safety Approval and RoHS compliant.Example 2:HCPL-M454 to order product of SO-5 Surface Mount package inTube packaging and non RoHS compliant. Option datasheets are available. Contact your Avago sales representative or authorized distributor for information.Remarks: The notation ‘#XXX’ is used for existing products, while (new) products launched since July 15, 2001 and RoHS compliant will use ‘–XXXE.’Absolute Maximum Ratings(No Derating Required up to 85°C)Storage Temperature .............................................................................-55°C to +125°C Operating Temperature ........................................................................-55°C to +100°C Average Input Current - I F .................................................................................25 mA [1]Peak Input Current - I F ........................................................................................50 mA [2](50% duty cycle, 1 ms pulse width)Peak Transient Input Current - I F ............................................................................1.0 A(≤1 µs pulse width, 300 pps)Reverse Input Voltage - V R (Pin 3-1) .............................................................................5 V Input Power Dissipation ......................................................................................45 mW [3]Average Output Current - I O (Pin 5) .......................................................................8 mA Peak Output Current .................................................................................................16 mA Output Voltage - V O (Pin 5-4) .....................................................................-0.5 V to 20 V Supply Voltage - V CC (Pin 6-4) ....................................................................-0.5 V to 30 V Output Power Dissipation ...............................................................................100 mW [4]Infrared and Vapor Phase Reflow Temperature .......................................see belowSolder Reflow Thermal ProfileTIME (SECONDS)T E M P E R A T U R E (°C )ROOMNote: Non-halide flux should be used.Insulation Related SpecificationsParameterSymbolValueUnitsConditionsMinimum External Air GapL(IO1) ≥ 5 mm Measured from input terminals (Clearance)to output terminalsMinimum External Tracking Path L(IO2) ≥ 5 mm Measured from input terminals (Creepage)to output terminals Minimum Internal Plastic Gap 0.08 mm Through insulation distance (Clearance) conductor to conductor Tracking ResistanceCTI 175 V DIN IEC 112/VDE 0303 Part 1 Isolation Group (per DIN VDE 0109)IIIaMaterial Group DIN VDE 0109SchematicLand Pattern RecommendationGNDV CCV OANODECATHODERecommended Pb-Free IR ProfileDIMENSION IN MILLIMETERS (INCHES)NOTES:THE TIME FROM 25 °C to PEAK TEMPERATURE = 8 MINUTES MAX.T smax = 200 °C, T smin = 150 °CNote: Non-halide flux should be used.Over recommended temperature (T A = 0°C to 70°C) unless otherwise specified. (See note 11)Parameter Symbol Min. Typ. Max. Units Test Conditions Fig. Note Current CTR 25 32 60 % T A = 25°C V O = 0.4 V I F = 16 mA 1,2,4 5 TransferRatio 21 34 V O = 0.5 V V CC = 4.5 VCurrent CTR 26 35 65 % T A = 25°C V O = 0.4 V I F = 12 mA 1,2,4 5 TransferRatio 22 37 V O = 0.5 V V CC = 4.5 VLogic Low V OL0.2 0.4 V T A = 25°C I O = 3.0 mA I F = 16 mAOutputVoltage 0.2 0.5 I O = 2.4 mA V CC = 4.5 VLogic High I OH0.003 0.5 µA T A = 25°C V O = V CC = 5.5 V I F = 0 mA 5 OutputCurrent 0.01 1.0 T A = 25°C V O = V CC = 15 V50Logic Low I CCL50 200 µA I F = 16 mA V CC = 15 V V O = open 11 SupplyCurrentLogic High I CCH0.02 1 µA T A = 25°C I F = 0 mA V CC = 15 V 11 Supply V O = openCurrent 0.02 2Input V F 1.5 1.7 V T A = 25°C I F = 16 mA 3 ForwardVoltage 1.5 1.8Input BV R 5 V I R = 10 µAReverseBreakdownCurrentTempera- ∆V F/∆T A-1.6 mV/°C I F = 16 mAture Co-efficient ofForwardVoltageInput C IN60 pF f = 1 MHz V F = 0 VCapacitanceInput- V ISO3750 V RMS RH < 50% t = 1 min 6,12 Output T A = 25°CInsulationVoltageResistance R I-O10[12]Ω V I-O = 500 Vdc 6 (Input-Output)Capacitance C I-O0.6 pF f = 1 MHz 6 (Input-Output)Over recommended temperature (T A = 0°C to 70°C) unless otherwise specifiedParameter Sym. Min. Typ. Max. Units Test Conditions Fig. Note Propagation t PHL0.2 0.3 µs T A = 25°C Pulse: f = 20 kHz 8, 9 9Delay Time Duty Cycle = 10%to Logic 0.2 0.5 I F = 16 mA V CC = 5.0 VLow at R L = 1.9 kΩ C L = 15 pFOutput V THHL = 1.5 V0.2 0.5 0.7 T A = 25°C Pulse: f = 10 kHz 10- 10Duty Cycle = 50% 140.1 0.5 1.0 I F = 12 mA V CC = 15.0 VR L = 20 kΩ C L = 100 pFV THHL = 1.5 VPropagation t PLH0.3 0.5 µs T A = 25°C Pulse: f = 20 kHz 8, 9 9Delay Time Duty Cycle = 10%to Logic 0.3 0.7 I F = 16 mA V CC = 5.0 VHigh at R L = 1.9 kΩ C L = 15 pFOutput V THLH = 1.5 V0.3 0.8 1.1 T A = 25°C Pulse: f = 10 kHz 10- 10Duty Cycle = 50% 140.2 0.8 1.4 I F = 12 mA V CC = 15.0 VR L = 20 kΩ C L = 100 pFV THLH = 2.0 VPropagation t PLH- -0.4 0.3 0.9 µs T A = 25°C Pulse: f = 10 kHz 10- 13Delay t PHL Duty Cycle = 50% 14Difference -0.7 0.3 1.3 I F = 12 mA V CC = 15.0 VBetween R L = 20 kΩ C L = 100 pFAny 2 Parts V THHL = 1.5 V V THLH = 2.0 VCommon |CM H| 15 30 kV/µs T A = 25°C V CC = 5.0 V R L = 1.9 kΩ 7 7,9 Mode C L = 15 pF I F = 0 mATransient V CM = 1500 V P-PImmunityat Logic 15 30 T A = 25°C V CC = 15.0 V R L = 20 kΩ 7 8,10 High Level C L = 100 pF I F = 0 mAOutput V CM = 1500 V P-PCommon |CM L| 15 30 kV/µs T A = 25°C V CC = 5.0 V R L = 1.9 kΩ 7 7,9 Mode C L = 15 pF I F = 16 mATransient V CM = 1500 V P-PImmunityat Logic 10 30 T A = 25°C V CC = 15.0 V R L = 20 kΩ 7 8,10 Low Level C L = 100 pF I F = 12 mAOutput V CM = 1500 V P-P15 30 T A = 25°C V CC = 15.0 V R L = 20 kΩ 7 8,10C L = 100 pF I F = 16 mAV CM = 1500 V P-PFigure 4. Current Transfer Ratio vs. Temperature.Figure 5. Logic High Output Current vs. Temperature.Figure 1. DC and Pulsed Transfer Characteristics.Figure 2. Current Transfer Ratio vs. Input Current.Figure 3. Input Current vs. Forward Voltage.Notes:1. Derate linearly above 70°C free-air temperature at a rate of 0.8 mA/°C.2. Derate linearly above 70°C free-air temperature at a rate of 1.6mA/°C.3. Derate linearly above 70°C free-air temperature at a rate of 0.9 mA/°C.4. Derate linearly above 70°C free-air temperature at a rate of 2.0 mA/°C.5. CURRENT TRANSFER RATIO in percent is defined as the ratio of output collector current (I O ), to the forward LED input current (I F ), times 100.6. Device