纳美芬基本介绍
盐酸纳美芬FDA的说明书

Revex (nalmefene hydrochloride injection)Rx onlyDESCRIPTIONREVEX (nalmefene hydrochloride injection), an opioid antagonist, is a 6-methylene analogue of naltrexone. The chemical structure is shown below:Molecular Formula: C21H25NO3•HClMolecular Weight: 375.9, CAS # 58895-64-0Chemical Name: 17-(Cyclopropylmethyl)-4,5〈-epoxy-6-methylenemorphinan-3,14-diol, hydrochloride salt.Nalmefene hydrochloride is a white to off-white crystalline powder which is freely soluble in water up to 130 mg/mL and slightly soluble in chloroform up to 0.13 mg/mL, with a pK a of 7.6.REVEX is available as a sterile solution for intravenous, intramuscular, and subcutaneous administration in two concentrations, containing 100 µg or 1.0 mg of nalmefene free base per mL. The 100 µg/mL concentration contains 110.8 µg of nalmefene hydrochloride and the 1.0 mg/mL concentration contains 1.108 mg of nalmefene hydrochloride per mL. Both concentrations contain 9.0 mg of sodium chloride per mL and the pH is adjusted to 3.9 with hydrochloric acid.Concentrations and dosages of REVEX are expressed as the free base equivalent of nalmefene.CLINICAL PHARMACOLOGYPharmacodynamicsREVEX prevents or reverses the effects of opioids, including respiratory depression, sedation, and hypotension. Pharmacodynamic studies have shown that REVEX has a longer duration of action than naloxone at fully reversing doses. REVEX has no opioid agonist activity.REVEX is not known to produce respiratory depression, psychotomimetic effects, or pupillary constriction. No pharmacological activity was observed when REVEX was administered in the absence of opioid agonists.REVEX has not been shown to produce tolerance, physical dependence, or abuse potential.REVEX can produce acute withdrawal symptoms in individuals who are opioid dependent.PharmacokineticsNalmefene exhibited dose proportional pharmacokinetics following intravenousadministration of 0.5 mg to 2.0 mg. Pharmacokinetic parameters for nalmefene after a 1mg intravenous administration in adult male volunteers are listed in Table 1.Table 1: Mean (CV%) Nalmefene Pharmacokinetic ParametersIn Adult Males Following a 1 mg Intravenous DoseParameter Young, N=18 Elderly, N=1162-80 Age 19-32Cp at 5 min. (ng/mL) 3.7 (29) 5.8 (38)Vdss (L/kg) 8.6 (19) 8.6 (29)Vc (L/kg) 3.9 (29) 2.8 (41)AUC0-inf (ng-hr/mL) 16.6 (27) 17.3 (14)Terminal T1/2 (hr) 10.8 (48) 9.4 (49)Clplasma (L/hr/kg) 0.8 (23) 0.8 (18)ABSORPTIONNalmefene was completely bioavailable following intramuscular or subcutaneousadministration in 12 male volunteers relative to intravenous nalmefene. The relativebioavailabilities of intramuscular and subcutaneous routes of administration were 101.5%± 8.1% (Mean ± SD) and 99.7% ± 6.9%, respectively. Nalmefene will be administeredprimarily as an intravenous bolus, however, nalmefene can be given intra-muscularly(IM) or subcutaneously (SC) if venous access cannot be established. While the time tomaximum plasma nalmefene concentration was 2.3 ± 1.1 hours following intramuscularand 1.5 ± 1.2 hours following subcutaneous administrations, therapeutic plasmaconcentrations are likely to be reached within 5-15 minutes after a 1 mg dose in anemergency. Because of the variability in the speed of absorption for IM & SC dosing, andthe inability to titrate to effect, great care should be taken if repeated doses must be givenby these routes.DISTRIBUTIONFollowing a 1 mg parenteral dose, nalmefene was rapidly distributed. In a study of brainreceptor occupancy, a 1 mg dose of nalmefene blocked over 80% of brain opioidreceptors within 5 minutes after administration. The apparent volumes of distributioncentrally (Vc) and at steady-state (Vdss) are 3.9 ± 1.1 L/kg and 8.6 ± 1.7 L/kg,respectively. Ultrafiltration studies of nalmefene have demonstrated that 45% (CV 4.1%)is bound to plasma proteins over a concentration range of 0.1 to 2 µg/mL. An in vitrodetermination of the distribution of nalmefene in human blood demonstrated thatnalmefene distributed 67% (CV 8.7%) into red blood cells and 39% (CV 6.4%) intoplasma. The whole blood to plasma ratio was 1.3 (CV 6.6%) over the nominalconcentration range in whole blood from 0.376 to 30 ng/mL.METABOLISMNalmefene is metabolized by the liver, primarily by glucuronide conjugation, andexcreted in the urine. Nalmefene is also metabolized to trace amounts of an N-dealkylated metabolite. Nalmefene glucuronide is inactive and the N-dealkylatedmetabolite has minimal pharmacological activity. Less than 5% of nalmefene is excretedin the urine unchanged. Seventeen percent (17%) of the nalmefene dose is excreted in thefeces. The plasma concentration-time profile in some subjects suggests that nalmefene undergoes enterohepatic recycling..ELIMINATIONAfter intravenous administration of 1 mg REVEX to normal males (ages 19-32), plasma concentrations declined biexponentially with a redistribution and a terminal elimination half-life of 41 ± 34 minutes and 10.8 ± 5.2 hours, respectively. The systemic clearance of nalmefene is 0.8 ± 0.2 L/hr/kg and the renal clearance is 0.08 ± 0.04 L/hr/kg.Special PopulationsELDERLYDose proportionality was observed in nalmefene AUC0-inf following 0.5 to 2 mg intravenous administration to elderly male subjects. Following a 1 mg intravenous nalmefene dose, there were no significant differences between young (19-32 years) and elderly (62-80 years) adult male subjects with respect to plasma clearance, steady-state volume of distribution, or half-life. There was an apparent age-related decrease in the central volume of distribution (young: 3.9 ± 1.1 L/kg, elderly: 2.8 ± 1.1 L/kg) that resulted in a greater initial nalmefene concentration in the elderly group. While initial nalmefene plasma concentrations were transiently higher in the elderly, it would not be anticipated that this population would require dosing adjustment. No clinical adverse events were noted in the elderly following the 1 mg intravenous nalmefene dose. PATIENTS WITH HEPATIC IMPAIRMENTSubjects with hepatic disease, when compared to matched normal controls, had a 28.3% decrease in plasma clearance of nalmefene (0.56 ± 0.21 L/hr/kg versus 0.78 ± 0.24L/hr/kg, respectively). Elimination halflife increased from 10.2 ± 2.2 hours to 11.9 ± 2.0 hours in the hepatically impaired. No dosage adjustment is recommended since nalmefene will be administered as an acute course of therapy.PATIENTS WITH RENAL IMPAIRMENTThere was a statistically significant 27% decrease in plasma clearance of nalmefene in the end-stage renal disease (ESRD) population during interdialysis (0.57 ± 0.20 L/hr/kg) and a 25% decreased plasma clearance in the ESRD population during intradialysis (0.59 ± 0.18 L/hr/kg) compared to normals (0.79 ± 0.24 L/hr/kg). The elimination half-life was prolonged in ESRD patients from 10.2 ± 2.2 hours in normals to 26.1 ± 9.9 hours. (See DOSAGE AND ADMINISTRATION.)GENDER DIFFERENCESThere has not been sufficient pharmacokinetic study to make a definitive statement as to whether the pharmacokinetics of nalmefene differs between the genders.CLINICAL TRIALSREVEX has been administered to reverse the effects of opioids after general anesthesia and in the treatment of overdose. It has also been used to reverse the systemic effects of intrathecal opioids.Reversal of Postoperative Opioid DepressionREVEX (nalmefene hydrochloride injection) (N=326) was studied in 5 controlled trials in patients who had received morphine or fentanyl intraoperatively. The primary efficacy criterion was the reversal of respiratory depression. A positive reversal was defined asboth an increase in respiratory rate by 5 breaths per minute and a minimum respiratory rate of 12 breaths per minute. Five minutes after administration, initial single REVEX doses of 0.1, 0.25, 0.5, or 1.0 µg/kg had effectively reversed respiratory depression in a dose-dependent manner. Twenty minutes after initial administration, respiratory depression had been effectively reversed in most patients receiving cumulative doses within the recommended range (0.1 to 1.0 µg/kg). Total doses of REVEX above 1.0µg/kg did not increase the therapeutic response. The postoperative administration of REVEX at the recommended doses did not prevent the analgesic response to subsequently administered opioids.Reversal of the Effect of Intrathecally Administered OpioidsIntravenous REVEX at doses of 0.5 and 1.0 µg/kg was administered to 47 patients given intrathecal morphine. One to 2 doses of 0.5 and 1.0 µg/kg REVEX reversed respiratory depression in most patients. The administration of REVEX at the recommended doses did not prevent the analgesic response to subsequently administered opioids. Management of Known or Suspected Opioid OverdoseREVEX (N=284) at doses of 0.5 mg to 2.0 mg was studied in 4 trials of patients who were presumed to have taken an opioid overdose. REVEX doses of 0.5 mg to 1.0 mg effectively reversed respiratory depression within 2 to 5 minutes in most patients subsequently confirmed to have opioid overdose. A total dose greater than 1.5 mg did not increase the therapeutic response.INDICATIONS AND USAGEREVEX is indicated for the complete or partial reversal of opioid drug effects, including respiratory depression, induced by either natural or synthetic opioids.REVEX is indicated in the management of known or suspected opioid overdose. CONTRAINDICATIONSREVEX is contraindicated in patients with a known hypersensitivity to the product. WARNINGSUse of REVEX in EmergenciesREVEX, like all drugs in this class, is not the primary treatment for ventilatory failure. In most emergency settings, treatment with REVEX should follow, not precede, the establishment of a patent airway, ventilatory assistance, administration of oxygen, and establishment of circulatory access.Risk of Recurrent Respiratory DepressionAccidental overdose with long acting opioids [such as methadone and levo-alpha-acetylmethadol (LAAM)] may result in prolonged respiratory depression. Respiratory depression in both the postoperative and overdose setting may be complex and involve the effects of anesthetic agents, neuromuscular blockers, and other drugs. While REVEX has a longer duration of action than naloxone in fully reversing doses, the physician should be aware that a recurrence of respiratory depression is possible, even after an apparently adequate initial response to REVEX treatment.Patients treated with REVEX should be observed until, in the opinion of the physician, there is no reasonable risk of recurrent respiratory depression. PRECAUTIONSGeneralCARDIOVASCULAR RISKS WITH NARCOTIC ANTAGONISTSPulmonary edema, cardiovascular instability, hypotension, hypertension, ventricular tachycardia, and ventricular fibrillation have been reported in connection with opioid reversal in both postoperative and emergency department settings. In many cases, these effects appear to be the result of abrupt reversal of opioid effects.Although REVEX has been used safely in patients with pre-existing cardiac disease, all drugs of this class should be used with caution in patients at high cardiovascular risk or who have received potentially cardiotoxic drugs. (See DOSAGE AND ADMINISTRATION.)RISK OF PRECIPITATED WITHDRAWALREVEX, like other opioid antagonists, is known to produce acute withdrawal symptoms and, therefore, should be used with extreme caution in patients with known physical dependence on opioids or following surgery involving high doses of opioids. Imprudent use or excessive doses of opioid antagonists in the postoperative setting has been associated with hypertension, tachycardia, and excessive mortality in patients at high risk for cardiovascular complications. (See PRECAUTIONS.)INCOMPLETE REVERSAL OF BUPRENORPHINEPreclinical studies have shown that nalmefene at doses up to 10 mg/kg (437 times the maximum recommended human dose) produced incomplete reversal of buprenorphine-induced analgesia in animal models. This appears to be a consequence of a high affinity and slow displacement of buprenorphine from the opioid receptors. Hence, REVEX may not completely reverse buprenorphine-induced respiratory depression.Drug InteractionsREVEX has been administered after benzodiazepines, inhalational anesthetics, muscle relaxants, and muscle relaxant antagonists administered in conjunction with general anesthesia. It also has been administered in outpatient settings, both in trials in conscious sedation and in the emergency management of overdose following a wide variety of agents. No deleterious interactions have been observed.Preclinical studies have shown that both flumazenil and nalmefene can induce seizures in animals. The coadministration of both flumazenil and nalmefene produced fewer seizures than expected in a study in rodents, based on the expected effects of each drug alone. Based on these data, an adverse interaction from the coadministration of the two drugs is not expected, but physicians should remain aware of the potential risk of seizures from agents in these classes.Carcinogenesis, Mutagenesis, Impairment of FertilityNalmefene did not have mutagenic activity in the Ames test with five bacterial strains or the mouse lymphoma assay. Clastogenic activity was not observed in the mouse micronucleus test or in the cytogenic bone marrow assay in rats. However, nalmefene did exhibit a weak but significant clastogenic activity in the human lymphocyte metaphase assay in the absence but not in the presence of exogenous metabolic activation. Oral administration of nalmefene up to 1200 mg/m2/day did not affect fertility, reproductive performance, and offspring survival in rats.Use in PregnancyPREGNANCY CATEGORY BReproduction studies have been performed in rats (up to 1200 mg/m2/day) and rabbits (up to 2400 mg/m2/day) by oral administration of nalmefene and in rabbits by intravenous administration up to 96 mg/m2/day (114 times the human dose). There was no evidence of impaired fertility or harm to the fetus. