Evaluation Indicators and Extraction Method for Pitting Corrosion of Structural Steel
药物分析专业英语

(dissolution) vessel 溶出杯(FTIR) 傅里叶变换红外光谱仪13C-NMR spectrum,13MR 碳-13核磁共振谱1ength basis 长度基准1H-NMR 氢谱2D-NMR 二维核磁共振谱:2D-NMR3D-spectrochromatogram 三维光谱-波谱图Aa stream of nitrogen 氮气流a wide temperature range 宽的温度围absolute detector response 检测器绝对响应(值)absolute entropy 绝对熵absolute error 绝对误差absolute reaction rate theory 绝对反应速率理论absolute temperature scale 绝对温标absorbance 吸光度,而不是吸收率(absorptance)。
当我们忽略反射光强时,透射率(T)与吸光度(A)满足如下关系式:A=lg(1/T)。
absorbance noise, absorbing noise 吸光度噪音。
也称光谱的稳定性,是指在确定的波长围对样品进行多次扫描,得到光谱的均方差。
吸光度噪音是体现仪器稳定性的重要指标。
将样品信号强度与吸光度噪音相比可计算出信噪比。
absorbed water 吸附水absorptance 吸收率absorptant 吸收剂absorption band 吸收带absorption cell 吸收池absorption curve 吸收光谱曲线/光吸收曲线absorption tube 吸收管abundance 丰度。
即具有某质荷比离子的数量accelerated solvent extraction(ASE) 加速溶剂萃取accelerated testing 加速试验accelerating deposition 加速破坏acceptance limit,acceptance criterion 验收限度,合格标准accidental error 随机误差accuracy 准确度。
USP401225药典的验证中英文对照

VALIDATION OF COMPENDIAL PROCEDURES药典方法的验证Test procedures for assessment of the quality levels of pharmaceutical articles are subject to various requirements. According to Section 501 of the Federal Food, Drug, and Cosmetic Act, assays and specifications in monographs of the United States Pharmacopeia and the National Formulary constitute legal standards. The Current Good Manufacturing Practice regulations [21 CFR 211.194(a)] require that test methods, which are used for assessing compliance of pharmaceutical articles with established specifications, must meet proper standards of accuracy and reliability. Also, according to these regulations [21 CFR 211.194(a)(2)], users of analytical methods described in USP NF are not required to validate the accuracy and reliability of these methods, but merely verify their suitability under actual conditions of use. Recognizing the legal status of USP and NF standards, it is essential, therefore, that proposals for adoption of new or revised compendial analytical procedures be supported by sufficient laboratory data to document their validity.用于评估药品质量的检验方法需要满足不同的要求。
试验室信用评价流程

试验室信用评价流程英文回答:Laboratory Credit Evaluation Process.The laboratory credit evaluation process is a crucial step in ensuring the quality and accuracy of laboratory testing results. The purpose is to assess the competence and reliability of a laboratory's operations and determine its suitability for performing specific tests or analyses. This process typically involves several key steps:1. Application and Documentation Review: The laboratory submits an application and supporting documentation outlining its capabilities, scope of accreditation, and quality management system.2. Site Assessment: Assessors conduct an on-site visit to evaluate the laboratory's facilities, equipment, personnel qualifications, documentation, and overalloperations. The assessment typically includes a review of the laboratory's testing procedures, quality control measures, and data management practices.3. Evaluation and Report: The assessment findings are analyzed, and a detailed report is prepared. The report evaluates the laboratory's strengths and weaknesses, identifies areas for improvement, and determines the laboratory's overall competence.4. Accreditation or Certification: Based on the evaluation report, the accrediting or certifying body may grant accreditation or certification to the laboratory. This signifies that the laboratory meets or exceeds the required standards and is deemed capable of performing specific tests or analyses.5. Ongoing Monitoring: Once accredited or certified, the laboratory undergoes periodic surveillance to ensure compliance with the accreditation or certification requirements. This typically includes regular audits and reviews of the laboratory's ongoing operations and qualitymanagement system.中文回答:实验室信用评价流程。
关系抽取研究综述

关系抽取研究综述母克东;万琪【摘要】信息抽取、自然语言理解、信息检索等应用需要更好地理解两个实体之间的语义关系,对关系抽取进行概况总结。
将关系抽取划分为两个阶段研究:特定领域的传统关系抽取和开放领域的关系抽取。
并对关系抽取从抽取算法、评估指标和未来发展趋势三个部分对关系抽取系统进行系统的分析总结。
%Many applications in natural language understanding, information extraction, information retrieval require an understanding of the seman-tic relations between entities. Carries on the summary to the relation extraction. There are two paradigms extracting the relation-ship be-tween two entities: the Traditional Relation Extraction and the Open Relation Extraction. Makes detailed introduction and analysis of the algorithm of relation extraction, evaluation indicators and the future of the relation extraction system.【期刊名称】《现代计算机(专业版)》【年(卷),期】2015(000)002【总页数】4页(P18-21)【关键词】关系抽取;机器学习;信息抽取;开放关系抽取【作者】母克东;万琪【作者单位】四川大学计算机学院,成都 610065;四川大学计算机学院,成都610065【正文语种】中文随着大数据的不断发展,海量信息以半结构或者纯原始文本的形式展现给信息使用者,如何采用自然语言处理和数据挖掘相关技术从中帮助用户获取有价值的信息,是当代计算机研究技术迫切的需求。
USP38通用章节目录中文

