材料科学基础 习题2
材料科学基础2复习题与参考答案

材料科学基础2复习题及部分参考答案一、名词解释1、再结晶:指经冷变形的金属在足够高的温度下加热时,通过新晶粒的形核及长大,以无畸变的等轴晶粒取代变形晶粒的过程。
2、交滑移:在晶体中,出现两个或多个滑移面沿着某个共同的滑移方向同时或交替滑移。
3、冷拉:在常温条件下,以超过原来屈服点强度的拉应力,强行拉伸聚合物,使其产生塑性变形以达到提高其屈服点强度和节约材料为目的。
(《笔记》聚合物拉伸时出现的细颈伸展过程。
)4、位错:指晶体材料的一种内部微观缺陷,即原子的局部不规则排列(晶体学缺陷)。
(《书》晶体中某处一列或者若干列原子发生了有规律的错排现象)5、柯氏气团:金属内部存在的大量位错线,在刃型位错线附近经常会吸附大量的异类溶质原子(大小不同吸附的位置有差别),形成所谓的“柯氏气团”。
(《书》溶质原子与位错弹性交互作用的结果,使溶质原子趋于聚集在位错周围,以减小畸变,降低体系的能量,使体系更加稳定。
)6、位错密度:单位体积晶体中所含的位错线的总长度或晶体中穿过单位截面面积的位错线数目。
7、二次再结晶:晶粒的不均匀长大就好像在再结晶后均匀、细小的等轴晶粒中又重新发生了再结晶。
8、滑移的临界分切应力:滑移系开动所需要的最小分切应力。
(《书》晶体开始滑移时,滑移方向上的分切应力。
)9、加工硬化:金属材料在再结晶温度以下塑性变形时强度和硬度升高,而塑性和韧性降低的现象,又称冷作硬化。
(《书》随塑性变形的增大,塑性变形抗力不断增加的现象。
)10、热加工:金属铸造、热扎、锻造、焊接和金属热处理等工艺的总称。
(《书》使金属在再结晶温度以上发生加工变形的工艺。
)11、柏氏矢量:是描述位错实质的重要物理量。
反映出柏氏回路包含的位错所引起点阵畸变的总积累。
(《书》揭示位错本质并描述位错行为的矢量。
)反映由位错引起的点阵畸变大小的物理量。
12、多滑移:晶体的滑移在两组或者更多的滑移面(系)上同时进行或者交替进行。
13、堆垛层错:晶体结构层正常的周期性重复堆垛顺序在某二层间出现了错误,从而导致的沿该层间平面(称为层错面)两侧附近原子的错排的一种面缺陷。
材料科学基础2复习题及参考答案

材料科学基础2复习题及部分参考答案一、名词解释1、再结晶:指经冷变形的金属在足够高的温度下加热时,通过新晶粒的形核及长大,以无畸变的等轴晶粒取代变形晶粒的过程。
2、交滑移:在晶体中,出现两个或多个滑移面沿着某个共同的滑移方向同时或交替滑移。
3、冷拉:在常温条件下,以超过原来屈服点强度的拉应力,强行拉伸聚合物,使其产生塑性变形以达到提高其屈服点强度和节约材料为目的。
(《笔记》聚合物拉伸时出现的细颈伸展过程。
)4、位错:指晶体材料的一种内部微观缺陷,即原子的局部不规则排列(晶体学缺陷)。
(《书》晶体中某处一列或者若干列原子发生了有规律的错排现象)5、柯氏气团:金属内部存在的大量位错线,在刃型位错线附近经常会吸附大量的异类溶质原子(大小不同吸附的位置有差别),形成所谓的“柯氏气团”。
(《书》溶质原子与位错弹性交互作用的结果,使溶质原子趋于聚集在位错周围,以减小畸变,降低体系的能量,使体系更加稳定。
)6、位错密度:单位体积晶体中所含的位错线的总长度或晶体中穿过单位截面面积的位错线数目。
7、二次再结晶:晶粒的不均匀长大就好像在再结晶后均匀、细小的等轴晶粒中又重新发生了再结晶。
8、滑移的临界分切应力:滑移系开动所需要的最小分切应力。
(《书》晶体开始滑移时,滑移方向上的分切应力。
)9、加工硬化:金属材料在再结晶温度以下塑性变形时强度和硬度升高,而塑性和韧性降低的现象,又称冷作硬化。
(《书》随塑性变形的增大,塑性变形抗力不断增加的现象。
)10、热加工:金属铸造、热扎、锻造、焊接和金属热处理等工艺的总称。
(《书》使金属在再结晶温度以上发生加工变形的工艺。
)11、柏氏矢量:是描述位错实质的重要物理量。
反映出柏氏回路包含的位错所引起点阵畸变的总积累。
(《书》揭示位错本质并描述位错行为的矢量。
)反映由位错引起的点阵畸变大小的物理量。
12、多滑移:晶体的滑移在两组或者更多的滑移面(系)上同时进行或者交替进行。
13、堆垛层错:晶体结构层正常的周期性重复堆垛顺序在某二层间出现了错误,从而导致的沿该层间平面(称为层错面)两侧附近原子的错排的一种面缺陷。
材料科学基础-张代东-习题答案(2)