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.Nursing MothersNalmefene and its metabolites were secreted into rat milk, reaching concentrations approximately three times those in plasma at one hour and decreasing to about half the corresponding plasma concentrations by 24 hours following bolus administration. As no clinical information is available, caution should be exercised when REVEX is administered to a nursing woman.Use in Pediatric PatientsSafety and effectiveness of REVEX in pediatric patients have not been established.Use in NeonatesThe safety and effectiveness of REVEX in neonates have not been established in clinical studies. In a preclinical study, nalmefene was administered by subcutaneous injection to rat pups at doses up to 205 mg/m2/day throughout maternal lactation without producing adverse effects. A preclinical study evaluating the irritancy of the dosage form following arterial and venous administration in animals showed no vascular irritancy.REVEX (nalmefene hydrochloride injection) should only be used in the resuscitation of the newborn when, in the opinion of the treating physician, the expected benefits outweigh the risks.Geriatric UseClinical studies of Revex did not include sufficient number of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.ADVERSE REACTIONSAdverse event information was obtained following administration of REVEX to 152 normal volunteers and in controlled clinical trials to 1127 patients for the treatment of opioid overdose or for postoperative opioid reversal.Nalmefene was well tolerated and showed no serious toxicity during experimental administration to healthy individuals, even when given at 15 times the highest recommended dose. In a small number of subjects, at doses exceeding the recommendedREVEX dose, nalmefene produced symptoms suggestive of reversal of endogenousopioids, such as have been reported for other narcotic antagonist drugs. These symptoms(nausea, chills, myalgia, dysphoria, abdominal cramps, and joint pain) were usuallytransient and occurred at very low frequency.Such symptoms of precipitated opioid withdrawal at the recommended clinical doseswere seen in both postoperative and overdose patients who were later found to have hadhistories of covert opioid use. Symptoms of precipitated withdrawal were similar to thoseseen with other opioid antagonists, were transient following the lower doses used in thepostoperative setting, and more prolonged following the administration of the largerdoses used in the treatment of overdose.Tachycardia and nausea following the use of nalmefene in the postoperative setting werereported at the same frequencies as for naloxone at equivalent doses. The risk of boththese adverse events was low at doses giving partial opioid reversal and increased withincreases in dose. Thus, total doses larger than 1.0 µg/kg in the postoperative setting and1.5 mg/70 kg in the treatment of overdose are not recommended.Relative Frequencies of Common Adverse ReactionsWith an Incidence Greater than 1%(all patients, all clinical settings)Adverse EventNalmefene Naloxone PlaceboN=1127N=369N=776% Nausea 18%18%Vomiting 9% 7% 4%- Tachycardia 5%8%- Hypertension 5%7%Postoperative pain 4% 4% N/A- Fever 3%4%Dizziness 3% 4% 1%Headache 1% 1% 4%Chills 1%-1%- Hypotension 1%1%- Vasodilatation 1%1%Incidence less than 1%CARDIOVASCULAR: Bradycardia, arrhythmiaDIGESTIVE: Diarrhea, dry mouthNERVOUS SYSTEM: Somnolence, depression, agitation, nervousness, tremor,confusion, withdrawal syndrome, myoclonusRESPIRATORY: PharyngitisSKIN: PruritusUROGENITAL: Urinary retentionThe incidence of adverse events was highest in patients who received more than therecommended dose of REVEX.Laboratory findings: Transient increases in CPK were reported as adverse events in 0.5%of the postoperative patients studied. These increases were believed to be related tosurgery and not believed to be related to the administration of REVEX. Increases in AST were reported as adverse events in 0.3% of the patients receiving either nalmefene or naloxone. The clinical significance of this finding is unknown. No cases of hepatitis or hepatic injury due to either nalmefene or naloxone were observed in the clinical trials. DRUG ABUSE AND DEPENDENCEREVEX is an opioid antagonist with no agonist activity. It has no demonstrated abuse potential, is not addictive, and is not a controlled substance.OVERDOSAGEIntravenous doses of up to 24 mg of nalmefene, administered to healthy volunteers in the absence of opioid agonists, produced no serious adverse reactions, severe signs or symptoms, or clinically significant laboratory abnormalities. As with all opioid antagonists, use in patients physically dependent on opioids can result in precipitated withdrawal reactions that may result in symptoms that require medical attention. Treatment of such cases should be symptomatic and supportive. Administration of large amounts of opioids to patients receiving opioid antagonists in an attempt to overcome a full blockade has resulted in adverse respiratory and circulatory reactions.DOSAGE AND ADMINISTRATIONImportant Information - Dosage FormsREVEX is supplied in two concentrations that can be identified by their color coded container labels: a concentration suitable for postoperative use (100 µg/mL) in a blue labeled ampul containing ONE (1) mL and a concentration suitable for the management of overdose (1 mg/mL, 10 times as concentrated, 20 times as much drug) in a green labeled ampul containing TWO (2) mL. Proper steps should be taken to prevent use of the incorrect concentration.General PrinciplesREVEX should be titrated to reverse the undesired effects of opioids. Once adequate reversal has been established, additional administration is not required and may actually be harmful due to unwanted reversal of analgesia or precipitated withdrawal.Duration of ActionThe duration of action of REVEX is as long as most opioid analgesics. The apparent duration of action of REVEX will vary, however, depending on the half-life and plasma concentration of the narcotic being reversed, the presence or absence of other drugs affecting the brain or muscles of respiration, and the dose of REVEX administered. Partially reversing doses of REVEX (1 µg/kg) lose their effect as the drug is redistributed through the body, and the effects of these low doses may not last more than 30-60 minutes in the presence of persistent opioid effects. Fully reversing doses (1 mg/70 kg) have been shown to last many hours in both experimental and clinical studies, but may complicate the management of patients who are in pain, at high cardiovascular risk, or who are physically dependent on opioids.The recommended doses represent a compromise between a desirable controlled reversal and the need for prompt response and adequate duration of action. Using higher dosages or shorter intervals between incremental doses is likely to increase the incidence and severity of symptoms related to acute withdrawal such as nausea, vomiting, elevated blood pressure, and anxiety.Patients Tolerant to or Physically Dependent on OpioidsREVEX may cause acute withdrawal symptoms in individuals who have some degree of tolerance to and dependence on opioids. These patients should be closely observed for symptoms of withdrawal following administration of the initial and subsequent injections of REVEX. Subsequent doses should be administered with intervals of at least 2-5 minutes between doses to allow the full effect of each incremental dose of REVEX to be reached.Recommended Doses for Reversal of Postoperative Opioid Depression Use 100 µg/mL dosage strength (blue label) and see Table 2 for initial doses.The goal of treatment with REVEX in the postoperative setting is to achieve reversal of excessive opioid effects without inducing a complete reversal and acute pain. This is best accomplished with an initial dose of 0.25 µg/kg followed by 0.25 µg/kg incremental doses at 2-5 minute intervals, stopping as soon as the desired degree of opioid reversal is obtained. A cumulative total dose above 1.0 µg/kg does not provide additional therapeutic effect.Table 2: Reversal of Postoperative Opioid DepressionmL of REVEXBody Weight100 µg/mL Solution50 kg 0.12560 kg 0.15070 kg 0.17580 kg 0.20090 kg 0.225100 kg 0.250In cases where the patient is known to be at increased cardiovascular risk, it may be desirable to dilute REVEX 1:1 with saline or sterile water and use smaller initial and incremental doses of 0.1 µg/kg.Management of Known or Suspected Opioid OverdoseUse 1.0 mg/mL dosage strength (green label).The recommended initial dose of REVEX for non-opioid dependent patients is 0.5 mg/70 kg. If needed, this may be followed by a second dose of 1.0 mg/70 kg, 2-5 minutes later. If a total dose of 1.5 mg /70 kg has been administered without clinical response, additional REVEX (nalmefene hydrochloride injection) is unlikely to have an effect. Patients should not be given more REVEX than is required to restore the respiratory rate to normal, thus minimizing the likelihood of cardiovascular stress and precipitated withdrawal syndrome.If there is a reasonable suspicion of opioid dependency, a challenge dose of REVEX 0.1 mg/70 kg should be administered initially. If there is no evidence of withdrawal in 2 minutes, the recommended dosing should be followed.REVEX had no effect in cases where opioids were not responsible for sedation and hypoventilation. Therefore, patients should only be treated with REVEX when thelikelihood of an opioid overdose is high, based on a history of opioid overdose or the clinical presentation of respiratory depression with concurrent pupillary constriction. Repeated DosingREVEX is the longest acting of the currently available parenteral opioid antagonists. If recurrence of respiratory depression does occur, the dose should again be titrated to clinical effect using incremental doses to avoid over-reversal.Hepatic and Renal DiseaseHepatic disease and renal failure substantially reduce the clearance of nalmefene (see Pharmacokinetics). For single episodes of opioid antagonism, adjustment of REVEX dosage is not required. However, in patients with renal failure, the incremental doses should be delivered slowly (over 60 seconds) to minimize the hypertension and dizziness reported following the abrupt administration of nalmefene to such patients.Loss of Intravenous AccessShould intravenous access be lost or not readily obtainable, a pharmacokinetic study has shown that a single dose of REVEX should be effective within 5-15 minutes after intramuscular or subcutaneous doses of 1.0 mg. (See Pharmacokinetics.)SAFETY AND HANDLINGREVEX is distributed in sealed ampuls which represent no known risk to health care workers. As with all parenterals, care should be taken to prevent the generation and inhalation of aerosols during preparation and use. Dermal absorption of spilled REVEX should be prevented by prompt removal of contaminated clothing and rinsing the skin thoroughly with cool water.Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.HOW SUPPLIEDREVEX (nalmefene hydrochloride injection) is available in the following presentations: An ampul containing 1 mL of 100 µg/mL nalmefene base (Blue Label) Box of 10 (NDC 10019-315-21)An ampul containing 2 mL of 1 mg/mL nalmefene base (Green Label) Box of 10 (NDC 10019-311-22)Store at controlled room temperature.REVEX is a registered trademark of Ivax Laboratories, Inc.Baxter is a trademark of Baxter International, Inc.Manufactured forBaxter Healthcare CorporationDeerfield, IL 60015 USAby: Taylor PharmaceuticalsDecatur, IL 62525For Product Inquiry 1 800 ANA DRUG (1-800-262-3784)U.S. Patent No. 4,535,157MLT-01167/1.011。
盐酸纳美芬用于神经外科血管介入手术麻醉复苏的临床观

