HACCP工艺风险评估报告模板
工艺风险评估范文

工艺风险评估范文1.识别潜在的风险点:通过评估工艺流程的各个环节,找出可能存在的风险点,并针对这些风险点进行重点分析。
2.评估风险等级:对各种风险进行评估,确定其对生产过程和产品质量的影响程度,以便进行相应的风险管理。
3.构建风险控制措施:根据评估结果,制定相应的风险控制措施,预防和控制风险的发生,确保生产过程的安全性和产品质量的稳定性。
4.提高工艺流程的效率:通过分析和评估,找出并消除生产过程中存在的风险隐患,提高工艺流程的效率和稳定性。
1.识别潜在风险点:对生产过程中可能存在的风险点进行识别和形成,包括物理、化学、生物等方面的风险。
2.评估风险等级:通过分析和研究,评估每个潜在风险点的风险等级,确定其对生产过程和产品质量的影响程度。
3.制定控制措施:针对每个风险点,制定相应的风险控制措施,包括技术、管理和操作等方面的措施。
4.实施控制措施:按照制定的控制措施,进行相应的改进和调整,确保控制措施的有效实施。
5.监测和验证效果:对实施后的控制措施进行监测和验证,确保其效果,并根据需要进行相应的调整和改进。
以食品企业的生产工艺为例进行实践。
该企业生产其中一种薯片产品,其中涉及到切片、炸制、调味等多个工艺环节。
1.识别潜在风险点:通过对每个工艺环节进行分析,确定可能存在的风险点。
比如在切片过程中,可能存在刀具伤害的风险;在炸制过程中,可能存在油温不稳定的风险等。
2.评估风险等级:对每个潜在风险点进行评估,确定其对产品质量和生产过程的影响程度。
比如对于刀具伤害的风险,在评估中确定其属于重要性较高的风险。
3.制定控制措施:针对每个风险点,制定相应的风险控制措施。
对于刀具伤害的风险,可以采取设置防护装置、加强培训等措施。
4.实施控制措施:根据制定的控制措施,对生产工艺进行相应改进和调整。
例如,设置防护装置并进行工人培训,确保切片过程中的刀具安全使用。
5.监测和验证效果:对实施后的控制措施进行监测和验证,并根据需要进行相应的调整和改进。
HACCP验证报告

HACCP验证和确认报告公司HACCP小组所有人员在安全体系建立运行后每年进行了确认和验证,从HACCP计划、变更控制、记录保持、CCP验证、校准验证、单项检测、不符合验证、安全投诉、模拟撤回、内审外审、CCP统计等进行评审,概括地看,建立的安全体系基本满足BRC/IOP要素要求,整个体系策划充分,执行有力,符合公司实际情况,采取的纠正或纠正措施正确有效。
由于体系运行时间较长,有部分文件及人员已更新,经过培训上岗后,未发现有潜在不安全产品或高事故风险的趋势,只是在下次人员绩效考核时更加全面详细一些。
以下为验证和确认过程。
一、清洁卫生控制确认:二、车间卫生硬件的确认三、HA(危害分析)的确认四、HACCP计划确认:五、变更验证六、记录保持验证七、CCP点验证CCP点验证记录CCP点编号:CCP1 监控人:关键限值的控制现场观测,并对员工能否对关键限值监控进行描述:员工能对监控方法和监控频率进行识别;当监控表明发生了关键心限值的偏离时,员工能采取纠偏行动,纠偏措施应纠正偏离的原因,确保无不安全物料进入。
见相关的《来料检验报告》、《不合格品控制程序》等监控人是否在岗是频率是否相符是监控工具是否正常使用是监控的实施监控对象是否正确是记录是否正确是记录是否真实是复核人是否及时审核是记录复查是否被及时收集归档是验证总结验证结论:验证人:验证日期:CCP点编号:CCP2 监控人:关键限值的控制现场观测,并对员工能否对关键限值监控进行描述:员工了解客户对产品的要求,如图文资料、客户签样的限值等监控人是否在岗是频率是否相符是监控工具是否正常使用是监控的实施监控对象是否正确是记录是否正确是记录是否真实是复核人是否及时审核是记录复查是否被及时收集归档是验证总结验证结论:验证人:验证日期:CCP点编号:CCP3 监控人:关键限值的控制现场观测,并对员工能否对关键限值监控进行描述:员工了解金属探测各种要求标准监控人是否在岗是频率是否相符是监控工具是否正常使用是监控的实施监控对象是否正确是记录是否正确是记录是否真实是复核人是否及时审核是记录复查是否被及时收集归档是验证总结验证结论:验证人:验证日期:八、测量仪器校准验证公司所有的测量仪器由实验室安排专人负责进行校准验证,经审核未见漏检现象。
HACCP验证报告 (范本)

产品类别:洗涤护肤类、蜡基类
CCP点编号:CCP1称料监控人:***,***,***
关键限值的控制
现场观测,并对员工能否对关键限值监控进行描述:
现场审核记录:
1.现场检测称料房两个消毒柜4个紫外灯强度,1号光源:377μW/cm²,2号光源:105μW/cm²,3号光源:400μW/cm²,4号光源:145μW/cm²,所有光源强度均大于要求的70μW/cm²;
