HA、Flag、Myc、His蛋白标签教学教材
蛋白纯化(his标签)说明书

Instruction ManualProBond TM Purification SystemFor purification of polyhistidine-containing recombinant proteinsCatalog nos. K850-01, K851-01, K852-01, K853-01, K854-01,R801-01, R801-15Version K2 September200425-0006iiTable of ContentsKit Contents and Storage (iv)Accessory Products (vi)Introduction (1)Overview (1)Methods (2)Preparing Cell Lysates (2)Purification Procedure—Native Conditions (7)Purification Procedure—Denaturing Conditions (11)Purification Procedure—Hybrid Conditions (13)Troubleshooting (15)Appendix (17)Additional Protocols (17)Recipes (18)Frequently Asked Questions (21)References (22)Technical Service (23)iiiKit Contents and StorageTypes of Products This manual is supplied with the following products:Product CatalogNo.ProBond™ Purification System K850-01ProBond™ Purification System with Antibodywith Anti-Xpress™ Antibody K851-01with Anti-myc-HRP Antibody K852-01with Anti-His(C-term)-HRP Antibody K853-01with Anti-V5-HRP Antibody K854-01ProBond™ Nickel-Chelating Resin (50 ml) R801-01ProBond™ Nickel Chelating Resin (150 ml) R801-15ProBond™Purification System Components The ProBond™ Purification System includes enough resin, reagents, and columns for six purifications. The components are listed below. See next page for resin specifications.Component Composition Quantity ProBond™ Resin 50% slurry in 20% ethanol 12 ml5X NativePurification Buffer250 mM NaH2PO4, pH 8.02.5 M NaCl1 × 125 ml bottleGuanidinium LysisBuffer6 M Guanidine HCl20 mM sodium phosphate, pH 7.8500 mM NaCl1 × 60 ml bottleDenaturingBinding Buffer8 M Urea20 mM sodium phosphate, pH 7.8500 mM NaCl2 × 125 ml bottlesDenaturing WashBuffer8 M Urea20 mM sodium phosphate, pH 6.0500 mM NaCl2 × 125 ml bottlesDenaturing ElutionBuffer8 M Urea20 mM NaH2PO4, pH 4.0500 mM NaCl1 × 60 ml bottle3 M Imidazole,20 mM sodium phosphate, pH 6.0500 mM NaCl1 × 8 ml bottlePurificationColumns10 ml columns 6Continued on next pageivKit Contents and Storage, ContinuedProBond™Purification System with Antibody The ProBond™ Purification System with Antibody includes resin, reagents, and columns as described for the ProBond™ Purification System (previous page) and 50 µl of the appropriate purified mouse monoclonal antibody. Sufficient reagents are included to perform six purifications and 25 Western blots with the antibody.For more details on the antibody specificity, subclass, and protocols for using the antibody, refer to the antibody manual supplied with the system.Storage Store ProBond™ resin at +4°C. Store buffer and columns at room temperature.Store the antibody at 4°C. Avoid repeated freezing and thawing of theantibody as it may result in loss of activity.The product is guaranteed for 6 months when stored properly.All native purification buffers are prepared from the 5X Native PurificationBuffer and the 3 M Imidazole, as described on page 7.The Denaturing Wash Buffer pH 5.3 is prepared from the Denaturing WashBuffer (pH 6.0), as described on page 11.Resin and ColumnSpecificationsProBond™ resin is precharged with Ni2+ ions and appears blue in color. It isprovided as a 50% slurry in 20% ethanol.ProBond™ resin and purification columns have the following specifications:• Binding capacity of ProBond™ resin: 1–5 mg of protein per ml of resin• Average bead size: 45–165 microns• Pore size of purification columns: 30–35 microns• Recommended flow rate: 0.5 ml/min• Maximum flow rate: 2 ml/min• Maximum linear flow rate: 700 cm/h• Column material: Polypropylene• pH stability (long term): pH 3–13• pH stability (short term): pH 2–14ProductQualificationThe ProBond™ Purification System is qualified by purifying 2 mg of myoglobinprotein on a column and performing a Bradford assay. Protein recovery mustbe 75% or higher.vAccessory ProductsAdditionalProductsThe following products are also available for order from Invitrogen:Product QuantityCatalogNo.ProBond™ Nickel-Chelating Resin 50 ml150 mlR801-01R801-15Polypropylene columns(empty)50 R640-50Ni-NTA Agarose 10 ml25 ml R901-01 R901-15Ni-NTA Purification System 6 purifications K950-01 Ni-NTA Purification Systemwith Antibodywith Anti-Xpress™ Antibody with Anti-myc-HRP Antibody with Anti-His(C-term)-HRP Antibodywith Anti-V5-HRP Antibody 1 kit1 kit1 kit1 kitK951-01K952-01K953-01K954-01Anti-myc Antibody 50 µl R950-25 Anti-V5 Antibody 50 µl R960-25 Anti-Xpress™ Antibody 50 µl R910-25 Anti-His(C-term) Antibody 50 µl R930-25 InVision™ His-tag In-gel Stain 500 ml LC6030 InVision™ His-tag In-gelStaining Kit1 kit LC6033Pre-Cast Gels and Pre-made Buffers A large variety of pre-cast gels for SDS-PAGE and pre-made buffers for your convenience are available from Invitrogen. For details, visit our web site at or contact Technical Service (page 23).viIntroductionOverviewIntroduction The ProBond™ Purification System is designed for purification of 6xHis-tagged recombinant proteins expressed in