盐酸纳美芬用于神经外科血管介入手术麻醉复苏的临床观背景介绍神经外科血管介入手术是一种常见的治疗脑血管疾病的方法。
该手术需要关闭相关的脑血管,在手术过程中需要进行全身麻醉。
复苏期是手术过程中非常关键的一环,如何使患者尽早恢复及时回复常规活动和工作是医生需要特别关注的问题。
盐酸纳美芬是一种镇痛剂,广泛应用于神经外科手术和其他麻醉的手术中。
在神经外科血管介入手术中,盐酸纳美芬被用于麻醉和复苏中。
盐酸纳美芬的特性盐酸纳美芬是一种非吗啡类的镇痛剂。
它主要通过抑制中枢神经系统的负责疼痛传导的负责体系来达到镇痛的效果。
因此,与其他类似药物相比,它没有明显的成瘾性和药物依赖性。
在注射盐酸纳美芬后,其作用会在15-30分钟内开始显现,并能持续6-8小时。
因此,它可以用于手术麻醉和复苏阶段,从而减轻术后疼痛和畏惧。
盐酸纳美芬在神经外科血管介入手术中的应用在神经外科血管介入手术中,盐酸纳美芬主要用于麻醉和复苏。
在手术前,医生会给患者注射适量的盐酸纳美芬,以使患者进入深度麻醉状态。
该状态通常持续数小时,直到神经外科医生完成手术并决定由麻醉医生停止麻醉。
在手术完成后,患者进入复苏阶段。
此时医生通常会给患者注射一定量的盐酸纳美芬,以帮助患者恢复。
在复苏过程中,医生需要密切关注患者的各项生命体征,并采取必要的措施确保患者安全康复。
盐酸纳美芬的适应症和注意事项盐酸纳美芬适用于各种类型的手术,包括神经外科血管介入手术。
该药物不建议使用于对盐酸纳美芬过敏的患者。
注射盐酸纳美芬的过程中,需要密切关注患者的生命体征,特别是呼吸和心跳。
在治疗期间,患者需要注射适量的药物,以确保患者始终处于安全的状态。
目前还没有确定盐酸纳美芬对于孕妇和哺乳期妇女的安全性,因此这类患者在使用该药物前应该谨慎使用,并听从医生的建议。
安全性和有效性盐酸纳美芬在神经外科血管介入手术中的应用已经被广泛地证实为安全和有效。
确保该药物的安全和有效性的关键是医生对于该药物的熟悉和合理的应用。
纳美芬-抒纳