considered a two-terminal device: Pins 1 and 3 shorted together and Pins 4, 5 and 6 shorted together.7. Under TTL load and drive conditions: Common mode transient immunity in a Logic High level is the maximum tolerable (positive) dV CM /dt onthe leading edge of the common mode pulse, V CM , to assure that the output will remain in a Logic High state (i.e., V O > 2.0 V). Common mode transient immunity in a Logic Low level is the maximum tolerable (negative) dV CM /dt on the trailing edge of the common mode pulse signal, V CM , to assure that the output will remain in a Logic Low state (i.e., V O < 0.8 V).8. Under IPM (Intelligent Power Module) load and LED drive conditions: Common mode transient immunity in a Logic High level is the maxi-mum tolerable dV CM /dt on the leading edge of the common mode pulse, V CM , to assure that the output will remain in a Logic High state (i.e., V O > 3.0 V). Common mode transient immunity in a Logic Low level is the maximum tolerable dV CM /dt on the trailing edge of the common mode pulse signal,V CM , to assure that the output will remain in a Logic Low state (i.e., V O < 1.0 V). 9. The 1.9 kΩ load represents 1 TTL unit load of 1.6 mA and the 5.6 kΩ pull-up resistor.10. The R L = 20 kΩ, C L = 100 pF load represents an IPM (Intelligent Power Mode) load.11. Use of a 0.1 µF bypass capacitor connected between pins 4 and 6 is recommended.12. In accordance with UL 1577, each optocoupler is proof tested by applying an insulation test voltage ≥4500 V RMS for 1 second (leakage detec-tion current limit, I i-e ≤ 5 µA).13. The difference between t PLH and t PHL , between any two HCPL-M454 parts under the same test condition. (See Power Inverter Dead Time and Propagation Delay Specifications section).1V O – OUTPUT VOLTAGE – VI O – O U T P U T C U R R E N T – m A105I F – INPUT CURRENT – mAN O R M AL I Z E D C U R R E N T T R A N S F E R R A T I OHCPL-M454 fig 2HCPL-M454 fig 3V F – FORWARD VOLTAGE – VOLTS10010I F – F O R W A R D C U R R E N T – m AT A – TEMPERATURE – °C N O R MA L I Z E D C U R R E N T T R A N S F E R R A T I OHCPL-M454 fig 4T A – TEMPERATURE – °CI O H – L O GI C H I G H O U T P U T C U R R E N T – n AHCPL-M454 fig 5101010101010-110-2Figure 6. Switching Test Circuit.Figure 7. Test Circuit for Transient Immunity and Typical Waveforms.Figure 8. Propagation Delay Time vs. Temperature.Figure 9. Propagation Delay Time vs. Load Resistance.Figure 10. Propagation Delay Time vs. Load Resis-tance.6O I F t V I F CC7O V FFCCV OV O0 VSWITCH AT B: I = 12 mA, 16 mA FV CMT A – TEMPERATURE – °C t p – P R O P A G A T I O N D E L A Y – µs0.500.450.400.350.300.250.200.150.10HCPL-M454 fig 8R L – LOAD RESISTANCE – k Ωt p – P R O P A G A T I O N D E L A Y – µs1.41.21.00.80.60.40.20.0HCPL-M454 fig 9R L – LOAD RESISTANCE – k Ωt p – P R O P A G A T I O N D E L A Y – µs1.41.21.00.80.60.40.20.0HCPL-M454 fig 101.61.82.02.22.42.6Figure 11. Propagation Delay Time vs. Temperature.Figure 12. Propagation Delay Time vs. Load Resis-tance.Figure 13. Propagation Delay Time vs. Load Capaci-tance.Figure 14. Propagation Delay Time vs. Supply Voltage.T A – TEMPERATURE – °Ct p – P R O P A G A T I O N D E L A Y – µs1.11.00.90.80.70.60.50.40.3HCPL-M454 fig 11R L – LOAD RESISTANCE – k Ωt p – P R O P A G A T I O N D E L A Y – µs1.61.41.21.00.60.20.0HCPL-M454 fig 121.80.40.8 R L – LOAD CAPACITANCE – pFt p – P R O P A G A T I O N D E L A Y – µs2.01.50.50.0HCPL-M454 fig 132.53.03.51.0 V CC – SUPPLY VOLTAGE – Vt p – P R O P A G A T I O N D E L A Y – µs0.90.80.60.2HCPL-M454 fig 141.01.11.20.70.50.40.3Figure 15. Typical Power Inverter.Figure 16. LED Delay and Dead Time Diagram.HCPL-M454 fig 15LED 1OUT 1LED 2OUT 2HCPL-M454 fig 16Power Inverter Dead Time and Propagation Delay Specifica-tionsThe HCPL-M454 includes a specification intended to help designers minimize “dead time” in their power inverter designs. The new “propagation delay difference” specifi-cation (t PLH - t PHL) is useful for determining not only how much optocoupler switching delay is needed to prevent “shoot-through” current, but also for determining the best achievable wort-case dead time for a given design. When inverter power transistors switch (Q1 and Q2 in Figure 15), it is essential that they never conduct at the same time. Extremely large currents will flow if there is any overlap in their conduction during switching transi-tions, potentially damaging the transistor and even the surrounding circuitry. This “shoot-through” current is eliminated by delaying the turn-on of one transistor (Q2) long enough to ensure that the opposing transistor (Q1) has completely turned off. This delay introduces a small amount of “dead time” at the output of the inverter dur-ing which both transistors are off during switching tran-sitions. Minimizing this dead time is an important design goal for an inverter designer.The amount of turn-on delay needed depends on the propagation delay characteristics of the optocoupler, as well as the characteristics of the transistor base/gate drive circuit. Considering only the delay characteristics of the optocoupler (the characteristics of the base/gate drive circuit can be analyzed in the same way), it is im-portant to know the minimum and maximum turn-on (t PHL) and turn-off (t PLH) propagation delay specifica-tions, preferably over the desired operating temperature range. The importance of these specifications is illustrat-ed in Figure 16. The waveforms labeled “LED1”, “LED2”, “OUT1”, and “OUT2” are the input and output voltages of the optocoupler circuits driving Q1 and Q2 respec-tively. Most inverters are designed such that the power transistor turns on when the optocoupler LED turns on; this ensures that both power transistors will be off in the event of a power loss in the control circuit. Inverters can also be designed such that the power transistor turns off when the optocoupler LED turns on; this type of design, however, requires additional fail-safe circuitry to turn off the power transistor if an over-current condition is de-tected. The timing illustrated in Figure 16 assumes that the power transistor turns on when the optocoupler LED turns on.The LED signal to turn on Q2 should be delayed enough so that an optocoupler with the very fastest turn-on propagation delay (t PHLmin) will never turn on before an optocoupler with the very slowest turn-off propaga-tion