Erastin_DataSheet_MedChemExpress
Erastin_CAS号571203-78-6说明书_AbMole中国
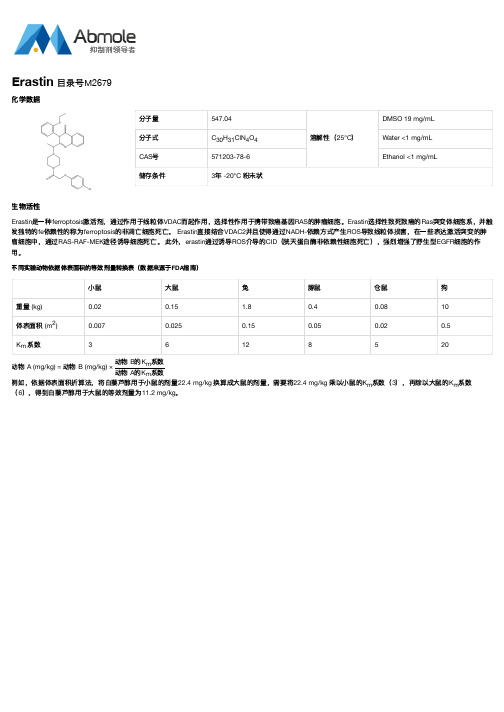
分子量547.04溶解性(25°C )DMSO 19 mg/mL 分子式C H ClN O Water <1 mg/mL CAS 号571203-78-6Ethanol <1 mg/mL储存条件3年 -20°C 粉末状生物活性Erastin 是一种ferroptosis 激活剂,通过作用于线粒体VDAC 而起作用,选择性作用于携带致癌基因RAS 的肿瘤细胞。
Erastin 选择性致死致癌的Ras 突变体细胞系,并触发独特的fe 依赖性的称为ferroptosis 的非凋亡细胞死亡。
Erastin 直接结合VDAC2并且使得通过NADH-依赖方式产生ROS 导致线粒体损害,在一些表达激活突变的肿瘤细胞中,通过RAS-RAF-MEK 途径诱导细胞死亡。
此外,erastin 通过诱导ROS 介导的CID (胱天蛋白酶非依赖性细胞死亡),强烈增强了野生型EGFR 细胞的作用。
不同实验动物依据体表面积的等效剂量转换表(数据来源于FDA 指南)小鼠大鼠兔豚鼠仓鼠狗重量 (kg)0.020.15 1.80.40.0810体表面积 (m )0.0070.0250.150.050.020.5K 系数36128520动物 A (mg/kg) = 动物 B (mg/kg) ×动物 B 的K 系数动物 A 的K 系数例如,依据体表面积折算法,将白藜芦醇用于小鼠的剂量22.4 mg/kg 换算成大鼠的剂量,需要将22.4 mg/kg 乘以小鼠的K 系数(3),再除以大鼠的K 系数(6),得到白藜芦醇用于大鼠的等效剂量为11.2 mg/kg 。
Erastin 目录号M2679化学数据3031442m m m m m。
Teriflunomide_DataSheet_MedChemExpress

Inhibitors, Agonists, Screening Libraries Data SheetBIOLOGICAL ACTIVITY:Teriflunomide is the active metabolite of leflunomide, which inhibits pyrimidine de novo synthesis by blocking the enzyme dihydroorotate dehydrogenase, used as an immunomodulatory agent.In Vitro: Teriflunomide primarily acts as an inhibitor of dihydroorotate dehydrogenase (DHODH), a key mitochondrial enzymeinvolved in the de novo synthesis of pyrimidines in rapidly proliferating cells. By reducing the activity of high–avidity proliferating T lymphocytes and B lymphocytes, teriflunomide likely attenuates the inflammatory response to autoantigens in MS. Thus,teriflunomide can be considered a cytostatic rather than a cytotoxic drug to leukocytes [1].In Vivo: Teriflunomide has demonstrated beneficial effects in two independent animal models of demyelinating disease. In the dark agouti rat model of experimental autoimmune encephalitis (EAE), teriflunomide administration results in clinical, histopathological,and electrophysiological evidence of efficacy both as a prophylactic and therapeutic agent. Similarly, in the female Lewis rat model of EAE, teriflunomide administration results in beneficial prophylactic and therapeutic clinical effects, with a delay in disease onset and symptom severity [1].References:[1]. Oh J, et al. An update of teriflunomide for treatment of multiple sclerosis. Ther Clin Risk Manag. 2013;9:177–90.Product Name:Teriflunomide Cat. No.:HY-15405CAS No.:163451-81-8Molecular Formula:C 12H 9F 3N 2O 2Molecular Weight:270.21Target:Others Pathway:Others Solubility:DMSO: 26 mg/mLCaution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@ Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
细胞死亡的类型

形态学:线粒体小且膜密度高,没有DNA断裂,脂质发生过氧化 激活剂: Erastin 抑制剂:ferrostatin-1 铁死亡无法被坏死抑制剂、 Caspase抑制剂等阻断 由于脂质发生过氧化,因此 可被C11-BODIPY(581/591)识别
Erastin诱导铁死亡,线粒体变小,膜密度增高
细胞坏死的常用检测方法 1、形态学(细胞肿大) 2、DNA检测分析(DNA Ladder实验呈弥散状) 3、流式细胞技术(Annexin V和PI双标记)
由基因调控的自杀程序性主动死亡
特点是核固缩、DNA降解、不引起炎症、质膜不破裂、产 生凋亡小体等
常用检测方法
1、形态学(核固缩等) 2、DNA检测分析(DNA ladder法或TUNEL法) 3、凋亡相关基因检测(Caspase剪切等)
Dale E.B. et al., Nature,2006. Cell death in the nervous system
经典的细胞死亡类型:
坏死 凋亡 自噬性死亡
其他死亡类型
Immunogenic Cell Death Ferroptosis等
极端的物理、化学性损害因子或严重的病理性刺激(如 缺氧、营养不良等)导致的细胞被动死亡,是非正常死 亡。
细胞器膨胀变形
染色质随机降解
细胞膜发生渗漏
导致细胞内容物释放到胞外,从而引起炎症反应
程,因此一般发生时间较晚。
最早来自化疗药物促使肿瘤细胞死 亡后,激活免疫反应,导致肿瘤二 次死亡。 坏死的神经元释放内容物,引起炎 症反应,激活小胶质细胞等。 小胶质细胞过度激活释放的炎症因 子等将造成神经元的继发性死亡。 能检测到免疫性细胞坏死相关信号, 如HMGB1、RAGE和DAMP 等升高
铁死亡激活剂Erastin的合成

第3 期
赵艳利等:铁死亡激活剂 Erastin 的合成
— 223 —
Scheme 1
代产物ꎬ所得产物不易纯化ꎻ3 倍量哌嗪与中间体 5 反应除生成中间体 6 外还易产生哌嗪双取代产 物(45% ) ꎻ制备终产物 Erastin 时ꎬ溶剂选择了毒 性较大 1ꎬ2 ̄二氯乙烷为反应溶剂ꎬ并未提供终产 物 Erastin 的纯化方法ꎬ粗品总收率仅为 8. 0% ꎮ
2019 年第 27 卷 第 3 期ꎬ 222 ~ 224
������制药技术������
ห้องสมุดไป่ตู้合成化学 Chinese Journal of Synthetic Chemistry
铁死亡激活剂 Erastin 的合成
Vol. 27ꎬ 2019 No. 3ꎬ 222 ~ 224
为了 获 得 适 合 实 验 室 研 究 的 小 规 模 制 备 Erastin 的合成方法ꎬ本文参考文献[12 - 15] 方法ꎬ建 立了一种简单有效、反应条件更加温和的 Erastin 的合成方法ꎮ 以 2 为起始原料通过 5 步反应合成 Erastin ( Scheme 1 ) ꎬ 其 结 构 经1 H NMR 和 MS ( ESI) 确证ꎬ产率 31. 4% ꎬ纯度 98. 33% ꎮ
Abstract: The preparation process of the ferroptosis ̄induced cell death activator Erastin was improved. Erastin was synthesized through five steps using 2 ̄aminobenzoic acid as starting material. The total yield was 31. 4% and the purity was 98. 33% . The structure was confirmed by 1H NMR and MS(ESI). Keywords: ferroptosisꎻ Erastinꎻ anti ̄tumor drugꎻ drug synthesisꎻ process optimization
Thiamet_G_LCMS_16482_MedChemExpress
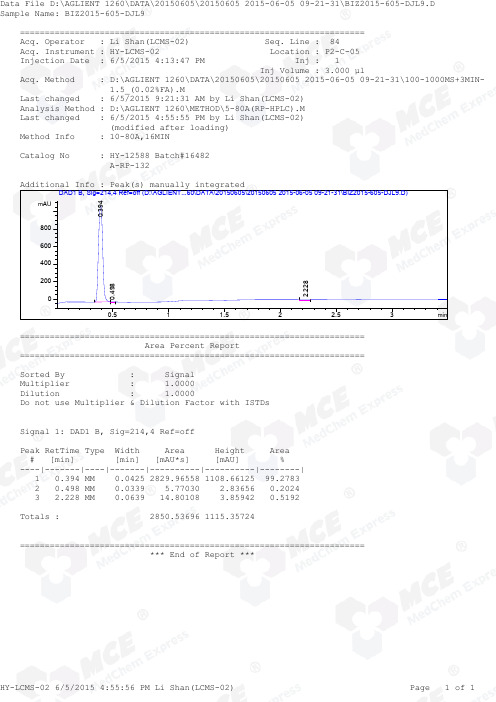
=====================================================================Acq. Operator : Li Shan(LCMS-02) Seq. Line : 84Acq. Instrument : HY-LCMS-02 Location : P2-C-05Injection Date : 6/5/2015 4:13:47 PM Inj : 1 Inj Volume : 3.000 µl Acq. Method : D:\AGLIENT 1260\DATA\20150605\20150605 2015-06-05 09-21-31\100-1000MS+3MIN- 1.5_(0.02%FA).M Last changed : 6/5/2015 9:21:31 AM by Li Shan(LCMS-02)Analysis Method : D:\AGLIENT 1260\METHOD\5-80A(RP-HPLC).M Last changed : 6/5/2015 4:55:55 PM by Li Shan(LCMS-02) (modified after loading)Method Info : 10-80A,16MIN Catalog No : HY-12588 Batch#16482 A-RP-132 Additional Info : Peak(s) manually integrated min0.51 1.52 2.53mAU200400600800DAD1 B, Sig=214,4 Ref=off (D:\AGLIENT...60\DATA\20150605\20150605 2015-06-05 09-21-31\BIZ2015-605-DJL9.D)0.394 0.498 2.228 ===================================================================== Area Percent Report ===================================================================== Sorted By : Signal Multiplier : 1.0000Dilution : 1.0000Do not use Multiplier & Dilution Factor with ISTDs Signal 1: DAD1 B, Sig=214,4 Ref=off Peak RetTime Type Width Area Height Area # [min] [min] [mAU*s] [mAU] %----|-------|----|-------|----------|----------|--------| 1 0.394 MM 0.0425 2829.96558 1108.66125 99.2783 2 0.498 MM 0.0339 5.77030 2.83656 0.2024 3 2.228 MM 0.0639 14.80108 3.85942 0.5192 Totals : 2850.53696 1115.35724 ===================================================================== *** End of Report ***=====================================================================Acq. Operator : Li Shan(LCMS-02) Seq. Line : 84Acq. Instrument : HY-LCMS-02 Location : P2-C-05Injection Date : 6/5/2015 4:13:47 PM Inj : 1 Inj Volume : 3.000 µl Acq. Method : D:\AGLIENT 1260\DATA\20150605\20150605 2015-06-05 09-21-31\100-1000MS+3MIN- 1.5_(0.02%FA).M Last changed : 6/5/2015 9:21:31 AM by Li Shan(LCMS-02)Analysis Method : D:\AGLIENT 1260\METHOD\5-80A(RP-HPLC).M Last changed : 6/5/2015 4:56:55 PM by Li Shan(LCMS-02) (modified after loading)Method Info : 10-80A,16MIN Catalog No : HY-12588 Batch#16482 A-RP-132 Additional Info : Peak(s) manually integrated min0.51 1.52 2.530100000200000300000400000500000MSD1 TIC, MS File (D:\AGLIENT 1260\DATA\20150605\20150605 2015-06-05 09-21-31\BIZ2015-605-DJL9.D) ES-API, Pos, Sca0.392MS Signal: MSD1 TIC, MS File, ES-API, Pos, Scan, Frag: 50 Spectra averaged over upper half of peaks. Noise Cutoff: 1000 counts. Reportable Ion Abundance: > 10%. Retention Mol. Weight Time (MS) MS Area or Ion 0.392 2110102 250.10 I 249.10 Im/z 100200300400500600020406080100*MSD1 SPC, time=0.377:0.413 of D:\AGLIENT 1260\DATA\20150605\20150605 2015-06-05 09-21-31\BIZ2015-605-DJL9.D ES-API,Max: 377429251.1 249.1 *** End of Report ***。
PF-562271-besylate-SDS-MedChemExpress

Inhibitors, Agonists, Screening LibrariesSafety Data Sheet Revision Date:Oct.-06-2018Print Date:Oct.-06-20181. PRODUCT AND COMPANY IDENTIFICATION1.1 Product identifierProduct name :PF-562271 (besylate)Catalog No. :HY-10458CAS No. :939791-38-51.2 Relevant identified uses of the substance or mixture and uses advised againstIdentified uses :Laboratory chemicals, manufacture of substances.1.3 Details of the supplier of the safety data sheetCompany:MedChemExpress USATel:609-228-6898Fax:609-228-5909E-mail:sales@1.4 Emergency telephone numberEmergency Phone #:609-228-68982. HAZARDS IDENTIFICATION2.1 Classification of the substance or mixtureNot a hazardous substance or mixture.2.2 GHS Label elements, including precautionary statementsNot a hazardous substance or mixture.2.3 Other hazardsNone.3. COMPOSITION/INFORMATION ON INGREDIENTS3.1 SubstancesSynonyms:PF562271 besylate;PF 562271 besylateFormula:C27H26F3N7O6S2Molecular Weight:665.66CAS No. :939791-38-54. FIRST AID MEASURES4.1 Description of first aid measuresEye contactRemove any contact lenses, locate eye-wash station, and flush eyes immediately with large amounts of water. Separate eyelids with fingers to ensure adequate flushing. Promptly call a physician.Skin contactRinse skin thoroughly with large amounts of water. Remove contaminated clothing and shoes and call a physician.InhalationImmediately relocate self or casualty to fresh air. If breathing is difficult, give cardiopulmonary resuscitation (CPR). Avoid mouth-to-mouth resuscitation.IngestionWash out mouth with water; Do NOT induce vomiting; call a physician.4.2 Most important symptoms and effects, both acute and delayedThe most important known symptoms and effects are described in the labelling (see section 2.2).4.3 Indication of any immediate medical attention and special treatment neededTreat symptomatically.5. FIRE FIGHTING MEASURES5.1 Extinguishing mediaSuitable extinguishing mediaUse water spray, dry chemical, foam, and carbon dioxide fire extinguisher.5.2 Special hazards arising from the substance or mixtureDuring combustion, may emit irritant fumes.5.3 Advice for firefightersWear self-contained breathing apparatus and protective clothing.6. ACCIDENTAL RELEASE MEASURES6.1 Personal precautions, protective equipment and emergency proceduresUse full personal protective equipment. Avoid breathing vapors, mist, dust or gas. Ensure adequate ventilation. Evacuate personnel to safe areas.Refer to protective measures listed in sections 8.6.2 Environmental precautionsTry to prevent further leakage or spillage. Keep the product away from drains or water courses.6.3 Methods and materials for containment and cleaning upAbsorb solutions with finely-powdered liquid-binding material (diatomite, universal binders); Decontaminate surfaces and equipment by scrubbing with alcohol; Dispose of contaminated material according to Section 13.7. HANDLING AND STORAGE7.1 Precautions for safe handlingAvoid inhalation, contact with eyes and skin. Avoid dust and aerosol formation. Use only in areas with appropriate exhaust ventilation.7.2 Conditions for safe storage, including any incompatibilitiesKeep container tightly sealed in cool, well-ventilated area. Keep away from direct sunlight and sources of ignition.Recommended storage temperature:Powder-20°C 3 years4°C 2 yearsIn solvent-80°C 6 months-20°C 1 monthShipping at room temperature if less than 2 weeks.7.3 Specific end use(s)No data available.8. EXPOSURE CONTROLS/PERSONAL PROTECTION8.1 Control parametersComponents with workplace control parametersThis product contains no substances with occupational exposure limit values.8.2 Exposure controlsEngineering controlsEnsure adequate ventilation. Provide accessible safety shower and eye wash station.Personal protective equipmentEye protection Safety goggles with side-shields.Hand protection Protective gloves.Skin and body protection Impervious clothing.Respiratory protection Suitable respirator.Environmental exposure controls Keep the product away from drains, water courses or the soil. Cleanspillages in a safe way as soon as possible.9. PHYSICAL AND CHEMICAL PROPERTIES9.1 Information on basic physical and chemical propertiesAppearance White to off-white (Solid)Odor No data availableOdor threshold No data availablepH No data availableMelting/freezing point No data availableBoiling point/range No data availableFlash point No data availableEvaporation rate No data availableFlammability (solid, gas)No data availableUpper/lower flammability or explosive limits No data availableVapor pressure No data availableVapor density No data availableRelative density No data availableWater Solubility No