电解质分析仪(presentation)讲义
电解质分析仪原理及临床应用ppt课件

引起的呼吸性酸中毒,因CO2潴留, 血浆[HCO3-]相应增加,Cl-自肾脏 排泄增加,血清Cl-减少 .
水,不适当地限制盐和应用袢
性利尿剂。如速尿等可使Cl-
丢失,而引起血清Cl-降低。
Page 14
Diagram
4、血清钙测定 及意义
血清钙水平相当稳定。血清中钙以两种形式存 在,一种为弥散性钙,以离子状态存在,为生 理活性部分;另一种为与蛋白质结合,不能通 过毛细血管壁,称为非弥散性钙,无生理功能。 血清钙的水平受甲状旁腺素、1,25-二羟维 生素D3[1,25-(OH)2-D3]及降钙素等调节,肾 脏亦是钙的调节器官。另外,离子钙测定逐渐 已为临床所重视,因为有些疾病血清总钙测定 并无变化,而离子钙有明显改变。
促进肾脏和肠道对钙的重吸收所致
④ 肾上腺皮质机能降低,常可出现高血钙。 骨髓增殖性疾病.
Page 16
(2)血清钙降低
① 甲状旁腺机能低下,如甲状腺手术中误切了甲状旁腺、特发性甲状旁腺机能 低下,或由于自身免疫和炎症等原因所引起,都可出现低钙血症。 ② 慢性肾功能衰竭,可因1,25(OH)2-D3生成不足而致血钙降低,引起继发性 PTH分泌亢进,可导致肾性佝偻病。 ③ 急性胰腺炎,亦可发生低血钙。
5 血清二氧化碳结合力测定的临床意义
(1)增高:提示碱储备过剩 ①代谢性碱中毒:幽门梗塞(胃酸大量丢失),小肠上部梗阻,缺钾,服碱性药 物过量(或中毒)。 ②呼吸性酸中毒:呼吸道阻塞,重症肺气肿,支气管扩张,气胸,肺气肿,肺性 脑病肺实变,肺纤维化,呼吸肌麻痹,代偿性呼吸性酸中毒。 ③高热,呼出二氧化碳过多。 ④肾上腺皮质功能亢进,使用肾上腺皮质激素过多。
(2)血清钠增高
① 体液容量减少,如脱水。
电解质分析仪原理及临床应用

电解质分析仪原理及临床应用目录一、概述 (2)1. 电解质分析仪定义及作用 (2)2. 电解质分析仪应用领域 (3)二、电解质分析仪原理 (4)1. 基本原理 (6)1.1 化学分析原理 (7)1.2 电化学分析原理 (8)2. 高级原理与技术 (9)2.1 电导滴定技术 (10)2.2 电位滴定技术 (11)三、电解质分析仪主要类型 (12)1. 离子选择性电极法电解质分析仪 (13)2. 干化学法电解质分析仪 (14)3. 血气电解质分析仪 (14)四、电解质分析仪的关键技术特点 (16)1. 高精度测量技术 (17)2. 快速响应技术 (18)3. 自动校准与质控技术 (19)五、电解质分析仪的临床应用 (20)1. 临床应用范围 (21)1.1 手术室与重症监护室应用 (22)1.2 急诊科应用 (23)1.3 其他科室应用 (24)2. 临床价值分析与应用实例解析 (25)六、电解质分析仪的操作流程与注意事项 (26)一、概述电解质分析仪是一种精密的医疗检测设备,用于测定体液中的电解质浓度,包括钠、钾、氯、钙、镁等。
这些电解质在人体内起着至关重要的作用,维持着正常的生理功能。
电解质分析仪利用电化学原理,通过测量电极之间的电压变化来确定电解质的浓度。
其临床应用广泛,对于诊断疾病、监测治疗效果以及评估患者的水盐平衡具有重要意义。
在现代医学中,电解质分析仪已经成为常规检查项目之一,尤其在急诊医学、重症监护、心血管疾病等领域发挥着重要作用。
通过电解质分析,医生可以迅速了解患者的体内电解质状况,从而做出准确的治疗决策。
电解质水平的变化也可能提示某些疾病的存在,如电解质紊乱、酸碱平衡失调等,因此定期进行电解质检测也是预防疾病发生和发展的重要措施。
1. 电解质分析仪定义及作用电解质分析仪是一种用于检测人体或其他生物样本中电解质浓度的医疗设备。
这些电解质包括钾(K+)、钠(Na+)、钙(Ca++)、氯(Cl)等,它们是维持人体正常生理功能的重要物质。
电解质测定演稿PPT课件

拓展应用领域
02
积极探索电解质测定技术在其他领域的应用,如农业、工业、
航天等,提高其应用价值。
