美国痛风指南
2020版美国痛风指南解读

2020版美国痛风指南解读2020年5月,最新版《美国风湿病学会痛风管理指南》因推荐内容变化较大,一经发布引发了广泛热议。
医脉通内分泌科于第一时间对指南的推荐部分进行了编译分享,很多老师留言发表了自己的看法。
本文中,余金泉老师对指南中的几个重要推荐变化进行了解读,小编进行了整理与各位老师分享,供大家参考。
2019年11月,美国风湿病学会(ACR)学术年会上发布了2020版ACR 痛风临床实践指南(草案),笔者也在第一时间撰文进行了分享,2020年5月,2020版ACR指南正式在Arthritis Care & Research刊发,目前已有不少分享。
前面看到了不少对新指南的解读,同样有不少同行认为有翻天覆地的变化,实则不然。
新版指南或许有些做法与过往推荐有了修订,但实在谈不上“翻天覆地”,而且不少推荐,笔者窃以为更重要的不是知其然,而是知其所以然——重要的不是指南怎么推荐,而是搞清楚指南推荐背后的逻辑,这样临床上决策时才游刃有余,以及可以做出最符合患者利益的治疗。
1痛风降尿酸治疗的指征以下三点为中高证据强推荐的指征(板上钉钉没错了!):➤≥1处皮下痛风石;➤有证据表明存在痛风引起的影像学破坏;➤频繁发作(≥2次/年)的痛风。
中等证据弱推荐:以往曾发作一次以上痛风,但属于非频繁发作(<2次/年)。
而对于首次发作的痛风患者,除了以下三种情况弱推荐应考虑降尿酸治疗:慢性肾脏病CKD3期以上;血尿酸≥9mg/dL(540μmol/L);存在泌尿系结石。
不符合以上三种情况的首次发作患者,应该谨慎推荐开始降尿酸治疗。
笔者注解1:中国指南推荐无症状高尿酸血症降尿酸治疗的指征为:血尿酸水平≥540μmol/L或≥480μmol/L,且有下列合并症之一:高血压、脂代谢异常、糖尿病、肥胖、脑卒中、冠心病、心功能不全、尿酸性肾石病、肾功能损害(≥CKD2期)。
中国指南的出发点是基于高尿酸血症的危害,而ACR的指征持反对意见,则主要基于目前无高质量证据支持无症状高尿酸血症长期降尿酸治疗获益超过治疗费用以及大量的患者发展成痛风的风险并不高,这与欧洲EULAR的推荐意见是一致的。
美国的痛风指南不推荐把苯溴马隆作为降尿酸首选!仅作为二线方案

美国的痛风指南不推荐把苯溴马隆作为降尿酸首选!仅作为二
线方案
痛风发作,首选什么药物控制?
1、抑制尿酸生成药:别嘌醇、非布司他
2、促进尿酸排泄药:目前上市的促尿酸排泄药物主要是苯溴马隆、丙磺舒。
(对肾结石者、尿酸肾病者而言,促尿酸排泄治疗并不适宜。
促尿酸排泄药物能增加结石、尿酸肾病风险,且适应面狭窄、副反应风险大。
美国的痛风指南不推荐把将促尿酸排泄药物作为降尿酸首选,只将其考虑为其他降尿酸药物不耐受时的二线方案。
)
实际上,降尿酸治疗首选推荐药物为抑制尿酸生成药:别嘌醇、非布司他。
不管什么类型的血尿酸偏高,抑制尿酸生成药都可以很好起到降血尿酸的作用,而且有研究表示:对慢性肾病患者采用别嘌醇和非布司他降尿酸治疗后,可以看到患者肾脏功能衰退的速度明显减缓,可以保护肾脏。
别嘌醇有很好的心脏保护效果。
长期使用可以提高了左室功能并防止了左室重构。
相对于非布司他的不良反应,别嘌醇更加安全。
一般首选抑制尿酸生成的药物,不推荐促进尿酸排泄药物。
因为:促尿酸排泄药物在使用前要依据患者的肾脏功能,肾脏不好的人,不推荐使用。
美国医学界建议的痛风菜谱
美国痛风斗士建议的菜谱如果禁食海鲜和啤酒,痛风病将会离你而去。
这个认识是不正确的。
在所有有关痛风的错误认知中,有关饮食的是最具有顽强生命力的。
就像所有善意的谎言一样,其中也包含一些科学依据。
海鲜和啤酒都被医学界证实,能够强有力的迅速提高尿酸水平,触发病痛的袭击。
在大部分病例中,患者严格地只食用低嘌呤食物(低尿酸)禁食海鲜和啤酒。
但是这样的饮食仅仅减少了病发的次数,并没有完全消灭病痛。
90%的痛风病例是遗传性的,饮食习惯无法战胜遗传基因。
假如你是痛风患者,你必须远离所有的酒精饮料。
这个认识也是片面的。
畅饮啤酒已经被证实对于痛风病是非常不利的,甚至很糟糕。
痛风病患必须远离啤酒,一滴也不能沾。
这条建议不会使我成名,但他是真理。
酒精类饮料如伏特加、威士忌,他们也会提高攞患痛风的风险,但只是啤酒危害的一半。
但是,假如您每天饮用不超过一杯,红酒可以轻微降低你攞患痛风的风险。
那么两杯呢,风险和一杯也不喝一样。
因此这里的关键是假如你想喝一杯,坚持选择红酒并限制自己最多两杯。
我的体重超重,但是这和痛风一点关系也没有。
完全错误。
体重超标与hyperuricemia(中文称为高尿酸血症)和痛风有密切联系。
为了防止痛风同时也保持你的身体健康,最佳方法就是减肥,向超重说拜拜。
假如你是痛风病患且体重严重超标,我可以保证你肯定患有上文提及的某一种致命的疾病。
当然,没有人愿意面对这个论断。
减轻体重和定期加强锻炼是避免此类疾病的最佳方式。
更有趣的是,痛风病人最常见的食疗法是一张“低嘌呤食谱”(low purine diet)。
但是几乎没有人,甚至包括医学专家,能够真正理解低嘌呤物质的意义。
的确,他们可能拿出一张旧图表,以告知你远离贝类海鲜、啤酒和芦笋等等。
但是,你需要了解这张列表仅仅是基于猜测的。
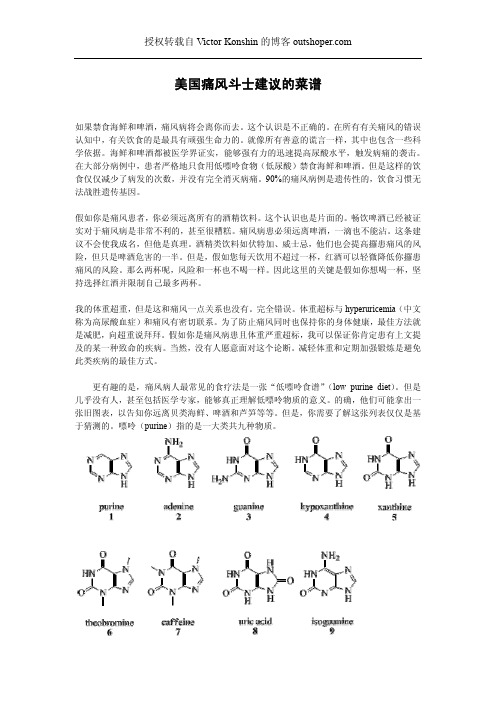
嘌呤(purine)指的是一大类共九种物质。
图1 嘌呤图2 腺嘌呤图3 鸟嘌呤图4 次黄嘌呤图5 黄嘌呤图6 可可碱图7 咖啡因图8 尿酸图9 异鸟嘌呤医学家对长期饮茶或者饮用咖啡的人进行了相关研究。
美国ACR的痛风2020指南(草案)修改解读

美国ACR的痛风2020指南(草案)修改解读11 月 13 日美国风湿病学会学术年会隆重发布了 2020ACR 痛风临床实践指南(草案)。
余金泉医生介绍了部分修改要点「1」。
今天我们一起来解读部分重要内容。
(帅气的余金泉医生在美国 ACR)为什么在痛风发作的急性期可降尿酸?我们已知痛风是尿酸盐结晶所致。
降低血尿酸水平则成为预防复发的关键措施。
在今天,我们提倡血尿酸的达标治疗。
即把血尿酸水平维持在特定偏低的浓度,从而达到几乎永久不复发的目的「2、3、4」。
但,我们习惯上不在痛风发作的时候降血尿酸治疗。
这一做法的依据是急性期降尿酸治疗可能会加重或延长炎症反应。
但是,实际上这更多是基于临床经验,并无高质量研究来支持该做法「5」。
在实际临床工作里,我们看到如下问题:1. 很多痛风病人几乎不间断的痛风发作或持续。
由于没有规范降尿酸治疗,痛风带来的关节炎症无法终止。
因为医生在等待病人痛风不再发作。
2. 由于痛风发作时的抗炎症效果有效,导致病人不再配合医生做降尿酸治疗。
从而带来痛风反复或持续存在。
基于此,我们能否在痛风发作的时候开始启动药物降尿酸治疗呢?一项小规模随机对照试验发现「6」:在痛风发作时启动药物降尿酸治疗并没有引发痛风持续存在。
此后有更多研究证实了该看法「7」。
更强的证据:一些系统回顾证实,在痛风发作时即启动药物降尿酸是可以的「8、9」。
在综合多个研究后,现在美国ACR 最新的痛风指南草案里提出「有条件」时可在痛风发作时启动药物降尿酸治疗。
笔者认为需注意如下风险:1. 警惕合并使用时的药物副反应。
2. 药物降尿酸的强度不宜大,避免血尿酸剧烈波动。
(痛风患者的特征性变化)为什么选择别嘌醇作为一线降尿酸药物?目前国内降尿酸治疗时,很多医生选择苯溴马隆。
但苯溴马隆的安全性值得怀疑----有潜在的严重肝毒性风险。
欧洲的研究证实约17000 人即可有 1 例,而日本研究证实东亚人的概率更高「10」。
除了肝毒性外,促尿酸排泄药物可能增加尿酸盐肾结石的风险。
2012美国ACR痛风指南(第一部分)

16North Mississippi Medical Center, Tupelo, MS 17Southern California Permanente Medical Group, Downey, CA 18University of Florida, Gainesville, FL 19Cleveland Clinic, Cleveland, OH 20University of Pennsylvania and VA Medical Center, Philadelphia, PA 21Harvard Vanguard Medical Associates/Atrius Health, Somerville, MA 22VA Medical Center. Birmingham, Alabama and University of Alabama, Birmingham, AL Keywords Allopurinol; Febuxostat; Probenecid; Pegloticase; Uricosuric; Xanthine Oxidase; Tophi INTRODUCTION Gout is a disorder that manifests as a spectrum of clinical and pathologic features built on a foundation of an excess body burden of uric acid, manifested in part by hyperuricemia,which is variably defined as serum urate greater than either 6.8 or 7.0 mg/dL (1;2). Tissue deposition of monosodium urate monohydrate crystals in supersaturated extracellular fluids of the joint, and certain other sites, mediates most of the clinical and pathologic features of gout. Typically, the disease first presents as arthritis that is acute and episodic, but can become recurrent in the majority of individuals. Gout also can manifest as chronic arthritis of one or more joints (1;2). Tophi, mainly in articular, periarticular, bursal, bone, auricular,and cutaneous tissues are a pathognomonic feature of gout, and are detectable by physical exam, and/or by imaging approaches and pathology examination (3;4;5). Renal manifestations of gout include urolithiasis, typically occurring with an acidic urine pH (1;2).Chronic interstitial nephropathy, mediated by monosodium urate monohydrate crystal deposition in the renal medulla, can occur in severe disease, but is currently considered to be an uncommon clinical manifestation of gout.Gout is one of the most common rheumatic diseases of adulthood, with self-reportedprevalence in the USA recently estimated at 3.9% of adults (~8.3 million people)(6).Prevalence of gout has risen in many countries (e.g., New Zealand), and especially in theUSA over the last few decades, mediated by factors such as