data availablePartition coefficient No data availableAuto-ignition temperature No data availableDecomposition temperature No data availableViscosity No data availableExplosive properties No data availableOxidizing properties No data available9.2 Other safety informationNo data available.10. STABILITY AND REACTIVITY10.1 ReactivityNo data available.10.2 Chemical stabilityStable under recommended storage conditions.10.3 Possibility of hazardous reactionsNo data available.10.4 Conditions to avoidNo data available.10.5 Incompatible materialsStrong acids/alkalis, strong oxidising/reducing agents.10.6 Hazardous decomposition productsUnder fire conditions, may decompose and emit toxic fumes.Other decomposition products - no data available.11.TOXICOLOGICAL INFORMATION11.1 Information on toxicological effectsAcute toxicityClassified based on available data. For more details, see section 2Skin corrosion/irritationClassified based on available data. For more details, see section 2Serious eye damage/irritationClassified based on available data. For more details, see section 2Respiratory or skin sensitizationClassified based on available data. For more details, see section 2Germ cell mutagenicityClassified based on available data. For more details, see section 2CarcinogenicityIARC: No component of this product present at a level equal to or greater than 0.1% is identified as probable, possible or confirmed human carcinogen by IARC.ACGIH: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by ACGIH.NTP: No component of this product present at a level equal to or greater than 0.1% is identified as a anticipated or confirmed carcinogen by NTP.OSHA: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by OSHA.Reproductive toxicityClassified based on available data. For more details, see section 2Specific target organ toxicity - single exposureClassified based on available data. For more details, see section 2Specific target organ toxicity - repeated exposureClassified based on available data. For more details, see section 2Aspiration hazardClassified based on available data. For more details, see section 212. ECOLOGICAL INFORMATION12.1 ToxicityNo data available.12.2 Persistence and degradabilityNo data available.12.3 Bioaccumlative potentialNo data available.12.4 Mobility in soilNo data available.12.5 Results of PBT and vPvB assessmentPBT/vPvB assessment unavailable as chemical safety assessment not required or not conducted.12.6 Other adverse effectsNo data available.13. DISPOSAL CONSIDERATIONS13.1 Waste treatment methodsProductDispose substance in accordance with prevailing country, federal, state and local regulations.Contaminated packagingConduct recycling or disposal in accordance with prevailing country, federal, state and local regulations.14. TRANSPORT INFORMATIONDOT (US)This substance is considered to be non-hazardous for transport.IMDGThis substance is considered to be non-hazardous for transport.IATAThis substance is considered to be non-hazardous for transport.15. REGULATORY INFORMATIONSARA 302 Components:No chemicals in this material are subject to the reporting requirements of SARA Title III, Section 302.SARA 313 Components:This material does not contain any chemical components with known CAS numbers that exceed the threshold (De Minimis) reporting levels established by SARA Title III, Section 313.SARA 311/312 Hazards:No SARA Hazards.Massachusetts Right To Know Components:No components are subject to the Massachusetts Right to Know Act.Pennsylvania Right To Know Components:No components are subject to the Pennsylvania Right to Know Act.New Jersey Right To Know Components:No components are subject to the New Jersey Right to Know Act.California Prop. 65 Components:This product does not contain any chemicals known to State of California to cause cancer, birth defects, or anyother reproductive harm.16. OTHER INFORMATIONCopyright 2018 MedChemExpress. The above information is correct to the best of our present knowledge but does not purport to be all inclusive and should be used only as a guide. The product is for research use only and for experienced personnel. It must only be handled by suitably qualified experienced scientists in appropriately equipped and authorized facilities. The burden of safe use of this material rests entirely with the user. MedChemExpress disclaims all liability for any damage resulting from handling or from contact with this product.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
Erastin在铁死亡中的作用

Erastin在铁死亡中的作用铁死亡是近几年新发现的一种细胞死亡方式,以铁依赖性脂质过氧化物累积为主要特征[1]。
铁死亡诱导剂主要有两大类:第一类包括Erastin,柳氮磺胺毗口定、谷氨酸盐等,可通过systemXC■发挥作用;第二类包括RS13、DP17等,可直接抑制谷胱甘肽过氧化物酶(GPX)活性起作用[2]。
其中,Erastin与其他铁死亡诱导剂通常介导单一通路不同,它可以介导多种分子,而且作用效果高效、迅速、持久[3]。
本文就Erastin的作用通路及抗肿瘤的特点进行综述。
1 Erastin的发现Do1ma等[4]在2003年发现一种小分子化合物,能够选择性杀死表达ST和RAS的工程肿瘤细胞,将其命名为Erastin o然而,与喜树碱可诱导表达ST和RAS的细胞凋亡不同,被EraStin 诱导死亡的细胞中,未发现线粒体细胞色素c的释放、Caspase-3的激活、DNA片段化等凋亡经典特征,并且这种死亡方式不能被凋亡抑制剂抑制[4,5,6]。
因此,EraStin所诱导的是一种新型的、非凋亡的细胞死亡形式⑼。
根据其特点,Dixon等[1]在2012年将Erastin诱导的细胞死亡方式命名为铁死亡。
2铁死亡的特征及相关通路1.1 铁死亡的概念及特征铁死亡是一种表现为铁依赖性的、细胞内脂质活性氧(1-ROS)累积的细胞死亡形式。
铁死亡与其他细胞死亡方式(如细胞凋亡、坏死、自噬等)在诸多方面均存在显著差异。
在形态学方面,铁死亡细胞表现为特有的线粒体皱缩和线粒体膜密度增加,而其他形式细胞死亡的典型特征在铁死亡中均不会出现[1,7]。
在生化代谢方面,铁死亡细胞内氧化还原稳态被打破,抗氧化能力降低,细胞内活性氧升高。
同时,这种死亡过程可以被抗氧化剂和铁螯合剂所抑制[1]。
尽管诱导铁死亡的上游通路有多个,最终都会直接或间接导致细胞内1-ROS生成与降解平衡失调,1-ROS增多,发生铁死亡[1,8]。
1.2 通过抑制胱氨酸-谷氨酸转运受体systemXC■诱导铁死亡SyStemXe-是一种反向转运蛋白,由S1C7A11和S1C3A2亚基组成,可将谷氨酸转出细胞的同时将胱氨酸转入细胞[9]。
AZD3839-free-base-SDS-MedChemExpress