第1章 习题解答1-1 解释下列基本概念金属键,离子键,共价键,范德华力,氢键,晶体,非晶体,理想晶体,单晶体,多晶体,晶体结构,空间点阵,阵点,晶胞,7个晶系,14种布拉菲点阵,晶向指数,晶面指数,晶向族,晶面族,晶带,晶带轴,晶带定理,晶面间距,面心立方,体心立方,密排立方,多晶型性,同素异构体,点阵常数,晶胞原子数,配位数,致密度,四面体间隙,八面体间隙,点缺陷,线缺陷,面缺陷,空位,间隙原子,肖脱基缺陷,弗兰克尔缺陷,点缺陷的平衡浓度,热缺陷,过饱和点缺陷,刃型位错,螺型位错,混合位错,柏氏回路,柏氏矢量,位错的应力场,位错的应变能,位错密度,晶界,亚晶界,小角度晶界,大角度晶界,对称倾斜晶界,不对称倾斜晶界,扭转晶界,晶界能,孪晶界,相界,共格相界,半共格相界,错配度,非共格相界(略)1-2 原子间的结合键共有几种?各自特点如何? 答:原子间的键合方式及其特点见下表。
类 型 特 点离子键 以离子为结合单位,无方向性和饱和性 共价键 共用电子对,有方向性键和饱和性 金属键 电子的共有化,无方向性键和饱和性分子键 借助瞬时电偶极矩的感应作用,无方向性和饱和性 氢 键依靠氢桥有方向性和饱和性1-3 问什么四方晶系中只有简单四方和体心四方两种点阵类型?答:如下图所示,底心四方点阵可取成更简单的简单四方点阵,面心四方点阵可取成更简单的体心四方点阵,故四方晶系中只有简单四方和体心四方两种点阵类型。
1-4 试证明在立方晶系中,具有相同指数的晶向和晶面必定相互垂直。
证明:根据晶面指数的确定规则并参照下图,(hkl )晶面ABC 在a 、b 、c 坐标轴上的截距分别为h a 、k b 、l c ,k h b a AB +-=,l h c a AC +-=,lk ca BC +-=;根据晶向指数的确定规则,[hkl ]晶向cb a L l k h ++=。
利用立方晶系中a=b=c , 90=γ=β=α的特点,有 0))((=+-++=⋅k h l k h ba cb a AB L 0))((=+-++=⋅lh l k h ca cb a AC L 由于L 与ABC 面上相交的两条直线垂直,所以L 垂直于ABC 面,从而在立方晶系具有相同指数的晶向和晶面相互垂直。
材料科学基础习题二

材料科学基础习题二1.指出下列概念的错误之处,并更正。
1)所谓过冷是指结晶过程中平台温度与冷却曲线上熔点之间的差异。
2)金属结晶时,原子从液相无序排列到固相有序排列,使体系熵值减小,因此是一个自发过程。
3)在任何温度下,液态金属中最大的结构波动是晶体胚。
4)在任何温度下,液相中最大的结构波动是原子核。
5)所谓临界晶核,就是体系自由能的减少完全补偿表面自由能的增加时的晶胚大小。
6)在液态金属中,任何小于临界核半径的晶体胚都不能形核,但只要有足够的能量波动来提供形核功,它就可以形核。
7)测定某纯金属铸件结晶时的最大过冷度,其实测值与用公式0.2tm计算值,基本一致。
8)当一些铸件结晶时,由于快速冷却速度,均匀形核率N1增加,非均匀形核率N2也增加,因此总形核率为n=N1+N2。
9)若在过冷液体中,外加10000颗形核剂,则结晶后就可以形成10000颗晶粒。
10)从非均匀形核功的计算公式中可以看出,当润湿角为0度时,非均匀形核的形核功最大。
11)为了生产出一批厚度大、粒度均匀的砂型铸件,可以采用在砂型铸造过程中加入成核剂的方法。
12)非均匀形核总是比均匀形核容易,因为前者是以外加质点为结晶核心,不像后者那样形成界面,而引起自由能的增加。
13)在研究金属晶粒细化过程时,我们主要寻找熔点低、晶格常数与金属相近的成核剂,它们的成核催化效率最高。
14)纯金属生长时,无论液固界面呈粗糙型还是光滑型,其液相原子都一个一个地沿着固相面得垂直方向连接上去。
15)无论温度分布如何,普通纯金属的生长都是树枝状界面。
16)氯化铵饱和水溶液与纯金属结晶终了时的组织形态一样,前者呈树枝状,后者也成树枝晶。
一17)人们无法观察到极纯金属的树枝状生长过程,所以关于树枝状的生长形态仅仅是一种推想。
18)在液态纯金属中加入成核剂时,其生长形式总是树枝状的。
19)纯金属结晶时,若呈垂直方式生长,其界面时而光滑,时而粗糙,交替生长。
20)从宏观上观察,若液固界面是平直的,称为光滑界面结构;若是呈金属锯齿形的,称为粗糙界面结构。
《材料科学基础》习题附答案

《材料科学基础》习题附答案第⼆章思考题与例题1. 离⼦键、共价键、分⼦键和⾦属键的特点,并解释⾦属键结合的固体材料的密度⽐离⼦键或共价键固体⾼的原因?2. 从结构、性能等⽅⾯描述晶体与⾮晶体的区别。
3. 何谓理想晶体?何谓单晶、多晶、晶粒及亚晶?为什么单晶体成各向异性⽽多晶体⼀般情况下不显⽰各向异性?何谓空间点阵、晶体结构及晶胞?晶胞有哪些重要的特征参数?4. ⽐较三种典型晶体结构的特征。
(Al 、α-Fe 、Mg 三种材料属何种晶体结构?描述它们的晶体结构特征并⽐较它们塑性的好坏并解释。
)何谓配位数?何谓致密度?⾦属中常见的三种晶体结构从原⼦排列紧密程度等⽅⾯⽐较有何异同?5. 固溶体和中间相的类型、特点和性能。
何谓间隙固溶体?它与间隙相、间隙化合物之间有何区别?(以⾦属为基的)固溶体与中间相的主要差异(如结构、键性、性能)是什么?6. 已知Cu 的原⼦直径为2.56A ,求Cu 的晶格常数,并计算1mm 3Cu 的原⼦数。
7. 已知Al 相对原⼦质量Ar (Al )=26.97,原⼦半径γ=0.143nm ,求Al 晶体的密度。
8 bcc 铁的单位晶胞体积,在912℃时是0.02464nm 3;fcc 铁在相同温度时其单位晶胞体积是0.0486nm 3。
当铁由bcc 转变为fcc 时,其密度改变的百分⽐为多少?9. 何谓⾦属化合物?常见⾦属化合物有⼏类?影响它们形成和结构的主要因素是什么?其性能如何?10. 在⾯⼼⽴⽅晶胞中画出[012]和[123]晶向。
在⾯⼼⽴⽅晶胞中画出(012)和(123)晶⾯。
11. 设晶⾯(152)和(034)属六⽅晶系的正交坐标表述,试给出其四轴坐标的表⽰。
反之,求(3121)及(2112)的正交坐标的表⽰。
(练习),上题中均改为相应晶向指数,求相互转换后结果。
12.在⼀个⽴⽅晶胞中确定6个表⾯⾯⼼位置的坐标,6个⾯⼼构成⼀个正⼋⾯体,指出这个⼋⾯体各个表⾯的晶⾯指数,各个棱边和对⾓线的晶向指数。
材料科学基础试卷(二)与参考答案

