chapter 13 Reaction of Carbanion
连锁反应英文版

Chain ReactionIntroductionChain reaction refers to a sequence of events that occurs as a result of a single initial action, causing a series of subsequent actions. It is a phenomenon that can be observed in various aspects of life, such as science, economics, and even human behavior. Understanding chain reactions and their impacts is crucial as they can have far-reaching consequences.ScienceIn the field of science, chain reactions are widely studied and observed. They occur when a single event triggers a series of subsequent events, often leading to a rapid and uncontrollable chain of reactions. One famous example is the nuclear chain reaction, where the fission of an atomic nucleus releases neutrons that go on to induce the fission of other nuclei in a self-sustaining manner. This process is the basis of nuclear power and atomic bombs.Another example of a chain reaction in science is the combustion of fuels. When a spark ignites a fuel, it releases energy which further raises the temperature and causes adjacent molecules to combust. This release of energy continues to propagate until the fuel source is exhausted or the reaction is somehow halted.EconomicsChain reactions also play a significant role in economics. A single event or policy change in one sector can have ripple effects on other sectors and the overall economy. For example, an increase in oil prices can lead to increased transportation costs, which in turn can raise the prices of goods and services. Higher prices can then result in reduced consumer spending, leading to decreased demand for products and services. This can have a domino effect on businesses, leading to layoffs, decreased investments, and ultimately a recession.On the other hand, positive chain reactions can occur in economics as well. An increase in consumer spending can lead to increased demand for products, prompting businesses to expand and hire more employees. This, in turn, leads to higher employment rates, increased incomes, and further boosts consumer spending, creating a cycle of economic growth.Human BehaviorChain reactions can also be observed in human behavior. An individual’s action can trigger a series of reactions in others, influencing their behavior and decisions. For example, a small act of kindness can inspire others to do the same, creating apositive chain reaction of goodwill. Similarly, negative behavior, such as aggression or hostility, can also trigger a chain reaction of negative responses.In social media platforms, chain reactions are prevalent. A single post can go viral and elicit a series of reactions from other users, often leading to debates, discussions, or even mass movements. The power of social media in spreading information and influencing public opinion has made chain reactions in human behavior even more significant in the modern age.ConclusionChain reactions are a fascinating phenomenon that can be observed in various aspects of life. Understanding how chain reactions occur and their potential impacts is essential in many fields, including science, economics, and human behavior. Whether it is a nuclear chain reaction, an economic ripple effect, or a series of actions influenced by human behavior, chain reactions have the power to shape our world in significant ways.By studying and carefully considering chain reactions, we can make informed decisions and harness their power for positive change. The awareness of these cascading actions allows us to anticipate their consequences and take appropriate measures to mitigate potential negative impacts. Ultimately, understanding chain reactions helps us navigate complex systems and facilitates progress in various aspects of life.Note: This document is created using Markdown, a lightweight formatting syntax that allows for easy conversion to other formats like HTML.。
酪蛋白肽锌螯合物的制备及体外消化分析

周桂成,肖珊,王波,等. 酪蛋白肽锌螯合物的制备及体外消化分析[J]. 食品工业科技,2023,44(23):270−279. doi:10.13386/j.issn1002-0306.2023020002ZHOU Guicheng, XIAO Shan, WANG Bo, et al. Preparation and in Vitro Digestive Analysis of Casein-Derived Peptide-Zinc Chelates[J]. Science and Technology of Food Industry, 2023, 44(23): 270−279. (in Chinese with English abstract). doi:10.13386/j.issn1002-0306.2023020002· 分析检测 ·酪蛋白肽锌螯合物的制备及体外消化分析周桂成1,2,肖 珊2,王 波2, *,王际辉2,*(1.大连工业大学生物工程学院,辽宁大连 116034;2.东莞理工学院生命健康技术学院,广东东莞 523808)摘 要:为开发安全高效且易吸收的补锌剂,利用碱性蛋白酶酶解和乳酸菌发酵相结合的方法制备生物活性肽,并以此多肽制备了酪蛋白肽锌螯合物。
采用光谱法对螯合物结构进行表征,利用体外消化模型和Caco-2细胞实验对其胃肠消化特性及生物安全性进行评价。
结果表明,制备酪蛋白肽的最优条件为酶解pH 为9、碱性蛋白酶添加量为0.3%(w/v ),乳酸菌发酵时间为12 h ,此时反应体系中多肽含量为142.39±0.95 mg/g ,对锌的螯合率为31.41%±0.97%。
与锌螯合后,酪蛋白肽表面的致密结构遭到破坏,形成疏松的状态;光谱学分析表明,Zn 2+能与酪蛋白肽上的活性基团进行结合,螯合位点为羧基氧、羟基氧和氨基。
体外模拟消化结果显示,酪蛋白肽锌螯合物在消化过程中锌溶解性优于硫酸锌;在胃肠消化后酪蛋白肽锌螯合物DPPH 和ABTS +自由基的清除能力分别提升了26.19%±3.30%和71.96%±7.06%,而铁还原力下降了36.26%±2.80%;同时,在消化过程中肽锌螯合物的β-转角与无规则卷曲含量减少,β-折叠结构增加,Zn 2+起到了维持多肽结构的作用。
碳负离子亲核取代反应的纵深思维导图

2019年第11期广东化工第46卷总第397期·217·碳负离子亲核取代反应的纵深思维导图庞韬,章超,付晨(安庆师范大学化学化工学院,安徽安庆246133)[摘要]亲核取代反应在有机化学中非常重要,已被广泛应用于有机合成尤其是在杂环化合物的合成等领域。
根据反应底物的不同,分为四类:以脂肪族氯代烃为代表的饱和碳原子上的亲核取代反应:S N1和S N2;以烯烃和芳环为底物的亲核取代反应;以苯炔中间体的亲核取代反应以及酰卤、酸酐、酯、酰胺等羧酸衍生物的亲核取代反应。
本论文重点阐述总结涉及碳负离子参与的亲核取代反应,找寻出反应的规律性,以帮助学生牢牢掌握该知识点。
[关键词]有机化学;碳负离子;亲核取代反应;思维导图[中图分类号]TQ[文献标识码]A[文章编号]1007-1865(2019)11-0217-02Deep Thinking Map of Nucleophilic Substitution Reaction of CarbanionPang Tao,Zhang Chao,Fu Chen(Chemistry and Chemical Industry Chemistry,Anqing Normal University,Anqing246133,China)Abstract:As a critical part of organic chemistry,nucleophilic substitution reactions have seen wide application in the field of organic synthesis,especially inthe synthesis of heterocyclic compounds.Based on different reaction substrates,nucleophilic substitution reaction can be classified into four types-S N1and S N2 which are nucleophilic substitution reaction on saturated carbon atoms represented by aliphatic chlorinated hydrocarbons;nucleophilic substitution reaction with olefin and aromatic ring as substrate;nucleophilic substitution reaction on benzyne intermediate and nucleophilic substitution reaction on carboxylic acid derivative such as acid halide,acid anhydride,ester and amide.By focusing on the nucleophilic substitution reaction involving carbanion,this paper intends to find the regularity of such reactions,helping students to understand this knowledge more deeply.Keywords:organic chemistry;carbanion;nucleophile substitution reaction;mind map正文亲核取代反应在有机化学这门课程中有着举足轻重的地位,在有机合成尤其是杂环的制备中被广泛应用。
高中化学竞赛【合成试剂】

3、Still Modification
CF3CH2O- groups make the betaine less stable, giving more Z-olefin.
2、亲核取代与Arbzuov 重排
3、脱氧脱硫
R
R
OH
S
+
N OH
N
N N
(+)
P(OR)3
C2H5
R = CH2CH(CH2)3CH3
O Ph
O
Ph SePh
NaIO4
O
Ph
PhSe O
63%
80%
54%
氧化 产生烯丙醇
5. 锡试剂
还原
(80% ee) (99.5% ee)
烷基硼作还原剂的选择性优于乙硼烷. 乙硼烷能使 C=O, CN, CO2H, CONR2 还原.
§2、磷试剂
(1) Wittig Reagent and Wittig Reaction
The Wittig Reaction allows the preparation of an alkene by the reaction of an aldehyde or ketone with the ylide generated from a phosphonium salt. The geometry of the resulting alkene depends on the reactivity of the ylide.
electrophillic character. Lactones may be effected by this reaction. Generally E stereoselectivity is observed when the ylide
常用化学反应英文单词

acetate 醋酸盐acid 酸Actinium(Ac) 锕aldehyde 醛alkali 碱,强碱alkalinity 碱性alkalinization 碱化alkaloid 生物碱alloy 合金Aluminium(Al) 铝Americium(Am) 镅ammonia 氨analysis 分解anhydride 酐anion 阴离子anode 阳极,正极Antimony(Sb) 锑apparatus 设备aqua fortis 王水Argon(Ar) 氩Arsenic(As) 砷asphalt 沥青Astatine(At) 砹atom 原子atomic mass 原子质量atomic number 原子数atomic weight 原子量Barium(Ba) 钡base 碱benzene 苯Berkelium(Bk) 锫Beryllium(Be) 铍Bismuth(Bi) 铋bivalent 二价body 物体bond 原子的聚合Boron(B) 硼Bromine(Br) 溴Bunsen burner 本生灯burette 滴定管butane 丁烷Cadmium(Cd) 镉Caesium(Cs) 铯Calcium(Ca) 钙Californium(Cf) 锎Carbon(C) 碳catalysis 催化作用catalyst 催化剂cathode 阴极,负极cation 阳离子caustic potash 苛性钾caustic soda 苛性钠Cerium(Ce) 铈chemical fiber 化学纤维Chlorine(Cl) 氯Chromium(Cr) 铬Cobalt(Co) 钴combination 合成作用combustion 燃烧compound 合成物compound 化合物Copper(Cu) 铜cracking 裂化crucible pot, melting pot 坩埚crude oil, crude 原油cupel 烤钵Curium(Cm) 锔derivative 衍生物dissolution 分解distillation column 分裂蒸馏塔distillation 蒸馏Dysprosium(Dy) 镝Einsteinium(Es) 锿electrode 电极electrolysis 电解electrolyte 电解质electron 电子element 元素endothermic reaction 吸热反应Erbium(Er) 铒ester 酯Europium(Eu) 铕exothermic reaction 放热反应fatty acid 脂肪酸fermentation 发酵Fermium(Fm) 镄filter 滤管flask 烧瓶Fluorine(F) 氟fractional distillation 分馏fractionating tower 分馏塔fractionation 分馏Francium(Fr) 钫fuel 燃料fusion, melting 熔解Gadolinium(Gd) 钆Gallium(Ga) 镓gas oil 柴油gel 凝胶体Germanium(Ge) 锗Gold(Au) 金graduate, graduated flask 量筒,量杯gram atom 克原子Hafnium(Hf) 铪halogen 成盐元素Helium(He) 氦high-grade petrol, high-octane petrol 高级汽油,高辛烷值汽油Holmium(Ho) 钬hydracid 氢酸hydrate 水合物hydrocarbon 碳氢化合物,羟hydrocarbon 烃,碳氢化合物hydrochloric acid 盐酸hydrogen sulfide 氢化硫Hydrogen(H) 氢hydrolysis 水解hydrosulphuric acid 氢硫酸hydroxide 氢氧化物,羟化物Indium(In) 铟inorganic chemistry 无机化学Iodine(I) 碘ion 离子Iridium(Ir) 铱Iron(Fe) 铁isomer 同分异物现象isomerism, isomery 同分异物现象isotope 同位素kerosene, karaffin oil 煤油Krypton(Kr) 氪Lanthanum(La) 镧Lawrencium(Lr) 铹Lead(Pb) 铅Lithium(Li) 锂litmus paper 石蕊试纸litmus 石蕊LNG, liquefied natural gas 液化天然气LPG, liquefied petroleum gas 液化石油气lubricating oil 润滑油Lutetium(Lu) 镥Magnesium(Mg) 镁Manganese(Mn) 锰matrass 卵形瓶Mendelevium(Md) 钔Mercury(Hg) 汞metal 金属metalloid 非金属methane 甲烷,沼气mixture 混合molecule 分子Molybdenum(Mo) 钼monovalent 单价natural gas 天然气Neodymium(Nd) 钕Neon(Ne) 氖Neptunium(Np) 镎Nickel(Ni) 镍Niobium(Nb) 铌nitric acid 硝酸Nitrogen(N) 氮Nobelium(No) 锘Nuclear Fusion 核聚变octane number 辛烷数,辛烷值olefin 烯烃organic acid 有机酸organic chemistry 有机化学Osmium(Os) 锇oxide 氧化物oxidization, oxidation 氧化Oxygen(O) 氧Palladium(Pd) 钯paraffin 石蜡petrol 汽油(美作:gasoline)PH indicator PH值指示剂,氢离子(浓度的)负指数指示剂phosphate 磷酸盐Phosphorus(P) 磷pipette 吸液管plastic 塑料Platinum(Pt) 铂Plutonium(Pu) 钚Polonium(Po) 钋polymer 聚合物polymerizing, polymerization 聚合potassium carbonate 碳酸钾Potassium(K) 钾Praseodymium(Pr) 镨precipitation 沉淀product 产物electrochemical analysis 电化学分析on-line analysis 在线分析macro analysis 常量分析characteristic 表征micro analysis 微量分析deformation analysis 形态分析semimicro analysis 半微量分析systematical error 系统误差routine analysis 常规分析random error 偶然误差arbitration analysis 仲裁分析gross error 过失误差normal distribution 正态分布accuracy 准确度deviation偏差precision 精密度relative standard deviation 相对标准偏差(RSD)coefficient variation 变异系数(CV)confidence level 置信水平confidence interval 置信区间significant test 显著性检验significant figure 有效数字standard solution 标准溶液titration 滴定stoichiometric point 化学计量点end point滴定终点titration error 滴定误差primary standard 基准物质amount of substance 物质的量standardization 标定chemical reaction 化学反应concentration浓度chemical equilibrium 化学平衡titer 滴定度general equation for a chemical reaction化学反应的通式proton theory of acid-base 酸碱质子理论acid-base titration 酸碱滴定法dissociation constant 解离常数conjugate acid-base pair 共轭酸碱对acetic acid 乙酸hydronium ion水合氢离子electrolyte 电解质ion-product constant of water 水的离子积ionization 电离proton condition 质子平衡zero level零水准buffer solution缓冲溶液methyl orange 甲基橙acid-base indicator 酸碱指示剂phenolphthalein 酚酞coordination compound 配位化合物center ion 中心离子cumulative stability constant 累积稳定常数alpha coefficient 酸效应系数overall stability constant 总稳定常数ligand 配位体ethylenediamine tetraacetic acid 乙二胺四乙酸side reaction coefficient 副反应系数coordination atom 配位原子coordination number 配位数lone pair electron 孤对电子chelate compound 螯合物metal indicator 金属指示剂chelating agent 螯合剂masking 掩蔽demasking 解蔽electron 电子catalysis 催化oxidation氧化catalyst 催化剂reduction 还原catalytic reaction 催化反应reaction rate 反应速率electrode potential 电极电势activation energy 反应的活化能redox couple 氧化还原电对potassium permanganate 高锰酸钾iodimetry碘量法potassium dichromate 重铬酸钾cerimetry 铈量法redox indicator 氧化还原指示oxygen consuming 耗氧量(OC)chemical oxygen demanded 化学需氧量(COD) dissolved oxygen 溶解氧(DO)precipitation 沉淀反应argentimetry 银量法heterogeneous equilibrium of ions 多相离子平衡aging 陈化postprecipitation 继沉淀coprecipitation 共沉淀ignition 灼烧fitration 过滤decantation 倾泻法chemical factor 化学因数spectrophotometry 分光光度法colorimetry 比色分析transmittance 透光率absorptivity 吸光率calibration curve 校正曲线standard curve 标准曲线monochromator 单色器source 光源wavelength dispersion 色散absorption cell吸收池detector 检测系统bathochromic shift 红移Molar absorptivity 摩尔吸光系数hypochromic shift 紫移acetylene 乙炔ethylene 乙烯acetylating agent 乙酰化剂acetic acid 乙酸adiethyl ether 乙醚ethyl alcohol 乙醇acetaldehtde 乙醛β-dicarbontl compound β–二羰基化合物bimolecular elimination 双分子消除反应bimolecular nucleophilic substitution 双分子亲核取代反应open chain compound 开链族化合物molecular orbital theory 分子轨道理论chiral molecule 手性分子tautomerism 互变异构现象reaction mechanism 反应历程chemical shift 化学位移Walden inversio 瓦尔登反转nEnantiomorph 对映体addition rea ction 加成反应dextro- 右旋levo- 左旋stereochemistry 立体化学stereo isomer 立体异构体Lucas reagent 卢卡斯试剂covalent bond 共价键conjugated diene 共轭二烯烃conjugated double bond 共轭双键conjugated system 共轭体系conjugated effect 共轭效应isomer 同分异构体isomerism 同分异构现象organic chemistry 有机化学hybridization 杂化hybrid orbital 杂化轨道heterocyclic compound 杂环化合物peroxide effect 过氧化物效应tvalence bond theory 价键理论sequence rule 次序规则electron-attracting grou p 吸电子基Huckel rule 休克尔规则Hinsberg test 兴斯堡试验infrared spectrum 红外光谱Michael reacton 麦克尔反应halogenated hydrocarbon 卤代烃haloform reaction 卤仿反应systematic nomenclatur 系统命名法eNewman projection 纽曼投影式aromatic compound 芳香族化合物aromatic character 芳香性rClaisen condensation reaction克莱森酯缩合反应Claisen rearrangement 克莱森重排Diels-Alder reation 狄尔斯-阿尔得反应Clemmensen reduction 克莱门森还原Cannizzaro reaction 坎尼扎罗反应positional isomers 位置异构体unimolecular elimination reaction 单分子消除反应unimolecular nucleophilic substitution 单分子亲核取代反应benzene 苯functional grou 官能团pconfiguration 构型conformation 构象confomational isome 构象异构体electrophilic addition 亲电加成electrophilic reagent 亲电试剂nucleophilic addition 亲核加成nucleophilic reagent 亲核试剂nucleophilic substitution reaction亲核取代反应active intermediate 活性中间体Saytzeff rule 查依采夫规则cis-trans isomerism 顺反异构inductive effect 诱导效应tFehling’s reagent 费林试剂phase transfer catalysis 相转移催化作用aliphatic compound 脂肪族化合物elimination reaction 消除反应Grignard reagent 格利雅试剂nuclear magnetic resonance 核磁共振alkene 烯烃allyl cation 烯丙基正离子leaving group 离去基团optical activity 旋光性boat confomation 船型构象silver mirror reaction 银镜反应Fischer projection 菲舍尔投影式Kekule structure 凯库勒结构式Friedel-Crafts reaction 傅列德尔-克拉夫茨反应Ketone 酮carboxylic acid 羧酸carboxylic acid derivative 羧酸衍生物hydroboration 硼氢化反应bond oength 键长bond energy 键能bond angle 键角carbohydrate 碳水化合物carbocation 碳正离子carbanion 碳负离子alcohol 醇Gofmann rule 霍夫曼规则Aldehyde 醛Ether 醚Polymer 聚合物product 化学反应产物Promethium(Pm) 钷Protactinium(Pa) 镤purification 净化qualitative analysis 定性分析quantitative analysis 定量分析chemical analysis 化学分析instrumental analysis 仪器分析titrimetry 滴定分析gravimetric analysis 重量分析法regent 试剂chromatographic analysis 色谱分析radical 基Radium(Ra) 镭Radon(Rn) 氡reagent 试剂reducer 还原剂refinery 炼油厂refining 炼油reforming 重整retort 曲颈甑reversible 可逆的Rhenium(Re) 铼Rhodium(Rh) 铑Rubidium(Rb) 铷Ruthenium(Ru) 钌salt 盐Samarium(Sm) 钐Scandium(Sc) 钪Selenium(Se) 硒separation 分离series 系列Silicon(Si) 硅Silver(Ag) 银soda 苏打sodium carbonate 碳酸钠Sodium(Na) 钠solution 溶解solvent 溶剂still 蒸馏釜stirring rod 搅拌棒Strontium(Sr) 锶structural formula 分子式Sulphur(S) 锍sulphuric acid 硫酸symbol 复合synthesis 合成synthetic rubber 合成橡胶Tantalum(Ta) 钽Technetium(Tc) 锝Tellurium(Te) 碲Terbium(Tb) 铽test tube 试管Thallium(Tl) 铊Thorium(Th) 钍Thulium(Tm) 铥Tin(Sn) 锡Titanium(Ti) 钛to calcine 煅烧to dehydrate 脱水to distil, to distill 蒸馏to hydrate 水合,水化to hydrogenate 氢化to neutralize 中和to oxidize 氧化to oxygenate, to oxidize 脱氧,氧化to precipitate 沉淀Tungsten(W) 钨Uranium(U) 铀valence, valency 价Vanadium(V) 钒vaseline 凡士林Xenon(Xe) 氙Ytterbium(Yb) 镱Yttrium(Y) 钇Zinc(Zn) 锌Zirconium(Zr) 锆理想气体状态方程Partial Pressures 分压Real Gases: Deviation from Ideal Behavior 真实气体:对理想气体行为的偏离The van der Waals Equation 范德华方程System and Surroundings 系统与环境State and State Functions 状态与状态函数Process 过程Phase 相The First Law of Thermodynamics 热力学第一定律Heat and Work 热与功Endothermic and Exothermic Processes 吸热与发热过程Enthalpies of Reactions 反应热Hess’s Law 盖斯定律Enthalpies of Formation 生成焓Reaction Rates 反应速率Reaction Order 反应级数Rate Constants 速率常数Activation Energy 活化能The Arrhenius Equation 阿累尼乌斯方程Reaction Mechanisms 反应机理Homogeneous Catalysis 均相催化剂Heterogeneous Catalysis 非均相催化剂Enzymes 酶The Equilibrium Constant 平衡常数the Direction of Reaction 反应方向Le Chatelier’s Principle 列·沙特列原理Effects of V olume, Pressure, Temperature Changes and Catalysts 体积,压力,温度变化以及催化剂的影响Spontaneous Processes 自发过程Entropy (Standard Entropy) 熵(标准熵)The Second Law of Thermodynamics 热力学第二定律Entropy Changes 熵变Standard Free-Energy Changes 标准自由能变Acid-Bases 酸碱The Dissociation of Water 水离解The Proton in Water 水合质子The pH Scales pH值Bronsted-Lowry Acids and Bases Bronsted-Lowry 酸和碱Proton-Transfer Reactions 质子转移反应Conjugate Acid-Base Pairs 共轭酸碱对Relative Strength of Acids and Bases 酸碱的相对强度Lewis Acids and Bases 路易斯酸碱Hydrolysis of Metal Ions 金属离子的水解Buffer Solutions 缓冲溶液The Common-Ion Effects 同离子效应Buffer Capacity 缓冲容量Formation of Complex Ions 配离子的形成Solubility 溶解度The Solubility-Product Constant Ksp 溶度积常数Precipitation and separation of Ions 离子的沉淀与分离Selective Precipitation of Ions 离子的选择沉淀Oxidation-Reduction Reactions 氧化还原反应Oxidation Number 氧化数Balancing Oxidation-Reduction Equations 氧化还原反应方程的配平Half-Reaction 半反应Galvani Cell 原电池V oltaic Cell 伏特电池Cell EMF 电池电动势Standard Electrode Potentials 标准电极电势Oxidizing and Reducing Agents 氧化剂和还原剂The Nernst Equation 能斯特方程Electrolysis 电解The Wave Behavior of Electrons 电子的波动性Bohr’s Model of The Hydrogen Atom 氢原子的波尔模型Line Spectra 线光谱Quantum Numbers 量子数Electron Spin 电子自旋Atomic Orbital 原子轨道The s (p, d, f) Orbital s(p,d,f)轨道Many-Electron Atoms 多电子原子Energies of Orbital 轨道能量The Pauli Exclusion Principle 泡林不相容原理Electron Configurations 电子构型The Periodic Table 周期表Row 行Group 族Isotopes, Atomic Numbers, and Mass Numbers 同位素,原子数,质量数Periodic Properties of the Elements 元素的周期律Radius of Atoms 原子半径Ionization Energy 电离能Electronegativity 电负性Effective Nuclear Charge 有效核电荷Electron Affinities 亲电性Metals 金属Nonmetals 非金属Valence Bond Theory 价键理论Covalence Bond 共价键Orbital Overlap 轨道重叠Multiple Bonds 重键Hybrid Orbital 杂化轨道The VSEPR Model 价层电子对互斥理论Molecular Geometries 分子空间构型Molecular Orbital 分子轨道Diatomic Molecules 双原子分子Bond Length 键长Bond Order 键级Bond Angles 键角Bond Enthalpies 键能Bond Polarity 键矩Dipole Moments 偶极矩Polarity Molecules 极性分子Polyatomic Molecules 多原子分子Crystal Structure 晶体结构Non-Crystal 非晶体Close Packing of Spheres 球密堆积Metallic Solids 金属晶体Metallic Bond 金属键Alloys 合金Ionic Solids 离子晶体Ion-Dipole Forces 离子偶极力Molecular Forces 分子间力Intermolecular Forces 分子间作用力Hydrogen Bonding 氢键Covalent-Network Solids 原子晶体Compounds 化合物The Nomenclature, Composition and Structure of Complexes 配合物的命名,组成和结构Charges, Coordination Numbers, and Geometries 电荷数、配位数、及几何构型Chelates 螯合物Isomerism 异构现象Structural Isomerism 结构异构Stereoisomerism 立体异构Magnetism 磁性Electron Configurations in Octahedral Complexes 八面体构型配合物的电子分布Tetrahedral and Square-planar Complexes 四面体和平面四边形配合物General Characteristics 共性s-Block Elements s区元素Alkali Metals 碱金属Alkaline Earth Metals 碱土金属Hydrides 氢化物Oxides 氧化物Peroxides and Superoxides 过氧化物和超氧化物Hydroxides 氢氧化物Salts 盐p-Block Elements p区元素Boron Group (Boron, Aluminium, Gallium, Indium, Thallium) 硼族(硼,铝,镓,铟,铊)Borane 硼烷Carbon Group (Carbon, Silicon, Germanium, Tin, Lead) 碳族(碳,硅,锗,锡,铅)Graphite, Carbon Monoxide, Carbon Dioxide 石墨,一氧化碳,二氧化碳Carbonic Acid, Carbonates and Carbides 碳酸,碳酸盐,碳化物Occurrence and Preparation of Silicon 硅的存在和制备Silicic Acid,Silicates 硅酸,硅酸盐Nitrogen Group (Phosphorus, Arsenic, Antimony, and Bismuth) 氮族(磷,砷,锑,铋)Ammonia, Nitric Acid, Phosphoric Acid 氨,硝酸,磷酸Phosphorates, phosphorus Halides 磷酸盐,卤化磷Oxygen Group (Oxygen, Sulfur, Selenium, and Tellurium) 氧族元素(氧,硫,硒,碲)Ozone, Hydrogen Peroxide 臭氧,过氧化氢Sulfides 硫化物Halogens (Fluorine, Chlorine, Bromine, Iodine) 卤素(氟,氯,溴,碘)Halides, Chloride 卤化物,氯化物The Noble Gases 稀有气体Noble-Gas Compounds 稀有气体化合物d-Block elements d区元素Transition Metals 过渡金属Potassium Dichromate 重铬酸钾Potassium Permanganate 高锰酸钾Iron Copper Zinc Mercury 铁,铜,锌,汞f-Block Elements f区元素Lanthanides 镧系元素Radioactivity 放射性Nuclear Chemistry 核化学Nuclear Fission 核裂变analytical chemistry 分析化学。
化学SUB的准备

