FDA最新工艺验证指南2011.1.1中英文对照版
FDA工艺验证指南

FDA工艺验证指南GUIDELINEON GENERAL PRINCIPLES OF ROCESS V ALIDATIONMay, 1987Prepared by: Center for Drugs and Biologics andCenter for Devices and Radiological HealthFood and Drug AdministrationMaintained by: Division of Manufacturing and Product Quality (HFN-320)Office of ComplianceCenter for Drugs and BiologicsFood and Drug Administration5600 Fishers LaneRockville, Maryland 20857General Principles of Process Validation May 1987GENERAL PRINCIPLES OF PROCESS VALIDATIONI. PURPOSEThis guideline outlines general principles that FDA considers to be acceptable elements of process validation for the preparation of human and animal drug products and medical devices.II. SCOPEThis guideline is issued under Section 10.90 (21 CFR 10.90) and is applicable to the manufacture of pharmaceuticals and medical devices. It states principles and practices of general applicability that are not legal requirements but are acceptable to the FDA. A person may rely upon this guideline with the assurance of its acceptability to FDA, or may follow different procedures. When different procedures are used, a person may, but is not required to, discuss the matter in advance with FDA to prevent the expenditure of money and effort on activities that may later be determined to be unacceptable. In short, this guideline lists principles and practices which are acceptable to the FDA for the process validation of drug products and medical devices; it does not list the principles and practices that must, in all instances, be used to comply withlaw.This guideline may be amended from time to time. Interested persons are invited to submit comments on this document and any subsequent revisions. Written comments should be submitted to the Dockets Management Branch (HFA-305), Food and Drug Administration, Room 4-62, 5600 Fishers Lane, Rockville, Maryland 20857. Received comments may be seen in that office between 9\a.m. and 4\p.m., Monday through Friday.III. INTRODUCTIONProcess validation is a requirement of the Current Good Manufacturing Practices Regulations for Finished Pharmaceuticals, 21 CFR Parts 210 and 211, and of the Good Manufacturing Practice Regulations for Medical Devices, 21 CFR Part 820, and therefore, is applicable to the manufacture of pharamaceuticals and medical devices.Several firms have asked FDA for specific guidance on what FDA expects firms to do to assure compliance with the requirements for process validation. This guideline discusses process validation elements and concepts that are considered by FDA as acceptable parts of a validation program. The constituents of validation presented in this document are not intended to be all-inclusive. FDA recognizes that, because of the great variety of medical products (drug products and medical devices), processes and manufacturing facilities, it is not possible to state in one document all of the specific validation elements that are applicable. Several broad concepts, however, have general applicability which manufacturers can use successfully as a guide in validating a manufacturing process. Although the particular requirements of process validation will vary according to such factors as the nature of the medical product (e.g., sterile vs non-sterile) and the complexity of the process, the broad concepts stated in this document have general applicability and provide an acceptable framework for building a comprehensive approach to process validation.DefinitionsInstallation qualification - Establishing confidence that process equipment and ancillary systems are capable of consistently operating within established limits and tolerances.Process performance qualification - Establishing confidence that the process is effective and reproducible.Product performance qualification - Establishing confidence through appropriate testing that the finished product produced by a specified process meets all release requirements for functionality and safety.Prospective validation - Validation conducted prior to the distribution of either a new product, or product made under a revised manufacturing process, where the revisions may affect the product's characteristics.Retrospective validation - Validation of a process for a product already in distribution based upon accumulated production, testing and control data.Validation - Establishing documented evidence which provides a high degree of assurance that a specific process will consistently produce a product meeting its pre-determined specifications andquality attributes.Validation protocol - A written plan stating how validation will be conducted, including test parameters, product characteristics, production equipment, and decision points on what constitutes acceptable test results.Worst case - A set of conditions encompassing upper and lower processing limits and circumstances, including those within standard operating procedures, which pose the greatest chance of process or product failure when compared to ideal conditions. Such conditions do not necessarily induce product or process failure.IV. GENERAL CONCEPTSAssurance of product quality is derived from careful attention to a number of factors including selection of quality parts and materials, adequate product and process design, control of the process, and in-process and end-product testing. Due to the complexity of today's medical products, routine end-product testing alone often is not sufficient to assure product quality for several reasons. Some end-product tests have limited sensitivity.1 In some cases, destructive testing would be required to show that the manufacturing process was adequate, and in other situations end-product testing does not reveal all variations that may occur in the product that may impact on safety and effectiveness.2The basic principles of quality assurance have as their goal the production of articles that are fit for their intended use. These1 For example, USP XXI states: "No sampling plan for applying sterility tests to a specified proportion of discrete units selected from a sterilization load is capable of demonstrating with complete assurance that all of the untested units are in fact sterile."2 As an example, in one instance a visual inspection failed to detect a defective structural weld which resulted in the failure of an infant warmer. The defect could only have been detected by using destructive testing or expensive test equipment.principles may be stated as follows:(1)quality, safety, and effectiveness must be designed and built into the product;(2)quality cannot be inspected or tested into the finished product;(3)and (3) each step of the manufacturing process must be controlled to maximize the probability thatthe finished product meets all quality and design specifications. Process validation is a keyelement in assuring that these quality assurance goals are met.It is through careful design and validation of both the process and process controls that a manufacturer can establish a high degree of confidence that all manufactured units from successive lots will be acceptable. Successfully validating a process may reduce the dependence upon intensive in-process and finished product testing.It should be noted that in most all cases, end-product testing plays a major role in assuring that quality assurance goals are met; i.e., validation and end-product testing are not mutually exclusive.The FDA defines process validation as follows:Process validation is establishing documented evidence which provides a high degree of assurance that a specific process will consistently produce a product meeting its pre-determined specifications and qualitycharacteristics.It is important that the manufacturer prepare a written validation protocol which specifies the procedures (and tests) to be conducted and the data to be collected. The purpose for which data are collected must be clear, the data must reflect facts and be collected carefully and accurately. The protocol should specify a sufficient number of replicate process runs to demonstrate reproducibility and provide an accurate measure of variability among successive runs. The test conditions for these runs should encompass upper and lower processing limits and circumstances, including those within standard operating procedures, which pose the greatest chance of process or product failure compared to ideal conditions; such conditions have become widely known as "worst case" conditions. (They are sometimes called "most appropriate challenge" conditions.) Validation documentation should include evidence of the suitability of materials and the performance and reliability of equipment and systems.Key process variables should be monitored and documented. Analysis of the data collected from monitoring will establish the variability of process parameters for individual runs and will establish whether or not the equipment and process controls are adequate to assure that product specifications are met.Finished product and in-process test data can be of value in process validation, particularly in those situations where quality attributes and variabilities can be readily measured. Where finished (or in-process) testing cannot adequately measure certain attributes, process validation should be derived primarily from qualification of each system used in production and from consideration of the interaction of the various systems.V. CGMP REGULATIONS FOR FINISHED PHARMACEUTICALSProcess validation is required, in both general and specific terms, by the Current Good Manufacturing Practice Regulations for Finished Pharmaceuticals, 21 CFR Parts 210 and 211. Examples of such requirements are listed below for informational purposes, and are not all-inclusive.A requirement for process validation is set forth in general terms in section\211.100 -- Written procedures; deviations -- which states, in part:"There shall be written procedures for production and process control designed to assure that the drug products have the identity, strength, quality, and purity they purport or are represented to possess."Several sections of the CGMP regulations state validation requirements in more specific terms. Excerpts from some of these sections are:Section 211.110, Sampling and testing of in-process materials and drug products.(a) "....control procedures shall be established to monitor the output and V ALIDATE the performance of those manufacturing processes that may be responsible for causing variability in the characteristics of in-process material and the drug product." (emphasis added) Section 211.113, Control of Microbiological Contamination.(b) "Appropriate written procedures, designed to prevent microbiological contamination of drug products purporting to besterile, shall be established and followed. Such proceduresshall include V ALIDATION of any sterilization process."(emphasis added)VI. GMP REGULATION FOR MEDICAL DEVICESProcess validation is required by the medical device GMP Regulations, 21 CFR Part\820. Section 820.5 requires every finished device manufacturer to:"...prepare and implement a quality assurance program that is appropriate to the specific device manufactured..."Section 820.3(n) defines quality assurance as:"...all activities necessary to verify confidence in the quality of the process used to manufacture a finished device."When applicable to a specific process, process validation is an essential element in establishing confidence that a process will consistently produce a product meeting the designed quality characteristics.A generally stated requirement for process validation is contained in section\820.100:"Written manufacturing specifications and processing procedures shall be established, implemented, and controlled to assure that the device conforms to its original design or any approved changes in that design."Validation is an essential element in the establishment and implementation of a process procedure, as well as in determining what process controls are required in order to assure conformance to specifications.Section 820.100(a)(1) states:"...control measures shall be established to assure that the design basis for the device, components and packaging is correctly translated into approved specifications."Validation is an essential control for assuring that the specifications for the device and manufacturing process are adequate to produce a device that will conform to the approveddesign characteristics.VII. PRELIMINARY CONSIDERA TIONSA manufacturer should evaluate all factors that affect product quality when designing and undertaking a process validation study. These factors may vary considerably among different products and manufacturing technologies and could include, for example, component specifications, air and water handling systems, environmental controls, equipment functions, and process control operations. No single approach to process validation will be appropriate and complete in all cases; however, the following quality activities should be undertaken in most situations.During the research and development (R&D) phase, the desired product should be carefully defined in terms of its characteristics, such as physical, chemical, electrical and performance characteristics.3 It is important to translate the product characteristics into specifications as a basis for description and control of the product.Documentation of changes made during development provide traceability which can later be used to pinpoint solutions to future problems.The product's end use should be a determining factor in the development of product (and component) characteristics and specifications. All pertinent aspects of the product which impact on safety andeffectiveness should be considered. These aspectsFor example, in the case