bacteria, insect, and mammalian cells. Thesystem is designed around the high affinity and selectivity of ProBond™Nickel-Chelating Resin for recombinant fusion proteins containing six tandemhistidine residues.The ProBond™ Purification System is a complete system that includespurification buffers and resin for purifying proteins under native, denaturing,or hybrid conditions. The resulting proteins are ready for use in many targetapplications.This manual is designed to provide generic protocols that can be adapted foryour particular proteins. The optimal purification parameters will vary witheach protein being purified.ProBond™ Nickel-Chelating Resin ProBond™ Nickel-Chelating Resin is used for purification of recombinant proteins expressed in bacteria, insect, and mammalian cells from any 6xHis-tagged vector. ProBond™ Nickel-Chelating Resin exhibits high affinity and selectivity for 6xHis-tagged recombinant fusion proteins.Proteins can be purified under native, denaturing, or hybrid conditions using the ProBond™ Nickel-Chelating Resin. Proteins bound to the resin are eluted with low pH buffer or by competition with imidazole or histidine. The resulting proteins are ready for use in target applications.Binding Characteristics ProBond™ Nickel-Chelating Resin uses the chelating ligand iminodiacetic acid (IDA) in a highly cross-linked agarose matrix. IDA binds Ni2+ ions by three coordination sites.The protocols provided in this manual are generic, and may not result in 100%pure protein. These protocols should be optimized based on the bindingcharacteristics of your particular proteins.Native VersusDenaturingConditionsThe decision to purify your 6xHis-tagged fusion proteins under native ordenaturing conditions depends on the solubility of the protein and the need toretain biological activity for downstream applications.• Use native conditions if your protein is soluble (in the supernatant afterlysis) and you want to preserve protein activity.• Use denaturing conditions if the protein is insoluble (in the pellet afterlysis) or if your downstream application does not depend on proteinactivity.• Use hybrid protocol if your protein is insoluble but you want to preserveprotein activity. Using this protocol, you prepare the lysate and columnsunder denaturing conditions and then use native buffers during the washand elution steps to refold the protein. Note that this protocol may notrestore activity for all proteins. See page 14.1MethodsPreparing Cell LysatesIntroduction Instructions for preparing lysates from bacteria, insect, and mammalian cellsusing native or denaturing conditions are described below.Materials Needed You will need the following items:• Native Binding Buffer (recipe is on page 8) for preparing lysates undernative conditions• Sonicator• 10 µg/ml RNase and 5 µg/ml DNase I (optional)• Guanidinium Lysis Buffer (supplied with the system) for preparing lysatesunder denaturing conditions• 18-gauge needle• Centrifuge• Sterile, distilled water• SDS-PAGE sample buffer• Lysozyme for preparing bacterial cell lysates• Bestatin or Leupeptin, for preparing mammalian cell lysatesProcessing Higher Amount of Starting Material Instructions for preparing lysates from specific amount of starting material (bacteria, insect, and mammalian cells) and purification with 2 ml resin under native or denaturing conditions are described in this manual.If you wish to purify your protein of interest from higher amounts of starting material, you may need to optimize the lysis protocol and purification conditions (amount of resin used for binding). The optimization depends on the expected yield of your protein and amount of resin to use for purification. Perform a pilot experiment to optimize the purification conditions and then based on the pilot experiment results, scale-up accordingly.Continued on next page2Preparing Bacterial Cell Lysate—Native Conditions Follow the procedure below to prepare bacterial cell lysate under native conditions. Scale up or down as necessary.1. Harvest cells from a 50 ml culture by centrifugation (e.g., 5000 rpm for5 minutes in a Sorvall SS-34 rotor). Resuspend the cells in 8 ml NativeBinding Buffer (recipe on page 8).2. Add 8 mg lysozyme and incubate on ice for 30 minutes.3. Using a sonicator equipped with a microtip, sonicate the solution on iceusing six 10-second bursts at high intensity with a 10-second coolingperiod between each burst.Alternatively, sonicate the solution on ice using two or three 10-secondbursts at medium intensity, then flash freeze the lysate in liquid nitrogen or a methanol dry ice slurry. Quickly thaw the lysate at 37°C andperform two more rapid sonicate-freeze-thaw cycles.4. Optional: If the lysate is very viscous, add RNase A (10 µg/ml) andDNase I (5 µg/ml) and incubate on ice for 10–15 minutes. Alternatively,draw the lysate through a 18-gauge syringe needle several times.5. Centrifuge