有机化学习题福山组会题2005年104
有机化学习题福山组会题2005年107
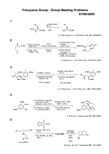
15234COCl 1) SnCl 4, CH 2Cl 2;DBU, 67%2) LDA, THF;TMSCl, 75%SnCl 4methyl vinyl ketoneCH 2Cl 261%J. Clardy et al ., J. Am. Chem. Soc., 116, 8378 (1994)1) CH 3CO 3H(> 2 eq)AcONaAcOH, 70°C 2) KOH, MeOH; HCl (pH < 1)3) TBDPSCl, imidazole DMF, 33%(3 steps)O OTBDPSOOMeMeOOHCHO1) di-tert -butyl malonate piperidine, AcOH benzene, reflux, 91%2) 3-methyl-1-butene h ν, benzene, 88%3) Me 3S +(O)·I -, NaH DMF, rt, 94%4) p -TsOH, toluene reflux, 55%MeOMeOO H HOi -Pr S. Ohta et al ., Heterocycles , 65, 1099 (2005)OOMeO OMeMeOOMe1) DIBAL, toluene 2) (MeO)2P(O)CHN 2 t -BuOK, THF 70% (2 steps)3) MsCl, 2,6-lutidine 50 °C4) CaCO 3, wet MeOH 50 °C 72% (2 steps)MeOOMeCO 2MeV. H. Rawal et al ., J. Am. Chem. Soc., 125, 13022 (2003)HN OHBr N MeN O OSePh 1) Bu 3P, THF, 0 °C 81%2) xs. NaNH 2, liq. NH 3 86%C 16H 21NSeBu 3SnH cat. AIBN PhH reflux 40%Shipman, M. et al ., Tetrahedron 58, 7165 (2002)C 10H 18BrNOAllocN HCO 2MePh I 2, MeCN-H 2O 60 ˚C 92%H 2NCO 2MePhR. H.Szumigala et al, Tetrahedron Lett ., 46, 4403(2005)15234A. G. Martinez et al ., Tetrahedron Lett , 46, 5157 (2005)OH1) O 3, CH 2Cl 2, –78 °C 2) Me 2S 74% (ca. 4/1)OH+OCO 2HO OPh MePh PhMeMeH OOMePhMePhMeTiCl 4 (3 eq)CDCl 3rt 93%T. Oshima et al ., J. Org. Chem., 69, 4577 (2004)R. P. Hsung et al ., J . Am . Chem . Soc ., 125, 12694 (2003)NBnO 2Ct -BuOKTHF 46%DMDO CH 2Cl 2, –45 °C75%OO ONBnO 2C single diastereomerH MeO MeONHTsSnBu 3OTIPSMeO MeONO1) PhI(CN)OTf CH 2Cl 2 –40 °C 2) LHMDS DME –40 °CKF Al 2O 3K. S. Feldman et al., J. Am. Chem. Soc., 124, 11600 (2002)N O H H CHOPhONNH H CHOOPhONH H OPhOO Me HMeNHOH toluene rt to reflux1) MeNHOH benzene reflux 2) H 3O + CHCl 3NOH H CHOPhOB. Alcaide et al ., Tetrahedron Lett., 41, 1647 (2000)OFukuyama Group - Group Meeting Problems107/27/20055Solution:Uchida234C. H. Heathcock et al ., J. Org. Chem., 60, 1120 (1995)HO Phas above150 °C 35%O Ph R. L. Danheiser et al ., J. Am. Chem. Soc. 120, 9378 (1998)O Phγ-terpinene (radical inhibitor)toluene, 180 °C (sealed tube)70%O PhK. Harano et al., Tetrahedron , 52, 13909 (1996)O SS SHHo -dichlorobenzenereflux 46%(cis /trans = 65/35)OHTBSO1) NaHphenyl vinyl sulfoxide THF rt, 3 h 100%2) NaHCO 3 Ph 2O260 ºC, 25 min3) TBAF THF rt, 2.5 h60% (2 steps)4) PCC CH 2Cl 2 79%OO K. Ogasawara et al ., Synlett 319 (1996)T. Mandai et al ., Tetrahedron Lett . 4041 (1990)HNONHO1) DIBAL, toluene, reflux 2) PhNCO, CH 2Cl 2, rt 3) HCO 2H, 100 °C 4) KOH, MeOH, refluxErick Cuevas-Yanez et al ., Tetrahedron., 60, 1505 (2004)1) BrCH 2CO 2Et, Zn, THF, reflux, 8h 2) TsN 3, Et 3N, CH 2Cl 2, rt, 12h 3) Rh 2(OAc)4, ClCH 2CH 2Cl, reflux, 3hN CH 3CH 2CN N CH 3OCO 2Et。