increased prevalence of co-morbidities that promote hyperuricemia, including hypertension, obesity, metabolicsyndrome, type 2 diabetes, and chronic kidney disease (CKD)(7–10). Other factors in therising prevalence of gout include certain dietary trends and widespread prescription ofthiazide and loop diuretics for cardiovascular diseases (11). Many gout patients, includingthe growing subset of affected elderly, have complex co-morbidities and medication profilesthat complicate overall management (12). Long-term morbidity and impairment of health-related quality of life are now better appreciated in many gout patients, particularly thosewith multiple co-morbidities and/or chronic gouty arthritis (13;14). Despite advancedunderstanding of the molecular bases of hyperuricemia and gouty inflammation, and theextensive practice experience of many providers, substantial quality of care gaps exist ingout management (15). Moreover, significant shortfalls in patient education and adherencehave been identified in gout (16).On behalf of the American College of Rheumatology (ACR), we were charged withdeveloping systematic non-pharmacologic and pharmacologic recommendations foreffective treatments in gout with acceptable risk-benefit ratio. Our assignment was to focuson four specific domains in gout management. Two of these domains are addressed herein,(i) Urate-lowering therapy (ULT), and (ii) chronic gouty arthritis with tophaceous diseasedetected on physical examination (designated by the ACR with the terminology “chronictophaceous gouty arthropathy” (abbreviated CTGA), and specifically represented in theNIH-PA Author ManuscriptNIH-PA Author ManuscriptNIH-PA Author Manuscriptfundamental case scenarios 7–9 described herein). Domains iii-iv (analgesic and anti-inflammatory management of acute gouty arthritis, and pharmacologic anti-inflammatory prophylaxis of attacks of gouty arthritis, respectively) are addressed in a separate manuscript (Part II of the guidelines)(17).There are multiple lines of epidemiologic and experimental evidence that hyperuricemia, via effects of excess soluble urate, may play a role in some human renal, cardiovascular, and metabolic co-morbidities also frequently associated with gout (7–10). We did not address pharmacologic management of asymptomatic hyperuricemia, due to a paucity of prospective, randomized, controlled human research trials in that area (18).We were charged by the ACR with developing gout recommendations based on evidence as available, at an international level, for rheumatologists and other health care providers,including other subspecialists, primary care practitioners, nurse practitioners, physician assistants, and allied health professionals. The ACR requested that we apply the established Research and Development/University of California at Los Angeles (RAND/UCLA)Appropriateness Method (19) to generate recommendations, and engaged a diverse,international panel of experts. Creating novel classification of gout as a disease, new gout diagnostic criteria, or definition of treatment outcomes were beyond the scope of this work.Instead, we generated multifaceted case scenarios to elucidate decision-making based primarily on clinical and laboratory test-based data that can be obtained on a gout patient in an office practice setting.Guidelines for gout management have been generated in the last decade, at the national or multinational society level, by the European League Against Rheumatism (EULAR)(20;21),the Dutch College of General Practitioners (22), the Japanese Society of Gout and Nucleic Acid Metabolism (23), and the British Society for Rheumatology (BSR)(24). Moreover, the National Institute for Health and Clinical Excellence (NICE) single technology appraisal (STA) process has been applied to ULT in gout using febuxostat (25). New guidelines wererequested by the ACR, as the understanding of gout risk factors has been greatly augmented by recent clinical research (12). Moreover, ULT options recently increased via clinicaldevelopment, and drug regulatory agency approval of new pharmacologic agents (febuxostat and the biologic drug pegloticase)(26;27). New imaging approaches for gout that can detect radiographic changes of early disease not visualized by plain radiography (e.g., highresolution ultrasound, dual energy computed tomography (DECT)(28;29), are beinginvestigated for impact on gout diagnosis, and assessment of disease burden and severity,and choices and effectiveness of management. Developments such as these are considered in the work of this committee, which was built on several key assumptions (Table 1).The ACR gout guidelines are designed to emphasize safety, and quality of therapy, and to reflect best practice, as evaluated by a diverse group of experts that examine the level ofevidence available at the time. Importantly, societal cost of health care, and cost and cost-effectiveness differences between therapies are excluded from analysis by the RAND/UCLA Appropriateness Methodology (19) (Table 1). Individual results of this work are designated as “recommendations” rather than guidelines, in order to reflect the non-prescriptive nature of decision-making evaluated by experts, and based on available evidence at the time. The recommendations cannot substitute for