DMH-1_COA_12446_MedChemExpress
UPLC-DAD波长切换法同时测定复方红衣补血口服液中6种多酚类化合物的含量

中图分类号:R 921 2 文献标识码:A 文章编号:1009 - 3656(2021)03 - 0283 - 06
doi:10 19778 / j chp 2021 03 018
Simultaneous determination of six polyphenols in Compound Hongyi
30、43 ng mL - 1 ꎻ 精 密 度、 稳 定 性、 重 复 性 试 验 的 RSD 均 小 于 2 0% ꎻ 平 均 加 样 回 收 率 分 别 为 98 56%
( RSD = 2 26% ꎬn = 9 ) 、 97 94% ( RSD = 1 54% ꎬ n = 9 ) 、 97 23 % ( RSD = 2 19% ꎬ n = 9 ) 、 98 54%
摘要 目的: 建立超高效液相色谱 ̄DAD 波长切换法同时测定复方红衣补血口服液中咖啡酸、没食子酸、对
羟基肉桂酸、阿魏酸、儿茶素、表儿茶素含量的方法ꎮ 方法:采用高效液相色谱法ꎮ 色谱柱为 Waters Symme ̄
try C18 (4 6 mm × 150 mmꎬ3 5 μm) ꎬ流动相为甲醇 ̄0 1% 甲酸溶液( 梯度洗脱) ꎬ流速为 0 5 mLmin - 1 ꎬ检测
2 方法与结果
2 1 色谱条件与系统适用性
色谱 柱: Waters Symmetry C18 ( 4 6 mm × 150
的质量研究是以儿茶素 [10] 、白藜芦醇 [11] 单一成分
mmꎬ3 5 μm) ꎻ 流 动 相: 甲 醇 ( A)  ̄0 1% 甲 酸 溶 液
定ꎮ 本研究建立 UPLC 法同时测定复方红衣补血口
3_种常用碳青霉烯类抗生素血药浓度UPLC-MS

3种常用碳青霉烯类抗生素血药浓度UPLC-MS/MS检测方法的建立Δ秦怡1*,张瑞霞2,吕雅瑶2,翁莉莉1,张弋2 #(1.天津医科大学一中心临床学院,天津 300192;2.天津市第一中心医院药学部,天津 300192)中图分类号 R917;R978.1文献标志码 A 文章编号 1001-0408(2024)03-0343-05DOI 10.6039/j.issn.1001-0408.2024.03.14摘要目的建立3种临床常用碳青霉烯类抗生素——厄他培南(ETP)、亚胺培南(IPM)、美罗培南(MEM)血药浓度检测的超高效液相色谱-质谱联用(UPLC-MS/MS)法。
方法血浆样品经甲醇沉淀蛋白后,以3种抗生素的稳定性同位素(ETP-D4、IPM-D4、MEM-D6)为内标,采用ACQUITY UPLC BEH C18(2.1 mm×50 mm,1.7μm)色谱柱分离;流动相为98%乙腈+2%水+0.1%甲酸和98%水+2%乙腈+0.1%甲酸,梯度洗脱;流速为0.3 mL/min;柱温为40 ℃;采用正离子、多反应监测模式进行扫描分析。
结果该方法专属性良好,在ETP、IPM、MEM 0.2~200、0.1~100、0.1~100μg/mL范围内线性良好(r2≥0.993),批内、批间精密度和准确度良好(RE均≤5.14%,RSD均≤11.15%),基质效应、提取回收率较一致(RSD≤12.99%)。
结论本实验建立了一种可以同时定量ETP、IPM、MEM血药浓度的UPLC-MS/MS法,该方法样品前处理简单、检测时间短、所需样品量少,可满足临床需求。
关键词碳青霉烯类抗生素;超高效液相色谱-质谱联用;血药浓度;厄他培南;亚胺培南;美罗培南Establishment of UPLC-MS/MS method for the determination of plasma concentration of three common carbapenem antibioticsQIN Yi1,ZHANG Ruixia2,LYU Yayao2,WENG Lili1,ZHANG Yi2(1. First Central Clinical College of Tianjin Medical University,Tianjin 300192,China;2. Dept. of Pharmacy,Tianjin First Central Clinical Hospital,Tianjin 300192, China)ABSTRACT OBJECTIVE To establish a UPLC-MS/MS method for the determination of plasma concentration of three carbapenem antibiotics,i.e. ertapenem (ETP),imipenem (IPM)and meropenem (MEM).METHODS After protein precipitation with methanol,the plasma samples were separated by ACQUITY UPLC BEH C18column (2.1mm×50mm,1.7μm)using stable isotopes of three antibiotics (ETP-D4,IPM-D4,MEM-D6)as the internal standard. The mobile phases were 98%acetonitrile +2% water +0.1%formic acid and 98%water +2%acetonitrile +0.1%formic acid,by gradient elution. The flow rate was 0.3mL/min and the column temperature was 40 ℃. Scanning analysis was performed in the positive ion and multiple reaction monitoring mode. RESULTS The method had good specificity,good linearity (r2≥0.993)in the range of 0.2-200,0.1-100and 0.1-100μg/mL of ETP,IPM and MEM,and good intra-batch and inter-batch precision and accuracy (all RE≤5.14%,all RSD≤11.15%),the matrix effect and extraction recovery were consistent (RSD≤12.99%). CONCLUSIONS This study establishes the UPLC-MS/MS method to simultaneously quantify the plasma concentration of ETP,IPM and MEM. The method has the advantages of simple pretreatment, short detection time and small sample quantity to meet clinical requirement.KEYWORDS carbapenem antibiotics; UPLC-MS/MS; plasma concentration; ertapenem; imipenem; meropenem碳青霉烯类抗生素具有抗菌谱广、抗菌活性强、耐药率低的特点,已成为治疗重症感染的主要选择。
超高效液相色谱法测定辛伐他汀胶囊中叔丁基-4-羟基茴香醚与2,6-二叔丁基对甲酚的含量

超高效液相色谱法测定辛伐他汀胶囊中叔丁基-4-羟基茴香醚与2,6-二叔丁基对甲酚的含量张红;李婕;王学涛【摘要】Objective To establish a method for analysis of butylated hydroxyanisole (BHA) and butylated hydroxytoluene (BHT) in Simvastatin Capsules. Methods The chromatographic conditions included ACQUITY UPLCTM BEH C18 column (50 mm X 2. 1 mm, 1. 7 μm) ,mobile phase of acetonitrile(B) ;0. 005 mol o L-1 ammonium acetate(A) with gradient elution:0 min,60 : 40 ;2 min, 60 = 40; 5 min, 90 = 10; 8 min, 90 = 10; 9 min, 60 = 40; 10 min, 60 = 40 at a flow rate of 0. 25 mL o min-1 , the detection wavelength 280 nm,and the column temperature 40 °C. Results Good resolution for BHA,BHT and vitamin C was obtained. The limit of detection for BHA was 0. 5 ng,the linear range was 0. 203 5-50. 88 μg o mL-1 (r=0. 999 9) ,and the average recovery was 98. 3% (RSD=1. 0%). The limit of detection for BHT was 0. 5 ng.the linear range was 0. 211 4-52. 84 μg o mL-1 (r=0. 999 9) ,and the average recovery was 97. 2%(RSD=0. 5%). Conclusion The method is rapid,specific,sensitive and economic.%目的建立超高效液相色谱法同时测定辛伐他汀胶囊中的抗氧剂叔丁基-4-羟基茴香醚(BHA)与2,6-二叔丁基对甲酚(BHT).方法色谱柱为ACQUITY UPLCTM BEH C18 (50 mm×2.1 mm,1.7 μm).以乙腈(A)-0.005 mol·L-1醋酸铵(B)为流动相,梯度洗脱程序为:0 min,60∶40;2 min,60∶40;5 min,90∶10;8 min,90∶10;9 min,60∶40;10 min,60∶40.检测波长为280 nm,流速为0.25 mL·m in-1,柱温为40 ℃.结果在该色谱条件下,BHA和BHT与维生素C峰均能良好分离.BHA的检出限为0.5 ng;质量浓度在0.203 5~50.88 μg·mL-1范围内与峰面积呈良好的线性关系,相关系数r=0.999 9;回收率为98.3%,RSD为1.0%.BHT的检出限为0.5 ng;质量浓度在0.211 4~52.84 μg·mL-1范围内与峰面积呈良好的线性关系,相关系数r=0.999 9;回收率为97.2%,RSD为0.5%.结论该方法快速、专属、灵敏度高,并且节能环保.【期刊名称】《西北药学杂志》【年(卷),期】2012(027)005【总页数】3页(P420-422)【关键词】超高效液相色谱法;辛伐他汀;BHA;BHT【作者】张红;李婕;王学涛【作者单位】中国食品药品检定研究院,北京,100050;中国食品药品检定研究院,北京,100050;秦皇岛市药品检验所,秦皇岛,053000【正文语种】中文【中图分类】R927.2辛伐他汀是唯一列入国家基本药物目录的调脂及抗动脉粥样硬化药,国内有多家企业生产,由于其结构中的4-羟基-2 H-吡喃环易氧化,故常在制剂处方中加入抗氧剂以提高其稳定性,其中叔丁基-4-羟基茴香醚(BHA)和2,6-二叔丁基对甲酚(BHT)是最为常用的2种抗氧剂。