delay (t PLHmax) turns off. To ensure this, the turn-on of the optocoupler should be delayed by an amount no less than (t PLHmax - t PHLmin), which also happens to be the maximum data sheet value for the propagation delay dif-ference specification, (t PLH - t PHL). The HCPL-M454 speci-fies a maximum (t PLH - t PHL) of 1.3 µs over an operating temperature range of 0-70°C.Although (t PLH- t PHL)max tells the designer how much delay is needed to prevent shoot-through current, it is insufficient to tell the designer how much dead time a design will have. Assuming that the optocoupler turn-on delay is exactly equal to (t PLH - t PHL)max, the minimum dead time is zero (i.e., there is zero time between the turn-off of the very slowest optocoupler and the turn-on of the very fastest optocoupler).Calculating the maximum dead time is slightly more complicated. Assuming that the LED turn-on delay is still exactly equal to (t PLH - t PHL)max, it can be seen in Figure 16 that the maximum dead time is the sum of the maximum difference in turn-on delay plus the maximum difference in turn-off delay,[(t PLHmax-t PLHmin) + (t PHLmax-t PHLmin)],This expression can be rearranged to obtain[(t PLHmax-t PHLmin) - (t PHLmin-t PHLmax)],and further rearranged to obtain[(t PLH-t PHL)max - (t PLH-t PHL)min],which is the maximum minus the minimum data sheet values of (t PLH - t PHL). The difference between the maxi-mum and minimum values depends directly on the to-tal spread of propagation delays and sets the limit on how good the worst-case dead time can be for a given design. Therefore, optocouplers with tight propagation delay specifications (and not just shorter delays or lower pulse-width distortion) can achieve short dead times in power inverters. The HCPL-M454 specifies a minimum (t PLH - t PHL) of -0.7 µs over an operating temeprature range of 0-70°C, resulting in a maximum dead time of 2.0 µs when the LED turn-on delay is equal to (t PLH - t PHL)max, or 1.3 µs.It is important to maintain accurate LED turn-on delays because delays shorter than (t PLH- t PHL)max may allow shoot-through currents, while longer delays will increase the worst-case dead time.For product information and a complete list of distributors, please go to our website: Avago, Avago Technologies, and the A logo are trademarks of Avago Technologies Limited in the United States and other countries. Data subject to change. Copyright © 2007 Avago Technologies Limited. All rights reserved. Obsoletes AV01-0553ENAV02-0967EN January 11, 2008。
Antec HCP-1000 Platinum 电源用户手册说明书

HCP-1000 Platinum POWER SUPPLY USER’S MANUALHCP-1000 PlatinumAntec’s High Current Pro Platinum series is the pinnacle of power supplies. High Current Pro Platinum is fully modular with a revolutionary 20+8-pin MBU socket for the needs of tomorrow. By using a PSU that is 80 PLUS® PLATINUM certified & ErP Lot 6: 2013, operating up to 94% efficient, you can reduce your electricity bill by up to 25% when compared to many other power supplies. HCP Platinum’s innovative 16-pin sockets create a new level of flexibility by doubling the modular connectivity, supporting two different 8-pins connectors and even future