individualized, direct assessment of the patient,coupled with clinical decision making by a competent health care practitioner. Treatment recommendations also assume appropriate attention to potential drug interactions (eg, with anticoagulants, azathioprine, amoxicillin), and effects of co-morbidities such as diabetes,and renal, cardiac, gastrointestinal, and hepatic disease (Table 1). The motivation, financial circumstances, and preferences of the gout patient play a very important role. Moreover, the NIH-PA Author ManuscriptNIH-PA Author ManuscriptNIH-PA Author Manuscriptrecommendations for gout management presented here are not intended to limit or denythird party payer coverage of health care costs for groups, or individual patients, with gout.METHODSProject design and development of recommendations and grading of evidence The overall design of the project is schematized in Supplemental Figure 1. The RAND/UCLA consensus methodology, developed in the 1980s, incorporates both Delphi and nominal group methods (19;30), and was successfully used to develop other guidelines commissioned by the ACR. The purpose of this methodology is to reach a consensus among experts, with an understanding that published literature may not be adequate to provide sufficient evidence for day-to-day clinical decision-making. The RAND/UCLA method requires 2 groups of experts—a core expert panel (CEP) that provides input into case scenario development and preparation of a scientific evidence report, and a task force panel (TFP) that votes on these case scenarios. Our CEP consisted of leaders for each domain (Supplemental Figure 2). Pharmacologic approaches, and diet, lifestyle and non-pharmacologic measures (e.g., weight loss, exercise) were addressed within each domain.The CEP leaders communicated with an international panel of gout experts, and the PIs, to develop initial case scenarios that reflect broad differences in severity of the disease and its clinical manifestations. In addition, there were weekly interactive teleconferences between domain leaders and PIs to refine case scenarios. Though previous systematic review for gout has been performed by EULAR, as a prime example, we performed our own systematic review of pertinent literature. The resultant scientific evidence report given to the TFP in conjunction with clinical scenarios representing a broad scope of disease, with multiple questions of interest, and alternative options, for each case scenario.By ACR mandate, the TFP had a majority of members without perceived potential conflict of interest (COI), and had diverse experience and expertise, as described in detail inSupplemental Figure 2. The TFP included 7 rheumatologists (one of whom is a Chair ofInternal Medicine, and one an Internal Medicine Residency Training Program Director), 2primary care physicians, a nephrologist, and a patient representative. There were 2 rounds of ratings, the first anonymously with the members of the TFP instructed to rank each of the potential elements of the guidelines on a risk-benefit basis ranging from 1 to 9 on a Likert scale using Delphi process, followed by a face-to-face group discussion and then re-voting of the same scenarios. A vote of 1–3 on the Likert Scale was rated as Inappropriate = risks clearly outweigh the benefits. A vote of 4–6 on the Likert Scale was considered Uncertain =risk-benefit ratio is uncertain. A vote of 7–9 on the Likert Scale was rated as Appropriate =benefits clearly outweigh the risks. Samples of votes taken and results are provided inSupplemental Figure 3. Votes on case scenarios were translated into recommendations if the median voting score was graded 7–9 (“appropriate”) and if there was no significantdisagreement, defined as no more than 1/3 of the votes graded (“inappropriate”) for thescenario. The final rating was done anonymously in a 2-day face-to-face meeting, facilitated by an experienced moderator (Neil Wenger). During the face-to-face TFP meeting, some case scenarios were clarified for content or verbiage, and re-voted on by the TFP.The level of evidence supporting each recommendation was ranked based on previousmethods used by the American College of Cardiology (31) and applied to recent ACRrecommendations (32;33). Level A grading was assigned to recommendations supported by multiple (i.e., more than one) randomized clinical trials or meta-analyses. Level B grading was assigned to the recommendations derived from a single randomized trial, ornonrandomized studies. Level C grading was assigned to consensus opinion of experts, case studies, or standard-of-care.NIH-PA Author ManuscriptNIH-PA Author ManuscriptNIH-PA Author ManuscriptSystematic reviewPubMed and Cochrane Central Register of Controlled Trials (CENTRAL) from the 1950s to the present were searched to find articles on gout with help of an experienced librarian(Rikke Ogawa). We used a search strategy based on the Cochrane Highly Sensitive Search Strategy for identifying randomized trials. The search was expanded to include articlesdiscussing research designs such as cohort, case control and cross sectional studies. Limits included English Language and the exclusion of “animal only” studies. The exact terms,process and results of the search are summarized in Supplemental Figure 4.Clinical