the lysate at 3,000 ×g for 15 minutes to pellet the cellulardebris. Transfer the supernatant to a fresh tube.Note: Some 6xHis-tagged protein may remain insoluble in the pellet, and can be recovered by preparing a denatured lysate (page 4) followed bythe denaturing purification protocol (page 12). To recover this insolubleprotein while preserving its biological activity, you can prepare thedenatured lysate and then follow the hybrid protocol on page 14. Notethat the hybrid protocol may not restore activity in all cases, and should be tested with your particular protein.6. Remove 5 µl of the lysate for SDS-PAGE analysis. Store the remaininglysate on ice or freeze at -20°C. When ready to use, proceed to theprotocol on page 7.Continued on next page3Preparing Bacterial Cell Lysate—Denaturing Conditions Follow the procedure below to prepare bacterial cell lysate under denaturing conditions:1. Equilibrate the Guanidinium Lysis Buffer, pH 7.8 (supplied with thesystem or see page 19 for recipe) to 37°C.2. Harvest cells from a 50 ml culture by centrifugation (e.g., 5000 rpm for5 minutes in a Sorvall SS-34 rotor).3. Resuspend the cell pellet in 8 ml Guanidinium Lysis Buffer from Step 1.4. Slowly rock the cells for 5–10 minutes at room temperature to ensurethorough cell lysis.5. Sonicate the cell lysate on ice with three 5-second pulses at high intensity.6. Centrifuge the lysate at 3,000 ×g for 15 minutes to pellet the cellulardebris.Transfer the supernatant to a fresh tube.7. Remove 5 µl of the lysate for SDS-PAGE analysis. Store the remaininglysate on ice or at -20°C. When ready to use, proceed to the denaturingprotocol on page 11 or hybrid protocol on page 13.Note: To perform SDS-PAGE with samples in Guanidinium Lysis Buffer, you need to dilute the samples, dialyze the samples, or perform TCAprecipitation prior to SDS-PAGE to prevent the precipitation of SDS.Harvesting Insect Cells For detailed protocols dealing with insect cell expression, consult the manual for your particular system. The following lysate protocols are for baculovirus-infected cells and are intended to be highly generic. They should be optimized for your cell lines.For baculovirus-infected insect cells, when the time point of maximal expression has been determined, large scale protein expression can be carried out. Generally, the large-scale expression is performed in 1 liter flasks seeded with cells at a density of 2 × 106 cells/ml in a total volume of 500 ml and infected with high titer viral stock at an MOI of 10 pfu/cell. At the point of maximal expression, harvest cells in 50 ml aliquots. Pellet the cells by centrifugation and store at -70°C until needed. Proceed to preparing cell lysates using native or denaturing conditions as described on the next page.Continued on next page4Preparing Insect Cell Lysate—Native Condition 1. Prepare 8 ml Native Binding Buffer (recipe on page 8) containingLeupeptin (a protease inhibitor) at a concentration of 0.5 µg/ml.2. After harvesting the cells (previous page), resuspend the cell pellet in8 ml Native Binding Buffer containing 0.5 µg/ml Leupeptin.3. Lyse the cells by two freeze-thaw cycles using a liquid nitrogen or dryice/ethanol bath and a 42°C water bath.4. Shear DNA by passing the preparation through an 18-gauge needle fourtimes.5. Centrifuge the lysate at 3,000 ×g for 15 minutes to pellet the cellulardebris.Transfer the supernatant to a fresh tube.6. Remove 5 µl of the lysate for SDS-PAGE analysis. Store remaining lysateon ice or freeze at -20°C. When ready to use, proceed to the protocol on page 7.Preparing Insect Cell Lysate—Denaturing Condition 1. After harvesting insect cells (previous page), resuspend the cell pellet in8 ml Guanidinium Lysis Buffer (supplied with the system or see page 19for recipe).2. Pass the preparation through an 18-gauge needle four times.3. Centrifuge the lysate at 3,000 ×g for 15 minutes to pellet the cellulardebris. Transfer the supernatant to a fresh tube.4. Remove 5 µl of the lysate for SDS-PAGE analysis. Store remaining lysateon ice or freeze at -20° C. When ready to use, proceed to the denaturingprotocol on page 11 or hybrid protocol on page 13.Note: To perform SDS-PAGE with samples in Guanidinium Lysis Buffer, you need to dilute the samples, dialyze the samples, or perform TCAprecipitation prior to SDS-PAGE to prevent the precipitation of SDS.Continued on next pagePreparing Mammalian Cell Lysate—Native Conditions For detailed protocols dealing with mammalian expression, consult the manual for your particular system. The following protocols are intended to be highly generic, and should be optimized for your cell lines.To produce recombinant protein, you need between 5 x 106and 1 x 107 cells. Seed cells and grow in the appropriate medium until they are 80–90% confluent. Harvest cells by trypsinization. You can freeze the cell pellet in liquid nitrogen and store