Inhibitors, Agonists, Screening LibrariesSafety Data Sheet Revision Date:Oct.-02-2018Print Date:Oct.-02-20181. PRODUCT AND COMPANY IDENTIFICATION1.1 Product identifierProduct name :AZD3839 (free base)Catalog No. :HY-13438CAS No. :1227163-84-91.2 Relevant identified uses of the substance or mixture and uses advised againstIdentified uses :Laboratory chemicals, manufacture of substances.1.3 Details of the supplier of the safety data sheetCompany:MedChemExpress USATel:609-228-6898Fax:609-228-5909E-mail:sales@1.4 Emergency telephone numberEmergency Phone #:609-228-68982. HAZARDS IDENTIFICATION2.1 Classification of the substance or mixtureGHS Classification in accordance with 29 CFR 1910 (OSHA HCS)Acute toxicity, Oral (Category 4),H302Acute aquatic toxicity (Category 1),H400Chronic aquatic toxicity (Category 1),H4102.2 GHS Label elements, including precautionary statementsPictogramSignal word WarningHazard statement(s)H302 Harmful if swallowed.H410 Very toxic to aquatic life with long lasting effects.Precautionary statement(s)P264 Wash skin thoroughly after handling.P270 Do not eat, drink or smoke when using this product.P273 Avoid release to the environment.P301 + P312 IF SWALLOWED: Call a POISON CENTER or doctor ⁄ physician if you feel unwell.P330 Rinse mouth.P391 Collect spillage.P501 Dispose of contents ⁄ container to an approved waste disposal plant.2.3 Other hazardsNone.3. COMPOSITION/INFORMATION ON INGREDIENTS3.1 SubstancesSynonyms:AZD-3839 free base;AZD 3839 free baseFormula:C24H16F3N5Molecular Weight:431.41CAS No. :1227163-84-94. FIRST AID MEASURES4.1 Description of first aid measuresEye contactRemove any contact lenses, locate eye-wash station, and flush eyes immediately with large amounts of water. Separate eyelids with fingers to ensure adequate flushing. Promptly call a physician.Skin contactRinse skin thoroughly with large amounts of water. Remove contaminated clothing and shoes and call a physician.InhalationImmediately relocate self or casualty to fresh air. If breathing is difficult, give cardiopulmonary resuscitation (CPR). Avoid mouth-to-mouth resuscitation.IngestionWash out mouth with water; Do NOT induce vomiting; call a physician.4.2 Most important symptoms and effects, both acute and delayedThe most important known symptoms and effects are described in the labelling (see section 2.2).4.3 Indication of any immediate medical attention and special treatment neededTreat symptomatically.5. FIRE FIGHTING MEASURES5.1 Extinguishing mediaSuitable extinguishing mediaUse water spray, dry chemical, foam, and carbon dioxide fire extinguisher.5.2 Special hazards arising from the substance or mixtureDuring combustion, may emit irritant fumes.5.3 Advice for firefightersWear self-contained breathing apparatus and protective clothing.6. ACCIDENTAL RELEASE MEASURES6.1 Personal precautions, protective equipment and emergency proceduresUse full personal protective equipment. Avoid breathing vapors, mist, dust or gas. Ensure adequate ventilation. Evacuate personnel to safe areas.Refer to protective measures listed in sections 8.6.2 Environmental precautionsTry to prevent further leakage or spillage. Keep the product away from drains or water courses.6.3 Methods and materials for containment and cleaning upAbsorb solutions with finely-powdered liquid-binding material (diatomite, universal binders); Decontaminate surfaces and equipment by scrubbing with alcohol; Dispose of contaminated material according to Section 13.7. HANDLING AND STORAGE7.1 Precautions for safe handlingAvoid inhalation, contact with eyes and skin. Avoid dust and aerosol formation. Use only in areas with appropriate exhaust ventilation.7.2 Conditions for safe storage, including any incompatibilitiesKeep container tightly sealed in cool, well-ventilated area. Keep away from direct sunlight and sources of ignition.Recommended storage temperature:Powder-20°C 3 years4°C 2 yearsIn solvent-80°C 6 months-20°C 1 monthShipping at room temperature if less than 2 weeks.7.3 Specific end use(s)No data available.8. EXPOSURE CONTROLS/PERSONAL PROTECTION8.1 Control parametersComponents with workplace control parametersThis product contains no substances with occupational exposure limit values.8.2 Exposure controlsEngineering controlsEnsure adequate ventilation. Provide accessible safety shower and eye wash station.Personal protective equipmentEye protection Safety goggles with side-shields.Hand protection Protective gloves.Skin and body protection Impervious clothing.Respiratory protection Suitable respirator.Environmental exposure controls Keep the product away from drains, water courses or the soil. Cleanspillages in a safe way as soon as possible.9. PHYSICAL AND CHEMICAL PROPERTIES9.1 Information on basic physical and chemical propertiesAppearance White to off-white (Solid)Odor No data availableOdor threshold No data availablepH No data availableMelting/freezing point No data availableBoiling point/range No data availableFlash point No data availableEvaporation rate No data availableFlammability (solid, gas)No data availableUpper/lower flammability or explosive limits No data availableVapor pressure No data availableVapor density No data availableRelative density No data availableWater Solubility No data availablePartition coefficient No data availableAuto-ignition temperature No data availableDecomposition temperature No data availableViscosity No data availableExplosive properties No data availableOxidizing properties No data available9.2 Other safety informationNo data available.10. STABILITY AND REACTIVITY10.1 ReactivityNo data available.10.2 Chemical stabilityStable under recommended storage conditions.10.3 Possibility of hazardous reactionsNo data available.10.4 Conditions to avoidNo data available.10.5 Incompatible materialsStrong acids/alkalis, strong oxidising/reducing agents.10.6 Hazardous decomposition productsUnder fire conditions, may decompose and emit toxic fumes.Other decomposition products - no data available.11.TOXICOLOGICAL INFORMATION11.1 Information on toxicological effectsAcute toxicityClassified based on available data. For more details, see section 2Skin corrosion/irritationClassified based on available data. For more details, see section 2Serious eye damage/irritationClassified based on