2.1阿片受体拮抗药
纳洛酮(naloxone);纳曲酮(naltrexone),主 要用于对已解除阿片类药物毒瘾者的康复期辅 助治疗 ,以口服为主;纳美芬(Nalmefene, NMF)等。 对各型阿片受体有拮抗作用,其本身无明显药 理效应及毒性。
2.2.1盐酸纳美芬优越的阿片受体拮抗剂
2.2.2盐酸纳美芬与纳曲酮、纳洛酮的比较
2.2.3纳洛酮的缺陷
①对κ受体的低选择性 ——脑干保护剂 由于κ受体主要分布于大脑内,所以脑保护和脑复 苏的关键是阻断大脑内的κ受体。 纳洛酮主要为μ受体拮抗剂,对μ受体的结合力强, 对κ、δ受体的结合力很弱,所以用于脑保护和脑 复苏时,纳洛酮主要治疗靶点在脑干而不在全脑。
——J. Neurotrauma. 1997 Dec;14(12):931-41.
2.3.5安全性研究
对健康用药者,即使剂量达到推荐剂量的15 倍或以上,没有出现严重的不良反应。 对未使用过阿片类拮抗剂的健康志愿者静注 24mg纳美芬(=240支),未产生严重不良反应。 美国临床试验时,试用1750例病人未发现其 导致疾病或死亡; 本公司产品在我国218例临床试验中,仅1例 出现轻度头痛,且继续用药症状消失。
损伤:脑外伤和出血,失血 70% 治疗:纳美芬/特尼札特/CG3703/多巴胺/安慰剂 监测:复苏0、60和120min 时脑血流量及脑电图 结果:纳美芬和CG3703组在复苏0时脑血流量和 氧输送明显增加,其它组明显降低。在60和120分 钟时,纳美芬组脑电图活性保持不变,但是其它组 变化显著。 结论:纳美芬阻止脑外伤和脑出血后的大脑氧输送 和脑电图活性的降低。
内源性阿片肽与脑损伤
一种盐酸纳美芬合成方法

一种盐酸纳美芬合成方法
一种盐酸纳美芬合成方法
盐酸纳美芬是一种常用的非甾体抗炎药,具有镇痛、退热、抗炎等作用。
本文将介绍一种盐酸纳美芬的合成方法。
需要准备的原料有苯甲酸、乙酰氯、氢氧化钠、盐酸、纳美芬等。
具体步骤如下:
1. 将苯甲酸和乙酰氯混合,加入氢氧化钠溶液中,反应生成苯乙酸乙酯。
2. 将苯乙酸乙酯和纳美芬混合,加入盐酸溶液中,反应生成盐酸纳美芬。
3. 将反应产物进行过滤、洗涤、干燥等处理,得到纯净的盐酸纳美芬。
这种合成方法具有简单、高效、成本低等优点,适用于工业生产。
同时,盐酸纳美芬也是一种常用的药物,广泛应用于临床治疗中。
需要注意的是,在合成过程中需要控制反应条件,避免产生副反应或产物不纯等问题。
同时,对于药物的生产和使用也需要严格遵守相关法规和规定,确保药品的质量和安全性。
盐酸纳美芬是一种重要的药物,其合成方法也具有重要的意义。
通过不断的研究和改进,我们可以更好地利用这种药物,为人类健康事业做出更大的贡献。
纳美芬说明书用法

纳美芬说明书用法
纳美芬是一种非处方药,通常用于缓解轻至中度疼痛和发热症状。
以下是纳美芬的使用说明:
1.用法:口服。
将药片整片吞下,并用足够的水服用。
2.剂量:根据年龄、体重和其他因素来确定剂量。
一般建议成人每次服用1-2片,每天2-3次,每次服用间隔至少4小时。
儿童剂量应根据其年龄和体重而定。
3.不宜过量:不要超过最大建议剂量,以避免毒性反应。
4.注意事项:患有肝脏、肾脏、心脏疾病、哮喘、消化道溃疡等疾病的人应在医生的指导下使用本药。
同时要避免饮酒、吸烟等刺激性物质。
5.儿童使用:儿童使用前需咨询医生,并根据医生的建议来确定剂量和用药频率。
6.孕妇和哺乳期妇女:孕妇和哺乳期妇女应遵循医生的指导,使用本药。
7.副作用:可能出现头痛、恶心、胃痛、皮疹等副作用。
如果出现严重反应,例如呼吸困难、过敏反应等,应立即就医。
请在使用本药物前仔细阅读并遵循药品说明书和医生的建议。
盐酸纳美芬注射液

盐酸纳美芬注射液【药品名称】通用名:盐酸纳美芬注射液汉语拼音:Yansuan Namei Zhusheye英文名:Nalmefene Hydrochloride Injection【成分】本品主要成分为盐酸纳美芬【性状】本品为无色成名液体【适应症】纳美芬用于完全或部分逆转阿片类药物的作用,包括由天然的或合成的阿片类药物引起的呼吸抑制。
【规格】1ml:0.1mg(以C21H25NO3计)【用法用量】纳美芬注射液一般为静注,也可肌注或皮下注射。
用于术后阿片样物质抑制:静脉注射,使用1ml∶0.1mg规格的制剂。
初始剂量0.25µg/kg,2~5min后再给0.25µg/kg补充,呈现阿片逆转作用后立即停止给药。
累计剂量超过1µg/kg不会增加治疗效应。
在已知可能增加患者心血管危险的情况下,可将本品用生理盐水或灭菌用水进行1∶1稀释,并使用0.1µg/ml这样较小的初始及补充剂量。
用于已知或怀疑使用阿片样物质过量:静脉注射,使用2ml∶2mg规格的制剂。
初始剂量0.5mg/70kg,如有必要,2~5min后给予第2个剂量。
如总剂量达到1.5mg/70kg仍无临床作用,则增加剂量也不会起作用。
当呼吸频率达到正常情况后,就应停止给药,以尽可能减少发生心血管危险与催促戒断综合征的几率。
对与阿片样物质无关的镇静及低血压的病例,本品不产生作用。
因此,只有根据患者使用阿片样物质过量的历史或呼吸抑制并伴随瞳孔收缩的临床特征判断阿片样物质过量的可能性较高情况下,才给患者使用本品进行治疗。
【药品详细说明】本品为纯粹的阿片受体拮抗剂,本身无内在活性,但能竞争性拮抗μ、κ、δ阿片受体,其中与μ受体的亲和力最强。
本品可预防或逆转阿片效应,包括呼吸抑制、镇静及低血压。
药效学研究显示纳美芬比纳洛酮的作用持续时间更长。
本品不具有产生呼吸抑制、拟精神或缩瞳作用。
本品不产生耐受性、躯体依赖性和滥用潜力。
纳美芬介绍