of a compressed tablet, physical characteristics would include size, weight, hardness, and freedom from defects, such as capping and splitting. Chemical characteristics would include quantitative formulation/potency; performance characteristics may include bioavailability (reflected by disintegration and dissolution). In the case of blood tubing, physical attributes would include internal and external diameters, length and color. Chemical characteristics would include raw material formulation. Mechanical properties would include hardness and tensile strength; performance characteristics would include biocompatibility and durability.include performance, reliability and stability. Acceptable ranges or limits should be established for each characteristic to set up allowable variations.4 These ranges should be expressed in readily measurable terms.The validity of acceptance specifications should be verified through testing and challenge of the product on a sound scientific basis during the initial development and production phase.Once a specification is demonstrated as acceptable it is important that any changes to the specification be made in accordance with documented change control procedures.VIII. ELEMENTS OF PROCESS V ALIDATIONA. Prospective ValidationProspective validation includes those considerations that should be made before an entirely new product is introduced by a firm or when there is a change in the manufacturing process which may affect the product's characteristics, such as uniformity and identity. The following are considered as key elements of prospective validation.4 For example, in order to assure that an oral, ophthalmic, or parenteral solution has an acceptable pH, a specification may be established by which a lot is released only if it has been shown to have a pH within a narrow established range. For a device, a specification for the electrical resistance of a pacemaker lead would be established so that the lead would be acceptable only if the resistance was within a specified range.1. Equipment and ProcessThe equipment and process(es) should be designed and/or selected so that product specifications are consistently achieved. This should be done with the participation of all appropriate groups that are concerned with assuring a quality product, e.g., engineering design, production operations, and quality assurance personnel.a. Equipment: Installation Qualification Installation qualification studies establish confidence that the process equipment and ancillary systems are capable of consistently operating within established limits and tolerances. After process equipment is designed or selected, it should be evaluated and tested to verify that it is capable of operating satisfactorily within the operating limits required by the process.5 This phase of validation includes examination of equipment design; determination of calibration, maintenance, and adjustment requirements; and identifying critical equipment features that could affect the process and product. Information obtained from these studies should be used to establish written procedures covering equipment calibration, maintenance, monitoring, and control.5 Examples of equipment performance characteristics which may be measured include temperature and pressure of injection molding machines, uniformity of speed for mixers, temperature, speed and pressure for packaging machines, and temperature and pressure of sterilization chambers.In assessing the suitability of a given piece of equipment, it is usually insufficient to rely solely upon the representations of the equipment supplier, or upon experience in producing some other product.6 Sound theoretical and practical engineering principles and considerations are a first step in the assessment.It is important that equipment qualification simulate actual production conditions, including those which are "worst case" situations.6 The importance of assessing equipment suitability based upon how it will be used to attain desired product attributes is illustrated in the case of deionizers used to produce Purified Water, USP. In one case, a firm used such water to make a topical drug product solution which, in view of its intended use, should have been free from objectionable microorganisms. However, the product was found to be contaminated with a pathogenic microorganism. The apparent cause of the problem was failure to assess the performance of the deionizer from a microbiological standpoint. It is fairly well recognized that the deionizers are prone to build-up of microorganisms--especially if the flow rates are low and the deionizers are not recharged and sanitized at suitable intervals. Therefore, these factors should have been considered. In this case, however, the firm relied upon the representations of the equipment itself, namely the "recharge" (i.e., conductivity) indicator, to signal the time for regeneration and cleaning. Considering the desired product characteristics, the firm should have determined the need for such procedures based upon pre-use testing, taking into account such factors as the length of time the equipment could produce deionized water of acceptable quality, flow rate, temperature, raw water quality, frequency of use, and surface area of deionizing resins.Tests and challenges should be repeated a sufficient number of times to assure reliable and meaningful results. All acceptance criteria must be met during the test or challenge. If any test or challenge shows that the equipment does not perform within its specifications, an evaluation should be performed to identify the cause of the failure. Corrections should be made and additional test runs performed, as needed, to verify that the equipment performs within specifications. The observed variability of the equipment between and within runs can be used as a basis for determining the total number of trials selected for the subsequent performance qualification studies of the process.7Once the equipment configuration and performance characteristics are established and qualified, they should be documented. The installation qualification should include a review of pertinent maintenance procedures, repair parts lists, and calibration methods for each piece of equipment. The objective is to assure that all repairs can be performed in such a way that will not affect the7 For example, the AAMI Guideline for Industrial Ethylene Oxide Sterilization of Medical Devices approved 2 December 1981, states: "The performance qualification should include a minimum of 3 successful, planned qualification runs, in which all of the acceptance criteria are met.....(5.3.1.2.).characteristics of material processed after the repair. In addition, special post-repair cleaning and calibration requirements should be developed to prevent inadvertent manufacture a of non-conforming product. Planning during the qualification phase can prevent confusion during emergency repairs which could lead touse of the wrong replacement part.b. Process: Performance Qualification The purpose of performance qualification is to provide rigorous testing to demonstrate the effectiveness and reproducibility of the process. In entering the performance qualification phase of validation, it is understood that the process specifications have been established and essentially proven acceptable through laboratory or other trial methods and that the equipment has been judged acceptable on the basis of suitable installation studies.Each process should be defined and described with sufficient specificity so that employees understand what is required.Parts of the process which may vary so as to affect important product quality should be challenged.8In challenging a process to assess its adequacy, it is important that challenge conditions simulate those that will be encountered during actual production, including "worst case" conditions. The challenges should be repeated enough times to assure that the results are meaningful and consistent.8 For example, in electroplating the metal case of an implantable pacemaker, the significant process steps to define, describe, and challenge include establishment and control of current density and temperature values for assuring adequate composition of electrolyte and for assuring cleanliness of the metal to be plated. In the production of parenteral solutions by aseptic filling, the significant aseptic filling process steps to define and challenge should include the sterilization and depyrogenation of containers/closures, sterilization of solutions, filling equipment and product contact surfaces, and the filling and closing of containers.Each specific manufacturing process should be appropriately qualified and validated. There is an inherent danger in relying on what are perceived to be similarities between products, processes, and equipment without appropriate challenge.9c. Product: Performance Qualification For purposes of this guideline, product performance qualification activities apply only to medical devices. These steps should be viewed as pre-production quality assurance activities.9 For example, in the production of a compressed tablet, a firm may switch from one type of granulation blender to another with the erroneous assumption that both types have similar performance characteristics, and, therefore, granulation mixing times and procedures need not be altered. However, if the blenders are substantially different, use of the new blender with procedures used for the previous blender may result in a granulation with poor content uniformity. This, in turn, may lead to tablets having significantly differing potencies. This situation may be averted if the quality assurance system detects the equipment change in the first place, challenges the blender performance, precipitates a revalidation of the process, and initiates appropriate changes. In this example, revalidation comprises installation qualification of the new equipment and performance qualification of the process intended for use in the new blender.Before reaching the conclusion that a process has been successfully validated, it is necessary to demonstrate that the specified process has not adversely affected the finished product. Where possible, product performance qualification testing should include performance testing under conditions that simulate actual use.Product performance qualification testing should be conducted using product manufactured from the same type of production equipment, methods and procedures that will be used for routine production. Otherwise, the qualified product may not be representative of production units and cannot be used as evidence that the manufacturing process will produce a product that meets the pre-determined specifications and quality attributes.10 For example, a manufacturer of heart valves received complaints that the valve-support structure was fracturing under use. Investigation by the manufacturer revealed that all material and dimensional specifications had been met but the production machining process created microscopic scratches on the valve supporting wireform. These scratches caused metal fatigue and subsequent fracture. Comprehensive fatigue testing of production units under simulated use conditions could have detected the process deficiency.In another example, a manufacturer recalled insulin syringes because of complaints that the needles were clogged. Investigation revealed that the needles were clogged by silicone oil which was employed as a lubricant during manufacturing. Investigation further revealed that the method used to extract the silicone oil was only partially effective. Although visual inspection of the syringes seemed to support that the cleaning method was effective, actual use proved otherwise.After actual production units have sucessfully passed product performance qualification, a formal technical review should be conducted and should include:Comparison of the approved product specifications and the actual qualified product.Determination of the validity of test methods used to determine compliance with the approved specifications.Determination of the adequacy of the specification change control program.2. System to Assure Timely Revalidation There should be a quality assurance system in place which requires revalidation whenever there are changes in packaging, formulation, equipment, or processes which could impact on product effectiveness or product characteristics, and whenever there are changes in product characteristics. Furthermore, when a change is made in raw material supplier, the manufacturer should consider subtle, potentially adverse differences in the raw material characteristics. A determination of adverse differences in raw material indicates a need to revalidate the process.One way of detecting the kind of changes that should initiate revalidation is the use of tests and methods of analysis which are capable of measuring characteristics which may vary. Such tests and methods usually yield specific results which go beyond the mere pass/fail basis, thereby detecting variations within product and process specifications and allowing determination of whether a process is slipping out of control.The quality assurance procedures should establish the circumstances under which revalidation is required. These may be based upon equipment, process, and product performance observed during the initial validation challenge studies. It is desirable to designate individuals who have the responsibility to review product, process, equipment and personnel changes to determine if and when revalidation is warranted.The extent of revalidation will depend upon the nature of the changes and how they impact upon。
美国FDA分析方法验证指南中英文对照