2.查看操作员…将清洗好的称量工具放置在消毒柜中,消毒时长60分钟;
3.查看称料员…,称料时从消毒柜中取用称量工具,符合要求。
监控的实施
监控人是否在岗?
是,现场监控人员…。
频率是否相符?
是,紫外灯开启时间固定1小时。
监控工具是否正常使用?
是,现场检测紫外灯强度,符合要求。
监控对象是否正确?
是,对紫外灯强度及照射时长进行监控。
6
加工过程所有危害是否全部识别出来,对识别出的危害是否进行风险评估?
查《HACCP计划》已建立第四部分“危害风险评估表”,且对各步骤进行了危害风险评估。
7
是否对每一危害制定了相应的控制措施,措施是否适当,可使危害降低、减小或消除到可接受水平?
查《HACCP计划》已建立第五部分“危害分析工作表”,对显著危害判定提出依据,并制定了相应的控制措施。
查《HACCP计划》已建立第七部分“CCP监控纠偏验证程序”,但未发现CCP控制偏离的情况。
10
HACCP计划是否对CCP建立了监控程序,监控程序的对象、频率和责任人是否合理,监控方式是否可行,监控设备是否满足监控要求?
查《HACCP计划》,已对各CCP点建立了监控程序,包括监控程序的对象、频率和责任人,且均能有效落实。
5.工艺验证风险评估报告

工艺验证风险评估报告药业有限公司1 目的1.1利用风险管理方法和工具,对药品生产过程中影响药品生产质量的各要素进行分析评估,提出风险控制建议和意见。
为验证确认活动提供风险分析参考,根据评估结果确定验证范围及程度,最大可能的降低风险因素保证产品质量。
1.2在评估过程中,建立完善质量风险降低的控制措施及再评价风险的可接受程度,指导在各环节开展质量风险控制与质量风险管理活动。
2 依据2.1 药品生产质量管理规范(2010修订)关于风险的要求2.2 本公司风险管理规程;文件编号:SMP-QA-00-003 成立风险评估管理小组风险评估小组成员包括质量授权人、QA主管、QC负责人、生产部部长、设备部部长等管理人员和专业技术人员。
表1 风险评估管理小组成员及职责4 分析识别4.1工艺验证是确认在正常的生产条件下,可能影响产品质量的各种生产系统要素和生产工艺变化因素对生产过程的稳定性及生产系统的可靠性不产生影响,能生产出符合企业内控质量标准的产品。
4.2由风险评估管理小组开展风险调查,召集生产管理人员、生产车间主任、设备管理人员和QC人员,通过总结历史经验、查找资料及对照GMP及实施指南等方法,找出影响工艺验证效果的因素,经过对风险的调查与分析,现将可能出现的风险和可能出现的危害统计如下,见表2表2 风险识别表5 风险分析采用ICH Q9推荐的方法FMEA(失效模式及效应分析)进行风险评估和管理。
风险RPN值=风险发生的可能性(O)×严重性(S)×可检测性(D)接受标准RPN值≤8,评分标准如表3所示。
表3 FMEA各项评分标准表根据以上评分标准,对表2列出的各项风险因素进行打分,结果见表4。
表4 风险因素FMEA表6 风险控制通过对上述12项风险进行评估,RPN值≤8的为可接受风险,RPN值>8为不可接受风险,由部门管理人员和QA制定专门的管理制度和操作程序予以控制,措施如下:表5 不可接受风险控制表7 风险总结通过风险管理小组对生产工艺风险管理过程中的调查、分析和评估,共查找出可能出现的风险12项,分析评估判断不可接受的风险共4项,针对这4项风险,风险管理小组制定了控制措施,相关部门根据控制措施,修改相应的文件,并组织相关人员进行培训,让岗位上的每一员工清楚存在的风险点。
生产工艺验证风险评估报告

生产工艺验证风险评估报告制药有限公司GMP管理文件生产工艺验证风险评估报告一、目的:建立一个生产工艺验证的质量风险管理报告,为生产工艺验证的风险管理提供指导和参考,并为生产工艺验证质量风险管理提供通用性的文件范例。
二、适用范围:适用于生产工艺验证的风险管理。
三、职责:质管部负责组织和实施质量风险管理,质量管理体系相关部门负责本规程的具体实施。
四、正文:1.风险评估计划:1.1风险评估名称:生产工艺验证的质量风险评估;1.2风险评估范围:本次风险管理计划主要是对本公司生产工艺验证进行风险评估活动的策划,包括参与人员和职责,风险分析、风险评价、风险控制,风险改进措施与支持活动,风险管理评审等;1.3参与人员和职责:1.3.1生产工艺验证质量风险评估小组包括质管部负责人,生产部负责人,检验室主管,工程部,QA,设备操作人员;1.3.2生产工艺验证质量风险评估小组负责组织实施质量风险评估;1.3.3质管部负责人负责制定质量风险评估计划,风险评估组织,风险评估后改进措施监督落实等;1.3.4质管部负责整理质量风险管理文档。
