Bienzymatic amperometric biosensor for glucose based
生物分子固定化方法

生物传感器中生物组分的固定化方法生物传感器由两部分组成: 生物敏感元件与信号转换器。
生物传感器的选择性主要取决于敏感材料的选取,而灵敏度的高低则与转换器的类型、生物组分的固定化技术等有很大的关系。
因此固定化技术的发展就是提高传感器性能的关键因素之一。
生物传感器要呈现良好的工作性能, 其固定化技术应满足以下条件:(1) 固定化后的生物组分仍能维持良好的生物活性;(2) 生物膜与转换器须紧密接触,且能适应多种测试环境;(3) 固定化层要有良好的稳定性与耐用性;(4) 减少生物膜中生物组分的相互作用以保持其原有的高度选择性。
为了研制廉价、灵敏度高而且选择性好的生物传感器,固定化技术已成为研究者们努力探求的目标。
经过近20年的不懈探索,已建立了对各种不同生物功能材料的固定化方法。
1、1、3、1 物理吸附法此法就是通过生物分子的极性键、氢键、疏水键的作用将生物组分吸附于不溶性的惰性载体上。
文献已经报道了一些材料可用作吸附其它材料的载体,比如,石墨粉[25]、石墨-聚四氟乙烯[26]、活性碳[27]、离子交换树脂[28]等。
物理吸附法的特点就是方法简便、操作条件温与,缺点就是生物分子与载体表面的结合力弱,在表面进行任意取向的不规则分布,因此使制得的生物传感器容易发生生物分子的脱落与泄漏,从而造成传感器的灵敏度低,重现性差。
1、1、3、2 包埋法将生物组分与合成高分子经溶剂混合而使生物组分包埋于其中,制成敏感膜的方法称作包埋法。
采取的包埋方式通常包括凝胶包埋法与胶囊包埋法二种形式[29,30]。
包埋法的优点就是操作条件比较温与,膜的孔径与形状可随意控制,对生物组分活性的影响较小,缺点就是需控制很多实验因素,而且生物组分在聚合物膜内的活性会受到影响。
1、1、3、3 共价键合法将生物组分通过共价键与电极表面结合而固定的方法称作共价键合法。
该法就是利用基体表面进行活化处理,然后与生物组分偶联,从而使生物组分结合到基体表面。
博舒替尼片-日本说明书

2.重要な基本的注意 ⑴AST(GOT)、ALT(GPT)、γ-GTP、ビリルビン等の上昇を
伴う肝機能障害があらわれることがあるので、投与 3 ヵ月目は 1 回、また、患者の状態
に応じて肝機能検査を行い、患者の状態を十分に観察すること。
⑵白血球減少、好中球減少、顆粒球減少、血小板減少、貧血等の
に不耐容の患者に本剤を投与する際には、投与中止の原因と
なった副作用と同様の副作用が起こるおそれがあるので、前治
療の副作用の内容を確認してから投与すること。
⑹浮動性めまい、疲労、視力障害等があらわれることがあるので、
このような場合には自動車の運転等危険を伴う機械の操作に従
事させないよう注意させること。
( ク ラ リ ス ロ マ イ シ ン、 併用する際には本剤の減量
エリスロマイシン等) を考慮するとともに、患者
HIVプロテアーゼ阻害剤 の状態を慎重に観察し、副
(リトナビル等)
作用発現に十分注意するこ
カルシウム拮抗薬(ジル と。
チアゼム、ベラパミル等)
抗がん剤(イマチニブ等)
アプレピタント、トフィ
アゾール系抗真菌剤(イ 副作用の発現頻度及び重症 の代謝活性を阻害するた
トラコナゾール、ケトコ 度が増加するおそれがある め、本剤の血中濃度が上
ナゾール、フルコナゾー ので、CYP3A阻害作用のな 昇する可能性がある。
ル、ボリコナゾール等) い又は弱い薬剤への代替を
マクロライド系抗生物質 考慮すること。やむを得ず
1
⑶心疾患又はその既往歴のある患者[心疾患が悪化することがあ
る。]
⑷QT間隔延長のおそれ又はその既往歴のある患者[QT間隔延長
が起こるおそれがある。]
美国留学的常用非处方药

美国留学的常用非处方药美国留学必备的常用非处方药美国留学必备常用非处方药。
在美国留学难免有个痛疼脑热,今天店铺给大家介绍一下美国常用非处方药。
Loperamide 是最有效的治疗腹泻的非处方药。
药片和液体形式都适宜儿童服用。
但该药物不能减缓消化不良症状,建议搭配Pepto Bismol服用。
推荐品牌:Imodium, Pepto BismolPseudoephedrine 是唯一投放在市场上,用于治疗由过敏或上呼吸道感染引发的鼻塞、镇咳、祛痰药。
该药的口服溶液也适宜六岁以上的患病儿童。
它最常见的副作用是刺激肾上腺素升高,从而导致心率过快,心悸,高血压等症状。
推荐品牌:SudafedMeclizine是一款常见止吐产品,可以作为处方药或者成药。
它对于治疗晕动病(指晕船、晕车、晕飞机)非常有效。
最常见的不良反应是导致困乏嗜睡。
推荐品牌:Bonine, DramamineRanitidine 是一款非常有效的缓解胃酸过多所致的胃痛、胃灼热(烧心)非处方药。
市面上同类用于减轻胃酸或者反胃的.药物,还有Prilosec、Prevacid、Zegerid等。
其中,Prevacid适合孕期服用,缓解孕妇胃灼热。
推荐品牌:ZantacHydrocortisone cream里百分之一的药物成分是类固醇膏。
它对于治疗由感染或过敏引起的轻微皮肤不适非常有效。
患病成人或儿童均宜使用。
同类产品还推荐diphenhydramine cream。
推荐品牌:CortizoneNeosporin ointment是一款常用的抗菌药膏,主要用于治疗轻微皮肤割伤裂伤,避免再度感染。
推荐品牌:NeosporinClotrimazole主要用于治疗真菌感染。
一般通过局部涂抹来治疗皮肤上的真菌感染,比如癣菌病,尿布疹等。
用于治疗女性宫颈感染时,可以选择其栓剂或者膏药形式。
推荐品牌:LotriminIbuprofen是一种非类固醇的消炎药,也是世界卫生组织指定的必备药品之一。
比阿培南药动学