at -70°C until use.1. Resuspend the cell pellet in 8 ml of Native Binding Buffer (page 8). Theaddition of protease inhibitors such as bestatin and leupeptin may benecessary depending on the cell line and expressed protein.2. Lyse the cells by two freeze-thaw cycles using a liquid nitrogen or dryice/ethanol bath and a 42°C water bath.3. Shear the DNA by passing the preparation through an 18-gauge needlefour times.4. Centrifuge the lysate at 3,000 ×g for 15 minutes to pellet the cellulardebris. Transfer the supernatant to a fresh tube.5. Remove 5 µl of the lysate for SDS-PAGE analysis. Store the remaininglysate on ice or freeze at -20° C. When ready to use, proceed to theprotocol on page 7.Preparing Mammalian Cell Lysates—Denaturing Conditions For detailed protocols dealing with mammalian expression, consult the manual for your particular system. The following protocols are intended to be highly generic, and should be optimized for your cell lines.To produce recombinant protein, you need between 5 x 106and 1 x 107 cells. Seed cells and grow in the appropriate medium until they are 80–90% confluent. Harvest cells by trypsinization. You can freeze the cell pellet in liquid nitrogen and store at -70°C until use.1. Resuspend the cell pellet in 8 ml Guanidinium Lysis Buffer (suppliedwith the system or see page 19 for recipe).2. Shear the DNA by passing the preparation through an 18-gauge needlefour times.3. Centrifuge the lysate at 3,000 ×g for 15 minutes to pellet the cellulardebris. Transfer the supernatant to a fresh tube.4. Remove 5 µl of the lysate for SDS-PAGE analysis. Store the remaininglysate on ice or freeze at -20° C until use. When ready to use, proceed to the denaturing protocol on page 11 or hybrid protocol on page 13.Note: To perform SDS-PAGE with samples in Guanidinium Lysis Buffer, you need to dilute the samples, dialyze the samples, or perform TCAprecipitation prior to SDS-PAGE to prevent the precipitation of SDS.Purification Procedure—Native ConditionsIntroduction In the following procedure, use the prepared Native Binding Buffer, NativeWash Buffer, and Native Elution Buffer, columns, and cell lysate preparedunder native conditions. Be sure to check the pH of your buffers before starting.Buffers for Native Purification All buffers for purification under native conditions are prepared from the5X Native Purification Buffer supplied with the system. Dilute and adjust the pH of the 5X Native Purification Buffer to create 1X Native Purification Buffer (page 8). From this, you can create the following buffers:• Native Binding Buffer• Native Wash Buffer• Native Elution BufferThe recipes described in this section will create sufficient buffers to perform one native purification using one kit-supplied purification column. Scale up accordingly.If you are preparing your own buffers, see page 18 for recipe.Materials Needed You will need the following items:• 5X Native Purification Buffer (supplied with the system or see page 18 forrecipe)• 3 M Imidazole (supplied with the system or see page 18 for recipe)• NaOH• HCl• Sterile distilled water• Prepared ProBond™ columns with native buffers (next page)• Lysate prepared under native conditions (page 2)Imidazole Concentration in Native Buffers Imidazole is included in the Native Wash and Elution Buffers to minimize the binding of untagged, contaminating proteins and increase the purity of the target protein with fewer wash steps. Note that, if your level of contaminating proteins is high, you may add imidazole to the Native Binding Buffer.If your protein does not bind well under these conditions, you can experiment with lowering or eliminating the imidazole in the buffers and increasing the number of wash and elution steps.Continued on next page1X Native Purification Buffer To prepare 100 ml 1X Native Purification Buffer, combine:• 80 ml of sterile distilled water• 20 ml of 5X Native Purification Buffer (supplied with the system or see page 18 for recipe)Mix well and adjust pH to 8.0 with NaOH or HCl.Native Binding Buffer Without ImidazoleUse 30 ml of the 1X Native Purification Buffer (see above for recipe) for use as the Native Binding Buffer (used for column preparation, cell lysis, and binding).With Imidazole (Optional):You can prepare the Native Binding Buffer with imidazole to reduce the binding of contaminating proteins. (Note that some His-tagged proteins may not bind under these conditions.).To prepare 30 ml Native Binding Buffer with 10 mM imidazole, combine: • 30 ml of 1X Native Purification Buffer• 100 µl of 3 M Imidazole, pH 6.0Mix well and adjust pH to 8.0 with NaOH or HCl.Native Wash Buffer To prepare 50 ml Native Wash Buffer with 20 mM imidazole, combine:• 50 ml of 1X Native Purification Buffer• 335 µl of 3 M Imidazole, pH 6.0Mix well and adjust pH to 8.0 with NaOH or HCl.Native Elution Buffer To prepare 15 ml Native Elution Buffer with 250 