Gelucire-14-44-SDS-MedChemExpress

Inhibitors, Agonists, Screening LibrariesSafety Data Sheet Revision Date:Nov.-23-2018Print Date:Nov.-23-20181. PRODUCT AND COMPANY IDENTIFICATION1.1 Product identifierProduct name :Gelucire 14/44Catalog No. :HY-Y1892CAS No. :121548-04-71.2 Relevant identified uses of the substance or mixture and uses advised againstIdentified uses :Laboratory chemicals, manufacture of substances.1.3 Details of the supplier of the safety data sheetCompany:MedChemExpress USATel:609-228-6898Fax:609-228-5909E-mail:sales@1.4 Emergency telephone numberEmergency Phone #:609-228-68982. HAZARDS IDENTIFICATION2.1 Classification of the substance or mixtureNot a hazardous substance or mixture.2.2 GHS Label elements, including precautionary statementsNot a hazardous substance or mixture.2.3 Other hazardsNone.3. COMPOSITION/INFORMATION ON INGREDIENTS3.1 SubstancesSynonyms:NoneFormula:N/AMolecular Weight:N/ACAS No. :121548-04-74. FIRST AID MEASURES4.1 Description of first aid measuresEye contactRemove any contact lenses, locate eye-wash station, and flush eyes immediately with large amounts of water. Separate eyelids with fingers to ensure adequate flushing. Promptly call a physician.Skin contactRinse skin thoroughly with large amounts of water. Remove contaminated clothing and shoes and call a physician.InhalationImmediately relocate self or casualty to fresh air. If breathing is difficult, give cardiopulmonary resuscitation (CPR). Avoid mouth-to-mouth resuscitation.IngestionWash out mouth with water; Do NOT induce vomiting; call a physician.4.2 Most important symptoms and effects, both acute and delayedThe most important known symptoms and effects are described in the labelling (see section 2.2).4.3 Indication of any immediate medical attention and special treatment neededTreat symptomatically.5. FIRE FIGHTING MEASURES5.1 Extinguishing mediaSuitable extinguishing mediaUse water spray, dry chemical, foam, and carbon dioxide fire extinguisher.5.2 Special hazards arising from the substance or mixtureDuring combustion, may emit irritant fumes.5.3 Advice for firefightersWear self-contained breathing apparatus and protective clothing.6. ACCIDENTAL RELEASE MEASURES6.1 Personal precautions, protective equipment and emergency proceduresUse full personal protective equipment. Avoid breathing vapors, mist, dust or gas. Ensure adequate ventilation. Evacuate personnel to safe areas.Refer to protective measures listed in sections 8.6.2 Environmental precautionsTry to prevent further leakage or spillage. Keep the product away from drains or water courses.6.3 Methods and materials for containment and cleaning upAbsorb solutions with finely-powdered liquid-binding material (diatomite, universal binders); Decontaminate surfaces and equipment by scrubbing with alcohol; Dispose of contaminated material according to Section 13.7. HANDLING AND STORAGE7.1 Precautions for safe handlingAvoid inhalation, contact with eyes and skin. Avoid dust and aerosol formation. Use only in areas with appropriate exhaust ventilation.7.2 Conditions for safe storage, including any incompatibilitiesKeep container tightly sealed in cool, well-ventilated area. Keep away from direct sunlight and sources of ignition.Recommended storage temperature:Pure form-20°C 3 years4°C 2 yearsIn solvent-80°C 6 months-20°C 1 monthShipping at room temperature if less than 2 weeks.7.3 Specific end use(s)No data available.8. EXPOSURE CONTROLS/PERSONAL PROTECTION8.1 Control parametersComponents with workplace control parametersThis product contains no substances with occupational exposure limit values.8.2 Exposure controlsEngineering controlsEnsure adequate ventilation. Provide accessible safety shower and eye wash station.Personal protective equipmentEye protection Safety goggles with side-shields.Hand protection Protective gloves.Skin and body protection Impervious clothing.Respiratory protection Suitable respirator.Environmental exposure controls Keep the product away from drains, water courses or the soil. Cleanspillages in a safe way as soon as possible.9. PHYSICAL AND CHEMICAL PROPERTIES9.1 Information on basic physical and chemical propertiesAppearance White to off-white (Oil)Odor No data availableOdor threshold No data availablepH No data availableMelting/freezing point No data availableBoiling point/range No data availableFlash point No data availableEvaporation rate No data availableFlammability (solid, gas)No data availableUpper/lower flammability or explosive limits No data availableVapor pressure No data availableVapor density No data availableRelative density No data availableWater Solubility No data availablePartition coefficient No data availableAuto-ignition temperature No data availableDecomposition temperature