比阿培南药动学/药效学和治疗药物监测的研究进展Δ陶兴隆 1*,张宇 2,武玺坤 1,马晓松 3,张甜甜 1,吴瑕 1,董维冲 1,宋宁 2,张志清 1 #(1.河北医科大学第二医院药学部,石家庄 050200;2.河北医科大学第二医院感染性疾病科,石家庄 050200;3.河北医科大学第二医院预防保健处,石家庄 050200)中图分类号 R 969;R 978.1 文献标志码 A 文章编号 1001-0408(2023)15-1915-06DOI 10.6039/j.issn.1001-0408.2023.15.23摘要 比阿培南是一种碳青霉烯类抗菌药物,用于治疗败血症、肺炎、肺脓肿、慢性呼吸道病变继发感染、复杂尿路感染、肾盂肾炎等疾病。
本文对比阿培南药动学、药效学和治疗药物监测(TDM )方面的研究进行了综述,发现该药的药动学参数在健康受试者中无明显差异,多次给药无蓄积,但在重症患者以及肾功能异常患者中的药动学参数与健康受试者相比存在较大差异,导致常规治疗方案不能达到预期效果。
在药效学方面,可通过增加给药频次、延长滴注时间来提高该药靶目标值的达标率;对于终末期肾病无尿患者的给药,可以延长间隔时间以避免药物蓄积;但对于重症感染患者,每日1.2 g 的剂量仍不能很好地控制鲍曼不动杆菌、铜绿假单胞菌引起的感染,这限制了其在重症患者中的应用。
建议在重症或肾功能异常患者中对该药实施TDM 并结合药动学模型探索最佳的给药方案,以保证该药游离血药浓度保持在最低抑菌浓度以上的时间占给药间隔时间的百分比(%fT >MIC )在有效范围内,使该药在重症或肾功能异常患者中发挥更大疗效;对于无法进行TDM 的医疗机构,可通过增加给药频次和延长滴注时间来使该药疗效最大化;针对耐药率较高的铜绿假单胞菌、鲍曼不动杆菌及黏质沙雷菌引起的感染,可联合或者更换其他抗菌药物进行治疗。
关键词 比阿培南;药动学;药效学;治疗药物监测Research progress in pharmacokinetics/pharmacodynamics and therapeutic drug monitoring of biapenem TAO Xinglong 1,ZHANG Yu 2,WU Xikun 1,MA Xiaosong 3,ZHANG Tiantian 1,WU Xia 1,DONG Weichong 1,SONG Ning 2,ZHANG Zhiqing 1(1. Dept. of Pharmacy , the Second Hospital of Hebei Medical University , Shijiazhuang 050200, China ;2. Dept. of Infectious Diseases , the Second Hospital of Hebei Medical University , Shijiazhuang 050200, China ;3. Dept. of Preventive Health Care , the Second Hospital of Hebei Medical University , Shijiazhuang 050200, China )ABSTRACTBiapenem is a carbapenem antibiotic , and can be used for the treatment of sepsis , pneumonia , lung abscess ,chronic respiratory lesions secondary infection , complex urinary tract infection and pyelonephritis , etc. This article reviewed the studies on the pharmacokinetics , pharmacodynamics and therapeutic drug monitoring (TDM ) of biapenem. The pharmacokinetic parameters of biapenem are not significantly different in healthy subjects , and there is no accumulation after multiple doses of biapenem. However , there are large differences in pharmacokinetic parameters in patients with severe disease and patients with abnormal renal function compared with healthy subjects , which leads to conventional treatment regimens not achieving the desired outcome. In terms of pharmacodynamics , biapenem can improve the rate of reaching the target value by increasing the frequency of administration and prolonging the infusion time. For patients with anuria in end-stage renal disease , dosing intervals can be extended to avoid drug accumulation. However, for patients with severe infection, a daily dose of 1.2 g still can not control infections caused by Acinetobacter baumannii or Pseudomonas aeruginosa , which limits its use in patients with severe disease. It is recommended to implement TDM in severe patients and patients with abnormal renal function , and explore the best dosing regimen for biapenem in combination with pharmacokinetic models to ensure that the time that the free blood concentration of biapenem remains above minimum inhibitory concentration as a percentage of the time between doses (%fT >MIC ) is within the effective range ,so that biapenem can exert a greater efficacy in severe patients and patients with abnormal renal function. For medical institutions that cannot carry out TDM , the efficacy of biapenem can be maximized by increasing the frequency of administrationand prolonging the infusion time. For infections caused by P .aeruginosa , A. baumannii and Serratia marcescens with high drug resistance rates , it is recommended to combine or replace other antibiotics.KEYWORDSbiapenem ;pharmacokinetics ; pharmacodyna-mics ;therapeutic drug monitoringΔ 基金项目北京医卫健康公益基金会医学科学研究基金项目(No.YWJKJJHKYJJ-ZLTC 2301)*第一作者主管药师,硕士。
新型非格司亭生物仿制药 Nivestim(TM) 获准在欧洲用于预防因化疗导致的发热性嗜中性白血球减少症.docx

新型非格司亭生物仿制药Nivestim(TM) 获准在欧洲用于预防因化疗导致的发热性嗜中性白血球减少症- Hospira 的非格司亭Nivestim(TM) 已经获得欧盟委员会(EC) 的批准,用于预防发热性嗜中性白血球减少症(FN) 和化疗后嗜中性白血球减少症(CIN) 引起的存活期的缩短- Nivestim 是一种新型非格司亭,该药集给药便利性、便于储藏性和安全性与一体- 嗜中性白血球减少症是由癌症化疗引起的最严重的血液中毒症,可导致化疗剂量相对常规疗程的减少和/或推迟(1)英格兰LEAMINGTON SPA 2010年6月10日电/美通社亚洲/ --Hospira 今天宣布,欧盟委员会已经批准将Nivestim(TM)(非格司亭)用于预防发热性嗜中性白血球减少症,这种病症是由癌症化疗引起的最严重的血液中毒症(1)。
目前Nivestim 已在欧盟各国获得营销授权。
Nivestim 预计将可降低嗜中性白血球减少症的治疗成本。
德国Freiburg University Medical Center(弗赖堡大学医学中心)内科医学副教授Cornelius Waller 博士表示:“Nivestim 的获批为医疗卫生专业人员和患者带来了切实的好处。
因癌症化疗导致的嗜中性白血球减少症可导致患者无法完成全部的化学疗程。
Nivestim 为医疗卫生专业人员提供了一种具有成本效益且易于使用的选择,帮助患者坚持到底。
”Nivestim 是Hospira 的第二种生物仿制药。
该公司的促红细胞生成素生物仿制药Retacrit(TM) 目前在17个欧洲国家销售。
Hospira 是首个在欧洲销售生物仿制药的美国公司。
该公司的生物仿制药产品线还包括非格司亭的长效版聚乙二醇非格司亭,是该行业最大的产品线之一。
Hospira 首席商务官Ron Squarer 表示:“作为Hospira 扩大生物仿制药产品组合持久承诺的一部分,我们很自豪地宣布,Nivestim 已经获得欧盟委员会的批准。
百普乐(培哚普利吲达帕胺片)