纳美芬(nalmefene)作为一种特异性吗啡受体阻断剂,结构为6位亚甲基的纳曲酮类似物,该药系1975年合成,并于1995年上市,现已渐成为纳洛酮的替代产品。
新一代用于神经保护治疗的阿片受体拮抗剂Nalmetrene与纳洛酮相比,纳美芬具有作用时间长、给药途径多、生物利用度高、不良反应小等特点,临床用途广泛,已用于拮抗麻醉性镇痛剂引起的呼吸抑制、镇静和低血压等症状,并应用于酒精中毒和海洛因依赖等的治疗。
1药理学作用纳美芬是μ、κ、α阿片受体阻断剂,能竞争性拮抗各类阿片受体,尤其对μ受体有很强的亲和力。
在人体的脑内和外周组织都存在着β-内啡肽、脑啡肽等阿片样内源性物质。
这些物质对神经、内分泌、呼吸及心血管等生理功能起着重要的调节作用。
纳美芬在与外周阿片受体结合后,还能与脑干等部位的阿片受体结合,从而阻断内源性阿片样物质在身体应激状态下引起的中枢神经呼吸和循环系统等产生的一系列症状。
纳美芬透过血脑屏障的能力与它所成的盐的种类有很大的关系,甲碘纳美芬不能透过血脑屏障,而盐酸纳美芬较易透过血脑屏障。
纳美芬静注2min即可产生受体拮抗作用,5min之内可阻断80%的大脑阿片受体。
它广泛分布于组织中,在肝脏与葡糖苷酸结合并缓慢代谢形成非活性物质,从尿中以原形排泄的量不足5%,但纳美芬的排泄对于肾末期疾病患者而言有很大改变,晚期肾病患者的清除半衰期从正常者的(10.2±2.2)h增至(26.1±9.9)h。
纳美芬的t1/2约为11h,口服吸收迅速,生物利用度高达40%~50%,而纳络酮的t1/2仅为1~2h,纳美芬的作用持续时间比多数阿片受体激动剂(除美沙酮和右丙氧芬)都长。
2药物制剂注射剂纳美芬注射剂(REVEX(是)由Ohmeda制剂公司开发,于1995年4月17日获得FDA批准上市的,是该药目前唯一的上市剂型。
该制剂有两种规格0.1g·L-1和1g·L-1的生理盐水溶液(pH3.9)。
浅谈盐酸纳美芬的临床应用

浅谈盐酸纳美芬的临床应用盐酸纳美芬是一种具有高选择性和特异性的新一代内源性阿片受体拮抗剂。
它于1975年合成,在1995年上市,现已渐成为纳洛酮的替代品。
与纳洛酮相比,本药具有肝脏毒性低,作用时间长,活性强,生物利用度高,不良反应少等特点。
临床用途较广。
1 药理作用纳美芬是纳曲酮的6-亚基类似物,作为内源性的阿片物质的阻断剂能与κ,μ 和δ各型阿片受体结合,尤其是能与μ受体结合作用最强,在人体脑内和外周组织,都存在着脑啡肽、内啡肽等阿片样内源性物质,这些物质对人体神经、内分泌、呼吸和心血管等生理功能起重要的调节作用,纳美芬与脑内和外周的阿片受体结合后,可阻断这些物质在身体应激状态下引起的中枢神经呼吸和循环系统等产生的一系列症状[1]具有广泛的药理作用。
纳美芬在体内分布迅速,用药后5分钟内可阻断80﹪的大脑阿片类体,在浓度为0.1-2ug/ml时其血浆蛋白的结合率为45﹪,主要通过肝脏代谢,与葡萄糖醛酸化合物结合形成无活性的代谢物随尿排出,5﹪以下的原型药物随尿液排出,17﹪的纳美芬通过粪便排出。
2 临床应用在临床上,纳美芬除了具有传统阿片受体拮抗剂的用途如抗休克,治疗酒精中毒;吗啡类药物急性中毒的治疗以及对戒毒患者复吸的预防;麻醉催醒;还用于心力衰竭;治疗脊髓损伤;胃肠功能紊乱;脑保护;减肥;治疗男性性功能障碍及一些功能紊乱、肿瘤等。
2.1 抗休克纳美芬为特异性的阿片受体拮抗剂,可阻断抗体应急状态下释放过量的β内啡肽的作用,提高左心室收缩压及增高血压作用,从而提高休克存活率2.2 手术后的催醒和吗啡類药物中毒的解救当纳美芬剂量在0.1~1μg?kg-1范围内,能有效地逆转手术后阿片样镇痛剂引起的呼吸抑制作用,而不产生麻醉药物带来的痛觉迟钝的烧灼感。
当给予高剂量(0.5~1.6mg)时,纳美芬能有效地逆转麻醉品服用过量的危重患者的呼吸抑制[2]。
2.3 治疗酒精中毒当酒精量过大,超过个人肝脏代谢而形成蓄积,并进入大脑,此时机体处于应激状态,下丘脑释放因子存世线垂体释放内源性阿片肽样物质,其中作用最强的是β-内啡肽,另外乙醇代谢物乙醛在体内与多巴胺缩合成阿片样物质,直接或间接作用于脑内阿片肽受体,使患者处于兴奋状态,渐转入抑制状态,继之皮质下中枢,小脑延髓血管运动中枢和呼吸中枢相继受抑制,严重中毒可发生呼吸循环衰竭。
盐酸纳美芬注射液说明书乐萌

盐酸纳美芬注射液说明书【药品名称】通用名称:盐酸纳美芬注射液汉语拼音:Yan sua n Nameife n Zhusheye商品名:乐萌英文名:Nalmefe ne Hydrochloride Injection【成份】1.本品主要成份为盐酸纳美芬,化学名称:二醇盐酸盐化学结构式:分子式:C21H25NO3 HCI分子量:375.9CAS No. : 58895-64-02.辅料:氯化钠、注射用水【性状】本品为无色的澄明液体。
【适应症】纳美芬用于完全或部分逆转阿片类药物的作用,包括由天然的或合成的阿片类药物引起的呼吸抑制。
【规格】1ml:0.1mg (以C21H25NO3 计)【用法用量】纳美芬注射液一般为静注,也可肌注或皮下注射。
一般原则:本品可通过剂量滴定逆转不期望的阿片类作用。
因为不期望逆转痛觉缺失而引起危害或产生撤药反应,一旦达到了足够的逆转效果,就不应继续用药。
逆转术后阿片类药物抑制的推荐剂量:使用100卩g/mL的剂量浓度,见表1的初始剂量。
术后使用纳美芬治疗的目的是为了逆转阿片类药物过度的抑制作用,而不是引起完全的逆转和急性疼痛。
初始剂量为0.25卩g/kg 2-5分钟后可增加剂量0.25卩g/kg当达到了预期的阿片类药物逆转作用后立即停药。
累积剂量大于1.0卩g/k环会增加疗效。
0. L2&(\ inf)0. 175u jjn0.2250.25017-环丙甲基-4,5 «环氧-6-亚甲基吗啡喃-3,14-对已知的心血管高危患者用药时,应将本品与氯化钠注射液或无菌注射用水按1:1 的比例稀释,并使用0.1卩g/kg乍为初始剂量和增加剂量。
对阿片类药物耐受或产生躯体依赖的患者:纳美芬对阿片类药物耐受或躯体依赖的患者能引起急性戒断症状。
在初次或持续用药时应密切观察这些患者是否出现戒断症状。
至少应在2-5 分钟后再次用药,以增加剂量达到最大疗效。
重复用药:如果复发呼吸抑制,应再增加剂量来达到临床治疗效果,增加剂量时应避免过度逆转。
纳美芬氮氧化物