Case DescriptionsThe TFP evaluated scenarios with a broad spectrum of clinical gout, similar to what aclinician might see in a busy practice, and divided into mild, moderate, and severe disease activity in each of three distinct “treatment groups” (Figure 1A–B). In generating these nine fundamental clinical case scenarios, mild disease activity levels in each “treatment group”were meant to represent patients at the lowest disease activity level for which mostclinicians would consider initiating or altering a specific medication regimen. Conversely,severe disease activity level was intended to represent patients with disease activity equal or greater to that of the “average” subject studied in a clinical trial. The case scenarios were not intended to serve as classification criteria. To allow the TFP to focus on managementdecisions, each case scenario had the assumption not only that the diagnosis of gout was correct, and that there was some clinical evidence of gout disease activity. This included intermittent symptoms of variable frequency, specifically presented to the TFP as episodes of acute gouty arthritis of at least moderate to severe pain intensity (17). Clinical evidence of gout disease activity, presented to the TFP, also included one or more tophi detected byphysical exam, or alternatively, chronic symptomatic arthritis (ie, “chronic arthropathy” or “synovitis”) due to gout, with or without confirmed joint damage (e.g., deformity, erosion due to gout on imaging study). Hyperuricemia was defined here as serum urate >6.8 mg/dL(2). We determined all aspects of case scenario definitions by a structured iterative process,using regular electronic mail, and teleconferences at least once per month. Multiplerevisions to the proposed parameters were carried out, until accepted by the CEP domain leaders.Definitions of pharmacologic therapeutic agentsMedication classes evaluated in the case scenarios were defined as follows: Xanthineoxidase inhibitor (XOI) refers to allopurinol or febuxostat; uricosuric agents were defined to include agents available in the USA (probenecid, and off-label use (as uricosuric therapy) of fenofibrate and losartan), but did not include sulfinpyrazone or benzbromarone. Other agents and modalities were self-explanatory. Evaluation by the TFP of effectiveness of a giventherapeutic option assumed that patients in case scenarios received the maximum tolerated typical dose for a period of time sufficient to accurately assess therapeutic response, unless otherwise indicated.Managing perceived potential COIPerceived potential COI was managed in a prospective and structured manner. Specifically,all participants intellectually involved in the project, whether authors or not, were required to fully, and prospectively disclose relationships with pharmaceutical companies with amaterial interest in gout (Supplemental Material Discussion). Disclosures were updatedevery 6 months, and for the PIs, CEP and TFP, updated just prior to the face-to-facemeeting. A summary listing of all perceived potential COI was disseminated to allparticipants in the project, and is available in the supplemental materials. Based on thepolicies of the ACR, which are aligned with those of many medical societies, no more than NIH-PA Author Manuscript NIH-PA Author Manuscript NIH-PA Author Manuscript49% of project participants could have COI at any given time. It was required that theproject PI (John FitzGerald) remain without perceived potential COI prior to and during the process.RESULTS Primary principles of management for all gout case scenarios The TFP generated recommendations for a systematic non-pharmacologic and pharmacologic management approach intended to be applicable to all patients with gout,which is summarized in Figure 3. This was based on the assumption that the diagnosis of gout was correct before initiation of management. The approach highlighted patient education on the disease and treatments and their objectives, and initiation of diet and lifestyle recommendations. The TFP also recommended elimination of prescription medication that were non-essential for the optimal management of co-morbidities (eg,hypertension, CHF, hyperlipidemia, or major organ transplant) in an individual patient,where such medication elevated serum urate levels; with prime examples being thiazide and loop diuretics, niacin, and calcineurin inhibitors (Evidence C). Though low dose acetylsalicylic acid (aspirin ≤ 325 mg daily) elevates serum urate, the TFP did not recommend discontinuation of this modality as cardiovascular disease prophylaxis in gout patients. In discussion, without a specific vote, the TFP viewed the relative risks specifically attributable to the modest effects of low dose aspirin on serum urate as negligible in gout management.The TFP recommended that clinicians consider causes of hyperuricemia for all gout patients,and recommended a specific co-morbidity checklist (Evidence C)(Table 2). In doing so, the TFP specially recommended consideration, and if indicated, medical evaluation of certain agents and disorders that cause uric acid underexcretion or overproduction, and thereby could merit laboratory investigations such as urinalysis, renal ultrasound, a completehemogram, or urine uric acid quantification as