No data availableViscosity No data availableExplosive properties No data availableOxidizing properties No data available9.2 Other safety informationNo data available.10. STABILITY AND REACTIVITY10.1 ReactivityNo data available.10.2 Chemical stabilityStable under recommended storage conditions.10.3 Possibility of hazardous reactionsNo data available.10.4 Conditions to avoidNo data available.10.5 Incompatible materialsStrong acids/alkalis, strong oxidising/reducing agents.10.6 Hazardous decomposition productsUnder fire conditions, may decompose and emit toxic fumes.Other decomposition products - no data available.11.TOXICOLOGICAL INFORMATION11.1 Information on toxicological effectsAcute toxicityClassified based on available data. For more details, see section 2Skin corrosion/irritationClassified based on available data. For more details, see section 2Serious eye damage/irritationClassified based on available data. For more details, see section 2Respiratory or skin sensitizationClassified based on available data. For more details, see section 2Germ cell mutagenicityClassified based on available data. For more details, see section 2CarcinogenicityIARC: No component of this product present at a level equal to or greater than 0.1% is identified as probable, possible or confirmed human carcinogen by IARC.ACGIH: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by ACGIH.NTP: No component of this product present at a level equal to or greater than 0.1% is identified as a anticipated or confirmed carcinogen by NTP.OSHA: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by OSHA.Reproductive toxicityClassified based on available data. For more details, see section 2Specific target organ toxicity - single exposureClassified based on available data. For more details, see section 2Specific target organ toxicity - repeated exposureClassified based on available data. For more details, see section 2Aspiration hazardClassified based on available data. For more details, see section 212. ECOLOGICAL INFORMATION12.1 ToxicityNo data available.12.2 Persistence and degradabilityNo data available.12.3 Bioaccumlative potentialNo data available.12.4 Mobility in soilNo data available.12.5 Results of PBT and vPvB assessmentPBT/vPvB assessment unavailable as chemical safety assessment not required or not conducted.12.6 Other adverse effectsNo data available.13. DISPOSAL CONSIDERATIONS13.1 Waste treatment methodsProductDispose substance in accordance with prevailing country, federal, state and local regulations.Contaminated packagingConduct recycling or disposal in accordance with prevailing country, federal, state and local regulations.14. TRANSPORT INFORMATIONDOT (US)This substance is considered to be non-hazardous for transport.IMDGThis substance is considered to be non-hazardous for transport.IATAThis substance is considered to be non-hazardous for transport.15. REGULATORY INFORMATIONSARA 302 Components:No chemicals in this material are subject to the reporting requirements of SARA Title III, Section 302.SARA 313 Components:This material does not contain any chemical components with known CAS numbers that exceed the threshold (De Minimis) reporting levels established by SARA Title III, Section 313.SARA 311/312 Hazards:No SARA Hazards.Massachusetts Right To Know Components:No components are subject to the Massachusetts Right to Know Act.Pennsylvania Right To Know Components:No components are subject to the Pennsylvania Right to Know Act.New Jersey Right To Know Components:No components are subject to the New Jersey Right to Know Act.California Prop. 65 Components:This product does not contain any chemicals known to State of California to cause cancer, birth defects, or anyother reproductive harm.16. OTHER INFORMATIONCopyright 2018 MedChemExpress. The above information is correct to the best of our present knowledge but does not purport to be all inclusive and should be used only as a guide. The product is for research use only and for experienced personnel. It must only be handled by suitably qualified experienced scientists in appropriately equipped and authorized facilities. The burden of safe use of this material rests entirely with the user. MedChemExpress disclaims all liability for any damage resulting from handling or from contact with this product.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
CWHM-12_DataSheet_MedChemExpress
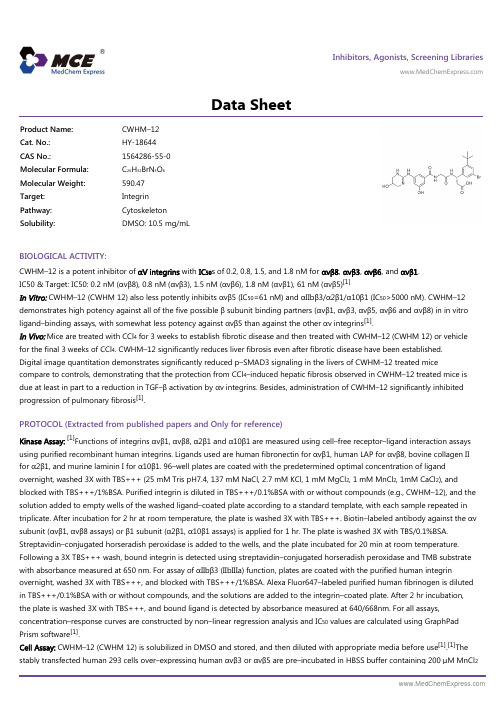
