Nanoparticles in cancertherapy and diagnosis
载抗肿瘤药物的壳聚糖纳米粒氨基化靶向修饰物的研究进展

载抗肿瘤药物的壳聚糖纳米粒氨基化靶向修饰物的研究进展目的:为寻找或开发更优的载抗肿瘤药物的壳聚糖纳米粒靶向修饰物提供参考。
方法:以“壳聚糖”“纳米粒”“氨基”“靶向修饰”“抗肿瘤”“Chitosan”“Nanoparticles”“Amino”“Targeting modification”“Antitumor”等为关键词,组合查询2005年1月-2018年3月在中国知网、万方、维普、PubMed、Web of Science、Elsevier、SpringerLink等数据库中的相关文献,对载抗肿瘤药物的壳聚糖纳米粒氨基化靶向修饰物从大分子和小分子配体两方面进行论述。
结果与结论:共检索到相关文献300篇,其中有效文献36篇。
对壳聚糖纳米粒表面的氨基进行修饰,可以获得具有主动寻靶作用的壳聚糖纳米粒,现有的靶向修饰配体有小分子的叶酸、生物素、乳糖酸、甘草酸等,大分子的透明质酸、鱼精蛋白、转铁蛋白、缬氨酸-精氨酸-甘氨酸-天冬氨酸-谷氨酸环肽、CD59特异性配体肽、促黄体生成素释放激素及MUC1等。
未来研究重点应利用壳聚糖纳米粒氨基這一表面特性,寻找或开发出更优的壳聚糖纳米粒的靶向修饰物,进一步提高壳聚糖纳米粒对肿瘤细胞的靶向效率。
关键词壳聚糖;纳米粒;氨基;靶向修饰;抗肿瘤主动靶向纳米给药系统,即对纳米粒表面进行靶向特异性修饰,使其靶向于细胞表面特异性表达或过表达的某种受体而实现靶向,该靶向系统可将药物定向递送于病变部位,达到降低毒副作用、增强疗效的目的[1-2]。
壳聚糖是目前自然界中发现的唯一一个碱性多糖[3],含量仅次于维生素。
壳聚糖纳米粒作为一种新型的给药系统,能够保护药物的稳定性,延长药物在体内的循环时间,有效提高药物利用度,并且具有良好的生物相容性和生物可降解性,因此,在药剂学中备受青睐[4]。
此外,壳聚糖分子结构中的氨基使其具有许多特殊功能,可进行多功能基化学反应和立体结构修饰,如将具有主动寻靶作用的单抗或配体通过化学修饰与之相结合,可获得具有定位传输功能的主动靶向制剂[5]。
一氧化氮治疗肿瘤的研究进展

一氧化氮治疗肿瘤的研究进展作者:王丽凯,田娅,吴惠霞来源:《上海师范大学学报·自然科学版》2022年第04期摘要:一氧化氮(NO)是一种半衰期很短的气体分子,对细胞膜具有高穿透性,能在人体内传递重要信息,并具有调节细胞的功能.NO气体分子既能维持正常细胞的生理功能和活性,又能选择性地快速耗尽肿瘤细胞的能量,诱导肿瘤细胞凋亡.研究表明:NO可以通过多种机制實现肿瘤治疗.已有一些NO供体药物表现出良好的抗肿瘤活性,精确控制NO在肿瘤部位的释放,可杀死肿瘤细胞.因此,NO气体疗法作为一种肿瘤治疗策略具有一定的应用前景.文章简述了NO的生理学特性和几种典型的NO供体,以及释放NO的生物材料在生物医学领域的应用进展.关键词:一氧化氮(NO); NO供体; 肿瘤; 气体治疗; 生物材料中图分类号: O 613.6 文献标志码: A 文章编号: 1000-5137(2022)04-0443-09Research progress of nitric oxide in the treatment of tumorWANG Likai, TIAN Ya, WU Huixia*(College of Chemistry and Materials Science, Shanghai Normal University, Shanghai 200234, China)Abstract: Nitric oxide (NO) is a ubiquitous gas molecule with a short half-life. It is highly permeable to cell membranes and can transmit important information and regulate cellular functions in the human body. NO molecules can not only maintain the physiological function and activity of normal cells, but also selectively and rapidly deplete the energy of tumor cells and induce their apoptosis. Studies have shown that NO may achieve tumor therapy through a variety of mechanisms. Some NO donor drugs have shown good anti-tumor activity and can be used to precisely control the release of NO at tumor sites and kill tumor cells. Therefore, NO gas therapy is a promising tumor treatment strategy. This review covers the physiological characteristics of NO, several typical NO donors, and the application progress of NO releasing biomaterials in biomedical field.Key words: nitric oxide(NO); NO donors; tumor; gas therapy; biomaterials0 引言一氧化氮(NO)是一氧化氮合酶(NOS)作用产生的半衰期仅为3-5 s的分子.NO分子中有一个未成对电子,可形成自由基,对多种生物分子具有很高的反应性.NO具有脂溶性,可以快速透过生物膜扩散,在体内极不稳定,能迅速被血红蛋白、氧自由基或氢醌等灭活.NO可以对血管生成和舒张、细胞周期、细胞凋亡、侵袭和转移等过程进行调节,从而影响细胞功能.NO还能与二氧化氮(NO2)反应生成三氧化二氮(N2O3),并能与超氧化物反应生成过氧亚硝酸盐(ONOO-).N2O3和ONOO-这2种分子均可通过亚硝化或氧化应激引起DNA损伤:N2O3可以通过胺的亚硝化作用导致N‒亚硝胺的形成,进而损伤DNA;过氧亚硝酸盐可以氧化和硝化DNA,并导致单链DNA断裂[1].NO的生物效应通常取决于分子的形成、代谢、NOS的类型和NO的浓度等.在过去的几十年中,人们一直在努力研究NO对癌生物学的影响.多年来,NO在致癌和抗肿瘤进展中有着较大的误解和争议,因为它同时具有促进肿瘤细胞生长和杀死肿瘤细胞的能力.然而,确定哪种作用占优势是很复杂的,包括但不限于NO存在的时间、位置、浓度和肿瘤微环境[2].NO生成过多或者生成不足都会引起基因突变、肿瘤等.近年来,许多气体纳米发生器已经能够通过被动或主动靶向聚集在肿瘤部位,在内源性或外源性刺激下有效控制气体分子的释放.因此,无论是单独使用NO还是与其他治疗方式联合使用,这些发现都使NO广泛应用于抗癌剂[3].目前,气体治疗已成为一种新兴的、安全有效的抗癌治疗策略.1 NO的生理学特性1.1 NO的生物合成细胞合成NO的主要途径是通过NOS的酶促作用将L‒精氨酸转化为L‒瓜氨酸,并释放出NO,如图1所示[4].NOS是一种同工酶,选择性分布在不同脑区的神经元中,其同工酶有3种亚型,即神经型一氧化氮合酶(nNOS)、诱导型一氧化氮合酶(iNOS)和内皮型一氧化氮合酶(eNOS).其中,eNOS和nNOS在细胞处于生理状态下即可组成性表达,并可因细胞内钙增加而被钙调蛋白激活;iNOS是非钙依赖型的,当细胞受到内源性或外源性刺激时,可在较短的时间内产生高浓度的NO[4].此外,还可以通过硝酸盐→亚硝酸盐→NO途径合成NO.体内的硝酸盐主要来自膳食和自身合成.在生物体内,循环的硝酸盐被唾液腺主动摄取,并被口腔中的细菌还原为亚硝酸盐,在血液和组织中进一步代谢为NO和其他生物活性氮氧化物[5].亚硝酸盐是氮氧化物的氧化还原过程中的中间产物,在血液和组织中比较稳定,且可被多种物质还原成NO,包括肌红蛋白、血红蛋白、抗坏血酸、黄嘌呤氧化还原酶、质子和多酚[5].这些途径产生的NO会因缺氧和酸中毒条件而增加,因而可以保证NO的产量.1.2 NO的生物学作用NO可以自由地通过生物膜并参与一系列生理和病理过程,如神经信号传递、血管扩张、血小板黏附和聚集等,在生物体内发挥着至关重要的作用.NO的生物学作用是通过直接或间接的化学反应产生的.例如,NO直接与不同蛋白质的金属配合物结合形成金属亚硝酰基配合物来调节靶蛋白的生物学活性.NO还可以与多种内源性自由基反应,产生活性氮氧化合物,这些强毒性的活性氮氧化合物将导致线粒体损伤,进而诱导细胞凋亡.NO在生物体内像一把双刃剑,因为它既具有杀死肿瘤细胞的作用,又具有促进肿瘤细胞生长的作用.在低生理水平下,NO可作为抗氧化剂,减少芬顿反应,终止自由基链式反应,并抑制过氧化物酶和氧化酶的活性.较高浓度的NO能够舒张血管,改善组织缺氧状态,有利于化疗药物的渗透,对肿瘤细胞具有杀伤作用[6].但是,持续过量的NO将产生神经毒性,影响体内平衡和改变蛋白质功能,从而导致基因突变,最终使正常黏膜癌变[7].不同组织中的生理过程对NO的需求量各不相同,浓度过高或者过低都会对组织造成一定的损伤,引起疾病的发生[8].只要将适当浓度的NO递送至肿瘤部位,NO的靶向释放也可能增强化学疗法和放射疗法的疗效.因此,如何将适当浓度的NO靶向释放至肿瘤,已成为近年来生物医学领域的研究重点.2 NO供体直接使用外源性或内源性NO的缺点是其半衰期极短,且易受各种谷胱甘肽(GSH)、超氧化物和血红蛋白等物质的影响.因此,将NO供体载入纳米平台中,直接和精确地控制NO的靶向释放,有很好的应用前景.NO供体是指在体内经酶促反应或非酶促反应释放NO的一类化合物,如有机硝酸盐、有机亚硝酸盐、S‒亚硝基硫醇(RSNO)、金属配合物等多种化学物质已被用作NO供体,用于各种生物或医学领域[9],如图2所示.2.1 有机硝酸酯(RONO2)及有机亚硝酸酯类RONO2是醇的硝酸酯,是最早的、目前最常用的NO供体.它们可以通过相应醇的酯化反应或烷基卤化物与AgNO3的反应来合成,如图3所示[10].这类供体的优点是给药途径比较广,但容易产生耐药性.硝酸甘油(GTN)和单硝酸异山梨酯(ISMN)是临床研究中使用最广泛的NO供体类药物.它们有几个既定的临床应用:GTN是一種廉价又有效的、能快速逆转与急性心绞痛有关疼痛的药物;ISMN是RONO2中释放NO较慢的一种,已被用于治疗慢性心绞痛[11].与RONO2类似,有机亚硝酸酯是醇类和亚硝酸酯化形成的酯.它们主要通过醇与亚硝酰氯(NOCl)反应或醇与NO和氧气(O2)经过酯化反应来合成,如图3所示[12].有机亚硝酸酯的主要作用是舒张静脉和降低血压,例如,亚硝酸丁酯(BN)、亚硝酸异丁酯(ISBN)和亚硝酸叔丁酯(TBN)已在临床上用作血管扩张剂[13].与GTN等RONO2相比,它们对酶的依赖性更低、作用效力更高,且不易引起耐药性.但是,它们缺乏选择性和生物利用度,以及细胞毒性和致癌性较高,因此不如RONO2常用[14].2.2 RSNO类RSNO是贮存、运输和释放NO的重要载体,在生物体内具有重要的生理作用.RSNO普遍存在于生物体的血液和组织中,只需要一个电子就能引发NO的释放,因此,可通过光、热、碱性pH值、过渡金属离子、抗坏血酸和酶等促使RSNO自发均裂反应产生NO[15].人工合成的RSNO是新型的NO供体类药物,通过静脉等途径进入体内后,可以参与呼吸、心血管、消化等多个系统疾病的诊断和治疗[16].2.3 金属-一氧化氮配合物NO是金属配合物中的强配体,它的结合常数比一氧化碳(CO)和O2高得多,具有多种氧化态,氧化价态的高低决定了配合物中NO的反应性.NO调节信号通路的主要机制是与金属中心原子(如铁(Fe)、钌(Ru)等)结合,如图4所示[12],如血红素基团或蛋白质的铁硫簇.硝普钠(SNP)已经广泛应用于急性降压药物和动静脉血管扩张剂,其血管舒张作用是由NO的产生而造成的[17].SNP晶体在避光且干燥的条件下可以长时间保存,光和O2会促使其水溶液分解,并释放出NO和氰化物,从而导致“氰化物毒性”,对机体造成伤害[18].除了Fe之外,Ru对NO也有很高的亲和力,且Ru对NO的亲和力可以随着其他配体的改变而变化,以便调节NO的释放.光活性Ru配合物热稳定性好,且能在紫外光照射下释放NO.然而,NO的有效释放需要使用对组织有害的高功率紫外线,这一缺陷阻碍了该类NO供体的临床应用[19].2.4 其他供体1956年MAGEE等[20]发现了二甲基亚硝胺和亚硝胺二甲胺均可致大鼠肝癌.其致癌作用是由于N-亚硝基化合物会导致蛋白质和核酸的烷基化.但是,N-亚硝胺却是一种能舒张血管的NO供体.链脲霉素(STZ)含有N-亚硝胺基团,具有抗肿瘤、致糖尿病和致癌作用[21].胰腺β细胞具有低水平的活性氧(ROS)清除酶,对NO和ROS比较敏感,STZ能在胰岛β细胞中释放NO,使细胞的DNA受到损害[22].因此,可将此类NO供体作为抗癌药物进行研究.偶氮二醇烯鎓盐(NONOates)释放NO的机制遵循动力学且不受细胞代谢产物或酶的催化.它们以固体形态稳定存在,但在生理条件下会自发分解生成NO,分解速率会因结构、温度和pH值而改变[23].因此,可以通过它们在体外的分解速率直接预测药物的持续作用时间.研究证明:NONOates能够降低多种肿瘤细胞的增长速率,抑制肿瘤细胞的生长[24].此外,还可以通过硝酸盐→亚硝酸盐→NO途径合成NO.体内的硝酸盐主要来自膳食和自身合成.在生物体内,循环的硝酸盐被唾液腺主动摄取,并被口腔中的细菌还原为亚硝酸盐,在血液和组织中进一步代谢为NO和其他生物活性氮氧化物[5].亚硝酸盐是氮氧化物的氧化还原过程中的中间产物,在血液和组织中比较稳定,且可被多种物质还原成NO,包括肌红蛋白、血红蛋白、抗坏血酸、黄嘌呤氧化还原酶、质子和多酚[5].这些途径产生的NO會因缺氧和酸中毒条件而增加,因而可以保证NO的产量.1.2 NO的生物学作用NO可以自由地通过生物膜并参与一系列生理和病理过程,如神经信号传递、血管扩张、血小板黏附和聚集等,在生物体内发挥着至关重要的作用.NO的生物学作用是通过直接或间接的化学反应产生的.例如,NO直接与不同蛋白质的金属配合物结合形成金属亚硝酰基配合物来调节靶蛋白的生物学活性.NO还可以与多种内源性自由基反应,产生活性氮氧化合物,这些强毒性的活性氮氧化合物将导致线粒体损伤,进而诱导细胞凋亡.NO在生物体内像一把双刃剑,因为它既具有杀死肿瘤细胞的作用,又具有促进肿瘤细胞生长的作用.在低生理水平下,NO可作为抗氧化剂,减少芬顿反应,终止自由基链式反应,并抑制过氧化物酶和氧化酶的活性.较高浓度的NO能够舒张血管,改善组织缺氧状态,有利于化疗药物的渗透,对肿瘤细胞具有杀伤作用[6].但是,持续过量的NO将产生神经毒性,影响体内平衡和改变蛋白质功能,从而导致基因突变,最终使正常黏膜癌变[7].不同组织中的生理过程对NO的需求量各不相同,浓度过高或者过低都会对组织造成一定的损伤,引起疾病的发生[8].只要将适当浓度的NO递送至肿瘤部位,NO的靶向释放也可能增强化学疗法和放射疗法的疗效.因此,如何将适当浓度的NO靶向释放至肿瘤,已成为近年来生物医学领域的研究重点.2 NO供体直接使用外源性或内源性NO的缺点是其半衰期极短,且易受各种谷胱甘肽(GSH)、超氧化物和血红蛋白等物质的影响.因此,将NO供体载入纳米平台中,直接和精确地控制NO的靶向释放,有很好的应用前景.NO供体是指在体内经酶促反应或非酶促反应释放NO的一类化合物,如有机硝酸盐、有机亚硝酸盐、S‒亚硝基硫醇(RSNO)、金属配合物等多种化学物质已被用作NO供体,用于各种生物或医学领域[9],如图2所示.2.1 有机硝酸酯(RONO2)及有机亚硝酸酯类RONO2是醇的硝酸酯,是最早的、目前最常用的NO供体.它们可以通过相应醇的酯化反应或烷基卤化物与AgNO3的反应来合成,如图3所示[10].这类供体的优点是给药途径比较广,但容易产生耐药性.硝酸甘油(GTN)和单硝酸异山梨酯(ISMN)是临床研究中使用最广泛的NO供体类药物.它们有几个既定的临床应用:GTN是一种廉价又有效的、能快速逆转与急性心绞痛有关疼痛的药物;ISMN是RONO2中释放NO较慢的一种,已被用于治疗慢性心绞痛[11].与RONO2类似,有机亚硝酸酯是醇类和亚硝酸酯化形成的酯.它们主要通过醇与亚硝酰氯(NOCl)反应或醇与NO和氧气(O2)经过酯化反应来合成,如图3所示[12].有机亚硝酸酯的主要作用是舒张静脉和降低血压,例如,亚硝酸丁酯(BN)、亚硝酸异丁酯(ISBN)和亚硝酸叔丁酯(TBN)已在临床上用作血管扩张剂[13].与GTN等RONO2相比,它们对酶的依赖性更低、作用效力更高,且不易引起耐药性.但是,它们缺乏选择性和生物利用度,以及细胞毒性和致癌性较高,因此不如RONO2常用[14].2.2 RSNO类RSNO是贮存、运输和释放NO的重要载体,在生物体内具有重要的生理作用.RSNO普遍存在于生物体的血液和组织中,只需要一个电子就能引发NO的释放,因此,可通过光、热、碱性pH值、过渡金属离子、抗坏血酸和酶等促使RSNO自发均裂反应产生NO[15].人工合成的RSNO是新型的NO供体类药物,通过静脉等途径进入体内后,可以参与呼吸、心血管、消化等多个系统疾病的诊断和治疗[16].2.3 金属-一氧化氮配合物NO是金属配合物中的强配体,它的结合常数比一氧化碳(CO)和O2高得多,具有多种氧化态,氧化价态的高低决定了配合物中NO的反应性.NO调节信号通路的主要机制是与金属中心原子(如铁(Fe)、钌(Ru)等)结合,如图4所示[12],如血红素基团或蛋白质的铁硫簇.硝普钠(SNP)已经广泛应用于急性降压药物和动静脉血管扩张剂,其血管舒张作用是由NO的产生而造成的[17].SNP晶体在避光且干燥的条件下可以长时间保存,光和O2会促使其水溶液分解,并释放出NO和氰化物,从而导致“氰化物毒性”,对机体造成伤害[18].除了Fe之外,Ru对NO也有很高的亲和力,且Ru对NO的亲和力可以随着其他配体的改变而变化,以便调节NO的释放.光活性Ru配合物热稳定性好,且能在紫外光照射下释放NO.然而,NO的有效释放需要使用对组织有害的高功率紫外线,这一缺陷阻碍了该类NO供体的临床应用[19].2.4 其他供体1956年MAGEE等[20]发现了二甲基亚硝胺和亚硝胺二甲胺均可致大鼠肝癌.其致癌作用是由于N-亚硝基化合物会导致蛋白质和核酸的烷基化.但是,N-亚硝胺却是一种能舒张血管的NO供体.链脲霉素(STZ)含有N-亚硝胺基团,具有抗肿瘤、致糖尿病和致癌作用[21].胰腺β细胞具有低水平的活性氧(ROS)清除酶,对NO和ROS比较敏感,STZ能在胰岛β细胞中释放NO,使细胞的DNA受到损害[22].因此,可将此类NO供体作为抗癌药物进行研究.偶氮二醇烯鎓盐(NONOates)释放NO的机制遵循动力学且不受细胞代谢产物或酶的催化.它们以固体形态稳定存在,但在生理条件下会自发分解生成NO,分解速率会因结构、温度和pH值而改变[23].因此,可以通过它们在体外的分解速率直接预测药物的持续作用时间.研究证明:NONOates能够降低多种肿瘤细胞的增长速率,抑制肿瘤细胞的生长[24].此外,还可以通过硝酸盐→亚硝酸盐→NO途径合成NO.体内的硝酸盐主要来自膳食和自身合成.在生物体内,循环的硝酸盐被唾液腺主动摄取,并被口腔中的细菌还原为亚硝酸盐,在血液和组织中进一步代谢为NO和其他生物活性氮氧化物[5].亚硝酸盐是氮氧化物的氧化还原过程中的中间产物,在血液和组织中比较稳定,且可被多种物质还原成NO,包括肌红蛋白、血红蛋白、抗坏血酸、黄嘌呤氧化还原酶、质子和多酚[5].这些途径产生的NO会因缺氧和酸中毒条件而增加,因而可以保证NO的产量.1.2 NO的生物学作用NO可以自由地通过生物膜并参与一系列生理和病理过程,如神经信号传递、血管扩张、血小板黏附和聚集等,在生物体内发挥着至关重要的作用.NO的生物学作用是通过直接或间接的化学反应产生的.例如,NO直接与不同蛋白质的金属配合物结合形成金属亚硝酰基配合物来调节靶蛋白的生物学活性.NO还可以与多种内源性自由基反应,产生活性氮氧化合物,这些强毒性的活性氮氧化合物將导致线粒体损伤,进而诱导细胞凋亡.NO在生物体内像一把双刃剑,因为它既具有杀死肿瘤细胞的作用,又具有促进肿瘤细胞生长的作用.在低生理水平下,NO可作为抗氧化剂,减少芬顿反应,终止自由基链式反应,并抑制过氧化物酶和氧化酶的活性.较高浓度的NO能够舒张血管,改善组织缺氧状态,有利于化疗药物的渗透,对肿瘤细胞具有杀伤作用[6].但是,持续过量的NO将产生神经毒性,影响体内平衡和改变蛋白质功能,从而导致基因突变,最终使正常黏膜癌变[7].不同组织中的生理过程对NO的需求量各不相同,浓度过高或者过低都会对组织造成一定的损伤,引起疾病的发生[8].只要将适当浓度的NO递送至肿瘤部位,NO的靶向释放也可能增强化学疗法和放射疗法的疗效.因此,如何将适当浓度的NO靶向释放至肿瘤,已成为近年来生物医学领域的研究重点.2 NO供体直接使用外源性或内源性NO的缺点是其半衰期极短,且易受各种谷胱甘肽(GSH)、超氧化物和血红蛋白等物质的影响.因此,将NO供体载入纳米平台中,直接和精确地控制NO的靶向释放,有很好的应用前景.NO供体是指在体内经酶促反应或非酶促反应释放NO的一类化合物,如有机硝酸盐、有机亚硝酸盐、S‒亚硝基硫醇(RSNO)、金属配合物等多种化学物质已被用作NO供体,用于各种生物或医学领域[9],如图2所示.2.1 有机硝酸酯(RONO2)及有机亚硝酸酯类RONO2是醇的硝酸酯,是最早的、目前最常用的NO供体.它们可以通过相应醇的酯化反应或烷基卤化物与AgNO3的反应来合成,如图3所示[10].这类供体的优点是给药途径比较广,但容易产生耐药性.硝酸甘油(GTN)和单硝酸异山梨酯(ISMN)是临床研究中使用最广泛的NO供体类药物.它们有几个既定的临床应用:GTN是一种廉价又有效的、能快速逆转与急性心绞痛有关疼痛的药物;ISMN是RONO2中释放NO较慢的一种,已被用于治疗慢性心绞痛[11].与RONO2类似,有机亚硝酸酯是醇类和亚硝酸酯化形成的酯.它们主要通过醇与亚硝酰氯(NOCl)反应或醇与NO和氧气(O2)经过酯化反应来合成,如图3所示[12].有机亚硝酸酯的主要作用是舒张静脉和降低血压,例如,亚硝酸丁酯(BN)、亚硝酸异丁酯(ISBN)和亚硝酸叔丁酯(TBN)已在临床上用作血管扩张剂[13].与GTN等RONO2相比,它们对酶的依赖性更低、作用效力更高,且不易引起耐药性.但是,它们缺乏选择性和生物利用度,以及细胞毒性和致癌性较高,因此不如RONO2常用[14].2.2 RSNO类RSNO是贮存、运输和释放NO的重要载体,在生物体内具有重要的生理作用.RSNO普遍存在于生物体的血液和组织中,只需要一个电子就能引发NO的释放,因此,可通过光、热、碱性pH值、过渡金属离子、抗坏血酸和酶等促使RSNO自发均裂反应产生NO[15].人工合成的RSNO是新型的NO供体类药物,通过静脉等途径进入体内后,可以参与呼吸、心血管、消化等多个系统疾病的诊断和治疗[16].2.3 金属-一氧化氮配合物NO是金属配合物中的强配体,它的结合常数比一氧化碳(CO)和O2高得多,具有多种氧化态,氧化价态的高低决定了配合物中NO的反应性.NO调节信号通路的主要机制是与金属中心原子(如铁(Fe)、钌(Ru)等)结合,如图4所示[12],如血红素基团或蛋白质的铁硫簇.硝普钠(SNP)已经广泛应用于急性降压药物和动静脉血管扩张剂,其血管舒张作用是由NO的产生而造成的[17].SNP晶体在避光且干燥的条件下可以长时间保存,光和O2会促使其水溶液分解,并释放出NO和氰化物,从而导致“氰化物毒性”,对机体造成伤害[18].除了Fe之外,Ru对NO也有很高的亲和力,且Ru对NO的亲和力可以随着其他配体的改变而变化,以便调节NO的释放.光活性Ru配合物热稳定性好,且能在紫外光照射下释放NO.然而,NO的有效释放需要使用对组织有害的高功率紫外线,这一缺陷阻碍了该类NO供体的临床应用[19].2.4 其他供体1956年MAGEE等[20]发现了二甲基亚硝胺和亚硝胺二甲胺均可致大鼠肝癌.其致癌作用是由于N-亚硝基化合物会导致蛋白质和核酸的烷基化.但是,N-亚硝胺却是一种能舒张血管的NO供体.链脲霉素(STZ)含有N-亚硝胺基团,具有抗肿瘤、致糖尿病和致癌作用[21].胰腺β细胞具有低水平的活性氧(ROS)清除酶,对NO和ROS比较敏感,STZ能在胰岛β细胞中释放NO,使细胞的DNA受到损害[22].因此,可将此类NO供体作为抗癌药物进行研究.偶氮二醇烯鎓盐(NONOates)释放NO的机制遵循动力学且不受细胞代谢产物或酶的催化.它们以固体形态稳定存在,但在生理条件下会自发分解生成NO,分解速率会因结构、温度和pH值而改变[23].因此,可以通过它们在体外的分解速率直接预测药物的持续作用时间.研究证明:NONOates能够降低多种肿瘤细胞的增长速率,抑制肿瘤细胞的生长[24].此外,还可以通过硝酸盐→亚硝酸盐→NO途径合成NO.体内的硝酸盐主要来自膳食和自身合成.在生物体内,循环的硝酸盐被唾液腺主动摄取,并被口腔中的细菌还原为亚硝酸盐,在血液和组织中进一步代谢为NO和其他生物活性氮氧化物[5].亚硝酸盐是氮氧化物的氧化还原过程中的中间产物,在血液和组织中比较稳定,且可被多种物质还原成NO,包括肌红蛋白、血红蛋白、抗坏血酸、黄嘌呤氧化还原酶、质子和多酚[5].这些途径产生的NO会因缺氧和酸中毒条件而增加,因而可以保证NO的产量.1.2 NO的生物学作用。
不可切除胰腺癌的分子靶向药物治疗进展