特殊警告 与培哚普利相关:
● 在免疫功能低下患者发生中性白细胞减少症 / 粒细胞缺乏症的危险 中性粒细胞减少症的危险与剂量及患者类型相关,并取决于患者的临床情况。没有并发症的患者极少会出现这种情况,但是与胶原血管性疾病相关的肾 功能不全的患者可能发生,如系统性红斑狼疮或硬皮病患者以及使用免疫抑制剂治疗的患者。 停止使用血管紧张素转化酶抑制剂治疗,危险性可消失。 严格遵守预先规定的剂量用药可能是防止事件发生的最好办法。但是,如果这些患者需要使用血管紧张素转化酶抑制剂,应慎重评估风险 / 效益比值。
儿童 百普乐不能用于儿童,因为儿童单独应用或联合应用培哚普利的疗效和耐受性尚未确定。
[不良反应]
服用培哚普利可抑制肾素-血管紧张素-醛固酮轴而使吲达帕胺所致的失钾减少。服用百普乐的 2%患者出现低钾血症(钾离子水平< 3.4mmol/l)。
胃肠道 - 通常发生(> 1/100, < 1/10):便秘、口干、恶心、上腹痛、厌食、腹痛、味觉障碍。 - 极少发生(< 1/10, 000):胰腺炎。 - 在肝功能不全病例中,有引发肝性脑病的可能性(见禁忌和注意事项)。
一个月内血压即出现下降,无急速抗药反应;停药后无反弹作用。在临床试验中,同时给予培哚普利和吲达帕胺,与分别单独使用这二种药物相比,可 产生具有协同作用的抗高血压疗效。
与培哚普利相关: 培哚普利可以治疗各种程度的高血压:轻度到中度或重度。可以降低卧位和立位的收缩压和舒张压。 最大降压作用出现在服用单一剂量后 4-6 小时,降压作用可持续 24 小时以上。
培哚普利在低或正常肾素水平的患者中也可产生抗高血压作用。
培哚普利通过它的活性代谢产物—培哚普利拉产生作用。其它的代谢产物均无活性。
培哚普利可减轻心脏负荷: - 通过改变前列腺素代谢产生扩张静脉的作用:减轻前负荷, - 通过降低总外周血管阻力:减轻后负荷。
中国痴呆与认知障碍诊治指南(三)_神经心理评估的量表选择

万方数据
万方数据
万方数据
万方数据
万方数据
万方数据
万方数据
中国痴呆与认知障碍诊治指南(三):神经心理评估的量表选择
作者:贾建平, 王荫华, 张振馨, 肖世富, 周爱红, 汪凯, 丁新生, 张晓君, 张朝东,李焰生, 杨莘, 陈晓春, 罗本燕, 唐牟尼, 徐江涛, 章军建, 彭丹涛, 蔡晓杰,
魏翠柏
作者单位:贾建平,周爱红,魏翠柏(首都医科大学宣武医院神经科,北京,100053), 王荫华(北京大学第一医院神经科), 张振馨(北京协和医学院北京协和医院神经内科), 肖世富(上海市精神卫
生中心), 汪凯(安徽医科大学第一附属医院神经科), 丁新生(南京医科大学第一附属医院
神经内科), 张晓君(北京同仁医院神经内科), 张朝东(中国医科大学第一临床医学院神经
内科), 李焰生(上海交通大学医学院附属仁济医院神经内科), 杨莘(首都医科大学宣武医
院护理部,北京,100053), 陈晓春(福建医科大学附属协和医院神经内科), 罗本燕(浙江大
学医学院附属第一医院神经内科), 唐牟尼(广州脑科医院精神科), 徐江涛(兰州军区乌鲁
木齐总医院神经内科), 章军建(武汉大学中南医院神经科), 彭丹涛,蔡晓杰(卫生部北京
医院神经内科)
刊名:
中华医学杂志
英文刊名:NATIONAL MEDICAL JOURNAL OF CHINA
年,卷(期):2011,91(11)
本文链接:/Periodical_zhyx201111007.aspx。
biosensor

Nose
Small molecules / olfactory membrane proteins / nerve cells / brain
Eye
Visible light / rods and cones (proteins) / nerve cells / brain
2. Biosensor components
• small molecules
glucose, alcohol, CO, CO2, urea, penicillin, TNT, cholesterol, amino acids pesticides, aspirin,
• bio-macromolecules
DNA, RNA, enzymes, proteins, hormones, viruses
Immobilization of biological receptor onto transducer surface
Biol. Receptor Transducer
Requirements:
- Correct orientation
- Accessibility to receptor by analyte - Retention of biological activity - Analyte diffusion to biological receptor - Stability over time
• 2.2.2.Antibodies/ antigens
Highly selective interactions and very tight binding. Antibodies can be raised against almost any antigen. Usually needs to be linked to other probe for detection.
bimekizumab成分

bimekizumab成分
Bimekizumab是一种新型的生物制剂,属于单克隆抗体类药物。
其主要成分是一种叫做bimekizumab的单克隆抗体。
这种单克隆抗
体是针对IL-17A和IL-17F的,这两种细胞因子在炎症过程中起着
重要作用。
bimekizumab通过结合IL-17A和IL-17F,阻止它们与受
体结合,从而抑制炎症反应。
这种药物被用于治疗类风湿性关节炎、牛皮癣和脊柱关节炎等自身免疫性疾病。
从化学结构上来看,bimekizumab是一种重组的人源IgG1kappa
单克隆抗体,它是通过基因工程技术在体外培养的哺乳动物细胞中
生产的。
这种单克隆抗体具有特异性和高亲和力,能够精确地识别
并结合IL-17A和IL-17F,从而发挥治疗作用。
总的来说,bimekizumab的主要成分是一种针对IL-17A和IL-
17F的单克隆抗体,通过干预炎症反应的关键细胞因子,达到治疗
自身免疫性疾病的效果。
希望这样的回答对你有所帮助。
植物维生素B1生物合成及生物强化的研究进展

SUN Ya⁃li, TANG Jia⁃qi, MAO Xin⁃chen et al ( Agricultural College of Yangzhou University / Jiangsu Key Laboratory of Crop Genetics
也会增加[19] 。 对向日葵根部进行外源施加维生素 B1 ,其可
剂
[4-5]
缺乏症。 若严重缺乏维生素 B1 ,则会干扰中枢神经和循环系
以高碳水化合物为主食的国家中普遍存在[8] 。
维生素 B1 在植物的生长发育、非生物和生物胁迫的响
应中发挥着重要的作用
[9]
。 维生素 B1 参与许多细胞代谢途
加[17-18] ,维生素 B1 生物合成途径关键酶的 mRNA 转录水平
酸合成酶(thiamine phosphate synthase,TH1) 催化 HMP -P 而
完成
[22]
。
噻唑 部 分 的 生 物 合 成 是 通 过 噻 唑 合 成 酶 ( HEP - T
synthase,THI1)催化底物形成腺苷二磷酸-5-( β-乙基) -4-
催化,耦联形成 TMP。 TMP 在原核生物中可以直接转化为
5-β-羟乙基噻唑)和嘧啶环(4-氨基-5-羟甲基嘧啶)2 个部
分组成。 2 个部分在质体中单独合成,然后结合在一起,最终
形成 TPP 的形式(图 2)。
嘧啶是通过嘧啶合成酶(HMP -P synthase,THIC) 催化底
6
安徽农业科学 2024 年
互联网医疗英文热词解读:Biosensor