Inhibitors, Agonists, Screening Libraries Data SheetBIOLOGICAL ACTIVITY:CWHM–12 is a potent inhibitor of αV integrins with IC 50s of 0.2, 0.8, 1.5, and 1.8 nM for αvβ8, αvβ3, αvβ6, and αvβ1.IC50 & Target: IC50: 0.2 nM (αvβ8), 0.8 nM (αvβ3), 1.5 nM (αvβ6), 1.8 nM (αvβ1), 61 nM (αvβ5)[1]In Vitro: CWHM–12 (CWHM 12) also less potently inhibits αvβ5 (IC 50=61 nM) and αIIbβ3/α2β1/α10β1 (IC 50>5000 nM). CWHM–12demonstrates high potency against all of the five possible β subunit binding partners (αvβ1, αvβ3, αvβ5, αvβ6 and αvβ8) in in vitro ligand–binding assays, with somewhat less potency against αvβ5 than against the other αv integrins [1].In Vivo: Mice are treated with CCl 4 for 3 weeks to establish fibrotic disease and then treated with CWHM–12 (CWHM 12) or vehicle for the final 3 weeks of CCl 4. CWHM–12 significantly reduces liver fibrosis even after fibrotic disease have been established.Digital image quantitation demonstrates significantly reduced p–SMAD3 signaling in the livers of CWHM–12 treated micecompare to controls, demonstrating that the protection from CCl 4–induced hepatic fibrosis observed in CWHM–12 treated mice is due at least in part to a reduction in TGF–β activation by αv integrins. Besides, administration of CWHM–12 significantly inhibited progression of pulmonary fibrosis [1].PROTOCOL (Extracted from published papers and Only for reference)Kinase Assay:[1]Functions of integrins αvβ1, αvβ8, α2β1 and α10β1 are measured using cell–free receptor–ligand interaction assays using purified recombinant human integrins. Ligands used are human fibronectin for αvβ1, human LAP for αvβ8, bovine collagen II for α2β1, and murine laminin I for α10β1. 96–well plates are coated with the predetermined optimal concentration of ligand overnight, washed 3X with TBS+++ (25 mM Tris pH7.4, 137 mM NaCl, 2.7 mM KCl, 1 mM MgCl 2, 1 mM MnCl 2, 1mM CaCl 2), andblocked with TBS+++/1%BSA. Purified integrin is diluted in TBS+++/0.1%BSA with or without compounds (e.g., CWHM–12), and the solution added to empty wells of the washed ligand–coated plate according to a standard template, with each sample repeated in triplicate. After incubation for 2 hr at room temperature, the plate is washed 3X with TBS+++. Biotin–labeled antibody against the αv subunit (αvβ1, αvβ8 assays) or β1 subunit (α2β1, α10β1 assays) is applied for 1 hr. The plate is washed 3X with TBS/0.1%BSA.Streptavidin–conjugated horseradish peroxidase is added to the wells, and the plate incubated for 20 min at room temperature.Following a 3X TBS+++ wash, bound integrin is detected using streptavidin–conjugated horseradish peroxidase and TMB substrate with absorbance measured at 650 nm. For assay of αIIbβ3 (IIbIIIa) function, plates are coated with the purified human integrinovernight, washed 3X with TBS+++, and blocked with TBS+++/1%BSA. Alexa Fluor647–labeled purified human fibrinogen is diluted in TBS+++/0.1%BSA with or without compounds, and the solutions are added to the integrin–coated plate. After 2 hr incubation,the plate is washed 3X with TBS+++, and bound ligand is detected by absorbance measured at 640/668nm. For all assays,concentration–response curves are constructed by non–linear regression analysis and IC 50 values are calculated using GraphPad Prism software [1].Cell Assay: CWHM–12 (CWHM 12) is solubilized in DMSO and stored, and then diluted with appropriate media before use [1].[1]The stably transfected human 293 cells over–expressing human αvβ3 or αvβ5 are pre–incubated in HBSS buffer containing 200 μM MnCl 2Product Name:CWHM–12Cat. No.:HY-18644CAS No.:1564286-55-0Molecular Formula:C 26H 32BrN 5O 6Molecular Weight:590.47Target:Integrin Pathway:Cytoskeleton Solubility:DMSO: 10.5 mg/mLfor 30 min at 37°C with 3–fold dilutions of compound (e.g., CWHM–12). Each sample is then added to triplicate wells of a 96–well plate which has been coated overnight at 4°C with a predetermined optimal concentration of purified vitronectin, washed, blocked by 1 hr incubation with BSA, and washed again. Cells are allowed to attach for 30 min at 37°C, and non–adherent cells are removed by washing. Remaining attached cells are measured by endogenous alkaline phosphatase activity using para–nitrophenyl phosphate and reading absorbance signal at 405 nM. The same procedure is used to measure adhesion of αvβ6–expressing human HT–29 cells to purified human latency associated peptide, and α5β1–expressing human K562 cells to human plasma fibronectin. In all cell–based assays, binding by the expected integrin is verified by testing activity of corresponding isotype–matched positive (function–blocking) and negative control antibodies[1].Animal Administration: CWHM–12 (CWHM 12) is solubilized in 50% DMSO (in sterile water) (Mice)[1].[1]Mice[1]The mTmG (Td tomato/EGFP) and Ai14 (Rosa–CAG–LSL–tdTomato–WPRE) mice are used and crossed with Pdgfrb–Cre mice. Wild type C57/BL6 mice, Itgav flox/flox mice and itgb8flox/flox mice are used. Mice used for all experiments are 8–12 weeks old and are housed under specific pathogen–free conditions. For all studies CWHM–12 and CWHM–96 are solubilized in 50% DMSO (insterile water) and dosed to 100 mg/kg/day. Drug or vehicle (50% DMSO) are delivered by implantable ALZET osmotic minipumps. For CCl4–induced fibrosis, pumps are inserted subcutaneously either before the firReferences:[1]. Henderson NC, et al. Targeting of αv integrin identifies a core molecular pathway that regulates fibrosis in several organs. Nat Med. 2013 Dec;19(12):1617–24.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