加强国际合作与交流
03
积极参与国际学术交流与合作,引进先进技术和管理经验,推
动电解质测定技术的全球发展。
THANKS FOR WATCHING
感谢您的观看
电解质测定演稿ppt课件
contents
目录
• 电解质测定简介 • 电解质测定的应用领域 • 电解质测定的实验操作 • 电解质测定的数据分析 • 电解质测定中的问题与解决方案 • 电解质测定的未来展望
01 电解质测定简介
定义与重要性
定义
电解质测定是指通过实验室检测,对血液、尿液等生物样本中的离子浓度进行定 量分析,以评估机体内电解质平衡状态的过程。
精度较低。
02 03
自动化分析
随着科技的发展,自动化分析仪逐渐取代手工操作,提高了测定效率和 准确性。目前市面上有多种型号的自动化电解质分析仪,广泛应用于各 级医疗机构。
未来展望
随着生物技术和信息技术的不断进步,电解质测定的准确性和效率有望 进一步提高。同时,新型检测方法的研发和应用也将为临床诊断和治疗 提供更多选择和依据。
微纳技术与生物技术的结合
利用微纳技术对电解质进行高灵敏度、高分辨率的检测,结合生物 技术对特定离子进行选择性识别。
远程监测与实时反馈
通过物联网和云计算技术,实现远程实时监测电解质状态,为医疗 保健和环境监测等领域提供有力支持。
应用前景展望
1 2ห้องสมุดไป่ตู้
医疗诊断
电解质测定在医疗诊断中具有重要作用,未来将 更加广泛应用于临床,为疾病诊断和治疗提供依 据。
重要性
电解质分析仪检定、校准培训课件

15
检定/校准方法 示值误差及重复性校准
用人血清无机成分分析国家有证标准物质在 仪器上连续测定六次,记录每次的测定值xi, 重复性r 按以下公式计算:
16
检定/校准方法 示值误差及重复性校准
csi xi
5
b 4000 0.94525 a 117.1471200.94525 3.7173
3781
24
检定/校准方法 交叉污染率
由测量系统将一个检测样品反应携带到另一 个检测样品反应的分析物不连续量,由此错误 地影响了另一个检测样品的表现量。通常以 给定的已知标称定值的低值样品和高值样品 交叉测量多次,用特定公式计算得到。
±0.1s/h,±0.5s/d。
12
检定/校准环境条件
1。环境温度:(15~30)℃。 2.相对湿度:≤85%。 3.电源:交流电压 (220±22)V,频率 (50±1)Hz,并具有 良好的接地。 4.仪器的周围应无冲击和振动,并不得有强电磁场的干 扰,无强光的照射。
13
检定/校准项目
14
检定/校准方法 外观及工作正常性检查
19
检定/校准方法 稳定性
20
检定/校准方法
线性误差
1.对检定用标准溶液(1~5)号,每一个浓 度分别测量三次,取平均值用于计算。 2.按照线性回归方法求得标准工作曲线的截 距、斜率 (计算方法见附录 A)及标准工作曲 线的线性方程,然后按照下列公式计算标准曲 线各对应点测量的线性误差Δxi,取每种离子 Δxi 最大值作为该离子测量的线性误差。
10
计量器具控制 检定用标准器及配套设备
电解质分析仪
第九章电解质分析仪概述电解质分析仪又叫离子计。
是采用离子选择性电极来测量溶液中离子浓度的仪器。
在生化检验中,电解质分析仪表主要用于测量体液中内钾、钠、氯、钙、锂等离子浓度。
人体内电解质的紊乱,会引起各器官、脏器生理功能失调,特别对心脏和神经系统影响最大。
因此,电解质分析仪表在临床上应用十分广泛,已成为评价人体内环境的主要工具之一。
按测定项目来分,电解质分析仪表可分为三项、四项及五项等。
有的公司采用模块式设计,可根据需要,自动组合测定项目。
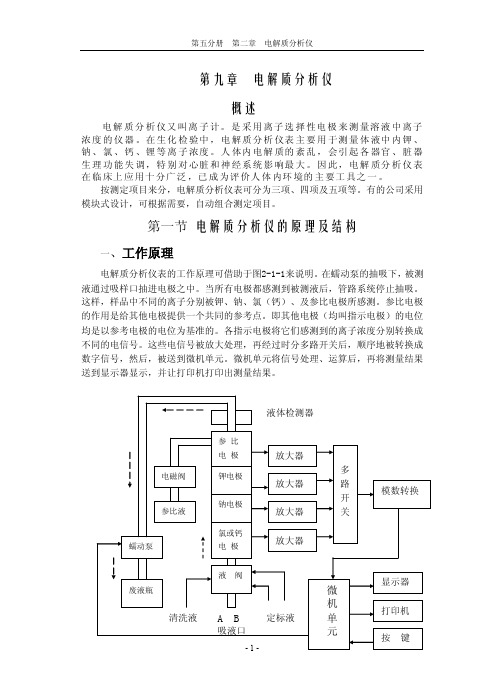
第一节电解质分析仪的原理及结构一、工作原理电解质分析仪表的工作原理可借助于图2-1-1来说明。
在蠕动泵的抽吸下,被测液通过吸样口抽进电极之中。
当所有电极都感测到被测液后,管路系统停止抽吸。
这样,样品中不同的离子分别被钾、钠、氯(钙)、及参比电极所感测。