indicated. In this context, the TFPspecifically recommended screening for uric acid overproduction (by urine uric acidevaluation for uric acid overproduction), in patient subsets with gout clinical disease onset before age 25 (Evidence C), or a history of urolithiasis (Evidence C).The TFP provided guidance for referral to a specialist, with caution to avoid appearing self-serving. Though limited by the absence of outcomes data on potential benefits of referral,the TFP recommended that gout case scenarios including any of the following should be amongst those where referral to a specialist is considered (Evidence C for all): (i) Unclear etiology of hyperuricemia; (ii) Refractory signs or symptoms of gout; (iii) Difficulty inreaching the target serum urate level, particularly with renal impairment and a trial of XOI treatment; (iv) Multiple and/or serious adverse events from pharmacologic ULT.Clinical evaluation of gout disease activity and burdenThe TFP recommended clinical evaluation of gout disease symptom severity and burden in individual patients by history and thorough physical exam for symptoms of arthritis, and signs such as tophi and acute and chronic synovitis (Evidence C). To be actionable byclinicians, the authors, without a specific TFP vote, suggest that clinicians can work with patients to record and estimate the number per year, and severity (17) of acute attacks of gouty arthritis per year.Core recommendations for non-pharmacologic ULT measures in goutThe TFP recommended certain diet and lifestyle measures for the vast majority of patients with gout (Evidence B-C for individual measures) (Figure 4). Many of the diet and lifestyle NIH-PA Author ManuscriptNIH-PA Author ManuscriptNIH-PA Author Manuscriptmeasures were recommended for decreasing the risk and frequency of acute gout attacks(12) and lowering serum urate, but the primary emphasis of the TFP recommendations in Figure 4 was on diet and lifestyle choices for promotion and maintenance of ideal health,and prevention and optimal management of life-threatening comorbidities in gout patients,including coronary artery disease (34,35), and obesity, metabolic syndrome, diabetes,hyperlipidemia, and hypertension.Dietary recommendations were grouped into 3 simple qualitative categories, termed “limit”,“avoid”, or “encourage” (Figure 4). This approach, with rare exceptions (36,37), reflected a general lack of specific evidence from prospective, blinded, randomized clinical intervention trials that linked consumed quantities of individual dietary components to changes in either serum urate levels or gout outcomes. Notably, the replication of hazardous lifestyle riskfactors in a conventional clinical research trial would potentially pose both design andethical difficulties. As such, the TFP deliberated on evidence regarding the impact ofexposures to alcohol or purine-rich foods in a short time frame. The evidence sources were epidemiologic studies of hyperuricemia and incident gout, including long-term prospective analyses and internet-based case-crossover studies. The TFP recommended that goutpatients limit their consumption of purine-rich meat and seafood (Evidence B) as well as high fructose corn syrup sweetened soft drinks and energy drinks (Evidence C), andencouraged the consumption of low-fat or non-fat dairy products (Evidence B) (38)(Figure4). The TFP also recommended reduced consumption of alcohol (particularly beer, but also wine and spirits), and avoidance of alcohol overuse in all gout patients (Evidence B) (Figure4). The TFP further recommended abstinence from alcohol consumption for gout patients during periods of active arthritis, especially with inadequate medical control of the disorder and in CTGA (Evidence C)(39). Significantly, in discussion by the TFP, without a specific vote, the TFP recognized that diet and lifestyle measures alone provide therapeuticallyinsufficient serum urate-lowering effects and/or gout attack prophylaxis for a large fraction of individuals with gout (12). For example, some clinical trials on diet and fitness havereported only ~10–18% decrease in serum urate (38). In further discussion by the TFP, again without a specific vote, the TFP viewed this degree of serum urate-lowering as beneficial for all case scenarios, but insufficient to achieve an effective serum urate target in those with sustained hyperuricemia substantially above 7 mg/dL.Core recommendations for pharmacologic ULT, including the serum urate targetHere, and with all other recommendations for drug therapy in Parts I and II of the 2012 ACR Guidelines for gout, the recommendations assumed a lack of contra-indications, intolerance,serious adverse events, or drug-drug interactions for given agents. The TFP recommended gout with CKD stage 2–5, or end stage renal disease (ESRD), as an appropriate indication,by itself, for pharmacologic ULT (Evidence C) in patients with prior gout attacks andcurrent hyperuricemia. In pharmacologic ULT, certain treatment choices (e.g., probenecid)and drug dosing decisions (e.g., allopurinol) are impacted by the creatinine clearance. The TFP, without a direct vote, discussed and recognized the clinical value of accuratemeasurement of creatinine clearance, not simply the serum creatinine, in ascertaining the degree of