不可切除胰腺癌的分子靶向药物治疗进展胡润,李俊蒽,姚沛,桂仁捷,段华新湖南师范大学附属第一医院,湖南省人民医院肿瘤科,长沙 410005通信作者:段华新,****************(ORCID: 0000-0001-9596-5013)摘要:胰腺癌作为消化系统最常见的恶性肿瘤之一,其发病率及死亡率正逐年上升,大多数胰腺癌患者因分期较晚而失去了手术机会。
尽管以吉西他滨、氟尿嘧啶为主的化疗方案在一定程度上延长了患者的生存期,但仍有部分患者因无法耐受化疗而失去治疗机会。
随着精准医疗时代的来临,分子靶向药物治疗展现出的优异疗效使其成为对抗肿瘤的重要治疗手段之一,但由于胰腺癌高度的异质性及复杂的免疫微环境,针对胰腺癌的分子靶向治疗并未取得显著效果,因此亟需探寻新的治疗靶点及药物攻克这一难题。
本综述基于胰腺癌常见分子靶点及肿瘤免疫相关靶点探究在不可切除胰腺癌中分子靶向药物治疗研究的最新进展,为胰腺癌患者提供新的治疗策略。
关键词:胰腺肿瘤;分子靶向治疗;免疫疗法基金项目:湖南省自然科学基金(2020JJ8084)Advances in molecular-targeted therapy for unresectable pancreatic cancerHU Run,LI Junen,YAO Pei,GUI Renjie,DUAN Huaxin.(Department of Oncology,The First Affiliated Hospital of Hunan Normal University, Hunan Provincial People’s Hospital, Changsha 410005, China)Corresponding author: DUAN Huaxin,****************(ORCID: 0000-0001-9596-5013)Abstract:Pancreatic cancer is one of the most prevalent malignant tumors of the digestive system, and its incidence and mortality rates are increasing year by year. Most patients with pancreatic cancer are unable to receive surgery due to the advanced stage. Although chemotherapy regimens based on gemcitabine and fluorouracil have prolonged the survival time of patients to some extent,some patients cannot tolerate chemotherapy and hence lose the opportunity for treatment. With the advent of the era of precision medicine, molecular-targeted therapy has exhibited an excellent therapeutic efficacy and has thus become one of the most important treatment techniques for tumors; however, due to the high heterogeneity of pancreatic cancer and its complicated tumor microenvironment, molecular-targeted therapy for pancreatic cancer has not achieved notable results. Therefore, it is imperative to seek new therapeutic targets and medications to overcome this issue. This article reviews the latest advances in the research on molecular-targeted therapy for unresectable pancreatic cancer based on common molecular targets and tumor immunity-related therapeutic targets, in order to provide new treatment strategies for patients with pancreatic cancer.Key words:Pancreatic Neoplasms; Molecular Targeted Therapy; ImmunotherapyResearch funding:Natural Science Foundation of Hunan Province of China (2020JJ8084)胰腺癌是一种起病隐匿、进展迅速、疗效及预后极差的恶性肿瘤,大多数患者确诊时已经属于晚期。
生物医学英文文献导读