available data. For more details, see section 2Respiratory or skin sensitizationClassified based on available data. For more details, see section 2Germ cell mutagenicityClassified based on available data. For more details, see section 2CarcinogenicityIARC: No component of this product present at a level equal to or greater than 0.1% is identified as probable, possible or confirmed human carcinogen by IARC.ACGIH: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by ACGIH.NTP: No component of this product present at a level equal to or greater than 0.1% is identified as a anticipated or confirmed carcinogen by NTP.OSHA: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by OSHA.Reproductive toxicityClassified based on available data. For more details, see section 2Specific target organ toxicity - single exposureClassified based on available data. For more details, see section 2Specific target organ toxicity - repeated exposureClassified based on available data. For more details, see section 2Aspiration hazardClassified based on available data. For more details, see section 212. ECOLOGICAL INFORMATION12.1 ToxicityNo data available.12.2 Persistence and degradabilityNo data available.12.3 Bioaccumlative potentialNo data available.12.4 Mobility in soilNo data available.12.5 Results of PBT and vPvB assessmentPBT/vPvB assessment unavailable as chemical safety assessment not required or not conducted.12.6 Other adverse effectsNo data available.13. DISPOSAL CONSIDERATIONS13.1 Waste treatment methodsProductDispose substance in accordance with prevailing country, federal, state and local regulations.Contaminated packagingConduct recycling or disposal in accordance with prevailing country, federal, state and local regulations.14. TRANSPORT INFORMATIONDOT (US)This substance is considered to be non-hazardous for transport.IMDGUN number: 3077Class: 9Packing group: IIIEMS-No: F-A, S-FProper shipping name: ENVIRONMENTALLY HAZARDOUS SUBSTANCE, SOLID, N.O.S.Marine pollutant: Marine pollutant.IATAUN number: 3077Class: 9Packing group: IIIProper shipping name: Environmentally hazardous substance, solid, n.o.s.15. REGULATORY INFORMATIONSARA 302 Components:No chemicals in this material are subject to the reporting requirements of SARA Title III, Section 302.SARA 313 Components:This material does not contain any chemical components with known CAS numbers that exceed the threshold (De Minimis) reporting levels established by SARA Title III, Section 313.SARA 311/312 Hazards:No SARA Hazards.Massachusetts Right To Know Components:No components are subject to the Massachusetts Right to Know Act.Pennsylvania Right To Know Components:No components are subject to the Pennsylvania Right to Know Act.New Jersey Right To Know Components:No components are subject to the New Jersey Right to Know Act.California Prop. 65 Components:This product does not contain any chemicals known to State of California to cause cancer, birth defects, or anyother reproductive harm.16. OTHER INFORMATIONCopyright 2018 MedChemExpress. The above information is correct to the best of our present knowledge but does not purport to be all inclusive and should be used only as a guide. The product is for research use only and for experienced personnel. It must only be handled by suitably qualified experienced scientists in appropriately equipped and authorized facilities. The burden of safe use of this material rests entirely with the user. MedChemExpress disclaims all liability for any damage resulting from handling or from contact with this product.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
erastin色谱条件

erastin色谱条件
erastin是一种用于分离和纯化蛋白质和多肽的色谱柱。
其色谱条件通常包括以下几个方面:
1. 流动相:通常使用0.05-0.2M的醋酸铵或0.05-0.2M的氯化钠溶液作为流动相,以保持蛋白质和多肽的稳定性和溶解度。
2. 流速:流速的选择取决于色谱柱的规格和填料类型,通常在0.5-5mL/min的范围内。
3. 温度:温度对色谱分离效果有一定影响,通常选择室温进行操作,但可以根据需要调节温度。
4. 洗脱方式:可以使用连续洗脱或梯度洗脱的方式进行分离,根据具体情况选择适合的洗脱方式。
5. 检测器:可以使用紫外可见吸收光谱、电导或质谱等检测器进行检测,根据具体需求选择适合的检测器。
在实际操作中,可以根据具体的分离需求和色谱柱的规格进行条件优化,以达到最佳的分离效果。
Exendin-4-Acetate-DataSheet-MedChemExpress

Exendin-4 Acetatepancreatic acinar inflammation, more pyknotic nuclei and weigh significantly less than control rats. Exendin-4 treatment is associated with lower insulin and leptin levels as well as lower HOMA values in rats [5]. Exenatide causes dose-dependent relaxation of rat thoracic aorta, which is evoked via the GLP-1 receptor and is mediated mainly by H 2S but also by NO and CO [6].PROTOCOLAnimalAdministration [4][5]Rats: 20 Sprague-Dawley male rats, ten of which are treated with exendin-4 (10 μg/kg) and ten of which are used as controls. The study period is 75 days. Serum and pancreatic tissue are removed for biochemical and histological study. Blood glucose, amylase, lipase, insulin and adipocytokines are compared between the two groups [5].Mice: The exendin-4 treatment groups are treated with 10 μg/kg every 24 hours for the first 14 days. This treatment is the induction phase. Respective control mice (lean and ob/ob) receive saline every 24 hours. After 14 days Exendin-4-treated mice are randomly divided into two groups: one group receives high dose exendin-4 (20 μg/kg) every 12 hours, while the second group continues with low dose exendin-4 (10 μg/kg) every 12 hours. The control mice continue to receive saline every 12 hours. The mice are weighed daily for the 60-day treatment period [4].MCE has not independently confirmed the accuracy of these methods. They are for reference only.CUSTOMER VALIDATIONSee more customer validations on REFERENCES[1]. Doyle ME, et al. The importance of the nine-amino acid C-terminal sequence of exendin-4 for binding to the GLP-1 receptor and for biological activity. Regul Pept. 2003 Jul 15;114(2-3):153-8.[2]. Wei R, et al. Exenatide exerts direct protective effects on endothelial cells through the AMPK/Akt/eNOS pathway in a GLP-1 receptor-dependent manner. Am J Physiol Endocrinol Metab. 