renal impairment. However, the scope of the project did allow for detailed,prescriptive recommendations regarding specific ULT drug doses, or usage of individual agents in the presence of a given degree of either renal impairment, or other co-morbidities such as hepatic impairment.TFP recommendations for pharmacologic ULT, presented graphically in Figure 3, included recommendation of xanthine oxidase inhibitor (XOI) therapy with either allopurinol orfebuxostat as the first line pharmacologic approach (Evidence A). The panel did notpreferentially recommend either XOI over the other XOI drug. In doing so, the TFP weighed the lack of published safety data for febuxostat in the setting of stage 4 or worse CKD.NIH-PA Author Manuscript NIH-PA Author Manuscript NIH-PA Author ManuscriptProbenecid was recommended as an alternative first line pharmacologic ULT option, in the setting of contra-indication or intolerance to at least one XOI agent (Evidence B). However,the TFP did not recommend probenecid as a first line ULT monotherapy in those with acreatinine clearance below 50 ml/min.The TFP recommended that pharmacologic ULT could be started during an acute goutattack, providing that effective anti-inflammatory management has been instituted (EvidenceC). The TFP recommended regular monitoring of serum urate (every 2–5 weeks) duringULT titration, including continuing measurements once the serum urate target is achieved (every 6 months) (Evidence C). The TFP weighed this measure as particularly useful tomonitor adherence, given that poor adherence to ULT is a common problem in gout patients(16).The TFP recommended that the goal of ULT is to achieve a serum urate target, at aminimum, of < 6 mg/dL in all gout case scenarios (Evidence A). Moreover, the TFPrecommended that the target serum urate should be lowered sufficiently to durably improve signs and symptoms of gout, including palpable and visible tophi detected by physicalexamination, and that this may involve therapeutic serum urate-lowering to below 5 mg/dL (Evidence B).Recommendations specific to allopurinol dosing and pharmacogenetics TFP recommendations for use of allopurinol in gout are summarized in Table 3A.Importantly, the TFP recommended that the starting dose of allopurinol be no greater than 100 mg per day (Evidence B)(40), consistent with prior FDA and EULAR guidelines (21).The rationale of the TFP was partly that a low allopurinol starting dose could reduce early gout flares after ULT initiation, (26), and partly as a component of risk management with respect to the potential for severe hypersensitivity reaction to allopurinol (40), discussed in further detail below. The TFP recommended gradual upward titration of the allopurinolmaintenance dose every 2–5 weeks to an appropriate maximum dose for gout, in order to treat to the serum urate target appropriate for the individual patient (Evidence C).The TFP weighed robust evidence that allopurinol monotherapy at doses of 300 mg daily or less failed to achieve the serum urate target of <6 mg/dL (26,41), or < 5 mg/dL (42,43), in more than half of subjects with gout. The TFP reviewed small studies in which theallopurinol dose was titrated above 300 mg daily in gout with overall success in achieving the serum urate target (43,44). Importantly, in doing so, the TFP also recommended that the maintenance dose of allopurinol can be raised above 300 mg per day, even in those withrenal impairment, provided there is adequate patient education and regular monitoring for drug hypersensitivity and other adverse events, such as pruritis, rash, and elevated hepatic transaminases, as well as attention to potential development of eosinophilia (Evidence B).The TFP next considered the issue of measures to reduce the incidence of severe allopurinol hypersensitivity reactions, here termed allopurinol hypersensitivity syndrome (AHS). TFP discussion recognized the potential for hospitalization and severe morbidity, and thereported mortality rate of 20–25% in AHS (45,46). The estimated incidence of AHS is~1:1000 in the USA and its spectrum includes not only Stevens-Johnson Syndrome andToxic Epidermal Necrolysis, but also systemic disease with a clinical constellation offeatures such as eosinophilia, vasculitis, rash, and major end-organ disease (47). Concurrent thiazide use and renal impairment have been implicated as risk factors for AHS (48–50). A widely employed risk management strategy has been a non-evidence-based algorithm for allopurinol maintenance dosing, calibrated to renal impairment (51)(Evidence C);importantly, the TFP did not recommend this strategy,NIH-PA Author ManuscriptNIH-PA Author ManuscriptNIH-PA Author Manuscript。
2020美国风湿病学会ACR痛风管理指南解读

2020美国风湿病学会ACR痛风管理指南解读Abstract•核心团队、专家小组和投票小组(由风湿病学家、普通内科医生、肾病学家、医师助理和患者代表组成)提出了57个PICO问题,以解决以下问题:对痛风患者(9个问题)和无症状高尿酸血症患者(8个问题)进行ULT适应症(5个问题)、开始ULT的方法(7个问题)、持续ULT管理(18个问题)、痛风发作(10个问题)、生活方式和其他药物治疗策略。
目录• 1.起始降尿酸治疗(ULT)指征• 2.痛风患者初始ULT的建议• 3.ULT起始时机• 4.ULT持续时间• 5.ULT具体药物的使用建议• 6.何时考虑更改ULT策略•7.痛风急性期管理•8.生活方式管理•9.同用药物的管理推荐强度分为“强烈推荐”和“有条件推荐”两类•➤强烈推荐:有中、高级别证据支持,获益大于风险。
•➤有条件推荐:获益和风险可能更为平衡,和/或证据级别较低或无相关数据。
•本指南中的建议适用于痛风患者,除外一项对无症状高尿酸血症患者[定义为血清尿酸(SU)≥6.8mg/dL(405umol/L),无痛风发作史或痛风石)]的降尿酸治疗建议。
•强烈建议对具有以下任一特征的痛风患者起始降尿酸治疗:–1个或多个皮下痛风石。
(证据级别:高)–有证据表明存在痛风引起的任何形式的影像学损伤。
(证据级别:中)–痛风频发(≥2次/年)。