纳美芬是一种阿片受体拮抗剂,主要用于拮抗阿片类药物引起的呼吸抑制、镇静和欣快感,常用于术后镇痛和分娩镇痛。
纳美芬对μ、κ、δ三种阿片受体均有阻断作用,其中对μ受体的亲和力最强。
纳美芬可以拮抗吗啡类药物的镇痛、镇静、呼吸抑制和欣快作用,但对非阿片受体的作用较弱。
纳美芬可以用于治疗急性酒精中毒,通过拮抗阿片受体,逆转中枢抑制作用,加速患者清醒。
此外,纳美芬还可以用于阿片类药物过量中毒的解救,与阿片类药物产生竞争性拮抗作用,逆转阿片类药物引起的中枢抑制。
需要注意的是,纳美芬也存在一些副作用和注意事项。
例如,纳美芬可能会引起恶心、呕吐、血压下降、心动过缓等不良反应,因此在使用时需要严格控制剂量和适应症。
此外,纳美芬可能会引起呼吸抑制和窒息等严重后果,因此在使用时需要密切观察患者的呼吸情况,确保呼吸通畅。
总的来说,纳美芬是一种阿片受体拮抗剂,具有拮抗阿片类药物引起的呼吸抑制、镇静和欣快感的作用,常用于术后镇痛和分娩镇痛。
在使用纳美芬时需要严格控制剂量和适应症,并密切观察患者的呼吸情况,确保呼吸通畅。
纳美芬在小儿复合麻醉中的应用

纳美芬在小儿麻醉中的应用
01
02
03
04
05
在小儿麻醉中,纳美芬 常用于以下几个方面
辅助麻醉:在全身麻醉 中,纳美芬常作为辅助 麻醉药物,与其他麻醉 药物联合使用,提高患 儿的耐受性和安全性。
术后镇痛:纳美芬可以 用于术后镇痛,减轻患 儿的疼痛和不适感。
拮抗阿片类药物的呼吸 抑制:在小儿麻醉中, 阿片类药物的使用是常 见的,但会引起呼吸抑 制等不良反应。纳美芬 可以拮抗阿片类药物的 作用,恢复自主呼吸。
减少阿片类药物的用量
纳美芬可以替代部分阿片类药物在麻醉中的作用,从而减少 了阿片类药物的用量。
纳美芬与阿片类药物联合使用,可以增强麻醉效果,减少阿 片类药物的剂量需求,降低术后呼吸抑制等不良反应的风险 。
03
纳美芬在小儿复合 麻醉中的实践应用
纳美芬与丙泊酚联合应用
总结词
纳美芬与丙泊酚联合应用在小儿麻醉中具有协同作用,可减少各自的用量和不良反应,提供更平稳、安全的麻醉 效果。
镇静作用:纳美芬可以引起轻度镇静, 有助于患儿在麻醉过程中的合作和配合 。
镇痛作用:纳美芬可以减轻疼痛,提高 患儿的舒适度和耐受性。
抑制阿片类药物引起的呼吸抑制:纳美 芬可以拮抗阿片类药物对呼吸中枢的抑 制作用,从而恢复自主呼吸。
抑制恶心呕吐:纳美芬可以抑制手术和 麻醉引起的恶心呕吐,降低术后恶心呕 吐的发生率。
拮抗恶心呕吐:纳美芬 可以拮抗手术和麻醉引 起的恶心呕吐,降低术 后恶心呕吐的发生率。
02
纳美芬在小儿复合 麻醉中的优势
缩短麻醉苏醒时间
01
纳美芬可以促进小儿在麻醉状态 下的苏醒过程,使他们在手术后 能够更快地恢复意识,减少因长 时间麻醉而引起的并发症风险。
盐酸纳美芬注射液说明书(乐萌)

盐酸纳美芬注射液说明书【药品名称】通用名称:盐酸纳美芬注射液汉语拼音:Yansuan Nameifen Zhusheye商品名:乐萌英文名:Nalmefene Hydrochloride Injection【成份】本品主要成份为盐酸纳美芬,辅料为氯化钠、注射用水化学名称:17-环丙甲基-4,5α-环氧-6-亚甲基吗啡喃-3,14-二醇盐酸盐化学结构式:分子式:C21H25NO3·HCl分子量:375.9【性状】本品为无色的澄明液体。
【适应症】纳美芬用于完全或部分逆转阿片类药物的作用,包括由天然的或合成的阿片类药物引起的呼吸抑制。
【规格】1ml:0.1mg(以C21H25NO3计)【用法用量】纳美芬注射液一般为静注,也可肌注或皮下注射。
一般原则:本品可通过剂量滴定逆转不期望的阿片类作用。
因为不期望逆转痛觉缺失而引起危害或产生撤药反应,一旦达到了足够的逆转效果,就不应继续用药。
逆转术后阿片类药物抑制的推荐剂量:使用100μg/mL的剂量浓度,见表1的初始剂量。
术后使用纳美芬治疗的目的是为了逆转阿片类药物过度的抑制作用,而不是引起完全的逆转和急性疼痛。
初始剂量为0.25μg/kg,2-5分钟后可增加剂量0.25μg/kg,当达到了预期的阿片类药物逆转作用后立即停药。
累积剂量大于1.0μg/kg不会增加疗效。
表1对已知的心血管高危患者用药时,应将本品与氯化钠注射液或无菌注射用水按1:1的比例稀释,并使用0.1μg/kg作为初始剂量和增加剂量。
对阿片类药物耐受或产生躯体依赖的患者:纳美芬对阿片类药物耐受或躯体依赖的患者能引起急性戒断症状。
在初次或持续用药时应密切观察这些患者是否出现戒断症状。
至少应在2-5分钟后再次用药,以增加剂量达到最大疗效。
重复用药:如果复发呼吸抑制,应再增加剂量来达到临床治疗效果,增加剂量时应避免过度逆转。
【不良反应】对健康者用药者,即使剂量达到推荐剂量的15倍或15倍以上,纳美芬的耐受性都很好、没有出现严重的不良反应。
纳美芬有何特点

纳美芬有何特点
【术语与解答】
①纳美芬是纳曲酮的衍生物,主要成分为盐酸纳美芬,该药为无色澄明液体,辅料为氯化钠和注射用水;
②纳美芬对μ受体的亲和力强于к受体或δ受体,故可与阿片受体激动药相竞争;
③纳美芬主要用于逆转阿片类药物的药理作用,包括由天然或合成的阿片类药物引起的呼吸抑制;
④临床将纳美芬稀释后静脉注射,也可肌注或皮下注射,一般初始剂量为0.25μg/kg(心血管疾病患者先以0.1μg/kg稀释剂量缓慢注射为妥),如仍需要应用,可先观察5分钟,必要时再给予0.1~
0.2μg/kg;
⑤纳美芬体内分布迅速,用药后5分钟内可阻断80%的脑内阿片受体,但并非完全逆转阿片的镇痛作用或逆转后出现急性疼痛。
【麻醉与实践】
纳美芬临床主要用于全麻术毕拮抗芬太尼类镇痛药的呼吸抑制作用,以避免术后患者机体缺氧或产生低氧血症及高碳酸血症。
此外,纳美芬其独特优点在于拮抗阿片类镇痛药的呼吸抑制作用后,仍能保留其部分原有的镇痛作用。
【提示与注意】
①纳美芬对阿片类药物耐受或躯体依赖的患者能引起急性戒断症状,在初次或持续用药时应密切观察该类患者是否出现戒断症状;
②对阿片类药物所致的复发性呼吸抑制,应再适当增加剂量以达到临床治疗效果,但增加剂量时应避免过度逆转;
③纳美芬过量应用可出现恶心、寒战、肌痛、烦躁不安及关节疼痛等;
④年老体弱或伴有心血管疾病患者如采用纳美芬拮抗阿片类药物应慎重,以很小剂量为适宜,避免发生肺水肿、低血压或高血压,以及室性心动过速等;
⑤对纳美芬过敏者禁用。
纳美芬的注意事项