化学SUB的准备经验今天SUB很受打击,倒不是题目出的很难,主要是自己复习的不好或是说学的不好了,题目的回忆别人已经做的差不多了,我想起来什么再补充吧。
主要说一下复习的一些心得,我已经提前说了考的很不好,所以化学的XDJM们暂且一听吧。
wien师姐两周就可以考一perfect的成绩,但是我想给我两个月我也做不到。
谁让我学的时候太不扎实,最后又迟迟不愿意碰化学的东西,而sub本身的特点就是广,这个广的程度真是无法形容,所以有时间的话还是把书彻彻底底的看一遍为好(我觉得是),比如有机今天考了一个什么磷酸酯,我根本没见过只好瞎蒙,可是回来后发现就在有机书上最后几页,我没有看到是因为老师没讲那个部分,所以也就懒的看了。
可能有人觉得没有必要把书全看了,可以理解,因为现在也正好是申请的时候,没人能拿出两个月的时候来专心看书,毕竟考到的东西就那么136道题,没考到的知识点比考到的要多得多,这又要看个人衡量了,呵呵原则上说Practice book上的范围绝对是最好最全的,但是看完之后你会觉得它什么都没说,比如有机部分,它用两行大约20个单词说考哪些物质的性质和反应,我们却要把500多页的有机书细细看一遍,而且它经常提到什么东西的应用,这个真的太难去找了。
所以只能结合题目来看书。
我手头上有四套题目,估计现在能找到的也就这些了,现在一一说一下model test 共5套,好像是ETS给的题,年代好像比较久远,而且题目跟现在的考试有了很多区别,比如那里有好多非常陌生的人名试剂和人名反应,害我整理了一大堆东西,结果考的时候一个也没涉及到,当然我也最后也没记住几个,呵呵。
还有这里的有机物往往是给英文名,而不是给分子式的,所以要掌握很多单词,但是今天考试时我观察了一下好像大部分反应都给分子式了,所以能记住最好,象我一样记不住的话就算了,呵呵。
之所以把这套题目放第一位是因为它的后面有比较详细的解释,有的甚至在其他的任何地方都查不到的,所以先做会比较容易上手,也比较省时间。
二氯环己烷消去反应方程式