互联网医疗英文热词解读:BiosensorVcbeat医生诊脉造型象牙制根付(一种用于和服上的挂钩),ちかあき,日本,19世纪什么是Biosensor?Biosensor即Biological Sensor生物传感器,是一种对生物物质敏感并将其浓度转换为电信号进行检测的仪器。
传统生物传感器由生物分子识别物质和一个换能器组成。
生物分子和待检测物质相互反应作用,生物反应通过换能器转化为电信号,运用于各个领域。
Biosensor的构成生物传感器主要由两个部分构成:分子识别物质充当感应器:分子识别物质用于识别被测目标,是可以引起某种物理变化或化学变化的主要功能元件。
它可以是•核酸•蛋白质类,包括酶和抗体•植物蛋白质或植物凝集素•组织切片、微生物、细胞器等复杂物质换能器:用于检测并传递信号的电子设备当分子识别物质与特定的待检测物质发生物理或化学反应(如抗体抗原的结合,酶与基质的结合等)时会产生信号,信号可能是电子的、光学的或者热学的,通过采用适当的换能器将这些信号转化为可量取的电信号(通常为电流或电压),从而达到不同的目的。
Biosensor的分类按照生物传感器中分子识别元件可分为五类:•酶传感器•微生物传感器•细胞传感器•组织传感器•免疫传感器Biosensor的工作原理我们拿酶传感器为例。
显而易见,酶传感器是由酶作为生物识别分子的生物传感器。
当我们吃下汉堡、薯条等食物后,它们在我们的身体里会通过一系列的反应步骤变为小分子,这个过程叫做分解代谢,接下来这些小分子将合成蛋白质等人体的重要物质,这个过程叫做合成代谢。
每一个分解代谢和合成代谢进程都由一种特定的酶催化发生(新陈代谢)。
因此这一种特定的酶就能够识别这样一个目标分子或其反应进程。
又例如,在测量病人血糖浓度变化的过程中需要用到3中生物传感器:•氧传感器测量氧浓度•酸碱度传感器测量葡萄糖酸产生过程•过氧化氢传感器测量过氧化氢浓度在这个过程中,氧传感器和过氧化氢传感器将转化为电流,酸碱度传感器转化为电压。
治疗阿尔茨海默病!非侵入性神经刺激器获FDA突破性医疗器械认定
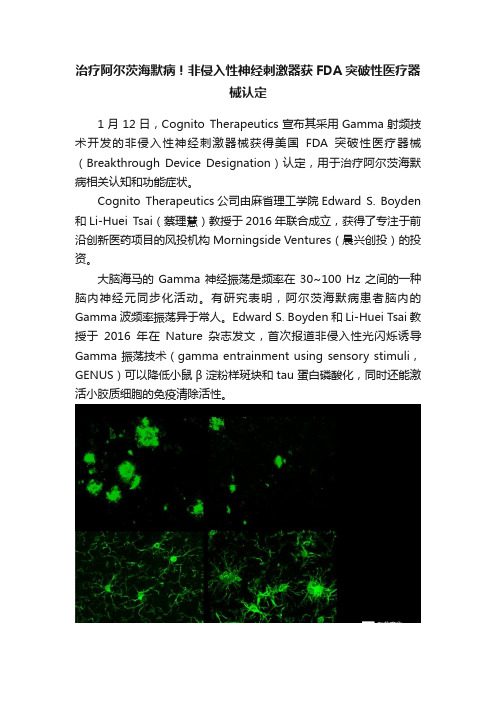
治疗阿尔茨海默病!非侵入性神经刺激器获FDA突破性医疗器械认定1月12日,Cognito Therapeutics宣布其采用Gamma射频技术开发的非侵入性神经刺激器械获得美国FDA突破性医疗器械(Breakthrough Device Designation)认定,用于治疗阿尔茨海默病相关认知和功能症状。
Cognito Therapeutics公司由麻省理工学院Edward S. Boyden 和Li-Huei Tsai(蔡理慧)教授于2016年联合成立,获得了专注于前沿创新医药项目的风投机构Morningside Ventures(晨兴创投)的投资。
大脑海马的 Gamma 神经振荡是频率在 30~100 Hz 之间的一种脑内神经元同步化活动。
有研究表明,阿尔茨海默病患者脑内的Gamma波频率振荡异于常人。
Edward S. Boyden和Li-Huei Tsai教授于2016年在Nature杂志发文,首次报道非侵入性光闪烁诱导Gamma 振荡技术(gamma entrainment using sensory stimuli,GENUS)可以降低小鼠β淀粉样斑块和tau蛋白磷酸化,同时还能激活小胶质细胞的免疫清除活性。
非侵入性脑刺激技术治疗1周可减少小鼠Aβ斑块数量,改善小胶质细胞功能形态2019年蔡理慧团队在Cell上发表一篇题为Multi-sensory Gamma Stimulation Ameliorates Alzheimer’s-Associated Pathology and Improves Cognition的文章,揭示在阿尔茨海默病动物模型中,听觉刺激与海马CA1和大脑听觉皮层区的GENUS诱导伽马振荡相结合,可降低淀粉样蛋白水平并改善记忆力。
来源:Cell。
生物传感器-文献综述-3110100122-邵建智

检测葡萄糖浓度的酶传感器研究文献1题目:Real-Time Noninvasive Measurement of Glucose Concentration Using a Microwave Biosensor检测机理:通过微波生物传感器,用探头尖端和葡萄糖溶液之间的实时电磁相互作用来检测葡萄糖浓度,微波生物传感器包括一个耦合到探针尖端的电解质谐振器,由于微波谐振器和葡萄糖溶液之间的电磁相互作用,葡萄糖浓度的变化与微波的反射系数直接相关,并且检测分辨率达1毫克/毫升。
检测仪器:如图所示的微波传感器。
分子识别元件:镀金探针尖端检测步骤:微波生物传感器包括一个耦合到探针尖端的电解质谐振器,其共振频率约为4.6GHz,为了获得高的灵敏度,有圆顶点的镀金探针尖端和圆筒形端部需要连接到谐振器的内部循环当中,硅管壁厚TT = 0.4毫米和内径TG = 2.5毫米被安装在圆筒形探针尖端的端部,如图所示。
整个系统放置在机械振动隔离台,测量全部在电磁内进行,其内环境,温度与湿度均自动控制,管内葡萄糖的流速保持着2毫米/秒的速度,利用网络分析仪,可以测得微波谐振器的反射系数,从而得出葡萄糖的浓度。
检测限:0.003dB/(mg/ml)检测时间:实时监控并检测创新性:可以进行无创实时检测不足:微波遥感平台应用不够广泛文献2题目:Measurement of Glucose Concentration in Blood Plasma Based on a Wireless Magnetoelastic Biosensor检测机理:血浆中的无线磁弹性葡萄糖生物传感器描述的基础上,使用质量敏感的磁传感器作为传感器。
葡萄糖生物传感器的制作是用pH敏感的聚合物和葡萄糖氧化酶(葡萄糖氧化酶)和过氧化氢酶的生物层涂布的带状,磁致弹性传感器。
将pH响应聚合物溶胀或收缩,从而改变传感器质量负荷,分别响应于增加或减少的pH值。
在血浆中的葡萄糖氧化酶催化的氧化反应产生葡糖酸,从而使pH敏感聚合物收缩,这反过来又降低了传感器的质量负荷。
维贝格龙获FDA批准用于膀胱过度活动症

