China2010
2010_上海世博会_中国馆屋顶花园“_新九州清晏”

SHANGHAI, CHINA, 2010中国 上海2010上海世博会中国馆屋顶花园“新九州清晏”"XIN-JIU-ZHOU-QING-YAN" ROOF GARDEN, CHINA PAVILION 2010 EXPODesigned and built for post-expo public activities, the roof garden below the giant red umbrella of China Pavilion is the largest in Shanghai. The main idea is to soften the monumentality of the China Pavilion and to provide more public space. It is based on the old Chinese diagram of “Jiu-Zhou-Qing-Yan” (Nine Continents in Harmony), which was first implemented in the Grand Summer Palace of the Qing dynasty. “Jiu-Zhou,” or Nine Continents, is an ancient Chinese system that represents the universe. The “New Jiu-Zhou-Qing-Yan” re-interprets it with a matrix of contemporary Chinese gardens, each representing a unique climatic and geological situation, including: farmland, wetland, water, mountain, forest, savanna, rupture, desert, and town. The original “Jiu-Zhou-Qing-Yan” is one of the main scenic spots in the Grand Summer Palace. It has nine islands/continents, each with its own name, including a major one and eight supporting ones. Although all the structures are burnt out now, the forms and atmosphere still exist. Seeing reproduction of these places, we can realize the power of its design concept. Therefore, the architects decided to relocate the islands to the areas surrounding the China Pavilion to provide a culturally significant landscape. Each “continent,” except “town,” is surrounded by slightly undulating ground, and the theme is conveyed in the center by the arrangement of plants, paving and furniture. The “continents” are separated by water surface and connected by footpaths. Four groups of structures and furniture are scattered in these “continents,”forming tea room, café, restaurant and so on, in order to enrich the public service function of the roof garden.上海世博会中国馆屋顶花园“新九州清晏”在世博会期间用于密集人流的休憩与疏散,在世博会后作为上海市重要的屋顶开放空间,为市民与游客提供长久的休闲与公共活动平台。
中国2010年上海世界博览会(中英对照)

中国2010年上海世界博览会世界博览会是人们灵感和思想的展示区。
自从1851年在伦敦举办的所有国家的工业盛展,世界博览会作为一项关于经济、科技和文化交流的盛大活动,其作用日益突出,为展示历史经验,交流创新的意念,发扬团队精神,展望未来提供了一个重要平台。
中国有着悠久的文明,促进国际交流并热爱世界和平。
由于国际社会的支持和中国改革开放的成功,中国赢得2010年世界博览会的举办权。
这将是第一次在发展中国家举办的世博会,这也寄予了全球人民对中国未来发展的美好期待。
那么2010的中国上海世博会将会向世界呈现什么呢?毫无疑问中国人民将会呈现出一个成功,壮观而难忘的展览会。
2010年世博会是将会是一项全面开发21世纪城市生活潜力以及城市进展重要阶段的一项盛事。
预计在2010年将有50%的世界人口会居住在城市。
未来的城市生活,是全球关注的话题,是世界各国,发达或欠发达国家和人民所关注的。
城市第一次作为世界博览会的主题, “城市,让生活更美好”将会在2010博览会中吸引世界各国政府和人民的关注。
为其184天,参加各国将全力显示其在新世纪的城市文明程度,充分交流城市发展的经验、传播先进城市发展概念和城市人居环境探索新思路、生活和工作条件。
他们将会学习如何创造一个经济友好型社会和如何维持人类的可持续发展。
2010世博会的核心是创新和互动。
创新是灵魂,而文化交流也是世博会的一项重要任务。
在新纪元,2010世博会将致力于以人为本的发展,科技创新,文化差异以及双赢的未来合作,因此在新世纪高度的创新和互动将会是组成这一主旋律的重要音符。
2010年世博会也是一个盛大的国际集会。
一方面,我们要努力吸引大约200个国家和国际组织和7千万国内外的参观者来参加世博会,以确保是史上最盛大的世博会。
另一方面,我们要以全球视野来看世博会,尽力让更多的人参与,获得各国人民的支持和理解;把2010世博会变成一场世界各地人民欢聚的盛会。
此外,2010世博会使跨文化的对话成为可能。
2010世博会中国馆介绍中英文对照

2010世博会中国馆介绍中英文对照The China Pavilion represents the Chinese presence in Expo 2010. China Pavilion is located at the projecting area near the main entrance of Pudong Site, within Zone B of the Enclosed Area. It comprises the Chinese National Pavilion, Chinese Provinces Pavilion and Pavilion of Hong Kong, Macao and Taiwan.中国馆是代表中国参展上海世博会的展览场馆,位于浦东世博园区主入口的突出位置,世博会规划园区中围栏区的B片区,由中国国家馆、中国地区馆、港澳台馆三部分组成。
The contour design of China Pavilion is based on the concept of “Oriental Crown”, to express the spirit and disposition of Chinese culture. Rising from the Chinese National Pavilion is supported by traditional Dougong brackets fixed layer upon layer, concentrating Chinese elements and embodying Chinese spirit. The Chinese Provinces (Joint) Pavilion extends in flat under the Chinese National Pavilion, serving as a reliable platform, to build an open, mild, compatible and rich layered city square. The Chinese National Pavilion and Chinese Provinces Pavilion will create a spectacular urban space series, with well-defined functions up and down, delicately fitted major and subordinate architectural formation、and the spatial layout focused on the main axis in a north-south direction. Pavilion of Hong Kong, Macao and Taiwan will be self-build pavilion.中国馆建筑外观以“东方之冠”的构想主题,表达中国文化的精神与气质。
bauma china 2010将火爆登场

还有20 O 多家企业依然在等待展位。 本届展会与
往 届相 比 , 国大 量 跨 行 企 业 涌 入 工 程机 械 行 中
业。 这~方面显示了国家4 万亿投 资中大量 资金
最大工作压 力为2 M a 装在柴油机飞轮 中的弹性联轴 器 5 P。 进入铁路等基础建设 . 力拉动了 大 工程机械的 全功率 变量主泵与发动机用法兰直接连接,
被 四川 客户看 好 . 一次 性 实现 销 售2 台 。 0
5 0元 0亿
9 曰 月7 安徽省交通运输厅与国家开发银 行安徽分行签订战略合作协议 十二五 期间
国开行安徽省分行将向安徽省交通 建设提供 5 0 0 亿元贷款 。 其中高速公路3O 5 亿元 . 国省道改
造 10 元 . O亿 水运 行 业5 亿 元 。 0
如何实现真正的自主创新将是bu hn 00 amaC i 2 1 a 最值得探索的课题之一。 bu h a21吸引了逾10 家展商在超 amaC i 00 n 70
过 2 万平方 米 的超 大 展 示空 间诠 释最 新 产 品和 3
【 r 数 字】
技术. 同时将吸引来自全球逾1万专业观众共聚 3
徐工平地机 北上助推 吉林公 路建设
9j 日 , 6 在一阵阵热烈的鞭炮声 中. E 徐Z4 台GR ∞ 平地机整装待发 . 3 2 即将进入东北市场服务当地 公路及养护建设 这也是进入2 1年 以来, 00 徐工筑路第五次实现大批量发车。
此次徐工平地机中标的是吉林省高等级公路建设局及吉林省高速公路管理局养护设备招标采购
l 2 中国 咯10 01 2 2 1 9
市 场 需 求 同时 表 明 工程 机 械 行业 是 个 开 放 程
驱动主泵 工作. 可以有效消除柴油机的振动对主泵的影响。
MEDTEC China 2010为中国医疗设备设计与制造业带来创新的解决方案

( 上接第 5 8页 ) 作的态 度,每 次维修 工作时都 能尽善 尽美 的完成 ,养 遇 还没 有 光顾 体 ,那就 耐心 准 备。我 们 身处 在 一个 成追求卓越的 习惯 。好的习惯是成功 的基石 。 越来 越开放 、越 来越公平 的社会,只要 自身能力足够 ,
总之 .机遇总 是 留给那些有 准备 的人 的,如果机 总是台 有机遇可以把握 的。
需的一 系列组件 设备 及技术 ,包括 从医疗级 原材料 “ a iWo l” 网罗展 示快 速碌型设计 、快速 制造 R pd r d
到制造 设备组 件、 电子,组 装、生产 及加工 机械、包 及捺加 制造 技术的参 展商。 这些技 术已被欧 洲医疗设 装材料 、杀菌 、制造技术、分包和 外包服务。 备制 造商广 泛应用 ,可缩短 了产 品生产时 间、减 少消 耗或降低运 行成本, 制造商从中获益 。而 D s n d 让 e i Me g
可与所有国际 品 会面 牌
Chi 则为 从事设 计 与生 产电子 医疗 系统的 电子 设 ia l
作 为 相 关 行 业 的 国 际 领 先 展 会. 一 年 一 度 的 计和 制造工 程师介绍 全新 的产品 ቤተ መጻሕፍቲ ባይዱ制造技 术。这 两个
疗 ME DTE ia发 挥着 重要 的 桥梁 作用 .拉近 国 内 元素将 会为中国 医 设备制造 商提供机会 .学习技术 , C Ch n
外 医 疗 设 备 制 造 商 的 差 距。 例 如 ,已 经 4次 参展 ME DTE i a C Ch n 布朗克 工业公司表 示,在展会上能 与 很 多有潜质 的客户 建立联 系,^ 而拓 展公 司在中国 的 研讨会让 参观者的展会之旅更蕾成效 L 业务。另一参展 商 S ba也认 为,展 会能帮助他们吸 引 er 许多潜在的客 户并带来满意的结 果。 除 了展会外.ME DTE hn C C ia还设置全面的研讨会 项目.帮助参观者处 于行业领先 地位。夸年,研 讨台由
IC CHINA 2010参展商名单

27、展讯通信有限公司、Spreadtrum Communications Inc.、3B04
28、苏州国芯科技有限公司、C*Core Technology (Suzhou) Co.,Ltd、3B05-2
93、宁波康强电子股份有限公司、Ninbo Kangqiang Electronics Co.,Ltd、3B27-2
94、中国科学院微电子研究所、Institute of Microelectronics of China Academy of Sciences、3B28-1
4、北京华大智宝电子系统有限公司、Beijing Huada Zhibao Electronic System Co.,Ltd、3A03
5、国民技术股份有限公司、Nationz Technologies、3A03
6、成都华微电子科技有限公司、Chengdu Sino Microelectronics Technology Co.,Ltd、3A03
19、北方微电子、Beijing NMC Co.,Ltd.、3A11-1
20、北京七星华创电子股份有限公司、Beijing Sevenstar Electronics Co.,Ltd、3A11-2
21、沈阳芯源微电子设备有限公司、KingSemi Co.,Ltd、3A12
22、沈阳新松机器人自动化股份有限公司、SIASUN ROBOT & AUTOMATION CO.,LTD、3A12
43、西安翔腾微电子科技有限公司、、3B12
44、北京半导体行业协会、、3B15
全球经济止跌回升,医药行业喜迎契机 世界制药原料中国展(CPhI China2010)再度“引爆”全球医药市场

)\ 一 — / 一 — — ~ L / —
3 医药行业喜迎契机
世界制 药原料 中国展( P I h a 0 0 ̄度 “ C h i 1) - C n2 4 引爆”全球 医药市场焦点
l 年之前 , 0 当首届世界制药原料 中国展 ( h h n 0 1 C IC ia 2 0 )的帷幕刚网拉开时 , P 4 正是中国制药工业整体起步发展 ,
4 陆 周平 . 医药 物流 模式 的转 变 和 “ S ” 的新课 题 [ ]. GP J
3 结 语
发展 医药现代物流是 国家 医药流通体制改革 的重 大 举措 ,对企业 而言如何结合实际完成好物流体 系的流程
』— / ) ~L — 一 \ L/ 一 3 ^ —— — \ 一L — — — \ 土 — — — 3 丑/ 3 工 — 一 —卫/
2 叶桦 ,陆 国平 . 浅谈 现代 医药物流背景下药 品经营监管 的
几个 问题 [ ] 巾国药事 ,20 ,2 4 : 9 . J . 0 6 0() 5 1 3 宋友华 . 代医药物流建设与药 品质量 管理 [ . 现 Jj 中国医
药 指 南 ,2 0 ,4 6 : 0 . 0 6 f) 12
量管理能级 ,是需要不断探讨和逐步完善的 。 参考文献
1 洪钢 . 浅析我国药品现代物流的发展现状 、内涵和特点 [ ] J.
中同药事,20 ,2 3 :9 . 08 2( 17 )
检查记录 、内部审核记录 ,顾客投诉记 录等 ,实 际上是 对不规范和随意性 的分析和评判 。 分析不合格项产生的原 因, 出整改方案 , 提 下发 “ 纠 正和预防措施通知” ,并督促 、验证责任部门对措施的实 施情况及效果 ,同时配套相应 的考核措施 。完善过程 质 量控制 ,制止和避免不合格 的发生 ,既是 医药 现代物 流 的标准化管垃需要 ,也是药品质量管理体 系的推进 。
Finetech参展NEPCONChina2010-在德国展团展示其高端返修设备

2 S t n , . R. r ly J L u K. n wd n D. h n g a , . e s n I M e s D. o e W . u s e , n S l v n “ a — r e . mea a J , Ho se , . a , S o o , S a g u n J Gl a o , . mi, L v , Da k h r a d B. u l a , d F e i De in Ma e il, n r c s f g n i a k g s , r c e i g f EX, a em, sg , t r s a d P o e so Hi h De st P c a e ” P o e d n so AP a y An h i CA, CD- ROM , a c 0 3 M rh 2 0 . 3 La , .N. o R. rly J S ea a D. h n g a , u s e , Lo e I M e i, n S l v , “Reib ly T s d Daa . uJ , Ho , Ho s , . m t n , e S a g u n W Da k h r D. v , . n s a d B. u l a in l i t e t a i n a t
a dT c oo n ee c , 0 1 P . 97 . n e h l g Co f rn e 2 0 , P 6 - 5 n y 6 La , . C. o g N. h n , n L e E e to i sM a u a trn t e d F e , l g n F e , n n u t eAd e i eM ae as . u J, W n , C e g a d R. e , lc r n c n fc u i gwi L a - r e Hao e - r e a d Co d ci - h s tr l, h v v i
2010年度中国十大类行业电子商务网站调查数据