材料科学基础试卷(二)与参考答案一、名词解释(每小题1分,共10分)1.晶胞2.间隙固溶体3.临界晶核4.枝晶偏析5.离异共晶6.反应扩散7.临界分切应力8.回复9.调幅分解10. 二次硬化二、判断正误(每小题1分,共10分)正确的在括号内画“√”, 错误的画“×”1. 金属中典型的空间点阵有体心立方、面心立方和密排六方三种。
( )2. 作用在位错线上的力F 的方向永远垂直于位错线并指向滑移面 上的未滑移区。
( )3. 只有置换固溶体的两个组元之间才能无限互溶,间隙固溶体则不能。
( )4. 金属结晶时,原子从液相无序排列到固相有序排列,使体系熵值减小,因此是一个自发过程。
( )5. 固溶体凝固形核的必要条件同样是ΔG B <0、结构起伏和能量起伏。
( )6. 三元相图垂直截面的两相区内不适用杠杆定律。
( )7. 物质的扩散方向总是与浓度梯度的方向相反。
( )8. 塑性变形时,滑移面总是晶体的密排面,滑移方向也总是密排方向。
( )9. 和液固转变一样,固态相变也有驱动力并要克服阻力,因此两种转变的难易程度相似。
( )10.除Co 以外,几乎所有溶入奥氏体中的合金元素都能使C 曲线 左移,从而增加钢的淬透性。
( )三、作图题(每小题5分,共15分)1. 在简单立方晶胞中标出具有下列密勒指数的晶面和晶向:a)立方晶系 (421),(231),[112];b)六方晶系(1112),[3112]。
2. 设面心立方晶体中的(111)为滑移面,位错滑移后的滑移矢量为2a [110]。
(1)在晶胞中画出柏氏矢量b的方向并计算出其大小。
(2)在晶胞中画出引起该滑移的刃型位错和螺型位错的位错线方向,并写出此二位错线的晶向指数。
3.如下图所示,将一锲形铜片置于间距恒定的两轧辊间轧制。
试画出轧制后铜片经再结晶后晶粒大小沿片长方向变化的示意图。
四、相图分析(共20分)(1) 就Fe-Fe3C相图,回答下列问题:1. 默画出Fe-Fe3C相图,用相组成物填写相图;2. 分析含碳量为1.0wt%的过共析钢的平衡结晶过程,并绘出室温组织示意图。
材料科学基础-张代东-习题问题详解(2)
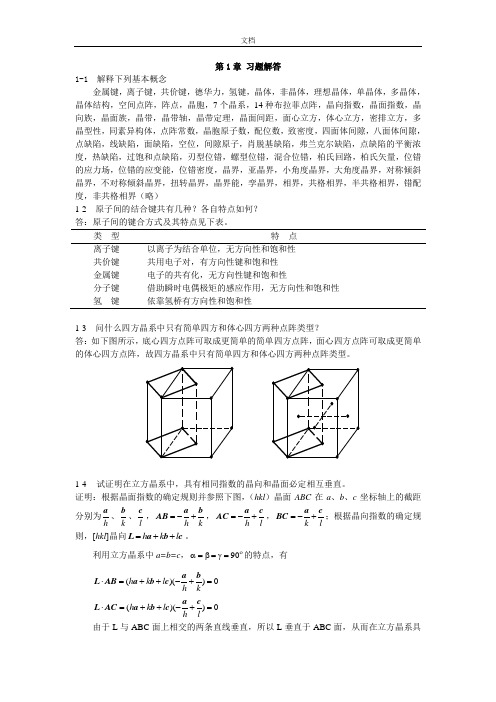
第1章 习题解答1-1 解释下列基本概念金属键,离子键,共价键,德华力,氢键,晶体,非晶体,理想晶体,单晶体,多晶体,晶体结构,空间点阵,阵点,晶胞,7个晶系,14种布拉菲点阵,晶向指数,晶面指数,晶向族,晶面族,晶带,晶带轴,晶带定理,晶面间距,面心立方,体心立方,密排立方,多晶型性,同素异构体,点阵常数,晶胞原子数,配位数,致密度,四面体间隙,八面体间隙,点缺陷,线缺陷,面缺陷,空位,间隙原子,肖脱基缺陷,弗兰克尔缺陷,点缺陷的平衡浓度,热缺陷,过饱和点缺陷,刃型位错,螺型位错,混合位错,柏氏回路,柏氏矢量,位错的应力场,位错的应变能,位错密度,晶界,亚晶界,小角度晶界,大角度晶界,对称倾斜晶界,不对称倾斜晶界,扭转晶界,晶界能,孪晶界,相界,共格相界,半共格相界,错配度,非共格相界(略)1-2 原子间的结合键共有几种?各自特点如何? 答:原子间的键合方式及其特点见下表。
类 型 特 点离子键 以离子为结合单位,无方向性和饱和性 共价键 共用电子对,有方向性键和饱和性 金属键 电子的共有化,无方向性键和饱和性分子键 借助瞬时电偶极矩的感应作用,无方向性和饱和性 氢 键依靠氢桥有方向性和饱和性1-3 问什么四方晶系中只有简单四方和体心四方两种点阵类型?答:如下图所示,底心四方点阵可取成更简单的简单四方点阵,面心四方点阵可取成更简单的体心四方点阵,故四方晶系中只有简单四方和体心四方两种点阵类型。
1-4 试证明在立方晶系中,具有相同指数的晶向和晶面必定相互垂直。
证明:根据晶面指数的确定规则并参照下图,(hkl )晶面ABC 在a 、b 、c 坐标轴上的截距分别为h a 、k b 、l c ,k h b a AB +-=,l h c a AC +-=,lk ca BC +-=;根据晶向指数的确定规则,[hkl ]晶向cb a L l k h ++=。
利用立方晶系中a=b=c ,ο90=γ=β=α的特点,有0))((=+-++=⋅kh l k h ba cb a AB L 0))((=+-++=⋅lh l k h ca cb a AC L 由于L 与ABC 面上相交的两条直线垂直,所以L 垂直于ABC 面,从而在立方晶系具有相同指数的晶向和晶面相互垂直。
材料科学基础复习题第二部分