参比电极的作用是给其他电极提供一个共同的参考点。
即其他电极(均叫指示电极)的电位均是以参考电极的电位为基准的。
各指示电极将它们感测到的离子浓度分别转换成不同的电信号。
这些电信号被放大处理,再经过时分多路开关后,顺序地被转换成数字信号,然后,被送到微机单元。
微机单元将信号处理、运算后,再将测量结果送到显示器显示,并让打印机打印出测量结果。
图2-1-1 电解质分析仪表方框图为了完成对样品的自动定标、自动测量和自动冲洗等功能,一般的电解质分析仪表均设有一套管路系统以及配合管路工作的蠕动泵和电磁阀。
泵和电磁阀的转、停、开、闭,清洗液、定标液的供、停等等,均由微机单元来进行控制或监测。
电解质分析方法也是一种相对测量方法。
所以,在进行测量之前,先要用标准液来确定电极的工作曲线。
通常把确定电极系统工作曲线的过程叫做定标或校准(Calibration)。
电极要有A、B两种液体来进行定标,以便确定建立工作曲线最少所需要的两个工作点。
清洗液是清洁管路用的。
为了防止交叉污染,每测量一次,都要用清洗液将管路清洗一次。
由此可知,无论何种型号的电解质分析仪表,都需要先对电极进行两点定标,建立了工作曲线之后,才能进行测量工作。
电解质分析仪讲解

2.电极系统
电极系统是测定样品结果的关键,决定测 定结果的准确度和灵敏度。
指示电极 电极系统 参比电极
指示电极:
pH、Na+、K+、Li+、Cl-、Ca2+、Mg2+等
离子选择性电极。
Yes No
参比电极: 银/氯化银电极
AVL 9180
新型仪器的测量电极 指示电极:流动式离子感应透明膜电极 参比电极:流动式透明接头电极
液路系统中的通路:由定标液(calibration solutions )/冲洗液(Rinse)通路、标本通路、 废液通路、回水通路、电磁阀通路等组成。
液路系统直接影响到样品浓度测定的准确 性和稳定性。
标本盘、三通阀和蠕动泵的转动、转换均
由微机自动控制。
MEDICA全自动 电解质分析仪
❖ 自动进样器的结构框图
测量样品中的指标:
pH、PCO2、PO2、 AB、SB、BB、TCO2、 BE blood、BEECF、SO2等。
电解质分析仪
一、电解质分析仪的分类
(一)按自动化程度分类 半自动电解质分析仪 全自动电解质分析仪
(二)按工作方式分类 湿式电解质分析仪 – 临床上常用 干式电解质分析仪
(三)常见电解质分析的仪器分类 电解质分析仪 — 只进行单独的电解质分
37℃,pH值: 6.84
(二)离子选择性电极工作原理
( principal of Ion selective electrode )
1. 离子选择性电极的结构
离子选择性电极又称膜电极(membrane electrodes )
特点:仅对溶液中特定离子有选择性响应。
膜电极的关键:是一个称为选择膜的敏感元件。
电解质分析仪

第九章电解质分析仪概述电解质分析仪又叫离子计。
是采用离子选择性电极来测量溶液中离子浓度的仪器。
在生化检验中,电解质分析仪表主要用于测量体液中内钾、钠、氯、钙、锂等离子浓度。
人体内电解质的紊乱,会引起各器官、脏器生理功能失调,特别对心脏和神经系统影响最大。
因此,电解质分析仪表在临床上应用十分广泛,已成为评价人体内环境的主要工具之一。
按测定项目来分,电解质分析仪表可分为三项、四项及五项等。
有的公司采用模块式设计,可根据需要,自动组合测定项目。
第一节电解质分析仪的原理及结构一、工作原理电解质分析仪表的工作原理可借助于图2-1-1来说明。
在蠕动泵的抽吸下,被测液通过吸样口抽进电极之中。
当所有电极都感测到被测液后,管路系统停止抽吸。
这样,样品中不同的离子分别被钾、钠、氯(钙)、及参比电极所感测。
参比电极的作用是给其他电极提供一个共同的参考点。
即其他电极(均叫指示电极)的电位均是以参考电极的电位为基准的。
各指示电极将它们感测到的离子浓度分别转换成不同的电信号。
这些电信号被放大处理,再经过时分多路开关后,顺序地被转换成数字信号,然后,被送到微机单元。