1.4风险评估小组人员2.风险分析:本次采用失败模式效果分析(FMEA),识别潜在的失败模式,按照风险评估操作规程,对生产工艺验证的严重程度、发生的几率、发现的可能性评分,其评分结果见表1-1、表1-2 、表1-3表1-1 生产工艺验证中的关键控制点失败影响的严重程度表1-2 生产工艺验证中的关键控制点失败影响的发生慨率表1-3 生产工艺验证中的关键控制点失败影响的可检测性3.风险评价3.1按照风险评估操作规程,对生产工艺验证质量风险进行评价,见表2。
3.2风险评价标准按照风险等级确定,可接受的风险不需要改进措施即可接受;合理可降低的风险与不经过风险/收益分析即判定为不可接受的风险,需制定相关的改进措施并有效执行以减少其质量风险后才能予以接受,(表3)。
表 2 生产工艺验证风险评价第5 页表3 生产工艺验证风险评价第6 页注:风险值(RPN):RPN≦8时风险程度低,不需要通过改进措施就能接受;56≧RPN﹥8时风险程度中,需要通过制定改进措施,实施后RPN≦8时,才能接受;RPN﹥56时风险程度高,制定相应的改进措施,并有效执行,确认无质量风险时,才能接受;4.风险控制:4.1按照风险评估操作规程对生产工艺验证质量风险中、高程度,制定详细的改进措施,并监督实施。
HACCP危害分析评估和控制措施范例
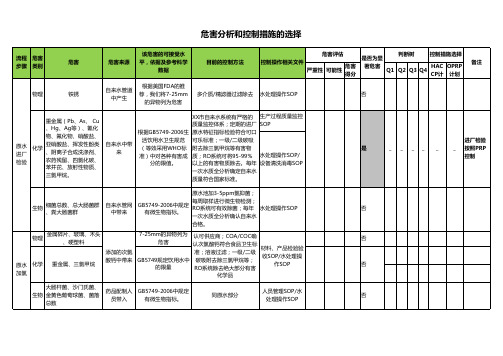
流程 危害 步骤 类别
物理
危害 铁锈
危害来源
该危害的可接受水 平,依据及参考国FDA的推 荐,我们将7-25mm
的异物列为危害
目前的控制方法
危害评估 控制操作相关文件 严重性 可能性 危害
得分
是否为显
判断树
控制措施选择
著危害 Q1 Q2 Q3 Q4 HAC OPRP CP计 计划
根据GB5749-2006生 原水特征指标检验符合可口
活饮用水卫生规范 可乐标准;一级/二级碳吸
(等效采用WHO标 附去除三氯甲烷等有害物 准)中对各种有害成 质;RO系统可将95-99% 水处理操作SOP/
分的限值。
以上的有害物质除去。每年 设备清洗消毒SOP
一次水质全分析确定自来水
质量符合国家标准。
GB5749-2006中规定 有微生物指标。
同原水部分
人员管理SOP/水 处理操作SOP
进厂检验
是
_ _ __ _
_ 按照PRP
控制
否 否 否 否
7-25mm的异物列为 认可供应商;COA/COC确
危害
GB5749规定饮用水中 的限量
认次氯酸钙符合食品卫生标 准;溶液过滤;一级/二级 碳吸附去除三氯甲烷等; RO系统除去绝大部分有害
材料、产品检验验 收SOP/水处理操
作SOP
化学品
大肠杆菌、沙门氏菌、 生物 金黄色葡萄球菌、菌落
总数
药品配制人 员带入
多介质/精滤器过滤除去 水处理操作SOP
否
备注
原水 进厂 检验
重金属(Pb、As、 Cu
、Hg、Ag等)、氰化
物、氟化物、硝酸盐、
haccp调研报告模板

haccp调研报告模板HACCP调研报告一、引言(100字)HACCP(Hazard Analysis and Critical Control Points)是一种食品安全管理系统,旨在预防并控制食品生产过程中的潜在危害因素。
本调研旨在了解HACCP在食品行业的应用情况,以及其对食品安全的贡献和影响。
二、调研背景(150字)食品安全是每个人都关心的重要问题。
随着食品供应链的全球化和消费者对食品安全的日益关注,各国政府和企业都加强了对食品安全的管理和控制。
HACCP作为一种国际通用的食品安全管理系统,被广泛采用和推广。
因此,本调研将有助于了解HACCP在食品行业的实际应用情况和意义。
三、调研方法(100字)本调研采用问卷调查的方式,针对食品生产企业进行调查。
调查问题包括企业是否实施HACCP系统、实施HACCP的具体步骤和内容、HACCP对食品安全的影响等。
我们通过发放调查问卷并进行个别访谈,获取了实际数据并进行分析。
四、调研结果(400字)调研结果显示,超过80%的企业表示已经实施了HACCP系统。
这些企业普遍认为HACCP有助于提高食品安全水平和降低食品安全风险。
同时,他们还提到HACCP的实施需要全面规划和培训,并且需要持续监测和改进。
调研结果还显示,企业在实施HACCP系统时主要遇到以下困难:1)缺乏专业知识和人员培训;2)高成本和资源投入;3)企业规模较小,缺乏实施HACCP系统的条件。
这些困难使得一些企业尚未实施HACCP系统或者仅实施了部分步骤。
此外,调研结果还发现HACCP系统的实施对食品企业的经营模式和市场竞争力有着积极的影响。
那些实施HACCP系统的企业更容易通过质量认证和检验,提高了产品质量和信誉度,从而在市场竞争中占据优势地位。
五、结论(150字)通过本次调研,我们了解到HACCP在食品行业的广泛应用和积极意义。
企业实施HACCP系统可以提高食品安全水平,降低食品安全风险,并增强企业的市场竞争力。
haccp范例范文