复习题(下)第六章空位与位错本章的主要内容:晶体中的缺陷,晶体缺陷的分类晶体缺陷的形成点缺陷:点缺陷的种类,点缺陷的形成,点缺陷的运动,点缺陷的平衡浓度,点缺陷对材料性能的影响位错:位错理论的起源:理论切变强度,位错学说位错的观察位错基本类型及特征:刃型位错,螺型位错,混合位错柏氏矢量:确定方法,柏氏矢量的模,实际晶体中的柏氏矢量,柏氏矢量的特性,位错密度外力场中作用在位错线上的力位错运动:滑移,攀移,派一纳力,混合位错的运动位错的弹性性质:直螺错的应力场,直刃错的应力场,混合直位错的应力场位错的应变能及位错线张力位错间的交互作用:两根平行螺位错的交互作用,两根平行刃位错的交互作用,位错的相互交截:螺型位错与螺型位错,刃错与刃错,螺错与刃错位错的塞积位错的增殖实际晶体中的位错:单位位错,堆垛层错,不全位错:肖克莱,弗兰克不全位错位错反应及汤普逊四面体位错与溶质原子的交互作用:弹性交互作用,柯垂尔气团,斯诺克气团,静电交互作用化学交互作用1 填空1 空位是热力学_______________的缺陷,而位错是热力学_____________的缺陷。
2 fcc晶体中单位位错(全位错)的柏氏矢量是_________________;bcc晶体中单位位错(全位错)的柏氏矢量是_________________;hcp晶体中单位位错(全位错)的柏氏矢量是_________________;fcc中Frank位错的柏氏矢量是___________。
3 一根柏氏矢量b=a/2<110>的扩展位错滑出晶体后,在晶体表面产生的台阶的高度为_____________________。
4 在某温度下,晶体中的空位数与点阵数的比值称为__________________。
2ξ为位错线单位矢量,b为柏氏矢量,则bξ=0时为_______位错,bξ=b时为________________位错,bξ =-b时为______________位错。
无机材料科学基础课后习题答案2

2-1 名词解释:配位数与配位体,同质多晶与多晶转变,位移性转变与重建性转变,晶体场理论与配位场理论。
答:配位数:晶体结构中与一个离子直接相邻的异号离子数。
配位体:晶体结构中与某一个阳离子直接相邻、形成配位关系的各个阴离子中心连线所构成的多面体。
同质多晶:同一化学组成在不同外界条件下(温度、压力、pH值等),结晶成为两种以上不同结构晶体的现象。
多晶转变:当外界条件改变到一定程度时,各种变体之间发生结构转变,从一种变体转变成为另一种变体的现象。
位移性转变:不打开任何键,也不改变原子最邻近的配位数,仅仅使结构发生畸变,原子从原来位置发生少许位移,使次级配位有所改变的一种多晶转变形式。
重建性转变:破坏原有原子间化学键,改变原子最邻近配位数,使晶体结构完全改变原样的一种多晶转变形式。
晶体场理论:认为在晶体结构中,中心阳离子与配位体之间是离子键,不存在电子轨道的重迭,并将配位体作为点电荷来处理的理论。
配位场理论:除了考虑到由配位体所引起的纯静电效应以外,还考虑了共价成键的效应的理论。
图2-1 MgO晶体中不同晶面的氧离子排布示意图2-2 面排列密度的定义为:在平面上球体所占的面积分数。
(a)画出MgO(NaCl型)晶体(111)、(110)和(100)晶面上的原子排布图;(b)计算这三个晶面的面排列密度。
解:MgO晶体中O2-做紧密堆积,Mg2+填充在八面体空隙中。
(a)(111)、(110)和(100)晶面上的氧离子排布情况如图2-1所示。
(b)在面心立方紧密堆积的单位晶胞中,(111)面:面排列密度=(110)面:面排列密度=(100)面:面排列密度=2-3 试证明等径球体六方紧密堆积的六方晶胞的轴比c/a≈1.633。
证明:六方紧密堆积的晶胞中,a轴上两个球直接相邻,a0=2r;c轴方向上,中间的一个球分别与上、下各三个球紧密接触,形成四面体,如图2-2所示:图2-2 六方紧密堆积晶胞中有关尺寸关系示意图2-4 设原子半径为R,试计算体心立方堆积结构的(100)、(110)、(111)面的面排列密度和晶面族的面间距。
【材料科学基础经典习题及答案】考试试题2