2010年度中国十大类行业电子商务网站调查数据化工行业中国化工网以70.0%市场占有率位稳居第一,据报告监测调查显示:2010年化工行业电子商务网站的市场份额中(按年营收状况),中国化工网以70.0%的市场份额占有率位居第一,排在第二的则为隆众石化商务网,市场份额为5.5%,中国万维化工城以3.4%的市场占有率排在第三,紧随其后的为中国化工信息网、易贸网分别占有2.7%、1.8%的市场份额。
调查显示:作为国内第一家行业电子商务网站的中国化工网在化工领域以其绝对的优势多年稳居第一位,该网是目前国内客户量最大、数据最丰富、访问量最高的化工网站,并且建有国内最大的化工专业数据库,内含40多个国家和地区的11万多个化工站点,含51万多家化工企业,150多万条化工产品记录。
调研显示:主要客户群为化工行业制造和流通企业,大客户包括中石化、中石油、中海油等;客户广泛分布于长三角、珠三角等经济发达区域与中西部地区。
报告研究表明:化工行业信息化程度与国内其他行业相比,属于前列水平,化工行业也是国民经济中最适合电子商务的产业之一。
对此,报告建议:在欧盟REACH背景下,我国化工企业正面临贸易壁垒困境,而借助第三方电子商务平台是较为有效方式之一。
钢铁行业我的钢铁网以55.5%市场占有率排名第一,近年来,我国钢铁行业发展形势严峻,产能严重过剩已成为行业一大痼疾。
另外,整个钢铁行业内仍存在着信息不畅、上下游脱节、资源分散等种种问题。
在此背景下,电子商务以其快捷的交易速度、便捷的交易方式、广阔的交易范围和低廉的交易成本受到了各大钢企的广泛关注,为它们提供了一条能够有效实现“产供销”一体化,快速响应市场变化,提升企业核心竞争力的崭新的发展之路。
调查数据显示:2010年我国钢铁行业电子商务网站市场占有率方面(按年营收状况),我的钢铁网以55.5%的市场占有率排名第一,排在第二的为中国联合钢铁网市场占有率为6.9%、排名第三的则为兰格钢铁网市场份额为5.2%,而海鑫钢材信息网、钢之家、今日钢铁网等紧随其后。
1929年-2010年中国历年GDP和美国历年GDP、日本历年GDP比较

1929年-2010年中国历年GDP和美国历年GDP、日本历年GDP比较20111001630.90234420112011 USA GDP USD$159241.8亿美元2010974743.720102010 USA GDP USD$146578亿美元2009963342.781520092009 USA GDP USD$141190.5亿美元20081048799.12639120082008 USA GDP USD$143690.8亿美元20071098648.43420072007 USA GDP USD$140618亿美元20061081427.61507820062006 USA GDP USD$133989.3亿美元20051028168.9420052005 USA GDP USD$124220亿美元2004967250.2220042004 USA GDP USD$116860亿美元2003907241.97 20032003 USA GDP USD$109610亿美元2002866601.9 20022002 USA GDP USD$104700亿美元2001838294.56 20012001 USA GDP USD$101280亿美元2000812749.43 20002000 USA GDP USD$98170亿美元1999767205.04 19991999 USA GDP USD$92680亿美元1998724164.13 19981998 USA GDP USD$87470亿美元1997688509.513 19971997 USA GDP USD$83043亿美元1996649897.066 19961996 USA GDP USD$78169亿美元1995617781.927 19951995 USA GDP USD$73977亿美元1994609552.918 19941994 USA GDP USD$70722亿美元1993383599.388 19931993 USA GDP USD$66574亿美元1992349587.532 19921992 USA GDP USD$63377亿美元1991319161.757 19911991 USA GDP USD$59959亿美元1990277562.273 19901990 USA GDP USD$58031亿美元1989206487.66 19891989 USA GDP USD$54844亿美元1988189963.436 19881988 USA GDP USD$51038亿美元1987176404.19 19871987 USA GDP USD$47395亿美元1986154100.484 19861986 USA GDP USD$44628亿美元1985123950.211 19851985 USA GDP USD$42203亿美元198491289.572 19841984 USA GDP USD$39332亿美元198369885.192 19831983 USA GDP USD$35367亿美元198261617.15 19821982 USA GDP USD$32550亿美元198153339.22 19811981 USA GDP USD$31284亿美元198041786.71 19801980 USA GDP USD$27895亿美元197939859.315 19791979 USA GDP USD$25633亿美元197838642.748 19781978 USA GDP USD$22947亿美元197737734.122 19771977 USA GDP USD$20309亿美元197635429.073 19761976 USA GDP USD$18253亿美元197530455.997 19751975 USA GDP USD$16383亿美元197429415 19741974 USA GDP USD$15000亿美元197327501.903 19731973 USA GDP USD$13827亿美元197227799.835 19721972 USA GDP USD$12383亿美元197127749.202 19711971 USA GDP USD$11271亿美元197025640.565 19701970 USA GDP USD$10385亿美元196924309.774 19691969 USA GDP USD$9846亿美元196822467.9 19681968 USA GDP USD$9100亿美元196720556.894 19671967 USA GDP USD$8326亿美元196619450.782 19661966 USA GDP USD$7878亿美元196517754.579 19651965 USA GDP USD$7191亿美元196416384.284 19641964 USA GDP USD$6636亿美元196315251.013 19631963 USA GDP USD$6177亿美元196214458.464 19621962 USA GDP USD$5856亿美元196113448.643 19611961 USA GDP USD$5447亿美元196012996.816 19601960 USA GDP USD$5264亿美元195912507.954 19591959 USA GDP USD$5066亿美元195811535.168 19581958 USA GDP USD$4672亿美元195711384.559 19571957 USA GDP USD$4611亿美元195610801.875 19561956 USA GDP USD$4375亿美元195510241.412 19551955 USA GDP USD$4148亿美元19549955.068 19541954 USA GDP USD$3804亿美元195319531953 USA GDP USD$3794亿美元19529376.711 19521952 USA GDP USD$3583亿美元19517593.534 19511951 USA GDP USD$3393亿美元19508079.5 19501950 USA GDP USD$2938亿美元19496147.9 19491949 USA GDP USD$2673亿美元194819481948 USA GDP USD$2692亿美元194719471947 USA GDP USD$2442亿美元194619461946 USA GDP USD$2223亿美元194519451945 USA GDP USD$2231亿美元194419441944 USA GDP USD$2198亿美元194319431943 USA GDP USD$1986亿美元194219421942 USA GDP USD$1619亿美元194119411941 USA GDP USD$1267亿美元194019401940 USA GDP USD$1014亿美元193919391939 USA GDP USD$922亿美元193819381938 USA GDP USD$861亿美元193719371937 USA GDP USD$919亿美元193619361936 USA GDP USD$838亿美元193519351935 USA GDP USD$733亿美元193419341934 USA GDP USD$660亿美元193319331933 USA GDP USD$564亿美元193219321932 USA GDP USD$587亿美元193119311931 USA GDP USD$765亿美元193019301930 USA GDP USD$912亿美元192919291929 USA GDP USD$1036亿美元201147156420112011 CHINA GDP USD$74970.429253亿美元(国家统计局CPI:5.4%,GDP增长率:9.2%,人口:134735万人)2011年中国真实CPI:9.3%,2011年中国人均GDP:34999.4元201039798320102010 CHINA GDP USD$59847.067669亿美元(国家统计局CPI:3.3%,GDP增长率:10.4%,人口:134100万人)2010年中国真实CPI:6.3%,2010年中国人均GDP:29678.1元2009340903.120092009 CHINA GDP USD$49963.812656亿美元(国家统计局CPI:-1%,GDP增长率:9.1%,人口:133474万人)2009年中国真实CPI:-0.5%,2009年中国人均GDP:25540.8元2008314045.420082008 CHINA GDP USD$43025.812611亿美元(国家统计局CPI:5.9%,GDP增长率:9.6%,人口:132802万人)2008年中国真实CPI:8.5%,2008年中国人均GDP:23647.6元2007265810.320072007 CHINA GDP USD$34021.542621亿美元(国家统计局CPI:4.8%,GDP增长率:11.4%,人口:132129万人)2007年中国真实CPI:11.5%,2007年中国人均GDP:20117.5元2006216314.420062006 CHINA GDP USD$26801.438019亿美元(国家统计局CPI:1.5%,GDP增长率:10.7%,人口:131448万人)2006年中国真实CPI:6.3%,2006年中国人均GDP:16456.3元2005184937.420052005 CHINA GDP USD$22343.53102亿美元(国家统计局CPI:1.8%,GDP增长率:10.4%,人口:130756万人)2005年中国真实CPI:5.3%,2005年中国人均GDP:14143.7元2004159878.320042004 CHINA GDP USD$19315.971593亿美元(国家统计局CPI:3.9%,GDP增长率:10.1%,人口:129988万人)2004年中国真实CPI:7.6%,2004年中国人均GDP:12299.5元2003135822.820032003 CHINA GDP USD$16409.66496亿美元(国家统计局CPI:1.2%,GDP增长率:10%,人口:129227万人)2003年中国真实CPI:2.9%,2003年中国人均GDP:10510.4元2002120332.720022002 CHINA GDP USD$14538.202625亿美元(国家统计局CPI:-.8%,GDP增长率:9.1%,人口:128453万人)2002年中国真实CPI:0.6%,2002年中国人均GDP:9367.8元2001109655.220012001 CHINA GDP USD$13248.182086亿美元(国家统计局CPI:.7%,GDP增长率:8.3%,人口:127627万人)2001年中国真实CPI:2.2%,2001年中国人均GDP:8591.8元200099214.620002000 CHINA GDP USD$11983.887132亿美元(国家统计局CPI:.4%,GDP增长率:8.4%,人口:126743万人)2000年中国真实CPI:2.2%,2000年中国人均GDP:7828元199989677.119991999 CHINA GDP USD$10833.184533亿美元(国家统计局CPI:-1.4%,GDP增长率:7.6%,人口:125786万人)1999年中国真实CPI:-1.4%,1999年中国人均GDP:7129.3元199884402.319981998 CHINA GDP USD$10194.745365亿美元(国家统计局CPI:-.8%,GDP增长率:7.8%,人口:124761万人)1998年中国真实CPI:-0.9%,1998年中国人均GDP:6765.1元19977897319971997 CHINA GDP USD$9525.147751亿美元(国家统计局CPI:2.8%,GDP增长率:9.3%,人口:123626万人)1997年中国真实CPI:1.7%,1997年中国人均GDP:6388.1元199671176.619961996 CHINA GDP USD$8561.053832亿美元(国家统计局CPI:8.3%,GDP增长率:10%,人口:122389万人)1996年中国真实CPI:7.1%,1996年中国人均GDP:5815.6元199560793.719951995 CHINA GDP USD$7279.810708亿美元(国家统计局CPI:17.1%,GDP增长率:10.9%,人口:121121万人)1995年中国真实CPI:15.2%,1995年中国人均GDP:5019.3元199448197.919941994 CHINA GDP USD$5592.052261亿美元(国家统计局CPI:24.1%,GDP增长率:13.1%,人口:119850万人)1994年中国真实CPI:23.3%,1994年中国人均GDP:4021.5元199335333.919931993 CHINA GDP USD$6132.228122亿美元(国家统计局CPI:14.7%,GDP增长率:14%,人口:118517万人)1993年中国真实CPI:17.2%,1993年中国人均GDP:2981.3元199226923.519921992 CHINA GDP USD$4880.982596亿美元(国家统计局CPI:6.4%,GDP增长率:14.2%,人口:117171万人)1992年中国真实CPI:9.4%,1992年中国人均GDP:2297.8元199121781.519911991 CHINA GDP USD$4091.959421亿美元(国家统计局CPI:3.4%,GDP增长率:9.2%,人口:115823万人)1991年中国真实CPI:7.5%,1991年中国人均GDP:1880.6元199018667.819901990 CHINA GDP USD$3902.948104亿美元(国家统计局CPI:3.1%,GDP增长率:3.8%,人口:114333万人)1990年中国真实CPI:6.1%,1990年中国人均GDP:1632.8元198916992.319891989 CHINA GDP USD$4513.227299亿美元(国家统计局CPI:18%,GDP增长率:4.1%,人口:112704万人)1989年中国真实CPI:8.9%,1989年中国人均GDP:1507.7元198815042.819881988 CHINA GDP USD$4041.59049亿美元(国家统计局CPI:18.8%,GDP增长率:11.3%,人口:111026万人)1988年中国真实CPI:13.4%,1988年中国人均GDP:1354.9元198712058.619871987 CHINA GDP USD$3239.817198亿美元(国家统计局CPI:7.3%,GDP增长率:11.6%,人口:109300万人)1987年中国真实CPI:5.8%,1987年中国人均GDP:1103.3元198610275.219861986 CHINA GDP USD$2975.731305亿美元(国家统计局CPI:6.5%,GDP增长率:8.8%,人口:107507万人)1986年中国真实CPI:5.2%,1986年中国人均GDP:955.8元1985901619851985 CHINA GDP USD$3069.799115亿美元(国家统计局CPI:9.3%,GDP增长率:13.5%,人口:105851万人)1985年中国真实CPI:11.6%,1985年中国人均GDP:851.8元19847208.119841984 CHINA GDP USD$3105.601076亿美元(国家统计局CPI:2.8%,GDP增长率:15.2%,人口:104357万人)1984年中国真实CPI:5.7%,1984年中国人均GDP:690.7元19835962.719831983 CHINA GDP USD$3017.560828亿美元(国家统计局CPI:1.5%,GDP增长率:10.9%,人口:103008万人)1983年中国真实CPI:1.1%,1983年中国人均GDP:578.9元19825323.419821982 CHINA GDP USD$2812.149975亿美元(国家统计局CPI:1.9%,GDP增长率:9.1%,人口:101654万人)1982年中国真实CPI:-0.3%,1982年中国人均GDP:523.7元19814891.619811981 CHINA GDP USD$2868.973664亿美元(国家统计局CPI:2.4%,GDP增长率:5.2%,人口:100072万人)1981年中国真实CPI:2.4%,1981年中国人均GDP:488.8元19804545.619801980 CHINA GDP USD$3034.445993亿美元(国家统计局CPI:6%,GDP增长率:7.8%,人口:98705万人)1980年中国真实CPI:4.1%,1980年中国人均GDP:460.5元19794062.619791979 CHINA GDP USD$2612.604564亿美元(国家统计局CPI:%,GDP增长率:7.6%,人口:97542万人)1979年中国真实CPI:3.9%,1979年中国人均GDP:416.5元19783645.219781978 CHINA GDP USD$2164.608047亿美元(国家统计局CPI:%,GDP增长率:11.7%,人口:96259万人)1978年中国真实CPI:2.1%,1978年中国人均GDP:378.7元19773201.919771977 CHINA GDP USD$1723.304576亿美元(国家统计局CPI:%,GDP增长率:7.6%,人口:94974万人)1977年中国真实CPI:1.2%,1977年中国人均GDP:337.1元19762943.719761976 CHINA GDP USD$1516.589362亿美元(国家统计局CPI:%,GDP增长率:-1.6%,人口:93717万人)1976年中国真实CPI:-0.2%,1976年中国人均GDP:314.1元19752997.319751975 CHINA GDP USD$1612.318477亿美元(国家统计局CPI:%,GDP增长率:8.7%,人口:92420万人)1975年中国真实CPI:-1.3%,1975年中国人均GDP:324.3元19742789.919741974 CHINA GDP USD$1422.692454亿美元(国家统计局CPI:%,GDP增长率:2.3%,人口:90859万人)1974年中国真实CPI:0.2%,1974年中国人均GDP:307.1元19732720.919731973 CHINA GDP USD$1367.973807亿美元(国家统计局CPI:%,GDP增长率:7.9%,人口:89211万人)1973年中国真实CPI:0.2%,1973年中国人均GDP:305元19722518.119721972 CHINA GDP USD$1121.64815亿美元(国家统计局CPI:%,GDP增长率:3.8%,人口:87177万人)1972年中国真实CPI:0.0%,1972年中国人均GDP:288.8元19712426.419711971 CHINA GDP USD$985.540172亿美元(国家统计局CPI:%,GDP增长率:7%,人口:85229万人)1971年中国真实CPI:0.7%,1971年中国人均GDP:284.7元19702252.719701970 CHINA GDP USD$912.393662亿美元(国家统计局CPI:%,GDP增长率:19.4%,人口:82992万人)1970年中国真实CPI:-3.2%,1970年中国人均GDP:271.4元19691937.919691969 CHINA GDP USD$784.892679亿美元(国家统计局CPI:%,GDP增长率:16.9%,人口:80671万人)1969年中国真实CPI:-4.4%,1969年中国人均GDP:240.2元19681723.119681968 CHINA GDP USD$697.893874亿美元(国家统计局CPI:%,GDP增长率:-4.1%,人口:78534万人)1968年中国真实CPI:1.2%,1968年中国人均GDP:219.4元19671773.919671967 CHINA GDP USD$718.469026亿美元(国家统计局CPI:%,GDP增长率:-5.7%,人口:76368万人)1967年中国真实CPI:0.7%,1967年中国人均GDP:232.3元1966186819661966 CHINA GDP USD$756.581612亿美元(国家统计局CPI:%,GDP增长率:10.7%,人口:74542万人)1966年中国真实CPI:-1.8%,1966年中国人均GDP:250.6元19651716.119651965 CHINA GDP USD$695.058718亿美元(国家统计局CPI:%,GDP增长率:17%,人口:72537万人)1965年中国真实CPI:1.0%,1965年中国人均GDP:236.6元1964145419641964 CHINA GDP USD$588.90239亿美元(国家统计局CPI:%,GDP增长率:18.3%,人口:70499万人)1964年中国真实CPI:-0.4%,1964年中国人均GDP:206.2元19631233.319631963 CHINA GDP USD$499.513993亿美元(国家统计局CPI:%,GDP增长率:10.2%,人口:69172万人)1963年中国真实CPI:-2.9%,1963年中国人均GDP:178.3元19621149.319621962 CHINA GDP USD$465.492122亿美元(国家统计局CPI:%,GDP增长率:-5.6%,人口:67295万人)1962年中国真实CPI:-0.2%,1962年中国人均GDP:170.8元1961122019611961 CHINA GDP USD$494.127177亿美元(国家统计局CPI:%,GDP增长率:-27.3%,人口:65859万人)1961年中国真实CPI:11.0%,1961年中国人均GDP:185.2元1960145719601960 CHINA GDP USD$590.117456亿美元(国家统计局CPI:%,GDP增长率:-.3%,人口:66207万人)1960年中国真实CPI:1.6%,1960年中国人均GDP:220.1元1959143919591959 CHINA GDP USD$582.827055亿美元(国家统计局CPI:%,GDP增长率:8.8%,人口:67206万人)1959年中国真实CPI:1.3%,1959年中国人均GDP:214.1元1958130719581958 CHINA GDP USD$529.364115亿美元(国家统计局CPI:%,GDP增长率:21.3%,人口:65994万人)1958年中国真实CPI:1.1%,1958年中国人均GDP:198元1957106819571957 CHINA GDP USD$432.563791亿美元(国家统计局CPI:%,GDP增长率:5.1%,人口:64653万人)1957年中国真实CPI:-1.2%,1957年中国人均GDP:165.2元1956102819561956 CHINA GDP USD$416.3629亿美元(国家统计局CPI:%,GDP增长率:15%,人口:62828万人)1956年中国真实CPI:-2.0%,1956年中国人均GDP:163.6元195591019551955 CHINA GDP USD$368.570271亿美元(国家统计局CPI:%,GDP增长率:6.8%,人口:61465万人)1955年中国真实CPI:-0.9%,1955年中国人均GDP:148.1元195485919541954 CHINA GDP USD$328.238441亿美元(国家统计局CPI:%,GDP增长率:4.2%,人口:60266万人)1954年中国真实CPI:0.0%,1954年中国人均GDP:142.5元195382419531953 CHINA GDP USD$亿美元/GDP/#经济数据2011375783.27343820112011 JAPAN GDP USD$59742.97亿美元2010363014.84980520102010 JAPAN GDP USD$54588.7亿美元2009343400.2307320092009 JAPAN GDP USD$50329.8亿美元2008356698.480520082008 JAPAN GDP USD$48869.5亿美元2007342050.027008 20072007 JAPAN GDP USD$43779.6亿美元2006352103.838105 20062006 JAPAN GDP USD$43625.8亿美元2005376784.753367 20052005 JAPAN GDP USD$45521.9亿美元2004381233.640867 20042004 JAPAN GDP USD$46059.4亿美元2003350042.607 20032003 JAPAN GDP USD$42291亿美元2002324320.180566 20022002 JAPAN GDP USD$39183.3亿美元2001338982.886066 20012001 JAPAN GDP USD$40954.8亿美元2000386418.1855 20002000 JAPAN GDP USD$46674.5亿美元1999361643.475867 19991999 JAPAN GDP USD$43687.3亿美元1998319323.520168 19981998 JAPAN GDP USD$38570.3亿美元1997353349.141445 19971997 JAPAN GDP USD$42618.4亿美元1996385981.607 19961996 JAPAN GDP USD$46425.5亿美元1995439628.380324 19951995 JAPAN GDP USD$52643.8亿美元1994411901.134633 19941994 JAPAN GDP USD$47789.9亿美元1993250122.072797 19931993 JAPAN GDP USD$43408.9亿美元1992208602.989109 19921992 JAPAN GDP USD$37817.8亿美元1991184438.228059 19911991 JAPAN GDP USD$34649.3亿美元1990144927.2915 19901990 JAPAN GDP USD$30300.5亿美元1989111134.137559 19891989 JAPAN GDP USD$29517.7亿美元198810979919881988 JAPAN GDP USD$29500亿美元198790429.712 19871987 JAPAN GDP USD$24296亿美元198669174.636902 19861986 JAPAN GDP USD$20033.2亿美元198539710.001053 19851985 JAPAN GDP USD$13520.6亿美元198429313.765347 19841984 JAPAN GDP USD$12629.8亿美元198323453.340828 19831983 JAPAN GDP USD$11869.1亿美元198220598.11197 19821982 JAPAN GDP USD$10881.2亿美元198119962.822666 19811981 JAPAN GDP USD$11708.4亿美元1980年日本GDP(国内生产总值):CNY¥15869.512107亿元1980年汇率1.498:1美元,1980年GDP实际增长%1980 JAPAN GDP USD$10593.8亿美元。
中国2010版gmp指南《厂房设施与设备》英文