生物医学英文文献导读Title: Biomedical Applications of Nanoparticles: An OverviewAuthors: John Smith, Emily Johnson, David Wilson Journal: Journal of Biomedical Materials Research Part AYear: 2018Summary:This review article provides an overview of the biomedical applications of nanoparticles. Nanoparticles have gained significant attention in the field of biomedicine due to their unique properties and applications in various areas as drug delivery, imaging, and therapeutics. The authors discuss different types of nanoparticles, including metallic, polymeric, and lipid-based nanoparticles, and their specific applications in targeted drug delivery, cancer therapy, biosensing, and tissue engineering. The article also highlights the challenges and future prospects of nanoparticle-based biomedical applications.Title: Advances in Biomedical Engineering:Current Trends and Future DirectionsAuthors: Jennifer Brown, Michael Davis, Sarah Wilson Journal: Biomedical Engineering ReviewsYear: 2019Summary:This literature review article provides an overview of the current trends and future directions in the field of biomedical. The authors discuss recent advancements in various areas of biomedical engineering, such as biomaterials, medical imaging, tissue engineering, regenerative medicine, and artificial intelligence in healthcare. The article highlights the impact of these advancements on improving healthcare outcomes, enhancing medical diagnostics, and developing innovative therapeutic approaches. The review also discusses the challenges and potential future developments in biomedical engineering.Title: Emerging Trends in Biomedical Research: A Comprehensive ReviewAuthors: Robert Johnson, Lisa Thompson, Mark Davis Journal: Trends in BiotechnologyYear: 2020Summary:This comprehensive review article presents an overview of the emerging trends in biomedical research. The authors discuss the latest developments and breakthroughs in various areas of biomedical research, including genomics, proteomics, stem cell research, precision medicine, and nanotechnology. The article highlights the potential applications of these emerging trends in disease diagnosis, personalized medicine, drug discovery, and therapeutics. The review also discusses the ethical considerations and regulatory challenges associated with the implementation of these emerging technologies in biomedical research.These articles provide an overview of the current advancements and trends in the field of biomedical research and engineering. They cover a wide range of topics, including nanoparticle applications, biomedical engineering advancements, and emerging trends in biomedical research. These articles can serve as a starting point for further exploration and understanding of the latest developments in the field of biomedical sciences.。
聚苯胺纳米复合材料用于癌症诊疗的研究进展
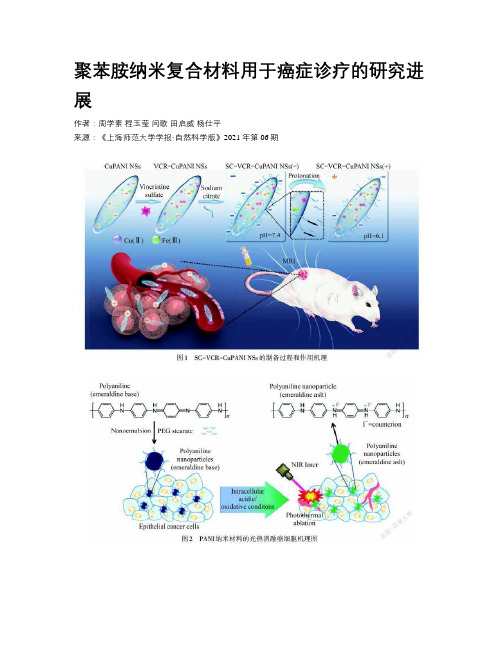
聚苯胺纳米复合材料用于癌症诊疗的研究进展作者:周学素程玉莹闫歌田启威杨仕平来源:《上海师范大学学报·自然科学版》2021年第06期摘要:共轭聚合物以其独特的结构和性能得到了广泛的关注.聚苯胺(PANI)纳米复合材料制备工艺简单、成本低廉、毒性低、易于功能化,从而在癌症治疗方面取得了巨大的进展.通过不同功能的化合物修饰制备的PANI纳米复合材料极大地拓宽了癌症治疗领域.基于PANI 纳米复合材料,文章总结了其在癌症诊疗领域的光热治疗、协同治疗、多模态成像引导治疗和智能响应治疗的研究进展,并分析了其发展趋势.关键词:聚苯胺(PANI)纳米复合材料; 光热治疗; 协同治疗; 多模态成像; 智能响应中图分类号: O 611.3 文献标志码: A 文章编号: 1000-5137(2021)06-0721-07Abstract: Conjugated polymers have attracted much attention due to their unique structure and properties. Polyaniline(PANI) nanocomposites have the advantages of simple preparation process, low cost, low toxicity, easy functionalization, etc., and have made great progress in tumor treatment. PANI nanocomposites prepared by modifying different functional compounds have greatly broadened the field of tumor treatment. Based on PANI nanocomposites, this article reviews their research progress in photothermal therapy, collaborative therapy, multimodal imaging guided therapy, and intelligent response therapy in the field of cancer diagnosis and treatment, and analyzes its development trends.Key words: polyaniline(PANI) nanocomposite; photothermal therapy; synergy therapy; multimodal imaging; intelligent response0 引言癌症作为世界上高死亡率的疾病之一,一直以来都是人类面临的最严重的健康问题之一.在2018年,全世界因癌症死亡的人数就达到960万.目前在癌症临床治疗中应用比较多的是传统方法,包括手术、化学疗法和放射疗法.虽在杀死癌细胞方面具有明显效果,但传统疗法也会杀死正常细胞及组织,给病人带来副作用大、缺乏特异性等问题.光热疗法是一种通过外部刺激从而杀死癌细胞,侵入性小的微创疗法[1].由于近红外光具有很好的组织穿透能力,光能量可以充分传递到作用部位,光热疗法采用能够吸收近红外光的纳米材料,将光能转化为热能,从而在体内实现局部高温,杀死癌细胞,并且不损害其他正常组织[2].共轭聚合物是一类特殊的聚合物,存在π电子共轭主链和高度离域化的结构,具有很高的光热转换效率,可以作为近红外光诱导的光热转换纳米材料.目前,共轭聚合物已广泛用于癌症治疗的研究领域[3],主要分为两大类:一类是有机共轭聚合物纳米粒子,例如聚吡咯(Pyy)、聚苯胺(PANI)、聚多巴胺[4]等;第二类是基于π共轭和离域电子的供体-受体(DA)体系设计的供体-受体共轭聚合物纳米粒子.共轭聚合物纳米粒子具有很高的光热转换效率,远远超过其他的纳米粒子,且具有优异的光稳定性和良好的生物降解性.聚苯胺掺杂后其离域轨道电子易发生迁移,导带与价带之间的能隙减小,导致紫外可见吸收峰发生红移.当受到近红外光(NIR)照射时,PANI价带中的电子将受激发,跃迁至导带,具有显著的光电转换效应,实现光热转换,使得PANI可作为诊疗一体化试剂进行癌症治疗[5].PANI具有制备工艺简单、成本低廉、毒性低,以及可调节的结构和表面形态,有增强肿瘤治疗的效果.本文主要概述了PANI纳米复合材料的制备和改性,以及其在肿瘤诊断领域的研究进展.1 PANI纳米复合材料的制备及改性1.1 制备PANI纳米粒子合成的常用方法有化学氧化聚合法[6-7].化学氧化法是在酸性条件下使用氧化剂将苯胺单体氧化聚合成PANI,广泛应用的氧化剂有过硫酸铵、过氧化氢、氯化铁、高锰酸钾[8]等.通过改变质子酸的种类、氧化剂、原料浓度、反应时间、反应环境等因素,获得不同结构和形貌的PANI纳米粒子,从而实现不同的功能.例如,LIU等[7]采用氧化铁作为氧化剂,并改变氧化剂与苯胺之间的浓度比,制备得到纳米梭状的PANI. MONDAL等[9]将掺杂剂改为芳族羧酸,并改变有机酸中的-COOH基团数目,获得不同长径比的管状PANI纤维,其吸附效果良好,可用于油水分离.1.2 表面改性对纳米粒子表面改性,可以减少材料在体内的聚集,降低细胞毒性,阻止免疫系统对材料的清除,延長材料在血液的循环周期等,从而实现功能化治疗癌症.由于纳米材料进入体内后,在其表面会形成蛋白冠,粒子破坏蛋白冠后被体内的免疫系统识别,并当作有害物质从体内清除,降低了治疗效果[10].表面改性可以掩盖纳米粒子或阻止蛋白冠的形成,延长PANI纳米粒子在血液中的循环时间,并到达作用部位,提高治疗效果[11].目前对PANI纳米材料的表面改性,主要通过物理吸附或采用共价键将配体结合在纳米颗粒表面等方式以实现其功能化.较常用于纳米颗粒改性的化合物是聚乙二醇.通过聚乙二醇改性可改善材料的亲水性,改变材料药代动力学效果,同时具有实现其他功能的优势[12].为了改善PANI在水中的分散性,WANG等[13]设计了一种核-壳结构的复合材料聚苯胺-聚吡咯烷酮(PANi@PVP),PVP作为空间稳定剂对PANI进行改性,在水中有优异的溶解性和良好的细胞相容性.LIU等[7]提出了一种分级靶向策略,设计了pH敏感的柠檬酸钠修饰的铜掺杂PANI 纳米梭(SC-VCR-CuPANI NSs),如图1所示,该材料在血液中呈负电,显示出增强的隐身效果,血液循环周期延长,且在肿瘤微酸环境中实现质子化,提高细胞内在化效果.通过这种分级靶向的策略,材料在血液中的循环半衰期从4.35 h增加到7.33 h,磁共振成像分辨率和信号强度得到显著提高.为了提高肿瘤的治疗效果,基于透明质酸(HA)可以特异性结合受体(CD44)靶向肿瘤细胞[14], JIANG等[15]设计了一种水溶性透明质酸-杂交PANI纳米材料,靶向光热治疗肿瘤.通过细胞实验,材料可以针对性地杀死癌细胞HeLa和HCT-116,而对正常细胞HFF没有影响.将HA修饰的二氧化硅荧光纳米颗粒连接到PANI,在靶向肿瘤的同时,二氧化硅提供的荧光成像可以引导光热治疗.2 PANI纳米复合材料在肿瘤诊疗上的应用2.1 光热治疗PANI经掺杂后在近红外区(700~900 nm)有很强的吸收,PANI具有成为光热剂的可能[16].如图2所示,YANG等[5]首次报道了PANI在酸性条件下进行掺杂,具有有机光热剂的潜力.用808 nm,2.45 W·cm-2的NIR激光照射5 min,掺杂态PANI温度升高了54.8 ℃,采用相同条件,本征态PANI温度升高了15 ℃左右,表明了掺杂后的PANI光热效果明显.为评估PANI对癌细胞在体外和体内的光热消融能力,将本征态的PANI用上皮癌细胞A431处理后发现,PANI颜色从紫色变成绿色,并在808 nm NIR激光下照射,台盼蓝染色实验发现大量细胞被破坏.同样在体内实验中,经瘤内注射材料,用激光照射治疗后,肿瘤组织切片显示有严重的细胞损伤.这说明PANI可以在癌细胞中掺杂,并诱导产生光热效应,从而杀死癌细胞.2.2 协同治疗光热疗法是微创治疗技术,通过外部激光刺激富集到肿瘤部位的材料导致局部升温,致使细胞结构发生破坏,但研究表明:受制于纳米材料在肿瘤部位的富集程度及机体代谢的影响,光热治疗的效果并不佳,同时材料也会给机体带来一定的毒性.对于深部肿瘤,激光强度会由于深度而依赖性改变,这些因素迫使光热疗法同其他的疗法相结合,从而增强肿瘤治疗的效果,降低治疗对机体正常组织的损伤[17-18].将光热疗法和光动力疗法相结合,两种疗法均采用外部激光照射激发.光动力疗法是利用光敏剂和组织细胞中的氧气(O2)的共同存在,经激光照射产生活性氧(ROS),ROS会氧化生物大分子,从而破坏细胞结构,促使细胞死亡[19-20].由于肿瘤中低氧的限制,会降低光动力疗法的治疗效果,将光动力疗法和光热疗法相结合,可以提高整体治疗效果,同时光热疗法会促进血液的流动,提高肿瘤中的氧气浓度,得到1+1>2的效果.TAN等[21]合成了一种PANI包覆吲哚菁绿载银纳米复合材料(ICG-Ag@PANI),银/聚苯胺核壳纳米粒子(Ag@PANI)通过π-π堆积和疏水相互作用的方式负载光敏剂吲哚菁绿(ICG),实现单光触发的光热疗法和光动力疗法协同治疗.将光热剂与化疗药物结合,可控制药物在肿瘤部位的释放[22],降低药物对正常细胞的损伤,协同化疗和光热疗法能很好地提高癌症治疗能力.结合药物的方法有两种:一是将光热剂同可载药的载体结合;二是将光热剂自身设计为药物载体.SILVA等[23]设计了装载5-FU的二甲基咪唑结合PANI纳米粒子(PANI@ZIF-8),在NIR和pH=5.2的缓冲溶液中,5-FU的累计释放量达到80%,PANI吸收NIR会促使温度的升高,增强了5-FU的释放,具有很好的化学光热效应.如图3所示,XIA等[24]采用多孔硅包覆阿霉素(DOX),并将PANI共价接枝到其表面,通过pH和NIR响应控制药物释放,多孔硅可降解为無毒氢氧化硅(Si(OH)4)排出体外,解决了光热剂不可生物降解所带来的长期毒性问题,化学与光热结合疗法展现了巨大的潜力.2.3 多模式成像引导治疗通过成像的方式来引导治疗可以有效地提高肿瘤治疗效率.掺杂态PANI在近红外区具有独特的光吸收特性,是一种优异的光声成像剂[25].光声成像是探测激光照射产生的光声信号后,产生组织分布图像的成像方式,具有高信噪比和高分辨率的优点[26].但是单一的成像依旧存在缺陷,尤其对深部肿瘤,光声成像对激光的强度存在依赖,激光过强会损伤正常的组织细胞.多模式成像方式引导治疗来提高治疗效果是目前研究的趋势.如图4所示,WANG等[27]制备一种PANI包覆二硫化钼(MoS2@PANI-PEG)量子点复合材料,MoS2量子点可产生强荧光,可用于体内成像的探针,同时MoS2是放射增敏剂,结合PANI的光热疗法和光声成像能力,协同增强癌症的治疗效果.2.4 智能响应治疗基于PANI易被质子酸掺杂和肿瘤微环境具有微酸性的特点,设计智能响应探针用于癌症治疗.由于肿瘤微酸性环境(pH为5.0~7.4)低于PANI掺杂要求的pH值(pH<3.0),限制了PANI在癌症治疗中光热治疗和光声成像的应用.JU等[28]合成了金/聚苯胺核壳纳米材料(Au@PANI),基于Au到PANI的电荷转移以及PANI掺杂过程诱导的电子传递效率的提高,Au@PANI在pH=6.5时即可实现掺杂,显示出优异的光热效应.但金离子在体内具有长期毒性,对人体会造成很大的危害.为提高智能治疗剂的治疗性能,如图5所示,LI等[29]采用牛血清白蛋白(BSA)包覆PANI,设计了BSA-PANI纳米粒子,可以实现肿瘤内源性触发的诊断和治疗,光热转换效率达到37%,且纳米粒子具有很好的生物相容性,降低体内毒性.目前对PANI纳米材料的表面改性,主要通过物理吸附或采用共价键将配体结合在纳米颗粒表面等方式以实现其功能化.较常用于纳米颗粒改性的化合物是聚乙二醇.通过聚乙二醇改性可改善材料的亲水性,改变材料药代动力学效果,同时具有实现其他功能的优势[12].为了改善PANI在水中的分散性,WANG等[13]设计了一种核-壳结构的复合材料聚苯胺-聚吡咯烷酮(PANi@PVP),PVP作为空间稳定剂对PANI进行改性,在水中有优异的溶解性和良好的细胞相容性.LIU等[7]提出了一种分级靶向策略,设计了pH敏感的檸檬酸钠修饰的铜掺杂PANI 纳米梭(SC-VCR-CuPANI NSs),如图1所示,该材料在血液中呈负电,显示出增强的隐身效果,血液循环周期延长,且在肿瘤微酸环境中实现质子化,提高细胞内在化效果.通过这种分级靶向的策略,材料在血液中的循环半衰期从4.35 h增加到7.33 h,磁共振成像分辨率和信号强度得到显著提高.为了提高肿瘤的治疗效果,基于透明质酸(HA)可以特异性结合受体(CD44)靶向肿瘤细胞[14], JIANG等[15]设计了一种水溶性透明质酸-杂交PANI纳米材料,靶向光热治疗肿瘤.通过细胞实验,材料可以针对性地杀死癌细胞HeLa和HCT-116,而对正常细胞HFF没有影响.将HA修饰的二氧化硅荧光纳米颗粒连接到PANI,在靶向肿瘤的同时,二氧化硅提供的荧光成像可以引导光热治疗.2 PANI纳米复合材料在肿瘤诊疗上的应用2.1 光热治疗PANI经掺杂后在近红外区(700~900 nm)有很强的吸收,PANI具有成为光热剂的可能[16].如图2所示,YANG等[5]首次报道了PANI在酸性条件下进行掺杂,具有有机光热剂的潜力.用808 nm,2.45 W·cm-2的NIR激光照射5 min,掺杂态PANI温度升高了54.8 ℃,采用相同条件,本征态PANI温度升高了15 ℃左右,表明了掺杂后的PANI光热效果明显.为评估PANI对癌细胞在体外和体内的光热消融能力,将本征态的PANI用上皮癌细胞A431处理后发现,PANI颜色从紫色变成绿色,并在808 nm NIR激光下照射,台盼蓝染色实验发现大量细胞被破坏.同样在体内实验中,经瘤内注射材料,用激光照射治疗后,肿瘤组织切片显示有严重的细胞损伤.这说明PANI可以在癌细胞中掺杂,并诱导产生光热效应,从而杀死癌细胞.2.2 协同治疗光热疗法是微创治疗技术,通过外部激光刺激富集到肿瘤部位的材料导致局部升温,致使细胞结构发生破坏,但研究表明:受制于纳米材料在肿瘤部位的富集程度及机体代谢的影响,光热治疗的效果并不佳,同时材料也会给机体带来一定的毒性.对于深部肿瘤,激光强度会由于深度而依赖性改变,这些因素迫使光热疗法同其他的疗法相结合,从而增强肿瘤治疗的效果,降低治疗对机体正常组织的损伤[17-18].将光热疗法和光动力疗法相结合,两种疗法均采用外部激光照射激发.光动力疗法是利用光敏剂和组织细胞中的氧气(O2)的共同存在,经激光照射产生活性氧(ROS),ROS会氧化生物大分子,从而破坏细胞结构,促使细胞死亡[19-20].由于肿瘤中低氧的限制,会降低光动力疗法的治疗效果,将光动力疗法和光热疗法相结合,可以提高整体治疗效果,同时光热疗法会促进血液的流动,提高肿瘤中的氧气浓度,得到1+1>2的效果.TAN等[21]合成了一种PANI包覆吲哚菁绿载银纳米复合材料(ICG-Ag@PANI),银/聚苯胺核壳纳米粒子(Ag@PANI)通过π-π堆积和疏水相互作用的方式负载光敏剂吲哚菁绿(ICG),实现单光触发的光热疗法和光动力疗法协同治疗.将光热剂与化疗药物结合,可控制药物在肿瘤部位的释放[22],降低药物对正常细胞的损伤,协同化疗和光热疗法能很好地提高癌症治疗能力.结合药物的方法有两种:一是将光热剂同可载药的载体结合;二是将光热剂自身设计为药物载体.SILVA等[23]设计了装载5-FU的二甲基咪唑结合PANI纳米粒子(PANI@ZIF-8),在NIR和pH=5.2的缓冲溶液中,5-FU的累计释放量达到80%,PANI吸收NIR会促使温度的升高,增强了5-FU的释放,具有很好的化学光热效应.如图3所示,XIA等[24]采用多孔硅包覆阿霉素(DOX),并将PANI共价接枝到其表面,通过pH和NIR响应控制药物释放,多孔硅可降解为无毒氢氧化硅(Si(OH)4)排出体外,解决了光热剂不可生物降解所带来的长期毒性问题,化学与光热结合疗法展现了巨大的潜力.2.3 多模式成像引导治疗通过成像的方式来引导治疗可以有效地提高肿瘤治疗效率.掺杂态PANI在近红外区具有独特的光吸收特性,是一种优异的光声成像剂[25].光声成像是探测激光照射产生的光声信号后,产生组织分布图像的成像方式,具有高信噪比和高分辨率的优点[26].但是单一的成像依旧存在缺陷,尤其对深部肿瘤,光声成像对激光的强度存在依赖,激光过强会损伤正常的组织细胞.多模式成像方式引导治疗来提高治疗效果是目前研究的趋势.如图4所示,WANG等[27]制备一种PANI包覆二硫化钼(MoS2@PANI-PEG)量子点复合材料,MoS2量子点可产生强荧光,可用于体内成像的探针,同时MoS2是放射增敏剂,结合PANI的光热疗法和光声成像能力,协同增强癌症的治疗效果.2.4 智能响应治疗基于PANI易被质子酸掺杂和肿瘤微环境具有微酸性的特点,设计智能响应探针用于癌症治疗.由于肿瘤微酸性环境(pH为5.0~7.4)低于PANI掺杂要求的pH值(pH<3.0),限制了PANI在癌症治疗中光热治疗和光声成像的应用.JU等[28]合成了金/聚苯胺核壳纳米材料(Au@PANI),基于Au到PANI的电荷转移以及PANI掺杂过程诱导的电子传递效率的提高,Au@PANI在pH=6.5时即可实现掺杂,显示出优异的光热效应.但金离子在体内具有长期毒性,对人体会造成很大的危害.为提高智能治疗剂的治疗性能,如图5所示,LI等[29]采用牛血清白蛋白(BSA)包覆PANI,设计了BSA-PANI纳米粒子,可以实现肿瘤内源性触发的诊断和治疗,光热转换效率达到37%,且纳米粒子具有很好的生物相容性,降低体内毒性.目前对PANI纳米材料的表面改性,主要通过物理吸附或采用共价键将配体结合在纳米颗粒表面等方式以实现其功能化.较常用于纳米颗粒改性的化合物是聚乙二醇.通过聚乙二醇改性可改善材料的亲水性,改变材料药代动力学效果,同时具有实现其他功能的优势[12].为了改善PANI在水中的分散性,WANG等[13]设计了一种核-壳结构的复合材料聚苯胺-聚吡咯烷酮(PANi@PVP),PVP作为空间稳定剂对PANI进行改性,在水中有优异的溶解性和良好的细胞相容性.LIU等[7]提出了一种分级靶向策略,设计了pH敏感的柠檬酸钠修饰的铜掺杂PANI 纳米梭(SC-VCR-CuPANI NSs),如图1所示,该材料在血液中呈负电,显示出增强的隐身效果,血液循环周期延长,且在肿瘤微酸环境中实现质子化,提高细胞内在化效果.通过这种分级靶向的策略,材料在血液中的循环半衰期从4.35 h增加到7.33 h,磁共振成像分辨率和信号强度得到显著提高.为了提高肿瘤的治疗效果,基于透明质酸(HA)可以特异性结合受体(CD44)靶向肿瘤细胞[14], JIANG等[15]设计了一种水溶性透明质酸-杂交PANI纳米材料,靶向光热治疗肿瘤.通过细胞实验,材料可以针对性地杀死癌细胞HeLa和HCT-116,而对正常细胞HFF没有影响.将HA修饰的二氧化硅荧光纳米颗粒连接到PANI,在靶向肿瘤的同时,二氧化硅提供的荧光成像可以引导光热治疗.2 PANI纳米复合材料在肿瘤诊疗上的应用2.1 光热治疗PANI经掺杂后在近红外区(700~900 nm)有很强的吸收,PANI具有成为光热剂的可能[16].如图2所示,YANG等[5]首次报道了PANI在酸性条件下进行掺杂,具有有机光热剂的潜力.用808 nm,2.45 W·cm-2的NIR激光照射5 min,掺杂态PANI温度升高了54.8 ℃,采用相同条件,本征态PANI温度升高了15 ℃左右,表明了掺杂后的PANI光热效果明显.为评估PANI对癌细胞在体外和体内的光热消融能力,将本征态的PANI用上皮癌细胞A431处理后发现,PANI颜色从紫色变成绿色,并在808 nm NIR激光下照射,台盼蓝染色实验发现大量细胞被破坏.同样在体内实验中,经瘤内注射材料,用激光照射治疗后,肿瘤组织切片显示有严重的细胞损伤.这说明PANI可以在癌细胞中掺杂,并诱导产生光热效应,从而杀死癌细胞.2.2 协同治疗光热疗法是微创治疗技术,通过外部激光刺激富集到肿瘤部位的材料导致局部升温,致使细胞结构发生破坏,但研究表明:受制于纳米材料在肿瘤部位的富集程度及机体代谢的影响,光热治疗的效果并不佳,同时材料也会给机体带来一定的毒性.对于深部肿瘤,激光强度会由于深度而依赖性改变,这些因素迫使光热疗法同其他的疗法相结合,从而增强肿瘤治疗的效果,降低治疗对机体正常组织的损伤[17-18].将光热疗法和光动力疗法相结合,两种疗法均采用外部激光照射激发.光动力疗法是利用光敏剂和组织细胞中的氧气(O2)的共同存在,经激光照射产生活性氧(ROS),ROS会氧化生物大分子,从而破坏细胞结构,促使细胞死亡[19-20].由于肿瘤中低氧的限制,会降低光动力疗法的治疗效果,将光动力疗法和光热疗法相结合,可以提高整体治疗效果,同时光热疗法会促进血液的流动,提高肿瘤中的氧气浓度,得到1+1>2的效果.TAN等[21]合成了一种PANI包覆吲哚菁绿载银纳米复合材料(ICG-Ag@PANI),银/聚苯胺核壳纳米粒子(Ag@PANI)通过π-π堆积和疏水相互作用的方式负载光敏剂吲哚菁绿(ICG),实现单光触发的光热疗法和光动力疗法协同治疗.将光热剂与化疗药物结合,可控制药物在肿瘤部位的释放[22],降低药物对正常细胞的损伤,协同化疗和光热疗法能很好地提高癌症治疗能力.结合药物的方法有两种:一是将光热剂同可载药的载体结合;二是将光热剂自身设计为药物载体.SILVA等[23]设计了装载5-FU的二甲基咪唑结合PANI纳米粒子(PANI@ZIF-8),在NIR和pH=5.2的緩冲溶液中,5-FU的累计释放量达到80%,PANI吸收NIR会促使温度的升高,增强了5-FU的释放,具有很好的化学光热效应.如图3所示,XIA等[24]采用多孔硅包覆阿霉素(DOX),并将PANI共价接枝到其表面,通过pH和NIR响应控制药物释放,多孔硅可降解为无毒氢氧化硅(Si(OH)4)排出体外,解决了光热剂不可生物降解所带来的长期毒性问题,化学与光热结合疗法展现了巨大的潜力.2.3 多模式成像引导治疗通过成像的方式来引导治疗可以有效地提高肿瘤治疗效率.掺杂态PANI在近红外区具有独特的光吸收特性,是一种优异的光声成像剂[25].光声成像是探测激光照射产生的光声信号后,产生组织分布图像的成像方式,具有高信噪比和高分辨率的优点[26].但是单一的成像依旧存在缺陷,尤其对深部肿瘤,光声成像对激光的强度存在依赖,激光过强会损伤正常的组织细胞.多模式成像方式引导治疗来提高治疗效果是目前研究的趋势.如图4所示,WANG等[27]制备一种PANI包覆二硫化钼(MoS2@PANI-PEG)量子点复合材料,MoS2量子点可产生强荧光,可用于体内成像的探针,同时MoS2是放射增敏剂,结合PANI的光热疗法和光声成像能力,协同增强癌症的治疗效果.2.4 智能响应治疗基于PANI易被质子酸掺杂和肿瘤微环境具有微酸性的特点,设计智能响应探针用于癌症治疗.由于肿瘤微酸性环境(pH为5.0~7.4)低于PANI掺杂要求的pH值(pH<3.0),限制了PANI在癌症治疗中光热治疗和光声成像的应用.JU等[28]合成了金/聚苯胺核壳纳米材料(Au@PANI),基于Au到PANI的电荷转移以及PANI掺杂过程诱导的电子传递效率的提高,Au@PANI在pH=6.5时即可实现掺杂,显示出优异的光热效应.但金离子在体内具有长期毒性,对人体会造成很大的危害.为提高智能治疗剂的治疗性能,如图5所示,LI等[29]采用牛血清白蛋白(BSA)包覆PANI,设计了BSA-PANI纳米粒子,可以实现肿瘤内源性触发的诊断和治疗,光热转换效率达到37%,且纳米粒子具有很好的生物相容性,降低体内毒性.目前对PANI纳米材料的表面改性,主要通过物理吸附或采用共价键将配体结合在纳米颗粒表面等方式以实现其功能化.较常用于纳米颗粒改性的化合物是聚乙二醇.通过聚乙二醇改性可改善材料的亲水性,改变材料药代动力学效果,同时具有实现其他功能的优势[12].为了改善PANI在水中的分散性,WANG等[13]设计了一种核-壳结构的复合材料聚苯胺-聚吡咯烷酮(PANi@PVP),PVP作为空间稳定剂对PANI进行改性,在水中有优异的溶解性和良好的细胞相容性.LIU等[7]提出了一种分级靶向策略,设计了pH敏感的柠檬酸钠修饰的铜掺杂PANI 纳米梭(SC-VCR-CuPANI NSs),如图1所示,该材料在血液中呈负电,显示出增强的隐身效果,血液循环周期延长,且在肿瘤微酸环境中实现质子化,提高细胞内在化效果.通过这种分级靶向的策略,材料在血液中的循环半衰期从4.35 h增加到7.33 h,磁共振成像分辨率和信号强度得到显著提高.为了提高肿瘤的治疗效果,基于透明质酸(HA)可以特异性结合受体(CD44)靶向肿瘤细胞[14], JIANG等[15]设计了一种水溶性透明质酸-杂交PANI纳米材料,靶向光热治疗肿瘤.通过细胞实验,材料可以针对性地杀死癌细胞HeLa和HCT-116,而對正常细胞HFF没有影响.将HA修饰的二氧化硅荧光纳米颗粒连接到PANI,在靶向肿瘤的同时,二氧化硅提供的荧光成像可以引导光热治疗.2 PANI纳米复合材料在肿瘤诊疗上的应用2.1 光热治疗PANI经掺杂后在近红外区(700~900 nm)有很强的吸收,PANI具有成为光热剂的可能[16].如图2所示,YANG等[5]首次报道了PANI在酸性条件下进行掺杂,具有有机光热剂的潜力.用808 nm,2.45 W·cm-2的NIR激光照射5 min,掺杂态PANI温度升高了54.8 ℃,采用相同条件,本征态PANI温度升高了15 ℃左右,表明了掺杂后的PANI光热效果明显.为评估PANI对癌细胞在体外和体内的光热消融能力,将本征态的PANI用上皮癌细胞A431处理后发现,PANI颜色从紫色变成绿色,并在808 nm NIR激光下照射,台盼蓝染色实验发现大量细胞被破坏.同样在体内实验中,经瘤内注射材料,用激光照射治疗后,肿瘤组织切片显示有严重的细胞损伤.这说明PANI可以在癌细胞中掺杂,并诱导产生光热效应,从而杀死癌细胞.。
氧化石墨烯纳米颗粒在小鼠骨骼肌和人血液中诱导的免疫反应与毒性效应