13.设面心立方晶体中的 为滑移面,位错滑移后的滑移矢量为 。
1)在晶胞中画出柏氏矢量b的方向并计算出其大小。
2)在晶胞中画出引起该滑移的刃型位错和螺型位错的位错线方向,并写出此二位错线的晶向指数。
14.判断下列位错反应能否进行。
1) 2)
3) 4)
2)指出位错环上各段位错线的类型,并画出位错运动出晶体后,滑移方向及滑移量。
12.设所示立方晶体中的滑移面ABCD平行于晶体的上、下底面。晶体中有一条位错线 段在滑移面上并平行AB, 段与滑移面垂直。位错的柏氏矢量b与 平行而与 垂直。
试问:
1)欲使 段位错在ABCD滑移面上运动而 不动,应对晶体施加怎样的应力?
15.若面心立方晶体中有b= 的单位位错及b= 的不全位错,此二位错相遇产生位错反应。
1)问此反应能否进行?为什么?
2)写出合成位错的柏氏矢量,并说明合成位错的类型。
16.若已知某晶体中位错密度 。1)由实验测得F-R位错源的平均长度为 ,求位错网络中F-R位错源的数目。2)计算具有这种F-R位错源的镍晶体发生滑移时所需要的切应力。已知Ni的 Pa, 。
7.1.6l×l013个原子/mm2;1.14X1013个原子/mm2;1.86×1013个原子/mm2。
8.(1) 5.29×1028个矽原子/m3;(2) 0.33。
9.9. 0.4×10-18/个原子。
10.1.06×1014倍。
11.(1)这种看法不正确。在位错环运动移出晶体后,滑移面上、下两部分晶体相对移动的距离是由其柏氏矢量决定的。位错环的柏氏矢量为b,故其相对滑移了一个b的距离。(2) A'B'为右螺型位错,C'D'为左螺型位错;B'C'为正刃型位错,D'A'为负刃型位错。位错运动移出晶体后滑移方向及滑移量如附图2.3所示。
材料科学基础1-8章例题、作业题及其解答

第2章 例 题(A )1. 在面心立方晶胞中画出[012]和[123]晶向。
2. 在面心立方晶胞中画出(012)和(123)晶面。
3. 右图中所画晶面的晶面指数是多少?4. 设晶面(152)和(034)属六方晶系的正交坐标表述,试给出其四轴坐标的表示。
反之,求(3121)及(2112)的正交坐标的表示。
5. (练习),上题中均改为相应晶向指数,求相互转换后结果。
答案:2. (2110) 4. (1562), (0334) 5. [1322] [1214] (123) (212)[033] [302]第2章 例题答案(A)4. (152))2615(6)51()(⇒-=+-=+-=v u t (034))4303(3)30()(⇒-=+-=+-=v u t (1213)⇒ (123) (2112) ⇒ (212)5. [152]]2231[22)51(31)(313)152(31)2(311)512(31)2(31⇒⎪⎪⎪⎭⎪⎪⎪⎬⎫==-=+-=+-==-⨯=-=-=-⨯=-=W w V U t U V v V U u [034]]4121[41)30(31)(312)032(31)2(311)302(31)2(31⇒⎪⎪⎪⎭⎪⎪⎪⎬⎫==-=+-=+-==-⨯=-=-=-⨯=-=W w V U t U V v V U u]3121[]033[33)1(20)1(1⇒⎪⎭⎪⎬⎫===--=-==---=-=w W t v V t u U[2112]]302[20)1(13)1(2⇒⎪⎭⎪⎬⎫===---=-==--=-=w W t v V t u U第2章 例 题(B )1. 已知Cu 的原子直径为2.56A ,求Cu 的晶格常数,并计算1mm 3Cu 的原子数。
2. 已知Al 相对原子质量Ar (Al )=26.97,原子半径γ=0.143nm ,求Al 晶体的密度。
3. bcc 铁的单位晶胞体积,在912℃时是0.02464nm 3;fcc 铁在相同温度时其单位晶胞体积是0.0486nm 3。
武汉理工大学材料科学基础 第二章 部分习题

非化学计量结构缺陷(电荷缺陷) 非化学计量结构缺陷(电荷缺陷)
非化学计量化合物类型: 非化学计量化合物类型: 类型 阴离子缺位型 阳离子填隙型 阴离子间隙型 阳离子空位型
2-1 名词解释: 名词解释:
类质同晶:化学组成相似或相近的物质,在相同的热力学条件下, 类质同晶:化学组成相似或相近的物质,在相同的热力学条件下,形 成的晶体具有相同的结构,这种现象称为类质同晶现象。 类质同晶现象 成的晶体具有相同的结构,这种现象称为类质同晶现象。 同质多晶:化学组成相同的物质,在不同的热力学条件下, 同质多晶:化学组成相同的物质,在不同的热力学条件下,结晶成结 构不同的晶体的现象。 构不同的晶体的现象。 弗仑克尔Frankel缺陷 正常结点上的原子(离子)跳入间隙,形成 缺陷:正常结点上的原子 离子)跳入间隙 间隙, 弗仑克尔 缺陷 正常结点上的原子( 间隙原子。 间隙原子。 肖特基Schttky缺陷:正常结点上的原子离开平衡位置迁移到晶 缺陷: 肖特基 缺陷 体表面,在原来位置形成空位。 体表面,在原来位置形成空位。 刃位错:位错线与滑移矢量垂直的位错。 刃位错:位错线与滑移矢量垂直的位错。 螺旋位错:位错线与滑移方向相互平行, 螺旋位错:位错线与滑移方向相互平行,位错线周围的一组原子面形 成了一个连续的螺旋形坡面,故称为螺位错。 成了一个连续的螺旋形坡面,故称为螺位错。 正型尖晶石: 尖晶石)型结构O 按立方紧密堆积排列, 正型尖晶石: MgAl2O4(尖晶石)型结构 2-按立方紧密堆积排列, 二价离子A充填 充填1/8 四面体空隙,三价离子 充填 八面体空隙 四面体空隙,三价离子B充填 八面体空隙—— 充填1/2八面体空隙 二价离子 充填 正型尖晶石结构 反型尖晶石结构:二价阳离子充填八面体空隙, 反型尖晶石结构:二价阳离子充填八面体空隙,三价阳离子一半充填 四面体空隙,另一半充填八面体空隙中。 四面体空隙,另一半充填八面体空隙中。
材料科学基础第二章答案