connectors of 10, 12, 14 or 16-pins. Backed by a 7-year warranty and lifetime global 24/7 support, the HCP-1000 Platinum embodies everything a power supply can accomplish today.STANDARDS AND FEATURESThe connectors and power specifications of the HCP-1000 Platinum PSU are all compatible withATX12V v2.32 and EPS12V v2.92 specifications. The HCP-1000 Platinum features Universal Input, which automatically senses when you connect the power supply to any AC power source between 100 - 240V without setting a voltage switch. This power supply also features Active Power Factor Correction (PFC), which improves the power factor value of the power supply by altering the input current wave shape, helping to power transmission across the grid.SYSTEM PROTECTIONA variety of industrial-grade safety circuitry will help protect your computer: Over Current Protection (OCP), Over Voltage Protection (OVP), Under Voltage Protection (UVP), Short Circuit Protection (SCP), Over Power Protection (OPP), Over Temparature Protection (OTP), Surge & Inrush Protection (SIP), No Load Operation (NLO) & Brown-Out Protection (BOP). Sometimes the PSU will “latch” into a prote cted state. You will need to power off the PSU and clear the fault before it will function again. There are no user-replaceable fuses in your HCP-1000 Platinum.MODULAR CABLE JACKSThere are six 5-pin black jacks and five 16-pin black jacks on the back of your HCP-1000 Platinum PSU. These jacks are for the optional cables that come with your power supply.Caution: Please connect only cables provided with this HCP-1000 Platinum. Other cables may not be compatible!20+8-pin MBU connection You can connect 1 or 2 modular 8-pin cables into any of the 16-pin socketsOC Link™ System InstallationConnect the included OC Link™ cable to two Antec HCP Platinum power supplies. You can use any combination of models such as 850 + 1000 or 1300 + 1300. You can connect any system component to either PSU without restrictions. Please read the chapter “INSTALLATION” for general installati on help. Please turn the “I/O” switch on both power supplies to “I” (On). After completion of set-up, simply turn on the system.HCP-850 P. / HCP-1000 P. HCP-1300 P.80 PLUS® CERTIFICATION80 PLUS® certification is the most widely recognizedindependent standard in power supply efficiency. An 80PLUS® certified power supply uses less energy andgenerates less heat to stay cooler, run quieter and lastlonger. The HCP-1000 Platinum has been 80 PLUS®PLATINUM certified to be at least 89% efficient at a widerange of operating loads; this will lower your operatingcosts and help protect the environment.POWER OUTPUTTo see the output capacity and regulation for each different voltage, see table 1. TABLE 1ConnectorsINSTALLATIONInstall the PSU into either the top or bottom of your case with the four screws provided. Refer to your case manual if you are unsure where the power supply should be installed.Connect the 24-pin main power connector to your motherboard.Connect the 8-pin or 4+4-pin connector for the CPU. If your motherboard has an 8-pin socket with a cover on some of the openings, we recommend that you remove the cover and use the 8-pinconnector.Note: Please also refer to your motherboards manual for any special instructions.Connect the AC power cord to the power supply AC inlet. Be sure to use the heavy-duty cord suppliedwith your PSU.PCI-E graphics cards use different amounts of power. For some, a single 6-pin connector is sufficient, making the hardwired connector the preferred choice. More powerful cards use multiple connectors, including the advanced 8-pin PCI-E connector. The 8-pin PCI-E connector on the PSU can be used aseither a 6- or 8-pin connector.Hard drives, optical drives (CD/DVD/BluRay™) and other accessories will use either the older 4-pin Molex connector or the newer 15-pin SATA connector. 4-pin Molex connectors have two black, one yellow and one red wire. The SATA connector has an additional orange power wire.When you have all the connections secured, turn the switch on the PSU to the “|” position.Technical Support:/supportUSA & Kanada1-800-22ANTEC*******************Europe+31-(0)10-462 20 60*******************Asia+886 (0)800-060-696 *******************Visit us on Facebook for contests, information & supportUSA & Canada /AntecIncEurope /AntecEuropeUK /AntecUKAustralia & New Zealand /AntecAUIndia /AntecIndiaIsrael /AntecIsraelMalaysia /AntecMalaysiaPhilippines /AntecPHSingapore /AntecSGAntec, Inc.47900 Fremont Blvd., Fremont, CA 94538 / USATel: 510-770-1200 - Fax: 510-770-1288©2012-2013 Antec, Inc. All rights reserved.Specifications are subject to change without prior notice. Actual product(s) and accessories may differ from illustrations. Omissions and printing errors excepted. Content of delivery might differ in different countries or areas. Some trademarks may be claimed as the property of others.Reproduction in whole or in part without written permission is prohibited.。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
机械制造技术基础课程设计说明书设计题目设计“传动箱壳体”零件的机械加工工艺及工艺设备设计者胡春鹏指导教师黑龙江东方学院年月日目录设计任务书一.零件的分析--------------------------------- 3二.工艺过程设计------------------------------- 3(一)确定毛坯的制造形式---------------------3 (二)基面的选择---------------------------- 3 (三)制订工艺路线-------------------------- 4 (四)机械加工余量、工序尺寸及毛坯尺寸的确定-8 (五)确定切削用量及基本工时-----------------10 三.夹具设计------------------------------------14定位分析----------------------------------14夹紧装置设计------------------------------15夹具的平衡设计----------------------------15公差确定----------------------------------16夹紧力分析--------------------------------16 四.参考文献------------------------------------17 附录相关机械图1零件的分析1.1零件的作用题目所给零件是CA6140车床的上刀架,它位于车床床身的上半部,与夹持刀具的分刀架相连,通过一系列的构件相互作用实现刀具的快速更换。
零件上部有Φ82凸圆,用以安装方刀架并将其固定。
1.2零件的工艺分析上刀架属于车床床身上比较精密的部件,所以有一系列的形状与位置要求,现分析如下:1.上表面A对下表面的平行度公差为0.02㎜2.Φ82H7对上表面A的垂直度公差为0.02㎜2工艺规程设计2.1确定毛胚的制造形式零件材料为HT200考虑到车床在使用过程中零件的冲击载荷比较稳定;选用铸件即可满足使用要求;且零件为CA6140车床标准件,符合大批生产要求。
2.2基面的选择基面的选择是工艺规程中的重要工作之一;基面选择得正确与合理;可以使加工质量得到保证,生产率得以提高,否则加工工艺过程中会问题百出;更有甚者;还会造成零件大批报废,使生产无法进行。
2.2.1粗基准的选择对于一般轴类零件而言,以外圆作为粗基准是完全合理的,但对于板类零件上下表面均为重要表面,依据粗基准选择原则中便于装夹的原则;即粗基准尽可能平整光洁;选择表面B为粗基准;主要考虑的是不加工表面与加工表面间的位置要求。
2.2.2精基准的选择主要考虑基准重合的问题,当基准与工序基准不重合时,应该进行尺寸换算;这在以后要专门计算。
2.3制定工艺路线制定工艺路线的出发点应该是使零件的几何形状,尺寸精度以及位置精度与技术要求得到合理的保证,在生产纲领已确定为批量生产的条件下;可以考虑采用设计夹具来保证,使生产成本尽量下降。
(1)工艺路线方案一工序1、粗铣上端面保证尺寸工序2、车Φ82的上端面再车,再车Φ82的外圆,倒角Φ72的圆,再钻Φ72 Φ76 Φ52,再车Φ32的圆锥孔保证尺寸工序3、先钻Φ68 Φ84的孔,再倒角,保证尺寸工序4、钻孔至Φ12,攻丝2xM12保证尺寸工序5、去毛刺;检查。