有机化学习题福山组会题2007年06
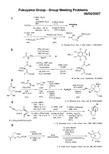
54123OOH CO 2EtFEtO 2CR 2NN Cl ClClClO ONR 2 =NH NH 2O HPPh 3 (2.5 eq.)CCl 4 (3.0 eq.)DBU (3.0 eq.)MeCN A(COCl)2, DMF (cat.)DCE, 40 min;evaporation;A , DCE, 2 h; H 2O, overnightZnBr 2 (cat.)i-Pr 2NH CH 2Cl 235 minOCO 2EtFEtO 2CR 2N N 2M. M. Bio, et al ., Synthesis , 19 (2005)A. Papagni et al ., Eur. J. Org. Chem., 1149 (2001)3) MsCl, Et 3N, 0 °C4) NaOH, EtOH 70% (3 steps)Cr(CO)61) MeLi, Et 2O; MeI;pyrrolidine, Et 2O 86%2) n -BuLi, –78 °C;MeCHO, –78 °CCHOC 6H 44-NO 21) LDA, THF –78 °C; 74%IncludingCrBurgess rgt. (3 eq.)THF 25%ONH 4-NO 2C 6H 4MeO 2CCO 2MeMeMeMeO BrMgMeMe (2 eq)(Cp)2TiCl 2THF –40 °C to rt51%CHOOBn LDA THF –78 °C 81%1) Ac 2OEt 3N, DMAP CH 2Cl 2 rt2) NaCN DMSO 130 °C51% (2 steps)[Rh(CO)2Cl]2 (0.5 mol%)toluene 110 °C 93%MeHMeMe OBnC 25H 36OP. A. Wender et al ., Org. Lett., 2, 2323 (2000)OCHO CO 2Et1) SmI 2MeOH-THF 2) TBSCl, Im DMF1) LiOH, MeOH 2) i -BuO 2CCl Et 3N, Et 2O; CH 2N 23) Cu(CF 3COCHCOCF 3)2 (5 mol%)CH 2Cl 2, refluxO H HOOTBSthree stereocentersJ. S. Clark et al ., Angew. Chem. Int. Ed., 46, 437 (2007)OTIPSORMe 1) LDA, HMPA THF, −78 °C; TMSCl, to 0 °C; HCl, H 2O, 74%2) s -BuLi, PPh 3CH 3Br THF, −78 °C to 0 °C 85%1) TBAF, THF quant2) Red-Al, Et 2O 0 °C; −78 °CNIS, 97%1) TBSCl, imidazole DMF, 97%2) PPh 3, Ag 2CO 3 Pd(OAc)2, 65 °C; TBAF, THF 90 %MeHOHRL. E. Overman et al ., J. Am. Chem. Soc., 121, 5467 (1999)R = (CH 2)3CH(OCH 2CH 2O)54123X P =1) NH 2OH·HCl NaOH EtOH-H 2O 2) Cl 2, CH 2Cl 2 78% (2 steps)NaHMDS THF, –78 °C;A ;1N HCl aq.64%1) NaBH 3CN, MeOH 2) NaH, toluene, reflux; n -BuLi, CeCl 3 –78 o C to rt 59% (2 steps)W. Oppolzer et al ., Tetrahedron Lett., 35, 7015 (1994)OA(C 6H 10ClNO)X POn -C 7H 15OON Hn -C 7H 15n -BuN SO 2OMeMeO MeONOMeMeO MeONO 1) KCN (6.0 eq) Boc 2O (3.0 eq) CH 2Cl 2-H 2O 15 h, 96%2) DIBAL toluene –78 °C, 3 h 63%TosMIC (1.0 eq)K 2CO 3 (1.0 eq)MeOH reflux, 1 h;KOH, reflux, 6 h82%C 14H 13NO 3tricyclic compoundC 22H 24N 2O 6o -dichlorobenzenereflux, 20 h90%J. K. Cha et al., J. Am. Chem. Soc ., 123, 3243 (2001)NO OMeO 2CSEtO Ac 2O, TsOH toluene 70%NOOCO 2Me MeO MeOMeO MeOA. Padwa et al., J. Org. Chem., 61, 4888 (1996)PhMeO 1) DMF, POCl 3CH 2Cl 2, 0 °C to rt 2) NaN 3, DMF, rt 3) hv (Pyrex filter) MeCN, rt1) Ph 3P, toluene reflux94% (E /Z = 92/8)2) DMAD, toluene reflux, 55%NCO 2MeCO 2Me PhT. Nakata et