2016 Jun 1;310(11):E947-57.[3]. Fidan-YaylalI G, et al. Antidiabetic exendin-4 activates apoptotic pathway and inhibits growth of breast cancer cells. Tumour Biol. 2016 Feb;37(2):2647-53.[4]. Ding X, et al. Exendin-4, a glucagon-like protein-1 (GLP-1) receptor agonist, reverses hepatic steatosis in ob/obmice. Hepatology. 2006 Jan;43(1):173-81.[5]. Nachnani JS, et al. Biochemical and histological effects of exendin-4 (exenatide) on the rat pancreas. Diabetologia. 2010 Jan;53(1):153-9.[6]. Sélley E, et al. Exenatide induces aortic vasodilation increasing hydrogen sulphide, carbon monoxide and nitric oxide production. Cardiovasc Diabetol. 2014 Apr 2;13:69.• Sci Rep . 2017 Jun 28;7(1):4351.• Acta Biochim Biophys Sin (Shanghai). 2017 May 5:1-8.Caution: Product has not been fully validated for medical applications. For research use only. Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@ Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
Erastin_LCMS_20203_MedChemExpress
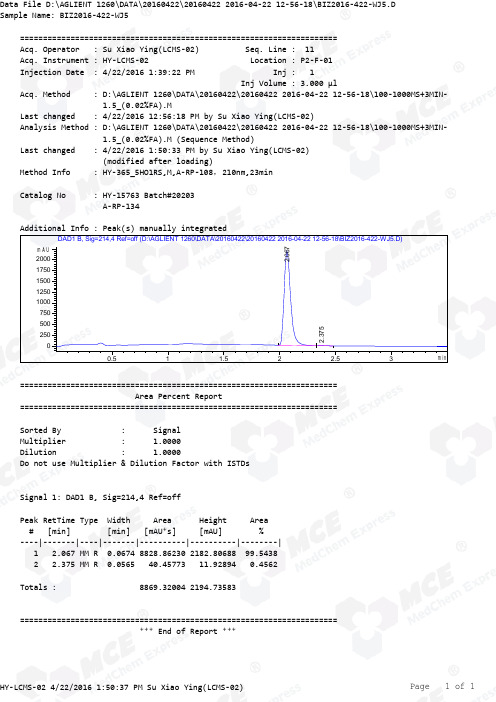
=====================================================================Acq. Operator : Su Xiao Ying(LCMS-02) Seq. Line : 11Acq. Instrument : HY-LCMS-02 Location : P2-F-01Injection Date : 4/22/2016 1:39:22 PM Inj : 1Inj Volume : 3.000 µlAcq. Method : D:\AGLIENT 1260\DATA\20160422\20160422 2016-04-22 12-56-18\100-1000MS+3MIN- 1.5_(0.02%FA).MLast changed : 4/22/2016 12:56:18 PM by Su Xiao Ying(LCMS-02)Analysis Method : D:\AGLIENT 1260\DATA\20160422\20160422 2016-04-22 12-56-18\100-1000MS+3MIN- 1.5_(0.02%FA).M (Sequence Method)Last changed : 4/22/2016 1:50:33 PM by Su Xiao Ying(LCMS-02) (modified after loading) M ethod Info : HY-365_5HO1RS,M,A-RP-108,210nm,23minCatalog No : HY-15763 Batch#20203 A-RP-134Additional Info : Peak(s) manually integratedmin0.511.522.53mAU 025050075010001250150017502000 DAD1 B, Sig=214,4 Ref=off (D:\AGLIENT 1260\DATA\20160422\20160422 2016-04-22 12-56-18\BIZ2016-422-WJ5.D)2.0672.375===================================================================== Area Percent Report =====================================================================Sorted By : Signal Multiplier : 1.0000Dilution : 1.0000Do not use Multiplier & Dilution Factor with ISTDsSignal 1: DAD1 B, Sig=214,4 Ref=offPeak RetTime Type Width Area Height Area # [min] [min] [mAU*s] [mAU] %----|-------|----|-------|----------|----------|--------| 1 2.067 MM R 0.0674 8828.86230 2182.80688 99.5438 2 2.375 MM R 0.0565 40.45773 11.92894 0.4562Totals : 8869.32004 2194.73583===================================================================== *** End of Report ***=====================================================================Acq. Operator : Su Xiao Ying(LCMS-02) Seq. Line : 11Acq. Instrument : HY-LCMS-02 Location : P2-F-01Injection Date : 4/22/2016 1:39:22 PM Inj : 1Inj Volume : 3.000 µlMethod : D:\AGLIENT 1260\DATA\20160422\20160422 2016-04-22 12-56-18\100-1000MS+3MIN- 1.5_(0.02%FA).M (Sequence Method)Last changed : 4/22/2016 12:56:18 PM by Su Xiao Ying(LCMS-02) M ethod Info : HY-365_5HO1RS,M,A-RP-108,210nm,23minCatalog No : HY-15763 Batch#20203 A-RP-134Additional Info : Peak(s) manually integratedmin0.511.522.53100000200000300000400000500000600000 MSD1 TIC, MS File (D:\AGLIENT 1260\DATA\20160422\20160422 2016-04-22 12-56-18\BIZ2016-422-WJ5.D) ES-API, Pos, Scan,2.076MS Signal: MSD1 TIC, MS File, ES-API, Pos, Scan, Frag: 50 Spectra averaged over upper half of peaks. Noise Cutoff: 1000 counts.Reportable Ion Abundance: > 10%.Retention Mol. Weight Time (MS) MS Area or Ion2.076 5245624 550.30 I 549.25 I 548.30 I 547.30 Im/z10020030040050060020406080100*MSD1 SPC, time=2.035:2.145 of D:\AGLIENT 1260\DATA\20160422\20160422 2016-04-22 12-56-18\BIZ2016-422-WJ5.D ES-API, Max: 263214550.3548.3 547.3*** End of Report ***。
Ebastine_SDS_MedChemExpress

Inhibitors, Agonists, Screening LibrariesSafety Data Sheet Revision Date:Mar.-30-2017Print Date:Mar.-30-20171. PRODUCT AND COMPANY IDENTIFICATION1.1 Product identifierProduct name :EbastineCatalog No. :HY-B0674CAS No. :90729-43-41.2 Relevant identified uses of the substance or mixture and uses advised againstIdentified uses :Laboratory chemicals, manufacture of substances.1.3 Details of the supplier of the safety data sheetCompany:MedChemExpress USATel:609-228-6898Fax:609-228-5909E-mail:sales@1.4 Emergency telephone numberEmergency Phone #:609-228-68982. HAZARDS IDENTIFICATION2.1 Classification of the substance or mixtureGHS Classification in accordance with 29 CFR 1910 (OSHA HCS)Acute toxicity, Oral (Category 4),H302Acute aquatic toxicity (Category 1),H400Chronic aquatic toxicity (Category 1),H4102.2 GHS Label elements, including precautionary statementsPictogramSignal word WarningHazard statement(s)H302 Harmful if swallowed.H410 Very toxic to aquatic life with long lasting effects.Precautionary statement(s)P264 Wash skin thoroughly after handling.P270 Do not eat, drink or smoke when using this product.P273 Avoid release to the environment.P301 + P312 IF SWALLOWED: Call a POISON CENTER or doctor/ physician if you feel unwell.P330 Rinse mouth.P391 Collect spillage.P501 Dispose of contents/ container to an approved waste disposal plant.2.3 Other hazardsNone.3. COMPOSITION/INFORMATION ON INGREDIENTS3.1 SubstancesSynonyms:LAS–W 090; RP64305Formula:C32H39NO2Molecular Weight:469.65CAS No. :90729-43-44. FIRST AID MEASURES4.1 Description of first aid measuresEye contactRemove any contact lenses, locate eye-wash station, and flush eyes immediately with large amounts of water. Separate eyelids with fingers to ensure adequate flushing. Promptly call a physician.Skin contactRinse skin thoroughly with large amounts of water. Remove contaminated clothing and shoes and call a physician.InhalationImmediately relocate self or casualty to fresh air. If breathing is difficult, give cardiopulmonary resuscitation (CPR). Avoid mouth-to-mouth resuscitation.IngestionWash