(2)工艺路线方案二工序1、粗铣上端面保证尺寸工序2、先钻Φ68 Φ84的孔,再倒角,保证尺寸工序3、车Φ72的上端面,再车Φ82的外圆,倒角Φ72的圆,再钻Φ72 Φ76 Φ52,再车Φ32的圆锥孔保证尺寸工序4、钻孔至Φ12,攻丝2xM12保证尺寸工序5、去毛刺、检查2.4工艺路线比较分析两方案的主要区别在于方案一先加工A面而方案二先加工B面;比较之下B面的平面较大,装夹比较方便;选B面为粗基准加工A面易于保证加工质量;且方案二中钻φ82H7圆孔是在车床上加工出来,紧接着工序一采用相同夹具;减少了装夹次数,定位基准相同;加工的精度较高;综上所述;最终采用工艺路线二工序Ⅰ:粗精铣B面;保证余量尺寸;以A面为基准选用X5032立式升降台铣床,专用夹具。
工序Ⅱ;粗精车φ82H7外圆、端面和上端面A;以B面为粗基准进行加工;选用CA6161卧式车床专用夹具。
工序Ⅲ:粗精铣D、E两端面保证余量尺寸;以A面φ82H7为基准实现定位;选用机床X5032立式升降台铣床专用夹具。
工序Ⅳ:粗精铣C、F两端面;以A面φ82H7圆柱圆及D、E 两端面实线定位。
工序Ⅴ;钻φ72H7 φ52H7 φ32H7孔,以外圆柱上平面及两侧面定位,选用机床Z5140A方柱立式钻床及专用夹具。
工序Ⅵ:钻φ68H7 φ84H7孔;以表面A,侧面E及圆柱面定位;选用机床Z5140A方柱式立式钻床,专用夹具;攻螺纹2-M12;选用S4010攻丝机;专用夹具。
工序Ⅶ;去毛刺;检查。
2.5机械加工余量,工序尺寸的确定CA6140车床上刀架材料为HT200,硬度170—220HBS,毛胚重量约为8kg,生产类型为大批生产,采用铸造毛胚。
根据上述原始资料及加工工艺,分别确定各加工表面的机械加工余量,工序尺寸,及毛胚尺寸如下.1:车φ82H7外圆,端面,A面。
2:φ82H7外圆要求与方刀架配合..是重要的加工表面,表面粗糙度要求Ra3.2,要求精加工,参照《切削加工简明实用手册》,表“轴的机械加工余量”得:毛胚:φ86mm粗车:φ83mm 2z=3mm精车:φ82mm 2z=1mm(1)上刀架A面上刀架A面是与方刀架底座配合的平面,实现应用中由于换刀要与方刀架摩擦,因此要求精度也较高,表面粗糙度压迫球Ra3.2,要求精加工参照《切削加工简明实用手册》表确定加工余量,工序尺寸及毛胚尺寸。
毛胚:86mm粗车:83.9mm z=2.1mm精车:82.5mm z=1.4mm(2)φ82H7端面表面粗糙度要求Rz=6.3,参照《切削加工简明实用手册》。
毛胚:35mm粗车:32.6mm z=2.4mm精车:32mm z=0.6mm(3)φ82H7圆柱体中心孔φ72mm加工要求为基孔制7级公差..参照《机械切削工艺参数速查手册》得:钻孔:φ70.0mm扩孔:φ71.8mm 2z=1.8mm粗铰:φ71.94mm 2z=0.14mm精铰:22.00mm 2z=0.06mm(4)粗精铣底面B加工B表面,粗糙度要求R=6.3,查《机械切削工艺参数速查手册》确定加工余量、工序尺寸。
粗铣;44.7mm z=1.8mm半精铣;43.2mm z=1.5mm精铣;43mm z=0.2mm(5)粗精铣D、E两侧面D、E两侧面粗糙度要求为Ra=6.3,且D、E两距离为125mm, 查《机械切削工艺参数速查手册》确定加工余量,毛胚尺寸。
粗铣;2z=3mm半精铣;2z=2.4mm(6)粗精铣C、F两端面C端面粗糙度要求Ra=6.3,F面粗糙度要求为Ra=6.3,简化工序内容. C、F两端面要求尺寸为330mm..查《机械切削工艺参数速查手册》确定加工余量,工序尺寸。
粗铣;2z=3mm半精铣;2z=2.4mm毛胚基本尺寸为346mm(7)攻螺纹2-M12(8)加工余量计算现以D,E两侧面的加工为例..加工余量计算长度为125mm毛胚的铸造精度为CT9级..查《公差与配合实用手册》可知.(1)毛胚铸造公差为2.5mm..取对称分布为1.25mm(2)粗铣时厚度偏差为-0.43—-0.63,取-0.5mm(3)精铣厚度偏差为-0.25mm(4)精铣时厚度偏差为-0.12mm由于本零件是大批生产,采用调整法加工,因此在计算最大..最小加工余量是,应按调整法加工方式予以确定。
DE两侧面尺寸加工余量和工序余量及公差分布如下图毛胚名义尺寸;125+2×3=131毛胚最大尺寸;131+1.25×2=133.5毛胚最小尺寸;131-1.25×2=128.5粗铣后最大尺寸;130.5+0.5=131粗铣后最小尺寸;128-0.5=127.5半精铣后最大尺寸;125+0.2=125.2半精铣后最小尺寸;125-0.2=125.2精铣后尺寸与零件图尺寸相符合即125mm(5)确定切削用量及基本工时工序1:粗精车φ82H7外圆,端面和上端面A1:加工条件加工材料:HT200铸铁.,a=200N/mm加工要求:粗精车φ82H7外圆,车上端面A,车φ482H7端面. Ra=3.2机床CA6161卧式车床刀具.刀片材料为YG6(2)计算切削用量粗车φ48H7外圆柱面(1)确定背吃刀量ap,单边余量2mm,留0.5mm作为精车余量,取粗车背吃刀量ap=1.5mm。
(2)确定进给量,查《切削加工简明实用手册》可得f=0.5—0.7mm/r,根据机床特性初步选定f=0.6mm/r。
(3)选择切削速度Vc查表可得Vc=1.333~1.83m/s,取Vc=1.6m/s。
(4)确定主轴转速n,由公式n=1000Vc/πd=1000×1.6/(π×52)=9.8(r/s)=588r/min..按机床说明书,与r=588r/min..相近转速600r/min取nw=600r/min。
则实际切削速度为v=98m/min。
(5)计算切削工时Tm=l/nwf=0.092..min.(6)精车φ82H7外圆柱面(1)背吃刀量取ap=0.5mm(2)确定进给量与粗加工工时相同f=0.6m/r .(3)切削速度v=98m/min(4)主轴转速nw=600r/min(5)切削工时t= l/nwf=0.092min(1)粗车端面A(1)背吃刀量取ap=2.1mm(2)确定进给量与粗加工工时相同f=1.0m/r .(3)切削速度v=96m/min(4)主轴转速nw=600r/min实际切削速度v=98m/min (5)切削工时t= l/nwf=0.1171min(2)精车端面A时(1)背吃刀量取ap=0.6mm(2)确定进给量与粗加工工时相同f=1.0m/r .(3)切削速度v=96m/min(4)主轴转速nw=600r/min实际切削速度v=98m/min (5)切削工时t= l/nwf=0.1171min(3)粗车φ82H7圆柱端面(1)背吃刀量取ap=2.4mm(2)确定进给量与粗加工工时相同f=0.6m/r .(3)切削速度v=96m/min(4)主轴转速nw=600r/min实际切削速度v=98m/min (5)切削工时t= l/nwf=0.067min(4)精车φ82H7圆柱端面(1)背吃刀量取ap=0.6mm(2)确定进给量与粗加工工时相同f=0.6m/r .(3)切削速度v=96m/min(4)主轴转速nw=600r/min实际切削速度v=98m/min (5)切削工时t= l/nwf=0.067min(5)检验机床功率因毛胚铸造等级为CT9级,加工余量较小,产生的切削力不大,因此不需要进行机床功率校核。