CAS号413611-93-5_10074-G5_MedBio_物理性质

1、产品物理参数:
常用名
10074-G5
英文名
10074-G5
CAS号
413611-93-5
分子量
332.313
密度
1.4±0.1 g/cm3
沸点
538.6±60.0 °C at 760 mmHg
分子式
C18H12N4O3
熔点
无资料
闪点
279.5±32.9 °C
2、技术资料:
体外研究
10074-G5抑制Daudi Burkitt淋巴瘤细胞的生长并破坏c-Myc / Max二聚化。针对Daudi和HL-60细胞的IC50值分别为15.6和13.5μM[1]。10074-G5在区域Arg363-Ile381中结合Myc肽Myc353-437,Kd值为2.8μM。10074-G5结合在由诱导螺旋结构域(Leu370-Arg378)的N末端的扭结(Asp379-Ile381)产生的空腔中[3]。
≥98%
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
MED11457
CDK inhibitor II
CDK inhibitor II
1269815-17-9
50mg
≥98%
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
MED11591
(S)-CCG-1423
(S)-CCG-1423
None
体内研究
静脉注射20 mg / kg小鼠的血浆半衰期为10074-G5,为37分钟,血药浓度峰值为58μM,比肿瘤峰值浓度高10倍[1]。
3、同类产品列表:
DMH-1_SDS_MedChemExpress
Inhibitors, Agonists, Screening LibrariesSafety Data Sheet Revision Date:Aug.-05-2017Print Date:Aug.-05-20171. PRODUCT AND COMPANY IDENTIFICATION1.1 Product identifierProduct name :DMH-1Catalog No. :HY-12273CAS No. :1206711-16-11.2 Relevant identified uses of the substance or mixture and uses advised againstIdentified uses :Laboratory chemicals, manufacture of substances.1.3 Details of the supplier of the safety data sheetCompany:MedChemExpress USATel:609-228-6898Fax:609-228-5909E-mail:sales@1.4 Emergency telephone numberEmergency Phone #:609-228-68982. HAZARDS IDENTIFICATION2.1 Classification of the substance or mixtureNot a hazardous substance or mixture.2.2 GHS Label elements, including precautionary statementsNot a hazardous substance or mixture.2.3 Other hazardsNone.3. COMPOSITION/INFORMATION ON INGREDIENTS3.1 SubstancesSynonyms:DMH1; DMH 1Formula:C24H20N4OMolecular Weight:380.44CAS No. :1206711-16-14. FIRST AID MEASURES4.1 Description of first aid measuresEye contactRemove any contact lenses, locate eye-wash station, and flush eyes immediately with large amounts of water. Separate eyelids with fingers to ensure adequate flushing. Promptly call a physician.Skin contactRinse skin thoroughly with large amounts of water. Remove contaminated clothing and shoes and call a physician.InhalationImmediately relocate self or casualty to fresh air. If breathing is difficult, give cardiopulmonary resuscitation (CPR). Avoid mouth-to-mouth resuscitation.IngestionWash out mouth with water; Do NOT induce vomiting; call a physician.4.2 Most important symptoms and effects, both acute and delayedThe most important known symptoms and effects are described in the labelling (see section 2.2).4.3 Indication of any immediate medical attention and special treatment neededTreat symptomatically.5. FIRE FIGHTING MEASURES5.1 Extinguishing mediaSuitable extinguishing mediaUse water spray, dry chemical, foam, and carbon dioxide fire extinguisher.5.2 Special hazards arising from the substance or mixtureDuring combustion, may emit irritant fumes.5.3 Advice for firefightersWear self-contained breathing apparatus and protective clothing.6. ACCIDENTAL RELEASE MEASURES6.1 Personal precautions, protective equipment and emergency proceduresUse full personal protective equipment. Avoid breathing vapors, mist, dust or gas. Ensure adequate ventilation. Evacuate personnel to safe areas.Refer to protective measures listed in sections 8.6.2 Environmental precautionsTry to prevent further leakage or spillage. Keep the product away from drains or water courses.6.3 Methods and materials for containment and cleaning upAbsorb solutions with finely-powdered liquid-binding material (diatomite, universal binders); Decontaminate surfaces and equipment by scrubbing with alcohol; Dispose of contaminated material according to Section 13.7. HANDLING AND STORAGE7.1 Precautions for safe handlingAvoid inhalation, contact with eyes and skin. Avoid dust and aerosol formation. Use only in areas with appropriate exhaust ventilation.7.2 Conditions for safe storage, including any incompatibilitiesKeep container tightly sealed in cool, well-ventilated area. Keep away from direct sunlight and sources of ignition.Recommended storage temperature:Powder-20°C 3 years4°C 2 yearsIn solvent-80°C 6 months-20°C 1 monthShipping at room temperature if less than 2 weeks.7.3 Specific end use(s)No data available.8. EXPOSURE CONTROLS/PERSONAL PROTECTION8.1 Control parametersComponents with workplace control parametersThis product contains no substances with occupational exposure limit values.8.2 Exposure controlsEngineering controlsEnsure adequate ventilation. Provide accessible safety shower and eye wash station.Personal protective equipmentEye protection Safety goggles with side-shields.Hand protection Protective gloves.Skin and body protection Impervious clothing.Respiratory protection Suitable respirator.Environmental exposure controls Keep the product away from drains, water courses or the soil. Cleanspillages in a safe way as soon as possible.9. PHYSICAL AND CHEMICAL PROPERTIES9.1 Information on basic physical and chemical