out mouth with water; Do NOT induce vomiting; call a physician.4.2 Most important symptoms and effects, both acute and delayedThe most important known symptoms and effects are described in the labelling (see section 2.2).4.3 Indication of any immediate medical attention and special treatment neededTreat symptomatically.5. FIRE FIGHTING MEASURES5.1 Extinguishing mediaSuitable extinguishing mediaUse water spray, dry chemical, foam, and carbon dioxide fire extinguisher.5.2 Special hazards arising from the substance or mixtureDuring combustion, may emit irritant fumes.5.3 Advice for firefightersWear self-contained breathing apparatus and protective clothing.6. ACCIDENTAL RELEASE MEASURES6.1 Personal precautions, protective equipment and emergency proceduresUse full personal protective equipment. Avoid breathing vapors, mist, dust or gas. Ensure adequate ventilation. Evacuate personnel to safe areas.Refer to protective measures listed in sections 8.6.2 Environmental precautionsTry to prevent further leakage or spillage. Keep the product away from drains or water courses.6.3 Methods and materials for containment and cleaning upAbsorb solutions with finely-powdered liquid-binding material (diatomite, universal binders); Decontaminate surfaces and equipment by scrubbing with alcohol; Dispose of contaminated material according to Section 13.7. HANDLING AND STORAGE7.1 Precautions for safe handlingAvoid inhalation, contact with eyes and skin. Avoid dust and aerosol formation. Use only in areas with appropriate exhaust ventilation.7.2 Conditions for safe storage, including any incompatibilitiesKeep container tightly sealed in cool, well-ventilated area. Keep away from direct sunlight and sources of ignition.Recommended storage temperature:Powder-20°C 3 years4°C 2 yearsIn solvent-80°C 6 months-20°C 1 monthShipping at room temperature if less than 2 weeks.7.3 Specific end use(s)No data available.8. EXPOSURE CONTROLS/PERSONAL PROTECTION8.1 Control parametersComponents with workplace control parametersThis product contains no substances with occupational exposure limit values.8.2 Exposure controlsEngineering controlsEnsure adequate ventilation. Provide accessible safety shower and eye wash station.Personal protective equipmentEye protection Safety goggles with side-shields.Hand protection Protective gloves.Skin and body protection Impervious clothing.Respiratory protection Suitable respirator.Environmental exposure controls Keep the product away from drains, water courses or the soil. Cleanspillages in a safe way as soon as possible.9. PHYSICAL AND CHEMICAL PROPERTIES9.1 Information on basic physical and chemical propertiesAppearance White to off-white (Solid)Odor No data availableOdor threshold No data availablepH No data availableMelting/freezing point No data availableBoiling point/range No data availableFlash point No data availableEvaporation rate No data availableFlammability (solid, gas)No data availableUpper/lower flammability or explosive limits No data availableVapor pressure No data availableVapor density No data availableRelative density No data availableWater Solubility No data availablePartition coefficient No data availableAuto-ignition temperature No data availableDecomposition temperature No data availableViscosity No data availableExplosive properties No data availableOxidizing properties No data available9.2 Other safety informationNo data available.10. STABILITY AND REACTIVITY10.1 ReactivityNo data available.10.2 Chemical stabilityStable under recommended storage conditions.10.3 Possibility of hazardous reactionsNo data available.10.4 Conditions to avoidNo data available.10.5 Incompatible materialsStrong acids/alkalis, strong oxidising/reducing agents.10.6 Hazardous decomposition productsUnder fire conditions, may decompose and emit toxic fumes.Other decomposition products - no data available.11.TOXICOLOGICAL INFORMATION11.1 Information on toxicological effectsAcute toxicityClassified based on available data. For more details, see section 2Skin corrosion/irritationClassified based on available data. For more details, see section 2Serious eye damage/irritationClassified based on available data. For more details, see section 2Respiratory or skin sensitizationClassified based on available data. For more details, see section 2Germ cell mutagenicityClassified based on available data. For more details, see section 2CarcinogenicityIARC: No component of this product present at a level equal to or greater than 0.1% is identified as probable, possible or confirmed human carcinogen by IARC.ACGIH: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by ACGIH.NTP: No component of this product present at a level equal to or greater than 0.1% is identified as a anticipated or confirmed carcinogen by NTP.OSHA: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by OSHA.Reproductive toxicityClassified based on available data. For more details, see section 2Specific target organ toxicity - single exposureClassified based on available data. For more details, see section 2Specific target organ toxicity - repeated exposureClassified based on available data. For more details, see section 2Aspiration hazardClassified based on available data. For more details, see section 212. ECOLOGICAL INFORMATION12.1 ToxicityNo data available.12.2 Persistence and degradabilityNo data available.12.3 Bioaccumlative potentialNo data available.12.4 Mobility in soilNo data available.12.5 Results of PBT and vPvB assessmentPBT/vPvB assessment unavailable as chemical safety assessment not required or not conducted.12.6 Other adverse effectsNo data available.13. DISPOSAL CONSIDERATIONS13.1 Waste treatment methodsProductDispose substance in accordance with prevailing country, federal, state and local regulations.Contaminated packagingConduct recycling or disposal in accordance with prevailing country, federal, state