mM imidazole, combine:• 13.75 ml of 1X Native Purification Buffer• 1.25 ml of 3 M Imidazole, pH 6.0Mix well and adjust pH to 8.0 with NaOH or HCl.Continued on next pageDo not use strong reducing agents such as DTT with ProBond™ columns. DTTreduces the nickel ions in the resin. In addition, do not use strong chelatingagents such as EDTA or EGTA in the loading buffers or wash buffers, as thesewill strip the nickel from the columns.Be sure to check the pH of your buffers before starting.PreparingProBond™ ColumnWhen preparing a column as described below, make sure that the snap-off capat the bottom of the column remains intact. To prepare a column:1. Resuspend the ProBond™ resin in its bottle by inverting and gentlytapping the bottle repeatedly.2. Pipet or pour 2 ml of the resin into a 10-ml Purification Columnsupplied with the kit. Allow the resin to settle completely by gravity(5-10 minutes) or gently pellet it by low-speed centrifugation (1 minuteat 800 ×g). Gently aspirate the supernatant.3. Add 6 ml of sterile, distilled water and resuspend the resin byalternately inverting and gently tapping the column.4. Allow the resin to settle using gravity or centrifugation as described inStep 2, and gently aspirate the supernatant.5. For purification under Native Conditions, add 6 ml Native BindingBuffer (recipe on page 8).6. Resuspend the resin by alternately inverting and gently tapping thecolumn.7. Allow the resin to settle using gravity or centrifugation as described inStep 2, and gently aspirate the supernatant.8. Repeat Steps 5 through 7.Storing PreparedColumnsTo store a column containing resin, add 0.02% azide or 20% ethanol as apreservative and cap or parafilm the column. Store at room temperature.Continued on next pagePurification Under Native Conditions Using the native buffers, columns and cell lysate, follow the procedure below to purify proteins under native conditions:1. Add 8 ml of lysate prepared under native conditions to a preparedPurification Column (page 9).2. Bind for 30–60 minutes using gentle agitation to keep the resinsuspended in the lysate solution.3. Settle the resin by gravity or low speed centrifugation (800 ×g), andcarefully aspirate the supernatant. Save supernatant at 4°C forSDS-PAGE analysis.4. Wash with 8 ml Native Wash Buffer (page 8). Settle the resin by gravityor low speed centrifugation (800 ×g), and carefully aspirate thesupernatant. Save supernatant at 4°C for SDS-PAGE analysis.5. Repeat Step 4 three more times.6. Clamp the column in a vertical position and snap off the cap on thelower end. Elute the protein with 8–12 ml Native Elution Buffer (seepage 2). Collect 1 ml fractions and analyze with SDS-PAGE.Note: Store the eluted fractions at 4°C. If -20°C storage is required, addglycerol to the fractions. For long term storage, add protease inhibitors to the fractions.If you wish to reuse the resin to purify the same recombinant protein, wash the resin with 0.5 M NaOH for 30 minutes and equilibrate the resin in a suitable binding buffer. If you need to recharge the resin, see page 17.Purification Procedure—Denaturing ConditionsIntroduction Instructions to perform purification using denaturing conditions with prepareddenaturing buffers, columns, and cell lysate are described below.Materials Needed You will need the following items:• Denaturing Binding Buffer (supplied with the system or see page 19 forrecipe)• Denaturing Wash Buffer, pH 6.0 (supplied with the system or see page 19 forrecipe) and Denaturing Wash Buffer, pH 5.3 (see recipe below)• Denaturing Elution Buffer (supplied with the system or see page 20 forrecipe)• Prepared ProBond™ columns with Denaturing buffers (see below)• Lysate prepared under denaturing conditions (page 11)Preparing the Denaturing Wash Buffer pH 5.3 Using a 10 ml aliquot of the kit-supplied Denaturing Wash Buffer (pH 6.0), mix well, and adjust the pH to 5.3 using HCl. Use this for the Denaturing Wash Buffer pH 5.3 in Step 5 next page.Be sure to check the pH of your buffers before starting. Note that thedenaturing buffers containing urea will become more basic over time. PreparingProBond™ ColumnWhen preparing a column as described below, make sure that the snap-off capat the bottom of the column remains intact.If you are reusing the ProBond™ resin, see page 17 for recharging protocol.To prepare a column:1. Resuspend the ProBond™ resin in its bottle by inverting and gentlytapping the bottle repeatedly.2. Pipet or pour 2 ml of the resin into a 10-ml Purification Columnsupplied with the kit. Allow the resin to settle completely by gravity(5-10 minutes) or gently pellet it by low-speed centrifugation (1 minuteat 800 ×g). Gently aspirate the supernatant.3. Add 6 ml of sterile, distilled water and resuspend the resin byalternately inverting and gently tapping the column.4. Allow the resin to settle using gravity or centrifugation as described inStep 