USP38通用章节目录中文USP38-通用章节指导目录(附录)Guide to General Chapters 通用章节指导General Requirements for Test and Assays检查与含量分析的一般要求<1>INJECTIONS AND IMPLANTED DRUG PRODUCTS (PARENTERALS)—PRODUCT QUALITY TESTS 注射和植入药物产品(注射用) —产品质量测试<1>INJECTIONS注射剂<2>ORAL DRUG PRODUCTS—PRODUCT QUALITY TESTS 口服药物产品质量测试<3>TOPICAL AND TRANSDERMAL DRUG PRODUCTS—PRODUCT QUALITY TESTS 局部和透皮药物产品—产品质量测试<4>MUCOSAL DRUG PRODUCTS—PRODUCT QUALITY TESTS 粘膜药物产品质量测试<5>INHALATION AND NASAL DRUG PRODUCTS—GENERAL INFORMATION AND PRODUCT QUALITY TESTS 吸入剂产品—产品质量测试<7>LABELING 标签<11>USP REFERENCE STANDARDS USP标准品Apparatus for Test and Assays用于检查与含量分析的器具<17>PRESCRIPTION CONTAINER LABELING处方容器标签<21>THERMOMETERS温度计<31>VOLUMETRIC APPARATUS容量器具<41>BALANCES天平Microbiological Tests 微生物检查法<51>ANTIMICROBIAL EFFECTIVENESS TESTING抗菌剂有效性检查法<55>BIOLOGICAL INDICATORS—RESISTANCE PERFORMANCE TESTS生物指示剂-耐药性实验<61>MICROBIOLOGICAL EXAMINATION OF NONSTERILE PRODUCTS: MICROBIAL ENUMERATION TESTS非无菌产品的微生物限度检查:微生物列举检查法<62>MICROBIOLOGICAL EXAMINATION OF NONSTERILE PRODUCTS: TESTS FOR SPECIFIED MICROORGANISMS 非无菌产品的微生物限度检查:特定微生物检查法<63>MYCOPLASMA TESTS 支原体检查法<71>STERILITY TESTS无菌检查法Biological tests and assays生物检查法与测定法<81>ANTIBIOTICS—MICROBIAL ASSAYS抗生素-微生物测定<85>BACTERIAL ENDOTOXINS TEST细菌内毒素检查法<87>BIOLOGICAL REACTIVITY TESTS, IN VITRO体外的生物反应性检查法<88>BIOLOGICAL REACTIVITY TESTS, IN VIVO 体内的生物反应性检查法<89>ENZYMES USED AS ANCILLARY MATERIALS IN PHARMACEUTICAL MANUFACTURING 药品生产中酶作为辅料所使用<90>FETAL BOVINE SERUM—QUALITY ATTRIBUTES AND FUNCTIONALITY TESTS 牛胎儿血清-质量品质和功能检查法<91>CALCIUM PANTOTHENATE ASSAY泛酸钙测定法<92>GROWTH FACTORS AND CYTOKINES USED IN CELL THERAPY MANUFACTURING 在细胞疗法中使用生长因子和细胞因子<111>DESIGN AND ANALYSIS OF BIOLOGICAL ASSAYS 生物测定法的设计与分析<115>DEXPANTHENOL ASSAY右泛醇(拟胆碱药)测定法<121>INSULIN ASSAYS胰岛素测定法<121.1>PHYSICOCHEMICAL ANALYTICAL PROCEDURES FOR INSULINS胰岛素的物理化学分析程序<123>GLUCAGON BIOIDENTITY TESTS 高血糖素的生物鉴别检查法<124>ERYTHROPOIETIN BIOASSAYS 红细胞生成素的微生物测定<126>SOMATROPIN BIOIDENTITY TESTS 生长激素的生物鉴别检查法<130>PROTEIN A QUALITY ATTRIBUTES 蛋白质A的质量特征<151>PYROGEN TEST热原检查法<161>TRANSFUSION AND INFUSION ASSEMBLIES AND SIMILAR MEDICAL DEVICES 输血输液用具以及相类似的医疗器械<171>VITAMIN B12 ACTIVITY ASSAY……2548维生素B12活性测定法Chemical Tests and assays化学实验检查与测定法鉴别检查<181>IDENTIFICATION—ORGANIC NITROGENOUS BASES 鉴别-有机氮碱化合物<191>IDENTIFICATION TESTS—GENERAL鉴别实验-通用<193>IDENTIFICATION—TETRACYCLINES鉴别-四环素类<197>SPECTROPHOTOMETRIC IDENTIFICATION TESTS分光光度计鉴别实验<201>THIN-LAYER CHROMATOGRAPHIC IDENTIFICATION TEST薄层色谱鉴别实验Limit Tests 限度检查法<206>ALUMINUM铝<207>TEST FOR 1,6-ANHYDRO DERIV ATIVE FOR ENOXAPARIN SODIUM依诺肝素钠的酐类衍生物实验<208>ANTI-FACTOR Xa AND ANTI-FACTOR IIa ASSAYS FOR UNFRACTIONATED AND LOW MOLECULAR WEIGHT HEPARINS 普通肝素和低分子肝素产品中抗体Xa和抗体IIa测定<209>LOW MOLECULAR WEIGHT HEPARIN MOLECULAR WEIGHT DETERMINATIONS 低分子肝素钠分子量测定<211>ARSENIC砷<221>CHLORIDE AND SULFATE氯和硫<223>DIMETHYLANILINE二甲基苯胺<226>4-EPIANHYDRO-TETRACYCLINE4-?-四环素<227>4-AMINOPHENOL IN ACETAMINOPHEN-CONTAINING DRUG PRODUCTS 对乙酰氨酚药物产品中氨基酚<228>ETHYLENE OXIDE AND DIOXANE 环氧乙烷和二氧六环<231>HEA VY METALS重金属(删除)<232>ELEMENTAL IMPURITIES—LIMITS 元素杂质-限度<233>ELEMENTAL IMPURITIES—PROCEDURES 元素杂质-规程<241>IRON铁<251>LEAD铅<261>MERCURY汞<267>POROSIMETRY BY MERCURY INTRUSION 水银孔隙仪<268>POROSITY BY NITROGEN ADSORPTION–DESORPTION 氮吸附-解吸测定孔隙率<271>READILY CARBONIZABLE SUBSTANCES TEST易碳化物检查法<281>RESIDUE ON IGNITION炽灼残渣<291>SELENIUM硒Other Tests and Assays 其它检查法与测定法<301>ACID-NEUTRALIZING CAPACITY酸中和容量<311>ALGINATES ASSAY藻酸盐测定法<341>ANTIMICROBIAL AGENTS—CONTENT 抗菌剂-含量<345>Assay for Citric Acid/Citrate and Phosphate 柠檬酸/柠檬酸盐和磷酸盐的测定<351>ASSAY FOR STEROIDS类固醇(甾类化合物)测定法<361> BARBITURATE ASSAY 巴比妥类药物测定法<371>COBALAMIN RADIOTRACER ASSAY钴铵素放射性跟踪剂测定法<381>ELASTOMERIC CLOSURES FOR INJECTIONS 注射剂的弹性密封件<391>EPINEPHRINE ASSAY肾上腺素测定法<401>FATS AND FIXED OILS脂肪与混合油<411>FOLIC ACID ASSAY叶酸测定法<413>IMPURITIES TESTING IN MEDICAL GASES 医用气体杂质检查<415>MEDICAL GASES ASSAY 医用气体含量检查<425>IODOMETRIC ASSAY—ANTIBIOTICS碘量检查法-抗生素<429>LIGHT DIFFRACTION MEASUREMENT OF PARTICLE SIZE粒径的光衍射测量法<431>METHOXY DETERMINATION甲氧基测定法<441>NIACIN OR NIACINAMIDE ASSAY 烟酰或烟酰胺测定法<451>NITRITE TITRATION亚硝酸盐滴定<461>NITROGEN DETERMINATION氮测定法<466>ORDINARY IMPURITIES一般杂质<467>RESIDUAL SOLVENTS残留溶剂<469>ETHYLENE GLYCOL, DIETHYLENE GLYCOL, AND TRIETHYLENE GLYCOL IN ETHOXYLATED SUBSTANCES乙氧基物质中乙二醇、二甘醇、三甘醇测定<471>OXYGEN FLASK COMBUSTION氧瓶燃烧法<481>RIBOFLAVIN ASSAY核黄素(维生素B2)测定法<501>SALTS OF ORGANIC NITROGENOUS BASES有机氮盐<503>ACETIC ACID IN PEPTIDES 多肽类中乙酸测定<511>SINGLE-STEROID ASSAY单一的类固醇测定法<525>SULFUR DIOXIDE 二氧化硫<531>THIAMINE ASSAY硫胺素测定法<541>TITRIMETRY滴定法<551>VITAMIN E ASSAY维生素E测定法<561>ARTICLES OF BOTANICAL ORIGIN植物起源的药品<563>IDENTIFICATION OF ARTICLES OF BOTANICAL ORIGIN植物药品的鉴别<565>BOTANICAL EXTRACTS植物提取<571>VITAMIN A ASSAY维生素A测定法<581>VITAMIN D ASSAY维生素D测定法<591>ZINC DETERMINATION锌的测定法Physical Test and Determinations物理检查与测定法<601>INHALATION AND NASAL DRUG PRODUCTS: AEROSOLS, SPRAYS, AND POWDERS—PERFORMANCE QUALITY TESTS吸入剂、鼻雾剂:气溶胶,喷雾,干粉-质量通则<602>PROPELLANTS 推进剂<603>TOPICAL AEROSOLS 局部喷雾剂<604>LEAK RATE 渗漏率<610>ALTERNATIVE MICROBIOLOGICAL SAMPLING METHODS FOR NONSTERILE INHALED AND NASAL PRODUCTS 非无菌吸入和鼻雾剂可供选择的微生物取样方法<611>ALCOHOL DETERMINATION乙醇测定法<616>BULK DENSITY AND TAPPED DENSITY堆密度与振实密度<621>CHROMATOGRAPHY色谱法<631>COLOR AND ACHROMICITY呈色与消色<641>COMPLETENESS OF SOLUTION溶解度<643>TOTAL ORGANIC CARBON总有机碳<645>W ATER CONDUCTIVITY水电导率<651>CONGEALING TEMPERATURE凝点温度<659>PACKAGING AND STORAGE REQUIREMENTS 包装和储藏要求<660>CONTAINERS—GLASS 容器-玻璃<661>CONTAINERS—PLASTICS容器-塑料<670>AUXILIARY PACKAGING COMPONENTS 辅助包装部件<671>CONTAINERS—PERFORMANCE TESTING容器-性能测试<691>COTTON棉花<695>CRYSTALLINITY结晶度<696>CHARACTERIZATION OF CRYSTALLINE SOLIDS BY MICROCALORIMETRY AND SOLUTION CALORIMETRY 通过溶液量热学测定结晶性<697>CONTAINER CONTENT FOR INJECTIONS 注射剂容器容积<698>DELIVERABLE VOLUME抽取体积<699>DENSITY OF SOLIDS固体密度<701>DISINTEGRATION崩解时限<705>QUALITY ATTRIBUTES OF TABLETS LABELED AS HA VING A FUNCTIONAL SCORE ?<711>DISSOLUTION 溶出度<721>DISTILLING RANGE馏程<724>DRUG RELEASE药物释放度<729>GLOBULE SIZE DISTRIBUTION IN LIPID INJECTABLEEMULSIONS脂类可注射的乳剂的粒径分布<730>Plasma Spectrochemistry 血浆光谱化学?<731>LOSS ON DRYING4干燥失重<733>LOSS ON IGNITION灼烧失重<735>X-RAY FLUORESCENCE SPECTROMETRY X射线光谱<736>MASS SPECTROMETRY 质谱<741>MELTING RANGE OR TEMPERATURE熔距或熔点<751>METAL PARTICLES IN OPHTHALMIC OINTMENTS眼用软膏中的金属粒子<755>MINIMUM FILL最低装量<761>NUCLEAR MAGNETIC RESONANCE核磁共振<771>OPHTHALMIC OINTMENTS眼用软膏<776>OPTICAL MICROSCOPY光学显微镜<781>OPTICAL ROTATION旋光度<785>OSMOLALITY AND OSMOLARITY渗透压<786>PARTICLE SIZE DISTRIBUTION ESTIMATION BY ANALYTICAL SIEVING 筛分法估算粒径分布<787>SUBVISIBLE PARTICULATE MATTER IN THERAPEUTIC PROTEIN INJECTIONS显微计数法在治疗性蛋白注射剂中应用<788>PARTICULATE MATTER IN INJECTIONS注射剂中的不溶性微粒<789>PARTICULATE MATTER IN OPHTHALMIC SOLUTIONS 眼用溶液中的不溶性微粒<790>VISIBLE PARTICULATES IN INJECTIONS 注射剂中可见异物<791>pH<795>PHARMACEUTICAL COMPOUNDING—NONSTERILE PREPARATIONS药物混合-非无菌制剂<797>PHARMACEUTICAL COMPOUNDING—STERILE PREPARATIONS药物混合-无菌制剂<801>POLAROGRAPHY极谱法<811>POWDER FINENESS粉剂细度<821>RADIOACTIVITY放射性<823>POSITRON EMISSION TOMOGRAPHY DRUGS FOR COMPOUNDING, INVESTIGATIONAL, AND RESEARCH USES用于正电子发射断层造影术的放射性药物<831>REFRACTIVE INDEX折光率<841>SPECIFIC GRAVITY比重<846>SPECIFIC SURFACE AREA 比表面积<851>SPECTROPHOTOMETRY AND LIGHT-SCATTERING分光光度计与光散射<852>ATOMIC ABSORPTION SPECTROSCOPY 原子吸收光谱<853>FLUORESCENCE SPECTROSCOPY 荧光光谱<854>MID-INFRARED SPECTROSCOPY 中红外光谱<857>ULTRAVIOLET-VISIBLE SPECTROSCOPY 紫外可见光谱<861>SUTURES—DIAMETER缝线-直径?<871>SUTURES—NEEDLE ATTACHMENT缝线-穿孔实验<881>TENSILE STRENGTH张力<891>THERMAL ANALYSIS热分析<905>UNIFORMITY OF DOSAGE UNITS制剂单位的含量均匀度<911>VISCOSITY—CAPILLARY METHODS黏度-毛细管法<912>VISCOSITY—ROTATIONAL METHODS 黏度-旋转法<913>VISCOSITY—ROLLING BALL METHOD 黏度-球法<921>W ATER DETERMINATION水分测定<941>CHARACTERIZATION OF CRYSTALLINE ANDPARTIALLY CRYSTALLINE SOLIDS BY X-RAY POWDER DIFFRACTION (XRPD)X光衍射General Information通用信息<1005>ACOUSTIC EMISSION 声频发射<1010>ANALYTICAL DATA—INTERPRETATION AND TREATMENT分析数据-解释与处理<1015>AUTOMATED RADIOCHEMICAL SYNTHESIS APPARATUS放射性自动合成装置<1024>BOVINE SERUM 牛血清<1027>FLOW CYTOMETRY 流式细胞仪<1030>BIOLOGICAL ASSAY CHAPTERS—OVERVIEW AND GLOSSARY生物测定章节-综述和术语<1031>THE BIOCOMPATIBILITY OF MATERIALS USED IN DRUG CONTAINERS, MEDICAL DEVICES, AND IMPLANTS 用于药物容器、医疗设施和植入剂的材料的生物相容性<1034>ANALYSIS OF BIOLOGICAL ASSAYS 生物测定分析<1035>BIOLOGICAL INDICATORS FOR STERILIZATION灭菌用生物指示剂<1041>BIOLOGICS生物制剂<1043>Ancillary Material for Cell, Gene, and Tissue-Engineered Products细胞,基因与组织设计产品的辅助材料<1044>CRYOPRESERV ATION OF CELLS 细胞低温保存<1045>BIOTECHNOLOGY-DERIVED ARTICLES生物技术提取产品<1046>CELLULAR AND TISSUE-BASED PRODUCTS细胞与组织产品<1047>GENE THERAPY PRODUCTS 基因治疗产品<1048>QUALITY OF BIOTECHNOLOGICAL PRODUCTS: ANALYSIS OF THE EXPRESSION CONSTRUCT IN CELLS USED FOR PRODUCTION OF r-DNA DERIVED PROTEIN PRODUCTS 生物技术产品的质量:从蛋白质产品中提取的r-DNA产品在细胞中表达结构的分析<1049>QUALITY OF BIOTECHNOLOGICAL PRODUCTS: STABILITY TESTING OF BIOTECHNOLOGICAL/BIOLOGICAL PRODUCTS生物技术产品的质量:生物技术/生物产品的稳定性实验<1050>VIRAL SAFETY EV ALUATION OF BIOTECHNOLOGY PRODUCTS DERIVED FROM CELL LINES OF HUMAN OR ANIMAL ORIGIN从人或动物细胞中提取的生物技术产品的病毒安全性评估<1051>CLEANING GLASS APPARATUS玻璃容器的清洗<1052>BIOTECHNOLOGY-DERIVED ARTICLES—AMINO ACID ANALYSIS生物技术提取法-氨基酸测定<1053>CAPILLARY ELECTROPHORESIS 毛细管电泳法<1054>BIOTECHNOLOGY-DERIVED ARTICLES—ISOELECTRIC FOCUSING生物技术提取法-等电点聚集<1055>BIOTECHNOLOGY-DERIVED ARTICLES—PEPTIDE MAPPING生物技术提取法-肽谱<1056>BIOTECHNOLOGY-DERIVED ARTICLES—POLYACRYLAMIDE GEL ELECTROPHORESIS 生物技术提取法-凝胶电泳<1057>BIOTECHNOLOGY-DERIVED ARTICLES—TOTAL PROTEIN ASSAY生物技术提取法-总蛋白测定<1058>ANALYTICAL INSTRUMENT QUALIFICATION 分析仪器要求<1059>EXCIPIENT PERFORMANCE 赋形剂<1061>COLOR—INSTRUMENTAL MEASUREMENT显色-仪器测量<1065>Ion Chromatography 离子色谱法<1066>PHYSICAL ENVIRONMENTS THAT PROMOTE SAFE MEDICATION USE物理环境促使安全使用药物<1072>DISINFECTANTS AND ANTISEPTICS 消毒剂和防腐剂<1074>EXCIPIENT BIOLOGICAL SAFETY EV ALUATION GUIDELINES赋形剂(辅料)生物安全性评估指导<1078>GOOD MANUFACTURING PRACTICES FOR BULK PHARMACEUTICAL EXCIPIENTS 批药品赋形剂的生产管理规范<1079>Good Storage and Shipping Practices 良好的贮存与运输规范<1080>BULK PHARMACEUTICAL EXCIPIENTS—CERTIFICATE OF ANALYSIS批药品赋形剂-COA<1084>GLYCOPROTEIN AND GLYCAN ANALYSIS—GENERAL CONSIDERATIONS 糖蛋白和多糖分析-一般通则<1086>IMPURITIES IN DRUG SUBSTANCES AND DRUG PRODUCTS药物和药物产品中的杂质<1087>APPARENT INTRINSIC DISSOLUTION—DISSOLUTION TESTING PROCEDURES FOR ROTATING DISK AND STATIONARY DISK内部的溶出度-旋转和静止溶出检测程序?<1088>IN VITRO AND IN VIVO EV ALUATION OF DOSAGEFORMS体内与体外的剂型的评估<1090>ASSESSMENT OF DRUG PRODUCT PERFORMANCE-BIOAV AILABILITY, BIOEQUIV ALENCE, AND DISSOLUTION药物产品性能评估:生物利用度、生物等效性和溶出<1091>LABELING OF INACTIVE INGREDIENTS非活性成分的标示<1092>THE DISSOLUTION PROCEDURE: DEVELOPMENT AND V ALIDATION溶出程序:开发与验证<1094>CAPSULES—DISSOLUTION TESTING AND RELATED QUALITY ATTRIBUTES 胶囊-关于产品质量的溶出测定<1097>BULK POWDER SAMPLING PROCEDURES:粉末样品取样程序<1102>IMMUNOLOGICAL TEST METHODS—GENERAL CONSIDERATIONS免疫测试方法-总则<1103>IMMUNOLOGICAL TEST METHODS—ENZYME-LINKED IMMUNOSORBENT ASSAY (ELISA) 免疫学测试方法-酶联免疫吸附测定<1104>IMMUNOLOGICAL TEST METHODS—IMMUNOBLOT ANALYSIS免疫测试方法-免疫印迹法<1105>IMMUNOLOGICAL TEST METHODS—SURFACE PLASMON RESONANCE 免疫测试方法-表面等离子体共振<1106>IMMUNOGENICITY ASSAYS—DESIGN AND VALIDATION OF IMMUNOASSAYS TO DETECT ANTI-DRUG ANTIBODIES<1111>MICROBIOLOGICAL EXAMINATION OF NONSTERILE PRODUCTS: ACCEPTANCE CRITERIA FORPHARMACEUTICAL PREPARATIONS AND SUBSTANCES FOR PHARMACEUTICAL USE非无菌产品的微生物学检查:药用制剂和制药过程使用的物质接受标准<1112>MICROBIAL CHARACTERIZATION, IDENTIFICATION, AND STRAIN TYPING 非无菌药物产品水活性测定应用<1113>MICROBIOLOGICAL ATTRIBUTES OF NONSTERILE PHARMACEUTICAL PRODUCTS 非无菌药品中的微生物分布<1115>BIOBURDEN CONTROL OF NONSTERILE DRUG SUBSTANCES AND PRODUCTS 非无菌药物和产品的生物负载控制<1116>MICROBIOLOGICAL CONTROL AND MONITORING OF ASEPTIC PROCESSING ENVIRONMENTS洁净的房间与其它可控环境的微生物评估<1117>MICROBIOLOGICAL BEST LABORATORY PRACTICES 微生物最优实验室规范<1118>MONITORING DEVICES—TIME, TEMPERATURE, AND HUMIDITY监控装置-时间、温度与湿度<1119>NEAR-INFRARED SPECTROPHOTOMETRY近红外分光光度测定法<1120>Raman Spectrophotometry 拉曼分光光度测定法<1121>NOMENCLATURE命名<1125>NUCLEIC ACID-BASED TECHNIQUES—GENERAL 核酸技术-通则<1126>NUCLEIC ACID-BASED TECHNIQUES—EXTRACTION, DETECTION, AND SEQUENCING 核酸技术-提取、检测、测序<1127>NUCLEIC ACID-BASED TECHNIQUES—AMPLIFICATION 核酸技术-扩增<1128>NUCLEIC ACID-BASED TECHNIQUES—MICROARRAY 核酸技术-微阵列<1129>NUCLEIC ACID-BASED TECHNIQUES—GENOTYPING 核酸技术-基因分型<1130>NUCLEIC ACID-BASED TECHNIQUES—APPROACHES FOR DETECTING TRACE NUCLEIC ACIDS (RESIDUAL DNA TESTING)核酸技术-探测微量核酸的应用(残留DNA测试)<1136>PACKAGING AND REPACKAGING—SINGLE-UNIT CONTAINERS包装和再包装-单一容器<1151>PHARMACEUTICAL DOSAGE FORMS药物剂型<1152>ANIMAL DRUGS FOR USE IN ANIMAL FEEDS兽药在动物饲料中的使用<1160>PHARMACEUTICAL CALCULATIONS IN PRESCRIPTION COMPOUNDING 按处方混合的药物的计算<1163>QUALITY ASSURANCE IN PHARMACEUTICAL COMPOUNDING按处方混合的药物的质量保证<1171>PHASE-SOLUBILITY ANALYSIS相溶解分析<1174>Powder Flow 粉末流动性<1176>PRESCRIPTION BALANCES AND VOLUMETRIC APPARATUS 处方天平与容量器具<1177>Good Packaging Practices 良好的包装操作<1178>Good Repackaging Practices 良好的再包装操作<1180>HUMAN PLASMA 人血浆<1181>SCANNING ELECTRON MICROSCOPY扫描电子显微镜<1184>SENSITIZATION TESTING 致敏测试<1191>STABILITY CONSIDERATIONS IN DISPENSING PRACTICE分装操作中稳定性考察<1195>SIGNIFICANT CHANGE GUIDE FOR BULK PHARMACEUTICAL EXCIPIENTS 散装药用辅料更换指导原则<1197>GOOD DISTRIBUTION PRACTICES FOR BULK PHARMACEUTICAL EXCIPIENTS 散装药用辅料良好的分装操作<1207>STERILE PRODUCT PACKAGING—INTEGRITY EV ALUATION无菌产品包装-完整性评估<1208>STERILITY TESTING—V ALIDATION OF ISOLATOR SYSTEMS无菌实验-隔离系统的验证<1209>STERILIZATION—CHEMICAL AND PHYSICOCHEMICAL INDICATORS AND INTEGRATORS灭菌-化学与物理化学的指示剂以及二者的综合<1211>STERILIZATION AND STERILITY ASSURANCE OF COMPENDIAL ARTICLES 药典物品中的灭菌与灭菌保证<1216>TABLET FRIABILITY片剂的脆碎度<1217>TABLET BREAKING FORCE 片剂断裂力<1222>TERMINALLY STERILIZED PHARMACEUTICAL PRODUCTS—PARAMETRIC RELEASE 药品终端灭菌-放行参数<1223>V ALIDATION OF ALTERNATIVE MICROBIOLOGICAL METHODS可供选择的微生物学方法的验证<1224>TRANSFER OF ANALYTICAL PROCEDURES 分析方法转移<1225>V ALIDATION OF COMPENDIAL PROCEDURES药典方法的验证<1226>VERIFICATION OF COMPENDIAL PROCEDURES 药典方法的确认<1227>V ALIDATION OF MICROBIAL RECOVERY FROM PHARMACOPEIAL ARTICLES从药物中回收微生物的验证<1229>STERILIZATION OF COMPENDIAL ARTICLES 药典灭菌过程<1229.1>STEAM STERILIZATION BY DIRECT CONTACT 直接蒸汽灭菌<1229.2>MOIST HEAT STERILIZATION OF AQUEOUS LIQUIDS 水溶液的湿热灭菌<1229.3>MONITORING OF BIOBURDEN 生物负载监控<1229.4>STERILIZING FILTRATION OF LIQUIDS 溶液的无菌过滤器<1229.6>LIQUID-PHASE STERILIZATION 液态灭菌<1229.7>GASEOUS STERILIZATION 气态灭菌<1229.8>DRY HEAT STERILIZATION 干热灭菌<1229.10>RADIATION STERILIZATION 辐射灭菌<1230>W ATER FOR HEMODIALYSIS APPLICATIONS 血液透析过程用水<1231>W ATER FOR PHARMACEUTICAL PURPOSES制药用水<1234>VACCINES FOR HUMAN USE—POLYSACCHARIDE AND GLYCOCONJUGATE VACCINES 人用疫苗-多糖和糖复合物疫苗<1235>V ACCINES FOR HUMAN USE—GENERAL CONSIDERATIONS 人用疫苗-通则<1237>VIROLOGY TEST METHODS 病毒测试方法<1238>V ACCINES FOR HUMAN USE—BACTERIAL V ACCINES 人用疫苗-细菌疫苗<1240>VIRUS TESTING OF HUMAN PLASMA FOR FURTHER MANUFACTURE下一步使用人血浆的病毒测试<1241>W ATER–SOLID INTERACTIONS IN PHARMACEUTICAL SYSTEMS在药物系统中水与固体的相互作用<1251>WEIGHING ON AN ANALYTICAL BALANCE关于分析天平的称重<1265>Written Prescription Drug Information-Guidelines 书面的处方药信息-指南<1285>PREPARATION OF BIOLOGICAL SPECIMENS FOR HISTOLOGIC AND IMMUNOHISTOCHEMICAL ANALYSIS 为了组织和免疫组织分析的生物标本制备<1285.1>HEMATOXYLIN AND EOSIN STAINING OF SECTIONED TISSUE FOR MICROSCOPIC EXAMINATION显微镜观察用苏木精和伊红染色的切片<1601>PRODUCTS FOR NEBULIZATION—CHARACTERIZATION TESTS 产品雾化状态-性状描述<1644>THEORY AND PRACTICE OF ELECTRICAL CONDUCTIVITY MEASUREMENTS OF SOLUTIONS溶液电导值测量方法的理论与实践<1660>EV ALUATION OF THE INNER SURFACE DURABILITY OF GLASS CONTAINERS 玻璃容器内表面耐久性评估<1724>SEMISOLID DRUG PRODUCTS—PERFORMANCE TESTS 半固态药物产品-性能测试<1736>APPLICATIONS OF MASS SPECTROMETRY 质谱应用<1761>APPLICATIONS OF NUCLEAR MAGNETIC RESONANCE SPECTROSCOPY核磁共振光谱应用<1787>MEASUREMENT OF SUBVISIBLE PARTICULATE MATTER IN THERAPEUTIC PROTEIN INJECTIONS用显微镜测量方法测量治疗性蛋白注射剂的不溶性微粒<1788>METHODS FOR THE DETERMINATION OF PARTICULATE MATTER IN INJECTIONS AND OPHTHALMIC SOLUTIONS注射剂和眼用溶液的不溶性微粒测定的方法选择<1852>ATOMIC ABSORPTION SPECTROSCOPY—THEORY AND PRACTICE原子吸收光谱-理论与实践<1853>FLUORESCENCE SPECTROSCOPY—THEORY AND PRACTICE荧光光谱-理论与实践<1854>MID-INFRARED SPECTROSCOPY—THEORY AND PRACTICE中红外光谱-理论与实践<1857>ULTRA VIOLET-VISIBLE SPECTROSCOPY—THEORY AND PRACTICE紫外可见光谱-理论与实践<1911>RHEOMETRY 流变测定Dietary Supplements营养补充剂General Tests and Assays 一般检查法与测定法<2021>MICROBIAL ENUMERATION TESTS—NUTRITIONAL AND DIETARY SUPPLEMENTS…3080微生物数量实验-营养与食品添加剂<2022>MICROBIOLOGICAL PROCEDURES FOR ABSENCE OF SPECIFIED MICROORGANISMS—NUTRITIONAL AND DIETARY SUPPLEMENTS (3083)不得检出特定微生物的程序-营养与营养补充剂<2023>MICROBIOLOGICAL ATTRIBUTES OF NONSTERILE NUTRITIONAL AND DIETARY SUPPLEMENTS……3087非无菌的营养与食品添加剂中的微生物分布<2040>DISINTEGRATION AND DISSOLUTION OF DIETARY SUPPLEMENTS (3089)食品添加剂的崩解与溶出<2091>WEIGHT V ARIATION OF DIETARYSUPPLEMENTS……3092食品添加剂的重量差异<2750>MANUFACTURING PRACTICES FOR DIETARY SUPPLEMENTS (3093)食品添加剂的生产操作。
基于Retinex理论的低光图像增强算法