and local regulations.14. TRANSPORT INFORMATIONDOT (US)This substance is considered to be non-hazardous for transport.IMDGUN number: 3077Class: 9Packing group: IIIEMS-No: F-A, S-FProper shipping name: ENVIRONMENTALLY HAZARDOUS SUBSTANCE, SOLID, N.O.S.Marine pollutant: Marine pollutant.IATAUN number: 3077Class: 9Packing group: IIIProper shipping name: Environmentally hazardous substance, solid, n.o.s.15. REGULATORY INFORMATIONSARA 302 Components:No chemicals in this material are subject to the reporting requirements of SARA Title III, Section 302.SARA 313 Components:This material does not contain any chemical components with known CAS numbers that exceed the threshold (De Minimis) reporting levels established by SARA Title III, Section 313.SARA 311/312 Hazards:No SARA Hazards.Massachusetts Right To Know Components:No components are subject to the Massachusetts Right to Know Act.Pennsylvania Right To Know Components:No components are subject to the Pennsylvania Right to Know Act.New Jersey Right To Know Components:No components are subject to the New Jersey Right to Know Act.California Prop. 65 Components:This product does not contain any chemicals known to State of California to cause cancer, birth defects, or anyother reproductive harm.16. OTHER INFORMATIONCopyright 2017 MedChemExpress. The above information is correct to the best of our present knowledge but does not purport to be all inclusive and should be used only as a guide. The product is for research use only and for experienced personnel. It must only be handled by suitably qualified experienced scientists in appropriately equipped and authorized facilities. The burden of safe use of this material rests entirely with the user. MedChemExpress disclaims all liability for any damage resulting from handling or from contact with this product.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
erastin分子量

erastin分子量
(原创版)
目录
1.Erastin 分子量的概念
2.Erastin 分子量的计算方法
3.Erastin 分子量的应用
正文
1.Erastin 分子量的概念
Erastin 是一种化学物质,其分子量指的是该物质分子中各个原子相对原子质量的总和。
分子量是衡量化学物质大小的重要参数,它可以用来判断物质的相对大小、物质的结构稳定性以及物质的物理化学性质等。
2.Erastin 分子量的计算方法
计算 Erastin 分子量的方法通常是通过各原子的相对原子质量相加得到。
在计算过程中,需要知道 Erastin 分子中各种原子的数量,然后将各种原子的相对原子质量乘以它们的数量,最后将这些值相加即可得到Erastin 的分子量。
需要注意的是,分子量的单位通常是“1”,因此在计算过程中需要将各原子的相对原子质量乘以它们的数量后进行相加,而不是直接将它们的数量相加。
3.Erastin 分子量的应用
Erastin 分子量在化学研究和工业生产中有着广泛的应用。
在化学研究中,通过计算 Erastin 的分子量,可以了解物质的大小、结构以及物理化学性质等信息,这对于研究物质的性质和行为具有重要意义。
在工业生产中,Erastin 分子量也可以用来设计和优化生产过程,例如通过改变生产过程中的温度、压力等条件,可以影响 Erastin 分子量的大小,从而影响产品的质量和性能。
erastin分子量

erastin分子量摘要:1.引言2.什么是Erastin 分子量3.Erastin 分子量在科学研究中的应用4.Erastin 分子量对药物研发的影响5.我国在Erastin 分子量研究方面的进展6.结论正文:Erastin 分子量是一种在药物研发中具有重要意义的参数。
药物的分子量对于药物的吸收、分布、代谢和排泄等过程有着重要的影响,因此对Erastin 分子量进行准确的研究和控制,对于药物的研发和应用具有重要意义。
Erastin 分子量是指Erastin 分子的大小,通常以道尔顿(Dalton)为单位表示。
Erastin 分子量的大小可以反映药物的溶解性和生物利用度,进而影响药物的效果和安全性。
在科学研究中,Erastin 分子量常被用于药物的定性、定量分析,以及药物代谢、药物动力学等研究。
此外,Erastin 分子量还可以用于药物的分离和纯化,以及药物的生物活性评价。
Erastin 分子量对药物研发的影响主要体现在以下几个方面:首先,药物的分子量决定了药物的溶解性和生物利用度,进而影响药物的效果和安全性;其次,药物的分子量也影响了药物的代谢和排泄,进而影响药物的药代动力学和药效学特性;最后,药物的分子量还影响了药物的制备和加工过程,包括药物的合成、纯化、制剂等。
我国在Erastin 分子量研究方面取得了显著的进展。
我国科学家们已经成功研发出了多种Erastin 分子量分析方法,包括质谱法、红外光谱法、X 射线衍射法等,这些方法不仅可以准确地测定Erastin 分子的分子量,还可以用于分析Erastin 分子的结构、纯度等特性。
总的来说,Erastin 分子量是药物研发中的一个重要参数,对药物的效果、安全性和制备过程都有着重要的影响。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Inhibitors, Agonists, Screening Libraries
Data Sheet
BIOLOGICAL ACTIVITY:
Erastin is a ferroptosis activator.
In Vitro: Erastin triggers oxidative, iron–dependent cell death. Treatment of NRAS–mutant HT–1080 fibrosarcoma cells with the RSL molecule erastin (10 μM) results in a time–dependent increase in cytosolic and lipid ROS beginning at 2 hours [1]. Cell death triggered by erastin is significantly inhibited by antioxidants (e.g., α–tocopherol, butylated hydroxytoluene, and β–carotene) and iron chelators (e.g., deferoxamine), suggesting that ROS– and iron–dependent signaling is required for erastin–induced
ferroptosis. Erastin can directly bind to VDAC2/3 in BJeLR cells. Knockdown of VDAC2 and VDAC3, but not VDAC1, leads to erastin resistance. Erastin has the ability to reduce glutathione level by directly inhibiting cystine/glutamate antiporter system Xc- activity,with activation of the ER stress response [2]. Erastin potently inhibits HT–29 cell survival. Erastin shows a dose–dependent effect,and 30 μM of erastin displays the most dramatic effect [3].
In Vivo: Intraperitoneal injection of erastin at well–tolerated doses dramatically inhibits HT–29 xenograft growth in severe combined
immunodeficient mice [3].PROTOCOL (Extracted from published papers and Only for reference)
Cell Assay:[3]To test erastin’s activity on colorectal cancer cell survival, HT–29 cells are treated with increasing concentrations of
erastin (0.1–30 μM). MTT assay was performed [3]. Animal Administration:[3]Mouse: Mice are treated daily with 10 or 30 mg/kg body weight of erastin (intraperitoneal injection, for 4weeks) or vehicle control (Saline). Tumor volumes are calculated. Mice body weights are also recorded every week [3].
References:
[1]. Dixon SJ, et al. Ferroptosis: an iron–dependent form of nonapoptotic cell death. Cell. 2012 May 25;149(5):1060–72.
[2]. Xie Y, et al. Ferroptosis: process and function. Cell Death Differ. 2016 Mar;23(3):369–79.
[3]. Huo H, et al. Erastin Disrupts Mitochondrial Permeability Transition Pore (mPTP) and Induces Apoptotic Death of Colorectal Cancer Cells. PLoS One. 2016May 12;11(5):e0154605.
Product Name:
Erastin Cat. No.:
HY-15763CAS No.:
571203-78-6Molecular Formula:
C 30H 31ClN 4O 4Molecular Weight:
547.04Target:
VDAC Pathway:
Membrane Transporter/Ion Channel Solubility:
DMSO: 6.4 mg/mL (Need ultrasonic)
Caution: Product has not been fully validated for medical applications. For research use only.
Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@ Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