al., Heterocycles , 48, 2551 (1998)monocyclic compoundC 9H 7NOClSMeBrO1) hexamethylenetetramine CHCl 3, rt2) conc. HCl, EtOH, rt (acidic workup)3) 2,5-dimethoxytetrahydrofuran NaOAc, AcOH, 100 °C 75% (3 steps)SN OCl4) NaIO 4 (1 eq) MeOH-H 2O, rt 89%5) TFAADMF, 0 °C 42%V. Nacci et al., J. Med. Chem., 41, 3763 (1998)54123OEt P(OEt)2O OH O Ba(OH)2 wet THF rt, 86%2) TFA Et 3SiH CH 2Cl 2 0 o C, 71%C 13H 19NO 21) Ac 2O, Et 3N, CH 2Cl 22) LHMDS, toluene, –78 to 80 o C 77% (2 steps)3) DIBAL, toluene, –78 o C, 59%1)NH 2MeO MeOTsOH (10 mol%)toluene refluxN HN. A . Magomedov et al., J. Org. Chem . 72, 3808 (2007)Me 2C NNHCO 2MeA C 6H 12N 2O 3N MeCO 2H1) DPPA, TEAPb(OAc)4MeOH 40-80%N MeNMeO MeOOJ. Warkentin, Synthesis , 279 (1970)James H. Rigby, Patrick J. Burke, Heterocycles, 67, 643 (2007)2) toluene, refluxA *toluene, reflux75%*Theoretically: 2.0 eqMeO OMeO H MeHTBSOMeOLiTMP (1.0 eq)*, TMSCl (1.0 eq)*THF, –78 to 0 ºC;Pd(OAc)2 (0.5 eq), CH 3CN68% (2 steps)6N NaOH (4.0 eq)aq. H 2O 2 (3.0 eq),MeOH, 0 ºC, 83%;Li, NH 3, THF, –33 ºC, 63%;aq. HF/CH 3CN (5:95 v/v)86%O HMeOHOMeOH* theoretical amountS. J. Danishefsky et al., Angew. Chem. Int. Ed., 46, 2199 (2007)N TrNHMeO MeOOOH1) SeO 2, TBHP, CH 2Cl 2, 90%2) NaH, AllylBr (theoretically 1 equiv) THF, 73%3) DBU, toluene, 220 °C, 75%4) TPAP, NMO (theoretically 1 equiv) MS4A, CH 2Cl 2, 70%OOL. Barriault et al., Org. Lett., 4, 1371 (2002)OTBSCONEt 22NMe CONEt 2Ph G. Bélanger et al., J. Org. Chem ., 72, 1104 (2007)1) Jones' reagent, acetone, 0 °C, 96%2) SOCl 2, CH 2Cl 2, 0 °C to rt; evaporation; PhNHCH(Me)CO 2Me, toluene, 89%3) LiOH·H 2O, THF-H 2O, reflux, 99%4) DCC, CH 2Cl 2, 89%。
有机化学习题福山组会题2006年03

15234J. D. Winkler et al., J. Am. Chem. Soc., 111, 4852 (1989)HNOn C 5H 13O CO 2t BuHNOOO n C 5H 131) acetone, TFA, TFAA, 84%2) h ν3) NaBH 4, EtOH4) NaH, THF, 60% (2 steps)L. E. Overman et al ., J. Am. Chem. Soc ., 127, 18054 (2005)L. E. Overman et al ., J. Org. Chem., 57, 1035 (1992)SEM NON1) MeOTf, CH 2Cl 22) Na 2Fe(CO)4 NMP, CO 50 °C 59% (2 steps)SEM NOMeNOA. Bhattacharjya et al ., Org. Lett., 7, 207 (2005)Mei -Pr1,3-dithiane n -BuLiTHF, –78ºCNaHBr HgCl 2CaCO 3MeCN-H 2O63%i -Pr MeMeHMe O1) isopropenyl lithium Et 2O, 0 °C 2) PBr 3Et 2O, 0 °CMeHMe MeSS3)MeCN 4) NaH, THFreflux, 6 hMeSSNaN NHTs D. A. Evans et al., Tetrahedron Lett., 47, 4691 (1973)n-Bu1) HBBr 2·SMe 2, CH 2Cl 22) , THFO 3) Bu 3SnH(0.01eq),NaBH 3CN(2.5 eq), THF 4)NN N OMe MeMe , benzene; H 2O, reflux 80 % (2steps)OHOH Bu HHR. A. Batey et al., Angew. Chem. Int. Ed., 38, 1798 (1999)15234OHOPOCl 3, DMF 75 °C;H 2O 80%C 10H 6O 31) p -anisidine p -TsOH·H 2Otol, reflux (Dean-Stark)2) CH 2=C=CHCO 2Et PPh 3 (cat)benzene, reflux, 55%ON OCO 2EtPMPP. S. Ishar et al ., Org. Lett., 2, 2023 (2000)O 3SNEtO FmocHNCO 2HNNHFmoc S Oi -Pr 2NEt MeCNCH 2Cl 2V. L. Boyd et al ., Tetrahedron Lett., 31, 3849 (1990)NCO 2Me N 2OCu(acac)2 (10 mol%)toluene, refluxNOMeO 2CF. G. West et al., Org. Lett., 7, 2949 (2005)cis : trans = 3.6 : 1Robert A. S. et al ., J. Org. Chem ., 69, 1598 (2004)1) NH 2OH.HCl NaOAc, MeOH rt, 24h 2) 190 °C(sealed tube) toluene, 3.5h 59% (2steps)ONC Tricyclic compound1) DIBAL, toluene -78 °C,2h, 100%2) n-PrP +Ph 3Br - KHMDS, THF -40 °C,24h, 62%3) H 2, 10%Pd/C MeOH, 69%NCHN HOt -BuO t -BuOTMS N C SOO OO OMs1) NH 2NH 2·H 2O neat, rt 2) TBSOTf, pyr. CH 2Cl 2, 0 °C 85 % (2 steps)3) LTA, toluene, rt 48 %OOTBSOOAcO A. Vassella et al ., Helv. Chim. Acta , 87, 2405 (2004)15234Y. Six et al ., Euro. J. Org . Chem ., 70, 4654 (2005)NO R R =MeOTi(O i -Pr)4 (1.4 eq)c C 5H 9MgCl (4.5 eq)THF, 20 ºC87%NRHOOOTBDPS O O SnCl 4CH 2Cl 2– 78 ºC 48%OO H OHHOTBDPSOL. Barriault et al., Org. Lett., 7, 5921 (2005)N OHNMe MeClPhO1)K 2CO 3, NaI 12 h, reflux 80%2) m CPBA (1.0eq) CH 2Cl 2 12 h, rt 90%3) MeOH 2 h, reflux 86%N OMeNOMePhO Me M. Abass et al ., Heterocycles , 65, 901 (2005)OEEOAc1) LAH, THF, rt 76% (2 steps)2) ClSO 3HCH 2Cl 2, –78 °C 3) H 2, Pd/C cHex, rt43% (2 steps)O +O+OHH 253045::R. L. Snowden et al., Helv. Chim. Acta , 88, 3055 (2005)OOPh Ph 1) NIS (2.5 eq) MeCN-H 2O 0 °C, 62%2) DDQMeCN-H 2O 60 °C, 58%3) NaClO 2, 2-methyl-2-butene NaH 2PO 4, t -BuOH-H 2O rt, 92%4) CAN, MeCN-H 2O, rt; K 2CO 3, rt, 80%OOIOY. Kita et al ., Angew. Chem. Int. Ed., 44, 734 (2005)。
有机化学习题福山组会题2011年09
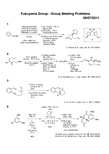
1) LiAlH4, THF 0 °C to reflux 95% 2) Tf2O CH2Cl2, rt; Et3N; n-PrMgBr 90%, 95% ee
n-Pr
Bn N
3 OBn
A. B. Charette et al., J. Org. Chem., 75, 7465 (2010)
3
O 1) PtCl2 (5 moห้องสมุดไป่ตู้%) toluene, 80 °C 95% 2) p-TsOH (10 mol%) benzene, reflux 91% O
O
K. Mikami et al., Synlett, 469 (1998)
3
O O Me O
O Cl N S O
CH2Cl2, rt 46%
A
OBn BnO O BnO
1) A Py, CHCl3, rt 43% 2) Ra-Ni benzene-toleune 0 °C to rt 45%
OBn BnO O BnO Me O O O
Fukuyama Group - Group Meeting Problems 09/07/2011
1
ethyl diazoacetate Cu(OTf)2 (2 mol%) Ligand A (2.5 mol%) PhNHNH2 (2 mol%) O CO2Me CH2Cl2 53%, >99% ee (after recrystallization)
Et N CO2Et
O HO
benzene, reflux 60%
B. C. Raju et al., Bioorg. Med. Chem. Lett., 21, 2855 (2011) M. P. S. Ishar et al., Tetrahedron, 65, 4593 (2009)