美国FDA分析方法验证指南中英文对照美国FDA分析方法验证指南中英文对照I. INTRODUCTIONThis guidance provides recommendations to applicants on submitting analytical procedures, validation data, and samples to support the documentation of the identity, strength, quality, purity, and potency of drug substances and drug products.1. 绪论本指南旨在为申请者提供建议,以帮助其提交分析方法,方法验证资料和样品用于支持原料药和制剂的认定,剂量,质量,纯度和效力方面的文件。
This guidance is intended to assist applicants in assembling information, submitting samples, and presenting data to support analytical methodologies. The recommendations apply to drug substances and drug products covered in new drug applications (NDAs), abbreviated new drug applications (ANDAs), biologics license applications (BLAs), product license applications (PLAs), and supplements to these applications. 本指南旨在帮助申请者收集资料,递交样品并资料以支持分析方法。
这些建议适用于NDA,ANDA,BLA,PLA及其它们的补充中所涉及的原料药和制剂。
FDA现场检查行业指南(中英文对照)

FDA行业指南-药品现场检查中被认为是延迟、否认、限制或拒绝的情形一、介绍2012年7月9日,《美国食品和药物管理局安全及创新法案》(FDASIA)被签署成为法律。
FDASIA章节707添加了501(j)到《食品、药品和化妆品法令》(FD&C Act),认为“任何从事生产、加工、包装或持有的生产企业、库房造成现场检查的延迟、否认、限制或拒绝的情况均被认为该产品为假劣药品”。
该指南的目的是对“延迟、否认、限制或拒绝”的情形进行定义。
二、定义1、延迟A、检查计划安排的延迟FDA将会根据当地的情况对检查计划进行适当的调整,例如天气、安保、节假日、其他非工作日、企业的生产计划等。
以下延迟的情况将会被认为产品是假劣药品,包括但不仅限于:●企业不同意建议的检查日期,但没有合理的解释。
●在检查安排后,企业要求延迟检查日期,但没有合理的解释。
●企业不能回答为什么FDA联系不上企业指定的联系人。
下面给出了将不会被认为是假劣药品的潜在合理解释的一个例子,但不仅限于:●企业没有正在生产,例如每个月只生产一次,企业要求检查日期另定,以便FDA检查时生产正在进行中。
B、检查期间的延迟以下检查期间的延迟情况将会被认为产品是假劣药品,包括但不仅限于:●企业不允许FDA检查官进入某个区域直至一段时间过去之后,即使这个区域是正在进行操作的并且是FDA有权检查的区域,对于这种行为没有合理的解释。
●企业长时间把FDA检查官单独撂在会议室,没有相应的文件或责任人供审查和询问,从而干扰检查官完成其相应的检查。
下面给出了将不会被认为是假劣药品的潜在合理解释的一个例子,但不仅限于:●企业不允许FDA检查官进入无菌工艺区域,直至检查官能满足企业的无菌更衣程序要求。
C、记录提供延迟以下记录提供延迟的情况将会被认为产品是假劣药品,包括但不仅限于:●在检查期间,FDA检查官要求在合理的时间内提供其有权查看的文件和记录,但是企业不能按时提供,且没有合理的解释。
美国FDA分析方法验证指南中文译稿[1]
![美国FDA分析方法验证指南中文译稿[1]](https://img.taocdn.com/s3/m/fa8be4d5ddccda38366baf5b.png)
1II. 背景 (2)III. 分析方法的类型 (3)A. 法定分析方法 (3)B. 可选择分析方法 (3)3 C. 稳定性指示分析 (3)IV. 对照品……………………………………………………………………………4A. 对照品的类型 (4)B. 分析报告单 (4)C. 对照品的界定 (4)V. IND 中的分析方法验证 (6)VI. NDA, ANDA, BLA 和PLA 中分析方法验证的内容和格式 (6)A. 原则 (6)B. 取样 (7)C. 仪器和仪器参数 (7)D. 试剂 (7)E. 系统适应性实验 (7)F. 对照品的制备 (7)G. 样品的制备 (8)H. 分析方法 (8)L. 计算 (8)J. 结果报告 (8)VII. NDA,ANDA,BLA 和PLA 中的分析方法验证 (9)A.非法定分析方法 (9)1.验证项目 (9)2. 其它分析方法验证信息 (10)a. 耐用性 (11)b. 强降解实验 (11)c. 仪器输出/原始资料 (11)3.各类检测的建议验证项目 (13)B.法定分析方法 (15)VIII. 统计分析…………………………………………………………………………15A. 总则 (15)C. 统计 (16)IX. 再验证 (16)X. 分析方法验证技术包:内容和过程……………………………………………17A. 分析方法验证技术包 (17)B. 样品的选择和运输 (18)C. 各方责任 (19)XI. 方法………………………………………………………………………………20A. 高效液相色谱(HPLC) (20)B. 气相色谱(GC) (22)C. 分光光度法,光谱学,光谱法和相关的物理方法 (23)D. 毛细管电泳 (23)E. 旋光度 (24)F. 粒径相关的分析方法 (25)G. 溶出度 (26)H. 其它仪器分析方法 (27)附件A:NDA,ANDA,BLA 和PLA 申请的内容 (28)附件B:分析方法验证的问题和延误 (29)参考文献……………………………………………………………………………………30术语表………………………………………………………………………………………32This guidance provides recommendations to applicants on submitting analytical procedures, validation data, and samples to support the documentation of the identity, strength, quality, purity, and potency of drug substances and drug products.1. 绪论本指南旨在为申请者提供建议,以帮助其提交分析方法,方法验证资料和样品用于支持原料药和制剂的认定,剂量,质量,纯度和效力方面的文件。
附录15和FDA工艺验证指南英文