微机单元将信号处理、运算后,再将测量结果送到显示器显示,并让打印机打印出测量结果。
图2-1-1 电解质分析仪表方框图为了完成对样品的自动定标、自动测量和自动冲洗等功能,一般的电解质分析仪表均设有一套管路系统以及配合管路工作的蠕动泵和电磁阀。
泵和电磁阀的转、停、开、闭,清洗液、定标液的供、停等等,均由微机单元来进行控制或监测。
电解质分析方法也是一种相对测量方法。
所以,在进行测量之前,先要用标准液来确定电极的工作曲线。
通常把确定电极系统工作曲线的过程叫做定标或校准(Calibration)。
电极要有A、B两种液体来进行定标,以便确定建立工作曲线最少所需要的两个工作点。
清洗液是清洁管路用的。
为了防止交叉污染,每测量一次,都要用清洗液将管路清洗一次。
由此可知,无论何种型号的电解质分析仪表,都需要先对电极进行两点定标,建立了工作曲线之后,才能进行测量工作。
电解质分析仪的工作原理

电解质分析仪的工作原理电解质分析仪是一种用于检测水或其他液体中电解质含量的仪器。
它的工作原理是利用电化学方法测定水样中的离子浓度。
1. 电解质分析仪的基本组成电解质分析仪主要由以下部分组成:•电解槽:用于容纳待测液体。
•电极:包括参比电极和工作电极两种,参比电极用于比较测定电极的电势变化,工作电极则用于测量待测液体中的离子含量。
•电位计:用于测量电极的电位差,从而计算出待测液体中的离子浓度。
•转换器:通过放大和转换电位计的输出信号,将其转化为电流信号,用于处理和转换离子浓度值。
2. 电解质分析仪的工作原理电解质分析仪利用将待测液体置于电解槽中,并引入适当的电极和电位计等装置来进行离子分析。
在这个过程中,待测液体中的离子会在电解槽中与电极反应,从而产生电位变化,这种变化会被电位计测量并记录下来。
测量得到的数据可以通过计算和转换器处理,得到待测液体中离子浓度的值。
具体地,电解质分析仪的工作过程如下:步骤1:准备电解质首先,需要将待测液体加入到电解槽中,并加入适量的电解质,使得电导率达到适当的程度。
步骤2:放置电极待测液体中加入电极对,包括参比电极和工作电极。
参比电极可以用于比较测定电极的电势变化,而工作电极则用于测定待测液体中的离子浓度。
步骤3:施加电压施加适当的电压或电流,在电极与待测液体之间形成电压梯度,从而引发离子的迁移和反应。
步骤4:测量电位差通过电位计测量参比电极和工作电极之间电势差,从而可以计算出待测液体中离子的浓度。
由于电位计的灵敏度比较高,因此需要用极高的精度来保证测量结果的准确性。
步骤5:分析处理数据在测量中,可以通过计算和转换器等装置处理数据,从而得到准确的离子浓度值。
这些数据可以被记录、保存和分析,用于判断水或其他液体是否符合某个标准。
3. 应用领域电解质分析仪作为一种离子分析仪,广泛应用于以下领域:•食品和饮料工业,测定水和其他液体中的离子含量,如钠、钾、镁等,以保证食品和饮料的质量和安全性。
9.电解质分析仪

(二)离子选择性电极的工作原理
离子选择性电极的电极电位表示为: 离子选择性电极的电极电位表示为:
2.303RT E =k± ln C f ISE x x nF
阳离子选择性电极为 +; 阴离子选择性电极为 -; 为离子电荷数; n 为离子电荷数; 为被测离子浓度; Cx 为被测离子浓度; 为被测离子活度系数; fx 为被测离子活度系数; 在测量条件恒定时为常数。 K 在测量条件恒定时为常数。
2.电极系统 2.电极系统 电极系统是测定样品结果的关键, 电极系统是测定样品结果的关键,决定测 准确度和灵敏度。 定结果的准确度和灵敏度 定结果的准确度和灵敏度。 指示电极 电极系统 参比电极 指示电极: 指示电极: pH、 pH、Na+、K+、Li+、Cl-、Ca2+、Mg2+等 Yes No 离子选择性电极。 离子选择性电极。 参比电极: 参比电极: 银/氯化银电极