纳美芬的注意事项纳美芬是一种消炎、抗菌的药物,主要成分是纳唑酮。
在使用纳美芬的过程中,我们需要注意以下几个方面:1. 切勿超量使用:使用纳美芬时,应按照医生的建议来使用,不可随意增加剂量。
过量使用纳美芬可能会引起肝脏问题,包括肝损伤。
2. 严禁酗酒:纳美芬与酒精不良反应严重,不推荐同时使用。
酒精与纳美芬一同使用可能会引起严重的肝脏问题,如肝炎、肝损伤等。
3. 个体差异:纳美芬的药效因人而异,因此必须根据个体差异调整剂量。
年龄、体重、身体状况等都会影响剂量的调整,因此在使用纳美芬前最好请医生根据个人条件给出正确的剂量指导。
4. 不适用人群:孕妇、哺乳期妇女、儿童、肾功能受损者和肝功能受损者等特殊人群应慎用纳美芬,必要时需要咨询医生的意见。
5. 注意过敏反应:使用纳美芬过程中,如出现过敏症状,如皮疹、荨麻疹、呼吸困难等,应及时停止使用,并咨询医生。
6. 长期使用:如果使用纳美芬时间较长,应定期进行相关检查,如血常规、肝功能检查等,以监控药物对身体的影响。
7. 注意并发症:使用纳美芬可能会引起一些并发症,如头晕、恶心、腹泻、食欲不振等,出现这些症状时应注意观察,如症状严重或持续时间较长,应立即咨询医生。
8. 不影响用药:纳美芬一般不会影响其他药物的代谢和排泄,但仍建议在使用其他药物时,事先咨询医生或药剂师,以免发生药物相互作用。
9. 存储注意:纳美芬应存放在阴凉、干燥的地方,避免阳光直射。
儿童不能接触到纳美芬,以防误服或误用。
10. 遵医嘱:在使用纳美芬过程中,应遵循医生的嘱咐,按时按量使用。
如有任何疑问或不适,应及时向医生咨询。
总之,纳美芬是一种消炎、抗菌的药物,但在使用过程中需要注意上述事项,合理用药,以避免不必要的健康问题。
如果有任何疑问或不适,建议立即咨询医生的建议。
盐酸纳美酚注射液(乐萌)阿片受体拮抗剂

全新一代阿片受体拮抗剂--纳美芬
1995年被美国医院药师联合会(ASHP)推荐为纳洛酮的取代药物
纳美芬的三大特点
速 强
效 效
“三大特点” 满足临床所需
安 全
速效
迅速解除中枢性呼吸抑制及循环抑制
脂溶性高,分子量小(375.9) 分布迅速,给药后迅速透过血脑屏障 2min起效,5min内可阻断80%的大脑 阿片受体
纳美芬改善感染性休克患者的预后
25 20.6 20 APACHEⅡ评分 15 10 5 0 治疗前
乐萌组
22.9
21.7 16.1
P<0.05
*
治疗后
生理盐水组
结论:乐萌显著降低了患者的APACHE Ⅱ评分。
注:APACHE Ⅱ——血流动力学、急性生理学与慢性健康状况评分系统Ⅱ
中国危重病急救医学,2010年
含 量
Байду номын сангаас**
P<0.05
*
0h
1h
3h
6h
12h
24h
休克发生后时间
中毒患者血浆β-内啡肽是正常人的2倍
250
农药中毒患者β-EP含量(pg/ml)
202 200 150 107.4 100 50 0
*
207.1
*
227.1
*
*P<0.05
对照组
轻度组
中度组
重度组
应激情况下患者血浆β-内啡肽含量明显升高,且中毒程度越严重,β-内啡肽 含量越高。
感知和运动功能障碍
呼吸抑制 循环抑制
μ受体
δ受体
心血管功能抑制
Cox BM. Life Sci 1982;31:1645-1658
纳美芬与纳洛酮区别
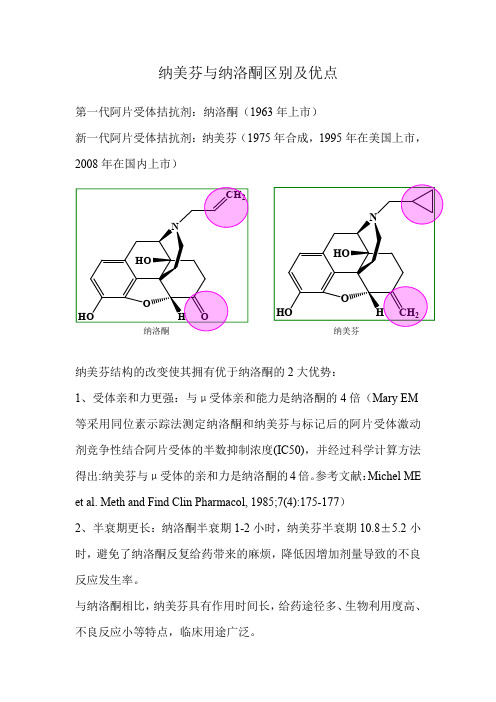
纳美芬与纳洛酮区别及优点
第一代阿片受体拮抗剂:纳洛酮(1963年上市)
新一代阿片受体拮抗剂:纳美芬(1975年合成,1995年在美国上市,2008年在国内上市)
纳美芬结构的改变使其拥有优于纳洛酮的2大优势:
1、受体亲和力更强:与μ受体亲和能力是纳洛酮的4倍(Mary EM 等采用同位素示踪法测定纳洛酮和纳美芬与标记后的阿片受体激动剂竞争性结合阿片受体的半数抑制浓度(IC50),并经过科学计算方法
得出:纳美芬与μ受体的亲和力是纳洛酮的4倍。
参考文献:
Michel ME et al.Meth and Find Clin Pharmacol,1985;7(4):175-177)
2、半衰期更长:纳洛酮半衰期1-2小时,纳美芬半衰期10.8±5.2小时,避免了纳洛酮反复给药带来的麻烦,降低因增加剂量导致的不良反应发生率。
与纳洛酮相比,纳美芬具有作用时间长,给药途径多、生物利用度高、不良反应小等特点,临床用途广泛。
纳美芬基本介绍资料