二氯环己烷消去反应方程式Answer: The elimination reaction of 1,2-dichlorocyclohexane can be represented by the following equation:1,2-dichlorocyclohexane -> cyclohexene + 2HCl.This reaction involves the removal of two chlorine atoms from the cyclohexane ring, resulting in the formation of cyclohexene and two molecules of hydrochloric acid.The mechanism of this reaction involves the use of a strong base, such as sodium hydroxide, which abstracts a proton from one of the adjacent carbon atoms, leading to the formation of a carbanion intermediate. This carbanion then undergoes an E2 elimination reaction, where the two chlorine atoms are removed as a leaving group, resulting in the formation of the double bond in cyclohexene.Overall, this elimination reaction is an importantmethod for the synthesis of alkenes from alkyl halides, and it is widely used in organic synthesis.中文回答:1,2-二氯环己烷的消去反应可以用以下方程式表示:1,2-二氯环己烷 -> 环己烯 + 2HCl.这个反应涉及从环己烷环中去除两个氯原子,导致环己烯和两分子盐酸的形成。
化学中英文对照表
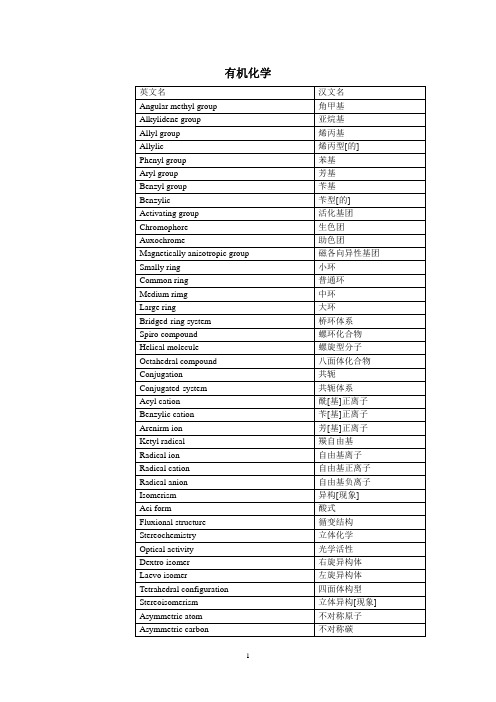
有机化学英文名汉文名Angular methyl group 角甲基Alkylidene group 亚烷基Allyl group 烯丙基Allylic 烯丙型[的] Phenyl group 苯基Aryl group 芳基Benzyl group 苄基Benzylic 苄型[的] Activating group 活化基团Chromophore 生色团Auxochrome 助色团Magnetically anisotropic group 磁各向异性基团Smally ring 小环Common ring 普通环Medium rimg 中环Large ring 大环Bridged-ring system 桥环体系Spiro compound 螺环化合物Helical molecule 螺旋型分子Octahedral compound 八面体化合物Conjugation 共轭Conjugated-system 共轭体系Acyl cation 酰[基]正离子Benzylic cation 苄[基]正离子Arenirm ion 芳[基]正离子Ketyl radical 羰自由基Radical ion 自由基离子Radical cation 自由基正离子Radical anion 自由基负离子Isomerism 异构[现象]Aci form 酸式Fluxional structure 循变结构Stereochemistry 立体化学Optical activity 光学活性Dextro isomer 右旋异构体Laevo isomer 左旋异构体Tetrahedral configuration 四面体构型Stereoisomerism 立体异构[现象] Asymmetric atom 不对称原子Asymmetric carbon 不对称碳Pseudoasymmetric carbon 假不对称碳Phantom atom 虚拟原子Homotopic 等位[的] Heterotopic 异位[的] Enantiotopic 对映异位[的] Diastereotopic 非对映异位[的] Configuration 构型Absolute configuration 绝对构型Chirality 手性Chiral 手性[的]Chiral center 手性中心Chiral molecule 手性分子Achiral 非手性[的] Fischer projection 费歇尔投影式Neoman projection 纽曼投影式D-L system of nomenclature D-L命名体系R-S syytem of nomenclature R-S命名体系Cahn-Ingold-Prelon sequence 顺序规则Symmetry factor 对称因素Plane of symmetry 对称面Mirror symmetry 镜面对称Enantiomer 对映[异构]体Diastereomer 非对映[异构]体Epimer 差向异构体Anomer 端基[差向]异构体Erythro configuration 赤型构型Erythro isomer 赤型异构体Threo configuration 苏型构型Threo isomer 苏型异构体Trigonal carbon 三角型碳Cis-trans isomerism 顺反异构E isomer E异构体Z isomer Z异构体Endo isomer 内型异构体Exo isomer 外型异构体Prochirality 前手性Pro-R group 前R基团Pro-S proup 前S基团Re face Re面Si face Si面Racemic mixture 外消旋混合物Racemic compound 外消旋化合物Racemic solid solution 外消旋固体溶液Meso compound 内消旋化合物Quasi recemate 准外消旋体Conformation 构象Conformational 构象分析Torsion angle 扭转角Rotamer 旋转异构体Anti conformation 反式构象Bisecting conformation 等分构象Anti periplanar conformation 反叠构象Synperiplanar conformation 顺叠构象Synclinal conformation 反错构象Synclinal conformation 顺错构象Eclipsed conformation 重叠构象Gauche conformation, skew con-formation 邻位交叉构象Staggered conformation 对位交叉构象Steric effect 空间效应Steric hindrance 位阻Atropismer 阻转异构体Puckered ring 折叠环Conformational inversion 构象反转Chair conformation 椅型构象Boat conformation 船型构象Twist conformation 扭型构象Skew boat conformation 扭船型构象Half-chair conformation 半椅型构象Pseudorotation 假旋转Envelope conformation 信封[型]构象Axial bond 直[立]键Equatorial bond 平[伏]键Cisoid conformation 顺向构象Transoid conformation 反向构象Retention of configuration 构型保持Regioselectivity 区域选择性Regiospecificity 区域专一性Stereocelectivity 立体选择性Stereospecificty 立体专一性Conformer 构象异构体Conformational effect 构象效应Cram’s rube 克拉姆规则Prelog’rule 普雷洛格规则Stereochemical orientation 立体[化学]取向Conformational transmission 构象传递Homolog 同系物Ipso position 本位Ortho position 邻位Meta position 间位Para position 对位Amphi position 远位Peri position 近位Trigonal hybridization 三角杂化Molecular orbiral method 分子轨道法Valence bond method 价键法Delocalezed bond 离域键Cross conjugation 交叉共轭Vinylog 插烯物Mesomeric effect 中介效应Resonance 共振Resonance effect 共振效应Hyperconjugation 超共轭Isovalent hyperconjugation 等价超共轭No-bond resonance 无键共振Aromaticity 芳香性Aromatic sexter 芳香六隅Huckel’rule 休克尔规则Paramagnetic ring current 顺磁环电流Diamagnetic ring cruuent 抗磁环电流Homoaromaticity 同芳香性Antiaromaticity 反芳香性Alternant hydrocarbon 交替烃Non-alternant hydrocarbon 非交替烷Pericyclic reaction 周环反应Electrocyclic rearrangement 电环[化]重排Conrotatory 顺旋Disroatatory 对旋Cycloaddition 环加成Symmetry forbidden-reaction 对称禁阻反应Synfacial reaction 同面反应Antarafacial reaction 异面反应Mobius system 默比乌斯体系Leois structure 路易斯结构Coordinate-covalent bond 配位共价键Banana bond 香蕉键Pauling electronegativity scale 鲍林电负性标度Polarizability 可极化性Inductive effect 诱导效应Field effect 场效应Electrical effect 电场效应tautomerism 互变异构Tautomerization 互变异构化Keto-enol tautomerism 酮-烯醇互变异构Phenol-keto tautomerism 酚-酮互变异构Imine-enamine atutomerism 亚胺-烯胺互变异构Ring-chain tautomerism 环-链互变异构Valence tautomerism 价互变异构Ambident 两可[的]Solvent effect 溶剂效应Acid-base catalyxed reaction 酸性溶剂Basic solvent 碱性溶剂Dielectric constant 介电常数Solvated electron 溶剂化电子Acid-base catalyzed reaction 酸碱催化反应Conjugate base 共轭酸Conjugate base 共轭碱Therm odynamic acidity 热力学酸度Kinetic acidity 动力学酸度Electron donof-acceptor complex,EDAcomplex电子给[体]受体络合物Host 主体Guest 客体Primary isotope effect 一级同位素效应Secondary isotope effect 二级同位数效应Inverse isotope effect 逆同位素效应Kinetic control 动力学控制Thermodynamic control 热力学控制Substrate 底物Intermediate 中间体Reactive intermediate 活泼中间体Microscopic reversibility 微观可逆性Hammond postulate 哈蒙德假说Linear free energy 线性自由能Non-bonded interaction 非键相互作用Torsional effect 扭转效应Pitzer strain 皮策张力Restricted rotation 阻碍旋转Eclipsing effect 重叠效应Eclipsing strain 重叠张力Small-angle strain 小角张力Large angle strain 大角张力Transannular interaction 跨环相互作用Transannular strain 跨环张力I strain 内张力 F strain 前张力 B strain 后张力 Anomeric effect 端基异构效应 Walden inversion 瓦尔登反转 Racemization 外消旋化 Isoinversion 等反转 Isoracemization 等消旋 Homochiral 纯手性[的] Mechanism机理Unimolecular nucleophilic单分子亲核取代 Bimolecular nucleophilic sub-stitution双分子亲核取代Bimolecular nucleophilic substi-tution(with allylic rearrange-ment) 双分子亲核取代(含烯丙型重排) Internal nucleophilic substiru-tion 分子内亲核取代 Aromatic nucleophilic substitu-tion 芳香亲核取代 Unimolecular electrophilic sub-stitution 单分子亲电取代 Bimolecular electrophilic substi-tution双分子亲电取代Nucleophile-assisted unimolecu-lar electrophilic substitution 亲核体协助单分子亲电取代 Unimolecular elimination 单分子消除 Bimolecular elimination双分子消除Unimolecular elimination through the conjugate base单分子共轭碱消除 Bimolecular elimination through the conjugate base双分子共轭碱消除 Bimolecular elimination with for-mation of a carbonyl group双分子羰基形成消除 Unimolecular acid-catalyzed acyl-oxygen cleavage单分子酸催化酰氧断裂 Bimolecular base-catalyzed acyl-oxygen cleavage双分子碱催化酰氧断裂 Unimolecular acid-catalyzed alkyl-oxygen cleavage单分子酸催化烷氧断裂 Bimllecular base-catalyzed al- kyl-oxygen cleavage双分子碱催化烷氧断裂 π-allyl complex mechanism π烯丙型络合机理 Borderline mechanism 边理机理 Homolysis 均裂 Heterolysis异裂 Heterolytic michanism 异裂机理 Counrer[gegen]ion 反荷离子 Ion pair离子对Carbocation 碳正离子Nonclassical carbocation 非经典碳正离子Carbanion 碳负离子Masked carbanion 掩蔽碳负离子Carbenoid 卡宾体Carbene 卡宾Nitrene 氮宾Carbine 碳炔Electrophilic addition 亲电加成Electrophile 亲电体Diaxial addition 双直键加成Markovnikov’s rube 马尔科夫尼科规则Anti-Markovnikov addition 反马氏加成Michael addition 迈克尔加成Substitution 取代Electrophilic substitution 亲电取代Addition-elimination mechanism 加成消除机理Electrophilic aromatic substitu-tion 亲电芳香取代Electron transfer 电子转移Electron-donating group 给电子基团Electron-Withdrawing group 吸电子基团Deactivating group 钝化基团Orinentation 取向Ortho-para directing group 邻对位定位基Meta directing group 间位定位基Ortho effect 邻位效应Partial rate factor 分速度系数Nucleophilic reaction 亲核反应Internal return 内返Nucleophilicity 亲核体Nucleophilicity 亲核性α-effect α-效应Backside attack 背面进攻Inversion 反转Umbrella effect 伞效应Push-pull effect 推拉效应Leaving group 离去基团Electrofuge 离电体Nucleofuge 离核体Phase-transfer catalysis 相转移催化Neighboring group participation 邻基基参与邻助作用Neighboring proup assistance,anchimericassistanceNeighboring group effect 邻基效应Apofacial reaction 反面反应Briddgehead displacement 桥头取代Aryl action 芳正离子Benzyne 苯炔Zaitsev rule 札依采夫规则Anti-Zaitsev orientation 反札依采夫定向Hofmann’s rule 霍夫曼规则Bredt rule 布雷特规则Initiation 引发Anionic cleavage 负离子裂解Partial bond fixation 键[的]部分固定化02.3有机化学反应Alkylation 烷基化C- alkylation C-烷基化O- alkylation O-烷基化N-alkylation N-烷基化Silylation 硅烷[基]化Exhaustive methylation 彻底甲基化Seco alkylation 断裂烷基化Demethylation 脱甲基化Ethylation 乙基化Arylation 芳基化Acylation 酰化Formylation 甲酰化Carbalkoxylation 烷氧羰基化Carboamidation 氨羰基化Carboxylation 羧基化Amination 氨基化Bisamination 双氨基化Cine substitution 移位取代Transamination 氨基交换Hydroxylation 羟基化acyloxyation 酰氧基化Decarboxylative nitration 脱羧卤化Allylic halogenation 烯丙型卤化Dehalogenation 脱卤Nitration 硝化Decarboxylative nitration 脱羧硝化Nitrosation 亚硝化Sulfonation 磺化Chlorosulfonation 氯磺酰化Desulfonation 脱磺酸基Sulfenylation 亚磺酰化Sulfonylation 磺酰化Chlorosulfenation 氯亚磺酰化Chlorocarbonylation 氯羰基化Diazotization 重氮化Diazo transfer 重氮基转移Coupling reaction 偶联反应Diazonium coupling 重氮偶联Cross-coupling reaction 交叉偶联反应1,4-addition 1,4-加成Conjugate addition 共轭加成Dimerization 二聚Trimefization 三聚Additive dimerization 加成二聚sulfurization 硫化Selenylation 硒化Hydroboration 硼氢化Oxyamination 羟氨基化Insertion 插入carbonylation 羧基化Hydroformylation 加氢甲酰基化Hydroacylation 加氢酰化Oxo process 羰基合成Decarbonylation 脱羰Hydrocarboxylation 氢羧基化Homologization 同系化Cyanoethylation 氰乙基化Decyanoethylation 脱氰乙基Ring clsure 环合Diene synthesis 双烯合成Dienophile 亲双烯体Endo addition 内型加成Exo addition 外型加成Diels-Alder reaction 第尔斯-尔德反应Retro Diels-Alder reaction 逆第尔斯-阿尔德反应Ene synthesis 单烯合成Anionic cycloaddition 负离子环加成Dipolar addition 偶极加成- elimination -消除- elimination -消除- elimination -消除-elimination -消除Dehydrohalogenation 脱卤化氢Deamination 脱氨基Pyrolytic elimination 热解消除Elimination-addition 消除-加成Decarboxylation 脱羧Decarboxamidation 脱酰胺Decyanation 脱氰基Alkylolysis,alkyl cleavage 烷基裂解Acylolysis,acyl cleavage 酰基裂解Flash pyrolysis 闪热裂Fragmentation 碎裂Chiletropic reaction 螯键反应Chelation 螯环化Esterification 酯化Transesterification 酯交换Saponification 皂化Alcoholysis 醇解Ethanolysis 乙醇解Cyanomethylation 氰甲基化Aminomethylation 氨甲基化Hydroxymethylation 羟甲基化Hydroxyalkylation 羟烷基化Cholromethylation 氯甲基化Haloalkylation 卤烷基化Transacetalation 缩醛交换Enolization 烯醇化Haloform reaction 卤仿反应Condensation 缩合Aldol condensation 羟醛缩合Cross aldol condensation 交叉羟醛缩合Retrograde aldol condensation 逆羟醛缩合Acyloin condensation 偶姻缩合Cyclization 环化Annulation,annelation 增环反应Spiroannulation 螺增环Autoxidation 自氧化Allylic hydroperoxylation 烯丙型氢过氧化Epoxidation 环氧化Oxonolysis 臭氧解Electrochemical oxidation 电化学氧化Oxidative decarboxylation 氧化脱羧Aromatization 芳构化Catalytic hydrogenation 催化氢化Heterogeneous hydrogenation 多相氢化Homogeneous hydrogenation 均相氢化Catalytic dehydrogenation 催化脱氢Transfer hydrogenation 转移氢化Hydrogenolysis 氢解Dissolving metal reduction 溶解金属还原Single electron transfer 单电子转移Bimolecular reduction 双分子还原Electrochemical reduction 电化学还原Reductive alkylation 还原烷基化Reductive acylation 还原酰化Reductive dimerization 还原二聚Deoxygenation 脱氧Desulfurization 脱硫Deselenization 脱硒Mitallation 金属化Lithiation 锂化Hydrometallation 氢金属化Mercuration 汞化Oxymercuration 羟汞化Aminomercuration 氨汞化Abstraction 夺取[反应] Internal abstraction 内夺取[反应] Rearrangement 重排Prototropic rearrangement 质了转移重排Double bond migration 双键移位Allylic migration 烯丙型重排Allylic migration 烯丙型迁移Ring contraction 环缩小[反应] Ring expansion,ring enlargement 扩环[反应]-ketol rearrangement -酮醇重排Pinacol rearrangement 频哪醇重排Retropinacol rearrangement 逆频哪醇重排Semipinacol rearrangement 半频哪醇重排Benzilic rearrangement 二苯乙醇酸重排Acyl rearrangement 酰基重排Migratory aptitude 迁移倾向Transannular insertion 跨环插入Transannular rearrangement 跨环重排Migration 迁移Prototropy 质子转移Cationotropic rearrangement 正离子转移重排Anionotropy 负离子转移Anionotropic rearrangement 负离子转移重排Sigmatropic rearrangement -迁移重排Homosigmatropic rearrangement 同迁移重排Electrophilic rearrangement 亲电重排Photosensitization 光敏化Forbidden transition 禁阻跃迁photooxidation 光氧化Photoisomerization 光异构化Photochemical rearrangement 光化学重排2.4 有机化合物类名Aliphatic compound 脂肪族化合物Hpdrocarbon 碳氢化合物Alkane 烷Wax 蜡Paraffin wax 石蜡Alkene 烯Alkyen 炔Acetylide 炔化物Active hydrogen compounds 活泼氢化合物Carbon acid 碳氢酸Super acid 超酸Diene 双烯Triene 三烯Allene 丙二烯Ccumulene 累积多烯Enyne 烯炔Diyne 二炔Alkyl halide 卤代烷Alcohol 醇Homoallylic alcohol 高烯丙醇Ether 醚Epoxide 环氧化物Cellosolve 溶纤剂Crown ether 冠醚Netro compound 硝基化合物Amine 胺Quaternaryammonium com-pound 季铵化合物Amine oxide 氧化胺Diazoalkane 重氮烷Mercaptan 硫醇Sulfonic acid 磺酸Sulfoxide 亚砜Sulfone 砜Aldehyde 醛Detone 酮Aldehyde hydrate 醛水合物Ketone hydrate 酮水合物Hemiacetal 半缩醛Acetal 缩醛Ketal 缩酮Dithiane 二噻烷Aminal 缩醛胺imine 亚胺Aldimine 醛亚胺Oxime 肟Aldimine 醛肟Oxime 亚硝基化合物aldoxime 硝酮Hydrazone 腙Azine 嗪Semicarbazone 缩氯基脲Cyanohydrin 羟腈Pinacol 频哪醇Enol 烯醇Enol ether 烯醇醚Enol ester 烯醇酯Enamine 烯胺Ynamine 炔胺Mannich base 曼尼希碱Carboxylic acid 羧酸Ester 酯orthoester 原酸酯Acyl halide 酰卤Acyl fluoride 酰氟Acyl chloride 酰氯Acyl rtomide 酰溴Acyl iodide 酰碘Carbobenzoxy chloride 苄氧甲酰氯Acyl tosylate 酰基对甲苯磺酸酐Ketene 乙烯酮Peracid 过酸Perester 过酸酯Acyl peroxide 酰基过氧化物Nitrile 腈Nitrile oxide 氧化腈Isonitrile 异腈Amide 酰胺Imide 二酰亚胺N-bromo compound N-溴化物Hydrazide 酰肼Acyl azide 酰叠氮Amidine 脒Keto ester 酮酸酯Acyl cyanide 酰腈Carbon suboxide 二氧化三碳Glycidic acid 环氧丙酸Carbammic acid 氨基甲酸Carbamate 氨基甲酸酯Urea 脲Cyanamide 氨腈Carbodiimide 碳二亚胺Allophanate 脲基甲酸酯Thioester 硫代酸酯Thiol acid 硫羰酸Lactone 内酯Lactol 内半缩醛Macrolide 大环内酯Amino acid 氨基酸Zwitterions 两性离子Inner salt 内盐Betaine 甜菜碱Lactam 内酰胺Hydantion 乙内酰脲Peptide 肽Glycol 二醇Aldol 羟醛Acyloin 偶姻Carbohydrate 碳水化合物Aldose 醛糖Ketose 酮糖Furanose 呋喃糖Pyranose 吡喃糖Glycoside 糖苷Glucoside 葡[萄]糖苷Aglycon 苷元Saccharide 糖类Oligosaccharide 寡糖Polysaccharide 多糖Alditol 糖醇Osazone 脎Alicyclic compound 脂环化合物Cycloalkene 环烷Spirane 环烯Cage compound 螺烷Propellane 笼型化合物Rotazane 螺桨烷Catenane 轮烷Rused ring 索烃Aromatic compound 稠环化合物Arene 芳香化合物Alkylbenzene 芳烃Bibenzyl 烷基苯Aiaryl 联苄Biphenyl 联芳Biphenyl 联苯Indene 茚Fluorene 芴fulvene 富烯cyclophane 环芳Acene 并苯Helicene 螺旋烃Aryne 芳炔Annulene 烨烯Dewar benzene 杜瓦苯Benzvalene 盆苯Barrelene 桶烯Azulene ?Diazo compound 重氮化合物diazonium salt 重氮盐Diazohydroxide 重氮氢氧化物Azo cimpound 偶氮化物Hydrazo compound 氢化偶氮化物Azoxy compound 氧化偶氮化合物Phenol 酚Hydroquinone 氢醌Quinhydrone 醌Quinhydrone 醌氢醌Semiquinone 半醌Benzoin 苯偶姻Benzil 偶苯酰Heterocyclic compound 杂环化合物Furan 呋喃Pyrrole 吡咯Thiophene 噻吩Porphyrin 卟啉Pyridene 吡啶Piperidine 哌啶Oxazole ?唑Azlactone 二氢?唑酮Pyrazole 吡唑Imidazole 咪唑Thiazole 噻唑Oxazine ?嗪Diazine 二嗪Diketopiperazine 哌嗪二酮Sydnone 悉尼酮Triazole 三唑Triazine 三嗪Indole 吲哚Quinoline 喹啉Isoquinoline 异喹啉Flavone 黄酮Isoflavone 异黄酮Chalcone 查耳酮Azepine 氮杂?Addition compound 加合化合物Organometallic 有机金属化合物Grignard reagent 格氏试剂Ferocene 二?铁Sandwich compound 夹心化合物Chloroborane 氯硼烷Phosphine 膦Phosphonium salt ?盐Arsine 胂Ylide 叶立德Nitrogen ylide 氮叶立德Sulfur ylide 硫叶立德Phosphorus ylide 磷叶立德Arsenic ylide 砷叶立德Lipid 类脂Phospholipid 磷脂Essential oil 精油Terpene 萜Monoterpene 单萜Sesquiterpene 倍半萜Diterpene 二萜Triterpene 三萜carotene 胡萝卜素Steroid 甾族化合物Sex hormone 性激素Pheromone 信息素Phytohormone 植物激素Alkaloid 生物碱2.5 有机化学分析和方法Charge-transfer spectrum 电荷转移光谱Chemical shift reagent 化学位移试剂Polarized light 偏振光Specific rotation 比旋光Molar rotation 摩尔旋光Circularly polarized light 圆偏振光Optical rotatory dispersion 旋光色散Circular dichroism 圆二色性Octant rule 八区规则Cotton effect 卡滕效应Plain curve 平坦曲线Resolution 拆分Optical purity 光学纯度Enantiomeric excess,ee 对映体过量Diasteromeric excess,de 非对映体过量Synthesis 合成Retrosynthesis 逆合成Total synthesis 全合成Formal synthesis 中继合成Partial synthesis 部分合成Relay synthesis 接替合成Tandem reaction sequence 连续反应过程Synthon 合成子Chiron,chiral building block 手性子Asymmetric synthesis 不对称合成Asymmetric induction 不对称诱导Optical induction 光学诱导Chiral induction 手性诱导Chiral reagent 手性试剂Chiral catalyst 手性催化剂Chiral solvent 手性溶剂Chiral auxiliary [reagent] 手性助剂Topochemistry 拓扑化学Biomimetic synthesis 仿生合成Protecting group 保护基Umpolung 极反转Linear synthesis 线性合成Convergent synthesis 汇集合成03.分析化学03.1 一般术语Qualitative analysis 定性分析Quantitative analysis 定量分析Chemical analysis 化学分析Instrumental analysis 仪器分析Classical analysis 经典分析Systematic analysis 系统分析Routine analysis 常规分析Referee analysis,arbitration ana-lysis 仲裁分析Macro analysis 常量分析Semimicro analysis,meso analysis 半微量分析Mcro analysis 微量分析Ultramicro analyisis,submicro analysis 超微量分析Trace analysis 痕量分析Ultratrace analysis 超痕量分析Wet method,wet way 湿法Dry mithod,dry way 干法Indirect mithod 间接法Reagent 试剂Reagent grade 试剂级别Guaranteed reagent,G.R. 保证试剂Analytical reagent,A.R. 分析纯Chimically pure,C.P. 化学纯Identification 鉴定Detection 检出Confirmatory test 证实试验Determination 测定Measurement 测量Separation 分离Calibration 校准Correction 校正Recovery 回收Mesh [筛]目Sampling 取样Quartering 四分[法] Sample 试样Reference material,RM 标准物质Primary standard 一级标准Secondary standard 二级标准Selectivity 选择性Selective reagent 选择[性]试剂Specific reagent 特效试剂Mole 摩尔Stock solution 储备溶液Test solution 试液Fusion 熔融Rlux 熔剂Air drying 风干Weighing 称量Constant weight 恒量Aliquot 等分部分Residue 残渣Ash 灰分Misture content 含湿量Cleaning solution 洗涤液Mauor constituent 主成分Minor constituent 少量成分Trace constituent 痕量成分Trial-and error method 尝试法Analytical balance 分析天平Single pan balance 单盘天平Air-damped balance [空气]阻尼天平Electronic balance 电子天平Semimicro [analytical]balance 半微量天平Micro[analytical]balance 微量天平Ultramicro[analytical ]balance 超微量天平Torsion balance 扭力天平Weights 砝码Rider 游码Filter paper 滤纸Test paper 试纸PH paper PH试纸Erlenmeyer flask 锥形瓶V olumetric flask [容]量瓶Weighing bottle 称量瓶Buchner funnel 布氏漏斗Sintered-glass filter crucible [烧结]玻璃砂[滤]?锅Iven,drying over 烘箱Water bath 水浴Hot plate 电热板Magnetic stirrer 洗瓶Iodine flask [电]磁搅拌器Iodine flaski 碘瓶Reagent bottle 试剂瓶03.2 化学计量学Chemometrics 化学计量学Accuracy 准确度Sensitivity 灵敏度Precision 精密度Repeatability 重复性Reproducibility 再现性Detection limit 检出限Determination limit 测定限Signal-noise ratio 信噪比Background 背景Blank 空白Uncertainty 不确定度Tolerance limitc 容许限Confidence limit 置信限Confidence interval 置信区间Confidence coefficient 置信系数Population 总体Sample 样本individual 个体Random variable 随机变量Fixed variable 固定变量Standardization 标准化Friquency 频数Histogram 直方图Frequency distribution 频数分布Class interval 组距Probability 概率Probability density 概率密度Nirmal distribution 正态分布Nonnormal distribution,abnormal distribution 非正态分布Log transformation 对数变换Normalization 正态化F-distribution F分布t-distribution T分布X2-distribution X2分布Binomial distribution 二项式分布Poisson’s distribution 泊松分布Uniform distritution 均匀平布True value 真值Value of expectation 期望值Observed value,measured value 观测值Unbiased estimator 无偏估计值Sample value 样本值Population mena 总体[平] 均值Sample mean 样本[平]均植Veighted mean 加权[平]均植Median 中位值Variability 变异性Variation within laboratory 组内变异性Variation between alboratories 组间变异性Error 误差Random error 随机误差Systematical error 系统误差Bias 偏倚Gross 过失误差Absolute error 绝对误差Relative error 相对误差Standard eror 标准误差Deviation 偏差Residual 残差Population deviation 总体偏差Sample deviation 样本偏差Arithmetic average deviation [算术]平均偏差Standardard deviation 标准[偏]差Absolute deviation 绝对偏差Relative deviation 相对偏差Relative standard deviation 相对标准[偏]差Pooled standard deviation 合并标准[偏]差Tolerance error 容许误差Variance 方差Population Variance 总体方差Sample variance 样本方差Pooled variance 合并方差Variance within laboratory 组内方差Variance between laboratories 组间方差Residual variance 残余方差Covariance 协方差Range 极差Statistical test 统计检验Hypothesis Test 假设检测Significance test 显著性检验Significance level 显著性水平Significant difference 显著性差异One-tailed test 单侧检验Two-atailed test 双侧检验Test statistic 检验统计量Parameter test 参数检验Nonparameter test 非参数检验Parameter estimation 参数估计Point estimation 点估计Interval estimation 区间估计Null hypothesis 零假设Alternative hypothesis 备择假设Critical value 临界值Acceptance region 接受域Rejection region 舍弃域Statistical inference 统计推断Error of the first kind,type 1error 第一类错误Error of the second kind,type 2error 第二类错误Extremum value 极值Outlier 异常值Sing test 符号检验Dixon’s test method 狄克松检验法Grubbs’test method 格鲁布斯检验法Cochrane’s test method 柯奇拉检验法t-test T检验F-test F检验X2-test,chi-square test X2检验Homoscedasticity,homogeneity of variance 方差齐性Sum of squares of residues,resi dual sum of残差平方和squaresRegression sum of squares 回归平方和Additivity of sum of squares 平方和加和性Analysis of variance,ANOV A 方差分析Cross classification 交叉分组Multiple comparisons 多重比较Paired comparison 成对比较Random factor 随机因素Fixed factor 固定因素Controllable factor 可控因素Level of factor 因素水平Pseudo leval 拟水平Factorial effect 因素效应Main effect 主效应Two-factor interaction,simple interaction 二因子交互效应Positive correlation 正相关Negative correlation 负相关Correlation test 相关性检验Correlation analysis 相关分析Correlation coefficient 相关系数Total correlationCorrelation 全相关系数Partial correlation coefficient 偏相关系数Regression analysis 回归分析Curve fitting 曲线拟合Least square fitting 最小二乘法拟合Weighted least square method 加权最小二乘法Goodness of fit 拟合优度Regression equation 回归方程Regression curve 回归曲线Regression surface 回归曲面Regression coefficient 回归系数Partial regression coefficient 偏回归系数Standar4dized regression coeffi-cient 标准回归系数Linear regression 线性回归Non-linear regression 非线性回归Stepwise regression 逐步回归Weighted regression 加权回归Polynomial regression 多项式回归Parallel displacement of curve 曲线平移Calibration curve 校正曲线Linearity range 线性范围Experimental design 实验设计Randomized blie 随机区组设计Factorial experiment 析因实验Latin square design 拉丁方设计Orthogonal table,orthogonal layout 正交表Homogeneous design 均匀设计Simplex oqtimization 单纯形优化Simple simplex 基本单纯形Modified simplex 改进单纯形Step sixe,step width 步长Variable step size 可变步长Reflection 反射Expansion 扩展Whole contraction 整体收缩Optimal estimate 量优估计Optimal value 最优值Optimal block design 最优区组设计Local optimization 局部优化Constrained optimization 有约束优化Constrained condition 约束条件Sequential search 序贯寻优Gradient search 梯度寻优Steepest ascent 最速上升法Steepest descent 最速下降法Holeen cut method 黄金分割法Mimimum residual method 最小残差法Iterative method 迭代法Recurrence method 递推法Successive approximate method 逐次近似法Monte Carlo method 蒙特卡罗法Quality control 质量控制Control chart 控制图Central line,CL 中心线X-control chart 平均值控制图R-control chart 极差控制图Upper alarm limit 上警告限Lower alarm limit 下警告限Upper control limit,UCL 下控制限Lower control limit,LCL 下控制限Rankom sampling 随机抽样Proportional sampling 比例抽样Systematic sampling 系统抽样Sequential sampling 序贯抽样Sequential sampling 序贯分析Sampling inspection 抽样检验Sample size,sample capacity 样本[容]量Random sample 随机样本Randomization 随机化Raw data 原始数据Coded data 编码数据Array 数组Data handling,data processing 数据处理Flow chart,flow diagram 程序框图Significant figrue 有效数字Rounding off method 修约方法Round-off error 修约误差Cluster analysis 聚类分析Discriminant analysis 判别分析Factor analysis 因子分析Generalized standard addition 广义标准加入法Method 模式识别Pattern recognition 矩阵Correlation matrix 相关矩阵Eigenvector 特征向量Eigenvalue 特征值Information 信息Information content 信息容量Information efficiency 信息效率Information profitability 信息效益Specific information price 信息比价0.33 化学分析Gravimetry,gravimetric analysis 重量分析法Titrimetry,titrimetric analysis 滴定[分析]法Titration 滴定Visual titration 目视滴定[法] Stepwise titration 分步滴定[法]Back titration 返滴定[法] Replacement titration 置换滴定[法]Linear titration 线性滴定[法] Logarithmic titration 对数滴定[法]Non-aqueous titration 非水滴定[法] Aquametry 测水[滴定]法Karl Fischer titration 卡尔·费歇尔滴定[法] Acid-base titration 酸碱滴定[法] Acidimetry 酸量法Alkalimetry 碱量法Precipitation titration 沉淀滴定[法] Compleximetry,complexometry,complexometric络合滴定[法] ietrationChil[at]ometry,chel[at]ometric 螯合商定[法]Redox titration 氧化还原滴定[法] Mohr mithod 莫尔法V olhard method 福尔哈德法Fajans method 法扬斯法Clear point 澄清点Argentimetry 银量法Mercurimetry 汞量法Cyanometric titration 氰量法Permanganate titration 高猛酸钾[滴定]法Dichromate titration 重铬酸钾[滴定]法Cerimetry,cerimetric titration 铈(IN)量法Iodimetry,iodometry 碘量法Bromometry 溴量法Priodate titration 高碘酸钾[滴]法Thermometric 热滴定[法] Thermometric titration 气体分析Elemental analysis 元素分析Flow in jection analysis,FIA 流动注射分析Vilatilization method,evolution method 挥发法Kjedahl determination 凯氏定氮法Automatic titration 自动滴定Ringoven method 环炉法Drop method 点滴法Spot test 斑点试验Brown ting test 棕环试验Blowpipe test 吹管试验Borax-bead test 吹管试验Borax-bead test 硼砂珠试验Flame test 焰色试验Bead test 熔珠试验Marsh test 马什试验Gutzeit test 古蔡试验Griess test 格里斯试验Silver mirror test 银镜试验Iodoform test 碘仿试验Organic reagent 有机试剂Eriochrome cyanine R 铬花青R Chromotropic acid 变色酸Diantipyrylmethane,4,4’-dianti-pyrinylmethane 二安替比林甲烷Diphenylcar bazide 二苯卡巴肼Diphenylcarbazone 二苯卡巴脘dithizone 二硫腙Cadion 镉试剂Chrome azurol S 铬天青S Chlorosulfophenol s 氯磺酚S2,2’-biquinoline,biquinolyl 联喹啉2,2’-bipyridine,2,2’-bipyridyl 联吡啶brilliant green 亮绿Chloranilic acid 氯冉酸Aluminon 铝试剂Arsenazo Ⅰ偶氮胂ⅠArsenazo Ⅲ偶氮胂ⅢChlorophosphonazo Ⅲ偶氮氯膦ⅢAlizarin 茜素Alizarin complexan,alizarin 茜素红S Neocupferron 新铜铁试剂Neocuproine 新亚铜试剂Bromopyrogallol red 溴[代]邻苯三酚红Cuproine 亚铜试剂Acetylacetone 乙酰丙铜Nessler reagent 奈斯勒试剂Organic precipitant 有机沉淀剂Arsonic acid 胂酸-benzoinoxime -安息香肟Benzotriazole 苯并三唑Tannin,tannic acid 单宁Isatinoxime 靛红肟Mandelic acid 苦杏仁酸8-quinolinecarboxylic acid,8-carboxyquinoline 8-喹啉羧酸Quinaldic acid 喹哪啶酸Benzidine 联苯胺Pyrogallol 连苯三酚Anthranilic acid 邻氨基苯甲酸5,6-naphthoquinoline 5,6-萘喹啉Tetraphenylarsonium chloride 氯化四苯砷8-hydroxyquinoline,oxine 8-羟基喹啉Metcaptobenzothiazole 巯基喹啉8-mercaptoquinoline 8-巯基喹啉Salicylaldoxime 四苯硼钠水杨醛肟Sodium tertraphenylborate,sodium tetraphenylboronCupferron 铜铁试剂Nitron 硝酸试剂Cinchonine 辛可宁1-nitroso-2-naphthol 1-亚硝基-2-萘酚N-N-benzoyl-N-phenyl hydroxylamine N-苯甲酰-N-苯基羟胺Diacetyldioxime,dimethylg-lyoxime 丁二酮肟Indicator 指示剂Acid-base indicator 酸碱指示剂Adsorption indicator 吸附指示剂Metal indicator ,metallochromic indicator 金属指示剂Oxidation-reduction indicator,redox indicator 氧化还原指示剂Mixed indicator 混合指示剂External indicator 外[用]指示剂Fluorescent indicator 荧光指示剂Metalfluorescent indicator 金属荧光指示剂Chemiluminescent indicator 化学发光指示剂Siloxene indicator 硅氧烯指示剂Litmus paper 石蕊试纸Turmeric paper 姜黄试纸Indicator constant 指示剂常数Indicator constant 指示剂空白Thymol blue,thymolsulfonphthal-ein 百里酚蓝Thymolphthalein 百里酚酞Phenol red ,phenolsulfonphthalein [苯]酚红Phenolphthalein 酚酞Cresol purple 甲酚紫Methyl orange 甲基橙Methyl red 甲基红Methyl yellow 甲基黄Chlorophenol red 氯酚红Alizarin yellow 茜素黄Bromothymol blue 溴百里酚蓝Btomophenol blue 溴酚蓝Btomocresol green 溴甲酚绿Neutral red 中性红Methyl violet 甲基紫Crystal violet 结晶紫Quinaldine red 喹哪啶红Malachite green 孔?[石]绿Nile blue A 尼罗蓝A Orange IV [酸性]四号橙p-ethoxychrysoidine 对乙氧基菊橙Diphenylamine blue 二苯胺蓝Dichlorofluorescein 二氯荧光黄Phenosafranine 酚藏花红Congo red 刚果红Rhodamine 6G 罗丹明6G Rose Bengal 玫瑰红Eosin 曙红Thorin 钍试剂Fluorescein 荧光黄Xylenol orange 二甲酚橙Calmagite 钙黄绿素Calconcarboxylic acid 钙指示剂Calcon 钙试剂Eriochrome black A 铬黑A Eriochrome black T 铬黑T Eriochrome blue black B 铬蓝黑B Eriochrome blue black F 铬蓝黑R Eriochrome violet B 铬紫B Methylthymol blue 甲基百里酚蓝Metaqlphthalen 金属酞Pyrocatechol violet 邻苯二酚紫1-(2-pyridylazo)-2napthol, PAN 1-(2-吡啶基偶氮)-2-蔡酚4-(2-pyridylazo)resorcinol, PAN 4-(2-吡啶基偶氮)间苯二酚Zincon 锌试剂Murexide 紫脲酸铵Sulfosalicylic acld 磺基水杨酸Tiron 钛试剂Vaariamine blue 变胺蓝Indigo monosulfonate 靛蓝一磺酸盐Indigo tetrasulfonate 靛蓝四磺酸盐p-nitrodiphenylamine 对硝基二苯胺Sodium diphenylaminesulfonate 二苯胺磺酸钠Forroin 邻菲咯啉亚铁离子Nitroferroin 硝基邻菲咯啉亚铁离子Methylene blue 亚甲蓝Erioglaucine A 罂红AN-phenylanthranilic acid N-苯基邻氨基苯甲酸Complexone 氨羧络合剂Chelating reahent,chelant 螯合试剂Ethylenediamineterraacetic acid,EDTA 乙二胺四乙酸Nitrilotriacetic acid,NTA 氨三乙酸Cyclohexanediaminetetraacetic acid,CyDTA 环已二胺四乙酸Ethyleneglycolbis(2-aminoethyl-ether)tetraacetic acid,EGTA 乙二醇双(2-氨基乙醚)四乙酸2-hydroxyethylethylenediamine-triaceticacid,HEDTA2-羟乙基乙二胺三乙酸Solvent 溶剂Non-apueous solvent 非水溶剂N,N-dimethylformamide,DMF 二甲基甲酰胺Sulfolane 环丁砜Inert solvent 惰性溶剂Apolar aprotic solvent 非极性非质子溶剂Non-polar solvent 非极性溶剂Aprotic solvent 非质子溶剂Differentiating solvent 区分溶剂Protogenic solvent 给质子溶剂Polar solvent 极性溶剂Leveling solvent 拉平溶剂Vaterlike solvent 类水溶剂Ionixing solvent 离子化溶剂Ionogen 可离子化基团Amphiprotic 两性的Amphiprotic solvent 两性溶剂Ampholyte 两性物Dipolar aprotic solvent 偶极非质子溶剂Dipolar protophilic solvent 偶极亲质子溶剂Dipolar protophobic solvent 偶极疏质子溶剂Protophilic solvent 亲质子溶剂Solvated proton 溶剂化质子Protophobic solvent 疏质子溶剂Protic solvent 质子溶剂Protolyte 质子传递物Protolysis 质子传递作用Neutral solvent 中性溶剂Muffle furnace 马弗炉Blast burnet 喷灯Meker burner 麦克灯Bunsen burner 本生灯Tirril burner 提利灯Desiccator 干燥器Desiccant,drying agent 干燥剂Hygrostat 恒湿器Ascarite,soda asbestos 烧碱石棉Combustion tube 燃烧管Boat 舟皿Crucible 坩埚Gooch crucible 古氏坩埚Mortar 研钵Evaporating dish 蒸发皿Vatch glass 表面皿Buret 滴定管Weighing buret,weight buret 称重滴定管Pipet,transfer pipet 称液管Measuring pipet 吸量管Titration thief 滴定阱Spot plate 点滴板Ring oven 环炉Policeman 淀帚Analyte 分析物Dry basis 干基Wet basis 湿基Parallel determination,replicate 平行测定Duplicate 双份Triplicate 三份。
有机化学术语(中英文对照)