第40卷第6期Vol.40㊀No.6重庆工商大学学报(自然科学版)J Chongqing Technol &Business Univ(Nat Sci Ed)2023年12月Dec.2023基于Retinex 理论的低光图像增强算法史宇飞,赵佰亭安徽理工大学电气与信息工程学院,安徽淮南232001摘㊀要:为了解决低光照图像存在的对比度低㊁噪声大等问题,提出一种基于Retinex 理论的卷积神经网络增强模型(Retinex-RANet )㊂它包括分解网络㊁降噪网络和亮度调整网络3部分:在分解网络中融入残差模块(RB )和跳跃连接,通过跳跃连接将第一个卷积层提取的特征与每一个RB 提取的特征融合,以确保图像特征的完整提取,从而得到更准确的反射分量和光照分量;降噪网络以U-Net 网络为基础,同时加入了空洞卷积和注意力机制,空洞卷积能提取更多的图像相关信息,注意力机制可以更好地去除反射分量中噪声,还原细节;亮度调整网络由卷积层和Sigmoid 层组成,用来提高光照分量的对比度;最后将降噪网络去噪后的反射分量和亮度调整网络增强后的光照分量融合,得到最终的增强结果㊂实验结果显示:Retinex-RANet 在主观视觉上不仅提高了低光图像的亮度,还提高了色彩深度和对比度,在客观评价指标上,相较于R2RNet ,PSNR 值上升了4.4%,SSIM 值上升了6.1%㊂结果表明:Retinex-RANet 具有更好的低光图像增强效果㊂关键词:低光增强;残差模块;注意力机制;Retinex 理论中图分类号:TP391㊀㊀文献标识码:A ㊀㊀doi:10.16055/j.issn.1672-058X.2023.0006.008㊀㊀㊀㊀㊀㊀㊀㊀㊀㊀㊀㊀㊀㊀㊀㊀㊀㊀㊀㊀㊀㊀㊀㊀㊀㊀㊀㊀㊀㊀㊀㊀㊀㊀㊀㊀㊀㊀㊀㊀㊀㊀㊀㊀㊀㊀㊀㊀㊀㊀㊀㊀㊀㊀㊀㊀㊀㊀㊀㊀㊀㊀㊀㊀㊀㊀㊀㊀㊀㊀㊀㊀㊀㊀㊀㊀㊀㊀㊀㊀㊀㊀㊀㊀㊀㊀㊀收稿日期:2022-06-09㊀修回日期:2022-07-20㊀文章编号:1672-058X(2023)06-0061-07基金项目:国家自然科学基金面上项目(52174141);安徽省重点研究与开发计划资助项目(202004A07020043);安徽省自然科学基金面上项目(2108085ME158);安徽高校协同创新项目(GXXT -2020-54).作者简介:史宇飞(1997 ),男,安徽安庆人,硕士研究生,从事图像处理研究.通讯作者:赵佰亭(1981 ),男,山东枣庄人,教授,博士,从事图像处理㊁智能控制研究.Email:btzhao@.引用格式:史宇飞,赵佰亭.基于Retinex 理论的低光图像增强算法[J].重庆工商大学学报(自然科学版),2023,40(6):61 67.SHI Yufei ZHAO Baiting.Low-light Image enhancement algorithm based on Retinex theory J .Journal of Chongqing Technology and Business University Natural Science Edition 2023 40 6 61 67.Low-light Image Enhancement Algorithm Based on Retinex Theory SHI Yufei ZHAO BaitingSchool of Electrical and Information Engineering Anhui University of Science and Technology Anhui Huainan 232001 ChinaAbstract In order to solve the problems of low contrast and high noise of low-light images a convolutional neural network enhancement model based on Retinex theory Retinex-RANet is proposed.It includes three parts the decomposition network the noise reduction network and the brightness adjustment network.The residual module RB and the jump connection were incorporated into the decomposition network and the features extracted by the first convolutional layer were fused with each RB extracted feature through the jump connection to ensure the complete extraction of the image features resulting in more accurate reflection and illumination components.The noise reduction network was based on the U-Net network and the cavity convolution and attention mechanism were added at the same time so as to extract more image-related information.The attention mechanism can better remove the noise in the reflected component and restore the details.The brightness adjustment network consists of a convolutional layer and a Sigmoid layer which is used to increase the contrast of the light components.Finally the reflection component after denoising by the noise reduction network and the light component after the brightness adjustment network were fused to obtain the final enhancement result.Experimental results show that Retinex-RANet not only improves the brightness of low-light images in subjective vision but also improves the color depth and contrast.In terms of objective evaluation indicators compared with R2RNet the PSNR value increased by 4.4% and the SSIM value increased by 6.1%.The results show that Retinex-RANet has better重庆工商大学学报(自然科学版)第40卷low-light image enhancement.Keywords low light enhancement residuals module attention mechanism Retinex theory1㊀引㊀言在光照不足㊁不均或者有阴影遮挡等条件下,采集的图像一般都存在噪声过多和对比度弱等问题,而这些问题不但会对图像的品质产生负面影响,还会妨碍一些机器视觉任务的进行㊂对低光照图像进行增强,有助于提高高级视觉性能,如图像识别㊁语义分割㊁目标检测等;也可以在一些实际应用中提高智能系统的性能,如视觉导航㊁自动驾驶等㊂因此,对低光图像增强进行研究是十分必要的㊂低光图像增强方法可分为以下4类:基于直方图均衡化的方法[1],其核心是通过改变图像部分区域的直方图来达到整体对比度提高的效果㊂此类方法可以起到提高图像对比度的作用,但是大多数不够灵活,部分区域仍会出现曝光不足和放大噪音等不好的视觉效果㊂基于去雾的方法[2-3],如一些研究人员[4]利用有雾图像和低光图像之间的相似性,通过已有的去雾算法来增强低光照图像㊂尽管此类方法得到了较好结果,但此类模型的物理解释不够充分,同时对增强后的图像进行去噪可能会导致图像细节模糊㊂基于Retinex理论[5]的方法,其将低光图像分解为光照和反射率两部分,在保持反射率一致性的前提下,增加光照的亮度,从而增强图像㊂此类方法不仅提高了图像的对比度,还降低了噪声带来的的影响,不足之处是要根据经验来人工设置算法的相关参数,并且不能对不同类型图像自适应增强㊂基于深度学习的方法,Lore等[6]提出的LLNet通过类深度神经网络来识别低光图像中的信号特征并对其自适应增强;Wei等[7]提出的Retinex-Net结合Retinex理论和神经网络进行图像增强;Wang等[8]提出的GLADNet先通过编解码网络对低光图像生成全局照明先验知识,然后结合全局照明先验知识和输入图像,采用卷积神经网络来增强图像的细节㊂此类基于深度学习的方法均取得了不错的效果,但是大多数方法在增强过程中并没有对噪声进行有效抑制,从而使得增强后的图像出现噪声大㊁颜色失真等问题㊂为解决这些问题,提出了Retinex-RANet(Retinex-Residuals Attention Net)方法㊂Retinex-RANet首先在分解阶段采用残差模块与跳跃连接,通过跳跃连接将第一个卷积层提取的特征与每一个RB提取的特征融合,从而得到更准确的反分量和光照分量㊂另外,还在降噪网络中加入通道注意力模块和空洞卷积,注意力机制可以更好地去除反射分量中的噪声,还原细节;而空洞卷积能获取更多的上下文信息特征㊂实验结果表明:Retinex-RANet具有更好的低光图像增强效果㊂2㊀模㊀型Retinex-RANet模型框图如图1所示㊂由图1可以看到:整个网络模型由3个子网络组成,即分解网络㊁降噪网络以及调整网络,分别用于分解图像㊁降低噪声和调整亮度㊂具体地说,首先该算法通过分解网络将低光照图像S l和正常光图像S h分解为反射分量(R l㊁R h)和光照分量(I l㊁I h),然后降噪网络将分解的反射分量R l作为输入,并使用光照分量I l作为约束来抑制反射分量中的噪声,同时将光照分量I l送入调整网络,来提高光照分量的对比度,最后融合Rᶄ和Iᶄ得到增强后的图像㊂输入Sh分解网络反射分量Rh光照分量Ih反射分量Rl光照分量Il分解网络输入Sl降噪网络调整网络输出R′I′Conv+ReluConv+ReluConv+ReluConvSigmoid图1㊀Retinex-RANet模型结构图Fig.1㊀Retinex-RANet model structure diagram2.1㊀分解网络基于Retinex理论方法的关键是在分解阶段如何得到高质量的光照分量和反射分量,而分解后的结果对后续的增强和降噪操作都会产生影响,因此,设计一个有效的网络对低光图像进行分解是很有必要的㊂分解网络结构如图2所示㊂输入Sl33Conv+Relu3?3Conv+Relu3?3Conv+Relu3?3Conv+Relu3?3Conv+Relu3?3Conv3?3Conv+Relu3?3Conv+Relu3?3Conv+Relu1?1Conv+Relu1?1Conv+Relu跳跃连接S C反射分量Rl光照分量IlS i g m o i d R BR B R B R B图2㊀分解网络Fig.2㊀Decomposition network在分解网络结构中,为了使深度神经网络在训练26第6期史宇飞,等:基于Retinex 理论的低光图像增强算法阶段更容易优化,使用3个残差块(RB)来获得更好的分解结果㊂首先使用3ˑ3卷积提取输入低光图像S l 的特征;然后再经过3个RB 模块提取更多的纹理㊁细节等特征,同时为了减少底层颜色㊁边缘线条等特征的丢失,引入了跳跃连接,即将第一个卷积层的输出连接到每一个RB 的输出,保证特征的充分提取;最后通过3ˑ3ˑ4的卷积层和sigmoid 函数激活,从而得到3通道的反射分量和1通道的光照分量㊂2.2㊀降噪网络在对低光图像进行增强的过程中,大多数基于Retinex 理论的方法在得到分解结果后都忽略了噪声的影响,这会导致最终的增强结果受到反射分量中噪声的干扰,出现模糊㊁失真等问题㊂为了解决这类问题,设计了如图3所示的降噪网络㊂输入R l 、I lS ES ES ES E S E (D i l a t e dC o n v +R e l u )2m a x p o o l i n g(C o n v +R e l u )?2m a x p o o l i n g(C o n v +R e l u )?2m a x p o o l i n g(C o n v +R e l u )?2m a x p o o l i n g(C o n v +R e l u )?2m a x p o o l i n g(C o n v +R e l u )?2m a x p o o l i n g(C o n v +R e l u )?2m a x p o o l i n g(C o n v +R e l u )?2m a x p o o l i n g(C o n v +R e l u )?2C o n vG l o b a lA v g .p o o lF CF CR e l uS i g m o i dS c a l eW ?H ?CW ?H ?C S i g m o i d输出R ′11C图3㊀降噪网络Fig.3㊀Denoising network㊀㊀在低光增强领域,U-Net 网络由于其优秀的结构设计,被大量网络作为其主要架构和部分架构,因此Retinex-RANet 也采用U-Net 作为降噪网络的基础网络部分㊂降噪网络包含编码和解码两个部分㊂在编码阶段,先融合输入的反射分量和光照分量,然后经过一组由两个3ˑ3的空洞卷积㊁RELU 函数激活和最大池化层组成的编码块,3组均由两个卷积核为3ˑ3的卷积激活层和一个最大池化层组成的编码块来提取特征,从而得到编码阶段的特征图,最后将其送入解码阶段㊂编码过程中,每次通过一个编码块,图像的通道数会翻倍,但是其尺寸会降低一半㊂在解码阶段,由4个相同的解码块组成,结构为3ˑ3的卷积层 RELU 函数激活 2ˑ2的反卷积层㊂受到图像识别中的SENet [9]的启发,将通道注意力模块嵌入到跳跃连接中,以便更好地降低噪声,恢复细节㊂如图3所示:首先将编码阶段采集到的图像特征进行全局平均池化操作,然后经过两个全连接层和两个激活函数,最后和解码阶段的特征图逐通道相乘,此过程可将更多的权重分配给有用的特征,如颜色㊁细节和纹理特征等,同时为噪声㊁阴影快和伪影等特征分配较少的权重;然后融合跳跃连接得到的特征图与反卷积后的特征,之后再进行卷积计算,解码过程中,每次通过一个解码块,图像的通道数会降低一半,但是其尺寸会翻倍;最后使用3ˑ3卷积得到一个3通道特征图,并对其进行sigmoid 函数激活,从而得到降噪后的反射分量㊂2.3㊀调整网络在得到分解后的光照分量后,需要提高其对比度,因此设计了图1中的调整网络㊂此调整网络是一个轻量级网络,包含3个卷积激活层㊁1个卷积层和1个Sigmoid 层,同时为了避免底层信息的损失,通过跳跃连接将输入连接到最后一个卷积层的输出㊂2.4㊀损失函数训练时,3个子网络均单独训练,因此,整个Retinex-RANet 的损失由分解损失L dc ㊁降噪损失L r 和调整损失L i 组成㊂2.4.1㊀分解损失为了更好地从低光图像中分解出反射分量和光照分量,设计了3个损失函数,即重建损失L rec ㊁反射分量36重庆工商大学学报(自然科学版)第40卷一致性损失L rs ㊁光照分量平滑损失L is ,如下所示:L dc =L rec +λ1L rs +λ2L isL rec = S l -R l I l 1+ S h -R h I h 1L rs = R l -R h 22L is =∇I lmax ∇S l ,ε()1+∇I hmax ∇S h ,ε()1其中,λ1和λ2分别为L rs 和L is 的权重系数,S l 和S h 为低光条件和正常光条件下的输入图像,R l ㊁R h 和I l ㊁I h 分别是低光和正常光图像分解后的反射分量和光照分量, 表示逐像素相乘操作, 1表示使用的是L 1范数约束损失, 2表示使用的是L 2范数约束损失,∇表示梯度,为水平梯度与垂直梯度之和,ɛ为一个小的正常数,取0.01㊂2.4.2㊀降噪损失为了保证经过降噪处理后的反射分量和正常光图像的反射分量在结构㊁纹理信息等方面能够保持一致,同时衡量降噪处理后图像与正常光图像之间的颜色差异,降噪网络的损失函数L r 如下所示:L r = R ᶄ-R h 22-SSIM R ᶄ,R h ()+ ∇R ᶄ-∇R h 22+L cR ᶄ为经过降噪处理后的反射率,SSIM ()为结构相似性度量,L c 为色彩损失函数,表达式如下:L c = ΓR ᶄ()-ΓS h () 22其具体含义为先对降噪后的图像R ᶄ和正常光图像S h 进行高斯模糊Γ(),再计算模糊后图像的均方误差㊂2.4.3㊀调整损失为了使调整过后的光照分量与正常光图像的光照分量尽可能相似,调整网络的损失函数L i 如下所示:L i = I ᶄ-I h 22+ ∇I ᶄ-∇I h 22其中,I ᶄ为I l 增强后的图像㊂3㊀实验结果和分析3.1㊀训练数据集实验中的训练集为LOL 数据集[7],该数据集包含500对图像:其中,训练集含485对图像,验证集为剩余15对图像㊂在训练过程中,分解模块和增强模块的批量化大小为16,块大小为48ˑ48,训练次数为2000次,分解网络损失函数的权重系数λ1=0.01,λ2=0.2㊂降噪模块的批量化大小为4,块大小为384ˑ384,训练次数为1000次㊂模型优化方法为随机梯度下降法㊂整个网络模型在CPU 型号为Intel (R )Core (TM )i7-10700K㊁GPU 型号为Nvidia GeForce RTX 2080Ti 的电脑上运行,同时训练框架为Tensorflow1.15,GPU 使用Nvidia CUDA10.0和CuDNN7.6.5加速㊂为了评估Retinex-RANet 的性能,将其与几种传统方法如BIMEF [10]㊁Dong [11]㊁LIME [12]㊁MF [13]㊁MSR [14]和SIRE [15]等,以及深度学习方法,如R2RNet [16]㊁Retinex-Net [7]㊁KinD [17]㊁Zero-Dce [18]等进行比较,并同时在多个数据集上评估了该算法,包括LOL㊁LIME㊁NPE [19]和MEF [20]数据集㊂在实验过程中,均采用原文献所提供的源代码对图像进行训练和测试㊂在评估过程中,采用峰值信噪比(R PSNR [21])㊁结构相似性(R SSIM [22])和自然图像质量评估(R NIQE [23])这3个指标来进行定量比较㊂R PSNR 和R SSIM 值越高,R NIQE 值越低,则增强后图像的质量越好㊂3.2㊀消融实验为了确定Retinex-RANet 的有效性,在KinD 网络的基础上进行消融实验㊂该实验使用LOL 数据集进行验证,同时采用R PSNR ㊁R SSIM 指标来评估增强后图像的质量㊂结果如表1所示,表中RB 表示残差模块,SC 表示跳跃连接,SE 表示注意力模块㊂表1㊀各改进模块的消融实验结果Table 1㊀Ablation experimental results of each improved module序号算法R PSNRR SSIM1KinD16.12450.71132KinD +RB 17.27750.76633KinD +RB +SC17.76050.77934KinD +RB +SC +SE19.01960.78395Ours19.77610.7922表1中序号2给出的是在KinD 网络基础上,使用残差模块作为分解网络时的结果㊂相比于KinD 网络,R PSNR 和R SSIM 均有显著的提升㊂在此基础上加入跳跃连接,见序号3,相较于序号2的结果又有了小幅提升㊂说明在使用残差模块和跳跃连接作为分解网络的情况下,得到了质量更高的分解结果,从而验证了残差模块和跳跃连接的有效性㊂由于3个子网络是单独进行训练的,确定改进的分解网络有用后,在此基础上确定在降噪网络中加入空洞卷积和注意力机制的有用性㊂从序号4的结果可以看出,在加入注意力机制后,图像指标明显上升,这是因为注意力模块能集中学习有用特征,如颜色㊁细节等,从而降低图像中的噪声,阴影等㊂为了获取更多的上下文信息,同时在降噪网络中加入空洞卷积(序号5),相较于序号4的结果有了小幅提升㊂从而确定了Retinex-RANet 的模型即为序号5的模型㊂3.3㊀实验评估各算法在不同数据集上的视觉对比如图4㊁图5所示㊂46第6期史宇飞,等:基于Retinex 理论的低光图像增强算法I n p u t D o n g R e t i n e x N e t Z e r o D ceM S R L I M E S I R E R 2R N etM F K i n DO u r sG r o u n d T r u th图4㊀LOL 数据集上各算法的视觉效果Fig.4㊀Visual effects of each algorithm on the LOL datasetI n p u t D o n g R e t i n e x N e t Z e r o D ceM S R L I M E S I R E R 2R N etM FB I M E F K i n DO u rs图5㊀其他数据集上各算法的视觉效果Fig.5㊀Visual effects of each algorithm on other datasets㊀㊀图4的输入来自LOL 数据集,是非常低亮度的真实世界图像㊂可以看出:Dong㊁Retinex-Net㊁Zero-Dce㊁MSR㊁LIME 的增强结果中存在明显的噪声㊁色差等问题,特别是对Retinex-Net 来说,看起来不像真实世界的56重庆工商大学学报(自然科学版)第40卷图像;SIRE㊁MF和KinD对图像的增量程度有限,增强结果偏暗;R2RNet的增强结果在整体上偏白,存在饱和度过低等问题;相比之下,Retinex-RANet增强后的图片更接近于真实世界图像,有效抑制了噪声,同时能很好地还原图像原有的色彩㊂此外,还在其他数据集上对本模型进行了测试,如图5所示㊂从左上角的细节图像中可以看到:虽然大多数方法都能在一定程度上改变输入图像的亮度,但仍然存在着一些严重的视觉缺陷,比如Dong和Retinex-Net存在噪声和颜色失真问题;Zero-Dce㊁R2RNet和MSR增强后的图像整体偏白,无法看清左上角图像的背景;SIRE和KinD增强后的图像总体偏暗,无法观察脸部细节;Retinex-RANet㊁LIME㊁MF和BIMEF 能相对清晰地观察到脸部细节,但比较左下角图的可知,Retinex-RANet相较于其他算法,增强的亮度适中,轮廓细节更加清晰,色彩更为真实㊂表2显示了在LOL数据集上各算法的评估对比,其中,加黑数字为最优数值㊂LOL数据集中的图像为成对的低光/正常光图像,因此可使用R PSNR和R SSIM 来衡量算法的优越性,同时还引用了R NIQE指标㊂从表中可以看出:在R PSNR和R SSIM指标上,Retinex-RANet相较于其他算法都取得了最高的值,而在R NIQE 指标上,所取得的值略高于KIND和R2RNet算法得到的值㊂因为LIME㊁NPE和MEF数据集只包含低光图像,无对应的正常光图像,所以只使用R NIQE指标来比较各算法之间的差异㊂从表3可以看出:在LIME和NPE数据集上,Retinex-RANet取得了最优值,而在MEF数据集上,所取得的值略高于SRIE算法得到的值㊂表2㊀LOL数据集上各算法的结果对比Table2㊀Comparison of the results of each algorithm on the LOL dataset指标SRIE MSR LIME Dong MF Zero-Dce Retinex-Net KinD R2RNet Ours R PSNR13.348612.097914.758315.263915.667616.361516.731716.124518.934219.7761R SSIM0.39760.36370.33610.34470.36890.52470.43090.71130.75250.7982 R NIQE7.28698.11368.37768.31578.77717.93138.8788 4.6724 3.7657 4.7465表3㊀不同数据集上的R NIQE对比Table3㊀Comparison of R NIQE on different datasets算法LIME-data NPE-data MEF-data SRIE 3.8596 4.1803 3.4456MSR 3.7642 4.0614 3.5654 LIME 3.7862 4.4466 3.7962 Dong 4.0516 4.6952 4.2759MF 4.0673 4.3506 3.5995 Zero-Dce 4.3421 4.6511 3.5532 Retinex-Net 4.8077 4.5712 5.1747 KinD 4.1441 3.933 4.7805 R2RNet 5.2291 4.0191 5.1082Ours 3.4064 3.4984 3.4621综上所述,虽然Retinex-RANet并没有在上述数据集上都取得最好的结果,但仍有一定优势㊂同时,在客观评判指标R SSIM和R PSNR上均取得了最高值㊂因此, Retinex-RANet相较于其他算法,对低光照图像增强后的效果更优㊂4㊀结束语针对低光图像在视觉效果上存在亮度低㊁噪声大以及对比度弱等问题,设计了Retinex-RANet网络模型㊂此模型在分解网络中结合残差模块(RB)和跳跃连接,充分提取图像特征和细节信息;在降噪网络中嵌入空洞卷积和注意力机制,可以获取更多的上下文信息,降低图像中的噪声㊁阴影等;最后将降噪网络去噪后的反射分量和亮度调整网络增强后的光照分量融合,得到最终的增强结果㊂实验表明:与LIME㊁Zero-Dce和R2RNet相比,Retinex-RANet在客观指标R PSNR 和R SSIM上均取得了最高的数值,Retinex-RANet在增强图像的视觉对比上,不仅提高了图像的对比度㊁抑制了噪声,而且明显消除了退化问题,达到了更好的视觉效果㊂66第6期史宇飞,等:基于Retinex理论的低光图像增强算法参考文献References1 ㊀SUBRAMANI B VELUCHAMY M.Fuzzy gray level differencehistogram equalization for medical image enhancement J .Journal of Medical Systems 2020 44 6 103 110.2 ㊀张驰谭南林李响等.基于改进型Retinex算法的雾天图像增强技术J .北京航空航天大学学报2019 452309 316.ZHANG Chi TAN Nan-lin LI Xiang et al.Foggy sky image enhancement technology based on the improved Retinex algorithm J .Journal of Beijing University of Aeronautics and Astronautics 2019 45 2 309 316.3 ㊀DONG X WANG G PANG Y et al.Fast efficient algorithmfor enhancement of low lighting video C .Barcelona ICME 2011 1 6.4 ㊀LI L WANG R WANG W et al.A low-light image enhancementmethod for both denoising and contrast enlarging C .QC Canda ICIP 2015 3730 3734.5 ㊀PARK S YU S KIM M et al.Dual autoencoder network forRetinex based low light image enhancement J .IEEE Access 2018 6 22084 22093.6 ㊀LORE K G AKINTAYO A SARKAR S.LLNet A deepautoencoder approach to natural low-light image enhancement J .Pattern Recognition 2017 61 650 662.7 ㊀CHEN W WANG W J YANG W H et al.Deep Retinexdecomposition for low-light enhancement C//Proceedings of British Machine Vision Conference BMVC .2018 155 158.8 ㊀WANG W J CHEN W YANG W H et al.GLADNet Low-light enhancement network with global awareness C .Xi anChina FG 2018 751 755.9 ㊀HU J SHEN L SUN G.Squeeze-and-excitation networks C .Salt Lake City UT USA CVPR 2018 7132 7141.10 YING Z GE L GAO W.A bio-inspired multi-exposurefusion framework for low-light image enhancement EB/OL .https ///abs/1711.0059/.2017.11 DONG J PANG Y WEN J.Fast efficient algorithm forenhancement of low lighting video C .Barcelona ICME 2011 1 6.12 GUO X LI Y LING H.Lime Low-light image enhancement via illumination map estimation J .IEEE Trans Image Process 2017 26 2 982 993.13 FU X ZENG D YUE H et al.A fusion-based enhancing method for weakly illuminated images J .Signal Processing 2016 129 82 96.14 JOBSON D J RAHMAN Z WOODELL G A.A multiscale Retinex for bridging the gap between color images and the human observation of scenes J .IEEE Transactions on Image processing 1997 6 7 965 976.15 FU X ZENG D HUANG Y et al.A weighted variational model for simultaneous reflectance and illumination estimation C .Las Vegas NV USA CVPR 2016 2782 2790.16 HAI J XUAN Z YANG R et al.R2RNet Low-light image enhancement via real-low to real-normal network EB/OL . https //arxivorg/ahs/2016.14501.2021.17 ZHANG Y ZHANG J GUO X.Kindling the darkness A practical low-light image enhancer C //27th ACM Multimedia. France Nice 2019 1632 1640.18 GUO C LI C GUO J et al.Zero-reference deep curve estimation for low-light image enhancement C .Seattle WA USA CVPR 2020 1780 1789.19 WANG S ZHENG J HU H M et al.Naturalness preserved enhancement algorithm for non-uniform illumination images J . IEEE Transactions on Image Processing 2013 229 3538 3548.20 MA J ZENG K WANG Z.Perceptual quality assessment for multi-exposure image fusion J .IEEE Transactions on Image Processing 2015 24 11 3345 3356.21 HUYNH-THU Q GHANBARI M.Scope of validity of PSNR in image/video quality assessment J .Electronics Letters 2008 44 13 800 801.22 WANG Z BOVIK A C SHEIKH H R et al.Image quality assessment From error visibility to structural similarity J .IEEE Transactions on Image Processing 2004 13 4 600 612.23 MITTAL A SOUNDARARAJAN R BOVIK A C.Making a completely blind image quality analyzer J .IEEE Signal Processing Letters 2012 20 3 209 212.责任编辑:李翠薇76。
智能工厂缩略词