习题:第一章第二章第三章第四章第五章第六章第七章第八章第九章第十章第十一章答案:第一章第二章第三章第四章第五章第六章第七章第八章第九章第十章第十一章2-1 略。
2-2 (1)一晶面在x、y、z轴上的截距分别为2a、3b、6c,求该晶面的晶面指数;(2)一晶面在x、y、z轴上的截距分别为a/3、b/2、c,求出该晶面的晶面指数。
答:(1)h:k:l==3:2:1,∴该晶面的晶面指数为(321);(2)h:k:l=3:2:1,∴该晶面的晶面指数为(321)。
2-3 在立方晶系晶胞中画出下列晶面指数和晶向指数:(001)与[],(111)与[],()与[111],()与[236],(257)与[],(123)与[],(102),(),(),[110],[],[]答:(001)与[]为:2-4 定性描述晶体结构的参量有哪些?定量描述晶体结构的参量又有哪些?答:定性:对称轴、对称中心、晶系、点阵。
定量:晶胞参数。
2-5 依据结合力的本质不同,晶体中的键合作用分为哪几类?其特点是什么?答:晶体中的键合作用可分为离子键、共价键、金属键、范德华键和氢键。
离子键的特点是没有方向性和饱和性,结合力很大。
共价键的特点是具有方向性和饱和性,结合力也很大。
金属键是没有方向性和饱和性的的共价键,结合力是离子间的静电库仑力。
范德华键是通过分子力而产生的键合,分子力很弱。
氢键是两个电负性较大的原子相结合形成的键,具有饱和性。
2-6 等径球最紧密堆积的空隙有哪两种?一个球的周围有多少个四面体空隙、多少个八面体空隙?答:等径球最紧密堆积有六方和面心立方紧密堆积两种,一个球的周围有8个四面体空隙、6个八面体空隙。
2-7 n 个等径球作最紧密堆积时可形成多少个四面体空隙、多少个八面体空隙?不等径球是如何进行堆积的?答:n 个等径球作最紧密堆积时可形成n 个八面体空隙、2n 个四面体空隙。
不等径球体进行紧密堆积时,可以看成由大球按等径球体紧密堆积后,小球按其大小分别填充到其空隙中,稍大的小球填充八面体空隙,稍小的小球填充四面体空隙,形成不等径球体紧密堆积。
材料科学基础 习题2答案
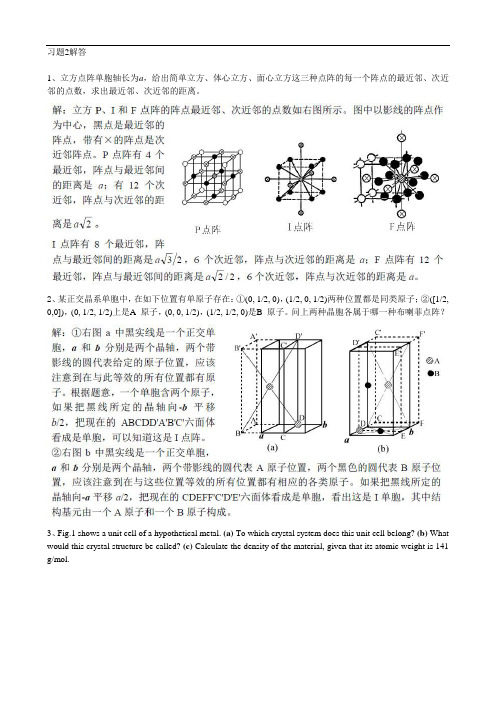
习题2解答1、立方点阵单胞轴长为a,给出简单立方、体心立方、面心立方这三种点阵的每一个阵点的最近邻、次近邻的点数,求出最近邻、次近邻的距离。
2、某正交晶系单胞中,在如下位置有单原子存在:①(0, 1/2, 0),(1/2, 0, 1/2)两种位置都是同类原子;②([1/2, 0,0]),(0, 1/2, 1/2)上是A 原子,(0, 0, 1/2),(1/2, 1/2, 0)是B 原子。
问上两种晶胞各属于哪一种布喇菲点阵?3、Fig.1 shows a unit cell of a hypothetical metal. (a) To which crystal system does this unit cell belong? (b) What would this crystal structure be called? (c) Calculate the density of the material, given that its atomic weight is 141 g/mol.Fig.1 Fig. 2体心正方,=8.5×1034、The unit cell for uranium has orthorhombic symmetry, with a, b, and c lattice parameters of 0.286, 0.587 and 0.495 nm, respectively. If its density, atomic weight, and atomic radius are 19.05 g/cm3, 238.03 g/mol, and 0.1385 nm, respectively, compute the atomic packing factor(APF).=0.5365、Three different crystallographic planes for a unit cell of a hypothetical metal are shown in Fig.2. The circles represent atoms. (a) To what crystal system does the unit cell belong? (b) What would this crystal structure be called? (c) If the density of this metal is 18.91 g/cm3, determine its atomic weight.正交,面心正交,42.7g/mol。
材料科学基础试题及答案