1.有机化合物的官能团和重要的基团官能团functional group双键double bond三键triple bond烃基hydroxy group琉基mercapto硫轻基sulfhydryl group羰基carbonyl group氨基amino group亚氨基imino group硝基nitro group亚硝基nitroso group氰基cyano group羧基carboxyl group磺基sulpho group烷基alkyl group苯基phenyl group卡基benzyl group芳基aryl group烯基allyl group烷氧基alkoxyl group酰基acyl group活性亚甲基active methylene group2.有机化合物的类型烃hydrocarbon石蜡paraffin脂肪烃aliphatic hydrocarbon烷烃alkane烯烃alkene炔烃alkyne共扼二烯烃conjugated diene脂环烃alicyclic hydrocarbon螺环化合物spiro compound桥环化合物bridged ring compound芳烃aromatic hydrocarbon非苯芳烃nonbenzenoid aromatic hydrocarbon 稠环芳烃condensed aromatics卤代烃halohydrocarbon醇alcohol酚phenol醚ether环氧化合物epoxide冠醚crown ether硫醇thiol硫酚thiophenol硫醚sulfide二硫化物disulfide亚磺酸sulfinic acid磺酸sulfonic acid 亚砜sulfoxide砜sulfone醛aldehyde酮ketone半缩醛hemiacetaI半缩酮hemiketal缩醛acetal缩酮ketal西佛碱shiff's base肟oxime腙hydrozone缩氨脲semicarbazoneα,β-不饱和酮α,β--unsaturated ketone 醌quinone羧酸carboxylic acid酰卤acid halide酸酐acid anhydride酯ester酰胺amide內酯lactone内酰胺lactam月青nitrile取代酸substituted acid羟基酸hydroxy acid醇酸alcoholic acid酚酸phenolic acid酮酸keto acidB-酮酸酯B-ketone ester乙酰乙酸乙醋ethyl acetoacetate亚硝基化合物nitroso compound硝基化合物njtro compound亚胺imine胺amine伯胺primary amine仲胺secondary amine叔胺tertiary amine季铵盐quaternary ammonium salt季铵碱quaternary ammonium hydroxide 重氮盐diazonium salt偶氮化合物azo compound胍guanidine氨基酸amino acid磷phosphine磷酸酯phosphate亚磷酸酯phosphite膦酸酯phosphonate膦酸phosphonic acid3.杂环化合物吡咯pyrrol呋喃furane噻吩thiophone吲哚indole卟吩porphine咪唑imidazole噻唑thioazole吡啶pyridine喹啉quinoline异喹啉isoquinoline吡喃鎓盐pyrylium salts 黄酮flavone嘧啶pirimidine嘌呤purine4.有机天然产物肽peptide多肽polypeptide核酸nucleic acid核苷nucleoside核苷酸nucleotide生物碱alkaloid碳水化合物carbohydrate单糖monosaccharide醛糖aldoses酮糖ketosesD-核糖ribose D-2-脱氧核糖deoxyribose 葡萄糖glucose果糖fructose糖脎osazone糖苷glucoside低聚糖oligosaccharide 麦芽糖maltose蔗糖sucrose纤维二糖cellobiose环糊精cyclodextrin多糖polysaccharide淀粉starch纤维素cellulose类脂lipid萜类化合物terpenoid甾族化合物steroid脂肪fat油oil脂肪酸fatty acid甘油三羧酸酯triglyceride磷脂phospholipid磷脂酸phosphalidic acid蜡wax5.有机化合物的结构理论价键理论valence-bond theory分子轨道理论molecular orbital theory 共振论resonance theory凯库勒式Kekule formula路易斯式Lewis formulaσ键σ bondπ键π bond键能bond energy键角bond angle键长bond Iength成键轨道bonding orbital反键轨道antibonding orbital最高已占轨道HOMO highest occupied molecular orbital 最低末占轨道LUMO lowest unoccupied molecular orbital 诱导效应inductive effect共轭效应conjugated effectπ,π-共轭π,π- conjugationp,π-共轭p,π- conjugation超共轭作用hyperconjugation离域能delocalization energy共振能resonance energy给电子基团electron donating group吸电子基团electron withdrawing group芳性aromaticity休克尔规律Huckel's rule两性离子Zwitterion6.有机化学中的同分异构异构体isomer构造constitution构型configuration构象conformation构造异构constitutional isomerism立体异构stereo isomerism构型异构configurational isomerism顺反异构cis-trans isomerism次序规则sequence ruIe同侧Zugammen Z异侧Entgegen E顺式cis反式trans对映异构enantiomerism = 光学异构旋光异构optical isomerism旋光性optical activity旋光度optical rotation比旋光度specific rotation对称面plane of symmetry对称中心center of symmetry对称轴axis of symmetry手性chirality手性分子chiral molecules对映异构体,对映体enantiomer 非对映体diastereomer外消旋体raceme左旋体leveisomer右旋体dextroisomer内消旋体mesomer费歇尔投影式Fischer projection相对构型relative configuration绝对构型absolute configurationR -构型R -configurationS -构型S -configuration赤式erythro苏式threo外消旋化racemization拆分resolution光学纯度Optical Purity对映体过量百分数enantiomeric excess立体专一性反应stereospecific reaction 立体选择性反应stereoselective reaction不对称合成asymmetric synthesis构象异构conformational isomerism构象分析conformational analysis锯架式perspective formula 纽曼投影式Newman projection formula椅式chair form船式boat form直立键a键axial bond 平伏键e键equatorial bond互变异构tautomerism酮式keto-form烯醇式enol-form差向异构化epimerization变旋现象mutamerism哈武斯式Haworth form7.有机反应的名称取代反应substitution reaction加成反应addition reaction马尔科夫尼可夫规律Markovnikov rule 共轭加成conjugate addition消去反应elemination reaction查依采夫规律Saytzeff rule霍夫曼规律Hofmann rule硼氢化反应hydroboration催化加氢catalytic hydrogenation 聚合反应polymerization单体monomer聚合物polymer硝化反应nitration卤化反应halogenation磺化反应sulfonation烷基化反应alkylation酰基化反应acylation酯化反应esterification酯交换反应transesterification脱羧反应decarboxylation 氯甲基化反应chloromethylation傅列德尔-克拉夫茨反应Friedel-Crafts reaction格利雅反应Grignard reaction 格利雅试剂(格氏试剂) Grignard reagent赖默-梯曼反应Reimer-Tiemann reaction 卤仿反应haloform reaction水解反应hydrolysis reaction醇解反应alcoholysis reaction氨解反应ammonolysisi reaction皂化saponification插烯作用vinylogy缩合condensation克莱森缩合Claisen condensation安息香缩合benzoin condensation羟醛缩合aldol condensation列弗尔马茨基反应Reformatsky reaction迈克尔反应Michael reaction诺文格尔反应Knoevenagel reaction加布里反应Gabriel reaction乙酰乙酸乙酯合成法acetoacetic ester synthesis 丙二酸酯合成法malonic ester synthesis 威廉逊合成法William Son synthesis海森堡试验Hinsberg test重氮化反应diazotization reaction偶联反应coupling reaction脱氨基反应deamination reaction维悌希反应Wittig reaction氧化反应oxidation reaction还原反应reduction reaction周环反应pericyclic reaction环加成反应cycloaddition reaction电环化反应electrocyclic reaction坎尼扎罗反应Cannizzaro reaction齐齐巴宾反应Chichibabin reaction狄尔斯-阿德尔反应Diels-alder reaction斐林试剂Fehling reagent托伦试剂Tollens reagent沃克还原Wolff-Kishner reduction罗森蒙德还原Rosenmund reduction克莱门森还原Clemmenson reduction考普重排Cope rearrangement霍夫曼重排Hofmann rearrangement嚬哪醇重排pinacol rearrangement弗里茨重排Fries rearrangement克莱森重排Claisen rearrangement二烯体diene亲二烯体dienophile分子轨道对称守恒原理conversation of orbital symmetry8.有机反应机理均裂homolytic异裂heterolytic活性中间体active intermediate碳正离子carbocation碳负离子carbanion烯醇负离子enolate anion自由基,游离基free radical卡宾,碳烯carbene氮烯nitrene速度决定步骤rate-determining step哈蒙特假定Hammond postulate能线图energy profile过渡状态transition state邻基参与neighboring group participation动力学控制kinetic control热力学控制thermodynamic control离去基团leaving group底物substrate亲电试剂electrofphile亲核试剂nucleophile亲电加成反应electrophilic addition亲电取代反应electrophilic substitution定位规律orientation rule亲核取代反应nucleophilic substitutionSN2 反应机理SN2 reaction mechanismSN1 反应机理SN1 reaction mechanism瓦尔登转化Walden inversion亲核加成反应nucleophilic addition亲核加成-消去反应nucleophilic addition-elimination reaction 消去反应机理elimination reaction mechanismE1 反应机理E1 reaction mechanismE2 反应机理E2 reaction mechanism反式消去anti elimination重排反应机理rearrangement reaction mechanism 自由基反应free radical reaction链引发chain initation链增长chain propagation链终止chain termination9.有机化合物的光谱红外光谱IR Infrared spectra傅立叶变换Fourier Transform指纹区finger print region吸收频率absorption frequency紫外光谱UV Ultraviolet spectra电子跃迁elctronic transition吸光度absorbance摩尔消光系数molar extinction coefficient发色团chromophore助色团auxochrome核磁共振NMR Nuclear Magnetic Resonance1HNMR 谱1HNMR spectra13CNMR 谱13CNMR spectra屏蔽效应shielding effect化学位移chemical shift自旋偶合spin-spin coupling自旋裂分spin-spin splitting偶合常数coupling constant质子去偶proton spin decoupling 质子偏共振去偶proton off-resonance decoupling质谱Mass Spectra(MS)电子流轰击election impact (EI)快原子轰击fast atom bombarment (FAB)分子离子峰molecular ion peak同位素峰isotopic peak基峰base peak质荷比(m/z) mass-to-charge ratio10.分子间作用力氢键hydrogen bond色散力dispersion force 范德华力Van Der Waals force 偶极-偶极作用力dipole-dipole interraction force11.物理性质熔点melting point沸点boiling point密度density溶解度solubility偶极矩dipole moment12.有机化合物的酸碱性酸性acidity碱性basicity<HTML>本站材料仅为本院教师与学生教学所用,请勿它用!</HTML>有机化合物编辑[yǒu jīhuàhéwù]有机化合物主要由氢元素、碳元素组成,含碳的化合物,但是不包括一氧化碳、二氧化碳和以碳酸根结尾的物质。
高等有机习题(答案)