维贝格龙获FDA批准用于膀胱过度活动症Urovant Sciences24日宣布,美国食品和药物管理局(FDA)已经批准了β-3肾上腺素能受体(β3)激动剂GEMTESA®(vibegron,维贝格龙)的每日一次75毫克的新药申请(NDA),用于治疗成人中出现尿急尿失禁(UUI)、尿急和尿频等症状的膀胱过度活动症(OAB)。
此次获批标志着自2012年以来FDA批准的第一个新的口服品牌OAB药物,这也是Urovant Sciences的第一个产品批准。
GEMTESA是一种口服、每日一次的片剂,含有75毫克的维贝琼,这是一种小分子β3受体激动剂,有助于放松膀胱肌肉,使膀胱能够容纳更多的尿液,从而减轻OAB的症状。
波士顿圣伊丽莎白医学中心的临床试验研究者和主要泌尿科医生David Staskin博士说:"GEMTESA是第一个以每日一次药片形式提供的β3受体激动剂,它不需要剂量滴定。
值得注意的是,在关键的EMPOWUR研究中,与安慰剂相比,GEMTESA没有增加任何高血压的不良事件,并且与CYP2D6代谢的药物没有相互作用,这一点很重要,因为许多常用药物都是由CYP2D6代谢的。
"FDA的批准是基于一项广泛的开发计划的结果,该计划涉及4000多名OAB患者,包括为期12周的双盲、安慰剂对照的3期EMPOWUR研究,剂量为75mg,以及双盲EMPOWUR长期扩展研究。
这些数据显示,在EMPOWUR中,与安慰剂相比,使用GEMTESA治疗可显著减少每日UUI、口渴和紧急发作,并增加排空量,具有统计学意义。
Vibegron第52周达到反应终点的患者比例更高在双盲、安慰剂对照的EMPOWUR研究中,GEMTESA最常见的不良反应(≥2%)是头痛、鼻咽炎、腹泻、恶心和上呼吸道感染。
GEMTESA表现出与安慰剂相同的高血压和血压升高的不良事件发生率。
关于膀胱过度活动症膀胱炎是一种临床症状,当膀胱肌肉不自主地收缩时,就会出现这种症状。
对β肾上腺素受体和M胆碱受体有双向调节作用的药物及其制法[发明专利]
![对β肾上腺素受体和M胆碱受体有双向调节作用的药物及其制法[发明专利]](https://img.taocdn.com/s3/m/d60f386084868762cbaed55a.png)
专利名称:对β肾上腺素受体和M胆碱受体有双向调节作用的药物及其制法
专利类型:发明专利
发明人:易宁育,夏宗勤,胡雅儿
申请号:CN93112451.4
申请日:19930531
公开号:CN1096031A
公开日:
19941207
专利内容由知识产权出版社提供
摘要:本发明是一种对β肾上腺素受体和M胆碱受 体有双向调节作用的药——知母皂苷元及其制法。
它具有对两种受体有双向调节作用的特殊优点,并且 不同于西药中的激动剂或拮抗剂,不存在停药“反跳” 现象。
其制造工艺亦具有成本低,得率高,操作简便, 适用于大规模生产等特点。
申请人:上海第二医科大学
地址:200025 上海市重庆南路280号
国籍:CN
代理机构:上海高校专利事务所
更多信息请下载全文后查看。
必思添(Biostim)--增强免疫功能,预防反复性呼吸道感染的有效药物

必思添(Biostim)--增强免疫功能,预防反复性呼吸道感染的有
效药物
俞森洋
【期刊名称】《中华保健医学杂志》
【年(卷),期】1999(001)003
【摘要】无
【总页数】1页(P62)
【作者】俞森洋
【作者单位】无
【正文语种】中文
【相关文献】
1.必思添用于防治反复呼吸道感染性疾病的临床意义 [J], 黄晓英;杨炯
2.Biostim(必思添)对慢性阻塞性肺病患者免疫功能的影响及其防治作用的研究 [J], 张宏;郭剑超;郭仓
3.必思添防治肺心病反复呼吸道感染疗效观察 [J], 任爱华;李大立
4.口服必思添预防53例小儿反复呼吸道感染 [J], 刘光华;郭新华
5.必思添治疗小儿反复呼吸道感染50例临床疗效观察 [J], 刘丹;司予倩;杨清华;陈剑
因版权原因,仅展示原文概要,查看原文内容请购买。
百士欣