GMP News28/10/2015Annex 15 and FDA Process Validation Guideline: Similarities/differences from the FDA perspective附录15和FDA工艺验证指南:与FDA预期的异同The "new" FDA Process Validation Guidance has been in force since January 2011. The revised Annex 15 has been valid since 1 October 2015. At a Conference in September 2015 which was co-sponsored by the FDA, Grace McNally, Senior FDA Official reported about similarities and differences between the two documents from the perspective of the FDA.“新”的FDA工艺验证已于2011年1月实施。
修订后的附录15在2015年10月1日生效。
在2015年9月的会议上,FDA提出了倡议,GRACE MCNALLY, FDA的资深官员报告了FDA角度所诠释的这两份文件的异同。
First to the similarities: both documents address a process validation life cycle and quality risk management across all stages of the life cycle. For Grace McNally there is also comparability with regard to a science-based process development and to the development of process understanding as the basis for stage 2 in accordance with the FDA Process Validation Guideline, resp. with the actual process validation in the sense of Annex 15. Prospective validation is favoured in both documents. Only in special cases one concurrent validation is possible, but is never favoured as a routine procedure. The FDA also sees similarities between the Annex 15 and the FDA Process Validation Guidelinerelative to the need for a rationale for determining the number of samples for PPQ/process validation, as well as in determining the number of PPQ - / validation runs. And this rationale should include, for example, the process variables and the complexity and experience with the process. For the FDA there are further similarities with regard to statistical methods and analyses as part of the process validation: mentioned are PAT, multivariate SPC, statistical methods regarding variability and process capabilities, trend analyses and methods for measuring/evaluating process stabilities and capabilities. Moreover, the authority considers stage 3 in the process validation life cycle (continued/ongoing process verification) as comparable. An exception is mentioned below in the differences. Finally, the requirements for change control in both FDA Process Validation Guideline and the revised Annex 15 are also similar from the perspective of the FDA.首先是相同的地方:两份文件均强调了工艺验证的生命周期和各阶段的质量风险管理。
准备FDA认证前检查中英文对照文档

2011-FDA行业指南_工艺验证(中英文对照):一般原则与规范

Guidance for Industry行业指南Process Validation: GeneralPrinciples and Practices工艺验证:一般原则与规范U.S. Department of Health and Human ServicesFood and Drug AdministrationCenter for Drug Evaluation and Research (CDER)Center for Biologics Evaluation and Research (CBER)Center for Veterinary Medicine (CVM)January 2011Current Good Manufacturing Practices (CGMP)Revision 1美国卫生与人类服务部食品药品管理局药物评价和研究中心(CDER)生物制品评价和研究中心(CBER)兽药中心(CVM)2011年1月现行药品质量生产管理规范(CGMP)修订版 1Guidance for Industry行业指南Process Validation: GeneralPrinciples and Practices工艺验证:一般原则与规范Additional copies are available from:Office of CommunicationsDivision of Drug Information, WO51, Room 220110903 New Hampshire Ave.Silver Spring, MD 20993Phone: 301-796-3400; Fax: 301-847-8714druginfo@/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/default.htmand/orOffice of Communication, Outreach and Development, HFM-40Center for Biologics Evaluation and ResearchFood and Drug Administration1401 Rockville Pike, Rockville, MD 20852-1448(Tel) 800-835-4709 or 301-827-1800/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/default.htmand/orCommunications Staff, HFV-12Center for Veterinary MedicineFood and Drug Administration7519 Standish Place,Rockville, MD 20855(Tel) 240-276-9300/AnimalVeterinary/GuidanceComplianceEnforcement/GuidanceforIndustry/default.htm另外的副本可从以下部门得到:马里兰州银泉市新罕布什尔大道10193号2201室药品信息处,对外信息办公室,邮政编码:20993电话:301-796-3400; 传真:301-847-8714druginfo@/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/default.htm和/或马里兰州洛克维尔市洛克维尔大道1401号 HFM-40 FDA生物制品评价和研究中心对外信息、外联与发展办公室邮政编码:20852-1448电话:800-835-4709 或 301-827-1800/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/default.htm和/或马里兰州洛克维尔市Standish Place 7519号食品药品管理局兽药中心HFV-12通讯处,邮政编码:20885电话:240-276-9300/AnimalVeterinary/GuidanceComplianceEnforcement/GuidanceforIndustry/default.htmU.S. Department of Health and Human ServicesFood and Drug AdministrationCenter for Drug Evaluation and Research (CDER)Center for Biologics Evaluation and Research (CBER)Center for Veterinary Medicine (CVM)January 2011Current Good Manufacturing Practices (CGMP)Revision 1美国卫生与人类服务部食品药品管理局药物评估和研究中心(CDER)生物制品评估和研究中心(CBER)兽药中心(CVM)2011年1月现行药品质量生产管理规范(CGMP)修订版 1Table of Contents目录I. INTRODUCTION (1)一. 简介 (1)II. BACKGROUND (3)二. 背景 (3)A. Process Validation and Drug Quality (4)A. 工艺验证与药品质量 (4)B. Approach to Process Validation (5)B. 工艺验证方法 (5)III. STATUTORY AND REGULATORY REQUIREMENTS FOR PROCESS VALIDATION (7)三. 对工艺验证的法规和监管要求 (7)IV. RECOMMENDATIONS (9)四. 建议 (9)A. General Considerations for Process Validation (9)A. 对工艺验证的总体考虑 (9)B. Stage 1 - Process Design (10)B. 第一阶段 - 工艺设计 (10)1. Building and Capturing Process Knowledge and Understanding (11)1. 建立和捕获工艺知识与理解 (11)2. Establishing a Strategy for Process Control (12)2. 建立工艺控制策略 (12)C. Stage 2 - Process Qualification (14)C. 第二阶段 - 工艺确认 (14)1. Design of a Facility and Qualification of Utilities and Equipment (14)1. 厂房设施设计以及公用设施与设备确认 (14)2. Process Performance Qualification (16)2. 工艺性能确认 (16)3. PPQ Protocol (17)3. 工艺性能确认方案 (17)4. PPQ Protocol Execution and Report (19)4. 工艺性能确认执行与报告 (19)D. Stage 3 - Continued Process Verification (20)D. 第三阶段 - 持续工艺验证 (20)V. CONCURRENT RELEASE OF PPQ BATCHES (22)五. 工艺性能确认批次的同时放行 (22)VI. DOCUMENTATION (24)六. 文件记录 (24)VII. ANALYTICAL METHODOLOGY (24)七. 分析方法 (24)GLOSSARY (26)术语表 (26)REFERENCES (28)参考资料 (28)Guidance for Industry1行业指南1Process Validation: General Principles and Practices工艺验证:一般原则与实施This guidance represents the Food and Drug Administration’s (FDA’s) current thinking on this topic. It does not create or confer any rights for or on any person and does not operate to bind FDA or the public. You can use an alternative approach if the approach satisfies the requirements of the applicable statutes and regulations. If you want to discuss an alternative approach, contact the FDA staff responsible for implementing this guidance. If you cannot identify the appropriate FDA staff, call the appropriate number listed on the title page of this guidance.本指南体现了食品药品管理局(FDA)关于这一主题的最新见解。
FDA最新工艺验证指南(2011.1版)北大中英对译-已打印