材料科学基础试题及答案一、选择题1. 材料科学中的“四要素”是指()。
A. 组成、结构、性能、加工B. 组成、结构、性能、应用C. 材料、工艺、设备、产品D. 材料、结构、性能、应用答案:B2. 下列哪种材料属于金属材料?A. 碳纤维B. 聚氯乙烯C. 铝合金D. 陶瓷答案:C3. 材料的屈服强度与抗拉强度之间的关系是()。
A. 屈服强度大于抗拉强度B. 屈服强度等于抗拉强度C. 屈服强度小于抗拉强度D. 无固定关系答案:A4. 非晶态材料的特点之一是()。
A. 高强度B. 各向同性C. 无长程有序D. 高导热性答案:C5. 下列关于纳米材料的描述,正确的是()。
A. 纳米材料仅指尺寸在纳米级别的材料B. 纳米材料具有宏观材料的所有性质C. 纳米材料因其尺寸效应表现出特殊性能D. 纳米材料的应用受到限制答案:C二、填空题1. 材料的______和______是决定其宏观性能的基本因素。
答案:组成、结构2. 金属材料的塑性变形主要是通过______和______来实现的。
答案:滑移、孪晶3. 陶瓷材料的主要特点是______、______和______。
答案:高硬度、高强度、耐磨损4. 复合材料是由两种或两种以上不同______、______和______的材料组合而成。
答案:材料类型、性能、形态5. 形状记忆合金在______作用下能够恢复到原始形状。
答案:温度三、简答题1. 简述材料的疲劳现象及其影响因素。
答:材料的疲劳现象是指在反复的应力作用下,材料逐渐产生并扩展裂纹,最终导致断裂的现象。
影响疲劳的因素包括应力的大小和作用方式、材料的微观结构、表面状态、环境条件等。
2. 说明金属材料的冷加工硬化现象及其应用。
答:冷加工硬化是指金属材料在冷加工过程中,由于晶粒变形和位错密度的增加,导致材料的硬度和强度提高,塑性降低的现象。
该现象在制造高强度、高硬度的零件和工具中具有重要应用。
3. 描述陶瓷材料的断裂机理。
材料科学基础II复习题

第四章扩散一、选择题1.在Kirkendall效应中,Zn的扩散通量在通过时大于Cu的通量扩散通量。
A 原始涂层(焊接)面B 俣野面C 标记面2.肖特基(Schottky)型空位表示形成的无序分布缺陷。
A 等量的阳离子和阴离子空位B 双空位C 等量的间隙阳离子和间隙阴离子3.作为塑料使用的高分子,在室温使用应处在。
A 高弹态B 玻璃态C黏流态4. 根据菲克第一定律,当扩散系数D为,表示发生上坡扩散。
A D=0B D<0C D>05. 原子的扩散是一种无规则行走,故扩散距离(x)和扩散时间(t)的关系为。
x∝Dt2 C x2∝DtABx∝Dt6. 高分子根据它们在高温时的力学特征可分为热塑性和热固性两类,在下列的高分子中属于热塑性高分子。
A聚苯乙烯B聚碳酸酯C聚乙烯7. 方铁矿(FeO)中部分Fe2+离子被氧化为Fe3+离子,此时晶体中的Fe原子数氧原子数。
A大于B小于C等于8. 在发生上坡扩散的系统中,扩散原子的化学势随其浓度的增加而。
A增加B减小C无关9. 俣野面指的是在该面两侧。
A扩散原子浓度相等B扩散原子化学势相等C扩散原子扩散通量相等但方向相反10. 高分子材料形成皮革态现象是。
A晶态和高弹态的综合效果B玻璃态和高弹态的综合效果C玻璃态和晶态的综合效果11. 以还原法由钛白粉(TiO2)制备的Ti2O3晶体结构中,最易出现的点缺陷为。
A氧离子空位B钛离子空位C间隙钛离子12. A-B合金与纯A形成扩散偶,经高温扩散退火后发现在纯A侧有空洞分布,则。
A扩散系数D A>D B B扩散时界面向A-B合金方向移动 C A、B答案都不对13. 柯肯达尔效应支持了扩散的机制。
A交换B间隙C空位14. 在高分子材料中,下面描述错误的是。
A热塑性高分子材料在温度交替变化时可以经历粘流态、高弹态和玻璃态的变化B交联会降低高分子的结晶能力C温度升高则链段长度增加15用CaO稳定ZrO2,如加入2%(摩尔比)的CaO,可形成。
材料科学基础练习题

第2章固体结构一、填空题1.所谓_______________是指晶体结构中任一原子周围最近邻且___________的原子数;而_______________是指晶体结构中原子体积占总体积的百分比。
面心立方和密排六方结构的致密度均为____________,是纯金属中最密集的结构。
2.组成合金的基本的___________的物质称为组元。
组元可以是金属和非金属元素,也可以是化合物。
固态下所形成的合金相基本上可分为_________和__________两大类。
3.固溶体是以某一组元为溶剂,在其晶体点阵中溶入其他组元原子(溶质原子)所形成的均匀混合的________________,它保持着_____________的晶体结构类型。
4.影响固体溶解度的因素很多,主要取决于四个因素:a._____________,b.__________,c._________________,d.___________________5. fcc结构的密排方向是_______,密排面是______,密排面的堆垛顺序是_______致密度为___________配位数是________________晶胞中原子数为___________,把原子视为刚性球时,若晶体点阵常数为a,原子的半径是____________;bcc结构的密排方向是_______,密排面是_____________致密度为___________配位数是________________ 晶胞中原子数为___________,若晶体点阵常数为a,原子的半径是____________;hcp结构的密排方向是_______,密排面是______,密排面的堆垛顺序是_______,致密度为___________配位数是________________,晶胞中原子数为___________,若晶体点阵常数为a和c,原子的半径是____________。
6.铝晶体属于_____________点阵。
材料科学基础试题及答案