INTRODUCTIONGraphene oxide (GO),a single -layer graphene ramification,is increasingly used as a biomedical nanomaterial in disease diagnosis,cancer treatment,tissue engineering,vaccine adjuvant,and drug delivery due to its large surface area,flexibility,excellent adsorption ability,hydrophilicity and good dispersibility in various solutions [1-4].But currently concerns over the biocompatibility of GO remain,as both in vitro or in vivo studies have shown GO -induced toxicities in diverse forms ranging from inflammation,DNA damage,pyroptosis,cell apoptosis,reactive oxygen formation to thrombus formation,pulmonary edema and granulomaformation [5-8].Carbon -based nanomaterials such as single -and multi -walled carbon nanotubes (SWCNTs and MWCNTs),fullerene and graphene were reported to induce immunotoxicity by interacting with the immune cells to cause either direct or immune pathway -mediateddamages [9,10].For example,exposure to these nanomaterials was reported to induce recruitment of mast cells and trigger immunotoxicity [9],and CNTs and fullerenes could be identified as pathogens by Toll -like receptors to elicit an immune response [11];SWCNTs have been shown to cause impairment of systemic immune function and promote allergic responses through inducing Th1-polarized immune responses [11-13].Inspite of the increasing evidences from in vitro studies showing immunotoxicities of GO nanoparticles [1,14,15],few in vivo studies have been conducted,except in adult zebrafish [16,17],to address this issue.Animal studies allow better understanding of the adsorption,distribution,metabolism,excretion and immunological response of nanomaterials [10].In particular,the skeletal muscle has been recognized in recent years as an ideal in vivo model for biocompatibility study of the biomedical materials [18,19],and GO as a vaccine adjuvant was reported to cause strong muscle inflammation at the injection site,but the precise mechanism mediating its immunotoxicity remainsunclear [20].The immunotoxicological effect of GO to cause muscular injuries has not been reported.In this study,we aimed to evaluate the immunotoxicity of GO nano -particles in mouse gastrocnemius muscles (GNs)and its hematotoxicity in human red blood cells (RBCs)in vitro .Immunogenic and toxic effects of graphene oxide nanoparticles in mouse skeletal muscles and human red blood cellsSUN Yiming 1,HUANGAilan 2,ZHAO Zhi 3,SONG Chen 4,LAI Guihua 11Department of Human Anatomy,2Department of Food Quality and Safety,3Department of Orthopedics of First Affiliated Hospital,BengbuMedical University,Bengbu 233030,China;4Department of Anatomy,Guangdong Provincial Key Laboratory of Construction and Detection in Tissue Engineering,Southern Medical University,Guangzhou 510515,ChinaOriginal ArticleReceived:2023-11-11Accepted:2024-03-05Supported by Key Science Research Project Fund of Education DepartmentofAnhuiProvince(KJ2021A0764,KJ2017A211).Corresponding author:LAI Guihua,associate professor,E-mail:*********************.cn.J South Med Univ,2024,44(4):617-626doi 10.12122/j.issn.1673-4254.2024.04.01··617METHODSPreparation and characterization of GO nanoparticles We used primary GO powder (XFNANO,China;sheet size of 0.5-5μm and thickness of 0.8-1.2nm)to prepare GO nanoparticles.Specifically,precisely weighed GO powder was dispersed in phosphate buffer solution (PBS,0.01mol/L,PH 7.4)or deionized water (H 2O)and sonicated using a probe sonicator (SONICS,VCX130,USA),which was programmed for cycles of sonication for 40s (ultrasonic power of 130W and 70%amplitude)at the interval of 20s in an ice bath for 1h to obtain the GO nanoparticles.The surface charge and particle size of GO nanoparticles in H 2O or PBS suspension was analyzed using a dynamic light scattering (DLS)apparatus (Zetasizer Nano ZS,Malvern,Malvern Instruments Ltd.,UK).Each sample was detected for at least 3times.Animal experimentThe protocols of animal experiments in this study were approved by the Animal Ethics Committees of Southern Medical University and Bengbu Medical College.A total of 120female C57BL/6mice (6-8weeks,20±2g)were provided by the Laboratory Animal Center of Southern Medical University and Bengbu Medical College.The mice were randomized into 5groups (n =24),and to investigate inflammatory and immune responses,4groups of mice were subjected to intramuscular injection with sterile PBS (negative control)or 0.5,1.0and 2.0mg/mL GO suspensions (0.5GO,1GO and 2GO groups,respectively).Specifically,the mice were anesthetized with intraperitoneal injection of 0.01mL/kg of 4%chloral hydrate,and the bilateral GNs were exposed for injections of 200μL sterile PBS or different concentrations of GO suspension at 8sites.The GO suspensions were all prepared within 3h before injection to avoid GO aggregation (which may occur beyond 3h after sonication at 37℃in vitro ).The left 24mice served as the sham -operated group,where the bilateral GNs were exposed only and then sutured without injecitons.At 2,4,6,and 8weeks following the injections,6mice from each group were euthanized at each time point and the bilateral GNs were dissected.The muscular tissues were embedded in OCT compound and imme -diately immersed in 4℃pre -cooled 98%isopentane (Aladdin,Shanghai,China)for 25to 60s.The tissues were stored at -80℃and frozen sections (6μm thick)were prepared for subsequent experiments.HE and immunofluorescence stainingFor HE staining,the cryosections were fixed with cold acetone,cleansed by water flushing,stained with hematoxylin and eosin (Leagene,China),dehydrated with gradient ethanol (80%,90%,95%,and 100%,V/V )and xylene (analytically purity),and mounted with neutral balsam.The sections were observed under an optical microscope (Olympus,BX53,Japan)to examine histological changes of the tissues.For immunofluorescence staining,the cryosectionswere fixed using pre -cooled acetone and washed with PBS for 5min.The specimens were incubated with 5%bovine serum albumin (Zhuosheng Biology,China)blocking buffer at room temperature for 30min before incubation with the primary antibodies including rat anti -mouse F4/80(1:200;eBioscience,USA),rat anti -mouse CD11b (1:200;eBioscience,USA),rabbit polyclonal anti -CD3e (1:100;Abcam,USA),rat anti -mouse CD4(1:100;eBioscience,USA)and mouse monoclonal anti -CD11c (1:200;Abcam,USA)for 24h at 4℃.After washing with PBS for 5min (4times),the sections were incubated with the secondary antibodies at room temperature for 40min in the dark,including Alexa Fluor488donkey anti -mouse IgG (H&L)(1:600;Invitrogen,USA),Alexa Fluor568donkey anti -mouse IgG (H&L)(1:600;Molecular probes,USA),and Alexa Fluor488donkey anti -rabbit IgG (H&L)(1:600;Invitrogen,USA).The slides were redyed with 4,6-diamidino -2-phenylindole (DAPI;Santa Cruz,USA),and then imaged under a fluorescence microscope (Olympus,BX53,Japan).For each group,at least 3unilateral GNs were included for examination.The positive expression of the target proteins was analyzed using Image -Pro -Plus software,and for slides with positive staining,the entire fluorescence area and positive fluorescence area were measured to calculate the mean percentage of the positive area relative to the entire fluorescence area.Scanning electron microscopy (SEM)To evaluate hematological toxicity of GO,we observed the morphological changes of human RBCs incubated with GO using SEM.All human hematological experiments were approved by the Medical Ethics Committee of Bengbu Medical College and the Second Affiliated Hospital ([2022]Number 96).Fresh human whole blood samples were obtained from the Physical Examination Center of the Second Affiliated Hospital of Bengbu Medical College.Nine tubes of fresh peripheral venous blood samples were collected in sodium citrate -anticoagulant vacuum blood tube from 3healthy volunteers and centrifuged at 1000×g for 5min to isolate the RBCs.The RBCs were washed with PBS (centrifuged at 1000×g for 5min)for 3times and then incubated for 30min at room temperature with GO suspensions (final concentrations of 0.002to 20mg/mL),with the RBCs treated with PBS as the control.After rinsing with PBS for 3times,the treated RBCs were fixed for 4h with 2.5%glutaraldehyde and stored at 4℃for 2or 3days.The fixed RBCs were dehydrated with gradient ethanol (70%,80%,90%,and 100%,V/V ),air dried,and then coated with gold for SEM observation (Hitachi,S -3000N,Japan).Measurement of hemoglobin absorbance valueTo assess the hemolytic activity of GO,2mL of whole blood sample was added into 4mL PBS,and the mixture was centrifuged at 1000×g for 5min to isolate the RBCs,which were resuspended in 10%volume fraction (V/V )with PBS.The RBC suspension (0.2mL,containing around 3.8×109cells/mL)and 0.8mL GO suspensionJ South Med Univ,2024,44(4):617-626··618(0.002to 20mg/mL)were mixed,with the cells treated with deionized water and PBS as the positive and negative controls,respectively.The mixtures were incubated at 37℃for 1or 3h and centrifuged (1000×g )for 5min,and the supernatant was collected for determine the absorbance values (A)of the released hemoglobin at 540nm (with 655nm as the reference)using a spectrum microplate spectrophotometer (Multiskan GO,Thermo Fisher Scientific,Finland).Percent hemolysis in the presence of the GO was calculated using the equation below:Percent hemolysis (%)=(A sample -A negative control )/(A positivecontrol -A negative control )×100%ThromboelastographyWe examined the effect of GO for inducing blood coagulation using thromboelastography.Briefly,whole blood sample was mixed in a volume ratio of 9:1with different concentrations (0.02to 200mg/mL)of GO suspension in kaolin -containing tubes and incubated for 5min at room temperature,with PBS as the control.The final concentration range of the GO suspension was 0.002-20mg/mL.In a TEG cup,20μL CaCl 2solution (0.2mol/L)and 340μL GO/blood mixture were successively added and the coagulation process was observed at 37℃using Thromboelastograph Hemostasis System 5000(Hemoscope Corporation,Niles,IL,USA).Each sample was measured in triplicates,and theexperiment was repeated for 3times.Statistical AnalysisThe data of continuous variables are presented as Mean ±SD and analyzed statistically using SPSS 25.0(IBM Corp.,USA)or GraphPad Prism (Graph Software,A).Multiple comparisons between different groups were performed using one -way ANOVA.A P value less than 0.05was considered to indicate a statistically significant difference.RESULTSParticle size and zeta potential of GO nanoparticles DLS analysis showed that approximately 80%of GO nanoparticles had a particle size ranging from 150nm to 250nm.The GO nanoparticles exhibited excellent colloidal dispersity and stability in H 2O within 24h at 37℃after sonication,and their average particle size was larger in PBS suspension than in H 2O suspension (330.29±54.87vs 188.93±34.82nm,Fig.1).Although a high salt content in PBS could cause nanoparticle aggregation due to the charge screening effect [6,10],the prepared GO nanoparticles showed homogenous dispersion in PBS within 3h at 37℃,suggesting their relatively good stability in high -salt microenvironment for biomedical applications.Fig.1Particle size of the prepared GO nanoparticles suspended in deionized water (H 2O)and PBS detected by dynamic light scattering.GO in H 2OP e r c e n t a g e (%)504030201000100200300400500Size (nm)P e r c e n t a g e (%)4035302520151050GO in PBS100200300400500Size (nm)We further analyzed surface charge of GO nano -particles suspended in H 2O and PBS by zeta -potential assessment.The results showed that GO nanoparticles carried negative surface charges in both H 2O (-43.6±6.4mV)and PBS suspensions (-24.6±2.1mV).GO nanoparticles induces inflammation in mouse GNs in vivoThe stability and biocompatibility of implanted biomaterials may affect the duration and extent of inflammatory and the subsequent immune responses and muscle regeneration [18,21,22].The results of HE staining showed that compared with those in PBS andsham -operated groups,the mouse GNs with injections of GO nanoparticles (0.5,1and 2mg/mL)presented with obvious and sustained inflammation and myofiber degeneration around the injection sites even till the 8th week (Fig.2).Muscular inflammation and myofiber degeneration were the most conspicuous in 2mg/mL GO group at 2weeks post -injection,and subsided gradually over time.Similar but milder inflammatory changes were also observed in 0.5and 1mg/mL GO groups,suggesting a dose -dependent effect of GO for inducing muscular inflammation and myofiber degeneration.Biomaterial implantation may cause activation of J South Med Univ,2024,44(4):617-626··619monocytes/macrophages and their recruitment to the implantation site to trigger inflammatory response [18].We investigated GO -induced intramuscular infiltration of monocytes/macrophages by fluorescence detection of the expression of the macrophage marker F4/80protein and the monocyte marker CD11b protein.As shown in Fig.3and Fig.4,F4/80+and CD11b +cells were mostly located in the GN around injection sites,and their intramuscular infiltration reached the peak levels at 2weeks post -injection,followed by gradual decrease over time.Numerous CD11b +and F4/80+cells were still present in the muscles even at 8weeks after injection of 1.0and 2.0mg/mL GO suspensions,while much fewer positive cells were detected in 0.5mg/mL GO group.The mean percentage of F4/80+and CD11b +areas remained significantly higher in GO groups than in PBS and sham operation groups across all the time points (P <0.05),and higher GO concentration induced more obvious inflammatory cell infiltration in the GNs.GO -induced intramuscular infiltration of dendritic cells (DCs)and CD4+T cellsAs shown in Fig.5and Fig.6,large numbers of mature DCs (CD11c +cells)and CD4+T cells (CD3+CD4+cells)were observed in the GNs following GO injections but not detected in normal GNs.In the GO -treated groups,CD11c +cell infiltration and CD3+CD4+T cell number increased dose -denpendently but decreased progressively with the post -injection time,suggesting that GO nanoparticles trigger inflammation -mediated adaptive immune response through DCs and CD4+T cells in mouse GNs.Hematotoxicity of GO nanoparticles in vitroFig.7A showed that at low concentrations (0.002and 0.02mg/mL),GO nanoparticles induced minimal morphological changes of the RBCs,while as GOFig.2HE staining of mouse gastrocnemius muscles (GNs)in Sham group (A ),PBS group (B ),and the 3GO treatment groups injected with 0.5,1.0and 2.0GO suspensions (C ,D ,and E ,respectively)at different time points post-injection (Scale bar=50μm).ABCDE2weeks4weeks6weeks8weeksJ South Med Univ,2024,44(4):617-626··620concentration increased to 0.2,2and 20mg/mL,the RBCs became crenated,and cell aggregation,cytolysis,and membrane disrupture occurred.The hematotoxicity of GO on RBCs was also confirmed by hemolysis experi -ment (Fig.7B and D),in which GO (0.002to 20mg/mL)exposure dose -and time -dependently increased hemolysis percentage of RBCs,leading to hemoglobin release in the supernatant (Fig.7C and E).Biomaterials may affect blood coagulation system through interaction with the coagulation components to cause potential detrimental effects on the host [23].Fig.8shows TEG traces of whole blood coagulation inducedbyFig.3Intramuscular infiltration of macrophages in mouse GNs at different time points following injections of GO nanoparticles.A :Immunofluorescence staining for F4/80expressions of in Sham group,PBS group,0.5GO group (0.5mg/mL),1GO group (1.0mg/mL)and 2GO group (2.0mg/mL)at different time points (Scale bar=50μm).The areas delineated by white lines are the implanted GO nanoparticles.B :Comparison of the mean percent of F4/80+fluorescent area in the muscles among the groups.*P <0.05,**P <0.01.Fig.4Intramuscular infiltration of monocytes in mouse GNs at different time points following injections of GO nanoparticles.A :Immunofluorescence staining for CD11b in different groups at different time points (Scale bar=50μm).The area delineated by white lines are the implanted GO nanoparticles.B :Comparison of mean percent of CD11b +fluorescent area in the muscles among the groups.*P <0.05,**P <0.01.2weeks4weeks6weeks8weeks2G O1G O0.5G OP B S S h a mABM e a n p e r c e n t o f F 4/80p o s i t i v e a r e a (%)50403020100S h a m P B S 0.5G O 1G O 2G OGROUP (2weeks)M e a n p e r c e n t o f F 4/80p o s i t i v e a r e a (%)403020100M e a n p e r c e n t o f F 4/80p o s i t i v e a r e a (%)151050M e a n p e r c e n t o f F 4/80p o s i t i v e a r e a (%)2520151050************************S h a m P B S 0.5G O 1G O 2G OGROUP (6weeks)S ha mP B S 0.5G O GROUP (8weeks)1G O 2G O S ha m P B S GROUP (4weeks)0.5G O 1G O 2G O2G O1G O0.5G OP B SS h a m2weeks 4weeks 6weeks 8weeksAM e a n p e r c e n t o f C D 11b p o s i t i v e a r e s (%)S ha m P B S 0.5G O 1G O 2G O GROUP (2weeks)403020100M e a n p e r c e n t o f C D 11b p o s i t i v e a r e s (%)3020100M e a n p e r c e n t o f C D 11b p o s i t i v e a r e s (%)1086420M e a n p e r c e n t o f C D 11b p o s i t i v e a r e s (%)20151050S ha m P B S 0.5G O 1G O 2G O GROUP (6weeks)S ha m P B S 0.5G O GROUP (8weeks)1G O 2G O S ha m P B S GROUP (4weeks).5G O 1G O 2G O ***********************B J South Med Univ,2024,44(4):617-626··621GO treatment (the corresponding TEG parameters are listed in Tab.1).In 0.002and 0.02mg/mL GO groups,the TEG traces were similar to that in PBS group.But at the concentrations of 0.2mg/mL or above,GO nanoparticles caused obvious abnormities in TEG traces and TEG parameters including coagulation time (K),reaction time(R),αangle (α),and maximum amplitude (MA)in a dose -dependent manner.These results demonstrate that GO can cause hematotoxicity by impairing the RBCs and blood coagulation,thereby indirectly affecting the immunesystem.Fig.5Dendritic cell (DC)infiltration in mouse GNs following GO nanoparticle injection.A :Immunofluorescence staining for CD11c in mouse GNs in the 5groups at different time points.The area labeled by white lines indicated the implanted GO nanoparticles (Scale bar=50μm).The area delineated by white lines are the implanted GO nanoparticles.B :Comparison of mean percent of CD11c +fluorescent area among the groups.*P <0.05,**P<0.01.Fig.6CD4+T cell infiltration in mouse GNs following GO nanoparticle injection.A :Immunofluorescence staining of CD3+CD4+cells in different groups at different time points.The area delineated by white lines are the implanted GO nanoparticles.B :Comparison of number of CD3+CD4+T cells in the muscles among the groups.*P <0.05,**P <0.01.M e a n p e r c e n t o f C D 11c p o s i t i v e a r e a (%)151050S h a m P B S 0.5G O 1G O 2G OGROUP (6weeks)M e a n p e r c e n t o f C D 11c p o s i t i v e a r e a (%)50403020100S ha m P B S 0.5G O 1G O 2G OGROUP (2weeks)******M e a n p e r c e n t o f C D 11c p o s i t i v e a r e a (%)3020100M e a n p e r c e n t o f C D 11c p o s i t i v e a r e a (%)1086420S ha m P B S 0.5G O GROUP (4weeks)1G O 2G OS ha mP B S 0.5G O GROUP (8weeks)1G O 2G O ***************2G O1G O0.5G OP B SS h a mAB2weeks4weeks6weeks8weeks2G O 1G O0.5G OP B SS h a mABS h a m P B S 0.5G O 1G O 2G OGROUP (6weeks)15105050403020100T h e n u m b e r o f C D 3+&C D 4+T c e l l sS h a m P B S 0.5G O 1G O 2G OGROUP (2weeks)T h e n u m b e r o f C D 3+&C D 4+T c e l l s2520151050S h a m P B S 0.5G O GROUP (4weeks)1G O 2G O S h a m P B S 0.5G O GROUP (8weeks)1G O 2G O T h e n u m b e r o f C D 3+&C D 4+T c e l l sT h e n u m b e r o f C D 3+&C D 4+T c e l l s86420**********************J South Med Univ,2024,44(4):617-6262weeks 4weeks 6weeks 8weeks··622DISCUSSIONHerein we demonstrated that different concentrations of GO could dose -and time -dependently cause sustained inflammatory response and myofiber degeneration in mouse GNs.GO -induced inflammation is probably attributed to the aggregation of GO triggered by thecharge screening effect between the surface oxygen groups of GO and ionic salt species in body fluids or high -salt solutions such as PBS or culture medium [24-26].In mouse GNs,we observed obvious aggregation of injected GO nanoparticles with massive inflammatory cell infiltration at the injection sites.The particle size of GO may also be a contributing factor,as a GO particlesize0.2mg/mL GO 2mg/mL GO 20mg/mL GOPBS0.002mg/mL GO0.02mg/mL GOAFig.7Hematological effects of GO on human RBCs in vitro .A :Morphological changes of human RBCs trerated with different concentrations of GO (0.002to 20mg/mL)observed by SEM (Scale bar=10μm).B ,D :Percent hemolysis of RBCs incubated with different concentrations of GO (0.002to 20mg/mL)for 1and 3h at 37℃.C ,E :RBCs with GO exposure at different concentrations (0.002to 20mg/mL)for 1and 3h.The presence of hemoglobin in the supernatant indicates membrane disrupture of the RBCs.(+)is the positive control (deionized water)and (-)the negative control (PBS).*P <0.05,**P <0.01.Concentration of grapheneoxide (mg/mL)0.0020.020.2220R e l a t i v e p e r c e n t h e m o l y s i s (%)c o m p a r e d t o c o n t r o l100806040200Concentration of grapheneoxide (mg/mL)GO nanoparticles concentration (mg.mL)-+0.0020.020.2220CR e l a t i v e p e r c e n t h e m o l y s i s (%)c o m p a r e d t o c o n t r o l1008060402000.0020.020.2220GO nanoparticles concentration (mg.mL)+-0.0020.020.2220EBD**************J South Med Univ,2024,44(4):617-626··623beyond 200nm was reported to significantly enhance particle aggregation and promote proinflammatoryresponses both in vitro and in vivo [24,25,27].Inflammation induced by foreign implants involvesboth innate and adaptive immune responses [21,28],in which monocytes and macrophages play critical roles [29,30].Studies have shown that implantation of biomaterials,including GO,causes significant activation and recruitment of monocytes and macrophages to trigger inflammatory response [18,25,26,31,32].In this study,we observed massive monocyte and macrophage infiltrations in necrotic myofibers in the GNs of mice at 2,4,6,and even 8weeks post -injection.GO nanoparticles was reported to promote macrophage polarization into M1phenotype and enhance pro -inflammatory response by stimulating the secretion of inflammatory cytokines and leukocyte recruitment [25].These observations suggest that M1polarization of macrophages very likely occurred in mouse GNs following GO injection,which,along with intramuscular aggregation of GO,induces muscle cell lysis,affects phagocytosis of cell debris,and thus induces persistent and spreading inflammation and myofiber necrosis.GO can be internalized by DCs in vitro probably by interacting with the cell membrane receptors related to the endocytic pathways [33],and mature DCs can facilitatepriming of CD4+T cells [18].In this study,we found that GO dose -and time -dependently induced obvious DC and CD4+T cell infiltration in the GNs,suggesting that inflammation triggered by GO promotes maturation and infiltration of DCs,and then directly activates naive CD4+T cells,leading to intramuscular adaptive immune response.In addition,macrophages can interact with graphene or its derivatives and internalize GO via phagocytosis.The activated macrophages present the antigens to T cells,thereby launching a adaptive immune response [19,25,34],and the T cells,in turn,facilitate macro -phage adhesion and fusion via paracrine effects [19,34].WeFig.8TEG traces of whole blood coagulation in vitro in the presence of different concentrations of GO nanoparticles (0.002to 20mg/mL).K:Coagulation time;R:Reaction time;α:αangle;MA:Maximum amplitude.Arrows indicate increase (↑)or reduction (↓)of the values relative to normal range provided by the TEG analyzer.PBS0.002mg/mL GO0.02mg/mL GO0.2mg/mL GO 2mg/mL GO 20mg/mL GOJ South Med Univ,2024,44(4):617-626··624hence inferred that GO might be recognized, phagocytized,and processed by macrophages,and then presented to CD4+T cells,thus triggering intramuscular adaptive immunity.The hydrosoluble elements from exogenous implants can enter blood circulation and even reach peripheral lymphoid organs,where they trigger an immunological response and impair the blood components including RBCs and blood coagulation system[20,18,35,36]to result in dysregulation of immunological function[37,38].But so far the mechanisms mediating hematotoxicity or blood-related immunotoxicity of GO remain poorly defined. Liao et al[37]proposed that the size and surface charge of GO particles both contributed to its RBC hemolytic activity,and they demonstrated that GO disrupted RBC membrane integrity to facilitate hemolysis through intense electrostatic attraction between the positively charged phosphatidylcholine lipids of RBC outer membrane and the negatively charged oxygen groups of GO surface.We observed obvious morphological changes and hemolysis of the RBCs after incubation with high-concentration GO nanoparticles,similar to the findings in previous studies[37,38].The results of TEG studies showed that GO also caused severe impairment of the blood coagulation,probably due to its interation with the blood coagulation componants.Overall,GO nanoparticles interact with the blood components and cause hematotoxicity by disrupting RBCs,promoting RBC hemolysis and impairing blood coagulation.In summary,GO nanoparticles elecit inflammatory and innate or adaptive immune respones in mice by causing myofiber degeneration and intramuscular immune cell infiltration possibly due to its hemotoxic and immunotoxic effects.REFERENCES:[1]Zare P,Aleemardani M,Seifalian A,et al.Graphene oxide: opportunities and challenges in biomedicine[J].Nanomaterials (Basel),2021,11(5):1083.[2]JiříčkováA,JankovskýO,Sofer Z,et al.Synthesis and applications of graphene oxide[J].Materials(Basel),2022,15(3):920.[3]Shahriari S,Sastry M,Panjikar S,et al.Graphene and graphene oxide as a support for biomolecules in the development of biosensors[J].Nanotechnol Sci Appl,2021,14:197-220.[4]Shafiee A,Iravani S,Varma RS,et al.Graphene and graphene oxide with anticancer applications:challenges and future perspectives[J].MedComm,2022,3(1):e118.[5]Raslan A,Burgo LSD,Ciriza J,et al.Graphene oxide and reduced graphene oxide-based scaffolds in regenerative medicine[J].Int J Pharm,2020,580:119226.[6]Ianni ED,Møller P,Vogel UB,et al.Pro-inflammatory response and genotoxicity caused by clay and graphene nanomaterials in A549and THP-1cells[J].Mutat Res Genet Toxicol Environ Mutagen,2021, 872:503405.[7]Mohamed HRH,Welson M,Yaseen AE,et al.Induction of chromosomal and DNA damage and histological alterations by graphene oxide nanoparticles in Swiss mice[J].Drug Chem Toxicol, 2021,44(6):631-41.[8]Bengtson S,Knudsen KB,Vogel U,et al.Differences in inflammation and acute phase response but similar genotoxicity in mice following pulmonary exposure to graphene oxide and reduced graphene oxide [J].PLoS ONE,2017,12(6):e0178355.[9]Hofer S,Hofstätter N,Punz B,et al.Immunotoxicity of nanomaterials in health and disease:current challenges and emerging approaches for identifying immune modifiers in susceptible populations[J].Wiley Interdiscip Rev Nanomed Nanobiotechnol,2022,14(6):e1804.[10]Dusinska M,Tulinska J,Smolkova B,et al.Immunotoxicity, genotoxicity and epigenetic toxicity of nanomaterials:new strategies for toxicity testing?[J].Food Chem Toxicol,2017,109(Pt1):797-811.[11]Park EJ,Choi J,Kim YH,et al.Subchronic immunotoxicity and screening of reproductive toxicity and developmental immunotoxicity following single instillation of HIPCO-single-walled carbon nanotubes:purity-based comparison[J].Nanotoxicology,2016,10(8): 1188-202.[12]Lee S,Khang D,Kim SH.High dispersity of carbon nanotubes diminishes immunotoxicity in spleen[J].Inter J Nanomedicine,2015, 1,(10):2697-710.[13]Minchenko OH,Tsymbal DO,Minchenko DO,et al.Single-walled carbon nanotubes affect the expression of genes associated with immune response in normal human astrocytes[J].Toxicol In Vitro, 2018,52:122-30.[14]Cho YC,Pak PJ,Chung NH,et al.In vitro and in vivo comparison of the immunotoxicity of single-and multi-layered graphene oxides with or without pluronic F-127[J].Sci Rep,2016,12(6):38884.[15]Yang ZW,Pan YN,Lin GM,et al.Cytotoxicity and immune dysfunction of dendritic cells caused by graphene oxide[J].Front Pharmacol,2020,17(11):1206.[16]Xiong GH,Deng YY,Lu HQ,et al.Graphene oxide nanoparticles induce hepatic dysfunction through the regulation of innate immune signaling in zebrafish(Danio rerio)[J].Nanotoxicology,2020,14(5): 667-82.[17]Yang XL,Yang QL,Fu ZW,et al.Developmental neurotoxicity and immunotoxicity induced by graphene oxide in zebrafish embryos[J].Environ Toxicol,2019,34(4):415-23.[18]Xiao JW,Huang C,Liao H,et al.Inflammatory and immuno-reactivity in mice induced by intramuscular implants of HSNGLPL peptide grafted-polyurethane[J].J Mater Chem B,2016,4(10):1898-907.[19]Yunus MA,Ramli MM,Mohamed R,et al.Stimulation of innate and adaptive immune cells with graphene oxide and reduced graphene oxide affect cancer progression[J].Arch Immunol Ther Exp(Warsz), 2021,69(1):20.[20]Meng CC,Zhi X,Cui DX,et al.Graphene oxides decorated with carnosine as an adjuvant to modulate innate immune and improve adaptive immunity in vivo[J].ACS Nano,2016,10(2):2203-13.[21]Tidball JG.Regulation of muscle growth and regeneration by the immune system[J].Nat Rev Immunol,2017,17(3):165-78.[22]Sousa NS,Brás MF,Antunes IB,et al.Aging disrupts MANF-mediated immune modulation during skeletal muscle regeneration[J].Nat Aging,2023,3(5):585-99.[23]Eivazzadeh-Keihan R,Radinekiyan F,Maleki A,et al.Graphene oxide/alginate/silk fibroin composite as a novel bionanostructure with improved blood compatibility,less toxicity and enhanced mechanical properties[J].Carbohydr Polym,2020,248:116802.[24]Han J,Kim YS,Kim BS,et al.Dual roles of graphene oxide to attenuate inflammation and elicit timely polarization of macrophage phenotypes for cardiac repair[J].ACS Nano,2018,12(2):1959-77.[25]Ma J,Liu R,Liu SJ,et al.Crucial role of lateral size for graphene oxide in activating macrophages and stimulating pro-inflammatory responses in cells and animals[J].ACS Nano,2015,9(10):10498-515.[26]Duch MC,Budinger GRS,Mutlu GM,et al.Minimizing oxidation and stable nanoscale dispersion improves the biocompatibility of graphene in the lung[J].Nano Lett,2011,11(12):5201-7.[27]Cicuéndez M,Fernandes M,Duarte IF,et al.Macrophage inflammatory and metabolic responses to graphene-based nanomaterials differing in size and functionalization[J].Colloids Surf B Biointerfaces,2020, 186:110709.[28]Liang F,LoréK.Local innate immune responses in the vaccine adjuvant-injected muscle[J].Clin Transl Immunol,2016,45(4):e74.[29]Yang WJ,Hu P.Hierarchical signaling transduction of the immune and muscle cell crosstalk in muscle regeneration[J].Cell Immunol, 2018,326:2-7.[30]Ziemkiewicz N,Hilliard G,Garg K,et al.The role of innate and adaptive immune cells in skeletal muscle regeneration[J].Int J Mol Sci,2021,22(6):3265.[31]Lin H,Ji DK,Bianco A,et parative effects of graphene and molybdenum disulfide on human macrophage toxicity[J].Small, 2020,16(35):e2002194.[32]Huang MJ,Xiao MH,Wang DQ,et al.Synergistic anti-inflammatory effects of graphene oxide quantum dots and trans-10-hydroxy-2-decenoic acid on LPS-stimulated RAW264.7macrophage cells[J].Biomater Adv,2022,136:212774.[33]Dudek I,Skoda M,Szukiewicz D,et al.The molecular influence of J South Med Univ,2024,44(4):617-626··625。
Nanoparticles for cancer therapy