《通过练习学习有机反应机理》 福山透 三氢剑魔汉化

A组题对于初学者来说,完全可以很轻松地自主挑战的。初级篇是参考于反应的简 单的步骤省略。另外,倘若一个问题20分钟也考虑不明白的话,还是乖乖自己翻答案比 较好,不懂的题目还可以等到以后再挑战。为此我们特地将问题分类,各种问题分为 三个阶段,为能力提高做准备。答案栏写的反应机理同时也著名了引用文献的作者,不 仅是福山研究室所考虑到的东西,也有与出版社双方考虑的因素。 一般来说,有机反应中不稳定的中间体很难确认,从而导致反应途径中有许多盲 区。因此,考虑真正的反应机构到底是什么是什么叫我们很是头疼。不过从逻辑上考 虑,还是反应机理更为重要。这本书的问题如果可以全部变成自己的东西。拥有相当 有机化学的实力不是问题。另外。答案栏的评语是用英语书写的,这种程度的英语对 于初学者来说不算什么问题,而对于英语简写,后文也有说明。 本考试中刊载的大部分问题,是当时研究室的团队会议出题。工作人员选择的问 题,是文部科学省的特定领域的研究“生物功能分子的创制”计划班的班成员承蒙提 供的问题,在这里对其表示感谢了。另外,这本书之前的显示的那几页是当时实验室 的工作人员和研究生百忙之余中执笔的,特别要感谢横岛聡助手的忘我的努力。 最后,这本书的企划、制作帮助您的化学同人编辑部平佑幸深深地对您表示感谢。
2005年7月东京大学研究生院药学研究科 天然物合成化学研究室福山透
目
录
● ● ● ●
序言 出版委员会名单与编者名单 前言 缩写表 问 题
■ 初级編
初级编,由有机化学的教科书中摘录而成 【例 題】2 問题数 78 題
■ 中级編
中级编,研究生入学考试上要求的题目 【例 題】20 問题数 128 題
■ 上级編
2005年7月东京大学研究生院药学研究科天然物合成化学研究室福山透目录序言出版委员会名单与编者名单前言缩写表问题初级編初级编由有机化学的教科书中摘录而成例題2問题数78題中级編中级编研究生入学考试上要求的题目例題20問题数128題上级編上级编历史上著名的反应例題48問题数109題答案答案初级編答案中级編答案上级編专栏福山研究室的小组会议的景象机理书写问题的解决方案附录有机反应的反应机理的考虑方法电负性和酸度常数索引日本及欧美的书籍引用目录缩写表加热ac乙酰基acac乙酰丙酮基aibn偶氮二异丁腈aq水溶液ar芳基bn苯甲基boc叔丁氧羰基bu正丁基cat催化cbz苯甲氧羰基csa樟脑磺酸csi磺酰氯异氰酸酯cy环己基dabco14二氮杂双环222辛烷liq液体m间位mcpba间氯过氧苯甲酸mememmommsmsnnbsncsnmmnmonsopphprrt甲基2甲氧基乙氧基甲基氯甲氧甲基甲磺酰基分子筛正某基n溴代丁二酰亚胺n氯代丁二酰亚胺n甲基吗啡啉dbadbudccddqdeaddmapdmedmfdmsodppbdppeedcieqethmpahvi己二酸二丁酯二环43015二氮5十一烯nn二环己基碳二亚胺23二氯56二氰14苯醌偶氮二甲酸二乙酯4二甲氨基吡啶二甲醚二甲基甲酰胺二甲亚砜14双二苯基膦丁烷双二苯基膦基乙烷1乙基3二甲基氨基丙基碳酰二亚胺等量物质乙基六甲基磷酸胺光照异某基ssettn甲基n氧化吗啉邻对硝基苯磺酰基邻位对位苯基丙基室温仲某基单电子转移叔某基tbaf四丁基氟化铵tbs叔丁基二甲基硅烷基tf三氟甲磺酸基tfa三氟乙酸tfaa三氟乙酸酐tfoh三氟甲磺酸thf四氢呋喃tips三异丙基甲硅烷基tms三甲基硅烷基tol苯甲基tosmic对甲基苯磺酰甲基异腈tr三苯基ts对甲苯磺酰基tsoh对甲苯磺酸khmds六甲基二硅基胺基钾lda二异丙基氨基锂初级编初级篇是以初学者的有机化学教科书中所提到的基础反应为主要成分
有机化学习题福山组会题2007年05

54123R. J. Stoodley et al ., Tetrahedron Lett ., 10, 941 (1967)NSH CO 2H H 2N ONaNO 2 (3 eq)* , HClMeOHN HS MeOOOOOOMe OMe1) p -TsOH·H 2O MeNO 270 °C, 90%2) DIBAL, toluene –78 °C, 100%3) BF 3·OEt 2MeCN, 0 °C, 99%tricycliccompoundOHO 2CCO 2HRuCl 3·nH 2O NaIO 4MeCN-CCl 40 °CS. Nagumo et al ., Tetrahedron Lett., 43, 5333 (2002)OMeOHCMe1) TMSC(Li)N 2,THF –78 °C to rt, 71%2) Bu 3SnCu(Bu)CNLi 2 THF, –78 °C; MeI, DMPU –78 °C to rt 3) I 2, Et 2O, 0 °C 80% (2 steps)C 11H 17IO1) vinyl magnesium bromide Pd(PPh 3)4, PhH, 70 °C 88%2) H 2, CO, Rh(acac)(CO)2 (S, R )-BINAPHOS PhH, 30-35 °C, 20 atm 3) CrCl 2, CHI 3, THF 72% (2 steps)OMeMeMeIMeE. N. Jacobsen et al., J. Am. Chem. Soc., 123, 10772 (2001)OMeLi THF, MW 120 °C;DMP CH 2Cl 2, 0 °C75%F. Serratosa, Acc. Chem. Res., 16, 170 (1983)D. C. Harrowven et al., Angew. Chem. Int. Ed., 46, 425 (2007)O t -But -BuO 1) benzene reflux, quant2) NBS, CCl 4 83%C 12H 18O 4C 10H 18O 2OMeO