2.血气分析仪 2.血气分析仪 利用电极对血样中的酸碱度( ) 利用电极对血样中的酸碱度(pH)、 酸碱度 二氧化碳分压( 氧分压( 二氧化碳分压(PCO )和氧分压(PO )进 行测定的仪器。 行测定的仪器。
2 2
测量样品中的指标: 测量样品中的指标: pH、PCO2、PO2、 、 AB、SB、BB、TCO2、 、 、 、 BE blood、BEECF、SO2等。
一、电化学分析法(Electrochemical analysis) analysis) 电化学分析法是建立在溶液电化学性 质基础上的一类分析方法。 质基础上的一类分析方法。 测 定 物理量 电位 电流 确定 电导 电量 参与反应的化 学物质的量
电位分析法
二、电化学临床分析仪器 电化学临床分析仪器 是利用电化学分析技术 是利用电化学分析技术而 电化学分析技术而 设计的临床分析仪器。 设计的临床分析仪器。 电解质分析仪 分类
电解质分析仪介绍和检测临床意义

电解质分析仪介绍和检测临床意义电解质分析仪器是用来从样本中检测钾离子,钠离子,氯离子,离子钙和锂离子的仪器.样本能够是全血,血清,血浆,尿液, 透析液,和水化液.工作原理:电解质分析仪有采用离子选择电极测量法来实现精准检测的.仪器上有六种电极:钠,钾,氯,离子钙,锂和参比电极.每一个电极都有一离子选择膜,会与被测样本中相应的离子产生反映,膜是一离子互换器,与离子电荷发生反映而改变了膜电势,就可检测液,样本和膜间的电势.膜两边被检测的两个电势差值会产生电流,样本,参考电极,参考电极液组成"回路"一边,膜,内部电极液,内部电极为另一边.内部电极液和样本间的离子浓度差会在工作电极的膜两边产生电化学电压,电压通太高传导性的内部电极引到到放大器,参考电极一样引到放大器的地址.通过检测一个精准的已知离子浓度的标准溶液取得定标曲线,从而检测样本中的离子浓度.溶液中被测离子接触电极时,在离子选择电极基质的含水层内发生离子迁移.迁移的离子的电荷改变存在着电势,因此使膜面间的电位发生转变,在测量电极与参比电极间产生一个电位差.一般常常利用电极结构:钠电极特点:钠电极是一种玻璃毛细管电极用来测定液体样本中的钠离子浓度,主要结构:电极套:透明塑料。
测量毛细管:钠敏感玻璃。
电极室:密封的,内充满钠电极液。
电极芯:Ag、Agcl钾电极特点:钾电极是一种膜电板,也是用来测量样本中的钾离子浓度。
主要结构:电极套:透明塑料。
测量毛细管:钾离子敏感膜。
电极室:密封的,内充满K+液。
电极芯:Ag/Agcl氯电极特点:氯电极也是一种膜电极,用来测量样本中的Cl离子浓度。
主要结构:电极套:透明塑料。
测量毛细管:Cl离子敏感膜。
电极室:密封的且充有Cl-液。
电极芯:Ag/Agcl参比电极特点:参比电极是连接样本和信号地的一个装置。
主要结构:参比电极由两部份组成:参比电极套和参比电极芯。
参比电极套中的参比液在以参比电极芯与样本之间形成一个盐桥,每次测量开始时,参比液被注入参比电极套中,同时有一小部份参比液由玻璃毛细管中渗入测量室,从而在样本和参比电极芯之间形成盐桥,参比电极芯在电信号地和参比液之间形成回路。
电解质分析仪介绍和检测临床意义

电解质分析仪介绍和检测临床意义电解质分析仪器是用来从样本中检测钾离子,钠离子,氯离子,离子钙和锂离子的仪器.样本可以是全血,血清,血浆,尿液, 透析液,和水化液.工作原理:电解质分析仪有采用离子选择电极测量法来实现精确检测的.仪器上有六种电极:钠,钾,氯,离子钙,锂和参比电极.每个电极都有一离子选择膜,会与被测样本中相应的离子产生反应,膜是一离子交换器,与离子电荷发生反应而改变了膜电势,就可检测液,样本和膜间的电势.膜两边被检测的两个电势差值会产生电流,样本,参考电极,参考电极液构成"回路"一边,膜,内部电极液,内部电极为另一边.内部电极液和样本间的离子浓度差会在工作电极的膜两边产生电化学电压,电压通过高传导性的内部电极引到到放大器,参考电极同样引到放大器的地点.通过检测一个精确的已知离子浓度的标准溶液获得定标曲线,从而检测样本中的离子浓度.