中文译稿:北京大学药物信息与工程研究中心 info@
另外的副本可从以下部门得到: 马里兰州银泉市新罕布什尔大道10193号2201室 药品信息处,对外信息办公室, 邮政编码:20993 电话:301-796-3400; 传真:301-847-8714
Guidance for Industry
行业指南 Process Validation: General Principles and Practices 工艺验证:一般原则与规范
U.S. Department of Health and Human Services Food and Drug Administration Center for Drug Evaluation and Research (CDER) Center for Biologics Evaluation and Research (CBER) Center for Veterinary Medicine (CVM) January 2011 Current Good Manufacturing Practices (CGMP) Revision 1 美国卫生与人类服务部 食品药品管理局 药物评价和研究中心(CDER) 生物制品评价和研究中心(CBER) 兽药中心(CVM) 2011年1月 现行药品质量生产管理规范(CGMP) 修订版 1
/BiologicsBloodVaccines/GuidanceComplianceRegulatoryInformation/Guidances/default.htm
and/or Communications Staff, HFV-12 Center for Veterinary Medicine Food and Drug Administration 7519 Standish Place, Rockville, MD 20855 (Tel) 240-276-9300
FDA清洁工艺验证指南中英文对照

Validation of Cleaning Processes (7/93)清洁工艺验证GUIDE TO INSPECTIONS VALIDATION OF CLEANING PROCESSES清洁工艺验证检查指南Note: This document is reference material for investigators and other FDA personnel. The document does not bind FDA, and does no confer any rights, privileges, benefits, or immunities for or on any person(s).注:此指南是FDA检查官和其他人员的参考资料,此文件不约束FDA,也不赋予任何人任何权利、特权、利益或豁免权I. INTRODUCTION一、介绍Validation of cleaning procedures has generated considerable discussion since agency documents, including the Inspection Guide for Bulk Pharmaceutical Chemicals and the Biotechnology Inspection Guide, have briefly addressed this issue. These Agency documents clearly establish the expectation that cleaning procedures (processes) be validated.自从FDA的各文件,包括化学原料药检查指南和生物技术检查指南,简单的提出了清洁验证的这个话题后,关于清洁工艺的验证已经引发了相当多的讨论,这些官方的文件,都清楚的确定了对清洁工艺需要被验证的期望。
美国FDA分析方法验证指南中文译稿

美国FDA分析方法验证指南中文译稿引言:分析方法的验证在确定该方法是否适合特定用途以及是否能够产生准确和可靠结果方面起着关键作用。
准确和可靠的分析结果对于药品和食品行业是至关重要的。
本指南旨在提供制药和食品行业关于分析方法验证的指导,以满足FDA的法规要求。
验证目标:分析方法的验证需要进行一系列的实验以确定其精确性、精密度、特异性、线性范围和灵敏度等参数。
验证的目标是确保分析方法能够产生准确、可重复和可靠的结果,以支持产品的质量控制和合规性。
验证过程:验证过程可以分为三个主要阶段:方法开发、验证实验和验证报告。
方法开发阶段包括确定分析参数、选择仪器和试剂、制定实验方案等。
验证实验阶段涵盖了一系列实验,如准确度实验、精确度实验、特异性实验等。
验证报告阶段需要详细记录所有实验结果,以便后续复查和审查。
实验设计:验证实验需要进行大量的实验设计,以覆盖所有可能的情况和变化。
例如,准确度实验应包括加标回收率实验和Spiked样品测试等。
精确度实验应包括系统反应的重复性和运行时间的稳定性等。
数据分析:验证实验收集到的数据需要进行统计分析,以确定分析方法的可信度。
数据分析应包括数学计算、图表绘制和合规性评估等。
分析结果应该足够准确和可靠,以支持产品的质量控制和合规性。
验证报告:验证报告是验证过程的最终产物,它需要详细记录实验设计、数据分析和结论等信息。
验证报告应包括验证实验的目的、方法、结果和结论等内容。
验证报告需要通过内部审查和外部审查,确保其准确性和可靠性。
结论:美国FDA的《分析方法验证指南》为制药和食品行业提供了详细的指导,以确保分析方法的有效性和可靠性。
通过正确进行方法的验证,可以保证分析结果的准确性,从而支持产品的质量控制和合规性。
制药和食品企业应密切遵循该指南,以便符合FDA的法规要求。
美国FDA生产过程(工艺)验证总则指南

美国FDA生产过程(工艺)验证总则指南1 9 8 7年I.目的 3II.范围 3III.序言 3Ⅳ. 总概念 4V.现行药品生产质量管理规范(CGMP)法规 6Ⅵ.医疗器械的生产质量管理规范法规7Ⅶ.验证预备阶段所需考虑的事情7Ⅷ.生产过程验证的内容8Ⅸ.产品检验的可接受性11I.目的本指南概述了人用和兽用药品和医疗器械的生产过程(工艺)验证的总则,其验证的基本原理是得到fdA认可的。
II.范围本指南是根据21CFR10-90颁布的,适用于药品和医疗器械的生产。
本指南阐述了一般适用范围的原则和方法,这些原则和方法在法律上未做规定要求,但是得到了fdA认可。
本指南可以作为依据,并保证可以得到FDA的批准,但也可以按照其他方法进行验证。
在使用不同方法进行验证时,可事前与(但也可以不与)fdA讨论所要进行的验证工作,以避免在以后被FDA认为不合格而浪费了财力和精力。
总而言之,本指南列述的有关药品和医疗器械的生产过程验证原则和方法,是得到FdA认可的。
但不是在所有情况下都必须使用本指南所列述的原则和方法以符合法律。
本指南是要经常进行修订的。
对此有兴趣的人士可对本文件及随后的任一版本提出意见。
书面意见应向FDA的Dockets Maragement Branch(HFA—305)上报。
地址为:Room 462,5600FishersLane,Rockville,Maryland20847。
在星期—至星期五,上午9:00到下午4:00可在该办公处查阅所收到的意见,III.序言生产过程验证是药品生产管理规范法规21CFR210•211和医疗器械生产管理规范法规21CFR820的规定要求,所以适用于药品和医疗器械的生产。
有些生产厂商曾向FDA要求提供具体的指导:关寸FDA要求生产商做些什么工作,以保证生产过程验证符合规定的要求。
本指南讨沦了生产过程验证的原理和概念,FDA认为这些原理和概念是符合验证方案要求的。
2020年(工艺技术)FDA最新工艺验证指南(版)(中文版)