Aapp的意思在手机中的意思就是application的简称的缩写,意即"自动导引运输车"BB2B(Business To Business),简写为B2B,将企业内部网,通过B2B网站与客户紧密结合起来,通过网络的快速反应,为客户提供更好的服务,从而促进企业的业务发展(Business Development)。
B2B发展(B2B Development,Directindustry B2B)势头迅猛,趋于成熟B2C是英文Business-to-Customer(商家对顾客)的缩写,而其中文简称为“商对客”。
B2C中的B是Business,意思是企业,2则是to的谐音,C是Customer,意思是消费者,所以B2C 是企业对消费者的电子商务模式BPM Business Process Management(BPM),即业务流程管理,是一套达成企业各种业务环节整合的全面管理模式CCRM(Customer Relationship Management)即客户关系管理。
是指企业用CRM技术来管理与客户之间的关系CPS Computer Process System 计算机处理系统Central Processing System 中央处理系统信息物理网络系统DDPM Data Processing Manager :数据处理管理程序DCS系统(DIstirbuted Control System,分散控制系统)是随着现代大型工业生产自动化的不断兴起和过程控制要求的日益复杂应运而生的综合控制系统,它是计算机技术、系统控制技术、网络通讯技术和多媒体技术相结合的产物,可提供窗口友好的人机界面和强大的通讯功能。
是完成过程控制、过程管理的现代化设备。
DMS数据库管理的空间(Database-ManagedSpace,DMS配电管理系统(DistributionManagementSystem,简称DMS)是一种对变电配电到用电过程进行监视控制管理的综合自动化系统,其中包括配电自动化(DA)地理信息系统(GIS)配电网络重构配电信息管理系统(MIS)需方管理(DSM)等几部分DDS是直接数字式频率合成器(Direct Digital Synthesizer)的英文缩写。
CMM术语缩写一览表