临床意义:钾K钾是细胞内液最主要的阳离子,在细胞间起最初的缓冲作用.90%的钾离子在细胞内,损坏的细胞会释放钾离子到血液中.钾在神经传导,肌肉功能,保持酸碱平衡和渗透压方面起着重要的作用.高钾值出现在少尿症,贫血,排尿障碍,肾炎或休克引起的肾功能不全,代谢性或呼吸性算毒症,带H离子和K离交换的肾管酸毒症,以及溶血症.低钾症往往是钾的过度流失,常见于:腹泻或呕吐,钾摄入不足, 吸收不良,严重的烧伤和醛固酮分泌的增加.钾值的高低会引起肌肉应激性变化,呼吸作用变化,以及心肌功能的变化.获得钾值常常用来在诊断和治疗以下情况时监测电解质的平衡,如临床注射,休克,心脏或循环功能不全,酸碱平衡,每日疗法,各种肾脏疾病,腹泻,肾上腺皮质功能过剩和不足,以及其它涉及电解质平衡的疾病.钠Na钠是细胞外液中最主要的阳离子. 其对人体的主要功能是通过化学作用维护渗透压和酸碱平衡以及传递神经冲动. 钠离子的功能是调节细胞膜内外的电位差以维护神经元兴奋传导.钠还作为因子参与一些酶催化反应.人体一直维持基本平衡,即便病理情况下一些细微的变化也会察觉.钠值低即低钠症,通常反映了体液相对体内总钠量过剩.钠水平的减少与以下相关:低钠流入;由于呕吐或腹泻造成钠流失,并补充充足的水分和不充足的盐,每日使用不当,或缺盐型肾病;渗透多尿,代谢性酸毒症;肾上腺皮质不足; 先天性肾上腺增生; 因水肿,心功能不全,肝功能不全,甲状腺机能减退引起的稀释.高钠值是水分的流失超过盐分的流失,例如大量得出汗,呼吸过度,剧烈的呕吐或腹泻,糖尿病或糖尿病性酸毒症; 醛固酮症,CUSHING综合引起的肾脏钠存量增加;因昏迷或中枢疾病造成水摄入不足; 脱水;或过度的碱治疗.获得钠值通常用来诊断或检测以下:所有的水平衡紊乱,临床注射,呕吐,腹泻,烧伤,心功能抯和肝功能不全,中枢或肾原来性糖尿病, 内分泌紊乱和原发性或继发性肾上腺皮质不足,或其它涉及电解质平衡的疾病.氯Cl氯是存在于细胞外的最主要的阴离子.通过它影响了细胞的渗透压.在监测酸碱平衡和水平衡中也起重要作用.在代谢性酸毒症中,当碳酸氢盐浓度下降时氯离子浓度会反向上升.氯降低发生在严重的呕吐,严重的腹泻,溃疡性结肠炎,幽门阻塞,严重烧伤,中暑,糖尿病酸毒症.Addison氏病,发烧, 象肺炎那样的急性感染,等.氯上升发生在脱水,Cushing综合症,换气过度,惊厥,贫血,心功能不全,等.离子钙血液中钙作为自由钙离子(50%)在蛋白质,大部分清蛋白(40%)和10%局限于如碳酸化,磷酸盐化和乳酸盐化阴离子.但是,仅离子钙能被在身体使用,如肌肉收缩,心脏的功能,传送神经冲动和血凝这样的重要过程.A VL 9180分析仪测量总钙的离子部分.诊断例如胰腺炎和甲状旁腺功能亢进,与总钙相比,离子钙是一更好的指标.钙nCa高血钙可以有各种各样的不良表现,钙值测量可以被生化学家作标记用.通常,在检测恶性肿瘤时,离子钙或总钙都有同样的作用,离子钙可能更敏感一些.高钙血症常常发生在酸碱调节和蛋白\白蛋白流失异常的危急病人中,通过检测离子钙可以很清晰有效地监视钙的状况.患肾脏血管球疾病的肾病患者通常会引起钙, 磷酸盐, 白蛋白,镁离子和PH的浓度异常.因为这些情况会改变总钙中离子钙的独立性,因此监测离子钙成为精确监护肾病患者钙状态的首选方法.(见附注3)离子钙对以下的诊断或监护有着重要意义:高血压控制,甲状旁腺,肾脏疾病,钙摄入不足,维生素监护,透析病人,癌症, 胰腺病,利尿剂作用,营养失调,肾结石,多发粘液瘤病,糖尿病等.尿液电解质电解质存在于人体,也从每日的食物中摄取,通过肾脏系统排泄到尿液中,这是一自然循环.从排泄物尿液中检测电解质,可以了解肾脏的状况和其它的病理状况等重要信息.检测可以从任意尿液样本中,也可从24小时收集的尿液样本作定量检测.每天排泄的电解质量可以通过检测一天尿液中排泄浓度的增加量(mmol/L)来获得.透析电解质在透析器中,动脉血和透析液在透析膜的两边进行透析.透析膜会防止蛋白质和红细胞的扩散.因为血液和透析液的成分不同,膜两边会产生压力梯度,小分子就可以通过膜进行弥漫.这种方法可以有效的滤除那些因肾功能低下而不能排泄的尿素,尿酸等物质.当血液和透析液中的电解质浓度有显著差异时,电解质就会从浓度高处理向低的弥散,如从血液向透析液扩散,或相反.)透析中电解质的透析对临床医生有着非常重要的意义,例如:* 为了维持透析前,透析时,透析后的电解质平衡,及时识别偏差,也可以及早纠正.* 控制透析液中电解质的浓度.一般混合定量的蒸馏水和适当浓度的物质来用.。