Guidance for Industry 行业指南Process Validation: General Principles and Practices工艺验证:一般原则与规范U.S. Department of Health and Human ServicesFood and Drug AdministrationCenter for Drug Evaluation and Research (CDER)Center for Biologics Evaluation and Research (CBER)Center for Veterinary Medicine (CVM)January 2011Current Good Manufacturing Practices (CGMP)Revision 1美国卫生与人类服务部食品药品管理局药物评价和研究中心(CDER)生物制品评价和研究中心(CBER)兽药中心(CVM)2011年1月现行药品质量生产管理规范(CGMP)修订版1包含不具约束力的建议中文译稿:北京大学药物信息与工程研究中心info@ Guidance for Industry 行业指南Process Validation: General Principles and Practices工艺验证:一般原则与规范Additional copies are available from:Office of CommunicationsDivision of Drug Information, WO51, Room 220110903 New Hampshire Ave.Silver Spring, MD 20993Phone: 301-796-3400; Fax: 301-847-8714druginfo@另外的副本可从以下部门得到:马里兰州银泉市新罕布什尔大道10193号2201室药品信息处,对外信息办公室,邮政编码:20993电话:301-796-3400; 传真:301-847-8714druginfo@Table of Contents目录I. INTRODUCTION (1)一. 简介 (1)II. BACKGROUND (3)二. 背景 (3)A. Process Validation and Drug Quality (4)A. 工艺验证与药品质量 (4)B. Approach to Process Validation (5)B. 工艺验证方法 (5)III. STATUTORY AND REGULATORY REQUIREMENTS FOR PROCESS VALIDATION (7)三. 对工艺验证的法规和监管要求 (7)IV. RECOMMENDATIONS (9)四. 建议 (9)A. General Considerations for Process Validation (9)A. 对工艺验证的总体考虑 (9)B. Stage 1 - Process Design (10)B. 第一阶段- 工艺设计 (10)1. Building and Capturing Process Knowledge and Understanding (11)1. 建立和捕获工艺知识与理解 (11)2. Establishing a Strategy for Process Control (12)2. 建立工艺控制策略 (12)C. Stage 2 - Process Qualification (14)C. 第二阶段- 工艺确认 (14)1. Design of a Facility and Qualification of Utilities and Equipment (14)1. 厂房设施设计以及公用设施与设备确认 (14)2. Process Performance Qualification (16)2. 工艺性能确认 (16)3. PPQ Protocol (17)3. 工艺性能确认方案 (17)4. PPQ Protocol Execution and Report (19)4. 工艺性能确认执行与报告 (19)D. Stage 3 - Continued Process Verification (20)D. 第三阶段- 持续工艺验证 (20)V. CONCURRENT RELEASE OF PPQ BATCHES (22)五. 工艺性能确认批次的同时放行 (22)VI. DOCUMENTATION (24)六. 文件记录 (24)VII. ANALYTICAL METHODOLOGY (24)七. 分析方法 (24)GLOSSARY (26)术语表 (26)REFERENCES (28)参考资料 (28)包含不具约束力的建议中文译稿:北京大学药物信息与工程研究中心info@1Guidance for Industry1行业指南1Process Validation: General Principles and Practices工艺验证:一般原则与实施This guidance represents the Food and Drug Administration’s (FDA’s) current thin king on this topic. It doesnot create or confer any rights for or on any person and does not operate to bind FDA or the public. You canuse an alternative approach if the approach satisfies the requirements of the applicable statutes and regulations. If you want to discuss an alternative approach, contact the FDA staff responsible for implementing this guidance. If you cannot identify the appropriate FDA staff, call the appropriate numberlisted on the title page of this guidance.本指南体现了食品药品管理局(FDA)关于这一主题的最新见解。
FDA灭菌工艺验证申报资料指南(中英文)

Guidance for Industry for the Submission Documentation for Sterilization Process Validation in Applications for Human and Veterinary Drug Products人药和兽药无菌工艺验证申报资料的工业指南Center for Drug Evaluation and Research (CDER)Center for Veterinary Medicine (CVM)November 1994CMC 2FDA药品评价与研究中心(CDER)FDA兽药中心(CVM)1994年11月TABLE OF CONTENTSI. INTRODUCTION (1)A. Purpose (1)B. Documenting Sterilization Process Validation (2)C. Remarks (2)II. INFORMATION FOR TERMINAL MOIST HEAT STERILIZATIONPROCESSES (3)A. Description of the Process and Product ........................ .. (3)1. The Drug Product and Container-Closure System (3)2. The Sterilization Process (3)3. The Autoclave Process and Performance Specifications (4)4. Autoclave Loading Patterns (4)5. Methods and Controls to Monitor Production Cycles (4)6. Requalification of Production Autoclaves (4)7. Reprocessing (4)B. Thermal Qualification of the Cycle (4)1. Heat Distribution and Penetration Studies (4)2. Thermal Monitors (5)3. The Effects of Loading on Thermal Input (5)4. Information Included in the Batch Record ........................................... . 5C. Microbiological Efficacy of the Cycle (5)1. Identification and Characterization of Bioburden Organisms (6)2. Specifications for Bioburden (6)3. Identification, Resistance, and Stability of BiologicalIndicators (6)4. The Resistance of the Biological Indicator Relative to That ofBioburden (6)5. Microbiological Challenge Studies (7)D. Microbiological Monitoring of the Environment (7)E. Container-Closure and Package Integrity (7)1. Simulation of the Stresses From Processing (7)2. Demonstrate Integrity Following the Maximum Exposure (8)3. Multiple Barriers (8)4. The Sensitivity of the Test (8)5. Integrity Over the Product Shelf Life (8)F. Bacterial Endotoxins Test and Method (8)G. Sterility Testing Methods and Release Criteria (8)H. Evidence of Formal, Written Procedures (9)III. OTHER TERMINAL STERILIZATION PROCESSES (9)A. Ethylene Oxide (9)1. Description of the Sterilizer (9)2. Cycle Parameters (10)3. Microbiological Methods (10)4. Stability (10)B. Radiation (10)1. The Facility and the Process (10)2. The Packaging of the Product (10)3. Multiple-Dose Mapping Studies (10)4. Microbiological Methods and Controls (11)5. Monitoring Stability (11)IV. INFORMATION FOR ASEPTIC FILL MANUFACTURING PROCESSES WHICH SHOULD BE INCLUDED IN DRUG APPLICATIONS (11)A. Buildings and Facilities (11)1. Floor Plan (11)2. Location of equipment (11)B. Overall Manufacturing Operation (11)1. Drug Product Solution Filtration (12)2. Specifications Concerning Holding Periods (12)3. Critical Operations (12)C. Sterilization and Depyrogenation of Containers, Closures, Equipment, andComponents (12)1. Bulk Drug Solution Components That are SterilizedSeparately (13)2. Sterilization Information in the Batch Records (13)D. Procedures and Specifications for Media Fills (13)E. Actions Concerning Product When Media Fills Fail (14)F. Microbiological monitoring of the environment (15)1. Microbiological Methods (15)2. Yeasts, Molds, and Anaerobic Microorganisms (15)3. Exceeded Limits (15)G. Container-Closure and Package Integrity (15)H. Sterility Testing Methods and Release Criteria (16)I. Bacterial Endotoxins Test and Method (16)J. Evidence of Formal Written Procedures (16)V. MAINTENANCE OF MICROBIOLOGICAL CONTROL AND QUALITY: STABILITY CONSIDERATIONS (16)A. Container-Closure Integrity (16)B. Preservative Effectiveness (17)C. Pyrogen or Endotoxin Testing (17)VI. ADDITIONAL INFORMATION (17)GUIDANCE FOR INDUSTRY1FOR THE SUBMISSION OF DOCUMENTATION FOR STERILIZATION PROCESS VALIDATION IN APPLICATIONS FOR HUMAN AND VETERINARY DRUGPRODUCTS人药和兽药无菌工艺验证申报资料的工业指南I. INTRODUCTION1、概述A. PurposeThis document is intended to provide guidance for the submission of information and data in support of the efficacy of sterilization processes in drug applications for both human and veterinary drugs. The recommendations in the guidance apply to applications for sterile drug products (new drug applications, new animal drug applications, abbreviated new drug applications, abbreviated antibiotic applications, and abbreviated new animal drug applications). These recommendations also apply to previously approved applications when supplements associated with the sterile processing of approved drugs are submitted. Information and data in support of sterility assurance may also be necessary in investigational new drug and investigational new animal drug applications.A. 目的本文件旨在为证明人药和兽药无菌工艺有效性申请上报的信息和资料提供指南。
FDA工艺验证指南(2023年.1版)(中文版)