百士欣药品介绍【百士欣商品名】百士欣【百士欣通用名】乌苯美司胶囊【本文来源】【百士欣英文通用名】 Ubenimex Capsules百士欣适用于:本品增强免疫功能,用于抗癌化疗、放疗的辅助治疗,老年性免疫功能缺陷等。
可配合化疗、放疗及联合应用于白血病、多发性骨髓瘤、骨髓增生异常综合症及造血干细胞移植后,以及其它实体瘤患者。
百士欣规格:10mg*15粒【百士欣适应症】本品可增强免疫功能,用于抗癌化疗、放疗的辅助治疗,老年性免疫功能缺陷等。
可配合化疗、放疗及联合应用于白血病、多发性骨髓瘤、骨髓增生异常综合症及造血干细胞移植后,以及其它实体瘤患者。
【百士欣用法用量】成人,一日30mg,1次(早晨空腹口服)或分3次口服;儿童酌减。
症状减轻或长期服用,也可每周服用2~3次,10个月为一疗程。
【百士欣不良反应】剂量超过200mg/日,可使T细胞减少。
偶有皮疹、瘙痒、头痛、面部浮肿和一些消化道反应,如恶心、呕吐、腹泻、软便。
个别可出现一过性轻度AST升高。
一般在口服过程中或停药后消失。
【百士欣规格包装】0.01g*15粒 15粒/盒【百士欣有效期】36个月百士欣药理本品从链霉菌属(Streptomyces ofivorecticuli)的培养液中分离所得的二肽化合物,可竞争性地抑制氨肽酶B(aminopeptidase B)及亮氨酸肽酶(Leucineamino Peptidase)和半胱天冬酶(Caspase)。
增强T细胞的功能,使NK细胞的杀伤活力增强,且可使集落刺激因子合成增加而刺激骨髓细胞的再生及分化。
本品能干扰肿瘤细胞的代谢,抑制肿瘤细胞增生,使肿瘤细胞凋亡,并激活人体细胞免疫功能,刺激细胞因子的生成和分泌,促进抗肿瘤效应细胞的产生和增殖。
百士欣适应症本品增强免疫功能,用于抗癌化疗、放疗的辅助治疗,老年性免疫功能缺陷等。
可配合化疗、放疗及联合应用于白血病、多发性骨髓瘤、骨髓增生异常综合症及造血干细胞移植后,以及其它实体瘤患者。
- 1、下载文档前请自行甄别文档内容的完整性,平台不提供额外的编辑、内容补充、找答案等附加服务。
- 2、"仅部分预览"的文档,不可在线预览部分如存在完整性等问题,可反馈申请退款(可完整预览的文档不适用该条件!)。
- 3、如文档侵犯您的权益,请联系客服反馈,我们会尽快为您处理(人工客服工作时间:9:00-18:30)。
Analytica Chimica Acta 451(2002)251–258Bienzymatic amperometric biosensor for glucose based on polypyrrole/ceramic carbon as electrode materialFaming Tian,Guoyi Zhu ∗Laboratory of Electroanalytical Chemistry,Changchun Institute of Applied Chemistry,Chinese Academy of Sciences,Changchun 130022,China Received 6April 2001;received in revised form 10September 2001;accepted 10September 2001AbstractA novel amperometric biosensor utilizing two enzymes,glucose oxidase (GOD)and horseradish peroxidase (HRP),was developed for the cathodic detection of glucose.The glucose biosensor was constructed by electrochemical formation of a polypyrrole (PPy)membrane in the presence of GOD on the surface of a HRP-modified sol–gel derived-mediated ceramic carbon electrode.Ferrocenecarboxylic acid (FCA)was used as mediator to transfer electron between enzyme and electrode.In the hetero-bilayer configuration of electrode,all enzymes were well immobilized in electrode matrices and showed favorable enzymatic activities.The amperometric detection of glucose was carried out at +0.16V (versus saturated calomel reference electrode (SCE))in 0.1M phosphate buffer solution (pH 6.9)with a linear response range between 8.0×10−5and 1.3×10−3M glucose.The biosensor showed a good suppression of interference in the amperometric detection.©2002Elsevier Science B.V .All rights reserved.Keywords:Biosensor;Glucose;Polypyrrole;Sol–gel;Ceramic carbon electrode1.IntroductionThe sol–gel technique has attracted much attention in the field of immobilization of different reagents [1–5].The excellent properties of sol–gel materi-als,such as chemical inertness,optical transparency,simplicity of preparation,negligible swelling in aque-ous,low temperature encapsulation and mechanical stability,endued these materials with an extensive role in preparation of chemical sensors and biosen-sors [6–12].The entrapped species such as chemical materials and biomolecules magnificently preserved their chemical properties or bioactivities.The most∗Corresponding author.Tel.:+86-431-5262071;fax:+86-431-5685653.E-mail address:zhuguoyi@ (G.Zhu).interesting thing is that the leaching of these en-trapped species did not occur or just occurred very slowly.The reason for this is that the sol–gel materials are usually formed by hydrolysis of an alkoxide precursor followed by condensation to yield a poly-meric oxo-bridged SiO 2network and the immobilized species are encapsulated within the physically rigid network [6].The enzymes and proteins can be immobilized within sol–gel matrices still maintaining their native properties and reactivities,this makes the technique a potential tool for the development of new biosensors.Sol–gel-derived electrochemical biosensors mainly relied on two basic configurations:conductive ceramic composites [13–15]and electrode surface coatings [8,16–18].Since the pioneering work of Lev and co-workers sol–gel derived composite carbon electrodes0003-2670/02/$–see front matter ©2002Elsevier Science B.V .All rights reserved.PII:S 0003-2670(01)01405-2252F.Tian,G.Zhu/Analytica Chimica Acta451(2002)251–258(CCEs)[13,19–22]have been widely used to deve-lop all kinds of amperometric biosensors.Catalysts, enzymes,electron transfer mediators can be readily incorporated into the matrices of CCEs for the deve-lopment of surface renewable amperometric ually mediators or electrocatalysts are used in CCEs to improve the performance of oxidase-based biosensor.Generally,the detection mode involved in oxidase-based biosensors is often based on the electrochemical detection of hydrogen peroxide,which is produced in the course of the enzyme-catalyzed oxidation of substrates by dissolved oxygen.However,the direct oxidation of hydrogen peroxide requires a relative high working potential(exceeding ca.0.6V versus saturated calomel reference electrode(SCE))in order to obtain a sufficiently high sensitivity.At such a potential many substances,usually present in biologic samples(such as urate,acetaminophen and ascorbic acid)can also be electrochemically oxidized leading to interfering response in quantitation of substrate concentration.General methods used to suppress the interferences have involved utilizing selective elec-trocatalysts as electron mediators[23]to reduce the overpotential of electrochemical oxidation of hydro-gen peroxide,or constructing an additional membrane [24–27]on the tip of the electrode to prevent the diffusion of interferences.An attractive alternative approach to suppress the interferences has been developed by the construction of bienzymatic peroxidase/hydrogen peroxide produ-cing oxidase amperometric biosensors in the past few years[26,28–31].In such configurations,hydrogen peroxide is selectively reduced by peroxidase.The mechanism of the bienzymatic biosensors may be expressed as followssubstrate+O2oxidase→product+H2O2H2O2+(POD)red→H2O+(POD)oxwhere(POD)red and(POD)ox are the reduced and oxi-dized forms of peroxidase,respectively.The(POD)ox is directly electroreduced by electrode in mediatorless biosensor or by the oxidized form of mediator in me-diated biosensor.In this paper,we takes advantages of sol–gel process to construct a bienzymatic amper-ometric biosensor for glucose by coating a glucose oxidase/polypyrrole(GOD/PPy)membrane on the surface of ferrocenecarboxylic acid(FCA)-mediated horseradish peroxidase(HRP)-modified CCE.2.Experimental2.1.ChemicalsMethyltrimethoxysilane(MTMOS)and FCA were purchased from Acros.Graphite powder was obtained from Aldrich.GOD(EC1.1.3.4,type VII-S,245.9 units mg−1from Aspergillus niger)was obtained from Sigma.HRP was purchased from Shanghai Lizhu Dongfeng Biotechnology Co.Ltd.Pyrrole was freshly distilled prior to use and stored under nitrogen atmosphere.Double distilled water was used for the preparation of all phosphate buffer solutions(0.1M, containing0.1M KCl).Fresh stock solution of urate, lactate and oxalate were prepared before use and kept in the dark.Ascorbate was prepared immediately be-fore use.All other reagents were of analytical grade and used without further purification.2.2.ApparatusAn EG&G PARC model273potentiostat driven by an IBM PC with270software was used for electro-polymerization,voltammetric and amperometric mea-surements.A three-electrode cell with a SCE and a platinum foil counter electrode was used.The solu-tions were deaerated thoroughly for at least15min with pure N2and kept under a