propertiesAppearance Light yellow to yellow (Solid)Odor No data availableOdor threshold No data availablepH No data availableMelting/freezing point No data availableBoiling point/range No data availableFlash point No data availableEvaporation rate No data availableFlammability (solid, gas)No data availableUpper/lower flammability or explosive limits No data availableVapor pressure No data availableVapor density No data availableRelative density No data availableWater Solubility No data availablePartition coefficient No data availableAuto-ignition temperature No data availableDecomposition temperature No data availableViscosity No data availableExplosive properties No data availableOxidizing properties No data available9.2 Other safety informationNo data available.10. STABILITY AND REACTIVITY10.1 ReactivityNo data available.10.2 Chemical stabilityStable under recommended storage conditions.10.3 Possibility of hazardous reactionsNo data available.10.4 Conditions to avoidNo data available.10.5 Incompatible materialsStrong acids/alkalis, strong oxidising/reducing agents.10.6 Hazardous decomposition productsUnder fire conditions, may decompose and emit toxic fumes.Other decomposition products - no data available.11.TOXICOLOGICAL INFORMATION11.1 Information on toxicological effectsAcute toxicityClassified based on available data. For more details, see section 2Skin corrosion/irritationClassified based on available data. For more details, see section 2Serious eye damage/irritationClassified based on available data. For more details, see section 2Respiratory or skin sensitizationClassified based on available data. For more details, see section 2Germ cell mutagenicityClassified based on available data. For more details, see section 2CarcinogenicityIARC: No component of this product present at a level equal to or greater than 0.1% is identified as probable, possible or confirmed human carcinogen by IARC.ACGIH: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by ACGIH.NTP: No component of this product present at a level equal to or greater than 0.1% is identified as a anticipated or confirmed carcinogen by NTP.OSHA: No component of this product present at a level equal to or greater than 0.1% is identified as a potential or confirmed carcinogen by OSHA.Reproductive toxicityClassified based on available data. For more details, see section 2Specific target organ toxicity - single exposureClassified based on available data. For more details, see section 2Specific target organ toxicity - repeated exposureClassified based on available data. For more details, see section 2Aspiration hazardClassified based on available data. For more details, see section 212. ECOLOGICAL INFORMATION12.1 ToxicityNo data available.12.2 Persistence and degradabilityNo data available.12.3 Bioaccumlative potentialNo data available.12.4 Mobility in soilNo data available.12.5 Results of PBT and vPvB assessmentPBT/vPvB assessment unavailable as chemical safety assessment not required or not conducted.12.6 Other adverse effectsNo data available.13. DISPOSAL CONSIDERATIONS13.1 Waste treatment methodsProductDispose substance in accordance with prevailing country, federal, state and local regulations.Contaminated packagingConduct recycling or disposal in accordance with prevailing country, federal, state and local regulations.14. TRANSPORT INFORMATIONDOT (US)This substance is considered to be non-hazardous for transport.IMDGThis substance is considered to be non-hazardous for transport.IATAThis substance is considered to be non-hazardous for transport.15. REGULATORY INFORMATIONSARA 302 Components:No chemicals in this material are subject to the reporting requirements of SARA Title III, Section 302.SARA 313 Components:This material does not contain any chemical components with known CAS numbers that exceed the threshold (De Minimis) reporting levels established by SARA Title III, Section 313.SARA 311/312 Hazards:No SARA Hazards.Massachusetts Right To Know Components:No components are subject to the Massachusetts Right to Know Act.Pennsylvania Right To Know Components:No components are subject to the Pennsylvania Right to Know Act.New Jersey Right To Know Components:No components are subject to the New Jersey Right to Know Act.California Prop. 65 Components:This product does not contain any chemicals known to State of California to cause cancer, birth defects, or anyother reproductive harm.16. OTHER INFORMATIONCopyright 2017 MedChemExpress. The above information is correct to the best of our present knowledge but does not purport to be all inclusive and should be used only as a guide. The product is for research use only and for experienced personnel. It must only be handled by suitably qualified experienced scientists in appropriately equipped and authorized facilities. The burden of safe use of this material rests entirely with the user. MedChemExpress disclaims all liability for any damage resulting from handling or from contact with this product.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