我国2010版gmp指南《厂房设施与设备》英文指的是Good Manufacturing Practice for Pharmaceutical Products,即《药品生产质量管理规范》的一部分。
这一部分主要涵盖了药品生产中的厂房设施和设备的管理规范,对药品生产的安全性、质量以及合规性具有重要意义。
让我们从我国2010版gmp指南《厂房设施与设备》规范的背景和意义开始。
在药品生产过程中,厂房设施和设备的良好管理对于保障药品质量至关重要。
符合规范的厂房设施和设备能够有效地减少污染物对药品质量的影响,确保药品的安全性和有效性,同时也能够提高生产效率,降低生产成本。
严格遵守我国2010版gmp指南《厂房设施与设备》规范是药品生产企业不可或缺的责任和义务。
接下来,我们需要重点关注我国2010版gmp指南《厂房设施与设备》规范的内容和要求。
根据该指南,厂房设施和设备的管理应包括但不限于以下几个方面:设计与布局、建筑结构、空气洁净度、污染控制、设备和用品的清洁与消毒、工程材料的选择等。
这些内容涵盖了药品生产过程中所涉及的关键环节,对于保障药品质量具有重要的作用。
在实践中,药品生产企业需要根据我国2010版gmp指南《厂房设施与设备》规范的要求,对厂房设施和设备进行严格管理和监控。
这包括严格执行设备清洁和消毒的操作规程,定期进行空气洁净度的检测和维护,加强对建筑结构和工程材料的管理等。
只有通过严格的管理和监控,药品生产企业才能确保厂房设施和设备的安全性和可靠性,保障药品质量的稳定性和一致性。
总结起来,我国2010版gmp指南《厂房设施与设备》规范对于药品生产企业而言具有重要的指导意义。
严格遵守该规范的要求,能够有效地保障药品的质量和安全性,提高生产效率,降低生产成本,最终实现药品生产的可持续发展。
作为药品生产质量管理的重要组成部分,我国2010版gmp指南《厂房设施与设备》规范的实施对于推动我国药品质量管理体系的建设和完善具有重要意义。
LS产电亮相EP China2010展