高等有机化学习题参考答案(注:本答案仅供参考,错误和不足之处敬请指正)Chapter 1 Effect of Substituted in Organic molecule1. 试判断下列各对基团,那一个具有强的-I 效应(即强的吸电子诱导效应): 答案:(1) -COOH > -COO -(2) C HN O CH 3<C H N N(CH 3)2CH 3+(3) C OCH 3 > C CH 2CH 3, (4) SO 2H <SO 3H 1(5) OCH 3 > SCH 3 (6) C H C H CH 3 <C C CH 3(7) N (CH 3)2 > P(CH 3)2(8) Si(CH 3)3 <Si(CH 3)2(9)N(CH 3)3+>NH 2 (10) CN > CH 2NH 2 (11) SiCH 3 <Cl(12) C C CH 3 , >C H C H CH 3与(6)同(13)>(14) NO 2>NO 2(15)O 2SCH 3<O 2SBr2. 指出下列各对酸中哪一个酸性强 答案:(1) H 3NCH 2CH 2COOH > HOCH 2CH 2COOH (2) HC C COOH >H 2C C H COOH(3) C 6H 5COCH 2COOH > C 6H 5CHOHCH 2COOH(4)C 4H 9CHCOOCOOH n<CCCH 3CH 3CH 3CH 3COOHOOC分子内氢键(5)COOHOH > COOH OH分子内氢键(6) BrCH 2CH 2COOH < CH 3CHBrCOOH(7)(H 3C)2CH 2CHCOOH>H 2CC HCH 2COOH(8) HC C COOH > SCOOH(9) CH 2(COOH)2 >HOOCH C COOCl(10) CH 3OCH 2CH 2COOH < CH 3SCH 2CH 2COOH 分子内氢键 (11) CH 3SCH 2COOH < CH 3SO 2COOH(12)OHCCOOH>COOHCHO(13)OHC(CH 3)3(H 3C)3C<OHC(CH 3)3CH 3(H 3C)3C(14) H 3COCOOH >OCH 3(15) C 6H 5CH 2SeH < H 3CSeH3. 预料以下各对化合物,何者具有更强的酸性? 答案:(1) CH 3NO 2 < (CH 3)2CHNO 2 (互变为酸式时的稳定性比较) (2) CH 2(SO 2C 6H 5)2 > CH 2(SOC 6H 5)2 (3)H 3CCH(C 6H 5)2> (C 6H 5)2CHCH 2C 6H 5(4) CH 3COCH 2COOCH 3 > CH 3COCH 2CONH 2(5) CH 3COCH 2COCH 2F < CH 3COCHFCOCH 3(6)NOCH 3<NO H 3C(7)SO 2O 2S >OO(8) NO 2CH 3CH 3H 3C >NO 2CH 3H 3CCH 3(场效应)(9)CH3>CH3(10) CH(C 6H 5)2< CH(C 6H 5)2(11) (CH 3)2Se < (CH 3)2O(12) <4. 解释以下现象:(1). 杯烯 (Calcene) 的偶极距很大,μ= 5.6 D.(答案:同时满足两个环的4n+2规则)(2). 吡咯 μ = 1.80 D ,吡啶 μ = 2.25 D ,且极性相反,如图:NN H(答案:孤对电子的排列方向)5. 比较下列化合物的碱性的强弱:NN(CH 3)2N(C 2H 5)2NH 2N答案:1>4>3>2>56. 9,10-二氢蒽-1-羧酸(A )和9,10-乙撑蒽-1-羧酸(B )的酸性取决于8-位上取代基X的性质。
碳负离子

(有机化合物中碳断开一个共价键得到相连原子的共用电子而形成的离子)
O R-CH2 C 碱性条件 OH直接与 -H
5/30/2015
8
The Typical Reaction of Carbanion–缩合反应
O O NaOC2H5 + R1-C-OR' R2-CH2-C-OR'
O O R1-C-CH-C-OR' R2
O X NaOC2H5 + R-C-R'(H) R"-CH-COOC2H5
O R-C C COOC2H5 R'(H) R"
Cannizzarro反应 不含-H的醛在浓碱作用下一半被还原为醇,另一半被氧 化为酸的反应,也称醛的歧化反应。
R2 R2 R1 C-CHO + R1 C-CHO R3 R3 浓OH
-
R2
R2
R1 C-CH2OH + R1 C-COO R3 R3
安息香缩合 芳香醛在CN-催化下二聚为-羟基酮的反应
R R'(H) C=O + R1 R2 CH-Pph3 X phLi R R'(H) C=C R1 R2 + ph3P=O
5/30/2015
11
The Typical Reaction of Carbanion-形成双键
Michael 加成 含活泼亚甲基的化合物在弱酸(碱)作用下形成的负碳离子 与,-不饱和醛(酮)的1,4-加成
物理有机化学 (浙江大学 ) 第7章 消去反应

Carbanion Mechanisms, the ElcB 如果与E1相反,不是有一个好的离去基,而是有一个酸性较大 的β-氢,就会先消除一个氢,再丢掉离去基。这就是碳负离子的 机理。根据反应速率的不同,以及所生成离子的溶剂化程度的 不同, E1cB机理有几种不同的类型。
Then the orientation of the double bond in the product depends more on the relative acidity of the two kinds of protons than on the relative stabilities of the possible double bonds. In the β-methyl protons are more acidic than the β-methylene ones and thus the more basic the counter-ion, the more terminal olefin results.
但如果碳正离子是处于紧密离子对中时,就可能不遵守Saytzeff 规则。例如,在醋酸中从下列化合物消除HX得到的产物是
X CH3 C CH2CH3 Ph AcOH CH3 C Ph C CH3 H
+
CH3 Ph
H C C
cis
trans
CH3
+ H2C
C Ph
CH2CH3
terminal
X Cl
%cis 68
烯烃mol%
X in t-Bu-X H2O 75°C
烯烃mol%
C2H5OH 75°C
烯烃mol%
CH3COOH 75°C
医学三级英语考试

一、选择题1.Which of the following is the primary function of the heart?A.To filter bloodB.To pump blood throughout the body(答案)C.To store oxygenD.To produce red blood cells2.What is the term used to describe the abnormal growth of cells that can invade andspread to other parts of the body?A.InfectionB.InflammationC.Cancer(答案)D.Allergy3.Which vitamin is essential for bone health and is primarily obtained from sunlight?A.Vitamin AB.Vitamin CC.Vitamin D(答案)D.Vitamin E4.The process of breaking down food into smaller, absorbable molecules is known as:A.Digestion(答案)B.MetabolismC.RespirationD.Circulation5.Which hormone is responsible for regulating blood sugar levels in the body?A.Insulin(答案)B.ThyroxineC.AdrenalineD.Estradiol6.The term used to describe the condition where the body's immune system attacks itsown tissues is:A.Autoimmune disease(答案)B.Allergic reactionC.Infectious diseaseD.Genetic disorder7.Which part of the brain is responsible for coordinating and controlling musclemovements?A.CerebrumB.Cerebellum(答案)C.Medulla oblongataD.Pons8.The process by which the kidneys filter blood, remove waste products, and produceurine is called:A.DialysisB.FiltrationC.UrinationD.Renal function(答案)。
关于兴趣实验的作文英语

When it comes to writing an essay about an interest experiment in English,its important to follow a clear structure and provide detailed descriptions of the experiment and its outcomes.Heres an example of how you might structure such an essay:Title:The Fascination of Chemical Reactions:An Experiment with Baking Soda and VinegarIntroduction:Hook the reader with a captivating statement about the wonders of science.Briefly introduce the topic of the experiment:a chemical reaction between baking soda and vinegar.Body Paragraph1:The Purpose of the ExperimentExplain the scientific curiosity that led to the experiment.Describe the hypothesis or expected outcome of the experiment.Body Paragraph2:Materials and MethodsList the materials used for the experiment,such as baking soda,vinegar,a measuring spoon,a balloon,and a funnel.Describe the stepbystep process of conducting the experiment,ensuring clarity and precision.Body Paragraph3:Observations and ResultsDetail the observations made during the experiment,such as the initial mixing of ingredients,the reaction,and any changes in the materials.Discuss the results of the experiment,including whether the hypothesis was confirmed or refuted.Body Paragraph4:Analysis and ConclusionAnalyze the results in the context of scientific principles,such as the release of carbon dioxide gas in the reaction.Reflect on the implications of the experiment,such as its relevance to everyday life or further scientific understanding.Conclusion:Summarize the key findings of the experiment.Reiterate the fascination with chemical reactions and the importance of scientific exploration.Heres a sample excerpt from the essay:The experiment began with a simple hypothesis:that the combination of baking soda and vinegar would produce a chemical reaction,resulting in the inflation of a balloon.Armed with a measuring spoon,a balloon,and a funnel,I carefully measured equal parts of baking soda and vinegar.As I poured the vinegar into the balloon,followed by the baking soda,I observed a rapid fizzing and bubbling.The balloon began to inflate,confirming my hypothesis and demonstrating the power of a chemical reaction.Remember to use clear and concise language,and to explain any scientific concepts in a way that is accessible to your readers.The goal is to convey your enthusiasm for the experiment and to share the knowledge you gained through the process.。
2-氨基-4甲基吡啶的设计合成