CMM/CMMI术语缩写一览表2008-09-11 16:55术语一览表(按字母排序)AB= Ability to perform (CMM KPA comon feature) AC=Activities to perform (CMM KPA comon featureAD/Software Group=Application DevelopmentAE=Adaptive Enterprise (HP) RTI AI=Assessment InstrumentAPW=Action Planning WorkshopARC=Appraisal Requirements for CMMIATQG=Aassessor Training and Qualifications Guide (ISO SPICE)ATW=Actiion Team WorkshopsBAM=Business Activity MonitoringBI=Business IntelligenceBpel=Business Process Execution LanguageBPFBPG=Baseline Practice Guide (ISO SPICE)BPM=Business Process ManagementBPM=Busiiness Process MaturityBPMM=Business Process Maturity ModeBPO=Business Process OutsourcingBPR=Business Process RedesignBSI=British Standards Institute (standard BS 15000) CAPM=Certified Associate in Project ManagementCAR=Causal Analysis and Resolution (CMMI process area)CBA=CMM-Based AssessmentCBA IPI=CMM-Based Assessment for Internal Process ImprovementCBP=Competency Based PracticesCCB=Configuration Control BoardC-CommerceCDG=Capability Determination Guide (ISO SPICE)CEP=Complex Event ProcessingCEU=Continuing Education UnitsCII=Confederation of Indian IndustriesCM=Configuration ManagementCMM=Capability Maturity Model (also referred to as SWCMM). A model for improving the capability of software organizations.CMMI=Capability Maturity Model-Integration (published by the Software engineering Institute at Carnegie Mellon University in Pittsburgh) /sei-home.html(integrates 3 source models the SW CMM, SE CMM and the IPD-CMM)CMU=Carnegie Mellon UniversityCO=Committement to perform (CMM KPA common feature)COBit=Control Objectives for Information and Related TechnologyCOCOMO II=COnstructive COst MOdel II is a model that allows one to estimate the cost, effort, and schedule when planning a new software development activity.CO=Commitment to PerformCOTS=Commercial off-the-shelfCPM=Corporate Permormance MonitoringCRADA=Cooperarive Research and Development AgreementCRD=Career Recommendations DevelopmentCRM=Customer Relationship ManagementDAR=Decision Analysis and Resolution (CMMI process area)DBA=Database AdministratorDELLTA=Danish Electronics Light & AcousticsDI=Directing ImplementationDoD=Department of DefenseDP=Defect Prevention (CMM Process area)DTIZC=Defense Technical Information CenterEAI=Enterprise Application IntegrationEDA=Event Driven ArchitectureEIA=Electronic Industries AllianceEIT=Enterprise Information IntegrationELG=Executive Leadership GroupEPG=Engineering Process GroupEPIG=Engineering Process Improvement GroupERP=Enterprise Resource PlanningESB=Enterprise Service BusesESP=External Service ProvidersETL=Extraction Transformation LoadingETVX format=Enty criteria, Tasks, Verification, and eXit criteria (CMMI)FAR=Functional Area Representative (term used in some assessments)FP=Function PointFTE=Full-time Equivalent (measure of personnel availability)GAO=General Accounting OfficeGESP=Global External Service ProvidersGG=Generic GoalGP=Generic PracticeG-Q-M Approach=Goal Queston Metric techniqueIC=Intergroup Coordination (CMM process Area)IDEAL=Initiating-Diagnosing-Establishing-Acting-Leveraging; an improvement cycle often used for process improvementIEC=International Electrotechnical CommissionIEEE=Institute of Electrical and Electronics Engineers.. A professional organizationIESP=Indian External Service ProvidersIG=Introductory Guide (ISO SPICE)IM=Integrated Management (CMM process area)IPD-CMM=Integrated Product Development Capability Maturity ModelIPM=Integrated Project Management (CMM process area)IPPD=Integrated Product and Process DevelopmentIPI=Internal Process ImprovementIPT=Integrated Product TeamISACA=Information Systems Audit and ControlAssociation ISM=Integrated Software Management (CMM process area)ISM=Integrated Supplier Management (CMMI process area)ISO=International Organization fro Standardization (International Standards Organization)IT=Integrated Teaming (CMM process area)ITIL=Information Technology Infrastructure LibraryJAD=Joint application designJIT=Just in TimeJTCI=Joint Technical Committee on Information TechnologyKGI=Key Goal IndicatorsKIPA=Korean IT Industry Promotion IndustryKP=Key practiceKPI's=Key Performance IndicatorKPA=Key Process AreaKSLOC=thousand source lines of codeMA (M&A)=Measurement and Analysis (CMM process area)MBNQA=Malcom Bridge National Quality AwardMDD=Method Description DocumentME=Measurement and Analysis (CMM KPA common feature)MOA=Memorandum of AgreementMOM=Message Orientated MiddlewareMQ=Maturity QuestionnaireMSG=Management Steering GroupMSMO=Microsoft Message Queue ServerMTBF-Mean Time Between FailuresOEI=Organizational Environment for Integration (CMMI process area)OID=Organizational Innovation and Deployment (CMMI process area)OO=Object OrientatedOOA&D=Object Orientated Analysis & DesignOoda=Observe-Orient-Decide-ActOPD=Organization Process Definition (CMM process area Level 3 KPA)OPF=Organizational Process Focus (CMM process area Level 3 KPA)OPF=Organizational Process Focus (CMMI process area)OPM3=Organizational Project Management Model (Published by PMI in January, 2004)OPP=Organizational Process Performance (OPF=Organizational Process Focus (CMMI process area)OSSP=Organization's Set of Standart PracticesOT=Organizational TrainingOPF=Organizational Process Focus (CMMI process area)OUSD/AT&L=Office of the Under Secretary of Defense, Acquisition , Technology and LogisticsPA=Process AreaPAIS=Process Appraisal Information Systems (Record of Entry Form for CBA IPIs)PAG=Process Assessment Guiide (ISO SPICE)PAT=Process Action TeamPC=Process Change (Management (CMM Level 5 KPA)PCA's=Pacaged Composite ApplicationsPCAR=People CMM Assessment Repository (Record of Entry Form for a PCMM Assessment)PCB's=Process Capability Baselines-a documented characterization of the range of expected resultsPCM=Process Change Management (CMM Level 5 KPA)PCMM=People Capability Maturity Model (CMM Level 3 KPA)PD=(Organization) Process DefinitionPDCA=Plan-Do-Check-Act; an improvement cycle often used for process improvementPDU=Professional Development UnitPE=(Software) Project Engineering (CMM Level 3 KPA)PF=(Organization) Process Focus (CMM Level 3 KPA)PI=Product IntegrationPII=Process Improvement IndicatorPII=Practice Implementation IndicatorsPIID=Practice Implementation Indicator Data (used for SCAMPI)PIG=Process Improvement Guide (ISO SPICE)PIP=Packaged Integration ProcessesPM=Project ManagementPMAT=appears in COCOMO II model shows the benefit of process maturity on and estimate of effort for a software project. CMM Level 2 to Level 3 noted improvements.4-11%PMBoK=Product Management Body of KnowledgePMC=Project Monitoring and Control (CMMI process area)PMC=Process Management CapabilityPMI=Project Management InstitutePMM=People Maturity ModelPMM=Process Maturity ModelPMP=Project Management ProfessionalPMO=Project Management OfficePP=(Software) Project Planning (CMM Level 2 KPA)PP=Project PlanningPI=Product Integration (CMMI process area)PPBs=Process Performance Baselines-a documented characterization of the actual results achieved by following a process.PPM=Process Performance Model (CMMI)PPQA=Process and Product Quality Assurance (CMMI process area)PSM= Practical Software and Systems ManagementPSM= Practical Software and Systems Measurement ()PSP/TSP=Personal Software Process/Team Software ProcessPT=(Software) Project Tracking (and Oversight) (CMM Level 2 KPA)PTO=Project Tracking and Oversight (CMM Level 2 KPA)QA=(Software) Quiality Assurance (CMM Level 2 KPA)QFD=Quality Function DeploymentQM=(Software) Quality Management (CMM Level 4 KPA)OO=Object OrientatedOoa&D=Object Orientation Analysis and DesignQP=Software Quality Process (Management (CMM Level 4 KPA) OPD=Organizational Process DefiinitionQPM=Quantitative Process Management (CMM process area) QPM=Quantitative Project Management (CMMI process area) RAI=Research Access Inc.RD=Requirements Development (CMMI process area)RE=Requirements EngineeringREQM=Requirements Management (CMMI process area)RM=Requirements Management (CMM Level 2 KPA)RM=Risk ManagmentROI=Return On InvestmentRPG=Report Program GeneratorRSKM=Risk Management (CMMI process area)RTE=Real Tme EnterpriseRTI=Real Time InfrastructureRTM=Requirements Traceability MatrixSA-CMM=Software Acquisition Capability Maturity Model SAM=Supplier Agreement Management (CMMI process area)Software Acquisition Management (CMM process area)SAP=Over the course of three decades, SAP has evolved from a small, regional enterprise into a world-class international company. Today, SAP is the global market leader incollaborative, inter-enterprise business solutions. The company now employs over 28,900 peopleSC7=Subcommittee 7 (ISO JTC1 subcommittee on software engineering)SCAMPI=Standard CMMI Appraisal Method for Process ImprovementSCCB=Software Configuration Control BoardSCE=Software Capability EvaluationsSCM=Software Configuration Management (CMM Level 2 KPA)SDD=Software Design DocumentSDF=Software Development FileSDLC=Software Development Life CycleSDP=Software Development Plan (also known as Project Plan)SE CAMM=Software Engineering Capability Assessment ModelSECM=Software Engineering Capability ModelSE CMM=Software Engineering Capability Maturity ModelSEI=Software Engineering Institute (at Carnegie Mellon University) 【软件工程学院】SEPG=Software Engineering Process GroupSEPI=Systems Engineering Process InitiativeSERP=Software Engineering Process GroupSG=Specific GoalSLA=Service Level AgreementSLOC=Source Line of CodeSM=Senior ManagementSM=(Software) Subcontract Management (CMM Level 2 KPA)SME=Subject Matter ExpertSOA=Service Orientated ArchitectureSOAP=Simple Object Access ProtocolSOW=Statement of WorkSP=Specific PracticeSPA=Software Process Assessment (SEI project; now CMM-based appraisals) software process assessment (method)SPC=Statistical Process ControlSPC=Software Product ConsortiumSPE=Software Product Engineering (CMM Level 3 KPA)SPI=Software Process ImprovementSPICE=Software Process Improvement and Capability DeterminationSPIN=Software Proess Improvement NetworkSPM=Software Process Mearurement (SEI project)SPP=Software Process Program; Software Project Planning (CMM Level 2 KPA)SPPT=Software Project Tracking and Oversight (CMM process area)SPTO=Software Project Tracking and OversightSQA=Software Quality Assurance (CMM Level 2 KPA)SQM=Software Quality Management (CMM Level 4 KPA)SRS=Software Requirements Specification (also known as RequirementsDocument)SS=Supplier SourcingSSM=Software Subcontract Management (CMM Level 2 KPA)STP=Straight Through ProcessingSW-CMM=Software Capability Maturity ModelTC176=Technical Committee 176 (ISO technical committee on quality managemetn systems)TCM=Technology Change ManagementTCO=Total Cost of OwnershipTM=Technology (Change) Management (CMM Level 5 KPA)TP=Training Program (CMM Level 3 KPA)TQM=Total Quality Management TQM can be defined as the application of quantitative methods and human resources to improve the materials and services provided asinputs to an organization an to improve all of the processes within the organization. The goal of TQM is to meet the needs of the customer, now and in the future.TS=Technical SolutionTTM=Time to MarketTTT=Train the TrainerTVO=Total Value of OpportunityUAN=Universal Application NetworkUAT=user Acceptance TestUDDI=Universal Desciption, Discovery and IntegrationVAL=Validation (CMMI process area)VB=Visual BasicVE=Verifying implementation (CMM KPA common feature)VER=Verification (CMMI process area)WBS=Work Breakdown StructureWG10=Working Group 10 (ISO/IEC JTC1/SC7 Working Group on software process assessment)WG7=Working Group 7 (ISO/IEC JTC1/SC7 Working Group on software life cycle processes)WiMAX=Worldwide Interoperability for Microwave Access. 802.11. 70MB Wireless connectivity over 30 milesWP=Workforce PlanningWS=Web ServicesWSDL=Web Services Descriptive LanguageXML=Extensible Markup LanguagexMM=Maturity Models for different business models (SW, I, P)XSL=Extensible Stylesheet LanguageZLE=Zero Latency Enterprise。
27152487_密西西比河谷型铅锌矿床成矿年代学研究进展:兼论金顶矿床成矿年龄分歧

1000 0569/2022/038(06) 1577 94ActaPetrologicaSinica 岩石学报doi:10 18654/1000 0569/2022 06 02密西西比河谷型铅锌矿床成矿年代学研究进展:兼论金顶矿床成矿年龄分歧王长明1,2 段泓羽1,2 李超3 祝佳萱1,2 石康兴1,2 陈奇1,2 刘俐君1,2 钱金龙1,2WANGChangMing1,2,DUANHongYu1,2,LIChao3,ZHUJiaXuan1,2,SHIKangXing1,2,CHENQi1,2,LIULiJun1,2andQIANJinLong1,21 中国地质大学(北京)地球科学与资源学院,北京 1000832 中国地质大学地质过程与矿产资源国家重点实验室,北京 1000833 国家地质实验测试中心,北京 1000371 SchoolofEarthScienceandResources,ChinaUniversityofGeosciences,Beijing100083,China2 StateKeyLaboratoryofGeologicalProcessesandMineralResources,ChinaUniversityofGeosciences,Beijing100083,China3 NationalResearchCenterforGeoanalysis,Beijing100037,China2022 02 14收稿,2022 04 15改回WangCM,DuanHY,LiC,ZhuJX,ShiKX,ChenQ,LiuLJandQianJL 2022 ResearchadvanceingeochronologyoftheMississippiValley typePb Zndeposits:DebateonthemetallogenicageoftheJindingdeposit ActaPetrologicaSinica,38(6):1577-1594,doi:10 18654/1000 0569/2022 06 02Abstract Precisegeochronologyofmetalliferousdepositsisofcriticalsignificancefortheunderstandingsofmetallogenicregularityandtheoptimizationandevaluationofresourceexplorationtargets,however,itisverydifficult Inpreviousstudies,exploratoryattemptswereapplicatedinMississippiValley type(MVT)Pb Zndepositsbyusingdifferentdatingmethods,suchasRe Osisotopicchronology,U Pbgeochronology,40Ar 39Arisotopicage,Rb Srdating,Sm Ndisochronage,paleomagneticdating,andfissiontrackages Re Osisotopicchronologyisstillaneffectivemethodtodirectlydatetheageofknottymetalliferousdeposits,althoughitcannotreachthecurrentlyavailablehigh precisionlevelcomparedwithzirconU Pbgeochronology TakingtheJindingPb Zn Cd TldepositintheLanpingBasinoftheSanjiangTethysasanexample,thisstudyfocusesontheaccurateidentificationandhigh purityextractionofsingle stagesulfide,andestablishesthemineralogicalindicatorsofsulfidesindifferentstagesbasedonthefinedissectionofthedeposit Previousstudiesmainlyusedgangueminerals,pyrite,andotherindirectmetallogenicmineralsfordatingoftheJindingdeposit,andtheagesvariedwidelyandconcentratedin114~23Ma Thisstudypresentshigh precisiondatingofmetallogenicminerals,andtheRe Osisochronageoffivesamplesofsphaleriteis129±10Ma(MSWD=126,n=5)whichisearlierthantheageofhostrocksoftheCretaceousJingxingFormationandthePaleoceneYunlongFormation Theageof129MacannotrepresenttherealmetallogenicageofPbandZn,anditmayberelatedtotheRe andOs richorganicmatter Alongwiththedevelopmentandinnovationofisotopicgeochronologyandtheprogressofhigh purityextractionmethods,precisedatingwouldprovidemorekeysupportsforthegeochronologyresearchoftheMVTPb ZndepositsKeywords MVTdeposits;Datingmethods;SphaleriteRe Osisotopicchronology;Jindingdeposit摘 要 金属矿床精确定年仍是国内外科学研究的难题,妨碍了对成矿规律的认识和资源勘探目标的优选评价。
GMP认证文件偏差中英文

STANDARD OPERATING PROCEDURE 标准操作规程Title : QUALITY DEVIATION MANAGEMENT 题目:质量偏差管理Procedure: 编号:Effective Date:生效日期:Supersedes:替代:Review Date:复审日期:Unmodified Review History:Affected Department / Area :受影响的部门/ 区域:1.PURPOSE 目的Whenever a product, material or system fails to meet the specifications or in the event of a failure to comply with relevant documentation or regulatory requirements, an appropriate investigation must be undertaken, the cause(s) identified and the necessary corrective actions taken当产品、物料或系统不符合质量标准要求或某事件不符合相关文件或法规要求时,必须进行适当的调查,查明原因并采取必要的改正措施。
2.SCOPE 范围This SOP covers all failures and unplanned incidents related to Chemical components, Packaging materials, Drug Products, Processes, Systems, Equipments, Utilities and Facilities used to produce and control them.本SOP适用于处理所有失误及非计划性故障事件,含概用于产品并控制产品的化学成分、包装材料、药品、工艺、系统、设备、公共设施和厂房等。
关于质量认证的英语词汇

关于质量认证的英语词汇很多学生学不好英语,不是智力问题,而主要是单词的问题,词汇量不够。
下面是分享的关于质量认证的,一起来看一下吧。
质量一致性检验inspecton of quality conformity仲裁性质质量监视quality supervision for arbitration 产品保护product protection产品定型product approval产品分等product classificatin产品标准product specification产品设计product design产品质量product quality产品合格率product percent of pass产品系列化product seriation产品管理标准product management standard产品计量单位unit of measurement of product产品售后效劳after service产品质量标志product quality mark产品质量标准product quality standard产品质量管理product quality management产品质量检验product quality inspection产品质量监视检验product quality supervision and inspection产品质量认证标志certification marking of product quality产品质量认证程序certification procedure of product quality产品质量认证制度certification system of product quality认证certification立法legislation自行认证self-certification认可areditation平安认证safety certification平安认证标志mark of safety certification强制性认证pulsory certificate实验室鉴定laboratory qualification实验室认证laboratory certification自愿认证制voluntary system of certification法规机构regulatory authorities认证活动certification activity认证体系certification system认证方案certification scheme认证机构certification body检验机构inspecton body许可证licence申请人applicant许可证持有者licensee合格证书certificate of conformity合格标记mark of conformity认证体系的利用aess to certification system认证体系的参与者participant in certification system 认证体系的成员member of certification system批准approval型式批准type approval测试实验室的认可areditation of testing laboratory认可areditation认可体系areditation system认可机构areditation body认可的实验室aredited laboratory认可准那么acreditatin criteria实验室评定laboratory assessment实验室评定者laboratory assessor认可的实验室的试验报告aredited laboratory test report 批准签署人approved signatory成认和批准协议recognition and approval arrangement成认协议recognition arrangement单边协议unilateral arrangement双边协议bilateral arrangement多边协议multilateral arrangemetn互利reciprocity平等待遇equal treatmetn国家待遇national treatment国家和平等待遇national and equal treatment合格及有关的通用概念conformity and related general concept合格conformity合格测试conformity testing合格aeptable合格品aeptable part合格标志mark of conformity合格认证conformity certification合格认证标志mark of conformity certification合格品标志mark of aeptable product合格证书certificate of conformity合格证检查inspection by certificate合格质量检查aeptable quality level评定合格assessment of conformity合格评定conformity assessment型式评价type evaluation合格监视conformity surveillance合格证明verification of conformity合格保证assurance of conformity供货商声明supplier's declaration生产许可证production licence一致consensus检验inspection不定期检验nonperiodic inspection抽样检验sampling inspection出厂检验exfactory inspection第一方检验first party inspection第二方检验second party inspection第三方检验third party inspection第三方认证制度third pary certification定期检验periodic inspection交收检验aeptance inspeciton例行检验routine inspeciton生产检验produciton inspeciton生产定型检验production approval inspeciton 验收检验aeptance inspeciton验证检验pliance test仲裁检验arbitration inspection制造商担保guarantee by manufacturer百分比抽样检查percent sampling inspection 不合格判定数rejection number不合格质量水平rejecton quality level抽查型质量监视sampling quality supervision 环境监测environmental monitoring抽样sampling屡次抽样multiple sampling分层抽样stratified sampling分层随机抽样stratified random smapling多级抽样multistage sampling二次抽样double sampling试件test piece试验报告test report试样test sample测试设备testing equipment测试试验室testing laboratority商标trademark商品名称trade-name测试testing试验test实验室间的试验比拟interlaboratory test parisons 熟练水平试验proficiency testing次品defective product代用产品substitute product定量试验quantitative test出口管理export control出口许可证制度export control进口许可证制度import licence system定额管理quota management非关税壁垒non-tarrif barrier关税壁垒tariff barrier关税普遍优惠制general preferential duties。
分光光度法测定水中挥发酚含量的不确定度评定