Nanoparticles for cancer therapy : A Promising ApproachCancer is one of the most deadly diseases affecting humans, with the World Health Organization predicting that it will soon become the leading cause of death worldwide. Despite the numerous available treatment options, such as chemotherapy and radiotherapy, the disease remains a significant challenge due to resistance, toxicity, and adverse side effects of current therapies. Hence, there is a quest for more efficient and targeted cancer treatments, and nanoparticles are emerging as promising tools for cancer therapy.Nanoparticles are tiny particles with sizes ranging from 1-100 nanometers and unique physicochemical properties that differ from their bulk counterparts. Due to their small sizes, nanoparticles can penetrate cancer cells' tissue and target tumors with precision. They can also be tailored to respond to specific signals, such as pH and temperature, allowing for controlled drug release and reduced toxicity.One of the most significant advantages of nanoparticles in cancer therapy is their ability to deliver drugs directly to cancer cells, avoiding healthy cells. Traditional chemotherapy drugs lack specificity and can kill both cancerous and healthy cells, resulting in adverse side effects such as hair loss, fatigue, and anemia. By encapsulating chemotherapeutic agents in nanoparticles, the drugs can selectively target cancer cells while leaving healthy ones unaffected, reducing toxicity and adverse side effects.Moreover, nanoparticles can improve drug's pharmacokinetics, allowing for sustained drug delivery and prolonged exposure to cancer cells. Traditional chemotherapy drugs have a narrow therapeutic window, and most are cleared from the body before reaching the tumor site. By using nanoparticles, drugs can circulate longer in the bloodstream, penetrate tumors' vasculature, and accumulate inside the tumor cells, leading to higher drug concentration at the tumor site and improved treatment outcomes.Another potential application of nanoparticles in cancer therapy is imaging and diagnosis. Imaging techniques such as magnetic resonance imaging (MRI) and computed tomography (CT) are invaluable in cancer diagnosis and staging, but they have limitations such as low spatial resolution and contrast. By attaching contrast agents or fluorescent tags to nanoparticles, they can enhance the imaging signal, allowing for improved diagnosis and more precise tumor margin definition during surgery.Despite the potential of nanoparticles in cancer therapy, several challenges need to be overcome before their routine clinical use. One major challenge is their safety profile since nanoparticles may interact with cells and tissues differently than larger particles, and their long-term effects on human health are unknown.Another challenge is manufacturing scalability and regulatory approval. Nanoparticles production often requires expensive and complex processes that may not be scalable for mass production. As such, regulatory agencies such as the FDA must enforce strict regulations to ensure the safety and effectiveness of nanoparticles before they can be approved for clinical use.In conclusion, nanoparticles are emerging as promising tools for cancer therapy, offering several advantages such as targeted drug delivery, improved pharmacokinetics, and better imaging and diagnosis. Although challenges exist, advancements in nanotechnology and improved regulatory approval processes promise significant benefits for cancer patients in the future.。
纳米材料靶向肿瘤相关巨噬细胞用于肿瘤成像及治疗的研究进展

纳米材料靶向肿瘤相关巨噬细胞用于肿瘤成像及治疗的研究进展郭峰亮;汤谷平;胡青莲【期刊名称】《浙江大学学报(医学版)》【年(卷),期】2017(046)002【摘要】肿瘤组织由肿瘤细胞和复杂的微环境构成.肿瘤相关巨噬细胞(TAM)是肿瘤微环境的重要组成成分,在肿瘤生长转移及微环境调控中扮演着重要的角色.近年来的研究表明,纳米材料作为新兴的技术平台,为肿瘤的成像和治疗提供了新的思路.一方面可以通过TAM成像为肿瘤发生、发展以及肿瘤治疗的效果提供直观的证据;另一方面通过TAM靶向杀伤或者促进TAM类型转化,调节肿瘤微环境的免疫抑制,提高肿瘤治疗效果.本文阐述了TAM的功能,同时对靶向TAM的纳米材料在肿瘤成像以及治疗方面的应用进行了综述.%Tumor tissues are composed of tumor cells and complicated microenvironment.Tumor associated macrophages (TAMs) as an important component in tumor microenvironment,play fundamental roles in tumor progression,metastasis and microenvironment regulation.Recently,studies have found that nanotechnology,as an emerging platform,provides unique potential for cancer imaging and therapy.With the nanotechnology,TAMs imaging presents direct evidence for cancer development,progression,and the effectiveness of cancer treatments;it also can regulate the immunosuppression of tumor microenvironment and improve therapeutic efficiency through TAMs targeted killing or phenotypic transformation.In this article,we illustrate thefunction of TAMs and review the latest development in nano-carriers and their applications in tumor associated macrophage targeting cancer imaging and therapy.【总页数】6页(P167-172)【作者】郭峰亮;汤谷平;胡青莲【作者单位】浙江工业大学生物工程学院,浙江杭州310032;浙江大学化学系,浙江杭州310028;浙江大学化学系,浙江杭州310028【正文语种】中文【中图分类】R730.5【相关文献】1.靶向肿瘤相关巨噬细胞的肿瘤治疗研究进展 [J], 夏莹;张岩;杨永广;刘文涛2.肿瘤相关巨噬细胞作用和靶向治疗的研究进展 [J], 王煦苏;徐娟(综述);贾雪梅(审校);3.纳米材料调控肿瘤相关巨噬细胞进行肿瘤免疫治疗的研究进展 [J], 徐睿; 刘晨光4.肿瘤相关巨噬细胞靶向治疗宫颈癌研究进展 [J], 陆杭铖;魏炜炜;陈继明;施如霞5.纳米载药系统靶向肿瘤相关巨噬细胞用于肿瘤治疗的研究进展 [J], 孙宏晨;李杏因版权原因,仅展示原文概要,查看原文内容请购买。
融合纳米囊泡在癌症治疗中的研究进展