haccp范例范文Hazard Analysis Critical Control Point (HACCP) 是一种食品安全管理系统,旨在确保食品在生产和加工过程中的安全性和卫生质量。
下面是一个关于HACCP的范例文档,涵盖了各个方面和要求。
第一部分:概述1.1介绍本文档旨在为使用HACCP系统进行食品生产和加工的组织提供指导和规范。
它包括了HACCP计划的各个步骤和相关文件,以确保食品安全,并满足相关法规和标准的要求。
1.2范围本HACCP计划适用于本公司的食品生产和加工过程。
它覆盖了从原材料采购到最终产品出货的各个环节。
第二部分:风险分析2.1风险评估本节列出了与食品生产和加工过程相关的可能风险。
包括物理、化学和生物性风险。
2.2紧急情况计划本节包括了针对可能出现的紧急情况所制定的应急计划,包括食品污染、供应链问题等。
第三部分:生产流程控制本节包括了原材料供应商的选择和评估标准,以确保其符合相关食品安全要求。
还包括了原材料的检验和接收程序。
3.2加工流程控制本节描述了食品的加工过程,包括温度控制、时间控制、卫生标准等。
还包括了员工培训和操作规程的制定。
3.3环境控制本节包括了食品加工环境的控制要求,包括温湿度控制、洁净度要求等。
还包括了设备维护和清洁程序。
第四部分:监控和纠正措施4.1监控措施本节包括了对食品生产和加工过程的监控措施,包括温度记录、检测方法等。
4.2纠正措施本节描述了当监控结果不符合预期时所采取的纠正措施。
包括产品召回、流程调整等。
4.3验证和验证本节包括对HACCP计划的验证和验证过程的描述。
包括抽样和分析方法等。
第五部分:文档和记录本节包括了对HACCP计划和相关文件的控制要求,包括版本控制、审查和批准程序等。
5.2记录控制本节描述了对HACCP计划中的记录的控制要求,包括记录保存时间、记录的查阅程序等。
5.3培训记录本节包括了对员工培训记录的要求,包括培训计划、培训内容和培训效果评估等。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Risk Assessment Report for XXX Process XXX工艺风险评估报告Revision History版本修订索引Index 目录1Overview概述 (4)2Purpose 目的 (4)3Scope范围 (4)4Responsibility 职责 (4)5Abbreviations缩略语 (6)6Regulation and Guidance 法规和指南 (7)7System Description系统描述 (9)7.1Plant Description车间概述 (9)7.2Product Information产品信息 (10)7.3Equipments/System List设备/系统清单 (15)8Reference Documents 参考文件 (16)9Risk Assessment Method 风险评估方法 (17)9.1Conduct a Hazard Analysis进行危害分析 (17)9.2Determine the Critical Control points (CCPs)关键控制点的确认 (20)9.3Establish Target Levels and Critical Limits建立目标水平和关键限值 (21)9.4Establish System(s) to monitoring CCP 建立CCP监测系统 (21)9.5Establish an appropriate Corrective Action Plan建立适当的纠正计划 (22)9.6Establish Procedures建立规程 (22)9.7Establish Documentation and keep records建立文件并保留记录 (23)10Hazard analysis Matrix危害分析矩阵 (25)11CCP Control Matrix关键控制点控制矩阵 (26)12Conclusion结论&建议 (27)1 Overview概述This document summarizes the quality risk management report for XXX at the XXX site. Thedocument embraces the principles of ICH Q9 (Quality Risk Management) and uses riskmanagement tools to support manufacturing strategies designed to minimize risks to product quality.本文件总结了位于XXX 的XXX的质量风险管理报告。