分析检测分光光度法测定水中挥发酚含量的不确定度评定张丽琴(山西省检验检测中心食品与粮食检验技术研究所,山西太原 030012)摘 要:本文依据《化学分析测量不确定度评定》(JJF1135—2005),按照《生活饮用水标准检验方法 感官性状和物理指标》(GB/T 5750.4—2006)中的4-氨基安替比林三氯甲烷萃取分光光度法,测定饮用 水中的挥发酚含量,对实验过程中的各因素引入的不确定度进行计算,得到该实验的扩展不确定度为 0.000 326 mg/L,影响该实验不确定度的主要因素为标准曲线的拟合过程。
关键词:不确定度;挥发酚;饮用水Evaluation of Uncertainty in Determination of volatile Phenol Content in water by PhotometerZHANG Liqin(Institute of Food and Grain Inspection Technology, Shanxi Inspection and Testing Center,Taiyuan 030012, China)Abstract: This paper determines the volatile phenol content of the factors introduced in drinking water by the 4-aminotebilin trichloromethane extraction method (JJF1135-2005), the sensory traits and physical indicators (GB/T 5750.4-2006), and the main factor of the standard curve.. The uncertainty introduced by each factor in the experiment process is calculated. The expanded uncertainty of this experiment is 0.000 326 mg/L. It is concluded that the main factor affecting the uncertainty of the experiment is the fitting process of the standard curve.Keywords: uncertainty; volatile phenol; drinking water依据《生活饮用水标准检验方法感官性状和物理指标》(GB/T 5750.4—2006)中的4-氨基安替比林三氯甲烷萃取分光光度法,测定饮用水中的挥发酚含量实验过程复杂,容易出现误差的环节较多,因此,对实验结果进行不确定度评价是很有必要的,测量不确定度是表示合理地赋予被测量之值的分散性,与测量结果相联系的参数[1],衡量检测结果是否准确,本文依据《化学分析测量不确定度评定》(JJF1135—2005),对一加标饮用水盲样进行了检测,分析了不确定度的来源及影响,为以后检验工作中误差规避和结果分析提供了依据。
OM-90系列温湿度数据记录器产品介绍说明书

OM-92OM-91Portable Temperature and Humidity Data LoggersOM-90 SeriesU M odels for Temperature (OM-91) and Temperature/Humidity (OM-92)U I mmediate, Delayed (Specific Date/Time) or Pushbutton Start U R eal-Time Mode U U ser-Configurable High and Low Alarms U L ED Status Indicators U U SB Interface for Fast Data Transfer U C ompact, Light Weight, Easy-to-Use U L ong Battery Life The OM-90 Series are portable, battery operated, temperature (OM-91) and temperature/humidity(OM-92) data loggers. The OM-90 Series data loggers offer accurate and repeatable logging for temperature and relative humidity. Temperature is measured to an accuracy of ±0.3°C over the range of 5 to 60°C (41 to 140°F) and ±2.0°C over the full range of -30 to 80°C (-22 to 176°F). Relative humidity is measured to an accuracy of ±3.0% RH over the 20 to 80% RH range and ±5.0% RH over the0 to 100% RH range. Both temperature and relative humidity are logged at a user configurable logging rate which is software selectable.These data loggers are ideal for transportationapplications where it is necessary to document that the material in-transit has stayed within particular environmental limits. High/low alarm limits can be set above or below which LED indicators will be activated so that the user can become aware that the desired transportation conditions have been exceeded.Each data logger can be assigned a 16 character name. This can be helpful in applications where multiple data loggers are used and there is a need to distinguish between them (for example based on location).The data logger includes a very easy to use software application. The application is used for configuration of the logger, extraction of logger data, graphically viewing data, spread sheet analysis of data and saving data to file (typically in comma separated value CSV file format) for third party applications such as Microsoft Excel. The graphical user interface is completely intuitive in terms of configuring new devices andextracting data from a field device. When the user has multiple devices requiring the same setup then a logger setup from a saved configuration file can be preloadedinto the application to help expedite the process and ensure all loggers get setup identically. The application does not require any driver installations to connect to the data logger. Just install the software, run it and then connect the data logger.OM-91temperature data logger.OM-92temperature/humidity data logger.Windows ® software used for data logger configuration and data display in graphical or tabular format.SPECIFICATIONSTemperatureRange: -30 to 80°C (-22 to 176°F) Resolution: 0.01°CAccuracy: ±0.3°C from 5 to 60°C (41 to 140°F) Calibration Accuracy @ 25°C ±20°C: ±0.1°COrdering Example: OM-92-NIST, temperature/humidity data logger with single point NIST calibration certificate.Humidity (OM-92 Only) Range: 0 to 100% RH Resolution: 0.01% RHA ccuracy: ±3% over 20 to 80% RH range; ±5% RH below 20% RH or above 80% RHC alibration Accuracy @ 25% RH: ±1.8% RH @ 25% RH MemoryOM-91: 65,520 temperature measurements O M-92: 65,520 temperature and 65,520 humidity measurementsStart Modes: Immediate start on disconnect from PC, delayed start (scheduled date/time) or pushbutton start Recording Mode: Stop on memory full; when datalogger operation is start/stopped by user intervention via button press, last memory location is retainedRecording Interval: 1 sec, 10 sec, 30 sec, 1 min, 10 min, 30 min, 1 hr (software selectable)Software Compatibility:Windows XP/Vista/7 and 8 (32-bit and 64-bit)Real Time Logging: Displays real time data when connected to a PCReal Time Clock: Time automatically synced to PC Battery Backup: Continues to keep time when off Accuracy: 0.50 sec/day (~3 min/year)Alarms: High and Low alarms for temperature and humidity; dedicated alarm LED indicator LED Indicators: Alarm and status LEDsDevice ID: Each data logger can have a 16 character name assignedConnections: 0.9 m (3') USB Micro-B to PC cable (included)Power: 3V lithium CR2450 battery included (user replaceable)Battery Life: Over 4 year battery life while logging Bypass Mode: Unit is powered from USB when connected to conserve the batteryDimensions: 53 H x 33 W x 15 mm D (1.38 x 2.36 x 0.6")Weight: 25 g (0.9 oz)OM-92OM-91。
Evaluation of five DNA extraction methods for comm