OO t -Bu 1) MeLi (1.05 eq) THF, –78 °C; TFAA, 82%2), THF 37% (dr = 3:2)N H N thionyl chloride (theoretically 1 eq)CH 2Cl 2,–10 °C to rt;filtration;NH 2MgBrN HMeMetoluene reflux 51%(1 eq)THF 96%96%Kim, Y. H. et al. Tetrahedron Lett. 26, 3821 (1995)Julia, S. A. et al. Tetrahedron Lett. 27, 837 (1996)(as 4 eq)* theoretical amount54123N HP. S. Baran et al., Nature , 446, 404 (2007)H NCMe 1) t -BuOCl (1.2 equiv) CH 2Cl 2, –78 °C;prenyl 9-BBN (2 equiv) –78 °C 60%2) h υ, Et 3N (xs) PhH, rt 41%(63% based on RSM)N HH NCMe Bprenyl 9-BBN:C 8H 8O 3isovanillin1) allyl bromide K 2CO 3, acetone reflux, 8 h, 92%2) 180º C, decalin 5 h, 95%3) MeI, K 2CO 3 acetone, reflux 8 h, 83%C 12H 14O 34) acrylonitrile, DABCO H 2O, rt 3-5 days 67%5) Grubbs' 2nd gen. (5 mol%) 0.05 M CH 2Cl 2, rt 5-8 h, 90%CNOCH 3H 3COE. Wang et al., Tetrahedron, 63, 2824 (2007)BocNSMeOOC1)105 ºC, 45h 2) Pb(OAc)4 (2.2 eq), benzene, reflux 3) NaOAc, MeOHN NCOOMe MeOOC BocNSMeOOCOH R. B. Woodward et al., J . Am . Chem . Soc ., 88, 852 (1966)MeMeOn BuOOOHHH n Bu1) MgBr(CH 2)2Br, THF, 1.5 h, 92%2) TsOH, acetone, reflux 11 h, 82%3) NH 2NH 2, KOH, HO(CH 2)2OH110 to 190 °C, 8 h 4) O 3, EtOAc, –78 °C, 4 h; PPh 3, overnight 51% (2 steps)D. Kim et al., J. Org. Chem., 65, 4846 (2000)Br OO OHHO O OOH H HOTBS 1) i BuO 2CCl Et 3N, Et 2O; CH 2N 2, Et 2O 0 °C to rt, 81%2) Cu(CF 3COCHCOCF 3)2CH 2Cl 2, reflux, 96%1) PhNTf 2 NaHMDS THF, –78 °C2) CH 2=C(OEt)SnBu 3 Pd(PPh 3)4, LiCl THF, reflux 3) methyl vinyl ketone toluene, reflux 67% (3 steps)bicyclic compoundO OH HOTBS EtOH J. S. Clark et al., Angew. Chem. Int. Ed., 46, 437 (2007)54123E. J. Enholm et al., J. Org. Chem., 55, 324 (1990)Ph 3P CH 3Brn -BuLi (1 eq)THF 0 °C to rt;On -BuLi (1 eq)cyclohexanone −23 °C to rt56%C 11H 18OCHOKH (1.3 eq)18-crown-6THF, reflux 76%OHOMe S OPh OOMe+TFAA (1.5 eq)CH 3CN −40 ºC 83%OMeOHCOMeOOC 23H 20O 4S1) m CPBA (1 eq) 98%2) n -BuLi (5 eq) THF, −78 ºC; DMF (5 eq) 56%Y. Kita et al., Org. Lett., 2, 2279 (2000)ON 3BF 3⋅OEt 2, O 2–78 to 0 ºC 40%tetracyclic compoundC 12H 17NO 3H 2, Pd/C THF 77%NO HOH HF. G. West et al., Org. Lett., 9, 703 (2007)OCHO MeMeNHOHDMAD rt 85%PhMe, reflux50%O NH HMeCO 2Me OCO 2Me Me S. Kanemasa et al., Chem. Lett., 797 (1984)OOH CNBzOPhI(OAc)2 (1.5 eq)I 2 (1.0 eq), h νbenzene1) conc. HCldioxane, 40 °C 2) (COCl)2, cat. DMF benzeneNOHSPy, DMAP, O 2toluene, 80 °C;P(OMe)3O O OHBzOS. Hatakeyama et al ., J . Am . Chem . Soc ., 116, 4081 (1994)。
有机化学习题福山组会题2008年10
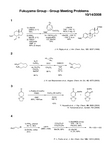