Design and Synthesis of2-Amino-4-methylpyridine Analogues as Inhibitors for Inducible Nitric Oxide Synthase and in Vivo Evaluation of [18F]6-(2-Fluoropropyl)-4-methyl-pyridin-2-amine asa Potential PET Tracer for Inducible Nitric Oxide SynthaseDong Zhou,†Hsiaoju Lee,†Justin M.Rothfuss,†Delphine L.Chen,†Datta E.Ponde,†,‡Michael J.Welch,†and Robert H.Mach*,†Di V ision of Radiological Sciences,Washington Uni V ersity School of Medicine,Campus Box 8225,510South Kingshighway Boule V ard,St.Louis,Missouri 63110Recei V ed December 9,2008A series of position-6substituted 2-amino-4-methylpyridine analogues was synthesized and compounds 9,18,and 20were identified as the inhibitors with the greatest potential to serve as PET tracers for imaging inducible nitric oxide synthase (iNOS).[18F]9was synthesized and evaluated in a mouse model oflipopolysaccharide (LPS)-induced iNOS activation.In vivo biodistribution studies of [18F]9indicate highertracer uptake in the lungs of the LPS-treated mice when compared to control mice.Tracer uptake at 60min postinjection was reduced in a blocking study using a known inhibitor of iNOS.The expression of iNOS was confirmed by Western blot analysis of lung samples from the LPS-treated mice.MicroPET studies also demonstrated accumulation of radiotracer in the lungs of the LPS-treated mice.Taken collectively,thesedata suggest that [18F]9shows favorable properties as a PET tracer to image iNOS activation with PET.IntroductionNitric oxide (NO a )is an important and unique mediator of avariety of physiological and pathological processes.1NO isgenerated from the oxidation of L -arginine to L -citrulline in a two-step process by nitric oxide synthase (NOS)enzymes.2Inthe NOS family,there are two constitutive isozymes of NOS,neuronal NOS (nNOS)and endothelial NOS (eNOS),and one inducible isozyme (iNOS).The three isozymes of NOS are expressed in different tissues to generate NO for specificphysiological roles.nNOS generates NO as a neurotransmitter and neuromodulator,mainly in brain and peripheral nerve cells;eNOS regulates blood pressure,primarily in vascular endothelial cells;3iNOS is induced by various inflammatory stimuli (e.g.,endotoxin)in activated macrophages and other types of cells and plays an crucial role in the host defense and the inflam-matory processes.Normally,the basal level of NO in all parts of the body is very low,mainly due to the constitutive nNOS and eNOS.In contrast,once expressed,iNOS can continue to generate NO in large amounts (up to µM concentrations)for a prolonged periodof time.4Studies have shown that production of NO by iNOS is implicated in a variety of acute and chronic inflammatory diseases (e.g.,sepsis,septic shock,vascular dysfunction in diabetes,asthma,arthritis,multiple sclerosis,and inflammato-ry diseases of the gut);5iNOS activity has also been found in many tumors.6Because of the central role of iNOS in NO-related diseases,numerous efforts have been made to developiNOS inhibitors as pharmaceuticals ranging from the nonselec-tive L -arginine analogues 7to the selective inhibitors reported recently.8Some inhibitors of iNOS have shown promising results in animal models of sepsis,lung inflammation,arthritis,and autoimmune diabetes.8c Therefore,the development of a radiolabeled iNOS inhibitor for probing iNOS expression in vivousing noninvasive positron emission tomography (PET)imaging will be of tremendous value to the study and treatment of NO-related diseases.PET is being used more frequently in clinical and researchstudies because of its high sensitivity,good spatial resolution,and ease in accurate quantification.Additionally,the absence of a physiologic effect from the radiotracers makes it a safe in vivo imaging tool.When short-lived positron-emitting radio-nuclides (18F t 1/2)109.8min and 11C t 1/2)20.4min)are incorporated into biologically active molecules (e.g.,iNOS inhibitors),they can be used as tracers that target those physiological pathways.2-Amino-4-methylpyridine (1)has been reported as a nonselective NOS inhibitor with good potency,9while the 6-substituted alkyl analogues of 1have slightlyimproved potency and selectivity over the parent compound;analogue 2has the best potency (IC 50against iNOS )28nM)(Chart 1).10Computational calculations suggest that the posi-tion-6is the most tolerant position to introduce a substitutent 11that would be suitable for radiolabeling with PET radionuclides 18F and 11C.In the past decade,the development of radiolabeled PET tracers for iNOS has been limited 12compared with the relatively rapid development of novel iNOS inhibitors as pharmaceuticals.In this paper,we describe the synthesis and screening of a series of position-6substituted 2-amino-4-methylpyridine analogues*To whom correspondence should be addressed.Phone:(314)362-8538.Fax:(314)362-0039.E-mail:rhmach@.†Division of Radiological Sciences,Washington University School of Medicine.‡Current address:Department of Radiology,University of Pennsylvania,420Curie Boulevard,Philadelphia,Pennsylvania 19104.aAbbreviations:NO,nitric oxide;NOS,nitric oxide synthase;iNOS,inducible nitric oxide synthase;eNOS,endothelial nitric oxide synthase;nNOS,neuronal nitric oxide synthase;LPS,lipopolysaccharide;PET,positron emission tomography;DAST,diethylaminosulfur trifluoride;PBSF,perfluorobutane sulfonyl fluoride;SEITU,S -ethylisothiourea;L-NIL,L -N 6-(1-iminoethyl)lysine;2-AP,2-aminopyridine.Chart 1J.Med.Chem.2009,52,2443–2453244310.1021/jm801556h CCC:$40.75 2009American Chemical SocietyPublished on Web 03/26/2009类似物2-氨基-4-甲基吡啶抑制剂可诱导的诱导型一氧化氮合成酶取代的丆代替的类似的一氧化氮合成酶活性评估聚对苯二甲酸乙二醇酯示踪物脂多糖吸收丆摄取蛋白质印迹放射示踪物反应传递者丆中介物生理的病理过程精氨酸瓜氨酸同工酶神经元内皮的组织产生丆生成特定的神经递质神经调制末梢神经细胞控制丆管理血管内皮细胞炎症性的刺激内毒素巨噬细胞宿主防御基态水平急性的丆激烈的慢性的丆长期的炎症的有关联丆关系无侵害的空间分辨率精确定量生物活性分子轻微的效能新奇的丆新颖的as potential PET tracers for imaging iNOS,the radiosynthesis of [18F]9,and the in vivo evaluation of [18F]9in a mouse model of lipopolysaccharide (LPS)-induced iNOS activation.Results and DiscussionChemistry.The previously reported method was applied to synthesizethe key intermediate 6(Scheme 1).10Compound 6reacted with acetaldehyde to afford 7in high yield (Scheme 2).Compound 7was converted to 8using diethylaminosulfur trifluoride (DAST)or perfluorobutane sulfonyl fluoride (PBSF)as the fluorinating pound 10was obtained as a byproduct in both cases and was formed as the major product when PBSF was used as the fluorinating agent.These results indicate the facile elimination to form a conjugated double bondadjacent to the pyridine ring.The conversion of the OH in 7to Br using PPh 3and CBr 4failed to give the expected product (data not shown).Compounds 12and 14were synthesized from 7via O-alkylation using CH 3I and BrCH 2CH 2F,respectively,in the presence of CaH 2(Scheme 2).The pyrrole protecting group in all the 2-amino pyridine analogues was removed byrefluxing in an aqueous ethanol solution of hydroxylamine hydrochloride as previous reported.11Although no details were given in the reference,we found that a 2:1mixture of ethanol and water (containing 4M NH 2OH ·HCl)at 110°C affordedgood results.The nucleophilic substitutions of BrCH 2CH 2F,BrCH 2CH 2CH 2F,and (CH 3)3SiCl by 6afforded 17,19,and 21,respectively,in high yields (Scheme 3).Among the compoundssynthesized,9,13,15,16,27,30,and 33are racemic mixtures,which were used without chiral resolution in the following studies.The Peterson olefination reaction 13using R -trimethylsilylcarbanion 22and the corresponding ketones was used to synthesize 23,25,28,and 31,respectively (Scheme 4).Compound 22was synthesized by reacting 21with 1equiv of n -butyl lithium in good yield,which was evidenced by the goodyield of 23following the reaction with acetone.E /Z isomers were observed in these reactions,and the isomers were reducedeither by ammonium formate/ethanol in the presence of pal-ladium on carbon (25and 28)or by magnesium in ethanol (31).The former method was unsuccessful for the synthesis of 32,most likely due to the poisoning of the palladium catalyst by the sulfur atom in 31.The synthesis of precursor 38for the nucleophilic labelingof [18F]9is shown in Scheme 5.The hydroxyl group in the 2-hydroxypropyl group had to be protected as the correspondingacetate ester during the reaction with di-tert -butyldicarbonate (Boc 2O).Without protection of the -OH,37was only a minor product,with the major product as the corresponding t -butylcarbonate and other byproduct (data not shown).Theacetylation to make 34from 7using acetyl chloride was slowand the yield was only 39%,probably due to steric hindrance from the secondary alcohol and the pyridine ring.A more efficient method of making 34involved treating 6with acetaldehyde followed by treatment with ethyl acetate to give 34in an overall yield of 43%.The synthesis of the mesylate and tosylate precursors required for the radiosynthesis of [18F]18failed to afford the desired products.It was found that the mesylate precursor formed initially but converted quickly even at room temperature to a cyclic 1-substituted pyridinium via an internal nucleophilic attack of the mesylate group by the pyridine nitrogen atom,andthis cyclized product was confirmed by mass spectrometry,1H NMR,and elemental analysis (data not shown).Therefore,attempts to radiolabel 18were not successful and our efforts focused on radiolabeling 9with 18F.Scheme 1aaReagents and conditions:(a)HOAc,toluene,reflux;(b)n -BuLi,Et 2O,-20to -10°C.Scheme 2aaReagents and conditions:(a)CH 3CHO,Et 2O,-78°C to RT;(b)DAST,CH 2Cl 2or PBSF,(NEt 3)(HF)3,Et 3N,CH 3CN;(c)NH 2OH ·HCl,EtOH,H 2O;(d)CH 3I or BrCH 2CH 2F,NaH,THF.Scheme 3aaReagents and conditions:(a)17:BrCH 2CH 2F;or 19:BrCH 2CH 2CH 2F;or 21:(CH 3)3SiCl,Et 2O.(b)NH 2OH ·HCl,EtOH,H 2O.2444Journal of Medicinal Chemistry,2009,Vol.52,No.8Zhou et al.乙醛三氟化物氟磺酰三氟化二乙胺基硫磺酰氟使与氟素化合共轭双键邻近的丆比邻的吡啶环吡咯保护乙醇水溶液羟胺盐酸化物外消旋化合物手性拆分三甲基硅烷基相应的酮丙酮同分异构体甲酸铵钯碳镁硫原子羟基羟丙基醋酸酯乙酰化作用乙酰氯位阻现象乙醛乙酸乙酯甲磺酸盐甲苯磺酸盐前体自身亲核进攻质谱分析法Radiosynthesis of [18F]9.[18F]9was synthesized via anucleophilic substitution of the mesylate precursor 38with [18F]fluoride,followed by deprotection with 1N HCl (Scheme 6).The incorporation of [18F]fluoride was only 10-20%at optimized conditions;however,after reversed-phase HPLC purification,[18F]9was obtained in high chemical and radio-chemical purity.The specific activity was >1000mCi/µmol at the end of synthesis.The total synthesis and purification time was 120min,and the isolated yield was up to 10%(decay corrected).The low incorporation of [18F]fluoride should be due to the slow rate of nucleophilic substitution on the secondary carbon and the base-catalyzed elimination to form the corre-sponding nonreactive olefin.The radiolabeling using acetonitrile as the solvent gave a higher yield than that in DMF;use of thecorresponding tosylate and triflate precursors gave a much lower yield or no incorporation of 18F at all.In Vitro Enzyme Assays.The inhibition potency for recombinant iNOS,eNOS,and nNOS was determined using a commercial nitric oxide synthase screening kit (GE HealthcareBiosciences Corporation,Piscataway,NJ)following the manu-facturer’s protocol with minor modifications.The assay was validated using standard NOS inhibitors.Only the most potent iNOS inhibitors were further evaluated for eNOS and nNOS potency to determine the selectivity between iNOS and eNOS or nNOS.As shown in Table 1,the most potent compound for iNOS was 18,with approximately 30-fold selectivity against eNOS and 10-fold against nNOS;9and 20showed less potency for iNOS and less selectivity against eNOS and nNOS but were comparable to the previously reported compound 2,which had a potency of IC 50)193nM for iNOS in our assay (The reported IC 50of 2is 28nM 10).We cannot determine the reasons for the difference between our value and the reported data,but slow time-dependent inhibition has been frequently reported.8b,11,14,15The IC 50value may be influenced by the incubation time;longer incubation time may deliver more potent IC 50values.Addition-ally,the measured inhibition potency may depend on the concentrations of NADPH and L -arginine.15Nevertheless,the assay was validated by standard iNOS inhibitors (Table 1),and 9,18,and 20were identified as potential PET tracers for imaging iNOS according to our assay results.It has been reported previously that the 6-position of the pyridine ring has the greatest amount of bulk tolerance with respect to potency for inhibiting iNOS.Therefore,several 6-substituted analogues were synthesized in order to further investigate this structure -activity relationship.When the 2′-methyl group in lead compound 2was replaced with a fluorineScheme 4aaReagents and conditions:(a)n -BuLi,Et 2O,-20°C.(b)Acetone,Et 2O,-78°C to RT.(c)1N HCl.(d)NH 2OH ·HCl,EtOH,H 2O.(e)25:CH 3COCH 2CH 2OCH 3;28:CH 3COCH 2CH 2F;or 31:CH 3COCH 2CH 2SCH 3,Et 2O,-78°C to RT.(f)HCOONH 4,Pd/C,EtOH,80°C (25,28);or Mg,EtOH (31).Scheme 5aaReagents and conditions:(a)6:CH 3CHO,Et 2O,-78°C to RT;then AcOEt,Et 2O.(b)7:AcCl,Et 3N,CH 2Cl 2.(c)NH 2OH ·HCl,EtOH,H 2O.(d)Boc 2O,t -BuOH.(e)K 2CO 3,MeOH,H 2O.(f)MsCl,Et 3N,CH 2Cl 2.Scheme 6aaReagents and conditions:(a)[18F]fluoride,K 2CO 3,K 222,CH 3CN,110°C;(b)1N HCl,microwave.2-Amino-4-methylpyridine Analogues as Inhibitors for iNOS Journal of Medicinal Chemistry,2009,Vol.52,No.82445亲核取代甲磺酸去保护最佳条件放射性比度烯烃乙腈甲苯磺酸盐三氟甲烷磺酸小改装确认培养时间atom (i.e.,9),the potency for iNOS remained the same.Introduction of a double bond to 2to give an alkene analogue,24,resulting in a diminished potency for iNOS.Removal of the cis methyl from 24(i.e.,11)regained the potency for iNOS (IC 50)282nM);this change in potency for iNOS from 24and 11impliesthat steric demand at the 6-position is high.The change from the methyl in 2or fluorine in 9to the hydroxy group in 16resulted in a large reduction in potency for iNOS,suggesting an adverse electronic substituent effect in this position.The corresponding methoxy (i.e.,13)and 2-fluoroet-hoxy (i.e.,15)analogues were pound 18,an isomer of 9with the fluorine at the terminal position,had enhanced potency and selectivity for iNOS;however,extension of the alkyl chain in 18by one methylene group (i.e.,20)resulted in a reduction of potency for iNOS.Addition of a methyl group in the 2′-position of 20to give 30resulted in a further decrease in potency for iNOS.The corresponding MeO or MeS ana-logues,27and 33,were inactive in the iNOS assay.In summary,there appear to be significant steric and electronic constraints for substituents at the 6-position of the pyridine ring with respect to potency for inhibiting iNOS.In Vitro Stability and in Vivo Metabolism Studies.An in vitro stability study was carried out using heparinized rat blood taken from an adult male Sprague -Dawley rat.[18F]9was relatively stable in the whole blood for the duration of the experiment (2h)at 37°C.At 1h,∼80%of the activity recovered from lysed whole blood was observed as [18F]9,and at 2h,∼75%of the recovered activity was still that of the parent compound.These data suggest that the blood is not a major site for compound degradation,which is in contrast to the previously reported 18F and 11C labeled isothiourea analogues.12aThe in vivo metabolic stability of [18F]9was evaluated in plasma samples obtained from an adult male Sprague -Dawleyrat at 5and 30min postinjection.The supernatant extracts wereanalyzed by silica gel radio-TLC and reversed-phase HPLC.After 30min,the percent parent compound was only 20.2%.Additionally,within 5min postinjection,only 40.3%of the activity in the blood was [18F]9,which was confirmed by HPLC coelution with nonradioactive 9.According to the HPLCanalysis,the major metabolite,constituting 50%of the activity in blood at 5min postinjection,was very polar but was not free [18F]fluoride.This observation is also consistent with the low bone uptake reported below in the biodistribution studies (Table 2).Because [18F]9demonstrated reasonable in vitro stability in whole blood,the metabolite observed in vivo at 5min in blood must be due to metabolism in peripheral organs.In Vivo Biodistribution Study.The biodistribution studies were performed in mature male C57BL/6mice:one group wastreated with bacterial lipopolysaccharide (LPS)(10mg/kg,iv)to induce iNOS expression and one group was without LPS-treatment as control.LPS has been well documented to induce iNOS mRNA and protein expression in both rats and mice,16and administration of LPS resulted in an elevated iNOS expression at 6-7h post-LPS-treatment.The iNOS distribution in organs was reported recently in male BALB/c mice.16c It has been demonstrated in that report that iNOS mRNA and protein expression 6h after LPS stimulation is observed in many organs,including lungs,heart,liver,spleen,gut,and kidneys.Among them,the highest iNOS expression was in the lungs,with moderate expression in the spleen and kidneys and the lowest in the heart,gut,and liver.16c Therefore,this endotoxin (LPS)lung injury mouse model was used to assess the efficiency of [18F]9as an iNOS radiotracer.The results of in vivo biodistribution studies in the normal and LPS-treated mice are shown in Table 2and Figure 1.The highest uptake in both groups was observed in the liver and kidneys,which are likely to be the major metabolic and/or excretory sites for the tracer.Excluding the potential metabolic sites,the highest uptake was observed in the lungs and blood,followed by muscle,heart,and brain in both groups.The low bone uptake suggested that [18F]9had a high degree of stability toward defluorination,considering the facile elimination to formTable 1.IC 50values of the 2-Amino-4-Methylpyridine analogues aIC 50(nM (standard deviation,n g 3)b NOS 2924111613151820302733iNOS 193(38(28)c 220(25685(127282(491776(395>5000>500057.6(5.3170(26731(87>5000>5000eNOS (150)c 1500(300-d ----1428(158----nNOS(100)c490(80-----514(83----aUsing recombinant human iNOS,eNOS,and nNOS.b Standard iNOS IC 50[measured (reported)]:SEITU )30.5(4.6(32),L-NIL )1465(148(1400),2-AP )108(35(170).c Reference 10.d -:Not determined.Table 2.Biodistribution of [18F]9in Control vs LPS Treated Mice a (Data Reported as Mean %ID/g (SD;n )4)b5min30min1h2h%ID/g LPS control LPS control LPS control LPS control blood 3.69(0.38 2.37(0.18 2.09(0.35 1.22(0.160.86(0.090.31(0.040.23(0.050.27(0.22lung 4.32(0.32 3.33(0.28 1.84(0.27 1.14(0.130.73(0.170.31(0.060.18(0.090.15(0.03liver 24.6(3.133.2(3.37.06(0.2810.7(0.3 2.63(0.52 2.59(0.490.42(0.150.74(0.23kidney 26.7(4.120.6(2.410.9(2.87.30(0.66 3.95(0.53 2.20(0.250.91(0.630.80(0.27muscle 1.64(0.09 1.25(0.06 1.06(0.400.71(0.310.78(0.470.27(0.180.23(0.130.34(0.27heart 2.03(0.06 1.50(0.12 1.02(0.160.60(0.050.41(0.100.19(0.020.12(0.040.08(0.02brain 1.81(0.09 1.60(0.170.52(0.150.28(0.020.19(0.030.11(0.020.07(0.030.07(0.03bone1.59(0.151.52(0.363.70(1.202.26(0.554.30(0.902.57(0.643.77(1.415.59(4.20aLPS mice were treated with lipopolysaccharide (LPS)10mg/kg iv 6h prior to tracer injection;all mice were injected with 50µCi/110µL [18F]9.b SD:standard deviation.2446Journal of Medicinal Chemistry,2009,Vol.52,No.8Zhou et al.上层清夜反相高效液相色谱代谢物脂多糖丆内毒素分配肺肝脏脾脏内脏丆肠子肾脏脱氟作用血浆样品aconjugated double bond at this pared to thecontrol group,the LPS-treated mice had a∼30%increase in tracer uptake in most of the organs at1h.In the organ expected to have the highest iNOS expression,the lungs,the increase in injected dose/g(ID/g)was even more pronounced,30%at5 min,60%at30min,and130%at1h.Because systemic LPS-induced acute lung injury results in pulmonary edema,the difference in total lung uptake between control and treatedanimals(i.e.,%ID)is more dramatic than the%ID/g in the lung (Figure1).The overall higher uptake in tissues of the LPS-treated mice compared to the controls is consistent with the previously reported systemic iNOS response with LPS treatment.16c From30min to1h,the uptake in the LPS-treated mice washed out at a slower rate than that of the control mice, which again can be attributed to the specific binding of[18F]9 to iNOS.On the basis of the in vivo metabolic stability study in the blood of normal rat(see above),there should be little circulating[18F]9left in the blood of the mice at2h postinjection.At2h postinjection,all the activity was washed out to the same low level and the uptake at2h postinjection should be due to the nonspecific uptake of the metabolites.In previously reported biodistribution studies in rats injected with less potent and less selective18F and11C labeled isothiourea analogues,about30%higher uptake of18F labeled analogue was observed in lungs,blood,liver,kidneys,and heart of LPS-treated rats than that of normal rats at10min postinjection. However,the difference in uptake between the two groups became insignificant at30min postinjection,possibly due to the rapid metabolism of these radiotracers.The more stable11C labeled analogue showed40%higher uptake at30min postin-jection in the lungs of the LPS-treated rats than that of the control.12a Compared to the previously described18F labeled isothiourea analogue,[18F]9,with prolonged retention in the LPS-treated mice,is a suitable PET tracer for iNOS.A standard Western blot was performed to compare the iNOS induction in lungs upon LPS stimulation versus untreated controls,and the results are shown in Figure2.Protein for the Western blot was obtained from the lungs harvested from treated and untreated animals in the biodistribution study.Thefirst lane is the purified iNOS protein as a positive control.Protein from two control lungs and six LPS-treated lungs were evaluated. As indicated in Figure2,neither of the control samples demonstrated iNOS expression,whereas the LPS-treated samples all showed some degree of iNOS induction due to the response toward LPS stimulation.This result is consistent with the higher tracer uptake in the lungs of the LPS-treated mice in the biodistribution studies,suggesting iNOS-specific uptake of [18F]9.Blocking Study.To further demonstrate that the increased uptake in the LPS-treated mice was due to specific binding of [18F]9to iNOS,blocking studies were carried out.First,the nonselective NOS inhibitor2-amino-4-methylpyridine(1)(10 mg/kg,iv)failed to block the increased uptake in the LPS-treated mice(data not shown).A failure to block tracer uptake using nonselective NOS inhibitors S-methyl or S-ethyl isothiourea has been reported previously;12a the failure was contributed to the blood pressure change caused by the blocking agents in the animal,which may alter the tracer uptake function.2-Amino-4-methylpyridine(1)elevates blood pressure9a,10,17and blocking of eNOS expression may also reduce the bloodflow.18 Therefore,a highly selectively iNOS inhibitor is preferred in order to avoid the side effects from nonselective inhibitors.N-(3-(Aminomethyl)benzyl)acetamidine(1400W)is a slow,tight binding,and highly selective inhibitor of iNOS in vitro and in vivo8b and has been used commonly as an iNOS inhibitor in many reported studies.However,due to its high toxicity,19only 5mg/kg of1400W was injected intravenously immediately before the injection of[18F]9.The results are shown in Figure 3.At1h post injection of[18F]9,32%of the tracer uptake in the lungs and blood of the LPS-treated mice was blocked by 1400W(Figure3).This reduced uptake in the lungs of the LPS-treated mice should be due to the specific blocking of iNOS by 1400W and is consistent with that the uptake of[18F]9in the LPS-treated mice is iNOS specific.MicroPET Study.A microPET imaging study using[18F]9 was carried out on an intratracheally LPS-treated mouse and a normal mouse as control.The microPET images(0-60min dynamic scan)are shown in Figure 4.As shown in the microPET images(Figure4),accumulation of[18F]9was observed in the target organ,the lungs,of the LPS-treated mouse,whereas no such accumulation was observed in the lungs of the normal mouse.The difference in the tracer uptake in the lungs of the LPS-treated and control mice is consistent withparison of total lung activity postinjection of[18F]9in LPS-pretreated mice vs control mice.Figure2.Representative Western blots showing the levels of iNOS expression in lungs from the control and LPS treated mice.Thefirst lane is purified iNOS as a positive control(iNOS);no iNOS expression was observed in the lungs of normal mice(C);different degrees of iNOS expression were found in the lungs of the LPS-treated mice(T). parison of activity in the lung1h postinjection of[18F]9 in the control,LPS treated,and LPS-treated-1400W-blocked mice(mean %ID/g(SD,n)4,p<0.05).2-Amino-4-methylpyridine Analogues as Inhibitors for iNOS Journal of Medicinal Chemistry,2009,Vol.52,No.82447急性肺损伤肺水肿特异性结合保留蛋白质印记精制的小路阳性对照乙脒毒性静脉注射iNOS expression induced by LPS treatment and is similar to the results of biodistribution study.ConclusionWe have identified6-(2-fluoropropyl)-4-methylpyridin-2-amine(9),6-(3-fluoropropyl)-4-methylpyridin-2-amine(18),and 6-(4-fluorobutyl)-4-methylpyridin-2-amine(20)as iNOS inhibi-tors with the greatest potential to serve as PET radiotracers. [18F]9was synthesized in modest yield(∼10%)but with high chemical and radiochemical purities.In the biodistribution study of a mouse model of LPS-induced iNOS activation,higher uptake of[18F]9was observed in the lungs of the LPS-treatedmice than those in the control mice.The higher uptake in the lungs of the LPS-treated animals correlated well with iNOS expression,which was confirmed by Western blot analysis of the lung samples from control and LPS-treated mice.The increased uptake in the lungs of the LPS-treated mice at60 min post injection of[18F]9was reduced by injection of the highly selective iNOS inhibitor,1400W.The blocking study suggests that the higher uptake of[18F]9in the lungs of the LPS-treated mice is due to specific binding of[18F]9to iNOS. MicroPET study of the LPS-treated mouse using[18F]9dem-onstrated an accumulation of[18F]9in the lungs of the LPS-treated mice,in sharp contrast to those of the control mouse.In conclusion,[18F]6-(2-Fluoropropyl)-4-methylpyridin-2-amine ([18F]9)is a potential radiotracer for PET imaging of iNOS expression.Experimental SectionGeneral Methods and Materials.All chemicals were obtained from standard commercial sources and used without further purification.All reactions were carried out by standard air-free and moisture-free techniques under an inert argon atmosphere with dry solvents unless otherwise stated.Flash column chromatography was conducted using Scientific Adsorbents,Inc.silica gel,60A,“40 Micron Flash”(32-63µm).Melting points were uncorrected. Routine1H NMR spectra were recorded at300MHz.All chemical shifts were reported as a part per million(ppm)downfield from tetramethylsilane(TMS)or when chloroform-d was used as solvent and the solvent peak atδ7.25ppm was used as an internal standard. All coupling constants(J)are given in Hz.Splitting patterns are typically described as follows:s,singlet;d,doublet;t,triplet;m, multiplet.19F NMR spectra were recorded at282.2MHz,and chemical shifts are reported as Hz upfield from an external CFCl3 standard.ESI/MS was performed on a Waters ZQ4000single quadrupole mass spectrometer equipped with an electrospray ionization(ESI)LC-MS interface.High-performance liquid chro-matography(HPLC)was performed with an ultraviolet detector operating at272nm and a well-scintillation NaI(Tl)detector and associated electronics for radioactivity detection.Alltech Platinum EPS C18250mm×10mm semipreparative column and Alltech Platinum EPS C18250mm×4.6mm analytical column were used for preparation and analysis,respectively.4-Methoxybutan-2-one was synthesized according to literature.20The purities offinal compounds are g95%,which were confirmed by examination of elemental analysis results or by reversed-phase HPLC(for com-pound13,15,18,24,and27).H218O was purchased from Rotem Industries(Israel).[18F]Fluo-ride was produced in Washington University by the18O(p,n)18F reaction through proton irradiation of enriched(95%)18O water using a RDS111cyclotron.Materials were heated using a custom-designed microwave cavity,model420BX(Micro-Now Instru-ments,Skokie,IL).Screw-cap test tubes used for microwave heating were purchased from Fisher Scientific(Pyrex no.9825).HLB Sep-Pak cartridges were purchased from Waters Corporation.For the TLC analyses,EM Science Silica Gel60F254TLC plates were purchased from Fisher Scientific(Pittsburgh,PA).Radio-TLC was accomplished using a Bioscan200imaging scanner(Bioscan,Inc., Washington,DC).Radioactivity was counted with a Beckman Gamma8000counter containing a NaI crystal(Beckman Instru-ments,Inc.,Irvine,CA).Typical Procedure for the Synthesis of6.Into a solution of5 (2.0g,10mmol)in dry Et2O(20mL)at-20°C was added dropwise n-BuLi(1.6M in hexane,6.9mL,10mmol)within5 min,then the reaction mixture was stirred at-20to-10°C for 1h to complete the reaction.Orange solids precipitated gradually during the experiment,and the reaction mixture of6was used directly for further reactions.1-(6-(2,5-Dimethyl-1H-pyrrol-1-yl)-4-methylpyridin-2-yl)pro-pan-2-ol(7).Into an Et2O solution of6at-78°C,which was made from5(5.6g,28.0mmol),n-BuLi(1.6M in hexane,19.5 mL,31.2mmol)in Et2O(30mL)was added CH3CHO(2mL,35.6 mmol)via a syringe.The reaction mixture was allowed to warm up to room temperature,and stirring was continued for10min. Then,the reaction mixture was treated with H2O(100mL)and extracted with ethyl acetate(3×50mL).The organic solution was washed with brine(2×30mL),dried over NaSO4,and the solvent was evaporated under reduced pressure.The crude product was purified with silica gel chromatograph using1:4ethyl acetate/ hexanes to afford7(5.6g)in80%yield as colorless liquid.1H NMR(CD3Cl,300MHz)δ6.97(s,1H),6.89(s,1H),5.87(s, 2H),4.51(s,br,1H),4.23(m,1H),2.94-2.80(m,2H),2.39(s, 3H),2.12(s,6H),1.25(d,3H,J)6.3Hz).2-(2,5-Dimethyl-1H-pyrrol-1-yl)-6-(2-fluoropropyl)-4-meth-ylpyridine(8).Using Diethylaminosulfur Trifluoride(DAST) as Fluorinating Agent.Into a solution of7(0.5g,2.05mmol)in CH2Cl2(5mL)at0°C was added DAST(0.4mL,3.05mmol) dropwise.The reaction mixture became brown in color,and the starting material was consumed within30min.Saturated NaHCO3 solution(10mL)was added to quench the reaction,and the aqueous solution was extracted with ethyl acetate(2×30mL).The organic layers was washed with brine(2×30mL)and dried over Na2SO4. Solvents were evaporated under reduced pressure,and the crude product was purified with silica gel chromatograph using1:10ethyl acetate/hexanes to afford8(0.13g)in26%yield as colorless liquid. 1H NMR(CD3Cl,300MHz)δ7.08(s,1H),6.93(s,1H),5.91(s, 2H),5.05-5.60(m,1H),3.20(m,2H),2.43(s,3H),2.15(s,6H), 1.43(dd,3H,J)23.4,6.3Hz),ESI/MS m/z247.06[M+H+].Using Perfluorobutane Sulfonyl Fluoride(PBSF)as Fluori-nating Agent.Into a solution of7(0.5g,2.05mmol)in CH3CN (5mL)were added PBSF(0.74mL,4.1mmol),Et3N(1.73mL, 12.4mmol),and(NEt3)(HF)3(0.67mL,4.11mmol).The reaction mixture was stirred quickly and became homogeneous after40min. The reaction was completed in2.5h according to TLC analysis, and the solvent was evaporated under reduced pressure.Silica gel chromatograph purification of the residue using1:10ethyl acetate/ hexanes afforded8(0.13g)in26%yield as colorless liquid.6-(2-Fluoropropyl)-4-methylpyridin-2-amine(9):Typical Pro-cedure for Deprotection.Into a50mL round-bottomflask equipped with a magnetic stirring bar and a condenser were addedFigure4.Whole-body MicroPET images of[18F]9in the LPS-treatedmice(left)and the control mice(right):(a)transaxal,(b)coronal.Imageswere summed from0to60min after injection of[18F]9.Accumulationof the activity was observed in the lungs of the LPS-treated mouse,whereas there is no such accumulation in the normal control.2448Journal of Medicinal Chemistry,2009,Vol.52,No.8Zhou et al.。
chemical_reaction_engineering(答案)