开放 式办公 区 中 地 板 下 或 者 天 花 板 上 对 于 多 信
息点 的需要 。
安普 布线 为 中 国人 民银 行 长沙 中 心 支行 省
级数 据 中心 机 房 的 网络 系 统 提 供 了高 性 能 的 产 品 以及完 整贴 合 的解决 方 案 , 个 布线 系统 为 今 整 后 的网络 系统 正常运 行 提供 了可靠 的保 障 。
展览 中心揭 开序 幕 。L s产 电携 旗 下 全 系列 最 新
款产 品及 解决 方案亮 相 此 次 展会 , 中涉及 了在 其 低压 电 力 方 面 拥 有 世 界 级 品 质 水 平 的 “ uo S sl /
及节 能理 念为 一体 的 电力 系 统设 备 、 系列再 生 能 源、 电动 汽车 零 部 件 和充 电 系统 、 电力 节 能 化 系 统、 自动 化设 备等 。
据 中心 “ 高性 能 、 高可靠 性 、 密度 、 维 护性 、 高 可 可 扩 展性 ” 的高 标 准性 能 要求 , 房 布 线 系统 选 用 机
安普 6类铜 缆 系 统 、 兆 光 纤 系 统 、 O高 密度 万 MP
布线 系统 及 Sg aLn i .ik预 端 接 布 线 系 统 。 中 国 m
・
信 息之窗 ・
L S产 电 亮 相 E hn 2 1 P C ia 0 0展
21 0 0年 1 0月 1 9日, 十三届 国际 电力 设备 第 及技 术 展览 会 ( P C i 0 0 在北 京 中 国 国际 E hn 2 1 ) a 保 电力装 备 、 超导 限流 器 以及作 为 新事 业 领域 的 绿 色汽 车零 部 件 、 电力 半 导 体 模 块 、 料 电 池 、 燃 L D、 能建 筑领域 。 E 节 在此 次展 会上 ,S产 电展 出了集 成 电力环 保 L
2010宝马展展商名录
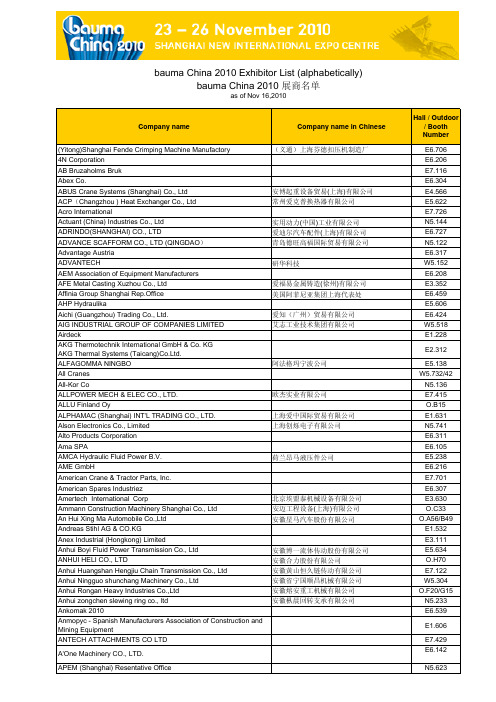
Company nameCompany name in ChineseHall / Outdoor/ Booth Number(Yitong)Shanghai Fende Crimping Machine Manufactory (义通)上海芬德扣压机制造厂E6.7064N Corporation E6.206AB Bruzaholms BrukE7.116Abex Co.E6.304ABUS Crane Systems (Shanghai) Co., Ltd 安博起重设备贸易(上海)有限公司E4.566ACP (Changzhou ) Heat Exchanger Co., Ltd 常州爱克普换热器有限公司E5.622Acro InternationalE7.726Actuant (China) Industries Co., Ltd 实用动力(中国)工业有限公司N5.144ADRINDO(SHANGHAI) CO., LTD爱迪尔汽车配件(上海)有限公司E6.727ADVANCE SCAFFORM CO., LTD (QINGDAO )青岛德旺高福国际贸易有限公司N5.122Advantage Austria E6.317ADVANTECH研华科技W5.152AEM Association of Equipment Manufacturers E6.208AFE Metal Casting Xuzhou Co., Ltd 爱福易金属铸造(徐州)有限公司E3.352Affinia Group Shanghai Rep.Office美国阿菲尼亚集团上海代表处E6.459AHP HydraulikaE5.606Aichi (Guangzhou) Trading Co., Ltd.爱知(广州)贸易有限公司E6.424AIG INDUSTRIAL GROUP OF COMPANIES LIMITED 艾志工业技术集团有限公司W5.518AirdeckE1.228AKG Thermotechnik International GmbH & Co. KG AKG Thermal Systems (Taicang)Co.Ltd.E2.312ALFAGOMMA NINGBO 阿法格玛宁波公司E5.138All CranesW5.732/42All-Kor CoN5.136ALLPOWER MECH & ELEC CO., LTD.欧杰实业有限公司E7.415ALLU Finland OyO.B15ALPHAMAC (Shanghai) INT'L TRADING CO., LTD.上海爱中国际贸易有限公司E1.631Alson Electronics Co., Limited 上海创烁电子有限公司N5.741Alto Products Corporation E6.311Ama SPAE6.105AMCA Hydraulic Fluid Power B.V.荷兰昂马液压件公司E5.238AME GmbHE6.216American Crane & Tractor Parts, Inc.E7.701American Spares Industriez E6.307Amertech International Corp北京埃盟泰机械设备有限公司E3.630Ammann Construction Machinery Shanghai Co., Ltd 安迈工程设备(上海)有限公司O.C33An Hui Xing Ma Automobile Co.,Ltd 安徽星马汽车股份有限公司O.A56/B49Andreas Stihl AG & CO.KG E1.532Anex Industrial (Hongkong) LimitedE3.111Anhui Boyi Fluid Power Transmission Co., Ltd 安徽博一流体传动股份有限公司E5.634ANHUI HELI CO., LTD安徽合力股份有限公司O.H70Anhui Huangshan Hengjiu Chain Transmission Co., Ltd 安徽黄山恒久链传动有限公司E7.122Anhui Ningguo shunchang Machinery Co., Ltd 安徽省宁国顺昌机械有限公司W5.304Anhui Rongan Heavy Industries Co.,Ltd 安徽熔安重工机械有限公司O.F20/G15Anhui zongchen slewing ring co., ltd 安徽枞晨回转支承有限公司N5.233Ankomak 2010E6.539Anmopyc - Spanish Manufacturers Association of Construction and Mining EquipmentE1.606ANTECH ATTACHMENTS CO LTD E7.429A'One Machinery CO., LTD.E6.142APEM (Shanghai) Resentative OfficeN5.623bauma China 2010 Exhibitor List (alphabetically)bauma China 2010 展商名单as of Nov 16,2010API Heat Transfer (Suzhou) Co., Ltd艾普尔换热器(苏州)有限公司E2.166 AR ELECTRONICS CO., LTD东莞采升电子有限公司N5.736 Argo-Hytos GmbH E2.122 AROMATIC INDUSTRIAL CO., LTD芳苑贸易(上海)有限公司E7.722 ARROW MACHINERY山西繁源路缘工程有限公司E1.466 ArvinMeritor E5.236 asa hydraulik GmbH E6.455 Ashine Diamond Tools Co., Ltd.厦门宇信金刚石工具有限公司E1.550 ASHUN FLUID POWER CO., LTD.E6.162 ASIA INFO SOURCES亚信资源W5.732/12Atlas Copco(Shenyang)Construction & Mining Equipment Ltd 阿特拉斯.科普柯(沈阳)建筑矿山设备有限公司E1.239AtlasCopco (Shanghai) Trading Co., Ltd阿特拉斯.科普柯(上海)贸易有限公司E4.106 ATOS SPA意大利阿托斯有限公司中国代表处E4.534 ATP CHINA Ltd广州英邦名车电子自动变速箱有限公司E5.547 Attachment Torque MFG. (China) Co., Ltd宁波市瑞基机械制造有限公司W5.766 Autec E1.419 AUTO & CONSTRUCTION EQUIPMENT CORORATION E7.735 Auto (Tianjin) International Trade Co., Ltd天津高拓国际贸易有限公司E4.462 AUTOEQUIPS TECH CO., LTD.帷享集团•深圳市奥凯普科技有限公司E7.537 Autol Vehicle Technology Co., Ltd.郑州奥特科技有限公司E3.452 AVIC HEBEI ANJI HONGYE MACHINERY CO., LTD.中航工业河北安吉宏业机械股份有限公司N5.250 AVIC Liyuan Hydraul ic Co., Ltd.中航力源液压股份有限公司E4.458 AWAKE LIONS ENTERPRISE CO., LTD上海乔锐精密有限公司W5.620 B.D.X. MACHINERY LTD.北京达新新创机械有限公司E3.446 B.S. Hydraulic Co., Ltd.保成(佛冈)机械有限公司E3.457 Baier + Köppel GmbH & Co.E2.220 Balama Prima Equipment Ltd百莱玛设备有限公司O.E23 BAOJIALI ENGINEERING MECHANISM PART CO.,LTD浙江宝佳利工程机械配件有限公司N5.119 Barloworld Equipment E7.349 Bauer Maschinen GmbH O.A06/B07 bauma / bC India E1.214 Baumer (China) Co., Ltd.堡盟电子(上海)有限公司W5.156 Beijing Kaiming Trade & industry Co.,Ltd北京凯铭工贸有限责任公司W5.248 Beijing BeiNei Diesel Engine Co., Ltd北京北内柴油机有限责任公司E1.212. Beijing Blastrac Equipment Trading Co., Ltd北京佰锐泰克机械设备销售有限公司O.F29 Beijing Broadbond Heavy Machinery Co., Ltd北京博邦重工机械有限公司W5.134 BEIJING BUILDING CONSTRUCTION RESEARCH INSTITUTE北京市建筑工程研究院N5.322 BEIJING CA-LONG ENGINEERING MACHINERY CO., LTD北京加隆工程机械有限公司O.A39 Beijing Chenshixiaosong Machinery Co., Ltd北京诚实小松机械有限责任公司N5.268 Beijing Digi Burner Technology Co., Ltd北京蒂吉博纳科技有限公司E1.119 Beijing Easysolution Electronic Co., Ltd北京易斯路电子有限公司E7.604 Beijing Engineering Technology Co.北京欣达立成贸易有限公司E7.338 Beijing Hella Automotive Lighting Ltd北京海拉车灯有限公司E6.506 Beijing HiLiQi Turbocharger Co., Ltd北京海力奇增压器有限公司E7.539 Beijing Huade Hydraulic Industrial Group Co., Ltd.北京华德液压工业集团有限公司W5.102 Beijing Hyundai Lube produce Co., LTD北京现代润滑油制造有限公司N5.419 Beijing InHand Networks Technology Co. Ltd北京映翰通网络技术有限公司N5.429 Beijing Jayu Xin Cheng Industrial and Trade Co., Ltd.北京嘉友心诚工贸有限公司O.F15 Beijing Jinfei Tianhong Construction Machinery Co. Ltd.北京金飞天虹建筑机械有限公司W5.145 Beijing Jingcheng Heavy Industry Co., Ltd北京京城重工机械有限责任公司O.D39 Beijing Jingkai Wanjia International Machinery City Co., Ltd.北京经开万佳国际机械城E6.636 Beijing Jinkeda Business & Trading Co., Ltd北京金克达经贸有限公司W5.752 Beijing Kaishang technical development Co., Ltd北京市凯商科技发展有限责任公司E7.110 Beijing Kanglu Trade Co., Ltd北京康路贸易有限公司N5.157 BEIJING NEW CHOICE CORPORATION北京浦然进出口有限公司E6.137B Beijing Rapid Energy Hydraulic Mechanery Co., Ltd.北京鼎力达液压机电有限公司W5.354 Beijing REIT Technology Development Co., Ltd北京瑞图科技发展有限公司E1.450 Beijing rigong construction machinery International import & exportco., Ltd日工建机(北京)国际进出口有限公司E6.144 Beijing Road-Hydraulic System Technology Co., Ltd北京海纳创为液压系统技术有限公司E6.715 Beijing Shidai Dianchuang International AD Ltd.北京时代点创国际广告有限公司E2.262 Beijing Shougang heavy Duty Track Manufactory Co., Ltd北京首钢重型汽车制造股份有限公司O.A47 Beijing Shunchengfu Machinery Equipment Co., Ltd北京顺成福机械设备有限公司W5.512 Beijing Shunyi Yongguang Cleaning Machine Factory北京顺义永光清洁机械厂E3.358 BEIJING SKD TECHNOLOGY CO., LTD.北京赛凯德科技有限公司N5.220Beijing Tempro Technologies Inc.北京天正通工贸有限公司E5.237 Beijing Tonsan Adhesive Co., Ltd北京天山新材料技术有限责任公司E6.434 BEIJING UNIVERSAL PIONEERING INDUSTRY TECHNOLOGYCO., LTD.北京博创兴工科技有限公司W5.141 Beijing XingHeRer Construction techique Co., Ltd北京星河人施工技术有限责任公司N5.130 Beijing Xinzhongnong Fuel Injection Pump Service Co.,Ltd北京欣中农油泵油嘴维修有限公司E6.630 Beijing Zhonghuan Kinetics Heavy Vehicles Co., Ltd.中环动力(北京)重型汽车有限公司O.C30/D23 Beijing Zhongnuo Jiaxin High Tech Co., Ltd.北京众诺嘉信高新科技有限公司E1.139 Beijing Zulin Formwork & Scaffolding Co., Ltd北京卓良模板有限公司N5.403 BEILITE MACHINERY CO., LTD.贝力特机械有限公司E6.516 BEML Limited E2.133 Bengbu Planet Engineering Machinery Co.,Ltd蚌埠市行星工程机械有限公司E1.750 Berco (Shanghai) Undercarriage Technologies Co., Ltd伯尔克(上海)底盘技术有限公司E7.522 Bergstrom (Changzhou) HVQS System Co., Ltd博格思众(常州)空调系统有限公司E3.262 Besser Machinery (Sanhe) Co., Ltd贝赛尔机械(三河)有限公司E1.106 Beta Industrial Products Trading (Shanghai) Co., Ltd百塔工业品贸易(上海)有限公司E3.137 Bezares E1.713 BFS BetonfertigteilsystemeGmbH E1.611 BHS-Sonthofen GmbH E1.506BICES 2011 / IVEX 2011中国(北京)国际工程机械、建材机械及矿山机械展览与技术交流会/ 中国国际商用车博览会E3.105Bimal SRL E6.205 Binder+Co.E6.214 Binic Industrial Corporation Limited上海邦佳实业有限公司E7.334 Bixen Hydraulic Co., Ltd E6.332 Black Cat Blades Ltd.E6.518 Black Whirlwind Engineering Machinery Development Co., Ltd黑旋风工程机械开发有限公司W5.502 BLK DIESEl CO., LTD北京汇百超机电有限公司W5.345 BLT MACHINERY LTD.比力特机械有限公司E7.738 BMWi E1.404 BO YU SEAL FACTORY YIBIN SICHUAN CHINA广州市迈特密封件有限责任公司E7.360 BOMAG GmbH E2.422 Bomax Engineering Inc.杭州利君机电设备有限公司W5.406 Bondioli & Pavesi Hydraulic and Mechanical Component(Hanghzhou) Co.,Ltd.邦贝液压机械(杭州)有限公司E4.239 Boneng Transmission(Suzhou) Co., Ltd博能传动(苏州)有限公司E5.235 Bonfiglioli Drives (Shanghai) Co., Ltd邦飞利传动设备(上海)有限公司E5.520 Bosch Rexroth China博世力士乐中国E4.430 Bossard Industrial Fasteners Int'l Trading (Shanghai) Co., Ltd柏中工业固定器国际贸易(上海)有限公司N5.528 BQ-Tadano (Beijing) Crane Co.,Ltd O.D28/E25 BRENNAN-ABEL MANUFACTURING CO., LTD上海光岳机械制造有限公司W5.212 Brevini China Shanghai Gearboxes Co. Ltd.E5.218 Brevini Fluid Power E5.218 Brevini Fluid Power SpA E5.218 Brevini Power Transmission SpA E5.218 Bridon International E7.257 Briggs & Stratton (Shanghai) International Trading Co., Ltd百力通(上海)国际贸易有限公司O.H10 Brokk AB Beijing Representative Office瑞典布鲁克有限公司北京代表处E2.622 BROSA AG E2.230 BSP International Foundations E7.311 BUCCMA ACCUMULATOR (TIANJIN) CO., LTD布柯玛蓄能器(天津)有限公司N5.105 Bucher Hydraulics GmbH E5.508 Builder's Association of India E3.147 Buma Ce. Ltd.E6.308 Bureau Veritas必维国际检验集团E7.451C&U Group Shanghai Bearing Co., Ltd人本集团上海轴承有限公司E6.463 CABR Construction Machinery Technology Co., Ltd廊坊凯博建设机械科技有限公司E1.144/O.H84 Calyca Hydraulic Industry Co., Ltd泉州利佳油压工业有限公司E1.332 Cangzhou Tianfeng Hydraulic Manufacturing Co., Ltd沧州田丰液压制造有限公司N5.630 Carlisle Engineered Transportation Solutions美国卡莱工程运输解决方案E4.642 Carraro Drive Tech SPA E5.126 CARWOOD (Shanghai) Limited卡活机械(上海)有限公司E5.133 Casagrande S.p.A.E2.104 Casappa Hydraulics (Shanghai) Co., Ltd E5.412 CASAPPA S.p.A.E5.412Casar Drahtseilwerk Saar GmbH E6.340 Caterpillar Inc., OEM solutions Group E4.230 Catsu Engineering Machinery Equipment Co., Ltd东莞市卡松机械设备有限公司E3.447 Cavotec China Ltd.凯伏特(上海)起重机动力技术有限公司E6.358 CCCC Xi'an Road Construction Machinery Co., Ltd.中交西安筑路机械有限公司E2.250 CCMA Maintenance Sub-Council中国工程机械工业协会工程机械维修分会E7.152 CCMA-China Construction Machinery Association中国工程机械工业协会E3.101 CEA E7.309 CEJN (Shanghai) Fluid System Co.,Ltd希恩流体系统(上海)有限公司E6.708 中国挖掘机械网W5.732/32 CENTA Antriebe Kirschey GmbH E2.217 CEP International Ltd.上海赛发国际贸易有限公司E6.600 CFS INDUSTRY LTD汕头市益广金属制品有限公司N5.415 CGR Cornelio Ghinassi Ricambi S.p.A.E7.701 Chang Shin International Co. Ltd.E1.262 Changchun Shi Huilong Friction Plate Co., Ltd长春市汇隆摩擦片有限公司N5.387 Changge Juba Machinery Co., Ltd长葛市巨霸机械有限公司E1.117 CHANGGE NEW CENTURY MACHINERY CO., LTD长葛市新世纪机电有限公司E2.127Changlin Company Ltd常林股份有限公司O.A40/B35 O.A42/B37Changsha BeOne Machine Technology Inc.长沙比一机械科技有限公司E7.747 Changsha Heijingang Industrial Co., Ltd长沙黑金刚实业有限公司E3.115 Changsha Joinfly Mechanical And Electrical Equipment ManufatureCo., Ltd.长沙金帆机电设备制造有限公司W5.607 Changsha LiJian Machinery Technology Co., Ltd长沙力健机械科技有限公司E3.113 Changsha Superlion Machinery Co., Ltd.长沙盛隆机械有限公司W5.242CHANGSHA TIANHE DRILLING TOOLS AND MACHINERY CO., LTD 长沙天和钻具机械有限公司W5.705Changsha Weiping Machinery Co., Ltd长沙威平机械有限公司E7.631Changsha Zoomlion Heavy Industry Science & Technology Development Co., Ltd 长沙中联重工科技发展股份有限公司O.C10/D03O.C18/D11O.D01Changshu Keenly Tools Co., Ltd常熟市坚力路面工具制造有限公司E2.267 CHANGZHI HYDRAULIC CO., LTD长治液压有限公司N5.536 Changzhi Qinghua Machinery Factory长治清华机械厂E2.123 Changzhou Cronos Special Bearing Manufacture Co., Ltd常州克劳诺斯特种轴承制造公司E3.236 Changzhou Aoxuan Slewing Ring Co., Ltd常州市奥旋回转支承有限公司E7.547 Changzhou Bestway International Co., Ltd常州得道国际贸易有限公司N5.437 Changzhou Boiler Co., Ltd常州锅炉有限公司E1.351 Changzhou Changlin Yongqing Casting Co., Ltd常州市常林永青铸造有限公司E7.364 Changzhou Chengshing Piston Co., Ltd.常州正兴活塞有限公司N5.238 Changzhou Donghai Rubber Co., Ltd常州市东海橡胶厂有限公司E7.463 Changzhou Haobang Automobile Spare Parts Co., Ltd常州昊邦汽车零部件有限公司E6.346 Changzhou ITC Power Equipment Manufacturing Co., Ltd常州大道机械有限公司E5.135 CHANGZHOU JIEHE MACHINERY CO., LTD常州杰和机械有限公司E2.642 CHANGZHOU JULING FOUNDRY CO. LTD常州钜苓铸造有限公司N5.726 Changzhou Laisai lighting technology Co., LTD常州市莱赛光电技术有限公司W5.125 CHANGZHOU QIMIN BEARING CO.,LTD常州市启民轴承有限公司N5.437 Changzhou Shuhua Electronic Instrument Co., Ltd常州市树华电子仪器有限公司W5.121 Changzhou Yelong Machinery Co., Ltd.常州市液龙机械有限公司W5.202 Chengdu Boshi Machinery Co., Ltd.成都博世机械有限公司N5.488 Chengdu Hi-tech crane safety Co., Ltd成都新泰起重安全系统有限公司W5.432 Chengdu KOBELCO Construction Machinery Group成都神钢工程机械(集团)有限公司O.B38 CHENGDU XIAOSONG DIAGNOSTIC TECH., INST成都小松检测技术研究所N5.632 Chengdu Xinzhu Road & Bridge Machinery Co., Ltd.成都市新筑路桥机械股份有限公司O.G34 Cheung Hing Lifting Components (S.Z.) Limited祥兴起重装备(深圳)有限公司E6.362 Chicon News W5.732/36 CHINA AUTOMEDIA中汽传媒W5.732/23China Coal & Mining Expo 2011第十四届中国国际煤炭采矿技术交流及设备展览会W5.544CHINA CONCRETE WEBSITE中国混凝土网W5.732/18 China Construction Machinery中国工程机械W5.624 CHINA CONSTRUCTION MACHINERY AND PATRS WEBSITE中国工程机械与配件网W5.732/25 China Construction Machinery Business Online中国工程机械商贸网W5.718 CHINA CONSTRUCTION MACHINERY INDUSTRY YEARBOOK中国机械工业年鉴社W5.732/28 CHINA CONSTRUCTION MACHINERY NETWORK中国工程机械网W5.732/26 CHINA ELECTRIC POWER CONSTRUCTION ASSOCIATION中国电力建设企业协会W5.730China Highway《中国公路》杂志社W5.724 CHINA INTERNATIONAL CONTRACTORS ASSOCIATION中国对外承包工程商会W5.747 CHINA LEEMIN HYDRAULIC CO., LTD黎明液压有限公司E5.302 CHINA NATIONAL BUILDING MATERIALS AND EQUIPMENTIMPORT & EXPORT CORP中建材集团进出口公司E6.707 China North Industries Group Corporation中国兵器工业集团公司O.C38 CHINA ROAD MACHINERY ONLINE中国路面机械网W5.655 China Tunnel International Limited.河北众鑫源桥隧设备制造有限公司E2.635 中国叉车网W5.732/19 CHONGQING CHONHCHI YONGJIN DRIVE EQUIPMENT CO.,LTD重庆重齿永进传动设备有限公司N5.646 Chongqing Clipper Industry Co., Ltd重庆文劲舟机电设备有限公司E2.131 Chongqing Construction Engineering Group Corporation Limited重庆建工工业有限公司W5.233 CHONGQING KINBULL CONSTRUCTION MACHINERY CO., LTD.重庆勤牛工程机械有限责任公司O.H54 CHUAN FU (SHANGHAI) CO., LTD上海泉福国际贸易有限公司E7.746 Chuangyu Kaiping Access & Scaffolding Ltd开平创誉栅架设备有限公司N5.106 Chyi Meang Machinery Co., Ltd.O.A15 CINTEC Heavy Equipment Co., Ltd.贵州成智重工科技有限公司O.E1/11 Cixi yulong Automobile Fan Manufacturing Co.,Ltd慈溪市玉龙汽车风叶有限公司W5.330 Cixi FANGJIA AUTO PARTS CO., LTD慈溪市方佳汽配有限公司W5.507 CLARCOR Filtration Commerce (Shanghai) Co., LTD克达克过滤器商业(上海)有限公司E1.630 CNBM International Corporation中建材国际贸易有限公司E7.337 CNBM International Corporation中建材国际贸易有限公司O.A53CNGC INNER MONGOLIA NORTH BARYVAL ENGINEERING SPECIAL VEHICLE CO., LTD.中国兵器工业集团内蒙古北方巴里巴工程专用车有限公司O.C38CNGC JIANGLU MACHINERY & ELECTRONICS TECHNOLOGY (GROUP) CO., LTD.中国兵器工业集团江麓机电科技(集团)有限公司O.C38COB BEARING ING.