1342HO 2C H 2NHNO N 3Tf CuSO 4aq. K 2CO 3CH 2Cl 2MeOH 99 %HSPPh 2BH 3DCC CH 2Cl 298 %DABCO THF H 2O 70 °C 80%J. H. van Maarseveen et al ., Angew. Chem. Int. Ed., 42, 4373 (2003)Y. Hayashi et al., J. Org. Chem., 69, 5966 (2004)H. Yamamoto et al., Synlett , 705 (2006)OOOL-Proline (5 mol%)PhNODMF86%, >99%eeONHPhOOOONHPhO+EuCl 3 (30 mol%)EtOH 105 °C43%OHO 2STMSCr(CO)3TMSOTBSHHTsO1) t-BuOKDMSO, 0 °C 78%2) KBH 4MeOH, 0 °C 3) TBSCl imidazole DMF, rt80% (2 steps)h ν, Cl(CH 2)2Cl;evaporation;CO, MeOH 70%t-BuOK THF, –105 °C;NCS (1 eq), rt;t-BuOK, –105 °C;–105 °C to rt60%J. H. Rigby et al ., J. Am. Chem. Soc ., 121, 8237 (1999)PhOBr1) PPh 3, phenol 90 °C, 48 h 2) EtOAc, reflux 24 h1) NaHMDS (4 eq) THF, –78 °C; PhCHO, 90%2) t -BuOK rt, 2 h, 60%PhCCl 3CO 2H CCl 3CO 2Na MeCN, 95%salt A (MW < 400)P. L. Fuchs et al., J. Am. Chem. Soc. 126, 14314 (2004)1342W. Oppolzer et al ., Tetrahedron Lett., 27, 5471 (1986)ClMg (5 equiv), THF –65 °C → rt → 65 °C;CuI (1.1 equiv)TMEDA (3 equiv)–65 to –40 °C;(0.95 equiv)–78 °C76%CO 2MeC 16H 26O 21) NaOHMeOH-H 2O, 65 °C 100%2) (COCl)2 toluene, rt 3) i -Pr 2NEttoluene, reflux 57% (2 steps)H H OMe dr = 3:1NBnO NCEtOMeCO 2MeTMSOTf, EtNO 274%1) Pd/Cmesitylene 98%2) NaOH, EtOH/H 2O (1:1) reflux 75%N HNBnO EtBrian L. Pagenkopf et al ., Org. Lett ., 10, 157 (2008)CO 2MeOO OEt OTMSZnCl 2, Et 2O CuBr·SMe 2HMPA, THF83%h νhexane 95%1) 10% HCl aq.2) (imid)2CS DMAP, THF 85% (2 steps)OHOCO 2Men Bu3SnHAIBNbenzene reflux 92%Tetracyclic CompoundM. T. Crimmins et al., Tetrahedron Lett., 37, 8703 (1996)OOHO Me1) PhI, CO, CO 2 Et 3N, Pd(PPh 3)4 100 °C, 79%2) BBr 3, CH 2Cl 2 95%OBn1) o -NO 2C 6H 4SeCN P(n-Bu)3, THF, 90%2) H 2O 2, THF, 85%O OOO PhMeK. C. Nicolaou et al., J. Am. Chem. Soc ., 130, 11114 (2008)1342NMeS Et 2NO S h υhexane reflux74%**mixture of two stereoisomersPh 2O reflux89%PhLi, 0 °C, THF;aq. NaOH 66%NMePhOR. S. Grainger et al., J. Org. Chem., 73, 8116 (2008)MeOOPOCl 3 (12.0 eq.)DMF 80 °C 20 min 69%POCl 3 (12.0 eq.)DMF 120 °C 20 min 78%MeOCHOClClMeOOOClM. Shi et al., J. Org. Chem., 73, 8317 (2008)Ti(O i -Pr)4 (0.1 equiv.)EtMgBr (4 equiv.)THF-Et 2O (4:1)51%PhCHO, Na 2SO 4Al(OTf)3 (0.3 equiv.)CH 2Cl 2, –10 °C;TiCl 4 (1 equiv.)55%OOPhOHCO 2EtK. E. O'Neil et al ., Org. Lett ., 7, 515 (2005)OTBS1) nBuLi (2.2 eq),THF, -78o C to rt ; ICH 2CH(OCH 3)2* HMPA, -78o C to rt, 81%2) TBAF, THF, 90%3) BocNHTs, PPh 3, DEAD, THF, 89%4) HCl, MeOH, 85%1) LHMDS (3.0 eq), nBu 3SnCl THF, 0o C, 66%2) PhI +CN -OTf, CH 2Cl 2, -40 o C ; tBuOK, THF, -40 o C 56%N TsOCH 3K. S. Feldman et al., . Chem., 61, 5440, (1996)* theoretical amount: 1 eq.。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