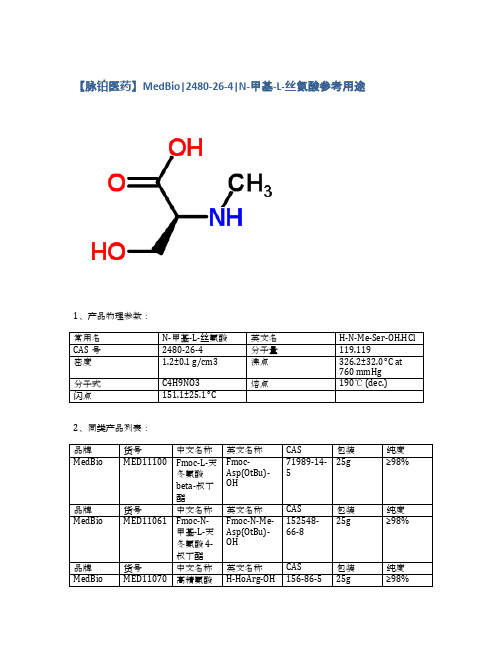
2480-26-4 N-甲基-L-丝氨酸_MedBio
≥98%
2、同类产品列表:
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
MED11100
Fmoc-L-天冬氨酸beta-叔丁酯
Fmoc-Asp(OtBu)-OH
71989-14-5
25g
≥98%
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
MED11061
Fmoc-N-甲基-L-天冬氨酸4-叔丁酯
Fmoc-N-Me-Asp(OtBu)-OH
【脉铂医药】
1、产品物理参数:
常用名
N-甲基-L-丝氨酸
英文名
H-N-Me-Ser-OH.HCl
CAS号
2480-26-4
分子量
119.119
密度
1.2±0.1 g/cm3
沸点
326.2±32.0 °C at 760 mmHg
分子式
C4H9NO3
熔点
190℃ (dec.)
闪点
151.1±25.1 °C
100g
≥98%
品牌
货号
中文名称
英文名称
CAS
包装
纯度
Moc-L-八氢吲哚-2-甲酸
Fmoc-Oic-OH
130309-37-4
250mg
≥98%
品牌
货号
中文名称
英文名称
CAS
包装
纯度
MedBio
MED11130
Fmoc-L-苏氨酸
Fmoc-Thr-OH
73731-37-0
100g
≥98%
品牌
常见中草药提取物及主要成分对细胞色素P4502C9抑制的研究进展

常见中草药提取物及主要成分对细胞色素 P4502C9抑制的研究进展陈悦悦1刘勇2李冬冬1黄豪1曹耘铭1丁倩3李巍1银1.扬州大学医学院转化医学研究院,江苏扬州225009;2.大连理工大学生命科学与药学学院,辽宁大连124221;3.扬州大学附属江都人民医院药剂科,江苏扬州225214[摘要]近年来中草药常与西药联合使用,用于疾病的预防和治疗。
中草药成分复杂,可能影响药物代谢酶活性,存 在引发中草药-药物相互作用(H D I)的潜在风险。
目前H D I尚未受到足够重视。
I相药物代谢酶细胞色素P450 2C9 (C Y P2C9)与超过15%的药物代谢相关,尤其是其底物包括几种治疗窗较窄的临床常用药物。
关注中草药对该酶 活性的影响,对H D I的评估至关重要。
本文综述了包括银杏、人参、丹参、生姜和大蒜等在内的常见中草药的提取 物及其主要活性成分对C Y P2C9的抑制作用,旨在为临床中草药与西药的合理使用提供理论依据。
[关键词]细胞色素P450 2C9;中草药;药物代谢;中药药物相互作用[中图分类号]R968 [文献标识码]A [文章编号]1674-4721(2021)6(a)-0025-07 Research progress in the investigation of inhibitory effect of common Chinese herbal extracts and major components on Cytochrome P450 2C9C H E N Y ue-yue' LIU Yong2L ID ong-dong HUANG Hao' CAO Yun-ming' DING Qian3L I Wei1银1. TranslaLional Medicine Research InsLiLuLe, College of Medicine, Yangzhou UniversiLy, Jiangsu Province, Yangzhou 225009, China;2. School of Life and PharmaceuLical Sciences, Dalian UniversiLy of Technology, Liaoning Province, Dalian 124221, China;3. DeparLmenL of Pharmacy, Jiangdu P eople's HospiLal AffiliaLed L o Yangzhou UniversiLy, Jiangsu Province, Yangzhou 225214, China[Abstract] Chinese herbs and WesLern medicine are frequenLly applied in combinaLion for disease LreaLmenL and pre- venLion currenLly. The complicaLed componenLs of Chinese Herbs may affecL Lhe acLiviLy of drug meLabolism enzymes, and Lhus lead L o herb-drug inLeracLion (HDI). However, HDI has been neglecLed. CyLochrome P450 2C9 (CYP2C9), a phase I drug meLabolism enzyme, is responsible for Lhe meLabolism of more Lhan 15%. drugs, including several narrow LherapeuLic window drugs. EvaluaLion of Lhe CYP2C9 acLiviLy alLeraLion is essenLial for HDI predicLion. The currenL arLi- cle reviewed Lhe inhibiLory effecL on CYP2C9 of Lhe common Chinese herbal exLracLs and major componenLs, such as Ginkgo Biloba, Ginseng, Danshen, Ginger and Garlic. The aim is L o provide LheoreLical basis for raLional use of Chinese herbs and WesLern medicine in clinic.[Key words] CyLochrome P450 2C9; Chinese herbs; Drug meLabolism; Herb-drug inLeracLion细胞色素P450(CYPs)是催化药物I相代谢最重 要的药物代谢酶之一,60%c的药物经由CYPs代谢清 除[1]。
顶空气相色谱法测定唑来膦酸中氯苯的残留量

顶空气相色谱法测定唑来膦酸中氯苯的残留量林生文【摘要】目的采用顶空气相色谱法测定唑来膦酸中氯苯的残留量.方法用DB-624毛细管柱,柱温:起始温度80 ℃,维持5 min,再以35 ℃·min-1的速率升温至220 ℃,维持5 min;进样口温度为250 ℃;氢火焰离子化检测器(FID);检测器温度为280 ℃.结果溶剂与氯苯的分离度良好,氯苯在所考察的质量浓度范围内线性关系良好,r=0.999 2,平均回收率为99.0%,检出限为0.007 μg·mL-1.结论该方法简单,结果准确,可用于唑来膦酸中有机溶剂的限度检查.【期刊名称】《西北药学杂志》【年(卷),期】2013(028)004【总页数】2页(P369-370)【关键词】顶空气相色谱法;氯苯;唑来膦酸【作者】林生文【作者单位】广东省食品药品检验所,广州,510180【正文语种】中文【中图分类】R927唑来膦酸是一种特异性地作用于骨的二磷酸化合物,它能抑制因破骨活性增加而导致的骨吸收,临床用于治疗恶性高钙血症,控制恶性肿瘤骨转移,能改善多发性骨髓瘤、前列腺癌、乳腺癌、肺癌等恶性肿瘤治疗的疗效,并且在治疗恶性肿瘤骨转移患者的过程中取得了较好的效果[1-3]。
目前主要有注射用唑来膦酸等制剂。
唑来膦酸的制备工艺比较复杂,各个厂家所用到的溶剂略有差异,但所有厂家的工艺中均采用了氯苯。
《中国药典》2010年版二部附录[4]规定氯苯的残留量限度为0.036%,氯苯对中枢神经系统有抑制和麻醉作用;对皮肤和黏膜有刺激性,毒性较大,其残留量应当得到有效控制。
本文建立了顶空毛细管气相色谱法测定唑来膦酸中的氯苯的残留量,方法快速,准确,灵敏度高。
1.1 仪器 Aglient 7890A气相色谱仪;Aglient G1888顶空进样器;氢火焰离子化检测器。
1.2 试药试剂均为分析纯;氯苯对照品(阿拉丁试剂,批号110503,质量分数≥99%);二甲基亚砜(默克公司,顶空级);唑来膦酸样品[山东新时代(批号:090901),北京诺华制药(批号:C0001),南京制药厂(批号:ZA20090801,ZA20090802,ZA20090803)]。
Alda-1_DataSheet_MedChemExpress

Inhibitors, Agonists, Screening Libraries Data SheetBIOLOGICAL ACTIVITY:Alda–1 is a potent ALDH2 agonist, which significantly improves ALDH2 activity.In Vivo: Alda–1 treatment results in a significant decrease of 4–HNE–protein content in the plasma of apoE -/- mice. Alda–1administration leads to a slight increase in gene expression related to neurogenesis (Nog ), mitochondrial biogenesis (CYTB , ND1),and apoptosis (Bax , Gsk3b ) in the Hp of apoE -/- mice. Alda–1 administration leads to 2 and 10 differentially expressed proteins in theFCx and Hp of apoE -/- mice, respectively [1]. Alda–1 (1.5 mg/kg, b.w., i.p.) administration significantly increases the climbing time,tends to reduce the immobility time and increases the swimming time of the prenatally stressed rats in the forced swim test.Moreover, treatment of prenatally stressed rats with Alda–1 significantly increases number of entries into the open arms of the maze and the time spent therein, as assessed by elevated plus–maze test [2]. Alda–1 (8.5 mg/kg, i.p.) with glucose significantly lowers 4–HNE and FJB–positive