浙江中达轴承有限公司E6.410 Cobo Group E6.605 Columbia Machine, Inc.E1.313 Comer Industries (Shaoxing) Co., Ltd, Shanghai Branch康迈尔机电(绍兴)有限公司上海分公司E5.320 Cometto Industrie SPA E2.334 ConBuild Vietnam 2010E1.105 Concrete混凝土杂志社W5.732/15 Conex 2010E6.334 CONSTRUCTION AND ARCHITECTURE建筑杂志社W5.732/22 Construction Enterprise Management《施工企业管理》杂志社W5.651 Construction Equipment Asia W5.732/37 Construction Machinery & Maintenance Magazine工程机械与维修杂志社E7.152 CONSTRUCTION MACHINERY AND EQUIPMENT《工程机械》杂志社W5.726 Construction Machinery Digest工程机械文摘E6.248 Construction Machinery Industry DM工程机械产业DM W5.732/6 Construction Machinery Magazine《建筑机械》E6.420 CONSTRUCTION MACHINERY TECHNOLOGY & MANAGEMENTMAGAZINE《建设机械技术与管理》杂志社E7.642 CONSTRUCTION MACHINERY TODAY MAGAZINE《今日工程机械》E7.152 CONSTRUCTION MACHINERY WEEKLY工程机械周刊W5.732/35 CONSTRUCTION MECHANIZATION MAGAZINE《建筑机械化》杂志社W5.725 Continental Automotive Asia Pacific Co., Ltd大陆汽车亚太管理(上海)有限公司N5.348 Continental Crane Ltd.O.A21 ContiTech AG Communication E1.629 Cooper Electric (Shanghai) Co., Ltd库柏电气(上海)有限公司N5.741 CORIMAG S.r.l.E3.209 Cormach SRL O.A21 Costex Tractor Parts (CTP)E6.347 CSB BEARING TECHNOLOGIES CO., LTD浙江长盛轴承技术有限公司E6.735 CSR TIMES Locomative & Rolling Stock Machinery Co., Ltd. OfBeijing北京南车时代机车车辆机械有限公司O.H86 Cummins (China) Investment Co., Ltd康明斯(中国)投资有限公司O.F01 D&G Machinery Co.,Ltd德基机械有限公司E2.164 Da Lian Forklift Co.,Ltd大连叉车有限责任公司O.A59 Dacame, S.L.E1.614 Daehan Heavy Industry E6.226 Daehan Tungsten Inc N5.234 Daemo Engineering Co., Ltd.O.A17 Dalian Fengshi Architectural Machinery Co., Ltd大连逢时建筑机械有限公司E3.247 Dalian Kailian Trading Co., Ltd大连凯联贸易有限公司E2.564Dalian Landtop parts大连蓝拓贸易有限公司W5.126 Dalian Shenglong Machinery Co., Ltd大连升隆机械有限公司E6.530 Dalian Yield Machinery Mfg Co., Ltd大连亿得机械制造有限公司E1.745B Dalian Yiliya Construction Machinery Co., Ltd大连益利亚工程机械有限公司N5.170 DANA HOLDING CORPORATION美国德纳控股公司E5.528 Danaher Setra ICG Co., Ltd丹纳赫西特传感工业控制(天津)有限公司E5.103 Danfo International Trade (Shenzhen) Co., LTD丹福国际贸易(深圳)有限公司E6.610 Danfoss (Shanghai) Automatic Controls Co.,Ltd丹佛斯(上海)自动控制有限公司E5.505 Datong Donghua Mine Machine Co., LTd.大同市东华矿机有限责任公司E7.608 Dawson Polymer Products (Shanghai) Co., Ltd.道森橡塑制品(上海)有限公司N5.442 Dayang Yangfan Electronics Co., Ltd.丹阳市扬帆电器有限公司W5.349 Dayi Changsheng heat Treatment Co., Ltd商丘市大一昌盛热处理有限公司E7.742 DB TECH Co., Ltd.E1.328 DELMAG Gmbh & Co. KG E2.320 Delta (Modena)E6.229 DELTA POWER TECNORD北京英德康自动化控制设备有限公司E5.418 DeltaTech Controls GmbH E2.309 DESA HEATING EQUIPMENT SHANGHAI CO., LTD.丹萨热能设备商贸(上海)有限公司E3.127 DESCH Drive Technology E2.128 Deutsch Connectors Trading (Shanghai) Co., Ltd德驰连接器贸易(上海)有限公司E6.246 DEUTZ AG E5.114 Deutz Dalian Engine Co. Ltd.E5.114 Dezhou Defu Hydraulic Machinery Limited Co., Ltd德州市德福液压机械设备有限公司N5.151 Dezhou Yuli Hydraulic Ltd德州宇力液压有限公司N5.376 DIEPA Drahtseilwerk Dietz GmbH & Co. KG E6.602 Dieseko BV O.A45 Diesel Parts of America E6.212 Diesel Progress E5.144 Dinamic Oil International Trading (Shanghai) Co., Ltd戴纳密克国际贸易(上海)有限公司E5.440 Dingsheng Tiangong Construction Machinery Co.,Ltd.鼎盛天工工程机械股份有限公司O.A36/B31 Dingzhou Jinhua Lantian Automotive Parts Co., Ltd定州市金华蓝天汽车零部件有限公司N5.634 Distributor Council of China Construction Machinery中国工程机械工业协会代理商工作委员会E7.152 DMI E2.125D-Mind Technology E6.324 Doka GmbH N5.330 Donaldson (Wuxi) Filters Co., Ltd唐纳森(无锡)过滤器有限公司E6.447 Dongguan DEF Seals Solutions Co.,Ltd.东莞市新志密封技术有限公司N5.240 Dongguan Liaobu Yaotai Filter Factory东莞市寮步耀泰滤芯厂W5.429 Dongguan Lvao Filter Co., Ltd东莞市律奥过滤器有限公司E6.440 Dongnam Heavy Industries Co., Ltd.E6.628 Dongtou HongDe Pump industry Co.,Ltd洞头县宏德电汽有限公司W5.748 Dongtou Country Xinda Electrical Apparatus Co., Ltd洞头县信达电器有限公司N5.636 Dongyang Heavy Industries Co., Ltd.O.A19 DongYang XinYaTe Precision Casting Co., Ltd东阳市新亚特精密铸造有限公司N5.444 Doosan Corporation E5.524 DOOSAN INFRACORE (CHINA).,LTD斗山工程机械(中国)有限公司O.D38 Double Coin Holding LTD.双钱集团股份有限公司E7.140 DOYLE PACIFIC INDUSTRIES LTD.美国多伊尔太平洋工业公司E4.625 Dr. Schulze GmbH E1.427 DRENNAN SHANG HAI COMPANY LTD卓尔能机电设备(上海)有限公司E5.312 Dropsa SpA E6.457 DV-B Drehverbindungen Bautzen GmbH E4.565 Dynapac (China) Compaction & Paving Equipment Co.,Ltd戴纳派克(中国)压实摊铺设备有限公司E4.106/E4.101 Dynaset Oy O.A23EA MACHINERY EQUIPMENT CO., LTD.欧亚机械设备有限公司O.H18 EARTH PRODUCTS CHINA LTD.欧美大地仪器设备中国有限公司N5.506 East Grace Corporation无锡新中润国际集团中润有限公司N5.316 EATON GROUP伊顿集团E5.402 Eberspächer Automotive Technology Co.Ltd.E2.225e-Build Innovations Pte. Ltd.E6.135 Economic Research India Ltd.W5.732/38 EDSV SEALS TECHNOLOGY CO., LTD.爱之福密封件-中国区运营本部N5.614 EFE COMPANY珠海市易仪五金有限公司N5.390 EGSTON ELECTORNICS ZHUHAI LIMITED益仕敦电子(珠海)有限公司E7.749EHE Machinery & Electronic Co., Ltd北京精海仪机电设备有限公司N5.638 EHWA DIAMOND IND. CO., LTD.E1.654 Eimco Elecon (India) Ltd.E2.150 Elematic GmbH E1.611 Element Six Hard Materials (Wuxi) Co., Ltd元素六硬质合金(无锡)有限公司E3.353 ELESA+GANTER China Co., Ltd.E2.222 Elettrotec SRL E6.103 Eme (Modena)E6.128 Emerson Industrial Automation艾默生工业自动化W5.616 Emiliana Serb (Modena)E6.225 Emmegi Heat Exchangers(Beijing)Co., Ltd依米奇热交换器(北京)有限公司E5.602 Emmequattro E6.119 Enarco, S.A.E1.622 Endeavour International Limited E4.640 Entre Marketing E1.433 era contact (Suzhou) Co., Ltd.爱乐联接(苏州)有限公司E6.634 ERKAT Spezialmaschinen und Service GmbH E2.120 Erma Rtmo SPA E6.125 ESCO (Xuzhou) Wearparts Co., Ltd爱斯科(徐州)耐磨件有限公司E6.160 Eurocomach E4.406 Eurodrill GmbH E3.557 Europe Tractor Parts s.r.l.E7.626 EVERDIGM CORP.韩宇工程机械(上海)有限公司E4.128 EVERPADS徐州奥得利科技有限公司N5.703 EXCAVATOR CHAMBER OF COMMERCE工商联挖掘机商会(中国挖掘机商会网)W5.732/11 Exen Corporation E1.636 Fangyuan Group Co.,Ltd方圆集团有限公司E3.156 FASTER HYDRAULICS (SHANGHAI) CO.,LTD速捷贸易(上海)有限公司E5.111 Fayat Group E2.422 FEDERAL MOGUL FRIEDBERG GMBH E2.101 Federal-Mogul (Shanghai) Automotive Parts Co., Ltd菲特尔莫古(上海)汽车零部件有限公司E5.345 Federal-Mogul Deva GmbH E2.101 FEICHENG JINCHENG AXLE CO., LTD肥城金城车桥有限公司E4.629 FEIGE GmbH Abfülltechnik E1.414 Feng cheng City ZhenQi Turbocharger Plant凤城市圳企增压器厂N5.737 Fenghua Chaori Hydraulic Co., Ltd奉化市朝日液压有限公司N5.101 Fenghua Ningdong Engineering Machinery Co., Ltd奉化市宁东工程机械有限公司E7.318 Fenner Sealing Technologies (Shanghai) Co., Ltd芬纳密封科技(上海)有限公司E7.455 Fiat Powertrain Technologies Management (Shanghai) Co., Ltd菲亚特动力科技管理(上海)有限公司E5.530 FILTERSUN FILTER (DONGGUAN) CO.,LTD.富滤盛滤清器(东莞)有限公司E6.739 Fine Form Co., Ltd.O.F02 First Forever Co., Ltd.E1.216 FIXATOR ASIA CO., LTD法适达(上海)机械设备有限公司E3.341 FLUID POWER WORLD行讯之《液气压世界》W5.732/29 Fluid Press (Modena)E6.122 FOSHAN ADVCORP SCAFFOLD LTD佛山安德沃脚手架有限公司N5.315Foshan NACRE Hydraulic Co., Ltd佛山市南海南曦液压机械有限公司E6.729 Foshan Shunde Guangshun Elevator Co., Ltd Zengcheng Branch佛山市顺德区广顺电梯有限公司增城分公司N5.577 Foshan Yunque Vibrator Co., Ltd.佛山市云雀振动器有限公司E2.456 FOSHAN-KAMUI HEAT EXCHANGER CO., LTD.佛山神威热交换器有限公司N5.743 FOTON LOVOL INTERNATIONAL HEAVY INDUSTRYCO., LTD福田雷沃国际重工股份有限公司O.G36 Foundation Associates Engineering Pte Ltd.E3.118 Fujian Everstrong Legpower Equipments Co., Ltd福建永强力加动力设备有限公司E4.143FUJIAN EXCELLENCE HONCHA BUILDING MATERIAL福建省卓越鸿昌建材装备股份有限公司E1.248 EQUIPMENT CO., LTDFUJIAN HAIYUAN AUTOMATIC EQUIPMENTS CO., LTD福建海源自动化机械股份有限公司E1.642 Fujian Jian Yong Machinery Equipment Co., Ltd福建建涌机械设备有限公司O.H26 Fujian Jingong Machinery Co.,Ltd福建晋工机械有限公司E4.422 Fujian Jinjiang Wantai Construction Machinery Co., Ltd福建省晋江市万泰工程机械有限公司N5.548 Fujian Province Jinjiang City Lijing Automobile Fittings Co.,Ltd福建省晋江市励精汽配有限公司W5.221 Fujian Quanzhou Huasheng Machinery Equipment Co., Ltd.福建泉州市华盛机械设备有限公司E7.136 Fujian Quanzhou Jinzheng Machinery Co ., Ltd福建泉州市金正机械有限公司E7.733 Fujian Quanzhou Licheng Jiaxing Engineering Machinery Co ., Ltd福建省泉州鲤城嘉兴工程机械有限公司N5.746FUJIAN QUANZHOU MACHINERY & EQUIPMENT IMPORT &EXPORT CORP福建省泉州机械设备进出口有限责任公司E7.422 FUJIAN SNOWMAN CO., LTD.福建雪人股份有限公司W5.324 Fujian Tietuo Machinery Co., Ltd福建铁拓机械有限公司O.D21 Fujian Wanxiang Auto Mobile Fittings Co.,Ltd福建万向汽配有限公司N5.550 Fujiang South Highway Machinery Co. Ltd.福建南方路面机械有限公司O.A52/B45 Fullness Lubrication Systems (Shanghai) Co., Ltd富鸟润滑设备(上海)有限公司E7.156 Furukawa Rock Drill (Shanghai) Co., Ltd古河凿岩机械(上海)有限公司O.D22 Fushun Bohui Industry Filter Cloth Co., Ltd抚顺市博晖产业滤布有限公司E6.645 Fushun Yongmao Construction Machinery Co., Ltd.抚顺永茂建筑机械有限公司O.C31 Fuwa Heavy Industry Co., Ltd.辽宁抚挖重工机械股份有限公司O.H09 Fuxin Beixinxing Hydraulic Compoment Co., Ltd阜新北鑫星液压有限公司N5.739 Fuxin Hemei Engineering Machinery Co., Ltd阜新市和美工程机械有限公司O.A26 Fuxin Hydraulic Pressure Pump Factory阜新液压油泵厂W5.715 Fuyao Glass Industry Group Co., Ltd福耀玻璃工业集团股份有限公司W5.754 Fuzhou Shenlong Rock Drill Factory福州神隆凿岩机厂W5.710 Fuzhou Skystone Diamond Tool Co., Ltd福州天石源超硬材料工具有限公司E1.664 Fuzhou Wonder Electric Co., Ltd福州万德电气有限公司E4.614 FW Murphy Instruments (Hangzhou) Co., Ltd摩菲仪器仪表(杭州)有限公司E5.338 G.A. SPA E7.149 Galtech (Modena)E6.126 GATES CHINA盖茨中国E5.112 GB Shanghai Construction Machinery Co., LTD工兵建筑工程机械(上海)有限公司O.A13 Gemels SRL E6.251 Geneset Powerplants E1.435 Giant Hydraulic Breaker Manufacturing Co.,Ltd安徽惊天液压智控股份有限公司O.G04 GKD E7.309 GKN Wheels (Liuzhou)吉凯恩车轮(柳州)有限公司E7.406 GLOBAL OVERSEAS GROUP CORP., LTD.欧沃斯集团有限公司E1.665 Global Project Logistics Network (GPLN)E7.724 Global Track Warehouse Europe GmbH E3.458 GLOBAL WIN VEHICLE CO., LTD广州家御汽车贸易有限公司W5.611 Globalmarket Group (Asia) Ltd.环球市场集团(亚洲)有限公司E3.246 Gocmaksan Makina Ltd.Sti E1.710 Goldhofer AG E2.116 GOLDPEAK ENGINEERING SPRING(WUXI) CO., LTD无锡金峰园弹簧制造有限公司E6.704 Graco Inc美国固瑞克公司E4.558 Grammer Interior (Tianjin) Co.,Ltd格拉默车辆内饰(天津)有限公司E7.504 GREEN ELECTRIC LTD. CO. BAZHOU CHINA霸州市格林电器有限公司E7.464 Groeneveld Transport Efficiency B.V.E7.611 Group Tower Electronics Limited E7.440 GSD INTERNATIONAL ENTERPRISES INC.北京贵诗迪商贸有限责任公司E2.565 Guang Shan Engineering Machine Ftting CO.,Ltd广山工程机械配件有限公司W5.101 Guang Zhou Halcyon-Hydraulic Co., Ltd广州市华欣液压有限公司W5.436 Guang Zhou Hui Yi Electromechanical Device Eiectyical Co.,Ltd广州市会亿机电设备有限公司O.H16 Guangdong Cimlineya Technology Industry Co., Ltd广东辛美来亚科技实业有限公司E2.551 Guangdong Liyuan Hydraulic Machinery Co., Ltd广东力源液压机械有限公司E2.135Guangdong Yuhuaxing Construction Machinery manufacture Co., Ltd 广东裕华兴建筑机械制造有限公司O.A29Guangxi Construction Engineering Group Construction MachineryManufacturing Co., Ltd广西建工集团建筑机械制造有限责任公司O.G33 Guangxi Nanning Jingxiang Instrument Co., Ltd广西南宁市精祥仪表有限责任公司W5.605 Guangxi Yuchai Heavy Industry Company Limited广西玉柴重工有限公司O.B21-A Guangzhou Changye Rubber Petroleum Equipment Co., LTD广州畅业橡胶石油设备有限公司E7.328 Guangzhou Dahua Desheng Scien-tech. Co., Ltd.广州大华德盛科技有限公司W5.346 Guangzhou Fine Sound M.&E. Co., Ltd广州泛音机电有限公司N5.733 Guangzhou HI-POWER Machine Co., Ltd广州明权机械设备有限公司E1.722 Guangzhou Hongfeng Machine Accessories广州鸿丰工程机械配件E7.434 Guangzhou Jcar Industrial Co., Ltd.广州巨康商贸有限公司N5.518 GUANGZHOU JIAMEI(CROWN)HYDRAULIC CO., LTD广州佳美(皇冠)油封专卖行N5.629 Guangzhou JingDa Construction Machinery Accessory Co., Ltd广州市景达工程机械配件N5.633 Guangzhou Jinlong Industry Development Co., Ltd广州市晋隆工贸发展有限公司N5.256 Guangzhou JIUMA Machinery CO., LTD广州玖玛机械有限公司W5.436 Guangzhou Jiye Auto Air Conditioner Co., Ltd广东增城市基业汽车空调有限公司N5.508 GUANGZHOU JUNDA MACHINERY EQUIPMENT CO., LTD广州市骏达工程机械配件公司W5.321 GUANGZHOU LEADER EQUIPMENT LTD.广州市艺达机械有限公司N5.225Guangzhou Ming Xiao Turbocharger广州市明晓涡轮增压器经营部E7.648 Guangzhou Ming Zhenengineering Machine Eitting Co.,Ltd广州市天河区东圃明振工程机械配件经营部W5.509 Guangzhou Mingzhen Engineering Machine Fitting Co., Ltd广州市天河东圃明真机械配件部N5.235 Guangzhou newsun Hydraulic Co., Ltd广州新光明液压N5.154 Guangzhou Qite Construction Machinery Manufacture Co., Ltd广州市奇特实业发展有限公司E4.626 GUANGZHOU SHENWEI SEALS REGIC SHOP广州市天河东圃神威油封经营部E7.730 GuangZhou ShiYou Mechanical Equipment Co.,LTD.广州市世有机械设备有限公司E4.464 Guangzhou Tianhe Dongpu Desheng Oil Seal Co., Ltd.广州市天河区东圃德升油封行E7.541 Guangzhou Tianhe Hongyou Machinery Equipments广州市天河宏铕机械设备经营部E7.743 Guangzhou Tiger Heavy Machinery Co., Ltd广州市泰戈机械设备有限公司E2.156 Guangzhou TSB Machinery Manufacturing Co., Ltd广州市华扬机械制造有限公司E1.138 Guangzhou Yanggui Manufacture Machine Co., Ltd广州洋贵优质发动机配件E6.725 Guangzhou Yifeng Autoparts Manufacturing Co., Ltd广州市毅峰汽配制造有限公司N5.513 Guangzhou zhanghong Machinery Equipment Co., Ltd广州市张宏机械设备有限公司E6.422 Guangzhou Zhengye Mechanical Engineering Accessory广州市天河区大观正野工程机械配件经营部N5.340 Guilin Huali Heavy Industries Co., Ltd桂林市华力重工机械有限责任公司O.E1/3 GUILIN TEBON SUPERHARD MATERIAL CO.,LTD桂林特邦新材料有限公司E1.445 Guiyang Xiaohe Tianzhu Precision Hydraulic Conponent Factory贵阳小河区天筑精密液压件厂E6.730 GuiYang YongQing Instruments & Electronic Science AndTechnology Co., Ltd贵阳永青仪电科技有限公司E6.158 Guizhou Fengyang Hydraulic Limited Liability Company贵州枫阳液压有限责任公司E7.536 Guizhou Jonyang Kinetics Co.,Ltd贵州詹阳动力重工有限公司O.C36/D29 GUIZHOU SINODRILLS EQUIPMENT CO.,LTD贵州中钻机械设备有限公司E7.646 GUIZHOU SUNCON GROUP CO., LTD贵州三占集团股份有限公司W5.511 Gunnebo Industries (Kunshan) Co., Ltd固力保起重设备(昆山)有限公司E3.528 H.E.A.D(Hydro Electronic Application Development Mfg.)E6.326 HAI YANG DELI FOUNDRY CO., LTD.海阳市得利铸造有限公司E7.633B Haining Nuosen Machinery Manufacturing Co., Ltd海宁诺森机械制造有限公司E7.132 Haion Caster Industrial Co., Ltd泰州圣佰轮业有限公司N5.417 HAIRISEN HYDRAULIC NINGBO CO., LTD宁波海日森液压有限公司W5.430 Haiyan Country Haiguan Pipe Fittings Manufacture Co., Ltd海盐县海管管件制造有限公司E5.137 HaiYan tonghui mining crusher Machinery Co., Ltd海盐县通惠地质矿山机械有限公司E1.648 Haiyan Zhongling Foundry Co., Ltd.海盐中凌铸造有限责任公司N5.622 HAMBITION DRIVETRAIN COMPONENTS & PARTS CO., LTD青岛天鸿动力传动设备有限公司E5.131Hamm AG E2.136 / E2.436Handan Fuye Hydraulic Machinery Co., Ltd邯郸市复液液压机械有限公司N5.472 Handanshi Kangmai Hydraulic Equipment Co., Ltd邯郸市康迈液压器材有限公司E1.241 Hangzhou Advance Gearbox Group Co.,Ltd杭州前进齿轮箱集团股份有限公司E5.538 Hangzhou Benfeng Auto Seat Co., Ltd杭州奔丰汽车座椅有限公司E7.111 Hangzhou Comansa Jie Construction Machinery Co., Ltd杭州科曼萨杰牌建设机械有限公司O.C44 HANGZHOU DONGJIANG FRICTION MATERIAL CO., LTD杭州东江摩擦材料有限公司N5.521 Hangzhou Dongyi Chuangzhan Excavator Parts Co., Ltd杭州东亦创展挖掘机配件制造有限公司E6.512 Hangzhou Fada Gearbox Co., Ltd杭州发达齿轮箱集团有限公司E7.555 Hangzhou Global Friend Precision Machinery Co., Ltd杭州友高精密机械有限公司E2.616 HANGZHOU HANGYU FRICTION MATERIAL CO., LTD.杭州杭宇摩擦材料有限公司E4.146 Hangzhou Heda electricity engineering Co., Ltd杭州和达机电工程有限公司E1.149 Hangzhou Henli friction material Co., Ltd杭州恒力摩擦材料有限公司N5.523 Hangzhou Hongyuan Textile Machinery Co.,Ltd杭州宏远纺织机械有限公司W5.310 HangZhou JDA Frictional Materal CO.,LTD杭州杰达摩擦材料有限公司N5.546 Hangzhou KleanAire Solutions Co., Ltd.杭州开颜科技有限公司E7.709 Hangzhou Nanhua Automobile Fitting Co.,Ltd杭州南华汽车配件有限公司N5.683 HANGZHOU NEWALLY VEHICLE AIR CONDITIONER CO., LTD杭州新都奥兰汽车空调有限公司E6.537 Hangzhou Rubber Group Company杭州橡胶集团公司E7.302 Hangzhou Truemax Machinery & Equipment Co.,Ltd.杭州骐瑞机电设备有限公司E1.341/O.A14 Hangzhou Xiaoshan Hongqi Friction Material Co., Ltd.杭州萧山红旗摩擦材料有限公司N5.384 Hangzhou Xinheng Machinery Co., Ltd.杭州信恒机械有限公司W5.453 Hangzhou Yunhe Rubber Plastic & Chemicals Co., Ltd浙江久运车辆部件有限公司E7.710 Hangzhou Zhenchuan Machinery Equipment Co., Ltd.杭州震川机械设备有限公司E3.554 Hangzhou Zhiyao Electronics Co., Ltd杭州智遥电子有限公司E3.550 Hanmi International Co., Ltd E6.533 Hansa (Modena)E6.132 HANSA-FLEX Hydraulik GmbH E2.213 HANWOOL (CHANGZHOU) HYDRAULIC SYSTEMMANUFACTURING CO., LTD汉湖(常州)液压系统制造有限公司N5.504 Hanyang S&C Co., Ltd.O.H66。
繁花似锦 群星璀璨——bauma china 2010再创辉煌