(证据级别:高)•对于经历过>1次痛风急性发作,但并不频繁(<2次/年)的患者,可推荐起始ULT治疗。
(证据级别:中)•对于首次痛风发作的痛风患者,推荐不要启动ULT治疗。
但对于以下患者,可推荐启动ULT:伴有中度至重度慢性肾病(CKD>3期)、血尿酸(SU)>9mg/dL(535.5umol/L)或尿石症的患者。
(证据级别:中)•对于无症状高尿酸血症患者(SU >6.8 mg/dl,无痛风发作或皮下痛风石),不推荐起始ULT。
(证据级别:高)中国高尿酸和痛风诊疗指南, 中国内分泌代谢杂志2020,36(1)•对于起始ULT,强烈推荐:–强烈推荐别嘌醇作为ULT的首选一线药物,包括在中、重度CKD患者中(CKD>3)。
《2020ACR痛风管理指南》.doc

《2020ACR痛风管理指南》2020ACR指南共包含以下9个部分:1.起始降尿酸治疗(ULT)指征2.痛风患者初始ULT的建议3.ULT起始时机4.ULT持续时间5.ULT具体药物的使用建议6.何时考虑更改ULT策略7.痛风急性期管理8.生活方式管理9.同用药物的管理推荐强度分为“强烈推荐”和“有条件推荐”两类:➤强烈推荐:有中、高级别证据支持,获益大于风险。
➤有条件推荐:获益和风险可能更为平衡,和/或证据级别较低或无相关数据。
本指南中的建议适用于痛风患者,除外一项对无症状高尿酸血症患者[定义为血清尿酸≥6.8mg/dL(405umol/L),无痛风发作史或痛风石)]的降尿酸治疗建议。
1.起始ULT的指征强烈建议对具有以下任一特征的痛风患者起始降尿酸治疗:➤1个或多个皮下痛风石。
(证据级别:高)➤有证据表明存在痛风引起的任何形式的放射学损伤。
(证据级别:中)➤痛风频发(>2次/年)。
(证据级别:高)对于经历过多于一次痛风急性发作,但并不频繁(<2次/年)的患者,有条件推荐起始ULT治疗。
(证据级别:中)对于首次痛风发作的痛风患者,有条件推荐不要启动ULT治疗。
但对于以下患者,有条件推荐启动ULT:伴有中度至重度慢性肾病(CKD>3期)、血尿酸(SU)>9mg/dL(535.5umol/L)或尿石症的患者。
(证据级别:中)对于无症状高尿酸血症患者,有条件推荐不起始ULT。
(证据级别:高)2.痛风患者初始ULT的建议对于起始ULT,强烈推荐:➤强烈推荐别嘌醇作为ULT的首选一线药物,包括在中、重度CKD患者中(CKD>3)。
(证据级别:中)➤强烈推荐在中、重度CKD患者中(CKD>3期),别嘌醇和非布司他的选择级别优先于丙磺舒。
(证据级别:中)➤强烈不建议将培戈洛酶(pegloticase)作为一线选择。
(证据级别:中)➤建议低剂量起始,随后逐步滴定:别嘌醇起始剂量<100mg/d(对于CKD >3期患者,剂量应更低);非布司他起始剂量<40mg/d;丙磺舒起始剂量为500mg,qd或bid(有关丙磺舒低剂量起始为有条件推荐)。
痛风管理指南acr指南

痛风管理指南: ACR指南简介痛风是一种常见的代谢性疾病,以高尿酸血症和尿酸盐沉积引起的关节炎为主要临床表现。
目前,美国风湿病学会(ACR)发布了一份痛风管理指南,旨在为临床医生和患者提供最新的痛风治疗和管理建议。
本文将以ACR指南为基础,介绍痛风的诊断、治疗和预防措施。
诊断根据ACR指南,痛风的诊断主要基于以下几个方面:1.临床症状:典型的痛风发作表现为急性关节炎,多累及大趾关节,常伴有剧烈疼痛和红、肿、热等症状。
2.尿酸水平:血尿酸水平升高是痛风的重要指标,但并非所有高尿酸血症患者都会发展为痛风。
ACR指南建议根据临床症状和尿酸水平来进行诊断。
3.关节液分析:关节液中尿酸结晶的检测是确诊痛风的金标准,但不是所有不明原因的急性关节炎都需要进行关节液分析。
治疗ACR指南给出了痛风治疗的详细建议,包括急性发作期的治疗和长期控制期的治疗。
急性发作期的治疗•非甾体类抗炎药(NSDs):对于急性发作期的疼痛和炎症,NSDs是首选的治疗药物。
•秋水仙碱:秋水仙碱是一种可用于急性痛风发作的药物,常用于NSDs不能耐受或禁用的患者。
•肾上腺皮质激素:对于严重症状或其他药物无效的患者,肾上腺皮质激素是有效的选择。
长期控制期的治疗•尿酸降低治疗:ACR指南推荐在持续高尿酸血症的患者中进行尿酸降低治疗,以减少痛风发作的风险。
•遵循药物治疗原则:ACR指南强调在进行药物治疗时应根据患者的肾功能和疗效来选择合适的药物,并定期监测尿酸水平。
•饮食和生活方式管理:控制体重、限制酒精和高嘌呤食物的摄入,以及适当的运动可以帮助预防痛风和减轻症状。
预防ACR指南认为痛风是可以预防的,以下是一些预防痛风的措施:•饮食调整:限制酒精和高嘌呤食物的摄入,增加低脂鱼类和植物蛋白的摄入。
•体重控制:减轻过重和肥胖可以降低痛风的发病风险。
•健康生活方式:适量运动、戒烟和控制血压等生活方式改变有助于预防痛风。
结论ACR痛风管理指南为临床医生和患者提供了最新的痛风诊断、治疗和预防的指南。
《2020ACR痛风管理指南》

《2020ACR痛风管理指南》2020ACR指南共包含以下9个部分:1.起始降尿酸治疗(ULT)指征2.痛风患者初始ULT的建议3.ULT起始时机4.ULT持续时间5.ULT具体药物的使用建议6.何时考虑更改ULT策略7.痛风急性期管理8.生活方式管理9.同用药物的管理推荐强度分为“强烈推荐”和“有条件推荐”两类:?强烈推荐:有中、高级别证据支持,获益大于风险。
?有条件推荐:获益和风险可能更为平衡,和/或证据级别较低或无相关数据。
本指南中的建议适用于痛风患者,除外一项对无症状高尿酸血症患者[定义为血清尿酸≥6.8mg/dL(405umol/L),无痛风发作史或痛风石)]的降尿酸治疗建议。
1.起始ULT的指征强烈建议对具有以下任一特征的痛风患者起始降尿酸治疗:?1个或多个皮下痛风石。
(证据级别:高)?有证据表明存在痛风引起的任何形式的放射学损伤。
(证据级别:中)?痛风频发(>2次/年)。
(证据级别:高)对于经历过多于一次痛风急性发作,但并不频繁(<2次/年)的患者,有条件推荐起始ULT治疗。
(证据级别:中)对于首次痛风发作的痛风患者,有条件推荐不要启动ULT治疗。
但对于以下患者,有条件推荐启动ULT:伴有中度至重度慢性肾病(CKD>3期)、血尿酸(SU)>9mg/dL(535.5umol/L)或尿石症的患者。
(证据级别:中)对于无症状高尿酸血症患者,有条件推荐不起始ULT。
(证据级别:高)2.痛风患者初始ULT的建议对于起始ULT,强烈推荐:?强烈推荐别嘌醇作为ULT的首选一线药物,包括在中、重度CKD患者中(CKD>3)。
(证据级别:中)?强烈推荐在中、重度CKD患者中(CKD>3期),别嘌醇和非布司他的选择级别优先于丙磺舒。
(证据级别:中)?强烈不建议将培戈洛酶(pegloticase)作为一线选择。
(证据级别:中)?建议低剂量起始,随后逐步滴定:别嘌醇起始剂量<100mg/d(对于CKD >3期患者,剂量应更低);非布司他起始剂量<40mg/d;丙磺舒起始剂量为500mg,qd或bid(有关丙磺舒低剂量起始为有条件推荐)。
《美国医师协会临床实践指南急性和复发性痛风管理》解读

·1645· E-mail:zgqkyx@ ·标准·指南·【编者按】 痛风是嘌呤代谢紊乱或尿酸排泄减少导致的尿酸盐在体内过多沉积而产生的疾病。
随着人类社会进步,物质生活丰富,痛风发病率逐年增高,发病人群呈年轻化趋势。
针对这一常见病,美国医师协会在2016年底推出《美国医师协会临床实践指南:急性和复发性痛风管理》指南。
本指南重点介绍了急性痛风发作的药物治疗和痛风患者的长期降尿酸药物策略,对于在我国基层医师推广并进行规范化痛风治疗具有重要参考价值。
《美国医师协会临床实践指南:急性和复发性痛风管理》解读熊逸凡,赵东宝*【摘要】 痛风作为一种常见的尿酸盐过度沉积导致的炎性关节疾病,其发病率在世界范围内逐年升高,如何进行相应的急性发作期控制和慢病管理成为重要的卫生问题。
至今,国内外相继推出多部相关指南,其中,2016年美国医师协会(ACP)推出《美国医师协会临床实践指南:急性和复发性痛风管理》指南较有权威性,主要分为急性痛风发作的管理和降尿酸治疗的管理方面。
本文对其进行深度解读,并选择近期推出的国内外痛风指南〔2016年欧洲风湿病防治联合会(EULAR)痛风指南、2017年英国风湿病学会(BSR)痛风指南和2016年中国痛风指南〕进行比较,并就不同点探讨了深层原因,值得我国广大基层医生学习借鉴。
【关键词】 痛风;高尿酸血症;治疗;指南【中图分类号】 R 589.7 【文献标识码】 A DOI:10.3969/j.issn.1007-9572.2018.00.132熊逸凡,赵东宝.《美国医师协会临床实践指南:急性和复发性痛风管理》解读[J].中国全科医学,2018,21(14):1645-1647.[]XIONG Y F ,ZHAO D B.Interpretation of management of acute and recurrent gout :a clinical practice guideline from the American College of Physicians [J ].Chinese General Practice ,2018,21(14):1645-1647.Interpretation of Management of Acute and Recurrent Gout :a Clinical Practice Guideline from the American College of Physicians XIONG Yi-fan ,ZHAO Dong-bao *Department of Rheumatology ,the Changhai Affiliated Hospital of the Second Military Medical University ,Shanghai 200433,China*Corresponding author :ZHAO Dong-bao ,Chief physician ;E-mail :dongbaozhao@【Abstract 】 Gout is a common inflammatory arthritis resulted from excessive accumulation of monosodium urate.Itsmorbidity is increasing worldwide ,so acute gout attack control and chronic gout management are very important.Of the guidelines for gout management published so far ,Management of Acute and Recurrent Gout ,a practice guideline developed by the American College of Physicians (ACP ) in 2016,mainly including management of acute gout and urate-lowering therapy ,is of authoritative significance.We intensively interpreted this guideline ,and compared it with other 3 latest guidelines for gout management generated by the European League Against Rheumatism (EULAR ) in 2016,British Society for Rheumatology (BSR ) in 2017 and Chinese Rheumatology Association in 2016.And for the differences between these guidelines ,we demonstrated the possible reasons.It is worth learning for Chinese primary care physicians.【Key words 】 Gout ;Hyperuricemia ;Therapy ;Guidebooks年的流行病学数据显示痛风发病率为0.86%~2.20%[1-2],给国民的健康、经济带来沉重的负担。