2, and gently aspirate the supernatant.5. For purification under Denaturing Conditions, add 6 ml of DenaturingBinding Buffer.6. Resuspend the resin by alternately inverting and gently tapping thecolumn.7. Allow the resin to settle using gravity or centrifugation as described inStep 2, and gently aspirate the supernatant. Repeat Steps 5 through 7.Continued on next pagePurification Procedure—Denaturing Conditions, ContinuedPurification Under Denaturing Conditions Using the denaturing buffers, columns, and cell lysate, follow the procedure below to purify proteins under denaturing conditions:1. Add 8 ml lysate prepared under denaturing conditions to a preparedPurification Column (page 11).2. Bind for 15–30 minutes at room temperature using gentle agitation (e.g.,using a rotating wheel) to keep the resin suspended in the lysatesolution. Settle the resin by gravity or low speed centrifugation (800 ×g), and carefully aspirate the supernatant.3. Wash the column with 4 ml Denaturing Binding Buffer supplied with thekit by resuspending the resin and rocking for two minutes. Settle theresin by gravity or low speed centrifugation (800 ×g), and carefullyaspirate the supernatant. Save supernatant at 4°C for SDS-PAGEanalysis. Repeat this step one more time.4. Wash the column with 4 ml Denaturing Wash Buffer, pH 6.0 supplied inthe kit by resuspending the resin and rocking for two minutes. Settle the resin by gravity or low speed centrifugation (800 ×g), and carefullyaspirate the supernatant. Save supernatant at 4°C for SDS-PAGEanalysis. Repeat this step one more time.5. Wash the column with 4 ml Denaturing Wash Buffer pH 5.3 (see recipeon previous page) by resuspending the resin and rocking for 2 minutes.Settle the resin by gravity or low speed centrifugation (800 ×g), andcarefully aspirate the supernatant. Save supernatant at 4°C for SDS-PAGE analysis. Repeat this step once more for a total of two washes with Denaturing Wash Buffer pH 5.3.6. Clamp the column in a vertical position and snap off the cap on thelower end. Elute the protein by adding 5 ml Denaturing Elution Buffersupplied with the kit. Collect 1 ml fractions and monitor the elution bytaking OD280readings of the fractions. Pool the fractions that contain the peak absorbance and dialyze against 10 mM Tris, pH 8.0, 0.1% Triton X-100 overnight at 4°C to remove the urea. Concentrate the dialyzedmaterial by any standard method (i.e., using 10,000 MW cut-off, low-protein binding centrifugal instruments or vacuum concentrationinstruments).If you wish to reuse the resin to purify the same recombinant protein, wash the resin with 0.5 M NaOH for 30 minutes and equilibrate the resin in a suitable binding buffer. If you need to recharge the resin, see page 17.。
常见蛋白质标签总结

常见蛋白质标签总结(Flag、HA、cMyc、CBP等)Protein tags are peptide sequences genetically grafted onto a recombinant protein. Often these tags are removable by chemical agents or by enzymatic means, such as proteolysis or intein splicing. Tags are attached to proteins for various purposes.一、氨基酸标签(含小肽标签)A stretch of amino acids is added to the protein and enables the recovery of the labelled protein by its unique affinity. Usually its easiest to add the tag to either end of the protein to ensure its accessibility and not to disturb the protein folding.1.组氨酸标签(His tag)一般为6个组氨酸,用Ni2+(Cu2+)亲和层析纯化2.FLAG tag :N-DYKDDDDK-C ,recovered with specific antibody3.HA tag: an epitope derived from the Influenza protein haemagglutinin(HA,禽流感病毒血凝素),e.g. N-YPYDVPDYA-C,recovery with anHA antibody4.MYC tag: an epitope derived from the human proto-oncoprotein MYC,e.g.N-ILKKATAYIL-C, N-EQKLISEEDL-C,recovery with an MYCantibody5.SBP tag:Streptavidin Binding Peptide,链霉亲合素结合肽,38 amino acidtag (MDEKTTGWRGGHVVEGLAGELEQLRARLEHHPQGQREP),更多参考在Sigma6.CBP tag:钙调蛋白结合肽(CBP; 26aa)钙调蛋白结合肽与钙调素结合是Ca2+依赖的,这种结合不受标签所处的位置影响(N端和C端均可),在中性pH条件下使用2mM EGTA可以很方便的将目标蛋白洗脱下来。
常见tag蛋白标签介绍讲课讲稿

常见t a g蛋白标签介绍蛋白标签蛋白标签(proteintag)是指利用DNA体外重组技术,与目的蛋白一起融合表达的一种多肽或者蛋白,以便于目的蛋白的表达、检测、示踪和纯化等。
随着技术的不断发展,研究人员相继开发出了具有各种不同功能的蛋白标签。
目前,这些蛋白标签已在基础研究和商业化产品生产等方面得到了广泛的应用。
美国GeneCopoeia(复能基因)为客户提供50多种蛋白标签,可以满足客户的不同需求,包括各种最新型的标签,如:SNAP-Tag™、Halo Tag™、AviTag™、Sumo等;也提供齐全的各种常用标签,如eGFP、His、Flag等等标签。
•标签的分子量小,只有~0.84KD,而GST和蛋白A分别为~26KD和~30KD,一般不影响目标蛋白的功能;•His标签融合蛋白可以在非离子型表面活性剂存在的条件下或变性条件下纯化,前者在纯化疏水性强的蛋白得到应用,后者在纯化包涵体蛋白时特别有用,用高浓度的变性剂溶解后通过金属螯和亲和层析去除杂蛋白,使复性不受其它蛋白的干扰,或进行金属螯和亲和层析复性;•His标签融合蛋白也被用于蛋白质-蛋白质、蛋白质-DNA相互作用研究;•His标签免疫原性相对较低,可将纯化的蛋白直接注射动物进行免疫并制备抗体。
•可应用于多种表达系统,纯化的条件温和;•可以和其它的亲和标签一起构建双亲和标签。
Flag标签蛋白Flag标签蛋白为编码8个氨基酸的亲水性多肽(DYKDDDDK),同时载体中构建的Kozak序列使得带有FLAG的融合蛋白在真核表达系统中表达效率更高。
FLAG作为标签蛋白,其融合表达目的蛋白后具有以下优点:•FLAG作为融合表达标签,其通常不会与目的蛋白相互作用并且通常不会影响目的蛋白的功能、性质,这样就有利用研究人员对融合蛋白进行下游研究。
•融合FLAG的目的蛋白,可以直接通过FLAG进行亲和层析,此层析为非变性纯化,可以纯化有活性的融合蛋白,并且纯化效率高。
flag磁珠说明书

flag磁珠说明书Anti-Flag磁珠,也称Anti-Flag免疫磁珠或Flag抗体磁珠,是由高品质的Flag 小鼠单克隆抗体与纳米级氨基磁珠共价偶联而成,可特异性地与动植物或微生物裂解液、血清、腹水等中含有Flag标签的蛋白结合,从而用于带有Flag标签的融合蛋白或其蛋白复合物的免疫沉淀(Immunoprecipitation, IP)、免疫共沉淀(Co-IP)或纯化。