综 述生命科学仪器 2023年第21卷/第6期22*共同通讯作者:张金凤(1987-年),女,博士研究生,副教授,主要研究领域:纳米生物医学,E m a i l :j f z h a n g@b i t .e d u .c n ㊂*胡敏(1985-年),男,博士研究生,助理研究员,主治医师,主要研究领域:肝癌的基础与临床应用研究和肝再生,E m a i l :h u -m i n 2019@jn u .e d u .c n ㊂基金项目:国家自然科学基金资助项目(项目编号:32001010),北京理工大学创新人才科技资助专项计划(项目编号:2022C X 01029),广东省基础与应用基础研究基金-面上项目(项目编号:2022A 1515011865),广东省医学科研基金-面上项目(项目编号:B 2022064)融合纳米囊泡在癌症治疗中的研究进展孙 梦1 樊玥芸1 杨佳妮1 胡 敏2* 张金凤1*(1.北京理工大学生命学院,北京1000812.暨南大学附属第一医院肝胆外科,广东广州510630)摘要 细胞外囊泡(E x t r a c e l l u l a r v e s i c l e s ,E V s)凭借其天然的生物学特性和后天获得的多功能优势,逐渐参与到机体调节的生理及病理过程中,成为疾病预防㊁治疗㊁评估的有力工具㊂癌症是目前危害人类健康的重大疾病之一,其临床治疗手段主要依靠放化疗㊁手术切除及新兴的免疫治疗㊂然而,以上治疗方式存在药物毒副作用强㊁生物利用度低㊁治疗成本昂贵㊁易复发等不足,极大影响癌症的治疗进展㊂因此,E V s 的出现为癌症治疗提供了更加安全㊁高效的治疗策略,但天然产生的单一E V s 产量低㊁自体靶向能力弱且载药能力低,在疾病治疗临床转化方面的发展受限㊂因此,建立普适性强且灵活性好的多功能融合纳米囊泡是扩大E V s 实际效用的关键㊂本综述系统地总结了融合纳米囊泡的制备方法㊁分类特点㊁在癌症治疗中的应用及面临的挑战,为开发有前途的精准医疗纳米平台提供理论指导㊂关键词 细胞外囊泡;外泌体;融合纳米囊泡;膜融合;癌症治疗R e s e a r c h A d v a n c e o f H y b r i d M e m b r a n e N a n o v e s i c l e s i n C a n c e r T h e r a p yM e n g S u n 1,Y u e y u n F a n 1,J i a n i Y a n g 1,M i n HU 2*,J i n f e n g Z h a n g1*(1B e i j i n g I n s t i t u t e o f T e c h n o l o g y ,B e i j i n g ,1000812T h e F i r s t A f f i l i a t e d H o s p i t a l o f J i n a n U n i v e r s i t y )ʌA b s t r a c t ɔE x t r a c e l l u l a r v e s i c l e s (E x t r a c e l l u l a r v e s i c l e s ,E V s )w i t h i t s n a t u r a l b i o l o gi c a l c h a r a c t e r i s t i c s a n d a c -q u i r e d m u l t i -f u n c t i o n a l a d v a n t a g e s ,g r a d u a l l y i n v o l v e d i n t h e b o d y 's r e g u l a t i o n o f t h e p h y s i o l o g i c a l a n d p a t h o l o gi -c a l p r o c e s s ,b e c o m e s a p o w e r f u l t o o l f o r d i s e a s e p r e v e n t i o n ,t r e a t m e n t ,a n d e v a l u a t i o n .C a n c e r i s o n e o f t h e m a jo r d i s e a s e s t h a t e n d a n g e r h u m a n h e a l t h a t p r e s e n t .I t s c l i n i c a l t r e a t m e n t m a i n l y r e l i e s o n r a d i o t h e r a p y an d c h e m o t h e r -a p y ,s u r g i c a l r e s e c t i o n a n d e m e r g i n g i mm u n o t h e r a p y.H o w e v e r ,t h e a b o v e t r e a t m e n t m e t h o d s s t i l l h a v e s h o r t c o m -i n g s s u c h a s s t r o n g d r u g s i d e e f f e c t s ,l o w b i o a v a i l a b i l i t y ,h i g h t r e a t m e n t c o s t ,a n d e a s y re c u r r e n c e ,w h i c h af f e c t t h e p r og r e s s o f c a n c e r t r e a t m e n t .Th e r e f o r e ,t h e e m e r ge n c e of E V s f o r c a n c e r t r e a t m e n t p r o v i d e s m o r e s e c u r e a n d e f f i c i e n t m e a n s .B u t t h e l o w y i e l d ,w e a k s e l f -t a rg e t i n g a b i l i t y a n d l o w d r u g l o a d i n g a b i l i t y o f n a t u r a l l y o c c u r r i n g s i n g l e E V sh a v e li m i t e d t h e d e v e l o pm e n t o f c l i n i c a l t r a n s l a t i o n o f d i s e a s e t r e a t m e n t .S o ,t h e e s t a b l i s h m e n t o f m u l t i -f u n c t i o n a l h y b r i d m e m b r a n e n a n o v e s i c l e s w i t h s t r o n g u n i v e r s a l i t y a n d g o o d f l e x i b i l i t y i s t h e k e y t o e x pa n d t h e p r a c -t i c a l u t i l i t y o f E V s .T h i s r e v i e w s y s t e m a t i c a l l y s u mm a r i z e s t h e p r e pa r a t i o n m e t h o d s ,c l a s s i f i c a t i o n c h a r a c t e r i s t i c s ,a p p l i c a t i o n a n d c h a l l e n g e s o f HMN V s i n c a n c e r t r e a t m e n t ,a n d p r o v i d e s t h e o r e t i c a l g u i d a n c e f o r t h e d e v e l o pm e n t o f p r o m i s i n g p r e c i s i o n m e d i c i n e n a n o pl a t f o r m s .ʌK e y wo r d s ɔe x t r a c e l l u l a r v e s i c l e ;e x o s o m e ;f u s i o n n a n o v e s i c l e s ;m e m b r a n e f u s i o n ;c a n c e r t r e a t m e n t 中图分类号:R 45 文献标识码:A D O I :10.11967/2023211204引言生物技术迅速崛起,细胞外囊泡(E x t r a c e l l u -l a r v e s i c l e s ,E V s )凭借着自身免疫原性低㊁可修饰性强㊁载药结构优良等多重天然优势,在临床前诊断㊁疾病治疗㊁预后评估等生物学医学应用中发挥着愈加重要的作用㊂分布于各种体液中的细胞外囊泡是活细胞分泌的天然囊泡样膜结构,其内部含有核酸(D N A 或R N A )㊁蛋白质㊁脂质,广泛参与着细胞间各种信息传递和物质交换[3]㊂E V s具有异质性,继承了亲本细胞的诸多特性,现阶段的研究综合分析了的E V s 的生物来源㊁分泌途径及尺寸大小等因素,并将其主要分为三大类:外泌生命科学仪器 2023年第21卷/第6期综 述23体(E x o s o m e s ,40-200n m )㊁微囊泡(M i c r o v e s i -c l e s ,200-2000n m )和凋亡小体(A p o p t o t i c b od ie s ,500-2000n m )㊂其中外泌体(E x o s o m e ,E x o)应用较为广泛,其发生过程较为明晰,制备方法比较成熟㊂通常情况下,研究者将外泌体的产生过程总结为细胞质膜的两次 内陷 :一是胞外物质与质膜接触结合后导致的第一次质膜内陷形成了早期内体,后逐步发展为晚期内体㊂随后晚期内体在胞内发生物质交换形成大量腔内囊泡(I L V s ),进一步形成多囊泡体(MV B ),MV B 内部包含大量I L V s 的过程被称为E x o 发生过程中的第二次内陷㊂最后MV B 或与溶酶体结合,发生降解,内含物在胞内重新循环;或与细胞质膜结合,释放出包含多种生物活性物的E x o [5]㊂微囊泡尺寸稍大,目前认为细胞内钙离子激发了质膜中磷脂的重新排布,使得细胞膜直接出芽释放出微囊泡,同时,m i c r o v e s i c l e s 内也含有多种细胞活性物质,其效用正逐步得到研究拓展㊂凋亡小体是在细胞凋亡过程中产生的,是胞外囊泡中尺寸最大的一类,其内部包含着大量破损的细胞器㊁核碎片等,但a p o pt o t i c b o d i e s 的产生机制及生物学作用仍需进一步的研究发展[6]㊂细胞外囊泡的生物发生过程如图1所示,产生的E V s 分布于各种体液中,通过受体与配体的相互结合㊁内吞途径以及膜融合等多样方式参与机体的各类调节㊂癌症是目前最为致命的疾病类型之一,发病比率逐年上升,严重危害着人类的生命健康㊂临床上治疗手段主要包括传统的化疗㊁放疗㊁手术治疗以及新兴的免疫治疗和靶向治疗,然而药物毒副作用强,生物利用度低;放疗靶向性差,易造成全身性损伤;手术治疗操作难度大,患者易复发;新兴的治疗手段成本高且存在一定的安全隐患㊂因此,亟需开发普适性强㊁安全性高㊁治疗效果佳的癌症治疗策略㊂纳米药物的出现极大地促进了疾病治疗的进展,并为癌症治疗新颖策略的开发带来了新希望㊂生物来源的纳米尺寸的细胞外囊泡具有良好的载药结构,可以极大程度降低药物的毒副作用㊁提高药物利用率;同时E V s 免疫原性低,具有较好的安全性;再者E V s 具有一定的靶向能力,如归巢靶向㊁趋炎靶向等都为开发更加安全高效的癌症治疗策略提供了可靠思路[12]㊂然而,天然单一细胞来源的E V s 在癌症的治疗过程中存在产量低㊁自身靶向性弱㊁载药量低等不足,基于此,融合纳米囊泡的出现极大的拓展了E V s 的应用范畴[13]㊂融合纳米囊泡(H y b r i d m e m b r a n e n a n o v e s i -c l e s ,HMN V s )通常指由天然分泌的或者人工合成的同源或异源膜等融合而成的囊泡㊂由于HMN V s 组成及来源广泛,制备方法多样,其解决了一些天然囊泡的固有问题,逐步成为天然单一囊泡的替代品并得到广泛研究发展㊂HMN V s 的优势主要总结为以下几点:(1)可大规模制备㊂相较于传统E V s 产量低㊁限制性强的不足,HMN V s 可通过多样的制备方法获得满足实验研究需求的足够产量㊂(2)靶向性强㊂囊泡融合,在 继承 双亲本细胞特性的同时,强化了双重靶向作用㊂(3)高生物安全性㊂制备成分明确,载生物药成分的HMN V s 减小了纯纳米药物的安全风险;载化药的HMN V s 提高了药物的生物利用度㊂(4)多功能性㊂打破了天然E V s 单一有限的作用限制,拓宽了HMN V s 的应用空间,提供了更多的治疗可能性㊂综上,本文主要总结了融合纳米囊泡的制备方法及分类特点,重点讲述了HMN V s 在癌症治疗中的应用,并提出了HMN V s 在目前临床转化应用中所面临的挑战和前景,旨在激发化学㊁生物工程㊁材料科学㊁纳米医学㊁药理学和临床医学等跨学科领域的广泛研究兴趣,为开发精准纳米医疗平台奠定理论基础㊂图1 细胞外囊泡的生物发生示意图F i g .1S c h e m a t i c d i a g r a m o f b i o ge n e s i s of e x t r a c e l l u l a r v e s i c l e1 融合纳米囊泡的制备策略具有自身膜结构特点及功能的物质如脂质体㊁细胞膜或细胞外囊泡等,通过适宜的方法被融合起来,构成完整结构单元,即称为融合㊂形成的融合结构单元同时具备参与融合物质的多种功能外,还强化了某些性能,如靶向性㊁诊疗一体化特综 述生命科学仪器 2023年第21卷/第6期24性等,发挥出更为突出的作用㊂随着融合纳米囊泡领域的迅速发展,标准化㊁可重复性好㊁损伤性小及融合率高的制备方法得到不断更新和丰富㊂HMN V s 的各种制备方法如图2所示㊂图2 融合纳米囊泡的制备方法示意图F i g .2S c h e m a t i c d i a g r a m o f p r e pa r a t i o n m e t h o d o f h yb r i d m e m b r a n e n a n o v e s ic l e s 1.1 物理制备策略 共挤压法是构建HMN V s最常见的方法,其通常是指多种物质基于微型挤压器等专业性仪器,借助挤压㊁推拉等外力作用,被迫通过孔径不同的滤膜滤孔,进而产生相互融合㊁特定尺寸的融合囊泡结构[15]㊂L i 等人利用共挤压法制备了一种融合囊泡(P -E V )可用于心肌缺血损伤的治疗㊂通过冻融法提取的血小板来源细胞膜(P MV )与差速离心法得到的间充质干细胞来源胞外囊泡(M S C-E x o),按照同等比例混合并依次经过孔径大小为400n m 和200n m 的聚碳酸酯多孔膜,最终得到尺寸约为138n m 的血小板-间充质干细胞融合囊泡,该杂化囊泡既保留了血小板的粘附蛋白靶向心脏损伤处的能力,又继承了间充质干细胞分泌活化因子促进血管再生的功能,提高了心肌缺血的治疗效果㊂过滤法与共挤压法的原理相同,经常借助自动装置提高制备效率,获得目标尺寸的纳米粒子[17]㊂冻融法除常被用于破碎细胞膜外,是另外一种常用的制备HMN V s 的简便方法㊂混合膜物质在温度的循环变化下发生破碎与重聚,S a t o 等人将等比例的R AW 264.7细胞来源的E V s 与人工合成且经P E G 修饰的脂质体混合,首先放入液氮中冷冻15分钟,取出后置于室温内解冻15分钟,反复循环多次,形成目标融合囊泡㊂该融合囊泡可以正常发挥药物递送及物质交换功能,并能借助脂质体的参与提升自身的载药量㊂冻融法虽然操作简单,但其形成的融合体直径尺寸通常不均一,需要进一步配合孵育㊁离心纯化㊁挤压控制等方法完善目标产物㊂同样,超声波也是一种基于打破-再重建的原理构建融合囊泡的方法,其操作简便,也常作为辅助性HMN V s 制备手段㊂在各类HMN V s 物理制备方法中,共孵育法是对质膜伤害性最小的一种制备方法,其通常是将不同膜物质混合在一起,在37ħ(或其他较高温度)下共同孵育一段时间,膜流动融合而形成融合囊泡㊂如L i n 等人制备了一种携带有基因编辑质粒的脂质体,并将其与人胚肾细胞H E K 293F T 细胞来源的E V s 按一定比例混合,37ħ孵育12小时,成功制备出可以高效表达C R I S P R /C a s 9系统的脂质体-人胚肾细胞融合体[19]㊂然而,仅依靠混合物质共孵育来制备HMN V s 不仅需要花费较多的时间,同时孵育过程易造成膜物质的聚集,导致形成的HMN V s 尺寸大小不均一,于是共孵育法也常作为辅助制备手段㊂1.2 化学诱导策略 化学诱导方法常借助化学试剂诱导促进膜物质相互融合来构建HMN V s ㊂在诸多化学试剂中,聚乙二醇(P E G )是细胞融合最常使用的辅助试剂,且不同相对分子量的P E G具有不同的功能或促融合效率㊂P i f f o u x 等人构建了一种生物响应性的融合囊泡㊂将人脐静脉内皮细胞来源的E V s 和脂质体混合,再经P E G 诱导后形成融合囊泡,其既保留了原有的生物特性,又提高了药物的递送效率㊂同时,该团队成员还探究了具有不同分子量的P E G 诱导膜融合的效率,发现在相同的膜融合构建条件下,P E G 8000的诱导效果最佳,这为该方法的后续使用提供了理论基础㊂P E G 诱导法通常配合其他的实验方法获得尺寸更理想的囊泡,M a 等人首先利用P E G 8000修饰了包载有光热剂的人工建构脂质体,随后将脂质体与具有靶向作用的血小板E x o 混合,紧接着利用100n m 滤膜的连续挤压混合物,得到尺寸均一的融合囊泡㊂制备的载药HMN V s 具有较好的光热转换效率,可用于肿瘤的光热治疗㊂1.3 生物转化策略 生物转化策略就是借助生物技术,包括基因转染㊁基因编辑及嵌合体生物技术等,开发出功能更为丰富㊁结构更加稳定的融合生命科学仪器 2023年第21卷/第6期综 述25纳米囊泡㊂研究发现,E x o 中的各类m i R N A s 可作为疾病诊断或预后评估的标志物,因此高效检测各类m i R N A s 的策略在临床中具有广阔的应用前景㊂包膜病毒的包膜可以协助病毒与细胞膜发生融合,进而促进病毒进入细胞进行增殖转化[22]㊂基于此,G a o 等人制备了一种病毒模拟囊泡(V i r -F V ),并利用囊泡与E x o 的融合,将检测E x o 中m i R N A s 种类的时间控制在两个小时之内㊂研究人员首先利用基因工程的方法制备了表达包膜病毒表面粘附蛋白的囊泡,随后与m i R N A 的标记分子共挤压,得到模拟病毒特性的囊泡V i r -F V ㊂然后V i r -F V 与E x o 表面过表达的唾液酸特异性结合并发生融合,融合后的V i r -F V 释放出其中的分子信号标记,发挥快速检测m i R -N A s 种类的作用㊂通过此检测手段对比肿瘤E x o与正常E x o 中m i R N A s 的种类,可以更好的发挥疾病前诊断与治疗后评估的作用,推动了E x o 临床转化应用的发展㊂制备策略优点缺点参考文献物理制备策略共挤压法融合率高;载药量高;尺寸合适;产量高操作复杂;膜物质重排;质膜损伤[16]冻融法操作简便;耗时短;成本低膜聚集;内容物泄露;分离纯化难[18]超声法操作简便;融合效率高;成本低结构破坏;载药量低尺寸控制难[24]嵌合体指的是借助核移植等生物技术合成的具有不同遗传性状的生物体(如组织㊁器官或人),其内部遗传物质发生较大变化[25]㊂于是,基于嵌合体技术制备的HMN V s 更接近自然分泌产生的细胞外囊泡,功能多样的同时具有更加显著的稳定性㊂例如:M a 和W e i 的团队创新性的利用嵌合体技术成功构建了可用于肿瘤免疫治疗的HMN -V s [26]㊂首先,研究人员利用核移植技术将肿瘤细胞的细胞核移植到去核的M 1型巨噬细胞中,随后通过进一步激活与培养形成了嵌合体细胞h -M P ,h -M P 自然分泌出有双重性状的嵌合体胞外囊泡(a MT-E x o s )㊂研究发现,a MT-E x o s 一方面可以靶向肿瘤,另一方面可以增强对肿瘤细胞的免疫杀伤作用㊂巧妙地使用嵌合体技术制备HMN V s,为制备功能更加优良㊁结构特性更加稳定的融合囊泡提供了新思路㊂综上所述,优缺点各异的HMN V s 制备方法显著增加了囊泡的产量,克服了E V s 在生物学应用及临床转化中的不足,具有广阔的应用前景㊂表1总结了上述HMN V s 制备策略的优缺点,研究人员可依据其选择合适的制备方法,进一步发挥HMN V s 的应用优势㊂表1 现阶段融合囊泡的制备策略及优缺点T a b .1A d v a n t a g e s a n d l i m i t a t i o n s o f t h e c u r r e n t m a j o r p r e p a r a t i o n t e c h n o l o gi e s o f H M N V s 物理制备策略共孵育法操作简便;作用条件简便;膜伤害性小;成本低质膜聚集;融合率低;耗时长;尺寸控制困难[19]化学诱导策略P E G诱导法融合率高;操作简便;成本低质膜聚集;潜在损伤及污染;尺寸控制困难[20,21]生物转化策略基因编辑法融合率高;载药量高;功能多样;应用广泛操作复杂;潜在生物安全问题;成本昂贵[23]嵌合体技术融合率高;载药量高;途径精准安全;制备可规模化操作复杂;成本昂贵;技术未成熟;伦理问题[26]2 融合纳米囊泡的分类HMN V s 在生理环境中更加稳定,循环时间延长,抗吞噬能力增强,特异性靶向能力增强,具有无穷的应用潜力㊂随着制备技术的日益完善,融合纳米囊泡的类型也逐渐增多,现阶段,研究以组成HMN V s 膜成分来源的不同,将其简要归纳为三大类:(1)同源或异源膜物质融合制备的HMN V s ;(2)细胞膜与脂质体融合制备的HMN -V s ;(3)嵌合体生物技术制备的HMN V s㊂2.1 同源或异源生物膜物质融合制备的HMN -V s 研究将均由细胞膜或均由细胞外囊泡融合构建的融合囊泡归纳为同源膜物质融合制备的HMN V s㊂研究发现,红细胞膜具有良好的生物相容性和较长的体内循环时间,因此常被用作药物的载体,并且衰老或损伤的红细胞可以促进抗原递呈细胞的摄取,进而可增强树突状细胞的免疫效应㊂于是H a n 等人将衰老的红细胞膜与肿瘤细胞(4T 1细胞)膜融合构建了HMN V s,随后HMN V s 表面的肿瘤抗原经抗原递呈细胞递呈,激活了树突细胞,最终发挥对肿瘤的免疫杀伤作用[24]㊂M e n g 的团队通过共挤压的方法先将4T 1综 述生命科学仪器 2023年第21卷/第6期26和B 16-F 10两种肿瘤细胞膜制备成均一的纳米囊泡,再将纳米囊泡融合成HMN V s,并将其应用于肿瘤的免疫治疗中㊂C h e n 等人将三种细胞膜来源的纳米囊泡进行融合,得到的HMN V s 可以发挥双重阻断C D 47/S I R P a 和P D-1/P D-L 1通路的作用,极大程度上防止了肿瘤的复发和转移㊂异源膜物质合成的HMN V s 则是指由细胞膜与细胞外囊泡融合制备的融合纳米囊泡,在实现结构稳定性的同时丰富了HMN V s 的效用㊂Z h a n g 等人融合了具有促再生作用的间充质干细胞外泌体(M S C-E x o s)与单核细胞膜,得到的HMN V s 在靶向受损心肌的同时还可以促进组织的再生,为心肌损伤修复提供了新颖的治疗策略㊂C h e n g 等研究人员将M S C-E x o s 与血小板膜杂交,制备的HMN V s 在减轻炎症的同时促进了血管生成,提高了心肌缺血再灌注损伤的治疗效果㊂2.2 细胞膜与脂质体融合制备的HMN V s 尽管天然细胞制备的HMN V s 已广泛应用于各种生物医学领域,但其载药量低㊁稳定性弱㊁制备工艺复杂等问题仍然阻碍着HMN V s 的进一步发展㊂幸运的是,脂质体的出现为HMN V s 的制备提供了新思路㊂脂质体是一种人工合成的小型囊泡,具有与E V s 类似的脂膜结构,其制备工艺成熟,载药性能优良,适应性强,现被逐步应用于HMN -V s 的构建㊂将天然细胞来源的生物膜与人工合成的脂质体结合,提升了HMN V s 的载药量㊁增强了HMN V s 的生物相容性,也赋予了HMN V s 的更多的功能㊂例如:巨噬细胞来源的E V s 具有靶向肿瘤的作用,R a y a m a j h i 等人将制备的脂质体同巨噬细胞来源的E V s 相结合制备了HMN V s ,并进一步向融合囊泡中装载了临床药物阿霉素,减少化药毒副作用的同时,有效提高了乳腺癌的治疗效果㊂L i n 等人制备了可以显著提高基因编辑效率的融合纳米囊泡[19]㊂研究人员将H E K 293F T 来源的E x o s 与脂质体共孵育初步制备了HMN V s ,然后将表达C R I S P R /C a s 9的质粒成功的装载入融合囊泡中,该HMN V s 可以被M S C s 成功内吞,并操纵靶基因在M S C s 中高效表达㊂2.3 嵌合体生物技术制备的HMN V s 与传统HMN V s 的制备方式不同,嵌合体技术制备的HMN V s 所含遗传信息发生了融合变化,以自然分泌的方式产生出的融合囊泡具有更加稳定的结构和优良的生物相容性,同时也具有更加自然贴切的功能㊂例如M a 和W e i 的团队联合构建的兼具肿瘤细胞归巢靶向作用以及巨噬细胞肿瘤杀伤作用的嵌合体HMN V s (a MT-E x o s)有效地激活了T 细胞,激发了肿瘤的免疫杀伤作用㊂图3为利用人体免疫细胞与肿瘤细胞制备嵌合体,进而自然分泌得到嵌合体HMN V s 的过程㊂现阶段,依据嵌合体技术创新性开发制备嵌合体HMN -V s,为肿瘤个性化㊁多级联治疗策略的开发提供了便利[26]㊂图3 用于进行肿瘤治疗的嵌合体融合囊泡的制备示意图F i g .3 S c h e m a t i c d i a g r a m o f t h e p r e pa r a t i o n o f c h i m e r i c f u s i o n v e s i c l e s f o r t u m o r t h e r a p y3 融合纳米囊泡在癌症治疗中的研究进展癌症严重影响着人们的生命健康,传统的治疗方法存在着全身毒副作用强㊁成本高㊁易复发等问题,虽然细胞外囊泡在癌症的治疗中取得了一定的进展,但仍需解决其产量低㊁靶向能力弱㊁载药量低㊁作用效果单一等不足[7]㊂因此,亟需在改进传统治疗方法的同时开发新的治疗策略,而具有创新性的HMN V s 凭借自身多重优于天然E V s 的特点,在癌症治疗中显现出愈加突出的潜力㊂为了克服腹腔热灌注化疗(H I P E C )治疗转移性腹膜癌(m P C )过程中存在的药物递送障碍和高耐药性的问题,L v 等人构建了由热敏脂质体和基因工程外泌体融合的融合纳米囊泡(gE T L N P s ),并且在g E T L N P s 中同时加载了治疗药物多西他赛(D T X )和粒细胞-巨噬细胞集落刺激因子(GM-C S F ),以配合H I P E C 治疗[36]㊂最终HMN V s 与H I P E C 联合治疗策略显著增加了药物的穿透力,提高了药物利用度,同时促进巨噬细胞向M 1的重极化,达到抑制C T 26来源的m P C 异种移植瘤生长的效果㊂Z h o u 的团队通过融合肿瘤来源的E V s 膜和磷脂膜合成了稳定的杂合脂质纳米囊泡(L E V s ),实现了高效递送s i R N A 到肿瘤部位的目的,推动了肿瘤的基因治疗的研究进生命科学仪器 2023年第21卷/第6期综 述27展[37]㊂值得关注的是,L E V s 不同于常见的脂质载体,其在递送核酸时主要通过高尔基体和内质网通道,绕过内吞途径,完成溶酶体逃逸,将s i R -N A 精确地转运到H C C 肿瘤细胞中,从而促进瘤内积累和s i R N A 的高效转染,大大增强抑癌效果㊂此外,针对产生耐药性的卵巢癌,L i 等人成功制备了脂质体与肿瘤外泌体融合的载药HMN -V s ,一方面借助肿瘤细胞膜过表达的C D 47进行肿瘤归巢靶向,减少纳米颗粒被吞噬的风险;另一方面,结合s i R N A 干扰疗法包载m i R 497及治疗药物雷公藤甲素,实现了阻断耐药通路和杀伤肿瘤细胞的双重功效㊂除了化疗和基因治疗,HMN V s 还常用于肿瘤的光热治疗(P T T )及免疫治疗㊂例如,J i a n g 等人在M C F-7细胞膜和红细胞膜融合构建的HMN V s 中加入了光热剂黑色素纳米粒子,增强光热转换效率的同时也提高了靶向肿瘤的能力,延长了药物的血液循环时间,极大地增加了对肿瘤的杀伤作用㊂同时,为了提高肿瘤P T T 治疗效果,B u 等人将四氧化三铁(F e 3O 4)包裹在血小板细胞膜与肿瘤干细胞膜融合制备的HMN V s 中,降低了纳米粒子被清除的风险,提高了肿瘤靶向性,同时结合核磁共振成像,增强了P T T 治疗头颈部鳞状细胞癌的疗效[35]㊂近年来,研究人员为了提高癌症的治疗效果通常会采取联合治疗策略,也取得了一定的突破性进展㊂W a n g 等人实现了化疗与P T T 的联合治疗㊂首先利用红细胞(R B C )膜与B 16-F 10质膜构建了融合R B C-B 16,接着制备了化疗药物阿霉素(D O X )和P T T 剂C u S 纳米颗粒的混合纳米颗粒D C u S ,随后D C u S N P s 被R B C-B 16融合囊泡进一步包裹,形成D C u @[R B C-B 16]N P s ,该HMN V s 的制备过程如图4A 所示㊂与未涂覆的C u S N P s 相比,仿生HMN V s 表现出更长的循环寿命和更好的同源靶向能力,表现出显著的黑色素瘤化学光热治疗效果㊂目前正在进行临床和临床前研究的免疫疗法正逐步改变着癌症治疗方式,其可能通过激活肿瘤部位的抗肿瘤免疫细胞群和重塑肿瘤免疫抑制微环境来实现根除癌症的目的㊂研究发现,P T T 可以诱导肿瘤的免疫治疗,如M a 等人将血小板膜与光热敏感脂质体融合,并向其中嵌入了铁铵(F A C )和葡萄糖氧化酶(G O x ),成功构建了可用于级联协同抗癌治疗的双靶向融合纳米囊泡(F G @P E L )㊂其中,脂质体的双分子层中加入了光热剂C y pa t e ,其引发的P T T 可以通过上调肿瘤浸润T 细胞和增加I N F γ的分泌来诱导体内免疫应答,进一步增强了G O x 和F A C 引起的铁死亡敏感性, 多管齐下 ,协同发挥抗肿瘤作用㊂此外,使用革兰氏阴性菌分泌的各种膜组件如外膜囊泡(OMV )或借助自体肿瘤细胞膜等,都可以作为肿瘤抗原或佐剂起到直接激活免疫系统的作用,实现个性化免疫治疗㊂基于此,Z h a n g 的团队将肿瘤细胞膜(m T )与细菌OMV 融合,开发出新型多功能融合纳米囊泡m T OMV ,可同时增强双侧肿瘤模型的先天性和适应性免疫反应[42]㊂同时,合成的m T OMV 具有良好的生物相容性,不仅能抑制原发肿瘤生长,还能抑制肿瘤肺转移,具有巨大的临床转化应用潜力(图4B )㊂图4 融合囊泡用于癌症治疗的制备及应用示意图㊂A :融合纳米囊泡用于化疗与P T T 的肿瘤联合治疗;B :融合纳米囊泡用于肿瘤的免疫治疗㊂F i g .4S c h e m a t i c d i a g r a m o f p r e p a r a t i o n a n d a p pl i c a -t i o n o f f u s i o n v e s i c l e s i n c a n c e r t h e r a p y.A :F u s i o n n a n o v e s i c l e s f o r t u m o r c o m b i n a t i o n t h e r a p y w i t h c h e m o -t h e r a p y an d P T T ;B :F u s i o n n a n o v e s i c l e s f o r i mm u n o t h e r -a p y of t u m o r s .4 融合纳米囊泡面临的挑战融合纳米囊泡(HMN V s )作为天然E V s 极富前途的生物医用替代品,已经成为靶向输送不同诊断试剂和治疗药物的个性化高效纳米平台㊂值得注意的是,与天然E V s 相比,HMN V s 整合了囊泡本身的生物学特性及人工修饰的丰富功能,具。
高效肿瘤靶向纳米药物_纳米特性协同与功能集成_申有青

高效肿瘤靶向纳米药物:纳米特性协同与功能集成申有青*,唐建斌,隋梅花,刘祥瑞浙江大学生物纳米工程中心、化学工程与生物工程学系,杭州,310027*Email: shenyq@肿瘤靶向纳米药物的体内输送过程,是一个从血液循环系统(C irculation)向肿瘤组织内蓄积(A ccumulation)、渗透(P enetration)、细胞内吞(I nternalization)、药物释(R elease) 放等五步“级联”的CAPIR 过程,只有每步都有高的效率时,纳米药物才能有高的输送效率和疗效。
CAPIR输送过程各步对纳米药物的主要特性(表面电荷性质、尺寸、稳定性)的要求不尽相同甚至相反,如何协同纳米药物的纳米特性使其满足各步的要求,是提高肿瘤纳米药物药效的关键。
本文将简要介绍肿瘤药物输送的CAPIR过程和2R2SP 纳米载体,以及作者在利用电荷翻转和系统组装方法、实现载体的功能及纳米特性协同性方面的工作。
关键词:肿瘤纳米药物;靶向输送过程;功能集成;纳米特性协同性参考文献[1] Sun Q.; Sun X.; Shen Y.Q.; Zhang Y.L. et al. Adv. Mater. Submitted.[2] Jin EL, Zhang B., Sun XR, Zhou ZX, Ma XP, Sun QH, Tang JB, Shen Y., Van Kirk EA, Murdoch WJ,Radosz M, J. Am. Chem. Soc. 2013, 135:933-40.[3] Sun Q.; Radosz M.; Shen Y. J. Controlled Release 2012, 164, 156-169Nanocharacter Synergization and Function Inegration for EffectiveCancer NanomedicineYouqing Shen*, Jianbin Tang, Meihua Sui, and Xiangrui Liu Center for Bionanoengineering and State Key Laboratory of Chemical Engineering, Department of Chemical and Biological Engineering, Zhejiang University, Hangzhou, China310027A cancer nanomedicine delivering active (free) drugs to the cytoplasm of cancer cells in a solid tumor must go through a cascade of five steps: circulation in the blood compartments, accumulation in the tumor from the hyperpermeable tumor vessels, penetration into the deep tumor tissue to reach all tumor cells and subsequent internalization by them, and finally intracellular drug release, or the ‘CAPIR’ cascade. The key to maximizing the efficacy is to design a nanosystem capable of fully accomplishing the CAPIR cascade -missing any step would make it unable to deliver active drug to all tumor cells, diminishing therapeutic efficacy and prognosis. Herein, we report our work on synergizing the nanocharacters of nanocarriers to integrate the needed functions for fulfilling the CAPIR process efficiently.。
写一篇纳米材料在肿瘤治疗应用中的英语综述

写一篇纳米材料在肿瘤治疗应用中的英语综述Nanotechnology has revolutionized the field of cancer therapy and has emerged as a promising tool in the diagnosis and treatment of cancer. Nanomaterials, in particular, have shown tremendous potential in the therapy of cancer due to their unique physicochemical properties and biocompatibility.Various types of nanomaterials such as metal nanoparticles, carbon nanotubes, and liposomes have been explored for cancer treatment. Metal nanoparticles such as gold and silver nanoparticles have attracted significant attention due to their ability to selectively accumulate in tumors and their photothermal properties. When exposed tonear-infrared radiation, these particles generate heat that can kill cancer cells selectively. Carbon nanotubes, on the other hand, have been shown to have excellent drug-loading capacity and can be used for targeted drug delivery. Liposomes, another type of nanomaterial, are biocompatible and can encapsulate both hydrophilic and hydrophobic drugs.Nanomaterials hold several advantages over conventional cancer therapies such as chemotherapy and radiation therapy. They can specifically target cancer cells without causing toxicity to healthy tissues,reducing side-effects. Additionally, they can overcome the problem of drug resistance, which is a significant challenge in cancer therapy. Nanomaterials can improve the pharmacokinetics of drugs, leading to higher drug concentration at the tumor site and therefore improving efficacy.Despite the vast potential of nanomaterials in cancer therapy, there are several challenges that need to be overcome for their successful clinical translation. One key challenge is the toxicity of certain nanomaterials, such as carbon nanotubes, which can accumulate in organs such as the liver and spleen. Additionally, long-term safety studies need to be conducted to understand the potential adverse effects of nanomaterials.In conclusion, nanomaterials have emerged as a promising tool in the treatment of cancer, providing a new avenue for the diagnosis and treatment of this devastating disease. However, further research and development are required to overcome the challenges and translate nanomaterial-based therapies into clinical practice.。
喜树碱聚前药纳米粒子包埋吲哚箐绿用于化疗与光动力联合抗肿瘤治疗