本文件包括ICH Q9(质量风险管理)的原则以及使用合适的风险管理工具来支持设计用于将对产品质量的风险降到最低的生产策略。
A formalized risk management approach was applied to the XXX manufacturing facility. Thisinvolved a holistic assessment that identified the potential hazards and risks to product quality for all products handled in the facility to ensure that appropriate controls were in place to manufacture these products safely. The assessment supported the development of a quality risk management (QRM) that addressed the manufacture of the following products: XXX.对XXXXX生产工厂采用了一种正式化的风险管理方法。
这涉及到能够确定对产品质量的可能危害和风险的全瞻性评估,以保证具有适宜的控制来以安全的方式生产这些产品。
这种评估行为针对XXXX 产品生产的质量风险管理(QRM)的制定提供了支持。
2 Purpose 目的The purpose of this process risk assessment is to evaluate, define, and document all potential hazard and critical control points for XXX Process of Oral Dosage Plant of XXX by applying the principle of ICH Q9 (Quality Risk Management) and risk management tool of Hazard Analysis and Critical Control Point (HACCP). This assessment activity is to ensure that the products can be manufactured under appropriate control and safety method, and provide support to define process critical control points of XXX Process.本工艺风险评估的目的是应用ICH Q9(质量风险管理)的原则以及使用危害分析和关键控制点(HACCP)的风险管理工具评估确定出XXX车间XXX生产工艺中所有的潜在危险和关键控制点,并记录在文件中。
以保证具有适宜的控制,并以安全的方式生产该产品。
这种评估行为针对XXX的生产工艺关键控制点的制定提供了支持。
3 Scope范围The risk assessment scope is the XXXX in XXX Plant of XXXX. The Product Code: XXX.本工艺风险评估的范围为XXXX车间XXX,产品代码为XXX。
4 Responsibility 职责XXX responsibility XXX的职责:✓Risk assessment execution进行风险评估✓Risk Assessment Report compilation风险评估报告的编写XXX responsibility XXX的职责:To assure that product-specific knowledge and expertise are available for the development of an effective HACCP plan by assembling a multidisciplinary team. Team members should represent all the relevant disciplines, such as research and development, production, quality control, quality assurance, microbiology, engineering and distribution or others as applicable with the ability to:确保具有产品的详细知识和专业技术,用以各专业组开发有效地危害分析和关键工艺控制点的计划。
小组成员应代表研发,生产,质量控制,质量保证,微生物学,工程和发货以及其他相关领域能力。