Flag标签(Flag-tag)、Myc标签(Myc-tag)、HA标签(HA-tag)、His标签(His-tag)和GST标签(GST-tag)等是表达载体上最常见的一些标签,通过与这些标签的融合表达可以非常方便地检测目的蛋白及与目的蛋白相互结合的蛋白,也可以非常方便地用于目的蛋白的纯化。
Flag-tag是8个氨基酸残基(DYKDDDDK)组成的多肽,常用的形式有Flag和3X Flag,通过基因重组技术把Flag-tag的核酸序列与目的基因的5’端或3’端连接,就可以最终表达形成Flag-tag的目的蛋白。
Flag-tag具有以下优点:Flag-tag通常不会与目的蛋白相互作用,并且大多数情况下不会影响目的蛋白的功能;Flag-tag作为标签蛋白,后续通过Flag抗体(AF519)、Anti-Flag磁珠或Anti-Flag亲和凝胶(P2271)即可对目的基因的表达、定位及功能进行检测或对目的蛋白进行纯化、免疫沉淀或免疫共沉淀等;融合在N端的Flag标签,可被肠激酶(Enterokinase, EK)切除(DDDK),从而得到完美的没有标签的目的蛋白。
基于以上优点,Flag标签已被广泛应用于蛋白表达、纯化、鉴定、相互作用和功能等多方面的研究。
Anti-Flag Magnetic Beads (Anti-Flag磁珠),也被称为Anti-DYKDDDDK Magnetic Beads,可以特异性地结合Flag标签融合蛋白,并可以借助磁力架等磁分离设备非常便捷地应用于带有Flag标签的融合蛋白或其蛋白复合物的免疫沉淀或纯化等实验。
蛋白标签技术简介及常用蛋白标签

蛋白标签技术简介及常用蛋白标签蛋白质作为生命活动的主要执行者,人们对其功能和生物学机能的研究逐步深入。
那么如何分离和研究某一特定蛋白呢?蛋白标签技术的广泛应用可以有效的解决这令许多研究者颇为头疼的问题。
目前,一些肽类和蛋白质被广泛的用于大量生产重组蛋白,它们与目的蛋白融合表达,以便于目的蛋白表达、检测、示踪和纯化。
这类多肽或蛋白,被称为蛋白标签(Protein Tag)。
例如MyC、His、GST、HA等。
而标签抗体可以高特异地结合对应的标签融合多肽或蛋白,籍以分离纯化和分析检测目的蛋白。
目前,云克隆推出了一系列蛋白标签抗体,让您从容面对蛋白实验。
先简单介绍一下系列蛋白标签。
HA标签蛋白,标签序列YPYDVPDYA,源于流感病毒的红细胞凝集素表面抗原决定簇,9个氨基酸,对外源靶蛋白的空间结构影响小,容易构建成标签蛋白融合到N端或者C端。
易于被Anti-HA抗体检测和ELISA检测。
MYC标签蛋白,MYC标签蛋白是一个含11个氨基酸的小标签,标签序列Glu-Gln-Lys-Leu-Ile-Ser-Glu-Glu-Asp-Leu,这11个氨基酸作为抗原表位表达在不同的蛋白质框架中仍可识别其相应抗体。
Myc tag已成功应用在Western-blot杂交技术、免疫沉淀和流式细胞计量术中,可用于检测重组蛋白质在靶细胞中的表达。
FLAG,Flag标签蛋白为编码8个氨基酸的亲水性多肽(DYKDDDDK),同时载体中构建的Kozak序列使得带有FLAG的融合蛋白在真核表达系统中表达效率更高。
GST,谷胱甘肽巯基转移酶在解毒过程中起到重要作用,它的天然大小为26KD。
由于GST高度可溶,可增加外源蛋白的可溶性,另外GST融合表达系统广泛应用于各种融合蛋白的表达,可提高表达量。
GST标签蛋白可直接从细菌裂解液中利用含有还原型谷胱甘肽琼脂糖凝胶(Glutathione sepharose)亲和树脂进行纯化。
而且,GST标签蛋白可在温和、非变性条件下洗脱,因此保留了蛋白的抗原性和生物活性。
常见蛋白质标签总结

/bbs/home.php?mod=space&uid =34800&do=blog&id=38530常见蛋白质标签总结(Flag、HA、cMyc、CBP等)Protein tags are peptide sequences genetically grafted onto a recombinant protein. Often these tags are removable by chemical agents or by enzymatic means, such as proteolysis or intein splicing. Tags are attached to proteins for various purposes.一、氨基酸标签(含小肽标签)A stretch of amino acids is added to the protein and enables the recovery of the labelled protein by its unique affinity. Usually its easiest to add the tag to either end of the protein to ensure its accessibility and not to disturb the protein folding.1.组氨酸标签(His tag)一般为6个组氨酸,用Ni2+(Cu2+)亲和层析纯化2.FLAG tag :N-DYKDDDDK-C ,recovered with specific antibody3.HA tag: an epitope derived from the Influenza protein haemagglutinin (HA,禽流感病毒血凝素),e.g. N-YPYDVPDYA-C,recovery with an HAantibody4.MYC tag: an epitope derived from the human proto-oncoprotein MYC,e.g.N-ILKKATAYIL-C, N-EQKLISEEDL-C,recovery with an MYCantibody5.SBP tag:Streptavidin Binding Peptide,链霉亲合素结合肽,38 amino acidtag (MDEKTTGWRGGHVVEGLAGELEQLRARLEHHPQGQREP),更多参考在Sigma6.CBP tag:钙调蛋白结合肽(CBP; 26aa)钙调蛋白结合肽与钙调素结合是Ca2+依赖的,这种结合不受标签所处的位置影响(N端和C端均可),在中性pH条件下使用2mM EGTA可以很方便的将目标蛋白洗脱下来。
HisTag_融合蛋白纯化(默克新版)

默克生命科学服务热线:400 820 8872 bioteam@
高纯度包涵体的制备 以下操作可用于任何 BugBuster®系列产品抽提的包涵体纯化。 1. 如可溶蛋白抽提步骤1-4进行操作。 2. 将步骤“4”所得到的沉淀重悬于BugBuster(货号70584),BugBuster的量与当初重悬细胞糊的体积相同。
默克生命科学服务热线:400 820 8872 bioteam@
一、亲和纯化样品的前处理
1. 菌液体积-起始目的蛋白量
纯化条件的优化需考虑多个因素,包括 His·Tag 融合蛋白表达水平和上样量。若目的蛋白未能高效表达,需要采 用一个较高的浓缩系数(concentration factor)进行菌的裂解,即在较大培养体系中,按一定比例加入一定体积 的裂解/结合缓冲液。浓缩系数定义为菌液体积与裂解/结合缓冲液体积之比。不同目的蛋白表达水平推荐使用的 裂解/结合缓冲液体积、对应的浓缩系数大小,请参考表 1。 例如,某蛋白表达水平约为 0.1mg/ml,需要在变性条件下进行小批提纯,则 100ml 的培养物离心获得的菌体, 按浓缩 100 倍比例重悬于含变性剂的 1ml 裂解/结合缓冲液中。 在非变性条件下进行纯化时,要准确预计裂解液中可溶蛋白的含量比较困难,一般建议采用 50-100 倍浓缩。
产品使用说明书
His·Tag 融合蛋白纯化操作手册
采用 pET 系统进行原核蛋白表达,蛋白的表达量达到 20mg/100ml 培养物并不是困难的事。 在大肠杆菌中表达的目的蛋白,其可溶性(可溶蛋白或包涵体)、细胞定位(细胞质、细胞周 质、培养基上清),都会对后续的纯化策略造成影响。我们建议研究者在蛋白表达后,首先进 行目的蛋白的细胞定位(请参考 pET 系统操作手册);在进行大量纯化之前,小量纯化蛋 白,摸索确定适合于具体蛋白的纯化条件,也是值得推荐的好方法。外源蛋白在大肠杆菌中表 达,可能以可溶形式存在,也可能以包涵体形式存在。尤其在高水平表达的条件下,更容易形 成包涵体。包涵体的形成与外源蛋白本身性质、载体、宿主菌、以及表达水平都有关系,可以 通过选择不同表达载体和 E.coli 宿主菌组合,摸索生长条件和适宜诱导条件,达到优化蛋白表 达的目的。His·Tag®融合蛋白,可以在天然条件或变性条件下用 NTA His·Bind 树脂或 IDA His·Bind 树脂进行纯化。
蛋白标签-基因克隆载体上的蛋白标签-齐全的各种常用标签

蛋白标签-基因克隆载体上的蛋白标签-齐全的各种常用标签蛋白标签蛋白标签(proteintag)是指利用DNA体外重组技术,与目的蛋白一起融合表达的一种多肽或者蛋白,以便于目的蛋白的表达、检测、示踪和纯化等。
蛋白表达载体按照表达宿主的不同新推出3类,分别为表达宿主为大肠杆菌,哺乳动物细胞的,以及慢病毒载体。
除了必要的复制和筛选的元件,协助表达和翻译的元件外,本文将各类载体分别按照功能标签的不同确定种类并将个标签的功能初步介绍如下:His6:His6是指六个组氨酸残基组成的融合标签,可插入在目的蛋白的C末端或N末端。
当某一个标签的使用,一是能构成表位利于纯化和检测;二是构成独特的结构特征(结合配体)利于纯化。
组氨酸残基侧链与固态的镍有强烈的吸引力,可用于固定化金属螯合层析(IMAC),对重组蛋白进行分离纯化。
使用His-tag有下面优点:1.标签的分子量小,只有~0.84KD,而GST和蛋白A分别为~26KD和~30KD,一般不影响目标蛋白的功能;2.His标签融合蛋白可以在非离子型表面活性剂存在的条件下或变性条件下纯化,前者在纯化疏水性强的蛋白得到应用,后者在纯化包涵体蛋白时特别有用,用高浓度的变性剂溶解后通过金属螯和亲和层析去除杂蛋白,使复性不受其它蛋白的干扰,或进行金属螯和亲和层析复性;3.His标签融合蛋白也被用于蛋白质-蛋白质、蛋白质-DNA相互作用研究;4.His标签免疫原性相对较低,可将纯化的蛋白直接注射动物进行免疫制备抗体;5.可应用于多种表达系统,纯化的条件温和;6.可以和其它的亲和标签一起构建双亲和标签。
Flag:Flag标签蛋白为编码8个氨基酸的亲水性多肽(DYKDDDDK),同时载体中构建的Kozak序列使得带有FLAG的融合蛋白在真核表达系统中表达效率更高。
FLAG作为标签蛋白,其融合表达目的蛋白后具有以下优点:1.FLAG作为融合表达标签,其通常不会与目的蛋白相互作用并且通常不会影响目的蛋白的功能、性质,这样就有利用研究人员对融合蛋白进行下游研究。
酵母杂交实验常见问题——单杂知识及自激活检测篇

酵母杂交实验常见问题——单杂知识及自激活检测篇下载提示:该文档是本店铺精心编制而成的,希望大家下载后,能够帮助大家解决实际问题。