IntroductionVegetable oils are important ingredient for human nutrition and widely used in food industry. In recent years, increasing attention to food safety has stimulated more interest in food product authenticity and presence of genetically modified (GM) ingredients (Consolandi et al., 2008; He et al., 2013; Fraiture et al., 2017). Methods have been developed to detect authenticity and GM ingredients in vegetable oils to ensure consumers’ awareness. Methods include high performance liquid chromatography (HPLC), gas chromatography (GC), proton transfer reaction mass spectrometry and specificsmall molecule analysis (Casale et al., 2008). However, many methods only discern physical and chemical properties of vegetable oils, but not the oil authenticity. Therefore, an effective method on detecting vegetable oil authenticity is urgently needed.Recently, scientists have focused on developing DNA based quantitative GMO ingredient detection method in vegetable oils. Unlike complicated chemicalpretreatment, which is time consuming and labor intensive, DNA based detection relies on highly specific amplification of DNA fragment using polymeraseYunjing Li 1,2§, Lin Shao 1,2§, Xiao Fang 1,2, Danfeng Wan 1,2, Yuhua Wu 1,2, Jun Li 1,2, Jun Li 1,2, Li Zhu 1,2, Gang Wu 1,2*1. Key Laboratory of Biology and Genetics improvement of Oil Crops, Ministry of Agriculture, Oil Crops Research Institute, Chinese Academy of Agricultural Sciences, Wuhan, China.2. Supervision and Test Center (Wuhan) for Environmental Safety of Genetically Modified Plants, Ministry of Agriculture, Wuhan, China.Evaluation of five DNA extraction methods for commercial vegetable oilsAbstract: To ensure food safety, it ’s vital to accurately detect genetically modified (GM) ingredient adulteration in food products and effectively detect the adulteration of vegetable oils from GM organisms (GMO). Therefore, it ’s essential to establish efficient DNA isolation method from vegetable oil. Here, we evaluate 5 DNA isolation methods using 25 commer -cial vegetable oils produced from soybean, peanut, corn, sunflower, rapeseed as well as blended oils. Quality of isolated DNA was determined by Nanodrop 2000 spectrophotom -etry. Real-time PCR and universal gene tRNA-Leu was used to assess resolution of meth-ods. Our results showed that only DNA samples isolated by modified emulsification method based on cetyl trimethylammonium bromide (CTAB) were able to amplify tRNA-Leu gene. Moreover, Ct values of species specific endogenous reference genes were greater than 36 in these samples. In summary, CTAB method showed the best resolution on GMO adulter -ation detection for commercial vegetable oils, especially in fully refined oils.Keywords: commercial vegetable oil, DNA extraction, DNA quality, quantitative PCR, GMOdetection123 Li et al. / Oil Crop Science 2018, 3 (2): 122-136DNA extraction methods for commercial vegetable oilschain reaction (PCR, Mafra et al., 2008). DNA based PCR method has been widely used to detect the presence of GMO (Morisset et al., 2008; Holst-Jensen et al., 2003; Michelini et al., 2008; Querci et al., 2010). Compared to RNA or protein based detection methods, DNA based PCR have several advantages, such as higher sensitivity, stability, reliability and better efficiency. The most critical and difficult aspect of DNA based detection method is DNA isolation efficiency from oils. Moreover, the rapid development of oil processing and refining technology brings new challenges for adulteration detection of vegetable oils. The difficulties of DNA extraction from vegetable oil come from refining. Crude oil contains many impurities and insoluble substances. For example, virgin rapeseed oil contains glucosinolate, which is harmful to human health. Therefore, crude vegetable oil must be processed into edible oil by refining, which includes degumming, neutralization, bleaching and deodorization (Gryson et al., 2002; Debode et al., 2012). In China, depending on their color, aroma, transparency, moisture and other physical properties, vegetable oils are classified into 4 grades (GB1536-2004). During refining, DNA molecules of vegetable oils are eliminated and degraded, so it is impossible to recover DNA from refined vegetable oils (Gryson et al., 2002; Pauli et al., 1998).Previous reports have shown that DNA can be extracted from crude vegetable oils and amplified by PCR through several techniques, including Wizard®Magnetic DNA purification system, DNeasy®Plant mini kit, NucleoSpin® food kit, DNeasy® Mericon food kit, CTAB based method, hexane based method and modified emulsification method (Testolin et al., 2005; Consolandi et al., 2008; Costa et al., 2010a; Gryson et al., 2002; Zorica et al., 2014; Zarzoso-Lacoste et al., 2013; Shao et al., 2016). For example, amplifiable DNA from refined vegetable oils has been extracted using NucleoSpin food kit and usual DNA extraction method (Costa et al., 2010b; Debode et al., 2012). However, few studies have focused on extracting and amplifying DNA from commercial refined oils.The development of DNA extraction and amplification method to detect GMO or adulteration in refined vegetable oils usually focuses on 1 type of oil. Present study has selected 5 methods to extract amplifiable DNA from both crude and refined vegetable oils. These methods include modified emulsification method based on CTAB, Resin type oil DNA extraction kit, Wizard® Magnetic DNA Purification for Food, NucleoSpin® Food kit and DNeasy® Mericon Food kit. Aim of this study is to select a suitable method to isolate DNA from commercial vegetable oils for real-time PCR detection of adulteration and GM ingredients. Materials and methodsMaterialsOil samples were purchased from Joybuy and Wal-Mart supermarkets, including 6 different commercial vegetable oils: soybean (Glycine max L.), peanut (Arachis hypogaea L.), corn (Zea mays L.), sunflower (Helianthus annuus), rapeseed (Brassica napus) and blended oils (Table 1).DNA extractionMethod A: Modified emulsification method based on CTABAccording to published protocol (Shao et al., 2016), 50 mL commercial vegetable oil and 50 mL n-hexane mixture was suspended for 3 h using electromagnetic stirrer. Subsequently, 50 mL of 1.5×CTAB (75 mmol/L tris-HCl, 1.05 M NaCl, 15 mM ethylenediaminetetraacetic acid (EDTA, 1.5% CTAB) plus 80 μg salmon sperm DNA (Sigma-Aldrich, St. Louis, MO, USA) was added, mixed and stirred for another 3 h. Mixed sample was then centrifuged at 12,000 g for 15 min. Top layer was carefully removed and discarded. Precipitate was transferred into 2 new sterile 50 mL centrifuge tubes, containing an equal volume of isopropanol and a 1/10 volume of 3 mol/L sodium acetate. Solution was mixed for 1 h and then incubated at -20°C overnight. Next day, it was centrifuged at 12,000 g for 25 min at 4°C and discarded the supernatant. Precipitate was dissolved in 600 μL 0.1 mol/L Tris-EDTA buffer solution (TE, 1 mmol/L Tris-HCl, pH 8.0, 0.1 mmol/L EDTA, pH 8.0) and transferred into a new sterile 2 mL tube124Li et al. / Oil Crop Science 2018, 3 (2): 122-136DNA extraction methods for commercial vegetable oilsbefore an equal volume of chloroform/isoamyl alcohol (24:1) was added. Mixture was well blended and centrifuged at 12,000 g for 10 min at 4°C. Supernatant was collected to a new sterile 2 mL tube and an equal volume of isopropanol, a 1/10 volume of 3 mol/L sodium acetate and 1/100 volume of Dr. GenTLE®Precipitation Carrier (TaKaRa Bio Inc., Shiga, Japan) were added. Solution was well mixed and incubated at -20°C for 2 h before centrifuging at 12,000 g for 25 min. To wash precipitate, 600 μL 75% ethanol was added and centrifuged at 12,000 g for 2 min. Finally, tubes were incubated at room temperature to dry and DNA precipitate was dissolved in 60 μL of 0.1×TE (1 mmol/L EDTA, 10 mmol/L Tris).Method B: Resin methodA resin type oil DNA extraction kit (Dingguo, NEP009, Beijing, China) was used to extract DNA following manufacturer’s instructions with some minor modifications. 10 mL refined oil sample was placed in a 50 mL sterile centrifuge tube. 10 mL of solution A and 5 mL of solutionB were added into tube and stirred thoroughly for 1 min. Mixture was incubated for 2 min at room temperature and the top layer was discarded carefully. A total of 50 μL solutionC was added to lower aqueous phase followed by gentle stirring. After 2 min incubation, mixtures were centrifuged at 8,000 rpm for 5 min at 4°C. Supernatant was discarded carefully, and pellet was homogenized in 1 mL solution B. Solution was subsequently transferred to 1.5 mL tubes and centrifuged at 8,000 r/min for 2 min to precipitate. Pellet was then homogenized in 1 mL solutionD and transferred to new 1.5 mL tubes followed by centrifuging at 8,000 rpm for 2 min. This step was then repeated once. Next, reaction tubes were centrifuged at 8,000 rpm for 1 min and supernatant was discarded. Finally, tubes were incubated for 5-10 minTable 1. Information obout type, grade, processing, origin and purchase location of 25 commercial vegetable oilsSample No.Edible oil Grade Processing Origin Purchase location DD3-1Soybean oil Third grade Pressing Harbin JoybuyDD3-2Soybean oil Third grade Pressing850farm JoybuyDD1-1Soybean oil First grade Solvent extraction Tianjin JoybuyDD1-2Soybean oil First grade Solvent extraction Wuhan JoybuyDD1-3Soybean oil First grade Solvent extraction Harbin JoybuyHS1-1Peanut oil First grade Pressing Shenzhen JoybuyHS1-2Peanut oil First grade Pressing Yantai JoybuyHS1-3Peanut oil First grade Pressing Liancheng JoybuyHS1-4Peanut oil First grade Pressing Suzhou JoybuyYM1-1Corn oil First grade Pressing Wuhan JoybuyYM1-2Corn oil First grade Pressing Tianjin JoybuyYM1-3Corn oil First grade Pressing Wuhan JoybuyYM1-4Corn oil First grade Pressing Binzhou JoybuyKH1-1Sunflower oil First grade Pressing Suzhou JoybuyKH1-2Sunflower oil First grade Pressing Tianjin JoybuyCZ4-1Rapeseed oil Fourth grade Pressing Xilin JoybuyCZ4-2Rapeseed oil Fourth grade Pressing Ma’anshan JoybuyCZ4-3Rapeseed oil Fourth grade Pressing Mianyang JoybuyCZ4-4Rapeseed oil Fourth grade Pressing Honghu JoybuyCZ3-1Rapeseed oil Third grade Pressing Chengdu JoybuyCZ3-2Rapeseed oil Third grade Pressing Mianyang JoybuyCZ1-1Rapeseed oil First grade Pressing Yueyang Wal-Mart supermarket CZ1-2Rapeseed oil First grade Pressing Zhumadian JoybuyTH1Blended oil//Wuhan JoybuyTH2Blended oil//Wuhan Joybuy Notes: /, blended oils which are not marked with grade level or processing method.125 Li et al. / Oil Crop Science 2018, 3 (2): 122-136DNA extraction methods for commercial vegetable oilsto dry DNA pellet and dissolved in 60 μL 0.1× TE with water bath at 50°C for 2-5 min.Method C: Wizard Magnetic methodWizard Magnetic DNA purification system for food (Promega, Madison, WI, USA) was used following manufacturer’s instructions with minor modifications. Oil sample (160 mL) was weighed and divided into 4 sterile 50 mL reaction tubes. A total of 2 mL lysis buffer A was added to each tube and mixed well by shaking. Subsequently, 1 mL of lysis buffer B was added to each tube and followed by shaking. After 10 min incubation at room temperature (mix twice during the time), 3 mL precipitate solution was added to each tube and mixed for 1 min. Mixture was centrifuged at 4,000 g for 30 min. The top layer was removed carefully using a 10 mL pipette and discarded. Pellets from the 4 tubes were collected in a new reaction tube. 50 μL resuspended MagneSil®Paramagnetic Particles (Promega) was added to the tube and then vortexed briefly. After adding a 0.9 volume of isopropanol, mixture was placed at room temperature for 1 h with intermittent mixing. Tube was then incubated in a PolyATtract®System 1000 Magnetic Separation Stand support (Promega) for approximately 2 min until all particles had adhered to the support. Particles were washed with 1.5 mL of 70% (V/V) ethanol for 3 times and then transferred to a 2 mL sterile centrifuge tube. After ethanol solution was carefully discarded, particles were dried in an uncapped tube for 20 min. Finally, 60 μL of 0.1×TE was added to elute DNA from magnetic particles for 5 min at 65°C. Final DNA sample was collected in a fresh tube and left in the stand for 1 min.Method D: NucleoSpin methodNucleoSpin food kit (Macherey-Nagel GmbH & Co. KG, Düren, Germany) was used according to manufacturer’s instructions and as described by Costa with minor modifications (Costa et al., 2010b). Oil samples (180 mL) were carefully weighed and divided into 4 sterile 50 mL reaction tubes. These tubes were then centrifuged at 18,514 g for 30 min at 4°C. Supernatant was discarded, and residual pellet was transferred into a set of four sterile 2 mL collection tubes. Reaction tubes were centrifuged at 18,514 g for 10 min at 4°C and supernatant was discarded. Pellet was homogenized with 550 μL buffer CF (preheated to 65°C) and 10 μL proteinase K (20 mg/mL). Subsequently, mixture was incubated at 65°C for 1 h with discontinuous overtaxing and then centrifuged at 18,514 g for 10 min at 4°C. The top layer was transferred to a new sterile 2 mL reaction tubes with addition of same volume buffer C4 and 100% ethanol. Mixture was vortexed for 30 s and then eluted through 1 spin column by centrifuging for 1 min at 11,000 g. A total of 400 μL buffer CQW was added to the column, and centrifuged for 1 min at 11,000 g. The column was then washed with 700 μL and 200 μL buffer C5 at 11,000 g for 1 min and 2 min, respectively. Finally, DNA sample was eluted using 60 μL 0.1×TE solution after 5 min incubation at room temperature. Method E: DNeasy Mericon methodDNeasy Mericon Food kit (Qiagen GmbH, Hilden, Germany) was used according to manufacturer’s instructions with minor modifications. Oil samples (180 mL) were carefully weighed and divided into 4 sterile 50 mL reaction tubes and centrifuged at 18,514 g for 30 min at 4°C. The top layer was carefully removed and pellet was transferred to 2 sterile 2 mL reaction tubes. Reaction tubes were then centrifuged at 18,514 g for 10 min at 4°C. The top layer was discarded, and 200 mg of homogenized pellet was weighed into a 2 mL microcentrifuge tube before the addition of 1 mL food lysis buffer and 2.5 μL of proteinase K solution (20 mg/mL). Mixture was vortexed briefly and incubated in a thermomixer for 30 min at 60°C with constant shaking (1,000 rpm). To enhance the precipitation, sample was cooled to room temperature on ice and then centrifuged for 5 min at 2,500 g. Subsequently, 500 μL chloroform was added into this 2 mL microcentrifuge tube. Clear supernatant was carefully drawn from each lysis tube without disturbing inhibitor precipitate at bottom. Supernatant aliquots from these 2 tubes were combined and then mixed by pipetting up and down several times. Afterwards, 700 μL of clear supernatant was transferred to a microcentrifuge tube containing chloroform, vortexed vigorously for 15 s, and then centrifuged at 14,000 g for 15 min. The upper aqueous phase (about 350 μL) was mixed thoroughly with 350 μL buffer PB and126Li et al . / Oil Crop Science 2018, 3 (2): 122-136DNA extraction methods for commercial vegetable oilstransferred into a fresh 2 mL microcentrifuge tube. Solution was pipetted into QIAquick spin column (Qiagen GmbH) and centrifuged at 17,900 g for 1 min. A total of 50 μL buffer AW2 was added to QIAquick spin column and centrifuged at 17,900 g for 1 min. Column was centrifuged again at 17,900 g for 1 min to dry membrane. QIAquick spin column was transferred into a new 1.5 mL or 2 mL microcentrifuge tube and 60 μL 0.1×TE buffer was added directly into QIAquick membrane. Finally, column was incubated for 1 min at room temperature and centrifuged at 17,900 g for 1 min to elute DNA.All extracted DNA was kept at -20°C until further analysis.DNA concentration and quality detectionQuality and purity of extracted DNA were analyzed using Nanodrop 2000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). DNA concentration and purity were determined by UV absorbance at 260 nm and A 260/280, respectively.Real-time PCR amplificationDNA extraction efficiency was evaluated by real-time PCR using a crop species specific endogenous reference gene, tRNA-Leu . Primers and probes used in this research were synthesized by Sangon Biotech Co. Ltd (Shanghai, China). Endogenous genes, CruA (rapeseed), Lectin (soybean), z SSIIb (maize), Arah2 (peanut) and HaG5 (sunflower) were chosen for species specific assays in vegetable oils (Table 2).PCR was performed using CFX96 Real-Time System (Bio-Rad Laboratories, Hercules, CA, USA) with a final volume of 25 μL. Reaction mixture consists of 5 μL template DNA, 400 nmol/L primers, 200 nmol/L probe, 12.5 μL 1×Premix Ex TaqTM (probe qPCR, Takara Bio Inc.) and ddH 2O to 25 µL. Real-time PCR program was as follow: an initial denaturation for 30 s at 95°C, followed by 50 cycles of 5 s at 95°C, then 60°C for 1 min. Fluorescence signals were measured and collected at the end of each cycle, and then processed using CFX Manager 3.1Table 2. Primers and probes used in this study CropTarget gene Primers/probesSequences (5’ - 3’)Size/bp Reference Rapeseed CruA qCruAFqCruARqCruAP GGCCAGGGTTTCCGTGAT CCGTCGTTGTAGAACCATTGGFAM-AGTCCTTATGTGCTCCACTTTCTGGTGCA-BHQ1101Wu et al., 2010SoybeanLectinLec-1215F GCCCTCTACTCCACCCCCA 118Zhang et al., 2013Lec-1332R GCCCATCTGCAAGCCTTTTTLec-1269P FAM-AGCTTCGCCGCTTCCTTCAACTTCAC-BHQ1CornZSSIIbzSS Ⅱb-3FCGGTGGATGCTAAGGCTGATG 88Yang et al., 2012zSS Ⅱb-4R AAAGGGCCAGGTTCATTATCCTCzSS Ⅱb-P FAM-TAAGGAGCACTCGCCGCCGCATCT-BHQ1PeanutArah2Arah2-FGCTCGAGAGGCCGAACCT ~100Hird et al., 2005Arah2-R TCCTCGTCACGTTGGATCTTCArah2-P FAM-AGGCCCTGCGAGCAACATCTCATG-BHQ1SunflowerHaG5hagFAGGGTCAGAACATTCCTCGC 151Zhang et al., 2009hagR TGGGCTTCTCGGAACACCThagP FAM-AACCCTCAGATGGGGCGGCAG-BHQ1CottonSad1BT1FTACTTGGTGGAGAACGCATTGAA 107Yang et al., 2005Bt2R GATGTCAACTAGTCCGAGAACGAABtP FAM-CACCTGGCACGAACTCGCTGAGCA-BHQ1PalmMT3-BMt1AGGGCAATTCCTAATGTATTGGAC 109Zhang et al., 2009Mt2CGCAGTTAGAGCCGCATTTMtp FAM-CGAGGAAGTCGTTGAGGTGGCAGC-BHQ1PlanttRNA-LeuPlant nes-2-fATTGAGCCTTGGTATGGAAACCT ~90Laube et al., 2010Plant nes-2-rGGATTTGGCTCAGGATTGCCPlant nes-2-p FAM-TTAATTCCAGGGTTTCTCTGAATTTGAAAGTT-BHQ1127Li et al . / Oil Crop Science 2018, 3 (2): 122-136DNA extraction methods for commercial vegetable oils Table 3. Comparison of 5 DNA extraction methods DNA extraction methodV olume (mL)Process durationCost (¥)Modified emulsification method (A)50 1.5 d 20Resin type oil DNA extraction kit (B)10 2 h 24Wizard ® Magnetic DNA Purification for Food (C)160 4 h 800NucleoSpin ® Food kit (D)180 4 h 30DNeasy ® Mericon Food kit (E)1804 h30(3.1.1517.0823) software (Bio-Rad Laboratories).ResultsComparison of five DNA extraction methods Three aspects of these DNA extraction methods were compared: sample volume, process duration and cost (Table 3). Our study revealed that the resin type (Method B) was the best protocol for its smallest sample volume, shortest process duration (2 h) and relatively low cost. The cheapest method was modified emulsification method (Method A), but it took 1.5 d to complete entire process. Wizard Magnetic DNA purification kit (Method C) for food was the most expensive method and it cost about 20 times more than others. NucleoSpin food kit (Method D) was similar to DNeasy Mericon food kit (Method C) regarding sample volume, process duration and cost.Quality and quantity of extracted DNAAll extracts were evaluated using Nanodrop 2000 spectrophotometer. DNA concentration was estimated by measuring the absorbance at A 260. Purity was measured by A 260/A 280 and A 260/A 230 ratios. DNA concentration and purity obtained by 5 methods were listed in Table 4. Wizard Magnetic DNA Purification for Food kit failed to extract any DNA from rapeseed oil in previous report (Shao et al., 2016), so it was not used to extract DNA from rapeseed oil samples in this study. NucleoSpin and DNeasy Mericon methods both successfully extracted DNA from 9 of total 25 commercial vegetable oils. These oil samples include third grade soybean oil (1), first grade peanut oil (2), fourth grade rapeseed oil (4), third grade rapeseed oil (1) and blended oil (1). Resin type and Wizard Magnetic methods successfully extracted DNA from 25 oil samples and 17 oil samples, respectively. Modified emulsification method extracted the highest concentrations of DNA from all 25 oil samples by adding salmon sperm DNA to CTAB buffer. These results indicated that NucleoSpin, DNeasy Mericon and Wizard Magnetic methods were inferior to emulsification and resin type methods.Modified emulsification method produced the highest DNA yield alomst in all oil samples (average yield 636.3 ng/μL), followed by resin type method (average yield 13.8 ng/μL). Wizard Magnetic, NucleoSpin and DNeasy Mericon methods produced lower yields of DNA, approximately 6.96, 6.45 and 4.85 ng/μL, respectively. The highest purity of DNA was also obtained using modified emulsification method, with A 260/A 280 ratio from 1.87 to 2.0 and A 260/A 230 ratio between 1.6 and 2.0. The other 4 protocols, especially resin type method, produced relatively low purity DNA.Real-time PCR amplificationTo further identify quality of isolated DNA andcompare efficiency of 5 extraction methods, all DNA extracts were amplified by real-time PCR using hydrolysis fluorescent probes. The 25 commercial vegetable oils used in present study included soybean oil (5 types), peanut oil (4 types), corn oil (4 types), sunflower oil (2 types), rapeseed oil (8 types) and blended oil (2 types). Plant reference gene tRNA-Leu was chosen for generic assay as proof for presence of plant DNA in oils, while species specific endogenous genes (Table 2) were chosen for detecting plant species in oils. Considering possible adulteration of commercial vegetable oil by unscrupulous producers, endogenous gene Sad1 for cotton, MT3-B for palm, CruA for rapeseed and Lectin for soybean were also chosen to check authenticity. All probes and primers referenced in national standards or literature are listed in Table 2. Amplification fragment length was about 100 bp due to128Li et al . / Oil Crop Science 2018, 3 (2): 122-136DNA extraction methods for commercial vegetable oilsT a b l e 4. C o n c e n t r a t i o n a n d p u r i t y o f D N A e x t r a c t e d f r o m 25 c o m m e r c i a l v e g e t a b l e o i l s a m p l e s b y 5 m e t h o d sS a m p l eM o d i fi e d e m u l s i fi c a t i o n m e t h o d (A )R e s i n t y p e (B )W i z a r d m a g n e t i c (C )N u c l e o s p i n (D )D N e a s y m e r i c o n (E )D N A (n g /μL )A 260/A 280 A 260/A 230D N A (n g /μL )A 260/A 280 A 260/A 230D N A (n g /μL )A 260/A 280 A 260/A 230D N A (n g /μL )A 260/A 280 A 260/A 230D N A (n g /μL )A 260/A 280 A 260/A 230D D 3-1892.5±2.11.931.934.5±0.32.280.033.9±0.12.460.01N A N A N A N A N A N AD D 3-21119.4±0.91.921.858.7±0.22.890.0120.3±0.25.050.0517.8±0.11.370.44.0±0.23.410.12D D 1-1561.0±0.21.901.806.4±0.12.040.039.2±0.11.710.10N A N A N A N A N AN AD D 1-2884.3±0.71.901.792.4±0.16.420.023.5±0.10.980.11N A N A N A N A N AN AD D 1-3448.2±1.81.881.8334.7±0.928.140.6011.3±0.31.490.02N A N A N A N AN AN AH S 1-11027.4±2.21.911.964.2±0.13.130.018.7±0.22.190.03N A N A N A7.4±0.12.200.14H S 1-2772.8±2.11.932.049.6±0.25.570.015.7±0.14.730.02N A N A N A N AN AN AH S 1-3688.8±1.51.921.885.7±0.12.920.035.5±0.25.770.034.8±0.12.270.134.5±0.12.130.14H S 1-4428.5±15.91.911.7610.8±0.28.100.028.0±0.11.830.055.9±0.11.910.19N AN AN AY M 1-1896.5±2.31.911.867.3±0.25.460.015.3±0.11.840.03N A N A N AN AN AN AY M 1-2473.8±1.91.911.9110.7±0.214.250.026.4±0.12.150.02N A N AN AN AN AN AY M 1-394.4±0.52.001.0528.7±0.442.300.547.8±0.21.780.02N A N AN AN AN AN AY M 1-4416.1±1.11.921.824.6±0.23.850.016.5±0.20.900.02N AN AN AN AN AN AK H 1-1694.9±2.91.901.9816.2±0.411.620.056.7±0.22.530.02N AN AN AN AN AN AK H 1-2285.7±3.91.891.6421.4±0.420.210.187.2±0.12.290.02N AN AN AN AN AN AC Z 4-1374.9±0.91.901.785.2±0.13.590.01///11.4±0.11.610.223.3±0.12.900.10C Z 4-263.3±1.51.960.954.8±0.22.480.03///4.4±0.15.690.124.8±0.12.270.13C Z 4-3313.4±0.81.901.637.0±0.114.250.01///2.6±0.14.260.087.0±0.12.370.15C Z 4-4848.7±1.11.891.952.5±0.118.600.01///3.7±0.11.980.102.3±0.13.130.08C Z 3-1927.7±0.61.912.0430.4±0.810.840.47///3.2±0.12.040.115.2±0.02.170.14C Z 3-2471.4±0.91.901.865.2±0.12.450.01///N AN AN AN AN AN AC Z 1-1558.0±1.71.891.885.0±0.12.940.01///N AN AN AN AN AN AC Z 1-2973.1±3.21.911.9211.8±0.32.930.02///N AN AN AN AN AN AT H 11026.8±1.01.892.0138.5±0.619.390.795.3±0.22.390.014.3±0.12.550.125.2±0.12.130.12T H 2665.8±1.31.871.8559.1±0.729.311.3510.9±0.12.800.01N AN AN AN AN AN AN o t e s : N A d e n o t e s n o D N A w a s e x t r a c t e d f r o m t h e o i l s a m p l e s ; / d e n o t e s W i z a r d M a g n e t i c m e t h o d w a s n o t u s e d o n r a p e s e e d o i l s a m p l e s .129Li et al . / Oil Crop Science 2018, 3 (2): 122-136DNA extraction methods for commercial vegetable oils T a b l e 5. R e a l -t i m e P C R r e s u l t s f o r D N A a m p l i fi c a t i o n f r o m v e g e t a b l e o i l e x t r a c t s u s i n g m o d i fi e d e m u l s i fi c a t i o n m e t h o dS a m p l et R N A -L e u C r u AL e c t i nz S S Ⅱb A r a h 2H a G 5S a d 1M T 3-BP C R (+/T o t a l )C tP C R (+/T o t a l )C tP C R (+/T o t a l )C t P C R (+/T o t a l )C t P C R (+/T o t a l )C t P C R (+/T o t a l )C t P C R (+/T o t a l )C t P C R (+/T o t a l )C t D D 3-14/431.60±0.884/438.82±1.650/4N A //////0/4N A 0/4N AD D 3-24/431.31±0.362/438.74±0.341/436.52±0.01//////1/437.47 ±0.010/4N AD D 1-14/430.82±0.753/439.61±3.860/4N A //////0/4N A 0/4N AD D 1-24/431.63±0.390/4N A0/4N A //////0/4N A 0/4N AD D 1-34/431.16±0.351/437.82±0.011/437.94±0.01//////2/437.54±0.570/4N AH S 1-14/432.73±0.582/439.49±0.721/437.53±0.01//0/4N A //0/4N A0/4N AH S 1-23/433.08±0.371/437.96±0.010/4N A //0/4N A //0/4N A0/4N AH S 1-34/433.38±1.050/4N A0/4N A //0/4N A //1/438.29 ±0.010/4N AH S 1-42/432.12±0.221/438.10±0.010/4N A //0/4N A //0/4N A0/4N AY M 1-14/432.89±0.202/440.91±0.010/4N A 1/437.71±0.01////0/4N A0/4N AY M 1-24/428.9±0.084/438.26±0.010/4N A 2/438.27±0.09////3/438.93±0.500/4N AY M 1-32/434.72±0.440/4N A0/4N A 2/440.42±1.23////0/4N A0/4N AY M 1-44/432.79±0.490/4N A0/4N A 4/438.37±0.49////0/4N A0/4N AK H 1-14/431.77±3.513/439.24±0.980/4N A ////0/4N A0/4N A0/4N AK H 1-24/431.63±0.412/436.97±1.300/4N A ////0/4N A0/4N A0/4N AC Z 4-14/431.11±0.222/438.62±0.500/4N A //////0/4N A0/4N AC Z 4-24/432.75±0.380/4N A0/4N A //////0/4N A0/4N AC Z 4-34/433.26±0.220/4N A0/4N A //////0/4N A0/4N AC Z 4-44/432.08±0.780/4N A0/4N A //////2/439.01±0.780/4N AC Z 3-14/431.25±0.191/438.57±0.010/4N A //////1/439.37 ±0.010/4N AC Z 3-24/433.14±3.100/4N A0/4N A //////3/437.00±0.870/4N AC Z 1-14/431.81±0.471/438.02±0.010/4N A //////0/4N A0/4N AC Z 1-24/430.64±0.660/4N A0/4N A //////3/437.03±0.680/4N AT H 14/432.75±0.280/4N A0/4N A 2/437.38±0.420/4N A0/4N A1/438.34 ±0.010/4N AT H 24/429.14±0.284/438.28±0.650/4N A 3/437.78±0.500/4N A0/4N A2/437.95±0.500/4N AM e a n 31.94±1.5538.63±1.4737.33±0.7338.32±1.06N AN A38.09±0.98N AN O D 25/2515/253/256/60/60/410/250/25A c c o u n t 59/141N o t e s : P C R (+/T o t a l ) i n d i c a t e s n u m b e r o f p o s i t i v e P C R r e p l i c a t e s i n t o t a l n u m b e r o f r e p l i c a t e s ; C t i n m e a n ± s t a n d a r d d e v i a t i o n ; N A d e n o t e s n o d e t e c t a b l e a m p l i fi c a t i o n ; / d e n o t e s n o d e t e c t i o n o f t h i s p a r a m e t e r ; N O D d e n o t e s n u m b e r o f a m p l i fi e d C t v a l u e s p e r t o t a l a m p l i fi c a t i o n n u m b e r s f o r e a c h g e n e ; A c c o u n t i s t o t a l n u m b e r o f a m p l i fi e d C t v a l u e s p e r t o t a l a m p l i fi c a t i o n n u m b e r s f o r a l l g e n e s .。
杠杆表测杆长短与转角对测量误差的影响