cells in the cerebral cortex of Alda–1–treated rats than in DMSO–treated rats 24 h after glucose administration [3]. Alda–1 (10 mg/kg per day) treatment prevents aldehydic overload, mitochondrial dysfunction and improves ventricular function in post–MI cardiomyopathy rats [4].PROTOCOL (Extracted from published papers and Only for reference)Cell Assay:[2]Spleen cells (4×106 cells/mL) are stimulated by optimal concentrations of concanavalin A (Con A; 2.5μg/mL and 0.6 μg/mL) and lipopolysaccharide (LPS, 5 μg/mL) and are incubated in 96–well plates at final volume of 0.2mL for 72 h. Cell proliferation is determined by adding 0.5 μCi of [3H]–thymidine per well at 16 h before the end of the incubation.The cultures are harvested with an automatic cell harvester, and [3H] thymidine incorporation is assessed using a liquid scintillationcounter.Animal Administration: Alda–1 is dissolved in 1 mL/kg b.w. DMSO/water 50/50.[2]After behavioral verification at three months of age,the animals are divided into the following four groups: control, control + Alda–1, prenatally stressed and prenatally stressed + Alda–1(6 animals per group). Alda–1 injections are given intraperitoneally (i.p.) once daily at a dose of 1.5 mg/kg b.w. (dissolved in 1 mL/kg b.w. DMSO/water 50/50) for 14 days. At the same time, the control and prenatally stressed rats receive 1 mL/kg b.w. DMSO/water 50/50. The injections of Alda–1 and vehicle are given between 10 a.m and 11 a.m. In the last five days of Alda–1 treatment the behavioral parameters in the elevated plus maze test and then in the forced swim test are measured.References:[1]. Stachowicz A, et al. Proteomic Analysis of Mitochondria–Enriched Fraction Isolated from the Frontal Cortex and Hippocampus of Apolipoprotein E Knockout Mice Treated with Alda–1, an Activator of Mitochondrial Aldehyde Dehydrogenase (ALDH2). Int J Mol Sci.[2]. Stachowicz A, et al. The impact of mitochondrial aldehyde dehydrogenase (ALDH2) activation by Alda–1 on the behavioral and biochemical disturbances in animal model of depression. Brain Behav Immun. 2016 Jan;51:144–53.Product Name:Alda–1Cat. No.:HY-18936CAS No.:349438-38-6Molecular Formula:C 15H 11Cl 2NO 3Molecular Weight:324.16Target:Aldehyde Dehydrogenase (ALDH)Pathway:Metabolic Enzyme/Protease Solubility:DMSO: ≥ 51 mg/mL[3]. Ikeda T, et al. Effects of Alda–1, an Aldehyde Dehydrogenase–2 Agonist, on Hypoglycemic Neuronal Death. PLoS One. 2015 Jun 17;10(6):e0128844.[4]. Gomes KM, et al. Aldehydic load and aldehyde dehydrogenase 2 profile during the progression of post–myocardial infarction cardiomyopathy: benefits of Alda–1. Int J Cardiol. 2015 Jan 20;179:129–138.Caution: Product has not been fully validated for medical applications. For research use only.Tel: 609-228-6898 Fax: 609-228-5909 E-mail: tech@Address: 1 Deer Park Dr, Suite Q, Monmouth Junction, NJ 08852, USA。
高效液相色谱法测定升血小板胶囊中靛玉红的含量

c o n c e n t r a t i o n o f i n d i r u b i n s t a y e d w i t h i n 0 . 2 0 _ 5 . 0 8
m l ;a l l t h e r e l a t i v e s t a n d a r d d e v i a t i o n s( R S D) o f p r e c i s i o n
d e g r e e,s t a b i l i t y a nd r e p e t i t i v e t e s t we r e <2 % :t h e r e e o v e r y r a t e o f s a mp l e a d d i t i o n wa s 98 . 9 7% . 1 0 0. 92 % . wi t h
WE I Y u j i e ,L I U J i n f e n g ,S O N G C h u n l i( D e p t . o f P h a r ma c y ,t h e F i r s t A f f i l i a t e d Ho s p i t a l o f B e n g b u Me d i c a l C o l l e g e , A n h u i B e n g b u 2 3 3 0 0 4, C h i n a )
高效液相色谱法测定生物样品中儿茶酚胺及其代谢产物

高效液相色谱法测定生物样品中儿茶酚胺及其代谢产物黄新华;陈本美;陈新【期刊名称】《中南大学学报(医学版)》【年(卷),期】2002(027)005【摘要】目的:建立一种测定生物中儿茶酚胺及其代谢产物的灵敏方法.方法:样品经过磺基水杨酸去蛋白后直接进样,测定各种生物样品中的儿茶酚胺及其相关的代谢产物;流动相为20 mmol*L-1柠檬酸三钠(pH4.50)-甲醇(95∶5,V/V),柱温35℃,检测器电压为0.75V.结果:该方法能同时检测7种化合物.最低检测限为10~25 pg*ml-1,回收率均在96%以上,线性范围为20 pg*ml-1~10 μg*ml-1.结论:该方法灵敏可靠,能同时测定生物样品中的儿茶酚胺及其代谢产物.【总页数】3页(P471-473)【作者】黄新华;陈本美;陈新【作者单位】中南大学资产管理处,长沙,410083;现代分析测试中心,长沙,410078;现代分析测试中心,长沙,410078【正文语种】中文【中图分类】R614.2【相关文献】1.高效液相色谱电化学检测生物样品中儿茶酚胺的研究 [J], 李炳源;黄慧伟;黄彦;李新玲;邱一华2.高效液相色谱在生物医药研究中的应用第七讲生物胺的分离和测定(上)——儿茶酚胺、5-羟基色胺及其代谢产物的分离和测定 [J], 唐琴梅3.高效液相色谱-电化学法测定尿儿茶酚胺及其甲氧基代谢产物在嗜铬细胞瘤诊断中的作用 [J], 李宏亮;余叶蓉;刘洪;张翔迅4.高效液相色谱-电化学法测定尿儿茶酚胺及其甲氧基代谢产物在嗜铬细胞瘤诊断中的作用 [J], 李宏亮; 余叶蓉; 刘洪; 张翔迅5.高效液相色谱-电化学检测法测定生物样品中单胺类神经递质及其代谢产物 [J], 杨佳凤;李娟;杨虹;潘桂湘;高秀梅因版权原因,仅展示原文概要,查看原文内容请购买。
用薄层层析法对鱼腥草注射液中醛酮等化合物的鉴定

用薄层层析法对鱼腥草注射液中醛酮等化合物的鉴定
陈国满;张黎明
【期刊名称】《中草药》
【年(卷),期】1979(0)5
【摘要】鱼腥草(Houttuynia cordata Thunb.)为我国民间传统用药。
近年来国内普遍采用水蒸气蒸馏法制得注射液,用于抗菌消炎疗效较好。
本文报道用薄层层析
法对不同产地的15批鱼腥草注射液进行了质量考察,其中5批不显任何斑点;另10批中主要含有甲基壬酮、癸酸以及癸醛、月桂醛等化合物,仅一批含少量癸酰乙醛。
本文还研究了薄层层析斑点面积法测定癸酰乙醛的含量。
【总页数】4页(P18-20)
【关键词】鱼腥草注射液;月桂醛;薄层层析法;化合物
【作者】陈国满;张黎明
【作者单位】湖南省药品检验所;中国人民解放军153医院
【正文语种】中文
【中图分类】R284.1;O658.1
【相关文献】
1.α-羰基烯酮环二硫代缩醛化学(Ⅺ)——α=羰基烯酮环二硫代缩醛类化合物与Reformatsky试剂的加成反应初步研究 [J], 刘群
2.α-羰基烯酮环二硫代缩醛化学(Ⅷ)——以叔丁醇钾为碱合成脂肪族α-羰基烯酮
环二硫代缩醛类化合物 [J], 刘群
3.含哒嗪酮的色酮醛腙类化合物的合成 [J], 陶晶;杨金凤
4.乙酰基取代的酮和酚酮化合物与芳醛了缩合反应──缩杂环酮化合物的合成(V) [J], 高文涛;王海妹;常明琴
5.乙酰基取代的酮和酚酮化合物与芳醛缩合反应的研究 [J], 高文涛;郑卓;陈惠麟
因版权原因,仅展示原文概要,查看原文内容请购买。