positive pressure of this gas during all experiments except the ampero-metric detection experiment.In the amperometric measurements,the buffer solution was magnetically stirred.All experiments were carried out at ambi-ent temperature.All potentials were measured and reported versus SCE.2.3.Preparation of HRP-modifiedFCA-mediated CCEGenerally high concentrations of methanol and/or strong acid are used in conventional sol–gel proce-dures employed in electroanalytical chemistry,which can cause enzymes to become denatured.Thus, the silica sols are usually prepared without adding methanol and with low concentration of acid as catalyst in constructing sol–gel derived biosensors.F .Tian,G.Zhu /Analytica Chimica Acta 451(2002)251–258253The silica sol–gel solutions used to construct CCEs were prepared as follows.A mixture of 1ml MTMOS,0.5ml water and 0.1ml HCl (0.01M)was sonicated for 10min,then,the homogeneous silica solution was stored over night at 4◦C in refrigerator.Twenty milligrams of HRP was dissolved in 0.5ml water and subsequently mixed with 160mg graphite powder in a mortar.The mix-ture was allowed to dry in a desiccator at 4◦C.The enzyme-modified graphite carbon,20mg FCA and 0.3ml silica sol–gel solution were thoroughly mixed.The mixture was packed into one end of a 3mm i.d.glass tube to a length of 5mm,subsequently allowed to dry and gel at least 5days at 4◦C.After being polished on weighing paper the surface of resulting HRP-modified FCA-mediated CCEs were smooth and shiny.Copper wire,inserted from the other end of glass tube,provided the electrical contact.2.4.Coating GOD/PPy on the surface of HRP-modified CCEThere are usually several electrochemical tech-niques used for direct electropolymerization including potentiostatic,galvanostatic and potentiodynamic.In the experiment,the galvanostatic mode was employed with a current density of 0.06mA cm −2in 0.1M phosphate buffer solution (pH 7.0)containing 0.25MFig.1.Cyclic voltammograms of HRP-modified FCA-mediated CCE in phosphate buffer solution (pH 7.0)at different scan rates:(1)5mV s −1;(2)20mV s −1and (3)50mV s −1.The inset shows the plot of anodic peak current vs.the square root of scan rate.pyrrole and 3.5mg ml −1GOD,which yielded films with more uniform thickness than in the potentiostatic mode.The solution was deaerated with N 2for 15min and left unstirred prior to electropolymerization.The bienzymatic biosensors were thoroughly washed after preparation and stored in phosphate buffer at 4◦C when not in use.3.Results and discussion3.1.Characteristics of bienzymatic glucose biosensor 3.1.1.The electrochemistry of HRP-modified FCA-mediated CCEThe performance of enzyme doped CCE was affected by the ratio of water to silicon in silica sol and the concentration of mediator.Under the optimized conditions in preparing enzyme doped CCE [32],the electrochemistry of HRP-modified FCA-mediated CCE was studied by cyclic voltam-metry.In the original several cycles,the peak to peak separation E p of FCA-mediated CCE was more than 350mV .With the diffusion of supporting electrolyte into the HRP-modified CCE,a thin active layer on the electrode surface was gradually come into being and reached equilibrium.Correspondingly,the redox couple of FCA became more reversible.Fig.1shows254F .Tian,G.Zhu /Analytica Chimica Acta 451(2002)251–258the cyclic voltammograms of HRP-modified FCA-mediated CCE at different scan rates.There are well-defined anodic and cathodic waves with mean peak potential E 1/2=(E pa +E pc )/2of 278mV .Inset shows the plot of peak current versus the square root of scan rate.A linear relation is recorded and the straight line does not pass though the origin,which suggests that the system is poorly diffusion controlled.3.1.2.Effect of PPy film thickness on the response of bienzymatic biosensorThe enzyme entrapment within polymer films can be accomplished non-covalently and covalently.Non-covalent entrapment is easily carried out by elec-tropolymerization of the monomers in the presence of enzyme.A relative large enzyme concentration is usually required in these non-covalent entrapments.As is known,PPy carries a net positive charge when being oxidatively polymerized and GOD is nega-tively charged in solutions with pH >4.2[33].Thus,GOD is prone to be entrapped within PPy membrane relying on the charge complimentarity between them.The amperometric response of PPy-based biosen-sor is largely affected by the amount of enzyme entrapped within the film and the thickness of film.These parameters can be controlled by manipulating the concentrations of enzyme,pyrrole in solution and the amount of charge duringelectropolymerization.Fig.2.Effect of passing charge during electropolymerization on the response of bienzymatic glucose biosensor towards 1.0×10−4M glucose.In this experiment,the electropolymerization was carried out in phosphate buffer solution (pH 7.0)con-taining 0.25M pyrrole and 3.5mg ml −1GOD accord-ing previous report [34].Fig.2shows the effect of electropolymerization charge on the response of bien-zymatic biosensor toward 10−4M glucose.It can be seen that a maximum response was achieved when the charge for electropolymerization was 10.18mC cm −2.The response increased with increasing of charge for electropolymerization during range from 4.60to 10.18mC cm −2.This may be due to the increasing of GOD within polymer membrane.When the passing charge was large than 10.18mC cm −2,because of the suppression of polymer membrane to the diffusion of analyte,the response decreased even though the amount of GOD entrapped within polymer membrane is known to be increased.Thus,all bienzymatic glu-cose biosensors were fabricated by coating GOD/PPy membrane on the surface of HRP-modified CCE with electropolymerization charge of 10.18mC cm −2in phosphate buffer solution (pH 7.0)containing 3.5mg ml −1GOD and 0.25M pyrrole.3.2.Response mechanism of bienzymatic biosensor for glucose detectionFig.3describes the proposed reaction mechanism of bienzymatic amperometric biosensor for the electro-F .Tian,G.Zhu /Analytica Chimica Acta 451(2002)251–258255Fig.3.Response mechanism of bienzymatic biosensor for glucose detection.enzymatic detection of glucose.Glucose,which dif-fuses from bulk solution into the GOD/PPy mem-brane,is catalyzed by GOD and dissolved dioxygen.The resulting hydrogen peroxide diffuses either to the surface of HRP-modified FCA-mediated CCE or back to the bulk buffer solution.The oxidized form of HRP (HRP ox )is produced by the enzymatic reaction of hydrogen peroxide and HRP,and subsequently reduced by the FCA to regenerate HRP.The resulting ferricinium ion (FCA +)is electroreduced to regene-rate FCA,producing the response current at the same time.The process involved in the glucose detection at the bienzymatic amperometric biosensor could be detailed as follows according to commonly recog-nized mechanism [35]glucose +GOD (FAD )→gluconolactone +GOD (FADH 2)Fig.4.Dependence of the bienzymatic biosensor response on applied potential to 1.0×10−4M glucose in phosphate buffer solution at pH 7.0.GOD (FADH 2)+O 2→GOD (FAD )+H 2O 2HRP +2H 2O 2→HRP ox +2H 2O HRP ox +2FCA →2FCA ++HRP 2FCA ++2e −→2FCAwhere FAD and FADH 2are the oxidized and reduced forms of flavine adenine dinucleotide,the active center of GOD,respectively.3.3.Effect of applied potential and buffer pH on the response of bienzymatic biosensorFig.4shows the effect of applied potential