电解质分析仪原理与临床应用PPT课件

06 电解质分析仪性能评价与 选购建议
性能指标与评价方法
精确度
通过测量标准溶液或质控物,评估仪器测量 结果的准确性。
重复性
对同一样本进行多次测量,观察测量结果的 重复性,评估仪器的一致性。
稳定性
长时间运行后,观察仪器测量结果的稳定性, 评估其可靠性。
线性范围
测试仪器在不同浓度范围内的线性表现,评 估其测量范围是否满足临床需求。
案例分析
结合具体案例,分析电解质分析仪在重症监护与 抢救治疗中的实际应用效果。
药物监测与剂量调整
01
药物对电解质的影响
介绍各类药物对电解质浓度的影响及其机制。
02
电解质分析仪在药物监测中的应用
通过监测患者用药前后的电解质变化,评估药物疗效和副解质分析仪提供的数据,为医生调整药物剂量提供科学依据。
原理比较与优缺点分析
01
02
03
04
离子选择性电极法
优点在于选择性好、灵敏度高 ;缺点在于电极易受干扰、需
定期校准。
电导法
优点在于测量范围宽、准确度 高;缺点在于受温度、粘度等
因素影响。
光学法
优点在于抗干扰能力强、测量 精度高;缺点在于仪器成本较
高。
综合比较
不同原理的电解质分析仪各有 优缺点,应根据实际需求选择
现状
目前市场上存在多种品牌 和型号的电解质分析仪, 广泛应用于医院、实验室 等场所。
市场需求与应用领域
市场需求
随着人们对健康关注的提高,电解质分析仪在医疗领域的需 求不断增加,同时,其在科研、教学等领域也具有一定的市 场需求。
应用领域
电解质分析仪主要应用于临床检验科、急诊科、重症医学科 等科室,用于监测患者体液中电解质浓度的变化,为医生提 供诊断和治疗依据。此外,还可用于科研实验、教学演示等 场合。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
9
3 Principle
Reference electrode
Key: membrane electrode is a known as the sensitive element of membrane choice.
Sensitive components: single crystal, mixed crystal, liquid membrane, membrane function and biological membrane, etc.
2 Denifition
Electrolyte analyzer
using ion selective electrode (ISE) measuring instrument for the ion concentration in the body fluids.
Measuring indicators of the sample: K+,Na+,Cl-, Ca+,pH, etc.
电解质分 析 仪
Electrolyte analyzers
作者xxx
1 Electrolyte chemical analyzers
2
1 Electrolyte chemical analyzers
What is the electrochemical analysis?