喜树碱聚前药纳米粒子包埋吲哚箐绿用于化疗与光动力联合抗肿瘤治疗张文佳;胡祥龙【期刊名称】《激光生物学报》【年(卷),期】2016(025)006【摘要】The therapeutic efficiency of tumors based on single chemotherapy is often limited,which would probably result in further drug resistance.Hence,the combination of chemotherapy with other therapeutic methods,such as photothermal therapy and photodynamic therapy has exhibited some advantages and attracted more and more attention.In the current work,reduction-responsive polyprodrug amphiphiles,PEG-b-PCPTM,were employed to encapsulate one kind ofphotosensitizer,indocyanine green (ICG),affording therapeutic polyprodrug nanoparticles,***********************'sreductive microenvironment,controlled CPT release was realized to activate its therapeutic efficiency,meanwhile,photodynamic therapy was achieved in the presence of ICG.Thus the combination of chemical and photodynamic therapy was given based on ICG@PEG-b-PCPTM,exhibiting excellent antitumor effect.%肿瘤单一药物化疗的效果往往达不到理想的肿瘤治疗效果,且容易导致耐药.因此,肿瘤的药物化疗与其他的抗肿瘤治疗方法,如光热治疗和光动力治疗等,联合治疗具有明显优势,并受到越来越多的关注.本工作构建了一种还原性响应的新型智能纳米体系,采用喜树碱聚前药两亲分子(PEG-b-PCPTM)物理包埋光敏剂吲哚箐绿(ICG).在肿瘤细胞的还原性微环境中,控制释放化疗药物喜树碱,激活化疗;同时,光敏剂ICG用于光动力治疗,从而实现化疗与光动力的联合治疗,表现出良好的抗肿瘤活性.【总页数】5页(P519-522,530)【作者】张文佳;胡祥龙【作者单位】华南师范大学生物光子学研究院,激光生命科学研究所暨激光生命科学教育部重点实验室,广东广州510631;华南师范大学生物光子学研究院,激光生命科学研究所暨激光生命科学教育部重点实验室,广东广州510631【正文语种】中文【中图分类】Q225【相关文献】1.叶酸介导的聚乙二醇-聚苹果酸-喜树碱聚合物前药的制备和性质初探 [J], 李伟;段晓;范黎;乔友备;吴红2.血红蛋白微粒装载吲哚菁绿用于光动力治疗 [J], 郑伟伟;仝维鋆3.吲哚箐绿荧光成像在甲状旁腺及甲状腺外科手术中的应用进展 [J], 张帆;何生;陈文青;姜增誉4.纳米炭与吲哚箐绿荧光两种示踪法对甲状腺微小乳头状癌术中淋巴结清扫和甲状旁腺保护作用研究 [J], 尚兴国; 张雪棉; 岳秀杰; 孙晋发; 徐凯5.吲哚箐绿衍生物结合荧光腹腔镜定量探测系统在胃肠肿瘤显像中的应用 [J], 郑晓明;刘琼;邵军;王娟丽;朱凝;赵灵智;魏波因版权原因,仅展示原文概要,查看原文内容请购买。
芹菜素丝素蛋白纳米粒的制备及其安全性、抗肿瘤活性研究
芹菜素丝素蛋白纳米粒的制备及其安全性、抗肿瘤活性研究作者:季鹏张进香王祥龙葛健文黄海琴来源:《中国药房》2022年第01期摘要目的制備芹菜素丝素蛋白(API@SF)纳米粒,并评价其安全性和抗肿瘤活性。
方法采用纳米沉淀法制备API@SF纳米粒,并对其形态、粒径、Zeta电位、载药量、体外释放等进行表征;采用溶血实验和HE染色法评价该纳米粒的安全性;采用MTT法评价该纳米粒对小鼠乳腺癌4T1细胞的抑制作用。
结果本研究所制得的API@SF纳米粒呈类球形,粒径分布均匀,平均粒径为406.61 nm,多分散性指数为0.154,Zeta电位为-18.4 mV,平均载药量为5.20%。
体外释放结果显示,该纳米粒在pH 5.0的释放介质中释放速率相对较快,在pH 7.4的释放介质中释放速率相对较慢。
溶血实验和HE染色结果显示,该纳米粒具有良好的生物相容性。
MTT实验结果显示,API@SF纳米粒对4T1细胞的抑制作用显著高于API原料药(P关键词芹菜素;丝素蛋白;纳米粒;制备;安全性;抗肿瘤活性中图分类号 R944 文献标志码 A 文章编号 1001-0408(2022)01-0058-06DOI 10.6039/j.issn.1001-0408.2022.01.10ABSTRACT OBJECTIVE To prepare apigenin silk fibroin (API@SF) nanoparticles and to evaluate their safety and anti-tumor activity. METHODS API@SF nanoparticles were prepared by nanoprecipitation method, and their morphology, particle size, Zeta potential, drug loading amount and in vitro release were characterized. The safety of nanoparticles was evaluated by hemolysis test and HE staining. MTT assay was adopted to evaluate inhibitory effects of API@SF nanoparticles on breast cancer 4T1 cells in mice. RESULTS The prepared API@SF nanoparticles were spherical with uniform distribution. The average particle size was 406.61 nm, the polydispersity index was 0.154, the Zeta potential was -18.4 mV, and the average drug-loading amount was 5.20%. The in vitro release results showed that the release rate of the nanoparticles was relatively fast in the release medium of pH 5.0 and relatively slow in the release medium of pH 7.4. Results of hemolysis test and HE staining showed that the nanoparticles had good biocompatibility.Results of MTT assay showed that the inhibitory effect of API@SF nanoparticles on 4T1 cells was significantly higher than that of API raw materials (PKEYWORDS apigenin; silk fibroin; nanoparticles; preparation; safety; anti-tumor activity癌症作为一种长期困扰人类的恶疾,其致死率仅次于心血管类疾病;且由于肿瘤细胞具有强大的代谢补偿和侵袭转移能力,导致在其治疗过程中会频繁复发并累及多个器官,严重降低了患者的生活质量[1]。
生物聚合物纳米微粒对人肺癌细胞凋亡的作用

Байду номын сангаас
双氢青 蒿素 (DHA)是青蒿 素的衍 生物 ,是从 天 然 药物青篙 中提取 的。DHA是 一类 临床奏效快 ,治愈 率 高且毒性低 的抗疟疾药物 ,尤其对易 耐药 的脑型疟 、 恶 性疟有效 。研 究表 明 ,DHA对 于各 种肿瘤 细胞 系的 癌 症 ,还具有抗 肿瘤活性 ,能促使肿瘤细 胞凋亡 [1-2]。 然 而 ,DHA治疗恶性 肿瘤方面 也存 在一些 问题 ,例如 在 溶液 中或血液 中溶解 度差 ,生 物利用度 低 , 口服 活 性 不佳 ,血 浆半衰 期短 ,从 而导致 在治疗过 程 中使 用 剂 量和次数 的增加 [ 。可 以通过制 备 DHA纳米微 粒 来增 加 DHA体外溶 出度 ,延长 释放 时间 ,提高其生物 利 用率 ,解决 DHA单独 给药 时存 在的问题。在前期研 究 中 ,作者 开发 了静 电场 系统制备 生物 聚合 物纳米 颗 粒 的方法 5。本 实验 采用 的肺腺 癌 A549细胞 是 目前 肺 癌研究 常用 的一种肺腺 癌细胞 系 ,通过制 备纳米 微 粒 作 为载体 来 包覆 DHA,探讨 DHA的生物 聚合物 纳 米 微 粒 的理化 性质 以及 对人 肺腺 癌 A549细胞 凋亡 作 用 的 影 响 。
浙江临床 医学2018年2月第 2O卷第 2期
· 249 ·
生 物聚合物纳米微粒对 人肺癌细胞凋亡的作用
孙倩 杨 丽君 周欢琴 ★
【摘 要 】 目的 制备包覆双氰青蒿素 (DHA)的纳米微粒,探讨DHA的生物聚合物纳米微粒的理化性质以及对人肺腺癌A549细胞凋亡
NANOPARTICLE CANCER THERAPY

专利名称:NANOPARTICLE CANCER THERAPY发明人:Ivan Mark KEMPSON,Tyron JamesTURNBULL申请号:US16758792申请日:20181026公开号:US20210000751A1公开日:20210107专利内容由知识产权出版社提供专利附图:摘要:Methods of potentiating chemotherapy or radiotherapy are disclosed. The methods comprise administering to a subject in need of chemotherapeutic orradiotherapeutic treatment an effective amount of a composition comprisingbiocompatible nanoparticles, particularly gold nanoparticles, under conditions in which the nanoparticles alter one or more cell regulatory mechanisms in cells in which the nanoparticles are localised or other cells. Then one or more doses of a chemotherapeutic or radiotherapeutic treatment are administered to the subject either concurrently with or after the nanoparticles have altered the one or more cell regulatory mechanisms in the cells in which the nanoparticles are localised or other cells. Also disclosed are methods of enhancing the effects of chemotherapy or radiotherapy on a cell population, methods of increasing the amount of strand breaks in DNA in a cell, and methods of inducing cancer cell death.申请人:UNIVERSITY OF SOUTH AUSTRALIA地址:Adelaide AU国籍:AU更多信息请下载全文后查看。
Nanomedicine for cancer therapy

Nanomedicine for cancer therapy癌症是目前世界上最为常见的疾病之一,而其中肺癌、胃癌、肝癌和乳腺癌等常见癌症的治疗难度一直是医学界攻坚的重点。
传统的化疗、放疗和手术治疗在治疗癌症时存在许多的局限性和副作用,而纳米医学技术的出现,为癌症治疗带来了全新的可能性。
本文将会介绍纳米医学在癌症治疗中的应用,重点介绍了纳米药物在癌症治疗中的作用以及其未来的发展前景。
一、纳米技术在癌症治疗中的应用纳米技术是一种用于设计、开发和应用纳米尺寸范围内的物质的科学。
纳米尺寸的物质具有比毛细血管更小的直径,能够穿过血管壁进入血流和细胞,并定向传递到肿瘤组织中。
基于这一优势,纳米技术可以用于癌症的诊断和治疗。
因此,纳米技术在癌症治疗中的应用越来越广泛,包括纳米诊断技术、纳米药物治疗和纳米光热治疗。
二、纳米药物在癌症治疗中的作用纳米药物具有许多优势,其尺寸和形状使其能够在体内逃脱免疫系统的攻击,并能够定向传递到肿瘤组织中,最大限度地减小了对健康组织的损伤,降低了副作用的发生率。
纳米药物具有高生物利用度和靶向性,这是传统药物所没有的,能够针对癌症的生物学特性,如肿瘤细胞表面的受体、转运蛋白等特性,针对性地进行选择性的作用,并高精确度地释放药剂,达到更好的疗效。
同时,纳米药物因为其特殊性质,还能够被选择性地富集在肿瘤组织中,从而增加药物的作用浓度,使治疗效果得到最大的提升。
纳米药物技术的应用范围很广,主要涉及到化学疗法、光动力疗法、磁动力疗法和放射性粒子疗法等多种方法。
使用铁氧化物纳米颗粒可以使药物在肿瘤处局部富集,使得药剂的活性提高了将近40%。
其中热敏纳米粒子则可以通过极短的激光作用将肿瘤组织瞬间烧灼,达到不需手术切除的治疗效果。
此外,美国食品和药品管理局还批准了纳米载药与化学疗法结合的药物治疗模式。
三、未来纳米药物的应用前景纳米技术的应用仍处于快速发展期,未来纳米药物会在路径选择、药物分子设计、体内监测、细胞靶向、自动调节和纳米机器人等多个方面进行技术突破和创新,为癌症研究和治疗开辟更为广阔的前景。
纳米给药系统的抗肿瘤药物联合治疗策略

纳米给药系统的抗肿瘤药物联合治疗策略∗叶鹏;张文典;杨坦;盖永康;项光亚【期刊名称】《医药导报》【年(卷),期】2016(035)007【摘要】Combination chemotherapy and nanoparticle drug delivery are two promising strategies in cancer treatment. The use of multiple therapeutic agents in combination provides synergistic effects among different drugs against cancer cells and suppresses drug resistance through distinct mechanisms of action.Nanocarriers can improve anti-tumor effects of drugs and reduce systemic toxicity through delivering drugs into the tumor tissue specially. Recently, many studies are aiming to encapsulate multiple agents into nanocarriers to optimize the anti-tumor effects. In the present review, the recent advances of nanoparticle platforms applied with co-delivering two or more drugs were summarized and the various combination strategies based on nanoparticles in oncology were discussed.%药物联合治疗和纳米给药系统的应用在肿瘤药物治疗领域均展现出广阔的前景。
纳米粒作为抗肿瘤药物载体的研究进展(英文)

纳米粒作为抗肿瘤药物载体的研究进展(英文) Yingying Sun;Huaqing Lin;Chuqin Yu;Suna Lin【期刊名称】《中德临床肿瘤学杂志:英文版》【年(卷),期】2014(0)10【摘要】Nanoparticles drug delivery system has sustained and controlled release features as well as targeted drug delivery, which can change the characteristics of drug distribution in vivo. It can increase the stability of the drug and enhance drug bioavailability. The selective targeting of nanoparticles can be achieved through enhanced permeability and retention effect and a conjugated specific ligand or through the effects of physiological conditions, such as pH and temperature. Nanoparticles can be prepared by using a wide range of materials and can be used to encapsulate chemotherapeutic agents to reduce toxicity, which can be used for imaging, therapy, and diagnosis. In this research, recent progress on nanoparticles as a targeted drug delivery system will be reviewed, including positive-targeting, negative-targeting, and physicochemical-targeting used as anticancer drug carriers.【总页数】5页(P489-493)【关键词】药物载体;纳米粒子;抗癌;靶向给药系统;纳米颗粒;生物利用度;生理条件;稳定性【作者】Yingying Sun;Huaqing Lin;Chuqin Yu;Suna Lin【作者单位】Department of Guangdong Pharmacy College, Guangdong Key Laboratory for New Pharmaceutical Dosage Forms【正文语种】中文【中图分类】TQ460.4;TB383【相关文献】1.纳米粒作为抗肿瘤药物载体的研究进展 [J], 林苏娜(综述);林华庆(审校)2.PLGA纳米粒抗肿瘤药物载体的研究进展 [J], 李方园;姜永莉;成颖3.纳米粒作为抗肿瘤药物载体的研究进展 [J], 章怡彬;梁文权;高建青4.普鲁兰多糖纳米粒作为靶向抗肿瘤药物载体的研究进展 [J], 郏亚静;南文滨;秦靖雯;魏向娟;郭双;李武山;徐佳迪;解丽芹;陈红丽5.多功能化乳酸-羟基乙酸共聚物纳米粒作为抗肿瘤药物载体研究进展 [J], 陈红丽;秦靖雯;南文滨;王永学;魏向娟;郏亚静因版权原因,仅展示原文概要,查看原文内容请购买。
Nanotechnology in Diagnosis: A Review

Nanotechnology in Diagnosis: A Review Tenderwealth Clement Jackson;Bernard Opatimidi Patani;Daniel Effiong Ekpa【期刊名称】《纳米粒子(英文)》【年(卷),期】2017(6)3【摘要】The fast pace of today’s world has presented several challenges in the area of healthcare. Depression, hypertension, diabetes, cancers and several infectious diseases are just some of the common outcomes associated with the high speed stress-filled lifestyle. Early diagnosis has been the goal for prompt arrest and management of these health conditions. This has been a challenge in recent times. However, great scientific advancement with improved potential in medical diagnosis has equally been a giant stride in times like these. Early disease detection even before symptoms’ presentation, improved imaging of i nternal body structure, as well as ease of diagnostic procedures, have been developed with the help of a new branch of laboratory medicine termed nanodiagnostics. Use of microchips, biosensors, nanorobots, nano identification of single celled structures, and microelectromechanical systems are current techniques being developed for use in nanodiagnostics. This piece of write up takes a panoramic view of available nanotechnological advances in current use for medical diagnosis and projecting into future possibilities and potentials for an improved health care delivery.【总页数】10页(P93-102)【关键词】Nanodiagnostics;Biosensors;Microelectromechanical;Systems 【作者】Tenderwealth Clement Jackson;Bernard Opatimidi Patani;Daniel Effiong Ekpa【作者单位】Department of Pharmaceutics & Pharmaceutical Technology, University of Uyo, Uyo, Nigeria;Medical Department, Nigerian Agip Oil Company, Port Harcourt, Nigeria【正文语种】中文【中图分类】R73【相关文献】1.Center of nanotechnology for cancer diagnosis and treatment launched in Tianjin [J],2.Diagnosis and Differential Diagnosis of Rhabdoid Meningioma: One Case Report and Literature Review [J], Huixia Han;Pine Du;Yan Zhang;Yongjian Deng3.Nanotechnology in Cancer Diagnosis and Treatment [J], Somaia Said Abd E1-Karim;Magdy Ibrahim E1-Zahar;Manal Mohamed Anwar;4.Nanotechnology for diagnosis and therapy of rheumatoidarthritis:Evolution towards theranostic approaches [J], Junkai Zhao;Xuan Chen;Kwun-Hei Ho;Chao Cai;Cheuk-Wing Li;Mo Yang;Changqing Yi5.Nanotechnology and Nanomedicine: A Promising Avenue for Lung Cancer Diagnosis and Therapy [J], Wei Yin;Feng Pan;Junjie Zhu;Junwu Xu;Diego Gonzalez-Rivas;Meinoshin Okumura;Zhiyong Tang;Yang Yang因版权原因,仅展示原文概要,查看原文内容请购买。
纳米技术在肿瘤治疗应用中的研究进展