Corresponding Solutions for Chemical Reaction EngineeringCHAPTER 1 OVERVIEW OF CHEMICAL REACTION ENGINEERING ..................... 错误!未定义书签。
CHAPTER 2 KINETICS OF HOMOGENEOUS REACTIONS ............................. 错误!未定义书签。
CHAPTER 3 INTERPRETATION OF BATCH REACTOR DATA .......................... 错误!未定义书签。
CHAPTER 4 INTRODUCTION TO REACTOR DESIGN ................................ 错误!未定义书签。
CHAPTER 5 IDEAL REACTOR FOR A SINGLE REACTOR ............................ 错误!未定义书签。
CHAPTER 6 DESIGN FOR SINGLE REACTIONS ................................... 错误!未定义书签。
CHAPTER 10 CHOOSING THE RIGHT KIND OF REACTOR ........................... 错误!未定义书签。
CHAPTER 11 BASICS OF NON-IDEAL FLOW ..................................... 错误!未定义书签。
CHAPTER 18 SOLID CATALYZED REACTIONS .................................... 错误!未定义书签。
Chapter 1 Overview of Chemical Reaction Engineering1.1 Municipal waste water treatment plant. Consider a municipal water treatment plant for a small community Waste water, 32000 m 3/day, flows through the treatment plant with a mean residence time of 8 hr, air is bubbled through the tanks, and microbes in the tank attack and break down the organic material (organic waste) +O 2 −−−→−microbes CO 2 + H 2OA typical entering feed has a BOD (biological oxygen demand) of 200 mg O 2/liter, while the effluent has a megligible BOD. Find the rate of reaction, or decrease in BOD in the treatment tanks.FigureSolution:)/(1017.2)/(75.183132/100010001)0200()(313200031320001343333s m mol day m mol day molgm L mg g L mg day day m dayday m VdtdN r AA ⋅⨯=⋅=-⨯⨯⨯-⨯-=-=--1.2 Coal burning electrical power station. Large central power stations (about 1000 MW electrical) using fluiding bed combustors may be built some day (see These giants would be fed 240 tons of coal/hr (90% C, 10%H 2), 50% of whichWaste water3Clean water3200 mg O 2Zero O 2would burn within the battery of primary fluidized beds, the other 50% elsewhere in the system. One suggested design would use a battery of 10 fluidized beds, each 20 m long, 4 m wide, and containing solids to a depth of 1 m. Find the rate of reaction within the beds, based on the oxygen used.Solution:380010)1420(m V =⨯⨯⨯=)/(9000101089.05.01024033hr bed molc hrkgckgcoal kgc hr coal t N c ⋅-=⨯-=⨯⨯⨯-=∆∆ )/(25.111900080011322hr m kmolO t N V r r c c O ⋅=-⨯-=∆∆-=-=)/(12000412000190002hr bed mol dt dO ⋅=+⨯= )/(17.4800)/(105.113422s m mol hr bed mol dt dO V r O ⋅=⋅⨯==-Chapter 2 Kinetics of Homogeneous ReactionsA reaction has the stoichiometric equation A +B =2R . What is the order of reactionSolution: Because we don’t know whether it is an elementary reaction or not, we can’t tell the index of th e reaction.2.2 Given the reaction 2NO 2 + 1/2 O 2 = N 2O 5 , what is the relation between therates of formation and disappearance of the three reaction components Solution: 522224O N O NO r r r =-=-2.3 A reaction with stoichiometric equation A + B = R + S has the followingrate expression-r A = 2 AC BWhat is the rate expression for this reaction if the stoichiometric equation is written asA + 2B = 2R + SSolution: No change. The stoichiometric equation can’t effect the rate equation, so it doesn’t chang e.For the enzyme-substrate reaction of Example 2, the rate of disappearance ofsubstrate is given by-r A =A06]][[1760C E A + , mol/m 3·sWhat are the units of the two constants Solution: ][]6[]][][[][03A A C E A k s m mol r +=⋅=-3/][]6[m mol C A ==∴sm mol m mol m mol s m mol k 1)/)(/(/][3333=⋅⋅=2.5 For the complex reaction with stoichiometry A + 3B → 2R + S and with second-order rate expression-r A = k 1[A][B]are the reaction rates related as follows: r A = r B = r R If the rates are notso related, then how are they related Please account for the sings , + or - .Solution: R B A r r r 2131=-=-A certain reaction has a rate given by-r A = C2 A , mol/cm 3·minIf the concentration is to be expressed in mol/liter and time in hours,what would be the value and units of the rate constant Solution:min)()(3'⋅⨯-=⋅⨯-cm molr hr L mol r A A 22443'300005.0106610)(minAA A A A C C r r cm mol mol hr L r =⨯⨯=⋅⨯=-⋅⋅⋅=-∴ AA A A A C C cmmol mol L C cmmolC L mol C 33'3'10)()(=⋅⋅=∴⨯=⨯2'42'32'103)10(300300)(AA A A C C C r --⨯=⨯==-∴ 4'103-⨯=∴kFor a gas reaction at 400 K the rate is reported as-dtdp A= p2 A, atm/hr (a) What are the units of the rate constant(b) What is the value of the rate constant for this reaction if the rateequation is expressed as-r A = - dtdN V A1 = k C2 A , mol/m 3·sSolution:(a)The unit of the rate constant is ]/1[hr atm ⋅ (b)dtdN V r AA 1-=-Because it’s a gas reaction occuring at the fined terperatuse, so V=constant, and T=constant, so the equation can be reduced to22)(66.366.3)(1RT C RTP RT dt dP RT dt dP VRT V r A A A A A ==-=-=-22)66.3(A A kC C RT ==So we can get that the value of1.12040008205.066.366.3=⨯⨯==RT kThe pyrolysis of ethane proceeds with an activation energy of about 300 kJ/mol.How much faster the decomposition at 650℃ than at 500℃ Solution:586.7)92311731()10/(314.8/300)11(3211212=-⋅⋅=-==KK K mol kJ mol kJ T T R E k k Ln r r Ln7.197012=∴r rIn the mid-nineteenth century the entomologist Henri Fabre noted that Frenchants (garden variety) busily bustled about their business on hot days but were rather sluggish on cool days. Checking his results with Oregon ants, I findRunning speed, m/hr150160230295370Temperature, ℃1316222428What activation energy represents this change in bustliness Solution:RTE RTE RTE ek eak t cons ion concentrat f let ion concentrat f ek r ---=⋅⋅=⋅='00tan )()(RET Lnk Lnr A 1'-=∴ Suppose Tx Lnr y A 1,==, so ,REslope -= intercept 'Lnk =)/(1-⋅h m r A150 160 230 295 370 A LnrC T o /13 16 22 24 28 3101-⨯T-y = x +Also K REslope 9.5147-=-=, intercept 'Lnk == , mol kJ K mol J K E /80.42)/(3145.89.5147=⋅⨯-=Chapter 3 Interpretation of Batch Reactor DataIf -r A = - (dC A /dt) = mol/liter·sec when C A = 1 mol/liter, what is the rateof reaction when C A = 10 mol/liter Note: the order of reaction is not known.Solution: I nformation is not enough, so we can’t answer this kind of question.3.2 Liquid a sedomposes by first-order kinetics, and in a batch reactor 50%of A is converted in a 5-minute run. How much longer would it take to reach 75% conversionSolution: Because the decomposition of A is a 1st -order reaction, so we can express the rate equation as:A A kC r =-We know that for 1st -order reaction, kt C C LnAAo=, 11kt C C LnA Ao =, 22kt C CLn A Ao = Ao A C C 5.01=, Ao A C C 25.02=So 21)24(1)(11212Ln kLn Ln k C C Ln C C Ln k t t A Ao A Ao =-=-=- equ(1) min 521)(111===Ln kC C Ln k t A Ao equ(2) So m in 5112==-t t t3.3 Repeat the previous problem for second-order kinetics. Solution: We know that for 2nd -order reaction,kt C C A A =-011,So we have two equations as follow:min 511211101k kt C C C C C AoAo Ao A A ===-=-, equ(1) 2123)1(31411kt kt C C C C C AoAo Ao Ao A ===-=-, equ(2) So m in 15312==t t , m in 1012=-t tA 10-minute experimental run shows that 75% of liquid reactant is converted to product by a 21-order rate. What would be the fraction converted in a half-hour run Solution: In a-21order reaction: 5.0AA A kC dt dC r =-=-, After integration, we can get:5.015.02A Ao C C kt -=, So we have two equations as follow:min)10(5.0)41(15.05.05.05.015.0k kt C C C C C Ao Ao AoA Ao ===-=-, equ(1) min)30(25.025.0k kt C C A Ao ==-, equ(2)Combining these two equations, we can get:25.05.1kt C Ao =, but this means 05.02<A C ,which is impossible, so we can conclude that less than half hours, all the reactant is consumed up. So the fraction converted 1=A X .In a hmogeneous isothermal liquid polymerization, 20% of the monomer disappears in 34 minutes for initial monomer concentration of and also for mol/liter. What rate equation represents the disappearance of the monomer Solution: The rate of reactant is independent of the initial concentration of monomers, so we know the order of reaction is first-order,monomer monomer kC r =-And k C C Lnoomin)34(8.0= 1min 00657.0-=kmonomer monomer C r )min 00657.0(1-=-After 8 minutes in a batch reactor, reactant (C A0 = 1 mol/liter) is 80% converted; after 18 minutes, conversion is 90%. Find a rate equation to represent this reaction. Solution:In 1st order reaction, 43.1511111111212==--=Ln Ln X Lnk X Ln k t t A A , dissatisfied.In 2nd order reaction, 49/4/912.0111.01)11(1)11(11212==--=--=Ao Ao Ao Ao Ao Ao Ao A Ao A C C C C C C C C k C C k t t, satisfied.According to the information, the reaction is a 2nd -order reaction.nake-Eyes Magoo is a man of habit. For instance, his Friday evenings are all alike —into the joint with his week’s salary of $180, steady gambling at “2-up” for two hours, then home to his family leaving $45 behind. Snake Eyes’s betting pattern is predictable. He always bets in amounts proportional to his cash at hand, and his losses are also predictable —at a rate proportional to his cash at hand. This week Snake-Eyes received a raise in salary, so he played for three hours, but as usual went home with $135. How much was his raise Solution:180=Ao n , 13=A n , h t 2=,135'=A n , h t 3;=, A A kn r α-So we obtain kt n n LnAAo=, ''')()(tn n Ln t n n Ln AAo A Ao= 3135213180'Ao n Ln Ln =, 28'=AnThe first-order reversible liquid reaction A ↔ R , C A0 = mol/liter, C R0=0takes place in a batch reactor. After 8 minutes, conversion of A is % while equilibrium conversion is %. Find the equation for the this reaction. Solution: Liquid reaction, which belongs to constant volume system, 1st order reversible reaction, according to page56 eq. 53b, we obtain121112102110)(1)(-+-+=+-==⎰⎰AX A A tX k k k k Lnk k X k k k dX dt t Amin 8sec 480==t Θ, 33.0=A X , so we obtain eq(1)33.0)(1min8sec 480211121k k k k Ln k k +-+= eq(1) Ae AeAe c X X M C C k k K -+===1Re 21, 0==AoRo C C M , so we obtain eq(2) 232132121=-=-==AeAe c X X k k K ,212k k =∴ eq(2)Combining eq(1) and eq(2), we obtain1412sec 108.4m in 02888.0---⨯==k 14121sec 1063.9m in 05776.02---⨯===k kSo the rate equation is )(21A Ao A AA C C k C k dtdC r --=-=- )(sec 1063.9sec 108.401414A A A C C C -⨯-⨯=----Aqueous A reacts to form R (A→R) and in the first minute in a batch reactorits concentration drops from C A0 = mol/liter to C Af = mol/liter. Find the rate equation from the reaction if the kinetics are second-order with respect to A.Solution: It’s a irreversible second -order reaction system, according to page44 eq 12, we obtainmin 103.2197.111⋅=-k , so min015.01⋅=mol Lkso the rate equation is 21)min 015.0(A A C r -=-At room temperature sucrose is hydrolyzed by the catalytic action of the enzymesucrase as follows:Aucrose −−→−sucraseproductsStarting with a sucrose concentration C A0 = millimol/liter and an enzymeconcentration C E0= millimol/liter, the following kinetic data are obtained in a batch reactor (concentrations calculated from optical rotation measurements):Determine whether these data can be reasonably fitted by a knietic equationC A ,millimol/lite rt,hr1234567891011of the Michaelis-Menten type, or -r A =MA E A C C C C k +03 where C M = Michaelis constantIf the fit is reasonable, evaluate the constants k 3 and C M . Solve by the integral method.Solution: Solve the question by the integral method:AA M A A Eo A A C k Ck C C C C k dt dC r 5431+=+=-=-, M Eo C C k k 34=, MC k 15= AAo A AoA Ao C C C C Ln k k k C C t -⋅+=-4451hr t ,A C ,mmol/LA Ao AAo C C C C Ln-AAo C C t-1 2 3 4 5 6 7 8 9 10 11Suppose y=A Ao C C t-, x=AAo A Ao C C C C Ln-, thus we obtain such straight line graph9879.0134===Eo M C k C k Slope , intercept=0497.545=k k So )/(1956.00497.59879.015L mmol k C M ===, 14380.1901.09879.01956.0-=⨯==hr C C k k Eo MEnzyme E catalyzes the transformation of reactant A to product R as follows:A −−→−enzymeR, -r A = min22000⋅+liter molC C C A E AIf we introduce enzyme (C E0 = mol/liter) and reactant (C A0 = 10mol/liter) into a batch rector and let the reaction proceed, find the time needed for the concentration of reactant to drop to mol/liter. Note that the concentration of enzyme remains unchanged during the reaction.. Solution:510001.020021+=⨯+=-=-AA A A A C C C dC dt r Rearranging and integrating, we obtain:10025.0025.010)(510)510(⎥⎦⎤⎢⎣⎡-+=+-==⎰⎰A Ao A Ao A A t C C C C Ln dC C dt t min 79.109)(5025.01010=-+=A Ao C C Lnand . Jungers, Bull. soc. chim. France, 386(1957), present the data in Table on thereaction of sulfuric acid with diethylsulfate in a aqueous solutionat ℃:H 2SO 4 + (C 2H 5)2SO 4 → 2C 2H 5SO 4HInitial concentrations of H 2SO 4 and (C 2H 5)2SO 4 are each mol/liter. Find a rate equation for this reaction.Tablet, minC 2H 5SO 4H,mol/litert, minC 2H 5SO 4H,mol/liter0 0 180 41 194 48 212 55 267 75 318 96 368 127 379 146 410 162∞ Solution: It’s a constant -volume system, so we can use X A solving the problem: i) We postulate it is a 2nd order reversible reaction system R B A 2⇔+ The rate equation is: 221R B A A A C k C C k dtdC r -=-=- L mol C C Bo Ao /5.5==, )1(A Ao A X C C -=,A A Ao BoBC X C C C =-=, A Ao R X C C 2=When ∞=t , L mol X C C Ae Ao /8.52Re == So 5273.05.528.5=⨯=Ae X , L mol X C C C Ae Ao Be Ae /6.2)5273.01(5.5)1(=-⨯=-== After integrating, we obtaint C X k X X X X X LnAo AeA Ae A Ae Ae )11(2)12(1-=--- eq (1)The calculating result is presented in following Table.t ,min L mol C R /,L mol C A /,A XA Ae A Ae Ae X X X X X Ln---)12( )1(AeA X XLn -0 0 0 0 0 41 48 55 75 96 127 146 162 180 194 212 267 318 368 379410 ∞——Draw AAe AAe Ae X X X X X Ln---)12(~ t plot, we obtain a straight line:0067.0)11(21=-=Ao AeC X k Slope , min)/(10794.65.5)15273.01(20067.041⋅⨯=⨯-=∴-mol L kWhen approach to equilibrium, BeAe c C C C k k K 2Re 21==, so min)/(10364.18.56.210794.642242Re 12⋅⨯=⨯⨯==--mol L C C C k k Be Ae So the rate equation ism in)/()10364.110794.6(244⋅⨯-⨯=---L mol C C C r R B A Aii) We postulate it is a 1st order reversible reaction system, so the rate equation isR A AA C k C k dtdC r 21-=-=- After rearranging and integrating, we obtaint k X X X Ln AeAe A '11)1(=-eq (2) Draw )1(AeAX X Ln -~ t plot, we obtain another straight line:0068.0'1-==AeX k Slope ,So 13'1m in 10586.35273.00068.0--⨯-=⨯-=k133Re '1'2min 10607.18.56.210586.3---⨯-=⨯⨯-==C C k k AeSo the rate equation ism in)/()10607.110586.3(33⋅⨯+⨯-=---L mol C C r R A AWe find that this reaction corresponds to both a 1st and 2nd order reversible reaction system, by comparing eq.(1) and eq.(2), especially when X Ae = , the two equations are identical. This means these two equations would have almost the same fitness of data when the experiment data of the reaction show that X Ae =.(The data that we use just have X Ae = approached to , so it causes to this.)In the presence of a homogeneous catalyst of given concentration, aqueous reactant A is converted to product at the following rates, and C A alone determinesthis rate:C A ,mol/liter 1 2 4 6 7 9 12 -r A , mol/liter·hrWe plan to run this reaction in a batch reactor at the same catelyst concentration as used in getting the above data. Find the time needed to lower the concentration of A from C A0 = 10 mol/liter to C Af = 2 mol/liter.Solution: By using graphical integration method, we obtain that the shaped area is 50 hr.The thermal decomposition of hydrogen iodide 2HI → H 2 + I 2 is reported by [ as follows:T,℃ 508 427 393 356 283 k,cm 3/mol ·s×10-6×10-6Find the complete rate equation for this reaction. Use units of joules, moles, cm 3, and seconds. According to Arrhenius’ Law,k = k 0e -E/R Ttransform it,0 4 8 1216 20 02468101214Ca-1/Ra- In(k) = E/R·(1/T) -In(k)Drawing the figure of the relationship between k and T as follows:From the figure, we getslope = E/R = intercept = - In(k) =E = 60851 J/mol k= 105556 cm3/mol·sFrom the unit [k] we obtain the thermal decomposition is second-order reaction, so the rate expression is- rA = 105556e-60851/R T·CA2Chapter 4 Introduction to Reactor DesignGiven a gaseous feed, C A0 = 100, C B0 = 200, A +B→ R + S, X A = . Find X B ,C A ,C B . Solution: Given a gaseous feed, 100=Ao C , 200=Bo C , S R B A +→+0=A X , find B X , A C , B C0==B A εε, 202.0100)1(=⨯=-=A Ao A X C C4.02008.01001=⨯⨯==Bo A Ao B C X bC X 1206.0200)1(=⨯=-=B Bo B X C CGiven a dilute aqueous feed, C A0 = C B0 =100, A +2B→ R + S, C A = 20. Find X A , X B , C B .Solution: Given a dilute aqueous feed, 100==Bo Ao C C ,S R B A +→+2, 20=A C , find A X , B X , B CAqueous reaction system, so 0==B A εε When 0=A X , 200=V When 1=A X , 100=VSo 21-=A ε, 41-==Ao Bo A B bC C εε8.01002011=-=-=Ao A A C C X , 16.11008.010012>=⨯⨯=⋅=Bo A Ao B C X C a b X , which is impossible. So 1=B X , 100==Bo B C CGiven a gaseous feed, C A0 =200, C B0 =100, A +B→ R, C A = 50. Find X A , X B , C B .Solution: Given a gaseous feed, 200=Ao C , 100=Bo C ,R B A →+, 50=A C .find A X , B X , B C75.02005011=-=-=Ao A A C C X , 15.1>==BoAAo B C X bC X , which is impossible. So 100==Bo B C CGiven a gaseous feed, C A0 = C B0 =100, A +2B→ R, C B = 20. Find X A , X B , C A . Solution: Given a gaseous feed, 100=+Bo Ao C C ,R B A →+2, 20=Bo C , Find A X , B X , A C0=B X , 200100100=+=B A V ,1=B X 15010050=+=R A V25.0200200150-=-=B ε, 5.01002110025.0-=⨯⨯-=-A ε842.02025.010020100=⨯--=B X , 421.0100842.010021=⨯⨯=A X34.73421.05.01421.0110011=⨯--⨯=+-=A A A AoA X X C C εGiven a gaseous feed, T 0 =1000 K, π0=5atm, C A0=100, C B0=200, A +B→5R,T =400K, π=4atm, C A =20. Find X A , X B , C B .Solution: Given a gaseous feed, K T o 1000=, atm 50=π, 100=Ao C , 200=Bo CR B A 5→+, K T 400=, atm 4=π, 20=A C , find A X , B X , B C .1300300600=-=A ε, 2==Ao Bo AB bC C a εε, 5.0410********=⨯⨯=ππT T According to eq page 87,818.05.010020115.0100201110000=⨯⨯+⨯-=+-=ππεππT T C C T T C C X Ao A AAo A A409.0200818.0100=⨯==Bo A Ao B aC X bC X130818.011200)818.0100200(1)(0=⨯+⨯-=+-=A A Ao A Ao Bo B X C T T X a b C C C εππA Commercial Popcorn Popping Popcorn Popper. We are constructing a 1-literpopcorn to be operatedin steady flow. First tests in this unit show that 1 liter/min of raw corn feed stream produces 28 liter/minof mixed exit stream. Independent tests show that when raw corn pops its volumegoes from 1 to 31.With this information determine what fraction of raw corn is popped in theunit.Solution: 301131=-=A ε, ..1u a C Ao =, ..281281u a C C Ao A == %5.462813012811=⨯+-=+-=∴AA Ao A Ao A C C C C X εChapter 5 Ideal Reactor for a single ReactorConsider a gas-phase reaction 2A → R + 2S with unknown kinetics. If a spacevelocity of 1/min is needed for 90% conversion of A in a plug flow reactor, find the corresponding space-time and mean residence time or holding time of fluid in the plug flow reactor. Solution: min 11==sτ, Varying volume system, so t can’t be found.In an isothermal batch reactor 70% of a liquid reactant is converted in 13min. What space-time and space-velocity are needed to effect this conversion in a plug flow reactor and in a mixed flow reactor Solution: Liquid reaction system, so 0=A ε According to on page 92, min 130=-=⎰AX AAAo r dC C t , AAAo A A Ao R F M r X C r C C -=--=..τ, R F M ..τ can’t be certain. , ⎰-=AX AAAo R F P r dX C 0..τ, so m in 13...==R B R F P t τWe plan to replace our present mixed flow reactor with one having double thebolume. For the same aqueous feed (10 mol A/liter) and the same feed rate find the new conversion. The reaction are represented byA → R, -r A = ASolution: Liquid reaction system, so 0=A εA A Ao Ao r X C F V -==τ, 5.1)]1([)(A Ao A A Ao A Ao X C k X r C C C -=--Now we know: V V 2=', Ao Ao F F =', Ao Ao C C =', 7.0=A X So we obtain5.15.15.15.1)1()2)1(2A Ao A A Ao A Ao Ao X kC X X kC X F VF V -='-'==''52.8)7.01(7.02)1(5.15.1=-⨯='-'∴A AX X 794.0='A XAn aqueous feed of A and B (400liter/min, 100 mmol A/liter, 200 mmol B/liter)is to be converted to product in a plug flow reactor. The kinetics of the reaction is represented byA +B→ R, -r A = 200C A C Bmin⋅liter molFind the volume of reactor needed for % conversion of A to product. Solution: Aqueous reaction system, so 0=A εAccording to page 102 ,⎰⎰-=-==Af AfX AA X A A AoAo Ao r dX r dC C C t F V 001⎰-==AfX AAAo or dX C Vντ, m in /400liter o =ν, L r dX r dX C V AAX A A o Ao Af3.1244001.0999.000=-⨯=-=∴⎰⎰ν5.9 A specific enzyme acts as catalyst in the fermentation of reactant A. Ata given enzyme concentration in the aqueous feed stream (25 liter/min) find the volume of plug flow reactor needed for 95% conversion of reactant A (C A0 =2 mol/liter ). The kinetics of the fermentation at this enzyme concentration is given byA −−→−enzymeR , -r A = litermolC C A A ⋅+min 5.011.0Solution: according to page 102 , aqueous reaction, 0=ε⎰-=A X AA Ao r dX F V 0 )11(21251.05.010A AX A A A Ao X X Ln dX C C F V A+-⨯=+=∴⎰\L Ln4.986)95.005.01(125=+=Enzyme E catalyses the fermentation of substrate A (the reactant) to productR. Find the size of mixed flow reactor needed for 95% conversion of reactant in a feed stream (25 liter/min ) of reactant (2 mol/liter) and enzyme. The kinetics of the fermentation at this enzyme concentration are given byA −−→−enzymeR , -r A = litermolC C A A ⋅+min 5.011.0Solution: min /25L o =ν, L mol C Ao /2=, m in /50mol F Ao =, 95.0=A X Constant volume system, so we obtainmin 5.199205.05.01205.01.095.02=⨯⨯+⨯⨯⨯=-==AAAo or X C Vντ,39875.4min /25min 5.199m L V o =⨯==τν5.14 A stream of pure gaseous reactant A (C A0 = 660 mmol/liter) enters a plugflow reactor at a flow rate of F A0 = 540 mmol/min and polymerizes the as follows3A → R, -r A = 54min⋅liter mmolHow large a reactor is needed to lower the concentration of A in the exit stream to C Af = 330 mmol/literSolution: 321131-=-=A ε, 75.0660330321660330111=⨯--=+-=Ao A A Ao A A C C C C X ε 0-order homogeneous reaction, according to page 103A Ao AoAooX C F VC kV kk ===ντ So we obtainL X k C C F V A Ao Ao Ao 5.75475.05401=⨯==Gaseous reactant A decomposes as follows:A → 3 R, -r A = C AFind the conversion of A in a 50% A – 50% inert feed (υ0 = 180 liter/min, C A0 =300 mmol/liter) to a 1 m 3 mixed flow reactor. Solution: 31m V =, 1224=-=A ε According to page 91 , AAAoAAo AAAo oX X C X C r X C V+-=-==116.0ντmin/1801000)1(6.0)1(L LX X X A A A =-+=So we obtain 667.0=A XChapter 6 Design for Single ReactionsA liquid reactant stream (1 mol/liter) passes through two mixed flow reactorsin a series. The concentration of A in the exit of the first reactor is mol/liter. Find the concentration in the exit stream of the second reactor.The reaction is second-order with respect to A and V2/V1=2.Solution:V 2/V1= 2, τ1 =011υV=AAArCC--10 ,2τ =022υV=221AAArCC--CA0=1mol/l , CA1=l ,0201υυ=, -r A1=kC2 A1 ,-rA2=kC2 A2 (2nd-order) , 2×211AAAkCCC-=2221AAAkCCC-So we obtain 2×/=/(kCA22)CA2= mol/lWater containing a short-lived radioactive species flows continuously through a well-mixed holdup tank. This gives time for the radioactive material to decay into harmless waste. As it now operates, the activity of the exit stream is 1/7 of the fee d stream. This is not bad, but we’d like to lower it still more.One of our office secretaries suggests that we insert a baffle down the middle of the tank so that the holdup tank acts as two well-mixed tanks in series. Do you think this would help If not, tell why; if so calculate the expected activity of the exit stream compared to the entering stream. Solution: 1st-order reaction, constant volume system. From the information offered about the first reaction,we obtain1τ=01100117171A A A A A A C k C C kC C C V ⋅-=-=υ If a baffle is added,022220212122212υυτττV V +=+==011υV =2222221210A A A A A A kC C C kC C C -+-=007176A A kC C =6/k …… ① 02112121021υV kC C C A A A =-=3/k=222221A A A kC C C - …… ②Combining equation ① and ② we obtain:C A21= ; C A22==0161A C So it will help, and the expected activity of the exit stream is 1/16 of the feed.An aqueous reactant stream (4 mol A/liter) passes through a mixed flow reactorfollowed by a plug flow reactor. Find the concentration at the exit of the plug flow reactor if in the mixed flow reactor C A = 1 mol/liter. The reaction is second-order with respect to A, and the volume of the plug flow unit is three times that of the mixed flow unit. Solution: Constant volume system and 2nd-order reaction:υτmm V ==110A A A r C C --=2110A A A kC C C ->k 14-=3/k …… ① 03υυτmpp V V ===9/k= -⎰-fA A C C AA r dC 1= -⎰-Af C A AdC k C 12=)11(1-Af C k …… ②Combining equation. ① and ② we obtain:C Af = mol/literReactant A (A → R,C A0=26 mol/m 3) passes in steady flow through fourequal-size mixed flow reactors in series (τtotal=2 min). When steady stateis achieved the concentration of A is found to be 11, 5, 2, 1 mol/m 3 in the four units. For this reaction, what must be τplugso as to reduce C Afrom C A0 = 26 to C Af = 1 mol/m 3Solution:4321m m m m m τττττ=====110A A A r C C --=221A A A r C C --=332A A A r C C --=443A A A r C C --C A0=26mol/liter, C A1=11 mol/liter, C A2=5 mol/liter, C A3= 2mol/liter, C A4=1mol/liter So we abtain: 15/(-r A1) = 6/(-r A2) = 3/(-r A3) = 1/(-r A4) We postalate the reaction rate is 1 unit when C A4=1 mol/liter So we obtainC A , mol 11 5 2 1 -r A 30 12 6 2 1/(-r A )1/301/121/61/2⎰--=AfA C C A A p r dC 0τ=⎰-0A Af C C AAr dC So we obtain =p τ min.At 100℃ pure gaseous A reacts away with stoichiometry 2A → R + S in a constantvolume batch reactor as follows:t, sec 0 20 40 60 80 100 120 140 160 p A , atmWhat size of plug flow reactor operating at 100℃ and 1 atm can treat 100 moles A/hr in a feed consisting of 20% inserts to obtain 95% conversion of A Solution:。
有机合成知识学习