文档下载后可定制修改,请根据实际需要进行调整和使用,谢谢!本店铺为大家提供各种类型的实用资料,如教育随笔、日记赏析、句子摘抄、古诗大全、经典美文、话题作文、工作总结、词语解析、文案摘录、其他资料等等,想了解不同资料格式和写法,敬请关注!Download tips: This document is carefully compiled by this editor. I hope that after you download it, it can help you solve practical problems. The document can be customized and modified after downloading, please adjust and use it according to actual needs, thank you! In addition, this shop provides you with various types of practical materials, such as educational essays, diary appreciation, sentence excerpts, ancient poems, classic articles, topic composition, work summary, word parsing, copy excerpts, other materials and so on, want to know different data formats and writing methods, please pay attention!题目:酵母杂交实验常见问题——单杂知识及自激活检测篇酵母杂交是一种广泛应用于蛋白质功能研究的实验方法,但在实际操作中仍会遇到一些常见问题。
几种常用的蛋白标签
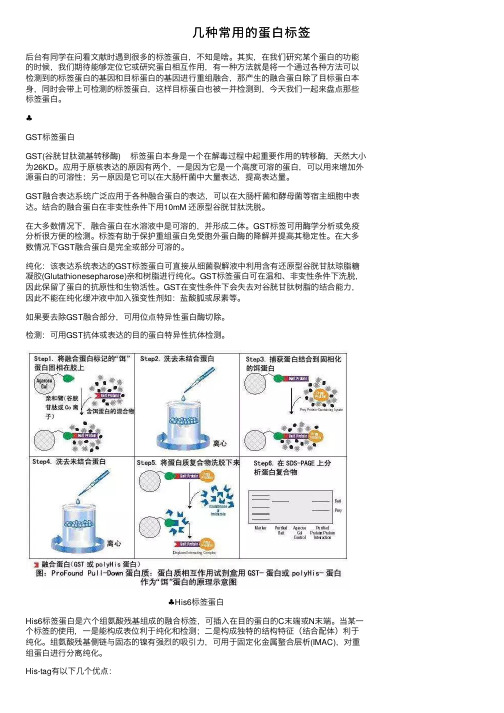
⼏种常⽤的蛋⽩标签后台有同学在问看⽂献时遇到很多的标签蛋⽩,不知是啥。
其实,在我们研究某个蛋⽩的功能的时候,我们期待能够定位它或研究蛋⽩相互作⽤,有⼀种⽅法就是将⼀个通过各种⽅法可以检测到的标签蛋⽩的基因和⽬标蛋⽩的基因进⾏重组融合,那产⽣的融合蛋⽩除了⽬标蛋⽩本⾝,同时会带上可检测的标签蛋⽩,这样⽬标蛋⽩也被⼀并检测到,今天我们⼀起来盘点那些标签蛋⽩。
♣GST标签蛋⽩GST(⾕胱⽢肽巯基转移酶) 标签蛋⽩本⾝是⼀个在解毒过程中起重要作⽤的转移酶,天然⼤⼩为26KD。
应⽤于原核表达的原因有两个,⼀是因为它是⼀个⾼度可溶的蛋⽩,可以⽤来增加外源蛋⽩的可溶性;另⼀原因是它可以在⼤肠杆菌中⼤量表达,提⾼表达量。
GST融合表达系统⼴泛应⽤于各种融合蛋⽩的表达,可以在⼤肠杆菌和酵母菌等宿主细胞中表达。
结合的融合蛋⽩在⾮变性条件下⽤10mM 还原型⾕胱⽢肽洗脱。
在⼤多数情况下,融合蛋⽩在⽔溶液中是可溶的,并形成⼆体。
GST标签可⽤酶学分析或免疫分析很⽅便的检测。
标签有助于保护重组蛋⽩免受胞外蛋⽩酶的降解并提⾼其稳定性。
在⼤多数情况下GST融合蛋⽩是完全或部分可溶的。
纯化:该表达系统表达的GST标签蛋⽩可直接从细菌裂解液中利⽤含有还原型⾕胱⽢肽琼脂糖凝胶(Glutathionesepharose)亲和树脂进⾏纯化。
GST标签蛋⽩可在温和、⾮变性条件下洗脱,因此保留了蛋⽩的抗原性和⽣物活性。
GST在变性条件下会失去对⾕胱⽢肽树脂的结合能⼒,因此不能在纯化缓冲液中加⼊强变性剂如:盐酸胍或尿素等。
如果要去除GST融合部分,可⽤位点特异性蛋⽩酶切除。
检测:可⽤GST抗体或表达的⽬的蛋⽩特异性抗体检测。
♣His6标签蛋⽩His6标签蛋⽩是六个组氨酸残基组成的融合标签,可插⼊在⽬的蛋⽩的C末端或N末端。
当某⼀个标签的使⽤,⼀是能构成表位利于纯化和检测;⼆是构成独特的结构特征(结合配体)利于纯化。
组氨酸残基侧链与固态的镍有强烈的吸引⼒,可⽤于固定化⾦属螯合层析(IMAC),对重组蛋⽩进⾏分离纯化。
免疫共沉淀研究生实验课

tag旳位置。将tag构建到蛋白旳N端还是C端,也是一种潜在 旳能够影响coIP成果旳原因。例如,分泌蛋白旳N端一般有 分泌信号肽,假如将tag构建到N端,则外分泌时会被信号肽 酶连同信号肽一起切除;所以这时必须构建在C端。再如, 多数蛋白旳C端是疏水区,有旳时候将tag构建在C端会影响 蛋白原来旳空间构造,如恰好C端同步是相互作用旳构造域, 某些时候还可能被遮蔽,从而干扰相互作用。所以,一般构 建质粒旳时候是N端、C端tagged同步分别构建,以备万一。
将裂解液转移至EP管中,4℃转鼓摇15min ,14000g 4℃离心15min ; 吸收上清液到新EP管中,每管加1μg 对照兔IgG,同步加入Protein
A agarose,4℃转鼓转30min,4℃ 10000g 离心5min; preclear及其作用 一抗和相应起源旳normal mouse, rat, rabbit or goat IgG中有相同或 相同旳成份(如无关IgG) , 用一抗相应起源旳IgG与蛋白裂解液和 protein A-agarose能够清除一抗中无关IgG对蛋白裂解液中物质旳 非特异吸附, 二是能够清除蛋白液中与protein A-agarose非特异结 合旳物质。做 preclear 旳IgG 是不针对特异性抗原旳。 琼脂糖珠旳选择 SEPHAROSE是注册商品名, 本身是agarose做旳凝胶。
4. 超声和冻融旳影响
诸多市售或自制旳裂解液过于温和,需要考虑采用机械或者超声等 手段提升裂解效率(超声要慎用,可能破坏弱旳相互作用)。
几种常用的蛋白标签的功能和优点

重组蛋白表达技术现已经广泛应用于生物学各个具体领域。
特别是体内功能研究和蛋白质的大规模生产都需要应用重组蛋白表达载体。
美国GeneCopoeia的蛋白表达载体按照表达宿主的不同新推出3类,分别为表达宿主为大肠杆菌,哺乳动物细胞的,以及慢病毒载体,宿主可以为哺乳动物细胞和原代细胞。
除了必要的复制和筛选的元件,协助表达和翻译的元件外,本文将各类载体分别按照功能标签的不同确定种类并将个标签的功能初步介绍如下:His6:His6是指六个组氨酸残基组成的融合标签,可插入在目的蛋白的C末端或N末端。
当某一个标签的使用,一是能构成表位利于纯化和检测;二是构成独特的结构特征(结合配体)利于纯化。
组氨酸残基侧链与固态的镍有强烈的吸引力,可用于固定化金属螯合层析(IMAC),对重组蛋白进行分离纯化。
使用His-tag有下面优点:1.标签的分子量小,只有~0.84KD,而GST和蛋白A分别为~26KD和~30KD,一般不影响目标蛋白的功能;2.His标签融合蛋白可以在非离子型表面活性剂存在的条件下或变性条件下纯化,前者在纯化疏水性强的蛋白得到应用,后者在纯化包涵体蛋白时特别有用,用高浓度的变性剂溶解后通过金属螯和亲和层析去除杂蛋白,使复性不受其它蛋白的干扰,或进行金属螯和亲和层析复性;3.His标签融合蛋白也被用于蛋白质-蛋白质、蛋白质-DNA相互作用研究;4.His标签免疫原性相对较低,可将纯化的蛋白直接注射动物进行免疫制备抗体;5.可应用于多种表达系统,纯化的条件温和;6.可以和其它的亲和标签一起构建双亲和标签。
Flag:Flag标签蛋白为编码8个氨基酸的亲水性多肽(DYKDDDDK),同时载体中构建的Kozak序列使得带有FLAG的融合蛋白在真核表达系统中表达效率更高。
FLAG作为标签蛋白,其融合表达目的蛋白后具有以下优点:1.FLAG作为融合表达标签,其通常不会与目的蛋白相互作用并且通常不会影响目的蛋白的功能、性质,这样就有利用研究人员对融合蛋白进行下游研究。
抗体标签

标签抗体简介标签抗体,别名为抗原表位,又称抗原决定簇,是抗原分子中决定抗原特异性的特殊区域或基团,是与抗体特异性结合的结构或序列。
随着生物技术的发展,科研人员可以通过DNA重组技术,构建包含目的基因以及表位标记的融合蛋白,进而通过特异性标签抗体对其鉴定与纯化,以达到研究的需求。
主要类别Flag抗体-抗Flag标签抗体融合标签,如Flag、GST等标签的使用可以简化蛋白质的纯化过程、控制蛋白质固定的空间取向及方便检测、使体内生物事件可视化、提高重组蛋白质的产量、增强重组蛋白质的可溶性和稳定性等。
常用的标签包括myc、HA、Flag、His、GST等。
其中Flag标签系统利用一个短的亲水性八氨基酸肽(DYKDDDDK)融合到目标蛋白。
Flag标签可位于蛋白质的C端或N端,该系统已广泛应用于各种细胞类型,包括细菌、酵母和哺乳动物细胞等,相应的Flag标签抗体也被广泛应用。
由于Flag标签系统的纯化条件是非变性的,因此可以纯化所有有活性的融合蛋白。
Flag标签可以通过加入肠激酶处理去除,肠激酶专一识别该肽序列C末端的5个氨基酸残基。
Flag抗体可以用于检测和Flag标签融合表达蛋白的表达、细胞内定位,以及纯化、定性或定量检测Flag融合表达蛋白等。
His抗体-抗His标签抗体融合标签根据其相对分子质量大小可以分为两大类:大的蛋白质分子和小的多肽片段。
融合标签的使用可以简化蛋白质的纯化过程、控制蛋白质固定的空间取向及方便检测、使体内生物事件可视化、提高重组蛋白质的产量、增强重组蛋白质的可溶性和稳定性等。
His 标签是由6个组氨酸(His-His-His-His-His-His)组成的短肽,专门设计用于重组蛋白质的吸附纯化。
由于分子量较小,并且较容易分离和纯化,His融合标签与其他标签相比有很多明显优势,是目前用于纯化的融合标签中使用最为广泛的一种。
利用His标签可以建立一个基于融合蛋白的高效检测和纯化系统。
各种蛋白标签汇总教学教材

各种蛋白标签汇总各种蛋白标签汇总蛋白标签蛋白标签(proteintag)是指利用DNA体外重组技术,与目的蛋白一起融合表达的一种多肽或者蛋白,以便于目的蛋白的表达、检测、示踪和纯化等。
随着技术的不断发展,研究人员相继开发出了具有各种不同功能的蛋白标签。
目前,这些蛋白标签已在基础研究和商业化产品生产等方面得到了广泛的应用。
TrxHISHis6是指六个组氨酸残基组成的融合标签,可插入在目的蛋白的C末端或N末端。
当某一个标签的使用,一是能构成表位利于纯化和检测;二是构成独特的结构特征(结合配体)利于纯化。
组氨酸残基侧链与固态的镍有强烈的吸引力,可用于固定化金属螯合层析(IMAC),对重组蛋白进行分离纯化。
使用His-tag有下面优点:•标签的分子量小,只有~0.84KD,而GST和蛋白A分别为~26KD和~30KD,一般不影响目标蛋白的功能;•His标签融合蛋白可以在非离子型表面活性剂存在的条件下或变性条件下纯化,前者在纯化疏水性强的蛋白得到应用,后者在纯化包涵体蛋白时特别有用,用高浓度的变性剂溶解后通过金属螯和亲和层析去除杂蛋白,使复性不受其它蛋白的干扰,或进行金属螯和亲和层析复性;•His标签融合蛋白也被用于蛋白质-蛋白质、蛋白质-DNA相互作用研究;•His标签免疫原性相对较低,可将纯化的蛋白直接注射动物进行免疫并制备抗体。
•可应用于多种表达系统,纯化的条件温和;•可以和其它的亲和标签一起构建双亲和标签。
Flag标签蛋白Flag标签蛋白为编码8个氨基酸的亲水性多肽(DYKDDDDK),同时载体中构建的Kozak序列使得带有FLAG的融合蛋白在真核表达系统中表达效率更高。
FLAG作为标签蛋白,其融合表达目的蛋白后具有以下优点:•FLAG作为融合表达标签,其通常不会与目的蛋白相互作用并且通常不会影响目的蛋白的功能、性质,这样就有利用研究人员对融合蛋白进行下游研究。
•融合FLAG的目的蛋白,可以直接通过FLAG进行亲和层析,此层析为非变性纯化,可以纯化有活性的融合蛋白,并且纯化效率高。