FDA最新工艺验证指南(2011.1版)(中文版)1000字食品药品监管局(FDA)发布了2011年1月的最新工艺验证指南(中文版),该指南包含了关于工艺验证的详细信息和建议。
工艺验证是一个验证过程,旨在确保产品的性能符合特定的规格要求。
以下是指南中重要的一些提要:工艺验证的定义工艺验证是一种验证过程,它通过控制和监测生产过程的关键要素(如温度、时间和物料)来证明产品性能的一致性,并确定任何很少出现的过程偏差的控制方法和监测程序。
此外,工艺验证有助于识别和消除潜在的质量问题,并确保产品的质量和安全性。
工艺验证的类型指南中提到了三种类型的工艺验证:安装资格验证、功能性验证和进程验证。
1.安装资格验证安装资格验证是确保关键设备和系统的安装、操作和性能符合要求的过程。
2.功能性验证功能性验证是证明产品在链接到现实的生产环境中,能够满足性能要求的过程。
3.进程验证进程验证是确保每次生产批次的过程参数符合规格要求的过程。
工艺验证的步骤指南中提到了四个主要的工艺验证步骤。
1.设计验证方案首先,必须设计一个包括验证示例、过程和程序检查和监测方法的验证方案。
2.执行验证执行验证时必须记录和监测所有的步骤和数据,特别是关键参数。
3.收集和分析数据通过收集和分析数据以确认生产过程的稳定性和一致性。
4.确定验证完成和维护验证状态一旦数据收集和分析完成,并验证的结果可以证明产品的一致性,就可以确定验证工作已经完成。
验证后,工厂必须确保维护验证状态,并定期进行监测。
总之,食品药品监管局(FDA)最新工艺验证指南(2011.1版)(中文版)提供了在生产过程中进行工艺验证的详细说明,这有助于确保生产的产品质量达到极高的水平,同时也有利于确保产品的安全性。
FDA 2011工艺验证指南培训
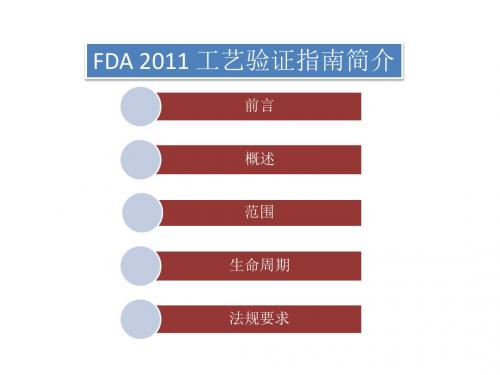
前言 概述 范围 生命周期 法规要求
前言
• 验证工作是实施 GMP 规范的基础,而工艺验证又是验证工作中的关键 性环节。1987年FDA发布了关于工艺验证的指南文件,FDA关于工艺验 证的要求和理解深深地影响着世界各国药政当局和制药行业。2008年 11月,FDA再次发布最新的工艺验证指南(草案),对工艺验证的概 念和文件要求进行了大幅度修改和更新。经过2年多的收集制药行业 的意见和激烈争论,2011年1月24日,FDA发布了工艺验证指南的正式版 指 南 文 件 《Process Validation: General Principles and Practices》。修订后的指南文件更加明确和具体 , 毫无疑问 , 这个指 南文件将对GMP实施产生巨大影响,尤其对于以国际市场为主的外向型 制药企业,影响必将是深远的。新指南对原来的观念有了颠覆性的转 变,尤其是将验证概念延伸到了研发阶段,这对中国企业是个很有难 度的课题 。最大的变化是将整个验证过程分成3个阶段,制药企业需 要在目前验证管理的基础上,将前期在研发和中试阶段的数据进行分 析整理,作为验证的第一部分。与上一版本不同的是,没有针对具体 的工艺验证进行规范,而是将ICH-Q8,Q9和Q10的理念整合到了新的指 南中。不再从技术指南文件上提出具体措施,而是鼓励制药企业在原 则范围内进行技术革新。
概述
• 此版指南将产品生命周期概念和工艺验证活动结合 起来,将工艺验证分为工艺设计、工艺确认、持续 的工艺核实三个阶段。 • 工艺验证是指从工艺设计阶段到商业生产的整个过 程中,对数据进行收集和评价,建立能够使工艺始 终如一的传递到优质产品中的科学证据。 • 对已经上市的产品则直接执行持续工艺核实这一阶 段的工作。制作商应该保持持续的信息收集和对工 艺的定期评价,以发现常见的工艺变异情况,进而 增加对工艺和变异的理解,评价和控制工艺参数, 并建立科学的参数评价方法,在商品生产这一阶段 内做到对工艺的逐步改进(如缩小参数范围等)。 在此阶段如发现有重大变异或工艺有较大改动,而 现有数据不足以进行分析时,可以回到工艺设计或 工艺确认阶段。
准备FDA认证前检查中英文对照

Purpose of a pre-Approval Inspection 认证前检验的目的
Ensure that data submitted in the ANDA or DMF submission is supported by raw data at the facility
确保在ANDA或DMF文件中提交的数据以设施的原始数据为依据
FDA什么时候审查ANDA
After the ANDA holder files the ANDA ANDA持有人将ANDA归档后
Because of backlogs, expect at least 6 month delay before reviewing 由于积压,预计至少延迟6个月才能被审查
The Company Presentation (30-45 minutes)
公司介绍(30-45分钟)
History of Business企业历史
When was it founded? By whom? 什么时候成立?由谁创建?
Important milestones during company development 公司发展中的重要里程碑
Name and title displayed at initial meeting 姓名和标题呈现在首次会议上
Name tags on uniform during inspection 检查期间所有姓名标签格式应统一
Consider also a handout with the following information for key people: 还考虑分发含有以下信息的资料给关键人员: ▪ Full name (and English surname if applicable) 全名(如果适用加上英文姓氏) ▪ Title 标题 ▪ Thumbnail photograph 照片缩图
FDA清洁工艺验证指南中英文对照

FDA清洁工艺验证指南中英文对照FDA(Food and Drug Administration)是美国食品药品监督管理局的简称。
FDA的清洁工艺验证指南提供了有关如何验证食品加工设备和工艺的清洁性的指导,确保产品的安全。
下面是FDA清洁工艺验证指南的英文全文对照。
FDA Cleaning Process Validation GuideIntroduction简介This document provides guidance on how to validate the cleaning processes used in food processing equipment to ensure their cleanliness. Cleaning validation is an essential step in preventing cross-contamination and ensuring the production of safe products.本文提供了关于如何验证食品加工设备中使用的清洁程序以确保其清洁度的指导。
清洁工艺验证是防止交叉污染和确保生产安全产品的关键步骤。
General Principles基本原则1. Validation should be based on a scientific and risk-based approach, taking into account the specific characteristics of the equipment and the product being manufactured.验证应基于科学和风险评估的方法,考虑到设备和正在生产的产品的特殊特性。
2. The validation process should be well-documented and include clear objectives, acceptance criteria, and a description of the methods used.验证过程应有良好的记录,并包括明确的目标、准入标准和方法描述。
▲FDA最新工艺验证指南深度解析