1
5
2
3
4
O O O
N 2
O
1) Rh 2(OAc)4 (3 mol%), CH 2Cl 2, 74%2) 6 N HCl, 1,4-dioxane, 93%3) MeI, NaH, DMF, 75%4) sealed tube
1-methylcyclohexene, 150 °C, 62%
O
O
Me H
OMe
J. E. Baldwin et al . Org . Lett . 1, 1933 (1999)
N S AcO
CO 2CH 3
Et Me
O
K 2CO 3
MeOH-H 2O
TsOH·H 2O toluene reflux 76%
N
S
Et CO 2CH 3
H.-G. Hahn et al . Heterocycles, 57, 1697 (2002)
O
OMe
NO 2Me
Me Me Me
OMe
O
H 2N O Me Me
Me
Me Boc 2O DMAP benzene-MeCN
Raney Ni H 2MeOH
tricyclo compound
D. G. J. Young et al . J. Org. Chem. 67, 3134 (2002)
OH H Me
1) Bu 3SnH (1.2 eq) AIBN (cat) PhH, 80 °C 2) TBAF, THF
81% (2 steps)
O
Me 3Si
Br
Tsai, Y. M. et al ., Tetrahedron Lett . 34, 1303 (1993)
O
O Ph
O Ph
OTMS
C 29H 40O 6SSi
∗
∗
∗
O Ph
O
O
O
Ph C 26H 32O 4
Stereochemistry C*?
"H +"C 21H 22O 3
SO 2, toluene Tf 2NH (cat.)
P. Vogel et al., Chem. Eur. J .,11,465 (2005)
78% d.r. 5:1
C 8H 18OSi
1
5
2
3
4
O H
1) TrisNHNH 2, THF;
t -BuLi (theoretically 2 eq),–78 °C;
, 36%
2) µwave, THF, 110°C; air, 80%3) µwave, toluene, 150 °C, 61%
O
O
t -BuO
H
O O
O t -Bu
H
D. C. Harrowven et al., Angew. Chem. Int. Ed. 44, 1221 (2005)
1) Ph 3P=C=C=O THF,reflux, 16h 60%
2) PhMe, 180 °C, 24h (sealed glass tube) 65%3) O 2 (air)
Rainer, Schobert. et al ., Tetrahedron Lett . 42, 4561 (2001)
HO
O
O
O
O O O
OH
N 3
Me
Ph
toluene, 100 °C;
TMSCN
NH H CN Ph
Me
K. S. Feldman et al., J. Am. Chem. Soc., 127, 4590 (2005)
OH
O
HO
1) SeO 2 TBHP CH 2Cl 22) AllylBr NaH THF
3) DBU (2.0 eq) toluene 220 ºC µwave 75%
L. Barriault etal., Org. Lett. 4, 1371 (2002)
H
O
O
CO 2Me
O
O
H
Co 2(CO)8, 4Å MS toluene;Me 3NO•2H 2O toluene
K 2CO 3MeOH
J.D. Winkler et al., Org. Lett. 7, 1489 (2005)
1
5
2
3
4
TBS
Ph
O O
TBSO
HO
Ph
NHMDS HMPA/THF –80 ºC to rt, 2 h
43%
K. Takeda et al., 125th Nihon Yakugakukai Abstract 4, 118 (2005)
N Boc
BocN
Ts
HO
Ts
I
HO
Bu 3SnH Et 3B, O 2CH 2Cl 20 °C 78%
80 °C 82%
D. H. Hodgson et al., Org. Lett. 7, 815 (2005)
O
O
OH
CyNC DMAD
PhH reflux 68%
O
O
O
NHCy
CO 2Me
CO 2Me
V. Nair et al., Heterocycles 58, 147 (2002)H
O OBn
C 9H 10O 2
D-Proline DMF,r.t.98% e.e.anti:syn 4:178%
C 18H 20O 4
TMSO OR MgBr 2.OEt 2
65%,d.r. 10:1
R=p-benzyloxy cinnamoyl
O BnO HO
OR
OBn
OH
C 21H 24O 4Si Protected D-Glucopyranose Stereoselectivities?
D. W. C. MacMillan et al., J. Am. Chem. Soc. 127, 3696 (2005)
S
S S Cl Cl
CO 2Me
MeO 2C (DMAD)
xylene, rt quant
DMAD xylene, reflux 55% (1:1)
S
S S
R
R
R
R +
S
S
R
R
R
S R
R = CO 2Me
V. A. Ogurtsov et al., Org. Lett. 7, 791 (2005)。