示面积、 展商数量和 观众数量都再创新高, 为全球 业界人士再次奉 献一场规模空前的工程机械 饕餮盛宴。 : . .
据主 办方的统计 数据 , 自 6 " 国家 的超过 1 0O o -_ 来 5i " ,o  ̄: t 5 e, k
观众参观了本届b u hn 。 a ma C ia 与上届展会 相比专业观众数量增长 了3 %; 3 展示面积扩大了1 %, 0 达到了2 Oo o 3 ,o 平方米 自钾 个国 来 家和地 区的18 8 ,5 家展商参加了本届b u hn , a ma e ’ a 展商数量增长 i 1 %, 5 展商的来源更是趋于国际化。 在18 8 ,5 家展商 中, 中国厂 商” 2 家 , 4 国际展商7 4 , 3 家 其中不
c u t i sa t n e h sy a b u i a o n r t e d d t i e r a ma Ch n e s
乏工程机械 行业 的新面孔 , 四天的展期 中, 在 总体来看 中国的展 商
更 加 出彩 。
( 0 8 1 26 4v s o sf m 1 4 c u tis. 2 0 : 1 ,7 i t r r o n r ) i o 2 e
尽管卡特彼 勒、 凯斯等美资企业依旧缺席b u a ma" ia 仍有 Chn , . 9 家美国企业参加了本届b u aG i 2 a m h a no “ 乱花渐欲迷人眼” 本届b u ;i ・ , a maE 1 , h3 不仅中 、 a I 展商的新产
品新技术精彩纷呈, 令人目不暇接 , 而且演绎出业内市场风云变幻
V ehiI san qu Pm en ,h sbr en a I ce d E i t a ok I
r or s wi h r ar o e hi ton s c , ec t eg d t x bii pa e d e h bt r u b r n ii r u b r g i x i i m e sa d v st m e s a n o n o n a
中国2010版GMP英文版译稿