Energized the electrochemical properties of the solution: the electrolyte solution, its potential, current and conductance and power electrochemical properties with the nature of the chemical composition and concentration. Electrochemical analysis: based on the electrochemical properties of solution of analysis method.
5
3 Principle
The basic principle
Electrolyte analyzer is based on the ion selective electrode and reference electrode potential measurement and developed.In an electrolyte, most salt ionization into ions, inserted in the electrolyte ion selective electrode (indicating electrode) as the positive pole of the battery, reference electrode as the cathode of batteries, form a galvanic cell.Such in the relevant between ion selective electrode and reference electrode electrode potential difference, namely the battery electromotive force.By measuring the battery electromotive force measured corresponding ion concentration.
CONTENTS
1 Electrolyte chemical analyzers
2 Denifition
5 Wet electrolyte analyzers
3 Principle
6 Dry electrolyte analyzers
4 Classification
7 Clinical significance
E=E0-R.T/F.lg[cl-]
10
3 Principle
Referenckinds of commonly used, one is the calomel electrode, the other is a Ag-AgCl electrode.
Ag-AgCl electrode: Agcl+e
Ag+cl-
the nernst equation
Electrochemical clinical analysis instrument is designed using electrochemical analysis technology and clinical analysis instrument.
Divided into: Blood gas analyzer Electrolyte analyzer
enzyme electrode
...
7
3 Principle
8
3 Principle
Ion selective electrode
Ion selective electrode is also called membrane electrode
Features: only the specific ion selective response in the solution.
6
3 Principle
Ion selective electrode
different material
solid ion exchange membrane electrode
liquid ion-exchange membrane electrode
gas sensitive electrode