最新一代用于神经保护治疗的阿片受体拮抗剂——纳美芬从第一代阿片受体拮抗剂——纳洛酮在临床应用以来,国内外研究者对纳洛酮在神经内外科的应用进行了持续的研究。
继1981年Baskin首次报道纳洛酮静注治疗脑梗塞,能明显改善神经功能后,纳洛酮陆续成功应用于蛛网膜下腔出血、颅脑损伤等神经功能性损伤疾病的治疗。
在国内,自2001年中华医学会神经外科学会组织完成511例盐酸纳洛酮治疗急性颅脑损伤病人随机双盲多中心前瞻性临床研究后,纳洛酮的应用日益受到临床医生的重视,神经保护作用的临床疗效卓越。
作为最新一代的阿片受体拮抗剂——纳美芬,和纳洛酮具有相同的作用机制,有着比纳洛酮更优越的药理、药代动力学参数,在国外已经成功应用于脑梗塞、脑外伤的治疗。
本文谨就纳美芬在神经保护方面的特点和应用作简要概述。
一、神经系统损伤的生理病理机制在人体中枢和外周系统,都存在着内啡肽、强啡肽、脑啡肽等内源性阿片类物质。
这类物质能与体内广泛存在的μ、κ和δ等阿片受体结合,对人体神经、内分泌、呼吸和心血管等生理功能起重要的调节作用。
神经系统损伤,是许多神经系统疾病共有的病理性过程。
外伤、卒中、中毒、感染、变性等疾病都会使神经系统受到致病因子的损害,导致不可逆性神经功能丧失。
中枢神经系统损伤后,兴奋性神经递质,如内源性阿片类物质、兴奋性氨基酸、单胺类神经递质等含量过度升高,且含量越高,损伤程度越严重。
这些神经递质的含量增高导致了相应受体(阿片受体、NMDA)过度兴奋,造成中枢神经系统的继发性损伤。
二、阿片受体拮抗剂的神经保护治疗作用阿片受体拮抗剂是能够与阿片受体竞争性结合,且自身无激动作用的一类物质。
它通过竞争性阻断内阿片肽与阿片受体的结合,从而阻断病理反应的通路来起到神经保护作用。
作为临床广泛应用的阿片受体拮抗剂,纳洛酮在神经保护方面的剂量和疗效是呈正比关系的,κ和δ受体对颅脑损伤具有保护作用。
小剂量纳洛酮只能作用于μ受体,用于解除术后呼吸抑制、酒精中毒、抗休克的治疗;大剂量纳洛酮能同时作用于μ、κ和δ三种阿片受体,已经广泛应用于神经内外科。
盐酸纳美芬注射液说明书
盐酸纳美芬注射液说明书一金美芬
逆转术后阿片类药物抑制的推荐剂量:
使用100 g/ml的剂量浓度,见表1的初始剂量
表1 :逆转术后阿片类药物的抑制作用
术后使用纳美芬治疗的目的是为了逆转阿片类药物过度的抑制作用,而不是引起完全的逆转和急性疼痛。
初始剂量为0.25 g/kg , 2〜5min后可增加剂量
0.25 g/kg,当达到了预期的阿片类药物逆转作用后立即停药。
累计剂量大于1.0 g/kg不会增加疗效。
对已知的心血管高危患者用药时,应将本品与氯化钠注射液或无菌注射用水按 1 :1的比例稀释,并使用0.1 g/kg作为初始剂量和增加剂量。
对阿片类药物耐受或产生躯体依赖的患者:
纳美芬对阿片类药物耐受或躯体依赖的患者能引起急性戒断症状。
在初次或持续用药时应密切观察这些患者是否出现戒断症状。
至少应在2〜5分钟后再次用药, 以增加剂量达到最大疗效。
床病例)。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
最新一代用于神经保护治疗的阿片受体拮抗剂——纳美芬
从第一代阿片受体拮抗剂——纳洛酮在临床应用以来,国内外研究者对纳洛酮在神经内外科的应用进行了持续的研究。
继1981年Baskin首次报道纳洛酮静注治疗脑梗塞,能明显改善神经功能后,纳洛酮陆续成功应用于蛛网膜下腔出血、颅脑损伤等神经功能性损伤疾病的治疗。
在国内,自2001年中华医学会神经外科学会组织完成511例盐酸纳洛酮治疗急性颅脑损伤病人随机双盲多中心前瞻性临床研究后,纳洛酮的应用日益受到临床医生的重视,神经保护作用的临床疗效卓越。
作为最新一代的阿片受体拮抗剂——纳美芬,和纳洛酮具有相同的作用机制,有着比纳洛酮更优越的药理、药代动力学参数,在国外已经成功应用于脑梗塞、脑外伤的治疗。
本文谨就纳美芬在神经保护方面的特点和应用作简要概述。
一、神经系统损伤的生理病理机制
在人体中枢和外周系统,都存在着内啡肽、强啡肽、脑啡肽等内源性阿片类物质。
这类物质能与体内广泛存在的μ、κ和δ等阿片受体结合,对人体神经、内分泌、呼吸和心血管等生理功能起重要的调节作用。
神经系统损伤,是许多神经系统疾病共有的病理性过程。
外伤、卒中、中毒、感染、变性等疾病都会使神经系统受到致病因子的损害,导致不可逆性神经功能丧失。
中枢神经系统损伤后,兴奋性神经递质,如内源性阿片类物质、兴奋性氨基酸、单胺类神经递质等含量过度升高,且含量越高,损伤程度越严重。
这些神经递质的含量增高导致了相应受体(阿片受体、NMDA)过度兴奋,造成中枢神经系统的继发性损伤。
二、阿片受体拮抗剂的神经保护治疗作用
阿片受体拮抗剂是能够与阿片受体竞争性结合,且自身无激动作用的一类物质。
它通过竞争性阻断内阿片肽与阿片受体的结合,从而阻断病理反应的通路来起到神经保护作用。
作为临床广泛应用的阿片受体拮抗剂,纳洛酮在神经保护方面的剂量和疗效是呈正比关系的,κ和δ受体对颅脑损伤具有保护作用。
小剂量纳洛酮只能作用于μ受体,用于解除术后呼吸抑制、酒精中毒、抗休克的治疗;大剂量纳洛酮能同时作用于μ、κ和δ三种阿片受体,已经广泛应用于神经内外科。
纳美芬与三种受体的亲和力比纳洛酮更强,能同时作用于三种受体,起到神经保护治疗作用。
三、纳美芬的临床作用特点
纳美芬作为最新一代的阿片受体拮抗剂,能与μ、κ和δ受体结合。
纳美芬与中枢
和外周的阿片受体结合后,可阻断应激状态下内阿片肽导致的中枢神经和循环系统等的一系列症状,具有广泛的药理作用。
和纳洛酮比较,纳美芬具有更优越的药理、药代动力学参数。
1、速效
血脑屏障的存在使得神经系统疾病的治疗不同于其他疾病。
人体的血脑屏障较低等动物更完善,所以在细胞培养和动物模型中疗效显着的药物,在临床试验中往往达不到预期效果。
不论何种药物,血脑屏障通透性直接影响它在脑部作用的强弱。
纳美芬能够快速突破血脑屏障,在体内分布迅速。
给药后在2min起效,5min内达血药浓度高峰,可阻断80%以上的大脑阿片受体。
2、强效
在中枢神经系统中,纳美芬与μ受体的结合力与纳曲酮相当,是纳洛酮的4倍。
纳美芬是三种阿片受体拮抗剂中与中枢κ、δ受体结合力最强的,其中与κ受体的亲合力是纳洛酮的28倍,能够更强效的阻断内阿片肽的病理效应。
注:Mary Ellen采用同位素示踪法测定纳洛酮、纳曲酮和纳美芬与标记后的双氢吗啡、乙基氧代环唑星以及丙氨酸-亮氨酸-脑啡肽竞争性结合μ、κ和δ受体。
测定出相应的值(半抑制浓度),表中数据显示,纳美芬与三种受体的亲和力最高。
IC
50
3、长效
纳美芬静注后的半衰期为10.8±5.2小时,而纳洛酮在成人血清中半衰期约为1小时;纳美芬的作用时间比纳洛酮更长,约为纳洛酮的10倍。
不需要在多次给药后连续监测病人的状况,更方便临床用药,节约治疗费用,减轻病人的痛苦。
4、安全
纳美芬安全性基本与纳洛酮相同,为纯阿片受体拮抗剂,无激动活性,不产生耐受性、依赖性。
给予健康志愿者推荐剂量的15倍或15倍以上,纳美芬的耐受性都很好,没有出现严重的不良反应;亦未显示严重毒性。
四、用法用量推荐
盐酸纳美芬注射液一般为静注,也可肌注或皮下注射。
1、逆转术后呼吸抑制的推荐剂量:
初始剂量0.25ug/kg,2-5min后再增加剂量0.25ug/kg,呈现阿片逆转作用后立即停止给药,累计剂量可达1ug/kg。
2、用于抗昏迷休克,酒精、CO、有机磷农药、安定、阿片类药物等中毒的治疗:
初始剂量0.1mg-0.2mg,如有必要在2-5min后给予第2个相同剂量。
3、用于血管性及外伤性中枢神经系统损伤的治疗:
静脉注射或静脉滴注,成人每日0.2mg~1.0mg,分两次给药,将本品与适量氯化钠注射液稀释后使用。
本品给药应在急性损伤早期开始,连用2周。
五、总结
神经保护在神经系统疾病的治疗中具有十分重要的意义。
纳美芬能够竞争性阻断过量内源性阿片肽对神经功能的损害作用,减少自由基的产生、抑制小胶质细胞活化和炎症介质的释放,改善神经细胞的能量代谢,逆转钙离子内流、兴奋性氨基酸升高等对神经系统的损害作用,并可能增强内源性脑保护因子的活性而达到神经保护的作用。
综上所述,作为最新一代的阿片受体拮抗剂,纳美芬是美国医院药师联合会(ASHP)推荐的纳洛酮替代品,具有“速效、强效、长效、安全”的作用特点,将在神经内外科的神经保护治疗中发挥重要的作用。