on the response of the biosensor.The amperometric detec-tion was carried out in 0.1M phosphate buffer solu-tion (pH 7.0)in the presence of 1.0×10−4M glucose.256F.Tian,G.Zhu/Analytica Chimica Acta451(2002)251–258Fig.5.Effect of pH on the bienzymatic glucose biosensor response to1.0×10−4M glucose.Applied potential160mV vs.SCE.It was found that the sensitivity of bienzymatic glu-cose biosensor increased with increasing potential from130to160mV and decreased during the po-tential range from160to190mV.The increased sensitivity with applied potential can be attributed to the increased driving force for the electroreduction of ferricinium ion,which is produced in enzymatic reac-tion.The highest sensitivity is acquired at potential of 160mV.The effect of phosphate buffer solution(con-taining1.0×10−4M glucose)pH on the response of bienzymatic glucose biosensor is shown in Fig.5, which shows the highest amperometric response at pH 6.9.On the basis of above results,the pH of6.9and potential of160mV were selected for the fol-lowing experiments in order to obtain the highest sensitivity.3.4.Selectivity of the bienzymaticglucose biosensorThe response from the bienzymatic glucose bio-sensor was also measured in the presence of some possible interfering substances(such as urate,lactate, oxalate,ascorbic acid,citrate and lactose).Table1 summarizes the effect of interferences on the biosen-sor response.The ratio of the amperometric response of mixtures of1.0×10−4M each interfering substance in the presence of1.0×10−4M glucose compared to that of1.0×10−4M glucose alone is taken as the criterion for the selectivity of the glucose biosensor. It can be seen that those substances cause hardly any interference on the response of the biosensor.In the hetero-bilayer configuration of the biosensor,the diffusion of interfering species from bulk solution to the electrode surface of CCE is slowed down by the PPy membrane.Especially,the selectively enzymatic reduction of hydrogen peroxide by HRP and the low operating potential for amperometric detection mainly contribute to the high selectivity of the glucose biosensor.Table1Effect of possible interfering substances on the bienzymatic glucose biosensor responsePossible interfering substance Current ratios aUrea0.99Lactate0.98Oxalate 1.00Ascorbic acid 1.05Citrate0.99Lactose 1.00a Current ratio=I mix/I glu.I mix:current of mixture of10−4M interference substance and10−4M glucose;I glu:current of10−4M glucose alone.F .Tian,G.Zhu /Analytica Chimica Acta 451(2002)251–258257Fig.6.Calibration curve of bienzymatic glucose biosensor in phosphate buffer solution (pH 6.9)at 160mV vs.SCE.The inset shows the linear range of the calibration curve.3.5.Calibration and stabilityThe calibration curve for the bienzymatic glu-cose biosensor (Fig.6)obtained by an amperometric response under optimized experimental conditions shows a linear response range between 8.0×10−5and 1.3×10−3M glucose with a sensitivity of 1.11A mM −1and a correlation coefficient of 0.998.It can be seen from Fig.6that the bienzymaticglucoseFig.7.Stability of the glucose biosensor stored at 4◦C.biosensor shows a high background current.This can be attributed to the hetero-bilayer configuration of the glucose ually the enzyme doped CCE shows a large background current because that the doped enzyme altered the hydrophilicity of the CCE surface [36].With the coating of PPy on the surface of enzyme doped CCE,the glucose biosen-sor shows a high background current.The apparentMichaelis–Menten constant (K appM ),which shows an258F.Tian,G.Zhu/Analytica Chimica Acta451(2002)251–258 indication of the enzyme–substrate kinetics,for theglucose biosensor is calculated to be0.25mM fromthe linear part of the calibration curve according to theEadie–Hoffstee form of the Michaelis–Menten equa-tionI ss=I max−K app MI ss Cwhere I ss is the steady-state current,I max maximum current under saturated substrate conditions and C bulk concentration of the substrate.The response time of biosensor towards glucose is less than25s.The detection limit,estimated as three-times the noise,is 1.0×10−5M for glucose.The stability of the bienzymatic glucose biosen-sor was studied by amperometric detection of1.0×10−4M glucose for every2–3days.Fig.7shows the effect of storage time on the relative response of the glucose biosensor.After1week,the biosensor retained 92%of the initial sensitivity when stored at4◦C.The biosensor showed a steady sensitivity during the fol-lowing time.After3weeks detection,the biosensor retained68%of initial sensitivity.Because of the rapid deactivation of enzymes and PPy membrane,the glu-cose biosensor lost its sensitivity rapidly when stored in air.4.ConclusionsA novel bienzymatic amperometric biosensor for glucose has been developed by electrodepositing GOD/PPy membrane on the surface of HRP-modified FCA-mediated CCE.The enzymes were well im-mobilized within the electrode matrices and retained satisfactory enzymatic catalytic activities.The hetero-bilayer glucose biosensor showed a good suppression of interferences(such as urate,oxalate,ascorbic acid, etc.)with favorable sensitivity towards glucose.The method combining electropolymerization with sol–gel derived CCE provides feasible choice for developing novel biosensors.References[1]L.L.Hench,J.K.West,Chem.Rev.90(1990)33.[2]M.D.Petit-Dominguez,H.Shen,W.R.Heineman, C.J.Seliskar,Anal.Chem.69(1997)703.[3]K.Kimura,T.Sunagawa,M.Yokoyama,Anal.Chem.69(1997)2379.[4]C.Y.Shen,N.M.Kostic,J.Am.Chem.Soc.119(1997)1304.[5]W.Song,N.Lu,Y.Jiang,Y.Liu,X.Wang,J.Liu,H.Xu,C.Sun,Microchem.J.62(1999)344.[6]B.Dave,B.Dunn,J.S.Valentine,J.Zink,Anal.Chem.66(1994)1120A.[7]O.Lev,M.Tsionsky,L.Rabinovich,V.Glezer,S.Sampath,I.Pankratov,J.Gun,Anal.Chem.67(1995)22A.[8]J.Lin,C.W.Brown,Trends Anal.Chem.16(1997)200.[9]M.M.Collinson, A.R.Howells,Anal.Chem.72(2000)702A.[10]D.Avnir,S.Braun,O.Lev,M.Ottolenghi,Chem.Mater.6(1994)1605.[11]J.Wang,Anal.Chim.Acta399(1999)21.[12]A.Walcarius,Electroanalysis10(1998)1217.[13]M.Tsionsky,G.Gun,V.Glezer,O.Lev,Anal.Chem.66(1994)1747.[14]J.Wang,P.V.A.Pamidi,Anal.Chem.69(1997)4490.[15]G.Oskam,P.C.Searson,J.Phys.Chem.B102(1998)2464.[16]J.Wang,P.V.A.Pamidi,D.R.Zanette,J.Am.Chem.Soc.120(1998)5852.[17]B.Wang,B.Li,Z.Wang,G.Xu,Q.Wang,S.Dong,Anal.Chem.71(1999)1935.[18]P.C.Pandy,S.Upadhyay,H.C.Pathak,Electroanalysis11(1999)59.[19]S.Sampath,O.Lev,Electroanalysis8(1996)1112.[20]S.Sampath,O.Lev,J.Electroanal.Chem.446(1998)57.[21]J.Wang,D.S.Park,P.V.A.Pamidi,J.Electroanal.Chem.434(1997)185.[22]J.Gun,O.Lev,Anal.Chim.Acta336(1996)95.[23]S.A.Wring,J.P.Hart,Analyst117(1992)1215.[24]G.G.Wallace,M.Smyth,H.Zhao,Trends Anal.Chem.18(1999)245.[25]S.Cosnier,A.Senillou,M.Grätzle,te,N.Vlacho-poulos,N.J.Renault,C.Martelet,J.Electroanal.Chem.469 (1999)176.[26]M.C.Shin,H.C.Yoon,H.S.Kim,Anal.Chim.Acta329(1996)223.[27]S.Hu,C.Xu,J.Luo,J.Luo,D.Cui,Anal.Chim.Acta412(2000)55.[28]L.Mao,K.Yamamoto,Anal.Chim.Acta415(2000)143.[29]E.F.Perez,G.Oliveira Neto,L.T.Kubota,Sens.Actuators B72(2001)80.[30]S.Cosnier,mbert,M.Stoytcheva,Electroanalysis12(2000)356.[31]M.Niculescu,C.Nistor,I.Frébort,P.Peˆc,B.Mattiasson,E.Csöregi,Anal.Chem.72(2000)1591.[32]J.Li,L.S.Chia,N.K.Goh,S.N.Tan,J.Electroanal.Chem.460(1999)234.[33]P.N.Barlett,J.M.Cooper,J.Electroanal.Chem.362(1993)1.[34]M.Umaña,J.Waller,Anal.Chem.58(1986)2979.[35]E.Csöregi,L.Gorton,G.Marko-Varga,Electroanalysis6(1994)925.[36]I.Pankratov,O.Lev,J.Electroanal.Chem.393(1995)35.。