纳米技术在肿瘤治疗应用中的研究进展张志凯;朴相浩;毕向军【期刊名称】《实用肿瘤学杂志》【年(卷),期】2012(26)2【摘要】肿瘤纳米治疗是一种新兴的肿瘤治疗手段,它是以纳米颗粒为载体,运载化疗药物,细胞因子或抗体等对肿瘤细胞进行靶向治疗,能够在提高疗效的同时减少治疗所带来的副作用.本文就近年来肿瘤纳米治疗的研究进展作一综述.%Nano therapy is a new treatment method for tumor. It is a nanoparticle as the carrier, carrying chemotherapy drugs, cytokines or antibody targeting therapy for tumor cells. It can improve the therapeutic effect and decrease the side effects. The research advancement of nano therapy for tumor in recent years will be summarized in this article.【总页数】5页(P178-182)【作者】张志凯;朴相浩;毕向军【作者单位】广州复大肿瘤医院,广州,510305;广州复大肿瘤医院,广州,510305;广州复大肿瘤医院,广州,510305【正文语种】中文【中图分类】R730.5【相关文献】1.纳米材料和纳米技术在肿瘤放疗增敏中的研究进展 [J], 魏常博;余东升;2.自杀基因在肿瘤治疗应用中的研究进展 [J], 杨章孺;吴敬波3.记忆性干细胞样T细胞在肿瘤治疗应用中的研究进展 [J], 邵洁;刘宝瑞4.CSE1L在恶性肿瘤中的表达及治疗应用研究进展 [J], 马晨博5.ATP1A1基因在肿瘤中的功能及治疗应用研究进展 [J], 冯晓异;李蓉涛因版权原因,仅展示原文概要,查看原文内容请购买。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Nanoparticles in cancer therapy and diagnosis ☆Irène Brigger,Catherine Dubernet,Patrick Couvreur ⁎University of Paris –Sud XI,UMR CNRS 8612,Faculty of Pharmacy,5rue J.B.Clément,92296Châtenay-Malabry,Francea b s t r a c ta r t i c l e i n f o Article history:Received 8January 2002Accepted 10May 2002Available online xxxxKeywords:Nanoparticles Nanospheres NanocapsulesConventional or long-circulating carriers Passive or active tumor targeting EPR effectMultidrug resistance (MDR)Oligonucleotide delivery Tumor imagingNumerous investigations have shown that both tissue and cell distribution pro files of anticancer drugs can be controlled by their entrapment in submicronic colloidal systems (nanoparticles).The rationale behind this approach is to increase antitumor ef ficacy,while reducing systemic side-effects.This review provides an up-date of tumor targeting with conventional or long-circulating nanoparticles.The in vivo fate of these systems,after intravascular or tumoral administration,is discussed,as well as the mechanism involved in tumor re-gression.Nanoparticles are also of bene fit for the selective delivery of oligonucleotides to tumor cells.More-over,certain types of nanoparticles showed some interesting capacity to reverse MDR resistance,which is a major problem in chemotherapy.The first experiments,aiming to decorate nanoparticles with molecular li-gand for ‘active ’targeting of cancerous cells,are also discussed here.The last part of this review focus on the application of nanoparticles in imaging for cancer diagnosis.©2012Published by Elsevier B.V.Contents 1.Introduction ..............................................................02.Nanoparticles for tumor tissues targeting and delivery ...........................................02.1.Systemic administration ......................................................02.1.1.Conventional nanoparticles ................................................02.1.2.Long-circulating nanoparticles ..............................................02.2.Local administration:subcutaneous or intratumoral .........................................03.Nanoparticles for tumor cells targeting and delivery ............................................03.1.Delivery of antisense oligonucleotides (ODNs)to cells ........................................03.2.Reversion of multidrug resistance in tumor cells ..........................................03.3.Molecular addressing of nanoparticles (‘active targeting ’)......................................04.Nanoparticles as tumor biomarkers ....................................................04.1.In vitro ..............................................................04.2.In vivo ..............................................................04.2.1.Radiodiagnosis (scintigraphy)puted tomography ..................................................04.2.3.Magnetic resonance imaging (MRI)............................................05.Conclusion ...............................................................0Acknowledgements ..............................................................0References ..................................................................1.IntroductionNeoplastic tissues may be divided into three subcompartments:vascular,interstitial and cellular [1].The vascularization of tumors is heterogeneous,showing regions of necrosis or hemorrhages as well as regions which are densely vas-cularized in order to sustain an adequate supply of nutrients and oxy-gen for rapid tumor growth (angiogenesis)[2].Tumor blood vessels present several abnormalities in comparison with normal physiological vessels,often including a relatively high proportion of proliferating en-dothelial cells,an increased tortuosity,a de ficiency in pericytes and an aberrant basement membrane formation [3,4].The resulting enhancedAdvanced Drug Delivery Reviews xxx (2012)xxx –xxx☆PII of original article:S0169-409X(02)00044-3.The article was originally published in Advanced Drug Delivery Reviews 54(2002)631–651.⁎Corresponding author.Tel.:+33146835396;fax:+33146619334.E-mail address:patrick.couvreur@cep.u-psud.fr (P.Couvreur).0169-409X/$–see front matter ©2012Published by Elsevier B.V./10.1016/j.addr.2012.09.006Contents lists available at SciVerse ScienceDirectAdvanced Drug Delivery Reviewsj o u r n a l h om e p a g e :ww w.e l s e v i e r.c o m /l o c a t e /a d d rpermeability of tumor vasculature is thought to be regulated by various mediators,such as vascular endothelium growth factor(VEGF),brady-kinin,nitric oxide,prostaglandins and matrix metalloproteinases[5]. Macromolecular transport pathways across tumor vessels have been shown to occur via open gaps(interendothelial junctions and trans-endothelial channels),vesicular vacuolar organelles(VVO)and fenes-trations[6].It remains,however,controversial as to which pathways are predominantly responsible for tumor hyperpermeability and mac-romolecular transvascular transport[6].Regardless of the transport mechanism,the pore cutoff size of several tumor models has been reported ranging between380and780nm[6,7].In vivofluores-cence microscopy has even permitted direct measurement of the ex-travasation of sterically stabilized liposomes into solid tumor tissue (neuroblastome C-1300),suggesting that the cutoff size of the pores lies around400nm[8].The tumor interstitial compartment is predominantly composed of a collagen and elasticfiber network[1].Interdispersed within this cross-linked structure are the interstitialfluid and macromolecular constituents(hyaluronate and proteoglycans),which form a hydro-philic gel[1].The interstitium,unlike most normal tissues,is also characterized by a high interstitial pressure leading to an outward convective interstitialfluidflow,as well as the absence of an anatom-ically well-defined functioning lymphatic network[1,2].Hence,the transport of an anticancer drug in the interstitium will be governed by physiological(i.e.pressure)and physicochemical(posi-tion,structure,charge)properties of the interstitium and by the phys-icochemical properties of the molecule(size,configuration,charge, hydrophobicity)itself[1].Thus,to deliver therapeutic agents to tumor cells in vivo,one must overcome the following problems:(i)drug resistance at the tumor level due to physiological barriers(non cellular based mechanisms), (ii)drug resistance at the cellular level(cellular mechanisms),and (iii)distribution,biotransformation and clearance of anticancer drugs in the body.In chemotherapy,clinical drug resistance may be defined either as a lack of tumor size reduction or as the occurrence of clinical relapse after an initial positive response to anti-tumor treatment[9].First,non-cellular drug resistance mechanisms could be due to poorly vascularized tumor regions which can effectively reduce drug access to the tumor and thus protect cancerous cells from cytotoxici-ty.The acidic environment in tumors can also confer a resistance mechanism against basic drugs.These compounds would be ionized, preventing their diffusion across cellular membrane.High interstitial pressure and low microvascular pressure may also retard or impede extravasation of molecules[10].Then,the resistance of tumors to therapeutic intervention may be due to cellular mechanisms,which are categorized in term of alter-ations in the biochemistry of malignant cells.They comprise altered activity of specific enzyme systems(for example topoisomerase ac-tivity),altered apoptosis regulation,or transport based mechanisms, like P-glycoprotein efflux system,responsible for the multi-drug resistance(MDR),or the multi-drug resistance associated protein (MRP)[9,10].Finally,anticancer drugs generally feature large volumes of distri-bution.As cancerfighting drugs are toxic to both tumor and normal cells,the efficacy of chemotherapy is often limited by important side-effects.A strategy could be to associate antitumor drugs with colloidal nanoparticles,with the aim to overcome non-cellular and cellular based mechanisms of resistance and to increase selectivity of drugs to-wards cancer cells while reducing their toxicity towards normal tissues.Nanoparticles may be defined as being submicronic(b1μm)col-loidal systems generally,but not necessarily,made of polymers(bio-degradable or not).According to the process used for the preparation of the nanoparticles,nanospheres or nanocapsules can be obtained. Unlike nanospheres(matrix systems in which the drug is dispersed throughout the particles),nanocapsules are vesicular systems in which the drug is confined to an aqueous or oily cavity surrounded by a single polymeric membrane.Nanocapsules may,thus,be consid-ered as a‘reservoir’system(Fig.1)[11].If designed appropriately,nanoparticles may act as a drug vehicle able to target tumor tissues or cells,to a certain extent,while pro-tecting the drug from premature inactivation during its transport.Indeed,at the tumor level,the accumulation mechanism of intra-venously injected nanoparticles relies on a passive diffusion or con-vection across the leaky,hyperpermeable tumor vasculature[12]. The uptake can also result from a specific recognition in case of ligand decorated nanoparticles(‘active targeting’)[13].Fig.1.Nanoparticles are:nanosphere(matrix systems)(top)or nanocapsule(reservoir system)(bottom).2I.Brigger et al./Advanced Drug Delivery Reviews xxx(2012)xxx–xxxHence,as the clearance via lymphatics is generally seriously compromised in neoplastic tissues,there will be an additional reten-tion of the colloidal particles(or macromolecules with a molecular weight above50kDa)in the tumor interstitium.This particular con-cept denominated‘enhanced permeability and retention effect’(EPR)results in an important intratumoral drug accumulation which is even higher than this observed in plasma and other tissues [5,14,15].Furthermore,a controlled release of the drug content inside of the tumoral interstitium may be achieved by controlling the nano-particulate structure:polymers used and the way by which the drug is associated with the carrier(adsorption or encapsulation).This paper willfirst review how nanoparticles loaded with anti-cancer drug successfully increase drug concentration in cancer tis-sues,enhancing antitumor efficacy.Moreover,nanoparticles may also act at the cellular level.They can be endocytosed/phagocytosed by cells,with a resulting cell inter-nalization of the encapsulated drug.Certain types of nanoparticles were also found to be able to overcome MDR resistance,which is due to the presence of the P-glycoprotein efflux system localized at the cancerous cell membrane.These points will be discussed in the second part of the paper.Finally,apart from therapeutic goals,nanoparticles have also proved to be useful for diagnostic purpose.This will be presented in the last part of this review.2.Nanoparticles for tumor tissues targeting and delivery2.1.Systemic administration2.1.1.Conventional nanoparticlesThe association of a cytostatic drug to conventional carriers leads to modifications of the drug biodistribution profile,as it is mainly de-livered to the mononuclear phagocytes system(MPS)(liver,spleen, lungs and bone marrow).Indeed,once in the bloodstream,surface non-modified nanoparticles(conventional nanoparticles)are rapid-ly opsonized and massively cleared by thefixed macrophages of the MPS organs[16].This was demonstrated in mice treated with doxorubicin incorpo-rated into poly(isohexylcyanoacrylate)(PIHCA)nanospheres,where higher concentrations of doxorubicin were found in the liver,spleen and lungs,as compared to the counterpart mice treated with free doxorubicin[17].At the same time,the concentration of doxorubicin in the heart and kidneys of mice were lower than when free doxoru-bicin was used.In much the same fashion,when actinomycin D was adsorbed on poly(methylcyanoacrylate)(PMCA)nanospheres,it concentrated main-ly in the lungs of the rats[18].However,when this compound was incor-porated into the more slowly biodegradable poly(ethylcyanoacrylate) (PECA)nanospheres,the drug accumulated mainly in the small in-testine of rats[19].Finally,when vinblastine was incorporated into the same PECA nanospheres,the drug concentrated highly in the spleen of rats[19].Thus,both the polymeric composition(type,hydrophobicity,bio-degradation profile)of the nanoparticles and the associated drug (molecular weight,charge,localization in the nanospheres:adsorbed or incorporated)have a great influence on the drug distribution pat-tern in the reticuloendothelial organs[19].However,the exact under-lying mechanism was not fully understood,but it was observed that this effect was rapid(within0.5or3h)and compatible with endocy-tosis[19].Such propensity of MPS macrophages for endocytosis/phagocyto-sis provides an opportunity to efficiently deliver therapeutic agents to these cells,using conventional nanoparticles.This biodistribution can be of benefit for the chemotherapeutic treatment of MPS local-ized tumors(for example,hepatocarcinoma or hepatic metastasis arising from digestive tract or gynaecological cancers,broncho-pulmonary tumors(primitive tumors or metastasis)including‘non small cells tumor’and‘small cells tumors’,myeloma and leukemia).The increased antitumor efficacy when dealing with drugs associ-ated to conventional nanoparticles was demonstrated on an hepatic metastases model in mice(M5076reticulum cell sarcoma)[20]. Doxorubicin–PIHCA nanoparticles had an improved antimetastatic efficacy,since their use resulted in a greater reduction of the number of metastases than when free doxorubicin was used[20].Additional-ly,it appeared to increase the life span of the metastasis-bearing mice[20].The underlying mechanism responsible for the increased therapeutic efficacy of the nanoparticle formulation was a transfer of doxorubicin from the healthy hepatic tissue,acting as a drug reservoir,to the malignant tissues[21].Histological examination allowed to visualize a considerable accumulation of nanoparticles in the lysosomal vesicles of Kupffer cells,whereas nanoparticles could not be clearly identified in tumoral cells[21].Thus,Kupffer cells,after a massive uptake of nanoparticles by phagocytosis,were able to induce the release of doxorubicin,leading to a gradient of drug concentration,favorable for a prolonged diffusion of the free and still active drug towards the neighboring metastatic cells[21]. Finally,in vitro experiments permitted to rule out the mechanism by which doxorubicin itself or PIHCA nanoparticles could have po-tentiated the intrinsic tumoricidal effect of macrophages[22].When conventional nanoparticles are used as carriers in chemo-therapy,some cytotoxicity against the Kupffer cells or other targeted macrophages can be expected,as the class of drugs being used is able to induce apoptosis in these cells[13,23].Treatments featuring fre-quent administrations(with intervals shorter than2weeks,period of restoration of Kupffer cells)could result in a deficiency of Kupffer cells,which in turn could lead to a decreased liver uptake,and a sub-sequent decreased therapeutic efficacy for hepatic tumors[13].A risk for bacteriemia can also not to be excluded[13,23].Moreover,conventional carriers also target the bone marrow(MPS organ),which is already an important but unfavorable site of action for most anticancer drugs.Hence,chemotherapy with such carriers may increase myelosuppressive effects.This was indeed observed with doxorubicin incorporated into poly(isobutylcyanoacrylate) (PIBCA)and PIHCA nanospheres,whose hematopoietic toxicity was generally more pronounced and long-lasting than that of free doxo-rubicin[24].Acute renal toxicity was another murine-reported doxorubicin toxicity,which was amplified by the association of the drug to PIBCA nanospheres.This toxicity(proteinuria)on the kidneys was probably the result of a modified biodistribution of the associated drug,leading to a strong uptake by mesangial cells,which resulted in glomerular damage[25].However,despite the amplification of these side-effects,it is like-ly that conventional nanoparticles have a better safety profile than free anticancer agents,when acting on normal tissues.For example, reduction of cardiac accumulation of drugs[26],as well as of genotoxicity of mitomycin C and farmorubicin[27]has been related. The former point is of great interest for chemotherapy with doxoru-bicin,as the limiting side effect of this compound is its cardiotoxity.Clinical pharmacokinetics after a single intravenous administra-tion of doxorubicin adsorbed onto polymethacrylate nanospheres has been investigated in hepatoma patients.This type of convention-al carrier,although of limited use in vivo because not biodegradable, allowed to reduce both the volume of distribution and the elimina-tion half-life of doxorubicin[28].Again,these data are consistent with the uptake by MPS organs acting as a drug reservoir.Another phase I clinical investigation has been carried out on21 assessable patients with doxorubicin associated to biodegradable PIHCA nanospheres.Pharmacokinetic studies conducted in3/21pa-tients revealed important interindividual variations for doxorubicin (encapsulated or not)plasma levels.Clinical toxicity of encapsulated3I.Brigger et al./Advanced Drug Delivery Reviews xxx(2012)xxx–xxxdoxorubicin consisted in dose-dependent myelosuppression of dif-ferent grades in all patients(limiting toxicity),in pseudo-allergic re-actions in3/21patients and in diffuse bone pain in3/21patients. However,neither cardiac toxicity nor hepatotoxicity were encoun-tered among the18patients treated with the nanoparticle formula-tion.The former could be explained by the rather low cumulative dose administered(180mg/m2).It was important to check the later,as the treatment schedule was a repeated administration with28days interval between each cycles.Unfortunately,according to WHO criteria,there were only2/21stable diseases lasting4–6months.All the other patients had progressive disease after the first course of doxorubicin-loaded PIHCA nanospheres[29].This could be due to tumor localization,as they were rarely located at MPS sites,resulting in subtherapeutic anticancer drug concentration exposure.Consequently,the contribution of conventional nanoparticles to en-hance anticancer drugs efficacy is limited to targeting tumors at the level of MPS organs.Addressing anticancer drug-loaded nanoparticles to other tumoral tissues is not feasible,due to their very short circu-lation time(the mean half-life of conventional nanoparticles is 3–5min after intravenous administration).Besides,penetration of such a carrier system across the leaky tumoral endothelium would be derisory,leading to subtherapeutic concentrations of the drug near the neoplastic cells.2.1.2.Long-circulating nanoparticlesSince the usefulness of conventional nanoparticles is limited by their massive capture by the macrophages of the MPS after intrave-nous administration,other nanoparticulate devices must be consid-ered to target tumors,which are not localized in the MPS area. Recently,a great deal of work has been devoted to developing so-called‘Stealth™’particles,which are‘invisible’to macrophages [30].These Stealth™nanoparticles have been shown to be character-ized by a prolonged half-life in the blood compartment[31,32].This allows them to selectively extravasate in pathological sites,like tu-mors or inflamed regions with a leaky vasculature(Fig.2)[13].As a result,such long-circulating nanoparticles are supposed to be able to directly target most tumors located outside the MPS regions[13].The size of the colloidal carriers as well as their surface character-istics are the key for the biological fate of nanoparticles,since these parameters can prevent their uptake by MPS macrophages.A high curvature(resulting in a small size:b100nm)and/or a hydrophilic surface(as opposed to the hydrophobic surface of conventional nanoparticles)are needed,in order to reduce opsonization reactions and subsequent clearance by macrophages[30].Chemotherapy with small-sized nanoparticles was performed in tumor-bearing animals.Taxol incorporated into polyvinylpyrrolidone nanospheres with a diameter of50–60nm were assayed on a B16F10murine melanoma transplanted subcutaneously in mice. Mice treated with repeated intravenous injections of taxol-loaded nanospheres showed a significant tumor regression and higher sur-vival rates than mice treated with free taxol[33].Similarly,the use in a murine tumor model(implanted subcutaneously J774A.1mac-rophages)of a dextran–doxorubicin conjugate incorporated into small chitosan nanospheres(100nm in diameter)was reported to outperform the free conjugate,especially in relation of life expectan-cy[34].In both cases,a higher tumor uptake,thanks to the small size and the hydrophilicity of the carrier device,as well as a sustained re-lease of the drug,were supposed to be the key factors in improving the efficacy of the chemotherapy[33,34].A major breakthrough in the nanoparticlesfield consisted in using hydrophilic polymers,(poly(ethylene glycol)(PEG),poloxamines, poloxamers,polysaccharides)to efficiently coat conventional nano-particle surface[30,35].These coatings provide a dynamic‘cloud’of hydrophilic and neutral chains at the particle surface,which repel plasma proteins,as modeled by Jeon et al.[36,37].Hydrophilic poly-mers can be introduced at the surface in two ways,either by adsorp-tion of surfactants or by use of block or branched copolymers[30,31].Coating conventional nanoparticles with surfactants,in order to obtain a long-circulating carrier,has been thefirst strategy used to di-rect tumor targeting in vivo.For instance,chemotherapy was performed in B16melanoma-bearing mice,with intravenous injection of mitoxantrone adsorbed onto PIBCA nanospheres,coated or not with poloxamine1508[38]. In both cases,the observed tumor concentrations of mitoxantrone were high.However,the influence of the hydrophilic coating of the nanoparticles on the biodistribution and pharmacokinetics were negli-gible and difficult to interpret,as the results showed important stan-dard deviations.Moreover,the non-adsorbed drug(which accounted for90%of the total drug)was not removed from the nanoparticles prep-aration and the hydrophilic coating was rapidly desorbed in vivo[38].Another study was done using Polysorbate80-coated PIBCA nanospheres[39].Unfortunately,the coating again did not provide major changes in biodistribution and in pharmacokinetic parameters in rats,when compared to those of conventional PIBCA nanospheres. However,the coated nanospheres did transport a significantamountFig.2.Extravasation of long-circulating(Stealth™)nanoparticles in the tumor interstitium by passive diffusion or convection across the altered and hyperpermeable neoplastic endothelium.4I.Brigger et al./Advanced Drug Delivery Reviews xxx(2012)xxx–xxxof incorporated doxorubicin to the brain of healthy rats,the highest drug levels(6μg/g)being reached in this organ2–4h after intrave-nous administration.At that time,plasma concentration laid around 0.1μg/g[39,40].Consequently,it was suspected that an active transport from the blood to the brain took place,since cerebral accumulation oc-curred against a concentration gradient.A mechanism involving an en-docytosis by brain endothelial cells was hypothesized,as the brain uptake was inhibited at4°C and after a pretreatment with cytochalasin B[39,40].Finally,a therapeutic effect with this‘sterically stabilized’carrier system was noted in rats bearing intracranial glioblastoma (101/8neoplastic cells)[41].Very recently,other results were obtained with the non-biodegradable poly(methylmethacrylate)(PMMA)nanospheres coated or not with different surfactants(Polysorbate80,Poloxamer 407and Poloxamine908).These systems were tested in mice,for their distribution in several tumor models,including a murine B16-melanoma(inoculated intramuscularly),a human breast cancer MaTu(engrafted subcutaneously)and an U-373glioblastoma (implanted intracerebrally)[42].The results showed a prolonged half life of the coated PMMA nanospheres in the circulation,especially with the Poloxamer407and the Poloxamine908coatings.Moreover an accumulation and retention of the coated PMMA nanospheres in the B-16and MaTu tumors were observed;this accumulation depended on the particles surface hydrophilicity and the specific growth difference of the tumors[42].Unfortunately,these experi-ments were performed without transcardiac perfusion with NaCl, which makes it difficult to separate the nanoparticles really accumu-lated in the tumor interstitium from those still present in the blood compartment.Despite of these interesting results,the second strategy consisting in the covalent linkage of amphiphilic copolymers is gen-erally preferred for obtaining a protective hydrophilic cloud on nanoparticles,as it avoids the possibility of rapid coating desorption upon dilution or after contact with blood components.This approach has been employed with poly(lactic acid)(PLA),poly(caprolactone) and poly(cyanoacrylate)polymers,which were chemically coupled to PEG[32,43–45].Although these copolymers have not been used until now as nanoparticles in cancer chemotherapy,it is likely that this type of construction will be extensively investigated in the near future.Another approach worth mentioning consists in tumor targeting with long-circulating carrier followed by irradiation of tumor site in case of photodynamic therapy(PDT)[46].This combination was aimed at improving anticancer efficacy in an EMT-6tumor-bearing mice model,while better avoiding systemic phototoxicity.Unfor-tunately,although the idea was clever,this system consisting in PLA nanospheres covered by adsorbed PEG did not allow a higher intratumoral accumulation of its incorporated photosensitizer (hexadecafluoro zinc phthalocyanine)[46].The fragility of the adsorbed coating and the large particle size(>900nm)could be re-sponsible for this failure.However,formulation of the photosensitiz-er in the biodegradable nanospheres improved PDT response(by possibly influencing the intratumoral distribution pattern of the photosensitizer),while providing prolonged tumor sensitivity to-wards PDT[46].Finally,one should expect a lower systemic photo-toxicity,as the carrier system successfully increased the tumor/skin and tumor/muscle uptake ratios for the drug.2.2.Local administration:subcutaneous or intratumoralUnlike water-soluble molecules,which are rapidly absorbed through the blood capillary wall and pass into circulation,small par-ticles injected locally infiltrate into the interstitial space around the injection site and are gradually absorbed by the lymphatic capillaries into the lymphatic system[47,48].For that reason,subcutaneously or locally injected(in the peri-tumoral region)nanoparticles can be used for lymphatic tar-geting,i.e.as a tool for chemotherapy against lymphatic tumors or metastases.For example,aclarubicin adsorbed onto activated carbon particles was tested after subcutaneous injection in mice,against a murine model(P388leukemia cells)of lymph node metastases [49].The same system was also used in patients after intratumoral and peritumoral injections,as a loco-regional chemotherapy adju-vant for breast cancer[50].In both applications,this carrier system distributed selectively high levels of free aclarubicin to the regional lymphatic system and low levels to the rest of the body[49,50].How-ever,this carrier system is open to criticism,as it is not biodegrad-able,and as it is rather big(>100nm),impeding the drainage from the injection site through the aqueous channels[47].Besides,the drug is associated to the particles by adsorption,leading to a rapid release with possible systemic absorption.It would seem that PIBCA nanocapsules with an oily core for hy-drophobic drugs[48]or biodegradable systems coated by adsorption of the surfactant Poloxamine904[47]offer interesting properties for future investigations in thisfield.Indeed,PIBCA nanocapsules,as compared to liposome or emulsion formulations,showed a potential to retain the lipophilic indicator12-(9-anthroxy)stearic acid(ASA) in the regional lymph nodes during168h after intramuscular ad-ministration[48].On the same way,Poloxamine904caused an in-creased sequestration of the particles in lymph nodes,which would probably reduce systemic absorption of any encapsulated drugs [47].Other systems regarded as promising in thefight against cancer are ultrasmall superparamagnetic iron oxides particles(USPIO)with an anticancer drug entrapped in their ferrous core(see below).A study combining intratumoral administration of gadolinium-loaded chitosan nanoparticles(gadopentetic acid–chitosan complex nano-particles)and neutron-capture therapy was performed on the B16F10melanoma model subcutaneously implanted in mice[51].Re-sults showed an outstanding gadolinium retention in tumor tissue when it was encapsulated,with respect to the free drug(a highly water-soluble compound with a rapid elimination).Irradiation of the tumors was performed8h after the last intratumoral injection of gado-linium nanoparticles and prevented further tumor growth in the ani-mals treated and increased their life expectancy[51].3.Nanoparticles for tumor cells targeting and delivery3.1.Delivery of antisense oligonucleotides(ODNs)to cellsAfter intratumoral injection,the bioavailability of ODNs is seri-ously reduced due to their fast degradation by ubiquitous exo-and endonucleases[52].Moreover,their negative charge seriously hin-ders the intracellular penetration of theses short fragments of nucleic acids[52].This leads to a somewhat reduced therapeutic ef-ficacy.In order to prevent the ODN degradation and improve their intracellular capture,it was proposed to associate them with nano-particles[53–55].In vitro growth inhibition was carried out on human tumorigenic cells(HBL100ras1,a clone obtained from the human mammary cell line HBL100)using anti-ras ODN-loaded PIHCA nanospheres[56]. In this study,the carrier system consisted of a cationic hydrophobic detergent(hexadecyltrimethylammoniumbromide(CTAB)),which interacted with the ODN by ion-pairing.This hydrophobic complex was then adsorbed onto the hydrophobic and negatively charged surfaces of cyanoacrylate nanospheres[56].In vivo,HBL100ras1cells implanted in nude mice were treated intratumorally with several formulations of ODNs targeted against Ha-ras oncogene.Tumor growth inhibition was achieved at concen-trations a100times lower than those needed with free ODN,when the ODN–CTAB complex was adsorbed onto the surface of the PIHCA nanospheres.Interestingly,the ODN–CTAB complex alone5I.Brigger et al./Advanced Drug Delivery Reviews xxx(2012)xxx–xxx。