✓Conduct a hazard analysis实施危害分析✓Identify potential hazards识别潜在的危害✓Identify hazards which should be controlled识别应该控制的危害✓Recommend controls and critical limits建议的控制和关键限度✓Devise procedures for monitoring and verification监测和确认的设计程序✓Recommend appropriate corrective action where deviations occur对发生的偏差推荐合适的纠正措施✓Establish Documentation and keep records建立文件并保留记录✓Verify the HACCP plan确认危害分析和关键控制点计划Other responsibilities:其它职责✓Information collection信息的收集✓Supply all procedure, data, manuals, drawing and documentation necessary for the completion of final report提供为报告编写所需要的所有的规程、数据、手册、图纸和文件✓Taking part in the RA参与风险评估✓Review and approve the report报告的审核和批准5 Abbreviations缩略语The abbreviations which will be used in this document are listed in the following form.在下面的表格中规定了本文件中使用的缩略语。
6 Regulation and Guidance 法规和指南To write this protocol the following reference documents have been used:为了编写本报告,参考了以下法规和指南:✓State Food and Drug Administration (CFDA), China, Good Manufacturing Practice (2010 Revision), March, 2011国家食品药品监督管理局(CFDA),中国,药品生产质量管理规范(2010年修订),2011年03月✓ICH Q9: Quality Risk ManagementICH Q9:质量风险管理,2005年11月✓ISPE Guideline Volume 5, Commissioning and Qualification, 1st Edition, 2001 ISPE指南5“调试和确认”,2001年第一版✓ISPE Guideline Volume 7, Risk Based Manufacture of Pharmaceutical Products, 1st Edition, 2010ISPE指南7“基于风险分析的制药产品生产”,2010年第一版✓ISPE Good Practice Guides for Applied Risk Management for Commissioning and Qualification,1st Edition, 2011ISPE良好实践指南,基于风险分析的调试和确认,2011年第一版✓ISPE GAMP 5 (Good Automated Manufacturing Practice 5)ISPE GAMP 5良好的自动化制造规范,2008年第五版✓ISPE Pharmaceutical Engineering Guides for New and Renovated facilities, Volume 4: Water and steam systemsISPE 新建和改造的工厂医药工程指南,第4卷-水和蒸汽系统✓WHO Technical Report series No. 908, 2003, Annex 7 WHO 技术报告系列No. 908,2003,附录77 System Description系统描述7.1 Plant Description车间概述7.2 Product Information产品信息7.2.1Product Name产品名称:General Name: XXX通用名称:XXXEnglish Name: XXX英文名称:XXXPinyin: XXX汉语拼音:XXXDosage: XXX剂型:XXX7.2.2 Description描述:Activity ingredients of this product is XXX.本品主要成份为XXX。