T D V N O N O F F I C I A L T R A N S L A T I O NGMP(Revised in 2010)Order from Ministry of Public Health, PR. ChinaNo. 79"Good Manufacturing Practice (revised in 2010)" was approved by the Ministry of Health on October 19, 2010. It will come into force from 1st, March of 2011.Minister: Chen Zhu17th, Jan, 2011T D V N O N O F F I C I A L T R A N S L A T I O NContents1. General Provisions (4)2. Quality Management (5)2.1 General rules (5)2.2 Quality assurance (5)2.3 Quality control (6)2.4 Quality Risk Management (7)3. Organization and personnel (8)3.1 General rules (8)3.2 Key personnel (8)3.3 Training (11)3.4 Personnel hygiene (11)4. Facility and utilities (13)4.1 General provisions (13)4.2 Production area (13)4.3 Warehousing area (15)4.4 Quality Control Area (16)4.5 Auxiliary area (17)5. Equipment (18)5.1 General rules (18)5.2 Design and installation (18)5.3 Maintenance and repair (18)5.4 Use and cleaning (19)5.5 Calibrations (20)5.6 Water for drug manufacturing (20)6. Materials and products (22)6.1 General rules (22)6.2 Raw materials and excipients (23)6.3 Intermediate products and bulk products (24)6.4 Packaging materials (24)6.5 Final products (25)6.6 Materials and products which need special management (25)6.7 Other (25)7. Validation and qualification (27)T D V N O N O F F I C I A L T R A N S L A T I O N8. Document management (28)8.1 Principles (28)8.2 Quality Standards (30)8.3 Process procedure (31)8.4 Batch production records (32)8.5 Batch Packaging Records (33)8.6 Operating procedures and records (33)9. Production Management (35)9.1 Principle (35)9.2 Prevent contamination and cross contamination in process of production (36)9.3 Production operations (37)9.4 Packaging operations (37)10. Quality control and quality assurance (40)10.1 Quality Control Laboratory Management Section (40)10.2 Release of materials and products (44)10.3 Continued stability study (45)10.4 Change Control (46)10.5 Deviation treatment (47)10.6 Corrective and preventive measures (48)10.7 Evaluation and approval of suppliers (49)10.8 Product quality review and analysis (50)10.9 Complaints and adverse reaction reports (51)11. Contractual production and analysis (53)11.1 Principle (53)11.2 The Company (53)11.3 The Contractor (53)11.4 Contract (54)12. Chapter12 Product shipment and recall (55)12.1 Principle (55)12.2 Delivery and transportation (55)12.3 Recall (55)13. Self-check (57)13.1 Principle (57)13.2 Self-analysis (57)14. Supplementary Provisions (57)T D V N O N O F F I C I A L T R A N S L A T I O N1. General ProvisionsArticle 1 This practice is established according to "Drug Administration Law of PRC" and "Implementation Regulations of the PRC Drug Administration Law" in order to regulate drug production quality control.Article 2 Pharmaceutical companies should establish a drug quality management system. The system should cover all factors that affect drug quality, including all of organized and planned activities that ensure the quality of medicines meet its intended use.Article 3 This practice, as a part of quality management system, acts as the basic requirements for drug production management and quality control with the aim of minimizing contamination, cross-contamination and confusion, error and other risks in the process of drug production to ensure Pharmaceutical companies can constantly produce drugs meeting its intended use and registration requirements.Article 4 Enterprises should strictly enforce the practice; adhere to the honest and trustworthy, and to prohibit any false, deceptive behavior.T D V N O N O F F I C I A L T R A N S L A T I O N2. Quality Management2.1 General rulesArticle 5 Enterprises should establish a quality target that meets pharmaceutical quality management requirements, applying all of requirements on safety, efficacy and quality control related with drug registration to whole process of drug production, control and product release, storage and shipment to ensure that production of pharmaceuticals meet their intended use and registration requirements.Article 6 Top management personnel should ensure implementation of established quality objectives; staff of different levels, suppliers and distributors should work together and assume their responsibilities.Article 7 Enterprises should be equipped with adequate, qualified staff, facilities, facilities and equipment to provide necessary conditions for achievement of quality objectives.2.2 Quality assuranceArticle 8 Quality assurance is part of the quality management system. Enterprises must establish a quality assurance system, while building a complete documentation system to ensure effective operation of the system.Article 9 Quality assurance system should ensure that:1. design and development of drugs reflects requirements of this practice;2. production management and quality control activities are consistent withrequirements of this Code;3. management responsibilities are clearly defined;4. raw materials and packaging materials purchased and used are correct;5. effective control of intermediate products;6. implementation of validation and verification;7. perform production, analysis, analyze and review strictly in accordance withprocedures;8. each batch can only be released with approval of qualified person;9. appropriate measures for drug quality assurance are available in process of storage,shipment and subsequent operation ;10. periodic analysis and evaluation of validity and applicability of quality assurancesystem in accordance with self-analysis operating proceduresArticle 10: The basic requirements for Pharmaceutical production and quality management:T D V N O N O F F I C I A L T R A N S L A T I O N(a) To develop production process, and perform systematic review to certify it can sustainably and stably manufacture qualified products(b) Production technology and major changes need to be validated;(c) Equipped with necessary resources, including at least the following:1. Personnel with appropriate qualifications and qualified by training;2. Adequate premises and facility;3. Proper equipment and maintenance support;4. Right raw materials, packaging materials and labels;5. Approved procedures and operation procedures;6. Appropriate storage conditions.(d) Accurate and understandable language should be used to develop operation procedures;(e) The operators are trained to operate in accordance with proper operating procedures;(f) Production process should be documented; deviations should be investigated and recorded;(g) Batch records and shipping records should allow traceability of complete history of batch products, and should be kept properly for easy reference;(h) Reduce quality risk in process of drug shipment;(i) Establishment of drug recall system should ensure any batch can be recalled after distribution;(j) Investigate complaints and quality defects and take measures to prevent recurrence of similar quality defects.2.3 Quality controlArticle11: Quality control, including related organization, documentation system, and sampling, and analysis to ensure materials or products complete their necessary analysis prior to release and to confirm quality meet requirements.Article 12: The basic requirements of quality control:(a) QC shall be equipped with appropriate facilities, equipment, instruments and trained personnel for effective and reliable completion of all related quality control activities; (b) Operating procedures should be available for sampling, analysis, test of raw materials, packaging materials, intermediate products, packaging and finished products as well as stability analysis of the product, and if necessary, carry out environmental monitoring to ensure compliance with the requirements of this practice;(c) Authorized personnel should sample raw materials, packaging materials, intermediate products, packaging and finished products in accordance with required method;(d) Analysis methods should be validated or confirmed;(e) Sampling, analysis, and check should be recorded, and deviations should be investigated and recorded(f) Materials, intermediate products, packaging and finished products must be checked and analyzed according to quality standards and should be recorded;T D V N O N O F F I C I A L T R A N S L A T I O N(g) Packaging materials and final product should have sufficient retained samples for necessary analysis or check except the final finished packaging container that is too large, retained samples for finished product should be packaged the same as final packaging.2.4 Quality Risk ManagementArticle 13 Quality risk management, a kind of prospective and retrospective method, is a systematic process to evaluate, control, communication, review the quality risk.Article 14 Quality risks should be evaluated on basis of scientific knowledge and experience to ensure product quality.Article 15: The methods, measures, forms and documents formed in the process of quality risk management process should be appropriate to the level of existing risk.T D V N O N O F F I C I A L T R A N S L A T I O N3. Organization and personnel3.1 General rulesArticle 16 Enterprises should establish a management structure appropriate to drug manufacturing and organizational structure chart.Enterprises should establish an independent quality control department to perform quality assurance and quality control responsibilities. Quality assurance department and quality control departments can be established separately within quality management department.Article 17 Quality control department should be involved in all quality related activities, and responsible for reviewing all documents relevant to this practice. Quality management personnel shall not delegate responsibilities to other departments.Article 18 Enterprises should be equipped with adequate and suitably qualified (including education, training and practical experience) management and operations staff; responsibilities of each department and each position should be clearly defined. Responsibilities cannot be missed, and overlapped responsibilities should be clarified. Responsibilities of each person should not be overburden.All personnel should be clear about and understand their responsibilities, familiar with requirements related to their duties and receive proper traiing, including pre-service training and continuing training.Article 19: Normally responsibilities should not be delegated to others. The responsibilities which are in definite need of delegation can be delegated to the qualified and designated personnel.3.2 Key personnelArticle 20 Key personnel should be full-time staff in the enterprise, at least including responsible person, person in charge of production management, quality management and qualified person.Person responsible for quality control and production management shall not serve each other. Person responsible for quality management and quality qualified can serve each other. Operating procedures should be developed to ensure qualified personnel perform their duties independently, free from interference from business leaders and other personnel.Article 21 Owners of enterprisesThe responsible person for enterprise, as the primary responsible person for the enterprise, should cover overall responsibility for daily management of enterprises. TheT D V N O N O F F I C I A L T R A N S L A T I O Nresponsible persons shall be responsible for providing necessary resources, rational planning, organization and coordination to ensure independent performance of responsibilities of quality management department in order to ensure realization of business objectives and production of pharmaceuticals in accordance with the regulatory requirements.Article 22 Person in charge of production managementQualifications:Person in charge of production management should at least have a degree in pharmacy (or intermediate professional titles or Licensed Pharmacist) with at least three years’ engagement in drug production and quality management experience, including at least one year of medicine production management experience, and have been trained on professional knowledge relating to manufactured product.Main responsibilities:1. To ensure drugs are manufactured and stored in accordance with approved process procedures to ensure drug quality;2. To ensure strict implementation of all operation procedures related to the operation and production;3. To ensure batch production and packaging records have been reviewed and sent to quality management department;4. To ensure implementation of facilities and equipment maintenance for good running condition;5. To ensure completion of all necessary validation;6. To ensure personnel related to production receive necessary pre-service training and continuing training, and adjust training contents basing on actual needs.Article 23 Personal in charge of quality managementQualifications: person in charge of quality management should have at least a degree in pharmacy or related (or intermediate professional titles or Licensed Pharmacist) with at least five years’ experience in pharmaceutical production and quality management, including at least one year in medicine Quality management, and have received professional knowledge training relating to manufactured products.Main responsibilities1. To ensure raw materials, packaging materials, intermediate products, packaging and finished products meet requirements and quality standards approval in registration;2. To ensure completion of batch record review before product release;3. To ensure completion of all necessary analysis;4. Approve operation procedures about quality standards, sampling methods, analysis methods and other quality management;5. Review and approve all quality-related changes;6. To ensure all significant deviations and OOS of analysis results have been timely investigated and handled;7. Approve and monitor contractual(entrusted) analysis;T D V N O N O F F I C I A L T R A N S L A T I O N 8. Supervision of facilities and equipment maintenance to ensure it keeps in good running condition;9. To ensure completion of necessary qualification or validation, review and approval of qualification or validation protocols and reports;10. To ensure complete self-analysis;11. Assessment and approval of materials suppliers;12. Ensure all quality-related complaints have been investigated, and timely and correctly treated;13. To ensure completion of quality continual stability study, and provide stability study data;14. To ensure completion of quality review;15. To ensure quality control and quality assurance personnel have received necessary pre-service training and continuing training, and training need to be adjusted according to actual needs.Article 24 Person in charge of production management and quality management normally share following responsibilities:(a) Review and approve products process procedures, operation procedures and other documents;(b) To supervise facilities hygiene status;(c) Ensure key equipment has been qualified;(d) Ensure completion of production process validation;(e) Ensure all related employees have received necessary pre-service training and continuing training, and training need to be adjusted basing on actual need;(f) Approve and supervise contractual production;(g) Identify and monitor the storage conditions of materials and products;(h) Record-keeping;(i)Monitoring implementation of the practice;(j) Monitoring factors that affect product quality.Article 25 Qualified peopleQualifications:The qualified person should have at least bachelor degree in pharmacy or related (or intermediate professional titles or Licensed Pharmacist) with at least five years’ practices in pharmaceutical production and quality management, and have engaged in pharmaceutical production process control and quality analysis.The qualified person shall have necessary professional knowledge, and shall only perform responsibilities after receive training related to product release.(B) Main responsibilities:1. Involving in establishment of the enterprise quality system, internal self-check and external quality audit, validation, and reports of adverse drug reaction, product recalls and other quality management activities;T D V N O N O F F I C I A L T R A N S L A T I O N2. Assume responsibilities of product release to ensure production and analysis for each batch released are in line with relevant laws and regulations, drug registration requirements and quality standards;3. The qualified person should present product release review record in accordance with the second article mentioned above before product release and should incorporate it in the batch record.3.3 TrainingArticle 26 Enterprise shall designate specific department or person responsible for training management; training or plan reviewed or approved by person in charge of production management and quality management should be available. Training records should be preserved.Article 27: All personnel related with pharmaceutical production and quality should be trained, and training should be appropriate to the requirements for corresponding position. In addition to theory and practice training of this practice, responsibilities and skills training about related positions and laws and regulation should be available. And actual result of training should be evaluated.Article 28 Operator engaged in high-risk operation areas (such as: production area for high activity, highly toxic, infectious, and sensitizing materials) should receive specialized training.3.4 Personnel hygieneArticle 29 All personnel should receive training on hygiene requirements; enterprises should establish personal hygiene rules to minimize risk of contamination caused by person.Article 30 Personal hygiene practice should include health, hygiene habits and dressing. Person in production areas and quality control should have proper understanding of personal hygiene practice. Enterprises should take measures to ensure implementation of personnel Hygienic Practice.Article 31 Enterprises should manage personnel health and establish health files. Production personnel in direct contact with drugs shall receive health checks before induction and later health check should be performed at least once a year.Article 32 Enterprises should take appropriate measures to keep person wound surface, infectious diseases or other diseases that may contaminate drug from being engaged in drug production.T D V N O N O F F I C I A L T R A N S L A T I O NArticle 33 Visitors and untrained personnel shall not have access to production and quality control areas. When there is need for access, instruction on personal hygiene, dressing and other matters should be given.Article 34: Any person entering the production area shall change clothes in accordance with provisions. Materials, style and dressing way of clothes should be appropriate to work engaged and air cleanliness level.Article 35 Personnel entering the clean production areas shall not wear make-up accessories.Article 36 There should be no smoking and eating, storage of food, beverages, cigarettes and drugs for personal use and other non-production items in production and storage area.Article 37 Operators should not directly touch drugs, and packaging materials and equipment surface that will be contact with drug with bare hands.T D V N O N O F F I C I A L T R A N S L A T I O N4. Facility and utilities4.1 General provisionsArticle 38 Facilities sites, design, layout, construction, renovation and maintenance must comply with requirements of pharmaceutical production to minimize contamination, cross contamination, confusion and errors and to facilitate cleaning, operation and maintenance.Article 39: The sites for facilities should be selected by taking protective measures for facilities and production into consideration. Facilities should be located in an environment able to minimize risk of contamination on materials and product.Article 40 Enterprises should have a clean production environment; factory floors, roads and transportation should not cause contamination to production of drugs; overall layout of production, administrative, living and support areas should be reasonable and not impede each other; facilities, personnel and materials flow within the facilities should be reasonable.Article 41 Facilities should be properly maintained and maintenance activities will not adversely affect drug quality. Facilities should be cleaned and sanitized according to detailed written operating procedures.Article 42 Facilities should be equipped with proper lighting, temperature, humidity and ventilation to ensure quality of product produced and stored as well as performance of related equipment will not be directly or indirectly affected.Article 43 Facilities and utilities should be designed and installed to prevent entrance of insects or other animals. Necessary measures should be taken to prevent use of rodenticides, pesticides and smoke agent will not cause contamination on equipment, materials, and products.Article 44 Appropriate measures should be taken to prevent access of unauthorized personnel. Production, storage and quality control areas should not be used as access for staff that not belongs to this area.Article 45 Facilities, utilities, fixed pipeline as-built drawings after completion of construction or reconstruction should be reserved.4.2 Production areaT D V N O N O F F I C I A L T R A N S L A T I O NArticle 46 Facilities, production utilities and equipment should be designed and used as required for drug properties, process flow and level of cleanliness in order to reduce risk of contamination and cross-contamination and also meet following requirements :(a) Feasibility of multi-drug facilities, production utilities and equipment should beevaluated by taking drug properties, process and intended use into consideration, and related evaluation report should be available.(b) Drugs with special properties, such as highly sensitizing drugs (e.g. penicillin) or biological products (such as BCG or other use of medicines prepared from microbial activity), must be produced with dedicated and independent facilities, production utilities and equipment. Operation area of penicillin with a large amount of dust should maintain a relatively negative pressure. The exhaust discharged into outdoors should be purified to meet requirements and air vents should be away from other air purification system inlet;(c) Production of β-lactam structure and sex hormone contraceptive drugs must use dedicated utilities (such as a independent air purification systems) and equipment and should be strictly separated from production areas for other drugs;(d) Certain hormones, cytotoxic class, highly active chemicals should be produced with dedicated utilities (such as a separate air purification systems) and equipment; special protective measures, if needed, should be validated. Drugs mentioned above can share same utilities and equipment through divided production.(e) The exhaust fan of air purification system mentioned in above b, c, and d should be purified.(f) Drug production facilities should not be used to produce non-medicinal products that may adversely affect drug quality.Article 47 Production areas and storage areas should be equipped with sufficient space to ensure orderly storage of equipment, materials, intermediate products, packaging and finished products and to avoid confusion of different products or materials, cross contamination, and omission and error caused by production or quality control operations.Article 48 Air purification systems should be installed according to drug variety, operation requirement as well as external environment in order that production area can be ventilated properly, temperature and humidity are controlled, and air is purified and filtered to ensure production environment for drug meet requirement.Pressures difference between clean areas and non-clean areas clean areas, among clean areas of different levels should not be less than 10 psi. When necessary, pressure difference should be maintained among areas with the same level of cleanliness but different functions (operating room).Areas where liquid and solid preparations, cavity medication (including rectal application), topical pharmaceutical skin preparations and other non-sterileT D V N O N O F F I C I A L T R A N S L A T I O Npreparations as well as final processing of packaging materials with direct contact with drug are exposed should be designed in accordance with requirements for D-level in Annex. Company can establish control on microorganism according to product specification and properties.Article 49 Inner surfaces of clean area (walls, floors, ceiling) should be smooth, with tight interface, free from cracks and fall-off particles to avoid dust and permit effective cleaning and if necessary, sanitization.Article 50 Various channels, lighting, air outlets and inlets and other public utilities should be designed and installed to facilitate cleaning. And maintenance should be performed from outside.Article 51 Drainage facilities should be appropriate to installation installed to avoid back-flow. Open drain should be avoided, and be as shallow as possible to facilitate cleaning and sanitization.Article 52 Raw materials and excipients for preparation should be weighed in preparation room specially designed.Article 53: Operation rooms that generate dust (operation rooms such as sampling, weighing, mixing, and packaging of dried material or product) should maintain relatively negative pressure or take special measures to prevent spread of dust, cross-contamination and to facilitate cleaning.Article 54 Facilities or areas used for dug packaging should be properly designed to avoid confusion or cross-contamination. Isolation measures should be taken when there are several packaging lines in the same area.Article 55 Production areas should be equipped with appropriate lighting; lighting in visual check area shall meet requirements.Article 56: In-process control can be located within production area when in-process control operation will not adversely affect product quality.4.3 Warehousing areaArticle 57 Storage areas should be equipped with sufficient space to ensure orderly storage of quarantined, accepted, rejected, return or recalled raw materials and excipients, packaging materials, intermediate products, packaging and finished products and other materials and products.Article 58 Storage areas should be designed and constructed to ensure good storage conditions and equipped with ventilation and lighting utilities. Storage area should be able to meet storage conditions for materials or products (such as temperature andT D V N O N O F F I C I A L T R A N S L A T I O Nhumidity, protection from light) and requirements for safe storage. The storage area needs to be analyzed and monitored.Article 59 Highly reactive materials or products and printed packaging materials should be stored in a secure area.Article 60 Reception, distribution and shipping areas should be able to protect materials, products from being impacted by outside weather (such as rain, snow). Layout and utilities of receiving area should be able to ensure outer packages for materials and product can be cleaned before warehousing.Article 61: If an independent area is used to store quarantine materials, this area should be labeled clearly and only restricted to authorized personnel.Rejected, returned or recalled materials or products should be quarantined.If other means are adopted to replace physical separation, this method should be of equivalent security.Article 62: Usually there should be separate material sampling area. Air cleanliness level in sampling area should be consistent with production requirements. If sampling is performed in other area or by other means, contamination or cross contamination should be avoided.4.4 Quality Control AreaArticle 63 Quality control laboratory should normally be separated from production areas. Biological analysis, microbiological and radioisotope laboratory should also be separated from each other.Article 64 Laboratory should be designed to meet its intended use and to avoid confusion and cross-contamination. And there should be enough area for sample disposal, storage of retained samples and samples for stability study as well as record keeping.Article 65: When necessary, specialized equipment room should be set to keep sensitive equipment from static electricity, vibration, humidity or other external disturbances.Article 66 Laboratory used to treat biological or radioactive samples and other special items should be consistent with relevant national requirements.Article 67 Experimental animal rooms should be strictly separated from other areas; its design and construction shall comply with relevant regulations and equipped with separate air-handling facilities and dedicated channels for animals.。
SCAN CHINA2010将于11月在京开幕