杠杆表主要用于工件的形状、位置和尺寸误差的测 量ꎬ是机械加工中常用的精密量具之一ꎬ因其结构小巧、
和齿轮 z1、z2、z3、z4 作为传动和放大机构的机械量仪ꎬ 也是杠杆表中最常用的杠杆齿轮式四级传动机构ꎮ
杠杆测头(以下简称测杆) 可任意扳转、使用灵活、操作
1 杠杆表机构与原理的误差分析
K左
=
(
b-c ac
) Z1Z3 Z2Z4
R
(1)
1������ 1 杠杆表的传动机构 由图 1 可知ꎬ杠杆表的机构是利用杠杆 a、b、c、d
K右
=
(
b+d ad
) Z1Z3 Z2Z4
R
(2)
������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������������
杠杆表测杆长短与转角对测量误差的影响
张 帆 田俊成
( 陕西工业职业技术学院ꎬ陕西 咸阳 712000)
摘 要: 针对杠杆表存在不可避免的原理误差ꎬ介绍了常用杠杆表的机构与原理ꎮ 并对杠杆表测杆长度、使 用时测杆报转的角度进行了分析计算ꎬ给出了不同的角度测量时误差修正值系数表ꎬ以及正确的使 用方法和应注意的问题ꎬ对提高检测效率和测量精度具有很高的参考价值ꎮ
撤机困难病人吸气肌训练的最佳证据总结

撤机困难病人吸气肌训练的最佳证据总结杨荟晶1,吕慧颐2*,杜婧2,吴亚文2,杨萍2,米同舟1,薛俊玲11.山西医科大学护理学院,山西 030001;2.山西医科大学第一医院Best evidence summary for inspiratory muscle training in patients with difficult weaningYANG Huijing, LYU Huiyi, DU Jing, WU Yawen, YANG Ping, MI Tongzhou, XUE JunlingNursing College of Shanxi Medical University, Shanxi 030001 ChinaCorresponding Author LYUHuiyi,E⁃mail:*********************Abstract Objective:To select, evaluate, and summarize the best evidence for inspiratory muscle training in patients with difficult weaning. Methods:Relevant content on inspiratory muscle training in patients with difficult weaning was systematically searched from March 31,2010 to March 31,2023.Evidence extraction and integration were carried out after evaluating the quality of literature.Results:A total of 11 articles were included,including 3 clinical decisions,1 guideline,4 expert consensuses,1 systematic evaluation,and 2 randomized controlled trials.Finally,the best evidence summary for inspiratory muscle training in patients with difficult weaning was formed,including 8 aspects (training objectives,respiratory assessment,training team,training population,training methods,training intensity,frequency and time,pause indicators and monitoring) with 22 recommended opinions.Conclusions:The best evidence summary for inspiratory muscle training in patients with difficult weaning summarized in this study can be applied individually and in a targeted manner by healthcare professionals in relation to clinical scenarios and patients' wishes,thus improving the success rate of weaning.Keywords difficult weaning; inspiratory muscle training; evidence summary; evidence⁃based nursing摘要目的:遴选、评价并归纳撤机困难病人吸气肌训练的最佳证据。
基于LSTM-CNN-CBAM模型的股票预测研究

2021,57(3) 203
基于 LSTM-CNN-CBAM 模型的股票预测研究
赵红蕊,薛 雷 上海大学 通信与信息工程学院,上海 200444
摘 要:为了更好地对股票价格进行预测 ,进而为股民提供合理化的建议 ,提出了一种在结合长短期记忆网络 (LSTM)和卷积神经网络(CNN)的基础上引入注意力机制的股票预测混合模型(LSTM-CNN-CBAM),该模型采用 的是端到端的网络结构,使用 LSTM 来提取数据中的时序特征,利用 CNN 挖掘数据中的深层特征,通过在网络结构 中加入注意力机制——Convolutional Attention Block Module(CBAM)卷积模块,可以有效地提升网络的特征提取 能力。基于上证指数进行对比实验,通过对比实验预测结果和评价指标,验证了在 LSTM 与 CNN 结合的网络模型中 加入 CBAM 模块的预测有效性和可行性。 关键词:长短期记忆网络(LSTM);卷积神经网络(CNN);注意力机制 ;股价预测 文献标志码:A 中图分类号:TP29 doi:10.3778/j.issn.1002-8331.1912-0448
1 LSTM 的结构和原理介绍
LSTM 是为了解决循环神经网络[12(] RNN)模型由
于输入序列过长而产生的梯度消失[13]问题而发展出来
的一种机器学习神经网络,主要由记忆细胞、输入门、输
出门、遗忘门组成,三个门的激活函数均为 Sigmoid。输
入门用来控制当前时刻神经单元的输入信息,遗忘门
用来控制上一时刻神经单元中存储的历史信息,输出门
赵红蕊,等:基于 LSTM-CNN-CBAM 模型的股票预测研究
成骨细胞源性外泌体的分离提取和鉴定

-1108-宁夏医学杂志2020年12月第42卷第12期Ningxia Med J,Dec.2020,Vol.42,No.12 Doi:10.13621/j.1001-5949.2020.12.1108•实验研究•成骨细胞源性外泌体的分离提取和鉴定蔡则成】,杨绍兵2,马荣3,张彦龙】,梁思敏3[摘要]目的分离成骨细胞来源的外泌体,并对其进行鉴定。
方法体外培养成骨细胞,用差速离心法分离、提取成骨细胞分泌的外泌体,经透射电子显微镜观察其形态及大小,采用蛋白质印迹法(WB)检测外泌体跨膜蛋白CD9和成骨细胞特异性碱性磷酸酶(AKP)的表达。
结果成骨细胞源性外泌体为直径30-100nm的圆形或椭圆形结构,高表达CD9和AKP蛋白。
结论差速离心法提取的成骨细胞源性外泌体,具有外泌体的一般特性,并高表达成骨细胞特异性蛋白AKP。
[关键词]结核分枝杆菌;外泌体;成骨细胞[中图分类号]Q2-33[文献标识码]AIsolation,extraction and identification of exosomes derived from osteoblasts CAI Zecheng,Y ANG Shaobing2,MA Rong3,ZHANG Yanlong1,LIANG Simin3. 1.Ningxia Medical University,Yinchun750004,China Department of Cardiology,General Hospital of Ningxia Medical University,Y inchun750004,C hina;3.Department of Orthopaedics,General Hospital of Ningxia Medical University,Y inchun750004,C hinaCorresponding author:L IANG Simin,Email:148376463@[Abstract]Objective The exosomes from osteoblasts were isolated and identified.Methods Osteoblasts were cultured in vitro.The exosomes secreted from osteoblasts were separated and extracted by differential centrifugation.The morphology and size of exosomes were observed by transmission electron microscopy.The expression of CD9and alkaline phosphatase in osteoblasts were detected by Western blotting.Results The exosomes of osteoblasts were round or oval structures with a diameter of30〜100nm,which highly expressed CD9and AKP protein.Conclusion The osteoblast derived from exosomes have the general characteristics of exosomes and highly express the osteoblast specific protein AKP.[Key words]Mycobacterium tuberculosis;E xosomes;Osteoblasts脊柱结核是由结核分枝杆菌引起的慢性感染性疾病,以进行性骨质破坏为主要病理特征[1],破骨细胞在脊柱结核骨质破坏的发生发展中起着极其重要的作用。
益肾固精暖脐贴制备工艺的研究
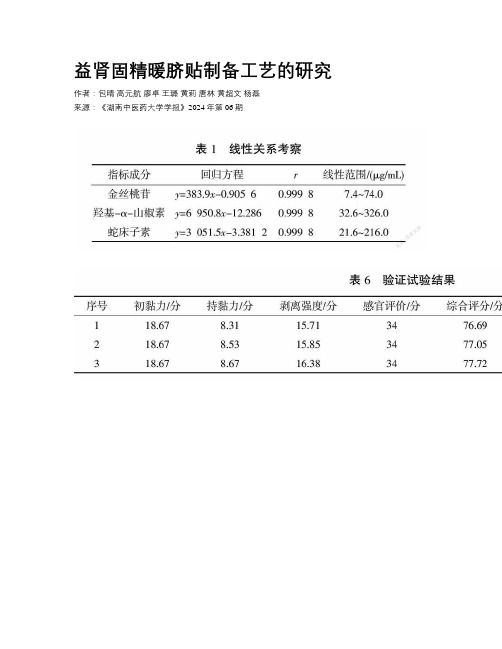
益肾固精暖脐贴制备工艺的研究作者:包晴高元航廖卓王璐黄莉唐林黄超文杨磊来源:《湖南中医药大学学报》2024年第06期〔摘要〕目的研究益肾固精暖脐贴的提取工艺及其成型工艺。
方法以蛇床子素、羟基-α-山椒素、金丝桃苷以及浸膏得率为考察指标,以提取时间、提取溶媒倍数、乙醇浓度为考察因素,运用Box-Behnken设计-响应面法优选出最佳提取工艺;以凝胶贴膏的初黏力、持黏力、剥离强度、感官评价为指标,采用D-最优混料设计优选益肾固精暖脐贴的最佳成型工艺。
结果益肾固精暖脐贴的最佳提取工艺为回流提取120 min,提取溶媒倍数为12,乙醇浓度为57%,同法提取2次;最佳成型工艺的质量比为NP700占6.00%、PVP-k90占0.60%、甘羥铝占0.15%、填充剂5.76%、EDTA-2Na占0.10%、甘油占21.76%、柠檬酸占0.20%、水和药液总量占65.43%。
结论该实验优选的益肾固精暖脐贴的提取工艺及其凝胶贴膏的制备工艺稳定可行,可为该产品的进一步开发利用提供参考。
〔关键词〕益肾固精暖脐贴;高效液相色谱法;Box-Behnken-设计响应面法;D-最优混料设计;凝胶贴膏〔中图分类号〕R283.6 〔文献标志码〕A 〔文章编号〕doi:10.3969/j.issn.1674-070X.2024.06.012Preparation process of Yishen Gujing Nuanqi PatchBAO Qing1, GAO Yuanhang1, LIAO Zhuo1, WANG Lu2, HUANG Li2,TANG Lin2, HUANG Chaowen3, YANG Lei2*1. The First Clinical School of Chinese Medicine, Hunan University of Chinese Medicine,Changsha, Hunan 410007, China;2. The First Hospital of Hunan University of Chinese Medicine, Changsha, Hunan 410007, China;3. The Hospital of Hunan Academy of Chinese Medicine, Changsha, Hunan 410006, China〔Abstract〕 Objective To study the extraction process and molding process of Yishen Gujing Nuanqi Patch (YSGJNQP). Methods Using osthole, hydroxy-α-sanshool,hyperoside, and extract yield as evaluation indicators, and extraction time, extraction solvent ratio, and ethanol concentration as factors, the Box-Behnken response surface method was used to optimize the optimal extraction process. Taking the initial adhesion, holding adhesion, peeling strength, and sensory evaluation of the gel patch as indicators, the D-optimal mixture design was used to optimize the optimal molding process for YSGJNQP. Results The optimal extraction process for YSGJNQP was reflux extraction for 120 minutes, with an extraction solvent ratio of 12 and an ethanol concentration of 57%, repeated twice using the same method. The optimal molding process had a mass ratio of 6.00% NP700, 0.60% PVP-k90, 0.15% glycoxyaluminium, 5.76% filler,0.10% EDTA-2Na, 21.76% glycerin, 0.20% citric acid, and 65.43% total amount of water and medicinal solution. Conclusion The optimized extraction process and gel patch preparation process forYSGJNQP in the study are stable and feasible, providing a reference for further development and utilization of this product.〔Keywords〕 Yishen Gujing Nuanqi Patch; high performance liquid chromatography; Box-Behnken response surface method; D-optimal mixture design; gel paste固精益肾暖脐膏出自《摄生秘剖》卷四,主要由花椒、蛇床子、韭菜子、肉桂、母丁香、麝香、附子、硫黄组成,用于治疗男子精寒,阳事痿弱,举而不坚,坚而不久,白浊遗精等症[1]。