材料科学基础试题及答案一、选择题1. 材料科学中,下列哪个不是材料的基本性能?A. 力学性能B. 热学性能C. 光学性能D. 化学性能答案:C2. 金属材料的塑性变形主要通过哪种机制进行?A. 位错运动B. 原子扩散C. 相变D. 晶界滑动答案:A3. 陶瓷材料通常具有哪些特性?A. 高韧性B. 高导电性C. 高熔点D. 高塑性答案:C二、填空题1. 材料科学是一门研究材料的________、________、________以及材料与环境相互作用的科学。
答案:组成、结构、性能2. 根据材料的组成和结构,材料可以分为________、________、________和复合材料。
答案:金属材料、无机非金属材料、有机高分子材料三、简答题1. 简述材料科学中的“相”的概念。
答案:在材料科学中,“相”指的是材料中具有相同化学成分和结构的均匀部分。
相可以是固体、液体或气体,并且可以在宏观上观察到。
材料的相可以决定其物理和化学性质。
2. 什么是材料的微观结构?它对材料性能有何影响?答案:材料的微观结构是指材料内部的原子、分子或晶粒的排列方式和分布状态。
微观结构对材料的力学性能、热学性能、电学性能等具有决定性影响,例如晶粒大小、晶界、位错密度等都会显著影响材料的强度、韧性和导电性。
四、计算题1. 已知某金属材料的屈服强度为300 MPa,弹性模量为200 GPa,求其在屈服点的应变。
答案:首先,根据胡克定律,σ = Eε,其中σ是应力,E是弹性模量,ε是应变。
将已知数值代入公式,可得ε = σ/E = 300 MPa / 200 GPa = 0.0015。
2. 若某材料的热膨胀系数为10^-6 K^-1,当温度从20°C升高到100°C时,计算该材料长度的变化百分比。
答案:材料长度的变化量ΔL可以通过公式ΔL = L0αΔT计算,其中L0是原始长度,α是热膨胀系数,ΔT是温度变化。
假设原始长度L0为1m,温度变化ΔT = 100°C - 20°C = 80°C,代入公式得ΔL = 1m * 10^-6 K^-1 * 80 = 8 * 10^-5 m。
材料科学基础课后习题答案第二章

第2章 习题2-1 a) 试证明均匀形核时,形成临界晶粒的△G K 与其临界晶核体积V K 之间的关系式为2K K V V G G ∆=-∆; b) 当非均匀形核形成球冠形晶核时,其△G K 与V K 之间的关系如何?a) 证明 因为临界晶核半径 2K Vr G σ=-∆ 临界晶核形成功 32163()K V G G πσ∆=∆ 故临界晶核的体积 3423K K K Vr G V G π∆==∆ 所以 2K K V V G G ∆=-∆ b) 当非均匀形核形成球冠形晶核时,SL 2K Vr G σ=-∆非 临界晶核形成功 3324(23cos cos )3()K SL V G G πσθθ∆=-+∆非故临界晶核的体积 331(23cos cos )3K K V r πθθ=-+非() 3333SL 3281(23cos cos )(23cos cos )33()SL K V V V V V G G G G σπσπθθθθ∆=--+∆=-+∆∆() 所以 2K K V V G G ∆=-∆非 2-2 如果临界晶核是边长为a 的正方体,试求出其△G K 与a 的关系。
为什么形成立方体晶核的△G K 比球形晶核要大?解:形核时的吉布斯自由能变化为326V V G V G A a G a σσ∆=∆+=∆+ 令()0d G da∆= 得临界晶核边长4K V a G σ=-∆ 临界形核功3333222244649632()6()()()()K tK V K V V V V V V G V G A G G G G G G σσσσσσσ∆=∆+=-∆+-=-+=∆∆∆∆∆ 2K Vr G σ=-∆,球形核胚的临界形核功 332242216()4()33()K bV V V V G G G G G σσπσππσ∆=-∆+=∆∆∆ 将两式相比较3232163()13262()K K b V t V G G G G πσπσ∆∆==≈∆∆ 可见形成球形晶核得临界形核功仅为形成立方形晶核的1/2。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
1、立方点阵单胞轴长为a,给出简单立方、体心立方、面心立方这三种点阵的每一个阵点的最近邻、次近邻的点数,求出最近邻、次近邻的距离。
2、某正交晶系单胞中,在如下位置有单原子存在:①(0, 1/2, 0),(1/2, 0, 1/2)两种位置都是同类原子;②([1/2, 0,0]),(0, 1/2, 1/2)上是A 原子,(0, 0, 1/2),(1/2, 1/2, 0)是B 原子。
问上两种晶胞各属于哪一种布喇菲点阵?
3、Fig.1 shows a unit cell of a hypothetical metal. (a) To which crystal system does this unit cell belong? (b) What would this crystal structure be called? (c) Calculate the density of the material, given that its atomic weight is 141 g/mol.
Fig.1 Fig. 2
4、The unit cell for uranium has orthorhombic symmetry, with a, b, and c lattice parameters of 0.286, 0.587 and 0.495 nm, respectively. If its density, atomic weight, and atomic radius are 19.05 g/cm3, 238.03 g/mol, and 0.1385 nm, respectively, compute the atomic packing factor.
5、Three different crystallographic planes for a unit cell of a hypothetical metal are shown in Fig.2. The circles represent atoms. (a) To what crystal system does the unit cell belong? (b) What would this crystal structure be called? (c) If the density of this metal is 18.91 g/cm3, determine its atomic weight.
1、立方点阵单胞轴长为a,给出简单立方、体心立方、面心立方这三种点阵的每一个阵点的最近邻、次近邻的点数,求出最近邻、次近邻的距离。
2、某正交晶系单胞中,在如下位置有单原子存在:①(0, 1/2, 0),(1/2, 0, 1/2)两种位置都是同类原子;②([1/2, 0,0]),(0, 1/2, 1/2)上是A 原子,(0, 0, 1/2),(1/2, 1/2, 0)是B 原子。
问上两种晶胞各属于哪一种布喇菲点阵?
3、Fig.1 shows a unit cell of a hypothetical metal. (a) To which crystal system does this unit cell belong? (b) What would this crystal structure be called? (c) Calculate the density of the material, given that its atomic weight is 141 g/mol.
Fig.1 Fig. 2
4、The unit cell for uranium has orthorhombic symmetry, with a, b, and c lattice parameters of 0.286, 0.587 and 0.495 nm, respectively. If its density, atomic weight, and atomic radius are 19.05 g/cm3, 238.03 g/mol, and 0.1385 nm, respectively, compute the atomic packing factor.
5、Three different crystallographic planes for a unit cell of a hypothetical metal are shown in Fig.2. The circles represent atoms. (a) To what crystal system does the unit cell belong? (b) What would this crystal structure be called? (c) If the density of this metal is 18.91 g/cm3, determine its atomic weight.。