丁香园FDA最新工艺验证指南深度解析验证工作是实施GMP规范的基础,而工艺验证又是验证工作中的关键性环节。
1987年,FDA发布了关于工艺验证的指南文件;FDA关于工艺验证的要求和理解深深地影响着世界各国药政当局和制药行业。
2008年11月,FDA再次发布最新的工艺验证指南(草案),对工艺验证的概念和文件要求进行了大幅度地修改和更新。
毫无疑问,这个指南文件将对GMP实施产生巨大影响,尤其对于以国际市场为主的外向型制药企业,影响也必将是深远的。
笔者通过查阅FDA网站资料,并结合其他著名制药企业和行业协会对于这个指南地评价意见,对这个指南文件进行系统的分析和解读。
一、工艺验证指南文件结构FDA工艺验证验证指南全名为《工艺验证:一般原则和规范》。
指南全文分 为7部分,依次是:简介、背景、工艺验证的法规和法规要求、建议、性能确认批次的同步放行、文件和分析方法学。
二、工艺验证指南文件解读为了方便读者阅读,本部分顺序按照FDA工艺验证指南正文顺序进行依次解读和评价,并且采用了表格形式进行比较。
第一部分:简介工艺验证指南 原文翻译 I.简介本指南概括了一般的原则与方法,这些原则与方法是FDA 认为进行工艺验证的恰当要素,这些工艺被用于生产人用药、动物用药以及生物制品,包括活性药物成分(API 或药用物质),在本指南中以上统称为药品或产品。
本指南整合了一般的原则和方法,所有的生产企业都可以将这些原则和方法应用于生产工艺的验证。
评价意见 应该在划线部分后面增加生物技术产品,应用范围和本指南后面描述保持一致。
工艺验证指南 原文翻译 本指南将工艺验证活动与产品生命周期概念以及现有FDA 相关指南(这里指得是Q8、Q9和Q10指南)进行了协调。
生命周期概念将产品与工艺开发、商业生产工艺的确认以及维护工艺在日常商业生产中处于受控状态连结在一起。
本指南可促进现代生产的原则、工艺改进、创新并且形成完善的科学。
评价意见 无工艺验证指南 原文翻译 以下类别的药品在本指南范围之内:• 人用药物• 兽药• 生物制品和生物技术产品• 制剂产品与活性药物成分(API 或药用物质)3 • 复合产品(药物和医疗设备)中的药物成分评价意见 1-建议从这个指南应用范围删除API术语,采用Q7的12.4部分关于工艺验证的要求;或者将Q7引入这个指南,作为一个支持文件。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
FDA工艺验证指南新旧版透彻比较解读【整理者提醒】1-左侧文本为2011年1月最新修订版本,右侧文本为2008年11月草案版本。
2-蓝色文本为修订后文本或者新增加文本。
3-下划线文本是比旧版本增加的部分内容。
4-删除线文本表示该部分存在于旧版本中,在新版本中删除。
5-注释前面加【注释】2字注明。
6-Zhulikou431关于FDA2008年11月草案彻底解读版本可以在丁香园论坛搜索到,欢迎下载阅读、讨论。
7-不得用于商业用途,转载请注明丁香园信息。
8-增加了新旧版本的中文译文。
9-欢迎各位朋友提出宝贵建议,联系邮箱zhulikou431@.Guidance for IndustryProcess Validation: General Principles and PracticesFinal Version January 2011 Draft 2008I. INTRODUCTIONI. INTRODUCTION简介This guidance outlines the general principlesand approaches that FDA considers appropriate elements of process validation for the manufacture of human and animal drug and biological products, including active pharmaceutical ingredients (APIs or drug substances), collectively referred to in thisguidance as drugs or products. This guidanceincorporates principles and approaches thatall manufacturers can use to validate manufacturing processes.本指南概括了一般的原则与方法,这些原则与方法是FDA 认为进行工艺验证的恰当要素,这些工艺被用于生产人用药、动物用药以及生物制品,包括活性药物成分(API 或药用物质),在本指南中以上统称为药品或产品。
本指南整合了一般的原则和方法,所有的生产企业都可以将这些原则和方法应用于生产工艺的验证。
This guidance outlines the general principles and approaches that FDA considers to be appropriate elements of process validation for the manufacture of human and animal drug and biological products, including active pharmaceutical ingredients (API or drug substance),collectively referred to in this guidance as drugs or products. This guidance incorporates principles and approaches that all manufacturers can use in validating amanufacturing process. 本指南概括了一般的原则与方法,这些原则与方法是FDA 认为进行工艺验证的恰当要素,这些工艺被用于生产人用药、动物用药以及生物制品,包括活性药物成分(API 或药用物质),在本指南中以上统称为药品或产品。
本指南整合了一般的原则和方法,所有的生产企业都可以将这些原则和方法应用于生产工艺的验证。
This guidance aligns process validation activities with a product lifecycle concept and with existing FDA guidance, including the FDA/International Conference on Harmonisation (ICH) guidances for industry, Q8(R2) Pharmaceutical Development, Q9 Quality Risk Management, and Q10 Pharmaceutical Quality System.2 Although this guidance does not repeat the concepts and principles explained in those guidances, FDA encourages the use of modern pharmaceutical development concepts, quality risk man- agement, and quality systems at all stages of the manufacturing process lifecycle. 本指南将工艺验证活动与产品生命周期概念以及现有FDA 相关指南进行了协调;这些存在的FDA指南包括FDA/ICH指南,Q8(R2)药品研发指南、Q9质量风险管理指南和Q10制药质量体系指南。
尽管本指南没有重复上述指南解释的概念和This guidance aligns process validation activities with the product lifecycle concept and with existing FDA guidance.2 2 See the FDA/International Conference on Harmonisation (ICH) guidances for industry: Q8 Pharmaceutical Development, Q9 Quality Risk Management, and when finalized, Q10 Pharmaceutical Quality System (a notice of availability for the May 2007 ICH draft guidance, Q10 Pharmaceutical Quality System, published in the Federal Register on July 13, 2007 (72 FR 38604)). We update guidance documents periodically. To make sure you have the most recent version of a guidance, check the CDER guidance page at /cder/guidance/index.htm, the CBER guidance page at原则,FDA鼓励在药品制造工艺生命周期的各个阶段使用现代药品研发概念、质量风险管理和质量体系概念。
注释2To make sure you have the most recent version of a guidance, check the CDER guidance page at /Drugs/GuidanceComplianceR egulatoryInformation/Guidances/default.htm, the CBER guidance page at/BiologicsBloodVaccines/Guida nceComplianceRegulatoryInformation/Guidances/default.htm, or the CVM guidance page at/AnimalVeterinary/GuidanceCo mplianceEnforcement/GuidanceforIndustry/default.htm/cber/guidelines.htm, or the CVM guidance page at /cvm/Guidance/published.htm . 本指南将工艺验证活动与产品生命周期概念以及现有FDA 相关指南(这里指得是Q8、Q9和Q10指南)进行了协调。
The lifecycle concept links product and process development, qualification of the commercial manufacturing process,3 andmaintenance of the process in a state of controlduring routine commercial production.This guidance supports process improvementand innovation through sound science.生命周期概念将产品与工艺开发、商业生产工艺的确认以及维护工艺在日常商业生产中处于受控状态连结在一起。
本指南通过足够科学知识来支持工艺优化和革新。
注释 3 In this guidance, the term commercialmanufacturingprocess refers to the manufacturingprocess resulting in commercial product (i.e., drugthat is marketed, distributed, and sold or intended to be sold). For the purposes of this guidance, the term commercial manufacturing process does not include clinical trial or treatment IND material. The lifecycle concept links product andprocess development, qualification of thecommercial manufacturing process, andmaintenance of the process in a state of controlduring routine commercial production.This guidance promotes modern manufacturingprinciples, process improvement, innovation,and sound science.生命周期概念将产品与工艺开发、商业生产工艺的确认以及维护工艺在日常商业生产中处于受控状态连结在一起。