2021/3/29
17
----Preparation of Amine----
1. Reductionofnitrocompounds
Ar NO2 metal,H+ or
R NO2 orH2,cat.
Nitrocompounds
E xam p les: CO2C2H5
Ar NH2 or
R NH2
1o amine
n -B u tyl alco h o l 1-B u tan o l
n -B u tylald eh yd e B u tan al
2021/3/29
22
----Reactions of Aldehyde & Ketone----
1. Oxidation 2. Reduction 3. Addition of Cyanide 4. Addition Derivatives of ammonia 5. Addition of Alcohols 6. Cannizzaro Reaction 7. Addition of Grignard Reagent
chiefly for aromatic amine
CO2C2H5
Pt, H2
NO2
Ethyl p-nitrobenzoate
NH2
Ethyl p-aminobenzoate
Fe,HCl
CH3CH2CH2NO2
CH3CH2CH2NH2
1 -N itro p ro p a n e
n -p ro p ylam in e
RO Ar
YieldfromRX: CH3>1o>2o(>3o)
E x a m p le s :
羰基alpha碳上的反应

所以
P(B) 1- P(B) 0.8
由减法公式,得 P(A - B) P(A) - P(AB) 0.6 - 0 0.6 .
古典概型
定义:设E 是一个随机试验,若它满足以下两个条件: (1)仅有有限多个基本事件; (2)每个基本事件发生的可能性相等。
这类随机试验的数学模型称为古典概型。
定义:设试验E为古典概型试验,Ai,i=1,2,…,n是基本事件, 则由
4. 有限可加性:若事件 A 与事件 B 互不相容,则 P(A B) P(A) P(B)
(1.3.9)
一般地,若 n 个事件 A1, A2, , An 互不相容,则
P(A1 A2
An ) P(A1) P(A2) P(An)
5. 概率的加法公式:对任意两个事件 A 与 B ,有
P(A B) P(A) P(B) - P(AB)
P(A - B) P(A) - P(AB) P(A B) - P(B)
(1.3.13)
例 1.3.1 若 AB , P(A) 0.6,P(A B) 0.8 ,求 P(B) 及 P(A - B) . 解 由可加性有
P(A B) P(A) P(B)
得
P(B) P(A B) - P(A) 0.8 - 0.6 0.2
几何概型
定义:设E是一个随机试验,若它满足以下两个条件:
(1)如果一个随机现象的样本空间充满某个
几何区域,其度量(长度、面积、体积等)大小可以 用S表示;
(2)任意样本点落在度量相同的子区域内是等 可能的。 这类随机试验的数学模型称为几何概型。
定义 1.3.4 设试验 E 为几何概型试验,若随机事件 A 为 中的某个子区域,其度量大小
解 设 A ={取到一个正品一个次品} (1)有放回抽取,样本点总数为 6 6 36 ,事件 A 包含的样本点个数为 2 4 A12 16 , 所以
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
ClCH2COONa
NaCN
H3O
HOOCCH2COOH
C2H5OH H3O
H2C
CO2C2H5 CO2C2H5
NaOC2H5
HC
CO2C2H5 RX CO2C2H5
CO2C2H5 CO2C2H5
O H5C2OCCH2
O C OC2H5
CO2C2H5 R CH NaOC2H5 CO2C2H5
C N OH
O C C NH C N OH N C OH
tautomerism-racemate (互变异构-消旋化)
O CH2CH CH2 CH3 10%KOH EtOH OK CH2CH CH2 CH3 O CH2CH CH2 CH3
>95%
C6H5 O H C C CH3 H3C
EtO EtOH
C4H9-n
Synthesis of dione
O O ClCH2Cl C2H5ONa CH3CCHCHO2C2H5 2CH3CCH2CO2C2H5
O O H2 O C CHCCH3 a. OH / H2O CH3CCH CH3CCH2 CO2C2H5 b. H3O CO2C2H5
O CH2CCH3
very strong base
(C6H5)3CNa
α-H and no α-H
O O C H C OC2H5 + H3C C OC2H5 1. 2H5ONa 2. H3O O C2H5O C OC2H5 + C6H5CH2 O C OC2H5 C 1. 2H5ONa C6H5CH(COOC2H5)2 2. H3O O O HC CH2C OC2H5
C6H5 O C C CH3 H3C
EtO EtOH
C6H5 O H3C C C CH3 H
(S)-
(R)-
(II). The aldol condensation-----β-hydroxyaldehyde
and
OH H O C C H C H CH3
ketone
H3CH2C O C H + H3CHC H O C H OH H3CH2C
con. OH
O CH3CCH CO2C2H5 C O O R X C R
dil. OH
con. OH
O HOCCH2
O C R
Using
O H O O 2 R-X NaOC2H5 H3C C C C OC2H5 H3C C CHCO2C2H5
O R H3C C CHCO2C2H5
a. OH / H2O + b . H3O
Preparation of acid
1. substituted acetic acid
H2C CO2C2H5 CH3(CH2)2CH(CO2C2H5)2 CO2C2H5 b. CH3(CH2)2Br CH2COOH a. OH / H2O (CH2)2 CH3(CH2)2 CH2COOH b. H3O/ CH2COOH
CH(CO2C2H5)2 b. H5C2O2CCHBr H3C CHCO2C2H5 CH3
a. NaOC2H5
CH2COOH a. OH / H2O H3C CHCOOH b. H3O/
VI. 烯胺的烷化和酰化(Reaction with secondary amine )
C O H
O HNR2 > N H N H > N H
couple properties: ketone: HCN, NaHSO3, NH2OH, C6H5NHNH2 enol form: Br2/ CCl4 , FeCl3
keto form decomposition and acid form decomposition(酮式分解和酸式分解 )
H2 H3C C C C OC2H5 O O
dil. NaOH O
H3C C CH3
con. NaOH
O H3C C OH
Mechanism
alkylation and acylation of α-carbon
O CH3CCH2 R O HOCCH2 R O O CH3CCH2 C R
O CH3CCH CO2C2H5 R RX
dil. OH
O
+ (CH3CO)2O CH3COONa CHO 150oC 7h
O
C CHCOOH H
Knoevenagel: Condensation of aldehyde/ketone with active CH2
R C O R' + H2C Y Y' B Y R C C R' Y'
Y or Y'=COR, COOR, COOH, CN,NO2 etc Mechanism:
CH2
Cyclic ketone
O CH2CH2CO2C2H5 CH2CH2CO2C2H5 a. C2H5ONa b. H3O CO2C2H5 a. C H ONa 2 5 b. C2H5Br
O CO2C2H5 C2H5 a. OH / H2O b. H3O/
O C2H5
Using of diethyl malonate
CH3COONH4
H3C H3C
CN COOC2H5
O C
+ H2C
COOC2H5
H3C C C COOH H3C H
(III). Darzen Reaction
O RCR'(H) + ClCH2CO2C2H5 C2H5ONa R (H)R' O CO2C2H5
O
+
ClCH2CO2C2H5
KOC(CH3)3
H3C
O C CH3
+
H3C
O C CH3
OH H3C
OH H2 C C CH3
O C CH3
Condensation of aryl aldehyde
Perkin
:aryl aldehyde/anhydride
CHO + (CH3CO)2O CH3COONa 180oC 5h
H C CHCOOH 55%
Dickman Condensation
intramolecular
CO2C2H5 CO2C2H5
C 1. 2H5ONa 2. H3O
O CO2C2H5
intermolecular O CO2C2H5 CO2C2H5 C2H5O2C + CHOC 2 5 2 1.C2H5ONa C2H5O2C 2. H3O O CO2C2H5
R C
R'X
R C R'CO2C2H5
CO2C2H5
a. OH / H2O b. H3O/ R CH2 CO2H
CO2C2H5 H2C CO2C2H5 b. Br(CH2)4Br a. NaOC2H5
H R C CO2H R'
CO2C2H5 CO2C2H5
a. OH / H2O b. H3O/
a. OH / H2O CO2H b. H3O/
78%
CHO + CH3COCH2COOC2H5
Et2NH 0oC
CH CCOOC2H5 COCH3
78%
CHO
H2C COOH COOH
H3CO H3CO
H C CHCOOH H3CO H3CO
H C CHCONH
OCH3 OCH3
Py/piperidine
HOOC
抗变态药物:曲尼司特
CH3 CH3 CN
H O a. OH/H2O C6H5 H3C b. H
O C O C6H5
OH C CH CH3 C6H5
H C CH CH3
O
II. 酯缩合--Claisen Condensation
O 2 H3C C OC2H5 1. NaOC2H5 2. H3O H3C O O
CCH2 C OC2H5
Mechanism
NaOC2H5
O R H3C C CCO2C2H5
O R H3C C CH2
O R H3C C CCO2C2H5 R'
R'-X
O HC C CH3
a. OH / H2O b . H+O 3
O R H3C C CH R'
O O O a. C2H5ONa CH CCHCO C H a. NaOH / H2O CH3CCH2CO2C2H5 CH3CCH2 3 2 2 5 b. n-C4H9Br b. H3O C4H9-n
O CO2C2H5
Mechanism
H ClCHCO2C2H5 RO O H R C R'(H) R C C CO2C2H5 ClCHCO2C2H5 Cl (H)R' R (H)R' O CO2C2H5 O
Using
O C6H5 C CH3 + Br CH2CO2C2H5 NaOC2H5 C6H5 O H3C CO2C2H5
CO2C2H5 CO2C2H5
CO2C2H5 + CO2C2H5
1. C2H5ONa 2.H3O
C2H5O2C C2H5O2C
O O
ketone and ester O O O 1.C2H5ONa C6H5CCH2CC6H5 C6H5CO2C2H5 + CH3CC6H5 2. H3O
O COCH3 1. C2H5ONa CO2C2H5 2. H3O O
Chapter 13 Reactions of Carbanion
Keto Form-Enol Form Tautomerism