2020年美国风湿病学会《痛风管理指南》解读

. 376 .CHINESE JOURNAL OF EVIDENCE-BASED MEDICINE, Apr. 2021, Vol. 21, No.4•指南解读•2020年美国风湿病学会《痛风管理指南》解读李秀,张姬慧,聂英坤哈尔滨医科大学附属第二医院风湿免疫科(哈尔滨150086)【摘要】痛风是最常见的炎性关节炎,其特征是尿酸盐增高和单钠尿酸盐(M SU)晶体沉积在组织中,引起关节炎、软组织肿块(即痛风石)、肾结石和尿酸盐肾病,对患者健康相关生活质量有重大影响。
美国风湿病学会(A C R)于2020年6月发布了《痛风管理指南》。
该指南提出了42条建议(包括16条强推荐建议),阐述了降尿酸治疗(U L T)的适应症、启动方法、持续管理、痛风发作、痛风患者和无症状高尿酸血症患者的生活方式和其他药物治疗策略。
.本文对其进行解读,以期为临床实践提供参考。
【关键词】痛风;降尿酸;治疗;管理;指南解读2020 American College of Rheumatology guideline for the management of gout: an interpretationLIXiu, ZHANG Jihui, NIE YingkunDepartment of R heumatology, The Second Affiliated Hospital of H arbin Medical University, Harbin 150086, P.R.ChinaCorrespondingauthor:LIXiu,Email:****************【A bstract】Gout is the most common inflammatory arthritis, which is characterized by elevated urate and monosodium urate (MSU) crystal deposition in tissues, leading to arthritis, soft-tissue masses (tophi), nephrolithiasis, andurate nephropathy. It has a major impact on health-related quality of life. The American College of Rheumatology (ACR) published ACR guidelines for the management of g out in June 2020, in which 42 recommendations (including 16 strong recommendations) were generated. The guideline described indications for urate-lowering therapy (ULT), approaches to initiating, ongoing management, gout flares, and lifestyle and other medication strategies in patients with gout and in individuals with asymptomatic hyperuricemia. This paper interprets it to provide references for clinical practice.【Key words 】Gout; Urate-lowering; Treatment; Management; Guideline interpretation痛风是最常见的炎性关节炎,在美国约有920 万成人患病(患病率3.9%) m,在我国患病率约为 1%~3%121。
美国风湿病学会痛风治疗指南,这些要点不容错过!(二)

在2016年EULAR、2017年英国风湿病协会(BSR)相继发表痛风治疗管理指南之后,新型治疗痛风的相关证据不断出现。
2020年ACR发布了新的痛风管理指南。
与之前的指南相比,该版指南中更新的观点颇多,共提出42条建议,其中包括16条强烈建议。
5改变降尿酸治疗的建议①对于首次以最大耐受剂量或FDA指示剂量接受黄嘌呤氧化酶抑制剂单一疗法但未达到血清尿酸目标和(或)出现频繁的痛风发作(≥2次/年)或无法解决的皮下痛风石的痛风患者,选择性建议将第一种黄嘌呤氧化酶抑制剂改为其他黄嘌呤氧化酶抑制剂药物,而不是加用促尿酸排泄药:证据等级极低,选择性推荐;②对于使用黄嘌呤氧化酶抑制剂、促尿酸排泄药和其他干预措施均未能达到血清尿酸目标,并且有频繁的痛风发作(≥2次/年)或无法解决的皮下痛风石的痛风患者,强烈建议改用培戈洛酶治疗:证据等级中,强烈推荐;③对于使用黄嘌呤氧化酶抑制剂、促尿酸排泄药和其他干预措施均未能达到血清尿酸目标,并且没有频繁的痛风发作(<2次/年)以及皮下痛风石的痛风患者,应继续当前降尿酸治疗,强烈建议反对培戈洛酶治疗:证据等级中,强烈反对。
6对于痛风急性发作的治疗①患者痛风发作时,强烈建议使用秋水仙碱、NSAIDs或糖皮质激素(口服、关节内或肌肉注射)作为治疗痛风发作的一线药物,而非IL-1抑制剂或促肾上腺皮质激素(ACTH)(秋水仙碱、NSAIDs或糖皮质激素的选择应基于患者的个体化因素):证据等级高,强烈推荐;②选择秋水仙碱治疗时,强烈推荐小剂量秋水仙碱(可带来的相似的疗效和较少的不良反应):证据等级中,强烈推荐;③对于其他抗炎疗法耐受性差或有禁忌证的痛风发作患者,选择性建议使用IL-1抑制剂而非其他治疗(支持/镇痛治疗除外):证据等级中,选择性推荐;④对于无法口服药物的患者,强烈推荐使用糖皮质激素(肌肉、静脉或关节内注射),而非IL-1抑制剂或促肾上腺皮质激素:证据等级高,选择性推荐;⑤对于痛风发作者的患者,选择性建议使用局部冰敷作为辅助治疗:证据等级低,选择性推荐。
2024年“美国风湿病学会痛风治疗指南”评析

2024年,“美国风湿病学会痛风治疗指南”在開始上路,無疑為痛
风患者提供了嶄新的治療指南,為改善患者的生命質量、痛浴陶行為提供
了建議與支持。
首先,本指南強調了患者主體性的權利,提倡患者制定自己的健康照
護計劃,尊重個人偏好,促進患者和治療者之間的信任和合作。
指南提出,患者應該參與治療的決策,國家應該確保患者獲得廣泛的可用藥物,醫師
和患者應該重視安全性和有效性,以及藥物的經濟性。
其次,本指南廣泛考慮了各種環境因素,考慮了患者的年齡、性別和
身體狀況等個人因素,並對不同病情的適應性治療提出了針對性的建議。
例如對大部分患者的治療計劃首先建議改變飲食習慣和活動水平,這可以
紓解其症狀,但不足以消除痛风,因此,還有適當的藥物治療,如激素、
抗炎和其他抗痛风藥物的應用。
此外,指南的發布具有重要的社會意義,對我國的痛风治療環境也會
有一定的影響。
《2020ACR痛风管理指南》要点

《2020ACR痛风管理指南》要点5月11 H ,《美国风湿病学会痛风管理指南(2020年版)》于Arthritis Rheumatol杂志和Arthritis Care /?es杂志同步在线发布。
距离2012年版ACR指南的发布(2012年10月)已过了9个年头,如同小编先前在《医脉"药"闻》栏目中报道的那样,2020年版ACR指南的—个重要更新是,仅保留别嗓醇为一线降尿酸用药选择,将非布司他从一线队伍中"踢〃了出来。
除此之外还有多项内容更新,小编逬行了编译整理,供各位老师参考(如需英文原版,请在留言处留下您的联系方式)。
2020ACR指南共包含以下9个部分:1 .起始降尿酸治疗(ULT )指征2. 痛风患者初始ULT的建议3. ULT起始时机4. ULT持续时间5. ULT具体药物的使用建议6•何时考虑更改ULT策略7.痛风急性期管理&生活方式管理9.同用药物的管理推荐强度分为"强烈推荐”和"有条件推荐〃两类:A强烈推荐:有中、高级别证据支持,获益大于风险。
A有条件推荐:获益和风险可能更为平衡,和/或证据级别较低或无相关数据。
本指南中的建议适用于痛风患者,除外一项对无症状高尿酸血症患者[定义为血清尿酸>6.8mg/dL ( 405umol/L ),无痛风发作史或痛风石)]的降尿酸治疗建议。
1起始ULT的指征>强烈建议对具有以下任一特征的痛风患者起始降尿酸治疗:(1) 1个或多个皮下痛风石。
(证据级别:高)(2)有证据表明存在痛风引起的任何形式的放射学损伤。
(证据级别:中)(3)痛风发作频繁(>2次/年)。
(证据级别:高)A对于经历过多于一次痛风急性发作,但并不频繁(< 2次/年)的患者,有条件推荐起始ULT治疗。
(证据级别:中)A对于首次痛风发作的痛风患者,有条件推荐不启动ULT治疗。
但对于以下患者,有条件推荐启动ULT :伴有中度至重度慢性肾痢CKD>3期)、血尿酸(SU ) >9mg/dL (535.5umol/L )或尿石症的患者。
最新《痛风管理指南》-降尿酸治疗的时机

最新《痛风管理指南》-降尿酸治疗的时机2020年5月美国风湿病学会(ACR)再次发布更新版痛风管理指南(以下简称本版指南),提出了42条推荐意见(包括16条强烈推荐意见),相对于2012版有何变化?一起来看一下吧。
初始降尿酸治疗时机1.当患者具备降尿酸治疗指征且正在经历痛风发作时,有条件推荐在痛风发作期即开始降尿酸治疗,并非痛风发作缓解后(证据级别:中)。
2.强烈推荐所有接受降尿酸治疗的痛风患者采用达标治疗策略,并依据连续血尿酸监测行药物剂量滴定及后续剂量调整,而不是固定剂量的降尿酸治疗(证据级别:中)。
3.强烈推荐所有接受降尿酸治疗的痛风患者,达到并维持血尿酸<6 mg/dl(证据级别:高)。
4.有条件推荐:由非临床医师、护理人员向接受降尿酸治疗的患者传递权威的剂量调整方案,包括患者教育、共享决策和达标治疗方案,以尽可能实现达标治疗策略(证据级别:中)。
新旧对比2012版指南提出,急性痛风性关节炎发作期的患者在有效抗炎治疗的基础上'可以'考虑启动降尿酸治疗。
本版指南依旧作为有条件推荐,主要基于两方面考虑,将降尿酸治疗推迟到关节炎缓解后启动,可能会导致部分患者失访,这也是由美国就诊制度(预约时间长)决定的;研究显示,在痛风发作期间启动降尿酸治疗不会显著增加痛风发作持续时间或严重程度。
我们建议两种选择皆可,可以结合患者意愿以及后续复诊的便捷性做出决定。
但必须牢记,痛风急性发作期只有在有效抗炎治疗基础上方能开始降尿酸治疗。
此次指南强调了非临床医生之外的卫生健康管理人员(如护士、助理医师等)对痛风慢病管理的优势,提倡患者教育、共享决策及达标治疗方案的重要性。
2012版指南提出,一般痛风患者的血尿酸目标值界定为<6 mg/dl(证据级别:A),对于明显痛风石患者,目标值为<5 mg/dl(证据级别:B)。
本版指南提出所有接受降尿酸治疗的患者,达到并维持血尿酸<6 mg/dl,并未提及痛风石患者降至血尿酸<5 mg/dl,可能缺乏足够的推荐证据。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
2020 美国痛风指南
美国风湿年会上,发布了2020 痛风临床实践指南(草案)
1.?急性期就开始降尿酸治疗
只要具有降尿酸治疗的指征,有条件推荐发作期间就应开始降尿酸治疗,而不是等急性发作缓解后再开始。
2.?推荐无限期使用降尿酸治疗药物
相对于停药,更推荐无限期使用降尿酸治疗药物。
以往无论患者还是医生都对是否需要长期降尿酸治疗持怀疑态度,而本版指南终于明确,疗程就是没有疗程。
3. ?反对查尿液尿酸水平,反对碱化尿液治疗
4.?秋水仙碱不要按说明书用
如果选择使用秋水仙碱,不推荐中国药物说明书推荐的用法,强烈推荐选择低剂量秋水仙碱(如0.5 mg,tid),而不是大剂量的秋水仙碱,在有一致治疗作用的前提下不良反应大大降低。
5.?降尿酸治疗一线用药推荐
慢性肾脏病CKD3 期以上者,强烈推荐选择黄嘌呤氧化酶抑制剂(XOI)-别嘌醇或非布司他,而不选择促尿酸排泄药物(丙磺舒)。
6.?限制高果糖玉米糖浆摄入
限制酒精和高嘌呤饮食接受度较高,限制高果糖玉米糖浆摄入应受到更大的重视。
7.?反对维生素C 补充剂
无论疾病活动情况,反对维生素C 补充剂应用。
8. 推荐亚裔人群使用别嘌醇前进行HLA-B*5801 基因检测,排除携带者(约为7.4%),以减少严重药物不良反应的发生。