SCAN CHINA2010将于11月在京开幕
作者:暂无
来源:《食品安全导刊》 2010年第10期
本刊讯(记者王崇民)由中国物品编码中心、中国自动识别技术协会主办的第十七届国际自动识别技术展览会(SCAN CHINA 2010)将于11月10日~12日在北京举行。
据介绍,SCAN CHINA 2010以“用智慧驾驭未来”为主题,通过开展“质量提升”主题活动、优秀会员颁奖晚会、参展商交流会、物联网与产品质量追溯论坛及物联网主题展区等为企业和观众全力奉献“年度大作”。
据了解,展览会将突出“展示产业新技术、新产品,倡导自主知识产权,推动产业健康发展”的特点,从应用推广、技术探讨、产业发展以及国际交流等方面着手,致力于促进中国自动识别技术的应用与发展。
SCAN CHINA以展览和论坛为载体,通过整合流通、连锁零售、制造、医疗、食品、制造业信息化、电子商务与现代物流等行业、领域资源,积极为自动识别技术研发商、系统集成商以及自动识别技术相关的信息化方案提供商构建交流、发展的平台。
第三届营养健康产业国际博览会
将于2011年4月在京举行本刊讯(记者王崇民)由国家发改委、外交部、商务部申报,经国务院主要领导同志批准,国家发改委宏观院公众营养与发展中心等机构主办的第三届《营养产业发展论坛暨营养产品国际博览会》(下称博览会),将于2011年4月19日至4月21日在北京举行。
据悉,博览会已经在上海成功举办了两届,其以独具的权威性、国际性、产业方向性、科技引导性等特点,受到了来自国内外经济、产业、健康等各领域领导、学者和企业的广泛好评。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
W,
vv
No. of Beekeepers No. of Beehives
r Introduced the use of natural disease prevention methods instead of chemicals r
173
450 78,30d Numb*r *f *e**ives
173
e Encouraged Tianzuo to explore new retail channels, including selJing to 14 university supermarkets
r
to provide an additional source of income
mushrooms with their ginseng
r
47
3 .Entrepreneurship
3,159
3,1
94
37,356
r ldentified a strategy for the ginseng processors to enter the end retail market r Increased the company's credibility and marketability by guiding the company owners
Project Period: 2 Years People Directly Benefited: 30 Organizations lnvolved: 2
find local wild bee honey suppliers. We also taught this business start-up how to create successful marketing
Annual Income Increase from Planting Mushrooms ($)
800 600 740
Organizations Involved: 2
**:-
400 200
0
Griteria
$lFE
Before
Entry
$irc fntry
After
{"",
,'tl,r
-
' - -- '.rj
1ru,,
HFF=G
*.*#t3.4*p
k
/*
{* i I 'r *tjniv$isity of I {- i 1""-l* /l \ *j *-1, '--*lAN
k
Before
SIFE Entqr
No. of beekeepers No. of beehives
10
36 150
The aim of the project was to improve the skills of local
involved in our project to 60 over the next year' and help the beekeepers establish a co-operative to sell their honey o llanzuo have continued to expand their retail outreach without SIFE assistance
Recommended Tianzuo invest in its suppliers and"consequently they purchased hives
to increase the beekeepers production capacity by 20%
$ep.2010
r Plan to double the number of beekeeperS
additional source of income
5. Environmental Sustainability
r Ant River use environmentally friendly recycled cardboard packaging
mushrooms alongside ginseng acts as a natural fertilizer, replacing chemical equivalents 6. Business Ethics t 1Ao/o of Ant River's profits are re-distributed to the poorest ginseng growers in the form of micro-finance loans
beekeepers that raised the Chinese wild bee. This species
K*y Stati*tics
SIFE Members Involved: 8 Hours Dedicated:
of bee, which pollinates over 500 wild flowers, is facing
process. Fuithermore, using the dividend we received from Ant River, we established a micro-finance fund to provide the poorest ginseng growers with loans. We also taught them how they could use this money to inter-pldnt mushrooms with their ginseng to provide a new regular sollrce of income.
Mountain how to inclease breeding rates and honey yields.
5j20
160
fdumb*r mf Beekt
We linked the beekeepers with a local, newly established
honey company called Tianzuo who had been struggling to
o Taught four ginseng processors how to establish and run a successful business
16s,375 218,575
r Equipped the ginseng growers with the skills to inter-plant
09
10
-Mav.
72 38
Mar.2010 2010 46,706
38,806
Jun" 2010
-Auq. 2010
45,M1 36,699
TOTAL
'.:,t'l .:..
l
1. Market Economics rTaught four ginseng processors how to by-pass intermediaries and sell directly to retailers 2. Success Skills
extinction.
Avg. annual income for keeping bees (g) Tianzuo's revenue from Chinese wild bee honey ($)
14.500
Working with a local beekeeping expert, we ran an education program' to teach beekeepers in Siming
SIFE Members Involved: 14 Hours Dedicated: 1 0.080 Project Period: 2 Years People Directly Benefited: 29
nevenG:-$zogi,zls
12,1
;:€,;
We helped four ginseng processors to establish a social enterprise called Ant River, which provided a direct sales channelto the end retail market. SIFE UNNC provided the company with training and guidance throughout the
e Equipped the beekeepers with the skills to effectively raise the Chinese wild bee,
leading to honey yields increasing by 100o/o Taught Tianzuo how to create marketing campaigns and approach new retailers, resulting in them independently securing contracts with Tesco, Carrefour and Watsons
Taught the beekeepers to grow their businesses organically through profit re-investment
r The beekeepers'improved techniques have now contributed to the wild bee poputation
